User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
COVID attacks DNA in heart, unlike flu, study says
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
FROM IMMUNOLOGY
Strong link found between enterovirus and type 1 diabetes
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Cutaneous Eruption in an Immunocompromised Patient
The Diagnosis: Secondary Syphilis
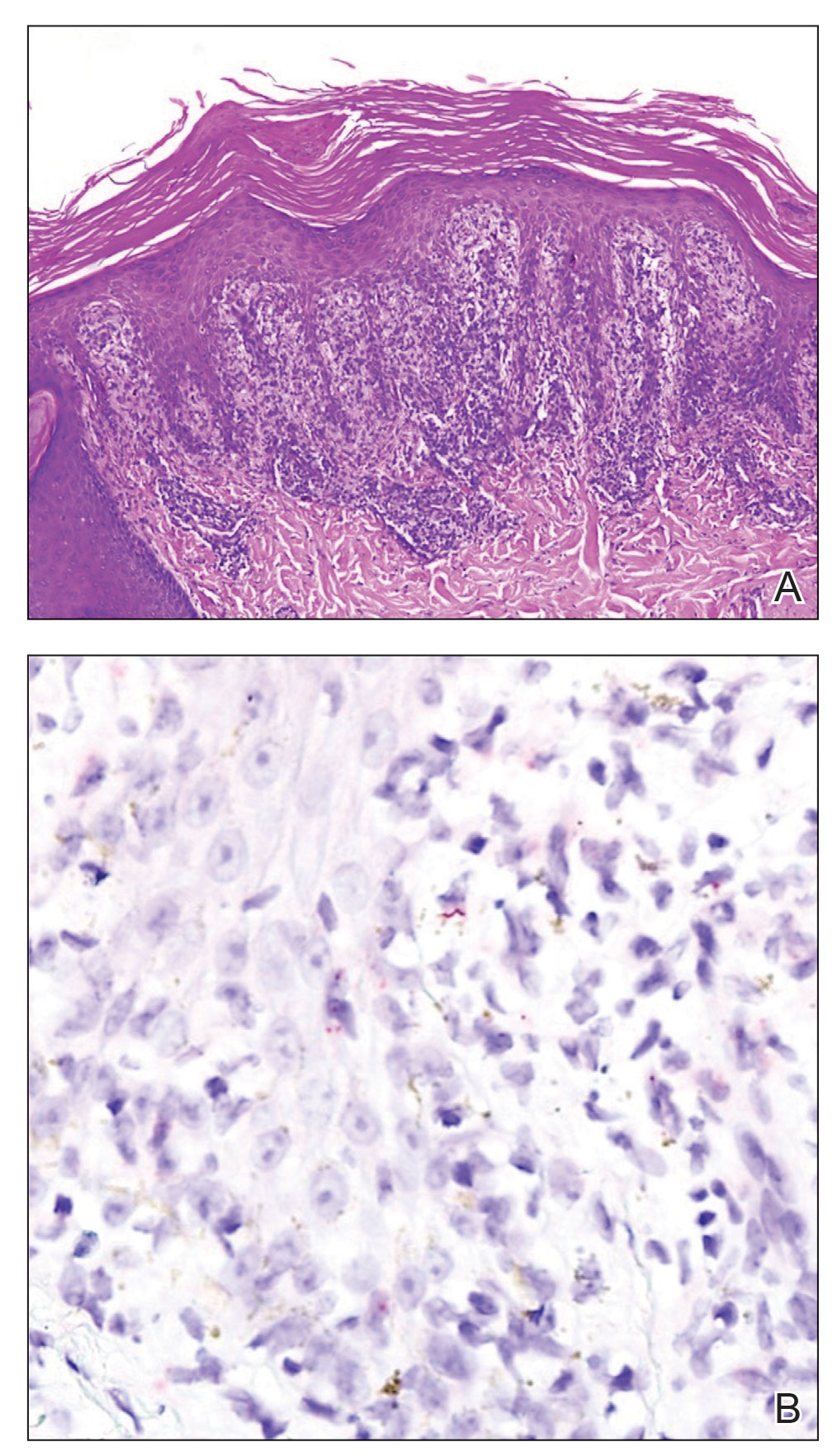
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.
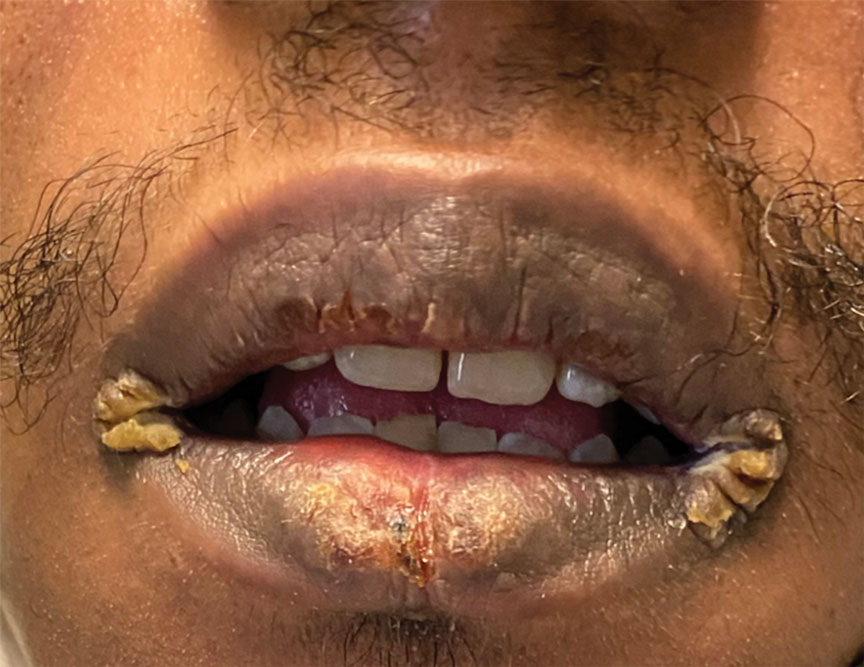
Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

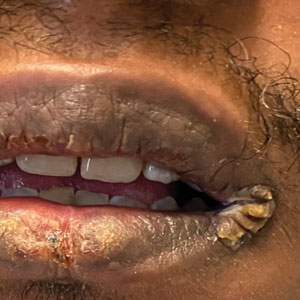
A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.

Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.

Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
A 29-year-old Black man with long-standing untreated HIV presented with mildly pruritic, scaly plaques on the palms and soles of 2 weeks’ duration. His medical history was notable for primary syphilis treated approximately 1 year prior. A review of symptoms was positive for blurry vision and floaters but negative for constitutional symptoms. Physical examination revealed well-defined scaly plaques over the palms, soles, and elbows with subungual hyperkeratosis. Patches of nonscarring alopecia over the scalp and split papules at the oral commissures also were noted. There were no palpable lymph nodes or genital involvement. Eye examination showed conjunctival injection and 20 cells per field in the vitreous humor. Laboratory evaluation revealed an HIV viral load of 31,623 copies/mL and a CD4 count of 47 cells/μL (reference range, 362–1531 cells/μL). A shave biopsy of the left elbow was performed for histopathologic evaluation.
BREEZE-AD-PEDS: First data for baricitinib in childhood eczema reported
The oral Janus kinase
After 16 weeks of treatment, the primary endpoint – an Investigators Global Assessment (IGA) score of 0 or 1 with at least a 2-point improvement from baseline – was met by 41.7% of patients given 2 mg (those younger than age 10) or 4 mg of baricitinib (those aged 10-17 years), the highest dose studied in each of those two age groups.
By comparison, the primary endpoint was met in 16.4% of children in the placebo group (P < .001).
Baricitinib is approved for the treatment of AD in adults in many countries, Antonio Torrelo, MD, of the Hospital Infantil Niño Jesús, Madrid, said at the annual congress of the European Academy of Dermatology and Venereology. It was approved by the U.S. Food and Drug Administration for treating adults with severe alopecia areata in June and is under FDA review for the treatment of AD.
The phase 3 BREEZE-AD-PEDS trial
BREEZE-AD-PEDS was a randomized, double-blind trial that evaluated the safety and efficacy of baricitinib in 483 children and adolescents with moderate to severe AD. Participants were aged 2-17 years. Those aged 2-5 years had been diagnosed with AD for at least 6 months; if they were older, they had been diagnosed for at least 12 months.
Three dosing levels of baricitinib were tested: 121 patients were given a low dose, which was 0.5 mg/day in children aged 2 to less than 10 years and 1 mg/day in those aged 10 to less than 18 years. A medium dose – 1 mg/day in the younger children and 2 mg/day in the older children – was given to 120 children, while a high dose – 2 mg/day and 4 mg/day, respectively – was given to another 120 children.
Topical treatments were permitted, although for entry into the trial, participants had to have had an inadequate response to steroids and an inadequate or no response to topical calcineurin inhibitors. In all groups, age, gender, race, geographic region, age at diagnosis of AD, and duration of AD “were more or less similar,” Dr. Torello said.
Good results, but only with highest dose
The primary IGA endpoint was reached by 25.8% of children in the medium-dose group and by 18.2% in the low-dose group. Neither result was statistically significant in comparison with placebo (16.4%).
When breaking down the results between different ages, “the results in the IGA scores are consistent in both age subgroups – below 10 years and over 10 years,” Dr. Torello noted. The results are also consistent across body weights (< 20 kg, 20-60 kg, and > 60 kg), he added.
Among those treated with the high dose of baricitinib, Eczema Area and Severity Index (EASI) 75% and 95% improvement scores were reached in 52.5% and 30% of patients, respectively. Corresponding figures for the medium dose were 40% and 21.7%; for the low baricitinib dose, 32.2% and 11.6%; and for placebo, 32% and 12.3%. Again, only the results for the highest baricitinib dose were significant in comparison with placebo.
A similar pattern was seen for improvement in itch, and there was a 75% improvement in Scoring Atopic Dermatitis (SCORAD75) results.
Safety of baricitinib in children
The labeling for JAK inhibitors that have been approved to date, including baricitinib, include a boxed warning regarding risks for thrombosis, major adverse cardiovascular events, and all-cause mortality. The warning is based on use by patients with rheumatoid arthritis.
Dr. Torello summarized baricitinib’s safety profile in the trial as being “consistent with the well-known safety profile for baricitinib in adults with moderate to severe atopic dermatitis.”
In the study, no severe adverse effects were noted, and no new safety signals were observed, he said. The rate of any treatment-emergent effect among patients was around 50% and was similar across all baricitinib and placebo groups. Study discontinuations because of a side effect were more frequent in the placebo arm (1.6% of patients) than in the baricitinib low-, medium-, and high-dose arms (0.8%, 0%, and 0.8%, respectively).
There were no cases of deep-vein thrombosis, pulmonary embolism, or other adverse effects of special interest, including major adverse cardiovascular events, gastrointestinal perforations, and opportunistic infections, Dr. Torrelo said.
No patient experienced elevations in liver enzyme levels, although there were some cases of elevated creatinine phosphokinase levels (16% in the placebo group and 19% in the baricitinib arms altogether) that were not from muscle injury. There was a possible increase in low-density cholesterol level (3.3% of those taking placebo vs. 10.1% of baricitinib-treated patients).
Is there a role for baricitinib?
“Baricitinib is a potential therapeutic option with a favorable benefit-to-risk profile for children between 2 and 18 years who have moderate to severe atopic dermatitis, and candidates for systemic therapy,” Dr. Torrelo said. “No single drug is capable to treat every patient with atopic dermatitis,” he added in discussing the possible place of baricitinib in pediatric practice.
“There are patients who do not respond to dupilumab, who apparently respond later to JAK inhibitors,” he noted.
“We are trying to work phenotypically, trying to learn what kind of patients – especially children who have a more heterogeneous disease than adults – can be better treated with JAK inhibitors or dupilumab.” There may be other important considerations in choosing a treatment in children, Dr. Torrelo said, including that JAK inhibitors can be given orally, while dupilumab is administered by injection.
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation in Irvine, Calif., pointed out that “only the higher dose is significantly more effective than placebo.”
In his view, “the potentially severe adverse events are not worth the risk compared to more effective agents, such as dupilumab, in this pediatric population,” added Dr. Wu, who recently authored a review of the role of JAK inhibitors in skin disease. He was not involved with the baricitinib study.
The study was funded by Eli Lilly in collaboration with Incyte. Dr. Torello has participated in advisory boards and/or has served as a principal investigator in clinical trials for AbbVie, Eli Lilly and Company, Novartis, Pfizer, Pierre Fabre, and Sanofi. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
The oral Janus kinase
After 16 weeks of treatment, the primary endpoint – an Investigators Global Assessment (IGA) score of 0 or 1 with at least a 2-point improvement from baseline – was met by 41.7% of patients given 2 mg (those younger than age 10) or 4 mg of baricitinib (those aged 10-17 years), the highest dose studied in each of those two age groups.
By comparison, the primary endpoint was met in 16.4% of children in the placebo group (P < .001).
Baricitinib is approved for the treatment of AD in adults in many countries, Antonio Torrelo, MD, of the Hospital Infantil Niño Jesús, Madrid, said at the annual congress of the European Academy of Dermatology and Venereology. It was approved by the U.S. Food and Drug Administration for treating adults with severe alopecia areata in June and is under FDA review for the treatment of AD.
The phase 3 BREEZE-AD-PEDS trial
BREEZE-AD-PEDS was a randomized, double-blind trial that evaluated the safety and efficacy of baricitinib in 483 children and adolescents with moderate to severe AD. Participants were aged 2-17 years. Those aged 2-5 years had been diagnosed with AD for at least 6 months; if they were older, they had been diagnosed for at least 12 months.
Three dosing levels of baricitinib were tested: 121 patients were given a low dose, which was 0.5 mg/day in children aged 2 to less than 10 years and 1 mg/day in those aged 10 to less than 18 years. A medium dose – 1 mg/day in the younger children and 2 mg/day in the older children – was given to 120 children, while a high dose – 2 mg/day and 4 mg/day, respectively – was given to another 120 children.
Topical treatments were permitted, although for entry into the trial, participants had to have had an inadequate response to steroids and an inadequate or no response to topical calcineurin inhibitors. In all groups, age, gender, race, geographic region, age at diagnosis of AD, and duration of AD “were more or less similar,” Dr. Torello said.
Good results, but only with highest dose
The primary IGA endpoint was reached by 25.8% of children in the medium-dose group and by 18.2% in the low-dose group. Neither result was statistically significant in comparison with placebo (16.4%).
When breaking down the results between different ages, “the results in the IGA scores are consistent in both age subgroups – below 10 years and over 10 years,” Dr. Torello noted. The results are also consistent across body weights (< 20 kg, 20-60 kg, and > 60 kg), he added.
Among those treated with the high dose of baricitinib, Eczema Area and Severity Index (EASI) 75% and 95% improvement scores were reached in 52.5% and 30% of patients, respectively. Corresponding figures for the medium dose were 40% and 21.7%; for the low baricitinib dose, 32.2% and 11.6%; and for placebo, 32% and 12.3%. Again, only the results for the highest baricitinib dose were significant in comparison with placebo.
A similar pattern was seen for improvement in itch, and there was a 75% improvement in Scoring Atopic Dermatitis (SCORAD75) results.
Safety of baricitinib in children
The labeling for JAK inhibitors that have been approved to date, including baricitinib, include a boxed warning regarding risks for thrombosis, major adverse cardiovascular events, and all-cause mortality. The warning is based on use by patients with rheumatoid arthritis.
Dr. Torello summarized baricitinib’s safety profile in the trial as being “consistent with the well-known safety profile for baricitinib in adults with moderate to severe atopic dermatitis.”
In the study, no severe adverse effects were noted, and no new safety signals were observed, he said. The rate of any treatment-emergent effect among patients was around 50% and was similar across all baricitinib and placebo groups. Study discontinuations because of a side effect were more frequent in the placebo arm (1.6% of patients) than in the baricitinib low-, medium-, and high-dose arms (0.8%, 0%, and 0.8%, respectively).
There were no cases of deep-vein thrombosis, pulmonary embolism, or other adverse effects of special interest, including major adverse cardiovascular events, gastrointestinal perforations, and opportunistic infections, Dr. Torrelo said.
No patient experienced elevations in liver enzyme levels, although there were some cases of elevated creatinine phosphokinase levels (16% in the placebo group and 19% in the baricitinib arms altogether) that were not from muscle injury. There was a possible increase in low-density cholesterol level (3.3% of those taking placebo vs. 10.1% of baricitinib-treated patients).
Is there a role for baricitinib?
“Baricitinib is a potential therapeutic option with a favorable benefit-to-risk profile for children between 2 and 18 years who have moderate to severe atopic dermatitis, and candidates for systemic therapy,” Dr. Torrelo said. “No single drug is capable to treat every patient with atopic dermatitis,” he added in discussing the possible place of baricitinib in pediatric practice.
“There are patients who do not respond to dupilumab, who apparently respond later to JAK inhibitors,” he noted.
“We are trying to work phenotypically, trying to learn what kind of patients – especially children who have a more heterogeneous disease than adults – can be better treated with JAK inhibitors or dupilumab.” There may be other important considerations in choosing a treatment in children, Dr. Torrelo said, including that JAK inhibitors can be given orally, while dupilumab is administered by injection.
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation in Irvine, Calif., pointed out that “only the higher dose is significantly more effective than placebo.”
In his view, “the potentially severe adverse events are not worth the risk compared to more effective agents, such as dupilumab, in this pediatric population,” added Dr. Wu, who recently authored a review of the role of JAK inhibitors in skin disease. He was not involved with the baricitinib study.
The study was funded by Eli Lilly in collaboration with Incyte. Dr. Torello has participated in advisory boards and/or has served as a principal investigator in clinical trials for AbbVie, Eli Lilly and Company, Novartis, Pfizer, Pierre Fabre, and Sanofi. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
The oral Janus kinase
After 16 weeks of treatment, the primary endpoint – an Investigators Global Assessment (IGA) score of 0 or 1 with at least a 2-point improvement from baseline – was met by 41.7% of patients given 2 mg (those younger than age 10) or 4 mg of baricitinib (those aged 10-17 years), the highest dose studied in each of those two age groups.
By comparison, the primary endpoint was met in 16.4% of children in the placebo group (P < .001).
Baricitinib is approved for the treatment of AD in adults in many countries, Antonio Torrelo, MD, of the Hospital Infantil Niño Jesús, Madrid, said at the annual congress of the European Academy of Dermatology and Venereology. It was approved by the U.S. Food and Drug Administration for treating adults with severe alopecia areata in June and is under FDA review for the treatment of AD.
The phase 3 BREEZE-AD-PEDS trial
BREEZE-AD-PEDS was a randomized, double-blind trial that evaluated the safety and efficacy of baricitinib in 483 children and adolescents with moderate to severe AD. Participants were aged 2-17 years. Those aged 2-5 years had been diagnosed with AD for at least 6 months; if they were older, they had been diagnosed for at least 12 months.
Three dosing levels of baricitinib were tested: 121 patients were given a low dose, which was 0.5 mg/day in children aged 2 to less than 10 years and 1 mg/day in those aged 10 to less than 18 years. A medium dose – 1 mg/day in the younger children and 2 mg/day in the older children – was given to 120 children, while a high dose – 2 mg/day and 4 mg/day, respectively – was given to another 120 children.
Topical treatments were permitted, although for entry into the trial, participants had to have had an inadequate response to steroids and an inadequate or no response to topical calcineurin inhibitors. In all groups, age, gender, race, geographic region, age at diagnosis of AD, and duration of AD “were more or less similar,” Dr. Torello said.
Good results, but only with highest dose
The primary IGA endpoint was reached by 25.8% of children in the medium-dose group and by 18.2% in the low-dose group. Neither result was statistically significant in comparison with placebo (16.4%).
When breaking down the results between different ages, “the results in the IGA scores are consistent in both age subgroups – below 10 years and over 10 years,” Dr. Torello noted. The results are also consistent across body weights (< 20 kg, 20-60 kg, and > 60 kg), he added.
Among those treated with the high dose of baricitinib, Eczema Area and Severity Index (EASI) 75% and 95% improvement scores were reached in 52.5% and 30% of patients, respectively. Corresponding figures for the medium dose were 40% and 21.7%; for the low baricitinib dose, 32.2% and 11.6%; and for placebo, 32% and 12.3%. Again, only the results for the highest baricitinib dose were significant in comparison with placebo.
A similar pattern was seen for improvement in itch, and there was a 75% improvement in Scoring Atopic Dermatitis (SCORAD75) results.
Safety of baricitinib in children
The labeling for JAK inhibitors that have been approved to date, including baricitinib, include a boxed warning regarding risks for thrombosis, major adverse cardiovascular events, and all-cause mortality. The warning is based on use by patients with rheumatoid arthritis.
Dr. Torello summarized baricitinib’s safety profile in the trial as being “consistent with the well-known safety profile for baricitinib in adults with moderate to severe atopic dermatitis.”
In the study, no severe adverse effects were noted, and no new safety signals were observed, he said. The rate of any treatment-emergent effect among patients was around 50% and was similar across all baricitinib and placebo groups. Study discontinuations because of a side effect were more frequent in the placebo arm (1.6% of patients) than in the baricitinib low-, medium-, and high-dose arms (0.8%, 0%, and 0.8%, respectively).
There were no cases of deep-vein thrombosis, pulmonary embolism, or other adverse effects of special interest, including major adverse cardiovascular events, gastrointestinal perforations, and opportunistic infections, Dr. Torrelo said.
No patient experienced elevations in liver enzyme levels, although there were some cases of elevated creatinine phosphokinase levels (16% in the placebo group and 19% in the baricitinib arms altogether) that were not from muscle injury. There was a possible increase in low-density cholesterol level (3.3% of those taking placebo vs. 10.1% of baricitinib-treated patients).
Is there a role for baricitinib?
“Baricitinib is a potential therapeutic option with a favorable benefit-to-risk profile for children between 2 and 18 years who have moderate to severe atopic dermatitis, and candidates for systemic therapy,” Dr. Torrelo said. “No single drug is capable to treat every patient with atopic dermatitis,” he added in discussing the possible place of baricitinib in pediatric practice.
“There are patients who do not respond to dupilumab, who apparently respond later to JAK inhibitors,” he noted.
“We are trying to work phenotypically, trying to learn what kind of patients – especially children who have a more heterogeneous disease than adults – can be better treated with JAK inhibitors or dupilumab.” There may be other important considerations in choosing a treatment in children, Dr. Torrelo said, including that JAK inhibitors can be given orally, while dupilumab is administered by injection.
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation in Irvine, Calif., pointed out that “only the higher dose is significantly more effective than placebo.”
In his view, “the potentially severe adverse events are not worth the risk compared to more effective agents, such as dupilumab, in this pediatric population,” added Dr. Wu, who recently authored a review of the role of JAK inhibitors in skin disease. He was not involved with the baricitinib study.
The study was funded by Eli Lilly in collaboration with Incyte. Dr. Torello has participated in advisory boards and/or has served as a principal investigator in clinical trials for AbbVie, Eli Lilly and Company, Novartis, Pfizer, Pierre Fabre, and Sanofi. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
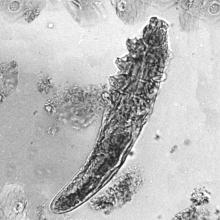
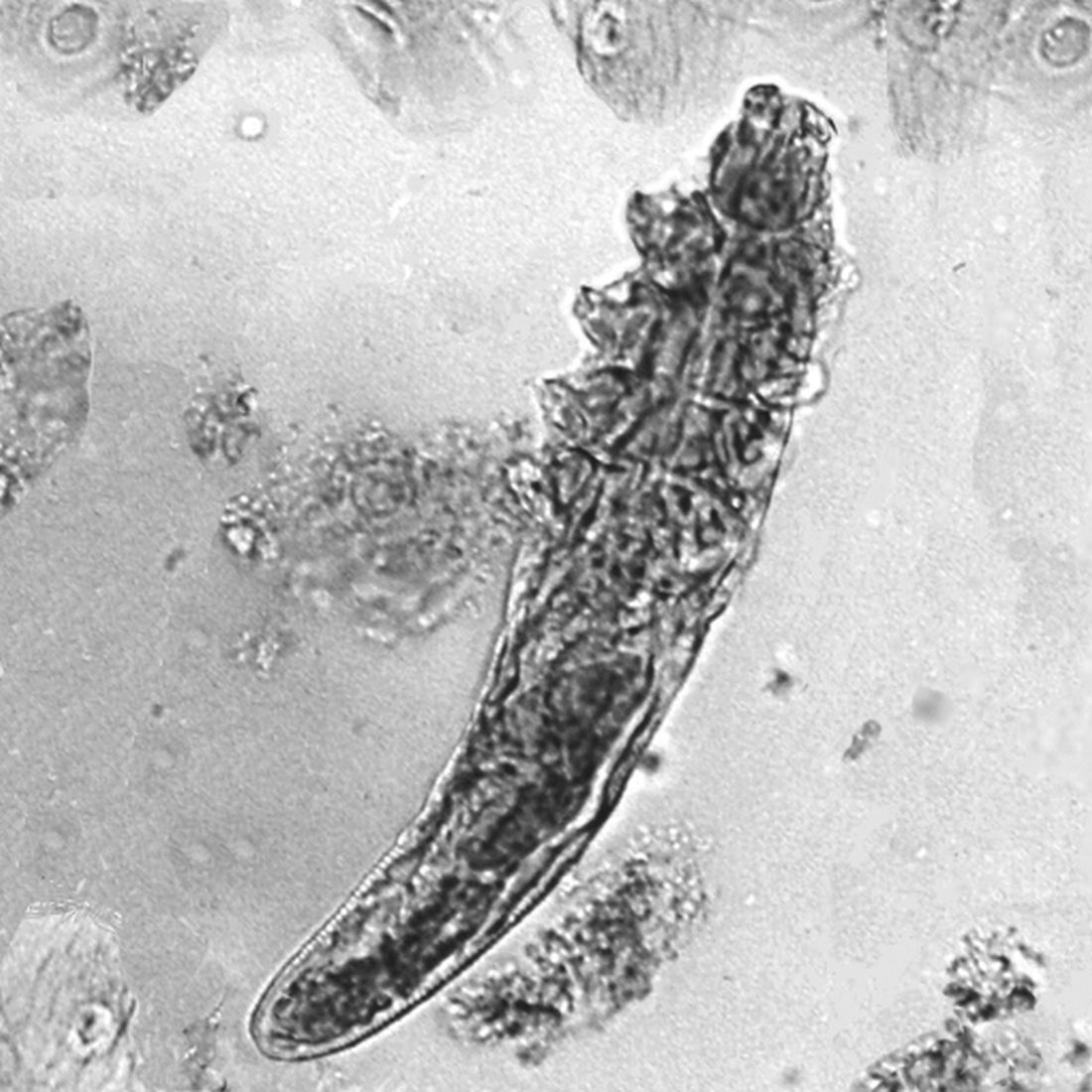
What’s the true role of Demodex mites in the development of papulopustular rosacea?
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
FROM JEADV
How to improve diagnosis of HFpEF, common in diabetes
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Risk-adapted screening strategy could reduce colonoscopy use
The Asia-Pacific Colorectal Screening (APCS) scoring system, combined with a stool DNA test, could improve the detection of advanced colorectal neoplasms and limit colonoscopy use, according to a new study published in Clinical Gastroenterology and Hepatology.
Although a colonoscopy can detect both colorectal cancer and precancerous lesions, using it as the primary screening tool can cause barriers due to high costs, limited resources, and low compliance, wrote Junfeng Xu of the gastroenterology department at the First Medical Center of Chinese PLA General Hospital, Beijing, and colleagues.
“Therefore, a more efficient risk-adapted screening approach for selection of colonoscopy is recommended,” the authors wrote. “This APCS-based algorithm for triaging subjects can significantly reduce the colonoscopy workload by half.”
Colorectal cancer is the third most common cancer worldwide, and in China, it was the third most diagnosed cancer and fourth most common cause of cancer-related death in 2016. In countries with limited health care resources, risk-adapted screening strategies could be more cost-effective and accessible than traditional screening strategies, the authors wrote.
Developed by the Asia-Pacific Working Group on CRC, the APCS score is based on age, sex, smoking status, and family history of colorectal cancer. Those with a score of 0-1 are considered low-risk, while scores of 2-3 are considered moderate-risk, and scores of 4-7 are considered high-risk.
In a cross-sectional, multicenter observational study, the investigators calculated APCS scores for 2,439 participants who visited eight outpatient clinics or cancer screening centers across four provinces in China between August 2017 and April 2019. Colonoscopy appointments were scheduled for all participants.
Participants provided test results for a stool DNA test (SDC2 and SFRP2 tests) and fecal immunochemical test (FIT), with colonoscopy outcomes used as the gold standard. The researchers used the manufacturer’s recommended hemoglobin threshold of 20 μg/g of dry stool for a positive result on FIT, in addition to a threshold of 4.4 μg/g to match the specificity of the stool DNA test.
Among all participants, 42 patients (1.9%) had colorectal cancer, 302 patients (13.5%) had advanced adenoma, and 551 patients (24.6%) had nonadvanced adenoma on colonoscopy.
Based on the APCS score, 946 participants (38.8%) were categorized as high risk, and they had a 1.8-fold increase in risk for advanced neoplasms (95% confidence interval, 1.4-2.3), as compared with the low- and moderate-risk groups.
Compared with direct colonoscopy, the combination of APCS score and stool DNA test detected 95.2% of invasive cancers (among 40 out of 42 patients) and 73.5% of advanced neoplasms (among 253 of 344 patients). The colonoscopy workload was 47.1% with this strategy. The risk-adapted screening approach required significantly fewer colonoscopies for detecting one advanced neoplasm than a direct colonoscopy screening, at 4.17 versus 6.51 (P < .001).
“Our findings provide critical references for designing effective population-based CRC screening strategies in the future, especially in resource-constrained countries and regions,” the authors concluded.
Avoiding complications
“Colonoscopy is expensive and time consuming for both the patient and the health care system. Colonoscopy is also not without risk since bowel perforation, post-procedural bleeding, and sedation-related complications all occur at low but measurable rates,” said Reid Ness, MD, associate professor of medicine and gastroenterologist at Vanderbilt University Medical Center, Nashville, Tenn.
“The primary strength of the study was that all patients eventually received colonoscopy, allowing for the best estimate of strategy sensitivity for advanced colorectal neoplasia,” he said.
At the same time, the cross-sectional study was unable to estimate the benefit of using this type of screening strategy over time, Dr. Ness said. With limited endoscopic resources available in many countries, however, clinicians need better modalities and strategies for noninvasive identification of advanced colorectal neoplasia, he added.
“Since less than 5% of the population will eventually develop colorectal cancer, the overwhelming majority can only be discomfited and possibly injured through colonoscopy screening,” he said. “For these reasons, the use of a CRC screening strategy that minimizes the use of colonoscopy without compromising the identification rate for advanced colorectal neoplasia is best for both the patient and the health care system.”
The study was supported by grants from the Beijing Municipal Science and Technology Commission. The authors declared no conflicts of interest. Dr. Ness reported no relevant disclosures.
This article was updated Oct. 4, 2022.
The Asia-Pacific Colorectal Screening (APCS) scoring system, combined with a stool DNA test, could improve the detection of advanced colorectal neoplasms and limit colonoscopy use, according to a new study published in Clinical Gastroenterology and Hepatology.
Although a colonoscopy can detect both colorectal cancer and precancerous lesions, using it as the primary screening tool can cause barriers due to high costs, limited resources, and low compliance, wrote Junfeng Xu of the gastroenterology department at the First Medical Center of Chinese PLA General Hospital, Beijing, and colleagues.
“Therefore, a more efficient risk-adapted screening approach for selection of colonoscopy is recommended,” the authors wrote. “This APCS-based algorithm for triaging subjects can significantly reduce the colonoscopy workload by half.”
Colorectal cancer is the third most common cancer worldwide, and in China, it was the third most diagnosed cancer and fourth most common cause of cancer-related death in 2016. In countries with limited health care resources, risk-adapted screening strategies could be more cost-effective and accessible than traditional screening strategies, the authors wrote.
Developed by the Asia-Pacific Working Group on CRC, the APCS score is based on age, sex, smoking status, and family history of colorectal cancer. Those with a score of 0-1 are considered low-risk, while scores of 2-3 are considered moderate-risk, and scores of 4-7 are considered high-risk.
In a cross-sectional, multicenter observational study, the investigators calculated APCS scores for 2,439 participants who visited eight outpatient clinics or cancer screening centers across four provinces in China between August 2017 and April 2019. Colonoscopy appointments were scheduled for all participants.
Participants provided test results for a stool DNA test (SDC2 and SFRP2 tests) and fecal immunochemical test (FIT), with colonoscopy outcomes used as the gold standard. The researchers used the manufacturer’s recommended hemoglobin threshold of 20 μg/g of dry stool for a positive result on FIT, in addition to a threshold of 4.4 μg/g to match the specificity of the stool DNA test.
Among all participants, 42 patients (1.9%) had colorectal cancer, 302 patients (13.5%) had advanced adenoma, and 551 patients (24.6%) had nonadvanced adenoma on colonoscopy.
Based on the APCS score, 946 participants (38.8%) were categorized as high risk, and they had a 1.8-fold increase in risk for advanced neoplasms (95% confidence interval, 1.4-2.3), as compared with the low- and moderate-risk groups.
Compared with direct colonoscopy, the combination of APCS score and stool DNA test detected 95.2% of invasive cancers (among 40 out of 42 patients) and 73.5% of advanced neoplasms (among 253 of 344 patients). The colonoscopy workload was 47.1% with this strategy. The risk-adapted screening approach required significantly fewer colonoscopies for detecting one advanced neoplasm than a direct colonoscopy screening, at 4.17 versus 6.51 (P < .001).
“Our findings provide critical references for designing effective population-based CRC screening strategies in the future, especially in resource-constrained countries and regions,” the authors concluded.
Avoiding complications
“Colonoscopy is expensive and time consuming for both the patient and the health care system. Colonoscopy is also not without risk since bowel perforation, post-procedural bleeding, and sedation-related complications all occur at low but measurable rates,” said Reid Ness, MD, associate professor of medicine and gastroenterologist at Vanderbilt University Medical Center, Nashville, Tenn.
“The primary strength of the study was that all patients eventually received colonoscopy, allowing for the best estimate of strategy sensitivity for advanced colorectal neoplasia,” he said.
At the same time, the cross-sectional study was unable to estimate the benefit of using this type of screening strategy over time, Dr. Ness said. With limited endoscopic resources available in many countries, however, clinicians need better modalities and strategies for noninvasive identification of advanced colorectal neoplasia, he added.
“Since less than 5% of the population will eventually develop colorectal cancer, the overwhelming majority can only be discomfited and possibly injured through colonoscopy screening,” he said. “For these reasons, the use of a CRC screening strategy that minimizes the use of colonoscopy without compromising the identification rate for advanced colorectal neoplasia is best for both the patient and the health care system.”
The study was supported by grants from the Beijing Municipal Science and Technology Commission. The authors declared no conflicts of interest. Dr. Ness reported no relevant disclosures.
This article was updated Oct. 4, 2022.
The Asia-Pacific Colorectal Screening (APCS) scoring system, combined with a stool DNA test, could improve the detection of advanced colorectal neoplasms and limit colonoscopy use, according to a new study published in Clinical Gastroenterology and Hepatology.
Although a colonoscopy can detect both colorectal cancer and precancerous lesions, using it as the primary screening tool can cause barriers due to high costs, limited resources, and low compliance, wrote Junfeng Xu of the gastroenterology department at the First Medical Center of Chinese PLA General Hospital, Beijing, and colleagues.
“Therefore, a more efficient risk-adapted screening approach for selection of colonoscopy is recommended,” the authors wrote. “This APCS-based algorithm for triaging subjects can significantly reduce the colonoscopy workload by half.”
Colorectal cancer is the third most common cancer worldwide, and in China, it was the third most diagnosed cancer and fourth most common cause of cancer-related death in 2016. In countries with limited health care resources, risk-adapted screening strategies could be more cost-effective and accessible than traditional screening strategies, the authors wrote.
Developed by the Asia-Pacific Working Group on CRC, the APCS score is based on age, sex, smoking status, and family history of colorectal cancer. Those with a score of 0-1 are considered low-risk, while scores of 2-3 are considered moderate-risk, and scores of 4-7 are considered high-risk.
In a cross-sectional, multicenter observational study, the investigators calculated APCS scores for 2,439 participants who visited eight outpatient clinics or cancer screening centers across four provinces in China between August 2017 and April 2019. Colonoscopy appointments were scheduled for all participants.
Participants provided test results for a stool DNA test (SDC2 and SFRP2 tests) and fecal immunochemical test (FIT), with colonoscopy outcomes used as the gold standard. The researchers used the manufacturer’s recommended hemoglobin threshold of 20 μg/g of dry stool for a positive result on FIT, in addition to a threshold of 4.4 μg/g to match the specificity of the stool DNA test.
Among all participants, 42 patients (1.9%) had colorectal cancer, 302 patients (13.5%) had advanced adenoma, and 551 patients (24.6%) had nonadvanced adenoma on colonoscopy.
Based on the APCS score, 946 participants (38.8%) were categorized as high risk, and they had a 1.8-fold increase in risk for advanced neoplasms (95% confidence interval, 1.4-2.3), as compared with the low- and moderate-risk groups.
Compared with direct colonoscopy, the combination of APCS score and stool DNA test detected 95.2% of invasive cancers (among 40 out of 42 patients) and 73.5% of advanced neoplasms (among 253 of 344 patients). The colonoscopy workload was 47.1% with this strategy. The risk-adapted screening approach required significantly fewer colonoscopies for detecting one advanced neoplasm than a direct colonoscopy screening, at 4.17 versus 6.51 (P < .001).
“Our findings provide critical references for designing effective population-based CRC screening strategies in the future, especially in resource-constrained countries and regions,” the authors concluded.
Avoiding complications
“Colonoscopy is expensive and time consuming for both the patient and the health care system. Colonoscopy is also not without risk since bowel perforation, post-procedural bleeding, and sedation-related complications all occur at low but measurable rates,” said Reid Ness, MD, associate professor of medicine and gastroenterologist at Vanderbilt University Medical Center, Nashville, Tenn.
“The primary strength of the study was that all patients eventually received colonoscopy, allowing for the best estimate of strategy sensitivity for advanced colorectal neoplasia,” he said.
At the same time, the cross-sectional study was unable to estimate the benefit of using this type of screening strategy over time, Dr. Ness said. With limited endoscopic resources available in many countries, however, clinicians need better modalities and strategies for noninvasive identification of advanced colorectal neoplasia, he added.
“Since less than 5% of the population will eventually develop colorectal cancer, the overwhelming majority can only be discomfited and possibly injured through colonoscopy screening,” he said. “For these reasons, the use of a CRC screening strategy that minimizes the use of colonoscopy without compromising the identification rate for advanced colorectal neoplasia is best for both the patient and the health care system.”
The study was supported by grants from the Beijing Municipal Science and Technology Commission. The authors declared no conflicts of interest. Dr. Ness reported no relevant disclosures.
This article was updated Oct. 4, 2022.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Monkeypox features include mucocutaneous involvement in almost all cases
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
AT THE EADV CONGRESS
Once-weekly insulin promising in phase 3 trial in type 2 diabetes
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Why can’t U.K. immunocompromised patients get Evusheld?
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.