User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
COVID-19 in children: Weekly cases trending downward
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
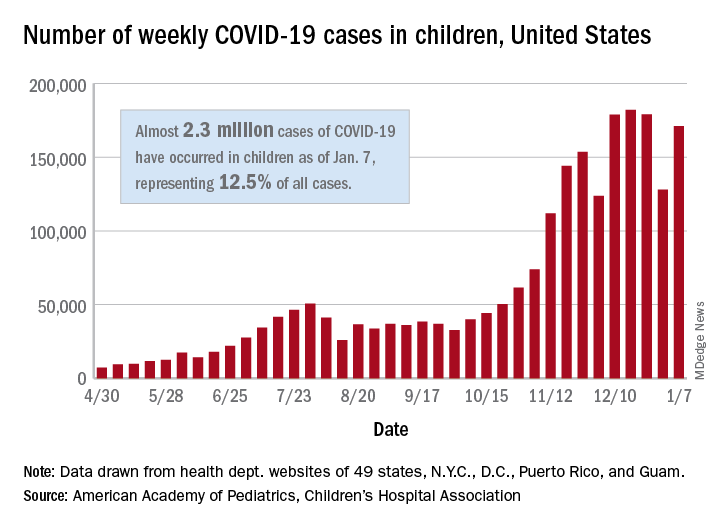
Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.
Updated USPSTF HBV screening recommendation may be a ‘lost opportunity’
An update of the U.S. Preventive Services Task Force recommendation for hepatitis B screening shows little change from the 2014 version, but some wonder if it should have gone farther than a risk-based approach.
The recommendation, which was published in JAMA, reinforces that screening should be conducted among adolescents and adults who are at increased risk of hepatitis B virus (HBV) infection. The USPSTF named six categories of individuals at increased risk of infection: Persons born in countries with a 2% or higher prevalence of hepatitis B, such as Asia, Africa, the Pacific Islands, and some areas of South America; unvaccinated individuals born in the United States to parents from regions with a very high prevalence of HBV (≥8%); HIV-positive individuals; those who use injected drugs; men who have sex with men; and people who live with people who have HBV or who have HBV-infected sexual partners. It also recommended that pregnant women be screened for HBV infection during their first prenatal visit.
“I view the updated recommendations as an important document because it validates the importance of HBV screening, and the Grade B recommendation supports mandated insurance coverage for the screening test,” said Joseph Lim, MD, who is a professor of medicine at Yale University and director of the Yale Viral Hepatitis Program, both in New Haven, Conn.
Still, the recommendation could have gone further. Notably absent from the USPSTF document, yet featured in recommendations from the Centers for Disease Control and Prevention and the American Association for the Study of Liver Disease, are patients who have diabetes, are on immunosuppressive therapy, or have elevated liver enzymes or liver disease. Furthermore, a single-center study found that, among physicians administering immunosuppressive therapy, a setting in which HBV reactivation is a concern, there were low rates of screening for HBV infection, and the physicians did not reliably identify high-risk patients.
“This may also be viewed as a lost opportunity. Evidence suggests that risk factor–based screening is ineffective for the identification of chronic conditions such as hepatitis B. Risk factor–based screening is difficult to implement across health systems and exacerbates the burden on community-based organizations that are motivated to address viral hepatitis. It may further exacerbate labeling, stigma, and discrimination within already marginalized communities that are deemed to be at high risk,” said Dr. Lim.
A similar view was expressed by Avegail Flores, MD, medical director of liver transplantation at the Michael E. DeBakey Veterans Affairs Medical Center and assistant professor of medicine at Baylor College of Medicine, both in Houston. “This is a good launching point, and with further evidence provided, hopefully it will also bring in a broader conversation about other persons who are at risk but not included in these criteria.” Neither Dr. Lim nor Dr. Flores were involved in the study.
She noted that resistance to universal screening may be caused by the relatively low prevalence of hepatitis B infection in the United States. However, the CDC estimates that only about 61% of people infected with HBV are aware of it. “I don’t think we have done a good job screening those who are at risk,” said Dr. Flores.
Universal screening could help, but would have a low yield. Dr. Flores suggested expansion into other at-risk groups, such as Baby Boomers. With respect to other risk groups that could be stigmatized or discriminated against, Dr. Flores recalled her medical school days when some students went directly into underserved communities to provide information and screening services. “We have to think of creative ways of how to reach out to people, not just relying on the usual physician-patient relationship.”
The issue is especially timely because the World Health Organization has declared a target to reduce new hepatitis B infections by 90% by 2030, and that will require addressing gaps in diagnosis. “That’s why these recommendations are so consequential. We are at a critical juncture in terms of global hepatitis elimination efforts. There is a time sensitive need to have multistakeholder engagement in ensuring that all aspects of the care cascade are addressed. Because of the central role of screening and diagnosis, it’s of critical importance that organizations such as USPSTF are in alignment with other organizations that have already issued clear guidance on who should be screened. It is (my) hope that further examination of the evidence-base will further support broadening USPSTF guidance to include a larger group of at-risk individuals, or ideally a universal screening strategy,” said Dr. Lim.
The recommendation’s authors received travel reimbursement for their involvement, and one author reported receiving grants and personal fees from Healthwise. Dr. Flores has no relevant financial disclosures. Dr. Lim is a member of the American Association for the Study of Liver Disease’s Viral Hepatitis Elimination Task Force.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2020 Dec 15. doi: 10.1001/jama.2020.22980.
Updated Jan. 20, 2021
An update of the U.S. Preventive Services Task Force recommendation for hepatitis B screening shows little change from the 2014 version, but some wonder if it should have gone farther than a risk-based approach.
The recommendation, which was published in JAMA, reinforces that screening should be conducted among adolescents and adults who are at increased risk of hepatitis B virus (HBV) infection. The USPSTF named six categories of individuals at increased risk of infection: Persons born in countries with a 2% or higher prevalence of hepatitis B, such as Asia, Africa, the Pacific Islands, and some areas of South America; unvaccinated individuals born in the United States to parents from regions with a very high prevalence of HBV (≥8%); HIV-positive individuals; those who use injected drugs; men who have sex with men; and people who live with people who have HBV or who have HBV-infected sexual partners. It also recommended that pregnant women be screened for HBV infection during their first prenatal visit.
“I view the updated recommendations as an important document because it validates the importance of HBV screening, and the Grade B recommendation supports mandated insurance coverage for the screening test,” said Joseph Lim, MD, who is a professor of medicine at Yale University and director of the Yale Viral Hepatitis Program, both in New Haven, Conn.
Still, the recommendation could have gone further. Notably absent from the USPSTF document, yet featured in recommendations from the Centers for Disease Control and Prevention and the American Association for the Study of Liver Disease, are patients who have diabetes, are on immunosuppressive therapy, or have elevated liver enzymes or liver disease. Furthermore, a single-center study found that, among physicians administering immunosuppressive therapy, a setting in which HBV reactivation is a concern, there were low rates of screening for HBV infection, and the physicians did not reliably identify high-risk patients.
“This may also be viewed as a lost opportunity. Evidence suggests that risk factor–based screening is ineffective for the identification of chronic conditions such as hepatitis B. Risk factor–based screening is difficult to implement across health systems and exacerbates the burden on community-based organizations that are motivated to address viral hepatitis. It may further exacerbate labeling, stigma, and discrimination within already marginalized communities that are deemed to be at high risk,” said Dr. Lim.
A similar view was expressed by Avegail Flores, MD, medical director of liver transplantation at the Michael E. DeBakey Veterans Affairs Medical Center and assistant professor of medicine at Baylor College of Medicine, both in Houston. “This is a good launching point, and with further evidence provided, hopefully it will also bring in a broader conversation about other persons who are at risk but not included in these criteria.” Neither Dr. Lim nor Dr. Flores were involved in the study.
She noted that resistance to universal screening may be caused by the relatively low prevalence of hepatitis B infection in the United States. However, the CDC estimates that only about 61% of people infected with HBV are aware of it. “I don’t think we have done a good job screening those who are at risk,” said Dr. Flores.
Universal screening could help, but would have a low yield. Dr. Flores suggested expansion into other at-risk groups, such as Baby Boomers. With respect to other risk groups that could be stigmatized or discriminated against, Dr. Flores recalled her medical school days when some students went directly into underserved communities to provide information and screening services. “We have to think of creative ways of how to reach out to people, not just relying on the usual physician-patient relationship.”
The issue is especially timely because the World Health Organization has declared a target to reduce new hepatitis B infections by 90% by 2030, and that will require addressing gaps in diagnosis. “That’s why these recommendations are so consequential. We are at a critical juncture in terms of global hepatitis elimination efforts. There is a time sensitive need to have multistakeholder engagement in ensuring that all aspects of the care cascade are addressed. Because of the central role of screening and diagnosis, it’s of critical importance that organizations such as USPSTF are in alignment with other organizations that have already issued clear guidance on who should be screened. It is (my) hope that further examination of the evidence-base will further support broadening USPSTF guidance to include a larger group of at-risk individuals, or ideally a universal screening strategy,” said Dr. Lim.
The recommendation’s authors received travel reimbursement for their involvement, and one author reported receiving grants and personal fees from Healthwise. Dr. Flores has no relevant financial disclosures. Dr. Lim is a member of the American Association for the Study of Liver Disease’s Viral Hepatitis Elimination Task Force.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2020 Dec 15. doi: 10.1001/jama.2020.22980.
Updated Jan. 20, 2021
An update of the U.S. Preventive Services Task Force recommendation for hepatitis B screening shows little change from the 2014 version, but some wonder if it should have gone farther than a risk-based approach.
The recommendation, which was published in JAMA, reinforces that screening should be conducted among adolescents and adults who are at increased risk of hepatitis B virus (HBV) infection. The USPSTF named six categories of individuals at increased risk of infection: Persons born in countries with a 2% or higher prevalence of hepatitis B, such as Asia, Africa, the Pacific Islands, and some areas of South America; unvaccinated individuals born in the United States to parents from regions with a very high prevalence of HBV (≥8%); HIV-positive individuals; those who use injected drugs; men who have sex with men; and people who live with people who have HBV or who have HBV-infected sexual partners. It also recommended that pregnant women be screened for HBV infection during their first prenatal visit.
“I view the updated recommendations as an important document because it validates the importance of HBV screening, and the Grade B recommendation supports mandated insurance coverage for the screening test,” said Joseph Lim, MD, who is a professor of medicine at Yale University and director of the Yale Viral Hepatitis Program, both in New Haven, Conn.
Still, the recommendation could have gone further. Notably absent from the USPSTF document, yet featured in recommendations from the Centers for Disease Control and Prevention and the American Association for the Study of Liver Disease, are patients who have diabetes, are on immunosuppressive therapy, or have elevated liver enzymes or liver disease. Furthermore, a single-center study found that, among physicians administering immunosuppressive therapy, a setting in which HBV reactivation is a concern, there were low rates of screening for HBV infection, and the physicians did not reliably identify high-risk patients.
“This may also be viewed as a lost opportunity. Evidence suggests that risk factor–based screening is ineffective for the identification of chronic conditions such as hepatitis B. Risk factor–based screening is difficult to implement across health systems and exacerbates the burden on community-based organizations that are motivated to address viral hepatitis. It may further exacerbate labeling, stigma, and discrimination within already marginalized communities that are deemed to be at high risk,” said Dr. Lim.
A similar view was expressed by Avegail Flores, MD, medical director of liver transplantation at the Michael E. DeBakey Veterans Affairs Medical Center and assistant professor of medicine at Baylor College of Medicine, both in Houston. “This is a good launching point, and with further evidence provided, hopefully it will also bring in a broader conversation about other persons who are at risk but not included in these criteria.” Neither Dr. Lim nor Dr. Flores were involved in the study.
She noted that resistance to universal screening may be caused by the relatively low prevalence of hepatitis B infection in the United States. However, the CDC estimates that only about 61% of people infected with HBV are aware of it. “I don’t think we have done a good job screening those who are at risk,” said Dr. Flores.
Universal screening could help, but would have a low yield. Dr. Flores suggested expansion into other at-risk groups, such as Baby Boomers. With respect to other risk groups that could be stigmatized or discriminated against, Dr. Flores recalled her medical school days when some students went directly into underserved communities to provide information and screening services. “We have to think of creative ways of how to reach out to people, not just relying on the usual physician-patient relationship.”
The issue is especially timely because the World Health Organization has declared a target to reduce new hepatitis B infections by 90% by 2030, and that will require addressing gaps in diagnosis. “That’s why these recommendations are so consequential. We are at a critical juncture in terms of global hepatitis elimination efforts. There is a time sensitive need to have multistakeholder engagement in ensuring that all aspects of the care cascade are addressed. Because of the central role of screening and diagnosis, it’s of critical importance that organizations such as USPSTF are in alignment with other organizations that have already issued clear guidance on who should be screened. It is (my) hope that further examination of the evidence-base will further support broadening USPSTF guidance to include a larger group of at-risk individuals, or ideally a universal screening strategy,” said Dr. Lim.
The recommendation’s authors received travel reimbursement for their involvement, and one author reported receiving grants and personal fees from Healthwise. Dr. Flores has no relevant financial disclosures. Dr. Lim is a member of the American Association for the Study of Liver Disease’s Viral Hepatitis Elimination Task Force.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2020 Dec 15. doi: 10.1001/jama.2020.22980.
Updated Jan. 20, 2021
FROM JAMA
Cloth masks provide inferior protection vs. medical masks, suggests evidence review
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
FROM ANNALS OF FAMILY MEDICINE
Feds authorize $3 billion to boost vaccine rollout
The CDC will send $3 billion to the states to boost a lagging national COVID-19 vaccination program.
The Department of Health and Human Services announced the new funding as only 30% of the more than 22 million doses of vaccine distributed in the U.S. has been injected into Americans’ arms.
Along with the $3 billion, HHS said another $19 billion is headed to states and jurisdictions to boost COVID-19 testing programs. The amount each state will receive will be determined by population.
The news comes days after President-elect Joe Biden said he planned to release all available doses of vaccine after he takes office on Jan. 20. The Trump administration has been holding back millions of doses to ensure supply of vaccine to provide the necessary second dose for those who received the first shot.
“This funding is another timely investment that will strengthen our nation’s efforts to stop the COVID-19 pandemic in America,” CDC Director Robert Redfield, MD, said in a statement. “Particularly now, it is crucial that states and communities have the resources they need to conduct testing, and to distribute and administer safe, high-quality COVID-19 vaccines safely and equitably.”
Federal officials and public health experts, however, expressed concerns this weekend about Biden’s plan.
Outgoing Trump administration officials and others said they worry that doing so will leave providers without enough second doses for people getting the two-shot vaccines.
If Biden releases all available doses and the vaccine-making process has an issue, they said, that could pose a supply risk.
“We have product that is going through QC right now – quality control – for sterility, identity check that we have tens and tens of millions of product. We always will. But batches fail. Sterility fails ... and then you don’t have a product for that second dose,” Alex Azar, secretary of health and human services, told the American Hospital Association on Jan. 8, according to CNN.
“And frankly, talking about that or encouraging that can really undermine a critical public health need, which is that people come back for their second vaccine,” he said.
One of the main roadblocks in the vaccine rollout has been administering the doses that have already been distributed. The U.S. has shipped 22.1 million doses, and 6.6 million first shots have been given, according to the latest CDC data updated Jan. 8. Mr. Azar and other federal health officials have encouraged states to use their current supply and expand vaccine access to more priority groups.
“We would be delighted to learn that jurisdictions have actually administered many more doses than they are presently reporting,” a spokesman for the U.S. Department of Health and Human Services told CNN. “We are encouraging jurisdictions to expand their priority groups as needed to ensure no vaccine is sitting on the shelf after having been delivered to the jurisdiction-directed locations.”
Releasing more vaccines for first doses could create ethical concerns as well, since people getting vaccines expect to get a second dose in the proper amount of time, according to The Week. Biden’s transition team said on Jan. 8 that he won’t delay the second dose but, instead, plans to ramp up production to stay on track.
To do this well, the federal government should create a coordinated vaccine strategy that sets expectations for an around-the-clock operation and help state and local vaccination programs meet their goals, Leana Wen, MD, a professor at George Washington University, wrote in an editorial for The Washington Post.
“The Biden team’s urgency around vaccinations is commendable,” she added in a Twitter post on Jan. 11. “I’d like to see a guarantee that every 1st dose given will be followed with a timely 2nd dose. Otherwise, there are ethical concerns that could add to vaccine hesitancy.”
Biden has pledged that 100 million doses will be administered in his first 100 days in office. He has grown frustrated as concerns grow that his administration could fall short of the promise, according to Politico. His coronavirus response team has noted several challenges, including what they say is a lack of long-term planning by the Trump administration and an initial refusal to share key information.
“We’re uncovering new information each day, and we’re unearthing – of course – more work to be done,” Vivek Murthy, MD, Biden’s nominee for surgeon general, told Politico.
The team has uncovered staffing shortages, technology problems, and issues with health care insurance coverage. The incoming Biden team has developed several initiatives, such as mobile vaccination units and new federal sites to give shots. It could take weeks to get the vaccine rollout on track, the news outlet reported.
“Will this be challenging? Absolutely,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Biden’s incoming chief medical adviser on the coronavirus, told Politico. “This is an unprecedented effort to vaccinate the entire country over a period of time that’s fighting against people dying at record numbers. To say it’s not a challenge would be unrealistic. Do I think it can be done? Yes.”
A version of this article first appeared on WebMD.com.
The CDC will send $3 billion to the states to boost a lagging national COVID-19 vaccination program.
The Department of Health and Human Services announced the new funding as only 30% of the more than 22 million doses of vaccine distributed in the U.S. has been injected into Americans’ arms.
Along with the $3 billion, HHS said another $19 billion is headed to states and jurisdictions to boost COVID-19 testing programs. The amount each state will receive will be determined by population.
The news comes days after President-elect Joe Biden said he planned to release all available doses of vaccine after he takes office on Jan. 20. The Trump administration has been holding back millions of doses to ensure supply of vaccine to provide the necessary second dose for those who received the first shot.
“This funding is another timely investment that will strengthen our nation’s efforts to stop the COVID-19 pandemic in America,” CDC Director Robert Redfield, MD, said in a statement. “Particularly now, it is crucial that states and communities have the resources they need to conduct testing, and to distribute and administer safe, high-quality COVID-19 vaccines safely and equitably.”
Federal officials and public health experts, however, expressed concerns this weekend about Biden’s plan.
Outgoing Trump administration officials and others said they worry that doing so will leave providers without enough second doses for people getting the two-shot vaccines.
If Biden releases all available doses and the vaccine-making process has an issue, they said, that could pose a supply risk.
“We have product that is going through QC right now – quality control – for sterility, identity check that we have tens and tens of millions of product. We always will. But batches fail. Sterility fails ... and then you don’t have a product for that second dose,” Alex Azar, secretary of health and human services, told the American Hospital Association on Jan. 8, according to CNN.
“And frankly, talking about that or encouraging that can really undermine a critical public health need, which is that people come back for their second vaccine,” he said.
One of the main roadblocks in the vaccine rollout has been administering the doses that have already been distributed. The U.S. has shipped 22.1 million doses, and 6.6 million first shots have been given, according to the latest CDC data updated Jan. 8. Mr. Azar and other federal health officials have encouraged states to use their current supply and expand vaccine access to more priority groups.
“We would be delighted to learn that jurisdictions have actually administered many more doses than they are presently reporting,” a spokesman for the U.S. Department of Health and Human Services told CNN. “We are encouraging jurisdictions to expand their priority groups as needed to ensure no vaccine is sitting on the shelf after having been delivered to the jurisdiction-directed locations.”
Releasing more vaccines for first doses could create ethical concerns as well, since people getting vaccines expect to get a second dose in the proper amount of time, according to The Week. Biden’s transition team said on Jan. 8 that he won’t delay the second dose but, instead, plans to ramp up production to stay on track.
To do this well, the federal government should create a coordinated vaccine strategy that sets expectations for an around-the-clock operation and help state and local vaccination programs meet their goals, Leana Wen, MD, a professor at George Washington University, wrote in an editorial for The Washington Post.
“The Biden team’s urgency around vaccinations is commendable,” she added in a Twitter post on Jan. 11. “I’d like to see a guarantee that every 1st dose given will be followed with a timely 2nd dose. Otherwise, there are ethical concerns that could add to vaccine hesitancy.”
Biden has pledged that 100 million doses will be administered in his first 100 days in office. He has grown frustrated as concerns grow that his administration could fall short of the promise, according to Politico. His coronavirus response team has noted several challenges, including what they say is a lack of long-term planning by the Trump administration and an initial refusal to share key information.
“We’re uncovering new information each day, and we’re unearthing – of course – more work to be done,” Vivek Murthy, MD, Biden’s nominee for surgeon general, told Politico.
The team has uncovered staffing shortages, technology problems, and issues with health care insurance coverage. The incoming Biden team has developed several initiatives, such as mobile vaccination units and new federal sites to give shots. It could take weeks to get the vaccine rollout on track, the news outlet reported.
“Will this be challenging? Absolutely,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Biden’s incoming chief medical adviser on the coronavirus, told Politico. “This is an unprecedented effort to vaccinate the entire country over a period of time that’s fighting against people dying at record numbers. To say it’s not a challenge would be unrealistic. Do I think it can be done? Yes.”
A version of this article first appeared on WebMD.com.
The CDC will send $3 billion to the states to boost a lagging national COVID-19 vaccination program.
The Department of Health and Human Services announced the new funding as only 30% of the more than 22 million doses of vaccine distributed in the U.S. has been injected into Americans’ arms.
Along with the $3 billion, HHS said another $19 billion is headed to states and jurisdictions to boost COVID-19 testing programs. The amount each state will receive will be determined by population.
The news comes days after President-elect Joe Biden said he planned to release all available doses of vaccine after he takes office on Jan. 20. The Trump administration has been holding back millions of doses to ensure supply of vaccine to provide the necessary second dose for those who received the first shot.
“This funding is another timely investment that will strengthen our nation’s efforts to stop the COVID-19 pandemic in America,” CDC Director Robert Redfield, MD, said in a statement. “Particularly now, it is crucial that states and communities have the resources they need to conduct testing, and to distribute and administer safe, high-quality COVID-19 vaccines safely and equitably.”
Federal officials and public health experts, however, expressed concerns this weekend about Biden’s plan.
Outgoing Trump administration officials and others said they worry that doing so will leave providers without enough second doses for people getting the two-shot vaccines.
If Biden releases all available doses and the vaccine-making process has an issue, they said, that could pose a supply risk.
“We have product that is going through QC right now – quality control – for sterility, identity check that we have tens and tens of millions of product. We always will. But batches fail. Sterility fails ... and then you don’t have a product for that second dose,” Alex Azar, secretary of health and human services, told the American Hospital Association on Jan. 8, according to CNN.
“And frankly, talking about that or encouraging that can really undermine a critical public health need, which is that people come back for their second vaccine,” he said.
One of the main roadblocks in the vaccine rollout has been administering the doses that have already been distributed. The U.S. has shipped 22.1 million doses, and 6.6 million first shots have been given, according to the latest CDC data updated Jan. 8. Mr. Azar and other federal health officials have encouraged states to use their current supply and expand vaccine access to more priority groups.
“We would be delighted to learn that jurisdictions have actually administered many more doses than they are presently reporting,” a spokesman for the U.S. Department of Health and Human Services told CNN. “We are encouraging jurisdictions to expand their priority groups as needed to ensure no vaccine is sitting on the shelf after having been delivered to the jurisdiction-directed locations.”
Releasing more vaccines for first doses could create ethical concerns as well, since people getting vaccines expect to get a second dose in the proper amount of time, according to The Week. Biden’s transition team said on Jan. 8 that he won’t delay the second dose but, instead, plans to ramp up production to stay on track.
To do this well, the federal government should create a coordinated vaccine strategy that sets expectations for an around-the-clock operation and help state and local vaccination programs meet their goals, Leana Wen, MD, a professor at George Washington University, wrote in an editorial for The Washington Post.
“The Biden team’s urgency around vaccinations is commendable,” she added in a Twitter post on Jan. 11. “I’d like to see a guarantee that every 1st dose given will be followed with a timely 2nd dose. Otherwise, there are ethical concerns that could add to vaccine hesitancy.”
Biden has pledged that 100 million doses will be administered in his first 100 days in office. He has grown frustrated as concerns grow that his administration could fall short of the promise, according to Politico. His coronavirus response team has noted several challenges, including what they say is a lack of long-term planning by the Trump administration and an initial refusal to share key information.
“We’re uncovering new information each day, and we’re unearthing – of course – more work to be done,” Vivek Murthy, MD, Biden’s nominee for surgeon general, told Politico.
The team has uncovered staffing shortages, technology problems, and issues with health care insurance coverage. The incoming Biden team has developed several initiatives, such as mobile vaccination units and new federal sites to give shots. It could take weeks to get the vaccine rollout on track, the news outlet reported.
“Will this be challenging? Absolutely,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Biden’s incoming chief medical adviser on the coronavirus, told Politico. “This is an unprecedented effort to vaccinate the entire country over a period of time that’s fighting against people dying at record numbers. To say it’s not a challenge would be unrealistic. Do I think it can be done? Yes.”
A version of this article first appeared on WebMD.com.
Physicians react: Doctors worry about patients reading their clinical notes
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.
VA Ramps up Vaccinations as COVID-19 Cases Continue to Rise
Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.

Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.

Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.
Over half of COVID-19 transmission may occur via asymptomatic people
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anaphylaxis cases after COVID-19 vaccine rising but still rare: CDC
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.
Study confirms key COVID-19 risk factors in children
Children and adolescents who receive positive COVID-19 test results are not only more likely to have been in close contact with someone with a confirmed case of the virus but also are less likely to have reported consistent mask use among students and staff inside the school they attended, reported Charlotte V. Hobbs, MD, and colleagues at the University of Mississippi, Jackson.
In partnership with the Centers for Disease Control and Prevention’s COVID-19 Response Team, Dr. Hobbs and colleagues conducted a case-control study of 397 children and adolescents under 18 years of age to assess school, community, and close contact exposures associated with pediatric COVID-19. Patients tested for COVID-19 at outpatient health centers or emergency departments affiliated with the University of Mississippi Medical Center between Sept. 1 and Nov. 5, 2020, were included in the study.
Nearly two-thirds reported that exposure came from family members
Of the total study participants observed, 82 (21%) were under 4 years of age; 214 (54%) were female; 217 (55%) were non-Hispanic black, and 145 (37%) were non-Hispanic white. More than half (53%) sought testing because of COVID-19 symptoms. Of those who tested positive, 66% reported having come into close contact with a COVID-19 case, and 64% reported that those contacts were family members, compared with 15% of contacts who were schoolmates and 27% who were child care classmates.
All participants completed in-person school or child care attendance less than 14 days before testing positive for the virus, including 62% of patients testing positive and 68% of those testing negative. The authors noted that school attendance itself was not found to be associated with any positive test results. In fact, parents in 64% of positive cases and 76% of negative cases reported mask wearing among children and staff inside places of learning.
Of those study participants testing positive who did come into close contact with someone with COVID-19, the contacts were more likely to be family members than school or child care classmates. Specifically, they were more likely, in the 2-week period preceding testing, to have attended gatherings with individuals outside their immediate households, including social events and activities with other children. Parents of students testing positive were also less likely to report consistent indoor mask use among their children older than 2 years and school staff members.
School attendance was not found to increase likelihood of testing positive
Attending in-person school or child care during the 2 weeks before the SARS-CoV-2 test was not associated with greater likelihood of testing positive, the study authors noted, adding that the majority of study respondents reported universal mask use inside school and child care facilities, consistent with Mississippi State Department of Health recommended guidelines.
Dr. Hobbs and colleagues reported at least four limitations of the study. They noted that the study participants may not be representative of youth in other geographic regions of the country. They considered the possibility of unmeasured confounding of participant behaviors that may not have been factored into the study. No attempt was made to verify parent claims of mask use at schools and child care programs. Lastly, they acknowledged that “case or control status might be subject to misclassification because of imperfect sensitivity or specificity of PCR-based testing.
As of Dec. 14, 2020, the CDC reported that 10.2% of all COVID-19 cases in the United States were in children and adolescents under the age of 18.
“Continued efforts to prevent transmission at schools and child care programs are important, as are assessments of various types of activities and exposures to identify risk factors for COVID-19 as children engage in classroom and social interactions.” Promoting behaviors to reduce exposures to the virus among youth in the household, the community, schools, and child care programs is important to preventing outbreaks of the virus at schools, the authors cautioned.
In a separate interview with this news organization, Karalyn Kinsella, MD, general pediatrician in a small group private practice in Cheshire, Conn., said, “What this report tells me is that COVID cases are more common when mask use is inconsistent in schools and at home and in schools that don’t properly adhere to CDC guidelines. Overall, so long as social distancing guidelines are followed, schools are pretty safe places for kids during this pandemic.”
This finding is important, since many families are keeping their children out of school over fears of contracting the virus, she added. Some of the consequences these children are suffering include a lack of social connection and structure, which in some cases is leading to worsening anxiety and depression, and for those with disabilities, such as those who receive physical therapy, occupational therapy, speech or have IEPs, they’re not getting the full benefit of the services that they would otherwise receive in person, she observed.
“I don’t think families really understand the risks of getting together with family or friends “in their bubble” or the risk of continuing sports participation. This is where the majority of COVID cases are coming from,” she said, adding that it is important to discuss this risk with them at appointments. So, when families ask us what we think of in-person learning, I think we should feel fairly confident that the benefit may outweigh the risk.”
Dr. Hobbs and colleagues, and Dr. Kinsella, had no conflicts of interest to report.
SOURCE: MMWR Morb Mortal Wkly Rep. 2020;69:1925-9. doi: 10.15585/mmwr.mm6950e3.
Children and adolescents who receive positive COVID-19 test results are not only more likely to have been in close contact with someone with a confirmed case of the virus but also are less likely to have reported consistent mask use among students and staff inside the school they attended, reported Charlotte V. Hobbs, MD, and colleagues at the University of Mississippi, Jackson.
In partnership with the Centers for Disease Control and Prevention’s COVID-19 Response Team, Dr. Hobbs and colleagues conducted a case-control study of 397 children and adolescents under 18 years of age to assess school, community, and close contact exposures associated with pediatric COVID-19. Patients tested for COVID-19 at outpatient health centers or emergency departments affiliated with the University of Mississippi Medical Center between Sept. 1 and Nov. 5, 2020, were included in the study.
Nearly two-thirds reported that exposure came from family members
Of the total study participants observed, 82 (21%) were under 4 years of age; 214 (54%) were female; 217 (55%) were non-Hispanic black, and 145 (37%) were non-Hispanic white. More than half (53%) sought testing because of COVID-19 symptoms. Of those who tested positive, 66% reported having come into close contact with a COVID-19 case, and 64% reported that those contacts were family members, compared with 15% of contacts who were schoolmates and 27% who were child care classmates.
All participants completed in-person school or child care attendance less than 14 days before testing positive for the virus, including 62% of patients testing positive and 68% of those testing negative. The authors noted that school attendance itself was not found to be associated with any positive test results. In fact, parents in 64% of positive cases and 76% of negative cases reported mask wearing among children and staff inside places of learning.
Of those study participants testing positive who did come into close contact with someone with COVID-19, the contacts were more likely to be family members than school or child care classmates. Specifically, they were more likely, in the 2-week period preceding testing, to have attended gatherings with individuals outside their immediate households, including social events and activities with other children. Parents of students testing positive were also less likely to report consistent indoor mask use among their children older than 2 years and school staff members.
School attendance was not found to increase likelihood of testing positive
Attending in-person school or child care during the 2 weeks before the SARS-CoV-2 test was not associated with greater likelihood of testing positive, the study authors noted, adding that the majority of study respondents reported universal mask use inside school and child care facilities, consistent with Mississippi State Department of Health recommended guidelines.
Dr. Hobbs and colleagues reported at least four limitations of the study. They noted that the study participants may not be representative of youth in other geographic regions of the country. They considered the possibility of unmeasured confounding of participant behaviors that may not have been factored into the study. No attempt was made to verify parent claims of mask use at schools and child care programs. Lastly, they acknowledged that “case or control status might be subject to misclassification because of imperfect sensitivity or specificity of PCR-based testing.
As of Dec. 14, 2020, the CDC reported that 10.2% of all COVID-19 cases in the United States were in children and adolescents under the age of 18.
“Continued efforts to prevent transmission at schools and child care programs are important, as are assessments of various types of activities and exposures to identify risk factors for COVID-19 as children engage in classroom and social interactions.” Promoting behaviors to reduce exposures to the virus among youth in the household, the community, schools, and child care programs is important to preventing outbreaks of the virus at schools, the authors cautioned.
In a separate interview with this news organization, Karalyn Kinsella, MD, general pediatrician in a small group private practice in Cheshire, Conn., said, “What this report tells me is that COVID cases are more common when mask use is inconsistent in schools and at home and in schools that don’t properly adhere to CDC guidelines. Overall, so long as social distancing guidelines are followed, schools are pretty safe places for kids during this pandemic.”
This finding is important, since many families are keeping their children out of school over fears of contracting the virus, she added. Some of the consequences these children are suffering include a lack of social connection and structure, which in some cases is leading to worsening anxiety and depression, and for those with disabilities, such as those who receive physical therapy, occupational therapy, speech or have IEPs, they’re not getting the full benefit of the services that they would otherwise receive in person, she observed.
“I don’t think families really understand the risks of getting together with family or friends “in their bubble” or the risk of continuing sports participation. This is where the majority of COVID cases are coming from,” she said, adding that it is important to discuss this risk with them at appointments. So, when families ask us what we think of in-person learning, I think we should feel fairly confident that the benefit may outweigh the risk.”
Dr. Hobbs and colleagues, and Dr. Kinsella, had no conflicts of interest to report.
SOURCE: MMWR Morb Mortal Wkly Rep. 2020;69:1925-9. doi: 10.15585/mmwr.mm6950e3.
Children and adolescents who receive positive COVID-19 test results are not only more likely to have been in close contact with someone with a confirmed case of the virus but also are less likely to have reported consistent mask use among students and staff inside the school they attended, reported Charlotte V. Hobbs, MD, and colleagues at the University of Mississippi, Jackson.
In partnership with the Centers for Disease Control and Prevention’s COVID-19 Response Team, Dr. Hobbs and colleagues conducted a case-control study of 397 children and adolescents under 18 years of age to assess school, community, and close contact exposures associated with pediatric COVID-19. Patients tested for COVID-19 at outpatient health centers or emergency departments affiliated with the University of Mississippi Medical Center between Sept. 1 and Nov. 5, 2020, were included in the study.
Nearly two-thirds reported that exposure came from family members
Of the total study participants observed, 82 (21%) were under 4 years of age; 214 (54%) were female; 217 (55%) were non-Hispanic black, and 145 (37%) were non-Hispanic white. More than half (53%) sought testing because of COVID-19 symptoms. Of those who tested positive, 66% reported having come into close contact with a COVID-19 case, and 64% reported that those contacts were family members, compared with 15% of contacts who were schoolmates and 27% who were child care classmates.
All participants completed in-person school or child care attendance less than 14 days before testing positive for the virus, including 62% of patients testing positive and 68% of those testing negative. The authors noted that school attendance itself was not found to be associated with any positive test results. In fact, parents in 64% of positive cases and 76% of negative cases reported mask wearing among children and staff inside places of learning.
Of those study participants testing positive who did come into close contact with someone with COVID-19, the contacts were more likely to be family members than school or child care classmates. Specifically, they were more likely, in the 2-week period preceding testing, to have attended gatherings with individuals outside their immediate households, including social events and activities with other children. Parents of students testing positive were also less likely to report consistent indoor mask use among their children older than 2 years and school staff members.
School attendance was not found to increase likelihood of testing positive
Attending in-person school or child care during the 2 weeks before the SARS-CoV-2 test was not associated with greater likelihood of testing positive, the study authors noted, adding that the majority of study respondents reported universal mask use inside school and child care facilities, consistent with Mississippi State Department of Health recommended guidelines.
Dr. Hobbs and colleagues reported at least four limitations of the study. They noted that the study participants may not be representative of youth in other geographic regions of the country. They considered the possibility of unmeasured confounding of participant behaviors that may not have been factored into the study. No attempt was made to verify parent claims of mask use at schools and child care programs. Lastly, they acknowledged that “case or control status might be subject to misclassification because of imperfect sensitivity or specificity of PCR-based testing.
As of Dec. 14, 2020, the CDC reported that 10.2% of all COVID-19 cases in the United States were in children and adolescents under the age of 18.
“Continued efforts to prevent transmission at schools and child care programs are important, as are assessments of various types of activities and exposures to identify risk factors for COVID-19 as children engage in classroom and social interactions.” Promoting behaviors to reduce exposures to the virus among youth in the household, the community, schools, and child care programs is important to preventing outbreaks of the virus at schools, the authors cautioned.
In a separate interview with this news organization, Karalyn Kinsella, MD, general pediatrician in a small group private practice in Cheshire, Conn., said, “What this report tells me is that COVID cases are more common when mask use is inconsistent in schools and at home and in schools that don’t properly adhere to CDC guidelines. Overall, so long as social distancing guidelines are followed, schools are pretty safe places for kids during this pandemic.”
This finding is important, since many families are keeping their children out of school over fears of contracting the virus, she added. Some of the consequences these children are suffering include a lack of social connection and structure, which in some cases is leading to worsening anxiety and depression, and for those with disabilities, such as those who receive physical therapy, occupational therapy, speech or have IEPs, they’re not getting the full benefit of the services that they would otherwise receive in person, she observed.
“I don’t think families really understand the risks of getting together with family or friends “in their bubble” or the risk of continuing sports participation. This is where the majority of COVID cases are coming from,” she said, adding that it is important to discuss this risk with them at appointments. So, when families ask us what we think of in-person learning, I think we should feel fairly confident that the benefit may outweigh the risk.”
Dr. Hobbs and colleagues, and Dr. Kinsella, had no conflicts of interest to report.
SOURCE: MMWR Morb Mortal Wkly Rep. 2020;69:1925-9. doi: 10.15585/mmwr.mm6950e3.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
IDSA panel updates guidelines on COVID molecular diagnostic tests
Saliva spit tests stack up well against the gold standard for molecular COVID-19 tests – the back-of-the-nose deep swab – without the discomfort and induced coughing or sneezing of the test taker, updated guidelines indicate.
In a press briefing on Jan. 6, the Infectious Diseases Society of America explained the findings of an expert panel that reviewed the literature since the IDSA released its first guidelines in May.
The panel found that saliva tests were especially effective if the test included instructions to cough or clear the throat before spitting into the tube, said panel chair Kimberly E. Hanson, MD, MHS, of University of Utah Health, Salt Lake City.
Throat swab alone less effective
Using a throat swab alone was less effective and missed more cases than the other methods, she said.
The IDSA has updated its recommendation: A saliva test or swabs from either the middle or front of the nose front are preferred to a throat swab alone.
A combination of saliva and swabs from the front and middle of the nose and throat together “looked pretty much equivalent” to the gold-standard deep swab, the panel found.
She acknowledged, however, that multiple swabs exacerbate already challenging supply issues.
Saliva samples do come with challenges, Dr. Hanson noted. A laboratory must validate that its systems can handle the stickier material. And asking a patient to cough necessitates more personal protective equipment for the health care professional.
Each center will have to tailor the specimen type it chooses, based on what resources it has available and the setting – whether in a hospital or a drive-through operation, for instance, she said.
Rapid testing vs. standard
Panel member Angela M. Caliendo, MD, PhD, of Brown University, Providence, R.I., said the panel preferred rapid polymerase chain reaction tests and standard, laboratory-based PCR tests over a rapid isothermal test.
The panel defined rapid tests as those for which results are available within an hour after a test provider has the specimen in hand. They excluded home tests for this category.
The only rapid isothermal test that had enough data on which to issue a recommendation was the ID NOW test (Abbott Labs), she noted.
Rapid PCR tests performed just as well as the standard laboratory-based tests, she said, with a high sensitivity of “97% on average and a very high specificity.”
But the rapid isothermal test had an average sensitivity of only about 80%, compared with the lab-based PCR test, Dr. Caliendo said, yielding a substantial number of false-negative results.
Testing centers will have to weigh the considerable advantages of having results in 15 minutes with a rapid isothermal test and being able to educate positive patients about immediate isolation against the potential for false negatives, which could send positive patients home thinking they don’t have the virus – and thus potentially spreading the disease.
And if a clinician gets a negative result with the rapid isothermal test, but has a strong suspicion the person has COVID or lives in an area with high prevalence, a backup test with a rapid PCR or laboratory-based test should be administered.
“You will miss a certain percentage of people using this rapid isothermal test,” she said.
However, Dr. Caliendo said, if the only available option is the isothermal test, “you should definitely use it because it’s certainly better than not testing at all.”
On a positive note, she said, all the varieties of tests have high specificity, so “you’re not going to see a lot of false-positive results.”
The guidelines back in May didn’t make recommendations on rapid tests, she said, because there weren’t enough data in the literature.
Dr. Caliendo noted that most of the available data were for symptomatic patients, but there are some data that show the amount of virus in the respiratory tract is similar for people with and without symptoms. The panel, therefore, expects that the performance of the various assays would be similar whether or not a person had symptoms.
Testing the immunocompromised
Dr. Hanson said the original recommendation in May was to do molecular testing for asymptomatic people who were awaiting a transplant or were waiting to start immunosuppressive therapy for cancer or an autoimmune disease. Now the current guidelines “make no recommendation for or against screening” in those cases.
Dr. Hanson added that the panel feels that patients awaiting bone marrow and solid organ transplants should have the testing because of the high risks that will result if patients have contracted the virus.
But for those with cancer or an autoimmune disease, the panel decided to leave it up to each physician to assess individual risk and determine whether the patient should be tested.
Home testing
The IDSA guidelines didn’t weigh in on home testing because the products are so new and studies so far have included fewer than 200 patients. But Dr. Caliendo said they clearly perform better earlier in the disease phase – the first 5-7 days – when the amount of the virus is higher.
Dr. Hanson and Dr. Caliendo also fielded a question about what the new virus variant, first discovered in the United Kingdom and now spreading to other countries (including the United States) means for diagnostic testing.
“So far we think with the majority of tests that are [emergency use] authorized, it doesn’t look like this new variant should really affect test performance,” Dr. Hanson said.
The variant has differences in the spike gene, and many of the current tests detect and identify SARS-CoV-2 without the spike gene so they wouldn’t be affected, she added.
Dr. Caliendo agreed: “I think the vast majority of our tests should be in good shape.”
Dr. Hanson and Dr. Caliendo disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Saliva spit tests stack up well against the gold standard for molecular COVID-19 tests – the back-of-the-nose deep swab – without the discomfort and induced coughing or sneezing of the test taker, updated guidelines indicate.
In a press briefing on Jan. 6, the Infectious Diseases Society of America explained the findings of an expert panel that reviewed the literature since the IDSA released its first guidelines in May.
The panel found that saliva tests were especially effective if the test included instructions to cough or clear the throat before spitting into the tube, said panel chair Kimberly E. Hanson, MD, MHS, of University of Utah Health, Salt Lake City.
Throat swab alone less effective
Using a throat swab alone was less effective and missed more cases than the other methods, she said.
The IDSA has updated its recommendation: A saliva test or swabs from either the middle or front of the nose front are preferred to a throat swab alone.
A combination of saliva and swabs from the front and middle of the nose and throat together “looked pretty much equivalent” to the gold-standard deep swab, the panel found.
She acknowledged, however, that multiple swabs exacerbate already challenging supply issues.
Saliva samples do come with challenges, Dr. Hanson noted. A laboratory must validate that its systems can handle the stickier material. And asking a patient to cough necessitates more personal protective equipment for the health care professional.
Each center will have to tailor the specimen type it chooses, based on what resources it has available and the setting – whether in a hospital or a drive-through operation, for instance, she said.
Rapid testing vs. standard
Panel member Angela M. Caliendo, MD, PhD, of Brown University, Providence, R.I., said the panel preferred rapid polymerase chain reaction tests and standard, laboratory-based PCR tests over a rapid isothermal test.
The panel defined rapid tests as those for which results are available within an hour after a test provider has the specimen in hand. They excluded home tests for this category.
The only rapid isothermal test that had enough data on which to issue a recommendation was the ID NOW test (Abbott Labs), she noted.
Rapid PCR tests performed just as well as the standard laboratory-based tests, she said, with a high sensitivity of “97% on average and a very high specificity.”
But the rapid isothermal test had an average sensitivity of only about 80%, compared with the lab-based PCR test, Dr. Caliendo said, yielding a substantial number of false-negative results.
Testing centers will have to weigh the considerable advantages of having results in 15 minutes with a rapid isothermal test and being able to educate positive patients about immediate isolation against the potential for false negatives, which could send positive patients home thinking they don’t have the virus – and thus potentially spreading the disease.
And if a clinician gets a negative result with the rapid isothermal test, but has a strong suspicion the person has COVID or lives in an area with high prevalence, a backup test with a rapid PCR or laboratory-based test should be administered.
“You will miss a certain percentage of people using this rapid isothermal test,” she said.
However, Dr. Caliendo said, if the only available option is the isothermal test, “you should definitely use it because it’s certainly better than not testing at all.”
On a positive note, she said, all the varieties of tests have high specificity, so “you’re not going to see a lot of false-positive results.”
The guidelines back in May didn’t make recommendations on rapid tests, she said, because there weren’t enough data in the literature.
Dr. Caliendo noted that most of the available data were for symptomatic patients, but there are some data that show the amount of virus in the respiratory tract is similar for people with and without symptoms. The panel, therefore, expects that the performance of the various assays would be similar whether or not a person had symptoms.
Testing the immunocompromised
Dr. Hanson said the original recommendation in May was to do molecular testing for asymptomatic people who were awaiting a transplant or were waiting to start immunosuppressive therapy for cancer or an autoimmune disease. Now the current guidelines “make no recommendation for or against screening” in those cases.
Dr. Hanson added that the panel feels that patients awaiting bone marrow and solid organ transplants should have the testing because of the high risks that will result if patients have contracted the virus.
But for those with cancer or an autoimmune disease, the panel decided to leave it up to each physician to assess individual risk and determine whether the patient should be tested.
Home testing
The IDSA guidelines didn’t weigh in on home testing because the products are so new and studies so far have included fewer than 200 patients. But Dr. Caliendo said they clearly perform better earlier in the disease phase – the first 5-7 days – when the amount of the virus is higher.
Dr. Hanson and Dr. Caliendo also fielded a question about what the new virus variant, first discovered in the United Kingdom and now spreading to other countries (including the United States) means for diagnostic testing.
“So far we think with the majority of tests that are [emergency use] authorized, it doesn’t look like this new variant should really affect test performance,” Dr. Hanson said.
The variant has differences in the spike gene, and many of the current tests detect and identify SARS-CoV-2 without the spike gene so they wouldn’t be affected, she added.
Dr. Caliendo agreed: “I think the vast majority of our tests should be in good shape.”
Dr. Hanson and Dr. Caliendo disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Saliva spit tests stack up well against the gold standard for molecular COVID-19 tests – the back-of-the-nose deep swab – without the discomfort and induced coughing or sneezing of the test taker, updated guidelines indicate.
In a press briefing on Jan. 6, the Infectious Diseases Society of America explained the findings of an expert panel that reviewed the literature since the IDSA released its first guidelines in May.
The panel found that saliva tests were especially effective if the test included instructions to cough or clear the throat before spitting into the tube, said panel chair Kimberly E. Hanson, MD, MHS, of University of Utah Health, Salt Lake City.
Throat swab alone less effective
Using a throat swab alone was less effective and missed more cases than the other methods, she said.
The IDSA has updated its recommendation: A saliva test or swabs from either the middle or front of the nose front are preferred to a throat swab alone.
A combination of saliva and swabs from the front and middle of the nose and throat together “looked pretty much equivalent” to the gold-standard deep swab, the panel found.
She acknowledged, however, that multiple swabs exacerbate already challenging supply issues.
Saliva samples do come with challenges, Dr. Hanson noted. A laboratory must validate that its systems can handle the stickier material. And asking a patient to cough necessitates more personal protective equipment for the health care professional.
Each center will have to tailor the specimen type it chooses, based on what resources it has available and the setting – whether in a hospital or a drive-through operation, for instance, she said.
Rapid testing vs. standard
Panel member Angela M. Caliendo, MD, PhD, of Brown University, Providence, R.I., said the panel preferred rapid polymerase chain reaction tests and standard, laboratory-based PCR tests over a rapid isothermal test.
The panel defined rapid tests as those for which results are available within an hour after a test provider has the specimen in hand. They excluded home tests for this category.
The only rapid isothermal test that had enough data on which to issue a recommendation was the ID NOW test (Abbott Labs), she noted.
Rapid PCR tests performed just as well as the standard laboratory-based tests, she said, with a high sensitivity of “97% on average and a very high specificity.”
But the rapid isothermal test had an average sensitivity of only about 80%, compared with the lab-based PCR test, Dr. Caliendo said, yielding a substantial number of false-negative results.
Testing centers will have to weigh the considerable advantages of having results in 15 minutes with a rapid isothermal test and being able to educate positive patients about immediate isolation against the potential for false negatives, which could send positive patients home thinking they don’t have the virus – and thus potentially spreading the disease.
And if a clinician gets a negative result with the rapid isothermal test, but has a strong suspicion the person has COVID or lives in an area with high prevalence, a backup test with a rapid PCR or laboratory-based test should be administered.
“You will miss a certain percentage of people using this rapid isothermal test,” she said.
However, Dr. Caliendo said, if the only available option is the isothermal test, “you should definitely use it because it’s certainly better than not testing at all.”
On a positive note, she said, all the varieties of tests have high specificity, so “you’re not going to see a lot of false-positive results.”
The guidelines back in May didn’t make recommendations on rapid tests, she said, because there weren’t enough data in the literature.
Dr. Caliendo noted that most of the available data were for symptomatic patients, but there are some data that show the amount of virus in the respiratory tract is similar for people with and without symptoms. The panel, therefore, expects that the performance of the various assays would be similar whether or not a person had symptoms.
Testing the immunocompromised
Dr. Hanson said the original recommendation in May was to do molecular testing for asymptomatic people who were awaiting a transplant or were waiting to start immunosuppressive therapy for cancer or an autoimmune disease. Now the current guidelines “make no recommendation for or against screening” in those cases.
Dr. Hanson added that the panel feels that patients awaiting bone marrow and solid organ transplants should have the testing because of the high risks that will result if patients have contracted the virus.
But for those with cancer or an autoimmune disease, the panel decided to leave it up to each physician to assess individual risk and determine whether the patient should be tested.
Home testing
The IDSA guidelines didn’t weigh in on home testing because the products are so new and studies so far have included fewer than 200 patients. But Dr. Caliendo said they clearly perform better earlier in the disease phase – the first 5-7 days – when the amount of the virus is higher.
Dr. Hanson and Dr. Caliendo also fielded a question about what the new virus variant, first discovered in the United Kingdom and now spreading to other countries (including the United States) means for diagnostic testing.
“So far we think with the majority of tests that are [emergency use] authorized, it doesn’t look like this new variant should really affect test performance,” Dr. Hanson said.
The variant has differences in the spike gene, and many of the current tests detect and identify SARS-CoV-2 without the spike gene so they wouldn’t be affected, she added.
Dr. Caliendo agreed: “I think the vast majority of our tests should be in good shape.”
Dr. Hanson and Dr. Caliendo disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.



