User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Alopecia areata: Positive results reported for two investigational JAK inhibitors
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Study identifies skin biomarkers that predict newborn eczema risk
It might be possible to develop a simple test to identify newborn children who are at risk of later developing atopic dermatitis (AD), according to findings from a Danish prospective birth cohort study.
but also for having more severe disease.
“We are able to identify predictive immune biomarkers of atopic dermatitis using a noninvasive method that was not associated with any pain,” one of the study’s investigators, Anne-Sofie Halling, MD, said at a press briefing at the annual congress of the European Academy of Dermatology and Venereology.
“Importantly, we were able to predict atopic dermatitis occurring months after [sample] collection,” said Dr. Halling, who works at Bispebjerg Hospital and is a PhD student at the University of Copenhagen.
These findings could hopefully be used to help identify children “so that preventive strategies can target these children ... and decrease the incidence of this common disease,” she added.
AD is caused “by a complex interplay between skin barrier dysfunction and immune dysregulation,” Dr. Halling said, and it is “the first step in the so-called atopic march, where children also develop food allergy, asthma, and rhinitis.” Almost all cases of AD begin during the first years of life. Approximately 15%-20% of children can be affected, she noted, emphasizing the high burden of the disease and pointing out that strategies are shifting toward trying to prevent the disease in those at risk.
Copenhagen BABY cohort
This is where the BABY study comes in, Dr. Halling said. The study enrolled 450 children at birth and followed them until age 2 years. Gene mutation testing was performed at enrollment. All children underwent skin examination, and skin samples were taken using tape strips. Tape strips were applied to the back of the hand of children born at term and between the shoulder blades on the back of children who were premature.
Skin examinations were repeated, and skin samples were obtained again at age 2 months. They were taken again only if there were any signs of AD. For those diagnosed with AD, disease severity was assessed using the Eczema Area and Severity Index (EASI) by the treating physician. Children were excluded if they had AD at the time the tape strip testing was due to be performed.
Comparing term and preterm children
Dr. Halling noted that analyses were performed separately for the 300 children born at term and for the 150 who were preterm.
The prevalence of AD was higher among children born at term than among the preterm children (34.6% vs. 21.2%), and the median time to onset was shorter (6 months vs. 8 months). There were also differences in the EASI scores among those who developed AD; median scores were higher in the children born at term than in the preterm children (4.1 vs. 1.6).
More children born at term than preterm children had moderate to severe AD (23.3% vs. 8%), Dr. Halling reported.
TARC, IL-8, and IL-18 predictive of AD
Multiple immune biomarkers were tested, including various cytokines and filaggrin degradation products. On examination of skin samples collected at birth, no particular biomarkers were found at higher levels among children who developed AD in comparison with those who did not develop AD.
With regard to biomarkers examined in skin samples at 2 months of age, however, the results were different, Dr. Halling said. One particular cytokine, thymus and activation-regulated chemokine (TARC), was seen to double the risk of AD in the first 2 years of a child’s life.
This doubled risk was seen not only among the children born at term but also among those born preterm, although the data were only significant with regard to the children born at term.
The unadjusted hazard ratios and adjusted HRs (adjusted for parental atopy and filaggrin gene mutations) in term children were 2.11 (95% confidence interval, 1.36-3.26; P = .0008) and 1.85 (95% CI, 1.18-2.89; P = .007), respectively.
For preterm children, the HRs were 2.23 (95% CI, 0.85-5.86; P = .1) and 2.60 (95% CI, 0.98-6.85; P =.05), respectively.
These findings were in line with findings of other studies, Dr. Halling said. “It is well recognized that TARC is currently the best biomarker in patients with established atopic dermatitis.” Moreover, she reported that TARC was associated with a cumulative increase in the risk for AD and that levels were found to be higher in children in whom onset occurred at a later age than among those diagnosed before 6 months of age.
“This is important, as these findings shows that TARC levels predict atopic dermatitis that occurred many months later,” Dr. Halling said.
And, in term-born children at least, TARC upped the chances that the severity of AD would be greater than had it not been present (adjusted HR, 4.65; 95% CI, 1.91-11.31; P = .0007).
Increased levels of interleukin-8 (IL-8) and IL-18 at 2 months of age were also found to be predictive of having moderate to severe AD. The risk was more than double in comparison with those in whom levels were not increased, again only in term-born children.
‘Stimulating and interesting findings’
These data are “very stimulating and interesting,” Dedee Murrell, MD, professor and head of the department of dermatology at St. George Hospital, University of New South Wales, Sydney, observed at the press briefing.
“You found this significant association mainly in the newborn children born at term, and the association in the preterm babies wasn’t as high. Is that anything to do with how they were taken care of in the hospital?” Dr. Murrell asked.
“That’s a really good question,” Dr. Halling said. “Maybe they need to be exposed for a month or two before we are actually able to identify which children will develop atopic dermatitis.”
The study was funded by the Lundbeck Foundation. Dr. Halling has acted as a consultant for Coloplast and as a speaker for Leo Pharma. Dr. Murrell has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It might be possible to develop a simple test to identify newborn children who are at risk of later developing atopic dermatitis (AD), according to findings from a Danish prospective birth cohort study.
but also for having more severe disease.
“We are able to identify predictive immune biomarkers of atopic dermatitis using a noninvasive method that was not associated with any pain,” one of the study’s investigators, Anne-Sofie Halling, MD, said at a press briefing at the annual congress of the European Academy of Dermatology and Venereology.
“Importantly, we were able to predict atopic dermatitis occurring months after [sample] collection,” said Dr. Halling, who works at Bispebjerg Hospital and is a PhD student at the University of Copenhagen.
These findings could hopefully be used to help identify children “so that preventive strategies can target these children ... and decrease the incidence of this common disease,” she added.
AD is caused “by a complex interplay between skin barrier dysfunction and immune dysregulation,” Dr. Halling said, and it is “the first step in the so-called atopic march, where children also develop food allergy, asthma, and rhinitis.” Almost all cases of AD begin during the first years of life. Approximately 15%-20% of children can be affected, she noted, emphasizing the high burden of the disease and pointing out that strategies are shifting toward trying to prevent the disease in those at risk.
Copenhagen BABY cohort
This is where the BABY study comes in, Dr. Halling said. The study enrolled 450 children at birth and followed them until age 2 years. Gene mutation testing was performed at enrollment. All children underwent skin examination, and skin samples were taken using tape strips. Tape strips were applied to the back of the hand of children born at term and between the shoulder blades on the back of children who were premature.
Skin examinations were repeated, and skin samples were obtained again at age 2 months. They were taken again only if there were any signs of AD. For those diagnosed with AD, disease severity was assessed using the Eczema Area and Severity Index (EASI) by the treating physician. Children were excluded if they had AD at the time the tape strip testing was due to be performed.
Comparing term and preterm children
Dr. Halling noted that analyses were performed separately for the 300 children born at term and for the 150 who were preterm.
The prevalence of AD was higher among children born at term than among the preterm children (34.6% vs. 21.2%), and the median time to onset was shorter (6 months vs. 8 months). There were also differences in the EASI scores among those who developed AD; median scores were higher in the children born at term than in the preterm children (4.1 vs. 1.6).
More children born at term than preterm children had moderate to severe AD (23.3% vs. 8%), Dr. Halling reported.
TARC, IL-8, and IL-18 predictive of AD
Multiple immune biomarkers were tested, including various cytokines and filaggrin degradation products. On examination of skin samples collected at birth, no particular biomarkers were found at higher levels among children who developed AD in comparison with those who did not develop AD.
With regard to biomarkers examined in skin samples at 2 months of age, however, the results were different, Dr. Halling said. One particular cytokine, thymus and activation-regulated chemokine (TARC), was seen to double the risk of AD in the first 2 years of a child’s life.
This doubled risk was seen not only among the children born at term but also among those born preterm, although the data were only significant with regard to the children born at term.
The unadjusted hazard ratios and adjusted HRs (adjusted for parental atopy and filaggrin gene mutations) in term children were 2.11 (95% confidence interval, 1.36-3.26; P = .0008) and 1.85 (95% CI, 1.18-2.89; P = .007), respectively.
For preterm children, the HRs were 2.23 (95% CI, 0.85-5.86; P = .1) and 2.60 (95% CI, 0.98-6.85; P =.05), respectively.
These findings were in line with findings of other studies, Dr. Halling said. “It is well recognized that TARC is currently the best biomarker in patients with established atopic dermatitis.” Moreover, she reported that TARC was associated with a cumulative increase in the risk for AD and that levels were found to be higher in children in whom onset occurred at a later age than among those diagnosed before 6 months of age.
“This is important, as these findings shows that TARC levels predict atopic dermatitis that occurred many months later,” Dr. Halling said.
And, in term-born children at least, TARC upped the chances that the severity of AD would be greater than had it not been present (adjusted HR, 4.65; 95% CI, 1.91-11.31; P = .0007).
Increased levels of interleukin-8 (IL-8) and IL-18 at 2 months of age were also found to be predictive of having moderate to severe AD. The risk was more than double in comparison with those in whom levels were not increased, again only in term-born children.
‘Stimulating and interesting findings’
These data are “very stimulating and interesting,” Dedee Murrell, MD, professor and head of the department of dermatology at St. George Hospital, University of New South Wales, Sydney, observed at the press briefing.
“You found this significant association mainly in the newborn children born at term, and the association in the preterm babies wasn’t as high. Is that anything to do with how they were taken care of in the hospital?” Dr. Murrell asked.
“That’s a really good question,” Dr. Halling said. “Maybe they need to be exposed for a month or two before we are actually able to identify which children will develop atopic dermatitis.”
The study was funded by the Lundbeck Foundation. Dr. Halling has acted as a consultant for Coloplast and as a speaker for Leo Pharma. Dr. Murrell has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It might be possible to develop a simple test to identify newborn children who are at risk of later developing atopic dermatitis (AD), according to findings from a Danish prospective birth cohort study.
but also for having more severe disease.
“We are able to identify predictive immune biomarkers of atopic dermatitis using a noninvasive method that was not associated with any pain,” one of the study’s investigators, Anne-Sofie Halling, MD, said at a press briefing at the annual congress of the European Academy of Dermatology and Venereology.
“Importantly, we were able to predict atopic dermatitis occurring months after [sample] collection,” said Dr. Halling, who works at Bispebjerg Hospital and is a PhD student at the University of Copenhagen.
These findings could hopefully be used to help identify children “so that preventive strategies can target these children ... and decrease the incidence of this common disease,” she added.
AD is caused “by a complex interplay between skin barrier dysfunction and immune dysregulation,” Dr. Halling said, and it is “the first step in the so-called atopic march, where children also develop food allergy, asthma, and rhinitis.” Almost all cases of AD begin during the first years of life. Approximately 15%-20% of children can be affected, she noted, emphasizing the high burden of the disease and pointing out that strategies are shifting toward trying to prevent the disease in those at risk.
Copenhagen BABY cohort
This is where the BABY study comes in, Dr. Halling said. The study enrolled 450 children at birth and followed them until age 2 years. Gene mutation testing was performed at enrollment. All children underwent skin examination, and skin samples were taken using tape strips. Tape strips were applied to the back of the hand of children born at term and between the shoulder blades on the back of children who were premature.
Skin examinations were repeated, and skin samples were obtained again at age 2 months. They were taken again only if there were any signs of AD. For those diagnosed with AD, disease severity was assessed using the Eczema Area and Severity Index (EASI) by the treating physician. Children were excluded if they had AD at the time the tape strip testing was due to be performed.
Comparing term and preterm children
Dr. Halling noted that analyses were performed separately for the 300 children born at term and for the 150 who were preterm.
The prevalence of AD was higher among children born at term than among the preterm children (34.6% vs. 21.2%), and the median time to onset was shorter (6 months vs. 8 months). There were also differences in the EASI scores among those who developed AD; median scores were higher in the children born at term than in the preterm children (4.1 vs. 1.6).
More children born at term than preterm children had moderate to severe AD (23.3% vs. 8%), Dr. Halling reported.
TARC, IL-8, and IL-18 predictive of AD
Multiple immune biomarkers were tested, including various cytokines and filaggrin degradation products. On examination of skin samples collected at birth, no particular biomarkers were found at higher levels among children who developed AD in comparison with those who did not develop AD.
With regard to biomarkers examined in skin samples at 2 months of age, however, the results were different, Dr. Halling said. One particular cytokine, thymus and activation-regulated chemokine (TARC), was seen to double the risk of AD in the first 2 years of a child’s life.
This doubled risk was seen not only among the children born at term but also among those born preterm, although the data were only significant with regard to the children born at term.
The unadjusted hazard ratios and adjusted HRs (adjusted for parental atopy and filaggrin gene mutations) in term children were 2.11 (95% confidence interval, 1.36-3.26; P = .0008) and 1.85 (95% CI, 1.18-2.89; P = .007), respectively.
For preterm children, the HRs were 2.23 (95% CI, 0.85-5.86; P = .1) and 2.60 (95% CI, 0.98-6.85; P =.05), respectively.
These findings were in line with findings of other studies, Dr. Halling said. “It is well recognized that TARC is currently the best biomarker in patients with established atopic dermatitis.” Moreover, she reported that TARC was associated with a cumulative increase in the risk for AD and that levels were found to be higher in children in whom onset occurred at a later age than among those diagnosed before 6 months of age.
“This is important, as these findings shows that TARC levels predict atopic dermatitis that occurred many months later,” Dr. Halling said.
And, in term-born children at least, TARC upped the chances that the severity of AD would be greater than had it not been present (adjusted HR, 4.65; 95% CI, 1.91-11.31; P = .0007).
Increased levels of interleukin-8 (IL-8) and IL-18 at 2 months of age were also found to be predictive of having moderate to severe AD. The risk was more than double in comparison with those in whom levels were not increased, again only in term-born children.
‘Stimulating and interesting findings’
These data are “very stimulating and interesting,” Dedee Murrell, MD, professor and head of the department of dermatology at St. George Hospital, University of New South Wales, Sydney, observed at the press briefing.
“You found this significant association mainly in the newborn children born at term, and the association in the preterm babies wasn’t as high. Is that anything to do with how they were taken care of in the hospital?” Dr. Murrell asked.
“That’s a really good question,” Dr. Halling said. “Maybe they need to be exposed for a month or two before we are actually able to identify which children will develop atopic dermatitis.”
The study was funded by the Lundbeck Foundation. Dr. Halling has acted as a consultant for Coloplast and as a speaker for Leo Pharma. Dr. Murrell has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
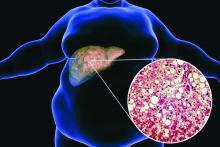
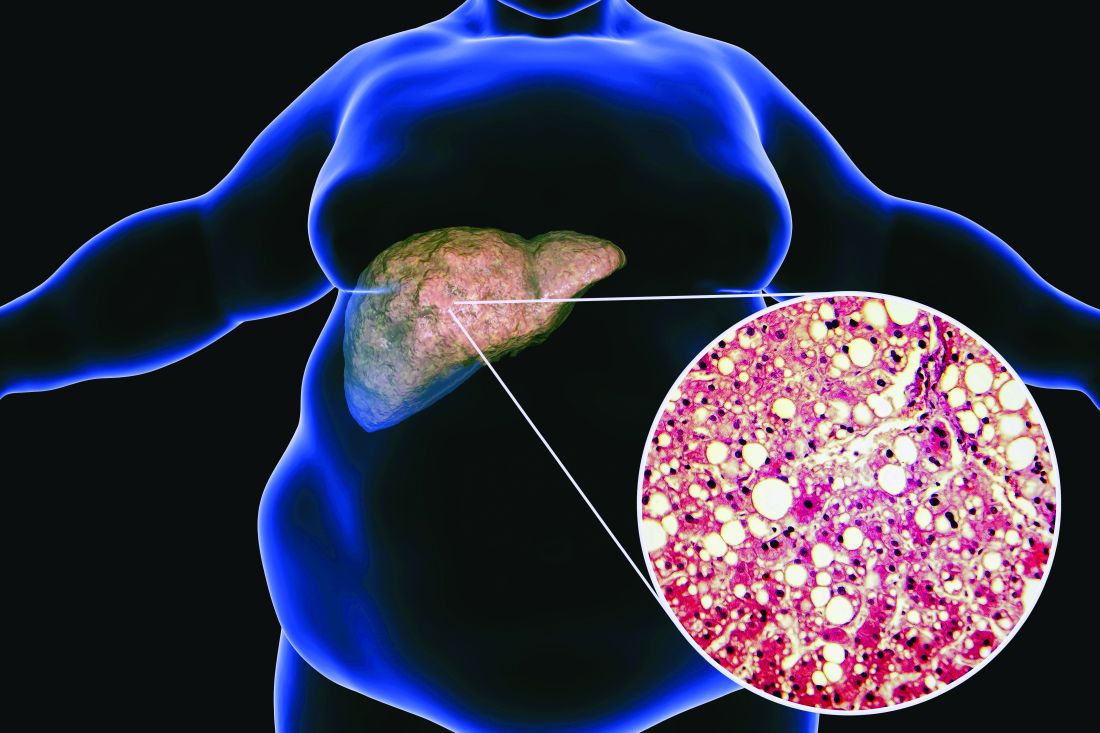
Ezetimibe-statin combo lowers liver fat in open-label trial
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
FROM EASD 2022
Does COVID-19 cause type 1 diabetes in children? Time will tell
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Severe COVID-19–related outcomes found worse in men with RA
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
FROM THE LANCET SUMMIT ON SEX AND GENDER IN RHEUMATOLOGY
COVID pandemic associated with anorexia in Canadian youth
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
The bionic pancreas triumphs in pivotal trial
This transcript of Impact Factor with F. Perry Wilson has been edited for clarity.
It was 100 years ago when Leonard Thompson, age 13, received a reprieve from a death sentence. Young master Thompson had type 1 diabetes, a disease that was uniformly fatal within months of diagnosis. But he received a new treatment, insulin, from a canine pancreas. He would live 13 more years before dying at age 26 of pneumonia.
The history of type 1 diabetes since that time has been a battle on two fronts: First, the search for a cause of and cure for the disease; second, the effort to make the administration of insulin safer, more reliable, and easier.
The past 2 decades have seen a technological revolution in type 1 diabetes care, with continuous glucose monitors decreasing the need for painful finger sticks, and insulin pumps allowing for more precise titration of doses.
The dream, of course, has been to combine those two technologies, continuous glucose monitoring and insulin pumps, to create so-called closed-loop systems – basically an artificial pancreas – that would obviate the need for any intervention on the part of the patient, save the occasional refilling of an insulin reservoir.
We aren’t there yet, but we are closer than ever.
Closed-loop systems for insulin delivery, like the Tandem Control IQ system, are a marvel of technology, but they are not exactly hands-free. Users need to dial in settings for their insulin usage, count carbohydrates at meals, and inform the system that they are about to eat those meals to allow the algorithm to administer an appropriate insulin dose.
The perceived complexity of these systems may be responsible for why there are substantial disparities in the prescription of closed-loop systems. Kids of lower socioeconomic status are dramatically less likely to receive these advanced technologies. Providers may feel that patients with lower health literacy or social supports are not “ideal” for these technologies, even though they lead to demonstrably better outcomes.
That means that easier might be better. And a “bionic pancreas,” as reported in an article from The New England Journal of Medicine, is exactly that.
Broadly, it’s another closed-loop system. The bionic pancreas integrates with a continuous glucose monitor and administers insulin when needed. But the algorithm appears to be a bit smarter than what we have in existing devices. For example, patients do not need to provide any information about their usual insulin doses – just their body weight. They don’t need to count carbohydrates at meals – just to inform the device when they are eating, and whether the meal is the usual amount they eat, more, or less. The algorithm learns and adapts as it is used. Easy.
And in this randomized trial, easy does it.
A total of 219 participants were randomized in a 2:1 ratio to the bionic pancreas or usual diabetes care, though it was required that control participants use a continuous glucose monitor. Participants were as young as 6 years old and up to 79 years old; the majority were White and had a relatively high household income. The mean A1c was around 7.8% at baseline.
By the end of the study, the A1c was significantly improved in the bionic pancreas group, with a mean of 7.3% vs. 7.7% in the usual-care group.
This effect was most pronounced in those with a higher A1c at baseline.
People randomized to the bionic pancreas also spent more time in the target glucose range of 70-180 mg/dL.
All in all, the technology that makes it easy to manage your blood sugar, well, made it easy to manage your blood sugar.
But new technology is never without its hiccups. Those randomized to the bionic pancreas had a markedly higher rate of adverse events (244 events in 126 people compared with 10 events in 8 people in the usual-care group.)
This is actually a little misleading, though. The vast majority of these events were hyperglycemic episodes due to infusion set failures, which were reportable only in the bionic pancreas group. In other words, the patients in the control group who had an infusion set failure (assuming they were using an insulin pump at all) would have just called their regular doctor to get things sorted and not reported it to the study team.
Nevertheless, these adverse events – not serious, but common – highlight the fact that good software is not the only key to solving the closed-loop problem. We need good hardware too, hardware that can withstand the very active lives that children with type 1 diabetes deserve to live.
In short, the dream of a functional cure to type 1 diabetes, a true artificial pancreas, is closer than ever, but it’s still just a dream. With iterative advances like this, though, the reality may be here before you know it.
Dr. Wilson is associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com. A version of this article first appeared on Medscape.com.
This transcript of Impact Factor with F. Perry Wilson has been edited for clarity.
It was 100 years ago when Leonard Thompson, age 13, received a reprieve from a death sentence. Young master Thompson had type 1 diabetes, a disease that was uniformly fatal within months of diagnosis. But he received a new treatment, insulin, from a canine pancreas. He would live 13 more years before dying at age 26 of pneumonia.
The history of type 1 diabetes since that time has been a battle on two fronts: First, the search for a cause of and cure for the disease; second, the effort to make the administration of insulin safer, more reliable, and easier.
The past 2 decades have seen a technological revolution in type 1 diabetes care, with continuous glucose monitors decreasing the need for painful finger sticks, and insulin pumps allowing for more precise titration of doses.
The dream, of course, has been to combine those two technologies, continuous glucose monitoring and insulin pumps, to create so-called closed-loop systems – basically an artificial pancreas – that would obviate the need for any intervention on the part of the patient, save the occasional refilling of an insulin reservoir.
We aren’t there yet, but we are closer than ever.
Closed-loop systems for insulin delivery, like the Tandem Control IQ system, are a marvel of technology, but they are not exactly hands-free. Users need to dial in settings for their insulin usage, count carbohydrates at meals, and inform the system that they are about to eat those meals to allow the algorithm to administer an appropriate insulin dose.
The perceived complexity of these systems may be responsible for why there are substantial disparities in the prescription of closed-loop systems. Kids of lower socioeconomic status are dramatically less likely to receive these advanced technologies. Providers may feel that patients with lower health literacy or social supports are not “ideal” for these technologies, even though they lead to demonstrably better outcomes.
That means that easier might be better. And a “bionic pancreas,” as reported in an article from The New England Journal of Medicine, is exactly that.
Broadly, it’s another closed-loop system. The bionic pancreas integrates with a continuous glucose monitor and administers insulin when needed. But the algorithm appears to be a bit smarter than what we have in existing devices. For example, patients do not need to provide any information about their usual insulin doses – just their body weight. They don’t need to count carbohydrates at meals – just to inform the device when they are eating, and whether the meal is the usual amount they eat, more, or less. The algorithm learns and adapts as it is used. Easy.
And in this randomized trial, easy does it.
A total of 219 participants were randomized in a 2:1 ratio to the bionic pancreas or usual diabetes care, though it was required that control participants use a continuous glucose monitor. Participants were as young as 6 years old and up to 79 years old; the majority were White and had a relatively high household income. The mean A1c was around 7.8% at baseline.
By the end of the study, the A1c was significantly improved in the bionic pancreas group, with a mean of 7.3% vs. 7.7% in the usual-care group.
This effect was most pronounced in those with a higher A1c at baseline.
People randomized to the bionic pancreas also spent more time in the target glucose range of 70-180 mg/dL.
All in all, the technology that makes it easy to manage your blood sugar, well, made it easy to manage your blood sugar.
But new technology is never without its hiccups. Those randomized to the bionic pancreas had a markedly higher rate of adverse events (244 events in 126 people compared with 10 events in 8 people in the usual-care group.)
This is actually a little misleading, though. The vast majority of these events were hyperglycemic episodes due to infusion set failures, which were reportable only in the bionic pancreas group. In other words, the patients in the control group who had an infusion set failure (assuming they were using an insulin pump at all) would have just called their regular doctor to get things sorted and not reported it to the study team.
Nevertheless, these adverse events – not serious, but common – highlight the fact that good software is not the only key to solving the closed-loop problem. We need good hardware too, hardware that can withstand the very active lives that children with type 1 diabetes deserve to live.
In short, the dream of a functional cure to type 1 diabetes, a true artificial pancreas, is closer than ever, but it’s still just a dream. With iterative advances like this, though, the reality may be here before you know it.
Dr. Wilson is associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com. A version of this article first appeared on Medscape.com.
This transcript of Impact Factor with F. Perry Wilson has been edited for clarity.
It was 100 years ago when Leonard Thompson, age 13, received a reprieve from a death sentence. Young master Thompson had type 1 diabetes, a disease that was uniformly fatal within months of diagnosis. But he received a new treatment, insulin, from a canine pancreas. He would live 13 more years before dying at age 26 of pneumonia.
The history of type 1 diabetes since that time has been a battle on two fronts: First, the search for a cause of and cure for the disease; second, the effort to make the administration of insulin safer, more reliable, and easier.
The past 2 decades have seen a technological revolution in type 1 diabetes care, with continuous glucose monitors decreasing the need for painful finger sticks, and insulin pumps allowing for more precise titration of doses.
The dream, of course, has been to combine those two technologies, continuous glucose monitoring and insulin pumps, to create so-called closed-loop systems – basically an artificial pancreas – that would obviate the need for any intervention on the part of the patient, save the occasional refilling of an insulin reservoir.
We aren’t there yet, but we are closer than ever.
Closed-loop systems for insulin delivery, like the Tandem Control IQ system, are a marvel of technology, but they are not exactly hands-free. Users need to dial in settings for their insulin usage, count carbohydrates at meals, and inform the system that they are about to eat those meals to allow the algorithm to administer an appropriate insulin dose.
The perceived complexity of these systems may be responsible for why there are substantial disparities in the prescription of closed-loop systems. Kids of lower socioeconomic status are dramatically less likely to receive these advanced technologies. Providers may feel that patients with lower health literacy or social supports are not “ideal” for these technologies, even though they lead to demonstrably better outcomes.
That means that easier might be better. And a “bionic pancreas,” as reported in an article from The New England Journal of Medicine, is exactly that.
Broadly, it’s another closed-loop system. The bionic pancreas integrates with a continuous glucose monitor and administers insulin when needed. But the algorithm appears to be a bit smarter than what we have in existing devices. For example, patients do not need to provide any information about their usual insulin doses – just their body weight. They don’t need to count carbohydrates at meals – just to inform the device when they are eating, and whether the meal is the usual amount they eat, more, or less. The algorithm learns and adapts as it is used. Easy.
And in this randomized trial, easy does it.
A total of 219 participants were randomized in a 2:1 ratio to the bionic pancreas or usual diabetes care, though it was required that control participants use a continuous glucose monitor. Participants were as young as 6 years old and up to 79 years old; the majority were White and had a relatively high household income. The mean A1c was around 7.8% at baseline.
By the end of the study, the A1c was significantly improved in the bionic pancreas group, with a mean of 7.3% vs. 7.7% in the usual-care group.
This effect was most pronounced in those with a higher A1c at baseline.
People randomized to the bionic pancreas also spent more time in the target glucose range of 70-180 mg/dL.
All in all, the technology that makes it easy to manage your blood sugar, well, made it easy to manage your blood sugar.
But new technology is never without its hiccups. Those randomized to the bionic pancreas had a markedly higher rate of adverse events (244 events in 126 people compared with 10 events in 8 people in the usual-care group.)
This is actually a little misleading, though. The vast majority of these events were hyperglycemic episodes due to infusion set failures, which were reportable only in the bionic pancreas group. In other words, the patients in the control group who had an infusion set failure (assuming they were using an insulin pump at all) would have just called their regular doctor to get things sorted and not reported it to the study team.
Nevertheless, these adverse events – not serious, but common – highlight the fact that good software is not the only key to solving the closed-loop problem. We need good hardware too, hardware that can withstand the very active lives that children with type 1 diabetes deserve to live.
In short, the dream of a functional cure to type 1 diabetes, a true artificial pancreas, is closer than ever, but it’s still just a dream. With iterative advances like this, though, the reality may be here before you know it.
Dr. Wilson is associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com. A version of this article first appeared on Medscape.com.
Long COVID could cost the economy trillions, experts predict
from restaurants struggling to replace low-wage workers, to airlines scrambling to replace crew, to overwhelmed hospitals, experts are predicting.
“There’s a lot we need to do to understand what it takes to enable disabled people to participate more in the economy,” said Katie Bach, a senior fellow with Brookings Institution and the author of a study looking into long COVID’s impact on the labor market.
Data from June 2022 from the Centers for Disease Control and Prevention shows that, of the 40% of American adults who contracted COVID-19, nearly one in five still have long COVID symptoms. That works out to 1 in 13, or 7.5%, of the overall U.S. adult population.
Drawing from the CDC data, Ms. Bach estimates in her August 2022 report that as many as 4 million working-age Americans are too sick with long COVID to perform their jobs. That works out to as much as $230 billion in lost wages, or almost 1% of the U.S. GDP.
“This is a big deal,” she said. “We’re talking potentially hundreds of billions of dollars a year and that this is big enough to have a measurable impact on the labor market.”
Other sources have suggested lower figures, but the conclusions are the same: Long COVID is an urgent issue that will cost tens of billions of dollars a year in lost wages alone, Ms. Bach said. But it’s not just lost income for workers. There is a cost for businesses and the public.
Throughout the pandemic, COVID-19’s crippling force could be felt across multiple industries. While business has picked up again, staffing shortages remain a challenge. At some airports this summer, air passengers spent hours in security lines; were stranded for days as flights were canceled, rebooked, and canceled again on short notice; and waited weeks for lost luggage. Restaurants have had to cut back their hours. Those seeking medical care had longer than usual wait times in EDs and urgent care clinics. Some EDs temporarily closed.
These challenges have been attributed in part to the “great resignation” and in part because so many infected workers were out, especially during the Omicron waves. But increasingly, economists and health care professionals alike worry about long COVID’s impact on employers and the broader economy.
David Cutler, PhD, a professor of economics at Harvard University, Cambridge, Mass., believes the total economic loss could be as high as $3.7 trillion, when factoring in the lost quality of life, the cost in lost earnings, and the cost of higher spending on medical care. His estimate is more than a trillion dollars higher than a previous projection he and fellow economist Lawrence Summers, PhD, made in 2020. The reason? Long COVID.
“The higher estimate is largely a result of the greater prevalence of long COVID than we had guessed at the time,” Dr. Cutler wrote in a paper released in July.
“There are about 10 times the number of people with long COVID as have died of COVID. Because long COVID is so new, there is uncertainty about all of the numbers involved in the calculations. Still, the costs here are conservative, based on only cases to date.”
In Ms. Bach’s Brookings report, she projected that, if recovery from long COVID does not pick up and the population of Americans with long COVID were to grow by 10% a year, the annual cost of lost wages alone could reach half a trillion dollars in a decade.
Meanwhile, a working paper by the National Bureau of Economic Research found that workers who missed an entire week of work because of probable COVID-19 illnesses were roughly 7 percentage points less likely to be working a year later, compared with those who did not miss work for health reasons.
“It’s not just individuals with long COVID who are suffering from this. It impacts their families, their livelihoods, and the economy on a global scale. So, we have to raise awareness about those ripple effects,” said Linda Geng, MD, a clinical assistant professor of medicine with Stanford (Calif.) University’s Primary Care and Population Health.
“I think it’s hard for the public to grasp ... and understand the scale of this public health crisis.”
Debilitating fatigue
Long COVID is roughly defined; the CDC defines it as symptoms that linger 3 or more months after a patient first catches the virus.
The symptoms vary and include profound fatigue and brain issues.
“It’s a new degree of extreme and debilitating fatigue and exhaustion, to the point where you can’t do your daily tasks,” said Dr. Geng, who is also the codirector of Stanford’s Post-Acute COVID-19 Syndrome Clinic.
“People can be so debilitated, they can’t even do basic things, like the activities of daily living, let alone do their job, particularly if it’s physically or mentally demanding.”
Patients can also have postexertional malaise, where they feel especially bad and symptoms worsen when they exert themselves physically or mentally, Dr. Geng said. Compounding the issue for many long COVID patients is their trouble getting restful sleep. Those with brain fog have issues with memory, processing information, focused concentration, confusion, making mistakes, and multitasking. Pain is another debilitating symptom that can disrupt daily life and ability to work.
Even people with relatively mild infections can end up with long COVID, Dr. Geng said, noting that many of the patients at the Stanford clinic were never hospitalized with their initial infections. While existing research and Dr. Geng’s clinical experience show that long COVID can hit any age, she most commonly sees patients from ages 20 to their 60s, with an average age in the 40s – people in their prime working ages.
Jason Furman, PhD, a former White House economic adviser who is now a professor at Harvard University, noted in August that the labor force participation rate was far below what could be explained by standard demographic changes like an aging population, with the decline evident across all age groups. Dr. Furman does not speculate about why, but others have.
“We are pessimistic: Both the aging of the population and the impact of long COVID imply that the participation rate will be slow to return to its prepandemic level,” Anna Wong, Yelena Shulyatyeva, Andrew Husby, and Eliza Winger, economists with Bloomberg Economics, wrote in a research note.
Supportive policies
There is some evidence that vaccination reduces the risk of long COVID, but not completely, and it is too early to know if repeat infections increase long COVID risks. There is also no definitive data on how fast or how many people are recovering. Economists often assume that those with long COVID will recover at some point, Ms. Bach noted, but she is careful not to make assumptions.
“If people aren’t recovering, then this group keeps getting bigger,” she said. “We’re still adding, and if people aren’t coming out of that group, this becomes a bigger and bigger problem.”
For now, the number of new people being diagnosed with long COVID appears to have slowed, Ms. Bach said, but it remains to be seen whether the trend can be sustained.
“If people are impaired longer than we think and if the impairment turns out to be severe, then we can have a lot of people who need services like disability insurance,” Dr. Cutler said.
“That could put a really big strain on public sector programs and our ability to meet those needs.”
Policies that support the research and clinical work necessary to prevent and treat long COVID are essential, experts say.
“To me, that is the biggest economic imperative, to say nothing of human suffering,” said Ms. Bach.
Employers also have a role, and experts say there are a number of accommodations businesses should consider. What happens when an employee has long COVID? Can accommodations be made that allow them to continue working productively? If they spend a great deal of time commuting, can they work from home? What can employers do so that family members do not have to drop out of the workforce to take care of loved ones with long COVID?
Disability insurance
To be sure, there is one piece of the puzzle that does not quite fit, according to Dr. Cutler and Ms. Bach. There is no sign yet of a large increase in federal disability insurance applications, and no one quite knows why. Publicly available government data shows that online applications actually dipped by about 4% each year between 2019 and 2021. Applications in 2022 appear on track to remain slightly below prepandemic levels.
To qualify for Social Security Disability Insurance (SSDI), people need to have a disability that lasts at least a year.
“If you’re disabled with long COVID, who knows, right? You don’t know,” said Ms. Bach. “Two of the most dominant symptoms of long COVID are fatigue and brain fog. So, I’ve heard from people that the process of going through an SSDI application is really hard.”
Some long COVID patients told Ms. Bach they simply assumed they would not get SSDI and did not even bother applying. She stressed that working Americans with debilitating long COVID should be aware that their condition is protected by the Americans with Disabilities Act. But the challenge, based on guidance issued by the government, is that not all cases of long COVID qualify as a disability and that individual assessments are necessary.
While more long COVID data are needed, Ms. Bach believes there is enough information for decisionmakers to go after the issue more aggressively. She pointed to the $1.15 billion in funding that Congress earmarked for the National Institutes of Health over the course of 4 years in support of research into the long-term health effects of COVID-19.
“Now, $250 million a year sounds like a lot of money until you start talking about the cost of lost wages – just lost wages,” Ms. Bach said. “That’s not lost productivity. That’s not the cost of people whose family members are sick. Who have to reduce their own labor force participation. That’s not medical costs. Suddenly, $250 million doesn’t really sound like that much.”
A version of this article first appeared on WebMD.com.
from restaurants struggling to replace low-wage workers, to airlines scrambling to replace crew, to overwhelmed hospitals, experts are predicting.
“There’s a lot we need to do to understand what it takes to enable disabled people to participate more in the economy,” said Katie Bach, a senior fellow with Brookings Institution and the author of a study looking into long COVID’s impact on the labor market.
Data from June 2022 from the Centers for Disease Control and Prevention shows that, of the 40% of American adults who contracted COVID-19, nearly one in five still have long COVID symptoms. That works out to 1 in 13, or 7.5%, of the overall U.S. adult population.
Drawing from the CDC data, Ms. Bach estimates in her August 2022 report that as many as 4 million working-age Americans are too sick with long COVID to perform their jobs. That works out to as much as $230 billion in lost wages, or almost 1% of the U.S. GDP.
“This is a big deal,” she said. “We’re talking potentially hundreds of billions of dollars a year and that this is big enough to have a measurable impact on the labor market.”
Other sources have suggested lower figures, but the conclusions are the same: Long COVID is an urgent issue that will cost tens of billions of dollars a year in lost wages alone, Ms. Bach said. But it’s not just lost income for workers. There is a cost for businesses and the public.
Throughout the pandemic, COVID-19’s crippling force could be felt across multiple industries. While business has picked up again, staffing shortages remain a challenge. At some airports this summer, air passengers spent hours in security lines; were stranded for days as flights were canceled, rebooked, and canceled again on short notice; and waited weeks for lost luggage. Restaurants have had to cut back their hours. Those seeking medical care had longer than usual wait times in EDs and urgent care clinics. Some EDs temporarily closed.
These challenges have been attributed in part to the “great resignation” and in part because so many infected workers were out, especially during the Omicron waves. But increasingly, economists and health care professionals alike worry about long COVID’s impact on employers and the broader economy.
David Cutler, PhD, a professor of economics at Harvard University, Cambridge, Mass., believes the total economic loss could be as high as $3.7 trillion, when factoring in the lost quality of life, the cost in lost earnings, and the cost of higher spending on medical care. His estimate is more than a trillion dollars higher than a previous projection he and fellow economist Lawrence Summers, PhD, made in 2020. The reason? Long COVID.
“The higher estimate is largely a result of the greater prevalence of long COVID than we had guessed at the time,” Dr. Cutler wrote in a paper released in July.
“There are about 10 times the number of people with long COVID as have died of COVID. Because long COVID is so new, there is uncertainty about all of the numbers involved in the calculations. Still, the costs here are conservative, based on only cases to date.”
In Ms. Bach’s Brookings report, she projected that, if recovery from long COVID does not pick up and the population of Americans with long COVID were to grow by 10% a year, the annual cost of lost wages alone could reach half a trillion dollars in a decade.
Meanwhile, a working paper by the National Bureau of Economic Research found that workers who missed an entire week of work because of probable COVID-19 illnesses were roughly 7 percentage points less likely to be working a year later, compared with those who did not miss work for health reasons.
“It’s not just individuals with long COVID who are suffering from this. It impacts their families, their livelihoods, and the economy on a global scale. So, we have to raise awareness about those ripple effects,” said Linda Geng, MD, a clinical assistant professor of medicine with Stanford (Calif.) University’s Primary Care and Population Health.
“I think it’s hard for the public to grasp ... and understand the scale of this public health crisis.”
Debilitating fatigue
Long COVID is roughly defined; the CDC defines it as symptoms that linger 3 or more months after a patient first catches the virus.
The symptoms vary and include profound fatigue and brain issues.
“It’s a new degree of extreme and debilitating fatigue and exhaustion, to the point where you can’t do your daily tasks,” said Dr. Geng, who is also the codirector of Stanford’s Post-Acute COVID-19 Syndrome Clinic.
“People can be so debilitated, they can’t even do basic things, like the activities of daily living, let alone do their job, particularly if it’s physically or mentally demanding.”
Patients can also have postexertional malaise, where they feel especially bad and symptoms worsen when they exert themselves physically or mentally, Dr. Geng said. Compounding the issue for many long COVID patients is their trouble getting restful sleep. Those with brain fog have issues with memory, processing information, focused concentration, confusion, making mistakes, and multitasking. Pain is another debilitating symptom that can disrupt daily life and ability to work.
Even people with relatively mild infections can end up with long COVID, Dr. Geng said, noting that many of the patients at the Stanford clinic were never hospitalized with their initial infections. While existing research and Dr. Geng’s clinical experience show that long COVID can hit any age, she most commonly sees patients from ages 20 to their 60s, with an average age in the 40s – people in their prime working ages.
Jason Furman, PhD, a former White House economic adviser who is now a professor at Harvard University, noted in August that the labor force participation rate was far below what could be explained by standard demographic changes like an aging population, with the decline evident across all age groups. Dr. Furman does not speculate about why, but others have.
“We are pessimistic: Both the aging of the population and the impact of long COVID imply that the participation rate will be slow to return to its prepandemic level,” Anna Wong, Yelena Shulyatyeva, Andrew Husby, and Eliza Winger, economists with Bloomberg Economics, wrote in a research note.
Supportive policies
There is some evidence that vaccination reduces the risk of long COVID, but not completely, and it is too early to know if repeat infections increase long COVID risks. There is also no definitive data on how fast or how many people are recovering. Economists often assume that those with long COVID will recover at some point, Ms. Bach noted, but she is careful not to make assumptions.
“If people aren’t recovering, then this group keeps getting bigger,” she said. “We’re still adding, and if people aren’t coming out of that group, this becomes a bigger and bigger problem.”
For now, the number of new people being diagnosed with long COVID appears to have slowed, Ms. Bach said, but it remains to be seen whether the trend can be sustained.
“If people are impaired longer than we think and if the impairment turns out to be severe, then we can have a lot of people who need services like disability insurance,” Dr. Cutler said.
“That could put a really big strain on public sector programs and our ability to meet those needs.”
Policies that support the research and clinical work necessary to prevent and treat long COVID are essential, experts say.
“To me, that is the biggest economic imperative, to say nothing of human suffering,” said Ms. Bach.
Employers also have a role, and experts say there are a number of accommodations businesses should consider. What happens when an employee has long COVID? Can accommodations be made that allow them to continue working productively? If they spend a great deal of time commuting, can they work from home? What can employers do so that family members do not have to drop out of the workforce to take care of loved ones with long COVID?
Disability insurance
To be sure, there is one piece of the puzzle that does not quite fit, according to Dr. Cutler and Ms. Bach. There is no sign yet of a large increase in federal disability insurance applications, and no one quite knows why. Publicly available government data shows that online applications actually dipped by about 4% each year between 2019 and 2021. Applications in 2022 appear on track to remain slightly below prepandemic levels.
To qualify for Social Security Disability Insurance (SSDI), people need to have a disability that lasts at least a year.
“If you’re disabled with long COVID, who knows, right? You don’t know,” said Ms. Bach. “Two of the most dominant symptoms of long COVID are fatigue and brain fog. So, I’ve heard from people that the process of going through an SSDI application is really hard.”
Some long COVID patients told Ms. Bach they simply assumed they would not get SSDI and did not even bother applying. She stressed that working Americans with debilitating long COVID should be aware that their condition is protected by the Americans with Disabilities Act. But the challenge, based on guidance issued by the government, is that not all cases of long COVID qualify as a disability and that individual assessments are necessary.
While more long COVID data are needed, Ms. Bach believes there is enough information for decisionmakers to go after the issue more aggressively. She pointed to the $1.15 billion in funding that Congress earmarked for the National Institutes of Health over the course of 4 years in support of research into the long-term health effects of COVID-19.
“Now, $250 million a year sounds like a lot of money until you start talking about the cost of lost wages – just lost wages,” Ms. Bach said. “That’s not lost productivity. That’s not the cost of people whose family members are sick. Who have to reduce their own labor force participation. That’s not medical costs. Suddenly, $250 million doesn’t really sound like that much.”
A version of this article first appeared on WebMD.com.
from restaurants struggling to replace low-wage workers, to airlines scrambling to replace crew, to overwhelmed hospitals, experts are predicting.
“There’s a lot we need to do to understand what it takes to enable disabled people to participate more in the economy,” said Katie Bach, a senior fellow with Brookings Institution and the author of a study looking into long COVID’s impact on the labor market.
Data from June 2022 from the Centers for Disease Control and Prevention shows that, of the 40% of American adults who contracted COVID-19, nearly one in five still have long COVID symptoms. That works out to 1 in 13, or 7.5%, of the overall U.S. adult population.
Drawing from the CDC data, Ms. Bach estimates in her August 2022 report that as many as 4 million working-age Americans are too sick with long COVID to perform their jobs. That works out to as much as $230 billion in lost wages, or almost 1% of the U.S. GDP.
“This is a big deal,” she said. “We’re talking potentially hundreds of billions of dollars a year and that this is big enough to have a measurable impact on the labor market.”
Other sources have suggested lower figures, but the conclusions are the same: Long COVID is an urgent issue that will cost tens of billions of dollars a year in lost wages alone, Ms. Bach said. But it’s not just lost income for workers. There is a cost for businesses and the public.
Throughout the pandemic, COVID-19’s crippling force could be felt across multiple industries. While business has picked up again, staffing shortages remain a challenge. At some airports this summer, air passengers spent hours in security lines; were stranded for days as flights were canceled, rebooked, and canceled again on short notice; and waited weeks for lost luggage. Restaurants have had to cut back their hours. Those seeking medical care had longer than usual wait times in EDs and urgent care clinics. Some EDs temporarily closed.
These challenges have been attributed in part to the “great resignation” and in part because so many infected workers were out, especially during the Omicron waves. But increasingly, economists and health care professionals alike worry about long COVID’s impact on employers and the broader economy.
David Cutler, PhD, a professor of economics at Harvard University, Cambridge, Mass., believes the total economic loss could be as high as $3.7 trillion, when factoring in the lost quality of life, the cost in lost earnings, and the cost of higher spending on medical care. His estimate is more than a trillion dollars higher than a previous projection he and fellow economist Lawrence Summers, PhD, made in 2020. The reason? Long COVID.
“The higher estimate is largely a result of the greater prevalence of long COVID than we had guessed at the time,” Dr. Cutler wrote in a paper released in July.
“There are about 10 times the number of people with long COVID as have died of COVID. Because long COVID is so new, there is uncertainty about all of the numbers involved in the calculations. Still, the costs here are conservative, based on only cases to date.”
In Ms. Bach’s Brookings report, she projected that, if recovery from long COVID does not pick up and the population of Americans with long COVID were to grow by 10% a year, the annual cost of lost wages alone could reach half a trillion dollars in a decade.
Meanwhile, a working paper by the National Bureau of Economic Research found that workers who missed an entire week of work because of probable COVID-19 illnesses were roughly 7 percentage points less likely to be working a year later, compared with those who did not miss work for health reasons.
“It’s not just individuals with long COVID who are suffering from this. It impacts their families, their livelihoods, and the economy on a global scale. So, we have to raise awareness about those ripple effects,” said Linda Geng, MD, a clinical assistant professor of medicine with Stanford (Calif.) University’s Primary Care and Population Health.
“I think it’s hard for the public to grasp ... and understand the scale of this public health crisis.”
Debilitating fatigue
Long COVID is roughly defined; the CDC defines it as symptoms that linger 3 or more months after a patient first catches the virus.
The symptoms vary and include profound fatigue and brain issues.
“It’s a new degree of extreme and debilitating fatigue and exhaustion, to the point where you can’t do your daily tasks,” said Dr. Geng, who is also the codirector of Stanford’s Post-Acute COVID-19 Syndrome Clinic.
“People can be so debilitated, they can’t even do basic things, like the activities of daily living, let alone do their job, particularly if it’s physically or mentally demanding.”
Patients can also have postexertional malaise, where they feel especially bad and symptoms worsen when they exert themselves physically or mentally, Dr. Geng said. Compounding the issue for many long COVID patients is their trouble getting restful sleep. Those with brain fog have issues with memory, processing information, focused concentration, confusion, making mistakes, and multitasking. Pain is another debilitating symptom that can disrupt daily life and ability to work.
Even people with relatively mild infections can end up with long COVID, Dr. Geng said, noting that many of the patients at the Stanford clinic were never hospitalized with their initial infections. While existing research and Dr. Geng’s clinical experience show that long COVID can hit any age, she most commonly sees patients from ages 20 to their 60s, with an average age in the 40s – people in their prime working ages.
Jason Furman, PhD, a former White House economic adviser who is now a professor at Harvard University, noted in August that the labor force participation rate was far below what could be explained by standard demographic changes like an aging population, with the decline evident across all age groups. Dr. Furman does not speculate about why, but others have.
“We are pessimistic: Both the aging of the population and the impact of long COVID imply that the participation rate will be slow to return to its prepandemic level,” Anna Wong, Yelena Shulyatyeva, Andrew Husby, and Eliza Winger, economists with Bloomberg Economics, wrote in a research note.
Supportive policies
There is some evidence that vaccination reduces the risk of long COVID, but not completely, and it is too early to know if repeat infections increase long COVID risks. There is also no definitive data on how fast or how many people are recovering. Economists often assume that those with long COVID will recover at some point, Ms. Bach noted, but she is careful not to make assumptions.
“If people aren’t recovering, then this group keeps getting bigger,” she said. “We’re still adding, and if people aren’t coming out of that group, this becomes a bigger and bigger problem.”
For now, the number of new people being diagnosed with long COVID appears to have slowed, Ms. Bach said, but it remains to be seen whether the trend can be sustained.
“If people are impaired longer than we think and if the impairment turns out to be severe, then we can have a lot of people who need services like disability insurance,” Dr. Cutler said.
“That could put a really big strain on public sector programs and our ability to meet those needs.”
Policies that support the research and clinical work necessary to prevent and treat long COVID are essential, experts say.
“To me, that is the biggest economic imperative, to say nothing of human suffering,” said Ms. Bach.
Employers also have a role, and experts say there are a number of accommodations businesses should consider. What happens when an employee has long COVID? Can accommodations be made that allow them to continue working productively? If they spend a great deal of time commuting, can they work from home? What can employers do so that family members do not have to drop out of the workforce to take care of loved ones with long COVID?
Disability insurance
To be sure, there is one piece of the puzzle that does not quite fit, according to Dr. Cutler and Ms. Bach. There is no sign yet of a large increase in federal disability insurance applications, and no one quite knows why. Publicly available government data shows that online applications actually dipped by about 4% each year between 2019 and 2021. Applications in 2022 appear on track to remain slightly below prepandemic levels.
To qualify for Social Security Disability Insurance (SSDI), people need to have a disability that lasts at least a year.
“If you’re disabled with long COVID, who knows, right? You don’t know,” said Ms. Bach. “Two of the most dominant symptoms of long COVID are fatigue and brain fog. So, I’ve heard from people that the process of going through an SSDI application is really hard.”
Some long COVID patients told Ms. Bach they simply assumed they would not get SSDI and did not even bother applying. She stressed that working Americans with debilitating long COVID should be aware that their condition is protected by the Americans with Disabilities Act. But the challenge, based on guidance issued by the government, is that not all cases of long COVID qualify as a disability and that individual assessments are necessary.
While more long COVID data are needed, Ms. Bach believes there is enough information for decisionmakers to go after the issue more aggressively. She pointed to the $1.15 billion in funding that Congress earmarked for the National Institutes of Health over the course of 4 years in support of research into the long-term health effects of COVID-19.
“Now, $250 million a year sounds like a lot of money until you start talking about the cost of lost wages – just lost wages,” Ms. Bach said. “That’s not lost productivity. That’s not the cost of people whose family members are sick. Who have to reduce their own labor force participation. That’s not medical costs. Suddenly, $250 million doesn’t really sound like that much.”
A version of this article first appeared on WebMD.com.
GERD linked to increased risk of nontuberculous mycobacterial pulmonary disease
Patients with gastrointestinal esophageal reflux disease (GERD) have more than three times the risk of developing nontuberculous mycobacterial pulmonary disease (NTM-PD), compared with those without GERD, according to a population-based retrospective cohort study.
“GERD is a common comorbidity of nontuberculous mycobacterial pulmonary disease [but] whether GERD is associated with an increased risk of developing NTM-PD is unknown,” Hayoung Choi, MD, PhD, Hallym University, Seoul, Republic of Korea, and colleagues reported.
Dr. Choi added in an email. “What needs to be understood is that GERD increases health care utilization in patients with NTM pulmonary disease; hence, clinicians who treat patients with NTM pulmonary disease need to be aware of the burden of GERD and treat the gastrointestinal illness simultaneously,” he added.
The study was published online in the journal CHEST.
Sample cohort
Data from the Korean National Health Insurance Service-National Sample Cohort between 2002 and 2015 were used to assess the impact of GERD on NTM-PD. The incidence and risk of NTM-PD were compared between 17,424 patients with GERD and 69,000 patients without GERD in a matched cohort. GERD was defined as patients having received more than 3 months of proton pump inhibitors (PPIs).
During a median follow-up of 5.1 years, the age- and sex-adjusted incidence of NTM-PD was significantly higher in the GERD cohort, at a rate of 34.8/100,000 person-years, than in the matched cohort, at a rate of only 10.5/100,000 person-years (P < .001), the authors reported.
As for risk factors for NTM-PD, being 60 years of age and older was associated with a 3.5-times higher risk of NTM-PD at an adjusted hazard ratio of 3.57 (95% confidence interval, 1.58-8.07), while bronchiectasis was associated with over an 18-times higher risk of NTM-PD in the GERD cohort at an adjusted HR of 18.69 (95% CI, 6.68-552.28). Those with GERD who developed NTM-PD had higher all-cause and respiratory disease–related emergency department visits or hospitalizations compared with patients with GERD who did not develop NTM-PD (P = .011), the investigators noted.
As the authors pointed out, the incidence of NTM-PD in the Korean population ranged from 6 to 19 cases/100,000 between 2008 and 2016; thus, the burden of incident NTM-PD associated with GERD appears to be considerable. As Dr. Choi explained, a combination of three factors influenced the development of NTM infections. The first is environmental, from water source, climate, or region; the second is patient influences, including such factors as immunodeficiency and comorbidities (including bronchiectasis); and the third is microbiological factors, including various NTM species.
Bile aspirating into the lung during GERD may be another possible pathway, as the authors suggested. Even if acid secretion is suppressed by PPI treatment in patients with GERD, NTM-PD may be induced or aggravated through mechanisms such as bile reflux. The fact that patients over the age of 60 were more prone to develop NTM-PD suggests that a decrease in gastric emptying and increased micro-aspiration or reflux associated with impaired swallowing (which are more common in elderly patients) may also be at play.
“Bronchiectasis is also a very well known risk factor for NTM pulmonary disease,” Dr. Choi emphasized. Thus, he recommends clinicians carefully observe clinical, radiological, and microbiological changes to detect NTM pulmonary disease when managing patients with bronchiectasis.
“The results of the present study have several potential clinical implications,” Dr. Choi and colleagues observed. First, NTM-PD should be suspected when new-onset worsening of respiratory symptoms develops during regular follow-up in patients with GERD. Second, because results indicate that older age and bronchiectasis significantly increase the risk of NTM-PD, “more active strategies (e.g., screening of symptoms and regular chest x-rays)” might be helpful in patients with GERD and these risk factors, the authors suggested. Because patients with GERD who developed NTM-PD had more respiratory disease–related ED visits and hospitalizations than those who did not develop NTM-PD, when GERD and NTM-PD are combined, clinicians should focus on the variations of respiratory symptoms, they suggested.
The authors cautioned, however, that because the study was one in a Korean population, studies in other countries and different ethnicities are needed before findings can be generalized.
More common than TB
Asked to comment on the findings, NTM-PD expert Theodore Marras, MD, clinician investigator, Krembil Research Institute, Toronto, noted that non-TB M-PD is about 10 times more common than TB and that could be an underestimate as there have been very large increases in the incidence of NTM-PD in recent years. “It’s an environmental germ – it’s in our water – and certain people are particularly susceptible to it, typically older age women who have underlying bronchiectasis,” Dr. Marras told this news organization. “And while there are ethnic differences in incidence rates between East Asian people and Black African people, immigration is not the main driver for the increase as far as we can tell,” he said.
He personally treats a lot of NTM-PD and he also believes that GERD is an important risk factor for all types of lung infections including NTM lung disease. “So without a doubt, I believe that GERD should be treated in patients with NTM-PD,” Dr. Marras emphasized. The big question is how to treat GERD, as there may be concerns with acid-suppressive agents such as proton pump inhibitors that “the reflux that comes back up may harbor more germs in it and if that reflux comes up high enough, we are at risk of aspirating some of that fluid into our lungs, especially when we’re asleep,” he said.
Some experts therefore argue in favor of using motility agents instead of PPIs. However, if Dr. Marras has a patient with heartburn, “you have to treat it,” he stressed. Similarly, if a patient has evidence of esophageal erosions, physicians need to treat those as well. However, if neither feature is present, “I tend to like the motility agents preferentially or use them in combination with a PPI,” Dr. Marras said.
Dr. Marras also thinks the study is encouraging physicians involved in treating these patients to think about controlling GERD both when they are treating patients and after they are treated to try to reduce recurrence.
The authors had no financial disclosures to make. Dr. Marras has given several talks on NTM lung disease, one sponsored by AstraZeneca and the other by Novartis.
Patients with gastrointestinal esophageal reflux disease (GERD) have more than three times the risk of developing nontuberculous mycobacterial pulmonary disease (NTM-PD), compared with those without GERD, according to a population-based retrospective cohort study.
“GERD is a common comorbidity of nontuberculous mycobacterial pulmonary disease [but] whether GERD is associated with an increased risk of developing NTM-PD is unknown,” Hayoung Choi, MD, PhD, Hallym University, Seoul, Republic of Korea, and colleagues reported.
Dr. Choi added in an email. “What needs to be understood is that GERD increases health care utilization in patients with NTM pulmonary disease; hence, clinicians who treat patients with NTM pulmonary disease need to be aware of the burden of GERD and treat the gastrointestinal illness simultaneously,” he added.
The study was published online in the journal CHEST.
Sample cohort
Data from the Korean National Health Insurance Service-National Sample Cohort between 2002 and 2015 were used to assess the impact of GERD on NTM-PD. The incidence and risk of NTM-PD were compared between 17,424 patients with GERD and 69,000 patients without GERD in a matched cohort. GERD was defined as patients having received more than 3 months of proton pump inhibitors (PPIs).
During a median follow-up of 5.1 years, the age- and sex-adjusted incidence of NTM-PD was significantly higher in the GERD cohort, at a rate of 34.8/100,000 person-years, than in the matched cohort, at a rate of only 10.5/100,000 person-years (P < .001), the authors reported.
As for risk factors for NTM-PD, being 60 years of age and older was associated with a 3.5-times higher risk of NTM-PD at an adjusted hazard ratio of 3.57 (95% confidence interval, 1.58-8.07), while bronchiectasis was associated with over an 18-times higher risk of NTM-PD in the GERD cohort at an adjusted HR of 18.69 (95% CI, 6.68-552.28). Those with GERD who developed NTM-PD had higher all-cause and respiratory disease–related emergency department visits or hospitalizations compared with patients with GERD who did not develop NTM-PD (P = .011), the investigators noted.
As the authors pointed out, the incidence of NTM-PD in the Korean population ranged from 6 to 19 cases/100,000 between 2008 and 2016; thus, the burden of incident NTM-PD associated with GERD appears to be considerable. As Dr. Choi explained, a combination of three factors influenced the development of NTM infections. The first is environmental, from water source, climate, or region; the second is patient influences, including such factors as immunodeficiency and comorbidities (including bronchiectasis); and the third is microbiological factors, including various NTM species.
Bile aspirating into the lung during GERD may be another possible pathway, as the authors suggested. Even if acid secretion is suppressed by PPI treatment in patients with GERD, NTM-PD may be induced or aggravated through mechanisms such as bile reflux. The fact that patients over the age of 60 were more prone to develop NTM-PD suggests that a decrease in gastric emptying and increased micro-aspiration or reflux associated with impaired swallowing (which are more common in elderly patients) may also be at play.
“Bronchiectasis is also a very well known risk factor for NTM pulmonary disease,” Dr. Choi emphasized. Thus, he recommends clinicians carefully observe clinical, radiological, and microbiological changes to detect NTM pulmonary disease when managing patients with bronchiectasis.
“The results of the present study have several potential clinical implications,” Dr. Choi and colleagues observed. First, NTM-PD should be suspected when new-onset worsening of respiratory symptoms develops during regular follow-up in patients with GERD. Second, because results indicate that older age and bronchiectasis significantly increase the risk of NTM-PD, “more active strategies (e.g., screening of symptoms and regular chest x-rays)” might be helpful in patients with GERD and these risk factors, the authors suggested. Because patients with GERD who developed NTM-PD had more respiratory disease–related ED visits and hospitalizations than those who did not develop NTM-PD, when GERD and NTM-PD are combined, clinicians should focus on the variations of respiratory symptoms, they suggested.
The authors cautioned, however, that because the study was one in a Korean population, studies in other countries and different ethnicities are needed before findings can be generalized.
More common than TB
Asked to comment on the findings, NTM-PD expert Theodore Marras, MD, clinician investigator, Krembil Research Institute, Toronto, noted that non-TB M-PD is about 10 times more common than TB and that could be an underestimate as there have been very large increases in the incidence of NTM-PD in recent years. “It’s an environmental germ – it’s in our water – and certain people are particularly susceptible to it, typically older age women who have underlying bronchiectasis,” Dr. Marras told this news organization. “And while there are ethnic differences in incidence rates between East Asian people and Black African people, immigration is not the main driver for the increase as far as we can tell,” he said.
He personally treats a lot of NTM-PD and he also believes that GERD is an important risk factor for all types of lung infections including NTM lung disease. “So without a doubt, I believe that GERD should be treated in patients with NTM-PD,” Dr. Marras emphasized. The big question is how to treat GERD, as there may be concerns with acid-suppressive agents such as proton pump inhibitors that “the reflux that comes back up may harbor more germs in it and if that reflux comes up high enough, we are at risk of aspirating some of that fluid into our lungs, especially when we’re asleep,” he said.
Some experts therefore argue in favor of using motility agents instead of PPIs. However, if Dr. Marras has a patient with heartburn, “you have to treat it,” he stressed. Similarly, if a patient has evidence of esophageal erosions, physicians need to treat those as well. However, if neither feature is present, “I tend to like the motility agents preferentially or use them in combination with a PPI,” Dr. Marras said.
Dr. Marras also thinks the study is encouraging physicians involved in treating these patients to think about controlling GERD both when they are treating patients and after they are treated to try to reduce recurrence.
The authors had no financial disclosures to make. Dr. Marras has given several talks on NTM lung disease, one sponsored by AstraZeneca and the other by Novartis.
Patients with gastrointestinal esophageal reflux disease (GERD) have more than three times the risk of developing nontuberculous mycobacterial pulmonary disease (NTM-PD), compared with those without GERD, according to a population-based retrospective cohort study.
“GERD is a common comorbidity of nontuberculous mycobacterial pulmonary disease [but] whether GERD is associated with an increased risk of developing NTM-PD is unknown,” Hayoung Choi, MD, PhD, Hallym University, Seoul, Republic of Korea, and colleagues reported.
Dr. Choi added in an email. “What needs to be understood is that GERD increases health care utilization in patients with NTM pulmonary disease; hence, clinicians who treat patients with NTM pulmonary disease need to be aware of the burden of GERD and treat the gastrointestinal illness simultaneously,” he added.
The study was published online in the journal CHEST.
Sample cohort
Data from the Korean National Health Insurance Service-National Sample Cohort between 2002 and 2015 were used to assess the impact of GERD on NTM-PD. The incidence and risk of NTM-PD were compared between 17,424 patients with GERD and 69,000 patients without GERD in a matched cohort. GERD was defined as patients having received more than 3 months of proton pump inhibitors (PPIs).
During a median follow-up of 5.1 years, the age- and sex-adjusted incidence of NTM-PD was significantly higher in the GERD cohort, at a rate of 34.8/100,000 person-years, than in the matched cohort, at a rate of only 10.5/100,000 person-years (P < .001), the authors reported.
As for risk factors for NTM-PD, being 60 years of age and older was associated with a 3.5-times higher risk of NTM-PD at an adjusted hazard ratio of 3.57 (95% confidence interval, 1.58-8.07), while bronchiectasis was associated with over an 18-times higher risk of NTM-PD in the GERD cohort at an adjusted HR of 18.69 (95% CI, 6.68-552.28). Those with GERD who developed NTM-PD had higher all-cause and respiratory disease–related emergency department visits or hospitalizations compared with patients with GERD who did not develop NTM-PD (P = .011), the investigators noted.
As the authors pointed out, the incidence of NTM-PD in the Korean population ranged from 6 to 19 cases/100,000 between 2008 and 2016; thus, the burden of incident NTM-PD associated with GERD appears to be considerable. As Dr. Choi explained, a combination of three factors influenced the development of NTM infections. The first is environmental, from water source, climate, or region; the second is patient influences, including such factors as immunodeficiency and comorbidities (including bronchiectasis); and the third is microbiological factors, including various NTM species.
Bile aspirating into the lung during GERD may be another possible pathway, as the authors suggested. Even if acid secretion is suppressed by PPI treatment in patients with GERD, NTM-PD may be induced or aggravated through mechanisms such as bile reflux. The fact that patients over the age of 60 were more prone to develop NTM-PD suggests that a decrease in gastric emptying and increased micro-aspiration or reflux associated with impaired swallowing (which are more common in elderly patients) may also be at play.
“Bronchiectasis is also a very well known risk factor for NTM pulmonary disease,” Dr. Choi emphasized. Thus, he recommends clinicians carefully observe clinical, radiological, and microbiological changes to detect NTM pulmonary disease when managing patients with bronchiectasis.
“The results of the present study have several potential clinical implications,” Dr. Choi and colleagues observed. First, NTM-PD should be suspected when new-onset worsening of respiratory symptoms develops during regular follow-up in patients with GERD. Second, because results indicate that older age and bronchiectasis significantly increase the risk of NTM-PD, “more active strategies (e.g., screening of symptoms and regular chest x-rays)” might be helpful in patients with GERD and these risk factors, the authors suggested. Because patients with GERD who developed NTM-PD had more respiratory disease–related ED visits and hospitalizations than those who did not develop NTM-PD, when GERD and NTM-PD are combined, clinicians should focus on the variations of respiratory symptoms, they suggested.
The authors cautioned, however, that because the study was one in a Korean population, studies in other countries and different ethnicities are needed before findings can be generalized.
More common than TB
Asked to comment on the findings, NTM-PD expert Theodore Marras, MD, clinician investigator, Krembil Research Institute, Toronto, noted that non-TB M-PD is about 10 times more common than TB and that could be an underestimate as there have been very large increases in the incidence of NTM-PD in recent years. “It’s an environmental germ – it’s in our water – and certain people are particularly susceptible to it, typically older age women who have underlying bronchiectasis,” Dr. Marras told this news organization. “And while there are ethnic differences in incidence rates between East Asian people and Black African people, immigration is not the main driver for the increase as far as we can tell,” he said.
He personally treats a lot of NTM-PD and he also believes that GERD is an important risk factor for all types of lung infections including NTM lung disease. “So without a doubt, I believe that GERD should be treated in patients with NTM-PD,” Dr. Marras emphasized. The big question is how to treat GERD, as there may be concerns with acid-suppressive agents such as proton pump inhibitors that “the reflux that comes back up may harbor more germs in it and if that reflux comes up high enough, we are at risk of aspirating some of that fluid into our lungs, especially when we’re asleep,” he said.
Some experts therefore argue in favor of using motility agents instead of PPIs. However, if Dr. Marras has a patient with heartburn, “you have to treat it,” he stressed. Similarly, if a patient has evidence of esophageal erosions, physicians need to treat those as well. However, if neither feature is present, “I tend to like the motility agents preferentially or use them in combination with a PPI,” Dr. Marras said.
Dr. Marras also thinks the study is encouraging physicians involved in treating these patients to think about controlling GERD both when they are treating patients and after they are treated to try to reduce recurrence.
The authors had no financial disclosures to make. Dr. Marras has given several talks on NTM lung disease, one sponsored by AstraZeneca and the other by Novartis.
FROM CHEST
Fast growing hand lesion
A scoop shave biopsy at the lower edge of the lesion revealed that this was a well-differentiated squamous cell carcinoma.
Squamous cell carcinoma is the second most common cancer in the United States and the most common skin cancer in Black people.1 A patient’s age and their accumulated UV radiation from sun exposure or artificial tanning is a major contributing factor. Lesions may manifest as precancers characterized as rough pink or brown papules with a sandpaper-like texture on sun-exposed skin. These lesions may clear spontaneously or develop into invasive disease, as occurred in this case.
Surgical treatment is often curative. Fusiform excision and Mohs micrographic surgery are 2 common options. More advanced squamous cell carcinomas that are large or found to have poorly differentiated cells or large perineural invasion carry a risk of metastasis.
In elderly patients, optimal treatment isn’t always straightforward.1 Nonsurgical options include radiation and intralesional chemotherapy. These nonsurgical choices may seem less aggressive, but total inconvenience, wound care, and discomfort can be equal to or worse than a single session of curative surgery.
This patient’s lesion was excised with a 5-mm margin. The patient tolerated an in-office procedure lasting about 45 minutes but would have struggled with a longer session with Mohs microsurgery. The postoperative period required limiting full use of his left hand for about 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178. 2. Renzi M Jr, Schimmel J, Decker A, et al. Management of skin cancer in the elderly. Dermatol Clin. 2019;37:279-286. doi: 10.1016/j.det.2019.02.003

A scoop shave biopsy at the lower edge of the lesion revealed that this was a well-differentiated squamous cell carcinoma.
Squamous cell carcinoma is the second most common cancer in the United States and the most common skin cancer in Black people.1 A patient’s age and their accumulated UV radiation from sun exposure or artificial tanning is a major contributing factor. Lesions may manifest as precancers characterized as rough pink or brown papules with a sandpaper-like texture on sun-exposed skin. These lesions may clear spontaneously or develop into invasive disease, as occurred in this case.
Surgical treatment is often curative. Fusiform excision and Mohs micrographic surgery are 2 common options. More advanced squamous cell carcinomas that are large or found to have poorly differentiated cells or large perineural invasion carry a risk of metastasis.
In elderly patients, optimal treatment isn’t always straightforward.1 Nonsurgical options include radiation and intralesional chemotherapy. These nonsurgical choices may seem less aggressive, but total inconvenience, wound care, and discomfort can be equal to or worse than a single session of curative surgery.
This patient’s lesion was excised with a 5-mm margin. The patient tolerated an in-office procedure lasting about 45 minutes but would have struggled with a longer session with Mohs microsurgery. The postoperative period required limiting full use of his left hand for about 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A scoop shave biopsy at the lower edge of the lesion revealed that this was a well-differentiated squamous cell carcinoma.
Squamous cell carcinoma is the second most common cancer in the United States and the most common skin cancer in Black people.1 A patient’s age and their accumulated UV radiation from sun exposure or artificial tanning is a major contributing factor. Lesions may manifest as precancers characterized as rough pink or brown papules with a sandpaper-like texture on sun-exposed skin. These lesions may clear spontaneously or develop into invasive disease, as occurred in this case.
Surgical treatment is often curative. Fusiform excision and Mohs micrographic surgery are 2 common options. More advanced squamous cell carcinomas that are large or found to have poorly differentiated cells or large perineural invasion carry a risk of metastasis.
In elderly patients, optimal treatment isn’t always straightforward.1 Nonsurgical options include radiation and intralesional chemotherapy. These nonsurgical choices may seem less aggressive, but total inconvenience, wound care, and discomfort can be equal to or worse than a single session of curative surgery.
This patient’s lesion was excised with a 5-mm margin. The patient tolerated an in-office procedure lasting about 45 minutes but would have struggled with a longer session with Mohs microsurgery. The postoperative period required limiting full use of his left hand for about 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178. 2. Renzi M Jr, Schimmel J, Decker A, et al. Management of skin cancer in the elderly. Dermatol Clin. 2019;37:279-286. doi: 10.1016/j.det.2019.02.003
1. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178. 2. Renzi M Jr, Schimmel J, Decker A, et al. Management of skin cancer in the elderly. Dermatol Clin. 2019;37:279-286. doi: 10.1016/j.det.2019.02.003