User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
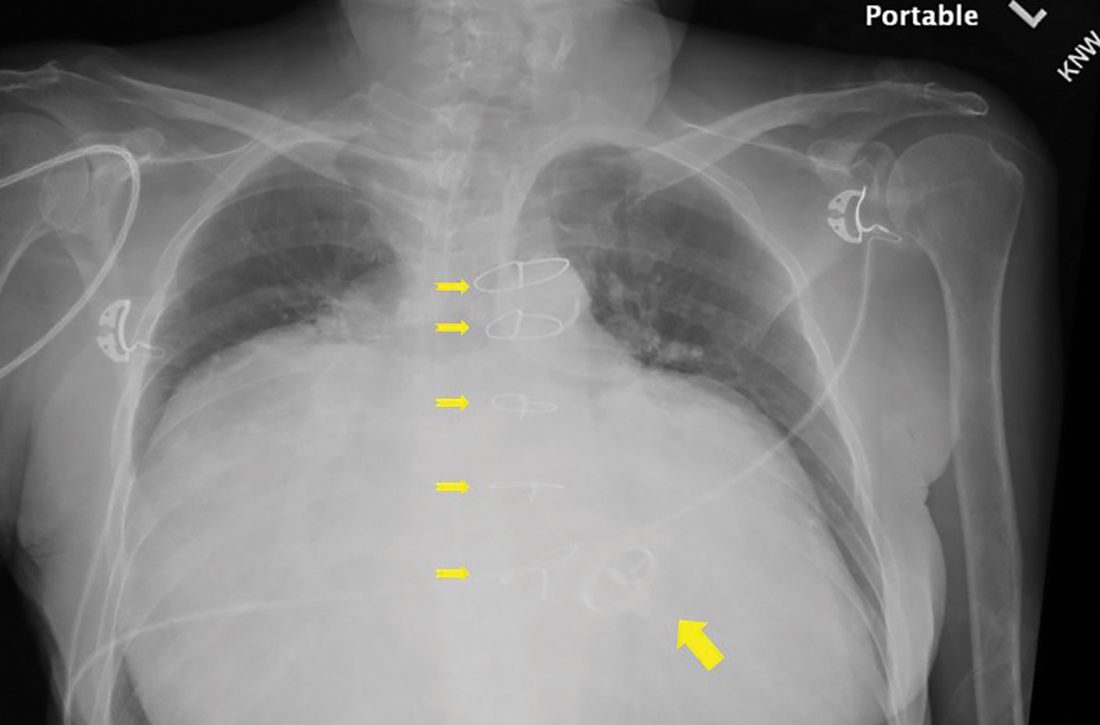
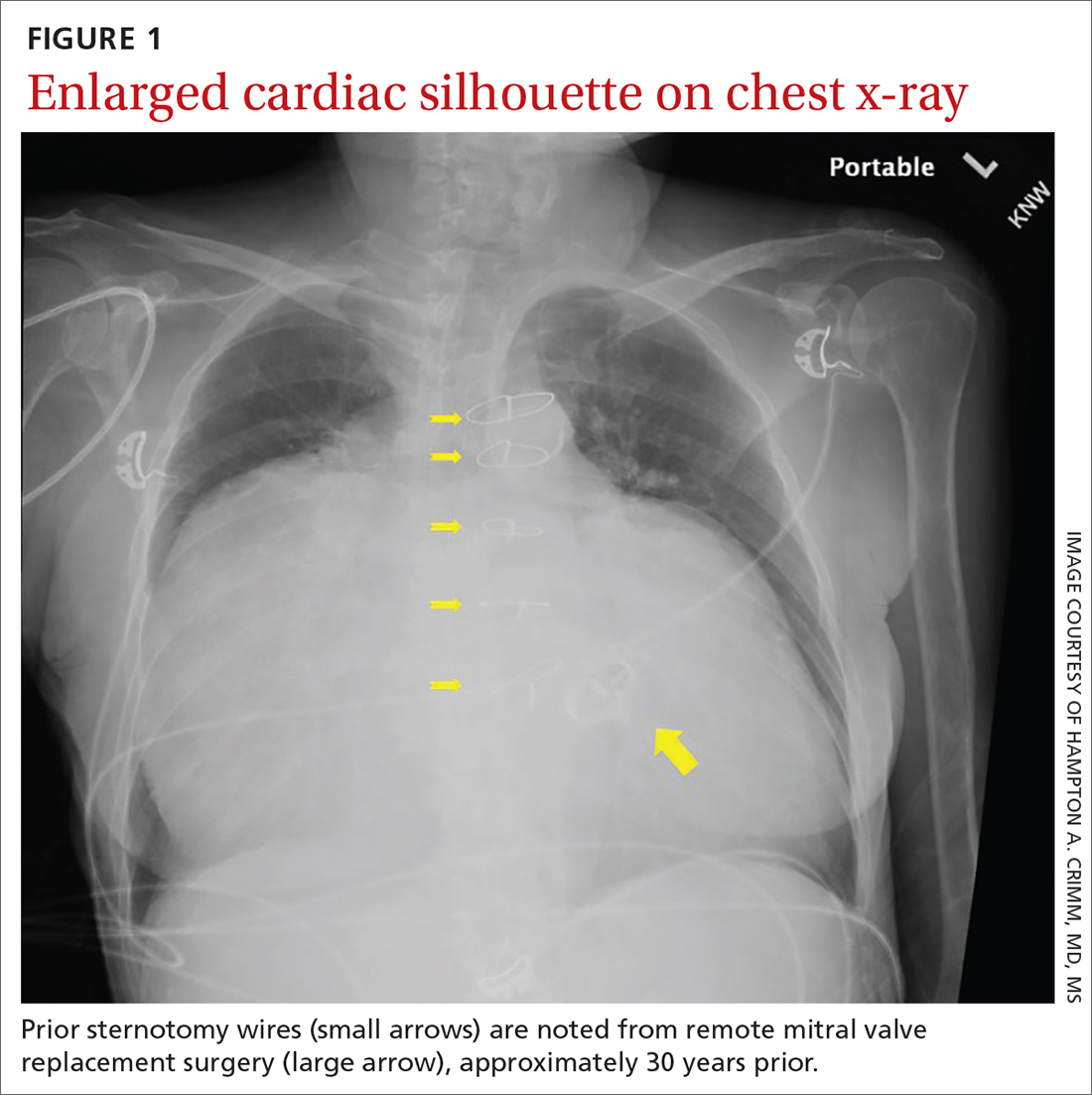
Substantially enlarged cardiac silhouette
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
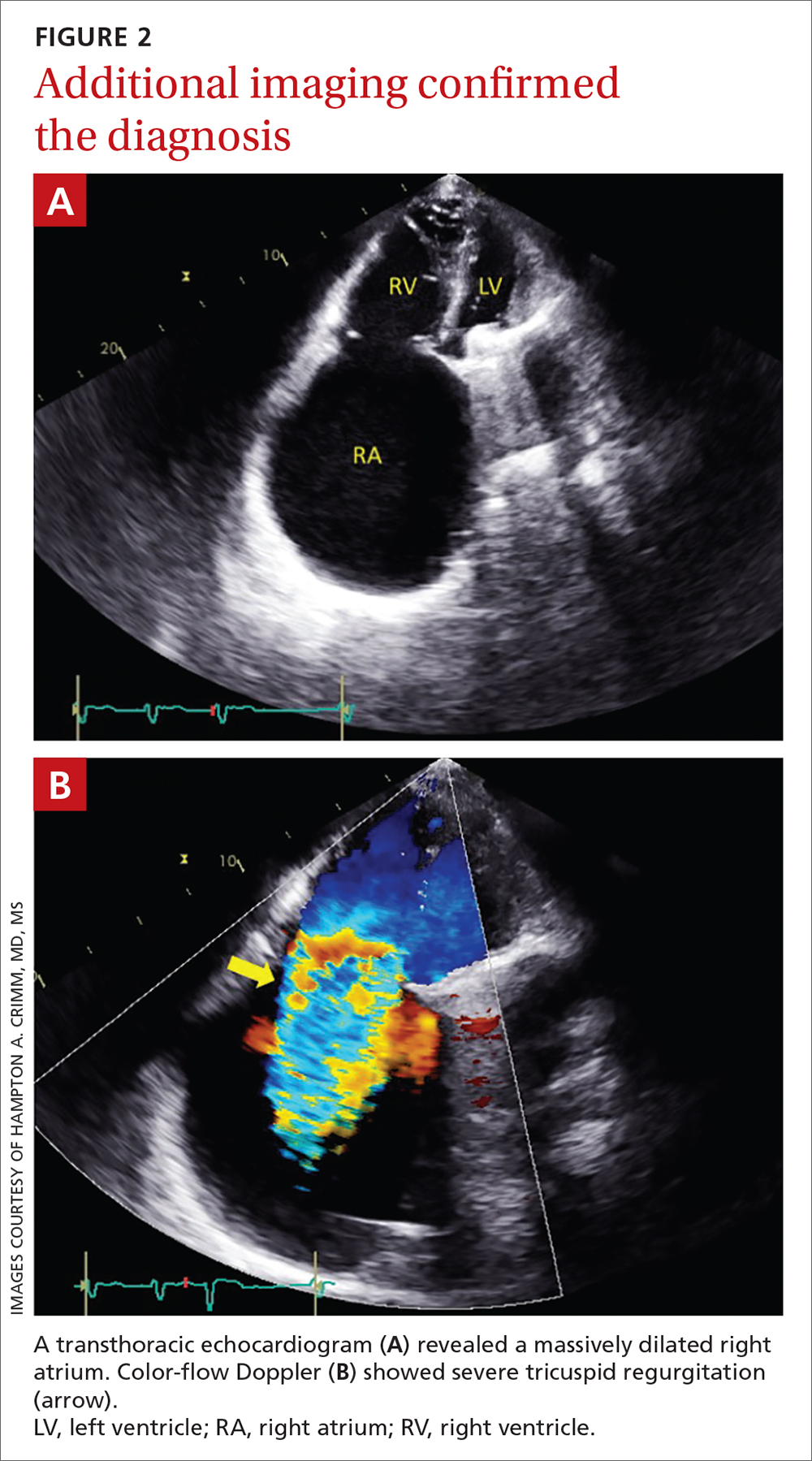
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.
RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.

RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.

RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
SARS-CoV-2 stays in GI tract long after it clears the lungs
New data present further evidence that SARS-CoV-2 infection can settle in the gastrointestinal (GI) tract and that it can persist long after the infection has cleared the lungs.
Infection of the GI tract may figure prominently in long COVID, the study authors suggest.
Led by Aravind Natarajan, PhD, with the departments of genetics and medicine at Stanford (Calif.) University, they analyzed fecal RNA shedding up to 10 months after a COVID-19 diagnosis in 673 stool samples from 113 patients with mild to moderate disease.
They found that in the week after diagnosis, COVID RNA remnants were present in the stool of approximately half (49.2%) of the patients. Seven months later, about 4% of them shed fecal viral RNA.
The authors note that there was no ongoing SARS-CoV-2 RNA shedding in respiratory samples of patients at the 4-month mark.
Using self-reported symptoms regularly collected by questionnaire, they also found a correlation of long-term fecal shedding of SARS-CoV-2 RNA with abdominal pain, nausea, and vomiting.
The findings were published online in Med.
Implications of long-term viral shedding
Previous studies have found SARS-CoV-2 RNA in respiratory and fecal samples and have documented viral replication in lung and intestinal tissue.
But before the current study, little had been known about long-term shedding, especially in those who have mild COVID. Most studies of viral shedding have been with severe COVID cases.
The authors note that most studies of this kind are cross-sectional. The few other longitudinal studies have focused on early time points just after diagnosis.
Senior author Ami S. Bhatt, MD, associate professor in the departments of medicine and hematology at Stanford, told this news organization that though the viral genetic material in the feces lingers, on the basis of available evidence, it is highly unlikely to be contagious in most cases.
She said that understanding the dynamics of fecal shedding of SARS-CoV-2 genetic material will help interpret wastewater-based studies that are trying to determine population prevalence of the virus.
“While we don’t know the exact clinical importance of the longer-term shedding of SARS-CoV-2 in individuals with COVID-19, some have speculated that those who have long-term shedding of SARS-CoV-2 may have ongoing infections that might benefit from treatment,” she said.
“Our data support the idea that the long-term GI-related symptoms in some people might be the consequence of an ongoing infection in the GI tract, even after the respiratory infection has cleared,” Dr. Bhatt said.
“Alternatively, the presence of ongoing viral genetic material in the gut might be a trigger for the immune system to continually be active against the virus, and our immune system reaction may be the reason for long-COVID type symptoms,” she added. “This area is ripe for additional studies.”
Dr. Bhatt and colleagues will continue studying viral shedding in fecal samples as part of the nationwide RECOVER Initiative.
When reached for comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology, Eastern Virginia Medical School, Norfolk, said in an interview that previous studies have indicated that the virus may be detected in the stool for a month or more and for about 2 weeks on average. Whether the virus is infectious has been in question.
But it’s not so much that the virus is infectious in the GI tract and causing symptoms, he said. Rather, there are biomic changes related to COVID, including a loss of diversity in the gut bacteria, which disrupts the balance.
“This may actually in some way predispose some patients to impaired clearance of their symptoms,” Dr. Johnson explained. “There seems to be a growing recognition that this entity called long-haul COVID may be related to specific bacterial disruptions, and the more rapidly you can resolve these disruptions, the less likely you are to continue with long-haul symptoms.”
He said that among people who have mild COVID, the virus typically clears and gut bacteria return to normal. With severe or persistent illness, gut dysbiosis persists, he said.
“People need to be aware that the GI tract is involved in a sizable percent of patients with COVID,” Dr. Johnson said. “The GI-tract testing may reflect that the virus is there, but persistence of the detectable test positivity is very unlikely to reflect active virus.”
The authors note in this study that they collected only six samples from the participants over the 10-month period.
“Follow-up studies with more frequent sampling, especially in the first 2 months after diagnosis, may help build a more nuanced model of decline of fecal viral RNA concentration over time,” they write.
The study was supported by a Stanford ChemH-IMA grant, fellowships from the AACR and the National Science Foundation, and the National Institutes of Health. The authors and Dr. Johnson report no relevant financial relationships. Dr. Johnson is a regular contributor to Medscape.
A version of this article first appeared to Medscape.com.
New data present further evidence that SARS-CoV-2 infection can settle in the gastrointestinal (GI) tract and that it can persist long after the infection has cleared the lungs.
Infection of the GI tract may figure prominently in long COVID, the study authors suggest.
Led by Aravind Natarajan, PhD, with the departments of genetics and medicine at Stanford (Calif.) University, they analyzed fecal RNA shedding up to 10 months after a COVID-19 diagnosis in 673 stool samples from 113 patients with mild to moderate disease.
They found that in the week after diagnosis, COVID RNA remnants were present in the stool of approximately half (49.2%) of the patients. Seven months later, about 4% of them shed fecal viral RNA.
The authors note that there was no ongoing SARS-CoV-2 RNA shedding in respiratory samples of patients at the 4-month mark.
Using self-reported symptoms regularly collected by questionnaire, they also found a correlation of long-term fecal shedding of SARS-CoV-2 RNA with abdominal pain, nausea, and vomiting.
The findings were published online in Med.
Implications of long-term viral shedding
Previous studies have found SARS-CoV-2 RNA in respiratory and fecal samples and have documented viral replication in lung and intestinal tissue.
But before the current study, little had been known about long-term shedding, especially in those who have mild COVID. Most studies of viral shedding have been with severe COVID cases.
The authors note that most studies of this kind are cross-sectional. The few other longitudinal studies have focused on early time points just after diagnosis.
Senior author Ami S. Bhatt, MD, associate professor in the departments of medicine and hematology at Stanford, told this news organization that though the viral genetic material in the feces lingers, on the basis of available evidence, it is highly unlikely to be contagious in most cases.
She said that understanding the dynamics of fecal shedding of SARS-CoV-2 genetic material will help interpret wastewater-based studies that are trying to determine population prevalence of the virus.
“While we don’t know the exact clinical importance of the longer-term shedding of SARS-CoV-2 in individuals with COVID-19, some have speculated that those who have long-term shedding of SARS-CoV-2 may have ongoing infections that might benefit from treatment,” she said.
“Our data support the idea that the long-term GI-related symptoms in some people might be the consequence of an ongoing infection in the GI tract, even after the respiratory infection has cleared,” Dr. Bhatt said.
“Alternatively, the presence of ongoing viral genetic material in the gut might be a trigger for the immune system to continually be active against the virus, and our immune system reaction may be the reason for long-COVID type symptoms,” she added. “This area is ripe for additional studies.”
Dr. Bhatt and colleagues will continue studying viral shedding in fecal samples as part of the nationwide RECOVER Initiative.
When reached for comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology, Eastern Virginia Medical School, Norfolk, said in an interview that previous studies have indicated that the virus may be detected in the stool for a month or more and for about 2 weeks on average. Whether the virus is infectious has been in question.
But it’s not so much that the virus is infectious in the GI tract and causing symptoms, he said. Rather, there are biomic changes related to COVID, including a loss of diversity in the gut bacteria, which disrupts the balance.
“This may actually in some way predispose some patients to impaired clearance of their symptoms,” Dr. Johnson explained. “There seems to be a growing recognition that this entity called long-haul COVID may be related to specific bacterial disruptions, and the more rapidly you can resolve these disruptions, the less likely you are to continue with long-haul symptoms.”
He said that among people who have mild COVID, the virus typically clears and gut bacteria return to normal. With severe or persistent illness, gut dysbiosis persists, he said.
“People need to be aware that the GI tract is involved in a sizable percent of patients with COVID,” Dr. Johnson said. “The GI-tract testing may reflect that the virus is there, but persistence of the detectable test positivity is very unlikely to reflect active virus.”
The authors note in this study that they collected only six samples from the participants over the 10-month period.
“Follow-up studies with more frequent sampling, especially in the first 2 months after diagnosis, may help build a more nuanced model of decline of fecal viral RNA concentration over time,” they write.
The study was supported by a Stanford ChemH-IMA grant, fellowships from the AACR and the National Science Foundation, and the National Institutes of Health. The authors and Dr. Johnson report no relevant financial relationships. Dr. Johnson is a regular contributor to Medscape.
A version of this article first appeared to Medscape.com.
New data present further evidence that SARS-CoV-2 infection can settle in the gastrointestinal (GI) tract and that it can persist long after the infection has cleared the lungs.
Infection of the GI tract may figure prominently in long COVID, the study authors suggest.
Led by Aravind Natarajan, PhD, with the departments of genetics and medicine at Stanford (Calif.) University, they analyzed fecal RNA shedding up to 10 months after a COVID-19 diagnosis in 673 stool samples from 113 patients with mild to moderate disease.
They found that in the week after diagnosis, COVID RNA remnants were present in the stool of approximately half (49.2%) of the patients. Seven months later, about 4% of them shed fecal viral RNA.
The authors note that there was no ongoing SARS-CoV-2 RNA shedding in respiratory samples of patients at the 4-month mark.
Using self-reported symptoms regularly collected by questionnaire, they also found a correlation of long-term fecal shedding of SARS-CoV-2 RNA with abdominal pain, nausea, and vomiting.
The findings were published online in Med.
Implications of long-term viral shedding
Previous studies have found SARS-CoV-2 RNA in respiratory and fecal samples and have documented viral replication in lung and intestinal tissue.
But before the current study, little had been known about long-term shedding, especially in those who have mild COVID. Most studies of viral shedding have been with severe COVID cases.
The authors note that most studies of this kind are cross-sectional. The few other longitudinal studies have focused on early time points just after diagnosis.
Senior author Ami S. Bhatt, MD, associate professor in the departments of medicine and hematology at Stanford, told this news organization that though the viral genetic material in the feces lingers, on the basis of available evidence, it is highly unlikely to be contagious in most cases.
She said that understanding the dynamics of fecal shedding of SARS-CoV-2 genetic material will help interpret wastewater-based studies that are trying to determine population prevalence of the virus.
“While we don’t know the exact clinical importance of the longer-term shedding of SARS-CoV-2 in individuals with COVID-19, some have speculated that those who have long-term shedding of SARS-CoV-2 may have ongoing infections that might benefit from treatment,” she said.
“Our data support the idea that the long-term GI-related symptoms in some people might be the consequence of an ongoing infection in the GI tract, even after the respiratory infection has cleared,” Dr. Bhatt said.
“Alternatively, the presence of ongoing viral genetic material in the gut might be a trigger for the immune system to continually be active against the virus, and our immune system reaction may be the reason for long-COVID type symptoms,” she added. “This area is ripe for additional studies.”
Dr. Bhatt and colleagues will continue studying viral shedding in fecal samples as part of the nationwide RECOVER Initiative.
When reached for comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology, Eastern Virginia Medical School, Norfolk, said in an interview that previous studies have indicated that the virus may be detected in the stool for a month or more and for about 2 weeks on average. Whether the virus is infectious has been in question.
But it’s not so much that the virus is infectious in the GI tract and causing symptoms, he said. Rather, there are biomic changes related to COVID, including a loss of diversity in the gut bacteria, which disrupts the balance.
“This may actually in some way predispose some patients to impaired clearance of their symptoms,” Dr. Johnson explained. “There seems to be a growing recognition that this entity called long-haul COVID may be related to specific bacterial disruptions, and the more rapidly you can resolve these disruptions, the less likely you are to continue with long-haul symptoms.”
He said that among people who have mild COVID, the virus typically clears and gut bacteria return to normal. With severe or persistent illness, gut dysbiosis persists, he said.
“People need to be aware that the GI tract is involved in a sizable percent of patients with COVID,” Dr. Johnson said. “The GI-tract testing may reflect that the virus is there, but persistence of the detectable test positivity is very unlikely to reflect active virus.”
The authors note in this study that they collected only six samples from the participants over the 10-month period.
“Follow-up studies with more frequent sampling, especially in the first 2 months after diagnosis, may help build a more nuanced model of decline of fecal viral RNA concentration over time,” they write.
The study was supported by a Stanford ChemH-IMA grant, fellowships from the AACR and the National Science Foundation, and the National Institutes of Health. The authors and Dr. Johnson report no relevant financial relationships. Dr. Johnson is a regular contributor to Medscape.
A version of this article first appeared to Medscape.com.
Children and COVID: New cases climb slowly but steadily
The current sustained increase in COVID-19 has brought the total number of cases in children to over 13 million since the start of the pandemic, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, when cases dropped to their lowest point since last summer. The cumulative number of cases in children is 13,052,988, which accounts for 19.0% of all cases reported in the United States, the AAP and CHA said in their weekly COVID-19 report.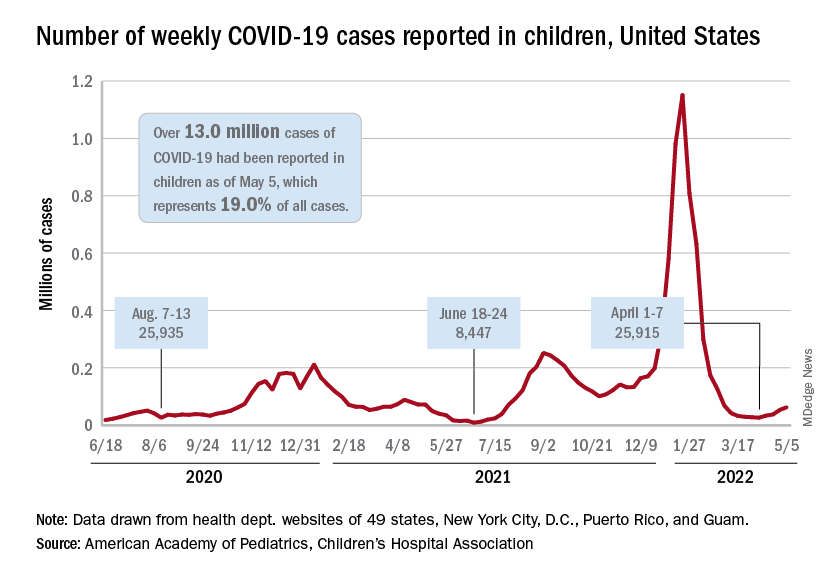
Other measures of incidence show the same steady rise. The rate of new admissions of children aged 0-17 with confirmed COVID-19, which had dipped as low as 0.13 per 100,000 population on April 11, was up to 0.19 per 100,000 on May 6, and the 7-day average for total admissions was 136 per day for May 1-7, compared with 118 for the last week of April, according to the Centers for Disease Control and Prevention.
At the state level, new admission rates for May 6 show wide variation, even regionally. Rhode Island came in with a 0.00 per 100,000 on that day, while Vermont recorded 0.88 admissions per 100,000, the highest of any state and lower only than the District of Columbia’s 1.23 per 100,000. Connecticut (0.45) and Massachusetts (0.33) also were in the highest group (see map), while Maine was in the lowest, CDC data show.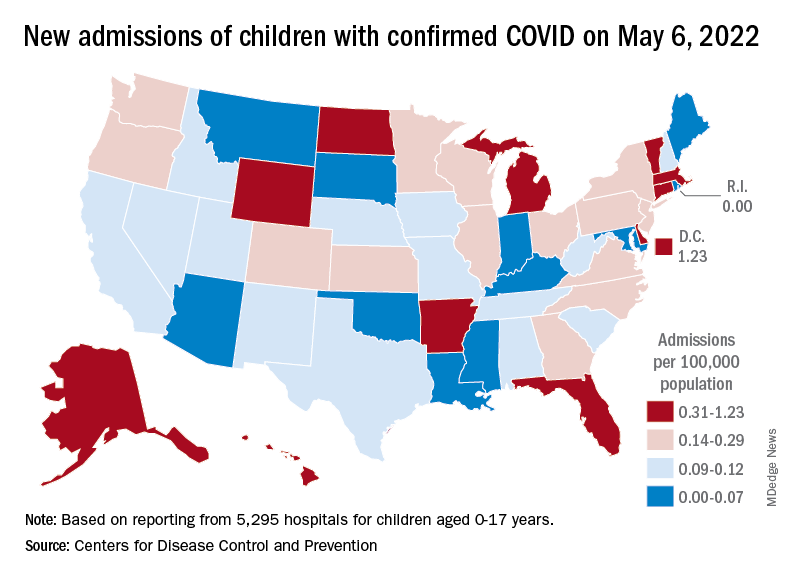
Nationally, emergency department visits also have been rising over the last month or so. Children aged 0-11 years, who were down to a 7-day average of 0.5% of ED visits with diagnosed COVID-19 in early April, saw that number rise to 1.4% on May 5. Children aged 12-15 years went from a rate of 0.3% in late March to the current 1.2%, as did 16- to 17-year-olds, the CDC said on its COVID Data Tracker.
The vaccination effort, meanwhile, continues to lose steam, at least among children who are currently eligible. Initial vaccinations in those aged 5-11 slipped to their lowest-ever 1-week total, 47,000 for April 28 to May 4, while children aged 16-17 continued a long-term slide that has the weekly count down to just 29,000, the AAP said in its weekly vaccination report.
Here’s how those latest recipients changed the populations of vaccinated children in the last week: 35.4% of all 5- to 11-year-olds had received at least one dose as of May 4, compared with 35.3% on April 27, with increases from 67.4% to 67.5% for 12- to 15-year-olds and 72.7% to 72.8% among those aged 16-17, the CDC reported.
The current sustained increase in COVID-19 has brought the total number of cases in children to over 13 million since the start of the pandemic, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, when cases dropped to their lowest point since last summer. The cumulative number of cases in children is 13,052,988, which accounts for 19.0% of all cases reported in the United States, the AAP and CHA said in their weekly COVID-19 report.
Other measures of incidence show the same steady rise. The rate of new admissions of children aged 0-17 with confirmed COVID-19, which had dipped as low as 0.13 per 100,000 population on April 11, was up to 0.19 per 100,000 on May 6, and the 7-day average for total admissions was 136 per day for May 1-7, compared with 118 for the last week of April, according to the Centers for Disease Control and Prevention.
At the state level, new admission rates for May 6 show wide variation, even regionally. Rhode Island came in with a 0.00 per 100,000 on that day, while Vermont recorded 0.88 admissions per 100,000, the highest of any state and lower only than the District of Columbia’s 1.23 per 100,000. Connecticut (0.45) and Massachusetts (0.33) also were in the highest group (see map), while Maine was in the lowest, CDC data show.
Nationally, emergency department visits also have been rising over the last month or so. Children aged 0-11 years, who were down to a 7-day average of 0.5% of ED visits with diagnosed COVID-19 in early April, saw that number rise to 1.4% on May 5. Children aged 12-15 years went from a rate of 0.3% in late March to the current 1.2%, as did 16- to 17-year-olds, the CDC said on its COVID Data Tracker.
The vaccination effort, meanwhile, continues to lose steam, at least among children who are currently eligible. Initial vaccinations in those aged 5-11 slipped to their lowest-ever 1-week total, 47,000 for April 28 to May 4, while children aged 16-17 continued a long-term slide that has the weekly count down to just 29,000, the AAP said in its weekly vaccination report.
Here’s how those latest recipients changed the populations of vaccinated children in the last week: 35.4% of all 5- to 11-year-olds had received at least one dose as of May 4, compared with 35.3% on April 27, with increases from 67.4% to 67.5% for 12- to 15-year-olds and 72.7% to 72.8% among those aged 16-17, the CDC reported.
The current sustained increase in COVID-19 has brought the total number of cases in children to over 13 million since the start of the pandemic, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, when cases dropped to their lowest point since last summer. The cumulative number of cases in children is 13,052,988, which accounts for 19.0% of all cases reported in the United States, the AAP and CHA said in their weekly COVID-19 report.
Other measures of incidence show the same steady rise. The rate of new admissions of children aged 0-17 with confirmed COVID-19, which had dipped as low as 0.13 per 100,000 population on April 11, was up to 0.19 per 100,000 on May 6, and the 7-day average for total admissions was 136 per day for May 1-7, compared with 118 for the last week of April, according to the Centers for Disease Control and Prevention.
At the state level, new admission rates for May 6 show wide variation, even regionally. Rhode Island came in with a 0.00 per 100,000 on that day, while Vermont recorded 0.88 admissions per 100,000, the highest of any state and lower only than the District of Columbia’s 1.23 per 100,000. Connecticut (0.45) and Massachusetts (0.33) also were in the highest group (see map), while Maine was in the lowest, CDC data show.
Nationally, emergency department visits also have been rising over the last month or so. Children aged 0-11 years, who were down to a 7-day average of 0.5% of ED visits with diagnosed COVID-19 in early April, saw that number rise to 1.4% on May 5. Children aged 12-15 years went from a rate of 0.3% in late March to the current 1.2%, as did 16- to 17-year-olds, the CDC said on its COVID Data Tracker.
The vaccination effort, meanwhile, continues to lose steam, at least among children who are currently eligible. Initial vaccinations in those aged 5-11 slipped to their lowest-ever 1-week total, 47,000 for April 28 to May 4, while children aged 16-17 continued a long-term slide that has the weekly count down to just 29,000, the AAP said in its weekly vaccination report.
Here’s how those latest recipients changed the populations of vaccinated children in the last week: 35.4% of all 5- to 11-year-olds had received at least one dose as of May 4, compared with 35.3% on April 27, with increases from 67.4% to 67.5% for 12- to 15-year-olds and 72.7% to 72.8% among those aged 16-17, the CDC reported.
COVID fallout: ‘Alarming’ dip in routine vax for pregnant women
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
FROM ACOG 2022
CDC predicts a rise in COVID-19 hospitalizations and deaths in coming weeks
, according to a national forecast used by the Centers for Disease Control and Prevention.
The national model also predicts that about 5,000 deaths will occur over the next two weeks, with Ohio, New Jersey, and New York projected to see the largest totals of daily deaths in upcoming weeks.
The numbers follow several weeks of steady increases in infections across the country. More than 67,000 new cases are being reported daily, according to the data tracker from The New York Times, marking a 59% increase in the past two weeks.
In the Northeast, infection rates have risen by nearly 65%. In the New York and New Jersey region, infection rates are up about 55% in the past two weeks.
Hospitalizations have already begun to climb as well, with about 19,000 COVID-19 patients hospitalized nationwide and 1,725 in intensive care, according to the latest data from the Department of Health and Human Services. In the last week, hospital admissions have jumped by 20%, and emergency department visits are up by 18%.
The CDC forecast shows that 42 states and territories will see increases in hospital admissions during the next two weeks. Florida, Minnesota, New York, and Wisconsin will see some of the largest increases.
On average, more than 2,200 COVID-19 patients are entering the hospital each day, which has increased about 20% in the last week, according to ABC News. This also marks the highest number of COVID-19 patients needing hospital care since mid-March.
Public health officials have cited several factors for the increase in cases, such as states lifting mask mandates and other safety restrictions, ABC News reported. Highly contagious Omicron subvariants, such as BA.2 and BA.2.12.1, continue to spread in the United States and escape immunity from previous infections.
The BA.2 subvariant accounts for 62% of new national cases, according to the latest CDC data. The BA.2.12.1 subvariant makes up about 36% of new cases across the United States but 62% in the New York area.
A version of this article first appeared on WebMD.com.
, according to a national forecast used by the Centers for Disease Control and Prevention.
The national model also predicts that about 5,000 deaths will occur over the next two weeks, with Ohio, New Jersey, and New York projected to see the largest totals of daily deaths in upcoming weeks.
The numbers follow several weeks of steady increases in infections across the country. More than 67,000 new cases are being reported daily, according to the data tracker from The New York Times, marking a 59% increase in the past two weeks.
In the Northeast, infection rates have risen by nearly 65%. In the New York and New Jersey region, infection rates are up about 55% in the past two weeks.
Hospitalizations have already begun to climb as well, with about 19,000 COVID-19 patients hospitalized nationwide and 1,725 in intensive care, according to the latest data from the Department of Health and Human Services. In the last week, hospital admissions have jumped by 20%, and emergency department visits are up by 18%.
The CDC forecast shows that 42 states and territories will see increases in hospital admissions during the next two weeks. Florida, Minnesota, New York, and Wisconsin will see some of the largest increases.
On average, more than 2,200 COVID-19 patients are entering the hospital each day, which has increased about 20% in the last week, according to ABC News. This also marks the highest number of COVID-19 patients needing hospital care since mid-March.
Public health officials have cited several factors for the increase in cases, such as states lifting mask mandates and other safety restrictions, ABC News reported. Highly contagious Omicron subvariants, such as BA.2 and BA.2.12.1, continue to spread in the United States and escape immunity from previous infections.
The BA.2 subvariant accounts for 62% of new national cases, according to the latest CDC data. The BA.2.12.1 subvariant makes up about 36% of new cases across the United States but 62% in the New York area.
A version of this article first appeared on WebMD.com.
, according to a national forecast used by the Centers for Disease Control and Prevention.
The national model also predicts that about 5,000 deaths will occur over the next two weeks, with Ohio, New Jersey, and New York projected to see the largest totals of daily deaths in upcoming weeks.
The numbers follow several weeks of steady increases in infections across the country. More than 67,000 new cases are being reported daily, according to the data tracker from The New York Times, marking a 59% increase in the past two weeks.
In the Northeast, infection rates have risen by nearly 65%. In the New York and New Jersey region, infection rates are up about 55% in the past two weeks.
Hospitalizations have already begun to climb as well, with about 19,000 COVID-19 patients hospitalized nationwide and 1,725 in intensive care, according to the latest data from the Department of Health and Human Services. In the last week, hospital admissions have jumped by 20%, and emergency department visits are up by 18%.
The CDC forecast shows that 42 states and territories will see increases in hospital admissions during the next two weeks. Florida, Minnesota, New York, and Wisconsin will see some of the largest increases.
On average, more than 2,200 COVID-19 patients are entering the hospital each day, which has increased about 20% in the last week, according to ABC News. This also marks the highest number of COVID-19 patients needing hospital care since mid-March.
Public health officials have cited several factors for the increase in cases, such as states lifting mask mandates and other safety restrictions, ABC News reported. Highly contagious Omicron subvariants, such as BA.2 and BA.2.12.1, continue to spread in the United States and escape immunity from previous infections.
The BA.2 subvariant accounts for 62% of new national cases, according to the latest CDC data. The BA.2.12.1 subvariant makes up about 36% of new cases across the United States but 62% in the New York area.
A version of this article first appeared on WebMD.com.
Screening for diabetes at normal BMIs could cut racial disparities
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
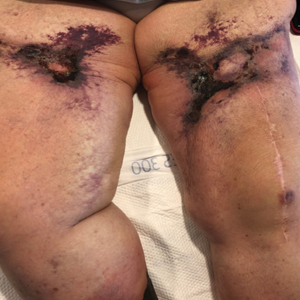
Retiform Purpura on the Legs
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2
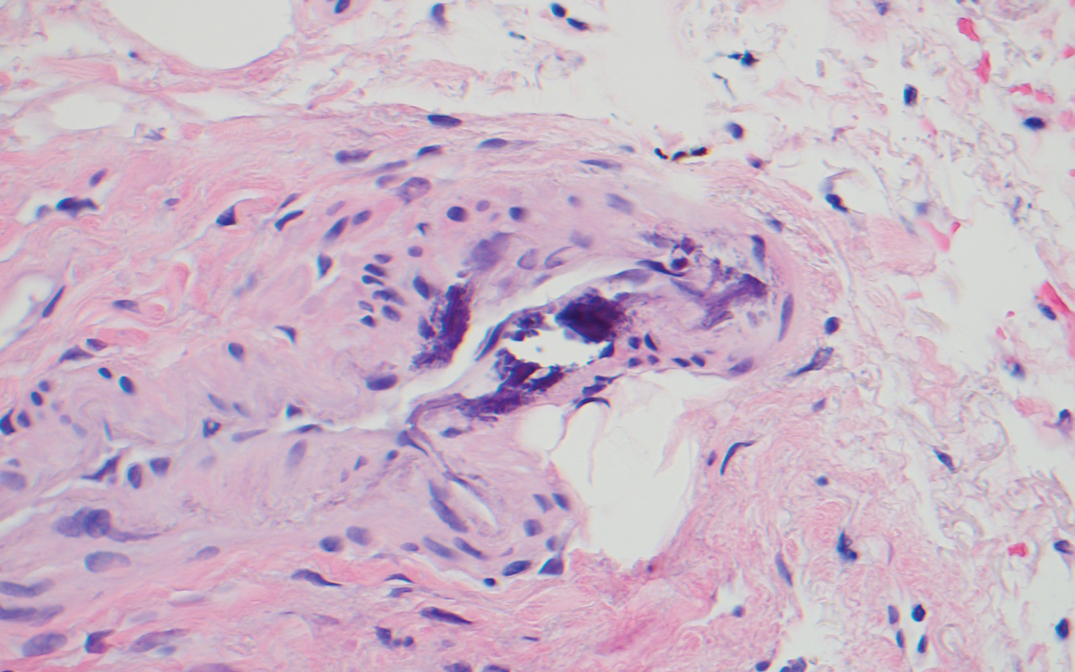
Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
A 70-year-old woman with a medical history of Takayasu arteritis, end-stage renal disease on peritoneal dialysis, coronary artery disease, hypertension, hypothyroidism, and anemia of chronic disease presented to the emergency department with enlarging painful stellate eschars of the legs with associated edema of 3 weeks’ duration. She denied a history of similar-appearing skin lesions. She initially thought the lesions were burns secondary to frequent hot showers for relief of uremic pruritus. For the treatment of these suspected burns prior to hospitalization, she had been applying over-the-counter antibiotic ointments to the affected areas and had completed a 2-week course of oral cephalexin without notable improvement. Physical examination revealed retiform purpura of the legs with large stellate eschars overlying the anteromedial thighs and right medial calf. Computed tomography angiogram of the abdomen and pelvis demonstrated diffuse calcifications of the aortic wall and its associated branches that were most pronounced in the legs without evidence of vessel wall thickening. Punch biopsies were performed, and nephrology, rheumatology, and wound care services were consulted.
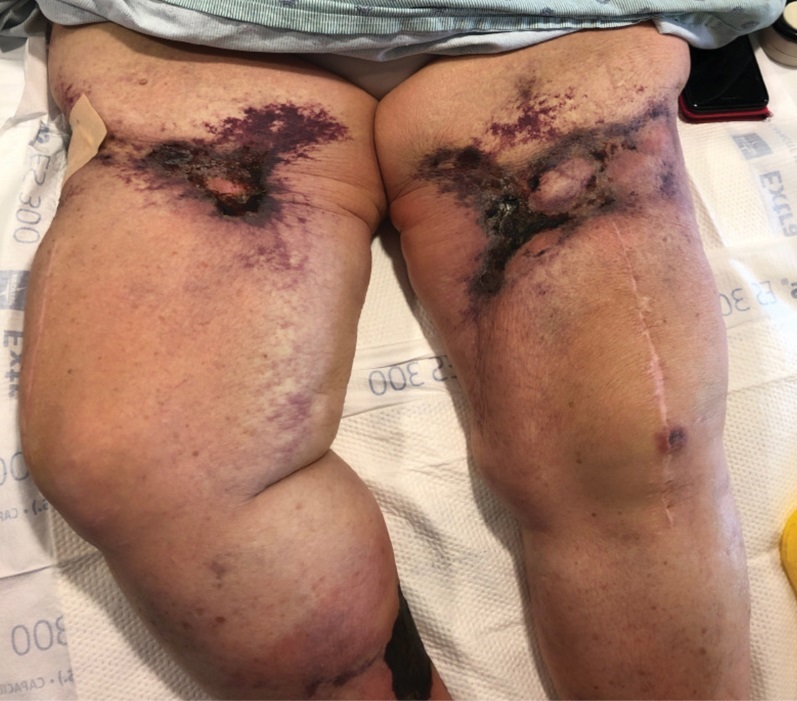
COPD screening for asymptomatic adults? USPSTF weighs in, again
Screening for chronic obstructive pulmonary disease (COPD) in asymptomatic adults has no net benefit, according to a U.S. Preventive Services Task Force (USPSTF) reassessment of its 2016 screening recommendations. The new recommendation is in line with the previous one and is made with moderate certainty (grade D evidence).
The USPSTF recommendation applies to adults who do not recognize or report respiratory symptoms. It does not apply to people with symptoms such as chronic cough, sputum production, difficulty breathing, or wheezing, or those known to be at very high risk for COPD. These latter include people with alpha-1 antitrypsin deficiency or workers exposed to certain toxins at their jobs, according to the report published in JAMA.
“Considering that the outcomes of several other chronic conditions, including cardiovascular disease and cancer, have been improved over the years with early detection and intervention, it is logical to ask whether screening to achieve early detection of COPD might also lead to better outcomes,” Surya P. Bhatt, MD, of the University of Alabama at Birmingham, and George T. O’Connor, MD, of the Boston University, explained in an editorial.
Task force assessment
The task force examined relevant publications after the 2016 deliberations and found no new studies that directly assessed the effects of screening for COPD in asymptomatic adults on morbidity, mortality, or health-related quality of life.
Although, as in their previous review, serious harms from treatment trials were not consistently reported, more recent large observational studies in screen-relevant populations suggested possible harms from the initiation of long-acting beta-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), and the use of inhaled corticosteroids.
“In addition to potential treatment harms, there are opportunity costs to screening that may include time spent on counseling and providing services and patient referrals for diagnostic testing,” the task force stated.
Because cigarette smoking is the leading cause of COPD, the USPSTF has reiterated its recommendations for physicians to address tobacco smoking cessation in adults, including pregnant persons, as well as tobacco use in children and adolescents.
Not the whole story?
“Truly asymptomatic individuals with airflow obstruction do not meet criteria for COPD therapy, but sensitive questionnaires may detect symptoms not previously reported by the patient. It may be more effective to redirect the focus from screening for asymptomatic COPD to case finding using sensitive and cost-effective tools,” Dr. Bhatt and Dr. O’Connor suggested in their editorial.
“Even though available data may not support screening asymptomatic adults for COPD, there is substantial rationale for further investigation of strategies to enhance earlier detection of this condition,” they concluded.
More research needed
Despite the recommendation, the USPSTF indicated that further studies are needed to fill in research gaps, including:
- The effectiveness of screening asymptomatic adults for COPD to reduce morbidity or mortality or improve health-related quality of life, with long-term follow-up.
- The effectiveness of early treatment for asymptomatic, minimally symptomatic, or screen-detected populations to slow disease progression and improve health outcomes, with long-term follow-up.
- The harms of screening in and treatment of persons with asymptomatic or minimally symptomatic COPD.
The USPSTF is an independent, voluntary body, and potential conflicts of interest of the members are on file with the organization. Dr. Bhatt reported serving on an advisory board for Boehringer Ingelheim and receiving consulting fees from Sanofi/Regeneron; and Dr. O’Connor reported receiving consulting fees from Grupo Menarini and Dicerna Pharmaceuticals.
Screening for chronic obstructive pulmonary disease (COPD) in asymptomatic adults has no net benefit, according to a U.S. Preventive Services Task Force (USPSTF) reassessment of its 2016 screening recommendations. The new recommendation is in line with the previous one and is made with moderate certainty (grade D evidence).
The USPSTF recommendation applies to adults who do not recognize or report respiratory symptoms. It does not apply to people with symptoms such as chronic cough, sputum production, difficulty breathing, or wheezing, or those known to be at very high risk for COPD. These latter include people with alpha-1 antitrypsin deficiency or workers exposed to certain toxins at their jobs, according to the report published in JAMA.
“Considering that the outcomes of several other chronic conditions, including cardiovascular disease and cancer, have been improved over the years with early detection and intervention, it is logical to ask whether screening to achieve early detection of COPD might also lead to better outcomes,” Surya P. Bhatt, MD, of the University of Alabama at Birmingham, and George T. O’Connor, MD, of the Boston University, explained in an editorial.
Task force assessment
The task force examined relevant publications after the 2016 deliberations and found no new studies that directly assessed the effects of screening for COPD in asymptomatic adults on morbidity, mortality, or health-related quality of life.
Although, as in their previous review, serious harms from treatment trials were not consistently reported, more recent large observational studies in screen-relevant populations suggested possible harms from the initiation of long-acting beta-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), and the use of inhaled corticosteroids.
“In addition to potential treatment harms, there are opportunity costs to screening that may include time spent on counseling and providing services and patient referrals for diagnostic testing,” the task force stated.
Because cigarette smoking is the leading cause of COPD, the USPSTF has reiterated its recommendations for physicians to address tobacco smoking cessation in adults, including pregnant persons, as well as tobacco use in children and adolescents.
Not the whole story?
“Truly asymptomatic individuals with airflow obstruction do not meet criteria for COPD therapy, but sensitive questionnaires may detect symptoms not previously reported by the patient. It may be more effective to redirect the focus from screening for asymptomatic COPD to case finding using sensitive and cost-effective tools,” Dr. Bhatt and Dr. O’Connor suggested in their editorial.
“Even though available data may not support screening asymptomatic adults for COPD, there is substantial rationale for further investigation of strategies to enhance earlier detection of this condition,” they concluded.
More research needed
Despite the recommendation, the USPSTF indicated that further studies are needed to fill in research gaps, including:
- The effectiveness of screening asymptomatic adults for COPD to reduce morbidity or mortality or improve health-related quality of life, with long-term follow-up.
- The effectiveness of early treatment for asymptomatic, minimally symptomatic, or screen-detected populations to slow disease progression and improve health outcomes, with long-term follow-up.
- The harms of screening in and treatment of persons with asymptomatic or minimally symptomatic COPD.
The USPSTF is an independent, voluntary body, and potential conflicts of interest of the members are on file with the organization. Dr. Bhatt reported serving on an advisory board for Boehringer Ingelheim and receiving consulting fees from Sanofi/Regeneron; and Dr. O’Connor reported receiving consulting fees from Grupo Menarini and Dicerna Pharmaceuticals.
Screening for chronic obstructive pulmonary disease (COPD) in asymptomatic adults has no net benefit, according to a U.S. Preventive Services Task Force (USPSTF) reassessment of its 2016 screening recommendations. The new recommendation is in line with the previous one and is made with moderate certainty (grade D evidence).
The USPSTF recommendation applies to adults who do not recognize or report respiratory symptoms. It does not apply to people with symptoms such as chronic cough, sputum production, difficulty breathing, or wheezing, or those known to be at very high risk for COPD. These latter include people with alpha-1 antitrypsin deficiency or workers exposed to certain toxins at their jobs, according to the report published in JAMA.
“Considering that the outcomes of several other chronic conditions, including cardiovascular disease and cancer, have been improved over the years with early detection and intervention, it is logical to ask whether screening to achieve early detection of COPD might also lead to better outcomes,” Surya P. Bhatt, MD, of the University of Alabama at Birmingham, and George T. O’Connor, MD, of the Boston University, explained in an editorial.
Task force assessment
The task force examined relevant publications after the 2016 deliberations and found no new studies that directly assessed the effects of screening for COPD in asymptomatic adults on morbidity, mortality, or health-related quality of life.
Although, as in their previous review, serious harms from treatment trials were not consistently reported, more recent large observational studies in screen-relevant populations suggested possible harms from the initiation of long-acting beta-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), and the use of inhaled corticosteroids.
“In addition to potential treatment harms, there are opportunity costs to screening that may include time spent on counseling and providing services and patient referrals for diagnostic testing,” the task force stated.
Because cigarette smoking is the leading cause of COPD, the USPSTF has reiterated its recommendations for physicians to address tobacco smoking cessation in adults, including pregnant persons, as well as tobacco use in children and adolescents.
Not the whole story?
“Truly asymptomatic individuals with airflow obstruction do not meet criteria for COPD therapy, but sensitive questionnaires may detect symptoms not previously reported by the patient. It may be more effective to redirect the focus from screening for asymptomatic COPD to case finding using sensitive and cost-effective tools,” Dr. Bhatt and Dr. O’Connor suggested in their editorial.
“Even though available data may not support screening asymptomatic adults for COPD, there is substantial rationale for further investigation of strategies to enhance earlier detection of this condition,” they concluded.
More research needed
Despite the recommendation, the USPSTF indicated that further studies are needed to fill in research gaps, including:
- The effectiveness of screening asymptomatic adults for COPD to reduce morbidity or mortality or improve health-related quality of life, with long-term follow-up.
- The effectiveness of early treatment for asymptomatic, minimally symptomatic, or screen-detected populations to slow disease progression and improve health outcomes, with long-term follow-up.
- The harms of screening in and treatment of persons with asymptomatic or minimally symptomatic COPD.
The USPSTF is an independent, voluntary body, and potential conflicts of interest of the members are on file with the organization. Dr. Bhatt reported serving on an advisory board for Boehringer Ingelheim and receiving consulting fees from Sanofi/Regeneron; and Dr. O’Connor reported receiving consulting fees from Grupo Menarini and Dicerna Pharmaceuticals.
FROM JAMA
Dermatology attracts more than its share of physician assistants
Dermatology added PAs at a mean rate of 11.6% annually over that 6-year period, compared with a mean of 7.8% for all other specialties (P <.001), as the National Commission on Certification of Physician Assistants (NCCPA) tallied 2,324 working in dermatology and 64,490 in all other specialties in 2013 and 3,938/94,616, respectively, in 2018, Justin D. Arnold, MD, of the University of California, Irvine, and associates reported in JAMA Dermatology.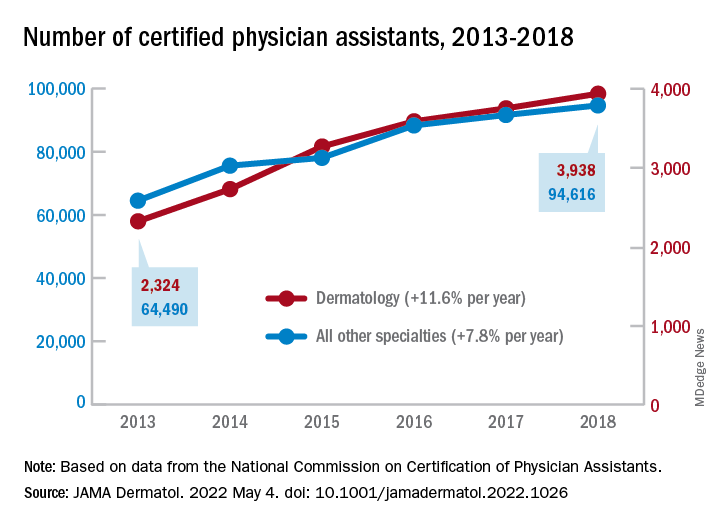
“There is, however, a lack of racial and ethnic diversity within the dermatology PA workforce,” they noted. A detailed comparison using the 2018 data showed that only 1.6% of dermatology PAs identified as Black, compared with 3.7% of those in all other specialties (P <.001), although “similar rates of Hispanic ethnicity were observed” in dermatology PAs (6.0%) and PAs in other fields (6.5%), the investigators added.
That was not the case for women in the profession, as 82% of PAs in dermatology were female in 2018, compared with 67% in the other specialties. Dermatology PAs also were significantly more likely to work in office-based practices than their nondermatology peers (93% vs. 37%, P < .001) and to reside in metropolitan areas (95% vs. 92%, P < .001), Dr. Arnold and associates said in the research letter.
The dermatology PAs also were more likely to work part time (30 or fewer hours per week) than those outside dermatology, 19.1% vs. 12.9% (P < .001). Despite that, the dermatology PAs reported seeing more patients per week (a mean of 119) than those in all of the other specialties (a mean of 71), the investigators said.
The total number of certified PAs was over 131,000 in 2018, but about 25% had not selected a principal specialty in their PA Professional Profiles and were not included in the study, they explained.
“Although this study did not assess the reasons for the substantial increase of dermatology PAs, numerous factors, such as a potential physician shortage or the expansion of private equity–owned practices, may contribute to the accelerating use of PAs within the field,” they wrote.
Dermatology added PAs at a mean rate of 11.6% annually over that 6-year period, compared with a mean of 7.8% for all other specialties (P <.001), as the National Commission on Certification of Physician Assistants (NCCPA) tallied 2,324 working in dermatology and 64,490 in all other specialties in 2013 and 3,938/94,616, respectively, in 2018, Justin D. Arnold, MD, of the University of California, Irvine, and associates reported in JAMA Dermatology.
“There is, however, a lack of racial and ethnic diversity within the dermatology PA workforce,” they noted. A detailed comparison using the 2018 data showed that only 1.6% of dermatology PAs identified as Black, compared with 3.7% of those in all other specialties (P <.001), although “similar rates of Hispanic ethnicity were observed” in dermatology PAs (6.0%) and PAs in other fields (6.5%), the investigators added.
That was not the case for women in the profession, as 82% of PAs in dermatology were female in 2018, compared with 67% in the other specialties. Dermatology PAs also were significantly more likely to work in office-based practices than their nondermatology peers (93% vs. 37%, P < .001) and to reside in metropolitan areas (95% vs. 92%, P < .001), Dr. Arnold and associates said in the research letter.
The dermatology PAs also were more likely to work part time (30 or fewer hours per week) than those outside dermatology, 19.1% vs. 12.9% (P < .001). Despite that, the dermatology PAs reported seeing more patients per week (a mean of 119) than those in all of the other specialties (a mean of 71), the investigators said.
The total number of certified PAs was over 131,000 in 2018, but about 25% had not selected a principal specialty in their PA Professional Profiles and were not included in the study, they explained.
“Although this study did not assess the reasons for the substantial increase of dermatology PAs, numerous factors, such as a potential physician shortage or the expansion of private equity–owned practices, may contribute to the accelerating use of PAs within the field,” they wrote.
Dermatology added PAs at a mean rate of 11.6% annually over that 6-year period, compared with a mean of 7.8% for all other specialties (P <.001), as the National Commission on Certification of Physician Assistants (NCCPA) tallied 2,324 working in dermatology and 64,490 in all other specialties in 2013 and 3,938/94,616, respectively, in 2018, Justin D. Arnold, MD, of the University of California, Irvine, and associates reported in JAMA Dermatology.
“There is, however, a lack of racial and ethnic diversity within the dermatology PA workforce,” they noted. A detailed comparison using the 2018 data showed that only 1.6% of dermatology PAs identified as Black, compared with 3.7% of those in all other specialties (P <.001), although “similar rates of Hispanic ethnicity were observed” in dermatology PAs (6.0%) and PAs in other fields (6.5%), the investigators added.
That was not the case for women in the profession, as 82% of PAs in dermatology were female in 2018, compared with 67% in the other specialties. Dermatology PAs also were significantly more likely to work in office-based practices than their nondermatology peers (93% vs. 37%, P < .001) and to reside in metropolitan areas (95% vs. 92%, P < .001), Dr. Arnold and associates said in the research letter.
The dermatology PAs also were more likely to work part time (30 or fewer hours per week) than those outside dermatology, 19.1% vs. 12.9% (P < .001). Despite that, the dermatology PAs reported seeing more patients per week (a mean of 119) than those in all of the other specialties (a mean of 71), the investigators said.
The total number of certified PAs was over 131,000 in 2018, but about 25% had not selected a principal specialty in their PA Professional Profiles and were not included in the study, they explained.
“Although this study did not assess the reasons for the substantial increase of dermatology PAs, numerous factors, such as a potential physician shortage or the expansion of private equity–owned practices, may contribute to the accelerating use of PAs within the field,” they wrote.
FROM JAMA DERMATOLOGY
Atypical knee pain
An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.
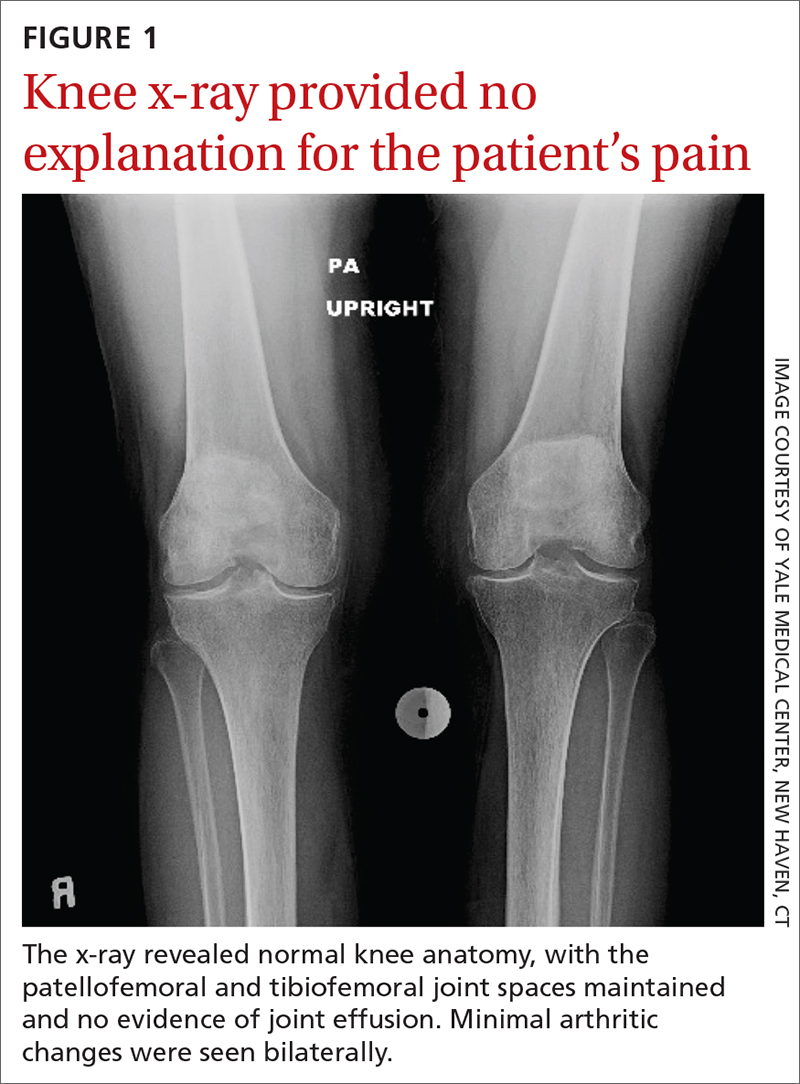
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.
Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.
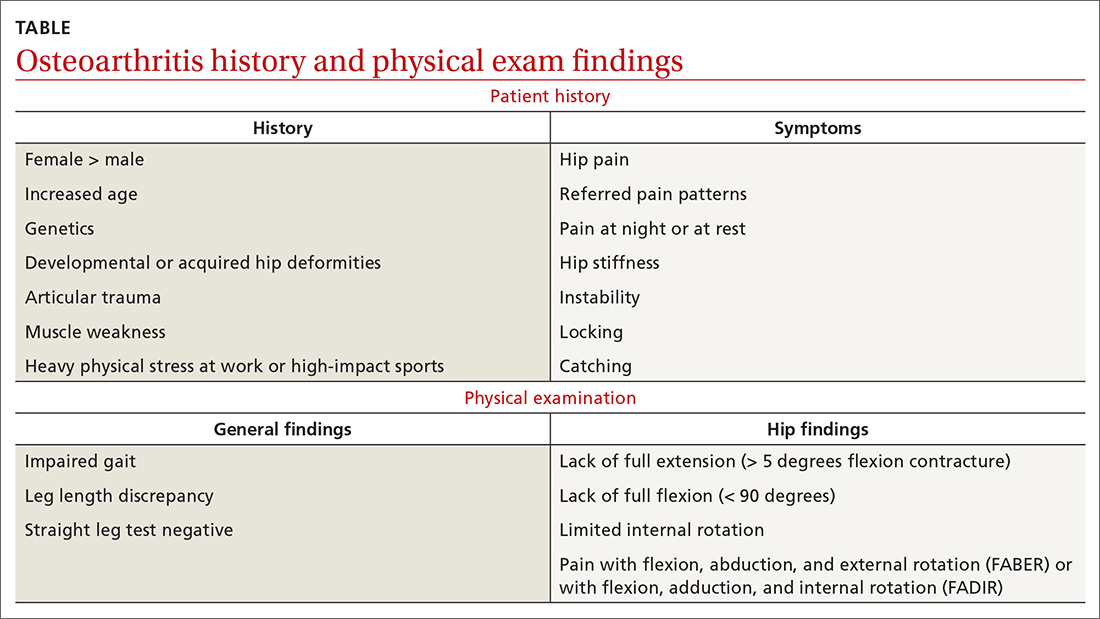
The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.
1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.

Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.

The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.

An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.

Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.

The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.

1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011