User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Match Day 2020: Online announcements replace celebrations, champagne
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
Feds tout drug candidates to treat COVID-19
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”
CD4 cells implicated in pathology of CCCA
in the lymphocytic inflammatory infiltrate, according to a histopathological study of biopsy specimens.
“Evaluation of the T-cell infiltrate may be a useful way to distinguish CCCA from lichen planopilaris or frontal fibrosing alopecia in some cases when histopathological features alone cannot be used to definitely distinguish between them,” reported Alexandra Flamm, MD, Ata Moshiri, MD, and coauthors from the departments of dermatology and pathology, University of Pennsylvania, Philadelphia.
The histopathological features of CCCA have been characterized previously, but the goal of this study was to go further in piecing together the pathophysiology, they noted.
Horizontal sections of 4-mm punch biopsy specimens were examined from 18 black women with a known diagnosis of CCCA. Both affected and unaffected follicles were evaluated with attention to the number and percentage of CD1a+ Langerhans cells, CD3+, CD4+, and CD8+ lymphocytes.
In this series, the lymphocytic infiltrate in both the affected and unaffected follicles was predominantly composed of CD4+ cells. The perifollicular ratio for CD4+ to CD8+ cells in affected follicles was 5.3:1. It was only modestly lower in unaffected follicles (4.3:1) and in the intrafollicular space of affected follicles (2.5:1).
Affected follicles had a higher number of CD1a+ Langerhans cells than unaffected follicles. This finding suggests, as others have hypothesized, that the antigen-presenting Langerhans cells draw lymphocytes to the follicle, according to the investigators. Elevated numbers of Langerhans cells have also been reported in other forms of scarring alopecia, such as lichen planopilaris (LPP).
In the case of CCCA, CD1a+ Langerhans cells appear to localize to the hair follicle in response to stimulus such as an injury. The CD4+ cells that follow the Langerhans cells participate in an inflammatory reaction that drives follicle destruction. In addition to this damage and scarring, the inflammatory response is also likely to be disrupting the blood supply.
“Fibroplasia associated with follicular scarring displaces blood vessels away from the outer root sheath epithelium,” the authors explained. Ultimately, “the mucinous fibroplasia and perifollicular fibrosis may disrupt and fragment blood vessels in the fibrous sheath, leaving only small clusters of vessels more distant to the keratinocytes in the outer root sheath.”
Prior studies of scarring alopecia diseases, including LLP, frontal fibrosing alopecia (FFA), and keratosis follicularis spinulosa decalvans (KFSD), have typically described a predominantly CD8+ lymphocytic infiltrate. The evidence from this study that the infiltrate is CD4+ predominant in CCCA supports the conclusion that the pathophysiologic features of this type of alopecia are unique, according to the authors.
Work by others has associated CCCA with mutations in the PAD13 gene, which suggests a defect in the formation of hair shaft structure, but this may speak to susceptibility but not the mechanism of hair follicle damage. Rather, this study suggests that it is the concentration of a CD4+ predominant lymphocytic infiltrate in the perifollicular space that induces the pathological events.
For determining the fundamental cause of CCCA, “it will be important to determine what recruits the Langerhans cells to affected follicles,” the investigators suggested. Meanwhile, they expressed hope that the progress being made into decoding the pathogenesis of CCCA will lead to novel therapeutic strategies.
The authors did not list any disclosures. The funding source was listed as the Center for Scientific Review (Grant/Award).
SOURCE: Flamm A et al. J Cutan Pathol. 2020 Feb 18.doi: 10.1111/cup.13666.
in the lymphocytic inflammatory infiltrate, according to a histopathological study of biopsy specimens.
“Evaluation of the T-cell infiltrate may be a useful way to distinguish CCCA from lichen planopilaris or frontal fibrosing alopecia in some cases when histopathological features alone cannot be used to definitely distinguish between them,” reported Alexandra Flamm, MD, Ata Moshiri, MD, and coauthors from the departments of dermatology and pathology, University of Pennsylvania, Philadelphia.
The histopathological features of CCCA have been characterized previously, but the goal of this study was to go further in piecing together the pathophysiology, they noted.
Horizontal sections of 4-mm punch biopsy specimens were examined from 18 black women with a known diagnosis of CCCA. Both affected and unaffected follicles were evaluated with attention to the number and percentage of CD1a+ Langerhans cells, CD3+, CD4+, and CD8+ lymphocytes.
In this series, the lymphocytic infiltrate in both the affected and unaffected follicles was predominantly composed of CD4+ cells. The perifollicular ratio for CD4+ to CD8+ cells in affected follicles was 5.3:1. It was only modestly lower in unaffected follicles (4.3:1) and in the intrafollicular space of affected follicles (2.5:1).
Affected follicles had a higher number of CD1a+ Langerhans cells than unaffected follicles. This finding suggests, as others have hypothesized, that the antigen-presenting Langerhans cells draw lymphocytes to the follicle, according to the investigators. Elevated numbers of Langerhans cells have also been reported in other forms of scarring alopecia, such as lichen planopilaris (LPP).
In the case of CCCA, CD1a+ Langerhans cells appear to localize to the hair follicle in response to stimulus such as an injury. The CD4+ cells that follow the Langerhans cells participate in an inflammatory reaction that drives follicle destruction. In addition to this damage and scarring, the inflammatory response is also likely to be disrupting the blood supply.
“Fibroplasia associated with follicular scarring displaces blood vessels away from the outer root sheath epithelium,” the authors explained. Ultimately, “the mucinous fibroplasia and perifollicular fibrosis may disrupt and fragment blood vessels in the fibrous sheath, leaving only small clusters of vessels more distant to the keratinocytes in the outer root sheath.”
Prior studies of scarring alopecia diseases, including LLP, frontal fibrosing alopecia (FFA), and keratosis follicularis spinulosa decalvans (KFSD), have typically described a predominantly CD8+ lymphocytic infiltrate. The evidence from this study that the infiltrate is CD4+ predominant in CCCA supports the conclusion that the pathophysiologic features of this type of alopecia are unique, according to the authors.
Work by others has associated CCCA with mutations in the PAD13 gene, which suggests a defect in the formation of hair shaft structure, but this may speak to susceptibility but not the mechanism of hair follicle damage. Rather, this study suggests that it is the concentration of a CD4+ predominant lymphocytic infiltrate in the perifollicular space that induces the pathological events.
For determining the fundamental cause of CCCA, “it will be important to determine what recruits the Langerhans cells to affected follicles,” the investigators suggested. Meanwhile, they expressed hope that the progress being made into decoding the pathogenesis of CCCA will lead to novel therapeutic strategies.
The authors did not list any disclosures. The funding source was listed as the Center for Scientific Review (Grant/Award).
SOURCE: Flamm A et al. J Cutan Pathol. 2020 Feb 18.doi: 10.1111/cup.13666.
in the lymphocytic inflammatory infiltrate, according to a histopathological study of biopsy specimens.
“Evaluation of the T-cell infiltrate may be a useful way to distinguish CCCA from lichen planopilaris or frontal fibrosing alopecia in some cases when histopathological features alone cannot be used to definitely distinguish between them,” reported Alexandra Flamm, MD, Ata Moshiri, MD, and coauthors from the departments of dermatology and pathology, University of Pennsylvania, Philadelphia.
The histopathological features of CCCA have been characterized previously, but the goal of this study was to go further in piecing together the pathophysiology, they noted.
Horizontal sections of 4-mm punch biopsy specimens were examined from 18 black women with a known diagnosis of CCCA. Both affected and unaffected follicles were evaluated with attention to the number and percentage of CD1a+ Langerhans cells, CD3+, CD4+, and CD8+ lymphocytes.
In this series, the lymphocytic infiltrate in both the affected and unaffected follicles was predominantly composed of CD4+ cells. The perifollicular ratio for CD4+ to CD8+ cells in affected follicles was 5.3:1. It was only modestly lower in unaffected follicles (4.3:1) and in the intrafollicular space of affected follicles (2.5:1).
Affected follicles had a higher number of CD1a+ Langerhans cells than unaffected follicles. This finding suggests, as others have hypothesized, that the antigen-presenting Langerhans cells draw lymphocytes to the follicle, according to the investigators. Elevated numbers of Langerhans cells have also been reported in other forms of scarring alopecia, such as lichen planopilaris (LPP).
In the case of CCCA, CD1a+ Langerhans cells appear to localize to the hair follicle in response to stimulus such as an injury. The CD4+ cells that follow the Langerhans cells participate in an inflammatory reaction that drives follicle destruction. In addition to this damage and scarring, the inflammatory response is also likely to be disrupting the blood supply.
“Fibroplasia associated with follicular scarring displaces blood vessels away from the outer root sheath epithelium,” the authors explained. Ultimately, “the mucinous fibroplasia and perifollicular fibrosis may disrupt and fragment blood vessels in the fibrous sheath, leaving only small clusters of vessels more distant to the keratinocytes in the outer root sheath.”
Prior studies of scarring alopecia diseases, including LLP, frontal fibrosing alopecia (FFA), and keratosis follicularis spinulosa decalvans (KFSD), have typically described a predominantly CD8+ lymphocytic infiltrate. The evidence from this study that the infiltrate is CD4+ predominant in CCCA supports the conclusion that the pathophysiologic features of this type of alopecia are unique, according to the authors.
Work by others has associated CCCA with mutations in the PAD13 gene, which suggests a defect in the formation of hair shaft structure, but this may speak to susceptibility but not the mechanism of hair follicle damage. Rather, this study suggests that it is the concentration of a CD4+ predominant lymphocytic infiltrate in the perifollicular space that induces the pathological events.
For determining the fundamental cause of CCCA, “it will be important to determine what recruits the Langerhans cells to affected follicles,” the investigators suggested. Meanwhile, they expressed hope that the progress being made into decoding the pathogenesis of CCCA will lead to novel therapeutic strategies.
The authors did not list any disclosures. The funding source was listed as the Center for Scientific Review (Grant/Award).
SOURCE: Flamm A et al. J Cutan Pathol. 2020 Feb 18.doi: 10.1111/cup.13666.
20% of U.S. COVID-19 deaths were aged 20-64 years
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
New melanoma treatments linked to mortality decline
Recent advances in treatment appear to have reversed the course of melanoma mortality since 2013, according to data published in the American Journal of Public Health.
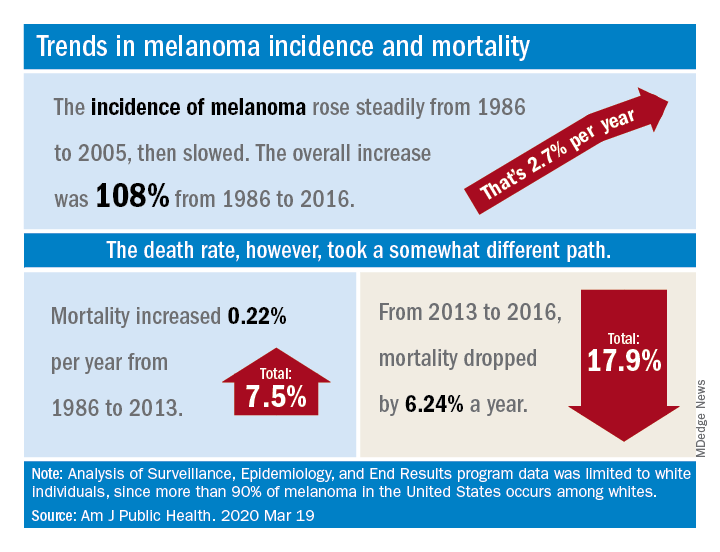
The U.S. death rate for melanoma, which had been rising at a rate of 0.22% a year for more than 2 decades, dropped by 17.9%, or 6.24% per year, during 2013-2016. That decline “coincides with the introduction of multiple new and efficacious treatments for metastatic melanoma,” such as BRAF inhibitors and immune checkpoint inhibitors, study author Juliana Berk-Krauss, MD, of the State University of New York Downstate Medical Center in Brooklyn and colleagues wrote.
The other possible explanation for the decline in deaths, “education and early detection resulting in migration toward earlier stage melanomas with a greater chance of surgical cure,” is unlikely, according to the investigators. That’s because the small decrease in median tumor thickness that occurred during 1989-2009 “is not associated with changes in prognosis.”
The investigators’ analysis encompassed data from the Surveillance, Epidemiology, and End Results registry recorded during 1986-2016. Nine registry areas were included (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah), which covered about 9.4% of the U.S. population. The analysis was limited to the white population, which accounts for more than 90% of melanoma cases in the United States.
The data showed a slight decline in annual percent change in melanoma incidence, from 3.24% for 1986-2005 to 1.72% for 2006-2016. However, over the whole period studied (1986-2016), melanoma incidence increased by 108%, or about 2.7% per year.
“Given the increased incidence of melanoma throughout this period and the lack of stage migration, these data strongly suggest that the mortality decline is due to the extended survival associated with these [newer] treatments,” the investigators wrote.
This study was funded by NYU Langone. Two investigators disclosed potential conflicts of interest, including relationships with Bio-Rad Laboratories, Novartis, Merck, and several other companies.
SOURCE: Berk-Krauss J et al. Am J Public Health. 2020 Mar 19. doi: 10.2105/AJPH.2020.305567.
Recent advances in treatment appear to have reversed the course of melanoma mortality since 2013, according to data published in the American Journal of Public Health.

The U.S. death rate for melanoma, which had been rising at a rate of 0.22% a year for more than 2 decades, dropped by 17.9%, or 6.24% per year, during 2013-2016. That decline “coincides with the introduction of multiple new and efficacious treatments for metastatic melanoma,” such as BRAF inhibitors and immune checkpoint inhibitors, study author Juliana Berk-Krauss, MD, of the State University of New York Downstate Medical Center in Brooklyn and colleagues wrote.
The other possible explanation for the decline in deaths, “education and early detection resulting in migration toward earlier stage melanomas with a greater chance of surgical cure,” is unlikely, according to the investigators. That’s because the small decrease in median tumor thickness that occurred during 1989-2009 “is not associated with changes in prognosis.”
The investigators’ analysis encompassed data from the Surveillance, Epidemiology, and End Results registry recorded during 1986-2016. Nine registry areas were included (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah), which covered about 9.4% of the U.S. population. The analysis was limited to the white population, which accounts for more than 90% of melanoma cases in the United States.
The data showed a slight decline in annual percent change in melanoma incidence, from 3.24% for 1986-2005 to 1.72% for 2006-2016. However, over the whole period studied (1986-2016), melanoma incidence increased by 108%, or about 2.7% per year.
“Given the increased incidence of melanoma throughout this period and the lack of stage migration, these data strongly suggest that the mortality decline is due to the extended survival associated with these [newer] treatments,” the investigators wrote.
This study was funded by NYU Langone. Two investigators disclosed potential conflicts of interest, including relationships with Bio-Rad Laboratories, Novartis, Merck, and several other companies.
SOURCE: Berk-Krauss J et al. Am J Public Health. 2020 Mar 19. doi: 10.2105/AJPH.2020.305567.
Recent advances in treatment appear to have reversed the course of melanoma mortality since 2013, according to data published in the American Journal of Public Health.

The U.S. death rate for melanoma, which had been rising at a rate of 0.22% a year for more than 2 decades, dropped by 17.9%, or 6.24% per year, during 2013-2016. That decline “coincides with the introduction of multiple new and efficacious treatments for metastatic melanoma,” such as BRAF inhibitors and immune checkpoint inhibitors, study author Juliana Berk-Krauss, MD, of the State University of New York Downstate Medical Center in Brooklyn and colleagues wrote.
The other possible explanation for the decline in deaths, “education and early detection resulting in migration toward earlier stage melanomas with a greater chance of surgical cure,” is unlikely, according to the investigators. That’s because the small decrease in median tumor thickness that occurred during 1989-2009 “is not associated with changes in prognosis.”
The investigators’ analysis encompassed data from the Surveillance, Epidemiology, and End Results registry recorded during 1986-2016. Nine registry areas were included (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah), which covered about 9.4% of the U.S. population. The analysis was limited to the white population, which accounts for more than 90% of melanoma cases in the United States.
The data showed a slight decline in annual percent change in melanoma incidence, from 3.24% for 1986-2005 to 1.72% for 2006-2016. However, over the whole period studied (1986-2016), melanoma incidence increased by 108%, or about 2.7% per year.
“Given the increased incidence of melanoma throughout this period and the lack of stage migration, these data strongly suggest that the mortality decline is due to the extended survival associated with these [newer] treatments,” the investigators wrote.
This study was funded by NYU Langone. Two investigators disclosed potential conflicts of interest, including relationships with Bio-Rad Laboratories, Novartis, Merck, and several other companies.
SOURCE: Berk-Krauss J et al. Am J Public Health. 2020 Mar 19. doi: 10.2105/AJPH.2020.305567.
FROM THE AMERICAN JOURNAL OF PUBLIC HEALTH
April 2020
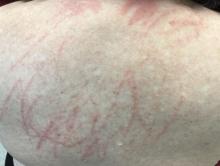
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Rheumatologists to share knowledge in COVID-19 patient-centered registry
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.
Real-world shortages not addressed in new COVID-19 guidance
Newly updated guidance on treating patients with the novel coronavirus (COVID-19) has been published by the World Health Organization.
While it can’t replace clinical judgment or specialist consultation, the new guidance may help strengthen the clinical management of patients when COVID-19 is suspected, according to its authors.
The guidance, adapted from an earlier edition focused on the management of suspected Middle East respiratory syndrome coronavirus (MERS-CoV), covers best practices for triage, infection prevention and control, and optimized supportive care for mild, severe, or critical coronavirus disease 2019 (COVID-19).
“This guidance should serve as a foundation for optimized supportive care to ensure the best possible chance for survival,” the authors wrote in the guidance.
While the WHO guidance does provide solid facts to support best practices for managing COVID-19, providers will also need to look beyond the document to tackle real-world issues, said David M. Ferraro, MD, FCCP, a pulmonary and critical care physician and associate professor of medicine at National Jewish Health in Denver.
For example, while the guidelines address the importance of screening and triage, limited COVID-19 testing may be a barrier to timely diagnoses that might compel more individuals to comply with social distancing recommendations, according to Dr. Ferraro, vice chair of the Fundamental Disaster Management Committee for the Society of Critical Care Medicine (SCCM).
“If we’re not providing people with confirmation that they have the virus, they may potentially continue to be spreaders of the disease, because they don’t have that absolute proof,” Dr. Ferraro said in an interview. “I think that’s where we are limited right now, because often we’re not able to tell the mild symptomatic people – or even the asymptomatic people – that they really need to play a role in preventing further spread.”
Likewise, the guidelines provide sound guidance on management of severe or critical COVID-19, according to Dr. Ferraro, yet they don’t address the potential for shortages of trained health care personnel to handle more severe cases requiring ventilation. That’s clearly an important issue, he said, especially with recent reports that the COVID-19 pandemic has pushed Italian intensive care units (ICUs) to the brink of collapse.
If the pandemic reaches crisis levels in the United States, nearly 1 million people would need ventilatory support, according to a recent report from SCCM on U.S. resource availability for COVID-19. And while there are an estimated 200,000 ventilators available in the United States, it’s estimated in that report that only 135,000 patients could be handled at a time, given the shortage of ICU physicians, advanced practice providers, nurses, and respiratory therapists with training in mechanical ventilation.
“If our ICUs get overwhelmed and swarmed, we may have the technology available, but we may not have enough resources and personnel to safely manage the number of patients,” Dr. Ferraro said.
The solution to that, according to the SCCM report, is to focus on expanding the pool of trained professionals who may be needed, not only to mechanically ventilate patients with COVID-19, but also to care for other critically ill patients routinely cared for in the ICU. They also suggest adopting a “tiered staffing strategy” in which non-ICU trained health care providers augment the capacity of experienced ICU staff.
With the prospect of untrained health care workers in mind, the WHO guidance could be a valuable resource for those who do have to jump into ICU roles, according to Dr. Ferraro.
The WHO also stresses immediate implementation of appropriate measures for infection prevention and control (IPC). According to their guidance, IPC needs to be initiated right at the point where the patient enters the hospital, with screening done at the first point of contact in the emergency department or outpatient clinics.
If patients are suspected to have COVID-19, they should receive a mask, and should be directed to a separate area where they are kept at least 1 meter apart from other individuals with suspected COVID-19, according to the WHO. (The Centers for Disease Control and Prevention recommends maintaining a distance of 6 feet to prevent spread of illness).
Beyond standard precautions such as hand washing and use of personal protective equipment, health care workers should do a point-of-care risk assessment at every patient contact to determine whether additional precautions are required.
Having standard IPC measures in place is “paramount,” according to Dr. Ferraro, for a disease that has no available vaccine, no proven treatments, and a stealthy spread fueled by asymptomatic carriers.
“Those are huge weapons against us, and the only thing we really have to knock this down is really infection prevention control, so that truly is at the cornerstone,” he said. “These are things that we must strictly follow.”
Newly updated guidance on treating patients with the novel coronavirus (COVID-19) has been published by the World Health Organization.
While it can’t replace clinical judgment or specialist consultation, the new guidance may help strengthen the clinical management of patients when COVID-19 is suspected, according to its authors.
The guidance, adapted from an earlier edition focused on the management of suspected Middle East respiratory syndrome coronavirus (MERS-CoV), covers best practices for triage, infection prevention and control, and optimized supportive care for mild, severe, or critical coronavirus disease 2019 (COVID-19).
“This guidance should serve as a foundation for optimized supportive care to ensure the best possible chance for survival,” the authors wrote in the guidance.
While the WHO guidance does provide solid facts to support best practices for managing COVID-19, providers will also need to look beyond the document to tackle real-world issues, said David M. Ferraro, MD, FCCP, a pulmonary and critical care physician and associate professor of medicine at National Jewish Health in Denver.
For example, while the guidelines address the importance of screening and triage, limited COVID-19 testing may be a barrier to timely diagnoses that might compel more individuals to comply with social distancing recommendations, according to Dr. Ferraro, vice chair of the Fundamental Disaster Management Committee for the Society of Critical Care Medicine (SCCM).
“If we’re not providing people with confirmation that they have the virus, they may potentially continue to be spreaders of the disease, because they don’t have that absolute proof,” Dr. Ferraro said in an interview. “I think that’s where we are limited right now, because often we’re not able to tell the mild symptomatic people – or even the asymptomatic people – that they really need to play a role in preventing further spread.”
Likewise, the guidelines provide sound guidance on management of severe or critical COVID-19, according to Dr. Ferraro, yet they don’t address the potential for shortages of trained health care personnel to handle more severe cases requiring ventilation. That’s clearly an important issue, he said, especially with recent reports that the COVID-19 pandemic has pushed Italian intensive care units (ICUs) to the brink of collapse.
If the pandemic reaches crisis levels in the United States, nearly 1 million people would need ventilatory support, according to a recent report from SCCM on U.S. resource availability for COVID-19. And while there are an estimated 200,000 ventilators available in the United States, it’s estimated in that report that only 135,000 patients could be handled at a time, given the shortage of ICU physicians, advanced practice providers, nurses, and respiratory therapists with training in mechanical ventilation.
“If our ICUs get overwhelmed and swarmed, we may have the technology available, but we may not have enough resources and personnel to safely manage the number of patients,” Dr. Ferraro said.
The solution to that, according to the SCCM report, is to focus on expanding the pool of trained professionals who may be needed, not only to mechanically ventilate patients with COVID-19, but also to care for other critically ill patients routinely cared for in the ICU. They also suggest adopting a “tiered staffing strategy” in which non-ICU trained health care providers augment the capacity of experienced ICU staff.
With the prospect of untrained health care workers in mind, the WHO guidance could be a valuable resource for those who do have to jump into ICU roles, according to Dr. Ferraro.
The WHO also stresses immediate implementation of appropriate measures for infection prevention and control (IPC). According to their guidance, IPC needs to be initiated right at the point where the patient enters the hospital, with screening done at the first point of contact in the emergency department or outpatient clinics.
If patients are suspected to have COVID-19, they should receive a mask, and should be directed to a separate area where they are kept at least 1 meter apart from other individuals with suspected COVID-19, according to the WHO. (The Centers for Disease Control and Prevention recommends maintaining a distance of 6 feet to prevent spread of illness).
Beyond standard precautions such as hand washing and use of personal protective equipment, health care workers should do a point-of-care risk assessment at every patient contact to determine whether additional precautions are required.
Having standard IPC measures in place is “paramount,” according to Dr. Ferraro, for a disease that has no available vaccine, no proven treatments, and a stealthy spread fueled by asymptomatic carriers.
“Those are huge weapons against us, and the only thing we really have to knock this down is really infection prevention control, so that truly is at the cornerstone,” he said. “These are things that we must strictly follow.”
Newly updated guidance on treating patients with the novel coronavirus (COVID-19) has been published by the World Health Organization.
While it can’t replace clinical judgment or specialist consultation, the new guidance may help strengthen the clinical management of patients when COVID-19 is suspected, according to its authors.
The guidance, adapted from an earlier edition focused on the management of suspected Middle East respiratory syndrome coronavirus (MERS-CoV), covers best practices for triage, infection prevention and control, and optimized supportive care for mild, severe, or critical coronavirus disease 2019 (COVID-19).
“This guidance should serve as a foundation for optimized supportive care to ensure the best possible chance for survival,” the authors wrote in the guidance.
While the WHO guidance does provide solid facts to support best practices for managing COVID-19, providers will also need to look beyond the document to tackle real-world issues, said David M. Ferraro, MD, FCCP, a pulmonary and critical care physician and associate professor of medicine at National Jewish Health in Denver.
For example, while the guidelines address the importance of screening and triage, limited COVID-19 testing may be a barrier to timely diagnoses that might compel more individuals to comply with social distancing recommendations, according to Dr. Ferraro, vice chair of the Fundamental Disaster Management Committee for the Society of Critical Care Medicine (SCCM).
“If we’re not providing people with confirmation that they have the virus, they may potentially continue to be spreaders of the disease, because they don’t have that absolute proof,” Dr. Ferraro said in an interview. “I think that’s where we are limited right now, because often we’re not able to tell the mild symptomatic people – or even the asymptomatic people – that they really need to play a role in preventing further spread.”
Likewise, the guidelines provide sound guidance on management of severe or critical COVID-19, according to Dr. Ferraro, yet they don’t address the potential for shortages of trained health care personnel to handle more severe cases requiring ventilation. That’s clearly an important issue, he said, especially with recent reports that the COVID-19 pandemic has pushed Italian intensive care units (ICUs) to the brink of collapse.
If the pandemic reaches crisis levels in the United States, nearly 1 million people would need ventilatory support, according to a recent report from SCCM on U.S. resource availability for COVID-19. And while there are an estimated 200,000 ventilators available in the United States, it’s estimated in that report that only 135,000 patients could be handled at a time, given the shortage of ICU physicians, advanced practice providers, nurses, and respiratory therapists with training in mechanical ventilation.
“If our ICUs get overwhelmed and swarmed, we may have the technology available, but we may not have enough resources and personnel to safely manage the number of patients,” Dr. Ferraro said.
The solution to that, according to the SCCM report, is to focus on expanding the pool of trained professionals who may be needed, not only to mechanically ventilate patients with COVID-19, but also to care for other critically ill patients routinely cared for in the ICU. They also suggest adopting a “tiered staffing strategy” in which non-ICU trained health care providers augment the capacity of experienced ICU staff.
With the prospect of untrained health care workers in mind, the WHO guidance could be a valuable resource for those who do have to jump into ICU roles, according to Dr. Ferraro.
The WHO also stresses immediate implementation of appropriate measures for infection prevention and control (IPC). According to their guidance, IPC needs to be initiated right at the point where the patient enters the hospital, with screening done at the first point of contact in the emergency department or outpatient clinics.
If patients are suspected to have COVID-19, they should receive a mask, and should be directed to a separate area where they are kept at least 1 meter apart from other individuals with suspected COVID-19, according to the WHO. (The Centers for Disease Control and Prevention recommends maintaining a distance of 6 feet to prevent spread of illness).
Beyond standard precautions such as hand washing and use of personal protective equipment, health care workers should do a point-of-care risk assessment at every patient contact to determine whether additional precautions are required.
Having standard IPC measures in place is “paramount,” according to Dr. Ferraro, for a disease that has no available vaccine, no proven treatments, and a stealthy spread fueled by asymptomatic carriers.
“Those are huge weapons against us, and the only thing we really have to knock this down is really infection prevention control, so that truly is at the cornerstone,” he said. “These are things that we must strictly follow.”
FROM THE WORLD HEALTH ORGANIZATION
Lopinavir-ritonavir trial results ‘disappointing’ for severe COVID-19
No difference in the primary endpoint of the time to clinical improvement was seen in an open-label trial of the antiretroviral drug lopinavir-ritonavir versus standard of care in adult patients hospitalized with severe COVID-19.
The median time to clinical improvement – defined as the time from randomization to either an improvement of two points on a seven-category ordinal scale or discharge from the hospital, whichever was first – was 16 days in both groups (hazard ratio, 1.31; 95% confidence interval 0.95-1.85; P = .09).
Although a numerically lower number of deaths were recorded at 28 days (19.2% vs. 25%) with the antiretroviral treatment versus standard of care, a similar percentage of patients had detectable levels of viral RNA in throat swabs taken at various time points during the study, Chinese researchers reported in the New England Journal of Medicine.
Bai Cao, M.D, from the China-Japan Friendship Hospital and Chinese Academy of Medical Sciences, both in Beijing, and associates, performed a randomized, controlled, trial of 199 adult patients hospitalized at the Jin Yin-Tan Hospital in Wuhan in Hubei Province, China.
For inclusion, patients had to have laboratory confirmed infection with SARS-Cov-2, the virus that causes COVID-19; pneumonia confirmed by chest imaging; and severely reduced oxygen saturation (94% or less while breathing ambient air or a ratio of the partial pressure of oxygen to the fraction of inspired oxygen of less than 300 mm Hg).
Patients were randomized to receive either a combination of lopinavir (400 mg) and ritonavir (100 mg) twice a day on top of standard care (n = 99) or to standard care alone (n = 100) for 14 days. “Because of the emergency nature of the trial, placebos of lopinavir-ritonavir were not prepared,” Dr. Cao et al. explained, noting that standard care consisted of supplemental oxygen, ventilation, antibiotic treatment, vasopressor support, renal-replacement therapy, and extracorporeal membrane oxygenation.
While the trial did not meet its primary endpoint, Dr. Cao et al. reported that patients in the lopinavir-ritonavir group had a shorter stay in the intensive care unit than did those in the standard-care group, at a median of 6 versus 11 days. They also reported that the duration from randomization to hospital discharge was numerically shorter, at a median of 12 versus 14 days. Furthermore, there was a higher percentage of patients with clinical improvement at day 14 in the lopinavir-ritonavir group than in the standard-care group (45.5% vs. 30.0%)
“The trial was initiated in rapid response to the COVID-19 public health emergency, at which time there was very limited information about clinical outcomes in hospitalized patients with COVID-19,” the researchers observed. “The question of whether earlier lopinavir-ritonavir treatment in COVID-19 could have clinical benefit is an important one that requires further study,” they wrote.
Among the trial’s limitations are it was not blinded and the researchers do not have data on the lopinavir exposure levels in the trial participants.
“This was a heroic effort” in a “particularly challenging population,” Lindsey R. Baden, MD, and Eric J. Rubin, MD, stated in an editorial accompanying the article (Baden LR and Rubin EJ. New Engl J Med. March 18, 2020. doi: 10.1056/NEJMe2005477). “Unfortunately, the trial results were disappointing,” they noted.
“The secondary end points provide both reason for hope and reason for discouragement,” wrote Dr. Baden and Dr. Rubin. The lack of effect on viral shedding, however, “strongly [suggests] that it did not have the activity desired” they observed.
Dr. Baden and Dr. Rubin commented that one of the important takeaways from the trial is that “the investigators appropriately prioritized speed, designing a trial that could rapidly produce an answer.” They continued that the investigators had shown that “rapidly initiated, high-quality randomized clinical trials are possible in epidemic conditions” and that results of such trials, whether they are positive or negative, “will be central to clinical care as the dangerous coronavirus outbreak continues.”
As it stands, more than 100 trials are listed in the ClinicalTrials.gov database as testing a wide range of different treatment approaches for COVID-19. These include trials investigating if sarilumab, hydroxychloroquine, fingolimod, bevacizumab, and losartan might have a role to play. There are also trials looking at the potential of other antiviral agents, such as Gilead’s investigational drug remdesivir, which has shown to have in vitro and in vivo activity against many emerging viral pathogens that cause Ebola, Middle Eastern Respiratory Syndrome, and Severe Acute Respiratory Syndrome.
Dr. Cao’s trial was supported by grants from Major Projects of National Science and Technology on New Drug Creation and Development and from the Chinese Academy of Medical Sciences (CAMS) Emergency Project of Covid-19, and a National Science Grant for Distinguished Young Scholars.
All authors had no financial conflicts of interest to disclose.
Dr. Baden is the director of clinical research in the Division of Infectious Diseases at the Brigham and Women’s Hospital and the director of infectious diseases at the Dana-Farber Cancer Institute. He is a deputy editor of the New England Journal of Medicine and chair of the FDA’s Antimicrobial Drug Advisory Committee. He is involved in HIV vaccine clinical trials and has received research grants from the Ragon Institute, the National Institutes of Health/National Institute of Allergy and Infectious Diseases, and the Gates Foundation.
Dr. Rubin is employed by the New England Journal of Medicine as editor-in-chief. He is an associate physician at Brigham and Women’s Hospital and is chair and Irene Heinz Given Professor of Immunology and Infectious Diseases at the Harvard T.H. Chan School of Public Health.
SOURCE: Cao B et al. New Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282.
No difference in the primary endpoint of the time to clinical improvement was seen in an open-label trial of the antiretroviral drug lopinavir-ritonavir versus standard of care in adult patients hospitalized with severe COVID-19.
The median time to clinical improvement – defined as the time from randomization to either an improvement of two points on a seven-category ordinal scale or discharge from the hospital, whichever was first – was 16 days in both groups (hazard ratio, 1.31; 95% confidence interval 0.95-1.85; P = .09).
Although a numerically lower number of deaths were recorded at 28 days (19.2% vs. 25%) with the antiretroviral treatment versus standard of care, a similar percentage of patients had detectable levels of viral RNA in throat swabs taken at various time points during the study, Chinese researchers reported in the New England Journal of Medicine.
Bai Cao, M.D, from the China-Japan Friendship Hospital and Chinese Academy of Medical Sciences, both in Beijing, and associates, performed a randomized, controlled, trial of 199 adult patients hospitalized at the Jin Yin-Tan Hospital in Wuhan in Hubei Province, China.
For inclusion, patients had to have laboratory confirmed infection with SARS-Cov-2, the virus that causes COVID-19; pneumonia confirmed by chest imaging; and severely reduced oxygen saturation (94% or less while breathing ambient air or a ratio of the partial pressure of oxygen to the fraction of inspired oxygen of less than 300 mm Hg).
Patients were randomized to receive either a combination of lopinavir (400 mg) and ritonavir (100 mg) twice a day on top of standard care (n = 99) or to standard care alone (n = 100) for 14 days. “Because of the emergency nature of the trial, placebos of lopinavir-ritonavir were not prepared,” Dr. Cao et al. explained, noting that standard care consisted of supplemental oxygen, ventilation, antibiotic treatment, vasopressor support, renal-replacement therapy, and extracorporeal membrane oxygenation.
While the trial did not meet its primary endpoint, Dr. Cao et al. reported that patients in the lopinavir-ritonavir group had a shorter stay in the intensive care unit than did those in the standard-care group, at a median of 6 versus 11 days. They also reported that the duration from randomization to hospital discharge was numerically shorter, at a median of 12 versus 14 days. Furthermore, there was a higher percentage of patients with clinical improvement at day 14 in the lopinavir-ritonavir group than in the standard-care group (45.5% vs. 30.0%)
“The trial was initiated in rapid response to the COVID-19 public health emergency, at which time there was very limited information about clinical outcomes in hospitalized patients with COVID-19,” the researchers observed. “The question of whether earlier lopinavir-ritonavir treatment in COVID-19 could have clinical benefit is an important one that requires further study,” they wrote.
Among the trial’s limitations are it was not blinded and the researchers do not have data on the lopinavir exposure levels in the trial participants.
“This was a heroic effort” in a “particularly challenging population,” Lindsey R. Baden, MD, and Eric J. Rubin, MD, stated in an editorial accompanying the article (Baden LR and Rubin EJ. New Engl J Med. March 18, 2020. doi: 10.1056/NEJMe2005477). “Unfortunately, the trial results were disappointing,” they noted.
“The secondary end points provide both reason for hope and reason for discouragement,” wrote Dr. Baden and Dr. Rubin. The lack of effect on viral shedding, however, “strongly [suggests] that it did not have the activity desired” they observed.
Dr. Baden and Dr. Rubin commented that one of the important takeaways from the trial is that “the investigators appropriately prioritized speed, designing a trial that could rapidly produce an answer.” They continued that the investigators had shown that “rapidly initiated, high-quality randomized clinical trials are possible in epidemic conditions” and that results of such trials, whether they are positive or negative, “will be central to clinical care as the dangerous coronavirus outbreak continues.”
As it stands, more than 100 trials are listed in the ClinicalTrials.gov database as testing a wide range of different treatment approaches for COVID-19. These include trials investigating if sarilumab, hydroxychloroquine, fingolimod, bevacizumab, and losartan might have a role to play. There are also trials looking at the potential of other antiviral agents, such as Gilead’s investigational drug remdesivir, which has shown to have in vitro and in vivo activity against many emerging viral pathogens that cause Ebola, Middle Eastern Respiratory Syndrome, and Severe Acute Respiratory Syndrome.
Dr. Cao’s trial was supported by grants from Major Projects of National Science and Technology on New Drug Creation and Development and from the Chinese Academy of Medical Sciences (CAMS) Emergency Project of Covid-19, and a National Science Grant for Distinguished Young Scholars.
All authors had no financial conflicts of interest to disclose.
Dr. Baden is the director of clinical research in the Division of Infectious Diseases at the Brigham and Women’s Hospital and the director of infectious diseases at the Dana-Farber Cancer Institute. He is a deputy editor of the New England Journal of Medicine and chair of the FDA’s Antimicrobial Drug Advisory Committee. He is involved in HIV vaccine clinical trials and has received research grants from the Ragon Institute, the National Institutes of Health/National Institute of Allergy and Infectious Diseases, and the Gates Foundation.
Dr. Rubin is employed by the New England Journal of Medicine as editor-in-chief. He is an associate physician at Brigham and Women’s Hospital and is chair and Irene Heinz Given Professor of Immunology and Infectious Diseases at the Harvard T.H. Chan School of Public Health.
SOURCE: Cao B et al. New Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282.
No difference in the primary endpoint of the time to clinical improvement was seen in an open-label trial of the antiretroviral drug lopinavir-ritonavir versus standard of care in adult patients hospitalized with severe COVID-19.
The median time to clinical improvement – defined as the time from randomization to either an improvement of two points on a seven-category ordinal scale or discharge from the hospital, whichever was first – was 16 days in both groups (hazard ratio, 1.31; 95% confidence interval 0.95-1.85; P = .09).
Although a numerically lower number of deaths were recorded at 28 days (19.2% vs. 25%) with the antiretroviral treatment versus standard of care, a similar percentage of patients had detectable levels of viral RNA in throat swabs taken at various time points during the study, Chinese researchers reported in the New England Journal of Medicine.
Bai Cao, M.D, from the China-Japan Friendship Hospital and Chinese Academy of Medical Sciences, both in Beijing, and associates, performed a randomized, controlled, trial of 199 adult patients hospitalized at the Jin Yin-Tan Hospital in Wuhan in Hubei Province, China.
For inclusion, patients had to have laboratory confirmed infection with SARS-Cov-2, the virus that causes COVID-19; pneumonia confirmed by chest imaging; and severely reduced oxygen saturation (94% or less while breathing ambient air or a ratio of the partial pressure of oxygen to the fraction of inspired oxygen of less than 300 mm Hg).
Patients were randomized to receive either a combination of lopinavir (400 mg) and ritonavir (100 mg) twice a day on top of standard care (n = 99) or to standard care alone (n = 100) for 14 days. “Because of the emergency nature of the trial, placebos of lopinavir-ritonavir were not prepared,” Dr. Cao et al. explained, noting that standard care consisted of supplemental oxygen, ventilation, antibiotic treatment, vasopressor support, renal-replacement therapy, and extracorporeal membrane oxygenation.
While the trial did not meet its primary endpoint, Dr. Cao et al. reported that patients in the lopinavir-ritonavir group had a shorter stay in the intensive care unit than did those in the standard-care group, at a median of 6 versus 11 days. They also reported that the duration from randomization to hospital discharge was numerically shorter, at a median of 12 versus 14 days. Furthermore, there was a higher percentage of patients with clinical improvement at day 14 in the lopinavir-ritonavir group than in the standard-care group (45.5% vs. 30.0%)
“The trial was initiated in rapid response to the COVID-19 public health emergency, at which time there was very limited information about clinical outcomes in hospitalized patients with COVID-19,” the researchers observed. “The question of whether earlier lopinavir-ritonavir treatment in COVID-19 could have clinical benefit is an important one that requires further study,” they wrote.
Among the trial’s limitations are it was not blinded and the researchers do not have data on the lopinavir exposure levels in the trial participants.
“This was a heroic effort” in a “particularly challenging population,” Lindsey R. Baden, MD, and Eric J. Rubin, MD, stated in an editorial accompanying the article (Baden LR and Rubin EJ. New Engl J Med. March 18, 2020. doi: 10.1056/NEJMe2005477). “Unfortunately, the trial results were disappointing,” they noted.
“The secondary end points provide both reason for hope and reason for discouragement,” wrote Dr. Baden and Dr. Rubin. The lack of effect on viral shedding, however, “strongly [suggests] that it did not have the activity desired” they observed.
Dr. Baden and Dr. Rubin commented that one of the important takeaways from the trial is that “the investigators appropriately prioritized speed, designing a trial that could rapidly produce an answer.” They continued that the investigators had shown that “rapidly initiated, high-quality randomized clinical trials are possible in epidemic conditions” and that results of such trials, whether they are positive or negative, “will be central to clinical care as the dangerous coronavirus outbreak continues.”
As it stands, more than 100 trials are listed in the ClinicalTrials.gov database as testing a wide range of different treatment approaches for COVID-19. These include trials investigating if sarilumab, hydroxychloroquine, fingolimod, bevacizumab, and losartan might have a role to play. There are also trials looking at the potential of other antiviral agents, such as Gilead’s investigational drug remdesivir, which has shown to have in vitro and in vivo activity against many emerging viral pathogens that cause Ebola, Middle Eastern Respiratory Syndrome, and Severe Acute Respiratory Syndrome.
Dr. Cao’s trial was supported by grants from Major Projects of National Science and Technology on New Drug Creation and Development and from the Chinese Academy of Medical Sciences (CAMS) Emergency Project of Covid-19, and a National Science Grant for Distinguished Young Scholars.
All authors had no financial conflicts of interest to disclose.
Dr. Baden is the director of clinical research in the Division of Infectious Diseases at the Brigham and Women’s Hospital and the director of infectious diseases at the Dana-Farber Cancer Institute. He is a deputy editor of the New England Journal of Medicine and chair of the FDA’s Antimicrobial Drug Advisory Committee. He is involved in HIV vaccine clinical trials and has received research grants from the Ragon Institute, the National Institutes of Health/National Institute of Allergy and Infectious Diseases, and the Gates Foundation.
Dr. Rubin is employed by the New England Journal of Medicine as editor-in-chief. He is an associate physician at Brigham and Women’s Hospital and is chair and Irene Heinz Given Professor of Immunology and Infectious Diseases at the Harvard T.H. Chan School of Public Health.
SOURCE: Cao B et al. New Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Dermatology therapies evolve as disease knowledge and investment grow
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.