User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Study confirms it’s possible to catch COVID-19 twice
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Research examines links between ‘long COVID’ and ME/CFS
Some patients who had COVID-19 continue to have symptoms weeks to months later, even after they no longer test positive for the virus. In two recent reports – one published in JAMA in July and another published in Morbidity and Mortality Weekly Report in August – chronic fatigue was listed as the top symptom among individuals still feeling unwell beyond 2 weeks after COVID-19 onset.
Although some of the reported persistent symptoms appear specific to SARS-CoV-2 – such as cough, chest pain, and dyspnea – others overlap with the diagnostic criteria for ME/CFS, which is defined by substantial, profound fatigue for at least 6 months, postexertional malaise, unrefreshing sleep, and one or both of orthostatic intolerance and/or cognitive impairment. Although the etiology of ME/CFS is unclear, the condition commonly arises following a viral illness.
At the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis August 21, the opening session was devoted to research documenting the extent to which COVID-19 survivors subsequently meet ME/CFS criteria, and to exploring underlying mechanisms.
“It offers a lot of opportunities for us to study potentially early ME/CFS and how it develops, but in addition, a lot of the research that has been done on ME/CFS may also provide answers for COVID-19,” IACFS/ME vice president Lily Chu, MD, said in an interview.
A hint from the SARS outbreak
This isn’t the first time researchers have seen a possible link between a coronavirus and ME/CFS, Harvey Moldofsky, MD, told attendees. To illustrate that point, Dr. Moldofsky, of the department of psychiatry (emeritus) at the University of Toronto, reviewed data from a previously published case-controlled study, which included 22 health care workers who had been infected in 2003 with SARS-CoV-1 and continued to report chronic fatigue, musculoskeletal pain, and disturbed and unrefreshing sleep with EEG-documented sleep disturbances 1-3 years following the illness. None had been able to return to work by 1 year.
“We’re looking at similar symptoms now” among survivors of COVID-19, Dr. Moldofsky said. “[T]he key issue is that we have no idea of its prevalence. … We need epidemiologic studies.”
Distinguishing ME/CFS from other post–COVID-19 symptoms
Not everyone who has persistent symptoms after COVID-19 will develop ME/CFS, and distinguishing between cases may be important.
Clinically, Dr. Chu said, one way to assess whether a patient with persistent COVID-19 symptoms might be progressing to ME/CFS is to ask him or her specifically about the level of fatigue following physical exertion and the timing of any fatigue. With ME/CFS, postexertional malaise often involves a dramatic exacerbation of symptoms such as fatigue, pain, and cognitive impairment a day or 2 after exertion rather than immediately following it. In contrast, shortness of breath during exertion isn’t typical of ME/CFS.
Objective measures of ME/CFS include low natural killer cell function (the test can be ordered from commercial labs but requires rapid transport of the blood sample), and autonomic dysfunction assessed by a tilt-table test.
While there is currently no cure for ME/CFS, diagnosing it allows for the patient to be taught “pacing” in which the person conserves his or her energy by balancing activity with rest. “That type of behavioral technique is valuable for everyone who suffers from a chronic disease with fatigue. It can help them be more functional,” Dr. Chu said.
If a patient appears to be exhibiting signs of ME/CFS, “don’t wait until they hit the 6-month mark to start helping them manage their symptoms,” she said. “Teaching pacing to COVID-19 patients who have a lot of fatigue isn’t going to harm them. As they get better they’re going to just naturally do more. But if they do have ME/CFS, [pacing] stresses their system less, since the data seem to be pointing to deficiencies in producing energy.”
Will COVID-19 unleash a new wave of ME/CFS patients?
Much of the session at the virtual meeting was devoted to ongoing studies. For example, Leonard Jason, PhD, of the Center for Community Research at DePaul University, Chicago, described a prospective study launched in 2014 that looked at risk factors for developing ME/CFS in college students who contracted infectious mononucleosis as a result of Epstein-Barr virus. Now, his team is also following students from the same cohort who develop COVID-19.
Because the study included collection of baseline biological samples, the results could help reveal predisposing factors associated with long-term illness from either virus.
Another project, funded by the Open Medicine Foundation, will follow patients who are discharged from the ICU following severe COVID-19 illness. Blood, urine, and cerebrospinal fluid will be collected from those with persistent symptoms at 6 months, along with questionnaire data. At 18-24 months, those who continue to report symptoms will undergo more intensive evaluation using genomics, metabolomics, and proteomics.
“We’re taking advantage of this horrible situation, hoping to understand how a serious viral infection might lead to ME/CFS,” said lead investigator Ronald Tompkins, MD, ScD, chief medical officer at the Open Medicine Foundation and a faculty member at Harvard Medical School, Boston. The results, he said, “might give us insight into potential drug targets or biomarkers useful for prevention and treatment strategies.”
Meanwhile, Sadie Whittaker, PhD, head of the Solve ME/CFS initiative, described her organization’s new plan to use their registry to prospectively track the impact of COVID-19 on people with ME/CFS.
She noted that they’ve also teamed up with “long-COVID” communities including Body Politic. “Our goal is to form a coalition to study together or at least harmonize data … and understand what’s going on through the power of bigger sample sizes,” Dr. Whittaker said.
None of the speakers disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Some patients who had COVID-19 continue to have symptoms weeks to months later, even after they no longer test positive for the virus. In two recent reports – one published in JAMA in July and another published in Morbidity and Mortality Weekly Report in August – chronic fatigue was listed as the top symptom among individuals still feeling unwell beyond 2 weeks after COVID-19 onset.
Although some of the reported persistent symptoms appear specific to SARS-CoV-2 – such as cough, chest pain, and dyspnea – others overlap with the diagnostic criteria for ME/CFS, which is defined by substantial, profound fatigue for at least 6 months, postexertional malaise, unrefreshing sleep, and one or both of orthostatic intolerance and/or cognitive impairment. Although the etiology of ME/CFS is unclear, the condition commonly arises following a viral illness.
At the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis August 21, the opening session was devoted to research documenting the extent to which COVID-19 survivors subsequently meet ME/CFS criteria, and to exploring underlying mechanisms.
“It offers a lot of opportunities for us to study potentially early ME/CFS and how it develops, but in addition, a lot of the research that has been done on ME/CFS may also provide answers for COVID-19,” IACFS/ME vice president Lily Chu, MD, said in an interview.
A hint from the SARS outbreak
This isn’t the first time researchers have seen a possible link between a coronavirus and ME/CFS, Harvey Moldofsky, MD, told attendees. To illustrate that point, Dr. Moldofsky, of the department of psychiatry (emeritus) at the University of Toronto, reviewed data from a previously published case-controlled study, which included 22 health care workers who had been infected in 2003 with SARS-CoV-1 and continued to report chronic fatigue, musculoskeletal pain, and disturbed and unrefreshing sleep with EEG-documented sleep disturbances 1-3 years following the illness. None had been able to return to work by 1 year.
“We’re looking at similar symptoms now” among survivors of COVID-19, Dr. Moldofsky said. “[T]he key issue is that we have no idea of its prevalence. … We need epidemiologic studies.”
Distinguishing ME/CFS from other post–COVID-19 symptoms
Not everyone who has persistent symptoms after COVID-19 will develop ME/CFS, and distinguishing between cases may be important.
Clinically, Dr. Chu said, one way to assess whether a patient with persistent COVID-19 symptoms might be progressing to ME/CFS is to ask him or her specifically about the level of fatigue following physical exertion and the timing of any fatigue. With ME/CFS, postexertional malaise often involves a dramatic exacerbation of symptoms such as fatigue, pain, and cognitive impairment a day or 2 after exertion rather than immediately following it. In contrast, shortness of breath during exertion isn’t typical of ME/CFS.
Objective measures of ME/CFS include low natural killer cell function (the test can be ordered from commercial labs but requires rapid transport of the blood sample), and autonomic dysfunction assessed by a tilt-table test.
While there is currently no cure for ME/CFS, diagnosing it allows for the patient to be taught “pacing” in which the person conserves his or her energy by balancing activity with rest. “That type of behavioral technique is valuable for everyone who suffers from a chronic disease with fatigue. It can help them be more functional,” Dr. Chu said.
If a patient appears to be exhibiting signs of ME/CFS, “don’t wait until they hit the 6-month mark to start helping them manage their symptoms,” she said. “Teaching pacing to COVID-19 patients who have a lot of fatigue isn’t going to harm them. As they get better they’re going to just naturally do more. But if they do have ME/CFS, [pacing] stresses their system less, since the data seem to be pointing to deficiencies in producing energy.”
Will COVID-19 unleash a new wave of ME/CFS patients?
Much of the session at the virtual meeting was devoted to ongoing studies. For example, Leonard Jason, PhD, of the Center for Community Research at DePaul University, Chicago, described a prospective study launched in 2014 that looked at risk factors for developing ME/CFS in college students who contracted infectious mononucleosis as a result of Epstein-Barr virus. Now, his team is also following students from the same cohort who develop COVID-19.
Because the study included collection of baseline biological samples, the results could help reveal predisposing factors associated with long-term illness from either virus.
Another project, funded by the Open Medicine Foundation, will follow patients who are discharged from the ICU following severe COVID-19 illness. Blood, urine, and cerebrospinal fluid will be collected from those with persistent symptoms at 6 months, along with questionnaire data. At 18-24 months, those who continue to report symptoms will undergo more intensive evaluation using genomics, metabolomics, and proteomics.
“We’re taking advantage of this horrible situation, hoping to understand how a serious viral infection might lead to ME/CFS,” said lead investigator Ronald Tompkins, MD, ScD, chief medical officer at the Open Medicine Foundation and a faculty member at Harvard Medical School, Boston. The results, he said, “might give us insight into potential drug targets or biomarkers useful for prevention and treatment strategies.”
Meanwhile, Sadie Whittaker, PhD, head of the Solve ME/CFS initiative, described her organization’s new plan to use their registry to prospectively track the impact of COVID-19 on people with ME/CFS.
She noted that they’ve also teamed up with “long-COVID” communities including Body Politic. “Our goal is to form a coalition to study together or at least harmonize data … and understand what’s going on through the power of bigger sample sizes,” Dr. Whittaker said.
None of the speakers disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Some patients who had COVID-19 continue to have symptoms weeks to months later, even after they no longer test positive for the virus. In two recent reports – one published in JAMA in July and another published in Morbidity and Mortality Weekly Report in August – chronic fatigue was listed as the top symptom among individuals still feeling unwell beyond 2 weeks after COVID-19 onset.
Although some of the reported persistent symptoms appear specific to SARS-CoV-2 – such as cough, chest pain, and dyspnea – others overlap with the diagnostic criteria for ME/CFS, which is defined by substantial, profound fatigue for at least 6 months, postexertional malaise, unrefreshing sleep, and one or both of orthostatic intolerance and/or cognitive impairment. Although the etiology of ME/CFS is unclear, the condition commonly arises following a viral illness.
At the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis August 21, the opening session was devoted to research documenting the extent to which COVID-19 survivors subsequently meet ME/CFS criteria, and to exploring underlying mechanisms.
“It offers a lot of opportunities for us to study potentially early ME/CFS and how it develops, but in addition, a lot of the research that has been done on ME/CFS may also provide answers for COVID-19,” IACFS/ME vice president Lily Chu, MD, said in an interview.
A hint from the SARS outbreak
This isn’t the first time researchers have seen a possible link between a coronavirus and ME/CFS, Harvey Moldofsky, MD, told attendees. To illustrate that point, Dr. Moldofsky, of the department of psychiatry (emeritus) at the University of Toronto, reviewed data from a previously published case-controlled study, which included 22 health care workers who had been infected in 2003 with SARS-CoV-1 and continued to report chronic fatigue, musculoskeletal pain, and disturbed and unrefreshing sleep with EEG-documented sleep disturbances 1-3 years following the illness. None had been able to return to work by 1 year.
“We’re looking at similar symptoms now” among survivors of COVID-19, Dr. Moldofsky said. “[T]he key issue is that we have no idea of its prevalence. … We need epidemiologic studies.”
Distinguishing ME/CFS from other post–COVID-19 symptoms
Not everyone who has persistent symptoms after COVID-19 will develop ME/CFS, and distinguishing between cases may be important.
Clinically, Dr. Chu said, one way to assess whether a patient with persistent COVID-19 symptoms might be progressing to ME/CFS is to ask him or her specifically about the level of fatigue following physical exertion and the timing of any fatigue. With ME/CFS, postexertional malaise often involves a dramatic exacerbation of symptoms such as fatigue, pain, and cognitive impairment a day or 2 after exertion rather than immediately following it. In contrast, shortness of breath during exertion isn’t typical of ME/CFS.
Objective measures of ME/CFS include low natural killer cell function (the test can be ordered from commercial labs but requires rapid transport of the blood sample), and autonomic dysfunction assessed by a tilt-table test.
While there is currently no cure for ME/CFS, diagnosing it allows for the patient to be taught “pacing” in which the person conserves his or her energy by balancing activity with rest. “That type of behavioral technique is valuable for everyone who suffers from a chronic disease with fatigue. It can help them be more functional,” Dr. Chu said.
If a patient appears to be exhibiting signs of ME/CFS, “don’t wait until they hit the 6-month mark to start helping them manage their symptoms,” she said. “Teaching pacing to COVID-19 patients who have a lot of fatigue isn’t going to harm them. As they get better they’re going to just naturally do more. But if they do have ME/CFS, [pacing] stresses their system less, since the data seem to be pointing to deficiencies in producing energy.”
Will COVID-19 unleash a new wave of ME/CFS patients?
Much of the session at the virtual meeting was devoted to ongoing studies. For example, Leonard Jason, PhD, of the Center for Community Research at DePaul University, Chicago, described a prospective study launched in 2014 that looked at risk factors for developing ME/CFS in college students who contracted infectious mononucleosis as a result of Epstein-Barr virus. Now, his team is also following students from the same cohort who develop COVID-19.
Because the study included collection of baseline biological samples, the results could help reveal predisposing factors associated with long-term illness from either virus.
Another project, funded by the Open Medicine Foundation, will follow patients who are discharged from the ICU following severe COVID-19 illness. Blood, urine, and cerebrospinal fluid will be collected from those with persistent symptoms at 6 months, along with questionnaire data. At 18-24 months, those who continue to report symptoms will undergo more intensive evaluation using genomics, metabolomics, and proteomics.
“We’re taking advantage of this horrible situation, hoping to understand how a serious viral infection might lead to ME/CFS,” said lead investigator Ronald Tompkins, MD, ScD, chief medical officer at the Open Medicine Foundation and a faculty member at Harvard Medical School, Boston. The results, he said, “might give us insight into potential drug targets or biomarkers useful for prevention and treatment strategies.”
Meanwhile, Sadie Whittaker, PhD, head of the Solve ME/CFS initiative, described her organization’s new plan to use their registry to prospectively track the impact of COVID-19 on people with ME/CFS.
She noted that they’ve also teamed up with “long-COVID” communities including Body Politic. “Our goal is to form a coalition to study together or at least harmonize data … and understand what’s going on through the power of bigger sample sizes,” Dr. Whittaker said.
None of the speakers disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FDA authorizes convalescent plasma for COVID-19
Convalescent plasma contains antibodies from the blood of recovered COVID-19 patients, which can be used to treat people with severe infections. Convalescent plasma has been used to treat patients for other infectious diseases. The authorization allows the plasma to be distributed in the United States and administered by health care providers.
“COVID-19 convalescent plasma is safe and shows promising efficacy,” Stephen Hahn, MD, commissioner of the FDA, said during a press briefing with President Donald Trump.
In April, the FDA approved a program to test convalescent plasma in COVID-19 patients at the Mayo Clinic, followed by other institutions. More than 90,000 patients have enrolled in the program, and 70,000 have received the treatment, Dr. Hahn said.
The data indicate that the plasma can reduce mortality in patients by 35%, particularly if patients are treated within 3 days of being diagnosed. Those who have benefited the most were under age 80 and not on artificial respiration, Alex Azar, the secretary for the Department of Health & Human Services, said during the briefing.
“We dream, in drug development, of something like a 35% mortality reduction,” he said.
But top scientists pushed back against the announcement.
Eric Topol, MD, director of the Scripps Research Translational Institute, professor of molecular medicine, and executive vice president of Scripps Research, said the data the FDA are relying on did not come from the rigorous randomized, double-blind placebo trials that best determine if a treatment is successful.
Still, convalescent plasma is “one more tool added to the arsenal” of combating COVID-19, Mr. Azar said. The FDA will continue to study convalescent plasma as a COVID-19 treatment, Dr. Hahn added.
“We’re waiting for more data. We’re going to continue to gather data,” Dr. Hahn said during the briefing, but the current results meet FDA criteria for issuing an emergency use authorization.
Convalescent plasma “may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients,” according to the FDA announcement. Potential side effects include allergic reactions, transfusion-transmitted infections, and transfusion-associated lung injury.
“We’ve seen a great deal of demand for this from doctors around the country,” Dr. Hahn said during the briefing. “The EUA … allows us to continue that and meet that demand.”
Dr. Topol, however, said it appears Trump and the FDA are playing politics with science.
“There’s no evidence to support any survival benefit,” Dr. Topol said on Twitter. “Two days ago [the] FDA’s website stated there was no evidence for an EUA.”
The American Red Cross and other blood centers put out a national call for blood donors in July, especially for patients who have recovered from COVID-19. Mr. Azar and Dr. Hahn emphasized the need for blood donors during the press briefing.
“If you donate plasma, you could save a life,” Mr. Azar said.
The study has not been peer reviewed and did not include a placebo group for comparison, STAT reported.
Last week several health officials warned that the scientific data were too weak to warrant an emergency authorization, the New York Times reported.
A version of this originally appeared on WebMD.com.
Convalescent plasma contains antibodies from the blood of recovered COVID-19 patients, which can be used to treat people with severe infections. Convalescent plasma has been used to treat patients for other infectious diseases. The authorization allows the plasma to be distributed in the United States and administered by health care providers.
“COVID-19 convalescent plasma is safe and shows promising efficacy,” Stephen Hahn, MD, commissioner of the FDA, said during a press briefing with President Donald Trump.
In April, the FDA approved a program to test convalescent plasma in COVID-19 patients at the Mayo Clinic, followed by other institutions. More than 90,000 patients have enrolled in the program, and 70,000 have received the treatment, Dr. Hahn said.
The data indicate that the plasma can reduce mortality in patients by 35%, particularly if patients are treated within 3 days of being diagnosed. Those who have benefited the most were under age 80 and not on artificial respiration, Alex Azar, the secretary for the Department of Health & Human Services, said during the briefing.
“We dream, in drug development, of something like a 35% mortality reduction,” he said.
But top scientists pushed back against the announcement.
Eric Topol, MD, director of the Scripps Research Translational Institute, professor of molecular medicine, and executive vice president of Scripps Research, said the data the FDA are relying on did not come from the rigorous randomized, double-blind placebo trials that best determine if a treatment is successful.
Still, convalescent plasma is “one more tool added to the arsenal” of combating COVID-19, Mr. Azar said. The FDA will continue to study convalescent plasma as a COVID-19 treatment, Dr. Hahn added.
“We’re waiting for more data. We’re going to continue to gather data,” Dr. Hahn said during the briefing, but the current results meet FDA criteria for issuing an emergency use authorization.
Convalescent plasma “may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients,” according to the FDA announcement. Potential side effects include allergic reactions, transfusion-transmitted infections, and transfusion-associated lung injury.
“We’ve seen a great deal of demand for this from doctors around the country,” Dr. Hahn said during the briefing. “The EUA … allows us to continue that and meet that demand.”
Dr. Topol, however, said it appears Trump and the FDA are playing politics with science.
“There’s no evidence to support any survival benefit,” Dr. Topol said on Twitter. “Two days ago [the] FDA’s website stated there was no evidence for an EUA.”
The American Red Cross and other blood centers put out a national call for blood donors in July, especially for patients who have recovered from COVID-19. Mr. Azar and Dr. Hahn emphasized the need for blood donors during the press briefing.
“If you donate plasma, you could save a life,” Mr. Azar said.
The study has not been peer reviewed and did not include a placebo group for comparison, STAT reported.
Last week several health officials warned that the scientific data were too weak to warrant an emergency authorization, the New York Times reported.
A version of this originally appeared on WebMD.com.
Convalescent plasma contains antibodies from the blood of recovered COVID-19 patients, which can be used to treat people with severe infections. Convalescent plasma has been used to treat patients for other infectious diseases. The authorization allows the plasma to be distributed in the United States and administered by health care providers.
“COVID-19 convalescent plasma is safe and shows promising efficacy,” Stephen Hahn, MD, commissioner of the FDA, said during a press briefing with President Donald Trump.
In April, the FDA approved a program to test convalescent plasma in COVID-19 patients at the Mayo Clinic, followed by other institutions. More than 90,000 patients have enrolled in the program, and 70,000 have received the treatment, Dr. Hahn said.
The data indicate that the plasma can reduce mortality in patients by 35%, particularly if patients are treated within 3 days of being diagnosed. Those who have benefited the most were under age 80 and not on artificial respiration, Alex Azar, the secretary for the Department of Health & Human Services, said during the briefing.
“We dream, in drug development, of something like a 35% mortality reduction,” he said.
But top scientists pushed back against the announcement.
Eric Topol, MD, director of the Scripps Research Translational Institute, professor of molecular medicine, and executive vice president of Scripps Research, said the data the FDA are relying on did not come from the rigorous randomized, double-blind placebo trials that best determine if a treatment is successful.
Still, convalescent plasma is “one more tool added to the arsenal” of combating COVID-19, Mr. Azar said. The FDA will continue to study convalescent plasma as a COVID-19 treatment, Dr. Hahn added.
“We’re waiting for more data. We’re going to continue to gather data,” Dr. Hahn said during the briefing, but the current results meet FDA criteria for issuing an emergency use authorization.
Convalescent plasma “may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients,” according to the FDA announcement. Potential side effects include allergic reactions, transfusion-transmitted infections, and transfusion-associated lung injury.
“We’ve seen a great deal of demand for this from doctors around the country,” Dr. Hahn said during the briefing. “The EUA … allows us to continue that and meet that demand.”
Dr. Topol, however, said it appears Trump and the FDA are playing politics with science.
“There’s no evidence to support any survival benefit,” Dr. Topol said on Twitter. “Two days ago [the] FDA’s website stated there was no evidence for an EUA.”
The American Red Cross and other blood centers put out a national call for blood donors in July, especially for patients who have recovered from COVID-19. Mr. Azar and Dr. Hahn emphasized the need for blood donors during the press briefing.
“If you donate plasma, you could save a life,” Mr. Azar said.
The study has not been peer reviewed and did not include a placebo group for comparison, STAT reported.
Last week several health officials warned that the scientific data were too weak to warrant an emergency authorization, the New York Times reported.
A version of this originally appeared on WebMD.com.
Serum cortisol testing for suspected adrenal insufficiency
Evaluating the hospitalized adult patient
Case
A 45-year-old female with moderate persistent asthma is admitted for right lower extremity cellulitis. She has hyponatremia with a sodium of 129 mEq/L and reports a history of longstanding fatigue and lightheadedness on standing. An early morning serum cortisol was 10 mcg/dL, normal per the reference range for the laboratory. Has adrenal insufficiency been excluded in this patient?
Overview
Adrenal insufficiency (AI) is a clinical syndrome characterized by a deficiency of cortisol. Presentation may range from nonspecific symptoms such as fatigue, weight loss, and gastrointestinal concerns to a fulminant adrenal crisis with severe weakness and hypotension (Table 1). The diagnosis of AI is commonly delayed, negatively impacting patients’ quality of life and risking dangerous complications.1,2
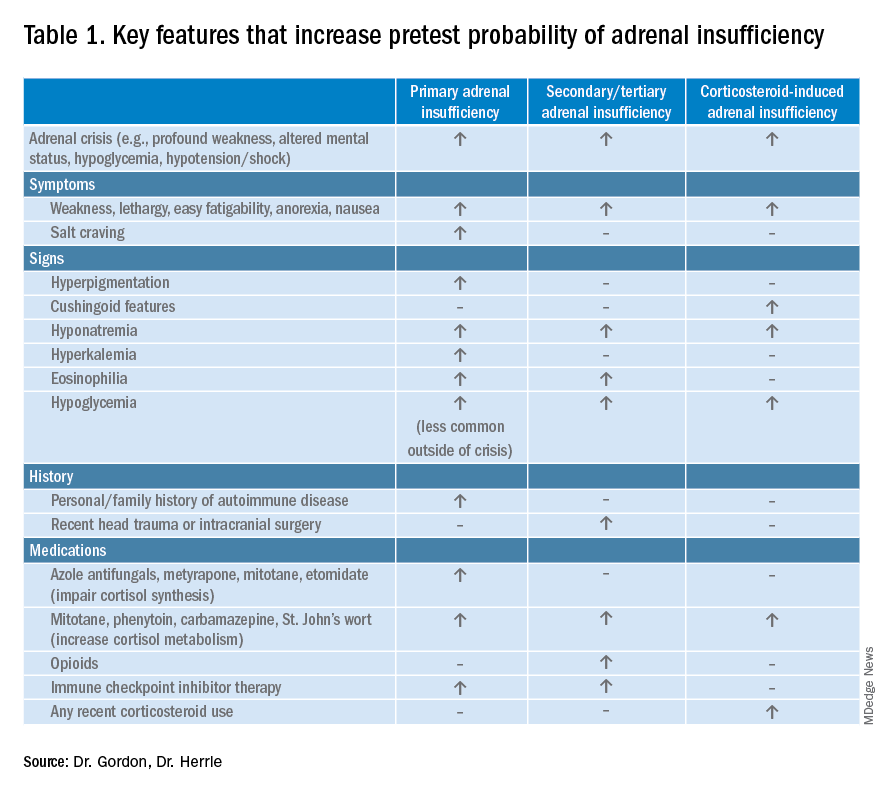
AI can occur due to diseases of the adrenal glands themselves (primary) or impairment of adrenocorticotropin (ACTH) secretion from the pituitary (secondary) or corticotropin-releasing hormone (CRH) secretion from the hypothalamus (tertiary). In the hospital setting, causes of primary AI may include autoimmune disease, infection, metastatic disease, hemorrhage, and adverse medication effects. Secondary and tertiary AI would be of particular concern for patients with traumatic brain injuries or pituitary surgery, but also are seen commonly as a result of adverse medication effects in the hospitalized patient, notably opioids and corticosteroids through suppression the hypothalamic-pituitary-adrenal (HPA) axis and immune checkpoint inhibitors via autoimmune hypophysitis.
Testing for AI in the hospitalized patient presents a host of challenges. Among these are the variability in presentation of different types of AI, high rates of exogenous corticosteroid use, the impact of critical illness on the HPA axis, medical illness altering protein binding of serum cortisol, interfering medications, the variation in assays used by laboratories, and the logistical challenges of obtaining appropriately timed phlebotomy.2,3
Cortisol testing
An intact HPA axis results in ACTH-dependent cortisol release from the adrenal glands. Cortisol secretion exhibits circadian rhythm, with the highest levels in the early morning (6 a.m. to 8 a.m.) and the lowest at night (12 a.m.). It also is pulsatile, which may explain the range of “normal” morning serum cortisol observed in a study of healthy volunteers.3 Note that serum cortisol is equivalent to plasma cortisol in current immunoassays, and will henceforth be called “cortisol” in this paper.3
There are instances when morning cortisol may strongly suggest a diagnosis of AI on its own. A meta-analysis found that morning cortisol of < 5 mcg/dL predicts AI and morning cortisol of > 13 mcg/dL ruled out AI.4 The Endocrine Society of America favors dynamic assessment of adrenal function for most patients.2
Historically, the gold standard for assessing dynamic adrenal function has been the insulin tolerance test (ITT), whereby cortisol is measured after inducing hypoglycemia to a blood glucose < 35 mg/dL. ITT is logistically difficult and poses some risk to the patient. The corticotropin (or cosyntropin) stimulation test (CST), in which a supraphysiologic dose of a synthetic ACTH analog is administered parenterally to a patient and resultant cortisol levels are measured, has been validated against the ITT and is generally preferred.5 CST is used to diagnose primary AI as well as chronic secondary and tertiary AI, given that longstanding lack of ACTH stimulation causes atrophy of the adrenal glands. The sensitivity for secondary and tertiary AI is likely lower than primary AI especially in acute onset of disease.6,7
In performance of the CST a baseline cortisol and ACTH are obtained, with subsequent cortisol testing at 30 and/or 60 minutes after administration of the ACTH analog (Figure 1). Currently, there is no consensus for which time point is preferred, but the 30-minute test is more sensitive for AI and the 60-minute test is more specific.2,7,8
CST is typically performed using a “standard high dose” of 250 mcg of the ACTH analog. There has been interest in the use of a “low-dose” 1 mcg test, which is closer to normal physiologic stimulation of the adrenal glands and may have better sensitivity for early secondary or partial AI. However, the 250-mcg dose is easier to prepare and has fewer technical pitfalls in administration as well as a lower risk for false positive testing. At this point the data do not compellingly favor the use of low-dose CST testing in general practice.2,3,7
Clinical decision making
Diagnostic evaluation should be guided by the likelihood of the disease (i.e., the pretest probability) (Figure 1). Begin with a review of the patient’s signs and symptoms, medical and family history, and medications with special consideration for opioids, exogenous steroids, and immune checkpoint inhibitors (Table 1).
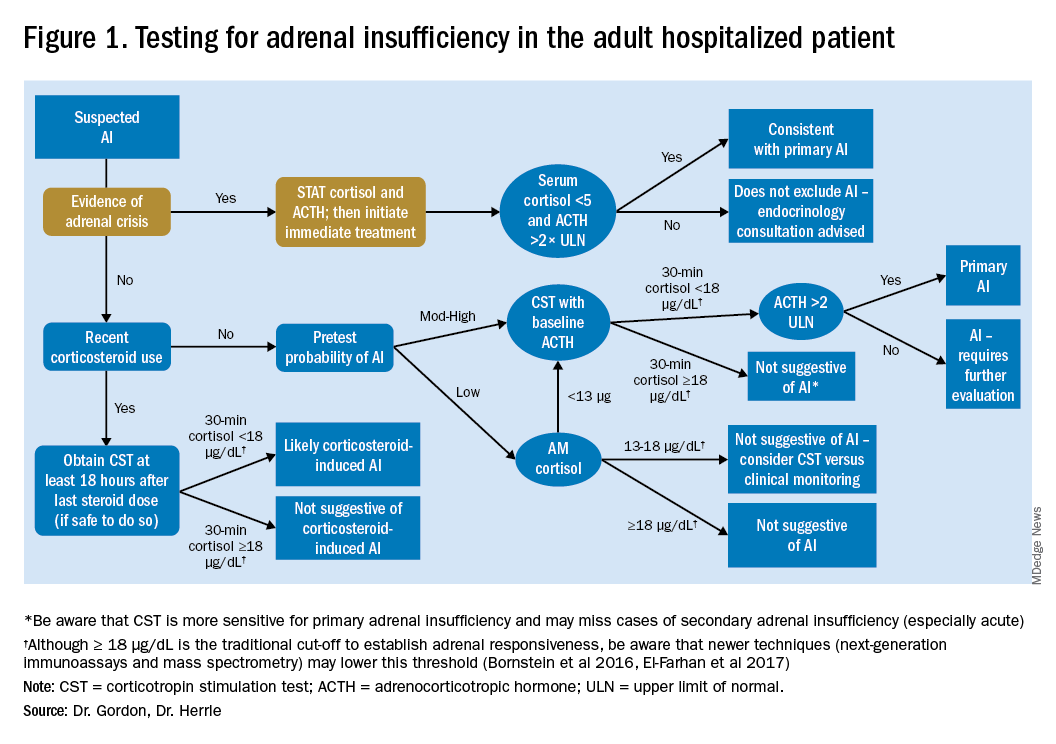
For patients with low pretest probability for AI, morning cortisol and ACTH is a reasonable first test (Figure 1). A cortisol value of 18 mcg/dL or greater does not support AI and no further testing is needed.2 Patients with morning cortisol of 13-18 mcg/dL could be followed clinically or could undergo further testing in the inpatient environment with CST, depending upon the clinical scenario.4 Patients with serum cortisol of <13 mcg/dL warrant CST.
For patients with moderate to high pretest probability for AI, we recommend initial testing with CST. While the results of high-dose CST are not necessarily impacted by time of day, if an a.m. cortisol has not yet been obtained and it is logistically feasible to do so, performing CST in the morning will provide the most useful data for clinical interpretation.
For patients presenting with possible adrenal crisis, it is essential not to delay treatment. In these patients, obtain a cortisol paired with ACTH and initiate treatment immediately. Further testing can be deferred until the time the patient is stable.2
Potential pitfalls
Interpreting cortisol requires awareness of multiple conditions that could directly impact the results.2,3 (Table 2).
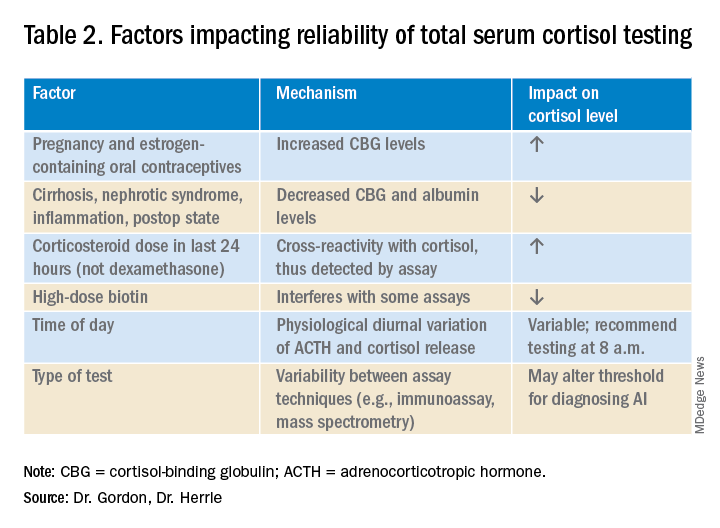
Currently available assays measure “total cortisol,” most of which is protein bound (cortisol-binding globulin as well as albumin). Therefore, conditions that lower serum protein (e.g., nephrotic syndrome, liver disease, inflammation) will lower the measured cortisol. Conversely, conditions that increase serum protein (e.g., estrogen excess in pregnancy and oral contraceptive use) will increase the measured cortisol.2,3
It is also important to recognize that existing immunoassay testing techniques informed the established cut-off for exclusion of AI at 18 mcg/dL. With newer immunoassays and emerging liquid chromatography/tandem mass spectrometry, this cut-off may be lowered; thus the assay should be confirmed with the performing laboratory. There is emerging evidence that serum or plasma free cortisol and salivary cortisol testing for AI may be useful in certain cases, but these techniques are not yet widespread or included in clinical practice guidelines.2,3,7
Population focus: Patients on exogenous steroids
Exogenous corticosteroids suppress the HPA axis via negative inhibition of CRH and ACTH release, often resulting in low endogenous cortisol levels which may or may not reflect true loss of adrenal function. In addition, many corticosteroids will be detected by standard serum cortisol tests that rely on immunoassays. For this reason, cortisol measurement and CST should be done at least 18-24 hours after the last dose of exogenous steroids.
Although the focus has been on higher doses and longer courses of steroids (e.g., chronic use of ≥ 5 mg prednisone daily, or ≥ 20 mg prednisone daily for > 3 weeks), there is increasing evidence that lower doses, shorter courses, and alternate routes (e.g., inhaled, intra-articular) can result in biochemical and clinical evidence of AI.9 Thus, a thorough history and exam should be obtained to determine all recent corticosteroid exposure and cushingoid features.
Application of the data to the case
To effectively assess the patient for adrenal insufficiency, we need additional information. First and foremost, is a description of the patient’s current clinical status. If she is demonstrating evidence of adrenal crisis, treatment should not be delayed for additional testing. If she is stable, a thorough history including use of corticosteroids by any route, pregnancy, oral contraceptives, recent surgery, and liver and kidney disease is essential.
Additional evaluation reveals the patient has been using her fluticasone inhaler daily. No other source of hyponatremia or lightheadedness is identified. The patient’s risk factors of corticosteroid use and unexplained hyponatremia with associated lightheadedness increase her pretest probability of AI and a single morning cortisol of 10 mcg/dL is insufficient to exclude adrenal insufficiency. The appropriate follow-up test is a standard high-dose cosyntropin stimulation test at least 18 hours after her last dose of fluticasone. A cortisol level > 18 mcg/dL at 30 minutes in the absence of other conditions that impact cortisol testing would not be suggestive of AI. A serum cortisol level of < 18 mcg/dL at 30 minutes would raise concern for abnormal adrenal reserve due to chronic corticosteroid therapy and would warrant referral to an endocrinologist.
Bottom line
An isolated serum cortisol is often insufficient to exclude adrenal insufficiency. Hospitalists should be aware of the many factors that impact the interpretation of this test.
Dr. Gordon is assistant professor of medicine at Tufts University, Boston, and a hospitalist at Maine Medical Center, Portland. She is the subspecialty education coordinator of inpatient medicine for the Internal Medicine Residency Program. Dr. Herrle is assistant professor of medicine at Tufts University and a hospitalist at Maine Medical Center. She is the associate director of medical student education for the department of internal medicine at MMC and a medical director for clinical informatics at MaineHealth.
References
1. Bleicken B et al. Delayed diagnosis of adrenal insufficiency is common: A cross-sectional study in 216 patients. Am J Med Sci. 2010;339(6):525-31. doi: 10.1097/MAJ.0b013e3181db6b7a.
2. Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
3. El-Farhan N et al. Measuring cortisol in serum, urine and saliva – Are our assays good enough? Ann Clin Biochem. 2017 May;54(3):308-22. doi: 10.1177/0004563216687335.
4. Kazlauskaite R et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: A metaanalysis. J Clin Endocrinol Metab. 2008;93:4245-53.
5. Wood JB et al. A rapid test of adrenocortical function. Lancet. 1965;191:243-5.
6. Singh Ospina N et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427-34.
7. Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
8. Odom DC et al. A Single, post-ACTH cortisol measurement to screen for adrenal insufficiency in the hospitalized patient. J Hosp Med. 2018;13(8):526-30. doi: 10.12788/jhm.2928.
9. Broersen LHA et al. Adrenal insufficiency in corticosteroids use: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(6): 2171-80.
Key points
• In general, random cortisol testing is of limited value and should be avoided.
• Serum cortisol testing in the hospitalized patient is impacted by a variety of patient and disease factors and should be interpreted carefully.
• For patients with low pretest probability of adrenal insufficiency, early morning serum cortisol testing may be sufficient to exclude the diagnosis.
• For patients with moderate to high pretest probability of adrenal insufficiency, standard high-dose (250 mcg) corticotropin stimulation testing is preferred.
Additional reading
Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
Quiz
An 82 y.o. woman with depression is admitted from her long-term care facility with worsening weakness and mild hypoglycemia. Her supine vital signs are stable, but she exhibits a drop in systolic blood pressure of 21 mm Hg upon standing. There is no evidence of infection by history, exam, or initial workup. She is not on chronic corticosteroids by any route.
What would be your initial workup for adrenal insufficiency?
A) Morning serum cortisol and ACTH
B) Insulin tolerance test
C) Corticotropin stimulation test
D) Would not test at this point
Answer: C. Although her symptom of weakness is nonspecific, her hypoglycemia and orthostatic hypotension are concerning enough that she would qualify as moderate to high pretest probability for AI. In this setting, one would acquire a basal serum total cortisol and ACTH then administer the standard high-dose corticotropin stimulation test (250 mcg) followed by repeat serum total cortisol at 30 or 60 minutes.
Evaluating the hospitalized adult patient
Evaluating the hospitalized adult patient
Case
A 45-year-old female with moderate persistent asthma is admitted for right lower extremity cellulitis. She has hyponatremia with a sodium of 129 mEq/L and reports a history of longstanding fatigue and lightheadedness on standing. An early morning serum cortisol was 10 mcg/dL, normal per the reference range for the laboratory. Has adrenal insufficiency been excluded in this patient?
Overview
Adrenal insufficiency (AI) is a clinical syndrome characterized by a deficiency of cortisol. Presentation may range from nonspecific symptoms such as fatigue, weight loss, and gastrointestinal concerns to a fulminant adrenal crisis with severe weakness and hypotension (Table 1). The diagnosis of AI is commonly delayed, negatively impacting patients’ quality of life and risking dangerous complications.1,2

AI can occur due to diseases of the adrenal glands themselves (primary) or impairment of adrenocorticotropin (ACTH) secretion from the pituitary (secondary) or corticotropin-releasing hormone (CRH) secretion from the hypothalamus (tertiary). In the hospital setting, causes of primary AI may include autoimmune disease, infection, metastatic disease, hemorrhage, and adverse medication effects. Secondary and tertiary AI would be of particular concern for patients with traumatic brain injuries or pituitary surgery, but also are seen commonly as a result of adverse medication effects in the hospitalized patient, notably opioids and corticosteroids through suppression the hypothalamic-pituitary-adrenal (HPA) axis and immune checkpoint inhibitors via autoimmune hypophysitis.
Testing for AI in the hospitalized patient presents a host of challenges. Among these are the variability in presentation of different types of AI, high rates of exogenous corticosteroid use, the impact of critical illness on the HPA axis, medical illness altering protein binding of serum cortisol, interfering medications, the variation in assays used by laboratories, and the logistical challenges of obtaining appropriately timed phlebotomy.2,3
Cortisol testing
An intact HPA axis results in ACTH-dependent cortisol release from the adrenal glands. Cortisol secretion exhibits circadian rhythm, with the highest levels in the early morning (6 a.m. to 8 a.m.) and the lowest at night (12 a.m.). It also is pulsatile, which may explain the range of “normal” morning serum cortisol observed in a study of healthy volunteers.3 Note that serum cortisol is equivalent to plasma cortisol in current immunoassays, and will henceforth be called “cortisol” in this paper.3
There are instances when morning cortisol may strongly suggest a diagnosis of AI on its own. A meta-analysis found that morning cortisol of < 5 mcg/dL predicts AI and morning cortisol of > 13 mcg/dL ruled out AI.4 The Endocrine Society of America favors dynamic assessment of adrenal function for most patients.2
Historically, the gold standard for assessing dynamic adrenal function has been the insulin tolerance test (ITT), whereby cortisol is measured after inducing hypoglycemia to a blood glucose < 35 mg/dL. ITT is logistically difficult and poses some risk to the patient. The corticotropin (or cosyntropin) stimulation test (CST), in which a supraphysiologic dose of a synthetic ACTH analog is administered parenterally to a patient and resultant cortisol levels are measured, has been validated against the ITT and is generally preferred.5 CST is used to diagnose primary AI as well as chronic secondary and tertiary AI, given that longstanding lack of ACTH stimulation causes atrophy of the adrenal glands. The sensitivity for secondary and tertiary AI is likely lower than primary AI especially in acute onset of disease.6,7
In performance of the CST a baseline cortisol and ACTH are obtained, with subsequent cortisol testing at 30 and/or 60 minutes after administration of the ACTH analog (Figure 1). Currently, there is no consensus for which time point is preferred, but the 30-minute test is more sensitive for AI and the 60-minute test is more specific.2,7,8
CST is typically performed using a “standard high dose” of 250 mcg of the ACTH analog. There has been interest in the use of a “low-dose” 1 mcg test, which is closer to normal physiologic stimulation of the adrenal glands and may have better sensitivity for early secondary or partial AI. However, the 250-mcg dose is easier to prepare and has fewer technical pitfalls in administration as well as a lower risk for false positive testing. At this point the data do not compellingly favor the use of low-dose CST testing in general practice.2,3,7
Clinical decision making
Diagnostic evaluation should be guided by the likelihood of the disease (i.e., the pretest probability) (Figure 1). Begin with a review of the patient’s signs and symptoms, medical and family history, and medications with special consideration for opioids, exogenous steroids, and immune checkpoint inhibitors (Table 1).

For patients with low pretest probability for AI, morning cortisol and ACTH is a reasonable first test (Figure 1). A cortisol value of 18 mcg/dL or greater does not support AI and no further testing is needed.2 Patients with morning cortisol of 13-18 mcg/dL could be followed clinically or could undergo further testing in the inpatient environment with CST, depending upon the clinical scenario.4 Patients with serum cortisol of <13 mcg/dL warrant CST.
For patients with moderate to high pretest probability for AI, we recommend initial testing with CST. While the results of high-dose CST are not necessarily impacted by time of day, if an a.m. cortisol has not yet been obtained and it is logistically feasible to do so, performing CST in the morning will provide the most useful data for clinical interpretation.
For patients presenting with possible adrenal crisis, it is essential not to delay treatment. In these patients, obtain a cortisol paired with ACTH and initiate treatment immediately. Further testing can be deferred until the time the patient is stable.2
Potential pitfalls
Interpreting cortisol requires awareness of multiple conditions that could directly impact the results.2,3 (Table 2).

Currently available assays measure “total cortisol,” most of which is protein bound (cortisol-binding globulin as well as albumin). Therefore, conditions that lower serum protein (e.g., nephrotic syndrome, liver disease, inflammation) will lower the measured cortisol. Conversely, conditions that increase serum protein (e.g., estrogen excess in pregnancy and oral contraceptive use) will increase the measured cortisol.2,3
It is also important to recognize that existing immunoassay testing techniques informed the established cut-off for exclusion of AI at 18 mcg/dL. With newer immunoassays and emerging liquid chromatography/tandem mass spectrometry, this cut-off may be lowered; thus the assay should be confirmed with the performing laboratory. There is emerging evidence that serum or plasma free cortisol and salivary cortisol testing for AI may be useful in certain cases, but these techniques are not yet widespread or included in clinical practice guidelines.2,3,7
Population focus: Patients on exogenous steroids
Exogenous corticosteroids suppress the HPA axis via negative inhibition of CRH and ACTH release, often resulting in low endogenous cortisol levels which may or may not reflect true loss of adrenal function. In addition, many corticosteroids will be detected by standard serum cortisol tests that rely on immunoassays. For this reason, cortisol measurement and CST should be done at least 18-24 hours after the last dose of exogenous steroids.
Although the focus has been on higher doses and longer courses of steroids (e.g., chronic use of ≥ 5 mg prednisone daily, or ≥ 20 mg prednisone daily for > 3 weeks), there is increasing evidence that lower doses, shorter courses, and alternate routes (e.g., inhaled, intra-articular) can result in biochemical and clinical evidence of AI.9 Thus, a thorough history and exam should be obtained to determine all recent corticosteroid exposure and cushingoid features.
Application of the data to the case
To effectively assess the patient for adrenal insufficiency, we need additional information. First and foremost, is a description of the patient’s current clinical status. If she is demonstrating evidence of adrenal crisis, treatment should not be delayed for additional testing. If she is stable, a thorough history including use of corticosteroids by any route, pregnancy, oral contraceptives, recent surgery, and liver and kidney disease is essential.
Additional evaluation reveals the patient has been using her fluticasone inhaler daily. No other source of hyponatremia or lightheadedness is identified. The patient’s risk factors of corticosteroid use and unexplained hyponatremia with associated lightheadedness increase her pretest probability of AI and a single morning cortisol of 10 mcg/dL is insufficient to exclude adrenal insufficiency. The appropriate follow-up test is a standard high-dose cosyntropin stimulation test at least 18 hours after her last dose of fluticasone. A cortisol level > 18 mcg/dL at 30 minutes in the absence of other conditions that impact cortisol testing would not be suggestive of AI. A serum cortisol level of < 18 mcg/dL at 30 minutes would raise concern for abnormal adrenal reserve due to chronic corticosteroid therapy and would warrant referral to an endocrinologist.
Bottom line
An isolated serum cortisol is often insufficient to exclude adrenal insufficiency. Hospitalists should be aware of the many factors that impact the interpretation of this test.
Dr. Gordon is assistant professor of medicine at Tufts University, Boston, and a hospitalist at Maine Medical Center, Portland. She is the subspecialty education coordinator of inpatient medicine for the Internal Medicine Residency Program. Dr. Herrle is assistant professor of medicine at Tufts University and a hospitalist at Maine Medical Center. She is the associate director of medical student education for the department of internal medicine at MMC and a medical director for clinical informatics at MaineHealth.
References
1. Bleicken B et al. Delayed diagnosis of adrenal insufficiency is common: A cross-sectional study in 216 patients. Am J Med Sci. 2010;339(6):525-31. doi: 10.1097/MAJ.0b013e3181db6b7a.
2. Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
3. El-Farhan N et al. Measuring cortisol in serum, urine and saliva – Are our assays good enough? Ann Clin Biochem. 2017 May;54(3):308-22. doi: 10.1177/0004563216687335.
4. Kazlauskaite R et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: A metaanalysis. J Clin Endocrinol Metab. 2008;93:4245-53.
5. Wood JB et al. A rapid test of adrenocortical function. Lancet. 1965;191:243-5.
6. Singh Ospina N et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427-34.
7. Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
8. Odom DC et al. A Single, post-ACTH cortisol measurement to screen for adrenal insufficiency in the hospitalized patient. J Hosp Med. 2018;13(8):526-30. doi: 10.12788/jhm.2928.
9. Broersen LHA et al. Adrenal insufficiency in corticosteroids use: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(6): 2171-80.
Key points
• In general, random cortisol testing is of limited value and should be avoided.
• Serum cortisol testing in the hospitalized patient is impacted by a variety of patient and disease factors and should be interpreted carefully.
• For patients with low pretest probability of adrenal insufficiency, early morning serum cortisol testing may be sufficient to exclude the diagnosis.
• For patients with moderate to high pretest probability of adrenal insufficiency, standard high-dose (250 mcg) corticotropin stimulation testing is preferred.
Additional reading
Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
Quiz
An 82 y.o. woman with depression is admitted from her long-term care facility with worsening weakness and mild hypoglycemia. Her supine vital signs are stable, but she exhibits a drop in systolic blood pressure of 21 mm Hg upon standing. There is no evidence of infection by history, exam, or initial workup. She is not on chronic corticosteroids by any route.
What would be your initial workup for adrenal insufficiency?
A) Morning serum cortisol and ACTH
B) Insulin tolerance test
C) Corticotropin stimulation test
D) Would not test at this point
Answer: C. Although her symptom of weakness is nonspecific, her hypoglycemia and orthostatic hypotension are concerning enough that she would qualify as moderate to high pretest probability for AI. In this setting, one would acquire a basal serum total cortisol and ACTH then administer the standard high-dose corticotropin stimulation test (250 mcg) followed by repeat serum total cortisol at 30 or 60 minutes.
Case
A 45-year-old female with moderate persistent asthma is admitted for right lower extremity cellulitis. She has hyponatremia with a sodium of 129 mEq/L and reports a history of longstanding fatigue and lightheadedness on standing. An early morning serum cortisol was 10 mcg/dL, normal per the reference range for the laboratory. Has adrenal insufficiency been excluded in this patient?
Overview
Adrenal insufficiency (AI) is a clinical syndrome characterized by a deficiency of cortisol. Presentation may range from nonspecific symptoms such as fatigue, weight loss, and gastrointestinal concerns to a fulminant adrenal crisis with severe weakness and hypotension (Table 1). The diagnosis of AI is commonly delayed, negatively impacting patients’ quality of life and risking dangerous complications.1,2

AI can occur due to diseases of the adrenal glands themselves (primary) or impairment of adrenocorticotropin (ACTH) secretion from the pituitary (secondary) or corticotropin-releasing hormone (CRH) secretion from the hypothalamus (tertiary). In the hospital setting, causes of primary AI may include autoimmune disease, infection, metastatic disease, hemorrhage, and adverse medication effects. Secondary and tertiary AI would be of particular concern for patients with traumatic brain injuries or pituitary surgery, but also are seen commonly as a result of adverse medication effects in the hospitalized patient, notably opioids and corticosteroids through suppression the hypothalamic-pituitary-adrenal (HPA) axis and immune checkpoint inhibitors via autoimmune hypophysitis.
Testing for AI in the hospitalized patient presents a host of challenges. Among these are the variability in presentation of different types of AI, high rates of exogenous corticosteroid use, the impact of critical illness on the HPA axis, medical illness altering protein binding of serum cortisol, interfering medications, the variation in assays used by laboratories, and the logistical challenges of obtaining appropriately timed phlebotomy.2,3
Cortisol testing
An intact HPA axis results in ACTH-dependent cortisol release from the adrenal glands. Cortisol secretion exhibits circadian rhythm, with the highest levels in the early morning (6 a.m. to 8 a.m.) and the lowest at night (12 a.m.). It also is pulsatile, which may explain the range of “normal” morning serum cortisol observed in a study of healthy volunteers.3 Note that serum cortisol is equivalent to plasma cortisol in current immunoassays, and will henceforth be called “cortisol” in this paper.3
There are instances when morning cortisol may strongly suggest a diagnosis of AI on its own. A meta-analysis found that morning cortisol of < 5 mcg/dL predicts AI and morning cortisol of > 13 mcg/dL ruled out AI.4 The Endocrine Society of America favors dynamic assessment of adrenal function for most patients.2
Historically, the gold standard for assessing dynamic adrenal function has been the insulin tolerance test (ITT), whereby cortisol is measured after inducing hypoglycemia to a blood glucose < 35 mg/dL. ITT is logistically difficult and poses some risk to the patient. The corticotropin (or cosyntropin) stimulation test (CST), in which a supraphysiologic dose of a synthetic ACTH analog is administered parenterally to a patient and resultant cortisol levels are measured, has been validated against the ITT and is generally preferred.5 CST is used to diagnose primary AI as well as chronic secondary and tertiary AI, given that longstanding lack of ACTH stimulation causes atrophy of the adrenal glands. The sensitivity for secondary and tertiary AI is likely lower than primary AI especially in acute onset of disease.6,7
In performance of the CST a baseline cortisol and ACTH are obtained, with subsequent cortisol testing at 30 and/or 60 minutes after administration of the ACTH analog (Figure 1). Currently, there is no consensus for which time point is preferred, but the 30-minute test is more sensitive for AI and the 60-minute test is more specific.2,7,8
CST is typically performed using a “standard high dose” of 250 mcg of the ACTH analog. There has been interest in the use of a “low-dose” 1 mcg test, which is closer to normal physiologic stimulation of the adrenal glands and may have better sensitivity for early secondary or partial AI. However, the 250-mcg dose is easier to prepare and has fewer technical pitfalls in administration as well as a lower risk for false positive testing. At this point the data do not compellingly favor the use of low-dose CST testing in general practice.2,3,7
Clinical decision making
Diagnostic evaluation should be guided by the likelihood of the disease (i.e., the pretest probability) (Figure 1). Begin with a review of the patient’s signs and symptoms, medical and family history, and medications with special consideration for opioids, exogenous steroids, and immune checkpoint inhibitors (Table 1).

For patients with low pretest probability for AI, morning cortisol and ACTH is a reasonable first test (Figure 1). A cortisol value of 18 mcg/dL or greater does not support AI and no further testing is needed.2 Patients with morning cortisol of 13-18 mcg/dL could be followed clinically or could undergo further testing in the inpatient environment with CST, depending upon the clinical scenario.4 Patients with serum cortisol of <13 mcg/dL warrant CST.
For patients with moderate to high pretest probability for AI, we recommend initial testing with CST. While the results of high-dose CST are not necessarily impacted by time of day, if an a.m. cortisol has not yet been obtained and it is logistically feasible to do so, performing CST in the morning will provide the most useful data for clinical interpretation.
For patients presenting with possible adrenal crisis, it is essential not to delay treatment. In these patients, obtain a cortisol paired with ACTH and initiate treatment immediately. Further testing can be deferred until the time the patient is stable.2
Potential pitfalls
Interpreting cortisol requires awareness of multiple conditions that could directly impact the results.2,3 (Table 2).

Currently available assays measure “total cortisol,” most of which is protein bound (cortisol-binding globulin as well as albumin). Therefore, conditions that lower serum protein (e.g., nephrotic syndrome, liver disease, inflammation) will lower the measured cortisol. Conversely, conditions that increase serum protein (e.g., estrogen excess in pregnancy and oral contraceptive use) will increase the measured cortisol.2,3
It is also important to recognize that existing immunoassay testing techniques informed the established cut-off for exclusion of AI at 18 mcg/dL. With newer immunoassays and emerging liquid chromatography/tandem mass spectrometry, this cut-off may be lowered; thus the assay should be confirmed with the performing laboratory. There is emerging evidence that serum or plasma free cortisol and salivary cortisol testing for AI may be useful in certain cases, but these techniques are not yet widespread or included in clinical practice guidelines.2,3,7
Population focus: Patients on exogenous steroids
Exogenous corticosteroids suppress the HPA axis via negative inhibition of CRH and ACTH release, often resulting in low endogenous cortisol levels which may or may not reflect true loss of adrenal function. In addition, many corticosteroids will be detected by standard serum cortisol tests that rely on immunoassays. For this reason, cortisol measurement and CST should be done at least 18-24 hours after the last dose of exogenous steroids.
Although the focus has been on higher doses and longer courses of steroids (e.g., chronic use of ≥ 5 mg prednisone daily, or ≥ 20 mg prednisone daily for > 3 weeks), there is increasing evidence that lower doses, shorter courses, and alternate routes (e.g., inhaled, intra-articular) can result in biochemical and clinical evidence of AI.9 Thus, a thorough history and exam should be obtained to determine all recent corticosteroid exposure and cushingoid features.
Application of the data to the case
To effectively assess the patient for adrenal insufficiency, we need additional information. First and foremost, is a description of the patient’s current clinical status. If she is demonstrating evidence of adrenal crisis, treatment should not be delayed for additional testing. If she is stable, a thorough history including use of corticosteroids by any route, pregnancy, oral contraceptives, recent surgery, and liver and kidney disease is essential.
Additional evaluation reveals the patient has been using her fluticasone inhaler daily. No other source of hyponatremia or lightheadedness is identified. The patient’s risk factors of corticosteroid use and unexplained hyponatremia with associated lightheadedness increase her pretest probability of AI and a single morning cortisol of 10 mcg/dL is insufficient to exclude adrenal insufficiency. The appropriate follow-up test is a standard high-dose cosyntropin stimulation test at least 18 hours after her last dose of fluticasone. A cortisol level > 18 mcg/dL at 30 minutes in the absence of other conditions that impact cortisol testing would not be suggestive of AI. A serum cortisol level of < 18 mcg/dL at 30 minutes would raise concern for abnormal adrenal reserve due to chronic corticosteroid therapy and would warrant referral to an endocrinologist.
Bottom line
An isolated serum cortisol is often insufficient to exclude adrenal insufficiency. Hospitalists should be aware of the many factors that impact the interpretation of this test.
Dr. Gordon is assistant professor of medicine at Tufts University, Boston, and a hospitalist at Maine Medical Center, Portland. She is the subspecialty education coordinator of inpatient medicine for the Internal Medicine Residency Program. Dr. Herrle is assistant professor of medicine at Tufts University and a hospitalist at Maine Medical Center. She is the associate director of medical student education for the department of internal medicine at MMC and a medical director for clinical informatics at MaineHealth.
References
1. Bleicken B et al. Delayed diagnosis of adrenal insufficiency is common: A cross-sectional study in 216 patients. Am J Med Sci. 2010;339(6):525-31. doi: 10.1097/MAJ.0b013e3181db6b7a.
2. Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
3. El-Farhan N et al. Measuring cortisol in serum, urine and saliva – Are our assays good enough? Ann Clin Biochem. 2017 May;54(3):308-22. doi: 10.1177/0004563216687335.
4. Kazlauskaite R et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: A metaanalysis. J Clin Endocrinol Metab. 2008;93:4245-53.
5. Wood JB et al. A rapid test of adrenocortical function. Lancet. 1965;191:243-5.
6. Singh Ospina N et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427-34.
7. Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
8. Odom DC et al. A Single, post-ACTH cortisol measurement to screen for adrenal insufficiency in the hospitalized patient. J Hosp Med. 2018;13(8):526-30. doi: 10.12788/jhm.2928.
9. Broersen LHA et al. Adrenal insufficiency in corticosteroids use: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(6): 2171-80.
Key points
• In general, random cortisol testing is of limited value and should be avoided.
• Serum cortisol testing in the hospitalized patient is impacted by a variety of patient and disease factors and should be interpreted carefully.
• For patients with low pretest probability of adrenal insufficiency, early morning serum cortisol testing may be sufficient to exclude the diagnosis.
• For patients with moderate to high pretest probability of adrenal insufficiency, standard high-dose (250 mcg) corticotropin stimulation testing is preferred.
Additional reading
Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
Quiz
An 82 y.o. woman with depression is admitted from her long-term care facility with worsening weakness and mild hypoglycemia. Her supine vital signs are stable, but she exhibits a drop in systolic blood pressure of 21 mm Hg upon standing. There is no evidence of infection by history, exam, or initial workup. She is not on chronic corticosteroids by any route.
What would be your initial workup for adrenal insufficiency?
A) Morning serum cortisol and ACTH
B) Insulin tolerance test
C) Corticotropin stimulation test
D) Would not test at this point
Answer: C. Although her symptom of weakness is nonspecific, her hypoglycemia and orthostatic hypotension are concerning enough that she would qualify as moderate to high pretest probability for AI. In this setting, one would acquire a basal serum total cortisol and ACTH then administer the standard high-dose corticotropin stimulation test (250 mcg) followed by repeat serum total cortisol at 30 or 60 minutes.
‘The pandemic within the pandemic’
The coronavirus has infected millions of Americans and killed over 174,000. But could it be worse? Maybe.
“Racism is the pandemic within the pandemic,” Marc H. Morial, president and CEO of the National Urban League, said in the 2020 “State of Black America, Unmasked” report.
“Black people with COVID-19 symptoms in February and March were less likely to get tested or treated than white patients,” he wrote.
After less testing and less treatment, the next step seems inevitable. The death rate from COVID-19 is 70 per 100,000 population among Black Americans, compared with 30 per 100,000 for Whites and 34 per 100,000 for Hispanics, the league said based on data from the Johns Hopkins Center for Health Equity.
Black and Hispanic patients with COVID-19 are more likely to have preexisting health conditions, but they “tend to receive less aggressive treatment than white patients,” the report noted. The lower death rate among Hispanics may be explained by the Black population’s greater age, although Hispanic Americans have a higher infection rate (73 per 10,000) than Blacks (62 per 10,000) or Whites (23 per 10,000).
Another possible explanation for the differences in infection rates: Blacks and Hispanics are less able to work at home because they “are overrepresented in low-wage jobs that offer the least flexibility and increase their risk of exposure to the coronavirus,” the league said.
Hispanics and Blacks also are more likely to be uninsured than Whites – 19.5% and 11.5%, respectively, vs. 7.5% – so “they tend to delay seeking treatment and are sicker than white patients when they finally do,” the league said. That may account for their much higher COVID-19 hospitalization rates: 213 per 100,000 for Blacks, 205 for Hispanics, and 46 for Whites.
“The silver lining during these dark times is that this pandemic has revealed our shared vulnerability and our interconnectedness. Many people are beginning to see that when others don’t have the opportunity to be healthy, it puts all of us at risk,” Lisa Cooper, MD, James F. Fries Professor of Medicine and Bloomberg Distinguished Professor in Health Equity at Johns Hopkins University, Baltimore, wrote in an essay accompanying the report.
The coronavirus has infected millions of Americans and killed over 174,000. But could it be worse? Maybe.

“Racism is the pandemic within the pandemic,” Marc H. Morial, president and CEO of the National Urban League, said in the 2020 “State of Black America, Unmasked” report.
“Black people with COVID-19 symptoms in February and March were less likely to get tested or treated than white patients,” he wrote.
After less testing and less treatment, the next step seems inevitable. The death rate from COVID-19 is 70 per 100,000 population among Black Americans, compared with 30 per 100,000 for Whites and 34 per 100,000 for Hispanics, the league said based on data from the Johns Hopkins Center for Health Equity.
Black and Hispanic patients with COVID-19 are more likely to have preexisting health conditions, but they “tend to receive less aggressive treatment than white patients,” the report noted. The lower death rate among Hispanics may be explained by the Black population’s greater age, although Hispanic Americans have a higher infection rate (73 per 10,000) than Blacks (62 per 10,000) or Whites (23 per 10,000).
Another possible explanation for the differences in infection rates: Blacks and Hispanics are less able to work at home because they “are overrepresented in low-wage jobs that offer the least flexibility and increase their risk of exposure to the coronavirus,” the league said.
Hispanics and Blacks also are more likely to be uninsured than Whites – 19.5% and 11.5%, respectively, vs. 7.5% – so “they tend to delay seeking treatment and are sicker than white patients when they finally do,” the league said. That may account for their much higher COVID-19 hospitalization rates: 213 per 100,000 for Blacks, 205 for Hispanics, and 46 for Whites.
“The silver lining during these dark times is that this pandemic has revealed our shared vulnerability and our interconnectedness. Many people are beginning to see that when others don’t have the opportunity to be healthy, it puts all of us at risk,” Lisa Cooper, MD, James F. Fries Professor of Medicine and Bloomberg Distinguished Professor in Health Equity at Johns Hopkins University, Baltimore, wrote in an essay accompanying the report.
The coronavirus has infected millions of Americans and killed over 174,000. But could it be worse? Maybe.

“Racism is the pandemic within the pandemic,” Marc H. Morial, president and CEO of the National Urban League, said in the 2020 “State of Black America, Unmasked” report.
“Black people with COVID-19 symptoms in February and March were less likely to get tested or treated than white patients,” he wrote.
After less testing and less treatment, the next step seems inevitable. The death rate from COVID-19 is 70 per 100,000 population among Black Americans, compared with 30 per 100,000 for Whites and 34 per 100,000 for Hispanics, the league said based on data from the Johns Hopkins Center for Health Equity.
Black and Hispanic patients with COVID-19 are more likely to have preexisting health conditions, but they “tend to receive less aggressive treatment than white patients,” the report noted. The lower death rate among Hispanics may be explained by the Black population’s greater age, although Hispanic Americans have a higher infection rate (73 per 10,000) than Blacks (62 per 10,000) or Whites (23 per 10,000).
Another possible explanation for the differences in infection rates: Blacks and Hispanics are less able to work at home because they “are overrepresented in low-wage jobs that offer the least flexibility and increase their risk of exposure to the coronavirus,” the league said.
Hispanics and Blacks also are more likely to be uninsured than Whites – 19.5% and 11.5%, respectively, vs. 7.5% – so “they tend to delay seeking treatment and are sicker than white patients when they finally do,” the league said. That may account for their much higher COVID-19 hospitalization rates: 213 per 100,000 for Blacks, 205 for Hispanics, and 46 for Whites.
“The silver lining during these dark times is that this pandemic has revealed our shared vulnerability and our interconnectedness. Many people are beginning to see that when others don’t have the opportunity to be healthy, it puts all of us at risk,” Lisa Cooper, MD, James F. Fries Professor of Medicine and Bloomberg Distinguished Professor in Health Equity at Johns Hopkins University, Baltimore, wrote in an essay accompanying the report.
Swab, spit, stay home? College coronavirus testing plans are all over the map
Yousuf El-Jayyousi, a junior engineering student at the University of Missouri, wanted guidance and reassurance that it would be safe to go back to school for the fall semester. He tuned into a pair of online town halls organized by the university hoping to find that.
He did not.

What he got instead from those town halls last month was encouragement to return to class at the institution affectionately known as Mizzou. The university, in Columbia, would be testing only people with symptoms, and at that point, the university said people who test positive off campus were under no obligation to inform the school.
“It feels like the university doesn’t really care whether we get sick or not,” said El-Jayyousi, who is scheduled for two in-person classes, and lives at home with his parents and 90-year-old grandmother.
He’s seen the studies from researchers at Yale and Harvard that suggest testing needs to be much more widespread. He asked his instructors if he could join lectures remotely once classes begin Monday. One was considering it; the other rejected it.
“It was kind of very dismissive, like ‘so what?’ ” El-Jayyousi said.
But it’s an enormous “so what?” packed with fear and unknowns for Jayyousi and some 20 million other students enrolled in some level of postsecondary education in America, if they are not already online only.
Policies for reentry onto campuses that were abruptly shut in March are all over the map.
Hundreds Undecided
According to the College Crisis Initiative, or C2i, a project of Davidson College that monitors how higher ed is responding to the pandemic, there is nothing resembling a common approach. Of 2,958 institutions it follows, 151 were planning to open fully online, 729 were mostly online and 433 were taking a hybrid approach. Just 75 schools were insisting on students attending fully in person, and 614 were aiming to be primarily in-person. Some 800 others were still deciding, just weeks before instruction was to start.
The decisions often have little correlation with the public health advisories in the region. Mizzou, which is in an area with recent COVID spikes, is holding some in-person instruction and has nearly 7,000 students signed up to live in dorms and other university-owned housing. Harvard, in a region with extremely low rates of viral spread, has opted to go all online and allowed students to defer a year.
The specific circumstances colleges and universities face are as much determined by local fiscal and political dictates as by medicine and epidemiology. It is often unclear who is making the call. So it’s every student for herself to chart these unknown waters, even as students (or their families) have written tuition checks for tens of thousands of dollars and signed leases for campus and off-campus housing.
And the risks – health, educational and financial – boomerang back on individual students: Two weeks after University of North Carolina students, as instructed, returned to the flagship campus in Chapel Hill with the promise of at least some in-person learning, all classes went online. Early outbreaks surged from a few students to more than 130 in a matter of days. Most undergrads have about a week to clear out of their dorms.
“It’s really tough,” said neuroscience major Luke Lawless, 20. “Chapel Hill is an amazing place, and as a senior it’s tough to know that my time’s running out – and the virus only adds to that.”
Location, location, location
C2i’s creator, Davidson education Assistant Professor Chris Marsicano, said the extreme diversity of approaches comes from the sheer diversity of schools, the penchant of many to follow the leads of more prestigious peers, and local politics.
“Some states have very strong and stringent mask requirements. Some have stronger stay-at-home orders. Others are sort of leaving it up to localities. So the confluence of politics, institutional isomorphism – that imitation – and different needs that the institutions have are driving the differences,” Marsicano said.
Location matters a lot, too, Marsicano said, pointing to schools like George Washington University and Boston University in urban settings where the environment is beyond the control of the school, versus a place like the University of the South in remote, rural Sewanee, Tennessee, where 90% of students will return to campus.
“It’s a lot easier to control an outbreak if you are a fairly isolated college campus than if you are in the middle of a city,” Marsicano said.
Student behavior is another wild card, Marsicano said, since even the best plans will fail if college kids “do something stupid, like have a massive frat party without masks.”
“You’ve got student affairs professionals across the country who are screaming at the top of their lungs, ‘We can’t control student behavior when they go off campus’” Marsicano said.
Another factor is a vacuum at the federal level. Although the Department of Education says Secretary Betsy DeVos has held dozens of calls with governors and state education superintendents, there’s no sign of an attempt to offer unified guidance to colleges beyond a webpage that links to relaxed regulatory requirements and anodyne fact sheets from the Centers for Disease Control and Prevention on preventing viral spread.
Even the money that the department notes it has dispensed – $30 billion from Congress’ CARES Act – is weighted toward K-12 schools, with about $13 billion for higher education, including student aid.
The U.S. Senate adjourned last week until Sept. 8, having never taken up a House-passed relief package that included some $30 billion for higher education. A trio of Democratic senators, including Sen. Elizabeth Warren, is calling for national reporting standards on college campuses.
No benchmarks
Campus communities with very different levels of contagion are making opposite calls about in-person learning. Mizzou’s Boone County has seen more than 1,400 confirmed COVID cases after a spike in mid-July. According to the Harvard Global Health Institute’s COVID risk map, Boone has accelerated spread, with 14 infections per day per 100,000 people. The institute advises stay-at-home orders or rigorous testing and tracing at such rates of infection. Two neighboring counties were in the red zone recently, with more than 25 cases per day per 100,000 people. Mizzou has left it up to deans whether classes will meet in person, making a strong argument for face-to-face instruction.
Meanwhile, Columbia University in New York City opted for all online instruction, even though the rate of infection there is a comparatively low 3.8 cases per day per 100,000 people.
Administrators at Mizzou considered and rejected mandatory testing. “All that does is provide one a snapshot of the situation,” University of Missouri system President Mun Choi said in one of the town halls.
Mizzou has an in-house team that will carry out case investigation and contact tracing with the local health department. This week, following questions from the press and pressure from the public, the university announced students will be required to report any positive COVID test to the school.
Who do you test? When?
CDC guidance for higher education suggests there’s not enough data to know whether testing everyone is effective, but some influential researchers, such as those at Harvard and Yale, disagree.
“This virus is subject to silent spreading and asymptomatic spreading, and it’s very hard to play catch-up,” said Yale professor David Paltiel, who studies public health policy. “And so thinking that you can keep your campus safe by simply waiting until students develop symptoms before acting, I think, is a very dangerous game.”
Simulation models conducted by Paltiel and his colleagues show that, of all the factors university administrators can control – including the sensitivity and specificity of COVID-19 tests – the frequency of testing is most important.
He’s “painfully aware” that testing everyone on campus every few days sets a very high bar – logistically, financially, behaviorally – that may be beyond what most schools can reach. But he says the consequences of reopening campuses without those measures are severe, not just for students, but for vulnerable populations among school workers and in the surrounding community.
“You really have to ask yourself whether you have any business reopening if you’re not going to commit to an aggressive program of high-frequency testing,” he said.
The fighting – and testing – Illini
Some institutions that desperately want students to return to campus are backing the goal with a maximal approach to safety and testing.
About a 4-hour drive east along the interstates from Mizzou is the University of Illinois at Urbana-Champaign, whose sports teams are known as the Fighting Illini.
Weeks ago, large white tents with signs reading “Walk-Up COVID-19 Testing” have popped up across campus; there students take a simple saliva test.
“This seems to be a lot easier than sticking a cotton swab up your nose,” graduate student Kristen Muñoz said after collecting a bit of her saliva in a plastic tube and sealing it in a bag labeled “Biohazard.”
In just a few hours, she got back her result: negative.
The school plans to offer free tests to the 50,000 students expected to return this month, as well as some 11,000 faculty and staff members.
“The exciting thing is, because we can test up to 10,000 per day, it allows the scientist to do what’s really the best for trying to protect the community as opposed to having to cut corners, because of the limitations of the testing,” said University of Illinois chemist Martin Burke, who helped develop the campus’s saliva test, which received emergency use authorization from the federal Food and Drug Administration this week.
The test is similar to one designed by Yale and funded by the NBA that cleared the FDA hurdle just before the Illinois test. Both Yale and Illinois hope aggressive testing will allow most undergraduate students to live on campus, even though most classes will be online.
University of Illinois epidemiologist Becky Smith said they are following data that suggest campuses need to test everyone every few days because the virus is not detectable in infected people for 3 or 4 days.
“But about two days after that, your infectiousness peaks,” she said. “So, we have a very small window of time in which to catch people before they have done most of the infection that they’re going to be doing.”
Campus officials accepted Smith’s recommendation that all faculty, staffers and students participating in any on-campus activities be required to get tested twice a week.
Illinois can do that because its test is convenient and not invasive, which spares the campus from using as much personal protective equipment as the more invasive tests require, Burke said. And on-site analysis avoids backlogs at public health and commercial labs.
Muddled in the middle
Most other colleges fall somewhere between the approaches of Mizzou and the University of Illinois, and many of their students still are uncertain how their fall semester will go.
At the University of Southern California, a private campus of about 48,500 students in Los Angeles, officials had hoped to have about 20% of classes in person – but the county government scaled that back, insisting on tougher rules for reopening than the statewide standards.
If students eventually are allowed back, they will have to show a recent coronavirus test result that they obtained on their own, said Dr. Sarah Van Orman, chief health officer of USC Student Health.
They will be asked to do daily health assessments, such as fever checks, and those who have been exposed to the virus or show symptoms will receive a rapid test, with about a 24-hour turnaround through the university medical center’s lab. “We believe it is really important to have very rapid access to those results,” Van Orman said.
At California State University – the nation’s largest 4-year system, with 23 campuses and nearly a half-million students – officials decided back in May to move nearly all its fall courses online.
“The first priority was really the health and safety of all of the campus community,” said Mike Uhlenkamp, spokesperson for the CSU Chancellor’s Office. About 10% of CSU students are expected to attend some in-person classes, such as nursing lab courses, fine art and dance classes, and some graduate classes.
Uhlenkamp said testing protocols are being left up to each campus, though all are required to follow local safety guidelines. And without a medical campus in the system, CSU campuses do not have the same capacity to take charge of their own testing, as the University of Illinois is doing.
For students who know they won’t be on campus this fall, there is regret at lost social experiences, networking and hands-on learning so important to college.
But the certainty also brings relief.
“I don’t think I would want to be indoors with a group of, you know, even just a handful of people, even if we have masks on,” said Haley Gray, a 28-year-old graduate student at the University of California-Berkeley starting the second year of her journalism program.
She knows she won’t have access to Berkeley’s advanced media labs or the collaborative sessions students experience there. And she said she realized the other day she probably won’t just sit around the student lounge and strike up unexpected friendships.
“That’s a pretty big bummer but, you know, I think overall we’re all just doing our best, and given the circumstances, I feel pretty OK about it,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story is part of a partnership that includes KBIA, Illinois Public Media, Side Effects Public Media, NPR and Kaiser Health News.
Yousuf El-Jayyousi, a junior engineering student at the University of Missouri, wanted guidance and reassurance that it would be safe to go back to school for the fall semester. He tuned into a pair of online town halls organized by the university hoping to find that.
He did not.

What he got instead from those town halls last month was encouragement to return to class at the institution affectionately known as Mizzou. The university, in Columbia, would be testing only people with symptoms, and at that point, the university said people who test positive off campus were under no obligation to inform the school.
“It feels like the university doesn’t really care whether we get sick or not,” said El-Jayyousi, who is scheduled for two in-person classes, and lives at home with his parents and 90-year-old grandmother.
He’s seen the studies from researchers at Yale and Harvard that suggest testing needs to be much more widespread. He asked his instructors if he could join lectures remotely once classes begin Monday. One was considering it; the other rejected it.
“It was kind of very dismissive, like ‘so what?’ ” El-Jayyousi said.
But it’s an enormous “so what?” packed with fear and unknowns for Jayyousi and some 20 million other students enrolled in some level of postsecondary education in America, if they are not already online only.
Policies for reentry onto campuses that were abruptly shut in March are all over the map.
Hundreds Undecided
According to the College Crisis Initiative, or C2i, a project of Davidson College that monitors how higher ed is responding to the pandemic, there is nothing resembling a common approach. Of 2,958 institutions it follows, 151 were planning to open fully online, 729 were mostly online and 433 were taking a hybrid approach. Just 75 schools were insisting on students attending fully in person, and 614 were aiming to be primarily in-person. Some 800 others were still deciding, just weeks before instruction was to start.
The decisions often have little correlation with the public health advisories in the region. Mizzou, which is in an area with recent COVID spikes, is holding some in-person instruction and has nearly 7,000 students signed up to live in dorms and other university-owned housing. Harvard, in a region with extremely low rates of viral spread, has opted to go all online and allowed students to defer a year.
The specific circumstances colleges and universities face are as much determined by local fiscal and political dictates as by medicine and epidemiology. It is often unclear who is making the call. So it’s every student for herself to chart these unknown waters, even as students (or their families) have written tuition checks for tens of thousands of dollars and signed leases for campus and off-campus housing.
And the risks – health, educational and financial – boomerang back on individual students: Two weeks after University of North Carolina students, as instructed, returned to the flagship campus in Chapel Hill with the promise of at least some in-person learning, all classes went online. Early outbreaks surged from a few students to more than 130 in a matter of days. Most undergrads have about a week to clear out of their dorms.
“It’s really tough,” said neuroscience major Luke Lawless, 20. “Chapel Hill is an amazing place, and as a senior it’s tough to know that my time’s running out – and the virus only adds to that.”
Location, location, location
C2i’s creator, Davidson education Assistant Professor Chris Marsicano, said the extreme diversity of approaches comes from the sheer diversity of schools, the penchant of many to follow the leads of more prestigious peers, and local politics.
“Some states have very strong and stringent mask requirements. Some have stronger stay-at-home orders. Others are sort of leaving it up to localities. So the confluence of politics, institutional isomorphism – that imitation – and different needs that the institutions have are driving the differences,” Marsicano said.
Location matters a lot, too, Marsicano said, pointing to schools like George Washington University and Boston University in urban settings where the environment is beyond the control of the school, versus a place like the University of the South in remote, rural Sewanee, Tennessee, where 90% of students will return to campus.
“It’s a lot easier to control an outbreak if you are a fairly isolated college campus than if you are in the middle of a city,” Marsicano said.
Student behavior is another wild card, Marsicano said, since even the best plans will fail if college kids “do something stupid, like have a massive frat party without masks.”
“You’ve got student affairs professionals across the country who are screaming at the top of their lungs, ‘We can’t control student behavior when they go off campus’” Marsicano said.
Another factor is a vacuum at the federal level. Although the Department of Education says Secretary Betsy DeVos has held dozens of calls with governors and state education superintendents, there’s no sign of an attempt to offer unified guidance to colleges beyond a webpage that links to relaxed regulatory requirements and anodyne fact sheets from the Centers for Disease Control and Prevention on preventing viral spread.
Even the money that the department notes it has dispensed – $30 billion from Congress’ CARES Act – is weighted toward K-12 schools, with about $13 billion for higher education, including student aid.
The U.S. Senate adjourned last week until Sept. 8, having never taken up a House-passed relief package that included some $30 billion for higher education. A trio of Democratic senators, including Sen. Elizabeth Warren, is calling for national reporting standards on college campuses.
No benchmarks
Campus communities with very different levels of contagion are making opposite calls about in-person learning. Mizzou’s Boone County has seen more than 1,400 confirmed COVID cases after a spike in mid-July. According to the Harvard Global Health Institute’s COVID risk map, Boone has accelerated spread, with 14 infections per day per 100,000 people. The institute advises stay-at-home orders or rigorous testing and tracing at such rates of infection. Two neighboring counties were in the red zone recently, with more than 25 cases per day per 100,000 people. Mizzou has left it up to deans whether classes will meet in person, making a strong argument for face-to-face instruction.
Meanwhile, Columbia University in New York City opted for all online instruction, even though the rate of infection there is a comparatively low 3.8 cases per day per 100,000 people.
Administrators at Mizzou considered and rejected mandatory testing. “All that does is provide one a snapshot of the situation,” University of Missouri system President Mun Choi said in one of the town halls.
Mizzou has an in-house team that will carry out case investigation and contact tracing with the local health department. This week, following questions from the press and pressure from the public, the university announced students will be required to report any positive COVID test to the school.
Who do you test? When?
CDC guidance for higher education suggests there’s not enough data to know whether testing everyone is effective, but some influential researchers, such as those at Harvard and Yale, disagree.
“This virus is subject to silent spreading and asymptomatic spreading, and it’s very hard to play catch-up,” said Yale professor David Paltiel, who studies public health policy. “And so thinking that you can keep your campus safe by simply waiting until students develop symptoms before acting, I think, is a very dangerous game.”
Simulation models conducted by Paltiel and his colleagues show that, of all the factors university administrators can control – including the sensitivity and specificity of COVID-19 tests – the frequency of testing is most important.
He’s “painfully aware” that testing everyone on campus every few days sets a very high bar – logistically, financially, behaviorally – that may be beyond what most schools can reach. But he says the consequences of reopening campuses without those measures are severe, not just for students, but for vulnerable populations among school workers and in the surrounding community.
“You really have to ask yourself whether you have any business reopening if you’re not going to commit to an aggressive program of high-frequency testing,” he said.
The fighting – and testing – Illini
Some institutions that desperately want students to return to campus are backing the goal with a maximal approach to safety and testing.
About a 4-hour drive east along the interstates from Mizzou is the University of Illinois at Urbana-Champaign, whose sports teams are known as the Fighting Illini.
Weeks ago, large white tents with signs reading “Walk-Up COVID-19 Testing” have popped up across campus; there students take a simple saliva test.
“This seems to be a lot easier than sticking a cotton swab up your nose,” graduate student Kristen Muñoz said after collecting a bit of her saliva in a plastic tube and sealing it in a bag labeled “Biohazard.”
In just a few hours, she got back her result: negative.
The school plans to offer free tests to the 50,000 students expected to return this month, as well as some 11,000 faculty and staff members.
“The exciting thing is, because we can test up to 10,000 per day, it allows the scientist to do what’s really the best for trying to protect the community as opposed to having to cut corners, because of the limitations of the testing,” said University of Illinois chemist Martin Burke, who helped develop the campus’s saliva test, which received emergency use authorization from the federal Food and Drug Administration this week.
The test is similar to one designed by Yale and funded by the NBA that cleared the FDA hurdle just before the Illinois test. Both Yale and Illinois hope aggressive testing will allow most undergraduate students to live on campus, even though most classes will be online.
University of Illinois epidemiologist Becky Smith said they are following data that suggest campuses need to test everyone every few days because the virus is not detectable in infected people for 3 or 4 days.
“But about two days after that, your infectiousness peaks,” she said. “So, we have a very small window of time in which to catch people before they have done most of the infection that they’re going to be doing.”
Campus officials accepted Smith’s recommendation that all faculty, staffers and students participating in any on-campus activities be required to get tested twice a week.
Illinois can do that because its test is convenient and not invasive, which spares the campus from using as much personal protective equipment as the more invasive tests require, Burke said. And on-site analysis avoids backlogs at public health and commercial labs.
Muddled in the middle
Most other colleges fall somewhere between the approaches of Mizzou and the University of Illinois, and many of their students still are uncertain how their fall semester will go.
At the University of Southern California, a private campus of about 48,500 students in Los Angeles, officials had hoped to have about 20% of classes in person – but the county government scaled that back, insisting on tougher rules for reopening than the statewide standards.
If students eventually are allowed back, they will have to show a recent coronavirus test result that they obtained on their own, said Dr. Sarah Van Orman, chief health officer of USC Student Health.
They will be asked to do daily health assessments, such as fever checks, and those who have been exposed to the virus or show symptoms will receive a rapid test, with about a 24-hour turnaround through the university medical center’s lab. “We believe it is really important to have very rapid access to those results,” Van Orman said.
At California State University – the nation’s largest 4-year system, with 23 campuses and nearly a half-million students – officials decided back in May to move nearly all its fall courses online.
“The first priority was really the health and safety of all of the campus community,” said Mike Uhlenkamp, spokesperson for the CSU Chancellor’s Office. About 10% of CSU students are expected to attend some in-person classes, such as nursing lab courses, fine art and dance classes, and some graduate classes.
Uhlenkamp said testing protocols are being left up to each campus, though all are required to follow local safety guidelines. And without a medical campus in the system, CSU campuses do not have the same capacity to take charge of their own testing, as the University of Illinois is doing.
For students who know they won’t be on campus this fall, there is regret at lost social experiences, networking and hands-on learning so important to college.
But the certainty also brings relief.
“I don’t think I would want to be indoors with a group of, you know, even just a handful of people, even if we have masks on,” said Haley Gray, a 28-year-old graduate student at the University of California-Berkeley starting the second year of her journalism program.
She knows she won’t have access to Berkeley’s advanced media labs or the collaborative sessions students experience there. And she said she realized the other day she probably won’t just sit around the student lounge and strike up unexpected friendships.
“That’s a pretty big bummer but, you know, I think overall we’re all just doing our best, and given the circumstances, I feel pretty OK about it,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story is part of a partnership that includes KBIA, Illinois Public Media, Side Effects Public Media, NPR and Kaiser Health News.
Yousuf El-Jayyousi, a junior engineering student at the University of Missouri, wanted guidance and reassurance that it would be safe to go back to school for the fall semester. He tuned into a pair of online town halls organized by the university hoping to find that.
He did not.

What he got instead from those town halls last month was encouragement to return to class at the institution affectionately known as Mizzou. The university, in Columbia, would be testing only people with symptoms, and at that point, the university said people who test positive off campus were under no obligation to inform the school.
“It feels like the university doesn’t really care whether we get sick or not,” said El-Jayyousi, who is scheduled for two in-person classes, and lives at home with his parents and 90-year-old grandmother.
He’s seen the studies from researchers at Yale and Harvard that suggest testing needs to be much more widespread. He asked his instructors if he could join lectures remotely once classes begin Monday. One was considering it; the other rejected it.
“It was kind of very dismissive, like ‘so what?’ ” El-Jayyousi said.
But it’s an enormous “so what?” packed with fear and unknowns for Jayyousi and some 20 million other students enrolled in some level of postsecondary education in America, if they are not already online only.
Policies for reentry onto campuses that were abruptly shut in March are all over the map.
Hundreds Undecided
According to the College Crisis Initiative, or C2i, a project of Davidson College that monitors how higher ed is responding to the pandemic, there is nothing resembling a common approach. Of 2,958 institutions it follows, 151 were planning to open fully online, 729 were mostly online and 433 were taking a hybrid approach. Just 75 schools were insisting on students attending fully in person, and 614 were aiming to be primarily in-person. Some 800 others were still deciding, just weeks before instruction was to start.
The decisions often have little correlation with the public health advisories in the region. Mizzou, which is in an area with recent COVID spikes, is holding some in-person instruction and has nearly 7,000 students signed up to live in dorms and other university-owned housing. Harvard, in a region with extremely low rates of viral spread, has opted to go all online and allowed students to defer a year.
The specific circumstances colleges and universities face are as much determined by local fiscal and political dictates as by medicine and epidemiology. It is often unclear who is making the call. So it’s every student for herself to chart these unknown waters, even as students (or their families) have written tuition checks for tens of thousands of dollars and signed leases for campus and off-campus housing.
And the risks – health, educational and financial – boomerang back on individual students: Two weeks after University of North Carolina students, as instructed, returned to the flagship campus in Chapel Hill with the promise of at least some in-person learning, all classes went online. Early outbreaks surged from a few students to more than 130 in a matter of days. Most undergrads have about a week to clear out of their dorms.
“It’s really tough,” said neuroscience major Luke Lawless, 20. “Chapel Hill is an amazing place, and as a senior it’s tough to know that my time’s running out – and the virus only adds to that.”
Location, location, location
C2i’s creator, Davidson education Assistant Professor Chris Marsicano, said the extreme diversity of approaches comes from the sheer diversity of schools, the penchant of many to follow the leads of more prestigious peers, and local politics.
“Some states have very strong and stringent mask requirements. Some have stronger stay-at-home orders. Others are sort of leaving it up to localities. So the confluence of politics, institutional isomorphism – that imitation – and different needs that the institutions have are driving the differences,” Marsicano said.
Location matters a lot, too, Marsicano said, pointing to schools like George Washington University and Boston University in urban settings where the environment is beyond the control of the school, versus a place like the University of the South in remote, rural Sewanee, Tennessee, where 90% of students will return to campus.
“It’s a lot easier to control an outbreak if you are a fairly isolated college campus than if you are in the middle of a city,” Marsicano said.
Student behavior is another wild card, Marsicano said, since even the best plans will fail if college kids “do something stupid, like have a massive frat party without masks.”
“You’ve got student affairs professionals across the country who are screaming at the top of their lungs, ‘We can’t control student behavior when they go off campus’” Marsicano said.
Another factor is a vacuum at the federal level. Although the Department of Education says Secretary Betsy DeVos has held dozens of calls with governors and state education superintendents, there’s no sign of an attempt to offer unified guidance to colleges beyond a webpage that links to relaxed regulatory requirements and anodyne fact sheets from the Centers for Disease Control and Prevention on preventing viral spread.
Even the money that the department notes it has dispensed – $30 billion from Congress’ CARES Act – is weighted toward K-12 schools, with about $13 billion for higher education, including student aid.
The U.S. Senate adjourned last week until Sept. 8, having never taken up a House-passed relief package that included some $30 billion for higher education. A trio of Democratic senators, including Sen. Elizabeth Warren, is calling for national reporting standards on college campuses.
No benchmarks
Campus communities with very different levels of contagion are making opposite calls about in-person learning. Mizzou’s Boone County has seen more than 1,400 confirmed COVID cases after a spike in mid-July. According to the Harvard Global Health Institute’s COVID risk map, Boone has accelerated spread, with 14 infections per day per 100,000 people. The institute advises stay-at-home orders or rigorous testing and tracing at such rates of infection. Two neighboring counties were in the red zone recently, with more than 25 cases per day per 100,000 people. Mizzou has left it up to deans whether classes will meet in person, making a strong argument for face-to-face instruction.
Meanwhile, Columbia University in New York City opted for all online instruction, even though the rate of infection there is a comparatively low 3.8 cases per day per 100,000 people.
Administrators at Mizzou considered and rejected mandatory testing. “All that does is provide one a snapshot of the situation,” University of Missouri system President Mun Choi said in one of the town halls.
Mizzou has an in-house team that will carry out case investigation and contact tracing with the local health department. This week, following questions from the press and pressure from the public, the university announced students will be required to report any positive COVID test to the school.
Who do you test? When?
CDC guidance for higher education suggests there’s not enough data to know whether testing everyone is effective, but some influential researchers, such as those at Harvard and Yale, disagree.
“This virus is subject to silent spreading and asymptomatic spreading, and it’s very hard to play catch-up,” said Yale professor David Paltiel, who studies public health policy. “And so thinking that you can keep your campus safe by simply waiting until students develop symptoms before acting, I think, is a very dangerous game.”
Simulation models conducted by Paltiel and his colleagues show that, of all the factors university administrators can control – including the sensitivity and specificity of COVID-19 tests – the frequency of testing is most important.
He’s “painfully aware” that testing everyone on campus every few days sets a very high bar – logistically, financially, behaviorally – that may be beyond what most schools can reach. But he says the consequences of reopening campuses without those measures are severe, not just for students, but for vulnerable populations among school workers and in the surrounding community.
“You really have to ask yourself whether you have any business reopening if you’re not going to commit to an aggressive program of high-frequency testing,” he said.
The fighting – and testing – Illini
Some institutions that desperately want students to return to campus are backing the goal with a maximal approach to safety and testing.
About a 4-hour drive east along the interstates from Mizzou is the University of Illinois at Urbana-Champaign, whose sports teams are known as the Fighting Illini.
Weeks ago, large white tents with signs reading “Walk-Up COVID-19 Testing” have popped up across campus; there students take a simple saliva test.
“This seems to be a lot easier than sticking a cotton swab up your nose,” graduate student Kristen Muñoz said after collecting a bit of her saliva in a plastic tube and sealing it in a bag labeled “Biohazard.”
In just a few hours, she got back her result: negative.
The school plans to offer free tests to the 50,000 students expected to return this month, as well as some 11,000 faculty and staff members.
“The exciting thing is, because we can test up to 10,000 per day, it allows the scientist to do what’s really the best for trying to protect the community as opposed to having to cut corners, because of the limitations of the testing,” said University of Illinois chemist Martin Burke, who helped develop the campus’s saliva test, which received emergency use authorization from the federal Food and Drug Administration this week.
The test is similar to one designed by Yale and funded by the NBA that cleared the FDA hurdle just before the Illinois test. Both Yale and Illinois hope aggressive testing will allow most undergraduate students to live on campus, even though most classes will be online.
University of Illinois epidemiologist Becky Smith said they are following data that suggest campuses need to test everyone every few days because the virus is not detectable in infected people for 3 or 4 days.
“But about two days after that, your infectiousness peaks,” she said. “So, we have a very small window of time in which to catch people before they have done most of the infection that they’re going to be doing.”
Campus officials accepted Smith’s recommendation that all faculty, staffers and students participating in any on-campus activities be required to get tested twice a week.
Illinois can do that because its test is convenient and not invasive, which spares the campus from using as much personal protective equipment as the more invasive tests require, Burke said. And on-site analysis avoids backlogs at public health and commercial labs.
Muddled in the middle
Most other colleges fall somewhere between the approaches of Mizzou and the University of Illinois, and many of their students still are uncertain how their fall semester will go.
At the University of Southern California, a private campus of about 48,500 students in Los Angeles, officials had hoped to have about 20% of classes in person – but the county government scaled that back, insisting on tougher rules for reopening than the statewide standards.
If students eventually are allowed back, they will have to show a recent coronavirus test result that they obtained on their own, said Dr. Sarah Van Orman, chief health officer of USC Student Health.
They will be asked to do daily health assessments, such as fever checks, and those who have been exposed to the virus or show symptoms will receive a rapid test, with about a 24-hour turnaround through the university medical center’s lab. “We believe it is really important to have very rapid access to those results,” Van Orman said.
At California State University – the nation’s largest 4-year system, with 23 campuses and nearly a half-million students – officials decided back in May to move nearly all its fall courses online.
“The first priority was really the health and safety of all of the campus community,” said Mike Uhlenkamp, spokesperson for the CSU Chancellor’s Office. About 10% of CSU students are expected to attend some in-person classes, such as nursing lab courses, fine art and dance classes, and some graduate classes.
Uhlenkamp said testing protocols are being left up to each campus, though all are required to follow local safety guidelines. And without a medical campus in the system, CSU campuses do not have the same capacity to take charge of their own testing, as the University of Illinois is doing.
For students who know they won’t be on campus this fall, there is regret at lost social experiences, networking and hands-on learning so important to college.
But the certainty also brings relief.
“I don’t think I would want to be indoors with a group of, you know, even just a handful of people, even if we have masks on,” said Haley Gray, a 28-year-old graduate student at the University of California-Berkeley starting the second year of her journalism program.
She knows she won’t have access to Berkeley’s advanced media labs or the collaborative sessions students experience there. And she said she realized the other day she probably won’t just sit around the student lounge and strike up unexpected friendships.
“That’s a pretty big bummer but, you know, I think overall we’re all just doing our best, and given the circumstances, I feel pretty OK about it,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story is part of a partnership that includes KBIA, Illinois Public Media, Side Effects Public Media, NPR and Kaiser Health News.
Population health can improve postdischarge care
With the United States spending the most per capita on health care among industrialized nations but having the worst aggregate health outcomes, there’s a stark need for improvement, according to an expert at HM20 Virtual, hosted by the Society of Hospital Medicine.
Broadening the focus beyond the four walls of the hospital can bring better results while also saving money, said Adam Myers, MD, chief of population health at Cleveland Clinic. Dr. Myers described the way his health system has begun to pay more careful attention to the needs of specific kinds of patients and tailoring posthospitalization care accordingly, with in-person and virtual home visits, and postdischarge clinics.
With an increasing attention to value, health care organizations have to change their structure or risk going the way of the Choluteca Bridge in Honduras, Dr. Myers said. The Choluteca Bridge was built to be hurricane proof, but was nonetheless rendered useless in 1998 after Hurricane Mitch shifted the very course of the river beneath it.
Similarly, the way health care is delivered often does not meet the needs of the population.
“Our national system has been focused almost entirely on inpatient care,” Dr. Myers said. “A lot of the transition in care is outside of facilities and outside the walls of our inpatient settings.”
Instead, he said a focus on population health – understanding and tending to the needs of people rather than just treating them when they show up at clinics – should involve more outpatient care that is less centralized, fees based on outcomes and patient experience rather than simply volume of services, team approaches rather than single-provider care, and a general attention to preserving health rather than treating sickness.
At Cleveland Clinic, care teams try to understand not just the care that is medically necessary, but what is wanted and justified, as well as how to deliver that care safely, reliably, and affordably with outcomes that patients and families desire.
The results are striking. After increasing the number of ambulatory patient “touches” for those with chronic disease, inpatient care – disliked by patients and costly to health centers – decreased. From the first quarter of 2018, outpatient visits increased 9%, while inpatient visits dropped 7.4%, Dr. Myers said.
“As we managed patients more effectively on an outpatient basis, their need for inpatient care diminished,” he said. “It works.”
Cleveland Clinic has also made changes designed to reduce costly readmissions, using virtual visits, house calls, time reserved for team meetings to identify patients with gaps in their care, and attention to nonmedical determinants of health, such as assessing fall risk at home and addressing lack of nutritious food options in a community.
The health system has seen a 28% reduction in the cost of care attributed to house calls, 12% cost reduction attributed to better care coordination, and a 49% decrease in hospital days for “superutilizers” of the ED, Dr. Myers said.
Postdischarge clinics – where patients can be seen for the first few visits after hospitalization – have also been valuable for many health systems, because they are closely in tune with what happened during the inpatient stay. These clinics are staffed by hospitalists, interns, residents, or ambulatory clinicians. Dr. Myers said hospitalists tend to have an improved perspective after working in a discharge clinic, with more concern about a patient’s needs once they leave the hospital bed.
“Those hospitalists that I know who have participated in programs like this start to act a bit more like primary care physicians,” he said.
In a Q&A session after Dr. Myers’ presentation, he discussed how hospitalists can affect the many layers of health care policy, factors that often overlap with population health.
He noted that medical care accounts for only about 20% of patient outcomes – the rest involve social and environmental factors.
“I don’t know about you , but I’m not satisfied only impacting 20% of health outcomes,” he said. First, physicians need to understand what is happening in their communities, and the health policies that are preventing improvement. Then, build partnerships to help fix these problems. He pointed to lead poisoning as an example.
“If you think about it, lead poisoning is a social housing problem that shows up as a health care issue. Unless we are getting out into the community and mitigating the root problem, we will have to treat it over and over again,” he said.
Dr. Myers reported no relevant financial disclosures.
With the United States spending the most per capita on health care among industrialized nations but having the worst aggregate health outcomes, there’s a stark need for improvement, according to an expert at HM20 Virtual, hosted by the Society of Hospital Medicine.
Broadening the focus beyond the four walls of the hospital can bring better results while also saving money, said Adam Myers, MD, chief of population health at Cleveland Clinic. Dr. Myers described the way his health system has begun to pay more careful attention to the needs of specific kinds of patients and tailoring posthospitalization care accordingly, with in-person and virtual home visits, and postdischarge clinics.
With an increasing attention to value, health care organizations have to change their structure or risk going the way of the Choluteca Bridge in Honduras, Dr. Myers said. The Choluteca Bridge was built to be hurricane proof, but was nonetheless rendered useless in 1998 after Hurricane Mitch shifted the very course of the river beneath it.
Similarly, the way health care is delivered often does not meet the needs of the population.
“Our national system has been focused almost entirely on inpatient care,” Dr. Myers said. “A lot of the transition in care is outside of facilities and outside the walls of our inpatient settings.”
Instead, he said a focus on population health – understanding and tending to the needs of people rather than just treating them when they show up at clinics – should involve more outpatient care that is less centralized, fees based on outcomes and patient experience rather than simply volume of services, team approaches rather than single-provider care, and a general attention to preserving health rather than treating sickness.
At Cleveland Clinic, care teams try to understand not just the care that is medically necessary, but what is wanted and justified, as well as how to deliver that care safely, reliably, and affordably with outcomes that patients and families desire.
The results are striking. After increasing the number of ambulatory patient “touches” for those with chronic disease, inpatient care – disliked by patients and costly to health centers – decreased. From the first quarter of 2018, outpatient visits increased 9%, while inpatient visits dropped 7.4%, Dr. Myers said.
“As we managed patients more effectively on an outpatient basis, their need for inpatient care diminished,” he said. “It works.”
Cleveland Clinic has also made changes designed to reduce costly readmissions, using virtual visits, house calls, time reserved for team meetings to identify patients with gaps in their care, and attention to nonmedical determinants of health, such as assessing fall risk at home and addressing lack of nutritious food options in a community.
The health system has seen a 28% reduction in the cost of care attributed to house calls, 12% cost reduction attributed to better care coordination, and a 49% decrease in hospital days for “superutilizers” of the ED, Dr. Myers said.
Postdischarge clinics – where patients can be seen for the first few visits after hospitalization – have also been valuable for many health systems, because they are closely in tune with what happened during the inpatient stay. These clinics are staffed by hospitalists, interns, residents, or ambulatory clinicians. Dr. Myers said hospitalists tend to have an improved perspective after working in a discharge clinic, with more concern about a patient’s needs once they leave the hospital bed.
“Those hospitalists that I know who have participated in programs like this start to act a bit more like primary care physicians,” he said.
In a Q&A session after Dr. Myers’ presentation, he discussed how hospitalists can affect the many layers of health care policy, factors that often overlap with population health.
He noted that medical care accounts for only about 20% of patient outcomes – the rest involve social and environmental factors.
“I don’t know about you , but I’m not satisfied only impacting 20% of health outcomes,” he said. First, physicians need to understand what is happening in their communities, and the health policies that are preventing improvement. Then, build partnerships to help fix these problems. He pointed to lead poisoning as an example.
“If you think about it, lead poisoning is a social housing problem that shows up as a health care issue. Unless we are getting out into the community and mitigating the root problem, we will have to treat it over and over again,” he said.
Dr. Myers reported no relevant financial disclosures.
With the United States spending the most per capita on health care among industrialized nations but having the worst aggregate health outcomes, there’s a stark need for improvement, according to an expert at HM20 Virtual, hosted by the Society of Hospital Medicine.
Broadening the focus beyond the four walls of the hospital can bring better results while also saving money, said Adam Myers, MD, chief of population health at Cleveland Clinic. Dr. Myers described the way his health system has begun to pay more careful attention to the needs of specific kinds of patients and tailoring posthospitalization care accordingly, with in-person and virtual home visits, and postdischarge clinics.
With an increasing attention to value, health care organizations have to change their structure or risk going the way of the Choluteca Bridge in Honduras, Dr. Myers said. The Choluteca Bridge was built to be hurricane proof, but was nonetheless rendered useless in 1998 after Hurricane Mitch shifted the very course of the river beneath it.
Similarly, the way health care is delivered often does not meet the needs of the population.
“Our national system has been focused almost entirely on inpatient care,” Dr. Myers said. “A lot of the transition in care is outside of facilities and outside the walls of our inpatient settings.”
Instead, he said a focus on population health – understanding and tending to the needs of people rather than just treating them when they show up at clinics – should involve more outpatient care that is less centralized, fees based on outcomes and patient experience rather than simply volume of services, team approaches rather than single-provider care, and a general attention to preserving health rather than treating sickness.
At Cleveland Clinic, care teams try to understand not just the care that is medically necessary, but what is wanted and justified, as well as how to deliver that care safely, reliably, and affordably with outcomes that patients and families desire.
The results are striking. After increasing the number of ambulatory patient “touches” for those with chronic disease, inpatient care – disliked by patients and costly to health centers – decreased. From the first quarter of 2018, outpatient visits increased 9%, while inpatient visits dropped 7.4%, Dr. Myers said.
“As we managed patients more effectively on an outpatient basis, their need for inpatient care diminished,” he said. “It works.”
Cleveland Clinic has also made changes designed to reduce costly readmissions, using virtual visits, house calls, time reserved for team meetings to identify patients with gaps in their care, and attention to nonmedical determinants of health, such as assessing fall risk at home and addressing lack of nutritious food options in a community.
The health system has seen a 28% reduction in the cost of care attributed to house calls, 12% cost reduction attributed to better care coordination, and a 49% decrease in hospital days for “superutilizers” of the ED, Dr. Myers said.
Postdischarge clinics – where patients can be seen for the first few visits after hospitalization – have also been valuable for many health systems, because they are closely in tune with what happened during the inpatient stay. These clinics are staffed by hospitalists, interns, residents, or ambulatory clinicians. Dr. Myers said hospitalists tend to have an improved perspective after working in a discharge clinic, with more concern about a patient’s needs once they leave the hospital bed.
“Those hospitalists that I know who have participated in programs like this start to act a bit more like primary care physicians,” he said.
In a Q&A session after Dr. Myers’ presentation, he discussed how hospitalists can affect the many layers of health care policy, factors that often overlap with population health.
He noted that medical care accounts for only about 20% of patient outcomes – the rest involve social and environmental factors.
“I don’t know about you , but I’m not satisfied only impacting 20% of health outcomes,” he said. First, physicians need to understand what is happening in their communities, and the health policies that are preventing improvement. Then, build partnerships to help fix these problems. He pointed to lead poisoning as an example.
“If you think about it, lead poisoning is a social housing problem that shows up as a health care issue. Unless we are getting out into the community and mitigating the root problem, we will have to treat it over and over again,” he said.
Dr. Myers reported no relevant financial disclosures.
How to get a position as a physician leader
The best ways to start
It’s been said that physicians tend to fall into leadership roles. Few physicians set out to become leaders, and then one day they realize that they desire to be a leader and an agent for change.
They may be rotating through the chairmanship of a clinical department or the management of a small practice and decide they like the work. In a large organization, doctors get assigned to committees, or specialists agree to run a new service line for a while, and it changes their lives.
Some physicians have a natural aptitude for managerial work. Often, colleagues tell them they are a good fit, but they may still have some reservations. In any case, it’s good to do a bit of soul-searching before taking the leap.
1. Weigh the pluses and minuses of a leadership role
When you stand at the precipice of a totally new career in physician leadership, it’s worthwhile to step back and consider the pluses and minuses of the work.
One plus is that there may be fewer work hours than on the clinical side, but being a physician leader is by no means a 9-to-5 job. In a large organization, a physician on the executive team can be on administrative call – dealing with institutional crises on off-hours – for a length of time. Board and strategic planning retreats tend to occur on weekends, and you may need to attend frequent dinner meetings.
Another plus is that the pay is pretty good. In 2016, physician leaders in large organizations earned an average of $350,000 a year, according to a survey by Cejka Executive Search and the American Association for Physician Leadership (AAPL).1
On the minus side, an executive probably won’t be as beloved as a clinician serving a host of grateful patients. And you will not have the kind of job security that most clinicians have. There may be frequent turnover among health care executives because of change of top leadership, pressure for more profitability, or a host of other reasons.
2. Try on different roles
To decide whether you want to make a career of being a physician leader, it’s useful to try out several different jobs. Volunteer for committees or take on a special project if it’s possible to do so in your organization.
You can also volunteer for posts outside the organization, such as joining the board of your local cancer or heart association or helping them out on a committee. You might volunteer for Little League or a school or civic organization. Your choices are wide open. The goal is to get a feel for directing an organization and whether that fits your lifestyle.
Also, talk to current physician leaders. Contact a cross-section of people, including those who are unhappy with their jobs and those who had to struggle with their new roles. This will give you some good perspective into whether the work is right for you, as well as tips on how to cope.
3. Find a mentor
This is also a good time to find a mentor for your new calling. Choose a seasoned physician leader who can help you over the long haul – someone who can get you up to speed and then advise you during crucial junctures in your career.
Good mentors should be willing to spend the time with you, have your best interests in mind, and be willing to provide honest assessments. They can also help you find opportunities for further learning and professional growth.
Some organizations assign mentors to physicians they want to develop for leadership roles. You can also choose specific mentors to help you in areas where you think you need more work, such as finance, quality improvement, or information systems.
Choose a path
There are many different paths you can take as a physician leader. In large organizations in particular, there are more leadership jobs open to physicians than ever before.
Jobs open to physicians can be found in the areas of clinical quality and safety, population health, managed care, and information technology. You can even look beyond these traditional roles to jobs that don’t usually attract physicians, such as in strategy, innovation, patient experience, and fundraising. In these roles, you are often expected to continue doing some clinical work.
Physician leaders now tend to have more influence than in the past. According to the Cejka-AAPL survey, 61% of physician executives said they had more strategic input currently than in the previous year.
A roster of potential physician leader jobs
1. Executive-level roles
Vice president for medical affairs. This is the traditional role for the physician executive, which involves acting as a liaison with the organization’s physicians. These officers oversee quality of care as well as hiring, training, and performance evaluation of physicians on staff.
Chief medical officer (CMO). This is now the typical term for the highest medical role in the organization. The CMO is part of the C-suite team and participates in governance, strategic planning, and business operation decisions. CMOs may be responsible for supporting value-based strategies and making sure that those strategies are efficient and medically necessary.
Physician-in-chief. This is a new term for the hospital’s top physician, who works with the senior leadership team to maintain standards of care and customer service. The physician-in-chief may also oversee operational efficiency and support organizational transformation.
Chief clinical officer (CCO). CCOs oversee patient engagement and clinical quality outcomes. They may lead initiatives to reduce waste and improve care quality, and they can be involved in implementation of electronic health records (EHRs) and data integration. They may also assist in medical staff development, clinical integration, and physician partnerships.
2. Quality, safety, and research roles
Chief patient safety officer (CPSO). CPSOs oversee the hospital or health system’s patient safety initiatives. Their goal is to reduce medical errors and near-misses.
Chief quality officer (CQO). CQOs are responsible for collecting quality data and supporting patient safety efforts. They advise on quality initiatives and hold clinicians accountable for meeting specific quality indicators. They may also be involved in developing a culture of continuous improvement in the organization.
Chief research officer (CRO). CROs oversee the organization’s research activities, including clinical trials, internal investigator-initiated research programs, and sponsored studies.
3. Technology
Chief medical information officer (CMIO). The CMIO is the information technology (IT) department’s liaison with the clinical staff, working on selection and improvement of EHR systems. The CMIO finds new ways for EHRs to improve healthcare delivery in the organization.
Chief health information officer (CHIO). CHIOs deal with EHR implementation and health informatics. They may report to the chief information officer, the chief operations officer, or another C-suite executive, and they manage health informatics, telehealth, business and clinical intelligence, and predictive analytics initiatives.
Chief technology officer (CTO). CTOs oversee the organization’s technology capabilities. They are responsible for leading the IT team and contributing to the organization’s strategic plan.
4. Jobs not usually for physicians
There are other leadership positions that may not traditionally appeal to physicians but could be worth considering:
Chief experience officer (CXO). This involves evaluating and improving the inpatient experience. CXOs work with physicians and staff on their performance in this area.
Chief innovation officer (CIO). CIOs keep up with industry trends, market disruptions, and new opportunities, and support policy innovations and training initiatives.
Chief transformation officer (CTO). CTOs are responsible for carrying out major changes in the organization. They are supposed to act as role models for change.
5. Salaries for selected physician executives
In addition to placing the average salary for a physician leader at $350,000, the 2016 Cejka-AAPL survey pinpointed average salaries for specific types of physician leaders. Chief medical officers earned $388,000, chief patient safety officers and chief quality officers $375,000, and chief medical information officers $372,500, the survey found.
Several emerging physician leader roles – physician-in-chief, chief strategy officer, chief transformation officer, chief innovation officer, and chief integration officer – earned on average $499,000 a year, according to the survey.
Those jobs provided even higher salaries than the $437,500 reported by Cejka-AAPL for physician CEOs. In comparison, a CEO at a medical group with fewer than 200 physicians had an average salary of $438,500 in 2018, according to SullivanCotter, a health care workforce strategy company.2
Some types of physician leaders have seen unusually high pay raises recently. From 2013 to 2016, the average salary for CMIOs rose 18%, and physician leaders working at the corporate level in a health system saw median compensation rise 67%, the Cejka-AAPL survey found.
Moving ahead
For physician leaders, moving up the ladder often means reinventing yourself. If you’re leaving clinical practice, be sure to develop a solid CV for your new role so that if your leadership position doesn’t work out, you are able to find an appropriate new position.
According to a 2003 assessment, CMOs typically lasted 18-24 months on the job.3
Expect to make mistakes and try to learn from them. If necessary, move on to the next job. There is always a market for seasoned physician executives who took a few punches, learned something from the experience, and found something new.
Start to network
One way to navigate the challenges of a new role is to have a strong network, a group of colleagues and mentors who can help you figure out your path forward. They can serve as sounding boards and contacts for new jobs in an industry that is constantly changing.
A well-functioning network takes constant maintenance.
You can find people for your network by attending a variety of different meetings that physician leaders and other healthcare executives attend. Make a point of keeping their contact information on file and periodically reaching out to them.
Learn in a dyad
Some healthcare organizations assign physician leaders to dyads, where they are matched with nonphysicians who have skills that the physician lacks, such as finance, data management, or organizational politics.
Dyads are less effective when the nonphysician has all the authority and the physician is basically a figurehead. But in an effective dyad, both partners share authority and they can teach skills to each other. While the physician in the dyad brings clinical insight, the nonphysician can provide managerial know-how.
Seek out coaching
There may be points in your leadership career when you become aware of areas where you need improvement. You may have gotten negative feedback on communication skills or political sensitivity. Consider hiring an executive coach; coaches provide concentrated sessions over limited periods of time.
Coaches can also help you prepare for the future. They can help you find ways to promote yourself for new projects or create a network of allies. They also can help you establish yourself as a thought leader in a particular field through writing and speaking engagements.
Some organizations provide in-house coaches. It is worthwhile to take advantage of this benefit. If you need to find a coach on your own, ask mentors or people in your network for recommendations.
Getting to the top
It can take years to rise to the level of the corporate C-suite or even to CEO of a large organization. At the top levels of management, you often have to cut back substantially on clinical work or even give it up entirely.
Becoming CEO of a hospital can be a logical fit for physicians. A physician CEO can relate to doctors on staff, who are a key constituency, and understands what clinical care is all about. However, physician CEOs also need to have a large degree of knowledge about finance, strategy, crisis management, quality improvement, and other nonclinical considerations, not to mention good people skills.
Physicians on boards
Some physicians would rather sit on the board of trustees than take the reins of CEO. Board membership allows you to continue practicing while still having a great deal of influence over the organization. Some physicians hold board seats for many years and enjoy a great deal of respect as the go-to person on clinical care.
Physicians are increasingly serving on the boards of hospitals and health systems. Trustees welcome physicians because they want more input from clinicians in decision-making. They tend to choose physicians who already have executive duties, such as having been a department head.
Which new skills should you learn?
Physician executives often put off learning business and management skills until after being appointed to a leadership position. Even then, they may prefer to take courses focused on a particular topic rather than earn a degree such as master of business administration (MBA).
Learning on the job
A number of executive skills can be learned on the job, such as dealing with quality measures, utilization, billing and coding, disease management, committees, and interpreting data. If you are not in a dyad model, you can ask someone knowledgeable in one of these skills to take you through the steps.
Many physicians could benefit from finance and business courses in order to learn some of the budgetary, accounting, and operational skills required to perform the job optimally.
In a survey of healthcare CEOs, only 30% said their most senior physician leader had a business or medical management degree, and only 21% required a degree.4
Taking classes
Physician executives who want to brush up on a particular topic can “mix and match,” taking short, focused classes on the particular topic whenever they feel the need. In addition to resources offered by SHM, courses are available from organizations such as the AAPL, American Hospital Association, the Medical Group Management Association, or the Healthcare Information and Management Systems Society, to name a few examples.
Pursuing degree programs
Degree programs like MBA, master of public health (MPH), and master of health care administration (MHA) are popular with many physician executives because they get a full overview of needed skills and the potential to earn more money with their new credentials. Physician leaders with an MBA earned 13% more in 2016 than did those with no MBA, according to the Cejka-AAPL survey.
Getting a master’s degree, however, takes time and money. For example, an MBA can cost $20,000 to as much as $100,000.5 MBA, MHA, and MPH degrees take 2 years to complete, while a master of medical management (MMM) and a physician-executive MBA – focusing specifically on what physician leaders need to learn – take 1 year.
Many part-time degree programs are available for those with full-time jobs. You can find them at nearby universities as well as far-off institutions. Much of the coursework is done online, but some on-site work is usually required. You’ll find that working directly with others enriches the learning experience and helps you build your network of colleagues.
Straight MBA or other degree?
In general, degree programs cover finance, communication, strategy, information systems, marketing, organizational behavior, operational management, and quality improvement. Straight MBA programs don’t focus on healthcare, but some physician executives still prefer this route, especially if it involves degrees from prestigious business schools.
MHA, MMM, physician-executive MBA, and other degree programs focused on healthcare are popular with many physicians on the executive track. The MPH is less business-oriented but may be preferable to some because of its focus on population-based health, which fits well with decision-making on health insurance and value-based care.
Conclusion
Physicians need to prepare for leadership because these roles are very different from clinical work. It’s easy to stumble and lose direction without mentors, a network of helpful colleagues, and at least some education in business principles.
Finding a mentor should start early in your new career. A seasoned physician executive can help you understand your options and point out your strengths and shortcomings. Beyond that, concentrated work with an executive coach can help you improve your skills and choose from among the many executive roles that are now available.
You can learn many skills on the job through dyads and other relationships with more seasoned colleagues, or take short classes on particular skills that need to be learned or sharpened. Many physician executives go a step further and get a master’s degree, such as an MBA, MHA, or MMM. This involves a year or two of study, but much of it can be done online.
This article is excerpted from the Medscape Physician Business Academy course “How to become an effective leader.” You can find more information on the course at www.medscape.com/courses/business/100018.
References
1. Cejka Executive Search. 2016 Physician Leadership Compensation Survey results released. Cejka and the American Association for Physician Leadership. Nov 3, 2016.
2. Knowles M. Salaries on upswing for physician executives. Becker’s Hospital Review. Sept 25, 2018.
3. Birrer RB. Becoming a physician executive. Health Progress: Journal of the Catholic Health Association of the United States. Jan-Feb 2003.
4. Witt/Kieffer. Transformation of physician executives: New accountability for quality, performance, integration. Fall 2010.
5. Jurica J. Does an executive salary stand up to a clinical salary? Vital Physician Executive. 2016.
The best ways to start
It’s been said that physicians tend to fall into leadership roles. Few physicians set out to become leaders, and then one day they realize that they desire to be a leader and an agent for change.
They may be rotating through the chairmanship of a clinical department or the management of a small practice and decide they like the work. In a large organization, doctors get assigned to committees, or specialists agree to run a new service line for a while, and it changes their lives.
Some physicians have a natural aptitude for managerial work. Often, colleagues tell them they are a good fit, but they may still have some reservations. In any case, it’s good to do a bit of soul-searching before taking the leap.
1. Weigh the pluses and minuses of a leadership role
When you stand at the precipice of a totally new career in physician leadership, it’s worthwhile to step back and consider the pluses and minuses of the work.
One plus is that there may be fewer work hours than on the clinical side, but being a physician leader is by no means a 9-to-5 job. In a large organization, a physician on the executive team can be on administrative call – dealing with institutional crises on off-hours – for a length of time. Board and strategic planning retreats tend to occur on weekends, and you may need to attend frequent dinner meetings.
Another plus is that the pay is pretty good. In 2016, physician leaders in large organizations earned an average of $350,000 a year, according to a survey by Cejka Executive Search and the American Association for Physician Leadership (AAPL).1
On the minus side, an executive probably won’t be as beloved as a clinician serving a host of grateful patients. And you will not have the kind of job security that most clinicians have. There may be frequent turnover among health care executives because of change of top leadership, pressure for more profitability, or a host of other reasons.
2. Try on different roles
To decide whether you want to make a career of being a physician leader, it’s useful to try out several different jobs. Volunteer for committees or take on a special project if it’s possible to do so in your organization.
You can also volunteer for posts outside the organization, such as joining the board of your local cancer or heart association or helping them out on a committee. You might volunteer for Little League or a school or civic organization. Your choices are wide open. The goal is to get a feel for directing an organization and whether that fits your lifestyle.
Also, talk to current physician leaders. Contact a cross-section of people, including those who are unhappy with their jobs and those who had to struggle with their new roles. This will give you some good perspective into whether the work is right for you, as well as tips on how to cope.
3. Find a mentor
This is also a good time to find a mentor for your new calling. Choose a seasoned physician leader who can help you over the long haul – someone who can get you up to speed and then advise you during crucial junctures in your career.
Good mentors should be willing to spend the time with you, have your best interests in mind, and be willing to provide honest assessments. They can also help you find opportunities for further learning and professional growth.
Some organizations assign mentors to physicians they want to develop for leadership roles. You can also choose specific mentors to help you in areas where you think you need more work, such as finance, quality improvement, or information systems.
Choose a path
There are many different paths you can take as a physician leader. In large organizations in particular, there are more leadership jobs open to physicians than ever before.
Jobs open to physicians can be found in the areas of clinical quality and safety, population health, managed care, and information technology. You can even look beyond these traditional roles to jobs that don’t usually attract physicians, such as in strategy, innovation, patient experience, and fundraising. In these roles, you are often expected to continue doing some clinical work.
Physician leaders now tend to have more influence than in the past. According to the Cejka-AAPL survey, 61% of physician executives said they had more strategic input currently than in the previous year.
A roster of potential physician leader jobs
1. Executive-level roles
Vice president for medical affairs. This is the traditional role for the physician executive, which involves acting as a liaison with the organization’s physicians. These officers oversee quality of care as well as hiring, training, and performance evaluation of physicians on staff.
Chief medical officer (CMO). This is now the typical term for the highest medical role in the organization. The CMO is part of the C-suite team and participates in governance, strategic planning, and business operation decisions. CMOs may be responsible for supporting value-based strategies and making sure that those strategies are efficient and medically necessary.
Physician-in-chief. This is a new term for the hospital’s top physician, who works with the senior leadership team to maintain standards of care and customer service. The physician-in-chief may also oversee operational efficiency and support organizational transformation.
Chief clinical officer (CCO). CCOs oversee patient engagement and clinical quality outcomes. They may lead initiatives to reduce waste and improve care quality, and they can be involved in implementation of electronic health records (EHRs) and data integration. They may also assist in medical staff development, clinical integration, and physician partnerships.
2. Quality, safety, and research roles
Chief patient safety officer (CPSO). CPSOs oversee the hospital or health system’s patient safety initiatives. Their goal is to reduce medical errors and near-misses.
Chief quality officer (CQO). CQOs are responsible for collecting quality data and supporting patient safety efforts. They advise on quality initiatives and hold clinicians accountable for meeting specific quality indicators. They may also be involved in developing a culture of continuous improvement in the organization.
Chief research officer (CRO). CROs oversee the organization’s research activities, including clinical trials, internal investigator-initiated research programs, and sponsored studies.
3. Technology
Chief medical information officer (CMIO). The CMIO is the information technology (IT) department’s liaison with the clinical staff, working on selection and improvement of EHR systems. The CMIO finds new ways for EHRs to improve healthcare delivery in the organization.
Chief health information officer (CHIO). CHIOs deal with EHR implementation and health informatics. They may report to the chief information officer, the chief operations officer, or another C-suite executive, and they manage health informatics, telehealth, business and clinical intelligence, and predictive analytics initiatives.
Chief technology officer (CTO). CTOs oversee the organization’s technology capabilities. They are responsible for leading the IT team and contributing to the organization’s strategic plan.
4. Jobs not usually for physicians
There are other leadership positions that may not traditionally appeal to physicians but could be worth considering:
Chief experience officer (CXO). This involves evaluating and improving the inpatient experience. CXOs work with physicians and staff on their performance in this area.
Chief innovation officer (CIO). CIOs keep up with industry trends, market disruptions, and new opportunities, and support policy innovations and training initiatives.
Chief transformation officer (CTO). CTOs are responsible for carrying out major changes in the organization. They are supposed to act as role models for change.
5. Salaries for selected physician executives
In addition to placing the average salary for a physician leader at $350,000, the 2016 Cejka-AAPL survey pinpointed average salaries for specific types of physician leaders. Chief medical officers earned $388,000, chief patient safety officers and chief quality officers $375,000, and chief medical information officers $372,500, the survey found.
Several emerging physician leader roles – physician-in-chief, chief strategy officer, chief transformation officer, chief innovation officer, and chief integration officer – earned on average $499,000 a year, according to the survey.
Those jobs provided even higher salaries than the $437,500 reported by Cejka-AAPL for physician CEOs. In comparison, a CEO at a medical group with fewer than 200 physicians had an average salary of $438,500 in 2018, according to SullivanCotter, a health care workforce strategy company.2
Some types of physician leaders have seen unusually high pay raises recently. From 2013 to 2016, the average salary for CMIOs rose 18%, and physician leaders working at the corporate level in a health system saw median compensation rise 67%, the Cejka-AAPL survey found.
Moving ahead
For physician leaders, moving up the ladder often means reinventing yourself. If you’re leaving clinical practice, be sure to develop a solid CV for your new role so that if your leadership position doesn’t work out, you are able to find an appropriate new position.
According to a 2003 assessment, CMOs typically lasted 18-24 months on the job.3
Expect to make mistakes and try to learn from them. If necessary, move on to the next job. There is always a market for seasoned physician executives who took a few punches, learned something from the experience, and found something new.
Start to network
One way to navigate the challenges of a new role is to have a strong network, a group of colleagues and mentors who can help you figure out your path forward. They can serve as sounding boards and contacts for new jobs in an industry that is constantly changing.
A well-functioning network takes constant maintenance.
You can find people for your network by attending a variety of different meetings that physician leaders and other healthcare executives attend. Make a point of keeping their contact information on file and periodically reaching out to them.
Learn in a dyad
Some healthcare organizations assign physician leaders to dyads, where they are matched with nonphysicians who have skills that the physician lacks, such as finance, data management, or organizational politics.
Dyads are less effective when the nonphysician has all the authority and the physician is basically a figurehead. But in an effective dyad, both partners share authority and they can teach skills to each other. While the physician in the dyad brings clinical insight, the nonphysician can provide managerial know-how.
Seek out coaching
There may be points in your leadership career when you become aware of areas where you need improvement. You may have gotten negative feedback on communication skills or political sensitivity. Consider hiring an executive coach; coaches provide concentrated sessions over limited periods of time.
Coaches can also help you prepare for the future. They can help you find ways to promote yourself for new projects or create a network of allies. They also can help you establish yourself as a thought leader in a particular field through writing and speaking engagements.
Some organizations provide in-house coaches. It is worthwhile to take advantage of this benefit. If you need to find a coach on your own, ask mentors or people in your network for recommendations.
Getting to the top
It can take years to rise to the level of the corporate C-suite or even to CEO of a large organization. At the top levels of management, you often have to cut back substantially on clinical work or even give it up entirely.
Becoming CEO of a hospital can be a logical fit for physicians. A physician CEO can relate to doctors on staff, who are a key constituency, and understands what clinical care is all about. However, physician CEOs also need to have a large degree of knowledge about finance, strategy, crisis management, quality improvement, and other nonclinical considerations, not to mention good people skills.
Physicians on boards
Some physicians would rather sit on the board of trustees than take the reins of CEO. Board membership allows you to continue practicing while still having a great deal of influence over the organization. Some physicians hold board seats for many years and enjoy a great deal of respect as the go-to person on clinical care.
Physicians are increasingly serving on the boards of hospitals and health systems. Trustees welcome physicians because they want more input from clinicians in decision-making. They tend to choose physicians who already have executive duties, such as having been a department head.
Which new skills should you learn?
Physician executives often put off learning business and management skills until after being appointed to a leadership position. Even then, they may prefer to take courses focused on a particular topic rather than earn a degree such as master of business administration (MBA).
Learning on the job
A number of executive skills can be learned on the job, such as dealing with quality measures, utilization, billing and coding, disease management, committees, and interpreting data. If you are not in a dyad model, you can ask someone knowledgeable in one of these skills to take you through the steps.
Many physicians could benefit from finance and business courses in order to learn some of the budgetary, accounting, and operational skills required to perform the job optimally.
In a survey of healthcare CEOs, only 30% said their most senior physician leader had a business or medical management degree, and only 21% required a degree.4
Taking classes
Physician executives who want to brush up on a particular topic can “mix and match,” taking short, focused classes on the particular topic whenever they feel the need. In addition to resources offered by SHM, courses are available from organizations such as the AAPL, American Hospital Association, the Medical Group Management Association, or the Healthcare Information and Management Systems Society, to name a few examples.
Pursuing degree programs
Degree programs like MBA, master of public health (MPH), and master of health care administration (MHA) are popular with many physician executives because they get a full overview of needed skills and the potential to earn more money with their new credentials. Physician leaders with an MBA earned 13% more in 2016 than did those with no MBA, according to the Cejka-AAPL survey.
Getting a master’s degree, however, takes time and money. For example, an MBA can cost $20,000 to as much as $100,000.5 MBA, MHA, and MPH degrees take 2 years to complete, while a master of medical management (MMM) and a physician-executive MBA – focusing specifically on what physician leaders need to learn – take 1 year.
Many part-time degree programs are available for those with full-time jobs. You can find them at nearby universities as well as far-off institutions. Much of the coursework is done online, but some on-site work is usually required. You’ll find that working directly with others enriches the learning experience and helps you build your network of colleagues.
Straight MBA or other degree?
In general, degree programs cover finance, communication, strategy, information systems, marketing, organizational behavior, operational management, and quality improvement. Straight MBA programs don’t focus on healthcare, but some physician executives still prefer this route, especially if it involves degrees from prestigious business schools.
MHA, MMM, physician-executive MBA, and other degree programs focused on healthcare are popular with many physicians on the executive track. The MPH is less business-oriented but may be preferable to some because of its focus on population-based health, which fits well with decision-making on health insurance and value-based care.
Conclusion
Physicians need to prepare for leadership because these roles are very different from clinical work. It’s easy to stumble and lose direction without mentors, a network of helpful colleagues, and at least some education in business principles.
Finding a mentor should start early in your new career. A seasoned physician executive can help you understand your options and point out your strengths and shortcomings. Beyond that, concentrated work with an executive coach can help you improve your skills and choose from among the many executive roles that are now available.
You can learn many skills on the job through dyads and other relationships with more seasoned colleagues, or take short classes on particular skills that need to be learned or sharpened. Many physician executives go a step further and get a master’s degree, such as an MBA, MHA, or MMM. This involves a year or two of study, but much of it can be done online.
This article is excerpted from the Medscape Physician Business Academy course “How to become an effective leader.” You can find more information on the course at www.medscape.com/courses/business/100018.
References
1. Cejka Executive Search. 2016 Physician Leadership Compensation Survey results released. Cejka and the American Association for Physician Leadership. Nov 3, 2016.
2. Knowles M. Salaries on upswing for physician executives. Becker’s Hospital Review. Sept 25, 2018.
3. Birrer RB. Becoming a physician executive. Health Progress: Journal of the Catholic Health Association of the United States. Jan-Feb 2003.
4. Witt/Kieffer. Transformation of physician executives: New accountability for quality, performance, integration. Fall 2010.
5. Jurica J. Does an executive salary stand up to a clinical salary? Vital Physician Executive. 2016.
The best ways to start
It’s been said that physicians tend to fall into leadership roles. Few physicians set out to become leaders, and then one day they realize that they desire to be a leader and an agent for change.
They may be rotating through the chairmanship of a clinical department or the management of a small practice and decide they like the work. In a large organization, doctors get assigned to committees, or specialists agree to run a new service line for a while, and it changes their lives.
Some physicians have a natural aptitude for managerial work. Often, colleagues tell them they are a good fit, but they may still have some reservations. In any case, it’s good to do a bit of soul-searching before taking the leap.
1. Weigh the pluses and minuses of a leadership role
When you stand at the precipice of a totally new career in physician leadership, it’s worthwhile to step back and consider the pluses and minuses of the work.
One plus is that there may be fewer work hours than on the clinical side, but being a physician leader is by no means a 9-to-5 job. In a large organization, a physician on the executive team can be on administrative call – dealing with institutional crises on off-hours – for a length of time. Board and strategic planning retreats tend to occur on weekends, and you may need to attend frequent dinner meetings.
Another plus is that the pay is pretty good. In 2016, physician leaders in large organizations earned an average of $350,000 a year, according to a survey by Cejka Executive Search and the American Association for Physician Leadership (AAPL).1
On the minus side, an executive probably won’t be as beloved as a clinician serving a host of grateful patients. And you will not have the kind of job security that most clinicians have. There may be frequent turnover among health care executives because of change of top leadership, pressure for more profitability, or a host of other reasons.
2. Try on different roles
To decide whether you want to make a career of being a physician leader, it’s useful to try out several different jobs. Volunteer for committees or take on a special project if it’s possible to do so in your organization.
You can also volunteer for posts outside the organization, such as joining the board of your local cancer or heart association or helping them out on a committee. You might volunteer for Little League or a school or civic organization. Your choices are wide open. The goal is to get a feel for directing an organization and whether that fits your lifestyle.
Also, talk to current physician leaders. Contact a cross-section of people, including those who are unhappy with their jobs and those who had to struggle with their new roles. This will give you some good perspective into whether the work is right for you, as well as tips on how to cope.
3. Find a mentor
This is also a good time to find a mentor for your new calling. Choose a seasoned physician leader who can help you over the long haul – someone who can get you up to speed and then advise you during crucial junctures in your career.
Good mentors should be willing to spend the time with you, have your best interests in mind, and be willing to provide honest assessments. They can also help you find opportunities for further learning and professional growth.
Some organizations assign mentors to physicians they want to develop for leadership roles. You can also choose specific mentors to help you in areas where you think you need more work, such as finance, quality improvement, or information systems.
Choose a path
There are many different paths you can take as a physician leader. In large organizations in particular, there are more leadership jobs open to physicians than ever before.
Jobs open to physicians can be found in the areas of clinical quality and safety, population health, managed care, and information technology. You can even look beyond these traditional roles to jobs that don’t usually attract physicians, such as in strategy, innovation, patient experience, and fundraising. In these roles, you are often expected to continue doing some clinical work.
Physician leaders now tend to have more influence than in the past. According to the Cejka-AAPL survey, 61% of physician executives said they had more strategic input currently than in the previous year.
A roster of potential physician leader jobs
1. Executive-level roles
Vice president for medical affairs. This is the traditional role for the physician executive, which involves acting as a liaison with the organization’s physicians. These officers oversee quality of care as well as hiring, training, and performance evaluation of physicians on staff.
Chief medical officer (CMO). This is now the typical term for the highest medical role in the organization. The CMO is part of the C-suite team and participates in governance, strategic planning, and business operation decisions. CMOs may be responsible for supporting value-based strategies and making sure that those strategies are efficient and medically necessary.
Physician-in-chief. This is a new term for the hospital’s top physician, who works with the senior leadership team to maintain standards of care and customer service. The physician-in-chief may also oversee operational efficiency and support organizational transformation.
Chief clinical officer (CCO). CCOs oversee patient engagement and clinical quality outcomes. They may lead initiatives to reduce waste and improve care quality, and they can be involved in implementation of electronic health records (EHRs) and data integration. They may also assist in medical staff development, clinical integration, and physician partnerships.
2. Quality, safety, and research roles
Chief patient safety officer (CPSO). CPSOs oversee the hospital or health system’s patient safety initiatives. Their goal is to reduce medical errors and near-misses.
Chief quality officer (CQO). CQOs are responsible for collecting quality data and supporting patient safety efforts. They advise on quality initiatives and hold clinicians accountable for meeting specific quality indicators. They may also be involved in developing a culture of continuous improvement in the organization.
Chief research officer (CRO). CROs oversee the organization’s research activities, including clinical trials, internal investigator-initiated research programs, and sponsored studies.
3. Technology
Chief medical information officer (CMIO). The CMIO is the information technology (IT) department’s liaison with the clinical staff, working on selection and improvement of EHR systems. The CMIO finds new ways for EHRs to improve healthcare delivery in the organization.
Chief health information officer (CHIO). CHIOs deal with EHR implementation and health informatics. They may report to the chief information officer, the chief operations officer, or another C-suite executive, and they manage health informatics, telehealth, business and clinical intelligence, and predictive analytics initiatives.
Chief technology officer (CTO). CTOs oversee the organization’s technology capabilities. They are responsible for leading the IT team and contributing to the organization’s strategic plan.
4. Jobs not usually for physicians
There are other leadership positions that may not traditionally appeal to physicians but could be worth considering:
Chief experience officer (CXO). This involves evaluating and improving the inpatient experience. CXOs work with physicians and staff on their performance in this area.
Chief innovation officer (CIO). CIOs keep up with industry trends, market disruptions, and new opportunities, and support policy innovations and training initiatives.
Chief transformation officer (CTO). CTOs are responsible for carrying out major changes in the organization. They are supposed to act as role models for change.
5. Salaries for selected physician executives
In addition to placing the average salary for a physician leader at $350,000, the 2016 Cejka-AAPL survey pinpointed average salaries for specific types of physician leaders. Chief medical officers earned $388,000, chief patient safety officers and chief quality officers $375,000, and chief medical information officers $372,500, the survey found.
Several emerging physician leader roles – physician-in-chief, chief strategy officer, chief transformation officer, chief innovation officer, and chief integration officer – earned on average $499,000 a year, according to the survey.
Those jobs provided even higher salaries than the $437,500 reported by Cejka-AAPL for physician CEOs. In comparison, a CEO at a medical group with fewer than 200 physicians had an average salary of $438,500 in 2018, according to SullivanCotter, a health care workforce strategy company.2
Some types of physician leaders have seen unusually high pay raises recently. From 2013 to 2016, the average salary for CMIOs rose 18%, and physician leaders working at the corporate level in a health system saw median compensation rise 67%, the Cejka-AAPL survey found.
Moving ahead
For physician leaders, moving up the ladder often means reinventing yourself. If you’re leaving clinical practice, be sure to develop a solid CV for your new role so that if your leadership position doesn’t work out, you are able to find an appropriate new position.
According to a 2003 assessment, CMOs typically lasted 18-24 months on the job.3
Expect to make mistakes and try to learn from them. If necessary, move on to the next job. There is always a market for seasoned physician executives who took a few punches, learned something from the experience, and found something new.
Start to network
One way to navigate the challenges of a new role is to have a strong network, a group of colleagues and mentors who can help you figure out your path forward. They can serve as sounding boards and contacts for new jobs in an industry that is constantly changing.
A well-functioning network takes constant maintenance.
You can find people for your network by attending a variety of different meetings that physician leaders and other healthcare executives attend. Make a point of keeping their contact information on file and periodically reaching out to them.
Learn in a dyad
Some healthcare organizations assign physician leaders to dyads, where they are matched with nonphysicians who have skills that the physician lacks, such as finance, data management, or organizational politics.
Dyads are less effective when the nonphysician has all the authority and the physician is basically a figurehead. But in an effective dyad, both partners share authority and they can teach skills to each other. While the physician in the dyad brings clinical insight, the nonphysician can provide managerial know-how.
Seek out coaching
There may be points in your leadership career when you become aware of areas where you need improvement. You may have gotten negative feedback on communication skills or political sensitivity. Consider hiring an executive coach; coaches provide concentrated sessions over limited periods of time.
Coaches can also help you prepare for the future. They can help you find ways to promote yourself for new projects or create a network of allies. They also can help you establish yourself as a thought leader in a particular field through writing and speaking engagements.
Some organizations provide in-house coaches. It is worthwhile to take advantage of this benefit. If you need to find a coach on your own, ask mentors or people in your network for recommendations.
Getting to the top
It can take years to rise to the level of the corporate C-suite or even to CEO of a large organization. At the top levels of management, you often have to cut back substantially on clinical work or even give it up entirely.
Becoming CEO of a hospital can be a logical fit for physicians. A physician CEO can relate to doctors on staff, who are a key constituency, and understands what clinical care is all about. However, physician CEOs also need to have a large degree of knowledge about finance, strategy, crisis management, quality improvement, and other nonclinical considerations, not to mention good people skills.
Physicians on boards
Some physicians would rather sit on the board of trustees than take the reins of CEO. Board membership allows you to continue practicing while still having a great deal of influence over the organization. Some physicians hold board seats for many years and enjoy a great deal of respect as the go-to person on clinical care.
Physicians are increasingly serving on the boards of hospitals and health systems. Trustees welcome physicians because they want more input from clinicians in decision-making. They tend to choose physicians who already have executive duties, such as having been a department head.
Which new skills should you learn?
Physician executives often put off learning business and management skills until after being appointed to a leadership position. Even then, they may prefer to take courses focused on a particular topic rather than earn a degree such as master of business administration (MBA).
Learning on the job
A number of executive skills can be learned on the job, such as dealing with quality measures, utilization, billing and coding, disease management, committees, and interpreting data. If you are not in a dyad model, you can ask someone knowledgeable in one of these skills to take you through the steps.
Many physicians could benefit from finance and business courses in order to learn some of the budgetary, accounting, and operational skills required to perform the job optimally.
In a survey of healthcare CEOs, only 30% said their most senior physician leader had a business or medical management degree, and only 21% required a degree.4
Taking classes
Physician executives who want to brush up on a particular topic can “mix and match,” taking short, focused classes on the particular topic whenever they feel the need. In addition to resources offered by SHM, courses are available from organizations such as the AAPL, American Hospital Association, the Medical Group Management Association, or the Healthcare Information and Management Systems Society, to name a few examples.
Pursuing degree programs
Degree programs like MBA, master of public health (MPH), and master of health care administration (MHA) are popular with many physician executives because they get a full overview of needed skills and the potential to earn more money with their new credentials. Physician leaders with an MBA earned 13% more in 2016 than did those with no MBA, according to the Cejka-AAPL survey.
Getting a master’s degree, however, takes time and money. For example, an MBA can cost $20,000 to as much as $100,000.5 MBA, MHA, and MPH degrees take 2 years to complete, while a master of medical management (MMM) and a physician-executive MBA – focusing specifically on what physician leaders need to learn – take 1 year.
Many part-time degree programs are available for those with full-time jobs. You can find them at nearby universities as well as far-off institutions. Much of the coursework is done online, but some on-site work is usually required. You’ll find that working directly with others enriches the learning experience and helps you build your network of colleagues.
Straight MBA or other degree?
In general, degree programs cover finance, communication, strategy, information systems, marketing, organizational behavior, operational management, and quality improvement. Straight MBA programs don’t focus on healthcare, but some physician executives still prefer this route, especially if it involves degrees from prestigious business schools.
MHA, MMM, physician-executive MBA, and other degree programs focused on healthcare are popular with many physicians on the executive track. The MPH is less business-oriented but may be preferable to some because of its focus on population-based health, which fits well with decision-making on health insurance and value-based care.
Conclusion
Physicians need to prepare for leadership because these roles are very different from clinical work. It’s easy to stumble and lose direction without mentors, a network of helpful colleagues, and at least some education in business principles.
Finding a mentor should start early in your new career. A seasoned physician executive can help you understand your options and point out your strengths and shortcomings. Beyond that, concentrated work with an executive coach can help you improve your skills and choose from among the many executive roles that are now available.
You can learn many skills on the job through dyads and other relationships with more seasoned colleagues, or take short classes on particular skills that need to be learned or sharpened. Many physician executives go a step further and get a master’s degree, such as an MBA, MHA, or MMM. This involves a year or two of study, but much of it can be done online.
This article is excerpted from the Medscape Physician Business Academy course “How to become an effective leader.” You can find more information on the course at www.medscape.com/courses/business/100018.
References
1. Cejka Executive Search. 2016 Physician Leadership Compensation Survey results released. Cejka and the American Association for Physician Leadership. Nov 3, 2016.
2. Knowles M. Salaries on upswing for physician executives. Becker’s Hospital Review. Sept 25, 2018.
3. Birrer RB. Becoming a physician executive. Health Progress: Journal of the Catholic Health Association of the United States. Jan-Feb 2003.
4. Witt/Kieffer. Transformation of physician executives: New accountability for quality, performance, integration. Fall 2010.
5. Jurica J. Does an executive salary stand up to a clinical salary? Vital Physician Executive. 2016.
Managing acute pain in inpatients on OUD therapy
“This is something we’re going to see more frequently, and many of us already have,” Theresa E. Vettese, MD, said at HM20 Virtual, hosted by the Society of Hospital Medicine.
The drastic drop in prescriptions for opioid pain medications in the last several years hasn’t curtailed the current opioid epidemic. Instead, the epidemic has to a great extent morphed into expanded use of illicit heroin and fentanyl, noted Dr. Vettese, an internist, hospitalist, and palliative care physician at Emory University and Grady Memorial Hospital in Atlanta.
Mythbusting
Treatment of acute pain in hospitalized patients on opioid agonist therapy for opioid use disorder (OUD) is actually pretty straightforward once a few common myths have been dispelled, she said.
One of these myths –common among both physicians and patients in treatment for OUD – is that prescribing opioids for management of acute pain will place such patients at risk for OUD relapse.
“In fact, the data really strongly suggest this is not the case,” Dr. Vettese said. “It will not worsen addiction. But if we don’t aggressively treat these patients’ acute pain, it puts them at higher risk for bad outcomes.”
Another myth – this one not uncommon among hospital pharmacy departments – is that only physicians with a special certification can prescribe methadone for inpatients.
“The federal laws are clear: Any physician who has a DEA license can prescribe methadone in the hospital acute care setting, not only for pain management, but also for treatment of opioid withdrawal. You can’t prescribe it in the outpatient setting for opioid withdrawal – that has to be dispensed through a federally regulated methadone outpatient treatment program. But in the hospital, we can feel safe that we can do so. You may need to educate your pharmacist about this,” she said.
Hospitalists also can prescribe buprenorphine in the acute care inpatient setting, both for pain and treatment of opioid withdrawal, without need for a DEA waiver.
“It’s useful to get some skills in using buprenorphine in the inpatient setting. You don’t need an X waiver, but I encourage everyone to do the X-waiver training because it’s a terrific educational session. It’s 8 hours for physicians and well worth it,” Dr. Vettese noted.
By federal law the inpatient physician also can prescribe 3 days of buprenorphine at discharge to get the patient to an outpatient provider.
Misconceptions also abound about NSAIDs as a nonopioid component of acute pain management in hospitalized patients. They actually are extremely effective for the treatment of musculoskeletal, orthopedic, procedural, migraine, and some types of cancer pain. The number needed to treat (NNT) for postoperative pain relief for ibuprofen or celecoxib is 2.5, and when used in conjunction with acetaminophen at 325 mg every 4 hours, that NNT drops to 1.5, similar to the NNT of 1.7 for oxycodone at 15 mg. It should be noted, however, that the bar defining effective pain relief in randomized studies is set rather low: A 50% greater reduction in pain than achieved with placebo.
Many hospitalists would like to use NSAIDs more often, but they’re leery of the associated risks of GI bleeding, ischemic cardiovascular events, and worsening kidney function. Dr. Vettese offered several risk-mitigation strategies to increase the use of NSAIDs as opioid-sparing agents for acute pain management.
She has changed her own clinical practice with regard to using NSAIDs in patients with chronic kidney disease in response to a 2019 systematic review by investigators at the University of Ottawa.
“This was a game changer for me because in this review, low-dose NSAIDs were safe in that they didn’t significantly increase the risk of worsening kidney failure even in patients with stage 3 chronic kidney disease. So this has expanded my use of NSAIDs in this population through stage 3 CKD. With a creatinine clearance below 30, however, kidney failure worsened rapidly, so I don’t do it in patients with CKD stage 4,” Dr. Vettese said.
Gastroenterologists categorize patients as being at high risk of GI bleeding related to NSAID use if they have a history of a complicated ulcer or they have at least three of the following risk factors: Age above 65 years, history of an uncomplicated ulcer, being on high-dose NSAID therapy, or concurrent use of aspirin, glucocorticoids, or anticoagulants. Patients are considered at moderate risk if they have one or two of the risk factors, and low risk if they have none. Dr. Vettese said that, while NSAIDs clearly should be avoided in the high-risk group, moderate-risk patients are a different matter.
“Many avoid the use of NSAIDs with moderate risk, but I think we can expand their use if we use the right NSAID and we use protective strategies,” Dr. Vettese said.
Celecoxib is the safest drug in terms of upper GI bleeding risk, but ibuprofen is close. They are associated with a 2.2-fold increased risk of bleeding when compared with risk in patients not on an NSAID. Naproxen or indeomethacin use carries a fourfold to fivefold increased risk.
“Celecoxib with a proton pump inhibitor is safest, followed by celecoxib alone, followed by ibuprofen with a proton pump inhibitor. So I advocate using NSAIDs more frequently in people who are at moderate risk by using them with a PPI,” she said.
There is persuasive evidence of increased cardiovascular risk in association with even short-duration NSAIDs, as the drugs are utilized in the treatment of acute pain in hospitalized patients. That being said, Dr. Vettese believes hospitalists can use these drugs safely in more patients by following a thoughtful cardiovascular risk-mitigation strategy developed by Italian investigators.
Communicating about pain management
“Communication is always the key to effective pain management in every situation,” Dr. Vettese emphasized.
“I talk to the patient about the goals of effective pain management. I’ll discourage the use of the 1-10 pain scale, and instead, I’ll be honest about expectations, saying, ‘You have a problem that will cause acute pain, and it’s unlikely that I will be able to completely relieve your pain. The goal is to improve your function so that you can get up and go the bathroom by yourself, and so that you can sleep for a few hours. That’s how we’re going to measure the efficacy of our pain-management program.’ ”
She explains to the patient that she’ll be using nonopioid medications and nondrug therapies along with oral opioid pain medications, which are less risky than IV opioids. She offers reassurance that this treatment strategy won’t cause an OUD relapse. She lets the patient know up-front that the opioids will be tapered as the acute pain improves.
For the patient who comes into the hospital on buprenorphine for OUD, she immediately checks with the state prescription drug monitoring program to make sure everything is above board and there’s no indication of doctor shopping for prescriptions. For in-hospital acute pain, it’s safe and effective to continue the outpatient dose. On an outpatient basis, however, the drug is given once daily. On that dosing schedule both the euphoric effect as well as the analgesic effect are lost, so for acute pain management in the hospital it’s recommended to split the dose into twice- or thrice-daily doses to achieve an analgesic effect.
Oral NSAIDs are part of the treatment strategy whenever possible. For severe acute pain, Dr. Vettese will prescribe an immediate-release opioid having a high affinity to the mu opioid receptor, such as oral hydromorphone, on an as-needed basis. The drug has onset of effect in 30 minutes, peak effect in 1 hour, and a duration of effect of 4-6 hours, although she recommends going with 4 hours to provide adequate analgesia.
“These patients will require much higher doses than the patients who are opioid naive,” she advised.
For the patient with acute pain who is admitted while on methadone for OUD, it’s important to call the outpatient treatment program to verify the dosage.
“You can split the dose of methadone to try to get better analgesia, although I can tell you that patients who are treated with methadone for OUD frequently don’t want to do that. And if they don’t want to, then I don’t,” the hospitalist said.
As with the patient on buprenorphine for OUD, she’ll use additional oral immediate-release opioids as needed for acute severe pain in a patient on methadone for medication-assisted OUD treatment.
Dr. Vettese reported having no financial conflicts regarding her presentation.
“This is something we’re going to see more frequently, and many of us already have,” Theresa E. Vettese, MD, said at HM20 Virtual, hosted by the Society of Hospital Medicine.
The drastic drop in prescriptions for opioid pain medications in the last several years hasn’t curtailed the current opioid epidemic. Instead, the epidemic has to a great extent morphed into expanded use of illicit heroin and fentanyl, noted Dr. Vettese, an internist, hospitalist, and palliative care physician at Emory University and Grady Memorial Hospital in Atlanta.
Mythbusting
Treatment of acute pain in hospitalized patients on opioid agonist therapy for opioid use disorder (OUD) is actually pretty straightforward once a few common myths have been dispelled, she said.
One of these myths –common among both physicians and patients in treatment for OUD – is that prescribing opioids for management of acute pain will place such patients at risk for OUD relapse.
“In fact, the data really strongly suggest this is not the case,” Dr. Vettese said. “It will not worsen addiction. But if we don’t aggressively treat these patients’ acute pain, it puts them at higher risk for bad outcomes.”
Another myth – this one not uncommon among hospital pharmacy departments – is that only physicians with a special certification can prescribe methadone for inpatients.
“The federal laws are clear: Any physician who has a DEA license can prescribe methadone in the hospital acute care setting, not only for pain management, but also for treatment of opioid withdrawal. You can’t prescribe it in the outpatient setting for opioid withdrawal – that has to be dispensed through a federally regulated methadone outpatient treatment program. But in the hospital, we can feel safe that we can do so. You may need to educate your pharmacist about this,” she said.
Hospitalists also can prescribe buprenorphine in the acute care inpatient setting, both for pain and treatment of opioid withdrawal, without need for a DEA waiver.
“It’s useful to get some skills in using buprenorphine in the inpatient setting. You don’t need an X waiver, but I encourage everyone to do the X-waiver training because it’s a terrific educational session. It’s 8 hours for physicians and well worth it,” Dr. Vettese noted.
By federal law the inpatient physician also can prescribe 3 days of buprenorphine at discharge to get the patient to an outpatient provider.
Misconceptions also abound about NSAIDs as a nonopioid component of acute pain management in hospitalized patients. They actually are extremely effective for the treatment of musculoskeletal, orthopedic, procedural, migraine, and some types of cancer pain. The number needed to treat (NNT) for postoperative pain relief for ibuprofen or celecoxib is 2.5, and when used in conjunction with acetaminophen at 325 mg every 4 hours, that NNT drops to 1.5, similar to the NNT of 1.7 for oxycodone at 15 mg. It should be noted, however, that the bar defining effective pain relief in randomized studies is set rather low: A 50% greater reduction in pain than achieved with placebo.
Many hospitalists would like to use NSAIDs more often, but they’re leery of the associated risks of GI bleeding, ischemic cardiovascular events, and worsening kidney function. Dr. Vettese offered several risk-mitigation strategies to increase the use of NSAIDs as opioid-sparing agents for acute pain management.
She has changed her own clinical practice with regard to using NSAIDs in patients with chronic kidney disease in response to a 2019 systematic review by investigators at the University of Ottawa.
“This was a game changer for me because in this review, low-dose NSAIDs were safe in that they didn’t significantly increase the risk of worsening kidney failure even in patients with stage 3 chronic kidney disease. So this has expanded my use of NSAIDs in this population through stage 3 CKD. With a creatinine clearance below 30, however, kidney failure worsened rapidly, so I don’t do it in patients with CKD stage 4,” Dr. Vettese said.
Gastroenterologists categorize patients as being at high risk of GI bleeding related to NSAID use if they have a history of a complicated ulcer or they have at least three of the following risk factors: Age above 65 years, history of an uncomplicated ulcer, being on high-dose NSAID therapy, or concurrent use of aspirin, glucocorticoids, or anticoagulants. Patients are considered at moderate risk if they have one or two of the risk factors, and low risk if they have none. Dr. Vettese said that, while NSAIDs clearly should be avoided in the high-risk group, moderate-risk patients are a different matter.
“Many avoid the use of NSAIDs with moderate risk, but I think we can expand their use if we use the right NSAID and we use protective strategies,” Dr. Vettese said.
Celecoxib is the safest drug in terms of upper GI bleeding risk, but ibuprofen is close. They are associated with a 2.2-fold increased risk of bleeding when compared with risk in patients not on an NSAID. Naproxen or indeomethacin use carries a fourfold to fivefold increased risk.
“Celecoxib with a proton pump inhibitor is safest, followed by celecoxib alone, followed by ibuprofen with a proton pump inhibitor. So I advocate using NSAIDs more frequently in people who are at moderate risk by using them with a PPI,” she said.
There is persuasive evidence of increased cardiovascular risk in association with even short-duration NSAIDs, as the drugs are utilized in the treatment of acute pain in hospitalized patients. That being said, Dr. Vettese believes hospitalists can use these drugs safely in more patients by following a thoughtful cardiovascular risk-mitigation strategy developed by Italian investigators.
Communicating about pain management
“Communication is always the key to effective pain management in every situation,” Dr. Vettese emphasized.
“I talk to the patient about the goals of effective pain management. I’ll discourage the use of the 1-10 pain scale, and instead, I’ll be honest about expectations, saying, ‘You have a problem that will cause acute pain, and it’s unlikely that I will be able to completely relieve your pain. The goal is to improve your function so that you can get up and go the bathroom by yourself, and so that you can sleep for a few hours. That’s how we’re going to measure the efficacy of our pain-management program.’ ”
She explains to the patient that she’ll be using nonopioid medications and nondrug therapies along with oral opioid pain medications, which are less risky than IV opioids. She offers reassurance that this treatment strategy won’t cause an OUD relapse. She lets the patient know up-front that the opioids will be tapered as the acute pain improves.
For the patient who comes into the hospital on buprenorphine for OUD, she immediately checks with the state prescription drug monitoring program to make sure everything is above board and there’s no indication of doctor shopping for prescriptions. For in-hospital acute pain, it’s safe and effective to continue the outpatient dose. On an outpatient basis, however, the drug is given once daily. On that dosing schedule both the euphoric effect as well as the analgesic effect are lost, so for acute pain management in the hospital it’s recommended to split the dose into twice- or thrice-daily doses to achieve an analgesic effect.
Oral NSAIDs are part of the treatment strategy whenever possible. For severe acute pain, Dr. Vettese will prescribe an immediate-release opioid having a high affinity to the mu opioid receptor, such as oral hydromorphone, on an as-needed basis. The drug has onset of effect in 30 minutes, peak effect in 1 hour, and a duration of effect of 4-6 hours, although she recommends going with 4 hours to provide adequate analgesia.
“These patients will require much higher doses than the patients who are opioid naive,” she advised.
For the patient with acute pain who is admitted while on methadone for OUD, it’s important to call the outpatient treatment program to verify the dosage.
“You can split the dose of methadone to try to get better analgesia, although I can tell you that patients who are treated with methadone for OUD frequently don’t want to do that. And if they don’t want to, then I don’t,” the hospitalist said.
As with the patient on buprenorphine for OUD, she’ll use additional oral immediate-release opioids as needed for acute severe pain in a patient on methadone for medication-assisted OUD treatment.
Dr. Vettese reported having no financial conflicts regarding her presentation.
“This is something we’re going to see more frequently, and many of us already have,” Theresa E. Vettese, MD, said at HM20 Virtual, hosted by the Society of Hospital Medicine.
The drastic drop in prescriptions for opioid pain medications in the last several years hasn’t curtailed the current opioid epidemic. Instead, the epidemic has to a great extent morphed into expanded use of illicit heroin and fentanyl, noted Dr. Vettese, an internist, hospitalist, and palliative care physician at Emory University and Grady Memorial Hospital in Atlanta.
Mythbusting
Treatment of acute pain in hospitalized patients on opioid agonist therapy for opioid use disorder (OUD) is actually pretty straightforward once a few common myths have been dispelled, she said.
One of these myths –common among both physicians and patients in treatment for OUD – is that prescribing opioids for management of acute pain will place such patients at risk for OUD relapse.
“In fact, the data really strongly suggest this is not the case,” Dr. Vettese said. “It will not worsen addiction. But if we don’t aggressively treat these patients’ acute pain, it puts them at higher risk for bad outcomes.”
Another myth – this one not uncommon among hospital pharmacy departments – is that only physicians with a special certification can prescribe methadone for inpatients.
“The federal laws are clear: Any physician who has a DEA license can prescribe methadone in the hospital acute care setting, not only for pain management, but also for treatment of opioid withdrawal. You can’t prescribe it in the outpatient setting for opioid withdrawal – that has to be dispensed through a federally regulated methadone outpatient treatment program. But in the hospital, we can feel safe that we can do so. You may need to educate your pharmacist about this,” she said.
Hospitalists also can prescribe buprenorphine in the acute care inpatient setting, both for pain and treatment of opioid withdrawal, without need for a DEA waiver.
“It’s useful to get some skills in using buprenorphine in the inpatient setting. You don’t need an X waiver, but I encourage everyone to do the X-waiver training because it’s a terrific educational session. It’s 8 hours for physicians and well worth it,” Dr. Vettese noted.
By federal law the inpatient physician also can prescribe 3 days of buprenorphine at discharge to get the patient to an outpatient provider.
Misconceptions also abound about NSAIDs as a nonopioid component of acute pain management in hospitalized patients. They actually are extremely effective for the treatment of musculoskeletal, orthopedic, procedural, migraine, and some types of cancer pain. The number needed to treat (NNT) for postoperative pain relief for ibuprofen or celecoxib is 2.5, and when used in conjunction with acetaminophen at 325 mg every 4 hours, that NNT drops to 1.5, similar to the NNT of 1.7 for oxycodone at 15 mg. It should be noted, however, that the bar defining effective pain relief in randomized studies is set rather low: A 50% greater reduction in pain than achieved with placebo.
Many hospitalists would like to use NSAIDs more often, but they’re leery of the associated risks of GI bleeding, ischemic cardiovascular events, and worsening kidney function. Dr. Vettese offered several risk-mitigation strategies to increase the use of NSAIDs as opioid-sparing agents for acute pain management.
She has changed her own clinical practice with regard to using NSAIDs in patients with chronic kidney disease in response to a 2019 systematic review by investigators at the University of Ottawa.
“This was a game changer for me because in this review, low-dose NSAIDs were safe in that they didn’t significantly increase the risk of worsening kidney failure even in patients with stage 3 chronic kidney disease. So this has expanded my use of NSAIDs in this population through stage 3 CKD. With a creatinine clearance below 30, however, kidney failure worsened rapidly, so I don’t do it in patients with CKD stage 4,” Dr. Vettese said.
Gastroenterologists categorize patients as being at high risk of GI bleeding related to NSAID use if they have a history of a complicated ulcer or they have at least three of the following risk factors: Age above 65 years, history of an uncomplicated ulcer, being on high-dose NSAID therapy, or concurrent use of aspirin, glucocorticoids, or anticoagulants. Patients are considered at moderate risk if they have one or two of the risk factors, and low risk if they have none. Dr. Vettese said that, while NSAIDs clearly should be avoided in the high-risk group, moderate-risk patients are a different matter.
“Many avoid the use of NSAIDs with moderate risk, but I think we can expand their use if we use the right NSAID and we use protective strategies,” Dr. Vettese said.
Celecoxib is the safest drug in terms of upper GI bleeding risk, but ibuprofen is close. They are associated with a 2.2-fold increased risk of bleeding when compared with risk in patients not on an NSAID. Naproxen or indeomethacin use carries a fourfold to fivefold increased risk.
“Celecoxib with a proton pump inhibitor is safest, followed by celecoxib alone, followed by ibuprofen with a proton pump inhibitor. So I advocate using NSAIDs more frequently in people who are at moderate risk by using them with a PPI,” she said.
There is persuasive evidence of increased cardiovascular risk in association with even short-duration NSAIDs, as the drugs are utilized in the treatment of acute pain in hospitalized patients. That being said, Dr. Vettese believes hospitalists can use these drugs safely in more patients by following a thoughtful cardiovascular risk-mitigation strategy developed by Italian investigators.
Communicating about pain management
“Communication is always the key to effective pain management in every situation,” Dr. Vettese emphasized.
“I talk to the patient about the goals of effective pain management. I’ll discourage the use of the 1-10 pain scale, and instead, I’ll be honest about expectations, saying, ‘You have a problem that will cause acute pain, and it’s unlikely that I will be able to completely relieve your pain. The goal is to improve your function so that you can get up and go the bathroom by yourself, and so that you can sleep for a few hours. That’s how we’re going to measure the efficacy of our pain-management program.’ ”
She explains to the patient that she’ll be using nonopioid medications and nondrug therapies along with oral opioid pain medications, which are less risky than IV opioids. She offers reassurance that this treatment strategy won’t cause an OUD relapse. She lets the patient know up-front that the opioids will be tapered as the acute pain improves.
For the patient who comes into the hospital on buprenorphine for OUD, she immediately checks with the state prescription drug monitoring program to make sure everything is above board and there’s no indication of doctor shopping for prescriptions. For in-hospital acute pain, it’s safe and effective to continue the outpatient dose. On an outpatient basis, however, the drug is given once daily. On that dosing schedule both the euphoric effect as well as the analgesic effect are lost, so for acute pain management in the hospital it’s recommended to split the dose into twice- or thrice-daily doses to achieve an analgesic effect.
Oral NSAIDs are part of the treatment strategy whenever possible. For severe acute pain, Dr. Vettese will prescribe an immediate-release opioid having a high affinity to the mu opioid receptor, such as oral hydromorphone, on an as-needed basis. The drug has onset of effect in 30 minutes, peak effect in 1 hour, and a duration of effect of 4-6 hours, although she recommends going with 4 hours to provide adequate analgesia.
“These patients will require much higher doses than the patients who are opioid naive,” she advised.
For the patient with acute pain who is admitted while on methadone for OUD, it’s important to call the outpatient treatment program to verify the dosage.
“You can split the dose of methadone to try to get better analgesia, although I can tell you that patients who are treated with methadone for OUD frequently don’t want to do that. And if they don’t want to, then I don’t,” the hospitalist said.
As with the patient on buprenorphine for OUD, she’ll use additional oral immediate-release opioids as needed for acute severe pain in a patient on methadone for medication-assisted OUD treatment.
Dr. Vettese reported having no financial conflicts regarding her presentation.
FROM HM20 VIRTUAL
NBA star Mason Plumlee on COVID and life inside the Orlando ‘bubble’
Editor’s Note: This transcript from the August 20 episode of the Blood & Cancer podcast has been edited for clarity. Click this link to listen to the full episode.
David Henry, MD: Welcome to this Blood & Cancer podcast. I’m your host, Dr. David Henry. This podcast airs on Thursday morning each week. This interview and others are archived with show notes from our residents at Pennsylvania Hospital at this link.
Each week we interview key opinion leaders involved in various aspects of blood and cancer. Mason was a first round pick in the NBA, a gold medalist for the U.S. men’s national team, and NBA All-Rookie first team honoree. He’s one of the top playmaking forwards in the country, if not the world, in my opinion. In his four-year college career at Duke University, he helped lead the Blue Devils to a National Collegiate Athletic Association (NCAA) championship and twice earned All-America first team academic honors at Duke. So he’s not just a basketball star, but an academic star as well. Mason, thanks so much for taking some time out from the bubble in Florida to talk with us today.
Mason Plumlee: Thanks for having me on. I’m happy to be here.
Henry: Beginning in March, the NBA didn’t know what to do about the COVID pandemic but finally decided to put you professional players in a ‘bubble.’ What did you have to go through to get there? You, your teammates, coaches, trainers, etc. And what’s the ongoing plan to be sure you continue to be safe?
Plumlee: Back to when the season shut down in March, the NBA shut down the practice facilities at the same time. Most people went home. I went back to Indiana. And then, as the idea of this bubble came up and the NBA formalized a plan to start the season again, players started to go back to market. I went back to Denver and was working out there.
About two weeks before we were scheduled to arrive in Orlando, they started testing us every other day. They used the deep nasal swab as well as the throat swab. But they were also taking two to three blood tests in that time period. You needed a certain number of consecutive negative tests before they would allow you to fly on the team plane down to Orlando. So there was an incredible amount of testing in the market. Once you got to Orlando, you went into a 48-hour quarantine. You had to have two negative tests with 48 hours between them before you could leave your hotel room.
Since then, it’s been quite strict down here. And although it’s annoying in a lot of ways, I think it’s one of the reasons our league has been able to pull this off. We’ve had no positive tests within the bubble and we are tested every day. A company called BioReference Laboratories has a setup in one of the meeting rooms here, and it’s like clockwork—we go in, we get our tests. One of my teammates missed a test and they made him stay in his room until he could get another test and get the results, so he missed a game because of that.
Henry: During this bubble time, no one has tested positive—players, coaches, staff?
Plumlee: Correct.
Henry: That’s incredible, and it’s allowed those of us who want to watch the NBA and those of you who are in it professionally to continue the sport. It must be a real nuisance for you and your family and friends, because no one can visit you, right?
Plumlee: Right. There’s no visitation. We had one false positive. It was our media relations person and the actions they took when that positive test came in -- they quarantined him in his room and interviewed everybody he had talked to; they tested anyone who had any interaction with him and those people had to go into quarantine. They’re on top of things down here. In addition to the testing, we each have a pulse oximeter and a thermometer, and we use these to check in everyday on an app. So, they’re getting all the insight they need. After the first round of the playoffs, they’re going to open the bubble to friends and family, but those friends and family will be subject to all the same protocols that we were coming in and once they’re here as well.
Henry: I’m sure you’ve heard about the Broadway star [Nick Cordero] who was healthy and suddenly got sick, lost a leg, and then lost his life. There have been some heart attacks that surprised us. Have your colleagues—players, coaches, etc.—been worried? Or are they thinking, what’s the big deal? Has the sense of how serious this is permeated through this sport?
Plumlee: The NBA is one of the groups that has heightened the understanding and awareness of this by shutting down. I think a lot of people were moving forward as is, and then, when the NBA decided to cancel the season, it let the world know, look, this is to be taken seriously.
Henry: A couple of players did test positive early on.
Plumlee: Exactly. A couple of people tested positive. I think at the outset, the unknown is always scarier. As we’ve learned more about the virus, the guys have become more comfortable. You know, I tested positive back in March. At the time, a loss of taste and smell was not a reported symptom.
Henry: And you had that?
Plumlee: I did have that, but I didn’t know what to think. More research has come out and we have a better understanding of that. I think most of the players are comfortable with the virus. We’re at a time in our lives where we’re healthy, we’re active, and we should be able to fight it off. We know the numbers for our age group. Even still, I think nobody wants to get it. Nobody wants to have to go through it. So why chance it?
Henry: Hats off to you and your sport. Other sports such as Major League Baseball haven’t been quite so successful. Of course, they’re wrestling with the players testing positive, and this has stopped games this season.
I was looking over your background prior to the interview and learned that your mother and father have been involved in the medical arena. Can you tell us about that and how it’s rubbed off on you?
Plumlee: Definitely. My mom is a pharmacist, so I spent a lot of time as a kid going to see her at work. And my dad is general counsel for an orthopedic company. My hometown is Warsaw, Ind. Some people refer to it as the “Orthopedic Capital of the World.” Zimmer Biomet is headquartered there. DePuy Synthes is there. Medtronic has offices there, as well as a lot of cottage businesses that support the orthopedic industry. In my hometown, the rock star was Dane Miller, who founded Biomet. I have no formal education in medicine or health care, but I’ve seen the impact of it. From my parents and some cousins, uncles who are doctors and surgeons, it’s been interesting to see their work and learn about what’s the latest and greatest in health care.
Henry: What’s so nice about you in particular is, with that background of interests from your family and your celebrity and accomplishments in professional basketball, you have used that to explore and promote ways to make progress in health care and help others who are less fortunate. For example, you’re involved in a telehealth platform for all-in-one practice management; affordable telehealth for pediatrics; health benefits for small businesses; prior authorization—if you can help with prior authorization, we will be in the stands for you at every game because it’s the bane of our existence; radiotherapy; and probably from mom’s background, pharmacy benefit management. Pick any of those you’d like to talk about, and tell us about your involvement and how it’s going.
Plumlee: My ticket into the arena is investment. Nobody’s calling me, asking for my expertise. But a lot of these visionary founders need financial support, and that’s where I get involved. Then also, with the celebrity angle from being an athlete, sometimes you can open doors for a start-up founder that they may not be able to open themselves.
I’m happy to speak about any of those companies. I am excited about the relaxed regulation that’s come from the pandemic; not that it’s like the Wild West out here, but I think it has allowed companies to implement solutions or think about problems in a way that they couldn’t before the pandemic. Take the prior authorization play, for example, and a company called Banjo Health, with one of my favorite founders, a guy named Saar Mahna. Medicare mandates that you turn around prior authorizations within three days. This company has an artificial intelligence and machine-learning play on prior authorizations that can deliver on that.
So efficiencies, things that increase access or affordability, better outcomes, those are the things that attract me. I lean on other people for the due diligence. The pediatric play that you referenced is a company called Blueberry Pediatrics. You have a monthly subscription for $15 that can be reimbursed by Medicaid. They send two devices to your home—an otoscope and an oximeter. The company is live in Florida right now, and it’s diverting a ton of emergency room (ER) visits. From home, for $15 a month, a mom has an otoscope and an oximeter, and she can chat or video conference with a pediatrician. There’s no additional fee. So that’s saving everyone time and saving the system money. Those are the kinds of things I’m attracted to.
Henry: You’ve touched on a couple of hot button issues for us. In oncology, unfortunately, most of our patients have pain. I am mystified every time I try to get a narcotic or a strong painkiller for a patient on a Friday night and I’m told it requires prior authorization and they’ll open up again on Monday. Well, that’s insane. These patients need something right away. So if you have a special interest in helping all of us with prior authorization, the artificial intelligence is a no brainer. If this kind of computer algorithm could happen overnight, that would be wonderful.
You mentioned the ER. Many people go to the ER as a default. They don’t know what else to do. In the COVID era, we’re trying to dial that down because we want to be able to see the sickest and have the non-sick get care elsewhere. If this particular person or people don’t know what to do, they go to the ER, it costs money, takes a lot of time, and others who may be sick are diverted from care. Families worry terribly about their children, so a device for mom and access to a pediatrician for $15 a month is another wonderful idea. These are both very interesting. Another company is in the pharmacy benefit management (PBM) space. Anything you could say about how that works?
Plumlee: I can give an overview of how I look at this as an investor in the PBM space. Three companies control about 75% of a multibillion dollar market. Several initiatives have been pursued politically to provide transparent pricing between these PBMs and pharmaceutical companies, and a lot of people are pointing fingers, but ultimately, drug prices just keep going up. Everybody knows it.
A couple of start-up founders are really set on bringing a competitive marketplace back to the pharmacy benefit manager. As an investor, when you see three people controlling a market, and you have small or medium PBMs that depend on aggregators to get competitive pricing with those big three, you get interested. It’s an interesting industry. My feeling is that somebody is going to disrupt it and bring competition back to that space. Ultimately, drug prices will come down because it’s not sustainable. The insurance companies just accommodate whatever the drug pricing is. If the drug prices go up, your premiums go up. I think these new companies will be level-setting.
Henry: In my world of oncology, we’re just a little more than halfway through 2020 and we’ve had five, six, seven new drugs approved. They all will be very expensive. One of the nicer things that’s happening and may help to tamp this down involves biosimilars. When you go to CVS or Rite Aid, you go down the aspirin aisle and see the generics, and they’re identical to the brand name aspirin. Well, these very complex molecules we used to treat cancer are antibodies or proteins, and they’re made in nature’s factories called cells. They’re not identical to the brand name drugs, but they’re called biosimilars. They work exactly the same as the branded drugs with exactly the same safety–our U.S. FDA has done a nice job of vetting that, to be sure. X, Y, Z Company has copied the brand drug after the patent expires. They were hoping for about a 30% discount in price but we’re seeing more like 15%. Nothing’s ever easy. So you make a very good point. This is not sustainable and the competition will be wonderful to tamp down these prices.
Plumlee: My hope is that those biosimilars and generics get placement in these formularies because the formularies are what’s valuable to the drug manufacturers. But they have to accommodate what the Big Three want in the PBM space. To me, making things affordable and accessible is what a lot of these startups are trying to do. And hopefully they will win.
Henry: What have you been going through, in terms of COVID? Have you recovered fully? Have your taste and smell returned, and you’re back to normal?
Plumlee: I’m all good. It caught me off guard but the symptoms weren’t too intense. For me, it was less than a flu, but more than a cold. And I’m all good today.
Henry: We’re so glad and wish you the best of luck.
Dr. Henry is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia and the host of the Blood & Cancer podcast. He has no relevant financial conflicts.
Mr. Plumlee is a board advisor to both Formsense and the Prysm Institute and a board observer with Voiceitt.
Editor’s Note: This transcript from the August 20 episode of the Blood & Cancer podcast has been edited for clarity. Click this link to listen to the full episode.
David Henry, MD: Welcome to this Blood & Cancer podcast. I’m your host, Dr. David Henry. This podcast airs on Thursday morning each week. This interview and others are archived with show notes from our residents at Pennsylvania Hospital at this link.
Each week we interview key opinion leaders involved in various aspects of blood and cancer. Mason was a first round pick in the NBA, a gold medalist for the U.S. men’s national team, and NBA All-Rookie first team honoree. He’s one of the top playmaking forwards in the country, if not the world, in my opinion. In his four-year college career at Duke University, he helped lead the Blue Devils to a National Collegiate Athletic Association (NCAA) championship and twice earned All-America first team academic honors at Duke. So he’s not just a basketball star, but an academic star as well. Mason, thanks so much for taking some time out from the bubble in Florida to talk with us today.
Mason Plumlee: Thanks for having me on. I’m happy to be here.
Henry: Beginning in March, the NBA didn’t know what to do about the COVID pandemic but finally decided to put you professional players in a ‘bubble.’ What did you have to go through to get there? You, your teammates, coaches, trainers, etc. And what’s the ongoing plan to be sure you continue to be safe?
Plumlee: Back to when the season shut down in March, the NBA shut down the practice facilities at the same time. Most people went home. I went back to Indiana. And then, as the idea of this bubble came up and the NBA formalized a plan to start the season again, players started to go back to market. I went back to Denver and was working out there.
About two weeks before we were scheduled to arrive in Orlando, they started testing us every other day. They used the deep nasal swab as well as the throat swab. But they were also taking two to three blood tests in that time period. You needed a certain number of consecutive negative tests before they would allow you to fly on the team plane down to Orlando. So there was an incredible amount of testing in the market. Once you got to Orlando, you went into a 48-hour quarantine. You had to have two negative tests with 48 hours between them before you could leave your hotel room.
Since then, it’s been quite strict down here. And although it’s annoying in a lot of ways, I think it’s one of the reasons our league has been able to pull this off. We’ve had no positive tests within the bubble and we are tested every day. A company called BioReference Laboratories has a setup in one of the meeting rooms here, and it’s like clockwork—we go in, we get our tests. One of my teammates missed a test and they made him stay in his room until he could get another test and get the results, so he missed a game because of that.
Henry: During this bubble time, no one has tested positive—players, coaches, staff?
Plumlee: Correct.
Henry: That’s incredible, and it’s allowed those of us who want to watch the NBA and those of you who are in it professionally to continue the sport. It must be a real nuisance for you and your family and friends, because no one can visit you, right?
Plumlee: Right. There’s no visitation. We had one false positive. It was our media relations person and the actions they took when that positive test came in -- they quarantined him in his room and interviewed everybody he had talked to; they tested anyone who had any interaction with him and those people had to go into quarantine. They’re on top of things down here. In addition to the testing, we each have a pulse oximeter and a thermometer, and we use these to check in everyday on an app. So, they’re getting all the insight they need. After the first round of the playoffs, they’re going to open the bubble to friends and family, but those friends and family will be subject to all the same protocols that we were coming in and once they’re here as well.
Henry: I’m sure you’ve heard about the Broadway star [Nick Cordero] who was healthy and suddenly got sick, lost a leg, and then lost his life. There have been some heart attacks that surprised us. Have your colleagues—players, coaches, etc.—been worried? Or are they thinking, what’s the big deal? Has the sense of how serious this is permeated through this sport?
Plumlee: The NBA is one of the groups that has heightened the understanding and awareness of this by shutting down. I think a lot of people were moving forward as is, and then, when the NBA decided to cancel the season, it let the world know, look, this is to be taken seriously.
Henry: A couple of players did test positive early on.
Plumlee: Exactly. A couple of people tested positive. I think at the outset, the unknown is always scarier. As we’ve learned more about the virus, the guys have become more comfortable. You know, I tested positive back in March. At the time, a loss of taste and smell was not a reported symptom.
Henry: And you had that?
Plumlee: I did have that, but I didn’t know what to think. More research has come out and we have a better understanding of that. I think most of the players are comfortable with the virus. We’re at a time in our lives where we’re healthy, we’re active, and we should be able to fight it off. We know the numbers for our age group. Even still, I think nobody wants to get it. Nobody wants to have to go through it. So why chance it?
Henry: Hats off to you and your sport. Other sports such as Major League Baseball haven’t been quite so successful. Of course, they’re wrestling with the players testing positive, and this has stopped games this season.
I was looking over your background prior to the interview and learned that your mother and father have been involved in the medical arena. Can you tell us about that and how it’s rubbed off on you?
Plumlee: Definitely. My mom is a pharmacist, so I spent a lot of time as a kid going to see her at work. And my dad is general counsel for an orthopedic company. My hometown is Warsaw, Ind. Some people refer to it as the “Orthopedic Capital of the World.” Zimmer Biomet is headquartered there. DePuy Synthes is there. Medtronic has offices there, as well as a lot of cottage businesses that support the orthopedic industry. In my hometown, the rock star was Dane Miller, who founded Biomet. I have no formal education in medicine or health care, but I’ve seen the impact of it. From my parents and some cousins, uncles who are doctors and surgeons, it’s been interesting to see their work and learn about what’s the latest and greatest in health care.
Henry: What’s so nice about you in particular is, with that background of interests from your family and your celebrity and accomplishments in professional basketball, you have used that to explore and promote ways to make progress in health care and help others who are less fortunate. For example, you’re involved in a telehealth platform for all-in-one practice management; affordable telehealth for pediatrics; health benefits for small businesses; prior authorization—if you can help with prior authorization, we will be in the stands for you at every game because it’s the bane of our existence; radiotherapy; and probably from mom’s background, pharmacy benefit management. Pick any of those you’d like to talk about, and tell us about your involvement and how it’s going.
Plumlee: My ticket into the arena is investment. Nobody’s calling me, asking for my expertise. But a lot of these visionary founders need financial support, and that’s where I get involved. Then also, with the celebrity angle from being an athlete, sometimes you can open doors for a start-up founder that they may not be able to open themselves.
I’m happy to speak about any of those companies. I am excited about the relaxed regulation that’s come from the pandemic; not that it’s like the Wild West out here, but I think it has allowed companies to implement solutions or think about problems in a way that they couldn’t before the pandemic. Take the prior authorization play, for example, and a company called Banjo Health, with one of my favorite founders, a guy named Saar Mahna. Medicare mandates that you turn around prior authorizations within three days. This company has an artificial intelligence and machine-learning play on prior authorizations that can deliver on that.
So efficiencies, things that increase access or affordability, better outcomes, those are the things that attract me. I lean on other people for the due diligence. The pediatric play that you referenced is a company called Blueberry Pediatrics. You have a monthly subscription for $15 that can be reimbursed by Medicaid. They send two devices to your home—an otoscope and an oximeter. The company is live in Florida right now, and it’s diverting a ton of emergency room (ER) visits. From home, for $15 a month, a mom has an otoscope and an oximeter, and she can chat or video conference with a pediatrician. There’s no additional fee. So that’s saving everyone time and saving the system money. Those are the kinds of things I’m attracted to.
Henry: You’ve touched on a couple of hot button issues for us. In oncology, unfortunately, most of our patients have pain. I am mystified every time I try to get a narcotic or a strong painkiller for a patient on a Friday night and I’m told it requires prior authorization and they’ll open up again on Monday. Well, that’s insane. These patients need something right away. So if you have a special interest in helping all of us with prior authorization, the artificial intelligence is a no brainer. If this kind of computer algorithm could happen overnight, that would be wonderful.
You mentioned the ER. Many people go to the ER as a default. They don’t know what else to do. In the COVID era, we’re trying to dial that down because we want to be able to see the sickest and have the non-sick get care elsewhere. If this particular person or people don’t know what to do, they go to the ER, it costs money, takes a lot of time, and others who may be sick are diverted from care. Families worry terribly about their children, so a device for mom and access to a pediatrician for $15 a month is another wonderful idea. These are both very interesting. Another company is in the pharmacy benefit management (PBM) space. Anything you could say about how that works?
Plumlee: I can give an overview of how I look at this as an investor in the PBM space. Three companies control about 75% of a multibillion dollar market. Several initiatives have been pursued politically to provide transparent pricing between these PBMs and pharmaceutical companies, and a lot of people are pointing fingers, but ultimately, drug prices just keep going up. Everybody knows it.
A couple of start-up founders are really set on bringing a competitive marketplace back to the pharmacy benefit manager. As an investor, when you see three people controlling a market, and you have small or medium PBMs that depend on aggregators to get competitive pricing with those big three, you get interested. It’s an interesting industry. My feeling is that somebody is going to disrupt it and bring competition back to that space. Ultimately, drug prices will come down because it’s not sustainable. The insurance companies just accommodate whatever the drug pricing is. If the drug prices go up, your premiums go up. I think these new companies will be level-setting.
Henry: In my world of oncology, we’re just a little more than halfway through 2020 and we’ve had five, six, seven new drugs approved. They all will be very expensive. One of the nicer things that’s happening and may help to tamp this down involves biosimilars. When you go to CVS or Rite Aid, you go down the aspirin aisle and see the generics, and they’re identical to the brand name aspirin. Well, these very complex molecules we used to treat cancer are antibodies or proteins, and they’re made in nature’s factories called cells. They’re not identical to the brand name drugs, but they’re called biosimilars. They work exactly the same as the branded drugs with exactly the same safety–our U.S. FDA has done a nice job of vetting that, to be sure. X, Y, Z Company has copied the brand drug after the patent expires. They were hoping for about a 30% discount in price but we’re seeing more like 15%. Nothing’s ever easy. So you make a very good point. This is not sustainable and the competition will be wonderful to tamp down these prices.
Plumlee: My hope is that those biosimilars and generics get placement in these formularies because the formularies are what’s valuable to the drug manufacturers. But they have to accommodate what the Big Three want in the PBM space. To me, making things affordable and accessible is what a lot of these startups are trying to do. And hopefully they will win.
Henry: What have you been going through, in terms of COVID? Have you recovered fully? Have your taste and smell returned, and you’re back to normal?
Plumlee: I’m all good. It caught me off guard but the symptoms weren’t too intense. For me, it was less than a flu, but more than a cold. And I’m all good today.
Henry: We’re so glad and wish you the best of luck.
Dr. Henry is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia and the host of the Blood & Cancer podcast. He has no relevant financial conflicts.
Mr. Plumlee is a board advisor to both Formsense and the Prysm Institute and a board observer with Voiceitt.
Editor’s Note: This transcript from the August 20 episode of the Blood & Cancer podcast has been edited for clarity. Click this link to listen to the full episode.
David Henry, MD: Welcome to this Blood & Cancer podcast. I’m your host, Dr. David Henry. This podcast airs on Thursday morning each week. This interview and others are archived with show notes from our residents at Pennsylvania Hospital at this link.
Each week we interview key opinion leaders involved in various aspects of blood and cancer. Mason was a first round pick in the NBA, a gold medalist for the U.S. men’s national team, and NBA All-Rookie first team honoree. He’s one of the top playmaking forwards in the country, if not the world, in my opinion. In his four-year college career at Duke University, he helped lead the Blue Devils to a National Collegiate Athletic Association (NCAA) championship and twice earned All-America first team academic honors at Duke. So he’s not just a basketball star, but an academic star as well. Mason, thanks so much for taking some time out from the bubble in Florida to talk with us today.
Mason Plumlee: Thanks for having me on. I’m happy to be here.
Henry: Beginning in March, the NBA didn’t know what to do about the COVID pandemic but finally decided to put you professional players in a ‘bubble.’ What did you have to go through to get there? You, your teammates, coaches, trainers, etc. And what’s the ongoing plan to be sure you continue to be safe?
Plumlee: Back to when the season shut down in March, the NBA shut down the practice facilities at the same time. Most people went home. I went back to Indiana. And then, as the idea of this bubble came up and the NBA formalized a plan to start the season again, players started to go back to market. I went back to Denver and was working out there.
About two weeks before we were scheduled to arrive in Orlando, they started testing us every other day. They used the deep nasal swab as well as the throat swab. But they were also taking two to three blood tests in that time period. You needed a certain number of consecutive negative tests before they would allow you to fly on the team plane down to Orlando. So there was an incredible amount of testing in the market. Once you got to Orlando, you went into a 48-hour quarantine. You had to have two negative tests with 48 hours between them before you could leave your hotel room.
Since then, it’s been quite strict down here. And although it’s annoying in a lot of ways, I think it’s one of the reasons our league has been able to pull this off. We’ve had no positive tests within the bubble and we are tested every day. A company called BioReference Laboratories has a setup in one of the meeting rooms here, and it’s like clockwork—we go in, we get our tests. One of my teammates missed a test and they made him stay in his room until he could get another test and get the results, so he missed a game because of that.
Henry: During this bubble time, no one has tested positive—players, coaches, staff?
Plumlee: Correct.
Henry: That’s incredible, and it’s allowed those of us who want to watch the NBA and those of you who are in it professionally to continue the sport. It must be a real nuisance for you and your family and friends, because no one can visit you, right?
Plumlee: Right. There’s no visitation. We had one false positive. It was our media relations person and the actions they took when that positive test came in -- they quarantined him in his room and interviewed everybody he had talked to; they tested anyone who had any interaction with him and those people had to go into quarantine. They’re on top of things down here. In addition to the testing, we each have a pulse oximeter and a thermometer, and we use these to check in everyday on an app. So, they’re getting all the insight they need. After the first round of the playoffs, they’re going to open the bubble to friends and family, but those friends and family will be subject to all the same protocols that we were coming in and once they’re here as well.
Henry: I’m sure you’ve heard about the Broadway star [Nick Cordero] who was healthy and suddenly got sick, lost a leg, and then lost his life. There have been some heart attacks that surprised us. Have your colleagues—players, coaches, etc.—been worried? Or are they thinking, what’s the big deal? Has the sense of how serious this is permeated through this sport?
Plumlee: The NBA is one of the groups that has heightened the understanding and awareness of this by shutting down. I think a lot of people were moving forward as is, and then, when the NBA decided to cancel the season, it let the world know, look, this is to be taken seriously.
Henry: A couple of players did test positive early on.
Plumlee: Exactly. A couple of people tested positive. I think at the outset, the unknown is always scarier. As we’ve learned more about the virus, the guys have become more comfortable. You know, I tested positive back in March. At the time, a loss of taste and smell was not a reported symptom.
Henry: And you had that?
Plumlee: I did have that, but I didn’t know what to think. More research has come out and we have a better understanding of that. I think most of the players are comfortable with the virus. We’re at a time in our lives where we’re healthy, we’re active, and we should be able to fight it off. We know the numbers for our age group. Even still, I think nobody wants to get it. Nobody wants to have to go through it. So why chance it?
Henry: Hats off to you and your sport. Other sports such as Major League Baseball haven’t been quite so successful. Of course, they’re wrestling with the players testing positive, and this has stopped games this season.
I was looking over your background prior to the interview and learned that your mother and father have been involved in the medical arena. Can you tell us about that and how it’s rubbed off on you?
Plumlee: Definitely. My mom is a pharmacist, so I spent a lot of time as a kid going to see her at work. And my dad is general counsel for an orthopedic company. My hometown is Warsaw, Ind. Some people refer to it as the “Orthopedic Capital of the World.” Zimmer Biomet is headquartered there. DePuy Synthes is there. Medtronic has offices there, as well as a lot of cottage businesses that support the orthopedic industry. In my hometown, the rock star was Dane Miller, who founded Biomet. I have no formal education in medicine or health care, but I’ve seen the impact of it. From my parents and some cousins, uncles who are doctors and surgeons, it’s been interesting to see their work and learn about what’s the latest and greatest in health care.
Henry: What’s so nice about you in particular is, with that background of interests from your family and your celebrity and accomplishments in professional basketball, you have used that to explore and promote ways to make progress in health care and help others who are less fortunate. For example, you’re involved in a telehealth platform for all-in-one practice management; affordable telehealth for pediatrics; health benefits for small businesses; prior authorization—if you can help with prior authorization, we will be in the stands for you at every game because it’s the bane of our existence; radiotherapy; and probably from mom’s background, pharmacy benefit management. Pick any of those you’d like to talk about, and tell us about your involvement and how it’s going.
Plumlee: My ticket into the arena is investment. Nobody’s calling me, asking for my expertise. But a lot of these visionary founders need financial support, and that’s where I get involved. Then also, with the celebrity angle from being an athlete, sometimes you can open doors for a start-up founder that they may not be able to open themselves.
I’m happy to speak about any of those companies. I am excited about the relaxed regulation that’s come from the pandemic; not that it’s like the Wild West out here, but I think it has allowed companies to implement solutions or think about problems in a way that they couldn’t before the pandemic. Take the prior authorization play, for example, and a company called Banjo Health, with one of my favorite founders, a guy named Saar Mahna. Medicare mandates that you turn around prior authorizations within three days. This company has an artificial intelligence and machine-learning play on prior authorizations that can deliver on that.
So efficiencies, things that increase access or affordability, better outcomes, those are the things that attract me. I lean on other people for the due diligence. The pediatric play that you referenced is a company called Blueberry Pediatrics. You have a monthly subscription for $15 that can be reimbursed by Medicaid. They send two devices to your home—an otoscope and an oximeter. The company is live in Florida right now, and it’s diverting a ton of emergency room (ER) visits. From home, for $15 a month, a mom has an otoscope and an oximeter, and she can chat or video conference with a pediatrician. There’s no additional fee. So that’s saving everyone time and saving the system money. Those are the kinds of things I’m attracted to.
Henry: You’ve touched on a couple of hot button issues for us. In oncology, unfortunately, most of our patients have pain. I am mystified every time I try to get a narcotic or a strong painkiller for a patient on a Friday night and I’m told it requires prior authorization and they’ll open up again on Monday. Well, that’s insane. These patients need something right away. So if you have a special interest in helping all of us with prior authorization, the artificial intelligence is a no brainer. If this kind of computer algorithm could happen overnight, that would be wonderful.
You mentioned the ER. Many people go to the ER as a default. They don’t know what else to do. In the COVID era, we’re trying to dial that down because we want to be able to see the sickest and have the non-sick get care elsewhere. If this particular person or people don’t know what to do, they go to the ER, it costs money, takes a lot of time, and others who may be sick are diverted from care. Families worry terribly about their children, so a device for mom and access to a pediatrician for $15 a month is another wonderful idea. These are both very interesting. Another company is in the pharmacy benefit management (PBM) space. Anything you could say about how that works?
Plumlee: I can give an overview of how I look at this as an investor in the PBM space. Three companies control about 75% of a multibillion dollar market. Several initiatives have been pursued politically to provide transparent pricing between these PBMs and pharmaceutical companies, and a lot of people are pointing fingers, but ultimately, drug prices just keep going up. Everybody knows it.
A couple of start-up founders are really set on bringing a competitive marketplace back to the pharmacy benefit manager. As an investor, when you see three people controlling a market, and you have small or medium PBMs that depend on aggregators to get competitive pricing with those big three, you get interested. It’s an interesting industry. My feeling is that somebody is going to disrupt it and bring competition back to that space. Ultimately, drug prices will come down because it’s not sustainable. The insurance companies just accommodate whatever the drug pricing is. If the drug prices go up, your premiums go up. I think these new companies will be level-setting.
Henry: In my world of oncology, we’re just a little more than halfway through 2020 and we’ve had five, six, seven new drugs approved. They all will be very expensive. One of the nicer things that’s happening and may help to tamp this down involves biosimilars. When you go to CVS or Rite Aid, you go down the aspirin aisle and see the generics, and they’re identical to the brand name aspirin. Well, these very complex molecules we used to treat cancer are antibodies or proteins, and they’re made in nature’s factories called cells. They’re not identical to the brand name drugs, but they’re called biosimilars. They work exactly the same as the branded drugs with exactly the same safety–our U.S. FDA has done a nice job of vetting that, to be sure. X, Y, Z Company has copied the brand drug after the patent expires. They were hoping for about a 30% discount in price but we’re seeing more like 15%. Nothing’s ever easy. So you make a very good point. This is not sustainable and the competition will be wonderful to tamp down these prices.
Plumlee: My hope is that those biosimilars and generics get placement in these formularies because the formularies are what’s valuable to the drug manufacturers. But they have to accommodate what the Big Three want in the PBM space. To me, making things affordable and accessible is what a lot of these startups are trying to do. And hopefully they will win.
Henry: What have you been going through, in terms of COVID? Have you recovered fully? Have your taste and smell returned, and you’re back to normal?
Plumlee: I’m all good. It caught me off guard but the symptoms weren’t too intense. For me, it was less than a flu, but more than a cold. And I’m all good today.
Henry: We’re so glad and wish you the best of luck.
Dr. Henry is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia and the host of the Blood & Cancer podcast. He has no relevant financial conflicts.
Mr. Plumlee is a board advisor to both Formsense and the Prysm Institute and a board observer with Voiceitt.



