User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
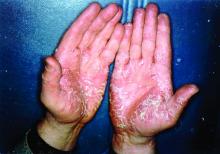
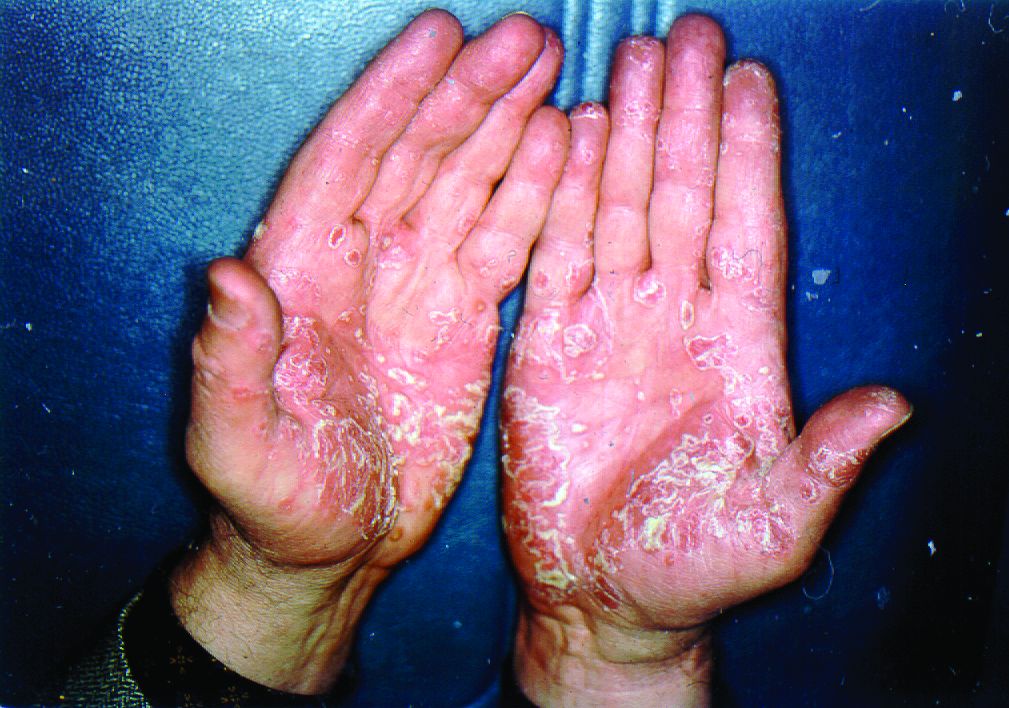
Pandemic puts patients with psoriatic disease off seeking medical help
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
FROM CARC 2021
Oxford launches COVID-19 vaccine study in children
Oxford University is starting a COVID-19 vaccine study with children and young adults aged between 6 and 17 years.
At Oxford and three partner sites in London, Southampton, and Bristol, the phase 2 clinical trial will test whether kids and teens have a good immune response to the AstraZeneca vaccine. Previous trials have shown that the shot is safe in children.
“While most children are relatively unaffected by coronavirus and are unlikely to become unwell with the infection, it is important to establish the safety and immune response to the vaccine in children and young people as some children may benefit from vaccination,” Andrew Pollard, PhD, the chief investigator for the trial and a professor of pediatric infection and immunity at Oxford, said in a statement.
The new trial will enroll 300 volunteers, with up to 240 receiving the vaccine. The control group will receive a meningitis vaccine, which is safe in children and produces similar side effects to the COVID-19 vaccine, such as a sore arm.
COVID-19 vaccine trials have included children over age 12, so this marks the youngest group to be tested so far. Pfizer, Moderna, and Janssen have announced plans to start trials in younger children this spring, according to the Washington Post. Widespread vaccination in children likely won’t occur until 2022, the newspaper reported.
The trial launched on Feb. 12, and the first vaccinations are expected by the end of the month. Parents can visit Oxford’s COVID-19 Vaccine Trial website to sign their children up for the study.
“This study will play an important role in helping to protect children in the future,” Grace Li, a pediatric clinical research fellow for the Oxford Vaccine Group, said in the statement.
“We’ve already seen that the vaccine is safe and effective in adults, and our understanding of how children are affected by the coronavirus continues to evolve,” she said.
A version of this article first appeared on WebMD.com.
Oxford University is starting a COVID-19 vaccine study with children and young adults aged between 6 and 17 years.
At Oxford and three partner sites in London, Southampton, and Bristol, the phase 2 clinical trial will test whether kids and teens have a good immune response to the AstraZeneca vaccine. Previous trials have shown that the shot is safe in children.
“While most children are relatively unaffected by coronavirus and are unlikely to become unwell with the infection, it is important to establish the safety and immune response to the vaccine in children and young people as some children may benefit from vaccination,” Andrew Pollard, PhD, the chief investigator for the trial and a professor of pediatric infection and immunity at Oxford, said in a statement.
The new trial will enroll 300 volunteers, with up to 240 receiving the vaccine. The control group will receive a meningitis vaccine, which is safe in children and produces similar side effects to the COVID-19 vaccine, such as a sore arm.
COVID-19 vaccine trials have included children over age 12, so this marks the youngest group to be tested so far. Pfizer, Moderna, and Janssen have announced plans to start trials in younger children this spring, according to the Washington Post. Widespread vaccination in children likely won’t occur until 2022, the newspaper reported.
The trial launched on Feb. 12, and the first vaccinations are expected by the end of the month. Parents can visit Oxford’s COVID-19 Vaccine Trial website to sign their children up for the study.
“This study will play an important role in helping to protect children in the future,” Grace Li, a pediatric clinical research fellow for the Oxford Vaccine Group, said in the statement.
“We’ve already seen that the vaccine is safe and effective in adults, and our understanding of how children are affected by the coronavirus continues to evolve,” she said.
A version of this article first appeared on WebMD.com.
Oxford University is starting a COVID-19 vaccine study with children and young adults aged between 6 and 17 years.
At Oxford and three partner sites in London, Southampton, and Bristol, the phase 2 clinical trial will test whether kids and teens have a good immune response to the AstraZeneca vaccine. Previous trials have shown that the shot is safe in children.
“While most children are relatively unaffected by coronavirus and are unlikely to become unwell with the infection, it is important to establish the safety and immune response to the vaccine in children and young people as some children may benefit from vaccination,” Andrew Pollard, PhD, the chief investigator for the trial and a professor of pediatric infection and immunity at Oxford, said in a statement.
The new trial will enroll 300 volunteers, with up to 240 receiving the vaccine. The control group will receive a meningitis vaccine, which is safe in children and produces similar side effects to the COVID-19 vaccine, such as a sore arm.
COVID-19 vaccine trials have included children over age 12, so this marks the youngest group to be tested so far. Pfizer, Moderna, and Janssen have announced plans to start trials in younger children this spring, according to the Washington Post. Widespread vaccination in children likely won’t occur until 2022, the newspaper reported.
The trial launched on Feb. 12, and the first vaccinations are expected by the end of the month. Parents can visit Oxford’s COVID-19 Vaccine Trial website to sign their children up for the study.
“This study will play an important role in helping to protect children in the future,” Grace Li, a pediatric clinical research fellow for the Oxford Vaccine Group, said in the statement.
“We’ve already seen that the vaccine is safe and effective in adults, and our understanding of how children are affected by the coronavirus continues to evolve,” she said.
A version of this article first appeared on WebMD.com.
Long-term CPAP use linked with more physical activity
in new research.
“The aim of this study was to determine whether long-term CPAP treatment affects self-reported physical activity among participants with moderate-severe OSA and comorbid CV disease,” wrote David Stevens, PhD, of Flinders University, Adelaide, Australia, and his colleagues. The findings were recently published in the Journal of Clinical Sleep Medicine.
Researchers conducted a secondary analysis of the Sleep apnea cardiovascular endpoints (SAVE) trial that enrolled 2,687 adults aged 45-75 years old with OSA and confirmed CVD. In the study, participants were randomized to receive either CPAP plus usual care or usual care alone.
Physical activity levels were self-reported using the Leisure-Time Exercise Questionnaire (LTEQ) at baseline and at 6-, 24-, and 48-month follow-up intervals. The physical functioning subscale of the 36-item short form questionnaire (SF-36) was used to determine if activity levels were consistent with expert recommendations and to evaluate the effects on any self-perceived limitation of physical activity.
Moderate physical activity was higher among CPAP users
After a mean follow-up duration of 3.7 years, participants in the CPAP arm had approximately 20% higher levels of moderate physical activity, compared with the control arm (adjusted mean scores]: 8.7 points vs. 7.3 points; 95% confidence interval, 7.5-9.9 vs. 6.1-8.5; P = .003).
However, no significant difference was observed between treatment arms for mild physical activity (adjusted mean scores, 14.4 points vs. 14.2 points; 95% CI, 13.5-15.3 vs. 13.3-15.1; P = 0.599) or vigorous physical activity (adjusted mean scores, 3.4 points vs. 2.9 points; 95% CI 2.6-4.2 vs. 2.1-3.7; P = .125).
In addition, participants in the CPAP group reported less limitation in physical activity (adjusted between-group difference in SF-36 physical functioning subscale score = 1.66; 95% CI, 0.87-2.45; P < .001) and were more likely to report activity levels consistent with guideline recommendations.
“We were pleasantly surprised to find that people assigned to CPAP reported more physical activity than their counterparts who received usual care, despite being given no specific exercise instructions,” Kelly A. Loffler, PhD, a coauthor of the study, said in an interview.
“While I don’t think this will result in any immediate changes to guidelines, it is a helpful reminder to clinicians who are treating such patients, that the symptomatic benefits people experience with CPAP present a window of opportunity to improve health more holistically,” Dr. Loffler explained.
The researchers acknowledged that a key limitation of the study was the use of self-reported outcome measures. In future studies, they recommended that recent technological innovations, such as the availability of activity tracking devices, should be used to measure physical activity.
They also noted that patients with excessive sleepiness and severe hypoxemia were excluded from the SAVE trial; thus, the findings may not be generalizable to all patients.
Study reinforces CPAP’s health benefits
Emerson M. Wickwire, PhD, associate professor of psychiatry and medicine at the University of Maryland, Baltimore, explained that CPAP treatment is associated with well-documented health benefits among patients with CVD, as well as enhanced quality of life.
“These results provide further evidence that treating OSA can provide direct and indirect health benefits, suggesting that increased physical activity can be a vital pathway to improved cardiovascular health and enjoyment of life,” Dr. Wickwire, who is also director of the Insomnia Program at the University of Maryland Midtown Medical Center, Baltimore, said in an interview.
Steven M. Scharf, MD, a pulmonologist who is director of the Sleep Disorders Center (Adults) at the University of Maryland, also said the study findings were consistent with previous research involving patients treated for OSA.
“It is no surprise that treatment of OSA improves patient’s daily physical functioning,” explained Dr. Scharf, who is also a clinical professor, in an interview. “These results are expected, but very welcome, and I was glad to see them.”
The study was funded by the National Health and Medical Research Council of Australia, the Respironics Sleep and Respiratory Research Foundation, and Philips Respironics. Some authors reported financial affiliations with medical device and pharmaceutical companies. Dr. Loffler, Dr. Wickwire, and Dr. Scharf reported no conflicts of interest related to this work.
in new research.
“The aim of this study was to determine whether long-term CPAP treatment affects self-reported physical activity among participants with moderate-severe OSA and comorbid CV disease,” wrote David Stevens, PhD, of Flinders University, Adelaide, Australia, and his colleagues. The findings were recently published in the Journal of Clinical Sleep Medicine.
Researchers conducted a secondary analysis of the Sleep apnea cardiovascular endpoints (SAVE) trial that enrolled 2,687 adults aged 45-75 years old with OSA and confirmed CVD. In the study, participants were randomized to receive either CPAP plus usual care or usual care alone.
Physical activity levels were self-reported using the Leisure-Time Exercise Questionnaire (LTEQ) at baseline and at 6-, 24-, and 48-month follow-up intervals. The physical functioning subscale of the 36-item short form questionnaire (SF-36) was used to determine if activity levels were consistent with expert recommendations and to evaluate the effects on any self-perceived limitation of physical activity.
Moderate physical activity was higher among CPAP users
After a mean follow-up duration of 3.7 years, participants in the CPAP arm had approximately 20% higher levels of moderate physical activity, compared with the control arm (adjusted mean scores]: 8.7 points vs. 7.3 points; 95% confidence interval, 7.5-9.9 vs. 6.1-8.5; P = .003).
However, no significant difference was observed between treatment arms for mild physical activity (adjusted mean scores, 14.4 points vs. 14.2 points; 95% CI, 13.5-15.3 vs. 13.3-15.1; P = 0.599) or vigorous physical activity (adjusted mean scores, 3.4 points vs. 2.9 points; 95% CI 2.6-4.2 vs. 2.1-3.7; P = .125).
In addition, participants in the CPAP group reported less limitation in physical activity (adjusted between-group difference in SF-36 physical functioning subscale score = 1.66; 95% CI, 0.87-2.45; P < .001) and were more likely to report activity levels consistent with guideline recommendations.
“We were pleasantly surprised to find that people assigned to CPAP reported more physical activity than their counterparts who received usual care, despite being given no specific exercise instructions,” Kelly A. Loffler, PhD, a coauthor of the study, said in an interview.
“While I don’t think this will result in any immediate changes to guidelines, it is a helpful reminder to clinicians who are treating such patients, that the symptomatic benefits people experience with CPAP present a window of opportunity to improve health more holistically,” Dr. Loffler explained.
The researchers acknowledged that a key limitation of the study was the use of self-reported outcome measures. In future studies, they recommended that recent technological innovations, such as the availability of activity tracking devices, should be used to measure physical activity.
They also noted that patients with excessive sleepiness and severe hypoxemia were excluded from the SAVE trial; thus, the findings may not be generalizable to all patients.
Study reinforces CPAP’s health benefits
Emerson M. Wickwire, PhD, associate professor of psychiatry and medicine at the University of Maryland, Baltimore, explained that CPAP treatment is associated with well-documented health benefits among patients with CVD, as well as enhanced quality of life.
“These results provide further evidence that treating OSA can provide direct and indirect health benefits, suggesting that increased physical activity can be a vital pathway to improved cardiovascular health and enjoyment of life,” Dr. Wickwire, who is also director of the Insomnia Program at the University of Maryland Midtown Medical Center, Baltimore, said in an interview.
Steven M. Scharf, MD, a pulmonologist who is director of the Sleep Disorders Center (Adults) at the University of Maryland, also said the study findings were consistent with previous research involving patients treated for OSA.
“It is no surprise that treatment of OSA improves patient’s daily physical functioning,” explained Dr. Scharf, who is also a clinical professor, in an interview. “These results are expected, but very welcome, and I was glad to see them.”
The study was funded by the National Health and Medical Research Council of Australia, the Respironics Sleep and Respiratory Research Foundation, and Philips Respironics. Some authors reported financial affiliations with medical device and pharmaceutical companies. Dr. Loffler, Dr. Wickwire, and Dr. Scharf reported no conflicts of interest related to this work.
in new research.
“The aim of this study was to determine whether long-term CPAP treatment affects self-reported physical activity among participants with moderate-severe OSA and comorbid CV disease,” wrote David Stevens, PhD, of Flinders University, Adelaide, Australia, and his colleagues. The findings were recently published in the Journal of Clinical Sleep Medicine.
Researchers conducted a secondary analysis of the Sleep apnea cardiovascular endpoints (SAVE) trial that enrolled 2,687 adults aged 45-75 years old with OSA and confirmed CVD. In the study, participants were randomized to receive either CPAP plus usual care or usual care alone.
Physical activity levels were self-reported using the Leisure-Time Exercise Questionnaire (LTEQ) at baseline and at 6-, 24-, and 48-month follow-up intervals. The physical functioning subscale of the 36-item short form questionnaire (SF-36) was used to determine if activity levels were consistent with expert recommendations and to evaluate the effects on any self-perceived limitation of physical activity.
Moderate physical activity was higher among CPAP users
After a mean follow-up duration of 3.7 years, participants in the CPAP arm had approximately 20% higher levels of moderate physical activity, compared with the control arm (adjusted mean scores]: 8.7 points vs. 7.3 points; 95% confidence interval, 7.5-9.9 vs. 6.1-8.5; P = .003).
However, no significant difference was observed between treatment arms for mild physical activity (adjusted mean scores, 14.4 points vs. 14.2 points; 95% CI, 13.5-15.3 vs. 13.3-15.1; P = 0.599) or vigorous physical activity (adjusted mean scores, 3.4 points vs. 2.9 points; 95% CI 2.6-4.2 vs. 2.1-3.7; P = .125).
In addition, participants in the CPAP group reported less limitation in physical activity (adjusted between-group difference in SF-36 physical functioning subscale score = 1.66; 95% CI, 0.87-2.45; P < .001) and were more likely to report activity levels consistent with guideline recommendations.
“We were pleasantly surprised to find that people assigned to CPAP reported more physical activity than their counterparts who received usual care, despite being given no specific exercise instructions,” Kelly A. Loffler, PhD, a coauthor of the study, said in an interview.
“While I don’t think this will result in any immediate changes to guidelines, it is a helpful reminder to clinicians who are treating such patients, that the symptomatic benefits people experience with CPAP present a window of opportunity to improve health more holistically,” Dr. Loffler explained.
The researchers acknowledged that a key limitation of the study was the use of self-reported outcome measures. In future studies, they recommended that recent technological innovations, such as the availability of activity tracking devices, should be used to measure physical activity.
They also noted that patients with excessive sleepiness and severe hypoxemia were excluded from the SAVE trial; thus, the findings may not be generalizable to all patients.
Study reinforces CPAP’s health benefits
Emerson M. Wickwire, PhD, associate professor of psychiatry and medicine at the University of Maryland, Baltimore, explained that CPAP treatment is associated with well-documented health benefits among patients with CVD, as well as enhanced quality of life.
“These results provide further evidence that treating OSA can provide direct and indirect health benefits, suggesting that increased physical activity can be a vital pathway to improved cardiovascular health and enjoyment of life,” Dr. Wickwire, who is also director of the Insomnia Program at the University of Maryland Midtown Medical Center, Baltimore, said in an interview.
Steven M. Scharf, MD, a pulmonologist who is director of the Sleep Disorders Center (Adults) at the University of Maryland, also said the study findings were consistent with previous research involving patients treated for OSA.
“It is no surprise that treatment of OSA improves patient’s daily physical functioning,” explained Dr. Scharf, who is also a clinical professor, in an interview. “These results are expected, but very welcome, and I was glad to see them.”
The study was funded by the National Health and Medical Research Council of Australia, the Respironics Sleep and Respiratory Research Foundation, and Philips Respironics. Some authors reported financial affiliations with medical device and pharmaceutical companies. Dr. Loffler, Dr. Wickwire, and Dr. Scharf reported no conflicts of interest related to this work.
FROM JOURNAL OF CLINICAL SLEEP MEDICINE
Large study finds trans men on testosterone at risk for blood clots
Over 10% of transgender men (females transitioning to male) who take testosterone develop high hematocrit levels that could put them at greater risk for a thrombotic event, and the largest increase in levels occurs in the first year after starting therapy, a new Dutch study indicates.
Erythrocytosis, defined as a hematocrit greater than 0.50 L/L, is a potentially serious side effect of testosterone therapy, say Milou Cecilia Madsen, MD, and colleagues in their article published online Feb. 18, 2021, in the Journal of Clinical Endocrinology & Metabolism.
When hematocrit was measured twice, 11.1% of the cohort of 1073 trans men had levels in excess of 0.50 L/L over a 20-year follow-up.
“Erythrocytosis is common in transgender men treated with testosterone, especially in those who smoke, have [a] high BMI [body mass index], and [who] use testosterone injections,” Dr. Madsen, of the VU University Medical Center Amsterdam, said in a statement from the Endocrine Society.
“A reasonable first step in the care of transgender men with high red blood cells while on testosterone is to advise them to quit smoking, switch injectable testosterone to gel, and, if BMI is high, to lose weight,” she added.
First large study of testosterone in trans men with 20-year follow-up
Transgender men often undergo testosterone therapy as part of gender-affirming treatment.
Secondary erythrocytosis, a condition where the body makes too many red blood cells, is a common side effect of testosterone therapy that can increase the risk of thrombolic events, heart attack, and stroke, Dr. Madsen and colleagues explained.
This is the first study of a large cohort of trans men taking testosterone therapy followed for up to 20 years. Because of the large sample size, statistical analysis with many determinants could be performed. And because of the long follow-up, a clear time relation between initiation of testosterone therapy and hematocrit could be studied, they noted.
Participants were part of the Amsterdam Cohort of Gender Dysphoria study, a large cohort of individuals seen at the Center of Expertise on Gender Dysphoria at Amsterdam University Medical Center between 1972 and 2015.
Laboratory measurements taken between 2004 and 2018 were available for analysis. Trans men visited the center every 3-6 months during their first year of testosterone therapy and were then monitored every year or every other year.
Long-acting undecanoate injection was associated with the highest risk of a hematocrit level greater than 0.50 L/L, and the risk of erythrocytosis in those who took long-acting intramuscular injections was about threefold higher, compared with testosterone gel (adjusted odds ratio, 3.1).
In contrast, short-acting ester injections and oral administration of testosterone had a similar risk for erythrocytosis, as did testosterone gel.
Other determinants of elevated hematocrit included smoking, medical history of a number of comorbid conditions, and older age on initiation of testosterone.
In contrast, “higher testosterone levels per se were not associated with an increased odds of hematocrit greater than 0.50 L/L”, the authors noted.
Current advice for trans men based on old guidance for hypogonadism
The authors said that current advice for trans men is based on recommendations for testosterone-treated hypogonadal cis men (those assigned male at birth) from 2008, which advises a hematocrit greater than 0.50 L/L has a moderate to high risk of adverse outcome. For levels greater than 0.54 L/L, cessation of testosterone therapy, a dose reduction, or therapeutic phlebotomy to reduce the risk of adverse events is advised. For levels 0.50-0.54 L/L, no clear advice is given.
But questions remain as to whether these guidelines are applicable to trans men because the duration of testosterone therapy is much longer in trans men and hormone treatment often cannot be discontinued without causing distress.
Meanwhile, hematology guidelines indicate an upper limit for hematocrit for cis females of 0.48 L/L.
“It could be argued that the upper limit for cis females should be applied, as trans men are born with female genetics,” the authors said. “This is a subject for further research.”
Duration of testosterone therapy impacts risk of erythrocytosis
In the study, the researchers found that longer duration of testosterone therapy increased the risk of developing hematocrit levels greater than 0.50 L/L. For example, after 1 year, the cumulative incidence of erythrocytosis was 8%; after 10 years, it was 38%; and after 14 years, it was 50%.
Until more specific guidance is developed for trans men, if hematocrit levels rise to 0.50-0.54 L/L, the researchers suggested taking “reasonable” steps to prevent a further increase:
- Consider switching patients who use injectable testosterone to transdermal products.
- Advise patients with a BMI greater than 25 kg/m2 to lose weight to attain a BMI of 18.5-25.
- Advise patients to stop smoking.
- Pursue treatment optimization for chronic lung disease or sleep apnea.
The study had no external funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over 10% of transgender men (females transitioning to male) who take testosterone develop high hematocrit levels that could put them at greater risk for a thrombotic event, and the largest increase in levels occurs in the first year after starting therapy, a new Dutch study indicates.
Erythrocytosis, defined as a hematocrit greater than 0.50 L/L, is a potentially serious side effect of testosterone therapy, say Milou Cecilia Madsen, MD, and colleagues in their article published online Feb. 18, 2021, in the Journal of Clinical Endocrinology & Metabolism.
When hematocrit was measured twice, 11.1% of the cohort of 1073 trans men had levels in excess of 0.50 L/L over a 20-year follow-up.
“Erythrocytosis is common in transgender men treated with testosterone, especially in those who smoke, have [a] high BMI [body mass index], and [who] use testosterone injections,” Dr. Madsen, of the VU University Medical Center Amsterdam, said in a statement from the Endocrine Society.
“A reasonable first step in the care of transgender men with high red blood cells while on testosterone is to advise them to quit smoking, switch injectable testosterone to gel, and, if BMI is high, to lose weight,” she added.
First large study of testosterone in trans men with 20-year follow-up
Transgender men often undergo testosterone therapy as part of gender-affirming treatment.
Secondary erythrocytosis, a condition where the body makes too many red blood cells, is a common side effect of testosterone therapy that can increase the risk of thrombolic events, heart attack, and stroke, Dr. Madsen and colleagues explained.
This is the first study of a large cohort of trans men taking testosterone therapy followed for up to 20 years. Because of the large sample size, statistical analysis with many determinants could be performed. And because of the long follow-up, a clear time relation between initiation of testosterone therapy and hematocrit could be studied, they noted.
Participants were part of the Amsterdam Cohort of Gender Dysphoria study, a large cohort of individuals seen at the Center of Expertise on Gender Dysphoria at Amsterdam University Medical Center between 1972 and 2015.
Laboratory measurements taken between 2004 and 2018 were available for analysis. Trans men visited the center every 3-6 months during their first year of testosterone therapy and were then monitored every year or every other year.
Long-acting undecanoate injection was associated with the highest risk of a hematocrit level greater than 0.50 L/L, and the risk of erythrocytosis in those who took long-acting intramuscular injections was about threefold higher, compared with testosterone gel (adjusted odds ratio, 3.1).
In contrast, short-acting ester injections and oral administration of testosterone had a similar risk for erythrocytosis, as did testosterone gel.
Other determinants of elevated hematocrit included smoking, medical history of a number of comorbid conditions, and older age on initiation of testosterone.
In contrast, “higher testosterone levels per se were not associated with an increased odds of hematocrit greater than 0.50 L/L”, the authors noted.
Current advice for trans men based on old guidance for hypogonadism
The authors said that current advice for trans men is based on recommendations for testosterone-treated hypogonadal cis men (those assigned male at birth) from 2008, which advises a hematocrit greater than 0.50 L/L has a moderate to high risk of adverse outcome. For levels greater than 0.54 L/L, cessation of testosterone therapy, a dose reduction, or therapeutic phlebotomy to reduce the risk of adverse events is advised. For levels 0.50-0.54 L/L, no clear advice is given.
But questions remain as to whether these guidelines are applicable to trans men because the duration of testosterone therapy is much longer in trans men and hormone treatment often cannot be discontinued without causing distress.
Meanwhile, hematology guidelines indicate an upper limit for hematocrit for cis females of 0.48 L/L.
“It could be argued that the upper limit for cis females should be applied, as trans men are born with female genetics,” the authors said. “This is a subject for further research.”
Duration of testosterone therapy impacts risk of erythrocytosis
In the study, the researchers found that longer duration of testosterone therapy increased the risk of developing hematocrit levels greater than 0.50 L/L. For example, after 1 year, the cumulative incidence of erythrocytosis was 8%; after 10 years, it was 38%; and after 14 years, it was 50%.
Until more specific guidance is developed for trans men, if hematocrit levels rise to 0.50-0.54 L/L, the researchers suggested taking “reasonable” steps to prevent a further increase:
- Consider switching patients who use injectable testosterone to transdermal products.
- Advise patients with a BMI greater than 25 kg/m2 to lose weight to attain a BMI of 18.5-25.
- Advise patients to stop smoking.
- Pursue treatment optimization for chronic lung disease or sleep apnea.
The study had no external funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over 10% of transgender men (females transitioning to male) who take testosterone develop high hematocrit levels that could put them at greater risk for a thrombotic event, and the largest increase in levels occurs in the first year after starting therapy, a new Dutch study indicates.
Erythrocytosis, defined as a hematocrit greater than 0.50 L/L, is a potentially serious side effect of testosterone therapy, say Milou Cecilia Madsen, MD, and colleagues in their article published online Feb. 18, 2021, in the Journal of Clinical Endocrinology & Metabolism.
When hematocrit was measured twice, 11.1% of the cohort of 1073 trans men had levels in excess of 0.50 L/L over a 20-year follow-up.
“Erythrocytosis is common in transgender men treated with testosterone, especially in those who smoke, have [a] high BMI [body mass index], and [who] use testosterone injections,” Dr. Madsen, of the VU University Medical Center Amsterdam, said in a statement from the Endocrine Society.
“A reasonable first step in the care of transgender men with high red blood cells while on testosterone is to advise them to quit smoking, switch injectable testosterone to gel, and, if BMI is high, to lose weight,” she added.
First large study of testosterone in trans men with 20-year follow-up
Transgender men often undergo testosterone therapy as part of gender-affirming treatment.
Secondary erythrocytosis, a condition where the body makes too many red blood cells, is a common side effect of testosterone therapy that can increase the risk of thrombolic events, heart attack, and stroke, Dr. Madsen and colleagues explained.
This is the first study of a large cohort of trans men taking testosterone therapy followed for up to 20 years. Because of the large sample size, statistical analysis with many determinants could be performed. And because of the long follow-up, a clear time relation between initiation of testosterone therapy and hematocrit could be studied, they noted.
Participants were part of the Amsterdam Cohort of Gender Dysphoria study, a large cohort of individuals seen at the Center of Expertise on Gender Dysphoria at Amsterdam University Medical Center between 1972 and 2015.
Laboratory measurements taken between 2004 and 2018 were available for analysis. Trans men visited the center every 3-6 months during their first year of testosterone therapy and were then monitored every year or every other year.
Long-acting undecanoate injection was associated with the highest risk of a hematocrit level greater than 0.50 L/L, and the risk of erythrocytosis in those who took long-acting intramuscular injections was about threefold higher, compared with testosterone gel (adjusted odds ratio, 3.1).
In contrast, short-acting ester injections and oral administration of testosterone had a similar risk for erythrocytosis, as did testosterone gel.
Other determinants of elevated hematocrit included smoking, medical history of a number of comorbid conditions, and older age on initiation of testosterone.
In contrast, “higher testosterone levels per se were not associated with an increased odds of hematocrit greater than 0.50 L/L”, the authors noted.
Current advice for trans men based on old guidance for hypogonadism
The authors said that current advice for trans men is based on recommendations for testosterone-treated hypogonadal cis men (those assigned male at birth) from 2008, which advises a hematocrit greater than 0.50 L/L has a moderate to high risk of adverse outcome. For levels greater than 0.54 L/L, cessation of testosterone therapy, a dose reduction, or therapeutic phlebotomy to reduce the risk of adverse events is advised. For levels 0.50-0.54 L/L, no clear advice is given.
But questions remain as to whether these guidelines are applicable to trans men because the duration of testosterone therapy is much longer in trans men and hormone treatment often cannot be discontinued without causing distress.
Meanwhile, hematology guidelines indicate an upper limit for hematocrit for cis females of 0.48 L/L.
“It could be argued that the upper limit for cis females should be applied, as trans men are born with female genetics,” the authors said. “This is a subject for further research.”
Duration of testosterone therapy impacts risk of erythrocytosis
In the study, the researchers found that longer duration of testosterone therapy increased the risk of developing hematocrit levels greater than 0.50 L/L. For example, after 1 year, the cumulative incidence of erythrocytosis was 8%; after 10 years, it was 38%; and after 14 years, it was 50%.
Until more specific guidance is developed for trans men, if hematocrit levels rise to 0.50-0.54 L/L, the researchers suggested taking “reasonable” steps to prevent a further increase:
- Consider switching patients who use injectable testosterone to transdermal products.
- Advise patients with a BMI greater than 25 kg/m2 to lose weight to attain a BMI of 18.5-25.
- Advise patients to stop smoking.
- Pursue treatment optimization for chronic lung disease or sleep apnea.
The study had no external funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pap test/cervical swab samples can reveal ovarian cancer biomarkers
Residual fixatives from liquid-based Pap tests and cervical swabs contain tumor-specific biomarkers for ovarian cancer, according to an analysis of proteins found in matched biospecimens from a woman with high grade serous ovarian cancer.
The findings suggest that Pap test fluid or cervical swabs could be used to detect ovarian cancer biomarker proteins to allow for earlier detection of ovarian cancer, reported Kristin L. M. Boylan, PhD, assistant director of the Ovarian Cancer Early Detection Program at the University of Minnesota, Minneapolis, and colleagues.
The investigators examined the biospecimens from a 72-year-old woman diagnosed with metastatic high-grade serous adenocarcinoma that did not encompass the cervix. The Pap test, obtained prior to surgery, was negative for malignancy, but nearly 5,000 proteins were detected in the three matched biospecimens, including more than 2,000 that were expressed in each of them.
These proteins included several known ovarian cancer biomarkers, such as CA125, HE4, and mesothelin, the investigators noted.
The findings were published online Feb. 9 in Clinical Proteomics.
“Our data demonstrate that ovarian cancer biomarkers can be detected in Pap test fluid or a cervical swab by MS-based proteomics,” the investigators wrote. “In addition to identifying multiple known biomarkers, over 2,000 proteins were detected in all three biospecimens, suggesting a potential role for novel biomarker discovery.”
Proteins from the cell-free supernatant of the patient’s liquid-based Pap test fixative were concentrated by acetone precipitation or eluted from the cervical swab, and protein was also extracted from the patient’s tumor. Analyses showed similarities in the Pap test fluid and cervical swab proteins, as well as the tumor extract.
The findings are notable, because while early detection of ovarian cancer increases survival, an adequately sensitive and specific screening tool for use in the general population is lacking, the investigators explained.
Pap test screening is widely accepted, suggesting that developing it as a screening tool for both cervical and ovarian cancers could improve testing for this “lethal but elusive disease,” they said, addding that “[W]hile our samples were from a single patient, the results are proof of concept: that Pap test fluid or cervical swabs could be used for detection of ovarian cancer biomarker proteins, and this approach warrants further investigation.”
Senior author Amy Skubitz, PhD, professor and director of the Ovarian Cancer Early Detection Program, stated in a press release that she “sees an opportunity for this method to be translated into a self-administered, at-home test, where swabs could be collected by women at home and sent to a central laboratory for analysis of proteins that would diagnose ovarian cancer.”
However, next steps include using quantitative mass spectrometry to determine if the proteins or peptides identified in this analysis are detected at higher levels in ovarian cancer Pap tests or swabs compared to controls.
“Their presence alone is not sufficient for diagnosis,” she stated.
This study was supported by the Minnesota Ovarian Cancer Alliance, the Cancurables Foundation, Charlene’s Light: A Foundation for Ovarian Cancer, and the Department of Defense Ovarian Cancer Research Program Pilot Award. The authors reported having no disclosures.
Residual fixatives from liquid-based Pap tests and cervical swabs contain tumor-specific biomarkers for ovarian cancer, according to an analysis of proteins found in matched biospecimens from a woman with high grade serous ovarian cancer.
The findings suggest that Pap test fluid or cervical swabs could be used to detect ovarian cancer biomarker proteins to allow for earlier detection of ovarian cancer, reported Kristin L. M. Boylan, PhD, assistant director of the Ovarian Cancer Early Detection Program at the University of Minnesota, Minneapolis, and colleagues.
The investigators examined the biospecimens from a 72-year-old woman diagnosed with metastatic high-grade serous adenocarcinoma that did not encompass the cervix. The Pap test, obtained prior to surgery, was negative for malignancy, but nearly 5,000 proteins were detected in the three matched biospecimens, including more than 2,000 that were expressed in each of them.
These proteins included several known ovarian cancer biomarkers, such as CA125, HE4, and mesothelin, the investigators noted.
The findings were published online Feb. 9 in Clinical Proteomics.
“Our data demonstrate that ovarian cancer biomarkers can be detected in Pap test fluid or a cervical swab by MS-based proteomics,” the investigators wrote. “In addition to identifying multiple known biomarkers, over 2,000 proteins were detected in all three biospecimens, suggesting a potential role for novel biomarker discovery.”
Proteins from the cell-free supernatant of the patient’s liquid-based Pap test fixative were concentrated by acetone precipitation or eluted from the cervical swab, and protein was also extracted from the patient’s tumor. Analyses showed similarities in the Pap test fluid and cervical swab proteins, as well as the tumor extract.
The findings are notable, because while early detection of ovarian cancer increases survival, an adequately sensitive and specific screening tool for use in the general population is lacking, the investigators explained.
Pap test screening is widely accepted, suggesting that developing it as a screening tool for both cervical and ovarian cancers could improve testing for this “lethal but elusive disease,” they said, addding that “[W]hile our samples were from a single patient, the results are proof of concept: that Pap test fluid or cervical swabs could be used for detection of ovarian cancer biomarker proteins, and this approach warrants further investigation.”
Senior author Amy Skubitz, PhD, professor and director of the Ovarian Cancer Early Detection Program, stated in a press release that she “sees an opportunity for this method to be translated into a self-administered, at-home test, where swabs could be collected by women at home and sent to a central laboratory for analysis of proteins that would diagnose ovarian cancer.”
However, next steps include using quantitative mass spectrometry to determine if the proteins or peptides identified in this analysis are detected at higher levels in ovarian cancer Pap tests or swabs compared to controls.
“Their presence alone is not sufficient for diagnosis,” she stated.
This study was supported by the Minnesota Ovarian Cancer Alliance, the Cancurables Foundation, Charlene’s Light: A Foundation for Ovarian Cancer, and the Department of Defense Ovarian Cancer Research Program Pilot Award. The authors reported having no disclosures.
Residual fixatives from liquid-based Pap tests and cervical swabs contain tumor-specific biomarkers for ovarian cancer, according to an analysis of proteins found in matched biospecimens from a woman with high grade serous ovarian cancer.
The findings suggest that Pap test fluid or cervical swabs could be used to detect ovarian cancer biomarker proteins to allow for earlier detection of ovarian cancer, reported Kristin L. M. Boylan, PhD, assistant director of the Ovarian Cancer Early Detection Program at the University of Minnesota, Minneapolis, and colleagues.
The investigators examined the biospecimens from a 72-year-old woman diagnosed with metastatic high-grade serous adenocarcinoma that did not encompass the cervix. The Pap test, obtained prior to surgery, was negative for malignancy, but nearly 5,000 proteins were detected in the three matched biospecimens, including more than 2,000 that were expressed in each of them.
These proteins included several known ovarian cancer biomarkers, such as CA125, HE4, and mesothelin, the investigators noted.
The findings were published online Feb. 9 in Clinical Proteomics.
“Our data demonstrate that ovarian cancer biomarkers can be detected in Pap test fluid or a cervical swab by MS-based proteomics,” the investigators wrote. “In addition to identifying multiple known biomarkers, over 2,000 proteins were detected in all three biospecimens, suggesting a potential role for novel biomarker discovery.”
Proteins from the cell-free supernatant of the patient’s liquid-based Pap test fixative were concentrated by acetone precipitation or eluted from the cervical swab, and protein was also extracted from the patient’s tumor. Analyses showed similarities in the Pap test fluid and cervical swab proteins, as well as the tumor extract.
The findings are notable, because while early detection of ovarian cancer increases survival, an adequately sensitive and specific screening tool for use in the general population is lacking, the investigators explained.
Pap test screening is widely accepted, suggesting that developing it as a screening tool for both cervical and ovarian cancers could improve testing for this “lethal but elusive disease,” they said, addding that “[W]hile our samples were from a single patient, the results are proof of concept: that Pap test fluid or cervical swabs could be used for detection of ovarian cancer biomarker proteins, and this approach warrants further investigation.”
Senior author Amy Skubitz, PhD, professor and director of the Ovarian Cancer Early Detection Program, stated in a press release that she “sees an opportunity for this method to be translated into a self-administered, at-home test, where swabs could be collected by women at home and sent to a central laboratory for analysis of proteins that would diagnose ovarian cancer.”
However, next steps include using quantitative mass spectrometry to determine if the proteins or peptides identified in this analysis are detected at higher levels in ovarian cancer Pap tests or swabs compared to controls.
“Their presence alone is not sufficient for diagnosis,” she stated.
This study was supported by the Minnesota Ovarian Cancer Alliance, the Cancurables Foundation, Charlene’s Light: A Foundation for Ovarian Cancer, and the Department of Defense Ovarian Cancer Research Program Pilot Award. The authors reported having no disclosures.
FROM CLINICAL PROTEOMICS
More from DAPA-HF: Dapagliflozin quickly reduces heart failure events
Dapagliflozin’s benefits in patients with heart failure with reduced ejection fraction appeared quickly after treatment began, and patients who had been hospitalized for heart failure within the prior year got the biggest boost from the drug, according to secondary analyses of the more than 4,700-patient DAPA-HF trial.
Dapagliflozin’s significant reduction of the incidence of cardiovascular death or worsening heart failure became apparent in DAPA-HF within 28 days after patients started treatment, by which time those on the study drug had a 49% cut in this combined endpoint, compared with patients on placebo, David D. Berg, MD, and associates said in a recent report published in JAMA Cardiology.
Their analyses also showed that the absolute reduction linked with dapagliflozin treatment for this primary endpoint of the study (which classified worsening heart failure as either hospitalization for heart failure or an urgent visit because of heart failure that required intravenous therapy) was greatest, 10% during 2 years of follow-up, among the roughly one-quarter of enrolled patients who had been hospitalized for heart failure within 12 months of entering the study. Patients previously hospitalized for heart failure more than 12 months before they entered DAPA-HF had a 4% absolute cut in their primary-outcome events during the trial, and those who had never been hospitalized for heart failure had a 2% absolute benefit, compared with placebo, during 2 years of follow-up.
These findings were consistent with the timing of benefits for patients with heart failure with reduced ejection fraction (HFrEF) in recent studies of two other drugs from the same class, the sodium-glucose cotransporter (SGLT) inhibitors, including empagliflozin (Jardiance, which inhibits SGLT-2) in the EMPEROR-Reduced trial, and sotagliflozin (Zynquista, which inhibits both SGLT1 and -2) in the SOLOIST-WHF trial, noted Gregg C. Fonarow, MD, and Clyde W. Yancy, MD, in an editor’s note that accompanied the new report.
The new findings show “the opportunity to expeditiously implement this remarkable class of therapy for HFrEF is now compelling and deserves disruptive efforts to ensure comprehensive treatment and the best patient outcomes,” wrote Dr. Fonarow, a professor of medicine at the University of California, Los Angeles, and Dr. Yancy, a professor of medicine at Northwestern University, Chicago.
But despite these new findings, their exact meaning remains unclear in terms of when to start dapagliflozin (or a different drug from the same class), compared with the other drug classes that have proven highly effective in patients with HFrEF, and exactly how long after hospitalization for heart failure dapagliflozin can safely and effectively begin.
Data needed on starting an SGLT inhibitor soon after hospitalization in patients without diabetes
“DAPA-HF showed that, in patients with or without diabetes, an SGLT2 inhibitor reduced the risk of cardiovascular death or worsening heart failure in patients with stable HFrEF. SOLOIST-WHF looked strictly at patients with diabetes, and showed that a combined SGLT1 and SGLT2 inhibitor could reduce the risk of cardiovascular death or worsening heart failure in patients with recently decompensated heart failure,” Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston, noted in an interview. “What we don’t have is a trial focused exclusively on enrolling patients while hospitalized with acute heart failure, irrespective of whether they have diabetes, and testing the immediate clinical efficacy and safety of starting an SGLT2 inhibitor. That is what we are testing with the ongoing DAPA ACT HF-TIMI 68 trial.”
In addition, updated recommendations from the American College of Cardiology on initiating drug therapy in patients newly diagnosed with HFrEF that appeared in early 2021 promoted a sequence that starts most patients on sacubitril/valsartan (Entresto) and a beta-blocker, followed by a diuretic (when needed), a mineralocorticoid receptor agonist, and then an SGLT inhibitor. The recommendations note that starting a patient on all these drug classes could take 3-6 months.
“There are intense debates about the optimal sequence for introducing these therapies, and I don’t think we have solid data to suggest that one sequence is clearly better than another,” noted Dr. Berg. “A one-size-fits-all approach probably doesn’t make sense. For example, each of these therapies has a different set of effects on heart rate and blood pressure, and each has a unique side effect profile, so clinicians will often need to tailor the treatment approach to the patient. And, of course, cost is an important consideration. Although the optimal time to start an SGLT2 inhibitor remains uncertain, the results of our analysis suggest that waiting may result in preventable adverse heart failure events.”
DAPA-HF randomized 4,744 patients with HFrEF and in New York Heart Association functional class II-IV at 410 sites in 20 countries. The incidence of the primary, combined endpoint fell by 26% with dapagliflozin treatment, compared with placebo, during a median 18-month follow-up. Among the study cohort 27% of patients had been hospitalized for heart failure within a year of their entry, 20% had been hospitalized for heart failure more than 1 year before entry, and 53% had no history of a hospitalization for heart failure.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Berg has received research support through his institution from AstraZeneca. Dr. Fonarow has received personal fees from AstraZeneca and from numerous other companies. Dr. Yancy’s spouse works for Abbott Laboratories.
Dapagliflozin’s benefits in patients with heart failure with reduced ejection fraction appeared quickly after treatment began, and patients who had been hospitalized for heart failure within the prior year got the biggest boost from the drug, according to secondary analyses of the more than 4,700-patient DAPA-HF trial.
Dapagliflozin’s significant reduction of the incidence of cardiovascular death or worsening heart failure became apparent in DAPA-HF within 28 days after patients started treatment, by which time those on the study drug had a 49% cut in this combined endpoint, compared with patients on placebo, David D. Berg, MD, and associates said in a recent report published in JAMA Cardiology.
Their analyses also showed that the absolute reduction linked with dapagliflozin treatment for this primary endpoint of the study (which classified worsening heart failure as either hospitalization for heart failure or an urgent visit because of heart failure that required intravenous therapy) was greatest, 10% during 2 years of follow-up, among the roughly one-quarter of enrolled patients who had been hospitalized for heart failure within 12 months of entering the study. Patients previously hospitalized for heart failure more than 12 months before they entered DAPA-HF had a 4% absolute cut in their primary-outcome events during the trial, and those who had never been hospitalized for heart failure had a 2% absolute benefit, compared with placebo, during 2 years of follow-up.
These findings were consistent with the timing of benefits for patients with heart failure with reduced ejection fraction (HFrEF) in recent studies of two other drugs from the same class, the sodium-glucose cotransporter (SGLT) inhibitors, including empagliflozin (Jardiance, which inhibits SGLT-2) in the EMPEROR-Reduced trial, and sotagliflozin (Zynquista, which inhibits both SGLT1 and -2) in the SOLOIST-WHF trial, noted Gregg C. Fonarow, MD, and Clyde W. Yancy, MD, in an editor’s note that accompanied the new report.
The new findings show “the opportunity to expeditiously implement this remarkable class of therapy for HFrEF is now compelling and deserves disruptive efforts to ensure comprehensive treatment and the best patient outcomes,” wrote Dr. Fonarow, a professor of medicine at the University of California, Los Angeles, and Dr. Yancy, a professor of medicine at Northwestern University, Chicago.
But despite these new findings, their exact meaning remains unclear in terms of when to start dapagliflozin (or a different drug from the same class), compared with the other drug classes that have proven highly effective in patients with HFrEF, and exactly how long after hospitalization for heart failure dapagliflozin can safely and effectively begin.
Data needed on starting an SGLT inhibitor soon after hospitalization in patients without diabetes
“DAPA-HF showed that, in patients with or without diabetes, an SGLT2 inhibitor reduced the risk of cardiovascular death or worsening heart failure in patients with stable HFrEF. SOLOIST-WHF looked strictly at patients with diabetes, and showed that a combined SGLT1 and SGLT2 inhibitor could reduce the risk of cardiovascular death or worsening heart failure in patients with recently decompensated heart failure,” Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston, noted in an interview. “What we don’t have is a trial focused exclusively on enrolling patients while hospitalized with acute heart failure, irrespective of whether they have diabetes, and testing the immediate clinical efficacy and safety of starting an SGLT2 inhibitor. That is what we are testing with the ongoing DAPA ACT HF-TIMI 68 trial.”
In addition, updated recommendations from the American College of Cardiology on initiating drug therapy in patients newly diagnosed with HFrEF that appeared in early 2021 promoted a sequence that starts most patients on sacubitril/valsartan (Entresto) and a beta-blocker, followed by a diuretic (when needed), a mineralocorticoid receptor agonist, and then an SGLT inhibitor. The recommendations note that starting a patient on all these drug classes could take 3-6 months.
“There are intense debates about the optimal sequence for introducing these therapies, and I don’t think we have solid data to suggest that one sequence is clearly better than another,” noted Dr. Berg. “A one-size-fits-all approach probably doesn’t make sense. For example, each of these therapies has a different set of effects on heart rate and blood pressure, and each has a unique side effect profile, so clinicians will often need to tailor the treatment approach to the patient. And, of course, cost is an important consideration. Although the optimal time to start an SGLT2 inhibitor remains uncertain, the results of our analysis suggest that waiting may result in preventable adverse heart failure events.”
DAPA-HF randomized 4,744 patients with HFrEF and in New York Heart Association functional class II-IV at 410 sites in 20 countries. The incidence of the primary, combined endpoint fell by 26% with dapagliflozin treatment, compared with placebo, during a median 18-month follow-up. Among the study cohort 27% of patients had been hospitalized for heart failure within a year of their entry, 20% had been hospitalized for heart failure more than 1 year before entry, and 53% had no history of a hospitalization for heart failure.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Berg has received research support through his institution from AstraZeneca. Dr. Fonarow has received personal fees from AstraZeneca and from numerous other companies. Dr. Yancy’s spouse works for Abbott Laboratories.
Dapagliflozin’s benefits in patients with heart failure with reduced ejection fraction appeared quickly after treatment began, and patients who had been hospitalized for heart failure within the prior year got the biggest boost from the drug, according to secondary analyses of the more than 4,700-patient DAPA-HF trial.
Dapagliflozin’s significant reduction of the incidence of cardiovascular death or worsening heart failure became apparent in DAPA-HF within 28 days after patients started treatment, by which time those on the study drug had a 49% cut in this combined endpoint, compared with patients on placebo, David D. Berg, MD, and associates said in a recent report published in JAMA Cardiology.
Their analyses also showed that the absolute reduction linked with dapagliflozin treatment for this primary endpoint of the study (which classified worsening heart failure as either hospitalization for heart failure or an urgent visit because of heart failure that required intravenous therapy) was greatest, 10% during 2 years of follow-up, among the roughly one-quarter of enrolled patients who had been hospitalized for heart failure within 12 months of entering the study. Patients previously hospitalized for heart failure more than 12 months before they entered DAPA-HF had a 4% absolute cut in their primary-outcome events during the trial, and those who had never been hospitalized for heart failure had a 2% absolute benefit, compared with placebo, during 2 years of follow-up.
These findings were consistent with the timing of benefits for patients with heart failure with reduced ejection fraction (HFrEF) in recent studies of two other drugs from the same class, the sodium-glucose cotransporter (SGLT) inhibitors, including empagliflozin (Jardiance, which inhibits SGLT-2) in the EMPEROR-Reduced trial, and sotagliflozin (Zynquista, which inhibits both SGLT1 and -2) in the SOLOIST-WHF trial, noted Gregg C. Fonarow, MD, and Clyde W. Yancy, MD, in an editor’s note that accompanied the new report.
The new findings show “the opportunity to expeditiously implement this remarkable class of therapy for HFrEF is now compelling and deserves disruptive efforts to ensure comprehensive treatment and the best patient outcomes,” wrote Dr. Fonarow, a professor of medicine at the University of California, Los Angeles, and Dr. Yancy, a professor of medicine at Northwestern University, Chicago.
But despite these new findings, their exact meaning remains unclear in terms of when to start dapagliflozin (or a different drug from the same class), compared with the other drug classes that have proven highly effective in patients with HFrEF, and exactly how long after hospitalization for heart failure dapagliflozin can safely and effectively begin.
Data needed on starting an SGLT inhibitor soon after hospitalization in patients without diabetes
“DAPA-HF showed that, in patients with or without diabetes, an SGLT2 inhibitor reduced the risk of cardiovascular death or worsening heart failure in patients with stable HFrEF. SOLOIST-WHF looked strictly at patients with diabetes, and showed that a combined SGLT1 and SGLT2 inhibitor could reduce the risk of cardiovascular death or worsening heart failure in patients with recently decompensated heart failure,” Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston, noted in an interview. “What we don’t have is a trial focused exclusively on enrolling patients while hospitalized with acute heart failure, irrespective of whether they have diabetes, and testing the immediate clinical efficacy and safety of starting an SGLT2 inhibitor. That is what we are testing with the ongoing DAPA ACT HF-TIMI 68 trial.”
In addition, updated recommendations from the American College of Cardiology on initiating drug therapy in patients newly diagnosed with HFrEF that appeared in early 2021 promoted a sequence that starts most patients on sacubitril/valsartan (Entresto) and a beta-blocker, followed by a diuretic (when needed), a mineralocorticoid receptor agonist, and then an SGLT inhibitor. The recommendations note that starting a patient on all these drug classes could take 3-6 months.
“There are intense debates about the optimal sequence for introducing these therapies, and I don’t think we have solid data to suggest that one sequence is clearly better than another,” noted Dr. Berg. “A one-size-fits-all approach probably doesn’t make sense. For example, each of these therapies has a different set of effects on heart rate and blood pressure, and each has a unique side effect profile, so clinicians will often need to tailor the treatment approach to the patient. And, of course, cost is an important consideration. Although the optimal time to start an SGLT2 inhibitor remains uncertain, the results of our analysis suggest that waiting may result in preventable adverse heart failure events.”
DAPA-HF randomized 4,744 patients with HFrEF and in New York Heart Association functional class II-IV at 410 sites in 20 countries. The incidence of the primary, combined endpoint fell by 26% with dapagliflozin treatment, compared with placebo, during a median 18-month follow-up. Among the study cohort 27% of patients had been hospitalized for heart failure within a year of their entry, 20% had been hospitalized for heart failure more than 1 year before entry, and 53% had no history of a hospitalization for heart failure.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Berg has received research support through his institution from AstraZeneca. Dr. Fonarow has received personal fees from AstraZeneca and from numerous other companies. Dr. Yancy’s spouse works for Abbott Laboratories.
FROM JAMA CARDIOLOGY
ASDSA warns of rogue insulin pen use for DIY fillers
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
Ivabradine knocks down heart rate, symptoms in POTS
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
Goldenseal may interfere with metformin absorption, jeopardizing glucose control
Goldenseal, a natural botanical product, may interfere with intestinal absorption of metformin, potentially compromising blood glucose control in patients with type 2 diabetes, according to investigators.
The study, which tested for interactions between goldenseal and several drugs in healthy volunteers, reveals that current models for predicting transporter-mediated drug-drug interactions may be insufficient to screen commonly used dietary supplements, reported lead investigator James T. Nguyen, PharmD, a PhD candidate at Washington State University, Spokane, and colleagues.
“Supplements containing goldenseal ... a perennial herb native to North America, have consistently ranked among the top 20 highest selling natural products during the last decade,” the investigators wrote in Clinical Pharmacology & Therapeutics . “As more patients continue to seek goldenseal and other natural products to self-treat their medical conditions, there is an increasing need to characterize their safety profiles, especially when co-consumed with prescribed medications, which can lead to adverse natural product-drug interactions.”
Previous clinical studies have shown that goldenseal inhibits cytochrome P450, with one study showing a roughly 40% increase in systemic midazolam exposure via CYP3A inhibition, “suggesting goldenseal could have prolonged inhibitory effects in vivo similar to grapefruit juice,” the investigators wrote.
Clinical and in vitro results for goldenseal-transporter interactions have been mixed, the investigators noted, specifically for P-glycoprotein, while other transporters remain clinically untested.
“Likewise, the effects of [goldenseal alkaloids], all of which are time-dependent inhibitors of CYP3A and/or CYP2D6, have not been tested on transporter function,” the investigators wrote.
To address this knowledge gap, the investigators first performed in vitro transporter inhibition assays and in vitro–in vivo predictions involving goldenseal, plus the alkaloids berberine, (−)-beta-hydrastine, and hydrastinine.
This analysis revealed that a number of transporters were sensitive to inhibition by goldenseal and its alkaloids.
“Using current [Food and Drug Administration]–recommended basic models, the goldenseal product was predicted to inhibit the intestinal efflux transporter BCRP [breast cancer resistance protein] and the hepatic uptake transporters OATP1B1 and OATP1B3,” the investigators wrote, which suggested that goldenseal would increase the area under the plasma concentration-time curve (AUC) of rosuvastatin acid and lactone.
This prediction was clinically tested in 16 healthy volunteers: 8 men and 8 nonpregnant women.
In the baseline portion of the study, each participant received an oral transporter probe cocktail consisting of 10 mg rosuvastatin (OATP1B1/3 and BCRP), 50 mg metformin (OCT1/2 and MATE1/2-K), 1 mg furosemide (OAT1/3), and 2.5 mg midazolam (CYP3A; positive control). Plasma and urine samples were collected before and after the cocktail, with urine collected up to 24 hours later, and plasma collected up to 96 hours later.
Following a minimum 9-day washout period, the same cohort received 1 gram of goldenseal every 8 hours for 5 days. On the day 6, the drug cocktail was given again, followed by two additional doses of goldenseal at 4-hour intervals. At the same time points used in the baseline protocol, urine and plasma samples were collected.
Plasma concentration vs. time profiles revealed that the model-based prediction was false, in that the presence of goldenseal did not alter the pharmacokinetics of rosuvastatin acid and lactone. The investigators suggested that this could be due to incomplete dissolution of goldenseal in the intestinal lumen, and/or low enterocyte concentrations of goldenseal stemming from “low permeability or extensive enterocyte metabolism or efflux.”
In contrast, and unpredicted by the basic model, goldenseal had a significant impact on apical efflux transporters MATE1 and MATE2-K, which mediate renal excretion of metformin. In consequence, AUC from zero to infinity and maximum plasma concentration of metformin were reduced by 23% and 27%, respectively.
“These observations, coupled with no change in half-life, suggested that goldenseal decreased metformin oral bioavailability by altering intestinal permeability, transport, and/or other processes involved in metformin absorption,” the investigators wrote.
According to principal author Mary Paine, PhD, of Washington State University, Spokane, this finding may have clinically significant implications for patients currently taking metformin for type 2 diabetes.
“Our study showed that goldenseal has an effect on the intestinal absorption of metformin, suggesting that the co-use of metformin and goldenseal may compromise blood glucose control in patients with type 2 diabetes and increase their risk of negative health outcomes,” Dr. Paine said. “While this finding warrants a degree of caution to be exercised among patients and their treating physicians, we have more work to do to confirm whether these findings in healthy volunteers in fact have clinical relevance in the management of diabetes. We are in the process of starting a follow-up study that should ultimately answer that question.”
The study was supported by the National Institutes of Health. The investigators reported no conflicts of interest.
Goldenseal, a natural botanical product, may interfere with intestinal absorption of metformin, potentially compromising blood glucose control in patients with type 2 diabetes, according to investigators.
The study, which tested for interactions between goldenseal and several drugs in healthy volunteers, reveals that current models for predicting transporter-mediated drug-drug interactions may be insufficient to screen commonly used dietary supplements, reported lead investigator James T. Nguyen, PharmD, a PhD candidate at Washington State University, Spokane, and colleagues.
“Supplements containing goldenseal ... a perennial herb native to North America, have consistently ranked among the top 20 highest selling natural products during the last decade,” the investigators wrote in Clinical Pharmacology & Therapeutics . “As more patients continue to seek goldenseal and other natural products to self-treat their medical conditions, there is an increasing need to characterize their safety profiles, especially when co-consumed with prescribed medications, which can lead to adverse natural product-drug interactions.”
Previous clinical studies have shown that goldenseal inhibits cytochrome P450, with one study showing a roughly 40% increase in systemic midazolam exposure via CYP3A inhibition, “suggesting goldenseal could have prolonged inhibitory effects in vivo similar to grapefruit juice,” the investigators wrote.
Clinical and in vitro results for goldenseal-transporter interactions have been mixed, the investigators noted, specifically for P-glycoprotein, while other transporters remain clinically untested.
“Likewise, the effects of [goldenseal alkaloids], all of which are time-dependent inhibitors of CYP3A and/or CYP2D6, have not been tested on transporter function,” the investigators wrote.
To address this knowledge gap, the investigators first performed in vitro transporter inhibition assays and in vitro–in vivo predictions involving goldenseal, plus the alkaloids berberine, (−)-beta-hydrastine, and hydrastinine.
This analysis revealed that a number of transporters were sensitive to inhibition by goldenseal and its alkaloids.
“Using current [Food and Drug Administration]–recommended basic models, the goldenseal product was predicted to inhibit the intestinal efflux transporter BCRP [breast cancer resistance protein] and the hepatic uptake transporters OATP1B1 and OATP1B3,” the investigators wrote, which suggested that goldenseal would increase the area under the plasma concentration-time curve (AUC) of rosuvastatin acid and lactone.
This prediction was clinically tested in 16 healthy volunteers: 8 men and 8 nonpregnant women.
In the baseline portion of the study, each participant received an oral transporter probe cocktail consisting of 10 mg rosuvastatin (OATP1B1/3 and BCRP), 50 mg metformin (OCT1/2 and MATE1/2-K), 1 mg furosemide (OAT1/3), and 2.5 mg midazolam (CYP3A; positive control). Plasma and urine samples were collected before and after the cocktail, with urine collected up to 24 hours later, and plasma collected up to 96 hours later.
Following a minimum 9-day washout period, the same cohort received 1 gram of goldenseal every 8 hours for 5 days. On the day 6, the drug cocktail was given again, followed by two additional doses of goldenseal at 4-hour intervals. At the same time points used in the baseline protocol, urine and plasma samples were collected.
Plasma concentration vs. time profiles revealed that the model-based prediction was false, in that the presence of goldenseal did not alter the pharmacokinetics of rosuvastatin acid and lactone. The investigators suggested that this could be due to incomplete dissolution of goldenseal in the intestinal lumen, and/or low enterocyte concentrations of goldenseal stemming from “low permeability or extensive enterocyte metabolism or efflux.”
In contrast, and unpredicted by the basic model, goldenseal had a significant impact on apical efflux transporters MATE1 and MATE2-K, which mediate renal excretion of metformin. In consequence, AUC from zero to infinity and maximum plasma concentration of metformin were reduced by 23% and 27%, respectively.
“These observations, coupled with no change in half-life, suggested that goldenseal decreased metformin oral bioavailability by altering intestinal permeability, transport, and/or other processes involved in metformin absorption,” the investigators wrote.
According to principal author Mary Paine, PhD, of Washington State University, Spokane, this finding may have clinically significant implications for patients currently taking metformin for type 2 diabetes.
“Our study showed that goldenseal has an effect on the intestinal absorption of metformin, suggesting that the co-use of metformin and goldenseal may compromise blood glucose control in patients with type 2 diabetes and increase their risk of negative health outcomes,” Dr. Paine said. “While this finding warrants a degree of caution to be exercised among patients and their treating physicians, we have more work to do to confirm whether these findings in healthy volunteers in fact have clinical relevance in the management of diabetes. We are in the process of starting a follow-up study that should ultimately answer that question.”
The study was supported by the National Institutes of Health. The investigators reported no conflicts of interest.
Goldenseal, a natural botanical product, may interfere with intestinal absorption of metformin, potentially compromising blood glucose control in patients with type 2 diabetes, according to investigators.
The study, which tested for interactions between goldenseal and several drugs in healthy volunteers, reveals that current models for predicting transporter-mediated drug-drug interactions may be insufficient to screen commonly used dietary supplements, reported lead investigator James T. Nguyen, PharmD, a PhD candidate at Washington State University, Spokane, and colleagues.
“Supplements containing goldenseal ... a perennial herb native to North America, have consistently ranked among the top 20 highest selling natural products during the last decade,” the investigators wrote in Clinical Pharmacology & Therapeutics . “As more patients continue to seek goldenseal and other natural products to self-treat their medical conditions, there is an increasing need to characterize their safety profiles, especially when co-consumed with prescribed medications, which can lead to adverse natural product-drug interactions.”
Previous clinical studies have shown that goldenseal inhibits cytochrome P450, with one study showing a roughly 40% increase in systemic midazolam exposure via CYP3A inhibition, “suggesting goldenseal could have prolonged inhibitory effects in vivo similar to grapefruit juice,” the investigators wrote.
Clinical and in vitro results for goldenseal-transporter interactions have been mixed, the investigators noted, specifically for P-glycoprotein, while other transporters remain clinically untested.
“Likewise, the effects of [goldenseal alkaloids], all of which are time-dependent inhibitors of CYP3A and/or CYP2D6, have not been tested on transporter function,” the investigators wrote.
To address this knowledge gap, the investigators first performed in vitro transporter inhibition assays and in vitro–in vivo predictions involving goldenseal, plus the alkaloids berberine, (−)-beta-hydrastine, and hydrastinine.
This analysis revealed that a number of transporters were sensitive to inhibition by goldenseal and its alkaloids.
“Using current [Food and Drug Administration]–recommended basic models, the goldenseal product was predicted to inhibit the intestinal efflux transporter BCRP [breast cancer resistance protein] and the hepatic uptake transporters OATP1B1 and OATP1B3,” the investigators wrote, which suggested that goldenseal would increase the area under the plasma concentration-time curve (AUC) of rosuvastatin acid and lactone.
This prediction was clinically tested in 16 healthy volunteers: 8 men and 8 nonpregnant women.
In the baseline portion of the study, each participant received an oral transporter probe cocktail consisting of 10 mg rosuvastatin (OATP1B1/3 and BCRP), 50 mg metformin (OCT1/2 and MATE1/2-K), 1 mg furosemide (OAT1/3), and 2.5 mg midazolam (CYP3A; positive control). Plasma and urine samples were collected before and after the cocktail, with urine collected up to 24 hours later, and plasma collected up to 96 hours later.
Following a minimum 9-day washout period, the same cohort received 1 gram of goldenseal every 8 hours for 5 days. On the day 6, the drug cocktail was given again, followed by two additional doses of goldenseal at 4-hour intervals. At the same time points used in the baseline protocol, urine and plasma samples were collected.
Plasma concentration vs. time profiles revealed that the model-based prediction was false, in that the presence of goldenseal did not alter the pharmacokinetics of rosuvastatin acid and lactone. The investigators suggested that this could be due to incomplete dissolution of goldenseal in the intestinal lumen, and/or low enterocyte concentrations of goldenseal stemming from “low permeability or extensive enterocyte metabolism or efflux.”
In contrast, and unpredicted by the basic model, goldenseal had a significant impact on apical efflux transporters MATE1 and MATE2-K, which mediate renal excretion of metformin. In consequence, AUC from zero to infinity and maximum plasma concentration of metformin were reduced by 23% and 27%, respectively.
“These observations, coupled with no change in half-life, suggested that goldenseal decreased metformin oral bioavailability by altering intestinal permeability, transport, and/or other processes involved in metformin absorption,” the investigators wrote.
According to principal author Mary Paine, PhD, of Washington State University, Spokane, this finding may have clinically significant implications for patients currently taking metformin for type 2 diabetes.
“Our study showed that goldenseal has an effect on the intestinal absorption of metformin, suggesting that the co-use of metformin and goldenseal may compromise blood glucose control in patients with type 2 diabetes and increase their risk of negative health outcomes,” Dr. Paine said. “While this finding warrants a degree of caution to be exercised among patients and their treating physicians, we have more work to do to confirm whether these findings in healthy volunteers in fact have clinical relevance in the management of diabetes. We are in the process of starting a follow-up study that should ultimately answer that question.”
The study was supported by the National Institutes of Health. The investigators reported no conflicts of interest.
FROM CLINICAL PHARMACOLOGY & THERAPEUTICS
Dried blood spot tests show sensitivity as cCMV screen
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
FROM JAMA PEDIATRICS