User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
PCOS equivalent in men: No ovaries required
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
FROM ENDO 2021
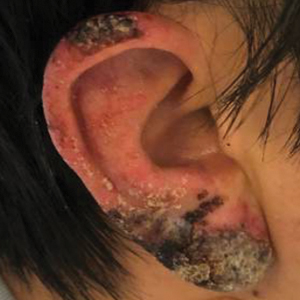
Crusted Papules on the Bilateral Helices and Lobules
The Diagnosis: Kikuchi-Fujimoto Disease
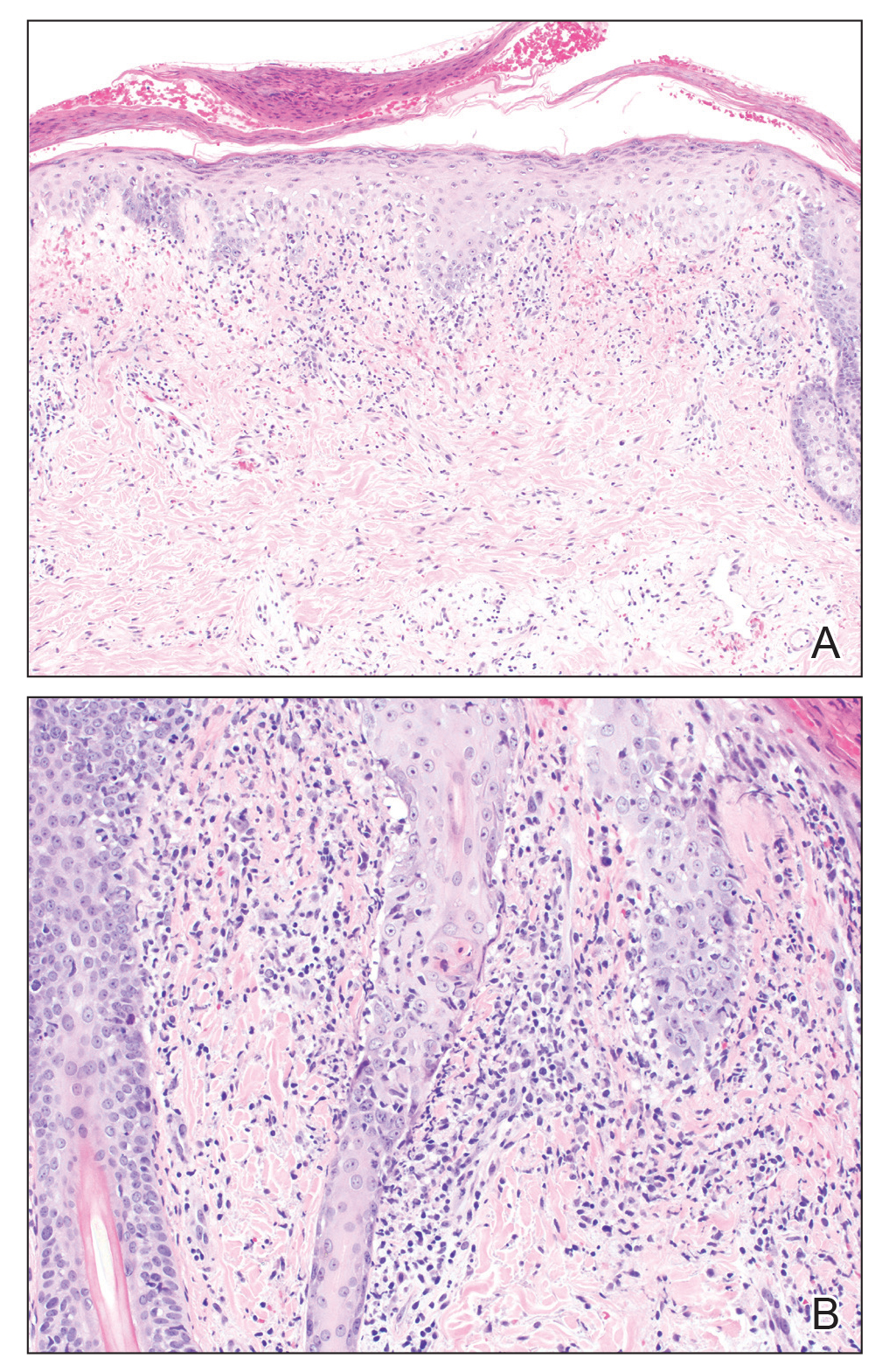
A skin biopsy from the left helix was obtained. Histopathologic examination revealed a vacuolar interface reaction with marked papillary dermal edema and a patchy perijunctional lymphocytic infiltrate. The dermis was free of increased mucin (Figure 1). Immunohistochemical staining for CD56 and Epstein-Barr virus (EBV)–encoded small nuclear RNA chromogenic in situ hybridization were negative. Laboratory workup was remarkable for elevated transaminases and inflammatory markers (eg, C-reactive protein, erythrocyte sedimentation rate) but negative for rheumatologic markers (eg, antinuclear antibodies, antineutrophil cytoplasmic antibodies, myeloperoxidase antibodies, serine protease IgG). An extensive infectious workup was unrevealing. Computed tomography highlighted prominent lymphadenopathy throughout the cervical and supraclavicular chains and a large necrotic lymph node in the porta hepatis (Figure 2). Right neck lymph node aspiration revealed necrotizing lymphadenitis in a background of histiocytes and mixed lymphocytes. Coupling the clinical presentation and histomorphology with imaging, a diagnosis of Kikuchi-Fujimoto disease (KD) was rendered.

Kikuchi-Fujimoto disease is a rare illness of unknown etiology characterized by cervical lymphadenopathy and fever. Originally described in Japan, KD affects all racial and ethnic groups1,2 but more commonly is seen in women and patients younger than 40 years.3 It can be associated with systemic lupus erythematosus (SLE) and other autoimmune diseases (eg, relapsing polychondritis, adult-onset Still disease),3 and lymphoma.4 Multiple infections have been implicated in the pathogenesis of KD, including EBV and other human herpesviruses; HIV; human T-cell leukemia virus type 1; dengue virus; parvovirus B19; and Yersinia enterocolitica, Bartonella, Brucella, and Toxoplasma infections.3,5,6
Kikuchi-Fujimoto disease classically presents with fever and cervical lymphadenopathy. In a retrospective review of 244 patients with KD, the 3 most common manifestations included lymphadenopathy, fever, and rash.7 A diagnosis of KD is rendered based on clinical presentation and lymph node histopathologic findings of paracortical necrosis and florid histiocytic infiltrate.1
The cutaneous manifestations of KD are heterogeneous yet mostly transient. Cutaneous involvement is reported in 16.6% to 40% of patients.3,5,6 Common cutaneous manifestations include erythematous macules, papules, patches, and plaques; erosions, nodules, and bullae less commonly can occur.6 A variety of cutaneous manifestations have been reported in KD, including lesions mimicking pigmented purpuric dermatoses, vasculitis, Sweet syndrome, drug eruptions, and viral exanthems.6 Signs and symptoms of KD usually resolve within 1 to 4 months. Although there are no established treatments for this disease, patients with severe or persistent symptoms can be treated with steroids or hydroxychloroquine. Recurrences after treatment have been reported.8
Systemic lupus erythematosus is a multiorgan disease with protean manifestations. Cutaneous manifestations of SLE include malar erythema and discoid, annular, and papulosquamous lesions. Histopathologic patterns frequently observed in cutaneous lesions associated with SLE include interface dermatitis with perivascular infiltrates, dermal mucin, and plasmacytoid dendritic cells (marked by CD123 staining); these findings were notably absent in our case.6
Lupus vulgaris is a form of cutaneous tuberculosis that results from reactivation of Mycobacterium tuberculosis in tubercles formed during preceding hematogenous dissemination. The head and neck region is the most common location, particularly the nose, cheeks, and earlobes. Small, brown-red, soft papules coalesce into gelatinous plaques, demonstrating a characteristic apple jelly appearance on diascopy. Other clinical manifestations include the plaque/plane, hypertrophic/tumorlike, and ulcerative/scarring forms.9 Delayed-type hypersensitivity testing by tuberculin skin test, interferon-gamma release assay, or polymerase chain reaction–based assays can detect Mycobacterium tuberculosis. Histopathology shows well-formed granulomas surrounded by chronic inflammatory cells and central necrosis.
Hydroa vacciniforme–like (HV-like) eruption is a rare photosensitive disorder characterized by vesiculopapules on sun-exposed areas. Hydroa vacciniforme–like eruptions rarely have been reported to progress to EBVassociated malignant lymphoma.10 Unlike typical hydroa vacciniforme, which resolves by early adulthood, HV-like eruptions can become more severe with age and are associated with systemic manifestations, including fevers, lymphadenopathy, and liver damage. Histopathologic examination reveals a dense infiltrate of atypical T lymphocytes or natural killer cells (CD56+), which stain positive for EBV-encoded small nuclear RNA,10 in contrast to the patchy perijunctional lymphocytic infiltrate seen in KD.
This case highlights the protean cutaneous manifestations of a rare rheumatologic entity. It demonstrates the importance of a full systemic workup when considering an enigmatic disease. Our patient was started on prednisone 20 mg and hydroxychloroquine 200 mg daily. Within 24 hours, the fevers and rash both improved.
- Turner RR, Martin J, Dorfman RF. Necrotizing lymphadenitis. a study of 30 cases. Am J Surg Pathol. 1983;7:115-123.
- Dorfman RF, Berry GJ. Kikuchi’s histiocytic necrotizing lymphadenitis: an analysis of 108 cases with emphasis on differential diagnosis. Semin Diagn Pathol. 1988;5:329-345.
- Atwater AR, Longley BJ, Aughenbaugh WD. Kikuchi’s disease: case report and systematic review of cutaneous and histopathologic presentations. J Am Acad Dermatol. 2008;59:130-136.
- Yoshino T, Mannami T, Ichimura K, et al. Two cases of histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto’s disease) following diffuse large B-cell lymphoma. Hum Pathol. 2000;31:1328-1331.
- Yen A, Fearneyhough P, Raimer SS, et al. EBV-associated Kikuchi’s histiocytic necrotizing lymphadenitis with cutaneous manifestations. J Am Acad Dermatol. 1997;36:342-346.
- Kim JH, Kim YB, In SI, et al. The cutaneous lesions of Kikuchi’s disease: a comprehensive analysis of 16 cases based on the clinicopathologic, immunohistochemical, and immunofluorescence studies with an emphasis on the differential diagnosis. Hum Pathol. 2010;41:1245-1254.
- Kucukardali Y, Solmazgul E, Kunter E, et al. Kikuchi-Fujimoto Disease: analysis of 244 cases. Clin Rheumatol. 2007;26:50-54.
- Smith KG, Becker GJ, Busmanis I. Recurrent Kikuchi’s disease. Lancet. 1992;340:124.
- Macgregor R. Cutaneous tuberculosis. Clin Dermatol. 1995;13:245-255.
- Iwatsuki K, Ohtsuka M, Harada H, et al. Clinicopathologic manifestations of Epstein-Barr virus–associated cutaneous lymphoproliferative disorders. Arch Dermatol. 1997;133:1081-1086.
The Diagnosis: Kikuchi-Fujimoto Disease
A skin biopsy from the left helix was obtained. Histopathologic examination revealed a vacuolar interface reaction with marked papillary dermal edema and a patchy perijunctional lymphocytic infiltrate. The dermis was free of increased mucin (Figure 1). Immunohistochemical staining for CD56 and Epstein-Barr virus (EBV)–encoded small nuclear RNA chromogenic in situ hybridization were negative. Laboratory workup was remarkable for elevated transaminases and inflammatory markers (eg, C-reactive protein, erythrocyte sedimentation rate) but negative for rheumatologic markers (eg, antinuclear antibodies, antineutrophil cytoplasmic antibodies, myeloperoxidase antibodies, serine protease IgG). An extensive infectious workup was unrevealing. Computed tomography highlighted prominent lymphadenopathy throughout the cervical and supraclavicular chains and a large necrotic lymph node in the porta hepatis (Figure 2). Right neck lymph node aspiration revealed necrotizing lymphadenitis in a background of histiocytes and mixed lymphocytes. Coupling the clinical presentation and histomorphology with imaging, a diagnosis of Kikuchi-Fujimoto disease (KD) was rendered.


Kikuchi-Fujimoto disease is a rare illness of unknown etiology characterized by cervical lymphadenopathy and fever. Originally described in Japan, KD affects all racial and ethnic groups1,2 but more commonly is seen in women and patients younger than 40 years.3 It can be associated with systemic lupus erythematosus (SLE) and other autoimmune diseases (eg, relapsing polychondritis, adult-onset Still disease),3 and lymphoma.4 Multiple infections have been implicated in the pathogenesis of KD, including EBV and other human herpesviruses; HIV; human T-cell leukemia virus type 1; dengue virus; parvovirus B19; and Yersinia enterocolitica, Bartonella, Brucella, and Toxoplasma infections.3,5,6
Kikuchi-Fujimoto disease classically presents with fever and cervical lymphadenopathy. In a retrospective review of 244 patients with KD, the 3 most common manifestations included lymphadenopathy, fever, and rash.7 A diagnosis of KD is rendered based on clinical presentation and lymph node histopathologic findings of paracortical necrosis and florid histiocytic infiltrate.1
The cutaneous manifestations of KD are heterogeneous yet mostly transient. Cutaneous involvement is reported in 16.6% to 40% of patients.3,5,6 Common cutaneous manifestations include erythematous macules, papules, patches, and plaques; erosions, nodules, and bullae less commonly can occur.6 A variety of cutaneous manifestations have been reported in KD, including lesions mimicking pigmented purpuric dermatoses, vasculitis, Sweet syndrome, drug eruptions, and viral exanthems.6 Signs and symptoms of KD usually resolve within 1 to 4 months. Although there are no established treatments for this disease, patients with severe or persistent symptoms can be treated with steroids or hydroxychloroquine. Recurrences after treatment have been reported.8
Systemic lupus erythematosus is a multiorgan disease with protean manifestations. Cutaneous manifestations of SLE include malar erythema and discoid, annular, and papulosquamous lesions. Histopathologic patterns frequently observed in cutaneous lesions associated with SLE include interface dermatitis with perivascular infiltrates, dermal mucin, and plasmacytoid dendritic cells (marked by CD123 staining); these findings were notably absent in our case.6
Lupus vulgaris is a form of cutaneous tuberculosis that results from reactivation of Mycobacterium tuberculosis in tubercles formed during preceding hematogenous dissemination. The head and neck region is the most common location, particularly the nose, cheeks, and earlobes. Small, brown-red, soft papules coalesce into gelatinous plaques, demonstrating a characteristic apple jelly appearance on diascopy. Other clinical manifestations include the plaque/plane, hypertrophic/tumorlike, and ulcerative/scarring forms.9 Delayed-type hypersensitivity testing by tuberculin skin test, interferon-gamma release assay, or polymerase chain reaction–based assays can detect Mycobacterium tuberculosis. Histopathology shows well-formed granulomas surrounded by chronic inflammatory cells and central necrosis.
Hydroa vacciniforme–like (HV-like) eruption is a rare photosensitive disorder characterized by vesiculopapules on sun-exposed areas. Hydroa vacciniforme–like eruptions rarely have been reported to progress to EBVassociated malignant lymphoma.10 Unlike typical hydroa vacciniforme, which resolves by early adulthood, HV-like eruptions can become more severe with age and are associated with systemic manifestations, including fevers, lymphadenopathy, and liver damage. Histopathologic examination reveals a dense infiltrate of atypical T lymphocytes or natural killer cells (CD56+), which stain positive for EBV-encoded small nuclear RNA,10 in contrast to the patchy perijunctional lymphocytic infiltrate seen in KD.
This case highlights the protean cutaneous manifestations of a rare rheumatologic entity. It demonstrates the importance of a full systemic workup when considering an enigmatic disease. Our patient was started on prednisone 20 mg and hydroxychloroquine 200 mg daily. Within 24 hours, the fevers and rash both improved.
The Diagnosis: Kikuchi-Fujimoto Disease
A skin biopsy from the left helix was obtained. Histopathologic examination revealed a vacuolar interface reaction with marked papillary dermal edema and a patchy perijunctional lymphocytic infiltrate. The dermis was free of increased mucin (Figure 1). Immunohistochemical staining for CD56 and Epstein-Barr virus (EBV)–encoded small nuclear RNA chromogenic in situ hybridization were negative. Laboratory workup was remarkable for elevated transaminases and inflammatory markers (eg, C-reactive protein, erythrocyte sedimentation rate) but negative for rheumatologic markers (eg, antinuclear antibodies, antineutrophil cytoplasmic antibodies, myeloperoxidase antibodies, serine protease IgG). An extensive infectious workup was unrevealing. Computed tomography highlighted prominent lymphadenopathy throughout the cervical and supraclavicular chains and a large necrotic lymph node in the porta hepatis (Figure 2). Right neck lymph node aspiration revealed necrotizing lymphadenitis in a background of histiocytes and mixed lymphocytes. Coupling the clinical presentation and histomorphology with imaging, a diagnosis of Kikuchi-Fujimoto disease (KD) was rendered.


Kikuchi-Fujimoto disease is a rare illness of unknown etiology characterized by cervical lymphadenopathy and fever. Originally described in Japan, KD affects all racial and ethnic groups1,2 but more commonly is seen in women and patients younger than 40 years.3 It can be associated with systemic lupus erythematosus (SLE) and other autoimmune diseases (eg, relapsing polychondritis, adult-onset Still disease),3 and lymphoma.4 Multiple infections have been implicated in the pathogenesis of KD, including EBV and other human herpesviruses; HIV; human T-cell leukemia virus type 1; dengue virus; parvovirus B19; and Yersinia enterocolitica, Bartonella, Brucella, and Toxoplasma infections.3,5,6
Kikuchi-Fujimoto disease classically presents with fever and cervical lymphadenopathy. In a retrospective review of 244 patients with KD, the 3 most common manifestations included lymphadenopathy, fever, and rash.7 A diagnosis of KD is rendered based on clinical presentation and lymph node histopathologic findings of paracortical necrosis and florid histiocytic infiltrate.1
The cutaneous manifestations of KD are heterogeneous yet mostly transient. Cutaneous involvement is reported in 16.6% to 40% of patients.3,5,6 Common cutaneous manifestations include erythematous macules, papules, patches, and plaques; erosions, nodules, and bullae less commonly can occur.6 A variety of cutaneous manifestations have been reported in KD, including lesions mimicking pigmented purpuric dermatoses, vasculitis, Sweet syndrome, drug eruptions, and viral exanthems.6 Signs and symptoms of KD usually resolve within 1 to 4 months. Although there are no established treatments for this disease, patients with severe or persistent symptoms can be treated with steroids or hydroxychloroquine. Recurrences after treatment have been reported.8
Systemic lupus erythematosus is a multiorgan disease with protean manifestations. Cutaneous manifestations of SLE include malar erythema and discoid, annular, and papulosquamous lesions. Histopathologic patterns frequently observed in cutaneous lesions associated with SLE include interface dermatitis with perivascular infiltrates, dermal mucin, and plasmacytoid dendritic cells (marked by CD123 staining); these findings were notably absent in our case.6
Lupus vulgaris is a form of cutaneous tuberculosis that results from reactivation of Mycobacterium tuberculosis in tubercles formed during preceding hematogenous dissemination. The head and neck region is the most common location, particularly the nose, cheeks, and earlobes. Small, brown-red, soft papules coalesce into gelatinous plaques, demonstrating a characteristic apple jelly appearance on diascopy. Other clinical manifestations include the plaque/plane, hypertrophic/tumorlike, and ulcerative/scarring forms.9 Delayed-type hypersensitivity testing by tuberculin skin test, interferon-gamma release assay, or polymerase chain reaction–based assays can detect Mycobacterium tuberculosis. Histopathology shows well-formed granulomas surrounded by chronic inflammatory cells and central necrosis.
Hydroa vacciniforme–like (HV-like) eruption is a rare photosensitive disorder characterized by vesiculopapules on sun-exposed areas. Hydroa vacciniforme–like eruptions rarely have been reported to progress to EBVassociated malignant lymphoma.10 Unlike typical hydroa vacciniforme, which resolves by early adulthood, HV-like eruptions can become more severe with age and are associated with systemic manifestations, including fevers, lymphadenopathy, and liver damage. Histopathologic examination reveals a dense infiltrate of atypical T lymphocytes or natural killer cells (CD56+), which stain positive for EBV-encoded small nuclear RNA,10 in contrast to the patchy perijunctional lymphocytic infiltrate seen in KD.
This case highlights the protean cutaneous manifestations of a rare rheumatologic entity. It demonstrates the importance of a full systemic workup when considering an enigmatic disease. Our patient was started on prednisone 20 mg and hydroxychloroquine 200 mg daily. Within 24 hours, the fevers and rash both improved.
- Turner RR, Martin J, Dorfman RF. Necrotizing lymphadenitis. a study of 30 cases. Am J Surg Pathol. 1983;7:115-123.
- Dorfman RF, Berry GJ. Kikuchi’s histiocytic necrotizing lymphadenitis: an analysis of 108 cases with emphasis on differential diagnosis. Semin Diagn Pathol. 1988;5:329-345.
- Atwater AR, Longley BJ, Aughenbaugh WD. Kikuchi’s disease: case report and systematic review of cutaneous and histopathologic presentations. J Am Acad Dermatol. 2008;59:130-136.
- Yoshino T, Mannami T, Ichimura K, et al. Two cases of histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto’s disease) following diffuse large B-cell lymphoma. Hum Pathol. 2000;31:1328-1331.
- Yen A, Fearneyhough P, Raimer SS, et al. EBV-associated Kikuchi’s histiocytic necrotizing lymphadenitis with cutaneous manifestations. J Am Acad Dermatol. 1997;36:342-346.
- Kim JH, Kim YB, In SI, et al. The cutaneous lesions of Kikuchi’s disease: a comprehensive analysis of 16 cases based on the clinicopathologic, immunohistochemical, and immunofluorescence studies with an emphasis on the differential diagnosis. Hum Pathol. 2010;41:1245-1254.
- Kucukardali Y, Solmazgul E, Kunter E, et al. Kikuchi-Fujimoto Disease: analysis of 244 cases. Clin Rheumatol. 2007;26:50-54.
- Smith KG, Becker GJ, Busmanis I. Recurrent Kikuchi’s disease. Lancet. 1992;340:124.
- Macgregor R. Cutaneous tuberculosis. Clin Dermatol. 1995;13:245-255.
- Iwatsuki K, Ohtsuka M, Harada H, et al. Clinicopathologic manifestations of Epstein-Barr virus–associated cutaneous lymphoproliferative disorders. Arch Dermatol. 1997;133:1081-1086.
- Turner RR, Martin J, Dorfman RF. Necrotizing lymphadenitis. a study of 30 cases. Am J Surg Pathol. 1983;7:115-123.
- Dorfman RF, Berry GJ. Kikuchi’s histiocytic necrotizing lymphadenitis: an analysis of 108 cases with emphasis on differential diagnosis. Semin Diagn Pathol. 1988;5:329-345.
- Atwater AR, Longley BJ, Aughenbaugh WD. Kikuchi’s disease: case report and systematic review of cutaneous and histopathologic presentations. J Am Acad Dermatol. 2008;59:130-136.
- Yoshino T, Mannami T, Ichimura K, et al. Two cases of histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto’s disease) following diffuse large B-cell lymphoma. Hum Pathol. 2000;31:1328-1331.
- Yen A, Fearneyhough P, Raimer SS, et al. EBV-associated Kikuchi’s histiocytic necrotizing lymphadenitis with cutaneous manifestations. J Am Acad Dermatol. 1997;36:342-346.
- Kim JH, Kim YB, In SI, et al. The cutaneous lesions of Kikuchi’s disease: a comprehensive analysis of 16 cases based on the clinicopathologic, immunohistochemical, and immunofluorescence studies with an emphasis on the differential diagnosis. Hum Pathol. 2010;41:1245-1254.
- Kucukardali Y, Solmazgul E, Kunter E, et al. Kikuchi-Fujimoto Disease: analysis of 244 cases. Clin Rheumatol. 2007;26:50-54.
- Smith KG, Becker GJ, Busmanis I. Recurrent Kikuchi’s disease. Lancet. 1992;340:124.
- Macgregor R. Cutaneous tuberculosis. Clin Dermatol. 1995;13:245-255.
- Iwatsuki K, Ohtsuka M, Harada H, et al. Clinicopathologic manifestations of Epstein-Barr virus–associated cutaneous lymphoproliferative disorders. Arch Dermatol. 1997;133:1081-1086.
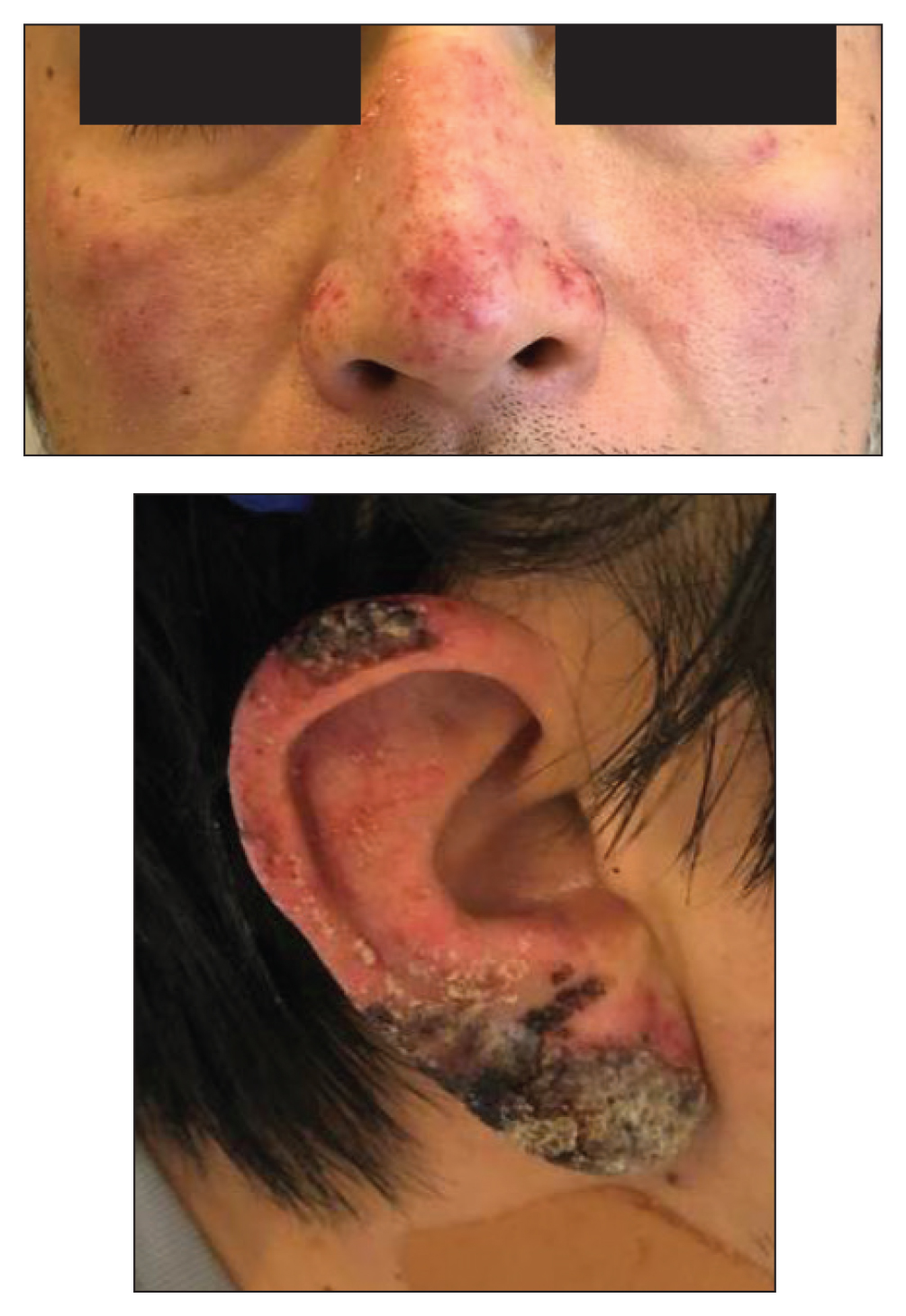
A healthy 42-year-old Japanese man presented with painful lymphadenopathy and fevers of 1 month’s duration as well as a pruritic rash and bilateral ear redness and crusting of 1 week’s duration. He initially was seen at an outside facility and was treated with antibiotics and supportive care for cervical adenitis. During clinical evaluation, he denied joint pain, photosensitivity, and oral lesions. His medical and family history were noncontributory. Although he reported recent travel to multiple countries, he denied exposure to animals, ticks, or sick individuals. Physical examination revealed erythematous blanching papules on the nose and cheeks (top) as well as crusted papules coalescing into plaques on the bilateral helices and lobules (bottom).
High-intensity interval training cuts cardiometabolic risks in women with PCOS
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
FROM ENDO 2021
Ruxolitinib cream for atopic dermatitis is in regulatory home stretch
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Comic books help explain type 1 diabetes to all ages
Overcoming the challenges in managing type 1 diabetes can sometimes feel like an unappreciated “superpower.” That was part of the thinking behind the creation of a comic book trilogy that aims to educate people of all ages – including health care providers – about the realities of living with this condition.
The series was initially launched by a team from Portsmouth (England) Hospitals University National Health Service Trust and University Hospital Southampton NHS Foundation Trust. It is now officially backed by the NHS. The first book in the trilogy, published in 2016, visually illustrates the challenges faced by a teenage boy who had recently been diagnosed with type 1 diabetes. The second volume, released in 2018, follows a young girl who is hospitalized with diabetic ketoacidosis. The third, published in December 2020, explores the stigma associated with diabetes and delves into hypoglycemia.
Available for free online, the three comic books are meant for adults, children, health care professionals, and laypeople. This news organization spoke with series cocreator Partha Kar, MBBS, MD, national specialty adviser, Diabetes for NHS England, about the series. This interview has been edited for length and clarity.
How did the idea for a comic book series about type 1 diabetes come about?Dr. Kar: My Southampton colleague Mayank Patel, BM, DM, FRCP, and I were discussing ways of reaching different audiences to raise awareness about type 1 diabetes, and we had the idea of comic books. After all, comic book movies are among the biggest blockbusters if one looks at popular culture, because it’s not just kids watching them.
One of our patients made an interesting observation that really resonated. He said having type 1 diabetes was like the Marvel Comics superhero Hulk.
Several scenes in the first publication, Type 1: Origins, were based on the Hulk, a scientist who gets a radioactive dose by accident. He doesn’t like turning green when he’s angry, even though he also becomes very strong. He basically spends the rest of his life trying to find the cure for himself, but he eventually makes the best of his two worlds – Professor and Hulk – rather than constantly fighting his situation.
The story line was primarily written by a group of patients with type 1 diabetes based on their own experiences. Mayank and I were mostly just supervising and financing the project. The graphics and layout were done by Revolve Comics, a publisher specializing in health education via the comic book medium.
Our aim was to bring awareness of type 1 diabetes to people who don’t have diabetes, including teachers, family members, and friends. At the end of Origins, we provide a list of online resources for more information and for social connection.
Since it launched in October 2016, Origins has been downloaded nearly 10,000 times. Lots of local charities and schools have picked it up. Parents and kids have come to us asking for more and giving us ideas. That’s what prompted the next one.
The second volume, Type 1: Attack of the Ketones, is more technical and somewhat surprising in that it portrays some hospital staff members as not well-informed about type 1 diabetes. Are they part of the intended audience?
Yes, this one was directed a little bit more towards professionals, hospitals, and staff. It’s also informed by patient feedback, and dovetails with my efforts to improve hospital care for people with type 1 diabetes. But of course, patients and interested laypeople can also learn from it.
A theme in volume 2 comes from another Marvel Comics superhero, Iron Man. In the movie, when Tony Stark’s heart is severely damaged with shrapnel, he acquires an arc reactor that keeps him alive and also powers the suit that gives him superpowers. After the reactor is taken away, he devises a way to replace the missing part and reassemble the suit.
Similarly, in type 1 diabetes, the ability to produce insulin has been taken away without permission. But what is missing can thankfully be replaced, albeit imperfectly. As we illustrate, things don’t always go to plan despite best efforts to administer insulin in the right dose at the right time.
At the end of Attack of the Ketones, we provide two pages of text about recognizing and managing hyperglycemia and preventing diabetic ketoacidosis. This volume was funded by NHS England and then backed by JDRF and Diabetes UK, and many hospitals picked it up. It has had about 8,000 downloads.
In Volume 3, you explore stigma and the issue of language regarding type 1 diabetes. How did those topics come about?
Kar: Type 1 Mission 3: S.T.I.G.M.A. was also based on patient feedback, with input from some Indian diabetes groups I’ve worked with. Here, the protagonist is a young man with type 1 diabetes who goes on holiday to India, where diabetes stigma is widespread. The characters address language problems such as use of the word “diabetic” to label a person, and they counter misconceptions such as that diabetes is contagious. There’s an Indian comic book version of this volume out now.
The main character of this volume experiences severe hypoglycemia and is saved by a glucagon injection from a colleague, one of several presented as superheroes who help in the fight to end diabetes stigma. They are referred to as Guardians of the Glucose, a take on yet another Marvel franchise, Guardians of the Galaxy.
At the end of this volume, we provide two pages of text about recognizing, managing, and preventing hypoglycemia. Again, we hope to educate as wide an audience as possible.
At the end of volume 3, you also briefly mention the COVID-19 pandemic. Will there be a fourth volume dealing with that, or other topics, such as diabetes technology?
We’ve left it open. We want to see how volume 3 lands. Depending on that, we might take it forward. There are certainly plenty of topics to tackle. We’ve also discussed moving into gaming or virtual reality. Overall, we hope to educate people by engaging them in different ways.
Dr. Kar has been a consultant diabetologist/endocrinologist within the NHS since 2008. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Overcoming the challenges in managing type 1 diabetes can sometimes feel like an unappreciated “superpower.” That was part of the thinking behind the creation of a comic book trilogy that aims to educate people of all ages – including health care providers – about the realities of living with this condition.
The series was initially launched by a team from Portsmouth (England) Hospitals University National Health Service Trust and University Hospital Southampton NHS Foundation Trust. It is now officially backed by the NHS. The first book in the trilogy, published in 2016, visually illustrates the challenges faced by a teenage boy who had recently been diagnosed with type 1 diabetes. The second volume, released in 2018, follows a young girl who is hospitalized with diabetic ketoacidosis. The third, published in December 2020, explores the stigma associated with diabetes and delves into hypoglycemia.
Available for free online, the three comic books are meant for adults, children, health care professionals, and laypeople. This news organization spoke with series cocreator Partha Kar, MBBS, MD, national specialty adviser, Diabetes for NHS England, about the series. This interview has been edited for length and clarity.
How did the idea for a comic book series about type 1 diabetes come about?Dr. Kar: My Southampton colleague Mayank Patel, BM, DM, FRCP, and I were discussing ways of reaching different audiences to raise awareness about type 1 diabetes, and we had the idea of comic books. After all, comic book movies are among the biggest blockbusters if one looks at popular culture, because it’s not just kids watching them.
One of our patients made an interesting observation that really resonated. He said having type 1 diabetes was like the Marvel Comics superhero Hulk.
Several scenes in the first publication, Type 1: Origins, were based on the Hulk, a scientist who gets a radioactive dose by accident. He doesn’t like turning green when he’s angry, even though he also becomes very strong. He basically spends the rest of his life trying to find the cure for himself, but he eventually makes the best of his two worlds – Professor and Hulk – rather than constantly fighting his situation.
The story line was primarily written by a group of patients with type 1 diabetes based on their own experiences. Mayank and I were mostly just supervising and financing the project. The graphics and layout were done by Revolve Comics, a publisher specializing in health education via the comic book medium.
Our aim was to bring awareness of type 1 diabetes to people who don’t have diabetes, including teachers, family members, and friends. At the end of Origins, we provide a list of online resources for more information and for social connection.
Since it launched in October 2016, Origins has been downloaded nearly 10,000 times. Lots of local charities and schools have picked it up. Parents and kids have come to us asking for more and giving us ideas. That’s what prompted the next one.
The second volume, Type 1: Attack of the Ketones, is more technical and somewhat surprising in that it portrays some hospital staff members as not well-informed about type 1 diabetes. Are they part of the intended audience?
Yes, this one was directed a little bit more towards professionals, hospitals, and staff. It’s also informed by patient feedback, and dovetails with my efforts to improve hospital care for people with type 1 diabetes. But of course, patients and interested laypeople can also learn from it.
A theme in volume 2 comes from another Marvel Comics superhero, Iron Man. In the movie, when Tony Stark’s heart is severely damaged with shrapnel, he acquires an arc reactor that keeps him alive and also powers the suit that gives him superpowers. After the reactor is taken away, he devises a way to replace the missing part and reassemble the suit.
Similarly, in type 1 diabetes, the ability to produce insulin has been taken away without permission. But what is missing can thankfully be replaced, albeit imperfectly. As we illustrate, things don’t always go to plan despite best efforts to administer insulin in the right dose at the right time.
At the end of Attack of the Ketones, we provide two pages of text about recognizing and managing hyperglycemia and preventing diabetic ketoacidosis. This volume was funded by NHS England and then backed by JDRF and Diabetes UK, and many hospitals picked it up. It has had about 8,000 downloads.
In Volume 3, you explore stigma and the issue of language regarding type 1 diabetes. How did those topics come about?
Kar: Type 1 Mission 3: S.T.I.G.M.A. was also based on patient feedback, with input from some Indian diabetes groups I’ve worked with. Here, the protagonist is a young man with type 1 diabetes who goes on holiday to India, where diabetes stigma is widespread. The characters address language problems such as use of the word “diabetic” to label a person, and they counter misconceptions such as that diabetes is contagious. There’s an Indian comic book version of this volume out now.
The main character of this volume experiences severe hypoglycemia and is saved by a glucagon injection from a colleague, one of several presented as superheroes who help in the fight to end diabetes stigma. They are referred to as Guardians of the Glucose, a take on yet another Marvel franchise, Guardians of the Galaxy.
At the end of this volume, we provide two pages of text about recognizing, managing, and preventing hypoglycemia. Again, we hope to educate as wide an audience as possible.
At the end of volume 3, you also briefly mention the COVID-19 pandemic. Will there be a fourth volume dealing with that, or other topics, such as diabetes technology?
We’ve left it open. We want to see how volume 3 lands. Depending on that, we might take it forward. There are certainly plenty of topics to tackle. We’ve also discussed moving into gaming or virtual reality. Overall, we hope to educate people by engaging them in different ways.
Dr. Kar has been a consultant diabetologist/endocrinologist within the NHS since 2008. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Overcoming the challenges in managing type 1 diabetes can sometimes feel like an unappreciated “superpower.” That was part of the thinking behind the creation of a comic book trilogy that aims to educate people of all ages – including health care providers – about the realities of living with this condition.
The series was initially launched by a team from Portsmouth (England) Hospitals University National Health Service Trust and University Hospital Southampton NHS Foundation Trust. It is now officially backed by the NHS. The first book in the trilogy, published in 2016, visually illustrates the challenges faced by a teenage boy who had recently been diagnosed with type 1 diabetes. The second volume, released in 2018, follows a young girl who is hospitalized with diabetic ketoacidosis. The third, published in December 2020, explores the stigma associated with diabetes and delves into hypoglycemia.
Available for free online, the three comic books are meant for adults, children, health care professionals, and laypeople. This news organization spoke with series cocreator Partha Kar, MBBS, MD, national specialty adviser, Diabetes for NHS England, about the series. This interview has been edited for length and clarity.
How did the idea for a comic book series about type 1 diabetes come about?Dr. Kar: My Southampton colleague Mayank Patel, BM, DM, FRCP, and I were discussing ways of reaching different audiences to raise awareness about type 1 diabetes, and we had the idea of comic books. After all, comic book movies are among the biggest blockbusters if one looks at popular culture, because it’s not just kids watching them.
One of our patients made an interesting observation that really resonated. He said having type 1 diabetes was like the Marvel Comics superhero Hulk.
Several scenes in the first publication, Type 1: Origins, were based on the Hulk, a scientist who gets a radioactive dose by accident. He doesn’t like turning green when he’s angry, even though he also becomes very strong. He basically spends the rest of his life trying to find the cure for himself, but he eventually makes the best of his two worlds – Professor and Hulk – rather than constantly fighting his situation.
The story line was primarily written by a group of patients with type 1 diabetes based on their own experiences. Mayank and I were mostly just supervising and financing the project. The graphics and layout were done by Revolve Comics, a publisher specializing in health education via the comic book medium.
Our aim was to bring awareness of type 1 diabetes to people who don’t have diabetes, including teachers, family members, and friends. At the end of Origins, we provide a list of online resources for more information and for social connection.
Since it launched in October 2016, Origins has been downloaded nearly 10,000 times. Lots of local charities and schools have picked it up. Parents and kids have come to us asking for more and giving us ideas. That’s what prompted the next one.
The second volume, Type 1: Attack of the Ketones, is more technical and somewhat surprising in that it portrays some hospital staff members as not well-informed about type 1 diabetes. Are they part of the intended audience?
Yes, this one was directed a little bit more towards professionals, hospitals, and staff. It’s also informed by patient feedback, and dovetails with my efforts to improve hospital care for people with type 1 diabetes. But of course, patients and interested laypeople can also learn from it.
A theme in volume 2 comes from another Marvel Comics superhero, Iron Man. In the movie, when Tony Stark’s heart is severely damaged with shrapnel, he acquires an arc reactor that keeps him alive and also powers the suit that gives him superpowers. After the reactor is taken away, he devises a way to replace the missing part and reassemble the suit.
Similarly, in type 1 diabetes, the ability to produce insulin has been taken away without permission. But what is missing can thankfully be replaced, albeit imperfectly. As we illustrate, things don’t always go to plan despite best efforts to administer insulin in the right dose at the right time.
At the end of Attack of the Ketones, we provide two pages of text about recognizing and managing hyperglycemia and preventing diabetic ketoacidosis. This volume was funded by NHS England and then backed by JDRF and Diabetes UK, and many hospitals picked it up. It has had about 8,000 downloads.
In Volume 3, you explore stigma and the issue of language regarding type 1 diabetes. How did those topics come about?
Kar: Type 1 Mission 3: S.T.I.G.M.A. was also based on patient feedback, with input from some Indian diabetes groups I’ve worked with. Here, the protagonist is a young man with type 1 diabetes who goes on holiday to India, where diabetes stigma is widespread. The characters address language problems such as use of the word “diabetic” to label a person, and they counter misconceptions such as that diabetes is contagious. There’s an Indian comic book version of this volume out now.
The main character of this volume experiences severe hypoglycemia and is saved by a glucagon injection from a colleague, one of several presented as superheroes who help in the fight to end diabetes stigma. They are referred to as Guardians of the Glucose, a take on yet another Marvel franchise, Guardians of the Galaxy.
At the end of this volume, we provide two pages of text about recognizing, managing, and preventing hypoglycemia. Again, we hope to educate as wide an audience as possible.
At the end of volume 3, you also briefly mention the COVID-19 pandemic. Will there be a fourth volume dealing with that, or other topics, such as diabetes technology?
We’ve left it open. We want to see how volume 3 lands. Depending on that, we might take it forward. There are certainly plenty of topics to tackle. We’ve also discussed moving into gaming or virtual reality. Overall, we hope to educate people by engaging them in different ways.
Dr. Kar has been a consultant diabetologist/endocrinologist within the NHS since 2008. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with PCOS at increased risk for COVID-19
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Diabetes prevention moves toward reality as studies published
Two newly published studies highlight recent success toward delaying the onset of type 1 diabetes in people at high risk and slowing progression in those with recent onset of the condition.
Both studies were initially presented in June 2020 at the annual scientific sessions of the American Diabetes Association and reported by this news organization at the time.
As yet, neither of the two strategies – preserving insulin-producing pancreatic beta-cell function soon after diagnosis or delaying type 1 diabetes onset in those at high risk – represent a cure or certain disease prevention.
However, both can potentially lead to better long-term glycemic control with less hypoglycemia and a lower risk for diabetes-related complications.
Combination treatment prolongs beta-cell function in new-onset disease
The first study, entitled, “Anti–interleukin-21 antibody and liraglutide for the preservation of beta-cell function in adults with recent-onset type 1 diabetes,” was published online March 1, 2021, in The Lancet Diabetes & Endocrinology by Matthias von Herrath, MD, of Novo Nordisk, Søborg, Denmark, and colleagues.
The randomized, placebo-controlled, double-blind, phase 2 combination treatment trial involved 308 individuals aged 18-45 years who had been diagnosed with type 1 diabetes in the previous 20 weeks and still had residual beta-cell function.
Patients were randomized with 77 per group to receive monoclonal anti-IL-21 plus liraglutide, anti-IL-21 alone, liraglutide alone, or placebo. The antibody was given intravenously every 6 weeks and liraglutide or matching placebo were self-administered by daily injections.
Compared with placebo (ratio to baseline, 0.61; 39% decrease), the decrease in mixed meal tolerance test stimulated C-peptide concentration from baseline to week 54 – the primary outcome – was significantly smaller with combination treatment (0.90, 10% decrease; estimated treatment ratio, 1.48; P = .0017), but not with anti-IL-21 alone (1.23; P = .093) or liraglutide alone (1.12; P = .38).
Despite greater insulin use in the placebo group, the decrease in hemoglobin A1c (a key secondary outcome) at week 54 was greater with all active treatments (–0.50 percentage points) than with placebo (–0.10 percentage points), although the differences versus placebo were not significant.
“The combination of anti-IL-21 and liraglutide could preserve beta-cell function in recently diagnosed type 1 diabetes,” the researchers said.
“These results suggest that this combination has the potential to offer a novel and valuable disease-modifying therapy for patients with recently diagnosed type 1 diabetes. However, the efficacy and safety need to be further investigated in a phase 3 program,” Dr. von Herrath and colleagues concluded.
Teplizumab: 3-year data continue to show benefit
The other study looked at delaying the onset of type 1 diabetes. Entitled, “Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals,” the article was published online March 3, 2021, in Science Translational Medicine by Emily K. Sims, MD, of the department of pediatrics, Indiana University, Indianapolis, and colleagues.
This trial of the anti-CD3 monoclonal antibody adds an additional year of follow-up to the “game-changer” 2-year data reported in 2019.
Among the 76 individuals aged 8-49 years who were positive for two or more type 1 diabetes–related autoantibodies, 50% of those randomized to a single 14-day infusion course of teplizumab remained diabetes free at a median follow-up of 923 days, compared with only 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01).
The teplizumab group had a greater average C-peptide area under the curve, compared with placebo, reflecting improved beta-cell function (1.96 vs 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“It is very encouraging to see that a single course of teplizumab delayed insulin dependence in this high-risk population for approximately 3 years versus placebo,” said Frank Martin, PhD, JDRF director of research at Provention Bio, which is developing teplizumab.
“These exciting results have been made possible by the unwavering efforts of TrialNet and Provention Bio. Teplizumab, if approved by the FDA, could positively change the course of disease development for people at risk of developing T1D and their standard of care,” he concluded.
The teplizumab study was funded by TrialNet. Dr. von Herrath is an employee of Novo Nordisk, which funded the study involving its drug liraglutide. Dr. Sims reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two newly published studies highlight recent success toward delaying the onset of type 1 diabetes in people at high risk and slowing progression in those with recent onset of the condition.
Both studies were initially presented in June 2020 at the annual scientific sessions of the American Diabetes Association and reported by this news organization at the time.
As yet, neither of the two strategies – preserving insulin-producing pancreatic beta-cell function soon after diagnosis or delaying type 1 diabetes onset in those at high risk – represent a cure or certain disease prevention.
However, both can potentially lead to better long-term glycemic control with less hypoglycemia and a lower risk for diabetes-related complications.
Combination treatment prolongs beta-cell function in new-onset disease
The first study, entitled, “Anti–interleukin-21 antibody and liraglutide for the preservation of beta-cell function in adults with recent-onset type 1 diabetes,” was published online March 1, 2021, in The Lancet Diabetes & Endocrinology by Matthias von Herrath, MD, of Novo Nordisk, Søborg, Denmark, and colleagues.
The randomized, placebo-controlled, double-blind, phase 2 combination treatment trial involved 308 individuals aged 18-45 years who had been diagnosed with type 1 diabetes in the previous 20 weeks and still had residual beta-cell function.
Patients were randomized with 77 per group to receive monoclonal anti-IL-21 plus liraglutide, anti-IL-21 alone, liraglutide alone, or placebo. The antibody was given intravenously every 6 weeks and liraglutide or matching placebo were self-administered by daily injections.
Compared with placebo (ratio to baseline, 0.61; 39% decrease), the decrease in mixed meal tolerance test stimulated C-peptide concentration from baseline to week 54 – the primary outcome – was significantly smaller with combination treatment (0.90, 10% decrease; estimated treatment ratio, 1.48; P = .0017), but not with anti-IL-21 alone (1.23; P = .093) or liraglutide alone (1.12; P = .38).
Despite greater insulin use in the placebo group, the decrease in hemoglobin A1c (a key secondary outcome) at week 54 was greater with all active treatments (–0.50 percentage points) than with placebo (–0.10 percentage points), although the differences versus placebo were not significant.
“The combination of anti-IL-21 and liraglutide could preserve beta-cell function in recently diagnosed type 1 diabetes,” the researchers said.
“These results suggest that this combination has the potential to offer a novel and valuable disease-modifying therapy for patients with recently diagnosed type 1 diabetes. However, the efficacy and safety need to be further investigated in a phase 3 program,” Dr. von Herrath and colleagues concluded.
Teplizumab: 3-year data continue to show benefit
The other study looked at delaying the onset of type 1 diabetes. Entitled, “Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals,” the article was published online March 3, 2021, in Science Translational Medicine by Emily K. Sims, MD, of the department of pediatrics, Indiana University, Indianapolis, and colleagues.
This trial of the anti-CD3 monoclonal antibody adds an additional year of follow-up to the “game-changer” 2-year data reported in 2019.
Among the 76 individuals aged 8-49 years who were positive for two or more type 1 diabetes–related autoantibodies, 50% of those randomized to a single 14-day infusion course of teplizumab remained diabetes free at a median follow-up of 923 days, compared with only 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01).
The teplizumab group had a greater average C-peptide area under the curve, compared with placebo, reflecting improved beta-cell function (1.96 vs 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“It is very encouraging to see that a single course of teplizumab delayed insulin dependence in this high-risk population for approximately 3 years versus placebo,” said Frank Martin, PhD, JDRF director of research at Provention Bio, which is developing teplizumab.
“These exciting results have been made possible by the unwavering efforts of TrialNet and Provention Bio. Teplizumab, if approved by the FDA, could positively change the course of disease development for people at risk of developing T1D and their standard of care,” he concluded.
The teplizumab study was funded by TrialNet. Dr. von Herrath is an employee of Novo Nordisk, which funded the study involving its drug liraglutide. Dr. Sims reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two newly published studies highlight recent success toward delaying the onset of type 1 diabetes in people at high risk and slowing progression in those with recent onset of the condition.
Both studies were initially presented in June 2020 at the annual scientific sessions of the American Diabetes Association and reported by this news organization at the time.
As yet, neither of the two strategies – preserving insulin-producing pancreatic beta-cell function soon after diagnosis or delaying type 1 diabetes onset in those at high risk – represent a cure or certain disease prevention.
However, both can potentially lead to better long-term glycemic control with less hypoglycemia and a lower risk for diabetes-related complications.
Combination treatment prolongs beta-cell function in new-onset disease
The first study, entitled, “Anti–interleukin-21 antibody and liraglutide for the preservation of beta-cell function in adults with recent-onset type 1 diabetes,” was published online March 1, 2021, in The Lancet Diabetes & Endocrinology by Matthias von Herrath, MD, of Novo Nordisk, Søborg, Denmark, and colleagues.
The randomized, placebo-controlled, double-blind, phase 2 combination treatment trial involved 308 individuals aged 18-45 years who had been diagnosed with type 1 diabetes in the previous 20 weeks and still had residual beta-cell function.
Patients were randomized with 77 per group to receive monoclonal anti-IL-21 plus liraglutide, anti-IL-21 alone, liraglutide alone, or placebo. The antibody was given intravenously every 6 weeks and liraglutide or matching placebo were self-administered by daily injections.
Compared with placebo (ratio to baseline, 0.61; 39% decrease), the decrease in mixed meal tolerance test stimulated C-peptide concentration from baseline to week 54 – the primary outcome – was significantly smaller with combination treatment (0.90, 10% decrease; estimated treatment ratio, 1.48; P = .0017), but not with anti-IL-21 alone (1.23; P = .093) or liraglutide alone (1.12; P = .38).
Despite greater insulin use in the placebo group, the decrease in hemoglobin A1c (a key secondary outcome) at week 54 was greater with all active treatments (–0.50 percentage points) than with placebo (–0.10 percentage points), although the differences versus placebo were not significant.
“The combination of anti-IL-21 and liraglutide could preserve beta-cell function in recently diagnosed type 1 diabetes,” the researchers said.
“These results suggest that this combination has the potential to offer a novel and valuable disease-modifying therapy for patients with recently diagnosed type 1 diabetes. However, the efficacy and safety need to be further investigated in a phase 3 program,” Dr. von Herrath and colleagues concluded.
Teplizumab: 3-year data continue to show benefit
The other study looked at delaying the onset of type 1 diabetes. Entitled, “Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals,” the article was published online March 3, 2021, in Science Translational Medicine by Emily K. Sims, MD, of the department of pediatrics, Indiana University, Indianapolis, and colleagues.
This trial of the anti-CD3 monoclonal antibody adds an additional year of follow-up to the “game-changer” 2-year data reported in 2019.
Among the 76 individuals aged 8-49 years who were positive for two or more type 1 diabetes–related autoantibodies, 50% of those randomized to a single 14-day infusion course of teplizumab remained diabetes free at a median follow-up of 923 days, compared with only 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01).
The teplizumab group had a greater average C-peptide area under the curve, compared with placebo, reflecting improved beta-cell function (1.96 vs 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“It is very encouraging to see that a single course of teplizumab delayed insulin dependence in this high-risk population for approximately 3 years versus placebo,” said Frank Martin, PhD, JDRF director of research at Provention Bio, which is developing teplizumab.
“These exciting results have been made possible by the unwavering efforts of TrialNet and Provention Bio. Teplizumab, if approved by the FDA, could positively change the course of disease development for people at risk of developing T1D and their standard of care,” he concluded.
The teplizumab study was funded by TrialNet. Dr. von Herrath is an employee of Novo Nordisk, which funded the study involving its drug liraglutide. Dr. Sims reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most breast cancer screening centers not following guidelines
, say researchers reporting on a new analysis.
They assessed 606 centers and report that, among the centers that recommended a starting age for screening mammography, nearly 90% advised women to begin screening at age 40 years and to continue annually.
This contrasts with the current recommendations from the U.S. Preventive Services Task Force (USPSTF) on mammography screening, which stipulate starting at age 50 years and continuing every 2 years.
The new analysis was published online in JAMA Internal Medicine.
This may be doing “more harm than good,” warn the authors of an accompanying editorial.
“The recommendation for annual mammography in women younger than 50 years is, at best, confusing for patients and is likely to conflict with advice from their primary care physicians, which can create tension,” write Anand R. Habib, MD, MPhil; Deborah Grady, MD; and Rita F. Redberg, MD, all from the University of California, San Francisco.
“This recommendation can also produce unnecessary testing, invasive procedures, overdiagnosis, and anxiety among women who receive screening,” they write.
“Breast cancer centers with clear financial benefits from increased mammography rates may wish to reconsider offering recommendations that create greater referral volume but conflict with unbiased evidence-based USPSTF guidelines and have the potential to increase harms among women,” the editorialists add.
The age at which to start mammography screening has long been a contentious issue, with some experts and medical societies arguing that it should begin at 40.
The American College of Radiology, the Society of Breast Imaging, and the American Society of Breast Surgeons recommend that women start annual mammography screening at age 40.
The American Cancer Society’s guidelines recommend an initial screening mammogram between ages 45 and 55 and after that, screening every 1-2 years.
One expert who argues for starting at 40 years is Laurie Margolies, MD, chief of breast imaging, Mount Sinai Health System, and professor of radiology, Icahn School of Medicine at Mount Sinai, New York.
In a statement, she noted that 17% of all breast cancers are diagnosed in women in their 40s and that the majority of these women are not considered to be at high risk of developing the disease.
“Our own Mount Sinai research has shown that women with screen-detected breast cancers are less likely to need a mastectomy and are less likely to require chemotherapy or axillary node dissection,” Dr. Margolies said.
“Additionally, women who get regular breast cancer screening have a 47% lower risk of breast cancer death within 20 years of diagnosis than those not regularly screened. Skipping a mammogram can have lethal consequences,” she said.
Details of the analysis
The analysis of recommendations by breast cancer centers regarding screening mammography was carried out by Jennifer L. Marti, MD, from Weill Cornell Medicine, New York, and colleagues.
They examined 606 centers and found that 487 (80.4%) offered screening recommendations on their public websites.
Of 431 centers that recommended a starting age, 376 centers (87.2%) advised women to begin screening at age 40 years; 35 centers (8.1%) recommended beginning at age 45 years; and the remaining 20 centers (4.6%) recommended that screening begin at age 50 years.
A total of 429 centers recommended both a starting age and a screening interval. Of this group, 347 centers (80.9%) advised that annual screening begin at age 40 years. Only 16 centers (3.3%) recommended biennial mammography (as per the USPSTF guidelines). Almost three-quarters (72.7%, n = 354) recommended annual screening; 59 centers (12.1%) recommended annual or biennial screening; and 58 centers (11.9%) recommended a discussion with a physician.
The authors note that there were differences between centers according to National Cancer Institute designation, but these differences did not reach statistical significance.
Dr. Marti and coauthors, Dr. Habib and coauthors, and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, say researchers reporting on a new analysis.
They assessed 606 centers and report that, among the centers that recommended a starting age for screening mammography, nearly 90% advised women to begin screening at age 40 years and to continue annually.
This contrasts with the current recommendations from the U.S. Preventive Services Task Force (USPSTF) on mammography screening, which stipulate starting at age 50 years and continuing every 2 years.
The new analysis was published online in JAMA Internal Medicine.
This may be doing “more harm than good,” warn the authors of an accompanying editorial.
“The recommendation for annual mammography in women younger than 50 years is, at best, confusing for patients and is likely to conflict with advice from their primary care physicians, which can create tension,” write Anand R. Habib, MD, MPhil; Deborah Grady, MD; and Rita F. Redberg, MD, all from the University of California, San Francisco.
“This recommendation can also produce unnecessary testing, invasive procedures, overdiagnosis, and anxiety among women who receive screening,” they write.
“Breast cancer centers with clear financial benefits from increased mammography rates may wish to reconsider offering recommendations that create greater referral volume but conflict with unbiased evidence-based USPSTF guidelines and have the potential to increase harms among women,” the editorialists add.
The age at which to start mammography screening has long been a contentious issue, with some experts and medical societies arguing that it should begin at 40.
The American College of Radiology, the Society of Breast Imaging, and the American Society of Breast Surgeons recommend that women start annual mammography screening at age 40.
The American Cancer Society’s guidelines recommend an initial screening mammogram between ages 45 and 55 and after that, screening every 1-2 years.
One expert who argues for starting at 40 years is Laurie Margolies, MD, chief of breast imaging, Mount Sinai Health System, and professor of radiology, Icahn School of Medicine at Mount Sinai, New York.
In a statement, she noted that 17% of all breast cancers are diagnosed in women in their 40s and that the majority of these women are not considered to be at high risk of developing the disease.
“Our own Mount Sinai research has shown that women with screen-detected breast cancers are less likely to need a mastectomy and are less likely to require chemotherapy or axillary node dissection,” Dr. Margolies said.
“Additionally, women who get regular breast cancer screening have a 47% lower risk of breast cancer death within 20 years of diagnosis than those not regularly screened. Skipping a mammogram can have lethal consequences,” she said.
Details of the analysis
The analysis of recommendations by breast cancer centers regarding screening mammography was carried out by Jennifer L. Marti, MD, from Weill Cornell Medicine, New York, and colleagues.
They examined 606 centers and found that 487 (80.4%) offered screening recommendations on their public websites.
Of 431 centers that recommended a starting age, 376 centers (87.2%) advised women to begin screening at age 40 years; 35 centers (8.1%) recommended beginning at age 45 years; and the remaining 20 centers (4.6%) recommended that screening begin at age 50 years.
A total of 429 centers recommended both a starting age and a screening interval. Of this group, 347 centers (80.9%) advised that annual screening begin at age 40 years. Only 16 centers (3.3%) recommended biennial mammography (as per the USPSTF guidelines). Almost three-quarters (72.7%, n = 354) recommended annual screening; 59 centers (12.1%) recommended annual or biennial screening; and 58 centers (11.9%) recommended a discussion with a physician.
The authors note that there were differences between centers according to National Cancer Institute designation, but these differences did not reach statistical significance.
Dr. Marti and coauthors, Dr. Habib and coauthors, and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, say researchers reporting on a new analysis.
They assessed 606 centers and report that, among the centers that recommended a starting age for screening mammography, nearly 90% advised women to begin screening at age 40 years and to continue annually.
This contrasts with the current recommendations from the U.S. Preventive Services Task Force (USPSTF) on mammography screening, which stipulate starting at age 50 years and continuing every 2 years.
The new analysis was published online in JAMA Internal Medicine.
This may be doing “more harm than good,” warn the authors of an accompanying editorial.
“The recommendation for annual mammography in women younger than 50 years is, at best, confusing for patients and is likely to conflict with advice from their primary care physicians, which can create tension,” write Anand R. Habib, MD, MPhil; Deborah Grady, MD; and Rita F. Redberg, MD, all from the University of California, San Francisco.
“This recommendation can also produce unnecessary testing, invasive procedures, overdiagnosis, and anxiety among women who receive screening,” they write.
“Breast cancer centers with clear financial benefits from increased mammography rates may wish to reconsider offering recommendations that create greater referral volume but conflict with unbiased evidence-based USPSTF guidelines and have the potential to increase harms among women,” the editorialists add.
The age at which to start mammography screening has long been a contentious issue, with some experts and medical societies arguing that it should begin at 40.
The American College of Radiology, the Society of Breast Imaging, and the American Society of Breast Surgeons recommend that women start annual mammography screening at age 40.
The American Cancer Society’s guidelines recommend an initial screening mammogram between ages 45 and 55 and after that, screening every 1-2 years.
One expert who argues for starting at 40 years is Laurie Margolies, MD, chief of breast imaging, Mount Sinai Health System, and professor of radiology, Icahn School of Medicine at Mount Sinai, New York.
In a statement, she noted that 17% of all breast cancers are diagnosed in women in their 40s and that the majority of these women are not considered to be at high risk of developing the disease.
“Our own Mount Sinai research has shown that women with screen-detected breast cancers are less likely to need a mastectomy and are less likely to require chemotherapy or axillary node dissection,” Dr. Margolies said.
“Additionally, women who get regular breast cancer screening have a 47% lower risk of breast cancer death within 20 years of diagnosis than those not regularly screened. Skipping a mammogram can have lethal consequences,” she said.
Details of the analysis
The analysis of recommendations by breast cancer centers regarding screening mammography was carried out by Jennifer L. Marti, MD, from Weill Cornell Medicine, New York, and colleagues.
They examined 606 centers and found that 487 (80.4%) offered screening recommendations on their public websites.
Of 431 centers that recommended a starting age, 376 centers (87.2%) advised women to begin screening at age 40 years; 35 centers (8.1%) recommended beginning at age 45 years; and the remaining 20 centers (4.6%) recommended that screening begin at age 50 years.
A total of 429 centers recommended both a starting age and a screening interval. Of this group, 347 centers (80.9%) advised that annual screening begin at age 40 years. Only 16 centers (3.3%) recommended biennial mammography (as per the USPSTF guidelines). Almost three-quarters (72.7%, n = 354) recommended annual screening; 59 centers (12.1%) recommended annual or biennial screening; and 58 centers (11.9%) recommended a discussion with a physician.
The authors note that there were differences between centers according to National Cancer Institute designation, but these differences did not reach statistical significance.
Dr. Marti and coauthors, Dr. Habib and coauthors, and Dr. Margolies have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dose-related AFib risk with omega-3 fatty acids?
There may be a dose-related risk for atrial fibrillation (AFib) with omega-3 fatty acid intake, data from four randomized clinical trials suggest.
The latest trial to evaluate the association, the VITAL-RHYTHM study, showed that using a low dose of omega-3 fatty acids or a vitamin D supplement had no significant effect on the risks of developing incident AFib.
The trial, first reported at last year’s American Heart Association meeting, was published online March 16 in the Journal of the American Medical Association.
Together with three other randomized clinical trials, however, these results suggest a possible dose-related effect of omega-3 fatty acids on the risk for AFib, an accompanying “Editor’s Note” suggests.
The note, by JAMA deputy editor Gregory Curfman, MD, points out that in the past 2 years, four randomized clinical trials have provided data on the risk of AFib with omega-3 fatty acid intake.
In the STRENGTH and REDUCE-IT trials, both of which evaluated high doses (4 g/day) of omega-3 fatty acids in patients with heart disease (or at high risk for it), there was a highly statistically significant increase in risk for AFib in the omega-3 groups vs. controls in both trials.
In the OMEMI trial in elderly patients with a recent myocardial infarction, an intermediate dose (1.8 g/day) of omega-3 fatty acids also showed an increase in AFib risk (hazard ratio, 1.84) but this was not significant. And now, the VITAL-RHYTHM trial shows no significant effect of a low dose (840 mg/day) of omega-3 fatty acids on the risk of developing AFib in a primary prevention population.
“Patients who choose to take omega-3 fatty acids, especially in high doses, should be informed of the risk of AF [AFib] and followed up for the possible development of this common and potentially hazardous arrhythmia,” Dr. Curfman concludes.
The authors of the VITAL-RHYTHM trial, led by Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, Calif., explain that the trial was conducted after observational studies had shown that individuals with low blood levels of omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and vitamin D3 have higher risks of incident AFib, but data on dietary or supplemental intake of these nutrients on AFib risk were mixed.
“To our knowledge, this study is the first randomized, placebo-controlled trial to prospectively test the effect of any intervention on incident AF and is the only trial to test alternative upstream preventive agents for AF in a large enough population over a long enough time period to provide an assessment of the plausible benefits and risks,” they write.
The VITAL-RHYTHM study was an ancillary trial embedded within the Vitamin D and Omega-3 (VITAL) trial, which used a 2 x 2 factorial design to evaluate daily supplementation with 2,000 IU of vitamin D3 and/or 840 mg of marine omega-3 fatty acids (460 mg EPA and 380 mg DHA), in the primary prevention of cardiovascular disease and cancer in 25,871 men and women age 50 and older in the United States.
Results showed that over a median 5.3 years of treatment and follow-up, the primary endpoint of incident AFib occurred in 3.6% of the study population. For the omega-3 part of the trial, incident AFib events occurred in 3.7% of patients taking EPA/DHA vs. 3.4% of the placebo group, giving a hazard ratio of 1.09, which was not significant (P = .19).
For the vitamin D3 vs. placebo comparison, results were very similar, with incident AFib events occurring in 3.7% vs. 3.4% of participants, respectively, giving a hazard ratio of 1.09, which was again not significant (P = .19). There was no evidence for interaction between the two study agents.
“Overall, these findings do not support the use of supplemental EPA-DHA or vitamin D3 for the primary prevention of AFib and provide reassurance regarding lack of a major risk of AFib incidence associated with these commonly used supplements at these doses,” the authors conclude.
Noting that significant increases in AFib have been seen with much higher doses of omega-3 fatty acids in the REDUCE-IT and STRENGTH trials, they add: “Potentially, the adverse effect on AF risk may be dose related, and the higher dosages of EPA used in these other studies might account for the significant adverse effect on AF.”
The researchers say that, to their knowledge, this is the only randomized trial to assess the effect of vitamin D3 supplementation on AFib risk and results suggest a null effect. They add that subgroup analyses in patients with vitamin D levels considered deficient (<20 ng/mL) did not suggest a benefit; however, the power to detect a benefit in this much smaller subset of the population was limited.
They point out that, while there were no significant differences in incident AFib for either omega-3 fatty acid or vitamin D in the overall study population, an increased risk for incident AFib associated with randomized treatment was observed in selected subgroups.
For omega-3 fatty acids, AFib risk was modestly increased in taller individuals, and for vitamin D3, elevations in AFib risk were observed in younger individuals and participants who drank less alcohol.
“Although the hazard ratios and tests for interaction were significant, the P values associated with these subgroup analyses have not been adjusted for multiple comparisons. Thus, these findings should be interpreted with caution and considered hypothesis generating,” they warn.
The VITAL Rhythm Study was supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Albert reported receipt of grants from St Jude Medical, Abbott, and Roche Diagnostics. Dr. Curfman reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
There may be a dose-related risk for atrial fibrillation (AFib) with omega-3 fatty acid intake, data from four randomized clinical trials suggest.
The latest trial to evaluate the association, the VITAL-RHYTHM study, showed that using a low dose of omega-3 fatty acids or a vitamin D supplement had no significant effect on the risks of developing incident AFib.
The trial, first reported at last year’s American Heart Association meeting, was published online March 16 in the Journal of the American Medical Association.
Together with three other randomized clinical trials, however, these results suggest a possible dose-related effect of omega-3 fatty acids on the risk for AFib, an accompanying “Editor’s Note” suggests.
The note, by JAMA deputy editor Gregory Curfman, MD, points out that in the past 2 years, four randomized clinical trials have provided data on the risk of AFib with omega-3 fatty acid intake.
In the STRENGTH and REDUCE-IT trials, both of which evaluated high doses (4 g/day) of omega-3 fatty acids in patients with heart disease (or at high risk for it), there was a highly statistically significant increase in risk for AFib in the omega-3 groups vs. controls in both trials.
In the OMEMI trial in elderly patients with a recent myocardial infarction, an intermediate dose (1.8 g/day) of omega-3 fatty acids also showed an increase in AFib risk (hazard ratio, 1.84) but this was not significant. And now, the VITAL-RHYTHM trial shows no significant effect of a low dose (840 mg/day) of omega-3 fatty acids on the risk of developing AFib in a primary prevention population.
“Patients who choose to take omega-3 fatty acids, especially in high doses, should be informed of the risk of AF [AFib] and followed up for the possible development of this common and potentially hazardous arrhythmia,” Dr. Curfman concludes.
The authors of the VITAL-RHYTHM trial, led by Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, Calif., explain that the trial was conducted after observational studies had shown that individuals with low blood levels of omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and vitamin D3 have higher risks of incident AFib, but data on dietary or supplemental intake of these nutrients on AFib risk were mixed.
“To our knowledge, this study is the first randomized, placebo-controlled trial to prospectively test the effect of any intervention on incident AF and is the only trial to test alternative upstream preventive agents for AF in a large enough population over a long enough time period to provide an assessment of the plausible benefits and risks,” they write.
The VITAL-RHYTHM study was an ancillary trial embedded within the Vitamin D and Omega-3 (VITAL) trial, which used a 2 x 2 factorial design to evaluate daily supplementation with 2,000 IU of vitamin D3 and/or 840 mg of marine omega-3 fatty acids (460 mg EPA and 380 mg DHA), in the primary prevention of cardiovascular disease and cancer in 25,871 men and women age 50 and older in the United States.
Results showed that over a median 5.3 years of treatment and follow-up, the primary endpoint of incident AFib occurred in 3.6% of the study population. For the omega-3 part of the trial, incident AFib events occurred in 3.7% of patients taking EPA/DHA vs. 3.4% of the placebo group, giving a hazard ratio of 1.09, which was not significant (P = .19).
For the vitamin D3 vs. placebo comparison, results were very similar, with incident AFib events occurring in 3.7% vs. 3.4% of participants, respectively, giving a hazard ratio of 1.09, which was again not significant (P = .19). There was no evidence for interaction between the two study agents.
“Overall, these findings do not support the use of supplemental EPA-DHA or vitamin D3 for the primary prevention of AFib and provide reassurance regarding lack of a major risk of AFib incidence associated with these commonly used supplements at these doses,” the authors conclude.
Noting that significant increases in AFib have been seen with much higher doses of omega-3 fatty acids in the REDUCE-IT and STRENGTH trials, they add: “Potentially, the adverse effect on AF risk may be dose related, and the higher dosages of EPA used in these other studies might account for the significant adverse effect on AF.”
The researchers say that, to their knowledge, this is the only randomized trial to assess the effect of vitamin D3 supplementation on AFib risk and results suggest a null effect. They add that subgroup analyses in patients with vitamin D levels considered deficient (<20 ng/mL) did not suggest a benefit; however, the power to detect a benefit in this much smaller subset of the population was limited.
They point out that, while there were no significant differences in incident AFib for either omega-3 fatty acid or vitamin D in the overall study population, an increased risk for incident AFib associated with randomized treatment was observed in selected subgroups.
For omega-3 fatty acids, AFib risk was modestly increased in taller individuals, and for vitamin D3, elevations in AFib risk were observed in younger individuals and participants who drank less alcohol.
“Although the hazard ratios and tests for interaction were significant, the P values associated with these subgroup analyses have not been adjusted for multiple comparisons. Thus, these findings should be interpreted with caution and considered hypothesis generating,” they warn.
The VITAL Rhythm Study was supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Albert reported receipt of grants from St Jude Medical, Abbott, and Roche Diagnostics. Dr. Curfman reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
There may be a dose-related risk for atrial fibrillation (AFib) with omega-3 fatty acid intake, data from four randomized clinical trials suggest.
The latest trial to evaluate the association, the VITAL-RHYTHM study, showed that using a low dose of omega-3 fatty acids or a vitamin D supplement had no significant effect on the risks of developing incident AFib.
The trial, first reported at last year’s American Heart Association meeting, was published online March 16 in the Journal of the American Medical Association.
Together with three other randomized clinical trials, however, these results suggest a possible dose-related effect of omega-3 fatty acids on the risk for AFib, an accompanying “Editor’s Note” suggests.
The note, by JAMA deputy editor Gregory Curfman, MD, points out that in the past 2 years, four randomized clinical trials have provided data on the risk of AFib with omega-3 fatty acid intake.
In the STRENGTH and REDUCE-IT trials, both of which evaluated high doses (4 g/day) of omega-3 fatty acids in patients with heart disease (or at high risk for it), there was a highly statistically significant increase in risk for AFib in the omega-3 groups vs. controls in both trials.
In the OMEMI trial in elderly patients with a recent myocardial infarction, an intermediate dose (1.8 g/day) of omega-3 fatty acids also showed an increase in AFib risk (hazard ratio, 1.84) but this was not significant. And now, the VITAL-RHYTHM trial shows no significant effect of a low dose (840 mg/day) of omega-3 fatty acids on the risk of developing AFib in a primary prevention population.
“Patients who choose to take omega-3 fatty acids, especially in high doses, should be informed of the risk of AF [AFib] and followed up for the possible development of this common and potentially hazardous arrhythmia,” Dr. Curfman concludes.
The authors of the VITAL-RHYTHM trial, led by Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, Calif., explain that the trial was conducted after observational studies had shown that individuals with low blood levels of omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and vitamin D3 have higher risks of incident AFib, but data on dietary or supplemental intake of these nutrients on AFib risk were mixed.
“To our knowledge, this study is the first randomized, placebo-controlled trial to prospectively test the effect of any intervention on incident AF and is the only trial to test alternative upstream preventive agents for AF in a large enough population over a long enough time period to provide an assessment of the plausible benefits and risks,” they write.
The VITAL-RHYTHM study was an ancillary trial embedded within the Vitamin D and Omega-3 (VITAL) trial, which used a 2 x 2 factorial design to evaluate daily supplementation with 2,000 IU of vitamin D3 and/or 840 mg of marine omega-3 fatty acids (460 mg EPA and 380 mg DHA), in the primary prevention of cardiovascular disease and cancer in 25,871 men and women age 50 and older in the United States.
Results showed that over a median 5.3 years of treatment and follow-up, the primary endpoint of incident AFib occurred in 3.6% of the study population. For the omega-3 part of the trial, incident AFib events occurred in 3.7% of patients taking EPA/DHA vs. 3.4% of the placebo group, giving a hazard ratio of 1.09, which was not significant (P = .19).
For the vitamin D3 vs. placebo comparison, results were very similar, with incident AFib events occurring in 3.7% vs. 3.4% of participants, respectively, giving a hazard ratio of 1.09, which was again not significant (P = .19). There was no evidence for interaction between the two study agents.
“Overall, these findings do not support the use of supplemental EPA-DHA or vitamin D3 for the primary prevention of AFib and provide reassurance regarding lack of a major risk of AFib incidence associated with these commonly used supplements at these doses,” the authors conclude.
Noting that significant increases in AFib have been seen with much higher doses of omega-3 fatty acids in the REDUCE-IT and STRENGTH trials, they add: “Potentially, the adverse effect on AF risk may be dose related, and the higher dosages of EPA used in these other studies might account for the significant adverse effect on AF.”
The researchers say that, to their knowledge, this is the only randomized trial to assess the effect of vitamin D3 supplementation on AFib risk and results suggest a null effect. They add that subgroup analyses in patients with vitamin D levels considered deficient (<20 ng/mL) did not suggest a benefit; however, the power to detect a benefit in this much smaller subset of the population was limited.
They point out that, while there were no significant differences in incident AFib for either omega-3 fatty acid or vitamin D in the overall study population, an increased risk for incident AFib associated with randomized treatment was observed in selected subgroups.
For omega-3 fatty acids, AFib risk was modestly increased in taller individuals, and for vitamin D3, elevations in AFib risk were observed in younger individuals and participants who drank less alcohol.
“Although the hazard ratios and tests for interaction were significant, the P values associated with these subgroup analyses have not been adjusted for multiple comparisons. Thus, these findings should be interpreted with caution and considered hypothesis generating,” they warn.
The VITAL Rhythm Study was supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Albert reported receipt of grants from St Jude Medical, Abbott, and Roche Diagnostics. Dr. Curfman reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
Adherence to antireflux lifestyle factors shows benefit in women
Antireflux lifestyle factors may significantly reduce the risk of gastroesophageal reflux disease (GERD), according to an analysis involving almost 43,000 women.
Even alongside therapy with a proton-pump inhibitor (PPI) and/or a histamine-receptor antagonist (H2RA), adherence to five antireflux lifestyle factors had a meaningful impact on risk for GERD symptoms, possibly preventing nearly 40% of cases with weekly GERD symptoms, reported lead author Raaj S. Mehta, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues.
“Clinicians recommend dietary and lifestyle modifications to prevent GERD symptoms, but no prospective data are available to inform these recommendations,” Dr. Mehta and colleagues wrote in JAMA Internal Medicine.
To address this gap, the investigators turned to the Nurses’ Health Study II, a nationwide, prospective study involving 116,671 women. The study, which has a follow-up rate exceeding 90%, began in 1989 and is ongoing. Participants complete biennial questionnaires that include a variety of health and lifestyle factors. In 2005, 2009, 2013, and 2017, the questionnaire inquired about heartburn or acid reflux.
The present analysis included data from 42,955 women aged 42-62 years. Participants were excluded at baseline if they had cancer, lacked dietary data, were lost to follow-up, already had GERD symptoms at least weekly, or used a PPI and/or H2RA on a regular basis. The final dataset included 392,215 person-years of follow-up, with 9,291 incident cases of GERD symptoms.
For each participant, the presence of five possible antireflux lifestyle factors were added together for a score ranging from 0 to 5: no more than two cups of soda, tea, or coffee per day; never smoking; normal body weight (BMI ≥18.5 and <25.0 kg/m2); “prudent” diet, based on top 40% of dietary pattern score; and at least 30 minutes of moderate to vigorous physical activity each day.
Multivariate logistic regression modeling showed that women who reported all five antireflux lifestyle factors had a 50% decreased risk of GERD symptoms (hazard ratio, 0.50; 95% confidence interval, 0.42-0.59), compared with women who adhered to none of them. Further analysis suggested that the collective effect of all five factors could reduce GERD symptom case volume by 37% (95% CI, 28%-46%).
Nonadherence to each antireflux lifestyle factor was independently associated with an increased risk of GERD symptoms. After mutual adjustment for other variables, BMI was associated with the highest population-attributable risk (19%), followed by physical activity (8%), food intake (7%), beverage intake (4%), and nonsmoker status (3%).
Dr. Mehta and colleagues also explored the relationship between GERD symptoms, antireflux medications, and lifestyle factors. Presence of all five antireflux factors was associated with a 53% decreased risk of GERD symptoms or initiation of PPI and/or H2RA therapy (HR, 0.47; 95% CI, 0.41-0.54). Among a group of 3,625 women who reported regular use of a PPI and/or H2RA and were free of GERD symptoms at baseline, adherence to all five lifestyle factors reduced risk of GERD symptoms by 68% (HR, 0.32; 95% CI, 0.18-0.57).
One limitation of the study was that its population was primarily White women; however, the authors noted a study suggesting GERD is more common in White women aged 30-60 years.
“Adherence to an antireflux lifestyle, even among regular users of PPIs and/or H2RAs, was associated with a decreased risk of GERD symptoms,” the investigators concluded.
Lifestyle matters
According to Ronnie Fass, MD, medical director of the Digestive Health Center at Case Western Reserve University, Cleveland, “This is the first study to show the incremental effect and thus the benefit of lifestyle factors in reducing the risk of GERD symptoms. While only five lifestyle factors were assessed in this study, potentially others may further decrease the risk for symptoms.”
Dr. Fass suggested that the nature of the data, which was self-reported, and the entirely female patient population, should inform interpretation of the findings.
“While nonerosive reflux disease is relatively more common in women, erosive esophagitis and Barrett’s esophagus are more common in men,” he said. “Furthermore, male gender is associated with more severe GERD and GERD complications.”
Yet Dr. Fass concluded by again emphasizing the merit of the analysis: “This is an important study that further supports the value of certain lifestyle factors in reducing the risk of GERD symptoms,” he said. “What is challenging for practicing physicians is to get patients to follow these lifestyle factors long term.”
The study was funded by the National Institutes of Health and by a Stuart and Suzanne Steele Massachusetts General Hospital Research Scholar Award. The investigators and Dr. Fass disclosed no conflicts of interest.
Antireflux lifestyle factors may significantly reduce the risk of gastroesophageal reflux disease (GERD), according to an analysis involving almost 43,000 women.
Even alongside therapy with a proton-pump inhibitor (PPI) and/or a histamine-receptor antagonist (H2RA), adherence to five antireflux lifestyle factors had a meaningful impact on risk for GERD symptoms, possibly preventing nearly 40% of cases with weekly GERD symptoms, reported lead author Raaj S. Mehta, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues.
“Clinicians recommend dietary and lifestyle modifications to prevent GERD symptoms, but no prospective data are available to inform these recommendations,” Dr. Mehta and colleagues wrote in JAMA Internal Medicine.
To address this gap, the investigators turned to the Nurses’ Health Study II, a nationwide, prospective study involving 116,671 women. The study, which has a follow-up rate exceeding 90%, began in 1989 and is ongoing. Participants complete biennial questionnaires that include a variety of health and lifestyle factors. In 2005, 2009, 2013, and 2017, the questionnaire inquired about heartburn or acid reflux.
The present analysis included data from 42,955 women aged 42-62 years. Participants were excluded at baseline if they had cancer, lacked dietary data, were lost to follow-up, already had GERD symptoms at least weekly, or used a PPI and/or H2RA on a regular basis. The final dataset included 392,215 person-years of follow-up, with 9,291 incident cases of GERD symptoms.
For each participant, the presence of five possible antireflux lifestyle factors were added together for a score ranging from 0 to 5: no more than two cups of soda, tea, or coffee per day; never smoking; normal body weight (BMI ≥18.5 and <25.0 kg/m2); “prudent” diet, based on top 40% of dietary pattern score; and at least 30 minutes of moderate to vigorous physical activity each day.
Multivariate logistic regression modeling showed that women who reported all five antireflux lifestyle factors had a 50% decreased risk of GERD symptoms (hazard ratio, 0.50; 95% confidence interval, 0.42-0.59), compared with women who adhered to none of them. Further analysis suggested that the collective effect of all five factors could reduce GERD symptom case volume by 37% (95% CI, 28%-46%).
Nonadherence to each antireflux lifestyle factor was independently associated with an increased risk of GERD symptoms. After mutual adjustment for other variables, BMI was associated with the highest population-attributable risk (19%), followed by physical activity (8%), food intake (7%), beverage intake (4%), and nonsmoker status (3%).
Dr. Mehta and colleagues also explored the relationship between GERD symptoms, antireflux medications, and lifestyle factors. Presence of all five antireflux factors was associated with a 53% decreased risk of GERD symptoms or initiation of PPI and/or H2RA therapy (HR, 0.47; 95% CI, 0.41-0.54). Among a group of 3,625 women who reported regular use of a PPI and/or H2RA and were free of GERD symptoms at baseline, adherence to all five lifestyle factors reduced risk of GERD symptoms by 68% (HR, 0.32; 95% CI, 0.18-0.57).
One limitation of the study was that its population was primarily White women; however, the authors noted a study suggesting GERD is more common in White women aged 30-60 years.
“Adherence to an antireflux lifestyle, even among regular users of PPIs and/or H2RAs, was associated with a decreased risk of GERD symptoms,” the investigators concluded.
Lifestyle matters
According to Ronnie Fass, MD, medical director of the Digestive Health Center at Case Western Reserve University, Cleveland, “This is the first study to show the incremental effect and thus the benefit of lifestyle factors in reducing the risk of GERD symptoms. While only five lifestyle factors were assessed in this study, potentially others may further decrease the risk for symptoms.”
Dr. Fass suggested that the nature of the data, which was self-reported, and the entirely female patient population, should inform interpretation of the findings.
“While nonerosive reflux disease is relatively more common in women, erosive esophagitis and Barrett’s esophagus are more common in men,” he said. “Furthermore, male gender is associated with more severe GERD and GERD complications.”
Yet Dr. Fass concluded by again emphasizing the merit of the analysis: “This is an important study that further supports the value of certain lifestyle factors in reducing the risk of GERD symptoms,” he said. “What is challenging for practicing physicians is to get patients to follow these lifestyle factors long term.”
The study was funded by the National Institutes of Health and by a Stuart and Suzanne Steele Massachusetts General Hospital Research Scholar Award. The investigators and Dr. Fass disclosed no conflicts of interest.
Antireflux lifestyle factors may significantly reduce the risk of gastroesophageal reflux disease (GERD), according to an analysis involving almost 43,000 women.
Even alongside therapy with a proton-pump inhibitor (PPI) and/or a histamine-receptor antagonist (H2RA), adherence to five antireflux lifestyle factors had a meaningful impact on risk for GERD symptoms, possibly preventing nearly 40% of cases with weekly GERD symptoms, reported lead author Raaj S. Mehta, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues.
“Clinicians recommend dietary and lifestyle modifications to prevent GERD symptoms, but no prospective data are available to inform these recommendations,” Dr. Mehta and colleagues wrote in JAMA Internal Medicine.
To address this gap, the investigators turned to the Nurses’ Health Study II, a nationwide, prospective study involving 116,671 women. The study, which has a follow-up rate exceeding 90%, began in 1989 and is ongoing. Participants complete biennial questionnaires that include a variety of health and lifestyle factors. In 2005, 2009, 2013, and 2017, the questionnaire inquired about heartburn or acid reflux.
The present analysis included data from 42,955 women aged 42-62 years. Participants were excluded at baseline if they had cancer, lacked dietary data, were lost to follow-up, already had GERD symptoms at least weekly, or used a PPI and/or H2RA on a regular basis. The final dataset included 392,215 person-years of follow-up, with 9,291 incident cases of GERD symptoms.
For each participant, the presence of five possible antireflux lifestyle factors were added together for a score ranging from 0 to 5: no more than two cups of soda, tea, or coffee per day; never smoking; normal body weight (BMI ≥18.5 and <25.0 kg/m2); “prudent” diet, based on top 40% of dietary pattern score; and at least 30 minutes of moderate to vigorous physical activity each day.
Multivariate logistic regression modeling showed that women who reported all five antireflux lifestyle factors had a 50% decreased risk of GERD symptoms (hazard ratio, 0.50; 95% confidence interval, 0.42-0.59), compared with women who adhered to none of them. Further analysis suggested that the collective effect of all five factors could reduce GERD symptom case volume by 37% (95% CI, 28%-46%).
Nonadherence to each antireflux lifestyle factor was independently associated with an increased risk of GERD symptoms. After mutual adjustment for other variables, BMI was associated with the highest population-attributable risk (19%), followed by physical activity (8%), food intake (7%), beverage intake (4%), and nonsmoker status (3%).
Dr. Mehta and colleagues also explored the relationship between GERD symptoms, antireflux medications, and lifestyle factors. Presence of all five antireflux factors was associated with a 53% decreased risk of GERD symptoms or initiation of PPI and/or H2RA therapy (HR, 0.47; 95% CI, 0.41-0.54). Among a group of 3,625 women who reported regular use of a PPI and/or H2RA and were free of GERD symptoms at baseline, adherence to all five lifestyle factors reduced risk of GERD symptoms by 68% (HR, 0.32; 95% CI, 0.18-0.57).
One limitation of the study was that its population was primarily White women; however, the authors noted a study suggesting GERD is more common in White women aged 30-60 years.
“Adherence to an antireflux lifestyle, even among regular users of PPIs and/or H2RAs, was associated with a decreased risk of GERD symptoms,” the investigators concluded.
Lifestyle matters
According to Ronnie Fass, MD, medical director of the Digestive Health Center at Case Western Reserve University, Cleveland, “This is the first study to show the incremental effect and thus the benefit of lifestyle factors in reducing the risk of GERD symptoms. While only five lifestyle factors were assessed in this study, potentially others may further decrease the risk for symptoms.”
Dr. Fass suggested that the nature of the data, which was self-reported, and the entirely female patient population, should inform interpretation of the findings.
“While nonerosive reflux disease is relatively more common in women, erosive esophagitis and Barrett’s esophagus are more common in men,” he said. “Furthermore, male gender is associated with more severe GERD and GERD complications.”
Yet Dr. Fass concluded by again emphasizing the merit of the analysis: “This is an important study that further supports the value of certain lifestyle factors in reducing the risk of GERD symptoms,” he said. “What is challenging for practicing physicians is to get patients to follow these lifestyle factors long term.”
The study was funded by the National Institutes of Health and by a Stuart and Suzanne Steele Massachusetts General Hospital Research Scholar Award. The investigators and Dr. Fass disclosed no conflicts of interest.
FROM JAMA INTERNAL MEDICINE