User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
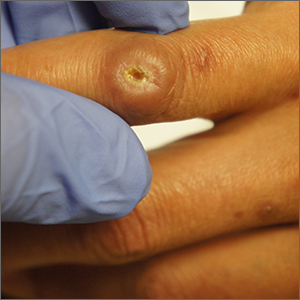
Ulcer on knuckle
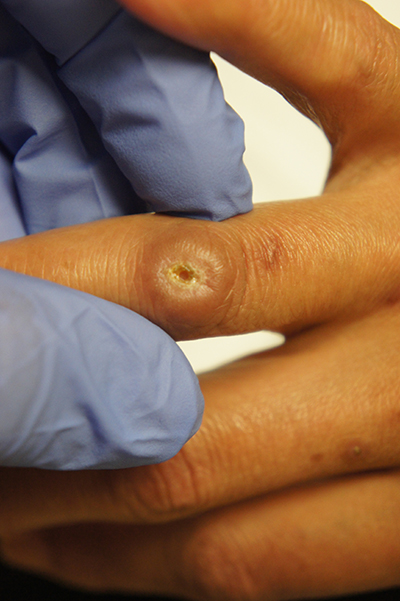
Since the papules were worrisome for vasculitis, 2 punch biopsies were performed on smaller, younger lesions on the hand and 1 was submitted for direct immunofluorescence. Findings revealed a leukocytoclastic vasculitis (LCV) with prominent immunoglobulin A (IgA) deposits around the vessel wall. The clinical and pathologic findings were consistent with a rare fibrosing LCV called erythema elevatum diutinum (EED). The practice of sampling nonblanching purpura or papules for both standard pathology (hematoxylin and eosin) and direct immunofluorescence can facilitate the diagnosis of unusual conditions that may occur only a few times in one’s career.
EED is rare, chronic, and may be associated with IgA gammopathy, IgA antineutrophil cytoplasmic antibodies, recent streptococcal or HIV infections, or myelodysplastic syndrome. A robust work-up to identify whether any of these factors are at work is critical.1 Distinct from other vasculitides, EED forms granulation tissue that becomes reinjured.
This patient was found to have an IgA monoclonal gammopathy that was monitored by Hematology. After checking her glucose-6-phosphate dehydrogenase activity, she was treated with oral dapsone 25 mg/d. Dapsone was titrated up to 100 mg/d, which improved her symptoms considerably and the ulcerated papule was surgically revised and closed. EED can last for years and ultimately clear or can persist indefinitely.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Sandhu JK, Albrecht J, Agnihotri G, et al. Erythema elevatum et diutinum as a systemic disease. Clin Dermatol. 2019;37:679-683. doi: 10.1016/j.clindermatol.2019.07.028

Since the papules were worrisome for vasculitis, 2 punch biopsies were performed on smaller, younger lesions on the hand and 1 was submitted for direct immunofluorescence. Findings revealed a leukocytoclastic vasculitis (LCV) with prominent immunoglobulin A (IgA) deposits around the vessel wall. The clinical and pathologic findings were consistent with a rare fibrosing LCV called erythema elevatum diutinum (EED). The practice of sampling nonblanching purpura or papules for both standard pathology (hematoxylin and eosin) and direct immunofluorescence can facilitate the diagnosis of unusual conditions that may occur only a few times in one’s career.
EED is rare, chronic, and may be associated with IgA gammopathy, IgA antineutrophil cytoplasmic antibodies, recent streptococcal or HIV infections, or myelodysplastic syndrome. A robust work-up to identify whether any of these factors are at work is critical.1 Distinct from other vasculitides, EED forms granulation tissue that becomes reinjured.
This patient was found to have an IgA monoclonal gammopathy that was monitored by Hematology. After checking her glucose-6-phosphate dehydrogenase activity, she was treated with oral dapsone 25 mg/d. Dapsone was titrated up to 100 mg/d, which improved her symptoms considerably and the ulcerated papule was surgically revised and closed. EED can last for years and ultimately clear or can persist indefinitely.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Since the papules were worrisome for vasculitis, 2 punch biopsies were performed on smaller, younger lesions on the hand and 1 was submitted for direct immunofluorescence. Findings revealed a leukocytoclastic vasculitis (LCV) with prominent immunoglobulin A (IgA) deposits around the vessel wall. The clinical and pathologic findings were consistent with a rare fibrosing LCV called erythema elevatum diutinum (EED). The practice of sampling nonblanching purpura or papules for both standard pathology (hematoxylin and eosin) and direct immunofluorescence can facilitate the diagnosis of unusual conditions that may occur only a few times in one’s career.
EED is rare, chronic, and may be associated with IgA gammopathy, IgA antineutrophil cytoplasmic antibodies, recent streptococcal or HIV infections, or myelodysplastic syndrome. A robust work-up to identify whether any of these factors are at work is critical.1 Distinct from other vasculitides, EED forms granulation tissue that becomes reinjured.
This patient was found to have an IgA monoclonal gammopathy that was monitored by Hematology. After checking her glucose-6-phosphate dehydrogenase activity, she was treated with oral dapsone 25 mg/d. Dapsone was titrated up to 100 mg/d, which improved her symptoms considerably and the ulcerated papule was surgically revised and closed. EED can last for years and ultimately clear or can persist indefinitely.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Sandhu JK, Albrecht J, Agnihotri G, et al. Erythema elevatum et diutinum as a systemic disease. Clin Dermatol. 2019;37:679-683. doi: 10.1016/j.clindermatol.2019.07.028
1. Sandhu JK, Albrecht J, Agnihotri G, et al. Erythema elevatum et diutinum as a systemic disease. Clin Dermatol. 2019;37:679-683. doi: 10.1016/j.clindermatol.2019.07.028
Takotsubo syndrome more deadly in men
Takotsubo syndrome occurs much more frequently in women than it does in men, but men are much more likely to die from it, according to the results of a new study.
In an analysis of almost 2,500 patients with Takotsubo syndrome (TSS) who were enrolled in an international registry, men, who made up just 11% of the sample, had significantly higher rates of cardiogenic shock and were more than twice as likely to die in the hospital than their female counterparts.
The authors concluded that TSS in males requires close in-hospital monitoring and long-term follow-up. Their study was published in the Journal of the American College of Cardiology.
Takotsubo syndrome is a condition characterized by acute heart failure and transient ventricular contractile dysfunction that can be precipitated by acute emotional or physical stress. It affects mostly women, particularly postmenopausal women, although the reasons for this are still not fully clear, Luca Arcari, MD, from the Institute of Cardiology, Madre Giuseppina Vannini Hospital, Rome, and colleagues wrote.
The syndrome also affects men, and recent data have identified that male sex is associated with worse outcomes. But, because it occurs relatively uncommonly in men, information about outcomes in men is limited.
To shed more light on the influence of gender on TTS, the investigators looked at 2,492 TTS patients (286 men, 2,206 women) who were participants in the GEIST (German Italian Spanish Takotsubo) registry and compared the clinical features and short- and long-term outcomes between the two.
Male patients were significantly younger (69 years) than women (71 years; P = .005) and had a higher prevalence of comorbid conditions, including diabetes (25% vs. 19%; P = .01); pulmonary diseases (21% vs. 15%; P = .006); malignancies (25% vs. 13%; P < .001).
In addition, TTS in men was more likely to be caused by physical triggers (55% vs. 32%; P < .01), whereas emotional triggers were more common in females (39% vs. 19%; P < 0.001).
The investigators then performed a propensity score analysis by matching men and women 1:1; this yielded 207 patients from each group.
After propensity matching, male patients had higher rates of cardiogenic shock (16% vs 6%), and in-hospital mortality (8% vs. 3%; both P < .05).
Men also had a higher mortality rate during the acute and long-term follow up. Male sex remained independently associated with both in-hospital mortality (odds ratio, 2.26; 95% confidence interval, 1.16-4.40) and long-term mortality (hazard ratio, 1.83; 95% CI, 1.32-2.52).
The study by Dr. Arcari and colleagues “shows convincingly that although men are far less likely to develop TTS than women, they have more serious complications and are more likely to die than women presenting with the syndrome, Ilan S. Wittstein, MD, of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
In an interview, Dr. Wittstein said one of the strengths of the study was its size.
“Over the years, there have been a lot of smaller, single center studies. This large registry had over 2,000 patients. So when the researchers say the rate of TTS is 10% in men and 90% in women, this is not necessarily surprising because that’s about the breakdown we’ve had since the very beginning, but it certainly validates that in a cohort that is large,” he said.
“I think what was novel about the paper is that the size of the cohort allowed the researchers to do propensity matching, so they were able not only to compare men versus women, they could do a 1:1 comparison. And they found even when you match men and women for various comorbidities, the men were much sicker
“What makes this a fascinating syndrome and different from most types of heart muscle problems is that, in the majority of patients, the condition is precipitated by an acute stressor,” said Dr. Wittstein.
“It can either be an emotional trigger, so for instance, getting some bad news that a loved one just died. That’s why we nicknamed the syndrome ‘broken heart syndrome’ many years ago. Or it can be a physical trigger, which can be a wide variety of things, such infection, a stroke, bad pneumonia, anything that stresses the body and causes a stress response. Regular heart attacks are not triggered in this way,” he said.
Dr. Arcari and Dr. Wittstein reported no relevant financial relationships.
Takotsubo syndrome occurs much more frequently in women than it does in men, but men are much more likely to die from it, according to the results of a new study.
In an analysis of almost 2,500 patients with Takotsubo syndrome (TSS) who were enrolled in an international registry, men, who made up just 11% of the sample, had significantly higher rates of cardiogenic shock and were more than twice as likely to die in the hospital than their female counterparts.
The authors concluded that TSS in males requires close in-hospital monitoring and long-term follow-up. Their study was published in the Journal of the American College of Cardiology.
Takotsubo syndrome is a condition characterized by acute heart failure and transient ventricular contractile dysfunction that can be precipitated by acute emotional or physical stress. It affects mostly women, particularly postmenopausal women, although the reasons for this are still not fully clear, Luca Arcari, MD, from the Institute of Cardiology, Madre Giuseppina Vannini Hospital, Rome, and colleagues wrote.
The syndrome also affects men, and recent data have identified that male sex is associated with worse outcomes. But, because it occurs relatively uncommonly in men, information about outcomes in men is limited.
To shed more light on the influence of gender on TTS, the investigators looked at 2,492 TTS patients (286 men, 2,206 women) who were participants in the GEIST (German Italian Spanish Takotsubo) registry and compared the clinical features and short- and long-term outcomes between the two.
Male patients were significantly younger (69 years) than women (71 years; P = .005) and had a higher prevalence of comorbid conditions, including diabetes (25% vs. 19%; P = .01); pulmonary diseases (21% vs. 15%; P = .006); malignancies (25% vs. 13%; P < .001).
In addition, TTS in men was more likely to be caused by physical triggers (55% vs. 32%; P < .01), whereas emotional triggers were more common in females (39% vs. 19%; P < 0.001).
The investigators then performed a propensity score analysis by matching men and women 1:1; this yielded 207 patients from each group.
After propensity matching, male patients had higher rates of cardiogenic shock (16% vs 6%), and in-hospital mortality (8% vs. 3%; both P < .05).
Men also had a higher mortality rate during the acute and long-term follow up. Male sex remained independently associated with both in-hospital mortality (odds ratio, 2.26; 95% confidence interval, 1.16-4.40) and long-term mortality (hazard ratio, 1.83; 95% CI, 1.32-2.52).
The study by Dr. Arcari and colleagues “shows convincingly that although men are far less likely to develop TTS than women, they have more serious complications and are more likely to die than women presenting with the syndrome, Ilan S. Wittstein, MD, of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
In an interview, Dr. Wittstein said one of the strengths of the study was its size.
“Over the years, there have been a lot of smaller, single center studies. This large registry had over 2,000 patients. So when the researchers say the rate of TTS is 10% in men and 90% in women, this is not necessarily surprising because that’s about the breakdown we’ve had since the very beginning, but it certainly validates that in a cohort that is large,” he said.
“I think what was novel about the paper is that the size of the cohort allowed the researchers to do propensity matching, so they were able not only to compare men versus women, they could do a 1:1 comparison. And they found even when you match men and women for various comorbidities, the men were much sicker
“What makes this a fascinating syndrome and different from most types of heart muscle problems is that, in the majority of patients, the condition is precipitated by an acute stressor,” said Dr. Wittstein.
“It can either be an emotional trigger, so for instance, getting some bad news that a loved one just died. That’s why we nicknamed the syndrome ‘broken heart syndrome’ many years ago. Or it can be a physical trigger, which can be a wide variety of things, such infection, a stroke, bad pneumonia, anything that stresses the body and causes a stress response. Regular heart attacks are not triggered in this way,” he said.
Dr. Arcari and Dr. Wittstein reported no relevant financial relationships.
Takotsubo syndrome occurs much more frequently in women than it does in men, but men are much more likely to die from it, according to the results of a new study.
In an analysis of almost 2,500 patients with Takotsubo syndrome (TSS) who were enrolled in an international registry, men, who made up just 11% of the sample, had significantly higher rates of cardiogenic shock and were more than twice as likely to die in the hospital than their female counterparts.
The authors concluded that TSS in males requires close in-hospital monitoring and long-term follow-up. Their study was published in the Journal of the American College of Cardiology.
Takotsubo syndrome is a condition characterized by acute heart failure and transient ventricular contractile dysfunction that can be precipitated by acute emotional or physical stress. It affects mostly women, particularly postmenopausal women, although the reasons for this are still not fully clear, Luca Arcari, MD, from the Institute of Cardiology, Madre Giuseppina Vannini Hospital, Rome, and colleagues wrote.
The syndrome also affects men, and recent data have identified that male sex is associated with worse outcomes. But, because it occurs relatively uncommonly in men, information about outcomes in men is limited.
To shed more light on the influence of gender on TTS, the investigators looked at 2,492 TTS patients (286 men, 2,206 women) who were participants in the GEIST (German Italian Spanish Takotsubo) registry and compared the clinical features and short- and long-term outcomes between the two.
Male patients were significantly younger (69 years) than women (71 years; P = .005) and had a higher prevalence of comorbid conditions, including diabetes (25% vs. 19%; P = .01); pulmonary diseases (21% vs. 15%; P = .006); malignancies (25% vs. 13%; P < .001).
In addition, TTS in men was more likely to be caused by physical triggers (55% vs. 32%; P < .01), whereas emotional triggers were more common in females (39% vs. 19%; P < 0.001).
The investigators then performed a propensity score analysis by matching men and women 1:1; this yielded 207 patients from each group.
After propensity matching, male patients had higher rates of cardiogenic shock (16% vs 6%), and in-hospital mortality (8% vs. 3%; both P < .05).
Men also had a higher mortality rate during the acute and long-term follow up. Male sex remained independently associated with both in-hospital mortality (odds ratio, 2.26; 95% confidence interval, 1.16-4.40) and long-term mortality (hazard ratio, 1.83; 95% CI, 1.32-2.52).
The study by Dr. Arcari and colleagues “shows convincingly that although men are far less likely to develop TTS than women, they have more serious complications and are more likely to die than women presenting with the syndrome, Ilan S. Wittstein, MD, of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
In an interview, Dr. Wittstein said one of the strengths of the study was its size.
“Over the years, there have been a lot of smaller, single center studies. This large registry had over 2,000 patients. So when the researchers say the rate of TTS is 10% in men and 90% in women, this is not necessarily surprising because that’s about the breakdown we’ve had since the very beginning, but it certainly validates that in a cohort that is large,” he said.
“I think what was novel about the paper is that the size of the cohort allowed the researchers to do propensity matching, so they were able not only to compare men versus women, they could do a 1:1 comparison. And they found even when you match men and women for various comorbidities, the men were much sicker
“What makes this a fascinating syndrome and different from most types of heart muscle problems is that, in the majority of patients, the condition is precipitated by an acute stressor,” said Dr. Wittstein.
“It can either be an emotional trigger, so for instance, getting some bad news that a loved one just died. That’s why we nicknamed the syndrome ‘broken heart syndrome’ many years ago. Or it can be a physical trigger, which can be a wide variety of things, such infection, a stroke, bad pneumonia, anything that stresses the body and causes a stress response. Regular heart attacks are not triggered in this way,” he said.
Dr. Arcari and Dr. Wittstein reported no relevant financial relationships.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
More evidence dementia not linked to PPI use in older people
Controversy regarding the purported link between the use of proton pump inhibitors (PPIs) or histamine H2 receptor antagonists (H2RAs) and risk for dementia continues.
Adding to the “no link” column comes new evidence from a study presented at the annual Digestive Disease Week® (DDW) .
Among almost 19,000 people, no association was found between the use of these agents and a greater likelihood of incident dementia, Alzheimer’s disease, or cognitive decline in people older than 65 years.
“We found that baseline PPI or H2RA use in older adults was not associated with dementia, with mild cognitive impairment, or declines in cognitive scores over time,” said lead author Raaj Shishir Mehta, MD, a gastroenterology fellow at Massachusetts General Hospital in Boston.
“While deprescribing efforts are important, especially when medications are not indicated, these data provide reassurance about the cognitive impacts of long-term use of PPIs in older adults,” he added.
Growing use, growing concern
As PPI use has increased worldwide, so too have concerns over the adverse effects from their long-term use, Dr. Mehta said.
“One particular area of concern, especially among older adults, is the link between long-term PPI use and risk for dementia,” he said.
Igniting the controversy was a February 2016 study published in JAMA Neurology that showed a positive association between PPI use and dementia in residents of Germany aged 75 years and older. Researchers linked PPI use to a 44% increased risk of dementia over 5 years.
The 2016 study was based on claims data, which can introduce “inaccuracy or bias in defining dementia cases,” Dr. Mehta said. He noted that it and other previous studies also were limited by an inability to account for concomitant medications or comorbidities.
To overcome these limitations in their study, Dr. Mehta and colleagues analyzed medication data collected during in-person visits and asked experts to confirm dementia outcomes. The research data come from ASPREE, a large aspirin study of 18,846 people older than 65 years in the United States and Australia. Participants were enrolled from 2010 to 2014. A total of 566 people developed incident dementia during follow-up.
The researchers had data on alcohol consumption and other lifestyle factors, as well as information on comorbidities, hospitalizations, and overall well-being.
“Perhaps the biggest strength of our study is our rigorous neurocognitive assessments,” Dr. Mehta said.
They assessed cognition at baseline and at years 1, 3, 5, and 7 using a battery of tests. An expert panel of neurologists, neuropsychologists, and geriatricians adjudicated cases of dementia, in accordance with DSM-IV criteria. If the diagnosis was unclear, they referred people for additional workup, including neuroimaging.
Cox proportional hazards, regression, and/or mixed effects modeling were used to relate medication use with cognitive scores.
All analyses were adjusted for age, sex, body mass index, alcohol use, family history of dementia, medications, and other medical comorbidities.
At baseline, PPI users were more likely to be White, have fewer years of education, and have higher rates of hypertension, diabetes, and kidney disease. This group also was more likely to be taking five or more medications.
Key points
During 80,976 person-years of follow-up, there were 566 incident cases of dementia, including 235 probable cases of Alzheimer’s disease and 331 other dementias.
Baseline PPI use, in comparison with nonuse, was not associated with incident dementia (hazard ratio, 0.86; 95% confidence interval, 0.70-1.05).
“Similarly, when we look specifically at Alzheimer’s disease or mixed types of dementia, we find no association between baseline PPI use and dementia,” Dr. Mehta said.
When they excluded people already taking PPIs at baseline, they found no association between starting PPIs and developing dementia over time.
Secondary aims of the study included looking for a link between PPI use and mild cognitive impairment or significant changes in cognition over time. In both cases, no association emerged. PPI use at baseline also was not associated with cognitive impairment/no dementia (also known as mild cognitive impairment) or with changes in overall cognitive test scores over time.
To determine whether any association could be a class effect of acid suppression medication, they assessed use of H2RA medications and development of incident dementia. Again, the researchers found no link.
A diverse multinational population from urban and rural areas was a strength of the study, as was the “very rigorous cognitive testing with expert adjudication of our endpoints,” Dr. Mehta said. In addition, fewer than 5% of patients were lost to follow-up.
In terms of limitations, this was an observational study “so residual confounding is always possible,” he added. “But I’ll emphasize that we are among the largest studies to date with wealth of covariates.”
Why the different findings?
The study was “really well done,” session moderator Paul Moayyedi, MD, said during the Q&A session at DDW 2022.
Dr. Moayyedi, a professor of medicine at McMaster University, Hamilton, Ont., asked Dr. Mehta why he “found absolutely no signal, whereas the German study did.”
“It’s a good question,” Dr. Mehta responded. “If you look across the board, there have been conflicting results.”
The disparity could be related to how researchers conducting claims data studies classify dementia, he noted.
“If you look at the nitty-gritty details over 5 years, almost 40% of participants [in those studies] end up with a diagnosis of dementia, which is quite high,” Dr. Mehta said. “That raises questions about whether the diagnosis of dementia is truly accurate.”
Dr. Mehta and Dr. Moayyedi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Controversy regarding the purported link between the use of proton pump inhibitors (PPIs) or histamine H2 receptor antagonists (H2RAs) and risk for dementia continues.
Adding to the “no link” column comes new evidence from a study presented at the annual Digestive Disease Week® (DDW) .
Among almost 19,000 people, no association was found between the use of these agents and a greater likelihood of incident dementia, Alzheimer’s disease, or cognitive decline in people older than 65 years.
“We found that baseline PPI or H2RA use in older adults was not associated with dementia, with mild cognitive impairment, or declines in cognitive scores over time,” said lead author Raaj Shishir Mehta, MD, a gastroenterology fellow at Massachusetts General Hospital in Boston.
“While deprescribing efforts are important, especially when medications are not indicated, these data provide reassurance about the cognitive impacts of long-term use of PPIs in older adults,” he added.
Growing use, growing concern
As PPI use has increased worldwide, so too have concerns over the adverse effects from their long-term use, Dr. Mehta said.
“One particular area of concern, especially among older adults, is the link between long-term PPI use and risk for dementia,” he said.
Igniting the controversy was a February 2016 study published in JAMA Neurology that showed a positive association between PPI use and dementia in residents of Germany aged 75 years and older. Researchers linked PPI use to a 44% increased risk of dementia over 5 years.
The 2016 study was based on claims data, which can introduce “inaccuracy or bias in defining dementia cases,” Dr. Mehta said. He noted that it and other previous studies also were limited by an inability to account for concomitant medications or comorbidities.
To overcome these limitations in their study, Dr. Mehta and colleagues analyzed medication data collected during in-person visits and asked experts to confirm dementia outcomes. The research data come from ASPREE, a large aspirin study of 18,846 people older than 65 years in the United States and Australia. Participants were enrolled from 2010 to 2014. A total of 566 people developed incident dementia during follow-up.
The researchers had data on alcohol consumption and other lifestyle factors, as well as information on comorbidities, hospitalizations, and overall well-being.
“Perhaps the biggest strength of our study is our rigorous neurocognitive assessments,” Dr. Mehta said.
They assessed cognition at baseline and at years 1, 3, 5, and 7 using a battery of tests. An expert panel of neurologists, neuropsychologists, and geriatricians adjudicated cases of dementia, in accordance with DSM-IV criteria. If the diagnosis was unclear, they referred people for additional workup, including neuroimaging.
Cox proportional hazards, regression, and/or mixed effects modeling were used to relate medication use with cognitive scores.
All analyses were adjusted for age, sex, body mass index, alcohol use, family history of dementia, medications, and other medical comorbidities.
At baseline, PPI users were more likely to be White, have fewer years of education, and have higher rates of hypertension, diabetes, and kidney disease. This group also was more likely to be taking five or more medications.
Key points
During 80,976 person-years of follow-up, there were 566 incident cases of dementia, including 235 probable cases of Alzheimer’s disease and 331 other dementias.
Baseline PPI use, in comparison with nonuse, was not associated with incident dementia (hazard ratio, 0.86; 95% confidence interval, 0.70-1.05).
“Similarly, when we look specifically at Alzheimer’s disease or mixed types of dementia, we find no association between baseline PPI use and dementia,” Dr. Mehta said.
When they excluded people already taking PPIs at baseline, they found no association between starting PPIs and developing dementia over time.
Secondary aims of the study included looking for a link between PPI use and mild cognitive impairment or significant changes in cognition over time. In both cases, no association emerged. PPI use at baseline also was not associated with cognitive impairment/no dementia (also known as mild cognitive impairment) or with changes in overall cognitive test scores over time.
To determine whether any association could be a class effect of acid suppression medication, they assessed use of H2RA medications and development of incident dementia. Again, the researchers found no link.
A diverse multinational population from urban and rural areas was a strength of the study, as was the “very rigorous cognitive testing with expert adjudication of our endpoints,” Dr. Mehta said. In addition, fewer than 5% of patients were lost to follow-up.
In terms of limitations, this was an observational study “so residual confounding is always possible,” he added. “But I’ll emphasize that we are among the largest studies to date with wealth of covariates.”
Why the different findings?
The study was “really well done,” session moderator Paul Moayyedi, MD, said during the Q&A session at DDW 2022.
Dr. Moayyedi, a professor of medicine at McMaster University, Hamilton, Ont., asked Dr. Mehta why he “found absolutely no signal, whereas the German study did.”
“It’s a good question,” Dr. Mehta responded. “If you look across the board, there have been conflicting results.”
The disparity could be related to how researchers conducting claims data studies classify dementia, he noted.
“If you look at the nitty-gritty details over 5 years, almost 40% of participants [in those studies] end up with a diagnosis of dementia, which is quite high,” Dr. Mehta said. “That raises questions about whether the diagnosis of dementia is truly accurate.”
Dr. Mehta and Dr. Moayyedi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Controversy regarding the purported link between the use of proton pump inhibitors (PPIs) or histamine H2 receptor antagonists (H2RAs) and risk for dementia continues.
Adding to the “no link” column comes new evidence from a study presented at the annual Digestive Disease Week® (DDW) .
Among almost 19,000 people, no association was found between the use of these agents and a greater likelihood of incident dementia, Alzheimer’s disease, or cognitive decline in people older than 65 years.
“We found that baseline PPI or H2RA use in older adults was not associated with dementia, with mild cognitive impairment, or declines in cognitive scores over time,” said lead author Raaj Shishir Mehta, MD, a gastroenterology fellow at Massachusetts General Hospital in Boston.
“While deprescribing efforts are important, especially when medications are not indicated, these data provide reassurance about the cognitive impacts of long-term use of PPIs in older adults,” he added.
Growing use, growing concern
As PPI use has increased worldwide, so too have concerns over the adverse effects from their long-term use, Dr. Mehta said.
“One particular area of concern, especially among older adults, is the link between long-term PPI use and risk for dementia,” he said.
Igniting the controversy was a February 2016 study published in JAMA Neurology that showed a positive association between PPI use and dementia in residents of Germany aged 75 years and older. Researchers linked PPI use to a 44% increased risk of dementia over 5 years.
The 2016 study was based on claims data, which can introduce “inaccuracy or bias in defining dementia cases,” Dr. Mehta said. He noted that it and other previous studies also were limited by an inability to account for concomitant medications or comorbidities.
To overcome these limitations in their study, Dr. Mehta and colleagues analyzed medication data collected during in-person visits and asked experts to confirm dementia outcomes. The research data come from ASPREE, a large aspirin study of 18,846 people older than 65 years in the United States and Australia. Participants were enrolled from 2010 to 2014. A total of 566 people developed incident dementia during follow-up.
The researchers had data on alcohol consumption and other lifestyle factors, as well as information on comorbidities, hospitalizations, and overall well-being.
“Perhaps the biggest strength of our study is our rigorous neurocognitive assessments,” Dr. Mehta said.
They assessed cognition at baseline and at years 1, 3, 5, and 7 using a battery of tests. An expert panel of neurologists, neuropsychologists, and geriatricians adjudicated cases of dementia, in accordance with DSM-IV criteria. If the diagnosis was unclear, they referred people for additional workup, including neuroimaging.
Cox proportional hazards, regression, and/or mixed effects modeling were used to relate medication use with cognitive scores.
All analyses were adjusted for age, sex, body mass index, alcohol use, family history of dementia, medications, and other medical comorbidities.
At baseline, PPI users were more likely to be White, have fewer years of education, and have higher rates of hypertension, diabetes, and kidney disease. This group also was more likely to be taking five or more medications.
Key points
During 80,976 person-years of follow-up, there were 566 incident cases of dementia, including 235 probable cases of Alzheimer’s disease and 331 other dementias.
Baseline PPI use, in comparison with nonuse, was not associated with incident dementia (hazard ratio, 0.86; 95% confidence interval, 0.70-1.05).
“Similarly, when we look specifically at Alzheimer’s disease or mixed types of dementia, we find no association between baseline PPI use and dementia,” Dr. Mehta said.
When they excluded people already taking PPIs at baseline, they found no association between starting PPIs and developing dementia over time.
Secondary aims of the study included looking for a link between PPI use and mild cognitive impairment or significant changes in cognition over time. In both cases, no association emerged. PPI use at baseline also was not associated with cognitive impairment/no dementia (also known as mild cognitive impairment) or with changes in overall cognitive test scores over time.
To determine whether any association could be a class effect of acid suppression medication, they assessed use of H2RA medications and development of incident dementia. Again, the researchers found no link.
A diverse multinational population from urban and rural areas was a strength of the study, as was the “very rigorous cognitive testing with expert adjudication of our endpoints,” Dr. Mehta said. In addition, fewer than 5% of patients were lost to follow-up.
In terms of limitations, this was an observational study “so residual confounding is always possible,” he added. “But I’ll emphasize that we are among the largest studies to date with wealth of covariates.”
Why the different findings?
The study was “really well done,” session moderator Paul Moayyedi, MD, said during the Q&A session at DDW 2022.
Dr. Moayyedi, a professor of medicine at McMaster University, Hamilton, Ont., asked Dr. Mehta why he “found absolutely no signal, whereas the German study did.”
“It’s a good question,” Dr. Mehta responded. “If you look across the board, there have been conflicting results.”
The disparity could be related to how researchers conducting claims data studies classify dementia, he noted.
“If you look at the nitty-gritty details over 5 years, almost 40% of participants [in those studies] end up with a diagnosis of dementia, which is quite high,” Dr. Mehta said. “That raises questions about whether the diagnosis of dementia is truly accurate.”
Dr. Mehta and Dr. Moayyedi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DDW 2022
Metformin bombs in breast cancer in landmark trial
Metformin, a common option for patients with type 2 diabetes, had previously been shown in observational studies to be associated with improved survival of cancer patients. Those studies mostly involved older patients with cancer who also had diabetes.
These findings have led to trials of the use of metformin for patients with cancer who do not have diabetes, but two lung cancer trials found no effect on survival.
Now this latest trial in breast cancer, which included 3,649 patients with hormone receptor–positive or –negative disease – who did not have diabetes – also found that metformin had no effect on survival.
These results “tell us that metformin is not effective against the most common types of breast cancer and any off-label use [of] this drug for the treatment of these common types of breast cancer should be stopped,” lead investigator and medical oncologist Pamela Goodwin, MD, a breast cancer researcher at the Lunenfeld-Tanenbaum Research Institute in Toronto, said in a press release.
The negative results “underscore the need for well-conducted randomized trials” before observational studies are put into practice, Dr. Goodwin and her team said.
However, the investigators cautioned against extrapolating their results to patients with diabetes, noting that “because metformin is effective in type 2 diabetes, the results ... should not affect the use of metformin” in breast cancer patients who have diabetes.
The study was published online in JAMA.
Patients were enrolled from 2010 to 2013 while undergoing adjuvant treatment – chemotherapy, radiotherapy, hormone therapy, and/or others – following complete resection of T1-3, N0-3 tumors. They were almost exclusively women (mean age, 52.4 years), and almost 90% were non-Hispanic White. They were primarily from the United States and Canada, with some patients from the United Kingdom and Switzerland.
Patients were randomly assigned equally to receive either metformin 850 mg twice daily or placebo for 5 years. Median follow-up was about 8 years.
Among 2,533 patients with estrogen receptor– and/or progesterone receptor–positive disease, the incidence of invasive disease–free survival events was 2.78 per 100 patient-years in the metformin group, vs. 2.74 per 100 patient-years in the placebo arm (hazard ratio [HR], 1.01, P = .93). There were 1.46 deaths per 100 patient-years with metformin, vs. 1.32 with placebo (HR, 1.10, P = .47).
Metformin was stopped early at about 3 years for the 1,116 hormone receptor–negative patients after futility was declared on interim analysis. The incidence of invasive disease–free survival events was 3.58 with metformin, vs. 3.60 with placebo per 100 patient-years (HR, 1.01, P = .92). There were 1.91 deaths per 100 patient-years in the metformin arm, vs. 2.15 in the group that received placebo (HR, 0.89, P = .46).
However, the findings were different and suggested a signal among the small subset of patients (17% of the total) who had HER2-positive disease. There were 1.93 disease-free survival events with metformin per 100 patient-years, vs. 3.05 events with placebo (HR, 0.64, P = .03), and 0.78 deaths in the metformin arm, vs. 1.43 deaths per 100 patient-years in the placebo arm (HR, 0.54, P = .04).
The benefit seen in this HER2-postive subgroup was limited to patients with any C allele of the rs11212617 single-nucleotide variant.
This was an exploratory analysis, so the results need to be confirmed in a randomized trial, but it’s possible that metformin “could provide an additional treatment option for HER2-positive breast cancer,” Dr. Goodwin said.
Grade 3 or higher adverse events were more common with metformin (21.5% vs. 17.5%). The most common such events were hypertension (2.4% vs. 1.9%), irregular menses (1.5% vs. 1.4%), and diarrhea (1.9% vs. 0.8%).
The study was conducted by the Canadian Cancer Trials Group and was funded by the Canadian Cancer Society, the National Cancer Institute, and others. Dr. Goodwin has disclosed no relevant financial relationships. Several coauthors reported ties to Pfizer, Eli Lilly, Roche, and a number of other companies. One coauthor is an AstraZeneca employee.
A version of this article first appeared on Medscape.com.
Metformin, a common option for patients with type 2 diabetes, had previously been shown in observational studies to be associated with improved survival of cancer patients. Those studies mostly involved older patients with cancer who also had diabetes.
These findings have led to trials of the use of metformin for patients with cancer who do not have diabetes, but two lung cancer trials found no effect on survival.
Now this latest trial in breast cancer, which included 3,649 patients with hormone receptor–positive or –negative disease – who did not have diabetes – also found that metformin had no effect on survival.
These results “tell us that metformin is not effective against the most common types of breast cancer and any off-label use [of] this drug for the treatment of these common types of breast cancer should be stopped,” lead investigator and medical oncologist Pamela Goodwin, MD, a breast cancer researcher at the Lunenfeld-Tanenbaum Research Institute in Toronto, said in a press release.
The negative results “underscore the need for well-conducted randomized trials” before observational studies are put into practice, Dr. Goodwin and her team said.
However, the investigators cautioned against extrapolating their results to patients with diabetes, noting that “because metformin is effective in type 2 diabetes, the results ... should not affect the use of metformin” in breast cancer patients who have diabetes.
The study was published online in JAMA.
Patients were enrolled from 2010 to 2013 while undergoing adjuvant treatment – chemotherapy, radiotherapy, hormone therapy, and/or others – following complete resection of T1-3, N0-3 tumors. They were almost exclusively women (mean age, 52.4 years), and almost 90% were non-Hispanic White. They were primarily from the United States and Canada, with some patients from the United Kingdom and Switzerland.
Patients were randomly assigned equally to receive either metformin 850 mg twice daily or placebo for 5 years. Median follow-up was about 8 years.
Among 2,533 patients with estrogen receptor– and/or progesterone receptor–positive disease, the incidence of invasive disease–free survival events was 2.78 per 100 patient-years in the metformin group, vs. 2.74 per 100 patient-years in the placebo arm (hazard ratio [HR], 1.01, P = .93). There were 1.46 deaths per 100 patient-years with metformin, vs. 1.32 with placebo (HR, 1.10, P = .47).
Metformin was stopped early at about 3 years for the 1,116 hormone receptor–negative patients after futility was declared on interim analysis. The incidence of invasive disease–free survival events was 3.58 with metformin, vs. 3.60 with placebo per 100 patient-years (HR, 1.01, P = .92). There were 1.91 deaths per 100 patient-years in the metformin arm, vs. 2.15 in the group that received placebo (HR, 0.89, P = .46).
However, the findings were different and suggested a signal among the small subset of patients (17% of the total) who had HER2-positive disease. There were 1.93 disease-free survival events with metformin per 100 patient-years, vs. 3.05 events with placebo (HR, 0.64, P = .03), and 0.78 deaths in the metformin arm, vs. 1.43 deaths per 100 patient-years in the placebo arm (HR, 0.54, P = .04).
The benefit seen in this HER2-postive subgroup was limited to patients with any C allele of the rs11212617 single-nucleotide variant.
This was an exploratory analysis, so the results need to be confirmed in a randomized trial, but it’s possible that metformin “could provide an additional treatment option for HER2-positive breast cancer,” Dr. Goodwin said.
Grade 3 or higher adverse events were more common with metformin (21.5% vs. 17.5%). The most common such events were hypertension (2.4% vs. 1.9%), irregular menses (1.5% vs. 1.4%), and diarrhea (1.9% vs. 0.8%).
The study was conducted by the Canadian Cancer Trials Group and was funded by the Canadian Cancer Society, the National Cancer Institute, and others. Dr. Goodwin has disclosed no relevant financial relationships. Several coauthors reported ties to Pfizer, Eli Lilly, Roche, and a number of other companies. One coauthor is an AstraZeneca employee.
A version of this article first appeared on Medscape.com.
Metformin, a common option for patients with type 2 diabetes, had previously been shown in observational studies to be associated with improved survival of cancer patients. Those studies mostly involved older patients with cancer who also had diabetes.
These findings have led to trials of the use of metformin for patients with cancer who do not have diabetes, but two lung cancer trials found no effect on survival.
Now this latest trial in breast cancer, which included 3,649 patients with hormone receptor–positive or –negative disease – who did not have diabetes – also found that metformin had no effect on survival.
These results “tell us that metformin is not effective against the most common types of breast cancer and any off-label use [of] this drug for the treatment of these common types of breast cancer should be stopped,” lead investigator and medical oncologist Pamela Goodwin, MD, a breast cancer researcher at the Lunenfeld-Tanenbaum Research Institute in Toronto, said in a press release.
The negative results “underscore the need for well-conducted randomized trials” before observational studies are put into practice, Dr. Goodwin and her team said.
However, the investigators cautioned against extrapolating their results to patients with diabetes, noting that “because metformin is effective in type 2 diabetes, the results ... should not affect the use of metformin” in breast cancer patients who have diabetes.
The study was published online in JAMA.
Patients were enrolled from 2010 to 2013 while undergoing adjuvant treatment – chemotherapy, radiotherapy, hormone therapy, and/or others – following complete resection of T1-3, N0-3 tumors. They were almost exclusively women (mean age, 52.4 years), and almost 90% were non-Hispanic White. They were primarily from the United States and Canada, with some patients from the United Kingdom and Switzerland.
Patients were randomly assigned equally to receive either metformin 850 mg twice daily or placebo for 5 years. Median follow-up was about 8 years.
Among 2,533 patients with estrogen receptor– and/or progesterone receptor–positive disease, the incidence of invasive disease–free survival events was 2.78 per 100 patient-years in the metformin group, vs. 2.74 per 100 patient-years in the placebo arm (hazard ratio [HR], 1.01, P = .93). There were 1.46 deaths per 100 patient-years with metformin, vs. 1.32 with placebo (HR, 1.10, P = .47).
Metformin was stopped early at about 3 years for the 1,116 hormone receptor–negative patients after futility was declared on interim analysis. The incidence of invasive disease–free survival events was 3.58 with metformin, vs. 3.60 with placebo per 100 patient-years (HR, 1.01, P = .92). There were 1.91 deaths per 100 patient-years in the metformin arm, vs. 2.15 in the group that received placebo (HR, 0.89, P = .46).
However, the findings were different and suggested a signal among the small subset of patients (17% of the total) who had HER2-positive disease. There were 1.93 disease-free survival events with metformin per 100 patient-years, vs. 3.05 events with placebo (HR, 0.64, P = .03), and 0.78 deaths in the metformin arm, vs. 1.43 deaths per 100 patient-years in the placebo arm (HR, 0.54, P = .04).
The benefit seen in this HER2-postive subgroup was limited to patients with any C allele of the rs11212617 single-nucleotide variant.
This was an exploratory analysis, so the results need to be confirmed in a randomized trial, but it’s possible that metformin “could provide an additional treatment option for HER2-positive breast cancer,” Dr. Goodwin said.
Grade 3 or higher adverse events were more common with metformin (21.5% vs. 17.5%). The most common such events were hypertension (2.4% vs. 1.9%), irregular menses (1.5% vs. 1.4%), and diarrhea (1.9% vs. 0.8%).
The study was conducted by the Canadian Cancer Trials Group and was funded by the Canadian Cancer Society, the National Cancer Institute, and others. Dr. Goodwin has disclosed no relevant financial relationships. Several coauthors reported ties to Pfizer, Eli Lilly, Roche, and a number of other companies. One coauthor is an AstraZeneca employee.
A version of this article first appeared on Medscape.com.
Births jump for first time since 2014
More than 3 million live births occurred in the United States in 2021, the largest increase in the nation’s birth rate since 2014, according to the U.S. Centers for Disease Control and Prevention.
Provisional data showed a 1% uptick in births, to 3.66 million, after 6 years of dropping by approximately 2% per year. The gains were concentrated among birthing people ages 25 and older. Teenage births, on the other hand, are at their lowest level since the 1990s, according to the CDC. The agency reported a record 6% decrease in births for teenagers aged 15 to 19 years between 2020 and 2021. Women ages 20 to 25 years also had a record decrease in births of 4% during that period.
Brady E. Hamilton, PhD, of the CDC’s National Center for Health Statistics, and the lead author of the new report, said the rise in births points to childbearing that was postponed during the pandemic. Data from 2021 showed a 4% drop in the nation’s birth rate between 2019 and 2020.
“The option to forgo birth is not always viable for older women, but you saw a lot of that during the pandemic,” Dr. Hamilton said. “Events happened related to job security and the economy that caused people to wait to have a child.”
Dr. Hamilton said more data are needed to determine the full impact of increased overall birth rates on individuals. The final report, which will be released in July, will delve deeper into the influence increased birth rates had on demographics and preterm births, which Dr. Hamilton and his team found have increased by 4%.
“For those beginning to have children, we see these trends, but it will be interesting to see what happens to younger women in the future,” Dr. Hamilton said. “Once we have the final data for 2021, we will be able to see a more detailed pattern emerge and draw conclusions from that.”
Dr. Hamilton has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 million live births occurred in the United States in 2021, the largest increase in the nation’s birth rate since 2014, according to the U.S. Centers for Disease Control and Prevention.
Provisional data showed a 1% uptick in births, to 3.66 million, after 6 years of dropping by approximately 2% per year. The gains were concentrated among birthing people ages 25 and older. Teenage births, on the other hand, are at their lowest level since the 1990s, according to the CDC. The agency reported a record 6% decrease in births for teenagers aged 15 to 19 years between 2020 and 2021. Women ages 20 to 25 years also had a record decrease in births of 4% during that period.
Brady E. Hamilton, PhD, of the CDC’s National Center for Health Statistics, and the lead author of the new report, said the rise in births points to childbearing that was postponed during the pandemic. Data from 2021 showed a 4% drop in the nation’s birth rate between 2019 and 2020.
“The option to forgo birth is not always viable for older women, but you saw a lot of that during the pandemic,” Dr. Hamilton said. “Events happened related to job security and the economy that caused people to wait to have a child.”
Dr. Hamilton said more data are needed to determine the full impact of increased overall birth rates on individuals. The final report, which will be released in July, will delve deeper into the influence increased birth rates had on demographics and preterm births, which Dr. Hamilton and his team found have increased by 4%.
“For those beginning to have children, we see these trends, but it will be interesting to see what happens to younger women in the future,” Dr. Hamilton said. “Once we have the final data for 2021, we will be able to see a more detailed pattern emerge and draw conclusions from that.”
Dr. Hamilton has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 million live births occurred in the United States in 2021, the largest increase in the nation’s birth rate since 2014, according to the U.S. Centers for Disease Control and Prevention.
Provisional data showed a 1% uptick in births, to 3.66 million, after 6 years of dropping by approximately 2% per year. The gains were concentrated among birthing people ages 25 and older. Teenage births, on the other hand, are at their lowest level since the 1990s, according to the CDC. The agency reported a record 6% decrease in births for teenagers aged 15 to 19 years between 2020 and 2021. Women ages 20 to 25 years also had a record decrease in births of 4% during that period.
Brady E. Hamilton, PhD, of the CDC’s National Center for Health Statistics, and the lead author of the new report, said the rise in births points to childbearing that was postponed during the pandemic. Data from 2021 showed a 4% drop in the nation’s birth rate between 2019 and 2020.
“The option to forgo birth is not always viable for older women, but you saw a lot of that during the pandemic,” Dr. Hamilton said. “Events happened related to job security and the economy that caused people to wait to have a child.”
Dr. Hamilton said more data are needed to determine the full impact of increased overall birth rates on individuals. The final report, which will be released in July, will delve deeper into the influence increased birth rates had on demographics and preterm births, which Dr. Hamilton and his team found have increased by 4%.
“For those beginning to have children, we see these trends, but it will be interesting to see what happens to younger women in the future,” Dr. Hamilton said. “Once we have the final data for 2021, we will be able to see a more detailed pattern emerge and draw conclusions from that.”
Dr. Hamilton has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Your grandmother, the metabolic influencer
“Grandma’s workouts may have made you healthier.” The title and accompanying photo of a pair of well-worn women’s running shoes caught my eye immediately. For whatever reason, we are a family of exercisers. My wife has competed in several triathlons and won two of them. With her I have cycled across the United States. It has not surprised us that all three of our children have run at least one marathon. I have always viewed their continued devotion to an active lifestyle and their healthy bodies as a tribute to the benefits of our attempts at parenting by example. We certainly didn’t coach them, lecture them, or run family boot camps on weekends and school vacations.
I had never really given much thought as to whether their grandparents also may have played any role in their affinity for physical activity until I read that article. Apparently, my mother was a gifted athlete as a young woman. I have seen photos of her playing tennis, skiing, and diving and heard stories, but I never saw her do any of these activities except a single perfect swan dive when I must have been 8 or 9 years old.
Similarly, scrapbooks reveal that my mother-in-law had an active sports life in high school. But we never saw any evidence of her athletic activity save a devotion to a gentle backstroke in the cold Maine waters during the summer. My wife and I and our children never saw these grandmothers do anything more sporting or physically taxing than single-handedly preparing a full Thanksgiving dinner. How could their exercise habits have influenced the health of their grandchildren?
A team of researchers at the Joslin Diabetes Center in Boston found that female mice who were given the opportunity to exercise produced offspring that had lower fat mass, higher bone mineral density, and insulin levels usually associated with a lower risk of type 2 diabetes. And, in a bit of a surprise, the next generation of offspring accrued a similar benefit even though its mothers were not exercising. The role of exercise in the fathers was eliminated by experimental design.
So it appears that the first-generation offspring’s gametes and hence the third generation was being exposed in utero to something generated by the grandmothers’ exercise. It does not appear to be a behavior pattern that is passed on. It may have to do with epigenetics. Searching for this unknown factor is ongoing and broad based.
Obviously, similar studies in humans are not on the drawing board. Our reproductive cycle is significantly longer than the 2 years of the mouse. However, looking at their current data, the researchers feel comfortable encouraging a mother to exercise during pregnancy as long as it is compatible with the particulars of her obstetrical course. It would be unkind and without basis in fact to blame your mother’s or your mother-in-law’s sedentary behavior for your child’s poor metabolic health. However, it is reasonable to point out to women considering pregnancy that, in addition to avoiding alcohol and smoking, a good dose of exercise during pregnancy will benefit their children. You can point out that it may even benefit their grandchildren. And of course, once the baby is born and a mother feels comfortable returning to her exercise regime, she should go for it. Remind her also that parenting by example is still the best way to do it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
“Grandma’s workouts may have made you healthier.” The title and accompanying photo of a pair of well-worn women’s running shoes caught my eye immediately. For whatever reason, we are a family of exercisers. My wife has competed in several triathlons and won two of them. With her I have cycled across the United States. It has not surprised us that all three of our children have run at least one marathon. I have always viewed their continued devotion to an active lifestyle and their healthy bodies as a tribute to the benefits of our attempts at parenting by example. We certainly didn’t coach them, lecture them, or run family boot camps on weekends and school vacations.
I had never really given much thought as to whether their grandparents also may have played any role in their affinity for physical activity until I read that article. Apparently, my mother was a gifted athlete as a young woman. I have seen photos of her playing tennis, skiing, and diving and heard stories, but I never saw her do any of these activities except a single perfect swan dive when I must have been 8 or 9 years old.
Similarly, scrapbooks reveal that my mother-in-law had an active sports life in high school. But we never saw any evidence of her athletic activity save a devotion to a gentle backstroke in the cold Maine waters during the summer. My wife and I and our children never saw these grandmothers do anything more sporting or physically taxing than single-handedly preparing a full Thanksgiving dinner. How could their exercise habits have influenced the health of their grandchildren?
A team of researchers at the Joslin Diabetes Center in Boston found that female mice who were given the opportunity to exercise produced offspring that had lower fat mass, higher bone mineral density, and insulin levels usually associated with a lower risk of type 2 diabetes. And, in a bit of a surprise, the next generation of offspring accrued a similar benefit even though its mothers were not exercising. The role of exercise in the fathers was eliminated by experimental design.
So it appears that the first-generation offspring’s gametes and hence the third generation was being exposed in utero to something generated by the grandmothers’ exercise. It does not appear to be a behavior pattern that is passed on. It may have to do with epigenetics. Searching for this unknown factor is ongoing and broad based.
Obviously, similar studies in humans are not on the drawing board. Our reproductive cycle is significantly longer than the 2 years of the mouse. However, looking at their current data, the researchers feel comfortable encouraging a mother to exercise during pregnancy as long as it is compatible with the particulars of her obstetrical course. It would be unkind and without basis in fact to blame your mother’s or your mother-in-law’s sedentary behavior for your child’s poor metabolic health. However, it is reasonable to point out to women considering pregnancy that, in addition to avoiding alcohol and smoking, a good dose of exercise during pregnancy will benefit their children. You can point out that it may even benefit their grandchildren. And of course, once the baby is born and a mother feels comfortable returning to her exercise regime, she should go for it. Remind her also that parenting by example is still the best way to do it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
“Grandma’s workouts may have made you healthier.” The title and accompanying photo of a pair of well-worn women’s running shoes caught my eye immediately. For whatever reason, we are a family of exercisers. My wife has competed in several triathlons and won two of them. With her I have cycled across the United States. It has not surprised us that all three of our children have run at least one marathon. I have always viewed their continued devotion to an active lifestyle and their healthy bodies as a tribute to the benefits of our attempts at parenting by example. We certainly didn’t coach them, lecture them, or run family boot camps on weekends and school vacations.
I had never really given much thought as to whether their grandparents also may have played any role in their affinity for physical activity until I read that article. Apparently, my mother was a gifted athlete as a young woman. I have seen photos of her playing tennis, skiing, and diving and heard stories, but I never saw her do any of these activities except a single perfect swan dive when I must have been 8 or 9 years old.
Similarly, scrapbooks reveal that my mother-in-law had an active sports life in high school. But we never saw any evidence of her athletic activity save a devotion to a gentle backstroke in the cold Maine waters during the summer. My wife and I and our children never saw these grandmothers do anything more sporting or physically taxing than single-handedly preparing a full Thanksgiving dinner. How could their exercise habits have influenced the health of their grandchildren?
A team of researchers at the Joslin Diabetes Center in Boston found that female mice who were given the opportunity to exercise produced offspring that had lower fat mass, higher bone mineral density, and insulin levels usually associated with a lower risk of type 2 diabetes. And, in a bit of a surprise, the next generation of offspring accrued a similar benefit even though its mothers were not exercising. The role of exercise in the fathers was eliminated by experimental design.
So it appears that the first-generation offspring’s gametes and hence the third generation was being exposed in utero to something generated by the grandmothers’ exercise. It does not appear to be a behavior pattern that is passed on. It may have to do with epigenetics. Searching for this unknown factor is ongoing and broad based.
Obviously, similar studies in humans are not on the drawing board. Our reproductive cycle is significantly longer than the 2 years of the mouse. However, looking at their current data, the researchers feel comfortable encouraging a mother to exercise during pregnancy as long as it is compatible with the particulars of her obstetrical course. It would be unkind and without basis in fact to blame your mother’s or your mother-in-law’s sedentary behavior for your child’s poor metabolic health. However, it is reasonable to point out to women considering pregnancy that, in addition to avoiding alcohol and smoking, a good dose of exercise during pregnancy will benefit their children. You can point out that it may even benefit their grandchildren. And of course, once the baby is born and a mother feels comfortable returning to her exercise regime, she should go for it. Remind her also that parenting by example is still the best way to do it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Children and COVID: Weekly cases keep rising past 100,000
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
Müllerian anomalies – old problem, new approach and classification
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at [email protected].
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at [email protected].
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at [email protected].
Vibrating pill counters constipation
A swallowable, vibrating capsule improved symptoms among patients with chronic idiopathic constipation in a phase 3 multicenter, randomized, controlled trial. The method represents a mechanical approach to the treatment of constipation.
The swallowable pill acts by vibrating during passage through the gut, where it is thought to augment colonic biorhythm and peristalsis. Traditional treatments for constipation generally increase motility or secretion.
“That’s how we have been managing constipation since time immemorial. Now we have come up with this novel approach, where there’s a pill that is designed to increase local oscillations, and probably induce local contractions of the colon to mimic what happens normally,” said Satish Rao, MD, PhD, who presented the results of the trial at the annual Digestive Disease Week® (DDW).
“We’re now seeing that local stimulation works, and the other neat thing seems to be a lack of side effects, which is really a huge plus. I think it will benefit people with both occasional or chronic constipation,” said Dr. Rao, professor of medicine at Medical College of Georgia, Augusta.
The capsules activate for two stimulation cycles, each lasting for about 2 hours. The cycle includes 3 seconds of vibration followed by 16 seconds of rest.
The researchers conducted a study with two active arms and a placebo. It included 312 patients age 22 or older who had an average of between 1 and 2.5 spontaneous bowel movements (SBM) per week.
Treatment lasted for 8 weeks, with patients ingesting a capsule between 9 and 10 p.m. In one treatment group, the device was activated at 6 a.m., and in the other group at about 2 p.m.
The placebo and treatment groups had similar baseline characteristics, except for a longer duration of constipation in the treatment groups (17.9 versus 14.5 years; P = .0253). In an intention-to-treat analysis, the treatment groups were more likely to achieve an increase of one complete SBM per week (39.26% versus 22.15%; P < .0001) and an increase of two complete SBMs per week (22.7% versus 11.41%; P < .0006).
The capsules also improved straining score, stool consistency, and quality of life. There was no significant difference between treatment and placebo groups with respect to bloating or rescue medication use.
The product had few adverse effects. The most common was a vibrating sensation or discomfort (11.0%, versus none in the placebo group).
Dr. Rao expects that the treatment could be widely applicable since many constipation patients don’t gain sufficient benefit from existing treatments, or find side effects intolerable.
Another benefit is that the therapy’s mimicry of natural cycles appears to grant patients more control of bowel movements. Laxatives and other pharmaceutical interventions may prompt the patient to go to the bathroom within an hour or two, but patients in the trial reported bowel movements at predictable times.
However, he noted that the pill is nondissolvable, which would make it contraindicated for patients who have had previous gut surgeries or narrowing of the gut. He noted that the sponsoring company, Vibrant Gastro, expects to obtain Food and Drug Administration approval by the end of 2022.
The results of the study were well received. “I think it’s an exciting new approach for managing patients with chronic constipation. It was a large sample size, and the treatment seems to be well tolerated. It may offer a promising option for patients who have not responded to many other medications,” said Adil E. Bharucha, MD, who comoderated the session where the research was presented.
However, he pointed out that the presentation did not indicate how many of the patients had previously tried other therapies. “We’d like to see the full paper, which will provide a better understanding of the role of this treatment in practice down the road,” said Dr. Bharucha, professor of medicine in the division of gastroenterology and hepatology and director of the office of clinical trials at Mayo Clinic, Rochester, Minn.
The capsule may not work for everyone, said Dr. Bharucha. He suspects that many refractory patients have an issue with pelvic floor muscles, which may restrict stool evacuation. “You wouldn’t expect those people to respond optimally to a laxative and I suspect perhaps not to a capsule, either. I think defecatory disorders are substantially underdiagnosed in patients who don’t respond to laxatives,” said Dr. Bharucha.
Asked why the capsule might benefit patients who don’t improve with laxatives, Dr. Bharucha responded: “I think we need more studies to understand how the capsule works.”
Dr. Rao consults for Vibrant Gastro. Dr. Bharucha has no relevant financial disclosures.
A swallowable, vibrating capsule improved symptoms among patients with chronic idiopathic constipation in a phase 3 multicenter, randomized, controlled trial. The method represents a mechanical approach to the treatment of constipation.
The swallowable pill acts by vibrating during passage through the gut, where it is thought to augment colonic biorhythm and peristalsis. Traditional treatments for constipation generally increase motility or secretion.
“That’s how we have been managing constipation since time immemorial. Now we have come up with this novel approach, where there’s a pill that is designed to increase local oscillations, and probably induce local contractions of the colon to mimic what happens normally,” said Satish Rao, MD, PhD, who presented the results of the trial at the annual Digestive Disease Week® (DDW).
“We’re now seeing that local stimulation works, and the other neat thing seems to be a lack of side effects, which is really a huge plus. I think it will benefit people with both occasional or chronic constipation,” said Dr. Rao, professor of medicine at Medical College of Georgia, Augusta.
The capsules activate for two stimulation cycles, each lasting for about 2 hours. The cycle includes 3 seconds of vibration followed by 16 seconds of rest.
The researchers conducted a study with two active arms and a placebo. It included 312 patients age 22 or older who had an average of between 1 and 2.5 spontaneous bowel movements (SBM) per week.
Treatment lasted for 8 weeks, with patients ingesting a capsule between 9 and 10 p.m. In one treatment group, the device was activated at 6 a.m., and in the other group at about 2 p.m.
The placebo and treatment groups had similar baseline characteristics, except for a longer duration of constipation in the treatment groups (17.9 versus 14.5 years; P = .0253). In an intention-to-treat analysis, the treatment groups were more likely to achieve an increase of one complete SBM per week (39.26% versus 22.15%; P < .0001) and an increase of two complete SBMs per week (22.7% versus 11.41%; P < .0006).
The capsules also improved straining score, stool consistency, and quality of life. There was no significant difference between treatment and placebo groups with respect to bloating or rescue medication use.
The product had few adverse effects. The most common was a vibrating sensation or discomfort (11.0%, versus none in the placebo group).
Dr. Rao expects that the treatment could be widely applicable since many constipation patients don’t gain sufficient benefit from existing treatments, or find side effects intolerable.
Another benefit is that the therapy’s mimicry of natural cycles appears to grant patients more control of bowel movements. Laxatives and other pharmaceutical interventions may prompt the patient to go to the bathroom within an hour or two, but patients in the trial reported bowel movements at predictable times.
However, he noted that the pill is nondissolvable, which would make it contraindicated for patients who have had previous gut surgeries or narrowing of the gut. He noted that the sponsoring company, Vibrant Gastro, expects to obtain Food and Drug Administration approval by the end of 2022.
The results of the study were well received. “I think it’s an exciting new approach for managing patients with chronic constipation. It was a large sample size, and the treatment seems to be well tolerated. It may offer a promising option for patients who have not responded to many other medications,” said Adil E. Bharucha, MD, who comoderated the session where the research was presented.
However, he pointed out that the presentation did not indicate how many of the patients had previously tried other therapies. “We’d like to see the full paper, which will provide a better understanding of the role of this treatment in practice down the road,” said Dr. Bharucha, professor of medicine in the division of gastroenterology and hepatology and director of the office of clinical trials at Mayo Clinic, Rochester, Minn.
The capsule may not work for everyone, said Dr. Bharucha. He suspects that many refractory patients have an issue with pelvic floor muscles, which may restrict stool evacuation. “You wouldn’t expect those people to respond optimally to a laxative and I suspect perhaps not to a capsule, either. I think defecatory disorders are substantially underdiagnosed in patients who don’t respond to laxatives,” said Dr. Bharucha.
Asked why the capsule might benefit patients who don’t improve with laxatives, Dr. Bharucha responded: “I think we need more studies to understand how the capsule works.”
Dr. Rao consults for Vibrant Gastro. Dr. Bharucha has no relevant financial disclosures.
A swallowable, vibrating capsule improved symptoms among patients with chronic idiopathic constipation in a phase 3 multicenter, randomized, controlled trial. The method represents a mechanical approach to the treatment of constipation.
The swallowable pill acts by vibrating during passage through the gut, where it is thought to augment colonic biorhythm and peristalsis. Traditional treatments for constipation generally increase motility or secretion.
“That’s how we have been managing constipation since time immemorial. Now we have come up with this novel approach, where there’s a pill that is designed to increase local oscillations, and probably induce local contractions of the colon to mimic what happens normally,” said Satish Rao, MD, PhD, who presented the results of the trial at the annual Digestive Disease Week® (DDW).
“We’re now seeing that local stimulation works, and the other neat thing seems to be a lack of side effects, which is really a huge plus. I think it will benefit people with both occasional or chronic constipation,” said Dr. Rao, professor of medicine at Medical College of Georgia, Augusta.
The capsules activate for two stimulation cycles, each lasting for about 2 hours. The cycle includes 3 seconds of vibration followed by 16 seconds of rest.
The researchers conducted a study with two active arms and a placebo. It included 312 patients age 22 or older who had an average of between 1 and 2.5 spontaneous bowel movements (SBM) per week.
Treatment lasted for 8 weeks, with patients ingesting a capsule between 9 and 10 p.m. In one treatment group, the device was activated at 6 a.m., and in the other group at about 2 p.m.
The placebo and treatment groups had similar baseline characteristics, except for a longer duration of constipation in the treatment groups (17.9 versus 14.5 years; P = .0253). In an intention-to-treat analysis, the treatment groups were more likely to achieve an increase of one complete SBM per week (39.26% versus 22.15%; P < .0001) and an increase of two complete SBMs per week (22.7% versus 11.41%; P < .0006).
The capsules also improved straining score, stool consistency, and quality of life. There was no significant difference between treatment and placebo groups with respect to bloating or rescue medication use.
The product had few adverse effects. The most common was a vibrating sensation or discomfort (11.0%, versus none in the placebo group).
Dr. Rao expects that the treatment could be widely applicable since many constipation patients don’t gain sufficient benefit from existing treatments, or find side effects intolerable.
Another benefit is that the therapy’s mimicry of natural cycles appears to grant patients more control of bowel movements. Laxatives and other pharmaceutical interventions may prompt the patient to go to the bathroom within an hour or two, but patients in the trial reported bowel movements at predictable times.
However, he noted that the pill is nondissolvable, which would make it contraindicated for patients who have had previous gut surgeries or narrowing of the gut. He noted that the sponsoring company, Vibrant Gastro, expects to obtain Food and Drug Administration approval by the end of 2022.
The results of the study were well received. “I think it’s an exciting new approach for managing patients with chronic constipation. It was a large sample size, and the treatment seems to be well tolerated. It may offer a promising option for patients who have not responded to many other medications,” said Adil E. Bharucha, MD, who comoderated the session where the research was presented.
However, he pointed out that the presentation did not indicate how many of the patients had previously tried other therapies. “We’d like to see the full paper, which will provide a better understanding of the role of this treatment in practice down the road,” said Dr. Bharucha, professor of medicine in the division of gastroenterology and hepatology and director of the office of clinical trials at Mayo Clinic, Rochester, Minn.
The capsule may not work for everyone, said Dr. Bharucha. He suspects that many refractory patients have an issue with pelvic floor muscles, which may restrict stool evacuation. “You wouldn’t expect those people to respond optimally to a laxative and I suspect perhaps not to a capsule, either. I think defecatory disorders are substantially underdiagnosed in patients who don’t respond to laxatives,” said Dr. Bharucha.
Asked why the capsule might benefit patients who don’t improve with laxatives, Dr. Bharucha responded: “I think we need more studies to understand how the capsule works.”
Dr. Rao consults for Vibrant Gastro. Dr. Bharucha has no relevant financial disclosures.
FROM DDW 2022
FDA, AMA prepare for potential COVID-19 shots for children younger than 6
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.