User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
U.S. tops 500,000 COVID-19 cases in children
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
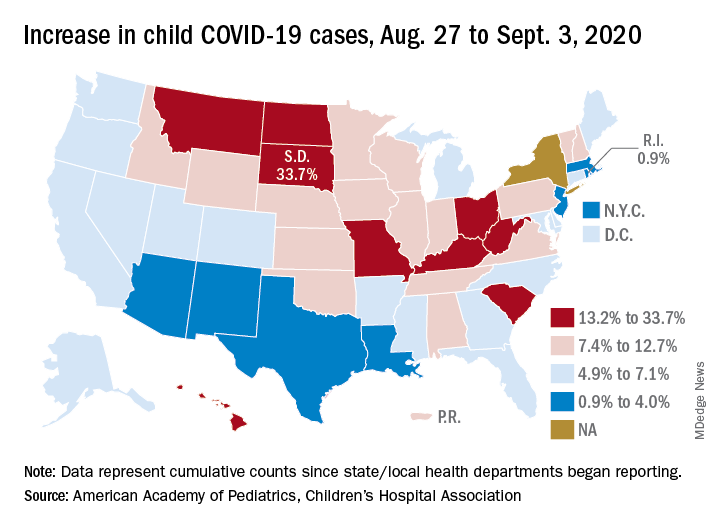
States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
Five reasons why medical meetings will never be the same
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
Deaths sky high in hospitalized COVID patients with kidney injury
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
Could these old drugs help fight COVID-19 and save lives?
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Unexpected results in new COVID-19 ‘cytokine storm’ data
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
HHS plan to improve rural health focuses on better broadband, telehealth services
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Novel smart needle system designed to reduce risk of filler complications
In the very near future, clinicians injecting
That is the goal of an experienced team composed of leading clinicians, academics, and researchers developing S3 Inject, a first-in-class safety innovation that has entered human trials.
“When physicians inject the fillers, they hope experience and technique will enable them to avoid adverse events,” Irina Erenburg, PhD, said during the virtual annual Masters of Aesthetics Symposium. “If they inadvertently hit a blood vessel, the filler can actually occlude that vessel and cause either an infarct of the skin or, in certain serious cases, blindness. This is a challenging adverse event that every injector is focused on avoiding. While hyaluronidase is used as a rescue [medication] in certain cases, the risk is real,” she added.
Vision abnormalities, including blindness, and necrosis are among the adverse events associated with dermal fillers that have been reported to the Food and Drug Administration.
S3 Inject is a sensing needle that can differentiate tissues such as fat, blood vessels, and muscle. Its proprietary algorithms provide immediate feedback via a micro LED light embedded in the needle hub. Results from recent human trials demonstrate that, as the needle tip passes through different biological tissues and fluids, “it senses changes in specific electrical properties and with that information sends a very precise signal to the needle hub,” said Dr. Erenburg, CEO and President of Waltham, Mass.–based Blossom Innovations, a company focused on developing early stage medical devices in dermatology. “With that information, the physician can make real-time treatment decisions.”
Currently, in order to determine if the needle is in a blood vessel, physicians pull back on the syringe and look for a flash of blood. “In speaking with physicians, the pull back technique has limitations, in part, because filler in the syringe can limit easy pull back to check the presence of a blood vessel,” she said. “Our needles provide an immediate response for a safer injection.”
Blossom Innovations has developed a proprietary manufacturing process that will initially target 27 gauge needles, but over time it plans to introduce multiple sizes, as well as cannulas.
“The physicians in our industry are committed to patient safety and they’re looking for better outcomes with a solution that does not impact their technique,” said Dr. Erenburg, who founded Blossom Innovations along with R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston; Dieter Manstein, MD, PhD, also at Massachusetts General Hospital; and Henry H.L. Chan, MD, PhD, of the Hong Kong Dermatology and Laser Center. During market research for S3 Inject, which was conducted with 15 leading injectors, thought leaders, and trend makers, the country’s leading injectors expressed strong interest in “solutions that allow them to provide additional safety for their patients and provide personal reassurance to the physician,” she said. “They definitely would want to train all their physicians and injectors on its use.”
As clinical testing continues, the company is preparing to submit data to the FDA’s Premarket Notification program, known as the 510(k) process. “Our intent is to create a scale-up manufacturing over the course of the coming year in time for our clearance, with a planned launch at the end of 2021,” Dr. Erenburg said. “Based on our clinical research and physician discussions, we are confident that S3 Inject is a breakthrough safety technology which will drive a better outcome for patients.”
Dr. Erenburg is an employee of Blossom Innovations.
In the very near future, clinicians injecting
That is the goal of an experienced team composed of leading clinicians, academics, and researchers developing S3 Inject, a first-in-class safety innovation that has entered human trials.
“When physicians inject the fillers, they hope experience and technique will enable them to avoid adverse events,” Irina Erenburg, PhD, said during the virtual annual Masters of Aesthetics Symposium. “If they inadvertently hit a blood vessel, the filler can actually occlude that vessel and cause either an infarct of the skin or, in certain serious cases, blindness. This is a challenging adverse event that every injector is focused on avoiding. While hyaluronidase is used as a rescue [medication] in certain cases, the risk is real,” she added.
Vision abnormalities, including blindness, and necrosis are among the adverse events associated with dermal fillers that have been reported to the Food and Drug Administration.
S3 Inject is a sensing needle that can differentiate tissues such as fat, blood vessels, and muscle. Its proprietary algorithms provide immediate feedback via a micro LED light embedded in the needle hub. Results from recent human trials demonstrate that, as the needle tip passes through different biological tissues and fluids, “it senses changes in specific electrical properties and with that information sends a very precise signal to the needle hub,” said Dr. Erenburg, CEO and President of Waltham, Mass.–based Blossom Innovations, a company focused on developing early stage medical devices in dermatology. “With that information, the physician can make real-time treatment decisions.”
Currently, in order to determine if the needle is in a blood vessel, physicians pull back on the syringe and look for a flash of blood. “In speaking with physicians, the pull back technique has limitations, in part, because filler in the syringe can limit easy pull back to check the presence of a blood vessel,” she said. “Our needles provide an immediate response for a safer injection.”
Blossom Innovations has developed a proprietary manufacturing process that will initially target 27 gauge needles, but over time it plans to introduce multiple sizes, as well as cannulas.
“The physicians in our industry are committed to patient safety and they’re looking for better outcomes with a solution that does not impact their technique,” said Dr. Erenburg, who founded Blossom Innovations along with R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston; Dieter Manstein, MD, PhD, also at Massachusetts General Hospital; and Henry H.L. Chan, MD, PhD, of the Hong Kong Dermatology and Laser Center. During market research for S3 Inject, which was conducted with 15 leading injectors, thought leaders, and trend makers, the country’s leading injectors expressed strong interest in “solutions that allow them to provide additional safety for their patients and provide personal reassurance to the physician,” she said. “They definitely would want to train all their physicians and injectors on its use.”
As clinical testing continues, the company is preparing to submit data to the FDA’s Premarket Notification program, known as the 510(k) process. “Our intent is to create a scale-up manufacturing over the course of the coming year in time for our clearance, with a planned launch at the end of 2021,” Dr. Erenburg said. “Based on our clinical research and physician discussions, we are confident that S3 Inject is a breakthrough safety technology which will drive a better outcome for patients.”
Dr. Erenburg is an employee of Blossom Innovations.
In the very near future, clinicians injecting
That is the goal of an experienced team composed of leading clinicians, academics, and researchers developing S3 Inject, a first-in-class safety innovation that has entered human trials.
“When physicians inject the fillers, they hope experience and technique will enable them to avoid adverse events,” Irina Erenburg, PhD, said during the virtual annual Masters of Aesthetics Symposium. “If they inadvertently hit a blood vessel, the filler can actually occlude that vessel and cause either an infarct of the skin or, in certain serious cases, blindness. This is a challenging adverse event that every injector is focused on avoiding. While hyaluronidase is used as a rescue [medication] in certain cases, the risk is real,” she added.
Vision abnormalities, including blindness, and necrosis are among the adverse events associated with dermal fillers that have been reported to the Food and Drug Administration.
S3 Inject is a sensing needle that can differentiate tissues such as fat, blood vessels, and muscle. Its proprietary algorithms provide immediate feedback via a micro LED light embedded in the needle hub. Results from recent human trials demonstrate that, as the needle tip passes through different biological tissues and fluids, “it senses changes in specific electrical properties and with that information sends a very precise signal to the needle hub,” said Dr. Erenburg, CEO and President of Waltham, Mass.–based Blossom Innovations, a company focused on developing early stage medical devices in dermatology. “With that information, the physician can make real-time treatment decisions.”
Currently, in order to determine if the needle is in a blood vessel, physicians pull back on the syringe and look for a flash of blood. “In speaking with physicians, the pull back technique has limitations, in part, because filler in the syringe can limit easy pull back to check the presence of a blood vessel,” she said. “Our needles provide an immediate response for a safer injection.”
Blossom Innovations has developed a proprietary manufacturing process that will initially target 27 gauge needles, but over time it plans to introduce multiple sizes, as well as cannulas.
“The physicians in our industry are committed to patient safety and they’re looking for better outcomes with a solution that does not impact their technique,” said Dr. Erenburg, who founded Blossom Innovations along with R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston; Dieter Manstein, MD, PhD, also at Massachusetts General Hospital; and Henry H.L. Chan, MD, PhD, of the Hong Kong Dermatology and Laser Center. During market research for S3 Inject, which was conducted with 15 leading injectors, thought leaders, and trend makers, the country’s leading injectors expressed strong interest in “solutions that allow them to provide additional safety for their patients and provide personal reassurance to the physician,” she said. “They definitely would want to train all their physicians and injectors on its use.”
As clinical testing continues, the company is preparing to submit data to the FDA’s Premarket Notification program, known as the 510(k) process. “Our intent is to create a scale-up manufacturing over the course of the coming year in time for our clearance, with a planned launch at the end of 2021,” Dr. Erenburg said. “Based on our clinical research and physician discussions, we are confident that S3 Inject is a breakthrough safety technology which will drive a better outcome for patients.”
Dr. Erenburg is an employee of Blossom Innovations.
REPORTING FROM MOA 2020
Study: 10% of pregnant women test positive for COVID-19, with most asymptomatic
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
FROM BMJ
Petition seeks to oust AAD president-elect, citing private equity conflicts
A make him too conflicted for the job.
The petition, created anonymously, appeared in early August and is seeking 1,500 signatures – calculated as the number needed to initiate a vote to recall Mark Kaufmann, MD. Dr. Kaufmann was voted president-elect of the AAD and its sister organization, the AAD Association, in April. He takes the position in 2021 and would become president in 2022.
The petition objects to the fact that Dr. Kaufmann is the chief medical officer of Advanced Dermatology and Cosmetic Surgery (ADCS), the nation’s largest private equity–backed dermatology group. It claims that he took the position in July, after being elected, which it portrays as dishonest.
The petition said Dr. Kaufmann “will have concurrent fiduciary responsibilities to both the AAD and a corporation whose interests may or may not be aligned with the AAD.” Even if Dr. Kaufmann chose to recuse himself from some decisions, that would be a disservice to the AAD, said the petition, which added that he “cannot serve two masters simultaneously.”
Several efforts were made to reach Dr. Kaufmann for a comment, with no responses at press time. At press time, ACDS had not responded to a request for comment.
In response to questions, Bruce H. Thiers, MD, president of the AAD/A, said that the organization has no policy barring officers from holding capital in a private equity (PE)–backed corporation, but that any elected officer is subject to the organization’s “fiduciary duties and obligations.”
The AAD/A shared a 7-page administrative regulation on disclosure of outside interests and management of conflicts of interest. The policy notes that employment by a private equity group is considered a potential conflict of interest, but so is employment by a government agency, solo practice, academic medical center, or multiple other models. Key leaders, including the president, president-elect, and immediate past president, are required to divest themselves of any direct financial relationships with industry during their entire term.
Members who do not disclose potential conflicts each year and who fail to update their disclosure within 30 days of acquiring a new financial relationship “will lose the right to hold office, serve in the governance structure and, except in unusual circumstances approved in advance by the Board of Directors, to participate in Academy programs,” according to the policy.
Petitioners lament conflicts, private equity
At press time, the petition had more than 1,100 signatures. Most who signed indicated in their comments that they believed that Dr. Kaufmann’s association with private equity disqualifies him.
“I believe private equity should not have a place at the table of the governing body of the AAD,” commented Cynthia Abbott, MD, an Atlanta dermatologist. In his comment, Ron Birnbaum, MD a Los Angeles–based dermatologist, wrote, “I oppose the movement to PE-based purchase and consolidation of practices,” adding that he had not voted for Dr. Kaufmann in April. Dr. Kaufmann “might resolve the conflict of interest by resigning from the job of CMO of ADCS,” wrote Dr. Birnbaum.
“I have great respect for Dr. Kaufmann but am very troubled by the influence of PE infiltration into dermatology,” Mark Gaughan, MD, a dermatologist in Durango, Colo., wrote in the petition.
The petition seeks 1,500 signatures – which is 10% of the AAD membership. That’s key because, as per AAD administrative rules, members can initiate the removal of a board member through a petition that is signed by 10% of voting members or by a two-thirds vote of entire board of directors.
Dr. Thiers said the AAD/A’s “administrative regulations do not cover the removal of an elected officer prior to the start of his or her term.” Thus, it’s not even clear that Dr. Kaufmann could be recalled before he took office in 2021.
Dr. Kaufmann previously served on the AAD/A board of directors for 4 years and is currently deputy chair of the AAD/A’s Patient Access and Payer Relations Committee. He is chair of the AAD’s Workgroup on Innovation in Payment and Delivery Actinic Keratosis Alternative Payment Model Development and is a member of numerous other AAD/A committees, including: the AAD budget committee; the AAD appointment selection committee; the Resource-Based Relative Value Scale and Current Procedural Terminology committee; the work group on innovation in payment and delivery; the work group on ICD-10; and the work group on rapid response team Medicare Physician Fee Schedule.
Since March 2019, Dr. Kaufmann has also served as the chief medical officer of Florham Park, N.J.–based MoleSafe, a company that provides full-body skin cancer screening directly to patients.
In an interview, former AAD president Daniel Siegel, MD, said that he believes the petition is an unwarranted attack. “Mark is an honest upstanding individual who has, in the almost 2 decades I’ve known and worked with him, always put the interests of the specialty and the AAD ahead of any personal interests,” said Dr. Siegel of the department of dermatology at State University of New York, Brooklyn. Dr. Siegel also disclosed that he is chief compliance officer for the Florida-based Skin and Cancer Associates, a large group of practices owned by private equity.
Dr. Kaufmann is “an objective person who has no difficulty declaring a conflict where there is one,” Dr. Siegel said.
But Sailesh Konda, MD, a critic of private equity in dermatology, who signed the petition, said in an interview that he believed the “membership is rightfully concerned and responding to these new conflicts of interest with a valid petition.”
“This is readily apparent with a petition signed by more than 1,000 dermatologists,” added Dr. Konda of the department of dermatology at the University of Florida in Gainesville.
It might be hard to erase all potential conflicts at the AAD. Some members work for industry, some for academia, and some for government agencies – all of which come with their own biases.
But the AAD will not publicly release disclosures for members, board members, or officers. Those disclosures are compiled in an internal AAD database, but the organization leaves it up to each individual to decide what they will make public.
Second anonymous conflict petition
It is not clear what will happen with the Dr. Kaufmann recall effort, but it is not the first time that a petition has taken aim at an AAD official. An October 2019 petition – also submitted by an anonymous dermatologist – sought to remove Scott M. Dinehart, MD, from the AAD board.
The petition noted Dr. Dinehart’s involvement in creating the American Dermatology Board of Physician Assistants, a group that aims to offer board certification to PAs who work with dermatologists. Dr. Dinehart’s “concurrent relationships with both organizations is a major conflict of interest,” said the petition, which garnered some 4,200 signatures – far in excess of what is required to initiate a removal vote.
That led to a unanimous decision by the AAD/A board to present the membership with a resolution to remove Dr. Dinehart. Some 6,400 votes were cast, with 97% approving Dr. Dinehart’s removal.
He is no longer a member of the AAD/A board.
A make him too conflicted for the job.
The petition, created anonymously, appeared in early August and is seeking 1,500 signatures – calculated as the number needed to initiate a vote to recall Mark Kaufmann, MD. Dr. Kaufmann was voted president-elect of the AAD and its sister organization, the AAD Association, in April. He takes the position in 2021 and would become president in 2022.
The petition objects to the fact that Dr. Kaufmann is the chief medical officer of Advanced Dermatology and Cosmetic Surgery (ADCS), the nation’s largest private equity–backed dermatology group. It claims that he took the position in July, after being elected, which it portrays as dishonest.
The petition said Dr. Kaufmann “will have concurrent fiduciary responsibilities to both the AAD and a corporation whose interests may or may not be aligned with the AAD.” Even if Dr. Kaufmann chose to recuse himself from some decisions, that would be a disservice to the AAD, said the petition, which added that he “cannot serve two masters simultaneously.”
Several efforts were made to reach Dr. Kaufmann for a comment, with no responses at press time. At press time, ACDS had not responded to a request for comment.
In response to questions, Bruce H. Thiers, MD, president of the AAD/A, said that the organization has no policy barring officers from holding capital in a private equity (PE)–backed corporation, but that any elected officer is subject to the organization’s “fiduciary duties and obligations.”
The AAD/A shared a 7-page administrative regulation on disclosure of outside interests and management of conflicts of interest. The policy notes that employment by a private equity group is considered a potential conflict of interest, but so is employment by a government agency, solo practice, academic medical center, or multiple other models. Key leaders, including the president, president-elect, and immediate past president, are required to divest themselves of any direct financial relationships with industry during their entire term.
Members who do not disclose potential conflicts each year and who fail to update their disclosure within 30 days of acquiring a new financial relationship “will lose the right to hold office, serve in the governance structure and, except in unusual circumstances approved in advance by the Board of Directors, to participate in Academy programs,” according to the policy.
Petitioners lament conflicts, private equity
At press time, the petition had more than 1,100 signatures. Most who signed indicated in their comments that they believed that Dr. Kaufmann’s association with private equity disqualifies him.
“I believe private equity should not have a place at the table of the governing body of the AAD,” commented Cynthia Abbott, MD, an Atlanta dermatologist. In his comment, Ron Birnbaum, MD a Los Angeles–based dermatologist, wrote, “I oppose the movement to PE-based purchase and consolidation of practices,” adding that he had not voted for Dr. Kaufmann in April. Dr. Kaufmann “might resolve the conflict of interest by resigning from the job of CMO of ADCS,” wrote Dr. Birnbaum.
“I have great respect for Dr. Kaufmann but am very troubled by the influence of PE infiltration into dermatology,” Mark Gaughan, MD, a dermatologist in Durango, Colo., wrote in the petition.
The petition seeks 1,500 signatures – which is 10% of the AAD membership. That’s key because, as per AAD administrative rules, members can initiate the removal of a board member through a petition that is signed by 10% of voting members or by a two-thirds vote of entire board of directors.
Dr. Thiers said the AAD/A’s “administrative regulations do not cover the removal of an elected officer prior to the start of his or her term.” Thus, it’s not even clear that Dr. Kaufmann could be recalled before he took office in 2021.
Dr. Kaufmann previously served on the AAD/A board of directors for 4 years and is currently deputy chair of the AAD/A’s Patient Access and Payer Relations Committee. He is chair of the AAD’s Workgroup on Innovation in Payment and Delivery Actinic Keratosis Alternative Payment Model Development and is a member of numerous other AAD/A committees, including: the AAD budget committee; the AAD appointment selection committee; the Resource-Based Relative Value Scale and Current Procedural Terminology committee; the work group on innovation in payment and delivery; the work group on ICD-10; and the work group on rapid response team Medicare Physician Fee Schedule.
Since March 2019, Dr. Kaufmann has also served as the chief medical officer of Florham Park, N.J.–based MoleSafe, a company that provides full-body skin cancer screening directly to patients.
In an interview, former AAD president Daniel Siegel, MD, said that he believes the petition is an unwarranted attack. “Mark is an honest upstanding individual who has, in the almost 2 decades I’ve known and worked with him, always put the interests of the specialty and the AAD ahead of any personal interests,” said Dr. Siegel of the department of dermatology at State University of New York, Brooklyn. Dr. Siegel also disclosed that he is chief compliance officer for the Florida-based Skin and Cancer Associates, a large group of practices owned by private equity.
Dr. Kaufmann is “an objective person who has no difficulty declaring a conflict where there is one,” Dr. Siegel said.
But Sailesh Konda, MD, a critic of private equity in dermatology, who signed the petition, said in an interview that he believed the “membership is rightfully concerned and responding to these new conflicts of interest with a valid petition.”
“This is readily apparent with a petition signed by more than 1,000 dermatologists,” added Dr. Konda of the department of dermatology at the University of Florida in Gainesville.
It might be hard to erase all potential conflicts at the AAD. Some members work for industry, some for academia, and some for government agencies – all of which come with their own biases.
But the AAD will not publicly release disclosures for members, board members, or officers. Those disclosures are compiled in an internal AAD database, but the organization leaves it up to each individual to decide what they will make public.
Second anonymous conflict petition
It is not clear what will happen with the Dr. Kaufmann recall effort, but it is not the first time that a petition has taken aim at an AAD official. An October 2019 petition – also submitted by an anonymous dermatologist – sought to remove Scott M. Dinehart, MD, from the AAD board.
The petition noted Dr. Dinehart’s involvement in creating the American Dermatology Board of Physician Assistants, a group that aims to offer board certification to PAs who work with dermatologists. Dr. Dinehart’s “concurrent relationships with both organizations is a major conflict of interest,” said the petition, which garnered some 4,200 signatures – far in excess of what is required to initiate a removal vote.
That led to a unanimous decision by the AAD/A board to present the membership with a resolution to remove Dr. Dinehart. Some 6,400 votes were cast, with 97% approving Dr. Dinehart’s removal.
He is no longer a member of the AAD/A board.
A make him too conflicted for the job.
The petition, created anonymously, appeared in early August and is seeking 1,500 signatures – calculated as the number needed to initiate a vote to recall Mark Kaufmann, MD. Dr. Kaufmann was voted president-elect of the AAD and its sister organization, the AAD Association, in April. He takes the position in 2021 and would become president in 2022.
The petition objects to the fact that Dr. Kaufmann is the chief medical officer of Advanced Dermatology and Cosmetic Surgery (ADCS), the nation’s largest private equity–backed dermatology group. It claims that he took the position in July, after being elected, which it portrays as dishonest.
The petition said Dr. Kaufmann “will have concurrent fiduciary responsibilities to both the AAD and a corporation whose interests may or may not be aligned with the AAD.” Even if Dr. Kaufmann chose to recuse himself from some decisions, that would be a disservice to the AAD, said the petition, which added that he “cannot serve two masters simultaneously.”
Several efforts were made to reach Dr. Kaufmann for a comment, with no responses at press time. At press time, ACDS had not responded to a request for comment.
In response to questions, Bruce H. Thiers, MD, president of the AAD/A, said that the organization has no policy barring officers from holding capital in a private equity (PE)–backed corporation, but that any elected officer is subject to the organization’s “fiduciary duties and obligations.”
The AAD/A shared a 7-page administrative regulation on disclosure of outside interests and management of conflicts of interest. The policy notes that employment by a private equity group is considered a potential conflict of interest, but so is employment by a government agency, solo practice, academic medical center, or multiple other models. Key leaders, including the president, president-elect, and immediate past president, are required to divest themselves of any direct financial relationships with industry during their entire term.
Members who do not disclose potential conflicts each year and who fail to update their disclosure within 30 days of acquiring a new financial relationship “will lose the right to hold office, serve in the governance structure and, except in unusual circumstances approved in advance by the Board of Directors, to participate in Academy programs,” according to the policy.
Petitioners lament conflicts, private equity
At press time, the petition had more than 1,100 signatures. Most who signed indicated in their comments that they believed that Dr. Kaufmann’s association with private equity disqualifies him.
“I believe private equity should not have a place at the table of the governing body of the AAD,” commented Cynthia Abbott, MD, an Atlanta dermatologist. In his comment, Ron Birnbaum, MD a Los Angeles–based dermatologist, wrote, “I oppose the movement to PE-based purchase and consolidation of practices,” adding that he had not voted for Dr. Kaufmann in April. Dr. Kaufmann “might resolve the conflict of interest by resigning from the job of CMO of ADCS,” wrote Dr. Birnbaum.
“I have great respect for Dr. Kaufmann but am very troubled by the influence of PE infiltration into dermatology,” Mark Gaughan, MD, a dermatologist in Durango, Colo., wrote in the petition.
The petition seeks 1,500 signatures – which is 10% of the AAD membership. That’s key because, as per AAD administrative rules, members can initiate the removal of a board member through a petition that is signed by 10% of voting members or by a two-thirds vote of entire board of directors.
Dr. Thiers said the AAD/A’s “administrative regulations do not cover the removal of an elected officer prior to the start of his or her term.” Thus, it’s not even clear that Dr. Kaufmann could be recalled before he took office in 2021.
Dr. Kaufmann previously served on the AAD/A board of directors for 4 years and is currently deputy chair of the AAD/A’s Patient Access and Payer Relations Committee. He is chair of the AAD’s Workgroup on Innovation in Payment and Delivery Actinic Keratosis Alternative Payment Model Development and is a member of numerous other AAD/A committees, including: the AAD budget committee; the AAD appointment selection committee; the Resource-Based Relative Value Scale and Current Procedural Terminology committee; the work group on innovation in payment and delivery; the work group on ICD-10; and the work group on rapid response team Medicare Physician Fee Schedule.
Since March 2019, Dr. Kaufmann has also served as the chief medical officer of Florham Park, N.J.–based MoleSafe, a company that provides full-body skin cancer screening directly to patients.
In an interview, former AAD president Daniel Siegel, MD, said that he believes the petition is an unwarranted attack. “Mark is an honest upstanding individual who has, in the almost 2 decades I’ve known and worked with him, always put the interests of the specialty and the AAD ahead of any personal interests,” said Dr. Siegel of the department of dermatology at State University of New York, Brooklyn. Dr. Siegel also disclosed that he is chief compliance officer for the Florida-based Skin and Cancer Associates, a large group of practices owned by private equity.
Dr. Kaufmann is “an objective person who has no difficulty declaring a conflict where there is one,” Dr. Siegel said.
But Sailesh Konda, MD, a critic of private equity in dermatology, who signed the petition, said in an interview that he believed the “membership is rightfully concerned and responding to these new conflicts of interest with a valid petition.”
“This is readily apparent with a petition signed by more than 1,000 dermatologists,” added Dr. Konda of the department of dermatology at the University of Florida in Gainesville.
It might be hard to erase all potential conflicts at the AAD. Some members work for industry, some for academia, and some for government agencies – all of which come with their own biases.
But the AAD will not publicly release disclosures for members, board members, or officers. Those disclosures are compiled in an internal AAD database, but the organization leaves it up to each individual to decide what they will make public.
Second anonymous conflict petition
It is not clear what will happen with the Dr. Kaufmann recall effort, but it is not the first time that a petition has taken aim at an AAD official. An October 2019 petition – also submitted by an anonymous dermatologist – sought to remove Scott M. Dinehart, MD, from the AAD board.
The petition noted Dr. Dinehart’s involvement in creating the American Dermatology Board of Physician Assistants, a group that aims to offer board certification to PAs who work with dermatologists. Dr. Dinehart’s “concurrent relationships with both organizations is a major conflict of interest,” said the petition, which garnered some 4,200 signatures – far in excess of what is required to initiate a removal vote.
That led to a unanimous decision by the AAD/A board to present the membership with a resolution to remove Dr. Dinehart. Some 6,400 votes were cast, with 97% approving Dr. Dinehart’s removal.
He is no longer a member of the AAD/A board.
Ten ways docs are cutting costs and saving money
“Some of our physician clients have seen their income decrease by as much as 50%,” says Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minnesota. “Many physicians had previously figured that whatever financial obligations they had wouldn’t be a problem because whatever amount they were making would continue, and if there were a decline it would be gradual.” However, assumption is now creating financial strain for many doctors.
Vikram Tarugu, MD, a gastroenterologist and CEO of Detox of South Florida in Okeechobee, Florida, says he has watched his budget for years, but has become even more careful with his spending in the past few months.
“It has helped me a lot to adjust to the new normal when it comes to the financial side of things,” Dr. Tarugu said. “Patients aren’t coming in as much as they used to, so my income has really been affected.”
Primary care physicians have seen a 55% decrease in revenue and a 20%-30% decrease in patient volume as a result of COVID-19. The impact has been even more severe for specialists. Even for physicians whose practices remain busy and whose family members haven’t lost their jobs or income, broader concerns about the economy may be reason enough for physicians to adopt cost-cutting measures.
In Medscape’s Physician Compensation Report 2020, we asked physicians to share their best cost-cutting tips. Many illustrate the lengths to which physicians are going to conserve money.
Here’s a look at some of the advice they shared, along with guidance from experts on how to make it work for you:
1. Create a written budget, even if you think it’s pointless.
Physicians said their most important piece of advice includes the following: “Use a formal budget to track progress,” “write out a budget,” “plan intermittent/large expenses in advance,” “Make sure all expenses are paid before you spend on leisure.”
Nearly 7 in 10 physicians say they have a budget for personal expenses, yet only one-quarter of those who do have a formal, written budget. Writing out a spending plan is key to being intentional about your spending, making sure that you’re living within your means, and identifying areas in which you may be able to cut back.
“Financial planning is all about cash flow, and everybody should know the amount of money coming in, how much is going out, and the difference between the two,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “That’s important in good times, but it’s even more important now when we see physicians taking pay cuts.”
Many physicians have found that budget apps or software programs are easier to work with than anticipated; some even walk you through the process of creating a budget. To get the most out of the apps, you’ll need to check them regularly and make changes based on their data.
“Sometimes there’s this false belief that just by signing up, you are automatically going to be better at budgeting,” says Scott Snider, CFP, a certified financial planner and partner with Mellen Money Management in Ponte Vedra Beach, Florida. “Basically, these apps are a great way to identify problem areas of spending. We have a tendency as humans to underestimate how much we spend on things like Starbucks, dining out, and Amazon shopping.”
One of the doctors’ tips that requires the most willpower is to “pay all expenses before spending on leisure.” That’s because we live in an instant gratification world, and want everything right away, Ms. Guerich said.
“I also think there’s an element of ‘keeping up with the Joneses’ and pressure associated with this profession,” she said. “The stereotype is that physicians are high-income earners so ‘We should be able to do that’ or ‘Mom and dad are doctors, so they can afford it.’ “
Creating and then revisiting your budget progress on a monthly or quarterly basis can give you a feeling of accomplishment and keep you motivated to stay with it.
Keep in mind that budgeting is a continual process rather than a singular event, and you’ll likely adjust it over time as your income and goals change.
2. Save more as you earn more.
Respondents to our Physician Compensation Report gave the following recommendations: “Pay yourself first,” “I put half of my bonus into an investment account no matter how much it is,” “I allocate extra money and put it into a savings account.”
Dr. Greenwald said, “I have a rule that every client needs to be saving 20% of their gross income toward retirement, including whatever the employer is putting in.”
Putting a portion of every paycheck into savings is key to making progress toward financial goals. Start by building an emergency fund with at least 3-6 months’ worth of expenses in it and making sure you’re saving at least enough for retirement to get any potential employer match.
Mr. Snider suggests increasing the percentage you save every time you get a raise.
“The thought behind that strategy is that when a doctor receives a pay raise – even if it’s just a cost-of-living raise of 3% – an extra 1% saved doesn’t reduce their take-home pay year-over-year,” he says. “In fact, they still take home more money, and they save more money. Win-win.”
3. Focus on paying down your debt.
Physicians told us how they were working to pay down debt with the following recommendations: “Accelerate debt reduction,” “I make additional principal payment to our home mortgage,” “We are aggressively attacking our remaining student loans.”
Reducing or eliminating debt is key to increasing cash flow, which can make it easier to meet all of your other financial goals. One-quarter of physicians have credit card debt, which typically carries interest rates higher than other types of debt, making it far more expensive. Focus on paying off such high-interest debt first, before moving on to other types of debt such as auto loans, student loans, or a mortgage.
“Credit card debt and any unsecured debt should be paid before anything else,” Mr. Snider says. “Getting rid of those high interest rates should be a priority. And that type of debt has less flexible terms than student debt.”
4. Great opportunity to take advantage of record-low interest rates.
Physicians said that, to save money, they are recommending the following: “Consolidating student debt into our mortgage,” “Accelerating payments of the principle on our mortgage,” “Making sure we have an affordable mortgage.”
With interest rates at an all-time low, even those who’ve recently refinanced might see significant savings by refinancing again. Given the associated fees, it typically makes sense to refinance if you can reduce your mortgage rate by at least a point, and you’re planning to stay in the home for at least 5 years.
“Depending on how much lower your rate is, refinancing can make a big difference in your monthly payments,” Ms. Guerich said. “For physicians who might need an emergency reserve but don’t have cash on hand, a HELOC [Home Equity Line of Credit] is a great way to accomplish that.”
5. Be wary of credit cards dangers; use cards wisely.
Physician respondents recommended the following: “Use 0% interest offers on credit cards,” “Only have one card and pay it off every month,” “Never carry over balance.”
Nearly 80% of physicians have three or more credit cards, with 18% reporting that they have seven or more. When used wisely, credit cards can be an important tool for managing finances. Many credit cards come with tools that can help with budgeting, and credit cards rewards and perks can offer real value to users. That said, rewards typically are not valuable enough to offset the cost of interest on balances (or the associated damage to your credit score) that aren’t paid off each month.
“If you’re paying a high rate on credit card balances that carry over every month, regardless of your income, that could be a symptom that you may be spending more than you should,” says Dan Keady, a CFP and chief financial planning strategist at financial services firm TIAA.
6. Give less to Uncle Sam: Keep it for yourself.
Physicians said that they do the following: “Maximize tax-free/deferred savings (401k, HSA, etc.),” “Give to charity to reduce tax,” “Use pre-tax dollars for childcare and healthcare.”
Not only does saving in workplace retirement accounts help you build your nest egg, but it also reduces the amount that you have to pay in taxes in a given year. Physicians should also take advantage of other ways to reduce their income for tax purposes, such as saving money in a health savings account or flexible savings account.
The 401(k) or 403(b) contribution limit for this year is $19,500 ($26,000) for those age 50 years and older. Self-employed physicians can save even more money via a Simplified Employee Pension (SEP) IRA, says Ms. Guerich said. They can save up to 25% of compensation, up to $57,000 in 2020.
7. Automate everything and spare yourself the headache.
Physicians said the following: “Designate money from your paycheck directly to tax deferred and taxable accounts automatically,” “Use automatic payment for credit card balance monthly,” “Automate your savings.”
You probably already automate your 401(k) contributions, but you can also automate bill payments, emergency savings contributions and other financial tasks. For busy physicians, this can make it easier to stick to your financial plan and achieve your goals.
“The older you get, the busier you get, said Mr. Snider says. “Automation can definitely help with that. But make sure you are checking in quarterly to make sure that everything is still in line with your plan. The problem with automation is when you forget about it completely and just let everything sit there.”
8. Save separately for big purchases.
Sometimes it’s the big major expenses that can start to derail a budget. Physicians told us the following tactics for large purchases: “We buy affordable cars and take budget vacations,” “I buy used cars,” “We save in advance for new cars and only buy cars with cash.”
The decision of which car to purchase or where to go on a family vacation is a personal one, and some physicians take great enjoyment and pride in driving a luxury vehicle or traveling to exotic locales. The key, experts say, is to factor the cost of that car into the rest of your budget, and make sure that it’s not preventing you from achieving other financial goals.
“I don’t like to judge or tell clients how they should spend their money,” said Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “Some people like cars, we have clients that have two planes, others want a second house or like to travel. Each person has their own interest where they may spend more relative to other people, but as long as they are meeting their savings targets, I encourage them to spend their money and enjoy what they enjoy most, guilt free.”
Mr. Snider suggests setting up a savings account separate from emergency or retirement accounts to set aside money if you have a goal for a large future purchase, such as a boat or a second home.
“That way, the funds don’t get commingled, and it’s explicitly clear whether or not the doctor is on target,” he says. “It also prevents them from treating their emergency savings account as an ATM machine.”
9. Start saving for college when the kids are little.
Respondents said the following: “We are buying less to save for the kids’ college education,” “We set up direct deposit into college and retirement savings plans,” “We have a 529 account for college savings.”
Helping pay for their children’s college education is an important financial goal for many physicians. The earlier that you start saving, the less you’ll have to save overall, thanks to compound interest. State 529 accounts are often a good place to start, especially if your state offers a tax incentive for doing so.
Mr. Snider recommends that physicians start small, with an initial investment of $1,000 per month and $100 per month contributions. Assuming a 7% rate of return and 17 years’ worth of savings, this would generate just over $42,000. (Note, current typical rates of return are less than 7%).
“Ideally, as other goals are accomplished and personal debt gets paid off, the doctor is ramping up their savings to have at least 50% of college expenses covered from their 529 college savings,” he says.
10. Watch out for the temptation of impulse purchases.
Physicians said the following: “Avoid impulse purchases,” “Avoid impulse shopping, make a list for the store and stick to it,” “Wait to buy things on sale.”
Nothing wrecks a budget like an impulse buy. More than half (54%) of U.S. shoppers have admitted to spending $100 or more on an impulse purchase. And 20% of shoppers have spent at least $1,000 on an impulse buy. Avoid buyers’ remorse by waiting a few days to make large purchase decisions or by limiting your unplanned spending to a certain dollar amount within your budget.
Online shopping may be a particular temptation. Dr. Tarugu, the Florida gastroenterologist, has focused on reducing those impulse buys as well, deleting all online shopping apps from his and his family’s phones.
“You won’t notice how much you have ordered online until it arrives at your doorstep,” he said. “It’s really important to keep it at bay.”
Mr. Keady, the TIAA chief planning strategist, recommended this tactic: Calculate the number of patients (or hours) you’d need to see in order to earn the cash required to make the purchase.
“Then, in a mindful way, figure out the amount of value derived from the purchase,” he said.
A version of this article originally appeared on Medscape.com.
“Some of our physician clients have seen their income decrease by as much as 50%,” says Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minnesota. “Many physicians had previously figured that whatever financial obligations they had wouldn’t be a problem because whatever amount they were making would continue, and if there were a decline it would be gradual.” However, assumption is now creating financial strain for many doctors.
Vikram Tarugu, MD, a gastroenterologist and CEO of Detox of South Florida in Okeechobee, Florida, says he has watched his budget for years, but has become even more careful with his spending in the past few months.
“It has helped me a lot to adjust to the new normal when it comes to the financial side of things,” Dr. Tarugu said. “Patients aren’t coming in as much as they used to, so my income has really been affected.”
Primary care physicians have seen a 55% decrease in revenue and a 20%-30% decrease in patient volume as a result of COVID-19. The impact has been even more severe for specialists. Even for physicians whose practices remain busy and whose family members haven’t lost their jobs or income, broader concerns about the economy may be reason enough for physicians to adopt cost-cutting measures.
In Medscape’s Physician Compensation Report 2020, we asked physicians to share their best cost-cutting tips. Many illustrate the lengths to which physicians are going to conserve money.
Here’s a look at some of the advice they shared, along with guidance from experts on how to make it work for you:
1. Create a written budget, even if you think it’s pointless.
Physicians said their most important piece of advice includes the following: “Use a formal budget to track progress,” “write out a budget,” “plan intermittent/large expenses in advance,” “Make sure all expenses are paid before you spend on leisure.”
Nearly 7 in 10 physicians say they have a budget for personal expenses, yet only one-quarter of those who do have a formal, written budget. Writing out a spending plan is key to being intentional about your spending, making sure that you’re living within your means, and identifying areas in which you may be able to cut back.
“Financial planning is all about cash flow, and everybody should know the amount of money coming in, how much is going out, and the difference between the two,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “That’s important in good times, but it’s even more important now when we see physicians taking pay cuts.”
Many physicians have found that budget apps or software programs are easier to work with than anticipated; some even walk you through the process of creating a budget. To get the most out of the apps, you’ll need to check them regularly and make changes based on their data.
“Sometimes there’s this false belief that just by signing up, you are automatically going to be better at budgeting,” says Scott Snider, CFP, a certified financial planner and partner with Mellen Money Management in Ponte Vedra Beach, Florida. “Basically, these apps are a great way to identify problem areas of spending. We have a tendency as humans to underestimate how much we spend on things like Starbucks, dining out, and Amazon shopping.”
One of the doctors’ tips that requires the most willpower is to “pay all expenses before spending on leisure.” That’s because we live in an instant gratification world, and want everything right away, Ms. Guerich said.
“I also think there’s an element of ‘keeping up with the Joneses’ and pressure associated with this profession,” she said. “The stereotype is that physicians are high-income earners so ‘We should be able to do that’ or ‘Mom and dad are doctors, so they can afford it.’ “
Creating and then revisiting your budget progress on a monthly or quarterly basis can give you a feeling of accomplishment and keep you motivated to stay with it.
Keep in mind that budgeting is a continual process rather than a singular event, and you’ll likely adjust it over time as your income and goals change.
2. Save more as you earn more.
Respondents to our Physician Compensation Report gave the following recommendations: “Pay yourself first,” “I put half of my bonus into an investment account no matter how much it is,” “I allocate extra money and put it into a savings account.”
Dr. Greenwald said, “I have a rule that every client needs to be saving 20% of their gross income toward retirement, including whatever the employer is putting in.”
Putting a portion of every paycheck into savings is key to making progress toward financial goals. Start by building an emergency fund with at least 3-6 months’ worth of expenses in it and making sure you’re saving at least enough for retirement to get any potential employer match.
Mr. Snider suggests increasing the percentage you save every time you get a raise.
“The thought behind that strategy is that when a doctor receives a pay raise – even if it’s just a cost-of-living raise of 3% – an extra 1% saved doesn’t reduce their take-home pay year-over-year,” he says. “In fact, they still take home more money, and they save more money. Win-win.”
3. Focus on paying down your debt.
Physicians told us how they were working to pay down debt with the following recommendations: “Accelerate debt reduction,” “I make additional principal payment to our home mortgage,” “We are aggressively attacking our remaining student loans.”
Reducing or eliminating debt is key to increasing cash flow, which can make it easier to meet all of your other financial goals. One-quarter of physicians have credit card debt, which typically carries interest rates higher than other types of debt, making it far more expensive. Focus on paying off such high-interest debt first, before moving on to other types of debt such as auto loans, student loans, or a mortgage.
“Credit card debt and any unsecured debt should be paid before anything else,” Mr. Snider says. “Getting rid of those high interest rates should be a priority. And that type of debt has less flexible terms than student debt.”
4. Great opportunity to take advantage of record-low interest rates.
Physicians said that, to save money, they are recommending the following: “Consolidating student debt into our mortgage,” “Accelerating payments of the principle on our mortgage,” “Making sure we have an affordable mortgage.”
With interest rates at an all-time low, even those who’ve recently refinanced might see significant savings by refinancing again. Given the associated fees, it typically makes sense to refinance if you can reduce your mortgage rate by at least a point, and you’re planning to stay in the home for at least 5 years.
“Depending on how much lower your rate is, refinancing can make a big difference in your monthly payments,” Ms. Guerich said. “For physicians who might need an emergency reserve but don’t have cash on hand, a HELOC [Home Equity Line of Credit] is a great way to accomplish that.”
5. Be wary of credit cards dangers; use cards wisely.
Physician respondents recommended the following: “Use 0% interest offers on credit cards,” “Only have one card and pay it off every month,” “Never carry over balance.”
Nearly 80% of physicians have three or more credit cards, with 18% reporting that they have seven or more. When used wisely, credit cards can be an important tool for managing finances. Many credit cards come with tools that can help with budgeting, and credit cards rewards and perks can offer real value to users. That said, rewards typically are not valuable enough to offset the cost of interest on balances (or the associated damage to your credit score) that aren’t paid off each month.
“If you’re paying a high rate on credit card balances that carry over every month, regardless of your income, that could be a symptom that you may be spending more than you should,” says Dan Keady, a CFP and chief financial planning strategist at financial services firm TIAA.
6. Give less to Uncle Sam: Keep it for yourself.
Physicians said that they do the following: “Maximize tax-free/deferred savings (401k, HSA, etc.),” “Give to charity to reduce tax,” “Use pre-tax dollars for childcare and healthcare.”
Not only does saving in workplace retirement accounts help you build your nest egg, but it also reduces the amount that you have to pay in taxes in a given year. Physicians should also take advantage of other ways to reduce their income for tax purposes, such as saving money in a health savings account or flexible savings account.
The 401(k) or 403(b) contribution limit for this year is $19,500 ($26,000) for those age 50 years and older. Self-employed physicians can save even more money via a Simplified Employee Pension (SEP) IRA, says Ms. Guerich said. They can save up to 25% of compensation, up to $57,000 in 2020.
7. Automate everything and spare yourself the headache.
Physicians said the following: “Designate money from your paycheck directly to tax deferred and taxable accounts automatically,” “Use automatic payment for credit card balance monthly,” “Automate your savings.”
You probably already automate your 401(k) contributions, but you can also automate bill payments, emergency savings contributions and other financial tasks. For busy physicians, this can make it easier to stick to your financial plan and achieve your goals.
“The older you get, the busier you get, said Mr. Snider says. “Automation can definitely help with that. But make sure you are checking in quarterly to make sure that everything is still in line with your plan. The problem with automation is when you forget about it completely and just let everything sit there.”
8. Save separately for big purchases.
Sometimes it’s the big major expenses that can start to derail a budget. Physicians told us the following tactics for large purchases: “We buy affordable cars and take budget vacations,” “I buy used cars,” “We save in advance for new cars and only buy cars with cash.”
The decision of which car to purchase or where to go on a family vacation is a personal one, and some physicians take great enjoyment and pride in driving a luxury vehicle or traveling to exotic locales. The key, experts say, is to factor the cost of that car into the rest of your budget, and make sure that it’s not preventing you from achieving other financial goals.
“I don’t like to judge or tell clients how they should spend their money,” said Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “Some people like cars, we have clients that have two planes, others want a second house or like to travel. Each person has their own interest where they may spend more relative to other people, but as long as they are meeting their savings targets, I encourage them to spend their money and enjoy what they enjoy most, guilt free.”
Mr. Snider suggests setting up a savings account separate from emergency or retirement accounts to set aside money if you have a goal for a large future purchase, such as a boat or a second home.
“That way, the funds don’t get commingled, and it’s explicitly clear whether or not the doctor is on target,” he says. “It also prevents them from treating their emergency savings account as an ATM machine.”
9. Start saving for college when the kids are little.
Respondents said the following: “We are buying less to save for the kids’ college education,” “We set up direct deposit into college and retirement savings plans,” “We have a 529 account for college savings.”
Helping pay for their children’s college education is an important financial goal for many physicians. The earlier that you start saving, the less you’ll have to save overall, thanks to compound interest. State 529 accounts are often a good place to start, especially if your state offers a tax incentive for doing so.
Mr. Snider recommends that physicians start small, with an initial investment of $1,000 per month and $100 per month contributions. Assuming a 7% rate of return and 17 years’ worth of savings, this would generate just over $42,000. (Note, current typical rates of return are less than 7%).
“Ideally, as other goals are accomplished and personal debt gets paid off, the doctor is ramping up their savings to have at least 50% of college expenses covered from their 529 college savings,” he says.
10. Watch out for the temptation of impulse purchases.
Physicians said the following: “Avoid impulse purchases,” “Avoid impulse shopping, make a list for the store and stick to it,” “Wait to buy things on sale.”
Nothing wrecks a budget like an impulse buy. More than half (54%) of U.S. shoppers have admitted to spending $100 or more on an impulse purchase. And 20% of shoppers have spent at least $1,000 on an impulse buy. Avoid buyers’ remorse by waiting a few days to make large purchase decisions or by limiting your unplanned spending to a certain dollar amount within your budget.
Online shopping may be a particular temptation. Dr. Tarugu, the Florida gastroenterologist, has focused on reducing those impulse buys as well, deleting all online shopping apps from his and his family’s phones.
“You won’t notice how much you have ordered online until it arrives at your doorstep,” he said. “It’s really important to keep it at bay.”
Mr. Keady, the TIAA chief planning strategist, recommended this tactic: Calculate the number of patients (or hours) you’d need to see in order to earn the cash required to make the purchase.
“Then, in a mindful way, figure out the amount of value derived from the purchase,” he said.
A version of this article originally appeared on Medscape.com.
“Some of our physician clients have seen their income decrease by as much as 50%,” says Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minnesota. “Many physicians had previously figured that whatever financial obligations they had wouldn’t be a problem because whatever amount they were making would continue, and if there were a decline it would be gradual.” However, assumption is now creating financial strain for many doctors.
Vikram Tarugu, MD, a gastroenterologist and CEO of Detox of South Florida in Okeechobee, Florida, says he has watched his budget for years, but has become even more careful with his spending in the past few months.
“It has helped me a lot to adjust to the new normal when it comes to the financial side of things,” Dr. Tarugu said. “Patients aren’t coming in as much as they used to, so my income has really been affected.”
Primary care physicians have seen a 55% decrease in revenue and a 20%-30% decrease in patient volume as a result of COVID-19. The impact has been even more severe for specialists. Even for physicians whose practices remain busy and whose family members haven’t lost their jobs or income, broader concerns about the economy may be reason enough for physicians to adopt cost-cutting measures.
In Medscape’s Physician Compensation Report 2020, we asked physicians to share their best cost-cutting tips. Many illustrate the lengths to which physicians are going to conserve money.
Here’s a look at some of the advice they shared, along with guidance from experts on how to make it work for you:
1. Create a written budget, even if you think it’s pointless.
Physicians said their most important piece of advice includes the following: “Use a formal budget to track progress,” “write out a budget,” “plan intermittent/large expenses in advance,” “Make sure all expenses are paid before you spend on leisure.”
Nearly 7 in 10 physicians say they have a budget for personal expenses, yet only one-quarter of those who do have a formal, written budget. Writing out a spending plan is key to being intentional about your spending, making sure that you’re living within your means, and identifying areas in which you may be able to cut back.
“Financial planning is all about cash flow, and everybody should know the amount of money coming in, how much is going out, and the difference between the two,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “That’s important in good times, but it’s even more important now when we see physicians taking pay cuts.”
Many physicians have found that budget apps or software programs are easier to work with than anticipated; some even walk you through the process of creating a budget. To get the most out of the apps, you’ll need to check them regularly and make changes based on their data.
“Sometimes there’s this false belief that just by signing up, you are automatically going to be better at budgeting,” says Scott Snider, CFP, a certified financial planner and partner with Mellen Money Management in Ponte Vedra Beach, Florida. “Basically, these apps are a great way to identify problem areas of spending. We have a tendency as humans to underestimate how much we spend on things like Starbucks, dining out, and Amazon shopping.”
One of the doctors’ tips that requires the most willpower is to “pay all expenses before spending on leisure.” That’s because we live in an instant gratification world, and want everything right away, Ms. Guerich said.
“I also think there’s an element of ‘keeping up with the Joneses’ and pressure associated with this profession,” she said. “The stereotype is that physicians are high-income earners so ‘We should be able to do that’ or ‘Mom and dad are doctors, so they can afford it.’ “
Creating and then revisiting your budget progress on a monthly or quarterly basis can give you a feeling of accomplishment and keep you motivated to stay with it.
Keep in mind that budgeting is a continual process rather than a singular event, and you’ll likely adjust it over time as your income and goals change.
2. Save more as you earn more.
Respondents to our Physician Compensation Report gave the following recommendations: “Pay yourself first,” “I put half of my bonus into an investment account no matter how much it is,” “I allocate extra money and put it into a savings account.”
Dr. Greenwald said, “I have a rule that every client needs to be saving 20% of their gross income toward retirement, including whatever the employer is putting in.”
Putting a portion of every paycheck into savings is key to making progress toward financial goals. Start by building an emergency fund with at least 3-6 months’ worth of expenses in it and making sure you’re saving at least enough for retirement to get any potential employer match.
Mr. Snider suggests increasing the percentage you save every time you get a raise.
“The thought behind that strategy is that when a doctor receives a pay raise – even if it’s just a cost-of-living raise of 3% – an extra 1% saved doesn’t reduce their take-home pay year-over-year,” he says. “In fact, they still take home more money, and they save more money. Win-win.”
3. Focus on paying down your debt.
Physicians told us how they were working to pay down debt with the following recommendations: “Accelerate debt reduction,” “I make additional principal payment to our home mortgage,” “We are aggressively attacking our remaining student loans.”
Reducing or eliminating debt is key to increasing cash flow, which can make it easier to meet all of your other financial goals. One-quarter of physicians have credit card debt, which typically carries interest rates higher than other types of debt, making it far more expensive. Focus on paying off such high-interest debt first, before moving on to other types of debt such as auto loans, student loans, or a mortgage.
“Credit card debt and any unsecured debt should be paid before anything else,” Mr. Snider says. “Getting rid of those high interest rates should be a priority. And that type of debt has less flexible terms than student debt.”
4. Great opportunity to take advantage of record-low interest rates.
Physicians said that, to save money, they are recommending the following: “Consolidating student debt into our mortgage,” “Accelerating payments of the principle on our mortgage,” “Making sure we have an affordable mortgage.”
With interest rates at an all-time low, even those who’ve recently refinanced might see significant savings by refinancing again. Given the associated fees, it typically makes sense to refinance if you can reduce your mortgage rate by at least a point, and you’re planning to stay in the home for at least 5 years.
“Depending on how much lower your rate is, refinancing can make a big difference in your monthly payments,” Ms. Guerich said. “For physicians who might need an emergency reserve but don’t have cash on hand, a HELOC [Home Equity Line of Credit] is a great way to accomplish that.”
5. Be wary of credit cards dangers; use cards wisely.
Physician respondents recommended the following: “Use 0% interest offers on credit cards,” “Only have one card and pay it off every month,” “Never carry over balance.”
Nearly 80% of physicians have three or more credit cards, with 18% reporting that they have seven or more. When used wisely, credit cards can be an important tool for managing finances. Many credit cards come with tools that can help with budgeting, and credit cards rewards and perks can offer real value to users. That said, rewards typically are not valuable enough to offset the cost of interest on balances (or the associated damage to your credit score) that aren’t paid off each month.
“If you’re paying a high rate on credit card balances that carry over every month, regardless of your income, that could be a symptom that you may be spending more than you should,” says Dan Keady, a CFP and chief financial planning strategist at financial services firm TIAA.
6. Give less to Uncle Sam: Keep it for yourself.
Physicians said that they do the following: “Maximize tax-free/deferred savings (401k, HSA, etc.),” “Give to charity to reduce tax,” “Use pre-tax dollars for childcare and healthcare.”
Not only does saving in workplace retirement accounts help you build your nest egg, but it also reduces the amount that you have to pay in taxes in a given year. Physicians should also take advantage of other ways to reduce their income for tax purposes, such as saving money in a health savings account or flexible savings account.
The 401(k) or 403(b) contribution limit for this year is $19,500 ($26,000) for those age 50 years and older. Self-employed physicians can save even more money via a Simplified Employee Pension (SEP) IRA, says Ms. Guerich said. They can save up to 25% of compensation, up to $57,000 in 2020.
7. Automate everything and spare yourself the headache.
Physicians said the following: “Designate money from your paycheck directly to tax deferred and taxable accounts automatically,” “Use automatic payment for credit card balance monthly,” “Automate your savings.”
You probably already automate your 401(k) contributions, but you can also automate bill payments, emergency savings contributions and other financial tasks. For busy physicians, this can make it easier to stick to your financial plan and achieve your goals.
“The older you get, the busier you get, said Mr. Snider says. “Automation can definitely help with that. But make sure you are checking in quarterly to make sure that everything is still in line with your plan. The problem with automation is when you forget about it completely and just let everything sit there.”
8. Save separately for big purchases.
Sometimes it’s the big major expenses that can start to derail a budget. Physicians told us the following tactics for large purchases: “We buy affordable cars and take budget vacations,” “I buy used cars,” “We save in advance for new cars and only buy cars with cash.”
The decision of which car to purchase or where to go on a family vacation is a personal one, and some physicians take great enjoyment and pride in driving a luxury vehicle or traveling to exotic locales. The key, experts say, is to factor the cost of that car into the rest of your budget, and make sure that it’s not preventing you from achieving other financial goals.
“I don’t like to judge or tell clients how they should spend their money,” said Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “Some people like cars, we have clients that have two planes, others want a second house or like to travel. Each person has their own interest where they may spend more relative to other people, but as long as they are meeting their savings targets, I encourage them to spend their money and enjoy what they enjoy most, guilt free.”
Mr. Snider suggests setting up a savings account separate from emergency or retirement accounts to set aside money if you have a goal for a large future purchase, such as a boat or a second home.
“That way, the funds don’t get commingled, and it’s explicitly clear whether or not the doctor is on target,” he says. “It also prevents them from treating their emergency savings account as an ATM machine.”
9. Start saving for college when the kids are little.
Respondents said the following: “We are buying less to save for the kids’ college education,” “We set up direct deposit into college and retirement savings plans,” “We have a 529 account for college savings.”
Helping pay for their children’s college education is an important financial goal for many physicians. The earlier that you start saving, the less you’ll have to save overall, thanks to compound interest. State 529 accounts are often a good place to start, especially if your state offers a tax incentive for doing so.
Mr. Snider recommends that physicians start small, with an initial investment of $1,000 per month and $100 per month contributions. Assuming a 7% rate of return and 17 years’ worth of savings, this would generate just over $42,000. (Note, current typical rates of return are less than 7%).
“Ideally, as other goals are accomplished and personal debt gets paid off, the doctor is ramping up their savings to have at least 50% of college expenses covered from their 529 college savings,” he says.
10. Watch out for the temptation of impulse purchases.
Physicians said the following: “Avoid impulse purchases,” “Avoid impulse shopping, make a list for the store and stick to it,” “Wait to buy things on sale.”
Nothing wrecks a budget like an impulse buy. More than half (54%) of U.S. shoppers have admitted to spending $100 or more on an impulse purchase. And 20% of shoppers have spent at least $1,000 on an impulse buy. Avoid buyers’ remorse by waiting a few days to make large purchase decisions or by limiting your unplanned spending to a certain dollar amount within your budget.
Online shopping may be a particular temptation. Dr. Tarugu, the Florida gastroenterologist, has focused on reducing those impulse buys as well, deleting all online shopping apps from his and his family’s phones.
“You won’t notice how much you have ordered online until it arrives at your doorstep,” he said. “It’s really important to keep it at bay.”
Mr. Keady, the TIAA chief planning strategist, recommended this tactic: Calculate the number of patients (or hours) you’d need to see in order to earn the cash required to make the purchase.
“Then, in a mindful way, figure out the amount of value derived from the purchase,” he said.
A version of this article originally appeared on Medscape.com.