User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Treatment paradigm for chronic HBV in flux
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
FROM GUILD 2021
Vaccine mismatch: What to do after dose 1 when plans change
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
COVID vaccines could lose their punch within a year, experts say
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
National Psoriasis Foundation recommends some stop methotrexate for 2 weeks after J&J vaccine
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
New COVID-19 cases rise again in children
The number of new COVID-19 cases in children increased for the second consecutive week in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
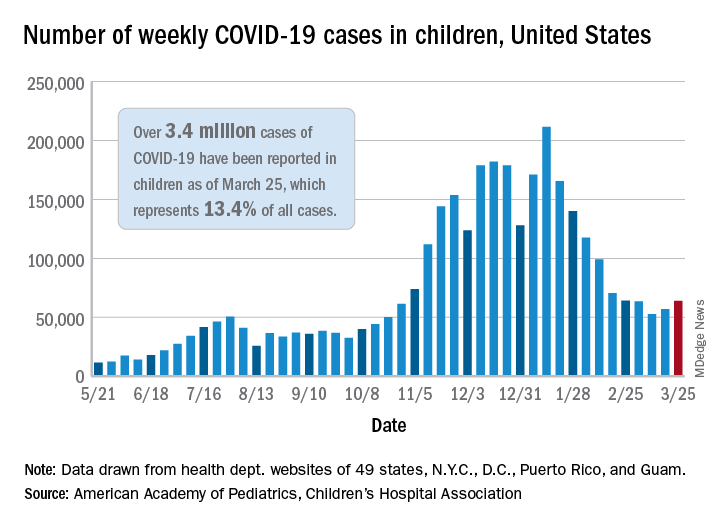
That brings the number of children infected with the coronavirus to over 3.4 million since the beginning of the pandemic, or 13.4% of all reported cases, the AAP and CHA said in their weekly COVID-19 report.
For just the week of March 19-25, however, the proportion of all cases occurring in children was quite a bit higher, 19.1%. That’s higher than at any other point during the pandemic, passing the previous high of 18.7% set just a week earlier, based on the data collected by AAP/CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The national infection rate was 4,525 cases per 100,000 children for the week of March 19-25, compared with 4,440 per 100,000 the previous week. States falling the farthest from that national mark were Hawaii at 1,101 per 100,000 and North Dakota at 8,848, the AAP and CHA said.
There was double-digit increase, 11, in the number of child deaths, as the total went from 268 to 279 despite Virginia’s revising its mortality data downward. The mortality rate for children remains 0.01%, and children represent only 0.06% of all COVID-19–related deaths in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting deaths by age, the report shows.
The state/local-level data show that Texas has the highest number of child deaths (48), followed by Arizona (26), New York City (22), California (16), and Illinois (16), while nine states and the District of Columbia have not yet reported a death, the AAP and CHA said.
The number of new COVID-19 cases in children increased for the second consecutive week in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That brings the number of children infected with the coronavirus to over 3.4 million since the beginning of the pandemic, or 13.4% of all reported cases, the AAP and CHA said in their weekly COVID-19 report.
For just the week of March 19-25, however, the proportion of all cases occurring in children was quite a bit higher, 19.1%. That’s higher than at any other point during the pandemic, passing the previous high of 18.7% set just a week earlier, based on the data collected by AAP/CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The national infection rate was 4,525 cases per 100,000 children for the week of March 19-25, compared with 4,440 per 100,000 the previous week. States falling the farthest from that national mark were Hawaii at 1,101 per 100,000 and North Dakota at 8,848, the AAP and CHA said.
There was double-digit increase, 11, in the number of child deaths, as the total went from 268 to 279 despite Virginia’s revising its mortality data downward. The mortality rate for children remains 0.01%, and children represent only 0.06% of all COVID-19–related deaths in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting deaths by age, the report shows.
The state/local-level data show that Texas has the highest number of child deaths (48), followed by Arizona (26), New York City (22), California (16), and Illinois (16), while nine states and the District of Columbia have not yet reported a death, the AAP and CHA said.
The number of new COVID-19 cases in children increased for the second consecutive week in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That brings the number of children infected with the coronavirus to over 3.4 million since the beginning of the pandemic, or 13.4% of all reported cases, the AAP and CHA said in their weekly COVID-19 report.
For just the week of March 19-25, however, the proportion of all cases occurring in children was quite a bit higher, 19.1%. That’s higher than at any other point during the pandemic, passing the previous high of 18.7% set just a week earlier, based on the data collected by AAP/CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The national infection rate was 4,525 cases per 100,000 children for the week of March 19-25, compared with 4,440 per 100,000 the previous week. States falling the farthest from that national mark were Hawaii at 1,101 per 100,000 and North Dakota at 8,848, the AAP and CHA said.
There was double-digit increase, 11, in the number of child deaths, as the total went from 268 to 279 despite Virginia’s revising its mortality data downward. The mortality rate for children remains 0.01%, and children represent only 0.06% of all COVID-19–related deaths in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting deaths by age, the report shows.
The state/local-level data show that Texas has the highest number of child deaths (48), followed by Arizona (26), New York City (22), California (16), and Illinois (16), while nine states and the District of Columbia have not yet reported a death, the AAP and CHA said.
COVID-19 ‘long-haul’ symptoms overlap with ME/CFS
People experiencing long-term symptoms following acute COVID-19 infection are increasingly meeting criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a phenomenon that highlights the need for unified research and clinical approaches, speakers said at a press briefing March 25 held by the advocacy group MEAction.
“Post-COVID lingering illness was predictable. Similar lingering fatigue syndromes have been reported in the scientific literature for nearly 100 years, following a variety of well-documented infections with viruses, bacteria, fungi, and even protozoa,” said Anthony Komaroff, MD, professor of medicine at Harvard Medical School, Boston.
Core criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for at least 6 months, postexertional malaise (PEM), or a worsening of symptoms following even minor exertion (often described as “crashes”), unrefreshing sleep, and cognitive impairment and/or orthostatic intolerance.
Patients with ME/CFS also commonly experience painful headaches, muscle or joint aches, and allergies/other sensitivities. Although many patients can trace their symptoms to an initiating infection, “the cause is often unclear because the diagnosis is often delayed for months or years after symptom onset,” said Lucinda Bateman, MD, founder of the Bateman Horne Center, Salt Lake City, who leads a clinician coalition that aims to improve ME/CFS management.
In an international survey of 3762 COVID-19 “long-haulers” published in a preprint in December of 2020, the most frequent symptoms reported at least 6 months after illness onset were fatigue in 78%, PEM in 72%, and cognitive dysfunction (“brain fog”) in 55%. At the time of the survey, 45% reported requiring reduced work schedules because of their illness, and 22% reported being unable to work at all.
Dr. Bateman said those findings align with her experience so far with 12 COVID-19 “long haulers” who self-referred to her ME/CFS and fibromyalgia specialty clinic. Nine of the 12 met criteria for postural orthostatic tachycardia syndrome (POTS) based on the 10-minute NASA Lean Test, she said, and half also met the 2016 American College of Rheumatology criteria for fibromyalgia.
“Some were severely impaired. We suspect a small fiber polyneuropathy in about half, and mast cell activation syndrome in more than half. We look forward to doing more testing,” Dr. Bateman said.
To be sure, Dr. Komaroff noted, there are some differences. “Long COVID” patients will often experience breathlessness and ongoing anosmia (loss of taste and smell), which aren’t typical of ME/CFS.
But, he said, “many of the symptoms are quite similar ... My guess is that ME/CFS is an illness with a final common pathway that can be triggered by different things,” said Dr. Komaroff, a senior physician at Brigham and Women’s Hospital in Boston, and editor-in-chief of the Harvard Health Letter.
Based on previous data about CFS suggesting a 10% rate of symptoms persisting at least a year following a variety of infectious agents and the predicted 200 million COVID-19 cases globally by the end of 2021, Dr. Komaroff estimated that about 20 million cases of “long COVID” would be expected in the next year.
‘A huge investment’
On the research side, the National Institutes of Health recently appropriated $1.15 billion dollars over the next 4 years to investigate “the heterogeneity in the recovery process after COVID and to develop treatments for those suffering from [postacute COVID-19 syndrome]” according to a Feb. 5, 2021, blog from the National Institute of Neurological Disorders and Stroke (NINDS).
That same day, another NINDS blog announced “new resources for large-scale ME/CFS research” and emphasized the tie-in with long–COVID-19 syndrome.
“That’s a huge investment. In my opinion, there will be several lingering illnesses following COVID,” Dr. Komaroff said, adding, “It’s my bet that long COVID will prove to be caused by certain kinds of abnormalities in the brain, some of the same abnormalities already identified in ME/CFS. Research will determine whether that’s right or wrong.”
In 2017, NINDS had announced a large increase in funding for ME/CFS research, including the creation of four dedicated research centers. In April 2019, NINDS held a 2-day conference highlighting that ongoing work, as reported by Medscape Medical News.
During the briefing, NINDS clinical director Avindra Nath, MD, described a comprehensive ongoing ME/CFS intramural study he’s been leading since 2016.
He’s now also overseeing two long–COVID-19 studies, one of which has a protocol similar to that of the ME/CFS study and will include individuals who are still experiencing long-term symptoms following confirmed cases of COVID-19. The aim is to screen about 1,300 patients. Several task forces are now examining all of these data together.
“Each aspect is now being analyzed … What we learn from one applies to the other,” Dr. Nath said.
Advice for clinicians
In interviews, Dr. Bateman and Dr. Nath offered clinical advice for managing patients who meet ME/CFS criteria, whether they had confirmed or suspected COVID-19, a different infection, or unknown trigger(s).
Dr. Bateman advised that clinicians assess patients for each of the symptoms individually. “Besides exercise intolerance and PEM, the most commonly missed is orthostatic intolerance. It really doesn’t matter what the cause is, it’s amenable to supportive treatment. It’s one aspect of the illness that contributes to severely impaired function. My plea to all physicians would be for sure to assess for [orthostatic intolerance], and gain an understanding about activity management and avoiding PEM symptoms.”
Dr. Nath noted that an often-challenging situation is when tests for the infectious agent and other blood work come back negative, yet the patient still reports multiple debilitating symptoms. This has been a particular issue with long COVID-19, since many patients became ill early in the pandemic before the polymerase chain reaction (PCR) tests for SARS-CoV-2 were widely available.
“The physician can only order tests that are available at their labs. I think what the physician should do is handle symptoms symptomatically but also refer patients to specialists who are taking care of these patients or to research studies,” he said.
Dr. Bateman added, “Whether they had a documented COVID infection – we just have to let go of that in 2020. Way too many people didn’t have access to a test or the timing wasn’t amenable. If people meet criteria for ME/CFS, it’s irrelevant … It’s mainly a clinical diagnosis. It’s not reliant on identifying the infectious trigger.”
Dr. Komaroff, who began caring for then-termed “chronic fatigue syndrome” patients and researching the condition more than 30 years ago, said that “every cloud has its silver lining. The increased focus on postinfectious fatigue syndrome is a silver lining in my mind around the terrible dark cloud that is the pandemic of COVID.”
Dr. Komaroff has received personal fees from Serimmune Inc., Ono Pharma, and Deallus, and grants from the NIH. Dr. Bateman is employed by the Bateman Horne Center, which receives grants from the NIH, and fees from Exagen Inc., and Teva Pharmaceutical. Dr. Nath is an NIH employee.
A version of this article first appeared on Medscape.com.
People experiencing long-term symptoms following acute COVID-19 infection are increasingly meeting criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a phenomenon that highlights the need for unified research and clinical approaches, speakers said at a press briefing March 25 held by the advocacy group MEAction.
“Post-COVID lingering illness was predictable. Similar lingering fatigue syndromes have been reported in the scientific literature for nearly 100 years, following a variety of well-documented infections with viruses, bacteria, fungi, and even protozoa,” said Anthony Komaroff, MD, professor of medicine at Harvard Medical School, Boston.
Core criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for at least 6 months, postexertional malaise (PEM), or a worsening of symptoms following even minor exertion (often described as “crashes”), unrefreshing sleep, and cognitive impairment and/or orthostatic intolerance.
Patients with ME/CFS also commonly experience painful headaches, muscle or joint aches, and allergies/other sensitivities. Although many patients can trace their symptoms to an initiating infection, “the cause is often unclear because the diagnosis is often delayed for months or years after symptom onset,” said Lucinda Bateman, MD, founder of the Bateman Horne Center, Salt Lake City, who leads a clinician coalition that aims to improve ME/CFS management.
In an international survey of 3762 COVID-19 “long-haulers” published in a preprint in December of 2020, the most frequent symptoms reported at least 6 months after illness onset were fatigue in 78%, PEM in 72%, and cognitive dysfunction (“brain fog”) in 55%. At the time of the survey, 45% reported requiring reduced work schedules because of their illness, and 22% reported being unable to work at all.
Dr. Bateman said those findings align with her experience so far with 12 COVID-19 “long haulers” who self-referred to her ME/CFS and fibromyalgia specialty clinic. Nine of the 12 met criteria for postural orthostatic tachycardia syndrome (POTS) based on the 10-minute NASA Lean Test, she said, and half also met the 2016 American College of Rheumatology criteria for fibromyalgia.
“Some were severely impaired. We suspect a small fiber polyneuropathy in about half, and mast cell activation syndrome in more than half. We look forward to doing more testing,” Dr. Bateman said.
To be sure, Dr. Komaroff noted, there are some differences. “Long COVID” patients will often experience breathlessness and ongoing anosmia (loss of taste and smell), which aren’t typical of ME/CFS.
But, he said, “many of the symptoms are quite similar ... My guess is that ME/CFS is an illness with a final common pathway that can be triggered by different things,” said Dr. Komaroff, a senior physician at Brigham and Women’s Hospital in Boston, and editor-in-chief of the Harvard Health Letter.
Based on previous data about CFS suggesting a 10% rate of symptoms persisting at least a year following a variety of infectious agents and the predicted 200 million COVID-19 cases globally by the end of 2021, Dr. Komaroff estimated that about 20 million cases of “long COVID” would be expected in the next year.
‘A huge investment’
On the research side, the National Institutes of Health recently appropriated $1.15 billion dollars over the next 4 years to investigate “the heterogeneity in the recovery process after COVID and to develop treatments for those suffering from [postacute COVID-19 syndrome]” according to a Feb. 5, 2021, blog from the National Institute of Neurological Disorders and Stroke (NINDS).
That same day, another NINDS blog announced “new resources for large-scale ME/CFS research” and emphasized the tie-in with long–COVID-19 syndrome.
“That’s a huge investment. In my opinion, there will be several lingering illnesses following COVID,” Dr. Komaroff said, adding, “It’s my bet that long COVID will prove to be caused by certain kinds of abnormalities in the brain, some of the same abnormalities already identified in ME/CFS. Research will determine whether that’s right or wrong.”
In 2017, NINDS had announced a large increase in funding for ME/CFS research, including the creation of four dedicated research centers. In April 2019, NINDS held a 2-day conference highlighting that ongoing work, as reported by Medscape Medical News.
During the briefing, NINDS clinical director Avindra Nath, MD, described a comprehensive ongoing ME/CFS intramural study he’s been leading since 2016.
He’s now also overseeing two long–COVID-19 studies, one of which has a protocol similar to that of the ME/CFS study and will include individuals who are still experiencing long-term symptoms following confirmed cases of COVID-19. The aim is to screen about 1,300 patients. Several task forces are now examining all of these data together.
“Each aspect is now being analyzed … What we learn from one applies to the other,” Dr. Nath said.
Advice for clinicians
In interviews, Dr. Bateman and Dr. Nath offered clinical advice for managing patients who meet ME/CFS criteria, whether they had confirmed or suspected COVID-19, a different infection, or unknown trigger(s).
Dr. Bateman advised that clinicians assess patients for each of the symptoms individually. “Besides exercise intolerance and PEM, the most commonly missed is orthostatic intolerance. It really doesn’t matter what the cause is, it’s amenable to supportive treatment. It’s one aspect of the illness that contributes to severely impaired function. My plea to all physicians would be for sure to assess for [orthostatic intolerance], and gain an understanding about activity management and avoiding PEM symptoms.”
Dr. Nath noted that an often-challenging situation is when tests for the infectious agent and other blood work come back negative, yet the patient still reports multiple debilitating symptoms. This has been a particular issue with long COVID-19, since many patients became ill early in the pandemic before the polymerase chain reaction (PCR) tests for SARS-CoV-2 were widely available.
“The physician can only order tests that are available at their labs. I think what the physician should do is handle symptoms symptomatically but also refer patients to specialists who are taking care of these patients or to research studies,” he said.
Dr. Bateman added, “Whether they had a documented COVID infection – we just have to let go of that in 2020. Way too many people didn’t have access to a test or the timing wasn’t amenable. If people meet criteria for ME/CFS, it’s irrelevant … It’s mainly a clinical diagnosis. It’s not reliant on identifying the infectious trigger.”
Dr. Komaroff, who began caring for then-termed “chronic fatigue syndrome” patients and researching the condition more than 30 years ago, said that “every cloud has its silver lining. The increased focus on postinfectious fatigue syndrome is a silver lining in my mind around the terrible dark cloud that is the pandemic of COVID.”
Dr. Komaroff has received personal fees from Serimmune Inc., Ono Pharma, and Deallus, and grants from the NIH. Dr. Bateman is employed by the Bateman Horne Center, which receives grants from the NIH, and fees from Exagen Inc., and Teva Pharmaceutical. Dr. Nath is an NIH employee.
A version of this article first appeared on Medscape.com.
People experiencing long-term symptoms following acute COVID-19 infection are increasingly meeting criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a phenomenon that highlights the need for unified research and clinical approaches, speakers said at a press briefing March 25 held by the advocacy group MEAction.
“Post-COVID lingering illness was predictable. Similar lingering fatigue syndromes have been reported in the scientific literature for nearly 100 years, following a variety of well-documented infections with viruses, bacteria, fungi, and even protozoa,” said Anthony Komaroff, MD, professor of medicine at Harvard Medical School, Boston.
Core criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for at least 6 months, postexertional malaise (PEM), or a worsening of symptoms following even minor exertion (often described as “crashes”), unrefreshing sleep, and cognitive impairment and/or orthostatic intolerance.
Patients with ME/CFS also commonly experience painful headaches, muscle or joint aches, and allergies/other sensitivities. Although many patients can trace their symptoms to an initiating infection, “the cause is often unclear because the diagnosis is often delayed for months or years after symptom onset,” said Lucinda Bateman, MD, founder of the Bateman Horne Center, Salt Lake City, who leads a clinician coalition that aims to improve ME/CFS management.
In an international survey of 3762 COVID-19 “long-haulers” published in a preprint in December of 2020, the most frequent symptoms reported at least 6 months after illness onset were fatigue in 78%, PEM in 72%, and cognitive dysfunction (“brain fog”) in 55%. At the time of the survey, 45% reported requiring reduced work schedules because of their illness, and 22% reported being unable to work at all.
Dr. Bateman said those findings align with her experience so far with 12 COVID-19 “long haulers” who self-referred to her ME/CFS and fibromyalgia specialty clinic. Nine of the 12 met criteria for postural orthostatic tachycardia syndrome (POTS) based on the 10-minute NASA Lean Test, she said, and half also met the 2016 American College of Rheumatology criteria for fibromyalgia.
“Some were severely impaired. We suspect a small fiber polyneuropathy in about half, and mast cell activation syndrome in more than half. We look forward to doing more testing,” Dr. Bateman said.
To be sure, Dr. Komaroff noted, there are some differences. “Long COVID” patients will often experience breathlessness and ongoing anosmia (loss of taste and smell), which aren’t typical of ME/CFS.
But, he said, “many of the symptoms are quite similar ... My guess is that ME/CFS is an illness with a final common pathway that can be triggered by different things,” said Dr. Komaroff, a senior physician at Brigham and Women’s Hospital in Boston, and editor-in-chief of the Harvard Health Letter.
Based on previous data about CFS suggesting a 10% rate of symptoms persisting at least a year following a variety of infectious agents and the predicted 200 million COVID-19 cases globally by the end of 2021, Dr. Komaroff estimated that about 20 million cases of “long COVID” would be expected in the next year.
‘A huge investment’
On the research side, the National Institutes of Health recently appropriated $1.15 billion dollars over the next 4 years to investigate “the heterogeneity in the recovery process after COVID and to develop treatments for those suffering from [postacute COVID-19 syndrome]” according to a Feb. 5, 2021, blog from the National Institute of Neurological Disorders and Stroke (NINDS).
That same day, another NINDS blog announced “new resources for large-scale ME/CFS research” and emphasized the tie-in with long–COVID-19 syndrome.
“That’s a huge investment. In my opinion, there will be several lingering illnesses following COVID,” Dr. Komaroff said, adding, “It’s my bet that long COVID will prove to be caused by certain kinds of abnormalities in the brain, some of the same abnormalities already identified in ME/CFS. Research will determine whether that’s right or wrong.”
In 2017, NINDS had announced a large increase in funding for ME/CFS research, including the creation of four dedicated research centers. In April 2019, NINDS held a 2-day conference highlighting that ongoing work, as reported by Medscape Medical News.
During the briefing, NINDS clinical director Avindra Nath, MD, described a comprehensive ongoing ME/CFS intramural study he’s been leading since 2016.
He’s now also overseeing two long–COVID-19 studies, one of which has a protocol similar to that of the ME/CFS study and will include individuals who are still experiencing long-term symptoms following confirmed cases of COVID-19. The aim is to screen about 1,300 patients. Several task forces are now examining all of these data together.
“Each aspect is now being analyzed … What we learn from one applies to the other,” Dr. Nath said.
Advice for clinicians
In interviews, Dr. Bateman and Dr. Nath offered clinical advice for managing patients who meet ME/CFS criteria, whether they had confirmed or suspected COVID-19, a different infection, or unknown trigger(s).
Dr. Bateman advised that clinicians assess patients for each of the symptoms individually. “Besides exercise intolerance and PEM, the most commonly missed is orthostatic intolerance. It really doesn’t matter what the cause is, it’s amenable to supportive treatment. It’s one aspect of the illness that contributes to severely impaired function. My plea to all physicians would be for sure to assess for [orthostatic intolerance], and gain an understanding about activity management and avoiding PEM symptoms.”
Dr. Nath noted that an often-challenging situation is when tests for the infectious agent and other blood work come back negative, yet the patient still reports multiple debilitating symptoms. This has been a particular issue with long COVID-19, since many patients became ill early in the pandemic before the polymerase chain reaction (PCR) tests for SARS-CoV-2 were widely available.
“The physician can only order tests that are available at their labs. I think what the physician should do is handle symptoms symptomatically but also refer patients to specialists who are taking care of these patients or to research studies,” he said.
Dr. Bateman added, “Whether they had a documented COVID infection – we just have to let go of that in 2020. Way too many people didn’t have access to a test or the timing wasn’t amenable. If people meet criteria for ME/CFS, it’s irrelevant … It’s mainly a clinical diagnosis. It’s not reliant on identifying the infectious trigger.”
Dr. Komaroff, who began caring for then-termed “chronic fatigue syndrome” patients and researching the condition more than 30 years ago, said that “every cloud has its silver lining. The increased focus on postinfectious fatigue syndrome is a silver lining in my mind around the terrible dark cloud that is the pandemic of COVID.”
Dr. Komaroff has received personal fees from Serimmune Inc., Ono Pharma, and Deallus, and grants from the NIH. Dr. Bateman is employed by the Bateman Horne Center, which receives grants from the NIH, and fees from Exagen Inc., and Teva Pharmaceutical. Dr. Nath is an NIH employee.
A version of this article first appeared on Medscape.com.
Step therapy: Inside the fight against insurance companies and fail-first medicine
Every day Melissa Fulton, RN, MSN, FNP, APRN-C, shows up to work, she’s ready for another fight. An advanced practice nurse who specializes in multiple sclerosis care, Ms. Fulton said she typically spends more than a third of her time battling it out with insurance companies over drugs she knows her patients need but that insurers don’t want to cover. Instead, they want the patient to first receive less expensive and often less efficacious drugs, even if that goes against recommendations and, in some cases, against the patient’s medical history.
The maddening protocol – familiar to health care providers everywhere – is known as “step therapy.” It forces patients to try alternative medications – medications that often fail – before receiving the one initially prescribed. The process can take weeks or months, which is time that some patients don’t have. Step therapy was sold as a way to lower costs. However, beyond the ethically problematic notion of forcing sick patients to receiver cheaper alternatives that are ineffective, research has also shown it may actually be more costly in the long run.
Ms. Fulton, who works at Saunders Medical Center in Wahoo, Neb., is a veteran in the war against step therapy. She is used to pushing her appeals up the insurance company chain of command, past nonmedical reviewers, until her patient’s case finally lands on the desk of someone with a neurology background. She said that can take three or four appeals – a judge might even get involved – and the patient could still lose. “This happens constantly,” she said, “but we fight like hell.”
Fortunately, life may soon get a little easier for Ms. Fulton. In late March, a bill to restrict step therapy made it through the Nebraska state legislature and is on its way to the governor’s desk. The Step Therapy Reform Act doesn’t outright ban the practice; however, it will put guardrails in place. It requires that insurers respond to appeals within certain time frames, and it creates key exemptions.
When the governor signs off, Nebraska will join more than two dozen other states that already have step therapy restrictions on the books, according to Hannah Lynch, MPS, associate director of federal government relations and health policy at the National Psoriasis Foundation, a leading advocate to reform and protect against the insurance practice. “There’s a lot of frustration out there,” Ms. Lynch said. “It really hinders providers’ ability to make decisions they think will have the best outcomes.”
Driven by coalitions of doctors, nurses, and patients, laws reining in step therapy have been adopted at a relatively quick clip, mostly within the past 5 years. Recent additions include South Dakota and North Carolina, which adopted step therapy laws in 2020, and Arkansas, which passed a law earlier this year.
Ms. Lynch attributed growing support to rising out-of-pocket drug costs and the introduction of biologic drugs, which are often more effective but also more expensive. Like Nebraska’s law, most step therapy reform legislation carves out exemptions and requires timely appeals processes; however, many of the laws still have significant gaps, such as not including certain types of insurance plans.
Ideally, Ms. Lynch said, the protections would apply to all types of health plans that are regulated at the state level, such as Medicaid, state employee health plans, and coverage sold through state insurance exchanges. Closing loopholes in the laws is a top priority for advocates, she added, pointing to work currently underway in Arkansas to extend its new protections to Medicaid expansion patients.
“With so many outside stakeholders, you have to compromise – it’s a give and take,” Ms. Lynch said. Still, when it comes to fighting step therapy, she says, “Any protection on the books is always our first goal when we go into a state.”
Putting patients first
Lisa Arkin, MD, a pediatric dermatologist at the University of Wisconsin–Madison, said she finds herself “swimming upstream every day in the fight with insurance.” Her patients are typically on their second or third stop and have more complex disorders. Dr. Arkin said that the problem with step therapy is that it tries to squeeze all patients into the same box, even if the circumstances don’t fit.
Her state passed restrictions on step therapy in 2019, but the measures only went into effect last year. Under the Wisconsin law, patients can be granted an exemption if an alternative treatment is contraindicated, likely to cause harm, or expected to be ineffective. Patients can also be exempt if their current treatment is working.
Dr. Arkin, an outspoken advocate for curbing step therapy, says the Wisconsin law is “very strong.” However, because it only applies to certain health plans – state employee health plans and those purchased in the state’s health insurance exchange – fewer than half the state’s patients benefit from its protections. She notes that some of the most severe presentations she treats occur in patients who rely on Medicaid coverage and already face barriers to care.
“I’m a doctor who puts up a fuss [with insurers], but that’s not fair – we shouldn’t have to do that,” Dr. Arkin said. “To me, it’s really critical to make this an even playing field so this law affords protection to everyone I see in the clinic.”
Major medical associations caution against step therapy as well. The American Society of Clinical Oncology and the American Medical Association have called out the risks to patient safety and health. In fact, in 2019, after the Centers for Medicare & Medicaid Services gave new authority to Medicare Advantage plans to start using step therapy, dozens of national medical groups called out the agency for allowing a practice that could potentially hurt patients and undercut the physician-patient decision-making process.
Last year, in a new position paper from the American College of Physicians, authors laid out recommendations for combating step therapy’s side effects. These recommendations included making related data transparent to the public and minimizing the policy’s disruptions to care. Jacqueline W. Fincher, MD, MACP, a member of the committee that issued the position paper and who is a primary care physician in Georgia, said such insurance practices need to be designed with “strong input from frontline physicians, not clipboard physicians.
“What we want from insurers is understanding, transparency, and the least burdensome protocol to provide patients the care they need at a cost-effective price they can afford,” said Dr. Fincher, who is also the current president of the ACP. “The focus needs to be on what’s in the patient’s best interest.”
Every year a new fight
“We all dread January,” said Dr. Fincher. That is the worst month, she added, because new health benefits go into effect, which means patients who are responding well to certain treatments may suddenly face new restrictions.
Another aggravating aspect of step therapy? It is often difficult – if not impossible – to access information on specific step therapy protocols in a patient’s health plan in real time in the exam room, where treatment conversations actually take place. In a more patient-centered world, Dr. Fincher said, she would be able to use the electronic health record system to quickly identify whether a patient’s plan covers a particular treatment and, if not, what the alternatives are.
Georgia’s new step therapy law went into effect last year. Like laws in other states, it spells out step therapy exemptions and sets time frames in which insurers must respond to exceptions and appeals. Dr. Fincher, who spoke in favor of the new law, said she’s “happy for any step forward.” Still, the growing burden of prior authorization rules are an utter “time sink” for her and her staff.
“I have to justify my decisions to nondoctors before I even get to a doctor, and that’s really frustrating,” she said. “We’re talking about people here, not widgets.”
Advocates in Nevada are hoping this is the year a step therapy bill will make it into law in their state. As of March, one had yet to be introduced in the state legislature. Tom McCoy, director of state government affairs at the Nevada Chronic Care Collaborative, said existing Nevada law already prohibits nonmedical drug switching during a policy year; however, insurers can still make changes the following year.
A bill to rein in step therapy was proposed previously, Mr. McCoy said, but it never got off the ground. The collaborative, as well as about two dozen organizations representing Nevada providers and patients, are now calling on state lawmakers to make the issue a priority in the current session.
“The health plans have a lot of power – a lot,” Mr. McCoy said. “We’re hoping to get a [legislative] sponsor in 2021 ... but it’s also been a really hard year to connect legislators with patients and doctors, and being able to hear their stories really does make a difference.”
In Nebraska, Marcus Snow, MD, a rheumatologist at Nebraska Medicine, in Omaha, said that the state’s new step therapy law will be a “great first step in helping to provide some guardrails” around the practice. He noted that turnaround requirements for insurer responses are “sorely needed.” However, he said that, because the bill doesn’t apply to all health plans, many Nebraskans still won’t benefit.
Dealing with step therapy is a daily “headache” for Dr. Snow, who says navigating the bureaucracy of prior authorization seems to be getting worse every year. Like his peers around the country, he spends an inordinate amount of time pushing appeals up the insurance company ranks to get access to treatments he believes will be most effective. But Snow says that, more than just being a mountain of tiresome red tape, these practices also intrude on the patient-provider relationship, casting an unsettling sense of uncertainty that the ultimate decision about the best course of action isn’t up to the doctor and patient at all.
“In the end, the insurance company is the judge and jury of my prescription,” Dr. Snow said. “They’d argue I can still prescribe it, but if it costs $70,000 a year – I don’t know who can afford that.”
Ms. Lynch, at the National Psoriasis Foundation, said their step therapy advocacy will continue to take a two-pronged approach. They will push for new and expanded protections at both state and federal levels. Protections are needed at both levels to make sure that all health plans regulated by all entities are covered. In the U.S. Senate and the House, step therapy bills were reintroduced this year. They would apply to health plans subject to the federal Employee Retirement Income Security Act, which governs employer-sponsored health coverage, and could close a big gap in existing protections. Oregon, New Jersey, and Arizona are at the top of the foundation’s advocacy list this year, according to Ms. Lynch.
“Folks are really starting to pay more attention to this issue,” she said. “And hearing those real-world stories and frustrations is definitely one of the most effective tools we have.”
A version of this article first appeared on Medscape.com.
Every day Melissa Fulton, RN, MSN, FNP, APRN-C, shows up to work, she’s ready for another fight. An advanced practice nurse who specializes in multiple sclerosis care, Ms. Fulton said she typically spends more than a third of her time battling it out with insurance companies over drugs she knows her patients need but that insurers don’t want to cover. Instead, they want the patient to first receive less expensive and often less efficacious drugs, even if that goes against recommendations and, in some cases, against the patient’s medical history.
The maddening protocol – familiar to health care providers everywhere – is known as “step therapy.” It forces patients to try alternative medications – medications that often fail – before receiving the one initially prescribed. The process can take weeks or months, which is time that some patients don’t have. Step therapy was sold as a way to lower costs. However, beyond the ethically problematic notion of forcing sick patients to receiver cheaper alternatives that are ineffective, research has also shown it may actually be more costly in the long run.
Ms. Fulton, who works at Saunders Medical Center in Wahoo, Neb., is a veteran in the war against step therapy. She is used to pushing her appeals up the insurance company chain of command, past nonmedical reviewers, until her patient’s case finally lands on the desk of someone with a neurology background. She said that can take three or four appeals – a judge might even get involved – and the patient could still lose. “This happens constantly,” she said, “but we fight like hell.”
Fortunately, life may soon get a little easier for Ms. Fulton. In late March, a bill to restrict step therapy made it through the Nebraska state legislature and is on its way to the governor’s desk. The Step Therapy Reform Act doesn’t outright ban the practice; however, it will put guardrails in place. It requires that insurers respond to appeals within certain time frames, and it creates key exemptions.
When the governor signs off, Nebraska will join more than two dozen other states that already have step therapy restrictions on the books, according to Hannah Lynch, MPS, associate director of federal government relations and health policy at the National Psoriasis Foundation, a leading advocate to reform and protect against the insurance practice. “There’s a lot of frustration out there,” Ms. Lynch said. “It really hinders providers’ ability to make decisions they think will have the best outcomes.”
Driven by coalitions of doctors, nurses, and patients, laws reining in step therapy have been adopted at a relatively quick clip, mostly within the past 5 years. Recent additions include South Dakota and North Carolina, which adopted step therapy laws in 2020, and Arkansas, which passed a law earlier this year.
Ms. Lynch attributed growing support to rising out-of-pocket drug costs and the introduction of biologic drugs, which are often more effective but also more expensive. Like Nebraska’s law, most step therapy reform legislation carves out exemptions and requires timely appeals processes; however, many of the laws still have significant gaps, such as not including certain types of insurance plans.
Ideally, Ms. Lynch said, the protections would apply to all types of health plans that are regulated at the state level, such as Medicaid, state employee health plans, and coverage sold through state insurance exchanges. Closing loopholes in the laws is a top priority for advocates, she added, pointing to work currently underway in Arkansas to extend its new protections to Medicaid expansion patients.
“With so many outside stakeholders, you have to compromise – it’s a give and take,” Ms. Lynch said. Still, when it comes to fighting step therapy, she says, “Any protection on the books is always our first goal when we go into a state.”
Putting patients first
Lisa Arkin, MD, a pediatric dermatologist at the University of Wisconsin–Madison, said she finds herself “swimming upstream every day in the fight with insurance.” Her patients are typically on their second or third stop and have more complex disorders. Dr. Arkin said that the problem with step therapy is that it tries to squeeze all patients into the same box, even if the circumstances don’t fit.
Her state passed restrictions on step therapy in 2019, but the measures only went into effect last year. Under the Wisconsin law, patients can be granted an exemption if an alternative treatment is contraindicated, likely to cause harm, or expected to be ineffective. Patients can also be exempt if their current treatment is working.
Dr. Arkin, an outspoken advocate for curbing step therapy, says the Wisconsin law is “very strong.” However, because it only applies to certain health plans – state employee health plans and those purchased in the state’s health insurance exchange – fewer than half the state’s patients benefit from its protections. She notes that some of the most severe presentations she treats occur in patients who rely on Medicaid coverage and already face barriers to care.
“I’m a doctor who puts up a fuss [with insurers], but that’s not fair – we shouldn’t have to do that,” Dr. Arkin said. “To me, it’s really critical to make this an even playing field so this law affords protection to everyone I see in the clinic.”
Major medical associations caution against step therapy as well. The American Society of Clinical Oncology and the American Medical Association have called out the risks to patient safety and health. In fact, in 2019, after the Centers for Medicare & Medicaid Services gave new authority to Medicare Advantage plans to start using step therapy, dozens of national medical groups called out the agency for allowing a practice that could potentially hurt patients and undercut the physician-patient decision-making process.
Last year, in a new position paper from the American College of Physicians, authors laid out recommendations for combating step therapy’s side effects. These recommendations included making related data transparent to the public and minimizing the policy’s disruptions to care. Jacqueline W. Fincher, MD, MACP, a member of the committee that issued the position paper and who is a primary care physician in Georgia, said such insurance practices need to be designed with “strong input from frontline physicians, not clipboard physicians.
“What we want from insurers is understanding, transparency, and the least burdensome protocol to provide patients the care they need at a cost-effective price they can afford,” said Dr. Fincher, who is also the current president of the ACP. “The focus needs to be on what’s in the patient’s best interest.”
Every year a new fight
“We all dread January,” said Dr. Fincher. That is the worst month, she added, because new health benefits go into effect, which means patients who are responding well to certain treatments may suddenly face new restrictions.
Another aggravating aspect of step therapy? It is often difficult – if not impossible – to access information on specific step therapy protocols in a patient’s health plan in real time in the exam room, where treatment conversations actually take place. In a more patient-centered world, Dr. Fincher said, she would be able to use the electronic health record system to quickly identify whether a patient’s plan covers a particular treatment and, if not, what the alternatives are.
Georgia’s new step therapy law went into effect last year. Like laws in other states, it spells out step therapy exemptions and sets time frames in which insurers must respond to exceptions and appeals. Dr. Fincher, who spoke in favor of the new law, said she’s “happy for any step forward.” Still, the growing burden of prior authorization rules are an utter “time sink” for her and her staff.
“I have to justify my decisions to nondoctors before I even get to a doctor, and that’s really frustrating,” she said. “We’re talking about people here, not widgets.”
Advocates in Nevada are hoping this is the year a step therapy bill will make it into law in their state. As of March, one had yet to be introduced in the state legislature. Tom McCoy, director of state government affairs at the Nevada Chronic Care Collaborative, said existing Nevada law already prohibits nonmedical drug switching during a policy year; however, insurers can still make changes the following year.
A bill to rein in step therapy was proposed previously, Mr. McCoy said, but it never got off the ground. The collaborative, as well as about two dozen organizations representing Nevada providers and patients, are now calling on state lawmakers to make the issue a priority in the current session.
“The health plans have a lot of power – a lot,” Mr. McCoy said. “We’re hoping to get a [legislative] sponsor in 2021 ... but it’s also been a really hard year to connect legislators with patients and doctors, and being able to hear their stories really does make a difference.”
In Nebraska, Marcus Snow, MD, a rheumatologist at Nebraska Medicine, in Omaha, said that the state’s new step therapy law will be a “great first step in helping to provide some guardrails” around the practice. He noted that turnaround requirements for insurer responses are “sorely needed.” However, he said that, because the bill doesn’t apply to all health plans, many Nebraskans still won’t benefit.
Dealing with step therapy is a daily “headache” for Dr. Snow, who says navigating the bureaucracy of prior authorization seems to be getting worse every year. Like his peers around the country, he spends an inordinate amount of time pushing appeals up the insurance company ranks to get access to treatments he believes will be most effective. But Snow says that, more than just being a mountain of tiresome red tape, these practices also intrude on the patient-provider relationship, casting an unsettling sense of uncertainty that the ultimate decision about the best course of action isn’t up to the doctor and patient at all.
“In the end, the insurance company is the judge and jury of my prescription,” Dr. Snow said. “They’d argue I can still prescribe it, but if it costs $70,000 a year – I don’t know who can afford that.”
Ms. Lynch, at the National Psoriasis Foundation, said their step therapy advocacy will continue to take a two-pronged approach. They will push for new and expanded protections at both state and federal levels. Protections are needed at both levels to make sure that all health plans regulated by all entities are covered. In the U.S. Senate and the House, step therapy bills were reintroduced this year. They would apply to health plans subject to the federal Employee Retirement Income Security Act, which governs employer-sponsored health coverage, and could close a big gap in existing protections. Oregon, New Jersey, and Arizona are at the top of the foundation’s advocacy list this year, according to Ms. Lynch.
“Folks are really starting to pay more attention to this issue,” she said. “And hearing those real-world stories and frustrations is definitely one of the most effective tools we have.”
A version of this article first appeared on Medscape.com.
Every day Melissa Fulton, RN, MSN, FNP, APRN-C, shows up to work, she’s ready for another fight. An advanced practice nurse who specializes in multiple sclerosis care, Ms. Fulton said she typically spends more than a third of her time battling it out with insurance companies over drugs she knows her patients need but that insurers don’t want to cover. Instead, they want the patient to first receive less expensive and often less efficacious drugs, even if that goes against recommendations and, in some cases, against the patient’s medical history.
The maddening protocol – familiar to health care providers everywhere – is known as “step therapy.” It forces patients to try alternative medications – medications that often fail – before receiving the one initially prescribed. The process can take weeks or months, which is time that some patients don’t have. Step therapy was sold as a way to lower costs. However, beyond the ethically problematic notion of forcing sick patients to receiver cheaper alternatives that are ineffective, research has also shown it may actually be more costly in the long run.
Ms. Fulton, who works at Saunders Medical Center in Wahoo, Neb., is a veteran in the war against step therapy. She is used to pushing her appeals up the insurance company chain of command, past nonmedical reviewers, until her patient’s case finally lands on the desk of someone with a neurology background. She said that can take three or four appeals – a judge might even get involved – and the patient could still lose. “This happens constantly,” she said, “but we fight like hell.”
Fortunately, life may soon get a little easier for Ms. Fulton. In late March, a bill to restrict step therapy made it through the Nebraska state legislature and is on its way to the governor’s desk. The Step Therapy Reform Act doesn’t outright ban the practice; however, it will put guardrails in place. It requires that insurers respond to appeals within certain time frames, and it creates key exemptions.
When the governor signs off, Nebraska will join more than two dozen other states that already have step therapy restrictions on the books, according to Hannah Lynch, MPS, associate director of federal government relations and health policy at the National Psoriasis Foundation, a leading advocate to reform and protect against the insurance practice. “There’s a lot of frustration out there,” Ms. Lynch said. “It really hinders providers’ ability to make decisions they think will have the best outcomes.”
Driven by coalitions of doctors, nurses, and patients, laws reining in step therapy have been adopted at a relatively quick clip, mostly within the past 5 years. Recent additions include South Dakota and North Carolina, which adopted step therapy laws in 2020, and Arkansas, which passed a law earlier this year.
Ms. Lynch attributed growing support to rising out-of-pocket drug costs and the introduction of biologic drugs, which are often more effective but also more expensive. Like Nebraska’s law, most step therapy reform legislation carves out exemptions and requires timely appeals processes; however, many of the laws still have significant gaps, such as not including certain types of insurance plans.
Ideally, Ms. Lynch said, the protections would apply to all types of health plans that are regulated at the state level, such as Medicaid, state employee health plans, and coverage sold through state insurance exchanges. Closing loopholes in the laws is a top priority for advocates, she added, pointing to work currently underway in Arkansas to extend its new protections to Medicaid expansion patients.
“With so many outside stakeholders, you have to compromise – it’s a give and take,” Ms. Lynch said. Still, when it comes to fighting step therapy, she says, “Any protection on the books is always our first goal when we go into a state.”
Putting patients first
Lisa Arkin, MD, a pediatric dermatologist at the University of Wisconsin–Madison, said she finds herself “swimming upstream every day in the fight with insurance.” Her patients are typically on their second or third stop and have more complex disorders. Dr. Arkin said that the problem with step therapy is that it tries to squeeze all patients into the same box, even if the circumstances don’t fit.
Her state passed restrictions on step therapy in 2019, but the measures only went into effect last year. Under the Wisconsin law, patients can be granted an exemption if an alternative treatment is contraindicated, likely to cause harm, or expected to be ineffective. Patients can also be exempt if their current treatment is working.
Dr. Arkin, an outspoken advocate for curbing step therapy, says the Wisconsin law is “very strong.” However, because it only applies to certain health plans – state employee health plans and those purchased in the state’s health insurance exchange – fewer than half the state’s patients benefit from its protections. She notes that some of the most severe presentations she treats occur in patients who rely on Medicaid coverage and already face barriers to care.
“I’m a doctor who puts up a fuss [with insurers], but that’s not fair – we shouldn’t have to do that,” Dr. Arkin said. “To me, it’s really critical to make this an even playing field so this law affords protection to everyone I see in the clinic.”
Major medical associations caution against step therapy as well. The American Society of Clinical Oncology and the American Medical Association have called out the risks to patient safety and health. In fact, in 2019, after the Centers for Medicare & Medicaid Services gave new authority to Medicare Advantage plans to start using step therapy, dozens of national medical groups called out the agency for allowing a practice that could potentially hurt patients and undercut the physician-patient decision-making process.
Last year, in a new position paper from the American College of Physicians, authors laid out recommendations for combating step therapy’s side effects. These recommendations included making related data transparent to the public and minimizing the policy’s disruptions to care. Jacqueline W. Fincher, MD, MACP, a member of the committee that issued the position paper and who is a primary care physician in Georgia, said such insurance practices need to be designed with “strong input from frontline physicians, not clipboard physicians.
“What we want from insurers is understanding, transparency, and the least burdensome protocol to provide patients the care they need at a cost-effective price they can afford,” said Dr. Fincher, who is also the current president of the ACP. “The focus needs to be on what’s in the patient’s best interest.”
Every year a new fight
“We all dread January,” said Dr. Fincher. That is the worst month, she added, because new health benefits go into effect, which means patients who are responding well to certain treatments may suddenly face new restrictions.
Another aggravating aspect of step therapy? It is often difficult – if not impossible – to access information on specific step therapy protocols in a patient’s health plan in real time in the exam room, where treatment conversations actually take place. In a more patient-centered world, Dr. Fincher said, she would be able to use the electronic health record system to quickly identify whether a patient’s plan covers a particular treatment and, if not, what the alternatives are.
Georgia’s new step therapy law went into effect last year. Like laws in other states, it spells out step therapy exemptions and sets time frames in which insurers must respond to exceptions and appeals. Dr. Fincher, who spoke in favor of the new law, said she’s “happy for any step forward.” Still, the growing burden of prior authorization rules are an utter “time sink” for her and her staff.
“I have to justify my decisions to nondoctors before I even get to a doctor, and that’s really frustrating,” she said. “We’re talking about people here, not widgets.”
Advocates in Nevada are hoping this is the year a step therapy bill will make it into law in their state. As of March, one had yet to be introduced in the state legislature. Tom McCoy, director of state government affairs at the Nevada Chronic Care Collaborative, said existing Nevada law already prohibits nonmedical drug switching during a policy year; however, insurers can still make changes the following year.
A bill to rein in step therapy was proposed previously, Mr. McCoy said, but it never got off the ground. The collaborative, as well as about two dozen organizations representing Nevada providers and patients, are now calling on state lawmakers to make the issue a priority in the current session.
“The health plans have a lot of power – a lot,” Mr. McCoy said. “We’re hoping to get a [legislative] sponsor in 2021 ... but it’s also been a really hard year to connect legislators with patients and doctors, and being able to hear their stories really does make a difference.”
In Nebraska, Marcus Snow, MD, a rheumatologist at Nebraska Medicine, in Omaha, said that the state’s new step therapy law will be a “great first step in helping to provide some guardrails” around the practice. He noted that turnaround requirements for insurer responses are “sorely needed.” However, he said that, because the bill doesn’t apply to all health plans, many Nebraskans still won’t benefit.
Dealing with step therapy is a daily “headache” for Dr. Snow, who says navigating the bureaucracy of prior authorization seems to be getting worse every year. Like his peers around the country, he spends an inordinate amount of time pushing appeals up the insurance company ranks to get access to treatments he believes will be most effective. But Snow says that, more than just being a mountain of tiresome red tape, these practices also intrude on the patient-provider relationship, casting an unsettling sense of uncertainty that the ultimate decision about the best course of action isn’t up to the doctor and patient at all.
“In the end, the insurance company is the judge and jury of my prescription,” Dr. Snow said. “They’d argue I can still prescribe it, but if it costs $70,000 a year – I don’t know who can afford that.”
Ms. Lynch, at the National Psoriasis Foundation, said their step therapy advocacy will continue to take a two-pronged approach. They will push for new and expanded protections at both state and federal levels. Protections are needed at both levels to make sure that all health plans regulated by all entities are covered. In the U.S. Senate and the House, step therapy bills were reintroduced this year. They would apply to health plans subject to the federal Employee Retirement Income Security Act, which governs employer-sponsored health coverage, and could close a big gap in existing protections. Oregon, New Jersey, and Arizona are at the top of the foundation’s advocacy list this year, according to Ms. Lynch.
“Folks are really starting to pay more attention to this issue,” she said. “And hearing those real-world stories and frustrations is definitely one of the most effective tools we have.”
A version of this article first appeared on Medscape.com.
Encephalopathy common, often lethal in hospitalized patients with COVID-19
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.
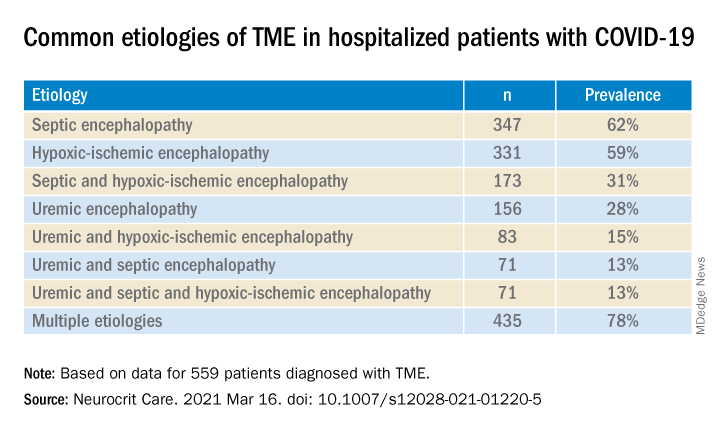
Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROCRITICAL CARE
New NAS report seeks to modernize STI paradigm
Approximately 68 million cases of sexually transmitted infections are reported in the United States each year, yet antiquated approaches to STI prevention, in addition to health care inequities and lack of funding, have substantially prevented providers and officials from curbing the spread. In response to rising case numbers, the National Academies of Sciences, Engineering, and Medicine released a report this week with recommendations to modernize the nation’s STI surveillance and monitoring systems, increase the capabilities of the STI workforce, and address structural barriers to STI prevention and access to care.
Given the rising rates of STIs and the urgent, unmet need for prevention and treatment, the Centers for Disease Control and Prevention’s National Association of County and City Health Officials commissioned the National Academies to develop actionable recommendations to control STIs. The new report marks a long road toward the public’s willingness to discuss STDs, or what a 1997 Institute of Medicine report described as a “hidden epidemic” that had been largely neglected in public discourse.
Jeffrey Crowley, MPH, committee member and an author of the new National Academies report, said in an interview that, despite the increased openness to discuss STIs in today’s society, STD rates since the late 1990s have gotten much worse. Lack of appropriate governmental funding for research and drug development, structural inequities, and persisting stigmatization are key drivers for rising rates, explained Mr. Crowley.
Addressing structural barriers to STI prevention
Playing a prominent role in the National Academies report are issues of structural and institutional barriers to STI prevention and care. In the report, the authors argued that a policy-based approach should seek to promote sexual health and eliminate structural racism and inequities to drive improvements in STI management.
“We think it’s these structural factors that are central to all the inequities that play out,” said Mr. Crowley, “and they either don’t get any attention or, if they do get attention, people don’t really speak concretely enough about how we address them.”
The concrete steps, as outlined in the report, begin with addressing factors that involve the health care industry at large. Automatic STI screening as part of routine visits, alerts in electronic health records that remind clinicians to screen patients, and reminders to test patients can be initial low-cost actions health care systems can take to improve STI testing, particularly in marginalized communities. Mr. Crowley noted that greater evidence is needed to support further steps to address structural factors that contribute to barriers in STI screening and treatment access.
Given the complexities inherent in structural barriers to STI care, the report calls on a whole-government response, in partnership with affected communities, to normalize discussions involving sexual well-being. “We have to ask ourselves how we can build healthier communities and how can we integrate sexual health into that dialogue in a way that improves our response to STI prevention and control,” Mr. Crowley explained.
Harnessing AI and dating apps
The report also addresses the power of artificial intelligence to predict STI rates and to discover trends in risk factors, both of which may improve STI surveillance and assist in the development of tailored interventions. The report calls for policy that will enable companies and the government to capitalize on AI to evaluate large collections of data in EHRs, insurance claims databases, social media, search engines, and even dating apps.
In particular, dating apps could be an avenue through which the public and private sectors could improve STI prevention, diagnosis, and treatment. “People want to focus on this idea of whether these apps increase transmission risk,” said Mr. Crowley. “But we would say that this is asking the wrong question, because these technologies are not going away.” He noted that private and public enterprises could work together to leverage these technologies to increase awareness of prevention and testing.
Unifying the STI/HIV and COVID-19 workforce
The report also recommends that the nation unify the STI/HIV workforce with the COVID-19 workforce. Given the high levels of expertise in these professional working groups, the report suggests unification could potentially address both the current crisis and possible future disease outbreaks. Combining COVID-19 response teams with underresourced STI/HIV programs may also improve the delivery of STI testing, considering that STI testing programs have had to compete for resources during the pandemic.
Addressing stigma
The National Academies report also addresses the ongoing issue of stigma, which results from “blaming” individuals and the choices they make so as to create shame, embarrassment, and discrimination. Because of stigma, sexually active people may be unwilling to seek recommended screening, which can lead to delays in diagnosis and treatment and can increase the risk for negative health outcomes.
“As a nation, we’ve almost focused too intently on individual-level factors in a way that’s driven stigma and really hasn’t been helpful for combating the problem,” said Mr. Crowley. He added that, instead of focusing solely on individual-level choices, the nation should instead work to reframe sexual health as a key aspect of overall physical, mental, and emotional well-being. Doing so could create more opportunities to address structural barriers to STI prevention and ensure that more prevention and screening services are available in stigma-free environments.
“I know what we’re recommending is ambitious, but it’s not too big to be achieved, and we’re not saying tomorrow we’re going to transform the world,” Mr. Crowley concluded. “It’s a puzzle with many pieces, but the long-term impact is really all of these pieces fitting together so that, over time, we can reduce the burden STIs have on the population.”
Implications for real-world change
H. Hunter Handsfield, MD, professor emeritus of medicine for the Center for AIDS and STD at the University of Washington, Seattle, said in an interview that this report essentially is a response to evolving societal changes, new and emerging means of social engagement, and increased focus on racial/ethnic disparities. “These features have all come to the forefront of health care and general policy discussions in recent years,” said Dr. Handsfield, who was not part of the committee that developed the NAS report.
Greater scrutiny on public health infrastructure and its relationship with health disparities in the United States makes the publication of these new recommendations especially appropriate during this era of enhanced focus on social justice. Although the report features the tone and quality needed to bolster bipartisan support, said Dr. Handsfield, it’s hard to predict whether such support will come to fruition in today’s political environment.
In terms of the effects the recommendations may have on STI rates, Dr. Handsfield noted that cherry-picking elements from the report to direct policy may result in its having only a trivial impact. “The report is really an appropriate and necessary response, and almost all the recommendations made can be helpful,” he said, “but for true effectiveness, all the elements need to be implemented to drive policy and funding.”
A version of this article first appeared on Medscape.com.
Approximately 68 million cases of sexually transmitted infections are reported in the United States each year, yet antiquated approaches to STI prevention, in addition to health care inequities and lack of funding, have substantially prevented providers and officials from curbing the spread. In response to rising case numbers, the National Academies of Sciences, Engineering, and Medicine released a report this week with recommendations to modernize the nation’s STI surveillance and monitoring systems, increase the capabilities of the STI workforce, and address structural barriers to STI prevention and access to care.
Given the rising rates of STIs and the urgent, unmet need for prevention and treatment, the Centers for Disease Control and Prevention’s National Association of County and City Health Officials commissioned the National Academies to develop actionable recommendations to control STIs. The new report marks a long road toward the public’s willingness to discuss STDs, or what a 1997 Institute of Medicine report described as a “hidden epidemic” that had been largely neglected in public discourse.
Jeffrey Crowley, MPH, committee member and an author of the new National Academies report, said in an interview that, despite the increased openness to discuss STIs in today’s society, STD rates since the late 1990s have gotten much worse. Lack of appropriate governmental funding for research and drug development, structural inequities, and persisting stigmatization are key drivers for rising rates, explained Mr. Crowley.
Addressing structural barriers to STI prevention
Playing a prominent role in the National Academies report are issues of structural and institutional barriers to STI prevention and care. In the report, the authors argued that a policy-based approach should seek to promote sexual health and eliminate structural racism and inequities to drive improvements in STI management.
“We think it’s these structural factors that are central to all the inequities that play out,” said Mr. Crowley, “and they either don’t get any attention or, if they do get attention, people don’t really speak concretely enough about how we address them.”
The concrete steps, as outlined in the report, begin with addressing factors that involve the health care industry at large. Automatic STI screening as part of routine visits, alerts in electronic health records that remind clinicians to screen patients, and reminders to test patients can be initial low-cost actions health care systems can take to improve STI testing, particularly in marginalized communities. Mr. Crowley noted that greater evidence is needed to support further steps to address structural factors that contribute to barriers in STI screening and treatment access.
Given the complexities inherent in structural barriers to STI care, the report calls on a whole-government response, in partnership with affected communities, to normalize discussions involving sexual well-being. “We have to ask ourselves how we can build healthier communities and how can we integrate sexual health into that dialogue in a way that improves our response to STI prevention and control,” Mr. Crowley explained.
Harnessing AI and dating apps
The report also addresses the power of artificial intelligence to predict STI rates and to discover trends in risk factors, both of which may improve STI surveillance and assist in the development of tailored interventions. The report calls for policy that will enable companies and the government to capitalize on AI to evaluate large collections of data in EHRs, insurance claims databases, social media, search engines, and even dating apps.
In particular, dating apps could be an avenue through which the public and private sectors could improve STI prevention, diagnosis, and treatment. “People want to focus on this idea of whether these apps increase transmission risk,” said Mr. Crowley. “But we would say that this is asking the wrong question, because these technologies are not going away.” He noted that private and public enterprises could work together to leverage these technologies to increase awareness of prevention and testing.
Unifying the STI/HIV and COVID-19 workforce
The report also recommends that the nation unify the STI/HIV workforce with the COVID-19 workforce. Given the high levels of expertise in these professional working groups, the report suggests unification could potentially address both the current crisis and possible future disease outbreaks. Combining COVID-19 response teams with underresourced STI/HIV programs may also improve the delivery of STI testing, considering that STI testing programs have had to compete for resources during the pandemic.
Addressing stigma
The National Academies report also addresses the ongoing issue of stigma, which results from “blaming” individuals and the choices they make so as to create shame, embarrassment, and discrimination. Because of stigma, sexually active people may be unwilling to seek recommended screening, which can lead to delays in diagnosis and treatment and can increase the risk for negative health outcomes.
“As a nation, we’ve almost focused too intently on individual-level factors in a way that’s driven stigma and really hasn’t been helpful for combating the problem,” said Mr. Crowley. He added that, instead of focusing solely on individual-level choices, the nation should instead work to reframe sexual health as a key aspect of overall physical, mental, and emotional well-being. Doing so could create more opportunities to address structural barriers to STI prevention and ensure that more prevention and screening services are available in stigma-free environments.
“I know what we’re recommending is ambitious, but it’s not too big to be achieved, and we’re not saying tomorrow we’re going to transform the world,” Mr. Crowley concluded. “It’s a puzzle with many pieces, but the long-term impact is really all of these pieces fitting together so that, over time, we can reduce the burden STIs have on the population.”
Implications for real-world change
H. Hunter Handsfield, MD, professor emeritus of medicine for the Center for AIDS and STD at the University of Washington, Seattle, said in an interview that this report essentially is a response to evolving societal changes, new and emerging means of social engagement, and increased focus on racial/ethnic disparities. “These features have all come to the forefront of health care and general policy discussions in recent years,” said Dr. Handsfield, who was not part of the committee that developed the NAS report.
Greater scrutiny on public health infrastructure and its relationship with health disparities in the United States makes the publication of these new recommendations especially appropriate during this era of enhanced focus on social justice. Although the report features the tone and quality needed to bolster bipartisan support, said Dr. Handsfield, it’s hard to predict whether such support will come to fruition in today’s political environment.
In terms of the effects the recommendations may have on STI rates, Dr. Handsfield noted that cherry-picking elements from the report to direct policy may result in its having only a trivial impact. “The report is really an appropriate and necessary response, and almost all the recommendations made can be helpful,” he said, “but for true effectiveness, all the elements need to be implemented to drive policy and funding.”
A version of this article first appeared on Medscape.com.
Approximately 68 million cases of sexually transmitted infections are reported in the United States each year, yet antiquated approaches to STI prevention, in addition to health care inequities and lack of funding, have substantially prevented providers and officials from curbing the spread. In response to rising case numbers, the National Academies of Sciences, Engineering, and Medicine released a report this week with recommendations to modernize the nation’s STI surveillance and monitoring systems, increase the capabilities of the STI workforce, and address structural barriers to STI prevention and access to care.
Given the rising rates of STIs and the urgent, unmet need for prevention and treatment, the Centers for Disease Control and Prevention’s National Association of County and City Health Officials commissioned the National Academies to develop actionable recommendations to control STIs. The new report marks a long road toward the public’s willingness to discuss STDs, or what a 1997 Institute of Medicine report described as a “hidden epidemic” that had been largely neglected in public discourse.
Jeffrey Crowley, MPH, committee member and an author of the new National Academies report, said in an interview that, despite the increased openness to discuss STIs in today’s society, STD rates since the late 1990s have gotten much worse. Lack of appropriate governmental funding for research and drug development, structural inequities, and persisting stigmatization are key drivers for rising rates, explained Mr. Crowley.
Addressing structural barriers to STI prevention
Playing a prominent role in the National Academies report are issues of structural and institutional barriers to STI prevention and care. In the report, the authors argued that a policy-based approach should seek to promote sexual health and eliminate structural racism and inequities to drive improvements in STI management.
“We think it’s these structural factors that are central to all the inequities that play out,” said Mr. Crowley, “and they either don’t get any attention or, if they do get attention, people don’t really speak concretely enough about how we address them.”
The concrete steps, as outlined in the report, begin with addressing factors that involve the health care industry at large. Automatic STI screening as part of routine visits, alerts in electronic health records that remind clinicians to screen patients, and reminders to test patients can be initial low-cost actions health care systems can take to improve STI testing, particularly in marginalized communities. Mr. Crowley noted that greater evidence is needed to support further steps to address structural factors that contribute to barriers in STI screening and treatment access.
Given the complexities inherent in structural barriers to STI care, the report calls on a whole-government response, in partnership with affected communities, to normalize discussions involving sexual well-being. “We have to ask ourselves how we can build healthier communities and how can we integrate sexual health into that dialogue in a way that improves our response to STI prevention and control,” Mr. Crowley explained.
Harnessing AI and dating apps
The report also addresses the power of artificial intelligence to predict STI rates and to discover trends in risk factors, both of which may improve STI surveillance and assist in the development of tailored interventions. The report calls for policy that will enable companies and the government to capitalize on AI to evaluate large collections of data in EHRs, insurance claims databases, social media, search engines, and even dating apps.
In particular, dating apps could be an avenue through which the public and private sectors could improve STI prevention, diagnosis, and treatment. “People want to focus on this idea of whether these apps increase transmission risk,” said Mr. Crowley. “But we would say that this is asking the wrong question, because these technologies are not going away.” He noted that private and public enterprises could work together to leverage these technologies to increase awareness of prevention and testing.
Unifying the STI/HIV and COVID-19 workforce
The report also recommends that the nation unify the STI/HIV workforce with the COVID-19 workforce. Given the high levels of expertise in these professional working groups, the report suggests unification could potentially address both the current crisis and possible future disease outbreaks. Combining COVID-19 response teams with underresourced STI/HIV programs may also improve the delivery of STI testing, considering that STI testing programs have had to compete for resources during the pandemic.
Addressing stigma
The National Academies report also addresses the ongoing issue of stigma, which results from “blaming” individuals and the choices they make so as to create shame, embarrassment, and discrimination. Because of stigma, sexually active people may be unwilling to seek recommended screening, which can lead to delays in diagnosis and treatment and can increase the risk for negative health outcomes.
“As a nation, we’ve almost focused too intently on individual-level factors in a way that’s driven stigma and really hasn’t been helpful for combating the problem,” said Mr. Crowley. He added that, instead of focusing solely on individual-level choices, the nation should instead work to reframe sexual health as a key aspect of overall physical, mental, and emotional well-being. Doing so could create more opportunities to address structural barriers to STI prevention and ensure that more prevention and screening services are available in stigma-free environments.
“I know what we’re recommending is ambitious, but it’s not too big to be achieved, and we’re not saying tomorrow we’re going to transform the world,” Mr. Crowley concluded. “It’s a puzzle with many pieces, but the long-term impact is really all of these pieces fitting together so that, over time, we can reduce the burden STIs have on the population.”
Implications for real-world change
H. Hunter Handsfield, MD, professor emeritus of medicine for the Center for AIDS and STD at the University of Washington, Seattle, said in an interview that this report essentially is a response to evolving societal changes, new and emerging means of social engagement, and increased focus on racial/ethnic disparities. “These features have all come to the forefront of health care and general policy discussions in recent years,” said Dr. Handsfield, who was not part of the committee that developed the NAS report.
Greater scrutiny on public health infrastructure and its relationship with health disparities in the United States makes the publication of these new recommendations especially appropriate during this era of enhanced focus on social justice. Although the report features the tone and quality needed to bolster bipartisan support, said Dr. Handsfield, it’s hard to predict whether such support will come to fruition in today’s political environment.
In terms of the effects the recommendations may have on STI rates, Dr. Handsfield noted that cherry-picking elements from the report to direct policy may result in its having only a trivial impact. “The report is really an appropriate and necessary response, and almost all the recommendations made can be helpful,” he said, “but for true effectiveness, all the elements need to be implemented to drive policy and funding.”
A version of this article first appeared on Medscape.com.
COVID-19 vaccination in RMD patients: Safety data “reassuring”
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
FROM ANNALS OF THE RHEUMATIC DISEASES










