User login
News and Views that Matter to Physicians
Experts emphasize scrupulous procedures for duodenoscopes, ERCP
Contaminated duodenoscopes have caused multiple outbreaks of multidrug-resistant infections with sometimes lethal consequences; until these instruments become easier to clean, personnel must strictly follow recommendations for sterilization, surveillance, and unit-by-unit quality control, according to an extensive commentary accompanying an American Gastroenterological Association Clinical Practice Update.
“Patients and physicians want and expect no transmission of infections by any medical instrument,” wrote Bret Petersen, M.D., of the Mayo Clinic, Rochester, Minn., Johannes Koch, M.D., of Virginia Mason Medical Center, Seattle, and Gregory Ginsberg, M.D., of the University of Pennsylvania, Philadelphia. It is the collective responsibility of endoscope manufacturers, health systems, and providers to ensure endoscope reprocessing is mistake proof, establishing systems to identify and eliminate the risk of infection for patients undergoing flexible endoscopy.”
More than 650,000 endoscopic retrograde cholangiopancreatographies (ERCPs) occur in the United States annually, and “even the lowest reported defect rate of 0.7% will expose 4,500 patients to a preventable risk,” the experts noted. Carbapenem-resistant Enterobacteriaceae (CRE) are becoming more prevalent and have been transmitted during ERCP, even when personnel seem to have followed sterilization protocols to the letter. Clinical CRE infections have a fatality rate of at least 50%, months may elapse between exposure and symptom onset, and infections may involve distant organs. These factors, along with the phenomenon of “silent carriers,” have linked duodenoscopes to at least 250 multidrug-resistant infections and at least 20 fatalities worldwide, the experts wrote (Gastroenterology 2016 May 27. doi: 10.1053/j.gastro.2016.05.040).
Current duodenoscopes can be tough to sterilize. Between 1 billion and 1 trillion organisms typically cover a used instrument. Bedside cleaning cuts this number about 1,000-fold, and manual washing kills about another million organisms, leaving up to 1 billion bugs to be killed by high-level disinfection. That’s “a tall order” that can strain space, time, and staffing resources, especially given the fact that duodenoscopes have “tight crevices and mechanical joints that are exposed repeatedly to highly infectious bioburden,” the experts wrote. Furthermore, slips in processing enable the formation of biofilms that resist both cleaning and high-level disinfection.
The key to stopping duodenoscopes from transmitting dangerous pathogens is manual cleaning, including wiping the outside of the duodenoscope, flushing its channels, and brushing the elevator lever “immediately after use and before the surfaces have become dried,” the experts stressed. Disinfectants should be used at the right concentration and temperature, and for the intended amount of time. Biofilms form on moist surfaces only, so channels should be flushed with alcohol (a desiccant), dried with forced air, and stored in a dry environment.
But recent outbreaks spurred the Food and Drug Administration to recommend further steps – including better oversight and training of reprocessing staff and closer attention to precleaning, manual cleaning, and manufacturer recommendations for use, including determining whether the company used its own “proprietary” cleaning brushes in its validation studies, the experts noted. “Optional supplemental measures” include surveillance cultures of duodenoscopes, ethylene oxide sterilization, and double reprocessing, in which each scope undergoes two cycles of manual cleaning and high-intensity sterilization between patients. Double reprocessing might be the simplest and most easily adopted of these measures, the experts said. The AGA, for its part, recommends active surveillance of patients who undergo ERCP, surveillance cultures of scopes, and recording of the serial number of every scope used in every procedure.
Surveillance culture makes sense, but can be costly and hard to conduct and interpret because sampling detects vast numbers of nonpathogenic organisms in addition to any pathogens, the experts noted. The Centers for Disease Control and Prevention recommends that each institution follow its own complex outbreak sampling protocol and quarantine duodenoscopes for 2-3 days, pending negative results. That may mean buying more duodenoscopes. A less costly option is to culture a subset of scopes at the end of every workweek, the experts said. Real-time tests that reliably reflect bacterial culture results remain “elusive,” but testing for adenosine triphosphate after manual washing is easiest and best studied, they added.
Clearly, industry is responsible for making endoscopes that can be reliably disinfected. “Recent submissions by all three manufacturers (Olympus, Pentax, and Fujinon) have validated current reprocessing outcomes in test environments, and the FDA has ruled that postmarket studies of reprocessing in clinical settings are expected, but these results will not be forthcoming for several years,” the experts wrote. Redesigning duodenoscopes may be “the ultimate solution,” but in the meantime, endoscopists should carefully review indications for ERCP and ensure thorough informed consent. Doing so “will uphold the trust that we must achieve and maintain with our patients,” the authors said.
They had no funding sources. Dr. Koch has consulted for Sedasys, and Dr. Ginsberg has consulted for Olympus.
Contaminated duodenoscopes have caused multiple outbreaks of multidrug-resistant infections with sometimes lethal consequences; until these instruments become easier to clean, personnel must strictly follow recommendations for sterilization, surveillance, and unit-by-unit quality control, according to an extensive commentary accompanying an American Gastroenterological Association Clinical Practice Update.
“Patients and physicians want and expect no transmission of infections by any medical instrument,” wrote Bret Petersen, M.D., of the Mayo Clinic, Rochester, Minn., Johannes Koch, M.D., of Virginia Mason Medical Center, Seattle, and Gregory Ginsberg, M.D., of the University of Pennsylvania, Philadelphia. It is the collective responsibility of endoscope manufacturers, health systems, and providers to ensure endoscope reprocessing is mistake proof, establishing systems to identify and eliminate the risk of infection for patients undergoing flexible endoscopy.”
More than 650,000 endoscopic retrograde cholangiopancreatographies (ERCPs) occur in the United States annually, and “even the lowest reported defect rate of 0.7% will expose 4,500 patients to a preventable risk,” the experts noted. Carbapenem-resistant Enterobacteriaceae (CRE) are becoming more prevalent and have been transmitted during ERCP, even when personnel seem to have followed sterilization protocols to the letter. Clinical CRE infections have a fatality rate of at least 50%, months may elapse between exposure and symptom onset, and infections may involve distant organs. These factors, along with the phenomenon of “silent carriers,” have linked duodenoscopes to at least 250 multidrug-resistant infections and at least 20 fatalities worldwide, the experts wrote (Gastroenterology 2016 May 27. doi: 10.1053/j.gastro.2016.05.040).
Current duodenoscopes can be tough to sterilize. Between 1 billion and 1 trillion organisms typically cover a used instrument. Bedside cleaning cuts this number about 1,000-fold, and manual washing kills about another million organisms, leaving up to 1 billion bugs to be killed by high-level disinfection. That’s “a tall order” that can strain space, time, and staffing resources, especially given the fact that duodenoscopes have “tight crevices and mechanical joints that are exposed repeatedly to highly infectious bioburden,” the experts wrote. Furthermore, slips in processing enable the formation of biofilms that resist both cleaning and high-level disinfection.
The key to stopping duodenoscopes from transmitting dangerous pathogens is manual cleaning, including wiping the outside of the duodenoscope, flushing its channels, and brushing the elevator lever “immediately after use and before the surfaces have become dried,” the experts stressed. Disinfectants should be used at the right concentration and temperature, and for the intended amount of time. Biofilms form on moist surfaces only, so channels should be flushed with alcohol (a desiccant), dried with forced air, and stored in a dry environment.
But recent outbreaks spurred the Food and Drug Administration to recommend further steps – including better oversight and training of reprocessing staff and closer attention to precleaning, manual cleaning, and manufacturer recommendations for use, including determining whether the company used its own “proprietary” cleaning brushes in its validation studies, the experts noted. “Optional supplemental measures” include surveillance cultures of duodenoscopes, ethylene oxide sterilization, and double reprocessing, in which each scope undergoes two cycles of manual cleaning and high-intensity sterilization between patients. Double reprocessing might be the simplest and most easily adopted of these measures, the experts said. The AGA, for its part, recommends active surveillance of patients who undergo ERCP, surveillance cultures of scopes, and recording of the serial number of every scope used in every procedure.
Surveillance culture makes sense, but can be costly and hard to conduct and interpret because sampling detects vast numbers of nonpathogenic organisms in addition to any pathogens, the experts noted. The Centers for Disease Control and Prevention recommends that each institution follow its own complex outbreak sampling protocol and quarantine duodenoscopes for 2-3 days, pending negative results. That may mean buying more duodenoscopes. A less costly option is to culture a subset of scopes at the end of every workweek, the experts said. Real-time tests that reliably reflect bacterial culture results remain “elusive,” but testing for adenosine triphosphate after manual washing is easiest and best studied, they added.
Clearly, industry is responsible for making endoscopes that can be reliably disinfected. “Recent submissions by all three manufacturers (Olympus, Pentax, and Fujinon) have validated current reprocessing outcomes in test environments, and the FDA has ruled that postmarket studies of reprocessing in clinical settings are expected, but these results will not be forthcoming for several years,” the experts wrote. Redesigning duodenoscopes may be “the ultimate solution,” but in the meantime, endoscopists should carefully review indications for ERCP and ensure thorough informed consent. Doing so “will uphold the trust that we must achieve and maintain with our patients,” the authors said.
They had no funding sources. Dr. Koch has consulted for Sedasys, and Dr. Ginsberg has consulted for Olympus.
Contaminated duodenoscopes have caused multiple outbreaks of multidrug-resistant infections with sometimes lethal consequences; until these instruments become easier to clean, personnel must strictly follow recommendations for sterilization, surveillance, and unit-by-unit quality control, according to an extensive commentary accompanying an American Gastroenterological Association Clinical Practice Update.
“Patients and physicians want and expect no transmission of infections by any medical instrument,” wrote Bret Petersen, M.D., of the Mayo Clinic, Rochester, Minn., Johannes Koch, M.D., of Virginia Mason Medical Center, Seattle, and Gregory Ginsberg, M.D., of the University of Pennsylvania, Philadelphia. It is the collective responsibility of endoscope manufacturers, health systems, and providers to ensure endoscope reprocessing is mistake proof, establishing systems to identify and eliminate the risk of infection for patients undergoing flexible endoscopy.”
More than 650,000 endoscopic retrograde cholangiopancreatographies (ERCPs) occur in the United States annually, and “even the lowest reported defect rate of 0.7% will expose 4,500 patients to a preventable risk,” the experts noted. Carbapenem-resistant Enterobacteriaceae (CRE) are becoming more prevalent and have been transmitted during ERCP, even when personnel seem to have followed sterilization protocols to the letter. Clinical CRE infections have a fatality rate of at least 50%, months may elapse between exposure and symptom onset, and infections may involve distant organs. These factors, along with the phenomenon of “silent carriers,” have linked duodenoscopes to at least 250 multidrug-resistant infections and at least 20 fatalities worldwide, the experts wrote (Gastroenterology 2016 May 27. doi: 10.1053/j.gastro.2016.05.040).
Current duodenoscopes can be tough to sterilize. Between 1 billion and 1 trillion organisms typically cover a used instrument. Bedside cleaning cuts this number about 1,000-fold, and manual washing kills about another million organisms, leaving up to 1 billion bugs to be killed by high-level disinfection. That’s “a tall order” that can strain space, time, and staffing resources, especially given the fact that duodenoscopes have “tight crevices and mechanical joints that are exposed repeatedly to highly infectious bioburden,” the experts wrote. Furthermore, slips in processing enable the formation of biofilms that resist both cleaning and high-level disinfection.
The key to stopping duodenoscopes from transmitting dangerous pathogens is manual cleaning, including wiping the outside of the duodenoscope, flushing its channels, and brushing the elevator lever “immediately after use and before the surfaces have become dried,” the experts stressed. Disinfectants should be used at the right concentration and temperature, and for the intended amount of time. Biofilms form on moist surfaces only, so channels should be flushed with alcohol (a desiccant), dried with forced air, and stored in a dry environment.
But recent outbreaks spurred the Food and Drug Administration to recommend further steps – including better oversight and training of reprocessing staff and closer attention to precleaning, manual cleaning, and manufacturer recommendations for use, including determining whether the company used its own “proprietary” cleaning brushes in its validation studies, the experts noted. “Optional supplemental measures” include surveillance cultures of duodenoscopes, ethylene oxide sterilization, and double reprocessing, in which each scope undergoes two cycles of manual cleaning and high-intensity sterilization between patients. Double reprocessing might be the simplest and most easily adopted of these measures, the experts said. The AGA, for its part, recommends active surveillance of patients who undergo ERCP, surveillance cultures of scopes, and recording of the serial number of every scope used in every procedure.
Surveillance culture makes sense, but can be costly and hard to conduct and interpret because sampling detects vast numbers of nonpathogenic organisms in addition to any pathogens, the experts noted. The Centers for Disease Control and Prevention recommends that each institution follow its own complex outbreak sampling protocol and quarantine duodenoscopes for 2-3 days, pending negative results. That may mean buying more duodenoscopes. A less costly option is to culture a subset of scopes at the end of every workweek, the experts said. Real-time tests that reliably reflect bacterial culture results remain “elusive,” but testing for adenosine triphosphate after manual washing is easiest and best studied, they added.
Clearly, industry is responsible for making endoscopes that can be reliably disinfected. “Recent submissions by all three manufacturers (Olympus, Pentax, and Fujinon) have validated current reprocessing outcomes in test environments, and the FDA has ruled that postmarket studies of reprocessing in clinical settings are expected, but these results will not be forthcoming for several years,” the experts wrote. Redesigning duodenoscopes may be “the ultimate solution,” but in the meantime, endoscopists should carefully review indications for ERCP and ensure thorough informed consent. Doing so “will uphold the trust that we must achieve and maintain with our patients,” the authors said.
They had no funding sources. Dr. Koch has consulted for Sedasys, and Dr. Ginsberg has consulted for Olympus.
FROM GASTROENTEROLOGY
Medicaid expansion linked to lower uninsured rates
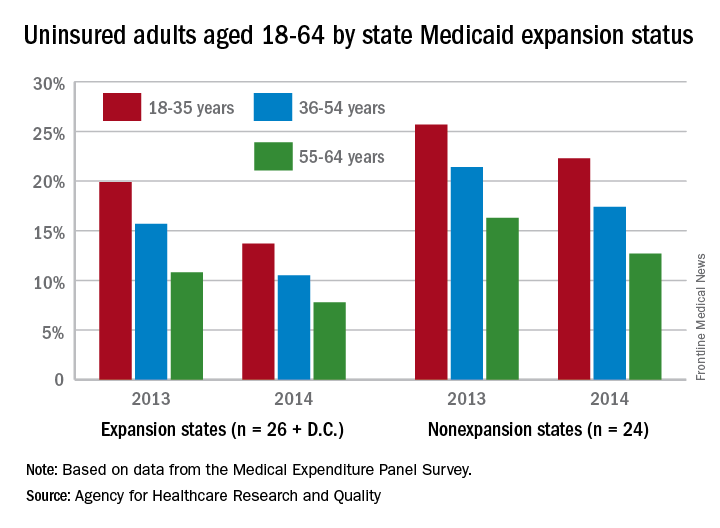
The overall uninsured rate for nonelderly adults took a significant drop from 18.8% in 2013 to 14.4% in 2014, with rates by age lower and declines generally larger among states that expanded Medicaid coverage, according to the Agency for Healthcare Research and Quality.
Among adults aged 18-35 years, states (and the District of Columbia) that expanded Medicaid had a larger drop in the percentage who were uninsured for the entire calendar year, going from 19.9% in 2013 to 13.7% in 2014 (6.2 percentage points), than did states that did not expand Medicaid, which dropped from 25.7% to 22.3% (3.4 percentage points), the AHRQ reported.
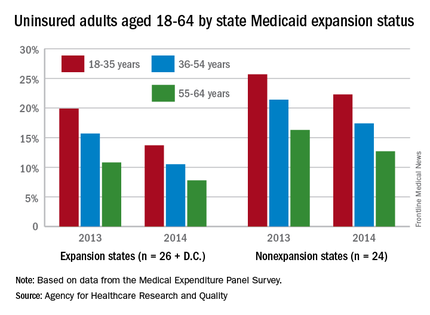
For adults aged 36-54 years, the situation was similar: The uninsured rate in states that expanded Medicaid went from 15.7% in 2013 to 10.5% in 2014, or 5.2 percentage points, while the rate dropped from 21.4% to 17.4%, or 4 percentage points, in states that did not expand Medicaid, the report noted.
Uninsured rates were lower among adults aged 56-64 in states that did expand Medicaid, but the absolute decrease was actually larger among states that did not expand it. The rate in nonexpanding states decreased by 3.6 percentage points, 16.3% to 12.7%, while expanding states saw a drop from 10.8% to 7.8%, 3 percentage points, according to data from the Medical Expenditure Panel Survey.
The overall uninsured rate for nonelderly adults took a significant drop from 18.8% in 2013 to 14.4% in 2014, with rates by age lower and declines generally larger among states that expanded Medicaid coverage, according to the Agency for Healthcare Research and Quality.
Among adults aged 18-35 years, states (and the District of Columbia) that expanded Medicaid had a larger drop in the percentage who were uninsured for the entire calendar year, going from 19.9% in 2013 to 13.7% in 2014 (6.2 percentage points), than did states that did not expand Medicaid, which dropped from 25.7% to 22.3% (3.4 percentage points), the AHRQ reported.

For adults aged 36-54 years, the situation was similar: The uninsured rate in states that expanded Medicaid went from 15.7% in 2013 to 10.5% in 2014, or 5.2 percentage points, while the rate dropped from 21.4% to 17.4%, or 4 percentage points, in states that did not expand Medicaid, the report noted.
Uninsured rates were lower among adults aged 56-64 in states that did expand Medicaid, but the absolute decrease was actually larger among states that did not expand it. The rate in nonexpanding states decreased by 3.6 percentage points, 16.3% to 12.7%, while expanding states saw a drop from 10.8% to 7.8%, 3 percentage points, according to data from the Medical Expenditure Panel Survey.
The overall uninsured rate for nonelderly adults took a significant drop from 18.8% in 2013 to 14.4% in 2014, with rates by age lower and declines generally larger among states that expanded Medicaid coverage, according to the Agency for Healthcare Research and Quality.
Among adults aged 18-35 years, states (and the District of Columbia) that expanded Medicaid had a larger drop in the percentage who were uninsured for the entire calendar year, going from 19.9% in 2013 to 13.7% in 2014 (6.2 percentage points), than did states that did not expand Medicaid, which dropped from 25.7% to 22.3% (3.4 percentage points), the AHRQ reported.

For adults aged 36-54 years, the situation was similar: The uninsured rate in states that expanded Medicaid went from 15.7% in 2013 to 10.5% in 2014, or 5.2 percentage points, while the rate dropped from 21.4% to 17.4%, or 4 percentage points, in states that did not expand Medicaid, the report noted.
Uninsured rates were lower among adults aged 56-64 in states that did expand Medicaid, but the absolute decrease was actually larger among states that did not expand it. The rate in nonexpanding states decreased by 3.6 percentage points, 16.3% to 12.7%, while expanding states saw a drop from 10.8% to 7.8%, 3 percentage points, according to data from the Medical Expenditure Panel Survey.
Continuing demand for internists drives up salaries
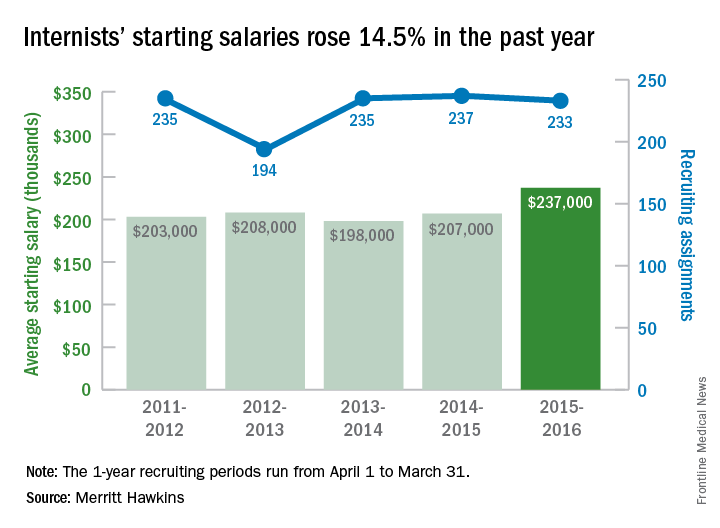
The average starting salary for general internists was up 14.5% in the last year, with growing physician shortages leading to increased demand, according to physician recruitment firm Merritt Hawkins.
The average starting salary was $237,000 among general internists recruited by the company in the 12 months from April 1, 2015, to March 31, 2016, compared with $207,000 the previous year. Of the 3,342 recruiting searches conducted in that year, 233 involved general internal medicine, third highest behind family medicine and psychiatry among the 19 medical specialties tracked in the company’s 2016 Review of Physician and Advanced Practitioner Recruiting Incentives.

Internal medicine had been second on the list of the most requested recruiting assignments for 9 consecutive years. The number of searches for 2015-2016 was down just slightly from the previous year, however, suggesting that the increased “focus health care providers are putting on addressing mental health challenges” does not represent a turn away from internal medicine, the report noted.
It is, possibly, “the migration of many general internists into hospitalist roles [that] has limited the supply of physicians willing to practice traditional internal medicine and increased salary offers to those who are willing to do so,” Merritt Hawkins said.
Starting salaries were up for 18 of the 19 specialties, with only emergency medicine showing a decease. “Demand for physicians is as intense as we have seen it in our 29-year history,” Travis Singleton, senior vice president of Merritt Hawkins, said in a separate statement. “The expansion of health insurance coverage, population growth, population aging, expanded care sites such as urgent care centers and other factors are driving demand for doctors through the roof, and salaries are spiking as a consequence.”
The average starting salary for general internists was up 14.5% in the last year, with growing physician shortages leading to increased demand, according to physician recruitment firm Merritt Hawkins.
The average starting salary was $237,000 among general internists recruited by the company in the 12 months from April 1, 2015, to March 31, 2016, compared with $207,000 the previous year. Of the 3,342 recruiting searches conducted in that year, 233 involved general internal medicine, third highest behind family medicine and psychiatry among the 19 medical specialties tracked in the company’s 2016 Review of Physician and Advanced Practitioner Recruiting Incentives.

Internal medicine had been second on the list of the most requested recruiting assignments for 9 consecutive years. The number of searches for 2015-2016 was down just slightly from the previous year, however, suggesting that the increased “focus health care providers are putting on addressing mental health challenges” does not represent a turn away from internal medicine, the report noted.
It is, possibly, “the migration of many general internists into hospitalist roles [that] has limited the supply of physicians willing to practice traditional internal medicine and increased salary offers to those who are willing to do so,” Merritt Hawkins said.
Starting salaries were up for 18 of the 19 specialties, with only emergency medicine showing a decease. “Demand for physicians is as intense as we have seen it in our 29-year history,” Travis Singleton, senior vice president of Merritt Hawkins, said in a separate statement. “The expansion of health insurance coverage, population growth, population aging, expanded care sites such as urgent care centers and other factors are driving demand for doctors through the roof, and salaries are spiking as a consequence.”
The average starting salary for general internists was up 14.5% in the last year, with growing physician shortages leading to increased demand, according to physician recruitment firm Merritt Hawkins.
The average starting salary was $237,000 among general internists recruited by the company in the 12 months from April 1, 2015, to March 31, 2016, compared with $207,000 the previous year. Of the 3,342 recruiting searches conducted in that year, 233 involved general internal medicine, third highest behind family medicine and psychiatry among the 19 medical specialties tracked in the company’s 2016 Review of Physician and Advanced Practitioner Recruiting Incentives.

Internal medicine had been second on the list of the most requested recruiting assignments for 9 consecutive years. The number of searches for 2015-2016 was down just slightly from the previous year, however, suggesting that the increased “focus health care providers are putting on addressing mental health challenges” does not represent a turn away from internal medicine, the report noted.
It is, possibly, “the migration of many general internists into hospitalist roles [that] has limited the supply of physicians willing to practice traditional internal medicine and increased salary offers to those who are willing to do so,” Merritt Hawkins said.
Starting salaries were up for 18 of the 19 specialties, with only emergency medicine showing a decease. “Demand for physicians is as intense as we have seen it in our 29-year history,” Travis Singleton, senior vice president of Merritt Hawkins, said in a separate statement. “The expansion of health insurance coverage, population growth, population aging, expanded care sites such as urgent care centers and other factors are driving demand for doctors through the roof, and salaries are spiking as a consequence.”
Newer insulin glargine formula curbs nocturnal hypoglycemia
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
NEW ORLEANS – Insulin glargine 300 U/mL provided comparable glycemic control to that seen with insulin glargine 100 U/mL and consistently reduced the risk of nocturnal hypoglycemia in patients with type 2 diabetes, regardless of their renal function, results from a large post hoc meta-analysis showed.
The EDITION I, II, and III studies showed that over a period of 6 months, Gla-300 provided comparable glycemic control to Gla-100 with less hypoglycemia in patients with type 2 diabetes. However, “renal impairment increases the risk of hypoglycemia in people with type 2 diabetes, and may limit glucose-lowering therapy options,” Javier Escalada, M.D., said at the annual scientific sessions of the American Diabetes Association. “Therefore, it may be more challenging to manage diabetes in this population than in people with normal renal function.”
Dr. Escalada of the department of endocrinology and nutrition at Clinic University of Navarra, Pamplona, Spain, and his associates set out to investigate the impact of renal function on hemoglobin A1c reduction and hypoglycemia in a post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies. Treatment consisted of once-daily evening doses of Gla-300 or Gla-100 titrated to a fasting self-measured plasma glucose of 80-100 mg/dL. Patients were classified by their renal function as having moderate loss (30 to less than 60 mL/min per 1.73 m3; 399 patients), mild loss (60 to less than 90; 1,386 patients), or normal function (at least 90; 683 patients).
Outcomes of interest were change in HbA1c from baseline to month 6, and the percentages of patients achieving an HbA1c target of lower than 7.0% and lower than 7.5% at month 6. The researchers also assessed the cumulative number of hypoglycemic events, the relative risk of at least one confirmed or severe hypoglycemic event, and the nocturnal and at any time event rate per participant year.
Slightly more than half of participants (56%) had a baseline estimated glomerular filtration rate of 60 to less than 90 mL/min per 1.73 m3. Dr. Escalada reported that noninferiority for HbA1c reduction was shown for Gla-300 and Gla-100 regardless of renal function, and that evidence of heterogeneity of treatment effect across subgroups was observed (P = .46). However, the risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group. Renal function did not affect the lower rate of nocturnal or anytime hypoglycemia. “Severe hypoglycemia was rare, and renal function did not affect the rate of severe events,” he said.
The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Insulin glargine 300 U/mL provided glycemic control comparable to insulin glargine 100 U/mL, but it reduced the risk of nocturnal hypoglycemia by a greater margin, regardless of renal function.
Major finding: The risk of confirmed or severe hypoglycemia was significantly lower for nocturnal events in the Gla-300 group, compared with the Gla-100 group (30% vs. 40% overall, respectively), while the risk of anytime hypoglycemia events in a 24-hour period was comparable to or lower in the Gla-300 group, compared with the Gla-100 group.
Data source: A post hoc meta-analysis of 2,468 patients aged 18 years and older with type 2 diabetes who were treated with Gla-300 or Gla-100 for 6 months in the EDITION I, II, and III studies.
Disclosures: The trial was sponsored by Sanofi. Dr. Escalada disclosed that he is a member of the advisory panel for Sanofi and for Merck Sharp & Dohme. He is also a member of the speakers bureau for both companies as well as for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
‘Clarion call’ to screen for, treat aldosteronism
The Endocrine Society’s updated Clinical Practice Guideline for managing primary aldosteronism is “a clarion call” for physicians to recognize the impact of this substantial public health problem and dramatically ramp up their screening and treatment efforts and was published in the Journal of Clinical Endocrinology and Metabolism.
This update differs from the previous (2008) version of the guideline in “the explicit recognition of primary aldosteronism as a major public health issue and not merely a matter of case detection, diagnosis, and treatment of individual patients,” wrote John W. Funder, MD, and his associates on the task force that compiled the guideline.
Many physicians in current practice were taught that the disorder “is a rare and benign cause of hypertension, [and] thus merely a footnote to the management of hypertension as a whole. Cardiologists usually write guidelines for hypertension with some input from nephrologists and clinical pharmacologists [but] little or none from endocrinologists,” they noted.
As a result, most patients with hypertension and occult aldosteronism are never screened for the disorder and receive suboptimal care. Primary care providers must be “made keenly aware” that the proportion of people with hypertension who have aldosteronism is much higher than previously thought (at roughly 10%), that another 20% of hypertensive people have “inappropriate aldosterone secretion,” and that both groups respond remarkably well to medical therapy, particularly to mineralocorticoid-receptor antagonists. This is critical because hypertensive patients with aldosteronism are at much greater risk for cardiovascular morbidity and mortality than their age-, sex-, and BP-matched counterparts who don’t have aldosteronism, said Dr. Funder of the Hudson Institute of Medical Research, Clayton (VIC), Australia, and his associates.
In addition to recommendations regarding screening and treatment and summaries of the evidence on which those recommendations are based, the new guideline offers a remarks section for each recommendation, which includes technical suggestions to help clinicians implement them in real-world practice.
Among the Guideline’s recommendations:
• Screen for primary aldosteronism all patients who have sustained BP above 150/100 mm Hg, hypertension resistant to three conventional antihypertensive drugs, hypertension that requires four or more drugs to control it, hypertension plus hypokalemia, hypertension plus adrenal incidentaloma, hypertension plus sleep apnea, hypertension plus a family history of early-onset hypertension or stroke at a young age, and hypertension plus a first-degree relative with primary aldosteronism.
Use the plasma aldosterone/renin ratio for this screening.
• Do one or more confirmatory tests to definitively confirm the diagnosis before proceeding to subtype classification. The exception to this recommendation is patients who develop spontaneous hypokalemia.
• Do adrenal CT as the initial step in subtype classification, to exclude large masses that may signal adrenocortical carcinoma and to help interventional radiologists and surgeons make anatomic assessments.
Before surgery, an experienced radiologist should determine whether adrenal disease is unilateral or bilateral using adrenal venous sampling.
Order genetic testing for patients with disease onset before age 20 years and for those who have a family history of either aldosteronism or early stroke.
• Laparoscopic adrenalectomy is the surgery of choice for most patients with unilateral adrenal disease. For patients unwilling or unable to undergo surgery or further investigations, prescribe a mineralocorticoid-receptor antagonist.
Medical therapy is the treatment of choice for bilateral adrenal disease. Spironolactone is the first-line agent, and eplerenone is an alternative agent to offer. This guideline is intended to be revised further as management evolves over the next 5 years. It is likely that by then, a rapid, inexpensive confirmatory test will be available to definitively establish the diagnosis and that third- and perhaps fourth-generation mineralocorticoid-receptor antagonists will be available for treatment. Simpler and more accurate methods of measuring plasma aldosterone concentration and direct renin concentration, which “would be a game changer for the primary care physician,” are currently being developed, Dr. Funder and his associates said (J. Clin. Endocrinol. Metab. 2016 May;101:1889-916).
In the meantime, “the main strategy is to convince primary-care physicians to screen for primary aldosteronism in all at-risk hypertensive patients,” they noted.
Copies of the full Guideline are available at [email protected] or by calling 202-971-3636.
The Endocrine Society’s updated Clinical Practice Guideline for managing primary aldosteronism is “a clarion call” for physicians to recognize the impact of this substantial public health problem and dramatically ramp up their screening and treatment efforts and was published in the Journal of Clinical Endocrinology and Metabolism.
This update differs from the previous (2008) version of the guideline in “the explicit recognition of primary aldosteronism as a major public health issue and not merely a matter of case detection, diagnosis, and treatment of individual patients,” wrote John W. Funder, MD, and his associates on the task force that compiled the guideline.
Many physicians in current practice were taught that the disorder “is a rare and benign cause of hypertension, [and] thus merely a footnote to the management of hypertension as a whole. Cardiologists usually write guidelines for hypertension with some input from nephrologists and clinical pharmacologists [but] little or none from endocrinologists,” they noted.
As a result, most patients with hypertension and occult aldosteronism are never screened for the disorder and receive suboptimal care. Primary care providers must be “made keenly aware” that the proportion of people with hypertension who have aldosteronism is much higher than previously thought (at roughly 10%), that another 20% of hypertensive people have “inappropriate aldosterone secretion,” and that both groups respond remarkably well to medical therapy, particularly to mineralocorticoid-receptor antagonists. This is critical because hypertensive patients with aldosteronism are at much greater risk for cardiovascular morbidity and mortality than their age-, sex-, and BP-matched counterparts who don’t have aldosteronism, said Dr. Funder of the Hudson Institute of Medical Research, Clayton (VIC), Australia, and his associates.
In addition to recommendations regarding screening and treatment and summaries of the evidence on which those recommendations are based, the new guideline offers a remarks section for each recommendation, which includes technical suggestions to help clinicians implement them in real-world practice.
Among the Guideline’s recommendations:
• Screen for primary aldosteronism all patients who have sustained BP above 150/100 mm Hg, hypertension resistant to three conventional antihypertensive drugs, hypertension that requires four or more drugs to control it, hypertension plus hypokalemia, hypertension plus adrenal incidentaloma, hypertension plus sleep apnea, hypertension plus a family history of early-onset hypertension or stroke at a young age, and hypertension plus a first-degree relative with primary aldosteronism.
Use the plasma aldosterone/renin ratio for this screening.
• Do one or more confirmatory tests to definitively confirm the diagnosis before proceeding to subtype classification. The exception to this recommendation is patients who develop spontaneous hypokalemia.
• Do adrenal CT as the initial step in subtype classification, to exclude large masses that may signal adrenocortical carcinoma and to help interventional radiologists and surgeons make anatomic assessments.
Before surgery, an experienced radiologist should determine whether adrenal disease is unilateral or bilateral using adrenal venous sampling.
Order genetic testing for patients with disease onset before age 20 years and for those who have a family history of either aldosteronism or early stroke.
• Laparoscopic adrenalectomy is the surgery of choice for most patients with unilateral adrenal disease. For patients unwilling or unable to undergo surgery or further investigations, prescribe a mineralocorticoid-receptor antagonist.
Medical therapy is the treatment of choice for bilateral adrenal disease. Spironolactone is the first-line agent, and eplerenone is an alternative agent to offer. This guideline is intended to be revised further as management evolves over the next 5 years. It is likely that by then, a rapid, inexpensive confirmatory test will be available to definitively establish the diagnosis and that third- and perhaps fourth-generation mineralocorticoid-receptor antagonists will be available for treatment. Simpler and more accurate methods of measuring plasma aldosterone concentration and direct renin concentration, which “would be a game changer for the primary care physician,” are currently being developed, Dr. Funder and his associates said (J. Clin. Endocrinol. Metab. 2016 May;101:1889-916).
In the meantime, “the main strategy is to convince primary-care physicians to screen for primary aldosteronism in all at-risk hypertensive patients,” they noted.
Copies of the full Guideline are available at [email protected] or by calling 202-971-3636.
The Endocrine Society’s updated Clinical Practice Guideline for managing primary aldosteronism is “a clarion call” for physicians to recognize the impact of this substantial public health problem and dramatically ramp up their screening and treatment efforts and was published in the Journal of Clinical Endocrinology and Metabolism.
This update differs from the previous (2008) version of the guideline in “the explicit recognition of primary aldosteronism as a major public health issue and not merely a matter of case detection, diagnosis, and treatment of individual patients,” wrote John W. Funder, MD, and his associates on the task force that compiled the guideline.
Many physicians in current practice were taught that the disorder “is a rare and benign cause of hypertension, [and] thus merely a footnote to the management of hypertension as a whole. Cardiologists usually write guidelines for hypertension with some input from nephrologists and clinical pharmacologists [but] little or none from endocrinologists,” they noted.
As a result, most patients with hypertension and occult aldosteronism are never screened for the disorder and receive suboptimal care. Primary care providers must be “made keenly aware” that the proportion of people with hypertension who have aldosteronism is much higher than previously thought (at roughly 10%), that another 20% of hypertensive people have “inappropriate aldosterone secretion,” and that both groups respond remarkably well to medical therapy, particularly to mineralocorticoid-receptor antagonists. This is critical because hypertensive patients with aldosteronism are at much greater risk for cardiovascular morbidity and mortality than their age-, sex-, and BP-matched counterparts who don’t have aldosteronism, said Dr. Funder of the Hudson Institute of Medical Research, Clayton (VIC), Australia, and his associates.
In addition to recommendations regarding screening and treatment and summaries of the evidence on which those recommendations are based, the new guideline offers a remarks section for each recommendation, which includes technical suggestions to help clinicians implement them in real-world practice.
Among the Guideline’s recommendations:
• Screen for primary aldosteronism all patients who have sustained BP above 150/100 mm Hg, hypertension resistant to three conventional antihypertensive drugs, hypertension that requires four or more drugs to control it, hypertension plus hypokalemia, hypertension plus adrenal incidentaloma, hypertension plus sleep apnea, hypertension plus a family history of early-onset hypertension or stroke at a young age, and hypertension plus a first-degree relative with primary aldosteronism.
Use the plasma aldosterone/renin ratio for this screening.
• Do one or more confirmatory tests to definitively confirm the diagnosis before proceeding to subtype classification. The exception to this recommendation is patients who develop spontaneous hypokalemia.
• Do adrenal CT as the initial step in subtype classification, to exclude large masses that may signal adrenocortical carcinoma and to help interventional radiologists and surgeons make anatomic assessments.
Before surgery, an experienced radiologist should determine whether adrenal disease is unilateral or bilateral using adrenal venous sampling.
Order genetic testing for patients with disease onset before age 20 years and for those who have a family history of either aldosteronism or early stroke.
• Laparoscopic adrenalectomy is the surgery of choice for most patients with unilateral adrenal disease. For patients unwilling or unable to undergo surgery or further investigations, prescribe a mineralocorticoid-receptor antagonist.
Medical therapy is the treatment of choice for bilateral adrenal disease. Spironolactone is the first-line agent, and eplerenone is an alternative agent to offer. This guideline is intended to be revised further as management evolves over the next 5 years. It is likely that by then, a rapid, inexpensive confirmatory test will be available to definitively establish the diagnosis and that third- and perhaps fourth-generation mineralocorticoid-receptor antagonists will be available for treatment. Simpler and more accurate methods of measuring plasma aldosterone concentration and direct renin concentration, which “would be a game changer for the primary care physician,” are currently being developed, Dr. Funder and his associates said (J. Clin. Endocrinol. Metab. 2016 May;101:1889-916).
In the meantime, “the main strategy is to convince primary-care physicians to screen for primary aldosteronism in all at-risk hypertensive patients,” they noted.
Copies of the full Guideline are available at [email protected] or by calling 202-971-3636.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Key clinical point: The Endocrine Society’s updated Clinical Practice Guideline for managing primary aldosteronism is “a clarion call” for physicians to ramp up screening for and treatment of this major public health problem.
Major finding: Primary care physicians must be convinced to screen all at-risk hypertensive patients for primary aldosteronism.
Data source: A comprehensive review of the literature and detailed update of a Clinical Practice Guideline for managing primary aldosteronism.
Disclosures: The Endocrine Society provided all the support for this Guideline. Dr. Funder’s and his associates’ conflicts of interest are available from the Endocrine Society.
New antibiotics targeting MDR pathogens are expensive, but not impressive
The U.S. Food and Drug Administration has approved a number of new antibiotics targeting multidrug-resistant bacteria in the past 5 years, but the new drugs have not led to a substantial improvement in patient outcomes when compared with existing antibiotics, according to a recent analysis in the Annals of Internal Medicine.
The eight new antibiotics approved by the FDA between January 2010 and December 2015 were ceftaroline, fidaxomicin, bedaquiline, dalbavancin, tedizolid, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam. Of those eight drugs, only three showed in vitro activity against the so-called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumonia, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species). Only one drug, fidaxomicin, demonstrated in vitro activity against an urgent-threat pathogen from the Centers for Disease Control and Prevention, Clostridium difficile. Bedaquiline was the only new antibiotic specifically indicated for a disease from a multidrug-resistant pathogen, although the investigators said most of the drugs demonstrated in vitro activity against gram-positive drug-resistant pathogens.
Importantly, the authors noted that in vitro activity does not necessarily reflect benefits on actual patient clinical outcomes, as exemplified by such drugs as tigecycline and doripenem.
The researchers found what they called “important deficiencies in the clinical trials leading to approval of these new antibiotic products.” Most pivotal trial designs were primarily noninferiority trials, and the antibiotics were not studied to evaluate whether they have substantial benefits in efficacy over what is currently available, they noted. Additionally, none of the trials evaluated direct patient outcomes as primary end points, and some drugs did not have confirmatory evidence from a second independent trial or did not have any confirmatory trials.
Researchers also examined the prices of a single dose of the new antibiotics. The prices ranged from $1,195 to $4,183 (4-14 days of ceftolozane/tazobactam for acute pyelonephritis and intra-abdominal infections) to $69,702 (24 weeks of bedaquiline) – quite a premium for antibiotics showing unclear evidence of additional benefit.
“As antibiotic innovation continues to move forward, greater attention needs to be paid to incentives for developing high-quality new products with demonstrated superiority to existing products on outcomes in patients with multidrug-resistant disease, replacing the current focus on quantity and presumed future benefits,” researchers concluded.
Read the full study in the Annals of Internal Medicine (doi: 10.7326/M16-0291).
The U.S. Food and Drug Administration has approved a number of new antibiotics targeting multidrug-resistant bacteria in the past 5 years, but the new drugs have not led to a substantial improvement in patient outcomes when compared with existing antibiotics, according to a recent analysis in the Annals of Internal Medicine.
The eight new antibiotics approved by the FDA between January 2010 and December 2015 were ceftaroline, fidaxomicin, bedaquiline, dalbavancin, tedizolid, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam. Of those eight drugs, only three showed in vitro activity against the so-called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumonia, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species). Only one drug, fidaxomicin, demonstrated in vitro activity against an urgent-threat pathogen from the Centers for Disease Control and Prevention, Clostridium difficile. Bedaquiline was the only new antibiotic specifically indicated for a disease from a multidrug-resistant pathogen, although the investigators said most of the drugs demonstrated in vitro activity against gram-positive drug-resistant pathogens.
Importantly, the authors noted that in vitro activity does not necessarily reflect benefits on actual patient clinical outcomes, as exemplified by such drugs as tigecycline and doripenem.
The researchers found what they called “important deficiencies in the clinical trials leading to approval of these new antibiotic products.” Most pivotal trial designs were primarily noninferiority trials, and the antibiotics were not studied to evaluate whether they have substantial benefits in efficacy over what is currently available, they noted. Additionally, none of the trials evaluated direct patient outcomes as primary end points, and some drugs did not have confirmatory evidence from a second independent trial or did not have any confirmatory trials.
Researchers also examined the prices of a single dose of the new antibiotics. The prices ranged from $1,195 to $4,183 (4-14 days of ceftolozane/tazobactam for acute pyelonephritis and intra-abdominal infections) to $69,702 (24 weeks of bedaquiline) – quite a premium for antibiotics showing unclear evidence of additional benefit.
“As antibiotic innovation continues to move forward, greater attention needs to be paid to incentives for developing high-quality new products with demonstrated superiority to existing products on outcomes in patients with multidrug-resistant disease, replacing the current focus on quantity and presumed future benefits,” researchers concluded.
Read the full study in the Annals of Internal Medicine (doi: 10.7326/M16-0291).
The U.S. Food and Drug Administration has approved a number of new antibiotics targeting multidrug-resistant bacteria in the past 5 years, but the new drugs have not led to a substantial improvement in patient outcomes when compared with existing antibiotics, according to a recent analysis in the Annals of Internal Medicine.
The eight new antibiotics approved by the FDA between January 2010 and December 2015 were ceftaroline, fidaxomicin, bedaquiline, dalbavancin, tedizolid, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam. Of those eight drugs, only three showed in vitro activity against the so-called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumonia, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species). Only one drug, fidaxomicin, demonstrated in vitro activity against an urgent-threat pathogen from the Centers for Disease Control and Prevention, Clostridium difficile. Bedaquiline was the only new antibiotic specifically indicated for a disease from a multidrug-resistant pathogen, although the investigators said most of the drugs demonstrated in vitro activity against gram-positive drug-resistant pathogens.
Importantly, the authors noted that in vitro activity does not necessarily reflect benefits on actual patient clinical outcomes, as exemplified by such drugs as tigecycline and doripenem.
The researchers found what they called “important deficiencies in the clinical trials leading to approval of these new antibiotic products.” Most pivotal trial designs were primarily noninferiority trials, and the antibiotics were not studied to evaluate whether they have substantial benefits in efficacy over what is currently available, they noted. Additionally, none of the trials evaluated direct patient outcomes as primary end points, and some drugs did not have confirmatory evidence from a second independent trial or did not have any confirmatory trials.
Researchers also examined the prices of a single dose of the new antibiotics. The prices ranged from $1,195 to $4,183 (4-14 days of ceftolozane/tazobactam for acute pyelonephritis and intra-abdominal infections) to $69,702 (24 weeks of bedaquiline) – quite a premium for antibiotics showing unclear evidence of additional benefit.
“As antibiotic innovation continues to move forward, greater attention needs to be paid to incentives for developing high-quality new products with demonstrated superiority to existing products on outcomes in patients with multidrug-resistant disease, replacing the current focus on quantity and presumed future benefits,” researchers concluded.
Read the full study in the Annals of Internal Medicine (doi: 10.7326/M16-0291).
FROM ANNALS OF INTERNAL MEDICINE
Not enough evidence to support LAA closure by device or surgery for atrial fib
FROM CIRCULATION: CARDIOVASCULAR QUALITY OUTCOMES
Insufficient evidence exists to routinely recommend surgical closure of the left atrial appendage, either surgically or with a LAA exclusion device, for patients who have atrial fibrillation, a large data review has concluded.
None of the devices so far examined is more effective than oral anticoagulation therapy in reducing AF-related stroke risk, but they appear to be riskier, with up to 1 in 15 patients experiencing a serious adverse event during or after percutaneous placement, North Noelck, MD, and his colleagues wrote (Circ Cardiovasc Qual Outcomes. 2016 Jul 12. doi: 10.1161/CIRCOUTCOMES.115.002539).
Surgical studies, which also have found no benefit, are poor in quality and provide no strong data in favor of any technique, wrote Dr. Noelck of Oregon Health and Science University, Portland.
The meta-analysis comprised 13 studies of the benefits and risks of percutaneous LAA exclusion and 7 of the benefits and harms of surgical LAA closure.
The team found “limited evidence” that one device, the Watchman (Boston Scientific), may be an effective alternative to long-term oral anticoagulation treatment in some patients. But the Watchman was also associated with “significant, procedural-related harms,” in almost 11% of patients in one large study, PROTECT-AF. These included pericardial effusions with and without associated tamponade, bleeding, device thrombus, and device embolization. However, the benefit conferred by the device appeared marginal. Neither PROTECT-AF nor the subsequent PREVAIL studies of the Watchman found that it conferred significant clinical benefit above the comparator arm of warfarin therapy. Indeed, in PREVAIL, a composite outcome of death, ischemic/hemorrhagic stroke, or systemic embolism occurred in 5.2% of the device group and in 2.9% of the warfarin group.
Three randomized studies and two observational studies examined surgical LAA closure relative to usual medical care. The authors said the randomized studies were underpowered to show clinical benefit. Neither of the observational studies showed significant advantages in stroke-free survival, but the team noted, “data such as information about anticoagulation use among the groups [were] lacking.”
“Overall, there is insufficient evidence to support the routine use of surgical LAA exclusion to reduce stroke risk or the future need for anticoagulant therapy,” wrote Dr. Noelck and his coinvestigators. However, they said, several ongoing studies “should add substantively to this body of evidence during the next several years.”
The study was funded by the Department of Veterans Affairs. None of the authors had any relevant financial disclosure.
FROM CIRCULATION: CARDIOVASCULAR QUALITY OUTCOMES
Insufficient evidence exists to routinely recommend surgical closure of the left atrial appendage, either surgically or with a LAA exclusion device, for patients who have atrial fibrillation, a large data review has concluded.
None of the devices so far examined is more effective than oral anticoagulation therapy in reducing AF-related stroke risk, but they appear to be riskier, with up to 1 in 15 patients experiencing a serious adverse event during or after percutaneous placement, North Noelck, MD, and his colleagues wrote (Circ Cardiovasc Qual Outcomes. 2016 Jul 12. doi: 10.1161/CIRCOUTCOMES.115.002539).
Surgical studies, which also have found no benefit, are poor in quality and provide no strong data in favor of any technique, wrote Dr. Noelck of Oregon Health and Science University, Portland.
The meta-analysis comprised 13 studies of the benefits and risks of percutaneous LAA exclusion and 7 of the benefits and harms of surgical LAA closure.
The team found “limited evidence” that one device, the Watchman (Boston Scientific), may be an effective alternative to long-term oral anticoagulation treatment in some patients. But the Watchman was also associated with “significant, procedural-related harms,” in almost 11% of patients in one large study, PROTECT-AF. These included pericardial effusions with and without associated tamponade, bleeding, device thrombus, and device embolization. However, the benefit conferred by the device appeared marginal. Neither PROTECT-AF nor the subsequent PREVAIL studies of the Watchman found that it conferred significant clinical benefit above the comparator arm of warfarin therapy. Indeed, in PREVAIL, a composite outcome of death, ischemic/hemorrhagic stroke, or systemic embolism occurred in 5.2% of the device group and in 2.9% of the warfarin group.
Three randomized studies and two observational studies examined surgical LAA closure relative to usual medical care. The authors said the randomized studies were underpowered to show clinical benefit. Neither of the observational studies showed significant advantages in stroke-free survival, but the team noted, “data such as information about anticoagulation use among the groups [were] lacking.”
“Overall, there is insufficient evidence to support the routine use of surgical LAA exclusion to reduce stroke risk or the future need for anticoagulant therapy,” wrote Dr. Noelck and his coinvestigators. However, they said, several ongoing studies “should add substantively to this body of evidence during the next several years.”
The study was funded by the Department of Veterans Affairs. None of the authors had any relevant financial disclosure.
FROM CIRCULATION: CARDIOVASCULAR QUALITY OUTCOMES
Insufficient evidence exists to routinely recommend surgical closure of the left atrial appendage, either surgically or with a LAA exclusion device, for patients who have atrial fibrillation, a large data review has concluded.
None of the devices so far examined is more effective than oral anticoagulation therapy in reducing AF-related stroke risk, but they appear to be riskier, with up to 1 in 15 patients experiencing a serious adverse event during or after percutaneous placement, North Noelck, MD, and his colleagues wrote (Circ Cardiovasc Qual Outcomes. 2016 Jul 12. doi: 10.1161/CIRCOUTCOMES.115.002539).
Surgical studies, which also have found no benefit, are poor in quality and provide no strong data in favor of any technique, wrote Dr. Noelck of Oregon Health and Science University, Portland.
The meta-analysis comprised 13 studies of the benefits and risks of percutaneous LAA exclusion and 7 of the benefits and harms of surgical LAA closure.
The team found “limited evidence” that one device, the Watchman (Boston Scientific), may be an effective alternative to long-term oral anticoagulation treatment in some patients. But the Watchman was also associated with “significant, procedural-related harms,” in almost 11% of patients in one large study, PROTECT-AF. These included pericardial effusions with and without associated tamponade, bleeding, device thrombus, and device embolization. However, the benefit conferred by the device appeared marginal. Neither PROTECT-AF nor the subsequent PREVAIL studies of the Watchman found that it conferred significant clinical benefit above the comparator arm of warfarin therapy. Indeed, in PREVAIL, a composite outcome of death, ischemic/hemorrhagic stroke, or systemic embolism occurred in 5.2% of the device group and in 2.9% of the warfarin group.
Three randomized studies and two observational studies examined surgical LAA closure relative to usual medical care. The authors said the randomized studies were underpowered to show clinical benefit. Neither of the observational studies showed significant advantages in stroke-free survival, but the team noted, “data such as information about anticoagulation use among the groups [were] lacking.”
“Overall, there is insufficient evidence to support the routine use of surgical LAA exclusion to reduce stroke risk or the future need for anticoagulant therapy,” wrote Dr. Noelck and his coinvestigators. However, they said, several ongoing studies “should add substantively to this body of evidence during the next several years.”
The study was funded by the Department of Veterans Affairs. None of the authors had any relevant financial disclosure.
Key clinical point: There is insufficient evidence to recommend LAA closure over oral anticoagulation for stroke prevention in patients with atrial fibrillation.
Major finding: Device closure was not more effective than oral anticoagulation but was associated with a 1 in 15 patient incidence of procedural adverse events.
Data source: The meta-analysis comprised 13 studies of LAA device closure and 7 studies of surgical closure.
Disclosures: The study was funded by the Department of Veterans Affairs. None of the authors had any relevant financial disclosure.
How practice changes us
I have learned a few things in my almost half-century of hurtling through space with all of you on this fragile blue-green rock. My most recent enlightenment is that the universe has a way of flipping our certitude on its head. Mental acrobatics requires flexibility, requiring us to bend so we don’t break.
On July 1, Minnesota began allowing clinicians to certify patients for “intractable pain” as a qualifying condition for the Minnesota Medical Cannabis Program. Recall that medical marijuana is a Schedule I drug, and we cannot prescribe it. These programs are set up in such a way that the only role a clinician plays is to certify patients with qualifying conditions. This allows a patient to pay a registration fee and visit a cannabis patient center, where a pharmacist will recommend cannabis dose and type.
Months before this, I was waxing professorial about our lack of certainty about dosing and efficacy of medical marijuana. Then I met a 30-year-old with chronic back pain.
She had been evaluated by every subspecialist. This patient was taking and failing supertherapeutic doses of NSAIDs, acetaminophen, and gabapentin. No more surgical options existed. She had been removed from opioid contracts for aberrant behavior. She had had a hysterectomy for severe bleeding. She was in pain and asking for help.
She relates to you that street marijuana has helped with the pain, but she is worried about being arrested and losing her job. Do we put her on another opioid contract? Do we throw up our hands in defeat, apologize, and show her the door?
Serendipitously, I ran across a study evaluating the relationship between cannabis use over a 20-year period and health conditions. The study by Madeline Meier, Ph.D., and her colleagues evaluated 1,037 New Zealanders followed into their late 30s. Laboratory measures were available, and tobacco use was determined (JAMA Psychiatry. 2016 Jul 1;73[7]:731-40).
Cannabis was associated with poorer periodontal health, but with no other health conditions in early midlife. In contrast, tobacco use was associated with significant adverse health consequences in multiple domains.
This study looked at smoked cannabis. In contrast, the cannabis that my patient would take is an oil, negating any potential respiratory health issues from by-products of burning. Furthermore, products with higher concentrations of or consisting exclusively of cannabidiol can be selected. Cannabidiol is proposed to possess health benefits and is not psychoactive.
As the opioid crisis rages, solutions are not readily presenting themselves. Will we be on the wrong side of medical history by providing patients with chronic pain access to medical marijuana? Perhaps we can avoid, at least for a short time, the all-too inevitable outcome of chronic pain patients in their 30s: ever-increasing opioid doses with the same amount of pain, frequent emergency department visits, a fractured patient-physician relationship, and drug overdose.
For our patients’ sake, I hope we can bend before we break.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
I have learned a few things in my almost half-century of hurtling through space with all of you on this fragile blue-green rock. My most recent enlightenment is that the universe has a way of flipping our certitude on its head. Mental acrobatics requires flexibility, requiring us to bend so we don’t break.
On July 1, Minnesota began allowing clinicians to certify patients for “intractable pain” as a qualifying condition for the Minnesota Medical Cannabis Program. Recall that medical marijuana is a Schedule I drug, and we cannot prescribe it. These programs are set up in such a way that the only role a clinician plays is to certify patients with qualifying conditions. This allows a patient to pay a registration fee and visit a cannabis patient center, where a pharmacist will recommend cannabis dose and type.
Months before this, I was waxing professorial about our lack of certainty about dosing and efficacy of medical marijuana. Then I met a 30-year-old with chronic back pain.
She had been evaluated by every subspecialist. This patient was taking and failing supertherapeutic doses of NSAIDs, acetaminophen, and gabapentin. No more surgical options existed. She had been removed from opioid contracts for aberrant behavior. She had had a hysterectomy for severe bleeding. She was in pain and asking for help.
She relates to you that street marijuana has helped with the pain, but she is worried about being arrested and losing her job. Do we put her on another opioid contract? Do we throw up our hands in defeat, apologize, and show her the door?
Serendipitously, I ran across a study evaluating the relationship between cannabis use over a 20-year period and health conditions. The study by Madeline Meier, Ph.D., and her colleagues evaluated 1,037 New Zealanders followed into their late 30s. Laboratory measures were available, and tobacco use was determined (JAMA Psychiatry. 2016 Jul 1;73[7]:731-40).
Cannabis was associated with poorer periodontal health, but with no other health conditions in early midlife. In contrast, tobacco use was associated with significant adverse health consequences in multiple domains.
This study looked at smoked cannabis. In contrast, the cannabis that my patient would take is an oil, negating any potential respiratory health issues from by-products of burning. Furthermore, products with higher concentrations of or consisting exclusively of cannabidiol can be selected. Cannabidiol is proposed to possess health benefits and is not psychoactive.
As the opioid crisis rages, solutions are not readily presenting themselves. Will we be on the wrong side of medical history by providing patients with chronic pain access to medical marijuana? Perhaps we can avoid, at least for a short time, the all-too inevitable outcome of chronic pain patients in their 30s: ever-increasing opioid doses with the same amount of pain, frequent emergency department visits, a fractured patient-physician relationship, and drug overdose.
For our patients’ sake, I hope we can bend before we break.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
I have learned a few things in my almost half-century of hurtling through space with all of you on this fragile blue-green rock. My most recent enlightenment is that the universe has a way of flipping our certitude on its head. Mental acrobatics requires flexibility, requiring us to bend so we don’t break.
On July 1, Minnesota began allowing clinicians to certify patients for “intractable pain” as a qualifying condition for the Minnesota Medical Cannabis Program. Recall that medical marijuana is a Schedule I drug, and we cannot prescribe it. These programs are set up in such a way that the only role a clinician plays is to certify patients with qualifying conditions. This allows a patient to pay a registration fee and visit a cannabis patient center, where a pharmacist will recommend cannabis dose and type.
Months before this, I was waxing professorial about our lack of certainty about dosing and efficacy of medical marijuana. Then I met a 30-year-old with chronic back pain.
She had been evaluated by every subspecialist. This patient was taking and failing supertherapeutic doses of NSAIDs, acetaminophen, and gabapentin. No more surgical options existed. She had been removed from opioid contracts for aberrant behavior. She had had a hysterectomy for severe bleeding. She was in pain and asking for help.
She relates to you that street marijuana has helped with the pain, but she is worried about being arrested and losing her job. Do we put her on another opioid contract? Do we throw up our hands in defeat, apologize, and show her the door?
Serendipitously, I ran across a study evaluating the relationship between cannabis use over a 20-year period and health conditions. The study by Madeline Meier, Ph.D., and her colleagues evaluated 1,037 New Zealanders followed into their late 30s. Laboratory measures were available, and tobacco use was determined (JAMA Psychiatry. 2016 Jul 1;73[7]:731-40).
Cannabis was associated with poorer periodontal health, but with no other health conditions in early midlife. In contrast, tobacco use was associated with significant adverse health consequences in multiple domains.
This study looked at smoked cannabis. In contrast, the cannabis that my patient would take is an oil, negating any potential respiratory health issues from by-products of burning. Furthermore, products with higher concentrations of or consisting exclusively of cannabidiol can be selected. Cannabidiol is proposed to possess health benefits and is not psychoactive.
As the opioid crisis rages, solutions are not readily presenting themselves. Will we be on the wrong side of medical history by providing patients with chronic pain access to medical marijuana? Perhaps we can avoid, at least for a short time, the all-too inevitable outcome of chronic pain patients in their 30s: ever-increasing opioid doses with the same amount of pain, frequent emergency department visits, a fractured patient-physician relationship, and drug overdose.
For our patients’ sake, I hope we can bend before we break.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
Retiring Baby Boomers leave fewer workers to pay for Medicare
The influx of aging Baby Boomers into the ranks of the retired will reduce the ratio of workers available to pay for each Medicare part A beneficiary by 40% from 2000 to 2030, according to the 2016 report of the Medicare Trustees.
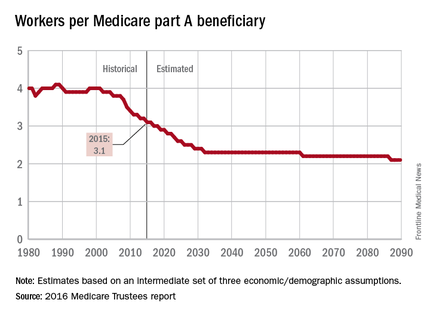
In 2015, there were 3.1 workers for each part A beneficiary, putting the United States in the middle of a projected drop from 4.0 workers per beneficiary in 2000 down to 2.4 in 2030. The Boomer-induced drop will largely be over by then, but the decline will continue until there are about 2.1 workers for each part A beneficiary by 2090, the report said.
“This reduction implies an increase in the [Medicare part A] cost rate of about 50% by 2090, relative to its current level, solely due to this demographic factor,” the trustees noted.
The projections are done using three sets – low-cost, intermediate, and high-cost – of economic and demographic assumptions. The figures presented here are from the intermediate assumption.
The influx of aging Baby Boomers into the ranks of the retired will reduce the ratio of workers available to pay for each Medicare part A beneficiary by 40% from 2000 to 2030, according to the 2016 report of the Medicare Trustees.

In 2015, there were 3.1 workers for each part A beneficiary, putting the United States in the middle of a projected drop from 4.0 workers per beneficiary in 2000 down to 2.4 in 2030. The Boomer-induced drop will largely be over by then, but the decline will continue until there are about 2.1 workers for each part A beneficiary by 2090, the report said.
“This reduction implies an increase in the [Medicare part A] cost rate of about 50% by 2090, relative to its current level, solely due to this demographic factor,” the trustees noted.
The projections are done using three sets – low-cost, intermediate, and high-cost – of economic and demographic assumptions. The figures presented here are from the intermediate assumption.
The influx of aging Baby Boomers into the ranks of the retired will reduce the ratio of workers available to pay for each Medicare part A beneficiary by 40% from 2000 to 2030, according to the 2016 report of the Medicare Trustees.

In 2015, there were 3.1 workers for each part A beneficiary, putting the United States in the middle of a projected drop from 4.0 workers per beneficiary in 2000 down to 2.4 in 2030. The Boomer-induced drop will largely be over by then, but the decline will continue until there are about 2.1 workers for each part A beneficiary by 2090, the report said.
“This reduction implies an increase in the [Medicare part A] cost rate of about 50% by 2090, relative to its current level, solely due to this demographic factor,” the trustees noted.
The projections are done using three sets – low-cost, intermediate, and high-cost – of economic and demographic assumptions. The figures presented here are from the intermediate assumption.
Statement warns of drugs causing or exacerbating heart failure
Many commonly used prescription drugs, many OTC agents, and also several complimentary or alternative medications, can either trigger heart failure or exacerbate the disease in patients with existing heart failure according to a Scientific Statement written by a committee of the American Heart Association and released on July 11.
This first-ever authoritative U.S. overview of what is known about drugs that can affect heart failure was compiled to address an important practice issue for the large and growing number of U.S. patients with heart failure, estimated to be nearly 6 million Americans, and “provide some guidance to health care providers in how to minimize polypharmacy, improve medication safety, as well as identify the medications that could exacerbate or cause heart failure,” said Robert L. Page II, PharmD, chair of the committee and a professor of clinical pharmacy at the University of Colorado at Denver, Aurora.
Although the comprehensive statement lists 88 distinct prescription drugs or drug classes as agents that pose major or moderate threats for causing or worsening heart failure, “from the American public’s perspective, importance should be placed on educating patients regarding the impact that OTC medications can have on their heart failure,” Dr. Page said in an interview. “For example, nonsteroidal anti-inflammatory drugs like ibuprofen or naproxen can cause sodium and water retention and antagonize the effects of evidence-based heart failure pharmacotherapies. Additionally, OTC medications like pseudoephedrine, which many cough and cold products contain, can increase blood pressure and afterload,” he noted. The risks these drugs pose becomes even greater when they are taken at higher doses.
NSAIDs
The statement cites already existing guidance from the American College of Cardiology and American Heart Association that for patients with existing heart failure, use of NSAIDs should either be avoided or withdrawn when possible. The statement advises educating patients to communicate with their health care provider before taking any OTC medication or complimentary or alternative medication, avoid these agents when their efficacy and safety is uncertain, and evaluate the labels of these products for their sodium content (although the sodium content from inactive ingredients may be difficult to find in labeling).
“Currently, we teach patients to read food labels for sodium content, but we also need to educate patients on how to read OTC medication labels for both ingredients and sodium content. Many OTC antacids may have a large sodium load,” Dr. Page said. The statement includes a list of 14 prescription drugs and also highlights several OTC formulations that have an especially high sodium content.
Metformin
Among the many prescription drugs listed, one notable entry is for the oral hypoglycemic agent metformin that today is among the most widely used drugs for treating type 2 diabetes and is especially relevant for heart failure patients because, as the statement notes, 38% also have diabetes. The statement details the long history of metformin and heart failure, noting that until a decade ago, the drug had a contraindication for patients with heart failure, that metformin’s label still carries a black box warning for cautious use in heart failure patients, and that earlier in 2016, the Food and Drug Administration cautioned that metformin should not be used in patients with an estimated glomerular filtration rate of less than 30 mL/minute per 1.73 m2. The statement also endorsed a recommendation from the American Diabetes Association that metformin not be used in patients with unstable heart failure or those hospitalized for heart failure.
Antihypertensives, biologics, and more
Other notable prescription drugs listed as potentially having a major impact on causing or worsening heart failure include the antihypertensive drugs diltiazem, verapamil, and moxonidine, the tumor necrosis factor–inhibitors that are widely used to treat rheumatologic and gastroenterologic diseases, the antipsychotic clozapine, and a long list of anticancer medications, including several anthracyclines and many types of newer biologic agents.
The statement also lists several specific recommendations to health care providers for improving oversight of the drugs taken by patients with heart failure or those at risk for heart failure. These include a comprehensive medication review during each clinical encounter. The statement also suggests a “medication flow sheet” for each patient that contains the basic information regarding the regimen for each medication taken by a patient: the brand and generic name, the purpose of the medication, and its dosage. “These medication flow sheets should be used by patients as a tool to enhance safety and adherence, and they should show their flow sheets at each provider visit,” Dr. Page said.
Managing myriad meds
The statement also calls for stopping medications without a well defined indication for a patient, avoid prescribing new drugs to address side effects of other drugs, and suggests establishing a “captain” among the health care providers seen by each patient who would be particularly responsible for overseeing and keeping track of the medications the patient takes.
“Ideally, this ‘captain’ would be the patient’s primary care provider, who should be in contact with the other specialists that the patient may be seeing. However, this does not always happen,” said Dr. Page. “Therefore, I encourage each patient with heart failure to contact both their primary care provider and their health care provider who is managing their heart failure before taking or stopping any new medication including prescription, OTC, herbal, complimentary or alternative medication or supplement. Health care providers need to encourage patients to be actively engaged in their medication management.”
Dr. Page had no disclosures.
On Twitter @mitchelzoler
Many commonly used prescription drugs, many OTC agents, and also several complimentary or alternative medications, can either trigger heart failure or exacerbate the disease in patients with existing heart failure according to a Scientific Statement written by a committee of the American Heart Association and released on July 11.
This first-ever authoritative U.S. overview of what is known about drugs that can affect heart failure was compiled to address an important practice issue for the large and growing number of U.S. patients with heart failure, estimated to be nearly 6 million Americans, and “provide some guidance to health care providers in how to minimize polypharmacy, improve medication safety, as well as identify the medications that could exacerbate or cause heart failure,” said Robert L. Page II, PharmD, chair of the committee and a professor of clinical pharmacy at the University of Colorado at Denver, Aurora.
Although the comprehensive statement lists 88 distinct prescription drugs or drug classes as agents that pose major or moderate threats for causing or worsening heart failure, “from the American public’s perspective, importance should be placed on educating patients regarding the impact that OTC medications can have on their heart failure,” Dr. Page said in an interview. “For example, nonsteroidal anti-inflammatory drugs like ibuprofen or naproxen can cause sodium and water retention and antagonize the effects of evidence-based heart failure pharmacotherapies. Additionally, OTC medications like pseudoephedrine, which many cough and cold products contain, can increase blood pressure and afterload,” he noted. The risks these drugs pose becomes even greater when they are taken at higher doses.
NSAIDs
The statement cites already existing guidance from the American College of Cardiology and American Heart Association that for patients with existing heart failure, use of NSAIDs should either be avoided or withdrawn when possible. The statement advises educating patients to communicate with their health care provider before taking any OTC medication or complimentary or alternative medication, avoid these agents when their efficacy and safety is uncertain, and evaluate the labels of these products for their sodium content (although the sodium content from inactive ingredients may be difficult to find in labeling).
“Currently, we teach patients to read food labels for sodium content, but we also need to educate patients on how to read OTC medication labels for both ingredients and sodium content. Many OTC antacids may have a large sodium load,” Dr. Page said. The statement includes a list of 14 prescription drugs and also highlights several OTC formulations that have an especially high sodium content.
Metformin
Among the many prescription drugs listed, one notable entry is for the oral hypoglycemic agent metformin that today is among the most widely used drugs for treating type 2 diabetes and is especially relevant for heart failure patients because, as the statement notes, 38% also have diabetes. The statement details the long history of metformin and heart failure, noting that until a decade ago, the drug had a contraindication for patients with heart failure, that metformin’s label still carries a black box warning for cautious use in heart failure patients, and that earlier in 2016, the Food and Drug Administration cautioned that metformin should not be used in patients with an estimated glomerular filtration rate of less than 30 mL/minute per 1.73 m2. The statement also endorsed a recommendation from the American Diabetes Association that metformin not be used in patients with unstable heart failure or those hospitalized for heart failure.
Antihypertensives, biologics, and more
Other notable prescription drugs listed as potentially having a major impact on causing or worsening heart failure include the antihypertensive drugs diltiazem, verapamil, and moxonidine, the tumor necrosis factor–inhibitors that are widely used to treat rheumatologic and gastroenterologic diseases, the antipsychotic clozapine, and a long list of anticancer medications, including several anthracyclines and many types of newer biologic agents.
The statement also lists several specific recommendations to health care providers for improving oversight of the drugs taken by patients with heart failure or those at risk for heart failure. These include a comprehensive medication review during each clinical encounter. The statement also suggests a “medication flow sheet” for each patient that contains the basic information regarding the regimen for each medication taken by a patient: the brand and generic name, the purpose of the medication, and its dosage. “These medication flow sheets should be used by patients as a tool to enhance safety and adherence, and they should show their flow sheets at each provider visit,” Dr. Page said.
Managing myriad meds
The statement also calls for stopping medications without a well defined indication for a patient, avoid prescribing new drugs to address side effects of other drugs, and suggests establishing a “captain” among the health care providers seen by each patient who would be particularly responsible for overseeing and keeping track of the medications the patient takes.
“Ideally, this ‘captain’ would be the patient’s primary care provider, who should be in contact with the other specialists that the patient may be seeing. However, this does not always happen,” said Dr. Page. “Therefore, I encourage each patient with heart failure to contact both their primary care provider and their health care provider who is managing their heart failure before taking or stopping any new medication including prescription, OTC, herbal, complimentary or alternative medication or supplement. Health care providers need to encourage patients to be actively engaged in their medication management.”
Dr. Page had no disclosures.
On Twitter @mitchelzoler
Many commonly used prescription drugs, many OTC agents, and also several complimentary or alternative medications, can either trigger heart failure or exacerbate the disease in patients with existing heart failure according to a Scientific Statement written by a committee of the American Heart Association and released on July 11.
This first-ever authoritative U.S. overview of what is known about drugs that can affect heart failure was compiled to address an important practice issue for the large and growing number of U.S. patients with heart failure, estimated to be nearly 6 million Americans, and “provide some guidance to health care providers in how to minimize polypharmacy, improve medication safety, as well as identify the medications that could exacerbate or cause heart failure,” said Robert L. Page II, PharmD, chair of the committee and a professor of clinical pharmacy at the University of Colorado at Denver, Aurora.
Although the comprehensive statement lists 88 distinct prescription drugs or drug classes as agents that pose major or moderate threats for causing or worsening heart failure, “from the American public’s perspective, importance should be placed on educating patients regarding the impact that OTC medications can have on their heart failure,” Dr. Page said in an interview. “For example, nonsteroidal anti-inflammatory drugs like ibuprofen or naproxen can cause sodium and water retention and antagonize the effects of evidence-based heart failure pharmacotherapies. Additionally, OTC medications like pseudoephedrine, which many cough and cold products contain, can increase blood pressure and afterload,” he noted. The risks these drugs pose becomes even greater when they are taken at higher doses.
NSAIDs
The statement cites already existing guidance from the American College of Cardiology and American Heart Association that for patients with existing heart failure, use of NSAIDs should either be avoided or withdrawn when possible. The statement advises educating patients to communicate with their health care provider before taking any OTC medication or complimentary or alternative medication, avoid these agents when their efficacy and safety is uncertain, and evaluate the labels of these products for their sodium content (although the sodium content from inactive ingredients may be difficult to find in labeling).
“Currently, we teach patients to read food labels for sodium content, but we also need to educate patients on how to read OTC medication labels for both ingredients and sodium content. Many OTC antacids may have a large sodium load,” Dr. Page said. The statement includes a list of 14 prescription drugs and also highlights several OTC formulations that have an especially high sodium content.
Metformin
Among the many prescription drugs listed, one notable entry is for the oral hypoglycemic agent metformin that today is among the most widely used drugs for treating type 2 diabetes and is especially relevant for heart failure patients because, as the statement notes, 38% also have diabetes. The statement details the long history of metformin and heart failure, noting that until a decade ago, the drug had a contraindication for patients with heart failure, that metformin’s label still carries a black box warning for cautious use in heart failure patients, and that earlier in 2016, the Food and Drug Administration cautioned that metformin should not be used in patients with an estimated glomerular filtration rate of less than 30 mL/minute per 1.73 m2. The statement also endorsed a recommendation from the American Diabetes Association that metformin not be used in patients with unstable heart failure or those hospitalized for heart failure.
Antihypertensives, biologics, and more
Other notable prescription drugs listed as potentially having a major impact on causing or worsening heart failure include the antihypertensive drugs diltiazem, verapamil, and moxonidine, the tumor necrosis factor–inhibitors that are widely used to treat rheumatologic and gastroenterologic diseases, the antipsychotic clozapine, and a long list of anticancer medications, including several anthracyclines and many types of newer biologic agents.
The statement also lists several specific recommendations to health care providers for improving oversight of the drugs taken by patients with heart failure or those at risk for heart failure. These include a comprehensive medication review during each clinical encounter. The statement also suggests a “medication flow sheet” for each patient that contains the basic information regarding the regimen for each medication taken by a patient: the brand and generic name, the purpose of the medication, and its dosage. “These medication flow sheets should be used by patients as a tool to enhance safety and adherence, and they should show their flow sheets at each provider visit,” Dr. Page said.
Managing myriad meds
The statement also calls for stopping medications without a well defined indication for a patient, avoid prescribing new drugs to address side effects of other drugs, and suggests establishing a “captain” among the health care providers seen by each patient who would be particularly responsible for overseeing and keeping track of the medications the patient takes.
“Ideally, this ‘captain’ would be the patient’s primary care provider, who should be in contact with the other specialists that the patient may be seeing. However, this does not always happen,” said Dr. Page. “Therefore, I encourage each patient with heart failure to contact both their primary care provider and their health care provider who is managing their heart failure before taking or stopping any new medication including prescription, OTC, herbal, complimentary or alternative medication or supplement. Health care providers need to encourage patients to be actively engaged in their medication management.”
Dr. Page had no disclosures.
On Twitter @mitchelzoler