User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Should patients with COVID-19 avoid ibuprofen or RAAS antagonists?
Researchers have hypothesized that treatments that increase angiotensin-converting enzyme 2 (ACE2) may also increase the risk of novel coronavirus disease (COVID-19). This speculation and other concerns have led some officials and organizations to question whether ibuprofen or other drugs such as renin angiotensin aldosterone system (RAAS) antagonists should be avoided as treatments in patients with COVID-19. Health agencies and professional organizations have said they are not recommending against these medications.
The Food and Drug Administration on March 19 advised patients that it was “not aware of scientific evidence connecting” nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen “with worsening COVID-19 symptoms.”
“The agency is investigating this issue further and will communicate publicly when more information is available,” the FDA said. “However, all prescription NSAID labels warn that ‘the pharmacological activity of NSAIDs in reducing inflammation, and possibly fever, may diminish the utility of diagnostic signs in detecting infections.’ ” The FDA also noted that other over-the-counter and prescription medications are available for pain relief and fever reduction, and patients who “are concerned about taking NSAIDs and rely on these medications to treat chronic diseases” should talk to a health care provider.
A World Health Organization spokesperson said during a press conference on March 17 that the organization was looking into concerns about ibuprofen use in patients with COVID-19 and suggested that in the meantime patients take acetaminophen for fever instead. On March 18, the WHO said that it was not recommending against the use of ibuprofen.
“At present, based on currently available information, WHO does not recommend against the use of ibuprofen,” the organization said. “We are also consulting with physicians treating COVID-19 patients and are not aware of reports of any negative effects of ibuprofen, beyond the usual known side effects that limit its use in certain populations. WHO is not aware of published clinical or population-based data on this topic.”
A spokesperson for the National Institute of Allergy and Infectious Diseases said on March 18, “More research is needed to evaluate reports that ibruprofen and other over-the-counter anti-inflammatory drugs may affect the course of COVID-19. Currently, there is no conclusive evidence that ibuprofen and other over-the-counter anti-inflammatory drugs increase the risk of serious complications or of acquiring the virus that causes COVID-19. There is also no conclusive evidence that taking over-the-counter anti-inflammatory drugs is harmful for other respiratory infections.”
The European Medicines Agency (EMA) on March 18 said, “There is currently no scientific evidence establishing a link between ibuprofen and worsening of COVID‑19. EMA is monitoring the situation closely and will review any new information that becomes available on this issue in the context of the pandemic.”
In correspondence published March 11 in the Lancet Respiratory Medicine, Lei Fang, MD, of the department of biomedicine at University Hospital Basel (Switzerland), and colleagues suggested that patients with hypertension and diabetes mellitus may be at increased risk of COVID-19 because these comorbidities “are often treated with angiotensin converting enzyme (ACE) inhibitors.” In addition, “ACE2 polymorphisms that have been linked to diabetes mellitus, cerebral stroke, and hypertension” also may play a role, the researchers said (Lancet Respir Med. 2020 Mar 11. https://doi.org/10.1016/S2213-2600(20)30116-8).
“ACE2 is substantially increased in patients with type 1 or type 2 diabetes, who are treated with ACE inhibitors and angiotensin II type-I receptor blockers (ARBs). Hypertension is also treated with ACE inhibitors and ARBs, which results in an upregulation of ACE2. ACE2 can also be increased by thiazolidinediones and ibuprofen.”
A March 16 statement from the Heart Failure Society of America (HSFC), American College of Cardiology (ACC), and American Heart Association (AHA) addressed concerns about using RAAS antagonists in COVID-19.
“Patients with underlying cardiovascular diseases appear to have an increased risk for adverse outcomes with [COVID-19],” the organizations said. “Although the clinical manifestations of COVID-19 are dominated by respiratory symptoms, some patients also may have severe cardiovascular damage. [ACE2] receptors have been shown to be the entry point into human cells for SARS-CoV-2, the virus that causes COVID-19. In a few experimental studies with animal models, both [ACE] inhibitors and angiotensin receptor blockers (ARBs) have been shown to upregulate ACE2 expression in the heart. Though these have not been shown in human studies, or in the setting of COVID-19, such potential upregulation of ACE2 by ACE inhibitors or ARBs has resulted in a speculation of potential increased risk for COVID-19 infection in patients with background treatment of these medications.”
ACE2, ACE, angiotensin II, and other RAAS system interactions “are quite complex, and at times, paradoxical,” the statement says. “In experimental studies, both ACE inhibitors and ARBs have been shown to reduce severe lung injury in certain viral pneumonias, and it has been speculated that these agents could be beneficial in COVID-19.
“Currently there are no experimental or clinical data demonstrating beneficial or adverse outcomes with background use of ACE inhibitors, ARBs or other RAAS antagonists in COVID-19 or among COVID-19 patients with a history of cardiovascular disease treated with such agents. The HFSA, ACC, and AHA recommend continuation of RAAS antagonists for those patients who are currently prescribed such agents for indications for which these agents are known to be beneficial, such as heart failure, hypertension, or ischemic heart disease. In the event patients with cardiovascular disease are diagnosed with COVID-19, individualized treatment decisions should be made according to each patient’s hemodynamic status and clinical presentation. Therefore, be advised not to add or remove any RAAS-related treatments, beyond actions based on standard clinical practice.
“These theoretical concerns and findings of cardiovascular involvement with COVID-19 deserve much more detailed research, and quickly. As further research and developments related to this issue evolve, we will update these recommendations as needed.”
Dr. Fang and colleagues had no competing interests.
SOURCE: Fang L et al. Lancet Respir Med. 2020 Mar 11. doi: 10.1016/S2213-2600(20)30116-8.
Researchers have hypothesized that treatments that increase angiotensin-converting enzyme 2 (ACE2) may also increase the risk of novel coronavirus disease (COVID-19). This speculation and other concerns have led some officials and organizations to question whether ibuprofen or other drugs such as renin angiotensin aldosterone system (RAAS) antagonists should be avoided as treatments in patients with COVID-19. Health agencies and professional organizations have said they are not recommending against these medications.
The Food and Drug Administration on March 19 advised patients that it was “not aware of scientific evidence connecting” nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen “with worsening COVID-19 symptoms.”
“The agency is investigating this issue further and will communicate publicly when more information is available,” the FDA said. “However, all prescription NSAID labels warn that ‘the pharmacological activity of NSAIDs in reducing inflammation, and possibly fever, may diminish the utility of diagnostic signs in detecting infections.’ ” The FDA also noted that other over-the-counter and prescription medications are available for pain relief and fever reduction, and patients who “are concerned about taking NSAIDs and rely on these medications to treat chronic diseases” should talk to a health care provider.
A World Health Organization spokesperson said during a press conference on March 17 that the organization was looking into concerns about ibuprofen use in patients with COVID-19 and suggested that in the meantime patients take acetaminophen for fever instead. On March 18, the WHO said that it was not recommending against the use of ibuprofen.
“At present, based on currently available information, WHO does not recommend against the use of ibuprofen,” the organization said. “We are also consulting with physicians treating COVID-19 patients and are not aware of reports of any negative effects of ibuprofen, beyond the usual known side effects that limit its use in certain populations. WHO is not aware of published clinical or population-based data on this topic.”
A spokesperson for the National Institute of Allergy and Infectious Diseases said on March 18, “More research is needed to evaluate reports that ibruprofen and other over-the-counter anti-inflammatory drugs may affect the course of COVID-19. Currently, there is no conclusive evidence that ibuprofen and other over-the-counter anti-inflammatory drugs increase the risk of serious complications or of acquiring the virus that causes COVID-19. There is also no conclusive evidence that taking over-the-counter anti-inflammatory drugs is harmful for other respiratory infections.”
The European Medicines Agency (EMA) on March 18 said, “There is currently no scientific evidence establishing a link between ibuprofen and worsening of COVID‑19. EMA is monitoring the situation closely and will review any new information that becomes available on this issue in the context of the pandemic.”
In correspondence published March 11 in the Lancet Respiratory Medicine, Lei Fang, MD, of the department of biomedicine at University Hospital Basel (Switzerland), and colleagues suggested that patients with hypertension and diabetes mellitus may be at increased risk of COVID-19 because these comorbidities “are often treated with angiotensin converting enzyme (ACE) inhibitors.” In addition, “ACE2 polymorphisms that have been linked to diabetes mellitus, cerebral stroke, and hypertension” also may play a role, the researchers said (Lancet Respir Med. 2020 Mar 11. https://doi.org/10.1016/S2213-2600(20)30116-8).
“ACE2 is substantially increased in patients with type 1 or type 2 diabetes, who are treated with ACE inhibitors and angiotensin II type-I receptor blockers (ARBs). Hypertension is also treated with ACE inhibitors and ARBs, which results in an upregulation of ACE2. ACE2 can also be increased by thiazolidinediones and ibuprofen.”
A March 16 statement from the Heart Failure Society of America (HSFC), American College of Cardiology (ACC), and American Heart Association (AHA) addressed concerns about using RAAS antagonists in COVID-19.
“Patients with underlying cardiovascular diseases appear to have an increased risk for adverse outcomes with [COVID-19],” the organizations said. “Although the clinical manifestations of COVID-19 are dominated by respiratory symptoms, some patients also may have severe cardiovascular damage. [ACE2] receptors have been shown to be the entry point into human cells for SARS-CoV-2, the virus that causes COVID-19. In a few experimental studies with animal models, both [ACE] inhibitors and angiotensin receptor blockers (ARBs) have been shown to upregulate ACE2 expression in the heart. Though these have not been shown in human studies, or in the setting of COVID-19, such potential upregulation of ACE2 by ACE inhibitors or ARBs has resulted in a speculation of potential increased risk for COVID-19 infection in patients with background treatment of these medications.”
ACE2, ACE, angiotensin II, and other RAAS system interactions “are quite complex, and at times, paradoxical,” the statement says. “In experimental studies, both ACE inhibitors and ARBs have been shown to reduce severe lung injury in certain viral pneumonias, and it has been speculated that these agents could be beneficial in COVID-19.
“Currently there are no experimental or clinical data demonstrating beneficial or adverse outcomes with background use of ACE inhibitors, ARBs or other RAAS antagonists in COVID-19 or among COVID-19 patients with a history of cardiovascular disease treated with such agents. The HFSA, ACC, and AHA recommend continuation of RAAS antagonists for those patients who are currently prescribed such agents for indications for which these agents are known to be beneficial, such as heart failure, hypertension, or ischemic heart disease. In the event patients with cardiovascular disease are diagnosed with COVID-19, individualized treatment decisions should be made according to each patient’s hemodynamic status and clinical presentation. Therefore, be advised not to add or remove any RAAS-related treatments, beyond actions based on standard clinical practice.
“These theoretical concerns and findings of cardiovascular involvement with COVID-19 deserve much more detailed research, and quickly. As further research and developments related to this issue evolve, we will update these recommendations as needed.”
Dr. Fang and colleagues had no competing interests.
SOURCE: Fang L et al. Lancet Respir Med. 2020 Mar 11. doi: 10.1016/S2213-2600(20)30116-8.
Researchers have hypothesized that treatments that increase angiotensin-converting enzyme 2 (ACE2) may also increase the risk of novel coronavirus disease (COVID-19). This speculation and other concerns have led some officials and organizations to question whether ibuprofen or other drugs such as renin angiotensin aldosterone system (RAAS) antagonists should be avoided as treatments in patients with COVID-19. Health agencies and professional organizations have said they are not recommending against these medications.
The Food and Drug Administration on March 19 advised patients that it was “not aware of scientific evidence connecting” nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen “with worsening COVID-19 symptoms.”
“The agency is investigating this issue further and will communicate publicly when more information is available,” the FDA said. “However, all prescription NSAID labels warn that ‘the pharmacological activity of NSAIDs in reducing inflammation, and possibly fever, may diminish the utility of diagnostic signs in detecting infections.’ ” The FDA also noted that other over-the-counter and prescription medications are available for pain relief and fever reduction, and patients who “are concerned about taking NSAIDs and rely on these medications to treat chronic diseases” should talk to a health care provider.
A World Health Organization spokesperson said during a press conference on March 17 that the organization was looking into concerns about ibuprofen use in patients with COVID-19 and suggested that in the meantime patients take acetaminophen for fever instead. On March 18, the WHO said that it was not recommending against the use of ibuprofen.
“At present, based on currently available information, WHO does not recommend against the use of ibuprofen,” the organization said. “We are also consulting with physicians treating COVID-19 patients and are not aware of reports of any negative effects of ibuprofen, beyond the usual known side effects that limit its use in certain populations. WHO is not aware of published clinical or population-based data on this topic.”
A spokesperson for the National Institute of Allergy and Infectious Diseases said on March 18, “More research is needed to evaluate reports that ibruprofen and other over-the-counter anti-inflammatory drugs may affect the course of COVID-19. Currently, there is no conclusive evidence that ibuprofen and other over-the-counter anti-inflammatory drugs increase the risk of serious complications or of acquiring the virus that causes COVID-19. There is also no conclusive evidence that taking over-the-counter anti-inflammatory drugs is harmful for other respiratory infections.”
The European Medicines Agency (EMA) on March 18 said, “There is currently no scientific evidence establishing a link between ibuprofen and worsening of COVID‑19. EMA is monitoring the situation closely and will review any new information that becomes available on this issue in the context of the pandemic.”
In correspondence published March 11 in the Lancet Respiratory Medicine, Lei Fang, MD, of the department of biomedicine at University Hospital Basel (Switzerland), and colleagues suggested that patients with hypertension and diabetes mellitus may be at increased risk of COVID-19 because these comorbidities “are often treated with angiotensin converting enzyme (ACE) inhibitors.” In addition, “ACE2 polymorphisms that have been linked to diabetes mellitus, cerebral stroke, and hypertension” also may play a role, the researchers said (Lancet Respir Med. 2020 Mar 11. https://doi.org/10.1016/S2213-2600(20)30116-8).
“ACE2 is substantially increased in patients with type 1 or type 2 diabetes, who are treated with ACE inhibitors and angiotensin II type-I receptor blockers (ARBs). Hypertension is also treated with ACE inhibitors and ARBs, which results in an upregulation of ACE2. ACE2 can also be increased by thiazolidinediones and ibuprofen.”
A March 16 statement from the Heart Failure Society of America (HSFC), American College of Cardiology (ACC), and American Heart Association (AHA) addressed concerns about using RAAS antagonists in COVID-19.
“Patients with underlying cardiovascular diseases appear to have an increased risk for adverse outcomes with [COVID-19],” the organizations said. “Although the clinical manifestations of COVID-19 are dominated by respiratory symptoms, some patients also may have severe cardiovascular damage. [ACE2] receptors have been shown to be the entry point into human cells for SARS-CoV-2, the virus that causes COVID-19. In a few experimental studies with animal models, both [ACE] inhibitors and angiotensin receptor blockers (ARBs) have been shown to upregulate ACE2 expression in the heart. Though these have not been shown in human studies, or in the setting of COVID-19, such potential upregulation of ACE2 by ACE inhibitors or ARBs has resulted in a speculation of potential increased risk for COVID-19 infection in patients with background treatment of these medications.”
ACE2, ACE, angiotensin II, and other RAAS system interactions “are quite complex, and at times, paradoxical,” the statement says. “In experimental studies, both ACE inhibitors and ARBs have been shown to reduce severe lung injury in certain viral pneumonias, and it has been speculated that these agents could be beneficial in COVID-19.
“Currently there are no experimental or clinical data demonstrating beneficial or adverse outcomes with background use of ACE inhibitors, ARBs or other RAAS antagonists in COVID-19 or among COVID-19 patients with a history of cardiovascular disease treated with such agents. The HFSA, ACC, and AHA recommend continuation of RAAS antagonists for those patients who are currently prescribed such agents for indications for which these agents are known to be beneficial, such as heart failure, hypertension, or ischemic heart disease. In the event patients with cardiovascular disease are diagnosed with COVID-19, individualized treatment decisions should be made according to each patient’s hemodynamic status and clinical presentation. Therefore, be advised not to add or remove any RAAS-related treatments, beyond actions based on standard clinical practice.
“These theoretical concerns and findings of cardiovascular involvement with COVID-19 deserve much more detailed research, and quickly. As further research and developments related to this issue evolve, we will update these recommendations as needed.”
Dr. Fang and colleagues had no competing interests.
SOURCE: Fang L et al. Lancet Respir Med. 2020 Mar 11. doi: 10.1016/S2213-2600(20)30116-8.
Give me an occupation, Miss Dashwood
“I’ve been watching YouTube videos on how to set a ventilator,” said one of our dermatologists. The absurdity, levity, and gravity of that statement captures in a single sentence where we are today.
None of us alive have experience with such a crisis. It is as if our planet passed through a wormhole and we’ve been transported to the late medieval period: We doctors fighting the Black Death donned in beaked masks filled with juniper berries, mint, and clove to protect us from the miasma. Now, though, we spray store-bought lavender disinfectant on surgical masks.
“A crisis shows you a person’s soul,” said New York Governor Andrew Cuomo, adding: “It shows you what they’re made of, the weaknesses explode and the strengths ... emboldened.” Most of us have traveled through life with no experience of peril. Such mortal danger explodes and emboldens us, dividing us in two, the fearful or the phlegmatic.
When President Trump proclaimed that plaquenil was a promising treatment for the virus, prescriptions for the drug soared so quickly that four of eight manufacturers reported being in shortage by the end of the day. Many of those prescriptions were written by physicians for themselves and their families. Private Facebook physician groups shared insider tips for how to get around constraints and find the drug – as hoardable as toilet paper. As a department chief and fellow human being, I understand why some of us might behave this way. We didn’t sign up to be dermatologists or nephrologists or surgeons or pulmonologists agreeing that, to do so, we might die. We are all afraid.
The track of this epic storm became clear last week and now, terrifyingly, it appears it will be a direct hit. I braced for an onslaught of anxiety from our doctors and staff. But as the forecast became more grim, the courage began to well up and creativity climbed. Doctors went to local stores and bought all the masks and shields on their own. Rolls of toilet paper and diapers began magically appearing in our mom-doctors’ offices, delivered by angels in scrubs. I’ve practically had to install a velvet rope at my door to organize the queue of people wanting to talk to me about their ideas to help – keep 6 feet apart please! Stories like this abound. Even at the EvergreenHealth hospital in Washington they’ve not had shortages of staff. Rather than calling out sick, they called in: “If you need me, I’m available.”
Doctors are afraid and frustrated. Some of the things we will do in the coming weeks will first do no good, perhaps even harm. But I believe it’s because we’ve yet to embolden our strengths. It’s our job as leaders, attendings, administrators to inform and enable them.
When Marianne fell deathly ill in “Sense and Sensibility,” Colonel Branden wrung his hands and paced the floor. “Give me an occupation, Miss Dashwood, or I shall run mad.” Doctors are running, mad. And, just in case, some dermatologists are relearning how to intubate, waiting for that occupation to be given.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He has no relevant conflicts of interest related to this column. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“I’ve been watching YouTube videos on how to set a ventilator,” said one of our dermatologists. The absurdity, levity, and gravity of that statement captures in a single sentence where we are today.
None of us alive have experience with such a crisis. It is as if our planet passed through a wormhole and we’ve been transported to the late medieval period: We doctors fighting the Black Death donned in beaked masks filled with juniper berries, mint, and clove to protect us from the miasma. Now, though, we spray store-bought lavender disinfectant on surgical masks.
“A crisis shows you a person’s soul,” said New York Governor Andrew Cuomo, adding: “It shows you what they’re made of, the weaknesses explode and the strengths ... emboldened.” Most of us have traveled through life with no experience of peril. Such mortal danger explodes and emboldens us, dividing us in two, the fearful or the phlegmatic.
When President Trump proclaimed that plaquenil was a promising treatment for the virus, prescriptions for the drug soared so quickly that four of eight manufacturers reported being in shortage by the end of the day. Many of those prescriptions were written by physicians for themselves and their families. Private Facebook physician groups shared insider tips for how to get around constraints and find the drug – as hoardable as toilet paper. As a department chief and fellow human being, I understand why some of us might behave this way. We didn’t sign up to be dermatologists or nephrologists or surgeons or pulmonologists agreeing that, to do so, we might die. We are all afraid.
The track of this epic storm became clear last week and now, terrifyingly, it appears it will be a direct hit. I braced for an onslaught of anxiety from our doctors and staff. But as the forecast became more grim, the courage began to well up and creativity climbed. Doctors went to local stores and bought all the masks and shields on their own. Rolls of toilet paper and diapers began magically appearing in our mom-doctors’ offices, delivered by angels in scrubs. I’ve practically had to install a velvet rope at my door to organize the queue of people wanting to talk to me about their ideas to help – keep 6 feet apart please! Stories like this abound. Even at the EvergreenHealth hospital in Washington they’ve not had shortages of staff. Rather than calling out sick, they called in: “If you need me, I’m available.”
Doctors are afraid and frustrated. Some of the things we will do in the coming weeks will first do no good, perhaps even harm. But I believe it’s because we’ve yet to embolden our strengths. It’s our job as leaders, attendings, administrators to inform and enable them.
When Marianne fell deathly ill in “Sense and Sensibility,” Colonel Branden wrung his hands and paced the floor. “Give me an occupation, Miss Dashwood, or I shall run mad.” Doctors are running, mad. And, just in case, some dermatologists are relearning how to intubate, waiting for that occupation to be given.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He has no relevant conflicts of interest related to this column. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“I’ve been watching YouTube videos on how to set a ventilator,” said one of our dermatologists. The absurdity, levity, and gravity of that statement captures in a single sentence where we are today.
None of us alive have experience with such a crisis. It is as if our planet passed through a wormhole and we’ve been transported to the late medieval period: We doctors fighting the Black Death donned in beaked masks filled with juniper berries, mint, and clove to protect us from the miasma. Now, though, we spray store-bought lavender disinfectant on surgical masks.
“A crisis shows you a person’s soul,” said New York Governor Andrew Cuomo, adding: “It shows you what they’re made of, the weaknesses explode and the strengths ... emboldened.” Most of us have traveled through life with no experience of peril. Such mortal danger explodes and emboldens us, dividing us in two, the fearful or the phlegmatic.
When President Trump proclaimed that plaquenil was a promising treatment for the virus, prescriptions for the drug soared so quickly that four of eight manufacturers reported being in shortage by the end of the day. Many of those prescriptions were written by physicians for themselves and their families. Private Facebook physician groups shared insider tips for how to get around constraints and find the drug – as hoardable as toilet paper. As a department chief and fellow human being, I understand why some of us might behave this way. We didn’t sign up to be dermatologists or nephrologists or surgeons or pulmonologists agreeing that, to do so, we might die. We are all afraid.
The track of this epic storm became clear last week and now, terrifyingly, it appears it will be a direct hit. I braced for an onslaught of anxiety from our doctors and staff. But as the forecast became more grim, the courage began to well up and creativity climbed. Doctors went to local stores and bought all the masks and shields on their own. Rolls of toilet paper and diapers began magically appearing in our mom-doctors’ offices, delivered by angels in scrubs. I’ve practically had to install a velvet rope at my door to organize the queue of people wanting to talk to me about their ideas to help – keep 6 feet apart please! Stories like this abound. Even at the EvergreenHealth hospital in Washington they’ve not had shortages of staff. Rather than calling out sick, they called in: “If you need me, I’m available.”
Doctors are afraid and frustrated. Some of the things we will do in the coming weeks will first do no good, perhaps even harm. But I believe it’s because we’ve yet to embolden our strengths. It’s our job as leaders, attendings, administrators to inform and enable them.
When Marianne fell deathly ill in “Sense and Sensibility,” Colonel Branden wrung his hands and paced the floor. “Give me an occupation, Miss Dashwood, or I shall run mad.” Doctors are running, mad. And, just in case, some dermatologists are relearning how to intubate, waiting for that occupation to be given.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He has no relevant conflicts of interest related to this column. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Preventable diseases could gain a foothold because of COVID-19
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
Milestone Match Day sees record highs; soar in DO applicants
Unifying allopathic (MD) and osteopathic (DO) applicants for the first time in a single matching program, 2020’s Match Day results underscored the continuing growth of DOs in the field, boosting numbers in primary care medicine and the Match as a whole.
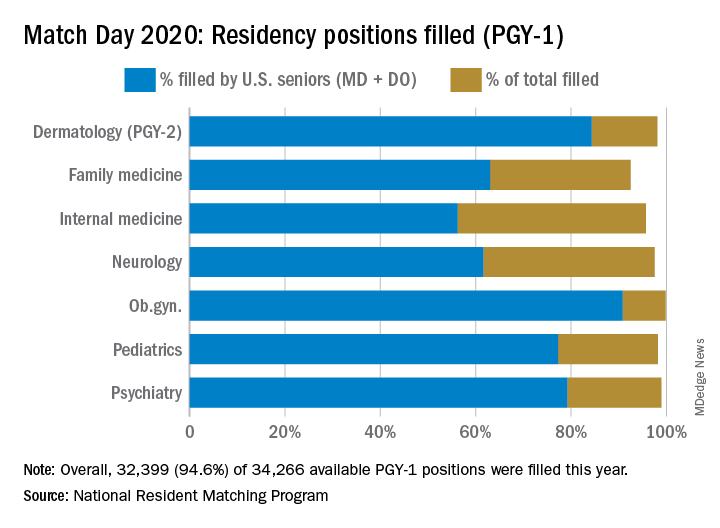
The 2020 Main Residency Match bested 2019’s record as the largest in the history of the National Resident Matching Program (NRMP), with 40,084 applicants submitting program choices for 37,256 positions. This compares with 38,376 applicants vying for 35,185 positions last year.
It’s the seventh consecutive year in which overall match numbers are up, according to the NRMP. Although the number of applicants increased, so did the number of positions, resulting in a slight drop in the percent of positions filled during 2019-2020.
Available first-year (PGY-1) positions rose to 34,266, an increase of 2,072 (6.4%) over 2019. “This was, in part, due to the last migration of osteopathic program positions into the Main Residency Match,” Donna L. Lamb, DHSc, NRMP president and CEO, said in an interview. An agreement the Accreditation Council for Graduate Medical Education, American Osteopathic Association and American Association of Colleges of Osteopathic Medicine reached in 2014 recognized ACGME as the primary accrediting body for graduate medical education programs by 2020.
This led to the first single match for U.S. MD and DO senior students and graduates and the inclusion of DO senior students as sponsored applicants in 2020, Dr. Lamb noted.
Gains, trends in 2020 match
Growth in U.S. DO senior participation also pushed this year’s Match to record highs. There were 6,581 U.S. DO medical school seniors who submitted rank order lists, 1,103 more than in 2019. Among those seniors, 90.7% matched to PGY-1 positions, driving the match rate for U.S. DO seniors up 2.6 percentage points from 2019.
Since 2016, the number of U.S. DO seniors seeking positions has risen by 3,599 or 120%. “Of course, the number of U.S. MD seniors who submitted program choices was also record-high: 19,326, an increase of 401 over 2019. The 93.7% match rate to first-year positions for this group has remained very consistent for many years,” Dr. Lamb said.
Among individual specialties, the NRMP reported extremely high fill rates for dermatology, medicine-emergency medicine, neurological surgery, physical medicine and rehabilitation (categorical), integrated plastic surgery, and thoracic surgery. Other competitive specialties included medicine-pediatrics, orthopedic surgery, otolaryngology, and vascular surgery.
Participation of international medical school students and graduates (IMGs) went up in 2020, breaking a 3-year cycle of decline. More than 61% matched to first-year positions, 2.5 percentage points higher than 2019 – and the highest match rate since 1990. “IMGs generally are having the most success matching to primary care specialties, including internal medicine, family medicine, and pediatrics,” Dr. Lamb said.
Primary care benefits from DO growth
DO candidates also helped drive up the numbers in primary care.
Internal medicine offered 8,697 categorical positions, 581 more than in 2019, reflecting a fill rate of 95.7%. More than 40% of these slots were filled by U.S. MD seniors, a category that’s seen decreases over the last 5 years, due in part to administrative and financial burdens associated with primary care internal medicine.
“In addition, the steady growth of internal medicine has increased the overall number of training positions available, and with the growth of other specialties in parallel, it has also likely had some effect on decreasing the percentage of U.S. graduates entering the field,” Phil Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in an interview.
However, fill rates for U.S. DO seniors reached 16% in 2020, a notable rise from 6.9% in 2016. “As the number of osteopathic trainees increases, we are happy that more are choosing internal medicine as a career path,” Dr. Masters said, adding that the slightly different training and practice orientation of osteopathic physicians “complements that of their allopathic colleagues, and add richness to the many different practice settings that internal medicine encompasses.”
A record number of DO seniors also matched in family medicine (1,392), accounting for nearly 30% of all applicants. The single match led to an important net increase in filled family medicine residency positions, Clif Knight, MD, senior vice president for education at the American Academy of Family Physicians, said in an interview.
Overall, family medicine filled 92.5% of its 4,662 positions, 555 more than in 2019. The results show that family medicine and primary care are on solid footing, Dr. Knight said. “We are excited that the number of filled family medicine residency positions increased from last year. This is important as we work to meet the significant primary care workforce shortage,” he added.
In other specialties:
- Pediatrics filled more than 98% of its 2,864 categorical positions, 17 more than in 2019. U.S. MD seniors filled 1,731 (60.4%) of those slots. “We’re very excited about our newly matched pediatricians,” Sara “Sally” H. Goza, MD, president of the American Academy of Pediatrics, said in an interview. “The coronavirus outbreak has shown us how valuable the pediatric workforce is and how much we’re needed.’’
- Dermatology offered 478 positions, achieving a fill rate of 98.1%. “Looking at our own program’s Match results, I feel very satisfied that we are accomplishing our specific aim to serve rural populations and to create a diverse workforce in dermatology,” Erik Stratman, MD, an expert on dermatologic education in U.S. medical schools/residency programs, and a member of the American Academy of Dermatology, said in an interview. “It’s nice to see the fruits of the specialty’s expanding efforts to get the right people in the specialty who reflect those populations we serve.”
- Obstetrics-gynecology offered 1,433 first-year positions – 48 more than in 2019 – achieving a fill rate of 99.8%, with U.S. MD seniors filling more than 75% of those slots.
- Neurology filled more than 97.5% of 682 offered positions in 2020. However, U.S. MD seniors represented just under half of those filled positions (46.5%).
- Psychiatry offered 1,858 positions in 2020, achieving an overall fill rate of 98.9%, 61.2% for U.S. MD seniors.
- Emergency Medicine filled 99.5% of the 2,665 positions offered this year. In this profession, the U.S. MD fill rate was 64.3%. These new interns are sorely needed at a time when EM physicians are on the front lines of a pandemic, Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association, said in an interview.
Unifying allopathic (MD) and osteopathic (DO) applicants for the first time in a single matching program, 2020’s Match Day results underscored the continuing growth of DOs in the field, boosting numbers in primary care medicine and the Match as a whole.

The 2020 Main Residency Match bested 2019’s record as the largest in the history of the National Resident Matching Program (NRMP), with 40,084 applicants submitting program choices for 37,256 positions. This compares with 38,376 applicants vying for 35,185 positions last year.
It’s the seventh consecutive year in which overall match numbers are up, according to the NRMP. Although the number of applicants increased, so did the number of positions, resulting in a slight drop in the percent of positions filled during 2019-2020.
Available first-year (PGY-1) positions rose to 34,266, an increase of 2,072 (6.4%) over 2019. “This was, in part, due to the last migration of osteopathic program positions into the Main Residency Match,” Donna L. Lamb, DHSc, NRMP president and CEO, said in an interview. An agreement the Accreditation Council for Graduate Medical Education, American Osteopathic Association and American Association of Colleges of Osteopathic Medicine reached in 2014 recognized ACGME as the primary accrediting body for graduate medical education programs by 2020.
This led to the first single match for U.S. MD and DO senior students and graduates and the inclusion of DO senior students as sponsored applicants in 2020, Dr. Lamb noted.
Gains, trends in 2020 match
Growth in U.S. DO senior participation also pushed this year’s Match to record highs. There were 6,581 U.S. DO medical school seniors who submitted rank order lists, 1,103 more than in 2019. Among those seniors, 90.7% matched to PGY-1 positions, driving the match rate for U.S. DO seniors up 2.6 percentage points from 2019.
Since 2016, the number of U.S. DO seniors seeking positions has risen by 3,599 or 120%. “Of course, the number of U.S. MD seniors who submitted program choices was also record-high: 19,326, an increase of 401 over 2019. The 93.7% match rate to first-year positions for this group has remained very consistent for many years,” Dr. Lamb said.
Among individual specialties, the NRMP reported extremely high fill rates for dermatology, medicine-emergency medicine, neurological surgery, physical medicine and rehabilitation (categorical), integrated plastic surgery, and thoracic surgery. Other competitive specialties included medicine-pediatrics, orthopedic surgery, otolaryngology, and vascular surgery.
Participation of international medical school students and graduates (IMGs) went up in 2020, breaking a 3-year cycle of decline. More than 61% matched to first-year positions, 2.5 percentage points higher than 2019 – and the highest match rate since 1990. “IMGs generally are having the most success matching to primary care specialties, including internal medicine, family medicine, and pediatrics,” Dr. Lamb said.
Primary care benefits from DO growth
DO candidates also helped drive up the numbers in primary care.
Internal medicine offered 8,697 categorical positions, 581 more than in 2019, reflecting a fill rate of 95.7%. More than 40% of these slots were filled by U.S. MD seniors, a category that’s seen decreases over the last 5 years, due in part to administrative and financial burdens associated with primary care internal medicine.
“In addition, the steady growth of internal medicine has increased the overall number of training positions available, and with the growth of other specialties in parallel, it has also likely had some effect on decreasing the percentage of U.S. graduates entering the field,” Phil Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in an interview.
However, fill rates for U.S. DO seniors reached 16% in 2020, a notable rise from 6.9% in 2016. “As the number of osteopathic trainees increases, we are happy that more are choosing internal medicine as a career path,” Dr. Masters said, adding that the slightly different training and practice orientation of osteopathic physicians “complements that of their allopathic colleagues, and add richness to the many different practice settings that internal medicine encompasses.”
A record number of DO seniors also matched in family medicine (1,392), accounting for nearly 30% of all applicants. The single match led to an important net increase in filled family medicine residency positions, Clif Knight, MD, senior vice president for education at the American Academy of Family Physicians, said in an interview.
Overall, family medicine filled 92.5% of its 4,662 positions, 555 more than in 2019. The results show that family medicine and primary care are on solid footing, Dr. Knight said. “We are excited that the number of filled family medicine residency positions increased from last year. This is important as we work to meet the significant primary care workforce shortage,” he added.
In other specialties:
- Pediatrics filled more than 98% of its 2,864 categorical positions, 17 more than in 2019. U.S. MD seniors filled 1,731 (60.4%) of those slots. “We’re very excited about our newly matched pediatricians,” Sara “Sally” H. Goza, MD, president of the American Academy of Pediatrics, said in an interview. “The coronavirus outbreak has shown us how valuable the pediatric workforce is and how much we’re needed.’’
- Dermatology offered 478 positions, achieving a fill rate of 98.1%. “Looking at our own program’s Match results, I feel very satisfied that we are accomplishing our specific aim to serve rural populations and to create a diverse workforce in dermatology,” Erik Stratman, MD, an expert on dermatologic education in U.S. medical schools/residency programs, and a member of the American Academy of Dermatology, said in an interview. “It’s nice to see the fruits of the specialty’s expanding efforts to get the right people in the specialty who reflect those populations we serve.”
- Obstetrics-gynecology offered 1,433 first-year positions – 48 more than in 2019 – achieving a fill rate of 99.8%, with U.S. MD seniors filling more than 75% of those slots.
- Neurology filled more than 97.5% of 682 offered positions in 2020. However, U.S. MD seniors represented just under half of those filled positions (46.5%).
- Psychiatry offered 1,858 positions in 2020, achieving an overall fill rate of 98.9%, 61.2% for U.S. MD seniors.
- Emergency Medicine filled 99.5% of the 2,665 positions offered this year. In this profession, the U.S. MD fill rate was 64.3%. These new interns are sorely needed at a time when EM physicians are on the front lines of a pandemic, Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association, said in an interview.
Unifying allopathic (MD) and osteopathic (DO) applicants for the first time in a single matching program, 2020’s Match Day results underscored the continuing growth of DOs in the field, boosting numbers in primary care medicine and the Match as a whole.

The 2020 Main Residency Match bested 2019’s record as the largest in the history of the National Resident Matching Program (NRMP), with 40,084 applicants submitting program choices for 37,256 positions. This compares with 38,376 applicants vying for 35,185 positions last year.
It’s the seventh consecutive year in which overall match numbers are up, according to the NRMP. Although the number of applicants increased, so did the number of positions, resulting in a slight drop in the percent of positions filled during 2019-2020.
Available first-year (PGY-1) positions rose to 34,266, an increase of 2,072 (6.4%) over 2019. “This was, in part, due to the last migration of osteopathic program positions into the Main Residency Match,” Donna L. Lamb, DHSc, NRMP president and CEO, said in an interview. An agreement the Accreditation Council for Graduate Medical Education, American Osteopathic Association and American Association of Colleges of Osteopathic Medicine reached in 2014 recognized ACGME as the primary accrediting body for graduate medical education programs by 2020.
This led to the first single match for U.S. MD and DO senior students and graduates and the inclusion of DO senior students as sponsored applicants in 2020, Dr. Lamb noted.
Gains, trends in 2020 match
Growth in U.S. DO senior participation also pushed this year’s Match to record highs. There were 6,581 U.S. DO medical school seniors who submitted rank order lists, 1,103 more than in 2019. Among those seniors, 90.7% matched to PGY-1 positions, driving the match rate for U.S. DO seniors up 2.6 percentage points from 2019.
Since 2016, the number of U.S. DO seniors seeking positions has risen by 3,599 or 120%. “Of course, the number of U.S. MD seniors who submitted program choices was also record-high: 19,326, an increase of 401 over 2019. The 93.7% match rate to first-year positions for this group has remained very consistent for many years,” Dr. Lamb said.
Among individual specialties, the NRMP reported extremely high fill rates for dermatology, medicine-emergency medicine, neurological surgery, physical medicine and rehabilitation (categorical), integrated plastic surgery, and thoracic surgery. Other competitive specialties included medicine-pediatrics, orthopedic surgery, otolaryngology, and vascular surgery.
Participation of international medical school students and graduates (IMGs) went up in 2020, breaking a 3-year cycle of decline. More than 61% matched to first-year positions, 2.5 percentage points higher than 2019 – and the highest match rate since 1990. “IMGs generally are having the most success matching to primary care specialties, including internal medicine, family medicine, and pediatrics,” Dr. Lamb said.
Primary care benefits from DO growth
DO candidates also helped drive up the numbers in primary care.
Internal medicine offered 8,697 categorical positions, 581 more than in 2019, reflecting a fill rate of 95.7%. More than 40% of these slots were filled by U.S. MD seniors, a category that’s seen decreases over the last 5 years, due in part to administrative and financial burdens associated with primary care internal medicine.
“In addition, the steady growth of internal medicine has increased the overall number of training positions available, and with the growth of other specialties in parallel, it has also likely had some effect on decreasing the percentage of U.S. graduates entering the field,” Phil Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in an interview.
However, fill rates for U.S. DO seniors reached 16% in 2020, a notable rise from 6.9% in 2016. “As the number of osteopathic trainees increases, we are happy that more are choosing internal medicine as a career path,” Dr. Masters said, adding that the slightly different training and practice orientation of osteopathic physicians “complements that of their allopathic colleagues, and add richness to the many different practice settings that internal medicine encompasses.”
A record number of DO seniors also matched in family medicine (1,392), accounting for nearly 30% of all applicants. The single match led to an important net increase in filled family medicine residency positions, Clif Knight, MD, senior vice president for education at the American Academy of Family Physicians, said in an interview.
Overall, family medicine filled 92.5% of its 4,662 positions, 555 more than in 2019. The results show that family medicine and primary care are on solid footing, Dr. Knight said. “We are excited that the number of filled family medicine residency positions increased from last year. This is important as we work to meet the significant primary care workforce shortage,” he added.
In other specialties:
- Pediatrics filled more than 98% of its 2,864 categorical positions, 17 more than in 2019. U.S. MD seniors filled 1,731 (60.4%) of those slots. “We’re very excited about our newly matched pediatricians,” Sara “Sally” H. Goza, MD, president of the American Academy of Pediatrics, said in an interview. “The coronavirus outbreak has shown us how valuable the pediatric workforce is and how much we’re needed.’’
- Dermatology offered 478 positions, achieving a fill rate of 98.1%. “Looking at our own program’s Match results, I feel very satisfied that we are accomplishing our specific aim to serve rural populations and to create a diverse workforce in dermatology,” Erik Stratman, MD, an expert on dermatologic education in U.S. medical schools/residency programs, and a member of the American Academy of Dermatology, said in an interview. “It’s nice to see the fruits of the specialty’s expanding efforts to get the right people in the specialty who reflect those populations we serve.”
- Obstetrics-gynecology offered 1,433 first-year positions – 48 more than in 2019 – achieving a fill rate of 99.8%, with U.S. MD seniors filling more than 75% of those slots.
- Neurology filled more than 97.5% of 682 offered positions in 2020. However, U.S. MD seniors represented just under half of those filled positions (46.5%).
- Psychiatry offered 1,858 positions in 2020, achieving an overall fill rate of 98.9%, 61.2% for U.S. MD seniors.
- Emergency Medicine filled 99.5% of the 2,665 positions offered this year. In this profession, the U.S. MD fill rate was 64.3%. These new interns are sorely needed at a time when EM physicians are on the front lines of a pandemic, Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association, said in an interview.
Emergency Rule: Docs can bill for telehealth and COVID-19 tests. Here’s how
Many medical practices have long wanted to use telehealth to perform office visits and other evaluation and management (E/M) services. The technology readily exists and many electronic health records are set up to do telehealth visits. The problem has been getting paid for those visits. Medicare limited telehealth services to patients in underserved areas, and commercial insurances wouldn’t pay. But amid the COVID-19 crisis, things have changed.
On March 17, Congress passed a law allowing Medicare to waive some telehealth restrictions during a government state of emergency only, which we are in now. Specifically, the patient no longer needs to be in a medically underserved area and no longer needs to go to an originating site, such as a hospital. The patient can be located anywhere in the country and be in their own home.
Further, the Centers for Medicare & Medicaid is waiving the requirement that the practitioner use a HIPAA-compliant platform for the telehealth service. The service must still be provided using a real-time audiovisual platform, but that could be via FaceTime or Skype, both of which are readily available via a patient’s smartphone or home computer. Audio alone – that is, phone calls between physician and patient – is still insufficient.
Billing for telemedicine
There are two lists of services that you can bill for telehealth. One of the lists is in Medicare’s telehealth fact sheet and includes both CPT and HCPCS codes. The second is in your CPT book, Appendix P, and lists only CPT codes.
Practices may bill all of the Medicare-covered telehealth services using these new rules. This includes new and established patient visits 99201–99215. It includes inpatient and skilled nursing services, for which CMS uses HCPCS codes in place of CPT codes.
Some notable additional services that you may bill via telehealth are: smoking cessation, transitional care management, advanced care planning, psychiatric diagnostic interviews and psychotherapy, and initial and subsequent Medicare wellness visits. The Welcome to Medicare visit is not on the list.
Report these services to Medicare with the correct CPT code and use place of service 02 (telehealth) on the claim. There is a CPT modifier for telehealth (Modifier -95 Synchronous Telemedicine Service Rendered Via a Real-Time Interactive Audio and Video Telecommunications System) but Medicare does not require it.
If you perform an office visit and also do smoking cessation, document those just as you would if you saw the patient in person. Document the history; observational exam, if relevant; and the assessment and plan. Note the additional time spent in smoking cessation counseling. If it was a level three established patient, code 99213-25 and 99406 (smoking and tobacco use cessation counseling visit, intermediate, 3-10 minutes).
The Office of Inspector General is allowing practices to reduce or waive copays and patient due amounts. However, a practice is not required to waive the copay or patient due amount for a telehealth service.
Medicare Advantage plans are required to cover all services that original Medicare covers. State Medicaid plans and Medicaid managed care organizations can set their own rules.
What about commercial payers?
While CMS has issued its Medicare guidelines, commercial insurance companies can also set their own rules about covering telehealth services. Many of them have rushed to update their policies to allow office visits to be billed via telehealth.
Unfortunately, each payer can set its own rules about whether to cover telehealth and if the place of service 02 and/or modifier -95 is needed. UnitedHealthcare is covering telehealth visits for all of its Medicare Advantage, Medicaid, and commercial accounts.
Humana also is covering telemedicine for urgent care needs. Some private insurers are continuing to offer virtual visits with their contracted telehealth provider, not with the patient’s own physician. It is likely that this will change in the days ahead, but it means practices must check their payer policies and pay attention to the emails they receive from the payers. If patient foot traffic is slow, this may be a good time to call each payer to not only find out their telehealth rules, but to also learn what else is being suspended during the COVID-19 pandemic.
This would also be a good job for an employee to do from home versus coming into the practice.
None of the payers are limiting the diagnosis code for telemedicine services. The patient does not need to have a cough or fever to have telemedicine covered. Any diagnosis or condition is eligible to be billed via telehealth.
The waived restrictions by Medicare are in place only as long as the government state of emergency. Commercial payers are also describing these as temporary. However, it may be hard to put the genie back in the bottle. Medical practices and patients may find that these visits are just what the doctor ordered.
COVID-19 testing
Although testing is still not widely available, the American Medical Association has developed a CPT code for the test:
- 87635: Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique
CMS has also developed codes for testing for this new coronavirus. One (U0001) is specifically for tests done in the CDC lab. The second (U0002) was for other labs, but it seems likely that the CPT code will replace it.
In February, the U.S. Food and Drug Administration issued a new policy for certain labs to develop their own validated COVID-19 diagnostics. This second HCPCS code could be used for such tests when submitting claims to Medicare or other insurers.
The hope by CMS is that having these specific codes will encourage further testing and improve tracking of the virus.
This article first appeared on Medscape.com.
Many medical practices have long wanted to use telehealth to perform office visits and other evaluation and management (E/M) services. The technology readily exists and many electronic health records are set up to do telehealth visits. The problem has been getting paid for those visits. Medicare limited telehealth services to patients in underserved areas, and commercial insurances wouldn’t pay. But amid the COVID-19 crisis, things have changed.
On March 17, Congress passed a law allowing Medicare to waive some telehealth restrictions during a government state of emergency only, which we are in now. Specifically, the patient no longer needs to be in a medically underserved area and no longer needs to go to an originating site, such as a hospital. The patient can be located anywhere in the country and be in their own home.
Further, the Centers for Medicare & Medicaid is waiving the requirement that the practitioner use a HIPAA-compliant platform for the telehealth service. The service must still be provided using a real-time audiovisual platform, but that could be via FaceTime or Skype, both of which are readily available via a patient’s smartphone or home computer. Audio alone – that is, phone calls between physician and patient – is still insufficient.
Billing for telemedicine
There are two lists of services that you can bill for telehealth. One of the lists is in Medicare’s telehealth fact sheet and includes both CPT and HCPCS codes. The second is in your CPT book, Appendix P, and lists only CPT codes.
Practices may bill all of the Medicare-covered telehealth services using these new rules. This includes new and established patient visits 99201–99215. It includes inpatient and skilled nursing services, for which CMS uses HCPCS codes in place of CPT codes.
Some notable additional services that you may bill via telehealth are: smoking cessation, transitional care management, advanced care planning, psychiatric diagnostic interviews and psychotherapy, and initial and subsequent Medicare wellness visits. The Welcome to Medicare visit is not on the list.
Report these services to Medicare with the correct CPT code and use place of service 02 (telehealth) on the claim. There is a CPT modifier for telehealth (Modifier -95 Synchronous Telemedicine Service Rendered Via a Real-Time Interactive Audio and Video Telecommunications System) but Medicare does not require it.
If you perform an office visit and also do smoking cessation, document those just as you would if you saw the patient in person. Document the history; observational exam, if relevant; and the assessment and plan. Note the additional time spent in smoking cessation counseling. If it was a level three established patient, code 99213-25 and 99406 (smoking and tobacco use cessation counseling visit, intermediate, 3-10 minutes).
The Office of Inspector General is allowing practices to reduce or waive copays and patient due amounts. However, a practice is not required to waive the copay or patient due amount for a telehealth service.
Medicare Advantage plans are required to cover all services that original Medicare covers. State Medicaid plans and Medicaid managed care organizations can set their own rules.
What about commercial payers?
While CMS has issued its Medicare guidelines, commercial insurance companies can also set their own rules about covering telehealth services. Many of them have rushed to update their policies to allow office visits to be billed via telehealth.
Unfortunately, each payer can set its own rules about whether to cover telehealth and if the place of service 02 and/or modifier -95 is needed. UnitedHealthcare is covering telehealth visits for all of its Medicare Advantage, Medicaid, and commercial accounts.
Humana also is covering telemedicine for urgent care needs. Some private insurers are continuing to offer virtual visits with their contracted telehealth provider, not with the patient’s own physician. It is likely that this will change in the days ahead, but it means practices must check their payer policies and pay attention to the emails they receive from the payers. If patient foot traffic is slow, this may be a good time to call each payer to not only find out their telehealth rules, but to also learn what else is being suspended during the COVID-19 pandemic.
This would also be a good job for an employee to do from home versus coming into the practice.
None of the payers are limiting the diagnosis code for telemedicine services. The patient does not need to have a cough or fever to have telemedicine covered. Any diagnosis or condition is eligible to be billed via telehealth.
The waived restrictions by Medicare are in place only as long as the government state of emergency. Commercial payers are also describing these as temporary. However, it may be hard to put the genie back in the bottle. Medical practices and patients may find that these visits are just what the doctor ordered.
COVID-19 testing
Although testing is still not widely available, the American Medical Association has developed a CPT code for the test:
- 87635: Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique
CMS has also developed codes for testing for this new coronavirus. One (U0001) is specifically for tests done in the CDC lab. The second (U0002) was for other labs, but it seems likely that the CPT code will replace it.
In February, the U.S. Food and Drug Administration issued a new policy for certain labs to develop their own validated COVID-19 diagnostics. This second HCPCS code could be used for such tests when submitting claims to Medicare or other insurers.
The hope by CMS is that having these specific codes will encourage further testing and improve tracking of the virus.
This article first appeared on Medscape.com.
Many medical practices have long wanted to use telehealth to perform office visits and other evaluation and management (E/M) services. The technology readily exists and many electronic health records are set up to do telehealth visits. The problem has been getting paid for those visits. Medicare limited telehealth services to patients in underserved areas, and commercial insurances wouldn’t pay. But amid the COVID-19 crisis, things have changed.
On March 17, Congress passed a law allowing Medicare to waive some telehealth restrictions during a government state of emergency only, which we are in now. Specifically, the patient no longer needs to be in a medically underserved area and no longer needs to go to an originating site, such as a hospital. The patient can be located anywhere in the country and be in their own home.
Further, the Centers for Medicare & Medicaid is waiving the requirement that the practitioner use a HIPAA-compliant platform for the telehealth service. The service must still be provided using a real-time audiovisual platform, but that could be via FaceTime or Skype, both of which are readily available via a patient’s smartphone or home computer. Audio alone – that is, phone calls between physician and patient – is still insufficient.
Billing for telemedicine
There are two lists of services that you can bill for telehealth. One of the lists is in Medicare’s telehealth fact sheet and includes both CPT and HCPCS codes. The second is in your CPT book, Appendix P, and lists only CPT codes.
Practices may bill all of the Medicare-covered telehealth services using these new rules. This includes new and established patient visits 99201–99215. It includes inpatient and skilled nursing services, for which CMS uses HCPCS codes in place of CPT codes.
Some notable additional services that you may bill via telehealth are: smoking cessation, transitional care management, advanced care planning, psychiatric diagnostic interviews and psychotherapy, and initial and subsequent Medicare wellness visits. The Welcome to Medicare visit is not on the list.
Report these services to Medicare with the correct CPT code and use place of service 02 (telehealth) on the claim. There is a CPT modifier for telehealth (Modifier -95 Synchronous Telemedicine Service Rendered Via a Real-Time Interactive Audio and Video Telecommunications System) but Medicare does not require it.
If you perform an office visit and also do smoking cessation, document those just as you would if you saw the patient in person. Document the history; observational exam, if relevant; and the assessment and plan. Note the additional time spent in smoking cessation counseling. If it was a level three established patient, code 99213-25 and 99406 (smoking and tobacco use cessation counseling visit, intermediate, 3-10 minutes).
The Office of Inspector General is allowing practices to reduce or waive copays and patient due amounts. However, a practice is not required to waive the copay or patient due amount for a telehealth service.
Medicare Advantage plans are required to cover all services that original Medicare covers. State Medicaid plans and Medicaid managed care organizations can set their own rules.
What about commercial payers?
While CMS has issued its Medicare guidelines, commercial insurance companies can also set their own rules about covering telehealth services. Many of them have rushed to update their policies to allow office visits to be billed via telehealth.
Unfortunately, each payer can set its own rules about whether to cover telehealth and if the place of service 02 and/or modifier -95 is needed. UnitedHealthcare is covering telehealth visits for all of its Medicare Advantage, Medicaid, and commercial accounts.
Humana also is covering telemedicine for urgent care needs. Some private insurers are continuing to offer virtual visits with their contracted telehealth provider, not with the patient’s own physician. It is likely that this will change in the days ahead, but it means practices must check their payer policies and pay attention to the emails they receive from the payers. If patient foot traffic is slow, this may be a good time to call each payer to not only find out their telehealth rules, but to also learn what else is being suspended during the COVID-19 pandemic.
This would also be a good job for an employee to do from home versus coming into the practice.
None of the payers are limiting the diagnosis code for telemedicine services. The patient does not need to have a cough or fever to have telemedicine covered. Any diagnosis or condition is eligible to be billed via telehealth.
The waived restrictions by Medicare are in place only as long as the government state of emergency. Commercial payers are also describing these as temporary. However, it may be hard to put the genie back in the bottle. Medical practices and patients may find that these visits are just what the doctor ordered.
COVID-19 testing
Although testing is still not widely available, the American Medical Association has developed a CPT code for the test:
- 87635: Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique
CMS has also developed codes for testing for this new coronavirus. One (U0001) is specifically for tests done in the CDC lab. The second (U0002) was for other labs, but it seems likely that the CPT code will replace it.
In February, the U.S. Food and Drug Administration issued a new policy for certain labs to develop their own validated COVID-19 diagnostics. This second HCPCS code could be used for such tests when submitting claims to Medicare or other insurers.
The hope by CMS is that having these specific codes will encourage further testing and improve tracking of the virus.
This article first appeared on Medscape.com.
New ASAM guideline released amid COVID-19 concerns
Home-based buprenorphine induction deemed safe for OUD
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
Home-based buprenorphine induction deemed safe for OUD
Home-based buprenorphine induction deemed safe for OUD
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
Coronavirus resources from AAD target safe office practices, new telemedicine guidance
The American Academy of Dermatology (
The guidance pages are publicly viewable. Additionally, AAD has made a collection of COVID-19 articles from the Journal of the American Academy of Dermatology freely available for the next 6 months.
George Hruza, MD, AAD president, detailed regulatory updates and other federal actions as well as guidance regarding telemedicine and clinical practice in a message to AAD members.
“While many questions still need answers, I have appointed an Ad Hoc Task Force to assess dermatology’s needs, share knowledge, and provide ongoing guidance and information throughout the crisis,” Dr. Hruza wrote.
“The situation is changing rapidly, and we are committed to keeping you updated with reliable and practical information to help you adapt to the circumstances,” he noted, referring dermatologists to the AAD’s information hub for the coronavirus outbreak. “We are keeping this page updated frequently, and it will serve as your primary source for what we know now,” he noted.
The Centers for Medicare & Medicaid Services has recently relaxed key regulations regarding technology to provide telemedicine so that physicians and patients can use existing platforms such as FaceTime and Skype for virtual visits. The usual fines for HIPAA noncompliance have been waived. Additionally, telemedicine visits can now be reimbursed at the same rate as in-person visits.
Private payers are beginning to follow suit, said Dr. Hruza, noting that the AAD Association is working to harmonize private coverage with public reimbursement. The AAD also is tracking which payers are coming in line with federal policies on its teledermatology page.
These changes in regulation around telemedicine apply to patient encounters for any purpose, not just coronavirus-related encounters, noted Dr. Hruza. “The good news is that the government has taken action to make it much easier for us to provide virtual consults to patients. Dermatology has always been a leader in telemedicine, and it will be an important way to offer care to patients who can’t or don’t need to come into the office or clinic,” he added.
Importantly, said Dr. Hruza, CMS is allowing practices to have discretion over whether copays are collected, or collected in full, so that these payments don’t present a barrier to patient care in the current crisis environment.
For dermatologists who are new to telemedicine, AAD has created an online resource that includes information about various telemedicine platforms, updated guidance regarding regulations, and best practices for accurate coding and documentation of telemedicine visits.
The Academy has also been developing dermatology-specific guidance, including how to address the concerns of patients who are receiving biologic therapies and how to conserve personal protective equipment while still protecting physicians, staff, and patients from COVID-19 infection.
For patients on biologic therapy who show no sign of coronavirus infection, the decision to continue or stop biologics should be made on a case-by-case basis. Factors to be considered include patient age, comorbidities, and the severity of the original indication for biologic use.
Initiation of biologics should only be done after a similar risk-benefit analysis, with a recommendation to consider deferring initiation for patients 60 and older and those with comorbidities that may portend a worse course in the event of coronavirus infection. Biologics should be discontinued for patients who test positive for COVID-19.
Dr. Hruza outlined some of the federal measures taken that may affect the business side of dermatology practices. These include a $20 million transfer to the Small Business Administration to offset administrative expenses associated with increased loan volumes related to the coronavirus outbreak. Eligible expenses for loans may include new devices and environmental adjustments to accommodate telehealth services.
Additionally, it is anticipated that as employers are required to provide paid sick and family leave, a payroll tax credit will be issued to employers. Some self-employed individuals will also be able to claim a tax credit for sick and family leave.
A recent news release from the AAD encourages the public to use high-emollient moisturizers after handwashing. The release also provides other tips, such as using petrolatum at bedtime for hands that are particularly dry and focusing on the fingertips when moisturizing, as these areas are prone to cracking. The release also reaffirms that the most effective way to clean hands is soap and water, and that moisturizing after handwashing does not negate the antiviral effects of cleansing, contrary to some social media reports.
[email protected]
The American Academy of Dermatology (
The guidance pages are publicly viewable. Additionally, AAD has made a collection of COVID-19 articles from the Journal of the American Academy of Dermatology freely available for the next 6 months.
George Hruza, MD, AAD president, detailed regulatory updates and other federal actions as well as guidance regarding telemedicine and clinical practice in a message to AAD members.
“While many questions still need answers, I have appointed an Ad Hoc Task Force to assess dermatology’s needs, share knowledge, and provide ongoing guidance and information throughout the crisis,” Dr. Hruza wrote.
“The situation is changing rapidly, and we are committed to keeping you updated with reliable and practical information to help you adapt to the circumstances,” he noted, referring dermatologists to the AAD’s information hub for the coronavirus outbreak. “We are keeping this page updated frequently, and it will serve as your primary source for what we know now,” he noted.
The Centers for Medicare & Medicaid Services has recently relaxed key regulations regarding technology to provide telemedicine so that physicians and patients can use existing platforms such as FaceTime and Skype for virtual visits. The usual fines for HIPAA noncompliance have been waived. Additionally, telemedicine visits can now be reimbursed at the same rate as in-person visits.
Private payers are beginning to follow suit, said Dr. Hruza, noting that the AAD Association is working to harmonize private coverage with public reimbursement. The AAD also is tracking which payers are coming in line with federal policies on its teledermatology page.
These changes in regulation around telemedicine apply to patient encounters for any purpose, not just coronavirus-related encounters, noted Dr. Hruza. “The good news is that the government has taken action to make it much easier for us to provide virtual consults to patients. Dermatology has always been a leader in telemedicine, and it will be an important way to offer care to patients who can’t or don’t need to come into the office or clinic,” he added.
Importantly, said Dr. Hruza, CMS is allowing practices to have discretion over whether copays are collected, or collected in full, so that these payments don’t present a barrier to patient care in the current crisis environment.
For dermatologists who are new to telemedicine, AAD has created an online resource that includes information about various telemedicine platforms, updated guidance regarding regulations, and best practices for accurate coding and documentation of telemedicine visits.
The Academy has also been developing dermatology-specific guidance, including how to address the concerns of patients who are receiving biologic therapies and how to conserve personal protective equipment while still protecting physicians, staff, and patients from COVID-19 infection.
For patients on biologic therapy who show no sign of coronavirus infection, the decision to continue or stop biologics should be made on a case-by-case basis. Factors to be considered include patient age, comorbidities, and the severity of the original indication for biologic use.
Initiation of biologics should only be done after a similar risk-benefit analysis, with a recommendation to consider deferring initiation for patients 60 and older and those with comorbidities that may portend a worse course in the event of coronavirus infection. Biologics should be discontinued for patients who test positive for COVID-19.
Dr. Hruza outlined some of the federal measures taken that may affect the business side of dermatology practices. These include a $20 million transfer to the Small Business Administration to offset administrative expenses associated with increased loan volumes related to the coronavirus outbreak. Eligible expenses for loans may include new devices and environmental adjustments to accommodate telehealth services.
Additionally, it is anticipated that as employers are required to provide paid sick and family leave, a payroll tax credit will be issued to employers. Some self-employed individuals will also be able to claim a tax credit for sick and family leave.
A recent news release from the AAD encourages the public to use high-emollient moisturizers after handwashing. The release also provides other tips, such as using petrolatum at bedtime for hands that are particularly dry and focusing on the fingertips when moisturizing, as these areas are prone to cracking. The release also reaffirms that the most effective way to clean hands is soap and water, and that moisturizing after handwashing does not negate the antiviral effects of cleansing, contrary to some social media reports.
[email protected]
The American Academy of Dermatology (
The guidance pages are publicly viewable. Additionally, AAD has made a collection of COVID-19 articles from the Journal of the American Academy of Dermatology freely available for the next 6 months.
George Hruza, MD, AAD president, detailed regulatory updates and other federal actions as well as guidance regarding telemedicine and clinical practice in a message to AAD members.
“While many questions still need answers, I have appointed an Ad Hoc Task Force to assess dermatology’s needs, share knowledge, and provide ongoing guidance and information throughout the crisis,” Dr. Hruza wrote.
“The situation is changing rapidly, and we are committed to keeping you updated with reliable and practical information to help you adapt to the circumstances,” he noted, referring dermatologists to the AAD’s information hub for the coronavirus outbreak. “We are keeping this page updated frequently, and it will serve as your primary source for what we know now,” he noted.
The Centers for Medicare & Medicaid Services has recently relaxed key regulations regarding technology to provide telemedicine so that physicians and patients can use existing platforms such as FaceTime and Skype for virtual visits. The usual fines for HIPAA noncompliance have been waived. Additionally, telemedicine visits can now be reimbursed at the same rate as in-person visits.
Private payers are beginning to follow suit, said Dr. Hruza, noting that the AAD Association is working to harmonize private coverage with public reimbursement. The AAD also is tracking which payers are coming in line with federal policies on its teledermatology page.
These changes in regulation around telemedicine apply to patient encounters for any purpose, not just coronavirus-related encounters, noted Dr. Hruza. “The good news is that the government has taken action to make it much easier for us to provide virtual consults to patients. Dermatology has always been a leader in telemedicine, and it will be an important way to offer care to patients who can’t or don’t need to come into the office or clinic,” he added.
Importantly, said Dr. Hruza, CMS is allowing practices to have discretion over whether copays are collected, or collected in full, so that these payments don’t present a barrier to patient care in the current crisis environment.
For dermatologists who are new to telemedicine, AAD has created an online resource that includes information about various telemedicine platforms, updated guidance regarding regulations, and best practices for accurate coding and documentation of telemedicine visits.
The Academy has also been developing dermatology-specific guidance, including how to address the concerns of patients who are receiving biologic therapies and how to conserve personal protective equipment while still protecting physicians, staff, and patients from COVID-19 infection.
For patients on biologic therapy who show no sign of coronavirus infection, the decision to continue or stop biologics should be made on a case-by-case basis. Factors to be considered include patient age, comorbidities, and the severity of the original indication for biologic use.
Initiation of biologics should only be done after a similar risk-benefit analysis, with a recommendation to consider deferring initiation for patients 60 and older and those with comorbidities that may portend a worse course in the event of coronavirus infection. Biologics should be discontinued for patients who test positive for COVID-19.
Dr. Hruza outlined some of the federal measures taken that may affect the business side of dermatology practices. These include a $20 million transfer to the Small Business Administration to offset administrative expenses associated with increased loan volumes related to the coronavirus outbreak. Eligible expenses for loans may include new devices and environmental adjustments to accommodate telehealth services.
Additionally, it is anticipated that as employers are required to provide paid sick and family leave, a payroll tax credit will be issued to employers. Some self-employed individuals will also be able to claim a tax credit for sick and family leave.
A recent news release from the AAD encourages the public to use high-emollient moisturizers after handwashing. The release also provides other tips, such as using petrolatum at bedtime for hands that are particularly dry and focusing on the fingertips when moisturizing, as these areas are prone to cracking. The release also reaffirms that the most effective way to clean hands is soap and water, and that moisturizing after handwashing does not negate the antiviral effects of cleansing, contrary to some social media reports.
[email protected]
DIY masks: Worth the risk? Researchers are conflicted
In the midst of the rapidly spreading COVID-19 pandemic, hospitals and clinics are running out of masks. Health care workers are going online to beg for more, the hashtags #GetMePPE and #WeNeedPPE are trending on Twitter, and some hospitals have even put out public calls for mask donations. Health providers are working scared: They know that the moment the masks run out, they’re at increased risk for disease. So instead of waiting for mask shipments that may be weeks off, some people are making their own.
Using a simple template, they cut green surgical sheeting into half-moons, which they pin and sew before attaching elastic straps. Deaconess Health System in Evansville, Indiana, has posted instructions for fabric masks on their website and asked the public to step up and sew.
Elsewhere, health care workers have turned to diapers, maxi pads and other products to create masks. Social media channels are full of tips and sewing patterns. It’s an innovative strategy that is also contentious. Limited evidence suggests that homemade masks can offer some protection. But the DIY approach has also drawn criticism for providing a false sense of security, potentially putting wearers at risk.
The conflict points to an immediate need for more protective equipment, says Christopher Friese, PhD, RN, professor of nursing and public health at the University of Michigan, Ann Arbor. Also needed, he says, are new ideas for reducing strain on limited supplies, like adopting gear from other industries and finding innovative ways to provide care so that less protective gear is needed.
“We don’t want clinicians inventing and ‘MacGyvering’ their own device because we don’t want to put them at risk if we can avoid it,” says Friese, referring to the TV character who could build and assemble a vast array of tools/devices. “We have options that have been tested, and we have experience, maybe not in health care, but in other settings. We want to try that first before that frontline doctor, nurse, respiratory therapist decides to take matters into their own hands.
Increasingly, though, health care workers are finding they have no other choice — something even the CDC has acknowledged. In new guidelines, the agency recommends a bandanna, scarf, or other type of covering in cases where face masks are not available.
N95 respirators or surgical masks?
There are two main types of masks generally used in health care. N95 respirators filter out 95% of airborne particles, including bacteria and viruses. The lighter surgical or medical face masks are made to prevent spit and mucous from getting on patients or equipment.
Both types reduce rates of infection among health care workers, though comparisons (at least for influenza) have yet to show that one is superior to the other. One 2020 review by Chinese researchers, for example, analyzed six randomly controlled trials that included more than 9000 participants and found no added benefits of N95 masks over ordinary surgical masks for health care providers treating patients with the flu.
But COVID-19 is not influenza, and evidence suggests it may require more intensive protection, says Friese, who coauthored a blog post for JAMA about the country’s unpreparedness for protecting health care workers during a pandemic. The virus can linger in the air for hours, suggesting that N95 respirators are health care providers’ best option when treating infected patients.
The problem is there’s not enough to go around — of either mask type. In a March 5 survey, National Nurses United reported that just 30% of more than 6500 US respondents said their organizations had enough PPE to respond to a surge in patients. Another 38% did not know if their organizations were prepared. In a tweet, Friese estimated that 12% of nurses and other providers are at risk from reusing equipment or using equipment that is not backed by evidence.
Physicians and providers around the world have been sharing strategies online for how to make their own masks. Techniques vary, as do materials and plans for how to use the homemade equipment. At Phoebe Putney Health, DIY masks are intended to be worn over N95 respirators and then disposed of so that the respirators can be reused more safely, says Amanda Clements, the hospital’s public relations coordinator. Providers might also wear them to greet people at the front door.
Some evidence suggests that homemade masks can help in a pinch, at least for some illnesses. For a 2013 study by researchers in the UK, volunteers made surgical masks from cotton T-shirts, then put them on and coughed into a chamber that measured how much bacterial content got through. The team also assessed the aerosol-filtering ability of a variety of household materials, including scarfs, antimicrobial pillowcases, vacuum-cleaner bags, and tea towels. They tested each material with an aerosol containing two types of bacteria similar in size to influenza.
Commercial surgical masks performed three times better than homemade ones in the filtration test. Surgical masks worked twice as well at blocking droplets on the cough test. But all the makeshift materials — which also included silk, linen, and regular pillowcases — blocked some microbes. Vacuum-cleaner bags blocked the most bacteria, but their stiffness and thickness made them unsuitable for use as masks, the researchers reported. Tea towels showed a similar pattern. But pillowcases and cotton T-shirts were stretchy enough to fit well, thereby reducing the particles that could get through or around them.
Homemade masks should be used only as a last resort if commercial masks become unavailable, the researchers concluded. “Probably something is better than nothing for trained health care workers — for droplet contact avoidance, if nothing else,” says Anna Davies, BSc, a research facilitator at the University of Cambridge, UK, who is a former public health microbiologist and one of the study’s authors.
She recommends that members of the general public donate any stockpiles they have to health care workers, and make their own if they want masks for personal use. She is working with collaborators in the US to develop guidance for how best to do it.
“If people are quarantined and looking for something worthwhile to do, it probably wouldn’t be the worst thing to apply themselves to,” she wrote by email. “My suggestion would be for something soft and cotton, ideally with a bit of stretch (although it’s a pain to sew), and in two layers, marked ‘inside’ and ‘outside.’ ”
The idea that something is better than nothing was also the conclusion of a 2008 study by researchers in the Netherlands and the US. The study enlisted 28 healthy individuals who performed a variety of tasks while wearing N95 masks, surgical masks, or homemade masks sewn from teacloths. Effectiveness varied among individuals, but over a 90-second period, N95 masks worked best, with 25 times more protection than surgical masks and about 50 times more protection than homemade ones. Surgical masks were twice as effective as homemade masks. But the homemade masks offered at least some protection against large droplets.
Researchers emphasize that it’s not yet clear whether those findings are applicable to aerosolized COVID-19. In an influenza pandemic, at least, the authors posit that homemade masks could reduce transmission for the general public enough for some immunity to build. “It is important not to focus on a single intervention in case of a pandemic,” the researchers write, “but to integrate all effective interventions for optimal protection.”
For health care workers on the frontlines of COVID-19, Friese says, homemade masks might do more than nothing but they also might not work. Instead, he would rather see providers using construction or nuclear-engineering masks. And his best suggestion is something many providers are already doing: reducing physical contact with patients through telemedicine and other creative solutions, which is cutting down the overwhelming need for PPE.
Homemade mask production emphasizes the urgent need for more supplies, Friese adds.
“The government needs to step up and do a variety of things to increase production, and that needs to happen now, immediately,” he says. “We don’t we don’t want our clinicians to have to come up with these decisions.”
This article first appeared on Medscape.com.
In the midst of the rapidly spreading COVID-19 pandemic, hospitals and clinics are running out of masks. Health care workers are going online to beg for more, the hashtags #GetMePPE and #WeNeedPPE are trending on Twitter, and some hospitals have even put out public calls for mask donations. Health providers are working scared: They know that the moment the masks run out, they’re at increased risk for disease. So instead of waiting for mask shipments that may be weeks off, some people are making their own.
Using a simple template, they cut green surgical sheeting into half-moons, which they pin and sew before attaching elastic straps. Deaconess Health System in Evansville, Indiana, has posted instructions for fabric masks on their website and asked the public to step up and sew.
Elsewhere, health care workers have turned to diapers, maxi pads and other products to create masks. Social media channels are full of tips and sewing patterns. It’s an innovative strategy that is also contentious. Limited evidence suggests that homemade masks can offer some protection. But the DIY approach has also drawn criticism for providing a false sense of security, potentially putting wearers at risk.
The conflict points to an immediate need for more protective equipment, says Christopher Friese, PhD, RN, professor of nursing and public health at the University of Michigan, Ann Arbor. Also needed, he says, are new ideas for reducing strain on limited supplies, like adopting gear from other industries and finding innovative ways to provide care so that less protective gear is needed.
“We don’t want clinicians inventing and ‘MacGyvering’ their own device because we don’t want to put them at risk if we can avoid it,” says Friese, referring to the TV character who could build and assemble a vast array of tools/devices. “We have options that have been tested, and we have experience, maybe not in health care, but in other settings. We want to try that first before that frontline doctor, nurse, respiratory therapist decides to take matters into their own hands.
Increasingly, though, health care workers are finding they have no other choice — something even the CDC has acknowledged. In new guidelines, the agency recommends a bandanna, scarf, or other type of covering in cases where face masks are not available.
N95 respirators or surgical masks?
There are two main types of masks generally used in health care. N95 respirators filter out 95% of airborne particles, including bacteria and viruses. The lighter surgical or medical face masks are made to prevent spit and mucous from getting on patients or equipment.
Both types reduce rates of infection among health care workers, though comparisons (at least for influenza) have yet to show that one is superior to the other. One 2020 review by Chinese researchers, for example, analyzed six randomly controlled trials that included more than 9000 participants and found no added benefits of N95 masks over ordinary surgical masks for health care providers treating patients with the flu.
But COVID-19 is not influenza, and evidence suggests it may require more intensive protection, says Friese, who coauthored a blog post for JAMA about the country’s unpreparedness for protecting health care workers during a pandemic. The virus can linger in the air for hours, suggesting that N95 respirators are health care providers’ best option when treating infected patients.
The problem is there’s not enough to go around — of either mask type. In a March 5 survey, National Nurses United reported that just 30% of more than 6500 US respondents said their organizations had enough PPE to respond to a surge in patients. Another 38% did not know if their organizations were prepared. In a tweet, Friese estimated that 12% of nurses and other providers are at risk from reusing equipment or using equipment that is not backed by evidence.
Physicians and providers around the world have been sharing strategies online for how to make their own masks. Techniques vary, as do materials and plans for how to use the homemade equipment. At Phoebe Putney Health, DIY masks are intended to be worn over N95 respirators and then disposed of so that the respirators can be reused more safely, says Amanda Clements, the hospital’s public relations coordinator. Providers might also wear them to greet people at the front door.
Some evidence suggests that homemade masks can help in a pinch, at least for some illnesses. For a 2013 study by researchers in the UK, volunteers made surgical masks from cotton T-shirts, then put them on and coughed into a chamber that measured how much bacterial content got through. The team also assessed the aerosol-filtering ability of a variety of household materials, including scarfs, antimicrobial pillowcases, vacuum-cleaner bags, and tea towels. They tested each material with an aerosol containing two types of bacteria similar in size to influenza.
Commercial surgical masks performed three times better than homemade ones in the filtration test. Surgical masks worked twice as well at blocking droplets on the cough test. But all the makeshift materials — which also included silk, linen, and regular pillowcases — blocked some microbes. Vacuum-cleaner bags blocked the most bacteria, but their stiffness and thickness made them unsuitable for use as masks, the researchers reported. Tea towels showed a similar pattern. But pillowcases and cotton T-shirts were stretchy enough to fit well, thereby reducing the particles that could get through or around them.
Homemade masks should be used only as a last resort if commercial masks become unavailable, the researchers concluded. “Probably something is better than nothing for trained health care workers — for droplet contact avoidance, if nothing else,” says Anna Davies, BSc, a research facilitator at the University of Cambridge, UK, who is a former public health microbiologist and one of the study’s authors.
She recommends that members of the general public donate any stockpiles they have to health care workers, and make their own if they want masks for personal use. She is working with collaborators in the US to develop guidance for how best to do it.
“If people are quarantined and looking for something worthwhile to do, it probably wouldn’t be the worst thing to apply themselves to,” she wrote by email. “My suggestion would be for something soft and cotton, ideally with a bit of stretch (although it’s a pain to sew), and in two layers, marked ‘inside’ and ‘outside.’ ”
The idea that something is better than nothing was also the conclusion of a 2008 study by researchers in the Netherlands and the US. The study enlisted 28 healthy individuals who performed a variety of tasks while wearing N95 masks, surgical masks, or homemade masks sewn from teacloths. Effectiveness varied among individuals, but over a 90-second period, N95 masks worked best, with 25 times more protection than surgical masks and about 50 times more protection than homemade ones. Surgical masks were twice as effective as homemade masks. But the homemade masks offered at least some protection against large droplets.
Researchers emphasize that it’s not yet clear whether those findings are applicable to aerosolized COVID-19. In an influenza pandemic, at least, the authors posit that homemade masks could reduce transmission for the general public enough for some immunity to build. “It is important not to focus on a single intervention in case of a pandemic,” the researchers write, “but to integrate all effective interventions for optimal protection.”
For health care workers on the frontlines of COVID-19, Friese says, homemade masks might do more than nothing but they also might not work. Instead, he would rather see providers using construction or nuclear-engineering masks. And his best suggestion is something many providers are already doing: reducing physical contact with patients through telemedicine and other creative solutions, which is cutting down the overwhelming need for PPE.
Homemade mask production emphasizes the urgent need for more supplies, Friese adds.
“The government needs to step up and do a variety of things to increase production, and that needs to happen now, immediately,” he says. “We don’t we don’t want our clinicians to have to come up with these decisions.”
This article first appeared on Medscape.com.
In the midst of the rapidly spreading COVID-19 pandemic, hospitals and clinics are running out of masks. Health care workers are going online to beg for more, the hashtags #GetMePPE and #WeNeedPPE are trending on Twitter, and some hospitals have even put out public calls for mask donations. Health providers are working scared: They know that the moment the masks run out, they’re at increased risk for disease. So instead of waiting for mask shipments that may be weeks off, some people are making their own.
Using a simple template, they cut green surgical sheeting into half-moons, which they pin and sew before attaching elastic straps. Deaconess Health System in Evansville, Indiana, has posted instructions for fabric masks on their website and asked the public to step up and sew.
Elsewhere, health care workers have turned to diapers, maxi pads and other products to create masks. Social media channels are full of tips and sewing patterns. It’s an innovative strategy that is also contentious. Limited evidence suggests that homemade masks can offer some protection. But the DIY approach has also drawn criticism for providing a false sense of security, potentially putting wearers at risk.
The conflict points to an immediate need for more protective equipment, says Christopher Friese, PhD, RN, professor of nursing and public health at the University of Michigan, Ann Arbor. Also needed, he says, are new ideas for reducing strain on limited supplies, like adopting gear from other industries and finding innovative ways to provide care so that less protective gear is needed.
“We don’t want clinicians inventing and ‘MacGyvering’ their own device because we don’t want to put them at risk if we can avoid it,” says Friese, referring to the TV character who could build and assemble a vast array of tools/devices. “We have options that have been tested, and we have experience, maybe not in health care, but in other settings. We want to try that first before that frontline doctor, nurse, respiratory therapist decides to take matters into their own hands.
Increasingly, though, health care workers are finding they have no other choice — something even the CDC has acknowledged. In new guidelines, the agency recommends a bandanna, scarf, or other type of covering in cases where face masks are not available.
N95 respirators or surgical masks?
There are two main types of masks generally used in health care. N95 respirators filter out 95% of airborne particles, including bacteria and viruses. The lighter surgical or medical face masks are made to prevent spit and mucous from getting on patients or equipment.
Both types reduce rates of infection among health care workers, though comparisons (at least for influenza) have yet to show that one is superior to the other. One 2020 review by Chinese researchers, for example, analyzed six randomly controlled trials that included more than 9000 participants and found no added benefits of N95 masks over ordinary surgical masks for health care providers treating patients with the flu.
But COVID-19 is not influenza, and evidence suggests it may require more intensive protection, says Friese, who coauthored a blog post for JAMA about the country’s unpreparedness for protecting health care workers during a pandemic. The virus can linger in the air for hours, suggesting that N95 respirators are health care providers’ best option when treating infected patients.
The problem is there’s not enough to go around — of either mask type. In a March 5 survey, National Nurses United reported that just 30% of more than 6500 US respondents said their organizations had enough PPE to respond to a surge in patients. Another 38% did not know if their organizations were prepared. In a tweet, Friese estimated that 12% of nurses and other providers are at risk from reusing equipment or using equipment that is not backed by evidence.
Physicians and providers around the world have been sharing strategies online for how to make their own masks. Techniques vary, as do materials and plans for how to use the homemade equipment. At Phoebe Putney Health, DIY masks are intended to be worn over N95 respirators and then disposed of so that the respirators can be reused more safely, says Amanda Clements, the hospital’s public relations coordinator. Providers might also wear them to greet people at the front door.
Some evidence suggests that homemade masks can help in a pinch, at least for some illnesses. For a 2013 study by researchers in the UK, volunteers made surgical masks from cotton T-shirts, then put them on and coughed into a chamber that measured how much bacterial content got through. The team also assessed the aerosol-filtering ability of a variety of household materials, including scarfs, antimicrobial pillowcases, vacuum-cleaner bags, and tea towels. They tested each material with an aerosol containing two types of bacteria similar in size to influenza.
Commercial surgical masks performed three times better than homemade ones in the filtration test. Surgical masks worked twice as well at blocking droplets on the cough test. But all the makeshift materials — which also included silk, linen, and regular pillowcases — blocked some microbes. Vacuum-cleaner bags blocked the most bacteria, but their stiffness and thickness made them unsuitable for use as masks, the researchers reported. Tea towels showed a similar pattern. But pillowcases and cotton T-shirts were stretchy enough to fit well, thereby reducing the particles that could get through or around them.
Homemade masks should be used only as a last resort if commercial masks become unavailable, the researchers concluded. “Probably something is better than nothing for trained health care workers — for droplet contact avoidance, if nothing else,” says Anna Davies, BSc, a research facilitator at the University of Cambridge, UK, who is a former public health microbiologist and one of the study’s authors.
She recommends that members of the general public donate any stockpiles they have to health care workers, and make their own if they want masks for personal use. She is working with collaborators in the US to develop guidance for how best to do it.
“If people are quarantined and looking for something worthwhile to do, it probably wouldn’t be the worst thing to apply themselves to,” she wrote by email. “My suggestion would be for something soft and cotton, ideally with a bit of stretch (although it’s a pain to sew), and in two layers, marked ‘inside’ and ‘outside.’ ”
The idea that something is better than nothing was also the conclusion of a 2008 study by researchers in the Netherlands and the US. The study enlisted 28 healthy individuals who performed a variety of tasks while wearing N95 masks, surgical masks, or homemade masks sewn from teacloths. Effectiveness varied among individuals, but over a 90-second period, N95 masks worked best, with 25 times more protection than surgical masks and about 50 times more protection than homemade ones. Surgical masks were twice as effective as homemade masks. But the homemade masks offered at least some protection against large droplets.
Researchers emphasize that it’s not yet clear whether those findings are applicable to aerosolized COVID-19. In an influenza pandemic, at least, the authors posit that homemade masks could reduce transmission for the general public enough for some immunity to build. “It is important not to focus on a single intervention in case of a pandemic,” the researchers write, “but to integrate all effective interventions for optimal protection.”
For health care workers on the frontlines of COVID-19, Friese says, homemade masks might do more than nothing but they also might not work. Instead, he would rather see providers using construction or nuclear-engineering masks. And his best suggestion is something many providers are already doing: reducing physical contact with patients through telemedicine and other creative solutions, which is cutting down the overwhelming need for PPE.
Homemade mask production emphasizes the urgent need for more supplies, Friese adds.
“The government needs to step up and do a variety of things to increase production, and that needs to happen now, immediately,” he says. “We don’t we don’t want our clinicians to have to come up with these decisions.”
This article first appeared on Medscape.com.
COVID-19 prompts ‘lifesaving’ policy change for opioid addiction
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
The apricot tree
My apricot tree has bloomed. It is a foolish tree planted by a foolish man since it blossoms, with beautiful pink then white flowers, at least 3 weeks too early in Northern Kentucky. Nonetheless, I am hopeful that it will produce fruit, maybe this year.
The apricot tree takes me back to my early childhood in Oklahoma City. We had a small apricot tree in the backyard of our rental house, and my dad would talk about how there was nothing finer than a sun ripened apricot. Those were happy times. My dad was a milkman and was home every day by late afternoon, though he was still taking classes at night to try to finish his degree. My mother was at home and my older brother in first grade down the street. My little sister was small and tried to keep up.
My time was unstructured, and I reveled in the backyard. In retrospect, the backyard was an open display of broken and hoped for dreams. There was a junked car my best friend Alvin and I would sit in, there was a huge tree stump we sat on and played around, we had an old slow dog named Pooch, gifted to us when my mom’s sister moved to Alaska. We ran around with no shirts or shoes, played and pretended, and carefully watched the apricot tree.
I remember one time when the apricots finally ripened. My father climbed up and got me one, and it was so sweet I did not notice that the juice ran down my face and my bare chest. It was the sweetest and most wonderful thing I have ever tasted. All the better for having to wait for it.
. I have had four major meetings canceled and though my livelihood and life are at risk, I feel oddly free and happy. I am no longer under those pressures to research, write, and present, and am spending at lot of time at home with my wife and daughter. I think I will clean out the garage (who knows what I will find?) and work in the backyard – and keep a close watch on the apricot tree.
As many of you have, I have awkwardly embraced telemedicine in the past. It is interesting now, how HIPAA regulations and state licensing requirements have finally been tossed aside, making it possible to practice telemedicine. I suspect things will stay that way if it is demonstrated they are unnecessary.
In my office, we are depopulating the waiting room and autoclaving face masks. I am cleaning out the stockroom and donating extra gloves, gowns, and masks to the local hospital. We may shut down altogether. There is little more I can do unless called to man a ventilator. I hope it doesn’t come to that, but I will serve if called.
I suggest you embrace your current unstructured time and use it to let your mind roam. It is a reprieve from today’s hyperconnected, hurly burly world. I also suggest you check COVID-19 news updates only once a day and turn off television news altogether. Other than following the recommendations and guidance of public health authorities, there is nothing you can do to speed up the resolution of this pandemic.
No matter how awful, this will pass. It is a warm spring and it is possible the apricot tree will not be bitten by frost, and we may have fruit this year. We should know in about 2 months. I am going to keep a close watch on it.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
My apricot tree has bloomed. It is a foolish tree planted by a foolish man since it blossoms, with beautiful pink then white flowers, at least 3 weeks too early in Northern Kentucky. Nonetheless, I am hopeful that it will produce fruit, maybe this year.
The apricot tree takes me back to my early childhood in Oklahoma City. We had a small apricot tree in the backyard of our rental house, and my dad would talk about how there was nothing finer than a sun ripened apricot. Those were happy times. My dad was a milkman and was home every day by late afternoon, though he was still taking classes at night to try to finish his degree. My mother was at home and my older brother in first grade down the street. My little sister was small and tried to keep up.
My time was unstructured, and I reveled in the backyard. In retrospect, the backyard was an open display of broken and hoped for dreams. There was a junked car my best friend Alvin and I would sit in, there was a huge tree stump we sat on and played around, we had an old slow dog named Pooch, gifted to us when my mom’s sister moved to Alaska. We ran around with no shirts or shoes, played and pretended, and carefully watched the apricot tree.
I remember one time when the apricots finally ripened. My father climbed up and got me one, and it was so sweet I did not notice that the juice ran down my face and my bare chest. It was the sweetest and most wonderful thing I have ever tasted. All the better for having to wait for it.
. I have had four major meetings canceled and though my livelihood and life are at risk, I feel oddly free and happy. I am no longer under those pressures to research, write, and present, and am spending at lot of time at home with my wife and daughter. I think I will clean out the garage (who knows what I will find?) and work in the backyard – and keep a close watch on the apricot tree.
As many of you have, I have awkwardly embraced telemedicine in the past. It is interesting now, how HIPAA regulations and state licensing requirements have finally been tossed aside, making it possible to practice telemedicine. I suspect things will stay that way if it is demonstrated they are unnecessary.
In my office, we are depopulating the waiting room and autoclaving face masks. I am cleaning out the stockroom and donating extra gloves, gowns, and masks to the local hospital. We may shut down altogether. There is little more I can do unless called to man a ventilator. I hope it doesn’t come to that, but I will serve if called.
I suggest you embrace your current unstructured time and use it to let your mind roam. It is a reprieve from today’s hyperconnected, hurly burly world. I also suggest you check COVID-19 news updates only once a day and turn off television news altogether. Other than following the recommendations and guidance of public health authorities, there is nothing you can do to speed up the resolution of this pandemic.
No matter how awful, this will pass. It is a warm spring and it is possible the apricot tree will not be bitten by frost, and we may have fruit this year. We should know in about 2 months. I am going to keep a close watch on it.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
My apricot tree has bloomed. It is a foolish tree planted by a foolish man since it blossoms, with beautiful pink then white flowers, at least 3 weeks too early in Northern Kentucky. Nonetheless, I am hopeful that it will produce fruit, maybe this year.
The apricot tree takes me back to my early childhood in Oklahoma City. We had a small apricot tree in the backyard of our rental house, and my dad would talk about how there was nothing finer than a sun ripened apricot. Those were happy times. My dad was a milkman and was home every day by late afternoon, though he was still taking classes at night to try to finish his degree. My mother was at home and my older brother in first grade down the street. My little sister was small and tried to keep up.
My time was unstructured, and I reveled in the backyard. In retrospect, the backyard was an open display of broken and hoped for dreams. There was a junked car my best friend Alvin and I would sit in, there was a huge tree stump we sat on and played around, we had an old slow dog named Pooch, gifted to us when my mom’s sister moved to Alaska. We ran around with no shirts or shoes, played and pretended, and carefully watched the apricot tree.
I remember one time when the apricots finally ripened. My father climbed up and got me one, and it was so sweet I did not notice that the juice ran down my face and my bare chest. It was the sweetest and most wonderful thing I have ever tasted. All the better for having to wait for it.
. I have had four major meetings canceled and though my livelihood and life are at risk, I feel oddly free and happy. I am no longer under those pressures to research, write, and present, and am spending at lot of time at home with my wife and daughter. I think I will clean out the garage (who knows what I will find?) and work in the backyard – and keep a close watch on the apricot tree.
As many of you have, I have awkwardly embraced telemedicine in the past. It is interesting now, how HIPAA regulations and state licensing requirements have finally been tossed aside, making it possible to practice telemedicine. I suspect things will stay that way if it is demonstrated they are unnecessary.
In my office, we are depopulating the waiting room and autoclaving face masks. I am cleaning out the stockroom and donating extra gloves, gowns, and masks to the local hospital. We may shut down altogether. There is little more I can do unless called to man a ventilator. I hope it doesn’t come to that, but I will serve if called.
I suggest you embrace your current unstructured time and use it to let your mind roam. It is a reprieve from today’s hyperconnected, hurly burly world. I also suggest you check COVID-19 news updates only once a day and turn off television news altogether. Other than following the recommendations and guidance of public health authorities, there is nothing you can do to speed up the resolution of this pandemic.
No matter how awful, this will pass. It is a warm spring and it is possible the apricot tree will not be bitten by frost, and we may have fruit this year. We should know in about 2 months. I am going to keep a close watch on it.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].