User login
The Journal of Family Practice is a peer-reviewed and indexed journal that provides its 95,000 family physician readers with timely, practical, and evidence-based information that they can immediately put into practice. Research and applied evidence articles, plus patient-oriented departments like Practice Alert, PURLs, and Clinical Inquiries can be found in print and at jfponline.com. The Web site, which logs an average of 125,000 visitors every month, also offers audiocasts by physician specialists and interactive features like Instant Polls and Photo Rounds Friday—a weekly diagnostic puzzle.
gambling
compulsive behaviors
ammunition
assault rifle
black jack
Boko Haram
bondage
child abuse
cocaine
Daech
drug paraphernalia
explosion
gun
human trafficking
ISIL
ISIS
Islamic caliphate
Islamic state
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
pedophile
pedophilia
poker
porn
pornography
psychedelic drug
recreational drug
sex slave rings
slot machine
terrorism
terrorist
Texas hold 'em
UFC
substance abuse
abuseed
abuseer
abusees
abuseing
abusely
abuses
aeolus
aeolused
aeoluser
aeoluses
aeolusing
aeolusly
aeoluss
ahole
aholeed
aholeer
aholees
aholeing
aholely
aholes
alcohol
alcoholed
alcoholer
alcoholes
alcoholing
alcoholly
alcohols
allman
allmaned
allmaner
allmanes
allmaning
allmanly
allmans
alted
altes
alting
altly
alts
analed
analer
anales
analing
anally
analprobe
analprobeed
analprobeer
analprobees
analprobeing
analprobely
analprobes
anals
anilingus
anilingused
anilinguser
anilinguses
anilingusing
anilingusly
anilinguss
anus
anused
anuser
anuses
anusing
anusly
anuss
areola
areolaed
areolaer
areolaes
areolaing
areolaly
areolas
areole
areoleed
areoleer
areolees
areoleing
areolely
areoles
arian
arianed
arianer
arianes
arianing
arianly
arians
aryan
aryaned
aryaner
aryanes
aryaning
aryanly
aryans
asiaed
asiaer
asiaes
asiaing
asialy
asias
ass
ass hole
ass lick
ass licked
ass licker
ass lickes
ass licking
ass lickly
ass licks
assbang
assbanged
assbangeded
assbangeder
assbangedes
assbangeding
assbangedly
assbangeds
assbanger
assbanges
assbanging
assbangly
assbangs
assbangsed
assbangser
assbangses
assbangsing
assbangsly
assbangss
assed
asser
asses
assesed
asseser
asseses
assesing
assesly
assess
assfuck
assfucked
assfucker
assfuckered
assfuckerer
assfuckeres
assfuckering
assfuckerly
assfuckers
assfuckes
assfucking
assfuckly
assfucks
asshat
asshated
asshater
asshates
asshating
asshatly
asshats
assholeed
assholeer
assholees
assholeing
assholely
assholes
assholesed
assholeser
assholeses
assholesing
assholesly
assholess
assing
assly
assmaster
assmastered
assmasterer
assmasteres
assmastering
assmasterly
assmasters
assmunch
assmunched
assmuncher
assmunches
assmunching
assmunchly
assmunchs
asss
asswipe
asswipeed
asswipeer
asswipees
asswipeing
asswipely
asswipes
asswipesed
asswipeser
asswipeses
asswipesing
asswipesly
asswipess
azz
azzed
azzer
azzes
azzing
azzly
azzs
babeed
babeer
babees
babeing
babely
babes
babesed
babeser
babeses
babesing
babesly
babess
ballsac
ballsaced
ballsacer
ballsaces
ballsacing
ballsack
ballsacked
ballsacker
ballsackes
ballsacking
ballsackly
ballsacks
ballsacly
ballsacs
ballsed
ballser
ballses
ballsing
ballsly
ballss
barf
barfed
barfer
barfes
barfing
barfly
barfs
bastard
bastarded
bastarder
bastardes
bastarding
bastardly
bastards
bastardsed
bastardser
bastardses
bastardsing
bastardsly
bastardss
bawdy
bawdyed
bawdyer
bawdyes
bawdying
bawdyly
bawdys
beaner
beanered
beanerer
beaneres
beanering
beanerly
beaners
beardedclam
beardedclamed
beardedclamer
beardedclames
beardedclaming
beardedclamly
beardedclams
beastiality
beastialityed
beastialityer
beastialityes
beastialitying
beastialityly
beastialitys
beatch
beatched
beatcher
beatches
beatching
beatchly
beatchs
beater
beatered
beaterer
beateres
beatering
beaterly
beaters
beered
beerer
beeres
beering
beerly
beeyotch
beeyotched
beeyotcher
beeyotches
beeyotching
beeyotchly
beeyotchs
beotch
beotched
beotcher
beotches
beotching
beotchly
beotchs
biatch
biatched
biatcher
biatches
biatching
biatchly
biatchs
big tits
big titsed
big titser
big titses
big titsing
big titsly
big titss
bigtits
bigtitsed
bigtitser
bigtitses
bigtitsing
bigtitsly
bigtitss
bimbo
bimboed
bimboer
bimboes
bimboing
bimboly
bimbos
bisexualed
bisexualer
bisexuales
bisexualing
bisexually
bisexuals
bitch
bitched
bitcheded
bitcheder
bitchedes
bitcheding
bitchedly
bitcheds
bitcher
bitches
bitchesed
bitcheser
bitcheses
bitchesing
bitchesly
bitchess
bitching
bitchly
bitchs
bitchy
bitchyed
bitchyer
bitchyes
bitchying
bitchyly
bitchys
bleached
bleacher
bleaches
bleaching
bleachly
bleachs
blow job
blow jobed
blow jober
blow jobes
blow jobing
blow jobly
blow jobs
blowed
blower
blowes
blowing
blowjob
blowjobed
blowjober
blowjobes
blowjobing
blowjobly
blowjobs
blowjobsed
blowjobser
blowjobses
blowjobsing
blowjobsly
blowjobss
blowly
blows
boink
boinked
boinker
boinkes
boinking
boinkly
boinks
bollock
bollocked
bollocker
bollockes
bollocking
bollockly
bollocks
bollocksed
bollockser
bollockses
bollocksing
bollocksly
bollockss
bollok
bolloked
bolloker
bollokes
bolloking
bollokly
bolloks
boner
bonered
bonerer
boneres
bonering
bonerly
boners
bonersed
bonerser
bonerses
bonersing
bonersly
bonerss
bong
bonged
bonger
bonges
bonging
bongly
bongs
boob
boobed
boober
boobes
boobies
boobiesed
boobieser
boobieses
boobiesing
boobiesly
boobiess
boobing
boobly
boobs
boobsed
boobser
boobses
boobsing
boobsly
boobss
booby
boobyed
boobyer
boobyes
boobying
boobyly
boobys
booger
boogered
boogerer
boogeres
boogering
boogerly
boogers
bookie
bookieed
bookieer
bookiees
bookieing
bookiely
bookies
bootee
booteeed
booteeer
booteees
booteeing
booteely
bootees
bootie
bootieed
bootieer
bootiees
bootieing
bootiely
booties
booty
bootyed
bootyer
bootyes
bootying
bootyly
bootys
boozeed
boozeer
boozees
boozeing
boozely
boozer
boozered
boozerer
boozeres
boozering
boozerly
boozers
boozes
boozy
boozyed
boozyer
boozyes
boozying
boozyly
boozys
bosomed
bosomer
bosomes
bosoming
bosomly
bosoms
bosomy
bosomyed
bosomyer
bosomyes
bosomying
bosomyly
bosomys
bugger
buggered
buggerer
buggeres
buggering
buggerly
buggers
bukkake
bukkakeed
bukkakeer
bukkakees
bukkakeing
bukkakely
bukkakes
bull shit
bull shited
bull shiter
bull shites
bull shiting
bull shitly
bull shits
bullshit
bullshited
bullshiter
bullshites
bullshiting
bullshitly
bullshits
bullshitsed
bullshitser
bullshitses
bullshitsing
bullshitsly
bullshitss
bullshitted
bullshitteded
bullshitteder
bullshittedes
bullshitteding
bullshittedly
bullshitteds
bullturds
bullturdsed
bullturdser
bullturdses
bullturdsing
bullturdsly
bullturdss
bung
bunged
bunger
bunges
bunging
bungly
bungs
busty
bustyed
bustyer
bustyes
bustying
bustyly
bustys
butt
butt fuck
butt fucked
butt fucker
butt fuckes
butt fucking
butt fuckly
butt fucks
butted
buttes
buttfuck
buttfucked
buttfucker
buttfuckered
buttfuckerer
buttfuckeres
buttfuckering
buttfuckerly
buttfuckers
buttfuckes
buttfucking
buttfuckly
buttfucks
butting
buttly
buttplug
buttpluged
buttpluger
buttpluges
buttpluging
buttplugly
buttplugs
butts
caca
cacaed
cacaer
cacaes
cacaing
cacaly
cacas
cahone
cahoneed
cahoneer
cahonees
cahoneing
cahonely
cahones
cameltoe
cameltoeed
cameltoeer
cameltoees
cameltoeing
cameltoely
cameltoes
carpetmuncher
carpetmunchered
carpetmuncherer
carpetmuncheres
carpetmunchering
carpetmuncherly
carpetmunchers
cawk
cawked
cawker
cawkes
cawking
cawkly
cawks
chinc
chinced
chincer
chinces
chincing
chincly
chincs
chincsed
chincser
chincses
chincsing
chincsly
chincss
chink
chinked
chinker
chinkes
chinking
chinkly
chinks
chode
chodeed
chodeer
chodees
chodeing
chodely
chodes
chodesed
chodeser
chodeses
chodesing
chodesly
chodess
clit
clited
cliter
clites
cliting
clitly
clitoris
clitorised
clitoriser
clitorises
clitorising
clitorisly
clitoriss
clitorus
clitorused
clitoruser
clitoruses
clitorusing
clitorusly
clitoruss
clits
clitsed
clitser
clitses
clitsing
clitsly
clitss
clitty
clittyed
clittyer
clittyes
clittying
clittyly
clittys
cocain
cocaine
cocained
cocaineed
cocaineer
cocainees
cocaineing
cocainely
cocainer
cocaines
cocaining
cocainly
cocains
cock
cock sucker
cock suckered
cock suckerer
cock suckeres
cock suckering
cock suckerly
cock suckers
cockblock
cockblocked
cockblocker
cockblockes
cockblocking
cockblockly
cockblocks
cocked
cocker
cockes
cockholster
cockholstered
cockholsterer
cockholsteres
cockholstering
cockholsterly
cockholsters
cocking
cockknocker
cockknockered
cockknockerer
cockknockeres
cockknockering
cockknockerly
cockknockers
cockly
cocks
cocksed
cockser
cockses
cocksing
cocksly
cocksmoker
cocksmokered
cocksmokerer
cocksmokeres
cocksmokering
cocksmokerly
cocksmokers
cockss
cocksucker
cocksuckered
cocksuckerer
cocksuckeres
cocksuckering
cocksuckerly
cocksuckers
coital
coitaled
coitaler
coitales
coitaling
coitally
coitals
commie
commieed
commieer
commiees
commieing
commiely
commies
condomed
condomer
condomes
condoming
condomly
condoms
coon
cooned
cooner
coones
cooning
coonly
coons
coonsed
coonser
coonses
coonsing
coonsly
coonss
corksucker
corksuckered
corksuckerer
corksuckeres
corksuckering
corksuckerly
corksuckers
cracked
crackwhore
crackwhoreed
crackwhoreer
crackwhorees
crackwhoreing
crackwhorely
crackwhores
crap
craped
craper
crapes
craping
craply
crappy
crappyed
crappyer
crappyes
crappying
crappyly
crappys
cum
cumed
cumer
cumes
cuming
cumly
cummin
cummined
cumminer
cummines
cumming
cumminged
cumminger
cumminges
cumminging
cummingly
cummings
cummining
cumminly
cummins
cums
cumshot
cumshoted
cumshoter
cumshotes
cumshoting
cumshotly
cumshots
cumshotsed
cumshotser
cumshotses
cumshotsing
cumshotsly
cumshotss
cumslut
cumsluted
cumsluter
cumslutes
cumsluting
cumslutly
cumsluts
cumstain
cumstained
cumstainer
cumstaines
cumstaining
cumstainly
cumstains
cunilingus
cunilingused
cunilinguser
cunilinguses
cunilingusing
cunilingusly
cunilinguss
cunnilingus
cunnilingused
cunnilinguser
cunnilinguses
cunnilingusing
cunnilingusly
cunnilinguss
cunny
cunnyed
cunnyer
cunnyes
cunnying
cunnyly
cunnys
cunt
cunted
cunter
cuntes
cuntface
cuntfaceed
cuntfaceer
cuntfacees
cuntfaceing
cuntfacely
cuntfaces
cunthunter
cunthuntered
cunthunterer
cunthunteres
cunthuntering
cunthunterly
cunthunters
cunting
cuntlick
cuntlicked
cuntlicker
cuntlickered
cuntlickerer
cuntlickeres
cuntlickering
cuntlickerly
cuntlickers
cuntlickes
cuntlicking
cuntlickly
cuntlicks
cuntly
cunts
cuntsed
cuntser
cuntses
cuntsing
cuntsly
cuntss
dago
dagoed
dagoer
dagoes
dagoing
dagoly
dagos
dagosed
dagoser
dagoses
dagosing
dagosly
dagoss
dammit
dammited
dammiter
dammites
dammiting
dammitly
dammits
damn
damned
damneded
damneder
damnedes
damneding
damnedly
damneds
damner
damnes
damning
damnit
damnited
damniter
damnites
damniting
damnitly
damnits
damnly
damns
dick
dickbag
dickbaged
dickbager
dickbages
dickbaging
dickbagly
dickbags
dickdipper
dickdippered
dickdipperer
dickdipperes
dickdippering
dickdipperly
dickdippers
dicked
dicker
dickes
dickface
dickfaceed
dickfaceer
dickfacees
dickfaceing
dickfacely
dickfaces
dickflipper
dickflippered
dickflipperer
dickflipperes
dickflippering
dickflipperly
dickflippers
dickhead
dickheaded
dickheader
dickheades
dickheading
dickheadly
dickheads
dickheadsed
dickheadser
dickheadses
dickheadsing
dickheadsly
dickheadss
dicking
dickish
dickished
dickisher
dickishes
dickishing
dickishly
dickishs
dickly
dickripper
dickrippered
dickripperer
dickripperes
dickrippering
dickripperly
dickrippers
dicks
dicksipper
dicksippered
dicksipperer
dicksipperes
dicksippering
dicksipperly
dicksippers
dickweed
dickweeded
dickweeder
dickweedes
dickweeding
dickweedly
dickweeds
dickwhipper
dickwhippered
dickwhipperer
dickwhipperes
dickwhippering
dickwhipperly
dickwhippers
dickzipper
dickzippered
dickzipperer
dickzipperes
dickzippering
dickzipperly
dickzippers
diddle
diddleed
diddleer
diddlees
diddleing
diddlely
diddles
dike
dikeed
dikeer
dikees
dikeing
dikely
dikes
dildo
dildoed
dildoer
dildoes
dildoing
dildoly
dildos
dildosed
dildoser
dildoses
dildosing
dildosly
dildoss
diligaf
diligafed
diligafer
diligafes
diligafing
diligafly
diligafs
dillweed
dillweeded
dillweeder
dillweedes
dillweeding
dillweedly
dillweeds
dimwit
dimwited
dimwiter
dimwites
dimwiting
dimwitly
dimwits
dingle
dingleed
dingleer
dinglees
dingleing
dinglely
dingles
dipship
dipshiped
dipshiper
dipshipes
dipshiping
dipshiply
dipships
dizzyed
dizzyer
dizzyes
dizzying
dizzyly
dizzys
doggiestyleed
doggiestyleer
doggiestylees
doggiestyleing
doggiestylely
doggiestyles
doggystyleed
doggystyleer
doggystylees
doggystyleing
doggystylely
doggystyles
dong
donged
donger
donges
donging
dongly
dongs
doofus
doofused
doofuser
doofuses
doofusing
doofusly
doofuss
doosh
dooshed
doosher
dooshes
dooshing
dooshly
dooshs
dopeyed
dopeyer
dopeyes
dopeying
dopeyly
dopeys
douchebag
douchebaged
douchebager
douchebages
douchebaging
douchebagly
douchebags
douchebagsed
douchebagser
douchebagses
douchebagsing
douchebagsly
douchebagss
doucheed
doucheer
douchees
doucheing
douchely
douches
douchey
doucheyed
doucheyer
doucheyes
doucheying
doucheyly
doucheys
drunk
drunked
drunker
drunkes
drunking
drunkly
drunks
dumass
dumassed
dumasser
dumasses
dumassing
dumassly
dumasss
dumbass
dumbassed
dumbasser
dumbasses
dumbassesed
dumbasseser
dumbasseses
dumbassesing
dumbassesly
dumbassess
dumbassing
dumbassly
dumbasss
dummy
dummyed
dummyer
dummyes
dummying
dummyly
dummys
dyke
dykeed
dykeer
dykees
dykeing
dykely
dykes
dykesed
dykeser
dykeses
dykesing
dykesly
dykess
erotic
eroticed
eroticer
erotices
eroticing
eroticly
erotics
extacy
extacyed
extacyer
extacyes
extacying
extacyly
extacys
extasy
extasyed
extasyer
extasyes
extasying
extasyly
extasys
fack
facked
facker
fackes
facking
fackly
facks
fag
faged
fager
fages
fagg
fagged
faggeded
faggeder
faggedes
faggeding
faggedly
faggeds
fagger
fagges
fagging
faggit
faggited
faggiter
faggites
faggiting
faggitly
faggits
faggly
faggot
faggoted
faggoter
faggotes
faggoting
faggotly
faggots
faggs
faging
fagly
fagot
fagoted
fagoter
fagotes
fagoting
fagotly
fagots
fags
fagsed
fagser
fagses
fagsing
fagsly
fagss
faig
faiged
faiger
faiges
faiging
faigly
faigs
faigt
faigted
faigter
faigtes
faigting
faigtly
faigts
fannybandit
fannybandited
fannybanditer
fannybandites
fannybanditing
fannybanditly
fannybandits
farted
farter
fartes
farting
fartknocker
fartknockered
fartknockerer
fartknockeres
fartknockering
fartknockerly
fartknockers
fartly
farts
felch
felched
felcher
felchered
felcherer
felcheres
felchering
felcherly
felchers
felches
felching
felchinged
felchinger
felchinges
felchinging
felchingly
felchings
felchly
felchs
fellate
fellateed
fellateer
fellatees
fellateing
fellately
fellates
fellatio
fellatioed
fellatioer
fellatioes
fellatioing
fellatioly
fellatios
feltch
feltched
feltcher
feltchered
feltcherer
feltcheres
feltchering
feltcherly
feltchers
feltches
feltching
feltchly
feltchs
feom
feomed
feomer
feomes
feoming
feomly
feoms
fisted
fisteded
fisteder
fistedes
fisteding
fistedly
fisteds
fisting
fistinged
fistinger
fistinges
fistinging
fistingly
fistings
fisty
fistyed
fistyer
fistyes
fistying
fistyly
fistys
floozy
floozyed
floozyer
floozyes
floozying
floozyly
floozys
foad
foaded
foader
foades
foading
foadly
foads
fondleed
fondleer
fondlees
fondleing
fondlely
fondles
foobar
foobared
foobarer
foobares
foobaring
foobarly
foobars
freex
freexed
freexer
freexes
freexing
freexly
freexs
frigg
frigga
friggaed
friggaer
friggaes
friggaing
friggaly
friggas
frigged
frigger
frigges
frigging
friggly
friggs
fubar
fubared
fubarer
fubares
fubaring
fubarly
fubars
fuck
fuckass
fuckassed
fuckasser
fuckasses
fuckassing
fuckassly
fuckasss
fucked
fuckeded
fuckeder
fuckedes
fuckeding
fuckedly
fuckeds
fucker
fuckered
fuckerer
fuckeres
fuckering
fuckerly
fuckers
fuckes
fuckface
fuckfaceed
fuckfaceer
fuckfacees
fuckfaceing
fuckfacely
fuckfaces
fuckin
fuckined
fuckiner
fuckines
fucking
fuckinged
fuckinger
fuckinges
fuckinging
fuckingly
fuckings
fuckining
fuckinly
fuckins
fuckly
fucknugget
fucknuggeted
fucknuggeter
fucknuggetes
fucknuggeting
fucknuggetly
fucknuggets
fucknut
fucknuted
fucknuter
fucknutes
fucknuting
fucknutly
fucknuts
fuckoff
fuckoffed
fuckoffer
fuckoffes
fuckoffing
fuckoffly
fuckoffs
fucks
fucksed
fuckser
fuckses
fucksing
fucksly
fuckss
fucktard
fucktarded
fucktarder
fucktardes
fucktarding
fucktardly
fucktards
fuckup
fuckuped
fuckuper
fuckupes
fuckuping
fuckuply
fuckups
fuckwad
fuckwaded
fuckwader
fuckwades
fuckwading
fuckwadly
fuckwads
fuckwit
fuckwited
fuckwiter
fuckwites
fuckwiting
fuckwitly
fuckwits
fudgepacker
fudgepackered
fudgepackerer
fudgepackeres
fudgepackering
fudgepackerly
fudgepackers
fuk
fuked
fuker
fukes
fuking
fukly
fuks
fvck
fvcked
fvcker
fvckes
fvcking
fvckly
fvcks
fxck
fxcked
fxcker
fxckes
fxcking
fxckly
fxcks
gae
gaeed
gaeer
gaees
gaeing
gaely
gaes
gai
gaied
gaier
gaies
gaiing
gaily
gais
ganja
ganjaed
ganjaer
ganjaes
ganjaing
ganjaly
ganjas
gayed
gayer
gayes
gaying
gayly
gays
gaysed
gayser
gayses
gaysing
gaysly
gayss
gey
geyed
geyer
geyes
geying
geyly
geys
gfc
gfced
gfcer
gfces
gfcing
gfcly
gfcs
gfy
gfyed
gfyer
gfyes
gfying
gfyly
gfys
ghay
ghayed
ghayer
ghayes
ghaying
ghayly
ghays
ghey
gheyed
gheyer
gheyes
gheying
gheyly
gheys
gigolo
gigoloed
gigoloer
gigoloes
gigoloing
gigololy
gigolos
goatse
goatseed
goatseer
goatsees
goatseing
goatsely
goatses
godamn
godamned
godamner
godamnes
godamning
godamnit
godamnited
godamniter
godamnites
godamniting
godamnitly
godamnits
godamnly
godamns
goddam
goddamed
goddamer
goddames
goddaming
goddamly
goddammit
goddammited
goddammiter
goddammites
goddammiting
goddammitly
goddammits
goddamn
goddamned
goddamner
goddamnes
goddamning
goddamnly
goddamns
goddams
goldenshower
goldenshowered
goldenshowerer
goldenshoweres
goldenshowering
goldenshowerly
goldenshowers
gonad
gonaded
gonader
gonades
gonading
gonadly
gonads
gonadsed
gonadser
gonadses
gonadsing
gonadsly
gonadss
gook
gooked
gooker
gookes
gooking
gookly
gooks
gooksed
gookser
gookses
gooksing
gooksly
gookss
gringo
gringoed
gringoer
gringoes
gringoing
gringoly
gringos
gspot
gspoted
gspoter
gspotes
gspoting
gspotly
gspots
gtfo
gtfoed
gtfoer
gtfoes
gtfoing
gtfoly
gtfos
guido
guidoed
guidoer
guidoes
guidoing
guidoly
guidos
handjob
handjobed
handjober
handjobes
handjobing
handjobly
handjobs
hard on
hard oned
hard oner
hard ones
hard oning
hard only
hard ons
hardknight
hardknighted
hardknighter
hardknightes
hardknighting
hardknightly
hardknights
hebe
hebeed
hebeer
hebees
hebeing
hebely
hebes
heeb
heebed
heeber
heebes
heebing
heebly
heebs
hell
helled
heller
helles
helling
hellly
hells
hemp
hemped
hemper
hempes
hemping
hemply
hemps
heroined
heroiner
heroines
heroining
heroinly
heroins
herp
herped
herper
herpes
herpesed
herpeser
herpeses
herpesing
herpesly
herpess
herping
herply
herps
herpy
herpyed
herpyer
herpyes
herpying
herpyly
herpys
hitler
hitlered
hitlerer
hitleres
hitlering
hitlerly
hitlers
hived
hiver
hives
hiving
hivly
hivs
hobag
hobaged
hobager
hobages
hobaging
hobagly
hobags
homey
homeyed
homeyer
homeyes
homeying
homeyly
homeys
homo
homoed
homoer
homoes
homoey
homoeyed
homoeyer
homoeyes
homoeying
homoeyly
homoeys
homoing
homoly
homos
honky
honkyed
honkyer
honkyes
honkying
honkyly
honkys
hooch
hooched
hoocher
hooches
hooching
hoochly
hoochs
hookah
hookahed
hookaher
hookahes
hookahing
hookahly
hookahs
hooker
hookered
hookerer
hookeres
hookering
hookerly
hookers
hoor
hoored
hoorer
hoores
hooring
hoorly
hoors
hootch
hootched
hootcher
hootches
hootching
hootchly
hootchs
hooter
hootered
hooterer
hooteres
hootering
hooterly
hooters
hootersed
hooterser
hooterses
hootersing
hootersly
hooterss
horny
hornyed
hornyer
hornyes
hornying
hornyly
hornys
houstoned
houstoner
houstones
houstoning
houstonly
houstons
hump
humped
humpeded
humpeder
humpedes
humpeding
humpedly
humpeds
humper
humpes
humping
humpinged
humpinger
humpinges
humpinging
humpingly
humpings
humply
humps
husbanded
husbander
husbandes
husbanding
husbandly
husbands
hussy
hussyed
hussyer
hussyes
hussying
hussyly
hussys
hymened
hymener
hymenes
hymening
hymenly
hymens
inbred
inbreded
inbreder
inbredes
inbreding
inbredly
inbreds
incest
incested
incester
incestes
incesting
incestly
incests
injun
injuned
injuner
injunes
injuning
injunly
injuns
jackass
jackassed
jackasser
jackasses
jackassing
jackassly
jackasss
jackhole
jackholeed
jackholeer
jackholees
jackholeing
jackholely
jackholes
jackoff
jackoffed
jackoffer
jackoffes
jackoffing
jackoffly
jackoffs
jap
japed
japer
japes
japing
japly
japs
japsed
japser
japses
japsing
japsly
japss
jerkoff
jerkoffed
jerkoffer
jerkoffes
jerkoffing
jerkoffly
jerkoffs
jerks
jism
jismed
jismer
jismes
jisming
jismly
jisms
jiz
jized
jizer
jizes
jizing
jizly
jizm
jizmed
jizmer
jizmes
jizming
jizmly
jizms
jizs
jizz
jizzed
jizzeded
jizzeder
jizzedes
jizzeding
jizzedly
jizzeds
jizzer
jizzes
jizzing
jizzly
jizzs
junkie
junkieed
junkieer
junkiees
junkieing
junkiely
junkies
junky
junkyed
junkyer
junkyes
junkying
junkyly
junkys
kike
kikeed
kikeer
kikees
kikeing
kikely
kikes
kikesed
kikeser
kikeses
kikesing
kikesly
kikess
killed
killer
killes
killing
killly
kills
kinky
kinkyed
kinkyer
kinkyes
kinkying
kinkyly
kinkys
kkk
kkked
kkker
kkkes
kkking
kkkly
kkks
klan
klaned
klaner
klanes
klaning
klanly
klans
knobend
knobended
knobender
knobendes
knobending
knobendly
knobends
kooch
kooched
koocher
kooches
koochesed
koocheser
koocheses
koochesing
koochesly
koochess
kooching
koochly
koochs
kootch
kootched
kootcher
kootches
kootching
kootchly
kootchs
kraut
krauted
krauter
krautes
krauting
krautly
krauts
kyke
kykeed
kykeer
kykees
kykeing
kykely
kykes
lech
leched
lecher
leches
leching
lechly
lechs
leper
lepered
leperer
leperes
lepering
leperly
lepers
lesbiansed
lesbianser
lesbianses
lesbiansing
lesbiansly
lesbianss
lesbo
lesboed
lesboer
lesboes
lesboing
lesboly
lesbos
lesbosed
lesboser
lesboses
lesbosing
lesbosly
lesboss
lez
lezbianed
lezbianer
lezbianes
lezbianing
lezbianly
lezbians
lezbiansed
lezbianser
lezbianses
lezbiansing
lezbiansly
lezbianss
lezbo
lezboed
lezboer
lezboes
lezboing
lezboly
lezbos
lezbosed
lezboser
lezboses
lezbosing
lezbosly
lezboss
lezed
lezer
lezes
lezing
lezly
lezs
lezzie
lezzieed
lezzieer
lezziees
lezzieing
lezziely
lezzies
lezziesed
lezzieser
lezzieses
lezziesing
lezziesly
lezziess
lezzy
lezzyed
lezzyer
lezzyes
lezzying
lezzyly
lezzys
lmaoed
lmaoer
lmaoes
lmaoing
lmaoly
lmaos
lmfao
lmfaoed
lmfaoer
lmfaoes
lmfaoing
lmfaoly
lmfaos
loined
loiner
loines
loining
loinly
loins
loinsed
loinser
loinses
loinsing
loinsly
loinss
lubeed
lubeer
lubees
lubeing
lubely
lubes
lusty
lustyed
lustyer
lustyes
lustying
lustyly
lustys
massa
massaed
massaer
massaes
massaing
massaly
massas
masterbate
masterbateed
masterbateer
masterbatees
masterbateing
masterbately
masterbates
masterbating
masterbatinged
masterbatinger
masterbatinges
masterbatinging
masterbatingly
masterbatings
masterbation
masterbationed
masterbationer
masterbationes
masterbationing
masterbationly
masterbations
masturbate
masturbateed
masturbateer
masturbatees
masturbateing
masturbately
masturbates
masturbating
masturbatinged
masturbatinger
masturbatinges
masturbatinging
masturbatingly
masturbatings
masturbation
masturbationed
masturbationer
masturbationes
masturbationing
masturbationly
masturbations
methed
mether
methes
mething
methly
meths
militaryed
militaryer
militaryes
militarying
militaryly
militarys
mofo
mofoed
mofoer
mofoes
mofoing
mofoly
mofos
molest
molested
molester
molestes
molesting
molestly
molests
moolie
moolieed
moolieer
mooliees
moolieing
mooliely
moolies
moron
moroned
moroner
morones
moroning
moronly
morons
motherfucka
motherfuckaed
motherfuckaer
motherfuckaes
motherfuckaing
motherfuckaly
motherfuckas
motherfucker
motherfuckered
motherfuckerer
motherfuckeres
motherfuckering
motherfuckerly
motherfuckers
motherfucking
motherfuckinged
motherfuckinger
motherfuckinges
motherfuckinging
motherfuckingly
motherfuckings
mtherfucker
mtherfuckered
mtherfuckerer
mtherfuckeres
mtherfuckering
mtherfuckerly
mtherfuckers
mthrfucker
mthrfuckered
mthrfuckerer
mthrfuckeres
mthrfuckering
mthrfuckerly
mthrfuckers
mthrfucking
mthrfuckinged
mthrfuckinger
mthrfuckinges
mthrfuckinging
mthrfuckingly
mthrfuckings
muff
muffdiver
muffdivered
muffdiverer
muffdiveres
muffdivering
muffdiverly
muffdivers
muffed
muffer
muffes
muffing
muffly
muffs
murdered
murderer
murderes
murdering
murderly
murders
muthafuckaz
muthafuckazed
muthafuckazer
muthafuckazes
muthafuckazing
muthafuckazly
muthafuckazs
muthafucker
muthafuckered
muthafuckerer
muthafuckeres
muthafuckering
muthafuckerly
muthafuckers
mutherfucker
mutherfuckered
mutherfuckerer
mutherfuckeres
mutherfuckering
mutherfuckerly
mutherfuckers
mutherfucking
mutherfuckinged
mutherfuckinger
mutherfuckinges
mutherfuckinging
mutherfuckingly
mutherfuckings
muthrfucking
muthrfuckinged
muthrfuckinger
muthrfuckinges
muthrfuckinging
muthrfuckingly
muthrfuckings
nad
naded
nader
nades
nading
nadly
nads
nadsed
nadser
nadses
nadsing
nadsly
nadss
nakeded
nakeder
nakedes
nakeding
nakedly
nakeds
napalm
napalmed
napalmer
napalmes
napalming
napalmly
napalms
nappy
nappyed
nappyer
nappyes
nappying
nappyly
nappys
nazi
nazied
nazier
nazies
naziing
nazily
nazis
nazism
nazismed
nazismer
nazismes
nazisming
nazismly
nazisms
negro
negroed
negroer
negroes
negroing
negroly
negros
nigga
niggaed
niggaer
niggaes
niggah
niggahed
niggaher
niggahes
niggahing
niggahly
niggahs
niggaing
niggaly
niggas
niggased
niggaser
niggases
niggasing
niggasly
niggass
niggaz
niggazed
niggazer
niggazes
niggazing
niggazly
niggazs
nigger
niggered
niggerer
niggeres
niggering
niggerly
niggers
niggersed
niggerser
niggerses
niggersing
niggersly
niggerss
niggle
niggleed
niggleer
nigglees
niggleing
nigglely
niggles
niglet
nigleted
nigleter
nigletes
nigleting
nigletly
niglets
nimrod
nimroded
nimroder
nimrodes
nimroding
nimrodly
nimrods
ninny
ninnyed
ninnyer
ninnyes
ninnying
ninnyly
ninnys
nooky
nookyed
nookyer
nookyes
nookying
nookyly
nookys
nuccitelli
nuccitellied
nuccitellier
nuccitellies
nuccitelliing
nuccitellily
nuccitellis
nympho
nymphoed
nymphoer
nymphoes
nymphoing
nympholy
nymphos
opium
opiumed
opiumer
opiumes
opiuming
opiumly
opiums
orgies
orgiesed
orgieser
orgieses
orgiesing
orgiesly
orgiess
orgy
orgyed
orgyer
orgyes
orgying
orgyly
orgys
paddy
paddyed
paddyer
paddyes
paddying
paddyly
paddys
paki
pakied
pakier
pakies
pakiing
pakily
pakis
pantie
pantieed
pantieer
pantiees
pantieing
pantiely
panties
pantiesed
pantieser
pantieses
pantiesing
pantiesly
pantiess
panty
pantyed
pantyer
pantyes
pantying
pantyly
pantys
pastie
pastieed
pastieer
pastiees
pastieing
pastiely
pasties
pasty
pastyed
pastyer
pastyes
pastying
pastyly
pastys
pecker
peckered
peckerer
peckeres
peckering
peckerly
peckers
pedo
pedoed
pedoer
pedoes
pedoing
pedoly
pedophile
pedophileed
pedophileer
pedophilees
pedophileing
pedophilely
pedophiles
pedophilia
pedophiliac
pedophiliaced
pedophiliacer
pedophiliaces
pedophiliacing
pedophiliacly
pedophiliacs
pedophiliaed
pedophiliaer
pedophiliaes
pedophiliaing
pedophilialy
pedophilias
pedos
penial
penialed
penialer
peniales
penialing
penially
penials
penile
penileed
penileer
penilees
penileing
penilely
peniles
penis
penised
peniser
penises
penising
penisly
peniss
perversion
perversioned
perversioner
perversiones
perversioning
perversionly
perversions
peyote
peyoteed
peyoteer
peyotees
peyoteing
peyotely
peyotes
phuck
phucked
phucker
phuckes
phucking
phuckly
phucks
pillowbiter
pillowbitered
pillowbiterer
pillowbiteres
pillowbitering
pillowbiterly
pillowbiters
pimp
pimped
pimper
pimpes
pimping
pimply
pimps
pinko
pinkoed
pinkoer
pinkoes
pinkoing
pinkoly
pinkos
pissed
pisseded
pisseder
pissedes
pisseding
pissedly
pisseds
pisser
pisses
pissing
pissly
pissoff
pissoffed
pissoffer
pissoffes
pissoffing
pissoffly
pissoffs
pisss
polack
polacked
polacker
polackes
polacking
polackly
polacks
pollock
pollocked
pollocker
pollockes
pollocking
pollockly
pollocks
poon
pooned
pooner
poones
pooning
poonly
poons
poontang
poontanged
poontanger
poontanges
poontanging
poontangly
poontangs
porn
porned
porner
pornes
porning
pornly
porno
pornoed
pornoer
pornoes
pornography
pornographyed
pornographyer
pornographyes
pornographying
pornographyly
pornographys
pornoing
pornoly
pornos
porns
prick
pricked
pricker
prickes
pricking
prickly
pricks
prig
priged
priger
priges
priging
prigly
prigs
prostitute
prostituteed
prostituteer
prostitutees
prostituteing
prostitutely
prostitutes
prude
prudeed
prudeer
prudees
prudeing
prudely
prudes
punkass
punkassed
punkasser
punkasses
punkassing
punkassly
punkasss
punky
punkyed
punkyer
punkyes
punkying
punkyly
punkys
puss
pussed
pusser
pusses
pussies
pussiesed
pussieser
pussieses
pussiesing
pussiesly
pussiess
pussing
pussly
pusss
pussy
pussyed
pussyer
pussyes
pussying
pussyly
pussypounder
pussypoundered
pussypounderer
pussypounderes
pussypoundering
pussypounderly
pussypounders
pussys
puto
putoed
putoer
putoes
putoing
putoly
putos
queaf
queafed
queafer
queafes
queafing
queafly
queafs
queef
queefed
queefer
queefes
queefing
queefly
queefs
queer
queered
queerer
queeres
queering
queerly
queero
queeroed
queeroer
queeroes
queeroing
queeroly
queeros
queers
queersed
queerser
queerses
queersing
queersly
queerss
quicky
quickyed
quickyer
quickyes
quickying
quickyly
quickys
quim
quimed
quimer
quimes
quiming
quimly
quims
racy
racyed
racyer
racyes
racying
racyly
racys
rape
raped
rapeded
rapeder
rapedes
rapeding
rapedly
rapeds
rapeed
rapeer
rapees
rapeing
rapely
raper
rapered
raperer
raperes
rapering
raperly
rapers
rapes
rapist
rapisted
rapister
rapistes
rapisting
rapistly
rapists
raunch
raunched
rauncher
raunches
raunching
raunchly
raunchs
rectus
rectused
rectuser
rectuses
rectusing
rectusly
rectuss
reefer
reefered
reeferer
reeferes
reefering
reeferly
reefers
reetard
reetarded
reetarder
reetardes
reetarding
reetardly
reetards
reich
reiched
reicher
reiches
reiching
reichly
reichs
retard
retarded
retardeded
retardeder
retardedes
retardeding
retardedly
retardeds
retarder
retardes
retarding
retardly
retards
rimjob
rimjobed
rimjober
rimjobes
rimjobing
rimjobly
rimjobs
ritard
ritarded
ritarder
ritardes
ritarding
ritardly
ritards
rtard
rtarded
rtarder
rtardes
rtarding
rtardly
rtards
rum
rumed
rumer
rumes
ruming
rumly
rump
rumped
rumper
rumpes
rumping
rumply
rumprammer
rumprammered
rumprammerer
rumprammeres
rumprammering
rumprammerly
rumprammers
rumps
rums
ruski
ruskied
ruskier
ruskies
ruskiing
ruskily
ruskis
sadism
sadismed
sadismer
sadismes
sadisming
sadismly
sadisms
sadist
sadisted
sadister
sadistes
sadisting
sadistly
sadists
scag
scaged
scager
scages
scaging
scagly
scags
scantily
scantilyed
scantilyer
scantilyes
scantilying
scantilyly
scantilys
schlong
schlonged
schlonger
schlonges
schlonging
schlongly
schlongs
scrog
scroged
scroger
scroges
scroging
scrogly
scrogs
scrot
scrote
scroted
scroteed
scroteer
scrotees
scroteing
scrotely
scroter
scrotes
scroting
scrotly
scrots
scrotum
scrotumed
scrotumer
scrotumes
scrotuming
scrotumly
scrotums
scrud
scruded
scruder
scrudes
scruding
scrudly
scruds
scum
scumed
scumer
scumes
scuming
scumly
scums
seaman
seamaned
seamaner
seamanes
seamaning
seamanly
seamans
seamen
seamened
seamener
seamenes
seamening
seamenly
seamens
seduceed
seduceer
seducees
seduceing
seducely
seduces
semen
semened
semener
semenes
semening
semenly
semens
shamedame
shamedameed
shamedameer
shamedamees
shamedameing
shamedamely
shamedames
shit
shite
shiteater
shiteatered
shiteaterer
shiteateres
shiteatering
shiteaterly
shiteaters
shited
shiteed
shiteer
shitees
shiteing
shitely
shiter
shites
shitface
shitfaceed
shitfaceer
shitfacees
shitfaceing
shitfacely
shitfaces
shithead
shitheaded
shitheader
shitheades
shitheading
shitheadly
shitheads
shithole
shitholeed
shitholeer
shitholees
shitholeing
shitholely
shitholes
shithouse
shithouseed
shithouseer
shithousees
shithouseing
shithousely
shithouses
shiting
shitly
shits
shitsed
shitser
shitses
shitsing
shitsly
shitss
shitt
shitted
shitteded
shitteder
shittedes
shitteding
shittedly
shitteds
shitter
shittered
shitterer
shitteres
shittering
shitterly
shitters
shittes
shitting
shittly
shitts
shitty
shittyed
shittyer
shittyes
shittying
shittyly
shittys
shiz
shized
shizer
shizes
shizing
shizly
shizs
shooted
shooter
shootes
shooting
shootly
shoots
sissy
sissyed
sissyer
sissyes
sissying
sissyly
sissys
skag
skaged
skager
skages
skaging
skagly
skags
skank
skanked
skanker
skankes
skanking
skankly
skanks
slave
slaveed
slaveer
slavees
slaveing
slavely
slaves
sleaze
sleazeed
sleazeer
sleazees
sleazeing
sleazely
sleazes
sleazy
sleazyed
sleazyer
sleazyes
sleazying
sleazyly
sleazys
slut
slutdumper
slutdumpered
slutdumperer
slutdumperes
slutdumpering
slutdumperly
slutdumpers
sluted
sluter
slutes
sluting
slutkiss
slutkissed
slutkisser
slutkisses
slutkissing
slutkissly
slutkisss
slutly
sluts
slutsed
slutser
slutses
slutsing
slutsly
slutss
smegma
smegmaed
smegmaer
smegmaes
smegmaing
smegmaly
smegmas
smut
smuted
smuter
smutes
smuting
smutly
smuts
smutty
smuttyed
smuttyer
smuttyes
smuttying
smuttyly
smuttys
snatch
snatched
snatcher
snatches
snatching
snatchly
snatchs
sniper
snipered
sniperer
sniperes
snipering
sniperly
snipers
snort
snorted
snorter
snortes
snorting
snortly
snorts
snuff
snuffed
snuffer
snuffes
snuffing
snuffly
snuffs
sodom
sodomed
sodomer
sodomes
sodoming
sodomly
sodoms
spic
spiced
spicer
spices
spicing
spick
spicked
spicker
spickes
spicking
spickly
spicks
spicly
spics
spik
spoof
spoofed
spoofer
spoofes
spoofing
spoofly
spoofs
spooge
spoogeed
spoogeer
spoogees
spoogeing
spoogely
spooges
spunk
spunked
spunker
spunkes
spunking
spunkly
spunks
steamyed
steamyer
steamyes
steamying
steamyly
steamys
stfu
stfued
stfuer
stfues
stfuing
stfuly
stfus
stiffy
stiffyed
stiffyer
stiffyes
stiffying
stiffyly
stiffys
stoneded
stoneder
stonedes
stoneding
stonedly
stoneds
stupided
stupider
stupides
stupiding
stupidly
stupids
suckeded
suckeder
suckedes
suckeding
suckedly
suckeds
sucker
suckes
sucking
suckinged
suckinger
suckinges
suckinging
suckingly
suckings
suckly
sucks
sumofabiatch
sumofabiatched
sumofabiatcher
sumofabiatches
sumofabiatching
sumofabiatchly
sumofabiatchs
tard
tarded
tarder
tardes
tarding
tardly
tards
tawdry
tawdryed
tawdryer
tawdryes
tawdrying
tawdryly
tawdrys
teabagging
teabagginged
teabagginger
teabagginges
teabagginging
teabaggingly
teabaggings
terd
terded
terder
terdes
terding
terdly
terds
teste
testee
testeed
testeeed
testeeer
testeees
testeeing
testeely
testeer
testees
testeing
testely
testes
testesed
testeser
testeses
testesing
testesly
testess
testicle
testicleed
testicleer
testiclees
testicleing
testiclely
testicles
testis
testised
testiser
testises
testising
testisly
testiss
thrusted
thruster
thrustes
thrusting
thrustly
thrusts
thug
thuged
thuger
thuges
thuging
thugly
thugs
tinkle
tinkleed
tinkleer
tinklees
tinkleing
tinklely
tinkles
tit
tited
titer
tites
titfuck
titfucked
titfucker
titfuckes
titfucking
titfuckly
titfucks
titi
titied
titier
tities
titiing
titily
titing
titis
titly
tits
titsed
titser
titses
titsing
titsly
titss
tittiefucker
tittiefuckered
tittiefuckerer
tittiefuckeres
tittiefuckering
tittiefuckerly
tittiefuckers
titties
tittiesed
tittieser
tittieses
tittiesing
tittiesly
tittiess
titty
tittyed
tittyer
tittyes
tittyfuck
tittyfucked
tittyfucker
tittyfuckered
tittyfuckerer
tittyfuckeres
tittyfuckering
tittyfuckerly
tittyfuckers
tittyfuckes
tittyfucking
tittyfuckly
tittyfucks
tittying
tittyly
tittys
toke
tokeed
tokeer
tokees
tokeing
tokely
tokes
toots
tootsed
tootser
tootses
tootsing
tootsly
tootss
tramp
tramped
tramper
trampes
tramping
tramply
tramps
transsexualed
transsexualer
transsexuales
transsexualing
transsexually
transsexuals
trashy
trashyed
trashyer
trashyes
trashying
trashyly
trashys
tubgirl
tubgirled
tubgirler
tubgirles
tubgirling
tubgirlly
tubgirls
turd
turded
turder
turdes
turding
turdly
turds
tush
tushed
tusher
tushes
tushing
tushly
tushs
twat
twated
twater
twates
twating
twatly
twats
twatsed
twatser
twatses
twatsing
twatsly
twatss
undies
undiesed
undieser
undieses
undiesing
undiesly
undiess
unweded
unweder
unwedes
unweding
unwedly
unweds
uzi
uzied
uzier
uzies
uziing
uzily
uzis
vag
vaged
vager
vages
vaging
vagly
vags
valium
valiumed
valiumer
valiumes
valiuming
valiumly
valiums
venous
virgined
virginer
virgines
virgining
virginly
virgins
vixen
vixened
vixener
vixenes
vixening
vixenly
vixens
vodkaed
vodkaer
vodkaes
vodkaing
vodkaly
vodkas
voyeur
voyeured
voyeurer
voyeures
voyeuring
voyeurly
voyeurs
vulgar
vulgared
vulgarer
vulgares
vulgaring
vulgarly
vulgars
wang
wanged
wanger
wanges
wanging
wangly
wangs
wank
wanked
wanker
wankered
wankerer
wankeres
wankering
wankerly
wankers
wankes
wanking
wankly
wanks
wazoo
wazooed
wazooer
wazooes
wazooing
wazooly
wazoos
wedgie
wedgieed
wedgieer
wedgiees
wedgieing
wedgiely
wedgies
weeded
weeder
weedes
weeding
weedly
weeds
weenie
weenieed
weenieer
weeniees
weenieing
weeniely
weenies
weewee
weeweeed
weeweeer
weeweees
weeweeing
weeweely
weewees
weiner
weinered
weinerer
weineres
weinering
weinerly
weiners
weirdo
weirdoed
weirdoer
weirdoes
weirdoing
weirdoly
weirdos
wench
wenched
wencher
wenches
wenching
wenchly
wenchs
wetback
wetbacked
wetbacker
wetbackes
wetbacking
wetbackly
wetbacks
whitey
whiteyed
whiteyer
whiteyes
whiteying
whiteyly
whiteys
whiz
whized
whizer
whizes
whizing
whizly
whizs
whoralicious
whoralicioused
whoraliciouser
whoraliciouses
whoraliciousing
whoraliciously
whoraliciouss
whore
whorealicious
whorealicioused
whorealiciouser
whorealiciouses
whorealiciousing
whorealiciously
whorealiciouss
whored
whoreded
whoreder
whoredes
whoreding
whoredly
whoreds
whoreed
whoreer
whorees
whoreface
whorefaceed
whorefaceer
whorefacees
whorefaceing
whorefacely
whorefaces
whorehopper
whorehoppered
whorehopperer
whorehopperes
whorehoppering
whorehopperly
whorehoppers
whorehouse
whorehouseed
whorehouseer
whorehousees
whorehouseing
whorehousely
whorehouses
whoreing
whorely
whores
whoresed
whoreser
whoreses
whoresing
whoresly
whoress
whoring
whoringed
whoringer
whoringes
whoringing
whoringly
whorings
wigger
wiggered
wiggerer
wiggeres
wiggering
wiggerly
wiggers
woody
woodyed
woodyer
woodyes
woodying
woodyly
woodys
wop
woped
woper
wopes
woping
woply
wops
wtf
wtfed
wtfer
wtfes
wtfing
wtfly
wtfs
xxx
xxxed
xxxer
xxxes
xxxing
xxxly
xxxs
yeasty
yeastyed
yeastyer
yeastyes
yeastying
yeastyly
yeastys
yobbo
yobboed
yobboer
yobboes
yobboing
yobboly
yobbos
zoophile
zoophileed
zoophileer
zoophilees
zoophileing
zoophilely
zoophiles
anal
ass
ass lick
balls
ballsac
bisexual
bleach
causas
cheap
cost of miracles
cunt
display network stats
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gfc
humira AND expensive
illegal
madvocate
masturbation
nuccitelli
overdose
porn
shit
snort
texarkana
abbvie
AbbVie
acid
addicted
addiction
adolescent
adult sites
Advocacy
advocacy
agitated states
AJO, postsurgical analgesic, knee, replacement, surgery
alcohol
amphetamine
androgen
antibody
apple cider vinegar
assistance
Assistance
association
at home
attorney
audit
ayurvedic
baby
ban
baricitinib
bed bugs
best
bible
bisexual
black
bleach
blog
bulimia nervosa
buy
cannabis
certificate
certification
certified
cervical cancer, concurrent chemoradiotherapy, intravoxel incoherent motion magnetic resonance imaging, MRI, IVIM, diffusion-weighted MRI, DWI
charlie sheen
cheap
cheapest
child
childhood
childlike
children
chronic fatigue syndrome
Cladribine Tablets
cocaine
cock
combination therapies, synergistic antitumor efficacy, pertuzumab, trastuzumab, ipilimumab, nivolumab, palbociclib, letrozole, lapatinib, docetaxel, trametinib, dabrafenib, carflzomib, lenalidomide
contagious
Cortical Lesions
cream
creams
crime
criminal
cure
dangerous
dangers
dasabuvir
Dasabuvir
dead
deadly
death
dementia
dependence
dependent
depression
dermatillomania
die
diet
Disability
Discount
discount
dog
drink
drug abuse
drug-induced
dying
eastern medicine
eat
ect
eczema
electroconvulsive therapy
electromagnetic therapy
electrotherapy
epa
epilepsy
erectile dysfunction
explosive disorder
fake
Fake-ovir
fatal
fatalities
fatality
fibromyalgia
financial
Financial
fish oil
food
foods
foundation
free
Gabriel Pardo
gaston
general hospital
genetic
geriatric
Giancarlo Comi
gilead
Gilead
glaucoma
Glenn S. Williams
Glenn Williams
Gloria Dalla Costa
gonorrhea
Greedy
greedy
guns
hallucinations
harvoni
Harvoni
herbal
herbs
heroin
herpes
Hidradenitis Suppurativa,
holistic
home
home remedies
home remedy
homeopathic
homeopathy
hydrocortisone
ice
image
images
job
kid
kids
kill
killer
laser
lawsuit
lawyer
ledipasvir
Ledipasvir
lesbian
lesions
lights
liver
lupus
marijuana
melancholic
memory loss
menopausal
mental retardation
military
milk
moisturizers
monoamine oxidase inhibitor drugs
MRI
MS
murder
national
natural
natural cure
natural cures
natural medications
natural medicine
natural medicines
natural remedies
natural remedy
natural treatment
natural treatments
naturally
Needy
needy
Neurology Reviews
neuropathic
nightclub massacre
nightclub shooting
nude
nudity
nutraceuticals
OASIS
oasis
off label
ombitasvir
Ombitasvir
ombitasvir/paritaprevir/ritonavir with dasabuvir
orlando shooting
overactive thyroid gland
overdose
overdosed
Paolo Preziosa
paritaprevir
Paritaprevir
pediatric
pedophile
photo
photos
picture
post partum
postnatal
pregnancy
pregnant
prenatal
prepartum
prison
program
Program
Protest
protest
psychedelics
pulse nightclub
puppy
purchase
purchasing
rape
recall
recreational drug
Rehabilitation
Retinal Measurements
retrograde ejaculation
risperdal
ritonavir
Ritonavir
ritonavir with dasabuvir
robin williams
sales
sasquatch
schizophrenia
seizure
seizures
sex
sexual
sexy
shock treatment
silver
sleep disorders
smoking
sociopath
sofosbuvir
Sofosbuvir
sovaldi
ssri
store
sue
suicidal
suicide
supplements
support
Support
Support Path
teen
teenage
teenagers
Telerehabilitation
testosterone
Th17
Th17:FoxP3+Treg cell ratio
Th22
toxic
toxin
tragedy
treatment resistant
V Pak
vagina
velpatasvir
Viekira Pa
Viekira Pak
viekira pak
violence
virgin
vitamin
VPak
weight loss
withdrawal
wrinkles
xxx
young adult
young adults
zoloft
financial
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
direct\-acting antivirals
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-jfp')]
div[contains(@class, 'pane-pub-home-jfp')]
div[contains(@class, 'pane-pub-topic-jfp')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
A tool to help limit patients’ sodium intake
The average American consumes about 3400 mg/d of sodium, which is more than double the 1500 mg recommended by the American Heart Association.1 Excess sodium added to foods during commercial processing and preparation represents the main source of sodium intake in American diets.2 Nevertheless, adding salt at the table is still very common, and people who add salt at the table have 1.5 g higher salt intakes than those who do not add salt.3 And as we know, high sodium intake has been associated with elevated blood pressure and an increased rate of cardiovascular disease.4
I have designed a self-produced “Salt Awareness—Limit Today” (SALT) label (FIGURE). This label is attached to the cap of a salt shaker in such a way that less salt flows through the openings of the cap. Moreover, the label serves as a reminder to limit salt intake in general. The feedback I have received from my patients has been extremely positive; they report increased awareness and decreased sodium intake. I mention it here so that others may benefit.

Zvi Weizman, MD
Beer-Sheva, Israel
1. Cobb LK, Anderson CA, Elliott P, et al; American Heart Association Council on Lifestyle and Metabolic Health. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173-1186.
2. Jackson SL, King SM, Zhao L, et al. Prevalence of excess sodium intake in the United States - NHANES, 2009-2012. MMWR Morb Mortal Wkly Rep. 2016;64:1393-1397.
3. Webster J, Su’a SA, Ieremia M, et al. Salt intakes, knowledge, and behavior in Samoa: Monitoring salt-consumption patterns through the World Health Organization’s surveillance of noncommunicable disease risk factors (STEPS). J Clin Hypertens (Greenwich). 2016.
4. Mozaffarian D, Fahimi S, Singh GM, et al; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624-634.
The average American consumes about 3400 mg/d of sodium, which is more than double the 1500 mg recommended by the American Heart Association.1 Excess sodium added to foods during commercial processing and preparation represents the main source of sodium intake in American diets.2 Nevertheless, adding salt at the table is still very common, and people who add salt at the table have 1.5 g higher salt intakes than those who do not add salt.3 And as we know, high sodium intake has been associated with elevated blood pressure and an increased rate of cardiovascular disease.4
I have designed a self-produced “Salt Awareness—Limit Today” (SALT) label (FIGURE). This label is attached to the cap of a salt shaker in such a way that less salt flows through the openings of the cap. Moreover, the label serves as a reminder to limit salt intake in general. The feedback I have received from my patients has been extremely positive; they report increased awareness and decreased sodium intake. I mention it here so that others may benefit.

Zvi Weizman, MD
Beer-Sheva, Israel
The average American consumes about 3400 mg/d of sodium, which is more than double the 1500 mg recommended by the American Heart Association.1 Excess sodium added to foods during commercial processing and preparation represents the main source of sodium intake in American diets.2 Nevertheless, adding salt at the table is still very common, and people who add salt at the table have 1.5 g higher salt intakes than those who do not add salt.3 And as we know, high sodium intake has been associated with elevated blood pressure and an increased rate of cardiovascular disease.4
I have designed a self-produced “Salt Awareness—Limit Today” (SALT) label (FIGURE). This label is attached to the cap of a salt shaker in such a way that less salt flows through the openings of the cap. Moreover, the label serves as a reminder to limit salt intake in general. The feedback I have received from my patients has been extremely positive; they report increased awareness and decreased sodium intake. I mention it here so that others may benefit.

Zvi Weizman, MD
Beer-Sheva, Israel
1. Cobb LK, Anderson CA, Elliott P, et al; American Heart Association Council on Lifestyle and Metabolic Health. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173-1186.
2. Jackson SL, King SM, Zhao L, et al. Prevalence of excess sodium intake in the United States - NHANES, 2009-2012. MMWR Morb Mortal Wkly Rep. 2016;64:1393-1397.
3. Webster J, Su’a SA, Ieremia M, et al. Salt intakes, knowledge, and behavior in Samoa: Monitoring salt-consumption patterns through the World Health Organization’s surveillance of noncommunicable disease risk factors (STEPS). J Clin Hypertens (Greenwich). 2016.
4. Mozaffarian D, Fahimi S, Singh GM, et al; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624-634.
1. Cobb LK, Anderson CA, Elliott P, et al; American Heart Association Council on Lifestyle and Metabolic Health. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173-1186.
2. Jackson SL, King SM, Zhao L, et al. Prevalence of excess sodium intake in the United States - NHANES, 2009-2012. MMWR Morb Mortal Wkly Rep. 2016;64:1393-1397.
3. Webster J, Su’a SA, Ieremia M, et al. Salt intakes, knowledge, and behavior in Samoa: Monitoring salt-consumption patterns through the World Health Organization’s surveillance of noncommunicable disease risk factors (STEPS). J Clin Hypertens (Greenwich). 2016.
4. Mozaffarian D, Fahimi S, Singh GM, et al; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624-634.
Point-of-care ultrasound: It’s no replacement for the stethoscope
In his August editorial, Dr. Hickner noted that an article in the issue prompted him to “wonder whether ultrasound might become the stethoscope of the future” (J Fam Pract. 2016;65:516). To that I say that we need to avoid conflating the stethoscope with point-of-care ultrasound (POCUS).
It is well documented that auscultation skills rapidly deteriorate (specifically in the cardiology realm) in clinical practice.1 This may occur because many physicians already think ultrasound can replace actually listening to their patients’ hearts. The motto has become, “I’ll just order an echo.”
POCUS is an imaging modality. Period. It can be used to auscultate, but Doppler ultrasound is not as precise as the stethoscope when used by a practiced listener for identifying the source and subtle characteristics of murmurs.2 The stethoscope remains an outstanding, inexpensive, and convenient screening tool and its use needs to be emphasized.
I strongly believe in training all medical students in POCUS—but as a complementary and adjunctive tool—not as something to replace a perfectly functional piece of equipment used around the world to provide good care.
Todd Fredricks, DO
Athens, Ohio
1. Vukanovic-Criley JM, Hovanesyan A, Criley SR, et al. Confidential testing of cardiac examination competency in cardiology and noncardiology faculty and trainees: a multicenter study. Clin Cardiol. 2010;33:738-745.
2. Tavel ME. Cardiac auscultation. A glorious past—but does it have a future? Circulation. 1996;93:1250-1253.
In his August editorial, Dr. Hickner noted that an article in the issue prompted him to “wonder whether ultrasound might become the stethoscope of the future” (J Fam Pract. 2016;65:516). To that I say that we need to avoid conflating the stethoscope with point-of-care ultrasound (POCUS).
It is well documented that auscultation skills rapidly deteriorate (specifically in the cardiology realm) in clinical practice.1 This may occur because many physicians already think ultrasound can replace actually listening to their patients’ hearts. The motto has become, “I’ll just order an echo.”
POCUS is an imaging modality. Period. It can be used to auscultate, but Doppler ultrasound is not as precise as the stethoscope when used by a practiced listener for identifying the source and subtle characteristics of murmurs.2 The stethoscope remains an outstanding, inexpensive, and convenient screening tool and its use needs to be emphasized.
I strongly believe in training all medical students in POCUS—but as a complementary and adjunctive tool—not as something to replace a perfectly functional piece of equipment used around the world to provide good care.
Todd Fredricks, DO
Athens, Ohio
In his August editorial, Dr. Hickner noted that an article in the issue prompted him to “wonder whether ultrasound might become the stethoscope of the future” (J Fam Pract. 2016;65:516). To that I say that we need to avoid conflating the stethoscope with point-of-care ultrasound (POCUS).
It is well documented that auscultation skills rapidly deteriorate (specifically in the cardiology realm) in clinical practice.1 This may occur because many physicians already think ultrasound can replace actually listening to their patients’ hearts. The motto has become, “I’ll just order an echo.”
POCUS is an imaging modality. Period. It can be used to auscultate, but Doppler ultrasound is not as precise as the stethoscope when used by a practiced listener for identifying the source and subtle characteristics of murmurs.2 The stethoscope remains an outstanding, inexpensive, and convenient screening tool and its use needs to be emphasized.
I strongly believe in training all medical students in POCUS—but as a complementary and adjunctive tool—not as something to replace a perfectly functional piece of equipment used around the world to provide good care.
Todd Fredricks, DO
Athens, Ohio
1. Vukanovic-Criley JM, Hovanesyan A, Criley SR, et al. Confidential testing of cardiac examination competency in cardiology and noncardiology faculty and trainees: a multicenter study. Clin Cardiol. 2010;33:738-745.
2. Tavel ME. Cardiac auscultation. A glorious past—but does it have a future? Circulation. 1996;93:1250-1253.
1. Vukanovic-Criley JM, Hovanesyan A, Criley SR, et al. Confidential testing of cardiac examination competency in cardiology and noncardiology faculty and trainees: a multicenter study. Clin Cardiol. 2010;33:738-745.
2. Tavel ME. Cardiac auscultation. A glorious past—but does it have a future? Circulation. 1996;93:1250-1253.
Rethinking A1C targets for patients with mental illness?
The article, “Diabetes update: Your guide to the latest ADA standards,” by Shubrook, et al (J Fam Pract. 2016;65:310-318) is a precise review of current recommendations for diabetes. We would like to draw attention, however, to comorbid diabetes and mental illness.
Diabetes and serious mental illness often coincide, making the treatment of both conditions difficult and leading to higher rates of complications.1
The American Diabetes Association (ADA)’s “Standards of Medical Care in Diabetes” recognizes that hemoglobin A1C targets for patients should be individualized.2 We consider it important to discus
For example, a more lenient A1C goal may be appropriate when:
- the assessment of the patient shows that he or she is struggling with active symptoms of mental illness
- new medications with undesirable metabolic effects are prescribed or titrated
- social support is poor
- patients have limited confidence in their ability to accomplish tasks and goals
- patients have cognitive limitations
- patients abuse substances.
We suggest that when factors are favorable (eg, younger patient, well-controlled serious mental illness, adequate support, good cognitive skills, no hazardous use of substances, good level of confidence in the ability to control diabetes), the A1C target can be set lower. When the factors are less favorable (eg, older patient, poorly controlled mental illness, abusing substances, cognitive impairment), the target should be set higher and incrementally reduced as care engagement, circumstances, and symptom control improve.
There is a need for further research to investigate the factors that can impact diabetes self-management in patients with comorbid mental illness.
Corinna Falck-Ytter, MD
Stephanie W. Kanuch, MEd
Richard McCormick, PhD
Michael Purdum, PhD
Neal V. Dawson, MD
Shari D. Bolen, MD, MPH
Martha Sajatovic, MD
Cleveland, Ohio
1. Ducat L, Philipson LH, Anderson BJ. The mental health comorbidities of diabetes. JAMA. 2014;312:691-692.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/content/diacare/suppl/2015/12/21/39.Supplement_1.DC2/2016-Standards-of-Care.pdf. Accessed May 18, 2016.
The article, “Diabetes update: Your guide to the latest ADA standards,” by Shubrook, et al (J Fam Pract. 2016;65:310-318) is a precise review of current recommendations for diabetes. We would like to draw attention, however, to comorbid diabetes and mental illness.
Diabetes and serious mental illness often coincide, making the treatment of both conditions difficult and leading to higher rates of complications.1
The American Diabetes Association (ADA)’s “Standards of Medical Care in Diabetes” recognizes that hemoglobin A1C targets for patients should be individualized.2 We consider it important to discus
For example, a more lenient A1C goal may be appropriate when:
- the assessment of the patient shows that he or she is struggling with active symptoms of mental illness
- new medications with undesirable metabolic effects are prescribed or titrated
- social support is poor
- patients have limited confidence in their ability to accomplish tasks and goals
- patients have cognitive limitations
- patients abuse substances.
We suggest that when factors are favorable (eg, younger patient, well-controlled serious mental illness, adequate support, good cognitive skills, no hazardous use of substances, good level of confidence in the ability to control diabetes), the A1C target can be set lower. When the factors are less favorable (eg, older patient, poorly controlled mental illness, abusing substances, cognitive impairment), the target should be set higher and incrementally reduced as care engagement, circumstances, and symptom control improve.
There is a need for further research to investigate the factors that can impact diabetes self-management in patients with comorbid mental illness.
Corinna Falck-Ytter, MD
Stephanie W. Kanuch, MEd
Richard McCormick, PhD
Michael Purdum, PhD
Neal V. Dawson, MD
Shari D. Bolen, MD, MPH
Martha Sajatovic, MD
Cleveland, Ohio
The article, “Diabetes update: Your guide to the latest ADA standards,” by Shubrook, et al (J Fam Pract. 2016;65:310-318) is a precise review of current recommendations for diabetes. We would like to draw attention, however, to comorbid diabetes and mental illness.
Diabetes and serious mental illness often coincide, making the treatment of both conditions difficult and leading to higher rates of complications.1
The American Diabetes Association (ADA)’s “Standards of Medical Care in Diabetes” recognizes that hemoglobin A1C targets for patients should be individualized.2 We consider it important to discus
For example, a more lenient A1C goal may be appropriate when:
- the assessment of the patient shows that he or she is struggling with active symptoms of mental illness
- new medications with undesirable metabolic effects are prescribed or titrated
- social support is poor
- patients have limited confidence in their ability to accomplish tasks and goals
- patients have cognitive limitations
- patients abuse substances.
We suggest that when factors are favorable (eg, younger patient, well-controlled serious mental illness, adequate support, good cognitive skills, no hazardous use of substances, good level of confidence in the ability to control diabetes), the A1C target can be set lower. When the factors are less favorable (eg, older patient, poorly controlled mental illness, abusing substances, cognitive impairment), the target should be set higher and incrementally reduced as care engagement, circumstances, and symptom control improve.
There is a need for further research to investigate the factors that can impact diabetes self-management in patients with comorbid mental illness.
Corinna Falck-Ytter, MD
Stephanie W. Kanuch, MEd
Richard McCormick, PhD
Michael Purdum, PhD
Neal V. Dawson, MD
Shari D. Bolen, MD, MPH
Martha Sajatovic, MD
Cleveland, Ohio
1. Ducat L, Philipson LH, Anderson BJ. The mental health comorbidities of diabetes. JAMA. 2014;312:691-692.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/content/diacare/suppl/2015/12/21/39.Supplement_1.DC2/2016-Standards-of-Care.pdf. Accessed May 18, 2016.
1. Ducat L, Philipson LH, Anderson BJ. The mental health comorbidities of diabetes. JAMA. 2014;312:691-692.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/content/diacare/suppl/2015/12/21/39.Supplement_1.DC2/2016-Standards-of-Care.pdf. Accessed May 18, 2016.
Recurrent right upper quadrant abdominal pain
An 88-year-old woman presented to our primary care clinic with recurrent right upper quadrant abdominal pain. Her history was negative for nausea, fever, vomiting, chest pain, heartburn, back pain, or changes in bowel movement patterns. There was no association between the pain and her eating patterns. She described the pain as dull, and rated it as a 4 to 5 out of 10. A physical examination was unremarkable except for a minimally tender mass in the right upper quadrant that was detected during palpation of the abdomen. A Murphy’s test was negative. A comprehensive metabolic panel, complete blood count, lipase test, amylase test, abdominal ultrasound, and abdominal x-ray (FIGURE) were ordered.
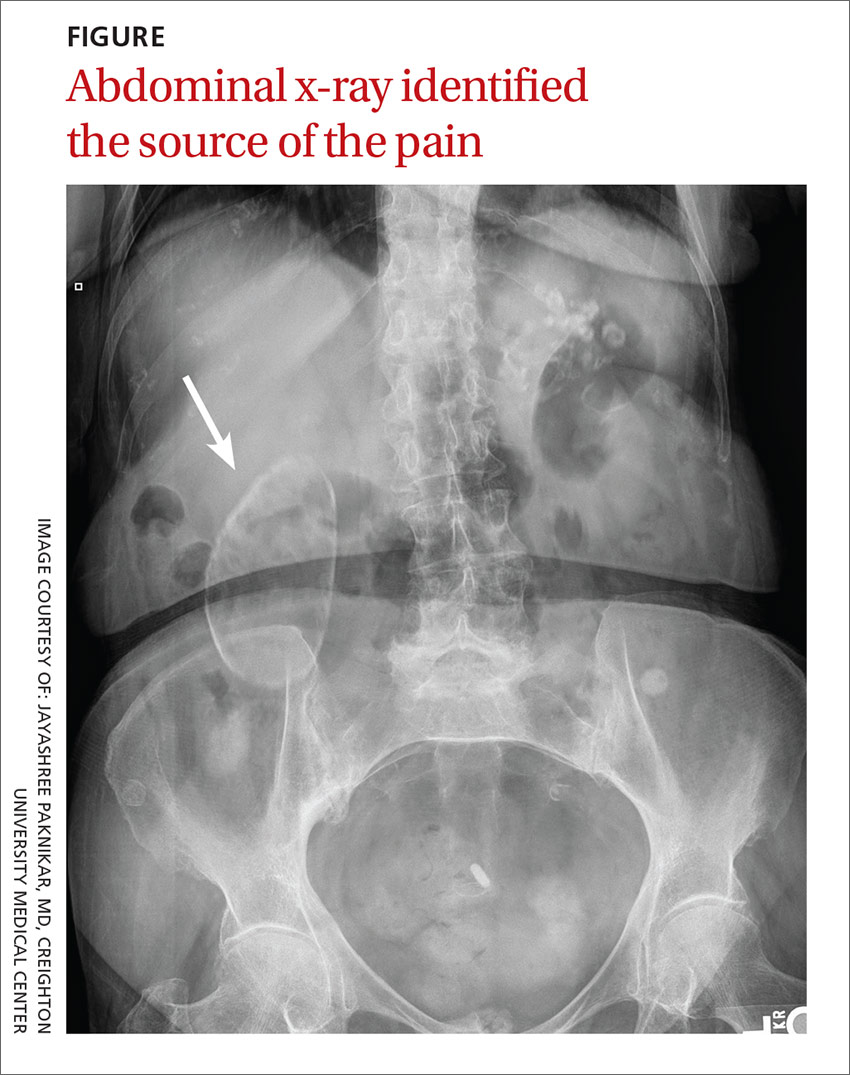
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porcelain gallbladder
We diagnosed this patient with porcelain gallbladder based on her history, physical exam, and x-ray, which revealed a well-defined air gas bubble encased in a calcified pouch. (The patient’s lab work was unremarkable and the ultrasound revealed the same findings as seen on the x-ray.)
Porcelain gallbladder is a rare condition.1 It is characterized by intramural calcifications of the gallbladder wall, which is rarely seen with chronic cholecystitis.2
There are a few theories behind the etiology of porcelain gallbladder. One theory is that gallstones can irritate the gallbladder wall, leading to inflammation and then calcification. Others believe that porcelain gallbladder is due to the obstruction of cystic ducts, which leads to bile stasis in the gallbladder, followed by the accumulation of calcium carbonate salts.3
Patients may present with biliary pain or a firm palpable mass in the right upper quadrant. However, patients are often asymptomatic.4
Cancer risk. There is about a 2% to 3% risk of gallbladder cancer in patients with porcelain gallbladder.5 The nature of the calcification has been linked to the probability that a patient will develop gallbladder cancer. Specifically, there is a higher probability of gallbladder cancer if discontinuous calcification is noted, or if only some portion of the gallbladder wall has calcified.6 About 80% of all gallbladder cancers are adenocarcinomas.6
Differential diagnosis includes GERD, cholecystitis
Right upper quadrant pain is associated with acute hepatitis, acute cholecystitis, acute pancreatitis, gastroesophageal reflex disease (GERD), ulcers, and umbilical hernias. In this patient’s case, her history and physical examination made a number of diagnoses less likely, including acute cholecystitis, GERD, ulcers, and an umbilical hernia. In addition, normal values on the patient’s lipase and amylase tests ruled out pancreatitis.
Imaging brought things into focus. The most significant finding in this case was the abdominal x-ray, which showed a well-defined air gas bubble encased in a calcified pouch.
In addition to an x-ray or an ultrasound, a computed tomography scan can confirm the diagnosis of porcelain gallbladder.
For most patients, cholecystectomy is recommended
Treatment and management is based on the pattern of calcification and the patient’s current health status. If there is incomplete calcification, cholecystectomy is warranted. Cholecystectomy may also be warranted when a patient is symptomatic and has complete calcification of the gallbladder. Regardless of the pattern of calcification, imaging surveillance of the patient is necessary. Moreover, cholecystectomy is preferred regardless of the status of the calcification if the patient is a good surgical candidate.7
It is important to send the gallbladder for histopathological examination after it is removed to determine the likelihood of malignancy.7 If cancer is ruled out, then no further work-up is necessary. If cancer is detected, then further evaluation, including additional surgery, may be necessary.
Our patient was a good surgical candidate, so we recommended cholecystectomy. The patient underwent surgery and the pathology report was negative for cancer.
CORRESPONDENCE
Pradeepa Vimalachandran, MD, MPH, 601 North 30th Street, Suite 6720, Omaha, NE 68131; [email protected].
1. Kane RA, Jacobs R, Katz J, et al. Porcelain gallbladder: ultrasound and CT appearance. Radiology. 1984;152:137-141.
2. Ochsner SF, Carrera GM. Calcification of the gallbladder (“porcelain gallbladder”). Am J Roentgenol Radium Ther Nucl Med. 1963;89:847-853.
3. Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129:699-703.
4. Geller SA, de Campos FP. Porcelain gallbladder. Autops Case Rep. 2015;5:5-7.
5. Towfigh S, McFadden DW, Cortina GR, et al. Porcelain gallbladder is not associated with gallbladder carcinoma. Am Surg. 2001;67:7-10.
6. Brown KM, Geller DA. Porcelain gallbladder and risk of gallbladder cancer. Arch Surg. 2011;146:1148.
7. Khan ZS, Livingston EH, Huerta S. Reassessing the need for prophylactic surgery in patients with porcelain gallbladder: case series and systematic review of the literature. Arch Surg. 2011;146:1143-1147.
An 88-year-old woman presented to our primary care clinic with recurrent right upper quadrant abdominal pain. Her history was negative for nausea, fever, vomiting, chest pain, heartburn, back pain, or changes in bowel movement patterns. There was no association between the pain and her eating patterns. She described the pain as dull, and rated it as a 4 to 5 out of 10. A physical examination was unremarkable except for a minimally tender mass in the right upper quadrant that was detected during palpation of the abdomen. A Murphy’s test was negative. A comprehensive metabolic panel, complete blood count, lipase test, amylase test, abdominal ultrasound, and abdominal x-ray (FIGURE) were ordered.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porcelain gallbladder
We diagnosed this patient with porcelain gallbladder based on her history, physical exam, and x-ray, which revealed a well-defined air gas bubble encased in a calcified pouch. (The patient’s lab work was unremarkable and the ultrasound revealed the same findings as seen on the x-ray.)
Porcelain gallbladder is a rare condition.1 It is characterized by intramural calcifications of the gallbladder wall, which is rarely seen with chronic cholecystitis.2
There are a few theories behind the etiology of porcelain gallbladder. One theory is that gallstones can irritate the gallbladder wall, leading to inflammation and then calcification. Others believe that porcelain gallbladder is due to the obstruction of cystic ducts, which leads to bile stasis in the gallbladder, followed by the accumulation of calcium carbonate salts.3
Patients may present with biliary pain or a firm palpable mass in the right upper quadrant. However, patients are often asymptomatic.4
Cancer risk. There is about a 2% to 3% risk of gallbladder cancer in patients with porcelain gallbladder.5 The nature of the calcification has been linked to the probability that a patient will develop gallbladder cancer. Specifically, there is a higher probability of gallbladder cancer if discontinuous calcification is noted, or if only some portion of the gallbladder wall has calcified.6 About 80% of all gallbladder cancers are adenocarcinomas.6
Differential diagnosis includes GERD, cholecystitis
Right upper quadrant pain is associated with acute hepatitis, acute cholecystitis, acute pancreatitis, gastroesophageal reflex disease (GERD), ulcers, and umbilical hernias. In this patient’s case, her history and physical examination made a number of diagnoses less likely, including acute cholecystitis, GERD, ulcers, and an umbilical hernia. In addition, normal values on the patient’s lipase and amylase tests ruled out pancreatitis.
Imaging brought things into focus. The most significant finding in this case was the abdominal x-ray, which showed a well-defined air gas bubble encased in a calcified pouch.
In addition to an x-ray or an ultrasound, a computed tomography scan can confirm the diagnosis of porcelain gallbladder.
For most patients, cholecystectomy is recommended
Treatment and management is based on the pattern of calcification and the patient’s current health status. If there is incomplete calcification, cholecystectomy is warranted. Cholecystectomy may also be warranted when a patient is symptomatic and has complete calcification of the gallbladder. Regardless of the pattern of calcification, imaging surveillance of the patient is necessary. Moreover, cholecystectomy is preferred regardless of the status of the calcification if the patient is a good surgical candidate.7
It is important to send the gallbladder for histopathological examination after it is removed to determine the likelihood of malignancy.7 If cancer is ruled out, then no further work-up is necessary. If cancer is detected, then further evaluation, including additional surgery, may be necessary.
Our patient was a good surgical candidate, so we recommended cholecystectomy. The patient underwent surgery and the pathology report was negative for cancer.
CORRESPONDENCE
Pradeepa Vimalachandran, MD, MPH, 601 North 30th Street, Suite 6720, Omaha, NE 68131; [email protected].
An 88-year-old woman presented to our primary care clinic with recurrent right upper quadrant abdominal pain. Her history was negative for nausea, fever, vomiting, chest pain, heartburn, back pain, or changes in bowel movement patterns. There was no association between the pain and her eating patterns. She described the pain as dull, and rated it as a 4 to 5 out of 10. A physical examination was unremarkable except for a minimally tender mass in the right upper quadrant that was detected during palpation of the abdomen. A Murphy’s test was negative. A comprehensive metabolic panel, complete blood count, lipase test, amylase test, abdominal ultrasound, and abdominal x-ray (FIGURE) were ordered.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porcelain gallbladder
We diagnosed this patient with porcelain gallbladder based on her history, physical exam, and x-ray, which revealed a well-defined air gas bubble encased in a calcified pouch. (The patient’s lab work was unremarkable and the ultrasound revealed the same findings as seen on the x-ray.)
Porcelain gallbladder is a rare condition.1 It is characterized by intramural calcifications of the gallbladder wall, which is rarely seen with chronic cholecystitis.2
There are a few theories behind the etiology of porcelain gallbladder. One theory is that gallstones can irritate the gallbladder wall, leading to inflammation and then calcification. Others believe that porcelain gallbladder is due to the obstruction of cystic ducts, which leads to bile stasis in the gallbladder, followed by the accumulation of calcium carbonate salts.3
Patients may present with biliary pain or a firm palpable mass in the right upper quadrant. However, patients are often asymptomatic.4
Cancer risk. There is about a 2% to 3% risk of gallbladder cancer in patients with porcelain gallbladder.5 The nature of the calcification has been linked to the probability that a patient will develop gallbladder cancer. Specifically, there is a higher probability of gallbladder cancer if discontinuous calcification is noted, or if only some portion of the gallbladder wall has calcified.6 About 80% of all gallbladder cancers are adenocarcinomas.6
Differential diagnosis includes GERD, cholecystitis
Right upper quadrant pain is associated with acute hepatitis, acute cholecystitis, acute pancreatitis, gastroesophageal reflex disease (GERD), ulcers, and umbilical hernias. In this patient’s case, her history and physical examination made a number of diagnoses less likely, including acute cholecystitis, GERD, ulcers, and an umbilical hernia. In addition, normal values on the patient’s lipase and amylase tests ruled out pancreatitis.
Imaging brought things into focus. The most significant finding in this case was the abdominal x-ray, which showed a well-defined air gas bubble encased in a calcified pouch.
In addition to an x-ray or an ultrasound, a computed tomography scan can confirm the diagnosis of porcelain gallbladder.
For most patients, cholecystectomy is recommended
Treatment and management is based on the pattern of calcification and the patient’s current health status. If there is incomplete calcification, cholecystectomy is warranted. Cholecystectomy may also be warranted when a patient is symptomatic and has complete calcification of the gallbladder. Regardless of the pattern of calcification, imaging surveillance of the patient is necessary. Moreover, cholecystectomy is preferred regardless of the status of the calcification if the patient is a good surgical candidate.7
It is important to send the gallbladder for histopathological examination after it is removed to determine the likelihood of malignancy.7 If cancer is ruled out, then no further work-up is necessary. If cancer is detected, then further evaluation, including additional surgery, may be necessary.
Our patient was a good surgical candidate, so we recommended cholecystectomy. The patient underwent surgery and the pathology report was negative for cancer.
CORRESPONDENCE
Pradeepa Vimalachandran, MD, MPH, 601 North 30th Street, Suite 6720, Omaha, NE 68131; [email protected].
1. Kane RA, Jacobs R, Katz J, et al. Porcelain gallbladder: ultrasound and CT appearance. Radiology. 1984;152:137-141.
2. Ochsner SF, Carrera GM. Calcification of the gallbladder (“porcelain gallbladder”). Am J Roentgenol Radium Ther Nucl Med. 1963;89:847-853.
3. Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129:699-703.
4. Geller SA, de Campos FP. Porcelain gallbladder. Autops Case Rep. 2015;5:5-7.
5. Towfigh S, McFadden DW, Cortina GR, et al. Porcelain gallbladder is not associated with gallbladder carcinoma. Am Surg. 2001;67:7-10.
6. Brown KM, Geller DA. Porcelain gallbladder and risk of gallbladder cancer. Arch Surg. 2011;146:1148.
7. Khan ZS, Livingston EH, Huerta S. Reassessing the need for prophylactic surgery in patients with porcelain gallbladder: case series and systematic review of the literature. Arch Surg. 2011;146:1143-1147.
1. Kane RA, Jacobs R, Katz J, et al. Porcelain gallbladder: ultrasound and CT appearance. Radiology. 1984;152:137-141.
2. Ochsner SF, Carrera GM. Calcification of the gallbladder (“porcelain gallbladder”). Am J Roentgenol Radium Ther Nucl Med. 1963;89:847-853.
3. Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129:699-703.
4. Geller SA, de Campos FP. Porcelain gallbladder. Autops Case Rep. 2015;5:5-7.
5. Towfigh S, McFadden DW, Cortina GR, et al. Porcelain gallbladder is not associated with gallbladder carcinoma. Am Surg. 2001;67:7-10.
6. Brown KM, Geller DA. Porcelain gallbladder and risk of gallbladder cancer. Arch Surg. 2011;146:1148.
7. Khan ZS, Livingston EH, Huerta S. Reassessing the need for prophylactic surgery in patients with porcelain gallbladder: case series and systematic review of the literature. Arch Surg. 2011;146:1143-1147.
Does knuckle popping lead to arthritis?
EVIDENCE-BASED ANSWER:
No, habitual knuckle popping, or cracking (over the course of several decades) isn’t associated with clinical or radiographic evidence of osteoarthritis (strength of recommendation [SOR]: B, retrospective cohort and case control studies). However, attempting to pop the knuckles can produce acute soft tissue injury (SOR: C, case reports).
Evidence summary
A cross-sectional study found no correlation between knuckle popping and osteoarthritis (OA) of the hand.1 Investigators recruited 300 consecutive patients (ages 45 years and older, mean age 63 years) and evaluated them for a history of habitual knuckle popping (74 of 300 patients, mean duration 35 years) and hand arthritis or dysfunction. Investigators excluded patients with neuromuscular, inflammatory, or malignant diseases.
Investigators found OA equally in both patients who did and didn’t pop their knuckles (12 of 74 vs 36 of 226, respectively; P nonsignificant); joint swelling was more common in participants with a history of knuckle popping (84% vs 6%; P<.01). Investigators didn’t describe how OA was diagnosed or specify which joints were affected.
Another cross-sectional study also found no correlation between habitual knuckle popping of the metacarpal phalangeal joint and the prevalence of OA in that joint.2 Investigators recruited 28 patients (mean age 78.5 years; 23 women and 5 men) from a Jewish home for the aged and asked them whether they had habitually cracked their knuckles during their lifetime. They then performed clinical and radiographic hand examinations (excluding patients with a history of traumatic injury, rheumatoid arthritis, gout, chondrocalcinosis, and hemochromatosis).
Knuckle popping didn’t correlate with OA of the metacarpal phalanges (1 of 15 knuckle popping patients vs 5 of 13 patients who didn’t pop their knuckles; P=.06). All 6 patients with radiographic evidence of OA showed involvement at the metacarpal phalangeal and distal interphalangeal joints, whether or not they popped their knuckles.
Years spent cracking knuckles doesn’t predict OA
A case control study found no correlation between OA in the hands and habitual knuckle popping.3 Investigators recruited 215 patients 50 to 89 years old who had received a radiograph of their right hand during the previous 5 years and divided them into cases with OA (135 patients), and controls without OA (80 patients). Patients completed questionnaires assessing the prevalence (20%), frequency (1 to 20 times per day), and duration (26 to 36 years) of knuckle popping.
Patients most commonly popped proximal interphalangeal joints (15.9%) followed by metacarpal phalangeal joints (13.5%), distal interphalangeal joints (6.1%), and first carpal metacarpal joints (2.3%). OA most often affected the distal interphalangeal joint (68.4%), followed by the first carpal metacarpal (57.1%), proximal interphalangeal (54.1%), and metacarpal phalangeal joints (28.6%). Investigators found no difference in the prevalence of knuckle popping between cases and controls (18% in cases vs 23.2% in controls; P=.361).
When investigators evaluated total knuckle popping exposure in “crack years” (number of times per day multiplied by years) in the distal interphalangeal or metacarpal phalangeal joints, they found no significant association between crack years and OA (distal interphalangeal joint, mean 108 crack years; metacarpal phalangeal joint, mean 75 crack years).
50 years of knuckle popping without ill effects
An n-of-1 case control study found similar results.4 The researcher, a physician, popped only the knuckles of his left hand, twice a day, for 50 years. He compared his hands at the end of the trial and found no arthritis in either hand and no visible differences.
But knuckle popping does have a downside
A paper described 2 case reports of acute injuries sustained during attempted knuckle popping—a partial tear of the ulnar collateral ligament of the thumb and subluxation of the extensor tendon of the fifth digit.5 Both injuries were associated with forceful manipulation of the digits, and both resolved with conservative management within 4 weeks.
1. Castellanos J, Axelrod D. Effect of habitual knuckle cracking on hand function. Ann Rheum Dis. 1990;49:308-309.
2. Swezey RL, Swezey SE. The consequences of habitual knuckle cracking. West J Med. 1975;122:377-379.
3. Deweber K, Olszewski M, Ortolano R. Knuckle cracking and hand osteoarthritis. J Am Board Fam Med. 2011;24:169-174.
4. Unger DL. Does knuckle cracking lead to arthritis of the fingers? Arthritis Rheum. 1998;41:949-950.
5. Chan PS, Steinberg DR, Bozentka DJ. Consequences of knuckle cracking: a report of two acute injuries. Am J Orthop (Belle Mead NJ). 1999;28:113-114.
EVIDENCE-BASED ANSWER:
No, habitual knuckle popping, or cracking (over the course of several decades) isn’t associated with clinical or radiographic evidence of osteoarthritis (strength of recommendation [SOR]: B, retrospective cohort and case control studies). However, attempting to pop the knuckles can produce acute soft tissue injury (SOR: C, case reports).
Evidence summary
A cross-sectional study found no correlation between knuckle popping and osteoarthritis (OA) of the hand.1 Investigators recruited 300 consecutive patients (ages 45 years and older, mean age 63 years) and evaluated them for a history of habitual knuckle popping (74 of 300 patients, mean duration 35 years) and hand arthritis or dysfunction. Investigators excluded patients with neuromuscular, inflammatory, or malignant diseases.
Investigators found OA equally in both patients who did and didn’t pop their knuckles (12 of 74 vs 36 of 226, respectively; P nonsignificant); joint swelling was more common in participants with a history of knuckle popping (84% vs 6%; P<.01). Investigators didn’t describe how OA was diagnosed or specify which joints were affected.
Another cross-sectional study also found no correlation between habitual knuckle popping of the metacarpal phalangeal joint and the prevalence of OA in that joint.2 Investigators recruited 28 patients (mean age 78.5 years; 23 women and 5 men) from a Jewish home for the aged and asked them whether they had habitually cracked their knuckles during their lifetime. They then performed clinical and radiographic hand examinations (excluding patients with a history of traumatic injury, rheumatoid arthritis, gout, chondrocalcinosis, and hemochromatosis).
Knuckle popping didn’t correlate with OA of the metacarpal phalanges (1 of 15 knuckle popping patients vs 5 of 13 patients who didn’t pop their knuckles; P=.06). All 6 patients with radiographic evidence of OA showed involvement at the metacarpal phalangeal and distal interphalangeal joints, whether or not they popped their knuckles.
Years spent cracking knuckles doesn’t predict OA
A case control study found no correlation between OA in the hands and habitual knuckle popping.3 Investigators recruited 215 patients 50 to 89 years old who had received a radiograph of their right hand during the previous 5 years and divided them into cases with OA (135 patients), and controls without OA (80 patients). Patients completed questionnaires assessing the prevalence (20%), frequency (1 to 20 times per day), and duration (26 to 36 years) of knuckle popping.
Patients most commonly popped proximal interphalangeal joints (15.9%) followed by metacarpal phalangeal joints (13.5%), distal interphalangeal joints (6.1%), and first carpal metacarpal joints (2.3%). OA most often affected the distal interphalangeal joint (68.4%), followed by the first carpal metacarpal (57.1%), proximal interphalangeal (54.1%), and metacarpal phalangeal joints (28.6%). Investigators found no difference in the prevalence of knuckle popping between cases and controls (18% in cases vs 23.2% in controls; P=.361).
When investigators evaluated total knuckle popping exposure in “crack years” (number of times per day multiplied by years) in the distal interphalangeal or metacarpal phalangeal joints, they found no significant association between crack years and OA (distal interphalangeal joint, mean 108 crack years; metacarpal phalangeal joint, mean 75 crack years).
50 years of knuckle popping without ill effects
An n-of-1 case control study found similar results.4 The researcher, a physician, popped only the knuckles of his left hand, twice a day, for 50 years. He compared his hands at the end of the trial and found no arthritis in either hand and no visible differences.
But knuckle popping does have a downside
A paper described 2 case reports of acute injuries sustained during attempted knuckle popping—a partial tear of the ulnar collateral ligament of the thumb and subluxation of the extensor tendon of the fifth digit.5 Both injuries were associated with forceful manipulation of the digits, and both resolved with conservative management within 4 weeks.
EVIDENCE-BASED ANSWER:
No, habitual knuckle popping, or cracking (over the course of several decades) isn’t associated with clinical or radiographic evidence of osteoarthritis (strength of recommendation [SOR]: B, retrospective cohort and case control studies). However, attempting to pop the knuckles can produce acute soft tissue injury (SOR: C, case reports).
Evidence summary
A cross-sectional study found no correlation between knuckle popping and osteoarthritis (OA) of the hand.1 Investigators recruited 300 consecutive patients (ages 45 years and older, mean age 63 years) and evaluated them for a history of habitual knuckle popping (74 of 300 patients, mean duration 35 years) and hand arthritis or dysfunction. Investigators excluded patients with neuromuscular, inflammatory, or malignant diseases.
Investigators found OA equally in both patients who did and didn’t pop their knuckles (12 of 74 vs 36 of 226, respectively; P nonsignificant); joint swelling was more common in participants with a history of knuckle popping (84% vs 6%; P<.01). Investigators didn’t describe how OA was diagnosed or specify which joints were affected.
Another cross-sectional study also found no correlation between habitual knuckle popping of the metacarpal phalangeal joint and the prevalence of OA in that joint.2 Investigators recruited 28 patients (mean age 78.5 years; 23 women and 5 men) from a Jewish home for the aged and asked them whether they had habitually cracked their knuckles during their lifetime. They then performed clinical and radiographic hand examinations (excluding patients with a history of traumatic injury, rheumatoid arthritis, gout, chondrocalcinosis, and hemochromatosis).
Knuckle popping didn’t correlate with OA of the metacarpal phalanges (1 of 15 knuckle popping patients vs 5 of 13 patients who didn’t pop their knuckles; P=.06). All 6 patients with radiographic evidence of OA showed involvement at the metacarpal phalangeal and distal interphalangeal joints, whether or not they popped their knuckles.
Years spent cracking knuckles doesn’t predict OA
A case control study found no correlation between OA in the hands and habitual knuckle popping.3 Investigators recruited 215 patients 50 to 89 years old who had received a radiograph of their right hand during the previous 5 years and divided them into cases with OA (135 patients), and controls without OA (80 patients). Patients completed questionnaires assessing the prevalence (20%), frequency (1 to 20 times per day), and duration (26 to 36 years) of knuckle popping.
Patients most commonly popped proximal interphalangeal joints (15.9%) followed by metacarpal phalangeal joints (13.5%), distal interphalangeal joints (6.1%), and first carpal metacarpal joints (2.3%). OA most often affected the distal interphalangeal joint (68.4%), followed by the first carpal metacarpal (57.1%), proximal interphalangeal (54.1%), and metacarpal phalangeal joints (28.6%). Investigators found no difference in the prevalence of knuckle popping between cases and controls (18% in cases vs 23.2% in controls; P=.361).
When investigators evaluated total knuckle popping exposure in “crack years” (number of times per day multiplied by years) in the distal interphalangeal or metacarpal phalangeal joints, they found no significant association between crack years and OA (distal interphalangeal joint, mean 108 crack years; metacarpal phalangeal joint, mean 75 crack years).
50 years of knuckle popping without ill effects
An n-of-1 case control study found similar results.4 The researcher, a physician, popped only the knuckles of his left hand, twice a day, for 50 years. He compared his hands at the end of the trial and found no arthritis in either hand and no visible differences.
But knuckle popping does have a downside
A paper described 2 case reports of acute injuries sustained during attempted knuckle popping—a partial tear of the ulnar collateral ligament of the thumb and subluxation of the extensor tendon of the fifth digit.5 Both injuries were associated with forceful manipulation of the digits, and both resolved with conservative management within 4 weeks.
1. Castellanos J, Axelrod D. Effect of habitual knuckle cracking on hand function. Ann Rheum Dis. 1990;49:308-309.
2. Swezey RL, Swezey SE. The consequences of habitual knuckle cracking. West J Med. 1975;122:377-379.
3. Deweber K, Olszewski M, Ortolano R. Knuckle cracking and hand osteoarthritis. J Am Board Fam Med. 2011;24:169-174.
4. Unger DL. Does knuckle cracking lead to arthritis of the fingers? Arthritis Rheum. 1998;41:949-950.
5. Chan PS, Steinberg DR, Bozentka DJ. Consequences of knuckle cracking: a report of two acute injuries. Am J Orthop (Belle Mead NJ). 1999;28:113-114.
1. Castellanos J, Axelrod D. Effect of habitual knuckle cracking on hand function. Ann Rheum Dis. 1990;49:308-309.
2. Swezey RL, Swezey SE. The consequences of habitual knuckle cracking. West J Med. 1975;122:377-379.
3. Deweber K, Olszewski M, Ortolano R. Knuckle cracking and hand osteoarthritis. J Am Board Fam Med. 2011;24:169-174.
4. Unger DL. Does knuckle cracking lead to arthritis of the fingers? Arthritis Rheum. 1998;41:949-950.
5. Chan PS, Steinberg DR, Bozentka DJ. Consequences of knuckle cracking: a report of two acute injuries. Am J Orthop (Belle Mead NJ). 1999;28:113-114.
Evidence-based answers from the Family Physicians Inquiries Network
Epistaxis, mass in right nostril • Dx?
THE CASE
A 49-year-old woman visited our family medicine clinic because she’d had 3 episodes of epistaxis during the previous month. She’d already visited the emergency department, and the doctor there had treated her symptomatically and referred her to our clinic.
On physical examination, we noted a whitish mass in the patient’s right nostril that was attached to the nasal septum. The patient’s vital signs were within normal limits. She had a history of hypertension, depression, anxiety, gastroesophageal reflux disease, and post-traumatic stress disorder. Her medications included amlodipine-benazepril, atenolol-chlorthalidone, citalopram, clonazepam, prazosin, and omeprazole. The patient lived alone and denied using tobacco or illicit drugs, but she drank one to 2 glasses of brandy every day. She denied any past medical or family history of similar complaints, autoimmune disorders, or skin rashes.
A complete blood count, international normalized ratio, sedimentation rate, anti-nuclear antibody test, and an anti-neutrophil cytoplasmic antibody panel were normal.
THE DIAGNOSIS
We referred the patient to an ear, nose, and throat doctor for a nasal endoscopy and a biopsy, which showed granulation tissue. A maxillofacial computed tomography (CT) scan revealed a 1.44 cm x 0.8 cm polypoid soft tissue mass in the right nasal cavity adherent to the nasal septum with no posterior extension (FIGURE 1).
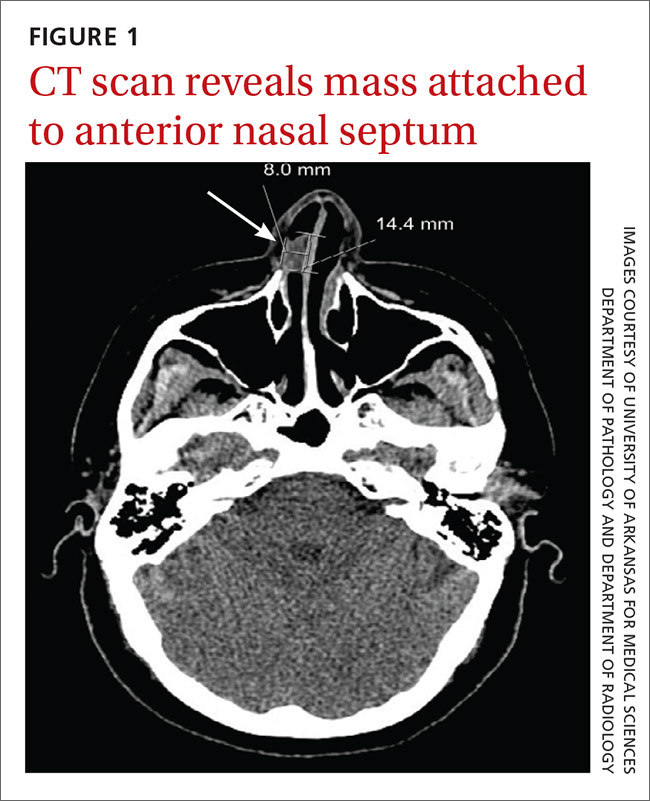
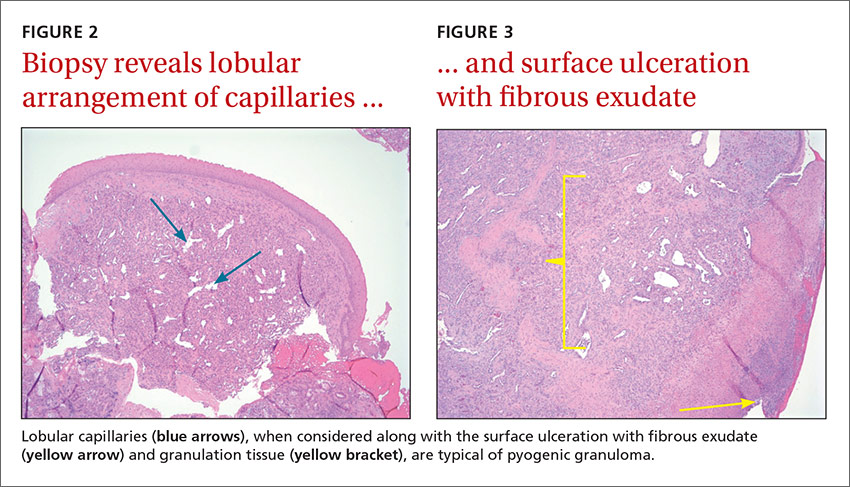
DISCUSSION
Pyogenic granuloma (PG) is a benign vascular tumor of the skin and mucous membranes that is not associated with an infection. Rather, it is a hyperplastic, neovascular, inflammatory response to an angiogenic stimulus. Several enhancers and inhibitors of angiogenesis have been shown to play a role in PG, including hormones, medications, and local injury. In fact, a local injury or hormonal factor is identified as a stimulus in more than half of PG patients.1
The hormone connection. Estrogen promotes production of nerve growth factor, granulocyte-macrophage colony-stimulating factor, basic fibroblast growth factor, vascular endothelial growth factor, and transforming growth factor beta 1. Progesterone enhances inflammatory mediators as well. Although there are no direct receptors for estrogen and progesterone in the oral and nasal mucosa, some of these pro-inflammatory effects create an environment conducive to the development of PG. This is supported by several studies documenting an increased incidence of PGs with oral contraceptive use and regression of PGs after childbirth.2-4
Medication may play a role. Drug-induced PG has also been described in several studies.5,6 Offending medications include systemic and topical retinoids, capecitabine, etoposide, 5-fluorouracil, cyclosporine, docetaxel, and human immunodeficiency virus protease inhibitors.
Local injury may also be a culprit. Nasal PGs are commonly attached to the anterior septum and typically result from nasal packing, habitual picking, or nose boring.7 In this particular case, however, we were unable to identify the irritant.
The classic presentation
PG classically presents as a painless mass that spontaneously develops over days to weeks. The mass can be sessile or pedunculated, and is frequently hemorrhagic. Intranasal PG usually presents with epiphora.7 While the prevalence of intraoral PG was found to be one in 25,000 individuals3, data for nasal lesions is scarce. Most cases of PG are seen in the second and third decades of life.1,3 In children, PG is slightly more predominant in males.1,3 Mucosal lesions, however, have a higher incidence in females.1,3 Granuloma gravidarum, the term used to describe mucosal PG in pregnant females, was found in 0.2% to 5% of pregnancies.2,3,8
Differential Dx includes warts, squamous cell carcinoma
The differential diagnosis of PG includes Spitz nevus, glomus tumors, common warts, amelanotic melanoma, squamous cell carcinoma, basal cell carcinoma, Kaposi’s sarcoma, bacillary angiomatosis, infantile hemangioma, and angiolymphoid hyperplasia, among others.3,5 Foreign bodies, nasal polyps, angiofibroma, meningocele, Wegener’s granulomatosis, and sarcoidosis should also be considered.
Radiologic evaluation may be beneficial—especially with nasal lesions—when looking for findings suggestive of malignancy. Both CT and magnetic resonance imaging with contrast identify PG as a soft tissue mass with lobulated contours,9,10 but histopathologic analysis is required to confirm the diagnosis. The histopathologic appearance of PG is characterized by a polypoid lesion with circumscribed anastomosing networks of capillaries arranged in one or more lobules at the base in an edematous and fibroblastic stroma.
Treatment is determined by the location and size of the lesion
The most suitable treatment is determined by considering the location of the lesion, the characteristics of the lesion (morphology/size), its amenability to surgery, risk of scar formation, and the presence or absence of a causative irritant. Excision is often preferred because it yields a specimen for pathologic analysis. Alternative treatments include electrocautery, cryotherapy, laser therapy, and intralesional and topical agents,3,6,7 but the recurrence rate is higher (up to 15%) with some of these modalities, when compared with excision (3.6%).3
Our patient underwent excision of the mass and was seen for an annual follow-up appointment. All of her symptoms resolved and no recurrence was noted.
THE TAKEAWAY
Although PG is a common and benign condition, it is rarely seen in the nasal cavity without an obvious history of a possible irritant. PG should be considered as a diagnosis for rapidly growing cutaneous or mucosal hemorrhagic lesions. Appropriate tissue pathology is essential to rule out malignancy and other serious conditions, such as bacillary angiomatosis and Wegener’s granulomatosis.
Treatment is usually required to avoid the frequent complications of ulceration and bleeding. Surgical treatments are preferred. The location of the lesion largely determines whether referral to a specialist is necessary.
1. Harris MN, Desai R, Chuang TY, et al. Lobular capillary hemangiomas: An epidemiologic report, with emphasis on cutaneous lesions. J Am Acad Dermatol. 2000;42:1012-1016.
2. Yuan K, Jin YT, Lin MT. The detection and comparison of angiogenesis-associated factors in pyogenic granuloma by immunohistochemistry. J Periodontol. 2000;71:701-709.
3. Giblin AV, Clover AJ, Athanassopoulos A, et al. Pyogenic granuloma–the quest for optimum treatment: audit of treatment of 408 cases. J Plast Reconstr Aesthet Surg. 2007;60:1030-1035.
4. Steelman R, Holmes D. Pregnancy tumor in a 16-year-old: case report and treatment considerations. J Clin Pediatr Dent. 1992;16:217-218.
5. Jafarzadeh H, Sanatkhani M, Mohtasham N. Oral pyogenic granuloma: a review. J Oral Sci. 2006;48:167-175.
6. Piraccini BM, Bellavista S, Misciali C, et al. Periungual and subungual pyogenic granuloma. Br J Dermatol. 2010;163:941-953.
7. Ozcan C, Apa DD, Görür K. Pediatric lobular capillary hemangioma of the nasal cavity. Eur Arch Otorhinolaryngol. 2004;261:449-451.
8. Henry F, Quatresooz P, Valverde-Lopez JC, et al. Blood vessel changes during pregnancy: a review. Am J Clin Dermatol. 2006;7:65-69.
9. Puxeddu R, Berlucchi M, Ledda GP, et al. Lobular capillary hemangioma of the nasal cavity: A retrospective study on 40 patients. Am J Rhinol. 2006;20:480-484.
10. Maroldi R, Berlucchi M, Farina D, et al. Benign neoplasms and tumor-like lesions. In: Maroldi R, Nicolai P, eds. Imaging in Treatment Planning for Sinonasal Diseases. Berlin, Heidelberg, New York: Springer-Verlag; 2005:107-158.
THE CASE
A 49-year-old woman visited our family medicine clinic because she’d had 3 episodes of epistaxis during the previous month. She’d already visited the emergency department, and the doctor there had treated her symptomatically and referred her to our clinic.
On physical examination, we noted a whitish mass in the patient’s right nostril that was attached to the nasal septum. The patient’s vital signs were within normal limits. She had a history of hypertension, depression, anxiety, gastroesophageal reflux disease, and post-traumatic stress disorder. Her medications included amlodipine-benazepril, atenolol-chlorthalidone, citalopram, clonazepam, prazosin, and omeprazole. The patient lived alone and denied using tobacco or illicit drugs, but she drank one to 2 glasses of brandy every day. She denied any past medical or family history of similar complaints, autoimmune disorders, or skin rashes.
A complete blood count, international normalized ratio, sedimentation rate, anti-nuclear antibody test, and an anti-neutrophil cytoplasmic antibody panel were normal.
THE DIAGNOSIS
We referred the patient to an ear, nose, and throat doctor for a nasal endoscopy and a biopsy, which showed granulation tissue. A maxillofacial computed tomography (CT) scan revealed a 1.44 cm x 0.8 cm polypoid soft tissue mass in the right nasal cavity adherent to the nasal septum with no posterior extension (FIGURE 1).


DISCUSSION
Pyogenic granuloma (PG) is a benign vascular tumor of the skin and mucous membranes that is not associated with an infection. Rather, it is a hyperplastic, neovascular, inflammatory response to an angiogenic stimulus. Several enhancers and inhibitors of angiogenesis have been shown to play a role in PG, including hormones, medications, and local injury. In fact, a local injury or hormonal factor is identified as a stimulus in more than half of PG patients.1
The hormone connection. Estrogen promotes production of nerve growth factor, granulocyte-macrophage colony-stimulating factor, basic fibroblast growth factor, vascular endothelial growth factor, and transforming growth factor beta 1. Progesterone enhances inflammatory mediators as well. Although there are no direct receptors for estrogen and progesterone in the oral and nasal mucosa, some of these pro-inflammatory effects create an environment conducive to the development of PG. This is supported by several studies documenting an increased incidence of PGs with oral contraceptive use and regression of PGs after childbirth.2-4
Medication may play a role. Drug-induced PG has also been described in several studies.5,6 Offending medications include systemic and topical retinoids, capecitabine, etoposide, 5-fluorouracil, cyclosporine, docetaxel, and human immunodeficiency virus protease inhibitors.
Local injury may also be a culprit. Nasal PGs are commonly attached to the anterior septum and typically result from nasal packing, habitual picking, or nose boring.7 In this particular case, however, we were unable to identify the irritant.
The classic presentation
PG classically presents as a painless mass that spontaneously develops over days to weeks. The mass can be sessile or pedunculated, and is frequently hemorrhagic. Intranasal PG usually presents with epiphora.7 While the prevalence of intraoral PG was found to be one in 25,000 individuals3, data for nasal lesions is scarce. Most cases of PG are seen in the second and third decades of life.1,3 In children, PG is slightly more predominant in males.1,3 Mucosal lesions, however, have a higher incidence in females.1,3 Granuloma gravidarum, the term used to describe mucosal PG in pregnant females, was found in 0.2% to 5% of pregnancies.2,3,8
Differential Dx includes warts, squamous cell carcinoma
The differential diagnosis of PG includes Spitz nevus, glomus tumors, common warts, amelanotic melanoma, squamous cell carcinoma, basal cell carcinoma, Kaposi’s sarcoma, bacillary angiomatosis, infantile hemangioma, and angiolymphoid hyperplasia, among others.3,5 Foreign bodies, nasal polyps, angiofibroma, meningocele, Wegener’s granulomatosis, and sarcoidosis should also be considered.
Radiologic evaluation may be beneficial—especially with nasal lesions—when looking for findings suggestive of malignancy. Both CT and magnetic resonance imaging with contrast identify PG as a soft tissue mass with lobulated contours,9,10 but histopathologic analysis is required to confirm the diagnosis. The histopathologic appearance of PG is characterized by a polypoid lesion with circumscribed anastomosing networks of capillaries arranged in one or more lobules at the base in an edematous and fibroblastic stroma.
Treatment is determined by the location and size of the lesion
The most suitable treatment is determined by considering the location of the lesion, the characteristics of the lesion (morphology/size), its amenability to surgery, risk of scar formation, and the presence or absence of a causative irritant. Excision is often preferred because it yields a specimen for pathologic analysis. Alternative treatments include electrocautery, cryotherapy, laser therapy, and intralesional and topical agents,3,6,7 but the recurrence rate is higher (up to 15%) with some of these modalities, when compared with excision (3.6%).3
Our patient underwent excision of the mass and was seen for an annual follow-up appointment. All of her symptoms resolved and no recurrence was noted.
THE TAKEAWAY
Although PG is a common and benign condition, it is rarely seen in the nasal cavity without an obvious history of a possible irritant. PG should be considered as a diagnosis for rapidly growing cutaneous or mucosal hemorrhagic lesions. Appropriate tissue pathology is essential to rule out malignancy and other serious conditions, such as bacillary angiomatosis and Wegener’s granulomatosis.
Treatment is usually required to avoid the frequent complications of ulceration and bleeding. Surgical treatments are preferred. The location of the lesion largely determines whether referral to a specialist is necessary.
THE CASE
A 49-year-old woman visited our family medicine clinic because she’d had 3 episodes of epistaxis during the previous month. She’d already visited the emergency department, and the doctor there had treated her symptomatically and referred her to our clinic.
On physical examination, we noted a whitish mass in the patient’s right nostril that was attached to the nasal septum. The patient’s vital signs were within normal limits. She had a history of hypertension, depression, anxiety, gastroesophageal reflux disease, and post-traumatic stress disorder. Her medications included amlodipine-benazepril, atenolol-chlorthalidone, citalopram, clonazepam, prazosin, and omeprazole. The patient lived alone and denied using tobacco or illicit drugs, but she drank one to 2 glasses of brandy every day. She denied any past medical or family history of similar complaints, autoimmune disorders, or skin rashes.
A complete blood count, international normalized ratio, sedimentation rate, anti-nuclear antibody test, and an anti-neutrophil cytoplasmic antibody panel were normal.
THE DIAGNOSIS
We referred the patient to an ear, nose, and throat doctor for a nasal endoscopy and a biopsy, which showed granulation tissue. A maxillofacial computed tomography (CT) scan revealed a 1.44 cm x 0.8 cm polypoid soft tissue mass in the right nasal cavity adherent to the nasal septum with no posterior extension (FIGURE 1).


DISCUSSION
Pyogenic granuloma (PG) is a benign vascular tumor of the skin and mucous membranes that is not associated with an infection. Rather, it is a hyperplastic, neovascular, inflammatory response to an angiogenic stimulus. Several enhancers and inhibitors of angiogenesis have been shown to play a role in PG, including hormones, medications, and local injury. In fact, a local injury or hormonal factor is identified as a stimulus in more than half of PG patients.1
The hormone connection. Estrogen promotes production of nerve growth factor, granulocyte-macrophage colony-stimulating factor, basic fibroblast growth factor, vascular endothelial growth factor, and transforming growth factor beta 1. Progesterone enhances inflammatory mediators as well. Although there are no direct receptors for estrogen and progesterone in the oral and nasal mucosa, some of these pro-inflammatory effects create an environment conducive to the development of PG. This is supported by several studies documenting an increased incidence of PGs with oral contraceptive use and regression of PGs after childbirth.2-4
Medication may play a role. Drug-induced PG has also been described in several studies.5,6 Offending medications include systemic and topical retinoids, capecitabine, etoposide, 5-fluorouracil, cyclosporine, docetaxel, and human immunodeficiency virus protease inhibitors.
Local injury may also be a culprit. Nasal PGs are commonly attached to the anterior septum and typically result from nasal packing, habitual picking, or nose boring.7 In this particular case, however, we were unable to identify the irritant.
The classic presentation
PG classically presents as a painless mass that spontaneously develops over days to weeks. The mass can be sessile or pedunculated, and is frequently hemorrhagic. Intranasal PG usually presents with epiphora.7 While the prevalence of intraoral PG was found to be one in 25,000 individuals3, data for nasal lesions is scarce. Most cases of PG are seen in the second and third decades of life.1,3 In children, PG is slightly more predominant in males.1,3 Mucosal lesions, however, have a higher incidence in females.1,3 Granuloma gravidarum, the term used to describe mucosal PG in pregnant females, was found in 0.2% to 5% of pregnancies.2,3,8
Differential Dx includes warts, squamous cell carcinoma
The differential diagnosis of PG includes Spitz nevus, glomus tumors, common warts, amelanotic melanoma, squamous cell carcinoma, basal cell carcinoma, Kaposi’s sarcoma, bacillary angiomatosis, infantile hemangioma, and angiolymphoid hyperplasia, among others.3,5 Foreign bodies, nasal polyps, angiofibroma, meningocele, Wegener’s granulomatosis, and sarcoidosis should also be considered.
Radiologic evaluation may be beneficial—especially with nasal lesions—when looking for findings suggestive of malignancy. Both CT and magnetic resonance imaging with contrast identify PG as a soft tissue mass with lobulated contours,9,10 but histopathologic analysis is required to confirm the diagnosis. The histopathologic appearance of PG is characterized by a polypoid lesion with circumscribed anastomosing networks of capillaries arranged in one or more lobules at the base in an edematous and fibroblastic stroma.
Treatment is determined by the location and size of the lesion
The most suitable treatment is determined by considering the location of the lesion, the characteristics of the lesion (morphology/size), its amenability to surgery, risk of scar formation, and the presence or absence of a causative irritant. Excision is often preferred because it yields a specimen for pathologic analysis. Alternative treatments include electrocautery, cryotherapy, laser therapy, and intralesional and topical agents,3,6,7 but the recurrence rate is higher (up to 15%) with some of these modalities, when compared with excision (3.6%).3
Our patient underwent excision of the mass and was seen for an annual follow-up appointment. All of her symptoms resolved and no recurrence was noted.
THE TAKEAWAY
Although PG is a common and benign condition, it is rarely seen in the nasal cavity without an obvious history of a possible irritant. PG should be considered as a diagnosis for rapidly growing cutaneous or mucosal hemorrhagic lesions. Appropriate tissue pathology is essential to rule out malignancy and other serious conditions, such as bacillary angiomatosis and Wegener’s granulomatosis.
Treatment is usually required to avoid the frequent complications of ulceration and bleeding. Surgical treatments are preferred. The location of the lesion largely determines whether referral to a specialist is necessary.
1. Harris MN, Desai R, Chuang TY, et al. Lobular capillary hemangiomas: An epidemiologic report, with emphasis on cutaneous lesions. J Am Acad Dermatol. 2000;42:1012-1016.
2. Yuan K, Jin YT, Lin MT. The detection and comparison of angiogenesis-associated factors in pyogenic granuloma by immunohistochemistry. J Periodontol. 2000;71:701-709.
3. Giblin AV, Clover AJ, Athanassopoulos A, et al. Pyogenic granuloma–the quest for optimum treatment: audit of treatment of 408 cases. J Plast Reconstr Aesthet Surg. 2007;60:1030-1035.
4. Steelman R, Holmes D. Pregnancy tumor in a 16-year-old: case report and treatment considerations. J Clin Pediatr Dent. 1992;16:217-218.
5. Jafarzadeh H, Sanatkhani M, Mohtasham N. Oral pyogenic granuloma: a review. J Oral Sci. 2006;48:167-175.
6. Piraccini BM, Bellavista S, Misciali C, et al. Periungual and subungual pyogenic granuloma. Br J Dermatol. 2010;163:941-953.
7. Ozcan C, Apa DD, Görür K. Pediatric lobular capillary hemangioma of the nasal cavity. Eur Arch Otorhinolaryngol. 2004;261:449-451.
8. Henry F, Quatresooz P, Valverde-Lopez JC, et al. Blood vessel changes during pregnancy: a review. Am J Clin Dermatol. 2006;7:65-69.
9. Puxeddu R, Berlucchi M, Ledda GP, et al. Lobular capillary hemangioma of the nasal cavity: A retrospective study on 40 patients. Am J Rhinol. 2006;20:480-484.
10. Maroldi R, Berlucchi M, Farina D, et al. Benign neoplasms and tumor-like lesions. In: Maroldi R, Nicolai P, eds. Imaging in Treatment Planning for Sinonasal Diseases. Berlin, Heidelberg, New York: Springer-Verlag; 2005:107-158.
1. Harris MN, Desai R, Chuang TY, et al. Lobular capillary hemangiomas: An epidemiologic report, with emphasis on cutaneous lesions. J Am Acad Dermatol. 2000;42:1012-1016.
2. Yuan K, Jin YT, Lin MT. The detection and comparison of angiogenesis-associated factors in pyogenic granuloma by immunohistochemistry. J Periodontol. 2000;71:701-709.
3. Giblin AV, Clover AJ, Athanassopoulos A, et al. Pyogenic granuloma–the quest for optimum treatment: audit of treatment of 408 cases. J Plast Reconstr Aesthet Surg. 2007;60:1030-1035.
4. Steelman R, Holmes D. Pregnancy tumor in a 16-year-old: case report and treatment considerations. J Clin Pediatr Dent. 1992;16:217-218.
5. Jafarzadeh H, Sanatkhani M, Mohtasham N. Oral pyogenic granuloma: a review. J Oral Sci. 2006;48:167-175.
6. Piraccini BM, Bellavista S, Misciali C, et al. Periungual and subungual pyogenic granuloma. Br J Dermatol. 2010;163:941-953.
7. Ozcan C, Apa DD, Görür K. Pediatric lobular capillary hemangioma of the nasal cavity. Eur Arch Otorhinolaryngol. 2004;261:449-451.
8. Henry F, Quatresooz P, Valverde-Lopez JC, et al. Blood vessel changes during pregnancy: a review. Am J Clin Dermatol. 2006;7:65-69.
9. Puxeddu R, Berlucchi M, Ledda GP, et al. Lobular capillary hemangioma of the nasal cavity: A retrospective study on 40 patients. Am J Rhinol. 2006;20:480-484.
10. Maroldi R, Berlucchi M, Farina D, et al. Benign neoplasms and tumor-like lesions. In: Maroldi R, Nicolai P, eds. Imaging in Treatment Planning for Sinonasal Diseases. Berlin, Heidelberg, New York: Springer-Verlag; 2005:107-158.
One lab finding, 2 vastly different causes
CASE 1
A 13-month-old boy who was recently adopted from Ethiopia presented to a primary care physician with a 3-week history of bloody diarrhea accompanied by flatulence and bloating. Stool cultures were positive for Campylobacter and Shigella. He was prescribed azithromycin but saw only moderate improvement. He was then referred to the Infectious Diseases Department. Neonatal, pregnancy, and immunization histories were unknown and a review of systems was unremarkable. On exam, the child looked well; he weighed 9.6 kg (15th percentile), was 69.5 cm long (<3rd percentile), and his head circumference was 45 cm (10th percentile). Head and neck, cardiorespiratory, and abdominal examinations were unremarkable.
A complete blood count (CBC) showed an elevated white blood cell (WBC) count of 26 x 109/L (normal: 4-10 x 109/L) with predominant eosinophilia (10.4 x 109/L or 40.1% of WBCs; normal: <0.45 x 109/L or 0%-8%). Hemoglobin and platelets were within normal limits. Stool testing for ova and parasites showed Strongyloides stercoralis larvae. Strongyloides serology was negative and Filaria serology was equivocal.
CASE 2
A 15-year-old boy was assessed for a 3-week history of fever and eosinophilia. He had enlarged cervical lymph nodes, a new rash, and had lost 4 pounds. He denied gastrointestinal symptoms, dyspnea, headaches, or chest pain. His past medical and family histories were unremarkable and he reported no drug use or allergies. He had traveled to Cuba with his family for 15 days 3 months prior to presentation. He recalled diarrhea while traveling, which resolved spontaneously. He and his family had traveled “off the beaten track,” eating foods prepared at local establishments and swimming in local rivers. He received pre-travel immunizations.
On examination, he appeared unwell, though his vital signs were normal. He had diffuse lymphadenopathy and a petechial rash on his chest, back, upper buttocks, legs, and feet. Cardiorespiratory and abdominal examinations were unremarkable. A CBC revealed an elevated WBC count of 76.9 x 109/L with predominant eosinophilia (71.5 x 109/L or 92% of WBCs). Hemoglobin, platelets, electrolytes, and liver function tests were normal. The patient was referred to a tertiary care center and was admitted to the hospital. Stool testing for ova and parasites, as well as serology for parasitic infections, was negative. A bone marrow aspiration and biopsy were performed and revealed the diagnosis of acute lymphoblastic leukemia (ALL).
DISCUSSION
These 2 cases highlight how the presentation of eosinophilia can vary and how important it is to maintain a broad differential diagnosis (TABLE 11-4). Causes of eosinophilia are numerous and can be divided into 3 categories: primary, secondary, and idiopathic.1,5 Hematologic malignancy, where eosinophilia is clonal, is an example of a primary etiology. Causes of secondary eosinophilia include infectious diseases, drugs (TABLE 25), autoimmune disorders, and allergic conditions. Prolonged eosinophilia that is >3 x 109/L is associated with end-organ damage. Dermatologic, pulmonary, gastrointestinal, and cardiac involvement is most common.2

Eosinophilia associated with parasitic infection

In returning travelers and international adoptees, multicellular helminthic parasites are the most common causes of eosinophilia, with eosinophilia occurring during tissue migration or penetration.1,3
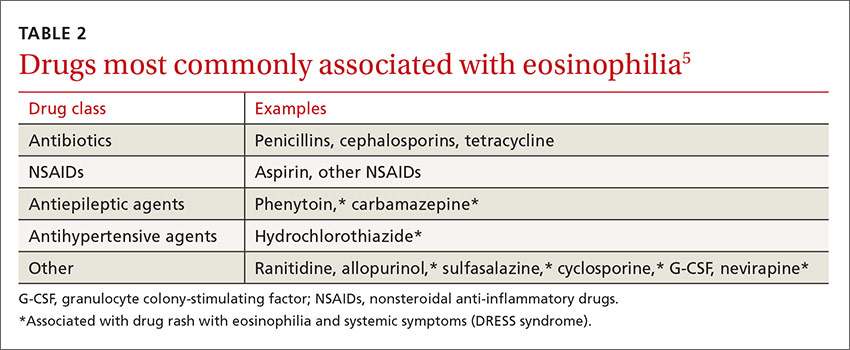
Schistosomiasis is a chronic parasitic infection of the human vascular system. It is transmitted by contact with contaminated fresh water, where cercariae penetrate the skin. High prevalence areas include Africa and Southeast Asia. Acute infection can result in Katayama fever—a febrile illness with prominent eosinophilia that occurs 4 to 7 weeks after exposure.4 Diagnosis is primarily clinical with appropriate epidemiology, as serology may be negative early in infection. Praziquantel is the treatment of choice, though dosing varies by species, so expert consultation should be considered.
Soil-transmitted helminths, such as Ascaris (Ascaris lumbricoides), whipworm (Trichuris trichiura), and hookworm (Ancyclostoma duodenale and Necator americanus), can also cause eosinophilia during larval tissue migration. Following infection by ingestion or skin penetration, an acute respiratory illness, termed Löffler’s syndrome, can develop with associated eosinophilia.1 Once the helminths reach the adult stage, eosinophilia subsides. Patients are most commonly treated with albendazole 400 mg orally for 3 days.4
Fascioliasis is common in sheep-rearing areas. Humans are infected through ingestion of aquatic plants (eg, watercress). Parasitic migration through the duodenal wall and liver parenchyma can lead to fever, right upper quadrant pain, and eosinophilia. The incubation period is 6 to 12 weeks. Diagnosis during acute infection is by serology.4
Filarial infections, eg lymphatic filariasis, loiasis, and onchocerciasis, can also cause eosinophilia. The rise in eosinophils can be triggered by either the adult worms or circulating microfilariae.4 Treatment of fascioliasis and filarial infections varies and expert consultation is recommended.
Eosinophilia associated with primary hematologic malignancy
Eosinophilia is a rare presentation of hematologic malignancy. Acute myeloid leukemia, acute lymphoblastic leukemia (ALL), chronic myeloid leukemia, and myeloproliferative disorders have all been associated with eosinophilia. Hepatosplenomegaly, generalized lymphadenopathy, and cytopenias in other cell lines are often noted. Also, the degree of eosinophilia is often more pronounced (>5 x 109/L). Patients with suspected hematologic malignancy should be urgently referred for expert consultation.5
A systematic approach to patients with eosinophilia
Consider the following approach in the assessment of patients with eosinophilia seen in the ambulatory care setting. Inpatients or patients being seen in developing areas may require a modified approach.
History. All patients with eosinophilia should have a thorough history taken, with particular attention paid to travel history. A travel history should make note of dates, duration and location of travel, and any relevant exposures, such as arthropod bites or swimming in freshwater. Dietary habits, such as ingestion of seafood, game, or undercooked meat can also be helpful in making a diagnosis.3,4
Physical exam. In addition to a general physical examination, the following features may be helpful in determining the etiology of eosinophilia. Wheeze is characteristic of parasites in a lung migration phase (eg, strongyloidiasis and ascariasis) or asthma. Hepatomegaly can be seen with liver flukes, visceral larva migrans, or schistosomiasis. Periorbital edema can be observed with Trichinella infection. Loa loa, a type of filarial infection, produces a transient, migratory angioedema, often localized to the wrists and large joints (termed Calabar swelling). Dermatitis of varying intensity may suggest filarial infection, schistosomiasis, or atopy. Perianal dermatitis is observed with strongyloidiasis. Cutaneous larva migrans is characterized by a linear, serpiginous rash.3,4
Laboratory investigations. Investigation will vary depending on the patient’s history, exposures, exam findings, and degree of eosinophilia. Any patient who is unwell or has significant eosinophilia (≥3 x 109/L) may warrant more urgent referral to infectious disease, travel medicine, or hematology. Basic laboratory investigations should include a CBC with differential, routine serum chemistries, and liver enzymes. In the setting of significant eosinophilia, an electrocardiogram, cardiac enzyme levels, and a chest x-ray should be obtained to screen for end-organ damage related to eosinophilia.3-5
In patients in whom you suspect hematologic malignancy, bone marrow aspiration and biopsy are often needed to make the diagnosis.5
Parasitic infections are most often diagnosed on stool examination for ova and parasites or by serology. Stool should be collected on 3 separate days to increase diagnostic yield. Certain species of Schistosoma can also be diagnosed on direct microscopy of urine specimens. Serologic assays are available for schistosomiasis, strongyloidiasis, Toxocara, fascioliasis, filariasis, and Trichinella. Further investigations for filiariasis, including blood films, eye exam, and skin snips will vary with filarial species, so expert consultation should be considered.3,4
Our patients. The first patient with strongyloidiasis was treated with ivermectin 200 µg/kg/day orally for 2 days and experienced symptomatic improvement and resolution of eosinophilia. The second patient with ALL was admitted and referred to hematology and received induction chemotherapy. Treatment was well tolerated and the patient was discharged one week later, with appropriate follow-up.
THE TAKEAWAY
Eosinophilia is commonly encountered in primary care. The approach to eosinophilia and the differential diagnosis can be challenging. The correct diagnosis was reached in both cases by maintaining a broad differential diagnosis. Obtaining a travel and exposure history is fundamental, although noninfectious causes, including allergy, malignancy, and drug reaction, must always be considered.
1. Moore TA, Nutman TB. Eosinophilia in the returning traveler. Infect Dis Clin North Am. 1998;12:503-521.
2. Tefferi A, Gotlib J, Pardanani A. Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin Proc. 2010;85:158-164.
3. Schulte C, Krebs B, Jelinek T, et al. Diagnostic significance of blood eosinophilia in returning travelers. Clin Infect Dis. 2002;34:407-411.
4. Checkley AM, Chiodini PL, Dockrell DH, et al; British Infection Society and Hospital for Tropical Diseases. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. J Infect. 2010;60:1-20.
5. Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006;133:468-492.
CASE 1
A 13-month-old boy who was recently adopted from Ethiopia presented to a primary care physician with a 3-week history of bloody diarrhea accompanied by flatulence and bloating. Stool cultures were positive for Campylobacter and Shigella. He was prescribed azithromycin but saw only moderate improvement. He was then referred to the Infectious Diseases Department. Neonatal, pregnancy, and immunization histories were unknown and a review of systems was unremarkable. On exam, the child looked well; he weighed 9.6 kg (15th percentile), was 69.5 cm long (<3rd percentile), and his head circumference was 45 cm (10th percentile). Head and neck, cardiorespiratory, and abdominal examinations were unremarkable.
A complete blood count (CBC) showed an elevated white blood cell (WBC) count of 26 x 109/L (normal: 4-10 x 109/L) with predominant eosinophilia (10.4 x 109/L or 40.1% of WBCs; normal: <0.45 x 109/L or 0%-8%). Hemoglobin and platelets were within normal limits. Stool testing for ova and parasites showed Strongyloides stercoralis larvae. Strongyloides serology was negative and Filaria serology was equivocal.
CASE 2
A 15-year-old boy was assessed for a 3-week history of fever and eosinophilia. He had enlarged cervical lymph nodes, a new rash, and had lost 4 pounds. He denied gastrointestinal symptoms, dyspnea, headaches, or chest pain. His past medical and family histories were unremarkable and he reported no drug use or allergies. He had traveled to Cuba with his family for 15 days 3 months prior to presentation. He recalled diarrhea while traveling, which resolved spontaneously. He and his family had traveled “off the beaten track,” eating foods prepared at local establishments and swimming in local rivers. He received pre-travel immunizations.
On examination, he appeared unwell, though his vital signs were normal. He had diffuse lymphadenopathy and a petechial rash on his chest, back, upper buttocks, legs, and feet. Cardiorespiratory and abdominal examinations were unremarkable. A CBC revealed an elevated WBC count of 76.9 x 109/L with predominant eosinophilia (71.5 x 109/L or 92% of WBCs). Hemoglobin, platelets, electrolytes, and liver function tests were normal. The patient was referred to a tertiary care center and was admitted to the hospital. Stool testing for ova and parasites, as well as serology for parasitic infections, was negative. A bone marrow aspiration and biopsy were performed and revealed the diagnosis of acute lymphoblastic leukemia (ALL).
DISCUSSION
These 2 cases highlight how the presentation of eosinophilia can vary and how important it is to maintain a broad differential diagnosis (TABLE 11-4). Causes of eosinophilia are numerous and can be divided into 3 categories: primary, secondary, and idiopathic.1,5 Hematologic malignancy, where eosinophilia is clonal, is an example of a primary etiology. Causes of secondary eosinophilia include infectious diseases, drugs (TABLE 25), autoimmune disorders, and allergic conditions. Prolonged eosinophilia that is >3 x 109/L is associated with end-organ damage. Dermatologic, pulmonary, gastrointestinal, and cardiac involvement is most common.2

Eosinophilia associated with parasitic infection

In returning travelers and international adoptees, multicellular helminthic parasites are the most common causes of eosinophilia, with eosinophilia occurring during tissue migration or penetration.1,3

Schistosomiasis is a chronic parasitic infection of the human vascular system. It is transmitted by contact with contaminated fresh water, where cercariae penetrate the skin. High prevalence areas include Africa and Southeast Asia. Acute infection can result in Katayama fever—a febrile illness with prominent eosinophilia that occurs 4 to 7 weeks after exposure.4 Diagnosis is primarily clinical with appropriate epidemiology, as serology may be negative early in infection. Praziquantel is the treatment of choice, though dosing varies by species, so expert consultation should be considered.
Soil-transmitted helminths, such as Ascaris (Ascaris lumbricoides), whipworm (Trichuris trichiura), and hookworm (Ancyclostoma duodenale and Necator americanus), can also cause eosinophilia during larval tissue migration. Following infection by ingestion or skin penetration, an acute respiratory illness, termed Löffler’s syndrome, can develop with associated eosinophilia.1 Once the helminths reach the adult stage, eosinophilia subsides. Patients are most commonly treated with albendazole 400 mg orally for 3 days.4
Fascioliasis is common in sheep-rearing areas. Humans are infected through ingestion of aquatic plants (eg, watercress). Parasitic migration through the duodenal wall and liver parenchyma can lead to fever, right upper quadrant pain, and eosinophilia. The incubation period is 6 to 12 weeks. Diagnosis during acute infection is by serology.4
Filarial infections, eg lymphatic filariasis, loiasis, and onchocerciasis, can also cause eosinophilia. The rise in eosinophils can be triggered by either the adult worms or circulating microfilariae.4 Treatment of fascioliasis and filarial infections varies and expert consultation is recommended.
Eosinophilia associated with primary hematologic malignancy
Eosinophilia is a rare presentation of hematologic malignancy. Acute myeloid leukemia, acute lymphoblastic leukemia (ALL), chronic myeloid leukemia, and myeloproliferative disorders have all been associated with eosinophilia. Hepatosplenomegaly, generalized lymphadenopathy, and cytopenias in other cell lines are often noted. Also, the degree of eosinophilia is often more pronounced (>5 x 109/L). Patients with suspected hematologic malignancy should be urgently referred for expert consultation.5
A systematic approach to patients with eosinophilia
Consider the following approach in the assessment of patients with eosinophilia seen in the ambulatory care setting. Inpatients or patients being seen in developing areas may require a modified approach.
History. All patients with eosinophilia should have a thorough history taken, with particular attention paid to travel history. A travel history should make note of dates, duration and location of travel, and any relevant exposures, such as arthropod bites or swimming in freshwater. Dietary habits, such as ingestion of seafood, game, or undercooked meat can also be helpful in making a diagnosis.3,4
Physical exam. In addition to a general physical examination, the following features may be helpful in determining the etiology of eosinophilia. Wheeze is characteristic of parasites in a lung migration phase (eg, strongyloidiasis and ascariasis) or asthma. Hepatomegaly can be seen with liver flukes, visceral larva migrans, or schistosomiasis. Periorbital edema can be observed with Trichinella infection. Loa loa, a type of filarial infection, produces a transient, migratory angioedema, often localized to the wrists and large joints (termed Calabar swelling). Dermatitis of varying intensity may suggest filarial infection, schistosomiasis, or atopy. Perianal dermatitis is observed with strongyloidiasis. Cutaneous larva migrans is characterized by a linear, serpiginous rash.3,4
Laboratory investigations. Investigation will vary depending on the patient’s history, exposures, exam findings, and degree of eosinophilia. Any patient who is unwell or has significant eosinophilia (≥3 x 109/L) may warrant more urgent referral to infectious disease, travel medicine, or hematology. Basic laboratory investigations should include a CBC with differential, routine serum chemistries, and liver enzymes. In the setting of significant eosinophilia, an electrocardiogram, cardiac enzyme levels, and a chest x-ray should be obtained to screen for end-organ damage related to eosinophilia.3-5
In patients in whom you suspect hematologic malignancy, bone marrow aspiration and biopsy are often needed to make the diagnosis.5
Parasitic infections are most often diagnosed on stool examination for ova and parasites or by serology. Stool should be collected on 3 separate days to increase diagnostic yield. Certain species of Schistosoma can also be diagnosed on direct microscopy of urine specimens. Serologic assays are available for schistosomiasis, strongyloidiasis, Toxocara, fascioliasis, filariasis, and Trichinella. Further investigations for filiariasis, including blood films, eye exam, and skin snips will vary with filarial species, so expert consultation should be considered.3,4
Our patients. The first patient with strongyloidiasis was treated with ivermectin 200 µg/kg/day orally for 2 days and experienced symptomatic improvement and resolution of eosinophilia. The second patient with ALL was admitted and referred to hematology and received induction chemotherapy. Treatment was well tolerated and the patient was discharged one week later, with appropriate follow-up.
THE TAKEAWAY
Eosinophilia is commonly encountered in primary care. The approach to eosinophilia and the differential diagnosis can be challenging. The correct diagnosis was reached in both cases by maintaining a broad differential diagnosis. Obtaining a travel and exposure history is fundamental, although noninfectious causes, including allergy, malignancy, and drug reaction, must always be considered.
CASE 1
A 13-month-old boy who was recently adopted from Ethiopia presented to a primary care physician with a 3-week history of bloody diarrhea accompanied by flatulence and bloating. Stool cultures were positive for Campylobacter and Shigella. He was prescribed azithromycin but saw only moderate improvement. He was then referred to the Infectious Diseases Department. Neonatal, pregnancy, and immunization histories were unknown and a review of systems was unremarkable. On exam, the child looked well; he weighed 9.6 kg (15th percentile), was 69.5 cm long (<3rd percentile), and his head circumference was 45 cm (10th percentile). Head and neck, cardiorespiratory, and abdominal examinations were unremarkable.
A complete blood count (CBC) showed an elevated white blood cell (WBC) count of 26 x 109/L (normal: 4-10 x 109/L) with predominant eosinophilia (10.4 x 109/L or 40.1% of WBCs; normal: <0.45 x 109/L or 0%-8%). Hemoglobin and platelets were within normal limits. Stool testing for ova and parasites showed Strongyloides stercoralis larvae. Strongyloides serology was negative and Filaria serology was equivocal.
CASE 2
A 15-year-old boy was assessed for a 3-week history of fever and eosinophilia. He had enlarged cervical lymph nodes, a new rash, and had lost 4 pounds. He denied gastrointestinal symptoms, dyspnea, headaches, or chest pain. His past medical and family histories were unremarkable and he reported no drug use or allergies. He had traveled to Cuba with his family for 15 days 3 months prior to presentation. He recalled diarrhea while traveling, which resolved spontaneously. He and his family had traveled “off the beaten track,” eating foods prepared at local establishments and swimming in local rivers. He received pre-travel immunizations.
On examination, he appeared unwell, though his vital signs were normal. He had diffuse lymphadenopathy and a petechial rash on his chest, back, upper buttocks, legs, and feet. Cardiorespiratory and abdominal examinations were unremarkable. A CBC revealed an elevated WBC count of 76.9 x 109/L with predominant eosinophilia (71.5 x 109/L or 92% of WBCs). Hemoglobin, platelets, electrolytes, and liver function tests were normal. The patient was referred to a tertiary care center and was admitted to the hospital. Stool testing for ova and parasites, as well as serology for parasitic infections, was negative. A bone marrow aspiration and biopsy were performed and revealed the diagnosis of acute lymphoblastic leukemia (ALL).
DISCUSSION
These 2 cases highlight how the presentation of eosinophilia can vary and how important it is to maintain a broad differential diagnosis (TABLE 11-4). Causes of eosinophilia are numerous and can be divided into 3 categories: primary, secondary, and idiopathic.1,5 Hematologic malignancy, where eosinophilia is clonal, is an example of a primary etiology. Causes of secondary eosinophilia include infectious diseases, drugs (TABLE 25), autoimmune disorders, and allergic conditions. Prolonged eosinophilia that is >3 x 109/L is associated with end-organ damage. Dermatologic, pulmonary, gastrointestinal, and cardiac involvement is most common.2

Eosinophilia associated with parasitic infection

In returning travelers and international adoptees, multicellular helminthic parasites are the most common causes of eosinophilia, with eosinophilia occurring during tissue migration or penetration.1,3

Schistosomiasis is a chronic parasitic infection of the human vascular system. It is transmitted by contact with contaminated fresh water, where cercariae penetrate the skin. High prevalence areas include Africa and Southeast Asia. Acute infection can result in Katayama fever—a febrile illness with prominent eosinophilia that occurs 4 to 7 weeks after exposure.4 Diagnosis is primarily clinical with appropriate epidemiology, as serology may be negative early in infection. Praziquantel is the treatment of choice, though dosing varies by species, so expert consultation should be considered.
Soil-transmitted helminths, such as Ascaris (Ascaris lumbricoides), whipworm (Trichuris trichiura), and hookworm (Ancyclostoma duodenale and Necator americanus), can also cause eosinophilia during larval tissue migration. Following infection by ingestion or skin penetration, an acute respiratory illness, termed Löffler’s syndrome, can develop with associated eosinophilia.1 Once the helminths reach the adult stage, eosinophilia subsides. Patients are most commonly treated with albendazole 400 mg orally for 3 days.4
Fascioliasis is common in sheep-rearing areas. Humans are infected through ingestion of aquatic plants (eg, watercress). Parasitic migration through the duodenal wall and liver parenchyma can lead to fever, right upper quadrant pain, and eosinophilia. The incubation period is 6 to 12 weeks. Diagnosis during acute infection is by serology.4
Filarial infections, eg lymphatic filariasis, loiasis, and onchocerciasis, can also cause eosinophilia. The rise in eosinophils can be triggered by either the adult worms or circulating microfilariae.4 Treatment of fascioliasis and filarial infections varies and expert consultation is recommended.
Eosinophilia associated with primary hematologic malignancy
Eosinophilia is a rare presentation of hematologic malignancy. Acute myeloid leukemia, acute lymphoblastic leukemia (ALL), chronic myeloid leukemia, and myeloproliferative disorders have all been associated with eosinophilia. Hepatosplenomegaly, generalized lymphadenopathy, and cytopenias in other cell lines are often noted. Also, the degree of eosinophilia is often more pronounced (>5 x 109/L). Patients with suspected hematologic malignancy should be urgently referred for expert consultation.5
A systematic approach to patients with eosinophilia
Consider the following approach in the assessment of patients with eosinophilia seen in the ambulatory care setting. Inpatients or patients being seen in developing areas may require a modified approach.
History. All patients with eosinophilia should have a thorough history taken, with particular attention paid to travel history. A travel history should make note of dates, duration and location of travel, and any relevant exposures, such as arthropod bites or swimming in freshwater. Dietary habits, such as ingestion of seafood, game, or undercooked meat can also be helpful in making a diagnosis.3,4
Physical exam. In addition to a general physical examination, the following features may be helpful in determining the etiology of eosinophilia. Wheeze is characteristic of parasites in a lung migration phase (eg, strongyloidiasis and ascariasis) or asthma. Hepatomegaly can be seen with liver flukes, visceral larva migrans, or schistosomiasis. Periorbital edema can be observed with Trichinella infection. Loa loa, a type of filarial infection, produces a transient, migratory angioedema, often localized to the wrists and large joints (termed Calabar swelling). Dermatitis of varying intensity may suggest filarial infection, schistosomiasis, or atopy. Perianal dermatitis is observed with strongyloidiasis. Cutaneous larva migrans is characterized by a linear, serpiginous rash.3,4
Laboratory investigations. Investigation will vary depending on the patient’s history, exposures, exam findings, and degree of eosinophilia. Any patient who is unwell or has significant eosinophilia (≥3 x 109/L) may warrant more urgent referral to infectious disease, travel medicine, or hematology. Basic laboratory investigations should include a CBC with differential, routine serum chemistries, and liver enzymes. In the setting of significant eosinophilia, an electrocardiogram, cardiac enzyme levels, and a chest x-ray should be obtained to screen for end-organ damage related to eosinophilia.3-5
In patients in whom you suspect hematologic malignancy, bone marrow aspiration and biopsy are often needed to make the diagnosis.5
Parasitic infections are most often diagnosed on stool examination for ova and parasites or by serology. Stool should be collected on 3 separate days to increase diagnostic yield. Certain species of Schistosoma can also be diagnosed on direct microscopy of urine specimens. Serologic assays are available for schistosomiasis, strongyloidiasis, Toxocara, fascioliasis, filariasis, and Trichinella. Further investigations for filiariasis, including blood films, eye exam, and skin snips will vary with filarial species, so expert consultation should be considered.3,4
Our patients. The first patient with strongyloidiasis was treated with ivermectin 200 µg/kg/day orally for 2 days and experienced symptomatic improvement and resolution of eosinophilia. The second patient with ALL was admitted and referred to hematology and received induction chemotherapy. Treatment was well tolerated and the patient was discharged one week later, with appropriate follow-up.
THE TAKEAWAY
Eosinophilia is commonly encountered in primary care. The approach to eosinophilia and the differential diagnosis can be challenging. The correct diagnosis was reached in both cases by maintaining a broad differential diagnosis. Obtaining a travel and exposure history is fundamental, although noninfectious causes, including allergy, malignancy, and drug reaction, must always be considered.
1. Moore TA, Nutman TB. Eosinophilia in the returning traveler. Infect Dis Clin North Am. 1998;12:503-521.
2. Tefferi A, Gotlib J, Pardanani A. Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin Proc. 2010;85:158-164.
3. Schulte C, Krebs B, Jelinek T, et al. Diagnostic significance of blood eosinophilia in returning travelers. Clin Infect Dis. 2002;34:407-411.
4. Checkley AM, Chiodini PL, Dockrell DH, et al; British Infection Society and Hospital for Tropical Diseases. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. J Infect. 2010;60:1-20.
5. Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006;133:468-492.
1. Moore TA, Nutman TB. Eosinophilia in the returning traveler. Infect Dis Clin North Am. 1998;12:503-521.
2. Tefferi A, Gotlib J, Pardanani A. Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin Proc. 2010;85:158-164.
3. Schulte C, Krebs B, Jelinek T, et al. Diagnostic significance of blood eosinophilia in returning travelers. Clin Infect Dis. 2002;34:407-411.
4. Checkley AM, Chiodini PL, Dockrell DH, et al; British Infection Society and Hospital for Tropical Diseases. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. J Infect. 2010;60:1-20.
5. Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006;133:468-492.
Monitoring home BP readings just got easier
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5
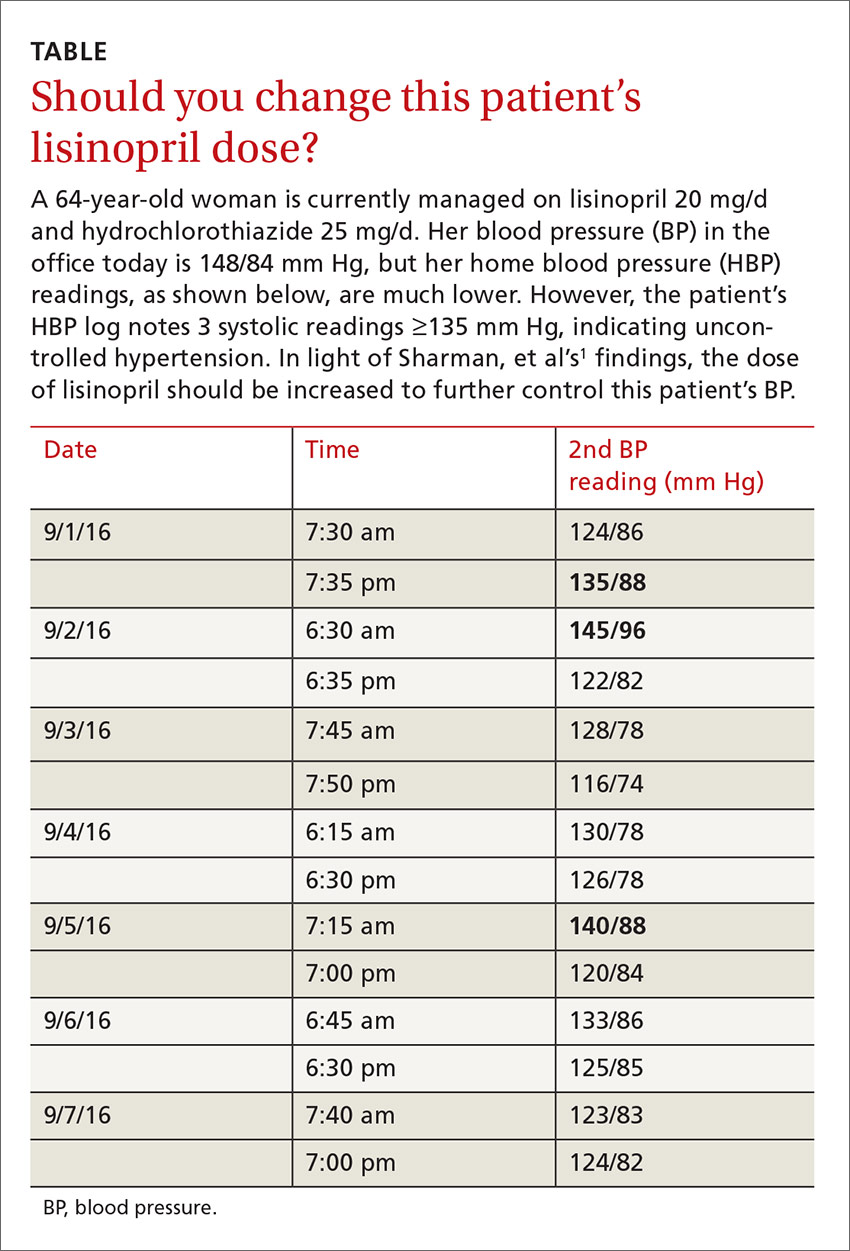
Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5

Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5

Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
A step-wise approach to exertional leg pain
PRACTICE RECOMMENDATIONS
› Consider the possibility of vascular and neurologic problems as the source of exertional leg pain (ELP). C
› Order magnetic resonance imaging to evaluate patients with ELP and negative x-rays for stress fractures. C
› Measure lower extremity intracompartmental pressures both before and after exercise when you suspect chronic exertional compartmental syndrome. Doing so is the gold standard for the diagnosis of this condition. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most family physicians are accustomed to treating active patients with shin splints and stress fractures. But many are less familiar with, and slower to recognize, other sources of exertional leg pain (ELP), defined as exercise-related pain that localizes in the lower extremity distal to the knee and proximal to the talocrural joint.1
ELP has a broad differential diagnosis that includes other musculoskeletal conditions—most notably chronic exertional compartment syndrome (CECS), which has been found to affect 33% of athletes with chronic ELP1—as well as a number of vascular and neurologic causes.2-4 In addition, etiologies may overlap. Greater awareness of the many causes of ELP can help you to avoid the unnecessary use of expensive diagnostic tests as well as delayed diagnosis and treatment.
A thorough medical and activity history, symptom review, and physical examination are your most important tools when patients present with ELP. When the cause is not obvious or the patient fails to respond to conservative measures, x-rays, magnetic resonance imaging (MRI), vascular studies, electromyography and nerve conduction studies, and/or intracompartmental pressure testing may be needed to find the source of the symptoms. In the text that follows, we review both common and relatively uncommon sources of ELP, using a stepwise diagnostic approach. You’ll find a diagnostic challenge, in which you can test your skills and a more comprehensive differential diagnosis in the TABLE.1-3,5-9
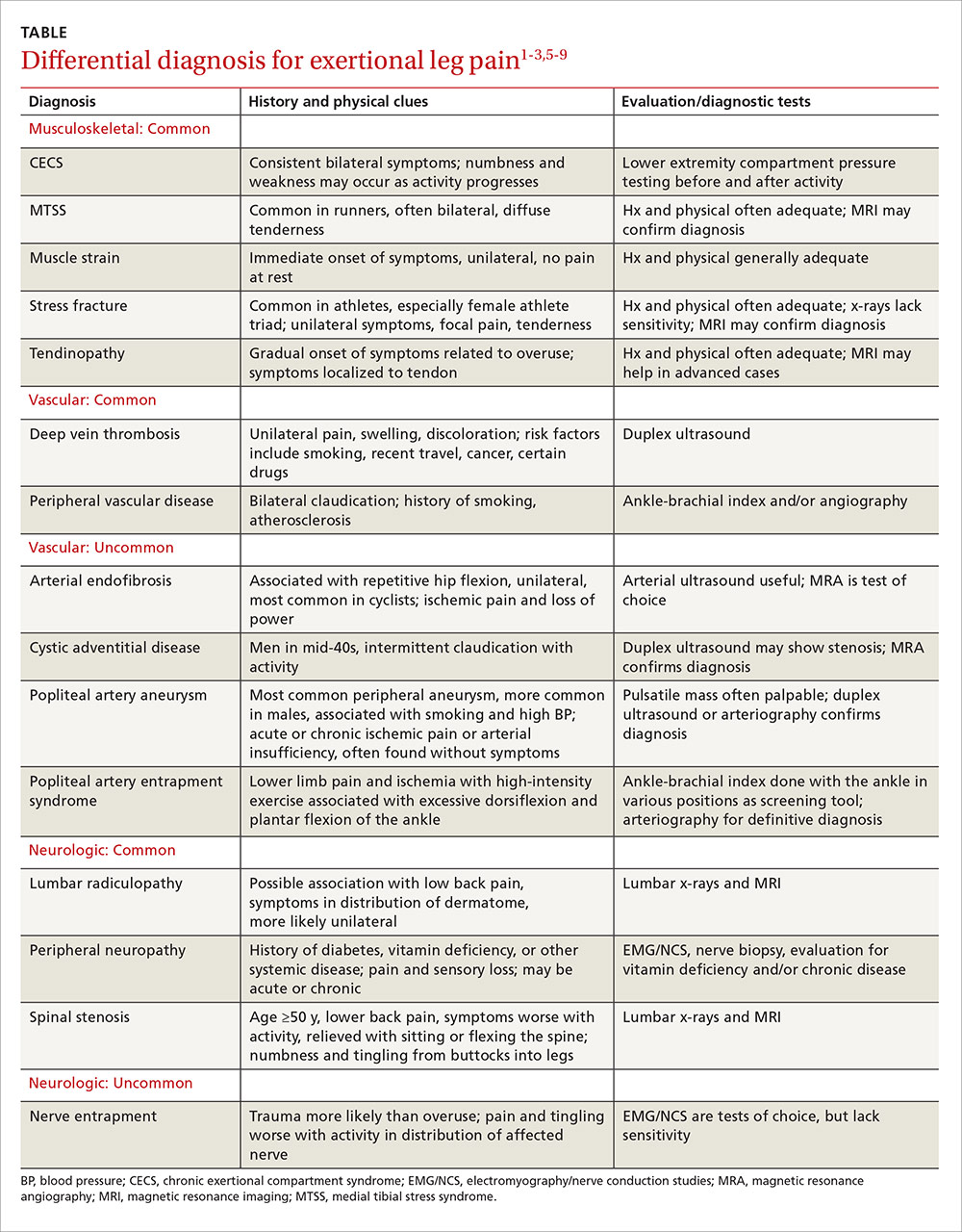
Musculoskeletal injuries: Shin splints and beyond

Medial tibial stress syndrome (MTSS), commonly known as shin splints, is characterized by pain and tenderness over the posteromedial aspect of the distal tibia.3 It typically results in diffuse pain that occurs with exercise, but may persist at rest in severe cases.3-6 Less often, localized swelling may also be present.2
MTSS accounts for between 6% and 16% of all running injuries.2,10 It is associated with a spectrum of tibial stress injuries, including periostitis, tendinopathy, and stress reaction, with dysfunction of the tibialis posterior, tibialis anterior, and soleus muscles thought to be contributing factors.2,11 Intrinsic factors include high body mass index (BMI), female sex, excessive internal and external hip rotation, hyperpronation, and hyper plantar flexion.2,10,12
X-rays of the leg are typically normal in patients with MTSS and should be considered only if the clinical presentation suggests the possibility of an alternative diagnosis, such as a stress fracture or tumor.2-4,13 Advanced imaging such as MRI or triple phase bone scans (TPBS) are useful when the diagnosis is in question and will reveal an abnormally high signal along the posterior medial tibial surface or the classic train-track appearance of nucleotide uptake in patients with MTSS.2 MRI readily shows periosteal reaction and bony edema and has a sensitivity of 78% to 89% and a specificity of 33% to 100% for the diagnosis of MTSS.14,15
Initial management of MTSS is conservative, with the mainstay of treatment consisting of rest, ice, and nonsteroidal anti-inflammatory drugs (NSAIDs).3,13,16 While ice, NSAIDs, proper conditioning, physical therapy to stretch and strengthen the calf musculature, rigid orthotics to correct foot hyperpronation, and activity modification are all appropriate treatments, randomized controlled trials have shown none of these interventions to be more effective than rest alone.2 Non-operative treatment is usually successful, but surgery may be required for severe or refractory cases. Procedures include posteromedial fasciotomy, release of the medial soleus fascial bridge, deep compartment fasciotomy, or removal of a section of the distal tibia periosteum.3,4
Lower extremity stress fracture. Stress fractures are caused by repetitive loading that results in microtrauma, including bony microfractures. The vast majority of cases—80% to 95% of stress fractures—affect the lower extremities, and most involve the tibia.2-4,6,13,17 The most common presentation is an insidious onset of pain over a specific bony area with a normal appearance, although localized swelling or erythema may occasionally be present.3,14,17,18 The pain may be reproduced or worsened by weight-bearing activities and relieved by rest.14,18
Consider the female athlete triad. In evaluating a patient with a stress fracture, pay close attention to dietary history, BMI, and, in female athletes, take a detailed menstrual history. Such patients are at risk for amenorrhea, low bone mineral density, and nutritional deficits—the “female athlete triad,” which carries an increased risk of stress fractures.3,14,17-19
Stress fractures can often be diagnosed with a thorough medical history and physical, with imaging used for confirmation.6,14,17,18 Historical features of a stress fracture that may differentiate it from MTSS include pain that is unilateral and absent at rest and occurs with more prolonged activity, as well as post-exercise and/or nocturnal pain. Notable physical exam features include pain that is reproduced in a focal area with a single leg hop or percussion with a tuning fork or ultrasound.5,11,17
Initially, sensitivity for a plain radiograph is as low as 10%.2,11 Abnormalities on x-ray are usually seen after 2 to 8 weeks of symptoms2,7,11 and may include a faint periosteal reaction, a fluffy area of callus, or a cortical lucency sometimes referred to as the “dreaded black line.”3,6,17 If a radiographic exam shows evidence of a stress fracture, further imaging is typically unnecessary. MRI or TPBS is suggested, however, when x-rays appear normal but suspicion of a stress fracture remains.3,17,18 MRI may show edema within 3 days of symptom onset and is more sensitive and specific than computed tomography (CT) or TPBS for diagnosing stress fractures of the tibia.2,16
Treatment of tibial stress fractures is typically non-operative and consists of alterations in activity (eg, non weight-bearing), correction of nutritional deficits, such as inadequate caloric intake or too little calcium or iron, and addressing problems with footwear, training regimen, and/or running surface.3,14,18 Fibular and posteromedial tibial stress fractures are considered low risk and heal with weight-bearing restrictions and rest, initially for a minimum of 2 to 4 weeks.3
Posteromedial tibia injuries tend to heal well because they are on the compression side of the bone. Anterior tibia stress fractures, which are located on the tension side of the bone2,7 and account for approximately 5% of all tibia stress fractures, are more prone to non-union or progression to a complete fracture.7,20 Thus, anterior tibia stress injuries warrant a more aggressive approach, with treatment options including non-weight bearing status that may last longer than 8 weeks, pneumatic brace casting, and/or orthopedic referral to evaluate for surgical intervention.7,20-22 Time for radiographic evidence of healing may exceed 8 months, so early surgical intervention should be considered, especially for high-level athletes.7,20,21
Test your skills: A diagnostic challenge
Janine T, a 24-year-old long-distance runner, presents with left lower leg pain that occurs with activity. There was no injury, Ms. T reports; the pain began about 6 weeks ago, shortly after she began training for a marathon and running more than 30 miles per week. The pain is not relieved with intermittent rest or over-the-counter analgesics, she says. But it usually abates within 15 to 30 minutes after she completes her run.
Ms. T is underweight, with a body mass index <17 kg/m2. She denies any dietary restrictions and has normal menstrual cycles. The patient reports taking oral contraceptives, but no other medications. An initial x-ray is normal, as is magnetic resonance imaging to evaluate for a stress fracture.
You suspect Ms. T has shin splints, advise her to rest for a few weeks and to consider getting orthotics for her running shoes, and schedule a follow-up visit.
When she comes in 6 weeks later, the patient reports that she resumed running after a 3-week rest; shortly after, she noticed pain in both legs. What’s more, she now experiences tingling in her feet after running a few miles.
What’s wrong with this patient?
Ms. T’s symptoms—bilateral persistent leg pain, with tingling in both feet, and little improvement with rest—strongly suggest that she has chronic exertional compartment syndrome. Intracompartmental pressure testing, which reveals pre-exercise values ≥15 mm Hg and post-exercise values of ≥30 mm Hg at one minute, confirms the diagnosis.
Activity avoidance or modification will allow Ms. T’s symptoms to subside, but they’re highly likely to recur when she resumes running. The definitive treatment is intracompartmental fasciotomy, which has a success rate of approximately 80%.1,28
When to suspect chronic exertional compartment syndrome
Leg pain in CECS results from increased pressure within the lower extremity fascial compartments temporally related to exercise.2,23,24 Its incidence in the general population is unknown, but CECS has been found to range from 14% to 27% in patients with previously undiagnosed leg pain1,14,25 and to affect about a third of athletes with chronic ELP. In addition, CECS has been found in 90% of patients who have both diabetes and ELP with normal findings on vascular studies.1,3,4,26
The anterior compartment is most commonly affected, followed by the lateral, deep posterior, and superficial posterior compartments.3,13,23,27 Symptoms are bilateral 60% to 95% of the time.2,13,14,25 Factors contributing to CECS include fixed muscular compartment constraints, muscle swelling, thickened fascia, muscle hypertrophy related to resistance training, dynamic muscular contraction patterns, and low muscle capillary supply. Stretching of fascial pain receptors and pressure fibers and inadequate myocyte response to increased metabolism may play a role, as well.14,28
The initial clinical presentation is usually predictable leg pain—ie, pain that begins at about the same time, distance, or intensity of a workout and resolves with rest; numbness and weakness may occur as the workout progresses. In time, leg pain associated with CECS may be present with everyday activity or at rest. The physical exam may be normal or reveal swelling, tenderness over the involved compartments, pain with passive digit or ankle motion, and palpable muscle herniation.14
Measurement of intracompartmental pressure before and after exercise is the gold standard for diagnosis of CECS.2,14,27 Pre-exercise values ≥15 mm Hg and post-exercise values ≥30 mm Hg at one minute or ≥20 mm Hg at 5 minutes are all considered diagnostic of CECS,11 although these widely accepted criteria for bilateral testing of all compartments yields a false-positive rate of 5%.27 CECS is almost always bilateral,29 and some clinicians advocate limiting the number of needle insertions by taking only post-exercise measurements and testing only symptomatic compartments in one limb.
Imaging has limited value, as both x-rays and MRIs are usually normal.14 However, post-exertional T2-weighted MRI findings of muscular edema correspond to increased intracompartmental pressures, with a sensitivity of 87% and a specificity of 62%.14,24,30,31 Infrared spectroscopy, which measures levels of oxygenated and deoxygenated blood, is sensitive for CECS when the post-exercise ratio of deoxygenated to oxygenated blood remains elevated.14,24 Neither of these screening modalities is routinely obtained or considered diagnostic, however. Their chief role is to exclude an alternative diagnosis.14
Treatment and symptom relief. Discontinuing or modifying the aggravating activity typically brings relief of CECS. But this is not a long-term solution, as symptoms are likely to recur when the patient returns to the activity in question.1 The definitive treatment is compartment release via fasciotomy. Success rates for anterior and lateral compartment releases are >80%.1,28 The success of fasciotomy of posterior compartments, however, is <50%—a finding attributed to more complex anatomy, difficult visualization, and the presence of additional compartments.1,32
When the cause is vascular
Arterial endofibrosis—the fibrotic thickening of the intima of an artery—is thought to be caused by repetitive hip flexion.8 This results in hyperplasia, wall thickening, and eventual stenosis of the vessel, with 90% of cases affecting the external iliac artery.8,33 The condition is most common in activities such as cycling, but is also seen in such activities as running, skiing, soccer, and rugby. Symptoms are typically unilateral, but an estimated 15% of patients experience bilateral symptoms.8,33
Loss of power in the affected leg, with intermittent claudication and pain due to presumed ischemia from the vascular defect, is the usual presentation, although some patients develop cramping of the buttocks and/or paresthesia of the affected leg and foot during uphill running or cycling.8,33 The physical exam is often normal, but there may be a post-exercise arterial bruit over the femoral artery when the hip is flexed.8,34
Pre-exercise ankle-brachial index (ABI) <0.5 and post-exercise ABI <0.66 at one minute is suggestive of moderate arterial endofibrosis, with 90% sensitivity and 87% specificity.8,33,34 Arterial ultrasound and color Doppler may also be used for diagnosis, but are often operator dependent. Magnetic resonance angiography (MRA), while more expensive, can detect excessive kinking or compression of the vessel and is not operator dependent.8,33 Angioplastic balloon catheter dilation and stenting, bypass surgery, vascular reconstruction and endarterectomy with vein patch are the options for treatment. The success rates of the various interventions are unknown due to a lack of head-to-head studies and long-term follow-up.8,33
Popliteal artery entrapment syndrome (PAES) is a constellation of symptoms caused by vascular impingement in the popliteal fossa of the knee.8,34 The typical presenting symptoms are lower limb ischemia and pain caused by intense exercise that resolves quickly afterwards. Symptoms correlate more with the intensity than the duration of exercise.3,8
PAES is usually caused by a variant of the gastrocnemius muscle in which a medial head passes behind the popliteal artery in males younger than 30 years.8,33-35 Less commonly, it is the result of an overuse or acute orthopedic injury that irritates structures surrounding the popliteal fossa.8,34 PAES affects football, basketball, and soccer players, as well as runners because of excessive dorsiflexion and plantar flexion of the ankle.3,4
The physical exam for a patient with PAES is typically normal, but a post-exercise popliteal bruit with weak peripheral pulses may be elicited.8,33 An ABI in the neutral, forced dorsiflexion and forced plantar flexion positions can serve as a useful screening tool. An ABI <0.9 is abnormal, with a sensitivity and specificity of 90% and 98%, respectively, for stenosis >50%.2,36
Arteriography is the gold standard for diagnosis of PAES. Contrast arteriography is most commonly used because of its availability and cost. But MRA better differentiates functional from anatomic entrapment—a differentiation that less invasive tests, such as duplex ultrasound studies, lack the specificity to reveal.8,34 Treatment requires either surgical removal of the offending musculotendinous structures or arterial bypass and grafting of the chronically impinged area, as conservative therapies lack efficacy.2,8,34
Cystic adventitial disease (CAD) is the narrowing of an artery by mucoid cysts in the arterial wall or adventitia.8,9 It is a rare condition, accounting for just 0.1% of all vascular diseases, most commonly occurring in men in their mid-40s.8,33 CAD is thought to be the result of mucin-producing cells being haphazardly incorporated into the adventitia during arterial development. About 85% of patients whose popliteal artery is affected in this way will experience intermittent claudication with activity.8,9
On exam, such patients often have diminished ankle-brachial pressure indices, and duplex ultrasound often reveals stenosis in the affected artery, as well as a collection of mucoid cysts in the adventitia.8,9
Diagnosis can be confirmed by MRA.8,37 Evidence for the treatment of CAD is largely anecdotal.9 Cysts may be aspirated but tend to recur, and stenting does not correct the cystic-induced narrowing of the vessel. Surgical removal of the cysts is the only successful treatment.8,9
Neurologic causes to consider
Spinal stenosis is caused by central canal narrowing secondary to congenital abnormalities, trauma, or, most commonly, degenerative changes in the lumbar spine. Spinal stenosis is generally seen in men or women ages 50 to 70 years.38 Patients experience unilateral or bilateral claudication that improves with sitting or flexion of the spine5 and may develop bilateral lower extremity numbness and tingling from the buttocks that radiates down the legs. Diagnosis is typically made with a combination of a lumbar x-ray and an MRI, which will show nerve compression and bony overgrowth.38 CT myelogram, another imaging option, isless sensitive in the acute phase, but can be used to monitor the disease course.
Initial treatment includes physical therapy and NSAIDs.5 If conservative therapy fails, epidural or nerve root corticosteroid injections and surgical decompression or laminectomy are options.38
Nerve entrapment is a less common source of lower extremity pain in which the superficial peroneal nerve is most often affected.4,12,17,39 Trauma is the usual cause of nerve entrapment, but it may also be associated with overuse, most notably related to dance, soccer, or tennis.2,14,40,41 The most likely anatomic site is where the nerve exits the deep fascia within the lateral compartment in the lower third of the leg.39,40 Less frequently, the common peroneal nerve at the fibular neck, the saphenous nerve as it passes through Hunter’s canal, the posterior tibial nerve at the tarsal tunnel, and the sural nerve in the posterior calf may be affected.3,4,12,17,20,40,41 Entrapment of the peroneal nerve may be associated with activities involving repetitive inversion and eversion, such as running and cycling. Injury of the saphenous nerve is seen in sports involving repetitive knee flexion like rowing and cycling. Sural nerve entrapment is a result of crural fascia compression of the nerve during activities like running and track.3,14,40,42,43
Patients typically experience burning, tingling, and radiation of pain with activity. Symptoms worsen with continued exercise. The physical exam is often normal, especially early in the disease process, but may reveal sensory loss, motor weakness, and a loss of reflexes.2,40 Patients with superficial peroneal nerve involvement may have distal lateral leg pain that radiates into the dorsum of the foot, often exacerbated by lower leg percussion and resulting in diminished sensation.1 Common peroneal nerve involvement may alter sensation of the lateral leg, as well, but may also cause foot drop.2 The saphenous nerve can cause medial knee or leg symptoms, while the sural nerve can yield pain in the lateral ankle and foot.2
To diagnose nerve entrapment, electromyography and nerve conduction velocities at the level of the lesion may yield positive results 3 to 4 weeks after symptom onset.2,13,40 There are wide ranges of sensitivity and specificity for these studies, but they are nonetheless considered the tests of choice for nerve entrapment.1,44 Conservative treatment with activity modification, physical therapy, massage, and NSAIDs is often sufficient,2 with surgical management warranted only for refractory cases.2,14,40,41
CORRESPONDENCE
Jonathan A. Becker, MD, CAQSM, University of Louisville Department of Family and Geriatric Medicine, 201 Abraham Flexner Way, Suite 690, Louisville, KY 40202; jon.becker@louisville@edu.
1. Rajasekaran S, Kvinlaug K, Finnoff JT. Exertional leg pain in the athlete. PM R. 2012;4:985-1000.
2. Brewer RB, Gregory AJ. Chronic lower leg pain in athletes: a guide for the differential diagnosis, evaluation, and treatment. Sports Health. 2012;4:121-127.
3. Edwards PH Jr, Wright ML, Hartman JF. A practical approach for the differential diagnosis of chronic leg pain in the athlete. Am J Sports Med. 2005;33:1241-1249.
4. Clanton TO, Solcher BW. Chronic leg pain in the athlete. Clin Sports Med. 1994;13:743-759.
5. Fredericson M, Wun C. Differential diagnosis of leg pain in the athlete. J Am Podiatr Med Assoc. 2003;93:321-324.
6. Pell RF 4th, Khanuja HS, Cooley GR. Leg pain in the running athlete. J Am Acad Orthop Surg. 2004;12:396-404.
7. Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8:344-353.
8. Pham TT, Kapur R, Harwood MI. Exertional leg pain: teasing out arterial entrapments. Curr Sports Med Rep. 2007;6:371-375.
9. Wright LB, Matchett WJ, Cruz CP, et al. Popliteal artery disease: diagnosis and treatment. Radiographics. 2004;24:467-479.
10. Yates B, White S. The incidence and risk factors in the development of medial tibial stress syndrome among naval recruits. Am J Sports Med. 2004;32:772-780.
11. Fredericson M, Jennings F, Beaulieu C, et al. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17:309-325.
12. Plisky MS, Rauh MJ, Heiderscheit B, et al. Medial tibial stress syndrome in high school cross-country runners: incidence and risk factors. J Orthop Sports Phys Ther. 2007;37:40-47.
13. Touliopolous S, Hershman EB. Lower leg pain. Diagnosis and treatment of compartment syndromes and other pain syndromes of the leg. Sports Med. 1999;27:193-204.
14. Burrus MT, Werner BC, Starman JS, et al. Chronic leg pain in athletes. Am J Sports Med. 2015;43:1538-1547.
15. Batt ME, Ugalde V, Anderson MW, et al. A prospective controlled study of diagnostic imaging for acute shin splints. Med Sci Sports Exerc. 1998;30:1564-1571.
16. Gaeta M, Minutoli F, Scribano E, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235:553-561.
17. Sterling JC, Edelstein DW, Calvo RD, et al. Stress fractures in the athlete. Diagnosis and management. Sports Med. 1992;14:336-346.
18. Brukner P. Exercise-related lower leg pain: bone. Med Sci Sports Exerc. 2000;32:S15-S26.
19. Tuan K, Wu S, Sennett B. Stress fractures in athletes: risk factors, diagnosis, and management. Orthopedics. 2004;27:583-591.
20. Harrast MA, Colonno D. Stress fractures in runners. Clin Sports Med. 2010;29:399-416.
21. Varner KE, Younas SA, Lintner DM, et al. Chronic anterior midtibial stress fractures in athletes treated with reamed intramedullary nailing. Am J Sports Med. 2005;33:1071-1076.
22. Kaeding CC, Yu JR, Wright R, et al. Management and return to play of stress fractures. Clin J Sport Med. 2005;15:442-447.
23. Blackman PG. A review of chronic exertional compartment syndrome in the lower leg. Med Sci Sports Exerc. 2000;32:S4-S10.
24. Brennan FH Jr, Kane SF. Diagnosis, treatment options, and rehabilitation of chronic lower leg exertional compartment syndrome. Curr Sports Med Rep. 2003;2:247-250.
25. Davis DE, Raikin S, Garras DN, et al. Characteristics of patients with chronic exertional compartment syndrome. Foot Ankle Int. 2013;34:1349-1354.
26. Edmundsson D, Toolanen G. Chronic exertional compartment syndrome in diabetes mellitus. Diabet Med. 2011;28:81-85.
27. Pedowitz RA, Hargens AR, Mubarak SJ, et al. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18:35-40.
28. Bong MR, Polatsch DB, Jazrawi LM, et al. Chronic exertional compartment syndrome: diagnosis and management. Bull Hosp Jt Dis. 2005;62:77-84.
29. Hislop M, Batt ME. Chronic exertional compartment syndrome testing: a minimalist approach. Br J Sports Med. 2011;45:954-955.
30. Brown RR, Rosenberg ZS. MR imaging of exercise-induced lower leg pain. Magn Reson Imaging Clin N Am. 2001;9:533-552.
31. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42:385-392.
32. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21:811-817.
33. Ehsan O, Darwish A, Edmundson C, et al. Non-traumatic lower limb vascular complications in endurance athletes. Review of literature. Eur J Vasc Endovasc Surg. 2004;28:1-8.
34. Turnipseed WD. Popliteal entrapment syndrome. J Vasc Surg. 2002;35:910-915.
35. Baltopoulos P, Filippou DK, Sigala F. Popliteal artery entrapment syndrome: anatomic or functional syndrome? Clin J Sport Med. 2004;14:8-12.
36. McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164-1171.
37. Elias DA, White LM, Rubenstein JD, et al. Clinical evaluation and MR imaging features of popliteal artery entrapment and cystic adventitial disease. AJR Am J Roentgenol. 2003;180:627-632.
38. Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol. 2010;24:253-265.
39. Korkola M, Amendola A. Exercise-induced leg pain: sifting through a broad differential. Phys Sportsmed. 2001;29:35-50.
40. McCrory P, Bell S, Bradshaw C. Nerve entrapments of the lower leg, ankle and foot in sport. Sports Med. 2002;32:371-391.
41. Schon LC. Nerve entrapment, neuropathy, and nerve dysfunction in athletes. Orthop Clin North Am. 1994;25:47-59.
42. Anselmi SJ. Common peroneal nerve compression. J Am Podiatr Med Assoc. 2006;96:413-417.
43. Maalla R, Youssef M, Ben Lassoued N, et al. Peroneal nerve entrapment at the fibular head: outcomes of neurolysis. Orthop Traumatol Surg Res. 2013;99:719-722.
44. Marciniak C, Armon C, Wilson J, et al. Practice parameter: utility of electrodiagnostic techniques in evaluating patients with suspected peroneal neuropathy: an evidence-based review. Muscle Nerve. 2005;31:520-527.
PRACTICE RECOMMENDATIONS
› Consider the possibility of vascular and neurologic problems as the source of exertional leg pain (ELP). C
› Order magnetic resonance imaging to evaluate patients with ELP and negative x-rays for stress fractures. C
› Measure lower extremity intracompartmental pressures both before and after exercise when you suspect chronic exertional compartmental syndrome. Doing so is the gold standard for the diagnosis of this condition. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most family physicians are accustomed to treating active patients with shin splints and stress fractures. But many are less familiar with, and slower to recognize, other sources of exertional leg pain (ELP), defined as exercise-related pain that localizes in the lower extremity distal to the knee and proximal to the talocrural joint.1
ELP has a broad differential diagnosis that includes other musculoskeletal conditions—most notably chronic exertional compartment syndrome (CECS), which has been found to affect 33% of athletes with chronic ELP1—as well as a number of vascular and neurologic causes.2-4 In addition, etiologies may overlap. Greater awareness of the many causes of ELP can help you to avoid the unnecessary use of expensive diagnostic tests as well as delayed diagnosis and treatment.
A thorough medical and activity history, symptom review, and physical examination are your most important tools when patients present with ELP. When the cause is not obvious or the patient fails to respond to conservative measures, x-rays, magnetic resonance imaging (MRI), vascular studies, electromyography and nerve conduction studies, and/or intracompartmental pressure testing may be needed to find the source of the symptoms. In the text that follows, we review both common and relatively uncommon sources of ELP, using a stepwise diagnostic approach. You’ll find a diagnostic challenge, in which you can test your skills and a more comprehensive differential diagnosis in the TABLE.1-3,5-9

Musculoskeletal injuries: Shin splints and beyond

Medial tibial stress syndrome (MTSS), commonly known as shin splints, is characterized by pain and tenderness over the posteromedial aspect of the distal tibia.3 It typically results in diffuse pain that occurs with exercise, but may persist at rest in severe cases.3-6 Less often, localized swelling may also be present.2
MTSS accounts for between 6% and 16% of all running injuries.2,10 It is associated with a spectrum of tibial stress injuries, including periostitis, tendinopathy, and stress reaction, with dysfunction of the tibialis posterior, tibialis anterior, and soleus muscles thought to be contributing factors.2,11 Intrinsic factors include high body mass index (BMI), female sex, excessive internal and external hip rotation, hyperpronation, and hyper plantar flexion.2,10,12
X-rays of the leg are typically normal in patients with MTSS and should be considered only if the clinical presentation suggests the possibility of an alternative diagnosis, such as a stress fracture or tumor.2-4,13 Advanced imaging such as MRI or triple phase bone scans (TPBS) are useful when the diagnosis is in question and will reveal an abnormally high signal along the posterior medial tibial surface or the classic train-track appearance of nucleotide uptake in patients with MTSS.2 MRI readily shows periosteal reaction and bony edema and has a sensitivity of 78% to 89% and a specificity of 33% to 100% for the diagnosis of MTSS.14,15
Initial management of MTSS is conservative, with the mainstay of treatment consisting of rest, ice, and nonsteroidal anti-inflammatory drugs (NSAIDs).3,13,16 While ice, NSAIDs, proper conditioning, physical therapy to stretch and strengthen the calf musculature, rigid orthotics to correct foot hyperpronation, and activity modification are all appropriate treatments, randomized controlled trials have shown none of these interventions to be more effective than rest alone.2 Non-operative treatment is usually successful, but surgery may be required for severe or refractory cases. Procedures include posteromedial fasciotomy, release of the medial soleus fascial bridge, deep compartment fasciotomy, or removal of a section of the distal tibia periosteum.3,4
Lower extremity stress fracture. Stress fractures are caused by repetitive loading that results in microtrauma, including bony microfractures. The vast majority of cases—80% to 95% of stress fractures—affect the lower extremities, and most involve the tibia.2-4,6,13,17 The most common presentation is an insidious onset of pain over a specific bony area with a normal appearance, although localized swelling or erythema may occasionally be present.3,14,17,18 The pain may be reproduced or worsened by weight-bearing activities and relieved by rest.14,18
Consider the female athlete triad. In evaluating a patient with a stress fracture, pay close attention to dietary history, BMI, and, in female athletes, take a detailed menstrual history. Such patients are at risk for amenorrhea, low bone mineral density, and nutritional deficits—the “female athlete triad,” which carries an increased risk of stress fractures.3,14,17-19
Stress fractures can often be diagnosed with a thorough medical history and physical, with imaging used for confirmation.6,14,17,18 Historical features of a stress fracture that may differentiate it from MTSS include pain that is unilateral and absent at rest and occurs with more prolonged activity, as well as post-exercise and/or nocturnal pain. Notable physical exam features include pain that is reproduced in a focal area with a single leg hop or percussion with a tuning fork or ultrasound.5,11,17
Initially, sensitivity for a plain radiograph is as low as 10%.2,11 Abnormalities on x-ray are usually seen after 2 to 8 weeks of symptoms2,7,11 and may include a faint periosteal reaction, a fluffy area of callus, or a cortical lucency sometimes referred to as the “dreaded black line.”3,6,17 If a radiographic exam shows evidence of a stress fracture, further imaging is typically unnecessary. MRI or TPBS is suggested, however, when x-rays appear normal but suspicion of a stress fracture remains.3,17,18 MRI may show edema within 3 days of symptom onset and is more sensitive and specific than computed tomography (CT) or TPBS for diagnosing stress fractures of the tibia.2,16
Treatment of tibial stress fractures is typically non-operative and consists of alterations in activity (eg, non weight-bearing), correction of nutritional deficits, such as inadequate caloric intake or too little calcium or iron, and addressing problems with footwear, training regimen, and/or running surface.3,14,18 Fibular and posteromedial tibial stress fractures are considered low risk and heal with weight-bearing restrictions and rest, initially for a minimum of 2 to 4 weeks.3
Posteromedial tibia injuries tend to heal well because they are on the compression side of the bone. Anterior tibia stress fractures, which are located on the tension side of the bone2,7 and account for approximately 5% of all tibia stress fractures, are more prone to non-union or progression to a complete fracture.7,20 Thus, anterior tibia stress injuries warrant a more aggressive approach, with treatment options including non-weight bearing status that may last longer than 8 weeks, pneumatic brace casting, and/or orthopedic referral to evaluate for surgical intervention.7,20-22 Time for radiographic evidence of healing may exceed 8 months, so early surgical intervention should be considered, especially for high-level athletes.7,20,21
Test your skills: A diagnostic challenge
Janine T, a 24-year-old long-distance runner, presents with left lower leg pain that occurs with activity. There was no injury, Ms. T reports; the pain began about 6 weeks ago, shortly after she began training for a marathon and running more than 30 miles per week. The pain is not relieved with intermittent rest or over-the-counter analgesics, she says. But it usually abates within 15 to 30 minutes after she completes her run.
Ms. T is underweight, with a body mass index <17 kg/m2. She denies any dietary restrictions and has normal menstrual cycles. The patient reports taking oral contraceptives, but no other medications. An initial x-ray is normal, as is magnetic resonance imaging to evaluate for a stress fracture.
You suspect Ms. T has shin splints, advise her to rest for a few weeks and to consider getting orthotics for her running shoes, and schedule a follow-up visit.
When she comes in 6 weeks later, the patient reports that she resumed running after a 3-week rest; shortly after, she noticed pain in both legs. What’s more, she now experiences tingling in her feet after running a few miles.
What’s wrong with this patient?
Ms. T’s symptoms—bilateral persistent leg pain, with tingling in both feet, and little improvement with rest—strongly suggest that she has chronic exertional compartment syndrome. Intracompartmental pressure testing, which reveals pre-exercise values ≥15 mm Hg and post-exercise values of ≥30 mm Hg at one minute, confirms the diagnosis.
Activity avoidance or modification will allow Ms. T’s symptoms to subside, but they’re highly likely to recur when she resumes running. The definitive treatment is intracompartmental fasciotomy, which has a success rate of approximately 80%.1,28
When to suspect chronic exertional compartment syndrome
Leg pain in CECS results from increased pressure within the lower extremity fascial compartments temporally related to exercise.2,23,24 Its incidence in the general population is unknown, but CECS has been found to range from 14% to 27% in patients with previously undiagnosed leg pain1,14,25 and to affect about a third of athletes with chronic ELP. In addition, CECS has been found in 90% of patients who have both diabetes and ELP with normal findings on vascular studies.1,3,4,26
The anterior compartment is most commonly affected, followed by the lateral, deep posterior, and superficial posterior compartments.3,13,23,27 Symptoms are bilateral 60% to 95% of the time.2,13,14,25 Factors contributing to CECS include fixed muscular compartment constraints, muscle swelling, thickened fascia, muscle hypertrophy related to resistance training, dynamic muscular contraction patterns, and low muscle capillary supply. Stretching of fascial pain receptors and pressure fibers and inadequate myocyte response to increased metabolism may play a role, as well.14,28
The initial clinical presentation is usually predictable leg pain—ie, pain that begins at about the same time, distance, or intensity of a workout and resolves with rest; numbness and weakness may occur as the workout progresses. In time, leg pain associated with CECS may be present with everyday activity or at rest. The physical exam may be normal or reveal swelling, tenderness over the involved compartments, pain with passive digit or ankle motion, and palpable muscle herniation.14
Measurement of intracompartmental pressure before and after exercise is the gold standard for diagnosis of CECS.2,14,27 Pre-exercise values ≥15 mm Hg and post-exercise values ≥30 mm Hg at one minute or ≥20 mm Hg at 5 minutes are all considered diagnostic of CECS,11 although these widely accepted criteria for bilateral testing of all compartments yields a false-positive rate of 5%.27 CECS is almost always bilateral,29 and some clinicians advocate limiting the number of needle insertions by taking only post-exercise measurements and testing only symptomatic compartments in one limb.
Imaging has limited value, as both x-rays and MRIs are usually normal.14 However, post-exertional T2-weighted MRI findings of muscular edema correspond to increased intracompartmental pressures, with a sensitivity of 87% and a specificity of 62%.14,24,30,31 Infrared spectroscopy, which measures levels of oxygenated and deoxygenated blood, is sensitive for CECS when the post-exercise ratio of deoxygenated to oxygenated blood remains elevated.14,24 Neither of these screening modalities is routinely obtained or considered diagnostic, however. Their chief role is to exclude an alternative diagnosis.14
Treatment and symptom relief. Discontinuing or modifying the aggravating activity typically brings relief of CECS. But this is not a long-term solution, as symptoms are likely to recur when the patient returns to the activity in question.1 The definitive treatment is compartment release via fasciotomy. Success rates for anterior and lateral compartment releases are >80%.1,28 The success of fasciotomy of posterior compartments, however, is <50%—a finding attributed to more complex anatomy, difficult visualization, and the presence of additional compartments.1,32
When the cause is vascular
Arterial endofibrosis—the fibrotic thickening of the intima of an artery—is thought to be caused by repetitive hip flexion.8 This results in hyperplasia, wall thickening, and eventual stenosis of the vessel, with 90% of cases affecting the external iliac artery.8,33 The condition is most common in activities such as cycling, but is also seen in such activities as running, skiing, soccer, and rugby. Symptoms are typically unilateral, but an estimated 15% of patients experience bilateral symptoms.8,33
Loss of power in the affected leg, with intermittent claudication and pain due to presumed ischemia from the vascular defect, is the usual presentation, although some patients develop cramping of the buttocks and/or paresthesia of the affected leg and foot during uphill running or cycling.8,33 The physical exam is often normal, but there may be a post-exercise arterial bruit over the femoral artery when the hip is flexed.8,34
Pre-exercise ankle-brachial index (ABI) <0.5 and post-exercise ABI <0.66 at one minute is suggestive of moderate arterial endofibrosis, with 90% sensitivity and 87% specificity.8,33,34 Arterial ultrasound and color Doppler may also be used for diagnosis, but are often operator dependent. Magnetic resonance angiography (MRA), while more expensive, can detect excessive kinking or compression of the vessel and is not operator dependent.8,33 Angioplastic balloon catheter dilation and stenting, bypass surgery, vascular reconstruction and endarterectomy with vein patch are the options for treatment. The success rates of the various interventions are unknown due to a lack of head-to-head studies and long-term follow-up.8,33
Popliteal artery entrapment syndrome (PAES) is a constellation of symptoms caused by vascular impingement in the popliteal fossa of the knee.8,34 The typical presenting symptoms are lower limb ischemia and pain caused by intense exercise that resolves quickly afterwards. Symptoms correlate more with the intensity than the duration of exercise.3,8
PAES is usually caused by a variant of the gastrocnemius muscle in which a medial head passes behind the popliteal artery in males younger than 30 years.8,33-35 Less commonly, it is the result of an overuse or acute orthopedic injury that irritates structures surrounding the popliteal fossa.8,34 PAES affects football, basketball, and soccer players, as well as runners because of excessive dorsiflexion and plantar flexion of the ankle.3,4
The physical exam for a patient with PAES is typically normal, but a post-exercise popliteal bruit with weak peripheral pulses may be elicited.8,33 An ABI in the neutral, forced dorsiflexion and forced plantar flexion positions can serve as a useful screening tool. An ABI <0.9 is abnormal, with a sensitivity and specificity of 90% and 98%, respectively, for stenosis >50%.2,36
Arteriography is the gold standard for diagnosis of PAES. Contrast arteriography is most commonly used because of its availability and cost. But MRA better differentiates functional from anatomic entrapment—a differentiation that less invasive tests, such as duplex ultrasound studies, lack the specificity to reveal.8,34 Treatment requires either surgical removal of the offending musculotendinous structures or arterial bypass and grafting of the chronically impinged area, as conservative therapies lack efficacy.2,8,34
Cystic adventitial disease (CAD) is the narrowing of an artery by mucoid cysts in the arterial wall or adventitia.8,9 It is a rare condition, accounting for just 0.1% of all vascular diseases, most commonly occurring in men in their mid-40s.8,33 CAD is thought to be the result of mucin-producing cells being haphazardly incorporated into the adventitia during arterial development. About 85% of patients whose popliteal artery is affected in this way will experience intermittent claudication with activity.8,9
On exam, such patients often have diminished ankle-brachial pressure indices, and duplex ultrasound often reveals stenosis in the affected artery, as well as a collection of mucoid cysts in the adventitia.8,9
Diagnosis can be confirmed by MRA.8,37 Evidence for the treatment of CAD is largely anecdotal.9 Cysts may be aspirated but tend to recur, and stenting does not correct the cystic-induced narrowing of the vessel. Surgical removal of the cysts is the only successful treatment.8,9
Neurologic causes to consider
Spinal stenosis is caused by central canal narrowing secondary to congenital abnormalities, trauma, or, most commonly, degenerative changes in the lumbar spine. Spinal stenosis is generally seen in men or women ages 50 to 70 years.38 Patients experience unilateral or bilateral claudication that improves with sitting or flexion of the spine5 and may develop bilateral lower extremity numbness and tingling from the buttocks that radiates down the legs. Diagnosis is typically made with a combination of a lumbar x-ray and an MRI, which will show nerve compression and bony overgrowth.38 CT myelogram, another imaging option, isless sensitive in the acute phase, but can be used to monitor the disease course.
Initial treatment includes physical therapy and NSAIDs.5 If conservative therapy fails, epidural or nerve root corticosteroid injections and surgical decompression or laminectomy are options.38
Nerve entrapment is a less common source of lower extremity pain in which the superficial peroneal nerve is most often affected.4,12,17,39 Trauma is the usual cause of nerve entrapment, but it may also be associated with overuse, most notably related to dance, soccer, or tennis.2,14,40,41 The most likely anatomic site is where the nerve exits the deep fascia within the lateral compartment in the lower third of the leg.39,40 Less frequently, the common peroneal nerve at the fibular neck, the saphenous nerve as it passes through Hunter’s canal, the posterior tibial nerve at the tarsal tunnel, and the sural nerve in the posterior calf may be affected.3,4,12,17,20,40,41 Entrapment of the peroneal nerve may be associated with activities involving repetitive inversion and eversion, such as running and cycling. Injury of the saphenous nerve is seen in sports involving repetitive knee flexion like rowing and cycling. Sural nerve entrapment is a result of crural fascia compression of the nerve during activities like running and track.3,14,40,42,43
Patients typically experience burning, tingling, and radiation of pain with activity. Symptoms worsen with continued exercise. The physical exam is often normal, especially early in the disease process, but may reveal sensory loss, motor weakness, and a loss of reflexes.2,40 Patients with superficial peroneal nerve involvement may have distal lateral leg pain that radiates into the dorsum of the foot, often exacerbated by lower leg percussion and resulting in diminished sensation.1 Common peroneal nerve involvement may alter sensation of the lateral leg, as well, but may also cause foot drop.2 The saphenous nerve can cause medial knee or leg symptoms, while the sural nerve can yield pain in the lateral ankle and foot.2
To diagnose nerve entrapment, electromyography and nerve conduction velocities at the level of the lesion may yield positive results 3 to 4 weeks after symptom onset.2,13,40 There are wide ranges of sensitivity and specificity for these studies, but they are nonetheless considered the tests of choice for nerve entrapment.1,44 Conservative treatment with activity modification, physical therapy, massage, and NSAIDs is often sufficient,2 with surgical management warranted only for refractory cases.2,14,40,41
CORRESPONDENCE
Jonathan A. Becker, MD, CAQSM, University of Louisville Department of Family and Geriatric Medicine, 201 Abraham Flexner Way, Suite 690, Louisville, KY 40202; jon.becker@louisville@edu.
PRACTICE RECOMMENDATIONS
› Consider the possibility of vascular and neurologic problems as the source of exertional leg pain (ELP). C
› Order magnetic resonance imaging to evaluate patients with ELP and negative x-rays for stress fractures. C
› Measure lower extremity intracompartmental pressures both before and after exercise when you suspect chronic exertional compartmental syndrome. Doing so is the gold standard for the diagnosis of this condition. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most family physicians are accustomed to treating active patients with shin splints and stress fractures. But many are less familiar with, and slower to recognize, other sources of exertional leg pain (ELP), defined as exercise-related pain that localizes in the lower extremity distal to the knee and proximal to the talocrural joint.1
ELP has a broad differential diagnosis that includes other musculoskeletal conditions—most notably chronic exertional compartment syndrome (CECS), which has been found to affect 33% of athletes with chronic ELP1—as well as a number of vascular and neurologic causes.2-4 In addition, etiologies may overlap. Greater awareness of the many causes of ELP can help you to avoid the unnecessary use of expensive diagnostic tests as well as delayed diagnosis and treatment.
A thorough medical and activity history, symptom review, and physical examination are your most important tools when patients present with ELP. When the cause is not obvious or the patient fails to respond to conservative measures, x-rays, magnetic resonance imaging (MRI), vascular studies, electromyography and nerve conduction studies, and/or intracompartmental pressure testing may be needed to find the source of the symptoms. In the text that follows, we review both common and relatively uncommon sources of ELP, using a stepwise diagnostic approach. You’ll find a diagnostic challenge, in which you can test your skills and a more comprehensive differential diagnosis in the TABLE.1-3,5-9

Musculoskeletal injuries: Shin splints and beyond

Medial tibial stress syndrome (MTSS), commonly known as shin splints, is characterized by pain and tenderness over the posteromedial aspect of the distal tibia.3 It typically results in diffuse pain that occurs with exercise, but may persist at rest in severe cases.3-6 Less often, localized swelling may also be present.2
MTSS accounts for between 6% and 16% of all running injuries.2,10 It is associated with a spectrum of tibial stress injuries, including periostitis, tendinopathy, and stress reaction, with dysfunction of the tibialis posterior, tibialis anterior, and soleus muscles thought to be contributing factors.2,11 Intrinsic factors include high body mass index (BMI), female sex, excessive internal and external hip rotation, hyperpronation, and hyper plantar flexion.2,10,12
X-rays of the leg are typically normal in patients with MTSS and should be considered only if the clinical presentation suggests the possibility of an alternative diagnosis, such as a stress fracture or tumor.2-4,13 Advanced imaging such as MRI or triple phase bone scans (TPBS) are useful when the diagnosis is in question and will reveal an abnormally high signal along the posterior medial tibial surface or the classic train-track appearance of nucleotide uptake in patients with MTSS.2 MRI readily shows periosteal reaction and bony edema and has a sensitivity of 78% to 89% and a specificity of 33% to 100% for the diagnosis of MTSS.14,15
Initial management of MTSS is conservative, with the mainstay of treatment consisting of rest, ice, and nonsteroidal anti-inflammatory drugs (NSAIDs).3,13,16 While ice, NSAIDs, proper conditioning, physical therapy to stretch and strengthen the calf musculature, rigid orthotics to correct foot hyperpronation, and activity modification are all appropriate treatments, randomized controlled trials have shown none of these interventions to be more effective than rest alone.2 Non-operative treatment is usually successful, but surgery may be required for severe or refractory cases. Procedures include posteromedial fasciotomy, release of the medial soleus fascial bridge, deep compartment fasciotomy, or removal of a section of the distal tibia periosteum.3,4
Lower extremity stress fracture. Stress fractures are caused by repetitive loading that results in microtrauma, including bony microfractures. The vast majority of cases—80% to 95% of stress fractures—affect the lower extremities, and most involve the tibia.2-4,6,13,17 The most common presentation is an insidious onset of pain over a specific bony area with a normal appearance, although localized swelling or erythema may occasionally be present.3,14,17,18 The pain may be reproduced or worsened by weight-bearing activities and relieved by rest.14,18
Consider the female athlete triad. In evaluating a patient with a stress fracture, pay close attention to dietary history, BMI, and, in female athletes, take a detailed menstrual history. Such patients are at risk for amenorrhea, low bone mineral density, and nutritional deficits—the “female athlete triad,” which carries an increased risk of stress fractures.3,14,17-19
Stress fractures can often be diagnosed with a thorough medical history and physical, with imaging used for confirmation.6,14,17,18 Historical features of a stress fracture that may differentiate it from MTSS include pain that is unilateral and absent at rest and occurs with more prolonged activity, as well as post-exercise and/or nocturnal pain. Notable physical exam features include pain that is reproduced in a focal area with a single leg hop or percussion with a tuning fork or ultrasound.5,11,17
Initially, sensitivity for a plain radiograph is as low as 10%.2,11 Abnormalities on x-ray are usually seen after 2 to 8 weeks of symptoms2,7,11 and may include a faint periosteal reaction, a fluffy area of callus, or a cortical lucency sometimes referred to as the “dreaded black line.”3,6,17 If a radiographic exam shows evidence of a stress fracture, further imaging is typically unnecessary. MRI or TPBS is suggested, however, when x-rays appear normal but suspicion of a stress fracture remains.3,17,18 MRI may show edema within 3 days of symptom onset and is more sensitive and specific than computed tomography (CT) or TPBS for diagnosing stress fractures of the tibia.2,16
Treatment of tibial stress fractures is typically non-operative and consists of alterations in activity (eg, non weight-bearing), correction of nutritional deficits, such as inadequate caloric intake or too little calcium or iron, and addressing problems with footwear, training regimen, and/or running surface.3,14,18 Fibular and posteromedial tibial stress fractures are considered low risk and heal with weight-bearing restrictions and rest, initially for a minimum of 2 to 4 weeks.3
Posteromedial tibia injuries tend to heal well because they are on the compression side of the bone. Anterior tibia stress fractures, which are located on the tension side of the bone2,7 and account for approximately 5% of all tibia stress fractures, are more prone to non-union or progression to a complete fracture.7,20 Thus, anterior tibia stress injuries warrant a more aggressive approach, with treatment options including non-weight bearing status that may last longer than 8 weeks, pneumatic brace casting, and/or orthopedic referral to evaluate for surgical intervention.7,20-22 Time for radiographic evidence of healing may exceed 8 months, so early surgical intervention should be considered, especially for high-level athletes.7,20,21
Test your skills: A diagnostic challenge
Janine T, a 24-year-old long-distance runner, presents with left lower leg pain that occurs with activity. There was no injury, Ms. T reports; the pain began about 6 weeks ago, shortly after she began training for a marathon and running more than 30 miles per week. The pain is not relieved with intermittent rest or over-the-counter analgesics, she says. But it usually abates within 15 to 30 minutes after she completes her run.
Ms. T is underweight, with a body mass index <17 kg/m2. She denies any dietary restrictions and has normal menstrual cycles. The patient reports taking oral contraceptives, but no other medications. An initial x-ray is normal, as is magnetic resonance imaging to evaluate for a stress fracture.
You suspect Ms. T has shin splints, advise her to rest for a few weeks and to consider getting orthotics for her running shoes, and schedule a follow-up visit.
When she comes in 6 weeks later, the patient reports that she resumed running after a 3-week rest; shortly after, she noticed pain in both legs. What’s more, she now experiences tingling in her feet after running a few miles.
What’s wrong with this patient?
Ms. T’s symptoms—bilateral persistent leg pain, with tingling in both feet, and little improvement with rest—strongly suggest that she has chronic exertional compartment syndrome. Intracompartmental pressure testing, which reveals pre-exercise values ≥15 mm Hg and post-exercise values of ≥30 mm Hg at one minute, confirms the diagnosis.
Activity avoidance or modification will allow Ms. T’s symptoms to subside, but they’re highly likely to recur when she resumes running. The definitive treatment is intracompartmental fasciotomy, which has a success rate of approximately 80%.1,28
When to suspect chronic exertional compartment syndrome
Leg pain in CECS results from increased pressure within the lower extremity fascial compartments temporally related to exercise.2,23,24 Its incidence in the general population is unknown, but CECS has been found to range from 14% to 27% in patients with previously undiagnosed leg pain1,14,25 and to affect about a third of athletes with chronic ELP. In addition, CECS has been found in 90% of patients who have both diabetes and ELP with normal findings on vascular studies.1,3,4,26
The anterior compartment is most commonly affected, followed by the lateral, deep posterior, and superficial posterior compartments.3,13,23,27 Symptoms are bilateral 60% to 95% of the time.2,13,14,25 Factors contributing to CECS include fixed muscular compartment constraints, muscle swelling, thickened fascia, muscle hypertrophy related to resistance training, dynamic muscular contraction patterns, and low muscle capillary supply. Stretching of fascial pain receptors and pressure fibers and inadequate myocyte response to increased metabolism may play a role, as well.14,28
The initial clinical presentation is usually predictable leg pain—ie, pain that begins at about the same time, distance, or intensity of a workout and resolves with rest; numbness and weakness may occur as the workout progresses. In time, leg pain associated with CECS may be present with everyday activity or at rest. The physical exam may be normal or reveal swelling, tenderness over the involved compartments, pain with passive digit or ankle motion, and palpable muscle herniation.14
Measurement of intracompartmental pressure before and after exercise is the gold standard for diagnosis of CECS.2,14,27 Pre-exercise values ≥15 mm Hg and post-exercise values ≥30 mm Hg at one minute or ≥20 mm Hg at 5 minutes are all considered diagnostic of CECS,11 although these widely accepted criteria for bilateral testing of all compartments yields a false-positive rate of 5%.27 CECS is almost always bilateral,29 and some clinicians advocate limiting the number of needle insertions by taking only post-exercise measurements and testing only symptomatic compartments in one limb.
Imaging has limited value, as both x-rays and MRIs are usually normal.14 However, post-exertional T2-weighted MRI findings of muscular edema correspond to increased intracompartmental pressures, with a sensitivity of 87% and a specificity of 62%.14,24,30,31 Infrared spectroscopy, which measures levels of oxygenated and deoxygenated blood, is sensitive for CECS when the post-exercise ratio of deoxygenated to oxygenated blood remains elevated.14,24 Neither of these screening modalities is routinely obtained or considered diagnostic, however. Their chief role is to exclude an alternative diagnosis.14
Treatment and symptom relief. Discontinuing or modifying the aggravating activity typically brings relief of CECS. But this is not a long-term solution, as symptoms are likely to recur when the patient returns to the activity in question.1 The definitive treatment is compartment release via fasciotomy. Success rates for anterior and lateral compartment releases are >80%.1,28 The success of fasciotomy of posterior compartments, however, is <50%—a finding attributed to more complex anatomy, difficult visualization, and the presence of additional compartments.1,32
When the cause is vascular
Arterial endofibrosis—the fibrotic thickening of the intima of an artery—is thought to be caused by repetitive hip flexion.8 This results in hyperplasia, wall thickening, and eventual stenosis of the vessel, with 90% of cases affecting the external iliac artery.8,33 The condition is most common in activities such as cycling, but is also seen in such activities as running, skiing, soccer, and rugby. Symptoms are typically unilateral, but an estimated 15% of patients experience bilateral symptoms.8,33
Loss of power in the affected leg, with intermittent claudication and pain due to presumed ischemia from the vascular defect, is the usual presentation, although some patients develop cramping of the buttocks and/or paresthesia of the affected leg and foot during uphill running or cycling.8,33 The physical exam is often normal, but there may be a post-exercise arterial bruit over the femoral artery when the hip is flexed.8,34
Pre-exercise ankle-brachial index (ABI) <0.5 and post-exercise ABI <0.66 at one minute is suggestive of moderate arterial endofibrosis, with 90% sensitivity and 87% specificity.8,33,34 Arterial ultrasound and color Doppler may also be used for diagnosis, but are often operator dependent. Magnetic resonance angiography (MRA), while more expensive, can detect excessive kinking or compression of the vessel and is not operator dependent.8,33 Angioplastic balloon catheter dilation and stenting, bypass surgery, vascular reconstruction and endarterectomy with vein patch are the options for treatment. The success rates of the various interventions are unknown due to a lack of head-to-head studies and long-term follow-up.8,33
Popliteal artery entrapment syndrome (PAES) is a constellation of symptoms caused by vascular impingement in the popliteal fossa of the knee.8,34 The typical presenting symptoms are lower limb ischemia and pain caused by intense exercise that resolves quickly afterwards. Symptoms correlate more with the intensity than the duration of exercise.3,8
PAES is usually caused by a variant of the gastrocnemius muscle in which a medial head passes behind the popliteal artery in males younger than 30 years.8,33-35 Less commonly, it is the result of an overuse or acute orthopedic injury that irritates structures surrounding the popliteal fossa.8,34 PAES affects football, basketball, and soccer players, as well as runners because of excessive dorsiflexion and plantar flexion of the ankle.3,4
The physical exam for a patient with PAES is typically normal, but a post-exercise popliteal bruit with weak peripheral pulses may be elicited.8,33 An ABI in the neutral, forced dorsiflexion and forced plantar flexion positions can serve as a useful screening tool. An ABI <0.9 is abnormal, with a sensitivity and specificity of 90% and 98%, respectively, for stenosis >50%.2,36
Arteriography is the gold standard for diagnosis of PAES. Contrast arteriography is most commonly used because of its availability and cost. But MRA better differentiates functional from anatomic entrapment—a differentiation that less invasive tests, such as duplex ultrasound studies, lack the specificity to reveal.8,34 Treatment requires either surgical removal of the offending musculotendinous structures or arterial bypass and grafting of the chronically impinged area, as conservative therapies lack efficacy.2,8,34
Cystic adventitial disease (CAD) is the narrowing of an artery by mucoid cysts in the arterial wall or adventitia.8,9 It is a rare condition, accounting for just 0.1% of all vascular diseases, most commonly occurring in men in their mid-40s.8,33 CAD is thought to be the result of mucin-producing cells being haphazardly incorporated into the adventitia during arterial development. About 85% of patients whose popliteal artery is affected in this way will experience intermittent claudication with activity.8,9
On exam, such patients often have diminished ankle-brachial pressure indices, and duplex ultrasound often reveals stenosis in the affected artery, as well as a collection of mucoid cysts in the adventitia.8,9
Diagnosis can be confirmed by MRA.8,37 Evidence for the treatment of CAD is largely anecdotal.9 Cysts may be aspirated but tend to recur, and stenting does not correct the cystic-induced narrowing of the vessel. Surgical removal of the cysts is the only successful treatment.8,9
Neurologic causes to consider
Spinal stenosis is caused by central canal narrowing secondary to congenital abnormalities, trauma, or, most commonly, degenerative changes in the lumbar spine. Spinal stenosis is generally seen in men or women ages 50 to 70 years.38 Patients experience unilateral or bilateral claudication that improves with sitting or flexion of the spine5 and may develop bilateral lower extremity numbness and tingling from the buttocks that radiates down the legs. Diagnosis is typically made with a combination of a lumbar x-ray and an MRI, which will show nerve compression and bony overgrowth.38 CT myelogram, another imaging option, isless sensitive in the acute phase, but can be used to monitor the disease course.
Initial treatment includes physical therapy and NSAIDs.5 If conservative therapy fails, epidural or nerve root corticosteroid injections and surgical decompression or laminectomy are options.38
Nerve entrapment is a less common source of lower extremity pain in which the superficial peroneal nerve is most often affected.4,12,17,39 Trauma is the usual cause of nerve entrapment, but it may also be associated with overuse, most notably related to dance, soccer, or tennis.2,14,40,41 The most likely anatomic site is where the nerve exits the deep fascia within the lateral compartment in the lower third of the leg.39,40 Less frequently, the common peroneal nerve at the fibular neck, the saphenous nerve as it passes through Hunter’s canal, the posterior tibial nerve at the tarsal tunnel, and the sural nerve in the posterior calf may be affected.3,4,12,17,20,40,41 Entrapment of the peroneal nerve may be associated with activities involving repetitive inversion and eversion, such as running and cycling. Injury of the saphenous nerve is seen in sports involving repetitive knee flexion like rowing and cycling. Sural nerve entrapment is a result of crural fascia compression of the nerve during activities like running and track.3,14,40,42,43
Patients typically experience burning, tingling, and radiation of pain with activity. Symptoms worsen with continued exercise. The physical exam is often normal, especially early in the disease process, but may reveal sensory loss, motor weakness, and a loss of reflexes.2,40 Patients with superficial peroneal nerve involvement may have distal lateral leg pain that radiates into the dorsum of the foot, often exacerbated by lower leg percussion and resulting in diminished sensation.1 Common peroneal nerve involvement may alter sensation of the lateral leg, as well, but may also cause foot drop.2 The saphenous nerve can cause medial knee or leg symptoms, while the sural nerve can yield pain in the lateral ankle and foot.2
To diagnose nerve entrapment, electromyography and nerve conduction velocities at the level of the lesion may yield positive results 3 to 4 weeks after symptom onset.2,13,40 There are wide ranges of sensitivity and specificity for these studies, but they are nonetheless considered the tests of choice for nerve entrapment.1,44 Conservative treatment with activity modification, physical therapy, massage, and NSAIDs is often sufficient,2 with surgical management warranted only for refractory cases.2,14,40,41
CORRESPONDENCE
Jonathan A. Becker, MD, CAQSM, University of Louisville Department of Family and Geriatric Medicine, 201 Abraham Flexner Way, Suite 690, Louisville, KY 40202; jon.becker@louisville@edu.
1. Rajasekaran S, Kvinlaug K, Finnoff JT. Exertional leg pain in the athlete. PM R. 2012;4:985-1000.
2. Brewer RB, Gregory AJ. Chronic lower leg pain in athletes: a guide for the differential diagnosis, evaluation, and treatment. Sports Health. 2012;4:121-127.
3. Edwards PH Jr, Wright ML, Hartman JF. A practical approach for the differential diagnosis of chronic leg pain in the athlete. Am J Sports Med. 2005;33:1241-1249.
4. Clanton TO, Solcher BW. Chronic leg pain in the athlete. Clin Sports Med. 1994;13:743-759.
5. Fredericson M, Wun C. Differential diagnosis of leg pain in the athlete. J Am Podiatr Med Assoc. 2003;93:321-324.
6. Pell RF 4th, Khanuja HS, Cooley GR. Leg pain in the running athlete. J Am Acad Orthop Surg. 2004;12:396-404.
7. Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8:344-353.
8. Pham TT, Kapur R, Harwood MI. Exertional leg pain: teasing out arterial entrapments. Curr Sports Med Rep. 2007;6:371-375.
9. Wright LB, Matchett WJ, Cruz CP, et al. Popliteal artery disease: diagnosis and treatment. Radiographics. 2004;24:467-479.
10. Yates B, White S. The incidence and risk factors in the development of medial tibial stress syndrome among naval recruits. Am J Sports Med. 2004;32:772-780.
11. Fredericson M, Jennings F, Beaulieu C, et al. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17:309-325.
12. Plisky MS, Rauh MJ, Heiderscheit B, et al. Medial tibial stress syndrome in high school cross-country runners: incidence and risk factors. J Orthop Sports Phys Ther. 2007;37:40-47.
13. Touliopolous S, Hershman EB. Lower leg pain. Diagnosis and treatment of compartment syndromes and other pain syndromes of the leg. Sports Med. 1999;27:193-204.
14. Burrus MT, Werner BC, Starman JS, et al. Chronic leg pain in athletes. Am J Sports Med. 2015;43:1538-1547.
15. Batt ME, Ugalde V, Anderson MW, et al. A prospective controlled study of diagnostic imaging for acute shin splints. Med Sci Sports Exerc. 1998;30:1564-1571.
16. Gaeta M, Minutoli F, Scribano E, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235:553-561.
17. Sterling JC, Edelstein DW, Calvo RD, et al. Stress fractures in the athlete. Diagnosis and management. Sports Med. 1992;14:336-346.
18. Brukner P. Exercise-related lower leg pain: bone. Med Sci Sports Exerc. 2000;32:S15-S26.
19. Tuan K, Wu S, Sennett B. Stress fractures in athletes: risk factors, diagnosis, and management. Orthopedics. 2004;27:583-591.
20. Harrast MA, Colonno D. Stress fractures in runners. Clin Sports Med. 2010;29:399-416.
21. Varner KE, Younas SA, Lintner DM, et al. Chronic anterior midtibial stress fractures in athletes treated with reamed intramedullary nailing. Am J Sports Med. 2005;33:1071-1076.
22. Kaeding CC, Yu JR, Wright R, et al. Management and return to play of stress fractures. Clin J Sport Med. 2005;15:442-447.
23. Blackman PG. A review of chronic exertional compartment syndrome in the lower leg. Med Sci Sports Exerc. 2000;32:S4-S10.
24. Brennan FH Jr, Kane SF. Diagnosis, treatment options, and rehabilitation of chronic lower leg exertional compartment syndrome. Curr Sports Med Rep. 2003;2:247-250.
25. Davis DE, Raikin S, Garras DN, et al. Characteristics of patients with chronic exertional compartment syndrome. Foot Ankle Int. 2013;34:1349-1354.
26. Edmundsson D, Toolanen G. Chronic exertional compartment syndrome in diabetes mellitus. Diabet Med. 2011;28:81-85.
27. Pedowitz RA, Hargens AR, Mubarak SJ, et al. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18:35-40.
28. Bong MR, Polatsch DB, Jazrawi LM, et al. Chronic exertional compartment syndrome: diagnosis and management. Bull Hosp Jt Dis. 2005;62:77-84.
29. Hislop M, Batt ME. Chronic exertional compartment syndrome testing: a minimalist approach. Br J Sports Med. 2011;45:954-955.
30. Brown RR, Rosenberg ZS. MR imaging of exercise-induced lower leg pain. Magn Reson Imaging Clin N Am. 2001;9:533-552.
31. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42:385-392.
32. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21:811-817.
33. Ehsan O, Darwish A, Edmundson C, et al. Non-traumatic lower limb vascular complications in endurance athletes. Review of literature. Eur J Vasc Endovasc Surg. 2004;28:1-8.
34. Turnipseed WD. Popliteal entrapment syndrome. J Vasc Surg. 2002;35:910-915.
35. Baltopoulos P, Filippou DK, Sigala F. Popliteal artery entrapment syndrome: anatomic or functional syndrome? Clin J Sport Med. 2004;14:8-12.
36. McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164-1171.
37. Elias DA, White LM, Rubenstein JD, et al. Clinical evaluation and MR imaging features of popliteal artery entrapment and cystic adventitial disease. AJR Am J Roentgenol. 2003;180:627-632.
38. Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol. 2010;24:253-265.
39. Korkola M, Amendola A. Exercise-induced leg pain: sifting through a broad differential. Phys Sportsmed. 2001;29:35-50.
40. McCrory P, Bell S, Bradshaw C. Nerve entrapments of the lower leg, ankle and foot in sport. Sports Med. 2002;32:371-391.
41. Schon LC. Nerve entrapment, neuropathy, and nerve dysfunction in athletes. Orthop Clin North Am. 1994;25:47-59.
42. Anselmi SJ. Common peroneal nerve compression. J Am Podiatr Med Assoc. 2006;96:413-417.
43. Maalla R, Youssef M, Ben Lassoued N, et al. Peroneal nerve entrapment at the fibular head: outcomes of neurolysis. Orthop Traumatol Surg Res. 2013;99:719-722.
44. Marciniak C, Armon C, Wilson J, et al. Practice parameter: utility of electrodiagnostic techniques in evaluating patients with suspected peroneal neuropathy: an evidence-based review. Muscle Nerve. 2005;31:520-527.
1. Rajasekaran S, Kvinlaug K, Finnoff JT. Exertional leg pain in the athlete. PM R. 2012;4:985-1000.
2. Brewer RB, Gregory AJ. Chronic lower leg pain in athletes: a guide for the differential diagnosis, evaluation, and treatment. Sports Health. 2012;4:121-127.
3. Edwards PH Jr, Wright ML, Hartman JF. A practical approach for the differential diagnosis of chronic leg pain in the athlete. Am J Sports Med. 2005;33:1241-1249.
4. Clanton TO, Solcher BW. Chronic leg pain in the athlete. Clin Sports Med. 1994;13:743-759.
5. Fredericson M, Wun C. Differential diagnosis of leg pain in the athlete. J Am Podiatr Med Assoc. 2003;93:321-324.
6. Pell RF 4th, Khanuja HS, Cooley GR. Leg pain in the running athlete. J Am Acad Orthop Surg. 2004;12:396-404.
7. Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8:344-353.
8. Pham TT, Kapur R, Harwood MI. Exertional leg pain: teasing out arterial entrapments. Curr Sports Med Rep. 2007;6:371-375.
9. Wright LB, Matchett WJ, Cruz CP, et al. Popliteal artery disease: diagnosis and treatment. Radiographics. 2004;24:467-479.
10. Yates B, White S. The incidence and risk factors in the development of medial tibial stress syndrome among naval recruits. Am J Sports Med. 2004;32:772-780.
11. Fredericson M, Jennings F, Beaulieu C, et al. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17:309-325.
12. Plisky MS, Rauh MJ, Heiderscheit B, et al. Medial tibial stress syndrome in high school cross-country runners: incidence and risk factors. J Orthop Sports Phys Ther. 2007;37:40-47.
13. Touliopolous S, Hershman EB. Lower leg pain. Diagnosis and treatment of compartment syndromes and other pain syndromes of the leg. Sports Med. 1999;27:193-204.
14. Burrus MT, Werner BC, Starman JS, et al. Chronic leg pain in athletes. Am J Sports Med. 2015;43:1538-1547.
15. Batt ME, Ugalde V, Anderson MW, et al. A prospective controlled study of diagnostic imaging for acute shin splints. Med Sci Sports Exerc. 1998;30:1564-1571.
16. Gaeta M, Minutoli F, Scribano E, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235:553-561.
17. Sterling JC, Edelstein DW, Calvo RD, et al. Stress fractures in the athlete. Diagnosis and management. Sports Med. 1992;14:336-346.
18. Brukner P. Exercise-related lower leg pain: bone. Med Sci Sports Exerc. 2000;32:S15-S26.
19. Tuan K, Wu S, Sennett B. Stress fractures in athletes: risk factors, diagnosis, and management. Orthopedics. 2004;27:583-591.
20. Harrast MA, Colonno D. Stress fractures in runners. Clin Sports Med. 2010;29:399-416.
21. Varner KE, Younas SA, Lintner DM, et al. Chronic anterior midtibial stress fractures in athletes treated with reamed intramedullary nailing. Am J Sports Med. 2005;33:1071-1076.
22. Kaeding CC, Yu JR, Wright R, et al. Management and return to play of stress fractures. Clin J Sport Med. 2005;15:442-447.
23. Blackman PG. A review of chronic exertional compartment syndrome in the lower leg. Med Sci Sports Exerc. 2000;32:S4-S10.
24. Brennan FH Jr, Kane SF. Diagnosis, treatment options, and rehabilitation of chronic lower leg exertional compartment syndrome. Curr Sports Med Rep. 2003;2:247-250.
25. Davis DE, Raikin S, Garras DN, et al. Characteristics of patients with chronic exertional compartment syndrome. Foot Ankle Int. 2013;34:1349-1354.
26. Edmundsson D, Toolanen G. Chronic exertional compartment syndrome in diabetes mellitus. Diabet Med. 2011;28:81-85.
27. Pedowitz RA, Hargens AR, Mubarak SJ, et al. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18:35-40.
28. Bong MR, Polatsch DB, Jazrawi LM, et al. Chronic exertional compartment syndrome: diagnosis and management. Bull Hosp Jt Dis. 2005;62:77-84.
29. Hislop M, Batt ME. Chronic exertional compartment syndrome testing: a minimalist approach. Br J Sports Med. 2011;45:954-955.
30. Brown RR, Rosenberg ZS. MR imaging of exercise-induced lower leg pain. Magn Reson Imaging Clin N Am. 2001;9:533-552.
31. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42:385-392.
32. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21:811-817.
33. Ehsan O, Darwish A, Edmundson C, et al. Non-traumatic lower limb vascular complications in endurance athletes. Review of literature. Eur J Vasc Endovasc Surg. 2004;28:1-8.
34. Turnipseed WD. Popliteal entrapment syndrome. J Vasc Surg. 2002;35:910-915.
35. Baltopoulos P, Filippou DK, Sigala F. Popliteal artery entrapment syndrome: anatomic or functional syndrome? Clin J Sport Med. 2004;14:8-12.
36. McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164-1171.
37. Elias DA, White LM, Rubenstein JD, et al. Clinical evaluation and MR imaging features of popliteal artery entrapment and cystic adventitial disease. AJR Am J Roentgenol. 2003;180:627-632.
38. Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol. 2010;24:253-265.
39. Korkola M, Amendola A. Exercise-induced leg pain: sifting through a broad differential. Phys Sportsmed. 2001;29:35-50.
40. McCrory P, Bell S, Bradshaw C. Nerve entrapments of the lower leg, ankle and foot in sport. Sports Med. 2002;32:371-391.
41. Schon LC. Nerve entrapment, neuropathy, and nerve dysfunction in athletes. Orthop Clin North Am. 1994;25:47-59.
42. Anselmi SJ. Common peroneal nerve compression. J Am Podiatr Med Assoc. 2006;96:413-417.
43. Maalla R, Youssef M, Ben Lassoued N, et al. Peroneal nerve entrapment at the fibular head: outcomes of neurolysis. Orthop Traumatol Surg Res. 2013;99:719-722.
44. Marciniak C, Armon C, Wilson J, et al. Practice parameter: utility of electrodiagnostic techniques in evaluating patients with suspected peroneal neuropathy: an evidence-based review. Muscle Nerve. 2005;31:520-527.
Preoperative evaluation: A time-saving algorithm
PRACTICE RECOMMENDATIONS
› Recommend that patients quit smoking 8 weeks before surgery; keep in mind, though, that quitting closer to the date of surgery does not increase the risk of complications. A
› Use the online American College of Surgeons/National Surgical Quality Improvement Program surgical risk calculator to estimate a patient’s surgical risk. C
› Send a patient directly to surgery if he or she has an estimated cardiac risk <1% or <2 risk factors of the Revised Cardiac Risk Index. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
About 27 million Americans undergo surgery every year1 and before doing so, they turn to you—their primary care physician—or their cardiologist for a preoperative evaluation. Of course, the goal of this evaluation is to determine an individual patient’s risk and compare it to procedural averages in an effort to identify opportunities for risk mitigation. But the preoperative evaluation is also an opportunity to make recommendations regarding perioperative management of medications. And certainly we want to conduct these evaluations in a way that is both expeditious and in keeping with the latest guidelines.
Current guidelines for preoperative evaluations are less complicated than they used to be and focus on cardiac and pulmonary risk stratification. While a risk calculator remains your primary tool, elements such as smoking cessation and identifying sleep apnea are important parts of the preop equation. In the review that follows, we present a simple algorithm (FIGURE 12-6) that we developed that can be completed in a single visit.

Cardiac assessment: A risk calculator is the primary tool

Cardiac risk estimation is perhaps the most important element in determining a patient’s overall surgical risk. But before you begin, you'll need to determine whether the preoperative evaluation of the patient is best handled by you or a specialist. Current guidelines recommend preoperative evaluation by a specialist when a patient has certain conditions, such as moderate or greater valvular stenosis/regurgitation, a cardiac implantable electronic device, pulmonary hypertension, congenital heart disease, or severe systemic disease.2
If these conditions are not present (and an immediate referral is not required), you can turn your attention to the cardiac assessment. The first step is to determine which cardiac risk calculator you’d like to use. In its comprehensive guideline on perioperative cardiovascular evaluation, the American College of Cardiology/American Heart Association (ACC/AHA) recommends the use of one of the 2 calculators described below.2
Revised Cardiac Risk Index. The most well-known cardiac risk calculator is the 6-element Revised Cardiac Risk Index (RCRI) (TABLE 1).7 Published in 1999, the RCRI was derived from a cohort of 2800 patients, verified in 1400 patients, and has been validated in numerous studies.2 Each element increases the odds of a cardiac complication by a factor of 2 to 3, and more than one positive response indicates the patient is at high risk for complications.7
The ACS NSQIP risk calculator has been criticized because it has not been validated in a group separate from the initial patient population used in its development.2 Another criticism is the inclusion of the American Society of Anesthesiologists' (ASA) classification of the overall health of the patient, a simple yet subjective and unreliable method of patient characterization.2
Choosing a calculator. The ACS NSQIP calculator may be more useful for primary care physicians because it provides individualized risks for numerous complications and is easy to use. The output page can be printed as documentation of the preoperative evaluation, and is useful for counseling patients about reconsideration of surgery or risk-reduction strategies. The RCRI is also simple to use, but considers only cardiac risk. Although the RCRI has been validated in numerous studies, the ACS NSQIP was derived from a more substantial 1.4 million patients.
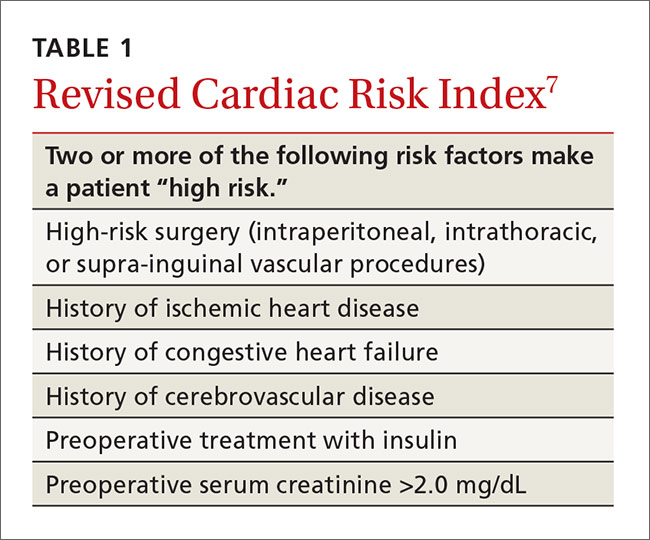
Mapping out next steps based on risk score
The next step in the preoperative evaluation process is to calculate your patient’s risk score and determine whether it is low or high. If the risk is determined to be low—either an RCRI score <2 or an ACS NSQIP cardiac complication risk <1%—the patient can be referred to surgery without further evaluation.2
If the calculator suggests higher risk, the patient’s functional status should be assessed. If the patient has a functional status of >4 metabolic equivalents (METs), then the patient can be recommended for surgery without further evaluation.2 Examples of activities that are greater than 4 METs are yard work such as raking leaves, weeding, or pushing a power mower; sexual relations; climbing a flight of stairs; walking up a hill; and participating in moderate recreational activities like golf, bowling, dancing, doubles tennis, or throwing a baseball or football.9
Patient can’t perform >4 METs? If the patient does not have a functional capacity of >4 METs, further risk stratification should be considered if the results would change management.2 Prior guidelines recommended either perioperative beta-blockers to mitigate risk or coronary interventions, but both are controversial due to lack of proven benefit.
Perioperative beta-blocker use. A recommendation to consider starting beta-blockers at least one day prior to surgery remains in the 2014 ACC/AHA guidelines for patients with 3 or more RCRI risk factors.2 But a group of studies supporting beta-blocker use has been discredited due to serious flaws and fabricated data. At the same time, a large study arguing against perioperative beta-blockers has been criticized for starting high doses of beta-blockers on the day of surgery.2,10,11 In the end, mortality benefit from perioperative beta-blockers is uncertain, and the suggested reduction in cardiac events is partially offset by an increased risk of stroke.2
Stress testing is of questionable value. A patient with high cardiac risk (as evaluated with a calculator) may need to forego the surgical procedure or undergo a modified procedure. Alternatively, he or she may need to be referred to a cardiologist for consultation and possible pharmacologic nuclear stress testing. Although a normal stress test has a high negative predictive value, an abnormal test often leads to percutaneous coronary intervention or bypass surgery, and neither has been shown to reduce cardiac surgical risk.2 Percutaneous coronary interventions require a period of dual antiplatelet therapy, delaying surgery for unproven benefit.2
EKGs and echocardiograms are of limited use. An anesthesia group or surgical center will often require an electrocardiogram (EKG) as part of a preoperative evaluation, but preoperative evaluation by EKG or echocardiogram is controversial due to unproven benefits and potential risks. The 2014 ACC/AHA guidelines recommend against a 12-lead EKG for patients with low cardiac risk using the RCRI or ACS NSQIP or those who are having a low-risk procedure, such as endoscopy or cataract surgery.2 The United States Preventive Health Services Task Force also recommends against screening low-risk patients and says that screening EKGs and stress testing in asymptomatic medium- to high-risk patients is of undetermined value.12 They noted no evidence of benefit from resting or exercise EKG, with harm from a 1.7% complication rate of angiography, which is performed after up to 2.9% of exercise EKG testing.12
There are no recommendations for preoperative echocardiogram in the asymptomatic patient. Only unexplained dyspnea or other clinical signs of heart failure require an echocardiogram. For patients with known heart failure that is clinically stable, the ACC/AHA guidelines suggest that an echocardiogram should be performed within the year prior to surgery, although this is based on expert opinion.2
Because of the controversy over both coronary interventions and perioperative beta-blocker therapy, consider cardiology referral for a patient with poor functional activity level who does not meet low-risk criteria. While stress testing is acceptable, it may not lead to improved patient outcomes.
Optimize preventive care
Begin by ensuring that blood pressure (BP) and cholesterol are managed according to ACC/AHA guidelines. Then consider whether to start preoperative medications. You'll also want to screen for sleep apnea and discuss smoking status and cessation, if appropriate.
Initiate medications preoperatively?
In addition to having value as long-term primary prevention, there is some evidence that statins help prevent cardiac events during surgery. A randomized trial of over 200 vascular surgery patients showed that starting statins an average of 30 days prior to surgery significantly reduced cardiac complications.13 Another systematic review demonstrated that preoperative statins significantly reduce acute kidney injury from surgery.14
Unlike statins, aspirin started prior to surgery does not confer benefit. Aspirin was shown to significantly increase bleeding risk without improving cardiac outcomes in a large trial.15
Screen for sleep apnea
Sleep apnea increases the rate of respiratory failure between 2 and 3 times and the rate of cardiac complications about 1.5 times.16 This is a potentially correctable risk factor. The European Society of Anaesthesiology recommends clinical screening for obstructive sleep apnea using the STOP-Bang screening tool5 (TABLE 24). For its part, the ASA combines screening questions from the STOP-Bang screening questionnaire with a review of the medical record and physical exam, because the STOP-Bang questionnaire alone has an insufficient negative predictive value, ranging from 30% and 82%.6 Obstructive sleep apnea is not addressed on published pulmonary and cardiac risk tools.2,3,7,8
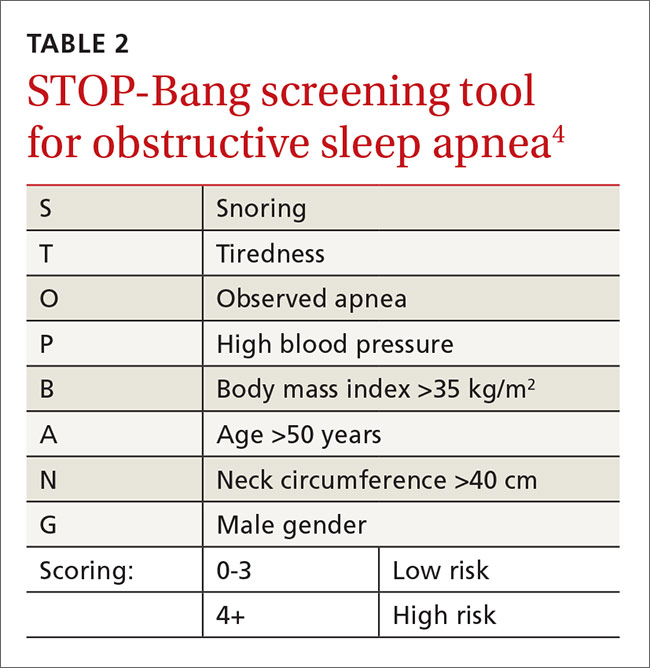
Is the patient a smoker?
Smoking is a reversible risk factor for pulmonary, cardiac, and infectious complications, as well as overall mortality. Perioperative smoking cessation counseling has been complicated by concerns that stopping smoking within 8 weeks of surgery might worsen postoperative outcomes. A study that looked at intraoperative sputum retrieved via tracheal suction showed that patients who had stopped smoking for 2 months or more prior to surgery had the same amount of sputum as non-smokers, while those that quit smoking <2 months before surgery were more likely to have increased intraoperative sputum volume.18 This study did not demonstrate a difference in postoperative pulmonary complications, likely because it included patients receiving minor surgeries only. But based on this study, a cessation period of 2 months was often recommended.
A recent systematic review showed that smoking cessation shortly before surgery does not increase risk.19 In fact, although the review did not show a statistically significant reduction in postoperative complications among recent quitters as compared with continued smokers, there was a trend toward a reduction in overall complications with only a slight increase in pulmonary complications.19
Address potential pulmonary complications
In addition to screening for issues that could lead to cardiac complications, it’s important to address the potential for pulmonary complications. Postoperative pulmonary complications are at least as common as cardiac complications, and include all possible respiratory related outcomes of surgery, from pneumonia to respiratory failure.
The seminal study on postoperative pulmonary complications is a systematic review published in 2006, which showed that the most important risk factors were surgery type, advanced age, ASA classification of overall health ≥II, and congestive heart failure.3 Chronic lung diseases and cigarette use were less predictive of pulmonary issues. All of these factors are included in the ACS NSQIP risk calculator.
Perioperative medication management
One aspect of the preoperative evaluation that should not be overlooked is a thorough medication reconciliation. Primary care providers can support the operative team by recommending medication adjustments prior to surgery. Several classes of medications have specific perioperative recommendations, which are summarized here.
Hypertension medications
- Beta-blockers. A patient who regularly takes a beta-blocker should continue the medication on the day of surgery and restart after surgery.2,20
- Calcium channel blockers. Calcium channel blockers can be continued through the day of surgery.2,20
- Renin-angiotensin system antagonists. Given the increased risk of hypotension following anesthesia induction, have patients refrain from taking angiotensin-converting enzyme inhibitors and angiotensin receptor blocker medications for at least 10 hours prior to surgery.20
- Diuretics. Diuretics can be given on the day of surgery, although they increase the risk of hypovolemia and electrolyte disturbances.20
Diabetes medications
- Insulin. For patients with type I diabetes, recommend basal insulin of 0.2 to 0.3 units/kg/day of long-acting insulin.21 If the patient is using an insulin pump, basal rate should be continued. For patients with type 2 diabetes, the simplest method is to use one-half the normal long-acting insulin dose on the morning of surgery.22
- Metformin. Discontinue metformin 24 hours prior to surgery because of the risk for lactic acidosis.21,22 While the risk of lactic acidosis from metformin is low, mortality rates as high as 50% have been documented after lactic acidosis occurred with similar medications.22
- Sulfonylureas. Sulfonylureas should be held on the day of surgery due to the risk of hypoglycemia and a possible increased risk of ischemia.21,22
- Thiazolidinediones, dipeptidyl peptidase-4 inhibitors, and glucagon-like peptide-1 agonists. All should be held on the day of surgery.21
Anticoagulant medications
- Vitamin K antagonists (warfarin). Discontinue warfarin 5 days prior to the procedure. The half-life is approximately 40 hours, requiring at least 5 days for the anticoagulant effect to be eliminated from the body.23 Use of bridging therapy with regular- or low-molecular weight heparin remains controversial due to increased surgical bleeding risk without evidence of a decrease in cardiovascular events.24 The patient’s risks of stroke and venous thromboembolism should be taken into account when deciding whether to use bridging therapy or not.
- Factor Xa inhibitor. Management of factor Xa inhibitors (rivaroxaban, apixaban) depends on the bleeding risk of the surgery and the patient’s renal function.24,25 For instance, a patient undergoing cataract surgery (low risk) needs a shorter cessation time than a patient undergoing hip arthroplasty (high risk). Discontinuation times are listed in TABLE 3.24
- Direct thrombin inhibitor. Management of direct thrombin inhibitors (dabigatran) is also dependent on surgical bleeding risk and renal function (TABLE 3).23,24
- Aspirin, clopidogrel, ticlopidine, prasugrel. All should be stopped 7 to 10 days prior to surgery to allow new platelet growth. Low-dose aspirin for secondary prevention of cardiovascular disease or primary prevention in a high-risk patient can be continued through surgery.23

Other

- Corticosteroids. Recent evidence suggests that stress-dose steroids are not needed to prevent adrenal insufficiency in patients taking corticosteroids chronically.26 These patients should continue maintenance therapy at regular dosing.26,27 Stress dosing of corticosteroids is only required when a patient has signs of adrenal insufficiency.26
- Statins. Statin medications should be continued on the day of surgery.2
- Nonsteroidal anti-inflammatory drugs. NSAIDs should be stopped 5 days prior to surgery to reverse antiplatelet effects.23
CORRESPONDENCE
CDR Michael J. Arnold, Naval Hospital, 2080 Child Street, Jacksonville, FL 32214; [email protected].
ACKNOWLEDGEMENT
The authors thank CDR Kristian Sanchack and LCDR Dustin Smith for their assistance with this manuscript.
1. Wier LM, Steiner CA, Owens PL. Surgeries in hospital-owned outpatient facilities, 2012. HCUP Statistical Brief #188. February 2015. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb188-Surgeries-Hospital-Outpatient-Facilities-2012.jsp. Accessed September 15, 2016.
2. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol. 2014;64:e77-e137.
3. Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581-595.
4. Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a prac tical approach to screen for obstructive sleep apnea. Chest. 2016;149:631-638.
5. De Hert S, Imberger G, Carlisle J, et al. Preoperative evaluation of the adult patient undergoing non-cardiac surgery: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:684-722.
6. American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea; an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology. 2014;120:268-286.
7. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049.
8. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aide and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833-842.
9. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651-654.
10. Bouri S, Shun-Shin MJ, Cole GD, et al. Meta-analysis of secure randomized controlled trials of B-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100:456-464.
11. Mounsey A, Roque JM, Egan M. Why you shouldn’t start beta-blockers before surgery. J Fam Pract. 2014;63:E15-E16.
12. Chou R, Arora B, Dana T, et al. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:375-385.
13. Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39:967-975.
14. Pan SY, Wu VC, Huang TM, et al. Effect of preoperative statin therapy on postoperative acute kidney injury in patients undergoing major surgery: systemic review and meta-analysis. Nephrology. 2014;19:750-763.
15. Devereux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494-1503.
16. Adesanya AO, Lee W, Greilich NB, et al. Perioperative management of obstructive sleep apnea. Chest. 2010;138:1489-1498.
17. Chung F, Nagappa M, Singh M, et al. CPAP in the perioperative setting: evidence of support. Chest. 2016;149:586-597.
18. Yamashita S, Yamaguchi H, Sakaguchi M, et al. Effect of smoking on intraoperative sputum and postoperative pulmonary complication in minor surgical patients. Respir Med. 2004;98:760-766.
19. Myers K, Hajek P, Hinds
20. Lonjaret L, Lairez O, Minville V, et al. Optimal perioperative management of arterial blood pressure. Integr Blood Press Control. 2014;7:49-59.
21. Sudhakaran S, Surani SR. Guidelines for perioperative management of the diabetic patient. Surg Res Pract. 2015;2015:284063.
22. Duncan AE. Hyperglycemia and perioperative glucose management. Curr Pharm Des. 2012;18:6195-6203.
23. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S-e350S.
24. Faraoni D, Levy JH, Albaladejo P, et al. Updates in the perioperative and emergency management of non-vitamin K antagonist oral anticoagulants. Crit Care. 2015;19:203.
25. Shamoun F, Obeid H, Ramakrishna H. Novel anticoagulants in atrial fibrillation: monitoring, reversal and perioperative management. Biomed Res Int. 2015;2015:424031.
26. Kelly KN, Domajnko B. Perioperative stress-dose steroids. Clin Colon Rectal Surg. 2013;26:163-167.
27. Scanzello CR, Nestor BJ. Perioperative management of medications used in the treatment of rheumatoid arthritis. HSSJ. 2006;2:141-147.
PRACTICE RECOMMENDATIONS
› Recommend that patients quit smoking 8 weeks before surgery; keep in mind, though, that quitting closer to the date of surgery does not increase the risk of complications. A
› Use the online American College of Surgeons/National Surgical Quality Improvement Program surgical risk calculator to estimate a patient’s surgical risk. C
› Send a patient directly to surgery if he or she has an estimated cardiac risk <1% or <2 risk factors of the Revised Cardiac Risk Index. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
About 27 million Americans undergo surgery every year1 and before doing so, they turn to you—their primary care physician—or their cardiologist for a preoperative evaluation. Of course, the goal of this evaluation is to determine an individual patient’s risk and compare it to procedural averages in an effort to identify opportunities for risk mitigation. But the preoperative evaluation is also an opportunity to make recommendations regarding perioperative management of medications. And certainly we want to conduct these evaluations in a way that is both expeditious and in keeping with the latest guidelines.
Current guidelines for preoperative evaluations are less complicated than they used to be and focus on cardiac and pulmonary risk stratification. While a risk calculator remains your primary tool, elements such as smoking cessation and identifying sleep apnea are important parts of the preop equation. In the review that follows, we present a simple algorithm (FIGURE 12-6) that we developed that can be completed in a single visit.

Cardiac assessment: A risk calculator is the primary tool

Cardiac risk estimation is perhaps the most important element in determining a patient’s overall surgical risk. But before you begin, you'll need to determine whether the preoperative evaluation of the patient is best handled by you or a specialist. Current guidelines recommend preoperative evaluation by a specialist when a patient has certain conditions, such as moderate or greater valvular stenosis/regurgitation, a cardiac implantable electronic device, pulmonary hypertension, congenital heart disease, or severe systemic disease.2
If these conditions are not present (and an immediate referral is not required), you can turn your attention to the cardiac assessment. The first step is to determine which cardiac risk calculator you’d like to use. In its comprehensive guideline on perioperative cardiovascular evaluation, the American College of Cardiology/American Heart Association (ACC/AHA) recommends the use of one of the 2 calculators described below.2
Revised Cardiac Risk Index. The most well-known cardiac risk calculator is the 6-element Revised Cardiac Risk Index (RCRI) (TABLE 1).7 Published in 1999, the RCRI was derived from a cohort of 2800 patients, verified in 1400 patients, and has been validated in numerous studies.2 Each element increases the odds of a cardiac complication by a factor of 2 to 3, and more than one positive response indicates the patient is at high risk for complications.7
The ACS NSQIP risk calculator has been criticized because it has not been validated in a group separate from the initial patient population used in its development.2 Another criticism is the inclusion of the American Society of Anesthesiologists' (ASA) classification of the overall health of the patient, a simple yet subjective and unreliable method of patient characterization.2
Choosing a calculator. The ACS NSQIP calculator may be more useful for primary care physicians because it provides individualized risks for numerous complications and is easy to use. The output page can be printed as documentation of the preoperative evaluation, and is useful for counseling patients about reconsideration of surgery or risk-reduction strategies. The RCRI is also simple to use, but considers only cardiac risk. Although the RCRI has been validated in numerous studies, the ACS NSQIP was derived from a more substantial 1.4 million patients.

Mapping out next steps based on risk score
The next step in the preoperative evaluation process is to calculate your patient’s risk score and determine whether it is low or high. If the risk is determined to be low—either an RCRI score <2 or an ACS NSQIP cardiac complication risk <1%—the patient can be referred to surgery without further evaluation.2
If the calculator suggests higher risk, the patient’s functional status should be assessed. If the patient has a functional status of >4 metabolic equivalents (METs), then the patient can be recommended for surgery without further evaluation.2 Examples of activities that are greater than 4 METs are yard work such as raking leaves, weeding, or pushing a power mower; sexual relations; climbing a flight of stairs; walking up a hill; and participating in moderate recreational activities like golf, bowling, dancing, doubles tennis, or throwing a baseball or football.9
Patient can’t perform >4 METs? If the patient does not have a functional capacity of >4 METs, further risk stratification should be considered if the results would change management.2 Prior guidelines recommended either perioperative beta-blockers to mitigate risk or coronary interventions, but both are controversial due to lack of proven benefit.
Perioperative beta-blocker use. A recommendation to consider starting beta-blockers at least one day prior to surgery remains in the 2014 ACC/AHA guidelines for patients with 3 or more RCRI risk factors.2 But a group of studies supporting beta-blocker use has been discredited due to serious flaws and fabricated data. At the same time, a large study arguing against perioperative beta-blockers has been criticized for starting high doses of beta-blockers on the day of surgery.2,10,11 In the end, mortality benefit from perioperative beta-blockers is uncertain, and the suggested reduction in cardiac events is partially offset by an increased risk of stroke.2
Stress testing is of questionable value. A patient with high cardiac risk (as evaluated with a calculator) may need to forego the surgical procedure or undergo a modified procedure. Alternatively, he or she may need to be referred to a cardiologist for consultation and possible pharmacologic nuclear stress testing. Although a normal stress test has a high negative predictive value, an abnormal test often leads to percutaneous coronary intervention or bypass surgery, and neither has been shown to reduce cardiac surgical risk.2 Percutaneous coronary interventions require a period of dual antiplatelet therapy, delaying surgery for unproven benefit.2
EKGs and echocardiograms are of limited use. An anesthesia group or surgical center will often require an electrocardiogram (EKG) as part of a preoperative evaluation, but preoperative evaluation by EKG or echocardiogram is controversial due to unproven benefits and potential risks. The 2014 ACC/AHA guidelines recommend against a 12-lead EKG for patients with low cardiac risk using the RCRI or ACS NSQIP or those who are having a low-risk procedure, such as endoscopy or cataract surgery.2 The United States Preventive Health Services Task Force also recommends against screening low-risk patients and says that screening EKGs and stress testing in asymptomatic medium- to high-risk patients is of undetermined value.12 They noted no evidence of benefit from resting or exercise EKG, with harm from a 1.7% complication rate of angiography, which is performed after up to 2.9% of exercise EKG testing.12
There are no recommendations for preoperative echocardiogram in the asymptomatic patient. Only unexplained dyspnea or other clinical signs of heart failure require an echocardiogram. For patients with known heart failure that is clinically stable, the ACC/AHA guidelines suggest that an echocardiogram should be performed within the year prior to surgery, although this is based on expert opinion.2
Because of the controversy over both coronary interventions and perioperative beta-blocker therapy, consider cardiology referral for a patient with poor functional activity level who does not meet low-risk criteria. While stress testing is acceptable, it may not lead to improved patient outcomes.
Optimize preventive care
Begin by ensuring that blood pressure (BP) and cholesterol are managed according to ACC/AHA guidelines. Then consider whether to start preoperative medications. You'll also want to screen for sleep apnea and discuss smoking status and cessation, if appropriate.
Initiate medications preoperatively?
In addition to having value as long-term primary prevention, there is some evidence that statins help prevent cardiac events during surgery. A randomized trial of over 200 vascular surgery patients showed that starting statins an average of 30 days prior to surgery significantly reduced cardiac complications.13 Another systematic review demonstrated that preoperative statins significantly reduce acute kidney injury from surgery.14
Unlike statins, aspirin started prior to surgery does not confer benefit. Aspirin was shown to significantly increase bleeding risk without improving cardiac outcomes in a large trial.15
Screen for sleep apnea
Sleep apnea increases the rate of respiratory failure between 2 and 3 times and the rate of cardiac complications about 1.5 times.16 This is a potentially correctable risk factor. The European Society of Anaesthesiology recommends clinical screening for obstructive sleep apnea using the STOP-Bang screening tool5 (TABLE 24). For its part, the ASA combines screening questions from the STOP-Bang screening questionnaire with a review of the medical record and physical exam, because the STOP-Bang questionnaire alone has an insufficient negative predictive value, ranging from 30% and 82%.6 Obstructive sleep apnea is not addressed on published pulmonary and cardiac risk tools.2,3,7,8

Is the patient a smoker?
Smoking is a reversible risk factor for pulmonary, cardiac, and infectious complications, as well as overall mortality. Perioperative smoking cessation counseling has been complicated by concerns that stopping smoking within 8 weeks of surgery might worsen postoperative outcomes. A study that looked at intraoperative sputum retrieved via tracheal suction showed that patients who had stopped smoking for 2 months or more prior to surgery had the same amount of sputum as non-smokers, while those that quit smoking <2 months before surgery were more likely to have increased intraoperative sputum volume.18 This study did not demonstrate a difference in postoperative pulmonary complications, likely because it included patients receiving minor surgeries only. But based on this study, a cessation period of 2 months was often recommended.
A recent systematic review showed that smoking cessation shortly before surgery does not increase risk.19 In fact, although the review did not show a statistically significant reduction in postoperative complications among recent quitters as compared with continued smokers, there was a trend toward a reduction in overall complications with only a slight increase in pulmonary complications.19
Address potential pulmonary complications
In addition to screening for issues that could lead to cardiac complications, it’s important to address the potential for pulmonary complications. Postoperative pulmonary complications are at least as common as cardiac complications, and include all possible respiratory related outcomes of surgery, from pneumonia to respiratory failure.
The seminal study on postoperative pulmonary complications is a systematic review published in 2006, which showed that the most important risk factors were surgery type, advanced age, ASA classification of overall health ≥II, and congestive heart failure.3 Chronic lung diseases and cigarette use were less predictive of pulmonary issues. All of these factors are included in the ACS NSQIP risk calculator.
Perioperative medication management
One aspect of the preoperative evaluation that should not be overlooked is a thorough medication reconciliation. Primary care providers can support the operative team by recommending medication adjustments prior to surgery. Several classes of medications have specific perioperative recommendations, which are summarized here.
Hypertension medications
- Beta-blockers. A patient who regularly takes a beta-blocker should continue the medication on the day of surgery and restart after surgery.2,20
- Calcium channel blockers. Calcium channel blockers can be continued through the day of surgery.2,20
- Renin-angiotensin system antagonists. Given the increased risk of hypotension following anesthesia induction, have patients refrain from taking angiotensin-converting enzyme inhibitors and angiotensin receptor blocker medications for at least 10 hours prior to surgery.20
- Diuretics. Diuretics can be given on the day of surgery, although they increase the risk of hypovolemia and electrolyte disturbances.20
Diabetes medications
- Insulin. For patients with type I diabetes, recommend basal insulin of 0.2 to 0.3 units/kg/day of long-acting insulin.21 If the patient is using an insulin pump, basal rate should be continued. For patients with type 2 diabetes, the simplest method is to use one-half the normal long-acting insulin dose on the morning of surgery.22
- Metformin. Discontinue metformin 24 hours prior to surgery because of the risk for lactic acidosis.21,22 While the risk of lactic acidosis from metformin is low, mortality rates as high as 50% have been documented after lactic acidosis occurred with similar medications.22
- Sulfonylureas. Sulfonylureas should be held on the day of surgery due to the risk of hypoglycemia and a possible increased risk of ischemia.21,22
- Thiazolidinediones, dipeptidyl peptidase-4 inhibitors, and glucagon-like peptide-1 agonists. All should be held on the day of surgery.21
Anticoagulant medications
- Vitamin K antagonists (warfarin). Discontinue warfarin 5 days prior to the procedure. The half-life is approximately 40 hours, requiring at least 5 days for the anticoagulant effect to be eliminated from the body.23 Use of bridging therapy with regular- or low-molecular weight heparin remains controversial due to increased surgical bleeding risk without evidence of a decrease in cardiovascular events.24 The patient’s risks of stroke and venous thromboembolism should be taken into account when deciding whether to use bridging therapy or not.
- Factor Xa inhibitor. Management of factor Xa inhibitors (rivaroxaban, apixaban) depends on the bleeding risk of the surgery and the patient’s renal function.24,25 For instance, a patient undergoing cataract surgery (low risk) needs a shorter cessation time than a patient undergoing hip arthroplasty (high risk). Discontinuation times are listed in TABLE 3.24
- Direct thrombin inhibitor. Management of direct thrombin inhibitors (dabigatran) is also dependent on surgical bleeding risk and renal function (TABLE 3).23,24
- Aspirin, clopidogrel, ticlopidine, prasugrel. All should be stopped 7 to 10 days prior to surgery to allow new platelet growth. Low-dose aspirin for secondary prevention of cardiovascular disease or primary prevention in a high-risk patient can be continued through surgery.23

Other

- Corticosteroids. Recent evidence suggests that stress-dose steroids are not needed to prevent adrenal insufficiency in patients taking corticosteroids chronically.26 These patients should continue maintenance therapy at regular dosing.26,27 Stress dosing of corticosteroids is only required when a patient has signs of adrenal insufficiency.26
- Statins. Statin medications should be continued on the day of surgery.2
- Nonsteroidal anti-inflammatory drugs. NSAIDs should be stopped 5 days prior to surgery to reverse antiplatelet effects.23
CORRESPONDENCE
CDR Michael J. Arnold, Naval Hospital, 2080 Child Street, Jacksonville, FL 32214; [email protected].
ACKNOWLEDGEMENT
The authors thank CDR Kristian Sanchack and LCDR Dustin Smith for their assistance with this manuscript.
PRACTICE RECOMMENDATIONS
› Recommend that patients quit smoking 8 weeks before surgery; keep in mind, though, that quitting closer to the date of surgery does not increase the risk of complications. A
› Use the online American College of Surgeons/National Surgical Quality Improvement Program surgical risk calculator to estimate a patient’s surgical risk. C
› Send a patient directly to surgery if he or she has an estimated cardiac risk <1% or <2 risk factors of the Revised Cardiac Risk Index. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
About 27 million Americans undergo surgery every year1 and before doing so, they turn to you—their primary care physician—or their cardiologist for a preoperative evaluation. Of course, the goal of this evaluation is to determine an individual patient’s risk and compare it to procedural averages in an effort to identify opportunities for risk mitigation. But the preoperative evaluation is also an opportunity to make recommendations regarding perioperative management of medications. And certainly we want to conduct these evaluations in a way that is both expeditious and in keeping with the latest guidelines.
Current guidelines for preoperative evaluations are less complicated than they used to be and focus on cardiac and pulmonary risk stratification. While a risk calculator remains your primary tool, elements such as smoking cessation and identifying sleep apnea are important parts of the preop equation. In the review that follows, we present a simple algorithm (FIGURE 12-6) that we developed that can be completed in a single visit.

Cardiac assessment: A risk calculator is the primary tool

Cardiac risk estimation is perhaps the most important element in determining a patient’s overall surgical risk. But before you begin, you'll need to determine whether the preoperative evaluation of the patient is best handled by you or a specialist. Current guidelines recommend preoperative evaluation by a specialist when a patient has certain conditions, such as moderate or greater valvular stenosis/regurgitation, a cardiac implantable electronic device, pulmonary hypertension, congenital heart disease, or severe systemic disease.2
If these conditions are not present (and an immediate referral is not required), you can turn your attention to the cardiac assessment. The first step is to determine which cardiac risk calculator you’d like to use. In its comprehensive guideline on perioperative cardiovascular evaluation, the American College of Cardiology/American Heart Association (ACC/AHA) recommends the use of one of the 2 calculators described below.2
Revised Cardiac Risk Index. The most well-known cardiac risk calculator is the 6-element Revised Cardiac Risk Index (RCRI) (TABLE 1).7 Published in 1999, the RCRI was derived from a cohort of 2800 patients, verified in 1400 patients, and has been validated in numerous studies.2 Each element increases the odds of a cardiac complication by a factor of 2 to 3, and more than one positive response indicates the patient is at high risk for complications.7
The ACS NSQIP risk calculator has been criticized because it has not been validated in a group separate from the initial patient population used in its development.2 Another criticism is the inclusion of the American Society of Anesthesiologists' (ASA) classification of the overall health of the patient, a simple yet subjective and unreliable method of patient characterization.2
Choosing a calculator. The ACS NSQIP calculator may be more useful for primary care physicians because it provides individualized risks for numerous complications and is easy to use. The output page can be printed as documentation of the preoperative evaluation, and is useful for counseling patients about reconsideration of surgery or risk-reduction strategies. The RCRI is also simple to use, but considers only cardiac risk. Although the RCRI has been validated in numerous studies, the ACS NSQIP was derived from a more substantial 1.4 million patients.

Mapping out next steps based on risk score
The next step in the preoperative evaluation process is to calculate your patient’s risk score and determine whether it is low or high. If the risk is determined to be low—either an RCRI score <2 or an ACS NSQIP cardiac complication risk <1%—the patient can be referred to surgery without further evaluation.2
If the calculator suggests higher risk, the patient’s functional status should be assessed. If the patient has a functional status of >4 metabolic equivalents (METs), then the patient can be recommended for surgery without further evaluation.2 Examples of activities that are greater than 4 METs are yard work such as raking leaves, weeding, or pushing a power mower; sexual relations; climbing a flight of stairs; walking up a hill; and participating in moderate recreational activities like golf, bowling, dancing, doubles tennis, or throwing a baseball or football.9
Patient can’t perform >4 METs? If the patient does not have a functional capacity of >4 METs, further risk stratification should be considered if the results would change management.2 Prior guidelines recommended either perioperative beta-blockers to mitigate risk or coronary interventions, but both are controversial due to lack of proven benefit.
Perioperative beta-blocker use. A recommendation to consider starting beta-blockers at least one day prior to surgery remains in the 2014 ACC/AHA guidelines for patients with 3 or more RCRI risk factors.2 But a group of studies supporting beta-blocker use has been discredited due to serious flaws and fabricated data. At the same time, a large study arguing against perioperative beta-blockers has been criticized for starting high doses of beta-blockers on the day of surgery.2,10,11 In the end, mortality benefit from perioperative beta-blockers is uncertain, and the suggested reduction in cardiac events is partially offset by an increased risk of stroke.2
Stress testing is of questionable value. A patient with high cardiac risk (as evaluated with a calculator) may need to forego the surgical procedure or undergo a modified procedure. Alternatively, he or she may need to be referred to a cardiologist for consultation and possible pharmacologic nuclear stress testing. Although a normal stress test has a high negative predictive value, an abnormal test often leads to percutaneous coronary intervention or bypass surgery, and neither has been shown to reduce cardiac surgical risk.2 Percutaneous coronary interventions require a period of dual antiplatelet therapy, delaying surgery for unproven benefit.2
EKGs and echocardiograms are of limited use. An anesthesia group or surgical center will often require an electrocardiogram (EKG) as part of a preoperative evaluation, but preoperative evaluation by EKG or echocardiogram is controversial due to unproven benefits and potential risks. The 2014 ACC/AHA guidelines recommend against a 12-lead EKG for patients with low cardiac risk using the RCRI or ACS NSQIP or those who are having a low-risk procedure, such as endoscopy or cataract surgery.2 The United States Preventive Health Services Task Force also recommends against screening low-risk patients and says that screening EKGs and stress testing in asymptomatic medium- to high-risk patients is of undetermined value.12 They noted no evidence of benefit from resting or exercise EKG, with harm from a 1.7% complication rate of angiography, which is performed after up to 2.9% of exercise EKG testing.12
There are no recommendations for preoperative echocardiogram in the asymptomatic patient. Only unexplained dyspnea or other clinical signs of heart failure require an echocardiogram. For patients with known heart failure that is clinically stable, the ACC/AHA guidelines suggest that an echocardiogram should be performed within the year prior to surgery, although this is based on expert opinion.2
Because of the controversy over both coronary interventions and perioperative beta-blocker therapy, consider cardiology referral for a patient with poor functional activity level who does not meet low-risk criteria. While stress testing is acceptable, it may not lead to improved patient outcomes.
Optimize preventive care
Begin by ensuring that blood pressure (BP) and cholesterol are managed according to ACC/AHA guidelines. Then consider whether to start preoperative medications. You'll also want to screen for sleep apnea and discuss smoking status and cessation, if appropriate.
Initiate medications preoperatively?
In addition to having value as long-term primary prevention, there is some evidence that statins help prevent cardiac events during surgery. A randomized trial of over 200 vascular surgery patients showed that starting statins an average of 30 days prior to surgery significantly reduced cardiac complications.13 Another systematic review demonstrated that preoperative statins significantly reduce acute kidney injury from surgery.14
Unlike statins, aspirin started prior to surgery does not confer benefit. Aspirin was shown to significantly increase bleeding risk without improving cardiac outcomes in a large trial.15
Screen for sleep apnea
Sleep apnea increases the rate of respiratory failure between 2 and 3 times and the rate of cardiac complications about 1.5 times.16 This is a potentially correctable risk factor. The European Society of Anaesthesiology recommends clinical screening for obstructive sleep apnea using the STOP-Bang screening tool5 (TABLE 24). For its part, the ASA combines screening questions from the STOP-Bang screening questionnaire with a review of the medical record and physical exam, because the STOP-Bang questionnaire alone has an insufficient negative predictive value, ranging from 30% and 82%.6 Obstructive sleep apnea is not addressed on published pulmonary and cardiac risk tools.2,3,7,8

Is the patient a smoker?
Smoking is a reversible risk factor for pulmonary, cardiac, and infectious complications, as well as overall mortality. Perioperative smoking cessation counseling has been complicated by concerns that stopping smoking within 8 weeks of surgery might worsen postoperative outcomes. A study that looked at intraoperative sputum retrieved via tracheal suction showed that patients who had stopped smoking for 2 months or more prior to surgery had the same amount of sputum as non-smokers, while those that quit smoking <2 months before surgery were more likely to have increased intraoperative sputum volume.18 This study did not demonstrate a difference in postoperative pulmonary complications, likely because it included patients receiving minor surgeries only. But based on this study, a cessation period of 2 months was often recommended.
A recent systematic review showed that smoking cessation shortly before surgery does not increase risk.19 In fact, although the review did not show a statistically significant reduction in postoperative complications among recent quitters as compared with continued smokers, there was a trend toward a reduction in overall complications with only a slight increase in pulmonary complications.19
Address potential pulmonary complications
In addition to screening for issues that could lead to cardiac complications, it’s important to address the potential for pulmonary complications. Postoperative pulmonary complications are at least as common as cardiac complications, and include all possible respiratory related outcomes of surgery, from pneumonia to respiratory failure.
The seminal study on postoperative pulmonary complications is a systematic review published in 2006, which showed that the most important risk factors were surgery type, advanced age, ASA classification of overall health ≥II, and congestive heart failure.3 Chronic lung diseases and cigarette use were less predictive of pulmonary issues. All of these factors are included in the ACS NSQIP risk calculator.
Perioperative medication management
One aspect of the preoperative evaluation that should not be overlooked is a thorough medication reconciliation. Primary care providers can support the operative team by recommending medication adjustments prior to surgery. Several classes of medications have specific perioperative recommendations, which are summarized here.
Hypertension medications
- Beta-blockers. A patient who regularly takes a beta-blocker should continue the medication on the day of surgery and restart after surgery.2,20
- Calcium channel blockers. Calcium channel blockers can be continued through the day of surgery.2,20
- Renin-angiotensin system antagonists. Given the increased risk of hypotension following anesthesia induction, have patients refrain from taking angiotensin-converting enzyme inhibitors and angiotensin receptor blocker medications for at least 10 hours prior to surgery.20
- Diuretics. Diuretics can be given on the day of surgery, although they increase the risk of hypovolemia and electrolyte disturbances.20
Diabetes medications
- Insulin. For patients with type I diabetes, recommend basal insulin of 0.2 to 0.3 units/kg/day of long-acting insulin.21 If the patient is using an insulin pump, basal rate should be continued. For patients with type 2 diabetes, the simplest method is to use one-half the normal long-acting insulin dose on the morning of surgery.22
- Metformin. Discontinue metformin 24 hours prior to surgery because of the risk for lactic acidosis.21,22 While the risk of lactic acidosis from metformin is low, mortality rates as high as 50% have been documented after lactic acidosis occurred with similar medications.22
- Sulfonylureas. Sulfonylureas should be held on the day of surgery due to the risk of hypoglycemia and a possible increased risk of ischemia.21,22
- Thiazolidinediones, dipeptidyl peptidase-4 inhibitors, and glucagon-like peptide-1 agonists. All should be held on the day of surgery.21
Anticoagulant medications
- Vitamin K antagonists (warfarin). Discontinue warfarin 5 days prior to the procedure. The half-life is approximately 40 hours, requiring at least 5 days for the anticoagulant effect to be eliminated from the body.23 Use of bridging therapy with regular- or low-molecular weight heparin remains controversial due to increased surgical bleeding risk without evidence of a decrease in cardiovascular events.24 The patient’s risks of stroke and venous thromboembolism should be taken into account when deciding whether to use bridging therapy or not.
- Factor Xa inhibitor. Management of factor Xa inhibitors (rivaroxaban, apixaban) depends on the bleeding risk of the surgery and the patient’s renal function.24,25 For instance, a patient undergoing cataract surgery (low risk) needs a shorter cessation time than a patient undergoing hip arthroplasty (high risk). Discontinuation times are listed in TABLE 3.24
- Direct thrombin inhibitor. Management of direct thrombin inhibitors (dabigatran) is also dependent on surgical bleeding risk and renal function (TABLE 3).23,24
- Aspirin, clopidogrel, ticlopidine, prasugrel. All should be stopped 7 to 10 days prior to surgery to allow new platelet growth. Low-dose aspirin for secondary prevention of cardiovascular disease or primary prevention in a high-risk patient can be continued through surgery.23

Other

- Corticosteroids. Recent evidence suggests that stress-dose steroids are not needed to prevent adrenal insufficiency in patients taking corticosteroids chronically.26 These patients should continue maintenance therapy at regular dosing.26,27 Stress dosing of corticosteroids is only required when a patient has signs of adrenal insufficiency.26
- Statins. Statin medications should be continued on the day of surgery.2
- Nonsteroidal anti-inflammatory drugs. NSAIDs should be stopped 5 days prior to surgery to reverse antiplatelet effects.23
CORRESPONDENCE
CDR Michael J. Arnold, Naval Hospital, 2080 Child Street, Jacksonville, FL 32214; [email protected].
ACKNOWLEDGEMENT
The authors thank CDR Kristian Sanchack and LCDR Dustin Smith for their assistance with this manuscript.
1. Wier LM, Steiner CA, Owens PL. Surgeries in hospital-owned outpatient facilities, 2012. HCUP Statistical Brief #188. February 2015. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb188-Surgeries-Hospital-Outpatient-Facilities-2012.jsp. Accessed September 15, 2016.
2. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol. 2014;64:e77-e137.
3. Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581-595.
4. Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a prac tical approach to screen for obstructive sleep apnea. Chest. 2016;149:631-638.
5. De Hert S, Imberger G, Carlisle J, et al. Preoperative evaluation of the adult patient undergoing non-cardiac surgery: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:684-722.
6. American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea; an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology. 2014;120:268-286.
7. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049.
8. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aide and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833-842.
9. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651-654.
10. Bouri S, Shun-Shin MJ, Cole GD, et al. Meta-analysis of secure randomized controlled trials of B-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100:456-464.
11. Mounsey A, Roque JM, Egan M. Why you shouldn’t start beta-blockers before surgery. J Fam Pract. 2014;63:E15-E16.
12. Chou R, Arora B, Dana T, et al. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:375-385.
13. Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39:967-975.
14. Pan SY, Wu VC, Huang TM, et al. Effect of preoperative statin therapy on postoperative acute kidney injury in patients undergoing major surgery: systemic review and meta-analysis. Nephrology. 2014;19:750-763.
15. Devereux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494-1503.
16. Adesanya AO, Lee W, Greilich NB, et al. Perioperative management of obstructive sleep apnea. Chest. 2010;138:1489-1498.
17. Chung F, Nagappa M, Singh M, et al. CPAP in the perioperative setting: evidence of support. Chest. 2016;149:586-597.
18. Yamashita S, Yamaguchi H, Sakaguchi M, et al. Effect of smoking on intraoperative sputum and postoperative pulmonary complication in minor surgical patients. Respir Med. 2004;98:760-766.
19. Myers K, Hajek P, Hinds
20. Lonjaret L, Lairez O, Minville V, et al. Optimal perioperative management of arterial blood pressure. Integr Blood Press Control. 2014;7:49-59.
21. Sudhakaran S, Surani SR. Guidelines for perioperative management of the diabetic patient. Surg Res Pract. 2015;2015:284063.
22. Duncan AE. Hyperglycemia and perioperative glucose management. Curr Pharm Des. 2012;18:6195-6203.
23. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S-e350S.
24. Faraoni D, Levy JH, Albaladejo P, et al. Updates in the perioperative and emergency management of non-vitamin K antagonist oral anticoagulants. Crit Care. 2015;19:203.
25. Shamoun F, Obeid H, Ramakrishna H. Novel anticoagulants in atrial fibrillation: monitoring, reversal and perioperative management. Biomed Res Int. 2015;2015:424031.
26. Kelly KN, Domajnko B. Perioperative stress-dose steroids. Clin Colon Rectal Surg. 2013;26:163-167.
27. Scanzello CR, Nestor BJ. Perioperative management of medications used in the treatment of rheumatoid arthritis. HSSJ. 2006;2:141-147.
1. Wier LM, Steiner CA, Owens PL. Surgeries in hospital-owned outpatient facilities, 2012. HCUP Statistical Brief #188. February 2015. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb188-Surgeries-Hospital-Outpatient-Facilities-2012.jsp. Accessed September 15, 2016.
2. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol. 2014;64:e77-e137.
3. Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581-595.
4. Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a prac tical approach to screen for obstructive sleep apnea. Chest. 2016;149:631-638.
5. De Hert S, Imberger G, Carlisle J, et al. Preoperative evaluation of the adult patient undergoing non-cardiac surgery: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:684-722.
6. American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea; an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology. 2014;120:268-286.
7. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049.
8. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aide and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833-842.
9. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651-654.
10. Bouri S, Shun-Shin MJ, Cole GD, et al. Meta-analysis of secure randomized controlled trials of B-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100:456-464.
11. Mounsey A, Roque JM, Egan M. Why you shouldn’t start beta-blockers before surgery. J Fam Pract. 2014;63:E15-E16.
12. Chou R, Arora B, Dana T, et al. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:375-385.
13. Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39:967-975.
14. Pan SY, Wu VC, Huang TM, et al. Effect of preoperative statin therapy on postoperative acute kidney injury in patients undergoing major surgery: systemic review and meta-analysis. Nephrology. 2014;19:750-763.
15. Devereux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494-1503.
16. Adesanya AO, Lee W, Greilich NB, et al. Perioperative management of obstructive sleep apnea. Chest. 2010;138:1489-1498.
17. Chung F, Nagappa M, Singh M, et al. CPAP in the perioperative setting: evidence of support. Chest. 2016;149:586-597.
18. Yamashita S, Yamaguchi H, Sakaguchi M, et al. Effect of smoking on intraoperative sputum and postoperative pulmonary complication in minor surgical patients. Respir Med. 2004;98:760-766.
19. Myers K, Hajek P, Hinds
20. Lonjaret L, Lairez O, Minville V, et al. Optimal perioperative management of arterial blood pressure. Integr Blood Press Control. 2014;7:49-59.
21. Sudhakaran S, Surani SR. Guidelines for perioperative management of the diabetic patient. Surg Res Pract. 2015;2015:284063.
22. Duncan AE. Hyperglycemia and perioperative glucose management. Curr Pharm Des. 2012;18:6195-6203.
23. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S-e350S.
24. Faraoni D, Levy JH, Albaladejo P, et al. Updates in the perioperative and emergency management of non-vitamin K antagonist oral anticoagulants. Crit Care. 2015;19:203.
25. Shamoun F, Obeid H, Ramakrishna H. Novel anticoagulants in atrial fibrillation: monitoring, reversal and perioperative management. Biomed Res Int. 2015;2015:424031.
26. Kelly KN, Domajnko B. Perioperative stress-dose steroids. Clin Colon Rectal Surg. 2013;26:163-167.
27. Scanzello CR, Nestor BJ. Perioperative management of medications used in the treatment of rheumatoid arthritis. HSSJ. 2006;2:141-147.