User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Bivalent Vaccines Protect Even Children Who’ve Had COVID
This transcript has been edited for clarity.
It was only 3 years ago when we called the pathogen we now refer to as the coronavirus “nCOV-19.” It was, in many ways, more descriptive than what we have today. The little “n” there stood for “novel” — and it was really that little “n” that caused us all the trouble.
You see, coronaviruses themselves were not really new to us. Understudied, perhaps, but with four strains running around the globe at any time giving rise to the common cold, these were viruses our bodies understood.
But Instead of acting like a cold, it acted like nothing we had seen before, at least in our lifetime. The story of the pandemic is very much a bildungsroman of our immune systems — a story of how our immunity grew up.
The difference between the start of 2020 and now, when infections with the coronavirus remain common but not as deadly, can be measured in terms of immune education. Some of our immune systems were educated by infection, some by vaccination, and many by both.
When the first vaccines emerged in December 2020, the opportunity to educate our immune systems was still huge. Though, at the time, an estimated 20 million had been infected in the US and 350,000 had died, there was a large population that remained immunologically naive. I was one of them.
If 2020 into early 2021 was the era of immune education, the postvaccine period was the era of the variant. From one COVID strain to two, to five, to innumerable, our immune memory — trained on a specific version of the virus or its spike protein — became imperfect again. Not naive; these variants were not “novel” in the way COVID-19 was novel, but they were different. And different enough to cause infection.
Following the playbook of another virus that loves to come dressed up in different outfits, the flu virus, we find ourselves in the booster era — a world where yearly doses of a vaccine, ideally matched to the variants circulating when the vaccine is given, are the recommendation if not the norm.
But questions remain about the vaccination program, particularly around who should get it. And two populations with big question marks over their heads are (1) people who have already been infected and (2) kids, because their risk for bad outcomes is so much lower.
This week, we finally have some evidence that can shed light on these questions. The study under the spotlight is this one, appearing in JAMA, which tries to analyze the ability of the bivalent vaccine — that’s the second one to come out, around September 2022 — to protect kids from COVID-19.
Now, right off the bat, this was not a randomized trial. The studies that established the viability of the mRNA vaccine platform were; they happened before the vaccine was authorized. But trials of the bivalent vaccine were mostly limited to proving immune response, not protection from disease.
Nevertheless, with some good observational methods and some statistics, we can try to tease out whether bivalent vaccines in kids worked.
The study combines three prospective cohort studies. The details are in the paper, but what you need to know is that the special sauce of these studies was that the kids were tested for COVID-19 on a weekly basis, whether they had symptoms or not. This is critical because asymptomatic infections can transmit COVID-19.
Let’s do the variables of interest. First and foremost, the bivalent vaccine. Some of these kids got the bivalent vaccine, some didn’t. Other key variables include prior vaccination with the monovalent vaccine. Some had been vaccinated with the monovalent vaccine before, some hadn’t. And, of course, prior infection. Some had been infected before (based on either nasal swabs or blood tests).
Let’s focus first on the primary exposure of interest: getting that bivalent vaccine. Again, this was not randomly assigned; kids who got the bivalent vaccine were different from those who did not. In general, they lived in smaller households, they were more likely to be White, less likely to have had a prior COVID infection, and quite a bit more likely to have at least one chronic condition.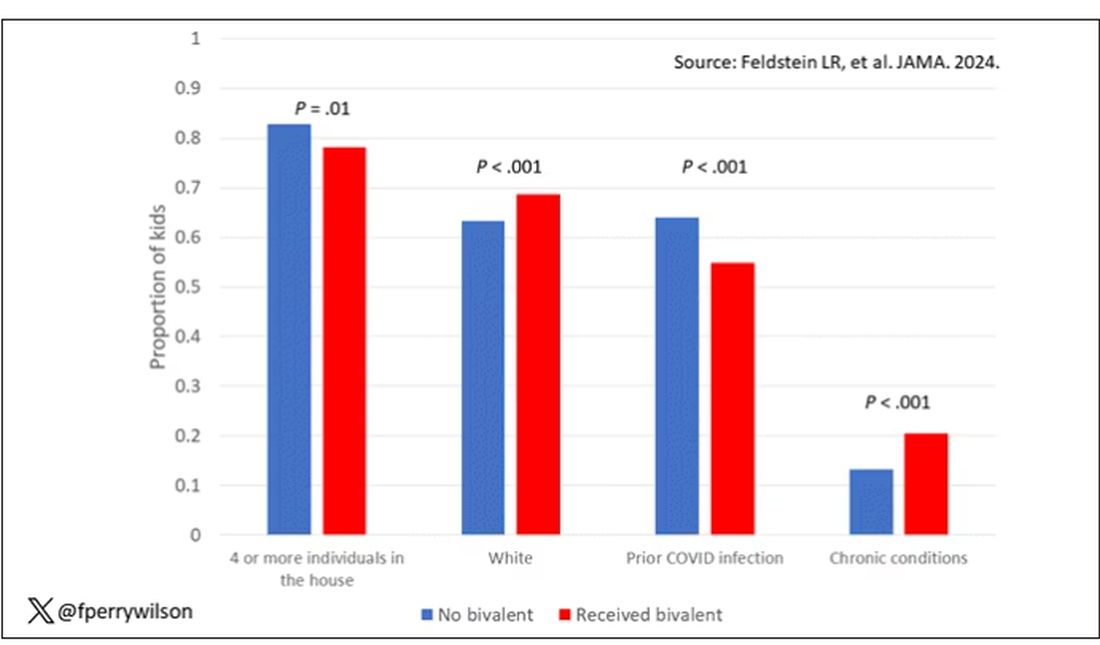
To me, this constellation of factors describes a slightly higher-risk group; it makes sense that they were more likely to get the second vaccine.
Given those factors, what were the rates of COVID infection? After nearly a year of follow-up, around 15% of the kids who hadn’t received the bivalent vaccine got infected compared with 5% of the vaccinated kids. Symptomatic infections represented roughly half of all infections in both groups.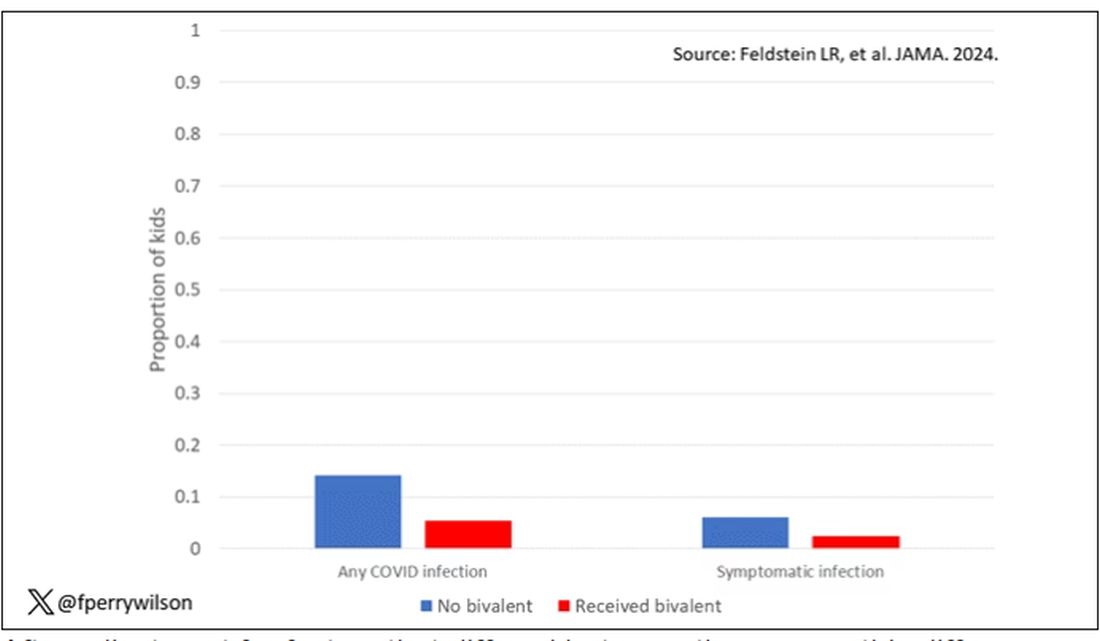
After adjustment for factors that differed between the groups, this difference translated into a vaccine efficacy of about 50% in this population. That’s our first data point. Yes, the bivalent vaccine worked. Not amazingly, of course. But it worked.
What about the kids who had had a prior COVID infection? Somewhat surprisingly, the vaccine was just as effective in this population, despite the fact that their immune systems already had some knowledge of COVID. Ten percent of unvaccinated kids got infected, even though they had been infected before. Just 2.5% of kids who received the bivalent vaccine got infected, suggesting some synergy between prior infection and vaccination.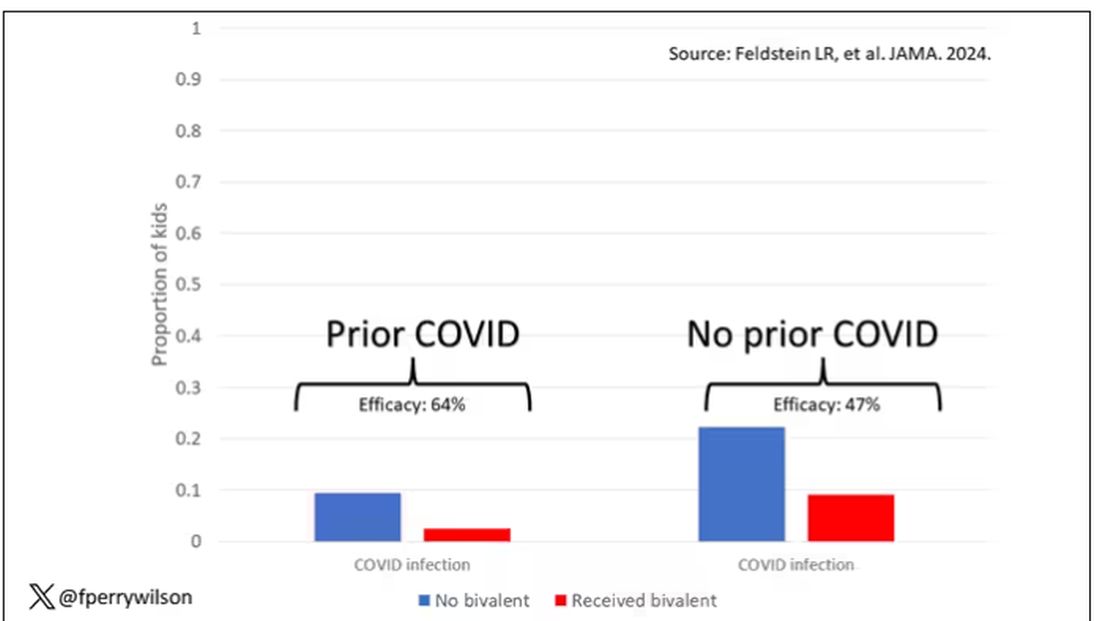
These data suggest that the bivalent vaccine did reduce the risk for COVID infection in kids. All good. But the piece still missing is how severe these infections were. It doesn’t appear that any of the 426 infections documented in this study resulted in hospitalization or death, fortunately. And no data are presented on the incidence of multisystem inflammatory syndrome of children, though given the rarity, I’d be surprised if any of these kids have this either.
So where are we? Well, it seems that the narrative out there that says “the vaccines don’t work” or “the vaccines don’t work if you’ve already been infected” is probably not true. They do work. This study and others in adults show that. If they work to reduce infections, as this study shows, they will also work to reduce deaths. It’s just that death is fortunately so rare in children that the number needed to vaccinate to prevent one death is very large. In that situation, the decision to vaccinate comes down to the risks associated with vaccination. So far, those risk seem very minimal.
Perhaps falling into a flu-like yearly vaccination schedule is not simply the result of old habits dying hard. Maybe it’s actually not a bad idea.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It was only 3 years ago when we called the pathogen we now refer to as the coronavirus “nCOV-19.” It was, in many ways, more descriptive than what we have today. The little “n” there stood for “novel” — and it was really that little “n” that caused us all the trouble.
You see, coronaviruses themselves were not really new to us. Understudied, perhaps, but with four strains running around the globe at any time giving rise to the common cold, these were viruses our bodies understood.
But Instead of acting like a cold, it acted like nothing we had seen before, at least in our lifetime. The story of the pandemic is very much a bildungsroman of our immune systems — a story of how our immunity grew up.
The difference between the start of 2020 and now, when infections with the coronavirus remain common but not as deadly, can be measured in terms of immune education. Some of our immune systems were educated by infection, some by vaccination, and many by both.
When the first vaccines emerged in December 2020, the opportunity to educate our immune systems was still huge. Though, at the time, an estimated 20 million had been infected in the US and 350,000 had died, there was a large population that remained immunologically naive. I was one of them.
If 2020 into early 2021 was the era of immune education, the postvaccine period was the era of the variant. From one COVID strain to two, to five, to innumerable, our immune memory — trained on a specific version of the virus or its spike protein — became imperfect again. Not naive; these variants were not “novel” in the way COVID-19 was novel, but they were different. And different enough to cause infection.
Following the playbook of another virus that loves to come dressed up in different outfits, the flu virus, we find ourselves in the booster era — a world where yearly doses of a vaccine, ideally matched to the variants circulating when the vaccine is given, are the recommendation if not the norm.
But questions remain about the vaccination program, particularly around who should get it. And two populations with big question marks over their heads are (1) people who have already been infected and (2) kids, because their risk for bad outcomes is so much lower.
This week, we finally have some evidence that can shed light on these questions. The study under the spotlight is this one, appearing in JAMA, which tries to analyze the ability of the bivalent vaccine — that’s the second one to come out, around September 2022 — to protect kids from COVID-19.
Now, right off the bat, this was not a randomized trial. The studies that established the viability of the mRNA vaccine platform were; they happened before the vaccine was authorized. But trials of the bivalent vaccine were mostly limited to proving immune response, not protection from disease.
Nevertheless, with some good observational methods and some statistics, we can try to tease out whether bivalent vaccines in kids worked.
The study combines three prospective cohort studies. The details are in the paper, but what you need to know is that the special sauce of these studies was that the kids were tested for COVID-19 on a weekly basis, whether they had symptoms or not. This is critical because asymptomatic infections can transmit COVID-19.
Let’s do the variables of interest. First and foremost, the bivalent vaccine. Some of these kids got the bivalent vaccine, some didn’t. Other key variables include prior vaccination with the monovalent vaccine. Some had been vaccinated with the monovalent vaccine before, some hadn’t. And, of course, prior infection. Some had been infected before (based on either nasal swabs or blood tests).
Let’s focus first on the primary exposure of interest: getting that bivalent vaccine. Again, this was not randomly assigned; kids who got the bivalent vaccine were different from those who did not. In general, they lived in smaller households, they were more likely to be White, less likely to have had a prior COVID infection, and quite a bit more likely to have at least one chronic condition.
To me, this constellation of factors describes a slightly higher-risk group; it makes sense that they were more likely to get the second vaccine.
Given those factors, what were the rates of COVID infection? After nearly a year of follow-up, around 15% of the kids who hadn’t received the bivalent vaccine got infected compared with 5% of the vaccinated kids. Symptomatic infections represented roughly half of all infections in both groups.
After adjustment for factors that differed between the groups, this difference translated into a vaccine efficacy of about 50% in this population. That’s our first data point. Yes, the bivalent vaccine worked. Not amazingly, of course. But it worked.
What about the kids who had had a prior COVID infection? Somewhat surprisingly, the vaccine was just as effective in this population, despite the fact that their immune systems already had some knowledge of COVID. Ten percent of unvaccinated kids got infected, even though they had been infected before. Just 2.5% of kids who received the bivalent vaccine got infected, suggesting some synergy between prior infection and vaccination.
These data suggest that the bivalent vaccine did reduce the risk for COVID infection in kids. All good. But the piece still missing is how severe these infections were. It doesn’t appear that any of the 426 infections documented in this study resulted in hospitalization or death, fortunately. And no data are presented on the incidence of multisystem inflammatory syndrome of children, though given the rarity, I’d be surprised if any of these kids have this either.
So where are we? Well, it seems that the narrative out there that says “the vaccines don’t work” or “the vaccines don’t work if you’ve already been infected” is probably not true. They do work. This study and others in adults show that. If they work to reduce infections, as this study shows, they will also work to reduce deaths. It’s just that death is fortunately so rare in children that the number needed to vaccinate to prevent one death is very large. In that situation, the decision to vaccinate comes down to the risks associated with vaccination. So far, those risk seem very minimal.
Perhaps falling into a flu-like yearly vaccination schedule is not simply the result of old habits dying hard. Maybe it’s actually not a bad idea.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It was only 3 years ago when we called the pathogen we now refer to as the coronavirus “nCOV-19.” It was, in many ways, more descriptive than what we have today. The little “n” there stood for “novel” — and it was really that little “n” that caused us all the trouble.
You see, coronaviruses themselves were not really new to us. Understudied, perhaps, but with four strains running around the globe at any time giving rise to the common cold, these were viruses our bodies understood.
But Instead of acting like a cold, it acted like nothing we had seen before, at least in our lifetime. The story of the pandemic is very much a bildungsroman of our immune systems — a story of how our immunity grew up.
The difference between the start of 2020 and now, when infections with the coronavirus remain common but not as deadly, can be measured in terms of immune education. Some of our immune systems were educated by infection, some by vaccination, and many by both.
When the first vaccines emerged in December 2020, the opportunity to educate our immune systems was still huge. Though, at the time, an estimated 20 million had been infected in the US and 350,000 had died, there was a large population that remained immunologically naive. I was one of them.
If 2020 into early 2021 was the era of immune education, the postvaccine period was the era of the variant. From one COVID strain to two, to five, to innumerable, our immune memory — trained on a specific version of the virus or its spike protein — became imperfect again. Not naive; these variants were not “novel” in the way COVID-19 was novel, but they were different. And different enough to cause infection.
Following the playbook of another virus that loves to come dressed up in different outfits, the flu virus, we find ourselves in the booster era — a world where yearly doses of a vaccine, ideally matched to the variants circulating when the vaccine is given, are the recommendation if not the norm.
But questions remain about the vaccination program, particularly around who should get it. And two populations with big question marks over their heads are (1) people who have already been infected and (2) kids, because their risk for bad outcomes is so much lower.
This week, we finally have some evidence that can shed light on these questions. The study under the spotlight is this one, appearing in JAMA, which tries to analyze the ability of the bivalent vaccine — that’s the second one to come out, around September 2022 — to protect kids from COVID-19.
Now, right off the bat, this was not a randomized trial. The studies that established the viability of the mRNA vaccine platform were; they happened before the vaccine was authorized. But trials of the bivalent vaccine were mostly limited to proving immune response, not protection from disease.
Nevertheless, with some good observational methods and some statistics, we can try to tease out whether bivalent vaccines in kids worked.
The study combines three prospective cohort studies. The details are in the paper, but what you need to know is that the special sauce of these studies was that the kids were tested for COVID-19 on a weekly basis, whether they had symptoms or not. This is critical because asymptomatic infections can transmit COVID-19.
Let’s do the variables of interest. First and foremost, the bivalent vaccine. Some of these kids got the bivalent vaccine, some didn’t. Other key variables include prior vaccination with the monovalent vaccine. Some had been vaccinated with the monovalent vaccine before, some hadn’t. And, of course, prior infection. Some had been infected before (based on either nasal swabs or blood tests).
Let’s focus first on the primary exposure of interest: getting that bivalent vaccine. Again, this was not randomly assigned; kids who got the bivalent vaccine were different from those who did not. In general, they lived in smaller households, they were more likely to be White, less likely to have had a prior COVID infection, and quite a bit more likely to have at least one chronic condition.
To me, this constellation of factors describes a slightly higher-risk group; it makes sense that they were more likely to get the second vaccine.
Given those factors, what were the rates of COVID infection? After nearly a year of follow-up, around 15% of the kids who hadn’t received the bivalent vaccine got infected compared with 5% of the vaccinated kids. Symptomatic infections represented roughly half of all infections in both groups.
After adjustment for factors that differed between the groups, this difference translated into a vaccine efficacy of about 50% in this population. That’s our first data point. Yes, the bivalent vaccine worked. Not amazingly, of course. But it worked.
What about the kids who had had a prior COVID infection? Somewhat surprisingly, the vaccine was just as effective in this population, despite the fact that their immune systems already had some knowledge of COVID. Ten percent of unvaccinated kids got infected, even though they had been infected before. Just 2.5% of kids who received the bivalent vaccine got infected, suggesting some synergy between prior infection and vaccination.
These data suggest that the bivalent vaccine did reduce the risk for COVID infection in kids. All good. But the piece still missing is how severe these infections were. It doesn’t appear that any of the 426 infections documented in this study resulted in hospitalization or death, fortunately. And no data are presented on the incidence of multisystem inflammatory syndrome of children, though given the rarity, I’d be surprised if any of these kids have this either.
So where are we? Well, it seems that the narrative out there that says “the vaccines don’t work” or “the vaccines don’t work if you’ve already been infected” is probably not true. They do work. This study and others in adults show that. If they work to reduce infections, as this study shows, they will also work to reduce deaths. It’s just that death is fortunately so rare in children that the number needed to vaccinate to prevent one death is very large. In that situation, the decision to vaccinate comes down to the risks associated with vaccination. So far, those risk seem very minimal.
Perhaps falling into a flu-like yearly vaccination schedule is not simply the result of old habits dying hard. Maybe it’s actually not a bad idea.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
How the New MRSA Antibiotic Cracked AI’s ‘Black Box’
“New antibiotics discovered using AI!”
That’s how headlines read in December 2023, when MIT researchers announced a new class of antibiotics that could wipe out the drug-resistant superbug methicillin-resistant Staphylococcus aureus (MRSA) in mice.
Powered by deep learning, the study was a significant breakthrough. Few new antibiotics have come out since the 1960s, and this one in particular could be crucial in fighting tough-to-treat MRSA, which kills more than 10,000 people annually in the United States.
But as remarkable as the antibiotic discovery was, it may not be the most impactful part of this study.
“Of course, we view the antibiotic-discovery angle to be very important,” said Felix Wong, PhD, a colead author of the study and postdoctoral fellow at the Broad Institute of MIT and Harvard, Cambridge, Massachusetts. “But I think equally important, or maybe even more important, is really our method of opening up the black box.”
The black box is generally thought of as impenetrable in complex machine learning models, and that poses a challenge in the drug discovery realm.
“A major bottleneck in AI-ML-driven drug discovery is that nobody knows what the heck is going on,” said Dr. Wong. Models have such powerful architectures that their decision-making is mysterious.
Researchers input data, such as patient features, and the model says what drugs might be effective. But researchers have no idea how the model arrived at its predictions — until now.
What the Researchers Did
Dr. Wong and his colleagues first mined 39,000 compounds for antibiotic activity against MRSA. They fed information about the compounds’ chemical structures and antibiotic activity into their machine learning model. With this, they “trained” the model to predict whether a compound is antibacterial.
Next, they used additional deep learning to narrow the field, ruling out compounds toxic to humans. Then, deploying their various models at once, they screened 12 million commercially available compounds. Five classes emerged as likely MRSA fighters. Further testing of 280 compounds from the five classes produced the final results: Two compounds from the same class. Both reduced MRSA infection in mouse models.
How did the computer flag these compounds? The researchers sought to answer that question by figuring out which chemical structures the model had been looking for.
A chemical structure can be “pruned” — that is, scientists can remove certain atoms and bonds to reveal an underlying substructure. The MIT researchers used the Monte Carlo Tree Search, a commonly used algorithm in machine learning, to select which atoms and bonds to edit out. Then they fed the pruned substructures into their model to find out which was likely responsible for the antibacterial activity.
“The main idea is we can pinpoint which substructure of a chemical structure is causative instead of just correlated with high antibiotic activity,” Dr. Wong said.
This could fuel new “design-driven” or generative AI approaches where these substructures become “starting points to design entirely unseen, unprecedented antibiotics,” Dr. Wong said. “That’s one of the key efforts that we’ve been working on since the publication of this paper.”
More broadly, their method could lead to discoveries in drug classes beyond antibiotics, such as antivirals and anticancer drugs, according to Dr. Wong.
“This is the first major study that I’ve seen seeking to incorporate explainability into deep learning models in the context of antibiotics,” said César de la Fuente, PhD, an assistant professor at the University of Pennsylvania, Philadelphia, Pennsylvania, whose lab has been engaged in AI for antibiotic discovery for the past 5 years.
“It’s kind of like going into the black box with a magnifying lens and figuring out what is actually happening in there,” Dr. de la Fuente said. “And that will open up possibilities for leveraging those different steps to make better drugs.”
How Explainable AI Could Revolutionize Medicine
In studies, explainable AI is showing its potential for informing clinical decisions as well — flagging high-risk patients and letting doctors know why that calculation was made. University of Washington researchers have used the technology to predict whether a patient will have hypoxemia during surgery, revealing which features contributed to the prediction, such as blood pressure or body mass index. Another study used explainable AI to help emergency medical services providers and emergency room clinicians optimize time — for example, by identifying trauma patients at high risk for acute traumatic coagulopathy more quickly.
A crucial benefit of explainable AI is its ability to audit machine learning models for mistakes, said Su-In Lee, PhD, a computer scientist who led the UW research.
For example, a surge of research during the pandemic suggested that AI models could predict COVID-19 infection based on chest x-rays. Dr. Lee’s research used explainable AI to show that many of the studies were not as accurate as they claimed. Her lab revealed that many models› decisions were based not on pathologies but rather on other aspects such as laterality markers in the corners of x-rays or medical devices worn by patients (like pacemakers). She applied the same model auditing technique to AI-powered dermatology devices, digging into the flawed reasoning in their melanoma predictions.
Explainable AI is beginning to affect drug development too. A 2023 study led by Dr. Lee used it to explain how to select complementary drugs for acute myeloid leukemia patients based on the differentiation levels of cancer cells. And in two other studies aimed at identifying Alzheimer’s therapeutic targets, “explainable AI played a key role in terms of identifying the driver pathway,” she said.
Currently, the US Food and Drug Administration (FDA) approval doesn’t require an understanding of a drug’s mechanism of action. But the issue is being raised more often, including at December’s Health Regulatory Policy Conference at MIT’s Jameel Clinic. And just over a year ago, Dr. Lee predicted that the FDA approval process would come to incorporate explainable AI analysis.
“I didn’t hesitate,” Dr. Lee said, regarding her prediction. “We didn’t see this in 2023, so I won’t assert that I was right, but I can confidently say that we are progressing in that direction.”
What’s Next?
The MIT study is part of the Antibiotics-AI project, a 7-year effort to leverage AI to find new antibiotics. Phare Bio, a nonprofit started by MIT professor James Collins, PhD, and others, will do clinical testing on the antibiotic candidates.
Even with the AI’s assistance, there’s still a long way to go before clinical approval.
But knowing which elements contribute to a candidate’s effectiveness against MRSA could help the researchers formulate scientific hypotheses and design better validation, Dr. Lee noted. In other words, because they used explainable AI, they could be better positioned for clinical trial success.
A version of this article appeared on Medscape.com.
“New antibiotics discovered using AI!”
That’s how headlines read in December 2023, when MIT researchers announced a new class of antibiotics that could wipe out the drug-resistant superbug methicillin-resistant Staphylococcus aureus (MRSA) in mice.
Powered by deep learning, the study was a significant breakthrough. Few new antibiotics have come out since the 1960s, and this one in particular could be crucial in fighting tough-to-treat MRSA, which kills more than 10,000 people annually in the United States.
But as remarkable as the antibiotic discovery was, it may not be the most impactful part of this study.
“Of course, we view the antibiotic-discovery angle to be very important,” said Felix Wong, PhD, a colead author of the study and postdoctoral fellow at the Broad Institute of MIT and Harvard, Cambridge, Massachusetts. “But I think equally important, or maybe even more important, is really our method of opening up the black box.”
The black box is generally thought of as impenetrable in complex machine learning models, and that poses a challenge in the drug discovery realm.
“A major bottleneck in AI-ML-driven drug discovery is that nobody knows what the heck is going on,” said Dr. Wong. Models have such powerful architectures that their decision-making is mysterious.
Researchers input data, such as patient features, and the model says what drugs might be effective. But researchers have no idea how the model arrived at its predictions — until now.
What the Researchers Did
Dr. Wong and his colleagues first mined 39,000 compounds for antibiotic activity against MRSA. They fed information about the compounds’ chemical structures and antibiotic activity into their machine learning model. With this, they “trained” the model to predict whether a compound is antibacterial.
Next, they used additional deep learning to narrow the field, ruling out compounds toxic to humans. Then, deploying their various models at once, they screened 12 million commercially available compounds. Five classes emerged as likely MRSA fighters. Further testing of 280 compounds from the five classes produced the final results: Two compounds from the same class. Both reduced MRSA infection in mouse models.
How did the computer flag these compounds? The researchers sought to answer that question by figuring out which chemical structures the model had been looking for.
A chemical structure can be “pruned” — that is, scientists can remove certain atoms and bonds to reveal an underlying substructure. The MIT researchers used the Monte Carlo Tree Search, a commonly used algorithm in machine learning, to select which atoms and bonds to edit out. Then they fed the pruned substructures into their model to find out which was likely responsible for the antibacterial activity.
“The main idea is we can pinpoint which substructure of a chemical structure is causative instead of just correlated with high antibiotic activity,” Dr. Wong said.
This could fuel new “design-driven” or generative AI approaches where these substructures become “starting points to design entirely unseen, unprecedented antibiotics,” Dr. Wong said. “That’s one of the key efforts that we’ve been working on since the publication of this paper.”
More broadly, their method could lead to discoveries in drug classes beyond antibiotics, such as antivirals and anticancer drugs, according to Dr. Wong.
“This is the first major study that I’ve seen seeking to incorporate explainability into deep learning models in the context of antibiotics,” said César de la Fuente, PhD, an assistant professor at the University of Pennsylvania, Philadelphia, Pennsylvania, whose lab has been engaged in AI for antibiotic discovery for the past 5 years.
“It’s kind of like going into the black box with a magnifying lens and figuring out what is actually happening in there,” Dr. de la Fuente said. “And that will open up possibilities for leveraging those different steps to make better drugs.”
How Explainable AI Could Revolutionize Medicine
In studies, explainable AI is showing its potential for informing clinical decisions as well — flagging high-risk patients and letting doctors know why that calculation was made. University of Washington researchers have used the technology to predict whether a patient will have hypoxemia during surgery, revealing which features contributed to the prediction, such as blood pressure or body mass index. Another study used explainable AI to help emergency medical services providers and emergency room clinicians optimize time — for example, by identifying trauma patients at high risk for acute traumatic coagulopathy more quickly.
A crucial benefit of explainable AI is its ability to audit machine learning models for mistakes, said Su-In Lee, PhD, a computer scientist who led the UW research.
For example, a surge of research during the pandemic suggested that AI models could predict COVID-19 infection based on chest x-rays. Dr. Lee’s research used explainable AI to show that many of the studies were not as accurate as they claimed. Her lab revealed that many models› decisions were based not on pathologies but rather on other aspects such as laterality markers in the corners of x-rays or medical devices worn by patients (like pacemakers). She applied the same model auditing technique to AI-powered dermatology devices, digging into the flawed reasoning in their melanoma predictions.
Explainable AI is beginning to affect drug development too. A 2023 study led by Dr. Lee used it to explain how to select complementary drugs for acute myeloid leukemia patients based on the differentiation levels of cancer cells. And in two other studies aimed at identifying Alzheimer’s therapeutic targets, “explainable AI played a key role in terms of identifying the driver pathway,” she said.
Currently, the US Food and Drug Administration (FDA) approval doesn’t require an understanding of a drug’s mechanism of action. But the issue is being raised more often, including at December’s Health Regulatory Policy Conference at MIT’s Jameel Clinic. And just over a year ago, Dr. Lee predicted that the FDA approval process would come to incorporate explainable AI analysis.
“I didn’t hesitate,” Dr. Lee said, regarding her prediction. “We didn’t see this in 2023, so I won’t assert that I was right, but I can confidently say that we are progressing in that direction.”
What’s Next?
The MIT study is part of the Antibiotics-AI project, a 7-year effort to leverage AI to find new antibiotics. Phare Bio, a nonprofit started by MIT professor James Collins, PhD, and others, will do clinical testing on the antibiotic candidates.
Even with the AI’s assistance, there’s still a long way to go before clinical approval.
But knowing which elements contribute to a candidate’s effectiveness against MRSA could help the researchers formulate scientific hypotheses and design better validation, Dr. Lee noted. In other words, because they used explainable AI, they could be better positioned for clinical trial success.
A version of this article appeared on Medscape.com.
“New antibiotics discovered using AI!”
That’s how headlines read in December 2023, when MIT researchers announced a new class of antibiotics that could wipe out the drug-resistant superbug methicillin-resistant Staphylococcus aureus (MRSA) in mice.
Powered by deep learning, the study was a significant breakthrough. Few new antibiotics have come out since the 1960s, and this one in particular could be crucial in fighting tough-to-treat MRSA, which kills more than 10,000 people annually in the United States.
But as remarkable as the antibiotic discovery was, it may not be the most impactful part of this study.
“Of course, we view the antibiotic-discovery angle to be very important,” said Felix Wong, PhD, a colead author of the study and postdoctoral fellow at the Broad Institute of MIT and Harvard, Cambridge, Massachusetts. “But I think equally important, or maybe even more important, is really our method of opening up the black box.”
The black box is generally thought of as impenetrable in complex machine learning models, and that poses a challenge in the drug discovery realm.
“A major bottleneck in AI-ML-driven drug discovery is that nobody knows what the heck is going on,” said Dr. Wong. Models have such powerful architectures that their decision-making is mysterious.
Researchers input data, such as patient features, and the model says what drugs might be effective. But researchers have no idea how the model arrived at its predictions — until now.
What the Researchers Did
Dr. Wong and his colleagues first mined 39,000 compounds for antibiotic activity against MRSA. They fed information about the compounds’ chemical structures and antibiotic activity into their machine learning model. With this, they “trained” the model to predict whether a compound is antibacterial.
Next, they used additional deep learning to narrow the field, ruling out compounds toxic to humans. Then, deploying their various models at once, they screened 12 million commercially available compounds. Five classes emerged as likely MRSA fighters. Further testing of 280 compounds from the five classes produced the final results: Two compounds from the same class. Both reduced MRSA infection in mouse models.
How did the computer flag these compounds? The researchers sought to answer that question by figuring out which chemical structures the model had been looking for.
A chemical structure can be “pruned” — that is, scientists can remove certain atoms and bonds to reveal an underlying substructure. The MIT researchers used the Monte Carlo Tree Search, a commonly used algorithm in machine learning, to select which atoms and bonds to edit out. Then they fed the pruned substructures into their model to find out which was likely responsible for the antibacterial activity.
“The main idea is we can pinpoint which substructure of a chemical structure is causative instead of just correlated with high antibiotic activity,” Dr. Wong said.
This could fuel new “design-driven” or generative AI approaches where these substructures become “starting points to design entirely unseen, unprecedented antibiotics,” Dr. Wong said. “That’s one of the key efforts that we’ve been working on since the publication of this paper.”
More broadly, their method could lead to discoveries in drug classes beyond antibiotics, such as antivirals and anticancer drugs, according to Dr. Wong.
“This is the first major study that I’ve seen seeking to incorporate explainability into deep learning models in the context of antibiotics,” said César de la Fuente, PhD, an assistant professor at the University of Pennsylvania, Philadelphia, Pennsylvania, whose lab has been engaged in AI for antibiotic discovery for the past 5 years.
“It’s kind of like going into the black box with a magnifying lens and figuring out what is actually happening in there,” Dr. de la Fuente said. “And that will open up possibilities for leveraging those different steps to make better drugs.”
How Explainable AI Could Revolutionize Medicine
In studies, explainable AI is showing its potential for informing clinical decisions as well — flagging high-risk patients and letting doctors know why that calculation was made. University of Washington researchers have used the technology to predict whether a patient will have hypoxemia during surgery, revealing which features contributed to the prediction, such as blood pressure or body mass index. Another study used explainable AI to help emergency medical services providers and emergency room clinicians optimize time — for example, by identifying trauma patients at high risk for acute traumatic coagulopathy more quickly.
A crucial benefit of explainable AI is its ability to audit machine learning models for mistakes, said Su-In Lee, PhD, a computer scientist who led the UW research.
For example, a surge of research during the pandemic suggested that AI models could predict COVID-19 infection based on chest x-rays. Dr. Lee’s research used explainable AI to show that many of the studies were not as accurate as they claimed. Her lab revealed that many models› decisions were based not on pathologies but rather on other aspects such as laterality markers in the corners of x-rays or medical devices worn by patients (like pacemakers). She applied the same model auditing technique to AI-powered dermatology devices, digging into the flawed reasoning in their melanoma predictions.
Explainable AI is beginning to affect drug development too. A 2023 study led by Dr. Lee used it to explain how to select complementary drugs for acute myeloid leukemia patients based on the differentiation levels of cancer cells. And in two other studies aimed at identifying Alzheimer’s therapeutic targets, “explainable AI played a key role in terms of identifying the driver pathway,” she said.
Currently, the US Food and Drug Administration (FDA) approval doesn’t require an understanding of a drug’s mechanism of action. But the issue is being raised more often, including at December’s Health Regulatory Policy Conference at MIT’s Jameel Clinic. And just over a year ago, Dr. Lee predicted that the FDA approval process would come to incorporate explainable AI analysis.
“I didn’t hesitate,” Dr. Lee said, regarding her prediction. “We didn’t see this in 2023, so I won’t assert that I was right, but I can confidently say that we are progressing in that direction.”
What’s Next?
The MIT study is part of the Antibiotics-AI project, a 7-year effort to leverage AI to find new antibiotics. Phare Bio, a nonprofit started by MIT professor James Collins, PhD, and others, will do clinical testing on the antibiotic candidates.
Even with the AI’s assistance, there’s still a long way to go before clinical approval.
But knowing which elements contribute to a candidate’s effectiveness against MRSA could help the researchers formulate scientific hypotheses and design better validation, Dr. Lee noted. In other words, because they used explainable AI, they could be better positioned for clinical trial success.
A version of this article appeared on Medscape.com.
SARS-CoV-2 a Possible Trigger for Achalasia
TOPLINE:
METHODOLOGY:
- The etiology of achalasia is unclear. Studies have suggested an immune reaction to viral infections, including SARS-CoV-2, as a potential cause.
- Researchers studied four adults who developed achalasia within 5 months of SARS-CoV-2 infection (group 1), six with longstanding achalasia predating SARS-CoV-2 infection (group 2), and two with longstanding achalasia with no known SARS-CoV-2 infection (group 3).
- They tested for the presence of SARS-CoV-2 nucleocapsid (N) and spike (S) proteins, as well as inflammatory markers, in esophageal muscle tissue isolated from the participants.
TAKEAWAY:
- Group 1 patients (confirmed or suspected post–COVID-19 achalasia) had the highest levels of the N protein in all four cases and higher levels of the S protein in the two confirmed cases. No N or S protein was detected in group 3.
- The presence of mRNA for SARS-CoV-2 N protein correlated with a significant increase in the inflammatory markers of NOD-like receptor family pyrin domain-containing 3 and tumor necrosis factor. There were no differences in interleukin 18 in groups 1 and 2.
- The S protein was detected in all muscle tissue samples from group 1. It was also detected in some (but not all) samples from group 2 and to a much lesser degree. The presence of S protein was irrespective of the SARS-CoV-2 vaccination status.
IN PRACTICE:
“Our findings not only show the continued presence of SARS-CoV-2 proteins in esophageal muscle tissue isolated from subjects with achalasia post infection, but they further correlate this with the presence of a sustained inflammatory response,” the authors wrote.
SOURCE:
The study, with first author Salih Samo, MD, MS, Division of Gastroenterology, Hepatology, and Motility, University of Kansas School of Medicine, Kansas City, Kansas, was published online on January 24, 2024, in the American Journal of Gastroenterology.
LIMITATIONS:
The sample size was small, and it was not known which SARS-CoV-2 variant each patient had. The study cannot definitively confirm that SARS-CoV-2 is causative for achalasia.
DISCLOSURES:
The study had no specific funding. Samo reported relationships with Castle Biosciences, Sanofi, Evoke, and EndoGastric Solutions.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The etiology of achalasia is unclear. Studies have suggested an immune reaction to viral infections, including SARS-CoV-2, as a potential cause.
- Researchers studied four adults who developed achalasia within 5 months of SARS-CoV-2 infection (group 1), six with longstanding achalasia predating SARS-CoV-2 infection (group 2), and two with longstanding achalasia with no known SARS-CoV-2 infection (group 3).
- They tested for the presence of SARS-CoV-2 nucleocapsid (N) and spike (S) proteins, as well as inflammatory markers, in esophageal muscle tissue isolated from the participants.
TAKEAWAY:
- Group 1 patients (confirmed or suspected post–COVID-19 achalasia) had the highest levels of the N protein in all four cases and higher levels of the S protein in the two confirmed cases. No N or S protein was detected in group 3.
- The presence of mRNA for SARS-CoV-2 N protein correlated with a significant increase in the inflammatory markers of NOD-like receptor family pyrin domain-containing 3 and tumor necrosis factor. There were no differences in interleukin 18 in groups 1 and 2.
- The S protein was detected in all muscle tissue samples from group 1. It was also detected in some (but not all) samples from group 2 and to a much lesser degree. The presence of S protein was irrespective of the SARS-CoV-2 vaccination status.
IN PRACTICE:
“Our findings not only show the continued presence of SARS-CoV-2 proteins in esophageal muscle tissue isolated from subjects with achalasia post infection, but they further correlate this with the presence of a sustained inflammatory response,” the authors wrote.
SOURCE:
The study, with first author Salih Samo, MD, MS, Division of Gastroenterology, Hepatology, and Motility, University of Kansas School of Medicine, Kansas City, Kansas, was published online on January 24, 2024, in the American Journal of Gastroenterology.
LIMITATIONS:
The sample size was small, and it was not known which SARS-CoV-2 variant each patient had. The study cannot definitively confirm that SARS-CoV-2 is causative for achalasia.
DISCLOSURES:
The study had no specific funding. Samo reported relationships with Castle Biosciences, Sanofi, Evoke, and EndoGastric Solutions.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The etiology of achalasia is unclear. Studies have suggested an immune reaction to viral infections, including SARS-CoV-2, as a potential cause.
- Researchers studied four adults who developed achalasia within 5 months of SARS-CoV-2 infection (group 1), six with longstanding achalasia predating SARS-CoV-2 infection (group 2), and two with longstanding achalasia with no known SARS-CoV-2 infection (group 3).
- They tested for the presence of SARS-CoV-2 nucleocapsid (N) and spike (S) proteins, as well as inflammatory markers, in esophageal muscle tissue isolated from the participants.
TAKEAWAY:
- Group 1 patients (confirmed or suspected post–COVID-19 achalasia) had the highest levels of the N protein in all four cases and higher levels of the S protein in the two confirmed cases. No N or S protein was detected in group 3.
- The presence of mRNA for SARS-CoV-2 N protein correlated with a significant increase in the inflammatory markers of NOD-like receptor family pyrin domain-containing 3 and tumor necrosis factor. There were no differences in interleukin 18 in groups 1 and 2.
- The S protein was detected in all muscle tissue samples from group 1. It was also detected in some (but not all) samples from group 2 and to a much lesser degree. The presence of S protein was irrespective of the SARS-CoV-2 vaccination status.
IN PRACTICE:
“Our findings not only show the continued presence of SARS-CoV-2 proteins in esophageal muscle tissue isolated from subjects with achalasia post infection, but they further correlate this with the presence of a sustained inflammatory response,” the authors wrote.
SOURCE:
The study, with first author Salih Samo, MD, MS, Division of Gastroenterology, Hepatology, and Motility, University of Kansas School of Medicine, Kansas City, Kansas, was published online on January 24, 2024, in the American Journal of Gastroenterology.
LIMITATIONS:
The sample size was small, and it was not known which SARS-CoV-2 variant each patient had. The study cannot definitively confirm that SARS-CoV-2 is causative for achalasia.
DISCLOSURES:
The study had no specific funding. Samo reported relationships with Castle Biosciences, Sanofi, Evoke, and EndoGastric Solutions.
A version of this article appeared on Medscape.com.
Switching From IV to Oral Antibiotics Safe for Patients, Study Shows
TOPLINE:
, according to a recent observational study published in JAMA Network Open.
METHODOLOGY:
- Patients receiving antibiotics through an IV line risk developing a secondary infection; antibiotics received orally are considered safer.
- Researchers analyzed observational data from 914 adults with uncomplicated gram-negative bacteremia who received care in four hospitals in Denmark between 2018 and 2021.
- The outcomes of patients who were switched to oral antibiotics within 4 days after a positive blood culture were compared with those who continued to receive IV antibiotics for at least 5 days after the blood culture; participants in both groups received antibiotics for 7-14 days.
- Researchers assessed mortality rates over a 90-day window and used a target trial emulation method to conduct the study.
TAKEAWAY:
- Overall, 14.3% of patients who received prolonged IV treatment died, compared with 6.9% in the oral antibiotics group.
- In an intention-to-treat analysis, patients who were switched to oral antibiotics had a 22% lower risk for death within 90 days of initiation of treatment (relative risk [RR], 0.78; 95% CI, 0.60-1.10).
- In a per-protocol analysis, patients who switched to the oral route had a 1% lower odds of dying within 90 days (RR, 0.99; 95% CI, 0.70-1.40).
- Individuals who were switched to oral antibiotic treatment were younger than those who continued to receive antibiotics via the IV route (median age, 73 vs 76 years, respectively), had fewer comorbidities (four vs five), and were more likely to have community-acquired gram-negative bacteremia (89.4% vs 80.9%).
IN PRACTICE:
“These findings suggest that the mortality associated with early antibiotic stepdown treatment is comparable to that associated with receiving prolonged IV antibiotic treatment for individuals with uncomplicated gram-negative bacteremia,” the authors of the study wrote.
SOURCE:
The study was led by Sandra Tingsgård, MD, of the Center of Research & Department of Infectious Diseases at Copenhagen University Hospital–Amager and Hvidovre in Denmark.
LIMITATIONS:
The study was based on data from electronic health records, so some factors may not have been recorded or considered. The researchers identified few cases of multidrug-resistant infections, and the findings may not apply to those cases. Complicated cases and people who were not stabilized by day 4 were excluded from the analysis.
DISCLOSURES:
The authors report no disclosures or sources of funding.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a recent observational study published in JAMA Network Open.
METHODOLOGY:
- Patients receiving antibiotics through an IV line risk developing a secondary infection; antibiotics received orally are considered safer.
- Researchers analyzed observational data from 914 adults with uncomplicated gram-negative bacteremia who received care in four hospitals in Denmark between 2018 and 2021.
- The outcomes of patients who were switched to oral antibiotics within 4 days after a positive blood culture were compared with those who continued to receive IV antibiotics for at least 5 days after the blood culture; participants in both groups received antibiotics for 7-14 days.
- Researchers assessed mortality rates over a 90-day window and used a target trial emulation method to conduct the study.
TAKEAWAY:
- Overall, 14.3% of patients who received prolonged IV treatment died, compared with 6.9% in the oral antibiotics group.
- In an intention-to-treat analysis, patients who were switched to oral antibiotics had a 22% lower risk for death within 90 days of initiation of treatment (relative risk [RR], 0.78; 95% CI, 0.60-1.10).
- In a per-protocol analysis, patients who switched to the oral route had a 1% lower odds of dying within 90 days (RR, 0.99; 95% CI, 0.70-1.40).
- Individuals who were switched to oral antibiotic treatment were younger than those who continued to receive antibiotics via the IV route (median age, 73 vs 76 years, respectively), had fewer comorbidities (four vs five), and were more likely to have community-acquired gram-negative bacteremia (89.4% vs 80.9%).
IN PRACTICE:
“These findings suggest that the mortality associated with early antibiotic stepdown treatment is comparable to that associated with receiving prolonged IV antibiotic treatment for individuals with uncomplicated gram-negative bacteremia,” the authors of the study wrote.
SOURCE:
The study was led by Sandra Tingsgård, MD, of the Center of Research & Department of Infectious Diseases at Copenhagen University Hospital–Amager and Hvidovre in Denmark.
LIMITATIONS:
The study was based on data from electronic health records, so some factors may not have been recorded or considered. The researchers identified few cases of multidrug-resistant infections, and the findings may not apply to those cases. Complicated cases and people who were not stabilized by day 4 were excluded from the analysis.
DISCLOSURES:
The authors report no disclosures or sources of funding.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a recent observational study published in JAMA Network Open.
METHODOLOGY:
- Patients receiving antibiotics through an IV line risk developing a secondary infection; antibiotics received orally are considered safer.
- Researchers analyzed observational data from 914 adults with uncomplicated gram-negative bacteremia who received care in four hospitals in Denmark between 2018 and 2021.
- The outcomes of patients who were switched to oral antibiotics within 4 days after a positive blood culture were compared with those who continued to receive IV antibiotics for at least 5 days after the blood culture; participants in both groups received antibiotics for 7-14 days.
- Researchers assessed mortality rates over a 90-day window and used a target trial emulation method to conduct the study.
TAKEAWAY:
- Overall, 14.3% of patients who received prolonged IV treatment died, compared with 6.9% in the oral antibiotics group.
- In an intention-to-treat analysis, patients who were switched to oral antibiotics had a 22% lower risk for death within 90 days of initiation of treatment (relative risk [RR], 0.78; 95% CI, 0.60-1.10).
- In a per-protocol analysis, patients who switched to the oral route had a 1% lower odds of dying within 90 days (RR, 0.99; 95% CI, 0.70-1.40).
- Individuals who were switched to oral antibiotic treatment were younger than those who continued to receive antibiotics via the IV route (median age, 73 vs 76 years, respectively), had fewer comorbidities (four vs five), and were more likely to have community-acquired gram-negative bacteremia (89.4% vs 80.9%).
IN PRACTICE:
“These findings suggest that the mortality associated with early antibiotic stepdown treatment is comparable to that associated with receiving prolonged IV antibiotic treatment for individuals with uncomplicated gram-negative bacteremia,” the authors of the study wrote.
SOURCE:
The study was led by Sandra Tingsgård, MD, of the Center of Research & Department of Infectious Diseases at Copenhagen University Hospital–Amager and Hvidovre in Denmark.
LIMITATIONS:
The study was based on data from electronic health records, so some factors may not have been recorded or considered. The researchers identified few cases of multidrug-resistant infections, and the findings may not apply to those cases. Complicated cases and people who were not stabilized by day 4 were excluded from the analysis.
DISCLOSURES:
The authors report no disclosures or sources of funding.
A version of this article appeared on Medscape.com.
Long COVID: Another Great Pretender
Has COVID replaced syphilis as the great imitator? At the turn of the 20th century, Sir William Osler was quoted as saying, “He who knows syphilis knows medicine.”
If you have any doubts about the “imitator” analogy, simply use any broad search engine or AI portal and enter “what is the differential diagnosis of primary syphilis, or secondary syphilis, or tertiary syphilis?” A plethora of diseases across many body systems appears.
Another Great Pretender?
Did you or do you now have long COVID? How do you know? Do you even know what long COVID is? How would you diagnosis it?
When asked about long COVID, this is what large language model source bard.google.com had to say:
Long COVID, also known as post-COVID conditions or PASC, is a complex condition that can occur in people who have had COVID-19, even if their initial illness was mild or even asymptomatic. It’s characterized by a wide range of symptoms that can last for weeks, months, or even years after the initial infection.
Symptoms of long COVID can include: fatigue, shortness of breath, brain fog (trouble thinking or concentrating), loss of smell or taste, sleep problems, muscle pain, joint pain, headache, dizziness, depression or anxiety, heart palpitations, gastrointestinal problems.
Duh!!! It reads much like a continuation of the symptoms of acute COVID. Bard continued:
The exact cause of long COVID is still unknown, but there are several theories. One possibility is that the virus itself can damage organs and tissues, leading to ongoing symptoms. Another possibility is that the immune system’s response to the virus can continue to cause inflammation even after the virus is gone.
Human intelligence source Wikipedia says this:
Long COVID or long-haul COVID is a group of health problems persisting or developing after an initial COVID-19 infection. Symptoms can last weeks, months or years and are often debilitating. The World Health Organization defines long COVID as starting three months after infection, but other definitions put the start of long COVID at four weeks.
Highly varied, including post-exertional malaise (symptoms made worse with effort), fatigue, muscle pain, shortness of breath, chest pain, and cognitive dysfunction (brain fog).
Acute COVID to Long COVID
The World Health Organization estimates that 36 million people in the European region have developed long COVID in the first 3 years of the pandemic. That›s a lot.
We all know that the common signs and symptoms of acute COVID-19 include fever or chills, a dry cough and shortness of breath, feeling very tired, muscle or body aches, headache, loss of taste or smell, sore throat, congestion, runny nose, nausea, vomiting, and diarrhea. Except for the taste and smell findings, every one of these symptoms or signs could indicate a different virus infection or even some type of allergy. My point is the nonspecificity in this list.
Uncommon signs and symptoms of acute COVID include a flat skin rash covered with small bumps, discolored swollen areas on the fingers and toes (COVID toes), and hives. The skin of hands, wrists, or ankles also can be affected. Blisters, itchiness, rough skin, or pus can be seen.
Severe confusion (delirium) might be the main or only symptom of COVID-19 in older people. This COVID-19 symptom is linked with a high risk for poor outcomes, including death. Pink eye (conjunctivitis) can be a COVID-19 symptom. Other eye problems linked to COVID-19 are light sensitivity, sore eyes, and itchy eyes. Acute myocarditis, tinnitus, vertigo, and hearing loss have been reported. And 1-4 weeks after the onset of COVID-19 infection, a patient may experience de novo reactive synovitis and arthritis of any joints.
So, take your pick. Myriad symptoms, signs, diseases, diagnoses, and organ systems — still present, recurring, just appearing, apparently de novo, or after asymptomatic infection. We have so much still to learn.
What big-time symptoms, signs, and major diseases are not on any of these lists? Obviously, cancer, atherosclerotic cardiovascular diseases, obesity, bone diseases, and competitive infections. But be patient; the lingering effects of direct tissue invasion by the virus as well as a wide range of immunologic reactions may just be getting started. Mitochondrial damage, especially in muscles, is increasingly a pathophysiologic suspect.
Human diseases can be physical or mental; and in COVID, that twain not only meet but mix and mingle freely, and may even merge into psychosoma. Don’t ever forget that. Consider “fatigue.” Who among us, COVID or NOVID, does not experience that from time to time?
Or consider brain fog as a common reported symptom of COVID. What on earth is that actually? How can a person know they have brain fog, or whether they had it and are over it?
We need one or more lab or other diagnostic tests that can objectively confirm the diagnosis of long COVID.
Useful Progress?
A recent research paper in Science reported intriguing chemical findings that seemed to point a finger at some form of complement dysregulation as a potential disease marker for long COVID. Unfortunately, some critics have pointed out that this entire study may be invalid or irrelevant because the New York cohort was recruited in 2020, before vaccines were available. The Zurich cohort was recruited up until April 2021, so some may have been vaccinated.
Then this news organization came along in early January 2024 with an article about COVID causing not only more than a million American deaths but also more than 5000 deaths from long COVID. We physicians don’t really know what long COVID even is, but we have to sign death certificates blaming thousands of deaths on it anyway? And rolling back the clock to 2020: Are patients dying from COVID or with COVID, according to death certificates?Now, armed with the knowledge that “documented serious post–COVID-19 conditions include cardiovascular, pulmonary, neurological, renal, endocrine, hematological, and gastrointestinal complications, as well as death,” CDC has published clear and fairly concise instructions on how to address post-acute COVID sequelae on death certificates.
In late January, this news organization painted a hopeful picture by naming four phenotypes of long COVID, suggesting that such divisions might further our understanding, including prognosis, and even therapy for this condition. Among the clinical phenotypes of (1) chronic fatigue–like syndrome, headache, and memory loss; (2) respiratory syndrome (which includes cough and difficulty breathing); (3) chronic pain; and (4) neurosensorial syndrome (which causes an altered sense of taste and smell), overlap is clearly possible but isn›t addressed.
I see these recent developments as needed and useful progress, but we are still left with…not much. So, when you tell me that you do or do not have long COVID, I will say to you, “How do you know?”
I also say: She/he/they who know COVID know medicine.
A version of this article first appeared on Medscape.com.
Has COVID replaced syphilis as the great imitator? At the turn of the 20th century, Sir William Osler was quoted as saying, “He who knows syphilis knows medicine.”
If you have any doubts about the “imitator” analogy, simply use any broad search engine or AI portal and enter “what is the differential diagnosis of primary syphilis, or secondary syphilis, or tertiary syphilis?” A plethora of diseases across many body systems appears.
Another Great Pretender?
Did you or do you now have long COVID? How do you know? Do you even know what long COVID is? How would you diagnosis it?
When asked about long COVID, this is what large language model source bard.google.com had to say:
Long COVID, also known as post-COVID conditions or PASC, is a complex condition that can occur in people who have had COVID-19, even if their initial illness was mild or even asymptomatic. It’s characterized by a wide range of symptoms that can last for weeks, months, or even years after the initial infection.
Symptoms of long COVID can include: fatigue, shortness of breath, brain fog (trouble thinking or concentrating), loss of smell or taste, sleep problems, muscle pain, joint pain, headache, dizziness, depression or anxiety, heart palpitations, gastrointestinal problems.
Duh!!! It reads much like a continuation of the symptoms of acute COVID. Bard continued:
The exact cause of long COVID is still unknown, but there are several theories. One possibility is that the virus itself can damage organs and tissues, leading to ongoing symptoms. Another possibility is that the immune system’s response to the virus can continue to cause inflammation even after the virus is gone.
Human intelligence source Wikipedia says this:
Long COVID or long-haul COVID is a group of health problems persisting or developing after an initial COVID-19 infection. Symptoms can last weeks, months or years and are often debilitating. The World Health Organization defines long COVID as starting three months after infection, but other definitions put the start of long COVID at four weeks.
Highly varied, including post-exertional malaise (symptoms made worse with effort), fatigue, muscle pain, shortness of breath, chest pain, and cognitive dysfunction (brain fog).
Acute COVID to Long COVID
The World Health Organization estimates that 36 million people in the European region have developed long COVID in the first 3 years of the pandemic. That›s a lot.
We all know that the common signs and symptoms of acute COVID-19 include fever or chills, a dry cough and shortness of breath, feeling very tired, muscle or body aches, headache, loss of taste or smell, sore throat, congestion, runny nose, nausea, vomiting, and diarrhea. Except for the taste and smell findings, every one of these symptoms or signs could indicate a different virus infection or even some type of allergy. My point is the nonspecificity in this list.
Uncommon signs and symptoms of acute COVID include a flat skin rash covered with small bumps, discolored swollen areas on the fingers and toes (COVID toes), and hives. The skin of hands, wrists, or ankles also can be affected. Blisters, itchiness, rough skin, or pus can be seen.
Severe confusion (delirium) might be the main or only symptom of COVID-19 in older people. This COVID-19 symptom is linked with a high risk for poor outcomes, including death. Pink eye (conjunctivitis) can be a COVID-19 symptom. Other eye problems linked to COVID-19 are light sensitivity, sore eyes, and itchy eyes. Acute myocarditis, tinnitus, vertigo, and hearing loss have been reported. And 1-4 weeks after the onset of COVID-19 infection, a patient may experience de novo reactive synovitis and arthritis of any joints.
So, take your pick. Myriad symptoms, signs, diseases, diagnoses, and organ systems — still present, recurring, just appearing, apparently de novo, or after asymptomatic infection. We have so much still to learn.
What big-time symptoms, signs, and major diseases are not on any of these lists? Obviously, cancer, atherosclerotic cardiovascular diseases, obesity, bone diseases, and competitive infections. But be patient; the lingering effects of direct tissue invasion by the virus as well as a wide range of immunologic reactions may just be getting started. Mitochondrial damage, especially in muscles, is increasingly a pathophysiologic suspect.
Human diseases can be physical or mental; and in COVID, that twain not only meet but mix and mingle freely, and may even merge into psychosoma. Don’t ever forget that. Consider “fatigue.” Who among us, COVID or NOVID, does not experience that from time to time?
Or consider brain fog as a common reported symptom of COVID. What on earth is that actually? How can a person know they have brain fog, or whether they had it and are over it?
We need one or more lab or other diagnostic tests that can objectively confirm the diagnosis of long COVID.
Useful Progress?
A recent research paper in Science reported intriguing chemical findings that seemed to point a finger at some form of complement dysregulation as a potential disease marker for long COVID. Unfortunately, some critics have pointed out that this entire study may be invalid or irrelevant because the New York cohort was recruited in 2020, before vaccines were available. The Zurich cohort was recruited up until April 2021, so some may have been vaccinated.
Then this news organization came along in early January 2024 with an article about COVID causing not only more than a million American deaths but also more than 5000 deaths from long COVID. We physicians don’t really know what long COVID even is, but we have to sign death certificates blaming thousands of deaths on it anyway? And rolling back the clock to 2020: Are patients dying from COVID or with COVID, according to death certificates?Now, armed with the knowledge that “documented serious post–COVID-19 conditions include cardiovascular, pulmonary, neurological, renal, endocrine, hematological, and gastrointestinal complications, as well as death,” CDC has published clear and fairly concise instructions on how to address post-acute COVID sequelae on death certificates.
In late January, this news organization painted a hopeful picture by naming four phenotypes of long COVID, suggesting that such divisions might further our understanding, including prognosis, and even therapy for this condition. Among the clinical phenotypes of (1) chronic fatigue–like syndrome, headache, and memory loss; (2) respiratory syndrome (which includes cough and difficulty breathing); (3) chronic pain; and (4) neurosensorial syndrome (which causes an altered sense of taste and smell), overlap is clearly possible but isn›t addressed.
I see these recent developments as needed and useful progress, but we are still left with…not much. So, when you tell me that you do or do not have long COVID, I will say to you, “How do you know?”
I also say: She/he/they who know COVID know medicine.
A version of this article first appeared on Medscape.com.
Has COVID replaced syphilis as the great imitator? At the turn of the 20th century, Sir William Osler was quoted as saying, “He who knows syphilis knows medicine.”
If you have any doubts about the “imitator” analogy, simply use any broad search engine or AI portal and enter “what is the differential diagnosis of primary syphilis, or secondary syphilis, or tertiary syphilis?” A plethora of diseases across many body systems appears.
Another Great Pretender?
Did you or do you now have long COVID? How do you know? Do you even know what long COVID is? How would you diagnosis it?
When asked about long COVID, this is what large language model source bard.google.com had to say:
Long COVID, also known as post-COVID conditions or PASC, is a complex condition that can occur in people who have had COVID-19, even if their initial illness was mild or even asymptomatic. It’s characterized by a wide range of symptoms that can last for weeks, months, or even years after the initial infection.
Symptoms of long COVID can include: fatigue, shortness of breath, brain fog (trouble thinking or concentrating), loss of smell or taste, sleep problems, muscle pain, joint pain, headache, dizziness, depression or anxiety, heart palpitations, gastrointestinal problems.
Duh!!! It reads much like a continuation of the symptoms of acute COVID. Bard continued:
The exact cause of long COVID is still unknown, but there are several theories. One possibility is that the virus itself can damage organs and tissues, leading to ongoing symptoms. Another possibility is that the immune system’s response to the virus can continue to cause inflammation even after the virus is gone.
Human intelligence source Wikipedia says this:
Long COVID or long-haul COVID is a group of health problems persisting or developing after an initial COVID-19 infection. Symptoms can last weeks, months or years and are often debilitating. The World Health Organization defines long COVID as starting three months after infection, but other definitions put the start of long COVID at four weeks.
Highly varied, including post-exertional malaise (symptoms made worse with effort), fatigue, muscle pain, shortness of breath, chest pain, and cognitive dysfunction (brain fog).
Acute COVID to Long COVID
The World Health Organization estimates that 36 million people in the European region have developed long COVID in the first 3 years of the pandemic. That›s a lot.
We all know that the common signs and symptoms of acute COVID-19 include fever or chills, a dry cough and shortness of breath, feeling very tired, muscle or body aches, headache, loss of taste or smell, sore throat, congestion, runny nose, nausea, vomiting, and diarrhea. Except for the taste and smell findings, every one of these symptoms or signs could indicate a different virus infection or even some type of allergy. My point is the nonspecificity in this list.
Uncommon signs and symptoms of acute COVID include a flat skin rash covered with small bumps, discolored swollen areas on the fingers and toes (COVID toes), and hives. The skin of hands, wrists, or ankles also can be affected. Blisters, itchiness, rough skin, or pus can be seen.
Severe confusion (delirium) might be the main or only symptom of COVID-19 in older people. This COVID-19 symptom is linked with a high risk for poor outcomes, including death. Pink eye (conjunctivitis) can be a COVID-19 symptom. Other eye problems linked to COVID-19 are light sensitivity, sore eyes, and itchy eyes. Acute myocarditis, tinnitus, vertigo, and hearing loss have been reported. And 1-4 weeks after the onset of COVID-19 infection, a patient may experience de novo reactive synovitis and arthritis of any joints.
So, take your pick. Myriad symptoms, signs, diseases, diagnoses, and organ systems — still present, recurring, just appearing, apparently de novo, or after asymptomatic infection. We have so much still to learn.
What big-time symptoms, signs, and major diseases are not on any of these lists? Obviously, cancer, atherosclerotic cardiovascular diseases, obesity, bone diseases, and competitive infections. But be patient; the lingering effects of direct tissue invasion by the virus as well as a wide range of immunologic reactions may just be getting started. Mitochondrial damage, especially in muscles, is increasingly a pathophysiologic suspect.
Human diseases can be physical or mental; and in COVID, that twain not only meet but mix and mingle freely, and may even merge into psychosoma. Don’t ever forget that. Consider “fatigue.” Who among us, COVID or NOVID, does not experience that from time to time?
Or consider brain fog as a common reported symptom of COVID. What on earth is that actually? How can a person know they have brain fog, or whether they had it and are over it?
We need one or more lab or other diagnostic tests that can objectively confirm the diagnosis of long COVID.
Useful Progress?
A recent research paper in Science reported intriguing chemical findings that seemed to point a finger at some form of complement dysregulation as a potential disease marker for long COVID. Unfortunately, some critics have pointed out that this entire study may be invalid or irrelevant because the New York cohort was recruited in 2020, before vaccines were available. The Zurich cohort was recruited up until April 2021, so some may have been vaccinated.
Then this news organization came along in early January 2024 with an article about COVID causing not only more than a million American deaths but also more than 5000 deaths from long COVID. We physicians don’t really know what long COVID even is, but we have to sign death certificates blaming thousands of deaths on it anyway? And rolling back the clock to 2020: Are patients dying from COVID or with COVID, according to death certificates?Now, armed with the knowledge that “documented serious post–COVID-19 conditions include cardiovascular, pulmonary, neurological, renal, endocrine, hematological, and gastrointestinal complications, as well as death,” CDC has published clear and fairly concise instructions on how to address post-acute COVID sequelae on death certificates.
In late January, this news organization painted a hopeful picture by naming four phenotypes of long COVID, suggesting that such divisions might further our understanding, including prognosis, and even therapy for this condition. Among the clinical phenotypes of (1) chronic fatigue–like syndrome, headache, and memory loss; (2) respiratory syndrome (which includes cough and difficulty breathing); (3) chronic pain; and (4) neurosensorial syndrome (which causes an altered sense of taste and smell), overlap is clearly possible but isn›t addressed.
I see these recent developments as needed and useful progress, but we are still left with…not much. So, when you tell me that you do or do not have long COVID, I will say to you, “How do you know?”
I also say: She/he/they who know COVID know medicine.
A version of this article first appeared on Medscape.com.
HPV Positive Test: How to Address Patients’ Anxieties
Faced with a positive human papillomavirus (HPV) test, patients are quickly overwhelmed by anxiety-inducing questions. It is crucial to provide them with adequate responses to reassure them, emphasized Jean-Louis Mergui, MD, president of the International Federation for Colposcopy, during the press conference of the Congress of the French Society of Colposcopy and Cervico-Vaginal Pathology.
“Do I have cancer? When did I catch this papillomavirus? Is it dangerous for my partner? How do I get rid of it?” “Not everyone is equipped to answer these four questions. However, it is extremely important that healthcare professionals provide correct answers to patients so that they stop worrying,” Dr. Mergui explained.
Papillomavirus and Cancer
One of the first instincts of patients who receive a positive HPV test is to turn to the Internet. There, they read about “high-risk HPV, which is potentially oncogenic,” and become completely panicked, said Dr. Mergui.
However, among women, the probability of having a high-grade CIN3 lesion or higher on the cervix when the HPV test is positive is about 7%, according to the ATHENA study. “About 93% of patients do not have a severe lesion on the cervix. That’s why colposcopy is not performed on all patients. They need to be reassured,” said Dr. Mergui. When the papillomavirus persists, there is a risk for a cervical lesion. After 11 years, between 20% and 30% of patients develop a high-grade lesion on the cervix. However, on average, a high-risk HPV is spontaneously eliminated within 1-2 years. “After 14 months, 50% of women will test negative for their papillomavirus,” Dr. Mergui noted.
“High-risk HPV does not mean there is a lesion; it means there is a risk of developing a lesion on the cervix one day. That’s why these patients need to be monitored and explored,” he added.
In practice, when a patient aged between 30 and 65 years has a positive HPV test, cytology is performed to look for lesions. Only in the case of an abnormal smear, ASC-US, is colposcopy recommended. In the absence of a lesion, a control HPV test is conducted 1 year later to monitor virus persistence.
It should be noted that patients who have been treated for a cervical lesion have a five times higher risk of developing invasive cervical, vaginal, or vulvar cancer. Therefore, treated patients must be monitored once every 3 years for life.
Time of Infection
Many patients ask, “When did I catch this papillomavirus?” In response, Dr. Mergui first emphasized that HPV infection is common. “Between ages 15 and 30 years, most of us are infected with a high-risk HPV. When we look at the incidence between ages 15 and 25 years, every year, 20% of all young girls are infected with HPV, including 17% with high-risk HPV. The virus is usually caught within the first 5 years of sexual activity, and typically disappears after about a year,” he explained.
However, the most disturbing scenario for patients is when their last examination was negative, and there is no apparent reason for having caught the virus since then. Suspicion often falls on the partner. Once again, the gynecologist seeks to reassure.
It is possible that the last time screening was conducted, the virus was not sought (HPV test), but rather cervical lesions were sought by smear. However, a normal smear does not mean that the papillomavirus is not present. A negative cytology does not mean a negative HPV test. As we have seen, the virus is not always associated with the presence of a lesion, explained Dr. Mergui.
Also, having had a negative HPV test a few years earlier does not mean that one was not already infected. The HPV test determines the quantity of virus. Therefore, it is possible that the virus was present in small quantities that were without clinical significance (hence, a negative test). However, a few years later, the virus may have multiplied, and the HPV test became positive.
“Sometimes, the virus re-emerges 40, 50 years after infection due to age-related immune decline,” said Dr. Mergui. “So, just because the smear was negative or the HPV test was negative at the last examination does not mean that one was infected between the two.” Moreover, only 15% of couples have the same virus present on the penis or vagina, he pointed out.
Protecting One’s Partner
Once the diagnosis is made, it is often too late to protect the partner because they have already been infected. “It is certain that the partner will be infected or has already been infected because when the patient comes to you with a positive HPV test, she has already had sexual intercourse. It is worth noting that the virus can be transmitted through digital touching, and condoms are not very effective in preventing virus transmission,” said Dr. Mergui.
The speaker further clarified that the risk for men is much lower than that for women. “In women, about 40,000 lesions linked to high-risk HPV types, precancerous or cancerous, are observed every year. In men, this number is 1900. So, this represents 20 times fewer neoplastic lesions in men. The problem in men is oropharyngeal lesions, which are three times more common than in women. However, there is no screening for oropharyngeal cancer.”
So, when should the partner consult? Dr. Mergui advised consulting when there are clinically visible lesions (small warts, bumps, or ear, nose, and throat symptoms). “I do not recommend systematic examination of male or female partners,” he added.
Clearing the Virus
There are treatments for cervical lesions but not for papillomavirus infection.
“The only thing that can be suggested is quitting smoking, which increases viral clearance, thus reducing viral load. Also, the use of condoms helps improve viral clearance, but when women have a stable relationship, it seems unrealistic to think they will constantly use condoms. Finally, the prophylactic vaccine has been proposed, but it does not treat the infection. In fact, the real solution is to tell patients that they need to continue regular monitoring,” said Dr. Mergui.
“It should be noted that an ongoing study at the European level seems to show that when women who have undergone surgical treatment for a high-grade cervical lesion are vaccinated at the time of treatment or just after treatment, it reduces the risk of recurrence by 50%. So, the risk of recurrence is around 7%-8%. This strategy could be interesting, but for now, there is no official recommendation,” Dr. Mergui concluded.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Faced with a positive human papillomavirus (HPV) test, patients are quickly overwhelmed by anxiety-inducing questions. It is crucial to provide them with adequate responses to reassure them, emphasized Jean-Louis Mergui, MD, president of the International Federation for Colposcopy, during the press conference of the Congress of the French Society of Colposcopy and Cervico-Vaginal Pathology.
“Do I have cancer? When did I catch this papillomavirus? Is it dangerous for my partner? How do I get rid of it?” “Not everyone is equipped to answer these four questions. However, it is extremely important that healthcare professionals provide correct answers to patients so that they stop worrying,” Dr. Mergui explained.
Papillomavirus and Cancer
One of the first instincts of patients who receive a positive HPV test is to turn to the Internet. There, they read about “high-risk HPV, which is potentially oncogenic,” and become completely panicked, said Dr. Mergui.
However, among women, the probability of having a high-grade CIN3 lesion or higher on the cervix when the HPV test is positive is about 7%, according to the ATHENA study. “About 93% of patients do not have a severe lesion on the cervix. That’s why colposcopy is not performed on all patients. They need to be reassured,” said Dr. Mergui. When the papillomavirus persists, there is a risk for a cervical lesion. After 11 years, between 20% and 30% of patients develop a high-grade lesion on the cervix. However, on average, a high-risk HPV is spontaneously eliminated within 1-2 years. “After 14 months, 50% of women will test negative for their papillomavirus,” Dr. Mergui noted.
“High-risk HPV does not mean there is a lesion; it means there is a risk of developing a lesion on the cervix one day. That’s why these patients need to be monitored and explored,” he added.
In practice, when a patient aged between 30 and 65 years has a positive HPV test, cytology is performed to look for lesions. Only in the case of an abnormal smear, ASC-US, is colposcopy recommended. In the absence of a lesion, a control HPV test is conducted 1 year later to monitor virus persistence.
It should be noted that patients who have been treated for a cervical lesion have a five times higher risk of developing invasive cervical, vaginal, or vulvar cancer. Therefore, treated patients must be monitored once every 3 years for life.
Time of Infection
Many patients ask, “When did I catch this papillomavirus?” In response, Dr. Mergui first emphasized that HPV infection is common. “Between ages 15 and 30 years, most of us are infected with a high-risk HPV. When we look at the incidence between ages 15 and 25 years, every year, 20% of all young girls are infected with HPV, including 17% with high-risk HPV. The virus is usually caught within the first 5 years of sexual activity, and typically disappears after about a year,” he explained.
However, the most disturbing scenario for patients is when their last examination was negative, and there is no apparent reason for having caught the virus since then. Suspicion often falls on the partner. Once again, the gynecologist seeks to reassure.
It is possible that the last time screening was conducted, the virus was not sought (HPV test), but rather cervical lesions were sought by smear. However, a normal smear does not mean that the papillomavirus is not present. A negative cytology does not mean a negative HPV test. As we have seen, the virus is not always associated with the presence of a lesion, explained Dr. Mergui.
Also, having had a negative HPV test a few years earlier does not mean that one was not already infected. The HPV test determines the quantity of virus. Therefore, it is possible that the virus was present in small quantities that were without clinical significance (hence, a negative test). However, a few years later, the virus may have multiplied, and the HPV test became positive.
“Sometimes, the virus re-emerges 40, 50 years after infection due to age-related immune decline,” said Dr. Mergui. “So, just because the smear was negative or the HPV test was negative at the last examination does not mean that one was infected between the two.” Moreover, only 15% of couples have the same virus present on the penis or vagina, he pointed out.
Protecting One’s Partner
Once the diagnosis is made, it is often too late to protect the partner because they have already been infected. “It is certain that the partner will be infected or has already been infected because when the patient comes to you with a positive HPV test, she has already had sexual intercourse. It is worth noting that the virus can be transmitted through digital touching, and condoms are not very effective in preventing virus transmission,” said Dr. Mergui.
The speaker further clarified that the risk for men is much lower than that for women. “In women, about 40,000 lesions linked to high-risk HPV types, precancerous or cancerous, are observed every year. In men, this number is 1900. So, this represents 20 times fewer neoplastic lesions in men. The problem in men is oropharyngeal lesions, which are three times more common than in women. However, there is no screening for oropharyngeal cancer.”
So, when should the partner consult? Dr. Mergui advised consulting when there are clinically visible lesions (small warts, bumps, or ear, nose, and throat symptoms). “I do not recommend systematic examination of male or female partners,” he added.
Clearing the Virus
There are treatments for cervical lesions but not for papillomavirus infection.
“The only thing that can be suggested is quitting smoking, which increases viral clearance, thus reducing viral load. Also, the use of condoms helps improve viral clearance, but when women have a stable relationship, it seems unrealistic to think they will constantly use condoms. Finally, the prophylactic vaccine has been proposed, but it does not treat the infection. In fact, the real solution is to tell patients that they need to continue regular monitoring,” said Dr. Mergui.
“It should be noted that an ongoing study at the European level seems to show that when women who have undergone surgical treatment for a high-grade cervical lesion are vaccinated at the time of treatment or just after treatment, it reduces the risk of recurrence by 50%. So, the risk of recurrence is around 7%-8%. This strategy could be interesting, but for now, there is no official recommendation,” Dr. Mergui concluded.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Faced with a positive human papillomavirus (HPV) test, patients are quickly overwhelmed by anxiety-inducing questions. It is crucial to provide them with adequate responses to reassure them, emphasized Jean-Louis Mergui, MD, president of the International Federation for Colposcopy, during the press conference of the Congress of the French Society of Colposcopy and Cervico-Vaginal Pathology.
“Do I have cancer? When did I catch this papillomavirus? Is it dangerous for my partner? How do I get rid of it?” “Not everyone is equipped to answer these four questions. However, it is extremely important that healthcare professionals provide correct answers to patients so that they stop worrying,” Dr. Mergui explained.
Papillomavirus and Cancer
One of the first instincts of patients who receive a positive HPV test is to turn to the Internet. There, they read about “high-risk HPV, which is potentially oncogenic,” and become completely panicked, said Dr. Mergui.
However, among women, the probability of having a high-grade CIN3 lesion or higher on the cervix when the HPV test is positive is about 7%, according to the ATHENA study. “About 93% of patients do not have a severe lesion on the cervix. That’s why colposcopy is not performed on all patients. They need to be reassured,” said Dr. Mergui. When the papillomavirus persists, there is a risk for a cervical lesion. After 11 years, between 20% and 30% of patients develop a high-grade lesion on the cervix. However, on average, a high-risk HPV is spontaneously eliminated within 1-2 years. “After 14 months, 50% of women will test negative for their papillomavirus,” Dr. Mergui noted.
“High-risk HPV does not mean there is a lesion; it means there is a risk of developing a lesion on the cervix one day. That’s why these patients need to be monitored and explored,” he added.
In practice, when a patient aged between 30 and 65 years has a positive HPV test, cytology is performed to look for lesions. Only in the case of an abnormal smear, ASC-US, is colposcopy recommended. In the absence of a lesion, a control HPV test is conducted 1 year later to monitor virus persistence.
It should be noted that patients who have been treated for a cervical lesion have a five times higher risk of developing invasive cervical, vaginal, or vulvar cancer. Therefore, treated patients must be monitored once every 3 years for life.
Time of Infection
Many patients ask, “When did I catch this papillomavirus?” In response, Dr. Mergui first emphasized that HPV infection is common. “Between ages 15 and 30 years, most of us are infected with a high-risk HPV. When we look at the incidence between ages 15 and 25 years, every year, 20% of all young girls are infected with HPV, including 17% with high-risk HPV. The virus is usually caught within the first 5 years of sexual activity, and typically disappears after about a year,” he explained.
However, the most disturbing scenario for patients is when their last examination was negative, and there is no apparent reason for having caught the virus since then. Suspicion often falls on the partner. Once again, the gynecologist seeks to reassure.
It is possible that the last time screening was conducted, the virus was not sought (HPV test), but rather cervical lesions were sought by smear. However, a normal smear does not mean that the papillomavirus is not present. A negative cytology does not mean a negative HPV test. As we have seen, the virus is not always associated with the presence of a lesion, explained Dr. Mergui.
Also, having had a negative HPV test a few years earlier does not mean that one was not already infected. The HPV test determines the quantity of virus. Therefore, it is possible that the virus was present in small quantities that were without clinical significance (hence, a negative test). However, a few years later, the virus may have multiplied, and the HPV test became positive.
“Sometimes, the virus re-emerges 40, 50 years after infection due to age-related immune decline,” said Dr. Mergui. “So, just because the smear was negative or the HPV test was negative at the last examination does not mean that one was infected between the two.” Moreover, only 15% of couples have the same virus present on the penis or vagina, he pointed out.
Protecting One’s Partner
Once the diagnosis is made, it is often too late to protect the partner because they have already been infected. “It is certain that the partner will be infected or has already been infected because when the patient comes to you with a positive HPV test, she has already had sexual intercourse. It is worth noting that the virus can be transmitted through digital touching, and condoms are not very effective in preventing virus transmission,” said Dr. Mergui.
The speaker further clarified that the risk for men is much lower than that for women. “In women, about 40,000 lesions linked to high-risk HPV types, precancerous or cancerous, are observed every year. In men, this number is 1900. So, this represents 20 times fewer neoplastic lesions in men. The problem in men is oropharyngeal lesions, which are three times more common than in women. However, there is no screening for oropharyngeal cancer.”
So, when should the partner consult? Dr. Mergui advised consulting when there are clinically visible lesions (small warts, bumps, or ear, nose, and throat symptoms). “I do not recommend systematic examination of male or female partners,” he added.
Clearing the Virus
There are treatments for cervical lesions but not for papillomavirus infection.
“The only thing that can be suggested is quitting smoking, which increases viral clearance, thus reducing viral load. Also, the use of condoms helps improve viral clearance, but when women have a stable relationship, it seems unrealistic to think they will constantly use condoms. Finally, the prophylactic vaccine has been proposed, but it does not treat the infection. In fact, the real solution is to tell patients that they need to continue regular monitoring,” said Dr. Mergui.
“It should be noted that an ongoing study at the European level seems to show that when women who have undergone surgical treatment for a high-grade cervical lesion are vaccinated at the time of treatment or just after treatment, it reduces the risk of recurrence by 50%. So, the risk of recurrence is around 7%-8%. This strategy could be interesting, but for now, there is no official recommendation,” Dr. Mergui concluded.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
New Evidence Suggests Long COVID Could Be a Brain Injury
Brain fog is one of the most common, persistent complaints in patients with long COVID. It affects as many as 46% of patients who also deal with other cognitive concerns like memory loss and difficulty concentrating.
Now, researchers believe they know why. A new study has found that these symptoms may be the result of a viral-borne brain injury that may cause cognitive and mental health issues that persist for years.
The findings were based on a series of cognitive tests, self-reported symptoms, brain scans, and biomarkers.
Brain Deficits Equal to 20 Years of Brain Aging
As part of the preprint study, participants took a cognition test with their scores age-matched to those who had not suffered a serious bout of COVID-19. Then a blood sample was taken to look for specific biomarkers, showing that elevated levels of certain biomarkers were consistent with a brain injury. Using brain scans, researchers also found that certain regions of the brain associated with attention were reduced in volume.
Patients who participated in the study were “less accurate and slower” in their cognition, and suffered from at least one mental health condition, such as depression, anxiety, or posttraumatic stress disorder, according to researchers.
The brain deficits found in COVID-19 patients were equivalent to 20 years of brain aging and provided proof of what doctors have feared: that this virus can damage the brain and result in ongoing mental health issues.
“We found global deficits across cognition,” said lead study author Benedict Michael, PhD, director of the Infection Neuroscience Lab at the University of Liverpool in Liverpool, England. “The cognitive and memory problems that patients complained of were associated with neuroanatomical changes to the brain.”
Proof That Symptoms Aren’t ‘Figment’ of Patients’ Imaginations
Cognitive deficits were common among all patients, but the researchers said they don’t yet know whether the brain damage causes permanent cognitive decline. But the research provides patients who have been overlooked by some clinicians with proof that their conditions aren’t a figment of their imaginations, said Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina School of Medicine’s COVID Recovery Clinic.
“Even though we’re several years into this pandemic, there are still a lot of providers who don’t believe that their patients are experiencing these residual symptoms,” said Dr. Thompson, “That’s why the use of biomarkers is important, because it provides an objective indication that the brain has been compromised in some way.”
Some patients with long COVID have said that getting their doctors to believe they have a physical ailment has been a persistent problem throughout the pandemic and especially as it relates to the sometimes-vague collection of symptoms associated with brain fog. One study found that as many as 79% of study respondents reported negative interactions with their healthcare providers when they sought treatment for their long-COVID symptoms.
How Do COVID-Related Brain Injuries Happen?
Researchers are unsure what’s causing these brain injuries, though they have identified some clues. Previous research has suggested that such injuries might be the result of a lack of oxygen to the brain, especially in patients who were hospitalized, like those in this study, and were put on ventilators.
Brain scans have previously shown atrophy to the brain›s gray matter in COVID-19 patients, likely caused by inflammation from a heightened immune response rather than the virus itself. This inflammatory response seems to affect the central nervous system. As part of the new study, researchers found some neuroprotective effects of using steroids during hospitalization to reduce brain inflammation.
The results suggest that clinicians should overcome their skepticism and consider the possibility that their patients have suffered a brain injury and should be treated appropriately, said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University School of Medicine. “The old saying is that if it walks like a duck and talks like a duck, it’s a duck,” said Dr. Jackson.
He contends that treatments used for patients who have brain injuries have also been shown to be effective in treating long COVID–related brain fog symptoms. These may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for the treatment of related mental health concerns.
A New Path Forward
Treating long-COVID brain fog like a brain injury can help patients get back to some semblance of normalcy, researchers said. “What we’re seeing in terms of brain injury biomarkers and differences in brain scans correlates to real-life problems that these patients are dealing with on a daily basis,” said Dr. Jackson. These include problems at work and in life with multitasking, remembering details, meeting deadlines, synthesizing large amounts of information, and maintaining focus on the task at hand, he said.
There’s also a fear that even with treatment, the aging of the brain caused by the virus might have long-term repercussions and that this enduring injury may cause the early onset of dementia and Alzheimer’s disease in those who were already vulnerable to it. One study, from the National Institute of Neurological Disorders and Stroke (NINDS), found that in those infected with COVID-19 who already had dementia, the virus “rapidly accelerated structural and functional brain deterioration.”
“We already know the role that neuroinflammation plays in the brains of patients with Alzheimer’s disease,” said Dr. Thompson. “If long COVID is involved in prolonged inflammation of the brain, it goes a long way in explaining the mechanism underlying [the study’s reported] brain aging.”
Still More to Learn
In some ways, this study raises nearly as many questions as it does answers. While it provides concrete evidence around the damage the virus is doing to the brains of patients who contracted severe COVID-19, researchers don’t know about the impact on those who had less serious cases of the virus.
For Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System, the concern is that some long-COVID patients may be suffering from cognitive deficits that are more subtle but still impacting their daily lives, and that they’re not getting the help they need.
What’s more, said Dr. Al-Aly, it’s unclear whether the impacts of the brain damage are permanent or how to stop them from worsening. Researchers and clinicians need a better understanding of the mechanism that allows this virus to enter the brain and do structural damage. If it’s inflammation, will anti-inflammatory or antiviral medications work at preventing it? Will steroids help to offset the damage? “It’s critical we find some answers,” he said.
“SARS-CoV-2 isn’t going anywhere. It will continue to infect the population, so if this is indeed a virus that damages the brain in the long term or permanently, we need to figure out what can be done to stop it,” said Dr. Al-Aly.
A version of this article appeared on Medscape.com.
Brain fog is one of the most common, persistent complaints in patients with long COVID. It affects as many as 46% of patients who also deal with other cognitive concerns like memory loss and difficulty concentrating.
Now, researchers believe they know why. A new study has found that these symptoms may be the result of a viral-borne brain injury that may cause cognitive and mental health issues that persist for years.
The findings were based on a series of cognitive tests, self-reported symptoms, brain scans, and biomarkers.
Brain Deficits Equal to 20 Years of Brain Aging
As part of the preprint study, participants took a cognition test with their scores age-matched to those who had not suffered a serious bout of COVID-19. Then a blood sample was taken to look for specific biomarkers, showing that elevated levels of certain biomarkers were consistent with a brain injury. Using brain scans, researchers also found that certain regions of the brain associated with attention were reduced in volume.
Patients who participated in the study were “less accurate and slower” in their cognition, and suffered from at least one mental health condition, such as depression, anxiety, or posttraumatic stress disorder, according to researchers.
The brain deficits found in COVID-19 patients were equivalent to 20 years of brain aging and provided proof of what doctors have feared: that this virus can damage the brain and result in ongoing mental health issues.
“We found global deficits across cognition,” said lead study author Benedict Michael, PhD, director of the Infection Neuroscience Lab at the University of Liverpool in Liverpool, England. “The cognitive and memory problems that patients complained of were associated with neuroanatomical changes to the brain.”
Proof That Symptoms Aren’t ‘Figment’ of Patients’ Imaginations
Cognitive deficits were common among all patients, but the researchers said they don’t yet know whether the brain damage causes permanent cognitive decline. But the research provides patients who have been overlooked by some clinicians with proof that their conditions aren’t a figment of their imaginations, said Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina School of Medicine’s COVID Recovery Clinic.
“Even though we’re several years into this pandemic, there are still a lot of providers who don’t believe that their patients are experiencing these residual symptoms,” said Dr. Thompson, “That’s why the use of biomarkers is important, because it provides an objective indication that the brain has been compromised in some way.”
Some patients with long COVID have said that getting their doctors to believe they have a physical ailment has been a persistent problem throughout the pandemic and especially as it relates to the sometimes-vague collection of symptoms associated with brain fog. One study found that as many as 79% of study respondents reported negative interactions with their healthcare providers when they sought treatment for their long-COVID symptoms.
How Do COVID-Related Brain Injuries Happen?
Researchers are unsure what’s causing these brain injuries, though they have identified some clues. Previous research has suggested that such injuries might be the result of a lack of oxygen to the brain, especially in patients who were hospitalized, like those in this study, and were put on ventilators.
Brain scans have previously shown atrophy to the brain›s gray matter in COVID-19 patients, likely caused by inflammation from a heightened immune response rather than the virus itself. This inflammatory response seems to affect the central nervous system. As part of the new study, researchers found some neuroprotective effects of using steroids during hospitalization to reduce brain inflammation.
The results suggest that clinicians should overcome their skepticism and consider the possibility that their patients have suffered a brain injury and should be treated appropriately, said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University School of Medicine. “The old saying is that if it walks like a duck and talks like a duck, it’s a duck,” said Dr. Jackson.
He contends that treatments used for patients who have brain injuries have also been shown to be effective in treating long COVID–related brain fog symptoms. These may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for the treatment of related mental health concerns.
A New Path Forward
Treating long-COVID brain fog like a brain injury can help patients get back to some semblance of normalcy, researchers said. “What we’re seeing in terms of brain injury biomarkers and differences in brain scans correlates to real-life problems that these patients are dealing with on a daily basis,” said Dr. Jackson. These include problems at work and in life with multitasking, remembering details, meeting deadlines, synthesizing large amounts of information, and maintaining focus on the task at hand, he said.
There’s also a fear that even with treatment, the aging of the brain caused by the virus might have long-term repercussions and that this enduring injury may cause the early onset of dementia and Alzheimer’s disease in those who were already vulnerable to it. One study, from the National Institute of Neurological Disorders and Stroke (NINDS), found that in those infected with COVID-19 who already had dementia, the virus “rapidly accelerated structural and functional brain deterioration.”
“We already know the role that neuroinflammation plays in the brains of patients with Alzheimer’s disease,” said Dr. Thompson. “If long COVID is involved in prolonged inflammation of the brain, it goes a long way in explaining the mechanism underlying [the study’s reported] brain aging.”
Still More to Learn
In some ways, this study raises nearly as many questions as it does answers. While it provides concrete evidence around the damage the virus is doing to the brains of patients who contracted severe COVID-19, researchers don’t know about the impact on those who had less serious cases of the virus.
For Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System, the concern is that some long-COVID patients may be suffering from cognitive deficits that are more subtle but still impacting their daily lives, and that they’re not getting the help they need.
What’s more, said Dr. Al-Aly, it’s unclear whether the impacts of the brain damage are permanent or how to stop them from worsening. Researchers and clinicians need a better understanding of the mechanism that allows this virus to enter the brain and do structural damage. If it’s inflammation, will anti-inflammatory or antiviral medications work at preventing it? Will steroids help to offset the damage? “It’s critical we find some answers,” he said.
“SARS-CoV-2 isn’t going anywhere. It will continue to infect the population, so if this is indeed a virus that damages the brain in the long term or permanently, we need to figure out what can be done to stop it,” said Dr. Al-Aly.
A version of this article appeared on Medscape.com.
Brain fog is one of the most common, persistent complaints in patients with long COVID. It affects as many as 46% of patients who also deal with other cognitive concerns like memory loss and difficulty concentrating.
Now, researchers believe they know why. A new study has found that these symptoms may be the result of a viral-borne brain injury that may cause cognitive and mental health issues that persist for years.
The findings were based on a series of cognitive tests, self-reported symptoms, brain scans, and biomarkers.
Brain Deficits Equal to 20 Years of Brain Aging
As part of the preprint study, participants took a cognition test with their scores age-matched to those who had not suffered a serious bout of COVID-19. Then a blood sample was taken to look for specific biomarkers, showing that elevated levels of certain biomarkers were consistent with a brain injury. Using brain scans, researchers also found that certain regions of the brain associated with attention were reduced in volume.
Patients who participated in the study were “less accurate and slower” in their cognition, and suffered from at least one mental health condition, such as depression, anxiety, or posttraumatic stress disorder, according to researchers.
The brain deficits found in COVID-19 patients were equivalent to 20 years of brain aging and provided proof of what doctors have feared: that this virus can damage the brain and result in ongoing mental health issues.
“We found global deficits across cognition,” said lead study author Benedict Michael, PhD, director of the Infection Neuroscience Lab at the University of Liverpool in Liverpool, England. “The cognitive and memory problems that patients complained of were associated with neuroanatomical changes to the brain.”
Proof That Symptoms Aren’t ‘Figment’ of Patients’ Imaginations
Cognitive deficits were common among all patients, but the researchers said they don’t yet know whether the brain damage causes permanent cognitive decline. But the research provides patients who have been overlooked by some clinicians with proof that their conditions aren’t a figment of their imaginations, said Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina School of Medicine’s COVID Recovery Clinic.
“Even though we’re several years into this pandemic, there are still a lot of providers who don’t believe that their patients are experiencing these residual symptoms,” said Dr. Thompson, “That’s why the use of biomarkers is important, because it provides an objective indication that the brain has been compromised in some way.”
Some patients with long COVID have said that getting their doctors to believe they have a physical ailment has been a persistent problem throughout the pandemic and especially as it relates to the sometimes-vague collection of symptoms associated with brain fog. One study found that as many as 79% of study respondents reported negative interactions with their healthcare providers when they sought treatment for their long-COVID symptoms.
How Do COVID-Related Brain Injuries Happen?
Researchers are unsure what’s causing these brain injuries, though they have identified some clues. Previous research has suggested that such injuries might be the result of a lack of oxygen to the brain, especially in patients who were hospitalized, like those in this study, and were put on ventilators.
Brain scans have previously shown atrophy to the brain›s gray matter in COVID-19 patients, likely caused by inflammation from a heightened immune response rather than the virus itself. This inflammatory response seems to affect the central nervous system. As part of the new study, researchers found some neuroprotective effects of using steroids during hospitalization to reduce brain inflammation.
The results suggest that clinicians should overcome their skepticism and consider the possibility that their patients have suffered a brain injury and should be treated appropriately, said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University School of Medicine. “The old saying is that if it walks like a duck and talks like a duck, it’s a duck,” said Dr. Jackson.
He contends that treatments used for patients who have brain injuries have also been shown to be effective in treating long COVID–related brain fog symptoms. These may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for the treatment of related mental health concerns.
A New Path Forward
Treating long-COVID brain fog like a brain injury can help patients get back to some semblance of normalcy, researchers said. “What we’re seeing in terms of brain injury biomarkers and differences in brain scans correlates to real-life problems that these patients are dealing with on a daily basis,” said Dr. Jackson. These include problems at work and in life with multitasking, remembering details, meeting deadlines, synthesizing large amounts of information, and maintaining focus on the task at hand, he said.
There’s also a fear that even with treatment, the aging of the brain caused by the virus might have long-term repercussions and that this enduring injury may cause the early onset of dementia and Alzheimer’s disease in those who were already vulnerable to it. One study, from the National Institute of Neurological Disorders and Stroke (NINDS), found that in those infected with COVID-19 who already had dementia, the virus “rapidly accelerated structural and functional brain deterioration.”
“We already know the role that neuroinflammation plays in the brains of patients with Alzheimer’s disease,” said Dr. Thompson. “If long COVID is involved in prolonged inflammation of the brain, it goes a long way in explaining the mechanism underlying [the study’s reported] brain aging.”
Still More to Learn
In some ways, this study raises nearly as many questions as it does answers. While it provides concrete evidence around the damage the virus is doing to the brains of patients who contracted severe COVID-19, researchers don’t know about the impact on those who had less serious cases of the virus.
For Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System, the concern is that some long-COVID patients may be suffering from cognitive deficits that are more subtle but still impacting their daily lives, and that they’re not getting the help they need.
What’s more, said Dr. Al-Aly, it’s unclear whether the impacts of the brain damage are permanent or how to stop them from worsening. Researchers and clinicians need a better understanding of the mechanism that allows this virus to enter the brain and do structural damage. If it’s inflammation, will anti-inflammatory or antiviral medications work at preventing it? Will steroids help to offset the damage? “It’s critical we find some answers,” he said.
“SARS-CoV-2 isn’t going anywhere. It will continue to infect the population, so if this is indeed a virus that damages the brain in the long term or permanently, we need to figure out what can be done to stop it,” said Dr. Al-Aly.
A version of this article appeared on Medscape.com.
Healthcare Workers Face Increased Risks During the Pandemic
Healthcare workers have been at an increased risk for SARS-CoV-2 infection and mental distress such as anxiety and depression during the pandemic, according to new research.
The risk for infection was higher among healthcare workers in the first two waves of the pandemic and again during the fifth wave.
“Previous publications, including ours, suggested that the main problem was in the early weeks and months of the pandemic, but this paper shows that it continued until the later stages,” senior author Nicola Cherry, MD, an occupational epidemiologist at the University of Alberta in Edmonton, Canada, told this news organization.
The findings were published in the Canadian Journal of Public Health.
Wave Upon Wave
In the current study, the investigators sought to compare the risk for SARS-CoV-2 infection and mental distress among healthcare workers and among community referents (CRs). They examined the following waves of the COVID-19 pandemic:
- Wave 1: From March to June 2020 (4 months).
- Wave 2: From July 2020 to February 2021 (8 months).
- Wave 3: From March to June 2021 (4 months).
- Wave 4: From July to October 2021 (4 months).
- Wave 5 (Omicron): From November 2021 to March 2022 (5 months).
Healthcare workers in Alberta were asked at recruitment for consent to match their individual records to the Alberta Administrative Health Database. As the pandemic progressed, participants were also asked for consent to be linked to COVID-19 immunization records maintained by the provinces, as well as for the results of all polymerase chain reaction (PCR) testing for the SARS-CoV-2 virus.
The investigators matched 2959 healthcare workers to 14,546 CRs according to their age, sex, geographic location in Alberta, and number of physician claims from April 1, 2019, to March 31, 2020.
Incident SARS-CoV-2 infection was examined using PCR testing and the first date of a physician consultation at which the code for SARS-CoV-2 infection had been recorded. Mental health disorders were identified from physician records. They included anxiety disorders, stress and adjustment reactions, and depressive disorders.
Most (79.5%) of the healthcare workers were registered nurses, followed by physicians (16.1%), healthcare aides (2.4%), and licensed practical nurses (2.0%). Most participants (87.5%) were female. The median age at recruitment was 44 years.
Healthcare workers were at a greater risk for COVID-19 overall, with the first SARS-CoV-2 infection defined from either PCR tests (odds ratio [OR], 1.96) or from physician records (OR, 1.33). They were also at an increased risk for anxiety (adjusted OR, 1.25; P < .001), stress/adjustment reaction (adjusted OR, 1.52; P < .001), and depressive condition (adjusted OR, 1.39; P < .001). Moreover, the excess risks for stress/adjustment reactions and depressive conditions increased with successive waves during the pandemic, peaking in the fourth wave and continuing in the fifth wave.
“Although the increase was less in the middle of the phases of the pandemic, it came back with a vengeance during the last phase, which was the Omicron phase,” said Dr. Cherry.
“Employers of healthcare workers can’t assume that everything is now under control, that they know what they’re doing, and that there is no risk. We are now having some increases in COVID. It’s going to go on. The pandemic is not over in that sense, and infection control continues to be major,” she added.
The finding that mental health worsened among healthcare workers was not surprising, Dr. Cherry said. Even before the pandemic, studies had shown that healthcare workers were at a greater risk for depression than the population overall.
“There is a lot of need for care in mental health support of healthcare workers, whether during a pandemic or not,” said Dr. Cherry.
Nurses Are Suffering
Commenting on the research for this news organization, Farinaz Havaei, PhD, RN, assistant professor of nursing at the University of British Columbia in Vancouver, Canada, said, “This is a very important and timely study that draws on objective clinical and administrative data, as opposed to healthcare workers’ subjective reports.” Dr. Havaei did not participate in the research.
Overall, the findings are consistent with previous research that drew upon healthcare workers’ reports. They speak to the chronic and cumulative impact of COVID-19 and its associated stressors on the mental health and well-being of healthcare workers, said Dr. Havaei.
“The likelihood of stress/adjustment reaction and depression showed a relatively steady increase with increasing COVID-19 waves. This increase can likely be explained by healthcare workers’ depleting emotional reserves for coping with chronic workplace stressors such as concerns about exposure to COVID-19, inadequate staffing, and work overload,” she said. Witnessing the suffering and trauma of patients and their families likely added to this risk.
Dr. Havaei also pointed out that most of the study participants were nurses. The findings are consistent with prepandemic research that showed that the suboptimal conditions that nurses increasingly faced resulted in high levels of exhaustion and burnout.
“While I agree with the authors’ call for more mental health support for healthcare workers, I think prevention efforts that address the root cause of the problem should be prioritized,” she said.
From Heroes to Zeros
The same phenomena have been observed in the United States, said John Q. Young, MD, MPP, PhD, professor and chair of psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. In various studies, Dr. Young and his colleagues have reported a strong association between exposure to the stressors of the pandemic and subsequent development of depression, anxiety, and posttraumatic stress disorder (PTSD) among healthcare workers.
“The findings from Alberta are remarkably consistent. In the beginning of the pandemic, there was a lot of acknowledgment of the work healthcare workers were doing. The fire department clapping as you leave work at night, being called heroes, even though a lot of healthcare workers feel uncomfortable with the hero language because they don’t feel like heroes. Yes, they’re afraid, but they are going to do what they need to do and help,” he said.
But as the pandemic continued, public sentiment changed, Dr. Young said. “They’ve gone from heroes to zeros. Now we are seeing the accumulated, chronic effects over months and years, and these are significant. Our healthcare workforce is vulnerable now. The reserves are low. There are serious shortages in nursing, with more retirements and more people leaving the field,” he said.
As part of a campaign to help healthcare workers cope, psychiatrists at Northwell Health have started a program called Stress First Aid at their Center for Traumatic Stress Response Resilience, where they train nurses, physicians, and other healthcare staff to use basic tools to recognize and respond to stress and distress in themselves and in their colleagues, said Dr. Young.
“For those healthcare workers who find that they are struggling and need more support, there is resilience coaching, which is one-on-one support. For those who need more clinical attention, there is a clinical program where our healthcare workers can meet with a psychologist, psychiatrist, or a therapist, to work through depression, PTSD, and anxiety. We didn’t have this before the pandemic, but it is now a big focus for our workforce,” he said. “We are trying to build resilience. The trauma is real.”
The study was supported by the College of Physicians and Surgeons of Alberta, the Canadian Institutes of Health Research, and the Canadian Immunology Task Force. Dr. Cherry and Dr. Havaei reported no relevant financial relationships. Dr. Young reported that he is senior vice president of behavioral health at Northwell.
A version of this article appeared on Medscape.com.
Healthcare workers have been at an increased risk for SARS-CoV-2 infection and mental distress such as anxiety and depression during the pandemic, according to new research.
The risk for infection was higher among healthcare workers in the first two waves of the pandemic and again during the fifth wave.
“Previous publications, including ours, suggested that the main problem was in the early weeks and months of the pandemic, but this paper shows that it continued until the later stages,” senior author Nicola Cherry, MD, an occupational epidemiologist at the University of Alberta in Edmonton, Canada, told this news organization.
The findings were published in the Canadian Journal of Public Health.
Wave Upon Wave
In the current study, the investigators sought to compare the risk for SARS-CoV-2 infection and mental distress among healthcare workers and among community referents (CRs). They examined the following waves of the COVID-19 pandemic:
- Wave 1: From March to June 2020 (4 months).
- Wave 2: From July 2020 to February 2021 (8 months).
- Wave 3: From March to June 2021 (4 months).
- Wave 4: From July to October 2021 (4 months).
- Wave 5 (Omicron): From November 2021 to March 2022 (5 months).
Healthcare workers in Alberta were asked at recruitment for consent to match their individual records to the Alberta Administrative Health Database. As the pandemic progressed, participants were also asked for consent to be linked to COVID-19 immunization records maintained by the provinces, as well as for the results of all polymerase chain reaction (PCR) testing for the SARS-CoV-2 virus.
The investigators matched 2959 healthcare workers to 14,546 CRs according to their age, sex, geographic location in Alberta, and number of physician claims from April 1, 2019, to March 31, 2020.
Incident SARS-CoV-2 infection was examined using PCR testing and the first date of a physician consultation at which the code for SARS-CoV-2 infection had been recorded. Mental health disorders were identified from physician records. They included anxiety disorders, stress and adjustment reactions, and depressive disorders.
Most (79.5%) of the healthcare workers were registered nurses, followed by physicians (16.1%), healthcare aides (2.4%), and licensed practical nurses (2.0%). Most participants (87.5%) were female. The median age at recruitment was 44 years.
Healthcare workers were at a greater risk for COVID-19 overall, with the first SARS-CoV-2 infection defined from either PCR tests (odds ratio [OR], 1.96) or from physician records (OR, 1.33). They were also at an increased risk for anxiety (adjusted OR, 1.25; P < .001), stress/adjustment reaction (adjusted OR, 1.52; P < .001), and depressive condition (adjusted OR, 1.39; P < .001). Moreover, the excess risks for stress/adjustment reactions and depressive conditions increased with successive waves during the pandemic, peaking in the fourth wave and continuing in the fifth wave.
“Although the increase was less in the middle of the phases of the pandemic, it came back with a vengeance during the last phase, which was the Omicron phase,” said Dr. Cherry.
“Employers of healthcare workers can’t assume that everything is now under control, that they know what they’re doing, and that there is no risk. We are now having some increases in COVID. It’s going to go on. The pandemic is not over in that sense, and infection control continues to be major,” she added.
The finding that mental health worsened among healthcare workers was not surprising, Dr. Cherry said. Even before the pandemic, studies had shown that healthcare workers were at a greater risk for depression than the population overall.
“There is a lot of need for care in mental health support of healthcare workers, whether during a pandemic or not,” said Dr. Cherry.
Nurses Are Suffering
Commenting on the research for this news organization, Farinaz Havaei, PhD, RN, assistant professor of nursing at the University of British Columbia in Vancouver, Canada, said, “This is a very important and timely study that draws on objective clinical and administrative data, as opposed to healthcare workers’ subjective reports.” Dr. Havaei did not participate in the research.
Overall, the findings are consistent with previous research that drew upon healthcare workers’ reports. They speak to the chronic and cumulative impact of COVID-19 and its associated stressors on the mental health and well-being of healthcare workers, said Dr. Havaei.
“The likelihood of stress/adjustment reaction and depression showed a relatively steady increase with increasing COVID-19 waves. This increase can likely be explained by healthcare workers’ depleting emotional reserves for coping with chronic workplace stressors such as concerns about exposure to COVID-19, inadequate staffing, and work overload,” she said. Witnessing the suffering and trauma of patients and their families likely added to this risk.
Dr. Havaei also pointed out that most of the study participants were nurses. The findings are consistent with prepandemic research that showed that the suboptimal conditions that nurses increasingly faced resulted in high levels of exhaustion and burnout.
“While I agree with the authors’ call for more mental health support for healthcare workers, I think prevention efforts that address the root cause of the problem should be prioritized,” she said.
From Heroes to Zeros
The same phenomena have been observed in the United States, said John Q. Young, MD, MPP, PhD, professor and chair of psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. In various studies, Dr. Young and his colleagues have reported a strong association between exposure to the stressors of the pandemic and subsequent development of depression, anxiety, and posttraumatic stress disorder (PTSD) among healthcare workers.
“The findings from Alberta are remarkably consistent. In the beginning of the pandemic, there was a lot of acknowledgment of the work healthcare workers were doing. The fire department clapping as you leave work at night, being called heroes, even though a lot of healthcare workers feel uncomfortable with the hero language because they don’t feel like heroes. Yes, they’re afraid, but they are going to do what they need to do and help,” he said.
But as the pandemic continued, public sentiment changed, Dr. Young said. “They’ve gone from heroes to zeros. Now we are seeing the accumulated, chronic effects over months and years, and these are significant. Our healthcare workforce is vulnerable now. The reserves are low. There are serious shortages in nursing, with more retirements and more people leaving the field,” he said.
As part of a campaign to help healthcare workers cope, psychiatrists at Northwell Health have started a program called Stress First Aid at their Center for Traumatic Stress Response Resilience, where they train nurses, physicians, and other healthcare staff to use basic tools to recognize and respond to stress and distress in themselves and in their colleagues, said Dr. Young.
“For those healthcare workers who find that they are struggling and need more support, there is resilience coaching, which is one-on-one support. For those who need more clinical attention, there is a clinical program where our healthcare workers can meet with a psychologist, psychiatrist, or a therapist, to work through depression, PTSD, and anxiety. We didn’t have this before the pandemic, but it is now a big focus for our workforce,” he said. “We are trying to build resilience. The trauma is real.”
The study was supported by the College of Physicians and Surgeons of Alberta, the Canadian Institutes of Health Research, and the Canadian Immunology Task Force. Dr. Cherry and Dr. Havaei reported no relevant financial relationships. Dr. Young reported that he is senior vice president of behavioral health at Northwell.
A version of this article appeared on Medscape.com.
Healthcare workers have been at an increased risk for SARS-CoV-2 infection and mental distress such as anxiety and depression during the pandemic, according to new research.
The risk for infection was higher among healthcare workers in the first two waves of the pandemic and again during the fifth wave.
“Previous publications, including ours, suggested that the main problem was in the early weeks and months of the pandemic, but this paper shows that it continued until the later stages,” senior author Nicola Cherry, MD, an occupational epidemiologist at the University of Alberta in Edmonton, Canada, told this news organization.
The findings were published in the Canadian Journal of Public Health.
Wave Upon Wave
In the current study, the investigators sought to compare the risk for SARS-CoV-2 infection and mental distress among healthcare workers and among community referents (CRs). They examined the following waves of the COVID-19 pandemic:
- Wave 1: From March to June 2020 (4 months).
- Wave 2: From July 2020 to February 2021 (8 months).
- Wave 3: From March to June 2021 (4 months).
- Wave 4: From July to October 2021 (4 months).
- Wave 5 (Omicron): From November 2021 to March 2022 (5 months).
Healthcare workers in Alberta were asked at recruitment for consent to match their individual records to the Alberta Administrative Health Database. As the pandemic progressed, participants were also asked for consent to be linked to COVID-19 immunization records maintained by the provinces, as well as for the results of all polymerase chain reaction (PCR) testing for the SARS-CoV-2 virus.
The investigators matched 2959 healthcare workers to 14,546 CRs according to their age, sex, geographic location in Alberta, and number of physician claims from April 1, 2019, to March 31, 2020.
Incident SARS-CoV-2 infection was examined using PCR testing and the first date of a physician consultation at which the code for SARS-CoV-2 infection had been recorded. Mental health disorders were identified from physician records. They included anxiety disorders, stress and adjustment reactions, and depressive disorders.
Most (79.5%) of the healthcare workers were registered nurses, followed by physicians (16.1%), healthcare aides (2.4%), and licensed practical nurses (2.0%). Most participants (87.5%) were female. The median age at recruitment was 44 years.
Healthcare workers were at a greater risk for COVID-19 overall, with the first SARS-CoV-2 infection defined from either PCR tests (odds ratio [OR], 1.96) or from physician records (OR, 1.33). They were also at an increased risk for anxiety (adjusted OR, 1.25; P < .001), stress/adjustment reaction (adjusted OR, 1.52; P < .001), and depressive condition (adjusted OR, 1.39; P < .001). Moreover, the excess risks for stress/adjustment reactions and depressive conditions increased with successive waves during the pandemic, peaking in the fourth wave and continuing in the fifth wave.
“Although the increase was less in the middle of the phases of the pandemic, it came back with a vengeance during the last phase, which was the Omicron phase,” said Dr. Cherry.
“Employers of healthcare workers can’t assume that everything is now under control, that they know what they’re doing, and that there is no risk. We are now having some increases in COVID. It’s going to go on. The pandemic is not over in that sense, and infection control continues to be major,” she added.
The finding that mental health worsened among healthcare workers was not surprising, Dr. Cherry said. Even before the pandemic, studies had shown that healthcare workers were at a greater risk for depression than the population overall.
“There is a lot of need for care in mental health support of healthcare workers, whether during a pandemic or not,” said Dr. Cherry.
Nurses Are Suffering
Commenting on the research for this news organization, Farinaz Havaei, PhD, RN, assistant professor of nursing at the University of British Columbia in Vancouver, Canada, said, “This is a very important and timely study that draws on objective clinical and administrative data, as opposed to healthcare workers’ subjective reports.” Dr. Havaei did not participate in the research.
Overall, the findings are consistent with previous research that drew upon healthcare workers’ reports. They speak to the chronic and cumulative impact of COVID-19 and its associated stressors on the mental health and well-being of healthcare workers, said Dr. Havaei.
“The likelihood of stress/adjustment reaction and depression showed a relatively steady increase with increasing COVID-19 waves. This increase can likely be explained by healthcare workers’ depleting emotional reserves for coping with chronic workplace stressors such as concerns about exposure to COVID-19, inadequate staffing, and work overload,” she said. Witnessing the suffering and trauma of patients and their families likely added to this risk.
Dr. Havaei also pointed out that most of the study participants were nurses. The findings are consistent with prepandemic research that showed that the suboptimal conditions that nurses increasingly faced resulted in high levels of exhaustion and burnout.
“While I agree with the authors’ call for more mental health support for healthcare workers, I think prevention efforts that address the root cause of the problem should be prioritized,” she said.
From Heroes to Zeros
The same phenomena have been observed in the United States, said John Q. Young, MD, MPP, PhD, professor and chair of psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. In various studies, Dr. Young and his colleagues have reported a strong association between exposure to the stressors of the pandemic and subsequent development of depression, anxiety, and posttraumatic stress disorder (PTSD) among healthcare workers.
“The findings from Alberta are remarkably consistent. In the beginning of the pandemic, there was a lot of acknowledgment of the work healthcare workers were doing. The fire department clapping as you leave work at night, being called heroes, even though a lot of healthcare workers feel uncomfortable with the hero language because they don’t feel like heroes. Yes, they’re afraid, but they are going to do what they need to do and help,” he said.
But as the pandemic continued, public sentiment changed, Dr. Young said. “They’ve gone from heroes to zeros. Now we are seeing the accumulated, chronic effects over months and years, and these are significant. Our healthcare workforce is vulnerable now. The reserves are low. There are serious shortages in nursing, with more retirements and more people leaving the field,” he said.
As part of a campaign to help healthcare workers cope, psychiatrists at Northwell Health have started a program called Stress First Aid at their Center for Traumatic Stress Response Resilience, where they train nurses, physicians, and other healthcare staff to use basic tools to recognize and respond to stress and distress in themselves and in their colleagues, said Dr. Young.
“For those healthcare workers who find that they are struggling and need more support, there is resilience coaching, which is one-on-one support. For those who need more clinical attention, there is a clinical program where our healthcare workers can meet with a psychologist, psychiatrist, or a therapist, to work through depression, PTSD, and anxiety. We didn’t have this before the pandemic, but it is now a big focus for our workforce,” he said. “We are trying to build resilience. The trauma is real.”
The study was supported by the College of Physicians and Surgeons of Alberta, the Canadian Institutes of Health Research, and the Canadian Immunology Task Force. Dr. Cherry and Dr. Havaei reported no relevant financial relationships. Dr. Young reported that he is senior vice president of behavioral health at Northwell.
A version of this article appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF PUBLIC HEALTH
Europe Needs to Get on Top of Its Measles Outbreak
“Measles should be a memory, not a present risk,” Quique Bassat, MBBS, PhD, director general of the Barcelona Institute of Global Health, told this news organization.
That is certainly not the case right now in some parts of Europe.
“What we are seeing currently is an almost 45-fold rise in measles cases in the WHO European Region,” Siddhartha Datta, MD, European regional advisor on vaccine-preventable diseases and immunization for the WHO, told this news organization. “In 2022, there were 940 cases, and in 2023 till November, it was around 42,000 plus. Between 2020 and 2022, we have seen 1.8 million children who have missed their measles vaccine doses.”
Lapses in Vaccinations
The overriding reason for the resurgence of measles is a backslide in vaccination coverage during the COVID-19 pandemic.
“During the COVID pandemic, we had a 5% decrease in coverage for most of the vaccines, and we are still seeing the consequences,” explained Dr. Bassat. “Measles is the perfect example of when you have a small drop of coverage you get outbreaks, as it’s extremely infectious and complicated to control.”
Reported national coverage with the first dose of measles-containing vaccine in the European Region fell from 96% in 2019 to 93% in 2022. Second-dose coverage fell from 92% in 2019 to 91% in 2022.
“You need to have 95% of the population vaccinated if you want herd immunity,” Dr. Bassat said.
Variation Across Europe
The WHO European Region comprises 53 countries, including Russia and some countries in central Asia. Its figures show Kazakhstan had the most recorded cases of measles last year, at more than 13,000, followed by the Russian Federation.
Romania declared a national epidemic in December 2023. Dr. Datta said there have also been outbreaks in Austria and France.
The UK Health Security Agency declared a major incident in January 2024 because of a surge in cases. From October 2023 to January 2024, there were 347 lab-confirmed cases of measles in England, with 127 of these confirmed in January. The West Midlands is an area of particular concern.
“It was not as though everything was rosy before COVID,” said Dr. Datta. “We saw wide variation in the coverage rates before the pandemic. Some countries weren’t doing as well. More particularly between some communities or municipalities, there were wide variations, and COVID-19 exacerbated the inequities in coverage. What we are seeing now is a combination of gaps before and after the pandemic, so it’s a compound problem.”
Belgium has also seen a measles resurgence, but not as many cases as the year before the pandemic. Laura Cornelissen, MD, works at the Belgian Public Health Institute, Sciensano, where she leads a team working on vaccine-preventable diseases.
She told this news organization: “We did observe a significant rise in cases and several clusters in 2023, compared to the very low numbers that were observed during the COVID-19 years. Preliminary figures indicate 85 measles cases for Belgium in 2023, leading to at least 26 hospitalizations. This is compared with eight cases for 2022, seven in 2021, and 47 in 2020; but 480 cases in the pre-pandemic year 2019.”
Sabrina Bacci, MD, head of vaccine-preventable diseases and immunization at the European Centre of Disease Control, told this news organization: “There have been a high number of cases in Romania and smaller outbreaks in other countries. However, there are a number of European countries which haven’t seen measles. Even though we have this variation between the different European countries, the tools to respond to outbreaks are the same.”
Vaccine Hesitance
Vaccine hesitance or even refusal is on the rise in Europe and elsewhere in the world.
“We can see from behavioral insights that, during COVID, people’s trust on vaccines, healthcare systems, and the government in general has gone down,” said Dr. Datta. “There had been skepticism before about the MMR jab causing autism, which was proved wrong, but vaccine skepticism shown throughout COVID is now showing its head in routine vaccine systems.”
The rise of so-called anti-vaxxers and associated fake conspiracy theories, including a mistrust of Big Pharma, hasn’t been helpful for encouraging essential childhood vaccination uptake, like measles, mumps, and rubella (MMR).
But the MMR vaccine backslide does not only originate in the pandemic.
Vanessa Saliba, consultant epidemiologist at the UK Health Security Agency, said: “MMR vaccine coverage has been falling for the last decade, with 1 out of 10 children starting school in England not protected.”
It could be that some people have religious concerns about the use of pork gelatin as a stabilizer in MMR vaccines. An alternative vaccine that does not contain pork gelatin can be requested.
Doctors and others in healthcare have a pivotal role to play when it comes to getting on top of the surges and educating patients, according to Dr. Bacci. “Healthcare professionals are the most precious resource we have, as they are the ones on the frontline explaining the importance of vaccination to their patients. It’s a very important dialogue.”
Clinics and Catch-Up Campaigns
Intensified routine immunization clinics and catch-up campaigns have been established in countries across Europe where they are needed.
Countries with large outbreaks are carrying out case investigations, identifying and vaccinating susceptible contacts, and generally raising awareness and implementing outbreak response immunization.
“Countries are really making good efforts and are systematically catching up the children who have missed their doses in the last 2 years. But the recovery to the 2019 levels has been slow, and more efforts and energy [need] to be put into this. We understand healthcare systems are stretched out from COVID, but this is not the time to lower our guard,” Dr. Datta said.
“Some countries are more proactive than others,” added Dr. Bassat. “Measles is an example of a disease where you typically organize catch-up campaigns. Measles has one of the highest reproductive numbers, as in the absence of preventive measures one infected person infects 14-16 others.”
All countries, even if they haven’t yet experienced measles outbreaks, are being urged by European healthcare authorities to look at potential immunity gaps and address them immediately.
When Will It Get Back to Normal?
“Measles was a disease that was targeted for elimination, but because of these outbreaks, we are seeing it almost everywhere again. We need to be careful and get on top of this,” warned Dr. Bassat.
Dr. Datta said it’s up to member states, decision-makers, healthcare leaders, and parents to come together to raise the immunity profiles of the European population. “Vaccination is a shared responsibility. The tools are effective. We just need to be ahead of the virus, and that is the challenge.”
Dr. Bacci added, “We have to remember we are entering the spring, which is a season when, traditionally, the disease can spread more easily, and it can find its way when people are susceptible. The vaccine is the tool that can help, and we have to act now and make sure it’s offered on time.”
A version of this article appeared on Medscape.com.
“Measles should be a memory, not a present risk,” Quique Bassat, MBBS, PhD, director general of the Barcelona Institute of Global Health, told this news organization.
That is certainly not the case right now in some parts of Europe.
“What we are seeing currently is an almost 45-fold rise in measles cases in the WHO European Region,” Siddhartha Datta, MD, European regional advisor on vaccine-preventable diseases and immunization for the WHO, told this news organization. “In 2022, there were 940 cases, and in 2023 till November, it was around 42,000 plus. Between 2020 and 2022, we have seen 1.8 million children who have missed their measles vaccine doses.”
Lapses in Vaccinations
The overriding reason for the resurgence of measles is a backslide in vaccination coverage during the COVID-19 pandemic.
“During the COVID pandemic, we had a 5% decrease in coverage for most of the vaccines, and we are still seeing the consequences,” explained Dr. Bassat. “Measles is the perfect example of when you have a small drop of coverage you get outbreaks, as it’s extremely infectious and complicated to control.”
Reported national coverage with the first dose of measles-containing vaccine in the European Region fell from 96% in 2019 to 93% in 2022. Second-dose coverage fell from 92% in 2019 to 91% in 2022.
“You need to have 95% of the population vaccinated if you want herd immunity,” Dr. Bassat said.
Variation Across Europe
The WHO European Region comprises 53 countries, including Russia and some countries in central Asia. Its figures show Kazakhstan had the most recorded cases of measles last year, at more than 13,000, followed by the Russian Federation.
Romania declared a national epidemic in December 2023. Dr. Datta said there have also been outbreaks in Austria and France.
The UK Health Security Agency declared a major incident in January 2024 because of a surge in cases. From October 2023 to January 2024, there were 347 lab-confirmed cases of measles in England, with 127 of these confirmed in January. The West Midlands is an area of particular concern.
“It was not as though everything was rosy before COVID,” said Dr. Datta. “We saw wide variation in the coverage rates before the pandemic. Some countries weren’t doing as well. More particularly between some communities or municipalities, there were wide variations, and COVID-19 exacerbated the inequities in coverage. What we are seeing now is a combination of gaps before and after the pandemic, so it’s a compound problem.”
Belgium has also seen a measles resurgence, but not as many cases as the year before the pandemic. Laura Cornelissen, MD, works at the Belgian Public Health Institute, Sciensano, where she leads a team working on vaccine-preventable diseases.
She told this news organization: “We did observe a significant rise in cases and several clusters in 2023, compared to the very low numbers that were observed during the COVID-19 years. Preliminary figures indicate 85 measles cases for Belgium in 2023, leading to at least 26 hospitalizations. This is compared with eight cases for 2022, seven in 2021, and 47 in 2020; but 480 cases in the pre-pandemic year 2019.”
Sabrina Bacci, MD, head of vaccine-preventable diseases and immunization at the European Centre of Disease Control, told this news organization: “There have been a high number of cases in Romania and smaller outbreaks in other countries. However, there are a number of European countries which haven’t seen measles. Even though we have this variation between the different European countries, the tools to respond to outbreaks are the same.”
Vaccine Hesitance
Vaccine hesitance or even refusal is on the rise in Europe and elsewhere in the world.
“We can see from behavioral insights that, during COVID, people’s trust on vaccines, healthcare systems, and the government in general has gone down,” said Dr. Datta. “There had been skepticism before about the MMR jab causing autism, which was proved wrong, but vaccine skepticism shown throughout COVID is now showing its head in routine vaccine systems.”
The rise of so-called anti-vaxxers and associated fake conspiracy theories, including a mistrust of Big Pharma, hasn’t been helpful for encouraging essential childhood vaccination uptake, like measles, mumps, and rubella (MMR).
But the MMR vaccine backslide does not only originate in the pandemic.
Vanessa Saliba, consultant epidemiologist at the UK Health Security Agency, said: “MMR vaccine coverage has been falling for the last decade, with 1 out of 10 children starting school in England not protected.”
It could be that some people have religious concerns about the use of pork gelatin as a stabilizer in MMR vaccines. An alternative vaccine that does not contain pork gelatin can be requested.
Doctors and others in healthcare have a pivotal role to play when it comes to getting on top of the surges and educating patients, according to Dr. Bacci. “Healthcare professionals are the most precious resource we have, as they are the ones on the frontline explaining the importance of vaccination to their patients. It’s a very important dialogue.”
Clinics and Catch-Up Campaigns
Intensified routine immunization clinics and catch-up campaigns have been established in countries across Europe where they are needed.
Countries with large outbreaks are carrying out case investigations, identifying and vaccinating susceptible contacts, and generally raising awareness and implementing outbreak response immunization.
“Countries are really making good efforts and are systematically catching up the children who have missed their doses in the last 2 years. But the recovery to the 2019 levels has been slow, and more efforts and energy [need] to be put into this. We understand healthcare systems are stretched out from COVID, but this is not the time to lower our guard,” Dr. Datta said.
“Some countries are more proactive than others,” added Dr. Bassat. “Measles is an example of a disease where you typically organize catch-up campaigns. Measles has one of the highest reproductive numbers, as in the absence of preventive measures one infected person infects 14-16 others.”
All countries, even if they haven’t yet experienced measles outbreaks, are being urged by European healthcare authorities to look at potential immunity gaps and address them immediately.
When Will It Get Back to Normal?
“Measles was a disease that was targeted for elimination, but because of these outbreaks, we are seeing it almost everywhere again. We need to be careful and get on top of this,” warned Dr. Bassat.
Dr. Datta said it’s up to member states, decision-makers, healthcare leaders, and parents to come together to raise the immunity profiles of the European population. “Vaccination is a shared responsibility. The tools are effective. We just need to be ahead of the virus, and that is the challenge.”
Dr. Bacci added, “We have to remember we are entering the spring, which is a season when, traditionally, the disease can spread more easily, and it can find its way when people are susceptible. The vaccine is the tool that can help, and we have to act now and make sure it’s offered on time.”
A version of this article appeared on Medscape.com.
“Measles should be a memory, not a present risk,” Quique Bassat, MBBS, PhD, director general of the Barcelona Institute of Global Health, told this news organization.
That is certainly not the case right now in some parts of Europe.
“What we are seeing currently is an almost 45-fold rise in measles cases in the WHO European Region,” Siddhartha Datta, MD, European regional advisor on vaccine-preventable diseases and immunization for the WHO, told this news organization. “In 2022, there were 940 cases, and in 2023 till November, it was around 42,000 plus. Between 2020 and 2022, we have seen 1.8 million children who have missed their measles vaccine doses.”
Lapses in Vaccinations
The overriding reason for the resurgence of measles is a backslide in vaccination coverage during the COVID-19 pandemic.
“During the COVID pandemic, we had a 5% decrease in coverage for most of the vaccines, and we are still seeing the consequences,” explained Dr. Bassat. “Measles is the perfect example of when you have a small drop of coverage you get outbreaks, as it’s extremely infectious and complicated to control.”
Reported national coverage with the first dose of measles-containing vaccine in the European Region fell from 96% in 2019 to 93% in 2022. Second-dose coverage fell from 92% in 2019 to 91% in 2022.
“You need to have 95% of the population vaccinated if you want herd immunity,” Dr. Bassat said.
Variation Across Europe
The WHO European Region comprises 53 countries, including Russia and some countries in central Asia. Its figures show Kazakhstan had the most recorded cases of measles last year, at more than 13,000, followed by the Russian Federation.
Romania declared a national epidemic in December 2023. Dr. Datta said there have also been outbreaks in Austria and France.
The UK Health Security Agency declared a major incident in January 2024 because of a surge in cases. From October 2023 to January 2024, there were 347 lab-confirmed cases of measles in England, with 127 of these confirmed in January. The West Midlands is an area of particular concern.
“It was not as though everything was rosy before COVID,” said Dr. Datta. “We saw wide variation in the coverage rates before the pandemic. Some countries weren’t doing as well. More particularly between some communities or municipalities, there were wide variations, and COVID-19 exacerbated the inequities in coverage. What we are seeing now is a combination of gaps before and after the pandemic, so it’s a compound problem.”
Belgium has also seen a measles resurgence, but not as many cases as the year before the pandemic. Laura Cornelissen, MD, works at the Belgian Public Health Institute, Sciensano, where she leads a team working on vaccine-preventable diseases.
She told this news organization: “We did observe a significant rise in cases and several clusters in 2023, compared to the very low numbers that were observed during the COVID-19 years. Preliminary figures indicate 85 measles cases for Belgium in 2023, leading to at least 26 hospitalizations. This is compared with eight cases for 2022, seven in 2021, and 47 in 2020; but 480 cases in the pre-pandemic year 2019.”
Sabrina Bacci, MD, head of vaccine-preventable diseases and immunization at the European Centre of Disease Control, told this news organization: “There have been a high number of cases in Romania and smaller outbreaks in other countries. However, there are a number of European countries which haven’t seen measles. Even though we have this variation between the different European countries, the tools to respond to outbreaks are the same.”
Vaccine Hesitance
Vaccine hesitance or even refusal is on the rise in Europe and elsewhere in the world.
“We can see from behavioral insights that, during COVID, people’s trust on vaccines, healthcare systems, and the government in general has gone down,” said Dr. Datta. “There had been skepticism before about the MMR jab causing autism, which was proved wrong, but vaccine skepticism shown throughout COVID is now showing its head in routine vaccine systems.”
The rise of so-called anti-vaxxers and associated fake conspiracy theories, including a mistrust of Big Pharma, hasn’t been helpful for encouraging essential childhood vaccination uptake, like measles, mumps, and rubella (MMR).
But the MMR vaccine backslide does not only originate in the pandemic.
Vanessa Saliba, consultant epidemiologist at the UK Health Security Agency, said: “MMR vaccine coverage has been falling for the last decade, with 1 out of 10 children starting school in England not protected.”
It could be that some people have religious concerns about the use of pork gelatin as a stabilizer in MMR vaccines. An alternative vaccine that does not contain pork gelatin can be requested.
Doctors and others in healthcare have a pivotal role to play when it comes to getting on top of the surges and educating patients, according to Dr. Bacci. “Healthcare professionals are the most precious resource we have, as they are the ones on the frontline explaining the importance of vaccination to their patients. It’s a very important dialogue.”
Clinics and Catch-Up Campaigns
Intensified routine immunization clinics and catch-up campaigns have been established in countries across Europe where they are needed.
Countries with large outbreaks are carrying out case investigations, identifying and vaccinating susceptible contacts, and generally raising awareness and implementing outbreak response immunization.
“Countries are really making good efforts and are systematically catching up the children who have missed their doses in the last 2 years. But the recovery to the 2019 levels has been slow, and more efforts and energy [need] to be put into this. We understand healthcare systems are stretched out from COVID, but this is not the time to lower our guard,” Dr. Datta said.
“Some countries are more proactive than others,” added Dr. Bassat. “Measles is an example of a disease where you typically organize catch-up campaigns. Measles has one of the highest reproductive numbers, as in the absence of preventive measures one infected person infects 14-16 others.”
All countries, even if they haven’t yet experienced measles outbreaks, are being urged by European healthcare authorities to look at potential immunity gaps and address them immediately.
When Will It Get Back to Normal?
“Measles was a disease that was targeted for elimination, but because of these outbreaks, we are seeing it almost everywhere again. We need to be careful and get on top of this,” warned Dr. Bassat.
Dr. Datta said it’s up to member states, decision-makers, healthcare leaders, and parents to come together to raise the immunity profiles of the European population. “Vaccination is a shared responsibility. The tools are effective. We just need to be ahead of the virus, and that is the challenge.”
Dr. Bacci added, “We have to remember we are entering the spring, which is a season when, traditionally, the disease can spread more easily, and it can find its way when people are susceptible. The vaccine is the tool that can help, and we have to act now and make sure it’s offered on time.”
A version of this article appeared on Medscape.com.
RNA Vaccines: Risk for Heavy Menstrual Bleeding Clarified
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.