User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Less sleep, more burnout linked to higher COVID-19 risk, study shows
among health care workers considered to be at high risk for exposure to patients with COVID-19, new evidence reveals.
For each additional hour of sleep at night, for example, risk for COVID-19 dropped by 12% in a study of 2844 frontline health care workers.
Furthermore, those who reported experiencing work-related burnout every day were 2.6 times more likely to report having COVID-19, to report having COVID-19 for a longer time, and to experience COVID-19 of more severity.
“This study underscores the importance of non–hygiene-related risk factors for COVID-19 and supports a holistic approach to health – including optimal sleep and job stress reduction to protect our health care workers from this and future pandemics,” senior author Sara B. Seidelmann, MD, said in an interview.
“Our findings add to the literature that sleep duration at night, sleep problems, and burnout may be risk factors for viral illnesses like COVID-19,” wrote Dr. Seidelmann and colleagues.
This is the first study to link COVID-19 risk to sleep habits – including number of hours of sleep at night, daytime napping hours, and severe sleep problems – among health care workers across multiple countries.
The study was published online March 22 in BMJ Nutrition, Prevention, and Health.
The researchers surveyed health care professionals in specialties considered to place personnel at high risk for exposure to SARS-CoV-2: critical care, emergency care, and internal medicine.
The association between sleep and burnout risk factors and COVID-19 did not vary significantly by specialty. “We didn’t detect any significant interactions between age, sex, specialty, or country,” said Dr. Seidelmann, assistant professor of clinical medicine at Columbia University College of Physicians and Surgeons, New York, and an internist at Stamford (Conn.) Hospital.
In addition to the 12% lower risk associated with each additional hour of sleep at night, each 1 additional hour of daytime napping was linked with a 6% increased risk for COVID-19 in an adjusted analysis (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.01-1.12).
Daytime napping slightly increased risk for COVID-19 in five of the six countries included in the study: France, Germany, Italy, the United Kingdom, and the United States. In contrast, in Spain, napping had a nonsignificant protective effect.
The survey asked health care workers to recall nighttime sleep duration, sleep disorders, and burnout in the year prior to onset of the COVID-19 pandemic.
‘Significant, close contact’ with COVID-19?
Lead author Hyunju Kim, NP, Dr. Seidelmann, and colleagues conducted the population-based, case-control study from July 17 to Sept. 25, 2020. They identified health care workers from the SurveyHealthcareGlobus (SHG) network.
Of the respondents, 72% were men. The mean age of the participants was 48 years, and the study population was 77% White, 12% Asian, 6% mixed background, 2% Black, and 1% other. (The remainder preferred not to say).
The 568 health care workers considered to have COVID-19 were classified on the basis of self-reported symptoms. Control participants had no symptoms associated with COVID-19.
All 2,844 participants answered yes to a question about having “significant close contact” with COVID-19 patients in their workplace.
Compared to reporting no sleep problems, having three such problems – difficulty sleeping at night, poor sleep continuity, and frequent use of sleeping pills – was associated with 88% greater odds of COVID-19 (OR, 1.88; 95% CI, 1.17–3.01).
Having one sleep problem was not associated with COVID-19.
More burnout, greater risk
The health care workers reported the severity of any work-related burnout. “There was a significant dose-response relationship between frequency of burnout and COVID-19,” the researchers noted.
Those who reported having burnout rarely or weekly had a 1.3-1.4 greater chance of reporting COVID-19 compared to those who reported having no burnout, for example.
In addition, reporting a high level of burnout was linked to about three times the risk for having COVID-19 of longer duration and of greater severity.
What drives the association between sleep problems, burnout, and higher risk for COVID-19 and severe COVID-19 remains unknown.
“The mechanism underlying these associations isn’t clear, but suboptimal sleep, sleep disorders, and stress may result in immune system dysregulation, increased inflammation, and alterations in hormones such as cortisol and melatonin that may increase vulnerability to viral infections,” Dr. Seidelmann said.
Strengths and limitations
Using a large network of health care workers in the early phase of the pandemic is a strength of the study. How generalizable the findings are outside the SHG database of 1.5 million health care workers remains unknown.
Another limitation was reliance on self-reporting of COVID-19 patient exposure, outcomes, and covariates, which could have introduced bias.
“However,” the researchers noted, “health care workers are likely a reliable source of information.”
Insomnia a common challenge
A 2020 meta-analysis examined the effect of insomnia and psychological factors on COVID-19 risk among health care workers. Lead author Kavita Batra, PhD, of the University of Nevada, Las Vegas (UNLV), and colleagues found that the pooled prevalence of insomnia was almost 28%.
“The recent six-country study by Kim and colleagues also underscores this relationship between lack of sleep and having higher odds of COVID-19 infection,” Manoj Sharma, MBBS, PhD, professor of social and behavioral health in the UNLV department of environmental and occupational health, and one of the study authors, said in an interview.
More research is warranted to learn the direction of the association, he said. Does reduced sleep lower immunity and make a health care worker more susceptible to SARS-CoV-2 infection, or does the anxiety associated with COVID-19 contribute to insomnia?
“Practicing sleep hygiene is a must not only for health workers but also for everyone,” Dr. Sharma added. Recommendations include having fixed hours of going to bed, fixed hours of waking up, not overdoing naps, having at least 30 minutes of winding down before sleeping, having a dark bedroom devoid of all electronics and other disturbances, avoiding smoking, alcohol, and stimulants (such as caffeine) before sleeping, and practicing relaxation right before sleeping, he said.
“It is hard for some health care workers, especially those who work night shifts, but it must be a priority to follow as many sleep hygiene measures as possible,” Dr. Sharma said. “After all, if you do not take care of yourself how can you take care of others?”
Dr. Seidelmann, Dr. Batra, and Dr. Sharma have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
among health care workers considered to be at high risk for exposure to patients with COVID-19, new evidence reveals.
For each additional hour of sleep at night, for example, risk for COVID-19 dropped by 12% in a study of 2844 frontline health care workers.
Furthermore, those who reported experiencing work-related burnout every day were 2.6 times more likely to report having COVID-19, to report having COVID-19 for a longer time, and to experience COVID-19 of more severity.
“This study underscores the importance of non–hygiene-related risk factors for COVID-19 and supports a holistic approach to health – including optimal sleep and job stress reduction to protect our health care workers from this and future pandemics,” senior author Sara B. Seidelmann, MD, said in an interview.
“Our findings add to the literature that sleep duration at night, sleep problems, and burnout may be risk factors for viral illnesses like COVID-19,” wrote Dr. Seidelmann and colleagues.
This is the first study to link COVID-19 risk to sleep habits – including number of hours of sleep at night, daytime napping hours, and severe sleep problems – among health care workers across multiple countries.
The study was published online March 22 in BMJ Nutrition, Prevention, and Health.
The researchers surveyed health care professionals in specialties considered to place personnel at high risk for exposure to SARS-CoV-2: critical care, emergency care, and internal medicine.
The association between sleep and burnout risk factors and COVID-19 did not vary significantly by specialty. “We didn’t detect any significant interactions between age, sex, specialty, or country,” said Dr. Seidelmann, assistant professor of clinical medicine at Columbia University College of Physicians and Surgeons, New York, and an internist at Stamford (Conn.) Hospital.
In addition to the 12% lower risk associated with each additional hour of sleep at night, each 1 additional hour of daytime napping was linked with a 6% increased risk for COVID-19 in an adjusted analysis (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.01-1.12).
Daytime napping slightly increased risk for COVID-19 in five of the six countries included in the study: France, Germany, Italy, the United Kingdom, and the United States. In contrast, in Spain, napping had a nonsignificant protective effect.
The survey asked health care workers to recall nighttime sleep duration, sleep disorders, and burnout in the year prior to onset of the COVID-19 pandemic.
‘Significant, close contact’ with COVID-19?
Lead author Hyunju Kim, NP, Dr. Seidelmann, and colleagues conducted the population-based, case-control study from July 17 to Sept. 25, 2020. They identified health care workers from the SurveyHealthcareGlobus (SHG) network.
Of the respondents, 72% were men. The mean age of the participants was 48 years, and the study population was 77% White, 12% Asian, 6% mixed background, 2% Black, and 1% other. (The remainder preferred not to say).
The 568 health care workers considered to have COVID-19 were classified on the basis of self-reported symptoms. Control participants had no symptoms associated with COVID-19.
All 2,844 participants answered yes to a question about having “significant close contact” with COVID-19 patients in their workplace.
Compared to reporting no sleep problems, having three such problems – difficulty sleeping at night, poor sleep continuity, and frequent use of sleeping pills – was associated with 88% greater odds of COVID-19 (OR, 1.88; 95% CI, 1.17–3.01).
Having one sleep problem was not associated with COVID-19.
More burnout, greater risk
The health care workers reported the severity of any work-related burnout. “There was a significant dose-response relationship between frequency of burnout and COVID-19,” the researchers noted.
Those who reported having burnout rarely or weekly had a 1.3-1.4 greater chance of reporting COVID-19 compared to those who reported having no burnout, for example.
In addition, reporting a high level of burnout was linked to about three times the risk for having COVID-19 of longer duration and of greater severity.
What drives the association between sleep problems, burnout, and higher risk for COVID-19 and severe COVID-19 remains unknown.
“The mechanism underlying these associations isn’t clear, but suboptimal sleep, sleep disorders, and stress may result in immune system dysregulation, increased inflammation, and alterations in hormones such as cortisol and melatonin that may increase vulnerability to viral infections,” Dr. Seidelmann said.
Strengths and limitations
Using a large network of health care workers in the early phase of the pandemic is a strength of the study. How generalizable the findings are outside the SHG database of 1.5 million health care workers remains unknown.
Another limitation was reliance on self-reporting of COVID-19 patient exposure, outcomes, and covariates, which could have introduced bias.
“However,” the researchers noted, “health care workers are likely a reliable source of information.”
Insomnia a common challenge
A 2020 meta-analysis examined the effect of insomnia and psychological factors on COVID-19 risk among health care workers. Lead author Kavita Batra, PhD, of the University of Nevada, Las Vegas (UNLV), and colleagues found that the pooled prevalence of insomnia was almost 28%.
“The recent six-country study by Kim and colleagues also underscores this relationship between lack of sleep and having higher odds of COVID-19 infection,” Manoj Sharma, MBBS, PhD, professor of social and behavioral health in the UNLV department of environmental and occupational health, and one of the study authors, said in an interview.
More research is warranted to learn the direction of the association, he said. Does reduced sleep lower immunity and make a health care worker more susceptible to SARS-CoV-2 infection, or does the anxiety associated with COVID-19 contribute to insomnia?
“Practicing sleep hygiene is a must not only for health workers but also for everyone,” Dr. Sharma added. Recommendations include having fixed hours of going to bed, fixed hours of waking up, not overdoing naps, having at least 30 minutes of winding down before sleeping, having a dark bedroom devoid of all electronics and other disturbances, avoiding smoking, alcohol, and stimulants (such as caffeine) before sleeping, and practicing relaxation right before sleeping, he said.
“It is hard for some health care workers, especially those who work night shifts, but it must be a priority to follow as many sleep hygiene measures as possible,” Dr. Sharma said. “After all, if you do not take care of yourself how can you take care of others?”
Dr. Seidelmann, Dr. Batra, and Dr. Sharma have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
among health care workers considered to be at high risk for exposure to patients with COVID-19, new evidence reveals.
For each additional hour of sleep at night, for example, risk for COVID-19 dropped by 12% in a study of 2844 frontline health care workers.
Furthermore, those who reported experiencing work-related burnout every day were 2.6 times more likely to report having COVID-19, to report having COVID-19 for a longer time, and to experience COVID-19 of more severity.
“This study underscores the importance of non–hygiene-related risk factors for COVID-19 and supports a holistic approach to health – including optimal sleep and job stress reduction to protect our health care workers from this and future pandemics,” senior author Sara B. Seidelmann, MD, said in an interview.
“Our findings add to the literature that sleep duration at night, sleep problems, and burnout may be risk factors for viral illnesses like COVID-19,” wrote Dr. Seidelmann and colleagues.
This is the first study to link COVID-19 risk to sleep habits – including number of hours of sleep at night, daytime napping hours, and severe sleep problems – among health care workers across multiple countries.
The study was published online March 22 in BMJ Nutrition, Prevention, and Health.
The researchers surveyed health care professionals in specialties considered to place personnel at high risk for exposure to SARS-CoV-2: critical care, emergency care, and internal medicine.
The association between sleep and burnout risk factors and COVID-19 did not vary significantly by specialty. “We didn’t detect any significant interactions between age, sex, specialty, or country,” said Dr. Seidelmann, assistant professor of clinical medicine at Columbia University College of Physicians and Surgeons, New York, and an internist at Stamford (Conn.) Hospital.
In addition to the 12% lower risk associated with each additional hour of sleep at night, each 1 additional hour of daytime napping was linked with a 6% increased risk for COVID-19 in an adjusted analysis (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.01-1.12).
Daytime napping slightly increased risk for COVID-19 in five of the six countries included in the study: France, Germany, Italy, the United Kingdom, and the United States. In contrast, in Spain, napping had a nonsignificant protective effect.
The survey asked health care workers to recall nighttime sleep duration, sleep disorders, and burnout in the year prior to onset of the COVID-19 pandemic.
‘Significant, close contact’ with COVID-19?
Lead author Hyunju Kim, NP, Dr. Seidelmann, and colleagues conducted the population-based, case-control study from July 17 to Sept. 25, 2020. They identified health care workers from the SurveyHealthcareGlobus (SHG) network.
Of the respondents, 72% were men. The mean age of the participants was 48 years, and the study population was 77% White, 12% Asian, 6% mixed background, 2% Black, and 1% other. (The remainder preferred not to say).
The 568 health care workers considered to have COVID-19 were classified on the basis of self-reported symptoms. Control participants had no symptoms associated with COVID-19.
All 2,844 participants answered yes to a question about having “significant close contact” with COVID-19 patients in their workplace.
Compared to reporting no sleep problems, having three such problems – difficulty sleeping at night, poor sleep continuity, and frequent use of sleeping pills – was associated with 88% greater odds of COVID-19 (OR, 1.88; 95% CI, 1.17–3.01).
Having one sleep problem was not associated with COVID-19.
More burnout, greater risk
The health care workers reported the severity of any work-related burnout. “There was a significant dose-response relationship between frequency of burnout and COVID-19,” the researchers noted.
Those who reported having burnout rarely or weekly had a 1.3-1.4 greater chance of reporting COVID-19 compared to those who reported having no burnout, for example.
In addition, reporting a high level of burnout was linked to about three times the risk for having COVID-19 of longer duration and of greater severity.
What drives the association between sleep problems, burnout, and higher risk for COVID-19 and severe COVID-19 remains unknown.
“The mechanism underlying these associations isn’t clear, but suboptimal sleep, sleep disorders, and stress may result in immune system dysregulation, increased inflammation, and alterations in hormones such as cortisol and melatonin that may increase vulnerability to viral infections,” Dr. Seidelmann said.
Strengths and limitations
Using a large network of health care workers in the early phase of the pandemic is a strength of the study. How generalizable the findings are outside the SHG database of 1.5 million health care workers remains unknown.
Another limitation was reliance on self-reporting of COVID-19 patient exposure, outcomes, and covariates, which could have introduced bias.
“However,” the researchers noted, “health care workers are likely a reliable source of information.”
Insomnia a common challenge
A 2020 meta-analysis examined the effect of insomnia and psychological factors on COVID-19 risk among health care workers. Lead author Kavita Batra, PhD, of the University of Nevada, Las Vegas (UNLV), and colleagues found that the pooled prevalence of insomnia was almost 28%.
“The recent six-country study by Kim and colleagues also underscores this relationship between lack of sleep and having higher odds of COVID-19 infection,” Manoj Sharma, MBBS, PhD, professor of social and behavioral health in the UNLV department of environmental and occupational health, and one of the study authors, said in an interview.
More research is warranted to learn the direction of the association, he said. Does reduced sleep lower immunity and make a health care worker more susceptible to SARS-CoV-2 infection, or does the anxiety associated with COVID-19 contribute to insomnia?
“Practicing sleep hygiene is a must not only for health workers but also for everyone,” Dr. Sharma added. Recommendations include having fixed hours of going to bed, fixed hours of waking up, not overdoing naps, having at least 30 minutes of winding down before sleeping, having a dark bedroom devoid of all electronics and other disturbances, avoiding smoking, alcohol, and stimulants (such as caffeine) before sleeping, and practicing relaxation right before sleeping, he said.
“It is hard for some health care workers, especially those who work night shifts, but it must be a priority to follow as many sleep hygiene measures as possible,” Dr. Sharma said. “After all, if you do not take care of yourself how can you take care of others?”
Dr. Seidelmann, Dr. Batra, and Dr. Sharma have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19’s impact on lupus inpatients examined in study
Severe COVID-19 infection was more likely in hospitalized patients with systemic lupus erythematosus (SLE) who had comorbidities and risk factors associated with severe infection in the general population, notably older age, male gender, and hypertension, based on data from a nationwide epidemiologic study of inpatients in France.
“Recently, anti-interferon antibodies have been implicated in severe SARS-CoV-2 infection while it has been known for decades that patients with SLE may produce such autoantibodies,” but large-scale data on the risk of severe COVID-19 infection in SLE patients are limited, Arthur Mageau, MD, of Bichat–Claude Bernard Hospital in Paris, and colleagues wrote.
In a research letter published in Annals of the Rheumatic Diseases, the researchers used the French health care database Programme de Médicalisation des Systèmes d’Information to identify 11,055 adult SLE patients who had at least one hospital stay between March 1, 2020, and Oct.31, 2020. Of these, 1,411 (12.8%) also were diagnosed with COVID-19, and these patients had a total of 1,721 hospital stays.
Overall, in-hospital mortality was approximately four times higher among SLE patients with COVID-19 infection, compared with SLE patients without COVID-19 infection (9.5% vs. 2.4%, P < .001), and 293 (17%) of the COVID-19 hospital stays involved an intensive care unit. In the ICU, 78 (26.7%) of the COVID-19 patients required invasive ventilation, and 71 (24.7%) required noninvasive mechanical ventilation.
The SLE patients with COVID-19 who died were significantly more likely than the SLE patients with COVID-19 who recovered to be older and male, and to have conditions including chronic kidney disease, high blood pressure, chronic pulmonary disease, and a history of cardiovascular events or lupus nephritis. The study findings were limited by the focus on hospitalized patients only, so the results cannot be generalized to all lupus patients, the researchers said.
“Interestingly, while the overall mortality rate was lower in SLE/COVID-19–positive inpatients as compared with the total population admitted for SARS-CoV-2 infection in France during the same period (9.5% vs 15.7%, P < .0001), the mortality rate at a younger age tended to be higher in patients with SLE,” the researchers wrote, but the difference for these younger patients was not statistically significant. This disparity may be caused by the reduced need for immunosuppressive drugs in SLE patients as they age, and the observed increased mortality in younger SLE patients, compared with the general population, suggests that SLE may promote poor outcomes from COVID-19 infection.
Dr. Mageau received PhD fellowship support from the Agence Nationale pour la recherche. He and the other researchers had no financial conflicts to disclose. The study received no outside funding.
Severe COVID-19 infection was more likely in hospitalized patients with systemic lupus erythematosus (SLE) who had comorbidities and risk factors associated with severe infection in the general population, notably older age, male gender, and hypertension, based on data from a nationwide epidemiologic study of inpatients in France.
“Recently, anti-interferon antibodies have been implicated in severe SARS-CoV-2 infection while it has been known for decades that patients with SLE may produce such autoantibodies,” but large-scale data on the risk of severe COVID-19 infection in SLE patients are limited, Arthur Mageau, MD, of Bichat–Claude Bernard Hospital in Paris, and colleagues wrote.
In a research letter published in Annals of the Rheumatic Diseases, the researchers used the French health care database Programme de Médicalisation des Systèmes d’Information to identify 11,055 adult SLE patients who had at least one hospital stay between March 1, 2020, and Oct.31, 2020. Of these, 1,411 (12.8%) also were diagnosed with COVID-19, and these patients had a total of 1,721 hospital stays.
Overall, in-hospital mortality was approximately four times higher among SLE patients with COVID-19 infection, compared with SLE patients without COVID-19 infection (9.5% vs. 2.4%, P < .001), and 293 (17%) of the COVID-19 hospital stays involved an intensive care unit. In the ICU, 78 (26.7%) of the COVID-19 patients required invasive ventilation, and 71 (24.7%) required noninvasive mechanical ventilation.
The SLE patients with COVID-19 who died were significantly more likely than the SLE patients with COVID-19 who recovered to be older and male, and to have conditions including chronic kidney disease, high blood pressure, chronic pulmonary disease, and a history of cardiovascular events or lupus nephritis. The study findings were limited by the focus on hospitalized patients only, so the results cannot be generalized to all lupus patients, the researchers said.
“Interestingly, while the overall mortality rate was lower in SLE/COVID-19–positive inpatients as compared with the total population admitted for SARS-CoV-2 infection in France during the same period (9.5% vs 15.7%, P < .0001), the mortality rate at a younger age tended to be higher in patients with SLE,” the researchers wrote, but the difference for these younger patients was not statistically significant. This disparity may be caused by the reduced need for immunosuppressive drugs in SLE patients as they age, and the observed increased mortality in younger SLE patients, compared with the general population, suggests that SLE may promote poor outcomes from COVID-19 infection.
Dr. Mageau received PhD fellowship support from the Agence Nationale pour la recherche. He and the other researchers had no financial conflicts to disclose. The study received no outside funding.
Severe COVID-19 infection was more likely in hospitalized patients with systemic lupus erythematosus (SLE) who had comorbidities and risk factors associated with severe infection in the general population, notably older age, male gender, and hypertension, based on data from a nationwide epidemiologic study of inpatients in France.
“Recently, anti-interferon antibodies have been implicated in severe SARS-CoV-2 infection while it has been known for decades that patients with SLE may produce such autoantibodies,” but large-scale data on the risk of severe COVID-19 infection in SLE patients are limited, Arthur Mageau, MD, of Bichat–Claude Bernard Hospital in Paris, and colleagues wrote.
In a research letter published in Annals of the Rheumatic Diseases, the researchers used the French health care database Programme de Médicalisation des Systèmes d’Information to identify 11,055 adult SLE patients who had at least one hospital stay between March 1, 2020, and Oct.31, 2020. Of these, 1,411 (12.8%) also were diagnosed with COVID-19, and these patients had a total of 1,721 hospital stays.
Overall, in-hospital mortality was approximately four times higher among SLE patients with COVID-19 infection, compared with SLE patients without COVID-19 infection (9.5% vs. 2.4%, P < .001), and 293 (17%) of the COVID-19 hospital stays involved an intensive care unit. In the ICU, 78 (26.7%) of the COVID-19 patients required invasive ventilation, and 71 (24.7%) required noninvasive mechanical ventilation.
The SLE patients with COVID-19 who died were significantly more likely than the SLE patients with COVID-19 who recovered to be older and male, and to have conditions including chronic kidney disease, high blood pressure, chronic pulmonary disease, and a history of cardiovascular events or lupus nephritis. The study findings were limited by the focus on hospitalized patients only, so the results cannot be generalized to all lupus patients, the researchers said.
“Interestingly, while the overall mortality rate was lower in SLE/COVID-19–positive inpatients as compared with the total population admitted for SARS-CoV-2 infection in France during the same period (9.5% vs 15.7%, P < .0001), the mortality rate at a younger age tended to be higher in patients with SLE,” the researchers wrote, but the difference for these younger patients was not statistically significant. This disparity may be caused by the reduced need for immunosuppressive drugs in SLE patients as they age, and the observed increased mortality in younger SLE patients, compared with the general population, suggests that SLE may promote poor outcomes from COVID-19 infection.
Dr. Mageau received PhD fellowship support from the Agence Nationale pour la recherche. He and the other researchers had no financial conflicts to disclose. The study received no outside funding.
FROM ANNALS OF THE RHEUMATIC DISEASES
Match Day 2021: Dermatology holds steady
Despite the pandemic, , according to the National Resident Matching Program (NRMP).
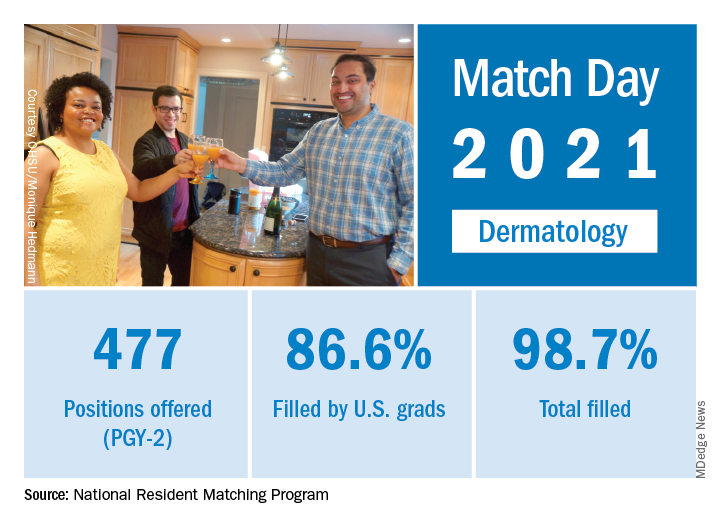
Available dermatology PGY-2 slots fell by 0.2% from 478 in 2020 to 477 in 2021, but 471 slots were filled in 2021, 2 more than last year, for an increase of 0.4%. The overall fill rate was 98.7% in 2021, with 86.6% being filled by U.S. graduates. Just under 88% of filled positions went to MD and DO seniors.
While Match Day 2021 set a record for positions offered and filled at 38,106 slots and 36,179, respectively, an overall total of 2,699 PGY-2 slots were offered in 2021, which is a decrease of 1.6% from last year’s total of 2,742 slots. Of the available PGY-2 slots, 97.6% were filled in 2021, compared with 96.6% in 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. We admire all the Match participants for their hard work and their commitment to train and serve alongside their peers. The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in a press release.
Despite the pandemic, , according to the National Resident Matching Program (NRMP).

Available dermatology PGY-2 slots fell by 0.2% from 478 in 2020 to 477 in 2021, but 471 slots were filled in 2021, 2 more than last year, for an increase of 0.4%. The overall fill rate was 98.7% in 2021, with 86.6% being filled by U.S. graduates. Just under 88% of filled positions went to MD and DO seniors.
While Match Day 2021 set a record for positions offered and filled at 38,106 slots and 36,179, respectively, an overall total of 2,699 PGY-2 slots were offered in 2021, which is a decrease of 1.6% from last year’s total of 2,742 slots. Of the available PGY-2 slots, 97.6% were filled in 2021, compared with 96.6% in 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. We admire all the Match participants for their hard work and their commitment to train and serve alongside their peers. The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in a press release.
Despite the pandemic, , according to the National Resident Matching Program (NRMP).

Available dermatology PGY-2 slots fell by 0.2% from 478 in 2020 to 477 in 2021, but 471 slots were filled in 2021, 2 more than last year, for an increase of 0.4%. The overall fill rate was 98.7% in 2021, with 86.6% being filled by U.S. graduates. Just under 88% of filled positions went to MD and DO seniors.
While Match Day 2021 set a record for positions offered and filled at 38,106 slots and 36,179, respectively, an overall total of 2,699 PGY-2 slots were offered in 2021, which is a decrease of 1.6% from last year’s total of 2,742 slots. Of the available PGY-2 slots, 97.6% were filled in 2021, compared with 96.6% in 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. We admire all the Match participants for their hard work and their commitment to train and serve alongside their peers. The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in a press release.
New analysis eyes the surgical landscape for hidradenitis suppurativa
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
FROM DERMATOLOGIC SURGERY
Ruxolitinib cream for atopic dermatitis is in regulatory home stretch
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
A young girl presents with ‘itchy, rashy’ hands
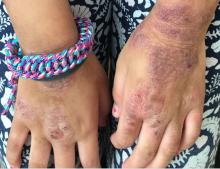
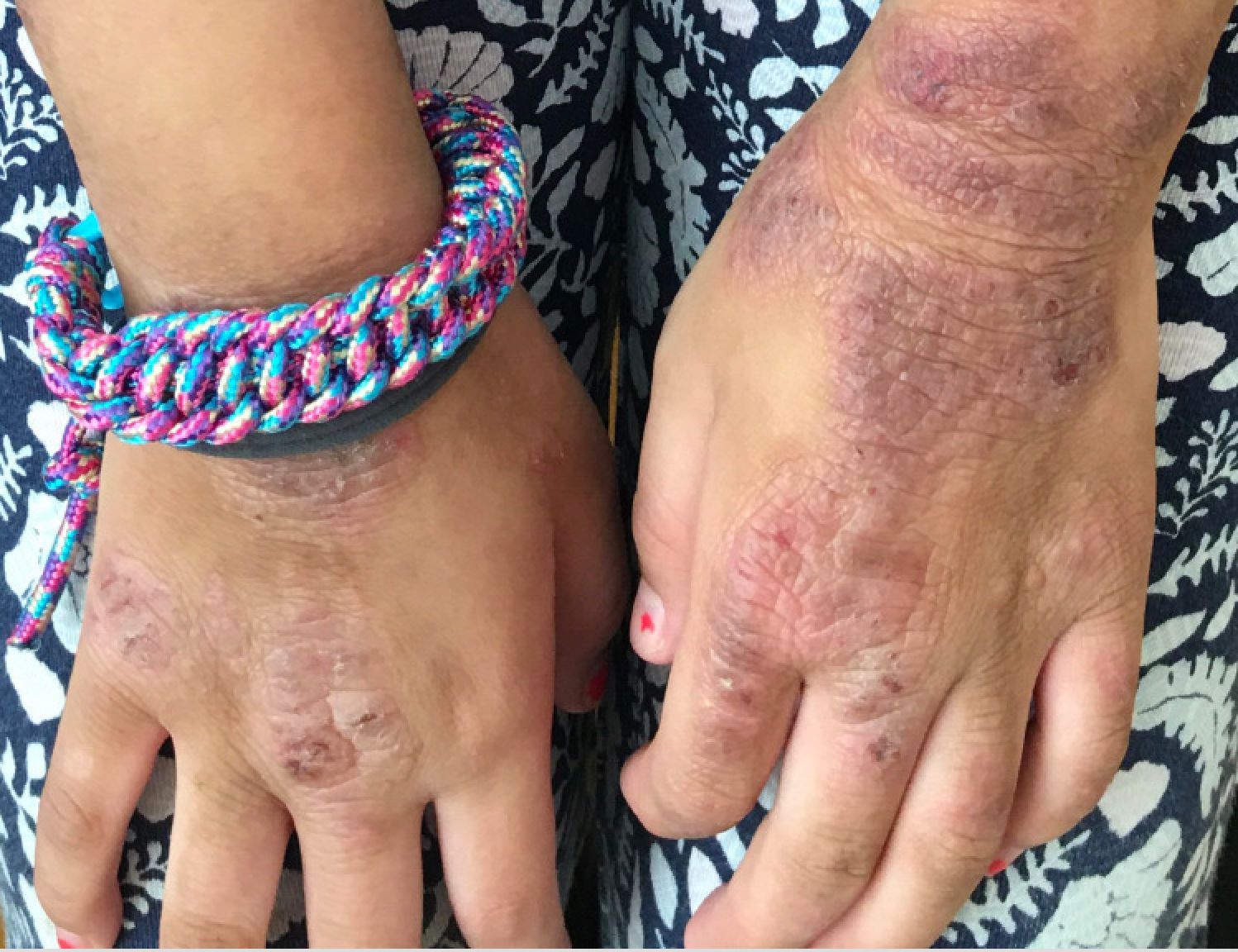
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Examination findings of the bilateral hands and wrists demonstrate plaques of erythema, lichenification, and scale of the dorsal surfaces of the hands and digits. Closer inspection reveals fissuring and erythematous crust of the affected skin but normal nails. The rest of the skin exam is unremarkable.
Update: U.S. regulators question AstraZeneca vaccine trial data
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
How to talk to patients reluctant to get a COVID-19 vaccine
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
2021 match sets records: Who matched and who didn’t?
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
Price transparency comes to medicine
There is a Chinese curse which says “May he live in interesting times.” Like it or not, we live in interesting times. They are times of danger and uncertainty; but they are also more open to the creative energy of men than any other time in history.
–Robert Kennedy, Cape Town, South Africa, 1966
Well, you may not know it, but price transparency is coming to medicine, including dermatology. . It has survived a challenge by the American Hospital Association in federal court, which generally means it is going to “stick.” Its effects should start to appear on Jan. 1, 2022.
The newly finalized rule will require insurers to publicly disclose in-network provider-negotiated rates, historical out-of-network allowed amounts, associated facility fees, and drug-pricing information in easily accessible machine-readable files. This information will be disclosed for the 500 most commonly billed physician services starting Jan. 1, 2022, and expanded to include all services the following year. Understand that you, as a practitioner, do not have to do anything, as insurers will do it for you, but your charge data will be on display. It is not clear if there is an appeal mechanism for physicians to correct erroneous data.
This should provide a fascinating look at just what things really cost, and may prove, as we suspect, small practices are less expensive. Important exemptions to reporting include emergency services, anesthesia, lab tests, and pathology fees, which will not be required, but recommended, to be disclosed.
Bear in mind that this rule was not designed to benefit physicians or hospitals, but rather to allow patients to comparison shop and drive down the cost of medical care. True price transparency may well accomplish this, particularly in our age of sky-high deductibles, if the information is accurate and readily accessible.
Although studies of patient behavior have shown that few patients actually use price comparison tools, the data required to be publicly disclosed and accessible will make this much easier. The Wall Street Journal or ProPublica will likely be all over this with applications to make comparisons easier. Still, many patients are price insensitive, particularly if they are Medicare recipients and only responsible for a nominal deductible.
Almost all the evaluation and management codes, as well as many dermatology procedure codes, are listed in the top 500 items and services included in the initial stage of the finalized rule. These include skin biopsies, destructions, drainages, several different benign and malignant excisions and, of course, Mohs surgery (but only the first stage, the 2nd stage will be listed in 2023).
While it is unlikely for patients to doctor shop for services that are performed on the same day as the office visit, such as a biopsies or destructions, we would expect comparisons for more expensive, planned procedures such as Mohs surgery and cancer excisions. Considering the rule, Mohs surgery may compare favorably to excisions performed in the hospital if the operating room charges are included, but not so well if the pathology and anesthesia charges are not included in the cost. It is inherently unfair to compare Mohs to excision in an operating room since the Mohs procedure has the anesthesia and pathology work embedded in the code (at 55% of the value of the code), and the multiple frozen sections taken by the surgeon in the operating room will not be listed as they are technically considered to be exempt additional pathology services.
This could put the Mohs surgeon in the interesting position of billing for excisions and frozen sections instead of Mohs surgery in order to compete with the hospital-based surgeon. This is not unbundling, if overall charges are lower and if distinctly different procedures are followed and different paperwork is generated. This is how I currently handle patients who demand Mohs surgery for inappropriate sites.
The effect on hospital groups that can charge facility fees could be quite dramatic, as it could be on large groups and on private equity groups who may have negotiated better rates. These increased costs will be revealed to consumers. In January 2023, the insurers will have to deploy a tool on their web site, updated monthly, that details rates for the 500 most common procedures for all in- and out-of-network providers and how much the patient can expect to pay out of pocket. All facility fees for procedures will be included. As noted earlier, we would expect third parties to already have done this. The historical and current costs for medications will also be included, which should make for interesting times in the pharmaceutical industry.
In January 2024, insurers will be required to post all the additional codes they cover, including complex closures, flaps, and grafts and any associated facility fees. Of course, a patient or a surgeon does not know what sort of repair a patient will need after Mohs surgery, but with high deductibles hitting harder, we would expect more patients requesting healing by second intent.
Whether these price comparisons will drive patients from relatively high-cost centers to less costly ones is unclear. This has certainly been the case for MRI and CT imaging. Price transparency for MRIs increased use of less costly providers and triggered provider competition.
Whether the price differentials will allow smaller practices some leverage in negotiating rates is also uncertain. Who knows, perhaps the out-of-network rate is greater than what your contract currently specifies, which could spur you to drop their network entirely. There may be great opportunity here for the smaller practitioner who has been boxed out of the big-group pricing and networks.
Be prepared in January 2022, to discuss these issues with patients and insurers, and be sure to check where you fall in cost comparisons. What possible logic could an insurer have for excluding you from a network where your average charges are less than their current panel? As noted before, this may be a boon for small practices that have been forced to the fringes of reimbursement and an opportunity to demonstrate that they are really much less expensive. We live in interesting times.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Bishop is doing a fellowship in micrographic surgery and dermatologic oncology with Dr. Coldiron at the Skin Cancer Center in Cincinnati. Write to Dr. Coldiron at [email protected].
There is a Chinese curse which says “May he live in interesting times.” Like it or not, we live in interesting times. They are times of danger and uncertainty; but they are also more open to the creative energy of men than any other time in history.
–Robert Kennedy, Cape Town, South Africa, 1966
Well, you may not know it, but price transparency is coming to medicine, including dermatology. . It has survived a challenge by the American Hospital Association in federal court, which generally means it is going to “stick.” Its effects should start to appear on Jan. 1, 2022.
The newly finalized rule will require insurers to publicly disclose in-network provider-negotiated rates, historical out-of-network allowed amounts, associated facility fees, and drug-pricing information in easily accessible machine-readable files. This information will be disclosed for the 500 most commonly billed physician services starting Jan. 1, 2022, and expanded to include all services the following year. Understand that you, as a practitioner, do not have to do anything, as insurers will do it for you, but your charge data will be on display. It is not clear if there is an appeal mechanism for physicians to correct erroneous data.
This should provide a fascinating look at just what things really cost, and may prove, as we suspect, small practices are less expensive. Important exemptions to reporting include emergency services, anesthesia, lab tests, and pathology fees, which will not be required, but recommended, to be disclosed.
Bear in mind that this rule was not designed to benefit physicians or hospitals, but rather to allow patients to comparison shop and drive down the cost of medical care. True price transparency may well accomplish this, particularly in our age of sky-high deductibles, if the information is accurate and readily accessible.
Although studies of patient behavior have shown that few patients actually use price comparison tools, the data required to be publicly disclosed and accessible will make this much easier. The Wall Street Journal or ProPublica will likely be all over this with applications to make comparisons easier. Still, many patients are price insensitive, particularly if they are Medicare recipients and only responsible for a nominal deductible.
Almost all the evaluation and management codes, as well as many dermatology procedure codes, are listed in the top 500 items and services included in the initial stage of the finalized rule. These include skin biopsies, destructions, drainages, several different benign and malignant excisions and, of course, Mohs surgery (but only the first stage, the 2nd stage will be listed in 2023).
While it is unlikely for patients to doctor shop for services that are performed on the same day as the office visit, such as a biopsies or destructions, we would expect comparisons for more expensive, planned procedures such as Mohs surgery and cancer excisions. Considering the rule, Mohs surgery may compare favorably to excisions performed in the hospital if the operating room charges are included, but not so well if the pathology and anesthesia charges are not included in the cost. It is inherently unfair to compare Mohs to excision in an operating room since the Mohs procedure has the anesthesia and pathology work embedded in the code (at 55% of the value of the code), and the multiple frozen sections taken by the surgeon in the operating room will not be listed as they are technically considered to be exempt additional pathology services.
This could put the Mohs surgeon in the interesting position of billing for excisions and frozen sections instead of Mohs surgery in order to compete with the hospital-based surgeon. This is not unbundling, if overall charges are lower and if distinctly different procedures are followed and different paperwork is generated. This is how I currently handle patients who demand Mohs surgery for inappropriate sites.
The effect on hospital groups that can charge facility fees could be quite dramatic, as it could be on large groups and on private equity groups who may have negotiated better rates. These increased costs will be revealed to consumers. In January 2023, the insurers will have to deploy a tool on their web site, updated monthly, that details rates for the 500 most common procedures for all in- and out-of-network providers and how much the patient can expect to pay out of pocket. All facility fees for procedures will be included. As noted earlier, we would expect third parties to already have done this. The historical and current costs for medications will also be included, which should make for interesting times in the pharmaceutical industry.
In January 2024, insurers will be required to post all the additional codes they cover, including complex closures, flaps, and grafts and any associated facility fees. Of course, a patient or a surgeon does not know what sort of repair a patient will need after Mohs surgery, but with high deductibles hitting harder, we would expect more patients requesting healing by second intent.
Whether these price comparisons will drive patients from relatively high-cost centers to less costly ones is unclear. This has certainly been the case for MRI and CT imaging. Price transparency for MRIs increased use of less costly providers and triggered provider competition.
Whether the price differentials will allow smaller practices some leverage in negotiating rates is also uncertain. Who knows, perhaps the out-of-network rate is greater than what your contract currently specifies, which could spur you to drop their network entirely. There may be great opportunity here for the smaller practitioner who has been boxed out of the big-group pricing and networks.
Be prepared in January 2022, to discuss these issues with patients and insurers, and be sure to check where you fall in cost comparisons. What possible logic could an insurer have for excluding you from a network where your average charges are less than their current panel? As noted before, this may be a boon for small practices that have been forced to the fringes of reimbursement and an opportunity to demonstrate that they are really much less expensive. We live in interesting times.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Bishop is doing a fellowship in micrographic surgery and dermatologic oncology with Dr. Coldiron at the Skin Cancer Center in Cincinnati. Write to Dr. Coldiron at [email protected].
There is a Chinese curse which says “May he live in interesting times.” Like it or not, we live in interesting times. They are times of danger and uncertainty; but they are also more open to the creative energy of men than any other time in history.
–Robert Kennedy, Cape Town, South Africa, 1966
Well, you may not know it, but price transparency is coming to medicine, including dermatology. . It has survived a challenge by the American Hospital Association in federal court, which generally means it is going to “stick.” Its effects should start to appear on Jan. 1, 2022.
The newly finalized rule will require insurers to publicly disclose in-network provider-negotiated rates, historical out-of-network allowed amounts, associated facility fees, and drug-pricing information in easily accessible machine-readable files. This information will be disclosed for the 500 most commonly billed physician services starting Jan. 1, 2022, and expanded to include all services the following year. Understand that you, as a practitioner, do not have to do anything, as insurers will do it for you, but your charge data will be on display. It is not clear if there is an appeal mechanism for physicians to correct erroneous data.
This should provide a fascinating look at just what things really cost, and may prove, as we suspect, small practices are less expensive. Important exemptions to reporting include emergency services, anesthesia, lab tests, and pathology fees, which will not be required, but recommended, to be disclosed.
Bear in mind that this rule was not designed to benefit physicians or hospitals, but rather to allow patients to comparison shop and drive down the cost of medical care. True price transparency may well accomplish this, particularly in our age of sky-high deductibles, if the information is accurate and readily accessible.
Although studies of patient behavior have shown that few patients actually use price comparison tools, the data required to be publicly disclosed and accessible will make this much easier. The Wall Street Journal or ProPublica will likely be all over this with applications to make comparisons easier. Still, many patients are price insensitive, particularly if they are Medicare recipients and only responsible for a nominal deductible.
Almost all the evaluation and management codes, as well as many dermatology procedure codes, are listed in the top 500 items and services included in the initial stage of the finalized rule. These include skin biopsies, destructions, drainages, several different benign and malignant excisions and, of course, Mohs surgery (but only the first stage, the 2nd stage will be listed in 2023).
While it is unlikely for patients to doctor shop for services that are performed on the same day as the office visit, such as a biopsies or destructions, we would expect comparisons for more expensive, planned procedures such as Mohs surgery and cancer excisions. Considering the rule, Mohs surgery may compare favorably to excisions performed in the hospital if the operating room charges are included, but not so well if the pathology and anesthesia charges are not included in the cost. It is inherently unfair to compare Mohs to excision in an operating room since the Mohs procedure has the anesthesia and pathology work embedded in the code (at 55% of the value of the code), and the multiple frozen sections taken by the surgeon in the operating room will not be listed as they are technically considered to be exempt additional pathology services.
This could put the Mohs surgeon in the interesting position of billing for excisions and frozen sections instead of Mohs surgery in order to compete with the hospital-based surgeon. This is not unbundling, if overall charges are lower and if distinctly different procedures are followed and different paperwork is generated. This is how I currently handle patients who demand Mohs surgery for inappropriate sites.
The effect on hospital groups that can charge facility fees could be quite dramatic, as it could be on large groups and on private equity groups who may have negotiated better rates. These increased costs will be revealed to consumers. In January 2023, the insurers will have to deploy a tool on their web site, updated monthly, that details rates for the 500 most common procedures for all in- and out-of-network providers and how much the patient can expect to pay out of pocket. All facility fees for procedures will be included. As noted earlier, we would expect third parties to already have done this. The historical and current costs for medications will also be included, which should make for interesting times in the pharmaceutical industry.
In January 2024, insurers will be required to post all the additional codes they cover, including complex closures, flaps, and grafts and any associated facility fees. Of course, a patient or a surgeon does not know what sort of repair a patient will need after Mohs surgery, but with high deductibles hitting harder, we would expect more patients requesting healing by second intent.
Whether these price comparisons will drive patients from relatively high-cost centers to less costly ones is unclear. This has certainly been the case for MRI and CT imaging. Price transparency for MRIs increased use of less costly providers and triggered provider competition.
Whether the price differentials will allow smaller practices some leverage in negotiating rates is also uncertain. Who knows, perhaps the out-of-network rate is greater than what your contract currently specifies, which could spur you to drop their network entirely. There may be great opportunity here for the smaller practitioner who has been boxed out of the big-group pricing and networks.
Be prepared in January 2022, to discuss these issues with patients and insurers, and be sure to check where you fall in cost comparisons. What possible logic could an insurer have for excluding you from a network where your average charges are less than their current panel? As noted before, this may be a boon for small practices that have been forced to the fringes of reimbursement and an opportunity to demonstrate that they are really much less expensive. We live in interesting times.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Bishop is doing a fellowship in micrographic surgery and dermatologic oncology with Dr. Coldiron at the Skin Cancer Center in Cincinnati. Write to Dr. Coldiron at [email protected].