User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Selective cooling technology being used to remove age spots
, according to Arisa E. Ortiz, MD.
“What’s unique about this device is that I can see results without any downtime,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said during a virtual course on laser and aesthetic skin therapy. “Most other devices are not like that. It was well tolerated; there was minimal pain. There was no postinflammatory hyperpigmentation; it really is customizable to the patients’ needs.”
First cleared by the Food and Drug Administration in 2016 to remove benign lesions of the skin, Glacial Rx received an expanded indication in 2020 to temporarily reduce pain, swelling, and inflammation. The device, which was developed by R2 Technologies, relies on cryomodulation, a concept developed at Massachusetts General Hospital and the Wellman Center for Photomedicine, Boston, to improve skin appearance and freeze melanin at the source. “Cryomodulation pauses melanin production, but the melanocyte function is preserved, the epidermal barrier is not disrupted, and there is no persistent inflammatory response, which is key, because it decreases the risk of postinflammatory hyperpigmentation, especially in darker skin types,” Dr. Ortiz said.
Here’s how it works: The handpiece of the device is placed on top of the skin and cooling is delivered to targeted solar lentigos and other benign lesions. Ice nucleation takes place within the dendrites. As cell turnover takes place, melanin-free cells migrate upward and appear as new skin. “Clinically, this appears as clearance of the lesion,” Dr. Ortiz said.
She discussed her clinical experience treating 15 patients with a beta version of the device. Since that time, Glacial Rx was redesigned to include a smaller-tipped handpiece, easier and faster prep time, and a proprietary water-based gel to facilitate ice crystal propagation, which is applied to the targeted lesions just prior to treatment.
For the trial at UCSD, the researchers performed 29 treatment sessions on 15 patients with Fitzpatrick skin types I-IV, to gain clinical experience and evaluate the effectiveness of the device. They found that the treatment was well tolerated, with minimal discomfort. The amount of heat extracted ranged from 107 to 166 kJ/cm2. No long-term dyschromia was observed, and some patients had lesion clearance after just one treatment.
“The settings are able to be titrated to where you have zero downtime, but you still get a lightening effect,” Dr. Ortiz said during the meeting, named “Laser & Aesthetic Skin Therapy: What’s the Truth?” sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “With other devices such as intense pulsed light, if you don’t see darkening than it probably didn’t work. With this device, you can titrate the length of the cooling and the temperature of the cooling.”
Posttreatment side effects commonly observed in the study were mild erythema, swelling, itching, and darkening. “There was minimal erythema in the higher settings, and some reports of itching and transient darkening in some of the higher settings,” she said.
Future indications for Glacial Rx may include psoriasis, acne, and rosacea. “We did try to use this for melasma,” she said. “It was effective, but I wouldn’t say it’s a cure for melasma. Melasma is very stubborn and requires a combination treatment, but it’s something we can use in our armamentarium.”
Dr. Ortiz reported having received consulting fees from R2 Technologies. She has been a paid consultant for and has received equipment from many device companies.
, according to Arisa E. Ortiz, MD.
“What’s unique about this device is that I can see results without any downtime,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said during a virtual course on laser and aesthetic skin therapy. “Most other devices are not like that. It was well tolerated; there was minimal pain. There was no postinflammatory hyperpigmentation; it really is customizable to the patients’ needs.”
First cleared by the Food and Drug Administration in 2016 to remove benign lesions of the skin, Glacial Rx received an expanded indication in 2020 to temporarily reduce pain, swelling, and inflammation. The device, which was developed by R2 Technologies, relies on cryomodulation, a concept developed at Massachusetts General Hospital and the Wellman Center for Photomedicine, Boston, to improve skin appearance and freeze melanin at the source. “Cryomodulation pauses melanin production, but the melanocyte function is preserved, the epidermal barrier is not disrupted, and there is no persistent inflammatory response, which is key, because it decreases the risk of postinflammatory hyperpigmentation, especially in darker skin types,” Dr. Ortiz said.
Here’s how it works: The handpiece of the device is placed on top of the skin and cooling is delivered to targeted solar lentigos and other benign lesions. Ice nucleation takes place within the dendrites. As cell turnover takes place, melanin-free cells migrate upward and appear as new skin. “Clinically, this appears as clearance of the lesion,” Dr. Ortiz said.
She discussed her clinical experience treating 15 patients with a beta version of the device. Since that time, Glacial Rx was redesigned to include a smaller-tipped handpiece, easier and faster prep time, and a proprietary water-based gel to facilitate ice crystal propagation, which is applied to the targeted lesions just prior to treatment.
For the trial at UCSD, the researchers performed 29 treatment sessions on 15 patients with Fitzpatrick skin types I-IV, to gain clinical experience and evaluate the effectiveness of the device. They found that the treatment was well tolerated, with minimal discomfort. The amount of heat extracted ranged from 107 to 166 kJ/cm2. No long-term dyschromia was observed, and some patients had lesion clearance after just one treatment.
“The settings are able to be titrated to where you have zero downtime, but you still get a lightening effect,” Dr. Ortiz said during the meeting, named “Laser & Aesthetic Skin Therapy: What’s the Truth?” sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “With other devices such as intense pulsed light, if you don’t see darkening than it probably didn’t work. With this device, you can titrate the length of the cooling and the temperature of the cooling.”
Posttreatment side effects commonly observed in the study were mild erythema, swelling, itching, and darkening. “There was minimal erythema in the higher settings, and some reports of itching and transient darkening in some of the higher settings,” she said.
Future indications for Glacial Rx may include psoriasis, acne, and rosacea. “We did try to use this for melasma,” she said. “It was effective, but I wouldn’t say it’s a cure for melasma. Melasma is very stubborn and requires a combination treatment, but it’s something we can use in our armamentarium.”
Dr. Ortiz reported having received consulting fees from R2 Technologies. She has been a paid consultant for and has received equipment from many device companies.
, according to Arisa E. Ortiz, MD.
“What’s unique about this device is that I can see results without any downtime,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said during a virtual course on laser and aesthetic skin therapy. “Most other devices are not like that. It was well tolerated; there was minimal pain. There was no postinflammatory hyperpigmentation; it really is customizable to the patients’ needs.”
First cleared by the Food and Drug Administration in 2016 to remove benign lesions of the skin, Glacial Rx received an expanded indication in 2020 to temporarily reduce pain, swelling, and inflammation. The device, which was developed by R2 Technologies, relies on cryomodulation, a concept developed at Massachusetts General Hospital and the Wellman Center for Photomedicine, Boston, to improve skin appearance and freeze melanin at the source. “Cryomodulation pauses melanin production, but the melanocyte function is preserved, the epidermal barrier is not disrupted, and there is no persistent inflammatory response, which is key, because it decreases the risk of postinflammatory hyperpigmentation, especially in darker skin types,” Dr. Ortiz said.
Here’s how it works: The handpiece of the device is placed on top of the skin and cooling is delivered to targeted solar lentigos and other benign lesions. Ice nucleation takes place within the dendrites. As cell turnover takes place, melanin-free cells migrate upward and appear as new skin. “Clinically, this appears as clearance of the lesion,” Dr. Ortiz said.
She discussed her clinical experience treating 15 patients with a beta version of the device. Since that time, Glacial Rx was redesigned to include a smaller-tipped handpiece, easier and faster prep time, and a proprietary water-based gel to facilitate ice crystal propagation, which is applied to the targeted lesions just prior to treatment.
For the trial at UCSD, the researchers performed 29 treatment sessions on 15 patients with Fitzpatrick skin types I-IV, to gain clinical experience and evaluate the effectiveness of the device. They found that the treatment was well tolerated, with minimal discomfort. The amount of heat extracted ranged from 107 to 166 kJ/cm2. No long-term dyschromia was observed, and some patients had lesion clearance after just one treatment.
“The settings are able to be titrated to where you have zero downtime, but you still get a lightening effect,” Dr. Ortiz said during the meeting, named “Laser & Aesthetic Skin Therapy: What’s the Truth?” sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “With other devices such as intense pulsed light, if you don’t see darkening than it probably didn’t work. With this device, you can titrate the length of the cooling and the temperature of the cooling.”
Posttreatment side effects commonly observed in the study were mild erythema, swelling, itching, and darkening. “There was minimal erythema in the higher settings, and some reports of itching and transient darkening in some of the higher settings,” she said.
Future indications for Glacial Rx may include psoriasis, acne, and rosacea. “We did try to use this for melasma,” she said. “It was effective, but I wouldn’t say it’s a cure for melasma. Melasma is very stubborn and requires a combination treatment, but it’s something we can use in our armamentarium.”
Dr. Ortiz reported having received consulting fees from R2 Technologies. She has been a paid consultant for and has received equipment from many device companies.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Disinclined to offer laser hair removal? An expert makes the case to think otherwise
Omar A. Ibrahimi, MD, PhD, hears some dermatology colleagues say they don’t bother to offer laser hair removal in their practices because they figure that the procedure is under the purview of medical spas, but he sees it differently.
“I offer laser hair removal in my practice as a way to protect my patients from being picked off by medical spas,” Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, said during a virtual course on laser and aesthetic skin therapy. “These patients are going to want to get laser hair removal. If they’re not going to have the opportunity to get it at your practice, they’re going to seek it elsewhere. When they go elsewhere, they’re going to be picked off for other procedures as well.”
First developed in 1995 by R. Rox Anderson, MD, and colleagues at The Wellman Center for Photomedicine, laser hair removal has become the gold standard for permanent hair destruction, and ranks as the most common energy-based procedure performed in the world, Dr. Ibrahimi said. “Results are very long lasting and durable beyond 2 years after treatment,” he said. “These patients tend to be highly satisfied and have permanence with these treatments.”
Treatment goal, patient selection
While the target chromophore for the procedure is melanin, the goal is to destroy the stem cells located in the hair bulge and the hair bulb. “This is technically called the extended theory of selective photothermolysis, but it’s the same concept except that our target chromophore and our desired target for destruction are slightly spatially separated,” he said.
Proper patient selection is key, so a focused medical history and physical exam are essential prior to the procedure. If unwanted hair is located on the face, jawline, or chest of a female, consider and ask about potential endocrine-related dysfunctions such as polycystic ovary syndrome (PCOS). “Getting those addressed can often help the hypertrichosis as well,” he said. “Another condition is explosive hypertrichosis where hair growth starts very suddenly. It’s uncommon but it’s something to think about.”
Pregnancy is not an absolute contraindication for laser hair removal, Dr. Ibrahimi continued, but he elects not to perform the procedure on pregnant patients. He also asks patients about any history of photosensitivity, active infection at the intended treatment site, keloids, or hypertrophic scarring. Past methods of hair removal also matter. “What we’re targeting is the pigment in the hair shafts,” he said. “So, if your patient is waxing or plucking or epilating or removing the hair in some manner, they’re actually removing the target chromophore.”
Patients with darker Fitzpatrick skin types can be treated safely but tanned individuals face a risk of complications because of active melanocytes. “As we approach summer in New England, we slow down the amount of hair removal we do because it’s a riskier procedure,” he said. “I recommend that my patients not get any significant amount of sun exposure a month before or after treatment.”
The color and quality of hair also drive treatment success. Black and brown terminal hairs absorb the millisecond laser energy, but white, gray, red, and light blond hairs lack adequate melanin to make them suitable target chromophores.
Excessive and unwanted body hair ranges in severity and can usually be classified as either hypertrichosis or hirsutism.
The desired clinical endpoint is perifollicular edema and erythema. Treatment parameters that can be varied with Food and Drug Administration–cleared devices include wavelength, fluence, pulse duration, spot size, and skin cooling. The most popular devices are the Alexandrite 755 nm laser; the diode 800 nm laser; and the 1064 nm Nd:YAG laser, which is safe for all skin types. “Often you have to use higher relative fluences to treat patients with the 1064 nm Nd:YAG because on the absorption spectrum, the 1064-nm wavelength has a relatively lower absorption for melanin compared to the alexandrite. However, you can still get effective, long-term hair reduction with the Nd:YAG laser,” he said (Arch Dermatol. 2008 Oct;144[10]:1323-7).
More recently, Dr. Ibrahimi and colleagues found that a 1060-nm diode laser system with multiple handpieces for permanent hair reduction was safe for all skin types, in an open label prospective study.
Higher fluences have been correlated with greater rates of permanent hair removal, but they also are more likely to cause undesired side effects. Dr. Ibrahimi advises clinicians new to laser hair removal to conduct a few different test spots and look for the desired clinical endpoint of perifollicular erythema and edema. “The highest fluence that gives you that endpoint without any adverse reactions is going to the best fluence for treatment,” he said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Do a few test spots, bring them back a week later and see which ones were tolerated well without any side effects and which weren’t. That gives you a good starting point for your treatment.”
Cooling down the epidermal melanin not only keeps the procedure safe, it’s a salve for pain. “There are a variety of methods of passive and active cooling,” said Dr. Ibrahimi, a member of the American Society for Dermatologic Surgery board of directors. “You can use something as simple as cold gel, but the active methods are better because once the method of passive application of cold gel warms up, you lose that cooling effect. You can use forced chilled air. Many commercial devices come with a cold tip which cools down the epidermal melanin. Others use dynamic cooling, which emit cryogen spray from a separate part of the handpiece. It hits right where the laser pulse is going to go, is absorbed by the skin, and it cools down the epidermal melanin.”
Treatment complications
Complications that can occur from treatment include pigmentary changes such as hyperpigmentation and hypopigmentation. “In lighter skinned individuals, sometimes excess fluence can lead to an erythematous appearance,” he said. “In darker-skinned individuals, this often manifests as hyperpigmentation and can be very long-lasting.” Dr. Ibrahimi ranks improper technique as a complication, “because ideally you want to lay your pulses down with 10%-15% overlap during treatment,” he explained. “If you don’t overlap, you’re going to have zones that don’t get any of the laser photons. If you do, then your patient is not going to be happy with you.”
Paradoxical hypertrichosis occurs in 1%-5% of patients, typically in women from Mediterranean, Middle Eastern, or South Asian ethnic backgrounds. This tends to develop on the lateral or jawline part of the face. “Often it occurs in the setting where they come in and want these vellus hairs treated,” he said. “Somehow the laser, instead of destroying the hair shaft, triggers it and stimulates it and can’t differentiate a vellus hair from a terminal hair. This is important to discuss during your informed consent, especially when you’re treating on the lateral jawline or the sideburn area. If this happens, you can treat through it.”
Transgender patients and future directions
Dr. Ibrahimi pointed out that increasing numbers of transgender patients are visiting dermatologists seeking laser hair removal. About 16 million Americans are estimated to have a gender identity that differs from the one assigned to them at birth, yet they face several barriers to care, “ranging from ignorance on our end to maybe our own biases being transposed onto these patients,” he said. “We really need to do a better job for them. We really have an obligation to provide good care for all of our patients.”
Transgender women typically seek hair removal on the face and neck as well as in the genital area to remove hairs in preparation for vaginoplasty. Transgender men typically seek hair reduction on the forearm or on the thigh, because those are graft sites in preparation for phalloplasty. As a resource for transgender care, he recommends the UCSF Transgender Care website.
As for future directions in the field, Dr. Ibrahimi predicted that hair removal devices for home use will continue to improve and become more widespread. “This raises a host of considerations, from the risk of eye damage to the risk for paradoxical hypertrichosis, and what happens when pigmented lesions get treated with these low-powered machines compared to the ones we have in our office,” he said. “I also think we’re going to see office-based devices with larger spot sizes, smarter devices that are capable of taking over more of the functions we do. I’m most excited about the potential for treating nonpigmented white hair or poorly pigmented blond or reddish hair in the future.”
Dr. Ibrahimi disclosed that he has received research funding and speaker honoraria from Lutronic, Lumenis, Cutera, and Syneron-Candela. He also holds stock in AVAVA Inc.
Omar A. Ibrahimi, MD, PhD, hears some dermatology colleagues say they don’t bother to offer laser hair removal in their practices because they figure that the procedure is under the purview of medical spas, but he sees it differently.
“I offer laser hair removal in my practice as a way to protect my patients from being picked off by medical spas,” Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, said during a virtual course on laser and aesthetic skin therapy. “These patients are going to want to get laser hair removal. If they’re not going to have the opportunity to get it at your practice, they’re going to seek it elsewhere. When they go elsewhere, they’re going to be picked off for other procedures as well.”
First developed in 1995 by R. Rox Anderson, MD, and colleagues at The Wellman Center for Photomedicine, laser hair removal has become the gold standard for permanent hair destruction, and ranks as the most common energy-based procedure performed in the world, Dr. Ibrahimi said. “Results are very long lasting and durable beyond 2 years after treatment,” he said. “These patients tend to be highly satisfied and have permanence with these treatments.”
Treatment goal, patient selection
While the target chromophore for the procedure is melanin, the goal is to destroy the stem cells located in the hair bulge and the hair bulb. “This is technically called the extended theory of selective photothermolysis, but it’s the same concept except that our target chromophore and our desired target for destruction are slightly spatially separated,” he said.
Proper patient selection is key, so a focused medical history and physical exam are essential prior to the procedure. If unwanted hair is located on the face, jawline, or chest of a female, consider and ask about potential endocrine-related dysfunctions such as polycystic ovary syndrome (PCOS). “Getting those addressed can often help the hypertrichosis as well,” he said. “Another condition is explosive hypertrichosis where hair growth starts very suddenly. It’s uncommon but it’s something to think about.”
Pregnancy is not an absolute contraindication for laser hair removal, Dr. Ibrahimi continued, but he elects not to perform the procedure on pregnant patients. He also asks patients about any history of photosensitivity, active infection at the intended treatment site, keloids, or hypertrophic scarring. Past methods of hair removal also matter. “What we’re targeting is the pigment in the hair shafts,” he said. “So, if your patient is waxing or plucking or epilating or removing the hair in some manner, they’re actually removing the target chromophore.”
Patients with darker Fitzpatrick skin types can be treated safely but tanned individuals face a risk of complications because of active melanocytes. “As we approach summer in New England, we slow down the amount of hair removal we do because it’s a riskier procedure,” he said. “I recommend that my patients not get any significant amount of sun exposure a month before or after treatment.”
The color and quality of hair also drive treatment success. Black and brown terminal hairs absorb the millisecond laser energy, but white, gray, red, and light blond hairs lack adequate melanin to make them suitable target chromophores.
Excessive and unwanted body hair ranges in severity and can usually be classified as either hypertrichosis or hirsutism.
The desired clinical endpoint is perifollicular edema and erythema. Treatment parameters that can be varied with Food and Drug Administration–cleared devices include wavelength, fluence, pulse duration, spot size, and skin cooling. The most popular devices are the Alexandrite 755 nm laser; the diode 800 nm laser; and the 1064 nm Nd:YAG laser, which is safe for all skin types. “Often you have to use higher relative fluences to treat patients with the 1064 nm Nd:YAG because on the absorption spectrum, the 1064-nm wavelength has a relatively lower absorption for melanin compared to the alexandrite. However, you can still get effective, long-term hair reduction with the Nd:YAG laser,” he said (Arch Dermatol. 2008 Oct;144[10]:1323-7).
More recently, Dr. Ibrahimi and colleagues found that a 1060-nm diode laser system with multiple handpieces for permanent hair reduction was safe for all skin types, in an open label prospective study.
Higher fluences have been correlated with greater rates of permanent hair removal, but they also are more likely to cause undesired side effects. Dr. Ibrahimi advises clinicians new to laser hair removal to conduct a few different test spots and look for the desired clinical endpoint of perifollicular erythema and edema. “The highest fluence that gives you that endpoint without any adverse reactions is going to the best fluence for treatment,” he said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Do a few test spots, bring them back a week later and see which ones were tolerated well without any side effects and which weren’t. That gives you a good starting point for your treatment.”
Cooling down the epidermal melanin not only keeps the procedure safe, it’s a salve for pain. “There are a variety of methods of passive and active cooling,” said Dr. Ibrahimi, a member of the American Society for Dermatologic Surgery board of directors. “You can use something as simple as cold gel, but the active methods are better because once the method of passive application of cold gel warms up, you lose that cooling effect. You can use forced chilled air. Many commercial devices come with a cold tip which cools down the epidermal melanin. Others use dynamic cooling, which emit cryogen spray from a separate part of the handpiece. It hits right where the laser pulse is going to go, is absorbed by the skin, and it cools down the epidermal melanin.”
Treatment complications
Complications that can occur from treatment include pigmentary changes such as hyperpigmentation and hypopigmentation. “In lighter skinned individuals, sometimes excess fluence can lead to an erythematous appearance,” he said. “In darker-skinned individuals, this often manifests as hyperpigmentation and can be very long-lasting.” Dr. Ibrahimi ranks improper technique as a complication, “because ideally you want to lay your pulses down with 10%-15% overlap during treatment,” he explained. “If you don’t overlap, you’re going to have zones that don’t get any of the laser photons. If you do, then your patient is not going to be happy with you.”
Paradoxical hypertrichosis occurs in 1%-5% of patients, typically in women from Mediterranean, Middle Eastern, or South Asian ethnic backgrounds. This tends to develop on the lateral or jawline part of the face. “Often it occurs in the setting where they come in and want these vellus hairs treated,” he said. “Somehow the laser, instead of destroying the hair shaft, triggers it and stimulates it and can’t differentiate a vellus hair from a terminal hair. This is important to discuss during your informed consent, especially when you’re treating on the lateral jawline or the sideburn area. If this happens, you can treat through it.”
Transgender patients and future directions
Dr. Ibrahimi pointed out that increasing numbers of transgender patients are visiting dermatologists seeking laser hair removal. About 16 million Americans are estimated to have a gender identity that differs from the one assigned to them at birth, yet they face several barriers to care, “ranging from ignorance on our end to maybe our own biases being transposed onto these patients,” he said. “We really need to do a better job for them. We really have an obligation to provide good care for all of our patients.”
Transgender women typically seek hair removal on the face and neck as well as in the genital area to remove hairs in preparation for vaginoplasty. Transgender men typically seek hair reduction on the forearm or on the thigh, because those are graft sites in preparation for phalloplasty. As a resource for transgender care, he recommends the UCSF Transgender Care website.
As for future directions in the field, Dr. Ibrahimi predicted that hair removal devices for home use will continue to improve and become more widespread. “This raises a host of considerations, from the risk of eye damage to the risk for paradoxical hypertrichosis, and what happens when pigmented lesions get treated with these low-powered machines compared to the ones we have in our office,” he said. “I also think we’re going to see office-based devices with larger spot sizes, smarter devices that are capable of taking over more of the functions we do. I’m most excited about the potential for treating nonpigmented white hair or poorly pigmented blond or reddish hair in the future.”
Dr. Ibrahimi disclosed that he has received research funding and speaker honoraria from Lutronic, Lumenis, Cutera, and Syneron-Candela. He also holds stock in AVAVA Inc.
Omar A. Ibrahimi, MD, PhD, hears some dermatology colleagues say they don’t bother to offer laser hair removal in their practices because they figure that the procedure is under the purview of medical spas, but he sees it differently.
“I offer laser hair removal in my practice as a way to protect my patients from being picked off by medical spas,” Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, said during a virtual course on laser and aesthetic skin therapy. “These patients are going to want to get laser hair removal. If they’re not going to have the opportunity to get it at your practice, they’re going to seek it elsewhere. When they go elsewhere, they’re going to be picked off for other procedures as well.”
First developed in 1995 by R. Rox Anderson, MD, and colleagues at The Wellman Center for Photomedicine, laser hair removal has become the gold standard for permanent hair destruction, and ranks as the most common energy-based procedure performed in the world, Dr. Ibrahimi said. “Results are very long lasting and durable beyond 2 years after treatment,” he said. “These patients tend to be highly satisfied and have permanence with these treatments.”
Treatment goal, patient selection
While the target chromophore for the procedure is melanin, the goal is to destroy the stem cells located in the hair bulge and the hair bulb. “This is technically called the extended theory of selective photothermolysis, but it’s the same concept except that our target chromophore and our desired target for destruction are slightly spatially separated,” he said.
Proper patient selection is key, so a focused medical history and physical exam are essential prior to the procedure. If unwanted hair is located on the face, jawline, or chest of a female, consider and ask about potential endocrine-related dysfunctions such as polycystic ovary syndrome (PCOS). “Getting those addressed can often help the hypertrichosis as well,” he said. “Another condition is explosive hypertrichosis where hair growth starts very suddenly. It’s uncommon but it’s something to think about.”
Pregnancy is not an absolute contraindication for laser hair removal, Dr. Ibrahimi continued, but he elects not to perform the procedure on pregnant patients. He also asks patients about any history of photosensitivity, active infection at the intended treatment site, keloids, or hypertrophic scarring. Past methods of hair removal also matter. “What we’re targeting is the pigment in the hair shafts,” he said. “So, if your patient is waxing or plucking or epilating or removing the hair in some manner, they’re actually removing the target chromophore.”
Patients with darker Fitzpatrick skin types can be treated safely but tanned individuals face a risk of complications because of active melanocytes. “As we approach summer in New England, we slow down the amount of hair removal we do because it’s a riskier procedure,” he said. “I recommend that my patients not get any significant amount of sun exposure a month before or after treatment.”
The color and quality of hair also drive treatment success. Black and brown terminal hairs absorb the millisecond laser energy, but white, gray, red, and light blond hairs lack adequate melanin to make them suitable target chromophores.
Excessive and unwanted body hair ranges in severity and can usually be classified as either hypertrichosis or hirsutism.
The desired clinical endpoint is perifollicular edema and erythema. Treatment parameters that can be varied with Food and Drug Administration–cleared devices include wavelength, fluence, pulse duration, spot size, and skin cooling. The most popular devices are the Alexandrite 755 nm laser; the diode 800 nm laser; and the 1064 nm Nd:YAG laser, which is safe for all skin types. “Often you have to use higher relative fluences to treat patients with the 1064 nm Nd:YAG because on the absorption spectrum, the 1064-nm wavelength has a relatively lower absorption for melanin compared to the alexandrite. However, you can still get effective, long-term hair reduction with the Nd:YAG laser,” he said (Arch Dermatol. 2008 Oct;144[10]:1323-7).
More recently, Dr. Ibrahimi and colleagues found that a 1060-nm diode laser system with multiple handpieces for permanent hair reduction was safe for all skin types, in an open label prospective study.
Higher fluences have been correlated with greater rates of permanent hair removal, but they also are more likely to cause undesired side effects. Dr. Ibrahimi advises clinicians new to laser hair removal to conduct a few different test spots and look for the desired clinical endpoint of perifollicular erythema and edema. “The highest fluence that gives you that endpoint without any adverse reactions is going to the best fluence for treatment,” he said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Do a few test spots, bring them back a week later and see which ones were tolerated well without any side effects and which weren’t. That gives you a good starting point for your treatment.”
Cooling down the epidermal melanin not only keeps the procedure safe, it’s a salve for pain. “There are a variety of methods of passive and active cooling,” said Dr. Ibrahimi, a member of the American Society for Dermatologic Surgery board of directors. “You can use something as simple as cold gel, but the active methods are better because once the method of passive application of cold gel warms up, you lose that cooling effect. You can use forced chilled air. Many commercial devices come with a cold tip which cools down the epidermal melanin. Others use dynamic cooling, which emit cryogen spray from a separate part of the handpiece. It hits right where the laser pulse is going to go, is absorbed by the skin, and it cools down the epidermal melanin.”
Treatment complications
Complications that can occur from treatment include pigmentary changes such as hyperpigmentation and hypopigmentation. “In lighter skinned individuals, sometimes excess fluence can lead to an erythematous appearance,” he said. “In darker-skinned individuals, this often manifests as hyperpigmentation and can be very long-lasting.” Dr. Ibrahimi ranks improper technique as a complication, “because ideally you want to lay your pulses down with 10%-15% overlap during treatment,” he explained. “If you don’t overlap, you’re going to have zones that don’t get any of the laser photons. If you do, then your patient is not going to be happy with you.”
Paradoxical hypertrichosis occurs in 1%-5% of patients, typically in women from Mediterranean, Middle Eastern, or South Asian ethnic backgrounds. This tends to develop on the lateral or jawline part of the face. “Often it occurs in the setting where they come in and want these vellus hairs treated,” he said. “Somehow the laser, instead of destroying the hair shaft, triggers it and stimulates it and can’t differentiate a vellus hair from a terminal hair. This is important to discuss during your informed consent, especially when you’re treating on the lateral jawline or the sideburn area. If this happens, you can treat through it.”
Transgender patients and future directions
Dr. Ibrahimi pointed out that increasing numbers of transgender patients are visiting dermatologists seeking laser hair removal. About 16 million Americans are estimated to have a gender identity that differs from the one assigned to them at birth, yet they face several barriers to care, “ranging from ignorance on our end to maybe our own biases being transposed onto these patients,” he said. “We really need to do a better job for them. We really have an obligation to provide good care for all of our patients.”
Transgender women typically seek hair removal on the face and neck as well as in the genital area to remove hairs in preparation for vaginoplasty. Transgender men typically seek hair reduction on the forearm or on the thigh, because those are graft sites in preparation for phalloplasty. As a resource for transgender care, he recommends the UCSF Transgender Care website.
As for future directions in the field, Dr. Ibrahimi predicted that hair removal devices for home use will continue to improve and become more widespread. “This raises a host of considerations, from the risk of eye damage to the risk for paradoxical hypertrichosis, and what happens when pigmented lesions get treated with these low-powered machines compared to the ones we have in our office,” he said. “I also think we’re going to see office-based devices with larger spot sizes, smarter devices that are capable of taking over more of the functions we do. I’m most excited about the potential for treating nonpigmented white hair or poorly pigmented blond or reddish hair in the future.”
Dr. Ibrahimi disclosed that he has received research funding and speaker honoraria from Lutronic, Lumenis, Cutera, and Syneron-Candela. He also holds stock in AVAVA Inc.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
FDA panel votes to approve Pfizer’s vaccine for children
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Major increase seen in cosmeceutical alternatives to topical hydroquinone
along with new strategies to improve their efficacy, according to a report at the Skin of Color Update 2021.
“Ten or 15 years ago, I was showing a slide with five [alternatives to hydroquinone]. Now there are dozens,” reported Heather Woolery-Lloyd, MD, director of the skin of color division in the department of dermatology at the University of Miami.
The growth in alternatives to hydroquinone is timely. After threats to do so for more than a decade, the Food and Drug Administration finally banned hydroquinone from OTC products in 2020. The ban was folded into the Coronavirus Aid, Relief, and Economic Security (CARES) Act passed in March of 2020 and then implemented the following September.
Until the ban of hydroquinone, OTC products with this compound were widely sought by many individuals with darker skin tones to self-treat melasma and other forms of hyperpigmentation, according to Dr. Woolery-Lloyd. Hydroquinone is still available in prescription products, but she is often asked for OTC alternatives, and she says the list is long and getting longer.
Niacinamide
Detailing the products she has been recommending most frequently as substitutes, Dr. Woolery-Lloyd reported that several are supported by high quality studies. One example is niacinamide.
Of the several controlled studies she cited, one double-blind randomized trial found niacinamide to be equivalent to hydroquinone for melasma on the basis of colorimetric measures. The study compared 4% niacinamide cream applied on one side of the face with 4% hydroquinone cream applied on the other side in 27 patients with melasma. Although the proportion of responses rated good or excellent on a subjective basis was lower with niacinamide (44% vs. 55%), the difference was not statistically significant and niacinamide cream was clearly active, producing objective improvements in mast cell infiltrate and solar elastosis in melasma skin as well. Both were well tolerated.
In other studies, niacinamide has been shown to be effective in the treatment of melasma when combined with other active agents such as tranexamic acid, said Dr. Woolery-Lloyd, who added that OTC products containing niacinamide are now “among my favorites” when directing patients to cosmeceuticals for hyperpigmentation.
Topical vitamin C
Topical vitamin C or ascorbic acid is another. Like niacinamide, topical vitamin C has also been compared with hydroquinone in a double-blind, randomized trial. Although the niacinamide trial and this study were performed 10 or more years ago, these data have new relevance with the ban of OTC hydroquinone.
In the study, 5% ascorbic acid cream on one side of the face was compared with 4% hydroquinone cream, applied on the other side, in 16 women with melasma. Again, there were no statistical differences in colorimetric measures, but good to excellent results were reported for 93% of the sides of the face treated with hydroquinone versus 62.5% of the sides treated with vitamin C (P < .05). “Hydroquinone performed better, but the vitamin C was active and very well tolerated,” Dr. Woolery-Lloyd said.
However, the ascorbic acid cream was better tolerated, with a far lower rate of adverse events (6.2% vs. 68.7%), an advantage that makes it easy to recommend to patients, said Dr. Woolery-Lloyd, who now uses it frequently in her own practice.
Liquiritin, a licorice extract, is another lightening agent increasingly included in OTC products that she also recommends. In two older studies in medical journals published in Pakistan, both the 2% and 4% strengths of liquiritin cream outperformed hydroquinone on the basis of a Melasma Area and Severity Index (MASI) rating. The liquiritin cream was well tolerated in both studies.
Azelaic acid, tranexamic acid
OTC products containing azelaic acid are also effective for hyperpigmentation based on published trials in which they were compared with hydroquinone for treating melasma. In one study of 29 women with melasma cited by Dr. Woolery-Lloyd, 20% azelaic acid cream was more effective than hydroquinone 4% cream after 2 months of treatment on the basis of the mean MASI score (6.2 vs. 3.8).
The list also includes cysteamine, silymarin, and tranexamic acid.
In the case of tranexamic acid, Dr. Woolery-Lloyd cited a relatively recent study of 60 patients with melasma, comparing two strategies for applying tranexamic acid to treatment with hydroquinone over 12 weeks. Compared with 2% hydroquinone (applied nightly) or 1.8% liposomal tranexamic acid (applied twice a day), 5% tranexamic acid solution with microneedling (weekly) had a slightly greater rate of success defined as more than a 50% improvement in hyperpigmentation in an Asian population (30%, 27.8%, and 33.3%, respectively).
“Microneedling is a newer technology that appears to be effective at improving absorption,” said Dr. Woolery-Lloyd. She predicts that microneedling will be used with increasing frequency in combination with topical cosmeceuticals.
She also predicted that these topical agents will be increasingly employed in combinations as the field of cosmeceuticals becomes increasingly more sophisticated. “When it comes to skin quality, cosmeceuticals remain our first-line therapy, especially in skin of color,” she said.
The rapid growth and utility of OTC cosmeceuticals is an area that dermatologists need to be following, according to Darius Mehregan, MD, chair of the department of dermatology, Wayne State University, Detroit, who was senior author of an article published last year that reviewed the ingredients of popular OTC cosmeceuticals.
“Our patients have a great interest in cosmeceuticals and are looking to us for guidance. I think we have a responsibility to help them identify products supported by evidence and to warn them about potential side effects,” Dr. Mehregan, who was not at the meeting, said in an interview.
He agreed that the removal of hydroquinone from OTC products will create a specific need in the area of cosmeceuticals.
“Hydroquinone has for a long time been one of the most effective agents in OTC products for melasma, so patients are going to be looking for alternatives. Identifying which drugs have shown efficacy in controlled studies will be very helpful,” he said.
Dr. Woolery-Lloyd reports financial relationships with Ortho Dermatologics, L’Oréal, Galderma, Allergan, and Somabella Laboratories. Dr. Mehregan reports no potential conflicts of interest.
along with new strategies to improve their efficacy, according to a report at the Skin of Color Update 2021.
“Ten or 15 years ago, I was showing a slide with five [alternatives to hydroquinone]. Now there are dozens,” reported Heather Woolery-Lloyd, MD, director of the skin of color division in the department of dermatology at the University of Miami.
The growth in alternatives to hydroquinone is timely. After threats to do so for more than a decade, the Food and Drug Administration finally banned hydroquinone from OTC products in 2020. The ban was folded into the Coronavirus Aid, Relief, and Economic Security (CARES) Act passed in March of 2020 and then implemented the following September.
Until the ban of hydroquinone, OTC products with this compound were widely sought by many individuals with darker skin tones to self-treat melasma and other forms of hyperpigmentation, according to Dr. Woolery-Lloyd. Hydroquinone is still available in prescription products, but she is often asked for OTC alternatives, and she says the list is long and getting longer.
Niacinamide
Detailing the products she has been recommending most frequently as substitutes, Dr. Woolery-Lloyd reported that several are supported by high quality studies. One example is niacinamide.
Of the several controlled studies she cited, one double-blind randomized trial found niacinamide to be equivalent to hydroquinone for melasma on the basis of colorimetric measures. The study compared 4% niacinamide cream applied on one side of the face with 4% hydroquinone cream applied on the other side in 27 patients with melasma. Although the proportion of responses rated good or excellent on a subjective basis was lower with niacinamide (44% vs. 55%), the difference was not statistically significant and niacinamide cream was clearly active, producing objective improvements in mast cell infiltrate and solar elastosis in melasma skin as well. Both were well tolerated.
In other studies, niacinamide has been shown to be effective in the treatment of melasma when combined with other active agents such as tranexamic acid, said Dr. Woolery-Lloyd, who added that OTC products containing niacinamide are now “among my favorites” when directing patients to cosmeceuticals for hyperpigmentation.
Topical vitamin C
Topical vitamin C or ascorbic acid is another. Like niacinamide, topical vitamin C has also been compared with hydroquinone in a double-blind, randomized trial. Although the niacinamide trial and this study were performed 10 or more years ago, these data have new relevance with the ban of OTC hydroquinone.
In the study, 5% ascorbic acid cream on one side of the face was compared with 4% hydroquinone cream, applied on the other side, in 16 women with melasma. Again, there were no statistical differences in colorimetric measures, but good to excellent results were reported for 93% of the sides of the face treated with hydroquinone versus 62.5% of the sides treated with vitamin C (P < .05). “Hydroquinone performed better, but the vitamin C was active and very well tolerated,” Dr. Woolery-Lloyd said.
However, the ascorbic acid cream was better tolerated, with a far lower rate of adverse events (6.2% vs. 68.7%), an advantage that makes it easy to recommend to patients, said Dr. Woolery-Lloyd, who now uses it frequently in her own practice.
Liquiritin, a licorice extract, is another lightening agent increasingly included in OTC products that she also recommends. In two older studies in medical journals published in Pakistan, both the 2% and 4% strengths of liquiritin cream outperformed hydroquinone on the basis of a Melasma Area and Severity Index (MASI) rating. The liquiritin cream was well tolerated in both studies.
Azelaic acid, tranexamic acid
OTC products containing azelaic acid are also effective for hyperpigmentation based on published trials in which they were compared with hydroquinone for treating melasma. In one study of 29 women with melasma cited by Dr. Woolery-Lloyd, 20% azelaic acid cream was more effective than hydroquinone 4% cream after 2 months of treatment on the basis of the mean MASI score (6.2 vs. 3.8).
The list also includes cysteamine, silymarin, and tranexamic acid.
In the case of tranexamic acid, Dr. Woolery-Lloyd cited a relatively recent study of 60 patients with melasma, comparing two strategies for applying tranexamic acid to treatment with hydroquinone over 12 weeks. Compared with 2% hydroquinone (applied nightly) or 1.8% liposomal tranexamic acid (applied twice a day), 5% tranexamic acid solution with microneedling (weekly) had a slightly greater rate of success defined as more than a 50% improvement in hyperpigmentation in an Asian population (30%, 27.8%, and 33.3%, respectively).
“Microneedling is a newer technology that appears to be effective at improving absorption,” said Dr. Woolery-Lloyd. She predicts that microneedling will be used with increasing frequency in combination with topical cosmeceuticals.
She also predicted that these topical agents will be increasingly employed in combinations as the field of cosmeceuticals becomes increasingly more sophisticated. “When it comes to skin quality, cosmeceuticals remain our first-line therapy, especially in skin of color,” she said.
The rapid growth and utility of OTC cosmeceuticals is an area that dermatologists need to be following, according to Darius Mehregan, MD, chair of the department of dermatology, Wayne State University, Detroit, who was senior author of an article published last year that reviewed the ingredients of popular OTC cosmeceuticals.
“Our patients have a great interest in cosmeceuticals and are looking to us for guidance. I think we have a responsibility to help them identify products supported by evidence and to warn them about potential side effects,” Dr. Mehregan, who was not at the meeting, said in an interview.
He agreed that the removal of hydroquinone from OTC products will create a specific need in the area of cosmeceuticals.
“Hydroquinone has for a long time been one of the most effective agents in OTC products for melasma, so patients are going to be looking for alternatives. Identifying which drugs have shown efficacy in controlled studies will be very helpful,” he said.
Dr. Woolery-Lloyd reports financial relationships with Ortho Dermatologics, L’Oréal, Galderma, Allergan, and Somabella Laboratories. Dr. Mehregan reports no potential conflicts of interest.
along with new strategies to improve their efficacy, according to a report at the Skin of Color Update 2021.
“Ten or 15 years ago, I was showing a slide with five [alternatives to hydroquinone]. Now there are dozens,” reported Heather Woolery-Lloyd, MD, director of the skin of color division in the department of dermatology at the University of Miami.
The growth in alternatives to hydroquinone is timely. After threats to do so for more than a decade, the Food and Drug Administration finally banned hydroquinone from OTC products in 2020. The ban was folded into the Coronavirus Aid, Relief, and Economic Security (CARES) Act passed in March of 2020 and then implemented the following September.
Until the ban of hydroquinone, OTC products with this compound were widely sought by many individuals with darker skin tones to self-treat melasma and other forms of hyperpigmentation, according to Dr. Woolery-Lloyd. Hydroquinone is still available in prescription products, but she is often asked for OTC alternatives, and she says the list is long and getting longer.
Niacinamide
Detailing the products she has been recommending most frequently as substitutes, Dr. Woolery-Lloyd reported that several are supported by high quality studies. One example is niacinamide.
Of the several controlled studies she cited, one double-blind randomized trial found niacinamide to be equivalent to hydroquinone for melasma on the basis of colorimetric measures. The study compared 4% niacinamide cream applied on one side of the face with 4% hydroquinone cream applied on the other side in 27 patients with melasma. Although the proportion of responses rated good or excellent on a subjective basis was lower with niacinamide (44% vs. 55%), the difference was not statistically significant and niacinamide cream was clearly active, producing objective improvements in mast cell infiltrate and solar elastosis in melasma skin as well. Both were well tolerated.
In other studies, niacinamide has been shown to be effective in the treatment of melasma when combined with other active agents such as tranexamic acid, said Dr. Woolery-Lloyd, who added that OTC products containing niacinamide are now “among my favorites” when directing patients to cosmeceuticals for hyperpigmentation.
Topical vitamin C
Topical vitamin C or ascorbic acid is another. Like niacinamide, topical vitamin C has also been compared with hydroquinone in a double-blind, randomized trial. Although the niacinamide trial and this study were performed 10 or more years ago, these data have new relevance with the ban of OTC hydroquinone.
In the study, 5% ascorbic acid cream on one side of the face was compared with 4% hydroquinone cream, applied on the other side, in 16 women with melasma. Again, there were no statistical differences in colorimetric measures, but good to excellent results were reported for 93% of the sides of the face treated with hydroquinone versus 62.5% of the sides treated with vitamin C (P < .05). “Hydroquinone performed better, but the vitamin C was active and very well tolerated,” Dr. Woolery-Lloyd said.
However, the ascorbic acid cream was better tolerated, with a far lower rate of adverse events (6.2% vs. 68.7%), an advantage that makes it easy to recommend to patients, said Dr. Woolery-Lloyd, who now uses it frequently in her own practice.
Liquiritin, a licorice extract, is another lightening agent increasingly included in OTC products that she also recommends. In two older studies in medical journals published in Pakistan, both the 2% and 4% strengths of liquiritin cream outperformed hydroquinone on the basis of a Melasma Area and Severity Index (MASI) rating. The liquiritin cream was well tolerated in both studies.
Azelaic acid, tranexamic acid
OTC products containing azelaic acid are also effective for hyperpigmentation based on published trials in which they were compared with hydroquinone for treating melasma. In one study of 29 women with melasma cited by Dr. Woolery-Lloyd, 20% azelaic acid cream was more effective than hydroquinone 4% cream after 2 months of treatment on the basis of the mean MASI score (6.2 vs. 3.8).
The list also includes cysteamine, silymarin, and tranexamic acid.
In the case of tranexamic acid, Dr. Woolery-Lloyd cited a relatively recent study of 60 patients with melasma, comparing two strategies for applying tranexamic acid to treatment with hydroquinone over 12 weeks. Compared with 2% hydroquinone (applied nightly) or 1.8% liposomal tranexamic acid (applied twice a day), 5% tranexamic acid solution with microneedling (weekly) had a slightly greater rate of success defined as more than a 50% improvement in hyperpigmentation in an Asian population (30%, 27.8%, and 33.3%, respectively).
“Microneedling is a newer technology that appears to be effective at improving absorption,” said Dr. Woolery-Lloyd. She predicts that microneedling will be used with increasing frequency in combination with topical cosmeceuticals.
She also predicted that these topical agents will be increasingly employed in combinations as the field of cosmeceuticals becomes increasingly more sophisticated. “When it comes to skin quality, cosmeceuticals remain our first-line therapy, especially in skin of color,” she said.
The rapid growth and utility of OTC cosmeceuticals is an area that dermatologists need to be following, according to Darius Mehregan, MD, chair of the department of dermatology, Wayne State University, Detroit, who was senior author of an article published last year that reviewed the ingredients of popular OTC cosmeceuticals.
“Our patients have a great interest in cosmeceuticals and are looking to us for guidance. I think we have a responsibility to help them identify products supported by evidence and to warn them about potential side effects,” Dr. Mehregan, who was not at the meeting, said in an interview.
He agreed that the removal of hydroquinone from OTC products will create a specific need in the area of cosmeceuticals.
“Hydroquinone has for a long time been one of the most effective agents in OTC products for melasma, so patients are going to be looking for alternatives. Identifying which drugs have shown efficacy in controlled studies will be very helpful,” he said.
Dr. Woolery-Lloyd reports financial relationships with Ortho Dermatologics, L’Oréal, Galderma, Allergan, and Somabella Laboratories. Dr. Mehregan reports no potential conflicts of interest.
FROM SOC 2021
Unvaccinated people likely to catch COVID repeatedly
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
Transgender use of dermatologic procedures has strong gender tilt
, according to the results of a recent survey.
Transfeminine persons – those assigned male at birth – were much more likely to report a previous dermatologic procedure, compared with transmasculine respondents, by a margin of 64.9%-7.5%, Laura Ragmanauskaite, MD, and associates reported.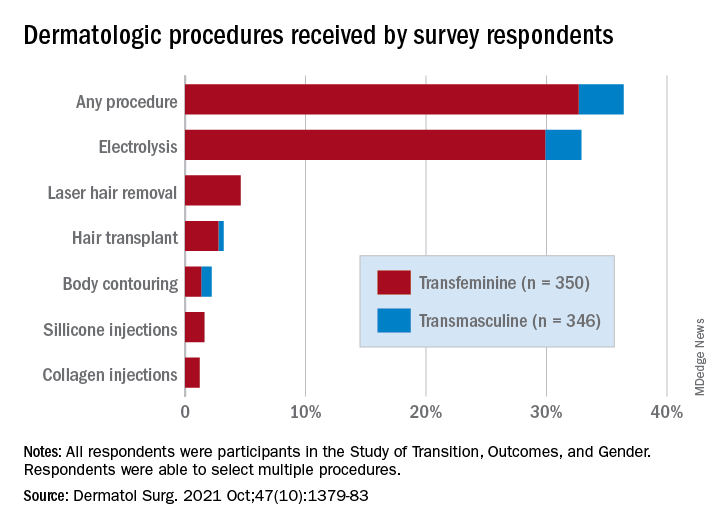
“Hair removal was the most frequently reported procedure type, with electrolysis being more common than laser hair removal,” they said, noting that “previous research on hair removal treatments among gender minority persons did not detect differences in the use of electrolysis and laser hair removal.”
Just under one-third of all respondents (32.9%) said that they had undergone electrolysis and 4.6% reported previous laser hair removal. For electrolysis, that works out to 59.4% of transfeminine and 6.1% of transmasculine respondents, while 9.1% of all transfeminine and no transmasculine persons had received laser hair removal, Dr. Ragmanauskaite of the department of dermatology, Emory University, Atlanta, and her coauthors said.
Those who had undergone gender-affirming surgery were significantly more likely to report electrolysis (78.6%) than were persons who had received no gender-affirming surgery or hormone therapy alone (47.4%), a statistically significant difference (P < .01). All of the other, less common procedures included in the online survey – 696 responses were received from 350 transfeminine and 346 transmasculine persons participating in the Study of Transition, Outcomes, and Gender – were reported more often by the transfeminine respondents. The procedure with the closest gender distribution was body contouring, reported by nine transfeminine and six transmasculine persons, the researchers said.
Use of dermal fillers was even less common (2.8% among all respondents, all transfeminine persons), with just 11 reporting having received silicone and 8 reporting having received collagen, although the survey did not ask about how the injections were obtained. In a previous study, the prevalence of illicit filler injection in transgender women was 16.9%, they pointed out.
These types of noninvasive, gender-affirming procedures “may contribute to higher levels of self-confidence and [reduce] gender dysphoria. Future studies should examine motivations, barriers, and optimal timing” for such procedures in transgender persons, Dr. Ragmanauskaite and associates wrote.
The authors reported that they had no relevant disclosures.
, according to the results of a recent survey.
Transfeminine persons – those assigned male at birth – were much more likely to report a previous dermatologic procedure, compared with transmasculine respondents, by a margin of 64.9%-7.5%, Laura Ragmanauskaite, MD, and associates reported.
“Hair removal was the most frequently reported procedure type, with electrolysis being more common than laser hair removal,” they said, noting that “previous research on hair removal treatments among gender minority persons did not detect differences in the use of electrolysis and laser hair removal.”
Just under one-third of all respondents (32.9%) said that they had undergone electrolysis and 4.6% reported previous laser hair removal. For electrolysis, that works out to 59.4% of transfeminine and 6.1% of transmasculine respondents, while 9.1% of all transfeminine and no transmasculine persons had received laser hair removal, Dr. Ragmanauskaite of the department of dermatology, Emory University, Atlanta, and her coauthors said.
Those who had undergone gender-affirming surgery were significantly more likely to report electrolysis (78.6%) than were persons who had received no gender-affirming surgery or hormone therapy alone (47.4%), a statistically significant difference (P < .01). All of the other, less common procedures included in the online survey – 696 responses were received from 350 transfeminine and 346 transmasculine persons participating in the Study of Transition, Outcomes, and Gender – were reported more often by the transfeminine respondents. The procedure with the closest gender distribution was body contouring, reported by nine transfeminine and six transmasculine persons, the researchers said.
Use of dermal fillers was even less common (2.8% among all respondents, all transfeminine persons), with just 11 reporting having received silicone and 8 reporting having received collagen, although the survey did not ask about how the injections were obtained. In a previous study, the prevalence of illicit filler injection in transgender women was 16.9%, they pointed out.
These types of noninvasive, gender-affirming procedures “may contribute to higher levels of self-confidence and [reduce] gender dysphoria. Future studies should examine motivations, barriers, and optimal timing” for such procedures in transgender persons, Dr. Ragmanauskaite and associates wrote.
The authors reported that they had no relevant disclosures.
, according to the results of a recent survey.
Transfeminine persons – those assigned male at birth – were much more likely to report a previous dermatologic procedure, compared with transmasculine respondents, by a margin of 64.9%-7.5%, Laura Ragmanauskaite, MD, and associates reported.
“Hair removal was the most frequently reported procedure type, with electrolysis being more common than laser hair removal,” they said, noting that “previous research on hair removal treatments among gender minority persons did not detect differences in the use of electrolysis and laser hair removal.”
Just under one-third of all respondents (32.9%) said that they had undergone electrolysis and 4.6% reported previous laser hair removal. For electrolysis, that works out to 59.4% of transfeminine and 6.1% of transmasculine respondents, while 9.1% of all transfeminine and no transmasculine persons had received laser hair removal, Dr. Ragmanauskaite of the department of dermatology, Emory University, Atlanta, and her coauthors said.
Those who had undergone gender-affirming surgery were significantly more likely to report electrolysis (78.6%) than were persons who had received no gender-affirming surgery or hormone therapy alone (47.4%), a statistically significant difference (P < .01). All of the other, less common procedures included in the online survey – 696 responses were received from 350 transfeminine and 346 transmasculine persons participating in the Study of Transition, Outcomes, and Gender – were reported more often by the transfeminine respondents. The procedure with the closest gender distribution was body contouring, reported by nine transfeminine and six transmasculine persons, the researchers said.
Use of dermal fillers was even less common (2.8% among all respondents, all transfeminine persons), with just 11 reporting having received silicone and 8 reporting having received collagen, although the survey did not ask about how the injections were obtained. In a previous study, the prevalence of illicit filler injection in transgender women was 16.9%, they pointed out.
These types of noninvasive, gender-affirming procedures “may contribute to higher levels of self-confidence and [reduce] gender dysphoria. Future studies should examine motivations, barriers, and optimal timing” for such procedures in transgender persons, Dr. Ragmanauskaite and associates wrote.
The authors reported that they had no relevant disclosures.
FROM DERMATOLOGIC SURGERY
How the pandemic prompted one clinic to embrace digital innovation
When the .
“Before COVID, we had zero use of teledermatology,” Dr. Afanasiev, a dermatologist at the practice, said during the annual meeting of the Pacific Dermatologic Association. “We didn’t do photography other than the required pre-biopsy photographs, and minimal evaluation of home photos, in response to the patients who say, ‘Wait, doc. Let me pull out my phone and show my photographs.”
During the very first days of the pandemic, in order to accommodate urgent patient requests, she and her colleagues used cloud services for patients to submit photos for concerning skin conditions or lesions, which were then discussed over commonly available video platforms. But they quickly realized that this would not work long-term so within two months, they created an electronic health record–integrated workflow that they are still using, she said.
Here’s how it works. If the patient request is deemed nonacute or does not require a full-body skin exam, the scheduling team offers that patient a store-and-video evaluation (SAVe) or an in-person visit. If a SAVe visit is requested, the patient is required to submit a photograph of his or her condition, then a medical assistant checks for the presence and quality of up to nine patient-submitted photos and contacts the patient if additional photos are required.
Immediately before the encounter, a medical assistant calls the patient to ensure video connectivity and performs a brief intake history. The patient and physician then connect via a video-capable platform – most commonly Vidyo, which is integrated with EPIC. After the visit, the provider notifies the scheduling team if any additional in-person or virtual follow up is required.
In a half day of practice, Dr. Afanasiev sees about 20 patients via video visits scheduled every 15 minutes. On a recent day, 55% of visits were related to acne and 10% were related to a mole check, which usually resulted in recommendation for biopsy. Most (70%) were existing patients, while 30% were new.
“There is no store-and-forward option at this time, meaning that patients can’t just submit a photo without a visit,” she said. “In addition, part of our consent is, if you don’t show up to your scheduled video visit, your photos will not be reviewed. The photos are linked to the video visit and uploaded to the patient’s chart. The rooming workflow is very similar to in clinic, except you’re at home and the medical assistant is remote.”
In an article recently published in the Journal of the American Academy of Dermatology, Dr. Afanasiev and her colleagues described their experience with this photo plus video teledermatology – SAVe – workflow between March 16, 2020, and Aug. 31, 2020. The researchers analyzed 74,411 dermatology cases encountered by 89 providers who cared for 46,024 patients during that time frame. Most of the encounters (79%) were in-person, while the remaining 21% were digital in nature – SAVe in 89% of cases, followed by telephone/message encounters.
At the initial peak of the COVID-19 pandemic in April 2020, SAVe encounters increased to 72% of all encounters from 0% prior to March 16, 2020, and were sustained at 12% when the clinic reopened in the summer of 2020. Over the study period, the clinic’s incorporation of SAVe increased care access to patients located in 731 unique ZIP codes in and near California. “We also have been able to retain many patients within our system,” Dr. Afanasiev said. “We have a large proportion of patients that require 2-3 hours of travel time to get to our clinic, so virtual visits allowed for increased rural access. It also allowed for flexibility for patients and providers.”
The new workflow also led to faster access to care. The time from referral to an in-person evaluation fell from an average of 56 days in 2019 to average of 27 days in 2020, while the wait time for a virtual visit was just 14 days in 2020. “We were able to see a diverse number of diagnostic categories with both in-person and virtual care, most commonly rashes, acne, dermal growths, and pigmentary disorders,” she said.
For clinicians interested in incorporating a SAVe-like system into their workflow, Dr. Afanasiev advises them to think seriously about consent. “You want to make sure patients understand what they’re getting themselves into,” she said. “You want to make sure they know that some diagnoses cannot be adequately addressed by teledermatology.” Photo quality is also important, she said. “Video quality is not good enough for most of our diagnoses, so photos are an important part of this evaluation. In this day and age, patients are actually pretty good photographers most of the time.”
She urges practices to carefully think about how they allow patients to submit photos, especially if photographs are not attached to a billable visit.
In her opinion, a good teledermatology platform should have trained support staff with the ability for patients to send photos prior to their visit, and should be safe, secure, and HIPAA compliant. It should also be app and browser compatible and have high resolution and low downtime.
“Into the future, I think it’s important to maintain teledermatology within our clinical practice, especially for remote monitoring of chronic skin diseases,” Dr. Afanasiev said. “Oftentimes we schedule 3- or 6-month follow-ups but often those do not correspond to the patients’ disease flare. They may have had an eczema or psoriatic flare 3 weeks prior, but we see them in clinic with clear skin, which makes it hard to judge how to tailor our treatment. It will also be important for us to understand the safety and security and legal implications of these new practice styles.”
She also referred to technological advances, which she said “will dovetail well with teledermatology, including robust at-home and commercial 3D virtual capture technology, machine learning algorithms for improved photos, and virtual biopsy technology.”
Dr. Afanasiev is a member of the American Academy of Dermatology Teledermatology Task Force. She had no relevant disclosures.
When the .
“Before COVID, we had zero use of teledermatology,” Dr. Afanasiev, a dermatologist at the practice, said during the annual meeting of the Pacific Dermatologic Association. “We didn’t do photography other than the required pre-biopsy photographs, and minimal evaluation of home photos, in response to the patients who say, ‘Wait, doc. Let me pull out my phone and show my photographs.”
During the very first days of the pandemic, in order to accommodate urgent patient requests, she and her colleagues used cloud services for patients to submit photos for concerning skin conditions or lesions, which were then discussed over commonly available video platforms. But they quickly realized that this would not work long-term so within two months, they created an electronic health record–integrated workflow that they are still using, she said.
Here’s how it works. If the patient request is deemed nonacute or does not require a full-body skin exam, the scheduling team offers that patient a store-and-video evaluation (SAVe) or an in-person visit. If a SAVe visit is requested, the patient is required to submit a photograph of his or her condition, then a medical assistant checks for the presence and quality of up to nine patient-submitted photos and contacts the patient if additional photos are required.
Immediately before the encounter, a medical assistant calls the patient to ensure video connectivity and performs a brief intake history. The patient and physician then connect via a video-capable platform – most commonly Vidyo, which is integrated with EPIC. After the visit, the provider notifies the scheduling team if any additional in-person or virtual follow up is required.
In a half day of practice, Dr. Afanasiev sees about 20 patients via video visits scheduled every 15 minutes. On a recent day, 55% of visits were related to acne and 10% were related to a mole check, which usually resulted in recommendation for biopsy. Most (70%) were existing patients, while 30% were new.
“There is no store-and-forward option at this time, meaning that patients can’t just submit a photo without a visit,” she said. “In addition, part of our consent is, if you don’t show up to your scheduled video visit, your photos will not be reviewed. The photos are linked to the video visit and uploaded to the patient’s chart. The rooming workflow is very similar to in clinic, except you’re at home and the medical assistant is remote.”
In an article recently published in the Journal of the American Academy of Dermatology, Dr. Afanasiev and her colleagues described their experience with this photo plus video teledermatology – SAVe – workflow between March 16, 2020, and Aug. 31, 2020. The researchers analyzed 74,411 dermatology cases encountered by 89 providers who cared for 46,024 patients during that time frame. Most of the encounters (79%) were in-person, while the remaining 21% were digital in nature – SAVe in 89% of cases, followed by telephone/message encounters.
At the initial peak of the COVID-19 pandemic in April 2020, SAVe encounters increased to 72% of all encounters from 0% prior to March 16, 2020, and were sustained at 12% when the clinic reopened in the summer of 2020. Over the study period, the clinic’s incorporation of SAVe increased care access to patients located in 731 unique ZIP codes in and near California. “We also have been able to retain many patients within our system,” Dr. Afanasiev said. “We have a large proportion of patients that require 2-3 hours of travel time to get to our clinic, so virtual visits allowed for increased rural access. It also allowed for flexibility for patients and providers.”
The new workflow also led to faster access to care. The time from referral to an in-person evaluation fell from an average of 56 days in 2019 to average of 27 days in 2020, while the wait time for a virtual visit was just 14 days in 2020. “We were able to see a diverse number of diagnostic categories with both in-person and virtual care, most commonly rashes, acne, dermal growths, and pigmentary disorders,” she said.
For clinicians interested in incorporating a SAVe-like system into their workflow, Dr. Afanasiev advises them to think seriously about consent. “You want to make sure patients understand what they’re getting themselves into,” she said. “You want to make sure they know that some diagnoses cannot be adequately addressed by teledermatology.” Photo quality is also important, she said. “Video quality is not good enough for most of our diagnoses, so photos are an important part of this evaluation. In this day and age, patients are actually pretty good photographers most of the time.”
She urges practices to carefully think about how they allow patients to submit photos, especially if photographs are not attached to a billable visit.
In her opinion, a good teledermatology platform should have trained support staff with the ability for patients to send photos prior to their visit, and should be safe, secure, and HIPAA compliant. It should also be app and browser compatible and have high resolution and low downtime.
“Into the future, I think it’s important to maintain teledermatology within our clinical practice, especially for remote monitoring of chronic skin diseases,” Dr. Afanasiev said. “Oftentimes we schedule 3- or 6-month follow-ups but often those do not correspond to the patients’ disease flare. They may have had an eczema or psoriatic flare 3 weeks prior, but we see them in clinic with clear skin, which makes it hard to judge how to tailor our treatment. It will also be important for us to understand the safety and security and legal implications of these new practice styles.”
She also referred to technological advances, which she said “will dovetail well with teledermatology, including robust at-home and commercial 3D virtual capture technology, machine learning algorithms for improved photos, and virtual biopsy technology.”
Dr. Afanasiev is a member of the American Academy of Dermatology Teledermatology Task Force. She had no relevant disclosures.
When the .
“Before COVID, we had zero use of teledermatology,” Dr. Afanasiev, a dermatologist at the practice, said during the annual meeting of the Pacific Dermatologic Association. “We didn’t do photography other than the required pre-biopsy photographs, and minimal evaluation of home photos, in response to the patients who say, ‘Wait, doc. Let me pull out my phone and show my photographs.”
During the very first days of the pandemic, in order to accommodate urgent patient requests, she and her colleagues used cloud services for patients to submit photos for concerning skin conditions or lesions, which were then discussed over commonly available video platforms. But they quickly realized that this would not work long-term so within two months, they created an electronic health record–integrated workflow that they are still using, she said.
Here’s how it works. If the patient request is deemed nonacute or does not require a full-body skin exam, the scheduling team offers that patient a store-and-video evaluation (SAVe) or an in-person visit. If a SAVe visit is requested, the patient is required to submit a photograph of his or her condition, then a medical assistant checks for the presence and quality of up to nine patient-submitted photos and contacts the patient if additional photos are required.
Immediately before the encounter, a medical assistant calls the patient to ensure video connectivity and performs a brief intake history. The patient and physician then connect via a video-capable platform – most commonly Vidyo, which is integrated with EPIC. After the visit, the provider notifies the scheduling team if any additional in-person or virtual follow up is required.
In a half day of practice, Dr. Afanasiev sees about 20 patients via video visits scheduled every 15 minutes. On a recent day, 55% of visits were related to acne and 10% were related to a mole check, which usually resulted in recommendation for biopsy. Most (70%) were existing patients, while 30% were new.
“There is no store-and-forward option at this time, meaning that patients can’t just submit a photo without a visit,” she said. “In addition, part of our consent is, if you don’t show up to your scheduled video visit, your photos will not be reviewed. The photos are linked to the video visit and uploaded to the patient’s chart. The rooming workflow is very similar to in clinic, except you’re at home and the medical assistant is remote.”
In an article recently published in the Journal of the American Academy of Dermatology, Dr. Afanasiev and her colleagues described their experience with this photo plus video teledermatology – SAVe – workflow between March 16, 2020, and Aug. 31, 2020. The researchers analyzed 74,411 dermatology cases encountered by 89 providers who cared for 46,024 patients during that time frame. Most of the encounters (79%) were in-person, while the remaining 21% were digital in nature – SAVe in 89% of cases, followed by telephone/message encounters.
At the initial peak of the COVID-19 pandemic in April 2020, SAVe encounters increased to 72% of all encounters from 0% prior to March 16, 2020, and were sustained at 12% when the clinic reopened in the summer of 2020. Over the study period, the clinic’s incorporation of SAVe increased care access to patients located in 731 unique ZIP codes in and near California. “We also have been able to retain many patients within our system,” Dr. Afanasiev said. “We have a large proportion of patients that require 2-3 hours of travel time to get to our clinic, so virtual visits allowed for increased rural access. It also allowed for flexibility for patients and providers.”
The new workflow also led to faster access to care. The time from referral to an in-person evaluation fell from an average of 56 days in 2019 to average of 27 days in 2020, while the wait time for a virtual visit was just 14 days in 2020. “We were able to see a diverse number of diagnostic categories with both in-person and virtual care, most commonly rashes, acne, dermal growths, and pigmentary disorders,” she said.
For clinicians interested in incorporating a SAVe-like system into their workflow, Dr. Afanasiev advises them to think seriously about consent. “You want to make sure patients understand what they’re getting themselves into,” she said. “You want to make sure they know that some diagnoses cannot be adequately addressed by teledermatology.” Photo quality is also important, she said. “Video quality is not good enough for most of our diagnoses, so photos are an important part of this evaluation. In this day and age, patients are actually pretty good photographers most of the time.”
She urges practices to carefully think about how they allow patients to submit photos, especially if photographs are not attached to a billable visit.
In her opinion, a good teledermatology platform should have trained support staff with the ability for patients to send photos prior to their visit, and should be safe, secure, and HIPAA compliant. It should also be app and browser compatible and have high resolution and low downtime.
“Into the future, I think it’s important to maintain teledermatology within our clinical practice, especially for remote monitoring of chronic skin diseases,” Dr. Afanasiev said. “Oftentimes we schedule 3- or 6-month follow-ups but often those do not correspond to the patients’ disease flare. They may have had an eczema or psoriatic flare 3 weeks prior, but we see them in clinic with clear skin, which makes it hard to judge how to tailor our treatment. It will also be important for us to understand the safety and security and legal implications of these new practice styles.”
She also referred to technological advances, which she said “will dovetail well with teledermatology, including robust at-home and commercial 3D virtual capture technology, machine learning algorithms for improved photos, and virtual biopsy technology.”
Dr. Afanasiev is a member of the American Academy of Dermatology Teledermatology Task Force. She had no relevant disclosures.
FROM PDA 2021
Better COVID-19 outcomes confirmed in TNF inhibitor users
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
CDC panel backs COVID-19 boosters for nearly all adults
Editor’s note: This story was updated with the CDC director’s endorsement.
Centers for Disease Control and Prevention (CDC) Director Rochelle Walensky, MD, has signed off on an advisory panel’s earlier unanimous vote to recommend boosters for the Moderna and Johnson and Johnson COVID vaccines.
The decision now means that millions of Americans are eligible to get a booster shot for either the Pfizer, Moderna, or J&J COVID vaccines.
“The evidence shows that all three COVID-19 vaccines authorized in the United States are safe – as demonstrated by the over 400 million vaccine doses already given. And, they are all highly effective in reducing the risk of severe disease, hospitalization, and death, even in the midst of the widely circulating Delta variant,” Dr. Walensky said in a CDC news release.
She also signed off on the panel’s suggestion that individuals can mix or match the booster from any one of the three available COVID-19 vaccines.
The Advisory Committee on Immunization Practices (ACIP) recommended in a late afternoon 15-0 vote that everyone over age 18 who are at least 2 months past their Johnson & Johnson vaccine should get a booster, an endorsement that affects an estimated 13 million Americans.
Those eligible for a booster at least 6 months after their last Moderna shot are the same groups who can get a Pfizer booster.
They are:
- Anyone over age 65.
- Those over age 18 with an underlying health condition that puts them at risk of severe COVID-19.
- Those over age 18 who may be at higher risk of a COVID-19 infection because they live or work in a risky setting.
These recommendations are in line with the Food and Drug Administration’s Oct. 20 authorization of the boosters, along with the ability to mix-and-match vaccines.
There are an estimated 47 million Pfizer recipients and 39 million people vaccinated with Moderna who are now eligible for a booster dose, according to data presented by the CDC.
Questions, concerns
Before voting, some committee members expressed discomfort in broadly recommending boosters, stressing that there is very little evidence supporting the need for boosters in people younger than age 50.
“I can’t say that I am comfortable that anybody under 50 – an otherwise healthy individual – needs a booster vaccine at this time with either Moderna or Pfizer,” said ACIP member Sarah Long, MD, professor of pediatrics at Drexel University in Philadelphia.
She said she would try to mitigate any potential harm by having some kind of age restriction on the otherwise worried well.
“We don’t usually have the vaccines [for] the worried well. We give it because we have a need that’s worth the risk, and there’s a burden of severity of disease,” Dr. Long said.
The evidence to date shows that all the vaccines authorized for use in the U.S. continue to protect people well against severe COVID-19 outcomes, including hospitalization and death.
But breakthrough infections are on the rise, especially for people who initially received the Johnson and Johnson one-dose vaccine.
On Oct. 21, Pfizer released data from a study of more than 10,000 fully vaccinated people. Half were randomly assigned to get a booster of their Comirnaty vaccine, the other half were given a placebo.
Over the ensuing 2.5 months, there were 5 COVID-19 cases in the boosted group, and 109 in the group that got a placebo.
The data were posted in a press release and have not yet been peer reviewed, but are the first to show clinical effectiveness of boosters at preventing COVID-19 infections.
Data recently considered by the FDA and CDC for booster doses come from studies that were mostly shorter and smaller. These studies looked at biomarkers of immunity like the concentration of antibodies in a person’s blood and the percentage of study participants who saw a boost to those antibodies.
The studies demonstrated that boosters indeed restore high levels of antibodies, but unlike the newest Pfizer data they were not able to show that these antibodies prevented COVID-19.
These studies also weren’t powered to pick up on any less common safety problems that might arise after another dose of the shots.
“Real world” recommendations
In the end, however, the panel felt it was more important to be permissive in allowing boosters so that individuals and their doctors could be free to make their own decisions.
“The decision made by the FDA and the ACIP recommendations, I think, reflects the real world. The public is going to do what they feel driven to do. This at least adds a scientific review of the currently available data,” said Jay Varkey, MD, an infectious disease physician and associate professor at Emory University in Atlanta, who was not involved in the ACIP’s deliberations.
Dr. Varkey said he would recommend that anyone who is younger than 65, and who has no underlying medical conditions such as diabetes or obesity, speak with their doctor about their individual benefits and risks before getting a booster.
The CDC is planning to release a detailed suite of clinical considerations to help people weigh the risks and benefits of getting a booster.
Safety updates presented at the meeting show that serious adverse events after vaccination are extremely rare, but in some cases, they may rise above the risk for those problems generally seen in the population.
Those rare events include the disabling autoimmune condition Guillain-Barré syndrome and the platelet disorder thrombosis with thrombocytopenia (TTS), which causes blood clots along with the risk of excess bleeding because of a low platelet count.
Both can occur after the J&J vaccine. Out of 15.3 million doses of the vaccine given to date, there have been 47 cases of TTS and five deaths. These events are more common in younger women.
The mRNA vaccines, such as those from Pfizer and Moderna, can cause heart inflammation called myocarditis or pericarditis. This side effect is more common in men 18-24 years old. The reported rate of myocarditis after vaccination is 39 cases for every 1 million doses.
In voting to permit boosters, committee member Wilbur Chen, MD, professor at the University of Maryland’s Center for Vaccine Development, said he hoped boosters wouldn’t give Americans false confidence.
Dr. Chen stressed that ending the pandemic would depend on “a multilayered approach” that includes masking, social distancing, avoiding large crowds indoors, and convincing more Americans to take their first doses of the vaccines.
“We’re not just going to vaccinate ourselves out of this situation,” Dr. Chen said.
A version of this article first appeared on WebMD.com.
Editor’s note: This story was updated with the CDC director’s endorsement.
Centers for Disease Control and Prevention (CDC) Director Rochelle Walensky, MD, has signed off on an advisory panel’s earlier unanimous vote to recommend boosters for the Moderna and Johnson and Johnson COVID vaccines.
The decision now means that millions of Americans are eligible to get a booster shot for either the Pfizer, Moderna, or J&J COVID vaccines.
“The evidence shows that all three COVID-19 vaccines authorized in the United States are safe – as demonstrated by the over 400 million vaccine doses already given. And, they are all highly effective in reducing the risk of severe disease, hospitalization, and death, even in the midst of the widely circulating Delta variant,” Dr. Walensky said in a CDC news release.
She also signed off on the panel’s suggestion that individuals can mix or match the booster from any one of the three available COVID-19 vaccines.
The Advisory Committee on Immunization Practices (ACIP) recommended in a late afternoon 15-0 vote that everyone over age 18 who are at least 2 months past their Johnson & Johnson vaccine should get a booster, an endorsement that affects an estimated 13 million Americans.
Those eligible for a booster at least 6 months after their last Moderna shot are the same groups who can get a Pfizer booster.
They are:
- Anyone over age 65.
- Those over age 18 with an underlying health condition that puts them at risk of severe COVID-19.
- Those over age 18 who may be at higher risk of a COVID-19 infection because they live or work in a risky setting.
These recommendations are in line with the Food and Drug Administration’s Oct. 20 authorization of the boosters, along with the ability to mix-and-match vaccines.
There are an estimated 47 million Pfizer recipients and 39 million people vaccinated with Moderna who are now eligible for a booster dose, according to data presented by the CDC.
Questions, concerns
Before voting, some committee members expressed discomfort in broadly recommending boosters, stressing that there is very little evidence supporting the need for boosters in people younger than age 50.
“I can’t say that I am comfortable that anybody under 50 – an otherwise healthy individual – needs a booster vaccine at this time with either Moderna or Pfizer,” said ACIP member Sarah Long, MD, professor of pediatrics at Drexel University in Philadelphia.
She said she would try to mitigate any potential harm by having some kind of age restriction on the otherwise worried well.
“We don’t usually have the vaccines [for] the worried well. We give it because we have a need that’s worth the risk, and there’s a burden of severity of disease,” Dr. Long said.
The evidence to date shows that all the vaccines authorized for use in the U.S. continue to protect people well against severe COVID-19 outcomes, including hospitalization and death.
But breakthrough infections are on the rise, especially for people who initially received the Johnson and Johnson one-dose vaccine.
On Oct. 21, Pfizer released data from a study of more than 10,000 fully vaccinated people. Half were randomly assigned to get a booster of their Comirnaty vaccine, the other half were given a placebo.
Over the ensuing 2.5 months, there were 5 COVID-19 cases in the boosted group, and 109 in the group that got a placebo.
The data were posted in a press release and have not yet been peer reviewed, but are the first to show clinical effectiveness of boosters at preventing COVID-19 infections.
Data recently considered by the FDA and CDC for booster doses come from studies that were mostly shorter and smaller. These studies looked at biomarkers of immunity like the concentration of antibodies in a person’s blood and the percentage of study participants who saw a boost to those antibodies.
The studies demonstrated that boosters indeed restore high levels of antibodies, but unlike the newest Pfizer data they were not able to show that these antibodies prevented COVID-19.
These studies also weren’t powered to pick up on any less common safety problems that might arise after another dose of the shots.
“Real world” recommendations
In the end, however, the panel felt it was more important to be permissive in allowing boosters so that individuals and their doctors could be free to make their own decisions.
“The decision made by the FDA and the ACIP recommendations, I think, reflects the real world. The public is going to do what they feel driven to do. This at least adds a scientific review of the currently available data,” said Jay Varkey, MD, an infectious disease physician and associate professor at Emory University in Atlanta, who was not involved in the ACIP’s deliberations.
Dr. Varkey said he would recommend that anyone who is younger than 65, and who has no underlying medical conditions such as diabetes or obesity, speak with their doctor about their individual benefits and risks before getting a booster.
The CDC is planning to release a detailed suite of clinical considerations to help people weigh the risks and benefits of getting a booster.
Safety updates presented at the meeting show that serious adverse events after vaccination are extremely rare, but in some cases, they may rise above the risk for those problems generally seen in the population.
Those rare events include the disabling autoimmune condition Guillain-Barré syndrome and the platelet disorder thrombosis with thrombocytopenia (TTS), which causes blood clots along with the risk of excess bleeding because of a low platelet count.
Both can occur after the J&J vaccine. Out of 15.3 million doses of the vaccine given to date, there have been 47 cases of TTS and five deaths. These events are more common in younger women.
The mRNA vaccines, such as those from Pfizer and Moderna, can cause heart inflammation called myocarditis or pericarditis. This side effect is more common in men 18-24 years old. The reported rate of myocarditis after vaccination is 39 cases for every 1 million doses.
In voting to permit boosters, committee member Wilbur Chen, MD, professor at the University of Maryland’s Center for Vaccine Development, said he hoped boosters wouldn’t give Americans false confidence.
Dr. Chen stressed that ending the pandemic would depend on “a multilayered approach” that includes masking, social distancing, avoiding large crowds indoors, and convincing more Americans to take their first doses of the vaccines.
“We’re not just going to vaccinate ourselves out of this situation,” Dr. Chen said.
A version of this article first appeared on WebMD.com.
Editor’s note: This story was updated with the CDC director’s endorsement.
Centers for Disease Control and Prevention (CDC) Director Rochelle Walensky, MD, has signed off on an advisory panel’s earlier unanimous vote to recommend boosters for the Moderna and Johnson and Johnson COVID vaccines.
The decision now means that millions of Americans are eligible to get a booster shot for either the Pfizer, Moderna, or J&J COVID vaccines.
“The evidence shows that all three COVID-19 vaccines authorized in the United States are safe – as demonstrated by the over 400 million vaccine doses already given. And, they are all highly effective in reducing the risk of severe disease, hospitalization, and death, even in the midst of the widely circulating Delta variant,” Dr. Walensky said in a CDC news release.
She also signed off on the panel’s suggestion that individuals can mix or match the booster from any one of the three available COVID-19 vaccines.
The Advisory Committee on Immunization Practices (ACIP) recommended in a late afternoon 15-0 vote that everyone over age 18 who are at least 2 months past their Johnson & Johnson vaccine should get a booster, an endorsement that affects an estimated 13 million Americans.
Those eligible for a booster at least 6 months after their last Moderna shot are the same groups who can get a Pfizer booster.
They are:
- Anyone over age 65.
- Those over age 18 with an underlying health condition that puts them at risk of severe COVID-19.
- Those over age 18 who may be at higher risk of a COVID-19 infection because they live or work in a risky setting.
These recommendations are in line with the Food and Drug Administration’s Oct. 20 authorization of the boosters, along with the ability to mix-and-match vaccines.
There are an estimated 47 million Pfizer recipients and 39 million people vaccinated with Moderna who are now eligible for a booster dose, according to data presented by the CDC.
Questions, concerns
Before voting, some committee members expressed discomfort in broadly recommending boosters, stressing that there is very little evidence supporting the need for boosters in people younger than age 50.
“I can’t say that I am comfortable that anybody under 50 – an otherwise healthy individual – needs a booster vaccine at this time with either Moderna or Pfizer,” said ACIP member Sarah Long, MD, professor of pediatrics at Drexel University in Philadelphia.
She said she would try to mitigate any potential harm by having some kind of age restriction on the otherwise worried well.
“We don’t usually have the vaccines [for] the worried well. We give it because we have a need that’s worth the risk, and there’s a burden of severity of disease,” Dr. Long said.
The evidence to date shows that all the vaccines authorized for use in the U.S. continue to protect people well against severe COVID-19 outcomes, including hospitalization and death.
But breakthrough infections are on the rise, especially for people who initially received the Johnson and Johnson one-dose vaccine.
On Oct. 21, Pfizer released data from a study of more than 10,000 fully vaccinated people. Half were randomly assigned to get a booster of their Comirnaty vaccine, the other half were given a placebo.
Over the ensuing 2.5 months, there were 5 COVID-19 cases in the boosted group, and 109 in the group that got a placebo.
The data were posted in a press release and have not yet been peer reviewed, but are the first to show clinical effectiveness of boosters at preventing COVID-19 infections.
Data recently considered by the FDA and CDC for booster doses come from studies that were mostly shorter and smaller. These studies looked at biomarkers of immunity like the concentration of antibodies in a person’s blood and the percentage of study participants who saw a boost to those antibodies.
The studies demonstrated that boosters indeed restore high levels of antibodies, but unlike the newest Pfizer data they were not able to show that these antibodies prevented COVID-19.
These studies also weren’t powered to pick up on any less common safety problems that might arise after another dose of the shots.
“Real world” recommendations
In the end, however, the panel felt it was more important to be permissive in allowing boosters so that individuals and their doctors could be free to make their own decisions.
“The decision made by the FDA and the ACIP recommendations, I think, reflects the real world. The public is going to do what they feel driven to do. This at least adds a scientific review of the currently available data,” said Jay Varkey, MD, an infectious disease physician and associate professor at Emory University in Atlanta, who was not involved in the ACIP’s deliberations.
Dr. Varkey said he would recommend that anyone who is younger than 65, and who has no underlying medical conditions such as diabetes or obesity, speak with their doctor about their individual benefits and risks before getting a booster.
The CDC is planning to release a detailed suite of clinical considerations to help people weigh the risks and benefits of getting a booster.
Safety updates presented at the meeting show that serious adverse events after vaccination are extremely rare, but in some cases, they may rise above the risk for those problems generally seen in the population.
Those rare events include the disabling autoimmune condition Guillain-Barré syndrome and the platelet disorder thrombosis with thrombocytopenia (TTS), which causes blood clots along with the risk of excess bleeding because of a low platelet count.
Both can occur after the J&J vaccine. Out of 15.3 million doses of the vaccine given to date, there have been 47 cases of TTS and five deaths. These events are more common in younger women.
The mRNA vaccines, such as those from Pfizer and Moderna, can cause heart inflammation called myocarditis or pericarditis. This side effect is more common in men 18-24 years old. The reported rate of myocarditis after vaccination is 39 cases for every 1 million doses.
In voting to permit boosters, committee member Wilbur Chen, MD, professor at the University of Maryland’s Center for Vaccine Development, said he hoped boosters wouldn’t give Americans false confidence.
Dr. Chen stressed that ending the pandemic would depend on “a multilayered approach” that includes masking, social distancing, avoiding large crowds indoors, and convincing more Americans to take their first doses of the vaccines.
“We’re not just going to vaccinate ourselves out of this situation,” Dr. Chen said.
A version of this article first appeared on WebMD.com.
The compass that points toward food
The new breakfast of champions
We love a good ranking system here at LOTME world headquarters, especially the food-based ones. Luckily for us (and our readers), a new study published in Nature Food offers a food-based ranking system.
Sadly, unlike the last food-related ranking we covered, the Food Compass doesn’t tell you how much life you gain or lose from each food you eat down to the precise minute. Instead, it favors a more simple rating system from 1 to 100, with healthier foods scoring higher, and even incorporates mixed foods, not just single ingredients. This makes it better at assessing and comparing food combinations, rather than trying to mix and match the many ingredients that go into even relatively simple recipes.
The top and bottom of the rankings contain the usual suspects. Legumes and nuts, at 78.6, had the highest average score among the broad food groups, followed by fruits and then vegetables. Rounding out the bottom were sweets and savory snacks at 16.4. Among the individual foods, there were perfect scores in both directions: 100 for raw raspberries, while instant noodle soup and nonchocolate, ready-to-eat, nonfat pudding (very specific there) each earned a 1.
There are a few surprises in between. Nonfat cappuccino received a green light from the investigators, great news for the coffee drinkers out there. A serving of sweet potato chips scored better than a simple grilled chicken breast, and a slice of pizza, loaded up with extra meat and a thick crust, is still more nutritious than a bowl of corn flakes.
Neither is good for you, of course, but we’re still going to take this as a sign that pizza is the ideal breakfast food. Add that to your morning coffee, and you’re ready to start the day. Move over Wheaties, there’s a new breakfast of champions.
COVID-19 resisters, please step forward
Some people have all the luck with good genes, both inside and out.
Genetically speaking, humans are 99.9% the same, but that 0.1% is where things get interesting. Because of that 0.1% difference, some people are more likely to contract diseases such as HIV, while others might be more resistant. These small differences in genetic code could be the key to finding treatments for COVID-19.
“The introduction of SARS-CoV-2 to a naive population, on a global scale, has provided yet another demonstration of the remarkable clinical variability between individuals in the course of infection, ranging from asymptomatic infections to life-threatening disease,” the researchers said in Nature Immunology.
The investigators have been scouring the world to find people who might be resistant to SARS-CoV-2 and have enrolled over 400 individuals in a “dedicated resistance study cohort,” according to ScienceAlert.
The investigators are looking at households in which families were infected but one member did not show severe symptoms, or for individuals who have been around the virus multiple times and haven’t contracted it. They are also looking at blood types.
Enrollment is ongoing, so if you’ve been in contact with COVID-19 multiple times and have not gotten sick, scientists would like to hear from you.
Better living through parasitization
How would you like to triple your life span, while maintaining a youthful appearance and gaining special social standing and privileges?
Sounds pretty good, right, so what’s the catch? Well, you have to be infected with a tapeworm ... and you have to be an ant.
If you are an ant, here’s the deal: Workers of the species Temnothorax nylanderi that have tapeworms live much longer than uninfected workers, and while living out those longer lives they do less work and receive gifts of food.
In a study conducted at Johannes Gutenberg University in Mainz, Germany, infected ants’ metabolic rates and lipid levels were similar to those of younger ants, and they appeared to remain in a permanent juvenile stage as a result of the infection, the investigators reported.
They tracked Temnothorax colonies for 3 years, at which point 95% of the uninfected workers had died but over half of the infected ants were still alive. Pretty great, right? Wrong. There was no joy in antville, for the uninfected workers had struck out. “Strained by the additional burden of their wormed-up nestmates, they seemed to be shunting care away from their queen. They were dying sooner than they might have if the colonies had remained parasite-free,” according to an article in the Atlantic.
Does this situation seem just a wee bit familiar? A small group lives longer, healthier lives and enjoys special privileges while the majority of that society works harder to support them? We’ll put it into the form of a chicken-and-egg argument: Which came first, the tapeworms or the one-percenters?
Laughing the pandemic stress away
Doomscrolling on social media has become one of the world’s favorite pastimes during the pandemic, but research shows that those memes about COVID-19 might combat the doom and gloom of the outside world.
A study recently published in Psychology of Popular Media showed that viewing memes, specifically those that were COVID-19 related, actually lessened the stress of the pandemic.
The researchers conducted a survey of 748 people aged 18-88 years. Each participant viewed three memes with text or three memes with text but no images. All three memes had similar cuteness levels (baby or adult), subject (animal or human), and caption (COVID-19–related or not). The participants were then asked to report on their stress levels and feelings before and after the memes.
The people who looked at memes felt less stressed and a higher humor level, especially the participants who received the COVID-19 memes. Study Finds said that they had more “pandemic-coping confidence” than those who got regular memes.
“While the World Health Organization recommended that people avoid too much COVID-related media for the benefit of their mental health, our research reveals that memes about COVID-19 could help people feel more confident in their ability to deal with the pandemic,” lead author Jessica Gall Myrick, PhD, said in a written statement. “The positive emotions associated with this type of content may make people feel psychologically safer and therefore better able to pay attention to the underlying messages related to health threats.”
So if you think you’ve been wasting time looking at memes during this pandemic, think again. It actually might keep you sane. Keep on scrolling!
Giving the gift of stress reduction
It’s a big week here at LOTME. You’ve just read our 100th edition, and to help celebrate that milestone – along with Count Your Buttons Day, Celebration of the Mind Day, and the International Day of the Nacho – we’re presenting an extra-special bonus feature, courtesy of Sad and Useless: The most depressive humor site on the Internet.
We hope you’ll stop your doomscrolling long enough to enjoy this stress-reducing meme. Thanks for reading!
The new breakfast of champions
We love a good ranking system here at LOTME world headquarters, especially the food-based ones. Luckily for us (and our readers), a new study published in Nature Food offers a food-based ranking system.
Sadly, unlike the last food-related ranking we covered, the Food Compass doesn’t tell you how much life you gain or lose from each food you eat down to the precise minute. Instead, it favors a more simple rating system from 1 to 100, with healthier foods scoring higher, and even incorporates mixed foods, not just single ingredients. This makes it better at assessing and comparing food combinations, rather than trying to mix and match the many ingredients that go into even relatively simple recipes.
The top and bottom of the rankings contain the usual suspects. Legumes and nuts, at 78.6, had the highest average score among the broad food groups, followed by fruits and then vegetables. Rounding out the bottom were sweets and savory snacks at 16.4. Among the individual foods, there were perfect scores in both directions: 100 for raw raspberries, while instant noodle soup and nonchocolate, ready-to-eat, nonfat pudding (very specific there) each earned a 1.
There are a few surprises in between. Nonfat cappuccino received a green light from the investigators, great news for the coffee drinkers out there. A serving of sweet potato chips scored better than a simple grilled chicken breast, and a slice of pizza, loaded up with extra meat and a thick crust, is still more nutritious than a bowl of corn flakes.
Neither is good for you, of course, but we’re still going to take this as a sign that pizza is the ideal breakfast food. Add that to your morning coffee, and you’re ready to start the day. Move over Wheaties, there’s a new breakfast of champions.
COVID-19 resisters, please step forward
Some people have all the luck with good genes, both inside and out.
Genetically speaking, humans are 99.9% the same, but that 0.1% is where things get interesting. Because of that 0.1% difference, some people are more likely to contract diseases such as HIV, while others might be more resistant. These small differences in genetic code could be the key to finding treatments for COVID-19.
“The introduction of SARS-CoV-2 to a naive population, on a global scale, has provided yet another demonstration of the remarkable clinical variability between individuals in the course of infection, ranging from asymptomatic infections to life-threatening disease,” the researchers said in Nature Immunology.
The investigators have been scouring the world to find people who might be resistant to SARS-CoV-2 and have enrolled over 400 individuals in a “dedicated resistance study cohort,” according to ScienceAlert.
The investigators are looking at households in which families were infected but one member did not show severe symptoms, or for individuals who have been around the virus multiple times and haven’t contracted it. They are also looking at blood types.
Enrollment is ongoing, so if you’ve been in contact with COVID-19 multiple times and have not gotten sick, scientists would like to hear from you.
Better living through parasitization
How would you like to triple your life span, while maintaining a youthful appearance and gaining special social standing and privileges?
Sounds pretty good, right, so what’s the catch? Well, you have to be infected with a tapeworm ... and you have to be an ant.
If you are an ant, here’s the deal: Workers of the species Temnothorax nylanderi that have tapeworms live much longer than uninfected workers, and while living out those longer lives they do less work and receive gifts of food.
In a study conducted at Johannes Gutenberg University in Mainz, Germany, infected ants’ metabolic rates and lipid levels were similar to those of younger ants, and they appeared to remain in a permanent juvenile stage as a result of the infection, the investigators reported.
They tracked Temnothorax colonies for 3 years, at which point 95% of the uninfected workers had died but over half of the infected ants were still alive. Pretty great, right? Wrong. There was no joy in antville, for the uninfected workers had struck out. “Strained by the additional burden of their wormed-up nestmates, they seemed to be shunting care away from their queen. They were dying sooner than they might have if the colonies had remained parasite-free,” according to an article in the Atlantic.
Does this situation seem just a wee bit familiar? A small group lives longer, healthier lives and enjoys special privileges while the majority of that society works harder to support them? We’ll put it into the form of a chicken-and-egg argument: Which came first, the tapeworms or the one-percenters?
Laughing the pandemic stress away
Doomscrolling on social media has become one of the world’s favorite pastimes during the pandemic, but research shows that those memes about COVID-19 might combat the doom and gloom of the outside world.
A study recently published in Psychology of Popular Media showed that viewing memes, specifically those that were COVID-19 related, actually lessened the stress of the pandemic.
The researchers conducted a survey of 748 people aged 18-88 years. Each participant viewed three memes with text or three memes with text but no images. All three memes had similar cuteness levels (baby or adult), subject (animal or human), and caption (COVID-19–related or not). The participants were then asked to report on their stress levels and feelings before and after the memes.
The people who looked at memes felt less stressed and a higher humor level, especially the participants who received the COVID-19 memes. Study Finds said that they had more “pandemic-coping confidence” than those who got regular memes.
“While the World Health Organization recommended that people avoid too much COVID-related media for the benefit of their mental health, our research reveals that memes about COVID-19 could help people feel more confident in their ability to deal with the pandemic,” lead author Jessica Gall Myrick, PhD, said in a written statement. “The positive emotions associated with this type of content may make people feel psychologically safer and therefore better able to pay attention to the underlying messages related to health threats.”
So if you think you’ve been wasting time looking at memes during this pandemic, think again. It actually might keep you sane. Keep on scrolling!
Giving the gift of stress reduction
It’s a big week here at LOTME. You’ve just read our 100th edition, and to help celebrate that milestone – along with Count Your Buttons Day, Celebration of the Mind Day, and the International Day of the Nacho – we’re presenting an extra-special bonus feature, courtesy of Sad and Useless: The most depressive humor site on the Internet.
We hope you’ll stop your doomscrolling long enough to enjoy this stress-reducing meme. Thanks for reading!
The new breakfast of champions
We love a good ranking system here at LOTME world headquarters, especially the food-based ones. Luckily for us (and our readers), a new study published in Nature Food offers a food-based ranking system.
Sadly, unlike the last food-related ranking we covered, the Food Compass doesn’t tell you how much life you gain or lose from each food you eat down to the precise minute. Instead, it favors a more simple rating system from 1 to 100, with healthier foods scoring higher, and even incorporates mixed foods, not just single ingredients. This makes it better at assessing and comparing food combinations, rather than trying to mix and match the many ingredients that go into even relatively simple recipes.
The top and bottom of the rankings contain the usual suspects. Legumes and nuts, at 78.6, had the highest average score among the broad food groups, followed by fruits and then vegetables. Rounding out the bottom were sweets and savory snacks at 16.4. Among the individual foods, there were perfect scores in both directions: 100 for raw raspberries, while instant noodle soup and nonchocolate, ready-to-eat, nonfat pudding (very specific there) each earned a 1.
There are a few surprises in between. Nonfat cappuccino received a green light from the investigators, great news for the coffee drinkers out there. A serving of sweet potato chips scored better than a simple grilled chicken breast, and a slice of pizza, loaded up with extra meat and a thick crust, is still more nutritious than a bowl of corn flakes.
Neither is good for you, of course, but we’re still going to take this as a sign that pizza is the ideal breakfast food. Add that to your morning coffee, and you’re ready to start the day. Move over Wheaties, there’s a new breakfast of champions.
COVID-19 resisters, please step forward
Some people have all the luck with good genes, both inside and out.
Genetically speaking, humans are 99.9% the same, but that 0.1% is where things get interesting. Because of that 0.1% difference, some people are more likely to contract diseases such as HIV, while others might be more resistant. These small differences in genetic code could be the key to finding treatments for COVID-19.
“The introduction of SARS-CoV-2 to a naive population, on a global scale, has provided yet another demonstration of the remarkable clinical variability between individuals in the course of infection, ranging from asymptomatic infections to life-threatening disease,” the researchers said in Nature Immunology.
The investigators have been scouring the world to find people who might be resistant to SARS-CoV-2 and have enrolled over 400 individuals in a “dedicated resistance study cohort,” according to ScienceAlert.
The investigators are looking at households in which families were infected but one member did not show severe symptoms, or for individuals who have been around the virus multiple times and haven’t contracted it. They are also looking at blood types.
Enrollment is ongoing, so if you’ve been in contact with COVID-19 multiple times and have not gotten sick, scientists would like to hear from you.
Better living through parasitization
How would you like to triple your life span, while maintaining a youthful appearance and gaining special social standing and privileges?
Sounds pretty good, right, so what’s the catch? Well, you have to be infected with a tapeworm ... and you have to be an ant.
If you are an ant, here’s the deal: Workers of the species Temnothorax nylanderi that have tapeworms live much longer than uninfected workers, and while living out those longer lives they do less work and receive gifts of food.
In a study conducted at Johannes Gutenberg University in Mainz, Germany, infected ants’ metabolic rates and lipid levels were similar to those of younger ants, and they appeared to remain in a permanent juvenile stage as a result of the infection, the investigators reported.
They tracked Temnothorax colonies for 3 years, at which point 95% of the uninfected workers had died but over half of the infected ants were still alive. Pretty great, right? Wrong. There was no joy in antville, for the uninfected workers had struck out. “Strained by the additional burden of their wormed-up nestmates, they seemed to be shunting care away from their queen. They were dying sooner than they might have if the colonies had remained parasite-free,” according to an article in the Atlantic.
Does this situation seem just a wee bit familiar? A small group lives longer, healthier lives and enjoys special privileges while the majority of that society works harder to support them? We’ll put it into the form of a chicken-and-egg argument: Which came first, the tapeworms or the one-percenters?
Laughing the pandemic stress away
Doomscrolling on social media has become one of the world’s favorite pastimes during the pandemic, but research shows that those memes about COVID-19 might combat the doom and gloom of the outside world.
A study recently published in Psychology of Popular Media showed that viewing memes, specifically those that were COVID-19 related, actually lessened the stress of the pandemic.
The researchers conducted a survey of 748 people aged 18-88 years. Each participant viewed three memes with text or three memes with text but no images. All three memes had similar cuteness levels (baby or adult), subject (animal or human), and caption (COVID-19–related or not). The participants were then asked to report on their stress levels and feelings before and after the memes.
The people who looked at memes felt less stressed and a higher humor level, especially the participants who received the COVID-19 memes. Study Finds said that they had more “pandemic-coping confidence” than those who got regular memes.
“While the World Health Organization recommended that people avoid too much COVID-related media for the benefit of their mental health, our research reveals that memes about COVID-19 could help people feel more confident in their ability to deal with the pandemic,” lead author Jessica Gall Myrick, PhD, said in a written statement. “The positive emotions associated with this type of content may make people feel psychologically safer and therefore better able to pay attention to the underlying messages related to health threats.”
So if you think you’ve been wasting time looking at memes during this pandemic, think again. It actually might keep you sane. Keep on scrolling!
Giving the gift of stress reduction
It’s a big week here at LOTME. You’ve just read our 100th edition, and to help celebrate that milestone – along with Count Your Buttons Day, Celebration of the Mind Day, and the International Day of the Nacho – we’re presenting an extra-special bonus feature, courtesy of Sad and Useless: The most depressive humor site on the Internet.
We hope you’ll stop your doomscrolling long enough to enjoy this stress-reducing meme. Thanks for reading!