User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
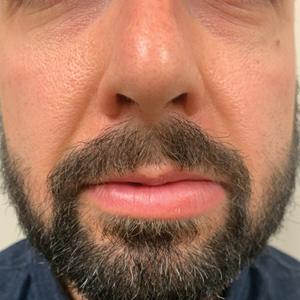
Persistent Lip Swelling
The Diagnosis: Granulomatous Cheilitis
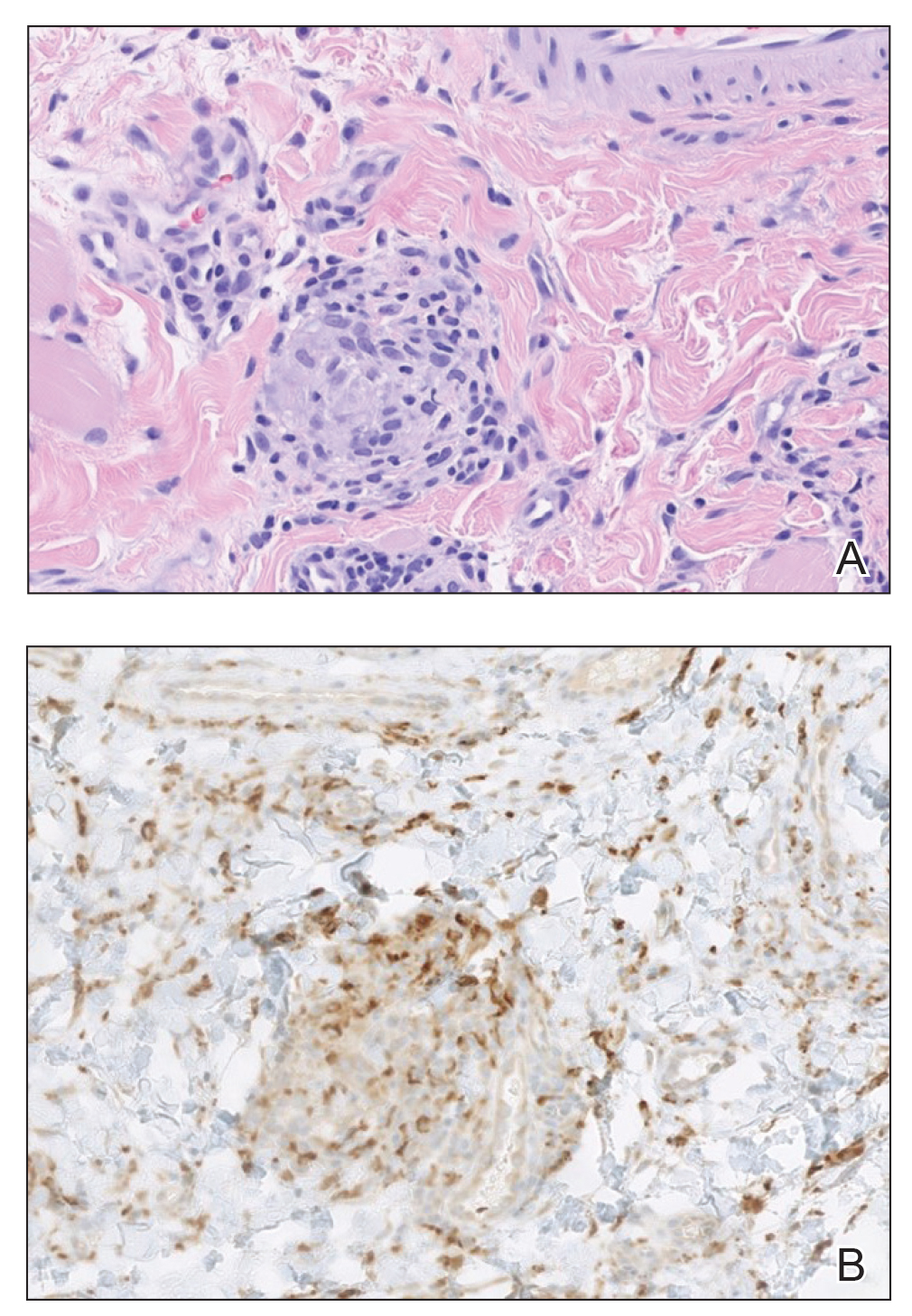
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.
Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
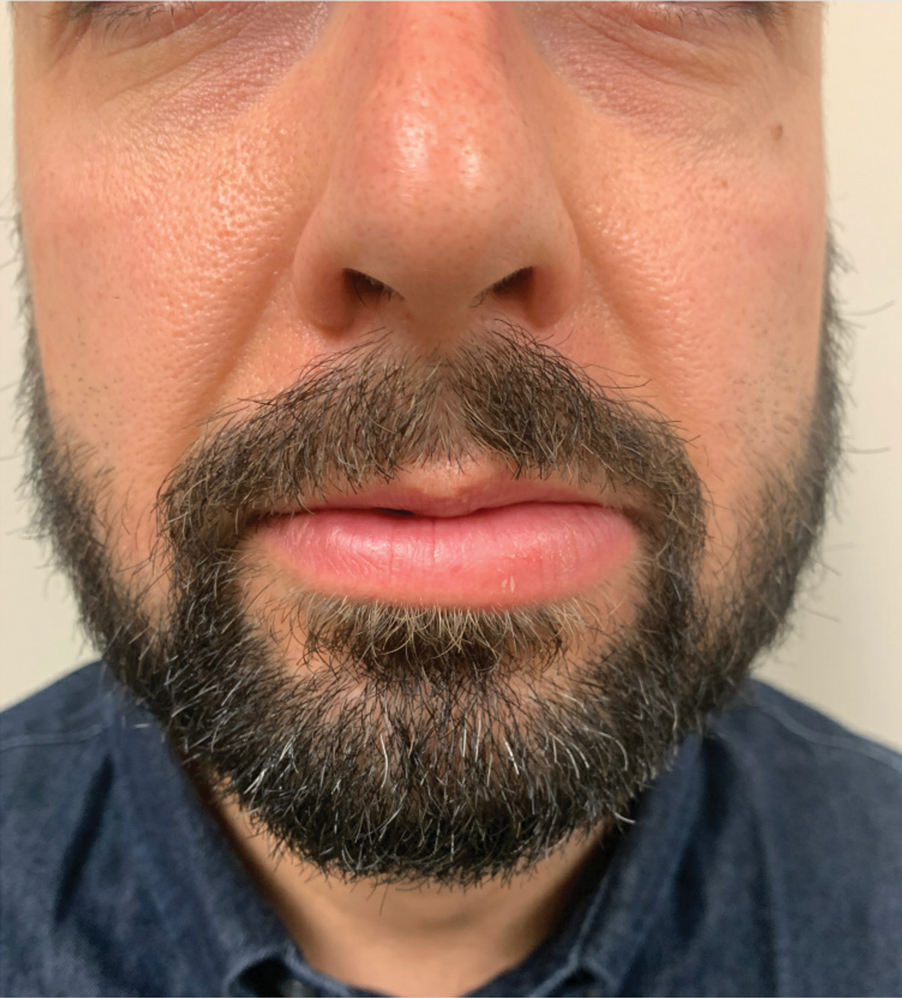
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.

Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.

Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
A 36-year-old man with allergic rhinitis presented with lower lip swelling of several months’ duration. The swelling was persistent and predominantly on the left side of the lower lip but occasionally spread to the entire lower lip. The episodes of increased swelling would last for several days and were not associated with any apparent triggers. He denied any pain, pruritus, or dryness. He noted more drooling from the affected side but denied any associated breathing difficulty or throat discomfort. Treatment with an oral antihistamine provided no relief. He denied any recent nonsteroidal anti-inflammatory drug or angiotensinconverting enzyme inhibitor use. His family history was notable for lupus in his maternal grandmother and maternal aunt. He denied any personal or family history of inflammatory bowel disease or recent gastrointestinal tract symptoms. Physical examination revealed nontender edema in the left side of the lower lip with no surface changes. No warmth or erythema were noted. The tongue and the rest of the oral cavity were unremarkable.
FDA approves topical tapinarof for plaque psoriasis
The the manufacturer announced.
Tapinarof is an aryl hydrocarbon receptor agonist and is the first FDA-approved steroid-free topical medication in this class, according to a press release from the manufacturer, Dermavant.
Approval was based on results of three studies in a phase 3 clinical trial program (PSOARING 1, PSOARING 2), and an open-label extension study, (PSOARING 3), the company release said. In PSOARING 1 and 2, approximately 1,000 adults aged 18-75 years (median age, 51 years) with plaque psoriasis were randomized to once-daily topical tapinarof or placebo for up to 12 weeks; 85% were White and 57% were men. The study findings were published in the New England Journal of Medicine in December 2021.
The primary endpoint for both trials was the proportion of patients who achieved Physician Global Assessment (PGA) scores score of “clear” (0) or “almost clear” (1) and improvement of at least two grades from baseline.
After 12 weeks, 36% of the patients in PSOARING 1 and 40% in PSOARING 2 who received tapinarof met the primary outcome, compared with 6% of patients on placebo (P < .001 for both studies). Of these, a total of 73 patients from both studies who achieved PGA scores of 0 were entered in PSOARING 3, a 40-week open-label extension study, in which they stopped tapinarof treatment and retained PGA scores of 0 or 1 for approximately 4 months off treatment. An additional 312 patients who were enrolled in the PSOARING 3 extension study achieved PGA scores of 0 at least once during the study period, with “remittive” effects lasting a mean of 130 days off of treatment.
In addition, patients who received tapinarof in the PSOARING 1 and 2 studies showed significant improvement from baseline, compared with patients on placebo, across a range of secondary endpoints including a 75% or greater improvement in Psoriasis Area and Severity Index score (PASI 75).
In PSOARING 1, and 2, respectively, 36.1% and 47.6% of those on tapinarof achieved a PASI 75 response at week 12, compared with 10.2% and 6.9% of those on the vehicle (P < .001 for both).
Across all three studies, the majority adverse events were mild to moderate, and limited to the application site.
The most common adverse events reported by patients in the tapinarof groups were folliculitis, nasopharyngitis, and contact dermatitis. Headaches were more common among those treated with tapinarof than those on vehicle in the studies (3.8% vs. 2.4% in PSOARING 1, and 3.8% vs. 0.6% in PSOARING 2), leading to only three treatment discontinuations.
At the end of the PSOARING 3 study (at either week 40 or early termination), 599 participants responded to satisfaction questionnaires. Of these, 83.6% said they were satisfied with the results of tapinarof treatment, and 81.7% said it was more effective than previous topical treatments they had used, according to the company’s release.
Tapinarof cream can be used on all areas of the body, including the face, skin folds, neck, genitalia, anal crux, inflammatory areas, and axillae, according to the company release.
Full prescribing information is available here.
The the manufacturer announced.
Tapinarof is an aryl hydrocarbon receptor agonist and is the first FDA-approved steroid-free topical medication in this class, according to a press release from the manufacturer, Dermavant.
Approval was based on results of three studies in a phase 3 clinical trial program (PSOARING 1, PSOARING 2), and an open-label extension study, (PSOARING 3), the company release said. In PSOARING 1 and 2, approximately 1,000 adults aged 18-75 years (median age, 51 years) with plaque psoriasis were randomized to once-daily topical tapinarof or placebo for up to 12 weeks; 85% were White and 57% were men. The study findings were published in the New England Journal of Medicine in December 2021.
The primary endpoint for both trials was the proportion of patients who achieved Physician Global Assessment (PGA) scores score of “clear” (0) or “almost clear” (1) and improvement of at least two grades from baseline.
After 12 weeks, 36% of the patients in PSOARING 1 and 40% in PSOARING 2 who received tapinarof met the primary outcome, compared with 6% of patients on placebo (P < .001 for both studies). Of these, a total of 73 patients from both studies who achieved PGA scores of 0 were entered in PSOARING 3, a 40-week open-label extension study, in which they stopped tapinarof treatment and retained PGA scores of 0 or 1 for approximately 4 months off treatment. An additional 312 patients who were enrolled in the PSOARING 3 extension study achieved PGA scores of 0 at least once during the study period, with “remittive” effects lasting a mean of 130 days off of treatment.
In addition, patients who received tapinarof in the PSOARING 1 and 2 studies showed significant improvement from baseline, compared with patients on placebo, across a range of secondary endpoints including a 75% or greater improvement in Psoriasis Area and Severity Index score (PASI 75).
In PSOARING 1, and 2, respectively, 36.1% and 47.6% of those on tapinarof achieved a PASI 75 response at week 12, compared with 10.2% and 6.9% of those on the vehicle (P < .001 for both).
Across all three studies, the majority adverse events were mild to moderate, and limited to the application site.
The most common adverse events reported by patients in the tapinarof groups were folliculitis, nasopharyngitis, and contact dermatitis. Headaches were more common among those treated with tapinarof than those on vehicle in the studies (3.8% vs. 2.4% in PSOARING 1, and 3.8% vs. 0.6% in PSOARING 2), leading to only three treatment discontinuations.
At the end of the PSOARING 3 study (at either week 40 or early termination), 599 participants responded to satisfaction questionnaires. Of these, 83.6% said they were satisfied with the results of tapinarof treatment, and 81.7% said it was more effective than previous topical treatments they had used, according to the company’s release.
Tapinarof cream can be used on all areas of the body, including the face, skin folds, neck, genitalia, anal crux, inflammatory areas, and axillae, according to the company release.
Full prescribing information is available here.
The the manufacturer announced.
Tapinarof is an aryl hydrocarbon receptor agonist and is the first FDA-approved steroid-free topical medication in this class, according to a press release from the manufacturer, Dermavant.
Approval was based on results of three studies in a phase 3 clinical trial program (PSOARING 1, PSOARING 2), and an open-label extension study, (PSOARING 3), the company release said. In PSOARING 1 and 2, approximately 1,000 adults aged 18-75 years (median age, 51 years) with plaque psoriasis were randomized to once-daily topical tapinarof or placebo for up to 12 weeks; 85% were White and 57% were men. The study findings were published in the New England Journal of Medicine in December 2021.
The primary endpoint for both trials was the proportion of patients who achieved Physician Global Assessment (PGA) scores score of “clear” (0) or “almost clear” (1) and improvement of at least two grades from baseline.
After 12 weeks, 36% of the patients in PSOARING 1 and 40% in PSOARING 2 who received tapinarof met the primary outcome, compared with 6% of patients on placebo (P < .001 for both studies). Of these, a total of 73 patients from both studies who achieved PGA scores of 0 were entered in PSOARING 3, a 40-week open-label extension study, in which they stopped tapinarof treatment and retained PGA scores of 0 or 1 for approximately 4 months off treatment. An additional 312 patients who were enrolled in the PSOARING 3 extension study achieved PGA scores of 0 at least once during the study period, with “remittive” effects lasting a mean of 130 days off of treatment.
In addition, patients who received tapinarof in the PSOARING 1 and 2 studies showed significant improvement from baseline, compared with patients on placebo, across a range of secondary endpoints including a 75% or greater improvement in Psoriasis Area and Severity Index score (PASI 75).
In PSOARING 1, and 2, respectively, 36.1% and 47.6% of those on tapinarof achieved a PASI 75 response at week 12, compared with 10.2% and 6.9% of those on the vehicle (P < .001 for both).
Across all three studies, the majority adverse events were mild to moderate, and limited to the application site.
The most common adverse events reported by patients in the tapinarof groups were folliculitis, nasopharyngitis, and contact dermatitis. Headaches were more common among those treated with tapinarof than those on vehicle in the studies (3.8% vs. 2.4% in PSOARING 1, and 3.8% vs. 0.6% in PSOARING 2), leading to only three treatment discontinuations.
At the end of the PSOARING 3 study (at either week 40 or early termination), 599 participants responded to satisfaction questionnaires. Of these, 83.6% said they were satisfied with the results of tapinarof treatment, and 81.7% said it was more effective than previous topical treatments they had used, according to the company’s release.
Tapinarof cream can be used on all areas of the body, including the face, skin folds, neck, genitalia, anal crux, inflammatory areas, and axillae, according to the company release.
Full prescribing information is available here.
Videos may not increase vaccinations in IBD
SAN DIEGO – Video and text messaging may not increase the proportion of people with inflammatory bowel disease (IBD) who get influenza vaccinations.
Although patients who received the messages expressed greater intention to get the vaccinations in a trial of the two methods, they didn’t follow through and get the shots, said Keren Appel, MD, a pediatric gastroenterologist at Children’s Hospital of Orange County in Orange, Calif.
“We found there was no difference in the uptake of the influenza vaccine between the two groups,” she said in an interview. Dr. Appel, who participated in the research while at Cedars-Sinai Medical Center in Los Angeles, presented the finding at the annual Digestive Diseases Week® (DDW) 2022.
People with IBD run an increased risk of complications such as infection, bone fractures, and cancer, said Dr. Appel. Previous research has suggested many people with IBD lack understanding or awareness or are skeptical of immunizations.
A previous trial with text-based email reminders did not result in more immunizations, according to Dr. Appel, so she and her colleagues decided to try promoting health prevention with videos. With feedback from patients, they created a series of animations encouraging patients to get influenza, pneumococcal, and zoster vaccinations and screening for bone health and skin cancer.
They randomly assigned 511 to receive videos and 545 patients to receive texts as a control group. After 6 months, 345 patients remained in the text group and 322 remained in the video group. The two groups had similar demographics, health status, and preventive health behaviors. They were mostly educated White women whose IBD was in remission.
The percentage of those who got flu vaccines increased from 59% (for the 2018-2019 season) to 63% (for the 2019-2020 flu season) in the group that watched the videos. However this change did not quite reach statistical significance (P = .07). The change in the text group, from 55% to 57%, was also not significant (P = .23).
The subjects did express more intention to get flu vaccines. The percentage with this intention increased from 59 to 75 in the video group, and from 55 to 72 in the text group. Both changes were statistically significant (P < .001).
Intentions to receive pneumonia and shingles vaccines, and bone and skin cancer screening, were not statistically different between the groups.
The researchers looked at age, immunosuppression, gender, and education to see if these factors could predict who was most likely to get the flu vaccine, but the only significant predictor was having received a previous flu shot.
Dr. Appel speculated that the videos might have been more effective in a more racially diverse, less educated population, or one where fewer people had previously received vaccinations.
“While we didn’t see a difference in this study, I think it opens up a lot of other questions that we can explore and answer,” she said. “It’s possible that patients may not have a one size fits all on their response. Some may respond better to video. Some may respond to text. Some may need more frequent reminders. Some might need to hear it from their doctor directly.”
Session comoderator Alyse Bedell, PhD, an assistant professor of psychiatry and behavioral neuroscience at the University of Chicago, agreed that a different patient population might have responded differently. “A population that may have lower access to educational resources, or has less educational attainment, or may have fewer people in their communities that are already receiving vaccines – those I think are going to be the populations where we’re going to be more likely to see the effects of an intervention like this,” she said in an interview.
Neither Dr. Appel nor Dr. Bedell reported any relevant financial interests. The study was funded by Pfizer.
SAN DIEGO – Video and text messaging may not increase the proportion of people with inflammatory bowel disease (IBD) who get influenza vaccinations.
Although patients who received the messages expressed greater intention to get the vaccinations in a trial of the two methods, they didn’t follow through and get the shots, said Keren Appel, MD, a pediatric gastroenterologist at Children’s Hospital of Orange County in Orange, Calif.
“We found there was no difference in the uptake of the influenza vaccine between the two groups,” she said in an interview. Dr. Appel, who participated in the research while at Cedars-Sinai Medical Center in Los Angeles, presented the finding at the annual Digestive Diseases Week® (DDW) 2022.
People with IBD run an increased risk of complications such as infection, bone fractures, and cancer, said Dr. Appel. Previous research has suggested many people with IBD lack understanding or awareness or are skeptical of immunizations.
A previous trial with text-based email reminders did not result in more immunizations, according to Dr. Appel, so she and her colleagues decided to try promoting health prevention with videos. With feedback from patients, they created a series of animations encouraging patients to get influenza, pneumococcal, and zoster vaccinations and screening for bone health and skin cancer.
They randomly assigned 511 to receive videos and 545 patients to receive texts as a control group. After 6 months, 345 patients remained in the text group and 322 remained in the video group. The two groups had similar demographics, health status, and preventive health behaviors. They were mostly educated White women whose IBD was in remission.
The percentage of those who got flu vaccines increased from 59% (for the 2018-2019 season) to 63% (for the 2019-2020 flu season) in the group that watched the videos. However this change did not quite reach statistical significance (P = .07). The change in the text group, from 55% to 57%, was also not significant (P = .23).
The subjects did express more intention to get flu vaccines. The percentage with this intention increased from 59 to 75 in the video group, and from 55 to 72 in the text group. Both changes were statistically significant (P < .001).
Intentions to receive pneumonia and shingles vaccines, and bone and skin cancer screening, were not statistically different between the groups.
The researchers looked at age, immunosuppression, gender, and education to see if these factors could predict who was most likely to get the flu vaccine, but the only significant predictor was having received a previous flu shot.
Dr. Appel speculated that the videos might have been more effective in a more racially diverse, less educated population, or one where fewer people had previously received vaccinations.
“While we didn’t see a difference in this study, I think it opens up a lot of other questions that we can explore and answer,” she said. “It’s possible that patients may not have a one size fits all on their response. Some may respond better to video. Some may respond to text. Some may need more frequent reminders. Some might need to hear it from their doctor directly.”
Session comoderator Alyse Bedell, PhD, an assistant professor of psychiatry and behavioral neuroscience at the University of Chicago, agreed that a different patient population might have responded differently. “A population that may have lower access to educational resources, or has less educational attainment, or may have fewer people in their communities that are already receiving vaccines – those I think are going to be the populations where we’re going to be more likely to see the effects of an intervention like this,” she said in an interview.
Neither Dr. Appel nor Dr. Bedell reported any relevant financial interests. The study was funded by Pfizer.
SAN DIEGO – Video and text messaging may not increase the proportion of people with inflammatory bowel disease (IBD) who get influenza vaccinations.
Although patients who received the messages expressed greater intention to get the vaccinations in a trial of the two methods, they didn’t follow through and get the shots, said Keren Appel, MD, a pediatric gastroenterologist at Children’s Hospital of Orange County in Orange, Calif.
“We found there was no difference in the uptake of the influenza vaccine between the two groups,” she said in an interview. Dr. Appel, who participated in the research while at Cedars-Sinai Medical Center in Los Angeles, presented the finding at the annual Digestive Diseases Week® (DDW) 2022.
People with IBD run an increased risk of complications such as infection, bone fractures, and cancer, said Dr. Appel. Previous research has suggested many people with IBD lack understanding or awareness or are skeptical of immunizations.
A previous trial with text-based email reminders did not result in more immunizations, according to Dr. Appel, so she and her colleagues decided to try promoting health prevention with videos. With feedback from patients, they created a series of animations encouraging patients to get influenza, pneumococcal, and zoster vaccinations and screening for bone health and skin cancer.
They randomly assigned 511 to receive videos and 545 patients to receive texts as a control group. After 6 months, 345 patients remained in the text group and 322 remained in the video group. The two groups had similar demographics, health status, and preventive health behaviors. They were mostly educated White women whose IBD was in remission.
The percentage of those who got flu vaccines increased from 59% (for the 2018-2019 season) to 63% (for the 2019-2020 flu season) in the group that watched the videos. However this change did not quite reach statistical significance (P = .07). The change in the text group, from 55% to 57%, was also not significant (P = .23).
The subjects did express more intention to get flu vaccines. The percentage with this intention increased from 59 to 75 in the video group, and from 55 to 72 in the text group. Both changes were statistically significant (P < .001).
Intentions to receive pneumonia and shingles vaccines, and bone and skin cancer screening, were not statistically different between the groups.
The researchers looked at age, immunosuppression, gender, and education to see if these factors could predict who was most likely to get the flu vaccine, but the only significant predictor was having received a previous flu shot.
Dr. Appel speculated that the videos might have been more effective in a more racially diverse, less educated population, or one where fewer people had previously received vaccinations.
“While we didn’t see a difference in this study, I think it opens up a lot of other questions that we can explore and answer,” she said. “It’s possible that patients may not have a one size fits all on their response. Some may respond better to video. Some may respond to text. Some may need more frequent reminders. Some might need to hear it from their doctor directly.”
Session comoderator Alyse Bedell, PhD, an assistant professor of psychiatry and behavioral neuroscience at the University of Chicago, agreed that a different patient population might have responded differently. “A population that may have lower access to educational resources, or has less educational attainment, or may have fewer people in their communities that are already receiving vaccines – those I think are going to be the populations where we’re going to be more likely to see the effects of an intervention like this,” she said in an interview.
Neither Dr. Appel nor Dr. Bedell reported any relevant financial interests. The study was funded by Pfizer.
AT DDW 2022
Newly approved tirzepatide’s retail price announced
Tirzepatide (Mounjaro) – the new twincretin approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes – was priced by Lilly, the company that will market the drug, at a list price of $974.33 for four weekly doses regardless of dose size, a cost that adds up to about $12,666 per year, according to a statement made on May 20 by a Lilly spokesperson.
This price puts tirzepatide, which combines the activity of two of the primary human incretins in one molecule, roughly in the same ballpark as what might be its main competitor, semaglutide (Ozempic) for type 2 diabetes, which retails at many U.S. pharmacies for about $925 for four weekly doses, or about $12,025 per year, although Ozempic’s posted retail price is about $100 higher for four doses.
According to the Lilly spokesperson, discount programs could reduce the monthly out-of-pocket cost for patients to as little as $25.
Tirzepatide, which received approval from the FDA on May 13, is a dual glucagonlike peptide–1 (GLP-1) receptor agonist and glucose-dependent insulinotropic polypeptide agonist. Several GLP-1 receptor agonists are already approved in the United States, including semaglutide, which is indicated as Wegovy for weight loss in patients with obesity regardless of diabetes status.
A version of this article first appeared on Medscape.com.
Tirzepatide (Mounjaro) – the new twincretin approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes – was priced by Lilly, the company that will market the drug, at a list price of $974.33 for four weekly doses regardless of dose size, a cost that adds up to about $12,666 per year, according to a statement made on May 20 by a Lilly spokesperson.
This price puts tirzepatide, which combines the activity of two of the primary human incretins in one molecule, roughly in the same ballpark as what might be its main competitor, semaglutide (Ozempic) for type 2 diabetes, which retails at many U.S. pharmacies for about $925 for four weekly doses, or about $12,025 per year, although Ozempic’s posted retail price is about $100 higher for four doses.
According to the Lilly spokesperson, discount programs could reduce the monthly out-of-pocket cost for patients to as little as $25.
Tirzepatide, which received approval from the FDA on May 13, is a dual glucagonlike peptide–1 (GLP-1) receptor agonist and glucose-dependent insulinotropic polypeptide agonist. Several GLP-1 receptor agonists are already approved in the United States, including semaglutide, which is indicated as Wegovy for weight loss in patients with obesity regardless of diabetes status.
A version of this article first appeared on Medscape.com.
Tirzepatide (Mounjaro) – the new twincretin approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes – was priced by Lilly, the company that will market the drug, at a list price of $974.33 for four weekly doses regardless of dose size, a cost that adds up to about $12,666 per year, according to a statement made on May 20 by a Lilly spokesperson.
This price puts tirzepatide, which combines the activity of two of the primary human incretins in one molecule, roughly in the same ballpark as what might be its main competitor, semaglutide (Ozempic) for type 2 diabetes, which retails at many U.S. pharmacies for about $925 for four weekly doses, or about $12,025 per year, although Ozempic’s posted retail price is about $100 higher for four doses.
According to the Lilly spokesperson, discount programs could reduce the monthly out-of-pocket cost for patients to as little as $25.
Tirzepatide, which received approval from the FDA on May 13, is a dual glucagonlike peptide–1 (GLP-1) receptor agonist and glucose-dependent insulinotropic polypeptide agonist. Several GLP-1 receptor agonists are already approved in the United States, including semaglutide, which is indicated as Wegovy for weight loss in patients with obesity regardless of diabetes status.
A version of this article first appeared on Medscape.com.
Contraceptive use boosted by enhanced counseling
Contraceptive counseling and interventions beyond usual care significantly increased the use of contraceptives with no accompanying increase in sexually transmitted infections or reduction in condom use, based on data from a new meta-analysis.
“Although effective contraception is available in the United States and guidelines support contraceptive care in clinical practice, providing contraceptive care has not been widely adopted across medical specialties as a preventive health service that is routinely offered to eligible patients, such as mammography screening,” lead author Heidi D. Nelson, MD, of Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, Calif., said in an interview.
“Access to and coverage of contraceptive care are frequently challenged by legislation and insurance policies, and influential preventive services guideline groups, such as the U.S. Preventive Services Task Force, have not issued recommendations for contraceptive care,” Dr. Nelson said.
“The evidence to determine the benefits and harms of contraceptive care as a preventive health service has not been examined using methods similar to those used for other preventive services and clinicians may lack guidance on the effectiveness of contraception services relevant to their practices,” she added.
In a study published in Annals of Internal Medicine, Dr. Nelson and colleagues reviewed data from 38 randomized, controlled trials with a total of 25,472 participants. The trials evaluated the effectiveness of various types of contraceptive counseling and provision interventions beyond usual care on subsequent contraception use, compared with nonintervention comparison groups.
Overall, higher contraceptive use was associated with counseling interventions (risk ratio, 1.39), advance provision of emergency contraception (RR, 2.12), counseling or provision of emergency contraception postpartum (RR, 1.15), or counseling or provision of emergency contraception at the time of abortion (RR, 1.19), compared with usual care or active controls across studies.
Most of the included trials were not powered to distinguish intended versus unintended pregnancy rates, but pregnancy rates were lower among intervention groups, compared with controls.
Five of the selected studies assessed the potential negative effect of contraceptive counseling with regard to increased rates of STIs and two studies examined decreased condom use. However, neither STI rates nor condom use were significantly different between study participants who received various contraceptive counseling interventions (such as advanced provision of emergency contraception, clinician training, and individual counseling) and those who did not (RR, 1.05 and RR, 1.03, respectively).
“These results indicate that additional efforts to assist patients with their contraception decisions improve its subsequent use,” and are not surprising, said Dr. Nelson.
“All clinicians providing health care to women, not only clinicians providing reproductive health care specifically, need to recognize contraceptive care as an essential preventive health service and assume responsibility for delivering contraceptive counseling and provision services appropriate for each patient,” Dr. Nelson emphasized. “Clinicians lacking contraceptive care clinical skills may require additional training or refer their patients if needed to assure high quality care.”
The study findings were limited by several factors including the variability of interventions across studies and the lack of data on unintended pregnancy outcomes, the researchers noted. However, the results suggest that various contraceptive counseling and interventions beyond usual care increased contraceptive use with no reduction in condom use or increase in STIs, they wrote.
“Additional research should further evaluate approaches to contraceptive counseling and provision to determine best practices,” Dr. Nelson said in an interview. “This is particularly important for medically high-risk populations, those with limited access to care, and additional populations and settings that have not yet been studied, including transgender and nonbinary patients. Research is needed to refine measures of pregnancy intention and planning; and create uniform definitions of contraceptive care, interventions, measures of use, and outcomes.”.
Make easy, effective contraception accessible to all
The news of a potential overturn of the 1973 Roe v. Wade Supreme Court decision that protects a pregnant person’s ability to choose abortion “shines a bright light on the importance of promoting the use of contraception,” and on the findings of the current review, Christine Laine, MD, editor-in-chief of Annals of Internal Medicine, wrote in an accompanying editorial. “Easy, effective, accessible, and affordable contraception becomes increasingly essential as ending unintended pregnancy becomes increasingly difficult, unsafe, inaccessible, and legally risky.”
The available evidence showed the benefits of enhanced counseling, providing emergency contraception in advance, and providing contraceptive interventions immediately after delivery or pregnancy termination, she wrote. The findings have strong clinical implications, especially with regard to the Healthy People 2030 goal of reducing unintended pregnancy from the current 43% to 36.5%.
Dr. Laine called on internal medicine physicians in particular to recognize the negative health consequences of unintended pregnancy, and to consider contraceptive counseling part of their responsibility to their patients.
“To expand the numbers of people who receive this essential preventive service, we must systematically incorporate contraceptive counseling into health care with the same fervor that we devote to other preventive services. The health of our patients – and their families – depends on it,” she concluded.
The study was supported by the Resources Legacy Fund. The researchers had no financial conflicts to disclose. Dr. Laine had no financial conflicts to disclose.
Contraceptive counseling and interventions beyond usual care significantly increased the use of contraceptives with no accompanying increase in sexually transmitted infections or reduction in condom use, based on data from a new meta-analysis.
“Although effective contraception is available in the United States and guidelines support contraceptive care in clinical practice, providing contraceptive care has not been widely adopted across medical specialties as a preventive health service that is routinely offered to eligible patients, such as mammography screening,” lead author Heidi D. Nelson, MD, of Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, Calif., said in an interview.
“Access to and coverage of contraceptive care are frequently challenged by legislation and insurance policies, and influential preventive services guideline groups, such as the U.S. Preventive Services Task Force, have not issued recommendations for contraceptive care,” Dr. Nelson said.
“The evidence to determine the benefits and harms of contraceptive care as a preventive health service has not been examined using methods similar to those used for other preventive services and clinicians may lack guidance on the effectiveness of contraception services relevant to their practices,” she added.
In a study published in Annals of Internal Medicine, Dr. Nelson and colleagues reviewed data from 38 randomized, controlled trials with a total of 25,472 participants. The trials evaluated the effectiveness of various types of contraceptive counseling and provision interventions beyond usual care on subsequent contraception use, compared with nonintervention comparison groups.
Overall, higher contraceptive use was associated with counseling interventions (risk ratio, 1.39), advance provision of emergency contraception (RR, 2.12), counseling or provision of emergency contraception postpartum (RR, 1.15), or counseling or provision of emergency contraception at the time of abortion (RR, 1.19), compared with usual care or active controls across studies.
Most of the included trials were not powered to distinguish intended versus unintended pregnancy rates, but pregnancy rates were lower among intervention groups, compared with controls.
Five of the selected studies assessed the potential negative effect of contraceptive counseling with regard to increased rates of STIs and two studies examined decreased condom use. However, neither STI rates nor condom use were significantly different between study participants who received various contraceptive counseling interventions (such as advanced provision of emergency contraception, clinician training, and individual counseling) and those who did not (RR, 1.05 and RR, 1.03, respectively).
“These results indicate that additional efforts to assist patients with their contraception decisions improve its subsequent use,” and are not surprising, said Dr. Nelson.
“All clinicians providing health care to women, not only clinicians providing reproductive health care specifically, need to recognize contraceptive care as an essential preventive health service and assume responsibility for delivering contraceptive counseling and provision services appropriate for each patient,” Dr. Nelson emphasized. “Clinicians lacking contraceptive care clinical skills may require additional training or refer their patients if needed to assure high quality care.”
The study findings were limited by several factors including the variability of interventions across studies and the lack of data on unintended pregnancy outcomes, the researchers noted. However, the results suggest that various contraceptive counseling and interventions beyond usual care increased contraceptive use with no reduction in condom use or increase in STIs, they wrote.
“Additional research should further evaluate approaches to contraceptive counseling and provision to determine best practices,” Dr. Nelson said in an interview. “This is particularly important for medically high-risk populations, those with limited access to care, and additional populations and settings that have not yet been studied, including transgender and nonbinary patients. Research is needed to refine measures of pregnancy intention and planning; and create uniform definitions of contraceptive care, interventions, measures of use, and outcomes.”.
Make easy, effective contraception accessible to all
The news of a potential overturn of the 1973 Roe v. Wade Supreme Court decision that protects a pregnant person’s ability to choose abortion “shines a bright light on the importance of promoting the use of contraception,” and on the findings of the current review, Christine Laine, MD, editor-in-chief of Annals of Internal Medicine, wrote in an accompanying editorial. “Easy, effective, accessible, and affordable contraception becomes increasingly essential as ending unintended pregnancy becomes increasingly difficult, unsafe, inaccessible, and legally risky.”
The available evidence showed the benefits of enhanced counseling, providing emergency contraception in advance, and providing contraceptive interventions immediately after delivery or pregnancy termination, she wrote. The findings have strong clinical implications, especially with regard to the Healthy People 2030 goal of reducing unintended pregnancy from the current 43% to 36.5%.
Dr. Laine called on internal medicine physicians in particular to recognize the negative health consequences of unintended pregnancy, and to consider contraceptive counseling part of their responsibility to their patients.
“To expand the numbers of people who receive this essential preventive service, we must systematically incorporate contraceptive counseling into health care with the same fervor that we devote to other preventive services. The health of our patients – and their families – depends on it,” she concluded.
The study was supported by the Resources Legacy Fund. The researchers had no financial conflicts to disclose. Dr. Laine had no financial conflicts to disclose.
Contraceptive counseling and interventions beyond usual care significantly increased the use of contraceptives with no accompanying increase in sexually transmitted infections or reduction in condom use, based on data from a new meta-analysis.
“Although effective contraception is available in the United States and guidelines support contraceptive care in clinical practice, providing contraceptive care has not been widely adopted across medical specialties as a preventive health service that is routinely offered to eligible patients, such as mammography screening,” lead author Heidi D. Nelson, MD, of Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, Calif., said in an interview.
“Access to and coverage of contraceptive care are frequently challenged by legislation and insurance policies, and influential preventive services guideline groups, such as the U.S. Preventive Services Task Force, have not issued recommendations for contraceptive care,” Dr. Nelson said.
“The evidence to determine the benefits and harms of contraceptive care as a preventive health service has not been examined using methods similar to those used for other preventive services and clinicians may lack guidance on the effectiveness of contraception services relevant to their practices,” she added.
In a study published in Annals of Internal Medicine, Dr. Nelson and colleagues reviewed data from 38 randomized, controlled trials with a total of 25,472 participants. The trials evaluated the effectiveness of various types of contraceptive counseling and provision interventions beyond usual care on subsequent contraception use, compared with nonintervention comparison groups.
Overall, higher contraceptive use was associated with counseling interventions (risk ratio, 1.39), advance provision of emergency contraception (RR, 2.12), counseling or provision of emergency contraception postpartum (RR, 1.15), or counseling or provision of emergency contraception at the time of abortion (RR, 1.19), compared with usual care or active controls across studies.
Most of the included trials were not powered to distinguish intended versus unintended pregnancy rates, but pregnancy rates were lower among intervention groups, compared with controls.
Five of the selected studies assessed the potential negative effect of contraceptive counseling with regard to increased rates of STIs and two studies examined decreased condom use. However, neither STI rates nor condom use were significantly different between study participants who received various contraceptive counseling interventions (such as advanced provision of emergency contraception, clinician training, and individual counseling) and those who did not (RR, 1.05 and RR, 1.03, respectively).
“These results indicate that additional efforts to assist patients with their contraception decisions improve its subsequent use,” and are not surprising, said Dr. Nelson.
“All clinicians providing health care to women, not only clinicians providing reproductive health care specifically, need to recognize contraceptive care as an essential preventive health service and assume responsibility for delivering contraceptive counseling and provision services appropriate for each patient,” Dr. Nelson emphasized. “Clinicians lacking contraceptive care clinical skills may require additional training or refer their patients if needed to assure high quality care.”
The study findings were limited by several factors including the variability of interventions across studies and the lack of data on unintended pregnancy outcomes, the researchers noted. However, the results suggest that various contraceptive counseling and interventions beyond usual care increased contraceptive use with no reduction in condom use or increase in STIs, they wrote.
“Additional research should further evaluate approaches to contraceptive counseling and provision to determine best practices,” Dr. Nelson said in an interview. “This is particularly important for medically high-risk populations, those with limited access to care, and additional populations and settings that have not yet been studied, including transgender and nonbinary patients. Research is needed to refine measures of pregnancy intention and planning; and create uniform definitions of contraceptive care, interventions, measures of use, and outcomes.”.
Make easy, effective contraception accessible to all
The news of a potential overturn of the 1973 Roe v. Wade Supreme Court decision that protects a pregnant person’s ability to choose abortion “shines a bright light on the importance of promoting the use of contraception,” and on the findings of the current review, Christine Laine, MD, editor-in-chief of Annals of Internal Medicine, wrote in an accompanying editorial. “Easy, effective, accessible, and affordable contraception becomes increasingly essential as ending unintended pregnancy becomes increasingly difficult, unsafe, inaccessible, and legally risky.”
The available evidence showed the benefits of enhanced counseling, providing emergency contraception in advance, and providing contraceptive interventions immediately after delivery or pregnancy termination, she wrote. The findings have strong clinical implications, especially with regard to the Healthy People 2030 goal of reducing unintended pregnancy from the current 43% to 36.5%.
Dr. Laine called on internal medicine physicians in particular to recognize the negative health consequences of unintended pregnancy, and to consider contraceptive counseling part of their responsibility to their patients.
“To expand the numbers of people who receive this essential preventive service, we must systematically incorporate contraceptive counseling into health care with the same fervor that we devote to other preventive services. The health of our patients – and their families – depends on it,” she concluded.
The study was supported by the Resources Legacy Fund. The researchers had no financial conflicts to disclose. Dr. Laine had no financial conflicts to disclose.
FROM ANNALS OF INTERNAL MEDICINE
Monkeypox quarantines not needed in U.S., Biden says
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
How to manage drug interactions with Paxlovid for COVID-19
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Does COVID-19 raise the risk for diabetes?
This transcript has been edited for clarity.
Does having had a COVID-19 infection increase your risk for the development of diabetes subsequently? Some data say yes and other data say no. No matter what, it’s obviously important to screen people for diabetes routinely, pandemic or not. Remember, screening should start at age 35.
For over a decade, we have known that SARS-type viruses bind to beta cells. This could cause either direct damage to the beta cell or in some way trigger beta cell autoimmunity. We also know that COVID-19 infection increases the levels of inflammatory mediators, which could cause damage to beta cells and potentially to insulin receptors. There is a potential that having had a COVID-19 infection could increase rates of developing type 1 and/or type 2 diabetes.
However, there are other possible causes for people to develop diabetes after having a COVID-19 infection. A COVID-19 infection could cause one to seek medical care, unmasking latent type 1 and/or type 2 diabetes by causing infection-related insulin resistance and worsening preexisting mild hypoglycemia. In addition, people could have sought more medical care in the years since the pandemic has been ebbing, which may make it look like cases have increased.
For example, during the worst of the pandemic, I had multiple referrals for “COVID-19–caused new-onset diabetes” only to find that the patient had an A1c level above 10% and a history of mildly elevated blood glucose levels. This suggests to me that COVID-19 did not cause the diabetes per se but rather worsened an underlying glucose abnormality.
Since the pandemic has improved, I have also seen people diagnosed with type 2 diabetes that I think is associated with pandemic-related weight gain and inactivity.
The bigger issue is what is happening to people after COVID-19 infection who lack risk factors. What about those who we didn’t think were at high risk to get diabetes to begin with and didn’t have prediabetes?
An article by Xie and Al-Aly in The Lancet Diabetes & Endocrinology showed an increase in rates of diabetes in a large VA cohort among those who had a COVID-19 infection compared with both a contemporaneous control who did not have COVID-19 and a historical control. The researchers looked at the patient data 1 year after they’d had COVID-19, so it wasn’t the immediate post–COVID-19 phase but several months later.
They found that the risk for incident type 2 diabetes development was increased by 40% after adjusting for many risk factors. This included individuals who didn’t have traditional risk factors before they developed type 2 diabetes.
What does this mean clinically? First, pandemic or not, people need screening for diabetes and encouragement to have a healthy lifestyle. There may be an increased risk for the diagnosis of type 2 diabetes after COVID-19 infection due to a variety of different mechanisms.
As for people with type 1 diabetes, we also don’t know if having a COVID-19 infection increases their risk. We do know that there was an increase in the severity of diabetic ketoacidosis presentation during the pandemic, so we need to be sure that we reinforce sick-day rules with our patients with type 1 diabetes and that all individuals with type 1 diabetes have the ability to test their ketone levels at home.
In people with new-onset diabetes, whether type 1 or type 2, caused by COVID-19 or not, we need to treat appropriately based on their clinical situation.
Data from registries started during the pandemic will provide more definitive answers and help us find out if there is a relationship between having had COVID-19 infection and developing diabetes.
Perhaps that can help us better understand the mechanisms behind the development of diabetes overall.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Does having had a COVID-19 infection increase your risk for the development of diabetes subsequently? Some data say yes and other data say no. No matter what, it’s obviously important to screen people for diabetes routinely, pandemic or not. Remember, screening should start at age 35.
For over a decade, we have known that SARS-type viruses bind to beta cells. This could cause either direct damage to the beta cell or in some way trigger beta cell autoimmunity. We also know that COVID-19 infection increases the levels of inflammatory mediators, which could cause damage to beta cells and potentially to insulin receptors. There is a potential that having had a COVID-19 infection could increase rates of developing type 1 and/or type 2 diabetes.
However, there are other possible causes for people to develop diabetes after having a COVID-19 infection. A COVID-19 infection could cause one to seek medical care, unmasking latent type 1 and/or type 2 diabetes by causing infection-related insulin resistance and worsening preexisting mild hypoglycemia. In addition, people could have sought more medical care in the years since the pandemic has been ebbing, which may make it look like cases have increased.
For example, during the worst of the pandemic, I had multiple referrals for “COVID-19–caused new-onset diabetes” only to find that the patient had an A1c level above 10% and a history of mildly elevated blood glucose levels. This suggests to me that COVID-19 did not cause the diabetes per se but rather worsened an underlying glucose abnormality.
Since the pandemic has improved, I have also seen people diagnosed with type 2 diabetes that I think is associated with pandemic-related weight gain and inactivity.
The bigger issue is what is happening to people after COVID-19 infection who lack risk factors. What about those who we didn’t think were at high risk to get diabetes to begin with and didn’t have prediabetes?
An article by Xie and Al-Aly in The Lancet Diabetes & Endocrinology showed an increase in rates of diabetes in a large VA cohort among those who had a COVID-19 infection compared with both a contemporaneous control who did not have COVID-19 and a historical control. The researchers looked at the patient data 1 year after they’d had COVID-19, so it wasn’t the immediate post–COVID-19 phase but several months later.
They found that the risk for incident type 2 diabetes development was increased by 40% after adjusting for many risk factors. This included individuals who didn’t have traditional risk factors before they developed type 2 diabetes.
What does this mean clinically? First, pandemic or not, people need screening for diabetes and encouragement to have a healthy lifestyle. There may be an increased risk for the diagnosis of type 2 diabetes after COVID-19 infection due to a variety of different mechanisms.
As for people with type 1 diabetes, we also don’t know if having a COVID-19 infection increases their risk. We do know that there was an increase in the severity of diabetic ketoacidosis presentation during the pandemic, so we need to be sure that we reinforce sick-day rules with our patients with type 1 diabetes and that all individuals with type 1 diabetes have the ability to test their ketone levels at home.
In people with new-onset diabetes, whether type 1 or type 2, caused by COVID-19 or not, we need to treat appropriately based on their clinical situation.
Data from registries started during the pandemic will provide more definitive answers and help us find out if there is a relationship between having had COVID-19 infection and developing diabetes.
Perhaps that can help us better understand the mechanisms behind the development of diabetes overall.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Does having had a COVID-19 infection increase your risk for the development of diabetes subsequently? Some data say yes and other data say no. No matter what, it’s obviously important to screen people for diabetes routinely, pandemic or not. Remember, screening should start at age 35.
For over a decade, we have known that SARS-type viruses bind to beta cells. This could cause either direct damage to the beta cell or in some way trigger beta cell autoimmunity. We also know that COVID-19 infection increases the levels of inflammatory mediators, which could cause damage to beta cells and potentially to insulin receptors. There is a potential that having had a COVID-19 infection could increase rates of developing type 1 and/or type 2 diabetes.
However, there are other possible causes for people to develop diabetes after having a COVID-19 infection. A COVID-19 infection could cause one to seek medical care, unmasking latent type 1 and/or type 2 diabetes by causing infection-related insulin resistance and worsening preexisting mild hypoglycemia. In addition, people could have sought more medical care in the years since the pandemic has been ebbing, which may make it look like cases have increased.
For example, during the worst of the pandemic, I had multiple referrals for “COVID-19–caused new-onset diabetes” only to find that the patient had an A1c level above 10% and a history of mildly elevated blood glucose levels. This suggests to me that COVID-19 did not cause the diabetes per se but rather worsened an underlying glucose abnormality.
Since the pandemic has improved, I have also seen people diagnosed with type 2 diabetes that I think is associated with pandemic-related weight gain and inactivity.
The bigger issue is what is happening to people after COVID-19 infection who lack risk factors. What about those who we didn’t think were at high risk to get diabetes to begin with and didn’t have prediabetes?
An article by Xie and Al-Aly in The Lancet Diabetes & Endocrinology showed an increase in rates of diabetes in a large VA cohort among those who had a COVID-19 infection compared with both a contemporaneous control who did not have COVID-19 and a historical control. The researchers looked at the patient data 1 year after they’d had COVID-19, so it wasn’t the immediate post–COVID-19 phase but several months later.
They found that the risk for incident type 2 diabetes development was increased by 40% after adjusting for many risk factors. This included individuals who didn’t have traditional risk factors before they developed type 2 diabetes.
What does this mean clinically? First, pandemic or not, people need screening for diabetes and encouragement to have a healthy lifestyle. There may be an increased risk for the diagnosis of type 2 diabetes after COVID-19 infection due to a variety of different mechanisms.
As for people with type 1 diabetes, we also don’t know if having a COVID-19 infection increases their risk. We do know that there was an increase in the severity of diabetic ketoacidosis presentation during the pandemic, so we need to be sure that we reinforce sick-day rules with our patients with type 1 diabetes and that all individuals with type 1 diabetes have the ability to test their ketone levels at home.
In people with new-onset diabetes, whether type 1 or type 2, caused by COVID-19 or not, we need to treat appropriately based on their clinical situation.
Data from registries started during the pandemic will provide more definitive answers and help us find out if there is a relationship between having had COVID-19 infection and developing diabetes.
Perhaps that can help us better understand the mechanisms behind the development of diabetes overall.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article first appeared on Medscape.com.
European committee recommends approval of baricitinib for severe alopecia areata
The European Medicines Agency’s ( (AA).
The development, which was announced in a May 20, 2022, press release from the manufacturer, Eli Lilly and Incyte, marks the first step toward European regulatory approval of baricitinib (Olumiant) for patients with severe AA, and it is now referred to the European Commission for final action. A decision is expected within the next 2 months.
The committee based its positive opinion on the results of the phase 3 BRAVE-AA1 and BRAVE-AA2 trials, recently published in the New England Journal of Medicine, which evaluated the efficacy and safety of baricitinib in 1,200 patients with severe AA, according to the press release. The primary endpoint was the proportion of patients achieving a Severity of Alopecia Tool (SALT) score of ≤20 at week 36. In both studies, 1 out of 3 patients treated with baricitinib 4-mg achieved 80% or more scalp hair coverage, compared with 1 out of 20 patients and 1 out of 50 patients taking placebo in BRAVE-AA1 and BRAVE-AA2, respectively (P ≤ .001 for all comparisons to placebo).
According to safety profile information from the phase 3 BRAVE-AA clinical program, few patients discontinued treatment because of adverse events (2.6% or less across both studies), and most treatment-emergent adverse events were mild or moderate in severity.
In February 2022, the Food and Drug Administration granted priority review for baricitinib in adults with severe AA. Lilly expects additional regulatory decisions in the United States and Japan in 2022.
Baricitinib is approved in the United States as a treatment for adults with moderate to severe rheumatoid arthritis. Prescribing information can be viewed here.
The European Medicines Agency’s ( (AA).
The development, which was announced in a May 20, 2022, press release from the manufacturer, Eli Lilly and Incyte, marks the first step toward European regulatory approval of baricitinib (Olumiant) for patients with severe AA, and it is now referred to the European Commission for final action. A decision is expected within the next 2 months.
The committee based its positive opinion on the results of the phase 3 BRAVE-AA1 and BRAVE-AA2 trials, recently published in the New England Journal of Medicine, which evaluated the efficacy and safety of baricitinib in 1,200 patients with severe AA, according to the press release. The primary endpoint was the proportion of patients achieving a Severity of Alopecia Tool (SALT) score of ≤20 at week 36. In both studies, 1 out of 3 patients treated with baricitinib 4-mg achieved 80% or more scalp hair coverage, compared with 1 out of 20 patients and 1 out of 50 patients taking placebo in BRAVE-AA1 and BRAVE-AA2, respectively (P ≤ .001 for all comparisons to placebo).
According to safety profile information from the phase 3 BRAVE-AA clinical program, few patients discontinued treatment because of adverse events (2.6% or less across both studies), and most treatment-emergent adverse events were mild or moderate in severity.
In February 2022, the Food and Drug Administration granted priority review for baricitinib in adults with severe AA. Lilly expects additional regulatory decisions in the United States and Japan in 2022.
Baricitinib is approved in the United States as a treatment for adults with moderate to severe rheumatoid arthritis. Prescribing information can be viewed here.
The European Medicines Agency’s ( (AA).
The development, which was announced in a May 20, 2022, press release from the manufacturer, Eli Lilly and Incyte, marks the first step toward European regulatory approval of baricitinib (Olumiant) for patients with severe AA, and it is now referred to the European Commission for final action. A decision is expected within the next 2 months.
The committee based its positive opinion on the results of the phase 3 BRAVE-AA1 and BRAVE-AA2 trials, recently published in the New England Journal of Medicine, which evaluated the efficacy and safety of baricitinib in 1,200 patients with severe AA, according to the press release. The primary endpoint was the proportion of patients achieving a Severity of Alopecia Tool (SALT) score of ≤20 at week 36. In both studies, 1 out of 3 patients treated with baricitinib 4-mg achieved 80% or more scalp hair coverage, compared with 1 out of 20 patients and 1 out of 50 patients taking placebo in BRAVE-AA1 and BRAVE-AA2, respectively (P ≤ .001 for all comparisons to placebo).
According to safety profile information from the phase 3 BRAVE-AA clinical program, few patients discontinued treatment because of adverse events (2.6% or less across both studies), and most treatment-emergent adverse events were mild or moderate in severity.
In February 2022, the Food and Drug Administration granted priority review for baricitinib in adults with severe AA. Lilly expects additional regulatory decisions in the United States and Japan in 2022.
Baricitinib is approved in the United States as a treatment for adults with moderate to severe rheumatoid arthritis. Prescribing information can be viewed here.
Rabies: CDC updates and simplifies preexposure prophylaxis vaccination recommendations
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.