User login
In Case You Missed It: COVID
COVID-19 may trigger irritable bowel syndrome
Gastrointestinal symptoms are common with long COVID, also known as post-acute COVID-19 syndrome, according to Walter Chan, MD, MPH, and Madhusudan Grover, MBBS.
Dr. Chan, an assistant professor at Harvard Medical School, Boston, and Dr. Grover, an associate professor of medicine and physiology at Mayo Clinic, Rochester, Minn., conducted a review of the literature on COVID-19’s long-term gastrointestinal effects. Their review was published in Clinical Gastroenterology and Hepatology.
Estimates of the prevalence of gastrointestinal symptoms with COVID-19 have ranged as high as 60%, Dr. Chan and Dr. Grover report, and the symptoms may be present in patients with long COVID, a syndrome that continues 4 weeks or longer.
In one survey of 749 COVID-19 survivors, 29% reported at least one new chronic gastrointestinal symptom. The most common were heartburn, constipation, diarrhea, and abdominal pain. Of those with abdominal pain, 39% had symptoms that met Rome IV criteria for irritable bowel syndrome.
People who have gastrointestinal symptoms after their initial SARS-CoV-2 infection are more likely to have them with long COVID. Psychiatric diagnoses, hospitalization, and the loss of smell and taste are predictors of gastrointestinal symptoms.
Infectious gastroenteritis can increase the risk for disorders of gut-brain interaction, especially postinfection IBS, Dr. Chan and Dr. Grover write.
COVID-19 likely causes gastrointestinal symptoms through multiple mechanisms. It may suppress angiotensin-converting enzyme 2, which protects intestinal cells. It can alter the microbiome. It can cause or worsen weight gain and diabetes. It may disrupt the immune system and trigger an autoimmune reaction. It can cause depression and anxiety, and it can alter dietary habits.
No specific treatments for gastrointestinal symptoms associated with long COVID have emerged, so clinicians should make use of established therapies for disorders of gut-brain interaction, Dr. Chan and Dr. Grover recommend.
Beyond adequate sleep and exercise, these may include high-fiber, low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), gluten-free, low-carbohydrate, or elimination diets.
For diarrhea, they list loperamide, ondansetron, alosetron, eluxadoline, antispasmodics, rifaximin, and bile acid sequestrants.
For constipation, they mention fiber supplements, polyethylene glycol, linaclotide, plecanatide, lubiprostone, tenapanor, tegaserod, and prucalopride.
For modulating intestinal permeability, they recommend glutamine.
Neuromodulation may be achieved with tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, azaperones, and delta ligands, they write.
For psychological therapy, they recommend cognitive-behavioral therapy and gut-directed hypnotherapy.
A handful of studies have suggested benefits from Lactiplantibacillus plantarum and Pediococcus acidilactici as probiotic therapies. Additionally, one study showed positive results with a high-fiber formula, perhaps by nourishing short-chain fatty acid-producing bacteria, Dr. Chan and Dr. Grover write.
Dr. Chan reported financial relationships with Ironwood, Takeda, and Phathom Pharmaceuticals. Dr. Grover reported financial relationships with Takeda, Donga, Alexza Pharmaceuticals, and Alfasigma.
A version of this article first appeared on Medscape.com.
Gastrointestinal symptoms are common with long COVID, also known as post-acute COVID-19 syndrome, according to Walter Chan, MD, MPH, and Madhusudan Grover, MBBS.
Dr. Chan, an assistant professor at Harvard Medical School, Boston, and Dr. Grover, an associate professor of medicine and physiology at Mayo Clinic, Rochester, Minn., conducted a review of the literature on COVID-19’s long-term gastrointestinal effects. Their review was published in Clinical Gastroenterology and Hepatology.
Estimates of the prevalence of gastrointestinal symptoms with COVID-19 have ranged as high as 60%, Dr. Chan and Dr. Grover report, and the symptoms may be present in patients with long COVID, a syndrome that continues 4 weeks or longer.
In one survey of 749 COVID-19 survivors, 29% reported at least one new chronic gastrointestinal symptom. The most common were heartburn, constipation, diarrhea, and abdominal pain. Of those with abdominal pain, 39% had symptoms that met Rome IV criteria for irritable bowel syndrome.
People who have gastrointestinal symptoms after their initial SARS-CoV-2 infection are more likely to have them with long COVID. Psychiatric diagnoses, hospitalization, and the loss of smell and taste are predictors of gastrointestinal symptoms.
Infectious gastroenteritis can increase the risk for disorders of gut-brain interaction, especially postinfection IBS, Dr. Chan and Dr. Grover write.
COVID-19 likely causes gastrointestinal symptoms through multiple mechanisms. It may suppress angiotensin-converting enzyme 2, which protects intestinal cells. It can alter the microbiome. It can cause or worsen weight gain and diabetes. It may disrupt the immune system and trigger an autoimmune reaction. It can cause depression and anxiety, and it can alter dietary habits.
No specific treatments for gastrointestinal symptoms associated with long COVID have emerged, so clinicians should make use of established therapies for disorders of gut-brain interaction, Dr. Chan and Dr. Grover recommend.
Beyond adequate sleep and exercise, these may include high-fiber, low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), gluten-free, low-carbohydrate, or elimination diets.
For diarrhea, they list loperamide, ondansetron, alosetron, eluxadoline, antispasmodics, rifaximin, and bile acid sequestrants.
For constipation, they mention fiber supplements, polyethylene glycol, linaclotide, plecanatide, lubiprostone, tenapanor, tegaserod, and prucalopride.
For modulating intestinal permeability, they recommend glutamine.
Neuromodulation may be achieved with tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, azaperones, and delta ligands, they write.
For psychological therapy, they recommend cognitive-behavioral therapy and gut-directed hypnotherapy.
A handful of studies have suggested benefits from Lactiplantibacillus plantarum and Pediococcus acidilactici as probiotic therapies. Additionally, one study showed positive results with a high-fiber formula, perhaps by nourishing short-chain fatty acid-producing bacteria, Dr. Chan and Dr. Grover write.
Dr. Chan reported financial relationships with Ironwood, Takeda, and Phathom Pharmaceuticals. Dr. Grover reported financial relationships with Takeda, Donga, Alexza Pharmaceuticals, and Alfasigma.
A version of this article first appeared on Medscape.com.
Gastrointestinal symptoms are common with long COVID, also known as post-acute COVID-19 syndrome, according to Walter Chan, MD, MPH, and Madhusudan Grover, MBBS.
Dr. Chan, an assistant professor at Harvard Medical School, Boston, and Dr. Grover, an associate professor of medicine and physiology at Mayo Clinic, Rochester, Minn., conducted a review of the literature on COVID-19’s long-term gastrointestinal effects. Their review was published in Clinical Gastroenterology and Hepatology.
Estimates of the prevalence of gastrointestinal symptoms with COVID-19 have ranged as high as 60%, Dr. Chan and Dr. Grover report, and the symptoms may be present in patients with long COVID, a syndrome that continues 4 weeks or longer.
In one survey of 749 COVID-19 survivors, 29% reported at least one new chronic gastrointestinal symptom. The most common were heartburn, constipation, diarrhea, and abdominal pain. Of those with abdominal pain, 39% had symptoms that met Rome IV criteria for irritable bowel syndrome.
People who have gastrointestinal symptoms after their initial SARS-CoV-2 infection are more likely to have them with long COVID. Psychiatric diagnoses, hospitalization, and the loss of smell and taste are predictors of gastrointestinal symptoms.
Infectious gastroenteritis can increase the risk for disorders of gut-brain interaction, especially postinfection IBS, Dr. Chan and Dr. Grover write.
COVID-19 likely causes gastrointestinal symptoms through multiple mechanisms. It may suppress angiotensin-converting enzyme 2, which protects intestinal cells. It can alter the microbiome. It can cause or worsen weight gain and diabetes. It may disrupt the immune system and trigger an autoimmune reaction. It can cause depression and anxiety, and it can alter dietary habits.
No specific treatments for gastrointestinal symptoms associated with long COVID have emerged, so clinicians should make use of established therapies for disorders of gut-brain interaction, Dr. Chan and Dr. Grover recommend.
Beyond adequate sleep and exercise, these may include high-fiber, low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), gluten-free, low-carbohydrate, or elimination diets.
For diarrhea, they list loperamide, ondansetron, alosetron, eluxadoline, antispasmodics, rifaximin, and bile acid sequestrants.
For constipation, they mention fiber supplements, polyethylene glycol, linaclotide, plecanatide, lubiprostone, tenapanor, tegaserod, and prucalopride.
For modulating intestinal permeability, they recommend glutamine.
Neuromodulation may be achieved with tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, azaperones, and delta ligands, they write.
For psychological therapy, they recommend cognitive-behavioral therapy and gut-directed hypnotherapy.
A handful of studies have suggested benefits from Lactiplantibacillus plantarum and Pediococcus acidilactici as probiotic therapies. Additionally, one study showed positive results with a high-fiber formula, perhaps by nourishing short-chain fatty acid-producing bacteria, Dr. Chan and Dr. Grover write.
Dr. Chan reported financial relationships with Ironwood, Takeda, and Phathom Pharmaceuticals. Dr. Grover reported financial relationships with Takeda, Donga, Alexza Pharmaceuticals, and Alfasigma.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Long COVID case study: persistent hormone deficiencies
A case study of a 65-year-old man in Japan with long COVID describes how he recovered from certain impaired hormone deficiencies that persisted for more than a year.
Days after the patient recovered from respiratory failure and came off a ventilator, he had a sudden drop in blood pressure, which responded to hydrocortisone.
The patient was found to have low levels of growth hormone and adrenocorticotropic hormone (ACTH), hypopituitarism, that persisted for more than a year. He also had low levels of testosterone that remained low at 15 months (the study end).
“An important finding in the present case is the eventual recovery from hypopituitarism over time but not from hypogonadism,” the researchers write in their study published in Endocrine Journal.
, which was confirmed using an insulin tolerance test, Kai Yoshimura, Kakogawa Medical Center, Japan, and colleagues report.
The findings show that “pituitary insufficiency should be considered in patients with prolonged symptoms of COVID-19,” they report, since it can be treated with hormone supplements that markedly improve symptoms and quality of life.
“It might be worthwhile to screen for endocrine dysfunction in patients with such persistent symptoms after their recovery from the acute disease,” the researchers conclude.
Case study timeline
The patient in this study was healthy without obesity, previous endocrine disease, or steroid use. He was admitted to hospital because he had dyspnea and fever for 8 days and a reverse transcription-polymerase chain reaction (RT-PCR) test that was positive for COVID-19.
He received ciclesonide 200 mcg/day for 2 days. Then he was put on a ventilator and the drug was discontinued and “favipiravir, ritonavir, and lopinavir, a standard regimen during the early phase of the COVID-19 pandemic, were initiated;” the researchers explain.
On day 25 of his hospital stay the patient had recovered from respiratory failure and was extubated.
On day 31, he had a negative PCR test for COVID-19.
On day 36, the patient’s blood pressure suddenly dropped from 120/80 mmHg to 80/50 mmHg. His plasma ACTH and serum cortisol levels were low, suggesting secondary adrenal insufficiency. The low blood pressure responded to hydrocortisone 100 mg, which was gradually tapered.
At day 96, the patient was discharged from hospital with a dose of 15 mg/day hydrocortisone.
At 3 months after discharge, an insulin tolerance test revealed that the patient’s ACTH and cortisol responses were blunted, suggestive of adrenal insufficiency. The patient also had moderate growth hormone deficiency and symptoms of hypogonadism.
At 6 months after discharge, the patient started testosterone therapy because his dysspermatism had worsened.
At 12 months after discharge, a repeat insulin tolerance test showed that both ACTH and cortisol responses were low but improved. The patient was no longer deficient in growth hormone.
At 15 months after discharge, early morning levels of ACTH and cortisol were now in the normal range. The patient discontinued testosterone treatment, but the symptoms returned, so he resumed it.
Long COVID symptoms, possible biological mechanism
The present case shows how certain COVID-19–associated conditions develop after the onset of, or the recovery from, respiratory disorders, the authors note.
Symptoms of long COVID-19 include fatigue, weakness, hair loss, diarrhea, arthralgia, and depression, and these symptoms are associated with pituitary insufficiency, especially secondary adrenocortical insufficiency.
In addition, an estimated 25% of sexually active men who recover from COVID have semen disorders such as azoospermia and oligospermia.
The underlying mechanism by which COVID-19 might trigger pituitary insufficiency is unknown, but other viral infections such as influenza-A and herpes simplex are also associated with transient hypopituitarism. An exaggerated immune response triggered by SARS-CoV-2 may explain the dysfunction of multiple endocrine organs, the researchers write.
The researchers have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
A case study of a 65-year-old man in Japan with long COVID describes how he recovered from certain impaired hormone deficiencies that persisted for more than a year.
Days after the patient recovered from respiratory failure and came off a ventilator, he had a sudden drop in blood pressure, which responded to hydrocortisone.
The patient was found to have low levels of growth hormone and adrenocorticotropic hormone (ACTH), hypopituitarism, that persisted for more than a year. He also had low levels of testosterone that remained low at 15 months (the study end).
“An important finding in the present case is the eventual recovery from hypopituitarism over time but not from hypogonadism,” the researchers write in their study published in Endocrine Journal.
, which was confirmed using an insulin tolerance test, Kai Yoshimura, Kakogawa Medical Center, Japan, and colleagues report.
The findings show that “pituitary insufficiency should be considered in patients with prolonged symptoms of COVID-19,” they report, since it can be treated with hormone supplements that markedly improve symptoms and quality of life.
“It might be worthwhile to screen for endocrine dysfunction in patients with such persistent symptoms after their recovery from the acute disease,” the researchers conclude.
Case study timeline
The patient in this study was healthy without obesity, previous endocrine disease, or steroid use. He was admitted to hospital because he had dyspnea and fever for 8 days and a reverse transcription-polymerase chain reaction (RT-PCR) test that was positive for COVID-19.
He received ciclesonide 200 mcg/day for 2 days. Then he was put on a ventilator and the drug was discontinued and “favipiravir, ritonavir, and lopinavir, a standard regimen during the early phase of the COVID-19 pandemic, were initiated;” the researchers explain.
On day 25 of his hospital stay the patient had recovered from respiratory failure and was extubated.
On day 31, he had a negative PCR test for COVID-19.
On day 36, the patient’s blood pressure suddenly dropped from 120/80 mmHg to 80/50 mmHg. His plasma ACTH and serum cortisol levels were low, suggesting secondary adrenal insufficiency. The low blood pressure responded to hydrocortisone 100 mg, which was gradually tapered.
At day 96, the patient was discharged from hospital with a dose of 15 mg/day hydrocortisone.
At 3 months after discharge, an insulin tolerance test revealed that the patient’s ACTH and cortisol responses were blunted, suggestive of adrenal insufficiency. The patient also had moderate growth hormone deficiency and symptoms of hypogonadism.
At 6 months after discharge, the patient started testosterone therapy because his dysspermatism had worsened.
At 12 months after discharge, a repeat insulin tolerance test showed that both ACTH and cortisol responses were low but improved. The patient was no longer deficient in growth hormone.
At 15 months after discharge, early morning levels of ACTH and cortisol were now in the normal range. The patient discontinued testosterone treatment, but the symptoms returned, so he resumed it.
Long COVID symptoms, possible biological mechanism
The present case shows how certain COVID-19–associated conditions develop after the onset of, or the recovery from, respiratory disorders, the authors note.
Symptoms of long COVID-19 include fatigue, weakness, hair loss, diarrhea, arthralgia, and depression, and these symptoms are associated with pituitary insufficiency, especially secondary adrenocortical insufficiency.
In addition, an estimated 25% of sexually active men who recover from COVID have semen disorders such as azoospermia and oligospermia.
The underlying mechanism by which COVID-19 might trigger pituitary insufficiency is unknown, but other viral infections such as influenza-A and herpes simplex are also associated with transient hypopituitarism. An exaggerated immune response triggered by SARS-CoV-2 may explain the dysfunction of multiple endocrine organs, the researchers write.
The researchers have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
A case study of a 65-year-old man in Japan with long COVID describes how he recovered from certain impaired hormone deficiencies that persisted for more than a year.
Days after the patient recovered from respiratory failure and came off a ventilator, he had a sudden drop in blood pressure, which responded to hydrocortisone.
The patient was found to have low levels of growth hormone and adrenocorticotropic hormone (ACTH), hypopituitarism, that persisted for more than a year. He also had low levels of testosterone that remained low at 15 months (the study end).
“An important finding in the present case is the eventual recovery from hypopituitarism over time but not from hypogonadism,” the researchers write in their study published in Endocrine Journal.
, which was confirmed using an insulin tolerance test, Kai Yoshimura, Kakogawa Medical Center, Japan, and colleagues report.
The findings show that “pituitary insufficiency should be considered in patients with prolonged symptoms of COVID-19,” they report, since it can be treated with hormone supplements that markedly improve symptoms and quality of life.
“It might be worthwhile to screen for endocrine dysfunction in patients with such persistent symptoms after their recovery from the acute disease,” the researchers conclude.
Case study timeline
The patient in this study was healthy without obesity, previous endocrine disease, or steroid use. He was admitted to hospital because he had dyspnea and fever for 8 days and a reverse transcription-polymerase chain reaction (RT-PCR) test that was positive for COVID-19.
He received ciclesonide 200 mcg/day for 2 days. Then he was put on a ventilator and the drug was discontinued and “favipiravir, ritonavir, and lopinavir, a standard regimen during the early phase of the COVID-19 pandemic, were initiated;” the researchers explain.
On day 25 of his hospital stay the patient had recovered from respiratory failure and was extubated.
On day 31, he had a negative PCR test for COVID-19.
On day 36, the patient’s blood pressure suddenly dropped from 120/80 mmHg to 80/50 mmHg. His plasma ACTH and serum cortisol levels were low, suggesting secondary adrenal insufficiency. The low blood pressure responded to hydrocortisone 100 mg, which was gradually tapered.
At day 96, the patient was discharged from hospital with a dose of 15 mg/day hydrocortisone.
At 3 months after discharge, an insulin tolerance test revealed that the patient’s ACTH and cortisol responses were blunted, suggestive of adrenal insufficiency. The patient also had moderate growth hormone deficiency and symptoms of hypogonadism.
At 6 months after discharge, the patient started testosterone therapy because his dysspermatism had worsened.
At 12 months after discharge, a repeat insulin tolerance test showed that both ACTH and cortisol responses were low but improved. The patient was no longer deficient in growth hormone.
At 15 months after discharge, early morning levels of ACTH and cortisol were now in the normal range. The patient discontinued testosterone treatment, but the symptoms returned, so he resumed it.
Long COVID symptoms, possible biological mechanism
The present case shows how certain COVID-19–associated conditions develop after the onset of, or the recovery from, respiratory disorders, the authors note.
Symptoms of long COVID-19 include fatigue, weakness, hair loss, diarrhea, arthralgia, and depression, and these symptoms are associated with pituitary insufficiency, especially secondary adrenocortical insufficiency.
In addition, an estimated 25% of sexually active men who recover from COVID have semen disorders such as azoospermia and oligospermia.
The underlying mechanism by which COVID-19 might trigger pituitary insufficiency is unknown, but other viral infections such as influenza-A and herpes simplex are also associated with transient hypopituitarism. An exaggerated immune response triggered by SARS-CoV-2 may explain the dysfunction of multiple endocrine organs, the researchers write.
The researchers have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
Large study amplifies evidence of COVID vaccine safety in pregnancy
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
FROM BMJ
Children and COVID: ED visits and new admissions change course
New child cases of COVID-19 made at least a temporary transition from slow increase to decrease, and emergency department visits and new admissions seem to be following a downward trend.
, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. For some historical perspective, the latest weekly count falls below last year’s Delta surge figure of 121,000 (Aug. 6-12) but above the summer 2020 total of 26,000 (Aug. 7-13).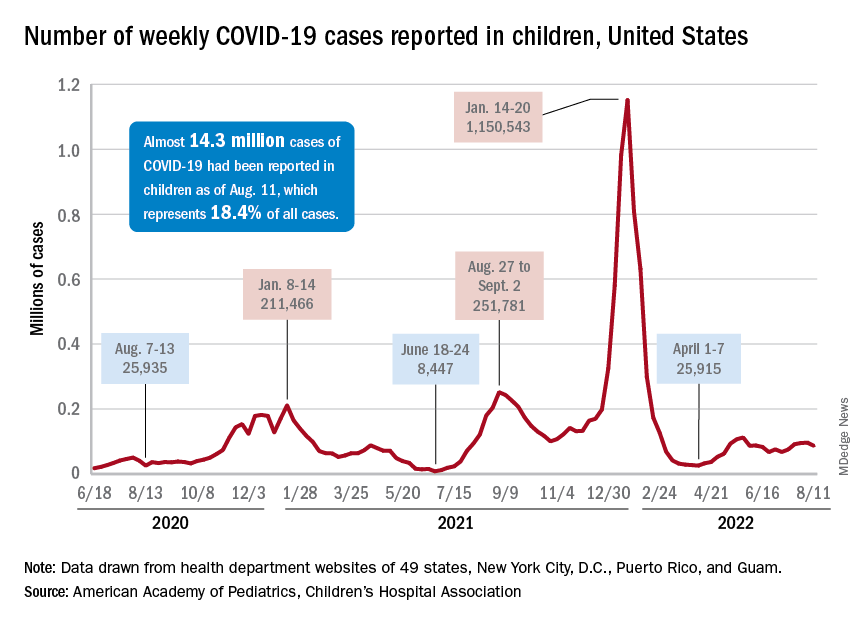
Measures of serious illness finally head downward
The prolonged rise in ED visits and new admissions over the last 5 months, which continued even through late spring when cases were declining, seems to have peaked, CDC data suggest.
That upward trend, driven largely by continued increases among younger children, peaked in late July, when 6.7% of all ED visits for children aged 0-11 years involved diagnosed COVID-19. The corresponding peaks for older children occurred around the same time but were only about half as high: 3.4% for 12- to 15-year-olds and 3.6% for those aged 16-17, the CDC reported.
The data for new admissions present a similar scenario: an increase starting in mid-April that continued unabated into late July despite the decline in new cases. By the time admissions among children aged 0-17 years peaked at 0.46 per 100,000 population in late July, they had reached the same level seen during the Delta surge. By Aug. 7, the rate of new hospitalizations was down to 0.42 per 100,000, the CDC said on its COVID Data Tracker.
The vaccine is ready for all students, but …
As children all over the country start or get ready to start a new school year, the only large-scale student vaccine mandate belongs to the District of Columbia. California has a mandate pending, but it will not go into effect until after July 1, 2023. There are, however, 20 states that have banned vaccine mandates for students, according to the National Academy for State Health Policy.
Nonmandated vaccination of the youngest children against COVID-19 continues to be slow. In the approximately 7 weeks (June 19 to Aug. 9) since the vaccine was approved for use in children younger than 5 years, just 4.4% of that age group has received at least one dose and 0.7% are fully vaccinated. Among those aged 5-11 years, who have been vaccine-eligible since early November of last year, 37.6% have received at least one dose and 30.2% are fully vaccinated, the CDC said.
New child cases of COVID-19 made at least a temporary transition from slow increase to decrease, and emergency department visits and new admissions seem to be following a downward trend.
, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. For some historical perspective, the latest weekly count falls below last year’s Delta surge figure of 121,000 (Aug. 6-12) but above the summer 2020 total of 26,000 (Aug. 7-13).
Measures of serious illness finally head downward
The prolonged rise in ED visits and new admissions over the last 5 months, which continued even through late spring when cases were declining, seems to have peaked, CDC data suggest.
That upward trend, driven largely by continued increases among younger children, peaked in late July, when 6.7% of all ED visits for children aged 0-11 years involved diagnosed COVID-19. The corresponding peaks for older children occurred around the same time but were only about half as high: 3.4% for 12- to 15-year-olds and 3.6% for those aged 16-17, the CDC reported.
The data for new admissions present a similar scenario: an increase starting in mid-April that continued unabated into late July despite the decline in new cases. By the time admissions among children aged 0-17 years peaked at 0.46 per 100,000 population in late July, they had reached the same level seen during the Delta surge. By Aug. 7, the rate of new hospitalizations was down to 0.42 per 100,000, the CDC said on its COVID Data Tracker.
The vaccine is ready for all students, but …
As children all over the country start or get ready to start a new school year, the only large-scale student vaccine mandate belongs to the District of Columbia. California has a mandate pending, but it will not go into effect until after July 1, 2023. There are, however, 20 states that have banned vaccine mandates for students, according to the National Academy for State Health Policy.
Nonmandated vaccination of the youngest children against COVID-19 continues to be slow. In the approximately 7 weeks (June 19 to Aug. 9) since the vaccine was approved for use in children younger than 5 years, just 4.4% of that age group has received at least one dose and 0.7% are fully vaccinated. Among those aged 5-11 years, who have been vaccine-eligible since early November of last year, 37.6% have received at least one dose and 30.2% are fully vaccinated, the CDC said.
New child cases of COVID-19 made at least a temporary transition from slow increase to decrease, and emergency department visits and new admissions seem to be following a downward trend.
, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. For some historical perspective, the latest weekly count falls below last year’s Delta surge figure of 121,000 (Aug. 6-12) but above the summer 2020 total of 26,000 (Aug. 7-13).
Measures of serious illness finally head downward
The prolonged rise in ED visits and new admissions over the last 5 months, which continued even through late spring when cases were declining, seems to have peaked, CDC data suggest.
That upward trend, driven largely by continued increases among younger children, peaked in late July, when 6.7% of all ED visits for children aged 0-11 years involved diagnosed COVID-19. The corresponding peaks for older children occurred around the same time but were only about half as high: 3.4% for 12- to 15-year-olds and 3.6% for those aged 16-17, the CDC reported.
The data for new admissions present a similar scenario: an increase starting in mid-April that continued unabated into late July despite the decline in new cases. By the time admissions among children aged 0-17 years peaked at 0.46 per 100,000 population in late July, they had reached the same level seen during the Delta surge. By Aug. 7, the rate of new hospitalizations was down to 0.42 per 100,000, the CDC said on its COVID Data Tracker.
The vaccine is ready for all students, but …
As children all over the country start or get ready to start a new school year, the only large-scale student vaccine mandate belongs to the District of Columbia. California has a mandate pending, but it will not go into effect until after July 1, 2023. There are, however, 20 states that have banned vaccine mandates for students, according to the National Academy for State Health Policy.
Nonmandated vaccination of the youngest children against COVID-19 continues to be slow. In the approximately 7 weeks (June 19 to Aug. 9) since the vaccine was approved for use in children younger than 5 years, just 4.4% of that age group has received at least one dose and 0.7% are fully vaccinated. Among those aged 5-11 years, who have been vaccine-eligible since early November of last year, 37.6% have received at least one dose and 30.2% are fully vaccinated, the CDC said.
Diagnosing children with long COVID can be tricky: Experts
When Spencer Siedlecki got COVID-19 in March 2021, he was sick for weeks with extreme fatigue, fevers, a sore throat, bad headaches, nausea, and eventually, pneumonia.
That was scary enough for the then-13-year-old and his parents, who live in Ohio. More than a year later, Spencer still had many of the symptoms and, more alarming, the once-healthy teen had postural orthostatic tachycardia syndrome, a condition that has caused dizziness, a racing heart when he stands, and fainting. Spencer missed most of the last few months of eighth grade because of long COVID.
“He gets sick very easily,” said his mother, Melissa Siedlecki, who works in technology sales. “The common cold that he would shake off in a few days takes weeks for him to feel better.”
The transformation from regular teen life to someone with a chronic illness “sucked,” said Spencer, who will turn 15 in August. “I felt like I was never going to get better.” Fortunately, after some therapy at a specialized clinic, Spencer is back to playing baseball and golf.
Spencer’s journey to better health was difficult; his regular pediatrician told the family at first that there were no treatments to help him – a reaction that is not uncommon. “I still get a lot of parents who heard of me through the grapevine,” said Amy Edwards, MD, director of the pediatric COVID clinic at University Hospitals Rainbow Babies & Children’s and an assistant professor of pediatrics at Case Western Reserve University, both in Cleveland. “The pediatricians either are unsure of what is wrong, or worse, tell children ‘there is nothing wrong with you. Stop faking it.’ ” Dr. Edwards treated Spencer after his mother found the clinic through an internet search.
Alexandra Yonts, MD, a pediatric infectious diseases doctor and director of the post-COVID program clinic at Children’s National Medical Center in Washington, has seen this too. she said.
But those who do get attention tend to be White and affluent, something Dr. Yonts said “doesn’t jibe with the epidemiologic data of who COVID has affected the most.” Black, Latino, and American Indian and Alaska Native children are more likely to be infected with COVID than White children, and have higher rates of hospitalization and death than White children.
It’s not clear whether these children have a particular risk factor, or if they are just the ones who have the resources to get to the clinics. But Dr. Yonts and Dr. Edwards believe many children are not getting the help they need. High-performing kids are coming in “because they are the ones whose symptoms are most obvious,” said Dr. Edwards. “I think there are kids out there who are getting missed because they’re already struggling because of socioeconomic reasons.”
Spencer is one of 14 million children who have tested positive for SARS-CoV-2 since the start of the pandemic. Many pediatricians are still grappling with how to address cases like Spencer’s. The American Academy of Pediatrics has issued only brief guidance on long COVID in children, in part because there have been so few studies to use as a basis for guidance.
The federal government is aiming to change that with a newly launched National Research Action Plan on Long COVID that includes speeding up research on how the condition affects children and youths, including their ability to learn and thrive.
A CDC study found children with COVID were significantly more likely to have smell and taste disturbances, circulatory system problems, fatigue and malaise, and pain. Those who had been infected had higher rates of acute blockage of a lung artery, myocarditis and weakening of the heart, kidney failure, and type 1 diabetes.
Difficult to diagnose
Even with increased media attention and more published studies on pediatric long COVID, it’s still hard for a busy primary care doctor “to sort through what could just be a cold or what could be a series of colds and trying to look at the bigger picture of what’s been going on in a 1- to 3-month period with a kid,” Dr. Yonts said.
Most children with potential or definite long COVID are still being seen by individual pediatricians, not in a specialized clinic with easy access to an army of specialists. It’s not clear how many of those pediatric clinics exist. Survivor Corps, an advocacy group for people with long COVID, has posted a map of locations providing care, but few are specialized or focus on pediatric long COVID.
Long COVID is different from multisystem inflammatory syndrome in children (MIS-C), which occurs within a month or so of infection, triggers high fevers and severe symptoms in the gut, and often results in hospitalization. MIS-C “is not subtle,” said Dr. Edwards.
The long COVID clinic doctors said most of their patients were not very sick at first. “Anecdotally, of the 83 kids that we’ve seen, most have had mild, very mild, or even asymptomatic infections initially,” and then went on to have long COVID, said Dr. Yonts.
“We see it even in children who have very mild disease or even are asymptomatic,” agreed Allison Eckard, MD, director of pediatric infectious diseases at the Medical University of South Carolina, Charleston.
Fatigue, mood problems
Dr. Yonts said 90% of her patients have fatigue, and many also have severe symptoms in their gut. Those and other long COVID symptoms will be looked at more closely in a 3-year study the Children’s National Medical Center is doing along with the National Institute of Allergy and Infectious Diseases.
There are no treatments for long COVID itself.
“Management is probably more the correct term for what we do in our clinic at this point,” said Dr. Yonts. That means dealing with fatigue and managing headache and digestive symptoms with medications or coping strategies. Guidelines from the American Academy of Physical Medicine and Rehabilitation help inform how to help kids safely resume exercise.
At the Children’s National Medical Center clinic, children will typically meet with a team of specialists including infectious diseases doctors on the same day, said Dr. Yonts. Psychologists help children with coping skills. Dr. Yonts is careful not to imply that long COVID is a psychological illness. Parents “will just shut down, because for so long, they’ve been told this is all a mental thing.”
In about a third of children, symptoms get better on their own, and most kids get better over time. But many still struggle. “We don’t talk about cure, because we don’t know what cure looks like,” said Dr. Edwards.
Vaccination may be best protection
Vaccination seems to help reduce the risk of long COVID, perhaps by as much as half. But parents have been slow to vaccinate children, especially the very young. The AAP reported that, as of Aug. 3, just 5% of children under age 5, 37% of those ages 5-11, and 69% of 12- to 17-year-olds have received at least one dose of a COVID-19 vaccine.
“We have tried to really push vaccine as one of the ways to help prevent some of these long COVID syndromes,” said Dr. Eckard. But that advice is not always welcome. Dr. Eckard told the story of a mother who refused to have her autistic son vaccinated, even as she tearfully pleaded for help with his long COVID symptoms, which had also worsened his autism. The woman told Dr. Eckard: “Nothing you can say will convince me to get him vaccinated.” She thought a vaccine could make his symptoms even worse.
The best prevention is to avoid being infected in the first place.
“The more times you get COVID, the more you increase your risk of getting long COVID,” said Dr. Yonts. “The more times you roll the dice, eventually your number could come up.
A version of this article first appeared on WebMD.com.
When Spencer Siedlecki got COVID-19 in March 2021, he was sick for weeks with extreme fatigue, fevers, a sore throat, bad headaches, nausea, and eventually, pneumonia.
That was scary enough for the then-13-year-old and his parents, who live in Ohio. More than a year later, Spencer still had many of the symptoms and, more alarming, the once-healthy teen had postural orthostatic tachycardia syndrome, a condition that has caused dizziness, a racing heart when he stands, and fainting. Spencer missed most of the last few months of eighth grade because of long COVID.
“He gets sick very easily,” said his mother, Melissa Siedlecki, who works in technology sales. “The common cold that he would shake off in a few days takes weeks for him to feel better.”
The transformation from regular teen life to someone with a chronic illness “sucked,” said Spencer, who will turn 15 in August. “I felt like I was never going to get better.” Fortunately, after some therapy at a specialized clinic, Spencer is back to playing baseball and golf.
Spencer’s journey to better health was difficult; his regular pediatrician told the family at first that there were no treatments to help him – a reaction that is not uncommon. “I still get a lot of parents who heard of me through the grapevine,” said Amy Edwards, MD, director of the pediatric COVID clinic at University Hospitals Rainbow Babies & Children’s and an assistant professor of pediatrics at Case Western Reserve University, both in Cleveland. “The pediatricians either are unsure of what is wrong, or worse, tell children ‘there is nothing wrong with you. Stop faking it.’ ” Dr. Edwards treated Spencer after his mother found the clinic through an internet search.
Alexandra Yonts, MD, a pediatric infectious diseases doctor and director of the post-COVID program clinic at Children’s National Medical Center in Washington, has seen this too. she said.
But those who do get attention tend to be White and affluent, something Dr. Yonts said “doesn’t jibe with the epidemiologic data of who COVID has affected the most.” Black, Latino, and American Indian and Alaska Native children are more likely to be infected with COVID than White children, and have higher rates of hospitalization and death than White children.
It’s not clear whether these children have a particular risk factor, or if they are just the ones who have the resources to get to the clinics. But Dr. Yonts and Dr. Edwards believe many children are not getting the help they need. High-performing kids are coming in “because they are the ones whose symptoms are most obvious,” said Dr. Edwards. “I think there are kids out there who are getting missed because they’re already struggling because of socioeconomic reasons.”
Spencer is one of 14 million children who have tested positive for SARS-CoV-2 since the start of the pandemic. Many pediatricians are still grappling with how to address cases like Spencer’s. The American Academy of Pediatrics has issued only brief guidance on long COVID in children, in part because there have been so few studies to use as a basis for guidance.
The federal government is aiming to change that with a newly launched National Research Action Plan on Long COVID that includes speeding up research on how the condition affects children and youths, including their ability to learn and thrive.
A CDC study found children with COVID were significantly more likely to have smell and taste disturbances, circulatory system problems, fatigue and malaise, and pain. Those who had been infected had higher rates of acute blockage of a lung artery, myocarditis and weakening of the heart, kidney failure, and type 1 diabetes.
Difficult to diagnose
Even with increased media attention and more published studies on pediatric long COVID, it’s still hard for a busy primary care doctor “to sort through what could just be a cold or what could be a series of colds and trying to look at the bigger picture of what’s been going on in a 1- to 3-month period with a kid,” Dr. Yonts said.
Most children with potential or definite long COVID are still being seen by individual pediatricians, not in a specialized clinic with easy access to an army of specialists. It’s not clear how many of those pediatric clinics exist. Survivor Corps, an advocacy group for people with long COVID, has posted a map of locations providing care, but few are specialized or focus on pediatric long COVID.
Long COVID is different from multisystem inflammatory syndrome in children (MIS-C), which occurs within a month or so of infection, triggers high fevers and severe symptoms in the gut, and often results in hospitalization. MIS-C “is not subtle,” said Dr. Edwards.
The long COVID clinic doctors said most of their patients were not very sick at first. “Anecdotally, of the 83 kids that we’ve seen, most have had mild, very mild, or even asymptomatic infections initially,” and then went on to have long COVID, said Dr. Yonts.
“We see it even in children who have very mild disease or even are asymptomatic,” agreed Allison Eckard, MD, director of pediatric infectious diseases at the Medical University of South Carolina, Charleston.
Fatigue, mood problems
Dr. Yonts said 90% of her patients have fatigue, and many also have severe symptoms in their gut. Those and other long COVID symptoms will be looked at more closely in a 3-year study the Children’s National Medical Center is doing along with the National Institute of Allergy and Infectious Diseases.
There are no treatments for long COVID itself.
“Management is probably more the correct term for what we do in our clinic at this point,” said Dr. Yonts. That means dealing with fatigue and managing headache and digestive symptoms with medications or coping strategies. Guidelines from the American Academy of Physical Medicine and Rehabilitation help inform how to help kids safely resume exercise.
At the Children’s National Medical Center clinic, children will typically meet with a team of specialists including infectious diseases doctors on the same day, said Dr. Yonts. Psychologists help children with coping skills. Dr. Yonts is careful not to imply that long COVID is a psychological illness. Parents “will just shut down, because for so long, they’ve been told this is all a mental thing.”
In about a third of children, symptoms get better on their own, and most kids get better over time. But many still struggle. “We don’t talk about cure, because we don’t know what cure looks like,” said Dr. Edwards.
Vaccination may be best protection
Vaccination seems to help reduce the risk of long COVID, perhaps by as much as half. But parents have been slow to vaccinate children, especially the very young. The AAP reported that, as of Aug. 3, just 5% of children under age 5, 37% of those ages 5-11, and 69% of 12- to 17-year-olds have received at least one dose of a COVID-19 vaccine.
“We have tried to really push vaccine as one of the ways to help prevent some of these long COVID syndromes,” said Dr. Eckard. But that advice is not always welcome. Dr. Eckard told the story of a mother who refused to have her autistic son vaccinated, even as she tearfully pleaded for help with his long COVID symptoms, which had also worsened his autism. The woman told Dr. Eckard: “Nothing you can say will convince me to get him vaccinated.” She thought a vaccine could make his symptoms even worse.
The best prevention is to avoid being infected in the first place.
“The more times you get COVID, the more you increase your risk of getting long COVID,” said Dr. Yonts. “The more times you roll the dice, eventually your number could come up.
A version of this article first appeared on WebMD.com.
When Spencer Siedlecki got COVID-19 in March 2021, he was sick for weeks with extreme fatigue, fevers, a sore throat, bad headaches, nausea, and eventually, pneumonia.
That was scary enough for the then-13-year-old and his parents, who live in Ohio. More than a year later, Spencer still had many of the symptoms and, more alarming, the once-healthy teen had postural orthostatic tachycardia syndrome, a condition that has caused dizziness, a racing heart when he stands, and fainting. Spencer missed most of the last few months of eighth grade because of long COVID.
“He gets sick very easily,” said his mother, Melissa Siedlecki, who works in technology sales. “The common cold that he would shake off in a few days takes weeks for him to feel better.”
The transformation from regular teen life to someone with a chronic illness “sucked,” said Spencer, who will turn 15 in August. “I felt like I was never going to get better.” Fortunately, after some therapy at a specialized clinic, Spencer is back to playing baseball and golf.
Spencer’s journey to better health was difficult; his regular pediatrician told the family at first that there were no treatments to help him – a reaction that is not uncommon. “I still get a lot of parents who heard of me through the grapevine,” said Amy Edwards, MD, director of the pediatric COVID clinic at University Hospitals Rainbow Babies & Children’s and an assistant professor of pediatrics at Case Western Reserve University, both in Cleveland. “The pediatricians either are unsure of what is wrong, or worse, tell children ‘there is nothing wrong with you. Stop faking it.’ ” Dr. Edwards treated Spencer after his mother found the clinic through an internet search.
Alexandra Yonts, MD, a pediatric infectious diseases doctor and director of the post-COVID program clinic at Children’s National Medical Center in Washington, has seen this too. she said.
But those who do get attention tend to be White and affluent, something Dr. Yonts said “doesn’t jibe with the epidemiologic data of who COVID has affected the most.” Black, Latino, and American Indian and Alaska Native children are more likely to be infected with COVID than White children, and have higher rates of hospitalization and death than White children.
It’s not clear whether these children have a particular risk factor, or if they are just the ones who have the resources to get to the clinics. But Dr. Yonts and Dr. Edwards believe many children are not getting the help they need. High-performing kids are coming in “because they are the ones whose symptoms are most obvious,” said Dr. Edwards. “I think there are kids out there who are getting missed because they’re already struggling because of socioeconomic reasons.”
Spencer is one of 14 million children who have tested positive for SARS-CoV-2 since the start of the pandemic. Many pediatricians are still grappling with how to address cases like Spencer’s. The American Academy of Pediatrics has issued only brief guidance on long COVID in children, in part because there have been so few studies to use as a basis for guidance.
The federal government is aiming to change that with a newly launched National Research Action Plan on Long COVID that includes speeding up research on how the condition affects children and youths, including their ability to learn and thrive.
A CDC study found children with COVID were significantly more likely to have smell and taste disturbances, circulatory system problems, fatigue and malaise, and pain. Those who had been infected had higher rates of acute blockage of a lung artery, myocarditis and weakening of the heart, kidney failure, and type 1 diabetes.
Difficult to diagnose
Even with increased media attention and more published studies on pediatric long COVID, it’s still hard for a busy primary care doctor “to sort through what could just be a cold or what could be a series of colds and trying to look at the bigger picture of what’s been going on in a 1- to 3-month period with a kid,” Dr. Yonts said.
Most children with potential or definite long COVID are still being seen by individual pediatricians, not in a specialized clinic with easy access to an army of specialists. It’s not clear how many of those pediatric clinics exist. Survivor Corps, an advocacy group for people with long COVID, has posted a map of locations providing care, but few are specialized or focus on pediatric long COVID.
Long COVID is different from multisystem inflammatory syndrome in children (MIS-C), which occurs within a month or so of infection, triggers high fevers and severe symptoms in the gut, and often results in hospitalization. MIS-C “is not subtle,” said Dr. Edwards.
The long COVID clinic doctors said most of their patients were not very sick at first. “Anecdotally, of the 83 kids that we’ve seen, most have had mild, very mild, or even asymptomatic infections initially,” and then went on to have long COVID, said Dr. Yonts.
“We see it even in children who have very mild disease or even are asymptomatic,” agreed Allison Eckard, MD, director of pediatric infectious diseases at the Medical University of South Carolina, Charleston.
Fatigue, mood problems
Dr. Yonts said 90% of her patients have fatigue, and many also have severe symptoms in their gut. Those and other long COVID symptoms will be looked at more closely in a 3-year study the Children’s National Medical Center is doing along with the National Institute of Allergy and Infectious Diseases.
There are no treatments for long COVID itself.
“Management is probably more the correct term for what we do in our clinic at this point,” said Dr. Yonts. That means dealing with fatigue and managing headache and digestive symptoms with medications or coping strategies. Guidelines from the American Academy of Physical Medicine and Rehabilitation help inform how to help kids safely resume exercise.
At the Children’s National Medical Center clinic, children will typically meet with a team of specialists including infectious diseases doctors on the same day, said Dr. Yonts. Psychologists help children with coping skills. Dr. Yonts is careful not to imply that long COVID is a psychological illness. Parents “will just shut down, because for so long, they’ve been told this is all a mental thing.”
In about a third of children, symptoms get better on their own, and most kids get better over time. But many still struggle. “We don’t talk about cure, because we don’t know what cure looks like,” said Dr. Edwards.
Vaccination may be best protection
Vaccination seems to help reduce the risk of long COVID, perhaps by as much as half. But parents have been slow to vaccinate children, especially the very young. The AAP reported that, as of Aug. 3, just 5% of children under age 5, 37% of those ages 5-11, and 69% of 12- to 17-year-olds have received at least one dose of a COVID-19 vaccine.
“We have tried to really push vaccine as one of the ways to help prevent some of these long COVID syndromes,” said Dr. Eckard. But that advice is not always welcome. Dr. Eckard told the story of a mother who refused to have her autistic son vaccinated, even as she tearfully pleaded for help with his long COVID symptoms, which had also worsened his autism. The woman told Dr. Eckard: “Nothing you can say will convince me to get him vaccinated.” She thought a vaccine could make his symptoms even worse.
The best prevention is to avoid being infected in the first place.
“The more times you get COVID, the more you increase your risk of getting long COVID,” said Dr. Yonts. “The more times you roll the dice, eventually your number could come up.
A version of this article first appeared on WebMD.com.
Guidelines: Convalescent plasma not recommended for most hospitalized with COVID
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
NYC switching children’s COVID vaccine sites to monkeypox
The city health department said demand for children’s COVID vaccines had been on the downswing at the clinics, which opened in late June. Meanwhile, monkeypox cases have increased, with the city declaring it a public health emergency July 30.
“We always planned to transition vaccination for very young children to providers,” the city’s health department said in a statement, according to Spectrum News NY1. “Due to the ongoing monkeypox emergency, we transitioned some of these sites to administer monkeypox vaccine.”
All the COVID vaccine sites for children will close by Aug. 14, Spectrum News NY1 said. It’s unclear if the other sites will transition to monkeypox vaccine.
No appointments for children’s COVID vaccinations had to be canceled, the city said. The plan is that children now needing the COVID vaccine can go to doctors, pharmacies, or the health department clinics.
Manhattan City Councilwoman Gale Brewer urged the health department to keep the kids’ COVID vaccine sites open through the fall.
“I strongly urge you to maintain these family-friendly sites, at least until mid-September so that children who are going to day care and school can get vaccinated,” Brewer wrote. City schools open Sept. 8
Ms. Brewer noted that the city-run sites administered the Moderna vaccines, while many doctors and neighborhood health clinics use the Pfizer vaccine. That could be a problem for a child that had not finished the Moderna regimen or for families that prefer Moderna.
According to the city health department, 2,130 people in New York City had tested positive for monkeypox as of Aug. 12.
On Friday, the city announced 9,000 additional monkeypox vaccines would be made available the morning of Aug. 13.
A version of this article first appeared on WebMD.com.
The city health department said demand for children’s COVID vaccines had been on the downswing at the clinics, which opened in late June. Meanwhile, monkeypox cases have increased, with the city declaring it a public health emergency July 30.
“We always planned to transition vaccination for very young children to providers,” the city’s health department said in a statement, according to Spectrum News NY1. “Due to the ongoing monkeypox emergency, we transitioned some of these sites to administer monkeypox vaccine.”
All the COVID vaccine sites for children will close by Aug. 14, Spectrum News NY1 said. It’s unclear if the other sites will transition to monkeypox vaccine.
No appointments for children’s COVID vaccinations had to be canceled, the city said. The plan is that children now needing the COVID vaccine can go to doctors, pharmacies, or the health department clinics.
Manhattan City Councilwoman Gale Brewer urged the health department to keep the kids’ COVID vaccine sites open through the fall.
“I strongly urge you to maintain these family-friendly sites, at least until mid-September so that children who are going to day care and school can get vaccinated,” Brewer wrote. City schools open Sept. 8
Ms. Brewer noted that the city-run sites administered the Moderna vaccines, while many doctors and neighborhood health clinics use the Pfizer vaccine. That could be a problem for a child that had not finished the Moderna regimen or for families that prefer Moderna.
According to the city health department, 2,130 people in New York City had tested positive for monkeypox as of Aug. 12.
On Friday, the city announced 9,000 additional monkeypox vaccines would be made available the morning of Aug. 13.
A version of this article first appeared on WebMD.com.
The city health department said demand for children’s COVID vaccines had been on the downswing at the clinics, which opened in late June. Meanwhile, monkeypox cases have increased, with the city declaring it a public health emergency July 30.
“We always planned to transition vaccination for very young children to providers,” the city’s health department said in a statement, according to Spectrum News NY1. “Due to the ongoing monkeypox emergency, we transitioned some of these sites to administer monkeypox vaccine.”
All the COVID vaccine sites for children will close by Aug. 14, Spectrum News NY1 said. It’s unclear if the other sites will transition to monkeypox vaccine.
No appointments for children’s COVID vaccinations had to be canceled, the city said. The plan is that children now needing the COVID vaccine can go to doctors, pharmacies, or the health department clinics.
Manhattan City Councilwoman Gale Brewer urged the health department to keep the kids’ COVID vaccine sites open through the fall.
“I strongly urge you to maintain these family-friendly sites, at least until mid-September so that children who are going to day care and school can get vaccinated,” Brewer wrote. City schools open Sept. 8
Ms. Brewer noted that the city-run sites administered the Moderna vaccines, while many doctors and neighborhood health clinics use the Pfizer vaccine. That could be a problem for a child that had not finished the Moderna regimen or for families that prefer Moderna.
According to the city health department, 2,130 people in New York City had tested positive for monkeypox as of Aug. 12.
On Friday, the city announced 9,000 additional monkeypox vaccines would be made available the morning of Aug. 13.
A version of this article first appeared on WebMD.com.
Seniors intend to receive variant-specific COVID booster in coming months
of 2022.
That finding comes from a new poll by researchers at the University of Michigan, Ann Arbor, who also report that when it comes to the shots, people appear to be putting more trust in their health care professionals than in public health authorities.
“When you are a doctor, you are a trusted source of medical information,” said Preeti Malani, MD, MSJ, an infectious disease specialist at the University of Michigan. “Use the ongoing conversation with your patient as an opportunity to answer their questions and counter any confusion.”
The vaccination campaign appears to be having a rub-off effect, too. More people say they’re likely to receive vaccines and boosters for other infections, such as flu, if they have already been vaccinated and boosted against COVID-19.
Inside the poll
Dr. Malani and her colleagues, who published their findings on the National Poll on Healthy Aging’s website, asked 1,024 adults older than 50 about their attitudes on COVID-19 vaccinations and their history of receiving the injections. The questions covered topics including whether the individual had contracted COVID, COVID vaccine doses, and the prevalence of a health care clinician’s opinion on vaccines and boosters. The poll was conducted July 21-26.
The researchers chose the age range of 50-65 years because this group is an important population for new booster shots that target specific variants of the SARS-CoV-2 virus that causes COVID-19.
Only 19% of people aged 50-64 and 44% of those older than 65 said they had received both their first and second COVID-19 booster shots. What’s more, 17% of people said they had not received any doses of a COVID-19 vaccine.
The vast majority (77%) of respondents said their clinician’s recommendations were “very important” or “somewhat important” in their decision to receive the vaccine.
Dr. Malani said that in her practice, patients have expressed hesitation about COVID-19 vaccines because of concerns about the potential side effects of the shots.
Monica Gandhi, MD, MPH, professor of medicine at the University of California, San Francisco, noted that Americans now appear to trust their physicians more than public health authorities such as the U.S. Centers for Disease Control and Prevention when it comes to COVID-19.
“More people are trusting their providers’ opinions [more] than the CDC or other public health agencies. That speaks volumes to me,” Dr. Gandhi said.
Among the more surprising findings of the poll, according to the researchers, was the number of people who said they had yet to contract COVID-19: 50% of those aged 50-64, and 69% of those older than 65. (Another 12% of those aged 50-64 said they were unsure if they’d ever had the infection.)
Dr. Malani said she hoped future studies would explore in depth the people who remain uninfected with COVID-19.
“We focus a lot on the science of COVID,” she said. “But we need to turn our attention to the behavioral aspects and how to address them.”
A version of this article first appeared on Medscape.com.
of 2022.
That finding comes from a new poll by researchers at the University of Michigan, Ann Arbor, who also report that when it comes to the shots, people appear to be putting more trust in their health care professionals than in public health authorities.
“When you are a doctor, you are a trusted source of medical information,” said Preeti Malani, MD, MSJ, an infectious disease specialist at the University of Michigan. “Use the ongoing conversation with your patient as an opportunity to answer their questions and counter any confusion.”
The vaccination campaign appears to be having a rub-off effect, too. More people say they’re likely to receive vaccines and boosters for other infections, such as flu, if they have already been vaccinated and boosted against COVID-19.
Inside the poll
Dr. Malani and her colleagues, who published their findings on the National Poll on Healthy Aging’s website, asked 1,024 adults older than 50 about their attitudes on COVID-19 vaccinations and their history of receiving the injections. The questions covered topics including whether the individual had contracted COVID, COVID vaccine doses, and the prevalence of a health care clinician’s opinion on vaccines and boosters. The poll was conducted July 21-26.
The researchers chose the age range of 50-65 years because this group is an important population for new booster shots that target specific variants of the SARS-CoV-2 virus that causes COVID-19.
Only 19% of people aged 50-64 and 44% of those older than 65 said they had received both their first and second COVID-19 booster shots. What’s more, 17% of people said they had not received any doses of a COVID-19 vaccine.
The vast majority (77%) of respondents said their clinician’s recommendations were “very important” or “somewhat important” in their decision to receive the vaccine.
Dr. Malani said that in her practice, patients have expressed hesitation about COVID-19 vaccines because of concerns about the potential side effects of the shots.
Monica Gandhi, MD, MPH, professor of medicine at the University of California, San Francisco, noted that Americans now appear to trust their physicians more than public health authorities such as the U.S. Centers for Disease Control and Prevention when it comes to COVID-19.
“More people are trusting their providers’ opinions [more] than the CDC or other public health agencies. That speaks volumes to me,” Dr. Gandhi said.
Among the more surprising findings of the poll, according to the researchers, was the number of people who said they had yet to contract COVID-19: 50% of those aged 50-64, and 69% of those older than 65. (Another 12% of those aged 50-64 said they were unsure if they’d ever had the infection.)
Dr. Malani said she hoped future studies would explore in depth the people who remain uninfected with COVID-19.
“We focus a lot on the science of COVID,” she said. “But we need to turn our attention to the behavioral aspects and how to address them.”
A version of this article first appeared on Medscape.com.
of 2022.
That finding comes from a new poll by researchers at the University of Michigan, Ann Arbor, who also report that when it comes to the shots, people appear to be putting more trust in their health care professionals than in public health authorities.
“When you are a doctor, you are a trusted source of medical information,” said Preeti Malani, MD, MSJ, an infectious disease specialist at the University of Michigan. “Use the ongoing conversation with your patient as an opportunity to answer their questions and counter any confusion.”
The vaccination campaign appears to be having a rub-off effect, too. More people say they’re likely to receive vaccines and boosters for other infections, such as flu, if they have already been vaccinated and boosted against COVID-19.
Inside the poll
Dr. Malani and her colleagues, who published their findings on the National Poll on Healthy Aging’s website, asked 1,024 adults older than 50 about their attitudes on COVID-19 vaccinations and their history of receiving the injections. The questions covered topics including whether the individual had contracted COVID, COVID vaccine doses, and the prevalence of a health care clinician’s opinion on vaccines and boosters. The poll was conducted July 21-26.
The researchers chose the age range of 50-65 years because this group is an important population for new booster shots that target specific variants of the SARS-CoV-2 virus that causes COVID-19.
Only 19% of people aged 50-64 and 44% of those older than 65 said they had received both their first and second COVID-19 booster shots. What’s more, 17% of people said they had not received any doses of a COVID-19 vaccine.
The vast majority (77%) of respondents said their clinician’s recommendations were “very important” or “somewhat important” in their decision to receive the vaccine.
Dr. Malani said that in her practice, patients have expressed hesitation about COVID-19 vaccines because of concerns about the potential side effects of the shots.
Monica Gandhi, MD, MPH, professor of medicine at the University of California, San Francisco, noted that Americans now appear to trust their physicians more than public health authorities such as the U.S. Centers for Disease Control and Prevention when it comes to COVID-19.
“More people are trusting their providers’ opinions [more] than the CDC or other public health agencies. That speaks volumes to me,” Dr. Gandhi said.
Among the more surprising findings of the poll, according to the researchers, was the number of people who said they had yet to contract COVID-19: 50% of those aged 50-64, and 69% of those older than 65. (Another 12% of those aged 50-64 said they were unsure if they’d ever had the infection.)
Dr. Malani said she hoped future studies would explore in depth the people who remain uninfected with COVID-19.
“We focus a lot on the science of COVID,” she said. “But we need to turn our attention to the behavioral aspects and how to address them.”
A version of this article first appeared on Medscape.com.
Sexual dysfunction, hair loss linked with long COVID
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
Regular fasting linked to less severe COVID: Study
, according to the findings of a new study.
The study was done on men and women in Utah who were, on average, in their 60s and got COVID before vaccines were available.
Roughly one in three people in Utah fast from time to time – higher than in other states. This is partly because more than 60% of people in Utah belong to the Church of Jesus Christ of Latter-day Saints, and roughly 40% of them fast – typically skipping two meals in a row.
Those who fasted, on average, for a day a month over the past 40 years were not less likely to get COVID, but they were less likely to be hospitalized or die from the virus.
“Intermittent fasting has already shown to lower inflammation and improve cardiovascular health,” lead study author Benjamin Horne, PhD, of Intermountain Medical Center Heart Institute in Salt Lake City, said in a statement.
“In this study, we’re finding additional benefits when it comes to battling an infection of COVID-19 in patients who have been fasting for decades,” he said.
The study was published in BMJ Nutrition, Prevention & Health.
Intermittent fasting not a substitute for a COVID-19 vaccine
Importantly, intermittent fasting shouldn’t be seen as a substitute for getting a COVID vaccine, the researchers stressed. Rather, periodic fasting might be a health habit to consider, since it is also linked to a lower risk of diabetes and heart disease, for example.
But anyone who wants to consider intermittent fasting should consult their doctor first, Dr. Horne stressed, especially if they are elderly, pregnant, or have diabetes, heart disease, or kidney disease.
Fasting didn’t prevent COVID-19 but made it less severe
In their study, the team looked at data from 1,524 adults who were seen in the cardiac catheterization lab at Intermountain Medical Center Heart Institute, completed a survey, and had a test for the virus that causes COVID-19 from March 16, 2020, to Feb. 25, 2021.
Of these patients, 205 tested positive for COVID, and of these, 73 reported that they had fasted regularly at least once a month.
Similar numbers of patients got COVID-19 whether they had, or had not, fasted regularly (14%, versus 13%).
But among those who tested positive for the virus, fewer patients were hospitalized for COVID or died during the study follow-up if they had fasted regularly (11%) than if they had not fasted regularly (29%).
Even when the analyses were adjusted for age, smoking, alcohol use, ethnicity, history of heart disease, and other factors, periodic fasting was still an independent predictor of a lower risk of hospitalization or death.
Several things may explain the findings, the researchers suggested.
A loss of appetite is a typical response to infection, they noted.
Fasting reduces inflammation, and after 12-14 hours of fasting, the body switches from using glucose in the blood to using ketones, including linoleic acid.
“There’s a pocket on the surface of SARS-CoV-2 that linoleic acid fits into – and can make the virus less able to attach to other cells,” Dr. Horne said.
Intermittent fasting also promotes autophagy, he noted, which is “the body’s recycling system that helps your body destroy and recycle damaged and infected cells.”
The researchers concluded that intermittent fasting plans should be investigated in further research “as a complementary therapy to vaccines to reduce COVID-19 severity, both during the pandemic and post pandemic, since repeat vaccinations cannot be performed every few months indefinitely for the entire world and vaccine access is limited in many nations.”
A version of this article first appeared on WebMD.com.
, according to the findings of a new study.
The study was done on men and women in Utah who were, on average, in their 60s and got COVID before vaccines were available.
Roughly one in three people in Utah fast from time to time – higher than in other states. This is partly because more than 60% of people in Utah belong to the Church of Jesus Christ of Latter-day Saints, and roughly 40% of them fast – typically skipping two meals in a row.
Those who fasted, on average, for a day a month over the past 40 years were not less likely to get COVID, but they were less likely to be hospitalized or die from the virus.
“Intermittent fasting has already shown to lower inflammation and improve cardiovascular health,” lead study author Benjamin Horne, PhD, of Intermountain Medical Center Heart Institute in Salt Lake City, said in a statement.
“In this study, we’re finding additional benefits when it comes to battling an infection of COVID-19 in patients who have been fasting for decades,” he said.
The study was published in BMJ Nutrition, Prevention & Health.
Intermittent fasting not a substitute for a COVID-19 vaccine
Importantly, intermittent fasting shouldn’t be seen as a substitute for getting a COVID vaccine, the researchers stressed. Rather, periodic fasting might be a health habit to consider, since it is also linked to a lower risk of diabetes and heart disease, for example.
But anyone who wants to consider intermittent fasting should consult their doctor first, Dr. Horne stressed, especially if they are elderly, pregnant, or have diabetes, heart disease, or kidney disease.
Fasting didn’t prevent COVID-19 but made it less severe
In their study, the team looked at data from 1,524 adults who were seen in the cardiac catheterization lab at Intermountain Medical Center Heart Institute, completed a survey, and had a test for the virus that causes COVID-19 from March 16, 2020, to Feb. 25, 2021.
Of these patients, 205 tested positive for COVID, and of these, 73 reported that they had fasted regularly at least once a month.
Similar numbers of patients got COVID-19 whether they had, or had not, fasted regularly (14%, versus 13%).
But among those who tested positive for the virus, fewer patients were hospitalized for COVID or died during the study follow-up if they had fasted regularly (11%) than if they had not fasted regularly (29%).
Even when the analyses were adjusted for age, smoking, alcohol use, ethnicity, history of heart disease, and other factors, periodic fasting was still an independent predictor of a lower risk of hospitalization or death.
Several things may explain the findings, the researchers suggested.
A loss of appetite is a typical response to infection, they noted.
Fasting reduces inflammation, and after 12-14 hours of fasting, the body switches from using glucose in the blood to using ketones, including linoleic acid.
“There’s a pocket on the surface of SARS-CoV-2 that linoleic acid fits into – and can make the virus less able to attach to other cells,” Dr. Horne said.
Intermittent fasting also promotes autophagy, he noted, which is “the body’s recycling system that helps your body destroy and recycle damaged and infected cells.”
The researchers concluded that intermittent fasting plans should be investigated in further research “as a complementary therapy to vaccines to reduce COVID-19 severity, both during the pandemic and post pandemic, since repeat vaccinations cannot be performed every few months indefinitely for the entire world and vaccine access is limited in many nations.”
A version of this article first appeared on WebMD.com.
, according to the findings of a new study.
The study was done on men and women in Utah who were, on average, in their 60s and got COVID before vaccines were available.
Roughly one in three people in Utah fast from time to time – higher than in other states. This is partly because more than 60% of people in Utah belong to the Church of Jesus Christ of Latter-day Saints, and roughly 40% of them fast – typically skipping two meals in a row.
Those who fasted, on average, for a day a month over the past 40 years were not less likely to get COVID, but they were less likely to be hospitalized or die from the virus.
“Intermittent fasting has already shown to lower inflammation and improve cardiovascular health,” lead study author Benjamin Horne, PhD, of Intermountain Medical Center Heart Institute in Salt Lake City, said in a statement.
“In this study, we’re finding additional benefits when it comes to battling an infection of COVID-19 in patients who have been fasting for decades,” he said.
The study was published in BMJ Nutrition, Prevention & Health.
Intermittent fasting not a substitute for a COVID-19 vaccine
Importantly, intermittent fasting shouldn’t be seen as a substitute for getting a COVID vaccine, the researchers stressed. Rather, periodic fasting might be a health habit to consider, since it is also linked to a lower risk of diabetes and heart disease, for example.
But anyone who wants to consider intermittent fasting should consult their doctor first, Dr. Horne stressed, especially if they are elderly, pregnant, or have diabetes, heart disease, or kidney disease.
Fasting didn’t prevent COVID-19 but made it less severe
In their study, the team looked at data from 1,524 adults who were seen in the cardiac catheterization lab at Intermountain Medical Center Heart Institute, completed a survey, and had a test for the virus that causes COVID-19 from March 16, 2020, to Feb. 25, 2021.
Of these patients, 205 tested positive for COVID, and of these, 73 reported that they had fasted regularly at least once a month.
Similar numbers of patients got COVID-19 whether they had, or had not, fasted regularly (14%, versus 13%).
But among those who tested positive for the virus, fewer patients were hospitalized for COVID or died during the study follow-up if they had fasted regularly (11%) than if they had not fasted regularly (29%).
Even when the analyses were adjusted for age, smoking, alcohol use, ethnicity, history of heart disease, and other factors, periodic fasting was still an independent predictor of a lower risk of hospitalization or death.
Several things may explain the findings, the researchers suggested.
A loss of appetite is a typical response to infection, they noted.
Fasting reduces inflammation, and after 12-14 hours of fasting, the body switches from using glucose in the blood to using ketones, including linoleic acid.
“There’s a pocket on the surface of SARS-CoV-2 that linoleic acid fits into – and can make the virus less able to attach to other cells,” Dr. Horne said.
Intermittent fasting also promotes autophagy, he noted, which is “the body’s recycling system that helps your body destroy and recycle damaged and infected cells.”
The researchers concluded that intermittent fasting plans should be investigated in further research “as a complementary therapy to vaccines to reduce COVID-19 severity, both during the pandemic and post pandemic, since repeat vaccinations cannot be performed every few months indefinitely for the entire world and vaccine access is limited in many nations.”
A version of this article first appeared on WebMD.com.
FROM BMJ NUTRITION, PREVENTION & HEALTH