User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Study Evaluates Factors Driving Fatigue in Patients With Psoriasis, PsA
TOPLINE:
Many factors may influence fatigue in patients with psoriasis and psoriatic arthritis (PsA), researchers report.
METHODOLOGY:
- The individual components of fatigue in psoriasis and PsA have not been examined thoroughly.
- Researchers drew from the nationwide prospective Danish Skin Cohort to identify 2741 adults with dermatologist-diagnosed psoriasis (of which 593 also had PsA) and 3788 controls in the general population.
- All adults in the analysis completed the multidimensional fatigue inventory (MIF-20), a validated 20-item tool that measures five dimensions of fatigue: General fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. A higher score indicates more severe fatigue.
- All adults were also asked about their current intensity of joint pain over the previous 7 days, severity of pruritus and skin pain over the previous 24 hours, and sleep problems over the previous 72 hours on a numerical rating scale (NRS). The researchers applied linear regression models to continuous outcomes and adjusted for age, sex, socioeconomic status, psoriasis severity, and joint pain intensity, and beta coefficients (β) for the slopes were estimated with 95% CIs.
TAKEAWAY:
- Compared with the general population, higher total MFI-20 scores were observed for psoriasis and PsA, respectively. However, on the adjusted analysis, the impact on total fatigue was greatest for those with PsA (β = 5.23; 95% CI, 3.55-6.90), followed by psoriasis (β = 2.10; 95% CI, 0.96-3.25) compared with the general population (P trend < .0001).
- Increasing age was associated with a lower impact on total fatigue in psoriasis (β = −0.13; 95% CI, −0.18 to −0.08) and in PsA (β = −0.10; 95% CI, −0.19 to −0.01).
- Among patients with psoriasis with or without PsA, increasing joint pain intensity was associated with overall fatigue (β = 2.23; 95% CI, 2.03-2.44) for each one-point increase in joint pain on the NRS.
- In other findings, greater intensity of itch was associated with higher fatigue scores for both psoriasis and PsA, while skin pain was significantly associated with fatigue in PsA (β = 0.65; 95% CI, 0.08-1.22) but not in psoriasis without PsA (P = .2043).
IN PRACTICE:
“The when treating psoriasis, rather than focusing on objective severity measures alone,” the authors wrote.
SOURCE:
Corresponding author Alexander Egeberg, MD, of the Department of Dermatology at Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark, and colleagues conducted the research, which was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The researchers were unable to assess whether the pain was inflammatory or noninflammatory or the number of affected joints. They also lacked information about the use of methotrexate, which commonly causes fatigue.
DISCLOSURES:
Dr. Egeberg is now an employee at LEO Pharma. He has received research funding from Pfizer, Eli Lilly, the Danish National Psoriasis Foundation, and the Royal Hofbundtmager Aage Bang Foundation, and honoraria as a consultant and/or speaker from AbbVie, Almirall, Bristol-Myers Squibb, Leo Pharma, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly, Novartis, UCB, Union Therapeutics, Horizon Therapeutics, Galderma, and Janssen Pharmaceuticals. Three of the coauthors reported being a consultant to, an adviser for, and/or having received research support from many pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
Many factors may influence fatigue in patients with psoriasis and psoriatic arthritis (PsA), researchers report.
METHODOLOGY:
- The individual components of fatigue in psoriasis and PsA have not been examined thoroughly.
- Researchers drew from the nationwide prospective Danish Skin Cohort to identify 2741 adults with dermatologist-diagnosed psoriasis (of which 593 also had PsA) and 3788 controls in the general population.
- All adults in the analysis completed the multidimensional fatigue inventory (MIF-20), a validated 20-item tool that measures five dimensions of fatigue: General fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. A higher score indicates more severe fatigue.
- All adults were also asked about their current intensity of joint pain over the previous 7 days, severity of pruritus and skin pain over the previous 24 hours, and sleep problems over the previous 72 hours on a numerical rating scale (NRS). The researchers applied linear regression models to continuous outcomes and adjusted for age, sex, socioeconomic status, psoriasis severity, and joint pain intensity, and beta coefficients (β) for the slopes were estimated with 95% CIs.
TAKEAWAY:
- Compared with the general population, higher total MFI-20 scores were observed for psoriasis and PsA, respectively. However, on the adjusted analysis, the impact on total fatigue was greatest for those with PsA (β = 5.23; 95% CI, 3.55-6.90), followed by psoriasis (β = 2.10; 95% CI, 0.96-3.25) compared with the general population (P trend < .0001).
- Increasing age was associated with a lower impact on total fatigue in psoriasis (β = −0.13; 95% CI, −0.18 to −0.08) and in PsA (β = −0.10; 95% CI, −0.19 to −0.01).
- Among patients with psoriasis with or without PsA, increasing joint pain intensity was associated with overall fatigue (β = 2.23; 95% CI, 2.03-2.44) for each one-point increase in joint pain on the NRS.
- In other findings, greater intensity of itch was associated with higher fatigue scores for both psoriasis and PsA, while skin pain was significantly associated with fatigue in PsA (β = 0.65; 95% CI, 0.08-1.22) but not in psoriasis without PsA (P = .2043).
IN PRACTICE:
“The when treating psoriasis, rather than focusing on objective severity measures alone,” the authors wrote.
SOURCE:
Corresponding author Alexander Egeberg, MD, of the Department of Dermatology at Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark, and colleagues conducted the research, which was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The researchers were unable to assess whether the pain was inflammatory or noninflammatory or the number of affected joints. They also lacked information about the use of methotrexate, which commonly causes fatigue.
DISCLOSURES:
Dr. Egeberg is now an employee at LEO Pharma. He has received research funding from Pfizer, Eli Lilly, the Danish National Psoriasis Foundation, and the Royal Hofbundtmager Aage Bang Foundation, and honoraria as a consultant and/or speaker from AbbVie, Almirall, Bristol-Myers Squibb, Leo Pharma, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly, Novartis, UCB, Union Therapeutics, Horizon Therapeutics, Galderma, and Janssen Pharmaceuticals. Three of the coauthors reported being a consultant to, an adviser for, and/or having received research support from many pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
Many factors may influence fatigue in patients with psoriasis and psoriatic arthritis (PsA), researchers report.
METHODOLOGY:
- The individual components of fatigue in psoriasis and PsA have not been examined thoroughly.
- Researchers drew from the nationwide prospective Danish Skin Cohort to identify 2741 adults with dermatologist-diagnosed psoriasis (of which 593 also had PsA) and 3788 controls in the general population.
- All adults in the analysis completed the multidimensional fatigue inventory (MIF-20), a validated 20-item tool that measures five dimensions of fatigue: General fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. A higher score indicates more severe fatigue.
- All adults were also asked about their current intensity of joint pain over the previous 7 days, severity of pruritus and skin pain over the previous 24 hours, and sleep problems over the previous 72 hours on a numerical rating scale (NRS). The researchers applied linear regression models to continuous outcomes and adjusted for age, sex, socioeconomic status, psoriasis severity, and joint pain intensity, and beta coefficients (β) for the slopes were estimated with 95% CIs.
TAKEAWAY:
- Compared with the general population, higher total MFI-20 scores were observed for psoriasis and PsA, respectively. However, on the adjusted analysis, the impact on total fatigue was greatest for those with PsA (β = 5.23; 95% CI, 3.55-6.90), followed by psoriasis (β = 2.10; 95% CI, 0.96-3.25) compared with the general population (P trend < .0001).
- Increasing age was associated with a lower impact on total fatigue in psoriasis (β = −0.13; 95% CI, −0.18 to −0.08) and in PsA (β = −0.10; 95% CI, −0.19 to −0.01).
- Among patients with psoriasis with or without PsA, increasing joint pain intensity was associated with overall fatigue (β = 2.23; 95% CI, 2.03-2.44) for each one-point increase in joint pain on the NRS.
- In other findings, greater intensity of itch was associated with higher fatigue scores for both psoriasis and PsA, while skin pain was significantly associated with fatigue in PsA (β = 0.65; 95% CI, 0.08-1.22) but not in psoriasis without PsA (P = .2043).
IN PRACTICE:
“The when treating psoriasis, rather than focusing on objective severity measures alone,” the authors wrote.
SOURCE:
Corresponding author Alexander Egeberg, MD, of the Department of Dermatology at Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark, and colleagues conducted the research, which was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The researchers were unable to assess whether the pain was inflammatory or noninflammatory or the number of affected joints. They also lacked information about the use of methotrexate, which commonly causes fatigue.
DISCLOSURES:
Dr. Egeberg is now an employee at LEO Pharma. He has received research funding from Pfizer, Eli Lilly, the Danish National Psoriasis Foundation, and the Royal Hofbundtmager Aage Bang Foundation, and honoraria as a consultant and/or speaker from AbbVie, Almirall, Bristol-Myers Squibb, Leo Pharma, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly, Novartis, UCB, Union Therapeutics, Horizon Therapeutics, Galderma, and Janssen Pharmaceuticals. Three of the coauthors reported being a consultant to, an adviser for, and/or having received research support from many pharmaceutical companies.
A version of this article appeared on Medscape.com.
Be Wary of TikTok Content on Infantile Hemangiomas: Study
TOPLINE:
.
METHODOLOGY:
- New parents may turn to TikTok for information about IHs, but little is known about the quality of videos on the social media platform related to the topic.
- Using the search term “hemangioma,” researchers reviewed the top 50 English-language TikTok videos that resulted from the query in November 2022.
- The researchers analyzed the videos for their content source, accuracy, and purpose and used Infantile Hemangioma Referral Score criteria to determine if the lesions pictured on the videos met criteria for referral to a specialist or not.
TAKEAWAY:
- Combined, the 50 videos were viewed 25.1 million times, had 2.6 million likes, and received 17,600 comments.
- Only 36 were considered likely to be IH. Of those 36 videos, the researchers deemed 33 (92%) to be potentially problematic, meriting referral to a specialist. The remaining three lesions could not be classified because of insufficient information.
- Of the 50 videos, 45 were created by individuals personally affected by IH (parents of a child with IH or young adults living with residual impacts), and only three were created by physicians (two by plastic surgeons and one by a neonatologist).
- In terms of content, 2 of the 45 videos created by someone personally affected by IH contained inaccurate information, while all three of videos created by physicians contained inaccurate information, such as oversimplification of the prognosis or incorrect nomenclature.
IN PRACTICE:
“Providers should be aware that TikTok may be useful for promoting birthmark awareness, but that it should not be relied on for accurate information about IHs,” the authors wrote.
SOURCE:
First author Sonora Yun, a medical student at Columbia University College of Physicians and Surgeons, New York City, conducted the research with Maria C. Garzon, MD, and Kimberly D. Morel, MD, who are board-certified pediatric dermatologists at Columbia. The study was published in Pediatric Dermatology.
LIMITATIONS:
The authors noted no specific limitations to the study.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- New parents may turn to TikTok for information about IHs, but little is known about the quality of videos on the social media platform related to the topic.
- Using the search term “hemangioma,” researchers reviewed the top 50 English-language TikTok videos that resulted from the query in November 2022.
- The researchers analyzed the videos for their content source, accuracy, and purpose and used Infantile Hemangioma Referral Score criteria to determine if the lesions pictured on the videos met criteria for referral to a specialist or not.
TAKEAWAY:
- Combined, the 50 videos were viewed 25.1 million times, had 2.6 million likes, and received 17,600 comments.
- Only 36 were considered likely to be IH. Of those 36 videos, the researchers deemed 33 (92%) to be potentially problematic, meriting referral to a specialist. The remaining three lesions could not be classified because of insufficient information.
- Of the 50 videos, 45 were created by individuals personally affected by IH (parents of a child with IH or young adults living with residual impacts), and only three were created by physicians (two by plastic surgeons and one by a neonatologist).
- In terms of content, 2 of the 45 videos created by someone personally affected by IH contained inaccurate information, while all three of videos created by physicians contained inaccurate information, such as oversimplification of the prognosis or incorrect nomenclature.
IN PRACTICE:
“Providers should be aware that TikTok may be useful for promoting birthmark awareness, but that it should not be relied on for accurate information about IHs,” the authors wrote.
SOURCE:
First author Sonora Yun, a medical student at Columbia University College of Physicians and Surgeons, New York City, conducted the research with Maria C. Garzon, MD, and Kimberly D. Morel, MD, who are board-certified pediatric dermatologists at Columbia. The study was published in Pediatric Dermatology.
LIMITATIONS:
The authors noted no specific limitations to the study.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- New parents may turn to TikTok for information about IHs, but little is known about the quality of videos on the social media platform related to the topic.
- Using the search term “hemangioma,” researchers reviewed the top 50 English-language TikTok videos that resulted from the query in November 2022.
- The researchers analyzed the videos for their content source, accuracy, and purpose and used Infantile Hemangioma Referral Score criteria to determine if the lesions pictured on the videos met criteria for referral to a specialist or not.
TAKEAWAY:
- Combined, the 50 videos were viewed 25.1 million times, had 2.6 million likes, and received 17,600 comments.
- Only 36 were considered likely to be IH. Of those 36 videos, the researchers deemed 33 (92%) to be potentially problematic, meriting referral to a specialist. The remaining three lesions could not be classified because of insufficient information.
- Of the 50 videos, 45 were created by individuals personally affected by IH (parents of a child with IH or young adults living with residual impacts), and only three were created by physicians (two by plastic surgeons and one by a neonatologist).
- In terms of content, 2 of the 45 videos created by someone personally affected by IH contained inaccurate information, while all three of videos created by physicians contained inaccurate information, such as oversimplification of the prognosis or incorrect nomenclature.
IN PRACTICE:
“Providers should be aware that TikTok may be useful for promoting birthmark awareness, but that it should not be relied on for accurate information about IHs,” the authors wrote.
SOURCE:
First author Sonora Yun, a medical student at Columbia University College of Physicians and Surgeons, New York City, conducted the research with Maria C. Garzon, MD, and Kimberly D. Morel, MD, who are board-certified pediatric dermatologists at Columbia. The study was published in Pediatric Dermatology.
LIMITATIONS:
The authors noted no specific limitations to the study.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
Unexpectedly Helpful Effects of Drugs Used For Other Reasons
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
Biological Sex Differences: Key to Understanding Long COVID?
Letícia Soares was infected with COVID-19 in April 2020, in the final year of postdoctoral studies in disease ecology at a Canadian University. What started with piercing migraines and severe fatigue in 2020 soon spiraled into a myriad of long COVID symptoms: Gastrointestinal issues, sleep problems, joint and muscle pain, along with unexpected menstrual changes.
After an absence of menstrual bleeding and its usual signs, she later suffered from severe periods and symptoms that worsened her long COVID condition. “It just baffled me,” said Soares, now 39. “It was debilitating.”
Cases like Soares’s are leading scientists to spend more time trying to understand the biological sex disparity in chronic illnesses such as long COVID that until recently have all but been ignored. According to the Centers for Disease Control and Prevention, long COVID affects nearly twice as many women as men.
What’s more, up to two thirds of female patients with long COVID report an increase in symptoms related to menstruation, which suggests a possible link between sex hormone fluctuations and immune dysfunction in the illness.
“These illnesses are underfunded and understudied relative to their disease burdens,” said Beth Pollack, a research scientist at the Massachusetts Institute of Technology, Cambridge, Massachusetts, who studies complex chronic illnesses.
Addressing knowledge gaps, especially around sex differences, could significantly improve our understanding of complex chronic illnesses, said Pollack, who coauthored a 2023 literature review of female reproductive health impacts of long COVID.
Emerging ‘Menstrual Science’ Could Be Key
There is a critical need, she said, for studies on these illnesses to include considerations of sex differences, hormones, reproductive phases, and reproductive conditions. This research could potentially inform doctors and other clinicians or lead to treatments, both for reproductive symptoms and for the illnesses themselves.
Pollack noted that reproductive symptoms are prevalent across a group of infection-associated chronic illnesses she studies, all of which disproportionately affect women. These associated conditions, traditionally studied in isolation, share pathologies like reproductive health concerns, signaling a need for focused research on their shared mechanisms.
Recognizing this critical gap, “menstrual science” is emerging as a pivotal area of study, aiming to connect these dots through focused research on hormonal influences.
Researchers at the University of Melbourne, Melbourne, Australia, for example, are studying whether hormones play a role in causing or worsening the symptoms of long COVID. By comparing hormone levels in people with these conditions with those in healthy people and by tracking how symptoms change with hormone levels over time and across menstrual cycles, scientists hope to find patterns that could help diagnose these conditions more easily and lead to new treatments. They’re also examining how hormonal life phases such as puberty, pregnancy, or perimenopause and hormone treatments like birth control might affect these illnesses.
How Gender and Long COVID Intertwine
The pathologies of long COVID, affecting at least 65 million people worldwide, currently focus on four hypotheses: Persistent viral infection, reactivation of dormant viruses (such as common herpes viruses), inflammation-related damage to tissues and organs, and autoimmunity (the body attacking itself).
It’s this last reason that holds some of the most interesting clues on biological sex differences, said Akiko Iwasaki, PhD, a Yale University, New Haven, Connecticut, immunologist who has led numerous research breakthroughs on long COVID since the start of the pandemic. Women have two X chromosomes, for example, and although one is inactivated, the inactivation is incomplete.
Some cells still express genes from the “inactivated genes” on the X chromosome, Iwasaki said. Those include key immune genes, which trigger a more robust response to infections and vaccinations but also predispose them to autoimmune reactions. “It comes at the cost of triggering too much immune response,” Iwasaki said.
Sex hormones also factor in. Testosterone, which is higher in males, is immunosuppressive, so it can dampen immune responses, Iwasaki said. That may contribute to making males more likely to get severe acute infections of COVID-19 but have fewer long-term effects.
Estrogen, on the other hand, is known to enhance the immune response. It can increase the production of antibodies and the activation of T cells, which are critical for fighting off infections. This heightened immune response, however, might also contribute to the persistent inflammation observed in long COVID, where the immune system continues to react even after the acute infection has resolved.
Sex-Specific Symptoms and Marginalized Communities
Of the more than 200 symptoms long haulers experience, Iwasaki said, several are also sex-specific. A recent draft study by Iwasaki and another leading COVID researcher, David Putrino, PhD, at Mount Sinai Health System in New York City, shows hair loss as one of the most female-dominant symptoms and sexual dysfunction among males.
In examining sex differences, another question is why long COVID rates in the trans community are disproportionately high. One of the reasons Iwasaki’s lab is looking at testosterone closely is because anecdotal evidence from female-to-male trans individuals indicates that testosterone therapy improved their long COVID symptoms significantly. It also raises the possibility that hormone therapy could help.
However, patients and advocates say it’s also important to consider socioeconomic factors in the trans community. “We need to start at this population and social structure level to understand why trans people over and over are put in harm’s way,” said JD Davids, a trans patient-researcher with long COVID and the cofounder and codirector of Strategies for High Impact and its Long COVID Justice project.
For trans people, said Davids, risk factors for both severe COVID and long COVID include being part of low-income groups, belonging to marginalized racial and ethnic communities, and living in crowded environments such as shelters or prisons.
The disproportionate impact of long COVID on marginalized communities, especially when seen through the lens of historical medical neglect, also demands attention, said Iwasaki. “Women used to be labeled hysteric when they complained about these kinds of symptoms.”
Where It All Leads
The possibility of diagnosing long COVID with a simple blood test could radically change some doctors’ false perceptions that it is not a real condition, Iwasaki said, ensuring it is recognized and treated with the seriousness it deserves.
“I feel like we need to get there with long COVID. If we can order a blood test and say somebody has a long COVID because of these values, then suddenly the diseases become medically explainable,” Iwasaki added. This advancement is critical for propelling research forward, she said, refining treatment approaches — including those that target sex-specific hormone, immunity, and inflammation issues — and improving the well-being of those living with long COVID.
This hope resonates with scientists like Pollack, who recently led the first National Institutes of Health-sponsored research webinar on less studied pathologies in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and long COVID, and with the experiences of individuals like Soares, who navigates through the unpredictable nature of both of these conditions with resilience.
“This illness never ceases to surprise me in how it changes my body. I feel like it’s a constant adaptation,” said Soares. Now living in Salvador, Brazil, her daily life has dramatically shifted to the confines of her home.
“It’s how I have more predictability in my symptoms,” she said, pointing out the pressing need for the scientific advancements that Iwasaki envisions and a deepening of our understanding of the disease’s impacts on patients’ lives.
A version of this article appeared on Medscape.com.
Letícia Soares was infected with COVID-19 in April 2020, in the final year of postdoctoral studies in disease ecology at a Canadian University. What started with piercing migraines and severe fatigue in 2020 soon spiraled into a myriad of long COVID symptoms: Gastrointestinal issues, sleep problems, joint and muscle pain, along with unexpected menstrual changes.
After an absence of menstrual bleeding and its usual signs, she later suffered from severe periods and symptoms that worsened her long COVID condition. “It just baffled me,” said Soares, now 39. “It was debilitating.”
Cases like Soares’s are leading scientists to spend more time trying to understand the biological sex disparity in chronic illnesses such as long COVID that until recently have all but been ignored. According to the Centers for Disease Control and Prevention, long COVID affects nearly twice as many women as men.
What’s more, up to two thirds of female patients with long COVID report an increase in symptoms related to menstruation, which suggests a possible link between sex hormone fluctuations and immune dysfunction in the illness.
“These illnesses are underfunded and understudied relative to their disease burdens,” said Beth Pollack, a research scientist at the Massachusetts Institute of Technology, Cambridge, Massachusetts, who studies complex chronic illnesses.
Addressing knowledge gaps, especially around sex differences, could significantly improve our understanding of complex chronic illnesses, said Pollack, who coauthored a 2023 literature review of female reproductive health impacts of long COVID.
Emerging ‘Menstrual Science’ Could Be Key
There is a critical need, she said, for studies on these illnesses to include considerations of sex differences, hormones, reproductive phases, and reproductive conditions. This research could potentially inform doctors and other clinicians or lead to treatments, both for reproductive symptoms and for the illnesses themselves.
Pollack noted that reproductive symptoms are prevalent across a group of infection-associated chronic illnesses she studies, all of which disproportionately affect women. These associated conditions, traditionally studied in isolation, share pathologies like reproductive health concerns, signaling a need for focused research on their shared mechanisms.
Recognizing this critical gap, “menstrual science” is emerging as a pivotal area of study, aiming to connect these dots through focused research on hormonal influences.
Researchers at the University of Melbourne, Melbourne, Australia, for example, are studying whether hormones play a role in causing or worsening the symptoms of long COVID. By comparing hormone levels in people with these conditions with those in healthy people and by tracking how symptoms change with hormone levels over time and across menstrual cycles, scientists hope to find patterns that could help diagnose these conditions more easily and lead to new treatments. They’re also examining how hormonal life phases such as puberty, pregnancy, or perimenopause and hormone treatments like birth control might affect these illnesses.
How Gender and Long COVID Intertwine
The pathologies of long COVID, affecting at least 65 million people worldwide, currently focus on four hypotheses: Persistent viral infection, reactivation of dormant viruses (such as common herpes viruses), inflammation-related damage to tissues and organs, and autoimmunity (the body attacking itself).
It’s this last reason that holds some of the most interesting clues on biological sex differences, said Akiko Iwasaki, PhD, a Yale University, New Haven, Connecticut, immunologist who has led numerous research breakthroughs on long COVID since the start of the pandemic. Women have two X chromosomes, for example, and although one is inactivated, the inactivation is incomplete.
Some cells still express genes from the “inactivated genes” on the X chromosome, Iwasaki said. Those include key immune genes, which trigger a more robust response to infections and vaccinations but also predispose them to autoimmune reactions. “It comes at the cost of triggering too much immune response,” Iwasaki said.
Sex hormones also factor in. Testosterone, which is higher in males, is immunosuppressive, so it can dampen immune responses, Iwasaki said. That may contribute to making males more likely to get severe acute infections of COVID-19 but have fewer long-term effects.
Estrogen, on the other hand, is known to enhance the immune response. It can increase the production of antibodies and the activation of T cells, which are critical for fighting off infections. This heightened immune response, however, might also contribute to the persistent inflammation observed in long COVID, where the immune system continues to react even after the acute infection has resolved.
Sex-Specific Symptoms and Marginalized Communities
Of the more than 200 symptoms long haulers experience, Iwasaki said, several are also sex-specific. A recent draft study by Iwasaki and another leading COVID researcher, David Putrino, PhD, at Mount Sinai Health System in New York City, shows hair loss as one of the most female-dominant symptoms and sexual dysfunction among males.
In examining sex differences, another question is why long COVID rates in the trans community are disproportionately high. One of the reasons Iwasaki’s lab is looking at testosterone closely is because anecdotal evidence from female-to-male trans individuals indicates that testosterone therapy improved their long COVID symptoms significantly. It also raises the possibility that hormone therapy could help.
However, patients and advocates say it’s also important to consider socioeconomic factors in the trans community. “We need to start at this population and social structure level to understand why trans people over and over are put in harm’s way,” said JD Davids, a trans patient-researcher with long COVID and the cofounder and codirector of Strategies for High Impact and its Long COVID Justice project.
For trans people, said Davids, risk factors for both severe COVID and long COVID include being part of low-income groups, belonging to marginalized racial and ethnic communities, and living in crowded environments such as shelters or prisons.
The disproportionate impact of long COVID on marginalized communities, especially when seen through the lens of historical medical neglect, also demands attention, said Iwasaki. “Women used to be labeled hysteric when they complained about these kinds of symptoms.”
Where It All Leads
The possibility of diagnosing long COVID with a simple blood test could radically change some doctors’ false perceptions that it is not a real condition, Iwasaki said, ensuring it is recognized and treated with the seriousness it deserves.
“I feel like we need to get there with long COVID. If we can order a blood test and say somebody has a long COVID because of these values, then suddenly the diseases become medically explainable,” Iwasaki added. This advancement is critical for propelling research forward, she said, refining treatment approaches — including those that target sex-specific hormone, immunity, and inflammation issues — and improving the well-being of those living with long COVID.
This hope resonates with scientists like Pollack, who recently led the first National Institutes of Health-sponsored research webinar on less studied pathologies in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and long COVID, and with the experiences of individuals like Soares, who navigates through the unpredictable nature of both of these conditions with resilience.
“This illness never ceases to surprise me in how it changes my body. I feel like it’s a constant adaptation,” said Soares. Now living in Salvador, Brazil, her daily life has dramatically shifted to the confines of her home.
“It’s how I have more predictability in my symptoms,” she said, pointing out the pressing need for the scientific advancements that Iwasaki envisions and a deepening of our understanding of the disease’s impacts on patients’ lives.
A version of this article appeared on Medscape.com.
Letícia Soares was infected with COVID-19 in April 2020, in the final year of postdoctoral studies in disease ecology at a Canadian University. What started with piercing migraines and severe fatigue in 2020 soon spiraled into a myriad of long COVID symptoms: Gastrointestinal issues, sleep problems, joint and muscle pain, along with unexpected menstrual changes.
After an absence of menstrual bleeding and its usual signs, she later suffered from severe periods and symptoms that worsened her long COVID condition. “It just baffled me,” said Soares, now 39. “It was debilitating.”
Cases like Soares’s are leading scientists to spend more time trying to understand the biological sex disparity in chronic illnesses such as long COVID that until recently have all but been ignored. According to the Centers for Disease Control and Prevention, long COVID affects nearly twice as many women as men.
What’s more, up to two thirds of female patients with long COVID report an increase in symptoms related to menstruation, which suggests a possible link between sex hormone fluctuations and immune dysfunction in the illness.
“These illnesses are underfunded and understudied relative to their disease burdens,” said Beth Pollack, a research scientist at the Massachusetts Institute of Technology, Cambridge, Massachusetts, who studies complex chronic illnesses.
Addressing knowledge gaps, especially around sex differences, could significantly improve our understanding of complex chronic illnesses, said Pollack, who coauthored a 2023 literature review of female reproductive health impacts of long COVID.
Emerging ‘Menstrual Science’ Could Be Key
There is a critical need, she said, for studies on these illnesses to include considerations of sex differences, hormones, reproductive phases, and reproductive conditions. This research could potentially inform doctors and other clinicians or lead to treatments, both for reproductive symptoms and for the illnesses themselves.
Pollack noted that reproductive symptoms are prevalent across a group of infection-associated chronic illnesses she studies, all of which disproportionately affect women. These associated conditions, traditionally studied in isolation, share pathologies like reproductive health concerns, signaling a need for focused research on their shared mechanisms.
Recognizing this critical gap, “menstrual science” is emerging as a pivotal area of study, aiming to connect these dots through focused research on hormonal influences.
Researchers at the University of Melbourne, Melbourne, Australia, for example, are studying whether hormones play a role in causing or worsening the symptoms of long COVID. By comparing hormone levels in people with these conditions with those in healthy people and by tracking how symptoms change with hormone levels over time and across menstrual cycles, scientists hope to find patterns that could help diagnose these conditions more easily and lead to new treatments. They’re also examining how hormonal life phases such as puberty, pregnancy, or perimenopause and hormone treatments like birth control might affect these illnesses.
How Gender and Long COVID Intertwine
The pathologies of long COVID, affecting at least 65 million people worldwide, currently focus on four hypotheses: Persistent viral infection, reactivation of dormant viruses (such as common herpes viruses), inflammation-related damage to tissues and organs, and autoimmunity (the body attacking itself).
It’s this last reason that holds some of the most interesting clues on biological sex differences, said Akiko Iwasaki, PhD, a Yale University, New Haven, Connecticut, immunologist who has led numerous research breakthroughs on long COVID since the start of the pandemic. Women have two X chromosomes, for example, and although one is inactivated, the inactivation is incomplete.
Some cells still express genes from the “inactivated genes” on the X chromosome, Iwasaki said. Those include key immune genes, which trigger a more robust response to infections and vaccinations but also predispose them to autoimmune reactions. “It comes at the cost of triggering too much immune response,” Iwasaki said.
Sex hormones also factor in. Testosterone, which is higher in males, is immunosuppressive, so it can dampen immune responses, Iwasaki said. That may contribute to making males more likely to get severe acute infections of COVID-19 but have fewer long-term effects.
Estrogen, on the other hand, is known to enhance the immune response. It can increase the production of antibodies and the activation of T cells, which are critical for fighting off infections. This heightened immune response, however, might also contribute to the persistent inflammation observed in long COVID, where the immune system continues to react even after the acute infection has resolved.
Sex-Specific Symptoms and Marginalized Communities
Of the more than 200 symptoms long haulers experience, Iwasaki said, several are also sex-specific. A recent draft study by Iwasaki and another leading COVID researcher, David Putrino, PhD, at Mount Sinai Health System in New York City, shows hair loss as one of the most female-dominant symptoms and sexual dysfunction among males.
In examining sex differences, another question is why long COVID rates in the trans community are disproportionately high. One of the reasons Iwasaki’s lab is looking at testosterone closely is because anecdotal evidence from female-to-male trans individuals indicates that testosterone therapy improved their long COVID symptoms significantly. It also raises the possibility that hormone therapy could help.
However, patients and advocates say it’s also important to consider socioeconomic factors in the trans community. “We need to start at this population and social structure level to understand why trans people over and over are put in harm’s way,” said JD Davids, a trans patient-researcher with long COVID and the cofounder and codirector of Strategies for High Impact and its Long COVID Justice project.
For trans people, said Davids, risk factors for both severe COVID and long COVID include being part of low-income groups, belonging to marginalized racial and ethnic communities, and living in crowded environments such as shelters or prisons.
The disproportionate impact of long COVID on marginalized communities, especially when seen through the lens of historical medical neglect, also demands attention, said Iwasaki. “Women used to be labeled hysteric when they complained about these kinds of symptoms.”
Where It All Leads
The possibility of diagnosing long COVID with a simple blood test could radically change some doctors’ false perceptions that it is not a real condition, Iwasaki said, ensuring it is recognized and treated with the seriousness it deserves.
“I feel like we need to get there with long COVID. If we can order a blood test and say somebody has a long COVID because of these values, then suddenly the diseases become medically explainable,” Iwasaki added. This advancement is critical for propelling research forward, she said, refining treatment approaches — including those that target sex-specific hormone, immunity, and inflammation issues — and improving the well-being of those living with long COVID.
This hope resonates with scientists like Pollack, who recently led the first National Institutes of Health-sponsored research webinar on less studied pathologies in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and long COVID, and with the experiences of individuals like Soares, who navigates through the unpredictable nature of both of these conditions with resilience.
“This illness never ceases to surprise me in how it changes my body. I feel like it’s a constant adaptation,” said Soares. Now living in Salvador, Brazil, her daily life has dramatically shifted to the confines of her home.
“It’s how I have more predictability in my symptoms,” she said, pointing out the pressing need for the scientific advancements that Iwasaki envisions and a deepening of our understanding of the disease’s impacts on patients’ lives.
A version of this article appeared on Medscape.com.
Climate Change and AD: New Review Shows Negative Impacts and Unknowns
A new . But it also showed the extent to which research is lacking.
“There’s not as much out there as one might expect, given that this is the most common dermatologic disease and one of the most burdensome diseases worldwide,” said Katrina Abuabara, MD, of the department of dermatology at the University of California, San Francisco, one of the senior authors of the review.
“There’s a genetic predisposition to AD, but it’s certainly very environmentally patterned,” she said in an interview. “Given that we know there are strong environmental influences, it’s an obvious example of how climate change affects our health ... It is one that may be underappreciated and that could give us near-term information.”
Indeed, she and her coauthors emphasized in their paper, “AD could serve as a case study for climatic impacts on health.” The review, which looked beyond the realm of air pollution, was published in Allergy, the journal of the European Academy of Allergy and Clinical Immunology.
Dr. Abuabara, UCSF dermatologist Sheng-Pei Wang, MD, MPH, and their coauthors — dermatologists and others from the United States, Europe, Brazil, and India — were convened by the International Eczema Council and teamed up with a biologist and climate science expert, Camilo Mora, PhD, of the University of Hawaii at Mānoa, Honolulu. Because research to date has focused on air pollution, with the impact of other hazards that Dr. Abuabara said were “a lot less developed and organized,” they used a framework and search strategy developed by Dr. Mora that looks at 10 climatic hazards related to greenhouse gas emissions, including heat waves, drought, precipitation, wildfires, and sea level rise.
“Given that this [framework] was already out there in the literature, we thought it would give us a structure and a nice way to organize the literature,” Dr. Abuabara said. While the literature is too heterogeneous for a systematic review and meta-analysis, the researchers used a systematic approach, she explained.
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and a coauthor of the paper, said in an e-mail that the review raises “our consciousness about how these [climate] changes may be impacting atopic dermatitis.”
Researchers have “much work to do to understand the evolving impact on AD development and course, and even more to figure out how to avoid extreme weather’s impact to minimize its effects on inflammatory skin diseases,” he said. “In the meantime, this paper is a call for the health care community to recognize a set of factors that can influence our patients’ dermatitis and lives.”
Mixed Results, But Negative Impacts Overall
The researchers identified 18 studies across most of the 10 climatic hazards with evidence for an impact on AD, the majority of which demonstrated harmful effects on various aspects of AD — most commonly on AD-related health care utilization and severity/flares. Only three of the studies examined AD prevalence and notably, none looked at incidence.
The impact of climatic hazards on AD appears to vary depending on the geographic region and its baseline climate, the authors said. A study in South Korea, for instance, found that in areas declared as disaster zones after storms and heavy rains, the number of AD-related outpatient visits increased for all ages. And a study in the United States showed an increased prevalence of childhood eczema in states with higher mean annual precipitation. However, some other studies on precipitation found no associations.
Just as published studies on precipitation yielded mixed results, so have studies on warming temperatures, Dr. Abuabara and her colleagues reported in their paper, with higher temperatures found to be positively associated with severity of AD symptoms in a study among patients with AD living in a region of Southern Italy, but decreased AD-related health care utilization in a study in Denmark.
In another study of over 5,500 children enrolled in an eczema registry in the United States between 2004 and 2012, higher temperature (odds ratio [OR] = 0.90, P < .001) and increased sun exposure (OR = 0.93, P = .009) were associated with poorly controlled eczema, after the researchers controlled for gender, race, income, and topical medication use.
Studies From 10 Countries Reviewed
Across the 18 studies identified in the review, data were collected in 10 countries. Five studies were conducted in the United States, one used global data, six were from Asia, and the others were from Europe and Africa. Data are lacking, the researchers wrote, in many parts of the world, including coastal regions of the tropics that are projected to experience the largest cumulative climatic hazards.
Future research should not only cover more geographic areas — especially those most impacted by climate change — but should examine impacts on AD incidence, prevalence, and “long-term monitoring of disease activity over time at the individual level,” the researchers recommended. Research should also aim to integrate multiple climatic factors and types of climate data, they said.
“As researchers, we always like to distill things down, but with climatic hazards like warming, you have to integrate other factors such as what the baseline temperature is and how precipitation is involved,” Dr. Abuabara said in the interview. With precipitation, similarly, associated factors such as outdoor humidity, pollen, and pollution exposure may also be at play for AD. Overall, she said, “you have to integrate many types of data.”
In addition to their literature review, the researchers created maps comparing the past, present, and future burden of climatic hazards to AD prevalence data. One pair of maps illustrates global cumulative exposure to climatic hazards in 2005 in parallel with the estimated annual change in AD prevalence in the subsequent decade. “It’s meant to be descriptive,” Dr. Abuabara said in the interview. The maps show alignment “between the areas experiencing the most climatic hazards and those where we subsequently saw the most rapid changes in AD.”
The paper also describes how climatic factors impact skin physiology and AD — exacerbating barrier impairment, immune dysregulation, dysbiosis, and pruritus — and how there are differential impacts on vulnerable and displaced populations with AD. It also briefly addresses air pollution, which was not included in the review framework but is impacted by wildfire and other included climatic factors.
The Need to Better Track AD, Anticipate Clinical Impact
“Outside of epidemiology, [clinicians and others] may not realize we actually have fairly poor measures of prevalence and severity of AD and disease flare over time,” Dr. Abuabara said. So “improving the ways we can measure this disease and getting more detailed data is important” for assessing the impact of climate changes.
More skin measures should be incorporated into large national health surveys, for one. “Skin doesn’t come to mind as much as diseases like heart disease and diabetes,” she said, and when surveys ask about AD, “they often don’t ask specific enough questions or ask about severity.” The clinical impacts of adverse climatic changes and extreme weather events — sudden therapy interruption, particularly of systemic agents, and delayed treatment, for instance — should be reflected in the planning and provision of dermatology services, Dr. Abuabara and her coauthors wrote.
There are currently no evidence-based recommendations for what patients with AD can do differently when faced with wildfire smoke or other climatic hazards, other than general recommendations, for instance, to reduce exposure to wildfire smoke and aeroallergens, she said in the interview. But “overall, the field has moved to more proactive treatment patterns ... toward providing anticipatory guidance and having individualized treatment plans that give people the tools to be ready to step things up or counteract [flares or worsening] if they need to.”
She and her San Francisco–based coauthors have already experienced the impact of wildfires firsthand. “It was amazing — in the period right after a major wildfire hundreds of miles away from the Bay area, we saw a huge spike in visits for itch and for eczema,” she said, referring to research on AD clinic visits after the 2018 California Camp Fire. “It showed up dramatically in the data,” said Dr. Abuabara, one of the authors of that study.
The new review adds to a growing body of literature documenting health impacts of climate change and advocating for action. In September 2021, more than 230 medical journals, including the New England Journal of Medicine — though not any dermatology journals — published an editorial calling for emergency action to limit global warming and protect health.
The following year, a commentary published across four dermatology journals discussed current and future impacts of climate change and urged dermatologists to become more engaged in finding solutions to help mitigate and adapt to climate change.
More recently, dermatologists have published about the environmental impact of professional practices such as print journals and meeting samples using single-use plastics.
Dr. Abuabara disclosed to Allergy that she is a consultant for TARGET RWE and Amgen and that her institution receives grants for research from Pfizer and LaRoche Posay. Dr. Eichenfield reported serving as a scientific adviser, consultant, and/or study investigator for Pfizer, AbbVie, Amgen and other companies. Dr. Wang disclosed that she is an International Eczema Council Fellow with financial support from Abbvie. Other authors had multiple disclosures.
A new . But it also showed the extent to which research is lacking.
“There’s not as much out there as one might expect, given that this is the most common dermatologic disease and one of the most burdensome diseases worldwide,” said Katrina Abuabara, MD, of the department of dermatology at the University of California, San Francisco, one of the senior authors of the review.
“There’s a genetic predisposition to AD, but it’s certainly very environmentally patterned,” she said in an interview. “Given that we know there are strong environmental influences, it’s an obvious example of how climate change affects our health ... It is one that may be underappreciated and that could give us near-term information.”
Indeed, she and her coauthors emphasized in their paper, “AD could serve as a case study for climatic impacts on health.” The review, which looked beyond the realm of air pollution, was published in Allergy, the journal of the European Academy of Allergy and Clinical Immunology.
Dr. Abuabara, UCSF dermatologist Sheng-Pei Wang, MD, MPH, and their coauthors — dermatologists and others from the United States, Europe, Brazil, and India — were convened by the International Eczema Council and teamed up with a biologist and climate science expert, Camilo Mora, PhD, of the University of Hawaii at Mānoa, Honolulu. Because research to date has focused on air pollution, with the impact of other hazards that Dr. Abuabara said were “a lot less developed and organized,” they used a framework and search strategy developed by Dr. Mora that looks at 10 climatic hazards related to greenhouse gas emissions, including heat waves, drought, precipitation, wildfires, and sea level rise.
“Given that this [framework] was already out there in the literature, we thought it would give us a structure and a nice way to organize the literature,” Dr. Abuabara said. While the literature is too heterogeneous for a systematic review and meta-analysis, the researchers used a systematic approach, she explained.
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and a coauthor of the paper, said in an e-mail that the review raises “our consciousness about how these [climate] changes may be impacting atopic dermatitis.”
Researchers have “much work to do to understand the evolving impact on AD development and course, and even more to figure out how to avoid extreme weather’s impact to minimize its effects on inflammatory skin diseases,” he said. “In the meantime, this paper is a call for the health care community to recognize a set of factors that can influence our patients’ dermatitis and lives.”
Mixed Results, But Negative Impacts Overall
The researchers identified 18 studies across most of the 10 climatic hazards with evidence for an impact on AD, the majority of which demonstrated harmful effects on various aspects of AD — most commonly on AD-related health care utilization and severity/flares. Only three of the studies examined AD prevalence and notably, none looked at incidence.
The impact of climatic hazards on AD appears to vary depending on the geographic region and its baseline climate, the authors said. A study in South Korea, for instance, found that in areas declared as disaster zones after storms and heavy rains, the number of AD-related outpatient visits increased for all ages. And a study in the United States showed an increased prevalence of childhood eczema in states with higher mean annual precipitation. However, some other studies on precipitation found no associations.
Just as published studies on precipitation yielded mixed results, so have studies on warming temperatures, Dr. Abuabara and her colleagues reported in their paper, with higher temperatures found to be positively associated with severity of AD symptoms in a study among patients with AD living in a region of Southern Italy, but decreased AD-related health care utilization in a study in Denmark.
In another study of over 5,500 children enrolled in an eczema registry in the United States between 2004 and 2012, higher temperature (odds ratio [OR] = 0.90, P < .001) and increased sun exposure (OR = 0.93, P = .009) were associated with poorly controlled eczema, after the researchers controlled for gender, race, income, and topical medication use.
Studies From 10 Countries Reviewed
Across the 18 studies identified in the review, data were collected in 10 countries. Five studies were conducted in the United States, one used global data, six were from Asia, and the others were from Europe and Africa. Data are lacking, the researchers wrote, in many parts of the world, including coastal regions of the tropics that are projected to experience the largest cumulative climatic hazards.
Future research should not only cover more geographic areas — especially those most impacted by climate change — but should examine impacts on AD incidence, prevalence, and “long-term monitoring of disease activity over time at the individual level,” the researchers recommended. Research should also aim to integrate multiple climatic factors and types of climate data, they said.
“As researchers, we always like to distill things down, but with climatic hazards like warming, you have to integrate other factors such as what the baseline temperature is and how precipitation is involved,” Dr. Abuabara said in the interview. With precipitation, similarly, associated factors such as outdoor humidity, pollen, and pollution exposure may also be at play for AD. Overall, she said, “you have to integrate many types of data.”
In addition to their literature review, the researchers created maps comparing the past, present, and future burden of climatic hazards to AD prevalence data. One pair of maps illustrates global cumulative exposure to climatic hazards in 2005 in parallel with the estimated annual change in AD prevalence in the subsequent decade. “It’s meant to be descriptive,” Dr. Abuabara said in the interview. The maps show alignment “between the areas experiencing the most climatic hazards and those where we subsequently saw the most rapid changes in AD.”
The paper also describes how climatic factors impact skin physiology and AD — exacerbating barrier impairment, immune dysregulation, dysbiosis, and pruritus — and how there are differential impacts on vulnerable and displaced populations with AD. It also briefly addresses air pollution, which was not included in the review framework but is impacted by wildfire and other included climatic factors.
The Need to Better Track AD, Anticipate Clinical Impact
“Outside of epidemiology, [clinicians and others] may not realize we actually have fairly poor measures of prevalence and severity of AD and disease flare over time,” Dr. Abuabara said. So “improving the ways we can measure this disease and getting more detailed data is important” for assessing the impact of climate changes.
More skin measures should be incorporated into large national health surveys, for one. “Skin doesn’t come to mind as much as diseases like heart disease and diabetes,” she said, and when surveys ask about AD, “they often don’t ask specific enough questions or ask about severity.” The clinical impacts of adverse climatic changes and extreme weather events — sudden therapy interruption, particularly of systemic agents, and delayed treatment, for instance — should be reflected in the planning and provision of dermatology services, Dr. Abuabara and her coauthors wrote.
There are currently no evidence-based recommendations for what patients with AD can do differently when faced with wildfire smoke or other climatic hazards, other than general recommendations, for instance, to reduce exposure to wildfire smoke and aeroallergens, she said in the interview. But “overall, the field has moved to more proactive treatment patterns ... toward providing anticipatory guidance and having individualized treatment plans that give people the tools to be ready to step things up or counteract [flares or worsening] if they need to.”
She and her San Francisco–based coauthors have already experienced the impact of wildfires firsthand. “It was amazing — in the period right after a major wildfire hundreds of miles away from the Bay area, we saw a huge spike in visits for itch and for eczema,” she said, referring to research on AD clinic visits after the 2018 California Camp Fire. “It showed up dramatically in the data,” said Dr. Abuabara, one of the authors of that study.
The new review adds to a growing body of literature documenting health impacts of climate change and advocating for action. In September 2021, more than 230 medical journals, including the New England Journal of Medicine — though not any dermatology journals — published an editorial calling for emergency action to limit global warming and protect health.
The following year, a commentary published across four dermatology journals discussed current and future impacts of climate change and urged dermatologists to become more engaged in finding solutions to help mitigate and adapt to climate change.
More recently, dermatologists have published about the environmental impact of professional practices such as print journals and meeting samples using single-use plastics.
Dr. Abuabara disclosed to Allergy that she is a consultant for TARGET RWE and Amgen and that her institution receives grants for research from Pfizer and LaRoche Posay. Dr. Eichenfield reported serving as a scientific adviser, consultant, and/or study investigator for Pfizer, AbbVie, Amgen and other companies. Dr. Wang disclosed that she is an International Eczema Council Fellow with financial support from Abbvie. Other authors had multiple disclosures.
A new . But it also showed the extent to which research is lacking.
“There’s not as much out there as one might expect, given that this is the most common dermatologic disease and one of the most burdensome diseases worldwide,” said Katrina Abuabara, MD, of the department of dermatology at the University of California, San Francisco, one of the senior authors of the review.
“There’s a genetic predisposition to AD, but it’s certainly very environmentally patterned,” she said in an interview. “Given that we know there are strong environmental influences, it’s an obvious example of how climate change affects our health ... It is one that may be underappreciated and that could give us near-term information.”
Indeed, she and her coauthors emphasized in their paper, “AD could serve as a case study for climatic impacts on health.” The review, which looked beyond the realm of air pollution, was published in Allergy, the journal of the European Academy of Allergy and Clinical Immunology.
Dr. Abuabara, UCSF dermatologist Sheng-Pei Wang, MD, MPH, and their coauthors — dermatologists and others from the United States, Europe, Brazil, and India — were convened by the International Eczema Council and teamed up with a biologist and climate science expert, Camilo Mora, PhD, of the University of Hawaii at Mānoa, Honolulu. Because research to date has focused on air pollution, with the impact of other hazards that Dr. Abuabara said were “a lot less developed and organized,” they used a framework and search strategy developed by Dr. Mora that looks at 10 climatic hazards related to greenhouse gas emissions, including heat waves, drought, precipitation, wildfires, and sea level rise.
“Given that this [framework] was already out there in the literature, we thought it would give us a structure and a nice way to organize the literature,” Dr. Abuabara said. While the literature is too heterogeneous for a systematic review and meta-analysis, the researchers used a systematic approach, she explained.
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and a coauthor of the paper, said in an e-mail that the review raises “our consciousness about how these [climate] changes may be impacting atopic dermatitis.”
Researchers have “much work to do to understand the evolving impact on AD development and course, and even more to figure out how to avoid extreme weather’s impact to minimize its effects on inflammatory skin diseases,” he said. “In the meantime, this paper is a call for the health care community to recognize a set of factors that can influence our patients’ dermatitis and lives.”
Mixed Results, But Negative Impacts Overall
The researchers identified 18 studies across most of the 10 climatic hazards with evidence for an impact on AD, the majority of which demonstrated harmful effects on various aspects of AD — most commonly on AD-related health care utilization and severity/flares. Only three of the studies examined AD prevalence and notably, none looked at incidence.
The impact of climatic hazards on AD appears to vary depending on the geographic region and its baseline climate, the authors said. A study in South Korea, for instance, found that in areas declared as disaster zones after storms and heavy rains, the number of AD-related outpatient visits increased for all ages. And a study in the United States showed an increased prevalence of childhood eczema in states with higher mean annual precipitation. However, some other studies on precipitation found no associations.
Just as published studies on precipitation yielded mixed results, so have studies on warming temperatures, Dr. Abuabara and her colleagues reported in their paper, with higher temperatures found to be positively associated with severity of AD symptoms in a study among patients with AD living in a region of Southern Italy, but decreased AD-related health care utilization in a study in Denmark.
In another study of over 5,500 children enrolled in an eczema registry in the United States between 2004 and 2012, higher temperature (odds ratio [OR] = 0.90, P < .001) and increased sun exposure (OR = 0.93, P = .009) were associated with poorly controlled eczema, after the researchers controlled for gender, race, income, and topical medication use.
Studies From 10 Countries Reviewed
Across the 18 studies identified in the review, data were collected in 10 countries. Five studies were conducted in the United States, one used global data, six were from Asia, and the others were from Europe and Africa. Data are lacking, the researchers wrote, in many parts of the world, including coastal regions of the tropics that are projected to experience the largest cumulative climatic hazards.
Future research should not only cover more geographic areas — especially those most impacted by climate change — but should examine impacts on AD incidence, prevalence, and “long-term monitoring of disease activity over time at the individual level,” the researchers recommended. Research should also aim to integrate multiple climatic factors and types of climate data, they said.
“As researchers, we always like to distill things down, but with climatic hazards like warming, you have to integrate other factors such as what the baseline temperature is and how precipitation is involved,” Dr. Abuabara said in the interview. With precipitation, similarly, associated factors such as outdoor humidity, pollen, and pollution exposure may also be at play for AD. Overall, she said, “you have to integrate many types of data.”
In addition to their literature review, the researchers created maps comparing the past, present, and future burden of climatic hazards to AD prevalence data. One pair of maps illustrates global cumulative exposure to climatic hazards in 2005 in parallel with the estimated annual change in AD prevalence in the subsequent decade. “It’s meant to be descriptive,” Dr. Abuabara said in the interview. The maps show alignment “between the areas experiencing the most climatic hazards and those where we subsequently saw the most rapid changes in AD.”
The paper also describes how climatic factors impact skin physiology and AD — exacerbating barrier impairment, immune dysregulation, dysbiosis, and pruritus — and how there are differential impacts on vulnerable and displaced populations with AD. It also briefly addresses air pollution, which was not included in the review framework but is impacted by wildfire and other included climatic factors.
The Need to Better Track AD, Anticipate Clinical Impact
“Outside of epidemiology, [clinicians and others] may not realize we actually have fairly poor measures of prevalence and severity of AD and disease flare over time,” Dr. Abuabara said. So “improving the ways we can measure this disease and getting more detailed data is important” for assessing the impact of climate changes.
More skin measures should be incorporated into large national health surveys, for one. “Skin doesn’t come to mind as much as diseases like heart disease and diabetes,” she said, and when surveys ask about AD, “they often don’t ask specific enough questions or ask about severity.” The clinical impacts of adverse climatic changes and extreme weather events — sudden therapy interruption, particularly of systemic agents, and delayed treatment, for instance — should be reflected in the planning and provision of dermatology services, Dr. Abuabara and her coauthors wrote.
There are currently no evidence-based recommendations for what patients with AD can do differently when faced with wildfire smoke or other climatic hazards, other than general recommendations, for instance, to reduce exposure to wildfire smoke and aeroallergens, she said in the interview. But “overall, the field has moved to more proactive treatment patterns ... toward providing anticipatory guidance and having individualized treatment plans that give people the tools to be ready to step things up or counteract [flares or worsening] if they need to.”
She and her San Francisco–based coauthors have already experienced the impact of wildfires firsthand. “It was amazing — in the period right after a major wildfire hundreds of miles away from the Bay area, we saw a huge spike in visits for itch and for eczema,” she said, referring to research on AD clinic visits after the 2018 California Camp Fire. “It showed up dramatically in the data,” said Dr. Abuabara, one of the authors of that study.
The new review adds to a growing body of literature documenting health impacts of climate change and advocating for action. In September 2021, more than 230 medical journals, including the New England Journal of Medicine — though not any dermatology journals — published an editorial calling for emergency action to limit global warming and protect health.
The following year, a commentary published across four dermatology journals discussed current and future impacts of climate change and urged dermatologists to become more engaged in finding solutions to help mitigate and adapt to climate change.
More recently, dermatologists have published about the environmental impact of professional practices such as print journals and meeting samples using single-use plastics.
Dr. Abuabara disclosed to Allergy that she is a consultant for TARGET RWE and Amgen and that her institution receives grants for research from Pfizer and LaRoche Posay. Dr. Eichenfield reported serving as a scientific adviser, consultant, and/or study investigator for Pfizer, AbbVie, Amgen and other companies. Dr. Wang disclosed that she is an International Eczema Council Fellow with financial support from Abbvie. Other authors had multiple disclosures.
FROM ALLERGY
National Rapid Genome Testing Program Benefits NICU Care
TOPLINE:
A national study in Israel demonstrates the feasibility and diagnostic benefits of rapid trio genome sequencing in critically ill neonates.
METHODOLOGY:
- Researchers conducted a prospective, multicenter cohort study from October 2021 to December 2022, involving all Israeli medical genetics institutes and neonatal intensive care units.
- A total of 130 critically ill neonates suspected of having a genetic disorder were enrolled, with rapid genome sequencing results expected within 10 days.
TAKEAWAY:
- Rapid trio genome sequencing diagnosed 50% of the neonates with disease-causing variants, including 12 chromosomal and 52 monogenic conditions.
- Another 11% had variants of unknown significance that were suspected to be disease-causing, and 1% had a novel gene suspected of causing disease.
- The mean turnaround time for the rapid reports was 7 days, demonstrating the feasibility of implementing rapid genome sequencing in a national healthcare setting, the researchers said.
- Genomic testing led to a change in clinical management for 22% of the neonates, which shows the clinical utility of this approach to diagnosis, they said.
IN PRACTICE:
Genetic testing may identify patients who are candidates for precision medical treatment and inform family planning, which is “critical for families with a severely affected or deceased child,” the study authors wrote.
SOURCE:
The corresponding author for the study was Daphna Marom, MD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. It was published online on February 22, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on voluntary participation may have introduced referral bias, potentially affecting the diagnostic rates. The long-term impact of diagnosis on survival, growth, and development remains to be evaluated. Bioinformatics tools have limitations, as shown by the missed detection of maternal uniparental disomy in one case of a hypotonic infant with Prader-Willi syndrome, the researchers noted. Clinical judgment is still essential, they said.
DISCLOSURES:
The study was sponsored by a collaboration between the Israeli Ministry of Health, Illumina, and the Genomics Center at the Tel Aviv Sourasky Medical Center. Illumina provided reagents, bioinformatics tools, and editorial assistance. Study authors disclosed financial ties to Illumina.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
A national study in Israel demonstrates the feasibility and diagnostic benefits of rapid trio genome sequencing in critically ill neonates.
METHODOLOGY:
- Researchers conducted a prospective, multicenter cohort study from October 2021 to December 2022, involving all Israeli medical genetics institutes and neonatal intensive care units.
- A total of 130 critically ill neonates suspected of having a genetic disorder were enrolled, with rapid genome sequencing results expected within 10 days.
TAKEAWAY:
- Rapid trio genome sequencing diagnosed 50% of the neonates with disease-causing variants, including 12 chromosomal and 52 monogenic conditions.
- Another 11% had variants of unknown significance that were suspected to be disease-causing, and 1% had a novel gene suspected of causing disease.
- The mean turnaround time for the rapid reports was 7 days, demonstrating the feasibility of implementing rapid genome sequencing in a national healthcare setting, the researchers said.
- Genomic testing led to a change in clinical management for 22% of the neonates, which shows the clinical utility of this approach to diagnosis, they said.
IN PRACTICE:
Genetic testing may identify patients who are candidates for precision medical treatment and inform family planning, which is “critical for families with a severely affected or deceased child,” the study authors wrote.
SOURCE:
The corresponding author for the study was Daphna Marom, MD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. It was published online on February 22, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on voluntary participation may have introduced referral bias, potentially affecting the diagnostic rates. The long-term impact of diagnosis on survival, growth, and development remains to be evaluated. Bioinformatics tools have limitations, as shown by the missed detection of maternal uniparental disomy in one case of a hypotonic infant with Prader-Willi syndrome, the researchers noted. Clinical judgment is still essential, they said.
DISCLOSURES:
The study was sponsored by a collaboration between the Israeli Ministry of Health, Illumina, and the Genomics Center at the Tel Aviv Sourasky Medical Center. Illumina provided reagents, bioinformatics tools, and editorial assistance. Study authors disclosed financial ties to Illumina.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
A national study in Israel demonstrates the feasibility and diagnostic benefits of rapid trio genome sequencing in critically ill neonates.
METHODOLOGY:
- Researchers conducted a prospective, multicenter cohort study from October 2021 to December 2022, involving all Israeli medical genetics institutes and neonatal intensive care units.
- A total of 130 critically ill neonates suspected of having a genetic disorder were enrolled, with rapid genome sequencing results expected within 10 days.
TAKEAWAY:
- Rapid trio genome sequencing diagnosed 50% of the neonates with disease-causing variants, including 12 chromosomal and 52 monogenic conditions.
- Another 11% had variants of unknown significance that were suspected to be disease-causing, and 1% had a novel gene suspected of causing disease.
- The mean turnaround time for the rapid reports was 7 days, demonstrating the feasibility of implementing rapid genome sequencing in a national healthcare setting, the researchers said.
- Genomic testing led to a change in clinical management for 22% of the neonates, which shows the clinical utility of this approach to diagnosis, they said.
IN PRACTICE:
Genetic testing may identify patients who are candidates for precision medical treatment and inform family planning, which is “critical for families with a severely affected or deceased child,” the study authors wrote.
SOURCE:
The corresponding author for the study was Daphna Marom, MD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. It was published online on February 22, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on voluntary participation may have introduced referral bias, potentially affecting the diagnostic rates. The long-term impact of diagnosis on survival, growth, and development remains to be evaluated. Bioinformatics tools have limitations, as shown by the missed detection of maternal uniparental disomy in one case of a hypotonic infant with Prader-Willi syndrome, the researchers noted. Clinical judgment is still essential, they said.
DISCLOSURES:
The study was sponsored by a collaboration between the Israeli Ministry of Health, Illumina, and the Genomics Center at the Tel Aviv Sourasky Medical Center. Illumina provided reagents, bioinformatics tools, and editorial assistance. Study authors disclosed financial ties to Illumina.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
FDA Clears Medical Grade Over-the-Counter Pulse Oximeter
The MightySat Medical, an over-the-counter medical fingertip pulse oximeter, has received clearance from the US Food and Drug Administration (FDA) for use without a prescription, according to a press release from manufacturer Masimo.
The device is the first medical fingertip pulse oximeter available directly to consumers without a prescription that includes the same technology used by many hospitals, according to the company.
According to the FDA, home pulse oximeters are currently generally of two classes: hospital-grade prescription devices which have been vetted for accuracy through clinical trials, and over-the-counter devices which are sold direct to consumers but often estimate oxygen saturation. FDA communication on pulse oximeter accuracy states "OTC oximeters that are sold as either general wellness or sporting/aviation products are not intended for medical purposes, so they do not undergo FDA review."
Pulse oximeter use is important for patients diagnosed with breathing problems or lung diseases such as asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, lung cancer, flu, pneumonia, or COVID-19 to collect accurate data on arterial blood oxygen saturation that they can share with their healthcare providers, according to the company. Patients with cardiac conditions, including pulmonary hypertension and heart failure may also benefit from pulse oximeter monitoring.
However, challenges of pulse oximeter use include measuring accuracy when patients are moving, measuring patients with poor circulation, and measuring patients with cool, thick, or darker skin. The MightySat Medical is designed to provide reliable measures of oxygen saturation and pulse rate across all patient groups, the manufacturers wrote in the press release.
Asked for additional comment, Diego J. Maselli, MD, FCCP, Professor and Chief in the division of Pulmonary Diseases and Critical Care at UT Health at San Antonio, noted, "Over the past decades, there has been an increased interest in home monitoring of medical conditions, particulrly with the development of more portable and accessible technology."
"This was heightended by the COVID-19 pandemic where telemedicine was frequently required as a means of delivering care," Dr. Maselli continued. "One of the important characteristics to monitor was the oxgen saturation in patients that had an active COVID-19 infection as it would dictate management and was part of the protocol for monitoring the clinical course of infection. Because of this need, many companies developed portable pulse oximeters for home use. This resulted in widespread use of pulse oximeters at home and other places outside clinic or hospital."
Other over-the-counter pulse oximeters that are not cleared by the FDA may create confusion among patients about the accuracy of their measurements, according to the company.
Dr. Maselli also commented that pulse oximeters' value can vary. "Unfortunately, these devices vary in quality and reliability and patients may not be fully aware of this. Most recently, the FDA approved a hospital-grade pulse oximeter that requires no prescription. This device may provide a more accurate reading in a wide range of clinical situations outside the healthcare setting. Patients should be aware that there are different grades of pulse oximeter before selecting one for home use. In addition, patients should work closely with their providers to better select the monitoring modaility that best fits their clinical situation," he said.
MightySat Medical is indicated for individuals aged 18 years and older who are well or poorly perfused under no motion conditions and is not intended as a diagnostic or screening tool for lung disease, according to the release. Treatment decisions based on data from the device should be made only in consultation with a healthcare provider, the company said. Dr. Maselli serves as a member of the CHEST Physician editorial board.
The FDA’s website offers further guidance related to at-home pulse oximeter use, with recommendations and limitations, as well as information on initiatives to ensure accurate and equitable pulse oximetry for all patients.
A version of this article appeared on Medscape.com.
The MightySat Medical, an over-the-counter medical fingertip pulse oximeter, has received clearance from the US Food and Drug Administration (FDA) for use without a prescription, according to a press release from manufacturer Masimo.
The device is the first medical fingertip pulse oximeter available directly to consumers without a prescription that includes the same technology used by many hospitals, according to the company.
According to the FDA, home pulse oximeters are currently generally of two classes: hospital-grade prescription devices which have been vetted for accuracy through clinical trials, and over-the-counter devices which are sold direct to consumers but often estimate oxygen saturation. FDA communication on pulse oximeter accuracy states "OTC oximeters that are sold as either general wellness or sporting/aviation products are not intended for medical purposes, so they do not undergo FDA review."
Pulse oximeter use is important for patients diagnosed with breathing problems or lung diseases such as asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, lung cancer, flu, pneumonia, or COVID-19 to collect accurate data on arterial blood oxygen saturation that they can share with their healthcare providers, according to the company. Patients with cardiac conditions, including pulmonary hypertension and heart failure may also benefit from pulse oximeter monitoring.
However, challenges of pulse oximeter use include measuring accuracy when patients are moving, measuring patients with poor circulation, and measuring patients with cool, thick, or darker skin. The MightySat Medical is designed to provide reliable measures of oxygen saturation and pulse rate across all patient groups, the manufacturers wrote in the press release.
Asked for additional comment, Diego J. Maselli, MD, FCCP, Professor and Chief in the division of Pulmonary Diseases and Critical Care at UT Health at San Antonio, noted, "Over the past decades, there has been an increased interest in home monitoring of medical conditions, particulrly with the development of more portable and accessible technology."
"This was heightended by the COVID-19 pandemic where telemedicine was frequently required as a means of delivering care," Dr. Maselli continued. "One of the important characteristics to monitor was the oxgen saturation in patients that had an active COVID-19 infection as it would dictate management and was part of the protocol for monitoring the clinical course of infection. Because of this need, many companies developed portable pulse oximeters for home use. This resulted in widespread use of pulse oximeters at home and other places outside clinic or hospital."
Other over-the-counter pulse oximeters that are not cleared by the FDA may create confusion among patients about the accuracy of their measurements, according to the company.
Dr. Maselli also commented that pulse oximeters' value can vary. "Unfortunately, these devices vary in quality and reliability and patients may not be fully aware of this. Most recently, the FDA approved a hospital-grade pulse oximeter that requires no prescription. This device may provide a more accurate reading in a wide range of clinical situations outside the healthcare setting. Patients should be aware that there are different grades of pulse oximeter before selecting one for home use. In addition, patients should work closely with their providers to better select the monitoring modaility that best fits their clinical situation," he said.
MightySat Medical is indicated for individuals aged 18 years and older who are well or poorly perfused under no motion conditions and is not intended as a diagnostic or screening tool for lung disease, according to the release. Treatment decisions based on data from the device should be made only in consultation with a healthcare provider, the company said. Dr. Maselli serves as a member of the CHEST Physician editorial board.
The FDA’s website offers further guidance related to at-home pulse oximeter use, with recommendations and limitations, as well as information on initiatives to ensure accurate and equitable pulse oximetry for all patients.
A version of this article appeared on Medscape.com.
The MightySat Medical, an over-the-counter medical fingertip pulse oximeter, has received clearance from the US Food and Drug Administration (FDA) for use without a prescription, according to a press release from manufacturer Masimo.
The device is the first medical fingertip pulse oximeter available directly to consumers without a prescription that includes the same technology used by many hospitals, according to the company.
According to the FDA, home pulse oximeters are currently generally of two classes: hospital-grade prescription devices which have been vetted for accuracy through clinical trials, and over-the-counter devices which are sold direct to consumers but often estimate oxygen saturation. FDA communication on pulse oximeter accuracy states "OTC oximeters that are sold as either general wellness or sporting/aviation products are not intended for medical purposes, so they do not undergo FDA review."
Pulse oximeter use is important for patients diagnosed with breathing problems or lung diseases such as asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, lung cancer, flu, pneumonia, or COVID-19 to collect accurate data on arterial blood oxygen saturation that they can share with their healthcare providers, according to the company. Patients with cardiac conditions, including pulmonary hypertension and heart failure may also benefit from pulse oximeter monitoring.
However, challenges of pulse oximeter use include measuring accuracy when patients are moving, measuring patients with poor circulation, and measuring patients with cool, thick, or darker skin. The MightySat Medical is designed to provide reliable measures of oxygen saturation and pulse rate across all patient groups, the manufacturers wrote in the press release.
Asked for additional comment, Diego J. Maselli, MD, FCCP, Professor and Chief in the division of Pulmonary Diseases and Critical Care at UT Health at San Antonio, noted, "Over the past decades, there has been an increased interest in home monitoring of medical conditions, particulrly with the development of more portable and accessible technology."
"This was heightended by the COVID-19 pandemic where telemedicine was frequently required as a means of delivering care," Dr. Maselli continued. "One of the important characteristics to monitor was the oxgen saturation in patients that had an active COVID-19 infection as it would dictate management and was part of the protocol for monitoring the clinical course of infection. Because of this need, many companies developed portable pulse oximeters for home use. This resulted in widespread use of pulse oximeters at home and other places outside clinic or hospital."
Other over-the-counter pulse oximeters that are not cleared by the FDA may create confusion among patients about the accuracy of their measurements, according to the company.
Dr. Maselli also commented that pulse oximeters' value can vary. "Unfortunately, these devices vary in quality and reliability and patients may not be fully aware of this. Most recently, the FDA approved a hospital-grade pulse oximeter that requires no prescription. This device may provide a more accurate reading in a wide range of clinical situations outside the healthcare setting. Patients should be aware that there are different grades of pulse oximeter before selecting one for home use. In addition, patients should work closely with their providers to better select the monitoring modaility that best fits their clinical situation," he said.
MightySat Medical is indicated for individuals aged 18 years and older who are well or poorly perfused under no motion conditions and is not intended as a diagnostic or screening tool for lung disease, according to the release. Treatment decisions based on data from the device should be made only in consultation with a healthcare provider, the company said. Dr. Maselli serves as a member of the CHEST Physician editorial board.
The FDA’s website offers further guidance related to at-home pulse oximeter use, with recommendations and limitations, as well as information on initiatives to ensure accurate and equitable pulse oximetry for all patients.
A version of this article appeared on Medscape.com.
Photoexposed Rash in an Older Adult
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3
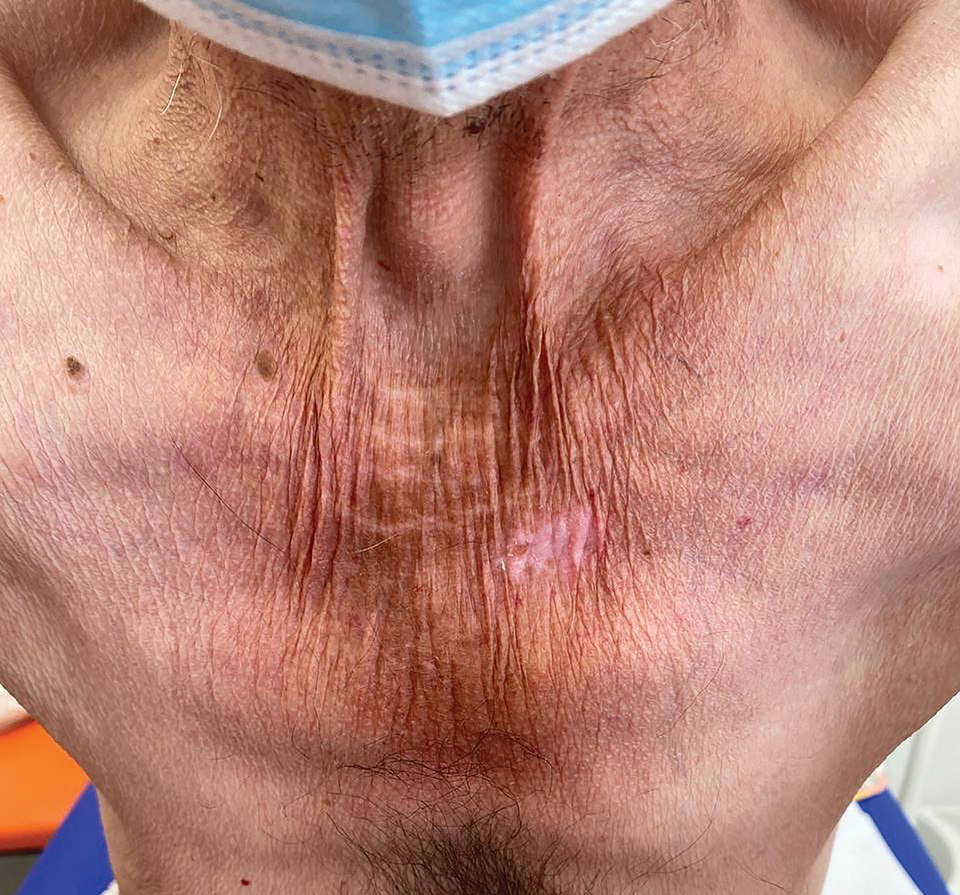
The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3

The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3

The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
A 66-year-old man presented with an intermittent pruriginous symmetric rash on the dorsal aspects of the arms, legs, and upper chest of 4 months' duration. The patient’s hands, forearms, and neck were diffusely hyperpigmented, dry, cracked, and scaling with a ring of peripheral erythema. He also experienced recurrent photosensitivity reactions on the legs. His poor clinical condition including confusion and diarrhea hindered intake of a balanced diet. He also reported a history of excessive alcohol use. The patient’s vital signs were normal, and Doppler ultrasonography ruled out deep venous thrombosis of the lower legs. A complete blood cell count showed anemia with decreased hemoglobin levels (117 g/L [reference range, 140–180 g/L]) and increased mean corpuscular volume (107.1 fL [reference range, 80–100 fL]). Additionally, low serum levels of albumin, folate, and vitamin B12 were noted. The patient had been taking hydrochlorothiazide and salicylic acid for hypertension with no recent changes in his medication regimen.
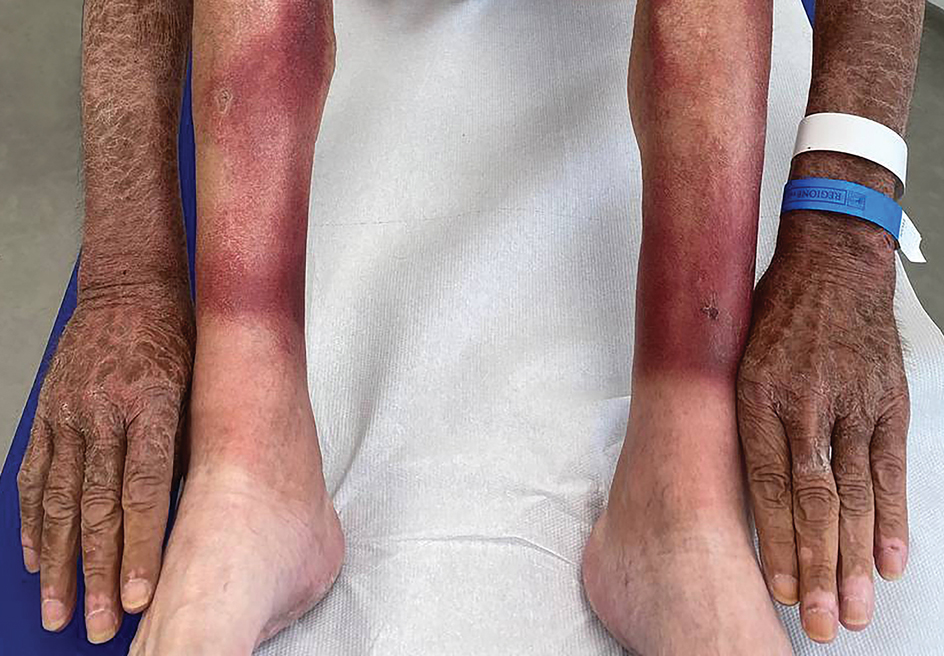
Gout Increases the Risk for a Wide Range of Cardiovascular Diseases
People with gout are 58% more likely to develop cardiovascular disease (CVD), according to a new analysis. This increased risk was observed across 12 different cardiovascular conditions, including heart failure, arrhythmias, and valve diseases.
“These findings suggest that the organ damage associated with gout is likely to be much broader than originally thought,” Nathalie Conrad, PhD, senior author of the research and cardiovascular epidemiologist at KU Leuven, Leuven, Belgium, said in an email. This could be useful for future research on underlying biological mechanisms driving CVD risk in gout, she added.
While previous research has tied gout to increased cardiovascular risk, these studies “largely focused on coronary heart disease, stroke, and thromboembolic outcomes,” she explained, and have been smaller in size.
This new study included more than 862,000 individuals, which permitted researchers to investigate rarer CVD outcomes such as myocarditis and pericarditis.
For the study, researchers used electronic health records from the UK Clinical Practice Research Datalink, a primary care database that contains anonymized health data for about 22 million individuals. Using these data, they identified more than 152,600 individuals with gout. Patients included in the analysis were diagnosed between 2000 and 2017, younger than 80 years at diagnosis, and free of CVD for at least 12 months after their gout diagnosis.
Patients with gout were compared with nearly 710,000 controls, matched on demographic factors such as age, sex, and geographic region.
Researchers then investigated the incidence of 12 CVDs, including atherosclerotic diseases, degenerative and thromboembolic diseases, and arrythmias, between the two groups from January 1, 2000, to June 30, 2019.
The findings were published in the March 2024 issue of The Lancet Rheumatology. Overall, patients with gout were 58% more likely to develop any CVD than their matched comparators without gout. There was a higher disease incidence among patients with gout for each of the 12 conditions. This association was more pronounced in women (hazard ratio [HR], 1.88) than in men (HR, 1.49), and gout amplified the risk for CVD in younger individuals to a greater extent.
Individuals younger than 45 years with gout were more than twice as likely to develop CVD compared with similarly aged individuals without gout. For comparison, individuals aged 45-54 years with gout were 84% more likely to develop CVD, and individuals aged 55-64 years were 57% more likely to develop CVD than matched controls.
Conduction system disease had the highest incident risk (HR, 1.88), followed by heart failure and valve disease (HR, 1.85 for both).
Individuals with gout had higher rates of comorbidities than the controls, including hypertension, obesity, and dyslipidemia. Overall, CVD risk was slightly attenuated after adjustment for traditional CVD risk factors such as smoking, blood pressure, and body mass index but still significant: Patients with gout had a 31% higher risk for CVD than comparators.
This shows “that known CVD risk factors only explain part of the CVD risks seen in patients with gout,” Dr. Conrad said. Other factors such as inflammation and other disease activity factors could be at play, she explained, which would need to be explored in future research.
The study “shows the whole landscape” of CVD and gout, Michael H. Pillinger, MD, rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Grossman School of Medicine in New York City, said in an interview. He was not involved with the research.
“Every possible cardiovascular disease that they could think of was something that gout patients had more of than the non-gout patients,” he added. “I think this is going to be a paper that gets cited a lot, at minimum when describing the background of risk when we look at gout patients.”
The study had some limitations, including that researchers were unable to account for how medications such as nonsteroidal anti-inflammatory drugs, corticosteroids, colchicine, or allopurinol may have affected the association between gout and CVD.
“This is because analyses of nonrandomized treatment can be confounded by indication, wherein it is difficult to differentiate the effects of the treatment from underlying disease severity,” the authors wrote.
There was also a large amount of missing data on blood pressure, body mass index, smoking status, and other health information relevant to cardiovascular risk, so sensitivity analyses adjusting for these factors “should be interpreted with caution,” they added.
Dr. Pillinger also noted that the rates of comorbidities in the gout study population were lower than what have been found in US study populations. For example, about 40% of patients with gout in the analysis had hypertension, while other studies have suggested higher rates of 60%-70%, he said. However, it’s not clear if these differences could have affected outcomes. He added that these limitations do not “in any way weaken [the authors’] conclusion.”
The findings call for better strategies to reduce CVD risk in patients with gout, Dr. Conrad noted.
“Further improvements could come from better recognition and intervention on CVD risk factors (eg, through lifestyle changes or drug therapies where they are indicated), as well as proactive screening for heart disease in patients with gout, which could allow early diagnosis and interventions to delay more severe outcomes,” she added.
This study was funded by Research Foundation Flanders. Dr. Conrad was funded by a personal fellowship from the Research Foundation Flanders and a European Society of Cardiology research grant. She received royalties from Oxford University Innovation. Four of Dr. Conrad’s eight coauthors also reported financial relationships with pharmaceutical companies. Dr. Pillinger served as a consultant to Amgen, Federation Bio, Fortress Biotech, and Scilex, and he holds an investigator-initiated grant from Hikma.
A version of this article appeared on Medscape.com.
People with gout are 58% more likely to develop cardiovascular disease (CVD), according to a new analysis. This increased risk was observed across 12 different cardiovascular conditions, including heart failure, arrhythmias, and valve diseases.
“These findings suggest that the organ damage associated with gout is likely to be much broader than originally thought,” Nathalie Conrad, PhD, senior author of the research and cardiovascular epidemiologist at KU Leuven, Leuven, Belgium, said in an email. This could be useful for future research on underlying biological mechanisms driving CVD risk in gout, she added.
While previous research has tied gout to increased cardiovascular risk, these studies “largely focused on coronary heart disease, stroke, and thromboembolic outcomes,” she explained, and have been smaller in size.
This new study included more than 862,000 individuals, which permitted researchers to investigate rarer CVD outcomes such as myocarditis and pericarditis.
For the study, researchers used electronic health records from the UK Clinical Practice Research Datalink, a primary care database that contains anonymized health data for about 22 million individuals. Using these data, they identified more than 152,600 individuals with gout. Patients included in the analysis were diagnosed between 2000 and 2017, younger than 80 years at diagnosis, and free of CVD for at least 12 months after their gout diagnosis.
Patients with gout were compared with nearly 710,000 controls, matched on demographic factors such as age, sex, and geographic region.
Researchers then investigated the incidence of 12 CVDs, including atherosclerotic diseases, degenerative and thromboembolic diseases, and arrythmias, between the two groups from January 1, 2000, to June 30, 2019.
The findings were published in the March 2024 issue of The Lancet Rheumatology. Overall, patients with gout were 58% more likely to develop any CVD than their matched comparators without gout. There was a higher disease incidence among patients with gout for each of the 12 conditions. This association was more pronounced in women (hazard ratio [HR], 1.88) than in men (HR, 1.49), and gout amplified the risk for CVD in younger individuals to a greater extent.
Individuals younger than 45 years with gout were more than twice as likely to develop CVD compared with similarly aged individuals without gout. For comparison, individuals aged 45-54 years with gout were 84% more likely to develop CVD, and individuals aged 55-64 years were 57% more likely to develop CVD than matched controls.
Conduction system disease had the highest incident risk (HR, 1.88), followed by heart failure and valve disease (HR, 1.85 for both).
Individuals with gout had higher rates of comorbidities than the controls, including hypertension, obesity, and dyslipidemia. Overall, CVD risk was slightly attenuated after adjustment for traditional CVD risk factors such as smoking, blood pressure, and body mass index but still significant: Patients with gout had a 31% higher risk for CVD than comparators.
This shows “that known CVD risk factors only explain part of the CVD risks seen in patients with gout,” Dr. Conrad said. Other factors such as inflammation and other disease activity factors could be at play, she explained, which would need to be explored in future research.
The study “shows the whole landscape” of CVD and gout, Michael H. Pillinger, MD, rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Grossman School of Medicine in New York City, said in an interview. He was not involved with the research.
“Every possible cardiovascular disease that they could think of was something that gout patients had more of than the non-gout patients,” he added. “I think this is going to be a paper that gets cited a lot, at minimum when describing the background of risk when we look at gout patients.”
The study had some limitations, including that researchers were unable to account for how medications such as nonsteroidal anti-inflammatory drugs, corticosteroids, colchicine, or allopurinol may have affected the association between gout and CVD.
“This is because analyses of nonrandomized treatment can be confounded by indication, wherein it is difficult to differentiate the effects of the treatment from underlying disease severity,” the authors wrote.
There was also a large amount of missing data on blood pressure, body mass index, smoking status, and other health information relevant to cardiovascular risk, so sensitivity analyses adjusting for these factors “should be interpreted with caution,” they added.
Dr. Pillinger also noted that the rates of comorbidities in the gout study population were lower than what have been found in US study populations. For example, about 40% of patients with gout in the analysis had hypertension, while other studies have suggested higher rates of 60%-70%, he said. However, it’s not clear if these differences could have affected outcomes. He added that these limitations do not “in any way weaken [the authors’] conclusion.”
The findings call for better strategies to reduce CVD risk in patients with gout, Dr. Conrad noted.
“Further improvements could come from better recognition and intervention on CVD risk factors (eg, through lifestyle changes or drug therapies where they are indicated), as well as proactive screening for heart disease in patients with gout, which could allow early diagnosis and interventions to delay more severe outcomes,” she added.
This study was funded by Research Foundation Flanders. Dr. Conrad was funded by a personal fellowship from the Research Foundation Flanders and a European Society of Cardiology research grant. She received royalties from Oxford University Innovation. Four of Dr. Conrad’s eight coauthors also reported financial relationships with pharmaceutical companies. Dr. Pillinger served as a consultant to Amgen, Federation Bio, Fortress Biotech, and Scilex, and he holds an investigator-initiated grant from Hikma.
A version of this article appeared on Medscape.com.
People with gout are 58% more likely to develop cardiovascular disease (CVD), according to a new analysis. This increased risk was observed across 12 different cardiovascular conditions, including heart failure, arrhythmias, and valve diseases.
“These findings suggest that the organ damage associated with gout is likely to be much broader than originally thought,” Nathalie Conrad, PhD, senior author of the research and cardiovascular epidemiologist at KU Leuven, Leuven, Belgium, said in an email. This could be useful for future research on underlying biological mechanisms driving CVD risk in gout, she added.
While previous research has tied gout to increased cardiovascular risk, these studies “largely focused on coronary heart disease, stroke, and thromboembolic outcomes,” she explained, and have been smaller in size.
This new study included more than 862,000 individuals, which permitted researchers to investigate rarer CVD outcomes such as myocarditis and pericarditis.
For the study, researchers used electronic health records from the UK Clinical Practice Research Datalink, a primary care database that contains anonymized health data for about 22 million individuals. Using these data, they identified more than 152,600 individuals with gout. Patients included in the analysis were diagnosed between 2000 and 2017, younger than 80 years at diagnosis, and free of CVD for at least 12 months after their gout diagnosis.
Patients with gout were compared with nearly 710,000 controls, matched on demographic factors such as age, sex, and geographic region.
Researchers then investigated the incidence of 12 CVDs, including atherosclerotic diseases, degenerative and thromboembolic diseases, and arrythmias, between the two groups from January 1, 2000, to June 30, 2019.
The findings were published in the March 2024 issue of The Lancet Rheumatology. Overall, patients with gout were 58% more likely to develop any CVD than their matched comparators without gout. There was a higher disease incidence among patients with gout for each of the 12 conditions. This association was more pronounced in women (hazard ratio [HR], 1.88) than in men (HR, 1.49), and gout amplified the risk for CVD in younger individuals to a greater extent.
Individuals younger than 45 years with gout were more than twice as likely to develop CVD compared with similarly aged individuals without gout. For comparison, individuals aged 45-54 years with gout were 84% more likely to develop CVD, and individuals aged 55-64 years were 57% more likely to develop CVD than matched controls.
Conduction system disease had the highest incident risk (HR, 1.88), followed by heart failure and valve disease (HR, 1.85 for both).
Individuals with gout had higher rates of comorbidities than the controls, including hypertension, obesity, and dyslipidemia. Overall, CVD risk was slightly attenuated after adjustment for traditional CVD risk factors such as smoking, blood pressure, and body mass index but still significant: Patients with gout had a 31% higher risk for CVD than comparators.
This shows “that known CVD risk factors only explain part of the CVD risks seen in patients with gout,” Dr. Conrad said. Other factors such as inflammation and other disease activity factors could be at play, she explained, which would need to be explored in future research.
The study “shows the whole landscape” of CVD and gout, Michael H. Pillinger, MD, rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Grossman School of Medicine in New York City, said in an interview. He was not involved with the research.
“Every possible cardiovascular disease that they could think of was something that gout patients had more of than the non-gout patients,” he added. “I think this is going to be a paper that gets cited a lot, at minimum when describing the background of risk when we look at gout patients.”
The study had some limitations, including that researchers were unable to account for how medications such as nonsteroidal anti-inflammatory drugs, corticosteroids, colchicine, or allopurinol may have affected the association between gout and CVD.
“This is because analyses of nonrandomized treatment can be confounded by indication, wherein it is difficult to differentiate the effects of the treatment from underlying disease severity,” the authors wrote.
There was also a large amount of missing data on blood pressure, body mass index, smoking status, and other health information relevant to cardiovascular risk, so sensitivity analyses adjusting for these factors “should be interpreted with caution,” they added.
Dr. Pillinger also noted that the rates of comorbidities in the gout study population were lower than what have been found in US study populations. For example, about 40% of patients with gout in the analysis had hypertension, while other studies have suggested higher rates of 60%-70%, he said. However, it’s not clear if these differences could have affected outcomes. He added that these limitations do not “in any way weaken [the authors’] conclusion.”
The findings call for better strategies to reduce CVD risk in patients with gout, Dr. Conrad noted.
“Further improvements could come from better recognition and intervention on CVD risk factors (eg, through lifestyle changes or drug therapies where they are indicated), as well as proactive screening for heart disease in patients with gout, which could allow early diagnosis and interventions to delay more severe outcomes,” she added.
This study was funded by Research Foundation Flanders. Dr. Conrad was funded by a personal fellowship from the Research Foundation Flanders and a European Society of Cardiology research grant. She received royalties from Oxford University Innovation. Four of Dr. Conrad’s eight coauthors also reported financial relationships with pharmaceutical companies. Dr. Pillinger served as a consultant to Amgen, Federation Bio, Fortress Biotech, and Scilex, and he holds an investigator-initiated grant from Hikma.
A version of this article appeared on Medscape.com.
Is Stretching Now Underrated? Accumulating Research Says Yes
For many, stretching is the fitness equivalent of awkward small talk. It’s the opening act, the thing you tolerate because you know it will be over soon.
Others have challenged the practice, suggesting that stretching isn’t necessary at all. Some research has found that a preworkout stretch may even be disadvantageous, weakening muscles and hindering performance.
To put it plainly, no one seems terribly enthusiastic about touching their toes.
That’s why a 2020 study on exercise and mortality was such a head-scratcher. The study found that stretching was uniquely associated with a lower risk for all-cause mortality among American adults. That’s after controlling for participation in other types of exercise.
The finding seemed like a fluke, until a 2023 study found essentially the same thing.
That was slightly better than the risk reduction associated with high volumes of aerobic exercise and resistance training.
How can that be ? It turns out, stretching is linked to several health benefits that you might not expect.
The Surprising Benefits of Stretching
When we talk about stretching, we usually mean static stretching — getting into and holding a position that challenges a muscle, with the goal of improving range of motion around a joint.
It doesn’t need to be a big challenge. “Research shows you can get increases in flexibility by stretching to the initial point of discomfort,” said David Behm, PhD, an exercise scientist at Memorial University of Newfoundland in Canada who’s published dozens of studies on stretching over the past quarter-century.
That brings us to the first benefit.
Stretching Benefit #1: More Strength
At first glance, flexibility training and strength training have little in common. You lengthen muscles in the former and contract them in the latter.
But in both cases, Dr. Behm said, you’re applying tension to muscles and connective tissues. Tension activates proteins called integrins, which send and receive signals across cellular membranes. Those signals are the start of a cascade that leads to protein synthesis. That’s how muscles get bigger and stronger when you lift weights.
That mechanism could explain the small gains in muscle strength and size associated with static stretching, Dr. Behm said.
But can you really stretch your way to muscle growth? Theoretically, yes. But strength training is far more time-efficient, Dr. Behm says. Studies showing increases in muscle mass have typically stretched a single muscle (usually the calves, using a specialized device) for > 30 min/session, 6 d/wk for 6 weeks. And that’s for just one leg.
Still, stretching may be more accessible for some patients — research suggested that older and more sedentary people are most likely to benefit from stretching-induced gains in strength.
Stretching Benefit #2: Reduced Arterial Stiffness
“Most people don’t think about the cardiovascular benefits of stretching,” Dr. Behm said. There are some big ones.
If your body doesn’t move well, it’s not unreasonable to assume your blood doesn’t flow well. That is indeed the case: Poor flexibility is associated with arterial stiffness.
Stretching is associated not only with improved arterial function but also with reductions in resting heart rate and blood pressure and increased vasodilation.
Mobility improvements may have an indirect benefit on cardiovascular health as well.
“Studies show runners are more economical when they’re more flexible,” Dr. Behm said. If your movement is more efficient, you’ll probably do more of it. Doing more, in turn, would lead to improved fitness.
Stretching Benefit #3: Improved Performance
Research is equivocal on whether stretching improves athletic performance, said Joe Yoon, a sports massage therapist in Orlando, Florida, and author of Better Stretching.
“But I’ve always taken the approach that if you can improve your range of motion and get into positions” required for your sport, you’ll probably perform better, with less risk for injury, Mr. Yoon said.
It’s worth noting that some research over the past 30 years has linked pre-exercise static stretching with a loss of strength, power, and/or speed.
But consider this: In a 2016 review, Dr. Behm and his coauthors showed that performance reductions were most likely to occur in two situations:
When participants did extremely long stretches (duration, ≥ 60 sec per muscle).
When researchers tested the participants’ strength, power, or speed immediately after they stretched.
Avoiding those problems is easy, Dr. Behm said: Stretch each muscle for < 60 sec, and combine static stretches with more active warm-up exercises.
“Stretching can impair your performance but only if you do it wrong,” he said.
Stretching Benefit #4: Fewer Injuries
When you stretch, the point where you feel tension is where the muscle is most vulnerable. “That’s where injuries usually happen,” Dr. Behm said.
More flexibility in those areas allows your muscles to safely generate force at longer lengths. For an athlete, that means fewer injuries when they’re doing explosive movements or changing direction.
For nonathletes, flexibility reduces injuries by improving balance. Better balance reduces the risk of falling and helps mitigate the damage if you do take a tumble.
Help Your Patients Get the Benefits of Stretching
Stretching, like training for endurance or strength, can be as complex as you want to make it. But Mr. Yoon advocates a simpler approach.
“You see this flashy stuff online,” he said. “But if you see those trainers in real life or you book a session with them, they go right back to the basics.”
Ideally, Mr. Yoon said, a flexibility routine will work the entire body. But if that’s too big a stretch for your patient, he recommends starting with one or two stretches for the most problematic area.
For example, for a stiff back, try doing the puppy pose at least once a day, although twice is better. Hold the position for 30 seconds to 2 minutes, said Mr. Yoon. Even if you combine it with a dynamic movement like the cat-cow, the two exercises would take just a few minutes a day.
“There’s this misconception that you have to do a lot of it to be successful,” Mr. Yoon said.
Consistency is far more important than volume. Mr. Yoon recommends “a little bit every day — the minimum viable dose.”
As a bonus, stretching an area like your upper back will probably improve your shoulder mobility, Mr. Yoon said. Same with your lower body: Stretches for your hips, over time, should also benefit your knees and lower back.
And thanks to a phenomenon called nonlocal flexibility transfer, lower-body stretches should improve upper-body flexibility, at least temporarily. Shoulder stretches can also have an immediate effect on hip mobility.
“It’s all connected,” Mr. Yoon said, which brings us back to where we started.
If stretching can indeed reduce mortality risk, it’s probably because of interconnected pathways, rather than any single mechanism.
Most obviously, stretching improves flexibility, which makes movement easier, improves balance, and reduces the risk for falls and other types of injuries. It can also lead to small improvements in strength. Less obviously, stretching improves several aspects of cardiovascular function, including circulation.
“There seems to be a global effect in everything we do,” Dr. Behm said. “Whether you’re stretching or weight training, the message is sent throughout your body."
A version of this article appeared on Medscape.com.
For many, stretching is the fitness equivalent of awkward small talk. It’s the opening act, the thing you tolerate because you know it will be over soon.
Others have challenged the practice, suggesting that stretching isn’t necessary at all. Some research has found that a preworkout stretch may even be disadvantageous, weakening muscles and hindering performance.
To put it plainly, no one seems terribly enthusiastic about touching their toes.
That’s why a 2020 study on exercise and mortality was such a head-scratcher. The study found that stretching was uniquely associated with a lower risk for all-cause mortality among American adults. That’s after controlling for participation in other types of exercise.
The finding seemed like a fluke, until a 2023 study found essentially the same thing.
That was slightly better than the risk reduction associated with high volumes of aerobic exercise and resistance training.
How can that be ? It turns out, stretching is linked to several health benefits that you might not expect.
The Surprising Benefits of Stretching
When we talk about stretching, we usually mean static stretching — getting into and holding a position that challenges a muscle, with the goal of improving range of motion around a joint.
It doesn’t need to be a big challenge. “Research shows you can get increases in flexibility by stretching to the initial point of discomfort,” said David Behm, PhD, an exercise scientist at Memorial University of Newfoundland in Canada who’s published dozens of studies on stretching over the past quarter-century.
That brings us to the first benefit.
Stretching Benefit #1: More Strength
At first glance, flexibility training and strength training have little in common. You lengthen muscles in the former and contract them in the latter.
But in both cases, Dr. Behm said, you’re applying tension to muscles and connective tissues. Tension activates proteins called integrins, which send and receive signals across cellular membranes. Those signals are the start of a cascade that leads to protein synthesis. That’s how muscles get bigger and stronger when you lift weights.
That mechanism could explain the small gains in muscle strength and size associated with static stretching, Dr. Behm said.
But can you really stretch your way to muscle growth? Theoretically, yes. But strength training is far more time-efficient, Dr. Behm says. Studies showing increases in muscle mass have typically stretched a single muscle (usually the calves, using a specialized device) for > 30 min/session, 6 d/wk for 6 weeks. And that’s for just one leg.
Still, stretching may be more accessible for some patients — research suggested that older and more sedentary people are most likely to benefit from stretching-induced gains in strength.
Stretching Benefit #2: Reduced Arterial Stiffness
“Most people don’t think about the cardiovascular benefits of stretching,” Dr. Behm said. There are some big ones.
If your body doesn’t move well, it’s not unreasonable to assume your blood doesn’t flow well. That is indeed the case: Poor flexibility is associated with arterial stiffness.
Stretching is associated not only with improved arterial function but also with reductions in resting heart rate and blood pressure and increased vasodilation.
Mobility improvements may have an indirect benefit on cardiovascular health as well.
“Studies show runners are more economical when they’re more flexible,” Dr. Behm said. If your movement is more efficient, you’ll probably do more of it. Doing more, in turn, would lead to improved fitness.
Stretching Benefit #3: Improved Performance
Research is equivocal on whether stretching improves athletic performance, said Joe Yoon, a sports massage therapist in Orlando, Florida, and author of Better Stretching.
“But I’ve always taken the approach that if you can improve your range of motion and get into positions” required for your sport, you’ll probably perform better, with less risk for injury, Mr. Yoon said.
It’s worth noting that some research over the past 30 years has linked pre-exercise static stretching with a loss of strength, power, and/or speed.
But consider this: In a 2016 review, Dr. Behm and his coauthors showed that performance reductions were most likely to occur in two situations:
When participants did extremely long stretches (duration, ≥ 60 sec per muscle).
When researchers tested the participants’ strength, power, or speed immediately after they stretched.
Avoiding those problems is easy, Dr. Behm said: Stretch each muscle for < 60 sec, and combine static stretches with more active warm-up exercises.
“Stretching can impair your performance but only if you do it wrong,” he said.
Stretching Benefit #4: Fewer Injuries
When you stretch, the point where you feel tension is where the muscle is most vulnerable. “That’s where injuries usually happen,” Dr. Behm said.
More flexibility in those areas allows your muscles to safely generate force at longer lengths. For an athlete, that means fewer injuries when they’re doing explosive movements or changing direction.
For nonathletes, flexibility reduces injuries by improving balance. Better balance reduces the risk of falling and helps mitigate the damage if you do take a tumble.
Help Your Patients Get the Benefits of Stretching
Stretching, like training for endurance or strength, can be as complex as you want to make it. But Mr. Yoon advocates a simpler approach.
“You see this flashy stuff online,” he said. “But if you see those trainers in real life or you book a session with them, they go right back to the basics.”
Ideally, Mr. Yoon said, a flexibility routine will work the entire body. But if that’s too big a stretch for your patient, he recommends starting with one or two stretches for the most problematic area.
For example, for a stiff back, try doing the puppy pose at least once a day, although twice is better. Hold the position for 30 seconds to 2 minutes, said Mr. Yoon. Even if you combine it with a dynamic movement like the cat-cow, the two exercises would take just a few minutes a day.
“There’s this misconception that you have to do a lot of it to be successful,” Mr. Yoon said.
Consistency is far more important than volume. Mr. Yoon recommends “a little bit every day — the minimum viable dose.”
As a bonus, stretching an area like your upper back will probably improve your shoulder mobility, Mr. Yoon said. Same with your lower body: Stretches for your hips, over time, should also benefit your knees and lower back.
And thanks to a phenomenon called nonlocal flexibility transfer, lower-body stretches should improve upper-body flexibility, at least temporarily. Shoulder stretches can also have an immediate effect on hip mobility.
“It’s all connected,” Mr. Yoon said, which brings us back to where we started.
If stretching can indeed reduce mortality risk, it’s probably because of interconnected pathways, rather than any single mechanism.
Most obviously, stretching improves flexibility, which makes movement easier, improves balance, and reduces the risk for falls and other types of injuries. It can also lead to small improvements in strength. Less obviously, stretching improves several aspects of cardiovascular function, including circulation.
“There seems to be a global effect in everything we do,” Dr. Behm said. “Whether you’re stretching or weight training, the message is sent throughout your body."
A version of this article appeared on Medscape.com.
For many, stretching is the fitness equivalent of awkward small talk. It’s the opening act, the thing you tolerate because you know it will be over soon.
Others have challenged the practice, suggesting that stretching isn’t necessary at all. Some research has found that a preworkout stretch may even be disadvantageous, weakening muscles and hindering performance.
To put it plainly, no one seems terribly enthusiastic about touching their toes.
That’s why a 2020 study on exercise and mortality was such a head-scratcher. The study found that stretching was uniquely associated with a lower risk for all-cause mortality among American adults. That’s after controlling for participation in other types of exercise.
The finding seemed like a fluke, until a 2023 study found essentially the same thing.
That was slightly better than the risk reduction associated with high volumes of aerobic exercise and resistance training.
How can that be ? It turns out, stretching is linked to several health benefits that you might not expect.
The Surprising Benefits of Stretching
When we talk about stretching, we usually mean static stretching — getting into and holding a position that challenges a muscle, with the goal of improving range of motion around a joint.
It doesn’t need to be a big challenge. “Research shows you can get increases in flexibility by stretching to the initial point of discomfort,” said David Behm, PhD, an exercise scientist at Memorial University of Newfoundland in Canada who’s published dozens of studies on stretching over the past quarter-century.
That brings us to the first benefit.
Stretching Benefit #1: More Strength
At first glance, flexibility training and strength training have little in common. You lengthen muscles in the former and contract them in the latter.
But in both cases, Dr. Behm said, you’re applying tension to muscles and connective tissues. Tension activates proteins called integrins, which send and receive signals across cellular membranes. Those signals are the start of a cascade that leads to protein synthesis. That’s how muscles get bigger and stronger when you lift weights.
That mechanism could explain the small gains in muscle strength and size associated with static stretching, Dr. Behm said.
But can you really stretch your way to muscle growth? Theoretically, yes. But strength training is far more time-efficient, Dr. Behm says. Studies showing increases in muscle mass have typically stretched a single muscle (usually the calves, using a specialized device) for > 30 min/session, 6 d/wk for 6 weeks. And that’s for just one leg.
Still, stretching may be more accessible for some patients — research suggested that older and more sedentary people are most likely to benefit from stretching-induced gains in strength.
Stretching Benefit #2: Reduced Arterial Stiffness
“Most people don’t think about the cardiovascular benefits of stretching,” Dr. Behm said. There are some big ones.
If your body doesn’t move well, it’s not unreasonable to assume your blood doesn’t flow well. That is indeed the case: Poor flexibility is associated with arterial stiffness.
Stretching is associated not only with improved arterial function but also with reductions in resting heart rate and blood pressure and increased vasodilation.
Mobility improvements may have an indirect benefit on cardiovascular health as well.
“Studies show runners are more economical when they’re more flexible,” Dr. Behm said. If your movement is more efficient, you’ll probably do more of it. Doing more, in turn, would lead to improved fitness.
Stretching Benefit #3: Improved Performance
Research is equivocal on whether stretching improves athletic performance, said Joe Yoon, a sports massage therapist in Orlando, Florida, and author of Better Stretching.
“But I’ve always taken the approach that if you can improve your range of motion and get into positions” required for your sport, you’ll probably perform better, with less risk for injury, Mr. Yoon said.
It’s worth noting that some research over the past 30 years has linked pre-exercise static stretching with a loss of strength, power, and/or speed.
But consider this: In a 2016 review, Dr. Behm and his coauthors showed that performance reductions were most likely to occur in two situations:
When participants did extremely long stretches (duration, ≥ 60 sec per muscle).
When researchers tested the participants’ strength, power, or speed immediately after they stretched.
Avoiding those problems is easy, Dr. Behm said: Stretch each muscle for < 60 sec, and combine static stretches with more active warm-up exercises.
“Stretching can impair your performance but only if you do it wrong,” he said.
Stretching Benefit #4: Fewer Injuries
When you stretch, the point where you feel tension is where the muscle is most vulnerable. “That’s where injuries usually happen,” Dr. Behm said.
More flexibility in those areas allows your muscles to safely generate force at longer lengths. For an athlete, that means fewer injuries when they’re doing explosive movements or changing direction.
For nonathletes, flexibility reduces injuries by improving balance. Better balance reduces the risk of falling and helps mitigate the damage if you do take a tumble.
Help Your Patients Get the Benefits of Stretching
Stretching, like training for endurance or strength, can be as complex as you want to make it. But Mr. Yoon advocates a simpler approach.
“You see this flashy stuff online,” he said. “But if you see those trainers in real life or you book a session with them, they go right back to the basics.”
Ideally, Mr. Yoon said, a flexibility routine will work the entire body. But if that’s too big a stretch for your patient, he recommends starting with one or two stretches for the most problematic area.
For example, for a stiff back, try doing the puppy pose at least once a day, although twice is better. Hold the position for 30 seconds to 2 minutes, said Mr. Yoon. Even if you combine it with a dynamic movement like the cat-cow, the two exercises would take just a few minutes a day.
“There’s this misconception that you have to do a lot of it to be successful,” Mr. Yoon said.
Consistency is far more important than volume. Mr. Yoon recommends “a little bit every day — the minimum viable dose.”
As a bonus, stretching an area like your upper back will probably improve your shoulder mobility, Mr. Yoon said. Same with your lower body: Stretches for your hips, over time, should also benefit your knees and lower back.
And thanks to a phenomenon called nonlocal flexibility transfer, lower-body stretches should improve upper-body flexibility, at least temporarily. Shoulder stretches can also have an immediate effect on hip mobility.
“It’s all connected,” Mr. Yoon said, which brings us back to where we started.
If stretching can indeed reduce mortality risk, it’s probably because of interconnected pathways, rather than any single mechanism.
Most obviously, stretching improves flexibility, which makes movement easier, improves balance, and reduces the risk for falls and other types of injuries. It can also lead to small improvements in strength. Less obviously, stretching improves several aspects of cardiovascular function, including circulation.
“There seems to be a global effect in everything we do,” Dr. Behm said. “Whether you’re stretching or weight training, the message is sent throughout your body."
A version of this article appeared on Medscape.com.