User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
AI detects ugly-duckling skin lesions for melanoma follow-up
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
How to convince patients muscle pain isn’t a statin Achilles heel: StatinWISE
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Study: Central sleep apnea is common in ticagrelor users post ACS
The prevalence of asymptomatic central sleep apnea after acute coronary syndrome is high and may be associated with the use of ticagrelor, a new study finds.
Prior studies have suggested that ticagrelor is associated with an increased likelihood of central sleep apnea. The drug’s label notes that two respiratory conditions – central sleep apnea and Cheyne-Stokes respiration – are adverse reactions that were identified after the drug’s approval in the United States in 2011. “Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the label says.
Among 80 patients receiving ticagrelor, 24 had central sleep apnea hypopnea syndrome (CSAHS), whereas of 41 patients not taking ticagrelor, 3 had this condition (30% vs. 7.3%, P = .004), in the new study published online Jan. 20, 2021, in Sleep Medicine. A multivariable analysis included in the paper found that age and ticagrelor administration were the only two factors associated with the occurrence of CSAHS.
Findings are ‘striking’
The different rates of central sleep apnea in the study are striking, but it is not clear that asymptomatic central sleep apnea in patients taking ticagrelor is a concern, Ofer Jacobowitz, MD, PhD, associate professor of otolaryngology at Hofstra University, Hempstead, N.Y, said in an interview.
“Whether this particular drug-induced central sleep apnea is consequential” is an open question, noted Dr. Jacobowitz. “There is no evidence that shows that this is definitely harmful.”
“The different types of central sleep apnea are caused by different mechanisms and this one, we don’t know,” Dr. Jacobwitz added.
Study author continues to prescribe ticagrelor
One of the study authors, Philippe Meurin, MD, said that he continues to prescribe ticagrelor every day and that the side effect is not necessarily important.
It is possible that central sleep apnea may resolve, although further studies would need to examine central sleep apnea over time to establish the duration of the condition, he added. Nevertheless, awareness of the association could have implications for clinical practice, Dr. Meurin said.
Central sleep apnea is rare, and if doctors detect it during a sleep study, they may perform extensive tests to assess for possible neurologic diseases, for example, when the cause may be attributed to the medication, he said. In addition, if a patient who is taking ticagrelor has dyspnea, the presence of central sleep apnea may suggest that dyspnea could be related to the drug, although this possibility needs further study, he noted.
Study included patients with ACS history, but no heart failure
Dr. Meurin, of Centre de Réadaptation Cardiaque de La Brie, Les Grands Prés, Villeneuve-Saint-Denis, France, and colleagues included in their study patients between 1 week and 1 year after acute coronary syndrome who did not have heart failure or a history of sleep apnea.
After an overnight sleep study, they classified patients as normal, as having CSAHS (i.e., an apnea-hypopnea index of 15 or greater, mostly with central sleep apneas), or as having obstructive sleep apnea hypopnea syndrome (OSAHS; i.e., an apnea-hypopnea index of 15 or greater, mostly with obstructive sleep apneas).
The prospective study included 121 consecutive patients between January 2018 and March 2020. Patients had a mean age of 56.8, and 88% were men.
Switching to another P2Y12 inhibitor ‘does not seem appropriate’
“CSAHS could be promoted by the use of ticagrelor, a relatively new drug that modifies the apneic threshold,” the study authors wrote. “Regarding underlying mechanisms, the most probable explanation seems to be increased chemosensitivity to hypercapnia by a direct P2Y12 inhibitory effect on the central nervous system.”
Doctors should not overestimate the severity of the adverse reaction or consider it the same way they do OSASH, they added.
Among patients with acute coronary syndrome in the PLATO study, ticagrelor, compared with clopidogrel, “significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke,” Dr. Meurin and colleagues said. “Because in this study more than 9,000 patients received ticagrelor for 12 months, CSAHS (even if it seems frequent in our study) did not seem to impair the good efficacy/tolerance balance of the drug. Therefore, in asymptomatic CSAHS patients, switching from ticagrelor to another P2Y12 inhibitor does not seem appropriate.”
A recent analysis of data from randomized, controlled trials with ticagrelor did not find excess cases of sleep apnea with the drug. But an asymptomatic adverse event such as central sleep apnea “cannot emerge from a post hoc analysis,” Dr. Meurin and colleagues said.
The analysis of randomized trial data was conducted by Marc S. Sabatine, MD, MPH, chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group at Brigham and Women’s Hospital, and coauthors. It was published in JACC: Cardiovascular Interventions in April 2020.
They “used the gold standard for medical evidence (randomized, placebo-controlled trials) and found 158 cases of sleep apnea reported, with absolutely no difference between ticagrelor and placebo,” Dr. Sabatine said in an interview. Their analysis examined clinically overt apnea, he noted.
“It is quite clear that when looking at large numbers in placebo-controlled trials, there is no excess,” Dr. Sabatine said. “Meurin et al. are examining a different outcome: the results of a lab test in what may be entirely asymptomatic patients.”
A randomized trial could confirm the association, he said.
“The association may be real, but also may be play of chance or confounded,” said Dr. Sabatine. “To convince the medical community, the next step would be for the investigators to do a randomized trial and test whether ticagrelor increases the risk of central sleep apnea.”
Dr. Meurin and the study coauthors had no disclosures. The analysis of randomized, controlled trial data by Dr. Sabatine and colleagues was funded by AstraZeneca, which distributes ticagrelor under the trade name Brilinta. Dr. Sabatine has been a consultant for AstraZeneca and received research grants through Brigham and Women’s Hospital from AstraZeneca. He has consulted for and received grants through the hospital from other companies as well. Dr. Jacobowitz had no relevant disclosures.
[email protected]
The prevalence of asymptomatic central sleep apnea after acute coronary syndrome is high and may be associated with the use of ticagrelor, a new study finds.
Prior studies have suggested that ticagrelor is associated with an increased likelihood of central sleep apnea. The drug’s label notes that two respiratory conditions – central sleep apnea and Cheyne-Stokes respiration – are adverse reactions that were identified after the drug’s approval in the United States in 2011. “Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the label says.
Among 80 patients receiving ticagrelor, 24 had central sleep apnea hypopnea syndrome (CSAHS), whereas of 41 patients not taking ticagrelor, 3 had this condition (30% vs. 7.3%, P = .004), in the new study published online Jan. 20, 2021, in Sleep Medicine. A multivariable analysis included in the paper found that age and ticagrelor administration were the only two factors associated with the occurrence of CSAHS.
Findings are ‘striking’
The different rates of central sleep apnea in the study are striking, but it is not clear that asymptomatic central sleep apnea in patients taking ticagrelor is a concern, Ofer Jacobowitz, MD, PhD, associate professor of otolaryngology at Hofstra University, Hempstead, N.Y, said in an interview.
“Whether this particular drug-induced central sleep apnea is consequential” is an open question, noted Dr. Jacobowitz. “There is no evidence that shows that this is definitely harmful.”
“The different types of central sleep apnea are caused by different mechanisms and this one, we don’t know,” Dr. Jacobwitz added.
Study author continues to prescribe ticagrelor
One of the study authors, Philippe Meurin, MD, said that he continues to prescribe ticagrelor every day and that the side effect is not necessarily important.
It is possible that central sleep apnea may resolve, although further studies would need to examine central sleep apnea over time to establish the duration of the condition, he added. Nevertheless, awareness of the association could have implications for clinical practice, Dr. Meurin said.
Central sleep apnea is rare, and if doctors detect it during a sleep study, they may perform extensive tests to assess for possible neurologic diseases, for example, when the cause may be attributed to the medication, he said. In addition, if a patient who is taking ticagrelor has dyspnea, the presence of central sleep apnea may suggest that dyspnea could be related to the drug, although this possibility needs further study, he noted.
Study included patients with ACS history, but no heart failure
Dr. Meurin, of Centre de Réadaptation Cardiaque de La Brie, Les Grands Prés, Villeneuve-Saint-Denis, France, and colleagues included in their study patients between 1 week and 1 year after acute coronary syndrome who did not have heart failure or a history of sleep apnea.
After an overnight sleep study, they classified patients as normal, as having CSAHS (i.e., an apnea-hypopnea index of 15 or greater, mostly with central sleep apneas), or as having obstructive sleep apnea hypopnea syndrome (OSAHS; i.e., an apnea-hypopnea index of 15 or greater, mostly with obstructive sleep apneas).
The prospective study included 121 consecutive patients between January 2018 and March 2020. Patients had a mean age of 56.8, and 88% were men.
Switching to another P2Y12 inhibitor ‘does not seem appropriate’
“CSAHS could be promoted by the use of ticagrelor, a relatively new drug that modifies the apneic threshold,” the study authors wrote. “Regarding underlying mechanisms, the most probable explanation seems to be increased chemosensitivity to hypercapnia by a direct P2Y12 inhibitory effect on the central nervous system.”
Doctors should not overestimate the severity of the adverse reaction or consider it the same way they do OSASH, they added.
Among patients with acute coronary syndrome in the PLATO study, ticagrelor, compared with clopidogrel, “significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke,” Dr. Meurin and colleagues said. “Because in this study more than 9,000 patients received ticagrelor for 12 months, CSAHS (even if it seems frequent in our study) did not seem to impair the good efficacy/tolerance balance of the drug. Therefore, in asymptomatic CSAHS patients, switching from ticagrelor to another P2Y12 inhibitor does not seem appropriate.”
A recent analysis of data from randomized, controlled trials with ticagrelor did not find excess cases of sleep apnea with the drug. But an asymptomatic adverse event such as central sleep apnea “cannot emerge from a post hoc analysis,” Dr. Meurin and colleagues said.
The analysis of randomized trial data was conducted by Marc S. Sabatine, MD, MPH, chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group at Brigham and Women’s Hospital, and coauthors. It was published in JACC: Cardiovascular Interventions in April 2020.
They “used the gold standard for medical evidence (randomized, placebo-controlled trials) and found 158 cases of sleep apnea reported, with absolutely no difference between ticagrelor and placebo,” Dr. Sabatine said in an interview. Their analysis examined clinically overt apnea, he noted.
“It is quite clear that when looking at large numbers in placebo-controlled trials, there is no excess,” Dr. Sabatine said. “Meurin et al. are examining a different outcome: the results of a lab test in what may be entirely asymptomatic patients.”
A randomized trial could confirm the association, he said.
“The association may be real, but also may be play of chance or confounded,” said Dr. Sabatine. “To convince the medical community, the next step would be for the investigators to do a randomized trial and test whether ticagrelor increases the risk of central sleep apnea.”
Dr. Meurin and the study coauthors had no disclosures. The analysis of randomized, controlled trial data by Dr. Sabatine and colleagues was funded by AstraZeneca, which distributes ticagrelor under the trade name Brilinta. Dr. Sabatine has been a consultant for AstraZeneca and received research grants through Brigham and Women’s Hospital from AstraZeneca. He has consulted for and received grants through the hospital from other companies as well. Dr. Jacobowitz had no relevant disclosures.
[email protected]
The prevalence of asymptomatic central sleep apnea after acute coronary syndrome is high and may be associated with the use of ticagrelor, a new study finds.
Prior studies have suggested that ticagrelor is associated with an increased likelihood of central sleep apnea. The drug’s label notes that two respiratory conditions – central sleep apnea and Cheyne-Stokes respiration – are adverse reactions that were identified after the drug’s approval in the United States in 2011. “Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the label says.
Among 80 patients receiving ticagrelor, 24 had central sleep apnea hypopnea syndrome (CSAHS), whereas of 41 patients not taking ticagrelor, 3 had this condition (30% vs. 7.3%, P = .004), in the new study published online Jan. 20, 2021, in Sleep Medicine. A multivariable analysis included in the paper found that age and ticagrelor administration were the only two factors associated with the occurrence of CSAHS.
Findings are ‘striking’
The different rates of central sleep apnea in the study are striking, but it is not clear that asymptomatic central sleep apnea in patients taking ticagrelor is a concern, Ofer Jacobowitz, MD, PhD, associate professor of otolaryngology at Hofstra University, Hempstead, N.Y, said in an interview.
“Whether this particular drug-induced central sleep apnea is consequential” is an open question, noted Dr. Jacobowitz. “There is no evidence that shows that this is definitely harmful.”
“The different types of central sleep apnea are caused by different mechanisms and this one, we don’t know,” Dr. Jacobwitz added.
Study author continues to prescribe ticagrelor
One of the study authors, Philippe Meurin, MD, said that he continues to prescribe ticagrelor every day and that the side effect is not necessarily important.
It is possible that central sleep apnea may resolve, although further studies would need to examine central sleep apnea over time to establish the duration of the condition, he added. Nevertheless, awareness of the association could have implications for clinical practice, Dr. Meurin said.
Central sleep apnea is rare, and if doctors detect it during a sleep study, they may perform extensive tests to assess for possible neurologic diseases, for example, when the cause may be attributed to the medication, he said. In addition, if a patient who is taking ticagrelor has dyspnea, the presence of central sleep apnea may suggest that dyspnea could be related to the drug, although this possibility needs further study, he noted.
Study included patients with ACS history, but no heart failure
Dr. Meurin, of Centre de Réadaptation Cardiaque de La Brie, Les Grands Prés, Villeneuve-Saint-Denis, France, and colleagues included in their study patients between 1 week and 1 year after acute coronary syndrome who did not have heart failure or a history of sleep apnea.
After an overnight sleep study, they classified patients as normal, as having CSAHS (i.e., an apnea-hypopnea index of 15 or greater, mostly with central sleep apneas), or as having obstructive sleep apnea hypopnea syndrome (OSAHS; i.e., an apnea-hypopnea index of 15 or greater, mostly with obstructive sleep apneas).
The prospective study included 121 consecutive patients between January 2018 and March 2020. Patients had a mean age of 56.8, and 88% were men.
Switching to another P2Y12 inhibitor ‘does not seem appropriate’
“CSAHS could be promoted by the use of ticagrelor, a relatively new drug that modifies the apneic threshold,” the study authors wrote. “Regarding underlying mechanisms, the most probable explanation seems to be increased chemosensitivity to hypercapnia by a direct P2Y12 inhibitory effect on the central nervous system.”
Doctors should not overestimate the severity of the adverse reaction or consider it the same way they do OSASH, they added.
Among patients with acute coronary syndrome in the PLATO study, ticagrelor, compared with clopidogrel, “significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke,” Dr. Meurin and colleagues said. “Because in this study more than 9,000 patients received ticagrelor for 12 months, CSAHS (even if it seems frequent in our study) did not seem to impair the good efficacy/tolerance balance of the drug. Therefore, in asymptomatic CSAHS patients, switching from ticagrelor to another P2Y12 inhibitor does not seem appropriate.”
A recent analysis of data from randomized, controlled trials with ticagrelor did not find excess cases of sleep apnea with the drug. But an asymptomatic adverse event such as central sleep apnea “cannot emerge from a post hoc analysis,” Dr. Meurin and colleagues said.
The analysis of randomized trial data was conducted by Marc S. Sabatine, MD, MPH, chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group at Brigham and Women’s Hospital, and coauthors. It was published in JACC: Cardiovascular Interventions in April 2020.
They “used the gold standard for medical evidence (randomized, placebo-controlled trials) and found 158 cases of sleep apnea reported, with absolutely no difference between ticagrelor and placebo,” Dr. Sabatine said in an interview. Their analysis examined clinically overt apnea, he noted.
“It is quite clear that when looking at large numbers in placebo-controlled trials, there is no excess,” Dr. Sabatine said. “Meurin et al. are examining a different outcome: the results of a lab test in what may be entirely asymptomatic patients.”
A randomized trial could confirm the association, he said.
“The association may be real, but also may be play of chance or confounded,” said Dr. Sabatine. “To convince the medical community, the next step would be for the investigators to do a randomized trial and test whether ticagrelor increases the risk of central sleep apnea.”
Dr. Meurin and the study coauthors had no disclosures. The analysis of randomized, controlled trial data by Dr. Sabatine and colleagues was funded by AstraZeneca, which distributes ticagrelor under the trade name Brilinta. Dr. Sabatine has been a consultant for AstraZeneca and received research grants through Brigham and Women’s Hospital from AstraZeneca. He has consulted for and received grants through the hospital from other companies as well. Dr. Jacobowitz had no relevant disclosures.
[email protected]
FROM SLEEP MEDICINE
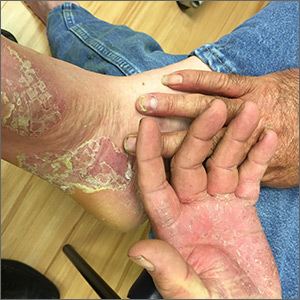
Painful hand and foot plaques
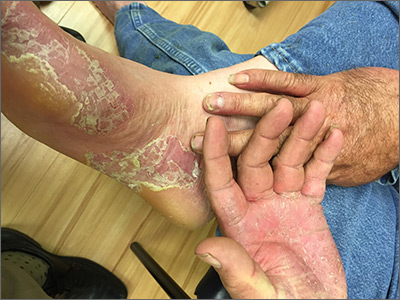
This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.

This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.
Emerging treatments for molluscum contagiosum and acne show promise
, but that could soon change, according to Leon H. Kircik, MD.
“The treatment of molluscum is still an unmet need,” Dr. Kircik, clinical professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said at the Orlando Dermatology Aesthetic and Clinical Conference. However, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been tested in phase 3 studies, is currently under FDA review. The manufacturer, Verrica Pharmaceuticals resubmitted a new drug application for the product, VP-102, in December 2020.
“VP-102 features a visualization agent so the injector can see which lesions have been treated, as well as a bittering agent to mitigate oral ingestion by children. Complete clearance at 12 weeks ranged from 46% to 54% of patients, while lesion count reduction compared with baseline ranged from 69% to 82%.”
Acne
In August, 2020, clascoterone 1% cream was approved for the treatment of acne in patients 12 years and older, a development that Dr. Kircik said “can be a game changer in acne treatment.” Clascoterone cream 1% exhibits strong, selective anti-androgen activity by targeting androgen receptors in the skin, not systemically. “It limits or blocks transcription of androgen responsive genes, but it also has an anti-inflammatory effect and an anti-sebum effect,” he explained.
According to results from two phase 3 trials of the product, a response of clear or almost clear on the IGA scale at week 12 was achieved in 18.4% of those on treatment vs. 9% of those on vehicle in one study (P less than .001) and 20.3% vs. 6.5%, respectively, in the second study (P less than .001). Clascoterone is also being evaluated for treating androgenetic alopecia.
In Dr. Kircik’s clinical experience, retinoids can be helpful for patients with moderate to severe acne. “We always use them for anticomedogenic effects, but we also know that they have anti-inflammatory effects,” he said. “They actually inhibit toll-like receptor activity. They also inhibit the AP-1 pathway by causing a reduction in inflammatory signaling associated with collagen degradation and scarring.”
The most recent retinoid to be approved for the topical treatment of acne was 0.005% trifarotene cream, in 2019, for patients aged 9 years and older. “But when we got the results, it was not that exciting,” a difference of about 3.6 (mean) inflammatory lesion reduction between the active and the vehicle arm, said Dr. Kircik, medical director of Physicians Skin Care in Louisville, Ky. “According to the package insert, treatment side effects included mild to moderate erythema in 59% of patients, scaling in 65%, dryness in 69%, and stinging/burning in 56%, which makes it difficult to use in our clinical practice.”
The drug was also tested for treating truncal acne. However, one comparative study showed that tazarotene 0.045% lotion spread an average of 36.7 square centimeters farther than the trifarotene cream, which makes the tazarotene lotion easier to use on the chest and back, he said.
Dr. Kircik also discussed 4% minocycline, a hydrophobic, topical foam formulation of minocycline that was approved by the FDA in 2019 for the treatment of moderate to severe acne, for patients aged 9 and older. In a 12-week study that involved 1,488 patients (mean age was about 20 years), investigators observed a 56% reduction in inflammatory lesion count among those treated with minocycline 4%, compared with 43% in the vehicle group.
Dr. Kircik, one of the authors of the study, noted that the hydrophobic composition of minocycline 4% allows for stable and efficient delivery of an inherently unstable active pharmaceutical ingredient such as minocycline. “It’s free of primary irritants such as surfactants and short chain alcohols, which makes it much more tolerable,” he said. “The unique physical foam characteristics facilitate ease of application and absorption at target sites.”
Dr. Kircik reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including Galderma, the manufacturer of trifarotene cream.
[email protected]
, but that could soon change, according to Leon H. Kircik, MD.
“The treatment of molluscum is still an unmet need,” Dr. Kircik, clinical professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said at the Orlando Dermatology Aesthetic and Clinical Conference. However, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been tested in phase 3 studies, is currently under FDA review. The manufacturer, Verrica Pharmaceuticals resubmitted a new drug application for the product, VP-102, in December 2020.
“VP-102 features a visualization agent so the injector can see which lesions have been treated, as well as a bittering agent to mitigate oral ingestion by children. Complete clearance at 12 weeks ranged from 46% to 54% of patients, while lesion count reduction compared with baseline ranged from 69% to 82%.”
Acne
In August, 2020, clascoterone 1% cream was approved for the treatment of acne in patients 12 years and older, a development that Dr. Kircik said “can be a game changer in acne treatment.” Clascoterone cream 1% exhibits strong, selective anti-androgen activity by targeting androgen receptors in the skin, not systemically. “It limits or blocks transcription of androgen responsive genes, but it also has an anti-inflammatory effect and an anti-sebum effect,” he explained.
According to results from two phase 3 trials of the product, a response of clear or almost clear on the IGA scale at week 12 was achieved in 18.4% of those on treatment vs. 9% of those on vehicle in one study (P less than .001) and 20.3% vs. 6.5%, respectively, in the second study (P less than .001). Clascoterone is also being evaluated for treating androgenetic alopecia.
In Dr. Kircik’s clinical experience, retinoids can be helpful for patients with moderate to severe acne. “We always use them for anticomedogenic effects, but we also know that they have anti-inflammatory effects,” he said. “They actually inhibit toll-like receptor activity. They also inhibit the AP-1 pathway by causing a reduction in inflammatory signaling associated with collagen degradation and scarring.”
The most recent retinoid to be approved for the topical treatment of acne was 0.005% trifarotene cream, in 2019, for patients aged 9 years and older. “But when we got the results, it was not that exciting,” a difference of about 3.6 (mean) inflammatory lesion reduction between the active and the vehicle arm, said Dr. Kircik, medical director of Physicians Skin Care in Louisville, Ky. “According to the package insert, treatment side effects included mild to moderate erythema in 59% of patients, scaling in 65%, dryness in 69%, and stinging/burning in 56%, which makes it difficult to use in our clinical practice.”
The drug was also tested for treating truncal acne. However, one comparative study showed that tazarotene 0.045% lotion spread an average of 36.7 square centimeters farther than the trifarotene cream, which makes the tazarotene lotion easier to use on the chest and back, he said.
Dr. Kircik also discussed 4% minocycline, a hydrophobic, topical foam formulation of minocycline that was approved by the FDA in 2019 for the treatment of moderate to severe acne, for patients aged 9 and older. In a 12-week study that involved 1,488 patients (mean age was about 20 years), investigators observed a 56% reduction in inflammatory lesion count among those treated with minocycline 4%, compared with 43% in the vehicle group.
Dr. Kircik, one of the authors of the study, noted that the hydrophobic composition of minocycline 4% allows for stable and efficient delivery of an inherently unstable active pharmaceutical ingredient such as minocycline. “It’s free of primary irritants such as surfactants and short chain alcohols, which makes it much more tolerable,” he said. “The unique physical foam characteristics facilitate ease of application and absorption at target sites.”
Dr. Kircik reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including Galderma, the manufacturer of trifarotene cream.
[email protected]
, but that could soon change, according to Leon H. Kircik, MD.
“The treatment of molluscum is still an unmet need,” Dr. Kircik, clinical professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said at the Orlando Dermatology Aesthetic and Clinical Conference. However, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been tested in phase 3 studies, is currently under FDA review. The manufacturer, Verrica Pharmaceuticals resubmitted a new drug application for the product, VP-102, in December 2020.
“VP-102 features a visualization agent so the injector can see which lesions have been treated, as well as a bittering agent to mitigate oral ingestion by children. Complete clearance at 12 weeks ranged from 46% to 54% of patients, while lesion count reduction compared with baseline ranged from 69% to 82%.”
Acne
In August, 2020, clascoterone 1% cream was approved for the treatment of acne in patients 12 years and older, a development that Dr. Kircik said “can be a game changer in acne treatment.” Clascoterone cream 1% exhibits strong, selective anti-androgen activity by targeting androgen receptors in the skin, not systemically. “It limits or blocks transcription of androgen responsive genes, but it also has an anti-inflammatory effect and an anti-sebum effect,” he explained.
According to results from two phase 3 trials of the product, a response of clear or almost clear on the IGA scale at week 12 was achieved in 18.4% of those on treatment vs. 9% of those on vehicle in one study (P less than .001) and 20.3% vs. 6.5%, respectively, in the second study (P less than .001). Clascoterone is also being evaluated for treating androgenetic alopecia.
In Dr. Kircik’s clinical experience, retinoids can be helpful for patients with moderate to severe acne. “We always use them for anticomedogenic effects, but we also know that they have anti-inflammatory effects,” he said. “They actually inhibit toll-like receptor activity. They also inhibit the AP-1 pathway by causing a reduction in inflammatory signaling associated with collagen degradation and scarring.”
The most recent retinoid to be approved for the topical treatment of acne was 0.005% trifarotene cream, in 2019, for patients aged 9 years and older. “But when we got the results, it was not that exciting,” a difference of about 3.6 (mean) inflammatory lesion reduction between the active and the vehicle arm, said Dr. Kircik, medical director of Physicians Skin Care in Louisville, Ky. “According to the package insert, treatment side effects included mild to moderate erythema in 59% of patients, scaling in 65%, dryness in 69%, and stinging/burning in 56%, which makes it difficult to use in our clinical practice.”
The drug was also tested for treating truncal acne. However, one comparative study showed that tazarotene 0.045% lotion spread an average of 36.7 square centimeters farther than the trifarotene cream, which makes the tazarotene lotion easier to use on the chest and back, he said.
Dr. Kircik also discussed 4% minocycline, a hydrophobic, topical foam formulation of minocycline that was approved by the FDA in 2019 for the treatment of moderate to severe acne, for patients aged 9 and older. In a 12-week study that involved 1,488 patients (mean age was about 20 years), investigators observed a 56% reduction in inflammatory lesion count among those treated with minocycline 4%, compared with 43% in the vehicle group.
Dr. Kircik, one of the authors of the study, noted that the hydrophobic composition of minocycline 4% allows for stable and efficient delivery of an inherently unstable active pharmaceutical ingredient such as minocycline. “It’s free of primary irritants such as surfactants and short chain alcohols, which makes it much more tolerable,” he said. “The unique physical foam characteristics facilitate ease of application and absorption at target sites.”
Dr. Kircik reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including Galderma, the manufacturer of trifarotene cream.
[email protected]
FROM ODAC 2021
Obesity pegged as diabetes cause in almost half of U.S. cases
Roughly 40% of all U.S. cases of incident diabetes during 2013-2016 were directly attributable to obesity, a finding that further solidifies the major etiologic role for obesity in the current American diabetes epidemic.
Researchers used data from a diverse cohort of 4,200 American adults in the MESA study during 2000-2017 to calculate a relative risk for developing diabetes of 2.7 in people with obesity compared with similar participants without obesity.
They then applied this relative risk estimate to obesity prevalence rates during serial iterations of NHANES, the recurring U.S.-wide survey of vital statistics in a representative cross-sectional population.
Their calculations showed that, during 2013-2016, 41% of U.S. adults who developed new onset diabetes did so because of obesity, after the researchers adjusted for potential confounders.
This “population attributable fraction,” or disease burden attributable to obesity, varied somewhat by sex, and by racial and ethnic subgrouping. Obesity was linked with the highest attributable rate among non-Hispanic White women, a rate of 53%, and with the lowest rate among non-Hispanic Black men, with an attributable fraction of 30%, Natalie A. Cameron, MD, and colleagues reported in their study, published online Feb. 10 in the Journal of the American Heart Association.
Potential for “meaningful impact” by reducing obesity
“Our study highlights the meaningful impact that reducing obesity could have on type 2 diabetes prevention in the United States. Decreasing obesity needs to be a priority,” Dr. Cameron, of the McGaw Medical Center of Northwestern University in Chicago, said in a statement issued by the American Heart Association.
“Public health efforts that support healthy lifestyles, such as increasing access to nutritious foods, promoting physical activity, and developing community programs to prevent obesity, could substantially reduce new cases of type 2 diabetes,” she added.
MESA (Multi-Ethnic Study of Atherosclerosis) enrolled adults aged 45-84 years and free from clinical cardiovascular disease at six U.S. sites during 2000-2002, and then followed them with four additional examinations through 2017.
For the current study, researchers narrowed the cohort down to 4,200 participants who were aged 45-79 years and free from diabetes at entry, and also restricted this subgroup to participants classified as non-Hispanic White (54% of the cohort), non-Hispanic Black (33%), or Mexican American (13%). At entry, 34% of the cohort had obesity, with a body mass index of at least 30 kg/m2.
During a median follow-up of just over 9 years, 12% of the cohort developed incident diabetes. After adjustment for possible confounders, a hazard ratio model showed an overall 2.7-fold higher rate of incident diabetes among people with obesity compared to those without.
The researchers then applied this hazard ratio to obesity prevalence statistics from NHANES (National Health and Nutrition Examination Survey) during the same time period, with data from the biennial NHANES project collapsed into four time strata: 2001-2004, 2005-2008, 2009-2012, and 2013-2016. They again limited their analysis to NHANES data collected from people aged 45-79 years who self-reported categorization as non-Hispanic White, non-Hispanic Black, or Mexican American.
During the period from 2001-2004 to 2013-2016, overall obesity prevalence tallied by NHANES data rose from 34% to 41%. Among people with type 2 diabetes during 2013-2016, obesity prevalence was 65%.
To calculate the population attributable fraction researchers combined the MESA and NHANES estimates and adjusted for potential confounders and found that, overall, in 41% of people with incident diabetes during 2013-2016, the disease was attributable to obesity.
The study received no commercial funding, and none of the authors had disclosures.
A version of this article first appeared on Medscape.com.
Roughly 40% of all U.S. cases of incident diabetes during 2013-2016 were directly attributable to obesity, a finding that further solidifies the major etiologic role for obesity in the current American diabetes epidemic.
Researchers used data from a diverse cohort of 4,200 American adults in the MESA study during 2000-2017 to calculate a relative risk for developing diabetes of 2.7 in people with obesity compared with similar participants without obesity.
They then applied this relative risk estimate to obesity prevalence rates during serial iterations of NHANES, the recurring U.S.-wide survey of vital statistics in a representative cross-sectional population.
Their calculations showed that, during 2013-2016, 41% of U.S. adults who developed new onset diabetes did so because of obesity, after the researchers adjusted for potential confounders.
This “population attributable fraction,” or disease burden attributable to obesity, varied somewhat by sex, and by racial and ethnic subgrouping. Obesity was linked with the highest attributable rate among non-Hispanic White women, a rate of 53%, and with the lowest rate among non-Hispanic Black men, with an attributable fraction of 30%, Natalie A. Cameron, MD, and colleagues reported in their study, published online Feb. 10 in the Journal of the American Heart Association.
Potential for “meaningful impact” by reducing obesity
“Our study highlights the meaningful impact that reducing obesity could have on type 2 diabetes prevention in the United States. Decreasing obesity needs to be a priority,” Dr. Cameron, of the McGaw Medical Center of Northwestern University in Chicago, said in a statement issued by the American Heart Association.
“Public health efforts that support healthy lifestyles, such as increasing access to nutritious foods, promoting physical activity, and developing community programs to prevent obesity, could substantially reduce new cases of type 2 diabetes,” she added.
MESA (Multi-Ethnic Study of Atherosclerosis) enrolled adults aged 45-84 years and free from clinical cardiovascular disease at six U.S. sites during 2000-2002, and then followed them with four additional examinations through 2017.
For the current study, researchers narrowed the cohort down to 4,200 participants who were aged 45-79 years and free from diabetes at entry, and also restricted this subgroup to participants classified as non-Hispanic White (54% of the cohort), non-Hispanic Black (33%), or Mexican American (13%). At entry, 34% of the cohort had obesity, with a body mass index of at least 30 kg/m2.
During a median follow-up of just over 9 years, 12% of the cohort developed incident diabetes. After adjustment for possible confounders, a hazard ratio model showed an overall 2.7-fold higher rate of incident diabetes among people with obesity compared to those without.
The researchers then applied this hazard ratio to obesity prevalence statistics from NHANES (National Health and Nutrition Examination Survey) during the same time period, with data from the biennial NHANES project collapsed into four time strata: 2001-2004, 2005-2008, 2009-2012, and 2013-2016. They again limited their analysis to NHANES data collected from people aged 45-79 years who self-reported categorization as non-Hispanic White, non-Hispanic Black, or Mexican American.
During the period from 2001-2004 to 2013-2016, overall obesity prevalence tallied by NHANES data rose from 34% to 41%. Among people with type 2 diabetes during 2013-2016, obesity prevalence was 65%.
To calculate the population attributable fraction researchers combined the MESA and NHANES estimates and adjusted for potential confounders and found that, overall, in 41% of people with incident diabetes during 2013-2016, the disease was attributable to obesity.
The study received no commercial funding, and none of the authors had disclosures.
A version of this article first appeared on Medscape.com.
Roughly 40% of all U.S. cases of incident diabetes during 2013-2016 were directly attributable to obesity, a finding that further solidifies the major etiologic role for obesity in the current American diabetes epidemic.
Researchers used data from a diverse cohort of 4,200 American adults in the MESA study during 2000-2017 to calculate a relative risk for developing diabetes of 2.7 in people with obesity compared with similar participants without obesity.
They then applied this relative risk estimate to obesity prevalence rates during serial iterations of NHANES, the recurring U.S.-wide survey of vital statistics in a representative cross-sectional population.
Their calculations showed that, during 2013-2016, 41% of U.S. adults who developed new onset diabetes did so because of obesity, after the researchers adjusted for potential confounders.
This “population attributable fraction,” or disease burden attributable to obesity, varied somewhat by sex, and by racial and ethnic subgrouping. Obesity was linked with the highest attributable rate among non-Hispanic White women, a rate of 53%, and with the lowest rate among non-Hispanic Black men, with an attributable fraction of 30%, Natalie A. Cameron, MD, and colleagues reported in their study, published online Feb. 10 in the Journal of the American Heart Association.
Potential for “meaningful impact” by reducing obesity
“Our study highlights the meaningful impact that reducing obesity could have on type 2 diabetes prevention in the United States. Decreasing obesity needs to be a priority,” Dr. Cameron, of the McGaw Medical Center of Northwestern University in Chicago, said in a statement issued by the American Heart Association.
“Public health efforts that support healthy lifestyles, such as increasing access to nutritious foods, promoting physical activity, and developing community programs to prevent obesity, could substantially reduce new cases of type 2 diabetes,” she added.
MESA (Multi-Ethnic Study of Atherosclerosis) enrolled adults aged 45-84 years and free from clinical cardiovascular disease at six U.S. sites during 2000-2002, and then followed them with four additional examinations through 2017.
For the current study, researchers narrowed the cohort down to 4,200 participants who were aged 45-79 years and free from diabetes at entry, and also restricted this subgroup to participants classified as non-Hispanic White (54% of the cohort), non-Hispanic Black (33%), or Mexican American (13%). At entry, 34% of the cohort had obesity, with a body mass index of at least 30 kg/m2.
During a median follow-up of just over 9 years, 12% of the cohort developed incident diabetes. After adjustment for possible confounders, a hazard ratio model showed an overall 2.7-fold higher rate of incident diabetes among people with obesity compared to those without.
The researchers then applied this hazard ratio to obesity prevalence statistics from NHANES (National Health and Nutrition Examination Survey) during the same time period, with data from the biennial NHANES project collapsed into four time strata: 2001-2004, 2005-2008, 2009-2012, and 2013-2016. They again limited their analysis to NHANES data collected from people aged 45-79 years who self-reported categorization as non-Hispanic White, non-Hispanic Black, or Mexican American.
During the period from 2001-2004 to 2013-2016, overall obesity prevalence tallied by NHANES data rose from 34% to 41%. Among people with type 2 diabetes during 2013-2016, obesity prevalence was 65%.
To calculate the population attributable fraction researchers combined the MESA and NHANES estimates and adjusted for potential confounders and found that, overall, in 41% of people with incident diabetes during 2013-2016, the disease was attributable to obesity.
The study received no commercial funding, and none of the authors had disclosures.
A version of this article first appeared on Medscape.com.
Safety profiles of hemophilia agents vary widely
Despite their similar functions, each current and emerging therapy for treating hemophilia has a unique safety profile, and each needs to be weighed apart from agents both within and outside its pharmacologic class, a hemophilia specialist said.
“My view is that each new molecule coming to the hemophilia space, including variant factor molecules, needs to be scrutinized separately, without class assumptions or extrapolations, and it’s clear that thrombosis risk has become a priority safety consideration,” said Dan Hart, MBChB, MRCP, FRCPath, PhD, from Barts and the London School of Medicine and Dentistry.
He reviewed the comparative safety of standard and novel therapies for hemophilia at the annual congress of the European Association for Haemophilia and Allied Disorders.
Factor inhibitors
Inhibitors occur in both hemophilia A and hemophilia B, and are primarily seen in patients with childhood exposure to factor concentrates. Inhibitors, which include anti–factor VIII and factor IX alloantibodies, are more common among patients with severe hemophilia and those with more disruptive factor VIII and factor IX mutations.
“There can be transient vs. persistent inhibitors, and arguably the more you look, the more you find, but clinically we never miss high-titer inhibitors that have a big impact on individuals and the subsequent decisions about management,” he said.
Hamster vs. human
It’s currently unclear whether there is an immunologic advantage for previously untreated patients to be started on factor VIII concentrates derived from recombinant human cells lines, or from products derived from Chinese hamster ovary (CHO) or baby hamster kidney (BHK) cell lines, Dr. Hart said.
“We need to ensure that we’re not selective about comparator choice for new products in the absence of head-to-head studies,” he said.
Route of administration matters
Inhibitors appear to be a more common occurrence among patients who received factor concentrates subcutaneously, compared with intravenously, Dr. Hart noted, pointing to a 2011 study indicating a background annual risk of 5 cases of inhibitor development per 1,000 treatment years in previously treated patients who received intravenous therapy (Blood. 2011 Jun 9;117[23]:6367-70).
In contrast, in a phase 1 trial of subcutaneous turoctocog alfa pegol, 5 out of 26 patients had detectable N8-GP–binding antibodies after 42-91 exposure days. Of these patients, one developed an inhibitor to factor VIII, and anti–N8-GP antibody appearance was associated with a decline in factor VIII plasma activity in four of the five patients. In addition, five patients reported a total of nine bleeding episodes requiring treatment during prophylaxis. As a result of this trial, further clinical development of the subcutaneous version was suspended. (J Thromb Haemost. 2020 Feb;18[2]:341-51).
Other subcutaneously administered factors are currently in development, Dr. Hart noted.
Nonfactor inhibitors?
“The nonfactor agents do have the risk of generating antibodies: Monoclonal antibodies outside the hemophilia setting provoke antidrug antibodies,” he said.
Although there is no consensus regarding which assay can best monitor antidrug antibodies (ADA), enzyme-linked immunosorbent assay (ELISA) can detect neutralizing antibodies and other antibodies.
In the hemophilia setting, surrogate markers for loss of drug efficacy include longer activated partial thromboplastin time (ATTP) or a drop in serum drug levels. Worsening bleeding phenotype can also be a marker for loss of efficacy, albeit an imperfect one.
Emicizumab (Hemlibara), the first nonfactor monoclonal agent to make it to market, has the largest dataset available, and evidence suggests a rate of neutralizing antibodies with this agent of less than 1% in the HAVEN clinical trial series, but 5.2% in the single-arm STASEY trial.
“We shouldn’t assume that other biophenotypics will have a similar ADA rate, and this needs to be evaluated for each molecule, as it will need to be for other monoclonals” such as anti–tissue factor pathway (TFPI) antibodies, Dr. Hart emphasized.
Pegylation
Pegylated compounds include polyethylene glycol, an inert polymer, covalently bound to the therapeutic protein to extend its half-life, and theoretically, reduce immunogenicity.
Many patients may already have exposure to pegylated products in the form of peginterferon to treat hepatitis C, consumer products such as toothpaste, cough medicine, and cosmetics, and, more recently, in vaccines against COVID-19.
Safety considerations with pegylated agents in hemophilia include concerns about accumulation of polyethylene glycol (PEG), although “some of the preclinical models looking at excretion of PEG are difficult to interpret in my view, and people debate about whether studies are long enough, but it’s undoubtedly the case that toxicology dosing is order of magnitude higher than the routine dosing in hemophilia,” he said.
After more than 5 years of experience with pegylated products there is no clinical evidence of concern, although “it’s not clear, actually, what we’re looking for, whether it’s a clinical parameter, or imaging or histological parameter.”
Patients may also not have lifelong exposure to pegylated products, as it is unlikely that they will stay on the same product for decades, Dr. Hart said.
Thrombosis
As of June 30, 2020, more than 7,200 persons with hemophilia have received emicizumab, and there have been 23 reported thrombotic events, 19 of which occurred in the postmarketing period. Of the reported cases, six patients had a myocardial infarction, and all of these patients had at least one cardiovascular risk factor.
The antithrombin agent fitusiran was associated with one fatal thrombotic event in a phase 2, open-label extension trial, leading to a pause and resumption with mitigation protocols, but that trial has since been paused again because of additional, nonfatal thrombotic events.
Nonfatal thrombotic events have also occurred in clinical trials for the investigational anti-TFPI monoclonal antibodies BAY 1093884 and concizumab, but none have thus far been reported in phase 3 trial of marstacimab.
“We need renewed efforts for prospective reporting and independent review of all adverse events of all agents, old and new: This will need some guidance nationally and internationally, and I think the relevant trial [serious adverse events] need to be reported in peer review literature, and clinicaltrials.gov updated in a timely manner, regardless of whether that strategy was successful or unsuccessful,” Dr. Hart said.
Risk with longer-acting agents?
In the question and answer following his presentation, Christoph Königs, MD, PhD, from University Hospital Frankfurt, asked whether there was potential for increased thrombosis risk with second-generation extended half-life (EHL) molecules in clinical trials.
“As we edge towards normalization of hemostasis, clearly the other non–hemophilia dependent issues of thrombosis risk come into play,” Dr. Hart acknowledged. “I think it will be an inevitability that there will be events, and we need to understand what the denominators are – hence my pitch for there being a renewed effort to try and collate sufficient data that we can really define events happening with people treated with standard half-life [products] through into the novel agents,” he said.
Dr. Hart disclosed grant/research support and speaker bureau activities for Bayer, Octapharma, Takeda, and others. Dr. Königs has reported no relevant disclosures.
Despite their similar functions, each current and emerging therapy for treating hemophilia has a unique safety profile, and each needs to be weighed apart from agents both within and outside its pharmacologic class, a hemophilia specialist said.
“My view is that each new molecule coming to the hemophilia space, including variant factor molecules, needs to be scrutinized separately, without class assumptions or extrapolations, and it’s clear that thrombosis risk has become a priority safety consideration,” said Dan Hart, MBChB, MRCP, FRCPath, PhD, from Barts and the London School of Medicine and Dentistry.
He reviewed the comparative safety of standard and novel therapies for hemophilia at the annual congress of the European Association for Haemophilia and Allied Disorders.
Factor inhibitors
Inhibitors occur in both hemophilia A and hemophilia B, and are primarily seen in patients with childhood exposure to factor concentrates. Inhibitors, which include anti–factor VIII and factor IX alloantibodies, are more common among patients with severe hemophilia and those with more disruptive factor VIII and factor IX mutations.
“There can be transient vs. persistent inhibitors, and arguably the more you look, the more you find, but clinically we never miss high-titer inhibitors that have a big impact on individuals and the subsequent decisions about management,” he said.
Hamster vs. human
It’s currently unclear whether there is an immunologic advantage for previously untreated patients to be started on factor VIII concentrates derived from recombinant human cells lines, or from products derived from Chinese hamster ovary (CHO) or baby hamster kidney (BHK) cell lines, Dr. Hart said.
“We need to ensure that we’re not selective about comparator choice for new products in the absence of head-to-head studies,” he said.
Route of administration matters
Inhibitors appear to be a more common occurrence among patients who received factor concentrates subcutaneously, compared with intravenously, Dr. Hart noted, pointing to a 2011 study indicating a background annual risk of 5 cases of inhibitor development per 1,000 treatment years in previously treated patients who received intravenous therapy (Blood. 2011 Jun 9;117[23]:6367-70).
In contrast, in a phase 1 trial of subcutaneous turoctocog alfa pegol, 5 out of 26 patients had detectable N8-GP–binding antibodies after 42-91 exposure days. Of these patients, one developed an inhibitor to factor VIII, and anti–N8-GP antibody appearance was associated with a decline in factor VIII plasma activity in four of the five patients. In addition, five patients reported a total of nine bleeding episodes requiring treatment during prophylaxis. As a result of this trial, further clinical development of the subcutaneous version was suspended. (J Thromb Haemost. 2020 Feb;18[2]:341-51).
Other subcutaneously administered factors are currently in development, Dr. Hart noted.
Nonfactor inhibitors?
“The nonfactor agents do have the risk of generating antibodies: Monoclonal antibodies outside the hemophilia setting provoke antidrug antibodies,” he said.
Although there is no consensus regarding which assay can best monitor antidrug antibodies (ADA), enzyme-linked immunosorbent assay (ELISA) can detect neutralizing antibodies and other antibodies.
In the hemophilia setting, surrogate markers for loss of drug efficacy include longer activated partial thromboplastin time (ATTP) or a drop in serum drug levels. Worsening bleeding phenotype can also be a marker for loss of efficacy, albeit an imperfect one.
Emicizumab (Hemlibara), the first nonfactor monoclonal agent to make it to market, has the largest dataset available, and evidence suggests a rate of neutralizing antibodies with this agent of less than 1% in the HAVEN clinical trial series, but 5.2% in the single-arm STASEY trial.
“We shouldn’t assume that other biophenotypics will have a similar ADA rate, and this needs to be evaluated for each molecule, as it will need to be for other monoclonals” such as anti–tissue factor pathway (TFPI) antibodies, Dr. Hart emphasized.
Pegylation
Pegylated compounds include polyethylene glycol, an inert polymer, covalently bound to the therapeutic protein to extend its half-life, and theoretically, reduce immunogenicity.
Many patients may already have exposure to pegylated products in the form of peginterferon to treat hepatitis C, consumer products such as toothpaste, cough medicine, and cosmetics, and, more recently, in vaccines against COVID-19.
Safety considerations with pegylated agents in hemophilia include concerns about accumulation of polyethylene glycol (PEG), although “some of the preclinical models looking at excretion of PEG are difficult to interpret in my view, and people debate about whether studies are long enough, but it’s undoubtedly the case that toxicology dosing is order of magnitude higher than the routine dosing in hemophilia,” he said.
After more than 5 years of experience with pegylated products there is no clinical evidence of concern, although “it’s not clear, actually, what we’re looking for, whether it’s a clinical parameter, or imaging or histological parameter.”
Patients may also not have lifelong exposure to pegylated products, as it is unlikely that they will stay on the same product for decades, Dr. Hart said.
Thrombosis
As of June 30, 2020, more than 7,200 persons with hemophilia have received emicizumab, and there have been 23 reported thrombotic events, 19 of which occurred in the postmarketing period. Of the reported cases, six patients had a myocardial infarction, and all of these patients had at least one cardiovascular risk factor.
The antithrombin agent fitusiran was associated with one fatal thrombotic event in a phase 2, open-label extension trial, leading to a pause and resumption with mitigation protocols, but that trial has since been paused again because of additional, nonfatal thrombotic events.
Nonfatal thrombotic events have also occurred in clinical trials for the investigational anti-TFPI monoclonal antibodies BAY 1093884 and concizumab, but none have thus far been reported in phase 3 trial of marstacimab.
“We need renewed efforts for prospective reporting and independent review of all adverse events of all agents, old and new: This will need some guidance nationally and internationally, and I think the relevant trial [serious adverse events] need to be reported in peer review literature, and clinicaltrials.gov updated in a timely manner, regardless of whether that strategy was successful or unsuccessful,” Dr. Hart said.
Risk with longer-acting agents?
In the question and answer following his presentation, Christoph Königs, MD, PhD, from University Hospital Frankfurt, asked whether there was potential for increased thrombosis risk with second-generation extended half-life (EHL) molecules in clinical trials.
“As we edge towards normalization of hemostasis, clearly the other non–hemophilia dependent issues of thrombosis risk come into play,” Dr. Hart acknowledged. “I think it will be an inevitability that there will be events, and we need to understand what the denominators are – hence my pitch for there being a renewed effort to try and collate sufficient data that we can really define events happening with people treated with standard half-life [products] through into the novel agents,” he said.
Dr. Hart disclosed grant/research support and speaker bureau activities for Bayer, Octapharma, Takeda, and others. Dr. Königs has reported no relevant disclosures.
Despite their similar functions, each current and emerging therapy for treating hemophilia has a unique safety profile, and each needs to be weighed apart from agents both within and outside its pharmacologic class, a hemophilia specialist said.
“My view is that each new molecule coming to the hemophilia space, including variant factor molecules, needs to be scrutinized separately, without class assumptions or extrapolations, and it’s clear that thrombosis risk has become a priority safety consideration,” said Dan Hart, MBChB, MRCP, FRCPath, PhD, from Barts and the London School of Medicine and Dentistry.
He reviewed the comparative safety of standard and novel therapies for hemophilia at the annual congress of the European Association for Haemophilia and Allied Disorders.
Factor inhibitors
Inhibitors occur in both hemophilia A and hemophilia B, and are primarily seen in patients with childhood exposure to factor concentrates. Inhibitors, which include anti–factor VIII and factor IX alloantibodies, are more common among patients with severe hemophilia and those with more disruptive factor VIII and factor IX mutations.
“There can be transient vs. persistent inhibitors, and arguably the more you look, the more you find, but clinically we never miss high-titer inhibitors that have a big impact on individuals and the subsequent decisions about management,” he said.
Hamster vs. human
It’s currently unclear whether there is an immunologic advantage for previously untreated patients to be started on factor VIII concentrates derived from recombinant human cells lines, or from products derived from Chinese hamster ovary (CHO) or baby hamster kidney (BHK) cell lines, Dr. Hart said.
“We need to ensure that we’re not selective about comparator choice for new products in the absence of head-to-head studies,” he said.
Route of administration matters
Inhibitors appear to be a more common occurrence among patients who received factor concentrates subcutaneously, compared with intravenously, Dr. Hart noted, pointing to a 2011 study indicating a background annual risk of 5 cases of inhibitor development per 1,000 treatment years in previously treated patients who received intravenous therapy (Blood. 2011 Jun 9;117[23]:6367-70).
In contrast, in a phase 1 trial of subcutaneous turoctocog alfa pegol, 5 out of 26 patients had detectable N8-GP–binding antibodies after 42-91 exposure days. Of these patients, one developed an inhibitor to factor VIII, and anti–N8-GP antibody appearance was associated with a decline in factor VIII plasma activity in four of the five patients. In addition, five patients reported a total of nine bleeding episodes requiring treatment during prophylaxis. As a result of this trial, further clinical development of the subcutaneous version was suspended. (J Thromb Haemost. 2020 Feb;18[2]:341-51).
Other subcutaneously administered factors are currently in development, Dr. Hart noted.
Nonfactor inhibitors?
“The nonfactor agents do have the risk of generating antibodies: Monoclonal antibodies outside the hemophilia setting provoke antidrug antibodies,” he said.
Although there is no consensus regarding which assay can best monitor antidrug antibodies (ADA), enzyme-linked immunosorbent assay (ELISA) can detect neutralizing antibodies and other antibodies.
In the hemophilia setting, surrogate markers for loss of drug efficacy include longer activated partial thromboplastin time (ATTP) or a drop in serum drug levels. Worsening bleeding phenotype can also be a marker for loss of efficacy, albeit an imperfect one.
Emicizumab (Hemlibara), the first nonfactor monoclonal agent to make it to market, has the largest dataset available, and evidence suggests a rate of neutralizing antibodies with this agent of less than 1% in the HAVEN clinical trial series, but 5.2% in the single-arm STASEY trial.
“We shouldn’t assume that other biophenotypics will have a similar ADA rate, and this needs to be evaluated for each molecule, as it will need to be for other monoclonals” such as anti–tissue factor pathway (TFPI) antibodies, Dr. Hart emphasized.
Pegylation
Pegylated compounds include polyethylene glycol, an inert polymer, covalently bound to the therapeutic protein to extend its half-life, and theoretically, reduce immunogenicity.
Many patients may already have exposure to pegylated products in the form of peginterferon to treat hepatitis C, consumer products such as toothpaste, cough medicine, and cosmetics, and, more recently, in vaccines against COVID-19.
Safety considerations with pegylated agents in hemophilia include concerns about accumulation of polyethylene glycol (PEG), although “some of the preclinical models looking at excretion of PEG are difficult to interpret in my view, and people debate about whether studies are long enough, but it’s undoubtedly the case that toxicology dosing is order of magnitude higher than the routine dosing in hemophilia,” he said.
After more than 5 years of experience with pegylated products there is no clinical evidence of concern, although “it’s not clear, actually, what we’re looking for, whether it’s a clinical parameter, or imaging or histological parameter.”
Patients may also not have lifelong exposure to pegylated products, as it is unlikely that they will stay on the same product for decades, Dr. Hart said.
Thrombosis
As of June 30, 2020, more than 7,200 persons with hemophilia have received emicizumab, and there have been 23 reported thrombotic events, 19 of which occurred in the postmarketing period. Of the reported cases, six patients had a myocardial infarction, and all of these patients had at least one cardiovascular risk factor.
The antithrombin agent fitusiran was associated with one fatal thrombotic event in a phase 2, open-label extension trial, leading to a pause and resumption with mitigation protocols, but that trial has since been paused again because of additional, nonfatal thrombotic events.
Nonfatal thrombotic events have also occurred in clinical trials for the investigational anti-TFPI monoclonal antibodies BAY 1093884 and concizumab, but none have thus far been reported in phase 3 trial of marstacimab.
“We need renewed efforts for prospective reporting and independent review of all adverse events of all agents, old and new: This will need some guidance nationally and internationally, and I think the relevant trial [serious adverse events] need to be reported in peer review literature, and clinicaltrials.gov updated in a timely manner, regardless of whether that strategy was successful or unsuccessful,” Dr. Hart said.
Risk with longer-acting agents?
In the question and answer following his presentation, Christoph Königs, MD, PhD, from University Hospital Frankfurt, asked whether there was potential for increased thrombosis risk with second-generation extended half-life (EHL) molecules in clinical trials.
“As we edge towards normalization of hemostasis, clearly the other non–hemophilia dependent issues of thrombosis risk come into play,” Dr. Hart acknowledged. “I think it will be an inevitability that there will be events, and we need to understand what the denominators are – hence my pitch for there being a renewed effort to try and collate sufficient data that we can really define events happening with people treated with standard half-life [products] through into the novel agents,” he said.
Dr. Hart disclosed grant/research support and speaker bureau activities for Bayer, Octapharma, Takeda, and others. Dr. Königs has reported no relevant disclosures.
FROM EAHAD 2021
COVID-19 vaccination recommended for rheumatology patients
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
7 key changes: The 2021 child and adolescent immunization schedules
Each February, the Centers for Disease Control and Prevention, along with multiple professional organizations, releases an updated Recommended Child and Adolescent Immunization Schedule.
Recent years have seen fewer changes in the vaccine schedule, mostly with adjustments based on products coming on or off the market, and sometimes with slight changes in recommendations. This year is no different, with mostly minor changes in store. As most practitioners know, having quick access to the tables that accompany the recommendations is always handy. Table 1 contains the typical, recommended immunization schedule. Table 2 contains the catch-up provisions, and Table 3 provides guidance on vaccines for special circumstances and for children with specific medical conditions.
2021 childhood and adolescent immunization schedule
One update is a recommendation that patients with egg allergies who had symptoms more extensive than hives should receive the influenza vaccine in a medical setting where severe allergic reactions or anaphylaxis can be recognized and treated, with the exclusion of two specific preparations, Flublok and Flucelvax.
In regard to the live attenuated influenza vaccine (LAIV), there are several points of reinforcement. First, the nomenclature has generally been changed to “LAIV4” throughout the document because only quadrivalent preparations are available. There are specific recommendations that patients should not receive LAIV4 if they recently took antiviral medication for influenza, with “lockout” periods lasting from 2 days to 17 days, depending on the antiviral preparation used. In addition, there is an emphasis on not using LAIV4 for children younger than 2 years.
Two updates to the meningococcal group B vaccine are worth reviewing. The first is that children aged 10 years or older with complement deficiency, complement inhibitor use, or asplenia should receive a meningitis B booster dose beginning 1 year after completion of the primary series, with boosters thereafter every 2 or 3 years as long as that patient remains at greater risk. Another recommendation for patients 10 years or older is that, even if they have received a primary series of meningitis B vaccines, they should receive a booster dose in the setting of an outbreak if it has been 1 year or more since completion of their primary series.
Recommendations have generally been relaxed for tetanus prophylaxis in older children, indicating that individuals requiring tetanus prophylaxis or their 10-year tetanus booster after receipt of at least one Tdap vaccine can receive either tetanus-diphtheria toxoid or Tdap.
COVID-19 vaccines
Although childhood vaccination against COVID-19 is still currently limited to adolescents involved in clinical trials, pediatricians surely are getting peppered with questions from parents about whether they should be vaccinated and what to make of the recent reports about allergic reactions. Fortunately, there are several resources for pediatricians. First, two reports point out that true anaphylactic reactions to COVID-19 vaccines appear quite rare. The reported data on Pfizer-developed mRNA vaccine demonstrated an anaphylaxis rate of approximately 2 cases per 1 million doses administered. Among the 21 recipients who experienced anaphylaxis (out of over 11 million total doses administered), fully one third had a history of anaphylaxis episodes. The report also reviews vaccine reactions that were reported but were not classified as anaphylaxis, pointing out that when reporting vaccine reactions, we should be very careful in the nomenclature we use.
Reporting on the Moderna mRNA vaccine showed anaphylaxis rates of about 2.5 per 1 million doses, with 50% of the recipients who experienced true anaphylaxis having a history of anaphylaxis. Most of those who experienced anaphylaxis (90% in the Moderna group and 86% in the Pfizer group) exhibited symptoms of anaphylaxis within 30 minutes of receiving the vaccine. The take-home point, and the current CDC recommendation, is that many individuals, even those with a history of anaphylaxis, can still receive COVID-19 vaccines. The rates of observed anaphylaxis after COVID vaccination are far below population rates of a history of allergy or severe allergic reactions. When coupled with an estimated mortality rate of 0.5%-1% for SARS-CoV-2 disease, that CDC recommends that we encourage people, even those with severe allergies, to get vaccinated.
One clear caveat is that individuals with a history of severe anaphylaxis, and even those concerned about allergies, should be observed for a longer period after vaccination (at least 30 minutes) than the 15 minutes recommended for the general population. In addition, individuals with a specific anaphylactic reaction or severe allergic reaction to any injectable vaccine should confer with an immunologist before considering vaccination.
Another useful resource is a column published by the American Medical Association that walks through some talking points for providers when discussing whether a patient should receive COVID-19 vaccination. Advice is offered on answering patient questions about which preparation to get, what side effects to watch for, and how to report an adverse reaction. Providers are reminded to urge patients to complete whichever series they begin (get that second dose!), and that they currently should not have to pay for a vaccine. FAQ resource pages are available for patients and health care providers.
More vaccine news: HPV and influenza
Meanwhile, published vaccine reports provide evidence from the field to demonstrate the benefits of vaccination. A study published in the New England Journal of Medicine reported on the effectiveness of human papillomavirus (HPV) vaccine in a Swedish cohort. The report evaluated females aged between 10 and 30 years beginning in 2006 and followed them through 2017, comparing rates of invasive cervical cancer among the group who received one or more HPV vaccine doses with the group who receive none. Even without adjustment, the raw rate of invasive cervical cancer in the vaccinated group was half of that in the unvaccinated group. After full adjustment, some populations experienced incident rate ratios that were greater than 80% reduced. The largest reduction, and therefore the biggest benefit, was among those who received the HPV vaccine before age 17.
A report from the United States looking at the 2018-2019 influenza season demonstrated a vaccine effectiveness rate against hospitalization of 41% and 51% against any ED visit related to influenza. The authors note that there was considerable drift in the influenza A type that appeared late in the influenza season, reducing the overall effectiveness, but that the vaccine was still largely effective.
William T. Basco Jr, MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
Each February, the Centers for Disease Control and Prevention, along with multiple professional organizations, releases an updated Recommended Child and Adolescent Immunization Schedule.
Recent years have seen fewer changes in the vaccine schedule, mostly with adjustments based on products coming on or off the market, and sometimes with slight changes in recommendations. This year is no different, with mostly minor changes in store. As most practitioners know, having quick access to the tables that accompany the recommendations is always handy. Table 1 contains the typical, recommended immunization schedule. Table 2 contains the catch-up provisions, and Table 3 provides guidance on vaccines for special circumstances and for children with specific medical conditions.
2021 childhood and adolescent immunization schedule
One update is a recommendation that patients with egg allergies who had symptoms more extensive than hives should receive the influenza vaccine in a medical setting where severe allergic reactions or anaphylaxis can be recognized and treated, with the exclusion of two specific preparations, Flublok and Flucelvax.
In regard to the live attenuated influenza vaccine (LAIV), there are several points of reinforcement. First, the nomenclature has generally been changed to “LAIV4” throughout the document because only quadrivalent preparations are available. There are specific recommendations that patients should not receive LAIV4 if they recently took antiviral medication for influenza, with “lockout” periods lasting from 2 days to 17 days, depending on the antiviral preparation used. In addition, there is an emphasis on not using LAIV4 for children younger than 2 years.
Two updates to the meningococcal group B vaccine are worth reviewing. The first is that children aged 10 years or older with complement deficiency, complement inhibitor use, or asplenia should receive a meningitis B booster dose beginning 1 year after completion of the primary series, with boosters thereafter every 2 or 3 years as long as that patient remains at greater risk. Another recommendation for patients 10 years or older is that, even if they have received a primary series of meningitis B vaccines, they should receive a booster dose in the setting of an outbreak if it has been 1 year or more since completion of their primary series.
Recommendations have generally been relaxed for tetanus prophylaxis in older children, indicating that individuals requiring tetanus prophylaxis or their 10-year tetanus booster after receipt of at least one Tdap vaccine can receive either tetanus-diphtheria toxoid or Tdap.
COVID-19 vaccines
Although childhood vaccination against COVID-19 is still currently limited to adolescents involved in clinical trials, pediatricians surely are getting peppered with questions from parents about whether they should be vaccinated and what to make of the recent reports about allergic reactions. Fortunately, there are several resources for pediatricians. First, two reports point out that true anaphylactic reactions to COVID-19 vaccines appear quite rare. The reported data on Pfizer-developed mRNA vaccine demonstrated an anaphylaxis rate of approximately 2 cases per 1 million doses administered. Among the 21 recipients who experienced anaphylaxis (out of over 11 million total doses administered), fully one third had a history of anaphylaxis episodes. The report also reviews vaccine reactions that were reported but were not classified as anaphylaxis, pointing out that when reporting vaccine reactions, we should be very careful in the nomenclature we use.
Reporting on the Moderna mRNA vaccine showed anaphylaxis rates of about 2.5 per 1 million doses, with 50% of the recipients who experienced true anaphylaxis having a history of anaphylaxis. Most of those who experienced anaphylaxis (90% in the Moderna group and 86% in the Pfizer group) exhibited symptoms of anaphylaxis within 30 minutes of receiving the vaccine. The take-home point, and the current CDC recommendation, is that many individuals, even those with a history of anaphylaxis, can still receive COVID-19 vaccines. The rates of observed anaphylaxis after COVID vaccination are far below population rates of a history of allergy or severe allergic reactions. When coupled with an estimated mortality rate of 0.5%-1% for SARS-CoV-2 disease, that CDC recommends that we encourage people, even those with severe allergies, to get vaccinated.
One clear caveat is that individuals with a history of severe anaphylaxis, and even those concerned about allergies, should be observed for a longer period after vaccination (at least 30 minutes) than the 15 minutes recommended for the general population. In addition, individuals with a specific anaphylactic reaction or severe allergic reaction to any injectable vaccine should confer with an immunologist before considering vaccination.
Another useful resource is a column published by the American Medical Association that walks through some talking points for providers when discussing whether a patient should receive COVID-19 vaccination. Advice is offered on answering patient questions about which preparation to get, what side effects to watch for, and how to report an adverse reaction. Providers are reminded to urge patients to complete whichever series they begin (get that second dose!), and that they currently should not have to pay for a vaccine. FAQ resource pages are available for patients and health care providers.
More vaccine news: HPV and influenza
Meanwhile, published vaccine reports provide evidence from the field to demonstrate the benefits of vaccination. A study published in the New England Journal of Medicine reported on the effectiveness of human papillomavirus (HPV) vaccine in a Swedish cohort. The report evaluated females aged between 10 and 30 years beginning in 2006 and followed them through 2017, comparing rates of invasive cervical cancer among the group who received one or more HPV vaccine doses with the group who receive none. Even without adjustment, the raw rate of invasive cervical cancer in the vaccinated group was half of that in the unvaccinated group. After full adjustment, some populations experienced incident rate ratios that were greater than 80% reduced. The largest reduction, and therefore the biggest benefit, was among those who received the HPV vaccine before age 17.
A report from the United States looking at the 2018-2019 influenza season demonstrated a vaccine effectiveness rate against hospitalization of 41% and 51% against any ED visit related to influenza. The authors note that there was considerable drift in the influenza A type that appeared late in the influenza season, reducing the overall effectiveness, but that the vaccine was still largely effective.
William T. Basco Jr, MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
Each February, the Centers for Disease Control and Prevention, along with multiple professional organizations, releases an updated Recommended Child and Adolescent Immunization Schedule.
Recent years have seen fewer changes in the vaccine schedule, mostly with adjustments based on products coming on or off the market, and sometimes with slight changes in recommendations. This year is no different, with mostly minor changes in store. As most practitioners know, having quick access to the tables that accompany the recommendations is always handy. Table 1 contains the typical, recommended immunization schedule. Table 2 contains the catch-up provisions, and Table 3 provides guidance on vaccines for special circumstances and for children with specific medical conditions.
2021 childhood and adolescent immunization schedule
One update is a recommendation that patients with egg allergies who had symptoms more extensive than hives should receive the influenza vaccine in a medical setting where severe allergic reactions or anaphylaxis can be recognized and treated, with the exclusion of two specific preparations, Flublok and Flucelvax.
In regard to the live attenuated influenza vaccine (LAIV), there are several points of reinforcement. First, the nomenclature has generally been changed to “LAIV4” throughout the document because only quadrivalent preparations are available. There are specific recommendations that patients should not receive LAIV4 if they recently took antiviral medication for influenza, with “lockout” periods lasting from 2 days to 17 days, depending on the antiviral preparation used. In addition, there is an emphasis on not using LAIV4 for children younger than 2 years.
Two updates to the meningococcal group B vaccine are worth reviewing. The first is that children aged 10 years or older with complement deficiency, complement inhibitor use, or asplenia should receive a meningitis B booster dose beginning 1 year after completion of the primary series, with boosters thereafter every 2 or 3 years as long as that patient remains at greater risk. Another recommendation for patients 10 years or older is that, even if they have received a primary series of meningitis B vaccines, they should receive a booster dose in the setting of an outbreak if it has been 1 year or more since completion of their primary series.
Recommendations have generally been relaxed for tetanus prophylaxis in older children, indicating that individuals requiring tetanus prophylaxis or their 10-year tetanus booster after receipt of at least one Tdap vaccine can receive either tetanus-diphtheria toxoid or Tdap.
COVID-19 vaccines
Although childhood vaccination against COVID-19 is still currently limited to adolescents involved in clinical trials, pediatricians surely are getting peppered with questions from parents about whether they should be vaccinated and what to make of the recent reports about allergic reactions. Fortunately, there are several resources for pediatricians. First, two reports point out that true anaphylactic reactions to COVID-19 vaccines appear quite rare. The reported data on Pfizer-developed mRNA vaccine demonstrated an anaphylaxis rate of approximately 2 cases per 1 million doses administered. Among the 21 recipients who experienced anaphylaxis (out of over 11 million total doses administered), fully one third had a history of anaphylaxis episodes. The report also reviews vaccine reactions that were reported but were not classified as anaphylaxis, pointing out that when reporting vaccine reactions, we should be very careful in the nomenclature we use.
Reporting on the Moderna mRNA vaccine showed anaphylaxis rates of about 2.5 per 1 million doses, with 50% of the recipients who experienced true anaphylaxis having a history of anaphylaxis. Most of those who experienced anaphylaxis (90% in the Moderna group and 86% in the Pfizer group) exhibited symptoms of anaphylaxis within 30 minutes of receiving the vaccine. The take-home point, and the current CDC recommendation, is that many individuals, even those with a history of anaphylaxis, can still receive COVID-19 vaccines. The rates of observed anaphylaxis after COVID vaccination are far below population rates of a history of allergy or severe allergic reactions. When coupled with an estimated mortality rate of 0.5%-1% for SARS-CoV-2 disease, that CDC recommends that we encourage people, even those with severe allergies, to get vaccinated.
One clear caveat is that individuals with a history of severe anaphylaxis, and even those concerned about allergies, should be observed for a longer period after vaccination (at least 30 minutes) than the 15 minutes recommended for the general population. In addition, individuals with a specific anaphylactic reaction or severe allergic reaction to any injectable vaccine should confer with an immunologist before considering vaccination.
Another useful resource is a column published by the American Medical Association that walks through some talking points for providers when discussing whether a patient should receive COVID-19 vaccination. Advice is offered on answering patient questions about which preparation to get, what side effects to watch for, and how to report an adverse reaction. Providers are reminded to urge patients to complete whichever series they begin (get that second dose!), and that they currently should not have to pay for a vaccine. FAQ resource pages are available for patients and health care providers.
More vaccine news: HPV and influenza
Meanwhile, published vaccine reports provide evidence from the field to demonstrate the benefits of vaccination. A study published in the New England Journal of Medicine reported on the effectiveness of human papillomavirus (HPV) vaccine in a Swedish cohort. The report evaluated females aged between 10 and 30 years beginning in 2006 and followed them through 2017, comparing rates of invasive cervical cancer among the group who received one or more HPV vaccine doses with the group who receive none. Even without adjustment, the raw rate of invasive cervical cancer in the vaccinated group was half of that in the unvaccinated group. After full adjustment, some populations experienced incident rate ratios that were greater than 80% reduced. The largest reduction, and therefore the biggest benefit, was among those who received the HPV vaccine before age 17.
A report from the United States looking at the 2018-2019 influenza season demonstrated a vaccine effectiveness rate against hospitalization of 41% and 51% against any ED visit related to influenza. The authors note that there was considerable drift in the influenza A type that appeared late in the influenza season, reducing the overall effectiveness, but that the vaccine was still largely effective.
William T. Basco Jr, MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
New cases of child COVID-19 drop for fifth straight week
The fifth consecutive week with a decline has the number of new COVID-19 cases in children at its lowest level since late October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

, when 61,000 cases were reported, the AAP and CHA said in their weekly COVID-19 report.
The cumulative number of COVID-19 cases in children is now just over 3.1 million, which represents 13.1% of cases among all ages in the United States, based on data gathered from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
More children in California (439,000) have been infected than in any other state, while Illinois (176,000), Florida (145,000), Tennessee (137,000), Arizona (127,000), Ohio (121,000), and Pennsylvania (111,000) are the only other states with more than 100,000 cases, the AAP/CHA report shows.
Proportionally, the children of Wyoming have been hardest hit: Pediatric cases represent 19.4% of all cases in the state. The other four states with proportions of 18% or more are Alaska, Vermont, South Carolina, and Tennessee. Cumulative rates, however, tell a somewhat different story, as North Dakota leads with just over 8,500 cases per 100,000 children, followed by Tennessee (7,700 per 100,000) and Rhode Island (7,000 per 100,000), the AAP and CHA said.
Deaths in children, which had not been following the trend of fewer new cases over the last few weeks, dropped below double digits for the first time in a month. The six deaths that occurred during the week of Feb. 12-18 bring the total to 247 since the start of the pandemic in the 43 states, along with New York City and Guam, that are reporting such data, according to the report.
The fifth consecutive week with a decline has the number of new COVID-19 cases in children at its lowest level since late October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

, when 61,000 cases were reported, the AAP and CHA said in their weekly COVID-19 report.
The cumulative number of COVID-19 cases in children is now just over 3.1 million, which represents 13.1% of cases among all ages in the United States, based on data gathered from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
More children in California (439,000) have been infected than in any other state, while Illinois (176,000), Florida (145,000), Tennessee (137,000), Arizona (127,000), Ohio (121,000), and Pennsylvania (111,000) are the only other states with more than 100,000 cases, the AAP/CHA report shows.
Proportionally, the children of Wyoming have been hardest hit: Pediatric cases represent 19.4% of all cases in the state. The other four states with proportions of 18% or more are Alaska, Vermont, South Carolina, and Tennessee. Cumulative rates, however, tell a somewhat different story, as North Dakota leads with just over 8,500 cases per 100,000 children, followed by Tennessee (7,700 per 100,000) and Rhode Island (7,000 per 100,000), the AAP and CHA said.
Deaths in children, which had not been following the trend of fewer new cases over the last few weeks, dropped below double digits for the first time in a month. The six deaths that occurred during the week of Feb. 12-18 bring the total to 247 since the start of the pandemic in the 43 states, along with New York City and Guam, that are reporting such data, according to the report.
The fifth consecutive week with a decline has the number of new COVID-19 cases in children at its lowest level since late October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

, when 61,000 cases were reported, the AAP and CHA said in their weekly COVID-19 report.
The cumulative number of COVID-19 cases in children is now just over 3.1 million, which represents 13.1% of cases among all ages in the United States, based on data gathered from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
More children in California (439,000) have been infected than in any other state, while Illinois (176,000), Florida (145,000), Tennessee (137,000), Arizona (127,000), Ohio (121,000), and Pennsylvania (111,000) are the only other states with more than 100,000 cases, the AAP/CHA report shows.
Proportionally, the children of Wyoming have been hardest hit: Pediatric cases represent 19.4% of all cases in the state. The other four states with proportions of 18% or more are Alaska, Vermont, South Carolina, and Tennessee. Cumulative rates, however, tell a somewhat different story, as North Dakota leads with just over 8,500 cases per 100,000 children, followed by Tennessee (7,700 per 100,000) and Rhode Island (7,000 per 100,000), the AAP and CHA said.
Deaths in children, which had not been following the trend of fewer new cases over the last few weeks, dropped below double digits for the first time in a month. The six deaths that occurred during the week of Feb. 12-18 bring the total to 247 since the start of the pandemic in the 43 states, along with New York City and Guam, that are reporting such data, according to the report.