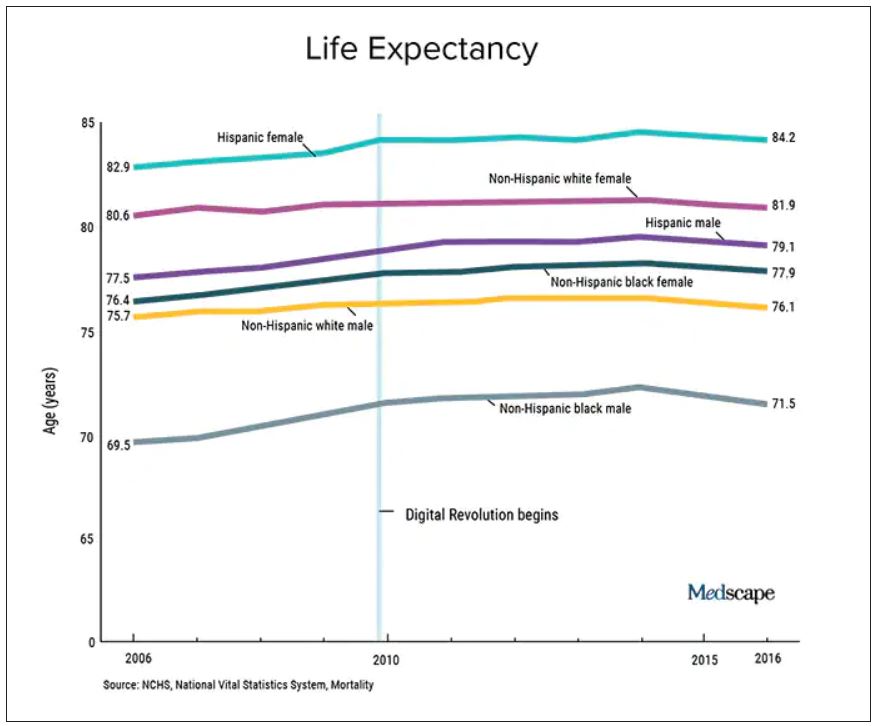
User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
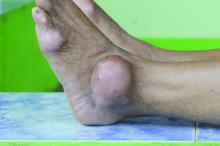
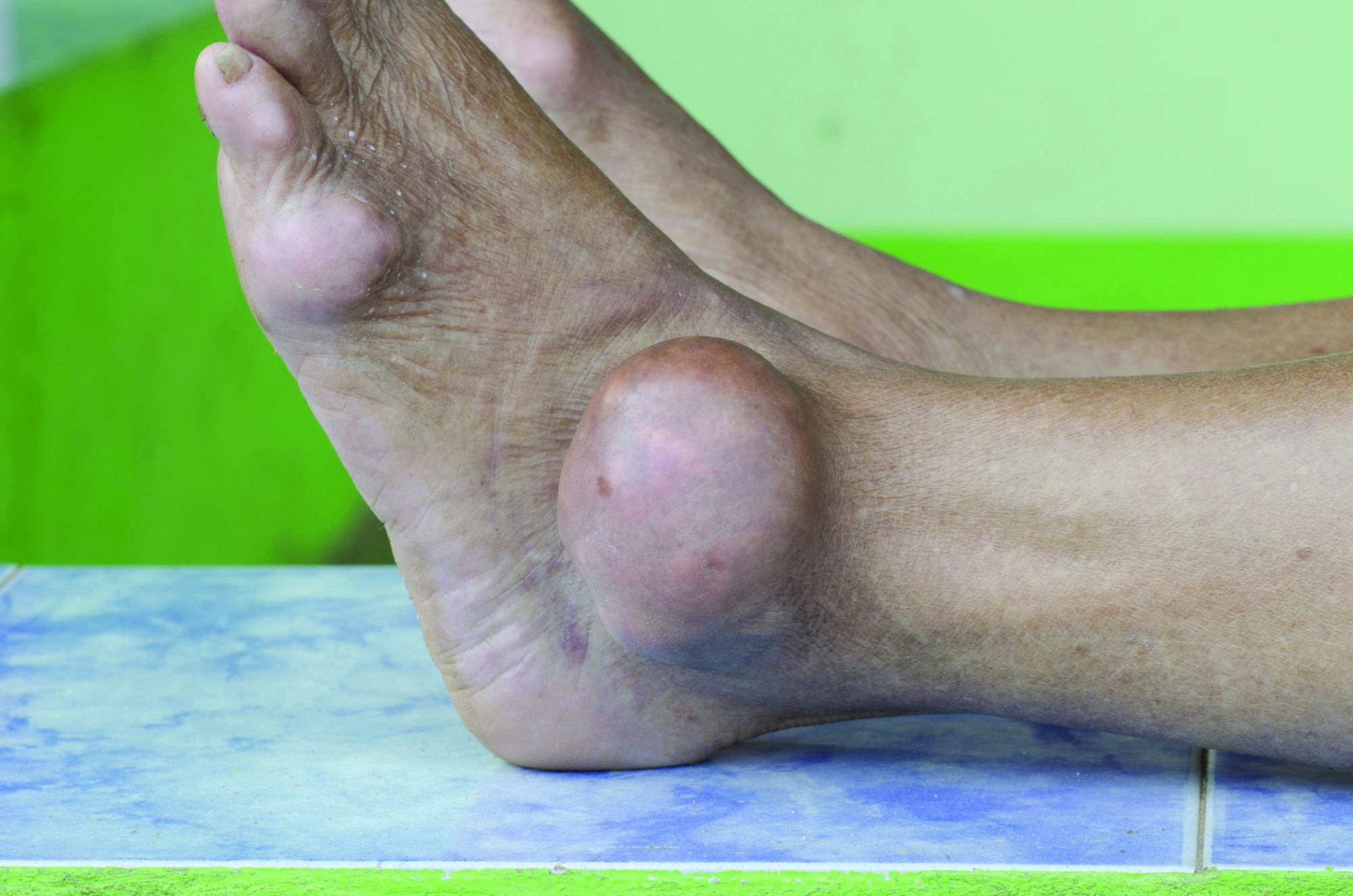
Potential new standard of care for biliary tract cancer
, according to interim results from the TOPAZ-1 trial.
The risk of death for those taking durvalumab plus chemotherapy was 20% lower than for patients on chemotherapy alone. At 18 months, overall survival was 35.1% in the durvalumab group versus 25.6% for chemotherapy alone. By 2 years, overall survival was 24.9% versus 10.4%.
“TOPAZ-1 is the first phase 3 trial to show that adding immunotherapy to standard chemotherapy can increase survival in biliary tract cancer, and importantly, does so without inducing any new serious side effects,” said lead author Do-Youn Oh, MD, PhD, professor in the Division of Medical Oncology at Seoul National University Hospital and Seoul National University College of Medicine, Korea.
“The study met its primary endpoint at a prespecified interim analysis, and durvalumab plus gemcitabine and cisplatin demonstrated statistically significant and clinically meaningful prolonged overall survival compared with placebo plus chemotherapy,” she said.
“This is an effective first-line therapy and could become a new standard of care for patients with advanced biliary tract cancer,” she added.
Dr. Oh presented the results at the Gastrointestinal Cancers Symposium (GICS) 2022.
In a discussion of the paper, Nilofer Saba Azad, MD, from the department of oncology, Johns Hopkins Sidney Kimmel Cancer Center, Baltimore, noted that overall, “we are seeing enticing benefit in survival and response rate.”
“There is moderately strong preliminary clinical data and biological rationale that immune checkpoint may have some activity in biliary tract cancer,” she said. “The trial was adequately powered and accounted for important known clinical subsets, and [it] was placebo controlled. The results suggest a meaningful benefit for patients.”
However, she pointed out that there are still open questions, mostly having to do with the subgroup analysis.
Biliary tract cancer: Incidence is rising
Biliary tract cancers are a relatively rare and heterogeneous group of cancers, and global incidence is rising. “Advanced unresectable biliary tract cancer is an area of high unmet need due to its aggressive nature, limited treatment options, and poor prognosis,” explained Dr. Oh. “The first-line standard of care for advanced biliary tract cancers, gemcitabine and cisplatin, has remained unchanged for over a decade.”
Previous research has suggested that checkpoint inhibition may result in antitumor immune responses, she commented. A previous phase 2 trial showed that durvalumab combined with gemcitabine and cisplatin showed promising antitumor activity in advanced biliary tract cancer. This latest study is a larger phase 3 trial to investigate this effect further.
The study involved 365 patients with unresectable locally advanced, recurrent, or metastatic biliary tract cancers. Patients had one of three types of biliary tract cancer: 55% had intrahepatic cancers; 19% had extrahepatic cancers; and 25% had gallbladder cancer.
The trial was conducted in the U.S. and 17 countries in Europe, South America, and Asia. Nearly 55% of the cohort was from Asia, including South Korea, Thailand, Japan, and China.
All patients received chemotherapy with gemcitabine (1,000 mg/m2) and cisplatin (25 mg/m2 on days 1 and 8 every 3 weeks) for up to eight cycles.
Patients were randomized to receive either durvalumab (1,500 mg every 3 weeks) or placebo before chemotherapy and also to receive durvalumab (1,500 mg every 4 weeks) or placebo after chemotherapy until disease progression or unacceptable toxicity.
At approximately 1 year, the authors found that adding durvalumab significantly improved overall survival (hazard ratio, 0.80; P = .021).
Progression-free survival was also significantly better with durvalumab compared to placebo: 7.2 months versus 5.7 months (HR, 0.75; P = .001).
The overall response rate (ORR) was 26.7% with durvalumab and 18.7% with placebo.
The most common adverse events were anemia (experienced by 48.2% of patients), neutropenia (31.7%), and nausea (40.2%). Grade 3/4 adverse events occurred in 75.7% of patients receiving durvalumab versus 77.8% for placebo, indicating that the majority of side effects in both arms were from chemotherapy, Dr. Oh commented. Discontinuation of any study medication because of toxicity occurred in 8.9% and 11.4% of patients, respectively.
Enticing benefit, but questions remain
In her discussion of the paper, Dr. Azad pointed out that Asian patients comprised more than half of the cohort and appeared to derive more benefit from the investigational treatment compared to other groups. “So the question is if that is driving the benefit or just an increased benefit,” she said. “That is going to be an open question for our research community.”
Dr. Azad also noted that patients with nonmetastatic disease at enrollment did a little better, so more data are needed on how that affected the outcomes.
“PDL-1 just missed statistical significance, but that is something that will be further explored,” she said. “And we still have open questions about viral hepatitis, liver fluke infection, and cirrhosis, and I do hope that these will be included in the final analysis of the study.”
The GICS meeting is organized by the American Society of Clinical Oncology (ASCO), and the society highlighted these data in a press release. Cathy Eng, MD, FACP, ASCO expert in gastrointestinal cancers, commented in the statement: “TOPAZ-1 is the first phase 3 trial to demonstrate the benefit of immunotherapy for improved overall survival, in combination with chemotherapy, creating a new standard of care.”
The study received funding from AstraZeneca, marker of durvalumab. Dr. Oh and Dr. Azad reported relationships with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
, according to interim results from the TOPAZ-1 trial.
The risk of death for those taking durvalumab plus chemotherapy was 20% lower than for patients on chemotherapy alone. At 18 months, overall survival was 35.1% in the durvalumab group versus 25.6% for chemotherapy alone. By 2 years, overall survival was 24.9% versus 10.4%.
“TOPAZ-1 is the first phase 3 trial to show that adding immunotherapy to standard chemotherapy can increase survival in biliary tract cancer, and importantly, does so without inducing any new serious side effects,” said lead author Do-Youn Oh, MD, PhD, professor in the Division of Medical Oncology at Seoul National University Hospital and Seoul National University College of Medicine, Korea.
“The study met its primary endpoint at a prespecified interim analysis, and durvalumab plus gemcitabine and cisplatin demonstrated statistically significant and clinically meaningful prolonged overall survival compared with placebo plus chemotherapy,” she said.
“This is an effective first-line therapy and could become a new standard of care for patients with advanced biliary tract cancer,” she added.
Dr. Oh presented the results at the Gastrointestinal Cancers Symposium (GICS) 2022.
In a discussion of the paper, Nilofer Saba Azad, MD, from the department of oncology, Johns Hopkins Sidney Kimmel Cancer Center, Baltimore, noted that overall, “we are seeing enticing benefit in survival and response rate.”
“There is moderately strong preliminary clinical data and biological rationale that immune checkpoint may have some activity in biliary tract cancer,” she said. “The trial was adequately powered and accounted for important known clinical subsets, and [it] was placebo controlled. The results suggest a meaningful benefit for patients.”
However, she pointed out that there are still open questions, mostly having to do with the subgroup analysis.
Biliary tract cancer: Incidence is rising
Biliary tract cancers are a relatively rare and heterogeneous group of cancers, and global incidence is rising. “Advanced unresectable biliary tract cancer is an area of high unmet need due to its aggressive nature, limited treatment options, and poor prognosis,” explained Dr. Oh. “The first-line standard of care for advanced biliary tract cancers, gemcitabine and cisplatin, has remained unchanged for over a decade.”
Previous research has suggested that checkpoint inhibition may result in antitumor immune responses, she commented. A previous phase 2 trial showed that durvalumab combined with gemcitabine and cisplatin showed promising antitumor activity in advanced biliary tract cancer. This latest study is a larger phase 3 trial to investigate this effect further.
The study involved 365 patients with unresectable locally advanced, recurrent, or metastatic biliary tract cancers. Patients had one of three types of biliary tract cancer: 55% had intrahepatic cancers; 19% had extrahepatic cancers; and 25% had gallbladder cancer.
The trial was conducted in the U.S. and 17 countries in Europe, South America, and Asia. Nearly 55% of the cohort was from Asia, including South Korea, Thailand, Japan, and China.
All patients received chemotherapy with gemcitabine (1,000 mg/m2) and cisplatin (25 mg/m2 on days 1 and 8 every 3 weeks) for up to eight cycles.
Patients were randomized to receive either durvalumab (1,500 mg every 3 weeks) or placebo before chemotherapy and also to receive durvalumab (1,500 mg every 4 weeks) or placebo after chemotherapy until disease progression or unacceptable toxicity.
At approximately 1 year, the authors found that adding durvalumab significantly improved overall survival (hazard ratio, 0.80; P = .021).
Progression-free survival was also significantly better with durvalumab compared to placebo: 7.2 months versus 5.7 months (HR, 0.75; P = .001).
The overall response rate (ORR) was 26.7% with durvalumab and 18.7% with placebo.
The most common adverse events were anemia (experienced by 48.2% of patients), neutropenia (31.7%), and nausea (40.2%). Grade 3/4 adverse events occurred in 75.7% of patients receiving durvalumab versus 77.8% for placebo, indicating that the majority of side effects in both arms were from chemotherapy, Dr. Oh commented. Discontinuation of any study medication because of toxicity occurred in 8.9% and 11.4% of patients, respectively.
Enticing benefit, but questions remain
In her discussion of the paper, Dr. Azad pointed out that Asian patients comprised more than half of the cohort and appeared to derive more benefit from the investigational treatment compared to other groups. “So the question is if that is driving the benefit or just an increased benefit,” she said. “That is going to be an open question for our research community.”
Dr. Azad also noted that patients with nonmetastatic disease at enrollment did a little better, so more data are needed on how that affected the outcomes.
“PDL-1 just missed statistical significance, but that is something that will be further explored,” she said. “And we still have open questions about viral hepatitis, liver fluke infection, and cirrhosis, and I do hope that these will be included in the final analysis of the study.”
The GICS meeting is organized by the American Society of Clinical Oncology (ASCO), and the society highlighted these data in a press release. Cathy Eng, MD, FACP, ASCO expert in gastrointestinal cancers, commented in the statement: “TOPAZ-1 is the first phase 3 trial to demonstrate the benefit of immunotherapy for improved overall survival, in combination with chemotherapy, creating a new standard of care.”
The study received funding from AstraZeneca, marker of durvalumab. Dr. Oh and Dr. Azad reported relationships with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
, according to interim results from the TOPAZ-1 trial.
The risk of death for those taking durvalumab plus chemotherapy was 20% lower than for patients on chemotherapy alone. At 18 months, overall survival was 35.1% in the durvalumab group versus 25.6% for chemotherapy alone. By 2 years, overall survival was 24.9% versus 10.4%.
“TOPAZ-1 is the first phase 3 trial to show that adding immunotherapy to standard chemotherapy can increase survival in biliary tract cancer, and importantly, does so without inducing any new serious side effects,” said lead author Do-Youn Oh, MD, PhD, professor in the Division of Medical Oncology at Seoul National University Hospital and Seoul National University College of Medicine, Korea.
“The study met its primary endpoint at a prespecified interim analysis, and durvalumab plus gemcitabine and cisplatin demonstrated statistically significant and clinically meaningful prolonged overall survival compared with placebo plus chemotherapy,” she said.
“This is an effective first-line therapy and could become a new standard of care for patients with advanced biliary tract cancer,” she added.
Dr. Oh presented the results at the Gastrointestinal Cancers Symposium (GICS) 2022.
In a discussion of the paper, Nilofer Saba Azad, MD, from the department of oncology, Johns Hopkins Sidney Kimmel Cancer Center, Baltimore, noted that overall, “we are seeing enticing benefit in survival and response rate.”
“There is moderately strong preliminary clinical data and biological rationale that immune checkpoint may have some activity in biliary tract cancer,” she said. “The trial was adequately powered and accounted for important known clinical subsets, and [it] was placebo controlled. The results suggest a meaningful benefit for patients.”
However, she pointed out that there are still open questions, mostly having to do with the subgroup analysis.
Biliary tract cancer: Incidence is rising
Biliary tract cancers are a relatively rare and heterogeneous group of cancers, and global incidence is rising. “Advanced unresectable biliary tract cancer is an area of high unmet need due to its aggressive nature, limited treatment options, and poor prognosis,” explained Dr. Oh. “The first-line standard of care for advanced biliary tract cancers, gemcitabine and cisplatin, has remained unchanged for over a decade.”
Previous research has suggested that checkpoint inhibition may result in antitumor immune responses, she commented. A previous phase 2 trial showed that durvalumab combined with gemcitabine and cisplatin showed promising antitumor activity in advanced biliary tract cancer. This latest study is a larger phase 3 trial to investigate this effect further.
The study involved 365 patients with unresectable locally advanced, recurrent, or metastatic biliary tract cancers. Patients had one of three types of biliary tract cancer: 55% had intrahepatic cancers; 19% had extrahepatic cancers; and 25% had gallbladder cancer.
The trial was conducted in the U.S. and 17 countries in Europe, South America, and Asia. Nearly 55% of the cohort was from Asia, including South Korea, Thailand, Japan, and China.
All patients received chemotherapy with gemcitabine (1,000 mg/m2) and cisplatin (25 mg/m2 on days 1 and 8 every 3 weeks) for up to eight cycles.
Patients were randomized to receive either durvalumab (1,500 mg every 3 weeks) or placebo before chemotherapy and also to receive durvalumab (1,500 mg every 4 weeks) or placebo after chemotherapy until disease progression or unacceptable toxicity.
At approximately 1 year, the authors found that adding durvalumab significantly improved overall survival (hazard ratio, 0.80; P = .021).
Progression-free survival was also significantly better with durvalumab compared to placebo: 7.2 months versus 5.7 months (HR, 0.75; P = .001).
The overall response rate (ORR) was 26.7% with durvalumab and 18.7% with placebo.
The most common adverse events were anemia (experienced by 48.2% of patients), neutropenia (31.7%), and nausea (40.2%). Grade 3/4 adverse events occurred in 75.7% of patients receiving durvalumab versus 77.8% for placebo, indicating that the majority of side effects in both arms were from chemotherapy, Dr. Oh commented. Discontinuation of any study medication because of toxicity occurred in 8.9% and 11.4% of patients, respectively.
Enticing benefit, but questions remain
In her discussion of the paper, Dr. Azad pointed out that Asian patients comprised more than half of the cohort and appeared to derive more benefit from the investigational treatment compared to other groups. “So the question is if that is driving the benefit or just an increased benefit,” she said. “That is going to be an open question for our research community.”
Dr. Azad also noted that patients with nonmetastatic disease at enrollment did a little better, so more data are needed on how that affected the outcomes.
“PDL-1 just missed statistical significance, but that is something that will be further explored,” she said. “And we still have open questions about viral hepatitis, liver fluke infection, and cirrhosis, and I do hope that these will be included in the final analysis of the study.”
The GICS meeting is organized by the American Society of Clinical Oncology (ASCO), and the society highlighted these data in a press release. Cathy Eng, MD, FACP, ASCO expert in gastrointestinal cancers, commented in the statement: “TOPAZ-1 is the first phase 3 trial to demonstrate the benefit of immunotherapy for improved overall survival, in combination with chemotherapy, creating a new standard of care.”
The study received funding from AstraZeneca, marker of durvalumab. Dr. Oh and Dr. Azad reported relationships with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM GICS 2022
Uptake uncertain for potent new LDL-lowerer inclisiran
As inclisiran, a first-in-class LDL-cholesterol lowering drug, enters the U.S. market following Food and Drug Administration approval in December 2021, several issues muddy how popular inclisiran will be in actual practice. That’s despite stellar phase 3 trial evidence for safety, tolerability, and a potent lipid-lowering effect.
The active ingredient of inclisiran (Leqvio) is a small interfering RNA (siRNA) molecule that shuts down production of the PCSK9 (proprotein convertase subtilisin/kexin type 9) protein, an enzyme that’s made and functions primarily in the liver and degrades cellular receptors for LDL cholesterol. Inhibiting PCSK9 production means LDL-cholesterol receptors accumulate and boost the ability of liver cells to pull more LDL cholesterol out of blood.
PCSK9 inhibition is the most potent LDL-cholesterol lowering method now available, and it works well in patients who have maxed out LDL reduction by diet and statin treatment. The siRNA of inclisiran is tweaked to target the molecule to the surface of liver cells following subcutaneous injection. Other modifications of the siRNA give it stability that allows twice-a-year dosing, although patients receive a third injection during their first year to hasten a maximum treatment effect.
Inclisiran’s FDA approval relied on results from three pivotal trials that together enrolled 3,660 patients with either atherosclerotic cardiovascular disease (ASCVD), ASCVD risk equivalents, or heterozygous familial hypercholesterolemia (HeFH), and LDL-cholesterol levels of at least 70 mg/dL in those with established ASCVD, or at least 100 mg/dL in other patients. (HeFH and ASCVD are the drug’s approved indications.) Pooled data from the three trials showed that inclisiran was safe and well tolerated during 18 months and produced an average LDL-cholesterol reduction after 510 days (1.4 years) of about 51% compared to baseline after correction for placebo effects (J Am Coll Cardiol. 2021 Mar 9;77 [9]:1182-93).
These data showed inclisiran was about as safe and effective for reducing LDL-cholesterol as agents from another class of PCSK9 inhibitors that rely on injected antibodies to inactivate PCSK9. Two agents from this class, alirocumab (Praluent) and evolocumab (Repatha), both came on the U.S. market in 2015. Although their performance in routine practice during the ensuing 6-plus years has been as safe and effective as what they showed in their respective registration trials, they have faced a rocky uptake road that’s been primarily hindered by the hefty price tag that both drugs carry.
Prior-authorization blues
When they first came out, evolocumab and alirocumab were burdened by annual drug costs of roughly $14,000, a fact that led to widespread prior-authorization and copay barriers set up by U.S. insurers. Although these barriers gradually lessened over time, in part aided by a substantial price cut for both drugs that led to annual drug costs more in the range of $6,000/year, they remain relatively pricey and are still not easy to start in patients because of prior-authorization requirements, said clinicians.
Recent penetration of the older PCSK9 inhibitors into eligible U.S. patients “is only about 1%-2%, based on the latest data,” said Michael H. Davidson, MD, a lipid specialist and director of Preventive Cardiology at the University of Chicago.
“We have these great, effective drugs, but they haven’t really made an impact over the past 5 years,” because of very limited uptake, a situation Dr. Davidson called “very disappointing,” during an interview.
Given this recent history, inclisiran, another expensive PCSK9 inhibitor, may face similar coverage pushback as it hits the U.S. market with a retail price, announced by its manufacturer Novartis, of $3,250/dose. This means that patients who start the drug and receive their initial dose, a second dose after 3 months, and then additional doses every 6 months, rack up a drug cost of close to $10,000 the first year on the drug and $6,500 each subsequent year.
This treatment schedule highlights the major logistical difference that distinguishes inclisiran from the antibody-based PCSK9 inhibitors, which are given by repeated subcutaneous injection every 2 or 4 weeks, usually with patients self-injecting the drugs at home. The less-frequent dosing schedule for inclisiran prompted the drug’s developers to schedule injections by a clinician in an office setting in the pivotal trials, which led to labeling for inclisiran that specifies administration only by a health care professional.
The ‘buy-and-bill’ coverage model
This difference in drug administration between inclisiran and the antibody-based PCSK9 inhibitors set up Novartis to promote insurance reimbursement for inclisiran using a “buy-and-bill” paradigm that was first developed for oncology drugs and which may provide a loophole around the prior-authorization roadblocks that hindered early uptake of the antibody-based PCSK9 inhibitors.
It’s also an approach that has made U.S. clinicians unsure how it will play out in practice. Infrequent inclisiran dosing may also boost patient compliance.
“Adherence is the greatest challenge in preventive cardiology, and thus inclisiran has the potential to be a game changer,” commented Christie M. Ballantyne, MD, professor and chief of cardiology at Baylor College of Medicine, Houston.
“Will it be easier for physicians to write a prescription and for patients to get the medication without a demanding and frustrating prior-authorization process?” he wondered during an interview. “I’m waiting to see how this unfolds, especially in systems where pharmacy is not fully integrated with the outpatient setting. In some ways, this is as big of an experiment as was development of the drug,” Dr. Ballantyne said.
Although the prior-authorization hoops for evolocumab and alirocumab have become easier to jump through, “most physicians don’t have the resources to handle it and don’t bother,” noted Dr. Davidson, and he’s concerned that infrastructure challenges will also hamper the buy-and-bill strategy for inclisiran.
He also expressed skepticism that the prior-authorization barrier will disappear. “Payers don’t want to open a large population to a very expensive drug without some gatekeeping,” he said, while acknowledging that in late January 2022 he did not yet have personal experience administering inclisiran or navigating its insurance reimbursement.
Boosting patient compliance
Dr. Davidson agreed that the prospect for enhanced patient compliance with inclisiran was intriguing and had already drawn the interest of some of his patients.
“There is a lot of appeal” to a treatment that’s only given once every 6 months, he said. “Compliance is a major issue, and this is less work for patients.”
“The biggest possible attraction of inclisiran is that it is given twice a year, but whether this plays out as anticipated in the real world need to be seen,” cautioned Vijay Nambi, MD, a cardiologist at the Michael E. DeBakey VA Hospital, Houston, and at Baylor College of Medicine who has written about inclisiran. He noted that while two doses a year is “on paper very attractive,” this scheme opens the door to missed or delayed appointments because of vacations, other patient travel, or events like a pandemic.
“The biggest pro for inclisiran is the dosing schedule,” said Chandni Bardolia, PharmD, a drug information specialist at Tabula Rasa Healthcare, Moorestown, N.J., who has analyzed and written about inclisiran and other lipid-lowering medications. “Twice yearly dosing following initiation will be a huge benefit to improve adherence and reduce the number of injections.”
However, inclisiran’s attractive dosing schedule as well as its safety and potent efficacy do not tell the whole story, she highlighted in an interview.
Inclisiran’s clinical evidence still cooking
“I see inclisiran as a last-line drug, mainly because the current alternatives have more safety and efficacy data,” Dr. Bardolia said.
Inclisiran’s “cost and the fact that there are other agents with clinical outcome data already available [alirocumab and evolocumab] means inclisiran is not a first-line agent after statins,” agreed Dr. Nambi.
The FDA based its inclisiran approval entirely on the drug’s demonstrated safety and LDL-lowering efficacy. The cardiovascular outcomes trial for inclisiran, ORION-4, with about 15,000 enrolled patients, started in 2018 and remains in progress with full results expected in 2026.
The lack of clinical outcomes data for inclisiran is a major limitation, said Neil J. Stone, MD, a cardiologist and professor at Northwestern University, Chicago, and vice chair of the panel that wrote the most recent cholesterol guideline for the American College of Cardiology and American Heart Association.
“My greatest concern is the lack of outcome trial data. That’s very important,” Dr. Stone said in an interview.
But others minimize this limitation given the overwhelming evidence that links lower levels of LDL-cholesterol to reduced clinical events.
Most clinicians “support lower LDL as a surrogate” for reduced clinical events, “just like blood pressure and hemoglobin A1c,” noted Dr. Davidson, although he conceded that a “substantial minority wants to wait to see inclisiran’s outcome benefits.”
It’s all about price
While opinions are mixed on the need for clinical outcomes data, experts are more uniform in seeing drug prices that run to several thousands per year as the main uptake issue.
“We need to look at the cost-efficacy with inclisiran, and we need benefit data to determine this,” said Dr. Stone.
“Outcomes data are central to characterizing value. I imagine that costs will impact adoption and dissemination” of inclisiran, commented Paul L. Hess, MD, a cardiologist at the Rocky Mountain Regional VA Medical Center, Denver.
Patient interest in less frequent dosing will be important for driving use, but “ultimately cost will be the most important driving factor,” for inclisiran uptake, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado School of Medicine, Aurora.
Dr. Davidson has ties to New Amsterdam Pharma and Amgen, which markets evolocumab (Repatha). Dr. Ballantyne is a consultant to numerous companies, including Amgen and Regeneron, which market alirocumab (Praluent). Dr. Nambi has been a site investigator for studies sponsored by Amgen, and by Merck, which markets the LDL-cholesterol drug ezetimibe (Zetia) and is developing an oral PCSK9 inhibitor (he said that the views he expressed are his own and don’t represent that of the department of Veterans Affairs or Baylor.) Dr. Bardolia had no disclosures beyond her employment at Tabula Rasa Healthcare. Dr. Stone, Dr. Hess, and Dr. Eckel had no relevant disclosures.
As inclisiran, a first-in-class LDL-cholesterol lowering drug, enters the U.S. market following Food and Drug Administration approval in December 2021, several issues muddy how popular inclisiran will be in actual practice. That’s despite stellar phase 3 trial evidence for safety, tolerability, and a potent lipid-lowering effect.
The active ingredient of inclisiran (Leqvio) is a small interfering RNA (siRNA) molecule that shuts down production of the PCSK9 (proprotein convertase subtilisin/kexin type 9) protein, an enzyme that’s made and functions primarily in the liver and degrades cellular receptors for LDL cholesterol. Inhibiting PCSK9 production means LDL-cholesterol receptors accumulate and boost the ability of liver cells to pull more LDL cholesterol out of blood.
PCSK9 inhibition is the most potent LDL-cholesterol lowering method now available, and it works well in patients who have maxed out LDL reduction by diet and statin treatment. The siRNA of inclisiran is tweaked to target the molecule to the surface of liver cells following subcutaneous injection. Other modifications of the siRNA give it stability that allows twice-a-year dosing, although patients receive a third injection during their first year to hasten a maximum treatment effect.
Inclisiran’s FDA approval relied on results from three pivotal trials that together enrolled 3,660 patients with either atherosclerotic cardiovascular disease (ASCVD), ASCVD risk equivalents, or heterozygous familial hypercholesterolemia (HeFH), and LDL-cholesterol levels of at least 70 mg/dL in those with established ASCVD, or at least 100 mg/dL in other patients. (HeFH and ASCVD are the drug’s approved indications.) Pooled data from the three trials showed that inclisiran was safe and well tolerated during 18 months and produced an average LDL-cholesterol reduction after 510 days (1.4 years) of about 51% compared to baseline after correction for placebo effects (J Am Coll Cardiol. 2021 Mar 9;77 [9]:1182-93).
These data showed inclisiran was about as safe and effective for reducing LDL-cholesterol as agents from another class of PCSK9 inhibitors that rely on injected antibodies to inactivate PCSK9. Two agents from this class, alirocumab (Praluent) and evolocumab (Repatha), both came on the U.S. market in 2015. Although their performance in routine practice during the ensuing 6-plus years has been as safe and effective as what they showed in their respective registration trials, they have faced a rocky uptake road that’s been primarily hindered by the hefty price tag that both drugs carry.
Prior-authorization blues
When they first came out, evolocumab and alirocumab were burdened by annual drug costs of roughly $14,000, a fact that led to widespread prior-authorization and copay barriers set up by U.S. insurers. Although these barriers gradually lessened over time, in part aided by a substantial price cut for both drugs that led to annual drug costs more in the range of $6,000/year, they remain relatively pricey and are still not easy to start in patients because of prior-authorization requirements, said clinicians.
Recent penetration of the older PCSK9 inhibitors into eligible U.S. patients “is only about 1%-2%, based on the latest data,” said Michael H. Davidson, MD, a lipid specialist and director of Preventive Cardiology at the University of Chicago.
“We have these great, effective drugs, but they haven’t really made an impact over the past 5 years,” because of very limited uptake, a situation Dr. Davidson called “very disappointing,” during an interview.
Given this recent history, inclisiran, another expensive PCSK9 inhibitor, may face similar coverage pushback as it hits the U.S. market with a retail price, announced by its manufacturer Novartis, of $3,250/dose. This means that patients who start the drug and receive their initial dose, a second dose after 3 months, and then additional doses every 6 months, rack up a drug cost of close to $10,000 the first year on the drug and $6,500 each subsequent year.
This treatment schedule highlights the major logistical difference that distinguishes inclisiran from the antibody-based PCSK9 inhibitors, which are given by repeated subcutaneous injection every 2 or 4 weeks, usually with patients self-injecting the drugs at home. The less-frequent dosing schedule for inclisiran prompted the drug’s developers to schedule injections by a clinician in an office setting in the pivotal trials, which led to labeling for inclisiran that specifies administration only by a health care professional.
The ‘buy-and-bill’ coverage model
This difference in drug administration between inclisiran and the antibody-based PCSK9 inhibitors set up Novartis to promote insurance reimbursement for inclisiran using a “buy-and-bill” paradigm that was first developed for oncology drugs and which may provide a loophole around the prior-authorization roadblocks that hindered early uptake of the antibody-based PCSK9 inhibitors.
It’s also an approach that has made U.S. clinicians unsure how it will play out in practice. Infrequent inclisiran dosing may also boost patient compliance.
“Adherence is the greatest challenge in preventive cardiology, and thus inclisiran has the potential to be a game changer,” commented Christie M. Ballantyne, MD, professor and chief of cardiology at Baylor College of Medicine, Houston.
“Will it be easier for physicians to write a prescription and for patients to get the medication without a demanding and frustrating prior-authorization process?” he wondered during an interview. “I’m waiting to see how this unfolds, especially in systems where pharmacy is not fully integrated with the outpatient setting. In some ways, this is as big of an experiment as was development of the drug,” Dr. Ballantyne said.
Although the prior-authorization hoops for evolocumab and alirocumab have become easier to jump through, “most physicians don’t have the resources to handle it and don’t bother,” noted Dr. Davidson, and he’s concerned that infrastructure challenges will also hamper the buy-and-bill strategy for inclisiran.
He also expressed skepticism that the prior-authorization barrier will disappear. “Payers don’t want to open a large population to a very expensive drug without some gatekeeping,” he said, while acknowledging that in late January 2022 he did not yet have personal experience administering inclisiran or navigating its insurance reimbursement.
Boosting patient compliance
Dr. Davidson agreed that the prospect for enhanced patient compliance with inclisiran was intriguing and had already drawn the interest of some of his patients.
“There is a lot of appeal” to a treatment that’s only given once every 6 months, he said. “Compliance is a major issue, and this is less work for patients.”
“The biggest possible attraction of inclisiran is that it is given twice a year, but whether this plays out as anticipated in the real world need to be seen,” cautioned Vijay Nambi, MD, a cardiologist at the Michael E. DeBakey VA Hospital, Houston, and at Baylor College of Medicine who has written about inclisiran. He noted that while two doses a year is “on paper very attractive,” this scheme opens the door to missed or delayed appointments because of vacations, other patient travel, or events like a pandemic.
“The biggest pro for inclisiran is the dosing schedule,” said Chandni Bardolia, PharmD, a drug information specialist at Tabula Rasa Healthcare, Moorestown, N.J., who has analyzed and written about inclisiran and other lipid-lowering medications. “Twice yearly dosing following initiation will be a huge benefit to improve adherence and reduce the number of injections.”
However, inclisiran’s attractive dosing schedule as well as its safety and potent efficacy do not tell the whole story, she highlighted in an interview.
Inclisiran’s clinical evidence still cooking
“I see inclisiran as a last-line drug, mainly because the current alternatives have more safety and efficacy data,” Dr. Bardolia said.
Inclisiran’s “cost and the fact that there are other agents with clinical outcome data already available [alirocumab and evolocumab] means inclisiran is not a first-line agent after statins,” agreed Dr. Nambi.
The FDA based its inclisiran approval entirely on the drug’s demonstrated safety and LDL-lowering efficacy. The cardiovascular outcomes trial for inclisiran, ORION-4, with about 15,000 enrolled patients, started in 2018 and remains in progress with full results expected in 2026.
The lack of clinical outcomes data for inclisiran is a major limitation, said Neil J. Stone, MD, a cardiologist and professor at Northwestern University, Chicago, and vice chair of the panel that wrote the most recent cholesterol guideline for the American College of Cardiology and American Heart Association.
“My greatest concern is the lack of outcome trial data. That’s very important,” Dr. Stone said in an interview.
But others minimize this limitation given the overwhelming evidence that links lower levels of LDL-cholesterol to reduced clinical events.
Most clinicians “support lower LDL as a surrogate” for reduced clinical events, “just like blood pressure and hemoglobin A1c,” noted Dr. Davidson, although he conceded that a “substantial minority wants to wait to see inclisiran’s outcome benefits.”
It’s all about price
While opinions are mixed on the need for clinical outcomes data, experts are more uniform in seeing drug prices that run to several thousands per year as the main uptake issue.
“We need to look at the cost-efficacy with inclisiran, and we need benefit data to determine this,” said Dr. Stone.
“Outcomes data are central to characterizing value. I imagine that costs will impact adoption and dissemination” of inclisiran, commented Paul L. Hess, MD, a cardiologist at the Rocky Mountain Regional VA Medical Center, Denver.
Patient interest in less frequent dosing will be important for driving use, but “ultimately cost will be the most important driving factor,” for inclisiran uptake, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado School of Medicine, Aurora.
Dr. Davidson has ties to New Amsterdam Pharma and Amgen, which markets evolocumab (Repatha). Dr. Ballantyne is a consultant to numerous companies, including Amgen and Regeneron, which market alirocumab (Praluent). Dr. Nambi has been a site investigator for studies sponsored by Amgen, and by Merck, which markets the LDL-cholesterol drug ezetimibe (Zetia) and is developing an oral PCSK9 inhibitor (he said that the views he expressed are his own and don’t represent that of the department of Veterans Affairs or Baylor.) Dr. Bardolia had no disclosures beyond her employment at Tabula Rasa Healthcare. Dr. Stone, Dr. Hess, and Dr. Eckel had no relevant disclosures.
As inclisiran, a first-in-class LDL-cholesterol lowering drug, enters the U.S. market following Food and Drug Administration approval in December 2021, several issues muddy how popular inclisiran will be in actual practice. That’s despite stellar phase 3 trial evidence for safety, tolerability, and a potent lipid-lowering effect.
The active ingredient of inclisiran (Leqvio) is a small interfering RNA (siRNA) molecule that shuts down production of the PCSK9 (proprotein convertase subtilisin/kexin type 9) protein, an enzyme that’s made and functions primarily in the liver and degrades cellular receptors for LDL cholesterol. Inhibiting PCSK9 production means LDL-cholesterol receptors accumulate and boost the ability of liver cells to pull more LDL cholesterol out of blood.
PCSK9 inhibition is the most potent LDL-cholesterol lowering method now available, and it works well in patients who have maxed out LDL reduction by diet and statin treatment. The siRNA of inclisiran is tweaked to target the molecule to the surface of liver cells following subcutaneous injection. Other modifications of the siRNA give it stability that allows twice-a-year dosing, although patients receive a third injection during their first year to hasten a maximum treatment effect.
Inclisiran’s FDA approval relied on results from three pivotal trials that together enrolled 3,660 patients with either atherosclerotic cardiovascular disease (ASCVD), ASCVD risk equivalents, or heterozygous familial hypercholesterolemia (HeFH), and LDL-cholesterol levels of at least 70 mg/dL in those with established ASCVD, or at least 100 mg/dL in other patients. (HeFH and ASCVD are the drug’s approved indications.) Pooled data from the three trials showed that inclisiran was safe and well tolerated during 18 months and produced an average LDL-cholesterol reduction after 510 days (1.4 years) of about 51% compared to baseline after correction for placebo effects (J Am Coll Cardiol. 2021 Mar 9;77 [9]:1182-93).
These data showed inclisiran was about as safe and effective for reducing LDL-cholesterol as agents from another class of PCSK9 inhibitors that rely on injected antibodies to inactivate PCSK9. Two agents from this class, alirocumab (Praluent) and evolocumab (Repatha), both came on the U.S. market in 2015. Although their performance in routine practice during the ensuing 6-plus years has been as safe and effective as what they showed in their respective registration trials, they have faced a rocky uptake road that’s been primarily hindered by the hefty price tag that both drugs carry.
Prior-authorization blues
When they first came out, evolocumab and alirocumab were burdened by annual drug costs of roughly $14,000, a fact that led to widespread prior-authorization and copay barriers set up by U.S. insurers. Although these barriers gradually lessened over time, in part aided by a substantial price cut for both drugs that led to annual drug costs more in the range of $6,000/year, they remain relatively pricey and are still not easy to start in patients because of prior-authorization requirements, said clinicians.
Recent penetration of the older PCSK9 inhibitors into eligible U.S. patients “is only about 1%-2%, based on the latest data,” said Michael H. Davidson, MD, a lipid specialist and director of Preventive Cardiology at the University of Chicago.
“We have these great, effective drugs, but they haven’t really made an impact over the past 5 years,” because of very limited uptake, a situation Dr. Davidson called “very disappointing,” during an interview.
Given this recent history, inclisiran, another expensive PCSK9 inhibitor, may face similar coverage pushback as it hits the U.S. market with a retail price, announced by its manufacturer Novartis, of $3,250/dose. This means that patients who start the drug and receive their initial dose, a second dose after 3 months, and then additional doses every 6 months, rack up a drug cost of close to $10,000 the first year on the drug and $6,500 each subsequent year.
This treatment schedule highlights the major logistical difference that distinguishes inclisiran from the antibody-based PCSK9 inhibitors, which are given by repeated subcutaneous injection every 2 or 4 weeks, usually with patients self-injecting the drugs at home. The less-frequent dosing schedule for inclisiran prompted the drug’s developers to schedule injections by a clinician in an office setting in the pivotal trials, which led to labeling for inclisiran that specifies administration only by a health care professional.
The ‘buy-and-bill’ coverage model
This difference in drug administration between inclisiran and the antibody-based PCSK9 inhibitors set up Novartis to promote insurance reimbursement for inclisiran using a “buy-and-bill” paradigm that was first developed for oncology drugs and which may provide a loophole around the prior-authorization roadblocks that hindered early uptake of the antibody-based PCSK9 inhibitors.
It’s also an approach that has made U.S. clinicians unsure how it will play out in practice. Infrequent inclisiran dosing may also boost patient compliance.
“Adherence is the greatest challenge in preventive cardiology, and thus inclisiran has the potential to be a game changer,” commented Christie M. Ballantyne, MD, professor and chief of cardiology at Baylor College of Medicine, Houston.
“Will it be easier for physicians to write a prescription and for patients to get the medication without a demanding and frustrating prior-authorization process?” he wondered during an interview. “I’m waiting to see how this unfolds, especially in systems where pharmacy is not fully integrated with the outpatient setting. In some ways, this is as big of an experiment as was development of the drug,” Dr. Ballantyne said.
Although the prior-authorization hoops for evolocumab and alirocumab have become easier to jump through, “most physicians don’t have the resources to handle it and don’t bother,” noted Dr. Davidson, and he’s concerned that infrastructure challenges will also hamper the buy-and-bill strategy for inclisiran.
He also expressed skepticism that the prior-authorization barrier will disappear. “Payers don’t want to open a large population to a very expensive drug without some gatekeeping,” he said, while acknowledging that in late January 2022 he did not yet have personal experience administering inclisiran or navigating its insurance reimbursement.
Boosting patient compliance
Dr. Davidson agreed that the prospect for enhanced patient compliance with inclisiran was intriguing and had already drawn the interest of some of his patients.
“There is a lot of appeal” to a treatment that’s only given once every 6 months, he said. “Compliance is a major issue, and this is less work for patients.”
“The biggest possible attraction of inclisiran is that it is given twice a year, but whether this plays out as anticipated in the real world need to be seen,” cautioned Vijay Nambi, MD, a cardiologist at the Michael E. DeBakey VA Hospital, Houston, and at Baylor College of Medicine who has written about inclisiran. He noted that while two doses a year is “on paper very attractive,” this scheme opens the door to missed or delayed appointments because of vacations, other patient travel, or events like a pandemic.
“The biggest pro for inclisiran is the dosing schedule,” said Chandni Bardolia, PharmD, a drug information specialist at Tabula Rasa Healthcare, Moorestown, N.J., who has analyzed and written about inclisiran and other lipid-lowering medications. “Twice yearly dosing following initiation will be a huge benefit to improve adherence and reduce the number of injections.”
However, inclisiran’s attractive dosing schedule as well as its safety and potent efficacy do not tell the whole story, she highlighted in an interview.
Inclisiran’s clinical evidence still cooking
“I see inclisiran as a last-line drug, mainly because the current alternatives have more safety and efficacy data,” Dr. Bardolia said.
Inclisiran’s “cost and the fact that there are other agents with clinical outcome data already available [alirocumab and evolocumab] means inclisiran is not a first-line agent after statins,” agreed Dr. Nambi.
The FDA based its inclisiran approval entirely on the drug’s demonstrated safety and LDL-lowering efficacy. The cardiovascular outcomes trial for inclisiran, ORION-4, with about 15,000 enrolled patients, started in 2018 and remains in progress with full results expected in 2026.
The lack of clinical outcomes data for inclisiran is a major limitation, said Neil J. Stone, MD, a cardiologist and professor at Northwestern University, Chicago, and vice chair of the panel that wrote the most recent cholesterol guideline for the American College of Cardiology and American Heart Association.
“My greatest concern is the lack of outcome trial data. That’s very important,” Dr. Stone said in an interview.
But others minimize this limitation given the overwhelming evidence that links lower levels of LDL-cholesterol to reduced clinical events.
Most clinicians “support lower LDL as a surrogate” for reduced clinical events, “just like blood pressure and hemoglobin A1c,” noted Dr. Davidson, although he conceded that a “substantial minority wants to wait to see inclisiran’s outcome benefits.”
It’s all about price
While opinions are mixed on the need for clinical outcomes data, experts are more uniform in seeing drug prices that run to several thousands per year as the main uptake issue.
“We need to look at the cost-efficacy with inclisiran, and we need benefit data to determine this,” said Dr. Stone.
“Outcomes data are central to characterizing value. I imagine that costs will impact adoption and dissemination” of inclisiran, commented Paul L. Hess, MD, a cardiologist at the Rocky Mountain Regional VA Medical Center, Denver.
Patient interest in less frequent dosing will be important for driving use, but “ultimately cost will be the most important driving factor,” for inclisiran uptake, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado School of Medicine, Aurora.
Dr. Davidson has ties to New Amsterdam Pharma and Amgen, which markets evolocumab (Repatha). Dr. Ballantyne is a consultant to numerous companies, including Amgen and Regeneron, which market alirocumab (Praluent). Dr. Nambi has been a site investigator for studies sponsored by Amgen, and by Merck, which markets the LDL-cholesterol drug ezetimibe (Zetia) and is developing an oral PCSK9 inhibitor (he said that the views he expressed are his own and don’t represent that of the department of Veterans Affairs or Baylor.) Dr. Bardolia had no disclosures beyond her employment at Tabula Rasa Healthcare. Dr. Stone, Dr. Hess, and Dr. Eckel had no relevant disclosures.
Presence of autoantibodies most predictive of long COVID in study
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
FROM CELL
Moderate-vigorous stepping seen to lower diabetes risk in older women
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
FROM DIABETES CARE
Allopurinol found safe in patients with concomitant gout, CKD
Allopurinol treatment is not associated with increased mortality in patients with gout and chronic kidney disease even at 5 years after starting treatment, a study has found.
Around one in five patients with gout also have chronic kidney disease, and previous research suggests that hyperuricemia is itself a contributor to renal disease, which is why there has been interest in the use of serum urate–lowering medication in patients with both conditions.
Since the publication of two earlier randomized controlled trials suggested a twofold increase in mortality among patients with renal disease who were treated with allopurinol in an attempt to slow progression, there has been wariness about the drug in patients with compromised renal function.
In a study published in Annals of Internal Medicine, Jie Wei, PhD, of Xiangya Hospital at Central South University in Changsha, China, and coauthors report the results of their retrospective, population-based study of 5,277 adults aged 40 and older with gout and moderate to severe chronic kidney disease who were initiated on allopurinol and 5,277 matched individuals not on allopurinol.
At 5 years after the patients started allopurinol, the study found that mortality was a statistically significant 15% lower (hazard ratio, 0.85; 95% confidence interval, 0.77-0.93) among those on allopurinol, compared with those not taking the drug. The rate was 4.9 deaths per 100 person-years among those on allopurinol, compared with 5.8 among those not taking it.
The researchers also created two simulated randomized clinical trials from the data for initiators of allopurinol, replicating each initiator twice. The first trial assigned patient replicates either to achieving a target serum urate level of less than 0.36 mmol/L within a year or not achieving it. The second assigned patient replicates to either an allopurinol dose-escalation group or no dose escalation.
For the target serum urate level study, 1,484 achieved the target, and this was associated with a 13% lower hazard ratio for mortality that just missed statistical significance (HR, 0.87; 95% confidence interval, 0.75-1.01).
In the dose-escalation study, there were 773 participants who increased their dose of allopurinol in the first year after initiation – from a median of 100 mg/day to a median final dose of 300 mg/day – and 2,923 who didn’t. Those who escalated their dose had a nonsignificant 12% lower risk of mortality (HR, 0.88; 95% CI, 0.73-1.07), compared with those who didn’t.
The authors suggest that this could be the result of confounding, as patients who achieved target serum urate levels may have been of better health generally than those who didn’t, which could also have contributed to lower mortality.
Coauthor of the study Yuqing Zhang, DSc, of Massachusetts General Hospital and Harvard Medical School, Boston, said there had previously been a theory that allopurinol could protect against progression of renal disease. However, the two randomized, controlled trials in patients with chronic kidney disease but not gout published in 2020 suggested that allopurinol was instead associated with a doubling of mortality in this group.
“This study really shows convincing evidence that among gout patients with renal disease, allopurinol does not increase mortality,” Dr. Zhang told this news organization. He suggested the reason that the earlier studies had found higher mortality among patients on allopurinol was because those patients did not have gout. Given that gout can increase mortality, treating it effectively with allopurinol may therefore reduce mortality even in patients with concurrent chronic kidney disease.
Commenting on the study, Angelo Gaffo, MD, from the Birmingham VA Medical Center and the division of rheumatology at the University of Alabama at Birmingham, said that, while there had been data suggesting increased mortality, the findings from this “very well-done” study were reassuring and even suggested a possible decrease in mortality associated with allopurinol.
“I wouldn’t scream it out loud because it needs confirmation, but it’s something also that we have a sense that could be true,” he said.
Dr. Gaffo noted that patients treated with allopurinol tended to be those with fewer comorbidities. “Patients who have a lot of comorbidities probably are less likely to have their dose of allopurinol started or increased because of some concerns that practitioners may have about putting them on another medicine or increasing the dose of that medicine,” he said.
He also stressed that the findings still need replication in other large database studies, given that a prospective, randomized clinical trial addressing such a question would be difficult to conduct.
The study was supported by the Project Program of National Clinical Research Center for Geriatric Disorders, the National Natural Science Foundation of China, and the U.S. National Institutes of Health. Two authors reported consulting fees from the pharmaceutical sector unrelated to the study. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
Allopurinol treatment is not associated with increased mortality in patients with gout and chronic kidney disease even at 5 years after starting treatment, a study has found.
Around one in five patients with gout also have chronic kidney disease, and previous research suggests that hyperuricemia is itself a contributor to renal disease, which is why there has been interest in the use of serum urate–lowering medication in patients with both conditions.
Since the publication of two earlier randomized controlled trials suggested a twofold increase in mortality among patients with renal disease who were treated with allopurinol in an attempt to slow progression, there has been wariness about the drug in patients with compromised renal function.
In a study published in Annals of Internal Medicine, Jie Wei, PhD, of Xiangya Hospital at Central South University in Changsha, China, and coauthors report the results of their retrospective, population-based study of 5,277 adults aged 40 and older with gout and moderate to severe chronic kidney disease who were initiated on allopurinol and 5,277 matched individuals not on allopurinol.
At 5 years after the patients started allopurinol, the study found that mortality was a statistically significant 15% lower (hazard ratio, 0.85; 95% confidence interval, 0.77-0.93) among those on allopurinol, compared with those not taking the drug. The rate was 4.9 deaths per 100 person-years among those on allopurinol, compared with 5.8 among those not taking it.
The researchers also created two simulated randomized clinical trials from the data for initiators of allopurinol, replicating each initiator twice. The first trial assigned patient replicates either to achieving a target serum urate level of less than 0.36 mmol/L within a year or not achieving it. The second assigned patient replicates to either an allopurinol dose-escalation group or no dose escalation.
For the target serum urate level study, 1,484 achieved the target, and this was associated with a 13% lower hazard ratio for mortality that just missed statistical significance (HR, 0.87; 95% confidence interval, 0.75-1.01).
In the dose-escalation study, there were 773 participants who increased their dose of allopurinol in the first year after initiation – from a median of 100 mg/day to a median final dose of 300 mg/day – and 2,923 who didn’t. Those who escalated their dose had a nonsignificant 12% lower risk of mortality (HR, 0.88; 95% CI, 0.73-1.07), compared with those who didn’t.
The authors suggest that this could be the result of confounding, as patients who achieved target serum urate levels may have been of better health generally than those who didn’t, which could also have contributed to lower mortality.
Coauthor of the study Yuqing Zhang, DSc, of Massachusetts General Hospital and Harvard Medical School, Boston, said there had previously been a theory that allopurinol could protect against progression of renal disease. However, the two randomized, controlled trials in patients with chronic kidney disease but not gout published in 2020 suggested that allopurinol was instead associated with a doubling of mortality in this group.
“This study really shows convincing evidence that among gout patients with renal disease, allopurinol does not increase mortality,” Dr. Zhang told this news organization. He suggested the reason that the earlier studies had found higher mortality among patients on allopurinol was because those patients did not have gout. Given that gout can increase mortality, treating it effectively with allopurinol may therefore reduce mortality even in patients with concurrent chronic kidney disease.
Commenting on the study, Angelo Gaffo, MD, from the Birmingham VA Medical Center and the division of rheumatology at the University of Alabama at Birmingham, said that, while there had been data suggesting increased mortality, the findings from this “very well-done” study were reassuring and even suggested a possible decrease in mortality associated with allopurinol.
“I wouldn’t scream it out loud because it needs confirmation, but it’s something also that we have a sense that could be true,” he said.
Dr. Gaffo noted that patients treated with allopurinol tended to be those with fewer comorbidities. “Patients who have a lot of comorbidities probably are less likely to have their dose of allopurinol started or increased because of some concerns that practitioners may have about putting them on another medicine or increasing the dose of that medicine,” he said.
He also stressed that the findings still need replication in other large database studies, given that a prospective, randomized clinical trial addressing such a question would be difficult to conduct.
The study was supported by the Project Program of National Clinical Research Center for Geriatric Disorders, the National Natural Science Foundation of China, and the U.S. National Institutes of Health. Two authors reported consulting fees from the pharmaceutical sector unrelated to the study. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
Allopurinol treatment is not associated with increased mortality in patients with gout and chronic kidney disease even at 5 years after starting treatment, a study has found.
Around one in five patients with gout also have chronic kidney disease, and previous research suggests that hyperuricemia is itself a contributor to renal disease, which is why there has been interest in the use of serum urate–lowering medication in patients with both conditions.
Since the publication of two earlier randomized controlled trials suggested a twofold increase in mortality among patients with renal disease who were treated with allopurinol in an attempt to slow progression, there has been wariness about the drug in patients with compromised renal function.
In a study published in Annals of Internal Medicine, Jie Wei, PhD, of Xiangya Hospital at Central South University in Changsha, China, and coauthors report the results of their retrospective, population-based study of 5,277 adults aged 40 and older with gout and moderate to severe chronic kidney disease who were initiated on allopurinol and 5,277 matched individuals not on allopurinol.
At 5 years after the patients started allopurinol, the study found that mortality was a statistically significant 15% lower (hazard ratio, 0.85; 95% confidence interval, 0.77-0.93) among those on allopurinol, compared with those not taking the drug. The rate was 4.9 deaths per 100 person-years among those on allopurinol, compared with 5.8 among those not taking it.
The researchers also created two simulated randomized clinical trials from the data for initiators of allopurinol, replicating each initiator twice. The first trial assigned patient replicates either to achieving a target serum urate level of less than 0.36 mmol/L within a year or not achieving it. The second assigned patient replicates to either an allopurinol dose-escalation group or no dose escalation.
For the target serum urate level study, 1,484 achieved the target, and this was associated with a 13% lower hazard ratio for mortality that just missed statistical significance (HR, 0.87; 95% confidence interval, 0.75-1.01).
In the dose-escalation study, there were 773 participants who increased their dose of allopurinol in the first year after initiation – from a median of 100 mg/day to a median final dose of 300 mg/day – and 2,923 who didn’t. Those who escalated their dose had a nonsignificant 12% lower risk of mortality (HR, 0.88; 95% CI, 0.73-1.07), compared with those who didn’t.
The authors suggest that this could be the result of confounding, as patients who achieved target serum urate levels may have been of better health generally than those who didn’t, which could also have contributed to lower mortality.
Coauthor of the study Yuqing Zhang, DSc, of Massachusetts General Hospital and Harvard Medical School, Boston, said there had previously been a theory that allopurinol could protect against progression of renal disease. However, the two randomized, controlled trials in patients with chronic kidney disease but not gout published in 2020 suggested that allopurinol was instead associated with a doubling of mortality in this group.
“This study really shows convincing evidence that among gout patients with renal disease, allopurinol does not increase mortality,” Dr. Zhang told this news organization. He suggested the reason that the earlier studies had found higher mortality among patients on allopurinol was because those patients did not have gout. Given that gout can increase mortality, treating it effectively with allopurinol may therefore reduce mortality even in patients with concurrent chronic kidney disease.
Commenting on the study, Angelo Gaffo, MD, from the Birmingham VA Medical Center and the division of rheumatology at the University of Alabama at Birmingham, said that, while there had been data suggesting increased mortality, the findings from this “very well-done” study were reassuring and even suggested a possible decrease in mortality associated with allopurinol.
“I wouldn’t scream it out loud because it needs confirmation, but it’s something also that we have a sense that could be true,” he said.
Dr. Gaffo noted that patients treated with allopurinol tended to be those with fewer comorbidities. “Patients who have a lot of comorbidities probably are less likely to have their dose of allopurinol started or increased because of some concerns that practitioners may have about putting them on another medicine or increasing the dose of that medicine,” he said.
He also stressed that the findings still need replication in other large database studies, given that a prospective, randomized clinical trial addressing such a question would be difficult to conduct.
The study was supported by the Project Program of National Clinical Research Center for Geriatric Disorders, the National Natural Science Foundation of China, and the U.S. National Institutes of Health. Two authors reported consulting fees from the pharmaceutical sector unrelated to the study. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Children and COVID: United States passes 10 million total cases
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.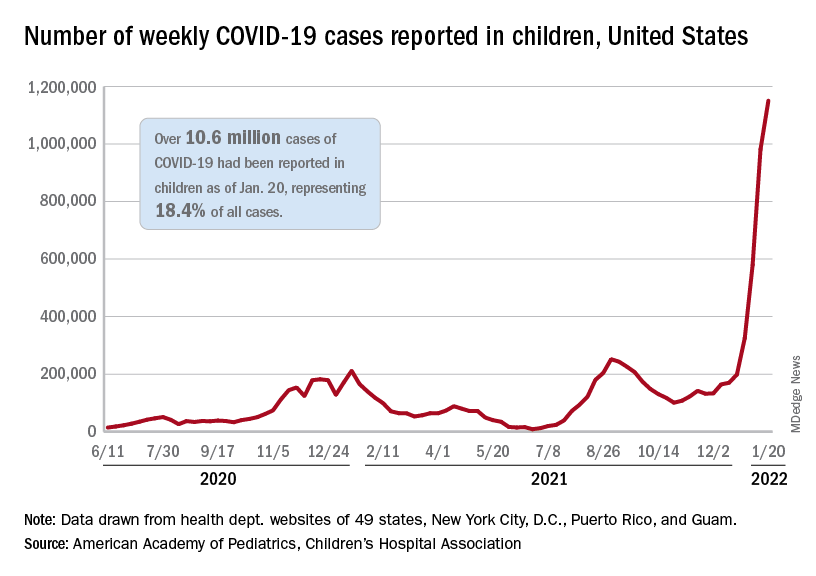
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.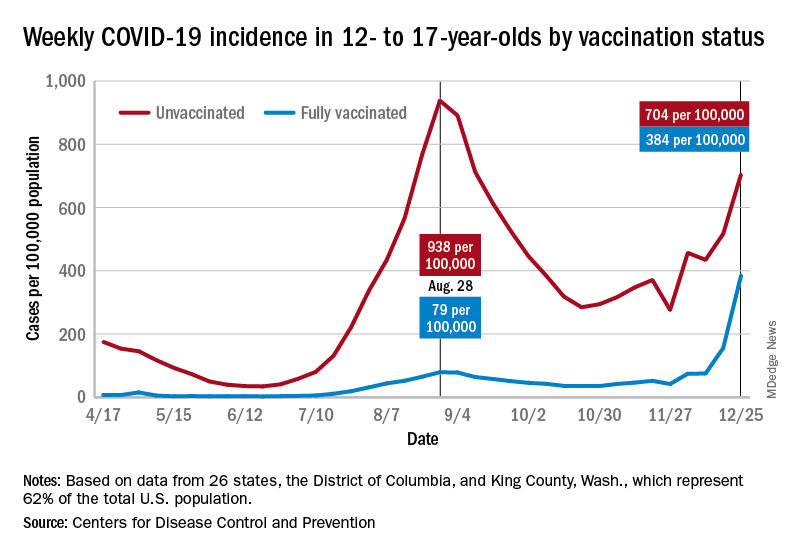
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
COVID brain fog is a ‘true neurologic condition’
early research suggests. Investigators found abnormalities in cerebrospinal fluid (CSF) and other risk factors, including diabetes and hypertension, present in individuals with mild COVID-19 experiencing persistent cognitive problems, often referred to as “brain fog.”
“We’re seeing changes to the [CSF] in the brain of most people who report cognitive changes,” said Joanna Hellmuth, MD, assistant professor of neurology, Memory and Aging Center, University of California, San Francisco. “We’re just in the beginning stages, but I hope this study will provide some legitimacy to this being a true neurologic condition.”
The study was published online Jan. 18, 2022, in Annals of Clinical and Translational Neurology.
No guidance
There is currently no guidance on how to identify patients with COVID-related cognitive changes, said Dr. Hellmuth. “The term ‘brain fog’ is not based in science or medicine, but that’s the most common term we use to describe this.”
The analysis included adults with confirmed SARS-CoV-2 infection not requiring hospitalization who were enrolled in the Long-term Impact of Infection with Novel Coronavirus study.
Participants underwent a structured interview that covered COVID-19 illness, past medical history, preexisting cognitive risk factors, medications, and cognitive symptoms following onset of COVID-19. They also completed an in-person battery of cognitive tests.
The analysis included 22 participants with at least one new cognitive symptom who had cognitive post-acute sequelae of SARS-CoV-2 infection (PASC). Ten cognitive controls reported no new cognitive symptoms after acute infection.
Participants were a median age of 41 years, had a median of 16 years of education, and were assessed a median of 10.1 months from their first COVID-19 symptom. There were no group differences in terms of age, gender, years of education, or distribution of race/ethnicity (all P > .05).
Among those with cognitive PASC, 43% reported cognitive symptoms starting 1 or more months after the first COVID symptom. About 29% reported cognitive changes started 2 or more months after their first COVID symptom.
“The immune system could be altered in some way after the infection, and perhaps that’s what’s contributing to these delayed onset cognitive changes,” said Dr. Hellmuth.
Compared with controls, participants with cognitive PASC had more preexisting cognitive risk factors (a median of 2.5 vs. 0; P = .03). These included hypertension and diabetes, which increase the risk of stroke, mild cognitive impairment, vascular dementia, traumatic brain injury, (TBI), learning disabilities, anxiety, depression, stimulant use, and ADHD, which may make the brain more vulnerable to executive functioning problems.
Dr. Hellmuth noted that the study wasn’t powered to determine whether any individual risk factor was associated with risk of cognitive changes.
As there are no published neuropsychological testing criteria for cognitive PASC, the researchers applied the equivalent criteria for HIV-associated neurocognitive disorder (HAND), a similar, virally associated cognitive disorder. Only 59% of those with cognitive PASC met equivalent HAND criteria for objective cognitive impairment versus 70% of cognitive controls. This, the investigators noted, highlights “the challenges and incongruities of using subjective, versus objective cognitive assessments for diagnosis.”
Is self-report enough?
While there is currently “nothing objective doctors can hang their hats on to say ‘you do’ or ‘you don’t’ have cognitive changes related to COVID,” using the HAND criteria is “not particularly helpful,” said Dr. Hellmuth. “Comparing an individual to a population-based norm in this case is really nuanced, and we shouldn’t rely on this solely to determine whether they do, or don’t, have cognitive changes.”
Perhaps self-reports in this case are “enough” said Dr. Hellmuth. “People know their brains better than anyone else, better than any doctor will.”
A total of 13 in the cognitive PASC group and 4 in the control group consented to a lumbar puncture. Cognitive PASC participants were older than controls (median of 47 vs. 28 years; P = .03) with no other between-group differences.
Overall, 77% of participants with cognitive PASC had a CSF abnormality, compared with 0% of cognitive controls (P = .01). CSF abnormalities included elevated protein levels with no other explainable cause in 2 of the 13 subjects with PASC, which Dr. Hellmuth said is typically a marker of inflammation.
Researchers also noted abnormal oligoclonal banding, a collection of antibodies, in the blood or brain fluid. These were identified in 69% of participants with cognitive PASC, compared with 0% of cognitive controls (P = .03).
“When we find this pattern in both blood and brain, it suggests a systemic inflammatory disorder,” although “we have no idea what these antibodies are targeting,” said Dr. Hellmuth.
The study represents “the very beginning stages” of PASC becoming a medical diagnosis “where doctors know what to call it, how to treat it, and how to do blood and cerebrospinal fluid tests to diagnose it,” said Dr. Hellmuth.
She hopes PASC will receive medical legitimacy just as TBI has. In years past, a player was hit on the head or had their “bell rung,” simply returned to the field. “Now that we understand the science, we call it a mild TBI or concussion, and we have a very different medical approach to it.”
A limitation of the study was the small sample size, which may hinder the results’ validity. In addition, the study demographics may not reflect the broader population of those impacted by PASC.
‘A first substantial step’
Commenting on the research, William Schaffner, MD, professor, division of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said the new results represent “a first substantial step on the road to trying to find out what’s going on” with COVID patients dealing with cognitive issues.
Dr. Schaffner noted that elevated protein levels, identified in some study subjects, “is usually a consequence of previous inflammation” and is “a very interesting” finding. “In people who are otherwise normal, if you do a lumbar puncture, you don’t find elevated proteins.”
However, he noted the “diversity of results” from CSF examinations. “A single pattern does not leap out.”
What the researchers are observing “is not just a phenomenon of the mind or just something psychological,” said Dr. Schaffner. “Something physical is going on here.”
The study was funded by grants from the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke. Dr. Hellmuth received grant support from the National Institutes of Health/NIMH supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner has disclosed not relevant financial relationships.
A version of this article first appeared on Medscape.com.
early research suggests. Investigators found abnormalities in cerebrospinal fluid (CSF) and other risk factors, including diabetes and hypertension, present in individuals with mild COVID-19 experiencing persistent cognitive problems, often referred to as “brain fog.”
“We’re seeing changes to the [CSF] in the brain of most people who report cognitive changes,” said Joanna Hellmuth, MD, assistant professor of neurology, Memory and Aging Center, University of California, San Francisco. “We’re just in the beginning stages, but I hope this study will provide some legitimacy to this being a true neurologic condition.”
The study was published online Jan. 18, 2022, in Annals of Clinical and Translational Neurology.
No guidance
There is currently no guidance on how to identify patients with COVID-related cognitive changes, said Dr. Hellmuth. “The term ‘brain fog’ is not based in science or medicine, but that’s the most common term we use to describe this.”
The analysis included adults with confirmed SARS-CoV-2 infection not requiring hospitalization who were enrolled in the Long-term Impact of Infection with Novel Coronavirus study.
Participants underwent a structured interview that covered COVID-19 illness, past medical history, preexisting cognitive risk factors, medications, and cognitive symptoms following onset of COVID-19. They also completed an in-person battery of cognitive tests.
The analysis included 22 participants with at least one new cognitive symptom who had cognitive post-acute sequelae of SARS-CoV-2 infection (PASC). Ten cognitive controls reported no new cognitive symptoms after acute infection.
Participants were a median age of 41 years, had a median of 16 years of education, and were assessed a median of 10.1 months from their first COVID-19 symptom. There were no group differences in terms of age, gender, years of education, or distribution of race/ethnicity (all P > .05).
Among those with cognitive PASC, 43% reported cognitive symptoms starting 1 or more months after the first COVID symptom. About 29% reported cognitive changes started 2 or more months after their first COVID symptom.
“The immune system could be altered in some way after the infection, and perhaps that’s what’s contributing to these delayed onset cognitive changes,” said Dr. Hellmuth.
Compared with controls, participants with cognitive PASC had more preexisting cognitive risk factors (a median of 2.5 vs. 0; P = .03). These included hypertension and diabetes, which increase the risk of stroke, mild cognitive impairment, vascular dementia, traumatic brain injury, (TBI), learning disabilities, anxiety, depression, stimulant use, and ADHD, which may make the brain more vulnerable to executive functioning problems.
Dr. Hellmuth noted that the study wasn’t powered to determine whether any individual risk factor was associated with risk of cognitive changes.
As there are no published neuropsychological testing criteria for cognitive PASC, the researchers applied the equivalent criteria for HIV-associated neurocognitive disorder (HAND), a similar, virally associated cognitive disorder. Only 59% of those with cognitive PASC met equivalent HAND criteria for objective cognitive impairment versus 70% of cognitive controls. This, the investigators noted, highlights “the challenges and incongruities of using subjective, versus objective cognitive assessments for diagnosis.”
Is self-report enough?
While there is currently “nothing objective doctors can hang their hats on to say ‘you do’ or ‘you don’t’ have cognitive changes related to COVID,” using the HAND criteria is “not particularly helpful,” said Dr. Hellmuth. “Comparing an individual to a population-based norm in this case is really nuanced, and we shouldn’t rely on this solely to determine whether they do, or don’t, have cognitive changes.”
Perhaps self-reports in this case are “enough” said Dr. Hellmuth. “People know their brains better than anyone else, better than any doctor will.”
A total of 13 in the cognitive PASC group and 4 in the control group consented to a lumbar puncture. Cognitive PASC participants were older than controls (median of 47 vs. 28 years; P = .03) with no other between-group differences.
Overall, 77% of participants with cognitive PASC had a CSF abnormality, compared with 0% of cognitive controls (P = .01). CSF abnormalities included elevated protein levels with no other explainable cause in 2 of the 13 subjects with PASC, which Dr. Hellmuth said is typically a marker of inflammation.
Researchers also noted abnormal oligoclonal banding, a collection of antibodies, in the blood or brain fluid. These were identified in 69% of participants with cognitive PASC, compared with 0% of cognitive controls (P = .03).
“When we find this pattern in both blood and brain, it suggests a systemic inflammatory disorder,” although “we have no idea what these antibodies are targeting,” said Dr. Hellmuth.
The study represents “the very beginning stages” of PASC becoming a medical diagnosis “where doctors know what to call it, how to treat it, and how to do blood and cerebrospinal fluid tests to diagnose it,” said Dr. Hellmuth.
She hopes PASC will receive medical legitimacy just as TBI has. In years past, a player was hit on the head or had their “bell rung,” simply returned to the field. “Now that we understand the science, we call it a mild TBI or concussion, and we have a very different medical approach to it.”
A limitation of the study was the small sample size, which may hinder the results’ validity. In addition, the study demographics may not reflect the broader population of those impacted by PASC.
‘A first substantial step’
Commenting on the research, William Schaffner, MD, professor, division of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said the new results represent “a first substantial step on the road to trying to find out what’s going on” with COVID patients dealing with cognitive issues.
Dr. Schaffner noted that elevated protein levels, identified in some study subjects, “is usually a consequence of previous inflammation” and is “a very interesting” finding. “In people who are otherwise normal, if you do a lumbar puncture, you don’t find elevated proteins.”
However, he noted the “diversity of results” from CSF examinations. “A single pattern does not leap out.”
What the researchers are observing “is not just a phenomenon of the mind or just something psychological,” said Dr. Schaffner. “Something physical is going on here.”
The study was funded by grants from the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke. Dr. Hellmuth received grant support from the National Institutes of Health/NIMH supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner has disclosed not relevant financial relationships.
A version of this article first appeared on Medscape.com.
early research suggests. Investigators found abnormalities in cerebrospinal fluid (CSF) and other risk factors, including diabetes and hypertension, present in individuals with mild COVID-19 experiencing persistent cognitive problems, often referred to as “brain fog.”
“We’re seeing changes to the [CSF] in the brain of most people who report cognitive changes,” said Joanna Hellmuth, MD, assistant professor of neurology, Memory and Aging Center, University of California, San Francisco. “We’re just in the beginning stages, but I hope this study will provide some legitimacy to this being a true neurologic condition.”
The study was published online Jan. 18, 2022, in Annals of Clinical and Translational Neurology.
No guidance
There is currently no guidance on how to identify patients with COVID-related cognitive changes, said Dr. Hellmuth. “The term ‘brain fog’ is not based in science or medicine, but that’s the most common term we use to describe this.”
The analysis included adults with confirmed SARS-CoV-2 infection not requiring hospitalization who were enrolled in the Long-term Impact of Infection with Novel Coronavirus study.
Participants underwent a structured interview that covered COVID-19 illness, past medical history, preexisting cognitive risk factors, medications, and cognitive symptoms following onset of COVID-19. They also completed an in-person battery of cognitive tests.
The analysis included 22 participants with at least one new cognitive symptom who had cognitive post-acute sequelae of SARS-CoV-2 infection (PASC). Ten cognitive controls reported no new cognitive symptoms after acute infection.
Participants were a median age of 41 years, had a median of 16 years of education, and were assessed a median of 10.1 months from their first COVID-19 symptom. There were no group differences in terms of age, gender, years of education, or distribution of race/ethnicity (all P > .05).
Among those with cognitive PASC, 43% reported cognitive symptoms starting 1 or more months after the first COVID symptom. About 29% reported cognitive changes started 2 or more months after their first COVID symptom.
“The immune system could be altered in some way after the infection, and perhaps that’s what’s contributing to these delayed onset cognitive changes,” said Dr. Hellmuth.
Compared with controls, participants with cognitive PASC had more preexisting cognitive risk factors (a median of 2.5 vs. 0; P = .03). These included hypertension and diabetes, which increase the risk of stroke, mild cognitive impairment, vascular dementia, traumatic brain injury, (TBI), learning disabilities, anxiety, depression, stimulant use, and ADHD, which may make the brain more vulnerable to executive functioning problems.
Dr. Hellmuth noted that the study wasn’t powered to determine whether any individual risk factor was associated with risk of cognitive changes.
As there are no published neuropsychological testing criteria for cognitive PASC, the researchers applied the equivalent criteria for HIV-associated neurocognitive disorder (HAND), a similar, virally associated cognitive disorder. Only 59% of those with cognitive PASC met equivalent HAND criteria for objective cognitive impairment versus 70% of cognitive controls. This, the investigators noted, highlights “the challenges and incongruities of using subjective, versus objective cognitive assessments for diagnosis.”
Is self-report enough?
While there is currently “nothing objective doctors can hang their hats on to say ‘you do’ or ‘you don’t’ have cognitive changes related to COVID,” using the HAND criteria is “not particularly helpful,” said Dr. Hellmuth. “Comparing an individual to a population-based norm in this case is really nuanced, and we shouldn’t rely on this solely to determine whether they do, or don’t, have cognitive changes.”
Perhaps self-reports in this case are “enough” said Dr. Hellmuth. “People know their brains better than anyone else, better than any doctor will.”
A total of 13 in the cognitive PASC group and 4 in the control group consented to a lumbar puncture. Cognitive PASC participants were older than controls (median of 47 vs. 28 years; P = .03) with no other between-group differences.
Overall, 77% of participants with cognitive PASC had a CSF abnormality, compared with 0% of cognitive controls (P = .01). CSF abnormalities included elevated protein levels with no other explainable cause in 2 of the 13 subjects with PASC, which Dr. Hellmuth said is typically a marker of inflammation.
Researchers also noted abnormal oligoclonal banding, a collection of antibodies, in the blood or brain fluid. These were identified in 69% of participants with cognitive PASC, compared with 0% of cognitive controls (P = .03).
“When we find this pattern in both blood and brain, it suggests a systemic inflammatory disorder,” although “we have no idea what these antibodies are targeting,” said Dr. Hellmuth.
The study represents “the very beginning stages” of PASC becoming a medical diagnosis “where doctors know what to call it, how to treat it, and how to do blood and cerebrospinal fluid tests to diagnose it,” said Dr. Hellmuth.
She hopes PASC will receive medical legitimacy just as TBI has. In years past, a player was hit on the head or had their “bell rung,” simply returned to the field. “Now that we understand the science, we call it a mild TBI or concussion, and we have a very different medical approach to it.”
A limitation of the study was the small sample size, which may hinder the results’ validity. In addition, the study demographics may not reflect the broader population of those impacted by PASC.
‘A first substantial step’
Commenting on the research, William Schaffner, MD, professor, division of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said the new results represent “a first substantial step on the road to trying to find out what’s going on” with COVID patients dealing with cognitive issues.
Dr. Schaffner noted that elevated protein levels, identified in some study subjects, “is usually a consequence of previous inflammation” and is “a very interesting” finding. “In people who are otherwise normal, if you do a lumbar puncture, you don’t find elevated proteins.”
However, he noted the “diversity of results” from CSF examinations. “A single pattern does not leap out.”
What the researchers are observing “is not just a phenomenon of the mind or just something psychological,” said Dr. Schaffner. “Something physical is going on here.”
The study was funded by grants from the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke. Dr. Hellmuth received grant support from the National Institutes of Health/NIMH supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner has disclosed not relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF CLINICAL AND TRANSLATIONAL NEUROLOGY
Watch, but don’t worry yet, about new Omicron subvariant
In the meantime, it’s worth watching BA.2, the World Health Organization said. The subvariant has been identified across at least 40 countries, including three cases reported in Houston and several in Washington state.
BA.2 accounts for only a small minority of reported cases so far, including 5% in India, 4% of those in the United Kingdom, and 2% each of cases in Sweden and Singapore.
The one exception is Denmark, a country with robust genetic sequencing abilities, where estimates range from 50% to 81% of cases.
The news throws a little more uncertainty into an already uncertain situation, including how close the world might be to a less life-altering infectious disease.
For example, the world is at an ideal point for a new variant to emerge, WHO Director General Tedros Adhanom Ghebreyesus, PhD, said during a Jan. 24 meeting of the WHO executive board. He also said it’s too early to call an “end game” to the pandemic.
Similarly, Anthony S. Fauci, MD, said on Jan. 19 that it remained “an open question” whether the Omicron variant could hasten endemic COVID-19, a situation where the virus still circulates but is much less disruptive to everyday life.
No Pi for you
This could be the first time a coronavirus subvariant rises to the level of a household name, or – if previous variants of the moment have shown us – it could recede from the spotlight.
For example, a lot of focus on the potential of the Mu variant to wreak havoc fizzled out a few weeks after the WHO listed it as a variant of interest on Aug. 30.
Subvariants can feature mutations and other small differences but are not distinct enough from an existing strain to be called a variant on their own and be named after the next letter in the Greek alphabet. That’s why BA.2 is not called the “Pi variant.”
Predicting what’s next for the coronavirus has puzzled many experts throughout the pandemic. That is why many public health officials wait for the WHO to officially designate a strain as a variant of interest or variant of concern before taking action.
At the moment with BA.2, it seems close monitoring is warranted.
Because it’s too early to call, expert predictions about BA.2 vary widely, from worry to cautious optimism.
For example, early data indicates that BA.2 could be more worrisome than original Omicron, Eric Feigl-Ding, ScD, an epidemiologist and health economist, said on Twitter.
Information from Denmark seems to show BA.2 either has “much faster transmission or it evades immunity even more,” he said.
The same day, Jan. 23, Dr. Feigl-Ding tweeted that other data shows the subvariant can spread twice as fast as Omicron, which was already much more contagious than previous versions of the virus.
At the same time, other experts appear less concerned. Robert Garry, PhD, a virologist at Tulane University, New Orleans, told the Washington Post that there is no reason to think BA.2 will be any worse than the original Omicron strain.
So which expert predictions will come closer to BA.2’s potential? For now, it’s just a watch-and-see situation.
For updated information, the website outbreak.info tracks BA.2’s average daily and cumulative prevalence in the United States and in other locations.
Also, if and when WHO experts decide to elevate BA.2 to a variant of interest or a variant of concern, it will be noted on its coronavirus variant tracking website.
A version of this article first appeared on WebMD.com.
In the meantime, it’s worth watching BA.2, the World Health Organization said. The subvariant has been identified across at least 40 countries, including three cases reported in Houston and several in Washington state.
BA.2 accounts for only a small minority of reported cases so far, including 5% in India, 4% of those in the United Kingdom, and 2% each of cases in Sweden and Singapore.
The one exception is Denmark, a country with robust genetic sequencing abilities, where estimates range from 50% to 81% of cases.
The news throws a little more uncertainty into an already uncertain situation, including how close the world might be to a less life-altering infectious disease.
For example, the world is at an ideal point for a new variant to emerge, WHO Director General Tedros Adhanom Ghebreyesus, PhD, said during a Jan. 24 meeting of the WHO executive board. He also said it’s too early to call an “end game” to the pandemic.
Similarly, Anthony S. Fauci, MD, said on Jan. 19 that it remained “an open question” whether the Omicron variant could hasten endemic COVID-19, a situation where the virus still circulates but is much less disruptive to everyday life.
No Pi for you
This could be the first time a coronavirus subvariant rises to the level of a household name, or – if previous variants of the moment have shown us – it could recede from the spotlight.
For example, a lot of focus on the potential of the Mu variant to wreak havoc fizzled out a few weeks after the WHO listed it as a variant of interest on Aug. 30.
Subvariants can feature mutations and other small differences but are not distinct enough from an existing strain to be called a variant on their own and be named after the next letter in the Greek alphabet. That’s why BA.2 is not called the “Pi variant.”
Predicting what’s next for the coronavirus has puzzled many experts throughout the pandemic. That is why many public health officials wait for the WHO to officially designate a strain as a variant of interest or variant of concern before taking action.
At the moment with BA.2, it seems close monitoring is warranted.
Because it’s too early to call, expert predictions about BA.2 vary widely, from worry to cautious optimism.
For example, early data indicates that BA.2 could be more worrisome than original Omicron, Eric Feigl-Ding, ScD, an epidemiologist and health economist, said on Twitter.
Information from Denmark seems to show BA.2 either has “much faster transmission or it evades immunity even more,” he said.
The same day, Jan. 23, Dr. Feigl-Ding tweeted that other data shows the subvariant can spread twice as fast as Omicron, which was already much more contagious than previous versions of the virus.
At the same time, other experts appear less concerned. Robert Garry, PhD, a virologist at Tulane University, New Orleans, told the Washington Post that there is no reason to think BA.2 will be any worse than the original Omicron strain.
So which expert predictions will come closer to BA.2’s potential? For now, it’s just a watch-and-see situation.
For updated information, the website outbreak.info tracks BA.2’s average daily and cumulative prevalence in the United States and in other locations.
Also, if and when WHO experts decide to elevate BA.2 to a variant of interest or a variant of concern, it will be noted on its coronavirus variant tracking website.
A version of this article first appeared on WebMD.com.
In the meantime, it’s worth watching BA.2, the World Health Organization said. The subvariant has been identified across at least 40 countries, including three cases reported in Houston and several in Washington state.
BA.2 accounts for only a small minority of reported cases so far, including 5% in India, 4% of those in the United Kingdom, and 2% each of cases in Sweden and Singapore.
The one exception is Denmark, a country with robust genetic sequencing abilities, where estimates range from 50% to 81% of cases.
The news throws a little more uncertainty into an already uncertain situation, including how close the world might be to a less life-altering infectious disease.
For example, the world is at an ideal point for a new variant to emerge, WHO Director General Tedros Adhanom Ghebreyesus, PhD, said during a Jan. 24 meeting of the WHO executive board. He also said it’s too early to call an “end game” to the pandemic.
Similarly, Anthony S. Fauci, MD, said on Jan. 19 that it remained “an open question” whether the Omicron variant could hasten endemic COVID-19, a situation where the virus still circulates but is much less disruptive to everyday life.
No Pi for you
This could be the first time a coronavirus subvariant rises to the level of a household name, or – if previous variants of the moment have shown us – it could recede from the spotlight.
For example, a lot of focus on the potential of the Mu variant to wreak havoc fizzled out a few weeks after the WHO listed it as a variant of interest on Aug. 30.
Subvariants can feature mutations and other small differences but are not distinct enough from an existing strain to be called a variant on their own and be named after the next letter in the Greek alphabet. That’s why BA.2 is not called the “Pi variant.”
Predicting what’s next for the coronavirus has puzzled many experts throughout the pandemic. That is why many public health officials wait for the WHO to officially designate a strain as a variant of interest or variant of concern before taking action.
At the moment with BA.2, it seems close monitoring is warranted.
Because it’s too early to call, expert predictions about BA.2 vary widely, from worry to cautious optimism.
For example, early data indicates that BA.2 could be more worrisome than original Omicron, Eric Feigl-Ding, ScD, an epidemiologist and health economist, said on Twitter.
Information from Denmark seems to show BA.2 either has “much faster transmission or it evades immunity even more,” he said.
The same day, Jan. 23, Dr. Feigl-Ding tweeted that other data shows the subvariant can spread twice as fast as Omicron, which was already much more contagious than previous versions of the virus.
At the same time, other experts appear less concerned. Robert Garry, PhD, a virologist at Tulane University, New Orleans, told the Washington Post that there is no reason to think BA.2 will be any worse than the original Omicron strain.
So which expert predictions will come closer to BA.2’s potential? For now, it’s just a watch-and-see situation.
For updated information, the website outbreak.info tracks BA.2’s average daily and cumulative prevalence in the United States and in other locations.
Also, if and when WHO experts decide to elevate BA.2 to a variant of interest or a variant of concern, it will be noted on its coronavirus variant tracking website.
A version of this article first appeared on WebMD.com.
This doc still supports NP/PA-led care ... with caveats
Two years ago, I argued that independent care from nurse practitioners (NPs) and physician assistants (PAs) would not have ill effects on health outcomes. To the surprise of no one, NPs and PAs embraced the argument; physicians clobbered it.
My case had three pegs: One was that medicine isn’t rocket science and clinicians control a lot less than we think we do. The second peg was that technology levels the playing field of clinical care. High-sensitivity troponin assays, for instance, make missing MI a lot less likely. The third peg was empirical: Studies have found little difference in MD versus non–MD-led care. Looking back, I now see empiricism as the weakest part of the argument because the studies had so many limitations.
I update this viewpoint now because health care is increasingly delivered by NPs and PAs. And there are two concerning trends regarding NP education and experience. First is that nurses are turning to advanced practitioner training earlier in their careers – without gathering much bedside experience. And these training programs are increasingly likely to be online, with minimal hands-on clinical tutoring.
Education and experience pop in my head often. Not every day, but many days I think back to my lucky 7 years in Indiana learning under the supervision of master clinicians – at a time when trainees were allowed the leeway to make decisions ... and mistakes. Then, when I joined private practice, I continued to learn from experienced practitioners.
It would be foolish to argue that training and experience aren’t important.
But here’s the thing:
I will make three points: First, I will bolster two of my old arguments as to why we shouldn’t be worried about non-MD clinicians, then I will propose some ideas to increase confidence in NP and PA care.
Health care does not equal health
On the matter of how much clinicians affect outcomes, a recently published randomized controlled trial performed in India found that subsidizing insurance care led to increased utilization of hospital services but had no significant effect on health outcomes. This follows the RAND and Oregon Health Insurance studies in the United States, which largely reported similar results.
We should also not dismiss the fact that – despite the massive technology gains over the past half-century in digital health and artificial intelligence and increased use of quality measures, new drugs and procedures, and mega-medical centers – the average lifespan of Americans is flat to declining (in most ethnic and racial groups). Worse than no gains in longevity, perhaps, is that death from diseases like dementia and Parkinson’s disease are on the rise.
A neutral Martian would look down and wonder why all this health care hasn’t translated to longer and better lives. The causes of this paradox remain speculative, and are for another column, but the point remains that – on average – more health care is clearly not delivering more health. And if that is true, one may deduce that much of U.S. health care is marginal when it comes to affecting major outcomes.
It’s about the delta
Logos trumps pathos. Sure, my physician colleagues can tell scary anecdotes of bad outcomes caused by an inexperienced NP or PA. I would counter that by saying I have sat on our hospital’s peer review committee for 2 decades, including the era before NPs or PAs were practicing, and I have plenty of stories of physician errors. These include, of course, my own errors.
Logos: We must consider the difference between non–MD-led care and MD-led care.
My arguments from 2020 remain relevant today. Most medical problems are not engineering puzzles. Many, perhaps most, patients fall into an easy protocol – say, chest pain, dyspnea, or atrial fibrillation. With basic training, a motivated serious person quickly gains skill in recognizing and treating everyday problems.
And just 2 years on, technology further levels the playing field. Consider radiology in 2022 – it’s easy to take for granted the speed of the CT scan, the fidelity of the MRI, and the easy access to both in the U.S. hospital system. Less experienced clinicians have never had more tools to assist with diagnostics and therapeutics.
The expansion of team-based care has also mitigated the effects of inexperience. It took Americans longer than Canadians to figure out how helpful pharmacists could be. Pharmacists in my hospital now help us dose complicated medicines and protect us against prescribing errors.
Then there is the immediate access to online information. Gone are the days when you had to memorize long-QT syndromes. Book knowledge – that I spent years acquiring – now comes in seconds. The other day an NP corrected me. I asked, Are you sure? Boom, she took out her phone and showed me the evidence.
In sum, if it were even possible to measure the clinical competence of care from NP and PA versus physicians, there would be two bell-shaped curves with a tremendous amount of overlap. And that overlap would steadily increase as a given NP or PA gathered experience. (The NP in our electrophysiology division has more than 25 years’ experience in heart rhythm care, and it is common for colleagues to call her before one of us docs. Rightly so.)
Three basic proposals regarding NP and PA care
To ensure quality of care, I have three proposals.
It has always seemed strange to me that an NP or PA can flip from one field to another without a period of training. I can’t just change practice from electrophysiology to dermatology without doing a residency. But NPs and PAs can.
My first proposal would be that NPs and PAs spend a substantial period of training in a field before practice – a legit apprenticeship. The duration of this period is a matter of debate, but it ought to be standardized.
My second proposal is that, if physicians are required to pass certification exams, so should NPs. (PAs have an exam every 10 years.) The exam should be the same as (or very similar to) the physician exam, and it should be specific to their field of practice.
While I have argued (and still feel) that the American Board of Internal Medicine brand of certification is dubious, the fact remains that physicians must maintain proficiency in their field. Requiring NPs and PAs to do the same would help foster specialization. And while I can’t cite empirical evidence, specialization seems super-important. We have NPs at my hospital who have been in the same area for years, and they exude clinical competence.
Finally, I have come to believe that the best way for nearly any clinician to practice medicine is as part of a team. (The exception being primary care in rural areas where there are clinician shortages.)
On the matter of team care, I’ve practiced for a long time, but nearly every day I run situations by a colleague; often this person is an NP. The economist Friedrich Hayek proposed that dispersed knowledge always outpaces the wisdom of any individual. That notion pertains well to the increasing complexities and specialization of modern medical practice.
A person who commits to learning one area of medicine, enjoys helping people, asks often for help, and has the support of colleagues is set up to be a successful clinician – whether the letters after their name are APRN, PA, DO, or MD.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky. He did not report any relevant financial disclosures. A version of this article first appeared on Medscape.com.
Two years ago, I argued that independent care from nurse practitioners (NPs) and physician assistants (PAs) would not have ill effects on health outcomes. To the surprise of no one, NPs and PAs embraced the argument; physicians clobbered it.
My case had three pegs: One was that medicine isn’t rocket science and clinicians control a lot less than we think we do. The second peg was that technology levels the playing field of clinical care. High-sensitivity troponin assays, for instance, make missing MI a lot less likely. The third peg was empirical: Studies have found little difference in MD versus non–MD-led care. Looking back, I now see empiricism as the weakest part of the argument because the studies had so many limitations.
I update this viewpoint now because health care is increasingly delivered by NPs and PAs. And there are two concerning trends regarding NP education and experience. First is that nurses are turning to advanced practitioner training earlier in their careers – without gathering much bedside experience. And these training programs are increasingly likely to be online, with minimal hands-on clinical tutoring.
Education and experience pop in my head often. Not every day, but many days I think back to my lucky 7 years in Indiana learning under the supervision of master clinicians – at a time when trainees were allowed the leeway to make decisions ... and mistakes. Then, when I joined private practice, I continued to learn from experienced practitioners.
It would be foolish to argue that training and experience aren’t important.
But here’s the thing:
I will make three points: First, I will bolster two of my old arguments as to why we shouldn’t be worried about non-MD clinicians, then I will propose some ideas to increase confidence in NP and PA care.
Health care does not equal health
On the matter of how much clinicians affect outcomes, a recently published randomized controlled trial performed in India found that subsidizing insurance care led to increased utilization of hospital services but had no significant effect on health outcomes. This follows the RAND and Oregon Health Insurance studies in the United States, which largely reported similar results.
We should also not dismiss the fact that – despite the massive technology gains over the past half-century in digital health and artificial intelligence and increased use of quality measures, new drugs and procedures, and mega-medical centers – the average lifespan of Americans is flat to declining (in most ethnic and racial groups). Worse than no gains in longevity, perhaps, is that death from diseases like dementia and Parkinson’s disease are on the rise.
A neutral Martian would look down and wonder why all this health care hasn’t translated to longer and better lives. The causes of this paradox remain speculative, and are for another column, but the point remains that – on average – more health care is clearly not delivering more health. And if that is true, one may deduce that much of U.S. health care is marginal when it comes to affecting major outcomes.
It’s about the delta
Logos trumps pathos. Sure, my physician colleagues can tell scary anecdotes of bad outcomes caused by an inexperienced NP or PA. I would counter that by saying I have sat on our hospital’s peer review committee for 2 decades, including the era before NPs or PAs were practicing, and I have plenty of stories of physician errors. These include, of course, my own errors.
Logos: We must consider the difference between non–MD-led care and MD-led care.
My arguments from 2020 remain relevant today. Most medical problems are not engineering puzzles. Many, perhaps most, patients fall into an easy protocol – say, chest pain, dyspnea, or atrial fibrillation. With basic training, a motivated serious person quickly gains skill in recognizing and treating everyday problems.
And just 2 years on, technology further levels the playing field. Consider radiology in 2022 – it’s easy to take for granted the speed of the CT scan, the fidelity of the MRI, and the easy access to both in the U.S. hospital system. Less experienced clinicians have never had more tools to assist with diagnostics and therapeutics.
The expansion of team-based care has also mitigated the effects of inexperience. It took Americans longer than Canadians to figure out how helpful pharmacists could be. Pharmacists in my hospital now help us dose complicated medicines and protect us against prescribing errors.
Then there is the immediate access to online information. Gone are the days when you had to memorize long-QT syndromes. Book knowledge – that I spent years acquiring – now comes in seconds. The other day an NP corrected me. I asked, Are you sure? Boom, she took out her phone and showed me the evidence.
In sum, if it were even possible to measure the clinical competence of care from NP and PA versus physicians, there would be two bell-shaped curves with a tremendous amount of overlap. And that overlap would steadily increase as a given NP or PA gathered experience. (The NP in our electrophysiology division has more than 25 years’ experience in heart rhythm care, and it is common for colleagues to call her before one of us docs. Rightly so.)
Three basic proposals regarding NP and PA care
To ensure quality of care, I have three proposals.
It has always seemed strange to me that an NP or PA can flip from one field to another without a period of training. I can’t just change practice from electrophysiology to dermatology without doing a residency. But NPs and PAs can.
My first proposal would be that NPs and PAs spend a substantial period of training in a field before practice – a legit apprenticeship. The duration of this period is a matter of debate, but it ought to be standardized.
My second proposal is that, if physicians are required to pass certification exams, so should NPs. (PAs have an exam every 10 years.) The exam should be the same as (or very similar to) the physician exam, and it should be specific to their field of practice.
While I have argued (and still feel) that the American Board of Internal Medicine brand of certification is dubious, the fact remains that physicians must maintain proficiency in their field. Requiring NPs and PAs to do the same would help foster specialization. And while I can’t cite empirical evidence, specialization seems super-important. We have NPs at my hospital who have been in the same area for years, and they exude clinical competence.
Finally, I have come to believe that the best way for nearly any clinician to practice medicine is as part of a team. (The exception being primary care in rural areas where there are clinician shortages.)
On the matter of team care, I’ve practiced for a long time, but nearly every day I run situations by a colleague; often this person is an NP. The economist Friedrich Hayek proposed that dispersed knowledge always outpaces the wisdom of any individual. That notion pertains well to the increasing complexities and specialization of modern medical practice.
A person who commits to learning one area of medicine, enjoys helping people, asks often for help, and has the support of colleagues is set up to be a successful clinician – whether the letters after their name are APRN, PA, DO, or MD.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky. He did not report any relevant financial disclosures. A version of this article first appeared on Medscape.com.
Two years ago, I argued that independent care from nurse practitioners (NPs) and physician assistants (PAs) would not have ill effects on health outcomes. To the surprise of no one, NPs and PAs embraced the argument; physicians clobbered it.
My case had three pegs: One was that medicine isn’t rocket science and clinicians control a lot less than we think we do. The second peg was that technology levels the playing field of clinical care. High-sensitivity troponin assays, for instance, make missing MI a lot less likely. The third peg was empirical: Studies have found little difference in MD versus non–MD-led care. Looking back, I now see empiricism as the weakest part of the argument because the studies had so many limitations.
I update this viewpoint now because health care is increasingly delivered by NPs and PAs. And there are two concerning trends regarding NP education and experience. First is that nurses are turning to advanced practitioner training earlier in their careers – without gathering much bedside experience. And these training programs are increasingly likely to be online, with minimal hands-on clinical tutoring.
Education and experience pop in my head often. Not every day, but many days I think back to my lucky 7 years in Indiana learning under the supervision of master clinicians – at a time when trainees were allowed the leeway to make decisions ... and mistakes. Then, when I joined private practice, I continued to learn from experienced practitioners.
It would be foolish to argue that training and experience aren’t important.
But here’s the thing:
I will make three points: First, I will bolster two of my old arguments as to why we shouldn’t be worried about non-MD clinicians, then I will propose some ideas to increase confidence in NP and PA care.
Health care does not equal health
On the matter of how much clinicians affect outcomes, a recently published randomized controlled trial performed in India found that subsidizing insurance care led to increased utilization of hospital services but had no significant effect on health outcomes. This follows the RAND and Oregon Health Insurance studies in the United States, which largely reported similar results.
We should also not dismiss the fact that – despite the massive technology gains over the past half-century in digital health and artificial intelligence and increased use of quality measures, new drugs and procedures, and mega-medical centers – the average lifespan of Americans is flat to declining (in most ethnic and racial groups). Worse than no gains in longevity, perhaps, is that death from diseases like dementia and Parkinson’s disease are on the rise.
A neutral Martian would look down and wonder why all this health care hasn’t translated to longer and better lives. The causes of this paradox remain speculative, and are for another column, but the point remains that – on average – more health care is clearly not delivering more health. And if that is true, one may deduce that much of U.S. health care is marginal when it comes to affecting major outcomes.
It’s about the delta
Logos trumps pathos. Sure, my physician colleagues can tell scary anecdotes of bad outcomes caused by an inexperienced NP or PA. I would counter that by saying I have sat on our hospital’s peer review committee for 2 decades, including the era before NPs or PAs were practicing, and I have plenty of stories of physician errors. These include, of course, my own errors.
Logos: We must consider the difference between non–MD-led care and MD-led care.
My arguments from 2020 remain relevant today. Most medical problems are not engineering puzzles. Many, perhaps most, patients fall into an easy protocol – say, chest pain, dyspnea, or atrial fibrillation. With basic training, a motivated serious person quickly gains skill in recognizing and treating everyday problems.
And just 2 years on, technology further levels the playing field. Consider radiology in 2022 – it’s easy to take for granted the speed of the CT scan, the fidelity of the MRI, and the easy access to both in the U.S. hospital system. Less experienced clinicians have never had more tools to assist with diagnostics and therapeutics.
The expansion of team-based care has also mitigated the effects of inexperience. It took Americans longer than Canadians to figure out how helpful pharmacists could be. Pharmacists in my hospital now help us dose complicated medicines and protect us against prescribing errors.
Then there is the immediate access to online information. Gone are the days when you had to memorize long-QT syndromes. Book knowledge – that I spent years acquiring – now comes in seconds. The other day an NP corrected me. I asked, Are you sure? Boom, she took out her phone and showed me the evidence.
In sum, if it were even possible to measure the clinical competence of care from NP and PA versus physicians, there would be two bell-shaped curves with a tremendous amount of overlap. And that overlap would steadily increase as a given NP or PA gathered experience. (The NP in our electrophysiology division has more than 25 years’ experience in heart rhythm care, and it is common for colleagues to call her before one of us docs. Rightly so.)
Three basic proposals regarding NP and PA care
To ensure quality of care, I have three proposals.
It has always seemed strange to me that an NP or PA can flip from one field to another without a period of training. I can’t just change practice from electrophysiology to dermatology without doing a residency. But NPs and PAs can.
My first proposal would be that NPs and PAs spend a substantial period of training in a field before practice – a legit apprenticeship. The duration of this period is a matter of debate, but it ought to be standardized.
My second proposal is that, if physicians are required to pass certification exams, so should NPs. (PAs have an exam every 10 years.) The exam should be the same as (or very similar to) the physician exam, and it should be specific to their field of practice.
While I have argued (and still feel) that the American Board of Internal Medicine brand of certification is dubious, the fact remains that physicians must maintain proficiency in their field. Requiring NPs and PAs to do the same would help foster specialization. And while I can’t cite empirical evidence, specialization seems super-important. We have NPs at my hospital who have been in the same area for years, and they exude clinical competence.
Finally, I have come to believe that the best way for nearly any clinician to practice medicine is as part of a team. (The exception being primary care in rural areas where there are clinician shortages.)
On the matter of team care, I’ve practiced for a long time, but nearly every day I run situations by a colleague; often this person is an NP. The economist Friedrich Hayek proposed that dispersed knowledge always outpaces the wisdom of any individual. That notion pertains well to the increasing complexities and specialization of modern medical practice.
A person who commits to learning one area of medicine, enjoys helping people, asks often for help, and has the support of colleagues is set up to be a successful clinician – whether the letters after their name are APRN, PA, DO, or MD.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky. He did not report any relevant financial disclosures. A version of this article first appeared on Medscape.com.
Does COVID-19 induce type 1 diabetes in kids? Jury still out
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.