User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
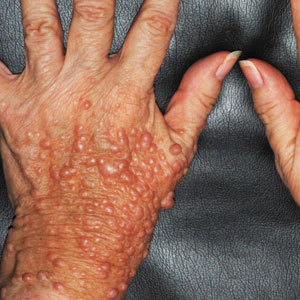
Margin Size for Unique Skin Tumors Treated With Mohs Micrographic Surgery: A Survey of Practice Patterns
Mohs micrographic surgery (MMS) is most commonly used for the surgical management of squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) in high-risk locations. The ability for 100% margin evaluation with MMS also has shown lower recurrence rates compared with wide local excision for less common and/or more aggressive tumors. However, there is a lack of standardization on initial and subsequent margin size when treating these less common skin tumors, such as dermatofibrosarcoma protuberans (DFSP), atypical fibroxanthoma (AFX), and sebaceous carcinoma.
Because Mohs surgeons must balance normal tissue preservation with the importance of tumor clearance in the context of comprehensive margin control, we aimed to assess the practice patterns of Mohs surgeons regarding margin size for these unique tumors. The average margin size for each Mohs layer has been reported to be 1 to 3 mm for BCC compared with 3 to 6 mm or larger for other skin cancers, such as melanoma in situ (MIS).1-3 We hypothesized that the initial margin size would vary among surgeons and likely be greater for more aggressive and rarer malignancies as well as for lesions on the trunk and extremities.
Methods
A descriptive survey was created using SurveyMonkey and distributed to members of the American College of Mohs Surgery (ACMS). Survey participants and their responses were anonymous. Demographic information on survey participants was collected in addition to initial and subsequent MMS margin size for DFSP, AFX, MIS, invasive melanoma, sebaceous carcinoma, microcystic adnexal carcinoma (MAC), poorly differentiated SCC, Merkel cell carcinoma, extramammary Paget disease, leiomyosarcoma, and endocrine mucin-producing sweat gland carcinoma. Survey participants were asked to choose from a range of margin sizes: 1 to 3 mm, 4 to 6 mm, 7 to 9 mm, and greater than 9 mm. This study was approved by the University of Texas Southwest Medical Center (Dallas, Texas) institutional review board.
Results
Eighty-seven respondents from the ACMS listserve completed the survey (response rate <10%). Of these, 58 respondents (66.7%) reported practicing for more than 5 years, and 58 (66.7%) were male. Practice setting was primarily private/community (71.3% [62/87]), and survey respondents were located across the United States. More than 50% of survey respondents treated the following tumors on the head and neck in their respective practices: DFSP (80.9% [55/68]), AFX (95.6% [65/68]), MIS (67.7% [46/68]), sebaceous carcinoma (92.7% [63/68]), MAC (83.8% [57/68]), poorly differentiated SCC (97.1% [66/68]), and endocrine mucin-producing sweat gland carcinoma (51.5% [35/68]). More than 50% of survey respondents treated the following tumors on the trunk and extremities: DFSP (90.3% [47/52]), AFX (86.4% [45/52]), MIS (55.8% [29/52]), sebaceous carcinoma (80.8% [42/52]), MAC (73.1% [38/52]), poorly differentiated SCC (94.2% [49/52]), and extramammary Paget disease (53.9% [28/52]). Invasive melanoma, Merkel cell carcinoma, and leiomyosarcoma were overall less commonly treated.
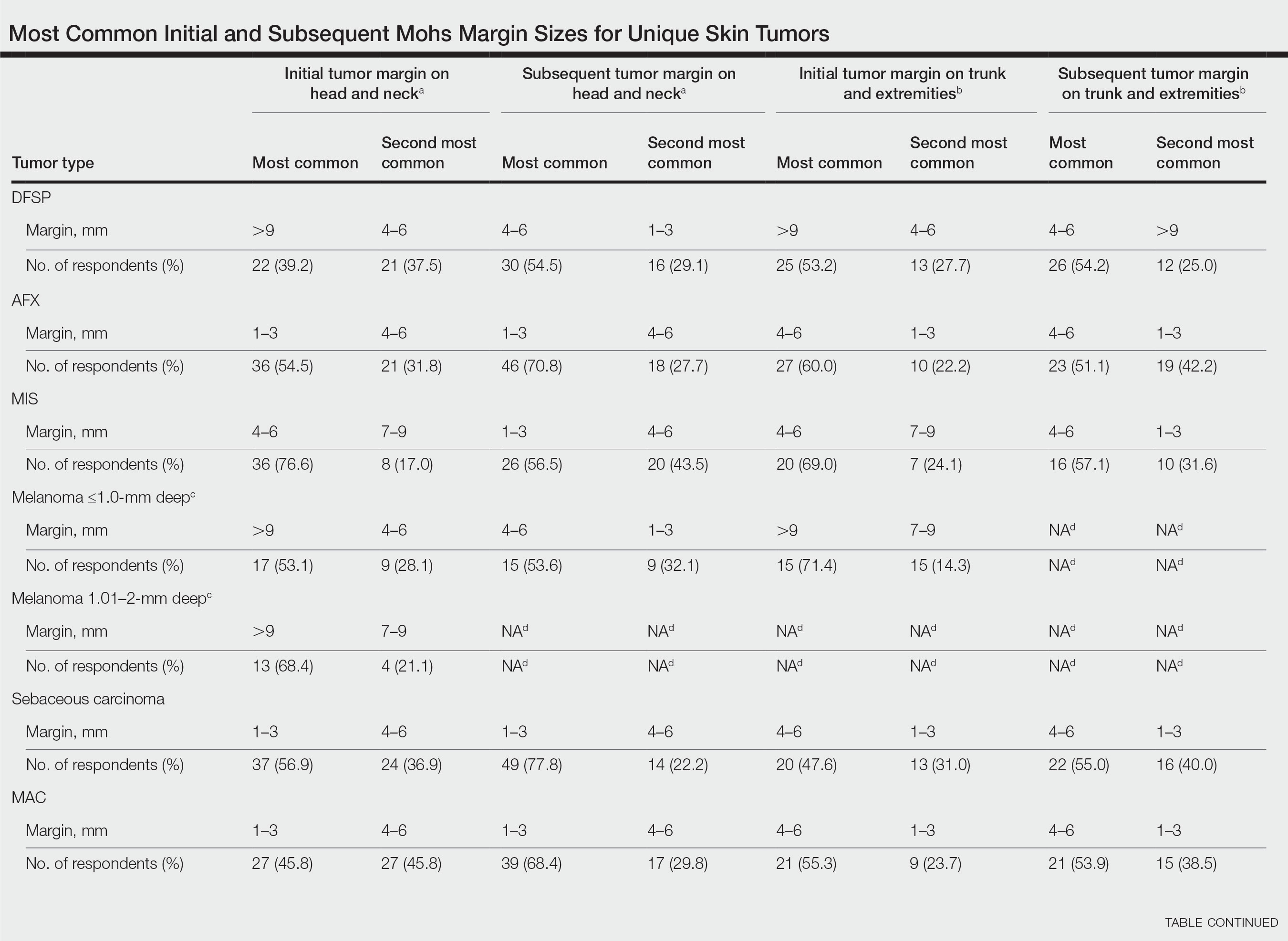
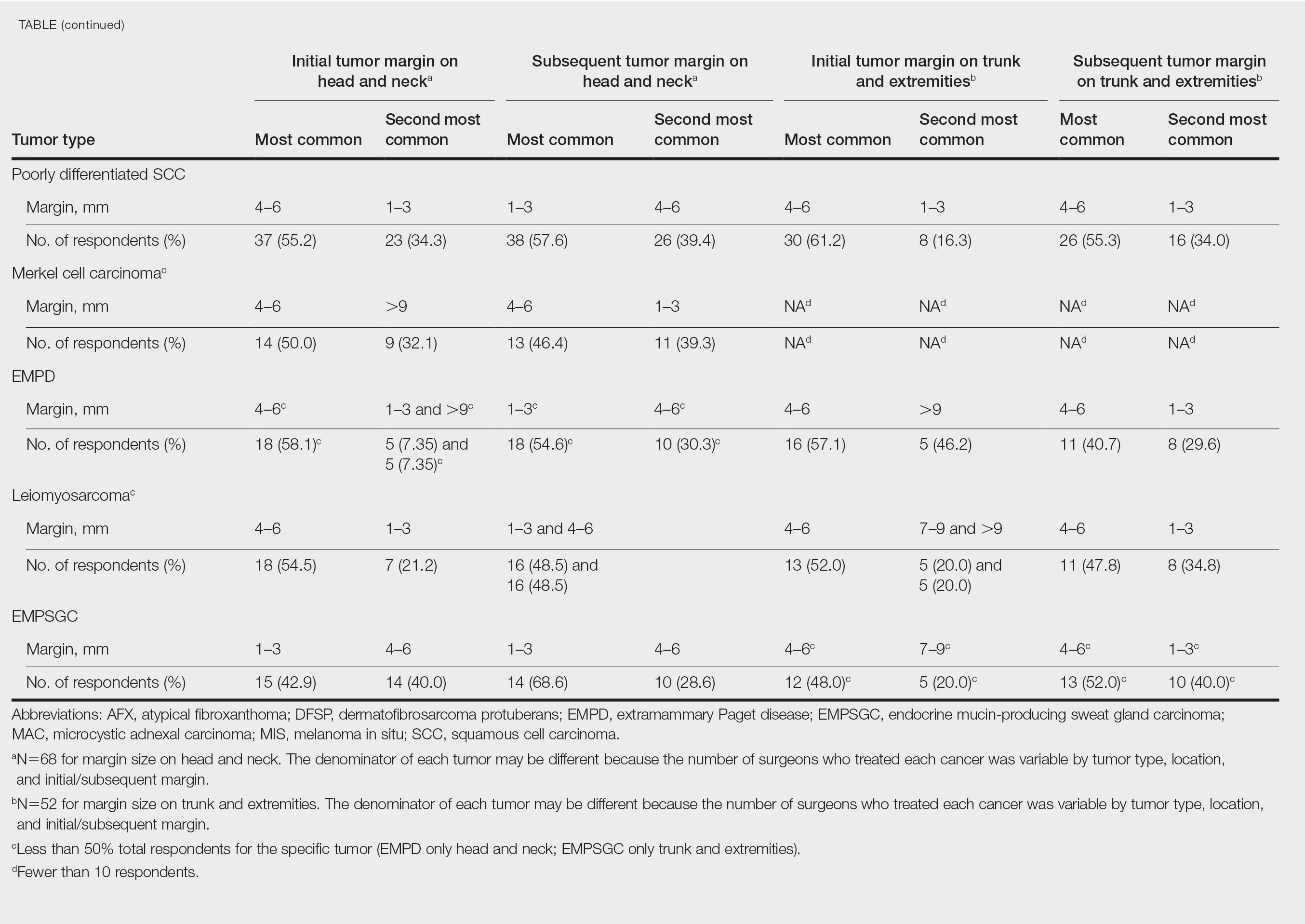
In general, respondent Mohs surgeons were more likely to take larger initial and subsequent margins for tumors treated on the trunk and extremities compared with the head and neck (Table). In addition, initial margin size often was larger than the 1- to 3-mm margin commonly used in Mohs surgery for BCCs and less aggressive SCCs (Table). A larger initial margin size (>9 mm) and subsequent margin size (4–6 mm) was more commonly reported for certain tumors known to be more aggressive and/or have extensive subclinical extension, such as DFSP and invasive melanoma. Of note, most respondents performed 4- to 6-mm margins (37/67 [55.2%]) for poorly differentiated SCC. Overall, there was a high range of margin size variability among Mohs surgeons for these unique and/or more aggressive skin tumors.
Comment
Given that no guidelines exist on margins with MMS for less commonly treated skin tumors, this study helps give Mohs surgeons perspective on current practice patterns for both initial and subsequent Mohs margin sizes. High margin-size variability among Mohs surgeons is expected, as surgeons also need to account for high-risk features of the tumor or specific locations where tissue sparing is critical. Overall, Mohs surgeons are more likely to take larger initial margins for these less common skin tumors compared with BCCs or SCCs. Initial margin size was consistently larger on the trunk and extremities where tissue sparing often is less critical.
Our survey was limited by a small sample size and incomplete response of the ACMS membership. In addition, most respondents practiced in a private/community setting, which may have led to bias, as academic centers may manage rare malignancies more commonly and/or have increased access to immunostains and multispecialty care. Future registries for rare skin malignancies will hopefully be developed that will allow for further consensus on standardized margins. Additional studies on the average number of stages required to clear these less common tumors also are warranted.
- Muller FM, Dawe RS, Moseley H, et al. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma: tissue‐sparing outcome. Dermatol Surg. 2009;35:1349-1354.
- van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
- Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: a prospective multicenter study. J Am Acad Dermatol. 2019;81:767-774.
Mohs micrographic surgery (MMS) is most commonly used for the surgical management of squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) in high-risk locations. The ability for 100% margin evaluation with MMS also has shown lower recurrence rates compared with wide local excision for less common and/or more aggressive tumors. However, there is a lack of standardization on initial and subsequent margin size when treating these less common skin tumors, such as dermatofibrosarcoma protuberans (DFSP), atypical fibroxanthoma (AFX), and sebaceous carcinoma.
Because Mohs surgeons must balance normal tissue preservation with the importance of tumor clearance in the context of comprehensive margin control, we aimed to assess the practice patterns of Mohs surgeons regarding margin size for these unique tumors. The average margin size for each Mohs layer has been reported to be 1 to 3 mm for BCC compared with 3 to 6 mm or larger for other skin cancers, such as melanoma in situ (MIS).1-3 We hypothesized that the initial margin size would vary among surgeons and likely be greater for more aggressive and rarer malignancies as well as for lesions on the trunk and extremities.
Methods
A descriptive survey was created using SurveyMonkey and distributed to members of the American College of Mohs Surgery (ACMS). Survey participants and their responses were anonymous. Demographic information on survey participants was collected in addition to initial and subsequent MMS margin size for DFSP, AFX, MIS, invasive melanoma, sebaceous carcinoma, microcystic adnexal carcinoma (MAC), poorly differentiated SCC, Merkel cell carcinoma, extramammary Paget disease, leiomyosarcoma, and endocrine mucin-producing sweat gland carcinoma. Survey participants were asked to choose from a range of margin sizes: 1 to 3 mm, 4 to 6 mm, 7 to 9 mm, and greater than 9 mm. This study was approved by the University of Texas Southwest Medical Center (Dallas, Texas) institutional review board.
Results
Eighty-seven respondents from the ACMS listserve completed the survey (response rate <10%). Of these, 58 respondents (66.7%) reported practicing for more than 5 years, and 58 (66.7%) were male. Practice setting was primarily private/community (71.3% [62/87]), and survey respondents were located across the United States. More than 50% of survey respondents treated the following tumors on the head and neck in their respective practices: DFSP (80.9% [55/68]), AFX (95.6% [65/68]), MIS (67.7% [46/68]), sebaceous carcinoma (92.7% [63/68]), MAC (83.8% [57/68]), poorly differentiated SCC (97.1% [66/68]), and endocrine mucin-producing sweat gland carcinoma (51.5% [35/68]). More than 50% of survey respondents treated the following tumors on the trunk and extremities: DFSP (90.3% [47/52]), AFX (86.4% [45/52]), MIS (55.8% [29/52]), sebaceous carcinoma (80.8% [42/52]), MAC (73.1% [38/52]), poorly differentiated SCC (94.2% [49/52]), and extramammary Paget disease (53.9% [28/52]). Invasive melanoma, Merkel cell carcinoma, and leiomyosarcoma were overall less commonly treated.
In general, respondent Mohs surgeons were more likely to take larger initial and subsequent margins for tumors treated on the trunk and extremities compared with the head and neck (Table). In addition, initial margin size often was larger than the 1- to 3-mm margin commonly used in Mohs surgery for BCCs and less aggressive SCCs (Table). A larger initial margin size (>9 mm) and subsequent margin size (4–6 mm) was more commonly reported for certain tumors known to be more aggressive and/or have extensive subclinical extension, such as DFSP and invasive melanoma. Of note, most respondents performed 4- to 6-mm margins (37/67 [55.2%]) for poorly differentiated SCC. Overall, there was a high range of margin size variability among Mohs surgeons for these unique and/or more aggressive skin tumors.


Comment
Given that no guidelines exist on margins with MMS for less commonly treated skin tumors, this study helps give Mohs surgeons perspective on current practice patterns for both initial and subsequent Mohs margin sizes. High margin-size variability among Mohs surgeons is expected, as surgeons also need to account for high-risk features of the tumor or specific locations where tissue sparing is critical. Overall, Mohs surgeons are more likely to take larger initial margins for these less common skin tumors compared with BCCs or SCCs. Initial margin size was consistently larger on the trunk and extremities where tissue sparing often is less critical.
Our survey was limited by a small sample size and incomplete response of the ACMS membership. In addition, most respondents practiced in a private/community setting, which may have led to bias, as academic centers may manage rare malignancies more commonly and/or have increased access to immunostains and multispecialty care. Future registries for rare skin malignancies will hopefully be developed that will allow for further consensus on standardized margins. Additional studies on the average number of stages required to clear these less common tumors also are warranted.
Mohs micrographic surgery (MMS) is most commonly used for the surgical management of squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) in high-risk locations. The ability for 100% margin evaluation with MMS also has shown lower recurrence rates compared with wide local excision for less common and/or more aggressive tumors. However, there is a lack of standardization on initial and subsequent margin size when treating these less common skin tumors, such as dermatofibrosarcoma protuberans (DFSP), atypical fibroxanthoma (AFX), and sebaceous carcinoma.
Because Mohs surgeons must balance normal tissue preservation with the importance of tumor clearance in the context of comprehensive margin control, we aimed to assess the practice patterns of Mohs surgeons regarding margin size for these unique tumors. The average margin size for each Mohs layer has been reported to be 1 to 3 mm for BCC compared with 3 to 6 mm or larger for other skin cancers, such as melanoma in situ (MIS).1-3 We hypothesized that the initial margin size would vary among surgeons and likely be greater for more aggressive and rarer malignancies as well as for lesions on the trunk and extremities.
Methods
A descriptive survey was created using SurveyMonkey and distributed to members of the American College of Mohs Surgery (ACMS). Survey participants and their responses were anonymous. Demographic information on survey participants was collected in addition to initial and subsequent MMS margin size for DFSP, AFX, MIS, invasive melanoma, sebaceous carcinoma, microcystic adnexal carcinoma (MAC), poorly differentiated SCC, Merkel cell carcinoma, extramammary Paget disease, leiomyosarcoma, and endocrine mucin-producing sweat gland carcinoma. Survey participants were asked to choose from a range of margin sizes: 1 to 3 mm, 4 to 6 mm, 7 to 9 mm, and greater than 9 mm. This study was approved by the University of Texas Southwest Medical Center (Dallas, Texas) institutional review board.
Results
Eighty-seven respondents from the ACMS listserve completed the survey (response rate <10%). Of these, 58 respondents (66.7%) reported practicing for more than 5 years, and 58 (66.7%) were male. Practice setting was primarily private/community (71.3% [62/87]), and survey respondents were located across the United States. More than 50% of survey respondents treated the following tumors on the head and neck in their respective practices: DFSP (80.9% [55/68]), AFX (95.6% [65/68]), MIS (67.7% [46/68]), sebaceous carcinoma (92.7% [63/68]), MAC (83.8% [57/68]), poorly differentiated SCC (97.1% [66/68]), and endocrine mucin-producing sweat gland carcinoma (51.5% [35/68]). More than 50% of survey respondents treated the following tumors on the trunk and extremities: DFSP (90.3% [47/52]), AFX (86.4% [45/52]), MIS (55.8% [29/52]), sebaceous carcinoma (80.8% [42/52]), MAC (73.1% [38/52]), poorly differentiated SCC (94.2% [49/52]), and extramammary Paget disease (53.9% [28/52]). Invasive melanoma, Merkel cell carcinoma, and leiomyosarcoma were overall less commonly treated.
In general, respondent Mohs surgeons were more likely to take larger initial and subsequent margins for tumors treated on the trunk and extremities compared with the head and neck (Table). In addition, initial margin size often was larger than the 1- to 3-mm margin commonly used in Mohs surgery for BCCs and less aggressive SCCs (Table). A larger initial margin size (>9 mm) and subsequent margin size (4–6 mm) was more commonly reported for certain tumors known to be more aggressive and/or have extensive subclinical extension, such as DFSP and invasive melanoma. Of note, most respondents performed 4- to 6-mm margins (37/67 [55.2%]) for poorly differentiated SCC. Overall, there was a high range of margin size variability among Mohs surgeons for these unique and/or more aggressive skin tumors.


Comment
Given that no guidelines exist on margins with MMS for less commonly treated skin tumors, this study helps give Mohs surgeons perspective on current practice patterns for both initial and subsequent Mohs margin sizes. High margin-size variability among Mohs surgeons is expected, as surgeons also need to account for high-risk features of the tumor or specific locations where tissue sparing is critical. Overall, Mohs surgeons are more likely to take larger initial margins for these less common skin tumors compared with BCCs or SCCs. Initial margin size was consistently larger on the trunk and extremities where tissue sparing often is less critical.
Our survey was limited by a small sample size and incomplete response of the ACMS membership. In addition, most respondents practiced in a private/community setting, which may have led to bias, as academic centers may manage rare malignancies more commonly and/or have increased access to immunostains and multispecialty care. Future registries for rare skin malignancies will hopefully be developed that will allow for further consensus on standardized margins. Additional studies on the average number of stages required to clear these less common tumors also are warranted.
- Muller FM, Dawe RS, Moseley H, et al. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma: tissue‐sparing outcome. Dermatol Surg. 2009;35:1349-1354.
- van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
- Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: a prospective multicenter study. J Am Acad Dermatol. 2019;81:767-774.
- Muller FM, Dawe RS, Moseley H, et al. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma: tissue‐sparing outcome. Dermatol Surg. 2009;35:1349-1354.
- van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
- Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: a prospective multicenter study. J Am Acad Dermatol. 2019;81:767-774.
Practice Points
- It is common for initial margin size for uncommon skin tumors to be larger than the 1 to 3 mm commonly used in Mohs surgery for basal cell carcinomas and less aggressive squamous cell carcinomas.
- Mohs surgeons commonly take larger starting and subsequent margins for uncommon skin tumors treated on the trunk and extremities compared with the head and neck.
Too old to practice medicine?
Unlike for many other professions, there is no age limit for practicing medicine. According to international standards, airplane pilots, for example, who are responsible for the safety of many human lives, must retire by the age of 60 if they work alone, or 65 if they have a copilot. In Brazil, however, this age limit does not exist for pilots or physicians.
The only restriction on professional practice within the medical context is the mandatory retirement imposed on medical professors who teach at public (state and federal) universities, starting at the age of 75. Nevertheless, these professionals can continue practicing administrative and research-related activities. After “expulsion,” as this mandatory retirement is often called, professors who stood out or contributed to the institution and science may receive the title of professor emeritus.
In the private sector, age limits are not formally set, but the hiring of middle-aged professionals is limited.
At the Heart Institute of the University of São Paulo (Brazil) School of Medicine Clinical Hospital (InCor/HCFMUSP), one of the world’s largest teaching and research centers for cardiovascular and pulmonary diseases, several octogenarian specialists lead studies and teams. One of these is Noedir Stolf, MD, an 82-year-old cardiovascular surgeon who operates almost every day and coordinates studies on transplants, mechanical circulatory support, and aortic surgery. There is also Protásio Lemos da Luz, MD, an 82-year-old clinical cardiologist who guides research on subjects including atherosclerosis, the endothelium, microbiota, and diabetes. The protective effect of wine on atherosclerosis is one of his best-known studies.
No longer working is also not in the cards for Angelita Habr-Gama, MD, who, at 89 years old, is one of the oldest physicians in current practice. With a career spanning more than 7 decades, she is a world reference in coloproctology. She was the first woman to become a surgical resident at the HCFMUSP, where she later founded the coloproctology specialty and created the first residency program for the specialty. In April 2022, Dr. Habr-Gama joined the ranks of the 100 most influential scientists in the world, nominated by researchers at Stanford (Calif.) University, and published in PLOS Biology.
In 2020, she was sedated, intubated, and hospitalized in the intensive care unit of the Oswaldo Cruz German Hospital for 54 days because of a SARS-CoV-2 infection. After her discharge, she went back to work in less than 10 days – and added chess classes to her routine. “To get up and go to work makes me very happy. Work is my greatest hobby. No one has ever heard me complain about my life,” Dr. Habr-Gama told this news organization after having rescheduled the interview twice because of emergency surgeries.
“Doctors have a professional longevity that does not exist for other professions in which the person retires and stops practicing their profession or goes on to do something else for entertainment. Doctors can retire from one place of employment or public practice and continue practicing medicine in the office as an administrator or consultant,” Ângelo Vattimo, first secretary of the state of São Paulo Regional Board of Medicine (CREMESP), stated. The board regularly organizes a ceremony to honor professionals who have been practicing for 50 years, awarding them a certificate and engraved medal. “Many of them are around 80 years old, working and teaching. This always makes us very happy. What profession has such exceptional compliance for so long?” said Mr. Vattimo.
In the medical field, the older the age range, the smaller the number of women. According to the 2020 Medical Demographics in Brazil survey, only 2 out of 10 practicing professionals older than 70 are women.
Not everyone over 80 has Dr. Habr-Gama’s vitality, because the impact of aging is not equal. “If you look at a group of 80-year-olds, there will be much more variability than within a group of 40-year-olds,” stated Mark Katlic, MD, chief of surgery at LifeBridge Health System in the United States, who has dedicated his life to studying the subject. Dr. Katlic spoke on the subject in an interview that was published in the article “How Old Is Too Old to Work as a Doctor?” published by this news organization in April of 2022. The article discusses the evaluations of elderly physicians’ skills and competences that U.S. companies conduct. The subject has been leading to profound debate.
Dr. Katlic defends screening programs for elderly physicians, which already are in effect at the company for which he works, LifeBridge Health, and various others in the United States. “We do [screen elderly physicians at LifeBridge Health], and so do a few dozen other [U.S. institutions], but there are hundreds [of health care institutions] that do not conduct this screening,” he pointed out.
Age-related assessment faces great resistance in the United States. One physician who is against the initiative is Frank Stockdale, MD, PhD, an 86-year-old practicing oncologist affiliated with Stanford (Calif.) University Health. “It’s age discrimination ... Physicians [in the United States] receive assessments throughout their careers as part of the accreditation process – there’s no need to change that as physicians reach a certain age,” Dr. Stockdale told this news organization.
The U.S. initiative of instituting physician assessment programs for those of a certain age has even been tested in court. According to an article published in Medscape, “in New Haven, Connecticut, for instance, the U.S. Equal Employment Opportunity Commission (EEOC) filed a suit in 2020 on behalf of the Yale New Haven Hospital staff, alleging a discriminatory late career practitioner policy.”
Also, according to the article, a similar case in Minnesota reached a settlement in 2021, providing monetary relief to staff impacted by out-of-pocket costs for the assessment, in addition to requiring that the hospital in question report to the EEOC any complaints related to age discrimination.
In Brazil, the subject is of interest to more than 34,571 physicians between 65 and 69 years of age and 34,237 physicians older than 70. In all, this population represents approximately 14.3% of the country’s active workforce, according to the 2020 Medical Demographics in Brazil survey.
The significant participation of health care professionals over age 50 in a survey conducted by this news organization to learn what physicians think about the age limit for practicing their professions is evidence that the subject is a present concern. Of a total of 1,641 participants, 57% were age 60 or older, 17% were between 50 and 59 years, and 12% were between 40 and 49 years. Among all participants, 51% were against these limitations, 17% approved of the idea for all specialties, and 32% believed the restriction was appropriate only for some specialties. Regarding the possibility of older physicians undergoing regular assessments, the opinions were divided: Thirty-one percent thought they should be assessed in all specialties. Furthermore, 31% believed that cognitive abilities should be regularly tested in all specialties, 31% thought this should take place for some specialties, and 38% were against this approach.
Professionals want to know, for example, how (and whether) advanced age can interfere with performance, what are the competences required to practice their activities, and if the criteria vary by specialty. “A psychiatrist doesn’t have to have perfect visual acuity, as required from a dermatologist, but it is important that they have good hearing, for example,” argued Clóvis Constantino, MD, former president of the São Paulo Regional Medical Board (CRM-SP) and former vice president of the Brazilian Federal Medical Board (CFM). “However, a surgeon has to stand for several hours in positions that may be uncomfortable. It’s not easy,” he told this news organization.
In the opinion of 82-year-old Henrique Klajner, MD, the oldest pediatrician in practice at the Albert Einstein Israeli Hospital in São Paulo, the physician cannot be subjected to the types of evaluations that have been applied in the United States. “Physicians should conduct constant self-evaluations to see if they have the competences and skills needed to practice their profession ... Moreover, this is not a matter of age. It is a matter of ethics,” said Dr. Klajner.
The ability to adapt to change and implement innovation is critical to professional longevity, he said. “Nowadays, when I admit patients, I no longer do hospital rounds, which requires a mobility equal to physical abuse for me. Therefore, I work with physicians who take care of my hospitalized patients.”
Dr. Klajner also feels there is a distinction between innovations learned through studies and what can be offered safely to patients. “If I have to care for a hospitalized patient with severe pneumonia, for example, since I am not up to date in this specialty, I am going to call upon a pulmonologist I trust and forgo my honorarium for this admission. But I will remain on the team, monitoring the patient’s progression,” he said.
During the COVID-19 pandemic, Dr. Klajner stopped seeing patients in person under the recommendation of his son, Sidney Klajner, MD, also a physician. The elder Dr. Klajner began exploring telemedicine, which opened a whole new world of possibilities. “I have conducted several online visits to provide educational instruction to mothers returning home post delivery, for example,” he told this news organization. The time to stop is not something that concerns Dr. Klajner. “I’m only going to stop when I have a really important reason to do so. For example, if I can no longer write or study, reading and rereading an article without being able to understand what is being said. At this time, none of that is happening.”
In the United States, as well as in Brazil, physicians rarely provide information to human resources departments on colleagues showing signs of cognitive or motor decline affecting their professional performance. “The expectation is that health care professionals will report colleagues with cognitive impairments, but that often does not happen,” Dr. Katlic said.
It is also not common for professionals to report their own deficits to their institutions. In large part, this is caused by a lack of well-defined policies for dealing with this issue. This news organization sought out several public and private hospitals in Brazil to see if there is any guidance on professional longevity: Most said that there is not. Only the A. C. Camargo Cancer Center reported, through its public relations team, that a committee is discussing the subject but that it is still in the early stages.
Brazilian specialist associations do not offer guidelines or instructions on the various aspects of professional longevity. Dr. Constantino tried to put the subject on the agenda during the years in which he was an administrator with the CFM. “We tried to open up discussions regarding truly elderly physicians, but the subject was not well received. I believe that it is precisely because there is a tradition of physicians working until they are no longer able that this is more difficult in Brazil ... No one exactly knows what to do in this respect.” Dr. Constantino is against the use of age as a criterion for quitting practice.
“Of course, this is a point that has to be considered, but I always defended the need for regular assessment of physicians, regardless of age range. And, although assessments are always welcome, in any profession, I also believe this would not be well received in Brazil.” He endorses an assessment of one’s knowledge and not of physical abilities, which are generally assessed through investigation when needed.
The absence of guidelines increases individual responsibility, as well as vulnerability. “Consciously, physicians will not put patients at risk if they do not have the competence to care for them or to perform a surgical procedure,” said Clystenes Odyr Soares Silva, MD, PhD, adjunct professor of pulmonology of the Federal University of São Paulo (Brazil) School of Medicine (UNIFESP). “Your peers will tell you if you are no longer able,” he added. The problem is that physicians rarely admit to or talk about their colleagues’ deficits, especially if they are in the spotlight because of advanced age. In this situation, the observation and opinion of family members regarding the health care professional’s competences and skills will hold more weight.
In case of health-related physical impairment, such as partial loss of hand movement, for example, “it is expected that this will set off an ethical warning in the person,” said Dr. Constantino. When this warning does not occur naturally, patients or colleagues can report the professional, and this may lead to the opening of an administrative investigation. If the report is found to be true, this investigation is used to suspend physicians who do not have the physical or mental ability to continue practicing medicine.
“If it’s something very serious, the physician’s license can be temporarily suspended while [the physician] is treated by a psychiatrist, with follow-up by the professional board. When discharged, the physician will get his or her [professional] license back and can go back to work,” Dr. Constantino explained. If an expert evaluation is needed, the physician will then be assessed by a forensic psychiatrist. One of the most in-demand forensic psychiatrists in Brazil is Guido Arturo Palomba, MD, 73 years old. “I have assessed some physicians for actions reported to see if they were normal people or not, but never for circumstances related to age,” Dr. Palomba said.
In practice, Brazilian medical entities do not have policies or programs to guide physicians who wish to grow old while they work or those who have started to notice they are not performing as they used to. “We have never lived as long; therefore, the quality of life in old age, as well as the concept of aging, are some of the most relevant questions of our time. These are subjects requiring additional discussion, broadening understanding and awareness in this regard,” observed Mr. Vattimo.
Dr. Constantino and Dr. Silva, who are completely against age-based assessments, believe that recertification of the specialist license every 5 years is the best path to confirming whether the physician is still able to practice. “A knowledge-based test every 5 years to recertify the specialist license has often been a topic of conversation. I think it’s an excellent idea. The person would provide a dossier of all they have done in terms of courses, conferences, and other activities, present it, and receive a score,” said Dr. Silva.
In practice, recertification of the specialist license is a topic of discussion that has been raised for years, and it is an idea that the Brazilian Medical Association (AMB) defends. In conjunction with the CFM, the association is studying a way to best implement this assessment. “It’s important to emphasize that this measure would not be retroactive at first. Instead, it would only be in effect for professionals licensed after the recertification requirement is established,” the AMB pointed out in a note sent to this news organization. Even so, the measure has faced significant resistance from a faction of the profession, and its enactment does not seem to be imminent.
The debate regarding professional longevity is taking place in various countries. In 2021, the American Medical Association Council on Medical Education released a report with a set of guidelines for the screening and assessment of physicians. The document is the product of a committee created in 2015 to study the subject. The AMA recommends that the assessment of elderly physicians be based on evidence and ethical, relevant, fair, equitable, transparent, verifiable, nonexhaustive principles, contemplating support and protecting against legal proceedings. In April of this year, a new AMA document highlighted the same principles.
Also in the United States, one of oldest initiatives created to support physicians in the process of recycling, the University of California San Diego Physician Assessment and Clinical Education Program (PACE), has a section focusing on the extended practice of medicine (Practicing Medicine Longer). For those wanting to learn more about discussions on this subject, there are online presentations on experiences in Quebec and Ontario with assessing aging physicians, neuropsychological perspectives on the aging medical population, and what to expect of healthy aging, among other subjects.
Created in 1996, PACE mostly provides services to physicians who need to address requirements of the state medical boards. Few physicians enroll on their own.
The first part of the program assesses knowledge and skills over approximately 2 days. In the second phase, the physician participates in a series of activities in a corresponding residency program. Depending on the results, the physician may have to go through a remedial program with varying activities to deal with performance deficiencies to clinical experiences at the residency level.
A version of this article first appeared on Medscape.com.
Unlike for many other professions, there is no age limit for practicing medicine. According to international standards, airplane pilots, for example, who are responsible for the safety of many human lives, must retire by the age of 60 if they work alone, or 65 if they have a copilot. In Brazil, however, this age limit does not exist for pilots or physicians.
The only restriction on professional practice within the medical context is the mandatory retirement imposed on medical professors who teach at public (state and federal) universities, starting at the age of 75. Nevertheless, these professionals can continue practicing administrative and research-related activities. After “expulsion,” as this mandatory retirement is often called, professors who stood out or contributed to the institution and science may receive the title of professor emeritus.
In the private sector, age limits are not formally set, but the hiring of middle-aged professionals is limited.
At the Heart Institute of the University of São Paulo (Brazil) School of Medicine Clinical Hospital (InCor/HCFMUSP), one of the world’s largest teaching and research centers for cardiovascular and pulmonary diseases, several octogenarian specialists lead studies and teams. One of these is Noedir Stolf, MD, an 82-year-old cardiovascular surgeon who operates almost every day and coordinates studies on transplants, mechanical circulatory support, and aortic surgery. There is also Protásio Lemos da Luz, MD, an 82-year-old clinical cardiologist who guides research on subjects including atherosclerosis, the endothelium, microbiota, and diabetes. The protective effect of wine on atherosclerosis is one of his best-known studies.
No longer working is also not in the cards for Angelita Habr-Gama, MD, who, at 89 years old, is one of the oldest physicians in current practice. With a career spanning more than 7 decades, she is a world reference in coloproctology. She was the first woman to become a surgical resident at the HCFMUSP, where she later founded the coloproctology specialty and created the first residency program for the specialty. In April 2022, Dr. Habr-Gama joined the ranks of the 100 most influential scientists in the world, nominated by researchers at Stanford (Calif.) University, and published in PLOS Biology.
In 2020, she was sedated, intubated, and hospitalized in the intensive care unit of the Oswaldo Cruz German Hospital for 54 days because of a SARS-CoV-2 infection. After her discharge, she went back to work in less than 10 days – and added chess classes to her routine. “To get up and go to work makes me very happy. Work is my greatest hobby. No one has ever heard me complain about my life,” Dr. Habr-Gama told this news organization after having rescheduled the interview twice because of emergency surgeries.
“Doctors have a professional longevity that does not exist for other professions in which the person retires and stops practicing their profession or goes on to do something else for entertainment. Doctors can retire from one place of employment or public practice and continue practicing medicine in the office as an administrator or consultant,” Ângelo Vattimo, first secretary of the state of São Paulo Regional Board of Medicine (CREMESP), stated. The board regularly organizes a ceremony to honor professionals who have been practicing for 50 years, awarding them a certificate and engraved medal. “Many of them are around 80 years old, working and teaching. This always makes us very happy. What profession has such exceptional compliance for so long?” said Mr. Vattimo.
In the medical field, the older the age range, the smaller the number of women. According to the 2020 Medical Demographics in Brazil survey, only 2 out of 10 practicing professionals older than 70 are women.
Not everyone over 80 has Dr. Habr-Gama’s vitality, because the impact of aging is not equal. “If you look at a group of 80-year-olds, there will be much more variability than within a group of 40-year-olds,” stated Mark Katlic, MD, chief of surgery at LifeBridge Health System in the United States, who has dedicated his life to studying the subject. Dr. Katlic spoke on the subject in an interview that was published in the article “How Old Is Too Old to Work as a Doctor?” published by this news organization in April of 2022. The article discusses the evaluations of elderly physicians’ skills and competences that U.S. companies conduct. The subject has been leading to profound debate.
Dr. Katlic defends screening programs for elderly physicians, which already are in effect at the company for which he works, LifeBridge Health, and various others in the United States. “We do [screen elderly physicians at LifeBridge Health], and so do a few dozen other [U.S. institutions], but there are hundreds [of health care institutions] that do not conduct this screening,” he pointed out.
Age-related assessment faces great resistance in the United States. One physician who is against the initiative is Frank Stockdale, MD, PhD, an 86-year-old practicing oncologist affiliated with Stanford (Calif.) University Health. “It’s age discrimination ... Physicians [in the United States] receive assessments throughout their careers as part of the accreditation process – there’s no need to change that as physicians reach a certain age,” Dr. Stockdale told this news organization.
The U.S. initiative of instituting physician assessment programs for those of a certain age has even been tested in court. According to an article published in Medscape, “in New Haven, Connecticut, for instance, the U.S. Equal Employment Opportunity Commission (EEOC) filed a suit in 2020 on behalf of the Yale New Haven Hospital staff, alleging a discriminatory late career practitioner policy.”
Also, according to the article, a similar case in Minnesota reached a settlement in 2021, providing monetary relief to staff impacted by out-of-pocket costs for the assessment, in addition to requiring that the hospital in question report to the EEOC any complaints related to age discrimination.
In Brazil, the subject is of interest to more than 34,571 physicians between 65 and 69 years of age and 34,237 physicians older than 70. In all, this population represents approximately 14.3% of the country’s active workforce, according to the 2020 Medical Demographics in Brazil survey.
The significant participation of health care professionals over age 50 in a survey conducted by this news organization to learn what physicians think about the age limit for practicing their professions is evidence that the subject is a present concern. Of a total of 1,641 participants, 57% were age 60 or older, 17% were between 50 and 59 years, and 12% were between 40 and 49 years. Among all participants, 51% were against these limitations, 17% approved of the idea for all specialties, and 32% believed the restriction was appropriate only for some specialties. Regarding the possibility of older physicians undergoing regular assessments, the opinions were divided: Thirty-one percent thought they should be assessed in all specialties. Furthermore, 31% believed that cognitive abilities should be regularly tested in all specialties, 31% thought this should take place for some specialties, and 38% were against this approach.
Professionals want to know, for example, how (and whether) advanced age can interfere with performance, what are the competences required to practice their activities, and if the criteria vary by specialty. “A psychiatrist doesn’t have to have perfect visual acuity, as required from a dermatologist, but it is important that they have good hearing, for example,” argued Clóvis Constantino, MD, former president of the São Paulo Regional Medical Board (CRM-SP) and former vice president of the Brazilian Federal Medical Board (CFM). “However, a surgeon has to stand for several hours in positions that may be uncomfortable. It’s not easy,” he told this news organization.
In the opinion of 82-year-old Henrique Klajner, MD, the oldest pediatrician in practice at the Albert Einstein Israeli Hospital in São Paulo, the physician cannot be subjected to the types of evaluations that have been applied in the United States. “Physicians should conduct constant self-evaluations to see if they have the competences and skills needed to practice their profession ... Moreover, this is not a matter of age. It is a matter of ethics,” said Dr. Klajner.
The ability to adapt to change and implement innovation is critical to professional longevity, he said. “Nowadays, when I admit patients, I no longer do hospital rounds, which requires a mobility equal to physical abuse for me. Therefore, I work with physicians who take care of my hospitalized patients.”
Dr. Klajner also feels there is a distinction between innovations learned through studies and what can be offered safely to patients. “If I have to care for a hospitalized patient with severe pneumonia, for example, since I am not up to date in this specialty, I am going to call upon a pulmonologist I trust and forgo my honorarium for this admission. But I will remain on the team, monitoring the patient’s progression,” he said.
During the COVID-19 pandemic, Dr. Klajner stopped seeing patients in person under the recommendation of his son, Sidney Klajner, MD, also a physician. The elder Dr. Klajner began exploring telemedicine, which opened a whole new world of possibilities. “I have conducted several online visits to provide educational instruction to mothers returning home post delivery, for example,” he told this news organization. The time to stop is not something that concerns Dr. Klajner. “I’m only going to stop when I have a really important reason to do so. For example, if I can no longer write or study, reading and rereading an article without being able to understand what is being said. At this time, none of that is happening.”
In the United States, as well as in Brazil, physicians rarely provide information to human resources departments on colleagues showing signs of cognitive or motor decline affecting their professional performance. “The expectation is that health care professionals will report colleagues with cognitive impairments, but that often does not happen,” Dr. Katlic said.
It is also not common for professionals to report their own deficits to their institutions. In large part, this is caused by a lack of well-defined policies for dealing with this issue. This news organization sought out several public and private hospitals in Brazil to see if there is any guidance on professional longevity: Most said that there is not. Only the A. C. Camargo Cancer Center reported, through its public relations team, that a committee is discussing the subject but that it is still in the early stages.
Brazilian specialist associations do not offer guidelines or instructions on the various aspects of professional longevity. Dr. Constantino tried to put the subject on the agenda during the years in which he was an administrator with the CFM. “We tried to open up discussions regarding truly elderly physicians, but the subject was not well received. I believe that it is precisely because there is a tradition of physicians working until they are no longer able that this is more difficult in Brazil ... No one exactly knows what to do in this respect.” Dr. Constantino is against the use of age as a criterion for quitting practice.
“Of course, this is a point that has to be considered, but I always defended the need for regular assessment of physicians, regardless of age range. And, although assessments are always welcome, in any profession, I also believe this would not be well received in Brazil.” He endorses an assessment of one’s knowledge and not of physical abilities, which are generally assessed through investigation when needed.
The absence of guidelines increases individual responsibility, as well as vulnerability. “Consciously, physicians will not put patients at risk if they do not have the competence to care for them or to perform a surgical procedure,” said Clystenes Odyr Soares Silva, MD, PhD, adjunct professor of pulmonology of the Federal University of São Paulo (Brazil) School of Medicine (UNIFESP). “Your peers will tell you if you are no longer able,” he added. The problem is that physicians rarely admit to or talk about their colleagues’ deficits, especially if they are in the spotlight because of advanced age. In this situation, the observation and opinion of family members regarding the health care professional’s competences and skills will hold more weight.
In case of health-related physical impairment, such as partial loss of hand movement, for example, “it is expected that this will set off an ethical warning in the person,” said Dr. Constantino. When this warning does not occur naturally, patients or colleagues can report the professional, and this may lead to the opening of an administrative investigation. If the report is found to be true, this investigation is used to suspend physicians who do not have the physical or mental ability to continue practicing medicine.
“If it’s something very serious, the physician’s license can be temporarily suspended while [the physician] is treated by a psychiatrist, with follow-up by the professional board. When discharged, the physician will get his or her [professional] license back and can go back to work,” Dr. Constantino explained. If an expert evaluation is needed, the physician will then be assessed by a forensic psychiatrist. One of the most in-demand forensic psychiatrists in Brazil is Guido Arturo Palomba, MD, 73 years old. “I have assessed some physicians for actions reported to see if they were normal people or not, but never for circumstances related to age,” Dr. Palomba said.
In practice, Brazilian medical entities do not have policies or programs to guide physicians who wish to grow old while they work or those who have started to notice they are not performing as they used to. “We have never lived as long; therefore, the quality of life in old age, as well as the concept of aging, are some of the most relevant questions of our time. These are subjects requiring additional discussion, broadening understanding and awareness in this regard,” observed Mr. Vattimo.
Dr. Constantino and Dr. Silva, who are completely against age-based assessments, believe that recertification of the specialist license every 5 years is the best path to confirming whether the physician is still able to practice. “A knowledge-based test every 5 years to recertify the specialist license has often been a topic of conversation. I think it’s an excellent idea. The person would provide a dossier of all they have done in terms of courses, conferences, and other activities, present it, and receive a score,” said Dr. Silva.
In practice, recertification of the specialist license is a topic of discussion that has been raised for years, and it is an idea that the Brazilian Medical Association (AMB) defends. In conjunction with the CFM, the association is studying a way to best implement this assessment. “It’s important to emphasize that this measure would not be retroactive at first. Instead, it would only be in effect for professionals licensed after the recertification requirement is established,” the AMB pointed out in a note sent to this news organization. Even so, the measure has faced significant resistance from a faction of the profession, and its enactment does not seem to be imminent.
The debate regarding professional longevity is taking place in various countries. In 2021, the American Medical Association Council on Medical Education released a report with a set of guidelines for the screening and assessment of physicians. The document is the product of a committee created in 2015 to study the subject. The AMA recommends that the assessment of elderly physicians be based on evidence and ethical, relevant, fair, equitable, transparent, verifiable, nonexhaustive principles, contemplating support and protecting against legal proceedings. In April of this year, a new AMA document highlighted the same principles.
Also in the United States, one of oldest initiatives created to support physicians in the process of recycling, the University of California San Diego Physician Assessment and Clinical Education Program (PACE), has a section focusing on the extended practice of medicine (Practicing Medicine Longer). For those wanting to learn more about discussions on this subject, there are online presentations on experiences in Quebec and Ontario with assessing aging physicians, neuropsychological perspectives on the aging medical population, and what to expect of healthy aging, among other subjects.
Created in 1996, PACE mostly provides services to physicians who need to address requirements of the state medical boards. Few physicians enroll on their own.
The first part of the program assesses knowledge and skills over approximately 2 days. In the second phase, the physician participates in a series of activities in a corresponding residency program. Depending on the results, the physician may have to go through a remedial program with varying activities to deal with performance deficiencies to clinical experiences at the residency level.
A version of this article first appeared on Medscape.com.
Unlike for many other professions, there is no age limit for practicing medicine. According to international standards, airplane pilots, for example, who are responsible for the safety of many human lives, must retire by the age of 60 if they work alone, or 65 if they have a copilot. In Brazil, however, this age limit does not exist for pilots or physicians.
The only restriction on professional practice within the medical context is the mandatory retirement imposed on medical professors who teach at public (state and federal) universities, starting at the age of 75. Nevertheless, these professionals can continue practicing administrative and research-related activities. After “expulsion,” as this mandatory retirement is often called, professors who stood out or contributed to the institution and science may receive the title of professor emeritus.
In the private sector, age limits are not formally set, but the hiring of middle-aged professionals is limited.
At the Heart Institute of the University of São Paulo (Brazil) School of Medicine Clinical Hospital (InCor/HCFMUSP), one of the world’s largest teaching and research centers for cardiovascular and pulmonary diseases, several octogenarian specialists lead studies and teams. One of these is Noedir Stolf, MD, an 82-year-old cardiovascular surgeon who operates almost every day and coordinates studies on transplants, mechanical circulatory support, and aortic surgery. There is also Protásio Lemos da Luz, MD, an 82-year-old clinical cardiologist who guides research on subjects including atherosclerosis, the endothelium, microbiota, and diabetes. The protective effect of wine on atherosclerosis is one of his best-known studies.
No longer working is also not in the cards for Angelita Habr-Gama, MD, who, at 89 years old, is one of the oldest physicians in current practice. With a career spanning more than 7 decades, she is a world reference in coloproctology. She was the first woman to become a surgical resident at the HCFMUSP, where she later founded the coloproctology specialty and created the first residency program for the specialty. In April 2022, Dr. Habr-Gama joined the ranks of the 100 most influential scientists in the world, nominated by researchers at Stanford (Calif.) University, and published in PLOS Biology.
In 2020, she was sedated, intubated, and hospitalized in the intensive care unit of the Oswaldo Cruz German Hospital for 54 days because of a SARS-CoV-2 infection. After her discharge, she went back to work in less than 10 days – and added chess classes to her routine. “To get up and go to work makes me very happy. Work is my greatest hobby. No one has ever heard me complain about my life,” Dr. Habr-Gama told this news organization after having rescheduled the interview twice because of emergency surgeries.
“Doctors have a professional longevity that does not exist for other professions in which the person retires and stops practicing their profession or goes on to do something else for entertainment. Doctors can retire from one place of employment or public practice and continue practicing medicine in the office as an administrator or consultant,” Ângelo Vattimo, first secretary of the state of São Paulo Regional Board of Medicine (CREMESP), stated. The board regularly organizes a ceremony to honor professionals who have been practicing for 50 years, awarding them a certificate and engraved medal. “Many of them are around 80 years old, working and teaching. This always makes us very happy. What profession has such exceptional compliance for so long?” said Mr. Vattimo.
In the medical field, the older the age range, the smaller the number of women. According to the 2020 Medical Demographics in Brazil survey, only 2 out of 10 practicing professionals older than 70 are women.
Not everyone over 80 has Dr. Habr-Gama’s vitality, because the impact of aging is not equal. “If you look at a group of 80-year-olds, there will be much more variability than within a group of 40-year-olds,” stated Mark Katlic, MD, chief of surgery at LifeBridge Health System in the United States, who has dedicated his life to studying the subject. Dr. Katlic spoke on the subject in an interview that was published in the article “How Old Is Too Old to Work as a Doctor?” published by this news organization in April of 2022. The article discusses the evaluations of elderly physicians’ skills and competences that U.S. companies conduct. The subject has been leading to profound debate.
Dr. Katlic defends screening programs for elderly physicians, which already are in effect at the company for which he works, LifeBridge Health, and various others in the United States. “We do [screen elderly physicians at LifeBridge Health], and so do a few dozen other [U.S. institutions], but there are hundreds [of health care institutions] that do not conduct this screening,” he pointed out.
Age-related assessment faces great resistance in the United States. One physician who is against the initiative is Frank Stockdale, MD, PhD, an 86-year-old practicing oncologist affiliated with Stanford (Calif.) University Health. “It’s age discrimination ... Physicians [in the United States] receive assessments throughout their careers as part of the accreditation process – there’s no need to change that as physicians reach a certain age,” Dr. Stockdale told this news organization.
The U.S. initiative of instituting physician assessment programs for those of a certain age has even been tested in court. According to an article published in Medscape, “in New Haven, Connecticut, for instance, the U.S. Equal Employment Opportunity Commission (EEOC) filed a suit in 2020 on behalf of the Yale New Haven Hospital staff, alleging a discriminatory late career practitioner policy.”
Also, according to the article, a similar case in Minnesota reached a settlement in 2021, providing monetary relief to staff impacted by out-of-pocket costs for the assessment, in addition to requiring that the hospital in question report to the EEOC any complaints related to age discrimination.
In Brazil, the subject is of interest to more than 34,571 physicians between 65 and 69 years of age and 34,237 physicians older than 70. In all, this population represents approximately 14.3% of the country’s active workforce, according to the 2020 Medical Demographics in Brazil survey.
The significant participation of health care professionals over age 50 in a survey conducted by this news organization to learn what physicians think about the age limit for practicing their professions is evidence that the subject is a present concern. Of a total of 1,641 participants, 57% were age 60 or older, 17% were between 50 and 59 years, and 12% were between 40 and 49 years. Among all participants, 51% were against these limitations, 17% approved of the idea for all specialties, and 32% believed the restriction was appropriate only for some specialties. Regarding the possibility of older physicians undergoing regular assessments, the opinions were divided: Thirty-one percent thought they should be assessed in all specialties. Furthermore, 31% believed that cognitive abilities should be regularly tested in all specialties, 31% thought this should take place for some specialties, and 38% were against this approach.
Professionals want to know, for example, how (and whether) advanced age can interfere with performance, what are the competences required to practice their activities, and if the criteria vary by specialty. “A psychiatrist doesn’t have to have perfect visual acuity, as required from a dermatologist, but it is important that they have good hearing, for example,” argued Clóvis Constantino, MD, former president of the São Paulo Regional Medical Board (CRM-SP) and former vice president of the Brazilian Federal Medical Board (CFM). “However, a surgeon has to stand for several hours in positions that may be uncomfortable. It’s not easy,” he told this news organization.
In the opinion of 82-year-old Henrique Klajner, MD, the oldest pediatrician in practice at the Albert Einstein Israeli Hospital in São Paulo, the physician cannot be subjected to the types of evaluations that have been applied in the United States. “Physicians should conduct constant self-evaluations to see if they have the competences and skills needed to practice their profession ... Moreover, this is not a matter of age. It is a matter of ethics,” said Dr. Klajner.
The ability to adapt to change and implement innovation is critical to professional longevity, he said. “Nowadays, when I admit patients, I no longer do hospital rounds, which requires a mobility equal to physical abuse for me. Therefore, I work with physicians who take care of my hospitalized patients.”
Dr. Klajner also feels there is a distinction between innovations learned through studies and what can be offered safely to patients. “If I have to care for a hospitalized patient with severe pneumonia, for example, since I am not up to date in this specialty, I am going to call upon a pulmonologist I trust and forgo my honorarium for this admission. But I will remain on the team, monitoring the patient’s progression,” he said.
During the COVID-19 pandemic, Dr. Klajner stopped seeing patients in person under the recommendation of his son, Sidney Klajner, MD, also a physician. The elder Dr. Klajner began exploring telemedicine, which opened a whole new world of possibilities. “I have conducted several online visits to provide educational instruction to mothers returning home post delivery, for example,” he told this news organization. The time to stop is not something that concerns Dr. Klajner. “I’m only going to stop when I have a really important reason to do so. For example, if I can no longer write or study, reading and rereading an article without being able to understand what is being said. At this time, none of that is happening.”
In the United States, as well as in Brazil, physicians rarely provide information to human resources departments on colleagues showing signs of cognitive or motor decline affecting their professional performance. “The expectation is that health care professionals will report colleagues with cognitive impairments, but that often does not happen,” Dr. Katlic said.
It is also not common for professionals to report their own deficits to their institutions. In large part, this is caused by a lack of well-defined policies for dealing with this issue. This news organization sought out several public and private hospitals in Brazil to see if there is any guidance on professional longevity: Most said that there is not. Only the A. C. Camargo Cancer Center reported, through its public relations team, that a committee is discussing the subject but that it is still in the early stages.
Brazilian specialist associations do not offer guidelines or instructions on the various aspects of professional longevity. Dr. Constantino tried to put the subject on the agenda during the years in which he was an administrator with the CFM. “We tried to open up discussions regarding truly elderly physicians, but the subject was not well received. I believe that it is precisely because there is a tradition of physicians working until they are no longer able that this is more difficult in Brazil ... No one exactly knows what to do in this respect.” Dr. Constantino is against the use of age as a criterion for quitting practice.
“Of course, this is a point that has to be considered, but I always defended the need for regular assessment of physicians, regardless of age range. And, although assessments are always welcome, in any profession, I also believe this would not be well received in Brazil.” He endorses an assessment of one’s knowledge and not of physical abilities, which are generally assessed through investigation when needed.
The absence of guidelines increases individual responsibility, as well as vulnerability. “Consciously, physicians will not put patients at risk if they do not have the competence to care for them or to perform a surgical procedure,” said Clystenes Odyr Soares Silva, MD, PhD, adjunct professor of pulmonology of the Federal University of São Paulo (Brazil) School of Medicine (UNIFESP). “Your peers will tell you if you are no longer able,” he added. The problem is that physicians rarely admit to or talk about their colleagues’ deficits, especially if they are in the spotlight because of advanced age. In this situation, the observation and opinion of family members regarding the health care professional’s competences and skills will hold more weight.
In case of health-related physical impairment, such as partial loss of hand movement, for example, “it is expected that this will set off an ethical warning in the person,” said Dr. Constantino. When this warning does not occur naturally, patients or colleagues can report the professional, and this may lead to the opening of an administrative investigation. If the report is found to be true, this investigation is used to suspend physicians who do not have the physical or mental ability to continue practicing medicine.
“If it’s something very serious, the physician’s license can be temporarily suspended while [the physician] is treated by a psychiatrist, with follow-up by the professional board. When discharged, the physician will get his or her [professional] license back and can go back to work,” Dr. Constantino explained. If an expert evaluation is needed, the physician will then be assessed by a forensic psychiatrist. One of the most in-demand forensic psychiatrists in Brazil is Guido Arturo Palomba, MD, 73 years old. “I have assessed some physicians for actions reported to see if they were normal people or not, but never for circumstances related to age,” Dr. Palomba said.
In practice, Brazilian medical entities do not have policies or programs to guide physicians who wish to grow old while they work or those who have started to notice they are not performing as they used to. “We have never lived as long; therefore, the quality of life in old age, as well as the concept of aging, are some of the most relevant questions of our time. These are subjects requiring additional discussion, broadening understanding and awareness in this regard,” observed Mr. Vattimo.
Dr. Constantino and Dr. Silva, who are completely against age-based assessments, believe that recertification of the specialist license every 5 years is the best path to confirming whether the physician is still able to practice. “A knowledge-based test every 5 years to recertify the specialist license has often been a topic of conversation. I think it’s an excellent idea. The person would provide a dossier of all they have done in terms of courses, conferences, and other activities, present it, and receive a score,” said Dr. Silva.
In practice, recertification of the specialist license is a topic of discussion that has been raised for years, and it is an idea that the Brazilian Medical Association (AMB) defends. In conjunction with the CFM, the association is studying a way to best implement this assessment. “It’s important to emphasize that this measure would not be retroactive at first. Instead, it would only be in effect for professionals licensed after the recertification requirement is established,” the AMB pointed out in a note sent to this news organization. Even so, the measure has faced significant resistance from a faction of the profession, and its enactment does not seem to be imminent.
The debate regarding professional longevity is taking place in various countries. In 2021, the American Medical Association Council on Medical Education released a report with a set of guidelines for the screening and assessment of physicians. The document is the product of a committee created in 2015 to study the subject. The AMA recommends that the assessment of elderly physicians be based on evidence and ethical, relevant, fair, equitable, transparent, verifiable, nonexhaustive principles, contemplating support and protecting against legal proceedings. In April of this year, a new AMA document highlighted the same principles.
Also in the United States, one of oldest initiatives created to support physicians in the process of recycling, the University of California San Diego Physician Assessment and Clinical Education Program (PACE), has a section focusing on the extended practice of medicine (Practicing Medicine Longer). For those wanting to learn more about discussions on this subject, there are online presentations on experiences in Quebec and Ontario with assessing aging physicians, neuropsychological perspectives on the aging medical population, and what to expect of healthy aging, among other subjects.
Created in 1996, PACE mostly provides services to physicians who need to address requirements of the state medical boards. Few physicians enroll on their own.
The first part of the program assesses knowledge and skills over approximately 2 days. In the second phase, the physician participates in a series of activities in a corresponding residency program. Depending on the results, the physician may have to go through a remedial program with varying activities to deal with performance deficiencies to clinical experiences at the residency level.
A version of this article first appeared on Medscape.com.
Analysis of PsA guidelines reveals much room for improvement on conflicts of interest
, according to a retrospective analysis of all authors on the most recent guidelines issued by the American College of Rheumatology (ACR) and the Japanese Dermatological Association (JDA).
In addition to finding that the majority of the authors of psoriatic arthritis (PsA) clinical practice guidelines (CPGs) issued by the JDA and ACR received substantial personal payments from pharmaceutical companies before and during CPG development, researchers led by Hanano Mamada and Anju Murayama of the Medical Governance Research Institute, Tokyo, wrote in Arthritis Care & Research that “several CPG authors self-cited their articles without the disclosure of NFCOI [nonfinancial conflicts of interest], and most of the recommendations were based on low or very low quality of evidence. Although the COI policies used by JDA and ACR are clearly inadequate, no significant revisions have been made for the last 3 years.”
Based on their findings, which were made using payment data from major Japanese pharmaceutical companies and the U.S. Open Payments Database from 2016 to 2018, the researchers suggested that the medical societies should:
- Adopt global standard COI policies from organizations such as the National Academy of Medicine and Guidelines International Network, including a 3-year lookback period for COI declaration.
- Consider a comprehensive definition and rigorous management with full disclosure of NFCOI.
- Publish a list of authors making each recommendation to grasp the implications of COI in clinical practice guidelines.
- Mention the detailed date of the COI disclosure, which should be close to the publication date as much as possible.
Financial conflicts of interest
The researchers used payment data published between 2016 and 2018 for all 83 companies belonging to the Japan Pharmaceutical Manufacturers Association, focusing on personal payments (for lecturing, writing, and consultancy) and excluding research payments, “since in Japan, the name, institution, and position of the author or researcher who received the research payment is not disclosed, which makes assessing research payments difficult.” To evaluate authors’ FCOI in the ACR’s CPG, the researchers analyzed the U.S. Open Payments Database “for all categories of general payments such as speaking, consulting, meals, and travel expenses 3 years from before the guideline’s first online publication on November 30, 2018.”
The 2018 ACR/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis had 36 authors and the JDA’s Clinical Practice Guideline for the Treatment of Psoriatic Arthritis 2019 had 23. Overall, 61% of JDA authors and half of ACR authors voluntarily declared FCOI with pharmaceutical companies; 25 of the ACR authors were U.S. physicians and could be included in the Open Payments Database search.
A total of 21 (91.3%) JDA authors and 21 (84.0%) ACR authors received at least one payment, with the combined total of $3,335,413 and $4,081,629 payments, respectively, over the 3 years. The average and median personal payments were $145,018 and $123,876 for JDA authors and $162,825 and $58,826 for ACR authors. When the payments to ACR authors were limited to lecturing, writing, and consulting fees that are required under the ACR’s COI policy, the mean was $130,102 and median was $39,375. The corresponding payments for JDA authors were $123,876 and $8,170, respectively,
The researchers found undisclosed payments for more than three-quarters of physician authors of the Japanese guideline, and nearly half of the doctors authoring the American guideline had undisclosed payments. These added up to $474,000 for the JDA, which amounted to 38% of the total for personal payments that must be reported to the JDA based on its COI policy for clinical practice guidelines, and $218,000 for the ACR, amounting to 18% of the total for personal payments that must be reported to the society based on its COI policy.
Of the 11 ACR authors who were not eligible for the U.S. Open Payments Database search, 5 declared FCOI with pharmaceutical companies in the guideline, meaning that 26 (72%) of the 36 authors had FCOI with pharmaceutical companies.
The ACR only required authors to declare FCOI covering 1 year before and during guideline development, and although the JDA required authors to declare their FCOI for the past 3 years of guideline development, the study authors noted that the JDA guideline disclosed them for only 2 years (between Jan. 1, 2017, and Dec. 31, 2018).
“It is true that influential doctors such as clinical practice guideline authors tend to receive various types of payments from pharmaceutical companies and that it is difficult to conduct research without funding from pharmaceutical companies. However, our current research mainly focuses on personal payments from pharmaceutical companies such as lecture fees and consulting fees. These payments are recognized as pocket money and are not used for research. Thus, it is questionable that the observed relationships are something evitable,” the researchers wrote.
Nonfinancial conflicts of interest
Many authors of the ACR’s CPG and the JDA’s CPG also had NFCOI, defined objectively in this study as self-citation rate. NFCOI have been more broadly defined by the International Committee of Medical Journal Editors (ICMJE) as “conflicts, such as personal relationships or rivalries, academic competition, and intellectual beliefs”; the ICMJE recommends reporting NFCOI on its COI form.
The JDA guideline included self-citations by 78% of its authors, compared with 32% of the ACR guideline authors, but this weighed differently among the two guidelines in that only 12 of the 354 (3.4%) citations in the JDA guideline were self-cited, compared with 46 of 137 (34%) citations in the ACR guideline.
The researchers noted that while the self-citation rates between JDA and ACR authors “differed remarkably,” the impact of ACR authors on CPG recommendations was much more direct. Three-quarters of JDA authors’ self-cited articles were about observational studies, whereas 52% of the ACR authors’ self-cited articles were clinical trials, most of which were randomized, controlled studies, and these NFCOI were not disclosed in the guideline.
Half of the strong recommendations in the JDA guideline were based on low or very low quality of evidence, whereas the ACR guideline had no strong recommendations based on low or very low quality of evidence.
This study was supported by the nonprofit Medical Governance Research Institute, which receives donations from Ain Pharmacies Inc., other organizations, and private individuals. The study also received support from the Tansa (formerly known as the Waseda Chronicle), an independent nonprofit news organization dedicated to investigative journalism. Three authors reported receiving personal fees from several pharmaceutical companies for work outside of the scope of this study.
, according to a retrospective analysis of all authors on the most recent guidelines issued by the American College of Rheumatology (ACR) and the Japanese Dermatological Association (JDA).
In addition to finding that the majority of the authors of psoriatic arthritis (PsA) clinical practice guidelines (CPGs) issued by the JDA and ACR received substantial personal payments from pharmaceutical companies before and during CPG development, researchers led by Hanano Mamada and Anju Murayama of the Medical Governance Research Institute, Tokyo, wrote in Arthritis Care & Research that “several CPG authors self-cited their articles without the disclosure of NFCOI [nonfinancial conflicts of interest], and most of the recommendations were based on low or very low quality of evidence. Although the COI policies used by JDA and ACR are clearly inadequate, no significant revisions have been made for the last 3 years.”
Based on their findings, which were made using payment data from major Japanese pharmaceutical companies and the U.S. Open Payments Database from 2016 to 2018, the researchers suggested that the medical societies should:
- Adopt global standard COI policies from organizations such as the National Academy of Medicine and Guidelines International Network, including a 3-year lookback period for COI declaration.
- Consider a comprehensive definition and rigorous management with full disclosure of NFCOI.
- Publish a list of authors making each recommendation to grasp the implications of COI in clinical practice guidelines.
- Mention the detailed date of the COI disclosure, which should be close to the publication date as much as possible.
Financial conflicts of interest
The researchers used payment data published between 2016 and 2018 for all 83 companies belonging to the Japan Pharmaceutical Manufacturers Association, focusing on personal payments (for lecturing, writing, and consultancy) and excluding research payments, “since in Japan, the name, institution, and position of the author or researcher who received the research payment is not disclosed, which makes assessing research payments difficult.” To evaluate authors’ FCOI in the ACR’s CPG, the researchers analyzed the U.S. Open Payments Database “for all categories of general payments such as speaking, consulting, meals, and travel expenses 3 years from before the guideline’s first online publication on November 30, 2018.”
The 2018 ACR/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis had 36 authors and the JDA’s Clinical Practice Guideline for the Treatment of Psoriatic Arthritis 2019 had 23. Overall, 61% of JDA authors and half of ACR authors voluntarily declared FCOI with pharmaceutical companies; 25 of the ACR authors were U.S. physicians and could be included in the Open Payments Database search.
A total of 21 (91.3%) JDA authors and 21 (84.0%) ACR authors received at least one payment, with the combined total of $3,335,413 and $4,081,629 payments, respectively, over the 3 years. The average and median personal payments were $145,018 and $123,876 for JDA authors and $162,825 and $58,826 for ACR authors. When the payments to ACR authors were limited to lecturing, writing, and consulting fees that are required under the ACR’s COI policy, the mean was $130,102 and median was $39,375. The corresponding payments for JDA authors were $123,876 and $8,170, respectively,
The researchers found undisclosed payments for more than three-quarters of physician authors of the Japanese guideline, and nearly half of the doctors authoring the American guideline had undisclosed payments. These added up to $474,000 for the JDA, which amounted to 38% of the total for personal payments that must be reported to the JDA based on its COI policy for clinical practice guidelines, and $218,000 for the ACR, amounting to 18% of the total for personal payments that must be reported to the society based on its COI policy.
Of the 11 ACR authors who were not eligible for the U.S. Open Payments Database search, 5 declared FCOI with pharmaceutical companies in the guideline, meaning that 26 (72%) of the 36 authors had FCOI with pharmaceutical companies.
The ACR only required authors to declare FCOI covering 1 year before and during guideline development, and although the JDA required authors to declare their FCOI for the past 3 years of guideline development, the study authors noted that the JDA guideline disclosed them for only 2 years (between Jan. 1, 2017, and Dec. 31, 2018).
“It is true that influential doctors such as clinical practice guideline authors tend to receive various types of payments from pharmaceutical companies and that it is difficult to conduct research without funding from pharmaceutical companies. However, our current research mainly focuses on personal payments from pharmaceutical companies such as lecture fees and consulting fees. These payments are recognized as pocket money and are not used for research. Thus, it is questionable that the observed relationships are something evitable,” the researchers wrote.
Nonfinancial conflicts of interest
Many authors of the ACR’s CPG and the JDA’s CPG also had NFCOI, defined objectively in this study as self-citation rate. NFCOI have been more broadly defined by the International Committee of Medical Journal Editors (ICMJE) as “conflicts, such as personal relationships or rivalries, academic competition, and intellectual beliefs”; the ICMJE recommends reporting NFCOI on its COI form.
The JDA guideline included self-citations by 78% of its authors, compared with 32% of the ACR guideline authors, but this weighed differently among the two guidelines in that only 12 of the 354 (3.4%) citations in the JDA guideline were self-cited, compared with 46 of 137 (34%) citations in the ACR guideline.
The researchers noted that while the self-citation rates between JDA and ACR authors “differed remarkably,” the impact of ACR authors on CPG recommendations was much more direct. Three-quarters of JDA authors’ self-cited articles were about observational studies, whereas 52% of the ACR authors’ self-cited articles were clinical trials, most of which were randomized, controlled studies, and these NFCOI were not disclosed in the guideline.
Half of the strong recommendations in the JDA guideline were based on low or very low quality of evidence, whereas the ACR guideline had no strong recommendations based on low or very low quality of evidence.
This study was supported by the nonprofit Medical Governance Research Institute, which receives donations from Ain Pharmacies Inc., other organizations, and private individuals. The study also received support from the Tansa (formerly known as the Waseda Chronicle), an independent nonprofit news organization dedicated to investigative journalism. Three authors reported receiving personal fees from several pharmaceutical companies for work outside of the scope of this study.
, according to a retrospective analysis of all authors on the most recent guidelines issued by the American College of Rheumatology (ACR) and the Japanese Dermatological Association (JDA).
In addition to finding that the majority of the authors of psoriatic arthritis (PsA) clinical practice guidelines (CPGs) issued by the JDA and ACR received substantial personal payments from pharmaceutical companies before and during CPG development, researchers led by Hanano Mamada and Anju Murayama of the Medical Governance Research Institute, Tokyo, wrote in Arthritis Care & Research that “several CPG authors self-cited their articles without the disclosure of NFCOI [nonfinancial conflicts of interest], and most of the recommendations were based on low or very low quality of evidence. Although the COI policies used by JDA and ACR are clearly inadequate, no significant revisions have been made for the last 3 years.”
Based on their findings, which were made using payment data from major Japanese pharmaceutical companies and the U.S. Open Payments Database from 2016 to 2018, the researchers suggested that the medical societies should:
- Adopt global standard COI policies from organizations such as the National Academy of Medicine and Guidelines International Network, including a 3-year lookback period for COI declaration.
- Consider a comprehensive definition and rigorous management with full disclosure of NFCOI.
- Publish a list of authors making each recommendation to grasp the implications of COI in clinical practice guidelines.
- Mention the detailed date of the COI disclosure, which should be close to the publication date as much as possible.
Financial conflicts of interest
The researchers used payment data published between 2016 and 2018 for all 83 companies belonging to the Japan Pharmaceutical Manufacturers Association, focusing on personal payments (for lecturing, writing, and consultancy) and excluding research payments, “since in Japan, the name, institution, and position of the author or researcher who received the research payment is not disclosed, which makes assessing research payments difficult.” To evaluate authors’ FCOI in the ACR’s CPG, the researchers analyzed the U.S. Open Payments Database “for all categories of general payments such as speaking, consulting, meals, and travel expenses 3 years from before the guideline’s first online publication on November 30, 2018.”
The 2018 ACR/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis had 36 authors and the JDA’s Clinical Practice Guideline for the Treatment of Psoriatic Arthritis 2019 had 23. Overall, 61% of JDA authors and half of ACR authors voluntarily declared FCOI with pharmaceutical companies; 25 of the ACR authors were U.S. physicians and could be included in the Open Payments Database search.
A total of 21 (91.3%) JDA authors and 21 (84.0%) ACR authors received at least one payment, with the combined total of $3,335,413 and $4,081,629 payments, respectively, over the 3 years. The average and median personal payments were $145,018 and $123,876 for JDA authors and $162,825 and $58,826 for ACR authors. When the payments to ACR authors were limited to lecturing, writing, and consulting fees that are required under the ACR’s COI policy, the mean was $130,102 and median was $39,375. The corresponding payments for JDA authors were $123,876 and $8,170, respectively,
The researchers found undisclosed payments for more than three-quarters of physician authors of the Japanese guideline, and nearly half of the doctors authoring the American guideline had undisclosed payments. These added up to $474,000 for the JDA, which amounted to 38% of the total for personal payments that must be reported to the JDA based on its COI policy for clinical practice guidelines, and $218,000 for the ACR, amounting to 18% of the total for personal payments that must be reported to the society based on its COI policy.
Of the 11 ACR authors who were not eligible for the U.S. Open Payments Database search, 5 declared FCOI with pharmaceutical companies in the guideline, meaning that 26 (72%) of the 36 authors had FCOI with pharmaceutical companies.
The ACR only required authors to declare FCOI covering 1 year before and during guideline development, and although the JDA required authors to declare their FCOI for the past 3 years of guideline development, the study authors noted that the JDA guideline disclosed them for only 2 years (between Jan. 1, 2017, and Dec. 31, 2018).
“It is true that influential doctors such as clinical practice guideline authors tend to receive various types of payments from pharmaceutical companies and that it is difficult to conduct research without funding from pharmaceutical companies. However, our current research mainly focuses on personal payments from pharmaceutical companies such as lecture fees and consulting fees. These payments are recognized as pocket money and are not used for research. Thus, it is questionable that the observed relationships are something evitable,” the researchers wrote.
Nonfinancial conflicts of interest
Many authors of the ACR’s CPG and the JDA’s CPG also had NFCOI, defined objectively in this study as self-citation rate. NFCOI have been more broadly defined by the International Committee of Medical Journal Editors (ICMJE) as “conflicts, such as personal relationships or rivalries, academic competition, and intellectual beliefs”; the ICMJE recommends reporting NFCOI on its COI form.
The JDA guideline included self-citations by 78% of its authors, compared with 32% of the ACR guideline authors, but this weighed differently among the two guidelines in that only 12 of the 354 (3.4%) citations in the JDA guideline were self-cited, compared with 46 of 137 (34%) citations in the ACR guideline.
The researchers noted that while the self-citation rates between JDA and ACR authors “differed remarkably,” the impact of ACR authors on CPG recommendations was much more direct. Three-quarters of JDA authors’ self-cited articles were about observational studies, whereas 52% of the ACR authors’ self-cited articles were clinical trials, most of which were randomized, controlled studies, and these NFCOI were not disclosed in the guideline.
Half of the strong recommendations in the JDA guideline were based on low or very low quality of evidence, whereas the ACR guideline had no strong recommendations based on low or very low quality of evidence.
This study was supported by the nonprofit Medical Governance Research Institute, which receives donations from Ain Pharmacies Inc., other organizations, and private individuals. The study also received support from the Tansa (formerly known as the Waseda Chronicle), an independent nonprofit news organization dedicated to investigative journalism. Three authors reported receiving personal fees from several pharmaceutical companies for work outside of the scope of this study.
FROM ARTHRITIS CARE & RESEARCH
Unusual Bilateral Distribution of Neurofibromatosis Type 5 on the Distal Upper Extremities
To the Editor:
Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosis type 1 (NF1)(also known as von Recklinghausen disease). Phenotypic manifestations of NF5 include café-au-lait macules, neurofibromas, or both in 1 or more adjacent dermatomes. In contrast to the systemic features of NF1, the dermatomal distribution of NF5 demonstrates mosaicism due to a spontaneous postzygotic mutation in the neurofibromin 1 gene, NF1. We describe an atypical presentation of NF5 with bilateral features on the upper extremities.
A 74-year-old woman presented with soft pink- to flesh-colored growths on the left dorsal forearm and hand that were observed incidentally during a Mohs procedure for treatment of a basal cell carcinoma on the upper cutaneous lip. The patient reported that the lesions initially appeared on the left dorsal hand at approximately 16 years of age and had since spread proximally up to the mid dorsal forearm over the course of her lifetime. She denied any pain but claimed the affected area could be itchy. The lesions did not interfere with her daily activities, but they negatively impacted her social life due to their cosmetic appearance as well as her fear that they could be contagious. She denied any family history of NF1.
Physical examination revealed innumerable soft, pink- to flesh-colored cutaneous nodules ranging from 3 to 9 mm in diameter clustered uniformly on the left dorsal hand and lower forearm within the C6, C7, and C8 dermatomal regions (Figure, A). A singular brown patch measuring 20 mm in diameter also was observed on the right dorsal hand within the C6 dermatome, which the patient reported had been present since birth (Figure, B). The nodules and pigmented patch were clinically diagnosed as cutaneous neurofibromas on the left arm and a café-au-lait macule on the right arm, each manifesting within the C6 dermatome on separate upper extremities. Lisch nodules, axillary freckling, and acoustic schwannomas were not observed. Because of the dermatomal distribution of the lesions and lack of family history of NF1, a diagnosis of bilateral NF5 was made. The patient stated she had declined treatment of the neurofibromas from her referring general dermatologist due to possible risk for recurrence.
Segmental neurofibromatosis was first described in 1931 by Gammel,1 and in 1982, segmental neurofibromatosis was classified as NF5 by Riccardi.2 After Tinschert et al3 later demonstrated NF5 to be a somatic mutation of NF1,3 Ruggieri and Huson4 proposed the term mosaic neurofibromatosis 1 in 2001.
While the prevalence of NF1 is 1 in 3000 individuals,5 NF5 is rare with an occurrence of 1 in 40,000.6 In NF5, a spontaneous NF1 gene mutation occurs on chromosome 17 in a dividing cell after conception.7 Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the NF1 gene.8 This contrasts with the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells. Patients with NF5 generally are not expected to have affected offspring because the spontaneous mutation usually arises in somatic cells; however, a postzygotic mutation in the gonadal region could potentially affect germline cells, resulting in vertical transmission, with documented cases of offspring with systemic NF1.4 Because of the risk for malignancy with systemic neurofibromatosis, early diagnosis with genetic counseling is imperative in patients with both NF1 and NF5.
Neurofibromatosis type 5 is a clinical diagnosis based on the presence of neurofibromas and/or café-au-lait macules in a dermatomal distribution. The clinical presentation depends on when and where the NF1 gene mutation occurs in utero as cells multiply, differentiate, and migrate.8 Earlier mutations result in a broader manifestation of NF5 in comparison to late mutations, which have more localized features. An NF1 gene mutation causes a loss of function of neurofibromin, a tumor suppressor protein, in Schwann cells and fibroblasts.8 This produces neurofibromas and café-au-lait macules, respectively.8
A large literature review on segmental neurofibromatosis by Garcia-Romero et al6 identified 320 individuals who did not meet full inclusion criteria for NF1 between 1977 and 2012. Overall, 76% of cases were unilaterally distributed. The investigators identified 157 individual case reports in which the most to least common presentation was pigmentary changes only, neurofibromas only, mixed pigmentary changes with neurofibromas, and plexiform neurofibromas only; however, many of these cases were children who may have later developed both neurofibromas and pigmentary changes during puberty.6 Additional features of NF5 may include freckling, Lisch nodules, optic gliomas, malignant peripheral nerve sheath tumors, skeletal abnormalities, precocious puberty, vascular malformations, hypertension, seizures, and/or learning difficulties based on the affected anatomy.
Segmental neurofibromatosis, or NF5, is a rare subtype of NF1. Our case demonstrates an unusual bilateral distribution of NF5 with cutaneous neurofibromas and a café-au-lait macule on the upper extremities. Awareness of variations of neurofibromatosis and their genetic implications is essential in establishing earlier clinical diagnoses in cases with subtle manifestations.
- Gammel JA. Localized neurofibromatosis. Arch Dermatol. 1931;24:712-713.
- Riccardi VM. Neurofibromatosis: clinical heterogeneity. Curr Probl Cancer. 1982;7:1-34.
- Tinschert S, Naumann I, Stegmann E, et al. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur J Hum Genet. 2000;8:455-459.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Crowe FW, Schull WJ, Neel JV. A Clinical, Pathological and Genetic Study of Multiple Neurofibromatosis. Charles C Thomas; 1956.
- García-Romero MT, Parkin P, Lara-Corrales I. Mosaic neurofibromatosis type 1: a systematic review. Pediatr Dermatol. 2016;33:9-17.
- Ledbetter DH, Rich DC, O’Connell P, et al. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989;44:20-24.
- Redlick FP, Shaw JC. Segmental neurofibromatosis follows Blaschko’s lines or dermatomes depending on the cell line affected: case report and literature review. J Cutan Med Surg. 2004;8:353-356.
To the Editor:
Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosis type 1 (NF1)(also known as von Recklinghausen disease). Phenotypic manifestations of NF5 include café-au-lait macules, neurofibromas, or both in 1 or more adjacent dermatomes. In contrast to the systemic features of NF1, the dermatomal distribution of NF5 demonstrates mosaicism due to a spontaneous postzygotic mutation in the neurofibromin 1 gene, NF1. We describe an atypical presentation of NF5 with bilateral features on the upper extremities.
A 74-year-old woman presented with soft pink- to flesh-colored growths on the left dorsal forearm and hand that were observed incidentally during a Mohs procedure for treatment of a basal cell carcinoma on the upper cutaneous lip. The patient reported that the lesions initially appeared on the left dorsal hand at approximately 16 years of age and had since spread proximally up to the mid dorsal forearm over the course of her lifetime. She denied any pain but claimed the affected area could be itchy. The lesions did not interfere with her daily activities, but they negatively impacted her social life due to their cosmetic appearance as well as her fear that they could be contagious. She denied any family history of NF1.
Physical examination revealed innumerable soft, pink- to flesh-colored cutaneous nodules ranging from 3 to 9 mm in diameter clustered uniformly on the left dorsal hand and lower forearm within the C6, C7, and C8 dermatomal regions (Figure, A). A singular brown patch measuring 20 mm in diameter also was observed on the right dorsal hand within the C6 dermatome, which the patient reported had been present since birth (Figure, B). The nodules and pigmented patch were clinically diagnosed as cutaneous neurofibromas on the left arm and a café-au-lait macule on the right arm, each manifesting within the C6 dermatome on separate upper extremities. Lisch nodules, axillary freckling, and acoustic schwannomas were not observed. Because of the dermatomal distribution of the lesions and lack of family history of NF1, a diagnosis of bilateral NF5 was made. The patient stated she had declined treatment of the neurofibromas from her referring general dermatologist due to possible risk for recurrence.

Segmental neurofibromatosis was first described in 1931 by Gammel,1 and in 1982, segmental neurofibromatosis was classified as NF5 by Riccardi.2 After Tinschert et al3 later demonstrated NF5 to be a somatic mutation of NF1,3 Ruggieri and Huson4 proposed the term mosaic neurofibromatosis 1 in 2001.
While the prevalence of NF1 is 1 in 3000 individuals,5 NF5 is rare with an occurrence of 1 in 40,000.6 In NF5, a spontaneous NF1 gene mutation occurs on chromosome 17 in a dividing cell after conception.7 Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the NF1 gene.8 This contrasts with the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells. Patients with NF5 generally are not expected to have affected offspring because the spontaneous mutation usually arises in somatic cells; however, a postzygotic mutation in the gonadal region could potentially affect germline cells, resulting in vertical transmission, with documented cases of offspring with systemic NF1.4 Because of the risk for malignancy with systemic neurofibromatosis, early diagnosis with genetic counseling is imperative in patients with both NF1 and NF5.
Neurofibromatosis type 5 is a clinical diagnosis based on the presence of neurofibromas and/or café-au-lait macules in a dermatomal distribution. The clinical presentation depends on when and where the NF1 gene mutation occurs in utero as cells multiply, differentiate, and migrate.8 Earlier mutations result in a broader manifestation of NF5 in comparison to late mutations, which have more localized features. An NF1 gene mutation causes a loss of function of neurofibromin, a tumor suppressor protein, in Schwann cells and fibroblasts.8 This produces neurofibromas and café-au-lait macules, respectively.8
A large literature review on segmental neurofibromatosis by Garcia-Romero et al6 identified 320 individuals who did not meet full inclusion criteria for NF1 between 1977 and 2012. Overall, 76% of cases were unilaterally distributed. The investigators identified 157 individual case reports in which the most to least common presentation was pigmentary changes only, neurofibromas only, mixed pigmentary changes with neurofibromas, and plexiform neurofibromas only; however, many of these cases were children who may have later developed both neurofibromas and pigmentary changes during puberty.6 Additional features of NF5 may include freckling, Lisch nodules, optic gliomas, malignant peripheral nerve sheath tumors, skeletal abnormalities, precocious puberty, vascular malformations, hypertension, seizures, and/or learning difficulties based on the affected anatomy.
Segmental neurofibromatosis, or NF5, is a rare subtype of NF1. Our case demonstrates an unusual bilateral distribution of NF5 with cutaneous neurofibromas and a café-au-lait macule on the upper extremities. Awareness of variations of neurofibromatosis and their genetic implications is essential in establishing earlier clinical diagnoses in cases with subtle manifestations.
To the Editor:
Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosis type 1 (NF1)(also known as von Recklinghausen disease). Phenotypic manifestations of NF5 include café-au-lait macules, neurofibromas, or both in 1 or more adjacent dermatomes. In contrast to the systemic features of NF1, the dermatomal distribution of NF5 demonstrates mosaicism due to a spontaneous postzygotic mutation in the neurofibromin 1 gene, NF1. We describe an atypical presentation of NF5 with bilateral features on the upper extremities.
A 74-year-old woman presented with soft pink- to flesh-colored growths on the left dorsal forearm and hand that were observed incidentally during a Mohs procedure for treatment of a basal cell carcinoma on the upper cutaneous lip. The patient reported that the lesions initially appeared on the left dorsal hand at approximately 16 years of age and had since spread proximally up to the mid dorsal forearm over the course of her lifetime. She denied any pain but claimed the affected area could be itchy. The lesions did not interfere with her daily activities, but they negatively impacted her social life due to their cosmetic appearance as well as her fear that they could be contagious. She denied any family history of NF1.
Physical examination revealed innumerable soft, pink- to flesh-colored cutaneous nodules ranging from 3 to 9 mm in diameter clustered uniformly on the left dorsal hand and lower forearm within the C6, C7, and C8 dermatomal regions (Figure, A). A singular brown patch measuring 20 mm in diameter also was observed on the right dorsal hand within the C6 dermatome, which the patient reported had been present since birth (Figure, B). The nodules and pigmented patch were clinically diagnosed as cutaneous neurofibromas on the left arm and a café-au-lait macule on the right arm, each manifesting within the C6 dermatome on separate upper extremities. Lisch nodules, axillary freckling, and acoustic schwannomas were not observed. Because of the dermatomal distribution of the lesions and lack of family history of NF1, a diagnosis of bilateral NF5 was made. The patient stated she had declined treatment of the neurofibromas from her referring general dermatologist due to possible risk for recurrence.

Segmental neurofibromatosis was first described in 1931 by Gammel,1 and in 1982, segmental neurofibromatosis was classified as NF5 by Riccardi.2 After Tinschert et al3 later demonstrated NF5 to be a somatic mutation of NF1,3 Ruggieri and Huson4 proposed the term mosaic neurofibromatosis 1 in 2001.
While the prevalence of NF1 is 1 in 3000 individuals,5 NF5 is rare with an occurrence of 1 in 40,000.6 In NF5, a spontaneous NF1 gene mutation occurs on chromosome 17 in a dividing cell after conception.7 Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the NF1 gene.8 This contrasts with the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells. Patients with NF5 generally are not expected to have affected offspring because the spontaneous mutation usually arises in somatic cells; however, a postzygotic mutation in the gonadal region could potentially affect germline cells, resulting in vertical transmission, with documented cases of offspring with systemic NF1.4 Because of the risk for malignancy with systemic neurofibromatosis, early diagnosis with genetic counseling is imperative in patients with both NF1 and NF5.
Neurofibromatosis type 5 is a clinical diagnosis based on the presence of neurofibromas and/or café-au-lait macules in a dermatomal distribution. The clinical presentation depends on when and where the NF1 gene mutation occurs in utero as cells multiply, differentiate, and migrate.8 Earlier mutations result in a broader manifestation of NF5 in comparison to late mutations, which have more localized features. An NF1 gene mutation causes a loss of function of neurofibromin, a tumor suppressor protein, in Schwann cells and fibroblasts.8 This produces neurofibromas and café-au-lait macules, respectively.8
A large literature review on segmental neurofibromatosis by Garcia-Romero et al6 identified 320 individuals who did not meet full inclusion criteria for NF1 between 1977 and 2012. Overall, 76% of cases were unilaterally distributed. The investigators identified 157 individual case reports in which the most to least common presentation was pigmentary changes only, neurofibromas only, mixed pigmentary changes with neurofibromas, and plexiform neurofibromas only; however, many of these cases were children who may have later developed both neurofibromas and pigmentary changes during puberty.6 Additional features of NF5 may include freckling, Lisch nodules, optic gliomas, malignant peripheral nerve sheath tumors, skeletal abnormalities, precocious puberty, vascular malformations, hypertension, seizures, and/or learning difficulties based on the affected anatomy.
Segmental neurofibromatosis, or NF5, is a rare subtype of NF1. Our case demonstrates an unusual bilateral distribution of NF5 with cutaneous neurofibromas and a café-au-lait macule on the upper extremities. Awareness of variations of neurofibromatosis and their genetic implications is essential in establishing earlier clinical diagnoses in cases with subtle manifestations.
- Gammel JA. Localized neurofibromatosis. Arch Dermatol. 1931;24:712-713.
- Riccardi VM. Neurofibromatosis: clinical heterogeneity. Curr Probl Cancer. 1982;7:1-34.
- Tinschert S, Naumann I, Stegmann E, et al. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur J Hum Genet. 2000;8:455-459.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Crowe FW, Schull WJ, Neel JV. A Clinical, Pathological and Genetic Study of Multiple Neurofibromatosis. Charles C Thomas; 1956.
- García-Romero MT, Parkin P, Lara-Corrales I. Mosaic neurofibromatosis type 1: a systematic review. Pediatr Dermatol. 2016;33:9-17.
- Ledbetter DH, Rich DC, O’Connell P, et al. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989;44:20-24.
- Redlick FP, Shaw JC. Segmental neurofibromatosis follows Blaschko’s lines or dermatomes depending on the cell line affected: case report and literature review. J Cutan Med Surg. 2004;8:353-356.
- Gammel JA. Localized neurofibromatosis. Arch Dermatol. 1931;24:712-713.
- Riccardi VM. Neurofibromatosis: clinical heterogeneity. Curr Probl Cancer. 1982;7:1-34.
- Tinschert S, Naumann I, Stegmann E, et al. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur J Hum Genet. 2000;8:455-459.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Crowe FW, Schull WJ, Neel JV. A Clinical, Pathological and Genetic Study of Multiple Neurofibromatosis. Charles C Thomas; 1956.
- García-Romero MT, Parkin P, Lara-Corrales I. Mosaic neurofibromatosis type 1: a systematic review. Pediatr Dermatol. 2016;33:9-17.
- Ledbetter DH, Rich DC, O’Connell P, et al. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989;44:20-24.
- Redlick FP, Shaw JC. Segmental neurofibromatosis follows Blaschko’s lines or dermatomes depending on the cell line affected: case report and literature review. J Cutan Med Surg. 2004;8:353-356.
Practice Points
- Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosistype 1 (NF1)(also known as von Recklinghausen disease).
- Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the neurofibromin 1 gene, NF1. This is in contrast to the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells.
Vedolizumab-Induced Acne Fulminans: An Uncommon and Severe Adverse Effect
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.
Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.
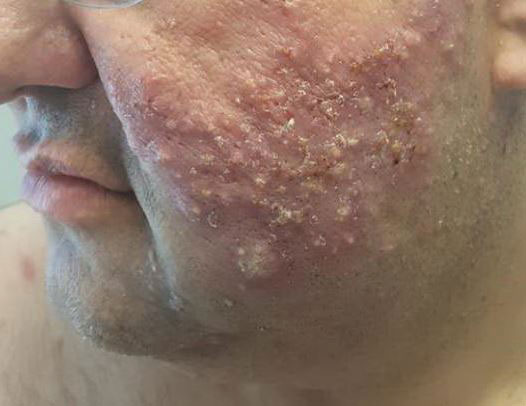
The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
Practice Points
- Vedolizumab, a monoclonal antibody for the treatment of refractory inflammatory bowel disease, was found to cause acne fulminans without systemic symptoms.
- Vedolizumab previously was believed to be a gut-limited immune modulator.
- Off-target cutaneous effects may indicate wider expression of the target integrin of vedolizumab and should be recognized as the drug becomes more widely used.
Malaria vaccine gets special delivery by tiny health personnel
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Reverse-Grip Technique of Scissors in Dermatologic Surgery: Tips to Improve Undermining Efficiency
Practice Gap
One of the most important elements of successful reconstruction is effective undermining prior to placement of buried sutures. The main benefit of an evenly undermined plane is that tension is reduced, thus permitting seamless tissue mobilization and wound edge approximation.1
However, achieving a consistent and appropriate plane can present challenges in certain blind spots within one’s field of work. A right hand–dominant surgeon might find it difficult to undermine tissue between the 3-o’clock and 6-o’clock positions (Figure 1) and often must resort to unnatural positioning to obtain adequate reach.
We propose a technique of reversing the grip on undermining scissors that improves efficiency without sacrificing technique.
Technique
The surgeon simply grasps the ring handles with the ring finger and thumb with the tip pointing to the wrist (Figure 2). Most of the control comes from rotating the wrist while spreading with the thumb (Figure 3).
The main advantage of the reverse-grip technique is that it prevents abduction of the arm at the shoulder joint, which reduces shoulder fatigue and keeps the elbow close to the trunk and away from the sterile surgical field. Achieving optimal ergonomics during surgery has been shown to reduce pain and likely prolong the surgeon’s career.2
A limitation of the reverse-grip technique is that direct visualization of the undermining plane is not achieved; however, direct visualization also is not obtained when undermining in the standard fashion unless the instruments are passed to the surgical assistant or the surgeon moves to the other side of the table.
Undermining can be performed safely without direct visualization as long as several rules are followed:
• The undermining plane is first established under direct visualization on the far side of the wound—at the 6-o’clock to 12-o’clock positions—and then followed to the area where direct visualization is not obtained.
• A blunt-tipped scissor is used to prevent penetrating trauma to neurovascular bundles. Blunt-tipped instruments allow more “feel” through tactile feedback to the surgeon and prevent accidental injury to these critical structures.
• A curved scissor is used with “tips up,” such that the surgeon does not unintentionally make the undermining plane deeper than anticipated.
Practice Implications
With practice, one can perform circumferential undermining independently with few alterations in stance and while maintaining a natural position throughout. Use of skin hooks to elevate the skin can further aid in visualizing the correct depth of undermining. If executed correctly, the reverse-grip technique can expand the surgeon’s work field, thus providing ease of dissection in difficult-to-reach areas.
- Chen DL, Carlson EO, Fathi R, et al. Undermining and hemostasis. Dermatol Surg. 2015;41(suppl 10):S201-S215. doi:10.1097/DSS.0000000000000489
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097/DSS.0000000000002295
Practice Gap
One of the most important elements of successful reconstruction is effective undermining prior to placement of buried sutures. The main benefit of an evenly undermined plane is that tension is reduced, thus permitting seamless tissue mobilization and wound edge approximation.1
However, achieving a consistent and appropriate plane can present challenges in certain blind spots within one’s field of work. A right hand–dominant surgeon might find it difficult to undermine tissue between the 3-o’clock and 6-o’clock positions (Figure 1) and often must resort to unnatural positioning to obtain adequate reach.

We propose a technique of reversing the grip on undermining scissors that improves efficiency without sacrificing technique.
Technique
The surgeon simply grasps the ring handles with the ring finger and thumb with the tip pointing to the wrist (Figure 2). Most of the control comes from rotating the wrist while spreading with the thumb (Figure 3).

The main advantage of the reverse-grip technique is that it prevents abduction of the arm at the shoulder joint, which reduces shoulder fatigue and keeps the elbow close to the trunk and away from the sterile surgical field. Achieving optimal ergonomics during surgery has been shown to reduce pain and likely prolong the surgeon’s career.2

A limitation of the reverse-grip technique is that direct visualization of the undermining plane is not achieved; however, direct visualization also is not obtained when undermining in the standard fashion unless the instruments are passed to the surgical assistant or the surgeon moves to the other side of the table.
Undermining can be performed safely without direct visualization as long as several rules are followed:
• The undermining plane is first established under direct visualization on the far side of the wound—at the 6-o’clock to 12-o’clock positions—and then followed to the area where direct visualization is not obtained.
• A blunt-tipped scissor is used to prevent penetrating trauma to neurovascular bundles. Blunt-tipped instruments allow more “feel” through tactile feedback to the surgeon and prevent accidental injury to these critical structures.
• A curved scissor is used with “tips up,” such that the surgeon does not unintentionally make the undermining plane deeper than anticipated.
Practice Implications
With practice, one can perform circumferential undermining independently with few alterations in stance and while maintaining a natural position throughout. Use of skin hooks to elevate the skin can further aid in visualizing the correct depth of undermining. If executed correctly, the reverse-grip technique can expand the surgeon’s work field, thus providing ease of dissection in difficult-to-reach areas.
Practice Gap
One of the most important elements of successful reconstruction is effective undermining prior to placement of buried sutures. The main benefit of an evenly undermined plane is that tension is reduced, thus permitting seamless tissue mobilization and wound edge approximation.1
However, achieving a consistent and appropriate plane can present challenges in certain blind spots within one’s field of work. A right hand–dominant surgeon might find it difficult to undermine tissue between the 3-o’clock and 6-o’clock positions (Figure 1) and often must resort to unnatural positioning to obtain adequate reach.

We propose a technique of reversing the grip on undermining scissors that improves efficiency without sacrificing technique.
Technique
The surgeon simply grasps the ring handles with the ring finger and thumb with the tip pointing to the wrist (Figure 2). Most of the control comes from rotating the wrist while spreading with the thumb (Figure 3).

The main advantage of the reverse-grip technique is that it prevents abduction of the arm at the shoulder joint, which reduces shoulder fatigue and keeps the elbow close to the trunk and away from the sterile surgical field. Achieving optimal ergonomics during surgery has been shown to reduce pain and likely prolong the surgeon’s career.2

A limitation of the reverse-grip technique is that direct visualization of the undermining plane is not achieved; however, direct visualization also is not obtained when undermining in the standard fashion unless the instruments are passed to the surgical assistant or the surgeon moves to the other side of the table.
Undermining can be performed safely without direct visualization as long as several rules are followed:
• The undermining plane is first established under direct visualization on the far side of the wound—at the 6-o’clock to 12-o’clock positions—and then followed to the area where direct visualization is not obtained.
• A blunt-tipped scissor is used to prevent penetrating trauma to neurovascular bundles. Blunt-tipped instruments allow more “feel” through tactile feedback to the surgeon and prevent accidental injury to these critical structures.
• A curved scissor is used with “tips up,” such that the surgeon does not unintentionally make the undermining plane deeper than anticipated.
Practice Implications
With practice, one can perform circumferential undermining independently with few alterations in stance and while maintaining a natural position throughout. Use of skin hooks to elevate the skin can further aid in visualizing the correct depth of undermining. If executed correctly, the reverse-grip technique can expand the surgeon’s work field, thus providing ease of dissection in difficult-to-reach areas.
- Chen DL, Carlson EO, Fathi R, et al. Undermining and hemostasis. Dermatol Surg. 2015;41(suppl 10):S201-S215. doi:10.1097/DSS.0000000000000489
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097/DSS.0000000000002295
- Chen DL, Carlson EO, Fathi R, et al. Undermining and hemostasis. Dermatol Surg. 2015;41(suppl 10):S201-S215. doi:10.1097/DSS.0000000000000489
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097/DSS.0000000000002295
Medicare Part D Prescription Claims for Brodalumab: Analysis of Annual Trends for 2017-2019
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).
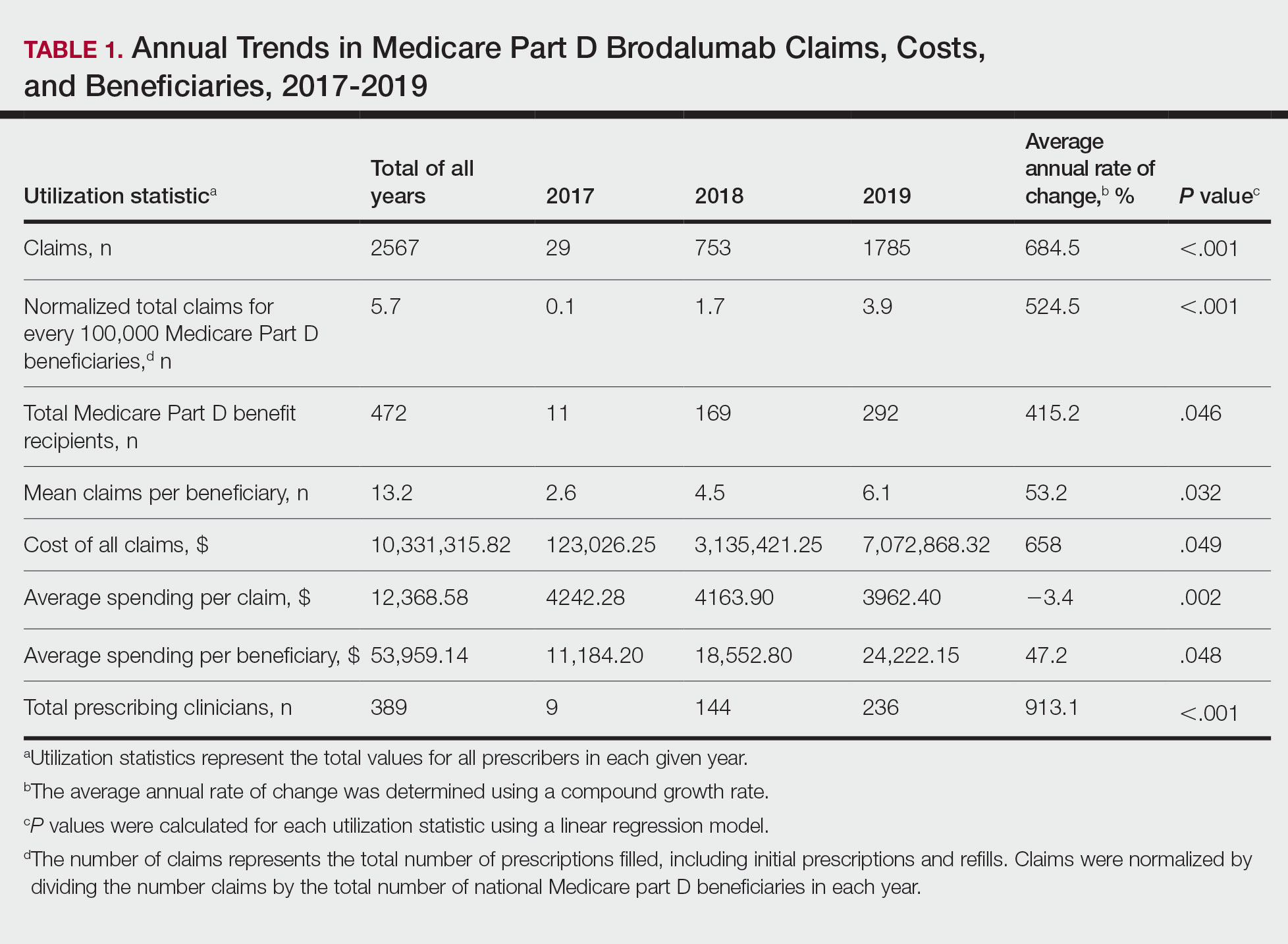
In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).
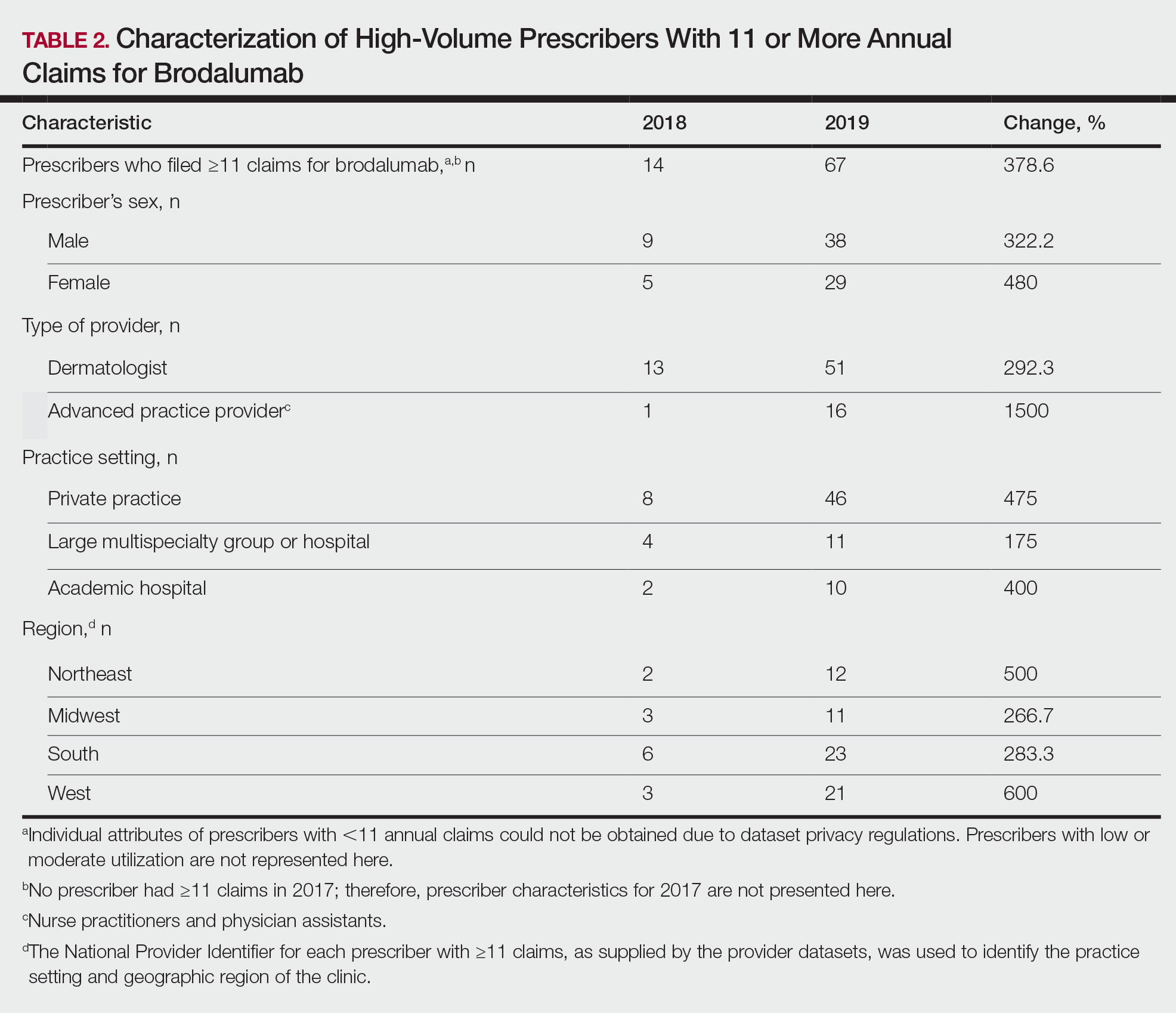
There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).

In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).

There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).

In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).

There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
Practice Points
- Brodalumab is associated with a boxed warning due to increased suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
- Brodalumab is underutilized compared to the other US Food and Drug Administration–approved IL-17 inhibitors used to treat psoriasis.
- Most experts agree that there is no increased risk for suicide associated with brodalumab. However, it remains imperative that prescribers assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services prior to initiating and during treatment with brodalumab.
Glucocorticoid-Induced Bone Loss: Dietary Supplementation Recommendations to Reduce the Risk for Osteoporosis and Osteoporotic Fractures
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6
Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6

Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6

Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
Practice Points
- Many long-term glucocorticoid (GC) users never receive therapy to prevent bone loss, and others are only started on therapy once they have sustained an insufficiency fracture.
- Oral GCs should be used at the lowest effective daily dose for the shortest duration possible.
- Patients using topical and intralesional corticosteroids are unlikely to develop GC-induced osteoporosis.
The CROWNing Event on Hair Loss in Women of Color: A Framework for Advocacy and Community Engagement (FACE) Survey Analysis
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.
We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.
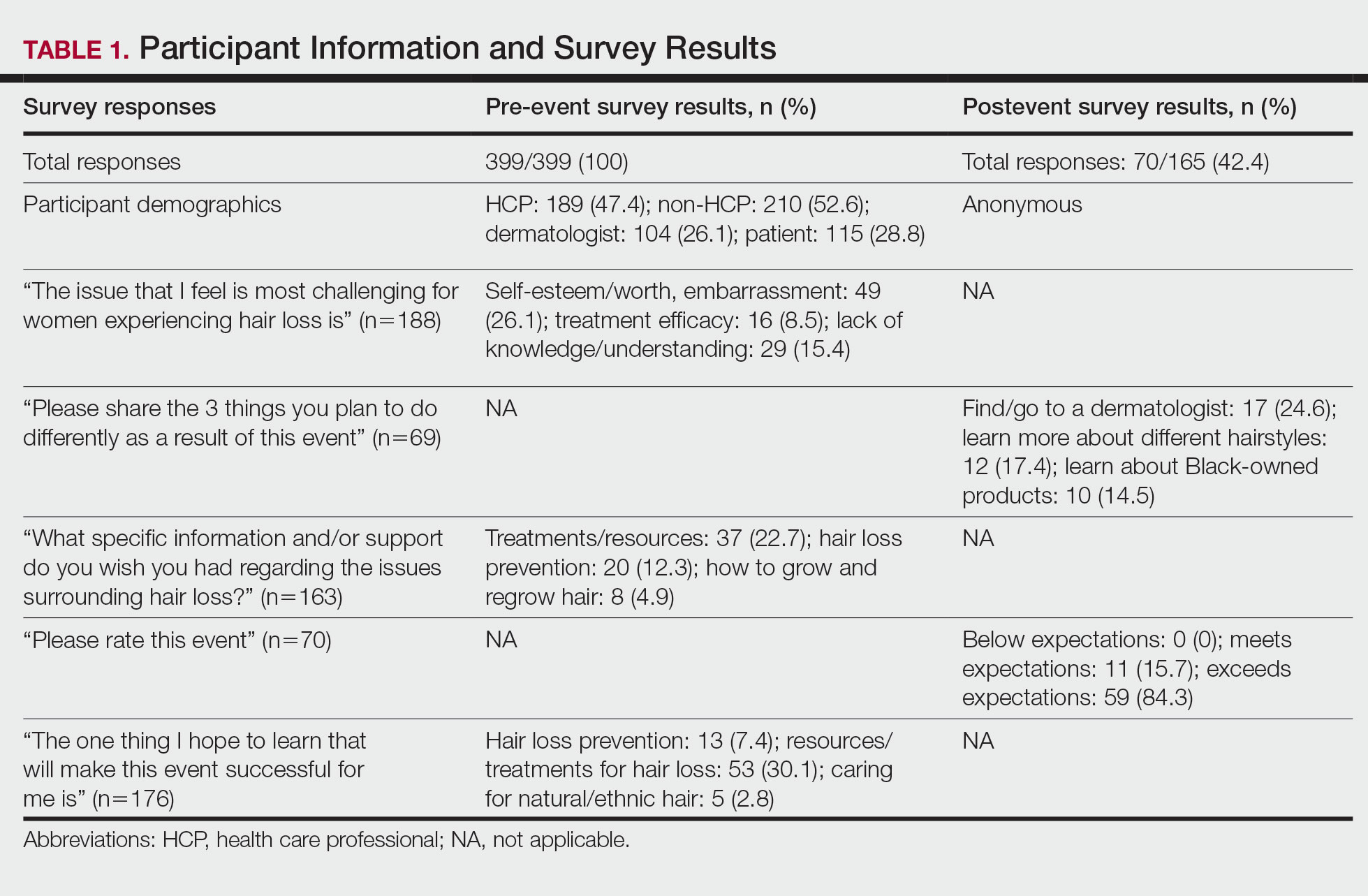
Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.
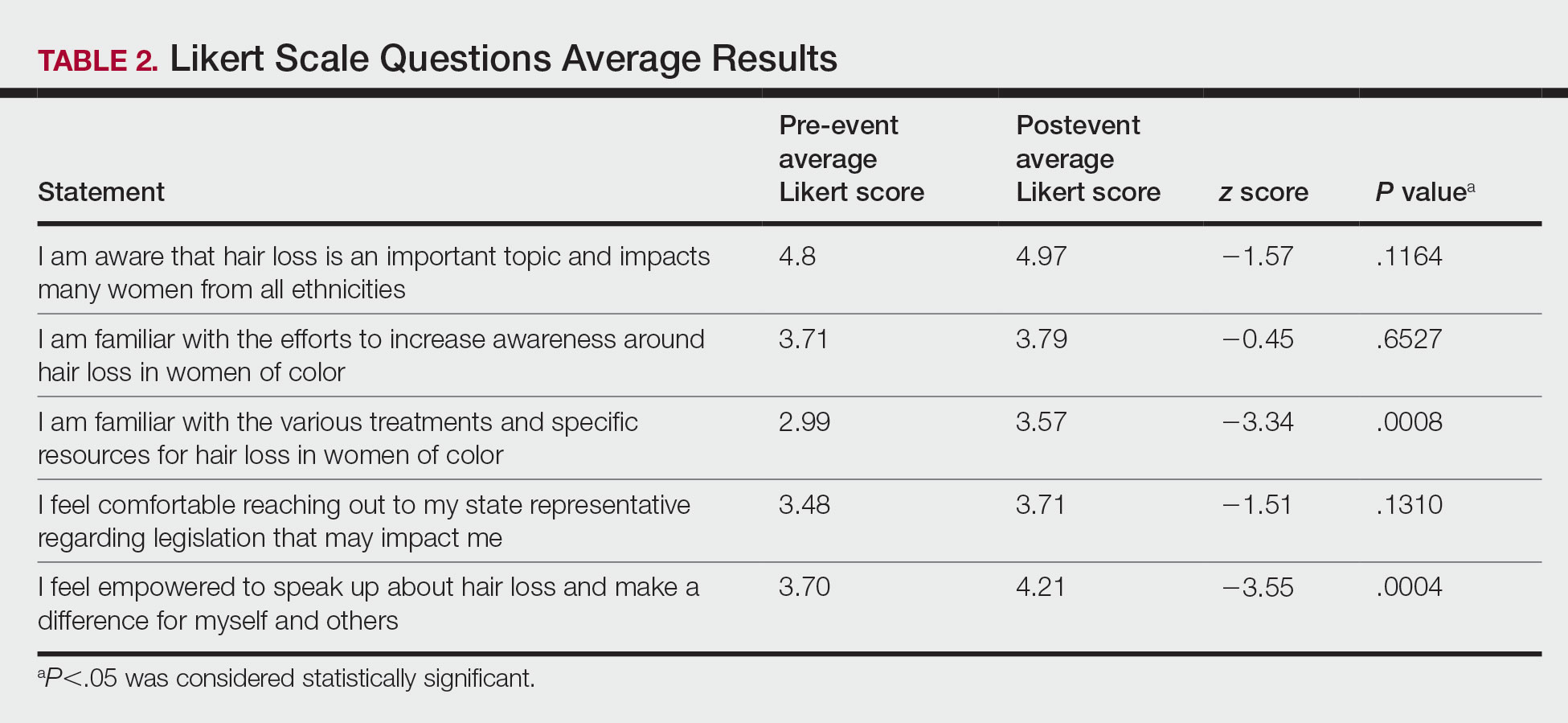
Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Practice Points
- Hair loss is associated with low self-esteem in women with skin of color; therefore, it is important to both acknowledge the social and psychological impacts of hair loss in this population and provide educational resources and community events that address patient concerns.
- There is a deficit of dermatology advocacy efforts that address conditions affecting patients with skin of color. Highlighting this disparity is the first step to catalyzing change.
- Dermatologists are responsible for advocating for women with skin of color and for addressing the social issues that impact their quality of life.
- The Framework for Advocacy and Community Efforts (FACE) model is a template for others to use when planning community engagement and advocacy efforts.