User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Heart failure drug a new treatment option for alcoholism?
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.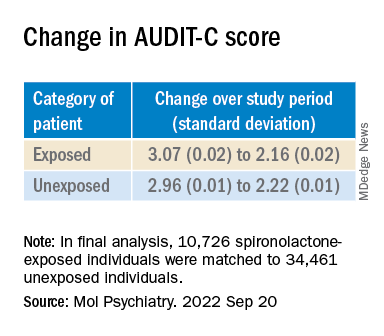
“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
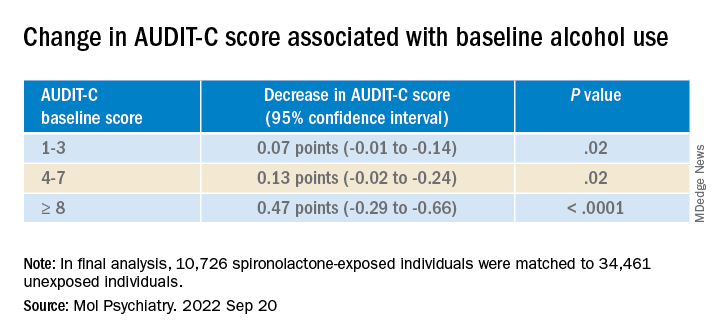
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.

“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.

“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY
Coffee linked to reduced cardiovascular disease and mortality risk
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
Tender Nonhealing Lesion on the Leg
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
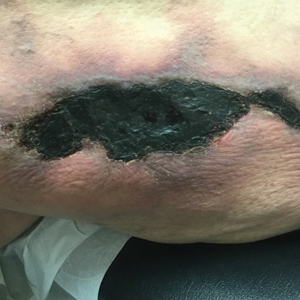
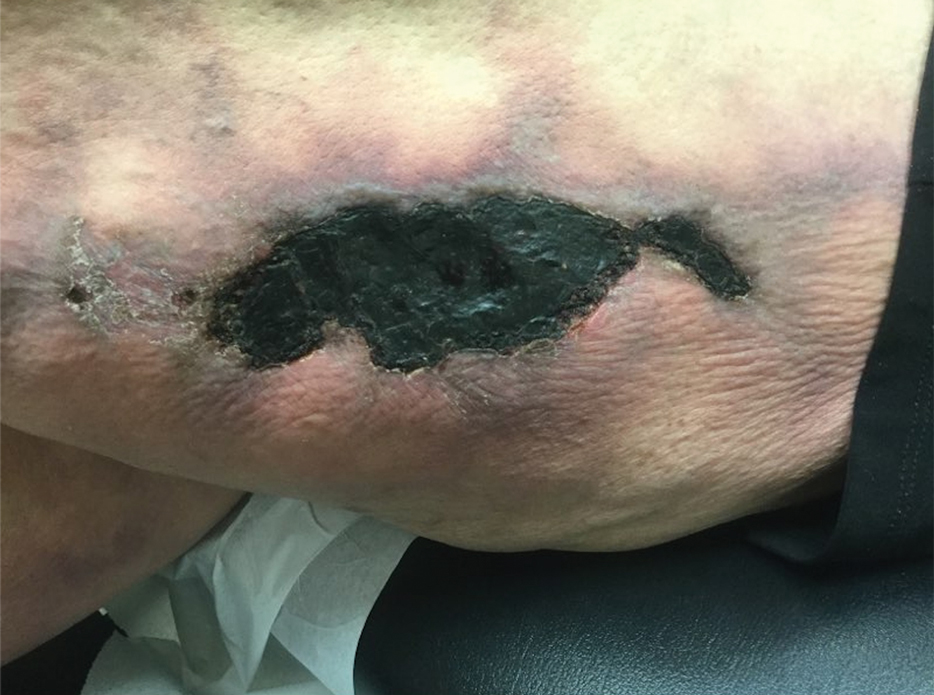
A 50-year-old woman presented to our dermatology clinic with an exquisitely tender, nonhealing lesion on the left leg of 2 weeks’ duration that began as a small red-purplish spot. She applied a triple antibiotic ointment and wrapped the area with gauze daily but reported that it continued to enlarge and darken in color before forming a “scab.” She noted occasional seropurulent discharge and denied any trauma or new exposures to the area. She was seen at a local emergency department 3 days prior to presentation and was prescribed oral clindamycin for suspected cellulitis, but she denied any improvement with the initiation of antibiotics. Her medical history was notable for obesity, depression, hypothyroidism, and liver disease secondary to alcohol use disorder. She reported that she drank a pint of vodka daily. Her medications included pantoprazole, spironolactone, bumetanide, citalopram, levothyroxine, naltrexone, tramadol, and a multivitamin. Physical examination revealed violaceous mottling with areas of superficial erythema and ulceration with necrotic eschars on the proximal left thigh that were extremely painful. A biopsy was obtained for confirmation of diagnosis, but the patient died before the results were returned.
What we know about long COVID so far
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Children and COVID: September slowdown continues
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.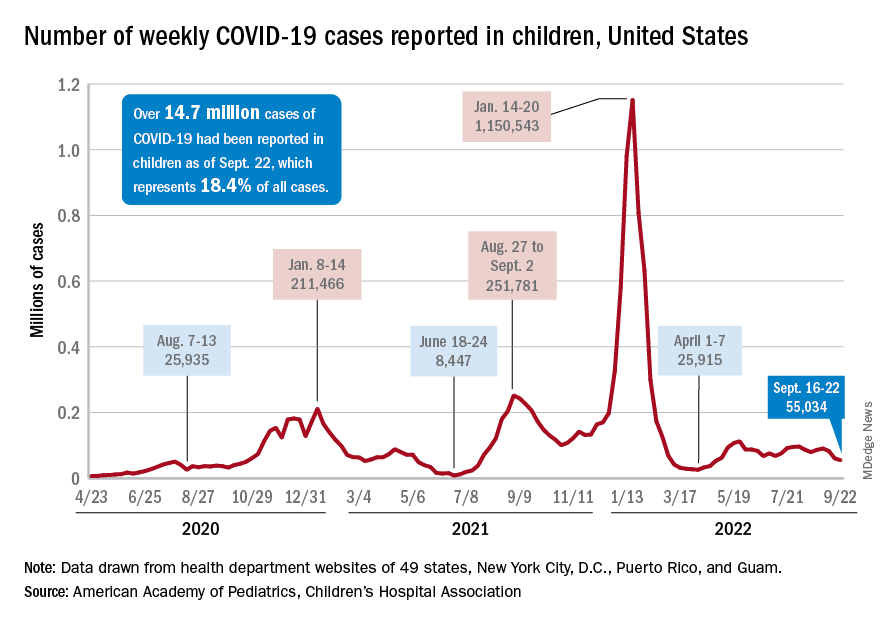
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
Many factors linked with higher, lower risk for hand eczema
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
FROM CONTACT DERMATITIS
Early emollient use reduces dermatitis in at-risk infants
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALLERGY
Type 1 diabetes cases poised to double worldwide by 2040
STOCKHOLM – The number of people living with type 1 diabetes worldwide is expected to double by 2040, with most new cases among adults living in low- and middle-income countries, new modeling data suggest.
The forecast, developed from available data collected in the newly established open-source Type 1 Diabetes Index, provides estimates for type 1 diabetes prevalence, incidence, associated mortality, and life expectancy for 201 countries for 2021.
The model also projects estimates for prevalent cases in 2040. It is the first type 1 diabetes dataset to account for the lack of prevalence because of premature mortality, particularly in low- and middle-income countries.
“The worldwide prevalence of type 1 diabetes is substantial and growing. Improved surveillance – particularly in adults who make up most of the population living with type 1 diabetes – is essential to enable improvements to care and outcomes. There is an opportunity to save millions of lives in the coming decades by raising the standard of care (including ensuring universal access to insulin and other essential supplies) and increasing awareness of the signs and symptoms of type 1 diabetes to enable a 100% rate of diagnosis in all countries,” the authors write.
“This work spells out the need for early diagnosis of type 1 diabetes and timely access to quality care,” said Chantal Mathieu, MD, at the European Association for the Study of Diabetes annual meeting.
One in five deaths from type 1 diabetes in under 25s
The new findings were published in Lancet Diabetes & Endocrinology by Gabriel A. Gregory, MD, of Life for a Child Program, New South Wales, Australia, and colleagues. The T1D Index Project database was published Sept. 21, 2022.
According to the model, about 8.4 million people were living with type 1 diabetes in 2021, with one-fifth from low- and middle-income countries. An additional 3.7 million died prematurely and would have been added to that count had they lived. One in five of all deaths caused by type 1 diabetes in 2021 is estimated to have occurred in people younger than age 25 years because of nondiagnosis.
“It is unacceptable that, in 2022, some 35,000 people worldwide are dying undiagnosed within a year of onset of symptoms. There also continues to be a huge disparity in life expectancy for people with type 1 diabetes, hitting those in the poorest countries hardest,” noted Dr. Mathieu, who is senior vice-president of EASD and an endocrinologist based at KU Leuven, Belgium.
By 2040, the model predicts that between 13.5 million and 17.4 million people will be living with the condition, with the largest relative increase from 2021 in low-income and lower-middle-income countries. The majority of incident and prevalent cases of type 1 diabetes are in adults, with an estimated 62% of 510,000 new diagnoses worldwide in 2021 occurring in people aged 20 years and older.
Type 1 diabetes is not predominantly a disease of childhood
Dr. Mathieu also noted that the data dispute the long-held view of type 1 diabetes as a predominantly pediatric condition. Indeed, worldwide, the median age for a person living with type 1 diabetes is 37 years.
“While type 1 diabetes is often referred to as ‘child-onset’ diabetes, this important study shows that only around one in five living with the condition are aged 20 years or younger, two-thirds are aged 20-64 years, and a further one in five are aged 65 years or older.”
“This condition does not stop at age 18 years – the children become adults, and the adults become elderly. All countries must examine and strengthen their diagnosis and care pathways for people of all ages living with type 1 diabetes,” Dr. Mathieu emphasized.
And in an accompanying editorial, Serena Jingchuan Guo, MD, PhD, and Hui Shao, MD, PhD, point out that most studies that estimate diabetes burden have focused on type 2 diabetes, noting, “type 1 diabetes faces the challenges of misdiagnosis, underdiagnosis, high risk of complications, and premature mortality.”
The insulin affordability issue is central, point out Dr. Guo and Dr. Shao of the Center for Drug Evaluation and Safety, department of pharmaceutical evaluation and policy, University of Florida College of Pharmacy, Gainesville.
“Countries need to strengthen the price regulation and reimbursement policy for insulin while building subsidy programs to ensure insulin access and to cope with the growing demand for insulin. Meanwhile, optimizing the insulin supply chain between manufacturers and patients while seeking alternative treatment options (for example, biosimilar products) will also improve the current situation,” they conclude.
The study was funded by JDRF, of which four coauthors are employees. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The number of people living with type 1 diabetes worldwide is expected to double by 2040, with most new cases among adults living in low- and middle-income countries, new modeling data suggest.
The forecast, developed from available data collected in the newly established open-source Type 1 Diabetes Index, provides estimates for type 1 diabetes prevalence, incidence, associated mortality, and life expectancy for 201 countries for 2021.
The model also projects estimates for prevalent cases in 2040. It is the first type 1 diabetes dataset to account for the lack of prevalence because of premature mortality, particularly in low- and middle-income countries.
“The worldwide prevalence of type 1 diabetes is substantial and growing. Improved surveillance – particularly in adults who make up most of the population living with type 1 diabetes – is essential to enable improvements to care and outcomes. There is an opportunity to save millions of lives in the coming decades by raising the standard of care (including ensuring universal access to insulin and other essential supplies) and increasing awareness of the signs and symptoms of type 1 diabetes to enable a 100% rate of diagnosis in all countries,” the authors write.
“This work spells out the need for early diagnosis of type 1 diabetes and timely access to quality care,” said Chantal Mathieu, MD, at the European Association for the Study of Diabetes annual meeting.
One in five deaths from type 1 diabetes in under 25s
The new findings were published in Lancet Diabetes & Endocrinology by Gabriel A. Gregory, MD, of Life for a Child Program, New South Wales, Australia, and colleagues. The T1D Index Project database was published Sept. 21, 2022.
According to the model, about 8.4 million people were living with type 1 diabetes in 2021, with one-fifth from low- and middle-income countries. An additional 3.7 million died prematurely and would have been added to that count had they lived. One in five of all deaths caused by type 1 diabetes in 2021 is estimated to have occurred in people younger than age 25 years because of nondiagnosis.
“It is unacceptable that, in 2022, some 35,000 people worldwide are dying undiagnosed within a year of onset of symptoms. There also continues to be a huge disparity in life expectancy for people with type 1 diabetes, hitting those in the poorest countries hardest,” noted Dr. Mathieu, who is senior vice-president of EASD and an endocrinologist based at KU Leuven, Belgium.
By 2040, the model predicts that between 13.5 million and 17.4 million people will be living with the condition, with the largest relative increase from 2021 in low-income and lower-middle-income countries. The majority of incident and prevalent cases of type 1 diabetes are in adults, with an estimated 62% of 510,000 new diagnoses worldwide in 2021 occurring in people aged 20 years and older.
Type 1 diabetes is not predominantly a disease of childhood
Dr. Mathieu also noted that the data dispute the long-held view of type 1 diabetes as a predominantly pediatric condition. Indeed, worldwide, the median age for a person living with type 1 diabetes is 37 years.
“While type 1 diabetes is often referred to as ‘child-onset’ diabetes, this important study shows that only around one in five living with the condition are aged 20 years or younger, two-thirds are aged 20-64 years, and a further one in five are aged 65 years or older.”
“This condition does not stop at age 18 years – the children become adults, and the adults become elderly. All countries must examine and strengthen their diagnosis and care pathways for people of all ages living with type 1 diabetes,” Dr. Mathieu emphasized.
And in an accompanying editorial, Serena Jingchuan Guo, MD, PhD, and Hui Shao, MD, PhD, point out that most studies that estimate diabetes burden have focused on type 2 diabetes, noting, “type 1 diabetes faces the challenges of misdiagnosis, underdiagnosis, high risk of complications, and premature mortality.”
The insulin affordability issue is central, point out Dr. Guo and Dr. Shao of the Center for Drug Evaluation and Safety, department of pharmaceutical evaluation and policy, University of Florida College of Pharmacy, Gainesville.
“Countries need to strengthen the price regulation and reimbursement policy for insulin while building subsidy programs to ensure insulin access and to cope with the growing demand for insulin. Meanwhile, optimizing the insulin supply chain between manufacturers and patients while seeking alternative treatment options (for example, biosimilar products) will also improve the current situation,” they conclude.
The study was funded by JDRF, of which four coauthors are employees. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The number of people living with type 1 diabetes worldwide is expected to double by 2040, with most new cases among adults living in low- and middle-income countries, new modeling data suggest.
The forecast, developed from available data collected in the newly established open-source Type 1 Diabetes Index, provides estimates for type 1 diabetes prevalence, incidence, associated mortality, and life expectancy for 201 countries for 2021.
The model also projects estimates for prevalent cases in 2040. It is the first type 1 diabetes dataset to account for the lack of prevalence because of premature mortality, particularly in low- and middle-income countries.
“The worldwide prevalence of type 1 diabetes is substantial and growing. Improved surveillance – particularly in adults who make up most of the population living with type 1 diabetes – is essential to enable improvements to care and outcomes. There is an opportunity to save millions of lives in the coming decades by raising the standard of care (including ensuring universal access to insulin and other essential supplies) and increasing awareness of the signs and symptoms of type 1 diabetes to enable a 100% rate of diagnosis in all countries,” the authors write.
“This work spells out the need for early diagnosis of type 1 diabetes and timely access to quality care,” said Chantal Mathieu, MD, at the European Association for the Study of Diabetes annual meeting.
One in five deaths from type 1 diabetes in under 25s
The new findings were published in Lancet Diabetes & Endocrinology by Gabriel A. Gregory, MD, of Life for a Child Program, New South Wales, Australia, and colleagues. The T1D Index Project database was published Sept. 21, 2022.
According to the model, about 8.4 million people were living with type 1 diabetes in 2021, with one-fifth from low- and middle-income countries. An additional 3.7 million died prematurely and would have been added to that count had they lived. One in five of all deaths caused by type 1 diabetes in 2021 is estimated to have occurred in people younger than age 25 years because of nondiagnosis.
“It is unacceptable that, in 2022, some 35,000 people worldwide are dying undiagnosed within a year of onset of symptoms. There also continues to be a huge disparity in life expectancy for people with type 1 diabetes, hitting those in the poorest countries hardest,” noted Dr. Mathieu, who is senior vice-president of EASD and an endocrinologist based at KU Leuven, Belgium.
By 2040, the model predicts that between 13.5 million and 17.4 million people will be living with the condition, with the largest relative increase from 2021 in low-income and lower-middle-income countries. The majority of incident and prevalent cases of type 1 diabetes are in adults, with an estimated 62% of 510,000 new diagnoses worldwide in 2021 occurring in people aged 20 years and older.
Type 1 diabetes is not predominantly a disease of childhood
Dr. Mathieu also noted that the data dispute the long-held view of type 1 diabetes as a predominantly pediatric condition. Indeed, worldwide, the median age for a person living with type 1 diabetes is 37 years.
“While type 1 diabetes is often referred to as ‘child-onset’ diabetes, this important study shows that only around one in five living with the condition are aged 20 years or younger, two-thirds are aged 20-64 years, and a further one in five are aged 65 years or older.”
“This condition does not stop at age 18 years – the children become adults, and the adults become elderly. All countries must examine and strengthen their diagnosis and care pathways for people of all ages living with type 1 diabetes,” Dr. Mathieu emphasized.
And in an accompanying editorial, Serena Jingchuan Guo, MD, PhD, and Hui Shao, MD, PhD, point out that most studies that estimate diabetes burden have focused on type 2 diabetes, noting, “type 1 diabetes faces the challenges of misdiagnosis, underdiagnosis, high risk of complications, and premature mortality.”
The insulin affordability issue is central, point out Dr. Guo and Dr. Shao of the Center for Drug Evaluation and Safety, department of pharmaceutical evaluation and policy, University of Florida College of Pharmacy, Gainesville.
“Countries need to strengthen the price regulation and reimbursement policy for insulin while building subsidy programs to ensure insulin access and to cope with the growing demand for insulin. Meanwhile, optimizing the insulin supply chain between manufacturers and patients while seeking alternative treatment options (for example, biosimilar products) will also improve the current situation,” they conclude.
The study was funded by JDRF, of which four coauthors are employees. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Is exercise effective for constipation?
I recently presented a clinical scenario about a patient of mine named Brenda. This 35-year-old woman came to me with symptoms that had been going on for a year already. I asked for readers’ comments about my management of Brenda.
I appreciate the comments I received regarding this case. The most common suggestion was to encourage Brenda to exercise, and a systematic review of randomized clinical trials published in 2019 supports this recommendation. This review included nine studies with a total of 680 participants, and the overall effect of exercise was a twofold improvement in symptoms associated with constipation. Walking was the most common exercise intervention, and along with qigong (which combines body position, breathing, and meditation), these two modes of exercise were effective in improving constipation. However, the one study evaluating resistance training failed to demonstrate a significant effect. Importantly, the reviewers considered the collective research to be at a high risk of bias.
Exercise will probably help Brenda, although some brainstorming might be necessary to help her fit exercise into her busy schedule. Another suggestion focused on her risk for colorectal cancer, and Dr. Cooke and Dr. Boboc both astutely noted that colorectal cancer is increasingly common among adults at early middle age. This stands in contrast to a steady decline in the prevalence of colorectal cancer among U.S. adults at age 65 years or older. Whereas colorectal cancer declined by 3.3% annually among U.S. older adults from 2011 to 2016, there was a reversal of this favorable trend among individuals between 50 and 64 years of age, with rates increasing by 1% annually.
The increase in the incidence of colorectal cancer among adults 50-64 years of age has been outpaced by the increase among adults younger than 50 years, who have experienced a 2.2% increase in the incidence of colorectal cancer annually between 2012 and 2016. Previously, the increase in colorectal cancer among early middle-aged adults was driven by higher rates of rectal cancer, but more recently this trend has included higher rates of proximal and distal colon tumors. In 2020, 12% of new cases of colorectal cancer were expected to be among individuals younger than 50 years.
So how do we act on this context in the case of Brenda? Her history suggests no overt warning signs for cancer. The history did not address a family history of gastrointestinal symptoms or colorectal cancer, which is an important omission.
Although the number of cases of cancer among persons younger than 50 years may be rising, the overall prevalence of colorectal cancer among younger adults is well under 1%. At 35 years of age, it is not necessary to evaluate Brenda for colorectal cancer. However, persistent or worsening symptoms could prompt a referral for colonoscopy at a later time.
Finally, let’s address how to practically manage Brenda’s case, because many options are available. I would begin with recommendations regarding her lifestyle, including regular exercise, adequate sleep, and whatever she can achieve in the FODMAP diet. I would also recommend psyllium as a soluble fiber and expect that these changes would help her constipation. But they might be less effective for abdominal cramping, so I would also recommend peppermint oil at this time.
If Brenda commits to these recommendations, she will very likely improve. If she does not, I will be more concerned regarding anxiety and depression complicating her illness. Treating those disorders can make a big difference.
In addition, if there is an inadequate response to initial therapy, I will initiate linaclotide or lubiprostone. Plecanatide is another reasonable option. At this point, I will also consider referral to a gastroenterologist for a recalcitrant case and will certainly refer if one of these specific treatments fails in Brenda. Conditions such as pelvic floor dysfunction can mimic irritable bowel syndrome with constipation and merit consideration.
However, I really believe that Brenda will feel better. Thanks for all of the insightful and interesting comments. It is easy to see how we are all invested in improving patients’ lives.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported disclosures with McNeil Pharmaceuticals. A version of this article first appeared on Medscape.com.
I recently presented a clinical scenario about a patient of mine named Brenda. This 35-year-old woman came to me with symptoms that had been going on for a year already. I asked for readers’ comments about my management of Brenda.
I appreciate the comments I received regarding this case. The most common suggestion was to encourage Brenda to exercise, and a systematic review of randomized clinical trials published in 2019 supports this recommendation. This review included nine studies with a total of 680 participants, and the overall effect of exercise was a twofold improvement in symptoms associated with constipation. Walking was the most common exercise intervention, and along with qigong (which combines body position, breathing, and meditation), these two modes of exercise were effective in improving constipation. However, the one study evaluating resistance training failed to demonstrate a significant effect. Importantly, the reviewers considered the collective research to be at a high risk of bias.
Exercise will probably help Brenda, although some brainstorming might be necessary to help her fit exercise into her busy schedule. Another suggestion focused on her risk for colorectal cancer, and Dr. Cooke and Dr. Boboc both astutely noted that colorectal cancer is increasingly common among adults at early middle age. This stands in contrast to a steady decline in the prevalence of colorectal cancer among U.S. adults at age 65 years or older. Whereas colorectal cancer declined by 3.3% annually among U.S. older adults from 2011 to 2016, there was a reversal of this favorable trend among individuals between 50 and 64 years of age, with rates increasing by 1% annually.
The increase in the incidence of colorectal cancer among adults 50-64 years of age has been outpaced by the increase among adults younger than 50 years, who have experienced a 2.2% increase in the incidence of colorectal cancer annually between 2012 and 2016. Previously, the increase in colorectal cancer among early middle-aged adults was driven by higher rates of rectal cancer, but more recently this trend has included higher rates of proximal and distal colon tumors. In 2020, 12% of new cases of colorectal cancer were expected to be among individuals younger than 50 years.
So how do we act on this context in the case of Brenda? Her history suggests no overt warning signs for cancer. The history did not address a family history of gastrointestinal symptoms or colorectal cancer, which is an important omission.
Although the number of cases of cancer among persons younger than 50 years may be rising, the overall prevalence of colorectal cancer among younger adults is well under 1%. At 35 years of age, it is not necessary to evaluate Brenda for colorectal cancer. However, persistent or worsening symptoms could prompt a referral for colonoscopy at a later time.
Finally, let’s address how to practically manage Brenda’s case, because many options are available. I would begin with recommendations regarding her lifestyle, including regular exercise, adequate sleep, and whatever she can achieve in the FODMAP diet. I would also recommend psyllium as a soluble fiber and expect that these changes would help her constipation. But they might be less effective for abdominal cramping, so I would also recommend peppermint oil at this time.
If Brenda commits to these recommendations, she will very likely improve. If she does not, I will be more concerned regarding anxiety and depression complicating her illness. Treating those disorders can make a big difference.
In addition, if there is an inadequate response to initial therapy, I will initiate linaclotide or lubiprostone. Plecanatide is another reasonable option. At this point, I will also consider referral to a gastroenterologist for a recalcitrant case and will certainly refer if one of these specific treatments fails in Brenda. Conditions such as pelvic floor dysfunction can mimic irritable bowel syndrome with constipation and merit consideration.
However, I really believe that Brenda will feel better. Thanks for all of the insightful and interesting comments. It is easy to see how we are all invested in improving patients’ lives.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported disclosures with McNeil Pharmaceuticals. A version of this article first appeared on Medscape.com.
I recently presented a clinical scenario about a patient of mine named Brenda. This 35-year-old woman came to me with symptoms that had been going on for a year already. I asked for readers’ comments about my management of Brenda.
I appreciate the comments I received regarding this case. The most common suggestion was to encourage Brenda to exercise, and a systematic review of randomized clinical trials published in 2019 supports this recommendation. This review included nine studies with a total of 680 participants, and the overall effect of exercise was a twofold improvement in symptoms associated with constipation. Walking was the most common exercise intervention, and along with qigong (which combines body position, breathing, and meditation), these two modes of exercise were effective in improving constipation. However, the one study evaluating resistance training failed to demonstrate a significant effect. Importantly, the reviewers considered the collective research to be at a high risk of bias.
Exercise will probably help Brenda, although some brainstorming might be necessary to help her fit exercise into her busy schedule. Another suggestion focused on her risk for colorectal cancer, and Dr. Cooke and Dr. Boboc both astutely noted that colorectal cancer is increasingly common among adults at early middle age. This stands in contrast to a steady decline in the prevalence of colorectal cancer among U.S. adults at age 65 years or older. Whereas colorectal cancer declined by 3.3% annually among U.S. older adults from 2011 to 2016, there was a reversal of this favorable trend among individuals between 50 and 64 years of age, with rates increasing by 1% annually.
The increase in the incidence of colorectal cancer among adults 50-64 years of age has been outpaced by the increase among adults younger than 50 years, who have experienced a 2.2% increase in the incidence of colorectal cancer annually between 2012 and 2016. Previously, the increase in colorectal cancer among early middle-aged adults was driven by higher rates of rectal cancer, but more recently this trend has included higher rates of proximal and distal colon tumors. In 2020, 12% of new cases of colorectal cancer were expected to be among individuals younger than 50 years.
So how do we act on this context in the case of Brenda? Her history suggests no overt warning signs for cancer. The history did not address a family history of gastrointestinal symptoms or colorectal cancer, which is an important omission.
Although the number of cases of cancer among persons younger than 50 years may be rising, the overall prevalence of colorectal cancer among younger adults is well under 1%. At 35 years of age, it is not necessary to evaluate Brenda for colorectal cancer. However, persistent or worsening symptoms could prompt a referral for colonoscopy at a later time.
Finally, let’s address how to practically manage Brenda’s case, because many options are available. I would begin with recommendations regarding her lifestyle, including regular exercise, adequate sleep, and whatever she can achieve in the FODMAP diet. I would also recommend psyllium as a soluble fiber and expect that these changes would help her constipation. But they might be less effective for abdominal cramping, so I would also recommend peppermint oil at this time.
If Brenda commits to these recommendations, she will very likely improve. If she does not, I will be more concerned regarding anxiety and depression complicating her illness. Treating those disorders can make a big difference.
In addition, if there is an inadequate response to initial therapy, I will initiate linaclotide or lubiprostone. Plecanatide is another reasonable option. At this point, I will also consider referral to a gastroenterologist for a recalcitrant case and will certainly refer if one of these specific treatments fails in Brenda. Conditions such as pelvic floor dysfunction can mimic irritable bowel syndrome with constipation and merit consideration.
However, I really believe that Brenda will feel better. Thanks for all of the insightful and interesting comments. It is easy to see how we are all invested in improving patients’ lives.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported disclosures with McNeil Pharmaceuticals. A version of this article first appeared on Medscape.com.
Emphasis on weight loss in new type 2 diabetes guidance
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
AT EASD 2022