User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
New antiobesity drugs will benefit many. Is that bad?
where some economists opined that their coverage would be disastrous for Medicare.
Among their concerns? The drugs need to be taken long term (just like drugs for any other chronic condition). The new drugs are more expensive than the old drugs (just like new drugs for any other chronic condition). Lots of people will want to take them (just like highly effective drugs for any other chronic condition that has a significant quality-of-life or clinical impact). The U.K. recommended that they be covered only for 2 years (unlike drugs for any other chronic condition). And the Institute for Clinical and Economic Review (ICER) on which they lean heavily decided that $13,618 annually was too expensive for a medication that leads to sustained 15%-20% weight losses and those losses’ consequential benefits.
As a clinician working with patients who sustain those levels of weight loss, I find that conclusion confusing. Whether by way of lifestyle alone, or more often by way of lifestyle efforts plus medication or lifestyle efforts plus surgery, the benefits reported and seen with 15%-20% weight losses are almost uniformly huge. Patients are regularly seen discontinuing or reducing the dosage of multiple medications as a result of improvements to multiple weight-responsive comorbidities, and they also report objective benefits to mood, sleep, mobility, pain, and energy. Losing that much weight changes lives. Not to mention the impact that that degree of loss has on the primary prevention of so many diseases, including plausible reductions in many common cancers – reductions that have been shown to occur after surgery-related weight losses and for which there’s no plausible reason to imagine that they wouldn’t occur with pharmaceutical-related losses.
Are those discussions found in the NEJM op-ed or in the ICER report? Well, yes, sort of. However, in the NEJM op-ed, the word “prevention” isn’t used once, and unlike with oral hypoglycemics or antihypertensives, the authors state that with antiobesity medications, additional research is needed to determine whether medication-induced changes to A1c, blood pressure, and waist circumference would have clinical benefits: “Antiobesity medications have been shown to improve the surrogate end points of weight, glycated hemoglobin levels, systolic blood pressure, and waist circumference. Long-term studies are needed, however, to clarify how medication-induced changes in these surrogate markers translate to health outcomes.”
Primary prevention is mentioned in the ICER review, but in the “limitations” section where the authors explain that they didn’t include it in their modeling: “The long-term benefits of preventing other comorbidities including cancer, chronic kidney disease, osteoarthritis, and sleep apnea were not explicitly modeled in the base case.”
And they pretended that the impact on existing weight-responsive comorbidities mostly didn’t exist, too: “To limit the complexity of the cost-effectiveness model and to prevent double-counting of treatment benefits, we limited the long-term effects of treatments for weight management to cardiovascular risk and delays in the onset and/or diagnosis of diabetes mellitus.”
As far as cardiovascular disease (CVD) benefits go, you might have thought that it would be a slam dunk on that basis alone, at least according to a recent simple back-of-the-envelope math exercise presented at a recent American College of Cardiology conference, which applied the semaglutide treatment group weight changes in the STEP 1 trial to estimate the population impact on weight and obesity in 30- to 74-year-olds without prior CVD, and estimated 10-year CVD risks utilizing the BMI-based Framingham CVD risk scores. By their accounting, semaglutide treatment in eligible American patients has the potential to prevent over 1.6 million CVD events over 10 years.
Finally, even putting aside ICER’s admittedly and exceedingly narrow base case, what lifestyle-alone studies could ICER possibly be comparing with drug efficacy? And what does “alone” mean? Does “alone” mean with a months- or years long interprofessional behavioral program? Does “alone” mean by way of diet books? Does “alone” mean by way of simply “moving more and eating less”? I’m not aware of robust studies demonstrating any long-term meaningful, predictable, reproducible, durable weight loss outcomes for any lifestyle-only approach, intensive or otherwise.
It’s difficult for me to imagine a situation in which a drug other than an antiobesity drug would be found to have too many benefits to include in your cost-effectiveness analysis but where you’d be comfortable to run that analysis anyhow, and then come out against the drug’s recommendation and fearmonger about its use.
But then again, systemic weight bias is a hell of a drug.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa. He disclosed ties with Constant Health and Novo Nordisk, and has shared opinions via Weighty Matters and social media.
A version of this article originally appeared on Medscape.com.
where some economists opined that their coverage would be disastrous for Medicare.
Among their concerns? The drugs need to be taken long term (just like drugs for any other chronic condition). The new drugs are more expensive than the old drugs (just like new drugs for any other chronic condition). Lots of people will want to take them (just like highly effective drugs for any other chronic condition that has a significant quality-of-life or clinical impact). The U.K. recommended that they be covered only for 2 years (unlike drugs for any other chronic condition). And the Institute for Clinical and Economic Review (ICER) on which they lean heavily decided that $13,618 annually was too expensive for a medication that leads to sustained 15%-20% weight losses and those losses’ consequential benefits.
As a clinician working with patients who sustain those levels of weight loss, I find that conclusion confusing. Whether by way of lifestyle alone, or more often by way of lifestyle efforts plus medication or lifestyle efforts plus surgery, the benefits reported and seen with 15%-20% weight losses are almost uniformly huge. Patients are regularly seen discontinuing or reducing the dosage of multiple medications as a result of improvements to multiple weight-responsive comorbidities, and they also report objective benefits to mood, sleep, mobility, pain, and energy. Losing that much weight changes lives. Not to mention the impact that that degree of loss has on the primary prevention of so many diseases, including plausible reductions in many common cancers – reductions that have been shown to occur after surgery-related weight losses and for which there’s no plausible reason to imagine that they wouldn’t occur with pharmaceutical-related losses.
Are those discussions found in the NEJM op-ed or in the ICER report? Well, yes, sort of. However, in the NEJM op-ed, the word “prevention” isn’t used once, and unlike with oral hypoglycemics or antihypertensives, the authors state that with antiobesity medications, additional research is needed to determine whether medication-induced changes to A1c, blood pressure, and waist circumference would have clinical benefits: “Antiobesity medications have been shown to improve the surrogate end points of weight, glycated hemoglobin levels, systolic blood pressure, and waist circumference. Long-term studies are needed, however, to clarify how medication-induced changes in these surrogate markers translate to health outcomes.”
Primary prevention is mentioned in the ICER review, but in the “limitations” section where the authors explain that they didn’t include it in their modeling: “The long-term benefits of preventing other comorbidities including cancer, chronic kidney disease, osteoarthritis, and sleep apnea were not explicitly modeled in the base case.”
And they pretended that the impact on existing weight-responsive comorbidities mostly didn’t exist, too: “To limit the complexity of the cost-effectiveness model and to prevent double-counting of treatment benefits, we limited the long-term effects of treatments for weight management to cardiovascular risk and delays in the onset and/or diagnosis of diabetes mellitus.”
As far as cardiovascular disease (CVD) benefits go, you might have thought that it would be a slam dunk on that basis alone, at least according to a recent simple back-of-the-envelope math exercise presented at a recent American College of Cardiology conference, which applied the semaglutide treatment group weight changes in the STEP 1 trial to estimate the population impact on weight and obesity in 30- to 74-year-olds without prior CVD, and estimated 10-year CVD risks utilizing the BMI-based Framingham CVD risk scores. By their accounting, semaglutide treatment in eligible American patients has the potential to prevent over 1.6 million CVD events over 10 years.
Finally, even putting aside ICER’s admittedly and exceedingly narrow base case, what lifestyle-alone studies could ICER possibly be comparing with drug efficacy? And what does “alone” mean? Does “alone” mean with a months- or years long interprofessional behavioral program? Does “alone” mean by way of diet books? Does “alone” mean by way of simply “moving more and eating less”? I’m not aware of robust studies demonstrating any long-term meaningful, predictable, reproducible, durable weight loss outcomes for any lifestyle-only approach, intensive or otherwise.
It’s difficult for me to imagine a situation in which a drug other than an antiobesity drug would be found to have too many benefits to include in your cost-effectiveness analysis but where you’d be comfortable to run that analysis anyhow, and then come out against the drug’s recommendation and fearmonger about its use.
But then again, systemic weight bias is a hell of a drug.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa. He disclosed ties with Constant Health and Novo Nordisk, and has shared opinions via Weighty Matters and social media.
A version of this article originally appeared on Medscape.com.
where some economists opined that their coverage would be disastrous for Medicare.
Among their concerns? The drugs need to be taken long term (just like drugs for any other chronic condition). The new drugs are more expensive than the old drugs (just like new drugs for any other chronic condition). Lots of people will want to take them (just like highly effective drugs for any other chronic condition that has a significant quality-of-life or clinical impact). The U.K. recommended that they be covered only for 2 years (unlike drugs for any other chronic condition). And the Institute for Clinical and Economic Review (ICER) on which they lean heavily decided that $13,618 annually was too expensive for a medication that leads to sustained 15%-20% weight losses and those losses’ consequential benefits.
As a clinician working with patients who sustain those levels of weight loss, I find that conclusion confusing. Whether by way of lifestyle alone, or more often by way of lifestyle efforts plus medication or lifestyle efforts plus surgery, the benefits reported and seen with 15%-20% weight losses are almost uniformly huge. Patients are regularly seen discontinuing or reducing the dosage of multiple medications as a result of improvements to multiple weight-responsive comorbidities, and they also report objective benefits to mood, sleep, mobility, pain, and energy. Losing that much weight changes lives. Not to mention the impact that that degree of loss has on the primary prevention of so many diseases, including plausible reductions in many common cancers – reductions that have been shown to occur after surgery-related weight losses and for which there’s no plausible reason to imagine that they wouldn’t occur with pharmaceutical-related losses.
Are those discussions found in the NEJM op-ed or in the ICER report? Well, yes, sort of. However, in the NEJM op-ed, the word “prevention” isn’t used once, and unlike with oral hypoglycemics or antihypertensives, the authors state that with antiobesity medications, additional research is needed to determine whether medication-induced changes to A1c, blood pressure, and waist circumference would have clinical benefits: “Antiobesity medications have been shown to improve the surrogate end points of weight, glycated hemoglobin levels, systolic blood pressure, and waist circumference. Long-term studies are needed, however, to clarify how medication-induced changes in these surrogate markers translate to health outcomes.”
Primary prevention is mentioned in the ICER review, but in the “limitations” section where the authors explain that they didn’t include it in their modeling: “The long-term benefits of preventing other comorbidities including cancer, chronic kidney disease, osteoarthritis, and sleep apnea were not explicitly modeled in the base case.”
And they pretended that the impact on existing weight-responsive comorbidities mostly didn’t exist, too: “To limit the complexity of the cost-effectiveness model and to prevent double-counting of treatment benefits, we limited the long-term effects of treatments for weight management to cardiovascular risk and delays in the onset and/or diagnosis of diabetes mellitus.”
As far as cardiovascular disease (CVD) benefits go, you might have thought that it would be a slam dunk on that basis alone, at least according to a recent simple back-of-the-envelope math exercise presented at a recent American College of Cardiology conference, which applied the semaglutide treatment group weight changes in the STEP 1 trial to estimate the population impact on weight and obesity in 30- to 74-year-olds without prior CVD, and estimated 10-year CVD risks utilizing the BMI-based Framingham CVD risk scores. By their accounting, semaglutide treatment in eligible American patients has the potential to prevent over 1.6 million CVD events over 10 years.
Finally, even putting aside ICER’s admittedly and exceedingly narrow base case, what lifestyle-alone studies could ICER possibly be comparing with drug efficacy? And what does “alone” mean? Does “alone” mean with a months- or years long interprofessional behavioral program? Does “alone” mean by way of diet books? Does “alone” mean by way of simply “moving more and eating less”? I’m not aware of robust studies demonstrating any long-term meaningful, predictable, reproducible, durable weight loss outcomes for any lifestyle-only approach, intensive or otherwise.
It’s difficult for me to imagine a situation in which a drug other than an antiobesity drug would be found to have too many benefits to include in your cost-effectiveness analysis but where you’d be comfortable to run that analysis anyhow, and then come out against the drug’s recommendation and fearmonger about its use.
But then again, systemic weight bias is a hell of a drug.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa. He disclosed ties with Constant Health and Novo Nordisk, and has shared opinions via Weighty Matters and social media.
A version of this article originally appeared on Medscape.com.
‘Excess’ deaths surging, but why?
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.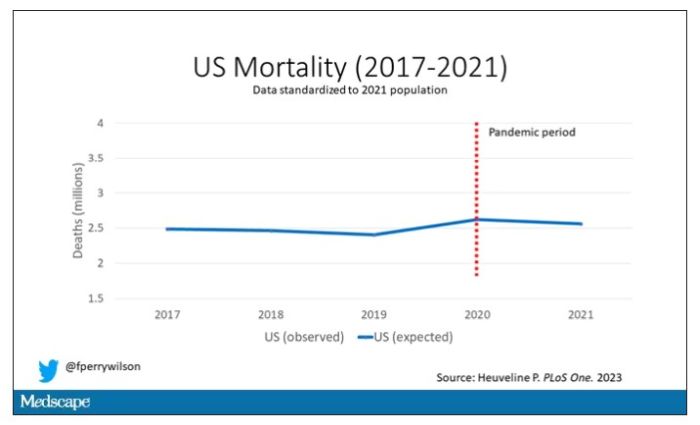
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.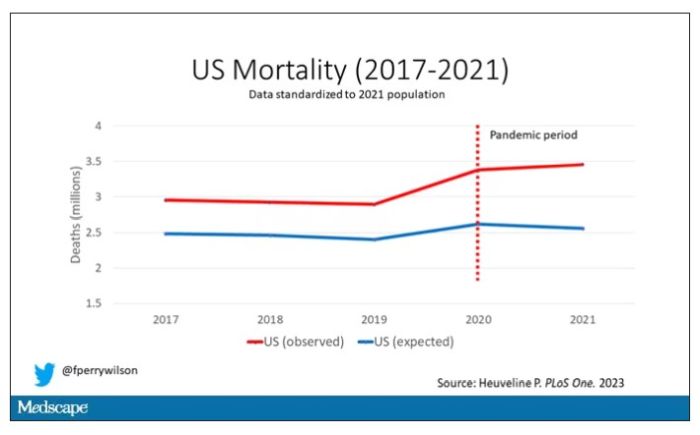
Highlighted here in green, then, is the excess mortality over time in the United States.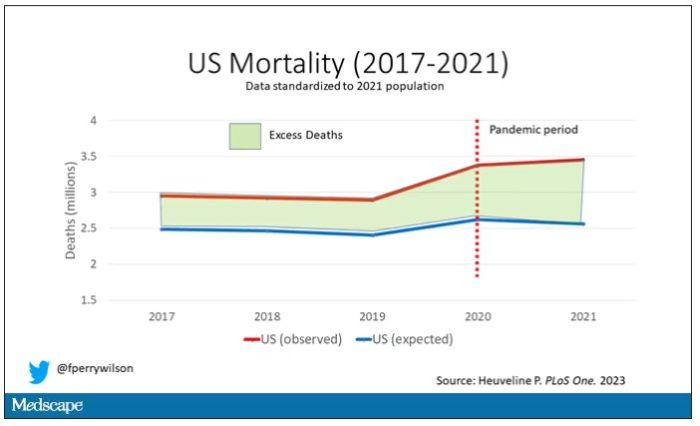
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
Is it time to stop treating high triglycerides?
The publication of the PROMINENT trial, where pemafibrate successfully lowered high levels but was not associated with a lower risk for cardiovascular events, reinforced the point. Is it time to stop measuring and treating high triglycerides?
There may be noncardiovascular reasons to treat hypertriglyceridemia. Pancreatitis is the most cited one, given that the risk for pancreatitis increases with increasing triglyceride levels, especially in patients with a prior episode.
There may also be practical reasons to lower trigs. Because most cholesterol panels use the Friedewald equation to calculate low-density lipoprotein cholesterol (LDL-C) rather than measuring it directly, very high triglyceride levels can invalidate the calculation and return error messages on lab reports.
But we now have alternatives to measuring LDL-C, including non–high-density lipoprotein cholesterol (HDL-C) and apolipoprotein B (apoB), that better predict risk and are usable even in the setting of nonfasting samples when triglycerides are elevated.
Independent cardiovascular risk factor?
If we are going to measure and treat high triglycerides for cardiovascular reasons, the relevant question is, are high triglycerides an independent risk factor for cardiovascular disease?
Proponents have a broad swath of supportive literature to point at. Multiple studies have shown an association between triglyceride levels and cardiovascular risk. The evidence even extends beyond traditional epidemiologic analyses, to genetic studies that should be free from some of the problems seen in observational cohorts.
But it is difficult to be certain whether these associations are causal or merely confounding. An unhealthy diet will increase triglycerides, as will alcohol. Patients with diabetes or metabolic syndrome have high triglycerides. So do patients with nephrotic syndrome or hypothyroidism, or hypertensive patients taking thiazide diuretics. Adjusting for these baseline factors is possible but imperfect, and residual confounding is always an issue. An analysis of the Reykjavik and the EPIC-Norfolk studies found an association between triglyceride levels and cardiovascular risk. That risk was attenuated, but not eliminated, when adjusted for traditional risk factors such as age, smoking, blood pressure, diabetes, and cholesterol.
Randomized trials of triglyceride-lowering therapies would help resolve the question of whether hypertriglyceridemia contributes to coronary disease or simply identifies high-risk patients. Early trials seemed to support the idea of a causal link. The Helsinki Heart Study randomized patients to gemfibrozil or placebo and found a 34% relative risk reduction in coronary artery disease with the fibrate. But gemfibrozil didn’t only reduce triglycerides. It also increased HDL-C and lowered LDL-C relative to placebo, which may explain the observed benefit.
Gemfibrozil is rarely used today because we can achieve much greater LDL-C reductions with statins, as well as ezetimibe and PCSK9 inhibitors. The success of these drugs may not leave any room for triglyceride-lowering medications.
The pre- vs. post-statin era
In the 2005 FIELD study, participants were randomized to receive fenofibrate or placebo. Although patients weren’t taking statin at study entry, 17% of the placebo group started taking one during the trial. Fenofibrate wasn’t associated with a reduction in the primary endpoint, a combination of coronary heart disease death or nonfatal myocardial infarction (MI). Among the many secondary endpoints, nonfatal MI was lower but cardiovascular mortality was not in the fibrate-treated patients. In the same vein, the 2010 ACCORD study randomized patients to receive simvastatin plus fenofibrate or simvastatin alone. The composite primary outcome of MI, stroke, and cardiovascular mortality was not lowered nor were any secondary outcomes with the combination therapy. In the statin era, triglyceride-lowering therapies have not shown much benefit.
The final nail in the coffin may very well be the aforementioned PROMINENT trial. The new agent, pemafibrate, fared no better than its predecessor fenofibrate. Pemafibrate had no impact on the study’s primary composite outcome of nonfatal MI, stroke, coronary revascularization, or cardiovascular death despite being very effective at lowering triglycerides (by more than 25%). Patients treated with pemafibrate had increased LDL-C and apoB compared with the placebo group. When you realize that, the results of the study are not very surprising.
Some point to the results of REDUCE-IT as proof that triglycerides are still a valid target for pharmacotherapy. The debate on whether REDUCE-IT tested a good drug or a bad placebo is one for another day. The salient point for today is that the benefits of eicosapentaenoic acid (EPA) were seen regardless of either baseline or final triglyceride level. EPA may lower cardiac risk, but there is no widespread consensus that it does so by lowering triglycerides. There may be other mechanisms at work.
You could still argue that high triglycerides have value as a risk prediction tool even if their role as a target for drug therapy is questionable. There was a time when medications to lower triglycerides had a benefit. But this is the post-statin era, and that time has passed.
If you see patients with high triglycerides, treating them with triglyceride-lowering medication probably isn’t going to reduce their cardiovascular risk. Dietary interventions, encouraging exercise, and reducing alcohol consumption are better options. Not only will they lead to lower cholesterol levels, but they’ll lower cardiovascular risk, too.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, with a degree in epidemiology. He has disclosed no relevant financial relationships. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal and is host of the award-winning podcast The Body of Evidence. The Body of Evidence.
A version of this article originally appeared on Medscape.com.
The publication of the PROMINENT trial, where pemafibrate successfully lowered high levels but was not associated with a lower risk for cardiovascular events, reinforced the point. Is it time to stop measuring and treating high triglycerides?
There may be noncardiovascular reasons to treat hypertriglyceridemia. Pancreatitis is the most cited one, given that the risk for pancreatitis increases with increasing triglyceride levels, especially in patients with a prior episode.
There may also be practical reasons to lower trigs. Because most cholesterol panels use the Friedewald equation to calculate low-density lipoprotein cholesterol (LDL-C) rather than measuring it directly, very high triglyceride levels can invalidate the calculation and return error messages on lab reports.
But we now have alternatives to measuring LDL-C, including non–high-density lipoprotein cholesterol (HDL-C) and apolipoprotein B (apoB), that better predict risk and are usable even in the setting of nonfasting samples when triglycerides are elevated.
Independent cardiovascular risk factor?
If we are going to measure and treat high triglycerides for cardiovascular reasons, the relevant question is, are high triglycerides an independent risk factor for cardiovascular disease?
Proponents have a broad swath of supportive literature to point at. Multiple studies have shown an association between triglyceride levels and cardiovascular risk. The evidence even extends beyond traditional epidemiologic analyses, to genetic studies that should be free from some of the problems seen in observational cohorts.
But it is difficult to be certain whether these associations are causal or merely confounding. An unhealthy diet will increase triglycerides, as will alcohol. Patients with diabetes or metabolic syndrome have high triglycerides. So do patients with nephrotic syndrome or hypothyroidism, or hypertensive patients taking thiazide diuretics. Adjusting for these baseline factors is possible but imperfect, and residual confounding is always an issue. An analysis of the Reykjavik and the EPIC-Norfolk studies found an association between triglyceride levels and cardiovascular risk. That risk was attenuated, but not eliminated, when adjusted for traditional risk factors such as age, smoking, blood pressure, diabetes, and cholesterol.
Randomized trials of triglyceride-lowering therapies would help resolve the question of whether hypertriglyceridemia contributes to coronary disease or simply identifies high-risk patients. Early trials seemed to support the idea of a causal link. The Helsinki Heart Study randomized patients to gemfibrozil or placebo and found a 34% relative risk reduction in coronary artery disease with the fibrate. But gemfibrozil didn’t only reduce triglycerides. It also increased HDL-C and lowered LDL-C relative to placebo, which may explain the observed benefit.
Gemfibrozil is rarely used today because we can achieve much greater LDL-C reductions with statins, as well as ezetimibe and PCSK9 inhibitors. The success of these drugs may not leave any room for triglyceride-lowering medications.
The pre- vs. post-statin era
In the 2005 FIELD study, participants were randomized to receive fenofibrate or placebo. Although patients weren’t taking statin at study entry, 17% of the placebo group started taking one during the trial. Fenofibrate wasn’t associated with a reduction in the primary endpoint, a combination of coronary heart disease death or nonfatal myocardial infarction (MI). Among the many secondary endpoints, nonfatal MI was lower but cardiovascular mortality was not in the fibrate-treated patients. In the same vein, the 2010 ACCORD study randomized patients to receive simvastatin plus fenofibrate or simvastatin alone. The composite primary outcome of MI, stroke, and cardiovascular mortality was not lowered nor were any secondary outcomes with the combination therapy. In the statin era, triglyceride-lowering therapies have not shown much benefit.
The final nail in the coffin may very well be the aforementioned PROMINENT trial. The new agent, pemafibrate, fared no better than its predecessor fenofibrate. Pemafibrate had no impact on the study’s primary composite outcome of nonfatal MI, stroke, coronary revascularization, or cardiovascular death despite being very effective at lowering triglycerides (by more than 25%). Patients treated with pemafibrate had increased LDL-C and apoB compared with the placebo group. When you realize that, the results of the study are not very surprising.
Some point to the results of REDUCE-IT as proof that triglycerides are still a valid target for pharmacotherapy. The debate on whether REDUCE-IT tested a good drug or a bad placebo is one for another day. The salient point for today is that the benefits of eicosapentaenoic acid (EPA) were seen regardless of either baseline or final triglyceride level. EPA may lower cardiac risk, but there is no widespread consensus that it does so by lowering triglycerides. There may be other mechanisms at work.
You could still argue that high triglycerides have value as a risk prediction tool even if their role as a target for drug therapy is questionable. There was a time when medications to lower triglycerides had a benefit. But this is the post-statin era, and that time has passed.
If you see patients with high triglycerides, treating them with triglyceride-lowering medication probably isn’t going to reduce their cardiovascular risk. Dietary interventions, encouraging exercise, and reducing alcohol consumption are better options. Not only will they lead to lower cholesterol levels, but they’ll lower cardiovascular risk, too.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, with a degree in epidemiology. He has disclosed no relevant financial relationships. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal and is host of the award-winning podcast The Body of Evidence. The Body of Evidence.
A version of this article originally appeared on Medscape.com.
The publication of the PROMINENT trial, where pemafibrate successfully lowered high levels but was not associated with a lower risk for cardiovascular events, reinforced the point. Is it time to stop measuring and treating high triglycerides?
There may be noncardiovascular reasons to treat hypertriglyceridemia. Pancreatitis is the most cited one, given that the risk for pancreatitis increases with increasing triglyceride levels, especially in patients with a prior episode.
There may also be practical reasons to lower trigs. Because most cholesterol panels use the Friedewald equation to calculate low-density lipoprotein cholesterol (LDL-C) rather than measuring it directly, very high triglyceride levels can invalidate the calculation and return error messages on lab reports.
But we now have alternatives to measuring LDL-C, including non–high-density lipoprotein cholesterol (HDL-C) and apolipoprotein B (apoB), that better predict risk and are usable even in the setting of nonfasting samples when triglycerides are elevated.
Independent cardiovascular risk factor?
If we are going to measure and treat high triglycerides for cardiovascular reasons, the relevant question is, are high triglycerides an independent risk factor for cardiovascular disease?
Proponents have a broad swath of supportive literature to point at. Multiple studies have shown an association between triglyceride levels and cardiovascular risk. The evidence even extends beyond traditional epidemiologic analyses, to genetic studies that should be free from some of the problems seen in observational cohorts.
But it is difficult to be certain whether these associations are causal or merely confounding. An unhealthy diet will increase triglycerides, as will alcohol. Patients with diabetes or metabolic syndrome have high triglycerides. So do patients with nephrotic syndrome or hypothyroidism, or hypertensive patients taking thiazide diuretics. Adjusting for these baseline factors is possible but imperfect, and residual confounding is always an issue. An analysis of the Reykjavik and the EPIC-Norfolk studies found an association between triglyceride levels and cardiovascular risk. That risk was attenuated, but not eliminated, when adjusted for traditional risk factors such as age, smoking, blood pressure, diabetes, and cholesterol.
Randomized trials of triglyceride-lowering therapies would help resolve the question of whether hypertriglyceridemia contributes to coronary disease or simply identifies high-risk patients. Early trials seemed to support the idea of a causal link. The Helsinki Heart Study randomized patients to gemfibrozil or placebo and found a 34% relative risk reduction in coronary artery disease with the fibrate. But gemfibrozil didn’t only reduce triglycerides. It also increased HDL-C and lowered LDL-C relative to placebo, which may explain the observed benefit.
Gemfibrozil is rarely used today because we can achieve much greater LDL-C reductions with statins, as well as ezetimibe and PCSK9 inhibitors. The success of these drugs may not leave any room for triglyceride-lowering medications.
The pre- vs. post-statin era
In the 2005 FIELD study, participants were randomized to receive fenofibrate or placebo. Although patients weren’t taking statin at study entry, 17% of the placebo group started taking one during the trial. Fenofibrate wasn’t associated with a reduction in the primary endpoint, a combination of coronary heart disease death or nonfatal myocardial infarction (MI). Among the many secondary endpoints, nonfatal MI was lower but cardiovascular mortality was not in the fibrate-treated patients. In the same vein, the 2010 ACCORD study randomized patients to receive simvastatin plus fenofibrate or simvastatin alone. The composite primary outcome of MI, stroke, and cardiovascular mortality was not lowered nor were any secondary outcomes with the combination therapy. In the statin era, triglyceride-lowering therapies have not shown much benefit.
The final nail in the coffin may very well be the aforementioned PROMINENT trial. The new agent, pemafibrate, fared no better than its predecessor fenofibrate. Pemafibrate had no impact on the study’s primary composite outcome of nonfatal MI, stroke, coronary revascularization, or cardiovascular death despite being very effective at lowering triglycerides (by more than 25%). Patients treated with pemafibrate had increased LDL-C and apoB compared with the placebo group. When you realize that, the results of the study are not very surprising.
Some point to the results of REDUCE-IT as proof that triglycerides are still a valid target for pharmacotherapy. The debate on whether REDUCE-IT tested a good drug or a bad placebo is one for another day. The salient point for today is that the benefits of eicosapentaenoic acid (EPA) were seen regardless of either baseline or final triglyceride level. EPA may lower cardiac risk, but there is no widespread consensus that it does so by lowering triglycerides. There may be other mechanisms at work.
You could still argue that high triglycerides have value as a risk prediction tool even if their role as a target for drug therapy is questionable. There was a time when medications to lower triglycerides had a benefit. But this is the post-statin era, and that time has passed.
If you see patients with high triglycerides, treating them with triglyceride-lowering medication probably isn’t going to reduce their cardiovascular risk. Dietary interventions, encouraging exercise, and reducing alcohol consumption are better options. Not only will they lead to lower cholesterol levels, but they’ll lower cardiovascular risk, too.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, with a degree in epidemiology. He has disclosed no relevant financial relationships. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal and is host of the award-winning podcast The Body of Evidence. The Body of Evidence.
A version of this article originally appeared on Medscape.com.
Could a baby’s gut health be an early predictor of future type 1 diabetes?
Microbial biomarkers for type 1 diabetes may be present in infants as young as 12 months old, suggesting the potential to mitigate disease onset by nurturing a healthy gut microbiome early, show data from the Swedish general population.
“Our findings indicate that the gut of infants who go on to develop type 1 diabetes is notably different from healthy babies,” said Malin Bélteky, MD, from the Crown Princess Victoria’s Children’s Hospital, Linköping, Sweden, who jointly led the work, which was recently published in Diabetologia, alongside Patricia L. Milletich, PhD candidate, from the University of Florida, Gainesville.
“This discovery could be used to help identity infants at [the] highest risk of developing type 1 diabetes before or during the first stage of disease and could offer the opportunity to bolster a healthy gut microbiome to prevent the disease from becoming established,” added Dr. Bélteky.
Currently, beta-cell autoantibodies are used to predict disease, which are usually only identifiable between 9 and 36 months of age.
Marian Rewers, MD, PhD, professor of pediatrics & medicine, University of Colorado, Denver, and principal investigator of The Environmental Determinants of Diabetes in the Young (TEDDY) study, welcomed the findings, saying it is a well-designed study from a strong group of investigators.
“While the effective number of cases was very small [n = 16], the results were apparently adjusted for multiple comparisons, and significant differences were noted in the microbiome of cases versus controls at 1 year of age. This was 12 years prior to the average age of type 1 diabetes diagnosis in the cases,” he said.
“The differences in diversity and abundances of specific bacteria need to be interpreted with caution; however, the study results are consistent with several previous reports,” he noted.
Differences in microbial diversity and function
Data were drawn from children participating in the longitudinal, general population All Babies In Southeast Sweden (ABIS) study. Microbiota from stool samples, taken at age 1 year, were sequenced and analyzed to establish diversity, abundance, and functional status of the component bacteria. Questionnaires were completed at birth and at 1 year of age, allowing for the study of environmental factors that might influence the microbiota or type 1 diabetes risk independently. Parent diaries provided information on pregnancy, nutrition, and lifestyle factors.
Of the cohort of 167 children who developed type 1 diabetes by 2020, stool samples were available for 16 of these participants, which were compared with 268 healthy controls. The microbiomes of the 16 infants who later developed type 1 diabetes were compared with 100 iterations of 32 matched control infants (matched by geographical region, siblings at birth, residence type, duration of breastfeeding, and month of stool collection) who didn’t develop type 1 diabetes by the age of 20.
Specific bacteria found in greater abundance in children who later developed type 1 diabetes, compared with those who didn’t, included Firmicutes (Enterococcus, Gemella, and Hungatella), as well as Bacteroides (Bacteroides and Porphyromonas), known to promote inflammation and be involved in the immune response.
Bacteria with greater abundance in children who didn’t develop type 1 diabetes, compared with those who did, were Firmicutes (Anaerostipes, Flavonifractor, and Ruminococcaceae UBA1819, and Eubacterium). These species help maintain metabolic and immune health and produce butyrate, an important short-chain fatty acid that helps prevent inflammation and fuels the cells of the gut lining.
Alistipes were more abundant in infants who didn’t develop type 1 diabetes, and various abundances of Fusicatenibacter were the strongest factors for differentiating future type 1 diabetes, reported the researchers.
“Gut microbial biomarkers at 12 months would benefit the prediction opportunity well before the onset of multiple autoantibodies,” write the authors.
The youngest age at type 1 diabetes diagnosis was aged 1 year, 4 months, and the oldest was aged 21 years, 4 months. The mean age at diagnosis was 13.3 years.
The microbial differences found between infants who go on to develop type 1 diabetes and those who don’t also shed light on interactions between the developing immune system and short-chain fatty acid production and metabolism in childhood autoimmunity, write the authors.
Prior studies have found fewer short-chain fatty acid–producing microbiota in the gut of children with early-onset autoantibody development. This study confirmed these data, finding a decrease in butyrate-producing bacteria (Anaerostipes, Flavonifractor, Ruminococcaceae UBA1819, and Eubacterium) in infants who went on to develop type 1 diabetes. Likewise, a reduction in pyruvate fermentation was found in those infants with future disease.
According to coauthor Eric Triplett, PhD, from the University of Florida, Gainesville: “The autoimmune processes usually begin long before any clinical signs of disease appear, highlighting how differences in the makeup of the infant gut microbiome could shed important light on the complex interaction between the developing immune system, environmental exposures in childhood, and autoimmunity. Studies with much larger cohorts of prospectively traced individuals will be required to establish which are the strongest biomarkers and how effectively they can predict disease.”
The authors and Dr. Rewers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Microbial biomarkers for type 1 diabetes may be present in infants as young as 12 months old, suggesting the potential to mitigate disease onset by nurturing a healthy gut microbiome early, show data from the Swedish general population.
“Our findings indicate that the gut of infants who go on to develop type 1 diabetes is notably different from healthy babies,” said Malin Bélteky, MD, from the Crown Princess Victoria’s Children’s Hospital, Linköping, Sweden, who jointly led the work, which was recently published in Diabetologia, alongside Patricia L. Milletich, PhD candidate, from the University of Florida, Gainesville.
“This discovery could be used to help identity infants at [the] highest risk of developing type 1 diabetes before or during the first stage of disease and could offer the opportunity to bolster a healthy gut microbiome to prevent the disease from becoming established,” added Dr. Bélteky.
Currently, beta-cell autoantibodies are used to predict disease, which are usually only identifiable between 9 and 36 months of age.
Marian Rewers, MD, PhD, professor of pediatrics & medicine, University of Colorado, Denver, and principal investigator of The Environmental Determinants of Diabetes in the Young (TEDDY) study, welcomed the findings, saying it is a well-designed study from a strong group of investigators.
“While the effective number of cases was very small [n = 16], the results were apparently adjusted for multiple comparisons, and significant differences were noted in the microbiome of cases versus controls at 1 year of age. This was 12 years prior to the average age of type 1 diabetes diagnosis in the cases,” he said.
“The differences in diversity and abundances of specific bacteria need to be interpreted with caution; however, the study results are consistent with several previous reports,” he noted.
Differences in microbial diversity and function
Data were drawn from children participating in the longitudinal, general population All Babies In Southeast Sweden (ABIS) study. Microbiota from stool samples, taken at age 1 year, were sequenced and analyzed to establish diversity, abundance, and functional status of the component bacteria. Questionnaires were completed at birth and at 1 year of age, allowing for the study of environmental factors that might influence the microbiota or type 1 diabetes risk independently. Parent diaries provided information on pregnancy, nutrition, and lifestyle factors.
Of the cohort of 167 children who developed type 1 diabetes by 2020, stool samples were available for 16 of these participants, which were compared with 268 healthy controls. The microbiomes of the 16 infants who later developed type 1 diabetes were compared with 100 iterations of 32 matched control infants (matched by geographical region, siblings at birth, residence type, duration of breastfeeding, and month of stool collection) who didn’t develop type 1 diabetes by the age of 20.
Specific bacteria found in greater abundance in children who later developed type 1 diabetes, compared with those who didn’t, included Firmicutes (Enterococcus, Gemella, and Hungatella), as well as Bacteroides (Bacteroides and Porphyromonas), known to promote inflammation and be involved in the immune response.
Bacteria with greater abundance in children who didn’t develop type 1 diabetes, compared with those who did, were Firmicutes (Anaerostipes, Flavonifractor, and Ruminococcaceae UBA1819, and Eubacterium). These species help maintain metabolic and immune health and produce butyrate, an important short-chain fatty acid that helps prevent inflammation and fuels the cells of the gut lining.
Alistipes were more abundant in infants who didn’t develop type 1 diabetes, and various abundances of Fusicatenibacter were the strongest factors for differentiating future type 1 diabetes, reported the researchers.
“Gut microbial biomarkers at 12 months would benefit the prediction opportunity well before the onset of multiple autoantibodies,” write the authors.
The youngest age at type 1 diabetes diagnosis was aged 1 year, 4 months, and the oldest was aged 21 years, 4 months. The mean age at diagnosis was 13.3 years.
The microbial differences found between infants who go on to develop type 1 diabetes and those who don’t also shed light on interactions between the developing immune system and short-chain fatty acid production and metabolism in childhood autoimmunity, write the authors.
Prior studies have found fewer short-chain fatty acid–producing microbiota in the gut of children with early-onset autoantibody development. This study confirmed these data, finding a decrease in butyrate-producing bacteria (Anaerostipes, Flavonifractor, Ruminococcaceae UBA1819, and Eubacterium) in infants who went on to develop type 1 diabetes. Likewise, a reduction in pyruvate fermentation was found in those infants with future disease.
According to coauthor Eric Triplett, PhD, from the University of Florida, Gainesville: “The autoimmune processes usually begin long before any clinical signs of disease appear, highlighting how differences in the makeup of the infant gut microbiome could shed important light on the complex interaction between the developing immune system, environmental exposures in childhood, and autoimmunity. Studies with much larger cohorts of prospectively traced individuals will be required to establish which are the strongest biomarkers and how effectively they can predict disease.”
The authors and Dr. Rewers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Microbial biomarkers for type 1 diabetes may be present in infants as young as 12 months old, suggesting the potential to mitigate disease onset by nurturing a healthy gut microbiome early, show data from the Swedish general population.
“Our findings indicate that the gut of infants who go on to develop type 1 diabetes is notably different from healthy babies,” said Malin Bélteky, MD, from the Crown Princess Victoria’s Children’s Hospital, Linköping, Sweden, who jointly led the work, which was recently published in Diabetologia, alongside Patricia L. Milletich, PhD candidate, from the University of Florida, Gainesville.
“This discovery could be used to help identity infants at [the] highest risk of developing type 1 diabetes before or during the first stage of disease and could offer the opportunity to bolster a healthy gut microbiome to prevent the disease from becoming established,” added Dr. Bélteky.
Currently, beta-cell autoantibodies are used to predict disease, which are usually only identifiable between 9 and 36 months of age.
Marian Rewers, MD, PhD, professor of pediatrics & medicine, University of Colorado, Denver, and principal investigator of The Environmental Determinants of Diabetes in the Young (TEDDY) study, welcomed the findings, saying it is a well-designed study from a strong group of investigators.
“While the effective number of cases was very small [n = 16], the results were apparently adjusted for multiple comparisons, and significant differences were noted in the microbiome of cases versus controls at 1 year of age. This was 12 years prior to the average age of type 1 diabetes diagnosis in the cases,” he said.
“The differences in diversity and abundances of specific bacteria need to be interpreted with caution; however, the study results are consistent with several previous reports,” he noted.
Differences in microbial diversity and function
Data were drawn from children participating in the longitudinal, general population All Babies In Southeast Sweden (ABIS) study. Microbiota from stool samples, taken at age 1 year, were sequenced and analyzed to establish diversity, abundance, and functional status of the component bacteria. Questionnaires were completed at birth and at 1 year of age, allowing for the study of environmental factors that might influence the microbiota or type 1 diabetes risk independently. Parent diaries provided information on pregnancy, nutrition, and lifestyle factors.
Of the cohort of 167 children who developed type 1 diabetes by 2020, stool samples were available for 16 of these participants, which were compared with 268 healthy controls. The microbiomes of the 16 infants who later developed type 1 diabetes were compared with 100 iterations of 32 matched control infants (matched by geographical region, siblings at birth, residence type, duration of breastfeeding, and month of stool collection) who didn’t develop type 1 diabetes by the age of 20.
Specific bacteria found in greater abundance in children who later developed type 1 diabetes, compared with those who didn’t, included Firmicutes (Enterococcus, Gemella, and Hungatella), as well as Bacteroides (Bacteroides and Porphyromonas), known to promote inflammation and be involved in the immune response.
Bacteria with greater abundance in children who didn’t develop type 1 diabetes, compared with those who did, were Firmicutes (Anaerostipes, Flavonifractor, and Ruminococcaceae UBA1819, and Eubacterium). These species help maintain metabolic and immune health and produce butyrate, an important short-chain fatty acid that helps prevent inflammation and fuels the cells of the gut lining.
Alistipes were more abundant in infants who didn’t develop type 1 diabetes, and various abundances of Fusicatenibacter were the strongest factors for differentiating future type 1 diabetes, reported the researchers.
“Gut microbial biomarkers at 12 months would benefit the prediction opportunity well before the onset of multiple autoantibodies,” write the authors.
The youngest age at type 1 diabetes diagnosis was aged 1 year, 4 months, and the oldest was aged 21 years, 4 months. The mean age at diagnosis was 13.3 years.
The microbial differences found between infants who go on to develop type 1 diabetes and those who don’t also shed light on interactions between the developing immune system and short-chain fatty acid production and metabolism in childhood autoimmunity, write the authors.
Prior studies have found fewer short-chain fatty acid–producing microbiota in the gut of children with early-onset autoantibody development. This study confirmed these data, finding a decrease in butyrate-producing bacteria (Anaerostipes, Flavonifractor, Ruminococcaceae UBA1819, and Eubacterium) in infants who went on to develop type 1 diabetes. Likewise, a reduction in pyruvate fermentation was found in those infants with future disease.
According to coauthor Eric Triplett, PhD, from the University of Florida, Gainesville: “The autoimmune processes usually begin long before any clinical signs of disease appear, highlighting how differences in the makeup of the infant gut microbiome could shed important light on the complex interaction between the developing immune system, environmental exposures in childhood, and autoimmunity. Studies with much larger cohorts of prospectively traced individuals will be required to establish which are the strongest biomarkers and how effectively they can predict disease.”
The authors and Dr. Rewers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA panels vote to modify isotretinoin iPLEDGE REMS
At a joint meeting of a drug for severe, nodular acne that is highly teratogenic.
The first vote was on whether to continue the 19-day lockout period for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the 7-day prescription window. Those patients currently have to wait 19 days to get their second pregnancy test and receive the medication.
Most (17) of the 22 voting members voted not to continue the 19-day period; 4 voted to keep it; and 1 abstained. But there was no consensus on when the second pregnancy test should occur if the 19-day lockout is changed.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among those voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
The second question concerned patients who cannot become pregnant, and it asked when REMS should require that the prescriber document counseling the patient in the iPLEDGE system. The current requirement is monthly.
Listed options and the number of votes for each were:
- Only with the first prescription as part of patient enrollment (10)
- Monthly (1)
- Every 120 days (6)
- Some other frequency (5)
For this question too, while the members largely agreed the current monthly requirement is too burdensome, there was little agreement on what the most appropriate interval should be.
Lack of data
On both questions, several advisory committee members cited a lack of data on which they could base their decision.
On the documentation question, Megha Tollefson, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., said she voted for the fourth option (some other frequency) with the thought of yearly attestation.
“As a part of this, providers have to provide monthly counseling,” Dr. Tollefson said. “This is just a documentation requirement in the iPLEDGE system. I think most prescribers do document their monthly counseling in their own medical records. I would say it would be okay not to redocument that in iPLEDGE.”
The two votes came at the end of the second day of a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee in which experts addressed ways to improve the iPLEDGE REMS for isotretinoin. A transition to a new platform for the iPLEDGE program caused chaos after its rollout at the end of 2021, resulting in extensive delays and denial of prescriptions.
The committees sought to balance reducing burden with maintaining safety and preventing fetal exposures to isotretinoin.
They were also tasked with discussing other REMS requirements without taking a vote on each topic.
Among those topics was whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most who spoke to the issue agreed that home tests should continue in an effort to increase access and decrease burden. Members suggested safeguards against falsified results that have been documented, including assigning names and barcodes to the test results and uploading the verification to the iPLEDGE website.
The advisory committees also discussed recommendations to encourage more participation in the iPLEDGE Pregnancy Registry.
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
A version of this article first appeared on Medscape.com.
At a joint meeting of a drug for severe, nodular acne that is highly teratogenic.
The first vote was on whether to continue the 19-day lockout period for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the 7-day prescription window. Those patients currently have to wait 19 days to get their second pregnancy test and receive the medication.
Most (17) of the 22 voting members voted not to continue the 19-day period; 4 voted to keep it; and 1 abstained. But there was no consensus on when the second pregnancy test should occur if the 19-day lockout is changed.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among those voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
The second question concerned patients who cannot become pregnant, and it asked when REMS should require that the prescriber document counseling the patient in the iPLEDGE system. The current requirement is monthly.
Listed options and the number of votes for each were:
- Only with the first prescription as part of patient enrollment (10)
- Monthly (1)
- Every 120 days (6)
- Some other frequency (5)
For this question too, while the members largely agreed the current monthly requirement is too burdensome, there was little agreement on what the most appropriate interval should be.
Lack of data
On both questions, several advisory committee members cited a lack of data on which they could base their decision.
On the documentation question, Megha Tollefson, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., said she voted for the fourth option (some other frequency) with the thought of yearly attestation.
“As a part of this, providers have to provide monthly counseling,” Dr. Tollefson said. “This is just a documentation requirement in the iPLEDGE system. I think most prescribers do document their monthly counseling in their own medical records. I would say it would be okay not to redocument that in iPLEDGE.”
The two votes came at the end of the second day of a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee in which experts addressed ways to improve the iPLEDGE REMS for isotretinoin. A transition to a new platform for the iPLEDGE program caused chaos after its rollout at the end of 2021, resulting in extensive delays and denial of prescriptions.
The committees sought to balance reducing burden with maintaining safety and preventing fetal exposures to isotretinoin.
They were also tasked with discussing other REMS requirements without taking a vote on each topic.
Among those topics was whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most who spoke to the issue agreed that home tests should continue in an effort to increase access and decrease burden. Members suggested safeguards against falsified results that have been documented, including assigning names and barcodes to the test results and uploading the verification to the iPLEDGE website.
The advisory committees also discussed recommendations to encourage more participation in the iPLEDGE Pregnancy Registry.
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
A version of this article first appeared on Medscape.com.
At a joint meeting of a drug for severe, nodular acne that is highly teratogenic.
The first vote was on whether to continue the 19-day lockout period for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the 7-day prescription window. Those patients currently have to wait 19 days to get their second pregnancy test and receive the medication.
Most (17) of the 22 voting members voted not to continue the 19-day period; 4 voted to keep it; and 1 abstained. But there was no consensus on when the second pregnancy test should occur if the 19-day lockout is changed.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among those voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
The second question concerned patients who cannot become pregnant, and it asked when REMS should require that the prescriber document counseling the patient in the iPLEDGE system. The current requirement is monthly.
Listed options and the number of votes for each were:
- Only with the first prescription as part of patient enrollment (10)
- Monthly (1)
- Every 120 days (6)
- Some other frequency (5)
For this question too, while the members largely agreed the current monthly requirement is too burdensome, there was little agreement on what the most appropriate interval should be.
Lack of data
On both questions, several advisory committee members cited a lack of data on which they could base their decision.
On the documentation question, Megha Tollefson, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., said she voted for the fourth option (some other frequency) with the thought of yearly attestation.
“As a part of this, providers have to provide monthly counseling,” Dr. Tollefson said. “This is just a documentation requirement in the iPLEDGE system. I think most prescribers do document their monthly counseling in their own medical records. I would say it would be okay not to redocument that in iPLEDGE.”
The two votes came at the end of the second day of a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee in which experts addressed ways to improve the iPLEDGE REMS for isotretinoin. A transition to a new platform for the iPLEDGE program caused chaos after its rollout at the end of 2021, resulting in extensive delays and denial of prescriptions.
The committees sought to balance reducing burden with maintaining safety and preventing fetal exposures to isotretinoin.
They were also tasked with discussing other REMS requirements without taking a vote on each topic.
Among those topics was whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most who spoke to the issue agreed that home tests should continue in an effort to increase access and decrease burden. Members suggested safeguards against falsified results that have been documented, including assigning names and barcodes to the test results and uploading the verification to the iPLEDGE website.
The advisory committees also discussed recommendations to encourage more participation in the iPLEDGE Pregnancy Registry.
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
A version of this article first appeared on Medscape.com.
Sweaty treatment for social anxiety could pass the sniff test
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
One or two high-step days may reduce mortality risks
Taking 8,000 steps or more for just 1 or 2 days a week was linked to a significant reduction in all-cause and cardiovascular mortality, according to a study of about 3,000 adults.
Previous research has shown lower mortality rates among individuals who walk consistently, especially those who log at least 8,000 steps daily, but the benefit of intense walking just once or twice a week on long-term health outcomes has not been examined, wrote Kosuke Inoue, MD, of Kyoto University, Japan, and colleagues.
In a study published in JAMA Network Open, the researchers reviewed 10-year follow-up data for 3,101 adults aged 20 years and older who were part of the 2005 and 2006 National Health and Nutrition Examination Survey (NHANES).
The participants were asked to wear accelerometers to track their steps for 7 consecutive days. The researchers assessed the dose-response relationship between days of taking 8,000 steps or more (about 4 miles) during 1 week, and the primary outcome of all-cause mortality risk after 10 years. Cardiovascular mortality risk after 10 years was a secondary outcome.
The mean age of the participants was 50.5 years and 51% were women. The breakdown by ethnicity was 51% White, 21% Black, 24% Hispanic, and 4% other races/ethnicities. A total of 632 individuals took 8,000 steps or more 0 days a week, 532 took at least 8,000 steps 1-2 days per week, and 1,937 took at least 8,000 steps 3-7 days a week.
During the 10-year follow-up period, overall all-cause mortality was 14.2% and cardiovascular mortality was 5.3% across all step groups.
In an adjusted analysis, individuals who took at least 8,000 steps 1-2 days a week had a 14.9% lower all-cause mortality risk compared with those who never reached 8,000 daily steps. This difference was similar to the 16.5% reduced mortality risk for those who took at least 8,000 steps 3-7 days a week.
Similarly, compared with the group with no days of at least 8,000 steps, cardiovascular mortality risk was 8.1% lower for those who took 8,000 steps 1-2 days per week and 8.4% lower for those who took at least 8,000 steps 3-7 days per week. The decreased mortality risk plateaued at 3-4 days.
These patterns in reduced all-cause mortality risk persisted in a stratified analysis by age (younger than 65 years and 65 years and older) and sex. Similar patterns in reduced mortality also emerged when the researchers used different thresholds of daily steps, such as a minimum of 10,000 steps instead of 8,000. The adjusted all-cause mortality for groups who took at least 10,000 steps 1-2 days a week, 3-7 days a week, and no days a week were 8.1%, 7.3%, and 16.7%, respectively, with corresponding cardiovascular mortality risks of 2.4%, 2.3%, and 7.0%, respectively.
“Given the simplicity and ease of counting daily steps, our findings indicate that the recommended number of steps taken on as few as 1 to 2 days per week may be a feasible option for individuals who are striving to achieve some health benefits through adhering to a recommended daily step count but are unable to accomplish this on a daily basis,” the researchers wrote in their discussion.
The findings were limited by several factors including the use daily step measures for 1 week only at baseline, with no data on how physical activity changes might impact mortality risk, the researchers noted. Other limitations included possible accelerometer error and misclassification of activity, possible selection bias, and lack of data on cause-specific mortality outside of cardiovascular death, they said.
However, the results were strengthened by the use of accelerometers as objective measures of activity and by the availability of 10-year follow-up data for nearly 100% of the participants, they said.
“Although our findings might suffer from residual confounding that should be addressed in future research, they suggest that people may receive substantial health benefits even if a sufficient number of steps are taken on only a couple days of the week,” they concluded.
Proceed with caution
The current study findings should be interpreted cautiously in light of the potential unmeasured confounding factors and selection bias that often occur in studies of physical activity, James Sawalla Guseh, MD, of Massachusetts General Hospital, and Jose F. Figueroa, MD, of Harvard T.H. Chan School of Public Health, Boston, wrote in an accompanying editorial.
The results support previous studies showing some longevity benefits with “weekend warrior” patterns of intense physical activity for only a couple of days; however, “the body of evidence for sporadic activity is not as robust as the evidence for sustained and regular aerobic activity,” the authors emphasized.
The editorial authors also highlighted the limitations of the current study, including the observational design and significant differences in demographics and comorbidities between the 1- to 2-days of 8,000 steps exercise group and the 0-day group, as well as the reliance on only a week’s worth of data to infer 10 years’ mortality.
Although the data are consistent with previous observations that increased exercise volume reduces mortality, more research is needed, as the current study findings may not reflect other dimensions of health, including neurological health, they said.
Despite the need for cautious interpretation of the results, the current study “supports the emerging and popular idea that step counting, which does not require consideration of exercise duration or intensity, can offer guidance toward robust and favorable health outcomes,” and may inform step-based activity goals to improve public health, the editorialists wrote.
The study was supported by the Japan Agency for Medical Research and Development, the Japan Society for the Promotion of Science, the Japan Endocrine Society, and the Meiji Yasuda Life Foundation of Health and Welfare. Dr. Inoue also was supported by the Program for the Development of Next-Generation Leading Scientists With Global Insight sponsored by the Ministry of Education, Culture, Sports, Science and Technology, Japan. The other researchers had no relevant financial conflicts to disclose. The editorial authors had no financial conflicts to disclose.
Taking 8,000 steps or more for just 1 or 2 days a week was linked to a significant reduction in all-cause and cardiovascular mortality, according to a study of about 3,000 adults.
Previous research has shown lower mortality rates among individuals who walk consistently, especially those who log at least 8,000 steps daily, but the benefit of intense walking just once or twice a week on long-term health outcomes has not been examined, wrote Kosuke Inoue, MD, of Kyoto University, Japan, and colleagues.
In a study published in JAMA Network Open, the researchers reviewed 10-year follow-up data for 3,101 adults aged 20 years and older who were part of the 2005 and 2006 National Health and Nutrition Examination Survey (NHANES).
The participants were asked to wear accelerometers to track their steps for 7 consecutive days. The researchers assessed the dose-response relationship between days of taking 8,000 steps or more (about 4 miles) during 1 week, and the primary outcome of all-cause mortality risk after 10 years. Cardiovascular mortality risk after 10 years was a secondary outcome.
The mean age of the participants was 50.5 years and 51% were women. The breakdown by ethnicity was 51% White, 21% Black, 24% Hispanic, and 4% other races/ethnicities. A total of 632 individuals took 8,000 steps or more 0 days a week, 532 took at least 8,000 steps 1-2 days per week, and 1,937 took at least 8,000 steps 3-7 days a week.
During the 10-year follow-up period, overall all-cause mortality was 14.2% and cardiovascular mortality was 5.3% across all step groups.
In an adjusted analysis, individuals who took at least 8,000 steps 1-2 days a week had a 14.9% lower all-cause mortality risk compared with those who never reached 8,000 daily steps. This difference was similar to the 16.5% reduced mortality risk for those who took at least 8,000 steps 3-7 days a week.
Similarly, compared with the group with no days of at least 8,000 steps, cardiovascular mortality risk was 8.1% lower for those who took 8,000 steps 1-2 days per week and 8.4% lower for those who took at least 8,000 steps 3-7 days per week. The decreased mortality risk plateaued at 3-4 days.
These patterns in reduced all-cause mortality risk persisted in a stratified analysis by age (younger than 65 years and 65 years and older) and sex. Similar patterns in reduced mortality also emerged when the researchers used different thresholds of daily steps, such as a minimum of 10,000 steps instead of 8,000. The adjusted all-cause mortality for groups who took at least 10,000 steps 1-2 days a week, 3-7 days a week, and no days a week were 8.1%, 7.3%, and 16.7%, respectively, with corresponding cardiovascular mortality risks of 2.4%, 2.3%, and 7.0%, respectively.
“Given the simplicity and ease of counting daily steps, our findings indicate that the recommended number of steps taken on as few as 1 to 2 days per week may be a feasible option for individuals who are striving to achieve some health benefits through adhering to a recommended daily step count but are unable to accomplish this on a daily basis,” the researchers wrote in their discussion.
The findings were limited by several factors including the use daily step measures for 1 week only at baseline, with no data on how physical activity changes might impact mortality risk, the researchers noted. Other limitations included possible accelerometer error and misclassification of activity, possible selection bias, and lack of data on cause-specific mortality outside of cardiovascular death, they said.
However, the results were strengthened by the use of accelerometers as objective measures of activity and by the availability of 10-year follow-up data for nearly 100% of the participants, they said.
“Although our findings might suffer from residual confounding that should be addressed in future research, they suggest that people may receive substantial health benefits even if a sufficient number of steps are taken on only a couple days of the week,” they concluded.
Proceed with caution
The current study findings should be interpreted cautiously in light of the potential unmeasured confounding factors and selection bias that often occur in studies of physical activity, James Sawalla Guseh, MD, of Massachusetts General Hospital, and Jose F. Figueroa, MD, of Harvard T.H. Chan School of Public Health, Boston, wrote in an accompanying editorial.
The results support previous studies showing some longevity benefits with “weekend warrior” patterns of intense physical activity for only a couple of days; however, “the body of evidence for sporadic activity is not as robust as the evidence for sustained and regular aerobic activity,” the authors emphasized.
The editorial authors also highlighted the limitations of the current study, including the observational design and significant differences in demographics and comorbidities between the 1- to 2-days of 8,000 steps exercise group and the 0-day group, as well as the reliance on only a week’s worth of data to infer 10 years’ mortality.
Although the data are consistent with previous observations that increased exercise volume reduces mortality, more research is needed, as the current study findings may not reflect other dimensions of health, including neurological health, they said.
Despite the need for cautious interpretation of the results, the current study “supports the emerging and popular idea that step counting, which does not require consideration of exercise duration or intensity, can offer guidance toward robust and favorable health outcomes,” and may inform step-based activity goals to improve public health, the editorialists wrote.
The study was supported by the Japan Agency for Medical Research and Development, the Japan Society for the Promotion of Science, the Japan Endocrine Society, and the Meiji Yasuda Life Foundation of Health and Welfare. Dr. Inoue also was supported by the Program for the Development of Next-Generation Leading Scientists With Global Insight sponsored by the Ministry of Education, Culture, Sports, Science and Technology, Japan. The other researchers had no relevant financial conflicts to disclose. The editorial authors had no financial conflicts to disclose.
Taking 8,000 steps or more for just 1 or 2 days a week was linked to a significant reduction in all-cause and cardiovascular mortality, according to a study of about 3,000 adults.
Previous research has shown lower mortality rates among individuals who walk consistently, especially those who log at least 8,000 steps daily, but the benefit of intense walking just once or twice a week on long-term health outcomes has not been examined, wrote Kosuke Inoue, MD, of Kyoto University, Japan, and colleagues.
In a study published in JAMA Network Open, the researchers reviewed 10-year follow-up data for 3,101 adults aged 20 years and older who were part of the 2005 and 2006 National Health and Nutrition Examination Survey (NHANES).
The participants were asked to wear accelerometers to track their steps for 7 consecutive days. The researchers assessed the dose-response relationship between days of taking 8,000 steps or more (about 4 miles) during 1 week, and the primary outcome of all-cause mortality risk after 10 years. Cardiovascular mortality risk after 10 years was a secondary outcome.
The mean age of the participants was 50.5 years and 51% were women. The breakdown by ethnicity was 51% White, 21% Black, 24% Hispanic, and 4% other races/ethnicities. A total of 632 individuals took 8,000 steps or more 0 days a week, 532 took at least 8,000 steps 1-2 days per week, and 1,937 took at least 8,000 steps 3-7 days a week.
During the 10-year follow-up period, overall all-cause mortality was 14.2% and cardiovascular mortality was 5.3% across all step groups.
In an adjusted analysis, individuals who took at least 8,000 steps 1-2 days a week had a 14.9% lower all-cause mortality risk compared with those who never reached 8,000 daily steps. This difference was similar to the 16.5% reduced mortality risk for those who took at least 8,000 steps 3-7 days a week.
Similarly, compared with the group with no days of at least 8,000 steps, cardiovascular mortality risk was 8.1% lower for those who took 8,000 steps 1-2 days per week and 8.4% lower for those who took at least 8,000 steps 3-7 days per week. The decreased mortality risk plateaued at 3-4 days.
These patterns in reduced all-cause mortality risk persisted in a stratified analysis by age (younger than 65 years and 65 years and older) and sex. Similar patterns in reduced mortality also emerged when the researchers used different thresholds of daily steps, such as a minimum of 10,000 steps instead of 8,000. The adjusted all-cause mortality for groups who took at least 10,000 steps 1-2 days a week, 3-7 days a week, and no days a week were 8.1%, 7.3%, and 16.7%, respectively, with corresponding cardiovascular mortality risks of 2.4%, 2.3%, and 7.0%, respectively.
“Given the simplicity and ease of counting daily steps, our findings indicate that the recommended number of steps taken on as few as 1 to 2 days per week may be a feasible option for individuals who are striving to achieve some health benefits through adhering to a recommended daily step count but are unable to accomplish this on a daily basis,” the researchers wrote in their discussion.
The findings were limited by several factors including the use daily step measures for 1 week only at baseline, with no data on how physical activity changes might impact mortality risk, the researchers noted. Other limitations included possible accelerometer error and misclassification of activity, possible selection bias, and lack of data on cause-specific mortality outside of cardiovascular death, they said.
However, the results were strengthened by the use of accelerometers as objective measures of activity and by the availability of 10-year follow-up data for nearly 100% of the participants, they said.
“Although our findings might suffer from residual confounding that should be addressed in future research, they suggest that people may receive substantial health benefits even if a sufficient number of steps are taken on only a couple days of the week,” they concluded.
Proceed with caution
The current study findings should be interpreted cautiously in light of the potential unmeasured confounding factors and selection bias that often occur in studies of physical activity, James Sawalla Guseh, MD, of Massachusetts General Hospital, and Jose F. Figueroa, MD, of Harvard T.H. Chan School of Public Health, Boston, wrote in an accompanying editorial.
The results support previous studies showing some longevity benefits with “weekend warrior” patterns of intense physical activity for only a couple of days; however, “the body of evidence for sporadic activity is not as robust as the evidence for sustained and regular aerobic activity,” the authors emphasized.
The editorial authors also highlighted the limitations of the current study, including the observational design and significant differences in demographics and comorbidities between the 1- to 2-days of 8,000 steps exercise group and the 0-day group, as well as the reliance on only a week’s worth of data to infer 10 years’ mortality.
Although the data are consistent with previous observations that increased exercise volume reduces mortality, more research is needed, as the current study findings may not reflect other dimensions of health, including neurological health, they said.
Despite the need for cautious interpretation of the results, the current study “supports the emerging and popular idea that step counting, which does not require consideration of exercise duration or intensity, can offer guidance toward robust and favorable health outcomes,” and may inform step-based activity goals to improve public health, the editorialists wrote.
The study was supported by the Japan Agency for Medical Research and Development, the Japan Society for the Promotion of Science, the Japan Endocrine Society, and the Meiji Yasuda Life Foundation of Health and Welfare. Dr. Inoue also was supported by the Program for the Development of Next-Generation Leading Scientists With Global Insight sponsored by the Ministry of Education, Culture, Sports, Science and Technology, Japan. The other researchers had no relevant financial conflicts to disclose. The editorial authors had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Limited treatment options exist for brittle nail syndrome
NEW ORLEANS – .
“The mainstay of treatment is irritant avoidance and moisturization,” Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the nail division at Weill Cornell Medicine, New York, said at the annual meeting of the American Academy of Dermatology. “This works well if patients are religious about doing it.”
Brittle nail syndrome affects about 20% of adults, she said, and is more common in females, particularly those older than age 50. Most cases are idiopathic, but some are secondary to dermatologic diseases including nail psoriasis and nail lichen planus, and systemic diseases such as hyperthyroidism and hypothyroidism. They are more common in patients in certain occupations such as carpentry. “The pathogenesis is poorly understood but is thought to be due to weakened intercellular keratinocyte bridges, decreased cholesterol sulphate in the nail plate, and reduced water content in the nail plate,” Dr. Lipner said.
Key clinical findings include onychoschizia (peeling of the nail plate), onychorrhexis (an increase in the longitudinal ridges and furrows, sometimes leading to splitting), and superficial granulation of keratin. Treatment involves general measures. “You want to treat the underlying cause and recommend that the patient avoid water and irritant exposure,” she said. Her general instructions for affected patients are to wear latex gloves for wet work and cotton gloves for dry work, avoid triclosan-based hand sanitizers, avoid nail cosmetics, minimize nail trauma, and foster moisturization.“It’s important to give these instructions verbally and in written form,” she said. “In our practice, we designed a QR code that links to our patient handout.”
According to Dr. Lipner, the promotion of vitamins and supplements such as biotin, vitamin D, amino acids, and chromium for treating brittle nail syndrome is rampant on the Internet and on social media, but no rigorously designed clinical trials have shown efficacy for any of them. “Very few people are deficient in biotin, except for those with inherited enzyme deficiencies,” and most people “can get all the biotin they need from a regular diet,” she said.
The initial rationale for using biotin for nails comes from the veterinary literature, she continued. In the 1940s, chickens with biotin deficiency developed fissures in their feet and parrot-like beaks. In the 1970s, pigs with biotin deficiency developed friable hooves, which was corrected with biotin supplementation. “By the 1980s it was standard practice to supplement the feet of pigs with biotin,” she said.
In a human trial from 1989, German researchers enrolled 71 patients with brittle nail syndrome who took oral biotin, 2.5 mg daily. Of the 45 patients evaluated, 41 (91%) showed improvement in firmness and hardness of the fingernails over the course of 5.5 months, but there was no good control group, Dr. Lipner said. In a follow-up study, the same German researchers used scanning electron microscopy to evaluate 22 patients with brittle nails who took oral biotin 2.5 mg daily and compared them with 10 patients with normal nails who did not take biotin. They found a 25% increase in nail plate thickness in the biotin group and onychoschizia resolved in 50% of patients who received biotin. “But again, there was no good control group,” Dr. Lipner said.
In a third study on the topic, researchers surveyed 46 patients who presented with onychorrhexis and/or onychoschizia on clinical exam and took 2.5 mg of biotin daily. Of the 35 survey respondents, 63% subjectively reported improvement in their nails at a mean of 2 months. “This is where we are today: There have been studies of only 80 patients that were done 25 years ago,” Dr. Lipner said. “That’s all of our evidence for biotin for the treatment of brittle nail syndrome.”
FDA warning about biotin
Additional cause for concern, she continued, is the safety communication issued by the FDA in 2017, stating that the use of biotin may interfere with certain lab tests such as thyroid tests and cardiac enzymes, in some cases leading to death. The safety communication was updated in 2019.
In 2018, Dr. Lipner and colleagues administered an anonymous survey to 447 patients at their clinic asking about their use of biotin supplements. Of the 447 patients, 34% reported current use of biotin. Among biotin users, 7% were aware of the FDA warning, 29% of respondents reported that it was recommended by either a primary care physician or a dermatologist, and 56% underwent laboratory testing while taking biotin. “It’s our duty to warn our patients about the evidence for biotin for treating brittle nails, and about this interference on laboratory tests,” Dr. Lipner said.
Other treatment options for brittle nail syndrome include two lacquers that are available by prescription. One contains hydroxypropyl chitosan, Equisetum arvense, and methylsulphonylmethane; the other contains 16% poly-ureaurethane, but has not been well studied. “These products can be very expensive if not covered by insurance,” Dr. Lipner said.
As an alternative, she recommends Nail Tek CITRA 2 Nail Strengthener, which is available for less than $10 from Walmart and other retailers.
Cyclosporine emulsion also has been studied for brittle nail syndrome, but results to date have been underwhelming. Dr. Lipner and colleagues are exploring the effect of platelet rich plasma for treating brittle nails on the premise that it will improve nail growth and promote healing, in a 16-week trial that has enrolled 10 patients and includes both a Physician Global Improvement Assessment (PGIA) and a Physician Global Assessment (PGA) score. “Our data is being analyzed by three independent nail experts, and we hope to report the findings next year,” she said.
Dr. Lipner reported having no disclosures relevant to her presentation.
NEW ORLEANS – .
“The mainstay of treatment is irritant avoidance and moisturization,” Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the nail division at Weill Cornell Medicine, New York, said at the annual meeting of the American Academy of Dermatology. “This works well if patients are religious about doing it.”
Brittle nail syndrome affects about 20% of adults, she said, and is more common in females, particularly those older than age 50. Most cases are idiopathic, but some are secondary to dermatologic diseases including nail psoriasis and nail lichen planus, and systemic diseases such as hyperthyroidism and hypothyroidism. They are more common in patients in certain occupations such as carpentry. “The pathogenesis is poorly understood but is thought to be due to weakened intercellular keratinocyte bridges, decreased cholesterol sulphate in the nail plate, and reduced water content in the nail plate,” Dr. Lipner said.
Key clinical findings include onychoschizia (peeling of the nail plate), onychorrhexis (an increase in the longitudinal ridges and furrows, sometimes leading to splitting), and superficial granulation of keratin. Treatment involves general measures. “You want to treat the underlying cause and recommend that the patient avoid water and irritant exposure,” she said. Her general instructions for affected patients are to wear latex gloves for wet work and cotton gloves for dry work, avoid triclosan-based hand sanitizers, avoid nail cosmetics, minimize nail trauma, and foster moisturization.“It’s important to give these instructions verbally and in written form,” she said. “In our practice, we designed a QR code that links to our patient handout.”
According to Dr. Lipner, the promotion of vitamins and supplements such as biotin, vitamin D, amino acids, and chromium for treating brittle nail syndrome is rampant on the Internet and on social media, but no rigorously designed clinical trials have shown efficacy for any of them. “Very few people are deficient in biotin, except for those with inherited enzyme deficiencies,” and most people “can get all the biotin they need from a regular diet,” she said.
The initial rationale for using biotin for nails comes from the veterinary literature, she continued. In the 1940s, chickens with biotin deficiency developed fissures in their feet and parrot-like beaks. In the 1970s, pigs with biotin deficiency developed friable hooves, which was corrected with biotin supplementation. “By the 1980s it was standard practice to supplement the feet of pigs with biotin,” she said.
In a human trial from 1989, German researchers enrolled 71 patients with brittle nail syndrome who took oral biotin, 2.5 mg daily. Of the 45 patients evaluated, 41 (91%) showed improvement in firmness and hardness of the fingernails over the course of 5.5 months, but there was no good control group, Dr. Lipner said. In a follow-up study, the same German researchers used scanning electron microscopy to evaluate 22 patients with brittle nails who took oral biotin 2.5 mg daily and compared them with 10 patients with normal nails who did not take biotin. They found a 25% increase in nail plate thickness in the biotin group and onychoschizia resolved in 50% of patients who received biotin. “But again, there was no good control group,” Dr. Lipner said.
In a third study on the topic, researchers surveyed 46 patients who presented with onychorrhexis and/or onychoschizia on clinical exam and took 2.5 mg of biotin daily. Of the 35 survey respondents, 63% subjectively reported improvement in their nails at a mean of 2 months. “This is where we are today: There have been studies of only 80 patients that were done 25 years ago,” Dr. Lipner said. “That’s all of our evidence for biotin for the treatment of brittle nail syndrome.”
FDA warning about biotin
Additional cause for concern, she continued, is the safety communication issued by the FDA in 2017, stating that the use of biotin may interfere with certain lab tests such as thyroid tests and cardiac enzymes, in some cases leading to death. The safety communication was updated in 2019.
In 2018, Dr. Lipner and colleagues administered an anonymous survey to 447 patients at their clinic asking about their use of biotin supplements. Of the 447 patients, 34% reported current use of biotin. Among biotin users, 7% were aware of the FDA warning, 29% of respondents reported that it was recommended by either a primary care physician or a dermatologist, and 56% underwent laboratory testing while taking biotin. “It’s our duty to warn our patients about the evidence for biotin for treating brittle nails, and about this interference on laboratory tests,” Dr. Lipner said.
Other treatment options for brittle nail syndrome include two lacquers that are available by prescription. One contains hydroxypropyl chitosan, Equisetum arvense, and methylsulphonylmethane; the other contains 16% poly-ureaurethane, but has not been well studied. “These products can be very expensive if not covered by insurance,” Dr. Lipner said.
As an alternative, she recommends Nail Tek CITRA 2 Nail Strengthener, which is available for less than $10 from Walmart and other retailers.
Cyclosporine emulsion also has been studied for brittle nail syndrome, but results to date have been underwhelming. Dr. Lipner and colleagues are exploring the effect of platelet rich plasma for treating brittle nails on the premise that it will improve nail growth and promote healing, in a 16-week trial that has enrolled 10 patients and includes both a Physician Global Improvement Assessment (PGIA) and a Physician Global Assessment (PGA) score. “Our data is being analyzed by three independent nail experts, and we hope to report the findings next year,” she said.
Dr. Lipner reported having no disclosures relevant to her presentation.
NEW ORLEANS – .
“The mainstay of treatment is irritant avoidance and moisturization,” Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the nail division at Weill Cornell Medicine, New York, said at the annual meeting of the American Academy of Dermatology. “This works well if patients are religious about doing it.”
Brittle nail syndrome affects about 20% of adults, she said, and is more common in females, particularly those older than age 50. Most cases are idiopathic, but some are secondary to dermatologic diseases including nail psoriasis and nail lichen planus, and systemic diseases such as hyperthyroidism and hypothyroidism. They are more common in patients in certain occupations such as carpentry. “The pathogenesis is poorly understood but is thought to be due to weakened intercellular keratinocyte bridges, decreased cholesterol sulphate in the nail plate, and reduced water content in the nail plate,” Dr. Lipner said.
Key clinical findings include onychoschizia (peeling of the nail plate), onychorrhexis (an increase in the longitudinal ridges and furrows, sometimes leading to splitting), and superficial granulation of keratin. Treatment involves general measures. “You want to treat the underlying cause and recommend that the patient avoid water and irritant exposure,” she said. Her general instructions for affected patients are to wear latex gloves for wet work and cotton gloves for dry work, avoid triclosan-based hand sanitizers, avoid nail cosmetics, minimize nail trauma, and foster moisturization.“It’s important to give these instructions verbally and in written form,” she said. “In our practice, we designed a QR code that links to our patient handout.”
According to Dr. Lipner, the promotion of vitamins and supplements such as biotin, vitamin D, amino acids, and chromium for treating brittle nail syndrome is rampant on the Internet and on social media, but no rigorously designed clinical trials have shown efficacy for any of them. “Very few people are deficient in biotin, except for those with inherited enzyme deficiencies,” and most people “can get all the biotin they need from a regular diet,” she said.
The initial rationale for using biotin for nails comes from the veterinary literature, she continued. In the 1940s, chickens with biotin deficiency developed fissures in their feet and parrot-like beaks. In the 1970s, pigs with biotin deficiency developed friable hooves, which was corrected with biotin supplementation. “By the 1980s it was standard practice to supplement the feet of pigs with biotin,” she said.
In a human trial from 1989, German researchers enrolled 71 patients with brittle nail syndrome who took oral biotin, 2.5 mg daily. Of the 45 patients evaluated, 41 (91%) showed improvement in firmness and hardness of the fingernails over the course of 5.5 months, but there was no good control group, Dr. Lipner said. In a follow-up study, the same German researchers used scanning electron microscopy to evaluate 22 patients with brittle nails who took oral biotin 2.5 mg daily and compared them with 10 patients with normal nails who did not take biotin. They found a 25% increase in nail plate thickness in the biotin group and onychoschizia resolved in 50% of patients who received biotin. “But again, there was no good control group,” Dr. Lipner said.
In a third study on the topic, researchers surveyed 46 patients who presented with onychorrhexis and/or onychoschizia on clinical exam and took 2.5 mg of biotin daily. Of the 35 survey respondents, 63% subjectively reported improvement in their nails at a mean of 2 months. “This is where we are today: There have been studies of only 80 patients that were done 25 years ago,” Dr. Lipner said. “That’s all of our evidence for biotin for the treatment of brittle nail syndrome.”
FDA warning about biotin
Additional cause for concern, she continued, is the safety communication issued by the FDA in 2017, stating that the use of biotin may interfere with certain lab tests such as thyroid tests and cardiac enzymes, in some cases leading to death. The safety communication was updated in 2019.
In 2018, Dr. Lipner and colleagues administered an anonymous survey to 447 patients at their clinic asking about their use of biotin supplements. Of the 447 patients, 34% reported current use of biotin. Among biotin users, 7% were aware of the FDA warning, 29% of respondents reported that it was recommended by either a primary care physician or a dermatologist, and 56% underwent laboratory testing while taking biotin. “It’s our duty to warn our patients about the evidence for biotin for treating brittle nails, and about this interference on laboratory tests,” Dr. Lipner said.
Other treatment options for brittle nail syndrome include two lacquers that are available by prescription. One contains hydroxypropyl chitosan, Equisetum arvense, and methylsulphonylmethane; the other contains 16% poly-ureaurethane, but has not been well studied. “These products can be very expensive if not covered by insurance,” Dr. Lipner said.
As an alternative, she recommends Nail Tek CITRA 2 Nail Strengthener, which is available for less than $10 from Walmart and other retailers.
Cyclosporine emulsion also has been studied for brittle nail syndrome, but results to date have been underwhelming. Dr. Lipner and colleagues are exploring the effect of platelet rich plasma for treating brittle nails on the premise that it will improve nail growth and promote healing, in a 16-week trial that has enrolled 10 patients and includes both a Physician Global Improvement Assessment (PGIA) and a Physician Global Assessment (PGA) score. “Our data is being analyzed by three independent nail experts, and we hope to report the findings next year,” she said.
Dr. Lipner reported having no disclosures relevant to her presentation.
AT AAD 2023
FDA Advisory panels consider easing isotretinoin requirements
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
COVID led to rise in pregnancy-related deaths: New research
The rise in deaths was most pronounced among Black mothers.
In 2021, 1,205 women died from pregnancy-related causes, making the year one of the worst for maternal mortality in U.S. history, according to newly released data from the Centers for Disease Control and Prevention. Maternal mortality is defined as occurring during pregnancy, at delivery, or soon after delivery.
COVID was the driver of the increased death rate, according to a study published in the journal Obstetrics & Gynecology. The researchers noted that unvaccinated pregnant people are more likely to get severe COVID, and that prenatal and postnatal care were disrupted during the early part of the pandemic. From July 2021 to March 2023, the rate of women being vaccinated before pregnancy has risen from 22% to 70%, CDC data show.
Maternal mortality rates jumped the most among Black women, who in 2021 had a maternal mortality rate of nearly 70 deaths per 100,000 live births, which was 2.6 times the rate for White women.
Existing risks based on a mother’s age also increased from 2020 to 2021. The maternal mortality rates by age in 2021 per 100,000 live births were:
- 20.4 for women under age 25.
- 31.3 for women ages 25 to 39.
- 138.5 for women ages 40 and older.
Iffath Abbasi Hoskins, MD, FACOG, president of the American College of Obstetricians and Gynecologists, called the situation “stunning” and “preventable.”
The findings “send a resounding message that maternal health and evidence-based efforts to eliminate racial health inequities need to be, and remain, a top public health priority,” Dr. Hoskins said in a statement.
“The COVID-19 pandemic had a dramatic and tragic effect on maternal death rates, but we cannot let that fact obscure that there was – and still is – already a maternal mortality crisis to compound,” she said.
A version of this article first appeared on WebMD.com.
The rise in deaths was most pronounced among Black mothers.
In 2021, 1,205 women died from pregnancy-related causes, making the year one of the worst for maternal mortality in U.S. history, according to newly released data from the Centers for Disease Control and Prevention. Maternal mortality is defined as occurring during pregnancy, at delivery, or soon after delivery.
COVID was the driver of the increased death rate, according to a study published in the journal Obstetrics & Gynecology. The researchers noted that unvaccinated pregnant people are more likely to get severe COVID, and that prenatal and postnatal care were disrupted during the early part of the pandemic. From July 2021 to March 2023, the rate of women being vaccinated before pregnancy has risen from 22% to 70%, CDC data show.
Maternal mortality rates jumped the most among Black women, who in 2021 had a maternal mortality rate of nearly 70 deaths per 100,000 live births, which was 2.6 times the rate for White women.
Existing risks based on a mother’s age also increased from 2020 to 2021. The maternal mortality rates by age in 2021 per 100,000 live births were:
- 20.4 for women under age 25.
- 31.3 for women ages 25 to 39.
- 138.5 for women ages 40 and older.
Iffath Abbasi Hoskins, MD, FACOG, president of the American College of Obstetricians and Gynecologists, called the situation “stunning” and “preventable.”
The findings “send a resounding message that maternal health and evidence-based efforts to eliminate racial health inequities need to be, and remain, a top public health priority,” Dr. Hoskins said in a statement.
“The COVID-19 pandemic had a dramatic and tragic effect on maternal death rates, but we cannot let that fact obscure that there was – and still is – already a maternal mortality crisis to compound,” she said.
A version of this article first appeared on WebMD.com.
The rise in deaths was most pronounced among Black mothers.
In 2021, 1,205 women died from pregnancy-related causes, making the year one of the worst for maternal mortality in U.S. history, according to newly released data from the Centers for Disease Control and Prevention. Maternal mortality is defined as occurring during pregnancy, at delivery, or soon after delivery.
COVID was the driver of the increased death rate, according to a study published in the journal Obstetrics & Gynecology. The researchers noted that unvaccinated pregnant people are more likely to get severe COVID, and that prenatal and postnatal care were disrupted during the early part of the pandemic. From July 2021 to March 2023, the rate of women being vaccinated before pregnancy has risen from 22% to 70%, CDC data show.
Maternal mortality rates jumped the most among Black women, who in 2021 had a maternal mortality rate of nearly 70 deaths per 100,000 live births, which was 2.6 times the rate for White women.
Existing risks based on a mother’s age also increased from 2020 to 2021. The maternal mortality rates by age in 2021 per 100,000 live births were:
- 20.4 for women under age 25.
- 31.3 for women ages 25 to 39.
- 138.5 for women ages 40 and older.
Iffath Abbasi Hoskins, MD, FACOG, president of the American College of Obstetricians and Gynecologists, called the situation “stunning” and “preventable.”
The findings “send a resounding message that maternal health and evidence-based efforts to eliminate racial health inequities need to be, and remain, a top public health priority,” Dr. Hoskins said in a statement.
“The COVID-19 pandemic had a dramatic and tragic effect on maternal death rates, but we cannot let that fact obscure that there was – and still is – already a maternal mortality crisis to compound,” she said.
A version of this article first appeared on WebMD.com.