User login
The Official Newspaper of the American Association for Thoracic Surgery
CANTOS sings of novel strategy for cardiovascular, cancer prevention
BARCELONA – Inhibiting the interleukin-1 beta innate immunity pathway with canakinumab reduced recurrent cardiovascular events and lung cancer in the groundbreaking phase III CANTOS trial, Paul M. Ridker, MD, reported at the annual congress of the European Society of Cardiology.
“These data provide the first proof that inflammation inhibition in the absence of lipid lowering can improve atherogenic outcomes and potentially alter progression of some fatal cancers,” declared Dr. Ridker, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
“Just like we’ve learned that lower LDL is better, I think we’re now learning that lower inflammation is better,” he said.
CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study) was a randomized, double-blind, placebo-controlled trial involving 10,061 patients in 39 countries, all of whom had a previous MI and a chronically high level of systemic inflammation as reflected in a median baseline high-sensitivity C-reactive protein (CRP) level of 4.1 mg/L. Ninety-one percent of participants were on statin therapy, with a median LDL cholesterol of 82 mg/dL when randomized to subcutaneous canakinumab at 50, 150, or 300 mg or to placebo once every 3 months.
Canakinumab is a fully human monoclonal antibody targeting IL-1B, a key player in systemic inflammation. The cytokine is activated by the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome, a part of the innate immune system. Canakinumab is approved as Ilaris for treatment of several uncommon rheumatologic diseases, including cryopryin-associated periodic syndrome and systemic juvenile idiopathic arthritis.
At a median follow-up of 3.7 years, the incidence of the primary composite efficacy endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death was 4.5 events per 100 person-years in the control group, significantly higher than the 3.86 and 3.9 events per 100 person-years in patients on canakinumab at 150 and 300 mg, respectively.
Since event rates were virtually identical in the 150- and 300-mg study arms, Dr. Ridker combined those two patient groups in his analysis. They showed a 15% reduction in the risk of the primary efficacy endpoint, compared with placebo-treated controls, along with a 39% reduction from baseline in CRP. They also were 30% less likely to undergo percutaneous coronary intervention or coronary artery bypass graft during follow-up.
“That’s quite important, because that’s a progression-of-atherosclerosis endpoint and also obviously a cost and financial endpoint,” he observed.
A key finding in CANTOS was that patients with a reduction in CRP at or exceeding the median decrease just 3 months into the study – that is, after a single injection – had a 27% reduction in major vascular events during follow-up. Patients with a lesser reduction in CRP at that point did not experience a significant reduction in the primary endpoint, compared with placebo.
“The clinician in me would say we probably ought to give a single dose of the drug, see what happens, and if you get a large inflammation reduction we could perhaps consider treating that patient, but if you did not get a large reduction perhaps this is not a therapy for that patient. Why not avoid the toxicity in people who aren’t going to respond?” Dr. Ridker said.
Side effects related to canakinumab consisted of mild leukopenia and a small but statistically significant increase in fatal infections, which he called “not surprising.”
“It’s in the same range as one gets in treating rheumatoid arthritis with a biologic drug, which rheumatologists are very comfortable doing. You would imagine that if this does become a treatment, physicians will get much better at bringing patients in early when they have signs and symptoms of infection,” the cardiologist continued.
Patients on canakinumab showed significant reductions in incident rheumatoid arthritis, gout, and osteoarthritis. The drug had no kidney or liver adverse events.
Cancer was a prespecified secondary outcome in CANTOS. The investigators saw the trial as an opportunity to test a longstanding hypothesis that inhibiting IL-1B would have a positive impact on lung cancer in particular.
“Smoking, exposure to diesel fuel, inhalation of asbestos or other silicates – these cause inflammation which activates the NLRP3 inflammasome, but in the pulmonary system rather than the arteries,” Dr. Ridker explained.
An entry requirement in CANTOS was that patients needed to be free of known cancer. During study follow-up, 129 patients were diagnosed with lung cancer. The risk was reduced in dose-dependent fashion with canakinumab: by 39% relative to placebo in the 150-mg group and by 67% in the 300-mg group. Lung cancer mortality was reduced by 77% in the canakinumab 300-mg group.
“I don’t think this is about oncogenesis per se. I think the tumors are already there, but they don’t progress because we’ve altered the tumor’s inflammatory microenvironment,” he continued.
Since CANTOS was first and foremost a study of atherosclerotic disease prevention, the cancer results need to be replicated on a high-priority basis. Dr. Ridker predicted that Novartis, which sponsored CANTOS, will quickly mount a clinical trial examining canakinumab’s potential as an adjunctive treatment to either chemotherapy or radiation following resection of lung cancer.
He stressed that CANTOS is only the beginning stanza in what will be an entirely new approach to preventive cardiology. Numerous other inflammatory pathways also might serve as targets.
“I think this is going to open up all kinds of approaches using a variety of agents that have really been in the rheumatology and immunology world,” the cardiologist predicted.
For example, he is principal investigator in the ongoing National Heart, Lung, and Blood Institute–sponsored Cardiovascular Inflammation Reduction Trial (CIRT), a randomized, double-blind, placebo-controlled study of low-dose methotrexate for prevention of cardiovascular events in a planned 7,000 patients with type 2 diabetes or metabolic syndrome who’ve had an MI or have multivessel CAD. Results are probably 4-6 years off.
“Right now, we know canakinumab works. If methotrexate were to work, then we’d have a generic, inexpensive approach as well,” Dr. Ridker noted.
Novartis officials indicated that, on the basis of the positive CANTOS results, the company plans to file for an expanded indication for canakinumab for cardiovascular prevention. The company also is gearing up for studies of the drug in oncology.
Simultaneous with Dr. Ridker’s presentation in Barcelona, both the atherosclerotic disease findings (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914) and the cancer findings (Lancet. 2017 Aug 27. doi: 10.1016/S0140-6736(17)32247-X) were published.
He reported serving as a consultant to Novartis.
BARCELONA – Inhibiting the interleukin-1 beta innate immunity pathway with canakinumab reduced recurrent cardiovascular events and lung cancer in the groundbreaking phase III CANTOS trial, Paul M. Ridker, MD, reported at the annual congress of the European Society of Cardiology.
“These data provide the first proof that inflammation inhibition in the absence of lipid lowering can improve atherogenic outcomes and potentially alter progression of some fatal cancers,” declared Dr. Ridker, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
“Just like we’ve learned that lower LDL is better, I think we’re now learning that lower inflammation is better,” he said.
CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study) was a randomized, double-blind, placebo-controlled trial involving 10,061 patients in 39 countries, all of whom had a previous MI and a chronically high level of systemic inflammation as reflected in a median baseline high-sensitivity C-reactive protein (CRP) level of 4.1 mg/L. Ninety-one percent of participants were on statin therapy, with a median LDL cholesterol of 82 mg/dL when randomized to subcutaneous canakinumab at 50, 150, or 300 mg or to placebo once every 3 months.
Canakinumab is a fully human monoclonal antibody targeting IL-1B, a key player in systemic inflammation. The cytokine is activated by the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome, a part of the innate immune system. Canakinumab is approved as Ilaris for treatment of several uncommon rheumatologic diseases, including cryopryin-associated periodic syndrome and systemic juvenile idiopathic arthritis.
At a median follow-up of 3.7 years, the incidence of the primary composite efficacy endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death was 4.5 events per 100 person-years in the control group, significantly higher than the 3.86 and 3.9 events per 100 person-years in patients on canakinumab at 150 and 300 mg, respectively.
Since event rates were virtually identical in the 150- and 300-mg study arms, Dr. Ridker combined those two patient groups in his analysis. They showed a 15% reduction in the risk of the primary efficacy endpoint, compared with placebo-treated controls, along with a 39% reduction from baseline in CRP. They also were 30% less likely to undergo percutaneous coronary intervention or coronary artery bypass graft during follow-up.
“That’s quite important, because that’s a progression-of-atherosclerosis endpoint and also obviously a cost and financial endpoint,” he observed.
A key finding in CANTOS was that patients with a reduction in CRP at or exceeding the median decrease just 3 months into the study – that is, after a single injection – had a 27% reduction in major vascular events during follow-up. Patients with a lesser reduction in CRP at that point did not experience a significant reduction in the primary endpoint, compared with placebo.
“The clinician in me would say we probably ought to give a single dose of the drug, see what happens, and if you get a large inflammation reduction we could perhaps consider treating that patient, but if you did not get a large reduction perhaps this is not a therapy for that patient. Why not avoid the toxicity in people who aren’t going to respond?” Dr. Ridker said.
Side effects related to canakinumab consisted of mild leukopenia and a small but statistically significant increase in fatal infections, which he called “not surprising.”
“It’s in the same range as one gets in treating rheumatoid arthritis with a biologic drug, which rheumatologists are very comfortable doing. You would imagine that if this does become a treatment, physicians will get much better at bringing patients in early when they have signs and symptoms of infection,” the cardiologist continued.
Patients on canakinumab showed significant reductions in incident rheumatoid arthritis, gout, and osteoarthritis. The drug had no kidney or liver adverse events.
Cancer was a prespecified secondary outcome in CANTOS. The investigators saw the trial as an opportunity to test a longstanding hypothesis that inhibiting IL-1B would have a positive impact on lung cancer in particular.
“Smoking, exposure to diesel fuel, inhalation of asbestos or other silicates – these cause inflammation which activates the NLRP3 inflammasome, but in the pulmonary system rather than the arteries,” Dr. Ridker explained.
An entry requirement in CANTOS was that patients needed to be free of known cancer. During study follow-up, 129 patients were diagnosed with lung cancer. The risk was reduced in dose-dependent fashion with canakinumab: by 39% relative to placebo in the 150-mg group and by 67% in the 300-mg group. Lung cancer mortality was reduced by 77% in the canakinumab 300-mg group.
“I don’t think this is about oncogenesis per se. I think the tumors are already there, but they don’t progress because we’ve altered the tumor’s inflammatory microenvironment,” he continued.
Since CANTOS was first and foremost a study of atherosclerotic disease prevention, the cancer results need to be replicated on a high-priority basis. Dr. Ridker predicted that Novartis, which sponsored CANTOS, will quickly mount a clinical trial examining canakinumab’s potential as an adjunctive treatment to either chemotherapy or radiation following resection of lung cancer.
He stressed that CANTOS is only the beginning stanza in what will be an entirely new approach to preventive cardiology. Numerous other inflammatory pathways also might serve as targets.
“I think this is going to open up all kinds of approaches using a variety of agents that have really been in the rheumatology and immunology world,” the cardiologist predicted.
For example, he is principal investigator in the ongoing National Heart, Lung, and Blood Institute–sponsored Cardiovascular Inflammation Reduction Trial (CIRT), a randomized, double-blind, placebo-controlled study of low-dose methotrexate for prevention of cardiovascular events in a planned 7,000 patients with type 2 diabetes or metabolic syndrome who’ve had an MI or have multivessel CAD. Results are probably 4-6 years off.
“Right now, we know canakinumab works. If methotrexate were to work, then we’d have a generic, inexpensive approach as well,” Dr. Ridker noted.
Novartis officials indicated that, on the basis of the positive CANTOS results, the company plans to file for an expanded indication for canakinumab for cardiovascular prevention. The company also is gearing up for studies of the drug in oncology.
Simultaneous with Dr. Ridker’s presentation in Barcelona, both the atherosclerotic disease findings (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914) and the cancer findings (Lancet. 2017 Aug 27. doi: 10.1016/S0140-6736(17)32247-X) were published.
He reported serving as a consultant to Novartis.
BARCELONA – Inhibiting the interleukin-1 beta innate immunity pathway with canakinumab reduced recurrent cardiovascular events and lung cancer in the groundbreaking phase III CANTOS trial, Paul M. Ridker, MD, reported at the annual congress of the European Society of Cardiology.
“These data provide the first proof that inflammation inhibition in the absence of lipid lowering can improve atherogenic outcomes and potentially alter progression of some fatal cancers,” declared Dr. Ridker, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
“Just like we’ve learned that lower LDL is better, I think we’re now learning that lower inflammation is better,” he said.
CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study) was a randomized, double-blind, placebo-controlled trial involving 10,061 patients in 39 countries, all of whom had a previous MI and a chronically high level of systemic inflammation as reflected in a median baseline high-sensitivity C-reactive protein (CRP) level of 4.1 mg/L. Ninety-one percent of participants were on statin therapy, with a median LDL cholesterol of 82 mg/dL when randomized to subcutaneous canakinumab at 50, 150, or 300 mg or to placebo once every 3 months.
Canakinumab is a fully human monoclonal antibody targeting IL-1B, a key player in systemic inflammation. The cytokine is activated by the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome, a part of the innate immune system. Canakinumab is approved as Ilaris for treatment of several uncommon rheumatologic diseases, including cryopryin-associated periodic syndrome and systemic juvenile idiopathic arthritis.
At a median follow-up of 3.7 years, the incidence of the primary composite efficacy endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death was 4.5 events per 100 person-years in the control group, significantly higher than the 3.86 and 3.9 events per 100 person-years in patients on canakinumab at 150 and 300 mg, respectively.
Since event rates were virtually identical in the 150- and 300-mg study arms, Dr. Ridker combined those two patient groups in his analysis. They showed a 15% reduction in the risk of the primary efficacy endpoint, compared with placebo-treated controls, along with a 39% reduction from baseline in CRP. They also were 30% less likely to undergo percutaneous coronary intervention or coronary artery bypass graft during follow-up.
“That’s quite important, because that’s a progression-of-atherosclerosis endpoint and also obviously a cost and financial endpoint,” he observed.
A key finding in CANTOS was that patients with a reduction in CRP at or exceeding the median decrease just 3 months into the study – that is, after a single injection – had a 27% reduction in major vascular events during follow-up. Patients with a lesser reduction in CRP at that point did not experience a significant reduction in the primary endpoint, compared with placebo.
“The clinician in me would say we probably ought to give a single dose of the drug, see what happens, and if you get a large inflammation reduction we could perhaps consider treating that patient, but if you did not get a large reduction perhaps this is not a therapy for that patient. Why not avoid the toxicity in people who aren’t going to respond?” Dr. Ridker said.
Side effects related to canakinumab consisted of mild leukopenia and a small but statistically significant increase in fatal infections, which he called “not surprising.”
“It’s in the same range as one gets in treating rheumatoid arthritis with a biologic drug, which rheumatologists are very comfortable doing. You would imagine that if this does become a treatment, physicians will get much better at bringing patients in early when they have signs and symptoms of infection,” the cardiologist continued.
Patients on canakinumab showed significant reductions in incident rheumatoid arthritis, gout, and osteoarthritis. The drug had no kidney or liver adverse events.
Cancer was a prespecified secondary outcome in CANTOS. The investigators saw the trial as an opportunity to test a longstanding hypothesis that inhibiting IL-1B would have a positive impact on lung cancer in particular.
“Smoking, exposure to diesel fuel, inhalation of asbestos or other silicates – these cause inflammation which activates the NLRP3 inflammasome, but in the pulmonary system rather than the arteries,” Dr. Ridker explained.
An entry requirement in CANTOS was that patients needed to be free of known cancer. During study follow-up, 129 patients were diagnosed with lung cancer. The risk was reduced in dose-dependent fashion with canakinumab: by 39% relative to placebo in the 150-mg group and by 67% in the 300-mg group. Lung cancer mortality was reduced by 77% in the canakinumab 300-mg group.
“I don’t think this is about oncogenesis per se. I think the tumors are already there, but they don’t progress because we’ve altered the tumor’s inflammatory microenvironment,” he continued.
Since CANTOS was first and foremost a study of atherosclerotic disease prevention, the cancer results need to be replicated on a high-priority basis. Dr. Ridker predicted that Novartis, which sponsored CANTOS, will quickly mount a clinical trial examining canakinumab’s potential as an adjunctive treatment to either chemotherapy or radiation following resection of lung cancer.
He stressed that CANTOS is only the beginning stanza in what will be an entirely new approach to preventive cardiology. Numerous other inflammatory pathways also might serve as targets.
“I think this is going to open up all kinds of approaches using a variety of agents that have really been in the rheumatology and immunology world,” the cardiologist predicted.
For example, he is principal investigator in the ongoing National Heart, Lung, and Blood Institute–sponsored Cardiovascular Inflammation Reduction Trial (CIRT), a randomized, double-blind, placebo-controlled study of low-dose methotrexate for prevention of cardiovascular events in a planned 7,000 patients with type 2 diabetes or metabolic syndrome who’ve had an MI or have multivessel CAD. Results are probably 4-6 years off.
“Right now, we know canakinumab works. If methotrexate were to work, then we’d have a generic, inexpensive approach as well,” Dr. Ridker noted.
Novartis officials indicated that, on the basis of the positive CANTOS results, the company plans to file for an expanded indication for canakinumab for cardiovascular prevention. The company also is gearing up for studies of the drug in oncology.
Simultaneous with Dr. Ridker’s presentation in Barcelona, both the atherosclerotic disease findings (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914) and the cancer findings (Lancet. 2017 Aug 27. doi: 10.1016/S0140-6736(17)32247-X) were published.
He reported serving as a consultant to Novartis.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: Canakinumab reduced the risk of recurrent cardiovascular events in a very-high-risk population by 15%, compared with placebo, while cutting incident lung cancer by 67% in a major clinical trial.
Data source: CANTOS was a phase III, randomized, double-blind, placebo-controlled trial involving 10,061 patients in 39 countries, all with a previous MI and chronically high systemic inflammation.
Disclosures: The study was sponsored by Novartis. The presenter reported serving as a consultant to the company.
Forgo supplemental oxygen in adequately perfused patients with acute MI, study suggests
Supplemental oxygen did not prevent mortality or rehospitalization among patients with suspected myocardial infarction whose oxygen saturation on room air exceeded 90%, investigators reported.
Rates of all-cause mortality at 1 year were 5% among patients who received supplemental oxygen through an open face mask (6 liters per minute for 6-12 hours) and 5.1% among patients who breathed room air, said Robin Hofmann, MD, of Karolinska Institutet, Stockholm, and his associates. In addition, rehospitalization for MI occurred in 3.8% of patients who received supplemental oxygen and 3.3% of those breathed room air. The findings of the randomized registry-based trial of 6,629 patients were presented at the annual congress of the European Society of Cardiology and published simultaneously in the New England Journal of Medicine.
Guidelines recommend oxygen supplementation in MI, and the practice has persisted for more than a century, but adequately powered trials of hard clinical endpoints are lacking. Above-normal oxygen saturation can potentially worsen reperfusion injury by causing coronary vasoconstriction and increasing production of reactive oxygen species, the researchers noted.
Notably, the Australian Air Versus Oxygen in Myocardial Infarction (AVOID) trial found that oxygen supplementation was associated with larger infarct sizes in patients with ST-segment elevation myocardial infarction, and a recent Cochrane report did not support routine oxygen supplementation for MI.
The current trial enrolled patients aged 30 years and older who had chest pain or shortness of breath lasting less than 6 hours, an oxygen saturation of at least 90% on pulse oximetry, and either electrocardiographic evidence of ischemia or elevated cardiac troponin T or I levels (N Engl J Med. 2017 Aug 28. doi: 10.1056/NEJMoa1706222).
Oxygen therapy lasted a median of 11.6 hours, after which median oxygen saturation levels were 99% in the intervention group and 97% in the control group.
A total of 62 patients (2%) who received oxygen developed hypoxemia, as did 254 patients (8%) who breathed room air. Median highest troponin levels during hospitalization were 946.5 ng per L and 983.0 ng per L, respectively. A total of 166 (5%) patients in the oxygen group and 168 (5.1%) control patients died from any cause by a year after treatment (hazard ratio, 0.97; P = .8). Likewise, supplemental oxygen did not prevent rehospitalization with MI within 1 year (HR, 1.13; P = .3).
“Because power for evaluation of the primary endpoint was lower than anticipated, we cannot completely rule out a small beneficial or detrimental effect of oxygen on mortality,” the researchers wrote. But clinical differences were unlikely, based on the superimposable time-to-event curves through 12 months, the consistent results across subgroups, and the neutral findings on secondary clinical endpoints, they added.
The Swedish Research Council, the Swedish Heart-Lung Foundation, and the Swedish Foundation for Strategic Research funded the study. Dr. Hofmann disclosed research grants from these entities.
The study by Hofmann and coworkers provides definitive evidence for a lack of benefit of supplemental oxygen therapy in patients with acute myocardial infarction who have normal oxygen saturation. Although the mechanisms underlying physiological and biochemical adaptation to myocardial ischemia are complex, the answer to the question is straightforward, and its implications for coronary care are indisputable: Supplemental oxygen provides no benefit to patients with acute coronary syndromes who do not have hypoxemia. It is clearly time for clinical practice to change to reflect this definitive evidence.
Joseph Loscalzo, MD, PhD, is in the department of medicine, Brigham and Women’s Hospital, Boston. He is an editor-at-large for the New England Journal of Medicine. He had no other disclosures. These comments are from his accompanying editorial (N Engl J Med. 2017 Aug 28. doi: 10.1056/NEJMe1709250).
The study by Hofmann and coworkers provides definitive evidence for a lack of benefit of supplemental oxygen therapy in patients with acute myocardial infarction who have normal oxygen saturation. Although the mechanisms underlying physiological and biochemical adaptation to myocardial ischemia are complex, the answer to the question is straightforward, and its implications for coronary care are indisputable: Supplemental oxygen provides no benefit to patients with acute coronary syndromes who do not have hypoxemia. It is clearly time for clinical practice to change to reflect this definitive evidence.
Joseph Loscalzo, MD, PhD, is in the department of medicine, Brigham and Women’s Hospital, Boston. He is an editor-at-large for the New England Journal of Medicine. He had no other disclosures. These comments are from his accompanying editorial (N Engl J Med. 2017 Aug 28. doi: 10.1056/NEJMe1709250).
The study by Hofmann and coworkers provides definitive evidence for a lack of benefit of supplemental oxygen therapy in patients with acute myocardial infarction who have normal oxygen saturation. Although the mechanisms underlying physiological and biochemical adaptation to myocardial ischemia are complex, the answer to the question is straightforward, and its implications for coronary care are indisputable: Supplemental oxygen provides no benefit to patients with acute coronary syndromes who do not have hypoxemia. It is clearly time for clinical practice to change to reflect this definitive evidence.
Joseph Loscalzo, MD, PhD, is in the department of medicine, Brigham and Women’s Hospital, Boston. He is an editor-at-large for the New England Journal of Medicine. He had no other disclosures. These comments are from his accompanying editorial (N Engl J Med. 2017 Aug 28. doi: 10.1056/NEJMe1709250).
Supplemental oxygen did not prevent mortality or rehospitalization among patients with suspected myocardial infarction whose oxygen saturation on room air exceeded 90%, investigators reported.
Rates of all-cause mortality at 1 year were 5% among patients who received supplemental oxygen through an open face mask (6 liters per minute for 6-12 hours) and 5.1% among patients who breathed room air, said Robin Hofmann, MD, of Karolinska Institutet, Stockholm, and his associates. In addition, rehospitalization for MI occurred in 3.8% of patients who received supplemental oxygen and 3.3% of those breathed room air. The findings of the randomized registry-based trial of 6,629 patients were presented at the annual congress of the European Society of Cardiology and published simultaneously in the New England Journal of Medicine.
Guidelines recommend oxygen supplementation in MI, and the practice has persisted for more than a century, but adequately powered trials of hard clinical endpoints are lacking. Above-normal oxygen saturation can potentially worsen reperfusion injury by causing coronary vasoconstriction and increasing production of reactive oxygen species, the researchers noted.
Notably, the Australian Air Versus Oxygen in Myocardial Infarction (AVOID) trial found that oxygen supplementation was associated with larger infarct sizes in patients with ST-segment elevation myocardial infarction, and a recent Cochrane report did not support routine oxygen supplementation for MI.
The current trial enrolled patients aged 30 years and older who had chest pain or shortness of breath lasting less than 6 hours, an oxygen saturation of at least 90% on pulse oximetry, and either electrocardiographic evidence of ischemia or elevated cardiac troponin T or I levels (N Engl J Med. 2017 Aug 28. doi: 10.1056/NEJMoa1706222).
Oxygen therapy lasted a median of 11.6 hours, after which median oxygen saturation levels were 99% in the intervention group and 97% in the control group.
A total of 62 patients (2%) who received oxygen developed hypoxemia, as did 254 patients (8%) who breathed room air. Median highest troponin levels during hospitalization were 946.5 ng per L and 983.0 ng per L, respectively. A total of 166 (5%) patients in the oxygen group and 168 (5.1%) control patients died from any cause by a year after treatment (hazard ratio, 0.97; P = .8). Likewise, supplemental oxygen did not prevent rehospitalization with MI within 1 year (HR, 1.13; P = .3).
“Because power for evaluation of the primary endpoint was lower than anticipated, we cannot completely rule out a small beneficial or detrimental effect of oxygen on mortality,” the researchers wrote. But clinical differences were unlikely, based on the superimposable time-to-event curves through 12 months, the consistent results across subgroups, and the neutral findings on secondary clinical endpoints, they added.
The Swedish Research Council, the Swedish Heart-Lung Foundation, and the Swedish Foundation for Strategic Research funded the study. Dr. Hofmann disclosed research grants from these entities.
Supplemental oxygen did not prevent mortality or rehospitalization among patients with suspected myocardial infarction whose oxygen saturation on room air exceeded 90%, investigators reported.
Rates of all-cause mortality at 1 year were 5% among patients who received supplemental oxygen through an open face mask (6 liters per minute for 6-12 hours) and 5.1% among patients who breathed room air, said Robin Hofmann, MD, of Karolinska Institutet, Stockholm, and his associates. In addition, rehospitalization for MI occurred in 3.8% of patients who received supplemental oxygen and 3.3% of those breathed room air. The findings of the randomized registry-based trial of 6,629 patients were presented at the annual congress of the European Society of Cardiology and published simultaneously in the New England Journal of Medicine.
Guidelines recommend oxygen supplementation in MI, and the practice has persisted for more than a century, but adequately powered trials of hard clinical endpoints are lacking. Above-normal oxygen saturation can potentially worsen reperfusion injury by causing coronary vasoconstriction and increasing production of reactive oxygen species, the researchers noted.
Notably, the Australian Air Versus Oxygen in Myocardial Infarction (AVOID) trial found that oxygen supplementation was associated with larger infarct sizes in patients with ST-segment elevation myocardial infarction, and a recent Cochrane report did not support routine oxygen supplementation for MI.
The current trial enrolled patients aged 30 years and older who had chest pain or shortness of breath lasting less than 6 hours, an oxygen saturation of at least 90% on pulse oximetry, and either electrocardiographic evidence of ischemia or elevated cardiac troponin T or I levels (N Engl J Med. 2017 Aug 28. doi: 10.1056/NEJMoa1706222).
Oxygen therapy lasted a median of 11.6 hours, after which median oxygen saturation levels were 99% in the intervention group and 97% in the control group.
A total of 62 patients (2%) who received oxygen developed hypoxemia, as did 254 patients (8%) who breathed room air. Median highest troponin levels during hospitalization were 946.5 ng per L and 983.0 ng per L, respectively. A total of 166 (5%) patients in the oxygen group and 168 (5.1%) control patients died from any cause by a year after treatment (hazard ratio, 0.97; P = .8). Likewise, supplemental oxygen did not prevent rehospitalization with MI within 1 year (HR, 1.13; P = .3).
“Because power for evaluation of the primary endpoint was lower than anticipated, we cannot completely rule out a small beneficial or detrimental effect of oxygen on mortality,” the researchers wrote. But clinical differences were unlikely, based on the superimposable time-to-event curves through 12 months, the consistent results across subgroups, and the neutral findings on secondary clinical endpoints, they added.
The Swedish Research Council, the Swedish Heart-Lung Foundation, and the Swedish Foundation for Strategic Research funded the study. Dr. Hofmann disclosed research grants from these entities.
FROM THE ESC CONGRESS 2017
Key clinical point: Supplemental oxygen did not benefit patients with suspected myocardial infarction who did not have hypoxemia.
Major finding: At 1 year, rates of all-cause mortality were 5% among patients who received supplemental oxygen and 5.1% among those who received no oxygen.
Data source: A registry-based, randomized clinical trial of 6,629 patients with suspected myocardial infarction without hypoxemia.
Disclosures: The Swedish Research Council, the Swedish Heart-Lung Foundation, and the Swedish Foundation for Strategic Research funded the study. Dr. Hofmann disclosed research grants from these entities.
Study finds bivalirudin efficacy for PCI no better than heparin
A large study of more than 6,000 heart patients in Sweden has found that patients having percutaneous coronary intervention who received bivalirudin did not have lower rates of deleterious outcomes – death, heart attack, or major bleeding – than did patients who received heparin monotherapy, a contrast to previous trials that found that bivalirudin had a lower bleeding risk than heparin alone after PCI.
The findings were presented at the annual congress of the European Society of Cardiology and published simultaneously in the New England Journal of Medicine.
The study sought to explain the conflicting findings of previous trials investigating the efficacy of bivalirudin vs. heparin monotherapy. The VALIDATE-SWEDEHEART trial evaluated 6,006 patients who had PCI from June 2014 to September 2016, 90.3% via radial-artery access. This trial differed from previous studies because it was conducted after radial-artery access was routine and potent P2Y12 inhibitors were available, and earlier trials did not compare bivalirudin to heparin monotherapy, said David Erlinge, MD, PhD, of Lund (Sweden) University, and 38 coauthors (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1706443).
The Swedish investigators evaluated the primary endpoint – the composite of any-cause death, MI or major bleeding – during 180 days of follow-up. Among the study patients, 3,005 had ST-segment elevation MI (STEMI) and 3,001 non-STEMI (NSTEMI). All had undergone urgent PCI and most were also on P2Y inhibitors. The P2Y12 inhibitors used were ticagrelor in 5,697 patients (94.9%), prasugrel in 125 (2.1%) and cangrelor in 21 (0.3%).
Study patients with STEMI were permitted to receive up to 5,000 U of intravenous unfractionated heparin before arrival in the catheterization laboratory, and both STEMI and non-STEMI patients who had not received heparin previously could receive up to 3,000 U of intra-arterial heparin before angiography. All patients received aspirin pretreatment, and 62% received potent P2Y12 inhibitors at least one hour before PCI.
After angiography, but before PCI, patients were randomized 1:1 to receive in an open-label fashion either intravenous bivalirudin (The Medicines Company), or intra-arterial unfractionated heparin (LEO Pharma). Bivalirudin was administered as a bolus of 0.75 mg/kg of body weight followed by an infusion of 1.7 mg/kg per hour.
Research nurses contacted patients by phone 7 and 180 days after PCI. Baseline characteristics were similar between the bivalirudin and heparin groups. For example, around 31% of both groups had hyperlipidemia, and 15.2% of the bivalirudin group and 14.2% of the heparin group had a previous PCI.
“The rate of the primary endpoint did not differ significantly between the treatment groups at 30 days after PCI,” Dr. Erlinge and his coauthors noted. At 30 days, 7.2% of the bivalirudin patients and 8% of the heparin group had one of the primary endpoint outcomes, a nonsignificant difference. At 180 days, 12.3% of the bivalirudin group and 12.8% of those receiving heparin had one of the primary endpoint outcomes, also a nonsignificant difference.
Specific outcomes in the bivalirudin vs. heparin patients, respectively, at 180 days were: MI, 2% vs. 2.4%; major bleeding, 8.6% in both groups; stent thrombosis, 0.4% vs. 0.7%; and death from any cause, 2.9% vs. 2.8%, all nonsignificant differences.
“Results were consistent between patients with STEMI and those with NSTEMI and across all other prespecified subgroups,” the researchers wrote. They noted that women in the bivalirudin group had a lower, although not statistically significant, primary endpoint rate than did women in the heparin group.
In this trial, the high rate of radial-artery access and the low use of glycoprotein IIb/IIIa inhibitors may explain the low bleeding rates, the researchers said.
Among the study limitations were that patients excluded from the trial were at higher risk for a primary endpoint than those enrolled, the open-label design may have biased participating physicians in identifying outcomes, the telephone call-based follow-up may have been inherently unreliable, and the fact that most patients received a small dose of heparin before randomization may have reconciled any differences between the two drugs.
Coauthors Stefan James, MD, and Ollie Ostlund, MD, disclosed receiving grants from Astra Zeneca, and The Medicines Company. Dr. Erlinge and other coauthors had no financial relationships relevant to the work.
After considering the findings of the VALIDATE-SWEDEHEART trial, Gregg W. Stone, MD, said in an accompanying editorial, “there is no definitive answer to the question of whether to use bivalirudin or heparin during PCI.”
Dr. Stone, of New York–Presbyterian Hospital, Columbia University Medical Center, and the Cardiovascular Research Foundation, New York, noted four potential flaws in the study findings. One, the 30-day interval may be a better for evaluating procedural anticoagulation than 180 days – and at 30 days the Swedish study showed “a nonsignificant trend in favor of bivalirudin.” Two, the composite primary endpoint could bias outcomes because individual measures could essentially cancel each other out. Three, differences between treatment groups could have been further minimized because 91% of patients who received bivalirudin also received a substantial dose of heparin before and during PCI. Finally, Dr. Stone said, the study was underpowered to examine the individual components of outcomes.
The data comparing outcomes in STEMI and NSTEMI patients did not show separate results for death, bleeding, and stent thrombosis. Dr. Stone pointed to a meta-analysis of six randomized trials of 14,095 patients with STEMI, showing that bivalirudin had lower rates of major bleeding and 30-day death but higher rates of stent thrombosis than heparin, and that mortality was lower regardless of the use of femoral artery or radial-artery access or other procedural factors. By contrast, previous trials did show similar rates of death, MI, and stent thrombosis between both treatment groups, although lower bleeding rates were seen with bivalirudin.
More definitive answers may lie in investigators from the large-scale randomized trials comparing the anticoagulant agents, including the Swedish authors, combining their data on more than 36,000 patients into a single database, as they have agreed to do, Dr. Stone said. That “should provide robust evidence to guide decisions regarding anticoagulation among patients with STEMI and NSTEMI,” he concluded.
Dr. Stone had no relevant financial relationships to disclose. He made his comments in an invited editorial in the New England Journal of Medicine (doi: 10.1056/NEJMe1709247).
After considering the findings of the VALIDATE-SWEDEHEART trial, Gregg W. Stone, MD, said in an accompanying editorial, “there is no definitive answer to the question of whether to use bivalirudin or heparin during PCI.”
Dr. Stone, of New York–Presbyterian Hospital, Columbia University Medical Center, and the Cardiovascular Research Foundation, New York, noted four potential flaws in the study findings. One, the 30-day interval may be a better for evaluating procedural anticoagulation than 180 days – and at 30 days the Swedish study showed “a nonsignificant trend in favor of bivalirudin.” Two, the composite primary endpoint could bias outcomes because individual measures could essentially cancel each other out. Three, differences between treatment groups could have been further minimized because 91% of patients who received bivalirudin also received a substantial dose of heparin before and during PCI. Finally, Dr. Stone said, the study was underpowered to examine the individual components of outcomes.
The data comparing outcomes in STEMI and NSTEMI patients did not show separate results for death, bleeding, and stent thrombosis. Dr. Stone pointed to a meta-analysis of six randomized trials of 14,095 patients with STEMI, showing that bivalirudin had lower rates of major bleeding and 30-day death but higher rates of stent thrombosis than heparin, and that mortality was lower regardless of the use of femoral artery or radial-artery access or other procedural factors. By contrast, previous trials did show similar rates of death, MI, and stent thrombosis between both treatment groups, although lower bleeding rates were seen with bivalirudin.
More definitive answers may lie in investigators from the large-scale randomized trials comparing the anticoagulant agents, including the Swedish authors, combining their data on more than 36,000 patients into a single database, as they have agreed to do, Dr. Stone said. That “should provide robust evidence to guide decisions regarding anticoagulation among patients with STEMI and NSTEMI,” he concluded.
Dr. Stone had no relevant financial relationships to disclose. He made his comments in an invited editorial in the New England Journal of Medicine (doi: 10.1056/NEJMe1709247).
After considering the findings of the VALIDATE-SWEDEHEART trial, Gregg W. Stone, MD, said in an accompanying editorial, “there is no definitive answer to the question of whether to use bivalirudin or heparin during PCI.”
Dr. Stone, of New York–Presbyterian Hospital, Columbia University Medical Center, and the Cardiovascular Research Foundation, New York, noted four potential flaws in the study findings. One, the 30-day interval may be a better for evaluating procedural anticoagulation than 180 days – and at 30 days the Swedish study showed “a nonsignificant trend in favor of bivalirudin.” Two, the composite primary endpoint could bias outcomes because individual measures could essentially cancel each other out. Three, differences between treatment groups could have been further minimized because 91% of patients who received bivalirudin also received a substantial dose of heparin before and during PCI. Finally, Dr. Stone said, the study was underpowered to examine the individual components of outcomes.
The data comparing outcomes in STEMI and NSTEMI patients did not show separate results for death, bleeding, and stent thrombosis. Dr. Stone pointed to a meta-analysis of six randomized trials of 14,095 patients with STEMI, showing that bivalirudin had lower rates of major bleeding and 30-day death but higher rates of stent thrombosis than heparin, and that mortality was lower regardless of the use of femoral artery or radial-artery access or other procedural factors. By contrast, previous trials did show similar rates of death, MI, and stent thrombosis between both treatment groups, although lower bleeding rates were seen with bivalirudin.
More definitive answers may lie in investigators from the large-scale randomized trials comparing the anticoagulant agents, including the Swedish authors, combining their data on more than 36,000 patients into a single database, as they have agreed to do, Dr. Stone said. That “should provide robust evidence to guide decisions regarding anticoagulation among patients with STEMI and NSTEMI,” he concluded.
Dr. Stone had no relevant financial relationships to disclose. He made his comments in an invited editorial in the New England Journal of Medicine (doi: 10.1056/NEJMe1709247).
A large study of more than 6,000 heart patients in Sweden has found that patients having percutaneous coronary intervention who received bivalirudin did not have lower rates of deleterious outcomes – death, heart attack, or major bleeding – than did patients who received heparin monotherapy, a contrast to previous trials that found that bivalirudin had a lower bleeding risk than heparin alone after PCI.
The findings were presented at the annual congress of the European Society of Cardiology and published simultaneously in the New England Journal of Medicine.
The study sought to explain the conflicting findings of previous trials investigating the efficacy of bivalirudin vs. heparin monotherapy. The VALIDATE-SWEDEHEART trial evaluated 6,006 patients who had PCI from June 2014 to September 2016, 90.3% via radial-artery access. This trial differed from previous studies because it was conducted after radial-artery access was routine and potent P2Y12 inhibitors were available, and earlier trials did not compare bivalirudin to heparin monotherapy, said David Erlinge, MD, PhD, of Lund (Sweden) University, and 38 coauthors (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1706443).
The Swedish investigators evaluated the primary endpoint – the composite of any-cause death, MI or major bleeding – during 180 days of follow-up. Among the study patients, 3,005 had ST-segment elevation MI (STEMI) and 3,001 non-STEMI (NSTEMI). All had undergone urgent PCI and most were also on P2Y inhibitors. The P2Y12 inhibitors used were ticagrelor in 5,697 patients (94.9%), prasugrel in 125 (2.1%) and cangrelor in 21 (0.3%).
Study patients with STEMI were permitted to receive up to 5,000 U of intravenous unfractionated heparin before arrival in the catheterization laboratory, and both STEMI and non-STEMI patients who had not received heparin previously could receive up to 3,000 U of intra-arterial heparin before angiography. All patients received aspirin pretreatment, and 62% received potent P2Y12 inhibitors at least one hour before PCI.
After angiography, but before PCI, patients were randomized 1:1 to receive in an open-label fashion either intravenous bivalirudin (The Medicines Company), or intra-arterial unfractionated heparin (LEO Pharma). Bivalirudin was administered as a bolus of 0.75 mg/kg of body weight followed by an infusion of 1.7 mg/kg per hour.
Research nurses contacted patients by phone 7 and 180 days after PCI. Baseline characteristics were similar between the bivalirudin and heparin groups. For example, around 31% of both groups had hyperlipidemia, and 15.2% of the bivalirudin group and 14.2% of the heparin group had a previous PCI.
“The rate of the primary endpoint did not differ significantly between the treatment groups at 30 days after PCI,” Dr. Erlinge and his coauthors noted. At 30 days, 7.2% of the bivalirudin patients and 8% of the heparin group had one of the primary endpoint outcomes, a nonsignificant difference. At 180 days, 12.3% of the bivalirudin group and 12.8% of those receiving heparin had one of the primary endpoint outcomes, also a nonsignificant difference.
Specific outcomes in the bivalirudin vs. heparin patients, respectively, at 180 days were: MI, 2% vs. 2.4%; major bleeding, 8.6% in both groups; stent thrombosis, 0.4% vs. 0.7%; and death from any cause, 2.9% vs. 2.8%, all nonsignificant differences.
“Results were consistent between patients with STEMI and those with NSTEMI and across all other prespecified subgroups,” the researchers wrote. They noted that women in the bivalirudin group had a lower, although not statistically significant, primary endpoint rate than did women in the heparin group.
In this trial, the high rate of radial-artery access and the low use of glycoprotein IIb/IIIa inhibitors may explain the low bleeding rates, the researchers said.
Among the study limitations were that patients excluded from the trial were at higher risk for a primary endpoint than those enrolled, the open-label design may have biased participating physicians in identifying outcomes, the telephone call-based follow-up may have been inherently unreliable, and the fact that most patients received a small dose of heparin before randomization may have reconciled any differences between the two drugs.
Coauthors Stefan James, MD, and Ollie Ostlund, MD, disclosed receiving grants from Astra Zeneca, and The Medicines Company. Dr. Erlinge and other coauthors had no financial relationships relevant to the work.
A large study of more than 6,000 heart patients in Sweden has found that patients having percutaneous coronary intervention who received bivalirudin did not have lower rates of deleterious outcomes – death, heart attack, or major bleeding – than did patients who received heparin monotherapy, a contrast to previous trials that found that bivalirudin had a lower bleeding risk than heparin alone after PCI.
The findings were presented at the annual congress of the European Society of Cardiology and published simultaneously in the New England Journal of Medicine.
The study sought to explain the conflicting findings of previous trials investigating the efficacy of bivalirudin vs. heparin monotherapy. The VALIDATE-SWEDEHEART trial evaluated 6,006 patients who had PCI from June 2014 to September 2016, 90.3% via radial-artery access. This trial differed from previous studies because it was conducted after radial-artery access was routine and potent P2Y12 inhibitors were available, and earlier trials did not compare bivalirudin to heparin monotherapy, said David Erlinge, MD, PhD, of Lund (Sweden) University, and 38 coauthors (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1706443).
The Swedish investigators evaluated the primary endpoint – the composite of any-cause death, MI or major bleeding – during 180 days of follow-up. Among the study patients, 3,005 had ST-segment elevation MI (STEMI) and 3,001 non-STEMI (NSTEMI). All had undergone urgent PCI and most were also on P2Y inhibitors. The P2Y12 inhibitors used were ticagrelor in 5,697 patients (94.9%), prasugrel in 125 (2.1%) and cangrelor in 21 (0.3%).
Study patients with STEMI were permitted to receive up to 5,000 U of intravenous unfractionated heparin before arrival in the catheterization laboratory, and both STEMI and non-STEMI patients who had not received heparin previously could receive up to 3,000 U of intra-arterial heparin before angiography. All patients received aspirin pretreatment, and 62% received potent P2Y12 inhibitors at least one hour before PCI.
After angiography, but before PCI, patients were randomized 1:1 to receive in an open-label fashion either intravenous bivalirudin (The Medicines Company), or intra-arterial unfractionated heparin (LEO Pharma). Bivalirudin was administered as a bolus of 0.75 mg/kg of body weight followed by an infusion of 1.7 mg/kg per hour.
Research nurses contacted patients by phone 7 and 180 days after PCI. Baseline characteristics were similar between the bivalirudin and heparin groups. For example, around 31% of both groups had hyperlipidemia, and 15.2% of the bivalirudin group and 14.2% of the heparin group had a previous PCI.
“The rate of the primary endpoint did not differ significantly between the treatment groups at 30 days after PCI,” Dr. Erlinge and his coauthors noted. At 30 days, 7.2% of the bivalirudin patients and 8% of the heparin group had one of the primary endpoint outcomes, a nonsignificant difference. At 180 days, 12.3% of the bivalirudin group and 12.8% of those receiving heparin had one of the primary endpoint outcomes, also a nonsignificant difference.
Specific outcomes in the bivalirudin vs. heparin patients, respectively, at 180 days were: MI, 2% vs. 2.4%; major bleeding, 8.6% in both groups; stent thrombosis, 0.4% vs. 0.7%; and death from any cause, 2.9% vs. 2.8%, all nonsignificant differences.
“Results were consistent between patients with STEMI and those with NSTEMI and across all other prespecified subgroups,” the researchers wrote. They noted that women in the bivalirudin group had a lower, although not statistically significant, primary endpoint rate than did women in the heparin group.
In this trial, the high rate of radial-artery access and the low use of glycoprotein IIb/IIIa inhibitors may explain the low bleeding rates, the researchers said.
Among the study limitations were that patients excluded from the trial were at higher risk for a primary endpoint than those enrolled, the open-label design may have biased participating physicians in identifying outcomes, the telephone call-based follow-up may have been inherently unreliable, and the fact that most patients received a small dose of heparin before randomization may have reconciled any differences between the two drugs.
Coauthors Stefan James, MD, and Ollie Ostlund, MD, disclosed receiving grants from Astra Zeneca, and The Medicines Company. Dr. Erlinge and other coauthors had no financial relationships relevant to the work.
FROM THE ESC CONGRESS 2017
Key clinical point: The rates of composite death, MI, or major bleeding for patients having PCI for MI were similar regardless of whether they received bivalirudin or heparin monotherapy.
Major finding: At 180 days, 12.3% of the bivalirudin patients and 12.8% of the heparin patients had one of the primary endpoint outcomes.
Data source: VALIDATE-SWEDEHEART, a registry-based, multicenter, randomized, controlled, open-label clinical trial of 6,006 patients who had PCI between June 2014 and September 2016.
Disclosure: Coauthors Stefan James, MD, and Ollie Ostlund, MD, disclosed receiving grants from AstraZeneca, and The Medicines Company. Dr. Erlinge and other coauthors had no financial relationships relevant to the work.
VIDEO: Inflammation’s role in atherosclerosis confirmed in CANTOS
BARCELONA – The results of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) mark the validation of many years of research on inflammation for Peter Libby, MD, Mallinckrodt Professor of Medicine, Harvard Medical School, Boston.
The CANTOS investigator said that, although some trials, most notably JUPITER, have linked reduced markers of inflammation with reduced cardiovascular events, none have been able to separate the effects of lowering LDL cholesterol from those of lowering the inflammatory marker interleukin-1B.
But using the monoclonal antibody canakinumab to target only interleukin-1B in CANTOS reduced the composite endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death by 15% at the highest dosage tested, compared with placebo, while lowering high-sensitivity C-reactive protein by 39 percentage points.
Dr. Libby has been studying interleukin-1B since the 1980s. “Now, today, for the first time, in a rigorous trial, we can show that an anti-inflammatory agent that is neutral for lipids (that doesn’t lower LDL) can provide a benefit for our patients, and that’s a real step forward,” Dr. Libby said in a video interview at the annual congress of the European Society of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Importantly, a dividend of the investigation was that “we found a decrease in fatal cancers, particularly lung cancer. So this again opens the door toward a whole new therapeutic window in patients not just in the cardiovascular space, but also in oncology. So it’s a doubly exciting day for us.”
CANTOS was presented at the meeting by Paul Ridker, MD, also of Harvard Medical School; the results were also published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
BARCELONA – The results of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) mark the validation of many years of research on inflammation for Peter Libby, MD, Mallinckrodt Professor of Medicine, Harvard Medical School, Boston.
The CANTOS investigator said that, although some trials, most notably JUPITER, have linked reduced markers of inflammation with reduced cardiovascular events, none have been able to separate the effects of lowering LDL cholesterol from those of lowering the inflammatory marker interleukin-1B.
But using the monoclonal antibody canakinumab to target only interleukin-1B in CANTOS reduced the composite endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death by 15% at the highest dosage tested, compared with placebo, while lowering high-sensitivity C-reactive protein by 39 percentage points.
Dr. Libby has been studying interleukin-1B since the 1980s. “Now, today, for the first time, in a rigorous trial, we can show that an anti-inflammatory agent that is neutral for lipids (that doesn’t lower LDL) can provide a benefit for our patients, and that’s a real step forward,” Dr. Libby said in a video interview at the annual congress of the European Society of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Importantly, a dividend of the investigation was that “we found a decrease in fatal cancers, particularly lung cancer. So this again opens the door toward a whole new therapeutic window in patients not just in the cardiovascular space, but also in oncology. So it’s a doubly exciting day for us.”
CANTOS was presented at the meeting by Paul Ridker, MD, also of Harvard Medical School; the results were also published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
BARCELONA – The results of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) mark the validation of many years of research on inflammation for Peter Libby, MD, Mallinckrodt Professor of Medicine, Harvard Medical School, Boston.
The CANTOS investigator said that, although some trials, most notably JUPITER, have linked reduced markers of inflammation with reduced cardiovascular events, none have been able to separate the effects of lowering LDL cholesterol from those of lowering the inflammatory marker interleukin-1B.
But using the monoclonal antibody canakinumab to target only interleukin-1B in CANTOS reduced the composite endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death by 15% at the highest dosage tested, compared with placebo, while lowering high-sensitivity C-reactive protein by 39 percentage points.
Dr. Libby has been studying interleukin-1B since the 1980s. “Now, today, for the first time, in a rigorous trial, we can show that an anti-inflammatory agent that is neutral for lipids (that doesn’t lower LDL) can provide a benefit for our patients, and that’s a real step forward,” Dr. Libby said in a video interview at the annual congress of the European Society of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Importantly, a dividend of the investigation was that “we found a decrease in fatal cancers, particularly lung cancer. So this again opens the door toward a whole new therapeutic window in patients not just in the cardiovascular space, but also in oncology. So it’s a doubly exciting day for us.”
CANTOS was presented at the meeting by Paul Ridker, MD, also of Harvard Medical School; the results were also published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
AT THE ESC CONGRESS 2017
Orsiro coronary DES outperforms Xience
BARCELONA – A new model of drug-eluting coronary stent outperformed the reigning benchmark Xience stent in a head-to-head, pivotal comparison with 1,334 patients.
The results “advance a new standard for drug eluting stents,” David E. Kandzari, MD, said at the annual congress of the European Society of Cardiology. The trial tested the Orsiro stent, which features very thin, 60-micron-thick cobalt chromium struts, a bioresorbable polymer, and sirolimus as the antiproliferative drug that’s released during the first 90 days of stent placement.
“To our knowledge, this is the only trial that has demonstrated superiority [of a new stent] to the Xience drug-eluting stent in a large, randomized trial. This is a landmark in interventional cardiology that raises the bar for future comparisons” of drug eluting coronary stents, Dr. Kandzari said in an interview.
The study’s primary endpoint of target lesion failure after 12 months of follow-up – a rate that combined the incidence of cardiovascular death, target-vessel related MI, and ischemia-driven target-lesion revascularization – stood at 6.2% for the 884 patients treated with the Orsiro stent and 9.6% of the 450 randomized to treatment with the Xience stent, which has struts that are 81 microns wide, and a durable polymer that releases everolimus as the antiproliferative drug.
The difference in the primary endpoint was driven primarily by a 3.6% absolute difference in the rate of target-vessel related MIs, a statistically significant difference, plus the Orsiro-treated patients showed numerically smaller rates of cardiac death and ischemia-driven target lesion revascularization, although the between-group differences for each of these two endpoints were not statistically significant. The Orsiro stent also showed a significantly reduced rate of late stent thrombosis, occurring during day 31 through 1 year, a 0.1% rate in the Orsiro-treated patients and a 0.9% rate in those who received Xience stents.
“These are remarkable results,” said Michael Haude, MD, an interventional cardiologist at Lukas Hospital in Neuss, Germany, and a cochair of the session in which Dr. Kandzari gave his report.
These results from the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in Subjects With Coronary Artery Lesions (BIOFLOW-V) trial will be the centerpiece of an application for U.S. marketing approval for the Orsiro stent, Dr. Kandzari said. The BIOFLOW-V trial enrolled patients at 90 centers in 13 countries including the United States.
Concurrently with his report, the results were published online (Lancet. 2017 Aug 26. doi: 10.1016/S0140-6736[17]32249-3).
The potential advantage of a thinner-strut drug eluting stent showed up during the initial treatment phase, with a procedural success rate of 94% using the Orsiro stent and 90% with the Xience stent. This difference in procedural success seemed largely the result of an increased rate of periprocedural MIs, a difference that might be explained by the difference in strut thickness, said Dr. Kandzari, of Piedmont Heart Institute, Atlanta. Based on this success, designers of future drug-eluting stents may focus on thinner-strut models, he suggested.
An additional analysis that Dr. Kandzari reported combined results from two prior randomized comparisons of the Orsiro and Xience stents, creating a pooled analysis with 2,208 patients. This Bayesian analysis calculated a 100% probability of noninferiority of the Orsiro stent compared with the Xience stent, and a 97% probability of superiority. This 97% probability of superiority fell just short of the 97.5% threshold for establishing superiority that Dr. Kandzari and his associates had prespecified for this analysis.
[email protected]
On Twitter @mitchelzoler
BARCELONA – A new model of drug-eluting coronary stent outperformed the reigning benchmark Xience stent in a head-to-head, pivotal comparison with 1,334 patients.
The results “advance a new standard for drug eluting stents,” David E. Kandzari, MD, said at the annual congress of the European Society of Cardiology. The trial tested the Orsiro stent, which features very thin, 60-micron-thick cobalt chromium struts, a bioresorbable polymer, and sirolimus as the antiproliferative drug that’s released during the first 90 days of stent placement.
“To our knowledge, this is the only trial that has demonstrated superiority [of a new stent] to the Xience drug-eluting stent in a large, randomized trial. This is a landmark in interventional cardiology that raises the bar for future comparisons” of drug eluting coronary stents, Dr. Kandzari said in an interview.
The study’s primary endpoint of target lesion failure after 12 months of follow-up – a rate that combined the incidence of cardiovascular death, target-vessel related MI, and ischemia-driven target-lesion revascularization – stood at 6.2% for the 884 patients treated with the Orsiro stent and 9.6% of the 450 randomized to treatment with the Xience stent, which has struts that are 81 microns wide, and a durable polymer that releases everolimus as the antiproliferative drug.
The difference in the primary endpoint was driven primarily by a 3.6% absolute difference in the rate of target-vessel related MIs, a statistically significant difference, plus the Orsiro-treated patients showed numerically smaller rates of cardiac death and ischemia-driven target lesion revascularization, although the between-group differences for each of these two endpoints were not statistically significant. The Orsiro stent also showed a significantly reduced rate of late stent thrombosis, occurring during day 31 through 1 year, a 0.1% rate in the Orsiro-treated patients and a 0.9% rate in those who received Xience stents.
“These are remarkable results,” said Michael Haude, MD, an interventional cardiologist at Lukas Hospital in Neuss, Germany, and a cochair of the session in which Dr. Kandzari gave his report.
These results from the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in Subjects With Coronary Artery Lesions (BIOFLOW-V) trial will be the centerpiece of an application for U.S. marketing approval for the Orsiro stent, Dr. Kandzari said. The BIOFLOW-V trial enrolled patients at 90 centers in 13 countries including the United States.
Concurrently with his report, the results were published online (Lancet. 2017 Aug 26. doi: 10.1016/S0140-6736[17]32249-3).
The potential advantage of a thinner-strut drug eluting stent showed up during the initial treatment phase, with a procedural success rate of 94% using the Orsiro stent and 90% with the Xience stent. This difference in procedural success seemed largely the result of an increased rate of periprocedural MIs, a difference that might be explained by the difference in strut thickness, said Dr. Kandzari, of Piedmont Heart Institute, Atlanta. Based on this success, designers of future drug-eluting stents may focus on thinner-strut models, he suggested.
An additional analysis that Dr. Kandzari reported combined results from two prior randomized comparisons of the Orsiro and Xience stents, creating a pooled analysis with 2,208 patients. This Bayesian analysis calculated a 100% probability of noninferiority of the Orsiro stent compared with the Xience stent, and a 97% probability of superiority. This 97% probability of superiority fell just short of the 97.5% threshold for establishing superiority that Dr. Kandzari and his associates had prespecified for this analysis.
[email protected]
On Twitter @mitchelzoler
BARCELONA – A new model of drug-eluting coronary stent outperformed the reigning benchmark Xience stent in a head-to-head, pivotal comparison with 1,334 patients.
The results “advance a new standard for drug eluting stents,” David E. Kandzari, MD, said at the annual congress of the European Society of Cardiology. The trial tested the Orsiro stent, which features very thin, 60-micron-thick cobalt chromium struts, a bioresorbable polymer, and sirolimus as the antiproliferative drug that’s released during the first 90 days of stent placement.
“To our knowledge, this is the only trial that has demonstrated superiority [of a new stent] to the Xience drug-eluting stent in a large, randomized trial. This is a landmark in interventional cardiology that raises the bar for future comparisons” of drug eluting coronary stents, Dr. Kandzari said in an interview.
The study’s primary endpoint of target lesion failure after 12 months of follow-up – a rate that combined the incidence of cardiovascular death, target-vessel related MI, and ischemia-driven target-lesion revascularization – stood at 6.2% for the 884 patients treated with the Orsiro stent and 9.6% of the 450 randomized to treatment with the Xience stent, which has struts that are 81 microns wide, and a durable polymer that releases everolimus as the antiproliferative drug.
The difference in the primary endpoint was driven primarily by a 3.6% absolute difference in the rate of target-vessel related MIs, a statistically significant difference, plus the Orsiro-treated patients showed numerically smaller rates of cardiac death and ischemia-driven target lesion revascularization, although the between-group differences for each of these two endpoints were not statistically significant. The Orsiro stent also showed a significantly reduced rate of late stent thrombosis, occurring during day 31 through 1 year, a 0.1% rate in the Orsiro-treated patients and a 0.9% rate in those who received Xience stents.
“These are remarkable results,” said Michael Haude, MD, an interventional cardiologist at Lukas Hospital in Neuss, Germany, and a cochair of the session in which Dr. Kandzari gave his report.
These results from the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in Subjects With Coronary Artery Lesions (BIOFLOW-V) trial will be the centerpiece of an application for U.S. marketing approval for the Orsiro stent, Dr. Kandzari said. The BIOFLOW-V trial enrolled patients at 90 centers in 13 countries including the United States.
Concurrently with his report, the results were published online (Lancet. 2017 Aug 26. doi: 10.1016/S0140-6736[17]32249-3).
The potential advantage of a thinner-strut drug eluting stent showed up during the initial treatment phase, with a procedural success rate of 94% using the Orsiro stent and 90% with the Xience stent. This difference in procedural success seemed largely the result of an increased rate of periprocedural MIs, a difference that might be explained by the difference in strut thickness, said Dr. Kandzari, of Piedmont Heart Institute, Atlanta. Based on this success, designers of future drug-eluting stents may focus on thinner-strut models, he suggested.
An additional analysis that Dr. Kandzari reported combined results from two prior randomized comparisons of the Orsiro and Xience stents, creating a pooled analysis with 2,208 patients. This Bayesian analysis calculated a 100% probability of noninferiority of the Orsiro stent compared with the Xience stent, and a 97% probability of superiority. This 97% probability of superiority fell just short of the 97.5% threshold for establishing superiority that Dr. Kandzari and his associates had prespecified for this analysis.
[email protected]
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: The target-lesion failure rate after 12 months was 6.2% with the Orsiro stent and 9.6% with the Xience stent.
Data source: BIOFLOW-V, a multicenter, randomized trial with 1,334 patients.
Disclosures: BIOFLOW-V was sponsored by Biotronik, the company that markets the Orsiro stent. Dr. Kandzari has been a consultant to and/or has received research funding from Biotronik, Boston Scientific, Medtronic, Micell Technologies, Abbott Vascular, St. Jude, Medinol, and OrbusNeich. Dr. Haude has been a consultant to and has received honoraria from Biotronik and from several other device and drug companies.
In T1 diabetes, CABG seems better than PCI
In patients with type 1 diabetes in need of multivessel revascularization, coronary artery bypass graft (CABG) may be a better choice than percutaneous coronary intervention (PCI), according to results from a new comparative study presented at the annual congress of the European Society of Cardiology by Martin J. Holzmann, PhD, of the Karolinska Institute, Stockholm.
The two procedures had similar mortality rates, but PCI patients fared worse with respect to mortality due to myocardial infarction and several cardiovascular outcomes.
Previous studies had also suggested better outcomes with CABG than with PCI, but they lumped together patients with type 1 and type 2 diabetes, while the current study focused only on patients with type 1 diabetes.
The study included patients in Sweden with type 1 diabetes who underwent CABG (683 patients) or PCI (1,863 patients) between 1995 and 2013. During follow-up, 44.6% of patients in the PCI group died, compared with 53.3% in the CABG group. After adjustment for between-group differences, however, there was no significant difference in mortality risk between the two groups.
However, assessments of cause-specific mortality told a different story. Subjects in the PCI group had a greater risk of death from coronary artery disease (hazard ratio, 1.45; 95% confidence interval, 1.21-1.74).
Subjects in the PCI group were also more likely to suffer myocardial infarction (HR, 1.47; 95% CI, 1.21-1.77) and were more than five times more likely to undergo repeat vascularization (adjusted HR, 5.64; 95% CI, 4.67-6.82). The CABG group had a higher 30-day stroke risk (1.9% vs. 0.8%), but there was no difference in long-term risk.
The two groups had similar risks of hospitalization for heart failure.
The researchers noted a large difference between the two groups with respect to risk during the first year of follow-up, which suggests that some patients underwent PCI because they were too ill to undergo CABG. This limitation is also suggested by the greater proportion of previous stroke, heart failure, active cancer, and end-stage renal disease in the PCI group. The researchers adjusted for these differences, but it remains possible that there were residual confounders.
No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
In patients with aggressive multivessel CAD and stable symptoms associated with diabetes or high SYNTAX score, the mechanisms of benefit of PCI and CABG are different, and this difference likely explains the superior results of CABG.
Better stents alone cannot change the superiority of CABG, compared with PCI for patients with aggressive CAD (diabetes or high SYNTAX score), because PCI addresses only a small portion of the coronary anatomy. This does not diminish the importance of continuing advances in stent technology, but rather, it puts into appropriate perspective what can be expected from these advances.
The findings of this important study help to better inform practice, and should influence decision-making for revascularization in patients with T1DM.
These remarks were taken from an editorial by Michael J. Domanski, MD, and Michael E. Farkouh, MD (J Am Coll Cardiol. 2017. doi: 10.1016/j.jacc.2017.07.781). Dr. Domanski is with the Peter Munk Cardiac Centre, Toronto, and the Heart and Stroke Richard Lewar Centre, University of Toronto. Dr. Farokouh is the director of clinical trials at the Peter Munk Cardiac Centre, University of Toronto.
In patients with aggressive multivessel CAD and stable symptoms associated with diabetes or high SYNTAX score, the mechanisms of benefit of PCI and CABG are different, and this difference likely explains the superior results of CABG.
Better stents alone cannot change the superiority of CABG, compared with PCI for patients with aggressive CAD (diabetes or high SYNTAX score), because PCI addresses only a small portion of the coronary anatomy. This does not diminish the importance of continuing advances in stent technology, but rather, it puts into appropriate perspective what can be expected from these advances.
The findings of this important study help to better inform practice, and should influence decision-making for revascularization in patients with T1DM.
These remarks were taken from an editorial by Michael J. Domanski, MD, and Michael E. Farkouh, MD (J Am Coll Cardiol. 2017. doi: 10.1016/j.jacc.2017.07.781). Dr. Domanski is with the Peter Munk Cardiac Centre, Toronto, and the Heart and Stroke Richard Lewar Centre, University of Toronto. Dr. Farokouh is the director of clinical trials at the Peter Munk Cardiac Centre, University of Toronto.
In patients with aggressive multivessel CAD and stable symptoms associated with diabetes or high SYNTAX score, the mechanisms of benefit of PCI and CABG are different, and this difference likely explains the superior results of CABG.
Better stents alone cannot change the superiority of CABG, compared with PCI for patients with aggressive CAD (diabetes or high SYNTAX score), because PCI addresses only a small portion of the coronary anatomy. This does not diminish the importance of continuing advances in stent technology, but rather, it puts into appropriate perspective what can be expected from these advances.
The findings of this important study help to better inform practice, and should influence decision-making for revascularization in patients with T1DM.
These remarks were taken from an editorial by Michael J. Domanski, MD, and Michael E. Farkouh, MD (J Am Coll Cardiol. 2017. doi: 10.1016/j.jacc.2017.07.781). Dr. Domanski is with the Peter Munk Cardiac Centre, Toronto, and the Heart and Stroke Richard Lewar Centre, University of Toronto. Dr. Farokouh is the director of clinical trials at the Peter Munk Cardiac Centre, University of Toronto.
In patients with type 1 diabetes in need of multivessel revascularization, coronary artery bypass graft (CABG) may be a better choice than percutaneous coronary intervention (PCI), according to results from a new comparative study presented at the annual congress of the European Society of Cardiology by Martin J. Holzmann, PhD, of the Karolinska Institute, Stockholm.
The two procedures had similar mortality rates, but PCI patients fared worse with respect to mortality due to myocardial infarction and several cardiovascular outcomes.
Previous studies had also suggested better outcomes with CABG than with PCI, but they lumped together patients with type 1 and type 2 diabetes, while the current study focused only on patients with type 1 diabetes.
The study included patients in Sweden with type 1 diabetes who underwent CABG (683 patients) or PCI (1,863 patients) between 1995 and 2013. During follow-up, 44.6% of patients in the PCI group died, compared with 53.3% in the CABG group. After adjustment for between-group differences, however, there was no significant difference in mortality risk between the two groups.
However, assessments of cause-specific mortality told a different story. Subjects in the PCI group had a greater risk of death from coronary artery disease (hazard ratio, 1.45; 95% confidence interval, 1.21-1.74).
Subjects in the PCI group were also more likely to suffer myocardial infarction (HR, 1.47; 95% CI, 1.21-1.77) and were more than five times more likely to undergo repeat vascularization (adjusted HR, 5.64; 95% CI, 4.67-6.82). The CABG group had a higher 30-day stroke risk (1.9% vs. 0.8%), but there was no difference in long-term risk.
The two groups had similar risks of hospitalization for heart failure.
The researchers noted a large difference between the two groups with respect to risk during the first year of follow-up, which suggests that some patients underwent PCI because they were too ill to undergo CABG. This limitation is also suggested by the greater proportion of previous stroke, heart failure, active cancer, and end-stage renal disease in the PCI group. The researchers adjusted for these differences, but it remains possible that there were residual confounders.
No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
In patients with type 1 diabetes in need of multivessel revascularization, coronary artery bypass graft (CABG) may be a better choice than percutaneous coronary intervention (PCI), according to results from a new comparative study presented at the annual congress of the European Society of Cardiology by Martin J. Holzmann, PhD, of the Karolinska Institute, Stockholm.
The two procedures had similar mortality rates, but PCI patients fared worse with respect to mortality due to myocardial infarction and several cardiovascular outcomes.
Previous studies had also suggested better outcomes with CABG than with PCI, but they lumped together patients with type 1 and type 2 diabetes, while the current study focused only on patients with type 1 diabetes.
The study included patients in Sweden with type 1 diabetes who underwent CABG (683 patients) or PCI (1,863 patients) between 1995 and 2013. During follow-up, 44.6% of patients in the PCI group died, compared with 53.3% in the CABG group. After adjustment for between-group differences, however, there was no significant difference in mortality risk between the two groups.
However, assessments of cause-specific mortality told a different story. Subjects in the PCI group had a greater risk of death from coronary artery disease (hazard ratio, 1.45; 95% confidence interval, 1.21-1.74).
Subjects in the PCI group were also more likely to suffer myocardial infarction (HR, 1.47; 95% CI, 1.21-1.77) and were more than five times more likely to undergo repeat vascularization (adjusted HR, 5.64; 95% CI, 4.67-6.82). The CABG group had a higher 30-day stroke risk (1.9% vs. 0.8%), but there was no difference in long-term risk.
The two groups had similar risks of hospitalization for heart failure.
The researchers noted a large difference between the two groups with respect to risk during the first year of follow-up, which suggests that some patients underwent PCI because they were too ill to undergo CABG. This limitation is also suggested by the greater proportion of previous stroke, heart failure, active cancer, and end-stage renal disease in the PCI group. The researchers adjusted for these differences, but it remains possible that there were residual confounders.
No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
FROM THE ESC CONGRESS 2017
Key clinical point: Patients undergoing PCI had worse cardiovascular outcomes than those receiving CABG.
Major finding: The PCI group had a 45% increased risk of death due to myocardial infarction.
Data source: Observational study (n = 2,546).
Disclosures: No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
Undiagnosed AF common in higher-risk patients
Over an 18-month period, small, insertable cardiac monitors detected atrial fibrillation in 29% of previously undiagnosed patients who were at high risk of both AF and stroke, and in 40% of patients over 30 months, according to investigators. The study was presented at the annual congress of the European Society of Cardiology and simultaneously published in JAMA Cardiology.
More than half (56%) of patients consequently started oral anticoagulation therapy, noted James A. Reiffel, MD, of Columbia University College of Physicians and Surgeons, New York, with his associates, for the REVEAL AF investigators.
“The incidence of previously undiagnosed atrial fibrillation may be substantial in patients with risk factors for AF and stroke,” they concluded. “Atrial fibrillation would have gone undetected in most patients had monitoring been limited to 30 days. Further trials regarding the value of detecting subclinical AF and of prophylactic therapies are warranted.”
Atrial fibrillation affects millions worldwide and is associated with older age, hypertension, diabetes, and heart failure, all of which also independently increase the risk of stroke. Minimally invasive prolonged electrocardiographic monitoring with insertable cardiac monitors might help hasten detection and treatment of AF, but diagnostic yield in high-risk patients has been unclear.
In this single-arm, multicenter, prospective study, researchers inserted Reveal XT or Reveal LINQ (Medtronic) cardiac monitors in 385 adults who had either CHAD2 scores of 3, or CHAD2 scores of 2 and one additional risk factor for AF, such as coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency. The primary endpoint was AF lasting at least 6 minutes (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3180). Median follow-up time was 22.5 months. Rates of detecting AF were 6% at 30 days compared with 20% at 6 months, 27% at 12 months, 34% at 24 months, and 40% at 30 months. Patients typically had their first AF episode about 4 months (median, 123 days) after the device was inserted. Among patients who had experienced AF by 18 months, 10% had one or more episodes lasting at least 24 hours, and 72 (56%) were prescribed oral anticoagulation therapy.
The recent PREDATE AF and ASSERT-II studies also found that previously undiagnosed AF was common among high-risk patients, the researchers noted. However, whether anticoagulating patients who have only brief episodes of AF significantly reduces their risk of stroke remains unclear, they added. Three trials (ARTESiA, NOAH, and LOOP) are underway to assess whether oral anticoagulation therapy improves outcomes in patients with device-detected AF.
Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
The availability of safe and effective oral anticoagulant therapy makes the findings of REVEAL AF highly relevant. This high rate of incident AF makes ICM-based screenings of high-risk individuals a potentially attractive stroke prevention strategy. More detailed subgroup analyses may help identify a patient population with an even higher risk of developing AF. It is also conceivable that this population could have a sufficiently high risk of AF and stroke that a strategy of empiric oral anticoagulation, without the need for AF monitoring, could prove beneficial.
The REVEAL AF study has shown that AF is extremely common among older individuals with stroke risk factors. Over the next 3-4 years, subgroup analyses, economic evaluations, and randomized clinical trials will help determine if this insight can be translated into a cost-effective stroke prevention strategy for high-risk individuals.
Jeff S. Healey, MD, MSc, is at the Population Health Research Institute, McMaster University, Hamilton, Ont. He is the principal investigator of the ASSERT-II and ARTESiA trials, and had no other relevant disclosures. These comments are from his editorial (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3203).
The availability of safe and effective oral anticoagulant therapy makes the findings of REVEAL AF highly relevant. This high rate of incident AF makes ICM-based screenings of high-risk individuals a potentially attractive stroke prevention strategy. More detailed subgroup analyses may help identify a patient population with an even higher risk of developing AF. It is also conceivable that this population could have a sufficiently high risk of AF and stroke that a strategy of empiric oral anticoagulation, without the need for AF monitoring, could prove beneficial.
The REVEAL AF study has shown that AF is extremely common among older individuals with stroke risk factors. Over the next 3-4 years, subgroup analyses, economic evaluations, and randomized clinical trials will help determine if this insight can be translated into a cost-effective stroke prevention strategy for high-risk individuals.
Jeff S. Healey, MD, MSc, is at the Population Health Research Institute, McMaster University, Hamilton, Ont. He is the principal investigator of the ASSERT-II and ARTESiA trials, and had no other relevant disclosures. These comments are from his editorial (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3203).
The availability of safe and effective oral anticoagulant therapy makes the findings of REVEAL AF highly relevant. This high rate of incident AF makes ICM-based screenings of high-risk individuals a potentially attractive stroke prevention strategy. More detailed subgroup analyses may help identify a patient population with an even higher risk of developing AF. It is also conceivable that this population could have a sufficiently high risk of AF and stroke that a strategy of empiric oral anticoagulation, without the need for AF monitoring, could prove beneficial.
The REVEAL AF study has shown that AF is extremely common among older individuals with stroke risk factors. Over the next 3-4 years, subgroup analyses, economic evaluations, and randomized clinical trials will help determine if this insight can be translated into a cost-effective stroke prevention strategy for high-risk individuals.
Jeff S. Healey, MD, MSc, is at the Population Health Research Institute, McMaster University, Hamilton, Ont. He is the principal investigator of the ASSERT-II and ARTESiA trials, and had no other relevant disclosures. These comments are from his editorial (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3203).
Over an 18-month period, small, insertable cardiac monitors detected atrial fibrillation in 29% of previously undiagnosed patients who were at high risk of both AF and stroke, and in 40% of patients over 30 months, according to investigators. The study was presented at the annual congress of the European Society of Cardiology and simultaneously published in JAMA Cardiology.
More than half (56%) of patients consequently started oral anticoagulation therapy, noted James A. Reiffel, MD, of Columbia University College of Physicians and Surgeons, New York, with his associates, for the REVEAL AF investigators.
“The incidence of previously undiagnosed atrial fibrillation may be substantial in patients with risk factors for AF and stroke,” they concluded. “Atrial fibrillation would have gone undetected in most patients had monitoring been limited to 30 days. Further trials regarding the value of detecting subclinical AF and of prophylactic therapies are warranted.”
Atrial fibrillation affects millions worldwide and is associated with older age, hypertension, diabetes, and heart failure, all of which also independently increase the risk of stroke. Minimally invasive prolonged electrocardiographic monitoring with insertable cardiac monitors might help hasten detection and treatment of AF, but diagnostic yield in high-risk patients has been unclear.
In this single-arm, multicenter, prospective study, researchers inserted Reveal XT or Reveal LINQ (Medtronic) cardiac monitors in 385 adults who had either CHAD2 scores of 3, or CHAD2 scores of 2 and one additional risk factor for AF, such as coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency. The primary endpoint was AF lasting at least 6 minutes (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3180). Median follow-up time was 22.5 months. Rates of detecting AF were 6% at 30 days compared with 20% at 6 months, 27% at 12 months, 34% at 24 months, and 40% at 30 months. Patients typically had their first AF episode about 4 months (median, 123 days) after the device was inserted. Among patients who had experienced AF by 18 months, 10% had one or more episodes lasting at least 24 hours, and 72 (56%) were prescribed oral anticoagulation therapy.
The recent PREDATE AF and ASSERT-II studies also found that previously undiagnosed AF was common among high-risk patients, the researchers noted. However, whether anticoagulating patients who have only brief episodes of AF significantly reduces their risk of stroke remains unclear, they added. Three trials (ARTESiA, NOAH, and LOOP) are underway to assess whether oral anticoagulation therapy improves outcomes in patients with device-detected AF.
Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
Over an 18-month period, small, insertable cardiac monitors detected atrial fibrillation in 29% of previously undiagnosed patients who were at high risk of both AF and stroke, and in 40% of patients over 30 months, according to investigators. The study was presented at the annual congress of the European Society of Cardiology and simultaneously published in JAMA Cardiology.
More than half (56%) of patients consequently started oral anticoagulation therapy, noted James A. Reiffel, MD, of Columbia University College of Physicians and Surgeons, New York, with his associates, for the REVEAL AF investigators.
“The incidence of previously undiagnosed atrial fibrillation may be substantial in patients with risk factors for AF and stroke,” they concluded. “Atrial fibrillation would have gone undetected in most patients had monitoring been limited to 30 days. Further trials regarding the value of detecting subclinical AF and of prophylactic therapies are warranted.”
Atrial fibrillation affects millions worldwide and is associated with older age, hypertension, diabetes, and heart failure, all of which also independently increase the risk of stroke. Minimally invasive prolonged electrocardiographic monitoring with insertable cardiac monitors might help hasten detection and treatment of AF, but diagnostic yield in high-risk patients has been unclear.
In this single-arm, multicenter, prospective study, researchers inserted Reveal XT or Reveal LINQ (Medtronic) cardiac monitors in 385 adults who had either CHAD2 scores of 3, or CHAD2 scores of 2 and one additional risk factor for AF, such as coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency. The primary endpoint was AF lasting at least 6 minutes (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3180). Median follow-up time was 22.5 months. Rates of detecting AF were 6% at 30 days compared with 20% at 6 months, 27% at 12 months, 34% at 24 months, and 40% at 30 months. Patients typically had their first AF episode about 4 months (median, 123 days) after the device was inserted. Among patients who had experienced AF by 18 months, 10% had one or more episodes lasting at least 24 hours, and 72 (56%) were prescribed oral anticoagulation therapy.
The recent PREDATE AF and ASSERT-II studies also found that previously undiagnosed AF was common among high-risk patients, the researchers noted. However, whether anticoagulating patients who have only brief episodes of AF significantly reduces their risk of stroke remains unclear, they added. Three trials (ARTESiA, NOAH, and LOOP) are underway to assess whether oral anticoagulation therapy improves outcomes in patients with device-detected AF.
Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
FROM THE ESC CONGRESS 2017
Key clinical point: Undiagnosed atrial fibrillation is common in high-risk patients.
Major finding: At 18 months, 29% of previously undiagnosed, high-risk patients had experienced atrial fibrillation lasting 6 or more minutes.
Data source: A single-arm, prospective, multicenter study of 446 patients with a CHADS2 score of at least 3, or a CHADS2 score of at least 2 plus at least one other risk factor (coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency).
Disclosures: Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
Intraoperative ketamine makes no dent in postop delirium or pain
Postoperative delirium remains a problem without an effective solution, wrote Michael S. Avidan, MBBCh, FCASA, of Washington University, Saint Louis, and his colleagues (Lancet 2017;390[10091]:267-75).
Recent guidelines published by the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council include ketamine as a recommended component of multimodal pain therapy for several commonly performed surgeries. “Before recommending widespread administration of an intraoperative bolus of subanaesthetic ketamine, demonstrating that ketamine decreases either delirium or pain, or both, without incurring adverse effects in a large, pragmatic trial was warranted,” the researchers said.
In the PODCAST (Prevention of Delirium and Complications Associated With Surgical Treatments) trial, the researchers randomized 672 patients over the age of 60 undergoing major open surgery under general anesthesia (such as open cardiac or noncardiac surgery, urological surgery, gynecologic surgery, or intra-abdominal surgery) to 0.5 mg/kg ketamine (227), 1.0 mg/kg ketamine (223), or placebo (222). The ketamine or placebo was given after anesthesia and before surgical incision.
Overall, no difference in the incidence of delirium occurred between patients in the combined ketamine groups (19.5%) and the placebo group (19.8%), and there was no significant difference in delirium across all three treatment groups.
No differences in pain based on visual analog scale scores were observed across the three groups, and overall adverse event rates were similar as well: approximately 40.8% in the 1.0-mg ketamine group, 39.6% in the 0.5-mg ketamine group, and 36.9% in the placebo group.
The study findings were limited by several factors, including a study population potentially too small to show an effect of ketamine on delirium, and a lack of data on other variables that might contribute to delirium and pain, the researchers noted. However, the results suggest that “despite present evidence and guidelines, the administration of a subanaesthetic ketamine dose during surgery is not useful for preventing postoperative delirium (primary outcome) or reducing postoperative pain and minimising opioid consumption (related secondary outcomes),” and appears to increase postoperative hallucinations and nightmares to an extent that might be prohibitive, they said.
The National Institutes of Health and Cancer Center Support funded the study. The researchers had no financial conflicts to disclose.
Postoperative delirium remains a problem without an effective solution, wrote Michael S. Avidan, MBBCh, FCASA, of Washington University, Saint Louis, and his colleagues (Lancet 2017;390[10091]:267-75).
Recent guidelines published by the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council include ketamine as a recommended component of multimodal pain therapy for several commonly performed surgeries. “Before recommending widespread administration of an intraoperative bolus of subanaesthetic ketamine, demonstrating that ketamine decreases either delirium or pain, or both, without incurring adverse effects in a large, pragmatic trial was warranted,” the researchers said.
In the PODCAST (Prevention of Delirium and Complications Associated With Surgical Treatments) trial, the researchers randomized 672 patients over the age of 60 undergoing major open surgery under general anesthesia (such as open cardiac or noncardiac surgery, urological surgery, gynecologic surgery, or intra-abdominal surgery) to 0.5 mg/kg ketamine (227), 1.0 mg/kg ketamine (223), or placebo (222). The ketamine or placebo was given after anesthesia and before surgical incision.
Overall, no difference in the incidence of delirium occurred between patients in the combined ketamine groups (19.5%) and the placebo group (19.8%), and there was no significant difference in delirium across all three treatment groups.
No differences in pain based on visual analog scale scores were observed across the three groups, and overall adverse event rates were similar as well: approximately 40.8% in the 1.0-mg ketamine group, 39.6% in the 0.5-mg ketamine group, and 36.9% in the placebo group.
The study findings were limited by several factors, including a study population potentially too small to show an effect of ketamine on delirium, and a lack of data on other variables that might contribute to delirium and pain, the researchers noted. However, the results suggest that “despite present evidence and guidelines, the administration of a subanaesthetic ketamine dose during surgery is not useful for preventing postoperative delirium (primary outcome) or reducing postoperative pain and minimising opioid consumption (related secondary outcomes),” and appears to increase postoperative hallucinations and nightmares to an extent that might be prohibitive, they said.
The National Institutes of Health and Cancer Center Support funded the study. The researchers had no financial conflicts to disclose.
Postoperative delirium remains a problem without an effective solution, wrote Michael S. Avidan, MBBCh, FCASA, of Washington University, Saint Louis, and his colleagues (Lancet 2017;390[10091]:267-75).
Recent guidelines published by the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council include ketamine as a recommended component of multimodal pain therapy for several commonly performed surgeries. “Before recommending widespread administration of an intraoperative bolus of subanaesthetic ketamine, demonstrating that ketamine decreases either delirium or pain, or both, without incurring adverse effects in a large, pragmatic trial was warranted,” the researchers said.
In the PODCAST (Prevention of Delirium and Complications Associated With Surgical Treatments) trial, the researchers randomized 672 patients over the age of 60 undergoing major open surgery under general anesthesia (such as open cardiac or noncardiac surgery, urological surgery, gynecologic surgery, or intra-abdominal surgery) to 0.5 mg/kg ketamine (227), 1.0 mg/kg ketamine (223), or placebo (222). The ketamine or placebo was given after anesthesia and before surgical incision.
Overall, no difference in the incidence of delirium occurred between patients in the combined ketamine groups (19.5%) and the placebo group (19.8%), and there was no significant difference in delirium across all three treatment groups.
No differences in pain based on visual analog scale scores were observed across the three groups, and overall adverse event rates were similar as well: approximately 40.8% in the 1.0-mg ketamine group, 39.6% in the 0.5-mg ketamine group, and 36.9% in the placebo group.
The study findings were limited by several factors, including a study population potentially too small to show an effect of ketamine on delirium, and a lack of data on other variables that might contribute to delirium and pain, the researchers noted. However, the results suggest that “despite present evidence and guidelines, the administration of a subanaesthetic ketamine dose during surgery is not useful for preventing postoperative delirium (primary outcome) or reducing postoperative pain and minimising opioid consumption (related secondary outcomes),” and appears to increase postoperative hallucinations and nightmares to an extent that might be prohibitive, they said.
The National Institutes of Health and Cancer Center Support funded the study. The researchers had no financial conflicts to disclose.
FROM THE LANCET
Key clinical point: Ketamine failed to reduce postoperative delirium in older adults.
Major finding: No difference was observed in the incidence of postoperative delirium between patients given ketamine before surgical incision and patients on placebo.
Data source: The Prevention of Delirium and Complications Associated With Surgical Treatments study, a randomized, multicenter trial of 672 adults older than 60 years.
Disclosures: The National Institutes of Health and Cancer Center Support funded the study. The researchers had no financial conflicts to disclose.
Insurance coverage gainers outnumber coverage losers
Fewer nonelderly adults lost their health insurance in 2015 than in 2013, while more gained coverage, according to the Agency for Healthcare Research and Quality.
The presence of chronic conditions played a part for those who lost coverage. From 2012 to 2013, 2.9% of adults aged 18-64 years with one or more chronic conditions lost their insurance, compared with 1.5% who lost coverage from 2014 to 2015. Those with no chronic conditions saw a corresponding drop from 4% to 3.2%, but that change was not significant, AHRQ investigators reported.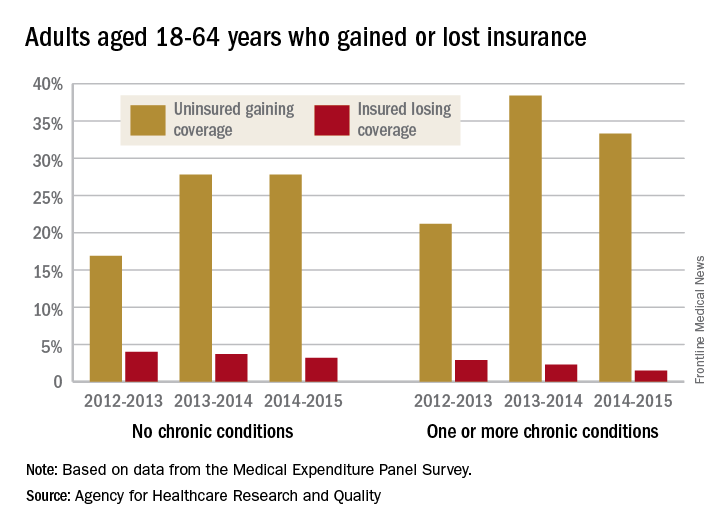
For this analysis, the chronic conditions were active asthma, arthritis, diabetes, emphysema, heart disease, high blood pressure, high cholesterol, bronchitis, and stroke. The source of the data was the Medical Expenditure Panel Survey.
Fewer nonelderly adults lost their health insurance in 2015 than in 2013, while more gained coverage, according to the Agency for Healthcare Research and Quality.
The presence of chronic conditions played a part for those who lost coverage. From 2012 to 2013, 2.9% of adults aged 18-64 years with one or more chronic conditions lost their insurance, compared with 1.5% who lost coverage from 2014 to 2015. Those with no chronic conditions saw a corresponding drop from 4% to 3.2%, but that change was not significant, AHRQ investigators reported.
For this analysis, the chronic conditions were active asthma, arthritis, diabetes, emphysema, heart disease, high blood pressure, high cholesterol, bronchitis, and stroke. The source of the data was the Medical Expenditure Panel Survey.
Fewer nonelderly adults lost their health insurance in 2015 than in 2013, while more gained coverage, according to the Agency for Healthcare Research and Quality.
The presence of chronic conditions played a part for those who lost coverage. From 2012 to 2013, 2.9% of adults aged 18-64 years with one or more chronic conditions lost their insurance, compared with 1.5% who lost coverage from 2014 to 2015. Those with no chronic conditions saw a corresponding drop from 4% to 3.2%, but that change was not significant, AHRQ investigators reported.
For this analysis, the chronic conditions were active asthma, arthritis, diabetes, emphysema, heart disease, high blood pressure, high cholesterol, bronchitis, and stroke. The source of the data was the Medical Expenditure Panel Survey.
Despite global decline, rheumatic heart disease persists in poorest regions
Global mortality due to rheumatic heart disease fell by about 48% during a recent 25-year-period, but some of the poorest areas of the world were left behind, according to a report in the New England Journal of Medicine.
Those regions included Oceania, South Asia, and central sub-Saharan Africa, where rheumatic heart disease remains endemic, wrote David A. Watkins, MD, MPH, of the University of Washington, Seattle, and his coinvestigators. “We estimate that 10 persons per 1,000 population living in South Asia and central sub-Saharan Africa and 15 persons per 1,000 population in Oceania were living with rheumatic heart disease in the year 2015,” they wrote. “Improvements in the measurement of the burden of rheumatic heart disease will assist in planning for its control and will help identify countries where further investments are needed.”
To better define the problem, Dr. Watkins and his associates analyzed epidemiologic studies of rheumatic heart disease from 1990 through 2015. They used the Cause of Death Ensemble model, which estimates mortality more reliably than older methods, and DisMod-MR (version 2.1), which sums epidemiologic data from multiple sources and corrects for gaps and inconsistencies (N Engl J Med. 2017;377:713-22).
Worldwide, about 319,400 individuals died of rheumatic heart disease in 2015, the researchers reported. Age-adjusted death rates fell by about 48% (95% confidence interval, 45%-51%), from 9.2 deaths per 100,000 population in 1990 to 4.8 deaths per 100,000 population in 2015. But this global trend masked striking regional disparities. In 1990, 77% of deaths from rheumatic heart disease occurred in endemic areas of Africa, South Asia, Oceania, and the Caribbean; by 2015, 82% of deaths occurred in endemic regions. Oceania, South Asia, and central sub-Saharan Africa had the highest death rates and were the only regions where the 95% confidence intervals for 1990 and 2015 overlapped, the investigators noted.
In 2015, age-standardized death rates exceeded 10 deaths per 100,000 population in the Solomon Islands, Pakistan, Papua New Guinea, Kiribati, Vanuatu, Fiji, India, Federated States of Micronesia, Marshall Islands, Central African Republic, and Lesotho, they reported. Estimated fatalities were highest in India (119,100 deaths), China (72,600), and Pakistan (18,900). They estimated that in 2015, there were 33.2 million cases of rheumatic heart disease and 10.5 million associated disability-adjusted life-years globally.
The study excluded “borderline” or subclinical rheumatic heart disease, which is detected by echocardiography and whose management remains unclear. “Better data for low-income and middle-income countries are needed to guide policies for the control of rheumatic heart disease,” the investigators wrote. They recommended studying death certificate misclassifications, disease prevalence among adults, and longitudinal trends in nonfatal outcomes and excess mortality.
Funders of the study included the Bill and Melinda Gates Foundation and the Medtronic Foundation. Dr. Watkins disclosed grants from the Medtronic Foundation during the conduct of the study and grants from the Bill and Melinda Gates Foundation outside the submitted work.
Rheumatic heart disease ranks as one of the most serious cardiovascular scourges of the past century. As a result of improvements in living conditions and the introduction of penicillin, the disease was almost eradicated in the developed world by the 1980s. However, it remains a force to be reckoned with in the developing world, as demonstrated by an assessment from the 2015 Global Burden of Disease study (GBD 2015), painstakingly performed by Dr. Watkins and his colleagues.
Several key messages emerge from this important study. It confirms the marked global heterogeneity of the burden of rheumatic heart disease, with near-zero prevalence in developed countries sharply contrasting with substantial prevalence and mortality in developing areas. In addition, however, the study documents the scarcity of accurately measured data in many locations, especially in areas with the highest prevalence (such as sub-Saharan Africa).
Although the “headline news” of a global decline in the prevalence of rheumatic heart disease described by Watkins et al. may give cause for optimism, the burden remains great for those parts of the world least able to afford it. Without sustained re-engagement of clinicians, researchers, funders, and public health bodies, the menace of rheumatic heart disease is unlikely to be eliminated in the near future. Rheumatic heart disease remains a problematic iceberg, yet undissolved, in warm tropical waters.
Eloi Marijon, MD, PhD, and Xavier Jouven, MD, PhD, are at European Georges Pompidou Hospital, Paris. David S. Celermajer, PhD, is at Sydney (Australia) Medical School. They reported having no conflicts of interest. Their editorial accompanied the report by Dr. Watkins and his colleagues (N Engl J Med. 2017;377:780-1).
Rheumatic heart disease ranks as one of the most serious cardiovascular scourges of the past century. As a result of improvements in living conditions and the introduction of penicillin, the disease was almost eradicated in the developed world by the 1980s. However, it remains a force to be reckoned with in the developing world, as demonstrated by an assessment from the 2015 Global Burden of Disease study (GBD 2015), painstakingly performed by Dr. Watkins and his colleagues.
Several key messages emerge from this important study. It confirms the marked global heterogeneity of the burden of rheumatic heart disease, with near-zero prevalence in developed countries sharply contrasting with substantial prevalence and mortality in developing areas. In addition, however, the study documents the scarcity of accurately measured data in many locations, especially in areas with the highest prevalence (such as sub-Saharan Africa).
Although the “headline news” of a global decline in the prevalence of rheumatic heart disease described by Watkins et al. may give cause for optimism, the burden remains great for those parts of the world least able to afford it. Without sustained re-engagement of clinicians, researchers, funders, and public health bodies, the menace of rheumatic heart disease is unlikely to be eliminated in the near future. Rheumatic heart disease remains a problematic iceberg, yet undissolved, in warm tropical waters.
Eloi Marijon, MD, PhD, and Xavier Jouven, MD, PhD, are at European Georges Pompidou Hospital, Paris. David S. Celermajer, PhD, is at Sydney (Australia) Medical School. They reported having no conflicts of interest. Their editorial accompanied the report by Dr. Watkins and his colleagues (N Engl J Med. 2017;377:780-1).
Rheumatic heart disease ranks as one of the most serious cardiovascular scourges of the past century. As a result of improvements in living conditions and the introduction of penicillin, the disease was almost eradicated in the developed world by the 1980s. However, it remains a force to be reckoned with in the developing world, as demonstrated by an assessment from the 2015 Global Burden of Disease study (GBD 2015), painstakingly performed by Dr. Watkins and his colleagues.
Several key messages emerge from this important study. It confirms the marked global heterogeneity of the burden of rheumatic heart disease, with near-zero prevalence in developed countries sharply contrasting with substantial prevalence and mortality in developing areas. In addition, however, the study documents the scarcity of accurately measured data in many locations, especially in areas with the highest prevalence (such as sub-Saharan Africa).
Although the “headline news” of a global decline in the prevalence of rheumatic heart disease described by Watkins et al. may give cause for optimism, the burden remains great for those parts of the world least able to afford it. Without sustained re-engagement of clinicians, researchers, funders, and public health bodies, the menace of rheumatic heart disease is unlikely to be eliminated in the near future. Rheumatic heart disease remains a problematic iceberg, yet undissolved, in warm tropical waters.
Eloi Marijon, MD, PhD, and Xavier Jouven, MD, PhD, are at European Georges Pompidou Hospital, Paris. David S. Celermajer, PhD, is at Sydney (Australia) Medical School. They reported having no conflicts of interest. Their editorial accompanied the report by Dr. Watkins and his colleagues (N Engl J Med. 2017;377:780-1).
Global mortality due to rheumatic heart disease fell by about 48% during a recent 25-year-period, but some of the poorest areas of the world were left behind, according to a report in the New England Journal of Medicine.
Those regions included Oceania, South Asia, and central sub-Saharan Africa, where rheumatic heart disease remains endemic, wrote David A. Watkins, MD, MPH, of the University of Washington, Seattle, and his coinvestigators. “We estimate that 10 persons per 1,000 population living in South Asia and central sub-Saharan Africa and 15 persons per 1,000 population in Oceania were living with rheumatic heart disease in the year 2015,” they wrote. “Improvements in the measurement of the burden of rheumatic heart disease will assist in planning for its control and will help identify countries where further investments are needed.”
To better define the problem, Dr. Watkins and his associates analyzed epidemiologic studies of rheumatic heart disease from 1990 through 2015. They used the Cause of Death Ensemble model, which estimates mortality more reliably than older methods, and DisMod-MR (version 2.1), which sums epidemiologic data from multiple sources and corrects for gaps and inconsistencies (N Engl J Med. 2017;377:713-22).
Worldwide, about 319,400 individuals died of rheumatic heart disease in 2015, the researchers reported. Age-adjusted death rates fell by about 48% (95% confidence interval, 45%-51%), from 9.2 deaths per 100,000 population in 1990 to 4.8 deaths per 100,000 population in 2015. But this global trend masked striking regional disparities. In 1990, 77% of deaths from rheumatic heart disease occurred in endemic areas of Africa, South Asia, Oceania, and the Caribbean; by 2015, 82% of deaths occurred in endemic regions. Oceania, South Asia, and central sub-Saharan Africa had the highest death rates and were the only regions where the 95% confidence intervals for 1990 and 2015 overlapped, the investigators noted.
In 2015, age-standardized death rates exceeded 10 deaths per 100,000 population in the Solomon Islands, Pakistan, Papua New Guinea, Kiribati, Vanuatu, Fiji, India, Federated States of Micronesia, Marshall Islands, Central African Republic, and Lesotho, they reported. Estimated fatalities were highest in India (119,100 deaths), China (72,600), and Pakistan (18,900). They estimated that in 2015, there were 33.2 million cases of rheumatic heart disease and 10.5 million associated disability-adjusted life-years globally.
The study excluded “borderline” or subclinical rheumatic heart disease, which is detected by echocardiography and whose management remains unclear. “Better data for low-income and middle-income countries are needed to guide policies for the control of rheumatic heart disease,” the investigators wrote. They recommended studying death certificate misclassifications, disease prevalence among adults, and longitudinal trends in nonfatal outcomes and excess mortality.
Funders of the study included the Bill and Melinda Gates Foundation and the Medtronic Foundation. Dr. Watkins disclosed grants from the Medtronic Foundation during the conduct of the study and grants from the Bill and Melinda Gates Foundation outside the submitted work.
Global mortality due to rheumatic heart disease fell by about 48% during a recent 25-year-period, but some of the poorest areas of the world were left behind, according to a report in the New England Journal of Medicine.
Those regions included Oceania, South Asia, and central sub-Saharan Africa, where rheumatic heart disease remains endemic, wrote David A. Watkins, MD, MPH, of the University of Washington, Seattle, and his coinvestigators. “We estimate that 10 persons per 1,000 population living in South Asia and central sub-Saharan Africa and 15 persons per 1,000 population in Oceania were living with rheumatic heart disease in the year 2015,” they wrote. “Improvements in the measurement of the burden of rheumatic heart disease will assist in planning for its control and will help identify countries where further investments are needed.”
To better define the problem, Dr. Watkins and his associates analyzed epidemiologic studies of rheumatic heart disease from 1990 through 2015. They used the Cause of Death Ensemble model, which estimates mortality more reliably than older methods, and DisMod-MR (version 2.1), which sums epidemiologic data from multiple sources and corrects for gaps and inconsistencies (N Engl J Med. 2017;377:713-22).
Worldwide, about 319,400 individuals died of rheumatic heart disease in 2015, the researchers reported. Age-adjusted death rates fell by about 48% (95% confidence interval, 45%-51%), from 9.2 deaths per 100,000 population in 1990 to 4.8 deaths per 100,000 population in 2015. But this global trend masked striking regional disparities. In 1990, 77% of deaths from rheumatic heart disease occurred in endemic areas of Africa, South Asia, Oceania, and the Caribbean; by 2015, 82% of deaths occurred in endemic regions. Oceania, South Asia, and central sub-Saharan Africa had the highest death rates and were the only regions where the 95% confidence intervals for 1990 and 2015 overlapped, the investigators noted.
In 2015, age-standardized death rates exceeded 10 deaths per 100,000 population in the Solomon Islands, Pakistan, Papua New Guinea, Kiribati, Vanuatu, Fiji, India, Federated States of Micronesia, Marshall Islands, Central African Republic, and Lesotho, they reported. Estimated fatalities were highest in India (119,100 deaths), China (72,600), and Pakistan (18,900). They estimated that in 2015, there were 33.2 million cases of rheumatic heart disease and 10.5 million associated disability-adjusted life-years globally.
The study excluded “borderline” or subclinical rheumatic heart disease, which is detected by echocardiography and whose management remains unclear. “Better data for low-income and middle-income countries are needed to guide policies for the control of rheumatic heart disease,” the investigators wrote. They recommended studying death certificate misclassifications, disease prevalence among adults, and longitudinal trends in nonfatal outcomes and excess mortality.
Funders of the study included the Bill and Melinda Gates Foundation and the Medtronic Foundation. Dr. Watkins disclosed grants from the Medtronic Foundation during the conduct of the study and grants from the Bill and Melinda Gates Foundation outside the submitted work.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Globally, age-adjusted death rates fell by about 48% between 1990 and 2015. Oceania, South Asia, and central sub-Saharan Africa had the highest death rates in 2015, and were the only regions where the 95% confidence intervals overlapped with those for 1990.
Data source: A systematic review and analysis of morbidity and mortality data from 1990 through 2015.
Disclosures: Funders included the Bill and Melinda Gates Foundation and the Medtronic Foundation. Dr. Watkins disclosed grants from the Medtronic Foundation during the conduct of the study and grants from the Bill and Melinda Gates Foundation outside the submitted work.