User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Coronavirus stays in aerosols for hours, on surfaces for days
according to a new study.
The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
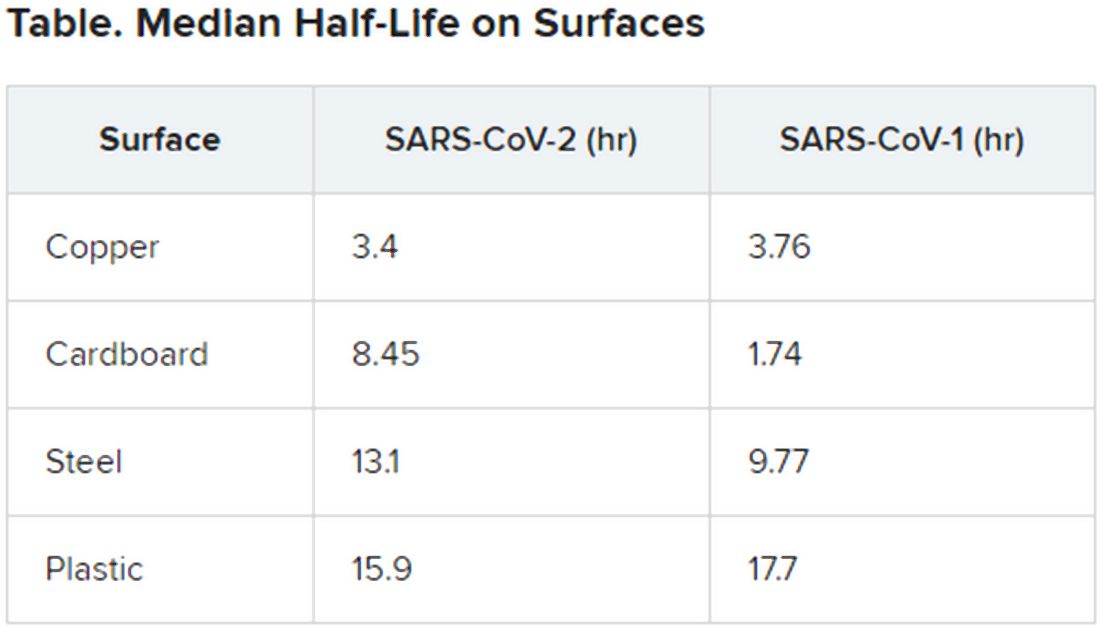
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
according to a new study.

The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
according to a new study.

The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Dupilumab approval sought for AD under age 12
LAHAINA, HAWAII – Reassuring evidence of the long-term effectiveness and safety of dupilumab in adolescents with moderate to severe atopic dermatitis comes from a phase 3 open-label extension study of the first teenagers in the world to have received the monoclonal antibody, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dupilumab (Dupixent), a monoclonal antibody directed against interleukins-4 and -13 initially approved in adults, received an expanded indication from the Food and Drug Administration in March 2019 for treatment of 12- to 17-year-olds with moderate to severe atopic dermatitis (AD) on the strength of a pivotal 251-patient, phase 3 randomized trial of 16 weeks’ duration (JAMA Dermatol. 2019 Nov 6. doi: 10.1001/jamadermatol.2019.3336). But since AD is a chronic disease, it was important to learn how dupilumab performs well beyond the 16-week mark in adolescents, observed Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
In addition to highlighting some of the emerging fine points of dupilumab therapy in adolescents, Dr. Eichenfield discussed the clinical implications of a potential further expanded indication for treatment of 6- to 12-year-olds, an event he considers likely in the coming months. He also described early data from an ongoing dupilumab clinical trials program in the 2- to 5-year-olds.
Long-term dupilumab in teens
Dr. Eichenfield was a coauthor of the recently published phase 3 international long-term extension study. The 40 participants experienced a mean 85% decrease from baseline at 52 weeks in EASI (Eczema Area and Severity Index) scores on 2 mg/kg per week dosing and an 84% reduction on 4 mg/kg per week dosing. This represented a substantial further improvement from week 2, when the EASI reductions were 34% and 51%, respectively.
The mean trough serum dupilumab concentrations over the course of the year were markedly lower in the 2 mg/kg group: 74 mg/L, as compared to 161 mg/L with dosing at 4 mg/kg per week (Br J Dermatol. 2020 Jan;182[1]:85-96).
“It’ll be interesting to see how this works out over time,” the dermatologist commented. “The issue of dose by weight becomes important as we start to treat younger patients because the pharmacokinetics are very different at 4 and 2 mg/kg, and it may have an impact on efficacy.”
The extension study also established the safety and effectiveness of utilizing dupilumab in combination with standard topical corticosteroid therapy, which wasn’t allowed in the pivotal 16-week trial.
Some have commented that dupilumab may be less effective in adolescents than in adults. They point to the 24% rate of an Investigator Global Assessment (IGA) of 0 or 1 – that is, clear or almost clear – at week 16 in the pivotal adolescent trial, a substantially lower rate than in the adult trials. However, Dr. Eichenfield noted that the adolescent study population was heavily skewed to the severe end of the disease spectrum, the placebo response rate was very low, and the absolute placebo-subtracted benefit turned out to be quite similar to what was seen in the adult trials. Moreover, he added, in a post hoc analysis of the pivotal trial data which utilized a different measure of clinically meaningful response – a composite of either a 50% reduction in EASI score, a 3-point or greater improvement on a 10-point pruritus scale, or at least a 6-point improvement from baseline on the Children’s Dermatology Quality Life Index – that outcome was achieved by 74% of adolescents who didn’t achieve clear or almost clear.
What’s next for dupilumab in pediatric AD
Approval of dupilumab in children under aged 12 years is eagerly awaited, Dr. Eichenfield said. The Food and Drug Administration is now analyzing as-yet unreleased data from completed clinical trials of dupilumab in 6- to 12-year-olds with moderate to severe AD with an eye toward a possible further expanded indication. The side effect profile appears to be the same as in 12- to 18-year-olds.
“I assume it will be approved,” Dr. Eichenfield said. “We don’t know what’s going to happen in 6- to 12-year-olds in terms of the ultimate dosing recommendations that will be put out, but be aware that the pharmacokinetics vary by weight over time.”
Early data in children aged 2-5 years with severe AD from the phase 2, open-label, single ascending dose Liberty AD PRESCHOOL study showed that weight-based dosing in that age group made a big difference in terms of pharmacokinetics. In terms of efficacy, the mean reduction in EASI scores 4 weeks after a single dose of dupilumab was 27% with 3 mg/kg and 49% with 6 mg/kg.
Avoidance of live vaccines while on dupilumab becomes more of a consideration in the under-12 population. The second dose of varicella is supposed to be administered at 4 to 6 years of age, as is the second dose of MMR. The nasal influenza vaccine is a live virus vaccine, as is the yellow fever vaccine.
“We don’t know if live vaccines are dangerous for someone on dupilumab, it’s just that it’s listed that you shouldn’t use them and they haven’t been studied,” Dr. Eichenfield observed.
He reported receiving research grants from or serving as a consultant to several dozen pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the long-term effectiveness and safety of dupilumab in adolescents with moderate to severe atopic dermatitis comes from a phase 3 open-label extension study of the first teenagers in the world to have received the monoclonal antibody, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dupilumab (Dupixent), a monoclonal antibody directed against interleukins-4 and -13 initially approved in adults, received an expanded indication from the Food and Drug Administration in March 2019 for treatment of 12- to 17-year-olds with moderate to severe atopic dermatitis (AD) on the strength of a pivotal 251-patient, phase 3 randomized trial of 16 weeks’ duration (JAMA Dermatol. 2019 Nov 6. doi: 10.1001/jamadermatol.2019.3336). But since AD is a chronic disease, it was important to learn how dupilumab performs well beyond the 16-week mark in adolescents, observed Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
In addition to highlighting some of the emerging fine points of dupilumab therapy in adolescents, Dr. Eichenfield discussed the clinical implications of a potential further expanded indication for treatment of 6- to 12-year-olds, an event he considers likely in the coming months. He also described early data from an ongoing dupilumab clinical trials program in the 2- to 5-year-olds.
Long-term dupilumab in teens
Dr. Eichenfield was a coauthor of the recently published phase 3 international long-term extension study. The 40 participants experienced a mean 85% decrease from baseline at 52 weeks in EASI (Eczema Area and Severity Index) scores on 2 mg/kg per week dosing and an 84% reduction on 4 mg/kg per week dosing. This represented a substantial further improvement from week 2, when the EASI reductions were 34% and 51%, respectively.
The mean trough serum dupilumab concentrations over the course of the year were markedly lower in the 2 mg/kg group: 74 mg/L, as compared to 161 mg/L with dosing at 4 mg/kg per week (Br J Dermatol. 2020 Jan;182[1]:85-96).
“It’ll be interesting to see how this works out over time,” the dermatologist commented. “The issue of dose by weight becomes important as we start to treat younger patients because the pharmacokinetics are very different at 4 and 2 mg/kg, and it may have an impact on efficacy.”
The extension study also established the safety and effectiveness of utilizing dupilumab in combination with standard topical corticosteroid therapy, which wasn’t allowed in the pivotal 16-week trial.
Some have commented that dupilumab may be less effective in adolescents than in adults. They point to the 24% rate of an Investigator Global Assessment (IGA) of 0 or 1 – that is, clear or almost clear – at week 16 in the pivotal adolescent trial, a substantially lower rate than in the adult trials. However, Dr. Eichenfield noted that the adolescent study population was heavily skewed to the severe end of the disease spectrum, the placebo response rate was very low, and the absolute placebo-subtracted benefit turned out to be quite similar to what was seen in the adult trials. Moreover, he added, in a post hoc analysis of the pivotal trial data which utilized a different measure of clinically meaningful response – a composite of either a 50% reduction in EASI score, a 3-point or greater improvement on a 10-point pruritus scale, or at least a 6-point improvement from baseline on the Children’s Dermatology Quality Life Index – that outcome was achieved by 74% of adolescents who didn’t achieve clear or almost clear.
What’s next for dupilumab in pediatric AD
Approval of dupilumab in children under aged 12 years is eagerly awaited, Dr. Eichenfield said. The Food and Drug Administration is now analyzing as-yet unreleased data from completed clinical trials of dupilumab in 6- to 12-year-olds with moderate to severe AD with an eye toward a possible further expanded indication. The side effect profile appears to be the same as in 12- to 18-year-olds.
“I assume it will be approved,” Dr. Eichenfield said. “We don’t know what’s going to happen in 6- to 12-year-olds in terms of the ultimate dosing recommendations that will be put out, but be aware that the pharmacokinetics vary by weight over time.”
Early data in children aged 2-5 years with severe AD from the phase 2, open-label, single ascending dose Liberty AD PRESCHOOL study showed that weight-based dosing in that age group made a big difference in terms of pharmacokinetics. In terms of efficacy, the mean reduction in EASI scores 4 weeks after a single dose of dupilumab was 27% with 3 mg/kg and 49% with 6 mg/kg.
Avoidance of live vaccines while on dupilumab becomes more of a consideration in the under-12 population. The second dose of varicella is supposed to be administered at 4 to 6 years of age, as is the second dose of MMR. The nasal influenza vaccine is a live virus vaccine, as is the yellow fever vaccine.
“We don’t know if live vaccines are dangerous for someone on dupilumab, it’s just that it’s listed that you shouldn’t use them and they haven’t been studied,” Dr. Eichenfield observed.
He reported receiving research grants from or serving as a consultant to several dozen pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the long-term effectiveness and safety of dupilumab in adolescents with moderate to severe atopic dermatitis comes from a phase 3 open-label extension study of the first teenagers in the world to have received the monoclonal antibody, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dupilumab (Dupixent), a monoclonal antibody directed against interleukins-4 and -13 initially approved in adults, received an expanded indication from the Food and Drug Administration in March 2019 for treatment of 12- to 17-year-olds with moderate to severe atopic dermatitis (AD) on the strength of a pivotal 251-patient, phase 3 randomized trial of 16 weeks’ duration (JAMA Dermatol. 2019 Nov 6. doi: 10.1001/jamadermatol.2019.3336). But since AD is a chronic disease, it was important to learn how dupilumab performs well beyond the 16-week mark in adolescents, observed Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
In addition to highlighting some of the emerging fine points of dupilumab therapy in adolescents, Dr. Eichenfield discussed the clinical implications of a potential further expanded indication for treatment of 6- to 12-year-olds, an event he considers likely in the coming months. He also described early data from an ongoing dupilumab clinical trials program in the 2- to 5-year-olds.
Long-term dupilumab in teens
Dr. Eichenfield was a coauthor of the recently published phase 3 international long-term extension study. The 40 participants experienced a mean 85% decrease from baseline at 52 weeks in EASI (Eczema Area and Severity Index) scores on 2 mg/kg per week dosing and an 84% reduction on 4 mg/kg per week dosing. This represented a substantial further improvement from week 2, when the EASI reductions were 34% and 51%, respectively.
The mean trough serum dupilumab concentrations over the course of the year were markedly lower in the 2 mg/kg group: 74 mg/L, as compared to 161 mg/L with dosing at 4 mg/kg per week (Br J Dermatol. 2020 Jan;182[1]:85-96).
“It’ll be interesting to see how this works out over time,” the dermatologist commented. “The issue of dose by weight becomes important as we start to treat younger patients because the pharmacokinetics are very different at 4 and 2 mg/kg, and it may have an impact on efficacy.”
The extension study also established the safety and effectiveness of utilizing dupilumab in combination with standard topical corticosteroid therapy, which wasn’t allowed in the pivotal 16-week trial.
Some have commented that dupilumab may be less effective in adolescents than in adults. They point to the 24% rate of an Investigator Global Assessment (IGA) of 0 or 1 – that is, clear or almost clear – at week 16 in the pivotal adolescent trial, a substantially lower rate than in the adult trials. However, Dr. Eichenfield noted that the adolescent study population was heavily skewed to the severe end of the disease spectrum, the placebo response rate was very low, and the absolute placebo-subtracted benefit turned out to be quite similar to what was seen in the adult trials. Moreover, he added, in a post hoc analysis of the pivotal trial data which utilized a different measure of clinically meaningful response – a composite of either a 50% reduction in EASI score, a 3-point or greater improvement on a 10-point pruritus scale, or at least a 6-point improvement from baseline on the Children’s Dermatology Quality Life Index – that outcome was achieved by 74% of adolescents who didn’t achieve clear or almost clear.
What’s next for dupilumab in pediatric AD
Approval of dupilumab in children under aged 12 years is eagerly awaited, Dr. Eichenfield said. The Food and Drug Administration is now analyzing as-yet unreleased data from completed clinical trials of dupilumab in 6- to 12-year-olds with moderate to severe AD with an eye toward a possible further expanded indication. The side effect profile appears to be the same as in 12- to 18-year-olds.
“I assume it will be approved,” Dr. Eichenfield said. “We don’t know what’s going to happen in 6- to 12-year-olds in terms of the ultimate dosing recommendations that will be put out, but be aware that the pharmacokinetics vary by weight over time.”
Early data in children aged 2-5 years with severe AD from the phase 2, open-label, single ascending dose Liberty AD PRESCHOOL study showed that weight-based dosing in that age group made a big difference in terms of pharmacokinetics. In terms of efficacy, the mean reduction in EASI scores 4 weeks after a single dose of dupilumab was 27% with 3 mg/kg and 49% with 6 mg/kg.
Avoidance of live vaccines while on dupilumab becomes more of a consideration in the under-12 population. The second dose of varicella is supposed to be administered at 4 to 6 years of age, as is the second dose of MMR. The nasal influenza vaccine is a live virus vaccine, as is the yellow fever vaccine.
“We don’t know if live vaccines are dangerous for someone on dupilumab, it’s just that it’s listed that you shouldn’t use them and they haven’t been studied,” Dr. Eichenfield observed.
He reported receiving research grants from or serving as a consultant to several dozen pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
COVID-19: Extra caution needed for patients with diabetes
Patients with diabetes may have an increased risk of developing coronavirus infection (COVID-19), along with increased risks of morbidity and mortality, according to researchers writing in Diabetes & Metabolic Syndrome.
Although relevant clinical data remain scarce, patients with diabetes should take extra precautions to avoid infection and, if infected, may require special care, reported Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues.
“The disease severity [with COVID-19] has varied from mild, self-limiting, flu-like illness to fulminant pneumonia, respiratory failure, and death,” the authors wrote.
As of March 16, 2020, the World Health Organization reported 167,515 confirmed cases of COVID-19 and 6,606 deaths from around the world, with a mortality rate of 3.9%. But the actual mortality rate may be lower, the authors suggested, because a study involving more than 1,000 confirmed cases reported a mortality rate of 1.4%.
“Considering that the number of unreported and unconfirmed cases is likely to be much higher than the reported cases, the actual mortality may be less than 1%, which is similar to that of severe seasonal influenza,” the authors said, in reference to an editorial by Anthony S. Fauci, MD, and colleagues in the New England Journal of Medicine. In addition, they noted, mortality rates may vary by region.
The largest study relevant to patients with diabetes, which involved 72,314 cases of COVID-19, showed that patients with diabetes had a threefold higher mortality rate than did those without diabetes (7.3% vs. 2.3%, respectively). These figures were reported by the Chinese Centre for Disease Control and Prevention.
However, data from smaller cohorts with diabetes and COVID-19 have yielded mixed results. For instance, one study, involving 140 patients from Wuhan, suggested that diabetes was not a risk factor for severe disease, and in an analysis of 11 studies reporting on laboratory abnormalities in patients with a diagnosis of COVID-19, raised blood sugar levels or diabetes were not mentioned among the predictors of severe disease.
“Our knowledge about the prevalence of COVID-19 and disease course in people with diabetes will evolve as more detailed analyses are carried out,” the authors wrote. “For now, it is reasonable to assume that people with diabetes are at increased risk of developing infection. Coexisting heart disease, kidney disease, advanced age, and frailty are likely to further increase the severity of disease.”
Prevention first
“It is important that people with diabetes maintain good glycemic control, because it might help in reducing the risk of infection and the severity,” the authors wrote.
In addition to more frequent monitoring of blood glucose levels, they recommended other preventive measures, such as getting adequate nutrition, exercising, and being current with vaccinations for influenza and pneumonia. The latter, they said, may also reduce the risk of secondary bacterial pneumonia after a respiratory viral infection.
In regard to nutrition, adequate protein intake is important and “any deficiencies of minerals and vitamins need to be taken care of,” they advised. Likewise, exercise is known to improve immunity and should continue, but they suggest avoiding gyms and swimming pools.
For patients with coexisting heart and/or kidney disease, they also recommended efforts to stabilize cardiac/renal status.
In addition, the general preventive measures, such as regular and thorough hand washing with soap and water, practicing good respiratory hygiene by sneezing and coughing into a bent elbow or a facial tissue, and avoiding contact with anyone who is infected, should be observed.
As with other patients with chronic diseases that are managed long-term medications, patients with diabetes should always ensure that they have a sufficient supply of their medications and refills, if possible.
After a diagnosis
If patients with diabetes develop COVID-19, then home management may still be possible, wrote the authors, who recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve.
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often. “Frequent changes in dosage and correctional bolus may be required to maintain normoglycemia,” they cautioned.
Concerning diabetic drug regimens, they suggest patients avoid antihyperglycemic agents that can cause volume depletion or hypoglycemia and, if necessary, that they reduce oral antidiabetic drugs and follow sick-day guidelines.
For hospitalized patients, the investigators strengthened that statement, advising that oral agents need to be stopped, particularly sodium-glucose cotransporter 2 inhibitors and metformin. “Insulin is the preferred agent for control of hyperglycemia in hospitalized sick patients,” they wrote.
Untested therapies
The authors also discussed a range of untested therapies that may help fight COVID-19, such as antiviral drugs (such as lopinavir and ritonavir), zinc nanoparticles, and vitamin C. Supplementing those recommendations, Dr. Gupta and colleagues provided a concise review of COVID-19 epidemiology and extant data relevant to patients with diabetes.
The investigators reported no conflicts of interest.
SOURCE: Gupta et al. Diabetes Metab Syndr. 2020;14(3):211-12.
Patients with diabetes may have an increased risk of developing coronavirus infection (COVID-19), along with increased risks of morbidity and mortality, according to researchers writing in Diabetes & Metabolic Syndrome.
Although relevant clinical data remain scarce, patients with diabetes should take extra precautions to avoid infection and, if infected, may require special care, reported Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues.
“The disease severity [with COVID-19] has varied from mild, self-limiting, flu-like illness to fulminant pneumonia, respiratory failure, and death,” the authors wrote.
As of March 16, 2020, the World Health Organization reported 167,515 confirmed cases of COVID-19 and 6,606 deaths from around the world, with a mortality rate of 3.9%. But the actual mortality rate may be lower, the authors suggested, because a study involving more than 1,000 confirmed cases reported a mortality rate of 1.4%.
“Considering that the number of unreported and unconfirmed cases is likely to be much higher than the reported cases, the actual mortality may be less than 1%, which is similar to that of severe seasonal influenza,” the authors said, in reference to an editorial by Anthony S. Fauci, MD, and colleagues in the New England Journal of Medicine. In addition, they noted, mortality rates may vary by region.
The largest study relevant to patients with diabetes, which involved 72,314 cases of COVID-19, showed that patients with diabetes had a threefold higher mortality rate than did those without diabetes (7.3% vs. 2.3%, respectively). These figures were reported by the Chinese Centre for Disease Control and Prevention.
However, data from smaller cohorts with diabetes and COVID-19 have yielded mixed results. For instance, one study, involving 140 patients from Wuhan, suggested that diabetes was not a risk factor for severe disease, and in an analysis of 11 studies reporting on laboratory abnormalities in patients with a diagnosis of COVID-19, raised blood sugar levels or diabetes were not mentioned among the predictors of severe disease.
“Our knowledge about the prevalence of COVID-19 and disease course in people with diabetes will evolve as more detailed analyses are carried out,” the authors wrote. “For now, it is reasonable to assume that people with diabetes are at increased risk of developing infection. Coexisting heart disease, kidney disease, advanced age, and frailty are likely to further increase the severity of disease.”
Prevention first
“It is important that people with diabetes maintain good glycemic control, because it might help in reducing the risk of infection and the severity,” the authors wrote.
In addition to more frequent monitoring of blood glucose levels, they recommended other preventive measures, such as getting adequate nutrition, exercising, and being current with vaccinations for influenza and pneumonia. The latter, they said, may also reduce the risk of secondary bacterial pneumonia after a respiratory viral infection.
In regard to nutrition, adequate protein intake is important and “any deficiencies of minerals and vitamins need to be taken care of,” they advised. Likewise, exercise is known to improve immunity and should continue, but they suggest avoiding gyms and swimming pools.
For patients with coexisting heart and/or kidney disease, they also recommended efforts to stabilize cardiac/renal status.
In addition, the general preventive measures, such as regular and thorough hand washing with soap and water, practicing good respiratory hygiene by sneezing and coughing into a bent elbow or a facial tissue, and avoiding contact with anyone who is infected, should be observed.
As with other patients with chronic diseases that are managed long-term medications, patients with diabetes should always ensure that they have a sufficient supply of their medications and refills, if possible.
After a diagnosis
If patients with diabetes develop COVID-19, then home management may still be possible, wrote the authors, who recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve.
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often. “Frequent changes in dosage and correctional bolus may be required to maintain normoglycemia,” they cautioned.
Concerning diabetic drug regimens, they suggest patients avoid antihyperglycemic agents that can cause volume depletion or hypoglycemia and, if necessary, that they reduce oral antidiabetic drugs and follow sick-day guidelines.
For hospitalized patients, the investigators strengthened that statement, advising that oral agents need to be stopped, particularly sodium-glucose cotransporter 2 inhibitors and metformin. “Insulin is the preferred agent for control of hyperglycemia in hospitalized sick patients,” they wrote.
Untested therapies
The authors also discussed a range of untested therapies that may help fight COVID-19, such as antiviral drugs (such as lopinavir and ritonavir), zinc nanoparticles, and vitamin C. Supplementing those recommendations, Dr. Gupta and colleagues provided a concise review of COVID-19 epidemiology and extant data relevant to patients with diabetes.
The investigators reported no conflicts of interest.
SOURCE: Gupta et al. Diabetes Metab Syndr. 2020;14(3):211-12.
Patients with diabetes may have an increased risk of developing coronavirus infection (COVID-19), along with increased risks of morbidity and mortality, according to researchers writing in Diabetes & Metabolic Syndrome.
Although relevant clinical data remain scarce, patients with diabetes should take extra precautions to avoid infection and, if infected, may require special care, reported Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues.
“The disease severity [with COVID-19] has varied from mild, self-limiting, flu-like illness to fulminant pneumonia, respiratory failure, and death,” the authors wrote.
As of March 16, 2020, the World Health Organization reported 167,515 confirmed cases of COVID-19 and 6,606 deaths from around the world, with a mortality rate of 3.9%. But the actual mortality rate may be lower, the authors suggested, because a study involving more than 1,000 confirmed cases reported a mortality rate of 1.4%.
“Considering that the number of unreported and unconfirmed cases is likely to be much higher than the reported cases, the actual mortality may be less than 1%, which is similar to that of severe seasonal influenza,” the authors said, in reference to an editorial by Anthony S. Fauci, MD, and colleagues in the New England Journal of Medicine. In addition, they noted, mortality rates may vary by region.
The largest study relevant to patients with diabetes, which involved 72,314 cases of COVID-19, showed that patients with diabetes had a threefold higher mortality rate than did those without diabetes (7.3% vs. 2.3%, respectively). These figures were reported by the Chinese Centre for Disease Control and Prevention.
However, data from smaller cohorts with diabetes and COVID-19 have yielded mixed results. For instance, one study, involving 140 patients from Wuhan, suggested that diabetes was not a risk factor for severe disease, and in an analysis of 11 studies reporting on laboratory abnormalities in patients with a diagnosis of COVID-19, raised blood sugar levels or diabetes were not mentioned among the predictors of severe disease.
“Our knowledge about the prevalence of COVID-19 and disease course in people with diabetes will evolve as more detailed analyses are carried out,” the authors wrote. “For now, it is reasonable to assume that people with diabetes are at increased risk of developing infection. Coexisting heart disease, kidney disease, advanced age, and frailty are likely to further increase the severity of disease.”
Prevention first
“It is important that people with diabetes maintain good glycemic control, because it might help in reducing the risk of infection and the severity,” the authors wrote.
In addition to more frequent monitoring of blood glucose levels, they recommended other preventive measures, such as getting adequate nutrition, exercising, and being current with vaccinations for influenza and pneumonia. The latter, they said, may also reduce the risk of secondary bacterial pneumonia after a respiratory viral infection.
In regard to nutrition, adequate protein intake is important and “any deficiencies of minerals and vitamins need to be taken care of,” they advised. Likewise, exercise is known to improve immunity and should continue, but they suggest avoiding gyms and swimming pools.
For patients with coexisting heart and/or kidney disease, they also recommended efforts to stabilize cardiac/renal status.
In addition, the general preventive measures, such as regular and thorough hand washing with soap and water, practicing good respiratory hygiene by sneezing and coughing into a bent elbow or a facial tissue, and avoiding contact with anyone who is infected, should be observed.
As with other patients with chronic diseases that are managed long-term medications, patients with diabetes should always ensure that they have a sufficient supply of their medications and refills, if possible.
After a diagnosis
If patients with diabetes develop COVID-19, then home management may still be possible, wrote the authors, who recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve.
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often. “Frequent changes in dosage and correctional bolus may be required to maintain normoglycemia,” they cautioned.
Concerning diabetic drug regimens, they suggest patients avoid antihyperglycemic agents that can cause volume depletion or hypoglycemia and, if necessary, that they reduce oral antidiabetic drugs and follow sick-day guidelines.
For hospitalized patients, the investigators strengthened that statement, advising that oral agents need to be stopped, particularly sodium-glucose cotransporter 2 inhibitors and metformin. “Insulin is the preferred agent for control of hyperglycemia in hospitalized sick patients,” they wrote.
Untested therapies
The authors also discussed a range of untested therapies that may help fight COVID-19, such as antiviral drugs (such as lopinavir and ritonavir), zinc nanoparticles, and vitamin C. Supplementing those recommendations, Dr. Gupta and colleagues provided a concise review of COVID-19 epidemiology and extant data relevant to patients with diabetes.
The investigators reported no conflicts of interest.
SOURCE: Gupta et al. Diabetes Metab Syndr. 2020;14(3):211-12.
FROM DIABETES & METABOLIC SYNDROME
The role of oleuropein, the primary phenol in olives, in skin health
Olives and olive oil have long been known to confer salutary effects to the skin.1 Leaves and fruits of the olive plant (Olea europaea) have been used as external emollients to treat skin ulcers and inflammatory wounds.2 The phenolic compound oleuropein, the most abundant phenolic found in olive leaves and oil, has been shown to exhibit antioxidant and free radical–scavenging activities.3,4 Also present in the stems and flowers of the plant, oleuropein, an ester of elenolic acid and 3,4-dihydroxyphenyl ethanol and the primary glycoside in olives,5 is thought to be the major contributor to its antioxidant and antimelanogenesis activities.6 Notably, olive leaves, which contain a copious supply of oleuropein, are thought to exert significantly more antioxidant activity than olive fruit.7
Hydroxytyrosol is an ortho-diphenolic substance and essential constituent of oleuropein that has been shown in vitro to prevent apoptotic cell death caused by UVB in HaCaT cells.8,9 Both oleuropein and hydroxytyrosol impart various anticancer properties at the initiation, promotion, and metastasis stages and yield protection against multiple cancers, including skin tumors.10 The antioxidant activity of both compounds, which has been found to be more potent than that of vitamin E, is attributed to their phenolic content.11,12 In addition, oleuropein and lipophilic olive mill wastewater derivatives have been useful as active ingredients for stabilizing cosmetic formulations.13 This column revisits oleuropein after 10 years to focus on its dermatologic potential.
Protection against UV damage
A hairless mouse study by Kimura and Sumiyoshi in 2009 revealed that olive leaf extract and its primary constituent oleuropein exert a skin-protective effect against chronic UVB-induced skin damage and carcinogenesis, as well as tumor growth. This is likely caused by reducing cutaneous cyclooxygenase (COX)-2 levels, thus suppressing the expression of vascular endothelial growth factor (VEGF) and various matrix metalloproteinases, specifically MMP-2, MMP-9, and MMP-13.14
A year later, the same researchers examined the potential protective effects of olive leaf extract and oleuropein on acute damage induced by UVB exposure in C57BL/6J mice. Both oral extract (300 mg/kg or 1,000 mg/kg) and oral oleuropein (25mg/kg or 85 mg/kg) hindered skin thickness increases engendered by daily doses of UVB (120 mJ/cm2 for 5 days, then every other day for 9 days). Olive leaf extract and oleuropein also suppressed increases in Ki-67- and 8-hydroxy-2’-deoxyguanosine–positive cell numbers, melanin granule area, and MMP-13 expression, the investigators noted.15 Preinitiation with oleuropein also appears to have prevented skin tumor formation in a two-stage carcinogenesis model in mice, which the investigators ascribed to the antioxidant and antiapoptotic properties of the olive protein.16
The cosmetic characteristics of oleuropein against UVB-induced erythema in healthy volunteers were assessed by Perugini et al. in 2008. Using an emulsion and emulgel containing oleuropein and vitamin E as a reference compound, the investigators found that the botanical ingredient was responsible for decreases in erythema (22%), transepidermal water loss (35%), and blood flow (30%). They suggested that the use of oleuropein in cosmetic formulations warrants further investigation for its potential to help mitigate UV damage.3
Wound healing
Koca et al. assessed the wound healing activity of O. europaea leaf extracts using in vivo wound models and the reference ointment Madecassol (Bayer; Istanbul) for comparison, in 2011. The results showed that the aqueous extract exhibited wound healing properties, with secoiridoid oleuropein (4.6059%) found to be the primary active constituent.2
In a 2014 skin wound–healing investigation in aged male Balb/c mice, Mehraein et al. divided 24 mice, 16 months of age, into control and experimental groups. On days 3 and 7 after incision, collagen fiber deposition was significantly increased and reepithelialization more advanced in the oleuropein group (administered via an intradermal injection once a day), which also experienced decreased cell infiltration. The investigators concluded that oleuropein speeds cutaneous wound healing in mice and may have potential for clinical applications in human would healing from surgery.17
Later that year, the same team investigated the therapeutic effects of oleuropein on the wounded skin of young male Balb/c mice, finding similar results, with the phenolic compound again accelerating reepithelialization, improving collagen fiber synthesis, and augmenting blood flow to wound areas via up-regulating VEGF protein expression.4
Hair growth
In 2015, Tong et al. reported that topically applied oleuropein spurred the anagen hair growth phase in telogenic C57BL/6N mouse skin.18 An O. europaea subcutaneous immunotherapy has also demonstrated reductions in cutaneous reactivity, safety, and tolerability in patients with rhinoconjunctivitis.19
Conclusion
The benefits of consuming olives and olive oil are well established and continue to be studied. backed by many years of anecdotal reporting and use in traditional medicine. While the emerging data on the dermatologic uses of the olive phenolic constituent oleuropein are encouraging, much more information, particularly derived from randomized, controlled trials in humans, is necessary to establish the full potential of oleuropein for indications such as wound healing and protection against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Baumann LS, Weisberg EM. “Olive oil in botanical cosmeceuticals.” Olives and Olive Oil in Health and Disease Prevention. New York: Academic Press, 2010.
2. Koca U et al. J Med Food. 2011 Jan-Feb;14(1-2):140-6.
3. Perugini P et al. Int J Cosmet Sci. 2008 Apr;30(2):113-20.
4. Mehraein F et al. Wounds. 2014 Mar;26(3):83-8.
5. Imran M et al. J Food Sci. 2018 Jul;83(7):1781-91.
6. Kishikawa A et al. Phytother Res. 2015 Jun;29(6):877-86.
7. Zheng J et al. Zhongguo Zhong Yao Za Zhi. 2016 Feb;41(4):613-8.
8. Salucci S et al. J Dermatol Sci. 2015 Oct;80(1):61-8.
9. Jeon S, Choi M. Biomed Dermatol. 2018;2:21.
10. Imran M et al. J Food Sci. 2018 Jul;83(7):1781-91.
11. Visioli F et al. Biochem Biophys Res Commun. 1998 Jun 9;247(1):60-4.
12. Polišak N et al. Phytother Res. 2019 Oct 27. doi: 10.1002/ptr.6524.
13. Aissa I et al. Biotechnol Appl Biochem. 2017 Jul;64(4):579-89.
14. Kimura Y, Sumiyoshi M. J Nutr. 2009 Nov;139(11):2079-86.
15. Sumiyoshi M, Kimura Y. Phytother Res. 2010 Jul;24(7):995-1003.
16. John DNS et al. JKIMSU. 2019 Jan-Mar;8(1):43-51.
17. Mehraein F et al. Cell J. 2014 Feb 3;16(1):25-30.
18. Tong T et al. PLoS One. 2015 Jun 10;10(6):e0129578.
19. Saenza De San Pedro B et al. Eur All Allergy Clin Immunol. 2019 Nov 27. doi: 10.23822/EurAnnACI.1764-1489.124.
Olives and olive oil have long been known to confer salutary effects to the skin.1 Leaves and fruits of the olive plant (Olea europaea) have been used as external emollients to treat skin ulcers and inflammatory wounds.2 The phenolic compound oleuropein, the most abundant phenolic found in olive leaves and oil, has been shown to exhibit antioxidant and free radical–scavenging activities.3,4 Also present in the stems and flowers of the plant, oleuropein, an ester of elenolic acid and 3,4-dihydroxyphenyl ethanol and the primary glycoside in olives,5 is thought to be the major contributor to its antioxidant and antimelanogenesis activities.6 Notably, olive leaves, which contain a copious supply of oleuropein, are thought to exert significantly more antioxidant activity than olive fruit.7
Hydroxytyrosol is an ortho-diphenolic substance and essential constituent of oleuropein that has been shown in vitro to prevent apoptotic cell death caused by UVB in HaCaT cells.8,9 Both oleuropein and hydroxytyrosol impart various anticancer properties at the initiation, promotion, and metastasis stages and yield protection against multiple cancers, including skin tumors.10 The antioxidant activity of both compounds, which has been found to be more potent than that of vitamin E, is attributed to their phenolic content.11,12 In addition, oleuropein and lipophilic olive mill wastewater derivatives have been useful as active ingredients for stabilizing cosmetic formulations.13 This column revisits oleuropein after 10 years to focus on its dermatologic potential.
Protection against UV damage
A hairless mouse study by Kimura and Sumiyoshi in 2009 revealed that olive leaf extract and its primary constituent oleuropein exert a skin-protective effect against chronic UVB-induced skin damage and carcinogenesis, as well as tumor growth. This is likely caused by reducing cutaneous cyclooxygenase (COX)-2 levels, thus suppressing the expression of vascular endothelial growth factor (VEGF) and various matrix metalloproteinases, specifically MMP-2, MMP-9, and MMP-13.14
A year later, the same researchers examined the potential protective effects of olive leaf extract and oleuropein on acute damage induced by UVB exposure in C57BL/6J mice. Both oral extract (300 mg/kg or 1,000 mg/kg) and oral oleuropein (25mg/kg or 85 mg/kg) hindered skin thickness increases engendered by daily doses of UVB (120 mJ/cm2 for 5 days, then every other day for 9 days). Olive leaf extract and oleuropein also suppressed increases in Ki-67- and 8-hydroxy-2’-deoxyguanosine–positive cell numbers, melanin granule area, and MMP-13 expression, the investigators noted.15 Preinitiation with oleuropein also appears to have prevented skin tumor formation in a two-stage carcinogenesis model in mice, which the investigators ascribed to the antioxidant and antiapoptotic properties of the olive protein.16
The cosmetic characteristics of oleuropein against UVB-induced erythema in healthy volunteers were assessed by Perugini et al. in 2008. Using an emulsion and emulgel containing oleuropein and vitamin E as a reference compound, the investigators found that the botanical ingredient was responsible for decreases in erythema (22%), transepidermal water loss (35%), and blood flow (30%). They suggested that the use of oleuropein in cosmetic formulations warrants further investigation for its potential to help mitigate UV damage.3
Wound healing
Koca et al. assessed the wound healing activity of O. europaea leaf extracts using in vivo wound models and the reference ointment Madecassol (Bayer; Istanbul) for comparison, in 2011. The results showed that the aqueous extract exhibited wound healing properties, with secoiridoid oleuropein (4.6059%) found to be the primary active constituent.2
In a 2014 skin wound–healing investigation in aged male Balb/c mice, Mehraein et al. divided 24 mice, 16 months of age, into control and experimental groups. On days 3 and 7 after incision, collagen fiber deposition was significantly increased and reepithelialization more advanced in the oleuropein group (administered via an intradermal injection once a day), which also experienced decreased cell infiltration. The investigators concluded that oleuropein speeds cutaneous wound healing in mice and may have potential for clinical applications in human would healing from surgery.17
Later that year, the same team investigated the therapeutic effects of oleuropein on the wounded skin of young male Balb/c mice, finding similar results, with the phenolic compound again accelerating reepithelialization, improving collagen fiber synthesis, and augmenting blood flow to wound areas via up-regulating VEGF protein expression.4
Hair growth
In 2015, Tong et al. reported that topically applied oleuropein spurred the anagen hair growth phase in telogenic C57BL/6N mouse skin.18 An O. europaea subcutaneous immunotherapy has also demonstrated reductions in cutaneous reactivity, safety, and tolerability in patients with rhinoconjunctivitis.19
Conclusion
The benefits of consuming olives and olive oil are well established and continue to be studied. backed by many years of anecdotal reporting and use in traditional medicine. While the emerging data on the dermatologic uses of the olive phenolic constituent oleuropein are encouraging, much more information, particularly derived from randomized, controlled trials in humans, is necessary to establish the full potential of oleuropein for indications such as wound healing and protection against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Baumann LS, Weisberg EM. “Olive oil in botanical cosmeceuticals.” Olives and Olive Oil in Health and Disease Prevention. New York: Academic Press, 2010.
2. Koca U et al. J Med Food. 2011 Jan-Feb;14(1-2):140-6.
3. Perugini P et al. Int J Cosmet Sci. 2008 Apr;30(2):113-20.
4. Mehraein F et al. Wounds. 2014 Mar;26(3):83-8.
5. Imran M et al. J Food Sci. 2018 Jul;83(7):1781-91.
6. Kishikawa A et al. Phytother Res. 2015 Jun;29(6):877-86.
7. Zheng J et al. Zhongguo Zhong Yao Za Zhi. 2016 Feb;41(4):613-8.
8. Salucci S et al. J Dermatol Sci. 2015 Oct;80(1):61-8.
9. Jeon S, Choi M. Biomed Dermatol. 2018;2:21.
10. Imran M et al. J Food Sci. 2018 Jul;83(7):1781-91.
11. Visioli F et al. Biochem Biophys Res Commun. 1998 Jun 9;247(1):60-4.
12. Polišak N et al. Phytother Res. 2019 Oct 27. doi: 10.1002/ptr.6524.
13. Aissa I et al. Biotechnol Appl Biochem. 2017 Jul;64(4):579-89.
14. Kimura Y, Sumiyoshi M. J Nutr. 2009 Nov;139(11):2079-86.
15. Sumiyoshi M, Kimura Y. Phytother Res. 2010 Jul;24(7):995-1003.
16. John DNS et al. JKIMSU. 2019 Jan-Mar;8(1):43-51.
17. Mehraein F et al. Cell J. 2014 Feb 3;16(1):25-30.
18. Tong T et al. PLoS One. 2015 Jun 10;10(6):e0129578.
19. Saenza De San Pedro B et al. Eur All Allergy Clin Immunol. 2019 Nov 27. doi: 10.23822/EurAnnACI.1764-1489.124.
Olives and olive oil have long been known to confer salutary effects to the skin.1 Leaves and fruits of the olive plant (Olea europaea) have been used as external emollients to treat skin ulcers and inflammatory wounds.2 The phenolic compound oleuropein, the most abundant phenolic found in olive leaves and oil, has been shown to exhibit antioxidant and free radical–scavenging activities.3,4 Also present in the stems and flowers of the plant, oleuropein, an ester of elenolic acid and 3,4-dihydroxyphenyl ethanol and the primary glycoside in olives,5 is thought to be the major contributor to its antioxidant and antimelanogenesis activities.6 Notably, olive leaves, which contain a copious supply of oleuropein, are thought to exert significantly more antioxidant activity than olive fruit.7
Hydroxytyrosol is an ortho-diphenolic substance and essential constituent of oleuropein that has been shown in vitro to prevent apoptotic cell death caused by UVB in HaCaT cells.8,9 Both oleuropein and hydroxytyrosol impart various anticancer properties at the initiation, promotion, and metastasis stages and yield protection against multiple cancers, including skin tumors.10 The antioxidant activity of both compounds, which has been found to be more potent than that of vitamin E, is attributed to their phenolic content.11,12 In addition, oleuropein and lipophilic olive mill wastewater derivatives have been useful as active ingredients for stabilizing cosmetic formulations.13 This column revisits oleuropein after 10 years to focus on its dermatologic potential.
Protection against UV damage
A hairless mouse study by Kimura and Sumiyoshi in 2009 revealed that olive leaf extract and its primary constituent oleuropein exert a skin-protective effect against chronic UVB-induced skin damage and carcinogenesis, as well as tumor growth. This is likely caused by reducing cutaneous cyclooxygenase (COX)-2 levels, thus suppressing the expression of vascular endothelial growth factor (VEGF) and various matrix metalloproteinases, specifically MMP-2, MMP-9, and MMP-13.14
A year later, the same researchers examined the potential protective effects of olive leaf extract and oleuropein on acute damage induced by UVB exposure in C57BL/6J mice. Both oral extract (300 mg/kg or 1,000 mg/kg) and oral oleuropein (25mg/kg or 85 mg/kg) hindered skin thickness increases engendered by daily doses of UVB (120 mJ/cm2 for 5 days, then every other day for 9 days). Olive leaf extract and oleuropein also suppressed increases in Ki-67- and 8-hydroxy-2’-deoxyguanosine–positive cell numbers, melanin granule area, and MMP-13 expression, the investigators noted.15 Preinitiation with oleuropein also appears to have prevented skin tumor formation in a two-stage carcinogenesis model in mice, which the investigators ascribed to the antioxidant and antiapoptotic properties of the olive protein.16
The cosmetic characteristics of oleuropein against UVB-induced erythema in healthy volunteers were assessed by Perugini et al. in 2008. Using an emulsion and emulgel containing oleuropein and vitamin E as a reference compound, the investigators found that the botanical ingredient was responsible for decreases in erythema (22%), transepidermal water loss (35%), and blood flow (30%). They suggested that the use of oleuropein in cosmetic formulations warrants further investigation for its potential to help mitigate UV damage.3
Wound healing
Koca et al. assessed the wound healing activity of O. europaea leaf extracts using in vivo wound models and the reference ointment Madecassol (Bayer; Istanbul) for comparison, in 2011. The results showed that the aqueous extract exhibited wound healing properties, with secoiridoid oleuropein (4.6059%) found to be the primary active constituent.2
In a 2014 skin wound–healing investigation in aged male Balb/c mice, Mehraein et al. divided 24 mice, 16 months of age, into control and experimental groups. On days 3 and 7 after incision, collagen fiber deposition was significantly increased and reepithelialization more advanced in the oleuropein group (administered via an intradermal injection once a day), which also experienced decreased cell infiltration. The investigators concluded that oleuropein speeds cutaneous wound healing in mice and may have potential for clinical applications in human would healing from surgery.17
Later that year, the same team investigated the therapeutic effects of oleuropein on the wounded skin of young male Balb/c mice, finding similar results, with the phenolic compound again accelerating reepithelialization, improving collagen fiber synthesis, and augmenting blood flow to wound areas via up-regulating VEGF protein expression.4
Hair growth
In 2015, Tong et al. reported that topically applied oleuropein spurred the anagen hair growth phase in telogenic C57BL/6N mouse skin.18 An O. europaea subcutaneous immunotherapy has also demonstrated reductions in cutaneous reactivity, safety, and tolerability in patients with rhinoconjunctivitis.19
Conclusion
The benefits of consuming olives and olive oil are well established and continue to be studied. backed by many years of anecdotal reporting and use in traditional medicine. While the emerging data on the dermatologic uses of the olive phenolic constituent oleuropein are encouraging, much more information, particularly derived from randomized, controlled trials in humans, is necessary to establish the full potential of oleuropein for indications such as wound healing and protection against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Baumann LS, Weisberg EM. “Olive oil in botanical cosmeceuticals.” Olives and Olive Oil in Health and Disease Prevention. New York: Academic Press, 2010.
2. Koca U et al. J Med Food. 2011 Jan-Feb;14(1-2):140-6.
3. Perugini P et al. Int J Cosmet Sci. 2008 Apr;30(2):113-20.
4. Mehraein F et al. Wounds. 2014 Mar;26(3):83-8.
5. Imran M et al. J Food Sci. 2018 Jul;83(7):1781-91.
6. Kishikawa A et al. Phytother Res. 2015 Jun;29(6):877-86.
7. Zheng J et al. Zhongguo Zhong Yao Za Zhi. 2016 Feb;41(4):613-8.
8. Salucci S et al. J Dermatol Sci. 2015 Oct;80(1):61-8.
9. Jeon S, Choi M. Biomed Dermatol. 2018;2:21.
10. Imran M et al. J Food Sci. 2018 Jul;83(7):1781-91.
11. Visioli F et al. Biochem Biophys Res Commun. 1998 Jun 9;247(1):60-4.
12. Polišak N et al. Phytother Res. 2019 Oct 27. doi: 10.1002/ptr.6524.
13. Aissa I et al. Biotechnol Appl Biochem. 2017 Jul;64(4):579-89.
14. Kimura Y, Sumiyoshi M. J Nutr. 2009 Nov;139(11):2079-86.
15. Sumiyoshi M, Kimura Y. Phytother Res. 2010 Jul;24(7):995-1003.
16. John DNS et al. JKIMSU. 2019 Jan-Mar;8(1):43-51.
17. Mehraein F et al. Cell J. 2014 Feb 3;16(1):25-30.
18. Tong T et al. PLoS One. 2015 Jun 10;10(6):e0129578.
19. Saenza De San Pedro B et al. Eur All Allergy Clin Immunol. 2019 Nov 27. doi: 10.23822/EurAnnACI.1764-1489.124.
FDA provides flexibility to improve COVID-19 test availability
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
CDC expert answers top COVID-19 questions
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”
Trump to governors: Don’t wait for feds on medical supplies
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.
President declares national emergency for COVID-19, ramps up testing capability
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
Emeritus
“So what do you do all day?”
.
Asking what an emeritus does all day sounds fair, but the question is harder to answer than it sounds. Last year, I asked Dave, who was already retired. He took a day to think about it.
“Sometimes I sit on the back porch and watch the birds,” he said.
Dave has long been an avid bird-watcher. Along with golf and Facebook, watching birds is a pursuit that really engages many people, but I never understood it. I still don’t.
Over the years, I’ve met people whose experience of retirement has ranged from, “I’m so busy, I don’t know how I had time to work!” to, “I miss the gang and I’m bored,” to everything in between. Before I (semi-) retired, I made a plan to not make plans, at least at first: No new hobbies, cooking lessons, or anthropology courses. I figured I would figure it out.
So I am figuring it out. No rush. After a lifetime of rushing, not rushing is part of the point.
One hobby I cultivate is napping. I always get up early, no alarm needed. By late morning I am sometimes inclined to lie down for a bit. Taking a midmorning nap has always struck me as one of life’s great pleasures, though one I could rarely enjoy, unless you count dozing off standing up while a patient described an itch that started 17 years before, on a Thursday.
Now I can shut my eyes for half an hour and wake up refreshed, ready for the rest of the day.
During which I will do ...
An older friend of mine, now long gone, wrote a witty essay on being embarrassed to work at home. He refused to answer the phone during the day and hid from the postman. Contemplating retirement, I was afraid I would also feel that way, picturing myself a pitiful pensioner shuffling abroad at mid-day, looking for a park to poison pigeons in. That of course was before “working remotely” became a goal for cool young strivers. You see them around at all hours, with things sticking out of their ears, talking urgently to no one you can see.
Now I also walk the streets proudly at 11 a.m. or 2:45 p.m. I may get one of those earbuds that stick out at 45 degrees, so people can think my ear fungus has grown branches. Maybe they’ll imagine me a mastermind of an international CBD cartel. What they think doesn’t really matter.
One thing that I actually do all day is wonder why I spent so much of my career worrying about what other people think. Dr. Smith used to refer patients. No longer. Did I fail to meet her expectations? Mr. Trelawney came in weekly with itches and pains. No more. Did I roll my eyes too obviously?
Questions like these used to trouble me. Now I can’t recall why. Instead I worry about more important things, like who will play right field for the Red Sox this year.
Though I never signed up, I am an enrolled Baby Boomer, that navel-gazing cohort now passing from the scene while pretending it won’t. I never understood my generation when it was claiming to overturn the universe in the 1960s. Now its members write and read books with chirpy titles like “Amazing Aging!” as though – because we are so wonderfully special – age, infirmity, and decline will repeal themselves just for us.
Well, anyone can dream.
I go into the office a couple of half-days a week, when I’m in town. I like bantering with the gang and chatting with old patients. They wish me well and hope I’ll refer them to someone worthy when I hang them up for good, as many of their (and my) doctors already have.
Here is one thing I don’t do all day – manage human resource issues in the office. What’s to miss?
Now and then, with lessening frequency, I muse, “Well, if I do get bored, I can always spend more time in the office.”
Time for another nap.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semi-retired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
“So what do you do all day?”
.
Asking what an emeritus does all day sounds fair, but the question is harder to answer than it sounds. Last year, I asked Dave, who was already retired. He took a day to think about it.
“Sometimes I sit on the back porch and watch the birds,” he said.
Dave has long been an avid bird-watcher. Along with golf and Facebook, watching birds is a pursuit that really engages many people, but I never understood it. I still don’t.
Over the years, I’ve met people whose experience of retirement has ranged from, “I’m so busy, I don’t know how I had time to work!” to, “I miss the gang and I’m bored,” to everything in between. Before I (semi-) retired, I made a plan to not make plans, at least at first: No new hobbies, cooking lessons, or anthropology courses. I figured I would figure it out.
So I am figuring it out. No rush. After a lifetime of rushing, not rushing is part of the point.
One hobby I cultivate is napping. I always get up early, no alarm needed. By late morning I am sometimes inclined to lie down for a bit. Taking a midmorning nap has always struck me as one of life’s great pleasures, though one I could rarely enjoy, unless you count dozing off standing up while a patient described an itch that started 17 years before, on a Thursday.
Now I can shut my eyes for half an hour and wake up refreshed, ready for the rest of the day.
During which I will do ...
An older friend of mine, now long gone, wrote a witty essay on being embarrassed to work at home. He refused to answer the phone during the day and hid from the postman. Contemplating retirement, I was afraid I would also feel that way, picturing myself a pitiful pensioner shuffling abroad at mid-day, looking for a park to poison pigeons in. That of course was before “working remotely” became a goal for cool young strivers. You see them around at all hours, with things sticking out of their ears, talking urgently to no one you can see.
Now I also walk the streets proudly at 11 a.m. or 2:45 p.m. I may get one of those earbuds that stick out at 45 degrees, so people can think my ear fungus has grown branches. Maybe they’ll imagine me a mastermind of an international CBD cartel. What they think doesn’t really matter.
One thing that I actually do all day is wonder why I spent so much of my career worrying about what other people think. Dr. Smith used to refer patients. No longer. Did I fail to meet her expectations? Mr. Trelawney came in weekly with itches and pains. No more. Did I roll my eyes too obviously?
Questions like these used to trouble me. Now I can’t recall why. Instead I worry about more important things, like who will play right field for the Red Sox this year.
Though I never signed up, I am an enrolled Baby Boomer, that navel-gazing cohort now passing from the scene while pretending it won’t. I never understood my generation when it was claiming to overturn the universe in the 1960s. Now its members write and read books with chirpy titles like “Amazing Aging!” as though – because we are so wonderfully special – age, infirmity, and decline will repeal themselves just for us.
Well, anyone can dream.
I go into the office a couple of half-days a week, when I’m in town. I like bantering with the gang and chatting with old patients. They wish me well and hope I’ll refer them to someone worthy when I hang them up for good, as many of their (and my) doctors already have.
Here is one thing I don’t do all day – manage human resource issues in the office. What’s to miss?
Now and then, with lessening frequency, I muse, “Well, if I do get bored, I can always spend more time in the office.”
Time for another nap.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semi-retired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
“So what do you do all day?”
.
Asking what an emeritus does all day sounds fair, but the question is harder to answer than it sounds. Last year, I asked Dave, who was already retired. He took a day to think about it.
“Sometimes I sit on the back porch and watch the birds,” he said.
Dave has long been an avid bird-watcher. Along with golf and Facebook, watching birds is a pursuit that really engages many people, but I never understood it. I still don’t.
Over the years, I’ve met people whose experience of retirement has ranged from, “I’m so busy, I don’t know how I had time to work!” to, “I miss the gang and I’m bored,” to everything in between. Before I (semi-) retired, I made a plan to not make plans, at least at first: No new hobbies, cooking lessons, or anthropology courses. I figured I would figure it out.
So I am figuring it out. No rush. After a lifetime of rushing, not rushing is part of the point.
One hobby I cultivate is napping. I always get up early, no alarm needed. By late morning I am sometimes inclined to lie down for a bit. Taking a midmorning nap has always struck me as one of life’s great pleasures, though one I could rarely enjoy, unless you count dozing off standing up while a patient described an itch that started 17 years before, on a Thursday.
Now I can shut my eyes for half an hour and wake up refreshed, ready for the rest of the day.
During which I will do ...
An older friend of mine, now long gone, wrote a witty essay on being embarrassed to work at home. He refused to answer the phone during the day and hid from the postman. Contemplating retirement, I was afraid I would also feel that way, picturing myself a pitiful pensioner shuffling abroad at mid-day, looking for a park to poison pigeons in. That of course was before “working remotely” became a goal for cool young strivers. You see them around at all hours, with things sticking out of their ears, talking urgently to no one you can see.
Now I also walk the streets proudly at 11 a.m. or 2:45 p.m. I may get one of those earbuds that stick out at 45 degrees, so people can think my ear fungus has grown branches. Maybe they’ll imagine me a mastermind of an international CBD cartel. What they think doesn’t really matter.
One thing that I actually do all day is wonder why I spent so much of my career worrying about what other people think. Dr. Smith used to refer patients. No longer. Did I fail to meet her expectations? Mr. Trelawney came in weekly with itches and pains. No more. Did I roll my eyes too obviously?
Questions like these used to trouble me. Now I can’t recall why. Instead I worry about more important things, like who will play right field for the Red Sox this year.
Though I never signed up, I am an enrolled Baby Boomer, that navel-gazing cohort now passing from the scene while pretending it won’t. I never understood my generation when it was claiming to overturn the universe in the 1960s. Now its members write and read books with chirpy titles like “Amazing Aging!” as though – because we are so wonderfully special – age, infirmity, and decline will repeal themselves just for us.
Well, anyone can dream.
I go into the office a couple of half-days a week, when I’m in town. I like bantering with the gang and chatting with old patients. They wish me well and hope I’ll refer them to someone worthy when I hang them up for good, as many of their (and my) doctors already have.
Here is one thing I don’t do all day – manage human resource issues in the office. What’s to miss?
Now and then, with lessening frequency, I muse, “Well, if I do get bored, I can always spend more time in the office.”
Time for another nap.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semi-retired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Your medical conference is canceled. Now what?
Khadija Hafidh, MD, was already booked on a 14-hour, direct flight from Dubai to Los Angeles, when the American College of Physicians (ACP) announced it was canceling its internal medicine meeting scheduled for April.
Canceling her hotel reservation was not a problem, and she was assured a refund for the conference fee, but her airline ticket was another matter, said Dr. Hafidh, an internist and diabetologist with the Dubai Health Authority.
“The airline I booked my ticket with is willing to waive the change fees, but will deduct a cancellation fee if I choose not to take the trip,” Dr. Hafidh said in an interview. “The cancellation fees is $300. A bit steep I must admit.”
Dr. Hafidh now faces a dilemma: Lose the $300 and cancel, or change her flight dates to June for the American Diabetes Association meeting in Chicago.
“But then again, we aren’t sure if that meeting will take place,” Dr. Hafidh said. “A few weeks ago I thought this whole thing was just a storm in a tea cup. However when it was declared a pandemic yesterday, it brought about another dimension.”
More than 25 medical meetings and conferences across the globe have been canceled or postponed because of COVID-19 concerns. The sudden cancellations have caused reservation woes and travel headaches for thousands of physicians who planned to attend the meetings. Some societies are considering the idea of virtual conferences, while other associations have scrapped their meetings until next year.
For physicians facing a canceled conference, the most likely question is, what now? Read on for tips and suggestions.
Reservation refunds vary
Refunds on airfare because of conference cancellations differ, depending on the airline and where you were traveling. Some airlines, such as United Airlines, have waived all change fees for tickets issued March 3, 2020, through March 31, 2020, and passengers can change their dates for up to 12 months after the ticket was issued.
Full refunds often depend on whether your ticket was nonrefundable when purchased. Many airlines, such as Delta, are providing full refunds if the airline canceled your flight. JetBlue is waiving all change and cancellation fees for customers scheduled to travel March 10, 2020, through April 30, 2020.
Las Vegas–based dermatologist H.L. Greenberg, MD, was satisfied with the credit he received from Southwest Airlines after the American Academy of Dermatology (AAD) canceled its Denver meeting. He and his staff were looking forward to the gathering, but he noted that the meeting would likely have been limited, even if it had take place as scheduled.
“I am disappointed that I won’t be able to meet with colleagues and industry to explore what the latest advances and interests are in dermatology,” he said. “Because many academic institutions were forbidding their faculty from traveling, the content of the meeting was going to be severely diminished. It’s just a rough time for everyone.”
Meanwhile, Asa Radix, MD, PhD, a New York–based internist, received a full refund for his Amtrak ticket to Boston when the Conference on Retroviruses and Opportunistic Infections (CROI) scheduled for early March was converted to a virtual meeting. Dr. Radix, senior director of research and education at the Callen-Lorde Community Health Center in New York, left another meeting in Brazil early to get to the Boston conference, he said.
“I was packed, but really that was a minor inconvenience,” he said in an interview. “I appreciate that they prioritized health concerns and changed to a virtual meeting. I received full refunds, no issues whatsoever. [It was] really great since I had no travel insurance.”
Check with your individual airline or train line for information about ticket refunds and credits. Many airlines are currently making special accommodations because of COVID-19. If your flight was covered by trip insurance, also called travel assistance, you are generally protected against unforeseen financial losses such as cancellations. The U.S. Department of Transportation provides this general online resource about airline refunds.
Hotel refunds probable
Most meeting organizations who have made the decision to cancel or postpone a conference also have canceled block hotel reservations reserved for the meeting. Medical associations are not directly refunding the hotel costs, but the majority of hotels are refunding reservations with no questions asked. Physicians interviewed for this story all reported no trouble getting refunds for their hotel reservations. However, attendees who did not book a hotel in official housing blocks should contact the hotel directly to cancel.
What about registration fees?
In response to COVID-19 cancellations, most conference leaders are refunding registration fees in full for both attendees and exhibitors. The refund may not be automatic, some associations such as ACP and the American College of Obstetricians and Gynecologists state it may take up to 45 days for the funds to be credited, depending on the payment used.
If the conference you planned to attend was postponed, the registration fee may be assigned to the new meeting dates and the money may not be refunded. Registration fees for the Minimally Invasive Surgery Symposium, for example, delayed until an unconfirmed date, and for the European Association of Urology (EAU) meeting, postponed until July, will be automatically credited to the rescheduled meeting, according to the websites. If attendees cannot attend the rescheduled EAU meeting, the association will not provide a refund and the registration will not apply to the 2021 meeting, according to its website. However, the group is providing registrants with a free access code for the EAU20 Resource Centre, which contains websites of sessions and scientific content.
A number of physicians have expressed disappointment with the EAU’s postponement on social media. On Twitter, some doctors wrote that the rescheduled dates were bad timing, while others lamented the refund refusal.
The EAU said it regrets that some delegates will experience financial losses, but that the organization has already experienced a significant outlay that cannot be recovered including venue, logistics, travel, and accommodation costs.
"We are doing what we can to absorb costs, but we need to be realistic about what is affordable; should the organization have to refund all or even most registrations, it would significantly jeopardize the viability of the organization," the EAU said in a statement. "These are difficult times, not only for the EAU, but on a global scale. Where there are specific cases of hardship or very extenuating financial circumstances, we will be willing to review individual cases. So far, we believe that we have done what we can do to meet the conflicting demands presented by the postponement of the congress, but this is a situation which changes from day to day, and we need to continuously evaluate what might be the best course of action." *
Contact your medical association directly for details on postponements.
What if I’m a presenter?
In an attempt to save the hard work and time that planners and presenters have invested into now-canceled meetings, some conferences are moving to a digital format. The Conference on Retroviruses and Opportunistic Infections (CROI) was the first to convert its in-person conference to a virtual meeting, held from March 8 to 11, 2020. At-home attendees logged onto CROI’s digital platform to hear plenaries, oral abstracts, themed discussion sessions, and symposia.
Dr. Radix was one of many CROI speakers who changed his presentation on HIV prevalence among transgender men to a virtual format.
“We were provided with detailed instructions from CROI about how to do this,” said Dr. Radix, who tweeted about the experience. “For my presentation, I used the video option in PowerPoint; it seemed the most straightforward and didn’t require buying additional software. It was fairly easy to follow the instructions to create the video but it was disappointing to present to an empty room.”
Matthew Spinelli, MD, an HIV researcher with the University of California, San Francisco, who also presented virtually, said it was remarkable that CROI leaders were able to put together the virtual program in such a short time. He delivered his presentation on the accuracy of a real-time urine tenofovir test using PowerPoint and a podcast microphone.
“It seemed to work pretty well,” he said in an interview. “It’s not the same as being there in person, there’s a lot of networking and chance conversations that happen when you’re all in the same place, but it was the right decision to cancel. If I have to be at home or at work doing social distancing, this was the best possible way of doing it.”
Following in CROI’s footsteps, the National Kidney Foundation’s spring conference has moved to a live virtual conference. The 2020 Healthcare Information and Management Systems Society (HIMSS) global health conference also will move to a digital format. Other societies are considering similar virtual options. Check with your meeting website for more details on digital options and attendee access.
*The article was updated on 03/16/2020.
Khadija Hafidh, MD, was already booked on a 14-hour, direct flight from Dubai to Los Angeles, when the American College of Physicians (ACP) announced it was canceling its internal medicine meeting scheduled for April.
Canceling her hotel reservation was not a problem, and she was assured a refund for the conference fee, but her airline ticket was another matter, said Dr. Hafidh, an internist and diabetologist with the Dubai Health Authority.
“The airline I booked my ticket with is willing to waive the change fees, but will deduct a cancellation fee if I choose not to take the trip,” Dr. Hafidh said in an interview. “The cancellation fees is $300. A bit steep I must admit.”
Dr. Hafidh now faces a dilemma: Lose the $300 and cancel, or change her flight dates to June for the American Diabetes Association meeting in Chicago.
“But then again, we aren’t sure if that meeting will take place,” Dr. Hafidh said. “A few weeks ago I thought this whole thing was just a storm in a tea cup. However when it was declared a pandemic yesterday, it brought about another dimension.”
More than 25 medical meetings and conferences across the globe have been canceled or postponed because of COVID-19 concerns. The sudden cancellations have caused reservation woes and travel headaches for thousands of physicians who planned to attend the meetings. Some societies are considering the idea of virtual conferences, while other associations have scrapped their meetings until next year.
For physicians facing a canceled conference, the most likely question is, what now? Read on for tips and suggestions.
Reservation refunds vary
Refunds on airfare because of conference cancellations differ, depending on the airline and where you were traveling. Some airlines, such as United Airlines, have waived all change fees for tickets issued March 3, 2020, through March 31, 2020, and passengers can change their dates for up to 12 months after the ticket was issued.
Full refunds often depend on whether your ticket was nonrefundable when purchased. Many airlines, such as Delta, are providing full refunds if the airline canceled your flight. JetBlue is waiving all change and cancellation fees for customers scheduled to travel March 10, 2020, through April 30, 2020.
Las Vegas–based dermatologist H.L. Greenberg, MD, was satisfied with the credit he received from Southwest Airlines after the American Academy of Dermatology (AAD) canceled its Denver meeting. He and his staff were looking forward to the gathering, but he noted that the meeting would likely have been limited, even if it had take place as scheduled.
“I am disappointed that I won’t be able to meet with colleagues and industry to explore what the latest advances and interests are in dermatology,” he said. “Because many academic institutions were forbidding their faculty from traveling, the content of the meeting was going to be severely diminished. It’s just a rough time for everyone.”
Meanwhile, Asa Radix, MD, PhD, a New York–based internist, received a full refund for his Amtrak ticket to Boston when the Conference on Retroviruses and Opportunistic Infections (CROI) scheduled for early March was converted to a virtual meeting. Dr. Radix, senior director of research and education at the Callen-Lorde Community Health Center in New York, left another meeting in Brazil early to get to the Boston conference, he said.
“I was packed, but really that was a minor inconvenience,” he said in an interview. “I appreciate that they prioritized health concerns and changed to a virtual meeting. I received full refunds, no issues whatsoever. [It was] really great since I had no travel insurance.”
Check with your individual airline or train line for information about ticket refunds and credits. Many airlines are currently making special accommodations because of COVID-19. If your flight was covered by trip insurance, also called travel assistance, you are generally protected against unforeseen financial losses such as cancellations. The U.S. Department of Transportation provides this general online resource about airline refunds.
Hotel refunds probable
Most meeting organizations who have made the decision to cancel or postpone a conference also have canceled block hotel reservations reserved for the meeting. Medical associations are not directly refunding the hotel costs, but the majority of hotels are refunding reservations with no questions asked. Physicians interviewed for this story all reported no trouble getting refunds for their hotel reservations. However, attendees who did not book a hotel in official housing blocks should contact the hotel directly to cancel.
What about registration fees?
In response to COVID-19 cancellations, most conference leaders are refunding registration fees in full for both attendees and exhibitors. The refund may not be automatic, some associations such as ACP and the American College of Obstetricians and Gynecologists state it may take up to 45 days for the funds to be credited, depending on the payment used.
If the conference you planned to attend was postponed, the registration fee may be assigned to the new meeting dates and the money may not be refunded. Registration fees for the Minimally Invasive Surgery Symposium, for example, delayed until an unconfirmed date, and for the European Association of Urology (EAU) meeting, postponed until July, will be automatically credited to the rescheduled meeting, according to the websites. If attendees cannot attend the rescheduled EAU meeting, the association will not provide a refund and the registration will not apply to the 2021 meeting, according to its website. However, the group is providing registrants with a free access code for the EAU20 Resource Centre, which contains websites of sessions and scientific content.
A number of physicians have expressed disappointment with the EAU’s postponement on social media. On Twitter, some doctors wrote that the rescheduled dates were bad timing, while others lamented the refund refusal.
The EAU said it regrets that some delegates will experience financial losses, but that the organization has already experienced a significant outlay that cannot be recovered including venue, logistics, travel, and accommodation costs.
"We are doing what we can to absorb costs, but we need to be realistic about what is affordable; should the organization have to refund all or even most registrations, it would significantly jeopardize the viability of the organization," the EAU said in a statement. "These are difficult times, not only for the EAU, but on a global scale. Where there are specific cases of hardship or very extenuating financial circumstances, we will be willing to review individual cases. So far, we believe that we have done what we can do to meet the conflicting demands presented by the postponement of the congress, but this is a situation which changes from day to day, and we need to continuously evaluate what might be the best course of action." *
Contact your medical association directly for details on postponements.
What if I’m a presenter?
In an attempt to save the hard work and time that planners and presenters have invested into now-canceled meetings, some conferences are moving to a digital format. The Conference on Retroviruses and Opportunistic Infections (CROI) was the first to convert its in-person conference to a virtual meeting, held from March 8 to 11, 2020. At-home attendees logged onto CROI’s digital platform to hear plenaries, oral abstracts, themed discussion sessions, and symposia.
Dr. Radix was one of many CROI speakers who changed his presentation on HIV prevalence among transgender men to a virtual format.
“We were provided with detailed instructions from CROI about how to do this,” said Dr. Radix, who tweeted about the experience. “For my presentation, I used the video option in PowerPoint; it seemed the most straightforward and didn’t require buying additional software. It was fairly easy to follow the instructions to create the video but it was disappointing to present to an empty room.”
Matthew Spinelli, MD, an HIV researcher with the University of California, San Francisco, who also presented virtually, said it was remarkable that CROI leaders were able to put together the virtual program in such a short time. He delivered his presentation on the accuracy of a real-time urine tenofovir test using PowerPoint and a podcast microphone.
“It seemed to work pretty well,” he said in an interview. “It’s not the same as being there in person, there’s a lot of networking and chance conversations that happen when you’re all in the same place, but it was the right decision to cancel. If I have to be at home or at work doing social distancing, this was the best possible way of doing it.”
Following in CROI’s footsteps, the National Kidney Foundation’s spring conference has moved to a live virtual conference. The 2020 Healthcare Information and Management Systems Society (HIMSS) global health conference also will move to a digital format. Other societies are considering similar virtual options. Check with your meeting website for more details on digital options and attendee access.
*The article was updated on 03/16/2020.
Khadija Hafidh, MD, was already booked on a 14-hour, direct flight from Dubai to Los Angeles, when the American College of Physicians (ACP) announced it was canceling its internal medicine meeting scheduled for April.
Canceling her hotel reservation was not a problem, and she was assured a refund for the conference fee, but her airline ticket was another matter, said Dr. Hafidh, an internist and diabetologist with the Dubai Health Authority.
“The airline I booked my ticket with is willing to waive the change fees, but will deduct a cancellation fee if I choose not to take the trip,” Dr. Hafidh said in an interview. “The cancellation fees is $300. A bit steep I must admit.”
Dr. Hafidh now faces a dilemma: Lose the $300 and cancel, or change her flight dates to June for the American Diabetes Association meeting in Chicago.
“But then again, we aren’t sure if that meeting will take place,” Dr. Hafidh said. “A few weeks ago I thought this whole thing was just a storm in a tea cup. However when it was declared a pandemic yesterday, it brought about another dimension.”
More than 25 medical meetings and conferences across the globe have been canceled or postponed because of COVID-19 concerns. The sudden cancellations have caused reservation woes and travel headaches for thousands of physicians who planned to attend the meetings. Some societies are considering the idea of virtual conferences, while other associations have scrapped their meetings until next year.
For physicians facing a canceled conference, the most likely question is, what now? Read on for tips and suggestions.
Reservation refunds vary
Refunds on airfare because of conference cancellations differ, depending on the airline and where you were traveling. Some airlines, such as United Airlines, have waived all change fees for tickets issued March 3, 2020, through March 31, 2020, and passengers can change their dates for up to 12 months after the ticket was issued.
Full refunds often depend on whether your ticket was nonrefundable when purchased. Many airlines, such as Delta, are providing full refunds if the airline canceled your flight. JetBlue is waiving all change and cancellation fees for customers scheduled to travel March 10, 2020, through April 30, 2020.
Las Vegas–based dermatologist H.L. Greenberg, MD, was satisfied with the credit he received from Southwest Airlines after the American Academy of Dermatology (AAD) canceled its Denver meeting. He and his staff were looking forward to the gathering, but he noted that the meeting would likely have been limited, even if it had take place as scheduled.
“I am disappointed that I won’t be able to meet with colleagues and industry to explore what the latest advances and interests are in dermatology,” he said. “Because many academic institutions were forbidding their faculty from traveling, the content of the meeting was going to be severely diminished. It’s just a rough time for everyone.”
Meanwhile, Asa Radix, MD, PhD, a New York–based internist, received a full refund for his Amtrak ticket to Boston when the Conference on Retroviruses and Opportunistic Infections (CROI) scheduled for early March was converted to a virtual meeting. Dr. Radix, senior director of research and education at the Callen-Lorde Community Health Center in New York, left another meeting in Brazil early to get to the Boston conference, he said.
“I was packed, but really that was a minor inconvenience,” he said in an interview. “I appreciate that they prioritized health concerns and changed to a virtual meeting. I received full refunds, no issues whatsoever. [It was] really great since I had no travel insurance.”
Check with your individual airline or train line for information about ticket refunds and credits. Many airlines are currently making special accommodations because of COVID-19. If your flight was covered by trip insurance, also called travel assistance, you are generally protected against unforeseen financial losses such as cancellations. The U.S. Department of Transportation provides this general online resource about airline refunds.
Hotel refunds probable
Most meeting organizations who have made the decision to cancel or postpone a conference also have canceled block hotel reservations reserved for the meeting. Medical associations are not directly refunding the hotel costs, but the majority of hotels are refunding reservations with no questions asked. Physicians interviewed for this story all reported no trouble getting refunds for their hotel reservations. However, attendees who did not book a hotel in official housing blocks should contact the hotel directly to cancel.
What about registration fees?
In response to COVID-19 cancellations, most conference leaders are refunding registration fees in full for both attendees and exhibitors. The refund may not be automatic, some associations such as ACP and the American College of Obstetricians and Gynecologists state it may take up to 45 days for the funds to be credited, depending on the payment used.
If the conference you planned to attend was postponed, the registration fee may be assigned to the new meeting dates and the money may not be refunded. Registration fees for the Minimally Invasive Surgery Symposium, for example, delayed until an unconfirmed date, and for the European Association of Urology (EAU) meeting, postponed until July, will be automatically credited to the rescheduled meeting, according to the websites. If attendees cannot attend the rescheduled EAU meeting, the association will not provide a refund and the registration will not apply to the 2021 meeting, according to its website. However, the group is providing registrants with a free access code for the EAU20 Resource Centre, which contains websites of sessions and scientific content.
A number of physicians have expressed disappointment with the EAU’s postponement on social media. On Twitter, some doctors wrote that the rescheduled dates were bad timing, while others lamented the refund refusal.
The EAU said it regrets that some delegates will experience financial losses, but that the organization has already experienced a significant outlay that cannot be recovered including venue, logistics, travel, and accommodation costs.
"We are doing what we can to absorb costs, but we need to be realistic about what is affordable; should the organization have to refund all or even most registrations, it would significantly jeopardize the viability of the organization," the EAU said in a statement. "These are difficult times, not only for the EAU, but on a global scale. Where there are specific cases of hardship or very extenuating financial circumstances, we will be willing to review individual cases. So far, we believe that we have done what we can do to meet the conflicting demands presented by the postponement of the congress, but this is a situation which changes from day to day, and we need to continuously evaluate what might be the best course of action." *
Contact your medical association directly for details on postponements.
What if I’m a presenter?
In an attempt to save the hard work and time that planners and presenters have invested into now-canceled meetings, some conferences are moving to a digital format. The Conference on Retroviruses and Opportunistic Infections (CROI) was the first to convert its in-person conference to a virtual meeting, held from March 8 to 11, 2020. At-home attendees logged onto CROI’s digital platform to hear plenaries, oral abstracts, themed discussion sessions, and symposia.
Dr. Radix was one of many CROI speakers who changed his presentation on HIV prevalence among transgender men to a virtual format.
“We were provided with detailed instructions from CROI about how to do this,” said Dr. Radix, who tweeted about the experience. “For my presentation, I used the video option in PowerPoint; it seemed the most straightforward and didn’t require buying additional software. It was fairly easy to follow the instructions to create the video but it was disappointing to present to an empty room.”
Matthew Spinelli, MD, an HIV researcher with the University of California, San Francisco, who also presented virtually, said it was remarkable that CROI leaders were able to put together the virtual program in such a short time. He delivered his presentation on the accuracy of a real-time urine tenofovir test using PowerPoint and a podcast microphone.
“It seemed to work pretty well,” he said in an interview. “It’s not the same as being there in person, there’s a lot of networking and chance conversations that happen when you’re all in the same place, but it was the right decision to cancel. If I have to be at home or at work doing social distancing, this was the best possible way of doing it.”
Following in CROI’s footsteps, the National Kidney Foundation’s spring conference has moved to a live virtual conference. The 2020 Healthcare Information and Management Systems Society (HIMSS) global health conference also will move to a digital format. Other societies are considering similar virtual options. Check with your meeting website for more details on digital options and attendee access.
*The article was updated on 03/16/2020.