User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
The CROWNing Event on Hair Loss in Women of Color: A Framework for Advocacy and Community Engagement (FACE) Survey Analysis
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
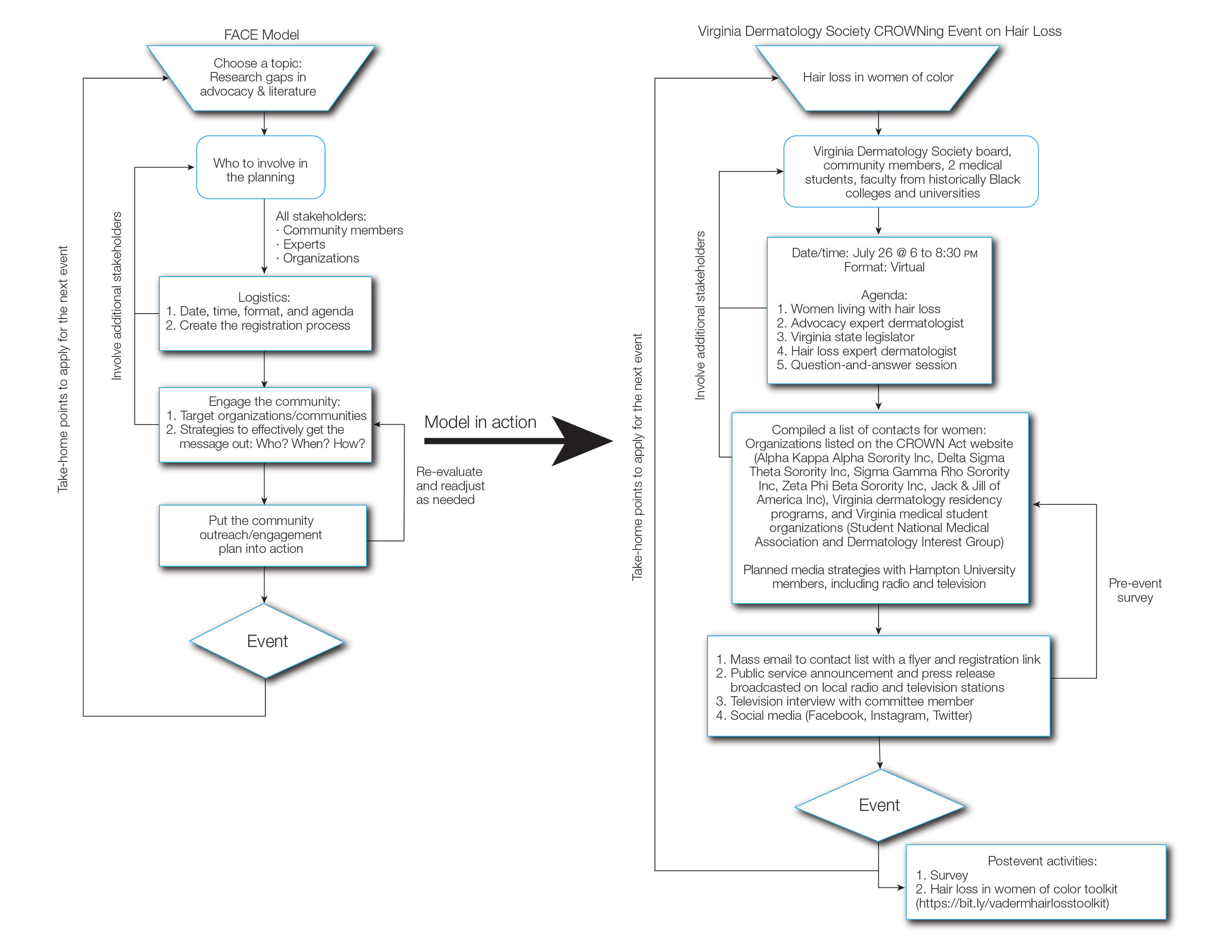
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.
We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.
Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
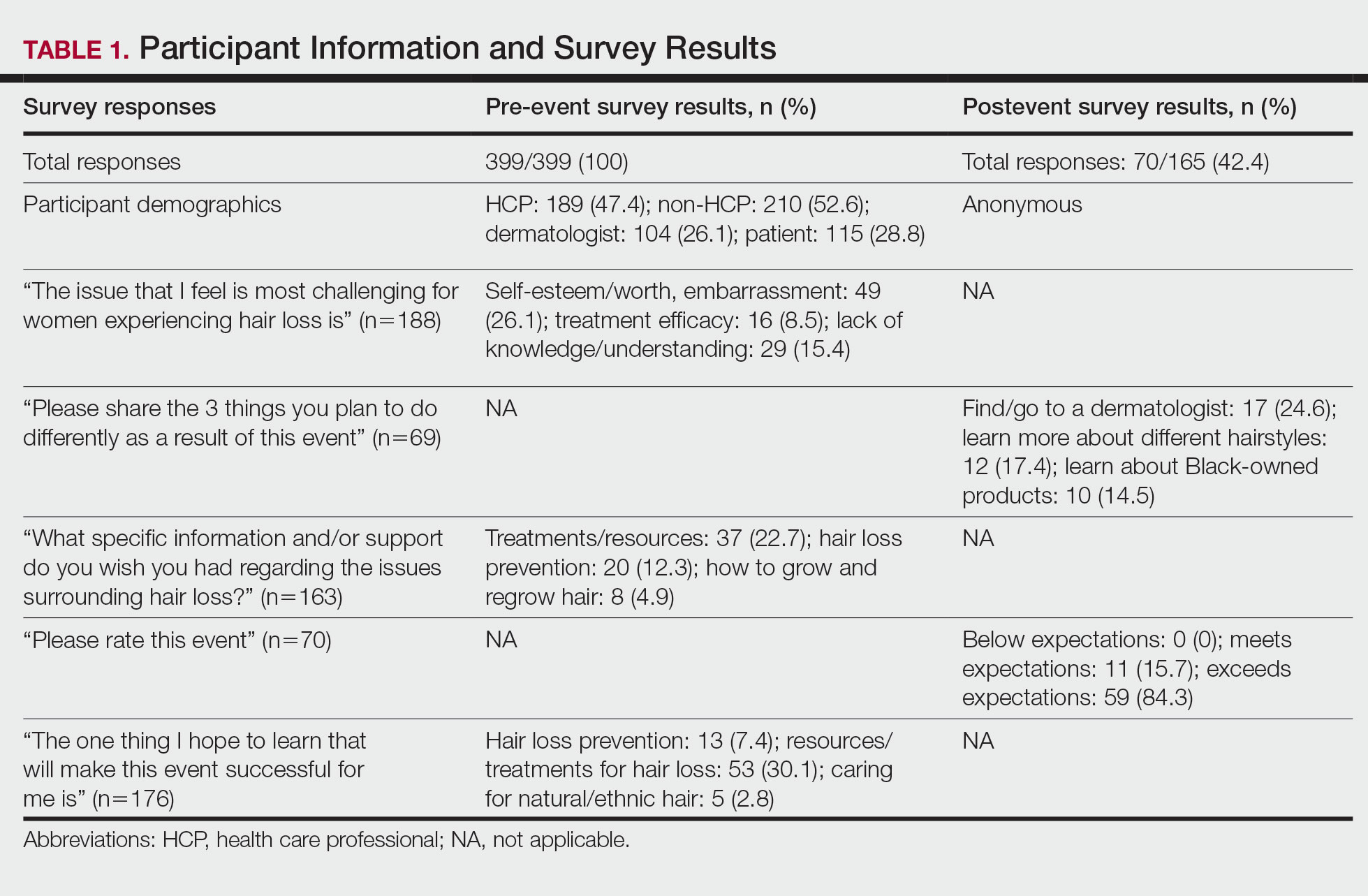
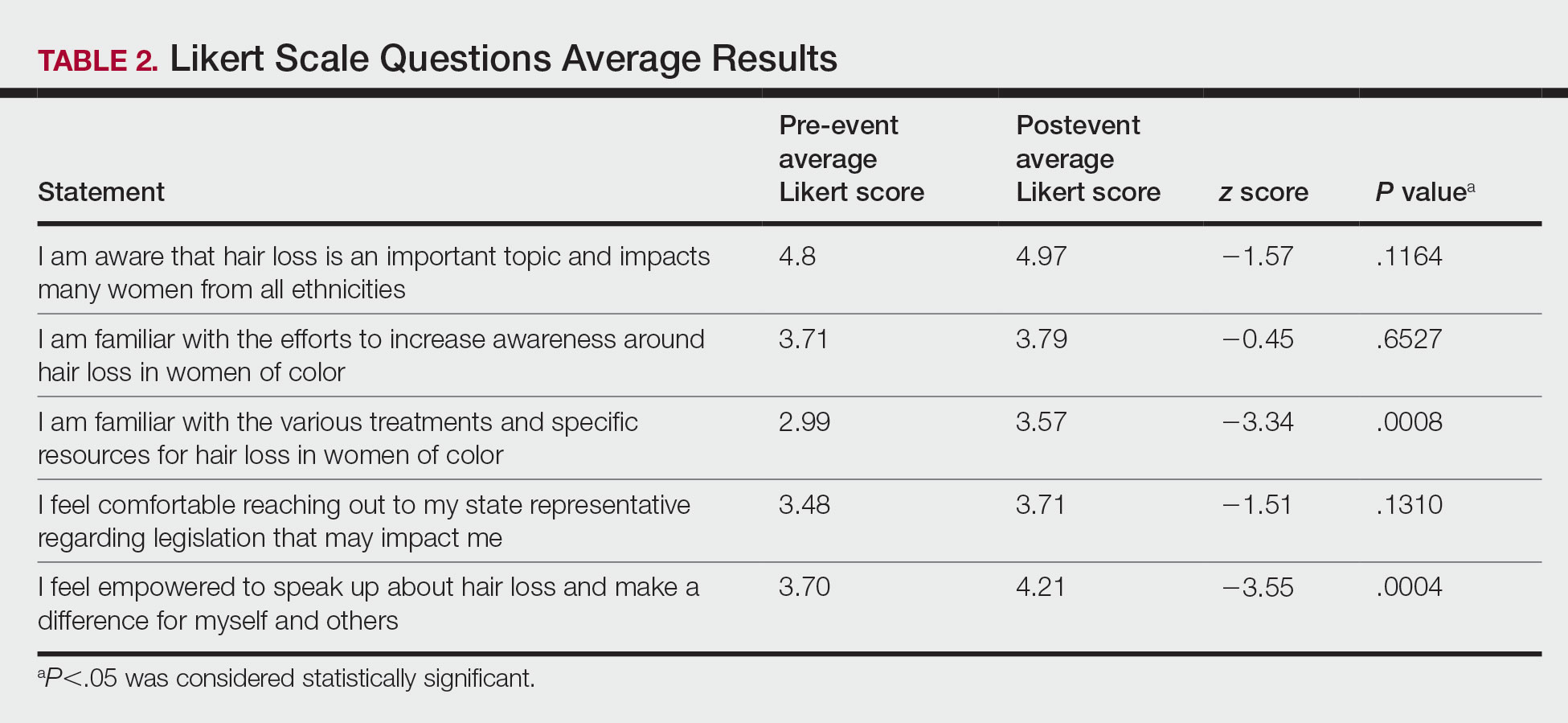
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.
Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Practice Points
- Hair loss is associated with low self-esteem in women with skin of color; therefore, it is important to both acknowledge the social and psychological impacts of hair loss in this population and provide educational resources and community events that address patient concerns.
- There is a deficit of dermatology advocacy efforts that address conditions affecting patients with skin of color. Highlighting this disparity is the first step to catalyzing change.
- Dermatologists are responsible for advocating for women with skin of color and for addressing the social issues that impact their quality of life.
- The Framework for Advocacy and Community Efforts (FACE) model is a template for others to use when planning community engagement and advocacy efforts.
Petrolatum Is Effective as a Moisturizer, But There Are More Uses for It
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Learning Experiences in LGBT Health During Dermatology Residency
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.
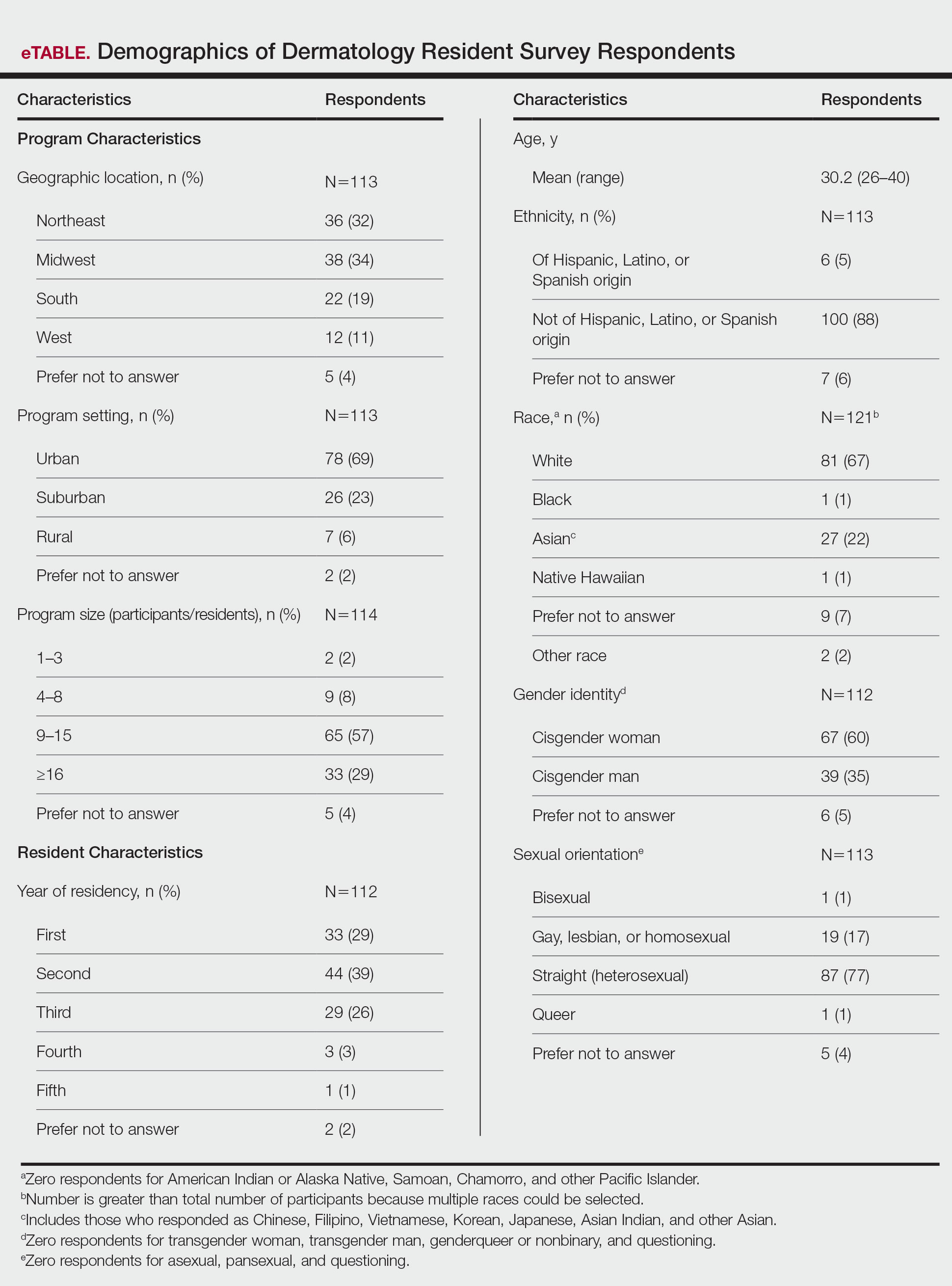
LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.
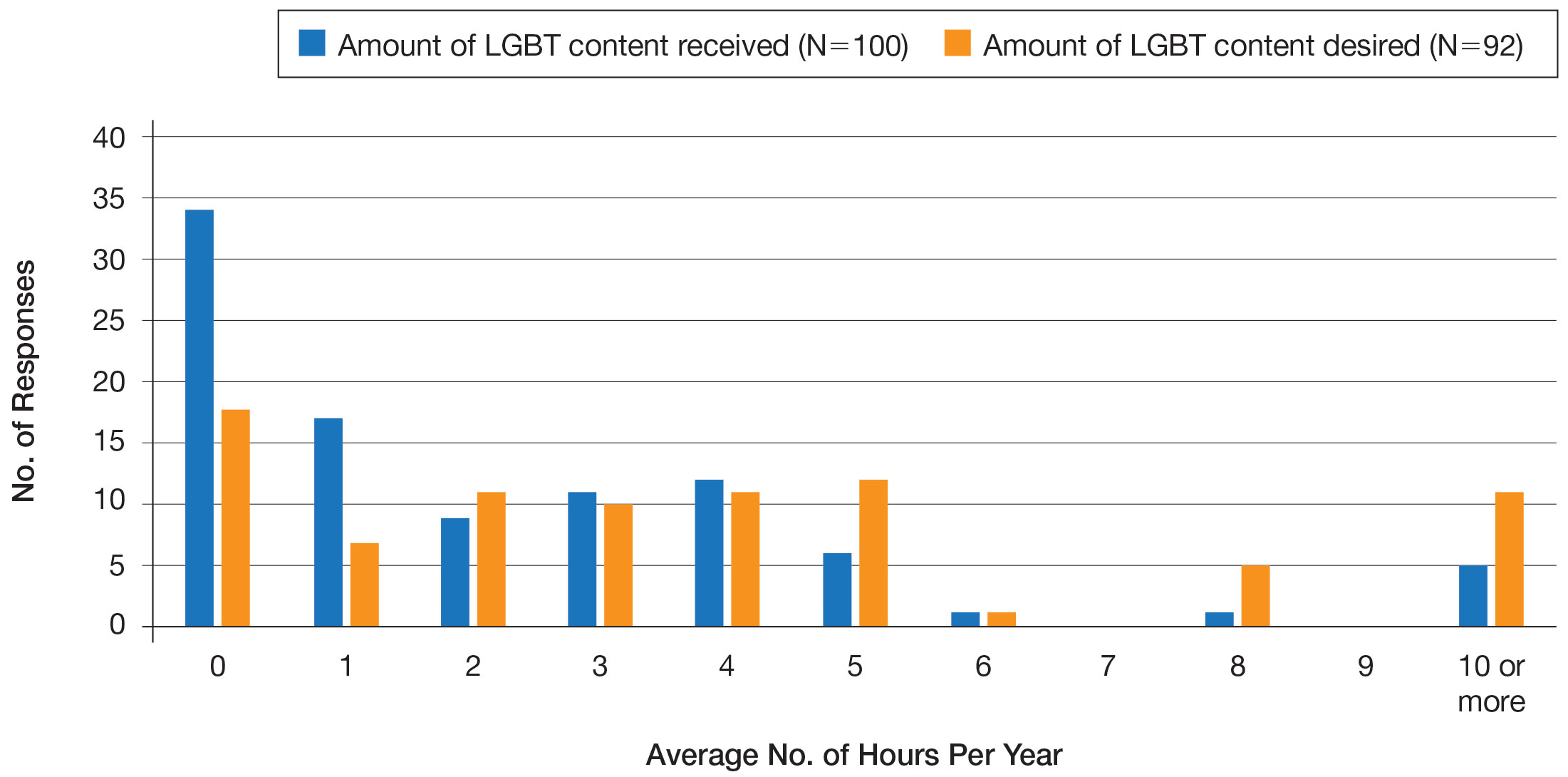
Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.
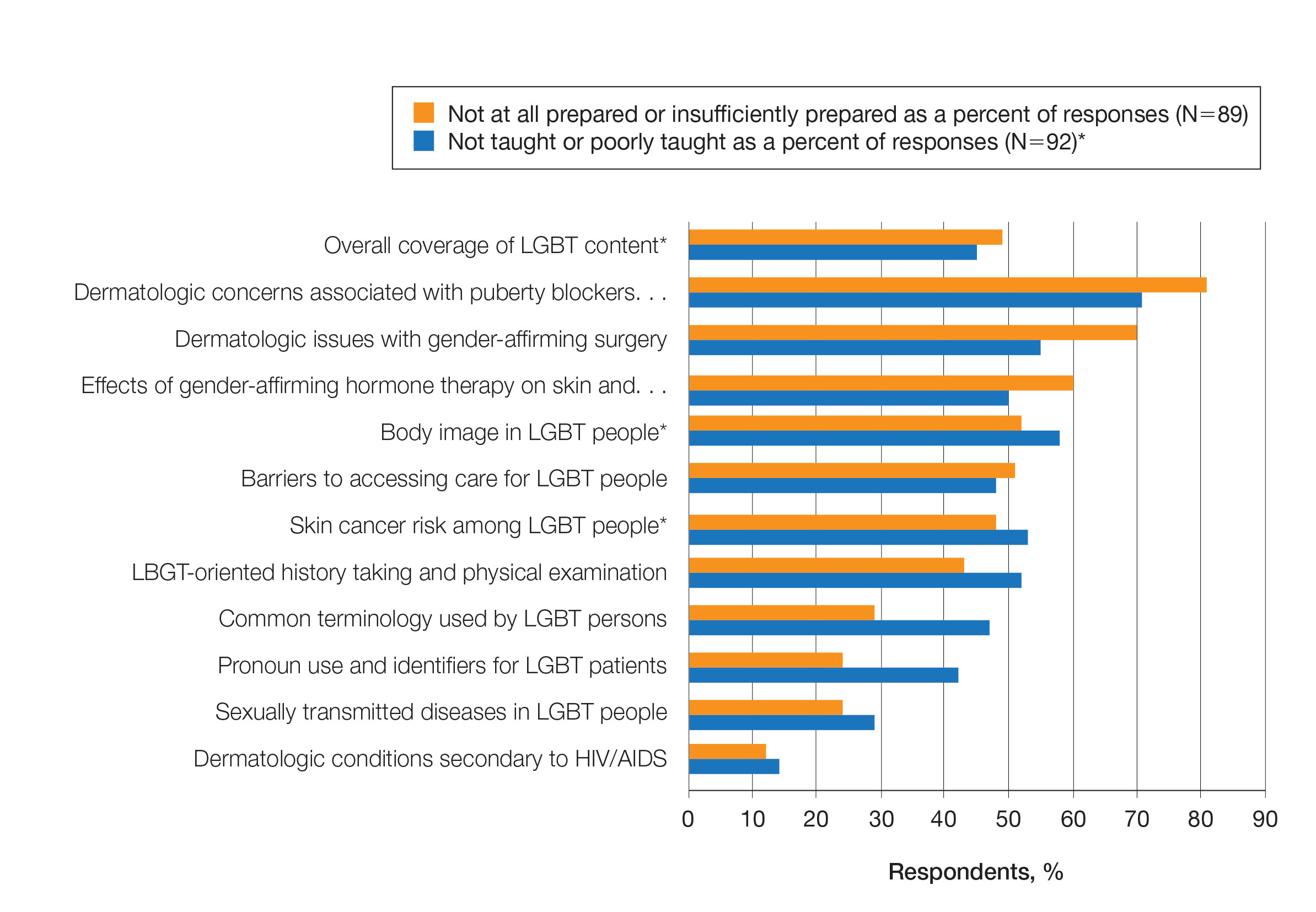
Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.

LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.

Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.

Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.

LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.

Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.

Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
Practice Points
- Dermatologists have the potential to greatly impact lesbian, gay, bisexual, transgender (LGBT) health since many health concerns in this population are cutaneous.
- Improving LGBT health education and training in dermatology residency likely will increase dermatology residents' comfort level in treating this population.
Temper tantrums, bullying colleagues: How to avert physician misbehavior?
Daniel Freedman, DO, a pediatric neurologist in Austin, Tex., remembers being flabbergasted when a surgeon threw an instrument across the room in medical school.
“I remember thinking, ‘I can’t believe people actually do this, a grown man in his 50s having a temper tantrum,’” Dr. Freedman said in an interview. But it certainly wasn’t the last time he witnessed bad behavior by one of his peers.
The results of Medscape’s recent report, Physicians Behaving Badly: Stress and Hardship Trigger Misconduct, suggest he has plenty of company. More than 4 in 10 respondents (41%) observed inappropriate behavior in the workplace in 2022, an uptick from 35% in 2021, according to the report, which polled more than 1,500 physicians about inappropriate behavior on and off the clock.
Of course, 38% of respondents have not seen any instances of misbehavior; and many of the instances that were seen were mild or infrequent. Additionally, instances of bad behavior have declined significantly over the past 5 years.
Dr. Freedman said he learned a lesson from his mentor and program director during training that has stuck with him throughout his career. “If you couldn’t act that way at any job, whether at McDonald’s or any other possible place, you shouldn’t act that way in medicine.” But he recognizes one limitation of that advice. “A lot of the people that behave badly may not have ever worked in a different environment before,” he said.
“They only perceive that they’re at the top of the food chain, so they can behave badly without repercussions.”
What Dr. Freedman described is formally called disruptive physician behavior, one of several categories of inappropriate behavior in medicine, according to Charles Samenow, MD, MPH, an associate professor of psychiatry and behavioral sciences at George Washington University, Washington, who has studied this phenomenon for years.
“Disruptive physician behavior compromises the safety of the workplace,” Dr. Samenow explained. The behavior can occur at work, outside of work, or on social media. It can hinder operations, threaten patient and staff safety, and affect workplace morale.
“The question is trying to understand where that bad behavior is coming from and the impact of that bad behavior,” Dr. Samenow said in an interview.
One reason is fairly simple: doctors are human, and humans have a wide range of behavior. Plus, as the Medscape survey showed,
Self-selecting traits become an Achilles heel
“Any human put in a position of power over other humans has the potential to be disruptive, harass, etc, if they have certain personality traits,” said David Gorski, MD, a professor of surgery at Wayne State University, Detroit. That jibes with Dr. Samenow’s research.
Classic disruptive behavior isn’t usually associated with depression, mania, psychosis, or similar characteristics, Dr. Samenow explained. Rather, it tends to be personality driven. “Physicians are not immune to the normal problems every human being faces,” he said.
In the Medscape report, physicians cited personal arrogance as one of the leading reasons physicians engaged in inappropriate behavior (56%), followed closely by personal problems outside of work (52%), a social shift in accepting more casual behavior (50%), and job-related stress (46%). (Respondents could choose more than one answer).
One factor contributing to misbehavior that Dr. Samenow has consistently identified in his research is a history of adverse childhood experiences or family dysfunction: People who grew up in homes with physical or verbal abuse learned anger as a coping skill instead of positive, assertive communication. It’s likely that some physicians, as well as the overall population, learned anger as a coping skill for that reason.
How to help avert disruptive behavior in medical settings
Dr. Samenow said that coaching is a “wonderful tool” in teaching the interpersonal skills that medical school often doesn’t address.
In some case, interventions can be very helpful. For example, programs that teach effective communication strategies and teamwork through a combination of culturally sensitive dialectical and cognitive-behavioral therapy and other modalities have been successful, Dr. Samenow said. Although they are more about treating an illness than addressing “misbehavior,” programs for substance use that have been developed by and for doctors are very effective, too.
Fewer resources are available, however, for addressing racism, classism, misogyny, and other forms of bigotry, Dr. Samenow noted. “There’s implicit bias training, but not at the level of what exists for disruptive physicians and those with addiction. “That’s an area we need to work on.” Racist language was the third most commonly observed bad behavior cited in the Medscape survey, behind only bullying of staff and mocking or disparaging of patients. It was reported frequently outside of work as well.
The Medscape report found an increase in observed behavior at work and on social media, although it’s hard to determine prevalence trends over time, Dr. Samenow said. “The tolerance for this behavior has really gone down,” likely leading to more reporting, he said, and more systems for reporting bad behavior exist today than in the past.
However, Dr. Freedman said inadequate regulation, disciplinary action, and follow-through remain a problem.
“There are lots of limitations to our reporting system and to our follow-through with those reports,” including hospitals that, whether for fear of litigation or other reasons, allow physicians to quietly resign and move to another institution, even with positive recommendations, Dr. Freedman said.
Indeed, only a third of observed misbehavior in the Medscape report resulted in disciplinary action. Half the respondents believed a verbal warning was a necessary consequence, followed by a conversation from management and being reported to a supervisor or human resources. Though only 10% thought a report to the medical board was warranted, it likely depends on the offense and its frequency.
“I think going from paternalism to more patient-centered care and having patients involved in those conversations is a nice shift that makes doctors more human and relatable, and hopefully makes the public more forgiving, that we’re going to make mistakes and nobody’s perfect,” Freedman said. But he added that physicians should be held accountable when a mistake or two becomes a pattern.
Misinformation is professional misconduct
Sufficient accountability is especially absent, these doctors said, for a subset of professional misconduct: spreading misinformation.
While more “conventional” bad behaviors include fraud, dishonesty, abuse of underlings, and incompetence, bad behavior should also include “selling quackery and antivaccine misinformation, the way some doctors did with various nostrums for COVID-19,” said Dr. Gorski, who frequently blogs about doctors’ spreading misinformation.
Taylor Nichols, MD, an emergency medicine physician based in Sacramento, cites the desire for attention and clout as motivations. “Saying things that are wildly, provably false is professional misconduct,” Nichols said. He distinguished such statements from scientific, academic, or clinical disagreement that is necessary within medicine.
Yet there’s been a “long tradition of looking the other way or letting people with fancy titles get away with saying nonsense just because they’re respected,” Jonathan Howard, MD, an associate professor of psychiatry and neurology at New York University said in an interview.
“We have a duty to be trusted members of the community,” Dr. Howard said. “People listen when we say things, and we have an obligation to try to be accurate and humble and as honest as possible and admit mistakes when we inevitably make them.”
That extends to social media, which Dr. Nichols said has magnified the problem of promoting quackery and misinformation. He thinks medical boards and professional credentialing bodies should pay attention to what’s happening in the public conversation and understand that our professional responsibility extends beyond the walls of the hospital or clinic. Physicians must represent themselves professionally and uphold the standards that the profession expects.
On the one hand, Medscape respondents agreed: 70% said one doctor’s misbehavior taints the whole profession. Yet, at the same time, 58% of respondents believed physicians should be able to “keep their private lives private” in 2022. But that’s not the reality of the profession when the lines between private life and behavior away from work get blurred, Dr. Samenow said.
“The way a physician behaves in public represents you,” he said. “What happens in Vegas doesn’t always stay in Vegas.”
A version of this article first appeared on Medscape.com.
Daniel Freedman, DO, a pediatric neurologist in Austin, Tex., remembers being flabbergasted when a surgeon threw an instrument across the room in medical school.
“I remember thinking, ‘I can’t believe people actually do this, a grown man in his 50s having a temper tantrum,’” Dr. Freedman said in an interview. But it certainly wasn’t the last time he witnessed bad behavior by one of his peers.
The results of Medscape’s recent report, Physicians Behaving Badly: Stress and Hardship Trigger Misconduct, suggest he has plenty of company. More than 4 in 10 respondents (41%) observed inappropriate behavior in the workplace in 2022, an uptick from 35% in 2021, according to the report, which polled more than 1,500 physicians about inappropriate behavior on and off the clock.
Of course, 38% of respondents have not seen any instances of misbehavior; and many of the instances that were seen were mild or infrequent. Additionally, instances of bad behavior have declined significantly over the past 5 years.
Dr. Freedman said he learned a lesson from his mentor and program director during training that has stuck with him throughout his career. “If you couldn’t act that way at any job, whether at McDonald’s or any other possible place, you shouldn’t act that way in medicine.” But he recognizes one limitation of that advice. “A lot of the people that behave badly may not have ever worked in a different environment before,” he said.
“They only perceive that they’re at the top of the food chain, so they can behave badly without repercussions.”
What Dr. Freedman described is formally called disruptive physician behavior, one of several categories of inappropriate behavior in medicine, according to Charles Samenow, MD, MPH, an associate professor of psychiatry and behavioral sciences at George Washington University, Washington, who has studied this phenomenon for years.
“Disruptive physician behavior compromises the safety of the workplace,” Dr. Samenow explained. The behavior can occur at work, outside of work, or on social media. It can hinder operations, threaten patient and staff safety, and affect workplace morale.
“The question is trying to understand where that bad behavior is coming from and the impact of that bad behavior,” Dr. Samenow said in an interview.
One reason is fairly simple: doctors are human, and humans have a wide range of behavior. Plus, as the Medscape survey showed,
Self-selecting traits become an Achilles heel
“Any human put in a position of power over other humans has the potential to be disruptive, harass, etc, if they have certain personality traits,” said David Gorski, MD, a professor of surgery at Wayne State University, Detroit. That jibes with Dr. Samenow’s research.
Classic disruptive behavior isn’t usually associated with depression, mania, psychosis, or similar characteristics, Dr. Samenow explained. Rather, it tends to be personality driven. “Physicians are not immune to the normal problems every human being faces,” he said.
In the Medscape report, physicians cited personal arrogance as one of the leading reasons physicians engaged in inappropriate behavior (56%), followed closely by personal problems outside of work (52%), a social shift in accepting more casual behavior (50%), and job-related stress (46%). (Respondents could choose more than one answer).
One factor contributing to misbehavior that Dr. Samenow has consistently identified in his research is a history of adverse childhood experiences or family dysfunction: People who grew up in homes with physical or verbal abuse learned anger as a coping skill instead of positive, assertive communication. It’s likely that some physicians, as well as the overall population, learned anger as a coping skill for that reason.
How to help avert disruptive behavior in medical settings
Dr. Samenow said that coaching is a “wonderful tool” in teaching the interpersonal skills that medical school often doesn’t address.
In some case, interventions can be very helpful. For example, programs that teach effective communication strategies and teamwork through a combination of culturally sensitive dialectical and cognitive-behavioral therapy and other modalities have been successful, Dr. Samenow said. Although they are more about treating an illness than addressing “misbehavior,” programs for substance use that have been developed by and for doctors are very effective, too.
Fewer resources are available, however, for addressing racism, classism, misogyny, and other forms of bigotry, Dr. Samenow noted. “There’s implicit bias training, but not at the level of what exists for disruptive physicians and those with addiction. “That’s an area we need to work on.” Racist language was the third most commonly observed bad behavior cited in the Medscape survey, behind only bullying of staff and mocking or disparaging of patients. It was reported frequently outside of work as well.
The Medscape report found an increase in observed behavior at work and on social media, although it’s hard to determine prevalence trends over time, Dr. Samenow said. “The tolerance for this behavior has really gone down,” likely leading to more reporting, he said, and more systems for reporting bad behavior exist today than in the past.
However, Dr. Freedman said inadequate regulation, disciplinary action, and follow-through remain a problem.
“There are lots of limitations to our reporting system and to our follow-through with those reports,” including hospitals that, whether for fear of litigation or other reasons, allow physicians to quietly resign and move to another institution, even with positive recommendations, Dr. Freedman said.
Indeed, only a third of observed misbehavior in the Medscape report resulted in disciplinary action. Half the respondents believed a verbal warning was a necessary consequence, followed by a conversation from management and being reported to a supervisor or human resources. Though only 10% thought a report to the medical board was warranted, it likely depends on the offense and its frequency.
“I think going from paternalism to more patient-centered care and having patients involved in those conversations is a nice shift that makes doctors more human and relatable, and hopefully makes the public more forgiving, that we’re going to make mistakes and nobody’s perfect,” Freedman said. But he added that physicians should be held accountable when a mistake or two becomes a pattern.
Misinformation is professional misconduct
Sufficient accountability is especially absent, these doctors said, for a subset of professional misconduct: spreading misinformation.
While more “conventional” bad behaviors include fraud, dishonesty, abuse of underlings, and incompetence, bad behavior should also include “selling quackery and antivaccine misinformation, the way some doctors did with various nostrums for COVID-19,” said Dr. Gorski, who frequently blogs about doctors’ spreading misinformation.
Taylor Nichols, MD, an emergency medicine physician based in Sacramento, cites the desire for attention and clout as motivations. “Saying things that are wildly, provably false is professional misconduct,” Nichols said. He distinguished such statements from scientific, academic, or clinical disagreement that is necessary within medicine.
Yet there’s been a “long tradition of looking the other way or letting people with fancy titles get away with saying nonsense just because they’re respected,” Jonathan Howard, MD, an associate professor of psychiatry and neurology at New York University said in an interview.
“We have a duty to be trusted members of the community,” Dr. Howard said. “People listen when we say things, and we have an obligation to try to be accurate and humble and as honest as possible and admit mistakes when we inevitably make them.”
That extends to social media, which Dr. Nichols said has magnified the problem of promoting quackery and misinformation. He thinks medical boards and professional credentialing bodies should pay attention to what’s happening in the public conversation and understand that our professional responsibility extends beyond the walls of the hospital or clinic. Physicians must represent themselves professionally and uphold the standards that the profession expects.
On the one hand, Medscape respondents agreed: 70% said one doctor’s misbehavior taints the whole profession. Yet, at the same time, 58% of respondents believed physicians should be able to “keep their private lives private” in 2022. But that’s not the reality of the profession when the lines between private life and behavior away from work get blurred, Dr. Samenow said.
“The way a physician behaves in public represents you,” he said. “What happens in Vegas doesn’t always stay in Vegas.”
A version of this article first appeared on Medscape.com.
Daniel Freedman, DO, a pediatric neurologist in Austin, Tex., remembers being flabbergasted when a surgeon threw an instrument across the room in medical school.
“I remember thinking, ‘I can’t believe people actually do this, a grown man in his 50s having a temper tantrum,’” Dr. Freedman said in an interview. But it certainly wasn’t the last time he witnessed bad behavior by one of his peers.
The results of Medscape’s recent report, Physicians Behaving Badly: Stress and Hardship Trigger Misconduct, suggest he has plenty of company. More than 4 in 10 respondents (41%) observed inappropriate behavior in the workplace in 2022, an uptick from 35% in 2021, according to the report, which polled more than 1,500 physicians about inappropriate behavior on and off the clock.
Of course, 38% of respondents have not seen any instances of misbehavior; and many of the instances that were seen were mild or infrequent. Additionally, instances of bad behavior have declined significantly over the past 5 years.
Dr. Freedman said he learned a lesson from his mentor and program director during training that has stuck with him throughout his career. “If you couldn’t act that way at any job, whether at McDonald’s or any other possible place, you shouldn’t act that way in medicine.” But he recognizes one limitation of that advice. “A lot of the people that behave badly may not have ever worked in a different environment before,” he said.
“They only perceive that they’re at the top of the food chain, so they can behave badly without repercussions.”
What Dr. Freedman described is formally called disruptive physician behavior, one of several categories of inappropriate behavior in medicine, according to Charles Samenow, MD, MPH, an associate professor of psychiatry and behavioral sciences at George Washington University, Washington, who has studied this phenomenon for years.
“Disruptive physician behavior compromises the safety of the workplace,” Dr. Samenow explained. The behavior can occur at work, outside of work, or on social media. It can hinder operations, threaten patient and staff safety, and affect workplace morale.
“The question is trying to understand where that bad behavior is coming from and the impact of that bad behavior,” Dr. Samenow said in an interview.
One reason is fairly simple: doctors are human, and humans have a wide range of behavior. Plus, as the Medscape survey showed,
Self-selecting traits become an Achilles heel
“Any human put in a position of power over other humans has the potential to be disruptive, harass, etc, if they have certain personality traits,” said David Gorski, MD, a professor of surgery at Wayne State University, Detroit. That jibes with Dr. Samenow’s research.
Classic disruptive behavior isn’t usually associated with depression, mania, psychosis, or similar characteristics, Dr. Samenow explained. Rather, it tends to be personality driven. “Physicians are not immune to the normal problems every human being faces,” he said.
In the Medscape report, physicians cited personal arrogance as one of the leading reasons physicians engaged in inappropriate behavior (56%), followed closely by personal problems outside of work (52%), a social shift in accepting more casual behavior (50%), and job-related stress (46%). (Respondents could choose more than one answer).
One factor contributing to misbehavior that Dr. Samenow has consistently identified in his research is a history of adverse childhood experiences or family dysfunction: People who grew up in homes with physical or verbal abuse learned anger as a coping skill instead of positive, assertive communication. It’s likely that some physicians, as well as the overall population, learned anger as a coping skill for that reason.
How to help avert disruptive behavior in medical settings
Dr. Samenow said that coaching is a “wonderful tool” in teaching the interpersonal skills that medical school often doesn’t address.
In some case, interventions can be very helpful. For example, programs that teach effective communication strategies and teamwork through a combination of culturally sensitive dialectical and cognitive-behavioral therapy and other modalities have been successful, Dr. Samenow said. Although they are more about treating an illness than addressing “misbehavior,” programs for substance use that have been developed by and for doctors are very effective, too.
Fewer resources are available, however, for addressing racism, classism, misogyny, and other forms of bigotry, Dr. Samenow noted. “There’s implicit bias training, but not at the level of what exists for disruptive physicians and those with addiction. “That’s an area we need to work on.” Racist language was the third most commonly observed bad behavior cited in the Medscape survey, behind only bullying of staff and mocking or disparaging of patients. It was reported frequently outside of work as well.
The Medscape report found an increase in observed behavior at work and on social media, although it’s hard to determine prevalence trends over time, Dr. Samenow said. “The tolerance for this behavior has really gone down,” likely leading to more reporting, he said, and more systems for reporting bad behavior exist today than in the past.
However, Dr. Freedman said inadequate regulation, disciplinary action, and follow-through remain a problem.
“There are lots of limitations to our reporting system and to our follow-through with those reports,” including hospitals that, whether for fear of litigation or other reasons, allow physicians to quietly resign and move to another institution, even with positive recommendations, Dr. Freedman said.
Indeed, only a third of observed misbehavior in the Medscape report resulted in disciplinary action. Half the respondents believed a verbal warning was a necessary consequence, followed by a conversation from management and being reported to a supervisor or human resources. Though only 10% thought a report to the medical board was warranted, it likely depends on the offense and its frequency.
“I think going from paternalism to more patient-centered care and having patients involved in those conversations is a nice shift that makes doctors more human and relatable, and hopefully makes the public more forgiving, that we’re going to make mistakes and nobody’s perfect,” Freedman said. But he added that physicians should be held accountable when a mistake or two becomes a pattern.
Misinformation is professional misconduct
Sufficient accountability is especially absent, these doctors said, for a subset of professional misconduct: spreading misinformation.
While more “conventional” bad behaviors include fraud, dishonesty, abuse of underlings, and incompetence, bad behavior should also include “selling quackery and antivaccine misinformation, the way some doctors did with various nostrums for COVID-19,” said Dr. Gorski, who frequently blogs about doctors’ spreading misinformation.
Taylor Nichols, MD, an emergency medicine physician based in Sacramento, cites the desire for attention and clout as motivations. “Saying things that are wildly, provably false is professional misconduct,” Nichols said. He distinguished such statements from scientific, academic, or clinical disagreement that is necessary within medicine.
Yet there’s been a “long tradition of looking the other way or letting people with fancy titles get away with saying nonsense just because they’re respected,” Jonathan Howard, MD, an associate professor of psychiatry and neurology at New York University said in an interview.
“We have a duty to be trusted members of the community,” Dr. Howard said. “People listen when we say things, and we have an obligation to try to be accurate and humble and as honest as possible and admit mistakes when we inevitably make them.”
That extends to social media, which Dr. Nichols said has magnified the problem of promoting quackery and misinformation. He thinks medical boards and professional credentialing bodies should pay attention to what’s happening in the public conversation and understand that our professional responsibility extends beyond the walls of the hospital or clinic. Physicians must represent themselves professionally and uphold the standards that the profession expects.
On the one hand, Medscape respondents agreed: 70% said one doctor’s misbehavior taints the whole profession. Yet, at the same time, 58% of respondents believed physicians should be able to “keep their private lives private” in 2022. But that’s not the reality of the profession when the lines between private life and behavior away from work get blurred, Dr. Samenow said.
“The way a physician behaves in public represents you,” he said. “What happens in Vegas doesn’t always stay in Vegas.”
A version of this article first appeared on Medscape.com.
52-week data show lebrikizumab atopic dermatitis effects maintained
from the phase 3 ADvocate1 and ADvocate2 trials.
“We’re focused on the responders,” said Andrew Blauvelt, MD, MBA, as he presented the positive findings at the annual congress of the European Academy of Dermatology and Venereology.
Responders were the 291 people whose atopic dermatitis greatly improved after an initial 16 weeks’ treatment with lebrikizumab in both trials and who were then randomly allocated to receive injections every 2 weeks (Q2W, n = 113) or every 4 weeks (Q4W, n = 118), or to receive placebo injections Q2W (n = 60).
“Very interestingly, for me, the Q4W maintenance dosing was just as good as the Q2W maintenance dosing,” said Dr. Blauvelt, president of Oregon Medical Research Center, Portland.
“Another highlight of these data is that the patients who went on to placebo, about 50% of the patients maintained good responses, despite no treatment from week 16 to week 52,” he added.
Most patients did not require topical steroids, and “there were no surprises here” in terms of the safety profile. Lebrikizumab, a monoclonal antibody, binds to soluble interleukin-13 and blocks IL-13 signaling.
“So, the study really shows that specific targeting of IL-13 with lebrikizumab, either Q2W or Q4W, has high maintenance of efficacy and is reasonably tolerated and safe in adolescents and adults with atopic dermatitis,” Dr. Blauvelt concluded.
“We know now that IL-13 is a critical cytokine in AD [atopic dermatitis] pathogenesis. The unique features of this drug I want to highlight is that it has high binding affinity for IL-13,” he said.
“It has a slow dissociation off rate, meaning it binds IL-13 tightly, very potently, and stays blocking and stays hold of IL-13 in a strong manner,” he added. The drug has a half-life of 25 days.
These features could be very important for long-term dosing of the drug, he argued.
Lebrikizumab phase 3 trials
ADvocate1 and ADvocate2 are two of several phase 3 trials evaluating the efficacy and safety of lebrikizumab for the treatment of atopic dermatitis.
These include the completed ADhere study, in which lebrikizumab was used in combination with topical steroids and showed positive results in skin improvement and relief of pruritus.
The ADore study, an open-label trial in adolescents, is yet to report. The ongoing ADjoin study, a long-term extension study, is actively recruiting.
ADvocate1 and ADvocate2 are two identically designed – multicenter, randomized, double-blind, placebo-controlled, parallel-group – monotherapy trials that initially pitched two dosing regimens of lebrikizumab (250 mg) against placebo with a double loading dose at baseline and week 2 and then one dose every 2 weeks. The pair of trials enrolled a total of 869 adolescents and adults.
After the 16-week induction period, all patients in the lebrikizumab arm who had responded to treatment were rerandomly assigned to receive lebrikizumab 250 mg Q2W or Q4W, or placebo Q2W during a 36-week long-term maintenance treatment period.
This brought the total treatment time to 52 weeks for those whose atopic dermatitis had initially responded to lebrikizumab, explained Blauvelt.
Responders were those who, at 16 weeks, had an Investigator’s Global Assessment score of 0 or 1 (IGA 0/1) with a 2-point improvement or who had a 75% improvement in the Eczema Area and Severity Index score (EASI 75) without the need for rescue medication, compared with baseline values.
Induction and maintenance phase results
At the end of the 16-week induction period, a greater proportion of patients who had been treated with lebrikizumab than placebo met a primary outcome of IGA 0/1 in each trial (43.1% vs. 12.7% in ADvocate1 and 33.2% vs. 10.8% in ADvocate2).
A similar result was seen for another primary outcome, EASI 75 (58.8% vs. 16.2% and 52.1% vs. 18.1%) and for a secondary outcome, improvement in pruritus using a numerical rating scale (45.9% vs. 13.0% and 39.8% vs. 11.5%).
In the maintenance phase, with respect to responders, Dr. Blauvelt reported “very similar results” between the QW2 and Q4W maintenance dosing, “and still a quite high response in [half] the patients who were randomized to placebo at week 16.”
In the ADvocate1 and ADvocate2 trials, respectively, an IGA 0/1 with at least a 2-point improvement was maintained at week 52 in 75.8% and 64.6% of patients treated with the Q2W lebrikizumab dose, 74.2% and 80.6% of those treated with the Q4W dose, and 46.5% and 49.8% of those given placebo.
EASI 75 was maintained at week 52 in a respective 79.2% and 77.4% of patients treated with the Q2W dose, 79.2% and 84.7% with the Q4W dose, and 61.3% and 72.0% with placebo.
As for maintenance of at least a 4-point improvement in pruritus score, results at 52 weeks were 81.2% and 90.3% for the 2-week dose, 80.4% and 88.1% for the 4-week dose, and 65.4% and 67.6% for placebo.
Although topical corticosteroid treatment was allowed during the maintenance phase, only about 15% of patients needed this, Dr. Blauvelt said.
Different dosing results questioned
During the discussion period, one delegate highlighted that the twice-weekly maintenance dosing schedule seemed to “do worse a little bit” than the 4-week dosing, with both “close to placebo,” although “the long-term effect is already very impressive.”
Dr. Blauvelt noted that a pooled analysis had been done and that “it’s very clear that being on lebrikizumab works better than not being on lebrikizumab.
“Now, Q2W versus Q4W. We believe that this may be due to the long half-life of the drug possibly. It could be due to the slow disassociation rate, it’s binding tightly,” he suggested.
“We also could talk about disease modification, right. So, it opens up the concept of hit hard, hit early for 16 weeks, and then maybe you can modify disease over time,” Dr. Blauvelt said.
He added: “That’s highly speculative, of course.”
Short-term safety data
The 52-week safety profile of lebrikizumab is consistent with previously published data at 16 weeks, Dr. Blauvelt said. The most common adverse events during the studies included atopic dermatitis, nasopharyngitis, conjunctivitis, conjunctivitis allergic, headache, and COVID-19.
“This drug has comparable efficacy with dupilumab and tralokinumab,” said Jashin J. Wu, MD, from the Dermatology Research and Education Foundation in Irvine, Calif., in an interview. He was not involved in the study.
“As it does not have any significant advantages with less long-term safety data, I do not see a place for it in my practice,” Dr. Wu said.
Dupilumab (Dupixent) and tralokinumab (Adbry) are monoclonal antibodies that also block IL-13. Both are already licensed for treating atopic dermatitis. Dupilumab was approved by the Food and Drug Administration in 2017, and tralokinumab was approved in 2021.
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. Eli Lilly has exclusive rights for the development and commercialization of lebrikizumab in the United States and all countries outside Europe; European rights belong to Almirall for all dermatology indications, including atopic dermatitis. Dr. Blauvelt acts as an investigator and adviser to these companies as well as many other pharmaceutical companies that are involved in developing new dermatologic treatments. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
from the phase 3 ADvocate1 and ADvocate2 trials.
“We’re focused on the responders,” said Andrew Blauvelt, MD, MBA, as he presented the positive findings at the annual congress of the European Academy of Dermatology and Venereology.
Responders were the 291 people whose atopic dermatitis greatly improved after an initial 16 weeks’ treatment with lebrikizumab in both trials and who were then randomly allocated to receive injections every 2 weeks (Q2W, n = 113) or every 4 weeks (Q4W, n = 118), or to receive placebo injections Q2W (n = 60).
“Very interestingly, for me, the Q4W maintenance dosing was just as good as the Q2W maintenance dosing,” said Dr. Blauvelt, president of Oregon Medical Research Center, Portland.
“Another highlight of these data is that the patients who went on to placebo, about 50% of the patients maintained good responses, despite no treatment from week 16 to week 52,” he added.
Most patients did not require topical steroids, and “there were no surprises here” in terms of the safety profile. Lebrikizumab, a monoclonal antibody, binds to soluble interleukin-13 and blocks IL-13 signaling.
“So, the study really shows that specific targeting of IL-13 with lebrikizumab, either Q2W or Q4W, has high maintenance of efficacy and is reasonably tolerated and safe in adolescents and adults with atopic dermatitis,” Dr. Blauvelt concluded.
“We know now that IL-13 is a critical cytokine in AD [atopic dermatitis] pathogenesis. The unique features of this drug I want to highlight is that it has high binding affinity for IL-13,” he said.
“It has a slow dissociation off rate, meaning it binds IL-13 tightly, very potently, and stays blocking and stays hold of IL-13 in a strong manner,” he added. The drug has a half-life of 25 days.
These features could be very important for long-term dosing of the drug, he argued.
Lebrikizumab phase 3 trials
ADvocate1 and ADvocate2 are two of several phase 3 trials evaluating the efficacy and safety of lebrikizumab for the treatment of atopic dermatitis.
These include the completed ADhere study, in which lebrikizumab was used in combination with topical steroids and showed positive results in skin improvement and relief of pruritus.
The ADore study, an open-label trial in adolescents, is yet to report. The ongoing ADjoin study, a long-term extension study, is actively recruiting.
ADvocate1 and ADvocate2 are two identically designed – multicenter, randomized, double-blind, placebo-controlled, parallel-group – monotherapy trials that initially pitched two dosing regimens of lebrikizumab (250 mg) against placebo with a double loading dose at baseline and week 2 and then one dose every 2 weeks. The pair of trials enrolled a total of 869 adolescents and adults.
After the 16-week induction period, all patients in the lebrikizumab arm who had responded to treatment were rerandomly assigned to receive lebrikizumab 250 mg Q2W or Q4W, or placebo Q2W during a 36-week long-term maintenance treatment period.
This brought the total treatment time to 52 weeks for those whose atopic dermatitis had initially responded to lebrikizumab, explained Blauvelt.
Responders were those who, at 16 weeks, had an Investigator’s Global Assessment score of 0 or 1 (IGA 0/1) with a 2-point improvement or who had a 75% improvement in the Eczema Area and Severity Index score (EASI 75) without the need for rescue medication, compared with baseline values.
Induction and maintenance phase results
At the end of the 16-week induction period, a greater proportion of patients who had been treated with lebrikizumab than placebo met a primary outcome of IGA 0/1 in each trial (43.1% vs. 12.7% in ADvocate1 and 33.2% vs. 10.8% in ADvocate2).
A similar result was seen for another primary outcome, EASI 75 (58.8% vs. 16.2% and 52.1% vs. 18.1%) and for a secondary outcome, improvement in pruritus using a numerical rating scale (45.9% vs. 13.0% and 39.8% vs. 11.5%).
In the maintenance phase, with respect to responders, Dr. Blauvelt reported “very similar results” between the QW2 and Q4W maintenance dosing, “and still a quite high response in [half] the patients who were randomized to placebo at week 16.”
In the ADvocate1 and ADvocate2 trials, respectively, an IGA 0/1 with at least a 2-point improvement was maintained at week 52 in 75.8% and 64.6% of patients treated with the Q2W lebrikizumab dose, 74.2% and 80.6% of those treated with the Q4W dose, and 46.5% and 49.8% of those given placebo.
EASI 75 was maintained at week 52 in a respective 79.2% and 77.4% of patients treated with the Q2W dose, 79.2% and 84.7% with the Q4W dose, and 61.3% and 72.0% with placebo.
As for maintenance of at least a 4-point improvement in pruritus score, results at 52 weeks were 81.2% and 90.3% for the 2-week dose, 80.4% and 88.1% for the 4-week dose, and 65.4% and 67.6% for placebo.
Although topical corticosteroid treatment was allowed during the maintenance phase, only about 15% of patients needed this, Dr. Blauvelt said.
Different dosing results questioned
During the discussion period, one delegate highlighted that the twice-weekly maintenance dosing schedule seemed to “do worse a little bit” than the 4-week dosing, with both “close to placebo,” although “the long-term effect is already very impressive.”
Dr. Blauvelt noted that a pooled analysis had been done and that “it’s very clear that being on lebrikizumab works better than not being on lebrikizumab.
“Now, Q2W versus Q4W. We believe that this may be due to the long half-life of the drug possibly. It could be due to the slow disassociation rate, it’s binding tightly,” he suggested.
“We also could talk about disease modification, right. So, it opens up the concept of hit hard, hit early for 16 weeks, and then maybe you can modify disease over time,” Dr. Blauvelt said.
He added: “That’s highly speculative, of course.”
Short-term safety data
The 52-week safety profile of lebrikizumab is consistent with previously published data at 16 weeks, Dr. Blauvelt said. The most common adverse events during the studies included atopic dermatitis, nasopharyngitis, conjunctivitis, conjunctivitis allergic, headache, and COVID-19.
“This drug has comparable efficacy with dupilumab and tralokinumab,” said Jashin J. Wu, MD, from the Dermatology Research and Education Foundation in Irvine, Calif., in an interview. He was not involved in the study.
“As it does not have any significant advantages with less long-term safety data, I do not see a place for it in my practice,” Dr. Wu said.
Dupilumab (Dupixent) and tralokinumab (Adbry) are monoclonal antibodies that also block IL-13. Both are already licensed for treating atopic dermatitis. Dupilumab was approved by the Food and Drug Administration in 2017, and tralokinumab was approved in 2021.
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. Eli Lilly has exclusive rights for the development and commercialization of lebrikizumab in the United States and all countries outside Europe; European rights belong to Almirall for all dermatology indications, including atopic dermatitis. Dr. Blauvelt acts as an investigator and adviser to these companies as well as many other pharmaceutical companies that are involved in developing new dermatologic treatments. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
from the phase 3 ADvocate1 and ADvocate2 trials.
“We’re focused on the responders,” said Andrew Blauvelt, MD, MBA, as he presented the positive findings at the annual congress of the European Academy of Dermatology and Venereology.
Responders were the 291 people whose atopic dermatitis greatly improved after an initial 16 weeks’ treatment with lebrikizumab in both trials and who were then randomly allocated to receive injections every 2 weeks (Q2W, n = 113) or every 4 weeks (Q4W, n = 118), or to receive placebo injections Q2W (n = 60).
“Very interestingly, for me, the Q4W maintenance dosing was just as good as the Q2W maintenance dosing,” said Dr. Blauvelt, president of Oregon Medical Research Center, Portland.
“Another highlight of these data is that the patients who went on to placebo, about 50% of the patients maintained good responses, despite no treatment from week 16 to week 52,” he added.
Most patients did not require topical steroids, and “there were no surprises here” in terms of the safety profile. Lebrikizumab, a monoclonal antibody, binds to soluble interleukin-13 and blocks IL-13 signaling.
“So, the study really shows that specific targeting of IL-13 with lebrikizumab, either Q2W or Q4W, has high maintenance of efficacy and is reasonably tolerated and safe in adolescents and adults with atopic dermatitis,” Dr. Blauvelt concluded.
“We know now that IL-13 is a critical cytokine in AD [atopic dermatitis] pathogenesis. The unique features of this drug I want to highlight is that it has high binding affinity for IL-13,” he said.
“It has a slow dissociation off rate, meaning it binds IL-13 tightly, very potently, and stays blocking and stays hold of IL-13 in a strong manner,” he added. The drug has a half-life of 25 days.
These features could be very important for long-term dosing of the drug, he argued.
Lebrikizumab phase 3 trials
ADvocate1 and ADvocate2 are two of several phase 3 trials evaluating the efficacy and safety of lebrikizumab for the treatment of atopic dermatitis.
These include the completed ADhere study, in which lebrikizumab was used in combination with topical steroids and showed positive results in skin improvement and relief of pruritus.
The ADore study, an open-label trial in adolescents, is yet to report. The ongoing ADjoin study, a long-term extension study, is actively recruiting.
ADvocate1 and ADvocate2 are two identically designed – multicenter, randomized, double-blind, placebo-controlled, parallel-group – monotherapy trials that initially pitched two dosing regimens of lebrikizumab (250 mg) against placebo with a double loading dose at baseline and week 2 and then one dose every 2 weeks. The pair of trials enrolled a total of 869 adolescents and adults.
After the 16-week induction period, all patients in the lebrikizumab arm who had responded to treatment were rerandomly assigned to receive lebrikizumab 250 mg Q2W or Q4W, or placebo Q2W during a 36-week long-term maintenance treatment period.
This brought the total treatment time to 52 weeks for those whose atopic dermatitis had initially responded to lebrikizumab, explained Blauvelt.
Responders were those who, at 16 weeks, had an Investigator’s Global Assessment score of 0 or 1 (IGA 0/1) with a 2-point improvement or who had a 75% improvement in the Eczema Area and Severity Index score (EASI 75) without the need for rescue medication, compared with baseline values.
Induction and maintenance phase results
At the end of the 16-week induction period, a greater proportion of patients who had been treated with lebrikizumab than placebo met a primary outcome of IGA 0/1 in each trial (43.1% vs. 12.7% in ADvocate1 and 33.2% vs. 10.8% in ADvocate2).
A similar result was seen for another primary outcome, EASI 75 (58.8% vs. 16.2% and 52.1% vs. 18.1%) and for a secondary outcome, improvement in pruritus using a numerical rating scale (45.9% vs. 13.0% and 39.8% vs. 11.5%).
In the maintenance phase, with respect to responders, Dr. Blauvelt reported “very similar results” between the QW2 and Q4W maintenance dosing, “and still a quite high response in [half] the patients who were randomized to placebo at week 16.”
In the ADvocate1 and ADvocate2 trials, respectively, an IGA 0/1 with at least a 2-point improvement was maintained at week 52 in 75.8% and 64.6% of patients treated with the Q2W lebrikizumab dose, 74.2% and 80.6% of those treated with the Q4W dose, and 46.5% and 49.8% of those given placebo.
EASI 75 was maintained at week 52 in a respective 79.2% and 77.4% of patients treated with the Q2W dose, 79.2% and 84.7% with the Q4W dose, and 61.3% and 72.0% with placebo.
As for maintenance of at least a 4-point improvement in pruritus score, results at 52 weeks were 81.2% and 90.3% for the 2-week dose, 80.4% and 88.1% for the 4-week dose, and 65.4% and 67.6% for placebo.
Although topical corticosteroid treatment was allowed during the maintenance phase, only about 15% of patients needed this, Dr. Blauvelt said.
Different dosing results questioned
During the discussion period, one delegate highlighted that the twice-weekly maintenance dosing schedule seemed to “do worse a little bit” than the 4-week dosing, with both “close to placebo,” although “the long-term effect is already very impressive.”
Dr. Blauvelt noted that a pooled analysis had been done and that “it’s very clear that being on lebrikizumab works better than not being on lebrikizumab.
“Now, Q2W versus Q4W. We believe that this may be due to the long half-life of the drug possibly. It could be due to the slow disassociation rate, it’s binding tightly,” he suggested.
“We also could talk about disease modification, right. So, it opens up the concept of hit hard, hit early for 16 weeks, and then maybe you can modify disease over time,” Dr. Blauvelt said.
He added: “That’s highly speculative, of course.”
Short-term safety data
The 52-week safety profile of lebrikizumab is consistent with previously published data at 16 weeks, Dr. Blauvelt said. The most common adverse events during the studies included atopic dermatitis, nasopharyngitis, conjunctivitis, conjunctivitis allergic, headache, and COVID-19.
“This drug has comparable efficacy with dupilumab and tralokinumab,” said Jashin J. Wu, MD, from the Dermatology Research and Education Foundation in Irvine, Calif., in an interview. He was not involved in the study.
“As it does not have any significant advantages with less long-term safety data, I do not see a place for it in my practice,” Dr. Wu said.
Dupilumab (Dupixent) and tralokinumab (Adbry) are monoclonal antibodies that also block IL-13. Both are already licensed for treating atopic dermatitis. Dupilumab was approved by the Food and Drug Administration in 2017, and tralokinumab was approved in 2021.
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. Eli Lilly has exclusive rights for the development and commercialization of lebrikizumab in the United States and all countries outside Europe; European rights belong to Almirall for all dermatology indications, including atopic dermatitis. Dr. Blauvelt acts as an investigator and adviser to these companies as well as many other pharmaceutical companies that are involved in developing new dermatologic treatments. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Ruxolitinib repigments many vitiligo-affected body areas
.
Those difficult areas include the hands and feet, said Thierry Passeron, MD, PhD, of Université Côte d’Azur and Centre Hospitalier Universitaire de Nice (France).
Indeed, a 50% or greater improvement in the Vitiligo Area Scoring Index (VASI-50) of the hands and feet was achieved with ruxolitinib cream (Opzelura) in around one-third of patients after 52 weeks’ treatment, and more than half of patients showed improvement in the upper and lower extremities.
During one of the late-breaking news sessions, Dr. Passeron presented a pooled analysis of the Topical Ruxolitinib Evaluation in Vitiligo Study 1 (TRuE-V1) and Study 2 (TruE-V2), which assessed VASI-50 data by body regions.
Similarly positive results were seen on the head and neck and the trunk, with VASI-50 being reached in a respective 68% and 48% of patients after a full year of treatment.
“VASI-50 response rates rose steadily through 52 weeks for both the head and trunk,” said Dr. Passeron. He noted that the trials were initially double-blinded for 24 weeks and that there was a further open-label extension phase through week 52.
In the latter phase, all patients were treated with ruxolitinib; those who originally received a vehicle agent as placebo crossed over to the active treatment.
First FDA-approved treatment for adults and adolescents with vitiligo
Ruxolitinib is a Janus kinase 1/2 inhibitor that has been available for the treatment for atopic dermatitis for more than a year. It was recently approved by the U.S. Food and Drug Administration for the treatment of vitiligo in adults and pediatric patients aged 12 years and older.
This approval was based on the positive findings of the TRuE-V1 and TRuE-V2 studies, which showed that after 24 weeks, 30% of patients treated with ruxolitinib had at least 75% improvement in the facial VASI, compared with 10% of placebo-treated patients.
“These studies demonstrated very nice results, especially on the face, which is the easiest part to repigment in vitiligo,” Dr. Passeron said.
“We know that the location is very important when it comes to repigmentation of vitiligo,” he added. He noted that other body areas, “the extremities, for example, are much more difficult.”
The analysis he presented specifically assessed the effect of ruxolitinib cream on repigmentation in other areas.
Pooled analysis performed
Data from the two TRuE-V trials were pooled. The new analysis included a total of 661 individuals; of those patients, 443 had been treated with topical ruxolitinib, and 218 had received a vehicle cream as a placebo.
For the first 24 weeks, patients received twice-daily 1.5% ruxolitinib cream or vehicle cream. This was followed by a 28-week extension phase in which everyone was treated with ruxolitinib cream, after which there was a 30-day final follow-up period.
Dr. Passeron reported data by body region for weeks 12, 24, and 52, which showed an increasing percentage of patients with VASI-50.
“We didn’t look at the face; that we know well, that is a very good result,” he said.
The best results were seen for the head and neck. VASI-50 was reached by 28.3%, 45.3%, and 68.1% of patients treated with ruxolitinib cream at weeks 12, 24, and 52, respectively. Corresponding rates for the placebo-crossover group were 19.8%, 23.8%, and 51%.
Repigmentation rates of the hand, upper extremities, trunk, lower extremities, and feet were about 9%-15% for both ruxolitinib and placebo at 12 weeks, but by 24 weeks, there was a clear increase in repigmentation rates in the ruxolitinib group for all body areas.
The 24-week VASI-50 rates for hand repigmentation were 24.9% for ruxolitinib cream and 14.4% for placebo. Corresponding rates for upper extremity repigmentation were 33.2% and 8.2%; for the trunk, 26.4% and 12.2%; for the lower extremities, 29.5% and 12.2%; and for the feet, 18.5% and 12.5%.
“The results are quite poor at 12 weeks,” Dr. Passeron said. “It’s very important to keep this in mind; it takes time to repigment vitiligo, it takes to 6-24 months. We have to explain to our patients that they will have to wait to see the results.”
Steady improvements, no new safety concerns
Regarding VASI-50 over time, there was a steady increase in total body scores; 47.7% of patients who received ruxolitinib and 23.3% of placebo-treated patients hit this target at 52 weeks.
“And what is also very important to see is that we didn’t reach the plateau,” Dr. Passeron reported.
Similar patterns were seen for all the other body areas. Again there was a suggestion that rates may continue to rise with continued long-term treatment.
“About one-third of the patients reached at least 50% repigmentation after 1 year of treatment in the hands and feet,” Dr. Passeron said. He noted that certain areas, such as the back of the hand or tips of the fingers, may be unresponsive.
“So, we have to also to warn the patient that probably on these areas we have to combine it with other treatment because it remains very, very difficult to treat.”
There were no new safety concerns regarding treatment-emergent adverse events, which were reported in 52% of patients who received ruxolitinib and in 36% of placebo-treated patients.
The most common adverse reactions included COVID-19 (6.1% vs. 3.1%), acne at the application site (5.3% vs. 1.3%), and pruritus at the application site (3.9% vs. 2.7%), although cases were “mild or moderate,” said Dr. Passeron.
An expert’s take-home
“The results of TRuE-V phase 3 studies are encouraging and exciting,” Viktoria Eleftheriadou, MD, MRCP(UK), SCE(Derm), PhD, said in providing an independent comment for this news organization.
“Although ruxolitinib cream is applied on the skin, this novel treatment for vitiligo is not without risks; therefore, careful monitoring of patients who are started on this topical treatment would be prudent,” said Dr. Eleftheriadou, who is a consultant dermatologist for Walsall Healthcare NHS Trust and the Royal Wolverhampton NHS Trust, Birmingham, United Kingdom.
“I would like to see how many patients achieved VASI-75 or VASI-80 score, which from patients’ perspectives is a more meaningful outcome, as well as how long these results will last for,” she added.
The study was funded by Incyte Corporation. Dr. Passeron has received grants, honoraria, or both from AbbVie, ACM Pharma, Almirall, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte Corporation, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceuticals, and UCB. Dr. Passeron is the cofounder of YUKIN Therapeutics and has patents on WNT agonists or GSK2b antagonist for repigmentation of vitiligo and on the use of CXCR3B blockers in vitiligo. Dr. Eleftheriadou is an investigator and trial development group member on the HI-Light Vitiligo Trial (specific), a lead investigator on the pilot HI-Light Vitiligo Trial, and a medical advisory panel member of the Vitiligo Society UK. Dr. Eleftheriadou also provides consultancy services to Incyte and Pfizer.
A version of this article first appeared on Medscape.com.
.
Those difficult areas include the hands and feet, said Thierry Passeron, MD, PhD, of Université Côte d’Azur and Centre Hospitalier Universitaire de Nice (France).
Indeed, a 50% or greater improvement in the Vitiligo Area Scoring Index (VASI-50) of the hands and feet was achieved with ruxolitinib cream (Opzelura) in around one-third of patients after 52 weeks’ treatment, and more than half of patients showed improvement in the upper and lower extremities.
During one of the late-breaking news sessions, Dr. Passeron presented a pooled analysis of the Topical Ruxolitinib Evaluation in Vitiligo Study 1 (TRuE-V1) and Study 2 (TruE-V2), which assessed VASI-50 data by body regions.
Similarly positive results were seen on the head and neck and the trunk, with VASI-50 being reached in a respective 68% and 48% of patients after a full year of treatment.
“VASI-50 response rates rose steadily through 52 weeks for both the head and trunk,” said Dr. Passeron. He noted that the trials were initially double-blinded for 24 weeks and that there was a further open-label extension phase through week 52.
In the latter phase, all patients were treated with ruxolitinib; those who originally received a vehicle agent as placebo crossed over to the active treatment.
First FDA-approved treatment for adults and adolescents with vitiligo
Ruxolitinib is a Janus kinase 1/2 inhibitor that has been available for the treatment for atopic dermatitis for more than a year. It was recently approved by the U.S. Food and Drug Administration for the treatment of vitiligo in adults and pediatric patients aged 12 years and older.
This approval was based on the positive findings of the TRuE-V1 and TRuE-V2 studies, which showed that after 24 weeks, 30% of patients treated with ruxolitinib had at least 75% improvement in the facial VASI, compared with 10% of placebo-treated patients.
“These studies demonstrated very nice results, especially on the face, which is the easiest part to repigment in vitiligo,” Dr. Passeron said.
“We know that the location is very important when it comes to repigmentation of vitiligo,” he added. He noted that other body areas, “the extremities, for example, are much more difficult.”
The analysis he presented specifically assessed the effect of ruxolitinib cream on repigmentation in other areas.
Pooled analysis performed
Data from the two TRuE-V trials were pooled. The new analysis included a total of 661 individuals; of those patients, 443 had been treated with topical ruxolitinib, and 218 had received a vehicle cream as a placebo.
For the first 24 weeks, patients received twice-daily 1.5% ruxolitinib cream or vehicle cream. This was followed by a 28-week extension phase in which everyone was treated with ruxolitinib cream, after which there was a 30-day final follow-up period.
Dr. Passeron reported data by body region for weeks 12, 24, and 52, which showed an increasing percentage of patients with VASI-50.
“We didn’t look at the face; that we know well, that is a very good result,” he said.
The best results were seen for the head and neck. VASI-50 was reached by 28.3%, 45.3%, and 68.1% of patients treated with ruxolitinib cream at weeks 12, 24, and 52, respectively. Corresponding rates for the placebo-crossover group were 19.8%, 23.8%, and 51%.
Repigmentation rates of the hand, upper extremities, trunk, lower extremities, and feet were about 9%-15% for both ruxolitinib and placebo at 12 weeks, but by 24 weeks, there was a clear increase in repigmentation rates in the ruxolitinib group for all body areas.
The 24-week VASI-50 rates for hand repigmentation were 24.9% for ruxolitinib cream and 14.4% for placebo. Corresponding rates for upper extremity repigmentation were 33.2% and 8.2%; for the trunk, 26.4% and 12.2%; for the lower extremities, 29.5% and 12.2%; and for the feet, 18.5% and 12.5%.
“The results are quite poor at 12 weeks,” Dr. Passeron said. “It’s very important to keep this in mind; it takes time to repigment vitiligo, it takes to 6-24 months. We have to explain to our patients that they will have to wait to see the results.”
Steady improvements, no new safety concerns
Regarding VASI-50 over time, there was a steady increase in total body scores; 47.7% of patients who received ruxolitinib and 23.3% of placebo-treated patients hit this target at 52 weeks.
“And what is also very important to see is that we didn’t reach the plateau,” Dr. Passeron reported.
Similar patterns were seen for all the other body areas. Again there was a suggestion that rates may continue to rise with continued long-term treatment.
“About one-third of the patients reached at least 50% repigmentation after 1 year of treatment in the hands and feet,” Dr. Passeron said. He noted that certain areas, such as the back of the hand or tips of the fingers, may be unresponsive.
“So, we have to also to warn the patient that probably on these areas we have to combine it with other treatment because it remains very, very difficult to treat.”
There were no new safety concerns regarding treatment-emergent adverse events, which were reported in 52% of patients who received ruxolitinib and in 36% of placebo-treated patients.
The most common adverse reactions included COVID-19 (6.1% vs. 3.1%), acne at the application site (5.3% vs. 1.3%), and pruritus at the application site (3.9% vs. 2.7%), although cases were “mild or moderate,” said Dr. Passeron.
An expert’s take-home
“The results of TRuE-V phase 3 studies are encouraging and exciting,” Viktoria Eleftheriadou, MD, MRCP(UK), SCE(Derm), PhD, said in providing an independent comment for this news organization.
“Although ruxolitinib cream is applied on the skin, this novel treatment for vitiligo is not without risks; therefore, careful monitoring of patients who are started on this topical treatment would be prudent,” said Dr. Eleftheriadou, who is a consultant dermatologist for Walsall Healthcare NHS Trust and the Royal Wolverhampton NHS Trust, Birmingham, United Kingdom.
“I would like to see how many patients achieved VASI-75 or VASI-80 score, which from patients’ perspectives is a more meaningful outcome, as well as how long these results will last for,” she added.
The study was funded by Incyte Corporation. Dr. Passeron has received grants, honoraria, or both from AbbVie, ACM Pharma, Almirall, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte Corporation, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceuticals, and UCB. Dr. Passeron is the cofounder of YUKIN Therapeutics and has patents on WNT agonists or GSK2b antagonist for repigmentation of vitiligo and on the use of CXCR3B blockers in vitiligo. Dr. Eleftheriadou is an investigator and trial development group member on the HI-Light Vitiligo Trial (specific), a lead investigator on the pilot HI-Light Vitiligo Trial, and a medical advisory panel member of the Vitiligo Society UK. Dr. Eleftheriadou also provides consultancy services to Incyte and Pfizer.
A version of this article first appeared on Medscape.com.
.
Those difficult areas include the hands and feet, said Thierry Passeron, MD, PhD, of Université Côte d’Azur and Centre Hospitalier Universitaire de Nice (France).
Indeed, a 50% or greater improvement in the Vitiligo Area Scoring Index (VASI-50) of the hands and feet was achieved with ruxolitinib cream (Opzelura) in around one-third of patients after 52 weeks’ treatment, and more than half of patients showed improvement in the upper and lower extremities.
During one of the late-breaking news sessions, Dr. Passeron presented a pooled analysis of the Topical Ruxolitinib Evaluation in Vitiligo Study 1 (TRuE-V1) and Study 2 (TruE-V2), which assessed VASI-50 data by body regions.
Similarly positive results were seen on the head and neck and the trunk, with VASI-50 being reached in a respective 68% and 48% of patients after a full year of treatment.
“VASI-50 response rates rose steadily through 52 weeks for both the head and trunk,” said Dr. Passeron. He noted that the trials were initially double-blinded for 24 weeks and that there was a further open-label extension phase through week 52.
In the latter phase, all patients were treated with ruxolitinib; those who originally received a vehicle agent as placebo crossed over to the active treatment.
First FDA-approved treatment for adults and adolescents with vitiligo
Ruxolitinib is a Janus kinase 1/2 inhibitor that has been available for the treatment for atopic dermatitis for more than a year. It was recently approved by the U.S. Food and Drug Administration for the treatment of vitiligo in adults and pediatric patients aged 12 years and older.
This approval was based on the positive findings of the TRuE-V1 and TRuE-V2 studies, which showed that after 24 weeks, 30% of patients treated with ruxolitinib had at least 75% improvement in the facial VASI, compared with 10% of placebo-treated patients.
“These studies demonstrated very nice results, especially on the face, which is the easiest part to repigment in vitiligo,” Dr. Passeron said.
“We know that the location is very important when it comes to repigmentation of vitiligo,” he added. He noted that other body areas, “the extremities, for example, are much more difficult.”
The analysis he presented specifically assessed the effect of ruxolitinib cream on repigmentation in other areas.
Pooled analysis performed
Data from the two TRuE-V trials were pooled. The new analysis included a total of 661 individuals; of those patients, 443 had been treated with topical ruxolitinib, and 218 had received a vehicle cream as a placebo.
For the first 24 weeks, patients received twice-daily 1.5% ruxolitinib cream or vehicle cream. This was followed by a 28-week extension phase in which everyone was treated with ruxolitinib cream, after which there was a 30-day final follow-up period.
Dr. Passeron reported data by body region for weeks 12, 24, and 52, which showed an increasing percentage of patients with VASI-50.
“We didn’t look at the face; that we know well, that is a very good result,” he said.
The best results were seen for the head and neck. VASI-50 was reached by 28.3%, 45.3%, and 68.1% of patients treated with ruxolitinib cream at weeks 12, 24, and 52, respectively. Corresponding rates for the placebo-crossover group were 19.8%, 23.8%, and 51%.
Repigmentation rates of the hand, upper extremities, trunk, lower extremities, and feet were about 9%-15% for both ruxolitinib and placebo at 12 weeks, but by 24 weeks, there was a clear increase in repigmentation rates in the ruxolitinib group for all body areas.
The 24-week VASI-50 rates for hand repigmentation were 24.9% for ruxolitinib cream and 14.4% for placebo. Corresponding rates for upper extremity repigmentation were 33.2% and 8.2%; for the trunk, 26.4% and 12.2%; for the lower extremities, 29.5% and 12.2%; and for the feet, 18.5% and 12.5%.
“The results are quite poor at 12 weeks,” Dr. Passeron said. “It’s very important to keep this in mind; it takes time to repigment vitiligo, it takes to 6-24 months. We have to explain to our patients that they will have to wait to see the results.”
Steady improvements, no new safety concerns
Regarding VASI-50 over time, there was a steady increase in total body scores; 47.7% of patients who received ruxolitinib and 23.3% of placebo-treated patients hit this target at 52 weeks.
“And what is also very important to see is that we didn’t reach the plateau,” Dr. Passeron reported.
Similar patterns were seen for all the other body areas. Again there was a suggestion that rates may continue to rise with continued long-term treatment.
“About one-third of the patients reached at least 50% repigmentation after 1 year of treatment in the hands and feet,” Dr. Passeron said. He noted that certain areas, such as the back of the hand or tips of the fingers, may be unresponsive.
“So, we have to also to warn the patient that probably on these areas we have to combine it with other treatment because it remains very, very difficult to treat.”
There were no new safety concerns regarding treatment-emergent adverse events, which were reported in 52% of patients who received ruxolitinib and in 36% of placebo-treated patients.
The most common adverse reactions included COVID-19 (6.1% vs. 3.1%), acne at the application site (5.3% vs. 1.3%), and pruritus at the application site (3.9% vs. 2.7%), although cases were “mild or moderate,” said Dr. Passeron.
An expert’s take-home
“The results of TRuE-V phase 3 studies are encouraging and exciting,” Viktoria Eleftheriadou, MD, MRCP(UK), SCE(Derm), PhD, said in providing an independent comment for this news organization.
“Although ruxolitinib cream is applied on the skin, this novel treatment for vitiligo is not without risks; therefore, careful monitoring of patients who are started on this topical treatment would be prudent,” said Dr. Eleftheriadou, who is a consultant dermatologist for Walsall Healthcare NHS Trust and the Royal Wolverhampton NHS Trust, Birmingham, United Kingdom.
“I would like to see how many patients achieved VASI-75 or VASI-80 score, which from patients’ perspectives is a more meaningful outcome, as well as how long these results will last for,” she added.
The study was funded by Incyte Corporation. Dr. Passeron has received grants, honoraria, or both from AbbVie, ACM Pharma, Almirall, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte Corporation, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceuticals, and UCB. Dr. Passeron is the cofounder of YUKIN Therapeutics and has patents on WNT agonists or GSK2b antagonist for repigmentation of vitiligo and on the use of CXCR3B blockers in vitiligo. Dr. Eleftheriadou is an investigator and trial development group member on the HI-Light Vitiligo Trial (specific), a lead investigator on the pilot HI-Light Vitiligo Trial, and a medical advisory panel member of the Vitiligo Society UK. Dr. Eleftheriadou also provides consultancy services to Incyte and Pfizer.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
How to handle pesky molluscum contagiosum lesions
.
“If you don’t treat them, they’re going to spread,” Dr. Smith, who practices dermatology in Fort Mill, S.C., said at Medscape Live’s annual Coastal Dermatology Symposium. “They’re going to be itchy, they can spread on the patient themselves and then to others, and they can cause scarring. The prevalence is anywhere from 5% to 11%. That means there are 6 million patients out there, just waiting to come into your clinics.”
To date, no treatment has been approved by the Food and Drug Administration for MC, although a laundry list of agents have been tried, including cantharidin; cryotherapy; curettage with and without imiquimod; sinecatechins ointment, 15%; imiquimod; and retinoids. And there are several treatments that are being investigated.
A 2017 Cochrane review of 22 studies involving 1,650 patients demonstrated that no single intervention has been consistently effective in treating MC. “Most of the studies were actually very low quality,” said Dr. Smith, who was not involved with the analysis. “The one high quality study showed that imiquimod did not work any better than its vehicle.”
Investigational treatments
One of the products in the pipeline is VP-102, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so that the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
VP-102, which is being developed by Verrica Pharmaceuticals, is applied once every 21 days in up to 4 applications, and multiple lesions can be treated with one applicator. “It’s a stable concentration with a good shelf life, and two phase 3 randomized studies have shown about a 50% complete clearance of new and existing lesions at day 84,” Dr. Smith said. Those studies enrolled children and adults.
A separate analysis of the same data presented at a meeting in 2019 showed that 77% of patients treated with VP-102 achieved greater than 75% clearance, while 65.8% achieved more than 90% clearance.
The new kid on the block is a gel formulation of a nitric oxide–releasing medication, berdazimer 10.3%, a first-in-class topical treatment being developed by Novan, which can be applied at home. In a multicenter study published in JAMA Dermatology, researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years and participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment.
At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
More recently, investigators evaluated autoinoculation vs. 35% trichloroacetic acid (TCA) for the treatment of MC. Autoinoculation involves puncturing the perilesional and lesional skin 5-7 times with an insulin syringe. “This gets a little bit of the virus into the dermis, and you hope to elicit an immune response,” explained Dr. Smith, who was not involved with the study. At 3 months, 80% of patients in the autoinoculation group achieved complete clearance, compared with 62% of those in the TCA group, while recurrence at 6 months was 3% vs. 40%, respectively.
Manual extraction of MC lesions is another option. “I love to pop the cores out with my thumbs,” Dr. Smith said. “You have to pick the patients who can tolerate this, and the MC lesions need to be ripe and ready.”
For ophthalmic lesions, watchful waiting is advisable unless the MC lesions are symptomatic or bothersome or large lesions form on the lid margin, which may cause ocular irritation or even a corneal abrasion. “If a patient presents with a multisite infection that includes ocular lesions, treat lesions on other parts of the body and keep your fingers crossed that a systemic immune response occurs,” she said.
The desired immune response is known as the “BOTE” sign (the beginning of the end), which heralds the clearance of the molluscum infection. This often appears as reddening of all the MC lesions and occasionally as a granulomatous “id-like” reaction especially on the extensor elbows and knees. “When this happens, it often scares the patients,” Dr. Smith said. But she explains that this is a positive development, and that “this means that the lesions are about to self-resolve.”
Dr. Smith disclosed that she serves as a speaker or a member of the speakers bureau for Amgen, CeraVe, EPI, Galderma, InCyte, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Sun. She also serves as an advisor or consultant for Janssen, Lilly, Regeneron, and Sanofi Genzyme.
Medscape Live and this news organization are owned by the same parent company.
.
“If you don’t treat them, they’re going to spread,” Dr. Smith, who practices dermatology in Fort Mill, S.C., said at Medscape Live’s annual Coastal Dermatology Symposium. “They’re going to be itchy, they can spread on the patient themselves and then to others, and they can cause scarring. The prevalence is anywhere from 5% to 11%. That means there are 6 million patients out there, just waiting to come into your clinics.”
To date, no treatment has been approved by the Food and Drug Administration for MC, although a laundry list of agents have been tried, including cantharidin; cryotherapy; curettage with and without imiquimod; sinecatechins ointment, 15%; imiquimod; and retinoids. And there are several treatments that are being investigated.
A 2017 Cochrane review of 22 studies involving 1,650 patients demonstrated that no single intervention has been consistently effective in treating MC. “Most of the studies were actually very low quality,” said Dr. Smith, who was not involved with the analysis. “The one high quality study showed that imiquimod did not work any better than its vehicle.”
Investigational treatments
One of the products in the pipeline is VP-102, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so that the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
VP-102, which is being developed by Verrica Pharmaceuticals, is applied once every 21 days in up to 4 applications, and multiple lesions can be treated with one applicator. “It’s a stable concentration with a good shelf life, and two phase 3 randomized studies have shown about a 50% complete clearance of new and existing lesions at day 84,” Dr. Smith said. Those studies enrolled children and adults.
A separate analysis of the same data presented at a meeting in 2019 showed that 77% of patients treated with VP-102 achieved greater than 75% clearance, while 65.8% achieved more than 90% clearance.
The new kid on the block is a gel formulation of a nitric oxide–releasing medication, berdazimer 10.3%, a first-in-class topical treatment being developed by Novan, which can be applied at home. In a multicenter study published in JAMA Dermatology, researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years and participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment.
At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
More recently, investigators evaluated autoinoculation vs. 35% trichloroacetic acid (TCA) for the treatment of MC. Autoinoculation involves puncturing the perilesional and lesional skin 5-7 times with an insulin syringe. “This gets a little bit of the virus into the dermis, and you hope to elicit an immune response,” explained Dr. Smith, who was not involved with the study. At 3 months, 80% of patients in the autoinoculation group achieved complete clearance, compared with 62% of those in the TCA group, while recurrence at 6 months was 3% vs. 40%, respectively.
Manual extraction of MC lesions is another option. “I love to pop the cores out with my thumbs,” Dr. Smith said. “You have to pick the patients who can tolerate this, and the MC lesions need to be ripe and ready.”
For ophthalmic lesions, watchful waiting is advisable unless the MC lesions are symptomatic or bothersome or large lesions form on the lid margin, which may cause ocular irritation or even a corneal abrasion. “If a patient presents with a multisite infection that includes ocular lesions, treat lesions on other parts of the body and keep your fingers crossed that a systemic immune response occurs,” she said.
The desired immune response is known as the “BOTE” sign (the beginning of the end), which heralds the clearance of the molluscum infection. This often appears as reddening of all the MC lesions and occasionally as a granulomatous “id-like” reaction especially on the extensor elbows and knees. “When this happens, it often scares the patients,” Dr. Smith said. But she explains that this is a positive development, and that “this means that the lesions are about to self-resolve.”
Dr. Smith disclosed that she serves as a speaker or a member of the speakers bureau for Amgen, CeraVe, EPI, Galderma, InCyte, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Sun. She also serves as an advisor or consultant for Janssen, Lilly, Regeneron, and Sanofi Genzyme.
Medscape Live and this news organization are owned by the same parent company.
.
“If you don’t treat them, they’re going to spread,” Dr. Smith, who practices dermatology in Fort Mill, S.C., said at Medscape Live’s annual Coastal Dermatology Symposium. “They’re going to be itchy, they can spread on the patient themselves and then to others, and they can cause scarring. The prevalence is anywhere from 5% to 11%. That means there are 6 million patients out there, just waiting to come into your clinics.”
To date, no treatment has been approved by the Food and Drug Administration for MC, although a laundry list of agents have been tried, including cantharidin; cryotherapy; curettage with and without imiquimod; sinecatechins ointment, 15%; imiquimod; and retinoids. And there are several treatments that are being investigated.
A 2017 Cochrane review of 22 studies involving 1,650 patients demonstrated that no single intervention has been consistently effective in treating MC. “Most of the studies were actually very low quality,” said Dr. Smith, who was not involved with the analysis. “The one high quality study showed that imiquimod did not work any better than its vehicle.”
Investigational treatments
One of the products in the pipeline is VP-102, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so that the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
VP-102, which is being developed by Verrica Pharmaceuticals, is applied once every 21 days in up to 4 applications, and multiple lesions can be treated with one applicator. “It’s a stable concentration with a good shelf life, and two phase 3 randomized studies have shown about a 50% complete clearance of new and existing lesions at day 84,” Dr. Smith said. Those studies enrolled children and adults.
A separate analysis of the same data presented at a meeting in 2019 showed that 77% of patients treated with VP-102 achieved greater than 75% clearance, while 65.8% achieved more than 90% clearance.
The new kid on the block is a gel formulation of a nitric oxide–releasing medication, berdazimer 10.3%, a first-in-class topical treatment being developed by Novan, which can be applied at home. In a multicenter study published in JAMA Dermatology, researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years and participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment.
At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
More recently, investigators evaluated autoinoculation vs. 35% trichloroacetic acid (TCA) for the treatment of MC. Autoinoculation involves puncturing the perilesional and lesional skin 5-7 times with an insulin syringe. “This gets a little bit of the virus into the dermis, and you hope to elicit an immune response,” explained Dr. Smith, who was not involved with the study. At 3 months, 80% of patients in the autoinoculation group achieved complete clearance, compared with 62% of those in the TCA group, while recurrence at 6 months was 3% vs. 40%, respectively.
Manual extraction of MC lesions is another option. “I love to pop the cores out with my thumbs,” Dr. Smith said. “You have to pick the patients who can tolerate this, and the MC lesions need to be ripe and ready.”
For ophthalmic lesions, watchful waiting is advisable unless the MC lesions are symptomatic or bothersome or large lesions form on the lid margin, which may cause ocular irritation or even a corneal abrasion. “If a patient presents with a multisite infection that includes ocular lesions, treat lesions on other parts of the body and keep your fingers crossed that a systemic immune response occurs,” she said.
The desired immune response is known as the “BOTE” sign (the beginning of the end), which heralds the clearance of the molluscum infection. This often appears as reddening of all the MC lesions and occasionally as a granulomatous “id-like” reaction especially on the extensor elbows and knees. “When this happens, it often scares the patients,” Dr. Smith said. But she explains that this is a positive development, and that “this means that the lesions are about to self-resolve.”
Dr. Smith disclosed that she serves as a speaker or a member of the speakers bureau for Amgen, CeraVe, EPI, Galderma, InCyte, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Sun. She also serves as an advisor or consultant for Janssen, Lilly, Regeneron, and Sanofi Genzyme.
Medscape Live and this news organization are owned by the same parent company.
FROM MEDSCAPE LIVE COASTAL DERM
Breakthrough COVID studies lend support to use of new boosters in immunosuppressed patients
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
Don’t make children with head lice leave school, report says
The American Academy of Pediatrics says children with head lice don’t need to be sent home from school.
Head lice infestations aren’t really a health hazard because of low transmission rates, a new report from the academy says, and sending students home “may stigmatize children suspected of having head lice.” The group says schools should instead offer education programs to help families understand how to manage head lice.
“Head lice are an unpleasant part of the human experience, but they can be successfully managed and are no reason for a child to miss school,” Dawn Nolt, MD, lead author of the report on head lice, said in a news release.
The report advises schools to abandon “no-nit” policies, which call for a student to be lice-free before being allowed back in class.
“A child or adolescent should not be restricted from school attendance because of head lice, given the low contagion within classrooms. ‘No-nit’ policies that exclude children or adolescents until all nits are removed may violate a child’s or adolescent’s civil liberties and are best addressed with legal counsel for schools,” the report says.
The report notes that lice almost always spread through head-to-head contact, not by “jumping” from one person to another. It’s possible for lice to spread by touching the belongings of a person with lice, such as combs or sports helmets, but the chances of that happening are very low, the academy said.
“Lice found on combs are likely to be injured or dead, and a louse is not likely to leave a healthy head unless there is a heavy infestation,” the report says.
The report lists new medications for treatment and gives an algorithm for managing head lice cases.
“The ideal treatment of head lice should be safe, free of toxic chemicals, readily available, simple to apply, effective, and inexpensive,” the report says.
This is the first updated guidance on head lice from the American Academy of Pediatrics since 2015. The CDC also says students with head lice don’t need to be sent home.
“Students diagnosed with live head lice do not need to be sent home early from school; they can go home at the end of the day, be treated, and return to class after appropriate treatment has begun. Nits may persist after treatment, but successful treatment should kill crawling lice,” the CDC says.
A version of this article first appeared on WebMD.com.
The American Academy of Pediatrics says children with head lice don’t need to be sent home from school.
Head lice infestations aren’t really a health hazard because of low transmission rates, a new report from the academy says, and sending students home “may stigmatize children suspected of having head lice.” The group says schools should instead offer education programs to help families understand how to manage head lice.
“Head lice are an unpleasant part of the human experience, but they can be successfully managed and are no reason for a child to miss school,” Dawn Nolt, MD, lead author of the report on head lice, said in a news release.
The report advises schools to abandon “no-nit” policies, which call for a student to be lice-free before being allowed back in class.
“A child or adolescent should not be restricted from school attendance because of head lice, given the low contagion within classrooms. ‘No-nit’ policies that exclude children or adolescents until all nits are removed may violate a child’s or adolescent’s civil liberties and are best addressed with legal counsel for schools,” the report says.
The report notes that lice almost always spread through head-to-head contact, not by “jumping” from one person to another. It’s possible for lice to spread by touching the belongings of a person with lice, such as combs or sports helmets, but the chances of that happening are very low, the academy said.
“Lice found on combs are likely to be injured or dead, and a louse is not likely to leave a healthy head unless there is a heavy infestation,” the report says.
The report lists new medications for treatment and gives an algorithm for managing head lice cases.
“The ideal treatment of head lice should be safe, free of toxic chemicals, readily available, simple to apply, effective, and inexpensive,” the report says.
This is the first updated guidance on head lice from the American Academy of Pediatrics since 2015. The CDC also says students with head lice don’t need to be sent home.
“Students diagnosed with live head lice do not need to be sent home early from school; they can go home at the end of the day, be treated, and return to class after appropriate treatment has begun. Nits may persist after treatment, but successful treatment should kill crawling lice,” the CDC says.
A version of this article first appeared on WebMD.com.
The American Academy of Pediatrics says children with head lice don’t need to be sent home from school.
Head lice infestations aren’t really a health hazard because of low transmission rates, a new report from the academy says, and sending students home “may stigmatize children suspected of having head lice.” The group says schools should instead offer education programs to help families understand how to manage head lice.
“Head lice are an unpleasant part of the human experience, but they can be successfully managed and are no reason for a child to miss school,” Dawn Nolt, MD, lead author of the report on head lice, said in a news release.
The report advises schools to abandon “no-nit” policies, which call for a student to be lice-free before being allowed back in class.
“A child or adolescent should not be restricted from school attendance because of head lice, given the low contagion within classrooms. ‘No-nit’ policies that exclude children or adolescents until all nits are removed may violate a child’s or adolescent’s civil liberties and are best addressed with legal counsel for schools,” the report says.
The report notes that lice almost always spread through head-to-head contact, not by “jumping” from one person to another. It’s possible for lice to spread by touching the belongings of a person with lice, such as combs or sports helmets, but the chances of that happening are very low, the academy said.
“Lice found on combs are likely to be injured or dead, and a louse is not likely to leave a healthy head unless there is a heavy infestation,” the report says.
The report lists new medications for treatment and gives an algorithm for managing head lice cases.
“The ideal treatment of head lice should be safe, free of toxic chemicals, readily available, simple to apply, effective, and inexpensive,” the report says.
This is the first updated guidance on head lice from the American Academy of Pediatrics since 2015. The CDC also says students with head lice don’t need to be sent home.
“Students diagnosed with live head lice do not need to be sent home early from school; they can go home at the end of the day, be treated, and return to class after appropriate treatment has begun. Nits may persist after treatment, but successful treatment should kill crawling lice,” the CDC says.
A version of this article first appeared on WebMD.com.
Physician bias may prevent quality care for patients with disabilities
For Tara Lagu, MD, the realization that the health care system was broken for patients with disabilities came when a woman she had been treating seemed to keep ignoring Dr. Lagu’s request to see a urologist.
When Dr. Lagu asked the patient’s two attentive daughters about the delay, their response surprised her. The women said they couldn’t find a urologist who was willing to see a patient in a wheelchair.
Surprised and a bit doubtful, Dr. Lagu checked around. She found that, indeed, the only way to get her patient in to see the type of physician required was to send her by ambulance.
“It opened my eyes to how hard it is for patients with disabilities to navigate the health care system,” Dr. Lagu said.
Dr. Lagu, director of the Center for Health Services and Outcomes Research at Northwestern University in Chicago, decided to take a closer look at how her colleagues in medicine care for – or not, as the case proved – the roughly one in four American adults, and millions of children, with disabilities.
In a series of three focus groups, Dr. Lagu and colleagues identified a range of obstacles – including some physician attitudes – that prevent people with disabilities from getting adequate care.
For the study, published in Health Affairs, the researchers interviewed 22 physicians in three groups: Nonrural primary care physicians, rural primary care physicians, and specialists in rheumatology, neurology, obstetrics/gynecology, orthopedics, and ophthalmology.
During the interviews, conducted in the fall of 2018, participants were asked about providing care for five specific types of disabilities: mobility, hearing, vision, mental health, and intellectual limitations.
Lack of experience, logistics often cited
Some physicians admitted that limited resources and training left them without the space and necessary knowledge to properly care for patients with disabilities. They felt they lacked the expertise or exposure to care for individuals with disabilities, nor did they have enough time and space to properly accommodate these patients, according to the researchers. Some said they struggled to coordinate care for individuals with disabilities and did not know which types of accessible equipment, such as adjustable tables and chair scales, were needed or how to use them.
Several physicians also noted that they are inadequately reimbursed for the special accommodations – including additional staff, equipment, and time – required to care for these patients. One primary care physician said he hired a sign-language interpreter for a patient but the bill for the services exceeded the amount insurance reimbursed. As a result, he said, he spent $30 of his own money per visit to see the patient.
Because of these limitations, some physicians in the focus groups said they try to turn away patients with disabilities. Both specialists and general practitioners said they had told patients with disabilities that they didn’t feel they could provide the care needed, and suggested they look elsewhere. A few were surprisingly – even upsettingly – honest, Dr. Lagu said, making statements such as: “I am not the doctor for you.”
‘We really need a rewrite’
Previous work has shown that people with disabilities have worse health outcomes, such as undetected cancer, obesity, and cardiovascular disease.
But “the disability itself isn’t what leads to worse outcomes,” said Allison Kessler, MD, section chief of the Renée Crown Center for Spinal Cord Innovation and associate director of the Shirley Ryan AbilityLab in Chicago*. This study does a good job at highlighting “the need for change on multiple levels,” said Dr. Kessler, who was not a member of the study team.
“People with disabilities have all these disparities in access and outcomes. We’ve never understood why. I think the why is complicated,” Dr. Lagu added. “I think this study suggests some of the negative outcomes are due to explicit bias.”
“It’s also clear that the current framework of health care in the United States does not lend to allowing physicians and medical providers the time needed to adequately address patient issues – those with disabilities or just multiple complex problems,” Colin O’Reilly, DO, vice president and chief medical officer at Children’s Specialized Hospital, an acute rehabilitation facility affiliated with RWJBarnabas Health, in New Brunswick, N.J. “We really need a rewrite.”
However, Dr. O’Reilly said, such a small study population with no control group and no mention of physician resources makes it difficult to come to a strong conclusion about physician bias and discriminatory attitudes against individuals with disabilities.
Dr. Lagu agreed, saying this research “is not conclusive in any way.” The excuses doctors use to discharge patients with disabilities, such as “we don’t accept your insurance,” “we aren’t taking new patients,” and “we can’t provide you with the appropriate care,” could be legitimate, the study authors wrote. But the “disparities in care for people with disabilities suggest that there is a pattern of more frequently denying care to them than people without a disability,” they added.
Dr. Kessler said many of her patients have told her they experience barriers to care. Some say finding an office with the necessary equipment is a challenge or that they often don’t feel welcome.
The Americans With Disabilities Act (ADA) is a federal civil rights law that prohibits discrimination against individuals with disabilities in all public and private places that are open to the general public, including medical offices.
“It is difficult to enforce the ADA in medical settings,” the researchers noted. “Explanations physicians gave in this study could, for any single case of denying care, be legitimate.” Knowing whether a particular instance of denial of care represents discrimination related to disability is “nearly impossible,” they wrote.
All the experts agreed that the study adds valuable insight into an ongoing health disparity. And while system and policy changes are required, Dr. Kessler said, individual physicians can take steps to improve the situation.
A physician in an academic setting can look at the curriculum and the medical school and see about increasing exposure to patients with disabilities earlier in training. In a practice, physicians can retrain staff to ask every patient if an accommodation is needed. “Each one of those changes can only help us move our system in the right direction,” Dr. Kessler said.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
*Correction, 10/5/22: This article includes a corrected title for Dr. Allison Kessler.
A version of this article first appeared on Medscape.com.
For Tara Lagu, MD, the realization that the health care system was broken for patients with disabilities came when a woman she had been treating seemed to keep ignoring Dr. Lagu’s request to see a urologist.
When Dr. Lagu asked the patient’s two attentive daughters about the delay, their response surprised her. The women said they couldn’t find a urologist who was willing to see a patient in a wheelchair.
Surprised and a bit doubtful, Dr. Lagu checked around. She found that, indeed, the only way to get her patient in to see the type of physician required was to send her by ambulance.
“It opened my eyes to how hard it is for patients with disabilities to navigate the health care system,” Dr. Lagu said.
Dr. Lagu, director of the Center for Health Services and Outcomes Research at Northwestern University in Chicago, decided to take a closer look at how her colleagues in medicine care for – or not, as the case proved – the roughly one in four American adults, and millions of children, with disabilities.
In a series of three focus groups, Dr. Lagu and colleagues identified a range of obstacles – including some physician attitudes – that prevent people with disabilities from getting adequate care.
For the study, published in Health Affairs, the researchers interviewed 22 physicians in three groups: Nonrural primary care physicians, rural primary care physicians, and specialists in rheumatology, neurology, obstetrics/gynecology, orthopedics, and ophthalmology.
During the interviews, conducted in the fall of 2018, participants were asked about providing care for five specific types of disabilities: mobility, hearing, vision, mental health, and intellectual limitations.
Lack of experience, logistics often cited
Some physicians admitted that limited resources and training left them without the space and necessary knowledge to properly care for patients with disabilities. They felt they lacked the expertise or exposure to care for individuals with disabilities, nor did they have enough time and space to properly accommodate these patients, according to the researchers. Some said they struggled to coordinate care for individuals with disabilities and did not know which types of accessible equipment, such as adjustable tables and chair scales, were needed or how to use them.
Several physicians also noted that they are inadequately reimbursed for the special accommodations – including additional staff, equipment, and time – required to care for these patients. One primary care physician said he hired a sign-language interpreter for a patient but the bill for the services exceeded the amount insurance reimbursed. As a result, he said, he spent $30 of his own money per visit to see the patient.
Because of these limitations, some physicians in the focus groups said they try to turn away patients with disabilities. Both specialists and general practitioners said they had told patients with disabilities that they didn’t feel they could provide the care needed, and suggested they look elsewhere. A few were surprisingly – even upsettingly – honest, Dr. Lagu said, making statements such as: “I am not the doctor for you.”
‘We really need a rewrite’
Previous work has shown that people with disabilities have worse health outcomes, such as undetected cancer, obesity, and cardiovascular disease.
But “the disability itself isn’t what leads to worse outcomes,” said Allison Kessler, MD, section chief of the Renée Crown Center for Spinal Cord Innovation and associate director of the Shirley Ryan AbilityLab in Chicago*. This study does a good job at highlighting “the need for change on multiple levels,” said Dr. Kessler, who was not a member of the study team.
“People with disabilities have all these disparities in access and outcomes. We’ve never understood why. I think the why is complicated,” Dr. Lagu added. “I think this study suggests some of the negative outcomes are due to explicit bias.”
“It’s also clear that the current framework of health care in the United States does not lend to allowing physicians and medical providers the time needed to adequately address patient issues – those with disabilities or just multiple complex problems,” Colin O’Reilly, DO, vice president and chief medical officer at Children’s Specialized Hospital, an acute rehabilitation facility affiliated with RWJBarnabas Health, in New Brunswick, N.J. “We really need a rewrite.”
However, Dr. O’Reilly said, such a small study population with no control group and no mention of physician resources makes it difficult to come to a strong conclusion about physician bias and discriminatory attitudes against individuals with disabilities.
Dr. Lagu agreed, saying this research “is not conclusive in any way.” The excuses doctors use to discharge patients with disabilities, such as “we don’t accept your insurance,” “we aren’t taking new patients,” and “we can’t provide you with the appropriate care,” could be legitimate, the study authors wrote. But the “disparities in care for people with disabilities suggest that there is a pattern of more frequently denying care to them than people without a disability,” they added.
Dr. Kessler said many of her patients have told her they experience barriers to care. Some say finding an office with the necessary equipment is a challenge or that they often don’t feel welcome.
The Americans With Disabilities Act (ADA) is a federal civil rights law that prohibits discrimination against individuals with disabilities in all public and private places that are open to the general public, including medical offices.
“It is difficult to enforce the ADA in medical settings,” the researchers noted. “Explanations physicians gave in this study could, for any single case of denying care, be legitimate.” Knowing whether a particular instance of denial of care represents discrimination related to disability is “nearly impossible,” they wrote.
All the experts agreed that the study adds valuable insight into an ongoing health disparity. And while system and policy changes are required, Dr. Kessler said, individual physicians can take steps to improve the situation.
A physician in an academic setting can look at the curriculum and the medical school and see about increasing exposure to patients with disabilities earlier in training. In a practice, physicians can retrain staff to ask every patient if an accommodation is needed. “Each one of those changes can only help us move our system in the right direction,” Dr. Kessler said.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
*Correction, 10/5/22: This article includes a corrected title for Dr. Allison Kessler.
A version of this article first appeared on Medscape.com.
For Tara Lagu, MD, the realization that the health care system was broken for patients with disabilities came when a woman she had been treating seemed to keep ignoring Dr. Lagu’s request to see a urologist.
When Dr. Lagu asked the patient’s two attentive daughters about the delay, their response surprised her. The women said they couldn’t find a urologist who was willing to see a patient in a wheelchair.
Surprised and a bit doubtful, Dr. Lagu checked around. She found that, indeed, the only way to get her patient in to see the type of physician required was to send her by ambulance.
“It opened my eyes to how hard it is for patients with disabilities to navigate the health care system,” Dr. Lagu said.
Dr. Lagu, director of the Center for Health Services and Outcomes Research at Northwestern University in Chicago, decided to take a closer look at how her colleagues in medicine care for – or not, as the case proved – the roughly one in four American adults, and millions of children, with disabilities.
In a series of three focus groups, Dr. Lagu and colleagues identified a range of obstacles – including some physician attitudes – that prevent people with disabilities from getting adequate care.
For the study, published in Health Affairs, the researchers interviewed 22 physicians in three groups: Nonrural primary care physicians, rural primary care physicians, and specialists in rheumatology, neurology, obstetrics/gynecology, orthopedics, and ophthalmology.
During the interviews, conducted in the fall of 2018, participants were asked about providing care for five specific types of disabilities: mobility, hearing, vision, mental health, and intellectual limitations.
Lack of experience, logistics often cited
Some physicians admitted that limited resources and training left them without the space and necessary knowledge to properly care for patients with disabilities. They felt they lacked the expertise or exposure to care for individuals with disabilities, nor did they have enough time and space to properly accommodate these patients, according to the researchers. Some said they struggled to coordinate care for individuals with disabilities and did not know which types of accessible equipment, such as adjustable tables and chair scales, were needed or how to use them.
Several physicians also noted that they are inadequately reimbursed for the special accommodations – including additional staff, equipment, and time – required to care for these patients. One primary care physician said he hired a sign-language interpreter for a patient but the bill for the services exceeded the amount insurance reimbursed. As a result, he said, he spent $30 of his own money per visit to see the patient.
Because of these limitations, some physicians in the focus groups said they try to turn away patients with disabilities. Both specialists and general practitioners said they had told patients with disabilities that they didn’t feel they could provide the care needed, and suggested they look elsewhere. A few were surprisingly – even upsettingly – honest, Dr. Lagu said, making statements such as: “I am not the doctor for you.”
‘We really need a rewrite’
Previous work has shown that people with disabilities have worse health outcomes, such as undetected cancer, obesity, and cardiovascular disease.
But “the disability itself isn’t what leads to worse outcomes,” said Allison Kessler, MD, section chief of the Renée Crown Center for Spinal Cord Innovation and associate director of the Shirley Ryan AbilityLab in Chicago*. This study does a good job at highlighting “the need for change on multiple levels,” said Dr. Kessler, who was not a member of the study team.
“People with disabilities have all these disparities in access and outcomes. We’ve never understood why. I think the why is complicated,” Dr. Lagu added. “I think this study suggests some of the negative outcomes are due to explicit bias.”
“It’s also clear that the current framework of health care in the United States does not lend to allowing physicians and medical providers the time needed to adequately address patient issues – those with disabilities or just multiple complex problems,” Colin O’Reilly, DO, vice president and chief medical officer at Children’s Specialized Hospital, an acute rehabilitation facility affiliated with RWJBarnabas Health, in New Brunswick, N.J. “We really need a rewrite.”
However, Dr. O’Reilly said, such a small study population with no control group and no mention of physician resources makes it difficult to come to a strong conclusion about physician bias and discriminatory attitudes against individuals with disabilities.
Dr. Lagu agreed, saying this research “is not conclusive in any way.” The excuses doctors use to discharge patients with disabilities, such as “we don’t accept your insurance,” “we aren’t taking new patients,” and “we can’t provide you with the appropriate care,” could be legitimate, the study authors wrote. But the “disparities in care for people with disabilities suggest that there is a pattern of more frequently denying care to them than people without a disability,” they added.
Dr. Kessler said many of her patients have told her they experience barriers to care. Some say finding an office with the necessary equipment is a challenge or that they often don’t feel welcome.
The Americans With Disabilities Act (ADA) is a federal civil rights law that prohibits discrimination against individuals with disabilities in all public and private places that are open to the general public, including medical offices.
“It is difficult to enforce the ADA in medical settings,” the researchers noted. “Explanations physicians gave in this study could, for any single case of denying care, be legitimate.” Knowing whether a particular instance of denial of care represents discrimination related to disability is “nearly impossible,” they wrote.
All the experts agreed that the study adds valuable insight into an ongoing health disparity. And while system and policy changes are required, Dr. Kessler said, individual physicians can take steps to improve the situation.
A physician in an academic setting can look at the curriculum and the medical school and see about increasing exposure to patients with disabilities earlier in training. In a practice, physicians can retrain staff to ask every patient if an accommodation is needed. “Each one of those changes can only help us move our system in the right direction,” Dr. Kessler said.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
*Correction, 10/5/22: This article includes a corrected title for Dr. Allison Kessler.
A version of this article first appeared on Medscape.com.