User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Ixekizumab deemed effective for pityriasis rubra pilaris
Teri Greiling, MD, PhD, said at the virtual annual meeting of the American Academy of Dermatology.
The interleukin-17A inhibitor induced long-term remission of pityriasis rubra pilaris (PRP) in 4 of the 11 participants in her open-label, single-arm, 24-week clinical trial of ixekizumab (Taltz) at the Food and Drug Administration–approved dosing for psoriasis. Of 11 patients, 7 experienced at least a 50% reduction in signs and symptoms of the disease. Marked quality-of-life improvements occurred as well, reported Dr. Greiling, a dermatologist at Oregon Health & Science University, Portland.
PRP is a rare papulosquamous disorder that’s challenging to diagnosis and often difficult to treat. Indeed, there is no FDA-approved treatment. The disorder is characterized by widespread follicular keratotic plaques with scale and notable islands of sparing, often with an accompanying waxy yellow palmoplantar keratoderma with fissuring. There are adult- and childhood-onset forms of PRP, as well as sporadic and familial subtypes. Some cases are associated with variants in the CARD14 gene, known to also play a role in familial psoriasis.
Patients with PRP say the disorder has a major adverse impact on their quality of life. Itching and pain are often prominent features.
The 11 study participants, drawn from throughout the United States, were adults with a mean 12-year history of symptoms. All were required to have both clinical and biopsy evidence of PRP. Five had classic adult-onset PRP, five had atypical adult-onset PRP, and one had classic juvenile-onset PRP. All had moderate or severe disease as reflected in a Physician’s Global Assessment score of 3 or 4 on the 0-4 scale. Of the 11, 10 had previously received various systemic therapies for their PRP without success.
Since there is as yet no established PRP severity grading tool, Dr. Greiling applied the principles of the Psoriasis Area and Severity Index (PASI). Participants had a mean baseline PASI score of 24.6, a Dermatology Life Quality Index (DLQI) score of 18, and itch and pain scores of 7 and 6, respectively, on a self-rated 10-point scale.
The primary outcome in the study was change in PASI score at week 24, which was 4 weeks after the final dose of ixekizumab. The mean score improved from 24.8 at week 0 to 9.7 at week 24. Five patients achieved at least a 75% decrease in PASI score, or PASI 75 response, and 2 had a PASI 90 response. The DLQI improved from 18 to 3, and both itch and pain scores dropped to a median of 1. There was no association between response to treatment and PRP clinical subtype.
The four top responders – those with a PASI 89 or better response at week 24 – remained clear or almost clear at week 36, fully 16 weeks after their last dose of ixekizumab. Patients with an intermediate week 24 response – that is, a 50%-85% reduction in PASI score – all relapsed before week 36. The patient with the worst PASI score at both baseline and 24 weeks decided to continue on ixekizumab dosed every 2 weeks independent of the study, rather than at the FDA-approved dosing every 4 weeks for psoriasis, with a resultant drop to a PASI score of 8 at week 36.
To look at mechanism of benefit, Dr. Greiling used quantitative polymerase chain reaction to examine key cytokine expression in the epidermis and dermis. Not surprisingly, IL-17A expression was markedly reduced at both sites, suggesting the importance of the Th17 axis in the pathophysiology of PRP. In contrast, there was no significant change in IL-23 expression.
No serious or unexpected adverse events occurred in the 24-week study.
“In terms of ixekizumab, compared to other treatments, I definitely think it is more effective than any conventional therapies, such as topical steroids, methotrexate, or acitretin,” she said in an interview.
Asked about other biologics, Dr. Greiling said she hasn’t found tumor necrosis factor inhibitors very helpful in her patients with PRP. A formal trial of the IL-17A inhibitor secukinumab (Cosentyx) has been done elsewhere, and although the results haven’t yet been published, her understanding is that the efficacy was similar to her ixekizumab trial.
“I’ve had some of my ixekizumab patients switch to secukinumab, for insurance reasons, though, and had it not be quite as effective, although still helpful,” she said.
Dr. Greiling is now enrolling patients with PRP in a trial of the IL-23 inhibitor guselkumab (Tremfya). It’s her early impression that this may prove to be another therapeutic option.
“I have not yet used brodalumab [Siliq], but I wonder if it would also be helpful, since it seems to have a stronger blockade, working on the IL-17 receptor A,” she said.
She cited two pressing needs that would advance PRP research: the lack of standard criteria for disease diagnosis and the absence of PRP-specific disease measurement tools. “We’re trying to remedy that,” the dermatologist said.
Her study was funded by Eli Lilly. She reported receiving research funding from that company and Janssen.
Teri Greiling, MD, PhD, said at the virtual annual meeting of the American Academy of Dermatology.
The interleukin-17A inhibitor induced long-term remission of pityriasis rubra pilaris (PRP) in 4 of the 11 participants in her open-label, single-arm, 24-week clinical trial of ixekizumab (Taltz) at the Food and Drug Administration–approved dosing for psoriasis. Of 11 patients, 7 experienced at least a 50% reduction in signs and symptoms of the disease. Marked quality-of-life improvements occurred as well, reported Dr. Greiling, a dermatologist at Oregon Health & Science University, Portland.
PRP is a rare papulosquamous disorder that’s challenging to diagnosis and often difficult to treat. Indeed, there is no FDA-approved treatment. The disorder is characterized by widespread follicular keratotic plaques with scale and notable islands of sparing, often with an accompanying waxy yellow palmoplantar keratoderma with fissuring. There are adult- and childhood-onset forms of PRP, as well as sporadic and familial subtypes. Some cases are associated with variants in the CARD14 gene, known to also play a role in familial psoriasis.
Patients with PRP say the disorder has a major adverse impact on their quality of life. Itching and pain are often prominent features.
The 11 study participants, drawn from throughout the United States, were adults with a mean 12-year history of symptoms. All were required to have both clinical and biopsy evidence of PRP. Five had classic adult-onset PRP, five had atypical adult-onset PRP, and one had classic juvenile-onset PRP. All had moderate or severe disease as reflected in a Physician’s Global Assessment score of 3 or 4 on the 0-4 scale. Of the 11, 10 had previously received various systemic therapies for their PRP without success.
Since there is as yet no established PRP severity grading tool, Dr. Greiling applied the principles of the Psoriasis Area and Severity Index (PASI). Participants had a mean baseline PASI score of 24.6, a Dermatology Life Quality Index (DLQI) score of 18, and itch and pain scores of 7 and 6, respectively, on a self-rated 10-point scale.
The primary outcome in the study was change in PASI score at week 24, which was 4 weeks after the final dose of ixekizumab. The mean score improved from 24.8 at week 0 to 9.7 at week 24. Five patients achieved at least a 75% decrease in PASI score, or PASI 75 response, and 2 had a PASI 90 response. The DLQI improved from 18 to 3, and both itch and pain scores dropped to a median of 1. There was no association between response to treatment and PRP clinical subtype.
The four top responders – those with a PASI 89 or better response at week 24 – remained clear or almost clear at week 36, fully 16 weeks after their last dose of ixekizumab. Patients with an intermediate week 24 response – that is, a 50%-85% reduction in PASI score – all relapsed before week 36. The patient with the worst PASI score at both baseline and 24 weeks decided to continue on ixekizumab dosed every 2 weeks independent of the study, rather than at the FDA-approved dosing every 4 weeks for psoriasis, with a resultant drop to a PASI score of 8 at week 36.
To look at mechanism of benefit, Dr. Greiling used quantitative polymerase chain reaction to examine key cytokine expression in the epidermis and dermis. Not surprisingly, IL-17A expression was markedly reduced at both sites, suggesting the importance of the Th17 axis in the pathophysiology of PRP. In contrast, there was no significant change in IL-23 expression.
No serious or unexpected adverse events occurred in the 24-week study.
“In terms of ixekizumab, compared to other treatments, I definitely think it is more effective than any conventional therapies, such as topical steroids, methotrexate, or acitretin,” she said in an interview.
Asked about other biologics, Dr. Greiling said she hasn’t found tumor necrosis factor inhibitors very helpful in her patients with PRP. A formal trial of the IL-17A inhibitor secukinumab (Cosentyx) has been done elsewhere, and although the results haven’t yet been published, her understanding is that the efficacy was similar to her ixekizumab trial.
“I’ve had some of my ixekizumab patients switch to secukinumab, for insurance reasons, though, and had it not be quite as effective, although still helpful,” she said.
Dr. Greiling is now enrolling patients with PRP in a trial of the IL-23 inhibitor guselkumab (Tremfya). It’s her early impression that this may prove to be another therapeutic option.
“I have not yet used brodalumab [Siliq], but I wonder if it would also be helpful, since it seems to have a stronger blockade, working on the IL-17 receptor A,” she said.
She cited two pressing needs that would advance PRP research: the lack of standard criteria for disease diagnosis and the absence of PRP-specific disease measurement tools. “We’re trying to remedy that,” the dermatologist said.
Her study was funded by Eli Lilly. She reported receiving research funding from that company and Janssen.
Teri Greiling, MD, PhD, said at the virtual annual meeting of the American Academy of Dermatology.
The interleukin-17A inhibitor induced long-term remission of pityriasis rubra pilaris (PRP) in 4 of the 11 participants in her open-label, single-arm, 24-week clinical trial of ixekizumab (Taltz) at the Food and Drug Administration–approved dosing for psoriasis. Of 11 patients, 7 experienced at least a 50% reduction in signs and symptoms of the disease. Marked quality-of-life improvements occurred as well, reported Dr. Greiling, a dermatologist at Oregon Health & Science University, Portland.
PRP is a rare papulosquamous disorder that’s challenging to diagnosis and often difficult to treat. Indeed, there is no FDA-approved treatment. The disorder is characterized by widespread follicular keratotic plaques with scale and notable islands of sparing, often with an accompanying waxy yellow palmoplantar keratoderma with fissuring. There are adult- and childhood-onset forms of PRP, as well as sporadic and familial subtypes. Some cases are associated with variants in the CARD14 gene, known to also play a role in familial psoriasis.
Patients with PRP say the disorder has a major adverse impact on their quality of life. Itching and pain are often prominent features.
The 11 study participants, drawn from throughout the United States, were adults with a mean 12-year history of symptoms. All were required to have both clinical and biopsy evidence of PRP. Five had classic adult-onset PRP, five had atypical adult-onset PRP, and one had classic juvenile-onset PRP. All had moderate or severe disease as reflected in a Physician’s Global Assessment score of 3 or 4 on the 0-4 scale. Of the 11, 10 had previously received various systemic therapies for their PRP without success.
Since there is as yet no established PRP severity grading tool, Dr. Greiling applied the principles of the Psoriasis Area and Severity Index (PASI). Participants had a mean baseline PASI score of 24.6, a Dermatology Life Quality Index (DLQI) score of 18, and itch and pain scores of 7 and 6, respectively, on a self-rated 10-point scale.
The primary outcome in the study was change in PASI score at week 24, which was 4 weeks after the final dose of ixekizumab. The mean score improved from 24.8 at week 0 to 9.7 at week 24. Five patients achieved at least a 75% decrease in PASI score, or PASI 75 response, and 2 had a PASI 90 response. The DLQI improved from 18 to 3, and both itch and pain scores dropped to a median of 1. There was no association between response to treatment and PRP clinical subtype.
The four top responders – those with a PASI 89 or better response at week 24 – remained clear or almost clear at week 36, fully 16 weeks after their last dose of ixekizumab. Patients with an intermediate week 24 response – that is, a 50%-85% reduction in PASI score – all relapsed before week 36. The patient with the worst PASI score at both baseline and 24 weeks decided to continue on ixekizumab dosed every 2 weeks independent of the study, rather than at the FDA-approved dosing every 4 weeks for psoriasis, with a resultant drop to a PASI score of 8 at week 36.
To look at mechanism of benefit, Dr. Greiling used quantitative polymerase chain reaction to examine key cytokine expression in the epidermis and dermis. Not surprisingly, IL-17A expression was markedly reduced at both sites, suggesting the importance of the Th17 axis in the pathophysiology of PRP. In contrast, there was no significant change in IL-23 expression.
No serious or unexpected adverse events occurred in the 24-week study.
“In terms of ixekizumab, compared to other treatments, I definitely think it is more effective than any conventional therapies, such as topical steroids, methotrexate, or acitretin,” she said in an interview.
Asked about other biologics, Dr. Greiling said she hasn’t found tumor necrosis factor inhibitors very helpful in her patients with PRP. A formal trial of the IL-17A inhibitor secukinumab (Cosentyx) has been done elsewhere, and although the results haven’t yet been published, her understanding is that the efficacy was similar to her ixekizumab trial.
“I’ve had some of my ixekizumab patients switch to secukinumab, for insurance reasons, though, and had it not be quite as effective, although still helpful,” she said.
Dr. Greiling is now enrolling patients with PRP in a trial of the IL-23 inhibitor guselkumab (Tremfya). It’s her early impression that this may prove to be another therapeutic option.
“I have not yet used brodalumab [Siliq], but I wonder if it would also be helpful, since it seems to have a stronger blockade, working on the IL-17 receptor A,” she said.
She cited two pressing needs that would advance PRP research: the lack of standard criteria for disease diagnosis and the absence of PRP-specific disease measurement tools. “We’re trying to remedy that,” the dermatologist said.
Her study was funded by Eli Lilly. She reported receiving research funding from that company and Janssen.
FROM AAD 2020
Lenalidomide may be an answer for refractory cutaneous lupus
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
FROM SPD 2020
Marked improvements seen for women in dermatology since the 1970s
Wilma F. Bergfeld, MD, one of only five women in her medical school class of 1964 and the third female in her dermatology residency program, had recently been appointed as a junior clinical dermatologist and head of dermatopathology at the Cleveland Clinic when she was told by a superior that she would not be promoted or invited to serve on any committee or decision-making group.
“I was told I should go home at night and take care of my husband and two children,” she recalled of that moment in the 1970s. The comment made her feel “outraged,” and it drove her, calmly and steadily, to work harder and to “challenge the system.”
Dr. Bergfeld not only was elected to the Cleveland Clinic’s board of governors and board of trustees and served as president of the Clinic’s staff in 1990, she also became the first woman president of the American Academy of Dermatology (1992) and led numerous other dermatologic organizations. Much earlier on, in 1973, to help fulfill her vision of “women helping women,” she had also founded the Women’s Dermatologic Society (WDS). Three years earlier, in 1970, 6.9% of the approximately 4,000 dermatologists in the United States were women, according to the American Medical Association.
Today, when she goes to work as the long-time director of the Clinic’s dermatopathology fellowship and professor of dermatology and pathology at the Cleveland Clinic Educational Foundation, she sees a transformed staff and, more broadly, a national physician workforce in which women made up almost 50% of active dermatologists in 2017 and almost 60% of dermatology residents in 2018, according to data from the American Association of Medical Colleges.
It’s a different and better world, she and other women dermatologists said, but one in which women must continue to mentor other women and continue to challenge the system. Achieving work-life balance, fairer compensation, and a greater proportion of women in the higher ranks of academia are all on their work list.
Women’s impact on the specialty
Dr. Bergfeld and Molly Hinshaw, MD, the current president of the WDS, said they believe women are drawn to dermatology for its visual nature, the growth in diagnostic tests and therapies, and the opportunity to diagnose early and prevent progression of disease in patients of all ages. “It’s a small but mighty specialty,” said Dr. Hinshaw, associate professor of dermatology and section chief of dermatopathology at the University of Wisconsin–Madison.
It’s also a versatile specialty with a variety of subspecialties and niches to pursue – and women have been stepping in to fill unmet needs, Dr. Hinshaw said. “Women dermatologists are directing vulvar specialty clinics across the country, for example. There aren’t that many, but they’re filling an important niche. We have one at [our university] and it is packed.”
Women have also been drawn to the in-demand subspecialty of pediatric dermatology, she noted. They now make up more than two-thirds of all pediatric dermatologists, and many in practice have trained the old-fashioned way, completing two residencies. “That’s [involved] self-selection into an additional year of years training and a commitment to caring for special populations that, quite honestly, takes more time,” said Dr. Hinshaw, who, as part of her dermatology practice, runs a nail clinic at UW Health in Madison.
Amy S. Paller, MD, who chairs the department of dermatology at Northwestern University, Chicago, where she is professor of dermatology and pediatrics and directs the Skin Biology & Diseases Resource-Based Center, is one of these women. She took a long and determined journey into the subspecialty, encountering bias and discouragement while actively seeking out mentors who helped her advance.
While in medical school at Stanford (Calif.) University in the late 1970s in a class “very progressively” made up of about one-third women, Dr. Paller met Alvin Jacobs, MD, who, in 1975, had founded the Society for Pediatric Dermatology. “There wasn’t much pediatric dermatology in the world at the time, and it was Al who helped [me realize] that it combined my love of genetic research with my [desire] to work with children,” she recalled.
Per Dr. Jacob’s advice, she went to Northwestern to train in both pediatrics and dermatology under Nancy Esterly, MD, who “is considered by many to be the mother of pediatric dermatology.” And knowing that she wanted to do research, Dr. Paller also worked with Ruth Freinkel, MD, who “was the strongest bench researcher” at Northwestern. (Dr. Freinkel had been one of the first female dermatology residents at Harvard and was the first full-time faculty member in dermatology at Northwestern).
After completing postdoctoral research at the University of North Carolina at Chapel Hill, Dr. Paller returned to Chicago and assumed Dr. Esterly’s position as chief of dermatology at the Children’s National Hospital of Chicago. It was there that “someone in a leadership position questioned me about how I could possibly be a scientist, a strong clinician, and a good mother to my three children – and suggested that I drop research,” Dr. Paller recalled.
“I think this person was trying to be helpful to me, but I was shocked,” she said. Just as Dr. Bergfeld had done, Dr. Paller channeled her frustration into new pursuits.
“It made me go home and think, how could I strengthen myself? What else could I do?” she said. “Soon after, with a highly supportive husband, I did a ‘pseudosabbatical,’ basically spending every ounce of spare time I had working with one of the premier female scientists in the country, Elaine Fuchs, and learning molecular biology” in her lab at the University of Chicago.
“I think we’ve all had discrimination along the way. Sometimes there’s implicit bias and sometimes there’s overt bias,” said Dr. Paller, who in 2004 led the society which her mentor Dr. Jacobs had founded several decades earlier. “I just jumped right in, and that’s enabled me to find good role models.”
Across dermatology broadly, the often holistic nature of the specialty – of the ability to peer into the body and its internal health – is another quality that women have been drawn to and advanced, Dr. Hinshaw said. “One of the reasons why I chose dermatology is because it’s a window to total patient health. Patients often see their dermatologists as physicians who help them identify next steps in their health care, who can help them address issues related to their overall health and well-being, including their mental health.”
In a WDS membership survey conducted in 2018, most respondents reported that they frequently or occasionally detect and diagnose systemic/internal diseases and conditions in their female patients, and that they consult and collaborate with different kinds of physicians (Int J Womens Dermatol. 2018 Nov 15;4[4]:189-92).
And in a March 2019 “Dialogues in Dermatology” podcast episode on the history and advancement of women in dermatology produced by the American Academy of Dermatology, Pearl Grimes, MD, a clinical professor of dermatology of the University of California, Los Angeles, and then-president of the WDS, described why “total women’s health” had become an additional focus for the society.
“We’re already gatekeepers” in many respects, Dr. Grimes said. “In addition to my addressing specific skin issues, my patients query me on hormone issues, on nutrition, on stress-related issues….and on [what other physicians they should see].”
Phoebe Rich, MD, who owns a small all-woman practice and a research center in Portland, Oregon, said that, in general, many women also communicate and practice in a way that facilitates holistic care. “These qualities aren’t exclusive to women, but women are very caring. We take time and are interested in [patients’] lives in general, not just their disease.”
Disparities in academia
Dermatology departments in academic medicine have burgeoned in size in the past 50 years, and women are well represented overall. In 2018, women comprised 51.2% of dermatology department faculty – up from 10.8% in 1970 – a current proportion that ranks fifth among specialties for the proportion of female faculty, according to a cross-sectional study of faculty diversity trends using data from the AAMC faculty roster (JAMA Dermatol. 2020 Jan 8;156[3]:280-7).
The AAMC data show the share of women dermatology faculty declining at each subsequent rank, however – a finding that suggests that women are not promoted as quickly or to the same levels of leadership as men, the report’s authors noted. (Dermatology isn’t alone: The AAMC issued a call to action on gender equity in medicine this year, citing this inverse association.)
Another recently published study of gender trends in academic dermatology – this one looking at a smaller sample of data from 15 institutions – similarly found that women dermatologists made up a majority of faculty (53.6%) and were well represented as assistant professors (60.7%) but underrepresented as full professors (17%).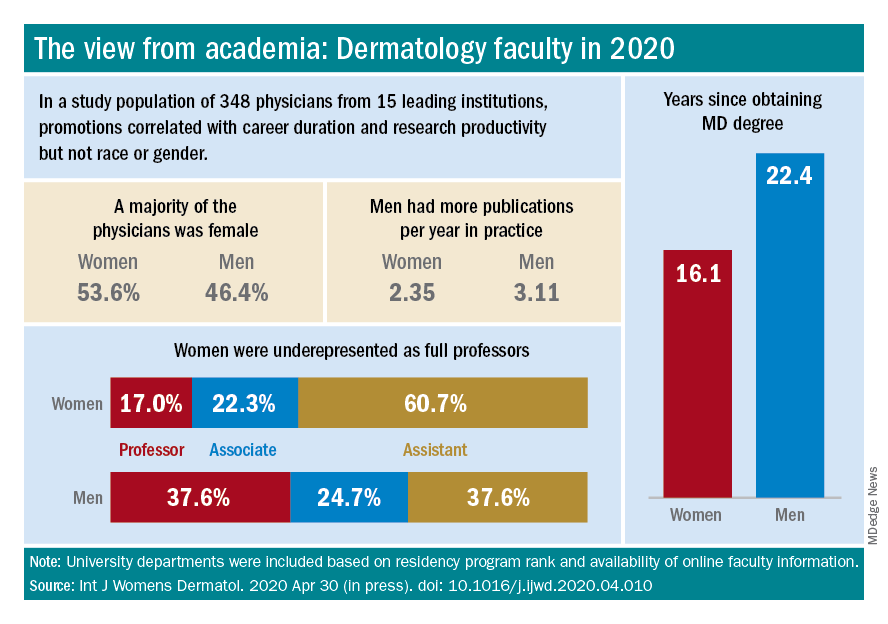
This study differed from the larger AAMC study, however, in that it controlled for “achievement indicators” – career duration, publications per year, and National Institutes of Health research funding – and found that gender alone was not associated with higher rank. Instead, promotions were correlated most significantly with NIH research funding and also with career duration and publications per year.
“If research achievement is to be used as a benchmark for academic promotion, increased efforts are needed to support the research activities of women,” the authors wrote, adding that recognition should be given to other factors as well.
Dr. Paller and Dr. Hinshaw both described the situation as complex and multifaceted. Some research on promotion in academia in general – but not all – has suggested that women do need to publish more than men in order to be promoted. But “the promotion process also has within it the ability to use judgment [about] the impact and merits of work,” said Dr. Hinshaw. “Not all publications [and levels of authorship] may be considered equal, for instance.”
Dr. Hinshaw said she is also concerned by data showing that women still perform the majority of household duties, “even in households in which both partners work outside the home equivalently.” As long as this is the case, women may be “inherently disadvantaged” in their ability to have adequate research time and to advance.
From where she sits, Dr. Paller sees several factors at play: “The pipeline, achievement during the pipeline, and decision-making about advancement” on the part of women themselves. Having served on search committees for top leadership in specialties in which women are well represented, she said, “I’ve seen fewer women who’ve come forward and been interested in rising into a chair or a dean position.”
And “having talked to so many women,” Dr. Paller added, “I think there’s a phenomenon where it’s harder for women to accept positions [that require] a significant change.”
Women “are nurturers, which makes them extremely good [leaders] and chairs, but it also makes it harder to make life changes that affect the people they love,” she said, noting that becoming a department chair or a dean often involves moving. “I also think that women in general are happier and committed to what they’re [currently] doing.”
Dr. Paller is optimistic that, with the support of department chairs and continued attention to role modeling and mentoring, the portrait of women in academic dermatology will continue to improve. Currently, 34 chairs of dermatology departments are female, she noted. “That number was 11 less 15 years ago.”
In the meantime, researchers are increasingly documenting trends in women’s editorships of journals as well as leadership and speaking opportunities at professional conferences.
The authors of one study published this year, for instance, reviewed the editorial boards of dermatology journals and found that women occupied 18% of editor in chief roles, 36% of deputy editor positions, and 22% of overall editorial board roles (Int J Womens Dermatol. 2019 Sep 12;6[1]:20-4). Other research shows women comprising 43% of all authorships across 23 dermatologic journals from 2008 to May 2017, 50.2% of first authorships, and 33.1% of last authorships (BMJ Open. 2018 Apr 13;8[4]:e020089).
Both in academic medicine and in practice, a gender pay gap still affects women physicians across the board. Medscape’s 2020 dermatologist compensation report shows male dermatologists earning about 12% more than their female peers (average, $435,000 vs. $387,000, respectively), while the average number of hours per week spent seeing patients is similar (36.2 vs. 35.6 hours, respectively).
And in its 2020 statement on gender equity, the AAMC said that women in academic medicine are offered less in starting salary, negotiated pay, and other forms of compensation than men “despite equal effort, rank, training, and experience.”
It’s complicated to tease apart all the factors that may be involved – but important to keep challenging the system, said Dr. Bergfeld, who was a long-time board adviser for Dermatology News. “I was underpaid,” she noted, and “this was only rectified in the last 10 years.”
Work-life balance
In the AAD podcast on women in dermatology, Dr. Grimes said that achieving a healthy and balanced work life remains one of the greatest challenges for women dermatologists – and it may be even greater than in the past given the growing numbers of group practices. “When women enter the realm of group practice, they have less flexibility in controlling their time and their own schedules.”
If Anna Hare, MD, is any indication, younger dermatologists may buck this trend. The daughter of Dr. Rich in Portland, Dr. Hare joined her mother’s dermatology practice and research center knowing that she’d have “the respect and flexibility for deciding how I want to practice.”
Younger dermatologists, she said, place “more of an emphasis on work-life balance and quality of life.”
Fortunately, said Dr. Bergfeld, women have advanced enough in the ranks of dermatology that, in networking, in mentorship, and in workplace settings, attention can be paid more fully to discussions about work-life management – “how to manage your life when you’re working with family and kids and parents.”
In the 1970s, at the Cleveland Clinic, “there were only five women on staff and we were fighting for [basic] rights,” she said. “We wanted equality – we were [perceived as] little worker bees….We needed to climb as the men did to positions of leadership and address the problems of women.”
In pursuing their goals and making further progress, women dermatologists today should be “steady and calm,” she advised. Formally acquiring leadership skills and communication skills is a timeless need. And when there are biases or conflicts, “you cannot have righteous indignation, you cannot have revenge. You have to calm yourself and move forward.”
Wilma F. Bergfeld, MD, one of only five women in her medical school class of 1964 and the third female in her dermatology residency program, had recently been appointed as a junior clinical dermatologist and head of dermatopathology at the Cleveland Clinic when she was told by a superior that she would not be promoted or invited to serve on any committee or decision-making group.
“I was told I should go home at night and take care of my husband and two children,” she recalled of that moment in the 1970s. The comment made her feel “outraged,” and it drove her, calmly and steadily, to work harder and to “challenge the system.”
Dr. Bergfeld not only was elected to the Cleveland Clinic’s board of governors and board of trustees and served as president of the Clinic’s staff in 1990, she also became the first woman president of the American Academy of Dermatology (1992) and led numerous other dermatologic organizations. Much earlier on, in 1973, to help fulfill her vision of “women helping women,” she had also founded the Women’s Dermatologic Society (WDS). Three years earlier, in 1970, 6.9% of the approximately 4,000 dermatologists in the United States were women, according to the American Medical Association.
Today, when she goes to work as the long-time director of the Clinic’s dermatopathology fellowship and professor of dermatology and pathology at the Cleveland Clinic Educational Foundation, she sees a transformed staff and, more broadly, a national physician workforce in which women made up almost 50% of active dermatologists in 2017 and almost 60% of dermatology residents in 2018, according to data from the American Association of Medical Colleges.
It’s a different and better world, she and other women dermatologists said, but one in which women must continue to mentor other women and continue to challenge the system. Achieving work-life balance, fairer compensation, and a greater proportion of women in the higher ranks of academia are all on their work list.
Women’s impact on the specialty
Dr. Bergfeld and Molly Hinshaw, MD, the current president of the WDS, said they believe women are drawn to dermatology for its visual nature, the growth in diagnostic tests and therapies, and the opportunity to diagnose early and prevent progression of disease in patients of all ages. “It’s a small but mighty specialty,” said Dr. Hinshaw, associate professor of dermatology and section chief of dermatopathology at the University of Wisconsin–Madison.
It’s also a versatile specialty with a variety of subspecialties and niches to pursue – and women have been stepping in to fill unmet needs, Dr. Hinshaw said. “Women dermatologists are directing vulvar specialty clinics across the country, for example. There aren’t that many, but they’re filling an important niche. We have one at [our university] and it is packed.”
Women have also been drawn to the in-demand subspecialty of pediatric dermatology, she noted. They now make up more than two-thirds of all pediatric dermatologists, and many in practice have trained the old-fashioned way, completing two residencies. “That’s [involved] self-selection into an additional year of years training and a commitment to caring for special populations that, quite honestly, takes more time,” said Dr. Hinshaw, who, as part of her dermatology practice, runs a nail clinic at UW Health in Madison.
Amy S. Paller, MD, who chairs the department of dermatology at Northwestern University, Chicago, where she is professor of dermatology and pediatrics and directs the Skin Biology & Diseases Resource-Based Center, is one of these women. She took a long and determined journey into the subspecialty, encountering bias and discouragement while actively seeking out mentors who helped her advance.
While in medical school at Stanford (Calif.) University in the late 1970s in a class “very progressively” made up of about one-third women, Dr. Paller met Alvin Jacobs, MD, who, in 1975, had founded the Society for Pediatric Dermatology. “There wasn’t much pediatric dermatology in the world at the time, and it was Al who helped [me realize] that it combined my love of genetic research with my [desire] to work with children,” she recalled.
Per Dr. Jacob’s advice, she went to Northwestern to train in both pediatrics and dermatology under Nancy Esterly, MD, who “is considered by many to be the mother of pediatric dermatology.” And knowing that she wanted to do research, Dr. Paller also worked with Ruth Freinkel, MD, who “was the strongest bench researcher” at Northwestern. (Dr. Freinkel had been one of the first female dermatology residents at Harvard and was the first full-time faculty member in dermatology at Northwestern).
After completing postdoctoral research at the University of North Carolina at Chapel Hill, Dr. Paller returned to Chicago and assumed Dr. Esterly’s position as chief of dermatology at the Children’s National Hospital of Chicago. It was there that “someone in a leadership position questioned me about how I could possibly be a scientist, a strong clinician, and a good mother to my three children – and suggested that I drop research,” Dr. Paller recalled.
“I think this person was trying to be helpful to me, but I was shocked,” she said. Just as Dr. Bergfeld had done, Dr. Paller channeled her frustration into new pursuits.
“It made me go home and think, how could I strengthen myself? What else could I do?” she said. “Soon after, with a highly supportive husband, I did a ‘pseudosabbatical,’ basically spending every ounce of spare time I had working with one of the premier female scientists in the country, Elaine Fuchs, and learning molecular biology” in her lab at the University of Chicago.
“I think we’ve all had discrimination along the way. Sometimes there’s implicit bias and sometimes there’s overt bias,” said Dr. Paller, who in 2004 led the society which her mentor Dr. Jacobs had founded several decades earlier. “I just jumped right in, and that’s enabled me to find good role models.”
Across dermatology broadly, the often holistic nature of the specialty – of the ability to peer into the body and its internal health – is another quality that women have been drawn to and advanced, Dr. Hinshaw said. “One of the reasons why I chose dermatology is because it’s a window to total patient health. Patients often see their dermatologists as physicians who help them identify next steps in their health care, who can help them address issues related to their overall health and well-being, including their mental health.”
In a WDS membership survey conducted in 2018, most respondents reported that they frequently or occasionally detect and diagnose systemic/internal diseases and conditions in their female patients, and that they consult and collaborate with different kinds of physicians (Int J Womens Dermatol. 2018 Nov 15;4[4]:189-92).
And in a March 2019 “Dialogues in Dermatology” podcast episode on the history and advancement of women in dermatology produced by the American Academy of Dermatology, Pearl Grimes, MD, a clinical professor of dermatology of the University of California, Los Angeles, and then-president of the WDS, described why “total women’s health” had become an additional focus for the society.
“We’re already gatekeepers” in many respects, Dr. Grimes said. “In addition to my addressing specific skin issues, my patients query me on hormone issues, on nutrition, on stress-related issues….and on [what other physicians they should see].”
Phoebe Rich, MD, who owns a small all-woman practice and a research center in Portland, Oregon, said that, in general, many women also communicate and practice in a way that facilitates holistic care. “These qualities aren’t exclusive to women, but women are very caring. We take time and are interested in [patients’] lives in general, not just their disease.”
Disparities in academia
Dermatology departments in academic medicine have burgeoned in size in the past 50 years, and women are well represented overall. In 2018, women comprised 51.2% of dermatology department faculty – up from 10.8% in 1970 – a current proportion that ranks fifth among specialties for the proportion of female faculty, according to a cross-sectional study of faculty diversity trends using data from the AAMC faculty roster (JAMA Dermatol. 2020 Jan 8;156[3]:280-7).
The AAMC data show the share of women dermatology faculty declining at each subsequent rank, however – a finding that suggests that women are not promoted as quickly or to the same levels of leadership as men, the report’s authors noted. (Dermatology isn’t alone: The AAMC issued a call to action on gender equity in medicine this year, citing this inverse association.)
Another recently published study of gender trends in academic dermatology – this one looking at a smaller sample of data from 15 institutions – similarly found that women dermatologists made up a majority of faculty (53.6%) and were well represented as assistant professors (60.7%) but underrepresented as full professors (17%).
This study differed from the larger AAMC study, however, in that it controlled for “achievement indicators” – career duration, publications per year, and National Institutes of Health research funding – and found that gender alone was not associated with higher rank. Instead, promotions were correlated most significantly with NIH research funding and also with career duration and publications per year.
“If research achievement is to be used as a benchmark for academic promotion, increased efforts are needed to support the research activities of women,” the authors wrote, adding that recognition should be given to other factors as well.
Dr. Paller and Dr. Hinshaw both described the situation as complex and multifaceted. Some research on promotion in academia in general – but not all – has suggested that women do need to publish more than men in order to be promoted. But “the promotion process also has within it the ability to use judgment [about] the impact and merits of work,” said Dr. Hinshaw. “Not all publications [and levels of authorship] may be considered equal, for instance.”
Dr. Hinshaw said she is also concerned by data showing that women still perform the majority of household duties, “even in households in which both partners work outside the home equivalently.” As long as this is the case, women may be “inherently disadvantaged” in their ability to have adequate research time and to advance.
From where she sits, Dr. Paller sees several factors at play: “The pipeline, achievement during the pipeline, and decision-making about advancement” on the part of women themselves. Having served on search committees for top leadership in specialties in which women are well represented, she said, “I’ve seen fewer women who’ve come forward and been interested in rising into a chair or a dean position.”
And “having talked to so many women,” Dr. Paller added, “I think there’s a phenomenon where it’s harder for women to accept positions [that require] a significant change.”
Women “are nurturers, which makes them extremely good [leaders] and chairs, but it also makes it harder to make life changes that affect the people they love,” she said, noting that becoming a department chair or a dean often involves moving. “I also think that women in general are happier and committed to what they’re [currently] doing.”
Dr. Paller is optimistic that, with the support of department chairs and continued attention to role modeling and mentoring, the portrait of women in academic dermatology will continue to improve. Currently, 34 chairs of dermatology departments are female, she noted. “That number was 11 less 15 years ago.”
In the meantime, researchers are increasingly documenting trends in women’s editorships of journals as well as leadership and speaking opportunities at professional conferences.
The authors of one study published this year, for instance, reviewed the editorial boards of dermatology journals and found that women occupied 18% of editor in chief roles, 36% of deputy editor positions, and 22% of overall editorial board roles (Int J Womens Dermatol. 2019 Sep 12;6[1]:20-4). Other research shows women comprising 43% of all authorships across 23 dermatologic journals from 2008 to May 2017, 50.2% of first authorships, and 33.1% of last authorships (BMJ Open. 2018 Apr 13;8[4]:e020089).
Both in academic medicine and in practice, a gender pay gap still affects women physicians across the board. Medscape’s 2020 dermatologist compensation report shows male dermatologists earning about 12% more than their female peers (average, $435,000 vs. $387,000, respectively), while the average number of hours per week spent seeing patients is similar (36.2 vs. 35.6 hours, respectively).
And in its 2020 statement on gender equity, the AAMC said that women in academic medicine are offered less in starting salary, negotiated pay, and other forms of compensation than men “despite equal effort, rank, training, and experience.”
It’s complicated to tease apart all the factors that may be involved – but important to keep challenging the system, said Dr. Bergfeld, who was a long-time board adviser for Dermatology News. “I was underpaid,” she noted, and “this was only rectified in the last 10 years.”
Work-life balance
In the AAD podcast on women in dermatology, Dr. Grimes said that achieving a healthy and balanced work life remains one of the greatest challenges for women dermatologists – and it may be even greater than in the past given the growing numbers of group practices. “When women enter the realm of group practice, they have less flexibility in controlling their time and their own schedules.”
If Anna Hare, MD, is any indication, younger dermatologists may buck this trend. The daughter of Dr. Rich in Portland, Dr. Hare joined her mother’s dermatology practice and research center knowing that she’d have “the respect and flexibility for deciding how I want to practice.”
Younger dermatologists, she said, place “more of an emphasis on work-life balance and quality of life.”
Fortunately, said Dr. Bergfeld, women have advanced enough in the ranks of dermatology that, in networking, in mentorship, and in workplace settings, attention can be paid more fully to discussions about work-life management – “how to manage your life when you’re working with family and kids and parents.”
In the 1970s, at the Cleveland Clinic, “there were only five women on staff and we were fighting for [basic] rights,” she said. “We wanted equality – we were [perceived as] little worker bees….We needed to climb as the men did to positions of leadership and address the problems of women.”
In pursuing their goals and making further progress, women dermatologists today should be “steady and calm,” she advised. Formally acquiring leadership skills and communication skills is a timeless need. And when there are biases or conflicts, “you cannot have righteous indignation, you cannot have revenge. You have to calm yourself and move forward.”
Wilma F. Bergfeld, MD, one of only five women in her medical school class of 1964 and the third female in her dermatology residency program, had recently been appointed as a junior clinical dermatologist and head of dermatopathology at the Cleveland Clinic when she was told by a superior that she would not be promoted or invited to serve on any committee or decision-making group.
“I was told I should go home at night and take care of my husband and two children,” she recalled of that moment in the 1970s. The comment made her feel “outraged,” and it drove her, calmly and steadily, to work harder and to “challenge the system.”
Dr. Bergfeld not only was elected to the Cleveland Clinic’s board of governors and board of trustees and served as president of the Clinic’s staff in 1990, she also became the first woman president of the American Academy of Dermatology (1992) and led numerous other dermatologic organizations. Much earlier on, in 1973, to help fulfill her vision of “women helping women,” she had also founded the Women’s Dermatologic Society (WDS). Three years earlier, in 1970, 6.9% of the approximately 4,000 dermatologists in the United States were women, according to the American Medical Association.
Today, when she goes to work as the long-time director of the Clinic’s dermatopathology fellowship and professor of dermatology and pathology at the Cleveland Clinic Educational Foundation, she sees a transformed staff and, more broadly, a national physician workforce in which women made up almost 50% of active dermatologists in 2017 and almost 60% of dermatology residents in 2018, according to data from the American Association of Medical Colleges.
It’s a different and better world, she and other women dermatologists said, but one in which women must continue to mentor other women and continue to challenge the system. Achieving work-life balance, fairer compensation, and a greater proportion of women in the higher ranks of academia are all on their work list.
Women’s impact on the specialty
Dr. Bergfeld and Molly Hinshaw, MD, the current president of the WDS, said they believe women are drawn to dermatology for its visual nature, the growth in diagnostic tests and therapies, and the opportunity to diagnose early and prevent progression of disease in patients of all ages. “It’s a small but mighty specialty,” said Dr. Hinshaw, associate professor of dermatology and section chief of dermatopathology at the University of Wisconsin–Madison.
It’s also a versatile specialty with a variety of subspecialties and niches to pursue – and women have been stepping in to fill unmet needs, Dr. Hinshaw said. “Women dermatologists are directing vulvar specialty clinics across the country, for example. There aren’t that many, but they’re filling an important niche. We have one at [our university] and it is packed.”
Women have also been drawn to the in-demand subspecialty of pediatric dermatology, she noted. They now make up more than two-thirds of all pediatric dermatologists, and many in practice have trained the old-fashioned way, completing two residencies. “That’s [involved] self-selection into an additional year of years training and a commitment to caring for special populations that, quite honestly, takes more time,” said Dr. Hinshaw, who, as part of her dermatology practice, runs a nail clinic at UW Health in Madison.
Amy S. Paller, MD, who chairs the department of dermatology at Northwestern University, Chicago, where she is professor of dermatology and pediatrics and directs the Skin Biology & Diseases Resource-Based Center, is one of these women. She took a long and determined journey into the subspecialty, encountering bias and discouragement while actively seeking out mentors who helped her advance.
While in medical school at Stanford (Calif.) University in the late 1970s in a class “very progressively” made up of about one-third women, Dr. Paller met Alvin Jacobs, MD, who, in 1975, had founded the Society for Pediatric Dermatology. “There wasn’t much pediatric dermatology in the world at the time, and it was Al who helped [me realize] that it combined my love of genetic research with my [desire] to work with children,” she recalled.
Per Dr. Jacob’s advice, she went to Northwestern to train in both pediatrics and dermatology under Nancy Esterly, MD, who “is considered by many to be the mother of pediatric dermatology.” And knowing that she wanted to do research, Dr. Paller also worked with Ruth Freinkel, MD, who “was the strongest bench researcher” at Northwestern. (Dr. Freinkel had been one of the first female dermatology residents at Harvard and was the first full-time faculty member in dermatology at Northwestern).
After completing postdoctoral research at the University of North Carolina at Chapel Hill, Dr. Paller returned to Chicago and assumed Dr. Esterly’s position as chief of dermatology at the Children’s National Hospital of Chicago. It was there that “someone in a leadership position questioned me about how I could possibly be a scientist, a strong clinician, and a good mother to my three children – and suggested that I drop research,” Dr. Paller recalled.
“I think this person was trying to be helpful to me, but I was shocked,” she said. Just as Dr. Bergfeld had done, Dr. Paller channeled her frustration into new pursuits.
“It made me go home and think, how could I strengthen myself? What else could I do?” she said. “Soon after, with a highly supportive husband, I did a ‘pseudosabbatical,’ basically spending every ounce of spare time I had working with one of the premier female scientists in the country, Elaine Fuchs, and learning molecular biology” in her lab at the University of Chicago.
“I think we’ve all had discrimination along the way. Sometimes there’s implicit bias and sometimes there’s overt bias,” said Dr. Paller, who in 2004 led the society which her mentor Dr. Jacobs had founded several decades earlier. “I just jumped right in, and that’s enabled me to find good role models.”
Across dermatology broadly, the often holistic nature of the specialty – of the ability to peer into the body and its internal health – is another quality that women have been drawn to and advanced, Dr. Hinshaw said. “One of the reasons why I chose dermatology is because it’s a window to total patient health. Patients often see their dermatologists as physicians who help them identify next steps in their health care, who can help them address issues related to their overall health and well-being, including their mental health.”
In a WDS membership survey conducted in 2018, most respondents reported that they frequently or occasionally detect and diagnose systemic/internal diseases and conditions in their female patients, and that they consult and collaborate with different kinds of physicians (Int J Womens Dermatol. 2018 Nov 15;4[4]:189-92).
And in a March 2019 “Dialogues in Dermatology” podcast episode on the history and advancement of women in dermatology produced by the American Academy of Dermatology, Pearl Grimes, MD, a clinical professor of dermatology of the University of California, Los Angeles, and then-president of the WDS, described why “total women’s health” had become an additional focus for the society.
“We’re already gatekeepers” in many respects, Dr. Grimes said. “In addition to my addressing specific skin issues, my patients query me on hormone issues, on nutrition, on stress-related issues….and on [what other physicians they should see].”
Phoebe Rich, MD, who owns a small all-woman practice and a research center in Portland, Oregon, said that, in general, many women also communicate and practice in a way that facilitates holistic care. “These qualities aren’t exclusive to women, but women are very caring. We take time and are interested in [patients’] lives in general, not just their disease.”
Disparities in academia
Dermatology departments in academic medicine have burgeoned in size in the past 50 years, and women are well represented overall. In 2018, women comprised 51.2% of dermatology department faculty – up from 10.8% in 1970 – a current proportion that ranks fifth among specialties for the proportion of female faculty, according to a cross-sectional study of faculty diversity trends using data from the AAMC faculty roster (JAMA Dermatol. 2020 Jan 8;156[3]:280-7).
The AAMC data show the share of women dermatology faculty declining at each subsequent rank, however – a finding that suggests that women are not promoted as quickly or to the same levels of leadership as men, the report’s authors noted. (Dermatology isn’t alone: The AAMC issued a call to action on gender equity in medicine this year, citing this inverse association.)
Another recently published study of gender trends in academic dermatology – this one looking at a smaller sample of data from 15 institutions – similarly found that women dermatologists made up a majority of faculty (53.6%) and were well represented as assistant professors (60.7%) but underrepresented as full professors (17%).
This study differed from the larger AAMC study, however, in that it controlled for “achievement indicators” – career duration, publications per year, and National Institutes of Health research funding – and found that gender alone was not associated with higher rank. Instead, promotions were correlated most significantly with NIH research funding and also with career duration and publications per year.
“If research achievement is to be used as a benchmark for academic promotion, increased efforts are needed to support the research activities of women,” the authors wrote, adding that recognition should be given to other factors as well.
Dr. Paller and Dr. Hinshaw both described the situation as complex and multifaceted. Some research on promotion in academia in general – but not all – has suggested that women do need to publish more than men in order to be promoted. But “the promotion process also has within it the ability to use judgment [about] the impact and merits of work,” said Dr. Hinshaw. “Not all publications [and levels of authorship] may be considered equal, for instance.”
Dr. Hinshaw said she is also concerned by data showing that women still perform the majority of household duties, “even in households in which both partners work outside the home equivalently.” As long as this is the case, women may be “inherently disadvantaged” in their ability to have adequate research time and to advance.
From where she sits, Dr. Paller sees several factors at play: “The pipeline, achievement during the pipeline, and decision-making about advancement” on the part of women themselves. Having served on search committees for top leadership in specialties in which women are well represented, she said, “I’ve seen fewer women who’ve come forward and been interested in rising into a chair or a dean position.”
And “having talked to so many women,” Dr. Paller added, “I think there’s a phenomenon where it’s harder for women to accept positions [that require] a significant change.”
Women “are nurturers, which makes them extremely good [leaders] and chairs, but it also makes it harder to make life changes that affect the people they love,” she said, noting that becoming a department chair or a dean often involves moving. “I also think that women in general are happier and committed to what they’re [currently] doing.”
Dr. Paller is optimistic that, with the support of department chairs and continued attention to role modeling and mentoring, the portrait of women in academic dermatology will continue to improve. Currently, 34 chairs of dermatology departments are female, she noted. “That number was 11 less 15 years ago.”
In the meantime, researchers are increasingly documenting trends in women’s editorships of journals as well as leadership and speaking opportunities at professional conferences.
The authors of one study published this year, for instance, reviewed the editorial boards of dermatology journals and found that women occupied 18% of editor in chief roles, 36% of deputy editor positions, and 22% of overall editorial board roles (Int J Womens Dermatol. 2019 Sep 12;6[1]:20-4). Other research shows women comprising 43% of all authorships across 23 dermatologic journals from 2008 to May 2017, 50.2% of first authorships, and 33.1% of last authorships (BMJ Open. 2018 Apr 13;8[4]:e020089).
Both in academic medicine and in practice, a gender pay gap still affects women physicians across the board. Medscape’s 2020 dermatologist compensation report shows male dermatologists earning about 12% more than their female peers (average, $435,000 vs. $387,000, respectively), while the average number of hours per week spent seeing patients is similar (36.2 vs. 35.6 hours, respectively).
And in its 2020 statement on gender equity, the AAMC said that women in academic medicine are offered less in starting salary, negotiated pay, and other forms of compensation than men “despite equal effort, rank, training, and experience.”
It’s complicated to tease apart all the factors that may be involved – but important to keep challenging the system, said Dr. Bergfeld, who was a long-time board adviser for Dermatology News. “I was underpaid,” she noted, and “this was only rectified in the last 10 years.”
Work-life balance
In the AAD podcast on women in dermatology, Dr. Grimes said that achieving a healthy and balanced work life remains one of the greatest challenges for women dermatologists – and it may be even greater than in the past given the growing numbers of group practices. “When women enter the realm of group practice, they have less flexibility in controlling their time and their own schedules.”
If Anna Hare, MD, is any indication, younger dermatologists may buck this trend. The daughter of Dr. Rich in Portland, Dr. Hare joined her mother’s dermatology practice and research center knowing that she’d have “the respect and flexibility for deciding how I want to practice.”
Younger dermatologists, she said, place “more of an emphasis on work-life balance and quality of life.”
Fortunately, said Dr. Bergfeld, women have advanced enough in the ranks of dermatology that, in networking, in mentorship, and in workplace settings, attention can be paid more fully to discussions about work-life management – “how to manage your life when you’re working with family and kids and parents.”
In the 1970s, at the Cleveland Clinic, “there were only five women on staff and we were fighting for [basic] rights,” she said. “We wanted equality – we were [perceived as] little worker bees….We needed to climb as the men did to positions of leadership and address the problems of women.”
In pursuing their goals and making further progress, women dermatologists today should be “steady and calm,” she advised. Formally acquiring leadership skills and communication skills is a timeless need. And when there are biases or conflicts, “you cannot have righteous indignation, you cannot have revenge. You have to calm yourself and move forward.”
Rapid drop of antibodies seen in those with mild COVID-19
The research was conducted by F. Javier Ibarrondo, PhD, and colleagues and was published online on July 21 in a letter to the editor of the New England Journal of Medicine. Ibarrondo is associate researcher at the University of California, Los Angeles. (The original letter incorrectly calculated the half-life at 73 days.)
Coauthor Otto Yang, MD, professor of medicine in the division of infectious diseases at UCLA, told Medscape Medical News that the rapidity in the antibody drop at 5 weeks “is striking compared to other infections.”
The phenomenon has been suspected and has been observed before but had not been quantified.
“Our paper is the first to put firm numbers on the dropping of antibodies after early infection,” he said.
The researchers evaluated 34 people (average age, 43 years) who had recovered from mild COVID-19 and had referred themselves to UCLA for observational research.
Previous report also found a quick fade
As Medscape Medical News reported, a previous study from China that was published in Nature Medicine also found that the antibodies fade quickly.
Interpreting the meaning of the current research comes with a few caveats, Dr. Yang said.
“One is that we don’t know for sure that antibodies are what protect people from getting infected,” he said. Although it’s a reasonable assumption, he said, that’s not always the case.
Another caveat is that even if antibodies do protect, the tests being used to measure them – including the test that was used in this study – may not measure them the right way, and it is not yet known how many antibodies are needed for protection, he explained.
The UCLA researchers used an enzyme-linked immunosorbent assay to detect anti–SARS-CoV-2 spike receptor–binding domain immunoglobulin G concentrations.
“No reason for anybody to be getting an antibody test medically”
The study provides further proof that “[t]here’s no reason for anybody to be getting an antibody test medically right now,” Dr. Yang said.
Additionally, “FDA-approved tests are not approved for quantitative measures, only qualitative,” he continued. He noted that the findings may have implications with respect to herd immunity.
“Herd immunity depends on a lot of people having immunity to the infection all at the same time. If infection is followed by only brief protection from infection, the natural infection is not going to reach herd immunity,” he explained.
Buddy Creech, MD, MPH, associate professor of pediatrics and director of the Vanderbilt Vaccine Research Program in Nashville, Tenn., pointed out that antibodies “are just part of the story.”
“When we make an immune response to any germ,” he said, “we not only make an immune response for the time being but for the future. The next time we’re exposed, we can call into action B cells and T cells who have been there and done that.”
So even though the antibodies fade over time, other arms of the immune system are being trained for future action, he said.
Herd immunity does not require that populations have a huge level of antibodies that remains forever, he explained.
“It requires that in general, we’re not going to get infected as easily, and we’re not going to have disease as easily, and we’re not going to transmit the virus for as long,” he said.
Dr. Creech said he and others researching COVID-19 find that studies that show that antibodies fade quickly provide more proof “that this coronavirus is going to be here to stay unless we can take care of it through very effective treatments to take it from potentially fatal disease to one that is nothing more than a cold” or until a vaccine is developed.
He noted there are four other coronaviruses in widespread circulation every year that “amount to about 25% of the common cold.”
This study may help narrow the window as to when convalescent plasma – plasma that is taken from people who have recovered from COVID-19 and that is used to help people who are acutely ill with the disease – will be most effective, Dr. Creech explained. He said the results suggest that it is important that plasma be collected within the first couple of months after recovery so as to capture the most antibodies.
This study is important as another snapshot “so we understand the differences between severe and mild disease, so we can study it over time, so we have all the tools we need as we start these pivotal vaccine studies to make sure we’re making the right immune response for the right duration of time so we can put an end to this pandemic,” Dr. Creech concluded.
The study was supported by grants from the AIDS Healthcare Foundation, the Doris Duke Charitable Foundation, the National Institutes of Health, the James B. Pendleton Charitable Trust, and the McCarthy Family Foundation. A coauthor reports receiving grants from Gilead outside the submitted work. Dr. Creech has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The research was conducted by F. Javier Ibarrondo, PhD, and colleagues and was published online on July 21 in a letter to the editor of the New England Journal of Medicine. Ibarrondo is associate researcher at the University of California, Los Angeles. (The original letter incorrectly calculated the half-life at 73 days.)
Coauthor Otto Yang, MD, professor of medicine in the division of infectious diseases at UCLA, told Medscape Medical News that the rapidity in the antibody drop at 5 weeks “is striking compared to other infections.”
The phenomenon has been suspected and has been observed before but had not been quantified.
“Our paper is the first to put firm numbers on the dropping of antibodies after early infection,” he said.
The researchers evaluated 34 people (average age, 43 years) who had recovered from mild COVID-19 and had referred themselves to UCLA for observational research.
Previous report also found a quick fade
As Medscape Medical News reported, a previous study from China that was published in Nature Medicine also found that the antibodies fade quickly.
Interpreting the meaning of the current research comes with a few caveats, Dr. Yang said.
“One is that we don’t know for sure that antibodies are what protect people from getting infected,” he said. Although it’s a reasonable assumption, he said, that’s not always the case.
Another caveat is that even if antibodies do protect, the tests being used to measure them – including the test that was used in this study – may not measure them the right way, and it is not yet known how many antibodies are needed for protection, he explained.
The UCLA researchers used an enzyme-linked immunosorbent assay to detect anti–SARS-CoV-2 spike receptor–binding domain immunoglobulin G concentrations.
“No reason for anybody to be getting an antibody test medically”
The study provides further proof that “[t]here’s no reason for anybody to be getting an antibody test medically right now,” Dr. Yang said.
Additionally, “FDA-approved tests are not approved for quantitative measures, only qualitative,” he continued. He noted that the findings may have implications with respect to herd immunity.
“Herd immunity depends on a lot of people having immunity to the infection all at the same time. If infection is followed by only brief protection from infection, the natural infection is not going to reach herd immunity,” he explained.
Buddy Creech, MD, MPH, associate professor of pediatrics and director of the Vanderbilt Vaccine Research Program in Nashville, Tenn., pointed out that antibodies “are just part of the story.”
“When we make an immune response to any germ,” he said, “we not only make an immune response for the time being but for the future. The next time we’re exposed, we can call into action B cells and T cells who have been there and done that.”
So even though the antibodies fade over time, other arms of the immune system are being trained for future action, he said.
Herd immunity does not require that populations have a huge level of antibodies that remains forever, he explained.
“It requires that in general, we’re not going to get infected as easily, and we’re not going to have disease as easily, and we’re not going to transmit the virus for as long,” he said.
Dr. Creech said he and others researching COVID-19 find that studies that show that antibodies fade quickly provide more proof “that this coronavirus is going to be here to stay unless we can take care of it through very effective treatments to take it from potentially fatal disease to one that is nothing more than a cold” or until a vaccine is developed.
He noted there are four other coronaviruses in widespread circulation every year that “amount to about 25% of the common cold.”
This study may help narrow the window as to when convalescent plasma – plasma that is taken from people who have recovered from COVID-19 and that is used to help people who are acutely ill with the disease – will be most effective, Dr. Creech explained. He said the results suggest that it is important that plasma be collected within the first couple of months after recovery so as to capture the most antibodies.
This study is important as another snapshot “so we understand the differences between severe and mild disease, so we can study it over time, so we have all the tools we need as we start these pivotal vaccine studies to make sure we’re making the right immune response for the right duration of time so we can put an end to this pandemic,” Dr. Creech concluded.
The study was supported by grants from the AIDS Healthcare Foundation, the Doris Duke Charitable Foundation, the National Institutes of Health, the James B. Pendleton Charitable Trust, and the McCarthy Family Foundation. A coauthor reports receiving grants from Gilead outside the submitted work. Dr. Creech has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The research was conducted by F. Javier Ibarrondo, PhD, and colleagues and was published online on July 21 in a letter to the editor of the New England Journal of Medicine. Ibarrondo is associate researcher at the University of California, Los Angeles. (The original letter incorrectly calculated the half-life at 73 days.)
Coauthor Otto Yang, MD, professor of medicine in the division of infectious diseases at UCLA, told Medscape Medical News that the rapidity in the antibody drop at 5 weeks “is striking compared to other infections.”
The phenomenon has been suspected and has been observed before but had not been quantified.
“Our paper is the first to put firm numbers on the dropping of antibodies after early infection,” he said.
The researchers evaluated 34 people (average age, 43 years) who had recovered from mild COVID-19 and had referred themselves to UCLA for observational research.
Previous report also found a quick fade
As Medscape Medical News reported, a previous study from China that was published in Nature Medicine also found that the antibodies fade quickly.
Interpreting the meaning of the current research comes with a few caveats, Dr. Yang said.
“One is that we don’t know for sure that antibodies are what protect people from getting infected,” he said. Although it’s a reasonable assumption, he said, that’s not always the case.
Another caveat is that even if antibodies do protect, the tests being used to measure them – including the test that was used in this study – may not measure them the right way, and it is not yet known how many antibodies are needed for protection, he explained.
The UCLA researchers used an enzyme-linked immunosorbent assay to detect anti–SARS-CoV-2 spike receptor–binding domain immunoglobulin G concentrations.
“No reason for anybody to be getting an antibody test medically”
The study provides further proof that “[t]here’s no reason for anybody to be getting an antibody test medically right now,” Dr. Yang said.
Additionally, “FDA-approved tests are not approved for quantitative measures, only qualitative,” he continued. He noted that the findings may have implications with respect to herd immunity.
“Herd immunity depends on a lot of people having immunity to the infection all at the same time. If infection is followed by only brief protection from infection, the natural infection is not going to reach herd immunity,” he explained.
Buddy Creech, MD, MPH, associate professor of pediatrics and director of the Vanderbilt Vaccine Research Program in Nashville, Tenn., pointed out that antibodies “are just part of the story.”
“When we make an immune response to any germ,” he said, “we not only make an immune response for the time being but for the future. The next time we’re exposed, we can call into action B cells and T cells who have been there and done that.”
So even though the antibodies fade over time, other arms of the immune system are being trained for future action, he said.
Herd immunity does not require that populations have a huge level of antibodies that remains forever, he explained.
“It requires that in general, we’re not going to get infected as easily, and we’re not going to have disease as easily, and we’re not going to transmit the virus for as long,” he said.
Dr. Creech said he and others researching COVID-19 find that studies that show that antibodies fade quickly provide more proof “that this coronavirus is going to be here to stay unless we can take care of it through very effective treatments to take it from potentially fatal disease to one that is nothing more than a cold” or until a vaccine is developed.
He noted there are four other coronaviruses in widespread circulation every year that “amount to about 25% of the common cold.”
This study may help narrow the window as to when convalescent plasma – plasma that is taken from people who have recovered from COVID-19 and that is used to help people who are acutely ill with the disease – will be most effective, Dr. Creech explained. He said the results suggest that it is important that plasma be collected within the first couple of months after recovery so as to capture the most antibodies.
This study is important as another snapshot “so we understand the differences between severe and mild disease, so we can study it over time, so we have all the tools we need as we start these pivotal vaccine studies to make sure we’re making the right immune response for the right duration of time so we can put an end to this pandemic,” Dr. Creech concluded.
The study was supported by grants from the AIDS Healthcare Foundation, the Doris Duke Charitable Foundation, the National Institutes of Health, the James B. Pendleton Charitable Trust, and the McCarthy Family Foundation. A coauthor reports receiving grants from Gilead outside the submitted work. Dr. Creech has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Is the presence of enanthem a clue for COVID-19?
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
FROM JAMA DERMATOLOGY
New developments in pustular psoriasis
It has various dermatologic and rheumatologic manifestations and sometimes overlaps with plaque psoriasis. Pustular palmoplantar psoriasis (PPP) affects the palmar and plantar areas of the skin, while generalized pustular psoriasis (GPP) can affect large areas of skin and tends to be more severe, even life threatening. PPP can accompany psoriatic arthritis or can be a side effect of tumor necrosis factor (TNF) inhibitor therapy, or a non–drug-induced component of rheumatologic syndromes, according to Kristina Callis Duffin, MD, an associate professor and chair of dermatology at the University of Utah, Salt Lake City.
“Each phenotype could be considered an orphan disease, and the response to therapy is often unpredictable,” Dr. Duffin said during a session on pustular psoriasis at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
But there is some positive news. A study in 2011 of several people with GPP opened the door to better understanding the pathophysiology of pustular psoriasis. Researchers identified a causal autosomal mutation in the IL36RN gene, which encodes an antagonist to the interleukin-36 receptor (Am J Hum Genet. 2011 Sep 9;89[3]:432-7). “As a result of this paper and others, drug development in this space has recently accelerated,” Dr. Duffin said.
In fact, she added,“it’s my opinion that pustular psoriasis is now where plaque psoriasis was 20 years ago, when accelerated drug development was driving a better understanding of the pathogenesis of psoriatic disease and its comorbidities, and also driving outcome measure development.”
In another presentation at the meeting, Hervé Bachelez, MD, PhD, professor of dermatology and immunologist at the University of Paris and Saint-Louis Hospital, Paris, discussed recent advances in drug development for pustular psoriasis. He noted other recent findings of genetic variants related to the disease, including AP1S3, CARD14, and SERPINA3.
For GPP, he said, the current algorithm for management is based on weak evidence for treatments like acitretin, cyclosporine, methotrexate, and infliximab. The story is similar for other biologics, with evidence in the form of case series; open-label studies; controlled, prospective studies; or retrospective analyses. Most of the evidence has been amassed for TNF inhibitors. A retrospective study of all TNF inhibitors suggested they may be effective as induction and maintenance therapy, he noted.
Among IL-17A inhibitors, a prospective study of 12 patients in Japan found secukinumab showed efficacy against GPP, as did studies of ixekizumab and brodalumab. A small phase 3 study in Japan demonstrated efficacy for the IL-23 inhibitor guselkumab in patients with erythrodermic psoriasis and GPP (J Dermatol. 2018 May;45[5]:529-39).
The limited data are a reflection in part of the difficulty in studying GPP, since its flares tend to be more self-remitting than with psoriasis vulgaris or PPP.
There are two monoclonal antibodies against the IL-36 receptor currently being developed. A proof-of-concept study of one of them, spesolimab, showed promise against GPP, with five of seven patients reaching “clear” or “almost clear” scores on the Generalized Pustular Psoriasis Physician Global Assessment within a week after infusion and in all seven by the fourth week (N Engl J Med. 2019 Mar 7;380[10]:981-3).
With respect to PPP, the strongest evidence for conventional therapies comes from two randomized, controlled trials of cyclosporine, with response rates of 48% and 89%, compared with 19% and 21%, respectively, in the placebo groups, although the primary endpoint was poorly designed, according to Dr. Bachelez. Retinoids like etretinate and acitretin, combined with psoralen and UVA, also have some supporting evidence regarding efficacy.
Among biologics, secukinumab did not fare well in a phase 3 study of patients with PPP. A subset of patients may benefit from it, but there are no biomarkers available to identify them, Dr. Bachelez said. A phase 2 study of guselkumab in Japan told a similar story, with only weak signs of efficacy. While there are many more ongoing clinical trials evaluating treatments for PPP, which is encouraging, PPP seems to be more challenging at this stage to tackle than GPP, Dr. Bachelez added. “The genetically inherited IL-36 antagonist abnormalities are clearly driving the advances regarding the pathogenesis of the disease, mainly for GPP rather than PPP.”
Part of the efforts to develop therapies for pustular psoriasis relies on the development of new outcome measures, or adaptation of existing ones. “We have a need to adapt or develop new investigator-reported measures, we need to adapt or develop new patient-reported outcomes,” Dr. Duffin said.
Many existing measures use inconsistent language and anchoring definitions, and some may be proprietary, she added. “The language varies by sponsor and is sometimes tweaked or modified by the agencies. Often synonyms are being used … it raises questions, does it change the validity of the instrument?”
Dr. Duffin called for the research community to use the pause in clinical research during the COVID-19 pandemic to reassess the research agenda, develop consensus on performing and training for GPP and PPP assessments, develop patient-reported outcomes, and strengthen connections to industry.
Dr. Duffin and Dr. Bachelez have consulted, served on the advisory board, been a speaker for, and/or received research support from a wide range of pharmaceutical companies, including those that manufacture and develop psoriasis treatments.
It has various dermatologic and rheumatologic manifestations and sometimes overlaps with plaque psoriasis. Pustular palmoplantar psoriasis (PPP) affects the palmar and plantar areas of the skin, while generalized pustular psoriasis (GPP) can affect large areas of skin and tends to be more severe, even life threatening. PPP can accompany psoriatic arthritis or can be a side effect of tumor necrosis factor (TNF) inhibitor therapy, or a non–drug-induced component of rheumatologic syndromes, according to Kristina Callis Duffin, MD, an associate professor and chair of dermatology at the University of Utah, Salt Lake City.
“Each phenotype could be considered an orphan disease, and the response to therapy is often unpredictable,” Dr. Duffin said during a session on pustular psoriasis at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
But there is some positive news. A study in 2011 of several people with GPP opened the door to better understanding the pathophysiology of pustular psoriasis. Researchers identified a causal autosomal mutation in the IL36RN gene, which encodes an antagonist to the interleukin-36 receptor (Am J Hum Genet. 2011 Sep 9;89[3]:432-7). “As a result of this paper and others, drug development in this space has recently accelerated,” Dr. Duffin said.
In fact, she added,“it’s my opinion that pustular psoriasis is now where plaque psoriasis was 20 years ago, when accelerated drug development was driving a better understanding of the pathogenesis of psoriatic disease and its comorbidities, and also driving outcome measure development.”
In another presentation at the meeting, Hervé Bachelez, MD, PhD, professor of dermatology and immunologist at the University of Paris and Saint-Louis Hospital, Paris, discussed recent advances in drug development for pustular psoriasis. He noted other recent findings of genetic variants related to the disease, including AP1S3, CARD14, and SERPINA3.
For GPP, he said, the current algorithm for management is based on weak evidence for treatments like acitretin, cyclosporine, methotrexate, and infliximab. The story is similar for other biologics, with evidence in the form of case series; open-label studies; controlled, prospective studies; or retrospective analyses. Most of the evidence has been amassed for TNF inhibitors. A retrospective study of all TNF inhibitors suggested they may be effective as induction and maintenance therapy, he noted.
Among IL-17A inhibitors, a prospective study of 12 patients in Japan found secukinumab showed efficacy against GPP, as did studies of ixekizumab and brodalumab. A small phase 3 study in Japan demonstrated efficacy for the IL-23 inhibitor guselkumab in patients with erythrodermic psoriasis and GPP (J Dermatol. 2018 May;45[5]:529-39).
The limited data are a reflection in part of the difficulty in studying GPP, since its flares tend to be more self-remitting than with psoriasis vulgaris or PPP.
There are two monoclonal antibodies against the IL-36 receptor currently being developed. A proof-of-concept study of one of them, spesolimab, showed promise against GPP, with five of seven patients reaching “clear” or “almost clear” scores on the Generalized Pustular Psoriasis Physician Global Assessment within a week after infusion and in all seven by the fourth week (N Engl J Med. 2019 Mar 7;380[10]:981-3).
With respect to PPP, the strongest evidence for conventional therapies comes from two randomized, controlled trials of cyclosporine, with response rates of 48% and 89%, compared with 19% and 21%, respectively, in the placebo groups, although the primary endpoint was poorly designed, according to Dr. Bachelez. Retinoids like etretinate and acitretin, combined with psoralen and UVA, also have some supporting evidence regarding efficacy.
Among biologics, secukinumab did not fare well in a phase 3 study of patients with PPP. A subset of patients may benefit from it, but there are no biomarkers available to identify them, Dr. Bachelez said. A phase 2 study of guselkumab in Japan told a similar story, with only weak signs of efficacy. While there are many more ongoing clinical trials evaluating treatments for PPP, which is encouraging, PPP seems to be more challenging at this stage to tackle than GPP, Dr. Bachelez added. “The genetically inherited IL-36 antagonist abnormalities are clearly driving the advances regarding the pathogenesis of the disease, mainly for GPP rather than PPP.”
Part of the efforts to develop therapies for pustular psoriasis relies on the development of new outcome measures, or adaptation of existing ones. “We have a need to adapt or develop new investigator-reported measures, we need to adapt or develop new patient-reported outcomes,” Dr. Duffin said.
Many existing measures use inconsistent language and anchoring definitions, and some may be proprietary, she added. “The language varies by sponsor and is sometimes tweaked or modified by the agencies. Often synonyms are being used … it raises questions, does it change the validity of the instrument?”
Dr. Duffin called for the research community to use the pause in clinical research during the COVID-19 pandemic to reassess the research agenda, develop consensus on performing and training for GPP and PPP assessments, develop patient-reported outcomes, and strengthen connections to industry.
Dr. Duffin and Dr. Bachelez have consulted, served on the advisory board, been a speaker for, and/or received research support from a wide range of pharmaceutical companies, including those that manufacture and develop psoriasis treatments.
It has various dermatologic and rheumatologic manifestations and sometimes overlaps with plaque psoriasis. Pustular palmoplantar psoriasis (PPP) affects the palmar and plantar areas of the skin, while generalized pustular psoriasis (GPP) can affect large areas of skin and tends to be more severe, even life threatening. PPP can accompany psoriatic arthritis or can be a side effect of tumor necrosis factor (TNF) inhibitor therapy, or a non–drug-induced component of rheumatologic syndromes, according to Kristina Callis Duffin, MD, an associate professor and chair of dermatology at the University of Utah, Salt Lake City.
“Each phenotype could be considered an orphan disease, and the response to therapy is often unpredictable,” Dr. Duffin said during a session on pustular psoriasis at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
But there is some positive news. A study in 2011 of several people with GPP opened the door to better understanding the pathophysiology of pustular psoriasis. Researchers identified a causal autosomal mutation in the IL36RN gene, which encodes an antagonist to the interleukin-36 receptor (Am J Hum Genet. 2011 Sep 9;89[3]:432-7). “As a result of this paper and others, drug development in this space has recently accelerated,” Dr. Duffin said.
In fact, she added,“it’s my opinion that pustular psoriasis is now where plaque psoriasis was 20 years ago, when accelerated drug development was driving a better understanding of the pathogenesis of psoriatic disease and its comorbidities, and also driving outcome measure development.”
In another presentation at the meeting, Hervé Bachelez, MD, PhD, professor of dermatology and immunologist at the University of Paris and Saint-Louis Hospital, Paris, discussed recent advances in drug development for pustular psoriasis. He noted other recent findings of genetic variants related to the disease, including AP1S3, CARD14, and SERPINA3.
For GPP, he said, the current algorithm for management is based on weak evidence for treatments like acitretin, cyclosporine, methotrexate, and infliximab. The story is similar for other biologics, with evidence in the form of case series; open-label studies; controlled, prospective studies; or retrospective analyses. Most of the evidence has been amassed for TNF inhibitors. A retrospective study of all TNF inhibitors suggested they may be effective as induction and maintenance therapy, he noted.
Among IL-17A inhibitors, a prospective study of 12 patients in Japan found secukinumab showed efficacy against GPP, as did studies of ixekizumab and brodalumab. A small phase 3 study in Japan demonstrated efficacy for the IL-23 inhibitor guselkumab in patients with erythrodermic psoriasis and GPP (J Dermatol. 2018 May;45[5]:529-39).
The limited data are a reflection in part of the difficulty in studying GPP, since its flares tend to be more self-remitting than with psoriasis vulgaris or PPP.
There are two monoclonal antibodies against the IL-36 receptor currently being developed. A proof-of-concept study of one of them, spesolimab, showed promise against GPP, with five of seven patients reaching “clear” or “almost clear” scores on the Generalized Pustular Psoriasis Physician Global Assessment within a week after infusion and in all seven by the fourth week (N Engl J Med. 2019 Mar 7;380[10]:981-3).
With respect to PPP, the strongest evidence for conventional therapies comes from two randomized, controlled trials of cyclosporine, with response rates of 48% and 89%, compared with 19% and 21%, respectively, in the placebo groups, although the primary endpoint was poorly designed, according to Dr. Bachelez. Retinoids like etretinate and acitretin, combined with psoralen and UVA, also have some supporting evidence regarding efficacy.
Among biologics, secukinumab did not fare well in a phase 3 study of patients with PPP. A subset of patients may benefit from it, but there are no biomarkers available to identify them, Dr. Bachelez said. A phase 2 study of guselkumab in Japan told a similar story, with only weak signs of efficacy. While there are many more ongoing clinical trials evaluating treatments for PPP, which is encouraging, PPP seems to be more challenging at this stage to tackle than GPP, Dr. Bachelez added. “The genetically inherited IL-36 antagonist abnormalities are clearly driving the advances regarding the pathogenesis of the disease, mainly for GPP rather than PPP.”
Part of the efforts to develop therapies for pustular psoriasis relies on the development of new outcome measures, or adaptation of existing ones. “We have a need to adapt or develop new investigator-reported measures, we need to adapt or develop new patient-reported outcomes,” Dr. Duffin said.
Many existing measures use inconsistent language and anchoring definitions, and some may be proprietary, she added. “The language varies by sponsor and is sometimes tweaked or modified by the agencies. Often synonyms are being used … it raises questions, does it change the validity of the instrument?”
Dr. Duffin called for the research community to use the pause in clinical research during the COVID-19 pandemic to reassess the research agenda, develop consensus on performing and training for GPP and PPP assessments, develop patient-reported outcomes, and strengthen connections to industry.
Dr. Duffin and Dr. Bachelez have consulted, served on the advisory board, been a speaker for, and/or received research support from a wide range of pharmaceutical companies, including those that manufacture and develop psoriasis treatments.
FROM THE GRAPPA 2020 VIRTUAL ANNUAL MEETING
Patch testing in children: An evolving science
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
FROM SPD 2020
Ob.gyns. struggle to keep pace with changing COVID-19 knowledge
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
OSHA in the COVID-19 era
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Medics with ‘long COVID’ call for clinical recognition
Thousands of coronavirus patients risk going without treatment and support for debilitating symptoms lasting months because of a lack of awareness of ‘long COVID’, according to a group formed by clinicians with extended serious after-effects of the virus.
Many members of the 100-strong Facebook group UK doctors: COVID “Long tail” have been unable to work for weeks after failing to recover from an episode of COVID-19. They warn of the need for clinical recognition of “long COVID,” along with systems to log symptoms and manage patients in the community. Without this, there could be major consequences for return to work across all professions, as well as implications for disease prevention.
‘Weird symptoms’
Three of the group: Dr Amali Lokugamage, consultant obstetrician at the Whittington Hospital; Dr Sharon Taylor, child psychiatrist at St Mary’s Hospital London, and Dr Clare Rayner, a retired occupational health physician and lecturer at the University of Manchester, have highlighted their concerns in The BMJ and on social media groups. They say colleagues are observing a range of symptoms of long COVID in their practices.
These include cardiac, gut and respiratory symptoms, skin manifestations, neurological and psychiatric symptoms, severe fatigue, and relapsing fevers, sometimes continuing for more than 16 weeks, and which they say go well beyond definitions of chronic fatigue. The authors are also aware of a pattern of symptom clusters recurring every third or fourth day, which in some cases are so severe that people are having to take extended periods of sick leave.
Writing in The BMJ the authors say: “Concerns have been raised about the lack of awareness among NHS doctors, nurses, paramedics, and other healthcare professionals with regard to the prolonged, varied, and weird symptoms [of COVID-19].”
Speaking to Medscape News UK, Dr. Clare Rayner said: “We see a huge need that is not being met, because these cases are just not being seen in hospital. All the attention has been on the acute illness.”
She pointed to the urgent need for government planning for a surge in people requiring support to return to work following long-term COVID-19 symptoms. According to occupational health research, only 10-40% of people who take 6 weeks off work return to work, dropping to 5%-10% after an absence of 6 months.
In her own case, she is recovering after 4 months of illness, including a hospital admission with gut symptoms and dehydration, and 2 weeks of social service home support. She has experienced a range of relapsing and remitting symptoms, which she describes as ‘bizarre and coming in phases’.
Stimulating recovery
The recently-announced NHS portal for COVID-19 patients has been welcomed by the authors as an opportunity for long-standing symptoms to reach the medical and Government radar. But Dr Taylor believes it should have been set up from the start with input from patients with symptoms, to make sure that any support provided reflects the nature of the problems experienced.
In her case, as a previously regular gym attender with a resting heart rate in the 50s, she has now been diagnosed as having multi-organ disease affecting her heart, spleen, lung, and autonomic system. She has fluid on the lungs and heart, and suffers from continuous chest pain and oxygen desaturation when lying down. She has not been able to work since she contracted COVID-19 in March.
“COVID patients with the chronic form of the disease need to be involved in research right from the start to ensure the right questions are asked - not just those who have had acute disease,” she insists to Medscape News UK. “We need to gather evidence, to inform the development of a multi-disciplinary approach and a range of rehabilitation options depending on the organs involved.
“The focus needs to be on stimulating recovery and preventing development of chronic problems. We still don’t know if those with chronic COVID disease are infectious, how long their prolonged cardio-respiratory and neurological complications will last, and crucially whether treatment will reduce the duration of their problems. The worry is that left unattended, these patients may develop irreversible damage leading to chronic illness.”
General practice
GPs have been at the forefront of management of the long-standing consequences of COVID-19. In its recent report General practice in the post-COVID world, the Royal College of General Practitioners highlights the need for urgent government planning and funding to prepare general practice services for facilitating the recovery of local communities.
The report calls on the four governments of the UK each to produce a comprehensive plan to support GPs in managing the longer-term effects of COVID-19 in the community, including costed proposals for additional funding for general practice; workforce solutions; reductions in regulatory burdens and ‘red tape’; a systematic approach for identifying patients most likely to need primary care support, and proposals for how health inequalities will be minimized to ensure all patients have access to the necessary post-COVID-19 care.
RCGP Chair Professor Martin Marshall said: “COVID-19 will leave a lingering and difficult legacy and it is GPs working with patients in their communities who will be picking up the pieces.”
One issue is the lack of a reliable estimate of the prevalence of post viral symptoms for other viruses, let alone for COVID-19. Even a 1% chance of long-term problems amongst survivors would suggest 2500 with a need for extra support, but experience with post-viral syndrome generally suggests the prevalence may be more like 3%.
The BMA has been carrying out tracker surveys of its own members at 2-week intervals since March. The most recent, involving more than 5000 doctors, indicated that around 30% of doctors who believed they’d had COVID-19 were still experiencing physical symptoms they thought were caused by the virus, 21% had taken sick leave, and a further 9% had taken annual leave to deal with ongoing symptoms.
Dr David Strain, chair of the BMA medical academic staff committee and clinical senior lecturer at the University of Exeter Medical School, has a particular interest in the after-effects of COVID-19. He said it was becoming evident that the virus was leaving a lasting legacy with a significant number of people, even younger ones.
He told Medscape News UK: “Once COVID-19 enters the nervous system, the lasting symptoms on people can range from a mild loss of sense of smell or taste, to more severe symptoms such as difficulties in concentration. A small number have also been left with chronic fatigue syndrome, which is poorly understood, and can be difficult to treat. This does not appear to be dependent on the initial severity of COVID-19 symptoms.
“Currently, it is impossible to predict the prevalence of longer-lasting effects. A full assessment of COVID-19’s impact will only be possible once people return to work on a regular basis and the effect on their physical health becomes evident. Of the doctors in the BMA survey who had experienced COVID-19, 15% took sick leave beyond their acute illness, and another 6% used annual leave allowance to extend their recovery time.
“Clearly, more research will be needed into the long-term consequences of COVID-19 and the future treatments needed to deal with them.”
Further research
The National Institute for Health Research (NIHR) has called for applications for research to enhance understanding and management of the health and social care consequences of the global COVID-19 pandemic beyond the acute phase, with a particular focus on ‘health outcomes, public health, social care and health service delivery and to mitigate the impact of subsequent phases and aftermath’.
The authors of The BMJ article stress the wide-ranging nature of ‘long COVID’ symptoms and warn of the dangers of treating them for research purposes under the banner of chronic fatigue. They say: “These wide-ranging, unusual, and potentially very serious symptoms can be anxiety-provoking, particularly secondary to a virus that has only been known to the world for 8 months and which we have barely begun to understand. However, it is dismissive solely to attribute such symptoms to anxiety in the thousands of patients like ourselves who have attended hospital or general practice with chronic COVID-19.”
This article first appeared on Medscape.com.
Thousands of coronavirus patients risk going without treatment and support for debilitating symptoms lasting months because of a lack of awareness of ‘long COVID’, according to a group formed by clinicians with extended serious after-effects of the virus.
Many members of the 100-strong Facebook group UK doctors: COVID “Long tail” have been unable to work for weeks after failing to recover from an episode of COVID-19. They warn of the need for clinical recognition of “long COVID,” along with systems to log symptoms and manage patients in the community. Without this, there could be major consequences for return to work across all professions, as well as implications for disease prevention.
‘Weird symptoms’
Three of the group: Dr Amali Lokugamage, consultant obstetrician at the Whittington Hospital; Dr Sharon Taylor, child psychiatrist at St Mary’s Hospital London, and Dr Clare Rayner, a retired occupational health physician and lecturer at the University of Manchester, have highlighted their concerns in The BMJ and on social media groups. They say colleagues are observing a range of symptoms of long COVID in their practices.
These include cardiac, gut and respiratory symptoms, skin manifestations, neurological and psychiatric symptoms, severe fatigue, and relapsing fevers, sometimes continuing for more than 16 weeks, and which they say go well beyond definitions of chronic fatigue. The authors are also aware of a pattern of symptom clusters recurring every third or fourth day, which in some cases are so severe that people are having to take extended periods of sick leave.
Writing in The BMJ the authors say: “Concerns have been raised about the lack of awareness among NHS doctors, nurses, paramedics, and other healthcare professionals with regard to the prolonged, varied, and weird symptoms [of COVID-19].”
Speaking to Medscape News UK, Dr. Clare Rayner said: “We see a huge need that is not being met, because these cases are just not being seen in hospital. All the attention has been on the acute illness.”
She pointed to the urgent need for government planning for a surge in people requiring support to return to work following long-term COVID-19 symptoms. According to occupational health research, only 10-40% of people who take 6 weeks off work return to work, dropping to 5%-10% after an absence of 6 months.
In her own case, she is recovering after 4 months of illness, including a hospital admission with gut symptoms and dehydration, and 2 weeks of social service home support. She has experienced a range of relapsing and remitting symptoms, which she describes as ‘bizarre and coming in phases’.
Stimulating recovery
The recently-announced NHS portal for COVID-19 patients has been welcomed by the authors as an opportunity for long-standing symptoms to reach the medical and Government radar. But Dr Taylor believes it should have been set up from the start with input from patients with symptoms, to make sure that any support provided reflects the nature of the problems experienced.
In her case, as a previously regular gym attender with a resting heart rate in the 50s, she has now been diagnosed as having multi-organ disease affecting her heart, spleen, lung, and autonomic system. She has fluid on the lungs and heart, and suffers from continuous chest pain and oxygen desaturation when lying down. She has not been able to work since she contracted COVID-19 in March.
“COVID patients with the chronic form of the disease need to be involved in research right from the start to ensure the right questions are asked - not just those who have had acute disease,” she insists to Medscape News UK. “We need to gather evidence, to inform the development of a multi-disciplinary approach and a range of rehabilitation options depending on the organs involved.
“The focus needs to be on stimulating recovery and preventing development of chronic problems. We still don’t know if those with chronic COVID disease are infectious, how long their prolonged cardio-respiratory and neurological complications will last, and crucially whether treatment will reduce the duration of their problems. The worry is that left unattended, these patients may develop irreversible damage leading to chronic illness.”
General practice
GPs have been at the forefront of management of the long-standing consequences of COVID-19. In its recent report General practice in the post-COVID world, the Royal College of General Practitioners highlights the need for urgent government planning and funding to prepare general practice services for facilitating the recovery of local communities.
The report calls on the four governments of the UK each to produce a comprehensive plan to support GPs in managing the longer-term effects of COVID-19 in the community, including costed proposals for additional funding for general practice; workforce solutions; reductions in regulatory burdens and ‘red tape’; a systematic approach for identifying patients most likely to need primary care support, and proposals for how health inequalities will be minimized to ensure all patients have access to the necessary post-COVID-19 care.
RCGP Chair Professor Martin Marshall said: “COVID-19 will leave a lingering and difficult legacy and it is GPs working with patients in their communities who will be picking up the pieces.”
One issue is the lack of a reliable estimate of the prevalence of post viral symptoms for other viruses, let alone for COVID-19. Even a 1% chance of long-term problems amongst survivors would suggest 2500 with a need for extra support, but experience with post-viral syndrome generally suggests the prevalence may be more like 3%.
The BMA has been carrying out tracker surveys of its own members at 2-week intervals since March. The most recent, involving more than 5000 doctors, indicated that around 30% of doctors who believed they’d had COVID-19 were still experiencing physical symptoms they thought were caused by the virus, 21% had taken sick leave, and a further 9% had taken annual leave to deal with ongoing symptoms.
Dr David Strain, chair of the BMA medical academic staff committee and clinical senior lecturer at the University of Exeter Medical School, has a particular interest in the after-effects of COVID-19. He said it was becoming evident that the virus was leaving a lasting legacy with a significant number of people, even younger ones.
He told Medscape News UK: “Once COVID-19 enters the nervous system, the lasting symptoms on people can range from a mild loss of sense of smell or taste, to more severe symptoms such as difficulties in concentration. A small number have also been left with chronic fatigue syndrome, which is poorly understood, and can be difficult to treat. This does not appear to be dependent on the initial severity of COVID-19 symptoms.
“Currently, it is impossible to predict the prevalence of longer-lasting effects. A full assessment of COVID-19’s impact will only be possible once people return to work on a regular basis and the effect on their physical health becomes evident. Of the doctors in the BMA survey who had experienced COVID-19, 15% took sick leave beyond their acute illness, and another 6% used annual leave allowance to extend their recovery time.
“Clearly, more research will be needed into the long-term consequences of COVID-19 and the future treatments needed to deal with them.”
Further research
The National Institute for Health Research (NIHR) has called for applications for research to enhance understanding and management of the health and social care consequences of the global COVID-19 pandemic beyond the acute phase, with a particular focus on ‘health outcomes, public health, social care and health service delivery and to mitigate the impact of subsequent phases and aftermath’.
The authors of The BMJ article stress the wide-ranging nature of ‘long COVID’ symptoms and warn of the dangers of treating them for research purposes under the banner of chronic fatigue. They say: “These wide-ranging, unusual, and potentially very serious symptoms can be anxiety-provoking, particularly secondary to a virus that has only been known to the world for 8 months and which we have barely begun to understand. However, it is dismissive solely to attribute such symptoms to anxiety in the thousands of patients like ourselves who have attended hospital or general practice with chronic COVID-19.”
This article first appeared on Medscape.com.
Thousands of coronavirus patients risk going without treatment and support for debilitating symptoms lasting months because of a lack of awareness of ‘long COVID’, according to a group formed by clinicians with extended serious after-effects of the virus.
Many members of the 100-strong Facebook group UK doctors: COVID “Long tail” have been unable to work for weeks after failing to recover from an episode of COVID-19. They warn of the need for clinical recognition of “long COVID,” along with systems to log symptoms and manage patients in the community. Without this, there could be major consequences for return to work across all professions, as well as implications for disease prevention.
‘Weird symptoms’
Three of the group: Dr Amali Lokugamage, consultant obstetrician at the Whittington Hospital; Dr Sharon Taylor, child psychiatrist at St Mary’s Hospital London, and Dr Clare Rayner, a retired occupational health physician and lecturer at the University of Manchester, have highlighted their concerns in The BMJ and on social media groups. They say colleagues are observing a range of symptoms of long COVID in their practices.
These include cardiac, gut and respiratory symptoms, skin manifestations, neurological and psychiatric symptoms, severe fatigue, and relapsing fevers, sometimes continuing for more than 16 weeks, and which they say go well beyond definitions of chronic fatigue. The authors are also aware of a pattern of symptom clusters recurring every third or fourth day, which in some cases are so severe that people are having to take extended periods of sick leave.
Writing in The BMJ the authors say: “Concerns have been raised about the lack of awareness among NHS doctors, nurses, paramedics, and other healthcare professionals with regard to the prolonged, varied, and weird symptoms [of COVID-19].”
Speaking to Medscape News UK, Dr. Clare Rayner said: “We see a huge need that is not being met, because these cases are just not being seen in hospital. All the attention has been on the acute illness.”
She pointed to the urgent need for government planning for a surge in people requiring support to return to work following long-term COVID-19 symptoms. According to occupational health research, only 10-40% of people who take 6 weeks off work return to work, dropping to 5%-10% after an absence of 6 months.
In her own case, she is recovering after 4 months of illness, including a hospital admission with gut symptoms and dehydration, and 2 weeks of social service home support. She has experienced a range of relapsing and remitting symptoms, which she describes as ‘bizarre and coming in phases’.
Stimulating recovery
The recently-announced NHS portal for COVID-19 patients has been welcomed by the authors as an opportunity for long-standing symptoms to reach the medical and Government radar. But Dr Taylor believes it should have been set up from the start with input from patients with symptoms, to make sure that any support provided reflects the nature of the problems experienced.
In her case, as a previously regular gym attender with a resting heart rate in the 50s, she has now been diagnosed as having multi-organ disease affecting her heart, spleen, lung, and autonomic system. She has fluid on the lungs and heart, and suffers from continuous chest pain and oxygen desaturation when lying down. She has not been able to work since she contracted COVID-19 in March.
“COVID patients with the chronic form of the disease need to be involved in research right from the start to ensure the right questions are asked - not just those who have had acute disease,” she insists to Medscape News UK. “We need to gather evidence, to inform the development of a multi-disciplinary approach and a range of rehabilitation options depending on the organs involved.
“The focus needs to be on stimulating recovery and preventing development of chronic problems. We still don’t know if those with chronic COVID disease are infectious, how long their prolonged cardio-respiratory and neurological complications will last, and crucially whether treatment will reduce the duration of their problems. The worry is that left unattended, these patients may develop irreversible damage leading to chronic illness.”
General practice
GPs have been at the forefront of management of the long-standing consequences of COVID-19. In its recent report General practice in the post-COVID world, the Royal College of General Practitioners highlights the need for urgent government planning and funding to prepare general practice services for facilitating the recovery of local communities.
The report calls on the four governments of the UK each to produce a comprehensive plan to support GPs in managing the longer-term effects of COVID-19 in the community, including costed proposals for additional funding for general practice; workforce solutions; reductions in regulatory burdens and ‘red tape’; a systematic approach for identifying patients most likely to need primary care support, and proposals for how health inequalities will be minimized to ensure all patients have access to the necessary post-COVID-19 care.
RCGP Chair Professor Martin Marshall said: “COVID-19 will leave a lingering and difficult legacy and it is GPs working with patients in their communities who will be picking up the pieces.”
One issue is the lack of a reliable estimate of the prevalence of post viral symptoms for other viruses, let alone for COVID-19. Even a 1% chance of long-term problems amongst survivors would suggest 2500 with a need for extra support, but experience with post-viral syndrome generally suggests the prevalence may be more like 3%.
The BMA has been carrying out tracker surveys of its own members at 2-week intervals since March. The most recent, involving more than 5000 doctors, indicated that around 30% of doctors who believed they’d had COVID-19 were still experiencing physical symptoms they thought were caused by the virus, 21% had taken sick leave, and a further 9% had taken annual leave to deal with ongoing symptoms.
Dr David Strain, chair of the BMA medical academic staff committee and clinical senior lecturer at the University of Exeter Medical School, has a particular interest in the after-effects of COVID-19. He said it was becoming evident that the virus was leaving a lasting legacy with a significant number of people, even younger ones.
He told Medscape News UK: “Once COVID-19 enters the nervous system, the lasting symptoms on people can range from a mild loss of sense of smell or taste, to more severe symptoms such as difficulties in concentration. A small number have also been left with chronic fatigue syndrome, which is poorly understood, and can be difficult to treat. This does not appear to be dependent on the initial severity of COVID-19 symptoms.
“Currently, it is impossible to predict the prevalence of longer-lasting effects. A full assessment of COVID-19’s impact will only be possible once people return to work on a regular basis and the effect on their physical health becomes evident. Of the doctors in the BMA survey who had experienced COVID-19, 15% took sick leave beyond their acute illness, and another 6% used annual leave allowance to extend their recovery time.
“Clearly, more research will be needed into the long-term consequences of COVID-19 and the future treatments needed to deal with them.”
Further research
The National Institute for Health Research (NIHR) has called for applications for research to enhance understanding and management of the health and social care consequences of the global COVID-19 pandemic beyond the acute phase, with a particular focus on ‘health outcomes, public health, social care and health service delivery and to mitigate the impact of subsequent phases and aftermath’.
The authors of The BMJ article stress the wide-ranging nature of ‘long COVID’ symptoms and warn of the dangers of treating them for research purposes under the banner of chronic fatigue. They say: “These wide-ranging, unusual, and potentially very serious symptoms can be anxiety-provoking, particularly secondary to a virus that has only been known to the world for 8 months and which we have barely begun to understand. However, it is dismissive solely to attribute such symptoms to anxiety in the thousands of patients like ourselves who have attended hospital or general practice with chronic COVID-19.”
This article first appeared on Medscape.com.















