User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Cardiovascular trials lose more women than men
A new analysis of 11 phase 3/4 cardiovascular clinical trials conducted by the Thrombolysis in Myocardial Infarction (TIMI) group shows that women are more likely than men to discontinue study medications, and to withdraw from trials. The differences could not be explained by different frequencies of reporting adverse events, or by baseline differences.
The findings are significant, since cardiovascular drugs are routinely prescribed to women based on clinical trials that are populated largely by men, according to lead study author Emily Lau, MD, who is an advanced cardiology fellow at Massachusetts General Hospital, Boston. “It highlights an important disparity in clinical research in cardiology, because if women are already not represented well in clinical trials, and if once in clinical trials they don’t complete the study, it’s very hard to extrapolate the clinical trial findings to our female population in an accurate way,” Dr. Lau said in an interview. She also noted that sex-specific and reproductive factors are increasingly recognized as being important in the development and progression of cardiovascular disease.
The study was published in the journal Circulation.
The study refutes previously advanced explanations for higher withdrawal among women, including sex difference and comorbidities, according to an accompanying editorial by Sofia Sederholm Lawesson, MD, PhD, Eva Swahn, MD, PhD, and Joakim Alfredsson, MD, PhD, of Linköping University, Sweden. They also pointed out that the study found a larger between-sex difference in failure to adhere to study drug in North America (odds ratio, 1.35; 95% confidence interval, 1.30-1.41), but a more moderate difference among participants in Europe/Middle East/Africa (OR, 1.13; 95% CI, 1.09-1.17) and Asia/Pacific (OR, 1.13; 95% CI, 1.03-1.23) regions. And there were no sex differences at all among South/Central American populations.
They noted that high rates of nonadherence increase the chances of a false negative finding and overestimation of drug safety. “We know the associations between nonadherence and clinical outcomes. The next step should be to better understand the underlying reasons for, as well as consistent reporting of, nonadherence, and discontinuation in RCTs,” the editorial authors wrote.
Dr. Lau suggested a simple method to better understand reasons for withdrawal: Addition of questions to the case report form that asks about reasons for drug discontinuation or study withdrawal. “Was it an adverse event? Was it because I’m a mother of three and I can’t get to the clinical trial site after work and also pick up my kids? Are there societal barriers for women, or was it the experience of the clinical trial that was maybe less favorable for women compared to men? Or maybe there are medical reasons we simply don’t know. Something as simple as asking those questions can help us better understand the barriers to female retention,” said Dr. Lau.
The analysis included data from 135,879 men (72%) and 51,812 women (28%) enrolled in the trials. After adjustment for baseline differences, women were more likely than were men to permanently discontinue study drug (adjusted odds ratio [aOR], 1.22: P < .001), which did not vary by study duration. The finding was consistent regardless of the type of drug studied, as well as across placebo and active study arms.
Women also were more likely to prematurely discontinue study drug (trial-adjusted OR, 1.18; P < .001). The rate of drug discontinuation due to adverse event was identical in both men and women, at 36%.
Women were more likely to withdraw consent than were men in a meta-analysis and when individual patient-level results were pooled (aOR, 1.26; P < .001 for both).
Dr. Lau received funding from the National Institutes of Health and has no relevant financial disclosures. The editorial authors had various disclosures, including lecture fees from Bayer, Pfizer, and Boehringer Ingelheim, and they served on advisory boards for AstraZeneca and MSD.
A new analysis of 11 phase 3/4 cardiovascular clinical trials conducted by the Thrombolysis in Myocardial Infarction (TIMI) group shows that women are more likely than men to discontinue study medications, and to withdraw from trials. The differences could not be explained by different frequencies of reporting adverse events, or by baseline differences.
The findings are significant, since cardiovascular drugs are routinely prescribed to women based on clinical trials that are populated largely by men, according to lead study author Emily Lau, MD, who is an advanced cardiology fellow at Massachusetts General Hospital, Boston. “It highlights an important disparity in clinical research in cardiology, because if women are already not represented well in clinical trials, and if once in clinical trials they don’t complete the study, it’s very hard to extrapolate the clinical trial findings to our female population in an accurate way,” Dr. Lau said in an interview. She also noted that sex-specific and reproductive factors are increasingly recognized as being important in the development and progression of cardiovascular disease.
The study was published in the journal Circulation.
The study refutes previously advanced explanations for higher withdrawal among women, including sex difference and comorbidities, according to an accompanying editorial by Sofia Sederholm Lawesson, MD, PhD, Eva Swahn, MD, PhD, and Joakim Alfredsson, MD, PhD, of Linköping University, Sweden. They also pointed out that the study found a larger between-sex difference in failure to adhere to study drug in North America (odds ratio, 1.35; 95% confidence interval, 1.30-1.41), but a more moderate difference among participants in Europe/Middle East/Africa (OR, 1.13; 95% CI, 1.09-1.17) and Asia/Pacific (OR, 1.13; 95% CI, 1.03-1.23) regions. And there were no sex differences at all among South/Central American populations.
They noted that high rates of nonadherence increase the chances of a false negative finding and overestimation of drug safety. “We know the associations between nonadherence and clinical outcomes. The next step should be to better understand the underlying reasons for, as well as consistent reporting of, nonadherence, and discontinuation in RCTs,” the editorial authors wrote.
Dr. Lau suggested a simple method to better understand reasons for withdrawal: Addition of questions to the case report form that asks about reasons for drug discontinuation or study withdrawal. “Was it an adverse event? Was it because I’m a mother of three and I can’t get to the clinical trial site after work and also pick up my kids? Are there societal barriers for women, or was it the experience of the clinical trial that was maybe less favorable for women compared to men? Or maybe there are medical reasons we simply don’t know. Something as simple as asking those questions can help us better understand the barriers to female retention,” said Dr. Lau.
The analysis included data from 135,879 men (72%) and 51,812 women (28%) enrolled in the trials. After adjustment for baseline differences, women were more likely than were men to permanently discontinue study drug (adjusted odds ratio [aOR], 1.22: P < .001), which did not vary by study duration. The finding was consistent regardless of the type of drug studied, as well as across placebo and active study arms.
Women also were more likely to prematurely discontinue study drug (trial-adjusted OR, 1.18; P < .001). The rate of drug discontinuation due to adverse event was identical in both men and women, at 36%.
Women were more likely to withdraw consent than were men in a meta-analysis and when individual patient-level results were pooled (aOR, 1.26; P < .001 for both).
Dr. Lau received funding from the National Institutes of Health and has no relevant financial disclosures. The editorial authors had various disclosures, including lecture fees from Bayer, Pfizer, and Boehringer Ingelheim, and they served on advisory boards for AstraZeneca and MSD.
A new analysis of 11 phase 3/4 cardiovascular clinical trials conducted by the Thrombolysis in Myocardial Infarction (TIMI) group shows that women are more likely than men to discontinue study medications, and to withdraw from trials. The differences could not be explained by different frequencies of reporting adverse events, or by baseline differences.
The findings are significant, since cardiovascular drugs are routinely prescribed to women based on clinical trials that are populated largely by men, according to lead study author Emily Lau, MD, who is an advanced cardiology fellow at Massachusetts General Hospital, Boston. “It highlights an important disparity in clinical research in cardiology, because if women are already not represented well in clinical trials, and if once in clinical trials they don’t complete the study, it’s very hard to extrapolate the clinical trial findings to our female population in an accurate way,” Dr. Lau said in an interview. She also noted that sex-specific and reproductive factors are increasingly recognized as being important in the development and progression of cardiovascular disease.
The study was published in the journal Circulation.
The study refutes previously advanced explanations for higher withdrawal among women, including sex difference and comorbidities, according to an accompanying editorial by Sofia Sederholm Lawesson, MD, PhD, Eva Swahn, MD, PhD, and Joakim Alfredsson, MD, PhD, of Linköping University, Sweden. They also pointed out that the study found a larger between-sex difference in failure to adhere to study drug in North America (odds ratio, 1.35; 95% confidence interval, 1.30-1.41), but a more moderate difference among participants in Europe/Middle East/Africa (OR, 1.13; 95% CI, 1.09-1.17) and Asia/Pacific (OR, 1.13; 95% CI, 1.03-1.23) regions. And there were no sex differences at all among South/Central American populations.
They noted that high rates of nonadherence increase the chances of a false negative finding and overestimation of drug safety. “We know the associations between nonadherence and clinical outcomes. The next step should be to better understand the underlying reasons for, as well as consistent reporting of, nonadherence, and discontinuation in RCTs,” the editorial authors wrote.
Dr. Lau suggested a simple method to better understand reasons for withdrawal: Addition of questions to the case report form that asks about reasons for drug discontinuation or study withdrawal. “Was it an adverse event? Was it because I’m a mother of three and I can’t get to the clinical trial site after work and also pick up my kids? Are there societal barriers for women, or was it the experience of the clinical trial that was maybe less favorable for women compared to men? Or maybe there are medical reasons we simply don’t know. Something as simple as asking those questions can help us better understand the barriers to female retention,” said Dr. Lau.
The analysis included data from 135,879 men (72%) and 51,812 women (28%) enrolled in the trials. After adjustment for baseline differences, women were more likely than were men to permanently discontinue study drug (adjusted odds ratio [aOR], 1.22: P < .001), which did not vary by study duration. The finding was consistent regardless of the type of drug studied, as well as across placebo and active study arms.
Women also were more likely to prematurely discontinue study drug (trial-adjusted OR, 1.18; P < .001). The rate of drug discontinuation due to adverse event was identical in both men and women, at 36%.
Women were more likely to withdraw consent than were men in a meta-analysis and when individual patient-level results were pooled (aOR, 1.26; P < .001 for both).
Dr. Lau received funding from the National Institutes of Health and has no relevant financial disclosures. The editorial authors had various disclosures, including lecture fees from Bayer, Pfizer, and Boehringer Ingelheim, and they served on advisory boards for AstraZeneca and MSD.
FROM CIRCULATION
Opioids prescribed for diabetic neuropathy pain, against advice
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
FDA clears novel daytime device for obstructive sleep apnea
eXciteOSA (Signifier Medical Technologies) is a prescription-only, neuromuscular stimulation device designed to improve tongue muscle function, which, over time, can help prevent the tongue from collapsing backwards and obstructing the airway during sleep, the FDA said.
The eXciteOSA mouthpiece has four electrodes that deliver a series of electrical pulses with rest periods in between. Two electrodes are located above the tongue and two are located below the tongue.
The patient uses the device for 20 minutes once a day while awake for 6 weeks, and once a week thereafter. It is indicated for adults aged 18 and older with snoring and mild OSA.
OSA is marked by the recurring collapse of the upper airways during sleep, intermittently reducing or completely blocking airflow. Common symptoms include snoring, restless sleep and daytime sleepiness. Untreated OSA can lead to serious complications such as cardiovascular disease and cognitive and behavioral disorders.
Continuous positive airway pressure therapy, administered through a face mask that is worn while asleep, is a first-line treatment for OSA.
The eXciteOSA device “offers a new option for the thousands of individuals who experience snoring or mild sleep apnea,” Malvina Eydelman, MD, director, FDA Office of Ophthalmic, Anesthesia, Respiratory, ENT, and Dental Devices, said in a news release.
The FDA reviewed data on the safety and effectiveness of the eXciteOSA device in 115 patients with snoring, including 48 patients with snoring and mild OSA. All patients used the device for 20 minutes once a day for 6 weeks, then stopped using it for 2 weeks before they were reassessed.
Overall, the percentage of time spent snoring at levels louder than 40 decibels was reduced by more than 20% in 87 out of the 115 patients.
In the subset of patients with snoring and mild OSA, the average apnea-hypopnea index score was reduced by 48%, from 10.21 to 5.27, in 41 of 48 patients. Mild OSA is defined as an AHI score greater than 5 but less than 15.
The most common adverse events were excessive salivation, tongue or tooth discomfort, tongue tingling, dental filling sensitivity, metallic taste, gagging, and tight jaw.
Before using the eXciteOSA device, patients should receive a comprehensive dental examination, the FDA said.
The device should not be used in patients with pacemakers or implanted pacing leads, or women who are pregnant. The device is also contraindicated in patients with temporary or permanent implants, dental braces, intraoral metal prosthesis/restorations, or ulcerations in or around the mouth.
The eXciteOSA device was approved under the de novo premarket review pathway for new low- to moderate-risk devices. More information on the device is available online.
A version of this article first appeared on Medscape.com.
eXciteOSA (Signifier Medical Technologies) is a prescription-only, neuromuscular stimulation device designed to improve tongue muscle function, which, over time, can help prevent the tongue from collapsing backwards and obstructing the airway during sleep, the FDA said.
The eXciteOSA mouthpiece has four electrodes that deliver a series of electrical pulses with rest periods in between. Two electrodes are located above the tongue and two are located below the tongue.
The patient uses the device for 20 minutes once a day while awake for 6 weeks, and once a week thereafter. It is indicated for adults aged 18 and older with snoring and mild OSA.
OSA is marked by the recurring collapse of the upper airways during sleep, intermittently reducing or completely blocking airflow. Common symptoms include snoring, restless sleep and daytime sleepiness. Untreated OSA can lead to serious complications such as cardiovascular disease and cognitive and behavioral disorders.
Continuous positive airway pressure therapy, administered through a face mask that is worn while asleep, is a first-line treatment for OSA.
The eXciteOSA device “offers a new option for the thousands of individuals who experience snoring or mild sleep apnea,” Malvina Eydelman, MD, director, FDA Office of Ophthalmic, Anesthesia, Respiratory, ENT, and Dental Devices, said in a news release.
The FDA reviewed data on the safety and effectiveness of the eXciteOSA device in 115 patients with snoring, including 48 patients with snoring and mild OSA. All patients used the device for 20 minutes once a day for 6 weeks, then stopped using it for 2 weeks before they were reassessed.
Overall, the percentage of time spent snoring at levels louder than 40 decibels was reduced by more than 20% in 87 out of the 115 patients.
In the subset of patients with snoring and mild OSA, the average apnea-hypopnea index score was reduced by 48%, from 10.21 to 5.27, in 41 of 48 patients. Mild OSA is defined as an AHI score greater than 5 but less than 15.
The most common adverse events were excessive salivation, tongue or tooth discomfort, tongue tingling, dental filling sensitivity, metallic taste, gagging, and tight jaw.
Before using the eXciteOSA device, patients should receive a comprehensive dental examination, the FDA said.
The device should not be used in patients with pacemakers or implanted pacing leads, or women who are pregnant. The device is also contraindicated in patients with temporary or permanent implants, dental braces, intraoral metal prosthesis/restorations, or ulcerations in or around the mouth.
The eXciteOSA device was approved under the de novo premarket review pathway for new low- to moderate-risk devices. More information on the device is available online.
A version of this article first appeared on Medscape.com.
eXciteOSA (Signifier Medical Technologies) is a prescription-only, neuromuscular stimulation device designed to improve tongue muscle function, which, over time, can help prevent the tongue from collapsing backwards and obstructing the airway during sleep, the FDA said.
The eXciteOSA mouthpiece has four electrodes that deliver a series of electrical pulses with rest periods in between. Two electrodes are located above the tongue and two are located below the tongue.
The patient uses the device for 20 minutes once a day while awake for 6 weeks, and once a week thereafter. It is indicated for adults aged 18 and older with snoring and mild OSA.
OSA is marked by the recurring collapse of the upper airways during sleep, intermittently reducing or completely blocking airflow. Common symptoms include snoring, restless sleep and daytime sleepiness. Untreated OSA can lead to serious complications such as cardiovascular disease and cognitive and behavioral disorders.
Continuous positive airway pressure therapy, administered through a face mask that is worn while asleep, is a first-line treatment for OSA.
The eXciteOSA device “offers a new option for the thousands of individuals who experience snoring or mild sleep apnea,” Malvina Eydelman, MD, director, FDA Office of Ophthalmic, Anesthesia, Respiratory, ENT, and Dental Devices, said in a news release.
The FDA reviewed data on the safety and effectiveness of the eXciteOSA device in 115 patients with snoring, including 48 patients with snoring and mild OSA. All patients used the device for 20 minutes once a day for 6 weeks, then stopped using it for 2 weeks before they were reassessed.
Overall, the percentage of time spent snoring at levels louder than 40 decibels was reduced by more than 20% in 87 out of the 115 patients.
In the subset of patients with snoring and mild OSA, the average apnea-hypopnea index score was reduced by 48%, from 10.21 to 5.27, in 41 of 48 patients. Mild OSA is defined as an AHI score greater than 5 but less than 15.
The most common adverse events were excessive salivation, tongue or tooth discomfort, tongue tingling, dental filling sensitivity, metallic taste, gagging, and tight jaw.
Before using the eXciteOSA device, patients should receive a comprehensive dental examination, the FDA said.
The device should not be used in patients with pacemakers or implanted pacing leads, or women who are pregnant. The device is also contraindicated in patients with temporary or permanent implants, dental braces, intraoral metal prosthesis/restorations, or ulcerations in or around the mouth.
The eXciteOSA device was approved under the de novo premarket review pathway for new low- to moderate-risk devices. More information on the device is available online.
A version of this article first appeared on Medscape.com.
New child COVID-19 cases decline as total passes 3 million
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
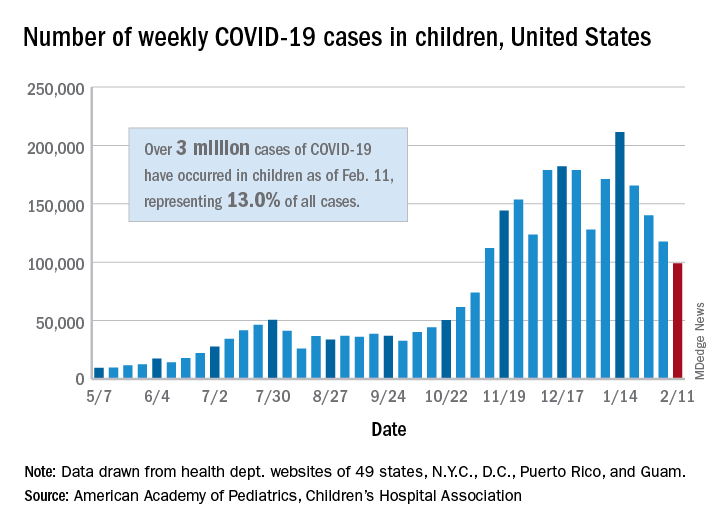
It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
FDA expands sacubitril/valsartan indication to embrace some HFpEF
The Food and Drug Administration has approved a groundbreaking expanded indication for sacubitril/valsartan (Entresto), making it the first drug in the United States indicated for chronic heart failure not specifically characterized by ejection fraction.
The new labeling, as provided by Novartis, grants physicians a good deal of discretion in prescribing sacubitril/valsartan for patients with HF beyond those with HF and reduced ejection fraction (HFrEF), for which the drug was approved in 2015 primarily on the basis of the PARADIGM-HF trial.
The indication now reads, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction (LVEF) below normal.”
Of note, the labeling cautions that “LVEF is a variable measure, so use clinical judgment in deciding whom to treat.”
The expanded indication essentially extends the sacubitril/valsartan option to many patients with HF and preserved LVEF (HFpEF), who in practice are most likely to have an LVEF in the range adjacent to “reduced,” long defined as “preserved” but lately categorized as “mid-range.”
But the FDA did not get so specific. In granting the expanded indication, which Novartis announced Feb. 16 in a press release, the agency accommodated the Dec. 15 majority recommendation of its Cardiovascular and Renal Drugs Advisory Committee that the PARAGON-HF trial “provided sufficient evidence to support” an indication beyond HFrEF.
The nature of the PARAGON-HF trial, along with detailed discussion among committee members after their vote tally, made it clear that the 12-to-1 majority favored an indication that would include clinically appropriate patients with “below normal” LVEF.
PARAGON-HF had assigned more than 4,800 patients whose LVEF was 45% or higher and were in NYHA class 2-4 to receive sacubitril/valsartan or valsartan only. Those taking the combo drug showed a 13% drop in risk for HF hospitalization or cardiovascular deaths over an average of 3 years, which narrowly missed significance (P = .059).
But a subgroup analysis garnered attention for its hint of benefit for patients with “mid-range” LVEF, in this case, below the median of 57%. The finding was supported by a later PARAGON-HF and PARADIGM-HF meta-analysis that pointed to a significant benefit for patients with HFpEF at its lowest LVEF levels, especially in women.
The expanded approval “is a significant advancement, providing a treatment to many patients who were not eligible for treatment before, because their ejection fraction was above the region we normally considered reduced,” Scott Solomon, MD, of Brigham and Women’s Hospital, Boston, said in the Novartis press release. “We can now offer a treatment to a wider range of patients who have an LVEF below normal,” added Dr. Solomon, PARAGON-HF executive committee cochair.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a groundbreaking expanded indication for sacubitril/valsartan (Entresto), making it the first drug in the United States indicated for chronic heart failure not specifically characterized by ejection fraction.
The new labeling, as provided by Novartis, grants physicians a good deal of discretion in prescribing sacubitril/valsartan for patients with HF beyond those with HF and reduced ejection fraction (HFrEF), for which the drug was approved in 2015 primarily on the basis of the PARADIGM-HF trial.
The indication now reads, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction (LVEF) below normal.”
Of note, the labeling cautions that “LVEF is a variable measure, so use clinical judgment in deciding whom to treat.”
The expanded indication essentially extends the sacubitril/valsartan option to many patients with HF and preserved LVEF (HFpEF), who in practice are most likely to have an LVEF in the range adjacent to “reduced,” long defined as “preserved” but lately categorized as “mid-range.”
But the FDA did not get so specific. In granting the expanded indication, which Novartis announced Feb. 16 in a press release, the agency accommodated the Dec. 15 majority recommendation of its Cardiovascular and Renal Drugs Advisory Committee that the PARAGON-HF trial “provided sufficient evidence to support” an indication beyond HFrEF.
The nature of the PARAGON-HF trial, along with detailed discussion among committee members after their vote tally, made it clear that the 12-to-1 majority favored an indication that would include clinically appropriate patients with “below normal” LVEF.
PARAGON-HF had assigned more than 4,800 patients whose LVEF was 45% or higher and were in NYHA class 2-4 to receive sacubitril/valsartan or valsartan only. Those taking the combo drug showed a 13% drop in risk for HF hospitalization or cardiovascular deaths over an average of 3 years, which narrowly missed significance (P = .059).
But a subgroup analysis garnered attention for its hint of benefit for patients with “mid-range” LVEF, in this case, below the median of 57%. The finding was supported by a later PARAGON-HF and PARADIGM-HF meta-analysis that pointed to a significant benefit for patients with HFpEF at its lowest LVEF levels, especially in women.
The expanded approval “is a significant advancement, providing a treatment to many patients who were not eligible for treatment before, because their ejection fraction was above the region we normally considered reduced,” Scott Solomon, MD, of Brigham and Women’s Hospital, Boston, said in the Novartis press release. “We can now offer a treatment to a wider range of patients who have an LVEF below normal,” added Dr. Solomon, PARAGON-HF executive committee cochair.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a groundbreaking expanded indication for sacubitril/valsartan (Entresto), making it the first drug in the United States indicated for chronic heart failure not specifically characterized by ejection fraction.
The new labeling, as provided by Novartis, grants physicians a good deal of discretion in prescribing sacubitril/valsartan for patients with HF beyond those with HF and reduced ejection fraction (HFrEF), for which the drug was approved in 2015 primarily on the basis of the PARADIGM-HF trial.
The indication now reads, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction (LVEF) below normal.”
Of note, the labeling cautions that “LVEF is a variable measure, so use clinical judgment in deciding whom to treat.”
The expanded indication essentially extends the sacubitril/valsartan option to many patients with HF and preserved LVEF (HFpEF), who in practice are most likely to have an LVEF in the range adjacent to “reduced,” long defined as “preserved” but lately categorized as “mid-range.”
But the FDA did not get so specific. In granting the expanded indication, which Novartis announced Feb. 16 in a press release, the agency accommodated the Dec. 15 majority recommendation of its Cardiovascular and Renal Drugs Advisory Committee that the PARAGON-HF trial “provided sufficient evidence to support” an indication beyond HFrEF.
The nature of the PARAGON-HF trial, along with detailed discussion among committee members after their vote tally, made it clear that the 12-to-1 majority favored an indication that would include clinically appropriate patients with “below normal” LVEF.
PARAGON-HF had assigned more than 4,800 patients whose LVEF was 45% or higher and were in NYHA class 2-4 to receive sacubitril/valsartan or valsartan only. Those taking the combo drug showed a 13% drop in risk for HF hospitalization or cardiovascular deaths over an average of 3 years, which narrowly missed significance (P = .059).
But a subgroup analysis garnered attention for its hint of benefit for patients with “mid-range” LVEF, in this case, below the median of 57%. The finding was supported by a later PARAGON-HF and PARADIGM-HF meta-analysis that pointed to a significant benefit for patients with HFpEF at its lowest LVEF levels, especially in women.
The expanded approval “is a significant advancement, providing a treatment to many patients who were not eligible for treatment before, because their ejection fraction was above the region we normally considered reduced,” Scott Solomon, MD, of Brigham and Women’s Hospital, Boston, said in the Novartis press release. “We can now offer a treatment to a wider range of patients who have an LVEF below normal,” added Dr. Solomon, PARAGON-HF executive committee cochair.
A version of this article first appeared on Medscape.com.
Romosozumab may not increase cardiovascular risk after all
The potent anabolic, antiosteoporosis agent romosozumab has been saddled with an Food and Drug Administration–mandated black-box warning for increased cardiovascular risk that may not be warranted, Glenn Haugeberg, MD, PhD, asserted at the 2021 Rheumatology Winter Clinical Symposium.
The black-box warning states that romosozumab (Evenity), a monoclonal antibody approved in 2019 for fracture prevention in patients with osteoporosis, may increase the risk of MI, stroke, and cardiovascular death. The warning arose from FDA concerns raised by the results of the phase 3 ARCH trial in which 4,093 postmenopausal women at high fracture risk were randomized to monthly subcutaneous injections of romosozumab or weekly dosing of the oral bisphosphonate alendronate (Fosamax) for 1 year, followed by 12 months of open-label alendronate for all. Alarm bells went off at the FDA because during year 1, the incidence of adjudicated major adverse cardiovascular events was 2.5% in the romosozumab arm, compared with 1.9% with alendronate.
Could a cardioprotective effect of bisphosphonates explain cardiovascular concerns?
However, evidence from multiple animal and human studies suggests that bisphosphonates actually have a cardioprotective effect. For example, a Taiwanese population-based cohort study of 1,548 patients on bisphosphonate therapy for osteoporotic fractures and 4,644 individuals with hip or vertebral fractures who were not on a bisphosphonate showed a 65% reduction in the risk of acute MI during 2 years of follow-up in those who received a bisphosphonate.
“That may explain the ARCH finding. It may – I say may – be that this concern in the ARCH study can be explained by the positive effect of the bisphosphonates on cardiovascular events,” according to Dr. Haugeberg, head of the division of rheumatology at the Southern Norway Hospital Trust, Kristiansand, and professor of medicine at the Norwegian University of Science and Technology, Trondheim.
He noted that, in the FRAME trial, another pivotal phase 3 trial of romosozumab, there was no signal of increased cardiovascular risk, compared with placebo. In FRAME, which included 7,180 osteoporotic postmenopausal women, rates of major adverse cardiovascular events and other adverse events were balanced between the two study arms at 12 months. Indeed, the incidence of adjudicated serious cardiovascular events was 0.5% with romosozumab and 0.4% with placebo injections. After 12 months, all participants were transitioned to denosumab (Prolia) for another 12 months. At 24 months, there remained no significant between-group difference in cardiovascular events, cancer, osteoarthritis, hyperostosis, or other major adverse events.
Potency of romosozumab
Romosozumab’s efficacy for fracture prevention in these two pivotal trials was striking. The risk of new vertebral fractures was reduced by 73% with romosozumab, compared with placebo at 12 months in FRAME, and by 75% at 24 months in the romosozumab-to-denosumab group.
“FRAME was a 12-month study for the primary endpoint. The bisphosphonate studies typically had a 3-year design in order to show benefit, but here you see only 12-month follow-up. This illustrates the potency of this drug. We saw rapid increase in bone density and a huge decrease in new vertebral fractures versus placebo in the first 12 months, then during follow-up with denosumab the reduction in fractures was maintained,” the rheumatologist commented.
In the ARCH trial, where romosozumab went head to head with a very effective oral bisphosphonate, the risk of new vertebral fractures was 48% lower at 24 months in the romosozumab-to-alendronate group than in women on alendronate for the full 24 months, while the risk of hip fractures was reduced by 38%.
Romosozumab is a humanized monoclonal antibody with a novel mechanism of anabolic action: This agent binds to sclerostin, which is produced in osteocytes. When sclerostin binds to receptors on osteoblasts it reduces their activity, thereby inhibiting bone formation. Romosozumab takes away this inhibition of osteoblasts, boosting their activity. The result is increased bone formation accompanied by decreased bone resorption. This allows for a logical treatment approach: first using an anabolic agent – in this instance, subcutaneously injected romosozumab at 210 mg once monthly for 12 months – then switching to an antiresorptive agent in order to maintain the gain in bone mineral density and decrease fracture risk. This is the same treatment strategy recommended when using the anabolic agents teriparatide (Forteo) and abaloparatide (Tymlos); however, those parathyroid hormone and parathyroid hormone–related protein analogs are seldom used in Norway because their cost is substantially greater than for romosozumab, he explained.
Updated Endocrine Society guidelines
Dr. Haugeberg called romosozumab “a new and wonderful drug.” The Endocrine Society also considers romosozumab an important new drug, as evidenced by the release of an 8-page update of the group’s clinical practice guideline on the pharmacologic management of osteoporosis in postmenopausal women; the update was devoted specifically to the use of romosozumab. The update, published in response to the biologic’s recent approval by U.S., Canadian, and European regulatory agencies, came just 10 months after release of the Endocrine Society’s comprehensive 28-page clinical practice guideline.
Dr. Haugeberg is a fan of the Endocrine Society guideline, which recommends romosozumab as a first-line therapy in postmenopausal women at very high risk of osteoporotic fracture, defined as those with a history of multiple vertebral fractures or severe osteoporosis with a T score of –2.5 or less at the hip or spine plus fractures. The updated guideline also recommends consideration of the antisclerostin biologic in high-risk patients who have failed on antiresorptive treatments.
The practice guideline states that the issue of a possible cardioprotective effect of alendronate in the ARCH trial “remains uncertain at this time.”
“Women at high risk of cardiovascular disease and stroke should not be considered for romosozumab pending further studies on cardiovascular risk associated with this treatment,” according to the Endocrine Society.
Dr. Haugeberg reported receiving research grants from Pfizer and Biogen and serving as a consultant to and/or on speakers’ bureaus for Amgen, which markets romosozumab, and more than a dozen other pharmaceutical companies.
The potent anabolic, antiosteoporosis agent romosozumab has been saddled with an Food and Drug Administration–mandated black-box warning for increased cardiovascular risk that may not be warranted, Glenn Haugeberg, MD, PhD, asserted at the 2021 Rheumatology Winter Clinical Symposium.
The black-box warning states that romosozumab (Evenity), a monoclonal antibody approved in 2019 for fracture prevention in patients with osteoporosis, may increase the risk of MI, stroke, and cardiovascular death. The warning arose from FDA concerns raised by the results of the phase 3 ARCH trial in which 4,093 postmenopausal women at high fracture risk were randomized to monthly subcutaneous injections of romosozumab or weekly dosing of the oral bisphosphonate alendronate (Fosamax) for 1 year, followed by 12 months of open-label alendronate for all. Alarm bells went off at the FDA because during year 1, the incidence of adjudicated major adverse cardiovascular events was 2.5% in the romosozumab arm, compared with 1.9% with alendronate.
Could a cardioprotective effect of bisphosphonates explain cardiovascular concerns?
However, evidence from multiple animal and human studies suggests that bisphosphonates actually have a cardioprotective effect. For example, a Taiwanese population-based cohort study of 1,548 patients on bisphosphonate therapy for osteoporotic fractures and 4,644 individuals with hip or vertebral fractures who were not on a bisphosphonate showed a 65% reduction in the risk of acute MI during 2 years of follow-up in those who received a bisphosphonate.
“That may explain the ARCH finding. It may – I say may – be that this concern in the ARCH study can be explained by the positive effect of the bisphosphonates on cardiovascular events,” according to Dr. Haugeberg, head of the division of rheumatology at the Southern Norway Hospital Trust, Kristiansand, and professor of medicine at the Norwegian University of Science and Technology, Trondheim.
He noted that, in the FRAME trial, another pivotal phase 3 trial of romosozumab, there was no signal of increased cardiovascular risk, compared with placebo. In FRAME, which included 7,180 osteoporotic postmenopausal women, rates of major adverse cardiovascular events and other adverse events were balanced between the two study arms at 12 months. Indeed, the incidence of adjudicated serious cardiovascular events was 0.5% with romosozumab and 0.4% with placebo injections. After 12 months, all participants were transitioned to denosumab (Prolia) for another 12 months. At 24 months, there remained no significant between-group difference in cardiovascular events, cancer, osteoarthritis, hyperostosis, or other major adverse events.
Potency of romosozumab
Romosozumab’s efficacy for fracture prevention in these two pivotal trials was striking. The risk of new vertebral fractures was reduced by 73% with romosozumab, compared with placebo at 12 months in FRAME, and by 75% at 24 months in the romosozumab-to-denosumab group.
“FRAME was a 12-month study for the primary endpoint. The bisphosphonate studies typically had a 3-year design in order to show benefit, but here you see only 12-month follow-up. This illustrates the potency of this drug. We saw rapid increase in bone density and a huge decrease in new vertebral fractures versus placebo in the first 12 months, then during follow-up with denosumab the reduction in fractures was maintained,” the rheumatologist commented.
In the ARCH trial, where romosozumab went head to head with a very effective oral bisphosphonate, the risk of new vertebral fractures was 48% lower at 24 months in the romosozumab-to-alendronate group than in women on alendronate for the full 24 months, while the risk of hip fractures was reduced by 38%.
Romosozumab is a humanized monoclonal antibody with a novel mechanism of anabolic action: This agent binds to sclerostin, which is produced in osteocytes. When sclerostin binds to receptors on osteoblasts it reduces their activity, thereby inhibiting bone formation. Romosozumab takes away this inhibition of osteoblasts, boosting their activity. The result is increased bone formation accompanied by decreased bone resorption. This allows for a logical treatment approach: first using an anabolic agent – in this instance, subcutaneously injected romosozumab at 210 mg once monthly for 12 months – then switching to an antiresorptive agent in order to maintain the gain in bone mineral density and decrease fracture risk. This is the same treatment strategy recommended when using the anabolic agents teriparatide (Forteo) and abaloparatide (Tymlos); however, those parathyroid hormone and parathyroid hormone–related protein analogs are seldom used in Norway because their cost is substantially greater than for romosozumab, he explained.
Updated Endocrine Society guidelines
Dr. Haugeberg called romosozumab “a new and wonderful drug.” The Endocrine Society also considers romosozumab an important new drug, as evidenced by the release of an 8-page update of the group’s clinical practice guideline on the pharmacologic management of osteoporosis in postmenopausal women; the update was devoted specifically to the use of romosozumab. The update, published in response to the biologic’s recent approval by U.S., Canadian, and European regulatory agencies, came just 10 months after release of the Endocrine Society’s comprehensive 28-page clinical practice guideline.
Dr. Haugeberg is a fan of the Endocrine Society guideline, which recommends romosozumab as a first-line therapy in postmenopausal women at very high risk of osteoporotic fracture, defined as those with a history of multiple vertebral fractures or severe osteoporosis with a T score of –2.5 or less at the hip or spine plus fractures. The updated guideline also recommends consideration of the antisclerostin biologic in high-risk patients who have failed on antiresorptive treatments.
The practice guideline states that the issue of a possible cardioprotective effect of alendronate in the ARCH trial “remains uncertain at this time.”
“Women at high risk of cardiovascular disease and stroke should not be considered for romosozumab pending further studies on cardiovascular risk associated with this treatment,” according to the Endocrine Society.
Dr. Haugeberg reported receiving research grants from Pfizer and Biogen and serving as a consultant to and/or on speakers’ bureaus for Amgen, which markets romosozumab, and more than a dozen other pharmaceutical companies.
The potent anabolic, antiosteoporosis agent romosozumab has been saddled with an Food and Drug Administration–mandated black-box warning for increased cardiovascular risk that may not be warranted, Glenn Haugeberg, MD, PhD, asserted at the 2021 Rheumatology Winter Clinical Symposium.
The black-box warning states that romosozumab (Evenity), a monoclonal antibody approved in 2019 for fracture prevention in patients with osteoporosis, may increase the risk of MI, stroke, and cardiovascular death. The warning arose from FDA concerns raised by the results of the phase 3 ARCH trial in which 4,093 postmenopausal women at high fracture risk were randomized to monthly subcutaneous injections of romosozumab or weekly dosing of the oral bisphosphonate alendronate (Fosamax) for 1 year, followed by 12 months of open-label alendronate for all. Alarm bells went off at the FDA because during year 1, the incidence of adjudicated major adverse cardiovascular events was 2.5% in the romosozumab arm, compared with 1.9% with alendronate.
Could a cardioprotective effect of bisphosphonates explain cardiovascular concerns?
However, evidence from multiple animal and human studies suggests that bisphosphonates actually have a cardioprotective effect. For example, a Taiwanese population-based cohort study of 1,548 patients on bisphosphonate therapy for osteoporotic fractures and 4,644 individuals with hip or vertebral fractures who were not on a bisphosphonate showed a 65% reduction in the risk of acute MI during 2 years of follow-up in those who received a bisphosphonate.
“That may explain the ARCH finding. It may – I say may – be that this concern in the ARCH study can be explained by the positive effect of the bisphosphonates on cardiovascular events,” according to Dr. Haugeberg, head of the division of rheumatology at the Southern Norway Hospital Trust, Kristiansand, and professor of medicine at the Norwegian University of Science and Technology, Trondheim.
He noted that, in the FRAME trial, another pivotal phase 3 trial of romosozumab, there was no signal of increased cardiovascular risk, compared with placebo. In FRAME, which included 7,180 osteoporotic postmenopausal women, rates of major adverse cardiovascular events and other adverse events were balanced between the two study arms at 12 months. Indeed, the incidence of adjudicated serious cardiovascular events was 0.5% with romosozumab and 0.4% with placebo injections. After 12 months, all participants were transitioned to denosumab (Prolia) for another 12 months. At 24 months, there remained no significant between-group difference in cardiovascular events, cancer, osteoarthritis, hyperostosis, or other major adverse events.
Potency of romosozumab
Romosozumab’s efficacy for fracture prevention in these two pivotal trials was striking. The risk of new vertebral fractures was reduced by 73% with romosozumab, compared with placebo at 12 months in FRAME, and by 75% at 24 months in the romosozumab-to-denosumab group.
“FRAME was a 12-month study for the primary endpoint. The bisphosphonate studies typically had a 3-year design in order to show benefit, but here you see only 12-month follow-up. This illustrates the potency of this drug. We saw rapid increase in bone density and a huge decrease in new vertebral fractures versus placebo in the first 12 months, then during follow-up with denosumab the reduction in fractures was maintained,” the rheumatologist commented.
In the ARCH trial, where romosozumab went head to head with a very effective oral bisphosphonate, the risk of new vertebral fractures was 48% lower at 24 months in the romosozumab-to-alendronate group than in women on alendronate for the full 24 months, while the risk of hip fractures was reduced by 38%.
Romosozumab is a humanized monoclonal antibody with a novel mechanism of anabolic action: This agent binds to sclerostin, which is produced in osteocytes. When sclerostin binds to receptors on osteoblasts it reduces their activity, thereby inhibiting bone formation. Romosozumab takes away this inhibition of osteoblasts, boosting their activity. The result is increased bone formation accompanied by decreased bone resorption. This allows for a logical treatment approach: first using an anabolic agent – in this instance, subcutaneously injected romosozumab at 210 mg once monthly for 12 months – then switching to an antiresorptive agent in order to maintain the gain in bone mineral density and decrease fracture risk. This is the same treatment strategy recommended when using the anabolic agents teriparatide (Forteo) and abaloparatide (Tymlos); however, those parathyroid hormone and parathyroid hormone–related protein analogs are seldom used in Norway because their cost is substantially greater than for romosozumab, he explained.
Updated Endocrine Society guidelines
Dr. Haugeberg called romosozumab “a new and wonderful drug.” The Endocrine Society also considers romosozumab an important new drug, as evidenced by the release of an 8-page update of the group’s clinical practice guideline on the pharmacologic management of osteoporosis in postmenopausal women; the update was devoted specifically to the use of romosozumab. The update, published in response to the biologic’s recent approval by U.S., Canadian, and European regulatory agencies, came just 10 months after release of the Endocrine Society’s comprehensive 28-page clinical practice guideline.
Dr. Haugeberg is a fan of the Endocrine Society guideline, which recommends romosozumab as a first-line therapy in postmenopausal women at very high risk of osteoporotic fracture, defined as those with a history of multiple vertebral fractures or severe osteoporosis with a T score of –2.5 or less at the hip or spine plus fractures. The updated guideline also recommends consideration of the antisclerostin biologic in high-risk patients who have failed on antiresorptive treatments.
The practice guideline states that the issue of a possible cardioprotective effect of alendronate in the ARCH trial “remains uncertain at this time.”
“Women at high risk of cardiovascular disease and stroke should not be considered for romosozumab pending further studies on cardiovascular risk associated with this treatment,” according to the Endocrine Society.
Dr. Haugeberg reported receiving research grants from Pfizer and Biogen and serving as a consultant to and/or on speakers’ bureaus for Amgen, which markets romosozumab, and more than a dozen other pharmaceutical companies.
FROM RWCS 2021
Outcomes have improved for PAH in connective tissue disease
Survival rates for patients with pulmonary arterial hypertension associated with connective tissue diseases have improved significantly in recent years, and there is growing evidence that treatments for idiopathic pulmonary arterial hypertension can also benefit this group.
In an article published online Feb. 3, 2021, in Arthritis & Rheumatology, researchers report the outcomes of a meta-analysis to explore the effect of more modern pulmonary arterial hypertension treatments on patients with conditions such as systemic sclerosis.
First author Dinesh Khanna, MBBS, MSc, of the division of rheumatology at the University of Michigan, Ann Arbor, said in an interview that connective tissue disease–associated pulmonary arterial hypertension (CTD-PAH) was a leading cause of death, but earlier clinical trials had found poor outcomes in patients with CTD, compared with those with idiopathic PAH.
“Recent clinical trial data show that aggressive, up-front PAH treatments have better outcomes in those with CTD-PAH, and we wanted to explore these observations carefully in a systematic review and meta-analysis,” Dr. Khanna said.
The analysis included 11 randomized, controlled trials, involving 4,329 patients with PAH (1,267 with CTD), and 19 registries with a total of 9,739 patients with PAH, including 4,008 with CTD. Trials were required to report long-term clinical outcomes with a median enrollment time of greater than 6 months, and outcomes measured between 3-6 months after the patients started treatment.
Patients with CTDs had an older mean age and a lower 6-minute walk distance than did those with idiopathic PAH.
Five randomized, controlled trials – involving 3,172 patients, 941 of whom had a CTD – found that additional PAH treatment was associated with a 36% reduction in the risk of morbidity or mortality events, compared with controls both in the overall PAH group and in those with CTD.
Additional therapy was also associated with a 34.6-meter increase in 6-minute walk distance in the general PAH population, and a 20.4-meter increase in those with CTD.
The authors commented that the smaller improvement in 6-minute walk distance among patients with CTD may be influenced by comorbidities such as musculoskeletal involvement that would be independent of their cardiopulmonary function.
Differential patient survival among PAH etiologies
“Our meta-analysis of RCTs demonstrated that patients with CTD-PAH derive a clinically significant benefit from currently available PAH therapies which, in many patients, comprised the addition of a drug targeting a second or third pathway involved in the pathophysiology of PAH,” the authors wrote.
When researchers analyzed data from nine registries that included a wide range of PAH etiologies, they found the overall survival rates were lower among patients with CTD, compared with the overall population. The analysis also suggested that patients with systemic sclerosis and PAH had lower survival rates than did those with systemic lupus erythematosus.
Dr. Khanna said this may relate to different pathophysiology of PAH in patients with CTDs, but could also be a reflection of other differences, such as older age and the involvement of other comorbidities, including lung fibrosis and heart involvement.
Data across all 19 registries also showed that survival rates among those with CTD were higher in registries where more than 50% of the registry study period was during or after 2010, compared with registries where 50% or more of the study period was before 2010.
The authors suggested the differences in survival rates may relate to increased screening for PAH, particularly among people with CTDs. They noted that increased screening leads to earlier diagnosis, which could introduce a lead-time bias such that later registries would have younger participants with less severe disease. However, their analysis found that the later registries had older patients but also with less severe disease, and they suggested that it wasn’t possible to determine if lead-time bias was playing a role in their results.
Improvements in treatment options could also account for differences in survival over time, although the authors commented that only six registries in the study included patients from 2015 or later, when currently available treatments came into use and early combination therapy was used more.
“These data also support the 2018 World Symposium on Pulmonary Hypertension recommendations to initiate up-front combination pulmonary arterial hypertension therapy in majority of cases with CTD-PAH,” Dr. Khanna said.
‘Still have to be aggressive at identifying the high-risk patients’
Commenting on the findings, Virginia Steen, MD, of the division of rheumatology at Georgetown University, Washington, said clinicians were finally seeing some significant changes over time in scleroderma-associated PAH.
“Although some of it may be just early diagnosis, I think that the combination of early diagnosis and more aggressive treatment with combination medication is definitely making a difference,” Dr. Steen said in an interview. “The bottom line is that we as rheumatologists still have to be aggressive at identifying the high-risk patients, making an early diagnosis, and working with our pulmonary hypertension colleagues and aggressively treating these patients so we can make a long-term difference.”
The authors of an accompanying editorial said the meta-analysis’ findings showed the positive impact of early combination therapy and early diagnosis through proactive screening.
“It is notable because the present analysis again confirms that outcomes are worse in CTD-PAH than in idiopathic or familial forms of PAH, the impact of treatments should no longer be regarded as insignificant,” the editorial’s authors wrote. “This is a practice changing observation, especially now that many of the drugs are available in generic formulations and so the cost of modern PAH treatment has fallen at the same time as its true value is convincingly demonstrated.”
They also argued there was strong evidence for the value of combination therapies, both for PAH-targeted drugs used in combination and concurrent use of immunosuppression and drugs specifically for PAH in some patients with CTD-PAH.
However, they pointed out that not all treatments for idiopathic PAH were suitable for patients with CTDs, highlighting the example of anticoagulation that can improve survival in the first but worsen it in the second.
The study was funded by Actelion. Six authors declared funding and grants from the pharmaceutical sector, including the study sponsor, and three authors were employees of Actelion.
Survival rates for patients with pulmonary arterial hypertension associated with connective tissue diseases have improved significantly in recent years, and there is growing evidence that treatments for idiopathic pulmonary arterial hypertension can also benefit this group.
In an article published online Feb. 3, 2021, in Arthritis & Rheumatology, researchers report the outcomes of a meta-analysis to explore the effect of more modern pulmonary arterial hypertension treatments on patients with conditions such as systemic sclerosis.
First author Dinesh Khanna, MBBS, MSc, of the division of rheumatology at the University of Michigan, Ann Arbor, said in an interview that connective tissue disease–associated pulmonary arterial hypertension (CTD-PAH) was a leading cause of death, but earlier clinical trials had found poor outcomes in patients with CTD, compared with those with idiopathic PAH.
“Recent clinical trial data show that aggressive, up-front PAH treatments have better outcomes in those with CTD-PAH, and we wanted to explore these observations carefully in a systematic review and meta-analysis,” Dr. Khanna said.
The analysis included 11 randomized, controlled trials, involving 4,329 patients with PAH (1,267 with CTD), and 19 registries with a total of 9,739 patients with PAH, including 4,008 with CTD. Trials were required to report long-term clinical outcomes with a median enrollment time of greater than 6 months, and outcomes measured between 3-6 months after the patients started treatment.
Patients with CTDs had an older mean age and a lower 6-minute walk distance than did those with idiopathic PAH.
Five randomized, controlled trials – involving 3,172 patients, 941 of whom had a CTD – found that additional PAH treatment was associated with a 36% reduction in the risk of morbidity or mortality events, compared with controls both in the overall PAH group and in those with CTD.
Additional therapy was also associated with a 34.6-meter increase in 6-minute walk distance in the general PAH population, and a 20.4-meter increase in those with CTD.
The authors commented that the smaller improvement in 6-minute walk distance among patients with CTD may be influenced by comorbidities such as musculoskeletal involvement that would be independent of their cardiopulmonary function.
Differential patient survival among PAH etiologies
“Our meta-analysis of RCTs demonstrated that patients with CTD-PAH derive a clinically significant benefit from currently available PAH therapies which, in many patients, comprised the addition of a drug targeting a second or third pathway involved in the pathophysiology of PAH,” the authors wrote.
When researchers analyzed data from nine registries that included a wide range of PAH etiologies, they found the overall survival rates were lower among patients with CTD, compared with the overall population. The analysis also suggested that patients with systemic sclerosis and PAH had lower survival rates than did those with systemic lupus erythematosus.
Dr. Khanna said this may relate to different pathophysiology of PAH in patients with CTDs, but could also be a reflection of other differences, such as older age and the involvement of other comorbidities, including lung fibrosis and heart involvement.
Data across all 19 registries also showed that survival rates among those with CTD were higher in registries where more than 50% of the registry study period was during or after 2010, compared with registries where 50% or more of the study period was before 2010.
The authors suggested the differences in survival rates may relate to increased screening for PAH, particularly among people with CTDs. They noted that increased screening leads to earlier diagnosis, which could introduce a lead-time bias such that later registries would have younger participants with less severe disease. However, their analysis found that the later registries had older patients but also with less severe disease, and they suggested that it wasn’t possible to determine if lead-time bias was playing a role in their results.
Improvements in treatment options could also account for differences in survival over time, although the authors commented that only six registries in the study included patients from 2015 or later, when currently available treatments came into use and early combination therapy was used more.
“These data also support the 2018 World Symposium on Pulmonary Hypertension recommendations to initiate up-front combination pulmonary arterial hypertension therapy in majority of cases with CTD-PAH,” Dr. Khanna said.
‘Still have to be aggressive at identifying the high-risk patients’
Commenting on the findings, Virginia Steen, MD, of the division of rheumatology at Georgetown University, Washington, said clinicians were finally seeing some significant changes over time in scleroderma-associated PAH.
“Although some of it may be just early diagnosis, I think that the combination of early diagnosis and more aggressive treatment with combination medication is definitely making a difference,” Dr. Steen said in an interview. “The bottom line is that we as rheumatologists still have to be aggressive at identifying the high-risk patients, making an early diagnosis, and working with our pulmonary hypertension colleagues and aggressively treating these patients so we can make a long-term difference.”
The authors of an accompanying editorial said the meta-analysis’ findings showed the positive impact of early combination therapy and early diagnosis through proactive screening.
“It is notable because the present analysis again confirms that outcomes are worse in CTD-PAH than in idiopathic or familial forms of PAH, the impact of treatments should no longer be regarded as insignificant,” the editorial’s authors wrote. “This is a practice changing observation, especially now that many of the drugs are available in generic formulations and so the cost of modern PAH treatment has fallen at the same time as its true value is convincingly demonstrated.”
They also argued there was strong evidence for the value of combination therapies, both for PAH-targeted drugs used in combination and concurrent use of immunosuppression and drugs specifically for PAH in some patients with CTD-PAH.
However, they pointed out that not all treatments for idiopathic PAH were suitable for patients with CTDs, highlighting the example of anticoagulation that can improve survival in the first but worsen it in the second.
The study was funded by Actelion. Six authors declared funding and grants from the pharmaceutical sector, including the study sponsor, and three authors were employees of Actelion.
Survival rates for patients with pulmonary arterial hypertension associated with connective tissue diseases have improved significantly in recent years, and there is growing evidence that treatments for idiopathic pulmonary arterial hypertension can also benefit this group.
In an article published online Feb. 3, 2021, in Arthritis & Rheumatology, researchers report the outcomes of a meta-analysis to explore the effect of more modern pulmonary arterial hypertension treatments on patients with conditions such as systemic sclerosis.
First author Dinesh Khanna, MBBS, MSc, of the division of rheumatology at the University of Michigan, Ann Arbor, said in an interview that connective tissue disease–associated pulmonary arterial hypertension (CTD-PAH) was a leading cause of death, but earlier clinical trials had found poor outcomes in patients with CTD, compared with those with idiopathic PAH.
“Recent clinical trial data show that aggressive, up-front PAH treatments have better outcomes in those with CTD-PAH, and we wanted to explore these observations carefully in a systematic review and meta-analysis,” Dr. Khanna said.
The analysis included 11 randomized, controlled trials, involving 4,329 patients with PAH (1,267 with CTD), and 19 registries with a total of 9,739 patients with PAH, including 4,008 with CTD. Trials were required to report long-term clinical outcomes with a median enrollment time of greater than 6 months, and outcomes measured between 3-6 months after the patients started treatment.
Patients with CTDs had an older mean age and a lower 6-minute walk distance than did those with idiopathic PAH.
Five randomized, controlled trials – involving 3,172 patients, 941 of whom had a CTD – found that additional PAH treatment was associated with a 36% reduction in the risk of morbidity or mortality events, compared with controls both in the overall PAH group and in those with CTD.
Additional therapy was also associated with a 34.6-meter increase in 6-minute walk distance in the general PAH population, and a 20.4-meter increase in those with CTD.
The authors commented that the smaller improvement in 6-minute walk distance among patients with CTD may be influenced by comorbidities such as musculoskeletal involvement that would be independent of their cardiopulmonary function.
Differential patient survival among PAH etiologies
“Our meta-analysis of RCTs demonstrated that patients with CTD-PAH derive a clinically significant benefit from currently available PAH therapies which, in many patients, comprised the addition of a drug targeting a second or third pathway involved in the pathophysiology of PAH,” the authors wrote.
When researchers analyzed data from nine registries that included a wide range of PAH etiologies, they found the overall survival rates were lower among patients with CTD, compared with the overall population. The analysis also suggested that patients with systemic sclerosis and PAH had lower survival rates than did those with systemic lupus erythematosus.
Dr. Khanna said this may relate to different pathophysiology of PAH in patients with CTDs, but could also be a reflection of other differences, such as older age and the involvement of other comorbidities, including lung fibrosis and heart involvement.
Data across all 19 registries also showed that survival rates among those with CTD were higher in registries where more than 50% of the registry study period was during or after 2010, compared with registries where 50% or more of the study period was before 2010.
The authors suggested the differences in survival rates may relate to increased screening for PAH, particularly among people with CTDs. They noted that increased screening leads to earlier diagnosis, which could introduce a lead-time bias such that later registries would have younger participants with less severe disease. However, their analysis found that the later registries had older patients but also with less severe disease, and they suggested that it wasn’t possible to determine if lead-time bias was playing a role in their results.
Improvements in treatment options could also account for differences in survival over time, although the authors commented that only six registries in the study included patients from 2015 or later, when currently available treatments came into use and early combination therapy was used more.
“These data also support the 2018 World Symposium on Pulmonary Hypertension recommendations to initiate up-front combination pulmonary arterial hypertension therapy in majority of cases with CTD-PAH,” Dr. Khanna said.
‘Still have to be aggressive at identifying the high-risk patients’
Commenting on the findings, Virginia Steen, MD, of the division of rheumatology at Georgetown University, Washington, said clinicians were finally seeing some significant changes over time in scleroderma-associated PAH.
“Although some of it may be just early diagnosis, I think that the combination of early diagnosis and more aggressive treatment with combination medication is definitely making a difference,” Dr. Steen said in an interview. “The bottom line is that we as rheumatologists still have to be aggressive at identifying the high-risk patients, making an early diagnosis, and working with our pulmonary hypertension colleagues and aggressively treating these patients so we can make a long-term difference.”
The authors of an accompanying editorial said the meta-analysis’ findings showed the positive impact of early combination therapy and early diagnosis through proactive screening.
“It is notable because the present analysis again confirms that outcomes are worse in CTD-PAH than in idiopathic or familial forms of PAH, the impact of treatments should no longer be regarded as insignificant,” the editorial’s authors wrote. “This is a practice changing observation, especially now that many of the drugs are available in generic formulations and so the cost of modern PAH treatment has fallen at the same time as its true value is convincingly demonstrated.”
They also argued there was strong evidence for the value of combination therapies, both for PAH-targeted drugs used in combination and concurrent use of immunosuppression and drugs specifically for PAH in some patients with CTD-PAH.
However, they pointed out that not all treatments for idiopathic PAH were suitable for patients with CTDs, highlighting the example of anticoagulation that can improve survival in the first but worsen it in the second.
The study was funded by Actelion. Six authors declared funding and grants from the pharmaceutical sector, including the study sponsor, and three authors were employees of Actelion.
FROM ARTHRITIS & RHEUMATOLOGY
Prospective data support delaying antibiotics for pediatric respiratory infections
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
FROM PEDIATRICS
Checkpoint inhibitors’ ‘big picture’ safety shown with preexisting autoimmune diseases
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
FROM ANNALS OF INTERNAL MEDICINE
The lost year – even for common respiratory viruses
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.
The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).

What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).

What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.