User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


COVID-19 shutdown fuels sharp rise in alcohol use
Americans sharply increased their alcohol intake last spring as many areas of the country shutdown because of the coronavirus pandemic, results of a national survey show.
The overall frequency of alcohol consumption increased by 14% among adults over age 30 in the spring of 2020 versus the same period a year earlier.
The increase was most evident in adults aged 30-59, women, and non-Hispanic Whites.
“Alcohol consumption can have significant negative health consequences, so this information suggests another way that the pandemic may be affecting the physical and mental health of Americans,” Michael Pollard, PhD, lead investigator and sociologist at Rand, said in a news release.
The results were published online as a research letter Sept. 29 in JAMA Network Open.
Booming business
After some U.S. states issued stay-at-home orders to fight the spread of SARS-CoV-2, one study noted a 54% increase in national sales of alcohol for the week ending March 21, 2020, relative to 1 year earlier and a 262% increase in online alcohol sales.
“We’ve had anecdotal information about people buying and consuming more alcohol,” Dr. Pollard said, but the Rand study provides the first survey-based information that shows how much alcohol consumption has increased during the pandemic.
The findings are based on 1,540 adults (mean age, 56.6 years; 57% women) from the Rand American Life Panel, a nationally representative sample of Americans who were surveyed about their alcohol consumption before the pandemic in the spring of 2019, and again in the spring of 2020 during the early months of the shutdown.
Overall, in spring 2020, respondents reported drinking alcohol 6.22 days in the prior month on average, a 14% increase from the monthly average of 5.48 days reported in spring 2019.
Among adults aged 30 to 59 years, the frequency of alcohol consumption increased from 4.98 days prepandemic to 5.91 days during the pandemic, a 19% increase.
Women reported drinking an average of 5.36 days in the prior month in the early pandemic period, a 17% increase from 4.58 monthly drinking days before the pandemic.
In addition, compared with spring 2019, in spring 2020 women reported a 41% increase in heavy drinking days – four or more drinks in a couple of hours.
Independent of consumption level, nearly 1 in 10 women had an increase in alcohol-related problems in the pandemic period, based on responses to the Short Inventory of Problems scale.
For non-Hispanic White individuals, the overall frequency of alcohol intake rose 10% during the early pandemic period.
“The population level changes for women, younger, and non-Hispanic White individuals highlight that health systems may need to educate consumers through print or online media about increased alcohol use during the pandemic and identify factors associated with susceptibility and resilience to the impacts of COVID-19,” write Dr. Pollard and colleagues.
The authors note , and whether psychological and physical well-being are subsequently affected.
The study was supported by the National Institute of Alcohol Abuse and Alcoholism. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Americans sharply increased their alcohol intake last spring as many areas of the country shutdown because of the coronavirus pandemic, results of a national survey show.
The overall frequency of alcohol consumption increased by 14% among adults over age 30 in the spring of 2020 versus the same period a year earlier.
The increase was most evident in adults aged 30-59, women, and non-Hispanic Whites.
“Alcohol consumption can have significant negative health consequences, so this information suggests another way that the pandemic may be affecting the physical and mental health of Americans,” Michael Pollard, PhD, lead investigator and sociologist at Rand, said in a news release.
The results were published online as a research letter Sept. 29 in JAMA Network Open.
Booming business
After some U.S. states issued stay-at-home orders to fight the spread of SARS-CoV-2, one study noted a 54% increase in national sales of alcohol for the week ending March 21, 2020, relative to 1 year earlier and a 262% increase in online alcohol sales.
“We’ve had anecdotal information about people buying and consuming more alcohol,” Dr. Pollard said, but the Rand study provides the first survey-based information that shows how much alcohol consumption has increased during the pandemic.
The findings are based on 1,540 adults (mean age, 56.6 years; 57% women) from the Rand American Life Panel, a nationally representative sample of Americans who were surveyed about their alcohol consumption before the pandemic in the spring of 2019, and again in the spring of 2020 during the early months of the shutdown.
Overall, in spring 2020, respondents reported drinking alcohol 6.22 days in the prior month on average, a 14% increase from the monthly average of 5.48 days reported in spring 2019.
Among adults aged 30 to 59 years, the frequency of alcohol consumption increased from 4.98 days prepandemic to 5.91 days during the pandemic, a 19% increase.
Women reported drinking an average of 5.36 days in the prior month in the early pandemic period, a 17% increase from 4.58 monthly drinking days before the pandemic.
In addition, compared with spring 2019, in spring 2020 women reported a 41% increase in heavy drinking days – four or more drinks in a couple of hours.
Independent of consumption level, nearly 1 in 10 women had an increase in alcohol-related problems in the pandemic period, based on responses to the Short Inventory of Problems scale.
For non-Hispanic White individuals, the overall frequency of alcohol intake rose 10% during the early pandemic period.
“The population level changes for women, younger, and non-Hispanic White individuals highlight that health systems may need to educate consumers through print or online media about increased alcohol use during the pandemic and identify factors associated with susceptibility and resilience to the impacts of COVID-19,” write Dr. Pollard and colleagues.
The authors note , and whether psychological and physical well-being are subsequently affected.
The study was supported by the National Institute of Alcohol Abuse and Alcoholism. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Americans sharply increased their alcohol intake last spring as many areas of the country shutdown because of the coronavirus pandemic, results of a national survey show.
The overall frequency of alcohol consumption increased by 14% among adults over age 30 in the spring of 2020 versus the same period a year earlier.
The increase was most evident in adults aged 30-59, women, and non-Hispanic Whites.
“Alcohol consumption can have significant negative health consequences, so this information suggests another way that the pandemic may be affecting the physical and mental health of Americans,” Michael Pollard, PhD, lead investigator and sociologist at Rand, said in a news release.
The results were published online as a research letter Sept. 29 in JAMA Network Open.
Booming business
After some U.S. states issued stay-at-home orders to fight the spread of SARS-CoV-2, one study noted a 54% increase in national sales of alcohol for the week ending March 21, 2020, relative to 1 year earlier and a 262% increase in online alcohol sales.
“We’ve had anecdotal information about people buying and consuming more alcohol,” Dr. Pollard said, but the Rand study provides the first survey-based information that shows how much alcohol consumption has increased during the pandemic.
The findings are based on 1,540 adults (mean age, 56.6 years; 57% women) from the Rand American Life Panel, a nationally representative sample of Americans who were surveyed about their alcohol consumption before the pandemic in the spring of 2019, and again in the spring of 2020 during the early months of the shutdown.
Overall, in spring 2020, respondents reported drinking alcohol 6.22 days in the prior month on average, a 14% increase from the monthly average of 5.48 days reported in spring 2019.
Among adults aged 30 to 59 years, the frequency of alcohol consumption increased from 4.98 days prepandemic to 5.91 days during the pandemic, a 19% increase.
Women reported drinking an average of 5.36 days in the prior month in the early pandemic period, a 17% increase from 4.58 monthly drinking days before the pandemic.
In addition, compared with spring 2019, in spring 2020 women reported a 41% increase in heavy drinking days – four or more drinks in a couple of hours.
Independent of consumption level, nearly 1 in 10 women had an increase in alcohol-related problems in the pandemic period, based on responses to the Short Inventory of Problems scale.
For non-Hispanic White individuals, the overall frequency of alcohol intake rose 10% during the early pandemic period.
“The population level changes for women, younger, and non-Hispanic White individuals highlight that health systems may need to educate consumers through print or online media about increased alcohol use during the pandemic and identify factors associated with susceptibility and resilience to the impacts of COVID-19,” write Dr. Pollard and colleagues.
The authors note , and whether psychological and physical well-being are subsequently affected.
The study was supported by the National Institute of Alcohol Abuse and Alcoholism. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Children’s share of new COVID-19 cases is on the rise
The cumulative percentage of COVID-19 cases reported in children continues to climb, but “the history behind that cumulative number shows substantial change,” according to a new analysis of state health department data.
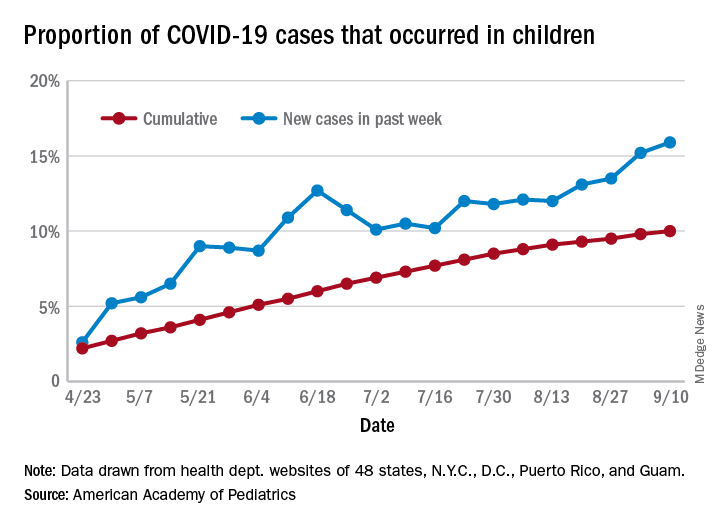
As of Sept. 10, the 549,432 cases in children represented 10.0% of all reported COVID-19 cases in the United States following a substantial rise over the course of the pandemic – the figure was 7.7% on July 16 and 3.2% on May 7, Blake Sisk, PhD, of the American Academy of Pediatrics and associates reported Sept. 29 in Pediatrics.
Unlike the cumulative number, the weekly proportion of cases in children fell early in the summer but then started climbing again in late July. Dr. Sisk and associates wrote.
Despite the increase, however, the proportion of pediatric COVID-19 cases is still well below children’s share of the overall population (22.6%). Also, “it is unclear how much of the increase in child cases is due to increased testing capacity, although CDC data from public and commercial laboratories show the share of all tests administered to children ages 0-17 has remained stable at 5%-7% since late April,” they said.
Data for the current report were drawn from 49 state health department websites (New York state does not report ages for COVID-19 cases), along with New York City, the District of Columbia, Puerto Rico, and Guam. Alabama changed its definition of a child case in August and was not included in the trend analysis (see graph), the investigators explained.
Those data show “substantial variation in case growth by region: in April, a preponderance of cases was in the Northeast. In June, cases surged in the South and West, followed by mid-July increases in the Midwest,” Dr. Sisk and associates said.
The increase among children in Midwest states is ongoing with the number of new cases reaching its highest level yet during the week ending Sept. 10, they reported.
SOURCE: Sisk B et al. Pediatrics. 2020 Sep 29. doi: 10.1542/peds.2020-027425.
The cumulative percentage of COVID-19 cases reported in children continues to climb, but “the history behind that cumulative number shows substantial change,” according to a new analysis of state health department data.

As of Sept. 10, the 549,432 cases in children represented 10.0% of all reported COVID-19 cases in the United States following a substantial rise over the course of the pandemic – the figure was 7.7% on July 16 and 3.2% on May 7, Blake Sisk, PhD, of the American Academy of Pediatrics and associates reported Sept. 29 in Pediatrics.
Unlike the cumulative number, the weekly proportion of cases in children fell early in the summer but then started climbing again in late July. Dr. Sisk and associates wrote.
Despite the increase, however, the proportion of pediatric COVID-19 cases is still well below children’s share of the overall population (22.6%). Also, “it is unclear how much of the increase in child cases is due to increased testing capacity, although CDC data from public and commercial laboratories show the share of all tests administered to children ages 0-17 has remained stable at 5%-7% since late April,” they said.
Data for the current report were drawn from 49 state health department websites (New York state does not report ages for COVID-19 cases), along with New York City, the District of Columbia, Puerto Rico, and Guam. Alabama changed its definition of a child case in August and was not included in the trend analysis (see graph), the investigators explained.
Those data show “substantial variation in case growth by region: in April, a preponderance of cases was in the Northeast. In June, cases surged in the South and West, followed by mid-July increases in the Midwest,” Dr. Sisk and associates said.
The increase among children in Midwest states is ongoing with the number of new cases reaching its highest level yet during the week ending Sept. 10, they reported.
SOURCE: Sisk B et al. Pediatrics. 2020 Sep 29. doi: 10.1542/peds.2020-027425.
The cumulative percentage of COVID-19 cases reported in children continues to climb, but “the history behind that cumulative number shows substantial change,” according to a new analysis of state health department data.

As of Sept. 10, the 549,432 cases in children represented 10.0% of all reported COVID-19 cases in the United States following a substantial rise over the course of the pandemic – the figure was 7.7% on July 16 and 3.2% on May 7, Blake Sisk, PhD, of the American Academy of Pediatrics and associates reported Sept. 29 in Pediatrics.
Unlike the cumulative number, the weekly proportion of cases in children fell early in the summer but then started climbing again in late July. Dr. Sisk and associates wrote.
Despite the increase, however, the proportion of pediatric COVID-19 cases is still well below children’s share of the overall population (22.6%). Also, “it is unclear how much of the increase in child cases is due to increased testing capacity, although CDC data from public and commercial laboratories show the share of all tests administered to children ages 0-17 has remained stable at 5%-7% since late April,” they said.
Data for the current report were drawn from 49 state health department websites (New York state does not report ages for COVID-19 cases), along with New York City, the District of Columbia, Puerto Rico, and Guam. Alabama changed its definition of a child case in August and was not included in the trend analysis (see graph), the investigators explained.
Those data show “substantial variation in case growth by region: in April, a preponderance of cases was in the Northeast. In June, cases surged in the South and West, followed by mid-July increases in the Midwest,” Dr. Sisk and associates said.
The increase among children in Midwest states is ongoing with the number of new cases reaching its highest level yet during the week ending Sept. 10, they reported.
SOURCE: Sisk B et al. Pediatrics. 2020 Sep 29. doi: 10.1542/peds.2020-027425.
FROM PEDIATRICS
Pandemic poses new challenges for rural doctors
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
HM20 Virtual: Combating racism in medicine
HM20 Virtual session title
When Grief and Crises Intersect: Perspectives of a Black Physician in the Time of Two Pandemics
Presenter
Kimberly Manning, MD, FACP, FAAP
Session summary
Dr. Kimberly Manning, associate vice chair of diversity, equity, and inclusion at Emory University, Atlanta, masterfully discussed the dual pandemics of COVID-19 and racism that we are currently experiencing and tried to describe the unique perspective of Black Americans.
Though it is easy to see that COVID-19 is a pandemic, racism is not always seen in this way. Dr. Manning demonstrated that when a pandemic is defined as “that which occurs over a wide geographic area and affects a high proportion of the population,” racism is absolutely a pandemic. She gave a great analogy: when sticking your hand into a bowl of Lucky Charms cereal, you do not expect to always end up with marshmallows alone, yet repeatedly, we see that Black Americans have been disproportionately affected by COVID-19. We often hear that we are in unprecedented times but as far as racism is concerned, there is nothing new about this.
Dr. Manning discussed the life stories of her grandfather, her father, and even her own life’s milestones such as starting college, getting into medical school, finishing residency – all the way to becoming a full professor. She described how each of these instances, though marked by something beautiful, was also marked by something truly awful. Each time she had a reason to smile and laugh, there was something awful happening in the country simultaneously that showed us how racism was still present. Though this was one person’s story, all Black Americans, not just those working in health care, can recount similar stories, emotions, and feelings of grief.
Dr. Manning concluded by telling us how we can “Do the Work” to combat the pandemic of racism:
- Broaden your fund of knowledge: Read books, listen to podcasts, watch documentaries.
- Remember that people are grieving.
- Explore your implicit biases.
- Be a brave bystander.
- Avoid performative allyship.
Key takeaways
- Though the COVID-19 pandemic is unprecedented, the pandemic of racism is not.
- The story of COVID-19 is the story of social determinants of health.
- We all must “Do the Work” to combat everyday racism and be cognizant of what our Black colleagues are going through every day.
Dr. Doraiswamy is an assistant professor of medicine and pediatrics and a med-peds hospitalist at The Ohio State University and Nationwide Children’s Hospital, Columbus.
HM20 Virtual session title
When Grief and Crises Intersect: Perspectives of a Black Physician in the Time of Two Pandemics
Presenter
Kimberly Manning, MD, FACP, FAAP
Session summary
Dr. Kimberly Manning, associate vice chair of diversity, equity, and inclusion at Emory University, Atlanta, masterfully discussed the dual pandemics of COVID-19 and racism that we are currently experiencing and tried to describe the unique perspective of Black Americans.
Though it is easy to see that COVID-19 is a pandemic, racism is not always seen in this way. Dr. Manning demonstrated that when a pandemic is defined as “that which occurs over a wide geographic area and affects a high proportion of the population,” racism is absolutely a pandemic. She gave a great analogy: when sticking your hand into a bowl of Lucky Charms cereal, you do not expect to always end up with marshmallows alone, yet repeatedly, we see that Black Americans have been disproportionately affected by COVID-19. We often hear that we are in unprecedented times but as far as racism is concerned, there is nothing new about this.
Dr. Manning discussed the life stories of her grandfather, her father, and even her own life’s milestones such as starting college, getting into medical school, finishing residency – all the way to becoming a full professor. She described how each of these instances, though marked by something beautiful, was also marked by something truly awful. Each time she had a reason to smile and laugh, there was something awful happening in the country simultaneously that showed us how racism was still present. Though this was one person’s story, all Black Americans, not just those working in health care, can recount similar stories, emotions, and feelings of grief.
Dr. Manning concluded by telling us how we can “Do the Work” to combat the pandemic of racism:
- Broaden your fund of knowledge: Read books, listen to podcasts, watch documentaries.
- Remember that people are grieving.
- Explore your implicit biases.
- Be a brave bystander.
- Avoid performative allyship.
Key takeaways
- Though the COVID-19 pandemic is unprecedented, the pandemic of racism is not.
- The story of COVID-19 is the story of social determinants of health.
- We all must “Do the Work” to combat everyday racism and be cognizant of what our Black colleagues are going through every day.
Dr. Doraiswamy is an assistant professor of medicine and pediatrics and a med-peds hospitalist at The Ohio State University and Nationwide Children’s Hospital, Columbus.
HM20 Virtual session title
When Grief and Crises Intersect: Perspectives of a Black Physician in the Time of Two Pandemics
Presenter
Kimberly Manning, MD, FACP, FAAP
Session summary
Dr. Kimberly Manning, associate vice chair of diversity, equity, and inclusion at Emory University, Atlanta, masterfully discussed the dual pandemics of COVID-19 and racism that we are currently experiencing and tried to describe the unique perspective of Black Americans.
Though it is easy to see that COVID-19 is a pandemic, racism is not always seen in this way. Dr. Manning demonstrated that when a pandemic is defined as “that which occurs over a wide geographic area and affects a high proportion of the population,” racism is absolutely a pandemic. She gave a great analogy: when sticking your hand into a bowl of Lucky Charms cereal, you do not expect to always end up with marshmallows alone, yet repeatedly, we see that Black Americans have been disproportionately affected by COVID-19. We often hear that we are in unprecedented times but as far as racism is concerned, there is nothing new about this.
Dr. Manning discussed the life stories of her grandfather, her father, and even her own life’s milestones such as starting college, getting into medical school, finishing residency – all the way to becoming a full professor. She described how each of these instances, though marked by something beautiful, was also marked by something truly awful. Each time she had a reason to smile and laugh, there was something awful happening in the country simultaneously that showed us how racism was still present. Though this was one person’s story, all Black Americans, not just those working in health care, can recount similar stories, emotions, and feelings of grief.
Dr. Manning concluded by telling us how we can “Do the Work” to combat the pandemic of racism:
- Broaden your fund of knowledge: Read books, listen to podcasts, watch documentaries.
- Remember that people are grieving.
- Explore your implicit biases.
- Be a brave bystander.
- Avoid performative allyship.
Key takeaways
- Though the COVID-19 pandemic is unprecedented, the pandemic of racism is not.
- The story of COVID-19 is the story of social determinants of health.
- We all must “Do the Work” to combat everyday racism and be cognizant of what our Black colleagues are going through every day.
Dr. Doraiswamy is an assistant professor of medicine and pediatrics and a med-peds hospitalist at The Ohio State University and Nationwide Children’s Hospital, Columbus.
DAPA-CKD resets eGFR floor for safe SGLT2 inhibitor use
The dramatically positive safety and efficacy results from the DAPA-CKD trial, which showed that treatment with the sodium-glucose transporter 2 (SGLT2) inhibitor dapagliflozin significantly cut both chronic kidney disease progression and all-cause death in patients with or without type 2 diabetes, were also notable for broadening the population of patients eligible for this treatment to those in the upper range of stage 4 CKD.
Of the 4,304 CKD patients enrolled in DAPA-CKD, 624 (14%) had an estimated glomerular filtration rate (eGFR) of 25-29 mL/min per 1.73m2, an unprecedented population to receive a drug from the SGLT2 inhibitor class in a reported study. The results provided definitive evidence for efficacy and safety in this range of renal function, said Hiddo J.L. Heerspink, Ph.D., at the virtual annual meeting of the European Association for the Study of Diabetes.
Until now, the widely accepted lowest level for starting an SGLT2 inhibitor in routine practice has been an eGFR as low as 30 mL/min per 1.73 m2.
Using SGLT2 inhibitors when eGFR is as low as 25
“It’s time to reduce the eGFR level for initiating an SGLT2 inhibitor to as low as 25,” said Dr. Heerspink, a professor of clinical pharmacology at the University of Groningen (the Netherlands).
While conceding that this is primarily a decision to be made by guideline writers and regulatory bodies, he declared what he believed was established by the DAPA-CKD findings: “We’ve shown that dapagliflozin can be safely used in these patients. It is effective across the spectrum of kidney function.”
Other experts not associated with the study agreed.
The trial researchers were “brave” to enroll patients with eGFRs as low as 25 mL/min per 1.73 m2, and “we urgently need these agents in patients with an eGFR this low,” commented Chantal Mathieu, MD, an endocrinologist and professor of medicine at Catholic University in Leuven, Belgium, and designated discussant for the report. Overall, she called the findings “spectacular,” a “landmark trial,” and a “winner.”
The study also set an new, lower floor for the level of albuminuria that can be usefully treated with dapagliflozin (Farxiga) by enrolling patients with a urinary albumin-to-creatinine ratio as low as 200 mg/g; the previous lower limit had been 300 mg/g, noted Dr. Mathieu. The new findings pose challenges to guideline writers, regulators who approve drug labels, and payers to a quickly make changes that will bring dapagliflozin to a wider number of patients with CKD.
Once the full DAPA-CKD results are reported, “it will change practice, and push the eGFR needle down” to as low as 25. It will also lower the albuminuria threshold for using dapagliflozin or other drugs in the class, commented David Z.I. Cherney, MD, a nephrologist at the University of Toronto. “It’s just one study,” he admitted, but the consistent renal benefits seen across several studies involving all four drugs in the SGLT2 inhibitor class will help hasten this change in identifying treatable patients, as well as expand the drug class to patients with CKD but no type 2 diabetes (T2D).
“I don’t think we’ve ever had stronger evidence” for drugs that can benefit both heart and renal function, plus the drug class is “very safe, and really easy to start” and maintain in patients, Dr. Cherney said in an interview. “It’s wonderful for these patients that we now have something new for treatment,” a drug with a “very favorable benefit-to-risk ratio.”
Results show many dapagliflozin benefits
While this broadening of the range of patients proven to tolerate and benefit from an SGLT2 inhibitor was an important consequence of DAPA-CKD, the study’s primary finding – that dapagliflozin was as safe and effective for slowing CKD progression in patients regardless of whether they also had T2D – will have an even bigger impact on expanding the target patient population. Showing efficacy in patients with CKD but without a T2D etiology, the status of about a third of the enrolled 4,304 patients, makes this treatment an option for “millions” of additional patients worldwide, said Dr. Heerspink. “These are the most common patients nephrologists see.” A major challenge now will be to do a better job finding patients with CKD who could benefit from dapagliflozin.
DAPA-CKD enrolled CKD patients based primarily on prespecified albuminuria and eGFR levels at more than 300 centers in 34 countries, including the United States. Virtually all patients, 97%, were on the only treatment now available with proven efficacy for slowing CKD, either an ACE inhibitor or an angiotensin receptor blocker. The small number of patients not on one of these drugs was because of poor tolerance.
The study’s primary endpoint was the combined rate of cardiovascular death, renal death, end-stage renal disease, or a drop in eGFR of at least 50% from baseline. This occurred in 14.5% of patients who received placebo and in 9.2% of those who received dapagliflozin during a median follow-up of 2.4 years, a highly significant 39% relative risk reduction. Concurrently with the report at the virtual meeting the results also appeared online in the New England Journal of Medicine. This 5.3% cut in the absolute rate of the combined, primary adverse outcome converted into a number needed to treat of 19 to prevent 1 event during 2.4 years, a “much lower” number needed to treat than reported for renin-angiotensin system inhibitors in these types of patients, Dr. Heerspink said.
Notable positive secondary outcomes included a significant 31% relative cut (a 2% absolute decline) in all-cause mortality, “a major highlight” of the findings, Dr. Heerspink said. Dapagliflozin treatment also linked with a significant 29% relative cut in the incidence of cardiovascular death or hospitalization for heart failure.
“Cardiovascular disease is the most common cause of death in patients with CKD,” explained David C. Wheeler, MD, a coinvestigator on the study and professor of kidney medicine at University College London. “The heart and kidney are intertwined. This is about cardiorenal disease.”
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin. Dr. Heerspink has been a consultant to and received research funding from AstraZeneca. He has also received personal fees from Mundipharma and Novo Nordisk, and he has also served as consultant to several other companies with the honoraria being paid to his institution. Dr. Mathieu has had relationships with AstraZeneca and several other companies. Dr. Cherney has been a consultant to and has received research funding from AstraZeneca and several other companies. Dr. Wheeler has received personal fees from AstraZeneca and from several other companies.
SOURCE: Heerspink HJL et al. EASD 2020 and N Engl J Med. 2020 Sep 24. doi: 10.1056/NEJMoa2024816.
The dramatically positive safety and efficacy results from the DAPA-CKD trial, which showed that treatment with the sodium-glucose transporter 2 (SGLT2) inhibitor dapagliflozin significantly cut both chronic kidney disease progression and all-cause death in patients with or without type 2 diabetes, were also notable for broadening the population of patients eligible for this treatment to those in the upper range of stage 4 CKD.
Of the 4,304 CKD patients enrolled in DAPA-CKD, 624 (14%) had an estimated glomerular filtration rate (eGFR) of 25-29 mL/min per 1.73m2, an unprecedented population to receive a drug from the SGLT2 inhibitor class in a reported study. The results provided definitive evidence for efficacy and safety in this range of renal function, said Hiddo J.L. Heerspink, Ph.D., at the virtual annual meeting of the European Association for the Study of Diabetes.
Until now, the widely accepted lowest level for starting an SGLT2 inhibitor in routine practice has been an eGFR as low as 30 mL/min per 1.73 m2.
Using SGLT2 inhibitors when eGFR is as low as 25
“It’s time to reduce the eGFR level for initiating an SGLT2 inhibitor to as low as 25,” said Dr. Heerspink, a professor of clinical pharmacology at the University of Groningen (the Netherlands).
While conceding that this is primarily a decision to be made by guideline writers and regulatory bodies, he declared what he believed was established by the DAPA-CKD findings: “We’ve shown that dapagliflozin can be safely used in these patients. It is effective across the spectrum of kidney function.”
Other experts not associated with the study agreed.
The trial researchers were “brave” to enroll patients with eGFRs as low as 25 mL/min per 1.73 m2, and “we urgently need these agents in patients with an eGFR this low,” commented Chantal Mathieu, MD, an endocrinologist and professor of medicine at Catholic University in Leuven, Belgium, and designated discussant for the report. Overall, she called the findings “spectacular,” a “landmark trial,” and a “winner.”
The study also set an new, lower floor for the level of albuminuria that can be usefully treated with dapagliflozin (Farxiga) by enrolling patients with a urinary albumin-to-creatinine ratio as low as 200 mg/g; the previous lower limit had been 300 mg/g, noted Dr. Mathieu. The new findings pose challenges to guideline writers, regulators who approve drug labels, and payers to a quickly make changes that will bring dapagliflozin to a wider number of patients with CKD.
Once the full DAPA-CKD results are reported, “it will change practice, and push the eGFR needle down” to as low as 25. It will also lower the albuminuria threshold for using dapagliflozin or other drugs in the class, commented David Z.I. Cherney, MD, a nephrologist at the University of Toronto. “It’s just one study,” he admitted, but the consistent renal benefits seen across several studies involving all four drugs in the SGLT2 inhibitor class will help hasten this change in identifying treatable patients, as well as expand the drug class to patients with CKD but no type 2 diabetes (T2D).
“I don’t think we’ve ever had stronger evidence” for drugs that can benefit both heart and renal function, plus the drug class is “very safe, and really easy to start” and maintain in patients, Dr. Cherney said in an interview. “It’s wonderful for these patients that we now have something new for treatment,” a drug with a “very favorable benefit-to-risk ratio.”
Results show many dapagliflozin benefits
While this broadening of the range of patients proven to tolerate and benefit from an SGLT2 inhibitor was an important consequence of DAPA-CKD, the study’s primary finding – that dapagliflozin was as safe and effective for slowing CKD progression in patients regardless of whether they also had T2D – will have an even bigger impact on expanding the target patient population. Showing efficacy in patients with CKD but without a T2D etiology, the status of about a third of the enrolled 4,304 patients, makes this treatment an option for “millions” of additional patients worldwide, said Dr. Heerspink. “These are the most common patients nephrologists see.” A major challenge now will be to do a better job finding patients with CKD who could benefit from dapagliflozin.
DAPA-CKD enrolled CKD patients based primarily on prespecified albuminuria and eGFR levels at more than 300 centers in 34 countries, including the United States. Virtually all patients, 97%, were on the only treatment now available with proven efficacy for slowing CKD, either an ACE inhibitor or an angiotensin receptor blocker. The small number of patients not on one of these drugs was because of poor tolerance.
The study’s primary endpoint was the combined rate of cardiovascular death, renal death, end-stage renal disease, or a drop in eGFR of at least 50% from baseline. This occurred in 14.5% of patients who received placebo and in 9.2% of those who received dapagliflozin during a median follow-up of 2.4 years, a highly significant 39% relative risk reduction. Concurrently with the report at the virtual meeting the results also appeared online in the New England Journal of Medicine. This 5.3% cut in the absolute rate of the combined, primary adverse outcome converted into a number needed to treat of 19 to prevent 1 event during 2.4 years, a “much lower” number needed to treat than reported for renin-angiotensin system inhibitors in these types of patients, Dr. Heerspink said.
Notable positive secondary outcomes included a significant 31% relative cut (a 2% absolute decline) in all-cause mortality, “a major highlight” of the findings, Dr. Heerspink said. Dapagliflozin treatment also linked with a significant 29% relative cut in the incidence of cardiovascular death or hospitalization for heart failure.
“Cardiovascular disease is the most common cause of death in patients with CKD,” explained David C. Wheeler, MD, a coinvestigator on the study and professor of kidney medicine at University College London. “The heart and kidney are intertwined. This is about cardiorenal disease.”
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin. Dr. Heerspink has been a consultant to and received research funding from AstraZeneca. He has also received personal fees from Mundipharma and Novo Nordisk, and he has also served as consultant to several other companies with the honoraria being paid to his institution. Dr. Mathieu has had relationships with AstraZeneca and several other companies. Dr. Cherney has been a consultant to and has received research funding from AstraZeneca and several other companies. Dr. Wheeler has received personal fees from AstraZeneca and from several other companies.
SOURCE: Heerspink HJL et al. EASD 2020 and N Engl J Med. 2020 Sep 24. doi: 10.1056/NEJMoa2024816.
The dramatically positive safety and efficacy results from the DAPA-CKD trial, which showed that treatment with the sodium-glucose transporter 2 (SGLT2) inhibitor dapagliflozin significantly cut both chronic kidney disease progression and all-cause death in patients with or without type 2 diabetes, were also notable for broadening the population of patients eligible for this treatment to those in the upper range of stage 4 CKD.
Of the 4,304 CKD patients enrolled in DAPA-CKD, 624 (14%) had an estimated glomerular filtration rate (eGFR) of 25-29 mL/min per 1.73m2, an unprecedented population to receive a drug from the SGLT2 inhibitor class in a reported study. The results provided definitive evidence for efficacy and safety in this range of renal function, said Hiddo J.L. Heerspink, Ph.D., at the virtual annual meeting of the European Association for the Study of Diabetes.
Until now, the widely accepted lowest level for starting an SGLT2 inhibitor in routine practice has been an eGFR as low as 30 mL/min per 1.73 m2.
Using SGLT2 inhibitors when eGFR is as low as 25
“It’s time to reduce the eGFR level for initiating an SGLT2 inhibitor to as low as 25,” said Dr. Heerspink, a professor of clinical pharmacology at the University of Groningen (the Netherlands).
While conceding that this is primarily a decision to be made by guideline writers and regulatory bodies, he declared what he believed was established by the DAPA-CKD findings: “We’ve shown that dapagliflozin can be safely used in these patients. It is effective across the spectrum of kidney function.”
Other experts not associated with the study agreed.
The trial researchers were “brave” to enroll patients with eGFRs as low as 25 mL/min per 1.73 m2, and “we urgently need these agents in patients with an eGFR this low,” commented Chantal Mathieu, MD, an endocrinologist and professor of medicine at Catholic University in Leuven, Belgium, and designated discussant for the report. Overall, she called the findings “spectacular,” a “landmark trial,” and a “winner.”
The study also set an new, lower floor for the level of albuminuria that can be usefully treated with dapagliflozin (Farxiga) by enrolling patients with a urinary albumin-to-creatinine ratio as low as 200 mg/g; the previous lower limit had been 300 mg/g, noted Dr. Mathieu. The new findings pose challenges to guideline writers, regulators who approve drug labels, and payers to a quickly make changes that will bring dapagliflozin to a wider number of patients with CKD.
Once the full DAPA-CKD results are reported, “it will change practice, and push the eGFR needle down” to as low as 25. It will also lower the albuminuria threshold for using dapagliflozin or other drugs in the class, commented David Z.I. Cherney, MD, a nephrologist at the University of Toronto. “It’s just one study,” he admitted, but the consistent renal benefits seen across several studies involving all four drugs in the SGLT2 inhibitor class will help hasten this change in identifying treatable patients, as well as expand the drug class to patients with CKD but no type 2 diabetes (T2D).
“I don’t think we’ve ever had stronger evidence” for drugs that can benefit both heart and renal function, plus the drug class is “very safe, and really easy to start” and maintain in patients, Dr. Cherney said in an interview. “It’s wonderful for these patients that we now have something new for treatment,” a drug with a “very favorable benefit-to-risk ratio.”
Results show many dapagliflozin benefits
While this broadening of the range of patients proven to tolerate and benefit from an SGLT2 inhibitor was an important consequence of DAPA-CKD, the study’s primary finding – that dapagliflozin was as safe and effective for slowing CKD progression in patients regardless of whether they also had T2D – will have an even bigger impact on expanding the target patient population. Showing efficacy in patients with CKD but without a T2D etiology, the status of about a third of the enrolled 4,304 patients, makes this treatment an option for “millions” of additional patients worldwide, said Dr. Heerspink. “These are the most common patients nephrologists see.” A major challenge now will be to do a better job finding patients with CKD who could benefit from dapagliflozin.
DAPA-CKD enrolled CKD patients based primarily on prespecified albuminuria and eGFR levels at more than 300 centers in 34 countries, including the United States. Virtually all patients, 97%, were on the only treatment now available with proven efficacy for slowing CKD, either an ACE inhibitor or an angiotensin receptor blocker. The small number of patients not on one of these drugs was because of poor tolerance.
The study’s primary endpoint was the combined rate of cardiovascular death, renal death, end-stage renal disease, or a drop in eGFR of at least 50% from baseline. This occurred in 14.5% of patients who received placebo and in 9.2% of those who received dapagliflozin during a median follow-up of 2.4 years, a highly significant 39% relative risk reduction. Concurrently with the report at the virtual meeting the results also appeared online in the New England Journal of Medicine. This 5.3% cut in the absolute rate of the combined, primary adverse outcome converted into a number needed to treat of 19 to prevent 1 event during 2.4 years, a “much lower” number needed to treat than reported for renin-angiotensin system inhibitors in these types of patients, Dr. Heerspink said.
Notable positive secondary outcomes included a significant 31% relative cut (a 2% absolute decline) in all-cause mortality, “a major highlight” of the findings, Dr. Heerspink said. Dapagliflozin treatment also linked with a significant 29% relative cut in the incidence of cardiovascular death or hospitalization for heart failure.
“Cardiovascular disease is the most common cause of death in patients with CKD,” explained David C. Wheeler, MD, a coinvestigator on the study and professor of kidney medicine at University College London. “The heart and kidney are intertwined. This is about cardiorenal disease.”
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin. Dr. Heerspink has been a consultant to and received research funding from AstraZeneca. He has also received personal fees from Mundipharma and Novo Nordisk, and he has also served as consultant to several other companies with the honoraria being paid to his institution. Dr. Mathieu has had relationships with AstraZeneca and several other companies. Dr. Cherney has been a consultant to and has received research funding from AstraZeneca and several other companies. Dr. Wheeler has received personal fees from AstraZeneca and from several other companies.
SOURCE: Heerspink HJL et al. EASD 2020 and N Engl J Med. 2020 Sep 24. doi: 10.1056/NEJMoa2024816.
FROM EASD 2020
Physicians, make a plan to vote
In March 2020, following the announcement of the United States’ first death related to COVID-19, many physicians began using their voices to discuss the shortage of personal protective equipment (PPE). Many physicians, myself included, petitioned elected leaders at the community, state, and federal levels to address the PPE shortage.
Historically, physicians have advocated for improved public health. From seat belt laws in the 1980s and 1990s to the Affordable Care Act in the 2000s, physicians have testified at the community, state, and federal levels to advocate for the health and safety of our patients and the public. Yet while we have been making our voices heard, we are often silent at the ballot box.
In the 1996 and 2000 elections, physicians voted 9% less often than the general public, and compared with lawyers – professionals with similar educational attainment and finances – physicians voted 22% less often.1 It is unclear why physicians are less likely to vote. In a 2016 article, David Grande, MD, and Katrina Armstrong, MD, postulated that physicians may not vote because our work hours create barriers to visiting polls.2
Despite our lack of engagement at the ballot box, voting is important to improving our patients’ social determinants of health. In a recently published systematic review, the authors found several studies supporting the association between voting and social determinants of health. Their review found that, when large numbers of people from communities participated in voting, it translated into greater influence over determining who held political power in that community. Those with power introduced and supported policies responding to their constituents’ needs, ultimately influencing their constituents’ social determinants of health.3 By voting, we as physicians are helping to address the social determinants of health in our communities.
Many medical students have been doing their part to improve the social determinants of health in their communities by pledging to vote. In 2018, the American Medical Student Association launched their “Med Out the Vote” initiative prior to the election. The organization called on all health care providers and providers in training to pledge to vote in the election.4 They are continuing these efforts for the 2020 elections.
We should join our nation’s medical students by also pledging to vote. To begin, we can all Make A Plan To Vote. Each plan should include the following:
- Register to vote: In many states eligible voters can register online.
- Request an absentee ballot: Many states require registered voters to request absentee ballots online or by mail.
- Vote: Submit an absentee ballot prior to election or vote in-person on election day. Some counties allow voting early in person.
In practice, our plans will differ slightly because each state has its own election laws.
This election season let us ensure all physician voices are heard. Make A Plan To Vote for your patients and communities.
Dr. Kumar is the pediatric editor of The Hospitalist. She is clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine at Case Western Reserve University and a pediatric hospitalist at Cleveland Clinic Children’s.
References
1. Grande D et al. Do Doctors Vote? J Gen Intern Med. 2007 May;22(5):585-9.
2. Grande D, Armstrong K. Will Physicians Vote? Ann Intern Med. 2016;165:814-5.
3. Brown CL et al. Voting, health and interventions in healthcare settings: A scoping review. Public Health Rev. 2020 Jul. doi: 10.1186/s40985-020-00133-6.
4. American Medical Student Association. AMSA Launches Med Out the Vote Campaign, Call to Action. 2018 Jul 29. Accessed 2020 Sep 14. https://www.amsa.org/about/amsa-press-room/amsa-launches-med-out-the-vote-campaign-call-to-action/
In March 2020, following the announcement of the United States’ first death related to COVID-19, many physicians began using their voices to discuss the shortage of personal protective equipment (PPE). Many physicians, myself included, petitioned elected leaders at the community, state, and federal levels to address the PPE shortage.
Historically, physicians have advocated for improved public health. From seat belt laws in the 1980s and 1990s to the Affordable Care Act in the 2000s, physicians have testified at the community, state, and federal levels to advocate for the health and safety of our patients and the public. Yet while we have been making our voices heard, we are often silent at the ballot box.
In the 1996 and 2000 elections, physicians voted 9% less often than the general public, and compared with lawyers – professionals with similar educational attainment and finances – physicians voted 22% less often.1 It is unclear why physicians are less likely to vote. In a 2016 article, David Grande, MD, and Katrina Armstrong, MD, postulated that physicians may not vote because our work hours create barriers to visiting polls.2
Despite our lack of engagement at the ballot box, voting is important to improving our patients’ social determinants of health. In a recently published systematic review, the authors found several studies supporting the association between voting and social determinants of health. Their review found that, when large numbers of people from communities participated in voting, it translated into greater influence over determining who held political power in that community. Those with power introduced and supported policies responding to their constituents’ needs, ultimately influencing their constituents’ social determinants of health.3 By voting, we as physicians are helping to address the social determinants of health in our communities.
Many medical students have been doing their part to improve the social determinants of health in their communities by pledging to vote. In 2018, the American Medical Student Association launched their “Med Out the Vote” initiative prior to the election. The organization called on all health care providers and providers in training to pledge to vote in the election.4 They are continuing these efforts for the 2020 elections.
We should join our nation’s medical students by also pledging to vote. To begin, we can all Make A Plan To Vote. Each plan should include the following:
- Register to vote: In many states eligible voters can register online.
- Request an absentee ballot: Many states require registered voters to request absentee ballots online or by mail.
- Vote: Submit an absentee ballot prior to election or vote in-person on election day. Some counties allow voting early in person.
In practice, our plans will differ slightly because each state has its own election laws.
This election season let us ensure all physician voices are heard. Make A Plan To Vote for your patients and communities.
Dr. Kumar is the pediatric editor of The Hospitalist. She is clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine at Case Western Reserve University and a pediatric hospitalist at Cleveland Clinic Children’s.
References
1. Grande D et al. Do Doctors Vote? J Gen Intern Med. 2007 May;22(5):585-9.
2. Grande D, Armstrong K. Will Physicians Vote? Ann Intern Med. 2016;165:814-5.
3. Brown CL et al. Voting, health and interventions in healthcare settings: A scoping review. Public Health Rev. 2020 Jul. doi: 10.1186/s40985-020-00133-6.
4. American Medical Student Association. AMSA Launches Med Out the Vote Campaign, Call to Action. 2018 Jul 29. Accessed 2020 Sep 14. https://www.amsa.org/about/amsa-press-room/amsa-launches-med-out-the-vote-campaign-call-to-action/
In March 2020, following the announcement of the United States’ first death related to COVID-19, many physicians began using their voices to discuss the shortage of personal protective equipment (PPE). Many physicians, myself included, petitioned elected leaders at the community, state, and federal levels to address the PPE shortage.
Historically, physicians have advocated for improved public health. From seat belt laws in the 1980s and 1990s to the Affordable Care Act in the 2000s, physicians have testified at the community, state, and federal levels to advocate for the health and safety of our patients and the public. Yet while we have been making our voices heard, we are often silent at the ballot box.
In the 1996 and 2000 elections, physicians voted 9% less often than the general public, and compared with lawyers – professionals with similar educational attainment and finances – physicians voted 22% less often.1 It is unclear why physicians are less likely to vote. In a 2016 article, David Grande, MD, and Katrina Armstrong, MD, postulated that physicians may not vote because our work hours create barriers to visiting polls.2
Despite our lack of engagement at the ballot box, voting is important to improving our patients’ social determinants of health. In a recently published systematic review, the authors found several studies supporting the association between voting and social determinants of health. Their review found that, when large numbers of people from communities participated in voting, it translated into greater influence over determining who held political power in that community. Those with power introduced and supported policies responding to their constituents’ needs, ultimately influencing their constituents’ social determinants of health.3 By voting, we as physicians are helping to address the social determinants of health in our communities.
Many medical students have been doing their part to improve the social determinants of health in their communities by pledging to vote. In 2018, the American Medical Student Association launched their “Med Out the Vote” initiative prior to the election. The organization called on all health care providers and providers in training to pledge to vote in the election.4 They are continuing these efforts for the 2020 elections.
We should join our nation’s medical students by also pledging to vote. To begin, we can all Make A Plan To Vote. Each plan should include the following:
- Register to vote: In many states eligible voters can register online.
- Request an absentee ballot: Many states require registered voters to request absentee ballots online or by mail.
- Vote: Submit an absentee ballot prior to election or vote in-person on election day. Some counties allow voting early in person.
In practice, our plans will differ slightly because each state has its own election laws.
This election season let us ensure all physician voices are heard. Make A Plan To Vote for your patients and communities.
Dr. Kumar is the pediatric editor of The Hospitalist. She is clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine at Case Western Reserve University and a pediatric hospitalist at Cleveland Clinic Children’s.
References
1. Grande D et al. Do Doctors Vote? J Gen Intern Med. 2007 May;22(5):585-9.
2. Grande D, Armstrong K. Will Physicians Vote? Ann Intern Med. 2016;165:814-5.
3. Brown CL et al. Voting, health and interventions in healthcare settings: A scoping review. Public Health Rev. 2020 Jul. doi: 10.1186/s40985-020-00133-6.
4. American Medical Student Association. AMSA Launches Med Out the Vote Campaign, Call to Action. 2018 Jul 29. Accessed 2020 Sep 14. https://www.amsa.org/about/amsa-press-room/amsa-launches-med-out-the-vote-campaign-call-to-action/
COVID-19 airway management: Expert tips on infection control
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
FROM AN SCCM VIRTUAL MEETING
J&J’s one-shot COVID-19 vaccine advances to phase 3 testing
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
CDC playbook prepares states for rollout of COVID-19 vaccine if one is approved
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
Social media and health information: Empowering or misleading?
The search engine giants, Dr. Google or Dr. Bing, are visited by most of our patients before seeking medical help. In 1976, medical student Tom Ferguson, MD, first coined the term e-Patient. It means a health consumer who uses the Internet to gather information about a medical condition for themselves or on behalf of family and friends and uses electronic communication tools to cope with medical conditions. Dr. Ferguson described e-Patients as “empowered medical consumers.”1
During the COVID-19 pandemic, social media and networking platforms – such as Facebook, Twitter, Instagram, Snapchat, YouTube, WhatsApp, online health support groups – are used increasingly by e-Patients to gather critical health information. Health care providers often take a conflicted stand on the use of social media. Though we want our patients to read about their illnesses and make informed choices, we often get frustrated by misdiagnoses, misinformation, and disinformation that comes with it.
According to a study investigating the differential diffusion of news stories distributed on Twitter from 2006 to 2017, fake news was considered more novel than true news, and people were more likely to share novel information.2 Bots accelerated the spread of true and fake news at the same rate, implying that fake news spreads more than the truth because humans, not robots, are more likely to spread it. Social media has promoted some of the best health campaigns, like public cancer awareness, the ALS Ice Bucket Challenge, World Heart Day, and others. At the same time, it has also provided a platform for antivaccination activists, dangerous and unproven alternative cancer therapies, weight loss pills, and nutrition plans.
According to a Pew Research Center survey, 72% of adult Internet users had searched online for information about a range of health issues of their own or for others in the past 12 months.3 A survey from 2019-2020 showed that those who relied on social media for news were among the least knowledgeable about key facts during the COVID-19 outbreak.4 About 74% of public posts about COVID-19 were linked to news organizations, while just 1% linked to health and science sites.5 While social media has emerged as one of the most significant health information sources, it famously has only a few safeguards in place against medical misinformation. Requiring responsibility and regulations for accurate or evidence-based information walks a thin line on infringing freedom of speech. Medical misinformation related to COVID-19 has become as contagious as the virus itself.
In February 2020, the World Health Organization warned that a massive ‘Infodemic’ had accompanied the COVID-19 outbreak, with an overabundance of information, some accurate and some not, making it difficult for people to find reliable sources and trustworthy information.6 The Black immunity myth, groups opposing vaccines, campaigns against 5G mobile phone networks, suggestions that SARS-CoV-2 was an engineered bioweapon, and online rumors leading to mob attacks in India and mass poisonings in Iran are some of the misleading health information that has circulated related to COVID-19.
In the Web 2.0 era, in which credible health information comes packaged with divisive and misleading information, social media’s full impact on health care, health outcomes, and mental health has yet to be explored. Social networks and media sharing networks have recently announced initiatives to stop misinformation and disinformation by fact-checking, flagging, issuing warnings, and deleting misinformation or misleading content. Providing links to more and correct information and partnering with health and science organizations can also encourage the spread of verifiable information.
While we have yet to see if social media safeguards are adequate, the medical community needs to proactively educate patients on the appropriate use of social media for health information, e-Health literacy, and media health literacy. Like health care providers evaluating scientific papers, we need to cultivate e-Patients’ ability to seek, evaluate, understand, and convey health information from electronic sources. Although the measurement and training tools for e-Health and media health literacy are still scarce, a good place to start could be to have simple conversations with patients. Encouraging patients to critically analyze online information, use credible social media sources, and recognizing the warnings, red flags, and links on unreliable information are some of the discussions worth considering. Equally important is to discourage patients from changing health behaviors or practices based on unverified social media resources and discussing the possible impact of medical misinformation.
A practical approach for e-Patients could be to ask the Five Ws, considered fundamental in information gathering: Who, What, Why, When, and Where.7,8
- Who runs the website? Examine the authors, sponsors, and sources. Federal agencies’ website addresses end in “.gov,” educational institutions maintain “.edu,” large professional or nonprofit organizations often use “.org,” and commercial websites use “.com.”
- What is offered, and What is the evidence? Does it provide unbelievable solutions or quick, miracle cures?
- Why was the site created? Is the mission or goal to inform, explain, or sell health or medical products? Check details on “About This Site” or “About Us.”
- When was the information written or the webpage last updated?
- Where are the privacy policies? Is your privacy protected?
The anonymity of sources, sponsors, financial interests, or the lack of medical credentials and reputable medical research, the use of testimonials as evidence, outdated or incomplete information, and emotional or exaggerated language should raise suspicion about the reliability of the information. Tools like the online tutorial and a checklist from the National Institute of Health’s National Library of Medicine can also be offered to e-Patients to learn how to evaluate health information online.9,10
Online health support groups widely used by patients can be an additional layer of support but can also be a source of misinformation. Since they have fewer gatekeepers than traditional face-to-face communication, keeping a check on the credibility of the information can be difficult. Support groups affiliated with local hospitals or national organizations, or those endorsed by well-known scientific societies, can be encouraged instead of less credible sources. Some online support groups, run by non–health care professionals but with experienced and reliable scientific panels, can be useful resources. However, patients must check for the credibility and reliability of the information.
Lastly, just as hospitalists take a social history of our patients, we could also ask for a “social media history” to understand patients’ sources of health information. We can then guide them toward more credible sources to make them truly empowered medical consumers.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Nelson R. Informatics: Empowering ePatients to drive health care reform - part I. Online J Issues Nurs. 2016 Sep 13;21(3):9.
2. Vosoughi S et al. The spread of true and false news online. Science. 2012;359(6380):1146-51.
3. Fox S. The social life of health information. Pew Research Center: Fact Tank. 2014 Jan 15. Accessed 2020 Jul 31.
4. Mitchel A, Jurkowitz M, Oliphant JB, Shearer E. Americans Who Mainly Get Their News on Social Media Are Less Engaged, Less Knowledgeable. Pew Research Center: Journalism & Media. 2020 Jul 30. Accessed 2020 Jul 31.
5. Stocking G, Matsa KE, Khuzam M. As COVID-19 Emerged in U.S., Facebook Posts About It Appeared in a Wide Range of Public Pages, Groups Pew Research Center: Journalism & Media. 2020 Jun 24. Accessed 2020 Jul 31.
6. Munich Security Conference. World Health Organization. 2020 Feb 15. Accessed 2020 Jul 31.
7. Levin-Zamir D, Bertschi I. Media health literacy, eHealth literacy, and the role of the social environment in context. Int J Environ Res Public Health. 2018 Aug 3;15(8):1643.
8. Online Health Information: Is It Reliable? National Institute on Aging, National Institutes of Health. 2018 Oct 31. Accessed 2020 Aug 10.
9. How To Evaluate Health Information on the Internet: Questions and Answers. Office of Dietary Supplements, National Institutes of Health. 2011 Jun 24. Accessed 2020 Aug 10.
10. Evaluating Internet Health Information: A Tutorial From the National Library of Medicine. Medline Plus. 2020 Mar 6. Accessed 2020 Aug 10.
The search engine giants, Dr. Google or Dr. Bing, are visited by most of our patients before seeking medical help. In 1976, medical student Tom Ferguson, MD, first coined the term e-Patient. It means a health consumer who uses the Internet to gather information about a medical condition for themselves or on behalf of family and friends and uses electronic communication tools to cope with medical conditions. Dr. Ferguson described e-Patients as “empowered medical consumers.”1
During the COVID-19 pandemic, social media and networking platforms – such as Facebook, Twitter, Instagram, Snapchat, YouTube, WhatsApp, online health support groups – are used increasingly by e-Patients to gather critical health information. Health care providers often take a conflicted stand on the use of social media. Though we want our patients to read about their illnesses and make informed choices, we often get frustrated by misdiagnoses, misinformation, and disinformation that comes with it.
According to a study investigating the differential diffusion of news stories distributed on Twitter from 2006 to 2017, fake news was considered more novel than true news, and people were more likely to share novel information.2 Bots accelerated the spread of true and fake news at the same rate, implying that fake news spreads more than the truth because humans, not robots, are more likely to spread it. Social media has promoted some of the best health campaigns, like public cancer awareness, the ALS Ice Bucket Challenge, World Heart Day, and others. At the same time, it has also provided a platform for antivaccination activists, dangerous and unproven alternative cancer therapies, weight loss pills, and nutrition plans.
According to a Pew Research Center survey, 72% of adult Internet users had searched online for information about a range of health issues of their own or for others in the past 12 months.3 A survey from 2019-2020 showed that those who relied on social media for news were among the least knowledgeable about key facts during the COVID-19 outbreak.4 About 74% of public posts about COVID-19 were linked to news organizations, while just 1% linked to health and science sites.5 While social media has emerged as one of the most significant health information sources, it famously has only a few safeguards in place against medical misinformation. Requiring responsibility and regulations for accurate or evidence-based information walks a thin line on infringing freedom of speech. Medical misinformation related to COVID-19 has become as contagious as the virus itself.
In February 2020, the World Health Organization warned that a massive ‘Infodemic’ had accompanied the COVID-19 outbreak, with an overabundance of information, some accurate and some not, making it difficult for people to find reliable sources and trustworthy information.6 The Black immunity myth, groups opposing vaccines, campaigns against 5G mobile phone networks, suggestions that SARS-CoV-2 was an engineered bioweapon, and online rumors leading to mob attacks in India and mass poisonings in Iran are some of the misleading health information that has circulated related to COVID-19.
In the Web 2.0 era, in which credible health information comes packaged with divisive and misleading information, social media’s full impact on health care, health outcomes, and mental health has yet to be explored. Social networks and media sharing networks have recently announced initiatives to stop misinformation and disinformation by fact-checking, flagging, issuing warnings, and deleting misinformation or misleading content. Providing links to more and correct information and partnering with health and science organizations can also encourage the spread of verifiable information.
While we have yet to see if social media safeguards are adequate, the medical community needs to proactively educate patients on the appropriate use of social media for health information, e-Health literacy, and media health literacy. Like health care providers evaluating scientific papers, we need to cultivate e-Patients’ ability to seek, evaluate, understand, and convey health information from electronic sources. Although the measurement and training tools for e-Health and media health literacy are still scarce, a good place to start could be to have simple conversations with patients. Encouraging patients to critically analyze online information, use credible social media sources, and recognizing the warnings, red flags, and links on unreliable information are some of the discussions worth considering. Equally important is to discourage patients from changing health behaviors or practices based on unverified social media resources and discussing the possible impact of medical misinformation.
A practical approach for e-Patients could be to ask the Five Ws, considered fundamental in information gathering: Who, What, Why, When, and Where.7,8
- Who runs the website? Examine the authors, sponsors, and sources. Federal agencies’ website addresses end in “.gov,” educational institutions maintain “.edu,” large professional or nonprofit organizations often use “.org,” and commercial websites use “.com.”
- What is offered, and What is the evidence? Does it provide unbelievable solutions or quick, miracle cures?
- Why was the site created? Is the mission or goal to inform, explain, or sell health or medical products? Check details on “About This Site” or “About Us.”
- When was the information written or the webpage last updated?
- Where are the privacy policies? Is your privacy protected?
The anonymity of sources, sponsors, financial interests, or the lack of medical credentials and reputable medical research, the use of testimonials as evidence, outdated or incomplete information, and emotional or exaggerated language should raise suspicion about the reliability of the information. Tools like the online tutorial and a checklist from the National Institute of Health’s National Library of Medicine can also be offered to e-Patients to learn how to evaluate health information online.9,10
Online health support groups widely used by patients can be an additional layer of support but can also be a source of misinformation. Since they have fewer gatekeepers than traditional face-to-face communication, keeping a check on the credibility of the information can be difficult. Support groups affiliated with local hospitals or national organizations, or those endorsed by well-known scientific societies, can be encouraged instead of less credible sources. Some online support groups, run by non–health care professionals but with experienced and reliable scientific panels, can be useful resources. However, patients must check for the credibility and reliability of the information.
Lastly, just as hospitalists take a social history of our patients, we could also ask for a “social media history” to understand patients’ sources of health information. We can then guide them toward more credible sources to make them truly empowered medical consumers.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Nelson R. Informatics: Empowering ePatients to drive health care reform - part I. Online J Issues Nurs. 2016 Sep 13;21(3):9.
2. Vosoughi S et al. The spread of true and false news online. Science. 2012;359(6380):1146-51.
3. Fox S. The social life of health information. Pew Research Center: Fact Tank. 2014 Jan 15. Accessed 2020 Jul 31.
4. Mitchel A, Jurkowitz M, Oliphant JB, Shearer E. Americans Who Mainly Get Their News on Social Media Are Less Engaged, Less Knowledgeable. Pew Research Center: Journalism & Media. 2020 Jul 30. Accessed 2020 Jul 31.
5. Stocking G, Matsa KE, Khuzam M. As COVID-19 Emerged in U.S., Facebook Posts About It Appeared in a Wide Range of Public Pages, Groups Pew Research Center: Journalism & Media. 2020 Jun 24. Accessed 2020 Jul 31.
6. Munich Security Conference. World Health Organization. 2020 Feb 15. Accessed 2020 Jul 31.
7. Levin-Zamir D, Bertschi I. Media health literacy, eHealth literacy, and the role of the social environment in context. Int J Environ Res Public Health. 2018 Aug 3;15(8):1643.
8. Online Health Information: Is It Reliable? National Institute on Aging, National Institutes of Health. 2018 Oct 31. Accessed 2020 Aug 10.
9. How To Evaluate Health Information on the Internet: Questions and Answers. Office of Dietary Supplements, National Institutes of Health. 2011 Jun 24. Accessed 2020 Aug 10.
10. Evaluating Internet Health Information: A Tutorial From the National Library of Medicine. Medline Plus. 2020 Mar 6. Accessed 2020 Aug 10.
The search engine giants, Dr. Google or Dr. Bing, are visited by most of our patients before seeking medical help. In 1976, medical student Tom Ferguson, MD, first coined the term e-Patient. It means a health consumer who uses the Internet to gather information about a medical condition for themselves or on behalf of family and friends and uses electronic communication tools to cope with medical conditions. Dr. Ferguson described e-Patients as “empowered medical consumers.”1
During the COVID-19 pandemic, social media and networking platforms – such as Facebook, Twitter, Instagram, Snapchat, YouTube, WhatsApp, online health support groups – are used increasingly by e-Patients to gather critical health information. Health care providers often take a conflicted stand on the use of social media. Though we want our patients to read about their illnesses and make informed choices, we often get frustrated by misdiagnoses, misinformation, and disinformation that comes with it.
According to a study investigating the differential diffusion of news stories distributed on Twitter from 2006 to 2017, fake news was considered more novel than true news, and people were more likely to share novel information.2 Bots accelerated the spread of true and fake news at the same rate, implying that fake news spreads more than the truth because humans, not robots, are more likely to spread it. Social media has promoted some of the best health campaigns, like public cancer awareness, the ALS Ice Bucket Challenge, World Heart Day, and others. At the same time, it has also provided a platform for antivaccination activists, dangerous and unproven alternative cancer therapies, weight loss pills, and nutrition plans.
According to a Pew Research Center survey, 72% of adult Internet users had searched online for information about a range of health issues of their own or for others in the past 12 months.3 A survey from 2019-2020 showed that those who relied on social media for news were among the least knowledgeable about key facts during the COVID-19 outbreak.4 About 74% of public posts about COVID-19 were linked to news organizations, while just 1% linked to health and science sites.5 While social media has emerged as one of the most significant health information sources, it famously has only a few safeguards in place against medical misinformation. Requiring responsibility and regulations for accurate or evidence-based information walks a thin line on infringing freedom of speech. Medical misinformation related to COVID-19 has become as contagious as the virus itself.
In February 2020, the World Health Organization warned that a massive ‘Infodemic’ had accompanied the COVID-19 outbreak, with an overabundance of information, some accurate and some not, making it difficult for people to find reliable sources and trustworthy information.6 The Black immunity myth, groups opposing vaccines, campaigns against 5G mobile phone networks, suggestions that SARS-CoV-2 was an engineered bioweapon, and online rumors leading to mob attacks in India and mass poisonings in Iran are some of the misleading health information that has circulated related to COVID-19.
In the Web 2.0 era, in which credible health information comes packaged with divisive and misleading information, social media’s full impact on health care, health outcomes, and mental health has yet to be explored. Social networks and media sharing networks have recently announced initiatives to stop misinformation and disinformation by fact-checking, flagging, issuing warnings, and deleting misinformation or misleading content. Providing links to more and correct information and partnering with health and science organizations can also encourage the spread of verifiable information.
While we have yet to see if social media safeguards are adequate, the medical community needs to proactively educate patients on the appropriate use of social media for health information, e-Health literacy, and media health literacy. Like health care providers evaluating scientific papers, we need to cultivate e-Patients’ ability to seek, evaluate, understand, and convey health information from electronic sources. Although the measurement and training tools for e-Health and media health literacy are still scarce, a good place to start could be to have simple conversations with patients. Encouraging patients to critically analyze online information, use credible social media sources, and recognizing the warnings, red flags, and links on unreliable information are some of the discussions worth considering. Equally important is to discourage patients from changing health behaviors or practices based on unverified social media resources and discussing the possible impact of medical misinformation.
A practical approach for e-Patients could be to ask the Five Ws, considered fundamental in information gathering: Who, What, Why, When, and Where.7,8
- Who runs the website? Examine the authors, sponsors, and sources. Federal agencies’ website addresses end in “.gov,” educational institutions maintain “.edu,” large professional or nonprofit organizations often use “.org,” and commercial websites use “.com.”
- What is offered, and What is the evidence? Does it provide unbelievable solutions or quick, miracle cures?
- Why was the site created? Is the mission or goal to inform, explain, or sell health or medical products? Check details on “About This Site” or “About Us.”
- When was the information written or the webpage last updated?
- Where are the privacy policies? Is your privacy protected?
The anonymity of sources, sponsors, financial interests, or the lack of medical credentials and reputable medical research, the use of testimonials as evidence, outdated or incomplete information, and emotional or exaggerated language should raise suspicion about the reliability of the information. Tools like the online tutorial and a checklist from the National Institute of Health’s National Library of Medicine can also be offered to e-Patients to learn how to evaluate health information online.9,10
Online health support groups widely used by patients can be an additional layer of support but can also be a source of misinformation. Since they have fewer gatekeepers than traditional face-to-face communication, keeping a check on the credibility of the information can be difficult. Support groups affiliated with local hospitals or national organizations, or those endorsed by well-known scientific societies, can be encouraged instead of less credible sources. Some online support groups, run by non–health care professionals but with experienced and reliable scientific panels, can be useful resources. However, patients must check for the credibility and reliability of the information.
Lastly, just as hospitalists take a social history of our patients, we could also ask for a “social media history” to understand patients’ sources of health information. We can then guide them toward more credible sources to make them truly empowered medical consumers.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Nelson R. Informatics: Empowering ePatients to drive health care reform - part I. Online J Issues Nurs. 2016 Sep 13;21(3):9.
2. Vosoughi S et al. The spread of true and false news online. Science. 2012;359(6380):1146-51.
3. Fox S. The social life of health information. Pew Research Center: Fact Tank. 2014 Jan 15. Accessed 2020 Jul 31.
4. Mitchel A, Jurkowitz M, Oliphant JB, Shearer E. Americans Who Mainly Get Their News on Social Media Are Less Engaged, Less Knowledgeable. Pew Research Center: Journalism & Media. 2020 Jul 30. Accessed 2020 Jul 31.
5. Stocking G, Matsa KE, Khuzam M. As COVID-19 Emerged in U.S., Facebook Posts About It Appeared in a Wide Range of Public Pages, Groups Pew Research Center: Journalism & Media. 2020 Jun 24. Accessed 2020 Jul 31.
6. Munich Security Conference. World Health Organization. 2020 Feb 15. Accessed 2020 Jul 31.
7. Levin-Zamir D, Bertschi I. Media health literacy, eHealth literacy, and the role of the social environment in context. Int J Environ Res Public Health. 2018 Aug 3;15(8):1643.
8. Online Health Information: Is It Reliable? National Institute on Aging, National Institutes of Health. 2018 Oct 31. Accessed 2020 Aug 10.
9. How To Evaluate Health Information on the Internet: Questions and Answers. Office of Dietary Supplements, National Institutes of Health. 2011 Jun 24. Accessed 2020 Aug 10.
10. Evaluating Internet Health Information: A Tutorial From the National Library of Medicine. Medline Plus. 2020 Mar 6. Accessed 2020 Aug 10.



















