User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
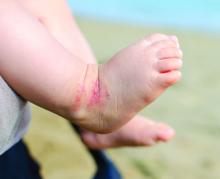
How Does Moderate to Severe Eczema Affect Growth in Children?
FROM AAD 2024
SAN DIEGO — , results from an ongoing 10-year observational study showed.
“We need to sort out whether this is reversed by newer treatments, especially in the 6- to 11-year-olds, as well as the factors that underlie it in atopic dermatitis,” said the study’s first author Amy S. Paller, MD, chair of dermatology, Northwestern University, Chicago, Illinois, following the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
Atopic Dermatitis Impacts Growth
In the ongoing international study called PEDISTAD, researchers enrolled 1326 children younger than 12 years with moderate to severe atopic dermatitis inadequately controlled by topical therapies who were candidates to receive systemic medications. They assessed the percentage of patients above the 50th percentile and the mean percentiles for height, weight, and body mass index (BMI) at baseline against the Centers for Disease Control and Prevention’s (CDC’s) Learning Management System reference healthy population, by age in months, and compared results to the CDC’s standardized growth curves for healthy children aged 0-12 years.
The investigators found that at baseline, compared with the age-specific population norms, 50% of men and 51% of women in the PEDISTAD study were above the 50th percentile for weight, but only 38% and 52%, respectively, were above the 50th percentile for height. Among patients aged 5-12 years, only 28% of men and 47% of women were above the 50th percentile for height, while 69% of men and 71% of women were above the 50th percentile for BMI.
Dr. Paller said that she was “not really surprised by the reduction in linear growth, since there are so many factors that may contribute,” including chronic inflammation, poor sleep, and the use of topical and systemic steroids. “But [it’s] good to have this data as an opportunity to see if our improved therapies can reverse this.”
She said that she was “a bit surprised by the increase in weight and body mass index, but this could reflect less physical activity/sports [participation and] deserves more investigation,” and added that the findings “mesh nicely with new attention on bone growth with good control of atopic dermatitis in this age group.”
Dr. Paller acknowledged certain limitations of the study, including the fact that those enrolled are a heterogeneous cohort with variable treatment regimens.
Some Answers, More Questions
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, who was asked to comment on the findings, said that atopic dermatitis “should be considered the cutaneous manifestations of a systemic inflammatory disease, though even if it were not, the impact on daily and nightly activities [such as sleep] could indirectly have systemic medical consequences.”
The data presented “highlights that children with moderate to severe disease have higher BMIs and shorter height than matched counterparts, likely owing to the treasure trove of direct and indirect consequences of uncontrolled type 2 inflammation,” he said. “What I would like to know, and as the authors astutely noted, could treatment, and especially early intervention, prevent or even alter this impact?”
Dr. Paller disclosed that she is a consultant for several pharmaceutical companies, including Sanofi and Regeneron, the study sponsor. She is also an investigator for AbbVie, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, LEO Pharma, and UCB and is a member of the data monitoring safety board for AbbVie, Abeona, Catawba, Galderma, and InMed. Dr. Friedman, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , results from an ongoing 10-year observational study showed.
“We need to sort out whether this is reversed by newer treatments, especially in the 6- to 11-year-olds, as well as the factors that underlie it in atopic dermatitis,” said the study’s first author Amy S. Paller, MD, chair of dermatology, Northwestern University, Chicago, Illinois, following the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
Atopic Dermatitis Impacts Growth
In the ongoing international study called PEDISTAD, researchers enrolled 1326 children younger than 12 years with moderate to severe atopic dermatitis inadequately controlled by topical therapies who were candidates to receive systemic medications. They assessed the percentage of patients above the 50th percentile and the mean percentiles for height, weight, and body mass index (BMI) at baseline against the Centers for Disease Control and Prevention’s (CDC’s) Learning Management System reference healthy population, by age in months, and compared results to the CDC’s standardized growth curves for healthy children aged 0-12 years.
The investigators found that at baseline, compared with the age-specific population norms, 50% of men and 51% of women in the PEDISTAD study were above the 50th percentile for weight, but only 38% and 52%, respectively, were above the 50th percentile for height. Among patients aged 5-12 years, only 28% of men and 47% of women were above the 50th percentile for height, while 69% of men and 71% of women were above the 50th percentile for BMI.
Dr. Paller said that she was “not really surprised by the reduction in linear growth, since there are so many factors that may contribute,” including chronic inflammation, poor sleep, and the use of topical and systemic steroids. “But [it’s] good to have this data as an opportunity to see if our improved therapies can reverse this.”
She said that she was “a bit surprised by the increase in weight and body mass index, but this could reflect less physical activity/sports [participation and] deserves more investigation,” and added that the findings “mesh nicely with new attention on bone growth with good control of atopic dermatitis in this age group.”
Dr. Paller acknowledged certain limitations of the study, including the fact that those enrolled are a heterogeneous cohort with variable treatment regimens.
Some Answers, More Questions
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, who was asked to comment on the findings, said that atopic dermatitis “should be considered the cutaneous manifestations of a systemic inflammatory disease, though even if it were not, the impact on daily and nightly activities [such as sleep] could indirectly have systemic medical consequences.”
The data presented “highlights that children with moderate to severe disease have higher BMIs and shorter height than matched counterparts, likely owing to the treasure trove of direct and indirect consequences of uncontrolled type 2 inflammation,” he said. “What I would like to know, and as the authors astutely noted, could treatment, and especially early intervention, prevent or even alter this impact?”
Dr. Paller disclosed that she is a consultant for several pharmaceutical companies, including Sanofi and Regeneron, the study sponsor. She is also an investigator for AbbVie, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, LEO Pharma, and UCB and is a member of the data monitoring safety board for AbbVie, Abeona, Catawba, Galderma, and InMed. Dr. Friedman, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , results from an ongoing 10-year observational study showed.
“We need to sort out whether this is reversed by newer treatments, especially in the 6- to 11-year-olds, as well as the factors that underlie it in atopic dermatitis,” said the study’s first author Amy S. Paller, MD, chair of dermatology, Northwestern University, Chicago, Illinois, following the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
Atopic Dermatitis Impacts Growth
In the ongoing international study called PEDISTAD, researchers enrolled 1326 children younger than 12 years with moderate to severe atopic dermatitis inadequately controlled by topical therapies who were candidates to receive systemic medications. They assessed the percentage of patients above the 50th percentile and the mean percentiles for height, weight, and body mass index (BMI) at baseline against the Centers for Disease Control and Prevention’s (CDC’s) Learning Management System reference healthy population, by age in months, and compared results to the CDC’s standardized growth curves for healthy children aged 0-12 years.
The investigators found that at baseline, compared with the age-specific population norms, 50% of men and 51% of women in the PEDISTAD study were above the 50th percentile for weight, but only 38% and 52%, respectively, were above the 50th percentile for height. Among patients aged 5-12 years, only 28% of men and 47% of women were above the 50th percentile for height, while 69% of men and 71% of women were above the 50th percentile for BMI.
Dr. Paller said that she was “not really surprised by the reduction in linear growth, since there are so many factors that may contribute,” including chronic inflammation, poor sleep, and the use of topical and systemic steroids. “But [it’s] good to have this data as an opportunity to see if our improved therapies can reverse this.”
She said that she was “a bit surprised by the increase in weight and body mass index, but this could reflect less physical activity/sports [participation and] deserves more investigation,” and added that the findings “mesh nicely with new attention on bone growth with good control of atopic dermatitis in this age group.”
Dr. Paller acknowledged certain limitations of the study, including the fact that those enrolled are a heterogeneous cohort with variable treatment regimens.
Some Answers, More Questions
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, who was asked to comment on the findings, said that atopic dermatitis “should be considered the cutaneous manifestations of a systemic inflammatory disease, though even if it were not, the impact on daily and nightly activities [such as sleep] could indirectly have systemic medical consequences.”
The data presented “highlights that children with moderate to severe disease have higher BMIs and shorter height than matched counterparts, likely owing to the treasure trove of direct and indirect consequences of uncontrolled type 2 inflammation,” he said. “What I would like to know, and as the authors astutely noted, could treatment, and especially early intervention, prevent or even alter this impact?”
Dr. Paller disclosed that she is a consultant for several pharmaceutical companies, including Sanofi and Regeneron, the study sponsor. She is also an investigator for AbbVie, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, LEO Pharma, and UCB and is a member of the data monitoring safety board for AbbVie, Abeona, Catawba, Galderma, and InMed. Dr. Friedman, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
Time to Lung Disease in Patients With Dermatomyositis Subtype Estimated
TOPLINE:
The time interval between onset of interstitial lung disease (ILD) and diagnosis of anti–melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis (DM) “has not been well described,” the authors say.
METHODOLOGY:
- , with the former having a particularly high mortality rate.
- In this retrospective cohort study using electronic medical records, researchers evaluated 774 patients with DM between 2008 and 2023 to learn more about the time interval between ILD and the time of an MDA5 antibody-positive DM diagnosis, which has not been well described.
- The primary outcome was ILD diagnosis and time in days between documented ILD and MDA5 antibody-positive DM diagnoses.
TAKEAWAY:
- Overall, 14 patients with DM (1.8%) were diagnosed with MDA5 antibody-positive DM in dermatology, rheumatology, or pulmonology departments (nine women and five men; age, 24-77 years; 79% were White and 7% were Black).
- ILD was diagnosed in 9 of the 14 patients (64%); 6 of the 14 (43%) met the criteria for RPILD. Two cases were diagnosed concurrently and two prior to MDA5 antibody-positive DM diagnosis.
- The median time between ILD and MDA5 antibody-positive DM diagnoses was 163 days.
- Gottron papules/sign and midfacial erythema were the most common dermatologic findings, and no association was seen between cutaneous signs and type of ILD.
IN PRACTICE:
“Establishing an accurate timeline between MDA5 antibody-positive DM and ILD can promote urgency among dermatologists to evaluate extracutaneous manifestations in their management of patients with DM for more accurate risk stratification and appropriate treatment,” the authors wrote.
SOURCE:
This study, led by Rachel R. Lin, from the University of Miami, Miami, Florida, was published online as a research letter in JAMA Dermatology.
LIMITATIONS:
Study limitations were the study’s retrospective design and small sample size.
DISCLOSURES:
No information on study funding was provided. One author reported personal fees from argenX outside this submitted work. Other authors did not disclose any competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
The time interval between onset of interstitial lung disease (ILD) and diagnosis of anti–melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis (DM) “has not been well described,” the authors say.
METHODOLOGY:
- , with the former having a particularly high mortality rate.
- In this retrospective cohort study using electronic medical records, researchers evaluated 774 patients with DM between 2008 and 2023 to learn more about the time interval between ILD and the time of an MDA5 antibody-positive DM diagnosis, which has not been well described.
- The primary outcome was ILD diagnosis and time in days between documented ILD and MDA5 antibody-positive DM diagnoses.
TAKEAWAY:
- Overall, 14 patients with DM (1.8%) were diagnosed with MDA5 antibody-positive DM in dermatology, rheumatology, or pulmonology departments (nine women and five men; age, 24-77 years; 79% were White and 7% were Black).
- ILD was diagnosed in 9 of the 14 patients (64%); 6 of the 14 (43%) met the criteria for RPILD. Two cases were diagnosed concurrently and two prior to MDA5 antibody-positive DM diagnosis.
- The median time between ILD and MDA5 antibody-positive DM diagnoses was 163 days.
- Gottron papules/sign and midfacial erythema were the most common dermatologic findings, and no association was seen between cutaneous signs and type of ILD.
IN PRACTICE:
“Establishing an accurate timeline between MDA5 antibody-positive DM and ILD can promote urgency among dermatologists to evaluate extracutaneous manifestations in their management of patients with DM for more accurate risk stratification and appropriate treatment,” the authors wrote.
SOURCE:
This study, led by Rachel R. Lin, from the University of Miami, Miami, Florida, was published online as a research letter in JAMA Dermatology.
LIMITATIONS:
Study limitations were the study’s retrospective design and small sample size.
DISCLOSURES:
No information on study funding was provided. One author reported personal fees from argenX outside this submitted work. Other authors did not disclose any competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
The time interval between onset of interstitial lung disease (ILD) and diagnosis of anti–melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis (DM) “has not been well described,” the authors say.
METHODOLOGY:
- , with the former having a particularly high mortality rate.
- In this retrospective cohort study using electronic medical records, researchers evaluated 774 patients with DM between 2008 and 2023 to learn more about the time interval between ILD and the time of an MDA5 antibody-positive DM diagnosis, which has not been well described.
- The primary outcome was ILD diagnosis and time in days between documented ILD and MDA5 antibody-positive DM diagnoses.
TAKEAWAY:
- Overall, 14 patients with DM (1.8%) were diagnosed with MDA5 antibody-positive DM in dermatology, rheumatology, or pulmonology departments (nine women and five men; age, 24-77 years; 79% were White and 7% were Black).
- ILD was diagnosed in 9 of the 14 patients (64%); 6 of the 14 (43%) met the criteria for RPILD. Two cases were diagnosed concurrently and two prior to MDA5 antibody-positive DM diagnosis.
- The median time between ILD and MDA5 antibody-positive DM diagnoses was 163 days.
- Gottron papules/sign and midfacial erythema were the most common dermatologic findings, and no association was seen between cutaneous signs and type of ILD.
IN PRACTICE:
“Establishing an accurate timeline between MDA5 antibody-positive DM and ILD can promote urgency among dermatologists to evaluate extracutaneous manifestations in their management of patients with DM for more accurate risk stratification and appropriate treatment,” the authors wrote.
SOURCE:
This study, led by Rachel R. Lin, from the University of Miami, Miami, Florida, was published online as a research letter in JAMA Dermatology.
LIMITATIONS:
Study limitations were the study’s retrospective design and small sample size.
DISCLOSURES:
No information on study funding was provided. One author reported personal fees from argenX outside this submitted work. Other authors did not disclose any competing interests.
A version of this article appeared on Medscape.com.
Botulinum Toxin, Dermal Fillers Safe in Skin of Color Patients, Review Finds
TOPLINE:
, and more data on Black and Latinx populations are needed, according to a literature review.
METHODOLOGY:
- Understanding the efficacy and safety of cosmetic injectables in diverse skin types is important because individuals identifying as racial and ethnic minorities accounted for 18% of neuromodulator procedures and 22% of soft tissue augmentation procedures in 2020 in the United States.
- Researchers reviewed available literature on the usability and efficacy of neuromodulators and soft tissue augmentation in individuals with SOC because of the limited data available in these populations, particularly non-Asian, SOC populations.
- Overall, 88 studies in English were included, which were either dedicated to discussing safety and/or efficacy of injectables in SOC populations or enrolled more than 20% of participants from SOC populations.
- High-quality level I and II evidence was found in 50 studies, and 9940 patients were analyzed in this review.
TAKEAWAY:
- Studies considered high quality indicated that botulinum toxin is safe and effective for treating glabellar lines in Asians; tailored guidelines recommended specific strategies; and adverse events, such as eyelid issues, were more common in Asians.
- Hyaluronic acid fillers showed significant improvement in moderate to severe cases of nasolabial folds in Asians, and adverse effects like swelling and pain were mild to moderate — some cases of granuloma formation and vascular compromise have been reported.
- In Black individuals, botulinum toxin was well tolerated; hyaluronic acid fillers showed favorable safety, with mild to moderate adverse events; and measures like slower injections and subdermal techniques minimized risks.
- In Latinx populations, there was a lack of robust study data on safety and efficacy of botulinum toxin, whereas hyaluronic acid and poly-L-lactic acid fillers were well tolerated.
IN PRACTICE:
“Neuromodulators and dermal fillers are useful and safe as cosmetic and antiaging treatments in SOC populations, with the greatest amount of data supporting its use in Asian populations,” although more data on Black and Latinx populations are needed, the authors concluded. “During cosmetic consultations, physicians should consider the impact of different cultural beauty norms on the aesthetic goals of diverse patient populations,” they added.
SOURCE:
This study led by Shanice McKenzie, MD, from the Department of Dermatology, University of Southern California, Los Angeles, California, was published online in the Journal of Cosmetic Dermatology.
LIMITATIONS:
Most of the recent data and formal consensus guidelines on injectables in the review came from Asian countries, and there was “a relative paucity of research on Black and Latinx populations,” the authors noted.
DISCLOSURES:
The study did not receive any funding. Two authors declared serving as a consultant, investigator, and/or speaker for various companies; the rest had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
, and more data on Black and Latinx populations are needed, according to a literature review.
METHODOLOGY:
- Understanding the efficacy and safety of cosmetic injectables in diverse skin types is important because individuals identifying as racial and ethnic minorities accounted for 18% of neuromodulator procedures and 22% of soft tissue augmentation procedures in 2020 in the United States.
- Researchers reviewed available literature on the usability and efficacy of neuromodulators and soft tissue augmentation in individuals with SOC because of the limited data available in these populations, particularly non-Asian, SOC populations.
- Overall, 88 studies in English were included, which were either dedicated to discussing safety and/or efficacy of injectables in SOC populations or enrolled more than 20% of participants from SOC populations.
- High-quality level I and II evidence was found in 50 studies, and 9940 patients were analyzed in this review.
TAKEAWAY:
- Studies considered high quality indicated that botulinum toxin is safe and effective for treating glabellar lines in Asians; tailored guidelines recommended specific strategies; and adverse events, such as eyelid issues, were more common in Asians.
- Hyaluronic acid fillers showed significant improvement in moderate to severe cases of nasolabial folds in Asians, and adverse effects like swelling and pain were mild to moderate — some cases of granuloma formation and vascular compromise have been reported.
- In Black individuals, botulinum toxin was well tolerated; hyaluronic acid fillers showed favorable safety, with mild to moderate adverse events; and measures like slower injections and subdermal techniques minimized risks.
- In Latinx populations, there was a lack of robust study data on safety and efficacy of botulinum toxin, whereas hyaluronic acid and poly-L-lactic acid fillers were well tolerated.
IN PRACTICE:
“Neuromodulators and dermal fillers are useful and safe as cosmetic and antiaging treatments in SOC populations, with the greatest amount of data supporting its use in Asian populations,” although more data on Black and Latinx populations are needed, the authors concluded. “During cosmetic consultations, physicians should consider the impact of different cultural beauty norms on the aesthetic goals of diverse patient populations,” they added.
SOURCE:
This study led by Shanice McKenzie, MD, from the Department of Dermatology, University of Southern California, Los Angeles, California, was published online in the Journal of Cosmetic Dermatology.
LIMITATIONS:
Most of the recent data and formal consensus guidelines on injectables in the review came from Asian countries, and there was “a relative paucity of research on Black and Latinx populations,” the authors noted.
DISCLOSURES:
The study did not receive any funding. Two authors declared serving as a consultant, investigator, and/or speaker for various companies; the rest had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
, and more data on Black and Latinx populations are needed, according to a literature review.
METHODOLOGY:
- Understanding the efficacy and safety of cosmetic injectables in diverse skin types is important because individuals identifying as racial and ethnic minorities accounted for 18% of neuromodulator procedures and 22% of soft tissue augmentation procedures in 2020 in the United States.
- Researchers reviewed available literature on the usability and efficacy of neuromodulators and soft tissue augmentation in individuals with SOC because of the limited data available in these populations, particularly non-Asian, SOC populations.
- Overall, 88 studies in English were included, which were either dedicated to discussing safety and/or efficacy of injectables in SOC populations or enrolled more than 20% of participants from SOC populations.
- High-quality level I and II evidence was found in 50 studies, and 9940 patients were analyzed in this review.
TAKEAWAY:
- Studies considered high quality indicated that botulinum toxin is safe and effective for treating glabellar lines in Asians; tailored guidelines recommended specific strategies; and adverse events, such as eyelid issues, were more common in Asians.
- Hyaluronic acid fillers showed significant improvement in moderate to severe cases of nasolabial folds in Asians, and adverse effects like swelling and pain were mild to moderate — some cases of granuloma formation and vascular compromise have been reported.
- In Black individuals, botulinum toxin was well tolerated; hyaluronic acid fillers showed favorable safety, with mild to moderate adverse events; and measures like slower injections and subdermal techniques minimized risks.
- In Latinx populations, there was a lack of robust study data on safety and efficacy of botulinum toxin, whereas hyaluronic acid and poly-L-lactic acid fillers were well tolerated.
IN PRACTICE:
“Neuromodulators and dermal fillers are useful and safe as cosmetic and antiaging treatments in SOC populations, with the greatest amount of data supporting its use in Asian populations,” although more data on Black and Latinx populations are needed, the authors concluded. “During cosmetic consultations, physicians should consider the impact of different cultural beauty norms on the aesthetic goals of diverse patient populations,” they added.
SOURCE:
This study led by Shanice McKenzie, MD, from the Department of Dermatology, University of Southern California, Los Angeles, California, was published online in the Journal of Cosmetic Dermatology.
LIMITATIONS:
Most of the recent data and formal consensus guidelines on injectables in the review came from Asian countries, and there was “a relative paucity of research on Black and Latinx populations,” the authors noted.
DISCLOSURES:
The study did not receive any funding. Two authors declared serving as a consultant, investigator, and/or speaker for various companies; the rest had no disclosures to report.
A version of this article appeared on Medscape.com.
Keratoacanthoma, SCC Relatively Rare With PD-1/PD-L1 Inhibitors, Study Suggests
TOPLINE:
(AEs) reported to the US Food and Drug Administration (FDA).
METHODOLOGY:
- The risk for dermatologic immune-related side effects may be increased with immunologic-modifying drugs.
- To determine if there are significant signals between keratoacanthomas and cSCCs and PD-1/PD-L1 inhibitors, researchers analyzed AEs associated with these agents reported to the FDA’s Adverse Event Reporting System (FAERS) between January 2004 and May 2023.
- Pharmacovigilance signals were identified, and a significant signal was defined as the lower 95% CI of a reporting odds ratio (ROR) greater than one or the lower 95% CI of an information component (IC) greater than 0.
TAKEAWAY:
- Of the 158,000 reports of PD-1/PD-L1 inhibitor use, 43 were in patients who developed a keratoacanthoma (mean age, 77 years; 39% women) and 83 were in patients who developed cSCC (mean age, 71 years; 41% women). Patients aged 60-79 years were most likely to develop keratoacanthomas and cSCC on these treatments.
- A PD-1/PD-L1 inhibitor was listed as the suspect drug in all 43 keratoacanthoma reports and in 70 of 83 cSCC reports (the remaining 13 listed them as the concomitant drug).
- Significant signals were reported for both keratoacanthoma (ROR, 9.7; IC, 1.9) and cSCC (ROR, 3.0; IC, 0.9) with PD-1/PD-L1 inhibitor use.
- Of the reports where this information was available, all 10 cases of PD-1/PD-L1 inhibitor–linked keratoacanthoma and 10 of 17 cases (59%) of PD-1/PD-L1 inhibitor–linked cSCC, resolution was noted following discontinuation or dose reduction of the inhibitor.
IN PRACTICE:
“Given the large number of patients receiving immunotherapy, FAERS recording only 43 patients developing keratoacanthoma and 83 patients developing cSCC highlights that these conditions are relatively rare adverse events,” the authors wrote but added that more studies are needed to confirm these results.
SOURCE:
The study, led by Pushkar Aggarwal, MD, MBA, of the Department of Dermatology, University of Cincinnati, Cincinnati, Ohio, was published online in JAMA Dermatology.
LIMITATIONS:
The data obtained from FAERS did not contain information on all AEs from drugs. In addition, a causal association could not be determined.
DISCLOSURES:
The funding source was not reported. The authors did not report any conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
(AEs) reported to the US Food and Drug Administration (FDA).
METHODOLOGY:
- The risk for dermatologic immune-related side effects may be increased with immunologic-modifying drugs.
- To determine if there are significant signals between keratoacanthomas and cSCCs and PD-1/PD-L1 inhibitors, researchers analyzed AEs associated with these agents reported to the FDA’s Adverse Event Reporting System (FAERS) between January 2004 and May 2023.
- Pharmacovigilance signals were identified, and a significant signal was defined as the lower 95% CI of a reporting odds ratio (ROR) greater than one or the lower 95% CI of an information component (IC) greater than 0.
TAKEAWAY:
- Of the 158,000 reports of PD-1/PD-L1 inhibitor use, 43 were in patients who developed a keratoacanthoma (mean age, 77 years; 39% women) and 83 were in patients who developed cSCC (mean age, 71 years; 41% women). Patients aged 60-79 years were most likely to develop keratoacanthomas and cSCC on these treatments.
- A PD-1/PD-L1 inhibitor was listed as the suspect drug in all 43 keratoacanthoma reports and in 70 of 83 cSCC reports (the remaining 13 listed them as the concomitant drug).
- Significant signals were reported for both keratoacanthoma (ROR, 9.7; IC, 1.9) and cSCC (ROR, 3.0; IC, 0.9) with PD-1/PD-L1 inhibitor use.
- Of the reports where this information was available, all 10 cases of PD-1/PD-L1 inhibitor–linked keratoacanthoma and 10 of 17 cases (59%) of PD-1/PD-L1 inhibitor–linked cSCC, resolution was noted following discontinuation or dose reduction of the inhibitor.
IN PRACTICE:
“Given the large number of patients receiving immunotherapy, FAERS recording only 43 patients developing keratoacanthoma and 83 patients developing cSCC highlights that these conditions are relatively rare adverse events,” the authors wrote but added that more studies are needed to confirm these results.
SOURCE:
The study, led by Pushkar Aggarwal, MD, MBA, of the Department of Dermatology, University of Cincinnati, Cincinnati, Ohio, was published online in JAMA Dermatology.
LIMITATIONS:
The data obtained from FAERS did not contain information on all AEs from drugs. In addition, a causal association could not be determined.
DISCLOSURES:
The funding source was not reported. The authors did not report any conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
(AEs) reported to the US Food and Drug Administration (FDA).
METHODOLOGY:
- The risk for dermatologic immune-related side effects may be increased with immunologic-modifying drugs.
- To determine if there are significant signals between keratoacanthomas and cSCCs and PD-1/PD-L1 inhibitors, researchers analyzed AEs associated with these agents reported to the FDA’s Adverse Event Reporting System (FAERS) between January 2004 and May 2023.
- Pharmacovigilance signals were identified, and a significant signal was defined as the lower 95% CI of a reporting odds ratio (ROR) greater than one or the lower 95% CI of an information component (IC) greater than 0.
TAKEAWAY:
- Of the 158,000 reports of PD-1/PD-L1 inhibitor use, 43 were in patients who developed a keratoacanthoma (mean age, 77 years; 39% women) and 83 were in patients who developed cSCC (mean age, 71 years; 41% women). Patients aged 60-79 years were most likely to develop keratoacanthomas and cSCC on these treatments.
- A PD-1/PD-L1 inhibitor was listed as the suspect drug in all 43 keratoacanthoma reports and in 70 of 83 cSCC reports (the remaining 13 listed them as the concomitant drug).
- Significant signals were reported for both keratoacanthoma (ROR, 9.7; IC, 1.9) and cSCC (ROR, 3.0; IC, 0.9) with PD-1/PD-L1 inhibitor use.
- Of the reports where this information was available, all 10 cases of PD-1/PD-L1 inhibitor–linked keratoacanthoma and 10 of 17 cases (59%) of PD-1/PD-L1 inhibitor–linked cSCC, resolution was noted following discontinuation or dose reduction of the inhibitor.
IN PRACTICE:
“Given the large number of patients receiving immunotherapy, FAERS recording only 43 patients developing keratoacanthoma and 83 patients developing cSCC highlights that these conditions are relatively rare adverse events,” the authors wrote but added that more studies are needed to confirm these results.
SOURCE:
The study, led by Pushkar Aggarwal, MD, MBA, of the Department of Dermatology, University of Cincinnati, Cincinnati, Ohio, was published online in JAMA Dermatology.
LIMITATIONS:
The data obtained from FAERS did not contain information on all AEs from drugs. In addition, a causal association could not be determined.
DISCLOSURES:
The funding source was not reported. The authors did not report any conflicts of interest.
A version of this article appeared on Medscape.com.
Combined Pediatric Derm-Rheum Clinics Supported by Survey Respondents
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.
Burnout
In last month’s column, I discussed employees who are “clock watchers” and how to address this issue in your practice if it exists. Here’s another scenario you may encounter from the Office Politics Forum at the recent American Academy of Dermatology annual meeting:
A 40-year-old dermatologist has practiced in the same office since residency and is loved by patients and staff. He remained with the practice through its takeover by a local hospital three years previously. Recently, over a 3-month period, everyone in the office notices a change in this dermatologist’s behavior. He no longer appears happy, is argumentative with staff and patients alike, often dismisses patients’ concerns, and calls in sick during the practice’s busiest days.
. According to the American Medical Association’s National Burnout Benchmarking report, over 50% of physicians report some characteristics of burnout, which include emotional exhaustion, depersonalization, and a feeling of decreased personal achievement.
The causes of physician burnout are multifactorial and vary in importance, depending on the individual and on which authorities you consult. Here are some of the most prevalent, based on my experience and research:
Bureaucratic and Administrative Tasks: The burden of paperwork and other administrative responsibilities has increased, consuming time that could be spent on patient care or personal well-being.
Electronic Health Record (EHR) Stress: As I (and many others) have predicted for decades, the demands of EHR documentation and the associated clerical tasks have become a major source of what is now called “technostress,” detracting from the efficiency and effectiveness of healthcare delivery.
Insurance and Regulatory Demands: Navigating insurance appeals and prior authorizations, meeting regulatory requirements, and dealing with the complexities of healthcare reimbursement systems add to the stress and frustration experienced by physicians.
Lack of Autonomy and Control: As small practices consolidate, physicians often face constraints on their professional autonomy, with limited control over their work environment, schedules, and clinical decision-making, leading to feelings of helplessness and dissatisfaction.
Emotional Exhaustion from Patient Care: The emotional toll of caring for patients, especially in high-stakes or emotionally charged specialties, can lead to compassion fatigue and burnout. This may account for the results of a 2023 Medscape report in which physicians reporting the most burnout worked in emergency medicine, internal medicine, pediatrics, obstetrics/gynecology, and infectious diseases.
Work-Life Imbalance: The demanding nature of the profession often leads to difficulties in balancing professional responsibilities with personal life, contributing to burnout.
Inadequate Support and Recognition: A lack of support from healthcare institutions and insufficient recognition of the challenges faced by physicians can exacerbate feelings of isolation and undervaluation.
Addressing physician burnout requires a systems-based approach that targets these root causes at all levels, from individual coping strategies to organizational and systemic changes in the healthcare industry. Here are some strategies that have worked for me and others:
Optimize Practice Efficiency: This is the consistent theme of this column over several decades: Streamline office processes to enhance the quality of care while reducing unnecessary workload. This can involve adopting efficient patient scheduling systems, improving clinic flow, and utilizing technology like patient portals judiciously to avoid increasing the task load without compensation.
Promote Work-Life Balance: Encourage a culture that values work-life balance. This can include flexible scheduling, respecting off-duty hours by limiting non-emergency work communications, and using your vacation time. Remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office.”
Implement Medical Scribes: I’ve written frequently about this, including a recent column on the new artificial intelligence (AI) scribes, such as DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, ScribeLink, and Amazon Web Services’ new HealthScribe product. Utilizing medical scribes to handle documentation can significantly reduce the administrative burden, allowing physicians to focus more on patient care rather than paperwork, potentially improving both physician and patient satisfaction. (As always, I have no financial interest in any product or service mentioned in this column.)
Provide Professional Development Opportunities: Offer opportunities for professional growth and development. This can include attending conferences, participating in research, or providing time and resources for continuing education. Such opportunities can reinvigorate a physician’s passion for medicine and improve job satisfaction.
Foster a Supportive Work Environment: Create a supportive work culture where staff and physicians feel comfortable discussing challenges and seeking support. Regular meetings or check-ins can help identify early signs of burnout and address them proactively.
Evaluate and Adjust Workloads: Regularly assess physician workloads to ensure they are manageable. Adjusting patient loads, redistributing tasks among team members, or hiring additional staff can help prevent burnout.
Leadership Training and Support: Provide training for leaders within the practice on recognizing signs of burnout and effective management strategies. Supportive leadership is crucial in creating an environment where physicians feel valued and heard.
Peer Support and Mentorship Programs: Establish peer support or mentorship programs where physicians can share experiences, offer advice, and provide emotional support to each other.
Feedback and Continuous Improvement: Managers should regularly solicit feedback from physicians regarding their workload, job satisfaction, and suggestions for improvements. Actively work on implementing feasible changes to address concerns.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In last month’s column, I discussed employees who are “clock watchers” and how to address this issue in your practice if it exists. Here’s another scenario you may encounter from the Office Politics Forum at the recent American Academy of Dermatology annual meeting:
A 40-year-old dermatologist has practiced in the same office since residency and is loved by patients and staff. He remained with the practice through its takeover by a local hospital three years previously. Recently, over a 3-month period, everyone in the office notices a change in this dermatologist’s behavior. He no longer appears happy, is argumentative with staff and patients alike, often dismisses patients’ concerns, and calls in sick during the practice’s busiest days.
. According to the American Medical Association’s National Burnout Benchmarking report, over 50% of physicians report some characteristics of burnout, which include emotional exhaustion, depersonalization, and a feeling of decreased personal achievement.
The causes of physician burnout are multifactorial and vary in importance, depending on the individual and on which authorities you consult. Here are some of the most prevalent, based on my experience and research:
Bureaucratic and Administrative Tasks: The burden of paperwork and other administrative responsibilities has increased, consuming time that could be spent on patient care or personal well-being.
Electronic Health Record (EHR) Stress: As I (and many others) have predicted for decades, the demands of EHR documentation and the associated clerical tasks have become a major source of what is now called “technostress,” detracting from the efficiency and effectiveness of healthcare delivery.
Insurance and Regulatory Demands: Navigating insurance appeals and prior authorizations, meeting regulatory requirements, and dealing with the complexities of healthcare reimbursement systems add to the stress and frustration experienced by physicians.
Lack of Autonomy and Control: As small practices consolidate, physicians often face constraints on their professional autonomy, with limited control over their work environment, schedules, and clinical decision-making, leading to feelings of helplessness and dissatisfaction.
Emotional Exhaustion from Patient Care: The emotional toll of caring for patients, especially in high-stakes or emotionally charged specialties, can lead to compassion fatigue and burnout. This may account for the results of a 2023 Medscape report in which physicians reporting the most burnout worked in emergency medicine, internal medicine, pediatrics, obstetrics/gynecology, and infectious diseases.
Work-Life Imbalance: The demanding nature of the profession often leads to difficulties in balancing professional responsibilities with personal life, contributing to burnout.
Inadequate Support and Recognition: A lack of support from healthcare institutions and insufficient recognition of the challenges faced by physicians can exacerbate feelings of isolation and undervaluation.
Addressing physician burnout requires a systems-based approach that targets these root causes at all levels, from individual coping strategies to organizational and systemic changes in the healthcare industry. Here are some strategies that have worked for me and others:
Optimize Practice Efficiency: This is the consistent theme of this column over several decades: Streamline office processes to enhance the quality of care while reducing unnecessary workload. This can involve adopting efficient patient scheduling systems, improving clinic flow, and utilizing technology like patient portals judiciously to avoid increasing the task load without compensation.
Promote Work-Life Balance: Encourage a culture that values work-life balance. This can include flexible scheduling, respecting off-duty hours by limiting non-emergency work communications, and using your vacation time. Remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office.”
Implement Medical Scribes: I’ve written frequently about this, including a recent column on the new artificial intelligence (AI) scribes, such as DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, ScribeLink, and Amazon Web Services’ new HealthScribe product. Utilizing medical scribes to handle documentation can significantly reduce the administrative burden, allowing physicians to focus more on patient care rather than paperwork, potentially improving both physician and patient satisfaction. (As always, I have no financial interest in any product or service mentioned in this column.)
Provide Professional Development Opportunities: Offer opportunities for professional growth and development. This can include attending conferences, participating in research, or providing time and resources for continuing education. Such opportunities can reinvigorate a physician’s passion for medicine and improve job satisfaction.
Foster a Supportive Work Environment: Create a supportive work culture where staff and physicians feel comfortable discussing challenges and seeking support. Regular meetings or check-ins can help identify early signs of burnout and address them proactively.
Evaluate and Adjust Workloads: Regularly assess physician workloads to ensure they are manageable. Adjusting patient loads, redistributing tasks among team members, or hiring additional staff can help prevent burnout.
Leadership Training and Support: Provide training for leaders within the practice on recognizing signs of burnout and effective management strategies. Supportive leadership is crucial in creating an environment where physicians feel valued and heard.
Peer Support and Mentorship Programs: Establish peer support or mentorship programs where physicians can share experiences, offer advice, and provide emotional support to each other.
Feedback and Continuous Improvement: Managers should regularly solicit feedback from physicians regarding their workload, job satisfaction, and suggestions for improvements. Actively work on implementing feasible changes to address concerns.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In last month’s column, I discussed employees who are “clock watchers” and how to address this issue in your practice if it exists. Here’s another scenario you may encounter from the Office Politics Forum at the recent American Academy of Dermatology annual meeting:
A 40-year-old dermatologist has practiced in the same office since residency and is loved by patients and staff. He remained with the practice through its takeover by a local hospital three years previously. Recently, over a 3-month period, everyone in the office notices a change in this dermatologist’s behavior. He no longer appears happy, is argumentative with staff and patients alike, often dismisses patients’ concerns, and calls in sick during the practice’s busiest days.
. According to the American Medical Association’s National Burnout Benchmarking report, over 50% of physicians report some characteristics of burnout, which include emotional exhaustion, depersonalization, and a feeling of decreased personal achievement.
The causes of physician burnout are multifactorial and vary in importance, depending on the individual and on which authorities you consult. Here are some of the most prevalent, based on my experience and research:
Bureaucratic and Administrative Tasks: The burden of paperwork and other administrative responsibilities has increased, consuming time that could be spent on patient care or personal well-being.
Electronic Health Record (EHR) Stress: As I (and many others) have predicted for decades, the demands of EHR documentation and the associated clerical tasks have become a major source of what is now called “technostress,” detracting from the efficiency and effectiveness of healthcare delivery.
Insurance and Regulatory Demands: Navigating insurance appeals and prior authorizations, meeting regulatory requirements, and dealing with the complexities of healthcare reimbursement systems add to the stress and frustration experienced by physicians.
Lack of Autonomy and Control: As small practices consolidate, physicians often face constraints on their professional autonomy, with limited control over their work environment, schedules, and clinical decision-making, leading to feelings of helplessness and dissatisfaction.
Emotional Exhaustion from Patient Care: The emotional toll of caring for patients, especially in high-stakes or emotionally charged specialties, can lead to compassion fatigue and burnout. This may account for the results of a 2023 Medscape report in which physicians reporting the most burnout worked in emergency medicine, internal medicine, pediatrics, obstetrics/gynecology, and infectious diseases.
Work-Life Imbalance: The demanding nature of the profession often leads to difficulties in balancing professional responsibilities with personal life, contributing to burnout.
Inadequate Support and Recognition: A lack of support from healthcare institutions and insufficient recognition of the challenges faced by physicians can exacerbate feelings of isolation and undervaluation.
Addressing physician burnout requires a systems-based approach that targets these root causes at all levels, from individual coping strategies to organizational and systemic changes in the healthcare industry. Here are some strategies that have worked for me and others:
Optimize Practice Efficiency: This is the consistent theme of this column over several decades: Streamline office processes to enhance the quality of care while reducing unnecessary workload. This can involve adopting efficient patient scheduling systems, improving clinic flow, and utilizing technology like patient portals judiciously to avoid increasing the task load without compensation.
Promote Work-Life Balance: Encourage a culture that values work-life balance. This can include flexible scheduling, respecting off-duty hours by limiting non-emergency work communications, and using your vacation time. Remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office.”
Implement Medical Scribes: I’ve written frequently about this, including a recent column on the new artificial intelligence (AI) scribes, such as DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, ScribeLink, and Amazon Web Services’ new HealthScribe product. Utilizing medical scribes to handle documentation can significantly reduce the administrative burden, allowing physicians to focus more on patient care rather than paperwork, potentially improving both physician and patient satisfaction. (As always, I have no financial interest in any product or service mentioned in this column.)
Provide Professional Development Opportunities: Offer opportunities for professional growth and development. This can include attending conferences, participating in research, or providing time and resources for continuing education. Such opportunities can reinvigorate a physician’s passion for medicine and improve job satisfaction.
Foster a Supportive Work Environment: Create a supportive work culture where staff and physicians feel comfortable discussing challenges and seeking support. Regular meetings or check-ins can help identify early signs of burnout and address them proactively.
Evaluate and Adjust Workloads: Regularly assess physician workloads to ensure they are manageable. Adjusting patient loads, redistributing tasks among team members, or hiring additional staff can help prevent burnout.
Leadership Training and Support: Provide training for leaders within the practice on recognizing signs of burnout and effective management strategies. Supportive leadership is crucial in creating an environment where physicians feel valued and heard.
Peer Support and Mentorship Programs: Establish peer support or mentorship programs where physicians can share experiences, offer advice, and provide emotional support to each other.
Feedback and Continuous Improvement: Managers should regularly solicit feedback from physicians regarding their workload, job satisfaction, and suggestions for improvements. Actively work on implementing feasible changes to address concerns.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
PCOS: Laser, Light Therapy Helpful for Hirsutism
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
What’s ‘Tried and True’ in Atopic Dermatitis? An Expert Reflects
SAN DIEGO — Whether you completed your dermatology residency training 20 years ago or 2 years ago, recent advances in treatments for atopic dermatitis (AD) have likely influenced your “go to” interventions when treating children with AD, according to Lawrence F. Eichenfield, MD.
“There have been many changes in the understanding of AD and recognition of the variable courses of the disease, and the associated allergic and nonallergic comorbidities,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego in California, said at the Society for Pediatric Dermatology meeting, held the day before the annual meeting of the American Academy of Dermatology. “With our revolutionary systemic and evolving topical therapies, we are in a new day of pediatric management.”
Drawing from 2023 American Academy of Dermatology guidelines of care on topical treatments of AD and his own clinical experience, he shared his perspective on “what’s tried and true” in care for patients with persistent eczema:
Both bathing and moisturizing leave skin moist. It’s well established that the use of moisturizers/emollients minimizes xerosis and the amount of prescription anti-inflammatory medications, but limited evidence exists to recommend a particular ingredient and formulation, said Dr. Eichenfield, also professor of dermatology and pediatrics at the University of California, San Diego. “Future studies may tell us whether specific moisturizers work better than others, and/or if early interventions may prevent AD, but that remains a big question mark,” he noted. In addition, applications may sometimes “mobilize” topical prescriptive residual absorption and activity.
As for baths, he said, “avoidance of bathing to avoid drying out skin is a practice without evidence basis. Bathing also may have many benefits in active eczema.”
Bleach baths may enhance skin barrier function, reduce itch, and improve eczema, but the practice remains controversial, he continued. Authors of a systematic review and meta-analysis concluded that while bleach baths are effective in reducing the severity of AD, they do not appear to be more effective than water bath alone. Authors of a more recent study found that bleach baths did not normalize dysbiosis, “but that study did not compare outcomes to bathing without bleach,” Dr. Eichenfield noted.“My sense is there is some benefit to regular bathing, especially in children with moderate to severe AD, especially those with colonized or infected eczema.”
He advises clinicians to be aware of other “standard AD interventions” from around the world, including black tea wet dressings and green tea bath therapy.
Topical corticosteroids. These are “tried and true” for their anti-inflammatory properties and rapid response, relatively low cost, and large range of potency, he said. Potential problems include the burden of topical application and the potential for stinging/burning, atrophy, telangiectasias, adrenal axis suppression, and concerns about withdrawal phenomena. “Being a proponent of topical corticosteroids, but explaining reasonable and appropriate use can be challenging,” Dr. Eichenfield said. “Social media has influenced concerns about topical corticosteroids, with steroid addiction and withdrawal being concerns influencing discomfort with therapies.”
Make sure to measure outcomes. The suggested core outcome measure for recording clinical signs in AD clinical trials is the Eczema Area and Severity Index (EASI) score, he said. In clinical practice, Dr. Eichenfield favors body surface area (BSA) and the Validated Global Assessment scale (v-IGA) to measure signs of moderate to severe AD. “Documenting extent of disease makes a big difference in families understanding how severe their child’s disease is and how it is doing over time.” Alternatively, he recommends the Atopic Dermatitis Control Tool (ADCT) or the Recap of Atopic Eczema (RECAP) as tools assessing long-term disease control.
Familiarize yourself with nonsteroidal anti-inflammatory medications for care regimens. Options include topical calcineurin inhibitors (TCIs) such as tacrolimus and pimecrolimus; phosphodiesterase 4 (PDE-4) inhibitors such as crisaborole and roflumilast; the aryl-hydrocarbon receptor agonist tapinarof; and topical Janus kinase (JAK) inhibitors such as delgocitinib and ruxolitinib as well as others in development. “There is variable status around the world in terms of whether these nonsteroidal options are approved or not,” Dr. Eichenfield said. “Issues of use include cost, availability, side effects, and concerns about potential absorption. I think there’s an evolution in how much we rely on these instead of topical corticosteroids. They’re more commonly used in maintenance regimens rather than for remission induction.”
Dr. Eichenfield encouraged dermatologists to share information about and experiences with evolving treatment options for AD, “because when the studies are done, they are done as monotherapy. We must translate that into clinical practice and figure out how they fit in. Our exchange of information is critical.”
Dr. Eichenfield disclosed conflicts of interest from many pharmaceutical companies, including those with AD treatments.
SAN DIEGO — Whether you completed your dermatology residency training 20 years ago or 2 years ago, recent advances in treatments for atopic dermatitis (AD) have likely influenced your “go to” interventions when treating children with AD, according to Lawrence F. Eichenfield, MD.
“There have been many changes in the understanding of AD and recognition of the variable courses of the disease, and the associated allergic and nonallergic comorbidities,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego in California, said at the Society for Pediatric Dermatology meeting, held the day before the annual meeting of the American Academy of Dermatology. “With our revolutionary systemic and evolving topical therapies, we are in a new day of pediatric management.”
Drawing from 2023 American Academy of Dermatology guidelines of care on topical treatments of AD and his own clinical experience, he shared his perspective on “what’s tried and true” in care for patients with persistent eczema:
Both bathing and moisturizing leave skin moist. It’s well established that the use of moisturizers/emollients minimizes xerosis and the amount of prescription anti-inflammatory medications, but limited evidence exists to recommend a particular ingredient and formulation, said Dr. Eichenfield, also professor of dermatology and pediatrics at the University of California, San Diego. “Future studies may tell us whether specific moisturizers work better than others, and/or if early interventions may prevent AD, but that remains a big question mark,” he noted. In addition, applications may sometimes “mobilize” topical prescriptive residual absorption and activity.
As for baths, he said, “avoidance of bathing to avoid drying out skin is a practice without evidence basis. Bathing also may have many benefits in active eczema.”
Bleach baths may enhance skin barrier function, reduce itch, and improve eczema, but the practice remains controversial, he continued. Authors of a systematic review and meta-analysis concluded that while bleach baths are effective in reducing the severity of AD, they do not appear to be more effective than water bath alone. Authors of a more recent study found that bleach baths did not normalize dysbiosis, “but that study did not compare outcomes to bathing without bleach,” Dr. Eichenfield noted.“My sense is there is some benefit to regular bathing, especially in children with moderate to severe AD, especially those with colonized or infected eczema.”
He advises clinicians to be aware of other “standard AD interventions” from around the world, including black tea wet dressings and green tea bath therapy.
Topical corticosteroids. These are “tried and true” for their anti-inflammatory properties and rapid response, relatively low cost, and large range of potency, he said. Potential problems include the burden of topical application and the potential for stinging/burning, atrophy, telangiectasias, adrenal axis suppression, and concerns about withdrawal phenomena. “Being a proponent of topical corticosteroids, but explaining reasonable and appropriate use can be challenging,” Dr. Eichenfield said. “Social media has influenced concerns about topical corticosteroids, with steroid addiction and withdrawal being concerns influencing discomfort with therapies.”
Make sure to measure outcomes. The suggested core outcome measure for recording clinical signs in AD clinical trials is the Eczema Area and Severity Index (EASI) score, he said. In clinical practice, Dr. Eichenfield favors body surface area (BSA) and the Validated Global Assessment scale (v-IGA) to measure signs of moderate to severe AD. “Documenting extent of disease makes a big difference in families understanding how severe their child’s disease is and how it is doing over time.” Alternatively, he recommends the Atopic Dermatitis Control Tool (ADCT) or the Recap of Atopic Eczema (RECAP) as tools assessing long-term disease control.
Familiarize yourself with nonsteroidal anti-inflammatory medications for care regimens. Options include topical calcineurin inhibitors (TCIs) such as tacrolimus and pimecrolimus; phosphodiesterase 4 (PDE-4) inhibitors such as crisaborole and roflumilast; the aryl-hydrocarbon receptor agonist tapinarof; and topical Janus kinase (JAK) inhibitors such as delgocitinib and ruxolitinib as well as others in development. “There is variable status around the world in terms of whether these nonsteroidal options are approved or not,” Dr. Eichenfield said. “Issues of use include cost, availability, side effects, and concerns about potential absorption. I think there’s an evolution in how much we rely on these instead of topical corticosteroids. They’re more commonly used in maintenance regimens rather than for remission induction.”
Dr. Eichenfield encouraged dermatologists to share information about and experiences with evolving treatment options for AD, “because when the studies are done, they are done as monotherapy. We must translate that into clinical practice and figure out how they fit in. Our exchange of information is critical.”
Dr. Eichenfield disclosed conflicts of interest from many pharmaceutical companies, including those with AD treatments.
SAN DIEGO — Whether you completed your dermatology residency training 20 years ago or 2 years ago, recent advances in treatments for atopic dermatitis (AD) have likely influenced your “go to” interventions when treating children with AD, according to Lawrence F. Eichenfield, MD.
“There have been many changes in the understanding of AD and recognition of the variable courses of the disease, and the associated allergic and nonallergic comorbidities,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego in California, said at the Society for Pediatric Dermatology meeting, held the day before the annual meeting of the American Academy of Dermatology. “With our revolutionary systemic and evolving topical therapies, we are in a new day of pediatric management.”
Drawing from 2023 American Academy of Dermatology guidelines of care on topical treatments of AD and his own clinical experience, he shared his perspective on “what’s tried and true” in care for patients with persistent eczema:
Both bathing and moisturizing leave skin moist. It’s well established that the use of moisturizers/emollients minimizes xerosis and the amount of prescription anti-inflammatory medications, but limited evidence exists to recommend a particular ingredient and formulation, said Dr. Eichenfield, also professor of dermatology and pediatrics at the University of California, San Diego. “Future studies may tell us whether specific moisturizers work better than others, and/or if early interventions may prevent AD, but that remains a big question mark,” he noted. In addition, applications may sometimes “mobilize” topical prescriptive residual absorption and activity.
As for baths, he said, “avoidance of bathing to avoid drying out skin is a practice without evidence basis. Bathing also may have many benefits in active eczema.”
Bleach baths may enhance skin barrier function, reduce itch, and improve eczema, but the practice remains controversial, he continued. Authors of a systematic review and meta-analysis concluded that while bleach baths are effective in reducing the severity of AD, they do not appear to be more effective than water bath alone. Authors of a more recent study found that bleach baths did not normalize dysbiosis, “but that study did not compare outcomes to bathing without bleach,” Dr. Eichenfield noted.“My sense is there is some benefit to regular bathing, especially in children with moderate to severe AD, especially those with colonized or infected eczema.”
He advises clinicians to be aware of other “standard AD interventions” from around the world, including black tea wet dressings and green tea bath therapy.
Topical corticosteroids. These are “tried and true” for their anti-inflammatory properties and rapid response, relatively low cost, and large range of potency, he said. Potential problems include the burden of topical application and the potential for stinging/burning, atrophy, telangiectasias, adrenal axis suppression, and concerns about withdrawal phenomena. “Being a proponent of topical corticosteroids, but explaining reasonable and appropriate use can be challenging,” Dr. Eichenfield said. “Social media has influenced concerns about topical corticosteroids, with steroid addiction and withdrawal being concerns influencing discomfort with therapies.”
Make sure to measure outcomes. The suggested core outcome measure for recording clinical signs in AD clinical trials is the Eczema Area and Severity Index (EASI) score, he said. In clinical practice, Dr. Eichenfield favors body surface area (BSA) and the Validated Global Assessment scale (v-IGA) to measure signs of moderate to severe AD. “Documenting extent of disease makes a big difference in families understanding how severe their child’s disease is and how it is doing over time.” Alternatively, he recommends the Atopic Dermatitis Control Tool (ADCT) or the Recap of Atopic Eczema (RECAP) as tools assessing long-term disease control.
Familiarize yourself with nonsteroidal anti-inflammatory medications for care regimens. Options include topical calcineurin inhibitors (TCIs) such as tacrolimus and pimecrolimus; phosphodiesterase 4 (PDE-4) inhibitors such as crisaborole and roflumilast; the aryl-hydrocarbon receptor agonist tapinarof; and topical Janus kinase (JAK) inhibitors such as delgocitinib and ruxolitinib as well as others in development. “There is variable status around the world in terms of whether these nonsteroidal options are approved or not,” Dr. Eichenfield said. “Issues of use include cost, availability, side effects, and concerns about potential absorption. I think there’s an evolution in how much we rely on these instead of topical corticosteroids. They’re more commonly used in maintenance regimens rather than for remission induction.”
Dr. Eichenfield encouraged dermatologists to share information about and experiences with evolving treatment options for AD, “because when the studies are done, they are done as monotherapy. We must translate that into clinical practice and figure out how they fit in. Our exchange of information is critical.”
Dr. Eichenfield disclosed conflicts of interest from many pharmaceutical companies, including those with AD treatments.
Consensus Statement Aims to Guide Use of Low-Dose Oral Minoxidil for Hair Loss
SAN DIEGO — .
Those are among the key recommendations that resulted from a modified eDelphi consensus of experts who convened to develop guidelines for LDOM prescribing and monitoring.
“Topical minoxidil is safe, effective, over-the-counter, and FDA-approved to treat the most common form of hair loss, androgenetic alopecia,” one of the study authors, Jennifer Fu, MD, a dermatologist who directs the Hair Disorders Clinic at the University of California, San Francisco, told this news organization following the annual meeting of the American Academy of Dermatology. The results of the expert consensus were presented during a poster session at the meeting. “It is often used off label for other types of hair loss, yet clinicians who treat hair loss know that patient compliance with topical minoxidil can be poor for a variety of reasons,” she said. “Patients report that it can be difficult to apply and complicate hair styling. For many patients, topical minoxidil can be drying or cause irritant or allergic contact reactions.”
LDOM has become a popular alternative for patients for whom topical minoxidil is logistically challenging, irritating, or ineffective, she continued. Although oral minoxidil is no longer a first-line antihypertensive agent given the risk of cardiovascular adverse effects at higher antihypertensive dosing (10-40 mg daily), a growing number of small studies have documented the use of LDOM at doses ranging from 0.25 mg to 5 mg daily as a safe, effective option for various types of hair loss.
“Given the current absence of larger trials on this topic, our research group identified a need for expert-based guidelines for prescribing and monitoring LDOM use in hair loss patients,” Dr. Fu said. “Our goal was to provide clinicians who treat hair loss patients a road map for using LDOM effectively, maximizing hair growth, and minimizing potential cardiovascular adverse effects.”
Arriving at a Consensus
The process involved 43 hair loss specialists from 12 countries with an average of 6.29 years of experience with LDOM for hair loss, who participated in a multi-round modified Delphi process. They considered questions that addressed LDOM safety, efficacy, dosing, and monitoring for hair loss, and consensus was reached if at least 70% of participants indicated “agree” or “strongly agree” on a five-point Likert scale. Round 1 consisted of 180 open-ended, multiple-choice, or Likert-scale questions, while round 2 involved 121 Likert-scale questions, round 3 consisted of 16 Likert-scale questions, and round 4 included 11 Likert-scale questions. In all, 94 items achieved Likert-scale consensus.
Specifically, experts on the panel found a direct benefit of LDOM for androgenetic alopecia, age-related patterned thinning, alopecia areata, telogen effluvium, traction alopecia, persistent chemotherapy-induced alopecia, and endocrine therapy-induced alopecia. They found a supportive benefit of LDOM for lichen planopilaris, frontal fibrosing alopecia, central centrifugal alopecia, and fibrosing alopecia in a patterned distribution.
“LDOM can be considered when topical minoxidil is more expensive, logistically challenging, has plateaued in efficacy, results in undesirable product residue/skin irritation,” or exacerbates inflammatory processes (ie eczema, psoriasis), they added.
Contraindications to LDOM listed in the consensus recommendations include hypersensitivity to minoxidil, significant drug-drug interactions with LDOM, a history of pericardial effusion/tamponade, pericarditis, heart failure, pulmonary hypertension associated with mitral stenosis, pheochromocytoma, and pregnancy/breastfeeding. Cited precautions of LDOM use include a history of tachycardia or arrhythmia, hypotension, renal impairment, and being on dialysis.
Dr. Fu and colleagues noted that the earliest time point at which LDOM should be expected to demonstrate efficacy is 3-6 months. “Baseline testing is not routine but may be considered in case of identified precautions,” they wrote. They also noted that LDOM can possibly be co-administered with beta-blockers with a specialty consultation, and with spironolactone in biologic female or transgender female patients with hirsutism, acne, polycystic ovary syndrome (PCOS), and with lower extremity and facial edema.
According to the consensus statement, the most frequently prescribed LDOM dosing regimen in adult females aged 18 years and older includes a starting dose of 1.25 mg daily, with a dosing range between 0.625 mg and 5 mg daily. For adult males, the most frequently prescribed dosing regimen is a starting dose of 2.5 daily, with a dosing range between 1.25 mg and 5 mg daily. The most frequently prescribed LDOM dosing regimen in adolescent females aged 12-17 years is a starting dose of 0.625 mg daily, with a dosing range of 0.625 to 2.5 mg daily. For adolescent males, the recommended regimen is a starting dose of 1.25 mg daily, with a dosing range of 1.25 mg to 5 mg daily.
“We hope that this consensus statement will guide our colleagues who would like to use LDOM to treat hair loss in their adult and adolescent patients,” Dr. Fu told this news organization. “These recommendations may be used to inform clinical practice until additional evidence-based data becomes available.”
She acknowledged certain limitations of the effort, including the fact that the expert panel was underrepresented in treating hair loss in pediatric patients, “and therefore failed to reach consensus on LDOM pediatric use and dosing,” she said. “We encourage our pediatric dermatology colleagues to further research LDOM in pediatric patients.”
In an interview, Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, who was asked to comment, but was not involved with the work, characterized the consensus as a “helpful, concise reference guide for dermatologists.”
The advantages of the study are the standardized methods used, “and the experience of the panel,” she said. “Study limitations include the response rate, which was less than 60%, and the risk of potential side effects are not stratified by age, sex, or comorbidities,” she added.
Dr. Fu disclosed that she is a consultant to Pfizer. Dr. Lipner reported having no relevant disclosures.
SAN DIEGO — .
Those are among the key recommendations that resulted from a modified eDelphi consensus of experts who convened to develop guidelines for LDOM prescribing and monitoring.
“Topical minoxidil is safe, effective, over-the-counter, and FDA-approved to treat the most common form of hair loss, androgenetic alopecia,” one of the study authors, Jennifer Fu, MD, a dermatologist who directs the Hair Disorders Clinic at the University of California, San Francisco, told this news organization following the annual meeting of the American Academy of Dermatology. The results of the expert consensus were presented during a poster session at the meeting. “It is often used off label for other types of hair loss, yet clinicians who treat hair loss know that patient compliance with topical minoxidil can be poor for a variety of reasons,” she said. “Patients report that it can be difficult to apply and complicate hair styling. For many patients, topical minoxidil can be drying or cause irritant or allergic contact reactions.”
LDOM has become a popular alternative for patients for whom topical minoxidil is logistically challenging, irritating, or ineffective, she continued. Although oral minoxidil is no longer a first-line antihypertensive agent given the risk of cardiovascular adverse effects at higher antihypertensive dosing (10-40 mg daily), a growing number of small studies have documented the use of LDOM at doses ranging from 0.25 mg to 5 mg daily as a safe, effective option for various types of hair loss.
“Given the current absence of larger trials on this topic, our research group identified a need for expert-based guidelines for prescribing and monitoring LDOM use in hair loss patients,” Dr. Fu said. “Our goal was to provide clinicians who treat hair loss patients a road map for using LDOM effectively, maximizing hair growth, and minimizing potential cardiovascular adverse effects.”
Arriving at a Consensus
The process involved 43 hair loss specialists from 12 countries with an average of 6.29 years of experience with LDOM for hair loss, who participated in a multi-round modified Delphi process. They considered questions that addressed LDOM safety, efficacy, dosing, and monitoring for hair loss, and consensus was reached if at least 70% of participants indicated “agree” or “strongly agree” on a five-point Likert scale. Round 1 consisted of 180 open-ended, multiple-choice, or Likert-scale questions, while round 2 involved 121 Likert-scale questions, round 3 consisted of 16 Likert-scale questions, and round 4 included 11 Likert-scale questions. In all, 94 items achieved Likert-scale consensus.
Specifically, experts on the panel found a direct benefit of LDOM for androgenetic alopecia, age-related patterned thinning, alopecia areata, telogen effluvium, traction alopecia, persistent chemotherapy-induced alopecia, and endocrine therapy-induced alopecia. They found a supportive benefit of LDOM for lichen planopilaris, frontal fibrosing alopecia, central centrifugal alopecia, and fibrosing alopecia in a patterned distribution.
“LDOM can be considered when topical minoxidil is more expensive, logistically challenging, has plateaued in efficacy, results in undesirable product residue/skin irritation,” or exacerbates inflammatory processes (ie eczema, psoriasis), they added.
Contraindications to LDOM listed in the consensus recommendations include hypersensitivity to minoxidil, significant drug-drug interactions with LDOM, a history of pericardial effusion/tamponade, pericarditis, heart failure, pulmonary hypertension associated with mitral stenosis, pheochromocytoma, and pregnancy/breastfeeding. Cited precautions of LDOM use include a history of tachycardia or arrhythmia, hypotension, renal impairment, and being on dialysis.
Dr. Fu and colleagues noted that the earliest time point at which LDOM should be expected to demonstrate efficacy is 3-6 months. “Baseline testing is not routine but may be considered in case of identified precautions,” they wrote. They also noted that LDOM can possibly be co-administered with beta-blockers with a specialty consultation, and with spironolactone in biologic female or transgender female patients with hirsutism, acne, polycystic ovary syndrome (PCOS), and with lower extremity and facial edema.
According to the consensus statement, the most frequently prescribed LDOM dosing regimen in adult females aged 18 years and older includes a starting dose of 1.25 mg daily, with a dosing range between 0.625 mg and 5 mg daily. For adult males, the most frequently prescribed dosing regimen is a starting dose of 2.5 daily, with a dosing range between 1.25 mg and 5 mg daily. The most frequently prescribed LDOM dosing regimen in adolescent females aged 12-17 years is a starting dose of 0.625 mg daily, with a dosing range of 0.625 to 2.5 mg daily. For adolescent males, the recommended regimen is a starting dose of 1.25 mg daily, with a dosing range of 1.25 mg to 5 mg daily.
“We hope that this consensus statement will guide our colleagues who would like to use LDOM to treat hair loss in their adult and adolescent patients,” Dr. Fu told this news organization. “These recommendations may be used to inform clinical practice until additional evidence-based data becomes available.”
She acknowledged certain limitations of the effort, including the fact that the expert panel was underrepresented in treating hair loss in pediatric patients, “and therefore failed to reach consensus on LDOM pediatric use and dosing,” she said. “We encourage our pediatric dermatology colleagues to further research LDOM in pediatric patients.”
In an interview, Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, who was asked to comment, but was not involved with the work, characterized the consensus as a “helpful, concise reference guide for dermatologists.”
The advantages of the study are the standardized methods used, “and the experience of the panel,” she said. “Study limitations include the response rate, which was less than 60%, and the risk of potential side effects are not stratified by age, sex, or comorbidities,” she added.
Dr. Fu disclosed that she is a consultant to Pfizer. Dr. Lipner reported having no relevant disclosures.
SAN DIEGO — .
Those are among the key recommendations that resulted from a modified eDelphi consensus of experts who convened to develop guidelines for LDOM prescribing and monitoring.
“Topical minoxidil is safe, effective, over-the-counter, and FDA-approved to treat the most common form of hair loss, androgenetic alopecia,” one of the study authors, Jennifer Fu, MD, a dermatologist who directs the Hair Disorders Clinic at the University of California, San Francisco, told this news organization following the annual meeting of the American Academy of Dermatology. The results of the expert consensus were presented during a poster session at the meeting. “It is often used off label for other types of hair loss, yet clinicians who treat hair loss know that patient compliance with topical minoxidil can be poor for a variety of reasons,” she said. “Patients report that it can be difficult to apply and complicate hair styling. For many patients, topical minoxidil can be drying or cause irritant or allergic contact reactions.”
LDOM has become a popular alternative for patients for whom topical minoxidil is logistically challenging, irritating, or ineffective, she continued. Although oral minoxidil is no longer a first-line antihypertensive agent given the risk of cardiovascular adverse effects at higher antihypertensive dosing (10-40 mg daily), a growing number of small studies have documented the use of LDOM at doses ranging from 0.25 mg to 5 mg daily as a safe, effective option for various types of hair loss.
“Given the current absence of larger trials on this topic, our research group identified a need for expert-based guidelines for prescribing and monitoring LDOM use in hair loss patients,” Dr. Fu said. “Our goal was to provide clinicians who treat hair loss patients a road map for using LDOM effectively, maximizing hair growth, and minimizing potential cardiovascular adverse effects.”
Arriving at a Consensus
The process involved 43 hair loss specialists from 12 countries with an average of 6.29 years of experience with LDOM for hair loss, who participated in a multi-round modified Delphi process. They considered questions that addressed LDOM safety, efficacy, dosing, and monitoring for hair loss, and consensus was reached if at least 70% of participants indicated “agree” or “strongly agree” on a five-point Likert scale. Round 1 consisted of 180 open-ended, multiple-choice, or Likert-scale questions, while round 2 involved 121 Likert-scale questions, round 3 consisted of 16 Likert-scale questions, and round 4 included 11 Likert-scale questions. In all, 94 items achieved Likert-scale consensus.
Specifically, experts on the panel found a direct benefit of LDOM for androgenetic alopecia, age-related patterned thinning, alopecia areata, telogen effluvium, traction alopecia, persistent chemotherapy-induced alopecia, and endocrine therapy-induced alopecia. They found a supportive benefit of LDOM for lichen planopilaris, frontal fibrosing alopecia, central centrifugal alopecia, and fibrosing alopecia in a patterned distribution.
“LDOM can be considered when topical minoxidil is more expensive, logistically challenging, has plateaued in efficacy, results in undesirable product residue/skin irritation,” or exacerbates inflammatory processes (ie eczema, psoriasis), they added.
Contraindications to LDOM listed in the consensus recommendations include hypersensitivity to minoxidil, significant drug-drug interactions with LDOM, a history of pericardial effusion/tamponade, pericarditis, heart failure, pulmonary hypertension associated with mitral stenosis, pheochromocytoma, and pregnancy/breastfeeding. Cited precautions of LDOM use include a history of tachycardia or arrhythmia, hypotension, renal impairment, and being on dialysis.
Dr. Fu and colleagues noted that the earliest time point at which LDOM should be expected to demonstrate efficacy is 3-6 months. “Baseline testing is not routine but may be considered in case of identified precautions,” they wrote. They also noted that LDOM can possibly be co-administered with beta-blockers with a specialty consultation, and with spironolactone in biologic female or transgender female patients with hirsutism, acne, polycystic ovary syndrome (PCOS), and with lower extremity and facial edema.
According to the consensus statement, the most frequently prescribed LDOM dosing regimen in adult females aged 18 years and older includes a starting dose of 1.25 mg daily, with a dosing range between 0.625 mg and 5 mg daily. For adult males, the most frequently prescribed dosing regimen is a starting dose of 2.5 daily, with a dosing range between 1.25 mg and 5 mg daily. The most frequently prescribed LDOM dosing regimen in adolescent females aged 12-17 years is a starting dose of 0.625 mg daily, with a dosing range of 0.625 to 2.5 mg daily. For adolescent males, the recommended regimen is a starting dose of 1.25 mg daily, with a dosing range of 1.25 mg to 5 mg daily.
“We hope that this consensus statement will guide our colleagues who would like to use LDOM to treat hair loss in their adult and adolescent patients,” Dr. Fu told this news organization. “These recommendations may be used to inform clinical practice until additional evidence-based data becomes available.”
She acknowledged certain limitations of the effort, including the fact that the expert panel was underrepresented in treating hair loss in pediatric patients, “and therefore failed to reach consensus on LDOM pediatric use and dosing,” she said. “We encourage our pediatric dermatology colleagues to further research LDOM in pediatric patients.”
In an interview, Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, who was asked to comment, but was not involved with the work, characterized the consensus as a “helpful, concise reference guide for dermatologists.”
The advantages of the study are the standardized methods used, “and the experience of the panel,” she said. “Study limitations include the response rate, which was less than 60%, and the risk of potential side effects are not stratified by age, sex, or comorbidities,” she added.
Dr. Fu disclosed that she is a consultant to Pfizer. Dr. Lipner reported having no relevant disclosures.
FROM AAD 2024
Port-Wine Birthmarks: Shorter Interval Laser Treatments Show Promise in Infants
TOPLINE:
METHODOLOGY:
- Early intervention of PWB in infants can significantly improve outcomes, and some studies suggest shorter intervals between laser treatments may be more effective. While laser treatment with PDL is the gold standard, the optimal treatment interval has not been determined.
- Researchers evaluated the records of 10 infants with PWB who received weekly PDL treatments from 2022 to 2023 at a single center. Treatment was initiated when the infants were 6 months old or younger, with the median age at the first treatment being 4 weeks. Of the 10 infants, eight had Fitzpatrick skin types I-III and two had skin type IV.
- Two dermatologists assessed photographs taken before and after laser treatment, and the primary outcome was the percentage improvement of PWB.
TAKEAWAY:
- Of the 10 patients, six achieved near-total (76%-95%) clearance, and one achieved total (96%-100%) clearance of PWB at a mean of 2 months after the first treatment.
- Marked improvement (51%-75%) in PWB was observed in the remaining three patients, who achieved near-total clearance with additional treatments.
- The median duration of treatment was 2 months (range, 0.2-5.1), and a median of eight treatments (range, 2-20) were needed to achieve near total or total clearance.
- No adverse events were reported, including pigmentary changes, scarring, burns, erosions, or infections.
IN PRACTICE:
The outcomes in the case series, the authors concluded, “are compelling and warrant attention and further investigation into the possibility that this novel and decreased treatment interval of 1 week ... is associated with potential improvement in outcomes and shorter overall treatment duration.”
SOURCE:
This study was led by Shirin Bajaj, MD, of the Laser & Skin Surgery Center of New York, where the infants were treated, and was published online on April 17, 2024, in JAMA Dermatology.
LIMITATIONS:
A small sample size and the lack of a comparison arm limited the ability to draw any conclusions or make treatment recommendations based on the results.
DISCLOSURES:
The authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Early intervention of PWB in infants can significantly improve outcomes, and some studies suggest shorter intervals between laser treatments may be more effective. While laser treatment with PDL is the gold standard, the optimal treatment interval has not been determined.
- Researchers evaluated the records of 10 infants with PWB who received weekly PDL treatments from 2022 to 2023 at a single center. Treatment was initiated when the infants were 6 months old or younger, with the median age at the first treatment being 4 weeks. Of the 10 infants, eight had Fitzpatrick skin types I-III and two had skin type IV.
- Two dermatologists assessed photographs taken before and after laser treatment, and the primary outcome was the percentage improvement of PWB.
TAKEAWAY:
- Of the 10 patients, six achieved near-total (76%-95%) clearance, and one achieved total (96%-100%) clearance of PWB at a mean of 2 months after the first treatment.
- Marked improvement (51%-75%) in PWB was observed in the remaining three patients, who achieved near-total clearance with additional treatments.
- The median duration of treatment was 2 months (range, 0.2-5.1), and a median of eight treatments (range, 2-20) were needed to achieve near total or total clearance.
- No adverse events were reported, including pigmentary changes, scarring, burns, erosions, or infections.
IN PRACTICE:
The outcomes in the case series, the authors concluded, “are compelling and warrant attention and further investigation into the possibility that this novel and decreased treatment interval of 1 week ... is associated with potential improvement in outcomes and shorter overall treatment duration.”
SOURCE:
This study was led by Shirin Bajaj, MD, of the Laser & Skin Surgery Center of New York, where the infants were treated, and was published online on April 17, 2024, in JAMA Dermatology.
LIMITATIONS:
A small sample size and the lack of a comparison arm limited the ability to draw any conclusions or make treatment recommendations based on the results.
DISCLOSURES:
The authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Early intervention of PWB in infants can significantly improve outcomes, and some studies suggest shorter intervals between laser treatments may be more effective. While laser treatment with PDL is the gold standard, the optimal treatment interval has not been determined.
- Researchers evaluated the records of 10 infants with PWB who received weekly PDL treatments from 2022 to 2023 at a single center. Treatment was initiated when the infants were 6 months old or younger, with the median age at the first treatment being 4 weeks. Of the 10 infants, eight had Fitzpatrick skin types I-III and two had skin type IV.
- Two dermatologists assessed photographs taken before and after laser treatment, and the primary outcome was the percentage improvement of PWB.
TAKEAWAY:
- Of the 10 patients, six achieved near-total (76%-95%) clearance, and one achieved total (96%-100%) clearance of PWB at a mean of 2 months after the first treatment.
- Marked improvement (51%-75%) in PWB was observed in the remaining three patients, who achieved near-total clearance with additional treatments.
- The median duration of treatment was 2 months (range, 0.2-5.1), and a median of eight treatments (range, 2-20) were needed to achieve near total or total clearance.
- No adverse events were reported, including pigmentary changes, scarring, burns, erosions, or infections.
IN PRACTICE:
The outcomes in the case series, the authors concluded, “are compelling and warrant attention and further investigation into the possibility that this novel and decreased treatment interval of 1 week ... is associated with potential improvement in outcomes and shorter overall treatment duration.”
SOURCE:
This study was led by Shirin Bajaj, MD, of the Laser & Skin Surgery Center of New York, where the infants were treated, and was published online on April 17, 2024, in JAMA Dermatology.
LIMITATIONS:
A small sample size and the lack of a comparison arm limited the ability to draw any conclusions or make treatment recommendations based on the results.
DISCLOSURES:
The authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.