User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
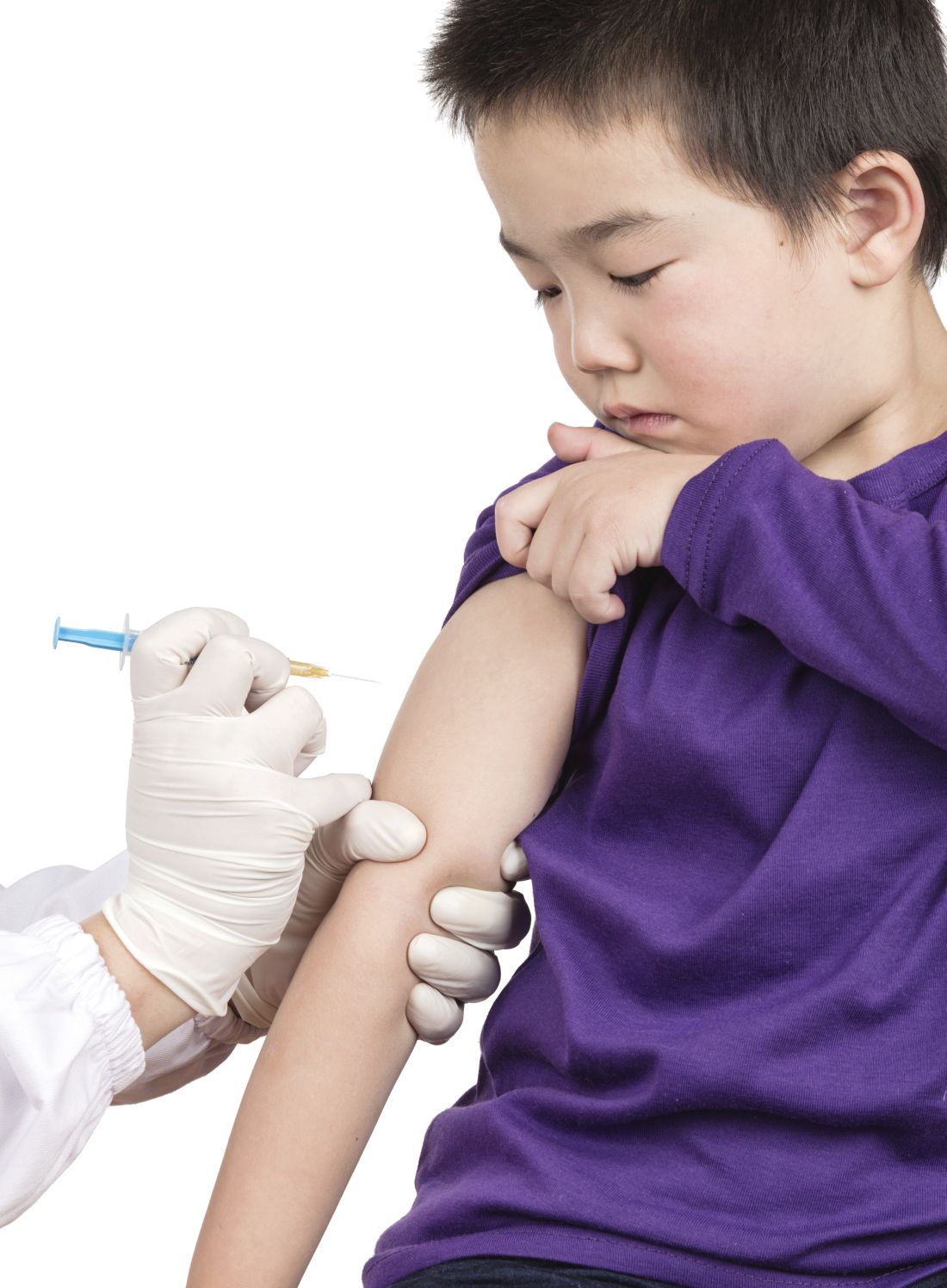
Is the WHO’s HPV vaccination target within reach?
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
FROM PREVENTIVE MEDICINE
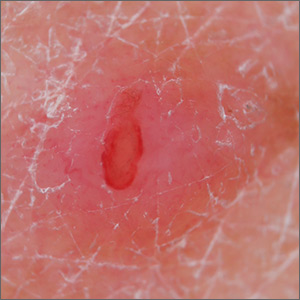
Pink papule on thigh

A deep-shave biopsy indicated that this was an inflamed/irritated solitary neurofibroma. Basal cell carcinoma, inflamed nevus, and Merkel cell carcinoma were also considered.
Most often manifesting in adults, solitary neurofibromas are common nonencapsulated, soft to firm papules that range in size from 2 mm to 2 cm. Solitary neurofibromas are benign and work-up for systemic neurofibromatosis is not indicated. However, if a patient presents with multiple neurofibromas, axillary freckling, or multiple café au lait macules, systemic disease should be considered, followed by molecular testing and/or referral to a medical geneticist or neurofibromatosis clinic.
Although both the triage amalgamated diagnostic algorithm and the 2-step dermoscopy algorithm suggested this lesion was higher risk, it was ultimately found to be benign. This case highlights areas in which dermoscopy and physical exam lack specificity, but this trade-off increases the sensitivity of an algorithmic approach. Solitary pink papules can include some subtle, but fearsome, diagnoses and deserve close attention. In this case, the biopsy not only helped confirm the diagnosis, but it also alleviated the discomfort caused by the neurofibroma.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Geller S, Pulitzer M, Brady MS, et al. Dermoscopic assessment of vascular structures in solitary small pink lesions—differentiating between good and evil. Dermatol Pract Concept. 2017;7:47-50. doi: 10.5826/dpc.0703a10

A deep-shave biopsy indicated that this was an inflamed/irritated solitary neurofibroma. Basal cell carcinoma, inflamed nevus, and Merkel cell carcinoma were also considered.
Most often manifesting in adults, solitary neurofibromas are common nonencapsulated, soft to firm papules that range in size from 2 mm to 2 cm. Solitary neurofibromas are benign and work-up for systemic neurofibromatosis is not indicated. However, if a patient presents with multiple neurofibromas, axillary freckling, or multiple café au lait macules, systemic disease should be considered, followed by molecular testing and/or referral to a medical geneticist or neurofibromatosis clinic.
Although both the triage amalgamated diagnostic algorithm and the 2-step dermoscopy algorithm suggested this lesion was higher risk, it was ultimately found to be benign. This case highlights areas in which dermoscopy and physical exam lack specificity, but this trade-off increases the sensitivity of an algorithmic approach. Solitary pink papules can include some subtle, but fearsome, diagnoses and deserve close attention. In this case, the biopsy not only helped confirm the diagnosis, but it also alleviated the discomfort caused by the neurofibroma.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

A deep-shave biopsy indicated that this was an inflamed/irritated solitary neurofibroma. Basal cell carcinoma, inflamed nevus, and Merkel cell carcinoma were also considered.
Most often manifesting in adults, solitary neurofibromas are common nonencapsulated, soft to firm papules that range in size from 2 mm to 2 cm. Solitary neurofibromas are benign and work-up for systemic neurofibromatosis is not indicated. However, if a patient presents with multiple neurofibromas, axillary freckling, or multiple café au lait macules, systemic disease should be considered, followed by molecular testing and/or referral to a medical geneticist or neurofibromatosis clinic.
Although both the triage amalgamated diagnostic algorithm and the 2-step dermoscopy algorithm suggested this lesion was higher risk, it was ultimately found to be benign. This case highlights areas in which dermoscopy and physical exam lack specificity, but this trade-off increases the sensitivity of an algorithmic approach. Solitary pink papules can include some subtle, but fearsome, diagnoses and deserve close attention. In this case, the biopsy not only helped confirm the diagnosis, but it also alleviated the discomfort caused by the neurofibroma.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Geller S, Pulitzer M, Brady MS, et al. Dermoscopic assessment of vascular structures in solitary small pink lesions—differentiating between good and evil. Dermatol Pract Concept. 2017;7:47-50. doi: 10.5826/dpc.0703a10
1. Geller S, Pulitzer M, Brady MS, et al. Dermoscopic assessment of vascular structures in solitary small pink lesions—differentiating between good and evil. Dermatol Pract Concept. 2017;7:47-50. doi: 10.5826/dpc.0703a10
Some with long COVID see relief after vaccination
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
We’re all vaccinated: Can we go back to the office (unmasked) now?
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Could pollen be driving COVID-19 infections?
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
This Rash Really Stinks!
ANSWER
The correct diagnosis is Darier disease (choice “d”).
DISCUSSION
Darier disease, also known as Darier-White disease or keratosis follicularis, is an inherited defect transmitted by autosomal dominant mode. The pathophysiologic process is a breakdown of cell adhesion that normally binds keratin filaments to tiny connecting fibers called desmosomes.
Darier disease manifests with a “branny” papulosquamous rash, typically arising in the third decade of life and affecting the chest, scalp, back, and intertriginous areas. The nail and intraoral findings noted in this patient are typical. In the author’s experience, the former is more commonly seen and is essentially pathognomic for the disease.
Darier disease is relatively rare, occurring in 1:30,000 to 1:100,000 population, depending on the geographic area studied. Men and women are equally affected, although it is more common in those with darker skin.
The differential outlined in the answer choices is reasonable, considering the condition’s rarity and how unlikely it is to manifest solely in the inframammary area. One could conclude that, just as with psoriasis (choice “b”) and seborrhea, intertrigo (choice “c”) is not always a primary process. And although yeast infection (choice “a”) can complicate any florid rash in this area, topical and oral anti-yeast treatment had utterly failed to help.
TREATMENT
Isotretinoin is used in cases such as this one, but it only offers temporary relief. For less severe cases, oral antibiotics (eg minocycline) or topical steroids (used with caution given the risk for atrophy in the inframammary area) often suffice. This patient’s prognosis is guarded at best, although control of the worst is certainly possible.
ANSWER
The correct diagnosis is Darier disease (choice “d”).
DISCUSSION
Darier disease, also known as Darier-White disease or keratosis follicularis, is an inherited defect transmitted by autosomal dominant mode. The pathophysiologic process is a breakdown of cell adhesion that normally binds keratin filaments to tiny connecting fibers called desmosomes.
Darier disease manifests with a “branny” papulosquamous rash, typically arising in the third decade of life and affecting the chest, scalp, back, and intertriginous areas. The nail and intraoral findings noted in this patient are typical. In the author’s experience, the former is more commonly seen and is essentially pathognomic for the disease.
Darier disease is relatively rare, occurring in 1:30,000 to 1:100,000 population, depending on the geographic area studied. Men and women are equally affected, although it is more common in those with darker skin.
The differential outlined in the answer choices is reasonable, considering the condition’s rarity and how unlikely it is to manifest solely in the inframammary area. One could conclude that, just as with psoriasis (choice “b”) and seborrhea, intertrigo (choice “c”) is not always a primary process. And although yeast infection (choice “a”) can complicate any florid rash in this area, topical and oral anti-yeast treatment had utterly failed to help.
TREATMENT
Isotretinoin is used in cases such as this one, but it only offers temporary relief. For less severe cases, oral antibiotics (eg minocycline) or topical steroids (used with caution given the risk for atrophy in the inframammary area) often suffice. This patient’s prognosis is guarded at best, although control of the worst is certainly possible.
ANSWER
The correct diagnosis is Darier disease (choice “d”).
DISCUSSION
Darier disease, also known as Darier-White disease or keratosis follicularis, is an inherited defect transmitted by autosomal dominant mode. The pathophysiologic process is a breakdown of cell adhesion that normally binds keratin filaments to tiny connecting fibers called desmosomes.
Darier disease manifests with a “branny” papulosquamous rash, typically arising in the third decade of life and affecting the chest, scalp, back, and intertriginous areas. The nail and intraoral findings noted in this patient are typical. In the author’s experience, the former is more commonly seen and is essentially pathognomic for the disease.
Darier disease is relatively rare, occurring in 1:30,000 to 1:100,000 population, depending on the geographic area studied. Men and women are equally affected, although it is more common in those with darker skin.
The differential outlined in the answer choices is reasonable, considering the condition’s rarity and how unlikely it is to manifest solely in the inframammary area. One could conclude that, just as with psoriasis (choice “b”) and seborrhea, intertrigo (choice “c”) is not always a primary process. And although yeast infection (choice “a”) can complicate any florid rash in this area, topical and oral anti-yeast treatment had utterly failed to help.
TREATMENT
Isotretinoin is used in cases such as this one, but it only offers temporary relief. For less severe cases, oral antibiotics (eg minocycline) or topical steroids (used with caution given the risk for atrophy in the inframammary area) often suffice. This patient’s prognosis is guarded at best, although control of the worst is certainly possible.

A 50-year-old woman is referred to dermatology with a “yeast” infection of several years’ duration. The condition causes considerable discomfort, especially during hot weather when the rash emits a very objectionable odor.
The florid, white, scaly rash under her breasts is a stark contrast to the patient’s type V skin. On both sides, the affected skin perfectly matches the inframammary fold. There are sharp margins and uniform moist scaling.
Looking elsewhere, 7 of 10 fingernails exhibit longitudinal white and red streaks, along with triangular nicks in the edges of several nails. The roof of the patient’s mouth is studded with fleshy nodules measuring 0.6 to 1.0 cm. Several pits are seen on her palms.
The patient is in no distress but is quite agitated by the lack of effective treatment. She reports trying a number of prescription and OTC anti-yeast creams, lotions, and oral medications, none of which resolved the problem.
History-taking reveals a family history of skin problems, although neither the patient nor anyone else in the family has ever been seen by a dermatologist. No one has ever suggested that a biopsy be done.
A punch biopsy is performed on the affected inframammary skin. The pathology report shows acantholysis with focal dyskeratotic keratinocytes. Intraepidermal separation is seen throughout the specimen.
ACG: CRC screening should start at age 45
The starting age was previously 50 years for most patients. However, for Black patients, the starting age was lowered to 45 years in 2005.
The new guidance brings the ACG in line with recommendations of the American Cancer Society, which lowered the starting age to 45 years for average-risk individuals in 2018.
However, the U.S. Preventive Services Task Force, the Multi-Specialty Task Force, and the American College of Physicians still recommend that CRC screening begin at the age of 50.
The new ACG guideline were published in March 2021 in the American Journal of Gastroenterology. The last time they were updated was in 2009.
The ACG said that the move was made in light of reports of an increase in the incidence of CRC in adults younger than 50.
“It has been estimated that [in the United States] persons born around 1990 have twice the risk of colon cancer and four times the risk of rectal cancer, compared with those born around 1950,” guideline author Aasma Shaukat, MD, MPH, University of Minnesota, Minneapolis, and colleagues pointed out.
“The fact that other developed countries are reporting similar increases in early-onset CRC and birth-cohort effects suggests that the Western lifestyle (especially exemplified by the obesity epidemic) is a significant contributor,” the authors added.
The new ACG guideline emphasize the importance of initiating CRC screening for average-risk patients aged 50-75 years. “Given that current rates of screening uptake are close to 60% (57.9% ages 50-64 and 62.4% ages 50-75), expanding the population to be screened may reduce these rates as emphasis shifts to screening 45- to 49-year-olds at the expense of efforts to screen the unscreened 50- to 75-year-olds,” the authors commented.
Now, however, the guideline suggests that the decision to continue screening after age 75 should be individualized. It notes that the benefits of screening are limited for those who are not expected to live for another 7-10 years. For patients with a family history of CRC, the guideline authors recommended initiating CRC screening at the age of 40 for patients with one or two first-degree relatives with either CRC or advanced colorectal polyps.
They also recommend screening colonoscopy over any other screening modality if the first-degree relative is younger than 60 or if two or more first-degree relatives of any age have CRC or advanced colorectal polyps. For such patients, screening should be repeated every 5 years.
For screening average-risk individuals, either colonoscopy or fecal immunochemical testing (FIT) is recommended. If colonoscopy is used, it should be repeated every 10 years. FIT should be conducted on an annual basis.
This is somewhat in contrast to recent changes proposed by the American Gastroenterological Association. The AGA recommends greater use of noninvasive testing, such as with fecal occult blood tests, initially. It recommends that initial colonoscopy be used only for patients at high risk for CRC.
For individuals unwilling or unable to undergo colonoscopy or FIT, the ACG suggests flexible sigmoidoscopy, multitarget stool DNA testing, CT colonography, or colon capsule. Only colonoscopy is a single-step test; all other screening modalities require a follow-up colonoscopy if test results are positive.
“We recommend against the use of aspirin as a substitute for CRC screening,” the ACG members emphasized. Rather, they suggest that the use of low-dose aspirin be considered only for patients aged 50-69 years whose risk for cardiovascular disease over the next 10 years is at least 10% and who are at low risk for bleeding.
To reduce their risk for CRC, patients need to take aspirin for at least 10 years, they pointed out.
Quality indicators
For endoscopists who perform colonoscopy, the ACG recommended that all operators determine their individual cecal intubation rates, adenoma detection rates, and withdrawal times. They also recommended that endoscopists spend at least 6 minutes inspecting the mucosa during withdrawal and achieve a cecal intubation rate of at least 95% for all patients screened.
The ACG recommended remedial training for any provider whose adenoma detection rate is less than 25%.
Screening rates dropped during pandemic
The authors of the new recommendations also pointed out that, despite public health initiatives to boost CRC screening in the United States and the availability of multiple screening modalities, almost one-third of individuals who are eligible for CRC screening do not undergo screening.
Moreover, the proportion of individuals not being screened has reportedly increased during the pandemic. In one report, claims data for colonoscopies dropped by 90% during April. “Colorectal cancer screening rates must be optimized to reach the aspirational target of >80%,” the authors emphasized.
“A recommendation to be screened by a PCP [primary care provider] – who is known and trusted by the person – is clearly effective in raising participation,” they added.
Dr. Shaukat has served as a scientific consultant for Iterative Scopes and Freenome. Other ACG guideline authors reported numerous financial relationships.
A version of this article first appeared on Medscape.com.
The starting age was previously 50 years for most patients. However, for Black patients, the starting age was lowered to 45 years in 2005.
The new guidance brings the ACG in line with recommendations of the American Cancer Society, which lowered the starting age to 45 years for average-risk individuals in 2018.
However, the U.S. Preventive Services Task Force, the Multi-Specialty Task Force, and the American College of Physicians still recommend that CRC screening begin at the age of 50.
The new ACG guideline were published in March 2021 in the American Journal of Gastroenterology. The last time they were updated was in 2009.
The ACG said that the move was made in light of reports of an increase in the incidence of CRC in adults younger than 50.
“It has been estimated that [in the United States] persons born around 1990 have twice the risk of colon cancer and four times the risk of rectal cancer, compared with those born around 1950,” guideline author Aasma Shaukat, MD, MPH, University of Minnesota, Minneapolis, and colleagues pointed out.
“The fact that other developed countries are reporting similar increases in early-onset CRC and birth-cohort effects suggests that the Western lifestyle (especially exemplified by the obesity epidemic) is a significant contributor,” the authors added.
The new ACG guideline emphasize the importance of initiating CRC screening for average-risk patients aged 50-75 years. “Given that current rates of screening uptake are close to 60% (57.9% ages 50-64 and 62.4% ages 50-75), expanding the population to be screened may reduce these rates as emphasis shifts to screening 45- to 49-year-olds at the expense of efforts to screen the unscreened 50- to 75-year-olds,” the authors commented.
Now, however, the guideline suggests that the decision to continue screening after age 75 should be individualized. It notes that the benefits of screening are limited for those who are not expected to live for another 7-10 years. For patients with a family history of CRC, the guideline authors recommended initiating CRC screening at the age of 40 for patients with one or two first-degree relatives with either CRC or advanced colorectal polyps.
They also recommend screening colonoscopy over any other screening modality if the first-degree relative is younger than 60 or if two or more first-degree relatives of any age have CRC or advanced colorectal polyps. For such patients, screening should be repeated every 5 years.
For screening average-risk individuals, either colonoscopy or fecal immunochemical testing (FIT) is recommended. If colonoscopy is used, it should be repeated every 10 years. FIT should be conducted on an annual basis.
This is somewhat in contrast to recent changes proposed by the American Gastroenterological Association. The AGA recommends greater use of noninvasive testing, such as with fecal occult blood tests, initially. It recommends that initial colonoscopy be used only for patients at high risk for CRC.
For individuals unwilling or unable to undergo colonoscopy or FIT, the ACG suggests flexible sigmoidoscopy, multitarget stool DNA testing, CT colonography, or colon capsule. Only colonoscopy is a single-step test; all other screening modalities require a follow-up colonoscopy if test results are positive.
“We recommend against the use of aspirin as a substitute for CRC screening,” the ACG members emphasized. Rather, they suggest that the use of low-dose aspirin be considered only for patients aged 50-69 years whose risk for cardiovascular disease over the next 10 years is at least 10% and who are at low risk for bleeding.
To reduce their risk for CRC, patients need to take aspirin for at least 10 years, they pointed out.
Quality indicators
For endoscopists who perform colonoscopy, the ACG recommended that all operators determine their individual cecal intubation rates, adenoma detection rates, and withdrawal times. They also recommended that endoscopists spend at least 6 minutes inspecting the mucosa during withdrawal and achieve a cecal intubation rate of at least 95% for all patients screened.
The ACG recommended remedial training for any provider whose adenoma detection rate is less than 25%.
Screening rates dropped during pandemic
The authors of the new recommendations also pointed out that, despite public health initiatives to boost CRC screening in the United States and the availability of multiple screening modalities, almost one-third of individuals who are eligible for CRC screening do not undergo screening.
Moreover, the proportion of individuals not being screened has reportedly increased during the pandemic. In one report, claims data for colonoscopies dropped by 90% during April. “Colorectal cancer screening rates must be optimized to reach the aspirational target of >80%,” the authors emphasized.
“A recommendation to be screened by a PCP [primary care provider] – who is known and trusted by the person – is clearly effective in raising participation,” they added.
Dr. Shaukat has served as a scientific consultant for Iterative Scopes and Freenome. Other ACG guideline authors reported numerous financial relationships.
A version of this article first appeared on Medscape.com.
The starting age was previously 50 years for most patients. However, for Black patients, the starting age was lowered to 45 years in 2005.
The new guidance brings the ACG in line with recommendations of the American Cancer Society, which lowered the starting age to 45 years for average-risk individuals in 2018.
However, the U.S. Preventive Services Task Force, the Multi-Specialty Task Force, and the American College of Physicians still recommend that CRC screening begin at the age of 50.
The new ACG guideline were published in March 2021 in the American Journal of Gastroenterology. The last time they were updated was in 2009.
The ACG said that the move was made in light of reports of an increase in the incidence of CRC in adults younger than 50.
“It has been estimated that [in the United States] persons born around 1990 have twice the risk of colon cancer and four times the risk of rectal cancer, compared with those born around 1950,” guideline author Aasma Shaukat, MD, MPH, University of Minnesota, Minneapolis, and colleagues pointed out.
“The fact that other developed countries are reporting similar increases in early-onset CRC and birth-cohort effects suggests that the Western lifestyle (especially exemplified by the obesity epidemic) is a significant contributor,” the authors added.
The new ACG guideline emphasize the importance of initiating CRC screening for average-risk patients aged 50-75 years. “Given that current rates of screening uptake are close to 60% (57.9% ages 50-64 and 62.4% ages 50-75), expanding the population to be screened may reduce these rates as emphasis shifts to screening 45- to 49-year-olds at the expense of efforts to screen the unscreened 50- to 75-year-olds,” the authors commented.
Now, however, the guideline suggests that the decision to continue screening after age 75 should be individualized. It notes that the benefits of screening are limited for those who are not expected to live for another 7-10 years. For patients with a family history of CRC, the guideline authors recommended initiating CRC screening at the age of 40 for patients with one or two first-degree relatives with either CRC or advanced colorectal polyps.
They also recommend screening colonoscopy over any other screening modality if the first-degree relative is younger than 60 or if two or more first-degree relatives of any age have CRC or advanced colorectal polyps. For such patients, screening should be repeated every 5 years.
For screening average-risk individuals, either colonoscopy or fecal immunochemical testing (FIT) is recommended. If colonoscopy is used, it should be repeated every 10 years. FIT should be conducted on an annual basis.
This is somewhat in contrast to recent changes proposed by the American Gastroenterological Association. The AGA recommends greater use of noninvasive testing, such as with fecal occult blood tests, initially. It recommends that initial colonoscopy be used only for patients at high risk for CRC.
For individuals unwilling or unable to undergo colonoscopy or FIT, the ACG suggests flexible sigmoidoscopy, multitarget stool DNA testing, CT colonography, or colon capsule. Only colonoscopy is a single-step test; all other screening modalities require a follow-up colonoscopy if test results are positive.
“We recommend against the use of aspirin as a substitute for CRC screening,” the ACG members emphasized. Rather, they suggest that the use of low-dose aspirin be considered only for patients aged 50-69 years whose risk for cardiovascular disease over the next 10 years is at least 10% and who are at low risk for bleeding.
To reduce their risk for CRC, patients need to take aspirin for at least 10 years, they pointed out.
Quality indicators
For endoscopists who perform colonoscopy, the ACG recommended that all operators determine their individual cecal intubation rates, adenoma detection rates, and withdrawal times. They also recommended that endoscopists spend at least 6 minutes inspecting the mucosa during withdrawal and achieve a cecal intubation rate of at least 95% for all patients screened.
The ACG recommended remedial training for any provider whose adenoma detection rate is less than 25%.
Screening rates dropped during pandemic
The authors of the new recommendations also pointed out that, despite public health initiatives to boost CRC screening in the United States and the availability of multiple screening modalities, almost one-third of individuals who are eligible for CRC screening do not undergo screening.
Moreover, the proportion of individuals not being screened has reportedly increased during the pandemic. In one report, claims data for colonoscopies dropped by 90% during April. “Colorectal cancer screening rates must be optimized to reach the aspirational target of >80%,” the authors emphasized.
“A recommendation to be screened by a PCP [primary care provider] – who is known and trusted by the person – is clearly effective in raising participation,” they added.
Dr. Shaukat has served as a scientific consultant for Iterative Scopes and Freenome. Other ACG guideline authors reported numerous financial relationships.
A version of this article first appeared on Medscape.com.
COVID-related immunization gaps portend return of preventable infections
Because of significant reduction in delivery of recommended childhood immunization during the pandemic, there is a risk for resurgence of vaccine preventable infections, including measles, pertussis, and polio, which can result in significant morbidity and mortality in children, reported Amy G. Feldman, MD, of Children’s Hospital Colorado, Aurora, and associates.
Will loss of herd immunity lead to vaccine deserts?
When asked to comment, pediatric infectious disease specialist Christopher J. Harrison, MD, said, “My concern is that we may see expansion of what I call ‘vaccine deserts.’ Vaccine deserts occur in underserved communities, areas with pockets of vaccine-hesitant families or among selected groups with difficult access to health care. These vaccine deserts have held a higher density of vulnerables due to low vaccine uptake, often giving rise to outbreaks of vaccine-preventable diseases, e.g., measles, mumps, pertussis. They are usually due to an index case arriving from another vaccine desert (a developing country or a developed country, U.S. or foreign) where the disease is still endemic or pockets of vaccine hesitancy/refusal exist. When detected, local outbreaks result in rapid responses from public/private health collaborations that limit the outbreak. But what if vaccine deserts became more generalized in the U.S. because of loss of vaccine-induced herd immunity in many more or larger areas of our communities because of pandemic-driven lack of vaccinations? That pandemic-driven indirect damage would further stress the health care system and the economy. And it may first show up in the older children whose vaccines were deferred in the first 4-6 months of the pandemic.”
Dr. Feldman and associates cited findings from a collaborative survey conducted by UNICEF, the World Health Organization, Gavi the Vaccine Alliance, the CDC, the Sabin Vaccine Institute, and the Johns Hopkins Bloomberg School of Public Health, which found that immunization programs experienced moderate to severe disruptions or terminations in at least 68 of 129 low and middle-income countries surveyed. According to the WHO, CDC, Red Cross, and GAVI, 94 million people presently are estimated to be at risk as a consequence of not receiving their measles vaccines following the suspensions.
“These national and international declines in routine immunizations have placed the global community at significant risk for outbreaks of vaccine-preventable infections (VPIs) including measles, polio, and pertussis, diseases which are more deadly, more contagious and have a higher reproductive factor (R0) amongst children than COVID-19,” the authors observed.
Dr. Feldman and associates outlined the horrible devastation that these VPI can cause in children, including significantly higher morbidity and mortality than adults, especially among those with immunodeficiencies. Neurologic deficits, paralysis, intellectual disabilities, and vision and hearing loss are just some of the permanent effects conveyed. “It is concerning to imagine how measles could spread across the United States when social distancing restriction[s] are relaxed and unvaccinated children return to school and usual community engagement,” they noted.
Collaborative engagement key to course correction
The authors found that primary care providers and public health communities are working not only to restore vaccine administration but also to restore confidence that vaccine delivery is safe in spite of COVID. In addition to recommending specific risk mitigation strategies for clinicians, they also suggested individual practitioners use electronic health records to identify patients with COVID-related lapses in vaccination, employ electronic health record–based parent notification of overdue immunizations, and offer distance-friendly vaccination options that include parking lot or drive-up window vaccine delivery.
Additionally, Dr. Feldman and colleagues recommended that local, state, regional, and national health systems use public service announcements via television and digital as well as social media platforms to convey important messages about the considerable health risks associated with vaccine avoidance and the availability of free or reduced-cost vaccination programs through the federally funded Vaccines For Children program for parents out of work or without insurance. Equally important is messaging around encouraging vaccine opportunities during all health care visits, whether they be subspecialty, urgent care, emergency room, or inpatient visits. In areas where access to clinics is limited, they urged the use of mobile clinics as well as additional focus on providing medical homes to children with poor access to care.
“A partial but expanding safety net may be developing spontaneously, i.e., practices and clinics based on a patient-centered medical home (PCMH) model,” noted Dr. Harrison, professor of pediatrics, University of Missouri-Kansas City, in an interview. “When lagging vaccinations were reported in mid-2020, we checked with a local hospital–based urban clinic and two suburban private practices modeled on PCMH. Each had noted a drastic drop in well checks in the first months of the pandemic. But with ill visits nearly nonexistent, they doubled down on maintaining health maintenance visits. Even though staff and provider work hours were limited, and families were less enthusiastic about well checks, momentum appears to have grown so that, by later in 2020, vaccine uptake rates were again comparable to 2019. So, some already seem to have answered the call, but practices/clinics remain hampered by months of reduced revenue needed to support staff, providers, PPE supplies, and added infection control needs,” he said.The study was funded by the Agency for Healthcare Research Quality. Dr. Isakov disclosed relationships with various pharmaceutical companies outside the submitted work. The other authors had no relevant disclosures. Dr. Harrison’s institution receives grant funding from GSK, Merck, and Pfizer for pediatric vaccine trials and pneumococcal seroprevalence studies on which he is an investigator.
Because of significant reduction in delivery of recommended childhood immunization during the pandemic, there is a risk for resurgence of vaccine preventable infections, including measles, pertussis, and polio, which can result in significant morbidity and mortality in children, reported Amy G. Feldman, MD, of Children’s Hospital Colorado, Aurora, and associates.
Will loss of herd immunity lead to vaccine deserts?
When asked to comment, pediatric infectious disease specialist Christopher J. Harrison, MD, said, “My concern is that we may see expansion of what I call ‘vaccine deserts.’ Vaccine deserts occur in underserved communities, areas with pockets of vaccine-hesitant families or among selected groups with difficult access to health care. These vaccine deserts have held a higher density of vulnerables due to low vaccine uptake, often giving rise to outbreaks of vaccine-preventable diseases, e.g., measles, mumps, pertussis. They are usually due to an index case arriving from another vaccine desert (a developing country or a developed country, U.S. or foreign) where the disease is still endemic or pockets of vaccine hesitancy/refusal exist. When detected, local outbreaks result in rapid responses from public/private health collaborations that limit the outbreak. But what if vaccine deserts became more generalized in the U.S. because of loss of vaccine-induced herd immunity in many more or larger areas of our communities because of pandemic-driven lack of vaccinations? That pandemic-driven indirect damage would further stress the health care system and the economy. And it may first show up in the older children whose vaccines were deferred in the first 4-6 months of the pandemic.”
Dr. Feldman and associates cited findings from a collaborative survey conducted by UNICEF, the World Health Organization, Gavi the Vaccine Alliance, the CDC, the Sabin Vaccine Institute, and the Johns Hopkins Bloomberg School of Public Health, which found that immunization programs experienced moderate to severe disruptions or terminations in at least 68 of 129 low and middle-income countries surveyed. According to the WHO, CDC, Red Cross, and GAVI, 94 million people presently are estimated to be at risk as a consequence of not receiving their measles vaccines following the suspensions.
“These national and international declines in routine immunizations have placed the global community at significant risk for outbreaks of vaccine-preventable infections (VPIs) including measles, polio, and pertussis, diseases which are more deadly, more contagious and have a higher reproductive factor (R0) amongst children than COVID-19,” the authors observed.
Dr. Feldman and associates outlined the horrible devastation that these VPI can cause in children, including significantly higher morbidity and mortality than adults, especially among those with immunodeficiencies. Neurologic deficits, paralysis, intellectual disabilities, and vision and hearing loss are just some of the permanent effects conveyed. “It is concerning to imagine how measles could spread across the United States when social distancing restriction[s] are relaxed and unvaccinated children return to school and usual community engagement,” they noted.
Collaborative engagement key to course correction
The authors found that primary care providers and public health communities are working not only to restore vaccine administration but also to restore confidence that vaccine delivery is safe in spite of COVID. In addition to recommending specific risk mitigation strategies for clinicians, they also suggested individual practitioners use electronic health records to identify patients with COVID-related lapses in vaccination, employ electronic health record–based parent notification of overdue immunizations, and offer distance-friendly vaccination options that include parking lot or drive-up window vaccine delivery.
Additionally, Dr. Feldman and colleagues recommended that local, state, regional, and national health systems use public service announcements via television and digital as well as social media platforms to convey important messages about the considerable health risks associated with vaccine avoidance and the availability of free or reduced-cost vaccination programs through the federally funded Vaccines For Children program for parents out of work or without insurance. Equally important is messaging around encouraging vaccine opportunities during all health care visits, whether they be subspecialty, urgent care, emergency room, or inpatient visits. In areas where access to clinics is limited, they urged the use of mobile clinics as well as additional focus on providing medical homes to children with poor access to care.
“A partial but expanding safety net may be developing spontaneously, i.e., practices and clinics based on a patient-centered medical home (PCMH) model,” noted Dr. Harrison, professor of pediatrics, University of Missouri-Kansas City, in an interview. “When lagging vaccinations were reported in mid-2020, we checked with a local hospital–based urban clinic and two suburban private practices modeled on PCMH. Each had noted a drastic drop in well checks in the first months of the pandemic. But with ill visits nearly nonexistent, they doubled down on maintaining health maintenance visits. Even though staff and provider work hours were limited, and families were less enthusiastic about well checks, momentum appears to have grown so that, by later in 2020, vaccine uptake rates were again comparable to 2019. So, some already seem to have answered the call, but practices/clinics remain hampered by months of reduced revenue needed to support staff, providers, PPE supplies, and added infection control needs,” he said.The study was funded by the Agency for Healthcare Research Quality. Dr. Isakov disclosed relationships with various pharmaceutical companies outside the submitted work. The other authors had no relevant disclosures. Dr. Harrison’s institution receives grant funding from GSK, Merck, and Pfizer for pediatric vaccine trials and pneumococcal seroprevalence studies on which he is an investigator.
Because of significant reduction in delivery of recommended childhood immunization during the pandemic, there is a risk for resurgence of vaccine preventable infections, including measles, pertussis, and polio, which can result in significant morbidity and mortality in children, reported Amy G. Feldman, MD, of Children’s Hospital Colorado, Aurora, and associates.
Will loss of herd immunity lead to vaccine deserts?
When asked to comment, pediatric infectious disease specialist Christopher J. Harrison, MD, said, “My concern is that we may see expansion of what I call ‘vaccine deserts.’ Vaccine deserts occur in underserved communities, areas with pockets of vaccine-hesitant families or among selected groups with difficult access to health care. These vaccine deserts have held a higher density of vulnerables due to low vaccine uptake, often giving rise to outbreaks of vaccine-preventable diseases, e.g., measles, mumps, pertussis. They are usually due to an index case arriving from another vaccine desert (a developing country or a developed country, U.S. or foreign) where the disease is still endemic or pockets of vaccine hesitancy/refusal exist. When detected, local outbreaks result in rapid responses from public/private health collaborations that limit the outbreak. But what if vaccine deserts became more generalized in the U.S. because of loss of vaccine-induced herd immunity in many more or larger areas of our communities because of pandemic-driven lack of vaccinations? That pandemic-driven indirect damage would further stress the health care system and the economy. And it may first show up in the older children whose vaccines were deferred in the first 4-6 months of the pandemic.”
Dr. Feldman and associates cited findings from a collaborative survey conducted by UNICEF, the World Health Organization, Gavi the Vaccine Alliance, the CDC, the Sabin Vaccine Institute, and the Johns Hopkins Bloomberg School of Public Health, which found that immunization programs experienced moderate to severe disruptions or terminations in at least 68 of 129 low and middle-income countries surveyed. According to the WHO, CDC, Red Cross, and GAVI, 94 million people presently are estimated to be at risk as a consequence of not receiving their measles vaccines following the suspensions.
“These national and international declines in routine immunizations have placed the global community at significant risk for outbreaks of vaccine-preventable infections (VPIs) including measles, polio, and pertussis, diseases which are more deadly, more contagious and have a higher reproductive factor (R0) amongst children than COVID-19,” the authors observed.
Dr. Feldman and associates outlined the horrible devastation that these VPI can cause in children, including significantly higher morbidity and mortality than adults, especially among those with immunodeficiencies. Neurologic deficits, paralysis, intellectual disabilities, and vision and hearing loss are just some of the permanent effects conveyed. “It is concerning to imagine how measles could spread across the United States when social distancing restriction[s] are relaxed and unvaccinated children return to school and usual community engagement,” they noted.
Collaborative engagement key to course correction
The authors found that primary care providers and public health communities are working not only to restore vaccine administration but also to restore confidence that vaccine delivery is safe in spite of COVID. In addition to recommending specific risk mitigation strategies for clinicians, they also suggested individual practitioners use electronic health records to identify patients with COVID-related lapses in vaccination, employ electronic health record–based parent notification of overdue immunizations, and offer distance-friendly vaccination options that include parking lot or drive-up window vaccine delivery.
Additionally, Dr. Feldman and colleagues recommended that local, state, regional, and national health systems use public service announcements via television and digital as well as social media platforms to convey important messages about the considerable health risks associated with vaccine avoidance and the availability of free or reduced-cost vaccination programs through the federally funded Vaccines For Children program for parents out of work or without insurance. Equally important is messaging around encouraging vaccine opportunities during all health care visits, whether they be subspecialty, urgent care, emergency room, or inpatient visits. In areas where access to clinics is limited, they urged the use of mobile clinics as well as additional focus on providing medical homes to children with poor access to care.
“A partial but expanding safety net may be developing spontaneously, i.e., practices and clinics based on a patient-centered medical home (PCMH) model,” noted Dr. Harrison, professor of pediatrics, University of Missouri-Kansas City, in an interview. “When lagging vaccinations were reported in mid-2020, we checked with a local hospital–based urban clinic and two suburban private practices modeled on PCMH. Each had noted a drastic drop in well checks in the first months of the pandemic. But with ill visits nearly nonexistent, they doubled down on maintaining health maintenance visits. Even though staff and provider work hours were limited, and families were less enthusiastic about well checks, momentum appears to have grown so that, by later in 2020, vaccine uptake rates were again comparable to 2019. So, some already seem to have answered the call, but practices/clinics remain hampered by months of reduced revenue needed to support staff, providers, PPE supplies, and added infection control needs,” he said.The study was funded by the Agency for Healthcare Research Quality. Dr. Isakov disclosed relationships with various pharmaceutical companies outside the submitted work. The other authors had no relevant disclosures. Dr. Harrison’s institution receives grant funding from GSK, Merck, and Pfizer for pediatric vaccine trials and pneumococcal seroprevalence studies on which he is an investigator.
FROM CLINICAL INFECTIOUS DISEASES
To improve psoriatic arthritis outcomes, address common comorbidities
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
FROM RWCS 2021
Don’t discontinue osteoporosis meds for COVID-19 vaccines, expert guidance says
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.