User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
AGA Clinical Practice Update: Expert Review on IBD dysplasia surveillance, management
The American Gastroenterological Association recently published an expert review and clinical practice update addressing endoscopic surveillance and management of colorectal dysplasia in patients with inflammatory bowel disease (IBD).
Because of practice-altering advances in therapy and surveillance over the past 2 decades, an updated approach is needed, according to authors led by Sanjay K. Murthy, MD, of Ottawa Hospital Research Institute and Fernando Velayos, MD, from Kaiser Permanente San Francisco Medical Center.
“Not long ago, notions of imperceptible CRC [colorectal cancer] development and urgent need for colectomy in the face of dysplasia dominated IBD practice,” the authors wrote in Gastroenterology. “However, improvements in disease management, as well as endoscopic technology and quality, have dramatically changed the way in which we conceptualize and manage IBD-related dysplasia over the past 20 years.”
Most notably, the authors called for a more conservative approach to sample collection and intervention.
“The practices of taking nontargeted biopsies and of referring patients for colectomy in the setting of low-grade or invisible dysplasia are being increasingly challenged in favor of ‘smart’ approaches that emphasize careful inspection and targeted sampling of visible and subtle lesions using newer technologies ... as well as endoscopic management of most lesions that appear endoscopically resectable,” the authors wrote. “Indeed, surgery is being increasingly reserved for lesions harboring strong risk factors for invasive cancer or when endoscopic clearance is not possible.”
The 14 best practice advice statements cover a variety of topics, including appropriate lesion terminology and characterization, endoscopy timing, and indications for biopsies, resection, and colectomy.
“The proposed conceptual model and best practice advice statements in this review are best used in conjunction with evolving literature and existing societal guidelines as part of a shared decision-making process,” the authors noted.
Lesion descriptions
First, the authors provided best practice advice for retirement of three older terms: “dysplasia-associated lesion or mass, adenoma-like mass, and flat dysplasia.” Instead, they advised sorting precancerous colorectal lesions into one of three categories: nonpolypoid (less than 2.5 mm tall), polypoid (at least 2.5 mm tall), or invisible (if detected by nontargeted biopsy).
According to the update, lesion descriptions should also include location, morphology, size, presence of ulceration, clarity of borders, presence within an area of past or current colitis, use of special visualization techniques, and perceived completeness of resection.
Surveillance timing
All patients with chronic IBD should undergo colonoscopy screening for dysplasia 8-10 years after diagnosis, the authors wrote. Subsequent colonoscopies should be performed every 1-5 years, depending on risk factors, such as family history of colorectal cancer and quality of prior surveillance exams.
Higher-risk patients may require colonoscopies earlier and more frequently, according to the update. Patients diagnosed with primary sclerosing cholangitis, for instance, should undergo immediate colonoscopy, while patients at high risk of dysplasia (such as those with prior CRC) should undergo annual pouch surveillance.
General principles and surveillance colonoscopy
“Conditions and practices for dysplasia detection should be optimized,” the authors wrote, “including control of inflammation, use of high-definition endoscopes, bowel preparation, careful washing and inspection of all colorectal mucosa, and targeted sampling of any suspicious mucosal irregularities.”
Endoscopists should consider use of dye spray chromoendoscopy, “particularly if a standard definition endoscope is used or if there is a history of dysplasia,” the authors wrote. Alternatively, virtual chromoendoscopy may be used in conjunction with high-definition endoscopy.
Biopsy, resection, and colectomy
According to the update, if chromoendoscopy is used, then biopsies should be targeted “where mucosal findings are suspicious for dysplasia or are inexplicably different from the surrounding mucosa.”
If chromoendoscopy isn’t used, then the authors advised clinicians to also perform nontargeted biopsies, ideally four per 10 cm of colon, in addition to targeted biopsies of suspicious areas.
When lesions are clearly demarcated and lack submucosal fibrosis or stigmata of invasive cancer, then endoscopic resection is preferred over biopsy. Following resection, mucosal biopsies are usually unnecessary, “unless there are concerns about resection completeness.”
“If the resectability of a lesion is in question, referral to a specialized endoscopist or inflammatory bowel disease center is suggested,” wrote the authors.
They noted that, if visible dysplasia is truly unresectable or if invisible multifocal/high-grade dysplasia is encountered, then colectomy should be performed.
IBD control
Finally, the authors emphasized the importance of adequately managing IBD activity to reduce dysplasia risk.
“Because CRC risk in IBD is primarily driven by inflammation, and available data do not demonstrate a clear independent chemopreventive effect of available agents, the focus of chemoprevention in IBD should be control of inflammation,” they wrote.
The expert review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed no conflicts of interest.
The American Gastroenterological Association recently published an expert review and clinical practice update addressing endoscopic surveillance and management of colorectal dysplasia in patients with inflammatory bowel disease (IBD).
Because of practice-altering advances in therapy and surveillance over the past 2 decades, an updated approach is needed, according to authors led by Sanjay K. Murthy, MD, of Ottawa Hospital Research Institute and Fernando Velayos, MD, from Kaiser Permanente San Francisco Medical Center.
“Not long ago, notions of imperceptible CRC [colorectal cancer] development and urgent need for colectomy in the face of dysplasia dominated IBD practice,” the authors wrote in Gastroenterology. “However, improvements in disease management, as well as endoscopic technology and quality, have dramatically changed the way in which we conceptualize and manage IBD-related dysplasia over the past 20 years.”
Most notably, the authors called for a more conservative approach to sample collection and intervention.
“The practices of taking nontargeted biopsies and of referring patients for colectomy in the setting of low-grade or invisible dysplasia are being increasingly challenged in favor of ‘smart’ approaches that emphasize careful inspection and targeted sampling of visible and subtle lesions using newer technologies ... as well as endoscopic management of most lesions that appear endoscopically resectable,” the authors wrote. “Indeed, surgery is being increasingly reserved for lesions harboring strong risk factors for invasive cancer or when endoscopic clearance is not possible.”
The 14 best practice advice statements cover a variety of topics, including appropriate lesion terminology and characterization, endoscopy timing, and indications for biopsies, resection, and colectomy.
“The proposed conceptual model and best practice advice statements in this review are best used in conjunction with evolving literature and existing societal guidelines as part of a shared decision-making process,” the authors noted.
Lesion descriptions
First, the authors provided best practice advice for retirement of three older terms: “dysplasia-associated lesion or mass, adenoma-like mass, and flat dysplasia.” Instead, they advised sorting precancerous colorectal lesions into one of three categories: nonpolypoid (less than 2.5 mm tall), polypoid (at least 2.5 mm tall), or invisible (if detected by nontargeted biopsy).
According to the update, lesion descriptions should also include location, morphology, size, presence of ulceration, clarity of borders, presence within an area of past or current colitis, use of special visualization techniques, and perceived completeness of resection.
Surveillance timing
All patients with chronic IBD should undergo colonoscopy screening for dysplasia 8-10 years after diagnosis, the authors wrote. Subsequent colonoscopies should be performed every 1-5 years, depending on risk factors, such as family history of colorectal cancer and quality of prior surveillance exams.
Higher-risk patients may require colonoscopies earlier and more frequently, according to the update. Patients diagnosed with primary sclerosing cholangitis, for instance, should undergo immediate colonoscopy, while patients at high risk of dysplasia (such as those with prior CRC) should undergo annual pouch surveillance.
General principles and surveillance colonoscopy
“Conditions and practices for dysplasia detection should be optimized,” the authors wrote, “including control of inflammation, use of high-definition endoscopes, bowel preparation, careful washing and inspection of all colorectal mucosa, and targeted sampling of any suspicious mucosal irregularities.”
Endoscopists should consider use of dye spray chromoendoscopy, “particularly if a standard definition endoscope is used or if there is a history of dysplasia,” the authors wrote. Alternatively, virtual chromoendoscopy may be used in conjunction with high-definition endoscopy.
Biopsy, resection, and colectomy
According to the update, if chromoendoscopy is used, then biopsies should be targeted “where mucosal findings are suspicious for dysplasia or are inexplicably different from the surrounding mucosa.”
If chromoendoscopy isn’t used, then the authors advised clinicians to also perform nontargeted biopsies, ideally four per 10 cm of colon, in addition to targeted biopsies of suspicious areas.
When lesions are clearly demarcated and lack submucosal fibrosis or stigmata of invasive cancer, then endoscopic resection is preferred over biopsy. Following resection, mucosal biopsies are usually unnecessary, “unless there are concerns about resection completeness.”
“If the resectability of a lesion is in question, referral to a specialized endoscopist or inflammatory bowel disease center is suggested,” wrote the authors.
They noted that, if visible dysplasia is truly unresectable or if invisible multifocal/high-grade dysplasia is encountered, then colectomy should be performed.
IBD control
Finally, the authors emphasized the importance of adequately managing IBD activity to reduce dysplasia risk.
“Because CRC risk in IBD is primarily driven by inflammation, and available data do not demonstrate a clear independent chemopreventive effect of available agents, the focus of chemoprevention in IBD should be control of inflammation,” they wrote.
The expert review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed no conflicts of interest.
The American Gastroenterological Association recently published an expert review and clinical practice update addressing endoscopic surveillance and management of colorectal dysplasia in patients with inflammatory bowel disease (IBD).
Because of practice-altering advances in therapy and surveillance over the past 2 decades, an updated approach is needed, according to authors led by Sanjay K. Murthy, MD, of Ottawa Hospital Research Institute and Fernando Velayos, MD, from Kaiser Permanente San Francisco Medical Center.
“Not long ago, notions of imperceptible CRC [colorectal cancer] development and urgent need for colectomy in the face of dysplasia dominated IBD practice,” the authors wrote in Gastroenterology. “However, improvements in disease management, as well as endoscopic technology and quality, have dramatically changed the way in which we conceptualize and manage IBD-related dysplasia over the past 20 years.”
Most notably, the authors called for a more conservative approach to sample collection and intervention.
“The practices of taking nontargeted biopsies and of referring patients for colectomy in the setting of low-grade or invisible dysplasia are being increasingly challenged in favor of ‘smart’ approaches that emphasize careful inspection and targeted sampling of visible and subtle lesions using newer technologies ... as well as endoscopic management of most lesions that appear endoscopically resectable,” the authors wrote. “Indeed, surgery is being increasingly reserved for lesions harboring strong risk factors for invasive cancer or when endoscopic clearance is not possible.”
The 14 best practice advice statements cover a variety of topics, including appropriate lesion terminology and characterization, endoscopy timing, and indications for biopsies, resection, and colectomy.
“The proposed conceptual model and best practice advice statements in this review are best used in conjunction with evolving literature and existing societal guidelines as part of a shared decision-making process,” the authors noted.
Lesion descriptions
First, the authors provided best practice advice for retirement of three older terms: “dysplasia-associated lesion or mass, adenoma-like mass, and flat dysplasia.” Instead, they advised sorting precancerous colorectal lesions into one of three categories: nonpolypoid (less than 2.5 mm tall), polypoid (at least 2.5 mm tall), or invisible (if detected by nontargeted biopsy).
According to the update, lesion descriptions should also include location, morphology, size, presence of ulceration, clarity of borders, presence within an area of past or current colitis, use of special visualization techniques, and perceived completeness of resection.
Surveillance timing
All patients with chronic IBD should undergo colonoscopy screening for dysplasia 8-10 years after diagnosis, the authors wrote. Subsequent colonoscopies should be performed every 1-5 years, depending on risk factors, such as family history of colorectal cancer and quality of prior surveillance exams.
Higher-risk patients may require colonoscopies earlier and more frequently, according to the update. Patients diagnosed with primary sclerosing cholangitis, for instance, should undergo immediate colonoscopy, while patients at high risk of dysplasia (such as those with prior CRC) should undergo annual pouch surveillance.
General principles and surveillance colonoscopy
“Conditions and practices for dysplasia detection should be optimized,” the authors wrote, “including control of inflammation, use of high-definition endoscopes, bowel preparation, careful washing and inspection of all colorectal mucosa, and targeted sampling of any suspicious mucosal irregularities.”
Endoscopists should consider use of dye spray chromoendoscopy, “particularly if a standard definition endoscope is used or if there is a history of dysplasia,” the authors wrote. Alternatively, virtual chromoendoscopy may be used in conjunction with high-definition endoscopy.
Biopsy, resection, and colectomy
According to the update, if chromoendoscopy is used, then biopsies should be targeted “where mucosal findings are suspicious for dysplasia or are inexplicably different from the surrounding mucosa.”
If chromoendoscopy isn’t used, then the authors advised clinicians to also perform nontargeted biopsies, ideally four per 10 cm of colon, in addition to targeted biopsies of suspicious areas.
When lesions are clearly demarcated and lack submucosal fibrosis or stigmata of invasive cancer, then endoscopic resection is preferred over biopsy. Following resection, mucosal biopsies are usually unnecessary, “unless there are concerns about resection completeness.”
“If the resectability of a lesion is in question, referral to a specialized endoscopist or inflammatory bowel disease center is suggested,” wrote the authors.
They noted that, if visible dysplasia is truly unresectable or if invisible multifocal/high-grade dysplasia is encountered, then colectomy should be performed.
IBD control
Finally, the authors emphasized the importance of adequately managing IBD activity to reduce dysplasia risk.
“Because CRC risk in IBD is primarily driven by inflammation, and available data do not demonstrate a clear independent chemopreventive effect of available agents, the focus of chemoprevention in IBD should be control of inflammation,” they wrote.
The expert review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
A boy went to a COVID-swamped ER. He waited for hours. Then his appendix burst.
Seth was finally diagnosed with appendicitis more than six hours after arriving at Cleveland Clinic Martin Health North Hospital in late July. Around midnight, he was taken by ambulance to a sister hospital about a half-hour away that was better equipped to perform pediatric emergency surgery, his father said.
But by the time the doctor operated in the early morning hours, Seth’s appendix had burst – a potentially fatal complication.
They, too, need emergency care, but the sheer number of COVID-19 cases is crowding them out. Treatment has often been delayed as ERs scramble to find a bed that may be hundreds of miles away.
Some health officials now worry about looming ethical decisions. Last week, Idaho activated a “crisis standard of care,” which one official described as a “last resort.” It allows overwhelmed hospitals to ration care, including “in rare cases, ventilator (breathing machines) or intensive care unit (ICU) beds may need to be used for those who are most likely to survive, while patients who are not likely to survive may not be able to receive one,” the state’s website said.
The federal government’s latest data shows Alabama is at 100% of its intensive care unit capacity, with Texas, Georgia, Mississippi and Arkansas at more than 90% ICU capacity. Florida is just under 90%.
It’s the COVID-19 cases that are dominating. In Georgia, 62% of the ICU beds are now filled with just COVID-19 patients. In Texas, the percentage is nearly half.
To have so many ICU beds pressed into service for a single diagnosis is “unheard of,” said Dr. Hasan Kakli, an emergency room physician at Bellville Medical Center in Bellville, Texas, about an hour from Houston. “It’s approaching apocalyptic.”
In Texas, state data released Monday showed there were only 319 adult and 104 pediatric staffed ICU beds available across a state of 29 million people.
Hospitals need to hold some ICU beds for other patients, such as those recovering from major surgery or other critical conditions such as stroke, trauma or heart failure.
“This is not just a COVID issue,” said Dr. Normaliz Rodriguez, pediatric emergency physician at Johns Hopkins All Children’s Hospital in St. Petersburg, Florida. “This is an everyone issue.”
While the latest hospital crisis echoes previous pandemic spikes, there are troubling differences this time around.
Before, localized COVID-19 hot spots led to bed shortages, but there were usually hospitals in the region not as affected that could accept a transfer.
Now, as the highly contagious delta variant envelops swaths of low-vaccination states all at once, it becomes harder to find nearby hospitals that are not slammed.
“Wait times can now be measured in days,” said Darrell Pile, CEO of the SouthEast Texas Regional Advisory Council, which helps coordinate patient transfers across a 25-county region.
Recently, Dr. Cedric Dark, a Houston emergency physician and assistant professor of emergency medicine at Baylor College of Medicine, said he saw a critically ill COVID-19 patient waiting in the emergency room for an ICU bed to open. The doctor worked eight hours, went home and came in the next day. The patient was still waiting.
Holding a seriously ill patient in an emergency room while waiting for an in-patient bed to open is known as boarding. The longer the wait, the more dangerous it can be for the patient, studies have found.
Not only do patients ultimately end up staying in the hospital or the ICU longer, some research suggests that long waits for a bed will worsen their condition and may increase the risk of in-hospital death.
That’s what happened last month in Texas.
On Aug. 21, around 11:30 a.m., Michelle Puget took her adult son, Daniel Wilkinson, to the Bellville Medical Center’s emergency room as a pain in his abdomen became unbearable. “Mama,” he said, “take me to the hospital.”
Wilkinson, a 46-year-old decorated Army veteran who did two tours of duty in Afghanistan, was ushered into an exam room about half an hour later. Kakli, the emergency room physician there, diagnosed gallstone pancreatitis, a serious but treatable condition that required a specialist to perform a surgical procedure and an ICU bed.
In other times, the transfer to a larger facility would be easy. But soon Kakli found himself on a frantic, six-hour quest to find a bed for his patient. Not only did he call hospitals across Texas, but he also tried Kansas, Missouri, Oklahoma and Colorado. It was like throwing darts at a map and hoping to get lucky, he told ProPublica. But no one could or would take the transfer.
By 2:30 p.m., Wilkinson’s condition was deteriorating. Kakli told Puget to come back to the hospital. “I have to tell you,” she said he told her, “Your son is a very, very sick man. If he doesn’t get this procedure he will die.” She began to weep.
Two hours later, Wilkinson’s blood pressure was dropping, signaling his organs were failing, she said.
Kakli went on Facebook and posted an all-caps plea to physician groups around the nation: “GETTING REJECTED BY ALL HOSPITALS IN TEXAS DUE TO NO ICU BEDS. PLEASE HELP. MESSAGE ME IF YOU HAVE A BED. PATIENT IS IN ER NOW. I AM THE ER DOC. WILL FLY ANYWHERE.”
The doctor tried Michael E. DeBakey VA Medical Center in Houston for a second time. This time he found a bed.
Around 7 p.m., Wilkinson, still conscious but in grave condition, was flown by helicopter to the hospital. He was put in a medically induced coma. Through the night and into the next morning, medical teams worked to stabilize him enough to perform the procedure. They could not.
Doctors told his family the internal damage was catastrophic. “We made the decision we had to let him go,” Puget said.
Time of death: 1:37 p.m. Aug. 22 – 26 hours after he first arrived in the emergency room.
The story was first reported by CBS News. Kakli told ProPublica last week he still sometimes does the math in his head: It should have been 40 minutes from diagnosis in Bellville to transfer to the ICU in Houston. “If he had 40 minutes to wait instead of six hours, I strongly believe he would have had a different outcome.”
Another difference with the latest surge is how it’s affecting children.
Last year, schools were closed, and children were more protected because they were mostly isolated at home. In fact, children’s hospitals were often so empty during previous spikes they opened beds to adult patients.
Now, families are out more. Schools have reopened, some with mask mandates, some without. Vaccines are not yet available to those under 12. Suddenly the numbers of hospitalized children are on the rise, setting up the same type of competition for resources between young COVID-19 patients and those with other illnesses such as new onset diabetes, trauma, pneumonia or appendicitis.
Dr. Rafael Santiago, a pediatric emergency physician in Central Florida, said at Lakeland Regional Health Medical Center, the average number of children coming into the emergency room is around 130 per day. During the lockdown last spring, that number dropped to 33. Last month – “the busiest month ever” – the average daily number of children in the emergency room was 160.
Pediatric transfers are not yet as fraught as adult ones, Santiago said, but it does take more calls than it once did to secure a bed.
Seth Osborn, the 12-year-old whose appendix burst after a long wait, spent five days and four nights in the hospital as doctors pumped his body full of antibiotics to stave off infection from the rupture. The typical hospitalization for a routine appendectomy is about 24 hours.
The initial hospital bill for the stay came to more than $48,000, Nathaniel Osborn said. Although insurance paid for most of it, he said the family still borrowed against its house to cover the more than $5,000 in out-of-pocket costs so far.
While the hospital system where Seth was treated declined to comment about his case because of patient privacy laws, it did email a statement about the strain the pandemic is creating.
“Since July 2021, we have seen a tremendous spike in COVID-19 patients needing care and hospitalization. In mid-August, we saw the highest number of patients hospitalized with COVID-19 across the Cleveland Clinic Florida region, a total of 395 COVID-19 patients in four hospitals. Those hospitals have approximately 1,000 total beds,” the email to ProPublica said. “We strongly encourage vaccination. Approximately 90% of our patients hospitalized due to COVID-19 are unvaccinated.”
On Sunday, The Washington Post reported that a hospital in Alabama called 43 others across three states before finding a bed for Ray DeMonia, a critically ill heart patient who later died. In his obituary his family wrote: “In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies. ... He would not want any other family to go through what his did.”
Today, Seth is mostly recovered. “Twelve-year-old boys bounce back,” his father said. Still, the experience has left Nathaniel Osborn shaken.
The high school history teacher said he likes to stay upbeat and apolitical in his social media musings, posting about Florida wildlife preservation and favorite books. But on Sept. 7, he tweeted: “My 12-year-old had appendicitis. The ER was overwhelmed with unvaccinated Covid patients and we had to wait 6+ hours. While waiting, his appendix ruptured and had to spend 5 days in hospital. ... So yeah, your decision to not vaccinate does affect others.”
It was retweeted 34,700 times, with 143,000 likes. Most comments were sympathetic and wished his child a speedy recovery. Some, though, went straight to hate, apparently triggered by his last line. He was attacked personally and accused of making up the story: “Good try with the guilt, jerk.”
Osborn, who is vaccinated, as are his wife and son, told ProPublica he only shared Seth’s story on Twitter to encourage vaccinations.
“I have no ill will towards the hospitals or the care received at either hospital,” he said this week, “but had these hospitals not been so crowded with COVID patients, we wouldn’t have had to wait so long and perhaps my son’s appendix would not have burst.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Seth was finally diagnosed with appendicitis more than six hours after arriving at Cleveland Clinic Martin Health North Hospital in late July. Around midnight, he was taken by ambulance to a sister hospital about a half-hour away that was better equipped to perform pediatric emergency surgery, his father said.
But by the time the doctor operated in the early morning hours, Seth’s appendix had burst – a potentially fatal complication.
They, too, need emergency care, but the sheer number of COVID-19 cases is crowding them out. Treatment has often been delayed as ERs scramble to find a bed that may be hundreds of miles away.
Some health officials now worry about looming ethical decisions. Last week, Idaho activated a “crisis standard of care,” which one official described as a “last resort.” It allows overwhelmed hospitals to ration care, including “in rare cases, ventilator (breathing machines) or intensive care unit (ICU) beds may need to be used for those who are most likely to survive, while patients who are not likely to survive may not be able to receive one,” the state’s website said.
The federal government’s latest data shows Alabama is at 100% of its intensive care unit capacity, with Texas, Georgia, Mississippi and Arkansas at more than 90% ICU capacity. Florida is just under 90%.
It’s the COVID-19 cases that are dominating. In Georgia, 62% of the ICU beds are now filled with just COVID-19 patients. In Texas, the percentage is nearly half.
To have so many ICU beds pressed into service for a single diagnosis is “unheard of,” said Dr. Hasan Kakli, an emergency room physician at Bellville Medical Center in Bellville, Texas, about an hour from Houston. “It’s approaching apocalyptic.”
In Texas, state data released Monday showed there were only 319 adult and 104 pediatric staffed ICU beds available across a state of 29 million people.
Hospitals need to hold some ICU beds for other patients, such as those recovering from major surgery or other critical conditions such as stroke, trauma or heart failure.
“This is not just a COVID issue,” said Dr. Normaliz Rodriguez, pediatric emergency physician at Johns Hopkins All Children’s Hospital in St. Petersburg, Florida. “This is an everyone issue.”
While the latest hospital crisis echoes previous pandemic spikes, there are troubling differences this time around.
Before, localized COVID-19 hot spots led to bed shortages, but there were usually hospitals in the region not as affected that could accept a transfer.
Now, as the highly contagious delta variant envelops swaths of low-vaccination states all at once, it becomes harder to find nearby hospitals that are not slammed.
“Wait times can now be measured in days,” said Darrell Pile, CEO of the SouthEast Texas Regional Advisory Council, which helps coordinate patient transfers across a 25-county region.
Recently, Dr. Cedric Dark, a Houston emergency physician and assistant professor of emergency medicine at Baylor College of Medicine, said he saw a critically ill COVID-19 patient waiting in the emergency room for an ICU bed to open. The doctor worked eight hours, went home and came in the next day. The patient was still waiting.
Holding a seriously ill patient in an emergency room while waiting for an in-patient bed to open is known as boarding. The longer the wait, the more dangerous it can be for the patient, studies have found.
Not only do patients ultimately end up staying in the hospital or the ICU longer, some research suggests that long waits for a bed will worsen their condition and may increase the risk of in-hospital death.
That’s what happened last month in Texas.
On Aug. 21, around 11:30 a.m., Michelle Puget took her adult son, Daniel Wilkinson, to the Bellville Medical Center’s emergency room as a pain in his abdomen became unbearable. “Mama,” he said, “take me to the hospital.”
Wilkinson, a 46-year-old decorated Army veteran who did two tours of duty in Afghanistan, was ushered into an exam room about half an hour later. Kakli, the emergency room physician there, diagnosed gallstone pancreatitis, a serious but treatable condition that required a specialist to perform a surgical procedure and an ICU bed.
In other times, the transfer to a larger facility would be easy. But soon Kakli found himself on a frantic, six-hour quest to find a bed for his patient. Not only did he call hospitals across Texas, but he also tried Kansas, Missouri, Oklahoma and Colorado. It was like throwing darts at a map and hoping to get lucky, he told ProPublica. But no one could or would take the transfer.
By 2:30 p.m., Wilkinson’s condition was deteriorating. Kakli told Puget to come back to the hospital. “I have to tell you,” she said he told her, “Your son is a very, very sick man. If he doesn’t get this procedure he will die.” She began to weep.
Two hours later, Wilkinson’s blood pressure was dropping, signaling his organs were failing, she said.
Kakli went on Facebook and posted an all-caps plea to physician groups around the nation: “GETTING REJECTED BY ALL HOSPITALS IN TEXAS DUE TO NO ICU BEDS. PLEASE HELP. MESSAGE ME IF YOU HAVE A BED. PATIENT IS IN ER NOW. I AM THE ER DOC. WILL FLY ANYWHERE.”
The doctor tried Michael E. DeBakey VA Medical Center in Houston for a second time. This time he found a bed.
Around 7 p.m., Wilkinson, still conscious but in grave condition, was flown by helicopter to the hospital. He was put in a medically induced coma. Through the night and into the next morning, medical teams worked to stabilize him enough to perform the procedure. They could not.
Doctors told his family the internal damage was catastrophic. “We made the decision we had to let him go,” Puget said.
Time of death: 1:37 p.m. Aug. 22 – 26 hours after he first arrived in the emergency room.
The story was first reported by CBS News. Kakli told ProPublica last week he still sometimes does the math in his head: It should have been 40 minutes from diagnosis in Bellville to transfer to the ICU in Houston. “If he had 40 minutes to wait instead of six hours, I strongly believe he would have had a different outcome.”
Another difference with the latest surge is how it’s affecting children.
Last year, schools were closed, and children were more protected because they were mostly isolated at home. In fact, children’s hospitals were often so empty during previous spikes they opened beds to adult patients.
Now, families are out more. Schools have reopened, some with mask mandates, some without. Vaccines are not yet available to those under 12. Suddenly the numbers of hospitalized children are on the rise, setting up the same type of competition for resources between young COVID-19 patients and those with other illnesses such as new onset diabetes, trauma, pneumonia or appendicitis.
Dr. Rafael Santiago, a pediatric emergency physician in Central Florida, said at Lakeland Regional Health Medical Center, the average number of children coming into the emergency room is around 130 per day. During the lockdown last spring, that number dropped to 33. Last month – “the busiest month ever” – the average daily number of children in the emergency room was 160.
Pediatric transfers are not yet as fraught as adult ones, Santiago said, but it does take more calls than it once did to secure a bed.
Seth Osborn, the 12-year-old whose appendix burst after a long wait, spent five days and four nights in the hospital as doctors pumped his body full of antibiotics to stave off infection from the rupture. The typical hospitalization for a routine appendectomy is about 24 hours.
The initial hospital bill for the stay came to more than $48,000, Nathaniel Osborn said. Although insurance paid for most of it, he said the family still borrowed against its house to cover the more than $5,000 in out-of-pocket costs so far.
While the hospital system where Seth was treated declined to comment about his case because of patient privacy laws, it did email a statement about the strain the pandemic is creating.
“Since July 2021, we have seen a tremendous spike in COVID-19 patients needing care and hospitalization. In mid-August, we saw the highest number of patients hospitalized with COVID-19 across the Cleveland Clinic Florida region, a total of 395 COVID-19 patients in four hospitals. Those hospitals have approximately 1,000 total beds,” the email to ProPublica said. “We strongly encourage vaccination. Approximately 90% of our patients hospitalized due to COVID-19 are unvaccinated.”
On Sunday, The Washington Post reported that a hospital in Alabama called 43 others across three states before finding a bed for Ray DeMonia, a critically ill heart patient who later died. In his obituary his family wrote: “In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies. ... He would not want any other family to go through what his did.”
Today, Seth is mostly recovered. “Twelve-year-old boys bounce back,” his father said. Still, the experience has left Nathaniel Osborn shaken.
The high school history teacher said he likes to stay upbeat and apolitical in his social media musings, posting about Florida wildlife preservation and favorite books. But on Sept. 7, he tweeted: “My 12-year-old had appendicitis. The ER was overwhelmed with unvaccinated Covid patients and we had to wait 6+ hours. While waiting, his appendix ruptured and had to spend 5 days in hospital. ... So yeah, your decision to not vaccinate does affect others.”
It was retweeted 34,700 times, with 143,000 likes. Most comments were sympathetic and wished his child a speedy recovery. Some, though, went straight to hate, apparently triggered by his last line. He was attacked personally and accused of making up the story: “Good try with the guilt, jerk.”
Osborn, who is vaccinated, as are his wife and son, told ProPublica he only shared Seth’s story on Twitter to encourage vaccinations.
“I have no ill will towards the hospitals or the care received at either hospital,” he said this week, “but had these hospitals not been so crowded with COVID patients, we wouldn’t have had to wait so long and perhaps my son’s appendix would not have burst.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Seth was finally diagnosed with appendicitis more than six hours after arriving at Cleveland Clinic Martin Health North Hospital in late July. Around midnight, he was taken by ambulance to a sister hospital about a half-hour away that was better equipped to perform pediatric emergency surgery, his father said.
But by the time the doctor operated in the early morning hours, Seth’s appendix had burst – a potentially fatal complication.
They, too, need emergency care, but the sheer number of COVID-19 cases is crowding them out. Treatment has often been delayed as ERs scramble to find a bed that may be hundreds of miles away.
Some health officials now worry about looming ethical decisions. Last week, Idaho activated a “crisis standard of care,” which one official described as a “last resort.” It allows overwhelmed hospitals to ration care, including “in rare cases, ventilator (breathing machines) or intensive care unit (ICU) beds may need to be used for those who are most likely to survive, while patients who are not likely to survive may not be able to receive one,” the state’s website said.
The federal government’s latest data shows Alabama is at 100% of its intensive care unit capacity, with Texas, Georgia, Mississippi and Arkansas at more than 90% ICU capacity. Florida is just under 90%.
It’s the COVID-19 cases that are dominating. In Georgia, 62% of the ICU beds are now filled with just COVID-19 patients. In Texas, the percentage is nearly half.
To have so many ICU beds pressed into service for a single diagnosis is “unheard of,” said Dr. Hasan Kakli, an emergency room physician at Bellville Medical Center in Bellville, Texas, about an hour from Houston. “It’s approaching apocalyptic.”
In Texas, state data released Monday showed there were only 319 adult and 104 pediatric staffed ICU beds available across a state of 29 million people.
Hospitals need to hold some ICU beds for other patients, such as those recovering from major surgery or other critical conditions such as stroke, trauma or heart failure.
“This is not just a COVID issue,” said Dr. Normaliz Rodriguez, pediatric emergency physician at Johns Hopkins All Children’s Hospital in St. Petersburg, Florida. “This is an everyone issue.”
While the latest hospital crisis echoes previous pandemic spikes, there are troubling differences this time around.
Before, localized COVID-19 hot spots led to bed shortages, but there were usually hospitals in the region not as affected that could accept a transfer.
Now, as the highly contagious delta variant envelops swaths of low-vaccination states all at once, it becomes harder to find nearby hospitals that are not slammed.
“Wait times can now be measured in days,” said Darrell Pile, CEO of the SouthEast Texas Regional Advisory Council, which helps coordinate patient transfers across a 25-county region.
Recently, Dr. Cedric Dark, a Houston emergency physician and assistant professor of emergency medicine at Baylor College of Medicine, said he saw a critically ill COVID-19 patient waiting in the emergency room for an ICU bed to open. The doctor worked eight hours, went home and came in the next day. The patient was still waiting.
Holding a seriously ill patient in an emergency room while waiting for an in-patient bed to open is known as boarding. The longer the wait, the more dangerous it can be for the patient, studies have found.
Not only do patients ultimately end up staying in the hospital or the ICU longer, some research suggests that long waits for a bed will worsen their condition and may increase the risk of in-hospital death.
That’s what happened last month in Texas.
On Aug. 21, around 11:30 a.m., Michelle Puget took her adult son, Daniel Wilkinson, to the Bellville Medical Center’s emergency room as a pain in his abdomen became unbearable. “Mama,” he said, “take me to the hospital.”
Wilkinson, a 46-year-old decorated Army veteran who did two tours of duty in Afghanistan, was ushered into an exam room about half an hour later. Kakli, the emergency room physician there, diagnosed gallstone pancreatitis, a serious but treatable condition that required a specialist to perform a surgical procedure and an ICU bed.
In other times, the transfer to a larger facility would be easy. But soon Kakli found himself on a frantic, six-hour quest to find a bed for his patient. Not only did he call hospitals across Texas, but he also tried Kansas, Missouri, Oklahoma and Colorado. It was like throwing darts at a map and hoping to get lucky, he told ProPublica. But no one could or would take the transfer.
By 2:30 p.m., Wilkinson’s condition was deteriorating. Kakli told Puget to come back to the hospital. “I have to tell you,” she said he told her, “Your son is a very, very sick man. If he doesn’t get this procedure he will die.” She began to weep.
Two hours later, Wilkinson’s blood pressure was dropping, signaling his organs were failing, she said.
Kakli went on Facebook and posted an all-caps plea to physician groups around the nation: “GETTING REJECTED BY ALL HOSPITALS IN TEXAS DUE TO NO ICU BEDS. PLEASE HELP. MESSAGE ME IF YOU HAVE A BED. PATIENT IS IN ER NOW. I AM THE ER DOC. WILL FLY ANYWHERE.”
The doctor tried Michael E. DeBakey VA Medical Center in Houston for a second time. This time he found a bed.
Around 7 p.m., Wilkinson, still conscious but in grave condition, was flown by helicopter to the hospital. He was put in a medically induced coma. Through the night and into the next morning, medical teams worked to stabilize him enough to perform the procedure. They could not.
Doctors told his family the internal damage was catastrophic. “We made the decision we had to let him go,” Puget said.
Time of death: 1:37 p.m. Aug. 22 – 26 hours after he first arrived in the emergency room.
The story was first reported by CBS News. Kakli told ProPublica last week he still sometimes does the math in his head: It should have been 40 minutes from diagnosis in Bellville to transfer to the ICU in Houston. “If he had 40 minutes to wait instead of six hours, I strongly believe he would have had a different outcome.”
Another difference with the latest surge is how it’s affecting children.
Last year, schools were closed, and children were more protected because they were mostly isolated at home. In fact, children’s hospitals were often so empty during previous spikes they opened beds to adult patients.
Now, families are out more. Schools have reopened, some with mask mandates, some without. Vaccines are not yet available to those under 12. Suddenly the numbers of hospitalized children are on the rise, setting up the same type of competition for resources between young COVID-19 patients and those with other illnesses such as new onset diabetes, trauma, pneumonia or appendicitis.
Dr. Rafael Santiago, a pediatric emergency physician in Central Florida, said at Lakeland Regional Health Medical Center, the average number of children coming into the emergency room is around 130 per day. During the lockdown last spring, that number dropped to 33. Last month – “the busiest month ever” – the average daily number of children in the emergency room was 160.
Pediatric transfers are not yet as fraught as adult ones, Santiago said, but it does take more calls than it once did to secure a bed.
Seth Osborn, the 12-year-old whose appendix burst after a long wait, spent five days and four nights in the hospital as doctors pumped his body full of antibiotics to stave off infection from the rupture. The typical hospitalization for a routine appendectomy is about 24 hours.
The initial hospital bill for the stay came to more than $48,000, Nathaniel Osborn said. Although insurance paid for most of it, he said the family still borrowed against its house to cover the more than $5,000 in out-of-pocket costs so far.
While the hospital system where Seth was treated declined to comment about his case because of patient privacy laws, it did email a statement about the strain the pandemic is creating.
“Since July 2021, we have seen a tremendous spike in COVID-19 patients needing care and hospitalization. In mid-August, we saw the highest number of patients hospitalized with COVID-19 across the Cleveland Clinic Florida region, a total of 395 COVID-19 patients in four hospitals. Those hospitals have approximately 1,000 total beds,” the email to ProPublica said. “We strongly encourage vaccination. Approximately 90% of our patients hospitalized due to COVID-19 are unvaccinated.”
On Sunday, The Washington Post reported that a hospital in Alabama called 43 others across three states before finding a bed for Ray DeMonia, a critically ill heart patient who later died. In his obituary his family wrote: “In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies. ... He would not want any other family to go through what his did.”
Today, Seth is mostly recovered. “Twelve-year-old boys bounce back,” his father said. Still, the experience has left Nathaniel Osborn shaken.
The high school history teacher said he likes to stay upbeat and apolitical in his social media musings, posting about Florida wildlife preservation and favorite books. But on Sept. 7, he tweeted: “My 12-year-old had appendicitis. The ER was overwhelmed with unvaccinated Covid patients and we had to wait 6+ hours. While waiting, his appendix ruptured and had to spend 5 days in hospital. ... So yeah, your decision to not vaccinate does affect others.”
It was retweeted 34,700 times, with 143,000 likes. Most comments were sympathetic and wished his child a speedy recovery. Some, though, went straight to hate, apparently triggered by his last line. He was attacked personally and accused of making up the story: “Good try with the guilt, jerk.”
Osborn, who is vaccinated, as are his wife and son, told ProPublica he only shared Seth’s story on Twitter to encourage vaccinations.
“I have no ill will towards the hospitals or the care received at either hospital,” he said this week, “but had these hospitals not been so crowded with COVID patients, we wouldn’t have had to wait so long and perhaps my son’s appendix would not have burst.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Want to see what COVID strain you have? The government says no
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.
The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.

The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.

The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Weight-loss surgery linked to fewer cardiovascular events, more so with RYGB
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
FROM DIABETES CARE
Urticaria and edema in a 2-year-old boy
A 2-YEAR-OLD BOY presented to the emergency room with a 1-day history of a diffuse, mildly pruritic rash and swelling of his knees, ankles, and feet following treatment of acute otitis media with amoxicillin for the previous 8 days. He was mildly febrile and consolable, but he was refusing to walk. His medical history was unremarkable.
Physical examination revealed erythematous annular wheals on his chest, face, back, and extremities. Lymphadenopathy and mucous membrane involvement were not present. A complete blood count (CBC) with differential, inflammatory marker tests, and a comprehensive metabolic panel were ordered. Given the joint swelling and rash, the patient was admitted for observation.
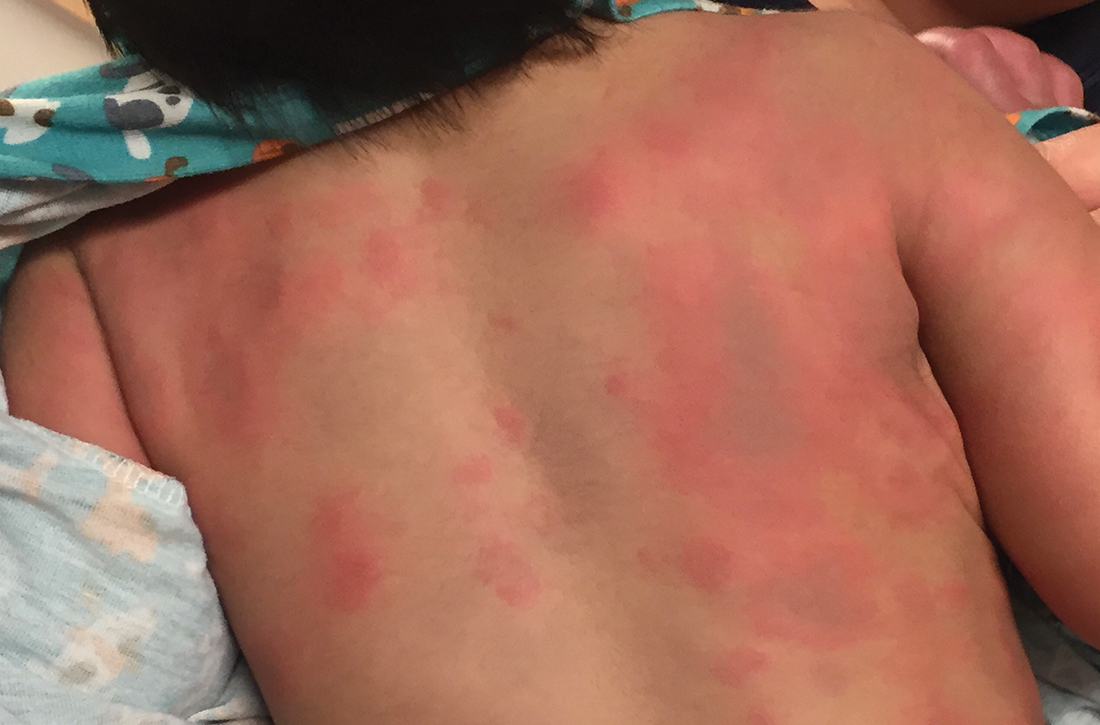
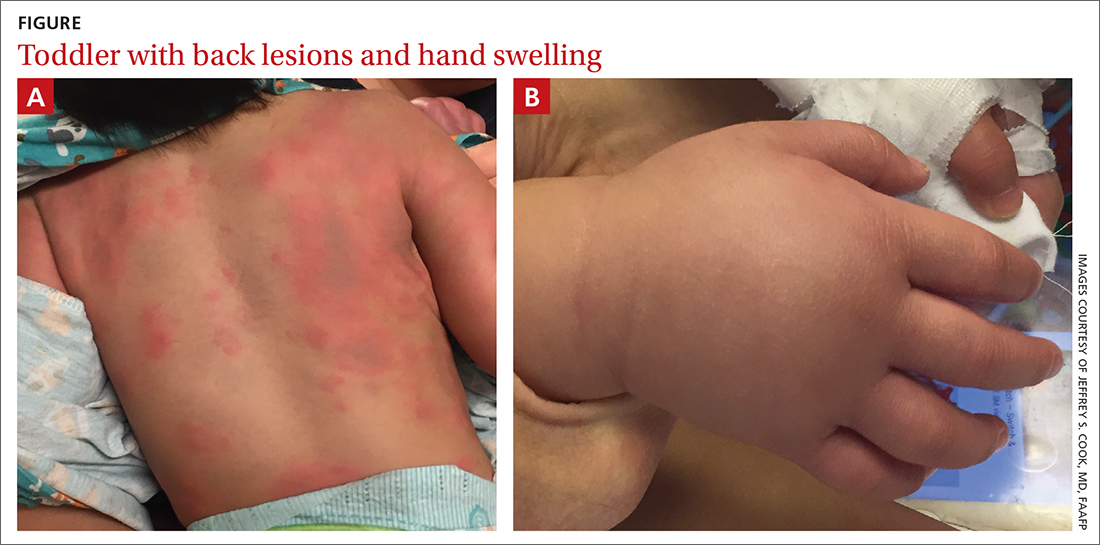
During his second day in the hospital, his skin lesions enlarged and several formed dusky blue centers (FIGURE 1A). He also developed swelling of his hands (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
The patient’s lab work came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. The incidence and prevalence are not known. Urticaria multiforme is considered common, but it is frequently misdiagnosed.1 It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 Individual lesions last less than 24 hours, but new ones may appear. The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Dermatographism due to mast cell–mediated cutaneous hypersensitivity at sites of minor skin trauma is common (44%).
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing.When performed, laboratory studies, including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis are routinely normal.
Erythema multiforme and urticarial vasculitis are part of the differential
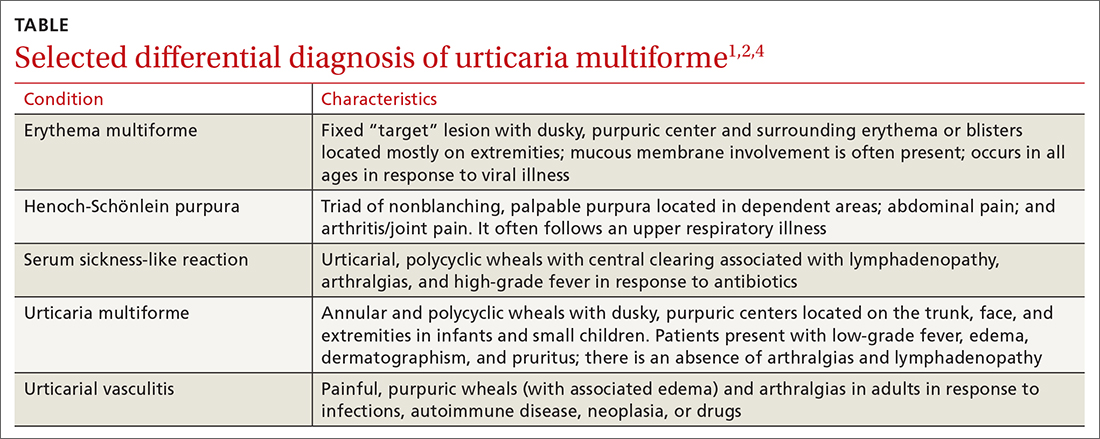
The differential diagnosis in this case includes erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis (TABLE1,2,4).
Continue to: Erythema multiforme
Erythema multiforme is a common misdiagnosis in patients with urticaria multiforme.1,2 The erythema multiforme rash has a “target” lesion with outer erythema and central ecchymosis, which may develop blisters or necrosis. Lesions are fixed and last 2 to 3 weeks. Unlike urticaria multiforme, patients with erythema multiforme commonly have mucous membrane erosions and occasionally ulcerations. Facial and acral edema is rare. Treatment is largely symptomatic and can include glucocorticoids. Antiviral medications may be used to treat recurrences.1,2
Henoch-Schönlein purpura is an immunoglobulin A–mediated vasculitis that affects the skin, gastrointestinal tract, and joints.4,5 Patients often present with arthralgias, gastrointestinal symptoms such as abdominal pain and bleeding, and a nonpruritic, erythematous rash that progresses to palpable purpura in dependent areas of the body. Treatment is generally symptomatic, but steroids may be used in severe cases.4,5
Serum sickness-like reaction can manifest with angioedema and a similar urticarial rash (with central clearing) that lasts 1 to 6 weeks.1,2,6,7 However, patients tend to have a high-grade fever, arthralgias, myalgias, and lymphadenopathy while dermatographism is absent. Treatment includes discontinuing the offending agent and the use of H1 and H2 antihistamines and steroids, in severe cases.
Urticarial vasculitis manifests as plaques or wheals lasting 1 to 7 days that may cause burning and pain but not pruritis.2,5 Purpura or hypopigmentation may develop as the hives resolve. Angioedema and arthralgias are common, but dermatographism is not present. Triggers include infections, autoimmune disease, malignancy, and the use of certain medications. H1 and H2 blockers and nonsteroidal anti-inflammatory agents are first-line therapy.2
Step 1: Discontinue offending agents; Step 2: Recommend antihistamines
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Continue to: Our patient's amoxicillin
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby, Elsevier Inc; 2016.
6. King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions. J Paediatr Child Health. 2003;39:677-681. doi: 10.1046/j.1440-1754.2003.00267.x
7. Misirlioglu ED, Duman H, Ozmen S, et al. Serum sickness-like reaction in children due to cefditoren. Pediatr Dermatol. 2011;29:327-328. doi: 10.1111/j.1525-1470.2011.01539.x
A 2-YEAR-OLD BOY presented to the emergency room with a 1-day history of a diffuse, mildly pruritic rash and swelling of his knees, ankles, and feet following treatment of acute otitis media with amoxicillin for the previous 8 days. He was mildly febrile and consolable, but he was refusing to walk. His medical history was unremarkable.
Physical examination revealed erythematous annular wheals on his chest, face, back, and extremities. Lymphadenopathy and mucous membrane involvement were not present. A complete blood count (CBC) with differential, inflammatory marker tests, and a comprehensive metabolic panel were ordered. Given the joint swelling and rash, the patient was admitted for observation.
During his second day in the hospital, his skin lesions enlarged and several formed dusky blue centers (FIGURE 1A). He also developed swelling of his hands (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
The patient’s lab work came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. The incidence and prevalence are not known. Urticaria multiforme is considered common, but it is frequently misdiagnosed.1 It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 Individual lesions last less than 24 hours, but new ones may appear. The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Dermatographism due to mast cell–mediated cutaneous hypersensitivity at sites of minor skin trauma is common (44%).
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing.When performed, laboratory studies, including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis are routinely normal.
Erythema multiforme and urticarial vasculitis are part of the differential
The differential diagnosis in this case includes erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis (TABLE1,2,4).

Continue to: Erythema multiforme
Erythema multiforme is a common misdiagnosis in patients with urticaria multiforme.1,2 The erythema multiforme rash has a “target” lesion with outer erythema and central ecchymosis, which may develop blisters or necrosis. Lesions are fixed and last 2 to 3 weeks. Unlike urticaria multiforme, patients with erythema multiforme commonly have mucous membrane erosions and occasionally ulcerations. Facial and acral edema is rare. Treatment is largely symptomatic and can include glucocorticoids. Antiviral medications may be used to treat recurrences.1,2
Henoch-Schönlein purpura is an immunoglobulin A–mediated vasculitis that affects the skin, gastrointestinal tract, and joints.4,5 Patients often present with arthralgias, gastrointestinal symptoms such as abdominal pain and bleeding, and a nonpruritic, erythematous rash that progresses to palpable purpura in dependent areas of the body. Treatment is generally symptomatic, but steroids may be used in severe cases.4,5
Serum sickness-like reaction can manifest with angioedema and a similar urticarial rash (with central clearing) that lasts 1 to 6 weeks.1,2,6,7 However, patients tend to have a high-grade fever, arthralgias, myalgias, and lymphadenopathy while dermatographism is absent. Treatment includes discontinuing the offending agent and the use of H1 and H2 antihistamines and steroids, in severe cases.
Urticarial vasculitis manifests as plaques or wheals lasting 1 to 7 days that may cause burning and pain but not pruritis.2,5 Purpura or hypopigmentation may develop as the hives resolve. Angioedema and arthralgias are common, but dermatographism is not present. Triggers include infections, autoimmune disease, malignancy, and the use of certain medications. H1 and H2 blockers and nonsteroidal anti-inflammatory agents are first-line therapy.2
Step 1: Discontinue offending agents; Step 2: Recommend antihistamines
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Continue to: Our patient's amoxicillin
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
A 2-YEAR-OLD BOY presented to the emergency room with a 1-day history of a diffuse, mildly pruritic rash and swelling of his knees, ankles, and feet following treatment of acute otitis media with amoxicillin for the previous 8 days. He was mildly febrile and consolable, but he was refusing to walk. His medical history was unremarkable.
Physical examination revealed erythematous annular wheals on his chest, face, back, and extremities. Lymphadenopathy and mucous membrane involvement were not present. A complete blood count (CBC) with differential, inflammatory marker tests, and a comprehensive metabolic panel were ordered. Given the joint swelling and rash, the patient was admitted for observation.
During his second day in the hospital, his skin lesions enlarged and several formed dusky blue centers (FIGURE 1A). He also developed swelling of his hands (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
The patient’s lab work came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. The incidence and prevalence are not known. Urticaria multiforme is considered common, but it is frequently misdiagnosed.1 It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 Individual lesions last less than 24 hours, but new ones may appear. The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Dermatographism due to mast cell–mediated cutaneous hypersensitivity at sites of minor skin trauma is common (44%).
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing.When performed, laboratory studies, including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis are routinely normal.
Erythema multiforme and urticarial vasculitis are part of the differential
The differential diagnosis in this case includes erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis (TABLE1,2,4).

Continue to: Erythema multiforme
Erythema multiforme is a common misdiagnosis in patients with urticaria multiforme.1,2 The erythema multiforme rash has a “target” lesion with outer erythema and central ecchymosis, which may develop blisters or necrosis. Lesions are fixed and last 2 to 3 weeks. Unlike urticaria multiforme, patients with erythema multiforme commonly have mucous membrane erosions and occasionally ulcerations. Facial and acral edema is rare. Treatment is largely symptomatic and can include glucocorticoids. Antiviral medications may be used to treat recurrences.1,2
Henoch-Schönlein purpura is an immunoglobulin A–mediated vasculitis that affects the skin, gastrointestinal tract, and joints.4,5 Patients often present with arthralgias, gastrointestinal symptoms such as abdominal pain and bleeding, and a nonpruritic, erythematous rash that progresses to palpable purpura in dependent areas of the body. Treatment is generally symptomatic, but steroids may be used in severe cases.4,5
Serum sickness-like reaction can manifest with angioedema and a similar urticarial rash (with central clearing) that lasts 1 to 6 weeks.1,2,6,7 However, patients tend to have a high-grade fever, arthralgias, myalgias, and lymphadenopathy while dermatographism is absent. Treatment includes discontinuing the offending agent and the use of H1 and H2 antihistamines and steroids, in severe cases.
Urticarial vasculitis manifests as plaques or wheals lasting 1 to 7 days that may cause burning and pain but not pruritis.2,5 Purpura or hypopigmentation may develop as the hives resolve. Angioedema and arthralgias are common, but dermatographism is not present. Triggers include infections, autoimmune disease, malignancy, and the use of certain medications. H1 and H2 blockers and nonsteroidal anti-inflammatory agents are first-line therapy.2
Step 1: Discontinue offending agents; Step 2: Recommend antihistamines
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Continue to: Our patient's amoxicillin
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby, Elsevier Inc; 2016.
6. King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions. J Paediatr Child Health. 2003;39:677-681. doi: 10.1046/j.1440-1754.2003.00267.x
7. Misirlioglu ED, Duman H, Ozmen S, et al. Serum sickness-like reaction in children due to cefditoren. Pediatr Dermatol. 2011;29:327-328. doi: 10.1111/j.1525-1470.2011.01539.x
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby, Elsevier Inc; 2016.
6. King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions. J Paediatr Child Health. 2003;39:677-681. doi: 10.1046/j.1440-1754.2003.00267.x
7. Misirlioglu ED, Duman H, Ozmen S, et al. Serum sickness-like reaction in children due to cefditoren. Pediatr Dermatol. 2011;29:327-328. doi: 10.1111/j.1525-1470.2011.01539.x
Children and COVID: New cases down slightly from record high
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Man dies after 43 full ICUs turn him away
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
PRESERVED-HF: Dapagliflozin improves physical limitations in patients with HFpEF
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
FROM HFSA 2021
USPSTF update: Screen young asymptomatic women for chlamydia and gonorrhea
But evidence for screening men remains insufficient, task force says
The U.S. Preventive Services Task Force has updated its 2014 statement on screening asymptomatic individuals for chlamydia and gonorrhea infection.
Published online in JAMA, the 2021 version recommends that all sexually active women aged 24 years or younger and at-risk women 25 years or older should be screened for chlamydia and gonorrhea.
As in 2014, the task force made no screening recommendation for men owing to inconclusive evidence of benefit.
With cases of sexually transmitted infections reaching all-time highs, Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues noted that chlamydia and gonorrhea are among the most common STIs in this country. According to the Centers for Disease Control and Prevention, 2019 saw approximately 1.8 million reported cases of chlamydia and more than 600,000 of gonorrhea.
In the current analysis of 27 observational and randomized studies comprising 179,515 patients, the USPSTF panel found that, compared with no screening, chlamydia screening was significantly associated with a reduced risk of pelvic inflammatory disease (PID) in young women in 2 out of 4 trials.
The authors cautioned, however, that the magnitude of benefit was relatively small. No studies reported on screening effectiveness in men, except for one reporting rates of epididymitis, and no studies were done on pregnant women for any outcome.
The largest and newest study, the Australian Chlamydia Control Effectiveness Pilot trial of 2018, assessed chlamydia screening against usual care in 180,355 men and women aged 16-29 years in 130 rural Australian primary care clinics. Screening was associated with a reduced risk of hospital-diagnosed PID: the absolute risk was 0.24% for screening versus 0.38% for usual care (unadjusted risk ratio, 0.6; 95% confidence interval, 0.4-1.0). It was not, however, significantly associated with a reduced risk of clinic-diagnosed PID, with an absolute risk of 0.45% versus 0.39% (RR, 1.1; 95% CI, 0.7-18). Nor did it correlate with a risk reduction for clinic-diagnosed epididymitis: 0.26% vs. 0.27% (RR, 0.9; 95% CI, 0.6-1.4).
While risk prediction criteria apart from age were only minimally accurate, testing for asymptomatic chlamydial and gonococcal infections was highly accurate at most anatomical sites, including urine and self-collected specimens, the investigators observed. Age 22 years or younger alone versus multi-item risk criteria demonstrated similar discrimination in a study that included symptomatic and asymptomatic women.
Sensitivity of chlamydial testing was similar at endocervical (89%-100%) and self- and clinician-collected vaginal (90%-100%) sites for women and at meatal (100%), urethral (99%), and rectal (92%) sites for men. It was lower, however, at pharyngeal sites (69.2%) for men who have sex with men (MSM).
Sensitivity of gonococcal testing was 89% or greater for all anatomical samples. False-positive and false-negative testing rates were low across anatomical sites and collection methods.
“Effectiveness of screening in men and during pregnancy, optimal screening intervals, and adverse effects of screening require further evaluation, Dr. Cantor and associates concluded.
In an accompanying editorial, Jeanne Marrazzo, MD, MPH, and Jodie Dionne-Odom, MD, MSPH, of the division of infectious diseases at the University of Alabama at Birmingham, called the guidelines “timely” and “powerful agents of change” that “influence a wide spectrum of health-based metrics, from quality assurance measures to criteria for financial reimbursement.”
They pointed out that men who have sex with men are experiencing historically high rates of gonorrhea, with most infections occurring extragenitally at the pharynx or rectum. In 2019 CDC data, MSM had substantially higher rates of gonorrhea than men who had sex only with women. They recommended that guidelines for men consider STI risk because of sexual relations with men, women, or both.
“Comprehensive screening guidelines for common STIs like chlamydia and gonorrhea could incorporate the limited evidence base for MSM, whether it is regular practice or not,” they wrote, with the same approach for women who have sex with women but may be at risk for chlamydia, particularly if they also have sex with men.
In their view, these latest guidelines appropriately prioritize high-level clinically based data. They pointed, however, to recent progress in understanding the pathogenesis of upper reproductive tract infection in women and the sexual networks behind the current resurgence of STIs in the United States in the failure to manage exposed sex partners.
“Considering these critical advances in the evolution of clinic-based screening guidelines is a work in progress,” they wrote, “the dialogue among basic scientists, clinical trial investigators, and public health professionals to inform the next version of updated USPSTF chlamydia and gonorrhea screening guidelines should start now.”
In the opinion of Jennifer L. Reed, MD, MS, a professor of pediatrics and an emergency medicine physician at Cincinnati Children’s Hospital Medical Center and not involved in the updated statement, the recommendations are very reasonable. “The highest rates of infection occur in females 15-24 years of age, and therefore asymptomatic screening for chlamydia and gonorrhea is imperative at least annually or more often if they are high risk,” she said in an interview.
“I would hope that providers increase their asymptomatic screening as a result of these recommendations and highly consider it in the younger men,” Dr. Reed added. “I see a very high rate of gonorrhea and chlamydia infections.” Her center is studying the implementation of gonorrhea and chlamydia asymptomatic screening for adolescents in the pediatric emergency department, a high-risk patient population that will benefit from STI screening opportunities in nontraditional settings.
This research was funded by the Agency for Healthcare Research and Quality and the Department of Health & Human Services under a contract to support the USPSTF. One statement coauthor reported personal fees from Insmed, Paratek, RedHill, and Spero, as well as grants from Insmed. No other disclosures were reported. Dr. Dionne-Odom reported grants from the National Institutes of Health/National Institute of Child Health and Development. Dr. Reed reported a grant from NIH/NICHD for a pragmatic trial of improving STI detection in the pediatric ED.
But evidence for screening men remains insufficient, task force says
But evidence for screening men remains insufficient, task force says
The U.S. Preventive Services Task Force has updated its 2014 statement on screening asymptomatic individuals for chlamydia and gonorrhea infection.
Published online in JAMA, the 2021 version recommends that all sexually active women aged 24 years or younger and at-risk women 25 years or older should be screened for chlamydia and gonorrhea.
As in 2014, the task force made no screening recommendation for men owing to inconclusive evidence of benefit.
With cases of sexually transmitted infections reaching all-time highs, Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues noted that chlamydia and gonorrhea are among the most common STIs in this country. According to the Centers for Disease Control and Prevention, 2019 saw approximately 1.8 million reported cases of chlamydia and more than 600,000 of gonorrhea.
In the current analysis of 27 observational and randomized studies comprising 179,515 patients, the USPSTF panel found that, compared with no screening, chlamydia screening was significantly associated with a reduced risk of pelvic inflammatory disease (PID) in young women in 2 out of 4 trials.
The authors cautioned, however, that the magnitude of benefit was relatively small. No studies reported on screening effectiveness in men, except for one reporting rates of epididymitis, and no studies were done on pregnant women for any outcome.
The largest and newest study, the Australian Chlamydia Control Effectiveness Pilot trial of 2018, assessed chlamydia screening against usual care in 180,355 men and women aged 16-29 years in 130 rural Australian primary care clinics. Screening was associated with a reduced risk of hospital-diagnosed PID: the absolute risk was 0.24% for screening versus 0.38% for usual care (unadjusted risk ratio, 0.6; 95% confidence interval, 0.4-1.0). It was not, however, significantly associated with a reduced risk of clinic-diagnosed PID, with an absolute risk of 0.45% versus 0.39% (RR, 1.1; 95% CI, 0.7-18). Nor did it correlate with a risk reduction for clinic-diagnosed epididymitis: 0.26% vs. 0.27% (RR, 0.9; 95% CI, 0.6-1.4).
While risk prediction criteria apart from age were only minimally accurate, testing for asymptomatic chlamydial and gonococcal infections was highly accurate at most anatomical sites, including urine and self-collected specimens, the investigators observed. Age 22 years or younger alone versus multi-item risk criteria demonstrated similar discrimination in a study that included symptomatic and asymptomatic women.
Sensitivity of chlamydial testing was similar at endocervical (89%-100%) and self- and clinician-collected vaginal (90%-100%) sites for women and at meatal (100%), urethral (99%), and rectal (92%) sites for men. It was lower, however, at pharyngeal sites (69.2%) for men who have sex with men (MSM).
Sensitivity of gonococcal testing was 89% or greater for all anatomical samples. False-positive and false-negative testing rates were low across anatomical sites and collection methods.
“Effectiveness of screening in men and during pregnancy, optimal screening intervals, and adverse effects of screening require further evaluation, Dr. Cantor and associates concluded.
In an accompanying editorial, Jeanne Marrazzo, MD, MPH, and Jodie Dionne-Odom, MD, MSPH, of the division of infectious diseases at the University of Alabama at Birmingham, called the guidelines “timely” and “powerful agents of change” that “influence a wide spectrum of health-based metrics, from quality assurance measures to criteria for financial reimbursement.”
They pointed out that men who have sex with men are experiencing historically high rates of gonorrhea, with most infections occurring extragenitally at the pharynx or rectum. In 2019 CDC data, MSM had substantially higher rates of gonorrhea than men who had sex only with women. They recommended that guidelines for men consider STI risk because of sexual relations with men, women, or both.
“Comprehensive screening guidelines for common STIs like chlamydia and gonorrhea could incorporate the limited evidence base for MSM, whether it is regular practice or not,” they wrote, with the same approach for women who have sex with women but may be at risk for chlamydia, particularly if they also have sex with men.
In their view, these latest guidelines appropriately prioritize high-level clinically based data. They pointed, however, to recent progress in understanding the pathogenesis of upper reproductive tract infection in women and the sexual networks behind the current resurgence of STIs in the United States in the failure to manage exposed sex partners.
“Considering these critical advances in the evolution of clinic-based screening guidelines is a work in progress,” they wrote, “the dialogue among basic scientists, clinical trial investigators, and public health professionals to inform the next version of updated USPSTF chlamydia and gonorrhea screening guidelines should start now.”
In the opinion of Jennifer L. Reed, MD, MS, a professor of pediatrics and an emergency medicine physician at Cincinnati Children’s Hospital Medical Center and not involved in the updated statement, the recommendations are very reasonable. “The highest rates of infection occur in females 15-24 years of age, and therefore asymptomatic screening for chlamydia and gonorrhea is imperative at least annually or more often if they are high risk,” she said in an interview.
“I would hope that providers increase their asymptomatic screening as a result of these recommendations and highly consider it in the younger men,” Dr. Reed added. “I see a very high rate of gonorrhea and chlamydia infections.” Her center is studying the implementation of gonorrhea and chlamydia asymptomatic screening for adolescents in the pediatric emergency department, a high-risk patient population that will benefit from STI screening opportunities in nontraditional settings.
This research was funded by the Agency for Healthcare Research and Quality and the Department of Health & Human Services under a contract to support the USPSTF. One statement coauthor reported personal fees from Insmed, Paratek, RedHill, and Spero, as well as grants from Insmed. No other disclosures were reported. Dr. Dionne-Odom reported grants from the National Institutes of Health/National Institute of Child Health and Development. Dr. Reed reported a grant from NIH/NICHD for a pragmatic trial of improving STI detection in the pediatric ED.
The U.S. Preventive Services Task Force has updated its 2014 statement on screening asymptomatic individuals for chlamydia and gonorrhea infection.
Published online in JAMA, the 2021 version recommends that all sexually active women aged 24 years or younger and at-risk women 25 years or older should be screened for chlamydia and gonorrhea.
As in 2014, the task force made no screening recommendation for men owing to inconclusive evidence of benefit.
With cases of sexually transmitted infections reaching all-time highs, Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues noted that chlamydia and gonorrhea are among the most common STIs in this country. According to the Centers for Disease Control and Prevention, 2019 saw approximately 1.8 million reported cases of chlamydia and more than 600,000 of gonorrhea.
In the current analysis of 27 observational and randomized studies comprising 179,515 patients, the USPSTF panel found that, compared with no screening, chlamydia screening was significantly associated with a reduced risk of pelvic inflammatory disease (PID) in young women in 2 out of 4 trials.
The authors cautioned, however, that the magnitude of benefit was relatively small. No studies reported on screening effectiveness in men, except for one reporting rates of epididymitis, and no studies were done on pregnant women for any outcome.
The largest and newest study, the Australian Chlamydia Control Effectiveness Pilot trial of 2018, assessed chlamydia screening against usual care in 180,355 men and women aged 16-29 years in 130 rural Australian primary care clinics. Screening was associated with a reduced risk of hospital-diagnosed PID: the absolute risk was 0.24% for screening versus 0.38% for usual care (unadjusted risk ratio, 0.6; 95% confidence interval, 0.4-1.0). It was not, however, significantly associated with a reduced risk of clinic-diagnosed PID, with an absolute risk of 0.45% versus 0.39% (RR, 1.1; 95% CI, 0.7-18). Nor did it correlate with a risk reduction for clinic-diagnosed epididymitis: 0.26% vs. 0.27% (RR, 0.9; 95% CI, 0.6-1.4).
While risk prediction criteria apart from age were only minimally accurate, testing for asymptomatic chlamydial and gonococcal infections was highly accurate at most anatomical sites, including urine and self-collected specimens, the investigators observed. Age 22 years or younger alone versus multi-item risk criteria demonstrated similar discrimination in a study that included symptomatic and asymptomatic women.
Sensitivity of chlamydial testing was similar at endocervical (89%-100%) and self- and clinician-collected vaginal (90%-100%) sites for women and at meatal (100%), urethral (99%), and rectal (92%) sites for men. It was lower, however, at pharyngeal sites (69.2%) for men who have sex with men (MSM).
Sensitivity of gonococcal testing was 89% or greater for all anatomical samples. False-positive and false-negative testing rates were low across anatomical sites and collection methods.
“Effectiveness of screening in men and during pregnancy, optimal screening intervals, and adverse effects of screening require further evaluation, Dr. Cantor and associates concluded.
In an accompanying editorial, Jeanne Marrazzo, MD, MPH, and Jodie Dionne-Odom, MD, MSPH, of the division of infectious diseases at the University of Alabama at Birmingham, called the guidelines “timely” and “powerful agents of change” that “influence a wide spectrum of health-based metrics, from quality assurance measures to criteria for financial reimbursement.”
They pointed out that men who have sex with men are experiencing historically high rates of gonorrhea, with most infections occurring extragenitally at the pharynx or rectum. In 2019 CDC data, MSM had substantially higher rates of gonorrhea than men who had sex only with women. They recommended that guidelines for men consider STI risk because of sexual relations with men, women, or both.
“Comprehensive screening guidelines for common STIs like chlamydia and gonorrhea could incorporate the limited evidence base for MSM, whether it is regular practice or not,” they wrote, with the same approach for women who have sex with women but may be at risk for chlamydia, particularly if they also have sex with men.
In their view, these latest guidelines appropriately prioritize high-level clinically based data. They pointed, however, to recent progress in understanding the pathogenesis of upper reproductive tract infection in women and the sexual networks behind the current resurgence of STIs in the United States in the failure to manage exposed sex partners.
“Considering these critical advances in the evolution of clinic-based screening guidelines is a work in progress,” they wrote, “the dialogue among basic scientists, clinical trial investigators, and public health professionals to inform the next version of updated USPSTF chlamydia and gonorrhea screening guidelines should start now.”
In the opinion of Jennifer L. Reed, MD, MS, a professor of pediatrics and an emergency medicine physician at Cincinnati Children’s Hospital Medical Center and not involved in the updated statement, the recommendations are very reasonable. “The highest rates of infection occur in females 15-24 years of age, and therefore asymptomatic screening for chlamydia and gonorrhea is imperative at least annually or more often if they are high risk,” she said in an interview.
“I would hope that providers increase their asymptomatic screening as a result of these recommendations and highly consider it in the younger men,” Dr. Reed added. “I see a very high rate of gonorrhea and chlamydia infections.” Her center is studying the implementation of gonorrhea and chlamydia asymptomatic screening for adolescents in the pediatric emergency department, a high-risk patient population that will benefit from STI screening opportunities in nontraditional settings.
This research was funded by the Agency for Healthcare Research and Quality and the Department of Health & Human Services under a contract to support the USPSTF. One statement coauthor reported personal fees from Insmed, Paratek, RedHill, and Spero, as well as grants from Insmed. No other disclosures were reported. Dr. Dionne-Odom reported grants from the National Institutes of Health/National Institute of Child Health and Development. Dr. Reed reported a grant from NIH/NICHD for a pragmatic trial of improving STI detection in the pediatric ED.
FROM JAMA
Are ESC’s new heart failure guidelines already outdated?
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”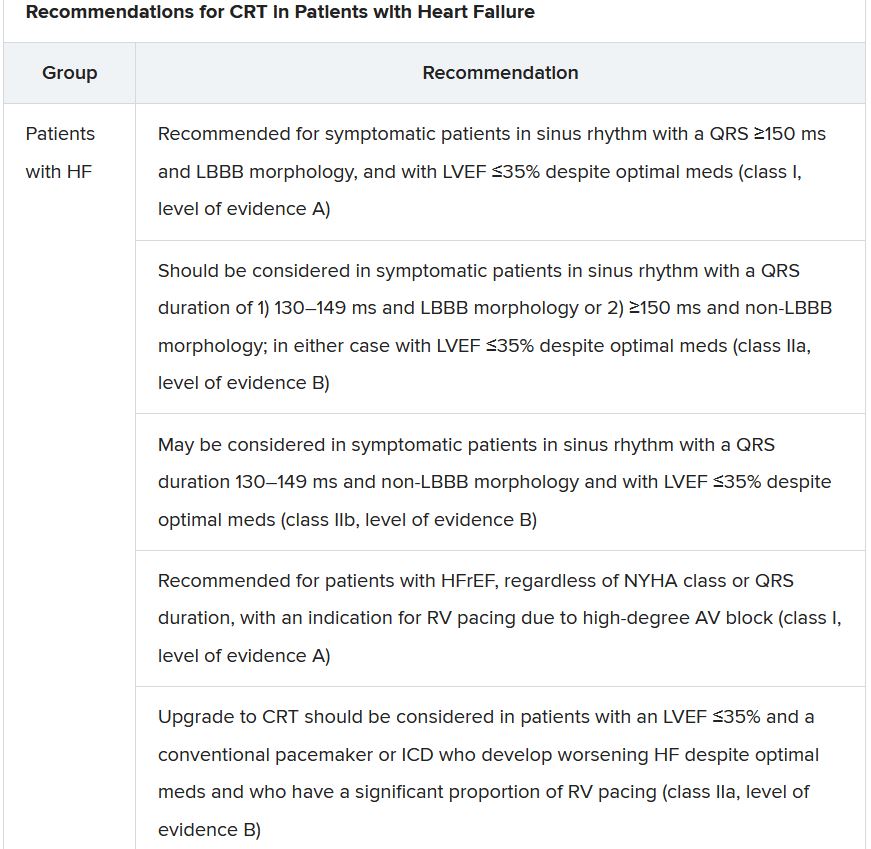
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.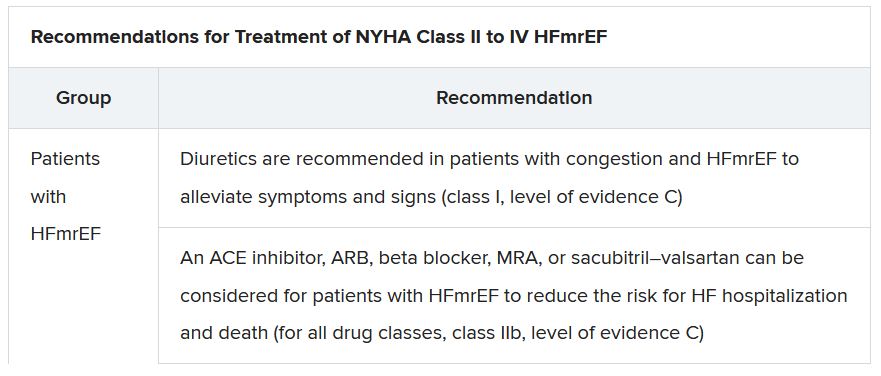
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.