User login
News and Views that Matter to Physicians
Prenatal exposure to TNF inhibitors does not increase infections in newborns
WASHINGTON – Prenatal exposure to tumor necrosis factor–inhibiting drugs does not significantly increase the risk of a serious antenatal infection in infants born to women taking the drugs for rheumatoid arthritis, according to a large database study.
Researchers from McGill University, Montreal, and the University of Alabama at Birmingham who conducted the study did find a higher rate of serious infections among infants born to users of a tumor necrosis factor inhibitor (TNFi), especially among those exposed to infliximab, but after adjustment for maternal age and other antirheumatic drugs, the risk was not statistically significant.
Infliximab is unique among the TNFi drugs in that it concentrates in cord blood, reaching levels that can exceed 150% of the maternal blood level, Dr. Vinet noted. Adalimumab concentrates similarly, although the current study did not find any significantly increased infection risk associated with that medication.
Dr. Vinet and her colleagues analyzed drug exposure in 2,455 infants born to mothers with rheumatoid arthritis (RA), who were included in the PregnAncies in RA mothers and Outcomes in offspring in the United States cohort (PAROUS) registry. This cohort is drawn from data in the national MarketScan commercial database. The infants were age- and gender-matched with more than 11,000 matched controls born to women without RA, and with no prenatal TNFi exposure. Among these drugs, she looked for exposure to adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab, as well as corticosteroids and other biologic and nonbiologic disease-modifying antirheumatic drugs (DMARDs).
Two exposures were considered: drugs taken during pregnancy and drugs taken before conception but not during pregnancy. These were compared with infants of mothers with RA who didn’t take TNFi drugs, and to the control infants. Serious infections were those that required a hospitalization during the first 12 months of life; only the index incident was counted.
Among the RA cohort, 290 (12%) were exposed to a TNFi during pregnancy and 109 (4%) were born to women who had taken a TNFi before conception. The remainder of the cohort was unexposed to those medications.
The mean maternal age was 32 years and similar in all RA categories and controls.
Corticosteroid use was common in women with RA, whether they took a TNFi during pregnancy (55%), before pregnancy (44%), or not at all (26%). Nonbiologic DMARDs were given to 19% of the TNFi cohort during pregnancy and 16% before pregnancy, as well as to 15% of those who didn’t take a TNFi.
The rate of serious neonatal infection was 2% among both infants born to RA mothers who didn’t take a TNFi and those born to RA mothers who took a TNFi before conception. Control infants born to women without RA had a serious infection rate of 0.2%.
Among infants exposed to a TNFi during pregnancy, the serious infection rate was 3%; it was also 3% among those exposed only in the third trimester.
A multivariate analysis that controlled for maternal age, prepregnancy diabetes, gestational diabetes, preterm birth, and exposure to the other drug categories determined that TNFi drugs did not significantly increase the risk of a serious infection in neonates with gestational exposure (odds ratio, 1.4) or whose mothers took the drugs before conception (OR, 0.9), compared with controls. The findings were similar when the analysis was restricted to TNFi exposure in the third trimester only.
When Dr. Vinet examined each drug independently, she found numerical differences in infection rates: golimumab and certolizumab pegol, 0%; adalimumab, 2.4%; etanercept 2.7%; and infliximab, 8.3%.
Because of the relatively small number of events, this portion of the regression analysis could not control for preterm birth and gestational diabetes. But after adjusting for maternal age and in utero corticosteroid exposure, Dr. Vinet found no significant associations with serious neonatal infection and any of the TNFi drugs, including infliximab (OR, 3.5; 95% CI, 0.8-15.0).
She and her colleagues had no financial disclosures.
[email protected]
On Twitter @alz_gal
WASHINGTON – Prenatal exposure to tumor necrosis factor–inhibiting drugs does not significantly increase the risk of a serious antenatal infection in infants born to women taking the drugs for rheumatoid arthritis, according to a large database study.
Researchers from McGill University, Montreal, and the University of Alabama at Birmingham who conducted the study did find a higher rate of serious infections among infants born to users of a tumor necrosis factor inhibitor (TNFi), especially among those exposed to infliximab, but after adjustment for maternal age and other antirheumatic drugs, the risk was not statistically significant.
Infliximab is unique among the TNFi drugs in that it concentrates in cord blood, reaching levels that can exceed 150% of the maternal blood level, Dr. Vinet noted. Adalimumab concentrates similarly, although the current study did not find any significantly increased infection risk associated with that medication.
Dr. Vinet and her colleagues analyzed drug exposure in 2,455 infants born to mothers with rheumatoid arthritis (RA), who were included in the PregnAncies in RA mothers and Outcomes in offspring in the United States cohort (PAROUS) registry. This cohort is drawn from data in the national MarketScan commercial database. The infants were age- and gender-matched with more than 11,000 matched controls born to women without RA, and with no prenatal TNFi exposure. Among these drugs, she looked for exposure to adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab, as well as corticosteroids and other biologic and nonbiologic disease-modifying antirheumatic drugs (DMARDs).
Two exposures were considered: drugs taken during pregnancy and drugs taken before conception but not during pregnancy. These were compared with infants of mothers with RA who didn’t take TNFi drugs, and to the control infants. Serious infections were those that required a hospitalization during the first 12 months of life; only the index incident was counted.
Among the RA cohort, 290 (12%) were exposed to a TNFi during pregnancy and 109 (4%) were born to women who had taken a TNFi before conception. The remainder of the cohort was unexposed to those medications.
The mean maternal age was 32 years and similar in all RA categories and controls.
Corticosteroid use was common in women with RA, whether they took a TNFi during pregnancy (55%), before pregnancy (44%), or not at all (26%). Nonbiologic DMARDs were given to 19% of the TNFi cohort during pregnancy and 16% before pregnancy, as well as to 15% of those who didn’t take a TNFi.
The rate of serious neonatal infection was 2% among both infants born to RA mothers who didn’t take a TNFi and those born to RA mothers who took a TNFi before conception. Control infants born to women without RA had a serious infection rate of 0.2%.
Among infants exposed to a TNFi during pregnancy, the serious infection rate was 3%; it was also 3% among those exposed only in the third trimester.
A multivariate analysis that controlled for maternal age, prepregnancy diabetes, gestational diabetes, preterm birth, and exposure to the other drug categories determined that TNFi drugs did not significantly increase the risk of a serious infection in neonates with gestational exposure (odds ratio, 1.4) or whose mothers took the drugs before conception (OR, 0.9), compared with controls. The findings were similar when the analysis was restricted to TNFi exposure in the third trimester only.
When Dr. Vinet examined each drug independently, she found numerical differences in infection rates: golimumab and certolizumab pegol, 0%; adalimumab, 2.4%; etanercept 2.7%; and infliximab, 8.3%.
Because of the relatively small number of events, this portion of the regression analysis could not control for preterm birth and gestational diabetes. But after adjusting for maternal age and in utero corticosteroid exposure, Dr. Vinet found no significant associations with serious neonatal infection and any of the TNFi drugs, including infliximab (OR, 3.5; 95% CI, 0.8-15.0).
She and her colleagues had no financial disclosures.
[email protected]
On Twitter @alz_gal
WASHINGTON – Prenatal exposure to tumor necrosis factor–inhibiting drugs does not significantly increase the risk of a serious antenatal infection in infants born to women taking the drugs for rheumatoid arthritis, according to a large database study.
Researchers from McGill University, Montreal, and the University of Alabama at Birmingham who conducted the study did find a higher rate of serious infections among infants born to users of a tumor necrosis factor inhibitor (TNFi), especially among those exposed to infliximab, but after adjustment for maternal age and other antirheumatic drugs, the risk was not statistically significant.
Infliximab is unique among the TNFi drugs in that it concentrates in cord blood, reaching levels that can exceed 150% of the maternal blood level, Dr. Vinet noted. Adalimumab concentrates similarly, although the current study did not find any significantly increased infection risk associated with that medication.
Dr. Vinet and her colleagues analyzed drug exposure in 2,455 infants born to mothers with rheumatoid arthritis (RA), who were included in the PregnAncies in RA mothers and Outcomes in offspring in the United States cohort (PAROUS) registry. This cohort is drawn from data in the national MarketScan commercial database. The infants were age- and gender-matched with more than 11,000 matched controls born to women without RA, and with no prenatal TNFi exposure. Among these drugs, she looked for exposure to adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab, as well as corticosteroids and other biologic and nonbiologic disease-modifying antirheumatic drugs (DMARDs).
Two exposures were considered: drugs taken during pregnancy and drugs taken before conception but not during pregnancy. These were compared with infants of mothers with RA who didn’t take TNFi drugs, and to the control infants. Serious infections were those that required a hospitalization during the first 12 months of life; only the index incident was counted.
Among the RA cohort, 290 (12%) were exposed to a TNFi during pregnancy and 109 (4%) were born to women who had taken a TNFi before conception. The remainder of the cohort was unexposed to those medications.
The mean maternal age was 32 years and similar in all RA categories and controls.
Corticosteroid use was common in women with RA, whether they took a TNFi during pregnancy (55%), before pregnancy (44%), or not at all (26%). Nonbiologic DMARDs were given to 19% of the TNFi cohort during pregnancy and 16% before pregnancy, as well as to 15% of those who didn’t take a TNFi.
The rate of serious neonatal infection was 2% among both infants born to RA mothers who didn’t take a TNFi and those born to RA mothers who took a TNFi before conception. Control infants born to women without RA had a serious infection rate of 0.2%.
Among infants exposed to a TNFi during pregnancy, the serious infection rate was 3%; it was also 3% among those exposed only in the third trimester.
A multivariate analysis that controlled for maternal age, prepregnancy diabetes, gestational diabetes, preterm birth, and exposure to the other drug categories determined that TNFi drugs did not significantly increase the risk of a serious infection in neonates with gestational exposure (odds ratio, 1.4) or whose mothers took the drugs before conception (OR, 0.9), compared with controls. The findings were similar when the analysis was restricted to TNFi exposure in the third trimester only.
When Dr. Vinet examined each drug independently, she found numerical differences in infection rates: golimumab and certolizumab pegol, 0%; adalimumab, 2.4%; etanercept 2.7%; and infliximab, 8.3%.
Because of the relatively small number of events, this portion of the regression analysis could not control for preterm birth and gestational diabetes. But after adjusting for maternal age and in utero corticosteroid exposure, Dr. Vinet found no significant associations with serious neonatal infection and any of the TNFi drugs, including infliximab (OR, 3.5; 95% CI, 0.8-15.0).
She and her colleagues had no financial disclosures.
[email protected]
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The rates of serious neonatal infection were 2% among infants born to RA mothers without exposure to TNFi drugs and 3% among those exposed to the drugs during gestation.
Data source: The case-control study comprised 2,455 cases and more than 11,000 controls.
Disclosures: Dr. Vinet and her colleagues had no financial disclosures.
Diabetes treatment costs doubled in Sweden since 2006
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
[email protected]
On Twitter @Alz_Gal
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
[email protected]
On Twitter @Alz_Gal
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
[email protected]
On Twitter @Alz_Gal
AT EASD 2016
Key clinical point:
Major finding: Treatment costs jumped from €608 million in 2006 to €1.27 billion in 2014.
Data source: The 8-year study used national health care data.
Disclosures: The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
AGA Guideline: Preventing Crohn’s recurrence after resection
Patients whose Crohn’s disease fully remits after resection should not wait for endoscopic recurrence to start tumor-necrosis-factor inhibitors or thiopurines, according to a new guideline from the American Gastroenterological Association.
Patients who are low risk or worried about side effects, however, “may reasonably select endoscopy-guided pharmacological treatment,” the guidelines state (doi: 10.1053/j.gastro.2016.10.038).
Early pharmacologic prophylaxis usually begins within 8 weeks of surgery, they noted. Whether this approach bests endoscopy-guided treatment is unclear: In one small trial (Gastroenterology. 2013;145[4]:766-74.e1), early azathioprine therapy failed to best endoscopy-guided therapy for preventing clinical or endoscopic recurrence.
Early prophylaxis, however, is usually reasonable because most Crohn’s patients who undergo surgery have at least one risk factor for recurrence, Dr. Nguyen and his associates emphasize. They suggest reserving endoscopy-guided therapy for patients who have real concerns about side effects and are at low risk, such as nonsmokers who were diagnosed within 10 years and have less than 10-20 cm of fibrostenotic disease.
For prophylaxis, a moderate amount of evidence supports anti–tumor necrosis factor (TNF) agents, thiopurines, or combined therapy over other agents, the guideline also states. In placebo-controlled clinical trials, anti-TNF therapy reduced the chances of clinical recurrence by 49% and endoscopic recurrence by 76%, while thiopurines cut these rates by 65% and 60%, respectively. Evidence favors anti-TNF agents over thiopurines for preventing recurrence, but it is of low quality, the guideline says. Furthermore, only indirect evidence supports combined therapy in patients at highest risk of recurrence.
Among the antibiotics, only nitroimidazoles such as metronidazole have been adequately studied, and they posted worse results than anti-TNF agents or thiopurines. Antibiotic therapy decreased the risk of endoscopic recurrence of Crohn’s disease by about 50%, but long-term use is associated with peripheral neuropathy and disease usually recurs within 2 years of stopping treatment. Accordingly, the guidelines suggest using a nitroimidazole for only 3-12 months, and only in lower-risk patients who are concerned about the adverse effects of anti-TNF agents and thiopurines.
The AGA made a conditional recommendation against the prophylactic use of budesonide, probiotics, and 5-aminosalicylates such as mesalamine. Only low-quality evidence supports their efficacy after resection, and by using these agents, clinicians may inadvertently boost the risk of recurrence by forgoing better therapies, the guideline states.
The initial endoscopy should be timed for 6-12 months after resection, regardless of whether patients are receiving pharmacologic prophylaxis, the guideline states. If there is endoscopic recurrence, then anti-TNF or thiopurine therapy should be started or optimized.
In the Postoperative Crohn’s Endoscopic Recurrence (POCER) trial, endoscopic monitoring and treatment escalation in the face of endoscopic recurrence cut the risk of subsequent clinical and endoscopic recurrence by about 18% and 27%, respectively, compared with continuing the original treatment regimen. Most patients received azathioprine or adalimumab with 3 months of metronidazole postoperatively, so “even [those] who were already on postoperative prophylaxis benefited from endoscopic monitoring with colonoscopy at 6-12 months,” the guideline notes. However, patients who elect early prophylaxis after resection can reasonably forego colonoscopy if endoscopic recurrence is unlikely to affect their treatment plan, the AGA states. The guideline strongly recommends ongoing surveillance endoscopies if patients decide against early postresection prophylaxis, but notes a lack of evidence on how far to space out these procedures.
None of the authors had relevant financial disclosures.
Patients whose Crohn’s disease fully remits after resection should not wait for endoscopic recurrence to start tumor-necrosis-factor inhibitors or thiopurines, according to a new guideline from the American Gastroenterological Association.
Patients who are low risk or worried about side effects, however, “may reasonably select endoscopy-guided pharmacological treatment,” the guidelines state (doi: 10.1053/j.gastro.2016.10.038).
Early pharmacologic prophylaxis usually begins within 8 weeks of surgery, they noted. Whether this approach bests endoscopy-guided treatment is unclear: In one small trial (Gastroenterology. 2013;145[4]:766-74.e1), early azathioprine therapy failed to best endoscopy-guided therapy for preventing clinical or endoscopic recurrence.
Early prophylaxis, however, is usually reasonable because most Crohn’s patients who undergo surgery have at least one risk factor for recurrence, Dr. Nguyen and his associates emphasize. They suggest reserving endoscopy-guided therapy for patients who have real concerns about side effects and are at low risk, such as nonsmokers who were diagnosed within 10 years and have less than 10-20 cm of fibrostenotic disease.
For prophylaxis, a moderate amount of evidence supports anti–tumor necrosis factor (TNF) agents, thiopurines, or combined therapy over other agents, the guideline also states. In placebo-controlled clinical trials, anti-TNF therapy reduced the chances of clinical recurrence by 49% and endoscopic recurrence by 76%, while thiopurines cut these rates by 65% and 60%, respectively. Evidence favors anti-TNF agents over thiopurines for preventing recurrence, but it is of low quality, the guideline says. Furthermore, only indirect evidence supports combined therapy in patients at highest risk of recurrence.
Among the antibiotics, only nitroimidazoles such as metronidazole have been adequately studied, and they posted worse results than anti-TNF agents or thiopurines. Antibiotic therapy decreased the risk of endoscopic recurrence of Crohn’s disease by about 50%, but long-term use is associated with peripheral neuropathy and disease usually recurs within 2 years of stopping treatment. Accordingly, the guidelines suggest using a nitroimidazole for only 3-12 months, and only in lower-risk patients who are concerned about the adverse effects of anti-TNF agents and thiopurines.
The AGA made a conditional recommendation against the prophylactic use of budesonide, probiotics, and 5-aminosalicylates such as mesalamine. Only low-quality evidence supports their efficacy after resection, and by using these agents, clinicians may inadvertently boost the risk of recurrence by forgoing better therapies, the guideline states.
The initial endoscopy should be timed for 6-12 months after resection, regardless of whether patients are receiving pharmacologic prophylaxis, the guideline states. If there is endoscopic recurrence, then anti-TNF or thiopurine therapy should be started or optimized.
In the Postoperative Crohn’s Endoscopic Recurrence (POCER) trial, endoscopic monitoring and treatment escalation in the face of endoscopic recurrence cut the risk of subsequent clinical and endoscopic recurrence by about 18% and 27%, respectively, compared with continuing the original treatment regimen. Most patients received azathioprine or adalimumab with 3 months of metronidazole postoperatively, so “even [those] who were already on postoperative prophylaxis benefited from endoscopic monitoring with colonoscopy at 6-12 months,” the guideline notes. However, patients who elect early prophylaxis after resection can reasonably forego colonoscopy if endoscopic recurrence is unlikely to affect their treatment plan, the AGA states. The guideline strongly recommends ongoing surveillance endoscopies if patients decide against early postresection prophylaxis, but notes a lack of evidence on how far to space out these procedures.
None of the authors had relevant financial disclosures.
Patients whose Crohn’s disease fully remits after resection should not wait for endoscopic recurrence to start tumor-necrosis-factor inhibitors or thiopurines, according to a new guideline from the American Gastroenterological Association.
Patients who are low risk or worried about side effects, however, “may reasonably select endoscopy-guided pharmacological treatment,” the guidelines state (doi: 10.1053/j.gastro.2016.10.038).
Early pharmacologic prophylaxis usually begins within 8 weeks of surgery, they noted. Whether this approach bests endoscopy-guided treatment is unclear: In one small trial (Gastroenterology. 2013;145[4]:766-74.e1), early azathioprine therapy failed to best endoscopy-guided therapy for preventing clinical or endoscopic recurrence.
Early prophylaxis, however, is usually reasonable because most Crohn’s patients who undergo surgery have at least one risk factor for recurrence, Dr. Nguyen and his associates emphasize. They suggest reserving endoscopy-guided therapy for patients who have real concerns about side effects and are at low risk, such as nonsmokers who were diagnosed within 10 years and have less than 10-20 cm of fibrostenotic disease.
For prophylaxis, a moderate amount of evidence supports anti–tumor necrosis factor (TNF) agents, thiopurines, or combined therapy over other agents, the guideline also states. In placebo-controlled clinical trials, anti-TNF therapy reduced the chances of clinical recurrence by 49% and endoscopic recurrence by 76%, while thiopurines cut these rates by 65% and 60%, respectively. Evidence favors anti-TNF agents over thiopurines for preventing recurrence, but it is of low quality, the guideline says. Furthermore, only indirect evidence supports combined therapy in patients at highest risk of recurrence.
Among the antibiotics, only nitroimidazoles such as metronidazole have been adequately studied, and they posted worse results than anti-TNF agents or thiopurines. Antibiotic therapy decreased the risk of endoscopic recurrence of Crohn’s disease by about 50%, but long-term use is associated with peripheral neuropathy and disease usually recurs within 2 years of stopping treatment. Accordingly, the guidelines suggest using a nitroimidazole for only 3-12 months, and only in lower-risk patients who are concerned about the adverse effects of anti-TNF agents and thiopurines.
The AGA made a conditional recommendation against the prophylactic use of budesonide, probiotics, and 5-aminosalicylates such as mesalamine. Only low-quality evidence supports their efficacy after resection, and by using these agents, clinicians may inadvertently boost the risk of recurrence by forgoing better therapies, the guideline states.
The initial endoscopy should be timed for 6-12 months after resection, regardless of whether patients are receiving pharmacologic prophylaxis, the guideline states. If there is endoscopic recurrence, then anti-TNF or thiopurine therapy should be started or optimized.
In the Postoperative Crohn’s Endoscopic Recurrence (POCER) trial, endoscopic monitoring and treatment escalation in the face of endoscopic recurrence cut the risk of subsequent clinical and endoscopic recurrence by about 18% and 27%, respectively, compared with continuing the original treatment regimen. Most patients received azathioprine or adalimumab with 3 months of metronidazole postoperatively, so “even [those] who were already on postoperative prophylaxis benefited from endoscopic monitoring with colonoscopy at 6-12 months,” the guideline notes. However, patients who elect early prophylaxis after resection can reasonably forego colonoscopy if endoscopic recurrence is unlikely to affect their treatment plan, the AGA states. The guideline strongly recommends ongoing surveillance endoscopies if patients decide against early postresection prophylaxis, but notes a lack of evidence on how far to space out these procedures.
None of the authors had relevant financial disclosures.
One in three older adults with epilepsy has poor adherence to antiepileptics
HOUSTON – One in three older Americans with epilepsy had poor adherence to antiepileptic drugs, according to a large retrospective study, and the numbers were even worse for members of minority populations.
In an analysis of Medicare claims, patients who were African American had an odds ratio of 1.56 (95% confidence interval, 1.46-1.68) for being non-adherent to antiepileptic drugs (AEDs). For Hispanic patients, the OR was 1.40 (95% CI, 1.28-1.54), while for Asians, the OR was 1.41 (95% CI, 1.25-1.54).
“Overall, 31.8% were non-adherent to AEDs; range was from 24.1% for whites to 34.3% for African Americans,” wrote Maria Pisu, PhD, and her collaborators in an abstract accompanying the poster presented at the annual meeting of the American Epilepsy Society.
The reasons for poor adherence are unknown, but warrant further investigation, said Dr. Pisu of the division of preventive medicine at the University of Alabama at Birmingham.
The sample included 36,912 patients with epilepsy. In the enhanced sample, 19.2% of patients were white, 62.5% were African American, 11.3% were Hispanic, 5.0% were Asian, and 2.0% were American Indian or Alaskan Native. Of the sample, 61.6% were female; 41.5% of patients were aged 66-74 years; 36.1% were aged 75-84 years; and 22.4% were 85 years or older.
In order to determine whether patients with epilepsy or seizures were adherent, investigators looked at the ratio of days that at least one AED prescription was in the database for a given patient, divided by the total days of follow-up on record (“proportion of days covered”). The primary outcome measure of nonadherence was defined as a proportion of days covered of less than 0.80.
Most patients had one to several comorbidities: only 8.3% had no other comorbidities, while 46.0% had more than four. The remainder fell somewhere in the middle.
A majority of patients (59.2%) had some form of copay or coinsurance for prescriptions. About half of patients (50.2%) lived in the South.
Through multivariable analysis, the investigators explored the relationships between nonadherence and race or ethnicity, demographics, geographic area of residence, comorbidities, and whether the prescribed AEDs were enzyme-inducing. Additionally, socioeconomic status was estimated by factoring in eligibility for Medicare Part D low income subsidies and using zip code–level poverty data.
The differences between ethnic groups, wrote Dr. Pisu, were significant “after accounting for several factors that affect prescription-taking factors, e.g., economic constraints,” so it was not just low socioeconomic status that accounted for the discrepancy.
The retrospective claims-based study was limited by several factors, wrote Dr. Pisu and her colleagues. True adherence is difficult to quantify, and may be overestimated by measuring filled prescriptions; on the other hand, some patients may have had other insurance coverage, and obtained medication through a means not captured in the study. Finally, dose adjustments may mean patients can “stretch” medications, thereby remaining adherent without refilling prescriptions.
Still, the study shows that nonadherence is pervasive among older Americans with epilepsy, with worse adherence among members of minority populations. “Interventions to promote adherence are important. These should account for the impact of drug cost-sharing and socioeconomic status on epilepsy treatment,” wrote Dr. Pisu.
The study was funded by the National Institute of Neurological Disorders and Stroke. Dr. Pisu reported no relevant financial disclosures.
[email protected]
On Twitter @karioakes
HOUSTON – One in three older Americans with epilepsy had poor adherence to antiepileptic drugs, according to a large retrospective study, and the numbers were even worse for members of minority populations.
In an analysis of Medicare claims, patients who were African American had an odds ratio of 1.56 (95% confidence interval, 1.46-1.68) for being non-adherent to antiepileptic drugs (AEDs). For Hispanic patients, the OR was 1.40 (95% CI, 1.28-1.54), while for Asians, the OR was 1.41 (95% CI, 1.25-1.54).
“Overall, 31.8% were non-adherent to AEDs; range was from 24.1% for whites to 34.3% for African Americans,” wrote Maria Pisu, PhD, and her collaborators in an abstract accompanying the poster presented at the annual meeting of the American Epilepsy Society.
The reasons for poor adherence are unknown, but warrant further investigation, said Dr. Pisu of the division of preventive medicine at the University of Alabama at Birmingham.
The sample included 36,912 patients with epilepsy. In the enhanced sample, 19.2% of patients were white, 62.5% were African American, 11.3% were Hispanic, 5.0% were Asian, and 2.0% were American Indian or Alaskan Native. Of the sample, 61.6% were female; 41.5% of patients were aged 66-74 years; 36.1% were aged 75-84 years; and 22.4% were 85 years or older.
In order to determine whether patients with epilepsy or seizures were adherent, investigators looked at the ratio of days that at least one AED prescription was in the database for a given patient, divided by the total days of follow-up on record (“proportion of days covered”). The primary outcome measure of nonadherence was defined as a proportion of days covered of less than 0.80.
Most patients had one to several comorbidities: only 8.3% had no other comorbidities, while 46.0% had more than four. The remainder fell somewhere in the middle.
A majority of patients (59.2%) had some form of copay or coinsurance for prescriptions. About half of patients (50.2%) lived in the South.
Through multivariable analysis, the investigators explored the relationships between nonadherence and race or ethnicity, demographics, geographic area of residence, comorbidities, and whether the prescribed AEDs were enzyme-inducing. Additionally, socioeconomic status was estimated by factoring in eligibility for Medicare Part D low income subsidies and using zip code–level poverty data.
The differences between ethnic groups, wrote Dr. Pisu, were significant “after accounting for several factors that affect prescription-taking factors, e.g., economic constraints,” so it was not just low socioeconomic status that accounted for the discrepancy.
The retrospective claims-based study was limited by several factors, wrote Dr. Pisu and her colleagues. True adherence is difficult to quantify, and may be overestimated by measuring filled prescriptions; on the other hand, some patients may have had other insurance coverage, and obtained medication through a means not captured in the study. Finally, dose adjustments may mean patients can “stretch” medications, thereby remaining adherent without refilling prescriptions.
Still, the study shows that nonadherence is pervasive among older Americans with epilepsy, with worse adherence among members of minority populations. “Interventions to promote adherence are important. These should account for the impact of drug cost-sharing and socioeconomic status on epilepsy treatment,” wrote Dr. Pisu.
The study was funded by the National Institute of Neurological Disorders and Stroke. Dr. Pisu reported no relevant financial disclosures.
[email protected]
On Twitter @karioakes
HOUSTON – One in three older Americans with epilepsy had poor adherence to antiepileptic drugs, according to a large retrospective study, and the numbers were even worse for members of minority populations.
In an analysis of Medicare claims, patients who were African American had an odds ratio of 1.56 (95% confidence interval, 1.46-1.68) for being non-adherent to antiepileptic drugs (AEDs). For Hispanic patients, the OR was 1.40 (95% CI, 1.28-1.54), while for Asians, the OR was 1.41 (95% CI, 1.25-1.54).
“Overall, 31.8% were non-adherent to AEDs; range was from 24.1% for whites to 34.3% for African Americans,” wrote Maria Pisu, PhD, and her collaborators in an abstract accompanying the poster presented at the annual meeting of the American Epilepsy Society.
The reasons for poor adherence are unknown, but warrant further investigation, said Dr. Pisu of the division of preventive medicine at the University of Alabama at Birmingham.
The sample included 36,912 patients with epilepsy. In the enhanced sample, 19.2% of patients were white, 62.5% were African American, 11.3% were Hispanic, 5.0% were Asian, and 2.0% were American Indian or Alaskan Native. Of the sample, 61.6% were female; 41.5% of patients were aged 66-74 years; 36.1% were aged 75-84 years; and 22.4% were 85 years or older.
In order to determine whether patients with epilepsy or seizures were adherent, investigators looked at the ratio of days that at least one AED prescription was in the database for a given patient, divided by the total days of follow-up on record (“proportion of days covered”). The primary outcome measure of nonadherence was defined as a proportion of days covered of less than 0.80.
Most patients had one to several comorbidities: only 8.3% had no other comorbidities, while 46.0% had more than four. The remainder fell somewhere in the middle.
A majority of patients (59.2%) had some form of copay or coinsurance for prescriptions. About half of patients (50.2%) lived in the South.
Through multivariable analysis, the investigators explored the relationships between nonadherence and race or ethnicity, demographics, geographic area of residence, comorbidities, and whether the prescribed AEDs were enzyme-inducing. Additionally, socioeconomic status was estimated by factoring in eligibility for Medicare Part D low income subsidies and using zip code–level poverty data.
The differences between ethnic groups, wrote Dr. Pisu, were significant “after accounting for several factors that affect prescription-taking factors, e.g., economic constraints,” so it was not just low socioeconomic status that accounted for the discrepancy.
The retrospective claims-based study was limited by several factors, wrote Dr. Pisu and her colleagues. True adherence is difficult to quantify, and may be overestimated by measuring filled prescriptions; on the other hand, some patients may have had other insurance coverage, and obtained medication through a means not captured in the study. Finally, dose adjustments may mean patients can “stretch” medications, thereby remaining adherent without refilling prescriptions.
Still, the study shows that nonadherence is pervasive among older Americans with epilepsy, with worse adherence among members of minority populations. “Interventions to promote adherence are important. These should account for the impact of drug cost-sharing and socioeconomic status on epilepsy treatment,” wrote Dr. Pisu.
The study was funded by the National Institute of Neurological Disorders and Stroke. Dr. Pisu reported no relevant financial disclosures.
[email protected]
On Twitter @karioakes
AT AES 2016
Key clinical point:
Major finding: For African-American patients, the odds ratio was 1.56 for being nonadherent (95% CI, 1.4-1.68).
Data source: Retrospective analysis of Medicare administrative claims data from 2008 to 2010, examining a 5% sample of patients with epilepsy aged 66 and over, and including all members of racial/ethnic minority populations who had claims for diagnoses of seizures or epilepsy.
Disclosures: The study was funded by the National Institute of Neurological Disorders and Stroke. Dr. Pisu reported no relevant financial disclosures.
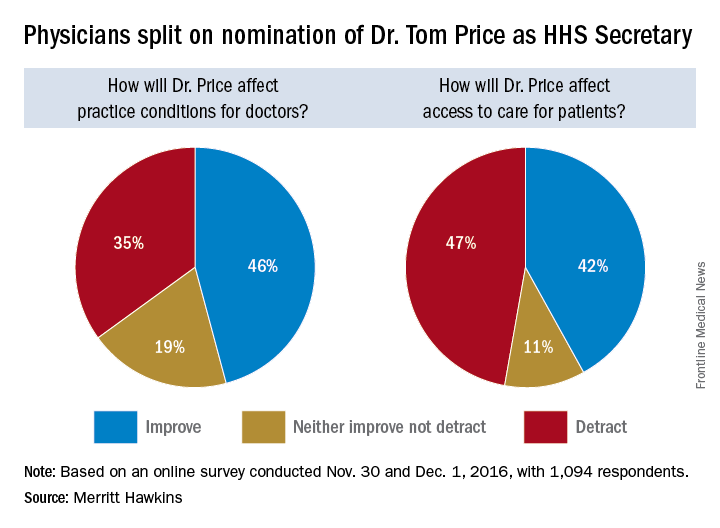
Trump HHS nominee could curb regulations, reshape health insurance
Opinions are mixed on what the nominations of Rep. Tom Price (R-Ga.) as Secretary of Health & Human Services will mean for medicine and health care.
An orthopedic surgeon and six-term congressman, Dr. Price is an outspoken critic of the Affordable Care Act and has sponsored or cosponsored numerous bills to replace it. President-elect Trump called Rep. Price “a renowned physician” who has “earned a reputation for being a tireless problem solver and the go-to expert on health care policy,” according to a statement.
Not everyone agrees.
But Adam Gaffney, MD, a pulmonologist at the Cambridge (Mass.) Health Alliance, said physicians’ ability to care for their patients would be compromised if Rep. Price succeeds with many of his proposals, such as the privatization of Medicare and block grants for Medicaid.
“If these reforms go through, we’re going to see the insurance protections of our patients get worse,” said Dr. Gaffney, a board member for Physicians for a National Health Program, which advocates for a single-payer health care system. “If [his] agenda is successful, I think it’s going to have a detrimental impact on our ability to provide the care that our patients need.”
ACA repeal, malpractice reform
In the House, Rep. Price has introduced the Empowering Patients First Act, legislation, which would allow doctors to opt out of Medicare and enter into private contracts with Medicare patients. The bill is seen by many as a potential blueprint for Trump administration health reform. Rep. Price is also a proponent of malpractice reform that would make it tougher for patients to sue doctors and would lower liability insurance premiums.
The Empowering Patients First Act would repeal the ACA and offer tax credits for the purchase of individual and family health insurance policies. It would also create incentives for patients to contribute to health savings accounts, offer state grants to subsidize coverage for high-risk patients, and authorize businesses to cover members through association health plans.
The American Medical Association praised Rep. Price’s nomination, expressing support for ability to lead HHS.
“Dr. Price has been a leader in the development of health policies to advance patient choice and market-based solutions as well as reduce excessive regulatory burdens that diminish time devoted to patient care and increase costs,” AMA Board of Trustees Chair Patrice A. Harris, MD, said in a statement.
The American College of Surgeons' Executive Director, David B. Hoyt, MD, FACS, issued a supportive statement about the nomination of Dr. Price. "“Dr. Price is a stalwart champion for patients and their surgeons, and the ACS looks forward to working with him on key issues, such as the implementation of the Medicare Access and CHIP Reauthorization Act,” said Dr. Hoyt in a statement. “The ACS encourages the Senate to swiftly confirm Dr. Price’s nomination as Secretary of HHS."
But thousands of physicians disagree. Rep. Price’s proposals on Medicaid and Medicare threaten to harm vulnerable patients and limit access to healthcare, according to an open letter to the AMA published on Medium and credited to Clinician Action Network, a nonpartisan group that supports evidence-based policies. The group was started in opposition to the nomination of Rep. Price.
“We cannot support the dismantling of Medicaid, which has helped 15 million Americans gain health coverage since 2014,” the letter states. “We oppose Dr. Price’s proposals to reduce funding for the Children’s Health Insurance Program, a critical mechanism by which poor children access preventative care.”
Value-based payment or fee for service?
Rep. Price’s experience as a physician fuels his efforts to reduce burdensome regulations for doctors and enhance care efficiency, according to one of his predecessors, Louis W. Sullivan, MD. If confirmed, Rep. Price will become the third physician to be HHS secretary; Dr. Sullivan served in the George H.W. Bush administration and Otis R. Bowen, MD, served in the Reagan administration.
“He is very much aware of the challenges that physicians face in trying to delivery care,” said Dr. Sullivan. “I know that he’ll be working to reduce regulation when feasible so that the cost and delays that some regulatory issues present will hopefully be relieved,”
Some of those regulatory modifications could affect value-based care programs, Dr. Rodriguez said. Rep. Price has been critical of the move from fee for service to quality-based care and has opposed some corresponding programs, such as bundled payment initiatives. Rep. Price and members of the GOP Doctors Caucus wrote to Centers for Medicare & Medicaid Services in October to protest the regulations to implement the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) as too burdensome for smaller practices and calling for flexibility in quality reporting.
Rep. Price voted for passage of MACRA.
“He has been cautious about some of the changes that are being promoted in health care,” Dr. Rodriguez said. “He could slow that down – the processes being put in place. That might delay the impact those systems have in bringing about the improved quality that we want. [This would be] enormous, given the amount of work that we’ve been doing.”
A fair medical liability system also is a priority for Rep. Price, Dr. Sullivan said. His Empowering Patients First bill would require collaboration between HHS and physician associations to develop best practice guidelines that would provide a litigation safe harbor to physicians who practiced in accordance with the standards.
“I know that he will be working to develop strategies to reduce litigation in the health space,” Dr. Sullivan said in an interview. “That is one of the challenges that adds to health care costs, adds tension, and enhances an adversarial relationship between physicians and patients.”
But Dr. Gaffney said that he believes Rep. Price’s views on reproductive rights and gay marriage are regressive and that his agenda regarding health policy issues is bad for medicine.
“The overall [theme] of that agenda can be summed up as ‘take from the poor and sick and give to the rich,’ ” Dr. Gaffney said in an interview. “I think the financing of this [new health reform] system will be much more aggressive, and the result will be greater health care inequity.”
Rep. Price also has supported a ban on federal funding for Planned Parenthood, calling some of their practices barbaric. He has also voted to prohibit the importation of prescription drugs by nonsanctioned importers and has voted to repeal the medical device excise tax.
[email protected]
On Twitter @legal_med
Opinions are mixed on what the nominations of Rep. Tom Price (R-Ga.) as Secretary of Health & Human Services will mean for medicine and health care.
An orthopedic surgeon and six-term congressman, Dr. Price is an outspoken critic of the Affordable Care Act and has sponsored or cosponsored numerous bills to replace it. President-elect Trump called Rep. Price “a renowned physician” who has “earned a reputation for being a tireless problem solver and the go-to expert on health care policy,” according to a statement.
Not everyone agrees.
But Adam Gaffney, MD, a pulmonologist at the Cambridge (Mass.) Health Alliance, said physicians’ ability to care for their patients would be compromised if Rep. Price succeeds with many of his proposals, such as the privatization of Medicare and block grants for Medicaid.
“If these reforms go through, we’re going to see the insurance protections of our patients get worse,” said Dr. Gaffney, a board member for Physicians for a National Health Program, which advocates for a single-payer health care system. “If [his] agenda is successful, I think it’s going to have a detrimental impact on our ability to provide the care that our patients need.”
ACA repeal, malpractice reform
In the House, Rep. Price has introduced the Empowering Patients First Act, legislation, which would allow doctors to opt out of Medicare and enter into private contracts with Medicare patients. The bill is seen by many as a potential blueprint for Trump administration health reform. Rep. Price is also a proponent of malpractice reform that would make it tougher for patients to sue doctors and would lower liability insurance premiums.
The Empowering Patients First Act would repeal the ACA and offer tax credits for the purchase of individual and family health insurance policies. It would also create incentives for patients to contribute to health savings accounts, offer state grants to subsidize coverage for high-risk patients, and authorize businesses to cover members through association health plans.
The American Medical Association praised Rep. Price’s nomination, expressing support for ability to lead HHS.
“Dr. Price has been a leader in the development of health policies to advance patient choice and market-based solutions as well as reduce excessive regulatory burdens that diminish time devoted to patient care and increase costs,” AMA Board of Trustees Chair Patrice A. Harris, MD, said in a statement.
The American College of Surgeons' Executive Director, David B. Hoyt, MD, FACS, issued a supportive statement about the nomination of Dr. Price. "“Dr. Price is a stalwart champion for patients and their surgeons, and the ACS looks forward to working with him on key issues, such as the implementation of the Medicare Access and CHIP Reauthorization Act,” said Dr. Hoyt in a statement. “The ACS encourages the Senate to swiftly confirm Dr. Price’s nomination as Secretary of HHS."
But thousands of physicians disagree. Rep. Price’s proposals on Medicaid and Medicare threaten to harm vulnerable patients and limit access to healthcare, according to an open letter to the AMA published on Medium and credited to Clinician Action Network, a nonpartisan group that supports evidence-based policies. The group was started in opposition to the nomination of Rep. Price.
“We cannot support the dismantling of Medicaid, which has helped 15 million Americans gain health coverage since 2014,” the letter states. “We oppose Dr. Price’s proposals to reduce funding for the Children’s Health Insurance Program, a critical mechanism by which poor children access preventative care.”
Value-based payment or fee for service?
Rep. Price’s experience as a physician fuels his efforts to reduce burdensome regulations for doctors and enhance care efficiency, according to one of his predecessors, Louis W. Sullivan, MD. If confirmed, Rep. Price will become the third physician to be HHS secretary; Dr. Sullivan served in the George H.W. Bush administration and Otis R. Bowen, MD, served in the Reagan administration.
“He is very much aware of the challenges that physicians face in trying to delivery care,” said Dr. Sullivan. “I know that he’ll be working to reduce regulation when feasible so that the cost and delays that some regulatory issues present will hopefully be relieved,”
Some of those regulatory modifications could affect value-based care programs, Dr. Rodriguez said. Rep. Price has been critical of the move from fee for service to quality-based care and has opposed some corresponding programs, such as bundled payment initiatives. Rep. Price and members of the GOP Doctors Caucus wrote to Centers for Medicare & Medicaid Services in October to protest the regulations to implement the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) as too burdensome for smaller practices and calling for flexibility in quality reporting.
Rep. Price voted for passage of MACRA.
“He has been cautious about some of the changes that are being promoted in health care,” Dr. Rodriguez said. “He could slow that down – the processes being put in place. That might delay the impact those systems have in bringing about the improved quality that we want. [This would be] enormous, given the amount of work that we’ve been doing.”
A fair medical liability system also is a priority for Rep. Price, Dr. Sullivan said. His Empowering Patients First bill would require collaboration between HHS and physician associations to develop best practice guidelines that would provide a litigation safe harbor to physicians who practiced in accordance with the standards.
“I know that he will be working to develop strategies to reduce litigation in the health space,” Dr. Sullivan said in an interview. “That is one of the challenges that adds to health care costs, adds tension, and enhances an adversarial relationship between physicians and patients.”
But Dr. Gaffney said that he believes Rep. Price’s views on reproductive rights and gay marriage are regressive and that his agenda regarding health policy issues is bad for medicine.
“The overall [theme] of that agenda can be summed up as ‘take from the poor and sick and give to the rich,’ ” Dr. Gaffney said in an interview. “I think the financing of this [new health reform] system will be much more aggressive, and the result will be greater health care inequity.”
Rep. Price also has supported a ban on federal funding for Planned Parenthood, calling some of their practices barbaric. He has also voted to prohibit the importation of prescription drugs by nonsanctioned importers and has voted to repeal the medical device excise tax.
[email protected]
On Twitter @legal_med
Opinions are mixed on what the nominations of Rep. Tom Price (R-Ga.) as Secretary of Health & Human Services will mean for medicine and health care.
An orthopedic surgeon and six-term congressman, Dr. Price is an outspoken critic of the Affordable Care Act and has sponsored or cosponsored numerous bills to replace it. President-elect Trump called Rep. Price “a renowned physician” who has “earned a reputation for being a tireless problem solver and the go-to expert on health care policy,” according to a statement.
Not everyone agrees.
But Adam Gaffney, MD, a pulmonologist at the Cambridge (Mass.) Health Alliance, said physicians’ ability to care for their patients would be compromised if Rep. Price succeeds with many of his proposals, such as the privatization of Medicare and block grants for Medicaid.
“If these reforms go through, we’re going to see the insurance protections of our patients get worse,” said Dr. Gaffney, a board member for Physicians for a National Health Program, which advocates for a single-payer health care system. “If [his] agenda is successful, I think it’s going to have a detrimental impact on our ability to provide the care that our patients need.”
ACA repeal, malpractice reform
In the House, Rep. Price has introduced the Empowering Patients First Act, legislation, which would allow doctors to opt out of Medicare and enter into private contracts with Medicare patients. The bill is seen by many as a potential blueprint for Trump administration health reform. Rep. Price is also a proponent of malpractice reform that would make it tougher for patients to sue doctors and would lower liability insurance premiums.
The Empowering Patients First Act would repeal the ACA and offer tax credits for the purchase of individual and family health insurance policies. It would also create incentives for patients to contribute to health savings accounts, offer state grants to subsidize coverage for high-risk patients, and authorize businesses to cover members through association health plans.
The American Medical Association praised Rep. Price’s nomination, expressing support for ability to lead HHS.
“Dr. Price has been a leader in the development of health policies to advance patient choice and market-based solutions as well as reduce excessive regulatory burdens that diminish time devoted to patient care and increase costs,” AMA Board of Trustees Chair Patrice A. Harris, MD, said in a statement.
The American College of Surgeons' Executive Director, David B. Hoyt, MD, FACS, issued a supportive statement about the nomination of Dr. Price. "“Dr. Price is a stalwart champion for patients and their surgeons, and the ACS looks forward to working with him on key issues, such as the implementation of the Medicare Access and CHIP Reauthorization Act,” said Dr. Hoyt in a statement. “The ACS encourages the Senate to swiftly confirm Dr. Price’s nomination as Secretary of HHS."
But thousands of physicians disagree. Rep. Price’s proposals on Medicaid and Medicare threaten to harm vulnerable patients and limit access to healthcare, according to an open letter to the AMA published on Medium and credited to Clinician Action Network, a nonpartisan group that supports evidence-based policies. The group was started in opposition to the nomination of Rep. Price.
“We cannot support the dismantling of Medicaid, which has helped 15 million Americans gain health coverage since 2014,” the letter states. “We oppose Dr. Price’s proposals to reduce funding for the Children’s Health Insurance Program, a critical mechanism by which poor children access preventative care.”
Value-based payment or fee for service?
Rep. Price’s experience as a physician fuels his efforts to reduce burdensome regulations for doctors and enhance care efficiency, according to one of his predecessors, Louis W. Sullivan, MD. If confirmed, Rep. Price will become the third physician to be HHS secretary; Dr. Sullivan served in the George H.W. Bush administration and Otis R. Bowen, MD, served in the Reagan administration.
“He is very much aware of the challenges that physicians face in trying to delivery care,” said Dr. Sullivan. “I know that he’ll be working to reduce regulation when feasible so that the cost and delays that some regulatory issues present will hopefully be relieved,”
Some of those regulatory modifications could affect value-based care programs, Dr. Rodriguez said. Rep. Price has been critical of the move from fee for service to quality-based care and has opposed some corresponding programs, such as bundled payment initiatives. Rep. Price and members of the GOP Doctors Caucus wrote to Centers for Medicare & Medicaid Services in October to protest the regulations to implement the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) as too burdensome for smaller practices and calling for flexibility in quality reporting.
Rep. Price voted for passage of MACRA.
“He has been cautious about some of the changes that are being promoted in health care,” Dr. Rodriguez said. “He could slow that down – the processes being put in place. That might delay the impact those systems have in bringing about the improved quality that we want. [This would be] enormous, given the amount of work that we’ve been doing.”
A fair medical liability system also is a priority for Rep. Price, Dr. Sullivan said. His Empowering Patients First bill would require collaboration between HHS and physician associations to develop best practice guidelines that would provide a litigation safe harbor to physicians who practiced in accordance with the standards.
“I know that he will be working to develop strategies to reduce litigation in the health space,” Dr. Sullivan said in an interview. “That is one of the challenges that adds to health care costs, adds tension, and enhances an adversarial relationship between physicians and patients.”
But Dr. Gaffney said that he believes Rep. Price’s views on reproductive rights and gay marriage are regressive and that his agenda regarding health policy issues is bad for medicine.
“The overall [theme] of that agenda can be summed up as ‘take from the poor and sick and give to the rich,’ ” Dr. Gaffney said in an interview. “I think the financing of this [new health reform] system will be much more aggressive, and the result will be greater health care inequity.”
Rep. Price also has supported a ban on federal funding for Planned Parenthood, calling some of their practices barbaric. He has also voted to prohibit the importation of prescription drugs by nonsanctioned importers and has voted to repeal the medical device excise tax.
[email protected]
On Twitter @legal_med
Aspirin use linked to increased ICH in trauma patients
WAIKOLOA, HAWAII – Among a group of anticoagulated trauma patients, those on aspirin had the highest rate and risk of intracranial hemorrhage (ICH), while those on novel oral anticoagulants were not at higher risk for ICH, ICH progression, or death, a multicenter study found.
“The number of patients on warfarin and antiplatelet agents has significantly increased over time,” Leslie Kobayashi, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “These oral antithrombotic agents have been associated with poor outcomes following traumatic injury, including increased rates of intracranial hemorrhage, increased progression of intracranial hemorrhage, and increased mortality.”
In a prospective, multicenter observational study conducted by the AAST’s Multi-institutional Trials Committee, Dr. Kobayashi and her associates set out identify injury patterns and outcomes in trauma patients taking the NOAs, and to test their hypothesis that patients taking NOAs would have higher rates of ICH, ICH progression, and death, compared with patients taking traditional oral anticoagulant therapies (OATs). Patients were included if they were admitted to the trauma service on warfarin, aspirin, clopidogrel, dabigatran, apixaban, or rivaroxaban. Pregnant patients, prisoners, and minors were excluded from the study. Data collected included demographics, mechanism of injury, vitals on admission, injuries/injury severity scores, labs, interventions, and reversal agents used such as vitamin K, prothrombin complexes, dialysis, and transfusion of fresh frozen plasma (FFP). Outcomes studied included ICH, ICH progression, and death.
In all, 16 Level 1 trauma centers enrolled 1,847 patients over a 2-year period. Their average age was 75 years, 46% were female, 77% were white, their median Injury Severity Score (ISS) was 9, and 99% sustained a blunt mechanism of trauma. The top two causes of injury were falls (71%) and motor vehicle crashes (15%). One-third of patients (33%) were on warfarin, while the remainder were on aspirin (26%), clopidogrel (24%), NOAs (10%), and 7% took multiple or other agents.
The mechanism of injury pattern was similar between patients taking NOAs and those taking OATs, with the exception of patients on aspirin being significantly less likely to have sustained a fall. Patients on aspirin also had a significantly higher median ISS. “Patients on NOAs presented more frequently in shock as defined by a systolic blood pressure of less than 90 mmHg, but this was not associated with increased need for packed red blood cell transfusion, bleeding requiring an intervention, need for surgical procedure, hospital LOS, complications, or death,” Dr. Kobayashi said.
About 30% of all patients studied underwent an attempt at reversal. The types of agents used to reverse the patients differed depending on drug agent, with antiplatelet patients more frequently getting platelets, and patients on warfarin more frequently receiving FFP, vitamin K, and prothrombin complex. “Interestingly, patients on the anti-Xa inhibitors more frequently received prothrombin complex as well,” she said. “This likely reflects some of the recent literature which suggests that there may be a therapeutic benefit to using prothrombin complex in patients taking the oral anti-Xa inhibitors but not in patients on dabigatran.”
Overall, bleeding, need for surgical procedure, need for neurosurgical procedure, complications, length of stay, and death were similar between those on NOAs and those on OATs. However, the rate of ICH was significantly higher in patients on aspirin. “What is even more surprising is that 89% of the patients in the aspirin-only group were on an 81-mg baby aspirin rather than the larger 325-mg dose,” Dr. Kobayashi said. This difference was significant on univariate analysis and was retained after multivariate logistic regression adjusted for differences between populations, with an OR for aspirin of 1.7 and a P value of .024. “This is not to suggest that patients on aspirin are doing markedly worse, compared to their counterparts, but I think most of us would have assumed that aspirin patients would have done better,” she commented. “I think we’ve definitively shown that is not the case.” Other independent predictors of ICH were advanced age (OR, 1.02), Asian race (OR, 3.1), ISS of 10 or greater (OR, 2.2), and a Glasgow coma score (GCS) of 8 or less (OR, 5.6).
Despite their increased risk for ICH, patients on aspirin were significantly less likely to undergo an attempt at reversal with any type of agent, at 16% with a P value of less than .001, on univariate analysis. “This was significantly lower than all other medications and was retained after multivariate logistic regression, with an OR of 0.3 and a P value of less than .001,” she said.
Progression of ICH did not differ by medication group. Other independent predictors included intraparenchymal location of hemorrhage (OR, 2.2), need for a neurosurgical procedure (OR, 5.1), an attempt at reversal (OR, 2.3) and a GCS of 8 or lower at admission (OR, 4.3). Similarly, multivariate analysis of death showed no significant differences between the different medication groups. Independent predictors included advanced age (OR, 1.06), GCS of 8 or less (OR, 13), progression of head injury (OR, 10), bleeding (OR, 2.3), and complications (OR, 2.1).
Dr. Kobayashi acknowledged that the study’s observational design is a limitation, as well as the fact that it lacked a control group of age-matched patients who were not taking anticoagulants. “Additionally, we had a relatively low number of patients on NOAs, at only 10% of the study population,” she said. “Lastly, there is potential for enrollment bias as all sites involved in this study were level one trauma centers.” She reported having no financial disclosures.
WAIKOLOA, HAWAII – Among a group of anticoagulated trauma patients, those on aspirin had the highest rate and risk of intracranial hemorrhage (ICH), while those on novel oral anticoagulants were not at higher risk for ICH, ICH progression, or death, a multicenter study found.
“The number of patients on warfarin and antiplatelet agents has significantly increased over time,” Leslie Kobayashi, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “These oral antithrombotic agents have been associated with poor outcomes following traumatic injury, including increased rates of intracranial hemorrhage, increased progression of intracranial hemorrhage, and increased mortality.”
In a prospective, multicenter observational study conducted by the AAST’s Multi-institutional Trials Committee, Dr. Kobayashi and her associates set out identify injury patterns and outcomes in trauma patients taking the NOAs, and to test their hypothesis that patients taking NOAs would have higher rates of ICH, ICH progression, and death, compared with patients taking traditional oral anticoagulant therapies (OATs). Patients were included if they were admitted to the trauma service on warfarin, aspirin, clopidogrel, dabigatran, apixaban, or rivaroxaban. Pregnant patients, prisoners, and minors were excluded from the study. Data collected included demographics, mechanism of injury, vitals on admission, injuries/injury severity scores, labs, interventions, and reversal agents used such as vitamin K, prothrombin complexes, dialysis, and transfusion of fresh frozen plasma (FFP). Outcomes studied included ICH, ICH progression, and death.
In all, 16 Level 1 trauma centers enrolled 1,847 patients over a 2-year period. Their average age was 75 years, 46% were female, 77% were white, their median Injury Severity Score (ISS) was 9, and 99% sustained a blunt mechanism of trauma. The top two causes of injury were falls (71%) and motor vehicle crashes (15%). One-third of patients (33%) were on warfarin, while the remainder were on aspirin (26%), clopidogrel (24%), NOAs (10%), and 7% took multiple or other agents.
The mechanism of injury pattern was similar between patients taking NOAs and those taking OATs, with the exception of patients on aspirin being significantly less likely to have sustained a fall. Patients on aspirin also had a significantly higher median ISS. “Patients on NOAs presented more frequently in shock as defined by a systolic blood pressure of less than 90 mmHg, but this was not associated with increased need for packed red blood cell transfusion, bleeding requiring an intervention, need for surgical procedure, hospital LOS, complications, or death,” Dr. Kobayashi said.
About 30% of all patients studied underwent an attempt at reversal. The types of agents used to reverse the patients differed depending on drug agent, with antiplatelet patients more frequently getting platelets, and patients on warfarin more frequently receiving FFP, vitamin K, and prothrombin complex. “Interestingly, patients on the anti-Xa inhibitors more frequently received prothrombin complex as well,” she said. “This likely reflects some of the recent literature which suggests that there may be a therapeutic benefit to using prothrombin complex in patients taking the oral anti-Xa inhibitors but not in patients on dabigatran.”
Overall, bleeding, need for surgical procedure, need for neurosurgical procedure, complications, length of stay, and death were similar between those on NOAs and those on OATs. However, the rate of ICH was significantly higher in patients on aspirin. “What is even more surprising is that 89% of the patients in the aspirin-only group were on an 81-mg baby aspirin rather than the larger 325-mg dose,” Dr. Kobayashi said. This difference was significant on univariate analysis and was retained after multivariate logistic regression adjusted for differences between populations, with an OR for aspirin of 1.7 and a P value of .024. “This is not to suggest that patients on aspirin are doing markedly worse, compared to their counterparts, but I think most of us would have assumed that aspirin patients would have done better,” she commented. “I think we’ve definitively shown that is not the case.” Other independent predictors of ICH were advanced age (OR, 1.02), Asian race (OR, 3.1), ISS of 10 or greater (OR, 2.2), and a Glasgow coma score (GCS) of 8 or less (OR, 5.6).
Despite their increased risk for ICH, patients on aspirin were significantly less likely to undergo an attempt at reversal with any type of agent, at 16% with a P value of less than .001, on univariate analysis. “This was significantly lower than all other medications and was retained after multivariate logistic regression, with an OR of 0.3 and a P value of less than .001,” she said.
Progression of ICH did not differ by medication group. Other independent predictors included intraparenchymal location of hemorrhage (OR, 2.2), need for a neurosurgical procedure (OR, 5.1), an attempt at reversal (OR, 2.3) and a GCS of 8 or lower at admission (OR, 4.3). Similarly, multivariate analysis of death showed no significant differences between the different medication groups. Independent predictors included advanced age (OR, 1.06), GCS of 8 or less (OR, 13), progression of head injury (OR, 10), bleeding (OR, 2.3), and complications (OR, 2.1).
Dr. Kobayashi acknowledged that the study’s observational design is a limitation, as well as the fact that it lacked a control group of age-matched patients who were not taking anticoagulants. “Additionally, we had a relatively low number of patients on NOAs, at only 10% of the study population,” she said. “Lastly, there is potential for enrollment bias as all sites involved in this study were level one trauma centers.” She reported having no financial disclosures.
WAIKOLOA, HAWAII – Among a group of anticoagulated trauma patients, those on aspirin had the highest rate and risk of intracranial hemorrhage (ICH), while those on novel oral anticoagulants were not at higher risk for ICH, ICH progression, or death, a multicenter study found.
“The number of patients on warfarin and antiplatelet agents has significantly increased over time,” Leslie Kobayashi, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “These oral antithrombotic agents have been associated with poor outcomes following traumatic injury, including increased rates of intracranial hemorrhage, increased progression of intracranial hemorrhage, and increased mortality.”
In a prospective, multicenter observational study conducted by the AAST’s Multi-institutional Trials Committee, Dr. Kobayashi and her associates set out identify injury patterns and outcomes in trauma patients taking the NOAs, and to test their hypothesis that patients taking NOAs would have higher rates of ICH, ICH progression, and death, compared with patients taking traditional oral anticoagulant therapies (OATs). Patients were included if they were admitted to the trauma service on warfarin, aspirin, clopidogrel, dabigatran, apixaban, or rivaroxaban. Pregnant patients, prisoners, and minors were excluded from the study. Data collected included demographics, mechanism of injury, vitals on admission, injuries/injury severity scores, labs, interventions, and reversal agents used such as vitamin K, prothrombin complexes, dialysis, and transfusion of fresh frozen plasma (FFP). Outcomes studied included ICH, ICH progression, and death.
In all, 16 Level 1 trauma centers enrolled 1,847 patients over a 2-year period. Their average age was 75 years, 46% were female, 77% were white, their median Injury Severity Score (ISS) was 9, and 99% sustained a blunt mechanism of trauma. The top two causes of injury were falls (71%) and motor vehicle crashes (15%). One-third of patients (33%) were on warfarin, while the remainder were on aspirin (26%), clopidogrel (24%), NOAs (10%), and 7% took multiple or other agents.
The mechanism of injury pattern was similar between patients taking NOAs and those taking OATs, with the exception of patients on aspirin being significantly less likely to have sustained a fall. Patients on aspirin also had a significantly higher median ISS. “Patients on NOAs presented more frequently in shock as defined by a systolic blood pressure of less than 90 mmHg, but this was not associated with increased need for packed red blood cell transfusion, bleeding requiring an intervention, need for surgical procedure, hospital LOS, complications, or death,” Dr. Kobayashi said.
About 30% of all patients studied underwent an attempt at reversal. The types of agents used to reverse the patients differed depending on drug agent, with antiplatelet patients more frequently getting platelets, and patients on warfarin more frequently receiving FFP, vitamin K, and prothrombin complex. “Interestingly, patients on the anti-Xa inhibitors more frequently received prothrombin complex as well,” she said. “This likely reflects some of the recent literature which suggests that there may be a therapeutic benefit to using prothrombin complex in patients taking the oral anti-Xa inhibitors but not in patients on dabigatran.”
Overall, bleeding, need for surgical procedure, need for neurosurgical procedure, complications, length of stay, and death were similar between those on NOAs and those on OATs. However, the rate of ICH was significantly higher in patients on aspirin. “What is even more surprising is that 89% of the patients in the aspirin-only group were on an 81-mg baby aspirin rather than the larger 325-mg dose,” Dr. Kobayashi said. This difference was significant on univariate analysis and was retained after multivariate logistic regression adjusted for differences between populations, with an OR for aspirin of 1.7 and a P value of .024. “This is not to suggest that patients on aspirin are doing markedly worse, compared to their counterparts, but I think most of us would have assumed that aspirin patients would have done better,” she commented. “I think we’ve definitively shown that is not the case.” Other independent predictors of ICH were advanced age (OR, 1.02), Asian race (OR, 3.1), ISS of 10 or greater (OR, 2.2), and a Glasgow coma score (GCS) of 8 or less (OR, 5.6).
Despite their increased risk for ICH, patients on aspirin were significantly less likely to undergo an attempt at reversal with any type of agent, at 16% with a P value of less than .001, on univariate analysis. “This was significantly lower than all other medications and was retained after multivariate logistic regression, with an OR of 0.3 and a P value of less than .001,” she said.
Progression of ICH did not differ by medication group. Other independent predictors included intraparenchymal location of hemorrhage (OR, 2.2), need for a neurosurgical procedure (OR, 5.1), an attempt at reversal (OR, 2.3) and a GCS of 8 or lower at admission (OR, 4.3). Similarly, multivariate analysis of death showed no significant differences between the different medication groups. Independent predictors included advanced age (OR, 1.06), GCS of 8 or less (OR, 13), progression of head injury (OR, 10), bleeding (OR, 2.3), and complications (OR, 2.1).
Dr. Kobayashi acknowledged that the study’s observational design is a limitation, as well as the fact that it lacked a control group of age-matched patients who were not taking anticoagulants. “Additionally, we had a relatively low number of patients on NOAs, at only 10% of the study population,” she said. “Lastly, there is potential for enrollment bias as all sites involved in this study were level one trauma centers.” She reported having no financial disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point:
Major finding: The rate of ICH was significantly higher in patients on aspirin, compared with those on novel oral anticoagulant therapies (OR, 1.7; P = .024).
Data source: A prospective evaluation of 1,847 patients treated at 16 level one trauma centers over a 2-year period.
Disclosures: Dr. Kobayashi reported having no financial disclosures.
Wide variation seen in treatment of infantile spasms
HOUSTON – The types of diagnostic tests ordered and medication used for treatment of infantile spasms vary considerably, a large study of children’s hospitals showed.
“Children with infantile spasms often require extensive diagnostic work-up to determine etiology, expensive medications for treatment, and hospitalization during the initiation of certain therapies,” researchers led by Sunita N. Misra, MD, PhD, wrote in an abstract presented at the annual meeting of the American Epilepsy Society. “The common diagnostic studies and therapies have evolved over the last several decades.”
The researchers collected patient demographics, hospital length of stay, hospital admission cost, use of various diagnostic studies (such as lumbar puncture, brain MRI, and EEG), and medications used for infantile spasms (including antiepileptic drugs, corticotropin, and steroids). Cost data, calculated as a ratio of cost to charges, were collected and adjusted to 2014 dollars.
A total of 6,183 patients were included in the analysis and their average age of infantile-spasm diagnosis was 9 months. The most common diagnostic test ordered was EEG (76%), followed by brain imaging (57%), organic acids (38%), and lumbar puncture (17%). Medications were started during inpatient hospitalization in two-thirds of patients, with 33% starting on corticotropin; 29% on topiramate; and fewer than 10% of patients on an oral or intravenous steroid, zonisamide, or vigabatrin (Sabril). Use of corticotropin decreased over time, while use of oral steroids trended upwards. “We were surprised that one-third of patients did not have a medication initiated as an inpatient, given the studies showing earlier use of effective therapy has better outcomes,” Dr. Misra said in an interview in advance of the meeting.
“The cost of taking care of children with infantile spasms has increased over the study period 2004-2014,” Dr. Misra said. “Although we identified a few contributors to rising cost, there are probably other factors that need to be considered in future studies.” She acknowledged certain limitations of the analysis, including its retrospective design and the fact that it only identified cost associated with the initial admission. “Several of the diagnostic studies and medications may be initiated as an outpatient, for which we do not have the data,” she said.
Dr. Misra reported having no financial disclosures.
HOUSTON – The types of diagnostic tests ordered and medication used for treatment of infantile spasms vary considerably, a large study of children’s hospitals showed.
“Children with infantile spasms often require extensive diagnostic work-up to determine etiology, expensive medications for treatment, and hospitalization during the initiation of certain therapies,” researchers led by Sunita N. Misra, MD, PhD, wrote in an abstract presented at the annual meeting of the American Epilepsy Society. “The common diagnostic studies and therapies have evolved over the last several decades.”
The researchers collected patient demographics, hospital length of stay, hospital admission cost, use of various diagnostic studies (such as lumbar puncture, brain MRI, and EEG), and medications used for infantile spasms (including antiepileptic drugs, corticotropin, and steroids). Cost data, calculated as a ratio of cost to charges, were collected and adjusted to 2014 dollars.
A total of 6,183 patients were included in the analysis and their average age of infantile-spasm diagnosis was 9 months. The most common diagnostic test ordered was EEG (76%), followed by brain imaging (57%), organic acids (38%), and lumbar puncture (17%). Medications were started during inpatient hospitalization in two-thirds of patients, with 33% starting on corticotropin; 29% on topiramate; and fewer than 10% of patients on an oral or intravenous steroid, zonisamide, or vigabatrin (Sabril). Use of corticotropin decreased over time, while use of oral steroids trended upwards. “We were surprised that one-third of patients did not have a medication initiated as an inpatient, given the studies showing earlier use of effective therapy has better outcomes,” Dr. Misra said in an interview in advance of the meeting.
“The cost of taking care of children with infantile spasms has increased over the study period 2004-2014,” Dr. Misra said. “Although we identified a few contributors to rising cost, there are probably other factors that need to be considered in future studies.” She acknowledged certain limitations of the analysis, including its retrospective design and the fact that it only identified cost associated with the initial admission. “Several of the diagnostic studies and medications may be initiated as an outpatient, for which we do not have the data,” she said.
Dr. Misra reported having no financial disclosures.
HOUSTON – The types of diagnostic tests ordered and medication used for treatment of infantile spasms vary considerably, a large study of children’s hospitals showed.
“Children with infantile spasms often require extensive diagnostic work-up to determine etiology, expensive medications for treatment, and hospitalization during the initiation of certain therapies,” researchers led by Sunita N. Misra, MD, PhD, wrote in an abstract presented at the annual meeting of the American Epilepsy Society. “The common diagnostic studies and therapies have evolved over the last several decades.”
The researchers collected patient demographics, hospital length of stay, hospital admission cost, use of various diagnostic studies (such as lumbar puncture, brain MRI, and EEG), and medications used for infantile spasms (including antiepileptic drugs, corticotropin, and steroids). Cost data, calculated as a ratio of cost to charges, were collected and adjusted to 2014 dollars.
A total of 6,183 patients were included in the analysis and their average age of infantile-spasm diagnosis was 9 months. The most common diagnostic test ordered was EEG (76%), followed by brain imaging (57%), organic acids (38%), and lumbar puncture (17%). Medications were started during inpatient hospitalization in two-thirds of patients, with 33% starting on corticotropin; 29% on topiramate; and fewer than 10% of patients on an oral or intravenous steroid, zonisamide, or vigabatrin (Sabril). Use of corticotropin decreased over time, while use of oral steroids trended upwards. “We were surprised that one-third of patients did not have a medication initiated as an inpatient, given the studies showing earlier use of effective therapy has better outcomes,” Dr. Misra said in an interview in advance of the meeting.
“The cost of taking care of children with infantile spasms has increased over the study period 2004-2014,” Dr. Misra said. “Although we identified a few contributors to rising cost, there are probably other factors that need to be considered in future studies.” She acknowledged certain limitations of the analysis, including its retrospective design and the fact that it only identified cost associated with the initial admission. “Several of the diagnostic studies and medications may be initiated as an outpatient, for which we do not have the data,” she said.
Dr. Misra reported having no financial disclosures.
AT AES 2016
Key clinical point:
Major finding: The most common diagnostic test ordered was EEG (76%), followed by brain imaging (57%), organic acids (38%), and lumbar puncture (17%).
Data source: Retrospective analysis of data on 6,183 patients with infantile spasms between 2004 and 2014.
Disclosures: Dr. Misra reported having no financial disclosures.
EMTALA – statutory law
This is the first of a two-part series.
Question: Which of the following statements regarding the Emergency Medical Treatment & Labor Act (EMTALA) is correct?
A. Deals with the standard of care in emergency medicine.
B. Provides safeguards for uninsured and nonpaying patients with an emergency medical condition.
C. Mandates uniform screening and treatment stabilization prior to transfer, irrespective of the hospital’s capability.
D. Is mostly directed at hospitals and emergency department staff doctors, but excludes on-call physicians.
E. Violations can result in fines, loss of Medicare provider participation, or even imprisonment.
Answer: B. In 1985, the CBS investigative news show “60 Minutes” ran an exposé on abuses in the emergency departments of U.S. hospitals, featuring the case of Eugene Barnes, a 32-year-old man brought to the Brookside Hospital emergency department (ED) in San Pablo, Calif., with a penetrating stab wound.
In another case, William Jenness, injured in an auto accident, died after a delayed transfer to a county hospital, because the original hospital required a $1,000 deposit in advance before initiating treatment.
In response to the widespread perception that uninsured patients were being denied treatment in the nation’s emergency departments, Congress enacted the Emergency Medical Treatment & Labor Act.1
Originally referred to as the “antidumping law,” EMTALA was designed to prevent hospitals from transferring financially undesirable patients to public hospitals without providing a medical screening examination and stabilizing treatment prior to transfer.
The purpose and intent of the law is to ensure that all patients who come to the ED have access to emergency services, although the statute itself is silent on payment ability.
EMTALA is not meant to replace or override state tort law, and does not deal with quality of care issues that may arise in the emergency department. Over the 30-year period since its enactment, EMTALA has received mixed reviews, with one scholar complaining that the statute is sloppily drafted and the premise of the statute, silly at best.2
EMTALA defines an emergency medical condition as:
1. A medical condition manifesting itself by acute symptoms of sufficient severity (including severe pain, psychiatric disturbances, and/or symptoms of substance abuse) such that the absence of immediate medical attention could reasonably be expected to result in placing the health of the individual (or, with respect to a pregnant woman, the health of the woman or her unborn child) in serious jeopardy, cause serious impairment to bodily functions, or result in serious dysfunction of any bodily organ or part.
2. With respect to a pregnant woman who is having contractions, there is inadequate time to effect a safe transfer to another hospital before delivery, or that transfer may pose a threat to the health or safety of the woman or the unborn child.3
Whether an emergency medical condition exists is determined by a medical screening exam (MSE). EMTALA is about a process directed at the well-being and safety of all patients with a medical emergency who come to the ED, defined as being licensed by the state or held out to the public as a place that provides care for emergency medical conditions. Hospital-based outpatient clinics that handle less than one-third of emergency visits and physician offices are exempt.
All patients who present to the ED seeking treatment are entitled to an MSE, and EDs are required to post such notification on their premises. A triage nurse may not be qualified to conduct the MSE unless he or she possesses special competencies, and has approval from the medical staff and the hospital’s governing body.
It is important that the MSE be documented soon after the patient’s arrival to determine if the medical condition warrants immediate treatment. It is definitely not acceptable to delay performing an MSE while awaiting information on insurance coverage, and one cannot “hold” the patient and delay stabilizing treatment because of the carrier’s insistence on using only certain approved facilities.
EMTALA requires that the screening exam be “appropriate,” but the statute does not define the term except to note that it is to be “within the capability of the hospital’s emergency department.” However, it is generally recognized that triage alone is insufficient, and the screening exam should be based on the patient’s symptoms and performed by a qualified person.
The important point is that it is uniformly applied, without discrimination, to all who seek treatment in the ED. The hospital itself is expected to have in place policies addressing the broad aspects of the screening process in a nondisparate manner.
The second key issue under the EMTALA statute concerns treatment and transfer.4 If an emergency medical condition exists, treatment must be provided until the emergency is resolved or stabilized.
Under the law, a patient is considered stable for transfer (or discharge) if the treating physician determines that no material deterioration is reasonably likely to occur during or as a result of the transfer between facilities. A receiving hospital is obligated to report any individual who has been transferred in an unstable condition in violation of EMTALA.
However, in the event the hospital does not have the capability to stabilize the emergency medical condition, EMTALA mandates an appropriate transfer, under prescribed conditions, to another hospital whose specialized capabilities obligate it to cooperate. The ED physician in the sending hospital will directly request acceptance of such a transfer. If the patient is unstable, the physician must certify that the medical benefits expected from the transfer outweigh the risks, unless the patient insists on a transfer in writing after being informed of the hospital’s obligations under EMTALA and the risks of transfer.
Furthermore, the transferring hospital must: 1. provide ongoing care within its capability until transfer to minimize transfer risks, 2. provide copies of medical records, 3. confirm that the receiving facility has space and qualified personnel to treat the condition and has agreed to accept the transfer, and 4. ensure that the transfer is made with qualified personnel and appropriate medical equipment.
On-call physicians at both transferring and accepting facilities are also subject to EMTALA. The U.S. Department of Health & Human Services’ Office of Inspector General (OIG) has promulgated rules regarding on-call physicians, even touching on reimbursement.
The American College of Emergency Physicians subscribes to the view that hospitals, medical staff, and payers share an ethical responsibility for the provision of emergency care, acknowledging that EDs require a reliable on-call system that provides for the availability of medical staff members for consultation and participation in the evaluation and treatment of emergency patients.5
Penalties for EMTALA violations include fines up to $50,000 per violation, and/or nullification of Medicare provider agreements. There is a 2-year statute of limitations for civil enforcement of any violation,6 carried out by the OIG and the Centers for Medicare & Medicaid Services (CMS).
A receiving facility, having suffered financial loss as a result of another hospital’s violation of EMTALA, can bring suit to recover damages, and patients may bring private lawsuits against hospitals, though not against physicians. EMTALA, being a civil rather than a criminal statute, does not impose any prison terms.
Investigations and citations by the OIG/CMS are common, with about half of all hospitals subjected to EMTALA investigations and a quarter receiving a violation citation over a recent 10-year period.
However, a recently published study covering 2002-2015 found that, despite 40% of investigations ending up with EMTALA violations, only 3% of investigations triggered fines – and none resulted in suspension of Medicare provider participation.7
There were a total of 192 settlements, or an average of 14 per year for the 4,000 hospitals in the United States. Most were for failing to provide screening (75%) and stabilization (42%). The vast majority of violations affected hospitals, and only eight physicians were involved.
Fines against hospitals and physicians totaled $6,357,000 (averages, $33,435 and $25,625, respectively). Patient dumping attributable to insurance or financial discrimination accounted for 15.6% of settlements.
References
1. 42 USC §1395dd et seq.
2. Chest. 2015 Jun;147(6):1691-6.
3. 42 USC §1395dd(a).
4. 42 USC §1395dd(b)(c).
5. “EMTALA and On-call Responsibility for Emergency Department Patients,” American College of Emergency Physicians.
6. 42 USC §1395dd(d).
7. West J Emerg Med. 2016 May;17(3):245-51.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
This is the first of a two-part series.
Question: Which of the following statements regarding the Emergency Medical Treatment & Labor Act (EMTALA) is correct?
A. Deals with the standard of care in emergency medicine.
B. Provides safeguards for uninsured and nonpaying patients with an emergency medical condition.
C. Mandates uniform screening and treatment stabilization prior to transfer, irrespective of the hospital’s capability.
D. Is mostly directed at hospitals and emergency department staff doctors, but excludes on-call physicians.
E. Violations can result in fines, loss of Medicare provider participation, or even imprisonment.
Answer: B. In 1985, the CBS investigative news show “60 Minutes” ran an exposé on abuses in the emergency departments of U.S. hospitals, featuring the case of Eugene Barnes, a 32-year-old man brought to the Brookside Hospital emergency department (ED) in San Pablo, Calif., with a penetrating stab wound.
In another case, William Jenness, injured in an auto accident, died after a delayed transfer to a county hospital, because the original hospital required a $1,000 deposit in advance before initiating treatment.
In response to the widespread perception that uninsured patients were being denied treatment in the nation’s emergency departments, Congress enacted the Emergency Medical Treatment & Labor Act.1
Originally referred to as the “antidumping law,” EMTALA was designed to prevent hospitals from transferring financially undesirable patients to public hospitals without providing a medical screening examination and stabilizing treatment prior to transfer.
The purpose and intent of the law is to ensure that all patients who come to the ED have access to emergency services, although the statute itself is silent on payment ability.
EMTALA is not meant to replace or override state tort law, and does not deal with quality of care issues that may arise in the emergency department. Over the 30-year period since its enactment, EMTALA has received mixed reviews, with one scholar complaining that the statute is sloppily drafted and the premise of the statute, silly at best.2
EMTALA defines an emergency medical condition as:
1. A medical condition manifesting itself by acute symptoms of sufficient severity (including severe pain, psychiatric disturbances, and/or symptoms of substance abuse) such that the absence of immediate medical attention could reasonably be expected to result in placing the health of the individual (or, with respect to a pregnant woman, the health of the woman or her unborn child) in serious jeopardy, cause serious impairment to bodily functions, or result in serious dysfunction of any bodily organ or part.
2. With respect to a pregnant woman who is having contractions, there is inadequate time to effect a safe transfer to another hospital before delivery, or that transfer may pose a threat to the health or safety of the woman or the unborn child.3
Whether an emergency medical condition exists is determined by a medical screening exam (MSE). EMTALA is about a process directed at the well-being and safety of all patients with a medical emergency who come to the ED, defined as being licensed by the state or held out to the public as a place that provides care for emergency medical conditions. Hospital-based outpatient clinics that handle less than one-third of emergency visits and physician offices are exempt.
All patients who present to the ED seeking treatment are entitled to an MSE, and EDs are required to post such notification on their premises. A triage nurse may not be qualified to conduct the MSE unless he or she possesses special competencies, and has approval from the medical staff and the hospital’s governing body.
It is important that the MSE be documented soon after the patient’s arrival to determine if the medical condition warrants immediate treatment. It is definitely not acceptable to delay performing an MSE while awaiting information on insurance coverage, and one cannot “hold” the patient and delay stabilizing treatment because of the carrier’s insistence on using only certain approved facilities.
EMTALA requires that the screening exam be “appropriate,” but the statute does not define the term except to note that it is to be “within the capability of the hospital’s emergency department.” However, it is generally recognized that triage alone is insufficient, and the screening exam should be based on the patient’s symptoms and performed by a qualified person.
The important point is that it is uniformly applied, without discrimination, to all who seek treatment in the ED. The hospital itself is expected to have in place policies addressing the broad aspects of the screening process in a nondisparate manner.
The second key issue under the EMTALA statute concerns treatment and transfer.4 If an emergency medical condition exists, treatment must be provided until the emergency is resolved or stabilized.
Under the law, a patient is considered stable for transfer (or discharge) if the treating physician determines that no material deterioration is reasonably likely to occur during or as a result of the transfer between facilities. A receiving hospital is obligated to report any individual who has been transferred in an unstable condition in violation of EMTALA.
However, in the event the hospital does not have the capability to stabilize the emergency medical condition, EMTALA mandates an appropriate transfer, under prescribed conditions, to another hospital whose specialized capabilities obligate it to cooperate. The ED physician in the sending hospital will directly request acceptance of such a transfer. If the patient is unstable, the physician must certify that the medical benefits expected from the transfer outweigh the risks, unless the patient insists on a transfer in writing after being informed of the hospital’s obligations under EMTALA and the risks of transfer.
Furthermore, the transferring hospital must: 1. provide ongoing care within its capability until transfer to minimize transfer risks, 2. provide copies of medical records, 3. confirm that the receiving facility has space and qualified personnel to treat the condition and has agreed to accept the transfer, and 4. ensure that the transfer is made with qualified personnel and appropriate medical equipment.
On-call physicians at both transferring and accepting facilities are also subject to EMTALA. The U.S. Department of Health & Human Services’ Office of Inspector General (OIG) has promulgated rules regarding on-call physicians, even touching on reimbursement.
The American College of Emergency Physicians subscribes to the view that hospitals, medical staff, and payers share an ethical responsibility for the provision of emergency care, acknowledging that EDs require a reliable on-call system that provides for the availability of medical staff members for consultation and participation in the evaluation and treatment of emergency patients.5
Penalties for EMTALA violations include fines up to $50,000 per violation, and/or nullification of Medicare provider agreements. There is a 2-year statute of limitations for civil enforcement of any violation,6 carried out by the OIG and the Centers for Medicare & Medicaid Services (CMS).
A receiving facility, having suffered financial loss as a result of another hospital’s violation of EMTALA, can bring suit to recover damages, and patients may bring private lawsuits against hospitals, though not against physicians. EMTALA, being a civil rather than a criminal statute, does not impose any prison terms.
Investigations and citations by the OIG/CMS are common, with about half of all hospitals subjected to EMTALA investigations and a quarter receiving a violation citation over a recent 10-year period.
However, a recently published study covering 2002-2015 found that, despite 40% of investigations ending up with EMTALA violations, only 3% of investigations triggered fines – and none resulted in suspension of Medicare provider participation.7
There were a total of 192 settlements, or an average of 14 per year for the 4,000 hospitals in the United States. Most were for failing to provide screening (75%) and stabilization (42%). The vast majority of violations affected hospitals, and only eight physicians were involved.
Fines against hospitals and physicians totaled $6,357,000 (averages, $33,435 and $25,625, respectively). Patient dumping attributable to insurance or financial discrimination accounted for 15.6% of settlements.
References
1. 42 USC §1395dd et seq.
2. Chest. 2015 Jun;147(6):1691-6.
3. 42 USC §1395dd(a).
4. 42 USC §1395dd(b)(c).
5. “EMTALA and On-call Responsibility for Emergency Department Patients,” American College of Emergency Physicians.
6. 42 USC §1395dd(d).
7. West J Emerg Med. 2016 May;17(3):245-51.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
This is the first of a two-part series.
Question: Which of the following statements regarding the Emergency Medical Treatment & Labor Act (EMTALA) is correct?
A. Deals with the standard of care in emergency medicine.
B. Provides safeguards for uninsured and nonpaying patients with an emergency medical condition.
C. Mandates uniform screening and treatment stabilization prior to transfer, irrespective of the hospital’s capability.
D. Is mostly directed at hospitals and emergency department staff doctors, but excludes on-call physicians.
E. Violations can result in fines, loss of Medicare provider participation, or even imprisonment.
Answer: B. In 1985, the CBS investigative news show “60 Minutes” ran an exposé on abuses in the emergency departments of U.S. hospitals, featuring the case of Eugene Barnes, a 32-year-old man brought to the Brookside Hospital emergency department (ED) in San Pablo, Calif., with a penetrating stab wound.
In another case, William Jenness, injured in an auto accident, died after a delayed transfer to a county hospital, because the original hospital required a $1,000 deposit in advance before initiating treatment.
In response to the widespread perception that uninsured patients were being denied treatment in the nation’s emergency departments, Congress enacted the Emergency Medical Treatment & Labor Act.1
Originally referred to as the “antidumping law,” EMTALA was designed to prevent hospitals from transferring financially undesirable patients to public hospitals without providing a medical screening examination and stabilizing treatment prior to transfer.
The purpose and intent of the law is to ensure that all patients who come to the ED have access to emergency services, although the statute itself is silent on payment ability.
EMTALA is not meant to replace or override state tort law, and does not deal with quality of care issues that may arise in the emergency department. Over the 30-year period since its enactment, EMTALA has received mixed reviews, with one scholar complaining that the statute is sloppily drafted and the premise of the statute, silly at best.2
EMTALA defines an emergency medical condition as:
1. A medical condition manifesting itself by acute symptoms of sufficient severity (including severe pain, psychiatric disturbances, and/or symptoms of substance abuse) such that the absence of immediate medical attention could reasonably be expected to result in placing the health of the individual (or, with respect to a pregnant woman, the health of the woman or her unborn child) in serious jeopardy, cause serious impairment to bodily functions, or result in serious dysfunction of any bodily organ or part.
2. With respect to a pregnant woman who is having contractions, there is inadequate time to effect a safe transfer to another hospital before delivery, or that transfer may pose a threat to the health or safety of the woman or the unborn child.3
Whether an emergency medical condition exists is determined by a medical screening exam (MSE). EMTALA is about a process directed at the well-being and safety of all patients with a medical emergency who come to the ED, defined as being licensed by the state or held out to the public as a place that provides care for emergency medical conditions. Hospital-based outpatient clinics that handle less than one-third of emergency visits and physician offices are exempt.
All patients who present to the ED seeking treatment are entitled to an MSE, and EDs are required to post such notification on their premises. A triage nurse may not be qualified to conduct the MSE unless he or she possesses special competencies, and has approval from the medical staff and the hospital’s governing body.
It is important that the MSE be documented soon after the patient’s arrival to determine if the medical condition warrants immediate treatment. It is definitely not acceptable to delay performing an MSE while awaiting information on insurance coverage, and one cannot “hold” the patient and delay stabilizing treatment because of the carrier’s insistence on using only certain approved facilities.
EMTALA requires that the screening exam be “appropriate,” but the statute does not define the term except to note that it is to be “within the capability of the hospital’s emergency department.” However, it is generally recognized that triage alone is insufficient, and the screening exam should be based on the patient’s symptoms and performed by a qualified person.
The important point is that it is uniformly applied, without discrimination, to all who seek treatment in the ED. The hospital itself is expected to have in place policies addressing the broad aspects of the screening process in a nondisparate manner.
The second key issue under the EMTALA statute concerns treatment and transfer.4 If an emergency medical condition exists, treatment must be provided until the emergency is resolved or stabilized.
Under the law, a patient is considered stable for transfer (or discharge) if the treating physician determines that no material deterioration is reasonably likely to occur during or as a result of the transfer between facilities. A receiving hospital is obligated to report any individual who has been transferred in an unstable condition in violation of EMTALA.
However, in the event the hospital does not have the capability to stabilize the emergency medical condition, EMTALA mandates an appropriate transfer, under prescribed conditions, to another hospital whose specialized capabilities obligate it to cooperate. The ED physician in the sending hospital will directly request acceptance of such a transfer. If the patient is unstable, the physician must certify that the medical benefits expected from the transfer outweigh the risks, unless the patient insists on a transfer in writing after being informed of the hospital’s obligations under EMTALA and the risks of transfer.
Furthermore, the transferring hospital must: 1. provide ongoing care within its capability until transfer to minimize transfer risks, 2. provide copies of medical records, 3. confirm that the receiving facility has space and qualified personnel to treat the condition and has agreed to accept the transfer, and 4. ensure that the transfer is made with qualified personnel and appropriate medical equipment.
On-call physicians at both transferring and accepting facilities are also subject to EMTALA. The U.S. Department of Health & Human Services’ Office of Inspector General (OIG) has promulgated rules regarding on-call physicians, even touching on reimbursement.
The American College of Emergency Physicians subscribes to the view that hospitals, medical staff, and payers share an ethical responsibility for the provision of emergency care, acknowledging that EDs require a reliable on-call system that provides for the availability of medical staff members for consultation and participation in the evaluation and treatment of emergency patients.5
Penalties for EMTALA violations include fines up to $50,000 per violation, and/or nullification of Medicare provider agreements. There is a 2-year statute of limitations for civil enforcement of any violation,6 carried out by the OIG and the Centers for Medicare & Medicaid Services (CMS).
A receiving facility, having suffered financial loss as a result of another hospital’s violation of EMTALA, can bring suit to recover damages, and patients may bring private lawsuits against hospitals, though not against physicians. EMTALA, being a civil rather than a criminal statute, does not impose any prison terms.
Investigations and citations by the OIG/CMS are common, with about half of all hospitals subjected to EMTALA investigations and a quarter receiving a violation citation over a recent 10-year period.
However, a recently published study covering 2002-2015 found that, despite 40% of investigations ending up with EMTALA violations, only 3% of investigations triggered fines – and none resulted in suspension of Medicare provider participation.7
There were a total of 192 settlements, or an average of 14 per year for the 4,000 hospitals in the United States. Most were for failing to provide screening (75%) and stabilization (42%). The vast majority of violations affected hospitals, and only eight physicians were involved.
Fines against hospitals and physicians totaled $6,357,000 (averages, $33,435 and $25,625, respectively). Patient dumping attributable to insurance or financial discrimination accounted for 15.6% of settlements.
References
1. 42 USC §1395dd et seq.
2. Chest. 2015 Jun;147(6):1691-6.
3. 42 USC §1395dd(a).
4. 42 USC §1395dd(b)(c).
5. “EMTALA and On-call Responsibility for Emergency Department Patients,” American College of Emergency Physicians.
6. 42 USC §1395dd(d).
7. West J Emerg Med. 2016 May;17(3):245-51.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
RSV is top cause of severe respiratory disease in preterm infants
Respiratory syncytial virus is the number one virus causing severe lower respiratory disease in preterm infants, while those of younger age and those exposed to young children are at greatest risk, Eric A. F. Simões, MD, of the University of Colorado at Denver, Aurora, and his coauthors reported in the Nov. 29 edition of PLOS ONE.
“These data demonstrate that higher risk for 32 to 35 wGA [weeks gestational age] infants can be easily identified by age or birth month and significant exposure to other young children,” they wrote. “These infants would benefit from targeted efforts to prevent severe RSV disease.”
The overall rates of lower respiratory infections per 100 infant-seasons – a season being 5 months of observation from November 1 to March 31 in 2009-2010 or 2010-2011 – were 13.7 for respiratory syncytial virus (RSV), 2.9 for adenovirus, 1.7 for parainfluenza virus type 2, 1.3 for human metapneumovirus, and 0.3 for parainfluenza virus type 2 (PLoS One. 2016 Nov 29. doi: 10.1371/journal.pone.0166226).
Infants who had been exposed to young children, either through attending day care or living with non–multiple birth preschool-age siblings, had a twofold higher risk of RSV and human metapneumovirus, and a 3.3-fold greater risk of adenovirus.
The youngest infants showed the highest rate of hospitalizations with RSV: the incidence ranged from 8.2 per 100 infant season in those aged less than 1 month to 2.3 per 100 infant seasons in those aged 10 months of age. Similarly, the incidence of admission to ICU was significantly higher among younger infants.
Infants born in May, before the RSV season, had a much lower incidence of hospitalization, compared with those born in the height of RSV season in February. ICU admission rates also were higher among those born in February, compared with those born in May.
The highest overall rates of hospitalization with RSV – 19 per 100 infant-seasons – were among those born in February, and also those who were exposed to other young children.
“The current results are unique in that they provide continuous age-based risk models for outpatient and inpatient disease for infants with and without young child exposure,” wrote Dr. Simões and his coauthors.
They argued that their findings refute earlier suggestions that the rate of RSV infection in preterm infants is similar to the rate in term infants, and suggested that the limitations of their study may have even underestimated the incidence in preterm babies.
The study was supported by AstraZeneca, parent company of MedImmune. Two authors declared grant support and research funding from AstraZeneca, one author was a former employee of AstraZeneca, one author was a former employee of MedImmune and now contractor to AstraZeneca. One author was a current employee of AstraZeneca and holds stock options. Two authors also declared funding and consultancies with AbbVie.
Respiratory syncytial virus is the number one virus causing severe lower respiratory disease in preterm infants, while those of younger age and those exposed to young children are at greatest risk, Eric A. F. Simões, MD, of the University of Colorado at Denver, Aurora, and his coauthors reported in the Nov. 29 edition of PLOS ONE.
“These data demonstrate that higher risk for 32 to 35 wGA [weeks gestational age] infants can be easily identified by age or birth month and significant exposure to other young children,” they wrote. “These infants would benefit from targeted efforts to prevent severe RSV disease.”
The overall rates of lower respiratory infections per 100 infant-seasons – a season being 5 months of observation from November 1 to March 31 in 2009-2010 or 2010-2011 – were 13.7 for respiratory syncytial virus (RSV), 2.9 for adenovirus, 1.7 for parainfluenza virus type 2, 1.3 for human metapneumovirus, and 0.3 for parainfluenza virus type 2 (PLoS One. 2016 Nov 29. doi: 10.1371/journal.pone.0166226).
Infants who had been exposed to young children, either through attending day care or living with non–multiple birth preschool-age siblings, had a twofold higher risk of RSV and human metapneumovirus, and a 3.3-fold greater risk of adenovirus.
The youngest infants showed the highest rate of hospitalizations with RSV: the incidence ranged from 8.2 per 100 infant season in those aged less than 1 month to 2.3 per 100 infant seasons in those aged 10 months of age. Similarly, the incidence of admission to ICU was significantly higher among younger infants.
Infants born in May, before the RSV season, had a much lower incidence of hospitalization, compared with those born in the height of RSV season in February. ICU admission rates also were higher among those born in February, compared with those born in May.
The highest overall rates of hospitalization with RSV – 19 per 100 infant-seasons – were among those born in February, and also those who were exposed to other young children.
“The current results are unique in that they provide continuous age-based risk models for outpatient and inpatient disease for infants with and without young child exposure,” wrote Dr. Simões and his coauthors.
They argued that their findings refute earlier suggestions that the rate of RSV infection in preterm infants is similar to the rate in term infants, and suggested that the limitations of their study may have even underestimated the incidence in preterm babies.
The study was supported by AstraZeneca, parent company of MedImmune. Two authors declared grant support and research funding from AstraZeneca, one author was a former employee of AstraZeneca, one author was a former employee of MedImmune and now contractor to AstraZeneca. One author was a current employee of AstraZeneca and holds stock options. Two authors also declared funding and consultancies with AbbVie.
Respiratory syncytial virus is the number one virus causing severe lower respiratory disease in preterm infants, while those of younger age and those exposed to young children are at greatest risk, Eric A. F. Simões, MD, of the University of Colorado at Denver, Aurora, and his coauthors reported in the Nov. 29 edition of PLOS ONE.
“These data demonstrate that higher risk for 32 to 35 wGA [weeks gestational age] infants can be easily identified by age or birth month and significant exposure to other young children,” they wrote. “These infants would benefit from targeted efforts to prevent severe RSV disease.”
The overall rates of lower respiratory infections per 100 infant-seasons – a season being 5 months of observation from November 1 to March 31 in 2009-2010 or 2010-2011 – were 13.7 for respiratory syncytial virus (RSV), 2.9 for adenovirus, 1.7 for parainfluenza virus type 2, 1.3 for human metapneumovirus, and 0.3 for parainfluenza virus type 2 (PLoS One. 2016 Nov 29. doi: 10.1371/journal.pone.0166226).
Infants who had been exposed to young children, either through attending day care or living with non–multiple birth preschool-age siblings, had a twofold higher risk of RSV and human metapneumovirus, and a 3.3-fold greater risk of adenovirus.
The youngest infants showed the highest rate of hospitalizations with RSV: the incidence ranged from 8.2 per 100 infant season in those aged less than 1 month to 2.3 per 100 infant seasons in those aged 10 months of age. Similarly, the incidence of admission to ICU was significantly higher among younger infants.
Infants born in May, before the RSV season, had a much lower incidence of hospitalization, compared with those born in the height of RSV season in February. ICU admission rates also were higher among those born in February, compared with those born in May.
The highest overall rates of hospitalization with RSV – 19 per 100 infant-seasons – were among those born in February, and also those who were exposed to other young children.
“The current results are unique in that they provide continuous age-based risk models for outpatient and inpatient disease for infants with and without young child exposure,” wrote Dr. Simões and his coauthors.
They argued that their findings refute earlier suggestions that the rate of RSV infection in preterm infants is similar to the rate in term infants, and suggested that the limitations of their study may have even underestimated the incidence in preterm babies.
The study was supported by AstraZeneca, parent company of MedImmune. Two authors declared grant support and research funding from AstraZeneca, one author was a former employee of AstraZeneca, one author was a former employee of MedImmune and now contractor to AstraZeneca. One author was a current employee of AstraZeneca and holds stock options. Two authors also declared funding and consultancies with AbbVie.
FROM PLOS ONE
Key clinical point: Respiratory syncytial virus is the leading viral cause of severe lower respiratory disease in preterm infants, with those of younger age and those exposed to young children at greatest risk.
Major finding: The rates of lower respiratory infections per 100 infant-seasons were 13.7 for RSV, 2.9 for adenovirus, 1.7 for parainfluenza virus type 2, and 1.3 for human metapneumovirus.
Data source: The prospective RSV Respiratory Events Among Preterm Infants Outcomes and Risk Tracking (REPORT) study, in 1,642 preterm infants with medically attended acute respiratory illness.
Disclosures: The study was supported by AstraZeneca, parent company of MedImmune. Two authors declared grant support and research funding from AstraZeneca, one author was a former employee of AstraZeneca, and one author was a former employee of MedImmune and now contractor to AstraZeneca. One author was a current employee of AstraZeneca and holds stock options. Two authors also declared funding and consultancies with AbbVie.
Empagliflozin first antidiabetes drug to gain cardioprotective indication
Empagliflozin is the first antidiabetes medication to be approved for reducing the risk of cardiovascular death in patients with type 2 diabetes and concomitant cardiovascular disease.
The Food and Drug Administration granted the new indication based on the EMPA-REG OUTCOME study, a postmarketing analysis that found that empagliflozin (Jardiance, Boehringer-Ingelheim) reduced the risk of cardiovascular death by 38% when added to standard-of-care type 2 diabetes therapy.
When empagliflozin was approved for type 2 diabetes in 2014, the FDA required an additional postmarketing study to examine its cardiovascular safety. The 48-month, open-label EMPA-REG enrolled more than 7,000 patients who had type 2 diabetes and a high risk of cardiovascular disease.
The study’s big surprise, however, was not empagliflozin’s safety, but its striking cardioprotective qualities. It reduced by 14% the risk of the primary endpoint, a composite of cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction (N Engl J Med. 2015;373:2117-28).
When examined as individual outcomes in a secondary analysis, empagliflozin significantly reduced the risk of cardiovascular death by 38%. However, risk reductions on the other endpoints were not significant. Nevertheless, experts called empagliflozin’s cardiovascular benefit a potential game-changer for the clinical challenge of managing patients with both disorders.
But the drug barely squeaked by its June FDA approval hearing for the cardioprotective indication, receiving a split 12-11 endorsement from the Endocrinologic and Metabolic Drugs Advisory Committee. The major sticking point was that EMPA-REG was a test of empagliflozin’s cardiovascular safety, not its efficacy, and that cardiovascular death was not a prespecified endpoint.
Although there were no significant cardiovascular safety issues, empagliflozin has been associated with hypotension, serious urinary tract infection, acute kidney injury, and genital infections.
“Cardiovascular disease is a leading cause of death in adults with type 2 diabetes mellitus,” Jean-Marc Guettier, MD, director of the Division of Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research, wrote in a press statement. “Availability of antidiabetes therapies that can help people live longer by reducing the risk of cardiovascular death is an important advance for adults with type 2 diabetes.”
[email protected]
On Twitter @alz_gal
Empagliflozin is the first antidiabetes medication to be approved for reducing the risk of cardiovascular death in patients with type 2 diabetes and concomitant cardiovascular disease.
The Food and Drug Administration granted the new indication based on the EMPA-REG OUTCOME study, a postmarketing analysis that found that empagliflozin (Jardiance, Boehringer-Ingelheim) reduced the risk of cardiovascular death by 38% when added to standard-of-care type 2 diabetes therapy.
When empagliflozin was approved for type 2 diabetes in 2014, the FDA required an additional postmarketing study to examine its cardiovascular safety. The 48-month, open-label EMPA-REG enrolled more than 7,000 patients who had type 2 diabetes and a high risk of cardiovascular disease.
The study’s big surprise, however, was not empagliflozin’s safety, but its striking cardioprotective qualities. It reduced by 14% the risk of the primary endpoint, a composite of cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction (N Engl J Med. 2015;373:2117-28).
When examined as individual outcomes in a secondary analysis, empagliflozin significantly reduced the risk of cardiovascular death by 38%. However, risk reductions on the other endpoints were not significant. Nevertheless, experts called empagliflozin’s cardiovascular benefit a potential game-changer for the clinical challenge of managing patients with both disorders.
But the drug barely squeaked by its June FDA approval hearing for the cardioprotective indication, receiving a split 12-11 endorsement from the Endocrinologic and Metabolic Drugs Advisory Committee. The major sticking point was that EMPA-REG was a test of empagliflozin’s cardiovascular safety, not its efficacy, and that cardiovascular death was not a prespecified endpoint.
Although there were no significant cardiovascular safety issues, empagliflozin has been associated with hypotension, serious urinary tract infection, acute kidney injury, and genital infections.
“Cardiovascular disease is a leading cause of death in adults with type 2 diabetes mellitus,” Jean-Marc Guettier, MD, director of the Division of Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research, wrote in a press statement. “Availability of antidiabetes therapies that can help people live longer by reducing the risk of cardiovascular death is an important advance for adults with type 2 diabetes.”
[email protected]
On Twitter @alz_gal
Empagliflozin is the first antidiabetes medication to be approved for reducing the risk of cardiovascular death in patients with type 2 diabetes and concomitant cardiovascular disease.
The Food and Drug Administration granted the new indication based on the EMPA-REG OUTCOME study, a postmarketing analysis that found that empagliflozin (Jardiance, Boehringer-Ingelheim) reduced the risk of cardiovascular death by 38% when added to standard-of-care type 2 diabetes therapy.
When empagliflozin was approved for type 2 diabetes in 2014, the FDA required an additional postmarketing study to examine its cardiovascular safety. The 48-month, open-label EMPA-REG enrolled more than 7,000 patients who had type 2 diabetes and a high risk of cardiovascular disease.
The study’s big surprise, however, was not empagliflozin’s safety, but its striking cardioprotective qualities. It reduced by 14% the risk of the primary endpoint, a composite of cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction (N Engl J Med. 2015;373:2117-28).
When examined as individual outcomes in a secondary analysis, empagliflozin significantly reduced the risk of cardiovascular death by 38%. However, risk reductions on the other endpoints were not significant. Nevertheless, experts called empagliflozin’s cardiovascular benefit a potential game-changer for the clinical challenge of managing patients with both disorders.
But the drug barely squeaked by its June FDA approval hearing for the cardioprotective indication, receiving a split 12-11 endorsement from the Endocrinologic and Metabolic Drugs Advisory Committee. The major sticking point was that EMPA-REG was a test of empagliflozin’s cardiovascular safety, not its efficacy, and that cardiovascular death was not a prespecified endpoint.
Although there were no significant cardiovascular safety issues, empagliflozin has been associated with hypotension, serious urinary tract infection, acute kidney injury, and genital infections.
“Cardiovascular disease is a leading cause of death in adults with type 2 diabetes mellitus,” Jean-Marc Guettier, MD, director of the Division of Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research, wrote in a press statement. “Availability of antidiabetes therapies that can help people live longer by reducing the risk of cardiovascular death is an important advance for adults with type 2 diabetes.”
[email protected]
On Twitter @alz_gal