User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
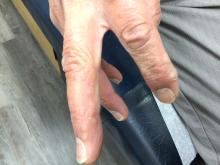
A 70-year-old man presents with firm papules on his hand and fingers
, although women are more often affected than men. GA most commonly appears in the first 3 decades of life. Although the etiology is not known, GA may represent a delayed hypersensitivity reaction. A link between GA and diabetes mellitus, autoimmune thyroiditis, dyslipidemia, and rarely, malignancy may exist.
GA is most commonly localized, presenting as an asymptomatic, erythematous, annular plaque with a firm border and central clearing localized to the wrists, ankles, and dorsal hands or feet. This form is the type most often seen in children. Generalized GA is far less common and presents later in life as multiple asymptomatic or pruritic papules and plaques on the trunk and extremities. Less common variants include subcutaneous GA, patch GA, atypical GA, and perforating GA. Perforating GA occurs on the dorsal hands and presents as (umbilicated) papules, and seems consistent with this patient’s clinical presentation. Histologically, transepidermal elimination of collagen is typically seen in perforating GA.1
Histology in this patient’s biopsy revealed a granulomatous dermatitis consistent with granuloma annulare. A palisaded arrangement of histiocytic cells surrounding altered collagen with increased dermal mucin was seen. There was associated perivascular mononuclear inflammatory infiltrates. The overlying epidermis was unremarkable.
Granuloma annulare often spontaneously resolves without sequelae. In some cases, atrophy may result. Lesions may also recur. Localized GA is often treated with high-potency topical corticosteroids or intralesional corticosteroids. For generalized GA, topical or intralesional corticosteroids may be used for select lesions. Topical calcineurin inhibitors, light therapy, cryotherapy, imiquimod, hydroxychloroquine, isotretinoin, and dapsone have also been reported in the literature as possible treatments.
This case and photo were provided by Dr. Berke, of Three Rivers Dermatology, Pittsburgh, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1 Alves J, Barreiros H, Bartolo E. Healthcare (Basel). 2014 Sep 4;2(3):338-45.
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
3. “Andrews’ Diseases of the Skin,” 13th ed. James W et al. Philadelphia: Saunders Elsevier, 2006.
, although women are more often affected than men. GA most commonly appears in the first 3 decades of life. Although the etiology is not known, GA may represent a delayed hypersensitivity reaction. A link between GA and diabetes mellitus, autoimmune thyroiditis, dyslipidemia, and rarely, malignancy may exist.
GA is most commonly localized, presenting as an asymptomatic, erythematous, annular plaque with a firm border and central clearing localized to the wrists, ankles, and dorsal hands or feet. This form is the type most often seen in children. Generalized GA is far less common and presents later in life as multiple asymptomatic or pruritic papules and plaques on the trunk and extremities. Less common variants include subcutaneous GA, patch GA, atypical GA, and perforating GA. Perforating GA occurs on the dorsal hands and presents as (umbilicated) papules, and seems consistent with this patient’s clinical presentation. Histologically, transepidermal elimination of collagen is typically seen in perforating GA.1
Histology in this patient’s biopsy revealed a granulomatous dermatitis consistent with granuloma annulare. A palisaded arrangement of histiocytic cells surrounding altered collagen with increased dermal mucin was seen. There was associated perivascular mononuclear inflammatory infiltrates. The overlying epidermis was unremarkable.
Granuloma annulare often spontaneously resolves without sequelae. In some cases, atrophy may result. Lesions may also recur. Localized GA is often treated with high-potency topical corticosteroids or intralesional corticosteroids. For generalized GA, topical or intralesional corticosteroids may be used for select lesions. Topical calcineurin inhibitors, light therapy, cryotherapy, imiquimod, hydroxychloroquine, isotretinoin, and dapsone have also been reported in the literature as possible treatments.
This case and photo were provided by Dr. Berke, of Three Rivers Dermatology, Pittsburgh, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1 Alves J, Barreiros H, Bartolo E. Healthcare (Basel). 2014 Sep 4;2(3):338-45.
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
3. “Andrews’ Diseases of the Skin,” 13th ed. James W et al. Philadelphia: Saunders Elsevier, 2006.
, although women are more often affected than men. GA most commonly appears in the first 3 decades of life. Although the etiology is not known, GA may represent a delayed hypersensitivity reaction. A link between GA and diabetes mellitus, autoimmune thyroiditis, dyslipidemia, and rarely, malignancy may exist.
GA is most commonly localized, presenting as an asymptomatic, erythematous, annular plaque with a firm border and central clearing localized to the wrists, ankles, and dorsal hands or feet. This form is the type most often seen in children. Generalized GA is far less common and presents later in life as multiple asymptomatic or pruritic papules and plaques on the trunk and extremities. Less common variants include subcutaneous GA, patch GA, atypical GA, and perforating GA. Perforating GA occurs on the dorsal hands and presents as (umbilicated) papules, and seems consistent with this patient’s clinical presentation. Histologically, transepidermal elimination of collagen is typically seen in perforating GA.1
Histology in this patient’s biopsy revealed a granulomatous dermatitis consistent with granuloma annulare. A palisaded arrangement of histiocytic cells surrounding altered collagen with increased dermal mucin was seen. There was associated perivascular mononuclear inflammatory infiltrates. The overlying epidermis was unremarkable.
Granuloma annulare often spontaneously resolves without sequelae. In some cases, atrophy may result. Lesions may also recur. Localized GA is often treated with high-potency topical corticosteroids or intralesional corticosteroids. For generalized GA, topical or intralesional corticosteroids may be used for select lesions. Topical calcineurin inhibitors, light therapy, cryotherapy, imiquimod, hydroxychloroquine, isotretinoin, and dapsone have also been reported in the literature as possible treatments.
This case and photo were provided by Dr. Berke, of Three Rivers Dermatology, Pittsburgh, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1 Alves J, Barreiros H, Bartolo E. Healthcare (Basel). 2014 Sep 4;2(3):338-45.
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
3. “Andrews’ Diseases of the Skin,” 13th ed. James W et al. Philadelphia: Saunders Elsevier, 2006.
FDA authorizes boosters for Moderna, J&J, allows mix-and-match
in people who are eligible to get them.
The move to amend the Emergency Use Authorization for these vaccines gives the vaccine experts on the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices latitude to recommend a mix-and-match strategy if they feel the science supports it.
The committee convenes Oct. 21 for a day-long meeting to make its recommendations for additional doses.
People who’ve previously received two doses of the Moderna mRNA vaccine, which is now called Spikevax, are eligible for a third dose of any COVID-19 vaccine if they are 6 months past their second dose and are:
- 65 years of age or older
- 18 to 64 years of age, but at high risk for severe COVID-19 because of an underlying health condition
- 18 to 64 years of age and at high risk for exposure to the SARS-CoV-2 virus because they live in a group setting, such as a prison or care home, or work in a risky occupation, such as healthcare
People who’ve previously received a dose of the Johnson & Johnson vaccine are eligible for a second dose of any COVID-19 vaccine if they are over the age of 18 and at least 2 months past their vaccination.
“Today’s actions demonstrate our commitment to public health in proactively fighting against the COVID-19 pandemic,” said Acting FDA Commissioner Janet Woodcock, MD, in a news release. “As the pandemic continues to impact the country, science has shown that vaccination continues to be the safest and most effective way to prevent COVID-19, including the most serious consequences of the disease, such as hospitalization and death.
“The available data suggest waning immunity in some populations who are fully vaccinated. The availability of these authorized boosters is important for continued protection against COVID-19 disease.”
A version of this article was first published on Medscape.com.
in people who are eligible to get them.
The move to amend the Emergency Use Authorization for these vaccines gives the vaccine experts on the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices latitude to recommend a mix-and-match strategy if they feel the science supports it.
The committee convenes Oct. 21 for a day-long meeting to make its recommendations for additional doses.
People who’ve previously received two doses of the Moderna mRNA vaccine, which is now called Spikevax, are eligible for a third dose of any COVID-19 vaccine if they are 6 months past their second dose and are:
- 65 years of age or older
- 18 to 64 years of age, but at high risk for severe COVID-19 because of an underlying health condition
- 18 to 64 years of age and at high risk for exposure to the SARS-CoV-2 virus because they live in a group setting, such as a prison or care home, or work in a risky occupation, such as healthcare
People who’ve previously received a dose of the Johnson & Johnson vaccine are eligible for a second dose of any COVID-19 vaccine if they are over the age of 18 and at least 2 months past their vaccination.
“Today’s actions demonstrate our commitment to public health in proactively fighting against the COVID-19 pandemic,” said Acting FDA Commissioner Janet Woodcock, MD, in a news release. “As the pandemic continues to impact the country, science has shown that vaccination continues to be the safest and most effective way to prevent COVID-19, including the most serious consequences of the disease, such as hospitalization and death.
“The available data suggest waning immunity in some populations who are fully vaccinated. The availability of these authorized boosters is important for continued protection against COVID-19 disease.”
A version of this article was first published on Medscape.com.
in people who are eligible to get them.
The move to amend the Emergency Use Authorization for these vaccines gives the vaccine experts on the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices latitude to recommend a mix-and-match strategy if they feel the science supports it.
The committee convenes Oct. 21 for a day-long meeting to make its recommendations for additional doses.
People who’ve previously received two doses of the Moderna mRNA vaccine, which is now called Spikevax, are eligible for a third dose of any COVID-19 vaccine if they are 6 months past their second dose and are:
- 65 years of age or older
- 18 to 64 years of age, but at high risk for severe COVID-19 because of an underlying health condition
- 18 to 64 years of age and at high risk for exposure to the SARS-CoV-2 virus because they live in a group setting, such as a prison or care home, or work in a risky occupation, such as healthcare
People who’ve previously received a dose of the Johnson & Johnson vaccine are eligible for a second dose of any COVID-19 vaccine if they are over the age of 18 and at least 2 months past their vaccination.
“Today’s actions demonstrate our commitment to public health in proactively fighting against the COVID-19 pandemic,” said Acting FDA Commissioner Janet Woodcock, MD, in a news release. “As the pandemic continues to impact the country, science has shown that vaccination continues to be the safest and most effective way to prevent COVID-19, including the most serious consequences of the disease, such as hospitalization and death.
“The available data suggest waning immunity in some populations who are fully vaccinated. The availability of these authorized boosters is important for continued protection against COVID-19 disease.”
A version of this article was first published on Medscape.com.
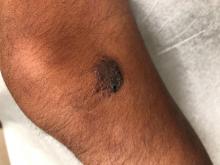
Teen boy’s knee lesion has changed
A biopsy of the lesion was performed which showed an increased number of eccrine glands and blood vessels within the dermis. Some areas showed an increase in adipocytes and smooth muscle bundles. The changes were consistent with eccrine angiomatous hamartoma (EAH).
The boy was referred to vascular laser therapy for treatment of the lesion.
EAH is a rare benign vascular growth characterized by an increased number of mature eccrine glands and blood vessels in the dermis and subcutis. The lesions are mostly present on the extremities, but cases of diffuse congenital lesions and lesions on the face and trunk have also been described. The lesions can be seen at birth or during the first years of life in about half of the cases, and the others tend to occur later in puberty and rarely in adulthood.1
Clinically, EAH lesions present as red, yellow to brown papules and plaques. Different dermoscopic patterns have been described which include the popcorn pattern that presents as yellow, confluent nodules with popcornlike shapes over a background of erythema, and linear arborizing vessels. The spitzoid pattern are brown globules on a background of erythema and pseudoreticular pigmentation around the globules. The verrucous hemangiomalike pattern has a bluish-white hue, reddish-blue or bluish lacunae, as seen in our patient.2-4
Most of the lesions are asymptomatic, but in some patients, they can be associated with pain, hyperhidrosis, and sometimes bleeding. Hyperhidrosis has been reported early in the presentation or during puberty or pregnancy. Our patient had started on amphetamines when hyperhidrosis occurred. Hyperhidrosis is a knowns side effect of this type of medication and may have had a role in the increased sweating noted on the hamartoma.
EAH can clinically look like verrucous hemangiomas, angiokeratomas, and vascular malformations, and histopathology may be needed to differentiate between them. Eccrine nevi and EAH can be similar. Hyperhidrosis is an early and predominant component of eccrine nevi, compared with one-third of EAH.
The exact etiology of this lesion is not known. It is thought to be caused by an abnormal differentiation of the epithelium, adnexal structure, and the mesenchyme during organogenesis.3 No other associated conditions have been described with EAH.
EAH are benign lesions that rarely require treatment. If the lesions are symptomatic or because of cosmetic reasons, they can be removed surgically. There are some reports of successful treatment with pulse dual-wavelength sequential 595- and 1064-nm lasers.5 Botulinum toxin has also been used in cases of symptomatic hyperhidrosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no conflicts. Email her at [email protected].
References
1. Smith SD et al. Pediatr Dermatol. 2019 Nov;36(6):909-12.
2. Patterson AT et al. Am J Dermatopathol. 2016;38:413-7.
3. Garcıa-Garcıa SC et al. JAAD Case Rep. 2018;4(2):165-7.
4. Awatef Kelati et al. JAAD Case Rep. 2018;4(8)835-6.
5. Felgueiras J et al. Dermatol Surg. 2015 Mar;41(3):428-30.
A biopsy of the lesion was performed which showed an increased number of eccrine glands and blood vessels within the dermis. Some areas showed an increase in adipocytes and smooth muscle bundles. The changes were consistent with eccrine angiomatous hamartoma (EAH).
The boy was referred to vascular laser therapy for treatment of the lesion.
EAH is a rare benign vascular growth characterized by an increased number of mature eccrine glands and blood vessels in the dermis and subcutis. The lesions are mostly present on the extremities, but cases of diffuse congenital lesions and lesions on the face and trunk have also been described. The lesions can be seen at birth or during the first years of life in about half of the cases, and the others tend to occur later in puberty and rarely in adulthood.1
Clinically, EAH lesions present as red, yellow to brown papules and plaques. Different dermoscopic patterns have been described which include the popcorn pattern that presents as yellow, confluent nodules with popcornlike shapes over a background of erythema, and linear arborizing vessels. The spitzoid pattern are brown globules on a background of erythema and pseudoreticular pigmentation around the globules. The verrucous hemangiomalike pattern has a bluish-white hue, reddish-blue or bluish lacunae, as seen in our patient.2-4
Most of the lesions are asymptomatic, but in some patients, they can be associated with pain, hyperhidrosis, and sometimes bleeding. Hyperhidrosis has been reported early in the presentation or during puberty or pregnancy. Our patient had started on amphetamines when hyperhidrosis occurred. Hyperhidrosis is a knowns side effect of this type of medication and may have had a role in the increased sweating noted on the hamartoma.
EAH can clinically look like verrucous hemangiomas, angiokeratomas, and vascular malformations, and histopathology may be needed to differentiate between them. Eccrine nevi and EAH can be similar. Hyperhidrosis is an early and predominant component of eccrine nevi, compared with one-third of EAH.
The exact etiology of this lesion is not known. It is thought to be caused by an abnormal differentiation of the epithelium, adnexal structure, and the mesenchyme during organogenesis.3 No other associated conditions have been described with EAH.
EAH are benign lesions that rarely require treatment. If the lesions are symptomatic or because of cosmetic reasons, they can be removed surgically. There are some reports of successful treatment with pulse dual-wavelength sequential 595- and 1064-nm lasers.5 Botulinum toxin has also been used in cases of symptomatic hyperhidrosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no conflicts. Email her at [email protected].
References
1. Smith SD et al. Pediatr Dermatol. 2019 Nov;36(6):909-12.
2. Patterson AT et al. Am J Dermatopathol. 2016;38:413-7.
3. Garcıa-Garcıa SC et al. JAAD Case Rep. 2018;4(2):165-7.
4. Awatef Kelati et al. JAAD Case Rep. 2018;4(8)835-6.
5. Felgueiras J et al. Dermatol Surg. 2015 Mar;41(3):428-30.
A biopsy of the lesion was performed which showed an increased number of eccrine glands and blood vessels within the dermis. Some areas showed an increase in adipocytes and smooth muscle bundles. The changes were consistent with eccrine angiomatous hamartoma (EAH).
The boy was referred to vascular laser therapy for treatment of the lesion.
EAH is a rare benign vascular growth characterized by an increased number of mature eccrine glands and blood vessels in the dermis and subcutis. The lesions are mostly present on the extremities, but cases of diffuse congenital lesions and lesions on the face and trunk have also been described. The lesions can be seen at birth or during the first years of life in about half of the cases, and the others tend to occur later in puberty and rarely in adulthood.1
Clinically, EAH lesions present as red, yellow to brown papules and plaques. Different dermoscopic patterns have been described which include the popcorn pattern that presents as yellow, confluent nodules with popcornlike shapes over a background of erythema, and linear arborizing vessels. The spitzoid pattern are brown globules on a background of erythema and pseudoreticular pigmentation around the globules. The verrucous hemangiomalike pattern has a bluish-white hue, reddish-blue or bluish lacunae, as seen in our patient.2-4
Most of the lesions are asymptomatic, but in some patients, they can be associated with pain, hyperhidrosis, and sometimes bleeding. Hyperhidrosis has been reported early in the presentation or during puberty or pregnancy. Our patient had started on amphetamines when hyperhidrosis occurred. Hyperhidrosis is a knowns side effect of this type of medication and may have had a role in the increased sweating noted on the hamartoma.
EAH can clinically look like verrucous hemangiomas, angiokeratomas, and vascular malformations, and histopathology may be needed to differentiate between them. Eccrine nevi and EAH can be similar. Hyperhidrosis is an early and predominant component of eccrine nevi, compared with one-third of EAH.
The exact etiology of this lesion is not known. It is thought to be caused by an abnormal differentiation of the epithelium, adnexal structure, and the mesenchyme during organogenesis.3 No other associated conditions have been described with EAH.
EAH are benign lesions that rarely require treatment. If the lesions are symptomatic or because of cosmetic reasons, they can be removed surgically. There are some reports of successful treatment with pulse dual-wavelength sequential 595- and 1064-nm lasers.5 Botulinum toxin has also been used in cases of symptomatic hyperhidrosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no conflicts. Email her at [email protected].
References
1. Smith SD et al. Pediatr Dermatol. 2019 Nov;36(6):909-12.
2. Patterson AT et al. Am J Dermatopathol. 2016;38:413-7.
3. Garcıa-Garcıa SC et al. JAAD Case Rep. 2018;4(2):165-7.
4. Awatef Kelati et al. JAAD Case Rep. 2018;4(8)835-6.
5. Felgueiras J et al. Dermatol Surg. 2015 Mar;41(3):428-30.
A 14-year-old male was referred to our pediatric dermatology clinic for evaluation of a lesion on the left knee that appeared at 1 year of age. The lesion has been growing with him and was not symptomatic until 6 months prior to the consultation, when it started bleeding and feeling wet.
He has a history of attention-deficit/hyperactivity disorder managed with dextroamphetamine-amphetamine. The changes noted on the knee lesion seem to occur at the same time that his ADHD medication was started.
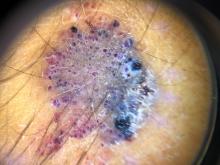
On physical exam he had a violaceous circular plaque on the left knee.
On dermoscopy the lesion showed multiple dilated red and violaceous lacunae and whitish blue hue.
Study finds plume generated during laser tattoo removal generally viewed as safe
Results of a new .
While tattoo removal plume has not been previously studied, an analysis from 2016 found that laser hair removal plume contains toxic compounds, including carcinogens and environmental toxins, underscoring the importance of using smoke evacuators, good ventilation, and respiratory protection. “Ultrafine particles can become lodged in human alveoli in the lungs,” the study’s senior author, Mathew M. Avram, MD, JD, said during a virtual course on laser and aesthetic skin therapy. “This travels over distances, so it is potentially affecting people in your waiting room and others in areas within the clinic.”
For the study of laser tattoo removal plume, Yakir S. Levin, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston; Dr. Avram, director of laser, cosmetics, and dermatologic surgery at MGH; and coinvestigators from NIOSH, conducted air sampling to determine the gaseous, particulate, and microbiological content of laser tattoo removal plume. They performed the study in ex vivo pig skin and in humans undergoing routine laser tattoo removal, and measured ultrafine particulate concentrations, metals, volatile organic compounds, and airborne bacteria.
For the swine portion of the study, they found that levels of metals including aluminum, copper, manganese, phosphorus, potassium, titanium, and zirconium were all below occupational exposure limits. All organic compounds including acetone and benzene were also below occupational exposure limits. “This is different than what we found in the study of laser plume generated during hair removal,” Dr. Avram said. “In laser hair removal, these were all elevated to a concerning extent.”
For the human part of the study, particle concentrations for ultrafine particulates were higher in the dermatologist’s breathing zone and near the tattoo removal site than in the rest of the treatment room or outside of the room. Concentrations were 30 times lower for human skin than for pig skin. “We’re not sure why, but there were higher levels of ultrafine particulates right around the area we treated,” Dr. Avram said. “Still, they were all below exposure limits that would be concerning in terms of NIOSH. So, although they were elevated, they were still considered safe. That was the case for organic compounds as well.”
He pointed out that the study, which was supported by a grant from the American Society for Dermatologic Surgery (ASDS), did not include an analysis of viral particles generated during later tattoo removal “so there is a question about that,” and it is something worth studying, he said.
Dr. Avram, the current president of ASDS, noted that 17% of the estimated 40 million-plus Americans with tattoos have “tattoo regret,” and many turn to dermatologic surgeons for removal, which requires multiple treatments, and is painful and expensive.
Picosecond lasers
“One thing that’s changed in the past several years is the development of picosecond lasers, which produce extraordinarily high energy for an extraordinarily short period of time,” he said at the meeting, named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. The desired endpoint is dermal whitening with cavitation and rupture. “You don’t want to see splatter with the epidermis flying off,” he said.
Several devices are commercially available with wavelengths of 532, 680, 755, 785, and 1064 nm, and pulse durations ranging from 300 to 750 picoseconds. Nd:Yag lasers target red and black ink, while alexandrite and ruby wavelengths target green and blue ink.
“After the treatment, we use simple Vaseline on top of the tattoo and a nonadherent Telfa dressing with paper tape over it,” Dr. Avram said. For patients with skin of color, he said, “occasionally I will add a steroid. Inflammation and redness can lead to hyperpigmentation. The steroid decreases some of that inflammation and therefore decreases the risk of hyperpigmentation.”
In his clinical experience, picosecond lasers are more effective at tattoo removal than Q-switched nanosecond lasers overall. With a picosecond laser, “you get some nonselective targeting of other pigments such as yellow to improve, even though you really don’t have the correct wavelength. I also think they are more effective for faded tattoos than the Q-switched nanosecond lasers, but they are significantly more expensive, so you need to think about that, and to what extent you are doing tattoo removal. In any event, it’s a multi-treatment process. You do it for multiple weeks between treatments and it takes time and patience. During the consultation, it is crucial to let patients know that.”
In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine, and colleagues first described the R20 method for tattoo removal, which consists of four consecutive treatment passes with a Q-switched alexandrite laser separated by 20 minutes. “On the first treatment pass, there was an immediate whitening reaction “with little or no whitening on subsequent passes,” said Dr. Avram, who was not involved with the study. “Three months later, treatment with the R20 method was much more effective than conventional single-pass laser treatment. Light microscopy showed greater dispersion of the ink with the R20 method.” A follow-up study conducted at the Wellman Center did not completely support these findings, but a subsequent study led by Suzanne L. Kilmer, MD, was more supportive.
This concept has led to new treatment paradigms for tattoo removal, including the Food and Drug Administration–cleared perfluorodecalin patch, a transparent PFD-infused silicone patch that helps reduce scatter and improves efficacy. “It also allows for performing of repeat laser treatments at the same visit without waiting 20 minutes as you would with the R20 method,” Dr. Avram said. In a pilot study, 11 of the 17 patients showed more rapid clearance with the PFD patch than the control side versus one pass without the PFD patch. “It’s important to note that they used only one wavelength, and some of the tattoos weren’t appropriate for that wavelength, so 11 out of 17 is actually better than it might seem,” he said.
Ablative fractional resurfacing can play a role with tattoo removal, but Dr. Avram typically limits this option to recalcitrant tattoos. “Remember: You’re creating a zone of ablation with a cuff of coagulation, so you’re going to remove some of the tattoo just by creating those areas of clearance and vaporization,” he said. “You can do that in combination with the Q-switched or picosecond laser, which has better efficacy. The best way to do this is to start with the pigment laser – the picosecond or nanosecond laser – and then do the ablative fractional resurfacing afterward. You should never use IPL or laser hair removal lasers to remove tattoos, though. I see that occasionally. You’re going to burn your patients.”
Another approach is to use an Nd:Yag picosecond laser followed by microneedling. “What we’re trying to do here is get an egress of the tattoo pigments,” he explained. “We’re trying to mobilize the ink, get it out of the skin, and get it out of the macrophages to get improvement.”
In 2019, Soliton’s Rapid Acoustic Pulse (RAP) device was cleared by the FDA for tattoo removal. The device is indicated as an accessory to the 1064-nm Q-switched laser for black ink tattoo removal on the arms, legs, and torso in Fitzpatrick skin type I-III individuals. “It’s an application for 1 minute and that allows for additional laser passes,” Dr. Avram said. “You do the laser treatment, you do the acoustic shock wave device, and you do this as multiple passes. This is getting back to the R20 method, the idea that you are going to treat repeatedly. The rapid acoustic pulses result in dispersion and destruction of dermal vacuoles, which enables multiple laser passes in a single treatment session. If you can see the ink, you can ablate the ink.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, and Galderma. He is a member of the scientific advisory board for Allergan and Soliton, is an investigator for Endo, and holds stock options in La Jolla NanoMedical Inc.
Results of a new .
While tattoo removal plume has not been previously studied, an analysis from 2016 found that laser hair removal plume contains toxic compounds, including carcinogens and environmental toxins, underscoring the importance of using smoke evacuators, good ventilation, and respiratory protection. “Ultrafine particles can become lodged in human alveoli in the lungs,” the study’s senior author, Mathew M. Avram, MD, JD, said during a virtual course on laser and aesthetic skin therapy. “This travels over distances, so it is potentially affecting people in your waiting room and others in areas within the clinic.”
For the study of laser tattoo removal plume, Yakir S. Levin, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston; Dr. Avram, director of laser, cosmetics, and dermatologic surgery at MGH; and coinvestigators from NIOSH, conducted air sampling to determine the gaseous, particulate, and microbiological content of laser tattoo removal plume. They performed the study in ex vivo pig skin and in humans undergoing routine laser tattoo removal, and measured ultrafine particulate concentrations, metals, volatile organic compounds, and airborne bacteria.
For the swine portion of the study, they found that levels of metals including aluminum, copper, manganese, phosphorus, potassium, titanium, and zirconium were all below occupational exposure limits. All organic compounds including acetone and benzene were also below occupational exposure limits. “This is different than what we found in the study of laser plume generated during hair removal,” Dr. Avram said. “In laser hair removal, these were all elevated to a concerning extent.”
For the human part of the study, particle concentrations for ultrafine particulates were higher in the dermatologist’s breathing zone and near the tattoo removal site than in the rest of the treatment room or outside of the room. Concentrations were 30 times lower for human skin than for pig skin. “We’re not sure why, but there were higher levels of ultrafine particulates right around the area we treated,” Dr. Avram said. “Still, they were all below exposure limits that would be concerning in terms of NIOSH. So, although they were elevated, they were still considered safe. That was the case for organic compounds as well.”
He pointed out that the study, which was supported by a grant from the American Society for Dermatologic Surgery (ASDS), did not include an analysis of viral particles generated during later tattoo removal “so there is a question about that,” and it is something worth studying, he said.
Dr. Avram, the current president of ASDS, noted that 17% of the estimated 40 million-plus Americans with tattoos have “tattoo regret,” and many turn to dermatologic surgeons for removal, which requires multiple treatments, and is painful and expensive.
Picosecond lasers
“One thing that’s changed in the past several years is the development of picosecond lasers, which produce extraordinarily high energy for an extraordinarily short period of time,” he said at the meeting, named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. The desired endpoint is dermal whitening with cavitation and rupture. “You don’t want to see splatter with the epidermis flying off,” he said.
Several devices are commercially available with wavelengths of 532, 680, 755, 785, and 1064 nm, and pulse durations ranging from 300 to 750 picoseconds. Nd:Yag lasers target red and black ink, while alexandrite and ruby wavelengths target green and blue ink.
“After the treatment, we use simple Vaseline on top of the tattoo and a nonadherent Telfa dressing with paper tape over it,” Dr. Avram said. For patients with skin of color, he said, “occasionally I will add a steroid. Inflammation and redness can lead to hyperpigmentation. The steroid decreases some of that inflammation and therefore decreases the risk of hyperpigmentation.”
In his clinical experience, picosecond lasers are more effective at tattoo removal than Q-switched nanosecond lasers overall. With a picosecond laser, “you get some nonselective targeting of other pigments such as yellow to improve, even though you really don’t have the correct wavelength. I also think they are more effective for faded tattoos than the Q-switched nanosecond lasers, but they are significantly more expensive, so you need to think about that, and to what extent you are doing tattoo removal. In any event, it’s a multi-treatment process. You do it for multiple weeks between treatments and it takes time and patience. During the consultation, it is crucial to let patients know that.”
In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine, and colleagues first described the R20 method for tattoo removal, which consists of four consecutive treatment passes with a Q-switched alexandrite laser separated by 20 minutes. “On the first treatment pass, there was an immediate whitening reaction “with little or no whitening on subsequent passes,” said Dr. Avram, who was not involved with the study. “Three months later, treatment with the R20 method was much more effective than conventional single-pass laser treatment. Light microscopy showed greater dispersion of the ink with the R20 method.” A follow-up study conducted at the Wellman Center did not completely support these findings, but a subsequent study led by Suzanne L. Kilmer, MD, was more supportive.
This concept has led to new treatment paradigms for tattoo removal, including the Food and Drug Administration–cleared perfluorodecalin patch, a transparent PFD-infused silicone patch that helps reduce scatter and improves efficacy. “It also allows for performing of repeat laser treatments at the same visit without waiting 20 minutes as you would with the R20 method,” Dr. Avram said. In a pilot study, 11 of the 17 patients showed more rapid clearance with the PFD patch than the control side versus one pass without the PFD patch. “It’s important to note that they used only one wavelength, and some of the tattoos weren’t appropriate for that wavelength, so 11 out of 17 is actually better than it might seem,” he said.
Ablative fractional resurfacing can play a role with tattoo removal, but Dr. Avram typically limits this option to recalcitrant tattoos. “Remember: You’re creating a zone of ablation with a cuff of coagulation, so you’re going to remove some of the tattoo just by creating those areas of clearance and vaporization,” he said. “You can do that in combination with the Q-switched or picosecond laser, which has better efficacy. The best way to do this is to start with the pigment laser – the picosecond or nanosecond laser – and then do the ablative fractional resurfacing afterward. You should never use IPL or laser hair removal lasers to remove tattoos, though. I see that occasionally. You’re going to burn your patients.”
Another approach is to use an Nd:Yag picosecond laser followed by microneedling. “What we’re trying to do here is get an egress of the tattoo pigments,” he explained. “We’re trying to mobilize the ink, get it out of the skin, and get it out of the macrophages to get improvement.”
In 2019, Soliton’s Rapid Acoustic Pulse (RAP) device was cleared by the FDA for tattoo removal. The device is indicated as an accessory to the 1064-nm Q-switched laser for black ink tattoo removal on the arms, legs, and torso in Fitzpatrick skin type I-III individuals. “It’s an application for 1 minute and that allows for additional laser passes,” Dr. Avram said. “You do the laser treatment, you do the acoustic shock wave device, and you do this as multiple passes. This is getting back to the R20 method, the idea that you are going to treat repeatedly. The rapid acoustic pulses result in dispersion and destruction of dermal vacuoles, which enables multiple laser passes in a single treatment session. If you can see the ink, you can ablate the ink.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, and Galderma. He is a member of the scientific advisory board for Allergan and Soliton, is an investigator for Endo, and holds stock options in La Jolla NanoMedical Inc.
Results of a new .
While tattoo removal plume has not been previously studied, an analysis from 2016 found that laser hair removal plume contains toxic compounds, including carcinogens and environmental toxins, underscoring the importance of using smoke evacuators, good ventilation, and respiratory protection. “Ultrafine particles can become lodged in human alveoli in the lungs,” the study’s senior author, Mathew M. Avram, MD, JD, said during a virtual course on laser and aesthetic skin therapy. “This travels over distances, so it is potentially affecting people in your waiting room and others in areas within the clinic.”
For the study of laser tattoo removal plume, Yakir S. Levin, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston; Dr. Avram, director of laser, cosmetics, and dermatologic surgery at MGH; and coinvestigators from NIOSH, conducted air sampling to determine the gaseous, particulate, and microbiological content of laser tattoo removal plume. They performed the study in ex vivo pig skin and in humans undergoing routine laser tattoo removal, and measured ultrafine particulate concentrations, metals, volatile organic compounds, and airborne bacteria.
For the swine portion of the study, they found that levels of metals including aluminum, copper, manganese, phosphorus, potassium, titanium, and zirconium were all below occupational exposure limits. All organic compounds including acetone and benzene were also below occupational exposure limits. “This is different than what we found in the study of laser plume generated during hair removal,” Dr. Avram said. “In laser hair removal, these were all elevated to a concerning extent.”
For the human part of the study, particle concentrations for ultrafine particulates were higher in the dermatologist’s breathing zone and near the tattoo removal site than in the rest of the treatment room or outside of the room. Concentrations were 30 times lower for human skin than for pig skin. “We’re not sure why, but there were higher levels of ultrafine particulates right around the area we treated,” Dr. Avram said. “Still, they were all below exposure limits that would be concerning in terms of NIOSH. So, although they were elevated, they were still considered safe. That was the case for organic compounds as well.”
He pointed out that the study, which was supported by a grant from the American Society for Dermatologic Surgery (ASDS), did not include an analysis of viral particles generated during later tattoo removal “so there is a question about that,” and it is something worth studying, he said.
Dr. Avram, the current president of ASDS, noted that 17% of the estimated 40 million-plus Americans with tattoos have “tattoo regret,” and many turn to dermatologic surgeons for removal, which requires multiple treatments, and is painful and expensive.
Picosecond lasers
“One thing that’s changed in the past several years is the development of picosecond lasers, which produce extraordinarily high energy for an extraordinarily short period of time,” he said at the meeting, named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. The desired endpoint is dermal whitening with cavitation and rupture. “You don’t want to see splatter with the epidermis flying off,” he said.
Several devices are commercially available with wavelengths of 532, 680, 755, 785, and 1064 nm, and pulse durations ranging from 300 to 750 picoseconds. Nd:Yag lasers target red and black ink, while alexandrite and ruby wavelengths target green and blue ink.
“After the treatment, we use simple Vaseline on top of the tattoo and a nonadherent Telfa dressing with paper tape over it,” Dr. Avram said. For patients with skin of color, he said, “occasionally I will add a steroid. Inflammation and redness can lead to hyperpigmentation. The steroid decreases some of that inflammation and therefore decreases the risk of hyperpigmentation.”
In his clinical experience, picosecond lasers are more effective at tattoo removal than Q-switched nanosecond lasers overall. With a picosecond laser, “you get some nonselective targeting of other pigments such as yellow to improve, even though you really don’t have the correct wavelength. I also think they are more effective for faded tattoos than the Q-switched nanosecond lasers, but they are significantly more expensive, so you need to think about that, and to what extent you are doing tattoo removal. In any event, it’s a multi-treatment process. You do it for multiple weeks between treatments and it takes time and patience. During the consultation, it is crucial to let patients know that.”
In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine, and colleagues first described the R20 method for tattoo removal, which consists of four consecutive treatment passes with a Q-switched alexandrite laser separated by 20 minutes. “On the first treatment pass, there was an immediate whitening reaction “with little or no whitening on subsequent passes,” said Dr. Avram, who was not involved with the study. “Three months later, treatment with the R20 method was much more effective than conventional single-pass laser treatment. Light microscopy showed greater dispersion of the ink with the R20 method.” A follow-up study conducted at the Wellman Center did not completely support these findings, but a subsequent study led by Suzanne L. Kilmer, MD, was more supportive.
This concept has led to new treatment paradigms for tattoo removal, including the Food and Drug Administration–cleared perfluorodecalin patch, a transparent PFD-infused silicone patch that helps reduce scatter and improves efficacy. “It also allows for performing of repeat laser treatments at the same visit without waiting 20 minutes as you would with the R20 method,” Dr. Avram said. In a pilot study, 11 of the 17 patients showed more rapid clearance with the PFD patch than the control side versus one pass without the PFD patch. “It’s important to note that they used only one wavelength, and some of the tattoos weren’t appropriate for that wavelength, so 11 out of 17 is actually better than it might seem,” he said.
Ablative fractional resurfacing can play a role with tattoo removal, but Dr. Avram typically limits this option to recalcitrant tattoos. “Remember: You’re creating a zone of ablation with a cuff of coagulation, so you’re going to remove some of the tattoo just by creating those areas of clearance and vaporization,” he said. “You can do that in combination with the Q-switched or picosecond laser, which has better efficacy. The best way to do this is to start with the pigment laser – the picosecond or nanosecond laser – and then do the ablative fractional resurfacing afterward. You should never use IPL or laser hair removal lasers to remove tattoos, though. I see that occasionally. You’re going to burn your patients.”
Another approach is to use an Nd:Yag picosecond laser followed by microneedling. “What we’re trying to do here is get an egress of the tattoo pigments,” he explained. “We’re trying to mobilize the ink, get it out of the skin, and get it out of the macrophages to get improvement.”
In 2019, Soliton’s Rapid Acoustic Pulse (RAP) device was cleared by the FDA for tattoo removal. The device is indicated as an accessory to the 1064-nm Q-switched laser for black ink tattoo removal on the arms, legs, and torso in Fitzpatrick skin type I-III individuals. “It’s an application for 1 minute and that allows for additional laser passes,” Dr. Avram said. “You do the laser treatment, you do the acoustic shock wave device, and you do this as multiple passes. This is getting back to the R20 method, the idea that you are going to treat repeatedly. The rapid acoustic pulses result in dispersion and destruction of dermal vacuoles, which enables multiple laser passes in a single treatment session. If you can see the ink, you can ablate the ink.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, and Galderma. He is a member of the scientific advisory board for Allergan and Soliton, is an investigator for Endo, and holds stock options in La Jolla NanoMedical Inc.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Timeless stories
Let me tell you a story. In 5 billion years the sun will run out of hydrogen, the fuel it is currently burning to power my solar panels amongst other things. At that time, the sun will no longer be able to keep its core contracted and will expand into a fiery, red giant, engulfing earth and obliterating any sign that we ever existed. No buildings. No blog posts. No mausoleums. No stories. Nothing of us will remain.
Well, here for a moment anyway, I’ve gotten you to think about something other than COVID. You’re welcome.
Fascinatingly, the image in your mind’s eye right now of a barren scorched landscape was put there by me. Simply by placing a few words together I have caused new thoughts in your head. You might even share this story with someone else – I would have actually changed your behavior through the power of language. This miraculous phenomenon seems to be unique to us humans; we are the only ones who can create whole worlds in another individual’s head just by making a few sounds. We in medicine have the privilege of experiencing this miracle every day.
Last week, a 97-year-old pale, frail, white man saw me for a basal cell carcinoma on his cheek. While performing a simple electrodesiccation and curettage, I asked if he remembers getting a lot of sunburns when he was young. He certainly remembered one. On a blustery sunny day, he fell asleep for hours on the deck of the USS West Virginia while in the Philippines. As a radio man, he was exhausted from days of conflict and he recalled how warm breezes lulled him asleep. He was so sunburned that for days he forgot how afraid he was of the Japanese.
After listening to his story, I had an image in my mind of palm trees swaying in the tropical winds while hundreds of hulking gray castles sat hidden in the vast surrounding oceans awaiting one of the greatest naval conflicts in history. I got to hear it from surely one of the last remaining people in existence to be able to tell that story. Listening to a patient’s tales is one of the benefits of being a physician. Not only do they help bond us with our patients, but also help lessen our burden of having to make diagnosis after diagnosis and write note after note for hours on end. Somehow performing yet another biopsy that day is made just a bit easier if I’m also learning about what it was like at the Battle of Leyte Gulf.
Encouraging patients to talk more can be risky. No physician, not even allergists, can afford to be waylaid by a retiree with nothing else to do today. But meaningful encounters can not only be a vaccine against burnout, they also lead to better patient adherence and satisfaction. Sometimes, there is simply not time. But often there is a little window during a procedure or when you’re reasonably caught up and don’t expect delays ahead. And like every story, they literally transform us, the listener. In a true physical sense, their stories live on in me, and now that I’ve shared this one in writing, also with you for perpetuity. That is at least for the next 5 billion years when it, too, will be swallowed by the sun, leaving only a crispy, smoking rock where we once existed.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Let me tell you a story. In 5 billion years the sun will run out of hydrogen, the fuel it is currently burning to power my solar panels amongst other things. At that time, the sun will no longer be able to keep its core contracted and will expand into a fiery, red giant, engulfing earth and obliterating any sign that we ever existed. No buildings. No blog posts. No mausoleums. No stories. Nothing of us will remain.
Well, here for a moment anyway, I’ve gotten you to think about something other than COVID. You’re welcome.
Fascinatingly, the image in your mind’s eye right now of a barren scorched landscape was put there by me. Simply by placing a few words together I have caused new thoughts in your head. You might even share this story with someone else – I would have actually changed your behavior through the power of language. This miraculous phenomenon seems to be unique to us humans; we are the only ones who can create whole worlds in another individual’s head just by making a few sounds. We in medicine have the privilege of experiencing this miracle every day.
Last week, a 97-year-old pale, frail, white man saw me for a basal cell carcinoma on his cheek. While performing a simple electrodesiccation and curettage, I asked if he remembers getting a lot of sunburns when he was young. He certainly remembered one. On a blustery sunny day, he fell asleep for hours on the deck of the USS West Virginia while in the Philippines. As a radio man, he was exhausted from days of conflict and he recalled how warm breezes lulled him asleep. He was so sunburned that for days he forgot how afraid he was of the Japanese.
After listening to his story, I had an image in my mind of palm trees swaying in the tropical winds while hundreds of hulking gray castles sat hidden in the vast surrounding oceans awaiting one of the greatest naval conflicts in history. I got to hear it from surely one of the last remaining people in existence to be able to tell that story. Listening to a patient’s tales is one of the benefits of being a physician. Not only do they help bond us with our patients, but also help lessen our burden of having to make diagnosis after diagnosis and write note after note for hours on end. Somehow performing yet another biopsy that day is made just a bit easier if I’m also learning about what it was like at the Battle of Leyte Gulf.
Encouraging patients to talk more can be risky. No physician, not even allergists, can afford to be waylaid by a retiree with nothing else to do today. But meaningful encounters can not only be a vaccine against burnout, they also lead to better patient adherence and satisfaction. Sometimes, there is simply not time. But often there is a little window during a procedure or when you’re reasonably caught up and don’t expect delays ahead. And like every story, they literally transform us, the listener. In a true physical sense, their stories live on in me, and now that I’ve shared this one in writing, also with you for perpetuity. That is at least for the next 5 billion years when it, too, will be swallowed by the sun, leaving only a crispy, smoking rock where we once existed.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Let me tell you a story. In 5 billion years the sun will run out of hydrogen, the fuel it is currently burning to power my solar panels amongst other things. At that time, the sun will no longer be able to keep its core contracted and will expand into a fiery, red giant, engulfing earth and obliterating any sign that we ever existed. No buildings. No blog posts. No mausoleums. No stories. Nothing of us will remain.
Well, here for a moment anyway, I’ve gotten you to think about something other than COVID. You’re welcome.
Fascinatingly, the image in your mind’s eye right now of a barren scorched landscape was put there by me. Simply by placing a few words together I have caused new thoughts in your head. You might even share this story with someone else – I would have actually changed your behavior through the power of language. This miraculous phenomenon seems to be unique to us humans; we are the only ones who can create whole worlds in another individual’s head just by making a few sounds. We in medicine have the privilege of experiencing this miracle every day.
Last week, a 97-year-old pale, frail, white man saw me for a basal cell carcinoma on his cheek. While performing a simple electrodesiccation and curettage, I asked if he remembers getting a lot of sunburns when he was young. He certainly remembered one. On a blustery sunny day, he fell asleep for hours on the deck of the USS West Virginia while in the Philippines. As a radio man, he was exhausted from days of conflict and he recalled how warm breezes lulled him asleep. He was so sunburned that for days he forgot how afraid he was of the Japanese.
After listening to his story, I had an image in my mind of palm trees swaying in the tropical winds while hundreds of hulking gray castles sat hidden in the vast surrounding oceans awaiting one of the greatest naval conflicts in history. I got to hear it from surely one of the last remaining people in existence to be able to tell that story. Listening to a patient’s tales is one of the benefits of being a physician. Not only do they help bond us with our patients, but also help lessen our burden of having to make diagnosis after diagnosis and write note after note for hours on end. Somehow performing yet another biopsy that day is made just a bit easier if I’m also learning about what it was like at the Battle of Leyte Gulf.
Encouraging patients to talk more can be risky. No physician, not even allergists, can afford to be waylaid by a retiree with nothing else to do today. But meaningful encounters can not only be a vaccine against burnout, they also lead to better patient adherence and satisfaction. Sometimes, there is simply not time. But often there is a little window during a procedure or when you’re reasonably caught up and don’t expect delays ahead. And like every story, they literally transform us, the listener. In a true physical sense, their stories live on in me, and now that I’ve shared this one in writing, also with you for perpetuity. That is at least for the next 5 billion years when it, too, will be swallowed by the sun, leaving only a crispy, smoking rock where we once existed.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
‘Multimorbidity’ more commonly seen in people with lupus
People with systemic lupus erythematosus (SLE) have a threefold greater likelihood of having up to five or more comorbidities in comparison with people in the general population, according to the results of two separate U.S. population-based studies.
The higher rate of comorbidities seen included many of those commonly reported before, such as cardiovascular and renal disease, but also some that may be less frequently associated with SLE, notably chronic obstructive pulmonary disease (COPD) and cardiac arrhythmias.
“In the past, the characterization of SLE comorbidities has relied on individual comorbidity assessment,” Alí Duarte García, MD, said at the 14th International Congress on Systemic Lupus Erythematosus, held together will the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“However, a patient-centric approach where a patient as a whole is seen and how many comorbidities they accrue has not been performed.” added Duarte García, who is a rheumatologist at the Mayo Clinic in Rochester, Minn.
Multiple conditions “overrepresented” in SLE patients
Dr. Duarte García reported the findings of one of the studies, both of which used data from the Rochester Epidemiology Project, a record-linkage system that collates clinical and hospital data from individuals who live in 19 counties in southeast Minnesota and eight counties in western Wisconsin; these patients have agreed to share their medical records for research.
The study population included 479 individuals diagnosed with SLE according to joint 2019 European Alliance of Associations for Rheumatology and American College of Rheumatology criteria. These were matched by age, sex, race, and county to 479 individuals without SLE.
The mean age of the study population was 53 years, 82% were women, and 86% were White.
“We defined multimorbidity as those patients who have two or more comorbidities and substantial multimorbidity as those patients who have five or more comorbidities,” Dr. Duarte García explained.
A previously published list of 44 categories of comorbidities was used to classify the multimorbidity seen, and 27 of these were “overrepresented” in patients with SLE.
Patients with SLE averaged 5.3 comorbidities, whereas control study subjects had 2.9. Comparing SLE with non-SLE individuals, the odds ratio for having two or more comorbid conditions was 2.96, and for five or more comorbidities it was 3.06.
The highest odds ratio comparing SLE with non-SLE individuals was seen for pulmonary disorders (39.0).
Dr. Duarte García highlighted four comorbidities that occurred in SLE patients that were perhaps more unusual: congestive heart failure (OR, 13.3), valvular heart disease (OR, 4.2), cardiac arrhythmias (OR, 2.85), and COPD (OR, 2.7).
“Given the association of multimorbidity with poor outcomes, care delivery strategies to manage multimorbidity are needed in SLE,” Dr. Duarte García concluded.
Similar findings seen in cutaneous lupus
There is also an excess of comorbid conditions in people with cutaneous lupus erythematosus (CLE), Mehmet Hocaoglu, MD, said in reporting the findings of the second study.
Dr. Hocaoglu, an internal medicine resident at the University of Maryland Medical Center in Baltimore, and part of the same team of researchers as Dr. Duarte García, noted that in skin-related lupus the risk of multimorbidity was about doubled.
For this separate analysis, a total of 303 patients with cutaneous lupus had been matched to 303 controls from the general population. Odds ratios for having two or more or five or more comorbidities were a respective 2.27 and 1.65.
Among the comorbidities seen that were higher in those with cutaneous lupus than in the general population subjects were fibromyalgia, liver disease, hypertension, anemia, hypothyroidism, and COPD.
“Further research is definitely needed to identify if the driver of this multimorbidity in CLE patients is the disease itself or the treatments CLE patients are receiving or a multifactorial cause that is driving the disease association,” Dr. Hocaoglu said.
Comment and perspective
“Comorbidities that are not appropriate to the general population, compared to SLE,” seem to have been included in the overall SLE and the cutaneous lupus analyses, Raquel Faria, MD, suggested.
Dr. Faria, an internal medicine consultant at Unidade de Imunologia Clínica – Centro Hospitalar Universitário Porto (Portugal), chaired the poster discussion session in which the two studies had been presented.
She wondered if the researchers had analyzed the data while accounting for “the comorbidities that you knew are due to activity in lupus, like anemia?”
The number of patients with SLE who had pulmonary circulation disorders – 7.5% vs. 0.2% of the general population – also caught Dr. Faria’s attention.
That’s “a really huge number,” Dr. Faria pointed out, “I think it is pretty overrepresented.”
Dr. Duarte García acknowledged that they “took a very broad approach” in using a “very large comorbidity index.”
“What we were observing initially is precisely what you’re mentioning,” he responded to Dr. Faria.
“We were pulling patients who were having disease manifestation rather than a comorbidity,” Dr. Duarte-García said.
These are initial and very exploratory data, he stressed. “We have now moved on to modify the index.” Some of the changes that they have made were to incorporate the SLICC Damage Index Score and tighten up the list of ICD codes used.
No outside funding was received for either of the studies. Dr. Duarte García and Dr. Hocaoglu individually stated that they had no actual or potential conflicts of interest in relation to their presentations.
A version of this article first appeared on Medscape.com.
People with systemic lupus erythematosus (SLE) have a threefold greater likelihood of having up to five or more comorbidities in comparison with people in the general population, according to the results of two separate U.S. population-based studies.
The higher rate of comorbidities seen included many of those commonly reported before, such as cardiovascular and renal disease, but also some that may be less frequently associated with SLE, notably chronic obstructive pulmonary disease (COPD) and cardiac arrhythmias.
“In the past, the characterization of SLE comorbidities has relied on individual comorbidity assessment,” Alí Duarte García, MD, said at the 14th International Congress on Systemic Lupus Erythematosus, held together will the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“However, a patient-centric approach where a patient as a whole is seen and how many comorbidities they accrue has not been performed.” added Duarte García, who is a rheumatologist at the Mayo Clinic in Rochester, Minn.
Multiple conditions “overrepresented” in SLE patients
Dr. Duarte García reported the findings of one of the studies, both of which used data from the Rochester Epidemiology Project, a record-linkage system that collates clinical and hospital data from individuals who live in 19 counties in southeast Minnesota and eight counties in western Wisconsin; these patients have agreed to share their medical records for research.
The study population included 479 individuals diagnosed with SLE according to joint 2019 European Alliance of Associations for Rheumatology and American College of Rheumatology criteria. These were matched by age, sex, race, and county to 479 individuals without SLE.
The mean age of the study population was 53 years, 82% were women, and 86% were White.
“We defined multimorbidity as those patients who have two or more comorbidities and substantial multimorbidity as those patients who have five or more comorbidities,” Dr. Duarte García explained.
A previously published list of 44 categories of comorbidities was used to classify the multimorbidity seen, and 27 of these were “overrepresented” in patients with SLE.
Patients with SLE averaged 5.3 comorbidities, whereas control study subjects had 2.9. Comparing SLE with non-SLE individuals, the odds ratio for having two or more comorbid conditions was 2.96, and for five or more comorbidities it was 3.06.
The highest odds ratio comparing SLE with non-SLE individuals was seen for pulmonary disorders (39.0).
Dr. Duarte García highlighted four comorbidities that occurred in SLE patients that were perhaps more unusual: congestive heart failure (OR, 13.3), valvular heart disease (OR, 4.2), cardiac arrhythmias (OR, 2.85), and COPD (OR, 2.7).
“Given the association of multimorbidity with poor outcomes, care delivery strategies to manage multimorbidity are needed in SLE,” Dr. Duarte García concluded.
Similar findings seen in cutaneous lupus
There is also an excess of comorbid conditions in people with cutaneous lupus erythematosus (CLE), Mehmet Hocaoglu, MD, said in reporting the findings of the second study.
Dr. Hocaoglu, an internal medicine resident at the University of Maryland Medical Center in Baltimore, and part of the same team of researchers as Dr. Duarte García, noted that in skin-related lupus the risk of multimorbidity was about doubled.
For this separate analysis, a total of 303 patients with cutaneous lupus had been matched to 303 controls from the general population. Odds ratios for having two or more or five or more comorbidities were a respective 2.27 and 1.65.
Among the comorbidities seen that were higher in those with cutaneous lupus than in the general population subjects were fibromyalgia, liver disease, hypertension, anemia, hypothyroidism, and COPD.
“Further research is definitely needed to identify if the driver of this multimorbidity in CLE patients is the disease itself or the treatments CLE patients are receiving or a multifactorial cause that is driving the disease association,” Dr. Hocaoglu said.
Comment and perspective
“Comorbidities that are not appropriate to the general population, compared to SLE,” seem to have been included in the overall SLE and the cutaneous lupus analyses, Raquel Faria, MD, suggested.
Dr. Faria, an internal medicine consultant at Unidade de Imunologia Clínica – Centro Hospitalar Universitário Porto (Portugal), chaired the poster discussion session in which the two studies had been presented.
She wondered if the researchers had analyzed the data while accounting for “the comorbidities that you knew are due to activity in lupus, like anemia?”
The number of patients with SLE who had pulmonary circulation disorders – 7.5% vs. 0.2% of the general population – also caught Dr. Faria’s attention.
That’s “a really huge number,” Dr. Faria pointed out, “I think it is pretty overrepresented.”
Dr. Duarte García acknowledged that they “took a very broad approach” in using a “very large comorbidity index.”
“What we were observing initially is precisely what you’re mentioning,” he responded to Dr. Faria.
“We were pulling patients who were having disease manifestation rather than a comorbidity,” Dr. Duarte-García said.
These are initial and very exploratory data, he stressed. “We have now moved on to modify the index.” Some of the changes that they have made were to incorporate the SLICC Damage Index Score and tighten up the list of ICD codes used.
No outside funding was received for either of the studies. Dr. Duarte García and Dr. Hocaoglu individually stated that they had no actual or potential conflicts of interest in relation to their presentations.
A version of this article first appeared on Medscape.com.
People with systemic lupus erythematosus (SLE) have a threefold greater likelihood of having up to five or more comorbidities in comparison with people in the general population, according to the results of two separate U.S. population-based studies.
The higher rate of comorbidities seen included many of those commonly reported before, such as cardiovascular and renal disease, but also some that may be less frequently associated with SLE, notably chronic obstructive pulmonary disease (COPD) and cardiac arrhythmias.
“In the past, the characterization of SLE comorbidities has relied on individual comorbidity assessment,” Alí Duarte García, MD, said at the 14th International Congress on Systemic Lupus Erythematosus, held together will the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“However, a patient-centric approach where a patient as a whole is seen and how many comorbidities they accrue has not been performed.” added Duarte García, who is a rheumatologist at the Mayo Clinic in Rochester, Minn.
Multiple conditions “overrepresented” in SLE patients
Dr. Duarte García reported the findings of one of the studies, both of which used data from the Rochester Epidemiology Project, a record-linkage system that collates clinical and hospital data from individuals who live in 19 counties in southeast Minnesota and eight counties in western Wisconsin; these patients have agreed to share their medical records for research.
The study population included 479 individuals diagnosed with SLE according to joint 2019 European Alliance of Associations for Rheumatology and American College of Rheumatology criteria. These were matched by age, sex, race, and county to 479 individuals without SLE.
The mean age of the study population was 53 years, 82% were women, and 86% were White.
“We defined multimorbidity as those patients who have two or more comorbidities and substantial multimorbidity as those patients who have five or more comorbidities,” Dr. Duarte García explained.
A previously published list of 44 categories of comorbidities was used to classify the multimorbidity seen, and 27 of these were “overrepresented” in patients with SLE.
Patients with SLE averaged 5.3 comorbidities, whereas control study subjects had 2.9. Comparing SLE with non-SLE individuals, the odds ratio for having two or more comorbid conditions was 2.96, and for five or more comorbidities it was 3.06.
The highest odds ratio comparing SLE with non-SLE individuals was seen for pulmonary disorders (39.0).
Dr. Duarte García highlighted four comorbidities that occurred in SLE patients that were perhaps more unusual: congestive heart failure (OR, 13.3), valvular heart disease (OR, 4.2), cardiac arrhythmias (OR, 2.85), and COPD (OR, 2.7).
“Given the association of multimorbidity with poor outcomes, care delivery strategies to manage multimorbidity are needed in SLE,” Dr. Duarte García concluded.
Similar findings seen in cutaneous lupus
There is also an excess of comorbid conditions in people with cutaneous lupus erythematosus (CLE), Mehmet Hocaoglu, MD, said in reporting the findings of the second study.
Dr. Hocaoglu, an internal medicine resident at the University of Maryland Medical Center in Baltimore, and part of the same team of researchers as Dr. Duarte García, noted that in skin-related lupus the risk of multimorbidity was about doubled.
For this separate analysis, a total of 303 patients with cutaneous lupus had been matched to 303 controls from the general population. Odds ratios for having two or more or five or more comorbidities were a respective 2.27 and 1.65.
Among the comorbidities seen that were higher in those with cutaneous lupus than in the general population subjects were fibromyalgia, liver disease, hypertension, anemia, hypothyroidism, and COPD.
“Further research is definitely needed to identify if the driver of this multimorbidity in CLE patients is the disease itself or the treatments CLE patients are receiving or a multifactorial cause that is driving the disease association,” Dr. Hocaoglu said.
Comment and perspective
“Comorbidities that are not appropriate to the general population, compared to SLE,” seem to have been included in the overall SLE and the cutaneous lupus analyses, Raquel Faria, MD, suggested.
Dr. Faria, an internal medicine consultant at Unidade de Imunologia Clínica – Centro Hospitalar Universitário Porto (Portugal), chaired the poster discussion session in which the two studies had been presented.
She wondered if the researchers had analyzed the data while accounting for “the comorbidities that you knew are due to activity in lupus, like anemia?”
The number of patients with SLE who had pulmonary circulation disorders – 7.5% vs. 0.2% of the general population – also caught Dr. Faria’s attention.
That’s “a really huge number,” Dr. Faria pointed out, “I think it is pretty overrepresented.”
Dr. Duarte García acknowledged that they “took a very broad approach” in using a “very large comorbidity index.”
“What we were observing initially is precisely what you’re mentioning,” he responded to Dr. Faria.
“We were pulling patients who were having disease manifestation rather than a comorbidity,” Dr. Duarte-García said.
These are initial and very exploratory data, he stressed. “We have now moved on to modify the index.” Some of the changes that they have made were to incorporate the SLICC Damage Index Score and tighten up the list of ICD codes used.
No outside funding was received for either of the studies. Dr. Duarte García and Dr. Hocaoglu individually stated that they had no actual or potential conflicts of interest in relation to their presentations.
A version of this article first appeared on Medscape.com.
Generalized Pustular Psoriasis: An Uncommon Diagnosis Carrying an Outsize Burden of Disease
In this supplement to Dermatology News, Alan Menter, MD discusses best practices for the rare, severe, and chronic autoinflammatory disease known as Generalized Pustular Psoriasis (GPP).
Read More
In this supplement to Dermatology News, Alan Menter, MD discusses best practices for the rare, severe, and chronic autoinflammatory disease known as Generalized Pustular Psoriasis (GPP).
Read More
In this supplement to Dermatology News, Alan Menter, MD discusses best practices for the rare, severe, and chronic autoinflammatory disease known as Generalized Pustular Psoriasis (GPP).
Read More
White House unveils plan to combat endocrine-disrupting PFAS pollution
The federal government is stepping up actions to protect Americans from per- and polyfluoroalkyl substances that continue to threaten health through pollution in the air, water, and foods, according to a statement from the White House on Oct. 18.
The comprehensive plan includes efforts to prevent per- and polyfluoroalkyl substances (PFAS) from being released into the air, drinking and ground water, and the food supply chain, according to the statement. Other efforts will expand cleanup and remediation of the impact of PFAS already present in the environment.
PFAS are a category of endocrine-disrupting chemicals (EDCs) that have been used for decades in a range of consumer products including cookware, stain-resistant clothes, fast food wrappers, treatments for carpets and furniture, and firefighting foams. PFAS can be released into the air, and also into surface water, drinking water, and ground water, because of how they are disposed, according to a 2020 report from the Endocrine Society and the International Pollutants Elimination Network. The report suggested that creation of more plastic products will likely increase exposure to PFAS and other EDCs.
The Environmental Protection Agency will take the lead on the Biden administration’s PFAS reduction efforts. The agency announced a PFAS Roadmap, which outlines actions to control PFAS over the next 3 years. The Roadmap’s goals include keeping PFAS out of the environment, holding polluters accountable for their actions, investing in scientific research to learn more about the impact of PFAS on human health, and prioritizing protection for disadvantaged communities. The EPA described its approach to PFAS as three pronged (Research, Restrict, Remediate). Planned actions noted on the EPA website include publication of a national PFAS testing strategy, establishing an improved review process for new PFAS, reviewing existing PFAS, and enhancing reporting to track sources and quantities of PFAS.
White House statement noted that other agencies committed to controlling PFAS include the Department of Defense, which will conduct cleanups and assessments at DOD and National Guard locations; the Food and Drug Administration, which will to expand its food supply testing to estimate dietary exposure to PFAS; and the Department of Agriculture, which is investigating causes and impacts of PFAS in the food system, and supporting research on environmental contaminants including PFAS.
The Department of Homeland Security has conducted an inventory of PFAS use, notably the use of PFAS in firefighting foams, and established an Emerging Contaminants Working Group to remediate PFAS and other contaminants. In addition, the Department of Health & Human Services monitors the evolving science on human health and PFAS and anticipates a report by the Centers for Disease Control and Prevention on the health effects of PFAS exposure, with data from eight states.
The American Chemistry Council (ACC), a trade association for American chemistry companies, issued a statement in response to the EPA’s PFAS Strategic Roadmap in which they supported the value of science-based regulation, but emphasized that PFAS are distinct from one another, and should not be grouped together for regulation purposes.
“According to EPA, approximately 600 PFAS substances are manufactured or in use today, each with its own unique properties and uses, from cellphones to solar panels, for which alternatives are not always available,” according to the ACC statement. “EPA’s Roadmap reinforces the differences between these chemistries and that they should not all be grouped together.” The newly formed Interagency Policy Committee on PFAS will coordinate PFAS response efforts across agencies and “help develop new policy strategies to support research, remediation, and removal of PFAS in communities across the country,” according to the White House statement.
The federal government is stepping up actions to protect Americans from per- and polyfluoroalkyl substances that continue to threaten health through pollution in the air, water, and foods, according to a statement from the White House on Oct. 18.
The comprehensive plan includes efforts to prevent per- and polyfluoroalkyl substances (PFAS) from being released into the air, drinking and ground water, and the food supply chain, according to the statement. Other efforts will expand cleanup and remediation of the impact of PFAS already present in the environment.
PFAS are a category of endocrine-disrupting chemicals (EDCs) that have been used for decades in a range of consumer products including cookware, stain-resistant clothes, fast food wrappers, treatments for carpets and furniture, and firefighting foams. PFAS can be released into the air, and also into surface water, drinking water, and ground water, because of how they are disposed, according to a 2020 report from the Endocrine Society and the International Pollutants Elimination Network. The report suggested that creation of more plastic products will likely increase exposure to PFAS and other EDCs.
The Environmental Protection Agency will take the lead on the Biden administration’s PFAS reduction efforts. The agency announced a PFAS Roadmap, which outlines actions to control PFAS over the next 3 years. The Roadmap’s goals include keeping PFAS out of the environment, holding polluters accountable for their actions, investing in scientific research to learn more about the impact of PFAS on human health, and prioritizing protection for disadvantaged communities. The EPA described its approach to PFAS as three pronged (Research, Restrict, Remediate). Planned actions noted on the EPA website include publication of a national PFAS testing strategy, establishing an improved review process for new PFAS, reviewing existing PFAS, and enhancing reporting to track sources and quantities of PFAS.
White House statement noted that other agencies committed to controlling PFAS include the Department of Defense, which will conduct cleanups and assessments at DOD and National Guard locations; the Food and Drug Administration, which will to expand its food supply testing to estimate dietary exposure to PFAS; and the Department of Agriculture, which is investigating causes and impacts of PFAS in the food system, and supporting research on environmental contaminants including PFAS.
The Department of Homeland Security has conducted an inventory of PFAS use, notably the use of PFAS in firefighting foams, and established an Emerging Contaminants Working Group to remediate PFAS and other contaminants. In addition, the Department of Health & Human Services monitors the evolving science on human health and PFAS and anticipates a report by the Centers for Disease Control and Prevention on the health effects of PFAS exposure, with data from eight states.
The American Chemistry Council (ACC), a trade association for American chemistry companies, issued a statement in response to the EPA’s PFAS Strategic Roadmap in which they supported the value of science-based regulation, but emphasized that PFAS are distinct from one another, and should not be grouped together for regulation purposes.
“According to EPA, approximately 600 PFAS substances are manufactured or in use today, each with its own unique properties and uses, from cellphones to solar panels, for which alternatives are not always available,” according to the ACC statement. “EPA’s Roadmap reinforces the differences between these chemistries and that they should not all be grouped together.” The newly formed Interagency Policy Committee on PFAS will coordinate PFAS response efforts across agencies and “help develop new policy strategies to support research, remediation, and removal of PFAS in communities across the country,” according to the White House statement.
The federal government is stepping up actions to protect Americans from per- and polyfluoroalkyl substances that continue to threaten health through pollution in the air, water, and foods, according to a statement from the White House on Oct. 18.
The comprehensive plan includes efforts to prevent per- and polyfluoroalkyl substances (PFAS) from being released into the air, drinking and ground water, and the food supply chain, according to the statement. Other efforts will expand cleanup and remediation of the impact of PFAS already present in the environment.
PFAS are a category of endocrine-disrupting chemicals (EDCs) that have been used for decades in a range of consumer products including cookware, stain-resistant clothes, fast food wrappers, treatments for carpets and furniture, and firefighting foams. PFAS can be released into the air, and also into surface water, drinking water, and ground water, because of how they are disposed, according to a 2020 report from the Endocrine Society and the International Pollutants Elimination Network. The report suggested that creation of more plastic products will likely increase exposure to PFAS and other EDCs.
The Environmental Protection Agency will take the lead on the Biden administration’s PFAS reduction efforts. The agency announced a PFAS Roadmap, which outlines actions to control PFAS over the next 3 years. The Roadmap’s goals include keeping PFAS out of the environment, holding polluters accountable for their actions, investing in scientific research to learn more about the impact of PFAS on human health, and prioritizing protection for disadvantaged communities. The EPA described its approach to PFAS as three pronged (Research, Restrict, Remediate). Planned actions noted on the EPA website include publication of a national PFAS testing strategy, establishing an improved review process for new PFAS, reviewing existing PFAS, and enhancing reporting to track sources and quantities of PFAS.
White House statement noted that other agencies committed to controlling PFAS include the Department of Defense, which will conduct cleanups and assessments at DOD and National Guard locations; the Food and Drug Administration, which will to expand its food supply testing to estimate dietary exposure to PFAS; and the Department of Agriculture, which is investigating causes and impacts of PFAS in the food system, and supporting research on environmental contaminants including PFAS.
The Department of Homeland Security has conducted an inventory of PFAS use, notably the use of PFAS in firefighting foams, and established an Emerging Contaminants Working Group to remediate PFAS and other contaminants. In addition, the Department of Health & Human Services monitors the evolving science on human health and PFAS and anticipates a report by the Centers for Disease Control and Prevention on the health effects of PFAS exposure, with data from eight states.
The American Chemistry Council (ACC), a trade association for American chemistry companies, issued a statement in response to the EPA’s PFAS Strategic Roadmap in which they supported the value of science-based regulation, but emphasized that PFAS are distinct from one another, and should not be grouped together for regulation purposes.
“According to EPA, approximately 600 PFAS substances are manufactured or in use today, each with its own unique properties and uses, from cellphones to solar panels, for which alternatives are not always available,” according to the ACC statement. “EPA’s Roadmap reinforces the differences between these chemistries and that they should not all be grouped together.” The newly formed Interagency Policy Committee on PFAS will coordinate PFAS response efforts across agencies and “help develop new policy strategies to support research, remediation, and removal of PFAS in communities across the country,” according to the White House statement.
COVID-19: Can doctors refuse to see unvaccinated patients?
In June, Gerald Bock, MD, a dermatologist in central California, instituted a new office policy: He would not be seeing any more patients who remain unvaccinated against COVID-19 in his practice.
“[It is] the height of self-centered and irresponsible behavior,” he told me. “People who come in unvaccinated, when vaccination is widely available, are stating that their personal preferences are more important than their health, and are more important than any risk that they may expose their friends and family to, and also to any risk they might present to my staff and me. We have gone to considerable effort and expense to diminish any risk that visiting our office might entail. I see no reason why we should tolerate this.”
Other doctors appear to be following in his footsteps. There is no question that physicians have the right to choose their patients, just as patients are free to choose their doctors, but That is a complicated question without a clear answer. In a statement on whether physicians can decline unvaccinated patients, the American Medical Association continues to maintain that “in general” a physician may not “ethically turn a patient away based solely on the individual’s infectious disease status,” but does concede that “the decision to accept or decline a patient must balance the urgency of the individual patient’s need; the risk the patient may pose to other patients in the physician’s practice; and the need for the physician and staff, to be available to provide care in the future.”
Medical ethics experts have offered varying opinions. Daniel Wikler, PhD, professor of ethics and population health at the Harvard School of Public Health, Boston, wrote in an op-ed in the Washington Post that “ignorance or other personal failing” should not be factors in the evaluation of patients for health care. He argues that “doctors and hospitals are not in the blame and punishment business. Nor should they be. That doctors treat sinners and responsible citizens alike is a noble tradition.”
Timothy Hoff, professor of management, healthcare systems, and health policy at Northeastern University, Boston, maintains that, in nonemergency situations, physicians are legally able to refuse patients for a variety of reasons, provided they are not doing so because of some aspect of the patient’s race, gender, sexuality, or religion. However, in the same Northeastern University news release,Robert Baginski, MD, the director of interdisciplinary affairs for the department of medical sciences at Northeastern, cautions that it is vital for health authorities to continue urging the public to get vaccinated, but not at the expense of care.
Arthur L. Caplan, PhD, the head of the division of medical ethics at New York University, said in a Medscape commentary, that the decision to refuse to see patients who can vaccinate, but choose not to, is justifiable. “If you’re trying to protect yourself, your staff, or other patients, I think you do have the right to not take on somebody who won’t vaccinate,” he writes. “This is somewhat similar to when pediatricians do not accept a family if they won’t give their kids the state-required shots to go to school. That’s been happening for many years now.
“I also think it is morally justified if they won’t take your advice,” he continues. “If they won’t follow what you think is the best healthcare for them [such as getting vaccinated], there’s not much point in building that relationship.”
The situation is different in ED and hospital settings, however. “It’s a little harder to use unvaccinated status when someone really is at death’s door,” Dr. Caplan pointed out. “When someone comes in very sick, or whatever the reason, I think we have to take care of them ethically, and legally we’re bound to get them stable in the emergency room. I do think different rules apply there.”
In the end, every private practitioner will have to make his or her own decision on this question. Dr. Bock feels he made the right one. “Since instituting the policy, we have written 55 refund checks for people who had paid for a series of cosmetic procedures. We have no idea how many people were deterred from making appointments. We’ve had several negative online reviews and one woman who wrote a letter to the Medical Board of California complaining that we were discriminating against her,” he said. He added, however, that “we’ve also had several patients who commented favorably about the policy. I have no regrets about instituting the policy, and would do it again.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In June, Gerald Bock, MD, a dermatologist in central California, instituted a new office policy: He would not be seeing any more patients who remain unvaccinated against COVID-19 in his practice.
“[It is] the height of self-centered and irresponsible behavior,” he told me. “People who come in unvaccinated, when vaccination is widely available, are stating that their personal preferences are more important than their health, and are more important than any risk that they may expose their friends and family to, and also to any risk they might present to my staff and me. We have gone to considerable effort and expense to diminish any risk that visiting our office might entail. I see no reason why we should tolerate this.”
Other doctors appear to be following in his footsteps. There is no question that physicians have the right to choose their patients, just as patients are free to choose their doctors, but That is a complicated question without a clear answer. In a statement on whether physicians can decline unvaccinated patients, the American Medical Association continues to maintain that “in general” a physician may not “ethically turn a patient away based solely on the individual’s infectious disease status,” but does concede that “the decision to accept or decline a patient must balance the urgency of the individual patient’s need; the risk the patient may pose to other patients in the physician’s practice; and the need for the physician and staff, to be available to provide care in the future.”
Medical ethics experts have offered varying opinions. Daniel Wikler, PhD, professor of ethics and population health at the Harvard School of Public Health, Boston, wrote in an op-ed in the Washington Post that “ignorance or other personal failing” should not be factors in the evaluation of patients for health care. He argues that “doctors and hospitals are not in the blame and punishment business. Nor should they be. That doctors treat sinners and responsible citizens alike is a noble tradition.”
Timothy Hoff, professor of management, healthcare systems, and health policy at Northeastern University, Boston, maintains that, in nonemergency situations, physicians are legally able to refuse patients for a variety of reasons, provided they are not doing so because of some aspect of the patient’s race, gender, sexuality, or religion. However, in the same Northeastern University news release,Robert Baginski, MD, the director of interdisciplinary affairs for the department of medical sciences at Northeastern, cautions that it is vital for health authorities to continue urging the public to get vaccinated, but not at the expense of care.
Arthur L. Caplan, PhD, the head of the division of medical ethics at New York University, said in a Medscape commentary, that the decision to refuse to see patients who can vaccinate, but choose not to, is justifiable. “If you’re trying to protect yourself, your staff, or other patients, I think you do have the right to not take on somebody who won’t vaccinate,” he writes. “This is somewhat similar to when pediatricians do not accept a family if they won’t give their kids the state-required shots to go to school. That’s been happening for many years now.
“I also think it is morally justified if they won’t take your advice,” he continues. “If they won’t follow what you think is the best healthcare for them [such as getting vaccinated], there’s not much point in building that relationship.”
The situation is different in ED and hospital settings, however. “It’s a little harder to use unvaccinated status when someone really is at death’s door,” Dr. Caplan pointed out. “When someone comes in very sick, or whatever the reason, I think we have to take care of them ethically, and legally we’re bound to get them stable in the emergency room. I do think different rules apply there.”
In the end, every private practitioner will have to make his or her own decision on this question. Dr. Bock feels he made the right one. “Since instituting the policy, we have written 55 refund checks for people who had paid for a series of cosmetic procedures. We have no idea how many people were deterred from making appointments. We’ve had several negative online reviews and one woman who wrote a letter to the Medical Board of California complaining that we were discriminating against her,” he said. He added, however, that “we’ve also had several patients who commented favorably about the policy. I have no regrets about instituting the policy, and would do it again.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In June, Gerald Bock, MD, a dermatologist in central California, instituted a new office policy: He would not be seeing any more patients who remain unvaccinated against COVID-19 in his practice.
“[It is] the height of self-centered and irresponsible behavior,” he told me. “People who come in unvaccinated, when vaccination is widely available, are stating that their personal preferences are more important than their health, and are more important than any risk that they may expose their friends and family to, and also to any risk they might present to my staff and me. We have gone to considerable effort and expense to diminish any risk that visiting our office might entail. I see no reason why we should tolerate this.”
Other doctors appear to be following in his footsteps. There is no question that physicians have the right to choose their patients, just as patients are free to choose their doctors, but That is a complicated question without a clear answer. In a statement on whether physicians can decline unvaccinated patients, the American Medical Association continues to maintain that “in general” a physician may not “ethically turn a patient away based solely on the individual’s infectious disease status,” but does concede that “the decision to accept or decline a patient must balance the urgency of the individual patient’s need; the risk the patient may pose to other patients in the physician’s practice; and the need for the physician and staff, to be available to provide care in the future.”
Medical ethics experts have offered varying opinions. Daniel Wikler, PhD, professor of ethics and population health at the Harvard School of Public Health, Boston, wrote in an op-ed in the Washington Post that “ignorance or other personal failing” should not be factors in the evaluation of patients for health care. He argues that “doctors and hospitals are not in the blame and punishment business. Nor should they be. That doctors treat sinners and responsible citizens alike is a noble tradition.”
Timothy Hoff, professor of management, healthcare systems, and health policy at Northeastern University, Boston, maintains that, in nonemergency situations, physicians are legally able to refuse patients for a variety of reasons, provided they are not doing so because of some aspect of the patient’s race, gender, sexuality, or religion. However, in the same Northeastern University news release,Robert Baginski, MD, the director of interdisciplinary affairs for the department of medical sciences at Northeastern, cautions that it is vital for health authorities to continue urging the public to get vaccinated, but not at the expense of care.
Arthur L. Caplan, PhD, the head of the division of medical ethics at New York University, said in a Medscape commentary, that the decision to refuse to see patients who can vaccinate, but choose not to, is justifiable. “If you’re trying to protect yourself, your staff, or other patients, I think you do have the right to not take on somebody who won’t vaccinate,” he writes. “This is somewhat similar to when pediatricians do not accept a family if they won’t give their kids the state-required shots to go to school. That’s been happening for many years now.
“I also think it is morally justified if they won’t take your advice,” he continues. “If they won’t follow what you think is the best healthcare for them [such as getting vaccinated], there’s not much point in building that relationship.”
The situation is different in ED and hospital settings, however. “It’s a little harder to use unvaccinated status when someone really is at death’s door,” Dr. Caplan pointed out. “When someone comes in very sick, or whatever the reason, I think we have to take care of them ethically, and legally we’re bound to get them stable in the emergency room. I do think different rules apply there.”
In the end, every private practitioner will have to make his or her own decision on this question. Dr. Bock feels he made the right one. “Since instituting the policy, we have written 55 refund checks for people who had paid for a series of cosmetic procedures. We have no idea how many people were deterred from making appointments. We’ve had several negative online reviews and one woman who wrote a letter to the Medical Board of California complaining that we were discriminating against her,” he said. He added, however, that “we’ve also had several patients who commented favorably about the policy. I have no regrets about instituting the policy, and would do it again.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Few JAK inhibitor users have diminished immune response to COVID-19 vaccines
Patients who are being treated with Janus kinase (JAK) inhibitors overall show a high immune response rate to COVID-19 vaccination, one that matches the rates seen in patients on other immunosuppressants, a new study has found.
The patients taking a JAK inhibitor who are most at risk of a diminished response may be those on upadacitinib (Rinvoq) and anyone 65 years or older, wrote Raphaèle Seror, MD, PhD, of Paris-Saclay (France) University and coauthors. The study was published in The Lancet Rheumatology.
To gauge the effectiveness of COVID-19 vaccines in this subset of immunosuppressed patients, the researchers analyzed 113 participants in the MAJIK-SFR Registry, a multicenter study of French patients with rheumatoid or psoriatic arthritis. The participants were treated at 13 centers throughout France; their mean age was 61.8 years (standard deviation, 12.5), and 72% were female. A total of 56 were taking baricitinib (Olumiant), 30 were taking tofacitinib (Xeljanz), and 27 were taking upadacitinib.
Serologic assessment was performed an average of 8.7 weeks (SD, 5.2) after the last dose of vaccine. The overall response rate – defined as the proportion of patients with detectable anti-spike antibodies per manufacturer’s cutoff values – was 88% (100 of 113). The nonresponse rate was higher with upadacitinib (7 of 27 patients, 26%) than with baricitinib (5 of 56, 9%) or tofacitinib (1 of 30, 3%). The only nonresponders who were not age 65 or older were four of the seven who received upadacitinib. The interval between the last vaccine dose and serologic assessment was somewhat longer in nonresponders (11.3 weeks) than in responders (8.3 weeks).
Earlier this year, the American College of Rheumatology recommended withholding JAK inhibitors for 1 week after each vaccine dose because of “concern related to the effects of this medication class on interferon signaling that may result in a diminished vaccine response Only two patients in the study had treatment with JAK inhibitors stopped before or after vaccination.
Questions about antibody levels remain difficult to answer
“This study does further confirm a big point,” said Alfred Kim, MD, PhD, of Washington University, St. Louis, in an interview. “Most people on any sort of immunosuppression, with rare exceptions, can mount responses to COVID-19 vaccination.”
“What level of response is going to be sufficient, of course, is not clear,” he added. “Even though most people generate responses, at the population level those responses seem lower than those in nonimmunosuppressed people. Particularly for those on upadacitinib, which is lower than patients on the other JAK inhibitors. Is that problematic? We don’t know yet.”
Dr. Kim, who was part of a separate, earlier study that assessed vaccine response in patients with chronic inflammatory disease who were being treated with immunosuppressive medications, noted that many of the questions patients are asking about their antibody levels cannot yet be answered.
“It’s kind of the Wild West of serologic testing out there right now,” he said. “Even though we’re recommending that people still don’t check their antibody levels because their results are largely inactionable, everyone is still getting them anyway. But each of these tests are slightly different, and the results and the interpretation are further clouded because of those slight performance differences between each platform.”
Dr. Kim highlighted the number of different tests as one of this study’s notable limitations: 11 different assays were used to determine patients’ immune responses. “The authors made the argument that these tests are FDA approved, and that’s true, but that doesn’t necessarily mean much. Approval does translate to technical reliability but not to comparisons between the tests.”
As for next steps, both the authors and Dr. Kim recognized the need for a prospective trial. “To do a vaccine effectiveness–type study and show clinical protection against either infection or hospitalization – those are going to take a while, simply because of the nature of how many people you need for each of these studies,” he said. “Time will tell whether or not the data that are being presented here will translate literally into protective outcomes downstream.”
The MAJIK Registry is supported by the French Rheumatology Society. The authors acknowledged numerous potential conflicts of interest, including receiving consulting fees, research support, and honoraria from various pharmaceutical companies.
Patients who are being treated with Janus kinase (JAK) inhibitors overall show a high immune response rate to COVID-19 vaccination, one that matches the rates seen in patients on other immunosuppressants, a new study has found.
The patients taking a JAK inhibitor who are most at risk of a diminished response may be those on upadacitinib (Rinvoq) and anyone 65 years or older, wrote Raphaèle Seror, MD, PhD, of Paris-Saclay (France) University and coauthors. The study was published in The Lancet Rheumatology.
To gauge the effectiveness of COVID-19 vaccines in this subset of immunosuppressed patients, the researchers analyzed 113 participants in the MAJIK-SFR Registry, a multicenter study of French patients with rheumatoid or psoriatic arthritis. The participants were treated at 13 centers throughout France; their mean age was 61.8 years (standard deviation, 12.5), and 72% were female. A total of 56 were taking baricitinib (Olumiant), 30 were taking tofacitinib (Xeljanz), and 27 were taking upadacitinib.
Serologic assessment was performed an average of 8.7 weeks (SD, 5.2) after the last dose of vaccine. The overall response rate – defined as the proportion of patients with detectable anti-spike antibodies per manufacturer’s cutoff values – was 88% (100 of 113). The nonresponse rate was higher with upadacitinib (7 of 27 patients, 26%) than with baricitinib (5 of 56, 9%) or tofacitinib (1 of 30, 3%). The only nonresponders who were not age 65 or older were four of the seven who received upadacitinib. The interval between the last vaccine dose and serologic assessment was somewhat longer in nonresponders (11.3 weeks) than in responders (8.3 weeks).
Earlier this year, the American College of Rheumatology recommended withholding JAK inhibitors for 1 week after each vaccine dose because of “concern related to the effects of this medication class on interferon signaling that may result in a diminished vaccine response Only two patients in the study had treatment with JAK inhibitors stopped before or after vaccination.
Questions about antibody levels remain difficult to answer
“This study does further confirm a big point,” said Alfred Kim, MD, PhD, of Washington University, St. Louis, in an interview. “Most people on any sort of immunosuppression, with rare exceptions, can mount responses to COVID-19 vaccination.”
“What level of response is going to be sufficient, of course, is not clear,” he added. “Even though most people generate responses, at the population level those responses seem lower than those in nonimmunosuppressed people. Particularly for those on upadacitinib, which is lower than patients on the other JAK inhibitors. Is that problematic? We don’t know yet.”
Dr. Kim, who was part of a separate, earlier study that assessed vaccine response in patients with chronic inflammatory disease who were being treated with immunosuppressive medications, noted that many of the questions patients are asking about their antibody levels cannot yet be answered.
“It’s kind of the Wild West of serologic testing out there right now,” he said. “Even though we’re recommending that people still don’t check their antibody levels because their results are largely inactionable, everyone is still getting them anyway. But each of these tests are slightly different, and the results and the interpretation are further clouded because of those slight performance differences between each platform.”
Dr. Kim highlighted the number of different tests as one of this study’s notable limitations: 11 different assays were used to determine patients’ immune responses. “The authors made the argument that these tests are FDA approved, and that’s true, but that doesn’t necessarily mean much. Approval does translate to technical reliability but not to comparisons between the tests.”
As for next steps, both the authors and Dr. Kim recognized the need for a prospective trial. “To do a vaccine effectiveness–type study and show clinical protection against either infection or hospitalization – those are going to take a while, simply because of the nature of how many people you need for each of these studies,” he said. “Time will tell whether or not the data that are being presented here will translate literally into protective outcomes downstream.”
The MAJIK Registry is supported by the French Rheumatology Society. The authors acknowledged numerous potential conflicts of interest, including receiving consulting fees, research support, and honoraria from various pharmaceutical companies.
Patients who are being treated with Janus kinase (JAK) inhibitors overall show a high immune response rate to COVID-19 vaccination, one that matches the rates seen in patients on other immunosuppressants, a new study has found.
The patients taking a JAK inhibitor who are most at risk of a diminished response may be those on upadacitinib (Rinvoq) and anyone 65 years or older, wrote Raphaèle Seror, MD, PhD, of Paris-Saclay (France) University and coauthors. The study was published in The Lancet Rheumatology.
To gauge the effectiveness of COVID-19 vaccines in this subset of immunosuppressed patients, the researchers analyzed 113 participants in the MAJIK-SFR Registry, a multicenter study of French patients with rheumatoid or psoriatic arthritis. The participants were treated at 13 centers throughout France; their mean age was 61.8 years (standard deviation, 12.5), and 72% were female. A total of 56 were taking baricitinib (Olumiant), 30 were taking tofacitinib (Xeljanz), and 27 were taking upadacitinib.
Serologic assessment was performed an average of 8.7 weeks (SD, 5.2) after the last dose of vaccine. The overall response rate – defined as the proportion of patients with detectable anti-spike antibodies per manufacturer’s cutoff values – was 88% (100 of 113). The nonresponse rate was higher with upadacitinib (7 of 27 patients, 26%) than with baricitinib (5 of 56, 9%) or tofacitinib (1 of 30, 3%). The only nonresponders who were not age 65 or older were four of the seven who received upadacitinib. The interval between the last vaccine dose and serologic assessment was somewhat longer in nonresponders (11.3 weeks) than in responders (8.3 weeks).
Earlier this year, the American College of Rheumatology recommended withholding JAK inhibitors for 1 week after each vaccine dose because of “concern related to the effects of this medication class on interferon signaling that may result in a diminished vaccine response Only two patients in the study had treatment with JAK inhibitors stopped before or after vaccination.
Questions about antibody levels remain difficult to answer
“This study does further confirm a big point,” said Alfred Kim, MD, PhD, of Washington University, St. Louis, in an interview. “Most people on any sort of immunosuppression, with rare exceptions, can mount responses to COVID-19 vaccination.”
“What level of response is going to be sufficient, of course, is not clear,” he added. “Even though most people generate responses, at the population level those responses seem lower than those in nonimmunosuppressed people. Particularly for those on upadacitinib, which is lower than patients on the other JAK inhibitors. Is that problematic? We don’t know yet.”
Dr. Kim, who was part of a separate, earlier study that assessed vaccine response in patients with chronic inflammatory disease who were being treated with immunosuppressive medications, noted that many of the questions patients are asking about their antibody levels cannot yet be answered.
“It’s kind of the Wild West of serologic testing out there right now,” he said. “Even though we’re recommending that people still don’t check their antibody levels because their results are largely inactionable, everyone is still getting them anyway. But each of these tests are slightly different, and the results and the interpretation are further clouded because of those slight performance differences between each platform.”
Dr. Kim highlighted the number of different tests as one of this study’s notable limitations: 11 different assays were used to determine patients’ immune responses. “The authors made the argument that these tests are FDA approved, and that’s true, but that doesn’t necessarily mean much. Approval does translate to technical reliability but not to comparisons between the tests.”
As for next steps, both the authors and Dr. Kim recognized the need for a prospective trial. “To do a vaccine effectiveness–type study and show clinical protection against either infection or hospitalization – those are going to take a while, simply because of the nature of how many people you need for each of these studies,” he said. “Time will tell whether or not the data that are being presented here will translate literally into protective outcomes downstream.”
The MAJIK Registry is supported by the French Rheumatology Society. The authors acknowledged numerous potential conflicts of interest, including receiving consulting fees, research support, and honoraria from various pharmaceutical companies.
FROM THE LANCET RHEUMATOLOGY