User login
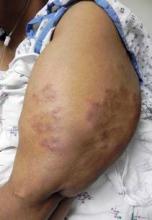
Morning Stiffness in Psoriasis Patient? Think Arthritis
SAN DIEGO – When psoriasis patients present to Dr. Abrar A. Qureshi with concomitant pain or stiffness, he routinely asks, "What is your worst time of day?"
"If patients say they experience an hour or more of profound morning stiffness, think inflammatory arthritis," Dr. Qureshi said at the annual meeting of the American Academy of Dermatology. "Some patients will be stiff in the morning for 5-15 minutes. That’s more suggestive of osteoarthritis, though there is no hard and fast rule."
Dr. Qureshi of the department of dermatology at Brigham and Women’s Hospital, Boston, also asks patients about joint swelling and if they have trouble getting into a car. His recommended physical exam consists of vitals, weight, height, nail changes, inverse psoriasis, swollen joints, warm joints, "sausage" digits, and the Schober test. "We do see patients with fatigue and malaise, occasionally low grade fever, and we always ask about TB exposure," he said.
He emphasized that dermatologists "are on the front line in the care of these patients. It behooves us to think about psoriatic arthritis, because the numbers suggest that 20%-25% of patients with psoriasis may develop psoriatic arthritis. If the diagnosis is missed, this can lead to debilitating pain, permanent joint damage, and disability."
If a psoriasis patient presents with pain or stiffness, consider inflammatory arthritis, including dactylitis and spondyloarthropathy, Dr. Qureshi said. Other differential diagnoses to consider include noninflammatory arthritis (osteoarthritis) or soft tissue problems such as enthesitis. "Osteoarthritis is probably the biggest red herring for dermatologists," he said.
In a study of patients referred by rheumatologists to the department of dermatology at Brigham and Women’s Hospital, the three most common diagnoses made by the rheumatologists prior to referral were psoriatic arthritis (41%), osteoarthritis (27%), and psoriatic arthritis plus osteoarthritis (15%).
Dr. Qureshi said that he had no relevant financial conflicts to disclose.
SAN DIEGO – When psoriasis patients present to Dr. Abrar A. Qureshi with concomitant pain or stiffness, he routinely asks, "What is your worst time of day?"
"If patients say they experience an hour or more of profound morning stiffness, think inflammatory arthritis," Dr. Qureshi said at the annual meeting of the American Academy of Dermatology. "Some patients will be stiff in the morning for 5-15 minutes. That’s more suggestive of osteoarthritis, though there is no hard and fast rule."
Dr. Qureshi of the department of dermatology at Brigham and Women’s Hospital, Boston, also asks patients about joint swelling and if they have trouble getting into a car. His recommended physical exam consists of vitals, weight, height, nail changes, inverse psoriasis, swollen joints, warm joints, "sausage" digits, and the Schober test. "We do see patients with fatigue and malaise, occasionally low grade fever, and we always ask about TB exposure," he said.
He emphasized that dermatologists "are on the front line in the care of these patients. It behooves us to think about psoriatic arthritis, because the numbers suggest that 20%-25% of patients with psoriasis may develop psoriatic arthritis. If the diagnosis is missed, this can lead to debilitating pain, permanent joint damage, and disability."
If a psoriasis patient presents with pain or stiffness, consider inflammatory arthritis, including dactylitis and spondyloarthropathy, Dr. Qureshi said. Other differential diagnoses to consider include noninflammatory arthritis (osteoarthritis) or soft tissue problems such as enthesitis. "Osteoarthritis is probably the biggest red herring for dermatologists," he said.
In a study of patients referred by rheumatologists to the department of dermatology at Brigham and Women’s Hospital, the three most common diagnoses made by the rheumatologists prior to referral were psoriatic arthritis (41%), osteoarthritis (27%), and psoriatic arthritis plus osteoarthritis (15%).
Dr. Qureshi said that he had no relevant financial conflicts to disclose.
SAN DIEGO – When psoriasis patients present to Dr. Abrar A. Qureshi with concomitant pain or stiffness, he routinely asks, "What is your worst time of day?"
"If patients say they experience an hour or more of profound morning stiffness, think inflammatory arthritis," Dr. Qureshi said at the annual meeting of the American Academy of Dermatology. "Some patients will be stiff in the morning for 5-15 minutes. That’s more suggestive of osteoarthritis, though there is no hard and fast rule."
Dr. Qureshi of the department of dermatology at Brigham and Women’s Hospital, Boston, also asks patients about joint swelling and if they have trouble getting into a car. His recommended physical exam consists of vitals, weight, height, nail changes, inverse psoriasis, swollen joints, warm joints, "sausage" digits, and the Schober test. "We do see patients with fatigue and malaise, occasionally low grade fever, and we always ask about TB exposure," he said.
He emphasized that dermatologists "are on the front line in the care of these patients. It behooves us to think about psoriatic arthritis, because the numbers suggest that 20%-25% of patients with psoriasis may develop psoriatic arthritis. If the diagnosis is missed, this can lead to debilitating pain, permanent joint damage, and disability."
If a psoriasis patient presents with pain or stiffness, consider inflammatory arthritis, including dactylitis and spondyloarthropathy, Dr. Qureshi said. Other differential diagnoses to consider include noninflammatory arthritis (osteoarthritis) or soft tissue problems such as enthesitis. "Osteoarthritis is probably the biggest red herring for dermatologists," he said.
In a study of patients referred by rheumatologists to the department of dermatology at Brigham and Women’s Hospital, the three most common diagnoses made by the rheumatologists prior to referral were psoriatic arthritis (41%), osteoarthritis (27%), and psoriatic arthritis plus osteoarthritis (15%).
Dr. Qureshi said that he had no relevant financial conflicts to disclose.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Botanicals on Par With Hydroquinone for Treating Melasma
SAN DIEGO – Expect botanicals to play an increasing role in the development of cosmeceuticals to treat melasma, Dr. Zoe D. Draelos said at the annual meeting of the American Academy of Dermatology.
"Combinations of kojic acid, emblica, and glycolic acid are now available in the cosmeceutical market," said Dr. Draelos, a clinical dermatologist and researcher in High Point, N.C. "This combination uses several mechanisms of pigment lightening, and clinical results are pretty much on par with hydroquinone."
Perhaps the most effective pigment-lightening agent in the over-the-counter market, she said, is kojic acid, a hydrophilic fungal derivative evolved from Acetobacter, Aspergillus, and Penicillium. Obtained from mushrooms, it inhibits tyrosinase by binding to copper. "Kojic acid is thought to be the most effective active [agent] behind hydroquinone," said Dr. Draelos, who is also a consulting professor of dermatology at Duke University in Durham, N.C.
Licorice extracts are also widely used for the treatment of melasma, particularly the licorice-derived flavonoids liquiritin and isoquercetin. "Glabridin is the most commonly used substance," Dr. Draelos said. "It is commonly combined with kojic acid and other active agents in order to create a sort of cocktail to multidimensionally inhibit pigment production."
Sometimes kojic acid and licorice extracts are combined with arbutin, which is obtained from the bearberry plant. "Interestingly enough, arbutin is very similar to glycosylated hydroquinone, and it has been used in Indian tribal medicine," she said. The most active form is deoxyarbutin, which is synthetic, inhibits tyrosinase, and has no cytotoxicity.
Soy, which interferes with melanosome transfer, is another natural substance that is being used to treat melasma. Fresh soy milk contains soybean trypsin inhibitor (STI), a polypeptide that decreases protease-activated receptor-2, a protein that is important in the regulation of pigmentation. STI reduces keratinocytes and decreases pigmentation.
The newcomer for the treatment of melasma is emblica, also known as Indian gooseberry. This substance is high in vitamin C and tannins, but also contains flavonoids, kaempferol, ellagic acid, and gallic acid. "It is thought that the tannins contained within emblica inhibit melanogenesis in human melanocyte cultures," Dr. Draelos said. "It is also a potent antioxidant."
She concluded her presentation by noting that although melasma is an important dermatologic need, "the depth of pigment is key to treatment success. Sunscreens, pharmaceuticals, cosmeceuticals, and surgical treatments may be used individually or in combination to treat melasma."
Dr. Draelos disclosed that she has received research consulting funds from Beiersdorf, L’Oréal, Neocutis, Neutrogena, Nu Skin, Procter & Gamble, and Syneron.
SAN DIEGO – Expect botanicals to play an increasing role in the development of cosmeceuticals to treat melasma, Dr. Zoe D. Draelos said at the annual meeting of the American Academy of Dermatology.
"Combinations of kojic acid, emblica, and glycolic acid are now available in the cosmeceutical market," said Dr. Draelos, a clinical dermatologist and researcher in High Point, N.C. "This combination uses several mechanisms of pigment lightening, and clinical results are pretty much on par with hydroquinone."
Perhaps the most effective pigment-lightening agent in the over-the-counter market, she said, is kojic acid, a hydrophilic fungal derivative evolved from Acetobacter, Aspergillus, and Penicillium. Obtained from mushrooms, it inhibits tyrosinase by binding to copper. "Kojic acid is thought to be the most effective active [agent] behind hydroquinone," said Dr. Draelos, who is also a consulting professor of dermatology at Duke University in Durham, N.C.
Licorice extracts are also widely used for the treatment of melasma, particularly the licorice-derived flavonoids liquiritin and isoquercetin. "Glabridin is the most commonly used substance," Dr. Draelos said. "It is commonly combined with kojic acid and other active agents in order to create a sort of cocktail to multidimensionally inhibit pigment production."
Sometimes kojic acid and licorice extracts are combined with arbutin, which is obtained from the bearberry plant. "Interestingly enough, arbutin is very similar to glycosylated hydroquinone, and it has been used in Indian tribal medicine," she said. The most active form is deoxyarbutin, which is synthetic, inhibits tyrosinase, and has no cytotoxicity.
Soy, which interferes with melanosome transfer, is another natural substance that is being used to treat melasma. Fresh soy milk contains soybean trypsin inhibitor (STI), a polypeptide that decreases protease-activated receptor-2, a protein that is important in the regulation of pigmentation. STI reduces keratinocytes and decreases pigmentation.
The newcomer for the treatment of melasma is emblica, also known as Indian gooseberry. This substance is high in vitamin C and tannins, but also contains flavonoids, kaempferol, ellagic acid, and gallic acid. "It is thought that the tannins contained within emblica inhibit melanogenesis in human melanocyte cultures," Dr. Draelos said. "It is also a potent antioxidant."
She concluded her presentation by noting that although melasma is an important dermatologic need, "the depth of pigment is key to treatment success. Sunscreens, pharmaceuticals, cosmeceuticals, and surgical treatments may be used individually or in combination to treat melasma."
Dr. Draelos disclosed that she has received research consulting funds from Beiersdorf, L’Oréal, Neocutis, Neutrogena, Nu Skin, Procter & Gamble, and Syneron.
SAN DIEGO – Expect botanicals to play an increasing role in the development of cosmeceuticals to treat melasma, Dr. Zoe D. Draelos said at the annual meeting of the American Academy of Dermatology.
"Combinations of kojic acid, emblica, and glycolic acid are now available in the cosmeceutical market," said Dr. Draelos, a clinical dermatologist and researcher in High Point, N.C. "This combination uses several mechanisms of pigment lightening, and clinical results are pretty much on par with hydroquinone."
Perhaps the most effective pigment-lightening agent in the over-the-counter market, she said, is kojic acid, a hydrophilic fungal derivative evolved from Acetobacter, Aspergillus, and Penicillium. Obtained from mushrooms, it inhibits tyrosinase by binding to copper. "Kojic acid is thought to be the most effective active [agent] behind hydroquinone," said Dr. Draelos, who is also a consulting professor of dermatology at Duke University in Durham, N.C.
Licorice extracts are also widely used for the treatment of melasma, particularly the licorice-derived flavonoids liquiritin and isoquercetin. "Glabridin is the most commonly used substance," Dr. Draelos said. "It is commonly combined with kojic acid and other active agents in order to create a sort of cocktail to multidimensionally inhibit pigment production."
Sometimes kojic acid and licorice extracts are combined with arbutin, which is obtained from the bearberry plant. "Interestingly enough, arbutin is very similar to glycosylated hydroquinone, and it has been used in Indian tribal medicine," she said. The most active form is deoxyarbutin, which is synthetic, inhibits tyrosinase, and has no cytotoxicity.
Soy, which interferes with melanosome transfer, is another natural substance that is being used to treat melasma. Fresh soy milk contains soybean trypsin inhibitor (STI), a polypeptide that decreases protease-activated receptor-2, a protein that is important in the regulation of pigmentation. STI reduces keratinocytes and decreases pigmentation.
The newcomer for the treatment of melasma is emblica, also known as Indian gooseberry. This substance is high in vitamin C and tannins, but also contains flavonoids, kaempferol, ellagic acid, and gallic acid. "It is thought that the tannins contained within emblica inhibit melanogenesis in human melanocyte cultures," Dr. Draelos said. "It is also a potent antioxidant."
She concluded her presentation by noting that although melasma is an important dermatologic need, "the depth of pigment is key to treatment success. Sunscreens, pharmaceuticals, cosmeceuticals, and surgical treatments may be used individually or in combination to treat melasma."
Dr. Draelos disclosed that she has received research consulting funds from Beiersdorf, L’Oréal, Neocutis, Neutrogena, Nu Skin, Procter & Gamble, and Syneron.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Propranolol Considered Standard of Care for Infantile Hemangiomas
SAN DIEGO – There are currently no Food and Drug Administration–approved treatments for infantile hemangiomas, but propranolol appears to be the current standard of care, Dr. Alfie Krol said at the annual meeting of the American Academy of Dermatology.
Since 2008, more than 200 journal articles have been published regarding the use of propranolol for hemangiomas in children, and "initial results are excellent – not just stabilization but shrinkage of the hemangioma occurs, and overall it’s safe," said Dr. Krol, professor of dermatology and pediatrics at Oregon Health and Science University in Portland. "There are some reports of hypoglycemia, which is the most common significant side effect."
Other adverse reactions reported include disturbed sleep, cool extremities, somnolence, hyperkalemia, gastrointestinal esophageal reflux disease, and dental caries. "Overall it’s been well tolerated," Dr. Krol said. "One of the concerning potential side effects is bronchospasm in patients who have a family history of asthma. We haven’t seen that in our center, but it’s something to watch out for."
The dose range is 1-3 mg/kg per day divided b.i.d. or t.i.d. The usual target dose is 2 mg/kg per day. "We don’t know the exact length of time that you need to treat," Dr. Krol said. "In younger patients, it’s likely that 6 months is the minimum because there are reports of regrowth if you discontinue prior to 6 months of active treatment. We have seen that in our center."
Dr. Krol noted that there is currently no clear consensus as to whether to initiate propranolol on an inpatient or outpatient basis. "There are some pediatric dermatologists who admit all patients to start treatment, and they usually do a rapid escalation of the dose from 1-2 mg/kg per day over 48 hours," Dr. Krol said. "Also, some centers have cardiologists see every patient and do echocardiograms on every patient prior to starting therapy. What is clear is that all patients need vital sign monitoring, EKG, cardiac auscultation, and monitoring of vital signs after initial dosing and dose escalations for a minimum of 2 hours on initial visits until the target dose is achieved."
At Oregon Health and Science University, outpatient initiation is done in the pediatric dermatology clinic in the majority of cases, "unless it’s a life- or sight-threatening lesion," Dr. Krol said. "We start at 0.5 mg/kg per day and increase weekly to 1 mg/kg per day and then 2 mg/kg per day over 2 weeks, with appropriate monitoring at each visit."
Rebound can occur, "but certainly not in all patients," he continued. "When it does occur, it often is only partial regrowth. [Propranolol] can shrink fully grown lesions that are no longer proliferating, and there seem to be site-specific effects. Periocular and parotid lesions seem to respond exceedingly well, while nasal tip lesions seem to be less responsive. Overall this drug works very well. The initial safety seems quite good, and overall [propranolol] seems to have replaced oral steroids as the initial therapy. Our comfort level and the overall efficacy of the drug would indicate that most pediatric dermatologists are using this as first-line therapy for hemangiomas of infancy."
In the largest published study to date, researchers in Spain and Argentina conducted a multicenter trial of 71 patients who received a propranolol dose of 2 mg/kg per day for at least 12 weeks (Pediatr. Dermatol. 2011;28:108-14). They found that the effect of propranolol occurred rapidly, usually within 4 weeks. The main side effect in this study was agitated sleep, which occurred in 10 of the 71 patients (14%).
The drug also appears to work beyond the proliferation phase. In a multicenter, retrospective study, researchers from eight centers enrolled 49 patients with hemangiomas that had ceased growth or patients aged 12 months or older (Pediatr. Dermatol.2011;28:94-8). A visual analog scale (VAS) of photographs was used to determine improvement.
The duration of therapy ranged from 1 to 8 months, for a mean of 3.6 months. The VAS score was reduced from 6.8 to 2.6. No significant side effects were reported, and the researchers concluded that propranolol is effective in treating hemangiomas in the postproliferative phase and should be considered first-line therapy in that setting.
In another published study conducted at the University of Miami and Miami Children’s Hospital, researchers compared the use of propranolol with corticosteroids in 110 patients with infantile hemangiomas (Arch. Dermatol. 2011;147:1371-6). They found that 82% of patients in the propranolol group improved by 75% or more, compared with 29% of patients in the steroid group. "That’s a significant improvement over the use of steroids alone," Dr. Krol commented.
One patient on propranolol had hypoglycemia, but all patients in the steroid group had at least one adverse event. The researchers also found propranolol therapy was about half the cost of steroid therapy ($205 for 7.9 months vs. $416 for 5.2 months, respectively). They concluded that propranolol should be considered a first-line therapy for infantile hemangiomas.
He said that larger, long-term studies "are needed to identify any potentially serious as well as long-term safety questions."
Dr. Krol disclosed that he is a paid investigator for Pierre Fabre.
SAN DIEGO – There are currently no Food and Drug Administration–approved treatments for infantile hemangiomas, but propranolol appears to be the current standard of care, Dr. Alfie Krol said at the annual meeting of the American Academy of Dermatology.
Since 2008, more than 200 journal articles have been published regarding the use of propranolol for hemangiomas in children, and "initial results are excellent – not just stabilization but shrinkage of the hemangioma occurs, and overall it’s safe," said Dr. Krol, professor of dermatology and pediatrics at Oregon Health and Science University in Portland. "There are some reports of hypoglycemia, which is the most common significant side effect."
Other adverse reactions reported include disturbed sleep, cool extremities, somnolence, hyperkalemia, gastrointestinal esophageal reflux disease, and dental caries. "Overall it’s been well tolerated," Dr. Krol said. "One of the concerning potential side effects is bronchospasm in patients who have a family history of asthma. We haven’t seen that in our center, but it’s something to watch out for."
The dose range is 1-3 mg/kg per day divided b.i.d. or t.i.d. The usual target dose is 2 mg/kg per day. "We don’t know the exact length of time that you need to treat," Dr. Krol said. "In younger patients, it’s likely that 6 months is the minimum because there are reports of regrowth if you discontinue prior to 6 months of active treatment. We have seen that in our center."
Dr. Krol noted that there is currently no clear consensus as to whether to initiate propranolol on an inpatient or outpatient basis. "There are some pediatric dermatologists who admit all patients to start treatment, and they usually do a rapid escalation of the dose from 1-2 mg/kg per day over 48 hours," Dr. Krol said. "Also, some centers have cardiologists see every patient and do echocardiograms on every patient prior to starting therapy. What is clear is that all patients need vital sign monitoring, EKG, cardiac auscultation, and monitoring of vital signs after initial dosing and dose escalations for a minimum of 2 hours on initial visits until the target dose is achieved."
At Oregon Health and Science University, outpatient initiation is done in the pediatric dermatology clinic in the majority of cases, "unless it’s a life- or sight-threatening lesion," Dr. Krol said. "We start at 0.5 mg/kg per day and increase weekly to 1 mg/kg per day and then 2 mg/kg per day over 2 weeks, with appropriate monitoring at each visit."
Rebound can occur, "but certainly not in all patients," he continued. "When it does occur, it often is only partial regrowth. [Propranolol] can shrink fully grown lesions that are no longer proliferating, and there seem to be site-specific effects. Periocular and parotid lesions seem to respond exceedingly well, while nasal tip lesions seem to be less responsive. Overall this drug works very well. The initial safety seems quite good, and overall [propranolol] seems to have replaced oral steroids as the initial therapy. Our comfort level and the overall efficacy of the drug would indicate that most pediatric dermatologists are using this as first-line therapy for hemangiomas of infancy."
In the largest published study to date, researchers in Spain and Argentina conducted a multicenter trial of 71 patients who received a propranolol dose of 2 mg/kg per day for at least 12 weeks (Pediatr. Dermatol. 2011;28:108-14). They found that the effect of propranolol occurred rapidly, usually within 4 weeks. The main side effect in this study was agitated sleep, which occurred in 10 of the 71 patients (14%).
The drug also appears to work beyond the proliferation phase. In a multicenter, retrospective study, researchers from eight centers enrolled 49 patients with hemangiomas that had ceased growth or patients aged 12 months or older (Pediatr. Dermatol.2011;28:94-8). A visual analog scale (VAS) of photographs was used to determine improvement.
The duration of therapy ranged from 1 to 8 months, for a mean of 3.6 months. The VAS score was reduced from 6.8 to 2.6. No significant side effects were reported, and the researchers concluded that propranolol is effective in treating hemangiomas in the postproliferative phase and should be considered first-line therapy in that setting.
In another published study conducted at the University of Miami and Miami Children’s Hospital, researchers compared the use of propranolol with corticosteroids in 110 patients with infantile hemangiomas (Arch. Dermatol. 2011;147:1371-6). They found that 82% of patients in the propranolol group improved by 75% or more, compared with 29% of patients in the steroid group. "That’s a significant improvement over the use of steroids alone," Dr. Krol commented.
One patient on propranolol had hypoglycemia, but all patients in the steroid group had at least one adverse event. The researchers also found propranolol therapy was about half the cost of steroid therapy ($205 for 7.9 months vs. $416 for 5.2 months, respectively). They concluded that propranolol should be considered a first-line therapy for infantile hemangiomas.
He said that larger, long-term studies "are needed to identify any potentially serious as well as long-term safety questions."
Dr. Krol disclosed that he is a paid investigator for Pierre Fabre.
SAN DIEGO – There are currently no Food and Drug Administration–approved treatments for infantile hemangiomas, but propranolol appears to be the current standard of care, Dr. Alfie Krol said at the annual meeting of the American Academy of Dermatology.
Since 2008, more than 200 journal articles have been published regarding the use of propranolol for hemangiomas in children, and "initial results are excellent – not just stabilization but shrinkage of the hemangioma occurs, and overall it’s safe," said Dr. Krol, professor of dermatology and pediatrics at Oregon Health and Science University in Portland. "There are some reports of hypoglycemia, which is the most common significant side effect."
Other adverse reactions reported include disturbed sleep, cool extremities, somnolence, hyperkalemia, gastrointestinal esophageal reflux disease, and dental caries. "Overall it’s been well tolerated," Dr. Krol said. "One of the concerning potential side effects is bronchospasm in patients who have a family history of asthma. We haven’t seen that in our center, but it’s something to watch out for."
The dose range is 1-3 mg/kg per day divided b.i.d. or t.i.d. The usual target dose is 2 mg/kg per day. "We don’t know the exact length of time that you need to treat," Dr. Krol said. "In younger patients, it’s likely that 6 months is the minimum because there are reports of regrowth if you discontinue prior to 6 months of active treatment. We have seen that in our center."
Dr. Krol noted that there is currently no clear consensus as to whether to initiate propranolol on an inpatient or outpatient basis. "There are some pediatric dermatologists who admit all patients to start treatment, and they usually do a rapid escalation of the dose from 1-2 mg/kg per day over 48 hours," Dr. Krol said. "Also, some centers have cardiologists see every patient and do echocardiograms on every patient prior to starting therapy. What is clear is that all patients need vital sign monitoring, EKG, cardiac auscultation, and monitoring of vital signs after initial dosing and dose escalations for a minimum of 2 hours on initial visits until the target dose is achieved."
At Oregon Health and Science University, outpatient initiation is done in the pediatric dermatology clinic in the majority of cases, "unless it’s a life- or sight-threatening lesion," Dr. Krol said. "We start at 0.5 mg/kg per day and increase weekly to 1 mg/kg per day and then 2 mg/kg per day over 2 weeks, with appropriate monitoring at each visit."
Rebound can occur, "but certainly not in all patients," he continued. "When it does occur, it often is only partial regrowth. [Propranolol] can shrink fully grown lesions that are no longer proliferating, and there seem to be site-specific effects. Periocular and parotid lesions seem to respond exceedingly well, while nasal tip lesions seem to be less responsive. Overall this drug works very well. The initial safety seems quite good, and overall [propranolol] seems to have replaced oral steroids as the initial therapy. Our comfort level and the overall efficacy of the drug would indicate that most pediatric dermatologists are using this as first-line therapy for hemangiomas of infancy."
In the largest published study to date, researchers in Spain and Argentina conducted a multicenter trial of 71 patients who received a propranolol dose of 2 mg/kg per day for at least 12 weeks (Pediatr. Dermatol. 2011;28:108-14). They found that the effect of propranolol occurred rapidly, usually within 4 weeks. The main side effect in this study was agitated sleep, which occurred in 10 of the 71 patients (14%).
The drug also appears to work beyond the proliferation phase. In a multicenter, retrospective study, researchers from eight centers enrolled 49 patients with hemangiomas that had ceased growth or patients aged 12 months or older (Pediatr. Dermatol.2011;28:94-8). A visual analog scale (VAS) of photographs was used to determine improvement.
The duration of therapy ranged from 1 to 8 months, for a mean of 3.6 months. The VAS score was reduced from 6.8 to 2.6. No significant side effects were reported, and the researchers concluded that propranolol is effective in treating hemangiomas in the postproliferative phase and should be considered first-line therapy in that setting.
In another published study conducted at the University of Miami and Miami Children’s Hospital, researchers compared the use of propranolol with corticosteroids in 110 patients with infantile hemangiomas (Arch. Dermatol. 2011;147:1371-6). They found that 82% of patients in the propranolol group improved by 75% or more, compared with 29% of patients in the steroid group. "That’s a significant improvement over the use of steroids alone," Dr. Krol commented.
One patient on propranolol had hypoglycemia, but all patients in the steroid group had at least one adverse event. The researchers also found propranolol therapy was about half the cost of steroid therapy ($205 for 7.9 months vs. $416 for 5.2 months, respectively). They concluded that propranolol should be considered a first-line therapy for infantile hemangiomas.
He said that larger, long-term studies "are needed to identify any potentially serious as well as long-term safety questions."
Dr. Krol disclosed that he is a paid investigator for Pierre Fabre.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Psoriasis Not Independent Cardiac Risk Factor, Study Finds
SAN DIEGO – A large study has determined that psoriasis is not an independent risk factor for ischemic heart disease, based on Framingham Risk Scores.
While previous studies have demonstrated that cardiac mortality is increased in patients with psoriasis, it is also known that people with the skin condition have a higher prevalence of smoking, alcohol consumption, obesity, diabetes, and dyslipidemia, researchers reported in a poster session at the annual meeting of the American Academy of Dermatology.
"Is this increase in ischemic heart disease due to traditional risk factors, or is psoriasis an additional independent risk factor?" wrote the investigators, who were led by Dr. Marian T. McEvoy, in the abstract.
They performed a population-based analysis of 1,338 adults with psoriasis who resided in Olmstead County, Minn., between 1998 and 2008 to evaluate the validity of the Framingham Risk Score (FRS) in predicting the incidence of ischemic heart disease in the study cohort. The FRS is a validated measure of standard ischemic heart disease risk factors.
Dr. McEvoy, a dermatologist at the Mayo Clinic in Rochester, Minn., and her associates compared the risk of cardiac death and myocardial infarction based on the FRS with the actual incidence of myocardial infarction and cardiac death in the study population, which was limited to patients older than age 30 but younger than age 80. They used Poisson regression models and standardized incidence ratios for statistical analysis.
The researchers reported that full Framingham risk factors were available for 974 of the 1,338 patients (73%). They predicted that the median 10-year risk of cardiac events based on the FRS was 3.8%, while the observed 10-year risk of cardiac events was 5.5%. However, there were 44 observed cardiac events compared with 47.7 FRS-predicted cardiac events, which translated into a standardized incidence ratio between the two groups of 0.9. Standardized incidence ratios also showed no statistically significant differences between the two groups when analyzed by gender, by age greater than 65 years, by age less than 65 years, and by whether patients were receiving systemic treatment or not.
"If psoriasis was an independent risk factor for ischemic heart disease, the observed incidence of cardiac events would have been in excess of predicted," the researchers wrote in their poster. "Since there was no statistical difference between actual and predicted events, psoriasis is not an independent risk factor for ischemic heart disease."
In a later interview, Dr. McEvoy acknowledged certain limitations of the study, including the fact that the small number of observed cardiac events (44) limited the statistical power of the study. "Since this is a retrospective study, we did not have a good tool to assess severity of psoriasis," she said. "We used surrogates based on therapy to identify those with ‘more severe disease.’ The Framingham Risk Score was valid for this group also."
The study was partially funded by a grant from Pfizer, and was made possible by a grant from Amgen/Wyeth and by the Rochester Epidemiology Project.
SAN DIEGO – A large study has determined that psoriasis is not an independent risk factor for ischemic heart disease, based on Framingham Risk Scores.
While previous studies have demonstrated that cardiac mortality is increased in patients with psoriasis, it is also known that people with the skin condition have a higher prevalence of smoking, alcohol consumption, obesity, diabetes, and dyslipidemia, researchers reported in a poster session at the annual meeting of the American Academy of Dermatology.
"Is this increase in ischemic heart disease due to traditional risk factors, or is psoriasis an additional independent risk factor?" wrote the investigators, who were led by Dr. Marian T. McEvoy, in the abstract.
They performed a population-based analysis of 1,338 adults with psoriasis who resided in Olmstead County, Minn., between 1998 and 2008 to evaluate the validity of the Framingham Risk Score (FRS) in predicting the incidence of ischemic heart disease in the study cohort. The FRS is a validated measure of standard ischemic heart disease risk factors.
Dr. McEvoy, a dermatologist at the Mayo Clinic in Rochester, Minn., and her associates compared the risk of cardiac death and myocardial infarction based on the FRS with the actual incidence of myocardial infarction and cardiac death in the study population, which was limited to patients older than age 30 but younger than age 80. They used Poisson regression models and standardized incidence ratios for statistical analysis.
The researchers reported that full Framingham risk factors were available for 974 of the 1,338 patients (73%). They predicted that the median 10-year risk of cardiac events based on the FRS was 3.8%, while the observed 10-year risk of cardiac events was 5.5%. However, there were 44 observed cardiac events compared with 47.7 FRS-predicted cardiac events, which translated into a standardized incidence ratio between the two groups of 0.9. Standardized incidence ratios also showed no statistically significant differences between the two groups when analyzed by gender, by age greater than 65 years, by age less than 65 years, and by whether patients were receiving systemic treatment or not.
"If psoriasis was an independent risk factor for ischemic heart disease, the observed incidence of cardiac events would have been in excess of predicted," the researchers wrote in their poster. "Since there was no statistical difference between actual and predicted events, psoriasis is not an independent risk factor for ischemic heart disease."
In a later interview, Dr. McEvoy acknowledged certain limitations of the study, including the fact that the small number of observed cardiac events (44) limited the statistical power of the study. "Since this is a retrospective study, we did not have a good tool to assess severity of psoriasis," she said. "We used surrogates based on therapy to identify those with ‘more severe disease.’ The Framingham Risk Score was valid for this group also."
The study was partially funded by a grant from Pfizer, and was made possible by a grant from Amgen/Wyeth and by the Rochester Epidemiology Project.
SAN DIEGO – A large study has determined that psoriasis is not an independent risk factor for ischemic heart disease, based on Framingham Risk Scores.
While previous studies have demonstrated that cardiac mortality is increased in patients with psoriasis, it is also known that people with the skin condition have a higher prevalence of smoking, alcohol consumption, obesity, diabetes, and dyslipidemia, researchers reported in a poster session at the annual meeting of the American Academy of Dermatology.
"Is this increase in ischemic heart disease due to traditional risk factors, or is psoriasis an additional independent risk factor?" wrote the investigators, who were led by Dr. Marian T. McEvoy, in the abstract.
They performed a population-based analysis of 1,338 adults with psoriasis who resided in Olmstead County, Minn., between 1998 and 2008 to evaluate the validity of the Framingham Risk Score (FRS) in predicting the incidence of ischemic heart disease in the study cohort. The FRS is a validated measure of standard ischemic heart disease risk factors.
Dr. McEvoy, a dermatologist at the Mayo Clinic in Rochester, Minn., and her associates compared the risk of cardiac death and myocardial infarction based on the FRS with the actual incidence of myocardial infarction and cardiac death in the study population, which was limited to patients older than age 30 but younger than age 80. They used Poisson regression models and standardized incidence ratios for statistical analysis.
The researchers reported that full Framingham risk factors were available for 974 of the 1,338 patients (73%). They predicted that the median 10-year risk of cardiac events based on the FRS was 3.8%, while the observed 10-year risk of cardiac events was 5.5%. However, there were 44 observed cardiac events compared with 47.7 FRS-predicted cardiac events, which translated into a standardized incidence ratio between the two groups of 0.9. Standardized incidence ratios also showed no statistically significant differences between the two groups when analyzed by gender, by age greater than 65 years, by age less than 65 years, and by whether patients were receiving systemic treatment or not.
"If psoriasis was an independent risk factor for ischemic heart disease, the observed incidence of cardiac events would have been in excess of predicted," the researchers wrote in their poster. "Since there was no statistical difference between actual and predicted events, psoriasis is not an independent risk factor for ischemic heart disease."
In a later interview, Dr. McEvoy acknowledged certain limitations of the study, including the fact that the small number of observed cardiac events (44) limited the statistical power of the study. "Since this is a retrospective study, we did not have a good tool to assess severity of psoriasis," she said. "We used surrogates based on therapy to identify those with ‘more severe disease.’ The Framingham Risk Score was valid for this group also."
The study was partially funded by a grant from Pfizer, and was made possible by a grant from Amgen/Wyeth and by the Rochester Epidemiology Project.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
New Results Challenge Laser Effectiveness for Onychomycosis
SAN DIEGO – Although previous studies of 1,064-nm Nd:YAG lasers for the treatment of onychomycosis have reported significant mycological cure rates, results from a newer, ongoing study challenge such findings.
At the annual meeting of the American Academy of Dermatology, Dr. Boni E. Elewski reported findings from a trial in which she and her colleagues set out to answer two questions: Can fungal organisms be killed by heat at temperatures tolerable to patients? And, can a laser be directly fungicidal on a growing fungal colony or dilute suspension?
Dr. Elewski and her colleagues conducted a heat kill study of three fungi: Trichophyton rubrum, Epidermophyton floccosum, and Scytalidium.
For T. rubrum, they found that growth was halted after the nail was treated at 50° C for 15 minutes. For E. floccosum, they found that growth was halted after the nail was treated at 50° C for 10 minutes, and for Scytalidium, growth was halted after the nail was treated at 55° C for 5 minutes. "However, nail temperatures that reach 40-41° C cause enough pain for patients to pull away, and the maximum nail temperature patients could tolerate was 45° C," said Dr. Elewski, professor of dermatology at the University of Alabama at Birmingham.
In part two of the study, the researchers used a 1,064-nm Nd:YAG laser to treat colonies and dilute solutions before growth occurred. They used various laser parameters, including spot sizes ranging from 3 to 5 mm, pulse durations that ranged from 100 to 300 milliseconds, fluences of 15 to 50 J/cm2, and frequencies of 2 to 10 Hz. "We found that there was no effect on fungal growth at numerous settings, and the temperature of the agar plate reached about 40° C," Dr. Elewski said. "We decided to take this knowledge and move it into our clinical practice."
In an ongoing study that has enrolled 10 patients to date, she and her associates use a 1,064-nm Nd:YAG laser for a protocol that involves five treatments at settings of 16 J/cm2, 0.3 microseconds, and 2 Hz. They use a 5-mm spot size with more than 300 pulses over the nail in a predetermined pattern. "You see some clearance," Dr. Elewski said of the results so far. "We have no mycological cures to date, but improvement as shown by other companies is noted. Some improvement may be seen, but in my opinion a durable response is needed to satisfy patients."
In a 2010 study from Yugoslavia, mycological cures were reported using a fluence of 35-40 J/cm2, Dr. Elewski said, "but our patients could not tolerate anything above 16 J/cm2, at least without a digital block. All I can assume is that patients in Yugoslavia are significantly heartier than patients in Alabama" (J. Laser and Health Academy 2010;1:1-8).
She concluded her remarks by noting that photodynamic therapy "may be an option for your patients who have failed other treatments or who could not tolerate another treatment. Laser treatment is still under investigation, but might be desirable for those who would be satisfied with an improvement."
Dr. Elewski disclosed that Cutera provided research funds for the study. She said that she had no financial conflicts of interest to disclose, and emphasized that she has never been a paid consultant to any laser device company.
SAN DIEGO – Although previous studies of 1,064-nm Nd:YAG lasers for the treatment of onychomycosis have reported significant mycological cure rates, results from a newer, ongoing study challenge such findings.
At the annual meeting of the American Academy of Dermatology, Dr. Boni E. Elewski reported findings from a trial in which she and her colleagues set out to answer two questions: Can fungal organisms be killed by heat at temperatures tolerable to patients? And, can a laser be directly fungicidal on a growing fungal colony or dilute suspension?
Dr. Elewski and her colleagues conducted a heat kill study of three fungi: Trichophyton rubrum, Epidermophyton floccosum, and Scytalidium.
For T. rubrum, they found that growth was halted after the nail was treated at 50° C for 15 minutes. For E. floccosum, they found that growth was halted after the nail was treated at 50° C for 10 minutes, and for Scytalidium, growth was halted after the nail was treated at 55° C for 5 minutes. "However, nail temperatures that reach 40-41° C cause enough pain for patients to pull away, and the maximum nail temperature patients could tolerate was 45° C," said Dr. Elewski, professor of dermatology at the University of Alabama at Birmingham.
In part two of the study, the researchers used a 1,064-nm Nd:YAG laser to treat colonies and dilute solutions before growth occurred. They used various laser parameters, including spot sizes ranging from 3 to 5 mm, pulse durations that ranged from 100 to 300 milliseconds, fluences of 15 to 50 J/cm2, and frequencies of 2 to 10 Hz. "We found that there was no effect on fungal growth at numerous settings, and the temperature of the agar plate reached about 40° C," Dr. Elewski said. "We decided to take this knowledge and move it into our clinical practice."
In an ongoing study that has enrolled 10 patients to date, she and her associates use a 1,064-nm Nd:YAG laser for a protocol that involves five treatments at settings of 16 J/cm2, 0.3 microseconds, and 2 Hz. They use a 5-mm spot size with more than 300 pulses over the nail in a predetermined pattern. "You see some clearance," Dr. Elewski said of the results so far. "We have no mycological cures to date, but improvement as shown by other companies is noted. Some improvement may be seen, but in my opinion a durable response is needed to satisfy patients."
In a 2010 study from Yugoslavia, mycological cures were reported using a fluence of 35-40 J/cm2, Dr. Elewski said, "but our patients could not tolerate anything above 16 J/cm2, at least without a digital block. All I can assume is that patients in Yugoslavia are significantly heartier than patients in Alabama" (J. Laser and Health Academy 2010;1:1-8).
She concluded her remarks by noting that photodynamic therapy "may be an option for your patients who have failed other treatments or who could not tolerate another treatment. Laser treatment is still under investigation, but might be desirable for those who would be satisfied with an improvement."
Dr. Elewski disclosed that Cutera provided research funds for the study. She said that she had no financial conflicts of interest to disclose, and emphasized that she has never been a paid consultant to any laser device company.
SAN DIEGO – Although previous studies of 1,064-nm Nd:YAG lasers for the treatment of onychomycosis have reported significant mycological cure rates, results from a newer, ongoing study challenge such findings.
At the annual meeting of the American Academy of Dermatology, Dr. Boni E. Elewski reported findings from a trial in which she and her colleagues set out to answer two questions: Can fungal organisms be killed by heat at temperatures tolerable to patients? And, can a laser be directly fungicidal on a growing fungal colony or dilute suspension?
Dr. Elewski and her colleagues conducted a heat kill study of three fungi: Trichophyton rubrum, Epidermophyton floccosum, and Scytalidium.
For T. rubrum, they found that growth was halted after the nail was treated at 50° C for 15 minutes. For E. floccosum, they found that growth was halted after the nail was treated at 50° C for 10 minutes, and for Scytalidium, growth was halted after the nail was treated at 55° C for 5 minutes. "However, nail temperatures that reach 40-41° C cause enough pain for patients to pull away, and the maximum nail temperature patients could tolerate was 45° C," said Dr. Elewski, professor of dermatology at the University of Alabama at Birmingham.
In part two of the study, the researchers used a 1,064-nm Nd:YAG laser to treat colonies and dilute solutions before growth occurred. They used various laser parameters, including spot sizes ranging from 3 to 5 mm, pulse durations that ranged from 100 to 300 milliseconds, fluences of 15 to 50 J/cm2, and frequencies of 2 to 10 Hz. "We found that there was no effect on fungal growth at numerous settings, and the temperature of the agar plate reached about 40° C," Dr. Elewski said. "We decided to take this knowledge and move it into our clinical practice."
In an ongoing study that has enrolled 10 patients to date, she and her associates use a 1,064-nm Nd:YAG laser for a protocol that involves five treatments at settings of 16 J/cm2, 0.3 microseconds, and 2 Hz. They use a 5-mm spot size with more than 300 pulses over the nail in a predetermined pattern. "You see some clearance," Dr. Elewski said of the results so far. "We have no mycological cures to date, but improvement as shown by other companies is noted. Some improvement may be seen, but in my opinion a durable response is needed to satisfy patients."
In a 2010 study from Yugoslavia, mycological cures were reported using a fluence of 35-40 J/cm2, Dr. Elewski said, "but our patients could not tolerate anything above 16 J/cm2, at least without a digital block. All I can assume is that patients in Yugoslavia are significantly heartier than patients in Alabama" (J. Laser and Health Academy 2010;1:1-8).
She concluded her remarks by noting that photodynamic therapy "may be an option for your patients who have failed other treatments or who could not tolerate another treatment. Laser treatment is still under investigation, but might be desirable for those who would be satisfied with an improvement."
Dr. Elewski disclosed that Cutera provided research funds for the study. She said that she had no financial conflicts of interest to disclose, and emphasized that she has never been a paid consultant to any laser device company.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
When to Consider PCOS in Female Acne Patients
SAN DIEGO – Correctly diagnosed and adequately treated polycystic ovary syndrome can appreciably improve acne in a select group of female patients, according to Dr. Anne W. Lucky.
Dr. Lucky offered tips on evaluating and treating acne and PCOS at the annual meeting of the American Academy of Dermatology. She said that PCOS should be considered in female patients with early onset acne, acne that is refractory to conventional therapy, relapse that occurs after treatment with isotretinoin, persistence of acne beyond adolescence, or late onset of acne, she said.
Several laboratory tests are typically used to diagnose PCOS but agreement on which tests to use is limited. "This is somewhat controversial," said Dr. Lucky, a professor of dermatology at the University of Cincinnati. She recommended the following tests: free testosterone, DHEAS (dehydroepiandrosterone sulfate), and the ratio of LH (luteinizing hormone) to FSH (follicle-stimulating hormone). "If I have a good suspicion or find abnormalities – [I include] fasting glucose and insulin and a fasting lipid profile," she said.
Treatment options include combination oral contraceptives, glucocorticoids (for specific adrenal abnormalities), and anti-androgens among others.
"By and large, oral contraceptives are the No. 1 choice. I mentioned combination contraceptives because it’s the estrogen that’s helpful. Progesterone-only contraceptives can actually worsen acne," Dr. Lucky said. "We don’t treat girls before menarche because they don’t have a risk for pregnancy and we might influence bone growth if we treat too early. You can suspect PCOS very early but you may have to suspend your treatment until they get a little older."
Using contraceptives – especially in girls or adolescents -- usually involves discussions with a gynecologist, a parent, and the child. "Our biggest hurdle is that the teenage population has heard the word on the street that the pill is going to make them fat and they don’t want to take it. Actually [the gynecologists that I work with] tell me that the studies show that some children gain weight, some lose weight, but most stay the same weight with the combination pills," she said.
In the United States, spironolactone is the only antiandrogen treatment option. It works by being a competitive inhibitor of androgen receptors. The drug does not lower serum androgen levels, but it prevents the action of androgen – increasing estrogenicity. However, the use of spironolactone for PCOS is considered off label. The drug is indicated for the treatment of hypertension and the label carries a warning that it should only be used for this indication, Dr. Lucky noted.
Dr. Lucky reported that she has financial relationships with Amgen, Galderma, and Johnson & Johnson.
SAN DIEGO – Correctly diagnosed and adequately treated polycystic ovary syndrome can appreciably improve acne in a select group of female patients, according to Dr. Anne W. Lucky.
Dr. Lucky offered tips on evaluating and treating acne and PCOS at the annual meeting of the American Academy of Dermatology. She said that PCOS should be considered in female patients with early onset acne, acne that is refractory to conventional therapy, relapse that occurs after treatment with isotretinoin, persistence of acne beyond adolescence, or late onset of acne, she said.
Several laboratory tests are typically used to diagnose PCOS but agreement on which tests to use is limited. "This is somewhat controversial," said Dr. Lucky, a professor of dermatology at the University of Cincinnati. She recommended the following tests: free testosterone, DHEAS (dehydroepiandrosterone sulfate), and the ratio of LH (luteinizing hormone) to FSH (follicle-stimulating hormone). "If I have a good suspicion or find abnormalities – [I include] fasting glucose and insulin and a fasting lipid profile," she said.
Treatment options include combination oral contraceptives, glucocorticoids (for specific adrenal abnormalities), and anti-androgens among others.
"By and large, oral contraceptives are the No. 1 choice. I mentioned combination contraceptives because it’s the estrogen that’s helpful. Progesterone-only contraceptives can actually worsen acne," Dr. Lucky said. "We don’t treat girls before menarche because they don’t have a risk for pregnancy and we might influence bone growth if we treat too early. You can suspect PCOS very early but you may have to suspend your treatment until they get a little older."
Using contraceptives – especially in girls or adolescents -- usually involves discussions with a gynecologist, a parent, and the child. "Our biggest hurdle is that the teenage population has heard the word on the street that the pill is going to make them fat and they don’t want to take it. Actually [the gynecologists that I work with] tell me that the studies show that some children gain weight, some lose weight, but most stay the same weight with the combination pills," she said.
In the United States, spironolactone is the only antiandrogen treatment option. It works by being a competitive inhibitor of androgen receptors. The drug does not lower serum androgen levels, but it prevents the action of androgen – increasing estrogenicity. However, the use of spironolactone for PCOS is considered off label. The drug is indicated for the treatment of hypertension and the label carries a warning that it should only be used for this indication, Dr. Lucky noted.
Dr. Lucky reported that she has financial relationships with Amgen, Galderma, and Johnson & Johnson.
SAN DIEGO – Correctly diagnosed and adequately treated polycystic ovary syndrome can appreciably improve acne in a select group of female patients, according to Dr. Anne W. Lucky.
Dr. Lucky offered tips on evaluating and treating acne and PCOS at the annual meeting of the American Academy of Dermatology. She said that PCOS should be considered in female patients with early onset acne, acne that is refractory to conventional therapy, relapse that occurs after treatment with isotretinoin, persistence of acne beyond adolescence, or late onset of acne, she said.
Several laboratory tests are typically used to diagnose PCOS but agreement on which tests to use is limited. "This is somewhat controversial," said Dr. Lucky, a professor of dermatology at the University of Cincinnati. She recommended the following tests: free testosterone, DHEAS (dehydroepiandrosterone sulfate), and the ratio of LH (luteinizing hormone) to FSH (follicle-stimulating hormone). "If I have a good suspicion or find abnormalities – [I include] fasting glucose and insulin and a fasting lipid profile," she said.
Treatment options include combination oral contraceptives, glucocorticoids (for specific adrenal abnormalities), and anti-androgens among others.
"By and large, oral contraceptives are the No. 1 choice. I mentioned combination contraceptives because it’s the estrogen that’s helpful. Progesterone-only contraceptives can actually worsen acne," Dr. Lucky said. "We don’t treat girls before menarche because they don’t have a risk for pregnancy and we might influence bone growth if we treat too early. You can suspect PCOS very early but you may have to suspend your treatment until they get a little older."
Using contraceptives – especially in girls or adolescents -- usually involves discussions with a gynecologist, a parent, and the child. "Our biggest hurdle is that the teenage population has heard the word on the street that the pill is going to make them fat and they don’t want to take it. Actually [the gynecologists that I work with] tell me that the studies show that some children gain weight, some lose weight, but most stay the same weight with the combination pills," she said.
In the United States, spironolactone is the only antiandrogen treatment option. It works by being a competitive inhibitor of androgen receptors. The drug does not lower serum androgen levels, but it prevents the action of androgen – increasing estrogenicity. However, the use of spironolactone for PCOS is considered off label. The drug is indicated for the treatment of hypertension and the label carries a warning that it should only be used for this indication, Dr. Lucky noted.
Dr. Lucky reported that she has financial relationships with Amgen, Galderma, and Johnson & Johnson.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Thorough Work-Up Crucial in Sarcoidosis Cases
SAN DIEGO – While it’s well known that sarcoidosis commonly affects pulmonary function, it’s perhaps less known that the disorder can be detrimental to cardiac function in approximately 5% of cases.
"A common way that patients present with cardiac sarcoidosis is with sudden cardiac death," Dr. Misha Rosenbach said at the annual meeting of the American Academy of Dermatology. "This is a terrible way to present to your doctor with a problem."
A multisystem disorder of unknown cause, sarcoidosis commonly affects young and middle-aged adults and frequently presents with bilateral hilar lymphadenopathy, pulmonary infiltration, and ocular and skin lesions. Other organs may be involved. The diagnosis is established when clinicoradiologic findings are supported by histologic evidence of noncaseating epithelioid cell granulomas.
"Sarcoidosis is primarily a pulmonary disease, but patients can also present with profound systemic symptoms," said Dr. Rosenbach of the departments of dermatology and internal medicine at the University of Pennsylvania, Philadelphia. "When you’re evaluating a patient with cutaneous sarcoidosis, and making a diagnosis of granulomatous disease of the skin, and looking for extracutaneous involvement, it’s important to know what else can be affected."
Although pulmonary function is affected in more than 90% of cases, other commonly affected sites include the eyes (25%-50% of cases), lymph nodes (about 33% of cases), musculoskeletal system (25%-40% of cases), endocrine system (10%-25% of cases), and liver (20%-50% of cases). The initial evaluation should consist of history and physical exam; chest x-ray; pulmonary function tests (including carbon monoxide diffusing capacity); ophthalmologic examination; complete blood count and serum chemistries (including calcium); urinalysis; EKG (plus additional testing if there is a history of palpitations); tuberculin skin test (TST) or interferon (IFN)–gamma release assay; and thyroid and vitamin D testing.
"Patients with sarcoidosis often have low levels of 25-hydroxyvitamin D, but elevated levels of 1,25-dihydroxyvitamin D3," Dr. Rosenbach said. "Inappropriate supplementation can lead to hypercalcemia."
For latent tuberculosis testing, he pointed out that the IFN-gamma release assay (IGRA) is thought to be more accurate than the TST. "IGRA significantly reduces false-positive results" in bacille Calmette-Guérin–vaccinated patients, said Dr. Rosenbach, who is also director of the cutaneous sarcoidosis clinic at the University of Pennsylvania. "Cost-benefit analyses suggest that IGRA [is] cost equivalent to TST, and the Centers for Disease Control and Prevention recommends that IGRA may be used in all circumstances in which the TST is currently used. However, both TST and IGRA have decreased responsiveness and lower sensitivity in patients with impaired immune systems."
In terms of the impact of sarcoidosis on the thyroid gland, a recent analysis of a large database in the United Kingdom found that hyper- and hypothyroidism were twice as common in patients with sarcoidosis, compared with a control population (Postgrad. Med. J. 2009;85:233-7).
A more recent study of 50 patients with cutaneous sarcoidosis conducted by Dr. Rosenbach and his colleagues found that 25% of patients had abnormal thyroid laboratory test results (J. Am. Acad. Dermatol. 2012;66:167-8).
The precise association between sarcoidosis and malignancy remains unclear, he said, but the best available studies suggest that the incidence of lymphoproliferative disorder may be increased in patients with sarcoidosis. Other granulomatous dermatitides may be associated with hematologic abnormalities. Authors of one review found that granulomatous dermatitides may be the first sign of underlying myelodysplastic syndrome (MDS), and recommended that clinicians consider looking for underlying MDS in patients with unexplained or atypical granulomatous skin eruptions (Arch. Dermatol. 2011;147:331-5).
A common stepwise approach for treating patients, Dr. Rosenbach said, begins with skin-directed therapies in the form of steroids or injections. The second step involves the use of antimalarials and tetracycline-class antibiotics; the third step involves methotrexate and/or prednisone, and the fourth step involves consideration for treatment with infliximab or adalimumab. "At this point, etanercept should probably not be used," Dr. Rosenbach said. "It appears to be less effective, and in a few reports has been associated with worsening of disease."
The data are strongest for infliximab, he said, at a recommended dosage of 5 mg/kg at 0, 2, and 6 weeks, and then with maintenance therapy every 6-8 weeks. Adalimumab appeared to work best at 40 mg every week, he said, "but the addition of low-dose methotrexate is sometimes necessary to either regimen."
Dr. Rosenbach disclosed that he was an investigator for a clinical trial sponsored by Centocor and Johnson & Johnson to investigate biologics for chronic/refractory sarcoidosis.
SAN DIEGO – While it’s well known that sarcoidosis commonly affects pulmonary function, it’s perhaps less known that the disorder can be detrimental to cardiac function in approximately 5% of cases.
"A common way that patients present with cardiac sarcoidosis is with sudden cardiac death," Dr. Misha Rosenbach said at the annual meeting of the American Academy of Dermatology. "This is a terrible way to present to your doctor with a problem."
A multisystem disorder of unknown cause, sarcoidosis commonly affects young and middle-aged adults and frequently presents with bilateral hilar lymphadenopathy, pulmonary infiltration, and ocular and skin lesions. Other organs may be involved. The diagnosis is established when clinicoradiologic findings are supported by histologic evidence of noncaseating epithelioid cell granulomas.
"Sarcoidosis is primarily a pulmonary disease, but patients can also present with profound systemic symptoms," said Dr. Rosenbach of the departments of dermatology and internal medicine at the University of Pennsylvania, Philadelphia. "When you’re evaluating a patient with cutaneous sarcoidosis, and making a diagnosis of granulomatous disease of the skin, and looking for extracutaneous involvement, it’s important to know what else can be affected."
Although pulmonary function is affected in more than 90% of cases, other commonly affected sites include the eyes (25%-50% of cases), lymph nodes (about 33% of cases), musculoskeletal system (25%-40% of cases), endocrine system (10%-25% of cases), and liver (20%-50% of cases). The initial evaluation should consist of history and physical exam; chest x-ray; pulmonary function tests (including carbon monoxide diffusing capacity); ophthalmologic examination; complete blood count and serum chemistries (including calcium); urinalysis; EKG (plus additional testing if there is a history of palpitations); tuberculin skin test (TST) or interferon (IFN)–gamma release assay; and thyroid and vitamin D testing.
"Patients with sarcoidosis often have low levels of 25-hydroxyvitamin D, but elevated levels of 1,25-dihydroxyvitamin D3," Dr. Rosenbach said. "Inappropriate supplementation can lead to hypercalcemia."
For latent tuberculosis testing, he pointed out that the IFN-gamma release assay (IGRA) is thought to be more accurate than the TST. "IGRA significantly reduces false-positive results" in bacille Calmette-Guérin–vaccinated patients, said Dr. Rosenbach, who is also director of the cutaneous sarcoidosis clinic at the University of Pennsylvania. "Cost-benefit analyses suggest that IGRA [is] cost equivalent to TST, and the Centers for Disease Control and Prevention recommends that IGRA may be used in all circumstances in which the TST is currently used. However, both TST and IGRA have decreased responsiveness and lower sensitivity in patients with impaired immune systems."
In terms of the impact of sarcoidosis on the thyroid gland, a recent analysis of a large database in the United Kingdom found that hyper- and hypothyroidism were twice as common in patients with sarcoidosis, compared with a control population (Postgrad. Med. J. 2009;85:233-7).
A more recent study of 50 patients with cutaneous sarcoidosis conducted by Dr. Rosenbach and his colleagues found that 25% of patients had abnormal thyroid laboratory test results (J. Am. Acad. Dermatol. 2012;66:167-8).
The precise association between sarcoidosis and malignancy remains unclear, he said, but the best available studies suggest that the incidence of lymphoproliferative disorder may be increased in patients with sarcoidosis. Other granulomatous dermatitides may be associated with hematologic abnormalities. Authors of one review found that granulomatous dermatitides may be the first sign of underlying myelodysplastic syndrome (MDS), and recommended that clinicians consider looking for underlying MDS in patients with unexplained or atypical granulomatous skin eruptions (Arch. Dermatol. 2011;147:331-5).
A common stepwise approach for treating patients, Dr. Rosenbach said, begins with skin-directed therapies in the form of steroids or injections. The second step involves the use of antimalarials and tetracycline-class antibiotics; the third step involves methotrexate and/or prednisone, and the fourth step involves consideration for treatment with infliximab or adalimumab. "At this point, etanercept should probably not be used," Dr. Rosenbach said. "It appears to be less effective, and in a few reports has been associated with worsening of disease."
The data are strongest for infliximab, he said, at a recommended dosage of 5 mg/kg at 0, 2, and 6 weeks, and then with maintenance therapy every 6-8 weeks. Adalimumab appeared to work best at 40 mg every week, he said, "but the addition of low-dose methotrexate is sometimes necessary to either regimen."
Dr. Rosenbach disclosed that he was an investigator for a clinical trial sponsored by Centocor and Johnson & Johnson to investigate biologics for chronic/refractory sarcoidosis.
SAN DIEGO – While it’s well known that sarcoidosis commonly affects pulmonary function, it’s perhaps less known that the disorder can be detrimental to cardiac function in approximately 5% of cases.
"A common way that patients present with cardiac sarcoidosis is with sudden cardiac death," Dr. Misha Rosenbach said at the annual meeting of the American Academy of Dermatology. "This is a terrible way to present to your doctor with a problem."
A multisystem disorder of unknown cause, sarcoidosis commonly affects young and middle-aged adults and frequently presents with bilateral hilar lymphadenopathy, pulmonary infiltration, and ocular and skin lesions. Other organs may be involved. The diagnosis is established when clinicoradiologic findings are supported by histologic evidence of noncaseating epithelioid cell granulomas.
"Sarcoidosis is primarily a pulmonary disease, but patients can also present with profound systemic symptoms," said Dr. Rosenbach of the departments of dermatology and internal medicine at the University of Pennsylvania, Philadelphia. "When you’re evaluating a patient with cutaneous sarcoidosis, and making a diagnosis of granulomatous disease of the skin, and looking for extracutaneous involvement, it’s important to know what else can be affected."
Although pulmonary function is affected in more than 90% of cases, other commonly affected sites include the eyes (25%-50% of cases), lymph nodes (about 33% of cases), musculoskeletal system (25%-40% of cases), endocrine system (10%-25% of cases), and liver (20%-50% of cases). The initial evaluation should consist of history and physical exam; chest x-ray; pulmonary function tests (including carbon monoxide diffusing capacity); ophthalmologic examination; complete blood count and serum chemistries (including calcium); urinalysis; EKG (plus additional testing if there is a history of palpitations); tuberculin skin test (TST) or interferon (IFN)–gamma release assay; and thyroid and vitamin D testing.
"Patients with sarcoidosis often have low levels of 25-hydroxyvitamin D, but elevated levels of 1,25-dihydroxyvitamin D3," Dr. Rosenbach said. "Inappropriate supplementation can lead to hypercalcemia."
For latent tuberculosis testing, he pointed out that the IFN-gamma release assay (IGRA) is thought to be more accurate than the TST. "IGRA significantly reduces false-positive results" in bacille Calmette-Guérin–vaccinated patients, said Dr. Rosenbach, who is also director of the cutaneous sarcoidosis clinic at the University of Pennsylvania. "Cost-benefit analyses suggest that IGRA [is] cost equivalent to TST, and the Centers for Disease Control and Prevention recommends that IGRA may be used in all circumstances in which the TST is currently used. However, both TST and IGRA have decreased responsiveness and lower sensitivity in patients with impaired immune systems."
In terms of the impact of sarcoidosis on the thyroid gland, a recent analysis of a large database in the United Kingdom found that hyper- and hypothyroidism were twice as common in patients with sarcoidosis, compared with a control population (Postgrad. Med. J. 2009;85:233-7).
A more recent study of 50 patients with cutaneous sarcoidosis conducted by Dr. Rosenbach and his colleagues found that 25% of patients had abnormal thyroid laboratory test results (J. Am. Acad. Dermatol. 2012;66:167-8).
The precise association between sarcoidosis and malignancy remains unclear, he said, but the best available studies suggest that the incidence of lymphoproliferative disorder may be increased in patients with sarcoidosis. Other granulomatous dermatitides may be associated with hematologic abnormalities. Authors of one review found that granulomatous dermatitides may be the first sign of underlying myelodysplastic syndrome (MDS), and recommended that clinicians consider looking for underlying MDS in patients with unexplained or atypical granulomatous skin eruptions (Arch. Dermatol. 2011;147:331-5).
A common stepwise approach for treating patients, Dr. Rosenbach said, begins with skin-directed therapies in the form of steroids or injections. The second step involves the use of antimalarials and tetracycline-class antibiotics; the third step involves methotrexate and/or prednisone, and the fourth step involves consideration for treatment with infliximab or adalimumab. "At this point, etanercept should probably not be used," Dr. Rosenbach said. "It appears to be less effective, and in a few reports has been associated with worsening of disease."
The data are strongest for infliximab, he said, at a recommended dosage of 5 mg/kg at 0, 2, and 6 weeks, and then with maintenance therapy every 6-8 weeks. Adalimumab appeared to work best at 40 mg every week, he said, "but the addition of low-dose methotrexate is sometimes necessary to either regimen."
Dr. Rosenbach disclosed that he was an investigator for a clinical trial sponsored by Centocor and Johnson & Johnson to investigate biologics for chronic/refractory sarcoidosis.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Study Finds Pruritus Common in Elderly Patients
SAN DIEGO – Nearly half of elderly patients admitted to a geriatric ward reported having symptoms of pruritus, results from a single-center study found.
"Pruritus is a common complaint among the elderly," Dr. Yee Leng Teoh and colleagues wrote in an abstract presented during a poster session at the annual meeting of the American Academy of Dermatology. "It may have a significant impact on quality of life, but may be underestimated and poorly addressed."
In a study of 194 patients admitted to the geriatric ward of Changi General Hospital, Singapore, between March and May of 2010, Dr. Teoh and associates used a structured questionnaire including the Dermatology Life Quality Index (DLQI) to assess the prevalence and severity of itch and its impact on quality of life. They reviewed the patients’ hospital records regarding comorbid conditions, prior skin disease, medication use, and social background.
The mean age of the patients was 85 years, and their mean DLQI score was 6.7. Nearly half (49%) reported having a problem with itch for a mean of 15.3 months. Negative impacts on quality of life included disruption of sleep (reported by 35% of respondents) and disruption of mental concentration (reported by 31% of respondents).
More than half of patients (61%) said they had informed a physician about the problem, yet 26% believed that it was not sufficiently addressed. Twenty patients (10%) were diagnosed with a specific dermatologic condition, most commonly eczema and dermatophyte infection.
Of the participants who had informed their physician about the problem, 58% were treated with topical agents, 4% with oral antihistamines, and 33% with topical agents and oral antihistamines; 5% did not receive any treatment.
Study participants with a history of cerebrovascular accidents or transient ischemic attacks were 3.5 times more likely to have itch, compared with those who did not have this history. And patients with diabetes were 2.2 times more likely to have itch, compared with those who did not have the condition.
This may be because "sympathetic dysfunction caused hypohidrosis and resulted in xerosis," they hypothesized. "Another possible explanation is that patients with diabetic polyneuropathy, usually as a result of poor diabetic control, have damaged sensory C fibers, which can cause pruritus."
The study also found that elderly patients being treated with laxatives were 2.1 times less likely to have itch, compared with patients not taking laxatives. "We postulate that laxatives may facilitate the clearing of bile acids, resulting in a reduced incidence of itch," Dr. Teoh and associates wrote. "Lactulose can inhibit formation of deoxycholic acid from primary bile acids. This effect is thought to be mediated through the acidification of the proximal bowel, leading to reduction in 7 alpha-dehydroxylase, which converts primary to secondary bile acids."
The researchers reported having no relevant financial disclosures.
SAN DIEGO – Nearly half of elderly patients admitted to a geriatric ward reported having symptoms of pruritus, results from a single-center study found.
"Pruritus is a common complaint among the elderly," Dr. Yee Leng Teoh and colleagues wrote in an abstract presented during a poster session at the annual meeting of the American Academy of Dermatology. "It may have a significant impact on quality of life, but may be underestimated and poorly addressed."
In a study of 194 patients admitted to the geriatric ward of Changi General Hospital, Singapore, between March and May of 2010, Dr. Teoh and associates used a structured questionnaire including the Dermatology Life Quality Index (DLQI) to assess the prevalence and severity of itch and its impact on quality of life. They reviewed the patients’ hospital records regarding comorbid conditions, prior skin disease, medication use, and social background.
The mean age of the patients was 85 years, and their mean DLQI score was 6.7. Nearly half (49%) reported having a problem with itch for a mean of 15.3 months. Negative impacts on quality of life included disruption of sleep (reported by 35% of respondents) and disruption of mental concentration (reported by 31% of respondents).
More than half of patients (61%) said they had informed a physician about the problem, yet 26% believed that it was not sufficiently addressed. Twenty patients (10%) were diagnosed with a specific dermatologic condition, most commonly eczema and dermatophyte infection.
Of the participants who had informed their physician about the problem, 58% were treated with topical agents, 4% with oral antihistamines, and 33% with topical agents and oral antihistamines; 5% did not receive any treatment.
Study participants with a history of cerebrovascular accidents or transient ischemic attacks were 3.5 times more likely to have itch, compared with those who did not have this history. And patients with diabetes were 2.2 times more likely to have itch, compared with those who did not have the condition.
This may be because "sympathetic dysfunction caused hypohidrosis and resulted in xerosis," they hypothesized. "Another possible explanation is that patients with diabetic polyneuropathy, usually as a result of poor diabetic control, have damaged sensory C fibers, which can cause pruritus."
The study also found that elderly patients being treated with laxatives were 2.1 times less likely to have itch, compared with patients not taking laxatives. "We postulate that laxatives may facilitate the clearing of bile acids, resulting in a reduced incidence of itch," Dr. Teoh and associates wrote. "Lactulose can inhibit formation of deoxycholic acid from primary bile acids. This effect is thought to be mediated through the acidification of the proximal bowel, leading to reduction in 7 alpha-dehydroxylase, which converts primary to secondary bile acids."
The researchers reported having no relevant financial disclosures.
SAN DIEGO – Nearly half of elderly patients admitted to a geriatric ward reported having symptoms of pruritus, results from a single-center study found.
"Pruritus is a common complaint among the elderly," Dr. Yee Leng Teoh and colleagues wrote in an abstract presented during a poster session at the annual meeting of the American Academy of Dermatology. "It may have a significant impact on quality of life, but may be underestimated and poorly addressed."
In a study of 194 patients admitted to the geriatric ward of Changi General Hospital, Singapore, between March and May of 2010, Dr. Teoh and associates used a structured questionnaire including the Dermatology Life Quality Index (DLQI) to assess the prevalence and severity of itch and its impact on quality of life. They reviewed the patients’ hospital records regarding comorbid conditions, prior skin disease, medication use, and social background.
The mean age of the patients was 85 years, and their mean DLQI score was 6.7. Nearly half (49%) reported having a problem with itch for a mean of 15.3 months. Negative impacts on quality of life included disruption of sleep (reported by 35% of respondents) and disruption of mental concentration (reported by 31% of respondents).
More than half of patients (61%) said they had informed a physician about the problem, yet 26% believed that it was not sufficiently addressed. Twenty patients (10%) were diagnosed with a specific dermatologic condition, most commonly eczema and dermatophyte infection.
Of the participants who had informed their physician about the problem, 58% were treated with topical agents, 4% with oral antihistamines, and 33% with topical agents and oral antihistamines; 5% did not receive any treatment.
Study participants with a history of cerebrovascular accidents or transient ischemic attacks were 3.5 times more likely to have itch, compared with those who did not have this history. And patients with diabetes were 2.2 times more likely to have itch, compared with those who did not have the condition.
This may be because "sympathetic dysfunction caused hypohidrosis and resulted in xerosis," they hypothesized. "Another possible explanation is that patients with diabetic polyneuropathy, usually as a result of poor diabetic control, have damaged sensory C fibers, which can cause pruritus."
The study also found that elderly patients being treated with laxatives were 2.1 times less likely to have itch, compared with patients not taking laxatives. "We postulate that laxatives may facilitate the clearing of bile acids, resulting in a reduced incidence of itch," Dr. Teoh and associates wrote. "Lactulose can inhibit formation of deoxycholic acid from primary bile acids. This effect is thought to be mediated through the acidification of the proximal bowel, leading to reduction in 7 alpha-dehydroxylase, which converts primary to secondary bile acids."
The researchers reported having no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Major Finding: Nearly half of elderly patients (49%) reported having a problem with itch for a mean of 15.3 months.
Data Source: The study involved 194 patients admitted to the geriatric ward of Changi General Hospital, Singapore, between March and May of 2010.
Disclosures: The researchers reported having no relevant financial disclosures.
Investigational Psoriasis Drug Reduces Vessel Inflammation
SAN DIEGO – An investigational first-in-class drug not only improved plaque psoriasis but reduced atherosclerotic inflammation in major vessels, a study has shown.
At 12 weeks there was a dose-related response in inflammation in the most-diseased vessels. Those in the placebo group had a 4% decrease in the target-to-baseline ratio (a validated PET/CT measure of changes in vessel inflammation), while those on 20 mg and 80 mg of VB-201 had decreases of 7% and 13%.
"As we look at our patients with psoriasis, where cardiovascular risk factors are a huge public health problem, this type of approach may have multiple benefits for them," said Dr. Alexa B. Kimball, who presented the results at the annual meeting of the American Academy of Dermatology.
The results come from a phase II trial of 184 patients with moderate to severe plaque psoriasis and a PET/CT substudy of 47 patients with cardiovascular risk factors. The patients in the double-blind, randomized, placebo-controlled study received either 20 or 80 mg of VB-201 or placebo once daily for 12 weeks.
VB-201, a phospholipid analogue, is being developed by VBL Therapeutics as an oral disease-modifying agent for chronic immunoinflammatory disease and atherosclerosis inflammation, and is the first in the lecinoxoid molecular class.
The compound’s novel mechanism of action for the control and attenuation of chronic immunoinflammatory diseases is thought to be through the highly selective modulation of components of the innate immune system. The drug is proposed to work by inhibiting cell-surface toll-like receptor signal cascade. "It’s mimicking some of the native molecules, but this turns out to be specific to antigen-presenting cells, endothelial cells, and monocytes," said Dr. Kimball. Thus, chemokine-mediated migration of monocytes to inflamed tissue is limited.
In the subanalysis, patients had to have a target-to-baseline ratio on PET/CT scan that was greater than 1.6 in order to be eligible.
PET/CT was used to evaluate inflammation in the vessels over time. The primary endpoint in this substudy included the mean of the maximum values of the target-to-baseline ratio in the most-diseased segment of vessel. The secondary endpoint involved looking at all of the inflammation in the vessels to determine a mean value for the vessels in a given patient; these mean values were then used to calculate an overall mean value of inflammation.
"One of the challenges in cardiovascular studies looking at atherosclerotic disease is that the endpoint that you’re most interested in is actually myocardial infarction, typically," said Dr. Kimball. "This form of PET/CT has been shown to be a pretty sensitive measure for inflammation in these vessels and is being used in investigational settings to be a surrogate marker for risk."
In addition, the technique correlates well with histopathologic inflammation and with cardiovascular risk factors. "It also seems to be predictive of future clinical vascular events," she said. High-fluorine-18fluorodeoxyglucose uptake appears to correlate with future vascular events.
"We did enhance the substudy with patients with additional cardiovascular risk factors to make sure that we had a population at risk where we actually could see inflammation to begin with," she said.
In the substudy, 13 patients were on placebo, 18 were on 20 mg VB-201, and 16 were taking 80 mg VB-201. These patients were a little older and had such risk factors as vascular disease dyslipidemia, diabetes, and obesity. Many were on statins.
At 12 weeks, there was a dose-related response in inflammation in the most-diseased vessel. Those in the placebo group had a 4% decrease in target-to-baseline ratio, while those on 20 mg and 80 mg of VB-201 had decreases of 7% and 13%.
"To put that into context with high-dose statin therapy, we would typically see these types of responses in the 7%-10% reduction range usually over 6 months rather than just 3 months," said Dr. Kimball of the dermatology department at Massachusetts General Hospital in Boston.
In the main study, the researchers looked at the effects of the drug on plaque psoriasis, compared with placebo. The primary endpoint was a Psoriasis Area and Severity Index (PASI) score of 75.
The 184 patients were randomized to receive 20 mg VB-201 (66), 80 mg VB-201 (59), or placebo (59). The study cohort was primarily composed of overweight men (BMI approximately 30 kg/m2) with an average age of 45 years. Overall, the group had a baseline PASI score close to 20.
Statistically significant improvements were achieved on the Physician Global Assessment and Patient Global Assessment. In terms of PASI 75, "there was a dose response as well," said Dr. Kimball. "This is not a drug that is achieving high levels of PASI 90 or PASI 100 ... but there is some benefit."
"This drug is moving people from substantial severity to reduced severity ... It may not be a drug that clears psoriasis to zero, but you can move patients out of the severe category," she said.
"VBL-201 is thought to be – at least in these initial studies – quite well tolerated. ... The study met its primary endpoint in the PET-CT substudy, demonstrating a statistically significant reduction in vascular inflammation," Dr. Kimball noted. "Importantly, these reductions occurred also in patients who were on statin therapy."
There were no treatment-related serious adverse events; the overall rates of adverse events were similar for both doses of VB-201 drug and placebo.
The study was funded by VBL Pharmaceuticals. Dr. Kimball is a consultant for VBL and several other pharmaceutical companies.
SAN DIEGO – An investigational first-in-class drug not only improved plaque psoriasis but reduced atherosclerotic inflammation in major vessels, a study has shown.
At 12 weeks there was a dose-related response in inflammation in the most-diseased vessels. Those in the placebo group had a 4% decrease in the target-to-baseline ratio (a validated PET/CT measure of changes in vessel inflammation), while those on 20 mg and 80 mg of VB-201 had decreases of 7% and 13%.
"As we look at our patients with psoriasis, where cardiovascular risk factors are a huge public health problem, this type of approach may have multiple benefits for them," said Dr. Alexa B. Kimball, who presented the results at the annual meeting of the American Academy of Dermatology.
The results come from a phase II trial of 184 patients with moderate to severe plaque psoriasis and a PET/CT substudy of 47 patients with cardiovascular risk factors. The patients in the double-blind, randomized, placebo-controlled study received either 20 or 80 mg of VB-201 or placebo once daily for 12 weeks.
VB-201, a phospholipid analogue, is being developed by VBL Therapeutics as an oral disease-modifying agent for chronic immunoinflammatory disease and atherosclerosis inflammation, and is the first in the lecinoxoid molecular class.
The compound’s novel mechanism of action for the control and attenuation of chronic immunoinflammatory diseases is thought to be through the highly selective modulation of components of the innate immune system. The drug is proposed to work by inhibiting cell-surface toll-like receptor signal cascade. "It’s mimicking some of the native molecules, but this turns out to be specific to antigen-presenting cells, endothelial cells, and monocytes," said Dr. Kimball. Thus, chemokine-mediated migration of monocytes to inflamed tissue is limited.
In the subanalysis, patients had to have a target-to-baseline ratio on PET/CT scan that was greater than 1.6 in order to be eligible.
PET/CT was used to evaluate inflammation in the vessels over time. The primary endpoint in this substudy included the mean of the maximum values of the target-to-baseline ratio in the most-diseased segment of vessel. The secondary endpoint involved looking at all of the inflammation in the vessels to determine a mean value for the vessels in a given patient; these mean values were then used to calculate an overall mean value of inflammation.
"One of the challenges in cardiovascular studies looking at atherosclerotic disease is that the endpoint that you’re most interested in is actually myocardial infarction, typically," said Dr. Kimball. "This form of PET/CT has been shown to be a pretty sensitive measure for inflammation in these vessels and is being used in investigational settings to be a surrogate marker for risk."
In addition, the technique correlates well with histopathologic inflammation and with cardiovascular risk factors. "It also seems to be predictive of future clinical vascular events," she said. High-fluorine-18fluorodeoxyglucose uptake appears to correlate with future vascular events.
"We did enhance the substudy with patients with additional cardiovascular risk factors to make sure that we had a population at risk where we actually could see inflammation to begin with," she said.
In the substudy, 13 patients were on placebo, 18 were on 20 mg VB-201, and 16 were taking 80 mg VB-201. These patients were a little older and had such risk factors as vascular disease dyslipidemia, diabetes, and obesity. Many were on statins.
At 12 weeks, there was a dose-related response in inflammation in the most-diseased vessel. Those in the placebo group had a 4% decrease in target-to-baseline ratio, while those on 20 mg and 80 mg of VB-201 had decreases of 7% and 13%.
"To put that into context with high-dose statin therapy, we would typically see these types of responses in the 7%-10% reduction range usually over 6 months rather than just 3 months," said Dr. Kimball of the dermatology department at Massachusetts General Hospital in Boston.
In the main study, the researchers looked at the effects of the drug on plaque psoriasis, compared with placebo. The primary endpoint was a Psoriasis Area and Severity Index (PASI) score of 75.
The 184 patients were randomized to receive 20 mg VB-201 (66), 80 mg VB-201 (59), or placebo (59). The study cohort was primarily composed of overweight men (BMI approximately 30 kg/m2) with an average age of 45 years. Overall, the group had a baseline PASI score close to 20.
Statistically significant improvements were achieved on the Physician Global Assessment and Patient Global Assessment. In terms of PASI 75, "there was a dose response as well," said Dr. Kimball. "This is not a drug that is achieving high levels of PASI 90 or PASI 100 ... but there is some benefit."
"This drug is moving people from substantial severity to reduced severity ... It may not be a drug that clears psoriasis to zero, but you can move patients out of the severe category," she said.
"VBL-201 is thought to be – at least in these initial studies – quite well tolerated. ... The study met its primary endpoint in the PET-CT substudy, demonstrating a statistically significant reduction in vascular inflammation," Dr. Kimball noted. "Importantly, these reductions occurred also in patients who were on statin therapy."
There were no treatment-related serious adverse events; the overall rates of adverse events were similar for both doses of VB-201 drug and placebo.
The study was funded by VBL Pharmaceuticals. Dr. Kimball is a consultant for VBL and several other pharmaceutical companies.
SAN DIEGO – An investigational first-in-class drug not only improved plaque psoriasis but reduced atherosclerotic inflammation in major vessels, a study has shown.
At 12 weeks there was a dose-related response in inflammation in the most-diseased vessels. Those in the placebo group had a 4% decrease in the target-to-baseline ratio (a validated PET/CT measure of changes in vessel inflammation), while those on 20 mg and 80 mg of VB-201 had decreases of 7% and 13%.
"As we look at our patients with psoriasis, where cardiovascular risk factors are a huge public health problem, this type of approach may have multiple benefits for them," said Dr. Alexa B. Kimball, who presented the results at the annual meeting of the American Academy of Dermatology.
The results come from a phase II trial of 184 patients with moderate to severe plaque psoriasis and a PET/CT substudy of 47 patients with cardiovascular risk factors. The patients in the double-blind, randomized, placebo-controlled study received either 20 or 80 mg of VB-201 or placebo once daily for 12 weeks.
VB-201, a phospholipid analogue, is being developed by VBL Therapeutics as an oral disease-modifying agent for chronic immunoinflammatory disease and atherosclerosis inflammation, and is the first in the lecinoxoid molecular class.
The compound’s novel mechanism of action for the control and attenuation of chronic immunoinflammatory diseases is thought to be through the highly selective modulation of components of the innate immune system. The drug is proposed to work by inhibiting cell-surface toll-like receptor signal cascade. "It’s mimicking some of the native molecules, but this turns out to be specific to antigen-presenting cells, endothelial cells, and monocytes," said Dr. Kimball. Thus, chemokine-mediated migration of monocytes to inflamed tissue is limited.
In the subanalysis, patients had to have a target-to-baseline ratio on PET/CT scan that was greater than 1.6 in order to be eligible.
PET/CT was used to evaluate inflammation in the vessels over time. The primary endpoint in this substudy included the mean of the maximum values of the target-to-baseline ratio in the most-diseased segment of vessel. The secondary endpoint involved looking at all of the inflammation in the vessels to determine a mean value for the vessels in a given patient; these mean values were then used to calculate an overall mean value of inflammation.
"One of the challenges in cardiovascular studies looking at atherosclerotic disease is that the endpoint that you’re most interested in is actually myocardial infarction, typically," said Dr. Kimball. "This form of PET/CT has been shown to be a pretty sensitive measure for inflammation in these vessels and is being used in investigational settings to be a surrogate marker for risk."
In addition, the technique correlates well with histopathologic inflammation and with cardiovascular risk factors. "It also seems to be predictive of future clinical vascular events," she said. High-fluorine-18fluorodeoxyglucose uptake appears to correlate with future vascular events.
"We did enhance the substudy with patients with additional cardiovascular risk factors to make sure that we had a population at risk where we actually could see inflammation to begin with," she said.
In the substudy, 13 patients were on placebo, 18 were on 20 mg VB-201, and 16 were taking 80 mg VB-201. These patients were a little older and had such risk factors as vascular disease dyslipidemia, diabetes, and obesity. Many were on statins.
At 12 weeks, there was a dose-related response in inflammation in the most-diseased vessel. Those in the placebo group had a 4% decrease in target-to-baseline ratio, while those on 20 mg and 80 mg of VB-201 had decreases of 7% and 13%.
"To put that into context with high-dose statin therapy, we would typically see these types of responses in the 7%-10% reduction range usually over 6 months rather than just 3 months," said Dr. Kimball of the dermatology department at Massachusetts General Hospital in Boston.
In the main study, the researchers looked at the effects of the drug on plaque psoriasis, compared with placebo. The primary endpoint was a Psoriasis Area and Severity Index (PASI) score of 75.
The 184 patients were randomized to receive 20 mg VB-201 (66), 80 mg VB-201 (59), or placebo (59). The study cohort was primarily composed of overweight men (BMI approximately 30 kg/m2) with an average age of 45 years. Overall, the group had a baseline PASI score close to 20.
Statistically significant improvements were achieved on the Physician Global Assessment and Patient Global Assessment. In terms of PASI 75, "there was a dose response as well," said Dr. Kimball. "This is not a drug that is achieving high levels of PASI 90 or PASI 100 ... but there is some benefit."
"This drug is moving people from substantial severity to reduced severity ... It may not be a drug that clears psoriasis to zero, but you can move patients out of the severe category," she said.
"VBL-201 is thought to be – at least in these initial studies – quite well tolerated. ... The study met its primary endpoint in the PET-CT substudy, demonstrating a statistically significant reduction in vascular inflammation," Dr. Kimball noted. "Importantly, these reductions occurred also in patients who were on statin therapy."
There were no treatment-related serious adverse events; the overall rates of adverse events were similar for both doses of VB-201 drug and placebo.
The study was funded by VBL Pharmaceuticals. Dr. Kimball is a consultant for VBL and several other pharmaceutical companies.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Major Finding: At 12 weeks there was a dose-related response in inflammation in the most-diseased vessels of patients with plaque psoriasis and cardiovascular risk factors: Those in the placebo group had a 4% decrease, while those on 20 mg and 80 mg of VB-201 had decreases of 7% and 13%.
Data Source: The phase II, double-blind, randomized placebo-controlled study enrolled 184 patients with moderate to severe psoriasis; a PET/CT substudy was done of 47 patients with cardiovascular risk factors.
Disclosures: The study was funded by VBL Pharmaceuticals. Dr. Kimball is a consultant for VBL and several other pharmaceutical companies.
Antibiotics Lead Outpatient Cutaneous Adverse Drug Events
SAN DIEGO – Antimicrobial agents were the most common identifiable causes of outpatient cutaneous adverse drug events, with amoxicillin being the most frequent single causative substance, in a study of data collected from 1995 to 2005.
Those are the key findings from the analysis of data from the National Ambulatory Medical Care Survey (NAMC) and the National Hospital and Ambulatory Medical Care Survey (NHAMCS).
While cutaneous reactions are thought to account for 16%-30% of reported adverse drug events, "there is limited information in the medical literature regarding the frequency of outpatient CADEs," Dr. Cheryl L. Gustafson wrote in a poster presented at the annual meeting of the American Academy of Dermatology.
Dr. Gustafson of the department of dermatology at Wake Forest University, Winston-Salem, N.C., and her associates queried the NAMCS and the NHAMC for data regarding CADEs (cutaneous adverse drug events) reported between 1995 and 2005. They used sample weights to estimate the national annual incidence of outpatient CADEs in the United States.
During the time period studied, a total of 635,982 CADE-related visits occurred, which translated into an annual incidence of 2.26 CADEs per 1,000 persons. Patients took an average of 2.2 medications in addition to the one causing the CADE. The incidence of CADEs increased with age, with a peak rate occurring in those aged 70-79 years.
Antibiotics were the most commonly implicated drug class (23%, chiefly amoxicillin), followed by cardiovascular agents (7%) and agents primarily affecting the skin and mucous membranes (6%). Unspecified or unknown agents accounted for a quarter of all CADEs.
Dermatitis and urticaria were the most common skin reactions reported (71% vs. 13%).
"We also found that patients with a dermatologic diagnosis experienced a CADE caused by a drug treating the initial skin condition in 11% of cases," Dr. Gustafson said in an interview.
Dr. Gustafson reported no relevant financial disclosures.
SAN DIEGO – Antimicrobial agents were the most common identifiable causes of outpatient cutaneous adverse drug events, with amoxicillin being the most frequent single causative substance, in a study of data collected from 1995 to 2005.
Those are the key findings from the analysis of data from the National Ambulatory Medical Care Survey (NAMC) and the National Hospital and Ambulatory Medical Care Survey (NHAMCS).
While cutaneous reactions are thought to account for 16%-30% of reported adverse drug events, "there is limited information in the medical literature regarding the frequency of outpatient CADEs," Dr. Cheryl L. Gustafson wrote in a poster presented at the annual meeting of the American Academy of Dermatology.
Dr. Gustafson of the department of dermatology at Wake Forest University, Winston-Salem, N.C., and her associates queried the NAMCS and the NHAMC for data regarding CADEs (cutaneous adverse drug events) reported between 1995 and 2005. They used sample weights to estimate the national annual incidence of outpatient CADEs in the United States.
During the time period studied, a total of 635,982 CADE-related visits occurred, which translated into an annual incidence of 2.26 CADEs per 1,000 persons. Patients took an average of 2.2 medications in addition to the one causing the CADE. The incidence of CADEs increased with age, with a peak rate occurring in those aged 70-79 years.
Antibiotics were the most commonly implicated drug class (23%, chiefly amoxicillin), followed by cardiovascular agents (7%) and agents primarily affecting the skin and mucous membranes (6%). Unspecified or unknown agents accounted for a quarter of all CADEs.
Dermatitis and urticaria were the most common skin reactions reported (71% vs. 13%).
"We also found that patients with a dermatologic diagnosis experienced a CADE caused by a drug treating the initial skin condition in 11% of cases," Dr. Gustafson said in an interview.
Dr. Gustafson reported no relevant financial disclosures.
SAN DIEGO – Antimicrobial agents were the most common identifiable causes of outpatient cutaneous adverse drug events, with amoxicillin being the most frequent single causative substance, in a study of data collected from 1995 to 2005.
Those are the key findings from the analysis of data from the National Ambulatory Medical Care Survey (NAMC) and the National Hospital and Ambulatory Medical Care Survey (NHAMCS).
While cutaneous reactions are thought to account for 16%-30% of reported adverse drug events, "there is limited information in the medical literature regarding the frequency of outpatient CADEs," Dr. Cheryl L. Gustafson wrote in a poster presented at the annual meeting of the American Academy of Dermatology.
Dr. Gustafson of the department of dermatology at Wake Forest University, Winston-Salem, N.C., and her associates queried the NAMCS and the NHAMC for data regarding CADEs (cutaneous adverse drug events) reported between 1995 and 2005. They used sample weights to estimate the national annual incidence of outpatient CADEs in the United States.
During the time period studied, a total of 635,982 CADE-related visits occurred, which translated into an annual incidence of 2.26 CADEs per 1,000 persons. Patients took an average of 2.2 medications in addition to the one causing the CADE. The incidence of CADEs increased with age, with a peak rate occurring in those aged 70-79 years.
Antibiotics were the most commonly implicated drug class (23%, chiefly amoxicillin), followed by cardiovascular agents (7%) and agents primarily affecting the skin and mucous membranes (6%). Unspecified or unknown agents accounted for a quarter of all CADEs.
Dermatitis and urticaria were the most common skin reactions reported (71% vs. 13%).
"We also found that patients with a dermatologic diagnosis experienced a CADE caused by a drug treating the initial skin condition in 11% of cases," Dr. Gustafson said in an interview.
Dr. Gustafson reported no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Major Finding: Antibiotics caused the most cutaneous adverse drug events (23%), followed by cardiovascular agents (7%) and agents primarily affecting the skin and mucous membranes (6%).
Data Source: A study of outpatient CADEs occurring between 1995 and 2005 was conducted using data from the National Ambulatory Medical Care Survey and the National Hospital and Ambulatory Medical Care Survey.
Disclosures: Dr. Gustafson said that she had no relevant financial disclosures.