User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Study finds Roux-en-Y safe, effective for older patients
LAS VEGAS – Older obese patients shouldn’t be excluded from undergoing a Roux-en-Y gastric bypass based on concern for their long-term survival.
A 30-year review has determined that patients 60 years and older who had the surgery lost most of their excess body weight, and lived just as long as an age- and weight- matched cohort.
“We found a major weight loss benefit and no long-term differences in survival,” Taryn Hassinger, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress. “Our data support the use of this surgery in the elderly to achieve safe and effective weight loss.”
These subjects were matched for age and baseline weight to a group of 425 who did not have any bariatric surgery. Survival data in the univariate analysis came from Social Security death records.
The groups were similar at baseline, with a mean age of 62 and a mean body mass index of 47 kg/m2. About half of each group had obstructive sleep apnea. Other comorbidities were osteoarthritis (63%), chronic obstructive pulmonary disease (24%), type 2 diabetes (58%), gastroesophageal reflux (52%), congestive heart failure (8%), and hypertension (78%). About a quarter of each group smoked.
Patients were followed for up to 6 years. At the end of follow-up, those who had the surgery had lost a mean of 84% of their excess body weight. There was hardly any weight loss evident in the control group – a mean reduction of 4.6%. At the end of the follow-up period, 90% of surgical patients and 93% of the control patients were still alive.
The study provides reassuring data in an area that has not been well explored, Dr. Hassinger added. The only extant studies have compared older and younger cohorts. Peter Muscarella, MD, who moderated the session, agreed.
“This is very interesting, and good to know as we continue to expand the use of Roux-en-Y into different populations,” said Dr. Muscarella, a surgeon at Montefiore Medical Center, New York. “We have already expanded it into the pediatric population and now we are looking at its use in older individuals. But one question is, are there epidemiologic data on obesity in elderly patients? In my own practice, I just don’t see a lot of obese elderly patients. Is this really a problem in our country?”
A 2012 paper published by the National Center for Health Statistics addressed this issue. Data from the National Health and Nutrition Examination Survey, 2007-2010, found that nearly one-third of U.S. adults aged 65 years and older were obese. Other key findings:
• Obesity prevalence was higher among those aged 65-74, compared with those aged 75 and over in both men and women.
• The prevalence of obesity in women aged 65-74 was higher than in women aged 75 and over in all racial and ethnic groups except non-Hispanic black women, where approximately one in two were obese among both age groups.
• Between 1999-2002 and 2007-2010, the prevalence of obesity among older men increased.
As the proportion of older adults increases in the U.S. population, surgeons are likely to see older patients who are candidates for bariatric surgery, Dr. Hassinger said.
“We believe that surgery may be an option for people who are in the 60-70 year range,” she said. “We do operate on those patients not infrequently.”
The investigator had no disclosures.
[email protected]
On Twitter @Alz_Gal
LAS VEGAS – Older obese patients shouldn’t be excluded from undergoing a Roux-en-Y gastric bypass based on concern for their long-term survival.
A 30-year review has determined that patients 60 years and older who had the surgery lost most of their excess body weight, and lived just as long as an age- and weight- matched cohort.
“We found a major weight loss benefit and no long-term differences in survival,” Taryn Hassinger, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress. “Our data support the use of this surgery in the elderly to achieve safe and effective weight loss.”
These subjects were matched for age and baseline weight to a group of 425 who did not have any bariatric surgery. Survival data in the univariate analysis came from Social Security death records.
The groups were similar at baseline, with a mean age of 62 and a mean body mass index of 47 kg/m2. About half of each group had obstructive sleep apnea. Other comorbidities were osteoarthritis (63%), chronic obstructive pulmonary disease (24%), type 2 diabetes (58%), gastroesophageal reflux (52%), congestive heart failure (8%), and hypertension (78%). About a quarter of each group smoked.
Patients were followed for up to 6 years. At the end of follow-up, those who had the surgery had lost a mean of 84% of their excess body weight. There was hardly any weight loss evident in the control group – a mean reduction of 4.6%. At the end of the follow-up period, 90% of surgical patients and 93% of the control patients were still alive.
The study provides reassuring data in an area that has not been well explored, Dr. Hassinger added. The only extant studies have compared older and younger cohorts. Peter Muscarella, MD, who moderated the session, agreed.
“This is very interesting, and good to know as we continue to expand the use of Roux-en-Y into different populations,” said Dr. Muscarella, a surgeon at Montefiore Medical Center, New York. “We have already expanded it into the pediatric population and now we are looking at its use in older individuals. But one question is, are there epidemiologic data on obesity in elderly patients? In my own practice, I just don’t see a lot of obese elderly patients. Is this really a problem in our country?”
A 2012 paper published by the National Center for Health Statistics addressed this issue. Data from the National Health and Nutrition Examination Survey, 2007-2010, found that nearly one-third of U.S. adults aged 65 years and older were obese. Other key findings:
• Obesity prevalence was higher among those aged 65-74, compared with those aged 75 and over in both men and women.
• The prevalence of obesity in women aged 65-74 was higher than in women aged 75 and over in all racial and ethnic groups except non-Hispanic black women, where approximately one in two were obese among both age groups.
• Between 1999-2002 and 2007-2010, the prevalence of obesity among older men increased.
As the proportion of older adults increases in the U.S. population, surgeons are likely to see older patients who are candidates for bariatric surgery, Dr. Hassinger said.
“We believe that surgery may be an option for people who are in the 60-70 year range,” she said. “We do operate on those patients not infrequently.”
The investigator had no disclosures.
[email protected]
On Twitter @Alz_Gal
LAS VEGAS – Older obese patients shouldn’t be excluded from undergoing a Roux-en-Y gastric bypass based on concern for their long-term survival.
A 30-year review has determined that patients 60 years and older who had the surgery lost most of their excess body weight, and lived just as long as an age- and weight- matched cohort.
“We found a major weight loss benefit and no long-term differences in survival,” Taryn Hassinger, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress. “Our data support the use of this surgery in the elderly to achieve safe and effective weight loss.”
These subjects were matched for age and baseline weight to a group of 425 who did not have any bariatric surgery. Survival data in the univariate analysis came from Social Security death records.
The groups were similar at baseline, with a mean age of 62 and a mean body mass index of 47 kg/m2. About half of each group had obstructive sleep apnea. Other comorbidities were osteoarthritis (63%), chronic obstructive pulmonary disease (24%), type 2 diabetes (58%), gastroesophageal reflux (52%), congestive heart failure (8%), and hypertension (78%). About a quarter of each group smoked.
Patients were followed for up to 6 years. At the end of follow-up, those who had the surgery had lost a mean of 84% of their excess body weight. There was hardly any weight loss evident in the control group – a mean reduction of 4.6%. At the end of the follow-up period, 90% of surgical patients and 93% of the control patients were still alive.
The study provides reassuring data in an area that has not been well explored, Dr. Hassinger added. The only extant studies have compared older and younger cohorts. Peter Muscarella, MD, who moderated the session, agreed.
“This is very interesting, and good to know as we continue to expand the use of Roux-en-Y into different populations,” said Dr. Muscarella, a surgeon at Montefiore Medical Center, New York. “We have already expanded it into the pediatric population and now we are looking at its use in older individuals. But one question is, are there epidemiologic data on obesity in elderly patients? In my own practice, I just don’t see a lot of obese elderly patients. Is this really a problem in our country?”
A 2012 paper published by the National Center for Health Statistics addressed this issue. Data from the National Health and Nutrition Examination Survey, 2007-2010, found that nearly one-third of U.S. adults aged 65 years and older were obese. Other key findings:
• Obesity prevalence was higher among those aged 65-74, compared with those aged 75 and over in both men and women.
• The prevalence of obesity in women aged 65-74 was higher than in women aged 75 and over in all racial and ethnic groups except non-Hispanic black women, where approximately one in two were obese among both age groups.
• Between 1999-2002 and 2007-2010, the prevalence of obesity among older men increased.
As the proportion of older adults increases in the U.S. population, surgeons are likely to see older patients who are candidates for bariatric surgery, Dr. Hassinger said.
“We believe that surgery may be an option for people who are in the 60-70 year range,” she said. “We do operate on those patients not infrequently.”
The investigator had no disclosures.
[email protected]
On Twitter @Alz_Gal
AT THE ACADEMIC SURGICAL CONGRESS
Key clinical point: Older patients can safely lose weight after Roux-en-Y gastric bypass without excess mortality risk.
Major finding: At the end of follow-up, patients had lost a mean of 84% of their excess body weight, compared with 4.6% loss in controls. Survival was similar (90% of surgical patients and 93% of controls).
Data source: The retrospective study comprised 107 patients and 425 controls.
Disclosures: The investigator had no disclosures.
CGM safe and effective without additional blood glucose testing
according to the authors of a noninferiority trial published in the April issue of Diabetes Care.
Before December 2016, continuous glucose monitoring (CGM) was approved by the Food and Drug Administration for use only as an adjunct to standard home blood glucose monitoring (BGM), which was required to confirm the continuous glucose monitoring reading before making a decision on insulin dosing.
In this randomized, noninferiority trial, 217 participants with type 1 diabetes who used an insulin pump were randomized either to CGM only (142 individuals) or to CGM with additional BGM before an insulin bolus was administered (75 individuals).
The primary outcome of the 26-week trial was time in the range of 70-180 mg/dL, according to findings from the CGM, and the study found that mean time in this range was the same for the two groups: 65%.
There were no severe hypoglycemic events in the CGM-only group, and only one in the CGM plus BGM group. There were also no significant changes from baseline to 26 weeks in other metrics of glucose control for mean glucose, hyperglycemia, hypoglycemia, and glycemic variability, and no significant differences between the two groups. The results were also similar for subgroups of age, duration of disease, education, use of CGM before study enrollment, baseline hemoglobin A1c, and baseline time in range.
“In addition to randomization and multiple center participation, the strengths of this study include a high degree of participant retention, CGM use, and treatment group adherence,” the authors wrote. “Notably, there was good separation between the treatment groups in the number of BGM tests per day, particularly when recognizing that two of the BGM measurements per day were required for CGM calibration and that, according to the protocol, the calibrations were performed at times such that they would not influence insulin bolusing.”
The authors said the results were likely to be equally applicable to individuals who use multiple daily insulin injections rather than an insulin pump, as the accuracy of the CGM sensor was just as relevant to this group.
They stressed that one major limitation of the trial was that it only included adults with well-controlled type 1 diabetes who were likely to adhere to the study protocol, and it excluded those with less well controlled disease.
Given this, they called for future studies to examine the safety of CGM alone in young people and adults who might be less compliant with their diabetes control, such as those with higher HbA1c levels, who test their blood glucose fewer than four times a day, and who are hypoglycemia-unaware.
“The application of this trial’s results to clinical practice can benefit people with T1D by reducing their burden of multiple daily fingersticks when using CGM and can enhance the cost-effectiveness of CGM therapy by reducing the number of daily BGM test strips,” they wrote. “Furthermore, the demonstration that insulin dosing based on CGM alone is safe has applicability to assessing risk involved with artificial pancreas systems that automate insulin delivery based on CGM sensor glucose measurements.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust, and Dexcom provided the CGM systems used in the trial. Authors declared speakers fees, consultancies, board positions and other funding from a range of pharmaceutical companies, including Dexcom. One author declared stock in Dexcom.
according to the authors of a noninferiority trial published in the April issue of Diabetes Care.
Before December 2016, continuous glucose monitoring (CGM) was approved by the Food and Drug Administration for use only as an adjunct to standard home blood glucose monitoring (BGM), which was required to confirm the continuous glucose monitoring reading before making a decision on insulin dosing.
In this randomized, noninferiority trial, 217 participants with type 1 diabetes who used an insulin pump were randomized either to CGM only (142 individuals) or to CGM with additional BGM before an insulin bolus was administered (75 individuals).
The primary outcome of the 26-week trial was time in the range of 70-180 mg/dL, according to findings from the CGM, and the study found that mean time in this range was the same for the two groups: 65%.
There were no severe hypoglycemic events in the CGM-only group, and only one in the CGM plus BGM group. There were also no significant changes from baseline to 26 weeks in other metrics of glucose control for mean glucose, hyperglycemia, hypoglycemia, and glycemic variability, and no significant differences between the two groups. The results were also similar for subgroups of age, duration of disease, education, use of CGM before study enrollment, baseline hemoglobin A1c, and baseline time in range.
“In addition to randomization and multiple center participation, the strengths of this study include a high degree of participant retention, CGM use, and treatment group adherence,” the authors wrote. “Notably, there was good separation between the treatment groups in the number of BGM tests per day, particularly when recognizing that two of the BGM measurements per day were required for CGM calibration and that, according to the protocol, the calibrations were performed at times such that they would not influence insulin bolusing.”
The authors said the results were likely to be equally applicable to individuals who use multiple daily insulin injections rather than an insulin pump, as the accuracy of the CGM sensor was just as relevant to this group.
They stressed that one major limitation of the trial was that it only included adults with well-controlled type 1 diabetes who were likely to adhere to the study protocol, and it excluded those with less well controlled disease.
Given this, they called for future studies to examine the safety of CGM alone in young people and adults who might be less compliant with their diabetes control, such as those with higher HbA1c levels, who test their blood glucose fewer than four times a day, and who are hypoglycemia-unaware.
“The application of this trial’s results to clinical practice can benefit people with T1D by reducing their burden of multiple daily fingersticks when using CGM and can enhance the cost-effectiveness of CGM therapy by reducing the number of daily BGM test strips,” they wrote. “Furthermore, the demonstration that insulin dosing based on CGM alone is safe has applicability to assessing risk involved with artificial pancreas systems that automate insulin delivery based on CGM sensor glucose measurements.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust, and Dexcom provided the CGM systems used in the trial. Authors declared speakers fees, consultancies, board positions and other funding from a range of pharmaceutical companies, including Dexcom. One author declared stock in Dexcom.
according to the authors of a noninferiority trial published in the April issue of Diabetes Care.
Before December 2016, continuous glucose monitoring (CGM) was approved by the Food and Drug Administration for use only as an adjunct to standard home blood glucose monitoring (BGM), which was required to confirm the continuous glucose monitoring reading before making a decision on insulin dosing.
In this randomized, noninferiority trial, 217 participants with type 1 diabetes who used an insulin pump were randomized either to CGM only (142 individuals) or to CGM with additional BGM before an insulin bolus was administered (75 individuals).
The primary outcome of the 26-week trial was time in the range of 70-180 mg/dL, according to findings from the CGM, and the study found that mean time in this range was the same for the two groups: 65%.
There were no severe hypoglycemic events in the CGM-only group, and only one in the CGM plus BGM group. There were also no significant changes from baseline to 26 weeks in other metrics of glucose control for mean glucose, hyperglycemia, hypoglycemia, and glycemic variability, and no significant differences between the two groups. The results were also similar for subgroups of age, duration of disease, education, use of CGM before study enrollment, baseline hemoglobin A1c, and baseline time in range.
“In addition to randomization and multiple center participation, the strengths of this study include a high degree of participant retention, CGM use, and treatment group adherence,” the authors wrote. “Notably, there was good separation between the treatment groups in the number of BGM tests per day, particularly when recognizing that two of the BGM measurements per day were required for CGM calibration and that, according to the protocol, the calibrations were performed at times such that they would not influence insulin bolusing.”
The authors said the results were likely to be equally applicable to individuals who use multiple daily insulin injections rather than an insulin pump, as the accuracy of the CGM sensor was just as relevant to this group.
They stressed that one major limitation of the trial was that it only included adults with well-controlled type 1 diabetes who were likely to adhere to the study protocol, and it excluded those with less well controlled disease.
Given this, they called for future studies to examine the safety of CGM alone in young people and adults who might be less compliant with their diabetes control, such as those with higher HbA1c levels, who test their blood glucose fewer than four times a day, and who are hypoglycemia-unaware.
“The application of this trial’s results to clinical practice can benefit people with T1D by reducing their burden of multiple daily fingersticks when using CGM and can enhance the cost-effectiveness of CGM therapy by reducing the number of daily BGM test strips,” they wrote. “Furthermore, the demonstration that insulin dosing based on CGM alone is safe has applicability to assessing risk involved with artificial pancreas systems that automate insulin delivery based on CGM sensor glucose measurements.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust, and Dexcom provided the CGM systems used in the trial. Authors declared speakers fees, consultancies, board positions and other funding from a range of pharmaceutical companies, including Dexcom. One author declared stock in Dexcom.
Key clinical point: Continuous glucose monitoring alone is safe and effective without the addition of confirmatory blood glucose monitoring.
Major finding: Mean time spent with blood glucose in the 70-180 mg/dL range was the same for individuals with continuous glucose monitoring and those with additional confirmatory blood glucose testing.
Data source: A randomized noninferiority trial in 217 adults with well-controlled type 1 diabetes.
Disclosures: The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust. Dexcom provided the continuous glucose monitoring systems used in the trial. Authors declared speaking fees, consultancies, board positions and other funding from a range of pharmaceutical companies, including Dexcom. One author declared stock ownership in Dexcom.
When to screen asymptomatic diabetics for CAD
SNOWMASS, COLO. – The use of coronary artery calcium screening in the subset of asymptomatic diabetes patients at higher clinical risk of CAD appears to offer a practical strategy for identifying a subgroup in whom costlier stress cardiac imaging may be justified, Marcelo F. di Carli, MD, said at the Annual Cardiovascular Conference at Snowmass.
The ultimate goal is to reliably identify those patients who have asymptomatic diabetes with significant CAD warranting revascularization or maximal medical therapy for primary cardiovascular prevention.
“Coronary artery calcium is a simple test that’s accessible and inexpensive and can give us a quick read on the extent of atherosclerosis in the coronary arteries,” said Dr. di Carli, professor of radiology and medicine at Harvard University in Boston. “There’s good data that in diabetic patients there’s a gradation of risk across the spectrum of calcium scores. Risk increases exponentially from a coronary artery calcium score of 0 to more than 400. The calcium score can also provide a snapshot of which patients are more likely to have flow-limiting coronary disease.”
Atherosclerotic cardiovascular disease is the biggest contributor to the direct and indirect costs of diabetes, and diabetes experts are eager to avoid jacking up those costs further by routinely ordering stress nuclear imaging, stress echocardiography, cardiac magnetic resonance, and other expensive noninvasive imaging methods unless they can be shown to lead to improved outcomes. There is general agreement on the value of noninvasive imaging in diabetic patients with CAD symptoms. However, the routine use of such testing in asymptomatic diabetic patients has been controversial.
Indeed, according to the 2017 American Diabetes Association Standards of Medical Care in Diabetes: “In asymptomatic patients, routine screening for coronary artery disease is not recommended as it does not improve outcomes as long as atherosclerotic cardiovascular disease risk factors are treated (Diabetes Care. 2017 Jan;40[Suppl. 1]:S75-87). That’s a Level A recommendation.
But Dr. di Carli is among many cardiologists who believe this statement paints with too broad a brush. He considers it an overgeneralization that’s based on the negative results of two randomized trials of routine screening in asymptomatic diabetics: DIAD, which utilized stress single-photon emission CT (SPECT) imaging (JAMA. 2009 Apr 15;301[15]:1547-55), and FACTOR-64, which relied upon coronary CT angiography (JAMA. 2014 Dec 3;312[21]: 2234-43). Both studies found relatively low yields of severe CAD and showed no survival benefit for screening. And of course, these are also costly and inconvenient tests.
The problem in generalizing from DIAD and FACTOR-64 to the overall population of asymptomatic diabetic patients is that both studies were conducted in asymptomatic patients at the lower end of the cardiovascular risk spectrum. They were young, with an average age of 60 years. They had a history of diabetes of less than 10 years, and their diabetes was reasonably well controlled. They had normal ECGs and preserved renal function. Peripheral artery disease (PAD) was present in only 9% of the DIAD population and no one in FACTOR-64. So this would not be expected to be a high-risk/high-yield population, according to Dr. di Carli, executive director of the cardiovascular imaging program at Brigham and Women’s Hospital, Boston.
An earlier study from the Mayo Clinic identified the clinical factors that can potentially be used to identify a higher-risk cohort of asymptomatic diabetic patients in whom high-tech noninvasive testing for significant CAD may be justified, he continued. This was a nonrandomized study of 1,427 asymptomatic diabetic patients without known CAD who underwent SPECT imaging. Compared with the study populations in DIAD and FACTOR-64, the Mayo Clinic patients had a longer duration of diabetes and substantially higher rates of poor diabetes control, renal dysfunction, hypertension, and dyslipidemia. One-third of them had PAD.
Fifty-eight percent of the 1,427 patients in the Mayo cohort proved to have an abnormal SPECT imaging scan, and 18% had a high-risk scan. In a multivariate analysis, the investigators identified several factors independently associated with a high-risk scan. Q waves were present on the ECGs of 9% of the asymptomatic diabetes patients, and 43% of that subgroup had a high-risk scan. Thirty-eight percent of patients had other ECG abnormalities, and 28% of them had a high-risk scan. Age greater than 65 was associated with an increased likelihood of a high-risk SPECT result. And 28% of patients with PAD had a high-risk scan.
On the other hand, the likelihood of a high-risk scan in the 69% of subjects without PAD was 14% (J Am Coll Cardiol. 2005 Jan 4;45[1]:43-9).
The 2017 ADA guidelines acknowledge this and similar evidence by providing as a relatively weak Level E recommendation: “Consider screening for CAD in the presence of any of the following: atypical cardiac symptoms (e.g., unexplained dyspnea, chest discomfort); signs of symptoms of associated vascular disease including carotid bruits, transient ischemic attack, stroke, claudication, or PAD; or electrogram abnormalities (e.g., Q waves).”
Dr. di Carli would add to that list age older than 65, diabetes duration of greater than 10 years, poor diabetes control, and a high burden of standard cardiovascular risk factors. And he proposed the coronary artery calcium (CAC) score as a sensible gateway to selective use of further screening tests, citing as support a report from the National Institutes of Health–sponsored Multi-Ethnic Study of Atherosclerosis (MESA).
The MESA investigators assessed CAC in 6,603 persons aged 45-84 free of known CAD at baseline, including 881 with diabetes. Participants were subsequently followed prospectively for an average of 6.4 years. Compared with diabetes patients who had a baseline CAC score of 0, those with a score of 1-99 were at a risk factor– and ethnicity-adjusted 2.9-fold increased risk for developing coronary heart disease during the follow-up period. The CHD risk climbed stepwise with an increasing CAC score such that subjects with a score of 400 or higher were at 9.5-fold increased risk (Diabetes Care. 2011 Oct;34[10]L2285-90).
Using CAC measurement in this way as a screening tool in asymptomatic diabetes patients with clinical factors placing them at higher risk of significant CAD is consistent with appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. The criteria were provided in a 2014 joint report by the American College of Cardiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
The report rates CAC testing as “May Be Appropriate” for asymptomatic patients of intermediate or high global risk. As such, CAC “can be an option for further evaluation of potential SIHD [stable ischemic heart disease] in an individual patient when deemed reasonable by the patient’s physician,” according to the appropriate use criteria guidance, which was created with the express purpose of developing standards to avoid overuse of costly cardiovascular testing (J Am Coll Cardiol. 2014 Feb 4;63[4]:380-406).
Dr. di Carli reported having no financial conflicts.
SNOWMASS, COLO. – The use of coronary artery calcium screening in the subset of asymptomatic diabetes patients at higher clinical risk of CAD appears to offer a practical strategy for identifying a subgroup in whom costlier stress cardiac imaging may be justified, Marcelo F. di Carli, MD, said at the Annual Cardiovascular Conference at Snowmass.
The ultimate goal is to reliably identify those patients who have asymptomatic diabetes with significant CAD warranting revascularization or maximal medical therapy for primary cardiovascular prevention.
“Coronary artery calcium is a simple test that’s accessible and inexpensive and can give us a quick read on the extent of atherosclerosis in the coronary arteries,” said Dr. di Carli, professor of radiology and medicine at Harvard University in Boston. “There’s good data that in diabetic patients there’s a gradation of risk across the spectrum of calcium scores. Risk increases exponentially from a coronary artery calcium score of 0 to more than 400. The calcium score can also provide a snapshot of which patients are more likely to have flow-limiting coronary disease.”
Atherosclerotic cardiovascular disease is the biggest contributor to the direct and indirect costs of diabetes, and diabetes experts are eager to avoid jacking up those costs further by routinely ordering stress nuclear imaging, stress echocardiography, cardiac magnetic resonance, and other expensive noninvasive imaging methods unless they can be shown to lead to improved outcomes. There is general agreement on the value of noninvasive imaging in diabetic patients with CAD symptoms. However, the routine use of such testing in asymptomatic diabetic patients has been controversial.
Indeed, according to the 2017 American Diabetes Association Standards of Medical Care in Diabetes: “In asymptomatic patients, routine screening for coronary artery disease is not recommended as it does not improve outcomes as long as atherosclerotic cardiovascular disease risk factors are treated (Diabetes Care. 2017 Jan;40[Suppl. 1]:S75-87). That’s a Level A recommendation.
But Dr. di Carli is among many cardiologists who believe this statement paints with too broad a brush. He considers it an overgeneralization that’s based on the negative results of two randomized trials of routine screening in asymptomatic diabetics: DIAD, which utilized stress single-photon emission CT (SPECT) imaging (JAMA. 2009 Apr 15;301[15]:1547-55), and FACTOR-64, which relied upon coronary CT angiography (JAMA. 2014 Dec 3;312[21]: 2234-43). Both studies found relatively low yields of severe CAD and showed no survival benefit for screening. And of course, these are also costly and inconvenient tests.
The problem in generalizing from DIAD and FACTOR-64 to the overall population of asymptomatic diabetic patients is that both studies were conducted in asymptomatic patients at the lower end of the cardiovascular risk spectrum. They were young, with an average age of 60 years. They had a history of diabetes of less than 10 years, and their diabetes was reasonably well controlled. They had normal ECGs and preserved renal function. Peripheral artery disease (PAD) was present in only 9% of the DIAD population and no one in FACTOR-64. So this would not be expected to be a high-risk/high-yield population, according to Dr. di Carli, executive director of the cardiovascular imaging program at Brigham and Women’s Hospital, Boston.
An earlier study from the Mayo Clinic identified the clinical factors that can potentially be used to identify a higher-risk cohort of asymptomatic diabetic patients in whom high-tech noninvasive testing for significant CAD may be justified, he continued. This was a nonrandomized study of 1,427 asymptomatic diabetic patients without known CAD who underwent SPECT imaging. Compared with the study populations in DIAD and FACTOR-64, the Mayo Clinic patients had a longer duration of diabetes and substantially higher rates of poor diabetes control, renal dysfunction, hypertension, and dyslipidemia. One-third of them had PAD.
Fifty-eight percent of the 1,427 patients in the Mayo cohort proved to have an abnormal SPECT imaging scan, and 18% had a high-risk scan. In a multivariate analysis, the investigators identified several factors independently associated with a high-risk scan. Q waves were present on the ECGs of 9% of the asymptomatic diabetes patients, and 43% of that subgroup had a high-risk scan. Thirty-eight percent of patients had other ECG abnormalities, and 28% of them had a high-risk scan. Age greater than 65 was associated with an increased likelihood of a high-risk SPECT result. And 28% of patients with PAD had a high-risk scan.
On the other hand, the likelihood of a high-risk scan in the 69% of subjects without PAD was 14% (J Am Coll Cardiol. 2005 Jan 4;45[1]:43-9).
The 2017 ADA guidelines acknowledge this and similar evidence by providing as a relatively weak Level E recommendation: “Consider screening for CAD in the presence of any of the following: atypical cardiac symptoms (e.g., unexplained dyspnea, chest discomfort); signs of symptoms of associated vascular disease including carotid bruits, transient ischemic attack, stroke, claudication, or PAD; or electrogram abnormalities (e.g., Q waves).”
Dr. di Carli would add to that list age older than 65, diabetes duration of greater than 10 years, poor diabetes control, and a high burden of standard cardiovascular risk factors. And he proposed the coronary artery calcium (CAC) score as a sensible gateway to selective use of further screening tests, citing as support a report from the National Institutes of Health–sponsored Multi-Ethnic Study of Atherosclerosis (MESA).
The MESA investigators assessed CAC in 6,603 persons aged 45-84 free of known CAD at baseline, including 881 with diabetes. Participants were subsequently followed prospectively for an average of 6.4 years. Compared with diabetes patients who had a baseline CAC score of 0, those with a score of 1-99 were at a risk factor– and ethnicity-adjusted 2.9-fold increased risk for developing coronary heart disease during the follow-up period. The CHD risk climbed stepwise with an increasing CAC score such that subjects with a score of 400 or higher were at 9.5-fold increased risk (Diabetes Care. 2011 Oct;34[10]L2285-90).
Using CAC measurement in this way as a screening tool in asymptomatic diabetes patients with clinical factors placing them at higher risk of significant CAD is consistent with appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. The criteria were provided in a 2014 joint report by the American College of Cardiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
The report rates CAC testing as “May Be Appropriate” for asymptomatic patients of intermediate or high global risk. As such, CAC “can be an option for further evaluation of potential SIHD [stable ischemic heart disease] in an individual patient when deemed reasonable by the patient’s physician,” according to the appropriate use criteria guidance, which was created with the express purpose of developing standards to avoid overuse of costly cardiovascular testing (J Am Coll Cardiol. 2014 Feb 4;63[4]:380-406).
Dr. di Carli reported having no financial conflicts.
SNOWMASS, COLO. – The use of coronary artery calcium screening in the subset of asymptomatic diabetes patients at higher clinical risk of CAD appears to offer a practical strategy for identifying a subgroup in whom costlier stress cardiac imaging may be justified, Marcelo F. di Carli, MD, said at the Annual Cardiovascular Conference at Snowmass.
The ultimate goal is to reliably identify those patients who have asymptomatic diabetes with significant CAD warranting revascularization or maximal medical therapy for primary cardiovascular prevention.
“Coronary artery calcium is a simple test that’s accessible and inexpensive and can give us a quick read on the extent of atherosclerosis in the coronary arteries,” said Dr. di Carli, professor of radiology and medicine at Harvard University in Boston. “There’s good data that in diabetic patients there’s a gradation of risk across the spectrum of calcium scores. Risk increases exponentially from a coronary artery calcium score of 0 to more than 400. The calcium score can also provide a snapshot of which patients are more likely to have flow-limiting coronary disease.”
Atherosclerotic cardiovascular disease is the biggest contributor to the direct and indirect costs of diabetes, and diabetes experts are eager to avoid jacking up those costs further by routinely ordering stress nuclear imaging, stress echocardiography, cardiac magnetic resonance, and other expensive noninvasive imaging methods unless they can be shown to lead to improved outcomes. There is general agreement on the value of noninvasive imaging in diabetic patients with CAD symptoms. However, the routine use of such testing in asymptomatic diabetic patients has been controversial.
Indeed, according to the 2017 American Diabetes Association Standards of Medical Care in Diabetes: “In asymptomatic patients, routine screening for coronary artery disease is not recommended as it does not improve outcomes as long as atherosclerotic cardiovascular disease risk factors are treated (Diabetes Care. 2017 Jan;40[Suppl. 1]:S75-87). That’s a Level A recommendation.
But Dr. di Carli is among many cardiologists who believe this statement paints with too broad a brush. He considers it an overgeneralization that’s based on the negative results of two randomized trials of routine screening in asymptomatic diabetics: DIAD, which utilized stress single-photon emission CT (SPECT) imaging (JAMA. 2009 Apr 15;301[15]:1547-55), and FACTOR-64, which relied upon coronary CT angiography (JAMA. 2014 Dec 3;312[21]: 2234-43). Both studies found relatively low yields of severe CAD and showed no survival benefit for screening. And of course, these are also costly and inconvenient tests.
The problem in generalizing from DIAD and FACTOR-64 to the overall population of asymptomatic diabetic patients is that both studies were conducted in asymptomatic patients at the lower end of the cardiovascular risk spectrum. They were young, with an average age of 60 years. They had a history of diabetes of less than 10 years, and their diabetes was reasonably well controlled. They had normal ECGs and preserved renal function. Peripheral artery disease (PAD) was present in only 9% of the DIAD population and no one in FACTOR-64. So this would not be expected to be a high-risk/high-yield population, according to Dr. di Carli, executive director of the cardiovascular imaging program at Brigham and Women’s Hospital, Boston.
An earlier study from the Mayo Clinic identified the clinical factors that can potentially be used to identify a higher-risk cohort of asymptomatic diabetic patients in whom high-tech noninvasive testing for significant CAD may be justified, he continued. This was a nonrandomized study of 1,427 asymptomatic diabetic patients without known CAD who underwent SPECT imaging. Compared with the study populations in DIAD and FACTOR-64, the Mayo Clinic patients had a longer duration of diabetes and substantially higher rates of poor diabetes control, renal dysfunction, hypertension, and dyslipidemia. One-third of them had PAD.
Fifty-eight percent of the 1,427 patients in the Mayo cohort proved to have an abnormal SPECT imaging scan, and 18% had a high-risk scan. In a multivariate analysis, the investigators identified several factors independently associated with a high-risk scan. Q waves were present on the ECGs of 9% of the asymptomatic diabetes patients, and 43% of that subgroup had a high-risk scan. Thirty-eight percent of patients had other ECG abnormalities, and 28% of them had a high-risk scan. Age greater than 65 was associated with an increased likelihood of a high-risk SPECT result. And 28% of patients with PAD had a high-risk scan.
On the other hand, the likelihood of a high-risk scan in the 69% of subjects without PAD was 14% (J Am Coll Cardiol. 2005 Jan 4;45[1]:43-9).
The 2017 ADA guidelines acknowledge this and similar evidence by providing as a relatively weak Level E recommendation: “Consider screening for CAD in the presence of any of the following: atypical cardiac symptoms (e.g., unexplained dyspnea, chest discomfort); signs of symptoms of associated vascular disease including carotid bruits, transient ischemic attack, stroke, claudication, or PAD; or electrogram abnormalities (e.g., Q waves).”
Dr. di Carli would add to that list age older than 65, diabetes duration of greater than 10 years, poor diabetes control, and a high burden of standard cardiovascular risk factors. And he proposed the coronary artery calcium (CAC) score as a sensible gateway to selective use of further screening tests, citing as support a report from the National Institutes of Health–sponsored Multi-Ethnic Study of Atherosclerosis (MESA).
The MESA investigators assessed CAC in 6,603 persons aged 45-84 free of known CAD at baseline, including 881 with diabetes. Participants were subsequently followed prospectively for an average of 6.4 years. Compared with diabetes patients who had a baseline CAC score of 0, those with a score of 1-99 were at a risk factor– and ethnicity-adjusted 2.9-fold increased risk for developing coronary heart disease during the follow-up period. The CHD risk climbed stepwise with an increasing CAC score such that subjects with a score of 400 or higher were at 9.5-fold increased risk (Diabetes Care. 2011 Oct;34[10]L2285-90).
Using CAC measurement in this way as a screening tool in asymptomatic diabetes patients with clinical factors placing them at higher risk of significant CAD is consistent with appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. The criteria were provided in a 2014 joint report by the American College of Cardiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
The report rates CAC testing as “May Be Appropriate” for asymptomatic patients of intermediate or high global risk. As such, CAC “can be an option for further evaluation of potential SIHD [stable ischemic heart disease] in an individual patient when deemed reasonable by the patient’s physician,” according to the appropriate use criteria guidance, which was created with the express purpose of developing standards to avoid overuse of costly cardiovascular testing (J Am Coll Cardiol. 2014 Feb 4;63[4]:380-406).
Dr. di Carli reported having no financial conflicts.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Osteoarthritis in hip or knee can increase diabetes risk
Individuals who have osteoarthritis in the hip or knee are significantly more likely to develop diabetes than are people without the condition, according to findings from a population-based cohort study.
The relationship between osteoarthritis (OA) and new-onset diabetes was largely explained by the walking limitations brought on by OA, which was unsurprising to lead author Tetyana Kendzerska, MD, PhD, of the University of Toronto, who presented the study at the annual meeting of the Canadian Rheumatology Association.
But Dr. Kendzerska noted that even though “the World Health Organization has determined that osteoarthritis is the fastest growing chronic disease and the single most common cause of disability in older adults [and] the majority of people with OA have at least one other chronic condition – usually diabetes, high blood pressure, [or] heart disease ... few studies have examined the impact of OA on these other conditions, including on the development of diabetes.”
Dr. Kendzerska and her coauthors used existing data to study a population of 16,362 adults aged 55 or older who did not have diabetes at baseline enrollment during 1996-1998 and were then followed until 2014 for a median period of 13 years. The adults’ median age was 68 years and median body mass index was 25.3 kg/m2; 61% were female. Cox regression modeling was used to quantify any association found between osteoarthritis and diabetes.
A total of 1,637 (10%) had hip osteoarthritis, 2,431 (15%) had knee osteoarthritis, and 3,908 (24%) had some type of walking limitation. Over the course of the follow-up period, 3,539 (22%) developed diabetes. The risk for diabetes was significantly elevated in particular for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees, after adjustment for baseline age, sex, income, body mass index, preexisting hypertension and cardiovascular disease, and prior primary care exposure. However, further adjustment to the comparisons for walking limitation attenuated the relationships so that they were no longer statistically significant.
The next steps for research may be to assess the impact of evidence-based osteoarthritis care on mobility and metabolic derangements, she said.
Previous “studies have been limited by a number of methodological limitations, [such as] cross-sectional design or failure to control for other factors that may explain a relationship. Our study has addressed these limitations and thus provides important evidence to suggest that osteoarthritis-related difficulty walking contributes causally to the development of diabetes [but] now we need studies to show if effective treatment of hip and knee osteoarthritis can reduce diabetes risk.”
The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
Individuals who have osteoarthritis in the hip or knee are significantly more likely to develop diabetes than are people without the condition, according to findings from a population-based cohort study.
The relationship between osteoarthritis (OA) and new-onset diabetes was largely explained by the walking limitations brought on by OA, which was unsurprising to lead author Tetyana Kendzerska, MD, PhD, of the University of Toronto, who presented the study at the annual meeting of the Canadian Rheumatology Association.
But Dr. Kendzerska noted that even though “the World Health Organization has determined that osteoarthritis is the fastest growing chronic disease and the single most common cause of disability in older adults [and] the majority of people with OA have at least one other chronic condition – usually diabetes, high blood pressure, [or] heart disease ... few studies have examined the impact of OA on these other conditions, including on the development of diabetes.”
Dr. Kendzerska and her coauthors used existing data to study a population of 16,362 adults aged 55 or older who did not have diabetes at baseline enrollment during 1996-1998 and were then followed until 2014 for a median period of 13 years. The adults’ median age was 68 years and median body mass index was 25.3 kg/m2; 61% were female. Cox regression modeling was used to quantify any association found between osteoarthritis and diabetes.
A total of 1,637 (10%) had hip osteoarthritis, 2,431 (15%) had knee osteoarthritis, and 3,908 (24%) had some type of walking limitation. Over the course of the follow-up period, 3,539 (22%) developed diabetes. The risk for diabetes was significantly elevated in particular for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees, after adjustment for baseline age, sex, income, body mass index, preexisting hypertension and cardiovascular disease, and prior primary care exposure. However, further adjustment to the comparisons for walking limitation attenuated the relationships so that they were no longer statistically significant.
The next steps for research may be to assess the impact of evidence-based osteoarthritis care on mobility and metabolic derangements, she said.
Previous “studies have been limited by a number of methodological limitations, [such as] cross-sectional design or failure to control for other factors that may explain a relationship. Our study has addressed these limitations and thus provides important evidence to suggest that osteoarthritis-related difficulty walking contributes causally to the development of diabetes [but] now we need studies to show if effective treatment of hip and knee osteoarthritis can reduce diabetes risk.”
The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
Individuals who have osteoarthritis in the hip or knee are significantly more likely to develop diabetes than are people without the condition, according to findings from a population-based cohort study.
The relationship between osteoarthritis (OA) and new-onset diabetes was largely explained by the walking limitations brought on by OA, which was unsurprising to lead author Tetyana Kendzerska, MD, PhD, of the University of Toronto, who presented the study at the annual meeting of the Canadian Rheumatology Association.
But Dr. Kendzerska noted that even though “the World Health Organization has determined that osteoarthritis is the fastest growing chronic disease and the single most common cause of disability in older adults [and] the majority of people with OA have at least one other chronic condition – usually diabetes, high blood pressure, [or] heart disease ... few studies have examined the impact of OA on these other conditions, including on the development of diabetes.”
Dr. Kendzerska and her coauthors used existing data to study a population of 16,362 adults aged 55 or older who did not have diabetes at baseline enrollment during 1996-1998 and were then followed until 2014 for a median period of 13 years. The adults’ median age was 68 years and median body mass index was 25.3 kg/m2; 61% were female. Cox regression modeling was used to quantify any association found between osteoarthritis and diabetes.
A total of 1,637 (10%) had hip osteoarthritis, 2,431 (15%) had knee osteoarthritis, and 3,908 (24%) had some type of walking limitation. Over the course of the follow-up period, 3,539 (22%) developed diabetes. The risk for diabetes was significantly elevated in particular for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees, after adjustment for baseline age, sex, income, body mass index, preexisting hypertension and cardiovascular disease, and prior primary care exposure. However, further adjustment to the comparisons for walking limitation attenuated the relationships so that they were no longer statistically significant.
The next steps for research may be to assess the impact of evidence-based osteoarthritis care on mobility and metabolic derangements, she said.
Previous “studies have been limited by a number of methodological limitations, [such as] cross-sectional design or failure to control for other factors that may explain a relationship. Our study has addressed these limitations and thus provides important evidence to suggest that osteoarthritis-related difficulty walking contributes causally to the development of diabetes [but] now we need studies to show if effective treatment of hip and knee osteoarthritis can reduce diabetes risk.”
The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
FROM THE CRA SCIENTIFIC CONFERENCE
Key clinical point:
Major finding: The risk for diabetes was significantly elevated for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees.
Data source: Population-based cohort study of 16,362 individuals without diabetes at baseline.
Disclosures: The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
Psoriatic arthritis raises diabetes risk
Psoriatic arthritis raises the risk of type 2 diabetes, and the risk correlates with higher disease activity, according to Canadian investigators.
They reviewed 1,065 patients free of diabetes when they entered treatment at the University of Toronto psoriatic arthritis (PsA) clinic during 1978-2014; 73 developed type 2 diabetes.
“This finding highlights the need to screen for DM [diabetes mellitis] in patients with PsA, especially in those with more active joint disease and elevated inflammatory markers. The control of inflammation may reduce the risk of developing DM,” said investigators led by Lihi Eder, MD, a research fellow at the University of Toronto (J Rheumatol. 2017 Feb 1; doi: 10.3899/jrheum.160861).
Psoriasis has been linked to diabetes before; the association with PsA, at least until now, has been less well supported.
Chronic inflammation could be part of the issue; it’s also been linked before to diabetes, independently of insulin resistance and obesity. More severe psoriasis, as indicated by higher Psoriasis Area and Severity Index scores, was associated with diabetes risk in the study, but fell out as an independent predictor after adjustment for obesity and other confounders.
Also, “part of the increased risk of cardiovascular in PsA may be attributed to the higher prevalence of DM, which was found in our study,” the investigators said.
Data about smoking, metabolic syndrome, and C-reactive protein weren’t routinely collected in the earlier years of the cohort, so could not be assessed. “It is possible that some of these variables could have modified the identified link between the extent of inflammation and DM risk,” they said.
Patients were 54 years old on average at baseline, and 56% were men. The mean duration of psoriasis at clinic presentation was 15.4 years, and of psoriatic arthritis 6.5 years. The mean duration of follow-up was 9.1 years.
Dr. Eder was supported by the Krembil Foundation and a Canadian Institutes of Health Research fellowship award.
Psoriatic arthritis raises the risk of type 2 diabetes, and the risk correlates with higher disease activity, according to Canadian investigators.
They reviewed 1,065 patients free of diabetes when they entered treatment at the University of Toronto psoriatic arthritis (PsA) clinic during 1978-2014; 73 developed type 2 diabetes.
“This finding highlights the need to screen for DM [diabetes mellitis] in patients with PsA, especially in those with more active joint disease and elevated inflammatory markers. The control of inflammation may reduce the risk of developing DM,” said investigators led by Lihi Eder, MD, a research fellow at the University of Toronto (J Rheumatol. 2017 Feb 1; doi: 10.3899/jrheum.160861).
Psoriasis has been linked to diabetes before; the association with PsA, at least until now, has been less well supported.
Chronic inflammation could be part of the issue; it’s also been linked before to diabetes, independently of insulin resistance and obesity. More severe psoriasis, as indicated by higher Psoriasis Area and Severity Index scores, was associated with diabetes risk in the study, but fell out as an independent predictor after adjustment for obesity and other confounders.
Also, “part of the increased risk of cardiovascular in PsA may be attributed to the higher prevalence of DM, which was found in our study,” the investigators said.
Data about smoking, metabolic syndrome, and C-reactive protein weren’t routinely collected in the earlier years of the cohort, so could not be assessed. “It is possible that some of these variables could have modified the identified link between the extent of inflammation and DM risk,” they said.
Patients were 54 years old on average at baseline, and 56% were men. The mean duration of psoriasis at clinic presentation was 15.4 years, and of psoriatic arthritis 6.5 years. The mean duration of follow-up was 9.1 years.
Dr. Eder was supported by the Krembil Foundation and a Canadian Institutes of Health Research fellowship award.
Psoriatic arthritis raises the risk of type 2 diabetes, and the risk correlates with higher disease activity, according to Canadian investigators.
They reviewed 1,065 patients free of diabetes when they entered treatment at the University of Toronto psoriatic arthritis (PsA) clinic during 1978-2014; 73 developed type 2 diabetes.
“This finding highlights the need to screen for DM [diabetes mellitis] in patients with PsA, especially in those with more active joint disease and elevated inflammatory markers. The control of inflammation may reduce the risk of developing DM,” said investigators led by Lihi Eder, MD, a research fellow at the University of Toronto (J Rheumatol. 2017 Feb 1; doi: 10.3899/jrheum.160861).
Psoriasis has been linked to diabetes before; the association with PsA, at least until now, has been less well supported.
Chronic inflammation could be part of the issue; it’s also been linked before to diabetes, independently of insulin resistance and obesity. More severe psoriasis, as indicated by higher Psoriasis Area and Severity Index scores, was associated with diabetes risk in the study, but fell out as an independent predictor after adjustment for obesity and other confounders.
Also, “part of the increased risk of cardiovascular in PsA may be attributed to the higher prevalence of DM, which was found in our study,” the investigators said.
Data about smoking, metabolic syndrome, and C-reactive protein weren’t routinely collected in the earlier years of the cohort, so could not be assessed. “It is possible that some of these variables could have modified the identified link between the extent of inflammation and DM risk,” they said.
Patients were 54 years old on average at baseline, and 56% were men. The mean duration of psoriasis at clinic presentation was 15.4 years, and of psoriatic arthritis 6.5 years. The mean duration of follow-up was 9.1 years.
Dr. Eder was supported by the Krembil Foundation and a Canadian Institutes of Health Research fellowship award.
FROM THE JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: Morning tender joint count (HR 1.53), morning erythrocyte sedimentation rate (HR 1.21), and body mass index (HR 1.09) significantly predicted diabetes.
Data source: A review of 1,065 patients free of diabetes when they entered treatment at the University of Toronto psoriatic arthritis (PsA) clinic during 1978-2014.
Disclosures: The lead investigator was supported by the Krembil Foundation and a Canadian Institutes of Health Research fellowship award.
Atherosclerosis severity in diabetes can be predicted by select biomarkers
in patient with type 2 diabetes, results from a long-term analysis of VA patients suggest.
Advanced glycation end products (AGEs) and oxidation products (OxPs) “can damage vascular cells by different mechanisms,” wrote researchers led by corresponding authors Aramesh Saremi, MD, and Peter D. Reaven, MD, and colleagues. The report appeared online Feb. 1 in Diabetes Care.
“One frequently reported pathway is AGE binding to their purported (and relatively promiscuous) receptors on cells, such as macrophages, vascular endothelial cells, and vascular smooth muscle cells, although this has not been consistent for all AGEs. Other mechanisms include, among others, binding to and altering the function of intracellular proteins, the activation of vascular NADPH [nicotinamide adenine dinucleotide phosphate] oxidase, and the uncoupling of endothelial nitric oxide synthase.”
Noting that data in the current medical literature are lacking with respect to long-term longitudinal associations between plasma levels of AGEs and OxPs on the extent of subclinical atherosclerosis in T2D patients, the researchers set out to determine whether baseline plasma levels of AGEs and OxPs are associated with the extent of carotid intima-media thickness (CIMT), coronary artery calcification (CAC), and abdominal aortic artery calcification (AAC) over an average of 10 years of follow-up in the VA Diabetes Trial (VADT). They also examined whether this relationship was altered by intervening improved glucose control (Diabetes Care 2017 Feb. 1. doi: 10.2337/dc16-1875]).
At baseline of the VADT, 411 study participants underwent plasma measurements of methylglyoxal hydroimidazolone, N epsilon–carboxymethyl lysine (CML), N epsilon–carboxyethyl lysine (CEL), 3-deoxyglucosone hydroimidazolone and glyoxal hydroimidazolone (G-H1), 2-aminoadipic acid (2-AAA), and methionine sulfoxide. The mean age of the study subjects was 58 years, 64% were non-Hispanic white, 96% were male, 69% had a history of hypertension, and they had diabetes for a mean of 11 years.
After a mean follow-up of 10 years, the 411 patients underwent ultrasound assessment of CIMT, and computed tomography scanning of CAC and AAC.
In risk factor–adjusted multivariable regression models, G-H1was associated with the extent of CIMT as well as with the extent of CAC (P = .01 for both associations). In addition, 2-AAA was strongly associated with the extent of CAC (P = .03 for continuous variables and P less than .01 for dichotomous variables), and CEL was strongly associated with the extent of AAC (P less than .01).
“These findings suggest that the effect of hyperglycemia and subsequent increased levels of AGEs and OxPs in patients with long-standing T2D may have long-lasting adverse effects on the development of macrovascular complications,” the researchers concluded. They acknowledged certain limitations of the analysis, including the fact that it was conducted in an older, primarily male population. Therefore, “extrapolation of the study findings to other populations must be done with caution,” they wrote. “This study also does not allow us to make a definite claim of causation between AGEs and OxPs with the extent of atherosclerosis.”
The Veterans Affairs Cooperative Studies Program of the U.S. Department of Veterans Affairs Office of Research and Development funded the study. Additional support was received from National Institutes of Health, the American Diabetes Association, and the National Institute of Diabetes and Digestive and Kidney Diseases. Two study authors, Scott Howell, MS, and Paul J. Beisswenger, MD, disclosed that they are affiliated with PreventAGE Healthcare. The other researchers reported having no financial disclosures.
[email protected]
For a long time the question about how glucose control relates to macrovascular disease has not been easy to answer. There are reports suggesting that glucose control correlates with macrovascular disease later in life, and others that do not make the association so convincing. This paper provides an important link between glucose control and the subsequent development of macrovascular disease. The link is by way of advanced glycation end products as well as oxidative byproducts and how they set the stage for macrovascular disease later on in life. It’s something that clinicians have postulated as being important, but to my knowledge this is one of the only studies to actually show the association.
The investigators used important endpoints like coronary artery calcification and carotid intima-media thickness. I was surprised at how well the relationship between glycosylated end products and oxidative products correlated, but there’s biologic plausibility; it makes sense. Glucose not only glycosates hemoglobin, but glycosates proteins throughout. This provides a logical, stepwise pathway for how the initial glycosylated protein will result years later in macrovascular disease as evidenced by the parameters that were used.
It was interesting to learn from this study that glycosylated end products and oxidative products interact with each other. That’s important, because as blood sugars rise acutely, both oxidative products and glycosylated products are produced. As a result of the oxidative stress that’s created by the sharp rise in blood sugar, endothelial function is affected and more glycosylated proteins are being formed.
Clinically, this study shows the importance of early, aggressive control of diabetes to not allow accumulation of both glycosylated end products and oxidative end products. It demonstrates that accumulation of these byproducts years later seems to relate strongly to macrovascular disease.
The study should be reproduced in younger, less sick patients. That may or may not further clarify the findings, but these findings need to be demonstrated in patients at a much earlier stage as well. We’ve been saying for a long time that early control of diabetes is so important because years later it makes a difference. This is a link to that rationale.
Paul S. Jellinger, MD, MACE, is professor of clinical medicine at the University of Miami Miller School of Medicine, Ft. Lauderdale. He is past president of the American Association of Clinical Endocrinologists (AACE) and past president of the American College of Endocrinology. Dr. Jellinger provided these comments in an interview.
For a long time the question about how glucose control relates to macrovascular disease has not been easy to answer. There are reports suggesting that glucose control correlates with macrovascular disease later in life, and others that do not make the association so convincing. This paper provides an important link between glucose control and the subsequent development of macrovascular disease. The link is by way of advanced glycation end products as well as oxidative byproducts and how they set the stage for macrovascular disease later on in life. It’s something that clinicians have postulated as being important, but to my knowledge this is one of the only studies to actually show the association.
The investigators used important endpoints like coronary artery calcification and carotid intima-media thickness. I was surprised at how well the relationship between glycosylated end products and oxidative products correlated, but there’s biologic plausibility; it makes sense. Glucose not only glycosates hemoglobin, but glycosates proteins throughout. This provides a logical, stepwise pathway for how the initial glycosylated protein will result years later in macrovascular disease as evidenced by the parameters that were used.
It was interesting to learn from this study that glycosylated end products and oxidative products interact with each other. That’s important, because as blood sugars rise acutely, both oxidative products and glycosylated products are produced. As a result of the oxidative stress that’s created by the sharp rise in blood sugar, endothelial function is affected and more glycosylated proteins are being formed.
Clinically, this study shows the importance of early, aggressive control of diabetes to not allow accumulation of both glycosylated end products and oxidative end products. It demonstrates that accumulation of these byproducts years later seems to relate strongly to macrovascular disease.
The study should be reproduced in younger, less sick patients. That may or may not further clarify the findings, but these findings need to be demonstrated in patients at a much earlier stage as well. We’ve been saying for a long time that early control of diabetes is so important because years later it makes a difference. This is a link to that rationale.
Paul S. Jellinger, MD, MACE, is professor of clinical medicine at the University of Miami Miller School of Medicine, Ft. Lauderdale. He is past president of the American Association of Clinical Endocrinologists (AACE) and past president of the American College of Endocrinology. Dr. Jellinger provided these comments in an interview.
For a long time the question about how glucose control relates to macrovascular disease has not been easy to answer. There are reports suggesting that glucose control correlates with macrovascular disease later in life, and others that do not make the association so convincing. This paper provides an important link between glucose control and the subsequent development of macrovascular disease. The link is by way of advanced glycation end products as well as oxidative byproducts and how they set the stage for macrovascular disease later on in life. It’s something that clinicians have postulated as being important, but to my knowledge this is one of the only studies to actually show the association.
The investigators used important endpoints like coronary artery calcification and carotid intima-media thickness. I was surprised at how well the relationship between glycosylated end products and oxidative products correlated, but there’s biologic plausibility; it makes sense. Glucose not only glycosates hemoglobin, but glycosates proteins throughout. This provides a logical, stepwise pathway for how the initial glycosylated protein will result years later in macrovascular disease as evidenced by the parameters that were used.
It was interesting to learn from this study that glycosylated end products and oxidative products interact with each other. That’s important, because as blood sugars rise acutely, both oxidative products and glycosylated products are produced. As a result of the oxidative stress that’s created by the sharp rise in blood sugar, endothelial function is affected and more glycosylated proteins are being formed.
Clinically, this study shows the importance of early, aggressive control of diabetes to not allow accumulation of both glycosylated end products and oxidative end products. It demonstrates that accumulation of these byproducts years later seems to relate strongly to macrovascular disease.
The study should be reproduced in younger, less sick patients. That may or may not further clarify the findings, but these findings need to be demonstrated in patients at a much earlier stage as well. We’ve been saying for a long time that early control of diabetes is so important because years later it makes a difference. This is a link to that rationale.
Paul S. Jellinger, MD, MACE, is professor of clinical medicine at the University of Miami Miller School of Medicine, Ft. Lauderdale. He is past president of the American Association of Clinical Endocrinologists (AACE) and past president of the American College of Endocrinology. Dr. Jellinger provided these comments in an interview.
in patient with type 2 diabetes, results from a long-term analysis of VA patients suggest.
Advanced glycation end products (AGEs) and oxidation products (OxPs) “can damage vascular cells by different mechanisms,” wrote researchers led by corresponding authors Aramesh Saremi, MD, and Peter D. Reaven, MD, and colleagues. The report appeared online Feb. 1 in Diabetes Care.
“One frequently reported pathway is AGE binding to their purported (and relatively promiscuous) receptors on cells, such as macrophages, vascular endothelial cells, and vascular smooth muscle cells, although this has not been consistent for all AGEs. Other mechanisms include, among others, binding to and altering the function of intracellular proteins, the activation of vascular NADPH [nicotinamide adenine dinucleotide phosphate] oxidase, and the uncoupling of endothelial nitric oxide synthase.”
Noting that data in the current medical literature are lacking with respect to long-term longitudinal associations between plasma levels of AGEs and OxPs on the extent of subclinical atherosclerosis in T2D patients, the researchers set out to determine whether baseline plasma levels of AGEs and OxPs are associated with the extent of carotid intima-media thickness (CIMT), coronary artery calcification (CAC), and abdominal aortic artery calcification (AAC) over an average of 10 years of follow-up in the VA Diabetes Trial (VADT). They also examined whether this relationship was altered by intervening improved glucose control (Diabetes Care 2017 Feb. 1. doi: 10.2337/dc16-1875]).
At baseline of the VADT, 411 study participants underwent plasma measurements of methylglyoxal hydroimidazolone, N epsilon–carboxymethyl lysine (CML), N epsilon–carboxyethyl lysine (CEL), 3-deoxyglucosone hydroimidazolone and glyoxal hydroimidazolone (G-H1), 2-aminoadipic acid (2-AAA), and methionine sulfoxide. The mean age of the study subjects was 58 years, 64% were non-Hispanic white, 96% were male, 69% had a history of hypertension, and they had diabetes for a mean of 11 years.
After a mean follow-up of 10 years, the 411 patients underwent ultrasound assessment of CIMT, and computed tomography scanning of CAC and AAC.
In risk factor–adjusted multivariable regression models, G-H1was associated with the extent of CIMT as well as with the extent of CAC (P = .01 for both associations). In addition, 2-AAA was strongly associated with the extent of CAC (P = .03 for continuous variables and P less than .01 for dichotomous variables), and CEL was strongly associated with the extent of AAC (P less than .01).
“These findings suggest that the effect of hyperglycemia and subsequent increased levels of AGEs and OxPs in patients with long-standing T2D may have long-lasting adverse effects on the development of macrovascular complications,” the researchers concluded. They acknowledged certain limitations of the analysis, including the fact that it was conducted in an older, primarily male population. Therefore, “extrapolation of the study findings to other populations must be done with caution,” they wrote. “This study also does not allow us to make a definite claim of causation between AGEs and OxPs with the extent of atherosclerosis.”
The Veterans Affairs Cooperative Studies Program of the U.S. Department of Veterans Affairs Office of Research and Development funded the study. Additional support was received from National Institutes of Health, the American Diabetes Association, and the National Institute of Diabetes and Digestive and Kidney Diseases. Two study authors, Scott Howell, MS, and Paul J. Beisswenger, MD, disclosed that they are affiliated with PreventAGE Healthcare. The other researchers reported having no financial disclosures.
[email protected]
in patient with type 2 diabetes, results from a long-term analysis of VA patients suggest.
Advanced glycation end products (AGEs) and oxidation products (OxPs) “can damage vascular cells by different mechanisms,” wrote researchers led by corresponding authors Aramesh Saremi, MD, and Peter D. Reaven, MD, and colleagues. The report appeared online Feb. 1 in Diabetes Care.
“One frequently reported pathway is AGE binding to their purported (and relatively promiscuous) receptors on cells, such as macrophages, vascular endothelial cells, and vascular smooth muscle cells, although this has not been consistent for all AGEs. Other mechanisms include, among others, binding to and altering the function of intracellular proteins, the activation of vascular NADPH [nicotinamide adenine dinucleotide phosphate] oxidase, and the uncoupling of endothelial nitric oxide synthase.”
Noting that data in the current medical literature are lacking with respect to long-term longitudinal associations between plasma levels of AGEs and OxPs on the extent of subclinical atherosclerosis in T2D patients, the researchers set out to determine whether baseline plasma levels of AGEs and OxPs are associated with the extent of carotid intima-media thickness (CIMT), coronary artery calcification (CAC), and abdominal aortic artery calcification (AAC) over an average of 10 years of follow-up in the VA Diabetes Trial (VADT). They also examined whether this relationship was altered by intervening improved glucose control (Diabetes Care 2017 Feb. 1. doi: 10.2337/dc16-1875]).
At baseline of the VADT, 411 study participants underwent plasma measurements of methylglyoxal hydroimidazolone, N epsilon–carboxymethyl lysine (CML), N epsilon–carboxyethyl lysine (CEL), 3-deoxyglucosone hydroimidazolone and glyoxal hydroimidazolone (G-H1), 2-aminoadipic acid (2-AAA), and methionine sulfoxide. The mean age of the study subjects was 58 years, 64% were non-Hispanic white, 96% were male, 69% had a history of hypertension, and they had diabetes for a mean of 11 years.
After a mean follow-up of 10 years, the 411 patients underwent ultrasound assessment of CIMT, and computed tomography scanning of CAC and AAC.
In risk factor–adjusted multivariable regression models, G-H1was associated with the extent of CIMT as well as with the extent of CAC (P = .01 for both associations). In addition, 2-AAA was strongly associated with the extent of CAC (P = .03 for continuous variables and P less than .01 for dichotomous variables), and CEL was strongly associated with the extent of AAC (P less than .01).
“These findings suggest that the effect of hyperglycemia and subsequent increased levels of AGEs and OxPs in patients with long-standing T2D may have long-lasting adverse effects on the development of macrovascular complications,” the researchers concluded. They acknowledged certain limitations of the analysis, including the fact that it was conducted in an older, primarily male population. Therefore, “extrapolation of the study findings to other populations must be done with caution,” they wrote. “This study also does not allow us to make a definite claim of causation between AGEs and OxPs with the extent of atherosclerosis.”
The Veterans Affairs Cooperative Studies Program of the U.S. Department of Veterans Affairs Office of Research and Development funded the study. Additional support was received from National Institutes of Health, the American Diabetes Association, and the National Institute of Diabetes and Digestive and Kidney Diseases. Two study authors, Scott Howell, MS, and Paul J. Beisswenger, MD, disclosed that they are affiliated with PreventAGE Healthcare. The other researchers reported having no financial disclosures.
[email protected]
Key clinical point:
Major finding: Glyoxal hydroimidazolone was associated with the extent of carotid intima-media thickness as well as with the extent of coronary artery calcification.
Data source: An analysis of 411 patients the VA Diabetes Trial.
Disclosures: The Veterans Affairs Cooperative Studies Program of the U.S. Department of Veterans Affairs Office of Research and Development funded the study. Additional support was received from National Institutes of Health, the American Diabetes Association, and the National Institute of Diabetes and Digestive and Kidney Diseases. Two study authors, Scott Howell, MS, and Paul J. Beisswenger, MD, disclosed that they are affiliated with PreventAGE Healthcare. The other researchers reported having no financial disclosures.
Updated guidelines offer insight into pediatric obesity
Among other things, the guidelines offer new insight into the role of genetics in childhood obesity, provide more extensive guidance regarding bariatric surgery in adolescents, and suggest that measurements of insulin concentrations aren’t useful barometers.
The guidelines also point to research gaps in several areas and call for more studies.
Why issue new guidelines now? “Eight years have passed since the last publication. We did a very thorough job, but there’s been an incredible amount of interest in child obesity, and more information is available,” lead author Dennis M. Styne, MD, professor of pediatrics and the Yocha Dehe endowed chair in pediatric endocrinology at the University of California at Davis, said in an interview. Indeed, recent years have produced hundreds of studies into pediatric obesity, he noted.
The 49-page guidelines are cosponsored by the European Society of Endocrinology and the Pediatric Endocrine Society (J Clin Endocrinol Metab. 2017 March;102[3]:1-49).
Pediatric obesity is of special interest to endocrinologists, Dr. Styne said. “While there’s interest from many specialists now, we are at the forefront of evaluation and treatment of complications like polycystic ovary syndrome, metabolic syndrome, dyslipidemia, and type 2 diabetes.”
The guidelines provide recommendations about a wide variety of obesity-related topics including screening, diagnosis, and treatment. The Endocrine Society commissioned two systematic reviews to support the guidelines: One examined longer randomized controlled trials into medication, surgery, lifestyle, and community-based intervention treatments. The other examined how changing body mass index levels corresponded to cardiometabolic changes.
Several updated areas in the guidelines should be of special interest to endocrinologists: guidance regarding the genetic causes of pediatric obesity, the use of weight-loss medication and surgery, and the roles of insulin tests and breast-feeding, according to Dr. Styne.
In regard to genetics, the guidelines note that research suggests 7% of patients with extreme pediatric obesity “may have rare chromosomal abnormalities and/or highly penetrant genetic mutations that drive their obesity. This percentage is likely to increase with newer methods for genetic testing.”
Dr. Styne calls this finding “remarkable.” As he put it, “we didn’t appreciate that so much in the past.”
The guidelines suggest genetic testing for patients who become obese before the age of 5 years, have a family history of extreme obesity, or show clinical signs of genetic obesity syndromes, especially extreme hyperphagia.
As for the most extreme treatments for the most obese children, the guidelines recommend against weight-loss medication outside of clinical trials and note that “increasing evidence” supports bariatric surgery in teens who haven’t been able to lose enough pounds through lifestyle modification. However, “the use of surgery requires experienced teams with resources for long-term follow-up.”
The guidelines also recommend against measuring serum insulin concentrations as part of pediatric screening for obesity. “A lot of people like to get insulin levels and think it tells them about the future of the child,” Dr. Styne said. “But it doesn’t work very well.”
In another area that reflects new information, the guidelines note that breast-feeding hasn’t been definitively shown to be effective in reducing childhood obesity. “The literature is contradictory,” Dr. Styne said, although he noted that breast-feeding still has many other benefits.
The guidelines point to research gaps in several areas, including the prevention and treatment of pediatric obesity. There’s also “uncharted territory” regarding how to “effectively transition to adult care, with continued necessary monitoring, support, and intervention.”
In regard to the best treatment programs, “we didn’t come up with an answer regarding overall effectiveness,” Dr. Styne said. “School- and community-based programs have promise, but I can’t give you the percentage of success.”
As for the overall picture of pediatric obesity in the United States, “we’re in a better situation than we were 8 years ago,” he said. “Everyone is talking about the problem, and when you talk to families, they’re more aware of it.”
Still, he said, the prevalence of obesity in kids is high, estimated at 17% of those aged 2-19 years in 2014. “That’s not a good place to be,” he said. “We still have to work harder.”
The Endocrine Society funded the guidelines. Dr. Styne reports ownership interests in Teva, Bristol Myers and Organovo. Other authors report various disclosures.
Among other things, the guidelines offer new insight into the role of genetics in childhood obesity, provide more extensive guidance regarding bariatric surgery in adolescents, and suggest that measurements of insulin concentrations aren’t useful barometers.
The guidelines also point to research gaps in several areas and call for more studies.
Why issue new guidelines now? “Eight years have passed since the last publication. We did a very thorough job, but there’s been an incredible amount of interest in child obesity, and more information is available,” lead author Dennis M. Styne, MD, professor of pediatrics and the Yocha Dehe endowed chair in pediatric endocrinology at the University of California at Davis, said in an interview. Indeed, recent years have produced hundreds of studies into pediatric obesity, he noted.
The 49-page guidelines are cosponsored by the European Society of Endocrinology and the Pediatric Endocrine Society (J Clin Endocrinol Metab. 2017 March;102[3]:1-49).
Pediatric obesity is of special interest to endocrinologists, Dr. Styne said. “While there’s interest from many specialists now, we are at the forefront of evaluation and treatment of complications like polycystic ovary syndrome, metabolic syndrome, dyslipidemia, and type 2 diabetes.”
The guidelines provide recommendations about a wide variety of obesity-related topics including screening, diagnosis, and treatment. The Endocrine Society commissioned two systematic reviews to support the guidelines: One examined longer randomized controlled trials into medication, surgery, lifestyle, and community-based intervention treatments. The other examined how changing body mass index levels corresponded to cardiometabolic changes.
Several updated areas in the guidelines should be of special interest to endocrinologists: guidance regarding the genetic causes of pediatric obesity, the use of weight-loss medication and surgery, and the roles of insulin tests and breast-feeding, according to Dr. Styne.
In regard to genetics, the guidelines note that research suggests 7% of patients with extreme pediatric obesity “may have rare chromosomal abnormalities and/or highly penetrant genetic mutations that drive their obesity. This percentage is likely to increase with newer methods for genetic testing.”
Dr. Styne calls this finding “remarkable.” As he put it, “we didn’t appreciate that so much in the past.”
The guidelines suggest genetic testing for patients who become obese before the age of 5 years, have a family history of extreme obesity, or show clinical signs of genetic obesity syndromes, especially extreme hyperphagia.
As for the most extreme treatments for the most obese children, the guidelines recommend against weight-loss medication outside of clinical trials and note that “increasing evidence” supports bariatric surgery in teens who haven’t been able to lose enough pounds through lifestyle modification. However, “the use of surgery requires experienced teams with resources for long-term follow-up.”
The guidelines also recommend against measuring serum insulin concentrations as part of pediatric screening for obesity. “A lot of people like to get insulin levels and think it tells them about the future of the child,” Dr. Styne said. “But it doesn’t work very well.”
In another area that reflects new information, the guidelines note that breast-feeding hasn’t been definitively shown to be effective in reducing childhood obesity. “The literature is contradictory,” Dr. Styne said, although he noted that breast-feeding still has many other benefits.
The guidelines point to research gaps in several areas, including the prevention and treatment of pediatric obesity. There’s also “uncharted territory” regarding how to “effectively transition to adult care, with continued necessary monitoring, support, and intervention.”
In regard to the best treatment programs, “we didn’t come up with an answer regarding overall effectiveness,” Dr. Styne said. “School- and community-based programs have promise, but I can’t give you the percentage of success.”
As for the overall picture of pediatric obesity in the United States, “we’re in a better situation than we were 8 years ago,” he said. “Everyone is talking about the problem, and when you talk to families, they’re more aware of it.”
Still, he said, the prevalence of obesity in kids is high, estimated at 17% of those aged 2-19 years in 2014. “That’s not a good place to be,” he said. “We still have to work harder.”
The Endocrine Society funded the guidelines. Dr. Styne reports ownership interests in Teva, Bristol Myers and Organovo. Other authors report various disclosures.
Among other things, the guidelines offer new insight into the role of genetics in childhood obesity, provide more extensive guidance regarding bariatric surgery in adolescents, and suggest that measurements of insulin concentrations aren’t useful barometers.
The guidelines also point to research gaps in several areas and call for more studies.
Why issue new guidelines now? “Eight years have passed since the last publication. We did a very thorough job, but there’s been an incredible amount of interest in child obesity, and more information is available,” lead author Dennis M. Styne, MD, professor of pediatrics and the Yocha Dehe endowed chair in pediatric endocrinology at the University of California at Davis, said in an interview. Indeed, recent years have produced hundreds of studies into pediatric obesity, he noted.
The 49-page guidelines are cosponsored by the European Society of Endocrinology and the Pediatric Endocrine Society (J Clin Endocrinol Metab. 2017 March;102[3]:1-49).
Pediatric obesity is of special interest to endocrinologists, Dr. Styne said. “While there’s interest from many specialists now, we are at the forefront of evaluation and treatment of complications like polycystic ovary syndrome, metabolic syndrome, dyslipidemia, and type 2 diabetes.”
The guidelines provide recommendations about a wide variety of obesity-related topics including screening, diagnosis, and treatment. The Endocrine Society commissioned two systematic reviews to support the guidelines: One examined longer randomized controlled trials into medication, surgery, lifestyle, and community-based intervention treatments. The other examined how changing body mass index levels corresponded to cardiometabolic changes.
Several updated areas in the guidelines should be of special interest to endocrinologists: guidance regarding the genetic causes of pediatric obesity, the use of weight-loss medication and surgery, and the roles of insulin tests and breast-feeding, according to Dr. Styne.
In regard to genetics, the guidelines note that research suggests 7% of patients with extreme pediatric obesity “may have rare chromosomal abnormalities and/or highly penetrant genetic mutations that drive their obesity. This percentage is likely to increase with newer methods for genetic testing.”
Dr. Styne calls this finding “remarkable.” As he put it, “we didn’t appreciate that so much in the past.”
The guidelines suggest genetic testing for patients who become obese before the age of 5 years, have a family history of extreme obesity, or show clinical signs of genetic obesity syndromes, especially extreme hyperphagia.
As for the most extreme treatments for the most obese children, the guidelines recommend against weight-loss medication outside of clinical trials and note that “increasing evidence” supports bariatric surgery in teens who haven’t been able to lose enough pounds through lifestyle modification. However, “the use of surgery requires experienced teams with resources for long-term follow-up.”
The guidelines also recommend against measuring serum insulin concentrations as part of pediatric screening for obesity. “A lot of people like to get insulin levels and think it tells them about the future of the child,” Dr. Styne said. “But it doesn’t work very well.”
In another area that reflects new information, the guidelines note that breast-feeding hasn’t been definitively shown to be effective in reducing childhood obesity. “The literature is contradictory,” Dr. Styne said, although he noted that breast-feeding still has many other benefits.
The guidelines point to research gaps in several areas, including the prevention and treatment of pediatric obesity. There’s also “uncharted territory” regarding how to “effectively transition to adult care, with continued necessary monitoring, support, and intervention.”
In regard to the best treatment programs, “we didn’t come up with an answer regarding overall effectiveness,” Dr. Styne said. “School- and community-based programs have promise, but I can’t give you the percentage of success.”
As for the overall picture of pediatric obesity in the United States, “we’re in a better situation than we were 8 years ago,” he said. “Everyone is talking about the problem, and when you talk to families, they’re more aware of it.”
Still, he said, the prevalence of obesity in kids is high, estimated at 17% of those aged 2-19 years in 2014. “That’s not a good place to be,” he said. “We still have to work harder.”
The Endocrine Society funded the guidelines. Dr. Styne reports ownership interests in Teva, Bristol Myers and Organovo. Other authors report various disclosures.
Even small weight loss can improve long-term atrial fib ablation success
ORLANDO – Excess body weight exerts a major negative impact on the likelihood of remaining free of atrial fibrillation long term after an ablation procedure, according to a new set of data and a review of published studies.
“Just losing 3 pounds can dramatically improve the long-term success of an ablation when compared to a weight gain,” reported John D. Day, MD, medical director, Intermountain Heart Rhythm Specialists, Salt Lake City, at the annual International AF Symposium.
“As BMI goes up, long-term success in controlling AF goes down. The difference at 1 year may not be a big deal, but if you follow patients for a long time, weight control is a very big deal,” Dr. Day advised. He emphasized repeatedly, “We are just talking about a few pounds” for a favorable effect.
New data presented at the meeting supported the message. In the study, ablation outcomes in relationship to body mass index (BMI) were evaluated in 2,715 AF patients undergoing 3,742 ablations. Patients were stratified into five groups by BMI: less than 25 kg/m2, 25 to less than 30; 30 to less than 35; 35 to less than 40, and at least 40.
As BMI increased from less than 25 to at least 40, there were significant increases in left atrial size (P less than .005), CHADS2 scores (P = .002), persistent AF (P less than .0001), and longstanding AF (P less than .0001). Unlike persistent and long-term AF, rates of paroxysmal AF fell (48% to 16.3%; P less than .0001).
Not surprisingly and consistent with other published reports, increasing BMI was associated with increases in many of the key risk factors for AF in the study.
Specifically, as BMI increased from less than 25 to at least 40, the proportion of patients with cardiomyopathy climbed from 7.6% to 12.4% (P less than .001), hypertension climbed from 41% to 72.9% (P less than .0001), diabetes climbed from 4.3% to 23.3% (P less than .0001), and sleep apnea climbed from 7.0% to 46.9% (P less than .0001).
Dr. Day cited the LEGACY trial as one of the most influential studies associating weight loss with a reduction in AF burden (J Am Coll Cardiol. 2015 May 26;65[20]:2159-69). In that study, weight loss of at least 10% resulted in a sixfold increased likelihood of AF-free survival. Independent of AF, Dr. Day also pointed out that the sense of well-being among patients who achieved weight loss improved 200%.
Recognizing that major weight loss is difficult to achieve, Dr. Day repeatedly returned to the theme of weight control.
He cited one study in which AF patients were randomized to a weight loss program or usual care. In the usual care group, which included physician advice to lose weight, there was a small but significant weight loss. Even though the effect of that weight loss on AF burden was a fraction of that achieved in the group that achieved greater reductions in weight on active management, it, too, was significant, according to Dr. Day.
“Even brief physician advice can have a meaningful influence on waist circumference,” said Dr. Day, who urged physicians to inform their AF patients about the benefits of weight loss. Failing to do so might deprive patients of achieving the very modest reductions in weight loss required to improve their likelihood of freedom from AF, he added.
Dr. Winkle had no relevant financial relationships. Dr. Day reported a financial relationship with St. Jude Medical.
ORLANDO – Excess body weight exerts a major negative impact on the likelihood of remaining free of atrial fibrillation long term after an ablation procedure, according to a new set of data and a review of published studies.
“Just losing 3 pounds can dramatically improve the long-term success of an ablation when compared to a weight gain,” reported John D. Day, MD, medical director, Intermountain Heart Rhythm Specialists, Salt Lake City, at the annual International AF Symposium.
“As BMI goes up, long-term success in controlling AF goes down. The difference at 1 year may not be a big deal, but if you follow patients for a long time, weight control is a very big deal,” Dr. Day advised. He emphasized repeatedly, “We are just talking about a few pounds” for a favorable effect.
New data presented at the meeting supported the message. In the study, ablation outcomes in relationship to body mass index (BMI) were evaluated in 2,715 AF patients undergoing 3,742 ablations. Patients were stratified into five groups by BMI: less than 25 kg/m2, 25 to less than 30; 30 to less than 35; 35 to less than 40, and at least 40.
As BMI increased from less than 25 to at least 40, there were significant increases in left atrial size (P less than .005), CHADS2 scores (P = .002), persistent AF (P less than .0001), and longstanding AF (P less than .0001). Unlike persistent and long-term AF, rates of paroxysmal AF fell (48% to 16.3%; P less than .0001).
Not surprisingly and consistent with other published reports, increasing BMI was associated with increases in many of the key risk factors for AF in the study.
Specifically, as BMI increased from less than 25 to at least 40, the proportion of patients with cardiomyopathy climbed from 7.6% to 12.4% (P less than .001), hypertension climbed from 41% to 72.9% (P less than .0001), diabetes climbed from 4.3% to 23.3% (P less than .0001), and sleep apnea climbed from 7.0% to 46.9% (P less than .0001).
Dr. Day cited the LEGACY trial as one of the most influential studies associating weight loss with a reduction in AF burden (J Am Coll Cardiol. 2015 May 26;65[20]:2159-69). In that study, weight loss of at least 10% resulted in a sixfold increased likelihood of AF-free survival. Independent of AF, Dr. Day also pointed out that the sense of well-being among patients who achieved weight loss improved 200%.
Recognizing that major weight loss is difficult to achieve, Dr. Day repeatedly returned to the theme of weight control.
He cited one study in which AF patients were randomized to a weight loss program or usual care. In the usual care group, which included physician advice to lose weight, there was a small but significant weight loss. Even though the effect of that weight loss on AF burden was a fraction of that achieved in the group that achieved greater reductions in weight on active management, it, too, was significant, according to Dr. Day.
“Even brief physician advice can have a meaningful influence on waist circumference,” said Dr. Day, who urged physicians to inform their AF patients about the benefits of weight loss. Failing to do so might deprive patients of achieving the very modest reductions in weight loss required to improve their likelihood of freedom from AF, he added.
Dr. Winkle had no relevant financial relationships. Dr. Day reported a financial relationship with St. Jude Medical.
ORLANDO – Excess body weight exerts a major negative impact on the likelihood of remaining free of atrial fibrillation long term after an ablation procedure, according to a new set of data and a review of published studies.
“Just losing 3 pounds can dramatically improve the long-term success of an ablation when compared to a weight gain,” reported John D. Day, MD, medical director, Intermountain Heart Rhythm Specialists, Salt Lake City, at the annual International AF Symposium.
“As BMI goes up, long-term success in controlling AF goes down. The difference at 1 year may not be a big deal, but if you follow patients for a long time, weight control is a very big deal,” Dr. Day advised. He emphasized repeatedly, “We are just talking about a few pounds” for a favorable effect.
New data presented at the meeting supported the message. In the study, ablation outcomes in relationship to body mass index (BMI) were evaluated in 2,715 AF patients undergoing 3,742 ablations. Patients were stratified into five groups by BMI: less than 25 kg/m2, 25 to less than 30; 30 to less than 35; 35 to less than 40, and at least 40.
As BMI increased from less than 25 to at least 40, there were significant increases in left atrial size (P less than .005), CHADS2 scores (P = .002), persistent AF (P less than .0001), and longstanding AF (P less than .0001). Unlike persistent and long-term AF, rates of paroxysmal AF fell (48% to 16.3%; P less than .0001).
Not surprisingly and consistent with other published reports, increasing BMI was associated with increases in many of the key risk factors for AF in the study.
Specifically, as BMI increased from less than 25 to at least 40, the proportion of patients with cardiomyopathy climbed from 7.6% to 12.4% (P less than .001), hypertension climbed from 41% to 72.9% (P less than .0001), diabetes climbed from 4.3% to 23.3% (P less than .0001), and sleep apnea climbed from 7.0% to 46.9% (P less than .0001).
Dr. Day cited the LEGACY trial as one of the most influential studies associating weight loss with a reduction in AF burden (J Am Coll Cardiol. 2015 May 26;65[20]:2159-69). In that study, weight loss of at least 10% resulted in a sixfold increased likelihood of AF-free survival. Independent of AF, Dr. Day also pointed out that the sense of well-being among patients who achieved weight loss improved 200%.
Recognizing that major weight loss is difficult to achieve, Dr. Day repeatedly returned to the theme of weight control.
He cited one study in which AF patients were randomized to a weight loss program or usual care. In the usual care group, which included physician advice to lose weight, there was a small but significant weight loss. Even though the effect of that weight loss on AF burden was a fraction of that achieved in the group that achieved greater reductions in weight on active management, it, too, was significant, according to Dr. Day.
“Even brief physician advice can have a meaningful influence on waist circumference,” said Dr. Day, who urged physicians to inform their AF patients about the benefits of weight loss. Failing to do so might deprive patients of achieving the very modest reductions in weight loss required to improve their likelihood of freedom from AF, he added.
Dr. Winkle had no relevant financial relationships. Dr. Day reported a financial relationship with St. Jude Medical.
Key clinical point: New data expand evidence that obesity reduces long-term success of ablation for atrial fibrillation.
Major finding: Freedom from AF 5 years after ablation fell from 70% in patients with a BMI of less than 35 kg/m2 to 57% in those with BMIs of at least 35.
Data source: A retrospective observational study.
Disclosures: Dr. Winkle had no relevant financial relationships; Dr. Day reported a financial relationship with St. Jude Medical.
Hypertension risk soars in offspring of early-HT parents
NEW ORLEANS – Young adults whose parents develop hypertension before age 55 years are themselves at sharply increased risk of developing the disease, according to a new report from the Framingham (Mass.) Heart Study.
“Our results demonstrate that early-onset but not late-onset hypertension in parents is a strong risk factor for incident hypertension. It may be important for physicians to distinguish between early- and late-onset hypertension as a familial trait when assessing an individual’s risk for hypertension,” Teemu J. Niiranen, MD, said at the American Heart Association scientific sessions.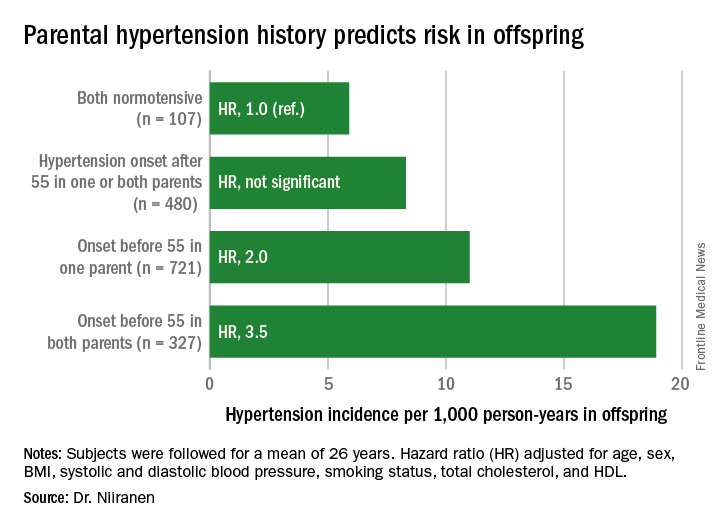
He reported on 1,635 participants in the Offspring cohort of the Framingham Heart Study who were normotensive when they enrolled in the prospective study beginning in 1972. At that time, they averaged 32 years of age. They were followed for a mean of 26 years. Like their parents who enrolled in the Original cohort of the landmark study beginning in 1948, they underwent meticulous blood pressure measurement roughly every 2 years.
Dr. Niiranen and his coinvestigators divided the Offspring cohort into four groups based upon parental hypertension status. There were 107 offspring with normotensive parents, 480 with one or both parents having developed late-onset hypertension after age 55 years, 721 offspring who had one parent with onset of hypertension before age 55 years, and 327 with both parents having early-onset hypertension.
The incidence rate of hypertension in the Offspring cohort climbed in concert with parental early hypertension status. So did the multivariate-adjusted relative risk of the disease, compared with children of normotensive parents.
Moreover, the earlier in life the parents developed hypertension, the earlier their offspring did, too.
Session moderator David J. Maron, MD, of Stanford (Calif.) University, commented, “Everybody’s thinking ‘genetics’ as we look at your findings. But do you have any way to tease out nature versus nurture in understanding the association?”
Dr. Niiranen replied that in a separate study of three generations of Framingham participants, the investigators incorporated two lifestyle factors in their analysis: level of exercise and sodium intake.
“Those didn’t have much effect on the results, so it seems like genetics is driving most of the outcome,” he said.
Dr. Niiranen reported having no financial conflicts of interest regarding his study, sponsored by the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Young adults whose parents develop hypertension before age 55 years are themselves at sharply increased risk of developing the disease, according to a new report from the Framingham (Mass.) Heart Study.
“Our results demonstrate that early-onset but not late-onset hypertension in parents is a strong risk factor for incident hypertension. It may be important for physicians to distinguish between early- and late-onset hypertension as a familial trait when assessing an individual’s risk for hypertension,” Teemu J. Niiranen, MD, said at the American Heart Association scientific sessions.
He reported on 1,635 participants in the Offspring cohort of the Framingham Heart Study who were normotensive when they enrolled in the prospective study beginning in 1972. At that time, they averaged 32 years of age. They were followed for a mean of 26 years. Like their parents who enrolled in the Original cohort of the landmark study beginning in 1948, they underwent meticulous blood pressure measurement roughly every 2 years.
Dr. Niiranen and his coinvestigators divided the Offspring cohort into four groups based upon parental hypertension status. There were 107 offspring with normotensive parents, 480 with one or both parents having developed late-onset hypertension after age 55 years, 721 offspring who had one parent with onset of hypertension before age 55 years, and 327 with both parents having early-onset hypertension.
The incidence rate of hypertension in the Offspring cohort climbed in concert with parental early hypertension status. So did the multivariate-adjusted relative risk of the disease, compared with children of normotensive parents.
Moreover, the earlier in life the parents developed hypertension, the earlier their offspring did, too.
Session moderator David J. Maron, MD, of Stanford (Calif.) University, commented, “Everybody’s thinking ‘genetics’ as we look at your findings. But do you have any way to tease out nature versus nurture in understanding the association?”
Dr. Niiranen replied that in a separate study of three generations of Framingham participants, the investigators incorporated two lifestyle factors in their analysis: level of exercise and sodium intake.
“Those didn’t have much effect on the results, so it seems like genetics is driving most of the outcome,” he said.
Dr. Niiranen reported having no financial conflicts of interest regarding his study, sponsored by the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Young adults whose parents develop hypertension before age 55 years are themselves at sharply increased risk of developing the disease, according to a new report from the Framingham (Mass.) Heart Study.
“Our results demonstrate that early-onset but not late-onset hypertension in parents is a strong risk factor for incident hypertension. It may be important for physicians to distinguish between early- and late-onset hypertension as a familial trait when assessing an individual’s risk for hypertension,” Teemu J. Niiranen, MD, said at the American Heart Association scientific sessions.
He reported on 1,635 participants in the Offspring cohort of the Framingham Heart Study who were normotensive when they enrolled in the prospective study beginning in 1972. At that time, they averaged 32 years of age. They were followed for a mean of 26 years. Like their parents who enrolled in the Original cohort of the landmark study beginning in 1948, they underwent meticulous blood pressure measurement roughly every 2 years.
Dr. Niiranen and his coinvestigators divided the Offspring cohort into four groups based upon parental hypertension status. There were 107 offspring with normotensive parents, 480 with one or both parents having developed late-onset hypertension after age 55 years, 721 offspring who had one parent with onset of hypertension before age 55 years, and 327 with both parents having early-onset hypertension.
The incidence rate of hypertension in the Offspring cohort climbed in concert with parental early hypertension status. So did the multivariate-adjusted relative risk of the disease, compared with children of normotensive parents.
Moreover, the earlier in life the parents developed hypertension, the earlier their offspring did, too.
Session moderator David J. Maron, MD, of Stanford (Calif.) University, commented, “Everybody’s thinking ‘genetics’ as we look at your findings. But do you have any way to tease out nature versus nurture in understanding the association?”
Dr. Niiranen replied that in a separate study of three generations of Framingham participants, the investigators incorporated two lifestyle factors in their analysis: level of exercise and sodium intake.
“Those didn’t have much effect on the results, so it seems like genetics is driving most of the outcome,” he said.
Dr. Niiranen reported having no financial conflicts of interest regarding his study, sponsored by the National Heart, Lung, and Blood Institute.
Key clinical point:
Major finding: Young adults with two parents who developed hypertension before age 55 years are at 3.5-fold greater risk of subsequently developing hypertension than if both parents were normotensive.
Data source: This was an analysis of the incidence of hypertension during a mean prospective follow-up of 26 years in 1,635 members of the Offspring cohort of the Framingham Heart Study who enrolled as young adults.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study sponsored by the National Heart, Lung, and Blood Institute.
Semaglutide compares well with sitagliptin
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.
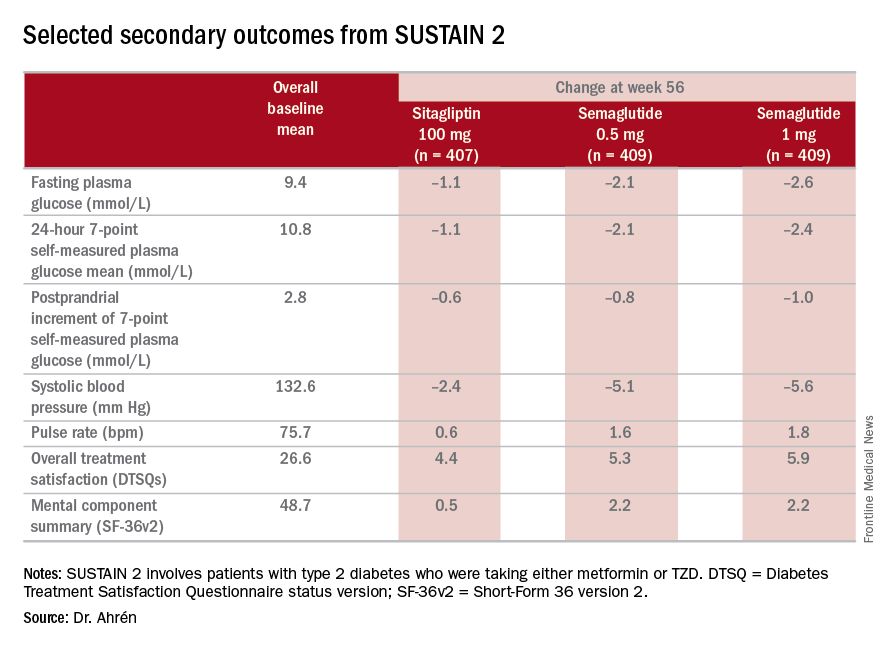
The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.

The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.

The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Investigators for a phase III trial have found weekly semaglutide superior to daily sitagliptin as add-on therapy for improving glycemic control and reducing body weight in type 2 diabetes.
Major finding: Semaglutide 0.5 and 1 mg showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c, compared with an average reduction of 0.5% for sitagliptin.
Data source: SUSTAIN 2 double-blind, randomized trial of 1,231 patients with type 2 diabetes taking either metformin or thiazolidinediones.
Disclosures: Dr. Ahrén disclosed relationships with Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Novo Nordisk, and Sanofi-Aventis Deutschland.














