User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Giving flu and COVID-19 shots at same time appears safe, effective: Study
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
New AMA president discusses pandemic during inaugural address
He has encountered “all manner of unexpected situations” and feels “more than prepared” to serve as president of the American Medical Association, he said.
At the same time, “I still find myself a little nervous about it,” Dr. Harmon said in an interview the day after he was sworn in as president. “I would be less than candid if I didn’t tell you that. I don’t mean intimidated. ... It’s almost like before an athletic event.”
Dr. Harmon was sworn in June 15 as the 176th president of the AMA during the virtual Special Meeting of the AMA House of Delegates. He follows Susan R. Bailey, MD, an allergist from Fort Worth, Tex., in leading the organization, which has more than 270,000 members.
Advancing health equity
During his inaugural address, Dr. Harmon discussed the pandemic and the AMA’s plan to advance health equity.
COVID-19 “has revealed enormous gaps in how we care for people and communities in America, demonstrated in the disproportionate impact of this pandemic on communities of color and in the weaknesses of our underfunded and underresourced public health infrastructure,” Dr. Harmon said.
He described medical professionals as being “at war against seemingly formidable adversaries,” including the pandemic, the effects of prolonged isolation on emotional and behavioral health, and political and racial tension. There is an “immense battle to rid our health system – and society – of health disparities and racism,” he said. “As we face these battles, we must remember that our actions as physicians and as leaders will have far-reaching consequences.”
Other challenges before the AMA include vaccinating patients, recovering from the ongoing pandemic, removing unnecessary obstacles to care, ending an epidemic of drug overdoses, improving outcomes for patients with chronic disease, incorporating technology in ways that benefit doctors and patients, and preparing future physicians, Dr. Harmon noted.
“We are going to embed the principles of equity and racial justice within the AMA and throughout our health system,” added Dr. Harmon, who has been an AMA board member since 2013 and served as board chair from 2017 to 2018. He highlighted the AMA’s strategic plan, released in May 2021, to advance health equity and justice and improve the quality of care for people who have been marginalized.
“Meaningful progress won’t happen until we, as doctors, recognize how profoundly systemic racism influences the health of our patients, and until we commit to taking action within our own spheres of influence,” Dr. Harmon said. “As a family doctor in a very diverse state, I have treated people from all backgrounds, and have seen inequities up close, inequities that understandably lead to distrust.”
Commenting in an interview on JAMA’s controversial tweet and podcast related to structural racism from earlier this year that have been deleted and removed from JAMA’s website, Dr. Harmon said, JAMA maintains editorial independence from the AMA, but that direction from a journal oversight committee could lead to changes at the journal that could help prevent similar incidents.
“We’ll support whatever the journal oversight committee suggests,” Dr. Harmon said.
“We had public statements about [the podcast]. I do think that we’ll be able to move very quickly in a stronger direction to address the issue of systemic racism,” Dr. Harmon said. “The AMA has acknowledged that it is a public health threat. We have acknowledged that it is ... a political description versus a biologic construct. So, I would anticipate that you’ll find changes.”
The AMA began developing its strategic plan to advance equity several years ago, Dr. Harmon noted. “I think we are very well poised to move forward and attack this enemy of health disparity.”
AAFP president supporting Dr. Harmon’s inauguration
Among those congratulating Dr. Harmon on his inauguration was Ada Stewart, MD, a fellow family physician and South Carolina resident who is the president of the American Academy of Family Physicians.
“We are very excited that family physician Dr. Gerald Harmon will serve as president of the AMA this coming year,” Dr. Stewart said. “Family medicine encompasses the very essence of medicine – treating the whole person, in the context of family, community, and each individual’s unique circumstances. As a family physician, Dr. Harmon brings important perspectives from the front lines of primary care. His commitment to health equity and evidence-based care, as well as his concern for practice sustainability and physician well-being, will serve him well as he leads the house of medicine into the future.”
Dr. Harmon has practiced as a family medicine specialist in Georgetown, S.C., for more than 30 years. He is a member of the clinical faculty for the Tidelands Health Medical University of South Carolina family medicine residency program, advises a community health system, and is vice president of a multispecialty physician practice. In addition, Dr. Harmon is the medical director of a nonprofit hospice and volunteers as medical supervisor for his local school district.
Dr. Harmon received his undergraduate degree in physics and mathematics from the University of South Carolina, Columbia, and received his medical degree from the Medical University of South Carolina, Charleston. He completed a residency training program in family medicine with the U.S. Air Force at Eglin (Fla.) AFB, Florida.
During a 35-year military career, Dr. Harmon served as chief surgeon for the National Guard Bureau and assistant surgeon general for the U.S. Air Force. He retired from the military as a major general.
Dr. Harmon and his wife, Linda, have three married children and eight grandchildren.
Every now and then, a bucket of tomatoes or even a half bushel of corn shows up in the back of Dr. Harmon’s pickup truck, with a note on the window thanking him. “That really touches you deeply,” Dr. Harmon said. “I practice that type of medicine and I’m honored to be able to do that every day.”
He has encountered “all manner of unexpected situations” and feels “more than prepared” to serve as president of the American Medical Association, he said.
At the same time, “I still find myself a little nervous about it,” Dr. Harmon said in an interview the day after he was sworn in as president. “I would be less than candid if I didn’t tell you that. I don’t mean intimidated. ... It’s almost like before an athletic event.”
Dr. Harmon was sworn in June 15 as the 176th president of the AMA during the virtual Special Meeting of the AMA House of Delegates. He follows Susan R. Bailey, MD, an allergist from Fort Worth, Tex., in leading the organization, which has more than 270,000 members.
Advancing health equity
During his inaugural address, Dr. Harmon discussed the pandemic and the AMA’s plan to advance health equity.
COVID-19 “has revealed enormous gaps in how we care for people and communities in America, demonstrated in the disproportionate impact of this pandemic on communities of color and in the weaknesses of our underfunded and underresourced public health infrastructure,” Dr. Harmon said.
He described medical professionals as being “at war against seemingly formidable adversaries,” including the pandemic, the effects of prolonged isolation on emotional and behavioral health, and political and racial tension. There is an “immense battle to rid our health system – and society – of health disparities and racism,” he said. “As we face these battles, we must remember that our actions as physicians and as leaders will have far-reaching consequences.”
Other challenges before the AMA include vaccinating patients, recovering from the ongoing pandemic, removing unnecessary obstacles to care, ending an epidemic of drug overdoses, improving outcomes for patients with chronic disease, incorporating technology in ways that benefit doctors and patients, and preparing future physicians, Dr. Harmon noted.
“We are going to embed the principles of equity and racial justice within the AMA and throughout our health system,” added Dr. Harmon, who has been an AMA board member since 2013 and served as board chair from 2017 to 2018. He highlighted the AMA’s strategic plan, released in May 2021, to advance health equity and justice and improve the quality of care for people who have been marginalized.
“Meaningful progress won’t happen until we, as doctors, recognize how profoundly systemic racism influences the health of our patients, and until we commit to taking action within our own spheres of influence,” Dr. Harmon said. “As a family doctor in a very diverse state, I have treated people from all backgrounds, and have seen inequities up close, inequities that understandably lead to distrust.”
Commenting in an interview on JAMA’s controversial tweet and podcast related to structural racism from earlier this year that have been deleted and removed from JAMA’s website, Dr. Harmon said, JAMA maintains editorial independence from the AMA, but that direction from a journal oversight committee could lead to changes at the journal that could help prevent similar incidents.
“We’ll support whatever the journal oversight committee suggests,” Dr. Harmon said.
“We had public statements about [the podcast]. I do think that we’ll be able to move very quickly in a stronger direction to address the issue of systemic racism,” Dr. Harmon said. “The AMA has acknowledged that it is a public health threat. We have acknowledged that it is ... a political description versus a biologic construct. So, I would anticipate that you’ll find changes.”
The AMA began developing its strategic plan to advance equity several years ago, Dr. Harmon noted. “I think we are very well poised to move forward and attack this enemy of health disparity.”
AAFP president supporting Dr. Harmon’s inauguration
Among those congratulating Dr. Harmon on his inauguration was Ada Stewart, MD, a fellow family physician and South Carolina resident who is the president of the American Academy of Family Physicians.
“We are very excited that family physician Dr. Gerald Harmon will serve as president of the AMA this coming year,” Dr. Stewart said. “Family medicine encompasses the very essence of medicine – treating the whole person, in the context of family, community, and each individual’s unique circumstances. As a family physician, Dr. Harmon brings important perspectives from the front lines of primary care. His commitment to health equity and evidence-based care, as well as his concern for practice sustainability and physician well-being, will serve him well as he leads the house of medicine into the future.”
Dr. Harmon has practiced as a family medicine specialist in Georgetown, S.C., for more than 30 years. He is a member of the clinical faculty for the Tidelands Health Medical University of South Carolina family medicine residency program, advises a community health system, and is vice president of a multispecialty physician practice. In addition, Dr. Harmon is the medical director of a nonprofit hospice and volunteers as medical supervisor for his local school district.
Dr. Harmon received his undergraduate degree in physics and mathematics from the University of South Carolina, Columbia, and received his medical degree from the Medical University of South Carolina, Charleston. He completed a residency training program in family medicine with the U.S. Air Force at Eglin (Fla.) AFB, Florida.
During a 35-year military career, Dr. Harmon served as chief surgeon for the National Guard Bureau and assistant surgeon general for the U.S. Air Force. He retired from the military as a major general.
Dr. Harmon and his wife, Linda, have three married children and eight grandchildren.
Every now and then, a bucket of tomatoes or even a half bushel of corn shows up in the back of Dr. Harmon’s pickup truck, with a note on the window thanking him. “That really touches you deeply,” Dr. Harmon said. “I practice that type of medicine and I’m honored to be able to do that every day.”
He has encountered “all manner of unexpected situations” and feels “more than prepared” to serve as president of the American Medical Association, he said.
At the same time, “I still find myself a little nervous about it,” Dr. Harmon said in an interview the day after he was sworn in as president. “I would be less than candid if I didn’t tell you that. I don’t mean intimidated. ... It’s almost like before an athletic event.”
Dr. Harmon was sworn in June 15 as the 176th president of the AMA during the virtual Special Meeting of the AMA House of Delegates. He follows Susan R. Bailey, MD, an allergist from Fort Worth, Tex., in leading the organization, which has more than 270,000 members.
Advancing health equity
During his inaugural address, Dr. Harmon discussed the pandemic and the AMA’s plan to advance health equity.
COVID-19 “has revealed enormous gaps in how we care for people and communities in America, demonstrated in the disproportionate impact of this pandemic on communities of color and in the weaknesses of our underfunded and underresourced public health infrastructure,” Dr. Harmon said.
He described medical professionals as being “at war against seemingly formidable adversaries,” including the pandemic, the effects of prolonged isolation on emotional and behavioral health, and political and racial tension. There is an “immense battle to rid our health system – and society – of health disparities and racism,” he said. “As we face these battles, we must remember that our actions as physicians and as leaders will have far-reaching consequences.”
Other challenges before the AMA include vaccinating patients, recovering from the ongoing pandemic, removing unnecessary obstacles to care, ending an epidemic of drug overdoses, improving outcomes for patients with chronic disease, incorporating technology in ways that benefit doctors and patients, and preparing future physicians, Dr. Harmon noted.
“We are going to embed the principles of equity and racial justice within the AMA and throughout our health system,” added Dr. Harmon, who has been an AMA board member since 2013 and served as board chair from 2017 to 2018. He highlighted the AMA’s strategic plan, released in May 2021, to advance health equity and justice and improve the quality of care for people who have been marginalized.
“Meaningful progress won’t happen until we, as doctors, recognize how profoundly systemic racism influences the health of our patients, and until we commit to taking action within our own spheres of influence,” Dr. Harmon said. “As a family doctor in a very diverse state, I have treated people from all backgrounds, and have seen inequities up close, inequities that understandably lead to distrust.”
Commenting in an interview on JAMA’s controversial tweet and podcast related to structural racism from earlier this year that have been deleted and removed from JAMA’s website, Dr. Harmon said, JAMA maintains editorial independence from the AMA, but that direction from a journal oversight committee could lead to changes at the journal that could help prevent similar incidents.
“We’ll support whatever the journal oversight committee suggests,” Dr. Harmon said.
“We had public statements about [the podcast]. I do think that we’ll be able to move very quickly in a stronger direction to address the issue of systemic racism,” Dr. Harmon said. “The AMA has acknowledged that it is a public health threat. We have acknowledged that it is ... a political description versus a biologic construct. So, I would anticipate that you’ll find changes.”
The AMA began developing its strategic plan to advance equity several years ago, Dr. Harmon noted. “I think we are very well poised to move forward and attack this enemy of health disparity.”
AAFP president supporting Dr. Harmon’s inauguration
Among those congratulating Dr. Harmon on his inauguration was Ada Stewart, MD, a fellow family physician and South Carolina resident who is the president of the American Academy of Family Physicians.
“We are very excited that family physician Dr. Gerald Harmon will serve as president of the AMA this coming year,” Dr. Stewart said. “Family medicine encompasses the very essence of medicine – treating the whole person, in the context of family, community, and each individual’s unique circumstances. As a family physician, Dr. Harmon brings important perspectives from the front lines of primary care. His commitment to health equity and evidence-based care, as well as his concern for practice sustainability and physician well-being, will serve him well as he leads the house of medicine into the future.”
Dr. Harmon has practiced as a family medicine specialist in Georgetown, S.C., for more than 30 years. He is a member of the clinical faculty for the Tidelands Health Medical University of South Carolina family medicine residency program, advises a community health system, and is vice president of a multispecialty physician practice. In addition, Dr. Harmon is the medical director of a nonprofit hospice and volunteers as medical supervisor for his local school district.
Dr. Harmon received his undergraduate degree in physics and mathematics from the University of South Carolina, Columbia, and received his medical degree from the Medical University of South Carolina, Charleston. He completed a residency training program in family medicine with the U.S. Air Force at Eglin (Fla.) AFB, Florida.
During a 35-year military career, Dr. Harmon served as chief surgeon for the National Guard Bureau and assistant surgeon general for the U.S. Air Force. He retired from the military as a major general.
Dr. Harmon and his wife, Linda, have three married children and eight grandchildren.
Every now and then, a bucket of tomatoes or even a half bushel of corn shows up in the back of Dr. Harmon’s pickup truck, with a note on the window thanking him. “That really touches you deeply,” Dr. Harmon said. “I practice that type of medicine and I’m honored to be able to do that every day.”
Supreme Court upholds Affordable Care Act
The challengers were comprised of 18 GOP-dominated states, led by Texas, that took issue with the ACA’s individual mandate – which required most Americans to have health insurance or pay a tax penalty.
But Congress reduced the penalty to zero in 2017. Challengers argued that without the mandate, the rest of the law should be scrapped, too. The court ruled that eliminated the harm the states were claiming.
“To have standing, a plaintiff must ‘allege personal injury fairly traceable to the defendant’s allegedly unlawful conduct and likely to be redressed by the requested relief,’” the majority wrote. “No plaintiff has shown such an injury ‘fairly traceable’ to the ‘allegedly unlawful conduct’ challenged here.”
Justice Stephen Breyer authored the opinion. Justices Samuel Alito and Neil Gorsuch dissented.
The decision said that the mandate in question did not require the 18 states that brought the complaint to pay anything, and therefore they had no standing.
President Joe Biden has said he plans to build on the ACA – which was enacted while he was vice president – to offer coverage to more Americans.
This marks the third time the Supreme Court spared the Obama-era law from GOP attacks. The mandate was also upheld in 2012 in a 5 to 4 ruling.
American Medical Association president Gerald Harmon, MD, also called for building on the ruling to expand the law.
“With yet another court decision upholding the ACA now behind us, we remain committed to strengthening the current law and look forward to policymakers advancing solutions to improve the ACA,” Dr. Harmon said in a statement. “The AMA will continue working to expand access to health care and ensure that all Americans have meaningful, comprehensive, and affordable health coverage to improve the health of the nation.”
House Speaker Nancy Pelosi (D-Calif.), a longtime advocate for the ACA, called the decision a “landmark victory for Democrats.”
“Thanks to the tireless advocacy of Americans across the country and Democrats in Congress, the Affordable Care Act endures as a pillar of American health and economic security alongside Medicare, Medicaid and Social Security,” she said in a statement.
Senate Majority Leader Chuck Schumer (D-N.Y.) also celebrated the ruling.
“The Affordable Care Act has won. The Supreme Court has just ruled: the ACA is here to stay and now we’re going to try to make it bigger and better,” he said, according to CNN. “For more than a decade, the assault on our health care law was relentless from Republicans in Congress, from the executive branch itself and from Republican attorneys general in the courts. Each time in each arena, the ACA has prevailed.”
This article was updated June 17, 2021.
A version of this article first appeared on WebMD.com.
The challengers were comprised of 18 GOP-dominated states, led by Texas, that took issue with the ACA’s individual mandate – which required most Americans to have health insurance or pay a tax penalty.
But Congress reduced the penalty to zero in 2017. Challengers argued that without the mandate, the rest of the law should be scrapped, too. The court ruled that eliminated the harm the states were claiming.
“To have standing, a plaintiff must ‘allege personal injury fairly traceable to the defendant’s allegedly unlawful conduct and likely to be redressed by the requested relief,’” the majority wrote. “No plaintiff has shown such an injury ‘fairly traceable’ to the ‘allegedly unlawful conduct’ challenged here.”
Justice Stephen Breyer authored the opinion. Justices Samuel Alito and Neil Gorsuch dissented.
The decision said that the mandate in question did not require the 18 states that brought the complaint to pay anything, and therefore they had no standing.
President Joe Biden has said he plans to build on the ACA – which was enacted while he was vice president – to offer coverage to more Americans.
This marks the third time the Supreme Court spared the Obama-era law from GOP attacks. The mandate was also upheld in 2012 in a 5 to 4 ruling.
American Medical Association president Gerald Harmon, MD, also called for building on the ruling to expand the law.
“With yet another court decision upholding the ACA now behind us, we remain committed to strengthening the current law and look forward to policymakers advancing solutions to improve the ACA,” Dr. Harmon said in a statement. “The AMA will continue working to expand access to health care and ensure that all Americans have meaningful, comprehensive, and affordable health coverage to improve the health of the nation.”
House Speaker Nancy Pelosi (D-Calif.), a longtime advocate for the ACA, called the decision a “landmark victory for Democrats.”
“Thanks to the tireless advocacy of Americans across the country and Democrats in Congress, the Affordable Care Act endures as a pillar of American health and economic security alongside Medicare, Medicaid and Social Security,” she said in a statement.
Senate Majority Leader Chuck Schumer (D-N.Y.) also celebrated the ruling.
“The Affordable Care Act has won. The Supreme Court has just ruled: the ACA is here to stay and now we’re going to try to make it bigger and better,” he said, according to CNN. “For more than a decade, the assault on our health care law was relentless from Republicans in Congress, from the executive branch itself and from Republican attorneys general in the courts. Each time in each arena, the ACA has prevailed.”
This article was updated June 17, 2021.
A version of this article first appeared on WebMD.com.
The challengers were comprised of 18 GOP-dominated states, led by Texas, that took issue with the ACA’s individual mandate – which required most Americans to have health insurance or pay a tax penalty.
But Congress reduced the penalty to zero in 2017. Challengers argued that without the mandate, the rest of the law should be scrapped, too. The court ruled that eliminated the harm the states were claiming.
“To have standing, a plaintiff must ‘allege personal injury fairly traceable to the defendant’s allegedly unlawful conduct and likely to be redressed by the requested relief,’” the majority wrote. “No plaintiff has shown such an injury ‘fairly traceable’ to the ‘allegedly unlawful conduct’ challenged here.”
Justice Stephen Breyer authored the opinion. Justices Samuel Alito and Neil Gorsuch dissented.
The decision said that the mandate in question did not require the 18 states that brought the complaint to pay anything, and therefore they had no standing.
President Joe Biden has said he plans to build on the ACA – which was enacted while he was vice president – to offer coverage to more Americans.
This marks the third time the Supreme Court spared the Obama-era law from GOP attacks. The mandate was also upheld in 2012 in a 5 to 4 ruling.
American Medical Association president Gerald Harmon, MD, also called for building on the ruling to expand the law.
“With yet another court decision upholding the ACA now behind us, we remain committed to strengthening the current law and look forward to policymakers advancing solutions to improve the ACA,” Dr. Harmon said in a statement. “The AMA will continue working to expand access to health care and ensure that all Americans have meaningful, comprehensive, and affordable health coverage to improve the health of the nation.”
House Speaker Nancy Pelosi (D-Calif.), a longtime advocate for the ACA, called the decision a “landmark victory for Democrats.”
“Thanks to the tireless advocacy of Americans across the country and Democrats in Congress, the Affordable Care Act endures as a pillar of American health and economic security alongside Medicare, Medicaid and Social Security,” she said in a statement.
Senate Majority Leader Chuck Schumer (D-N.Y.) also celebrated the ruling.
“The Affordable Care Act has won. The Supreme Court has just ruled: the ACA is here to stay and now we’re going to try to make it bigger and better,” he said, according to CNN. “For more than a decade, the assault on our health care law was relentless from Republicans in Congress, from the executive branch itself and from Republican attorneys general in the courts. Each time in each arena, the ACA has prevailed.”
This article was updated June 17, 2021.
A version of this article first appeared on WebMD.com.
AHA: Don’t delay COVID shot while CDC reviews myocarditis cases
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
Extensive limb swelling after vaccines – including SARS-CoV-2 vaccine
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.
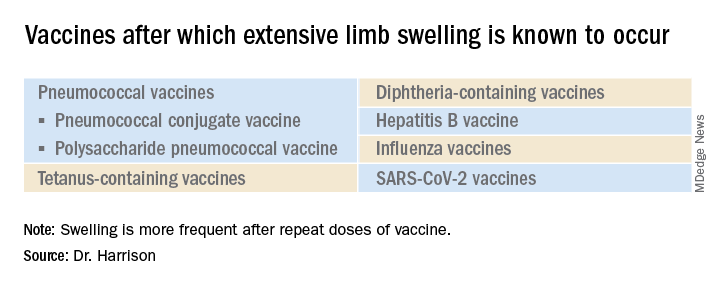
What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.

What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.

What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.
Back-to-school threat: Missed vaccinations in children, teens
U.S. children and adolescents may be at higher risk for vaccine-preventable diseases this fall as vaccination levels have not caught up with prepandemic coverage, according to a study published in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
“Pediatric outbreaks of vaccine-preventable diseases have the potential to derail efforts to reopen schools for the 2021-22 academic year and further delay nationwide efforts to return students to the classroom,” wrote Bhavini Patel Murthy, MD, with the immunization services division, National Center for Immunization and Respiratory Diseases, and colleagues.
The number of children getting routine vaccinations plummeted between March and May 2020, compared with the same months in 2019. Although vaccination rates increased again from June 2020 to September 2020, the rebound was not enough to reach prepandemic levels, according to the study.
At the beginning of the June–September 2020 period, the news was good, the authors wrote. After most stay-at-home orders were lifted, the number of weekly routine pediatric vaccinations started to approach, and even surpass, baseline prepandemic levels in most of the 10 jurisdictions studied.
“However,” the authors wrote, “across all age groups and across all vaccine types, none of the jurisdictions demonstrated a sustained or prolonged increase in the number of weekly doses administered above prepandemic administration levels, which would have been necessary to catch up children and adolescents who missed routine vaccinations.”
To overcome the gap, the authors said that clinicians should take the initiative. “Health care providers should assess the vaccination status of all pediatric patients, including adolescents, and contact those who are behind schedule to ensure that all children are fully vaccinated.”
As COVID-19 vaccinations become more readily available to children, the CDC recommends that providers consider giving COVID-19 shots along with other routinely recommended vaccines.
Martha Perry, MD, associate professor and medical director at the University of North Carolina Children’s Primary Care Clinic, Chapel Hill, said in an interview that getting the message out about the need to get children and adolescents caught up may require a national messaging campaign similar to that for COVID-19 vaccinations, as well as opening mass vaccination sites rather than families seeking vaccinations from individual providers.
She noted that, although schools may offer a checks and balances system for required vaccinations, children who are not yet school age depend on families getting individual appointments.
Size of the gaps
The MMWR article shows that the shortfall in vaccinations in June–September 2020, compared with those months the year before are striking.
For children younger than 2 years old and aged 2-6 years, diphtheria, tetanus, and acellular pertussis (DtaP) vaccinations declined an average of 9.1% and 6.7%, respectively.
Among children aged 12-23 months and 2-8 years, MMR vaccinations decreased 8.8% and 11.3%, respectively.
Among children aged 9-12 years and adolescents 13-17 years, human papillomavirus vaccinations decreased an average 12.2% and 28.1%, respectively. Among the same age groups, Tdap vaccinations dropped 21.3% and 30.0%, respectively.
Dr. Perry said that, although all the shortfalls are important, lags in vaccinations for measles and pertussis are particularly alarming in light of outbreaks in recent years.
Additionally, she said, as COVID-19 restrictions are lifting, some of the mitigation strategies, such as mask wearing, that kept other diseases at bay will not be in place, heightening the risk for infection.
The authors chose to measure weekly doses in March–May 2020, and June–September 2020 because many jurisdictions imposed and then lifted stay-at-home orders during these times. They analyzed data from 10 jurisdictions with high-performing information systems (Idaho, Iowa, Louisiana, Michigan, Minnesota, New York City, North Dakota, Oregon, Washington, and Wisconsin).
Adults missing vaccinations as well
Another analysis, commissioned by GlaxoSmithKline and conducted by Avalere Health, calculated 8.8 million missed adolescent vaccine doses and 17.2 million missed adult vaccine doses as a result of the pandemic and ongoing government restrictions and public health measures.
That study examined claims for CDC-recommended vaccines across commercial, managed Medicaid, Medicare Advantage, and Medicare fee-for-service Part B for January–November 2020, compared with the same period in 2019.
It also found that vaccine claims remain well below 2019 levels. Total noninfluenza vaccine claims submissions were down by between 13% and 35% among adolescents and 17% and 40% among adults, compared with the same period in 2019.
Dr. Perry said it will be critical for schools across the nation to enforce their policies on requiring up-to-date vaccinations even if online attendance is offered.
The workforce needed for this will be challenging, she noted.
“We’ve lost a lot of workforce in the health care field in the pandemic for a variety of reasons and it may be challenging to fill those positions,” she said.
She also said the study underlines the importance of each state having a vaccine registry so each provider can determine what vaccinations a child needs.
The study authors and Dr. Perry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S. children and adolescents may be at higher risk for vaccine-preventable diseases this fall as vaccination levels have not caught up with prepandemic coverage, according to a study published in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
“Pediatric outbreaks of vaccine-preventable diseases have the potential to derail efforts to reopen schools for the 2021-22 academic year and further delay nationwide efforts to return students to the classroom,” wrote Bhavini Patel Murthy, MD, with the immunization services division, National Center for Immunization and Respiratory Diseases, and colleagues.
The number of children getting routine vaccinations plummeted between March and May 2020, compared with the same months in 2019. Although vaccination rates increased again from June 2020 to September 2020, the rebound was not enough to reach prepandemic levels, according to the study.
At the beginning of the June–September 2020 period, the news was good, the authors wrote. After most stay-at-home orders were lifted, the number of weekly routine pediatric vaccinations started to approach, and even surpass, baseline prepandemic levels in most of the 10 jurisdictions studied.
“However,” the authors wrote, “across all age groups and across all vaccine types, none of the jurisdictions demonstrated a sustained or prolonged increase in the number of weekly doses administered above prepandemic administration levels, which would have been necessary to catch up children and adolescents who missed routine vaccinations.”
To overcome the gap, the authors said that clinicians should take the initiative. “Health care providers should assess the vaccination status of all pediatric patients, including adolescents, and contact those who are behind schedule to ensure that all children are fully vaccinated.”
As COVID-19 vaccinations become more readily available to children, the CDC recommends that providers consider giving COVID-19 shots along with other routinely recommended vaccines.
Martha Perry, MD, associate professor and medical director at the University of North Carolina Children’s Primary Care Clinic, Chapel Hill, said in an interview that getting the message out about the need to get children and adolescents caught up may require a national messaging campaign similar to that for COVID-19 vaccinations, as well as opening mass vaccination sites rather than families seeking vaccinations from individual providers.
She noted that, although schools may offer a checks and balances system for required vaccinations, children who are not yet school age depend on families getting individual appointments.
Size of the gaps
The MMWR article shows that the shortfall in vaccinations in June–September 2020, compared with those months the year before are striking.
For children younger than 2 years old and aged 2-6 years, diphtheria, tetanus, and acellular pertussis (DtaP) vaccinations declined an average of 9.1% and 6.7%, respectively.
Among children aged 12-23 months and 2-8 years, MMR vaccinations decreased 8.8% and 11.3%, respectively.
Among children aged 9-12 years and adolescents 13-17 years, human papillomavirus vaccinations decreased an average 12.2% and 28.1%, respectively. Among the same age groups, Tdap vaccinations dropped 21.3% and 30.0%, respectively.
Dr. Perry said that, although all the shortfalls are important, lags in vaccinations for measles and pertussis are particularly alarming in light of outbreaks in recent years.
Additionally, she said, as COVID-19 restrictions are lifting, some of the mitigation strategies, such as mask wearing, that kept other diseases at bay will not be in place, heightening the risk for infection.
The authors chose to measure weekly doses in March–May 2020, and June–September 2020 because many jurisdictions imposed and then lifted stay-at-home orders during these times. They analyzed data from 10 jurisdictions with high-performing information systems (Idaho, Iowa, Louisiana, Michigan, Minnesota, New York City, North Dakota, Oregon, Washington, and Wisconsin).
Adults missing vaccinations as well
Another analysis, commissioned by GlaxoSmithKline and conducted by Avalere Health, calculated 8.8 million missed adolescent vaccine doses and 17.2 million missed adult vaccine doses as a result of the pandemic and ongoing government restrictions and public health measures.
That study examined claims for CDC-recommended vaccines across commercial, managed Medicaid, Medicare Advantage, and Medicare fee-for-service Part B for January–November 2020, compared with the same period in 2019.
It also found that vaccine claims remain well below 2019 levels. Total noninfluenza vaccine claims submissions were down by between 13% and 35% among adolescents and 17% and 40% among adults, compared with the same period in 2019.
Dr. Perry said it will be critical for schools across the nation to enforce their policies on requiring up-to-date vaccinations even if online attendance is offered.
The workforce needed for this will be challenging, she noted.
“We’ve lost a lot of workforce in the health care field in the pandemic for a variety of reasons and it may be challenging to fill those positions,” she said.
She also said the study underlines the importance of each state having a vaccine registry so each provider can determine what vaccinations a child needs.
The study authors and Dr. Perry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S. children and adolescents may be at higher risk for vaccine-preventable diseases this fall as vaccination levels have not caught up with prepandemic coverage, according to a study published in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
“Pediatric outbreaks of vaccine-preventable diseases have the potential to derail efforts to reopen schools for the 2021-22 academic year and further delay nationwide efforts to return students to the classroom,” wrote Bhavini Patel Murthy, MD, with the immunization services division, National Center for Immunization and Respiratory Diseases, and colleagues.
The number of children getting routine vaccinations plummeted between March and May 2020, compared with the same months in 2019. Although vaccination rates increased again from June 2020 to September 2020, the rebound was not enough to reach prepandemic levels, according to the study.
At the beginning of the June–September 2020 period, the news was good, the authors wrote. After most stay-at-home orders were lifted, the number of weekly routine pediatric vaccinations started to approach, and even surpass, baseline prepandemic levels in most of the 10 jurisdictions studied.
“However,” the authors wrote, “across all age groups and across all vaccine types, none of the jurisdictions demonstrated a sustained or prolonged increase in the number of weekly doses administered above prepandemic administration levels, which would have been necessary to catch up children and adolescents who missed routine vaccinations.”
To overcome the gap, the authors said that clinicians should take the initiative. “Health care providers should assess the vaccination status of all pediatric patients, including adolescents, and contact those who are behind schedule to ensure that all children are fully vaccinated.”
As COVID-19 vaccinations become more readily available to children, the CDC recommends that providers consider giving COVID-19 shots along with other routinely recommended vaccines.
Martha Perry, MD, associate professor and medical director at the University of North Carolina Children’s Primary Care Clinic, Chapel Hill, said in an interview that getting the message out about the need to get children and adolescents caught up may require a national messaging campaign similar to that for COVID-19 vaccinations, as well as opening mass vaccination sites rather than families seeking vaccinations from individual providers.
She noted that, although schools may offer a checks and balances system for required vaccinations, children who are not yet school age depend on families getting individual appointments.
Size of the gaps
The MMWR article shows that the shortfall in vaccinations in June–September 2020, compared with those months the year before are striking.
For children younger than 2 years old and aged 2-6 years, diphtheria, tetanus, and acellular pertussis (DtaP) vaccinations declined an average of 9.1% and 6.7%, respectively.
Among children aged 12-23 months and 2-8 years, MMR vaccinations decreased 8.8% and 11.3%, respectively.
Among children aged 9-12 years and adolescents 13-17 years, human papillomavirus vaccinations decreased an average 12.2% and 28.1%, respectively. Among the same age groups, Tdap vaccinations dropped 21.3% and 30.0%, respectively.
Dr. Perry said that, although all the shortfalls are important, lags in vaccinations for measles and pertussis are particularly alarming in light of outbreaks in recent years.
Additionally, she said, as COVID-19 restrictions are lifting, some of the mitigation strategies, such as mask wearing, that kept other diseases at bay will not be in place, heightening the risk for infection.
The authors chose to measure weekly doses in March–May 2020, and June–September 2020 because many jurisdictions imposed and then lifted stay-at-home orders during these times. They analyzed data from 10 jurisdictions with high-performing information systems (Idaho, Iowa, Louisiana, Michigan, Minnesota, New York City, North Dakota, Oregon, Washington, and Wisconsin).
Adults missing vaccinations as well
Another analysis, commissioned by GlaxoSmithKline and conducted by Avalere Health, calculated 8.8 million missed adolescent vaccine doses and 17.2 million missed adult vaccine doses as a result of the pandemic and ongoing government restrictions and public health measures.
That study examined claims for CDC-recommended vaccines across commercial, managed Medicaid, Medicare Advantage, and Medicare fee-for-service Part B for January–November 2020, compared with the same period in 2019.
It also found that vaccine claims remain well below 2019 levels. Total noninfluenza vaccine claims submissions were down by between 13% and 35% among adolescents and 17% and 40% among adults, compared with the same period in 2019.
Dr. Perry said it will be critical for schools across the nation to enforce their policies on requiring up-to-date vaccinations even if online attendance is offered.
The workforce needed for this will be challenging, she noted.
“We’ve lost a lot of workforce in the health care field in the pandemic for a variety of reasons and it may be challenging to fill those positions,” she said.
She also said the study underlines the importance of each state having a vaccine registry so each provider can determine what vaccinations a child needs.
The study authors and Dr. Perry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AMA acknowledges medical education racism of past, vows better future
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
‘COVID toes’ chilblain-like lesions not related to COVID-19
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Prediction rule identifies low infection risk in febrile infants
A clinical prediction rule combining procalcitonin, absolute neutrophil count, and urinalysis effectively identified most febrile infants at low risk for serious bacterial infections, based on data from 702 individuals
The clinical prediction rule (CPR) described in 2019 in JAMA Pediatrics was developed by the Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN) to identify febrile infants at low risk for serious bacterial infections in order to reduce unnecessary procedures, antibiotics use, and hospitalization, according to April Clawson, MD, of Arkansas Children’s Hospital, Little Rock, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, the researchers conducted an external validation of the rule via a retrospective, observational study of febrile infants aged 60 days and younger who presented to an urban pediatric ED between October 2014 and June 2019. The study population included 702 infants with an average age of 36 days. Approximately 45% were female, and 60% were White. Fever was defined as 38° C or greater. Exclusion criteria were prematurity, receipt of antibiotics in the past 48 hours, presence of an indwelling medical device, and evidence of focal infection (not including otitis media); those who were critically ill at presentation or had a previous medical condition were excluded as well, the researchers said. A serious bacterial infection (SBI) was defined as a urinary tract infection (UTI), bacteremia, or bacterial meningitis.
Based on the CPR, a patient is considered low risk for an SBI if all the following criteria are met: normal urinalysis (defined as absence of leukocyte esterase, nitrite, and 5 or less white blood cells per high power field); an absolute neutrophil count of 4,090/mL or less; and procalcitonin of 1.71 ng/mL or less.
Overall, 62 infants (8.8%) were diagnosed with an SBI, similar to the 9.3% seen in the parent study of the CPR, Dr. Clawson said.
Of these, 42 had a UTI only (6%), 10 had bacteremia only (1.4%), and 1 had meningitis only (0.1%). Another five infants had UTI with bacteremia (0.7%), and four had bacteremia and meningitis (0.6%).
According to the CPR, 432 infants met criteria for low risk and 270 were considered high risk. A total of five infants who were classified as low risk had SBIs, including two with UTIs, two with bacteremia, and one with meningitis.
“The CPR derived and validated by Kupperman et al. had a decreased sensitivity for the patients in our study and missed some SBIs,” Dr. Clawson noted. “However, it had a strong negative predictive value, so it may still be a useful CPR.”
The sensitivity for the CPR in the parent study and the current study was 97.7 and 91.9, respectively; specificity was 60 and 66.7, respectively. The negative predictive values for the parent and current studies were 99.6 and 98.8, respectively, and the positive predictive values were 20.7 and 21.1.
The results support the potential of the CPR, but more external validation is needed, they said.
PECARN rule keeps it simple
“It has always been a challenge to identify infants with fever with serious bacterial infections when they are well-appearing,” Yashas Nathani, MD, of Oklahoma University, Oklahoma City, said in an interview. “The clinical prediction rule offers a simple, step-by-step approach for pediatricians and emergency medicine physicians to stratify infants in high or low risk categories for SBIs. However, as with everything, validation of protocols, guidelines and decision-making algorithms is extremely important, especially as more clinicians start to employ this CPR to their daily practice. This study objectively puts the CPR to the test and offers an independent external validation.
“Although this study had a lower sensitivity in identifying infants with SBI using the clinical prediction rule as compared to the original study, the robust validation of negative predictive value is extremely important and not surprising,” said Dr. Nathani. “The goal of this CPR is to identify infants with low-risk for SBI and the stated NPV helps clinicians in doing just that.”
Overall, “the clinical prediction rule is a fantastic resource for physicians to identify potentially sick infants with fever, especially the ones that appear well on initial evaluation,” said Dr. Nathani. However, “it is important to acknowledge that this is merely a guideline, and not an absolute rule. Clinicians also must remain cautious, as this rule does not incorporate the presence of viral pathogens as a factor.
“It is important to continue the scientific quest to refine our approach in identifying infants with serious bacterial infections when fever is the only presentation,” Dr. Nathani noted. “Additional research is needed to continue fine-tuning this CPR and the thresholds for procalcitonin and absolute neutrophil counts to improve the sensitivity and specificity.” Research also is needed to explore whether this CPR can be extended to incorporate viral testing, “as a large number of infants with fever have viral pathogens as the primary etiology,” he concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Nathani had no financial conflicts to disclose.
A clinical prediction rule combining procalcitonin, absolute neutrophil count, and urinalysis effectively identified most febrile infants at low risk for serious bacterial infections, based on data from 702 individuals
The clinical prediction rule (CPR) described in 2019 in JAMA Pediatrics was developed by the Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN) to identify febrile infants at low risk for serious bacterial infections in order to reduce unnecessary procedures, antibiotics use, and hospitalization, according to April Clawson, MD, of Arkansas Children’s Hospital, Little Rock, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, the researchers conducted an external validation of the rule via a retrospective, observational study of febrile infants aged 60 days and younger who presented to an urban pediatric ED between October 2014 and June 2019. The study population included 702 infants with an average age of 36 days. Approximately 45% were female, and 60% were White. Fever was defined as 38° C or greater. Exclusion criteria were prematurity, receipt of antibiotics in the past 48 hours, presence of an indwelling medical device, and evidence of focal infection (not including otitis media); those who were critically ill at presentation or had a previous medical condition were excluded as well, the researchers said. A serious bacterial infection (SBI) was defined as a urinary tract infection (UTI), bacteremia, or bacterial meningitis.
Based on the CPR, a patient is considered low risk for an SBI if all the following criteria are met: normal urinalysis (defined as absence of leukocyte esterase, nitrite, and 5 or less white blood cells per high power field); an absolute neutrophil count of 4,090/mL or less; and procalcitonin of 1.71 ng/mL or less.
Overall, 62 infants (8.8%) were diagnosed with an SBI, similar to the 9.3% seen in the parent study of the CPR, Dr. Clawson said.
Of these, 42 had a UTI only (6%), 10 had bacteremia only (1.4%), and 1 had meningitis only (0.1%). Another five infants had UTI with bacteremia (0.7%), and four had bacteremia and meningitis (0.6%).
According to the CPR, 432 infants met criteria for low risk and 270 were considered high risk. A total of five infants who were classified as low risk had SBIs, including two with UTIs, two with bacteremia, and one with meningitis.
“The CPR derived and validated by Kupperman et al. had a decreased sensitivity for the patients in our study and missed some SBIs,” Dr. Clawson noted. “However, it had a strong negative predictive value, so it may still be a useful CPR.”
The sensitivity for the CPR in the parent study and the current study was 97.7 and 91.9, respectively; specificity was 60 and 66.7, respectively. The negative predictive values for the parent and current studies were 99.6 and 98.8, respectively, and the positive predictive values were 20.7 and 21.1.
The results support the potential of the CPR, but more external validation is needed, they said.
PECARN rule keeps it simple
“It has always been a challenge to identify infants with fever with serious bacterial infections when they are well-appearing,” Yashas Nathani, MD, of Oklahoma University, Oklahoma City, said in an interview. “The clinical prediction rule offers a simple, step-by-step approach for pediatricians and emergency medicine physicians to stratify infants in high or low risk categories for SBIs. However, as with everything, validation of protocols, guidelines and decision-making algorithms is extremely important, especially as more clinicians start to employ this CPR to their daily practice. This study objectively puts the CPR to the test and offers an independent external validation.
“Although this study had a lower sensitivity in identifying infants with SBI using the clinical prediction rule as compared to the original study, the robust validation of negative predictive value is extremely important and not surprising,” said Dr. Nathani. “The goal of this CPR is to identify infants with low-risk for SBI and the stated NPV helps clinicians in doing just that.”
Overall, “the clinical prediction rule is a fantastic resource for physicians to identify potentially sick infants with fever, especially the ones that appear well on initial evaluation,” said Dr. Nathani. However, “it is important to acknowledge that this is merely a guideline, and not an absolute rule. Clinicians also must remain cautious, as this rule does not incorporate the presence of viral pathogens as a factor.
“It is important to continue the scientific quest to refine our approach in identifying infants with serious bacterial infections when fever is the only presentation,” Dr. Nathani noted. “Additional research is needed to continue fine-tuning this CPR and the thresholds for procalcitonin and absolute neutrophil counts to improve the sensitivity and specificity.” Research also is needed to explore whether this CPR can be extended to incorporate viral testing, “as a large number of infants with fever have viral pathogens as the primary etiology,” he concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Nathani had no financial conflicts to disclose.
A clinical prediction rule combining procalcitonin, absolute neutrophil count, and urinalysis effectively identified most febrile infants at low risk for serious bacterial infections, based on data from 702 individuals
The clinical prediction rule (CPR) described in 2019 in JAMA Pediatrics was developed by the Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN) to identify febrile infants at low risk for serious bacterial infections in order to reduce unnecessary procedures, antibiotics use, and hospitalization, according to April Clawson, MD, of Arkansas Children’s Hospital, Little Rock, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, the researchers conducted an external validation of the rule via a retrospective, observational study of febrile infants aged 60 days and younger who presented to an urban pediatric ED between October 2014 and June 2019. The study population included 702 infants with an average age of 36 days. Approximately 45% were female, and 60% were White. Fever was defined as 38° C or greater. Exclusion criteria were prematurity, receipt of antibiotics in the past 48 hours, presence of an indwelling medical device, and evidence of focal infection (not including otitis media); those who were critically ill at presentation or had a previous medical condition were excluded as well, the researchers said. A serious bacterial infection (SBI) was defined as a urinary tract infection (UTI), bacteremia, or bacterial meningitis.
Based on the CPR, a patient is considered low risk for an SBI if all the following criteria are met: normal urinalysis (defined as absence of leukocyte esterase, nitrite, and 5 or less white blood cells per high power field); an absolute neutrophil count of 4,090/mL or less; and procalcitonin of 1.71 ng/mL or less.
Overall, 62 infants (8.8%) were diagnosed with an SBI, similar to the 9.3% seen in the parent study of the CPR, Dr. Clawson said.
Of these, 42 had a UTI only (6%), 10 had bacteremia only (1.4%), and 1 had meningitis only (0.1%). Another five infants had UTI with bacteremia (0.7%), and four had bacteremia and meningitis (0.6%).
According to the CPR, 432 infants met criteria for low risk and 270 were considered high risk. A total of five infants who were classified as low risk had SBIs, including two with UTIs, two with bacteremia, and one with meningitis.
“The CPR derived and validated by Kupperman et al. had a decreased sensitivity for the patients in our study and missed some SBIs,” Dr. Clawson noted. “However, it had a strong negative predictive value, so it may still be a useful CPR.”
The sensitivity for the CPR in the parent study and the current study was 97.7 and 91.9, respectively; specificity was 60 and 66.7, respectively. The negative predictive values for the parent and current studies were 99.6 and 98.8, respectively, and the positive predictive values were 20.7 and 21.1.
The results support the potential of the CPR, but more external validation is needed, they said.
PECARN rule keeps it simple
“It has always been a challenge to identify infants with fever with serious bacterial infections when they are well-appearing,” Yashas Nathani, MD, of Oklahoma University, Oklahoma City, said in an interview. “The clinical prediction rule offers a simple, step-by-step approach for pediatricians and emergency medicine physicians to stratify infants in high or low risk categories for SBIs. However, as with everything, validation of protocols, guidelines and decision-making algorithms is extremely important, especially as more clinicians start to employ this CPR to their daily practice. This study objectively puts the CPR to the test and offers an independent external validation.
“Although this study had a lower sensitivity in identifying infants with SBI using the clinical prediction rule as compared to the original study, the robust validation of negative predictive value is extremely important and not surprising,” said Dr. Nathani. “The goal of this CPR is to identify infants with low-risk for SBI and the stated NPV helps clinicians in doing just that.”
Overall, “the clinical prediction rule is a fantastic resource for physicians to identify potentially sick infants with fever, especially the ones that appear well on initial evaluation,” said Dr. Nathani. However, “it is important to acknowledge that this is merely a guideline, and not an absolute rule. Clinicians also must remain cautious, as this rule does not incorporate the presence of viral pathogens as a factor.
“It is important to continue the scientific quest to refine our approach in identifying infants with serious bacterial infections when fever is the only presentation,” Dr. Nathani noted. “Additional research is needed to continue fine-tuning this CPR and the thresholds for procalcitonin and absolute neutrophil counts to improve the sensitivity and specificity.” Research also is needed to explore whether this CPR can be extended to incorporate viral testing, “as a large number of infants with fever have viral pathogens as the primary etiology,” he concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Nathani had no financial conflicts to disclose.
FROM PAS 2021
As new cases fall, U.S. passes 4 million children with COVID-19
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.





