User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
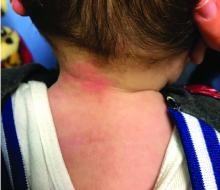
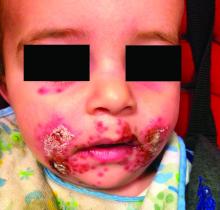
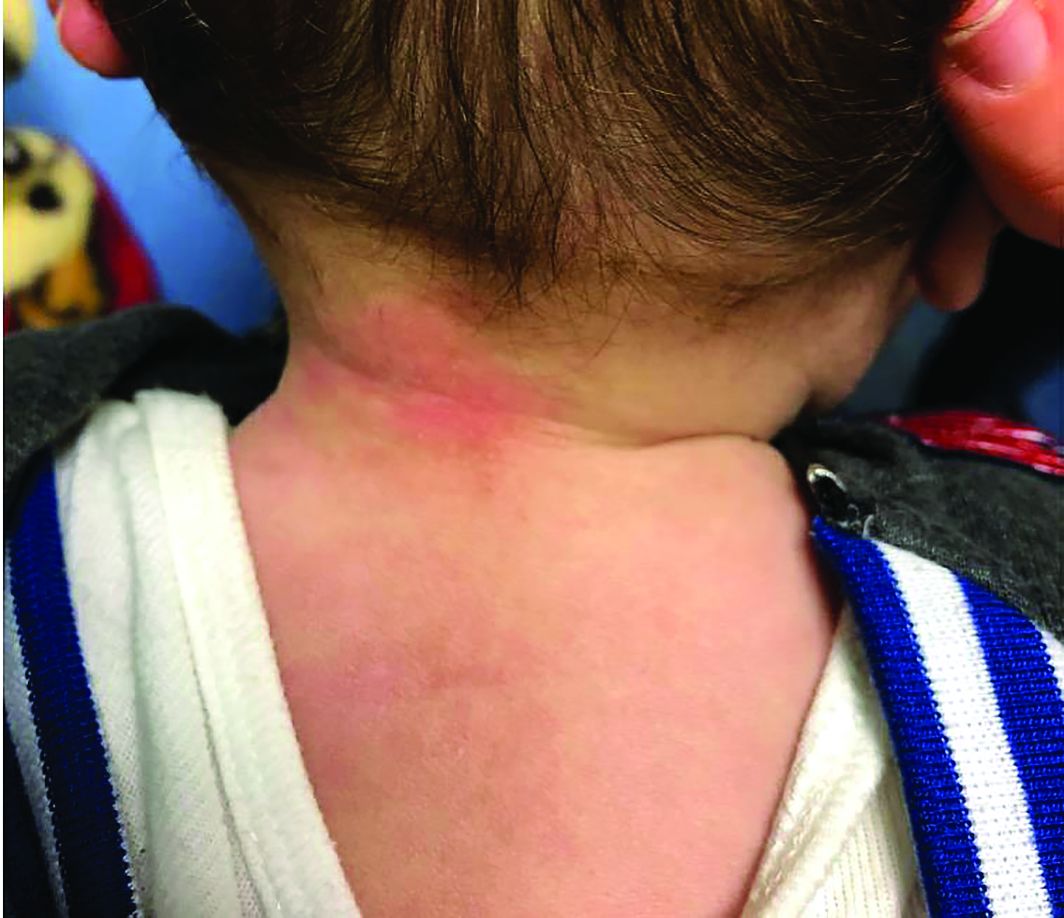
A 7-month-old male presents with perioral rash and fever
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at [email protected].
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at [email protected].
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at [email protected].
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Medical identity theft
In his book, “Scam Me If You Can,” fraud expert Frank Abagnale relates the case of a 5-year-old boy whose pediatrician’s computer was hacked, compromising his name, birth date, Social Security number, insurance information, and medical records. The result was a bureaucratic nightmare that may well continue for the rest of that unfortunate young patient’s life. One can only speculate on the difficulties he might have as adult in obtaining a line of credit, or in proving his medical identity to physicians and hospitals.
– your Social Security number, bank account numbers, etc. – sells for about $25 on the black market; add health insurance and medical records, and the price can jump to $1,000 or more. That’s because there is a far greater potential yield from medical identity theft – and once your personal information and medical records are breached, they are in the Cloud for the rest of your life, available to anyone who wants to buy them. Older patients are particularly vulnerable: Medicare billing scams cost taxpayers more than $60 billion a year.
If your office’s computer system does not have effective fraud protection, you could be held liable for any fraud committed with information stolen from it – and if the information is resold years later and reused to commit more fraud, you’ll be liable for that, too. That’s why I strongly recommend that you invest in high-quality security technology and software, so that in the event of a breach, the security company will at least share in the fault and the liability. (As always, I have no financial interest in any product or industry mentioned in this column.)
Even with adequate protection, breaches can still occur, so all medical offices should have a breach response plan in place, covering how to halt security breaches, and how to handle any lost or stolen data. Your computer and security vendors can help with formulating such a plan. Patients affected by a breach need to be contacted as well, so they may put a freeze on accounts or send out fraud alerts.
Patients also need to be aware of the risks. If your EHR includes an online portal to communicate protected information to patients, it may be secure on your end, but patients are unlikely to have similar protection on their home computers. If you offer online patient portal services, you should make your patients aware of measures they can take to protect their data once it arrives on their computers or phones.
Patients should also be warned of the risks that come with sharing medical information with others. If they are asked to reveal medical data via phone or email, they need to ask who is requesting it, and why. Any unsolicited calls inquiring about their medical information, from someone who can’t or won’t confirm their identity, should be considered extremely suspicious.
We tell our patients to protect their insurance numbers as carefully as they guard their Social Security number and other valuable data, and to shred any medical paperwork they no longer need, including labels on prescription bottles. And if they see something on an Explanation of Benefits that doesn’t look right, they should question it immediately. We encourage them to take advantage of the free services at MyMedicare.gov, including Medicare Summary Notices provided every 3 months (if any services or medical supplies are received during that period), to make sure they’re being billed only for services they have received.
Your staff should be made aware of the potential for “friendly fraud,” which is defined as theft of identity and medical information by patients’ friends or family members. (According to some studies, as much as 50% of all medical identity theft may be committed this way.) Staffers should never divulge insurance numbers, diagnoses, lab reports, or any other privileged information to family or friends, whether by phone, fax, mail, or in person, without written permission from the patient. And when callers claiming to be patients request information about themselves, your employees should be alert for “red flags.” For example, legitimate patients won’t stumble over simple questions (such as “What is your birth date?”) or request test results or diagnoses that they should already know about.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In his book, “Scam Me If You Can,” fraud expert Frank Abagnale relates the case of a 5-year-old boy whose pediatrician’s computer was hacked, compromising his name, birth date, Social Security number, insurance information, and medical records. The result was a bureaucratic nightmare that may well continue for the rest of that unfortunate young patient’s life. One can only speculate on the difficulties he might have as adult in obtaining a line of credit, or in proving his medical identity to physicians and hospitals.
– your Social Security number, bank account numbers, etc. – sells for about $25 on the black market; add health insurance and medical records, and the price can jump to $1,000 or more. That’s because there is a far greater potential yield from medical identity theft – and once your personal information and medical records are breached, they are in the Cloud for the rest of your life, available to anyone who wants to buy them. Older patients are particularly vulnerable: Medicare billing scams cost taxpayers more than $60 billion a year.
If your office’s computer system does not have effective fraud protection, you could be held liable for any fraud committed with information stolen from it – and if the information is resold years later and reused to commit more fraud, you’ll be liable for that, too. That’s why I strongly recommend that you invest in high-quality security technology and software, so that in the event of a breach, the security company will at least share in the fault and the liability. (As always, I have no financial interest in any product or industry mentioned in this column.)
Even with adequate protection, breaches can still occur, so all medical offices should have a breach response plan in place, covering how to halt security breaches, and how to handle any lost or stolen data. Your computer and security vendors can help with formulating such a plan. Patients affected by a breach need to be contacted as well, so they may put a freeze on accounts or send out fraud alerts.
Patients also need to be aware of the risks. If your EHR includes an online portal to communicate protected information to patients, it may be secure on your end, but patients are unlikely to have similar protection on their home computers. If you offer online patient portal services, you should make your patients aware of measures they can take to protect their data once it arrives on their computers or phones.
Patients should also be warned of the risks that come with sharing medical information with others. If they are asked to reveal medical data via phone or email, they need to ask who is requesting it, and why. Any unsolicited calls inquiring about their medical information, from someone who can’t or won’t confirm their identity, should be considered extremely suspicious.
We tell our patients to protect their insurance numbers as carefully as they guard their Social Security number and other valuable data, and to shred any medical paperwork they no longer need, including labels on prescription bottles. And if they see something on an Explanation of Benefits that doesn’t look right, they should question it immediately. We encourage them to take advantage of the free services at MyMedicare.gov, including Medicare Summary Notices provided every 3 months (if any services or medical supplies are received during that period), to make sure they’re being billed only for services they have received.
Your staff should be made aware of the potential for “friendly fraud,” which is defined as theft of identity and medical information by patients’ friends or family members. (According to some studies, as much as 50% of all medical identity theft may be committed this way.) Staffers should never divulge insurance numbers, diagnoses, lab reports, or any other privileged information to family or friends, whether by phone, fax, mail, or in person, without written permission from the patient. And when callers claiming to be patients request information about themselves, your employees should be alert for “red flags.” For example, legitimate patients won’t stumble over simple questions (such as “What is your birth date?”) or request test results or diagnoses that they should already know about.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In his book, “Scam Me If You Can,” fraud expert Frank Abagnale relates the case of a 5-year-old boy whose pediatrician’s computer was hacked, compromising his name, birth date, Social Security number, insurance information, and medical records. The result was a bureaucratic nightmare that may well continue for the rest of that unfortunate young patient’s life. One can only speculate on the difficulties he might have as adult in obtaining a line of credit, or in proving his medical identity to physicians and hospitals.
– your Social Security number, bank account numbers, etc. – sells for about $25 on the black market; add health insurance and medical records, and the price can jump to $1,000 or more. That’s because there is a far greater potential yield from medical identity theft – and once your personal information and medical records are breached, they are in the Cloud for the rest of your life, available to anyone who wants to buy them. Older patients are particularly vulnerable: Medicare billing scams cost taxpayers more than $60 billion a year.
If your office’s computer system does not have effective fraud protection, you could be held liable for any fraud committed with information stolen from it – and if the information is resold years later and reused to commit more fraud, you’ll be liable for that, too. That’s why I strongly recommend that you invest in high-quality security technology and software, so that in the event of a breach, the security company will at least share in the fault and the liability. (As always, I have no financial interest in any product or industry mentioned in this column.)
Even with adequate protection, breaches can still occur, so all medical offices should have a breach response plan in place, covering how to halt security breaches, and how to handle any lost or stolen data. Your computer and security vendors can help with formulating such a plan. Patients affected by a breach need to be contacted as well, so they may put a freeze on accounts or send out fraud alerts.
Patients also need to be aware of the risks. If your EHR includes an online portal to communicate protected information to patients, it may be secure on your end, but patients are unlikely to have similar protection on their home computers. If you offer online patient portal services, you should make your patients aware of measures they can take to protect their data once it arrives on their computers or phones.
Patients should also be warned of the risks that come with sharing medical information with others. If they are asked to reveal medical data via phone or email, they need to ask who is requesting it, and why. Any unsolicited calls inquiring about their medical information, from someone who can’t or won’t confirm their identity, should be considered extremely suspicious.
We tell our patients to protect their insurance numbers as carefully as they guard their Social Security number and other valuable data, and to shred any medical paperwork they no longer need, including labels on prescription bottles. And if they see something on an Explanation of Benefits that doesn’t look right, they should question it immediately. We encourage them to take advantage of the free services at MyMedicare.gov, including Medicare Summary Notices provided every 3 months (if any services or medical supplies are received during that period), to make sure they’re being billed only for services they have received.
Your staff should be made aware of the potential for “friendly fraud,” which is defined as theft of identity and medical information by patients’ friends or family members. (According to some studies, as much as 50% of all medical identity theft may be committed this way.) Staffers should never divulge insurance numbers, diagnoses, lab reports, or any other privileged information to family or friends, whether by phone, fax, mail, or in person, without written permission from the patient. And when callers claiming to be patients request information about themselves, your employees should be alert for “red flags.” For example, legitimate patients won’t stumble over simple questions (such as “What is your birth date?”) or request test results or diagnoses that they should already know about.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Coronavirus in dermatology: What steps to take
The novel coronavirus (2019-nCoV) is presenting a severe challenge to global health care, but its impact isn’t just felt in the emergency department. Specialists, including dermatologists, must also navigate the presence of the virus and its impact on patients and practices.
A new report from dermatologists in China’s Wuhan province, where the 2019-nCoV outbreak began, outlines initial experiences and provides a blueprint for triaging potential cases before they reach the dermatology clinic. Despite its presence in the epicenter of the outbreak, the hospital has not detected any 2019-nCoV-infected patients in any of its departments.
The commentary appeared in the British Journal of Dermatology and was authored by a group led by Juan Tao of Huazhong University of Science and Technology (Br J Dermatol. 2020 Mar 5. doi: 10.1111/bjd.19011).
The hospital triages all patients at the hospital entrance. Those who are suspected of having 2019-nCoV infection are sent to a designated department. Those with a skin condition who are not suspected of being infected are allowed to go to a dermatology triage center, where they are examined again. If the second examination raises suspicion, they are sent to the designated 2019-nCoV department. If no infection is suspected, or a patient from the 2019-nCoV department is cleared, they are allowed access to the dermatology clinic.
The team also suggested that skin lesions associated with dermatological conditions could lead to increased risk of 2019-nCoV infection. Contacted by email, Dr. Tao outlined a theoretical risk that the virus could lead to infection through contact with subcutaneous tissues, mucosal surfaces, or blood vessels. He did not respond to a request for evidence that such a route of transmission had occurred.
However, Adam Friedman, MD, professor of dermatology at George Washington University, Washington, said he doubted any such transmission would occur since the virus does not infect keratinocytes, and expressed concern that the suggestion could add to the stigma experienced by dermatological patients, whose noticeable rashes can sometimes lead to social avoidance. “I don’t want to add to that,” said Dr. Friedman in an interview.
A critical aspect of dermatology is the immunosuppressive agents often used in dermatology patients. Such drugs could make them more susceptible to infections, or to worse outcomes in the event of disease. Dr. Friedman recounted sending a letter to one patient on an immunosuppressive medication, suggesting that she work remotely. “I think that’s something we have to think about in at-risk individuals. I know there’s such a focus on the elderly, but there’s a large population of individuals on medications that lower their immune system who are going to be at risk for more severe infections,” said Dr. Friedman.
To reduce patient exposure, the commentary recommended that dermatologists perform online consultation for mild and nonemergency cases.
The authors also covered hospitalized patients with primary or secondary skin conditions. A dermatologist is on site at the dermatology triage station to conduct in-depth assessments if needed. If a patient has a fever that is believed to be caused by a dermatologic condition, the on-site dermatologist assists in the consult.
Because some patients may only become symptomatic after admission to a ward, the authors recommend hospitals have a COVID-19 trained contingency group on hand to prevent and control outbreaks within the institution. The team should be in communication with respiratory intensive care and radiology departments to exclude 2019-nCoV when cases develop in-hospital, and to ensure proper care infected patients who require it.
When a hospitalized 2019-nCoV-infected patient has a skin condition requiring treatment, the authors recommend that pictures be sent to the dermatologist for evaluation, along with teleconferences to further assess the patient. If necessary, the dermatologist should go to the patient’s bedside, with as much information as possible related in advance in order to minimize bedside exposure.
There was no funding source. Dr. Tao and Dr. Friedman have no relevant financial conflicts.
The novel coronavirus (2019-nCoV) is presenting a severe challenge to global health care, but its impact isn’t just felt in the emergency department. Specialists, including dermatologists, must also navigate the presence of the virus and its impact on patients and practices.
A new report from dermatologists in China’s Wuhan province, where the 2019-nCoV outbreak began, outlines initial experiences and provides a blueprint for triaging potential cases before they reach the dermatology clinic. Despite its presence in the epicenter of the outbreak, the hospital has not detected any 2019-nCoV-infected patients in any of its departments.
The commentary appeared in the British Journal of Dermatology and was authored by a group led by Juan Tao of Huazhong University of Science and Technology (Br J Dermatol. 2020 Mar 5. doi: 10.1111/bjd.19011).
The hospital triages all patients at the hospital entrance. Those who are suspected of having 2019-nCoV infection are sent to a designated department. Those with a skin condition who are not suspected of being infected are allowed to go to a dermatology triage center, where they are examined again. If the second examination raises suspicion, they are sent to the designated 2019-nCoV department. If no infection is suspected, or a patient from the 2019-nCoV department is cleared, they are allowed access to the dermatology clinic.
The team also suggested that skin lesions associated with dermatological conditions could lead to increased risk of 2019-nCoV infection. Contacted by email, Dr. Tao outlined a theoretical risk that the virus could lead to infection through contact with subcutaneous tissues, mucosal surfaces, or blood vessels. He did not respond to a request for evidence that such a route of transmission had occurred.
However, Adam Friedman, MD, professor of dermatology at George Washington University, Washington, said he doubted any such transmission would occur since the virus does not infect keratinocytes, and expressed concern that the suggestion could add to the stigma experienced by dermatological patients, whose noticeable rashes can sometimes lead to social avoidance. “I don’t want to add to that,” said Dr. Friedman in an interview.
A critical aspect of dermatology is the immunosuppressive agents often used in dermatology patients. Such drugs could make them more susceptible to infections, or to worse outcomes in the event of disease. Dr. Friedman recounted sending a letter to one patient on an immunosuppressive medication, suggesting that she work remotely. “I think that’s something we have to think about in at-risk individuals. I know there’s such a focus on the elderly, but there’s a large population of individuals on medications that lower their immune system who are going to be at risk for more severe infections,” said Dr. Friedman.
To reduce patient exposure, the commentary recommended that dermatologists perform online consultation for mild and nonemergency cases.
The authors also covered hospitalized patients with primary or secondary skin conditions. A dermatologist is on site at the dermatology triage station to conduct in-depth assessments if needed. If a patient has a fever that is believed to be caused by a dermatologic condition, the on-site dermatologist assists in the consult.
Because some patients may only become symptomatic after admission to a ward, the authors recommend hospitals have a COVID-19 trained contingency group on hand to prevent and control outbreaks within the institution. The team should be in communication with respiratory intensive care and radiology departments to exclude 2019-nCoV when cases develop in-hospital, and to ensure proper care infected patients who require it.
When a hospitalized 2019-nCoV-infected patient has a skin condition requiring treatment, the authors recommend that pictures be sent to the dermatologist for evaluation, along with teleconferences to further assess the patient. If necessary, the dermatologist should go to the patient’s bedside, with as much information as possible related in advance in order to minimize bedside exposure.
There was no funding source. Dr. Tao and Dr. Friedman have no relevant financial conflicts.
The novel coronavirus (2019-nCoV) is presenting a severe challenge to global health care, but its impact isn’t just felt in the emergency department. Specialists, including dermatologists, must also navigate the presence of the virus and its impact on patients and practices.
A new report from dermatologists in China’s Wuhan province, where the 2019-nCoV outbreak began, outlines initial experiences and provides a blueprint for triaging potential cases before they reach the dermatology clinic. Despite its presence in the epicenter of the outbreak, the hospital has not detected any 2019-nCoV-infected patients in any of its departments.
The commentary appeared in the British Journal of Dermatology and was authored by a group led by Juan Tao of Huazhong University of Science and Technology (Br J Dermatol. 2020 Mar 5. doi: 10.1111/bjd.19011).
The hospital triages all patients at the hospital entrance. Those who are suspected of having 2019-nCoV infection are sent to a designated department. Those with a skin condition who are not suspected of being infected are allowed to go to a dermatology triage center, where they are examined again. If the second examination raises suspicion, they are sent to the designated 2019-nCoV department. If no infection is suspected, or a patient from the 2019-nCoV department is cleared, they are allowed access to the dermatology clinic.
The team also suggested that skin lesions associated with dermatological conditions could lead to increased risk of 2019-nCoV infection. Contacted by email, Dr. Tao outlined a theoretical risk that the virus could lead to infection through contact with subcutaneous tissues, mucosal surfaces, or blood vessels. He did not respond to a request for evidence that such a route of transmission had occurred.
However, Adam Friedman, MD, professor of dermatology at George Washington University, Washington, said he doubted any such transmission would occur since the virus does not infect keratinocytes, and expressed concern that the suggestion could add to the stigma experienced by dermatological patients, whose noticeable rashes can sometimes lead to social avoidance. “I don’t want to add to that,” said Dr. Friedman in an interview.
A critical aspect of dermatology is the immunosuppressive agents often used in dermatology patients. Such drugs could make them more susceptible to infections, or to worse outcomes in the event of disease. Dr. Friedman recounted sending a letter to one patient on an immunosuppressive medication, suggesting that she work remotely. “I think that’s something we have to think about in at-risk individuals. I know there’s such a focus on the elderly, but there’s a large population of individuals on medications that lower their immune system who are going to be at risk for more severe infections,” said Dr. Friedman.
To reduce patient exposure, the commentary recommended that dermatologists perform online consultation for mild and nonemergency cases.
The authors also covered hospitalized patients with primary or secondary skin conditions. A dermatologist is on site at the dermatology triage station to conduct in-depth assessments if needed. If a patient has a fever that is believed to be caused by a dermatologic condition, the on-site dermatologist assists in the consult.
Because some patients may only become symptomatic after admission to a ward, the authors recommend hospitals have a COVID-19 trained contingency group on hand to prevent and control outbreaks within the institution. The team should be in communication with respiratory intensive care and radiology departments to exclude 2019-nCoV when cases develop in-hospital, and to ensure proper care infected patients who require it.
When a hospitalized 2019-nCoV-infected patient has a skin condition requiring treatment, the authors recommend that pictures be sent to the dermatologist for evaluation, along with teleconferences to further assess the patient. If necessary, the dermatologist should go to the patient’s bedside, with as much information as possible related in advance in order to minimize bedside exposure.
There was no funding source. Dr. Tao and Dr. Friedman have no relevant financial conflicts.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Early GI symptoms in COVID-19 may indicate fecal transmission
Fecal-oral transmission may be part of the COVID-19 clinical picture, according to two reports published in Gastroenterology. The researchers find that RNA and proteins from SARS-CoV-2, the viral cause of COVID-19, are shed in feces early in infection and persist after respiratory symptoms abate.
But the discovery is preliminary. “There is evidence of the virus in stool, but not evidence of infectious virus,” David A. Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine in Norfolk, told Medscape Medical News.
The findings are not entirely unexpected. Both of the coronaviruses behind SARS and MERS are shed in stool, Jinyang Gu, MD, from Shanghai Jiao Tong University School of Medicine in Shanghai, China, and colleagues, note in one of the newly published articles.
In addition, as COVID-19 spread beyond China, clinicians began noticing initial mild gastrointestinal (GI) symptoms in some patients, including diarrhea, nausea, vomiting, and abdominal pain, preceding the hallmark fever, dry cough, and dyspnea. The first patient diagnosed in the United States with COVID-19 reported having 2 days of nausea and vomiting, with viral RNA detected in fecal and respiratory specimens, according to an earlier report.
Gu and colleagues warn that initial investigations would likely have not considered cases that manifested initially only as mild gastrointestinal symptoms.
Although early reports indicated that only about 10% of people with COVID-19 have GI symptoms, it isn’t known whether some infected individuals have only GI symptoms, Johnson said.
The GI manifestations are consistent with the distribution of ACE2 receptors, which serve as entry points for SARS-CoV-2, as well as SARS-CoV-1, which causes SARS. The receptors are most abundant in the cell membranes of lung AT2 cells, as well as in enterocytes in the ileum and colon.
“Altogether, many efforts should be made to be alert on the initial digestive symptoms of COVID-19 for early detection, early diagnosis, early isolation and early intervention,” Gu and colleagues conclude.
But Johnson cautions, “gastroenterologists are not the ones managing diagnosis of COVID-19. It is diagnosed as a respiratory illness, but we are seeing concomitant gastrointestinal shedding in stool and saliva, and GI symptoms.”
Samples From 73 Patients Studied
In the second article published, Fei Xiao, MD, of Sun Yat-sen University in Guangdong Province, China, and colleagues report detecting viral RNA in samples from the mouths, noses, throats, urine, and feces of 73 patients hospitalized during the first 2 weeks of February.
Of the 73 hospitalized patients, 39 (53.24%; 25 males and 14 females) had viral RNA in their feces, present from 1 to 12 days. Seventeen (23.29%) of the patients continued to have viral RNA in their stool after respiratory symptoms had improved.
One patient underwent endoscopy. There was no evidence of damage to the GI epithelium, but the clinicians detected slightly elevated levels of lymphocytes and plasma cells.
The researcher used laser scanning confocal microscopy to analyze samples taken during the endoscopy. They found evidence of both ACE2 receptors and viral nucleocapsid proteins in the gastric, duodenal, and rectal glandular epithelial cells.
Finding evidence of SARS-CoV-2 throughout the GI system, if not direct infectivity, suggests a fecal-oral route of transmission, the researchers conclude. “Our immunofluorescent data showed that ACE2 protein, a cell receptor for SARS-CoV-2, is abundantly expressed in the glandular cells of gastric, duodenal and rectal epithelia, supporting the entry of SARS-CoV-2 into the host cells.”
Detection of viral RNA at different time points in infection, they write, suggests that the virions are continually secreted and therefore likely infectious, which is under investigation. “Prevention of fecal-oral transmission should be taken into consideration to control the spread of the virus,” they write.
Current recommendations do not require that patients’ fecal samples be tested before being considered noninfectious. However, given their findings and evidence from other studies, Xiao and colleagues recommend that real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) testing of fecal samples be added to current protocols.
Johnson offers practical suggestions based on the “potty hygiene” suggestions he gives to patients dealing with fecal shedding in Clostridioides difficile infection.
“To combat the microaerosolization of C. diff spores, I have patients do a complete bacteriocidal washing out of the toilet bowl, as well as clean surface areas and especially toothbrushes.” Keeping the bowl closed when not in use is important too in preventing “fecal-oral transmission of remnants” of toilet contents, he adds.
The new papers add to other reports suggesting that virus-bearing droplets may reach people in various ways, Johnson said. “Maybe the virus isn’t only spread by a cough or a sneeze.”
The researchers and commentator have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Fecal-oral transmission may be part of the COVID-19 clinical picture, according to two reports published in Gastroenterology. The researchers find that RNA and proteins from SARS-CoV-2, the viral cause of COVID-19, are shed in feces early in infection and persist after respiratory symptoms abate.
But the discovery is preliminary. “There is evidence of the virus in stool, but not evidence of infectious virus,” David A. Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine in Norfolk, told Medscape Medical News.
The findings are not entirely unexpected. Both of the coronaviruses behind SARS and MERS are shed in stool, Jinyang Gu, MD, from Shanghai Jiao Tong University School of Medicine in Shanghai, China, and colleagues, note in one of the newly published articles.
In addition, as COVID-19 spread beyond China, clinicians began noticing initial mild gastrointestinal (GI) symptoms in some patients, including diarrhea, nausea, vomiting, and abdominal pain, preceding the hallmark fever, dry cough, and dyspnea. The first patient diagnosed in the United States with COVID-19 reported having 2 days of nausea and vomiting, with viral RNA detected in fecal and respiratory specimens, according to an earlier report.
Gu and colleagues warn that initial investigations would likely have not considered cases that manifested initially only as mild gastrointestinal symptoms.
Although early reports indicated that only about 10% of people with COVID-19 have GI symptoms, it isn’t known whether some infected individuals have only GI symptoms, Johnson said.
The GI manifestations are consistent with the distribution of ACE2 receptors, which serve as entry points for SARS-CoV-2, as well as SARS-CoV-1, which causes SARS. The receptors are most abundant in the cell membranes of lung AT2 cells, as well as in enterocytes in the ileum and colon.
“Altogether, many efforts should be made to be alert on the initial digestive symptoms of COVID-19 for early detection, early diagnosis, early isolation and early intervention,” Gu and colleagues conclude.
But Johnson cautions, “gastroenterologists are not the ones managing diagnosis of COVID-19. It is diagnosed as a respiratory illness, but we are seeing concomitant gastrointestinal shedding in stool and saliva, and GI symptoms.”
Samples From 73 Patients Studied
In the second article published, Fei Xiao, MD, of Sun Yat-sen University in Guangdong Province, China, and colleagues report detecting viral RNA in samples from the mouths, noses, throats, urine, and feces of 73 patients hospitalized during the first 2 weeks of February.
Of the 73 hospitalized patients, 39 (53.24%; 25 males and 14 females) had viral RNA in their feces, present from 1 to 12 days. Seventeen (23.29%) of the patients continued to have viral RNA in their stool after respiratory symptoms had improved.
One patient underwent endoscopy. There was no evidence of damage to the GI epithelium, but the clinicians detected slightly elevated levels of lymphocytes and plasma cells.
The researcher used laser scanning confocal microscopy to analyze samples taken during the endoscopy. They found evidence of both ACE2 receptors and viral nucleocapsid proteins in the gastric, duodenal, and rectal glandular epithelial cells.
Finding evidence of SARS-CoV-2 throughout the GI system, if not direct infectivity, suggests a fecal-oral route of transmission, the researchers conclude. “Our immunofluorescent data showed that ACE2 protein, a cell receptor for SARS-CoV-2, is abundantly expressed in the glandular cells of gastric, duodenal and rectal epithelia, supporting the entry of SARS-CoV-2 into the host cells.”
Detection of viral RNA at different time points in infection, they write, suggests that the virions are continually secreted and therefore likely infectious, which is under investigation. “Prevention of fecal-oral transmission should be taken into consideration to control the spread of the virus,” they write.
Current recommendations do not require that patients’ fecal samples be tested before being considered noninfectious. However, given their findings and evidence from other studies, Xiao and colleagues recommend that real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) testing of fecal samples be added to current protocols.
Johnson offers practical suggestions based on the “potty hygiene” suggestions he gives to patients dealing with fecal shedding in Clostridioides difficile infection.
“To combat the microaerosolization of C. diff spores, I have patients do a complete bacteriocidal washing out of the toilet bowl, as well as clean surface areas and especially toothbrushes.” Keeping the bowl closed when not in use is important too in preventing “fecal-oral transmission of remnants” of toilet contents, he adds.
The new papers add to other reports suggesting that virus-bearing droplets may reach people in various ways, Johnson said. “Maybe the virus isn’t only spread by a cough or a sneeze.”
The researchers and commentator have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Fecal-oral transmission may be part of the COVID-19 clinical picture, according to two reports published in Gastroenterology. The researchers find that RNA and proteins from SARS-CoV-2, the viral cause of COVID-19, are shed in feces early in infection and persist after respiratory symptoms abate.
But the discovery is preliminary. “There is evidence of the virus in stool, but not evidence of infectious virus,” David A. Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine in Norfolk, told Medscape Medical News.
The findings are not entirely unexpected. Both of the coronaviruses behind SARS and MERS are shed in stool, Jinyang Gu, MD, from Shanghai Jiao Tong University School of Medicine in Shanghai, China, and colleagues, note in one of the newly published articles.
In addition, as COVID-19 spread beyond China, clinicians began noticing initial mild gastrointestinal (GI) symptoms in some patients, including diarrhea, nausea, vomiting, and abdominal pain, preceding the hallmark fever, dry cough, and dyspnea. The first patient diagnosed in the United States with COVID-19 reported having 2 days of nausea and vomiting, with viral RNA detected in fecal and respiratory specimens, according to an earlier report.
Gu and colleagues warn that initial investigations would likely have not considered cases that manifested initially only as mild gastrointestinal symptoms.
Although early reports indicated that only about 10% of people with COVID-19 have GI symptoms, it isn’t known whether some infected individuals have only GI symptoms, Johnson said.
The GI manifestations are consistent with the distribution of ACE2 receptors, which serve as entry points for SARS-CoV-2, as well as SARS-CoV-1, which causes SARS. The receptors are most abundant in the cell membranes of lung AT2 cells, as well as in enterocytes in the ileum and colon.
“Altogether, many efforts should be made to be alert on the initial digestive symptoms of COVID-19 for early detection, early diagnosis, early isolation and early intervention,” Gu and colleagues conclude.
But Johnson cautions, “gastroenterologists are not the ones managing diagnosis of COVID-19. It is diagnosed as a respiratory illness, but we are seeing concomitant gastrointestinal shedding in stool and saliva, and GI symptoms.”
Samples From 73 Patients Studied
In the second article published, Fei Xiao, MD, of Sun Yat-sen University in Guangdong Province, China, and colleagues report detecting viral RNA in samples from the mouths, noses, throats, urine, and feces of 73 patients hospitalized during the first 2 weeks of February.
Of the 73 hospitalized patients, 39 (53.24%; 25 males and 14 females) had viral RNA in their feces, present from 1 to 12 days. Seventeen (23.29%) of the patients continued to have viral RNA in their stool after respiratory symptoms had improved.
One patient underwent endoscopy. There was no evidence of damage to the GI epithelium, but the clinicians detected slightly elevated levels of lymphocytes and plasma cells.
The researcher used laser scanning confocal microscopy to analyze samples taken during the endoscopy. They found evidence of both ACE2 receptors and viral nucleocapsid proteins in the gastric, duodenal, and rectal glandular epithelial cells.
Finding evidence of SARS-CoV-2 throughout the GI system, if not direct infectivity, suggests a fecal-oral route of transmission, the researchers conclude. “Our immunofluorescent data showed that ACE2 protein, a cell receptor for SARS-CoV-2, is abundantly expressed in the glandular cells of gastric, duodenal and rectal epithelia, supporting the entry of SARS-CoV-2 into the host cells.”
Detection of viral RNA at different time points in infection, they write, suggests that the virions are continually secreted and therefore likely infectious, which is under investigation. “Prevention of fecal-oral transmission should be taken into consideration to control the spread of the virus,” they write.
Current recommendations do not require that patients’ fecal samples be tested before being considered noninfectious. However, given their findings and evidence from other studies, Xiao and colleagues recommend that real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) testing of fecal samples be added to current protocols.
Johnson offers practical suggestions based on the “potty hygiene” suggestions he gives to patients dealing with fecal shedding in Clostridioides difficile infection.
“To combat the microaerosolization of C. diff spores, I have patients do a complete bacteriocidal washing out of the toilet bowl, as well as clean surface areas and especially toothbrushes.” Keeping the bowl closed when not in use is important too in preventing “fecal-oral transmission of remnants” of toilet contents, he adds.
The new papers add to other reports suggesting that virus-bearing droplets may reach people in various ways, Johnson said. “Maybe the virus isn’t only spread by a cough or a sneeze.”
The researchers and commentator have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Dermatologists best at finding work satisfaction in the office
, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

About 41% of dermatologists reported being very happy at work, making their specialty the only one to break the 40% barrier. While dermatologists weren’t the happiest outside of work – that honor went to rheumatologists – dermatology was only 4 percentage points behind (60% vs. 56%).
Perhaps unsurprisingly, the percentage of dermatologists who were burned out was lower than that of physicians overall (36% vs. 41%). The biggest factors leading to burnout in dermatologists were an overabundance of bureaucratic tasks (58%), increased time devoted to EHRs (38%), and compliance with government regulations (35%).
Dermatologists dealt with burnout through a variety of ways, with the most common being exercise (44%), talk with family/friends (44%), and isolation from others (40%). In addition, dermatologists took slightly more vacation time than did physicians overall, with 51% of dermatologists taking 3-4 weeks of vacation, compared with 44% for physicians overall.
About 16% of dermatologists have contemplated suicide; however, none reported attempting suicide, and 72% of dermatologists have never felt suicidal. Most dermatologists also plan to deal with burnout or depression on their own, with only 31% reporting that they are currently seeking professional help, planning to seek help, or are not currently looking but have been treated in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

About 41% of dermatologists reported being very happy at work, making their specialty the only one to break the 40% barrier. While dermatologists weren’t the happiest outside of work – that honor went to rheumatologists – dermatology was only 4 percentage points behind (60% vs. 56%).
Perhaps unsurprisingly, the percentage of dermatologists who were burned out was lower than that of physicians overall (36% vs. 41%). The biggest factors leading to burnout in dermatologists were an overabundance of bureaucratic tasks (58%), increased time devoted to EHRs (38%), and compliance with government regulations (35%).
Dermatologists dealt with burnout through a variety of ways, with the most common being exercise (44%), talk with family/friends (44%), and isolation from others (40%). In addition, dermatologists took slightly more vacation time than did physicians overall, with 51% of dermatologists taking 3-4 weeks of vacation, compared with 44% for physicians overall.
About 16% of dermatologists have contemplated suicide; however, none reported attempting suicide, and 72% of dermatologists have never felt suicidal. Most dermatologists also plan to deal with burnout or depression on their own, with only 31% reporting that they are currently seeking professional help, planning to seek help, or are not currently looking but have been treated in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

About 41% of dermatologists reported being very happy at work, making their specialty the only one to break the 40% barrier. While dermatologists weren’t the happiest outside of work – that honor went to rheumatologists – dermatology was only 4 percentage points behind (60% vs. 56%).
Perhaps unsurprisingly, the percentage of dermatologists who were burned out was lower than that of physicians overall (36% vs. 41%). The biggest factors leading to burnout in dermatologists were an overabundance of bureaucratic tasks (58%), increased time devoted to EHRs (38%), and compliance with government regulations (35%).
Dermatologists dealt with burnout through a variety of ways, with the most common being exercise (44%), talk with family/friends (44%), and isolation from others (40%). In addition, dermatologists took slightly more vacation time than did physicians overall, with 51% of dermatologists taking 3-4 weeks of vacation, compared with 44% for physicians overall.
About 16% of dermatologists have contemplated suicide; however, none reported attempting suicide, and 72% of dermatologists have never felt suicidal. Most dermatologists also plan to deal with burnout or depression on their own, with only 31% reporting that they are currently seeking professional help, planning to seek help, or are not currently looking but have been treated in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
Testing times for epidermolysis bullosa topical therapies
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
EXPERT ANALYSIS FROM EB 2020
Merkel cell carcinoma management undergoes revolution
LAHAINA, HAWAII – The , Paul Nghiem, MD, PhD, declared at the SDEF Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
That’s because dermatologists are typically the physicians who make the diagnosis of Merkel cell carcinoma (MCC), so they’re on the scene from the outset and well positioned to help direct early management of this particularly aggressive malignancy, explained Dr. Nghiem, professor and head of dermatology at the University of Washington, Seattle.
“The management of Merkel is pretty high stakes, and if you get it right at the beginning it makes a huge difference in the side effects, as well as the chances that the patient will have the disease under control,” said Dr. Nghiem, who is sometimes called “the Merkel man” because of his many pioneering contributions to the field.
Better early management
Getting early management right, he added, hinges upon ordering a baseline PET-CT scan to search for metastases before performing definitive surgical excision of the primary tumor.
“There are really important prognostic and therapeutic implications for a baseline scan in almost any patient with early Merkel – and that’s a very different situation than with melanoma,” the dermatologist said. “There’s at least a threefold higher likelihood that the cancer has spread asymptomatically at baseline with Merkel cell carcinoma than with melanoma.”
In a soon-to-be-published study by Dr. Nghiem and coworkers, baseline imaging resulted in prognostically important upstaging that led to an altered management strategy in 12% of 584 patients with MCC, or 1 in 8.
“You don’t want to overtreat locally a lesion that has already spread distantly; you want to start focusing on the distant disease. The local disease is secondary,” he said.
The surgical excision of the primary lesion should be thoughtfully wide without being aggressive or mutilating, and it should involve primary closure. “Definitely avoid flaps and grafts, which delay your further management with radiotherapy by months and months,” Dr. Nghiem advised.
Adjuvant radiotherapy of the primary tumor site is extremely effective at preventing recurrent MCC. In Dr. Nghiem’s view, almost everyone is a candidate: In a series of 803 patients in the Seattle MCC cohort, 92% received local adjuvant radiotherapy. The national rate, in contrast, is only about 50%, highlighting the need for additional physician education.
“A little bit of radiation – one dose – appears to be just as effective as 6 weeks in controlling microscopic disease. That’s probably something we’re going to be moving towards as a field,” he predicted.
Indeed, local adjuvant radiotherapy is so effective in MCC that the surgical margins make no difference. This was demonstrated in a study by Dr. Nghiem and his coinvestigators involving 70 patients with margins greater than 1 cm who received radiotherapy, 70 others with smaller or even positive margins who received radiotherapy, and 35 patients with margins of 1 cm or less who did not receive radiotherapy. There were no MCC recurrences in any of the radiotherapy recipients, regardless of their margin status. In contrast, 7 of the 35 patients who didn’t receive radiation therapy developed a cancer recurrence. Of note, the recurrence rate of MCC is historically about 40% – far greater than for any other skin cancer. Most recurrences happen within the first 2-3 years, Dr. Nghiem observed.
Immune therapy takes center stage
Another major transformation in MCC management has been the emergence of immune therapy as first-line systemic therapy. It has replaced chemotherapy, which is more toxic and has a much shorter average duration of response. Avelumab (Bavencio) and pembrolizumab (Keytruda), the two monoclonal antibodies directed against the protein programmed death–ligand 1 (PD-L1) receptor which are approved for MCC and have been incorporated into the National Comprehensive Cancer Network (NCCN) guidelines, provide a sixfold improvement in survival, compared with chemotherapy. For example, Dr. Nghiem was first author of a multicenter phase 2 study of pembrolizumab in which the 12- and 24-month overall survival rates in pembrolizumab responders were 85% and 79%, compared with just 12% and 6%, respectively, in historical controls on first-line chemotherapy (J Clin Oncol. 2019 Mar 20;37[9]:693-702).
“Merkel cell carcinoma is the most responsive solid tumor to immune therapy,” Dr. Nghiem commented.
Why MCC matters
Although rare, MCC is important because it’s five times more lethal than melanoma. Moreover, its incidence has been rising at a rate roughly twice that of the increase in melanoma since the turn of the century. There are now more than 3,000 new cases of MCC annually, about the same as for cutaneous T-cell lymphoma (CTCL).
“It’s just that you live a long time with CTCL and you don’t with Merkel cell carcinoma. You either get rid of Merkel fast or it gets rid of you,” the dermatologist observed.
It’s a fascinating malignancy, he continued. Eight of 10 cases are caused by Merkel cell polyomavirus, discovered in 2008. The virus is ubiquitously acquired in childhood and then lies dormant on the skin for the next 6 or 7 decades, at which point MCC rates shoot up dramatically, probably due to immunosenescence. Immunosuppressed patients are at 10-fold increased risk for MCC.
Given the rarity of MCC, it doesn’t make sense to actively hunt for it. But Dr. Nghiem and coworkers have developed a handy vowel-based mnemonic that serves to raise the index of suspicion: the “AEIOU” features.
- A = asymptomatic.
- E = expanding rapidly within past 3 months.
- I = immune-mediated.
- O = older than age 50.
- U = UV-exposed skin.
The investigators found in a series of 195 MCC patients that 89% of them possessed three or more of these features (J Am Acad Dermatol. 2008 Mar;58[3]:375-81). But while the AEIOU guide is quite sensitive, it’s not specific.
“If you have any three or more of these features, that lesion probably deserves a biopsy if it’s not readily explained. Even if it’s not a Merkel, it may turn out to be a different nonmelanoma skin cancer, something you want to know about,” Dr. Nghiem said.
A shift in surveillance strategy
Dr. Nghiem was senior author of a major study that validated the clinical utility of a Merkel polyomavirus serology test for monitoring the disease status of patients treated for MCC (Cancer. 2017 Apr 15;123[8]:1464-74). The test, which measures antibodies to Merkel cell polyomavirus oncoproteins, has been incorporated in NCCN guidelines. The blood test is used initially in newly diagnosed MCC to stratify patients into two subgroups: the half who are seropositive at baseline, and the other half who are seronegative. The seropositive group undergoes surveillance via repeat blood testing every 3 months. If antibody levels are low, there is a high degree of certainty that immune therapy is working and remission is present. Thus, the blood test spares patients in this group the expense and radiation exposure entailed in repeated surveillance scans. However, rising antibody levels indicate the cancer has already recurred or will do so within the next several months.
Unfortunately, the blood test cannot be used serially to track disease status in patients who are seronegative at baseline. That group is at 42% increased risk of MCC recurrence.
Immune therapy works in only about two-thirds of MCC patients with distant disease. Leaving the visible primary tumor in place to serve as a real-time window into immune treatment effectiveness is a useful contemporary surveillance strategy.
“By leaving the visible primary there, you will rapidly know if that patient is in the favorable two-thirds group or not,” he explained.
Historically, surgery and surveillance of MCC were based upon the melanoma model, and medical oncologists were trained to treat the malignancy as they would small cell lung cancer. These are now outmoded approaches, Dr. Nghiem said. That’s why a multidisciplinary approach is highly desirable for management of MCC, including dermatologists, pathologists, surgeons, radiation oncologists, medical oncologists, and imaging experts.
Dr. Nghiem and his colleagues have created a comprehensive source of information about Merkel cell carcinoma for physicians and patients at merkelcell.org.
He reported receiving research grants from Bristol-Myers Squibb and serving as a consultant to EMD Serono, Merck, Sanofi/Regeneron, and 4SC.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The , Paul Nghiem, MD, PhD, declared at the SDEF Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
That’s because dermatologists are typically the physicians who make the diagnosis of Merkel cell carcinoma (MCC), so they’re on the scene from the outset and well positioned to help direct early management of this particularly aggressive malignancy, explained Dr. Nghiem, professor and head of dermatology at the University of Washington, Seattle.
“The management of Merkel is pretty high stakes, and if you get it right at the beginning it makes a huge difference in the side effects, as well as the chances that the patient will have the disease under control,” said Dr. Nghiem, who is sometimes called “the Merkel man” because of his many pioneering contributions to the field.
Better early management
Getting early management right, he added, hinges upon ordering a baseline PET-CT scan to search for metastases before performing definitive surgical excision of the primary tumor.
“There are really important prognostic and therapeutic implications for a baseline scan in almost any patient with early Merkel – and that’s a very different situation than with melanoma,” the dermatologist said. “There’s at least a threefold higher likelihood that the cancer has spread asymptomatically at baseline with Merkel cell carcinoma than with melanoma.”
In a soon-to-be-published study by Dr. Nghiem and coworkers, baseline imaging resulted in prognostically important upstaging that led to an altered management strategy in 12% of 584 patients with MCC, or 1 in 8.
“You don’t want to overtreat locally a lesion that has already spread distantly; you want to start focusing on the distant disease. The local disease is secondary,” he said.
The surgical excision of the primary lesion should be thoughtfully wide without being aggressive or mutilating, and it should involve primary closure. “Definitely avoid flaps and grafts, which delay your further management with radiotherapy by months and months,” Dr. Nghiem advised.
Adjuvant radiotherapy of the primary tumor site is extremely effective at preventing recurrent MCC. In Dr. Nghiem’s view, almost everyone is a candidate: In a series of 803 patients in the Seattle MCC cohort, 92% received local adjuvant radiotherapy. The national rate, in contrast, is only about 50%, highlighting the need for additional physician education.
“A little bit of radiation – one dose – appears to be just as effective as 6 weeks in controlling microscopic disease. That’s probably something we’re going to be moving towards as a field,” he predicted.
Indeed, local adjuvant radiotherapy is so effective in MCC that the surgical margins make no difference. This was demonstrated in a study by Dr. Nghiem and his coinvestigators involving 70 patients with margins greater than 1 cm who received radiotherapy, 70 others with smaller or even positive margins who received radiotherapy, and 35 patients with margins of 1 cm or less who did not receive radiotherapy. There were no MCC recurrences in any of the radiotherapy recipients, regardless of their margin status. In contrast, 7 of the 35 patients who didn’t receive radiation therapy developed a cancer recurrence. Of note, the recurrence rate of MCC is historically about 40% – far greater than for any other skin cancer. Most recurrences happen within the first 2-3 years, Dr. Nghiem observed.
Immune therapy takes center stage
Another major transformation in MCC management has been the emergence of immune therapy as first-line systemic therapy. It has replaced chemotherapy, which is more toxic and has a much shorter average duration of response. Avelumab (Bavencio) and pembrolizumab (Keytruda), the two monoclonal antibodies directed against the protein programmed death–ligand 1 (PD-L1) receptor which are approved for MCC and have been incorporated into the National Comprehensive Cancer Network (NCCN) guidelines, provide a sixfold improvement in survival, compared with chemotherapy. For example, Dr. Nghiem was first author of a multicenter phase 2 study of pembrolizumab in which the 12- and 24-month overall survival rates in pembrolizumab responders were 85% and 79%, compared with just 12% and 6%, respectively, in historical controls on first-line chemotherapy (J Clin Oncol. 2019 Mar 20;37[9]:693-702).
“Merkel cell carcinoma is the most responsive solid tumor to immune therapy,” Dr. Nghiem commented.
Why MCC matters
Although rare, MCC is important because it’s five times more lethal than melanoma. Moreover, its incidence has been rising at a rate roughly twice that of the increase in melanoma since the turn of the century. There are now more than 3,000 new cases of MCC annually, about the same as for cutaneous T-cell lymphoma (CTCL).
“It’s just that you live a long time with CTCL and you don’t with Merkel cell carcinoma. You either get rid of Merkel fast or it gets rid of you,” the dermatologist observed.
It’s a fascinating malignancy, he continued. Eight of 10 cases are caused by Merkel cell polyomavirus, discovered in 2008. The virus is ubiquitously acquired in childhood and then lies dormant on the skin for the next 6 or 7 decades, at which point MCC rates shoot up dramatically, probably due to immunosenescence. Immunosuppressed patients are at 10-fold increased risk for MCC.
Given the rarity of MCC, it doesn’t make sense to actively hunt for it. But Dr. Nghiem and coworkers have developed a handy vowel-based mnemonic that serves to raise the index of suspicion: the “AEIOU” features.
- A = asymptomatic.
- E = expanding rapidly within past 3 months.
- I = immune-mediated.
- O = older than age 50.
- U = UV-exposed skin.
The investigators found in a series of 195 MCC patients that 89% of them possessed three or more of these features (J Am Acad Dermatol. 2008 Mar;58[3]:375-81). But while the AEIOU guide is quite sensitive, it’s not specific.
“If you have any three or more of these features, that lesion probably deserves a biopsy if it’s not readily explained. Even if it’s not a Merkel, it may turn out to be a different nonmelanoma skin cancer, something you want to know about,” Dr. Nghiem said.
A shift in surveillance strategy
Dr. Nghiem was senior author of a major study that validated the clinical utility of a Merkel polyomavirus serology test for monitoring the disease status of patients treated for MCC (Cancer. 2017 Apr 15;123[8]:1464-74). The test, which measures antibodies to Merkel cell polyomavirus oncoproteins, has been incorporated in NCCN guidelines. The blood test is used initially in newly diagnosed MCC to stratify patients into two subgroups: the half who are seropositive at baseline, and the other half who are seronegative. The seropositive group undergoes surveillance via repeat blood testing every 3 months. If antibody levels are low, there is a high degree of certainty that immune therapy is working and remission is present. Thus, the blood test spares patients in this group the expense and radiation exposure entailed in repeated surveillance scans. However, rising antibody levels indicate the cancer has already recurred or will do so within the next several months.
Unfortunately, the blood test cannot be used serially to track disease status in patients who are seronegative at baseline. That group is at 42% increased risk of MCC recurrence.
Immune therapy works in only about two-thirds of MCC patients with distant disease. Leaving the visible primary tumor in place to serve as a real-time window into immune treatment effectiveness is a useful contemporary surveillance strategy.
“By leaving the visible primary there, you will rapidly know if that patient is in the favorable two-thirds group or not,” he explained.
Historically, surgery and surveillance of MCC were based upon the melanoma model, and medical oncologists were trained to treat the malignancy as they would small cell lung cancer. These are now outmoded approaches, Dr. Nghiem said. That’s why a multidisciplinary approach is highly desirable for management of MCC, including dermatologists, pathologists, surgeons, radiation oncologists, medical oncologists, and imaging experts.
Dr. Nghiem and his colleagues have created a comprehensive source of information about Merkel cell carcinoma for physicians and patients at merkelcell.org.
He reported receiving research grants from Bristol-Myers Squibb and serving as a consultant to EMD Serono, Merck, Sanofi/Regeneron, and 4SC.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The , Paul Nghiem, MD, PhD, declared at the SDEF Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
That’s because dermatologists are typically the physicians who make the diagnosis of Merkel cell carcinoma (MCC), so they’re on the scene from the outset and well positioned to help direct early management of this particularly aggressive malignancy, explained Dr. Nghiem, professor and head of dermatology at the University of Washington, Seattle.
“The management of Merkel is pretty high stakes, and if you get it right at the beginning it makes a huge difference in the side effects, as well as the chances that the patient will have the disease under control,” said Dr. Nghiem, who is sometimes called “the Merkel man” because of his many pioneering contributions to the field.
Better early management
Getting early management right, he added, hinges upon ordering a baseline PET-CT scan to search for metastases before performing definitive surgical excision of the primary tumor.
“There are really important prognostic and therapeutic implications for a baseline scan in almost any patient with early Merkel – and that’s a very different situation than with melanoma,” the dermatologist said. “There’s at least a threefold higher likelihood that the cancer has spread asymptomatically at baseline with Merkel cell carcinoma than with melanoma.”
In a soon-to-be-published study by Dr. Nghiem and coworkers, baseline imaging resulted in prognostically important upstaging that led to an altered management strategy in 12% of 584 patients with MCC, or 1 in 8.
“You don’t want to overtreat locally a lesion that has already spread distantly; you want to start focusing on the distant disease. The local disease is secondary,” he said.
The surgical excision of the primary lesion should be thoughtfully wide without being aggressive or mutilating, and it should involve primary closure. “Definitely avoid flaps and grafts, which delay your further management with radiotherapy by months and months,” Dr. Nghiem advised.
Adjuvant radiotherapy of the primary tumor site is extremely effective at preventing recurrent MCC. In Dr. Nghiem’s view, almost everyone is a candidate: In a series of 803 patients in the Seattle MCC cohort, 92% received local adjuvant radiotherapy. The national rate, in contrast, is only about 50%, highlighting the need for additional physician education.
“A little bit of radiation – one dose – appears to be just as effective as 6 weeks in controlling microscopic disease. That’s probably something we’re going to be moving towards as a field,” he predicted.
Indeed, local adjuvant radiotherapy is so effective in MCC that the surgical margins make no difference. This was demonstrated in a study by Dr. Nghiem and his coinvestigators involving 70 patients with margins greater than 1 cm who received radiotherapy, 70 others with smaller or even positive margins who received radiotherapy, and 35 patients with margins of 1 cm or less who did not receive radiotherapy. There were no MCC recurrences in any of the radiotherapy recipients, regardless of their margin status. In contrast, 7 of the 35 patients who didn’t receive radiation therapy developed a cancer recurrence. Of note, the recurrence rate of MCC is historically about 40% – far greater than for any other skin cancer. Most recurrences happen within the first 2-3 years, Dr. Nghiem observed.
Immune therapy takes center stage
Another major transformation in MCC management has been the emergence of immune therapy as first-line systemic therapy. It has replaced chemotherapy, which is more toxic and has a much shorter average duration of response. Avelumab (Bavencio) and pembrolizumab (Keytruda), the two monoclonal antibodies directed against the protein programmed death–ligand 1 (PD-L1) receptor which are approved for MCC and have been incorporated into the National Comprehensive Cancer Network (NCCN) guidelines, provide a sixfold improvement in survival, compared with chemotherapy. For example, Dr. Nghiem was first author of a multicenter phase 2 study of pembrolizumab in which the 12- and 24-month overall survival rates in pembrolizumab responders were 85% and 79%, compared with just 12% and 6%, respectively, in historical controls on first-line chemotherapy (J Clin Oncol. 2019 Mar 20;37[9]:693-702).
“Merkel cell carcinoma is the most responsive solid tumor to immune therapy,” Dr. Nghiem commented.
Why MCC matters
Although rare, MCC is important because it’s five times more lethal than melanoma. Moreover, its incidence has been rising at a rate roughly twice that of the increase in melanoma since the turn of the century. There are now more than 3,000 new cases of MCC annually, about the same as for cutaneous T-cell lymphoma (CTCL).
“It’s just that you live a long time with CTCL and you don’t with Merkel cell carcinoma. You either get rid of Merkel fast or it gets rid of you,” the dermatologist observed.
It’s a fascinating malignancy, he continued. Eight of 10 cases are caused by Merkel cell polyomavirus, discovered in 2008. The virus is ubiquitously acquired in childhood and then lies dormant on the skin for the next 6 or 7 decades, at which point MCC rates shoot up dramatically, probably due to immunosenescence. Immunosuppressed patients are at 10-fold increased risk for MCC.
Given the rarity of MCC, it doesn’t make sense to actively hunt for it. But Dr. Nghiem and coworkers have developed a handy vowel-based mnemonic that serves to raise the index of suspicion: the “AEIOU” features.
- A = asymptomatic.
- E = expanding rapidly within past 3 months.
- I = immune-mediated.
- O = older than age 50.
- U = UV-exposed skin.
The investigators found in a series of 195 MCC patients that 89% of them possessed three or more of these features (J Am Acad Dermatol. 2008 Mar;58[3]:375-81). But while the AEIOU guide is quite sensitive, it’s not specific.
“If you have any three or more of these features, that lesion probably deserves a biopsy if it’s not readily explained. Even if it’s not a Merkel, it may turn out to be a different nonmelanoma skin cancer, something you want to know about,” Dr. Nghiem said.
A shift in surveillance strategy
Dr. Nghiem was senior author of a major study that validated the clinical utility of a Merkel polyomavirus serology test for monitoring the disease status of patients treated for MCC (Cancer. 2017 Apr 15;123[8]:1464-74). The test, which measures antibodies to Merkel cell polyomavirus oncoproteins, has been incorporated in NCCN guidelines. The blood test is used initially in newly diagnosed MCC to stratify patients into two subgroups: the half who are seropositive at baseline, and the other half who are seronegative. The seropositive group undergoes surveillance via repeat blood testing every 3 months. If antibody levels are low, there is a high degree of certainty that immune therapy is working and remission is present. Thus, the blood test spares patients in this group the expense and radiation exposure entailed in repeated surveillance scans. However, rising antibody levels indicate the cancer has already recurred or will do so within the next several months.
Unfortunately, the blood test cannot be used serially to track disease status in patients who are seronegative at baseline. That group is at 42% increased risk of MCC recurrence.
Immune therapy works in only about two-thirds of MCC patients with distant disease. Leaving the visible primary tumor in place to serve as a real-time window into immune treatment effectiveness is a useful contemporary surveillance strategy.
“By leaving the visible primary there, you will rapidly know if that patient is in the favorable two-thirds group or not,” he explained.
Historically, surgery and surveillance of MCC were based upon the melanoma model, and medical oncologists were trained to treat the malignancy as they would small cell lung cancer. These are now outmoded approaches, Dr. Nghiem said. That’s why a multidisciplinary approach is highly desirable for management of MCC, including dermatologists, pathologists, surgeons, radiation oncologists, medical oncologists, and imaging experts.
Dr. Nghiem and his colleagues have created a comprehensive source of information about Merkel cell carcinoma for physicians and patients at merkelcell.org.
He reported receiving research grants from Bristol-Myers Squibb and serving as a consultant to EMD Serono, Merck, Sanofi/Regeneron, and 4SC.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
WHO declares COVID-19 outbreak a pandemic
The World Health Organization has formally declared the COVID-19 outbreak a pandemic.

“WHO has been assessing this outbreak around the clock and we are deeply concerned both by the alarming levels of spread and severity, and by the alarming levels of inaction,” WHO Director-General Tedros Adhanom Ghebreyesus said during a March 11 press briefing. “We therefore made the assessment that COVID-19 can be characterized as a pandemic.”
He noted that this is the first time a coronavirus has been seen as a pandemic.
The Director-General cautioned that just looking at the number of countries affected, 114 countries, “does not tell the full story. ... We cannot say this loudly enough, or clearly enough, or often enough: All countries can still change the course of this pandemic.”
He reiterated the need for a whole-of-government and a whole-of-society approach to dealing with this, including taking precautions such as isolating, testing, and treating every case and tracing every contact, as well as readying hospitals and health care professionals.
“Let’s look out for each other, because we need each other,” he said.
The World Health Organization has formally declared the COVID-19 outbreak a pandemic.

“WHO has been assessing this outbreak around the clock and we are deeply concerned both by the alarming levels of spread and severity, and by the alarming levels of inaction,” WHO Director-General Tedros Adhanom Ghebreyesus said during a March 11 press briefing. “We therefore made the assessment that COVID-19 can be characterized as a pandemic.”
He noted that this is the first time a coronavirus has been seen as a pandemic.
The Director-General cautioned that just looking at the number of countries affected, 114 countries, “does not tell the full story. ... We cannot say this loudly enough, or clearly enough, or often enough: All countries can still change the course of this pandemic.”
He reiterated the need for a whole-of-government and a whole-of-society approach to dealing with this, including taking precautions such as isolating, testing, and treating every case and tracing every contact, as well as readying hospitals and health care professionals.
“Let’s look out for each other, because we need each other,” he said.
The World Health Organization has formally declared the COVID-19 outbreak a pandemic.

“WHO has been assessing this outbreak around the clock and we are deeply concerned both by the alarming levels of spread and severity, and by the alarming levels of inaction,” WHO Director-General Tedros Adhanom Ghebreyesus said during a March 11 press briefing. “We therefore made the assessment that COVID-19 can be characterized as a pandemic.”
He noted that this is the first time a coronavirus has been seen as a pandemic.
The Director-General cautioned that just looking at the number of countries affected, 114 countries, “does not tell the full story. ... We cannot say this loudly enough, or clearly enough, or often enough: All countries can still change the course of this pandemic.”
He reiterated the need for a whole-of-government and a whole-of-society approach to dealing with this, including taking precautions such as isolating, testing, and treating every case and tracing every contact, as well as readying hospitals and health care professionals.
“Let’s look out for each other, because we need each other,” he said.
Patients accept artificial intelligence in skin cancer screening
In a small survey, 75% of dermatology patients said they would recommend the use of artificial intelligence (AI) for skin cancer screening to friends and family members, but 94% emphasized the need for symbiosis between doctors, patients, and AI.
AI under investigation in dermatology includes both direct-to-patient and clinician decision-support AI tools for skin cancer screening, but patients’ perceptions of AI in health care remains unclear, Caroline A. Nelson, MD, of Yale University in New Haven, Conn., and colleagues wrote in JAMA Dermatology.
“We sought to elucidate perceived benefits and risks, strengths and weaknesses, implementation, response to conflict between human and AI clinical decision making, and recommendation for or against AI,” the researchers wrote.
They identified 48 patients seen from May 6, 2019, to July 8, 2019, at general dermatology clinics and melanoma clinics. This included 16 patients with a history of melanoma, 16 with a history of nonmelanoma skin cancer, and 16 with no history of skin cancer. The average age of the patients was 53.3 years, 54% were women, and 94% were white.
The researchers interviewed 24 patients about a direct-to-patient AI tool and 24 patients about a clinician decision-support AI tool.
Overall, 36 patients (75%) said they would recommend the AI tool to family and friends, with 17 patients (71%) saying they would recommend the direct-to-patient tool and 19 (79%) saying they would recommend the clinician decision-support tool. Another nine patients (19%) were ambivalent about the AI tools, and three patients (6%) said they would not recommend the tools.
Diagnostic speed and health care access were the most common perceived benefits of AI (by 60% of patients for each), and increased patient anxiety was the most common perceived risk (by 40% of patients). In addition, 69% of patients perceived more accurate diagnosis to be the greatest strength of an AI tool, and 85% perceived less accurate diagnosis to be the greatest weakness.
The study findings were limited by several factors, including the small sample size, qualitative design, use of a hypothetical rather than real-world situation, and a homogeneous study population, the researchers noted. However, the results merit more studies to obtain perspectives from diverse populations, they said.
“This expansion is particularly important in light of concerns raised that AI tools may exacerbate health care disparities in dermatology,” the researchers wrote.
From the patient perspective, the use of AI “may improve health care quality but should be implemented in a manner that preserves the integrity of the human physician-patient relationship,” the authors concluded.
“Although AI technology has not been widely implemented in dermatology yet, it is the pivotal time to assess patients’ views on the subject to understand their knowledge base, as well as values, preferences, and concerns regarding AI,” wrote Carrie L. Kovarik, MD, of the University of Pennsylvania in Philadelphia, in an accompanying editorial.
“Vulnerable patients, including racial and ethnic minorities, the underinsured or uninsured, economically disadvantaged, and those with chronic health conditions, may be at risk for improper consent for or use of AI,” she wrote.
Dr. Kovarik cited the position statement on augmented intelligence from the American Academy of Dermatology, which states that, for both patients and clinicians, “there should be transparency and choice on how their medical information is gathered, utilized, and stored and when, what, and how augmented intelligence technologies are utilized in their care process. There should be clarity in the symbiotic and synergistic roles of augmented intelligence and human judgment so that it is clear to the patient and provider when and how this technology is utilized to augment human judgment and interpretation.”
Clinicians will need to understand the perspectives on AI from patients of a range of backgrounds to achieve this goal, Dr. Kovarik said.
Dr. Nelson had no financial conflicts to disclose, but her colleagues disclosed relationships with pharmaceutical companies, government agencies, and nonprofit organizations. Dr. Kovarik disclosed serving on the artificial intelligence task force for the American Academy of Dermatology.
SOURCES: Nelson CA et al. JAMA Dermatol. 2020 Mar 11. doi: 10.1001/jamadermatol.2019.5014; Kovarik CL. JAMA Dermatol. 2020 Mar 11. doi: 10.1001/jamadermatol.2019.5013.
In a small survey, 75% of dermatology patients said they would recommend the use of artificial intelligence (AI) for skin cancer screening to friends and family members, but 94% emphasized the need for symbiosis between doctors, patients, and AI.
AI under investigation in dermatology includes both direct-to-patient and clinician decision-support AI tools for skin cancer screening, but patients’ perceptions of AI in health care remains unclear, Caroline A. Nelson, MD, of Yale University in New Haven, Conn., and colleagues wrote in JAMA Dermatology.
“We sought to elucidate perceived benefits and risks, strengths and weaknesses, implementation, response to conflict between human and AI clinical decision making, and recommendation for or against AI,” the researchers wrote.
They identified 48 patients seen from May 6, 2019, to July 8, 2019, at general dermatology clinics and melanoma clinics. This included 16 patients with a history of melanoma, 16 with a history of nonmelanoma skin cancer, and 16 with no history of skin cancer. The average age of the patients was 53.3 years, 54% were women, and 94% were white.
The researchers interviewed 24 patients about a direct-to-patient AI tool and 24 patients about a clinician decision-support AI tool.
Overall, 36 patients (75%) said they would recommend the AI tool to family and friends, with 17 patients (71%) saying they would recommend the direct-to-patient tool and 19 (79%) saying they would recommend the clinician decision-support tool. Another nine patients (19%) were ambivalent about the AI tools, and three patients (6%) said they would not recommend the tools.
Diagnostic speed and health care access were the most common perceived benefits of AI (by 60% of patients for each), and increased patient anxiety was the most common perceived risk (by 40% of patients). In addition, 69% of patients perceived more accurate diagnosis to be the greatest strength of an AI tool, and 85% perceived less accurate diagnosis to be the greatest weakness.
The study findings were limited by several factors, including the small sample size, qualitative design, use of a hypothetical rather than real-world situation, and a homogeneous study population, the researchers noted. However, the results merit more studies to obtain perspectives from diverse populations, they said.
“This expansion is particularly important in light of concerns raised that AI tools may exacerbate health care disparities in dermatology,” the researchers wrote.
From the patient perspective, the use of AI “may improve health care quality but should be implemented in a manner that preserves the integrity of the human physician-patient relationship,” the authors concluded.
“Although AI technology has not been widely implemented in dermatology yet, it is the pivotal time to assess patients’ views on the subject to understand their knowledge base, as well as values, preferences, and concerns regarding AI,” wrote Carrie L. Kovarik, MD, of the University of Pennsylvania in Philadelphia, in an accompanying editorial.
“Vulnerable patients, including racial and ethnic minorities, the underinsured or uninsured, economically disadvantaged, and those with chronic health conditions, may be at risk for improper consent for or use of AI,” she wrote.
Dr. Kovarik cited the position statement on augmented intelligence from the American Academy of Dermatology, which states that, for both patients and clinicians, “there should be transparency and choice on how their medical information is gathered, utilized, and stored and when, what, and how augmented intelligence technologies are utilized in their care process. There should be clarity in the symbiotic and synergistic roles of augmented intelligence and human judgment so that it is clear to the patient and provider when and how this technology is utilized to augment human judgment and interpretation.”
Clinicians will need to understand the perspectives on AI from patients of a range of backgrounds to achieve this goal, Dr. Kovarik said.
Dr. Nelson had no financial conflicts to disclose, but her colleagues disclosed relationships with pharmaceutical companies, government agencies, and nonprofit organizations. Dr. Kovarik disclosed serving on the artificial intelligence task force for the American Academy of Dermatology.
SOURCES: Nelson CA et al. JAMA Dermatol. 2020 Mar 11. doi: 10.1001/jamadermatol.2019.5014; Kovarik CL. JAMA Dermatol. 2020 Mar 11. doi: 10.1001/jamadermatol.2019.5013.
In a small survey, 75% of dermatology patients said they would recommend the use of artificial intelligence (AI) for skin cancer screening to friends and family members, but 94% emphasized the need for symbiosis between doctors, patients, and AI.
AI under investigation in dermatology includes both direct-to-patient and clinician decision-support AI tools for skin cancer screening, but patients’ perceptions of AI in health care remains unclear, Caroline A. Nelson, MD, of Yale University in New Haven, Conn., and colleagues wrote in JAMA Dermatology.
“We sought to elucidate perceived benefits and risks, strengths and weaknesses, implementation, response to conflict between human and AI clinical decision making, and recommendation for or against AI,” the researchers wrote.
They identified 48 patients seen from May 6, 2019, to July 8, 2019, at general dermatology clinics and melanoma clinics. This included 16 patients with a history of melanoma, 16 with a history of nonmelanoma skin cancer, and 16 with no history of skin cancer. The average age of the patients was 53.3 years, 54% were women, and 94% were white.
The researchers interviewed 24 patients about a direct-to-patient AI tool and 24 patients about a clinician decision-support AI tool.
Overall, 36 patients (75%) said they would recommend the AI tool to family and friends, with 17 patients (71%) saying they would recommend the direct-to-patient tool and 19 (79%) saying they would recommend the clinician decision-support tool. Another nine patients (19%) were ambivalent about the AI tools, and three patients (6%) said they would not recommend the tools.
Diagnostic speed and health care access were the most common perceived benefits of AI (by 60% of patients for each), and increased patient anxiety was the most common perceived risk (by 40% of patients). In addition, 69% of patients perceived more accurate diagnosis to be the greatest strength of an AI tool, and 85% perceived less accurate diagnosis to be the greatest weakness.
The study findings were limited by several factors, including the small sample size, qualitative design, use of a hypothetical rather than real-world situation, and a homogeneous study population, the researchers noted. However, the results merit more studies to obtain perspectives from diverse populations, they said.
“This expansion is particularly important in light of concerns raised that AI tools may exacerbate health care disparities in dermatology,” the researchers wrote.
From the patient perspective, the use of AI “may improve health care quality but should be implemented in a manner that preserves the integrity of the human physician-patient relationship,” the authors concluded.
“Although AI technology has not been widely implemented in dermatology yet, it is the pivotal time to assess patients’ views on the subject to understand their knowledge base, as well as values, preferences, and concerns regarding AI,” wrote Carrie L. Kovarik, MD, of the University of Pennsylvania in Philadelphia, in an accompanying editorial.
“Vulnerable patients, including racial and ethnic minorities, the underinsured or uninsured, economically disadvantaged, and those with chronic health conditions, may be at risk for improper consent for or use of AI,” she wrote.
Dr. Kovarik cited the position statement on augmented intelligence from the American Academy of Dermatology, which states that, for both patients and clinicians, “there should be transparency and choice on how their medical information is gathered, utilized, and stored and when, what, and how augmented intelligence technologies are utilized in their care process. There should be clarity in the symbiotic and synergistic roles of augmented intelligence and human judgment so that it is clear to the patient and provider when and how this technology is utilized to augment human judgment and interpretation.”
Clinicians will need to understand the perspectives on AI from patients of a range of backgrounds to achieve this goal, Dr. Kovarik said.
Dr. Nelson had no financial conflicts to disclose, but her colleagues disclosed relationships with pharmaceutical companies, government agencies, and nonprofit organizations. Dr. Kovarik disclosed serving on the artificial intelligence task force for the American Academy of Dermatology.
SOURCES: Nelson CA et al. JAMA Dermatol. 2020 Mar 11. doi: 10.1001/jamadermatol.2019.5014; Kovarik CL. JAMA Dermatol. 2020 Mar 11. doi: 10.1001/jamadermatol.2019.5013.
FROM JAMA DERMATOLOGY
AAD-NPF releases first guidelines for nonbiologic treatments of psoriasis
It’s been 11 years since monotherapy and suggest a framework for a number of off-label treatments.
The guidelines, issued jointly with the National Psoriasis Foundation (NPF), were published in the Journal of the American Academy of Dermatology.
“I think we are way behind,” Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, and cochair of the guideline writing committee, said in an interview. “Most other countries update their guidelines every 1 or 2 years; we were 10 years behind.” The guidelines for systemic nonbiologic drugs follow up psoriasis guidelines issued by the AAD and the NPF on pediatric patients issued earlier this year, and on phototherapy, biologic treatments, and management of comorbidities issued last year.
“A lot has happened in the last 10 years,” said cochair Craig Elmets, MD, professor of dermatology at the University of Alabama at Birmingham. “While much of the interest is on biologic agents, nonbiologics are still used quite frequently, and the guidelines for their appropriate use have changed. Use of the guidelines provides people in the health profession with the most up to date evidence-based information so they can give their patients the best care.”
The guidelines acknowledge that the medications it covers are still widely used, either by themselves or in combination with biologic agents; readily available; easy to use; and, in the case of older therapies, relatively cheap.
Methotrexate has been available since the 1970s. Given as an injection or taken orally, the guidelines recommend supplementation with folic acid to counteract methotrexate’s side effects, particularly GI upset. The guidelines note that folic acid is less expensive than folinic acid. Combination therapy with methotrexate and tumor necrosis factor (TNF) inhibitors is more effective than methotrexate monotherapy, with a similar side effect profile, the guidelines state.
Methotrexate is more widely used outside the United States, “but it is a very good, quick fix and it’s much safer in children and young people than it is in people with cardiovascular disease,” Dr. Menter noted. “It’s still the most commonly used drug worldwide because it’s cheap, and you do have to worry about the long-term toxicity which is related the liver issues.”
The guidelines say that subcutaneous administration of methotrexate “may be particularly useful” for patients on higher doses, which when taken orally, are associated with a higher risk of GI effects.
Dr. Menter referred to a 2017 study, which reported 41% of patients treated with subcutaneous methotrexate once a week achieved a Psoriasis Area and Severity Index 75 score of 41% after a year of treatment, compared with 10% of those on placebo (Lancet. 2017 Feb 4;389[10068]:528-37).
The guidelines rate strength of recommendation as class A for methotrexate for moderate to severe psoriasis in adults, recommend supplementation with folic or folinic acid to counteract GI complications and liver problems, and note that adalimumab and infliximab are more effective than methotrexate for cutaneous psoriasis. Class B recommendations for methotrexate and psoriasis include statements that patients should begin with a test dose, especially if they have impaired kidney function; methotrexate is effective for peripheral, but not axial, psoriatic arthritis (PsA); and TNF inhibitors are more effective than methotrexate for PsA.
Approved by the FDA in 2014 for psoriasis, apremilast, which inhibits phosphodiesterase-4, is the newest drug in the recommendations. The guidelines recommend its use for moderate to severe psoriasis in adults, with a class A recommendation. Patients should start on a low dose and then build up to the 30-mg, twice-daily dose over 6 days and should be counseled about the risk of depression before starting treatment. Routine laboratory testing can be considered on an individual basis.
The guidelines also lay out three recommendations (and strength of recommendation) for cyclosporine, a drug that’s been around since the 1990s: for severe, recalcitrant cases (class A); for erythrodermic, general pustular, and palmoplantar psoriasis (class B); and as short-term therapy for psoriasis flare in patients already on another drug (class C).
Acitretin is another longstanding therapy used mostly for palmar-plantar psoriasis, but it can also be used as monotherapy for plaque psoriasis as well as erythrodermic and pustular disease. It can also be used in combination with psoralens with UVA for psoriasis and combined with broadband UVB phototherapy for plaque psoriasis. The acitretin recommendations are class B.
The oral Janus kinase (JAK) inhibitor tofacitinib isn’t specifically approved for psoriasis, but it is approved for RA, PsA, and ulcerative colitis. The drug targets the JAK-STAT signaling pathway that causes inflammation. The guidelines state that tofacitinib can be considered for moderate to severe psoriasis, but lists no strength of recommendation. The recommended dose is either 5 or 10 mg orally twice a day, with a caveat that the higher dose carries a higher risk of adverse events. Patients should be evaluated for getting a zoster vaccine before they begin therapy.
“We thought that, because there was probably a small chance that it might get approved for psoriasis, that we would discuss it briefly,” Dr. Menter said of tofacitinib.
Another off-label use the guidelines address is for fumaric and acid esters, also known as fumarates, which are used to in Europe to treat moderate to severe psoriasis. Dimethyl fumarate is approved for relapsing forms of multiple sclerosis in the United States. The guidelines state that fumarates can be used for psoriasis, but offer no strength of recommendation. Side effects include gastrointestinal disturbance and flushing.
Other treatments that are also addressed in the guidelines include a host of systemic immunosuppressants and antimetabolites: azathioprine, hydroxyurea, leflunomide, mycophenolate mofetil, thioguanine, and tacrolimus, none of which are FDA approved for psoriasis. They’re rarely used for psoriasis, but may have value in selected cases, the guidelines state.
Dr. Menter said that apremilast is the only oral drug in the guidelines, but they are the wave of the future for treating psoriasis. “I think there’s a tremendous potential for new oral drugs – TK2 [thymidine kinase], the JAK inhibitors, and other drugs coming down the pipelines. The majority of patients, if you ask them their preference, would like to take an oral drug rather than an injectable drug. And it would be much easier for dermatologists, they wouldn’t have to train patients on how to do the injections.”
Dr. Menter and Dr. Elmets disclosed financial relationships with numerous pharmaceutical companies. Other authors/work group members also had disclosures related to pharmaceutical manufacturers, and several had no disclosures.
SOURCE: Menter A et al. J Am Acad Dermatol. 2020 Feb 28. doi: 10.1016/j.jaad.2020.02.044.
It’s been 11 years since monotherapy and suggest a framework for a number of off-label treatments.
The guidelines, issued jointly with the National Psoriasis Foundation (NPF), were published in the Journal of the American Academy of Dermatology.
“I think we are way behind,” Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, and cochair of the guideline writing committee, said in an interview. “Most other countries update their guidelines every 1 or 2 years; we were 10 years behind.” The guidelines for systemic nonbiologic drugs follow up psoriasis guidelines issued by the AAD and the NPF on pediatric patients issued earlier this year, and on phototherapy, biologic treatments, and management of comorbidities issued last year.
“A lot has happened in the last 10 years,” said cochair Craig Elmets, MD, professor of dermatology at the University of Alabama at Birmingham. “While much of the interest is on biologic agents, nonbiologics are still used quite frequently, and the guidelines for their appropriate use have changed. Use of the guidelines provides people in the health profession with the most up to date evidence-based information so they can give their patients the best care.”
The guidelines acknowledge that the medications it covers are still widely used, either by themselves or in combination with biologic agents; readily available; easy to use; and, in the case of older therapies, relatively cheap.
Methotrexate has been available since the 1970s. Given as an injection or taken orally, the guidelines recommend supplementation with folic acid to counteract methotrexate’s side effects, particularly GI upset. The guidelines note that folic acid is less expensive than folinic acid. Combination therapy with methotrexate and tumor necrosis factor (TNF) inhibitors is more effective than methotrexate monotherapy, with a similar side effect profile, the guidelines state.
Methotrexate is more widely used outside the United States, “but it is a very good, quick fix and it’s much safer in children and young people than it is in people with cardiovascular disease,” Dr. Menter noted. “It’s still the most commonly used drug worldwide because it’s cheap, and you do have to worry about the long-term toxicity which is related the liver issues.”
The guidelines say that subcutaneous administration of methotrexate “may be particularly useful” for patients on higher doses, which when taken orally, are associated with a higher risk of GI effects.
Dr. Menter referred to a 2017 study, which reported 41% of patients treated with subcutaneous methotrexate once a week achieved a Psoriasis Area and Severity Index 75 score of 41% after a year of treatment, compared with 10% of those on placebo (Lancet. 2017 Feb 4;389[10068]:528-37).
The guidelines rate strength of recommendation as class A for methotrexate for moderate to severe psoriasis in adults, recommend supplementation with folic or folinic acid to counteract GI complications and liver problems, and note that adalimumab and infliximab are more effective than methotrexate for cutaneous psoriasis. Class B recommendations for methotrexate and psoriasis include statements that patients should begin with a test dose, especially if they have impaired kidney function; methotrexate is effective for peripheral, but not axial, psoriatic arthritis (PsA); and TNF inhibitors are more effective than methotrexate for PsA.
Approved by the FDA in 2014 for psoriasis, apremilast, which inhibits phosphodiesterase-4, is the newest drug in the recommendations. The guidelines recommend its use for moderate to severe psoriasis in adults, with a class A recommendation. Patients should start on a low dose and then build up to the 30-mg, twice-daily dose over 6 days and should be counseled about the risk of depression before starting treatment. Routine laboratory testing can be considered on an individual basis.
The guidelines also lay out three recommendations (and strength of recommendation) for cyclosporine, a drug that’s been around since the 1990s: for severe, recalcitrant cases (class A); for erythrodermic, general pustular, and palmoplantar psoriasis (class B); and as short-term therapy for psoriasis flare in patients already on another drug (class C).
Acitretin is another longstanding therapy used mostly for palmar-plantar psoriasis, but it can also be used as monotherapy for plaque psoriasis as well as erythrodermic and pustular disease. It can also be used in combination with psoralens with UVA for psoriasis and combined with broadband UVB phototherapy for plaque psoriasis. The acitretin recommendations are class B.
The oral Janus kinase (JAK) inhibitor tofacitinib isn’t specifically approved for psoriasis, but it is approved for RA, PsA, and ulcerative colitis. The drug targets the JAK-STAT signaling pathway that causes inflammation. The guidelines state that tofacitinib can be considered for moderate to severe psoriasis, but lists no strength of recommendation. The recommended dose is either 5 or 10 mg orally twice a day, with a caveat that the higher dose carries a higher risk of adverse events. Patients should be evaluated for getting a zoster vaccine before they begin therapy.
“We thought that, because there was probably a small chance that it might get approved for psoriasis, that we would discuss it briefly,” Dr. Menter said of tofacitinib.
Another off-label use the guidelines address is for fumaric and acid esters, also known as fumarates, which are used to in Europe to treat moderate to severe psoriasis. Dimethyl fumarate is approved for relapsing forms of multiple sclerosis in the United States. The guidelines state that fumarates can be used for psoriasis, but offer no strength of recommendation. Side effects include gastrointestinal disturbance and flushing.
Other treatments that are also addressed in the guidelines include a host of systemic immunosuppressants and antimetabolites: azathioprine, hydroxyurea, leflunomide, mycophenolate mofetil, thioguanine, and tacrolimus, none of which are FDA approved for psoriasis. They’re rarely used for psoriasis, but may have value in selected cases, the guidelines state.
Dr. Menter said that apremilast is the only oral drug in the guidelines, but they are the wave of the future for treating psoriasis. “I think there’s a tremendous potential for new oral drugs – TK2 [thymidine kinase], the JAK inhibitors, and other drugs coming down the pipelines. The majority of patients, if you ask them their preference, would like to take an oral drug rather than an injectable drug. And it would be much easier for dermatologists, they wouldn’t have to train patients on how to do the injections.”
Dr. Menter and Dr. Elmets disclosed financial relationships with numerous pharmaceutical companies. Other authors/work group members also had disclosures related to pharmaceutical manufacturers, and several had no disclosures.
SOURCE: Menter A et al. J Am Acad Dermatol. 2020 Feb 28. doi: 10.1016/j.jaad.2020.02.044.
It’s been 11 years since monotherapy and suggest a framework for a number of off-label treatments.
The guidelines, issued jointly with the National Psoriasis Foundation (NPF), were published in the Journal of the American Academy of Dermatology.
“I think we are way behind,” Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, and cochair of the guideline writing committee, said in an interview. “Most other countries update their guidelines every 1 or 2 years; we were 10 years behind.” The guidelines for systemic nonbiologic drugs follow up psoriasis guidelines issued by the AAD and the NPF on pediatric patients issued earlier this year, and on phototherapy, biologic treatments, and management of comorbidities issued last year.
“A lot has happened in the last 10 years,” said cochair Craig Elmets, MD, professor of dermatology at the University of Alabama at Birmingham. “While much of the interest is on biologic agents, nonbiologics are still used quite frequently, and the guidelines for their appropriate use have changed. Use of the guidelines provides people in the health profession with the most up to date evidence-based information so they can give their patients the best care.”
The guidelines acknowledge that the medications it covers are still widely used, either by themselves or in combination with biologic agents; readily available; easy to use; and, in the case of older therapies, relatively cheap.
Methotrexate has been available since the 1970s. Given as an injection or taken orally, the guidelines recommend supplementation with folic acid to counteract methotrexate’s side effects, particularly GI upset. The guidelines note that folic acid is less expensive than folinic acid. Combination therapy with methotrexate and tumor necrosis factor (TNF) inhibitors is more effective than methotrexate monotherapy, with a similar side effect profile, the guidelines state.
Methotrexate is more widely used outside the United States, “but it is a very good, quick fix and it’s much safer in children and young people than it is in people with cardiovascular disease,” Dr. Menter noted. “It’s still the most commonly used drug worldwide because it’s cheap, and you do have to worry about the long-term toxicity which is related the liver issues.”
The guidelines say that subcutaneous administration of methotrexate “may be particularly useful” for patients on higher doses, which when taken orally, are associated with a higher risk of GI effects.
Dr. Menter referred to a 2017 study, which reported 41% of patients treated with subcutaneous methotrexate once a week achieved a Psoriasis Area and Severity Index 75 score of 41% after a year of treatment, compared with 10% of those on placebo (Lancet. 2017 Feb 4;389[10068]:528-37).
The guidelines rate strength of recommendation as class A for methotrexate for moderate to severe psoriasis in adults, recommend supplementation with folic or folinic acid to counteract GI complications and liver problems, and note that adalimumab and infliximab are more effective than methotrexate for cutaneous psoriasis. Class B recommendations for methotrexate and psoriasis include statements that patients should begin with a test dose, especially if they have impaired kidney function; methotrexate is effective for peripheral, but not axial, psoriatic arthritis (PsA); and TNF inhibitors are more effective than methotrexate for PsA.
Approved by the FDA in 2014 for psoriasis, apremilast, which inhibits phosphodiesterase-4, is the newest drug in the recommendations. The guidelines recommend its use for moderate to severe psoriasis in adults, with a class A recommendation. Patients should start on a low dose and then build up to the 30-mg, twice-daily dose over 6 days and should be counseled about the risk of depression before starting treatment. Routine laboratory testing can be considered on an individual basis.
The guidelines also lay out three recommendations (and strength of recommendation) for cyclosporine, a drug that’s been around since the 1990s: for severe, recalcitrant cases (class A); for erythrodermic, general pustular, and palmoplantar psoriasis (class B); and as short-term therapy for psoriasis flare in patients already on another drug (class C).
Acitretin is another longstanding therapy used mostly for palmar-plantar psoriasis, but it can also be used as monotherapy for plaque psoriasis as well as erythrodermic and pustular disease. It can also be used in combination with psoralens with UVA for psoriasis and combined with broadband UVB phototherapy for plaque psoriasis. The acitretin recommendations are class B.
The oral Janus kinase (JAK) inhibitor tofacitinib isn’t specifically approved for psoriasis, but it is approved for RA, PsA, and ulcerative colitis. The drug targets the JAK-STAT signaling pathway that causes inflammation. The guidelines state that tofacitinib can be considered for moderate to severe psoriasis, but lists no strength of recommendation. The recommended dose is either 5 or 10 mg orally twice a day, with a caveat that the higher dose carries a higher risk of adverse events. Patients should be evaluated for getting a zoster vaccine before they begin therapy.
“We thought that, because there was probably a small chance that it might get approved for psoriasis, that we would discuss it briefly,” Dr. Menter said of tofacitinib.
Another off-label use the guidelines address is for fumaric and acid esters, also known as fumarates, which are used to in Europe to treat moderate to severe psoriasis. Dimethyl fumarate is approved for relapsing forms of multiple sclerosis in the United States. The guidelines state that fumarates can be used for psoriasis, but offer no strength of recommendation. Side effects include gastrointestinal disturbance and flushing.
Other treatments that are also addressed in the guidelines include a host of systemic immunosuppressants and antimetabolites: azathioprine, hydroxyurea, leflunomide, mycophenolate mofetil, thioguanine, and tacrolimus, none of which are FDA approved for psoriasis. They’re rarely used for psoriasis, but may have value in selected cases, the guidelines state.
Dr. Menter said that apremilast is the only oral drug in the guidelines, but they are the wave of the future for treating psoriasis. “I think there’s a tremendous potential for new oral drugs – TK2 [thymidine kinase], the JAK inhibitors, and other drugs coming down the pipelines. The majority of patients, if you ask them their preference, would like to take an oral drug rather than an injectable drug. And it would be much easier for dermatologists, they wouldn’t have to train patients on how to do the injections.”
Dr. Menter and Dr. Elmets disclosed financial relationships with numerous pharmaceutical companies. Other authors/work group members also had disclosures related to pharmaceutical manufacturers, and several had no disclosures.
SOURCE: Menter A et al. J Am Acad Dermatol. 2020 Feb 28. doi: 10.1016/j.jaad.2020.02.044.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY