User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
How can we make medical training less ‘toxic’?
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me to discuss ways to address and reform the toxic culture associated with medical training is Dr. Amy Faith Ho, senior vice president of clinical informatics and analytics at Integrative Emergency Services in Dallas. Also joining us is Dr. Júlia Loyola Ferreira, a pediatric surgeon originally from Brazil, now practicing at Montreal Children’s and focused on advocacy for gender equity and patient-centered care.
Welcome to both of you. Thanks so much for joining me.
Amy Faith Ho, MD, MPH: Thanks so much for having us, Rob.
Dr. Glatter: Amy, I noticed a tweet recently where you talked about how your career choice was affected by the toxic environment in medical school, affecting your choice of residency. Can you elaborate on that?
Dr. Ho: In this instance, what we’re talking about is gender, but it can be directed toward any number of other groups as well.
What you’re alluding to is a tweet by Stanford Surgery Group showing the next residency class, and what was really stunning about this residency class was that it was almost all females. And this was something that took off on social media.
When I saw this, I was really brought back to one of my personal experiences that I chose to share, which was basically that, as a medical student, I really wanted to be a surgeon. I’m an emergency medicine doctor now, so you know that didn’t happen.
The story that I was sharing was that when I was a third-year medical student rotating on surgery, we had a male attending who was very well known at that school at the time who basically would take the female medical students, and instead of clinic, he would round us up. He would have us sit around him in the workplace room while everyone else was seeing patients, and he would have you look at news clippings of himself. He would tell you stories about himself, like he was holding court for the ladies.
It was this very weird culture where my takeaway as a med student was like, “Wow, this is kind of abusive patriarchy that is supported,” because everyone knew about it and was complicit. Even though I really liked surgery, this was just one instance and one example of where you see this culture that really resonates into the rest of life that I didn’t really want to be a part of.
I went into emergency medicine and loved it. It’s also highly procedural, and I was very happy with where I was. What was really interesting about this tweet to me, though, is that it really took off and garnered hundreds of thousands of views on a very niche topic, because what was most revealing is that everyone has a story like this.
It is not just surgery. It is definitely not just one specialty and it is not just one school. It is an endemic problem in medicine. Not only does it change the lives of young women, but it also says so much about the complicity and the culture that we have in medicine that many people were upset about just the same way I was.
Medical training experience in other countries vs. the United States
Dr. Glatter: Júlia, I want to hear about your experience in medical school, surgery, and then fellowship training and up to the present, if possible.
Júlia Loyola Ferreira, MD: In Brazil, as in many countries now, women have made up the majority of the medical students since 2010. It’s a more female-friendly environment when you’re going through medical school, and I was lucky enough to do rotations in areas of surgery where people were friendly to women.
I lived in this tiny bubble that also gave me the privilege of not facing some things that I can imagine that people in Brazil in different areas and smaller towns face. In Brazil, people try to not talk about this gender agenda. This is something that’s being talked about outside Brazil. But in Brazil, we are years back. People are not really engaging on this conversation. I thought it was going to be hard for me as a woman, because Brazil has around 20% female surgeons.
I knew it was going to be challenging, but I had no idea how bad it was. When I started and things started happening, the list was big. I have an example of everything that is written about – microaggression, implicit bias, discrimination, harassment.
Every time I would try to speak about it and talk to someone, I would be strongly gaslighted. It was the whole training, the whole 5 years. People would say, “Oh, I don’t think it was like that. I think you were overreacting.” People would come with all these different answers for what I was experiencing, and that was frustrating. That was even harder because I had to cope with everything that was happening and I had no one to turn to. I had no mentors.
When I looked up to women who were in surgery, they would be tougher on us young surgeons than the men and they would tell us that we should not complain because in their time it was even harder. Now, it’s getting better and we are supposed to accept whatever comes.
That was at least a little bit of what I experienced in my training. It was only after I finished and started to do research about it that I really encountered a field of people who would echo what I was trying to say to many people in different hospitals that I attended to.
That was the key for me to get out of that situation of being gaslighted and of not being able to really talk about it. Suddenly, I started to publish things about Brazil that nobody was even writing or studying. That gave me a large amount of responsibility, but also motivation to keep going and to see the change.
Valuing women in medicine
Dr. Glatter: This is a very important point that you’re raising about the environment of women being hard on other women. We know that men can be very difficult on and also judgmental toward their trainees.
Amy, how would you respond to that? Was your experience similar in emergency medicine training?
Dr. Ho: I actually don’t feel like it was. I think what Júlia is alluding to is this “mean girls” idea, of “I went through it and thus you have to go through it.” I think you do see this in many specialties. One of the classic ones we hear about, and I don’t want to speak to it too much because it’s not my specialty, is ob.gyn., where it is a very female-dominant surgery group. There’s almost a hazing level that you hear about in some of the more malignant workplaces.
I think that you speak to two really important things. Number one is the numbers game. As you were saying, Brazil actually has many women. That’s awesome. That’s actually different from the United States, especially for the historic, existing workplace and less so for the medical students and for residents. I think step one is having minorities like women just present and there.
Step two is actually including and valuing them. While I think it’s really easy to move away from the women discussion, because there are women when you look around in medicine, it doesn’t mean that women are actually being heard, that they’re actually being accepted, or that their viewpoints are being listened to. A big part of it is normalizing not only seeing women in medicine but also normalizing the narrative of women in medicine.
It’s not just about motherhood; it’s about things like normalizing talking about advancement, academic promotions, pay, culture, being called things like “too reactive,” “anxious,” or “too assertive.” These are all classic things that we hear about when we talk about women.
That’s why we’re looking to not only conversations like this, but also structured ways for women to discuss being women in medicine. There are many women in medicine groups in emergency medicine, including: Females Working in Emergency Medicine (FemInEM); the American College of Emergency Physicians (ACEP) and Society for Academic Emergency Medicine (SAEM) women’s groups, which are American Association of Women Emergency Physicians (AAWEP) and Academy for Women in Academic Emergency Medicine (AWAEM), respectively; and the American Medical Women’s Association (AMWA), which is the American Medical Association’s offshoot.
All of these groups are geared toward normalizing women in medicine, normalizing the narrative of women in medicine, and then working on mentoring and educating so that we can advance our initiatives.
Gender balance is not gender equity
Dr. Glatter: Amy, you bring up a very critical point that mentoring is sort of the antidote to gender-based discrimination. Júlia had written a paper back in November of 2022 that was published in the Journal of Surgical Research talking exactly about this and how important it is to develop mentoring. Part of her research showed that about 20% of medical students who took the survey, about 1,000 people, had mentors, which was very disturbing.
Dr. Loyola Ferreira: Mentorship is one of the ways of changing the reality about gender-based discrimination. Amy’s comment was very strong and we need to really keep saying it, which is that gender balance is not gender equity.
The idea of having more women is not the same as women being recognized as equals, as able as men, and as valued as men. To change this very long culture of male domination, we need support, and this support comes from mentorship.
Although I didn’t have one, I feel that since I started being a mentor for some students, it changed not only them but myself. It gave me strength to keep going, studying, publishing, and going further with this discussion. I feel like the relationship was as good for them as it is for me. That’s how things change.
Diversity, equity, and inclusion training
Dr. Glatter: We’re talking about the reality of gender equity in terms of the ability to have equal respect, recognition, opportunities, and access. That’s really an important point to realize, and for our audience, to understand that gender equity is not gender balance.
Amy, I want to talk about medical school curriculums. Are there advances that you’re aware of being made at certain schools, programs, even in residencies, to enforce these things and make it a priority?
Dr. Ho: We’re really lucky that, as a culture in the United States, medical training is certainly very geared toward diversity. Some of that is certainly unofficial. Some of that just means when they’re looking at a medical school class or looking at rank lists for residency, that they’re cognizant of the different backgrounds that people have. That’s still a step. That is a step, that we’re at least acknowledging it.
There are multiple medical schools and residencies that have more formal unconscious-bias training or diversity, equity, and inclusion (DEI) training, both of which are excellent not only for us in the workplace but also for our patients. Almost all of us will see patients of highly diverse backgrounds. I think the biggest push is looking toward the criteria that we use for selecting trainees and students into our programs. Historically, it’s been MCAT, GPA, and so on.
We’ve really started to ask the question of, are these sorts of “objective criteria” actually biased in institutional ways? They talk about this all the time where GPAs will bias against students from underrepresented minorities (URM). I think all medical students and residencies have really acknowledged that. Although there are still test cutoffs, we are putting an inquisitive eye to what those mean, why they exist, and what are the other things that we should consider. This is all very heartening from what I’m seeing in medical training.
Dr. Glatter: There’s no formal rating system for DEI curriculums right now, like ranking of this school, or this program has more advanced recognition in terms of DEI?
Dr. Ho: No, but on the flip side, the U.S. News & World Report was classically one of the major rankings for medical schools. What we saw fairly recently was that very high-tier schools like Harvard and University of Chicago pulled out of that ranking because that ranking did not acknowledge the value of diversity. That was an incredible stance for medical schools to take, to say, “Hey, you are not evaluating an important criterion of ours.”
Dr. Glatter: That’s a great point. Júlia, where are we now in Brazil in terms of awareness of DEI and curriculum in schools and training programs?
Dr. Loyola Ferreira: Our reality is not as good as in the U.S., unfortunately. I don’t see much discussion on residency programs or medical schools at the moment. I see many students bringing it out and trying to make their schools engage in that discussion. This is something that is coming from the bottom up and not from the top down. I think it can lead to change as well. It is a step and it’s a beginning. Institutions should take the responsibility of doing this from the beginning. This is something where Brazil is still years behind you guys.
Dr. Glatter: It’s unfortunate, but certainly it’s important to hear that. What about in Canada and certainly your institution, McGill, where you just completed a master’s degree?
Dr. Loyola Ferreira: Canada is very much like the U.S. This is something that is really happening and it’s happening fast. I see, at least at McGill, a large amount of DEI inclusion and everything on this discussion. They have institutional courses for us to do as students, and we are all obliged to do many courses, which I think is really educating, especially for people with different cultures and backgrounds.
Dr. Glatter: Amy, where do you think we are in emergency medicine to look at the other side of it? Comparing surgery with emergency medicine, do you think we’re well advanced in terms of DEI, inclusion criteria, respect, and dignity, or are we really far off?
Dr. Ho: I may be biased, but I think emergency medicine is one of the best in terms of this, and I think there are a couple of reasons for it. One is that we are an inherently team-based organization. The attending, the residents, and the students all work in line with one another. There’s less of a hierarchy.
The same is true for our nurses, pharmacists, techs, and EMS. We all work together as a team. Because of that fairly flat structure, it’s really easy for us to value one another as individuals with our diverse backgrounds. In a way, that’s harder for specialties that are more hierarchical, and I think surgery is certainly one of the most hierarchical.
The second reason why emergency medicine is fairly well off in this is that we’re, by nature, a safety-net specialty. We see patients of all-comers, all walks, all backgrounds. I think we both recognize the value of physician-patient concordance. When we share characteristics with our patients, we recognize that value immediately at the bedside.
It exposes us to so much diversity. I see a refugee one day and the next patient is someone who is incarcerated. The next patient after that is an important businessman in society. That diversity and whiplash in the type of patients that we see back-to-back helps us see the playing field in a really flat, diverse way. Because of that, I think our culture is much better, as is our understanding of the value and importance of diversity not only for our programs, but also for our patients.
Do female doctors have better patient outcomes?
Dr. Glatter: Specialties working together in the emergency department is so important. Building that team and that togetherness is so critical. Júlia, would you agree?
Dr. Loyola Ferreira: Definitely. Something Amy said that is beautiful is that you recognize yourself in these patients. In surgery, we are taught to try to be away from the patients and not to put ourselves in the same position. We are taught to be less engaging, and this is not good. The good thing is when we really have patient-centered care, when we listen to them, and when we are involved with them.
I saw a publication showing that female and male surgeons treating similar patients had the same surgical outcomes. Women are as good as men technically to do surgery and have the same surgical outcomes. However, there is research showing that surgical teams with greater representation of women have improved surgical outcomes because of patient-centered care and the way women conduct bedside attention to patients. And they have better patient experience measures afterward. That is not only from the women who are treating the patients, but the whole environment. Women end up bringing men [into the conversation] and this better improves patient-centered care, and that makes the whole team a better team attending patients. Definitely, we are in the moment of patient experience and satisfaction, and increasing women is a way of achieving better patient satisfaction and experience.
Dr. Ho: There’s much to be said about having female clinicians available for patients. It doesn’t have to be just for female patients, although again, concordance between physicians and patients is certainly beneficial. Besides outcomes benefit, there’s even just a communication benefit. The way that women and men communicate is inherently different. The way women and men experience certain things is also inherently different.
A classic example of this is women who are experiencing a heart attack may not actually have chest pain but present with nausea. As a female who’s sensitive to this, when I see a woman throwing up, I am very attuned to something actually being wrong, knowing that they may not present with classic pain for a syndrome, but actually may be presenting with nausea instead. It doesn’t have to be a woman who takes that knowledge and turns it into something at the bedside. It certainly doesn’t have to, but it is just a natural, easy thing to step into as a female.
While I’m really careful to not step into this “women are better than men” or “men are better than women” argument, there’s something to be said about how the availability of female clinicians for all patients, not just female patients, can have benefit. Again, it’s shown in studies with cardiovascular outcomes and cardiologists, it’s certainly shown in ob.gyn., particularly for underrepresented minorities as well for maternal outcomes of Black mothers. It’s certainly shown again in patient satisfaction, which is concordance.
There is a profound level of research already on this that goes beyond just the idea of stacking the bench and putting more women in there. That’s not the value. We’re not just here to check off the box. We’re here to actually lend some value to our patients and, again, to one another as well.
Dr. Glatter: Absolutely. These are excellent points. The point you make about patient presentation is so vital. The fact that women have nausea sometimes in ACS presentations, the research never was really attentive to this. It was biased. The symptoms that women may have that are not “typical” for ACS weren’t included in patient presentations. Educating everyone about, overall, the types of presentations that we can recognize is vital and important.
Dr. Ho: Yes. It’s worth saying that, when you look at how medicine and research developed, classically, who were the research participants? They were often White men. They were college students who, historically, because women were not allowed to go to college, were men.
I say that not to fault the institution, because that was the culture of our history, but to just say it is okay to question things. It is okay to realize that someone’s presenting outside of the box and that maybe we actually need to reframe what even created the walls of the box in the first place.
Dr. Glatter: Thank you again for joining us. I truly appreciate your insight and expertise.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, Hofstra/Northwell, New York. Dr. Ho is senior vice president of clinical informatics & analytics, department of emergency medicine, Integrative Emergency Services, Dallas. Dr. Loyola Ferreira is a master of science candidate, department of experimental surgery, McGill University, Montreal. They reported that they had no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me to discuss ways to address and reform the toxic culture associated with medical training is Dr. Amy Faith Ho, senior vice president of clinical informatics and analytics at Integrative Emergency Services in Dallas. Also joining us is Dr. Júlia Loyola Ferreira, a pediatric surgeon originally from Brazil, now practicing at Montreal Children’s and focused on advocacy for gender equity and patient-centered care.
Welcome to both of you. Thanks so much for joining me.
Amy Faith Ho, MD, MPH: Thanks so much for having us, Rob.
Dr. Glatter: Amy, I noticed a tweet recently where you talked about how your career choice was affected by the toxic environment in medical school, affecting your choice of residency. Can you elaborate on that?
Dr. Ho: In this instance, what we’re talking about is gender, but it can be directed toward any number of other groups as well.
What you’re alluding to is a tweet by Stanford Surgery Group showing the next residency class, and what was really stunning about this residency class was that it was almost all females. And this was something that took off on social media.
When I saw this, I was really brought back to one of my personal experiences that I chose to share, which was basically that, as a medical student, I really wanted to be a surgeon. I’m an emergency medicine doctor now, so you know that didn’t happen.
The story that I was sharing was that when I was a third-year medical student rotating on surgery, we had a male attending who was very well known at that school at the time who basically would take the female medical students, and instead of clinic, he would round us up. He would have us sit around him in the workplace room while everyone else was seeing patients, and he would have you look at news clippings of himself. He would tell you stories about himself, like he was holding court for the ladies.
It was this very weird culture where my takeaway as a med student was like, “Wow, this is kind of abusive patriarchy that is supported,” because everyone knew about it and was complicit. Even though I really liked surgery, this was just one instance and one example of where you see this culture that really resonates into the rest of life that I didn’t really want to be a part of.
I went into emergency medicine and loved it. It’s also highly procedural, and I was very happy with where I was. What was really interesting about this tweet to me, though, is that it really took off and garnered hundreds of thousands of views on a very niche topic, because what was most revealing is that everyone has a story like this.
It is not just surgery. It is definitely not just one specialty and it is not just one school. It is an endemic problem in medicine. Not only does it change the lives of young women, but it also says so much about the complicity and the culture that we have in medicine that many people were upset about just the same way I was.
Medical training experience in other countries vs. the United States
Dr. Glatter: Júlia, I want to hear about your experience in medical school, surgery, and then fellowship training and up to the present, if possible.
Júlia Loyola Ferreira, MD: In Brazil, as in many countries now, women have made up the majority of the medical students since 2010. It’s a more female-friendly environment when you’re going through medical school, and I was lucky enough to do rotations in areas of surgery where people were friendly to women.
I lived in this tiny bubble that also gave me the privilege of not facing some things that I can imagine that people in Brazil in different areas and smaller towns face. In Brazil, people try to not talk about this gender agenda. This is something that’s being talked about outside Brazil. But in Brazil, we are years back. People are not really engaging on this conversation. I thought it was going to be hard for me as a woman, because Brazil has around 20% female surgeons.
I knew it was going to be challenging, but I had no idea how bad it was. When I started and things started happening, the list was big. I have an example of everything that is written about – microaggression, implicit bias, discrimination, harassment.
Every time I would try to speak about it and talk to someone, I would be strongly gaslighted. It was the whole training, the whole 5 years. People would say, “Oh, I don’t think it was like that. I think you were overreacting.” People would come with all these different answers for what I was experiencing, and that was frustrating. That was even harder because I had to cope with everything that was happening and I had no one to turn to. I had no mentors.
When I looked up to women who were in surgery, they would be tougher on us young surgeons than the men and they would tell us that we should not complain because in their time it was even harder. Now, it’s getting better and we are supposed to accept whatever comes.
That was at least a little bit of what I experienced in my training. It was only after I finished and started to do research about it that I really encountered a field of people who would echo what I was trying to say to many people in different hospitals that I attended to.
That was the key for me to get out of that situation of being gaslighted and of not being able to really talk about it. Suddenly, I started to publish things about Brazil that nobody was even writing or studying. That gave me a large amount of responsibility, but also motivation to keep going and to see the change.
Valuing women in medicine
Dr. Glatter: This is a very important point that you’re raising about the environment of women being hard on other women. We know that men can be very difficult on and also judgmental toward their trainees.
Amy, how would you respond to that? Was your experience similar in emergency medicine training?
Dr. Ho: I actually don’t feel like it was. I think what Júlia is alluding to is this “mean girls” idea, of “I went through it and thus you have to go through it.” I think you do see this in many specialties. One of the classic ones we hear about, and I don’t want to speak to it too much because it’s not my specialty, is ob.gyn., where it is a very female-dominant surgery group. There’s almost a hazing level that you hear about in some of the more malignant workplaces.
I think that you speak to two really important things. Number one is the numbers game. As you were saying, Brazil actually has many women. That’s awesome. That’s actually different from the United States, especially for the historic, existing workplace and less so for the medical students and for residents. I think step one is having minorities like women just present and there.
Step two is actually including and valuing them. While I think it’s really easy to move away from the women discussion, because there are women when you look around in medicine, it doesn’t mean that women are actually being heard, that they’re actually being accepted, or that their viewpoints are being listened to. A big part of it is normalizing not only seeing women in medicine but also normalizing the narrative of women in medicine.
It’s not just about motherhood; it’s about things like normalizing talking about advancement, academic promotions, pay, culture, being called things like “too reactive,” “anxious,” or “too assertive.” These are all classic things that we hear about when we talk about women.
That’s why we’re looking to not only conversations like this, but also structured ways for women to discuss being women in medicine. There are many women in medicine groups in emergency medicine, including: Females Working in Emergency Medicine (FemInEM); the American College of Emergency Physicians (ACEP) and Society for Academic Emergency Medicine (SAEM) women’s groups, which are American Association of Women Emergency Physicians (AAWEP) and Academy for Women in Academic Emergency Medicine (AWAEM), respectively; and the American Medical Women’s Association (AMWA), which is the American Medical Association’s offshoot.
All of these groups are geared toward normalizing women in medicine, normalizing the narrative of women in medicine, and then working on mentoring and educating so that we can advance our initiatives.
Gender balance is not gender equity
Dr. Glatter: Amy, you bring up a very critical point that mentoring is sort of the antidote to gender-based discrimination. Júlia had written a paper back in November of 2022 that was published in the Journal of Surgical Research talking exactly about this and how important it is to develop mentoring. Part of her research showed that about 20% of medical students who took the survey, about 1,000 people, had mentors, which was very disturbing.
Dr. Loyola Ferreira: Mentorship is one of the ways of changing the reality about gender-based discrimination. Amy’s comment was very strong and we need to really keep saying it, which is that gender balance is not gender equity.
The idea of having more women is not the same as women being recognized as equals, as able as men, and as valued as men. To change this very long culture of male domination, we need support, and this support comes from mentorship.
Although I didn’t have one, I feel that since I started being a mentor for some students, it changed not only them but myself. It gave me strength to keep going, studying, publishing, and going further with this discussion. I feel like the relationship was as good for them as it is for me. That’s how things change.
Diversity, equity, and inclusion training
Dr. Glatter: We’re talking about the reality of gender equity in terms of the ability to have equal respect, recognition, opportunities, and access. That’s really an important point to realize, and for our audience, to understand that gender equity is not gender balance.
Amy, I want to talk about medical school curriculums. Are there advances that you’re aware of being made at certain schools, programs, even in residencies, to enforce these things and make it a priority?
Dr. Ho: We’re really lucky that, as a culture in the United States, medical training is certainly very geared toward diversity. Some of that is certainly unofficial. Some of that just means when they’re looking at a medical school class or looking at rank lists for residency, that they’re cognizant of the different backgrounds that people have. That’s still a step. That is a step, that we’re at least acknowledging it.
There are multiple medical schools and residencies that have more formal unconscious-bias training or diversity, equity, and inclusion (DEI) training, both of which are excellent not only for us in the workplace but also for our patients. Almost all of us will see patients of highly diverse backgrounds. I think the biggest push is looking toward the criteria that we use for selecting trainees and students into our programs. Historically, it’s been MCAT, GPA, and so on.
We’ve really started to ask the question of, are these sorts of “objective criteria” actually biased in institutional ways? They talk about this all the time where GPAs will bias against students from underrepresented minorities (URM). I think all medical students and residencies have really acknowledged that. Although there are still test cutoffs, we are putting an inquisitive eye to what those mean, why they exist, and what are the other things that we should consider. This is all very heartening from what I’m seeing in medical training.
Dr. Glatter: There’s no formal rating system for DEI curriculums right now, like ranking of this school, or this program has more advanced recognition in terms of DEI?
Dr. Ho: No, but on the flip side, the U.S. News & World Report was classically one of the major rankings for medical schools. What we saw fairly recently was that very high-tier schools like Harvard and University of Chicago pulled out of that ranking because that ranking did not acknowledge the value of diversity. That was an incredible stance for medical schools to take, to say, “Hey, you are not evaluating an important criterion of ours.”
Dr. Glatter: That’s a great point. Júlia, where are we now in Brazil in terms of awareness of DEI and curriculum in schools and training programs?
Dr. Loyola Ferreira: Our reality is not as good as in the U.S., unfortunately. I don’t see much discussion on residency programs or medical schools at the moment. I see many students bringing it out and trying to make their schools engage in that discussion. This is something that is coming from the bottom up and not from the top down. I think it can lead to change as well. It is a step and it’s a beginning. Institutions should take the responsibility of doing this from the beginning. This is something where Brazil is still years behind you guys.
Dr. Glatter: It’s unfortunate, but certainly it’s important to hear that. What about in Canada and certainly your institution, McGill, where you just completed a master’s degree?
Dr. Loyola Ferreira: Canada is very much like the U.S. This is something that is really happening and it’s happening fast. I see, at least at McGill, a large amount of DEI inclusion and everything on this discussion. They have institutional courses for us to do as students, and we are all obliged to do many courses, which I think is really educating, especially for people with different cultures and backgrounds.
Dr. Glatter: Amy, where do you think we are in emergency medicine to look at the other side of it? Comparing surgery with emergency medicine, do you think we’re well advanced in terms of DEI, inclusion criteria, respect, and dignity, or are we really far off?
Dr. Ho: I may be biased, but I think emergency medicine is one of the best in terms of this, and I think there are a couple of reasons for it. One is that we are an inherently team-based organization. The attending, the residents, and the students all work in line with one another. There’s less of a hierarchy.
The same is true for our nurses, pharmacists, techs, and EMS. We all work together as a team. Because of that fairly flat structure, it’s really easy for us to value one another as individuals with our diverse backgrounds. In a way, that’s harder for specialties that are more hierarchical, and I think surgery is certainly one of the most hierarchical.
The second reason why emergency medicine is fairly well off in this is that we’re, by nature, a safety-net specialty. We see patients of all-comers, all walks, all backgrounds. I think we both recognize the value of physician-patient concordance. When we share characteristics with our patients, we recognize that value immediately at the bedside.
It exposes us to so much diversity. I see a refugee one day and the next patient is someone who is incarcerated. The next patient after that is an important businessman in society. That diversity and whiplash in the type of patients that we see back-to-back helps us see the playing field in a really flat, diverse way. Because of that, I think our culture is much better, as is our understanding of the value and importance of diversity not only for our programs, but also for our patients.
Do female doctors have better patient outcomes?
Dr. Glatter: Specialties working together in the emergency department is so important. Building that team and that togetherness is so critical. Júlia, would you agree?
Dr. Loyola Ferreira: Definitely. Something Amy said that is beautiful is that you recognize yourself in these patients. In surgery, we are taught to try to be away from the patients and not to put ourselves in the same position. We are taught to be less engaging, and this is not good. The good thing is when we really have patient-centered care, when we listen to them, and when we are involved with them.
I saw a publication showing that female and male surgeons treating similar patients had the same surgical outcomes. Women are as good as men technically to do surgery and have the same surgical outcomes. However, there is research showing that surgical teams with greater representation of women have improved surgical outcomes because of patient-centered care and the way women conduct bedside attention to patients. And they have better patient experience measures afterward. That is not only from the women who are treating the patients, but the whole environment. Women end up bringing men [into the conversation] and this better improves patient-centered care, and that makes the whole team a better team attending patients. Definitely, we are in the moment of patient experience and satisfaction, and increasing women is a way of achieving better patient satisfaction and experience.
Dr. Ho: There’s much to be said about having female clinicians available for patients. It doesn’t have to be just for female patients, although again, concordance between physicians and patients is certainly beneficial. Besides outcomes benefit, there’s even just a communication benefit. The way that women and men communicate is inherently different. The way women and men experience certain things is also inherently different.
A classic example of this is women who are experiencing a heart attack may not actually have chest pain but present with nausea. As a female who’s sensitive to this, when I see a woman throwing up, I am very attuned to something actually being wrong, knowing that they may not present with classic pain for a syndrome, but actually may be presenting with nausea instead. It doesn’t have to be a woman who takes that knowledge and turns it into something at the bedside. It certainly doesn’t have to, but it is just a natural, easy thing to step into as a female.
While I’m really careful to not step into this “women are better than men” or “men are better than women” argument, there’s something to be said about how the availability of female clinicians for all patients, not just female patients, can have benefit. Again, it’s shown in studies with cardiovascular outcomes and cardiologists, it’s certainly shown in ob.gyn., particularly for underrepresented minorities as well for maternal outcomes of Black mothers. It’s certainly shown again in patient satisfaction, which is concordance.
There is a profound level of research already on this that goes beyond just the idea of stacking the bench and putting more women in there. That’s not the value. We’re not just here to check off the box. We’re here to actually lend some value to our patients and, again, to one another as well.
Dr. Glatter: Absolutely. These are excellent points. The point you make about patient presentation is so vital. The fact that women have nausea sometimes in ACS presentations, the research never was really attentive to this. It was biased. The symptoms that women may have that are not “typical” for ACS weren’t included in patient presentations. Educating everyone about, overall, the types of presentations that we can recognize is vital and important.
Dr. Ho: Yes. It’s worth saying that, when you look at how medicine and research developed, classically, who were the research participants? They were often White men. They were college students who, historically, because women were not allowed to go to college, were men.
I say that not to fault the institution, because that was the culture of our history, but to just say it is okay to question things. It is okay to realize that someone’s presenting outside of the box and that maybe we actually need to reframe what even created the walls of the box in the first place.
Dr. Glatter: Thank you again for joining us. I truly appreciate your insight and expertise.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, Hofstra/Northwell, New York. Dr. Ho is senior vice president of clinical informatics & analytics, department of emergency medicine, Integrative Emergency Services, Dallas. Dr. Loyola Ferreira is a master of science candidate, department of experimental surgery, McGill University, Montreal. They reported that they had no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me to discuss ways to address and reform the toxic culture associated with medical training is Dr. Amy Faith Ho, senior vice president of clinical informatics and analytics at Integrative Emergency Services in Dallas. Also joining us is Dr. Júlia Loyola Ferreira, a pediatric surgeon originally from Brazil, now practicing at Montreal Children’s and focused on advocacy for gender equity and patient-centered care.
Welcome to both of you. Thanks so much for joining me.
Amy Faith Ho, MD, MPH: Thanks so much for having us, Rob.
Dr. Glatter: Amy, I noticed a tweet recently where you talked about how your career choice was affected by the toxic environment in medical school, affecting your choice of residency. Can you elaborate on that?
Dr. Ho: In this instance, what we’re talking about is gender, but it can be directed toward any number of other groups as well.
What you’re alluding to is a tweet by Stanford Surgery Group showing the next residency class, and what was really stunning about this residency class was that it was almost all females. And this was something that took off on social media.
When I saw this, I was really brought back to one of my personal experiences that I chose to share, which was basically that, as a medical student, I really wanted to be a surgeon. I’m an emergency medicine doctor now, so you know that didn’t happen.
The story that I was sharing was that when I was a third-year medical student rotating on surgery, we had a male attending who was very well known at that school at the time who basically would take the female medical students, and instead of clinic, he would round us up. He would have us sit around him in the workplace room while everyone else was seeing patients, and he would have you look at news clippings of himself. He would tell you stories about himself, like he was holding court for the ladies.
It was this very weird culture where my takeaway as a med student was like, “Wow, this is kind of abusive patriarchy that is supported,” because everyone knew about it and was complicit. Even though I really liked surgery, this was just one instance and one example of where you see this culture that really resonates into the rest of life that I didn’t really want to be a part of.
I went into emergency medicine and loved it. It’s also highly procedural, and I was very happy with where I was. What was really interesting about this tweet to me, though, is that it really took off and garnered hundreds of thousands of views on a very niche topic, because what was most revealing is that everyone has a story like this.
It is not just surgery. It is definitely not just one specialty and it is not just one school. It is an endemic problem in medicine. Not only does it change the lives of young women, but it also says so much about the complicity and the culture that we have in medicine that many people were upset about just the same way I was.
Medical training experience in other countries vs. the United States
Dr. Glatter: Júlia, I want to hear about your experience in medical school, surgery, and then fellowship training and up to the present, if possible.
Júlia Loyola Ferreira, MD: In Brazil, as in many countries now, women have made up the majority of the medical students since 2010. It’s a more female-friendly environment when you’re going through medical school, and I was lucky enough to do rotations in areas of surgery where people were friendly to women.
I lived in this tiny bubble that also gave me the privilege of not facing some things that I can imagine that people in Brazil in different areas and smaller towns face. In Brazil, people try to not talk about this gender agenda. This is something that’s being talked about outside Brazil. But in Brazil, we are years back. People are not really engaging on this conversation. I thought it was going to be hard for me as a woman, because Brazil has around 20% female surgeons.
I knew it was going to be challenging, but I had no idea how bad it was. When I started and things started happening, the list was big. I have an example of everything that is written about – microaggression, implicit bias, discrimination, harassment.
Every time I would try to speak about it and talk to someone, I would be strongly gaslighted. It was the whole training, the whole 5 years. People would say, “Oh, I don’t think it was like that. I think you were overreacting.” People would come with all these different answers for what I was experiencing, and that was frustrating. That was even harder because I had to cope with everything that was happening and I had no one to turn to. I had no mentors.
When I looked up to women who were in surgery, they would be tougher on us young surgeons than the men and they would tell us that we should not complain because in their time it was even harder. Now, it’s getting better and we are supposed to accept whatever comes.
That was at least a little bit of what I experienced in my training. It was only after I finished and started to do research about it that I really encountered a field of people who would echo what I was trying to say to many people in different hospitals that I attended to.
That was the key for me to get out of that situation of being gaslighted and of not being able to really talk about it. Suddenly, I started to publish things about Brazil that nobody was even writing or studying. That gave me a large amount of responsibility, but also motivation to keep going and to see the change.
Valuing women in medicine
Dr. Glatter: This is a very important point that you’re raising about the environment of women being hard on other women. We know that men can be very difficult on and also judgmental toward their trainees.
Amy, how would you respond to that? Was your experience similar in emergency medicine training?
Dr. Ho: I actually don’t feel like it was. I think what Júlia is alluding to is this “mean girls” idea, of “I went through it and thus you have to go through it.” I think you do see this in many specialties. One of the classic ones we hear about, and I don’t want to speak to it too much because it’s not my specialty, is ob.gyn., where it is a very female-dominant surgery group. There’s almost a hazing level that you hear about in some of the more malignant workplaces.
I think that you speak to two really important things. Number one is the numbers game. As you were saying, Brazil actually has many women. That’s awesome. That’s actually different from the United States, especially for the historic, existing workplace and less so for the medical students and for residents. I think step one is having minorities like women just present and there.
Step two is actually including and valuing them. While I think it’s really easy to move away from the women discussion, because there are women when you look around in medicine, it doesn’t mean that women are actually being heard, that they’re actually being accepted, or that their viewpoints are being listened to. A big part of it is normalizing not only seeing women in medicine but also normalizing the narrative of women in medicine.
It’s not just about motherhood; it’s about things like normalizing talking about advancement, academic promotions, pay, culture, being called things like “too reactive,” “anxious,” or “too assertive.” These are all classic things that we hear about when we talk about women.
That’s why we’re looking to not only conversations like this, but also structured ways for women to discuss being women in medicine. There are many women in medicine groups in emergency medicine, including: Females Working in Emergency Medicine (FemInEM); the American College of Emergency Physicians (ACEP) and Society for Academic Emergency Medicine (SAEM) women’s groups, which are American Association of Women Emergency Physicians (AAWEP) and Academy for Women in Academic Emergency Medicine (AWAEM), respectively; and the American Medical Women’s Association (AMWA), which is the American Medical Association’s offshoot.
All of these groups are geared toward normalizing women in medicine, normalizing the narrative of women in medicine, and then working on mentoring and educating so that we can advance our initiatives.
Gender balance is not gender equity
Dr. Glatter: Amy, you bring up a very critical point that mentoring is sort of the antidote to gender-based discrimination. Júlia had written a paper back in November of 2022 that was published in the Journal of Surgical Research talking exactly about this and how important it is to develop mentoring. Part of her research showed that about 20% of medical students who took the survey, about 1,000 people, had mentors, which was very disturbing.
Dr. Loyola Ferreira: Mentorship is one of the ways of changing the reality about gender-based discrimination. Amy’s comment was very strong and we need to really keep saying it, which is that gender balance is not gender equity.
The idea of having more women is not the same as women being recognized as equals, as able as men, and as valued as men. To change this very long culture of male domination, we need support, and this support comes from mentorship.
Although I didn’t have one, I feel that since I started being a mentor for some students, it changed not only them but myself. It gave me strength to keep going, studying, publishing, and going further with this discussion. I feel like the relationship was as good for them as it is for me. That’s how things change.
Diversity, equity, and inclusion training
Dr. Glatter: We’re talking about the reality of gender equity in terms of the ability to have equal respect, recognition, opportunities, and access. That’s really an important point to realize, and for our audience, to understand that gender equity is not gender balance.
Amy, I want to talk about medical school curriculums. Are there advances that you’re aware of being made at certain schools, programs, even in residencies, to enforce these things and make it a priority?
Dr. Ho: We’re really lucky that, as a culture in the United States, medical training is certainly very geared toward diversity. Some of that is certainly unofficial. Some of that just means when they’re looking at a medical school class or looking at rank lists for residency, that they’re cognizant of the different backgrounds that people have. That’s still a step. That is a step, that we’re at least acknowledging it.
There are multiple medical schools and residencies that have more formal unconscious-bias training or diversity, equity, and inclusion (DEI) training, both of which are excellent not only for us in the workplace but also for our patients. Almost all of us will see patients of highly diverse backgrounds. I think the biggest push is looking toward the criteria that we use for selecting trainees and students into our programs. Historically, it’s been MCAT, GPA, and so on.
We’ve really started to ask the question of, are these sorts of “objective criteria” actually biased in institutional ways? They talk about this all the time where GPAs will bias against students from underrepresented minorities (URM). I think all medical students and residencies have really acknowledged that. Although there are still test cutoffs, we are putting an inquisitive eye to what those mean, why they exist, and what are the other things that we should consider. This is all very heartening from what I’m seeing in medical training.
Dr. Glatter: There’s no formal rating system for DEI curriculums right now, like ranking of this school, or this program has more advanced recognition in terms of DEI?
Dr. Ho: No, but on the flip side, the U.S. News & World Report was classically one of the major rankings for medical schools. What we saw fairly recently was that very high-tier schools like Harvard and University of Chicago pulled out of that ranking because that ranking did not acknowledge the value of diversity. That was an incredible stance for medical schools to take, to say, “Hey, you are not evaluating an important criterion of ours.”
Dr. Glatter: That’s a great point. Júlia, where are we now in Brazil in terms of awareness of DEI and curriculum in schools and training programs?
Dr. Loyola Ferreira: Our reality is not as good as in the U.S., unfortunately. I don’t see much discussion on residency programs or medical schools at the moment. I see many students bringing it out and trying to make their schools engage in that discussion. This is something that is coming from the bottom up and not from the top down. I think it can lead to change as well. It is a step and it’s a beginning. Institutions should take the responsibility of doing this from the beginning. This is something where Brazil is still years behind you guys.
Dr. Glatter: It’s unfortunate, but certainly it’s important to hear that. What about in Canada and certainly your institution, McGill, where you just completed a master’s degree?
Dr. Loyola Ferreira: Canada is very much like the U.S. This is something that is really happening and it’s happening fast. I see, at least at McGill, a large amount of DEI inclusion and everything on this discussion. They have institutional courses for us to do as students, and we are all obliged to do many courses, which I think is really educating, especially for people with different cultures and backgrounds.
Dr. Glatter: Amy, where do you think we are in emergency medicine to look at the other side of it? Comparing surgery with emergency medicine, do you think we’re well advanced in terms of DEI, inclusion criteria, respect, and dignity, or are we really far off?
Dr. Ho: I may be biased, but I think emergency medicine is one of the best in terms of this, and I think there are a couple of reasons for it. One is that we are an inherently team-based organization. The attending, the residents, and the students all work in line with one another. There’s less of a hierarchy.
The same is true for our nurses, pharmacists, techs, and EMS. We all work together as a team. Because of that fairly flat structure, it’s really easy for us to value one another as individuals with our diverse backgrounds. In a way, that’s harder for specialties that are more hierarchical, and I think surgery is certainly one of the most hierarchical.
The second reason why emergency medicine is fairly well off in this is that we’re, by nature, a safety-net specialty. We see patients of all-comers, all walks, all backgrounds. I think we both recognize the value of physician-patient concordance. When we share characteristics with our patients, we recognize that value immediately at the bedside.
It exposes us to so much diversity. I see a refugee one day and the next patient is someone who is incarcerated. The next patient after that is an important businessman in society. That diversity and whiplash in the type of patients that we see back-to-back helps us see the playing field in a really flat, diverse way. Because of that, I think our culture is much better, as is our understanding of the value and importance of diversity not only for our programs, but also for our patients.
Do female doctors have better patient outcomes?
Dr. Glatter: Specialties working together in the emergency department is so important. Building that team and that togetherness is so critical. Júlia, would you agree?
Dr. Loyola Ferreira: Definitely. Something Amy said that is beautiful is that you recognize yourself in these patients. In surgery, we are taught to try to be away from the patients and not to put ourselves in the same position. We are taught to be less engaging, and this is not good. The good thing is when we really have patient-centered care, when we listen to them, and when we are involved with them.
I saw a publication showing that female and male surgeons treating similar patients had the same surgical outcomes. Women are as good as men technically to do surgery and have the same surgical outcomes. However, there is research showing that surgical teams with greater representation of women have improved surgical outcomes because of patient-centered care and the way women conduct bedside attention to patients. And they have better patient experience measures afterward. That is not only from the women who are treating the patients, but the whole environment. Women end up bringing men [into the conversation] and this better improves patient-centered care, and that makes the whole team a better team attending patients. Definitely, we are in the moment of patient experience and satisfaction, and increasing women is a way of achieving better patient satisfaction and experience.
Dr. Ho: There’s much to be said about having female clinicians available for patients. It doesn’t have to be just for female patients, although again, concordance between physicians and patients is certainly beneficial. Besides outcomes benefit, there’s even just a communication benefit. The way that women and men communicate is inherently different. The way women and men experience certain things is also inherently different.
A classic example of this is women who are experiencing a heart attack may not actually have chest pain but present with nausea. As a female who’s sensitive to this, when I see a woman throwing up, I am very attuned to something actually being wrong, knowing that they may not present with classic pain for a syndrome, but actually may be presenting with nausea instead. It doesn’t have to be a woman who takes that knowledge and turns it into something at the bedside. It certainly doesn’t have to, but it is just a natural, easy thing to step into as a female.
While I’m really careful to not step into this “women are better than men” or “men are better than women” argument, there’s something to be said about how the availability of female clinicians for all patients, not just female patients, can have benefit. Again, it’s shown in studies with cardiovascular outcomes and cardiologists, it’s certainly shown in ob.gyn., particularly for underrepresented minorities as well for maternal outcomes of Black mothers. It’s certainly shown again in patient satisfaction, which is concordance.
There is a profound level of research already on this that goes beyond just the idea of stacking the bench and putting more women in there. That’s not the value. We’re not just here to check off the box. We’re here to actually lend some value to our patients and, again, to one another as well.
Dr. Glatter: Absolutely. These are excellent points. The point you make about patient presentation is so vital. The fact that women have nausea sometimes in ACS presentations, the research never was really attentive to this. It was biased. The symptoms that women may have that are not “typical” for ACS weren’t included in patient presentations. Educating everyone about, overall, the types of presentations that we can recognize is vital and important.
Dr. Ho: Yes. It’s worth saying that, when you look at how medicine and research developed, classically, who were the research participants? They were often White men. They were college students who, historically, because women were not allowed to go to college, were men.
I say that not to fault the institution, because that was the culture of our history, but to just say it is okay to question things. It is okay to realize that someone’s presenting outside of the box and that maybe we actually need to reframe what even created the walls of the box in the first place.
Dr. Glatter: Thank you again for joining us. I truly appreciate your insight and expertise.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, Hofstra/Northwell, New York. Dr. Ho is senior vice president of clinical informatics & analytics, department of emergency medicine, Integrative Emergency Services, Dallas. Dr. Loyola Ferreira is a master of science candidate, department of experimental surgery, McGill University, Montreal. They reported that they had no conflicts of interest.
A version of this article first appeared on Medscape.com.
Acute diffuse rash on trunk
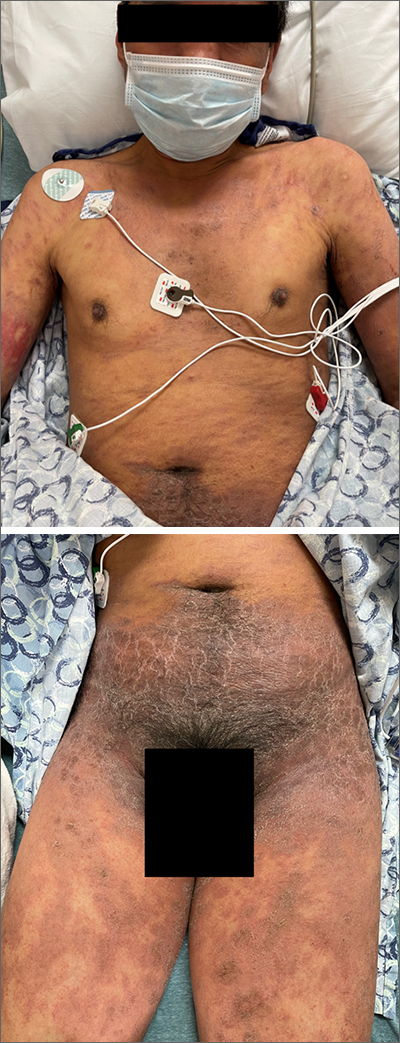
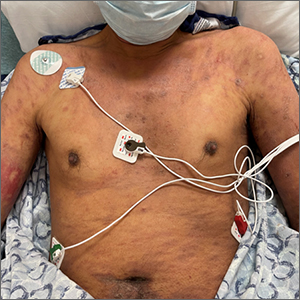
This patient’s diffusely erythematous and scaly rash, in association with recent antibiotic use, was a classic presentation of a drug eruption. Drug eruptions are adverse cutaneous reactions to various medications; they frequently involve antibiotics and anti-epileptics. They can manifest in a multitude of ways with different morphologies. Medication history and timing to onset of symptoms are paramount in making the diagnosis.
Classic reactions include those that are morbilliform (erythematous macules and papules), lichenoid (violaceous and hyperpigmented papules), exfoliative/erythrodermic, and/or urticarial.1 Petechiae and palpable purpura may also manifest.1 Severe reactions, while less common, must always be considered, given their significant morbidity and mortality. These include2:
- Stevens-Johnson syndrome/toxic epidermal necrolysis with diffuse erythema and areas of denuded, necrotic epidermis,
- Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and
- Acute, generalized, exanthematous pustulosis (AGEP) consisting of confluent, nonfollicular pustules.
A general principle in the management of drug eruptions is the discontinuation of the offending drug (if known) as soon as possible. If the agent is not known, it is important to discontinue all drugs that are not deemed as essential, particularly medications that are often associated with reactions, such as antibiotics and anti-epileptics. Additionally, evaluation of the oral mucosa, eyes, and genitourinary tract is helpful to diagnose Stevens-Johnson syndrome, if indicated by symptoms or history.
Wound care with cleansing and covering of denuded skin with emollients and wet dressings should be performed. Infections are common complications in these patients due to the increased inflammation, fissuring, and excoriations that accompany the rash, with sepsis from staphylococcal bacteria being the most concerning complication of infection. Additionally, the compromised skin barrier may lead to heat loss and hypothermia, a compensatory hypermetabolism with hyperthermia, and electrolyte imbalances from insensible water losses.2
Most mild eruptions can be treated with topical corticosteroids and antihistamines. However, in severe eruptions, systemic corticosteroids, or referral for immunosuppressive and anticytokine therapies, also should be considered.1
This patient was treated with both a short course of systemic corticosteroids (prednisone 40 mg/d for 5 days, then tapered over 15 days) and topical steroids (triamcinolone 0.1% ointment bid) for symptomatic care. He also was started on an antihistamine (cetirizine 10 mg bid) for itching. Doxycycline and Augmentin were added to his allergy list. At a 1-week follow up, the patient had near resolution of his rash.
Images courtesy of Jose L. Cortez, MD. Text courtesy of Jose L. Cortez, MD, Department of Dermatology, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
2. Zhang J, Lei Z, Xu C, et al. Current perspectives on severe drug eruption. Clin Rev Allergy Immunol. 2021;61:282-298. doi: 10.1007/s12016-021-08859-0

This patient’s diffusely erythematous and scaly rash, in association with recent antibiotic use, was a classic presentation of a drug eruption. Drug eruptions are adverse cutaneous reactions to various medications; they frequently involve antibiotics and anti-epileptics. They can manifest in a multitude of ways with different morphologies. Medication history and timing to onset of symptoms are paramount in making the diagnosis.
Classic reactions include those that are morbilliform (erythematous macules and papules), lichenoid (violaceous and hyperpigmented papules), exfoliative/erythrodermic, and/or urticarial.1 Petechiae and palpable purpura may also manifest.1 Severe reactions, while less common, must always be considered, given their significant morbidity and mortality. These include2:
- Stevens-Johnson syndrome/toxic epidermal necrolysis with diffuse erythema and areas of denuded, necrotic epidermis,
- Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and
- Acute, generalized, exanthematous pustulosis (AGEP) consisting of confluent, nonfollicular pustules.
A general principle in the management of drug eruptions is the discontinuation of the offending drug (if known) as soon as possible. If the agent is not known, it is important to discontinue all drugs that are not deemed as essential, particularly medications that are often associated with reactions, such as antibiotics and anti-epileptics. Additionally, evaluation of the oral mucosa, eyes, and genitourinary tract is helpful to diagnose Stevens-Johnson syndrome, if indicated by symptoms or history.
Wound care with cleansing and covering of denuded skin with emollients and wet dressings should be performed. Infections are common complications in these patients due to the increased inflammation, fissuring, and excoriations that accompany the rash, with sepsis from staphylococcal bacteria being the most concerning complication of infection. Additionally, the compromised skin barrier may lead to heat loss and hypothermia, a compensatory hypermetabolism with hyperthermia, and electrolyte imbalances from insensible water losses.2
Most mild eruptions can be treated with topical corticosteroids and antihistamines. However, in severe eruptions, systemic corticosteroids, or referral for immunosuppressive and anticytokine therapies, also should be considered.1
This patient was treated with both a short course of systemic corticosteroids (prednisone 40 mg/d for 5 days, then tapered over 15 days) and topical steroids (triamcinolone 0.1% ointment bid) for symptomatic care. He also was started on an antihistamine (cetirizine 10 mg bid) for itching. Doxycycline and Augmentin were added to his allergy list. At a 1-week follow up, the patient had near resolution of his rash.
Images courtesy of Jose L. Cortez, MD. Text courtesy of Jose L. Cortez, MD, Department of Dermatology, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This patient’s diffusely erythematous and scaly rash, in association with recent antibiotic use, was a classic presentation of a drug eruption. Drug eruptions are adverse cutaneous reactions to various medications; they frequently involve antibiotics and anti-epileptics. They can manifest in a multitude of ways with different morphologies. Medication history and timing to onset of symptoms are paramount in making the diagnosis.
Classic reactions include those that are morbilliform (erythematous macules and papules), lichenoid (violaceous and hyperpigmented papules), exfoliative/erythrodermic, and/or urticarial.1 Petechiae and palpable purpura may also manifest.1 Severe reactions, while less common, must always be considered, given their significant morbidity and mortality. These include2:
- Stevens-Johnson syndrome/toxic epidermal necrolysis with diffuse erythema and areas of denuded, necrotic epidermis,
- Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and
- Acute, generalized, exanthematous pustulosis (AGEP) consisting of confluent, nonfollicular pustules.
A general principle in the management of drug eruptions is the discontinuation of the offending drug (if known) as soon as possible. If the agent is not known, it is important to discontinue all drugs that are not deemed as essential, particularly medications that are often associated with reactions, such as antibiotics and anti-epileptics. Additionally, evaluation of the oral mucosa, eyes, and genitourinary tract is helpful to diagnose Stevens-Johnson syndrome, if indicated by symptoms or history.
Wound care with cleansing and covering of denuded skin with emollients and wet dressings should be performed. Infections are common complications in these patients due to the increased inflammation, fissuring, and excoriations that accompany the rash, with sepsis from staphylococcal bacteria being the most concerning complication of infection. Additionally, the compromised skin barrier may lead to heat loss and hypothermia, a compensatory hypermetabolism with hyperthermia, and electrolyte imbalances from insensible water losses.2
Most mild eruptions can be treated with topical corticosteroids and antihistamines. However, in severe eruptions, systemic corticosteroids, or referral for immunosuppressive and anticytokine therapies, also should be considered.1
This patient was treated with both a short course of systemic corticosteroids (prednisone 40 mg/d for 5 days, then tapered over 15 days) and topical steroids (triamcinolone 0.1% ointment bid) for symptomatic care. He also was started on an antihistamine (cetirizine 10 mg bid) for itching. Doxycycline and Augmentin were added to his allergy list. At a 1-week follow up, the patient had near resolution of his rash.
Images courtesy of Jose L. Cortez, MD. Text courtesy of Jose L. Cortez, MD, Department of Dermatology, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
2. Zhang J, Lei Z, Xu C, et al. Current perspectives on severe drug eruption. Clin Rev Allergy Immunol. 2021;61:282-298. doi: 10.1007/s12016-021-08859-0
1. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
2. Zhang J, Lei Z, Xu C, et al. Current perspectives on severe drug eruption. Clin Rev Allergy Immunol. 2021;61:282-298. doi: 10.1007/s12016-021-08859-0
Cell activity in psoriasis may predict disease severity and provide clues to comorbidities
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
FROM SCIENCE IMMUNOLOGY
Does weight loss surgery up the risk for bone fractures?
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Menopause and long COVID: What women should know
British researchers have noted that women at midlife who have long COVID seem to get specific, and severe, symptoms, including brain fog, fatigue, new-onset dizziness, and difficulty sleeping through the night.
Doctors also think it’s possible that long COVID worsens the symptoms of perimenopause and menopause. Lower levels of estrogen and testosterone appear to be the reason.
“A long COVID theory is that there is a temporary disruption to physiological ovarian steroid hormone production, which could [worsen] symptoms of perimenopause and menopause,” said JoAnn V. Pinkerton, MD, professor of obstetrics at the University of Virginia, Charlottesville, and executive director of the North American Menopause Society.
Long COVID symptoms and menopause symptoms can also be very hard to tell apart.
Another U.K. study cautions that because of this kind of symptom overlap, women at midlife may be misdiagnosed. Research from the North American Menopause Society shows that many women may have trouble recovering from long COVID unless their hormone deficiency is treated.
What are the symptoms of long COVID?
There are over 200 symptoms that have been associated with long COVID, according to the American Medical Association. Some common symptoms are currently defined as the following: feeling extremely tired, feeling depleted after exertion, cognitive issues such as brain fog, heart beating over 100 times a minute, and a loss of sense of smell and taste.
Long COVID symptoms begin a few weeks to a few months after a COVID infection. They can last an indefinite amount of time, but “the hope is that long COVID will not be lifelong,” said Clare Flannery, MD, an endocrinologist and associate professor in the departments of obstetrics, gynecology and reproductive sciences and internal medicine at Yale University, New Haven, Conn.
What are the symptoms of menopause?
Some symptoms of menopause include vaginal infections, irregular bleeding, urinary problems, and sexual problems.
Women in their middle years have other symptoms that can be the same as perimenopause/menopause symptoms.
“Common symptoms of perimenopause and menopause which may also be symptoms ascribed to long COVID include hot flashes, night sweats, disrupted sleep, low mood, depression or anxiety, decreased concentration, memory problems, joint and muscle pains, and headaches,” Dr. Pinkerton said.
Can long COVID actually bring on menopause?
In short: Possibly.
A new study from the Massachusetts Institute of Technology/Patient-Led Research Collaborative/University of California, San Francisco, found that long COVID can cause disruptions to a woman’s menstrual cycle, ovaries, fertility, and menopause itself.
This could be caused by chronic inflammation caused by long COVID on hormones as well. This kind of inflammatory response could explain irregularities in a woman’s menstrual cycle, according to the Newson Health Research and Education study. For instance, “when the body has inflammation, ovulation can happen,” Dr. Flannery said.
The mechanism for how long COVID could spur menopause can also involve a woman’s ovaries.
“Since the theory is that COVID affects the ovary with declines in ovarian reserve and ovarian function, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely and lengthen the symptoms of the perimenopause and menopausal transition,” Dr. Pinkerton said.
How can hormone replacement therapy benefit women dealing with long COVID during menopause?
Estradiol, the strongest estrogen hormone in a woman’s body, has already been shown to have a positive effect against COVID.
“Estradiol therapy treats symptoms more aggressively in the setting of long COVID,” said Dr. Flannery.
Estradiol is also a form of hormone therapy for menopause symptoms.
“Estradiol has been shown to help hot flashes, night sweats, and sleep and improve mood during perimenopause,” said Dr. Pinkerton. “So it’s likely that perimenopausal or menopausal women with long COVID would see improvements both due to the action of estradiol on the ovary seen during COVID and the improvements in symptoms.”
Estrogen-based hormone therapy has been linked to an increased risk for endometrial, breast, and ovarian cancer, according to the American Cancer Society. This means you should carefully consider how comfortable you are with those additional risks before starting this kind of therapy.
“Which of your symptoms are the most difficult to manage? You may see if you can navigate one to three of them. What are you willing to do for your symptoms? If a woman is willing to favor her sleep for the next 6 months to a year, she may be willing to change how she perceives her risk for cancer,” Dr. Flannery said. “What risk is a woman willing to take? I think if someone has a very low concern about a risk of cancer, and she’s suffering a disrupted life, then taking estradiol in a 1- to 2-year trial period could be critical to help.”
What else can help ease long COVID during menopause?
Getting the COVID vaccine, as well as getting a booster, could help. Not only will this help prevent people from being reinfected with COVID, which can worsen symptoms, but a new Swedish study says there is no evidence that it will cause postmenopausal problems like irregular bleeding.
“Weak and inconsistent associations were observed between SARS-CoV-2 vaccination and healthcare contacts for bleeding in women who are postmenopausal, and even less evidence was recorded of an association for menstrual disturbance or bleeding in women who were premenopausal,” said study coauthor Rickard Ljung, MD, PhD, MPH, professor and acting head of the pharmacoepidemiology and analysis department in the division of use and information of the Swedish Medical Products Agency in Uppsala.
A version of this article first appeared on WebMD.com.
British researchers have noted that women at midlife who have long COVID seem to get specific, and severe, symptoms, including brain fog, fatigue, new-onset dizziness, and difficulty sleeping through the night.
Doctors also think it’s possible that long COVID worsens the symptoms of perimenopause and menopause. Lower levels of estrogen and testosterone appear to be the reason.
“A long COVID theory is that there is a temporary disruption to physiological ovarian steroid hormone production, which could [worsen] symptoms of perimenopause and menopause,” said JoAnn V. Pinkerton, MD, professor of obstetrics at the University of Virginia, Charlottesville, and executive director of the North American Menopause Society.
Long COVID symptoms and menopause symptoms can also be very hard to tell apart.
Another U.K. study cautions that because of this kind of symptom overlap, women at midlife may be misdiagnosed. Research from the North American Menopause Society shows that many women may have trouble recovering from long COVID unless their hormone deficiency is treated.
What are the symptoms of long COVID?
There are over 200 symptoms that have been associated with long COVID, according to the American Medical Association. Some common symptoms are currently defined as the following: feeling extremely tired, feeling depleted after exertion, cognitive issues such as brain fog, heart beating over 100 times a minute, and a loss of sense of smell and taste.
Long COVID symptoms begin a few weeks to a few months after a COVID infection. They can last an indefinite amount of time, but “the hope is that long COVID will not be lifelong,” said Clare Flannery, MD, an endocrinologist and associate professor in the departments of obstetrics, gynecology and reproductive sciences and internal medicine at Yale University, New Haven, Conn.
What are the symptoms of menopause?
Some symptoms of menopause include vaginal infections, irregular bleeding, urinary problems, and sexual problems.
Women in their middle years have other symptoms that can be the same as perimenopause/menopause symptoms.
“Common symptoms of perimenopause and menopause which may also be symptoms ascribed to long COVID include hot flashes, night sweats, disrupted sleep, low mood, depression or anxiety, decreased concentration, memory problems, joint and muscle pains, and headaches,” Dr. Pinkerton said.
Can long COVID actually bring on menopause?
In short: Possibly.
A new study from the Massachusetts Institute of Technology/Patient-Led Research Collaborative/University of California, San Francisco, found that long COVID can cause disruptions to a woman’s menstrual cycle, ovaries, fertility, and menopause itself.
This could be caused by chronic inflammation caused by long COVID on hormones as well. This kind of inflammatory response could explain irregularities in a woman’s menstrual cycle, according to the Newson Health Research and Education study. For instance, “when the body has inflammation, ovulation can happen,” Dr. Flannery said.
The mechanism for how long COVID could spur menopause can also involve a woman’s ovaries.
“Since the theory is that COVID affects the ovary with declines in ovarian reserve and ovarian function, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely and lengthen the symptoms of the perimenopause and menopausal transition,” Dr. Pinkerton said.
How can hormone replacement therapy benefit women dealing with long COVID during menopause?
Estradiol, the strongest estrogen hormone in a woman’s body, has already been shown to have a positive effect against COVID.
“Estradiol therapy treats symptoms more aggressively in the setting of long COVID,” said Dr. Flannery.
Estradiol is also a form of hormone therapy for menopause symptoms.
“Estradiol has been shown to help hot flashes, night sweats, and sleep and improve mood during perimenopause,” said Dr. Pinkerton. “So it’s likely that perimenopausal or menopausal women with long COVID would see improvements both due to the action of estradiol on the ovary seen during COVID and the improvements in symptoms.”
Estrogen-based hormone therapy has been linked to an increased risk for endometrial, breast, and ovarian cancer, according to the American Cancer Society. This means you should carefully consider how comfortable you are with those additional risks before starting this kind of therapy.
“Which of your symptoms are the most difficult to manage? You may see if you can navigate one to three of them. What are you willing to do for your symptoms? If a woman is willing to favor her sleep for the next 6 months to a year, she may be willing to change how she perceives her risk for cancer,” Dr. Flannery said. “What risk is a woman willing to take? I think if someone has a very low concern about a risk of cancer, and she’s suffering a disrupted life, then taking estradiol in a 1- to 2-year trial period could be critical to help.”
What else can help ease long COVID during menopause?
Getting the COVID vaccine, as well as getting a booster, could help. Not only will this help prevent people from being reinfected with COVID, which can worsen symptoms, but a new Swedish study says there is no evidence that it will cause postmenopausal problems like irregular bleeding.
“Weak and inconsistent associations were observed between SARS-CoV-2 vaccination and healthcare contacts for bleeding in women who are postmenopausal, and even less evidence was recorded of an association for menstrual disturbance or bleeding in women who were premenopausal,” said study coauthor Rickard Ljung, MD, PhD, MPH, professor and acting head of the pharmacoepidemiology and analysis department in the division of use and information of the Swedish Medical Products Agency in Uppsala.
A version of this article first appeared on WebMD.com.
British researchers have noted that women at midlife who have long COVID seem to get specific, and severe, symptoms, including brain fog, fatigue, new-onset dizziness, and difficulty sleeping through the night.
Doctors also think it’s possible that long COVID worsens the symptoms of perimenopause and menopause. Lower levels of estrogen and testosterone appear to be the reason.
“A long COVID theory is that there is a temporary disruption to physiological ovarian steroid hormone production, which could [worsen] symptoms of perimenopause and menopause,” said JoAnn V. Pinkerton, MD, professor of obstetrics at the University of Virginia, Charlottesville, and executive director of the North American Menopause Society.
Long COVID symptoms and menopause symptoms can also be very hard to tell apart.
Another U.K. study cautions that because of this kind of symptom overlap, women at midlife may be misdiagnosed. Research from the North American Menopause Society shows that many women may have trouble recovering from long COVID unless their hormone deficiency is treated.
What are the symptoms of long COVID?
There are over 200 symptoms that have been associated with long COVID, according to the American Medical Association. Some common symptoms are currently defined as the following: feeling extremely tired, feeling depleted after exertion, cognitive issues such as brain fog, heart beating over 100 times a minute, and a loss of sense of smell and taste.
Long COVID symptoms begin a few weeks to a few months after a COVID infection. They can last an indefinite amount of time, but “the hope is that long COVID will not be lifelong,” said Clare Flannery, MD, an endocrinologist and associate professor in the departments of obstetrics, gynecology and reproductive sciences and internal medicine at Yale University, New Haven, Conn.
What are the symptoms of menopause?
Some symptoms of menopause include vaginal infections, irregular bleeding, urinary problems, and sexual problems.
Women in their middle years have other symptoms that can be the same as perimenopause/menopause symptoms.
“Common symptoms of perimenopause and menopause which may also be symptoms ascribed to long COVID include hot flashes, night sweats, disrupted sleep, low mood, depression or anxiety, decreased concentration, memory problems, joint and muscle pains, and headaches,” Dr. Pinkerton said.
Can long COVID actually bring on menopause?
In short: Possibly.
A new study from the Massachusetts Institute of Technology/Patient-Led Research Collaborative/University of California, San Francisco, found that long COVID can cause disruptions to a woman’s menstrual cycle, ovaries, fertility, and menopause itself.
This could be caused by chronic inflammation caused by long COVID on hormones as well. This kind of inflammatory response could explain irregularities in a woman’s menstrual cycle, according to the Newson Health Research and Education study. For instance, “when the body has inflammation, ovulation can happen,” Dr. Flannery said.
The mechanism for how long COVID could spur menopause can also involve a woman’s ovaries.
“Since the theory is that COVID affects the ovary with declines in ovarian reserve and ovarian function, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely and lengthen the symptoms of the perimenopause and menopausal transition,” Dr. Pinkerton said.
How can hormone replacement therapy benefit women dealing with long COVID during menopause?
Estradiol, the strongest estrogen hormone in a woman’s body, has already been shown to have a positive effect against COVID.
“Estradiol therapy treats symptoms more aggressively in the setting of long COVID,” said Dr. Flannery.
Estradiol is also a form of hormone therapy for menopause symptoms.
“Estradiol has been shown to help hot flashes, night sweats, and sleep and improve mood during perimenopause,” said Dr. Pinkerton. “So it’s likely that perimenopausal or menopausal women with long COVID would see improvements both due to the action of estradiol on the ovary seen during COVID and the improvements in symptoms.”
Estrogen-based hormone therapy has been linked to an increased risk for endometrial, breast, and ovarian cancer, according to the American Cancer Society. This means you should carefully consider how comfortable you are with those additional risks before starting this kind of therapy.
“Which of your symptoms are the most difficult to manage? You may see if you can navigate one to three of them. What are you willing to do for your symptoms? If a woman is willing to favor her sleep for the next 6 months to a year, she may be willing to change how she perceives her risk for cancer,” Dr. Flannery said. “What risk is a woman willing to take? I think if someone has a very low concern about a risk of cancer, and she’s suffering a disrupted life, then taking estradiol in a 1- to 2-year trial period could be critical to help.”
What else can help ease long COVID during menopause?
Getting the COVID vaccine, as well as getting a booster, could help. Not only will this help prevent people from being reinfected with COVID, which can worsen symptoms, but a new Swedish study says there is no evidence that it will cause postmenopausal problems like irregular bleeding.
“Weak and inconsistent associations were observed between SARS-CoV-2 vaccination and healthcare contacts for bleeding in women who are postmenopausal, and even less evidence was recorded of an association for menstrual disturbance or bleeding in women who were premenopausal,” said study coauthor Rickard Ljung, MD, PhD, MPH, professor and acting head of the pharmacoepidemiology and analysis department in the division of use and information of the Swedish Medical Products Agency in Uppsala.
A version of this article first appeared on WebMD.com.
COVID vaccines safe for young children, study finds
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
Medicaid patients with heart failure get poor follow-up after hospital discharge
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Investigational uricase-based gout drug meets primary endpoints in phase 3 trials
MILAN – Serum uric acid of less than 6 mg/dL was achieved and maintained for a substantial period of time with a once-monthly infusion of SEL-212 in patients with refractory gout, according to results of the two phase 3 DISSOLVE I and II trials.
Both trials met their primary endpoints. In DISSOLVE I – the U.S. study – 56% of patients on SEL-212 at 0.15 mg/kg (high dose) achieved a response, defined as achievement and maintenance of a reduction in serum urate to less than 6 mg/dL for at least 80% of the time during month 6 of treatment. In DISSOLVE II – the global study – 46% of patients on SEL-212 on the 0.15-mg/kg dose achieved response.
In participants aged 50 years or older, there was a statistically significant higher response rate at the high dose of SEL-212 in both DISSOLVE I and II of 65% and 47%, respectively, compared with placebo.
Herbert S.B. Baraf, MD, clinical professor of medicine at George Washington University, Washington, and principal investigator of the DISSOLVE program, presented results of the two phase 3 trials during a late-breaking session at the annual European Congress of Rheumatology.
“The top-line data from the two SEL-212 phase 3 studies are encouraging. They show that induction of immunotolerance with an infusion of a rapamycin-containing nanoparticle (SEL-110), followed immediately by an infusion of pegadricase, a potent but immunogenic uricase, allows for a strong and sustained uric acid–lowering effect without the development of anti-drug antibodies,” Dr. Baraf said in an interview.
SEL-212 is a monthly two-part infusion therapy – a combination of Selecta Biosciences’s ImmTOR immune tolerance platform, and a therapeutic uricase enzyme (pegadricase), designed to treat refractory gout. SEL-110 (ImmTOR) is an immune-tolerizing, nanoencapsulated rapamycin administered 30 minutes before pegadricase and inhibits anti-pegadricase antibodies. SEL-37 is a pegylated uricase (pegadricase) that converts uric acid to excretable allantoin.
SEL-212 was originally developed by Selecta. Swedish Orphan Biovitrum (Sobi) licensed SEL-212 from Selecta in June 2020 and is responsible for development, regulatory, and commercial activities in all markets outside of China. Selecta is responsible for ImmTOR manufacturing. The phase 3 program for SEL-212 was run by Selecta and funded by Sobi.
It is understood that a biologic license application will be submitted to the Food and Drug Administration, most likely next year, and if approved, “the SEL-212 two-component infusion treatment would provide a monthly alternative to twice-monthly pegloticase, for patients with refractory gout,” Dr. Baraf added.
Details of the trials
The two DISSOLVE studies replicate double-blind, placebo-controlled trials in patients with chronic refractory gout. DISSOLVE I was carried out in 112 patients across 29 sites in the United States, and DISSOLVE II tested the two-part treatment in 153 patients across 37 sites in the United States, Russia, Ukraine, Georgia, and Serbia.
Both studies randomized patients 1:1:1 to a high dose (SEL-110 of 0.15 mg/kg plus SEL-037 of 0.2 mg/kg), low dose (SEL-110 of 0.1 mg/kg plus SEL-037 of 0.2 mg/kg), or placebo (saline) infused every 28 days for 6 months. Prophylaxis against infusion reactions and gout flares were given to all participants.
Adult patients had a 10- to 14-year history of symptomatic gout, with three or more flares over the 18 months prior to screening, or one or more tophus, or a diagnosis of gouty arthritis. They were also required to have chronic refractory gout with a failure to normalize serum uric acid with any xanthine oxidase inhibitor (for example, allopurinol) and to have not been previously exposed to uricase-based therapy. Serum uric acid had to be at least 7 mg/dL. Participants were balanced for age, body mass index, and sex across treatment groups. Gout severity was greater in DISSOLVE II, Dr. Baraf reported.
Both studies treated patients for 6 months, but DISSOLVE 1 continued with a 6-month, blinded safety extension. The primary endpoint in both studies was serum urate control during month 6, and secondary endpoints included tender and swollen joint counts, tophus burden, patient-reported outcomes of activity limitation, quality of life, and gout flare incidence.
In DISSOLVE I, patients on SEL-212 had a statistically significant higher response rate during month 6 of 56% with the high dose (P < .0001) and 48% with the low dose (P < .0001), compared with 4% of patients randomized to receive placebo. In DISSOLVE II, participants on SEL-212 had a statistically significant higher response rate during month 6 of 46% with the high dose (P = .0002) and 40% with the low dose (P = .0008), compared with 11% of patients randomized to receive placebo.
“We also saw significant reductions in serum uric acid for all treatment groups, compared with placebo,” Dr. Baraf reported. Mean percentage change was –62.3% and –58.3% in the high- and low-dose groups, respectively, in DISSOLVE I, and –58.1% and –52.2% in DISSOLVE II, respectively.
SEL-212 had a favorable safety profile with adverse events as expected across both doses, including mild to moderate stomatitis (3.4% in the low-dose group and 9.2% in the high-dose group versus 0% in the placebo group), and a greater number of infusion reactions at 24 hours and 1 hour after drug administration in both treatment groups versus placebo. Six patients had treatment-related serious adverse events, including two cases of anaphylaxis and one gout flare in both the high- and low-dose treatment groups. The 6-month extension period in the DISSOLVE I trial showed that the majority (75%) of patients who completed 6 months of SEL-212 treatment as a responder continued to be successfully treated through 12 months with no infusion reactions or safety signals.
“I expect more data will be forthcoming on the important clinical secondary endpoints targeted by SEL-212 therapy,” Dr. Baraf noted.
Need control arm taking allopurinol?
Roy Fleischmann, MD, clinical professor of medicine at the University of Texas Southwestern Medical Center and codirector of the Metroplex Clinical Research Center, both in Dallas, commented on the study methods after the presentation. “The major problem with this study is that they say the patients had had insufficient response to allopurinol, and my guess is most had received 100-200 mg of allopurinol but were not titrated up to the maximum tolerated dose,” he said, adding: “they should have had a control arm of patients on allopurinol and titrated to the maximum tolerated dose. So, I don’t know what this is really telling us with respect to allopurinol, which is a relatively cheap drug.”
Dr. Baraf reported consulting with Horizon, Sobi, and Selecta; serving on Horizon’s speakers bureau, and receiving grant/research support from Horizon and Sobi. Dr. Fleischmann reported no financial relationship of relevance to this study.
MILAN – Serum uric acid of less than 6 mg/dL was achieved and maintained for a substantial period of time with a once-monthly infusion of SEL-212 in patients with refractory gout, according to results of the two phase 3 DISSOLVE I and II trials.
Both trials met their primary endpoints. In DISSOLVE I – the U.S. study – 56% of patients on SEL-212 at 0.15 mg/kg (high dose) achieved a response, defined as achievement and maintenance of a reduction in serum urate to less than 6 mg/dL for at least 80% of the time during month 6 of treatment. In DISSOLVE II – the global study – 46% of patients on SEL-212 on the 0.15-mg/kg dose achieved response.
In participants aged 50 years or older, there was a statistically significant higher response rate at the high dose of SEL-212 in both DISSOLVE I and II of 65% and 47%, respectively, compared with placebo.
Herbert S.B. Baraf, MD, clinical professor of medicine at George Washington University, Washington, and principal investigator of the DISSOLVE program, presented results of the two phase 3 trials during a late-breaking session at the annual European Congress of Rheumatology.
“The top-line data from the two SEL-212 phase 3 studies are encouraging. They show that induction of immunotolerance with an infusion of a rapamycin-containing nanoparticle (SEL-110), followed immediately by an infusion of pegadricase, a potent but immunogenic uricase, allows for a strong and sustained uric acid–lowering effect without the development of anti-drug antibodies,” Dr. Baraf said in an interview.
SEL-212 is a monthly two-part infusion therapy – a combination of Selecta Biosciences’s ImmTOR immune tolerance platform, and a therapeutic uricase enzyme (pegadricase), designed to treat refractory gout. SEL-110 (ImmTOR) is an immune-tolerizing, nanoencapsulated rapamycin administered 30 minutes before pegadricase and inhibits anti-pegadricase antibodies. SEL-37 is a pegylated uricase (pegadricase) that converts uric acid to excretable allantoin.
SEL-212 was originally developed by Selecta. Swedish Orphan Biovitrum (Sobi) licensed SEL-212 from Selecta in June 2020 and is responsible for development, regulatory, and commercial activities in all markets outside of China. Selecta is responsible for ImmTOR manufacturing. The phase 3 program for SEL-212 was run by Selecta and funded by Sobi.
It is understood that a biologic license application will be submitted to the Food and Drug Administration, most likely next year, and if approved, “the SEL-212 two-component infusion treatment would provide a monthly alternative to twice-monthly pegloticase, for patients with refractory gout,” Dr. Baraf added.
Details of the trials
The two DISSOLVE studies replicate double-blind, placebo-controlled trials in patients with chronic refractory gout. DISSOLVE I was carried out in 112 patients across 29 sites in the United States, and DISSOLVE II tested the two-part treatment in 153 patients across 37 sites in the United States, Russia, Ukraine, Georgia, and Serbia.
Both studies randomized patients 1:1:1 to a high dose (SEL-110 of 0.15 mg/kg plus SEL-037 of 0.2 mg/kg), low dose (SEL-110 of 0.1 mg/kg plus SEL-037 of 0.2 mg/kg), or placebo (saline) infused every 28 days for 6 months. Prophylaxis against infusion reactions and gout flares were given to all participants.
Adult patients had a 10- to 14-year history of symptomatic gout, with three or more flares over the 18 months prior to screening, or one or more tophus, or a diagnosis of gouty arthritis. They were also required to have chronic refractory gout with a failure to normalize serum uric acid with any xanthine oxidase inhibitor (for example, allopurinol) and to have not been previously exposed to uricase-based therapy. Serum uric acid had to be at least 7 mg/dL. Participants were balanced for age, body mass index, and sex across treatment groups. Gout severity was greater in DISSOLVE II, Dr. Baraf reported.
Both studies treated patients for 6 months, but DISSOLVE 1 continued with a 6-month, blinded safety extension. The primary endpoint in both studies was serum urate control during month 6, and secondary endpoints included tender and swollen joint counts, tophus burden, patient-reported outcomes of activity limitation, quality of life, and gout flare incidence.
In DISSOLVE I, patients on SEL-212 had a statistically significant higher response rate during month 6 of 56% with the high dose (P < .0001) and 48% with the low dose (P < .0001), compared with 4% of patients randomized to receive placebo. In DISSOLVE II, participants on SEL-212 had a statistically significant higher response rate during month 6 of 46% with the high dose (P = .0002) and 40% with the low dose (P = .0008), compared with 11% of patients randomized to receive placebo.
“We also saw significant reductions in serum uric acid for all treatment groups, compared with placebo,” Dr. Baraf reported. Mean percentage change was –62.3% and –58.3% in the high- and low-dose groups, respectively, in DISSOLVE I, and –58.1% and –52.2% in DISSOLVE II, respectively.
SEL-212 had a favorable safety profile with adverse events as expected across both doses, including mild to moderate stomatitis (3.4% in the low-dose group and 9.2% in the high-dose group versus 0% in the placebo group), and a greater number of infusion reactions at 24 hours and 1 hour after drug administration in both treatment groups versus placebo. Six patients had treatment-related serious adverse events, including two cases of anaphylaxis and one gout flare in both the high- and low-dose treatment groups. The 6-month extension period in the DISSOLVE I trial showed that the majority (75%) of patients who completed 6 months of SEL-212 treatment as a responder continued to be successfully treated through 12 months with no infusion reactions or safety signals.
“I expect more data will be forthcoming on the important clinical secondary endpoints targeted by SEL-212 therapy,” Dr. Baraf noted.
Need control arm taking allopurinol?
Roy Fleischmann, MD, clinical professor of medicine at the University of Texas Southwestern Medical Center and codirector of the Metroplex Clinical Research Center, both in Dallas, commented on the study methods after the presentation. “The major problem with this study is that they say the patients had had insufficient response to allopurinol, and my guess is most had received 100-200 mg of allopurinol but were not titrated up to the maximum tolerated dose,” he said, adding: “they should have had a control arm of patients on allopurinol and titrated to the maximum tolerated dose. So, I don’t know what this is really telling us with respect to allopurinol, which is a relatively cheap drug.”
Dr. Baraf reported consulting with Horizon, Sobi, and Selecta; serving on Horizon’s speakers bureau, and receiving grant/research support from Horizon and Sobi. Dr. Fleischmann reported no financial relationship of relevance to this study.
MILAN – Serum uric acid of less than 6 mg/dL was achieved and maintained for a substantial period of time with a once-monthly infusion of SEL-212 in patients with refractory gout, according to results of the two phase 3 DISSOLVE I and II trials.
Both trials met their primary endpoints. In DISSOLVE I – the U.S. study – 56% of patients on SEL-212 at 0.15 mg/kg (high dose) achieved a response, defined as achievement and maintenance of a reduction in serum urate to less than 6 mg/dL for at least 80% of the time during month 6 of treatment. In DISSOLVE II – the global study – 46% of patients on SEL-212 on the 0.15-mg/kg dose achieved response.
In participants aged 50 years or older, there was a statistically significant higher response rate at the high dose of SEL-212 in both DISSOLVE I and II of 65% and 47%, respectively, compared with placebo.
Herbert S.B. Baraf, MD, clinical professor of medicine at George Washington University, Washington, and principal investigator of the DISSOLVE program, presented results of the two phase 3 trials during a late-breaking session at the annual European Congress of Rheumatology.
“The top-line data from the two SEL-212 phase 3 studies are encouraging. They show that induction of immunotolerance with an infusion of a rapamycin-containing nanoparticle (SEL-110), followed immediately by an infusion of pegadricase, a potent but immunogenic uricase, allows for a strong and sustained uric acid–lowering effect without the development of anti-drug antibodies,” Dr. Baraf said in an interview.
SEL-212 is a monthly two-part infusion therapy – a combination of Selecta Biosciences’s ImmTOR immune tolerance platform, and a therapeutic uricase enzyme (pegadricase), designed to treat refractory gout. SEL-110 (ImmTOR) is an immune-tolerizing, nanoencapsulated rapamycin administered 30 minutes before pegadricase and inhibits anti-pegadricase antibodies. SEL-37 is a pegylated uricase (pegadricase) that converts uric acid to excretable allantoin.
SEL-212 was originally developed by Selecta. Swedish Orphan Biovitrum (Sobi) licensed SEL-212 from Selecta in June 2020 and is responsible for development, regulatory, and commercial activities in all markets outside of China. Selecta is responsible for ImmTOR manufacturing. The phase 3 program for SEL-212 was run by Selecta and funded by Sobi.
It is understood that a biologic license application will be submitted to the Food and Drug Administration, most likely next year, and if approved, “the SEL-212 two-component infusion treatment would provide a monthly alternative to twice-monthly pegloticase, for patients with refractory gout,” Dr. Baraf added.
Details of the trials
The two DISSOLVE studies replicate double-blind, placebo-controlled trials in patients with chronic refractory gout. DISSOLVE I was carried out in 112 patients across 29 sites in the United States, and DISSOLVE II tested the two-part treatment in 153 patients across 37 sites in the United States, Russia, Ukraine, Georgia, and Serbia.
Both studies randomized patients 1:1:1 to a high dose (SEL-110 of 0.15 mg/kg plus SEL-037 of 0.2 mg/kg), low dose (SEL-110 of 0.1 mg/kg plus SEL-037 of 0.2 mg/kg), or placebo (saline) infused every 28 days for 6 months. Prophylaxis against infusion reactions and gout flares were given to all participants.
Adult patients had a 10- to 14-year history of symptomatic gout, with three or more flares over the 18 months prior to screening, or one or more tophus, or a diagnosis of gouty arthritis. They were also required to have chronic refractory gout with a failure to normalize serum uric acid with any xanthine oxidase inhibitor (for example, allopurinol) and to have not been previously exposed to uricase-based therapy. Serum uric acid had to be at least 7 mg/dL. Participants were balanced for age, body mass index, and sex across treatment groups. Gout severity was greater in DISSOLVE II, Dr. Baraf reported.
Both studies treated patients for 6 months, but DISSOLVE 1 continued with a 6-month, blinded safety extension. The primary endpoint in both studies was serum urate control during month 6, and secondary endpoints included tender and swollen joint counts, tophus burden, patient-reported outcomes of activity limitation, quality of life, and gout flare incidence.
In DISSOLVE I, patients on SEL-212 had a statistically significant higher response rate during month 6 of 56% with the high dose (P < .0001) and 48% with the low dose (P < .0001), compared with 4% of patients randomized to receive placebo. In DISSOLVE II, participants on SEL-212 had a statistically significant higher response rate during month 6 of 46% with the high dose (P = .0002) and 40% with the low dose (P = .0008), compared with 11% of patients randomized to receive placebo.
“We also saw significant reductions in serum uric acid for all treatment groups, compared with placebo,” Dr. Baraf reported. Mean percentage change was –62.3% and –58.3% in the high- and low-dose groups, respectively, in DISSOLVE I, and –58.1% and –52.2% in DISSOLVE II, respectively.
SEL-212 had a favorable safety profile with adverse events as expected across both doses, including mild to moderate stomatitis (3.4% in the low-dose group and 9.2% in the high-dose group versus 0% in the placebo group), and a greater number of infusion reactions at 24 hours and 1 hour after drug administration in both treatment groups versus placebo. Six patients had treatment-related serious adverse events, including two cases of anaphylaxis and one gout flare in both the high- and low-dose treatment groups. The 6-month extension period in the DISSOLVE I trial showed that the majority (75%) of patients who completed 6 months of SEL-212 treatment as a responder continued to be successfully treated through 12 months with no infusion reactions or safety signals.
“I expect more data will be forthcoming on the important clinical secondary endpoints targeted by SEL-212 therapy,” Dr. Baraf noted.
Need control arm taking allopurinol?
Roy Fleischmann, MD, clinical professor of medicine at the University of Texas Southwestern Medical Center and codirector of the Metroplex Clinical Research Center, both in Dallas, commented on the study methods after the presentation. “The major problem with this study is that they say the patients had had insufficient response to allopurinol, and my guess is most had received 100-200 mg of allopurinol but were not titrated up to the maximum tolerated dose,” he said, adding: “they should have had a control arm of patients on allopurinol and titrated to the maximum tolerated dose. So, I don’t know what this is really telling us with respect to allopurinol, which is a relatively cheap drug.”
Dr. Baraf reported consulting with Horizon, Sobi, and Selecta; serving on Horizon’s speakers bureau, and receiving grant/research support from Horizon and Sobi. Dr. Fleischmann reported no financial relationship of relevance to this study.
AT EULAR 2023
Lower racial disparity in melanoma diagnoses in vets than U.S. men overall, study finds
a new analysis shows.
“The trend of a lower racial disparity in the VA in the proportion of melanomas with local disease and in the proportion of distant metastasis at presentation was observed across age groups,” wrote Martin A. Weinstock MD, PhD, and Rachel K. Lim, of the department of dermatology at Brown University, Providence, R.I., and the Center for Dermatoepidemiology at the VA Providence Healthcare System. The study was published online in the Journal of the American Academy of Dermatology.
“Melanoma was the fourth-most common cancer [diagnosed] in male VA patients in 2010,” wrote the authors, who also pointed out that “prior surveys found that 11%-13% of U.S. active-duty personnel routinely use sunscreen despite significant occupational sun exposure. Racial disparities are important concerns in the VA and elsewhere.”
To compare the stage of melanoma at presentation among White and non-Whites patients in the VA and in the general U.S. population, the researchers identified invasive cutaneous melanoma cases from 2000 to 2019 in the VA Corporate Data Warehouse and the Surveillance, Epidemiology and End Results Program (SEER).
They restricted the analysis to men because of the small proportion of women in the at-risk veteran population and excluded cases with an age younger than 20, those with unknown histology, and melanoma in situ. The researchers performed two-tailed z-tests to evaluate the difference in proportions of melanoma stages between the veteran population and the general population.
The analysis included 44,077 cases of invasive melanoma in the VA and 217,030 in SEER. Racial disparities in melanoma staging were substantially less pronounced in the VA than in SEER.
In the VA, localized disease represented 77.9% of melanomas among Whites versus 71.0% among non-Whites. But in SEER, localized disease represented 80.7% of melanomas among Whites versus 61.5% in non-Whites – over double the VA disparity (P < .0001).
Likewise, the disparity between Whites and nonwhites observed for regional or distant metastatic disease at presentation in the VA was lower than the disparity observed in SEER. For example, in the VA, distant metastatic disease at presentation represented 6.1% of melanomas among Whites versus 8.6% among non-Whites, while in SEER it represented 4.8% of melanomas among Whites versus 11.3% in non-Whites – again, more than double the VA disparity (P < .0001).
“These differences between the VA and SEER were less marked” among those older than 65 years, the researchers wrote. “Notably, the differences between VA and SEER in racial disparities among those greater than 65 in age were still significant for localized disease and for distant metastasis.”
The findings suggest that the VA “may be more effective in reducing racial disparities in melanoma stage at diagnosis, potentially due to all patients in the VA dataset having insured access to health care, regardless of socioeconomic status,” the researchers concluded. Similarly, the decreased difference in racial disparities observed in patients older than 65 across systems “may be related to the availability of Medicare to the older general populations. The authors acknowledged several study limitations, such as the predominantly elderly and male VA population, potentially underreported utilization of non-VA dermatologic care, and variation in geographic regions covered by each database.
Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the work, said in an interview he would have liked to see a more detailed breakdown of the younger patients, “for those in their 30s and 40s, to see if this trend held up.”
He would have also liked to see how the data trended over time, adding, “while this, broadly, may be good news for our veterans, attributing this finding to a reduction in access disparity or some other organizational intervention seems a little premature. Regardless, Dr. Weinstock has given us, once again, information from our veterans to probe for the betterment of all patients.”
The researchers reported having no relevant disclosures and the study had no funding. Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
a new analysis shows.
“The trend of a lower racial disparity in the VA in the proportion of melanomas with local disease and in the proportion of distant metastasis at presentation was observed across age groups,” wrote Martin A. Weinstock MD, PhD, and Rachel K. Lim, of the department of dermatology at Brown University, Providence, R.I., and the Center for Dermatoepidemiology at the VA Providence Healthcare System. The study was published online in the Journal of the American Academy of Dermatology.
“Melanoma was the fourth-most common cancer [diagnosed] in male VA patients in 2010,” wrote the authors, who also pointed out that “prior surveys found that 11%-13% of U.S. active-duty personnel routinely use sunscreen despite significant occupational sun exposure. Racial disparities are important concerns in the VA and elsewhere.”
To compare the stage of melanoma at presentation among White and non-Whites patients in the VA and in the general U.S. population, the researchers identified invasive cutaneous melanoma cases from 2000 to 2019 in the VA Corporate Data Warehouse and the Surveillance, Epidemiology and End Results Program (SEER).
They restricted the analysis to men because of the small proportion of women in the at-risk veteran population and excluded cases with an age younger than 20, those with unknown histology, and melanoma in situ. The researchers performed two-tailed z-tests to evaluate the difference in proportions of melanoma stages between the veteran population and the general population.
The analysis included 44,077 cases of invasive melanoma in the VA and 217,030 in SEER. Racial disparities in melanoma staging were substantially less pronounced in the VA than in SEER.
In the VA, localized disease represented 77.9% of melanomas among Whites versus 71.0% among non-Whites. But in SEER, localized disease represented 80.7% of melanomas among Whites versus 61.5% in non-Whites – over double the VA disparity (P < .0001).
Likewise, the disparity between Whites and nonwhites observed for regional or distant metastatic disease at presentation in the VA was lower than the disparity observed in SEER. For example, in the VA, distant metastatic disease at presentation represented 6.1% of melanomas among Whites versus 8.6% among non-Whites, while in SEER it represented 4.8% of melanomas among Whites versus 11.3% in non-Whites – again, more than double the VA disparity (P < .0001).
“These differences between the VA and SEER were less marked” among those older than 65 years, the researchers wrote. “Notably, the differences between VA and SEER in racial disparities among those greater than 65 in age were still significant for localized disease and for distant metastasis.”
The findings suggest that the VA “may be more effective in reducing racial disparities in melanoma stage at diagnosis, potentially due to all patients in the VA dataset having insured access to health care, regardless of socioeconomic status,” the researchers concluded. Similarly, the decreased difference in racial disparities observed in patients older than 65 across systems “may be related to the availability of Medicare to the older general populations. The authors acknowledged several study limitations, such as the predominantly elderly and male VA population, potentially underreported utilization of non-VA dermatologic care, and variation in geographic regions covered by each database.
Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the work, said in an interview he would have liked to see a more detailed breakdown of the younger patients, “for those in their 30s and 40s, to see if this trend held up.”
He would have also liked to see how the data trended over time, adding, “while this, broadly, may be good news for our veterans, attributing this finding to a reduction in access disparity or some other organizational intervention seems a little premature. Regardless, Dr. Weinstock has given us, once again, information from our veterans to probe for the betterment of all patients.”
The researchers reported having no relevant disclosures and the study had no funding. Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
a new analysis shows.
“The trend of a lower racial disparity in the VA in the proportion of melanomas with local disease and in the proportion of distant metastasis at presentation was observed across age groups,” wrote Martin A. Weinstock MD, PhD, and Rachel K. Lim, of the department of dermatology at Brown University, Providence, R.I., and the Center for Dermatoepidemiology at the VA Providence Healthcare System. The study was published online in the Journal of the American Academy of Dermatology.
“Melanoma was the fourth-most common cancer [diagnosed] in male VA patients in 2010,” wrote the authors, who also pointed out that “prior surveys found that 11%-13% of U.S. active-duty personnel routinely use sunscreen despite significant occupational sun exposure. Racial disparities are important concerns in the VA and elsewhere.”
To compare the stage of melanoma at presentation among White and non-Whites patients in the VA and in the general U.S. population, the researchers identified invasive cutaneous melanoma cases from 2000 to 2019 in the VA Corporate Data Warehouse and the Surveillance, Epidemiology and End Results Program (SEER).
They restricted the analysis to men because of the small proportion of women in the at-risk veteran population and excluded cases with an age younger than 20, those with unknown histology, and melanoma in situ. The researchers performed two-tailed z-tests to evaluate the difference in proportions of melanoma stages between the veteran population and the general population.
The analysis included 44,077 cases of invasive melanoma in the VA and 217,030 in SEER. Racial disparities in melanoma staging were substantially less pronounced in the VA than in SEER.
In the VA, localized disease represented 77.9% of melanomas among Whites versus 71.0% among non-Whites. But in SEER, localized disease represented 80.7% of melanomas among Whites versus 61.5% in non-Whites – over double the VA disparity (P < .0001).
Likewise, the disparity between Whites and nonwhites observed for regional or distant metastatic disease at presentation in the VA was lower than the disparity observed in SEER. For example, in the VA, distant metastatic disease at presentation represented 6.1% of melanomas among Whites versus 8.6% among non-Whites, while in SEER it represented 4.8% of melanomas among Whites versus 11.3% in non-Whites – again, more than double the VA disparity (P < .0001).
“These differences between the VA and SEER were less marked” among those older than 65 years, the researchers wrote. “Notably, the differences between VA and SEER in racial disparities among those greater than 65 in age were still significant for localized disease and for distant metastasis.”
The findings suggest that the VA “may be more effective in reducing racial disparities in melanoma stage at diagnosis, potentially due to all patients in the VA dataset having insured access to health care, regardless of socioeconomic status,” the researchers concluded. Similarly, the decreased difference in racial disparities observed in patients older than 65 across systems “may be related to the availability of Medicare to the older general populations. The authors acknowledged several study limitations, such as the predominantly elderly and male VA population, potentially underreported utilization of non-VA dermatologic care, and variation in geographic regions covered by each database.
Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the work, said in an interview he would have liked to see a more detailed breakdown of the younger patients, “for those in their 30s and 40s, to see if this trend held up.”
He would have also liked to see how the data trended over time, adding, “while this, broadly, may be good news for our veterans, attributing this finding to a reduction in access disparity or some other organizational intervention seems a little premature. Regardless, Dr. Weinstock has given us, once again, information from our veterans to probe for the betterment of all patients.”
The researchers reported having no relevant disclosures and the study had no funding. Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Abrocitinib remains effective at 96 weeks, in older as well as younger adults
WASHINGTON – A substantial proportion of , Andrew F. Alexis, MD, MPH, reported in a late-breaker abstract session at the annual Revolutionizing Atopic Dermatitis conference.
The analysis stratified patients by age – 18-50 and over 50 years – and found that the sustained improvement with the JAK-1 selective inhibitor as monotherapy was seen regardless of age. “In practice, patients who are older tend to have had AD for a longer period of time and tend to be more difficult to treat so it’s reassuring to see that even in the over-50 age group, they show substantial responses, even with more stringent endpoints,” said Dr. Alexis, professor of clinical dermatology at Weill Cornell Medical College, New York.
At week 96, for instance, the proportion of patients who achieved at least a 75% improvement from baseline on the Eczema Area and Severity Index (EASI-75) was 73% with the 100-mg dose and 85% with the 200-mg dose in the younger age group, and 86% and 89%, respectively, in the older age group.
An EASI-90 response – one of the more stringent outcomes – was achieved by 45% and 58% in the 18-50 group and 58% and 73% in the over 50 group (for 100-mg and 200-mg doses, respectively), Dr. Alexis reported.
The interim analysis also showed dose-dependent efficacy overall up to 96 weeks in the younger age group but only up to 48 weeks in the older age group. Response to some outcome measures in patients over age 50 years was “less clearly dose dependent after week 48” than earlier, Dr. Alexis said.
The ongoing JADE EXTEND trial enrolled patients who had participated in the phase 3 JADE clinical trials. This analysis covered 1,309 patients who were enrolled by a September 2021 cutoff. The patient population leaned young: Eighty percent (1,046) were aged 18-50, and 20% (263) were over 50.
Patients who were randomly assigned to abrocitinib 200 mg or 100 mg in the parent trials continued to receive the same dose in JADE EXTEND with blinding maintained. Those who received placebo in the qualifying trial were randomly assigned to abrocitinib 200 mg or 100 mg. And patients from JADE DARE continued with their dosing of 200 mg. Grouping by age for the analysis was made based on the age recorded at the screening visit of the qualifying trial.
IGA, PP-NRS, and DLQI results
At week 96, the proportion of patients 18-50 years of age who achieved the Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with at least a 2-grade improvement from baseline was 44% in the 100-mg group and 55% in the 200-mg group. Among patients over 50, these proportions were 51% and 58%, respectively.
The proportion of patients who achieved at least a 4-point improvement from baseline in the Peak Pruritus Numerical Rating Scale (PP-NRS) score was 54% and 66% (on 100 mg and 200 mg, respectively) among those aged 18-50, and 79% and 80%, respectively, among those over 50.
Looking at more stringent outcomes, 26% and 38% in the 18-50 group on 100 mg and 200 mg, respectively, achieved a PP-NRS of 0/1, as did 54% and 44% in the over-50 group.
Lastly, a score of less than 2 on the Dermatology Life Quality Index (DLQI 0/1) was achieved by 32% and 41% of patients aged 18-50 and by 51% and 48% of patients over 50, for the 100-mg and 200-mg doses, respectively.
The decline in dose-dependent efficacy in the older age group after 48 weeks may be due to the smaller sample of older patients and/or the fact that a higher proportion of older patients had moderate baseline disease per their IGA score, versus severe disease, compared with the younger patients, Dr. Alexis said. “We see a skewing toward a bit more severe [disease] in the younger age group compared to the older,” he noted.
Abrocitinib (Cibinqo) is approved for the treatment of moderate to severe AD in adolescents aged 12 and up and adults whose disease is not adequately controlled with other systemic treatments or those for whom the use of these drugs is not advised. It is available in a 50-mg dose for dose adjustments in special populations, but this dose was not studied in the clinical trials, Dr. Alexis noted. The interim analysis did not include safety data.
In a separate presentation in which he reviewed long-term data on AD medications, Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that most patients who meet defined endpoints at week 12 of treatment with abrocitinib maintain that response over time. “By and large, there’s a steep initial rise that flattens over the long run, which is what you want to see. People getting that response are generally staying there over the course of treatment,” he said, referring to the JADE EXTEND data up to week 48.
It’s important to also appreciate, however, that the proportion of patients meeting efficacy outcomes in the trials of abrocitinib has grown well beyond 12 weeks, Dr. Chovatiya said.
Pointing to data presented at a 2021 RAD meeting depicting the proportion of 12-week nonresponders achieving a response at weeks 24 and 48 on IGA 0/1, EASI-75, and PP-NRS, Dr. Chovatiya said the level of response grew at both time points. “You’re capturing a chunk of people well beyond the primary endpoint if you keep them on therapy continuously, suggesting that ... we may need to reframe how we’re thinking about oral JAK inhibitors,” he said. “Not only are they rapidly acting, but they are medications that can provide good control and changes in the long run.”
Dr. Alexis and Dr. Chovatiya disclosed ties with Pfizer, which funded the study.
WASHINGTON – A substantial proportion of , Andrew F. Alexis, MD, MPH, reported in a late-breaker abstract session at the annual Revolutionizing Atopic Dermatitis conference.
The analysis stratified patients by age – 18-50 and over 50 years – and found that the sustained improvement with the JAK-1 selective inhibitor as monotherapy was seen regardless of age. “In practice, patients who are older tend to have had AD for a longer period of time and tend to be more difficult to treat so it’s reassuring to see that even in the over-50 age group, they show substantial responses, even with more stringent endpoints,” said Dr. Alexis, professor of clinical dermatology at Weill Cornell Medical College, New York.
At week 96, for instance, the proportion of patients who achieved at least a 75% improvement from baseline on the Eczema Area and Severity Index (EASI-75) was 73% with the 100-mg dose and 85% with the 200-mg dose in the younger age group, and 86% and 89%, respectively, in the older age group.
An EASI-90 response – one of the more stringent outcomes – was achieved by 45% and 58% in the 18-50 group and 58% and 73% in the over 50 group (for 100-mg and 200-mg doses, respectively), Dr. Alexis reported.
The interim analysis also showed dose-dependent efficacy overall up to 96 weeks in the younger age group but only up to 48 weeks in the older age group. Response to some outcome measures in patients over age 50 years was “less clearly dose dependent after week 48” than earlier, Dr. Alexis said.
The ongoing JADE EXTEND trial enrolled patients who had participated in the phase 3 JADE clinical trials. This analysis covered 1,309 patients who were enrolled by a September 2021 cutoff. The patient population leaned young: Eighty percent (1,046) were aged 18-50, and 20% (263) were over 50.
Patients who were randomly assigned to abrocitinib 200 mg or 100 mg in the parent trials continued to receive the same dose in JADE EXTEND with blinding maintained. Those who received placebo in the qualifying trial were randomly assigned to abrocitinib 200 mg or 100 mg. And patients from JADE DARE continued with their dosing of 200 mg. Grouping by age for the analysis was made based on the age recorded at the screening visit of the qualifying trial.
IGA, PP-NRS, and DLQI results
At week 96, the proportion of patients 18-50 years of age who achieved the Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with at least a 2-grade improvement from baseline was 44% in the 100-mg group and 55% in the 200-mg group. Among patients over 50, these proportions were 51% and 58%, respectively.
The proportion of patients who achieved at least a 4-point improvement from baseline in the Peak Pruritus Numerical Rating Scale (PP-NRS) score was 54% and 66% (on 100 mg and 200 mg, respectively) among those aged 18-50, and 79% and 80%, respectively, among those over 50.
Looking at more stringent outcomes, 26% and 38% in the 18-50 group on 100 mg and 200 mg, respectively, achieved a PP-NRS of 0/1, as did 54% and 44% in the over-50 group.
Lastly, a score of less than 2 on the Dermatology Life Quality Index (DLQI 0/1) was achieved by 32% and 41% of patients aged 18-50 and by 51% and 48% of patients over 50, for the 100-mg and 200-mg doses, respectively.
The decline in dose-dependent efficacy in the older age group after 48 weeks may be due to the smaller sample of older patients and/or the fact that a higher proportion of older patients had moderate baseline disease per their IGA score, versus severe disease, compared with the younger patients, Dr. Alexis said. “We see a skewing toward a bit more severe [disease] in the younger age group compared to the older,” he noted.
Abrocitinib (Cibinqo) is approved for the treatment of moderate to severe AD in adolescents aged 12 and up and adults whose disease is not adequately controlled with other systemic treatments or those for whom the use of these drugs is not advised. It is available in a 50-mg dose for dose adjustments in special populations, but this dose was not studied in the clinical trials, Dr. Alexis noted. The interim analysis did not include safety data.
In a separate presentation in which he reviewed long-term data on AD medications, Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that most patients who meet defined endpoints at week 12 of treatment with abrocitinib maintain that response over time. “By and large, there’s a steep initial rise that flattens over the long run, which is what you want to see. People getting that response are generally staying there over the course of treatment,” he said, referring to the JADE EXTEND data up to week 48.
It’s important to also appreciate, however, that the proportion of patients meeting efficacy outcomes in the trials of abrocitinib has grown well beyond 12 weeks, Dr. Chovatiya said.
Pointing to data presented at a 2021 RAD meeting depicting the proportion of 12-week nonresponders achieving a response at weeks 24 and 48 on IGA 0/1, EASI-75, and PP-NRS, Dr. Chovatiya said the level of response grew at both time points. “You’re capturing a chunk of people well beyond the primary endpoint if you keep them on therapy continuously, suggesting that ... we may need to reframe how we’re thinking about oral JAK inhibitors,” he said. “Not only are they rapidly acting, but they are medications that can provide good control and changes in the long run.”
Dr. Alexis and Dr. Chovatiya disclosed ties with Pfizer, which funded the study.
WASHINGTON – A substantial proportion of , Andrew F. Alexis, MD, MPH, reported in a late-breaker abstract session at the annual Revolutionizing Atopic Dermatitis conference.
The analysis stratified patients by age – 18-50 and over 50 years – and found that the sustained improvement with the JAK-1 selective inhibitor as monotherapy was seen regardless of age. “In practice, patients who are older tend to have had AD for a longer period of time and tend to be more difficult to treat so it’s reassuring to see that even in the over-50 age group, they show substantial responses, even with more stringent endpoints,” said Dr. Alexis, professor of clinical dermatology at Weill Cornell Medical College, New York.
At week 96, for instance, the proportion of patients who achieved at least a 75% improvement from baseline on the Eczema Area and Severity Index (EASI-75) was 73% with the 100-mg dose and 85% with the 200-mg dose in the younger age group, and 86% and 89%, respectively, in the older age group.
An EASI-90 response – one of the more stringent outcomes – was achieved by 45% and 58% in the 18-50 group and 58% and 73% in the over 50 group (for 100-mg and 200-mg doses, respectively), Dr. Alexis reported.
The interim analysis also showed dose-dependent efficacy overall up to 96 weeks in the younger age group but only up to 48 weeks in the older age group. Response to some outcome measures in patients over age 50 years was “less clearly dose dependent after week 48” than earlier, Dr. Alexis said.
The ongoing JADE EXTEND trial enrolled patients who had participated in the phase 3 JADE clinical trials. This analysis covered 1,309 patients who were enrolled by a September 2021 cutoff. The patient population leaned young: Eighty percent (1,046) were aged 18-50, and 20% (263) were over 50.
Patients who were randomly assigned to abrocitinib 200 mg or 100 mg in the parent trials continued to receive the same dose in JADE EXTEND with blinding maintained. Those who received placebo in the qualifying trial were randomly assigned to abrocitinib 200 mg or 100 mg. And patients from JADE DARE continued with their dosing of 200 mg. Grouping by age for the analysis was made based on the age recorded at the screening visit of the qualifying trial.
IGA, PP-NRS, and DLQI results
At week 96, the proportion of patients 18-50 years of age who achieved the Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with at least a 2-grade improvement from baseline was 44% in the 100-mg group and 55% in the 200-mg group. Among patients over 50, these proportions were 51% and 58%, respectively.
The proportion of patients who achieved at least a 4-point improvement from baseline in the Peak Pruritus Numerical Rating Scale (PP-NRS) score was 54% and 66% (on 100 mg and 200 mg, respectively) among those aged 18-50, and 79% and 80%, respectively, among those over 50.
Looking at more stringent outcomes, 26% and 38% in the 18-50 group on 100 mg and 200 mg, respectively, achieved a PP-NRS of 0/1, as did 54% and 44% in the over-50 group.
Lastly, a score of less than 2 on the Dermatology Life Quality Index (DLQI 0/1) was achieved by 32% and 41% of patients aged 18-50 and by 51% and 48% of patients over 50, for the 100-mg and 200-mg doses, respectively.
The decline in dose-dependent efficacy in the older age group after 48 weeks may be due to the smaller sample of older patients and/or the fact that a higher proportion of older patients had moderate baseline disease per their IGA score, versus severe disease, compared with the younger patients, Dr. Alexis said. “We see a skewing toward a bit more severe [disease] in the younger age group compared to the older,” he noted.
Abrocitinib (Cibinqo) is approved for the treatment of moderate to severe AD in adolescents aged 12 and up and adults whose disease is not adequately controlled with other systemic treatments or those for whom the use of these drugs is not advised. It is available in a 50-mg dose for dose adjustments in special populations, but this dose was not studied in the clinical trials, Dr. Alexis noted. The interim analysis did not include safety data.
In a separate presentation in which he reviewed long-term data on AD medications, Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that most patients who meet defined endpoints at week 12 of treatment with abrocitinib maintain that response over time. “By and large, there’s a steep initial rise that flattens over the long run, which is what you want to see. People getting that response are generally staying there over the course of treatment,” he said, referring to the JADE EXTEND data up to week 48.
It’s important to also appreciate, however, that the proportion of patients meeting efficacy outcomes in the trials of abrocitinib has grown well beyond 12 weeks, Dr. Chovatiya said.
Pointing to data presented at a 2021 RAD meeting depicting the proportion of 12-week nonresponders achieving a response at weeks 24 and 48 on IGA 0/1, EASI-75, and PP-NRS, Dr. Chovatiya said the level of response grew at both time points. “You’re capturing a chunk of people well beyond the primary endpoint if you keep them on therapy continuously, suggesting that ... we may need to reframe how we’re thinking about oral JAK inhibitors,” he said. “Not only are they rapidly acting, but they are medications that can provide good control and changes in the long run.”
Dr. Alexis and Dr. Chovatiya disclosed ties with Pfizer, which funded the study.
AT RAD 2023