User login
Commentary: HER2+-targeted therapy, ovarian suppression, and adjuvant therapy in breast cancer, February 2023
The elevated risk for recurrence in young women with HR+ early breast cancer highlights the importance of aggressive endocrine therapy in the majority of patients in this population. Examples of approaches to maximize endocrine therapy benefit include the addition of ovarian suppression to either tamoxifen or an aromatase inhibitor (AI) as well as an extended duration of adjuvant endocrine therapy.4,5 Among 3047 premenopausal women included in SOFT study, at 12 years follow-up, the addition of ovarian function suppression (OFS) to tamoxifen significantly improved disease-free survival (DFS) compared with tamoxifen alone (hazard ratio 0.82; P = .03) with a more pronounced DFS benefit with exemestane plus OFS compared with tamoxifen (hazard ratio 0.69) (Francis et al). In the HER2- subgroup, those who received prior chemotherapy had 12-year OFS rates of 78.8% with tamoxifen, 81.1% with tamoxifen plus OFS and 84.4% with exemestane plus OFS. Furthermore, in the HER2- subgroup, women younger than 35 years had absolute improvements in 12-year OS of 9.1% with tamoxifen plus OFS and 16.5% with exemestane plus OFS compared with tamoxifen. These updated results provide further support for OFS added to tamoxifen or an AI (with more benefit seen with an AI) in the treatment of HR+ early breast cancer in young women who are at high risk for recurrence. Longer follow-up will be important to better define the treatment effect considering recurrence patterns for this subtype of breast cancer.
Various guidelines recommend the use of adjuvant bisphosphonates for postmenopausal patients with early breast cancer on the basis of disease-free and bone metastasis-free survival benefits.6 A regimen of zolendronic acid every 6 months for 3 years is commonly used in clinical practice. A substudy of ABCSG-12, including 725 premenopausal patients with HR+ early breast cancer on ovarian suppression randomly assigned to receive tamoxifen or anastrozole with or without zolendronic acid every 6 months, investigated the effect of shorter duration of bisphosphonate therapy on breast cancer outcomes (Beltran-Bless et al). After a median follow-up of 96 months, there was no statistically significant difference in DFS (hazard ratio 0.88; log-rank P = .642) or OS (stratified hazard ratio 1.16; log-rank P = .796) between patients who received ≤6 or ≥7 infusions. Rates of adverse events were increased in the patients who received ≥7 or ≤6 infusions (arthralgia, 20.1% vs 12.4%; nausea, 12.8% vs 7.3%; bone pain, 41.6% vs 34.9%). Modifications to adjuvant breast cancer regimens that can provide more ease for patients with less toxicity while maintaining efficacy are greatly desired to simultaneously support quality of life and disease outcomes.
Additional References
- Swain SM, Baselga J, Kim SB, et al; for the CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724-734. Doi: 10.1056/NEJMoa1413513
- Miles D, Ciruelos E, Schneeweiss A, et al; for the PERUSE investigators. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann Oncol. 2021;32:1245-1255. Doi: 10.1016/j.annonc.2021.06.024
- Hua X, Bi X-W, Zhao J-L, et al; for the South China Breast Cancer Group (SCBCG). Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for patients with hormone receptor-positive and HER2-positive metastatic breast cancer (SYSUCC-002). Clin Cancer Res. 2022;28:637-645. Doi: 10.1158/1078-0432.CCR-21-3435
- Kim H-A, Lee JW, Nam SJ, et al; for the Korean Breast Cancer Study Group. Adding ovarian suppression to tamoxifen for premenopausal breast Cancer: a randomized phase III trial. J Clin Oncol. 2020;38:434-443. Doi: 10.1200/JCO.19.00126
- Davies C, Pan H, Godwin J, et al; for the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-816. Doi: 10.1016/S0140-6736(12)61963-1
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353-1361. Doi: 10.1016/S0140-6736(15)60908-4
The elevated risk for recurrence in young women with HR+ early breast cancer highlights the importance of aggressive endocrine therapy in the majority of patients in this population. Examples of approaches to maximize endocrine therapy benefit include the addition of ovarian suppression to either tamoxifen or an aromatase inhibitor (AI) as well as an extended duration of adjuvant endocrine therapy.4,5 Among 3047 premenopausal women included in SOFT study, at 12 years follow-up, the addition of ovarian function suppression (OFS) to tamoxifen significantly improved disease-free survival (DFS) compared with tamoxifen alone (hazard ratio 0.82; P = .03) with a more pronounced DFS benefit with exemestane plus OFS compared with tamoxifen (hazard ratio 0.69) (Francis et al). In the HER2- subgroup, those who received prior chemotherapy had 12-year OFS rates of 78.8% with tamoxifen, 81.1% with tamoxifen plus OFS and 84.4% with exemestane plus OFS. Furthermore, in the HER2- subgroup, women younger than 35 years had absolute improvements in 12-year OS of 9.1% with tamoxifen plus OFS and 16.5% with exemestane plus OFS compared with tamoxifen. These updated results provide further support for OFS added to tamoxifen or an AI (with more benefit seen with an AI) in the treatment of HR+ early breast cancer in young women who are at high risk for recurrence. Longer follow-up will be important to better define the treatment effect considering recurrence patterns for this subtype of breast cancer.
Various guidelines recommend the use of adjuvant bisphosphonates for postmenopausal patients with early breast cancer on the basis of disease-free and bone metastasis-free survival benefits.6 A regimen of zolendronic acid every 6 months for 3 years is commonly used in clinical practice. A substudy of ABCSG-12, including 725 premenopausal patients with HR+ early breast cancer on ovarian suppression randomly assigned to receive tamoxifen or anastrozole with or without zolendronic acid every 6 months, investigated the effect of shorter duration of bisphosphonate therapy on breast cancer outcomes (Beltran-Bless et al). After a median follow-up of 96 months, there was no statistically significant difference in DFS (hazard ratio 0.88; log-rank P = .642) or OS (stratified hazard ratio 1.16; log-rank P = .796) between patients who received ≤6 or ≥7 infusions. Rates of adverse events were increased in the patients who received ≥7 or ≤6 infusions (arthralgia, 20.1% vs 12.4%; nausea, 12.8% vs 7.3%; bone pain, 41.6% vs 34.9%). Modifications to adjuvant breast cancer regimens that can provide more ease for patients with less toxicity while maintaining efficacy are greatly desired to simultaneously support quality of life and disease outcomes.
Additional References
- Swain SM, Baselga J, Kim SB, et al; for the CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724-734. Doi: 10.1056/NEJMoa1413513
- Miles D, Ciruelos E, Schneeweiss A, et al; for the PERUSE investigators. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann Oncol. 2021;32:1245-1255. Doi: 10.1016/j.annonc.2021.06.024
- Hua X, Bi X-W, Zhao J-L, et al; for the South China Breast Cancer Group (SCBCG). Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for patients with hormone receptor-positive and HER2-positive metastatic breast cancer (SYSUCC-002). Clin Cancer Res. 2022;28:637-645. Doi: 10.1158/1078-0432.CCR-21-3435
- Kim H-A, Lee JW, Nam SJ, et al; for the Korean Breast Cancer Study Group. Adding ovarian suppression to tamoxifen for premenopausal breast Cancer: a randomized phase III trial. J Clin Oncol. 2020;38:434-443. Doi: 10.1200/JCO.19.00126
- Davies C, Pan H, Godwin J, et al; for the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-816. Doi: 10.1016/S0140-6736(12)61963-1
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353-1361. Doi: 10.1016/S0140-6736(15)60908-4
The elevated risk for recurrence in young women with HR+ early breast cancer highlights the importance of aggressive endocrine therapy in the majority of patients in this population. Examples of approaches to maximize endocrine therapy benefit include the addition of ovarian suppression to either tamoxifen or an aromatase inhibitor (AI) as well as an extended duration of adjuvant endocrine therapy.4,5 Among 3047 premenopausal women included in SOFT study, at 12 years follow-up, the addition of ovarian function suppression (OFS) to tamoxifen significantly improved disease-free survival (DFS) compared with tamoxifen alone (hazard ratio 0.82; P = .03) with a more pronounced DFS benefit with exemestane plus OFS compared with tamoxifen (hazard ratio 0.69) (Francis et al). In the HER2- subgroup, those who received prior chemotherapy had 12-year OFS rates of 78.8% with tamoxifen, 81.1% with tamoxifen plus OFS and 84.4% with exemestane plus OFS. Furthermore, in the HER2- subgroup, women younger than 35 years had absolute improvements in 12-year OS of 9.1% with tamoxifen plus OFS and 16.5% with exemestane plus OFS compared with tamoxifen. These updated results provide further support for OFS added to tamoxifen or an AI (with more benefit seen with an AI) in the treatment of HR+ early breast cancer in young women who are at high risk for recurrence. Longer follow-up will be important to better define the treatment effect considering recurrence patterns for this subtype of breast cancer.
Various guidelines recommend the use of adjuvant bisphosphonates for postmenopausal patients with early breast cancer on the basis of disease-free and bone metastasis-free survival benefits.6 A regimen of zolendronic acid every 6 months for 3 years is commonly used in clinical practice. A substudy of ABCSG-12, including 725 premenopausal patients with HR+ early breast cancer on ovarian suppression randomly assigned to receive tamoxifen or anastrozole with or without zolendronic acid every 6 months, investigated the effect of shorter duration of bisphosphonate therapy on breast cancer outcomes (Beltran-Bless et al). After a median follow-up of 96 months, there was no statistically significant difference in DFS (hazard ratio 0.88; log-rank P = .642) or OS (stratified hazard ratio 1.16; log-rank P = .796) between patients who received ≤6 or ≥7 infusions. Rates of adverse events were increased in the patients who received ≥7 or ≤6 infusions (arthralgia, 20.1% vs 12.4%; nausea, 12.8% vs 7.3%; bone pain, 41.6% vs 34.9%). Modifications to adjuvant breast cancer regimens that can provide more ease for patients with less toxicity while maintaining efficacy are greatly desired to simultaneously support quality of life and disease outcomes.
Additional References
- Swain SM, Baselga J, Kim SB, et al; for the CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724-734. Doi: 10.1056/NEJMoa1413513
- Miles D, Ciruelos E, Schneeweiss A, et al; for the PERUSE investigators. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann Oncol. 2021;32:1245-1255. Doi: 10.1016/j.annonc.2021.06.024
- Hua X, Bi X-W, Zhao J-L, et al; for the South China Breast Cancer Group (SCBCG). Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for patients with hormone receptor-positive and HER2-positive metastatic breast cancer (SYSUCC-002). Clin Cancer Res. 2022;28:637-645. Doi: 10.1158/1078-0432.CCR-21-3435
- Kim H-A, Lee JW, Nam SJ, et al; for the Korean Breast Cancer Study Group. Adding ovarian suppression to tamoxifen for premenopausal breast Cancer: a randomized phase III trial. J Clin Oncol. 2020;38:434-443. Doi: 10.1200/JCO.19.00126
- Davies C, Pan H, Godwin J, et al; for the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-816. Doi: 10.1016/S0140-6736(12)61963-1
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353-1361. Doi: 10.1016/S0140-6736(15)60908-4
Commentary: A New Drug, and Pediatric Concerns, February 2023
I love registries! With large numbers of participants, registries can be very helpful to identify rare side effects and to assess the efficacy and safety of medications in populations that may not be fully represented in clinical trials. I also love dupilumab; it was revolutionary in the management of patients with AD.
Vittrup and colleagues have created a registry of 347 participants treated with dupilumab. This does not yet have the large number of participants needed to identify new issues that wouldn't have been detected in clinical trials, but the study is informative about real-life use. The dramatic improvement in the Eczema Area and Severity Index (EASI) score is consistent with the high efficacy of dupilumab. The high rate of treatment persistence is also consistent with dupilumab being a very effective and safe treatment (because if the drug wasn't working well or was causing a severe problem, patients would probably stop the treatment). Though the study reported persistent head and neck involvement, the residual involvement may be quite minimal.
The EASI-75 and Investigator Global Assessment response rates reported in dupilumab trials underestimate the value of this drug. With a 2-year persistence rate of nearly 90%, it's clear that dupilumab is making a huge difference in the lives of patients with AD.
Fatigue is a fascinating issue in AD. We might wonder if all the inflammation in patients with AD would directly cause fatigue. Almost certainly all the itching in AD adversely affects sleep and would cause tremendous fatigue. It surprised me that most of the children in the study by Rangel and colleagues were reported as having no or mild fatigue; severe fatigue was very uncommon. It leaves me wondering whether the assessments of fatigue fully capture what's happening. Also, since the fatigue score was reported by the parents, I (as the parent of a child with AD) am wondering whether the parents were projecting, with the score more reflective of the parents' fatigue than with that of the child; alternatively, perhaps the child's hyperactivity leaves parents thinking there is no fatigue when there actually is (and possibly even causing the perceived hyperactivity).
The lack of a control group without AD is another major limitation in our ability to interpret the study findings. Is fatigue more common or less common in children with AD than in children without AD? I cannot tell from these findings. Does fatigue warrant, as the authors suggest, more attention in clinical practice? I don't know. If we are already treating our patients based on patients' global impressions of how they are doing — combined, of course, with our observations of their objective disease severity — I'm not sure how asking about fatigue would change anything, even if future studies were to definitively show that AD is associated with fatigue.
I hate new drugs (well, maybe not hate, but I worry about unknown long-term risks). Clinical trials that help a drug get approved can tell us a lot about a drug's efficacy, but these studies are generally limited in what they tell us about a drug's safety. Clinical trials are generally not powered enough (not enough participants and not followed for long enough) to be informative about rare risks. I love long-term studies of new drugs in large numbers of people because those studies can be very reassuring about the risks of medications. Studying nearly 10,000 patients for 5 years is quite reassuring, confirming my impression that dupilumab has a remarkable, excellent safety profile (Owji et al). Blocking interleukin 4 and interleukin 13 seems to be very specific to AD. Finding no association to cancer is what I would have expected; being able to share this information with patients is likely to be reassuring to them.
Oh, lord help me, another study that claims we should change our disease management because they've identified an increased risk for something. When you compare 70,000 patients with 270,000 controls, you have huge power to detect statistically significant associations of no clinical consequence. Let's assume for the moment that the detected association the authors found between AD and juvenile idiopathic arthritis (JIA) is real. The odds ratio is 2; the odds ratio for smoking causing cancer is on the order of 100.
In this study, over 99% of individuals in both AD and control groups did not have JIA. The difference between rates of JIA in patients with AD compared with controls was 0.3%! The authors conclude "it is important to inquire actively about symptoms not directly linked to the patients' skin disease"; based on the findings of this study, I would conclude that we don't need to worry about JIA in patients with AD even if there is a (marginally) higher prevalence of JIA in this group.
I love registries! With large numbers of participants, registries can be very helpful to identify rare side effects and to assess the efficacy and safety of medications in populations that may not be fully represented in clinical trials. I also love dupilumab; it was revolutionary in the management of patients with AD.
Vittrup and colleagues have created a registry of 347 participants treated with dupilumab. This does not yet have the large number of participants needed to identify new issues that wouldn't have been detected in clinical trials, but the study is informative about real-life use. The dramatic improvement in the Eczema Area and Severity Index (EASI) score is consistent with the high efficacy of dupilumab. The high rate of treatment persistence is also consistent with dupilumab being a very effective and safe treatment (because if the drug wasn't working well or was causing a severe problem, patients would probably stop the treatment). Though the study reported persistent head and neck involvement, the residual involvement may be quite minimal.
The EASI-75 and Investigator Global Assessment response rates reported in dupilumab trials underestimate the value of this drug. With a 2-year persistence rate of nearly 90%, it's clear that dupilumab is making a huge difference in the lives of patients with AD.
Fatigue is a fascinating issue in AD. We might wonder if all the inflammation in patients with AD would directly cause fatigue. Almost certainly all the itching in AD adversely affects sleep and would cause tremendous fatigue. It surprised me that most of the children in the study by Rangel and colleagues were reported as having no or mild fatigue; severe fatigue was very uncommon. It leaves me wondering whether the assessments of fatigue fully capture what's happening. Also, since the fatigue score was reported by the parents, I (as the parent of a child with AD) am wondering whether the parents were projecting, with the score more reflective of the parents' fatigue than with that of the child; alternatively, perhaps the child's hyperactivity leaves parents thinking there is no fatigue when there actually is (and possibly even causing the perceived hyperactivity).
The lack of a control group without AD is another major limitation in our ability to interpret the study findings. Is fatigue more common or less common in children with AD than in children without AD? I cannot tell from these findings. Does fatigue warrant, as the authors suggest, more attention in clinical practice? I don't know. If we are already treating our patients based on patients' global impressions of how they are doing — combined, of course, with our observations of their objective disease severity — I'm not sure how asking about fatigue would change anything, even if future studies were to definitively show that AD is associated with fatigue.
I hate new drugs (well, maybe not hate, but I worry about unknown long-term risks). Clinical trials that help a drug get approved can tell us a lot about a drug's efficacy, but these studies are generally limited in what they tell us about a drug's safety. Clinical trials are generally not powered enough (not enough participants and not followed for long enough) to be informative about rare risks. I love long-term studies of new drugs in large numbers of people because those studies can be very reassuring about the risks of medications. Studying nearly 10,000 patients for 5 years is quite reassuring, confirming my impression that dupilumab has a remarkable, excellent safety profile (Owji et al). Blocking interleukin 4 and interleukin 13 seems to be very specific to AD. Finding no association to cancer is what I would have expected; being able to share this information with patients is likely to be reassuring to them.
Oh, lord help me, another study that claims we should change our disease management because they've identified an increased risk for something. When you compare 70,000 patients with 270,000 controls, you have huge power to detect statistically significant associations of no clinical consequence. Let's assume for the moment that the detected association the authors found between AD and juvenile idiopathic arthritis (JIA) is real. The odds ratio is 2; the odds ratio for smoking causing cancer is on the order of 100.
In this study, over 99% of individuals in both AD and control groups did not have JIA. The difference between rates of JIA in patients with AD compared with controls was 0.3%! The authors conclude "it is important to inquire actively about symptoms not directly linked to the patients' skin disease"; based on the findings of this study, I would conclude that we don't need to worry about JIA in patients with AD even if there is a (marginally) higher prevalence of JIA in this group.
I love registries! With large numbers of participants, registries can be very helpful to identify rare side effects and to assess the efficacy and safety of medications in populations that may not be fully represented in clinical trials. I also love dupilumab; it was revolutionary in the management of patients with AD.
Vittrup and colleagues have created a registry of 347 participants treated with dupilumab. This does not yet have the large number of participants needed to identify new issues that wouldn't have been detected in clinical trials, but the study is informative about real-life use. The dramatic improvement in the Eczema Area and Severity Index (EASI) score is consistent with the high efficacy of dupilumab. The high rate of treatment persistence is also consistent with dupilumab being a very effective and safe treatment (because if the drug wasn't working well or was causing a severe problem, patients would probably stop the treatment). Though the study reported persistent head and neck involvement, the residual involvement may be quite minimal.
The EASI-75 and Investigator Global Assessment response rates reported in dupilumab trials underestimate the value of this drug. With a 2-year persistence rate of nearly 90%, it's clear that dupilumab is making a huge difference in the lives of patients with AD.
Fatigue is a fascinating issue in AD. We might wonder if all the inflammation in patients with AD would directly cause fatigue. Almost certainly all the itching in AD adversely affects sleep and would cause tremendous fatigue. It surprised me that most of the children in the study by Rangel and colleagues were reported as having no or mild fatigue; severe fatigue was very uncommon. It leaves me wondering whether the assessments of fatigue fully capture what's happening. Also, since the fatigue score was reported by the parents, I (as the parent of a child with AD) am wondering whether the parents were projecting, with the score more reflective of the parents' fatigue than with that of the child; alternatively, perhaps the child's hyperactivity leaves parents thinking there is no fatigue when there actually is (and possibly even causing the perceived hyperactivity).
The lack of a control group without AD is another major limitation in our ability to interpret the study findings. Is fatigue more common or less common in children with AD than in children without AD? I cannot tell from these findings. Does fatigue warrant, as the authors suggest, more attention in clinical practice? I don't know. If we are already treating our patients based on patients' global impressions of how they are doing — combined, of course, with our observations of their objective disease severity — I'm not sure how asking about fatigue would change anything, even if future studies were to definitively show that AD is associated with fatigue.
I hate new drugs (well, maybe not hate, but I worry about unknown long-term risks). Clinical trials that help a drug get approved can tell us a lot about a drug's efficacy, but these studies are generally limited in what they tell us about a drug's safety. Clinical trials are generally not powered enough (not enough participants and not followed for long enough) to be informative about rare risks. I love long-term studies of new drugs in large numbers of people because those studies can be very reassuring about the risks of medications. Studying nearly 10,000 patients for 5 years is quite reassuring, confirming my impression that dupilumab has a remarkable, excellent safety profile (Owji et al). Blocking interleukin 4 and interleukin 13 seems to be very specific to AD. Finding no association to cancer is what I would have expected; being able to share this information with patients is likely to be reassuring to them.
Oh, lord help me, another study that claims we should change our disease management because they've identified an increased risk for something. When you compare 70,000 patients with 270,000 controls, you have huge power to detect statistically significant associations of no clinical consequence. Let's assume for the moment that the detected association the authors found between AD and juvenile idiopathic arthritis (JIA) is real. The odds ratio is 2; the odds ratio for smoking causing cancer is on the order of 100.
In this study, over 99% of individuals in both AD and control groups did not have JIA. The difference between rates of JIA in patients with AD compared with controls was 0.3%! The authors conclude "it is important to inquire actively about symptoms not directly linked to the patients' skin disease"; based on the findings of this study, I would conclude that we don't need to worry about JIA in patients with AD even if there is a (marginally) higher prevalence of JIA in this group.
Fluorescence-optical imaging may detect preclinical PsA
Fluorescence-optical imaging (FOI) identified early signs of psoriatic arthritis, based on data from 2 years of follow-up of a cohort of 389 adults at 14 rheumatology centers.
Approximately 25% of individuals with psoriasis go on to develop psoriatic arthritis (PsA), but there are no validated biomarkers to identify patients at risk for progression to PsA, Michaela Koehm, MD, of Goethe University, Frankfurt am Main, Germany, and colleagues wrote in RMD Open.
FOI is a technique that allows assessment of changes in microvascularization and subdermal skin inflammation, and because individuals with psoriasis who develop PsA have shown changes in blood vessel formation in the early stages of disease, the researchers sought to determine if FOI could be used to predict early PsA.
The researchers conducted a multicenter, two-part observational cohort study. The two parts, known as XCITING and XTEND, included 389 adults aged 18-75 years with plaque psoriasis deemed at increased risk for PsA. The patients were seen at rheumatology sites in Germany between Jan. 28, 2014, and March 16, 2017. The XTEND study included clinic visits 18-24 months after the XCITING study.
Participants underwent a complete clinical examination, with musculoskeletal ultrasound (MSUS) and FOI on both hands at a single visit. Those with positive FOI findings not seen with clinical exam or MSUS underwent MRI within 7 days. Patients with positive FOI but negative findings on clinical exam, MSUS, and MRI were followed for 2 years in the XTEND study.
The primary outcome was the ability of FOI to detect musculoskeletal inflammation, compared with clinical examination and MSUS.
Overall, 50% of the patients were diagnosed with PsA. A total of 116 (30%) had positive FOI findings; complete MRI data were available for 108 of these patients, including 68 negative MRIs and 40 positive MRIs.
In the XTEND study, another 12% of patients who were positive on FOI but not on MRI also developed PsA by the end of the 2-year follow-up. In comparison, the researchers noted that “literature data on yearly incidence rates [of PsA] in different national cohorts indicate an incidence rate of approximately 4.3% per year.”
A total of 149 of the 196 patients with PsA confirmed by either clinical exam or MSUS were also positive on FOI, yielding a sensitivity of 76.0%. The specificity of FOI was 39.5%.
The sensitive visualization of musculoskeletal inflammation possible with FOI “may exceed its ability to detect clinically manifest PsA at high sensitivity or specificity, but early visualization is arguably of greater value as other imaging methods are currently available for detection of later stages of PsA,” the researchers wrote in their discussion. “A technique allowing early identification of PsA may be especially valuable for nonrheumatologists, including dermatologists and general practitioners, and help expedite more efficient referral to specialists.”
The findings were limited by several factors, including the nonrandomized design and small subgroup numbers, the researchers noted. Other limitations include the presence of alternative conditions such as osteoarthritis that might have complicated the imaging; the focus only on the hands; and potential variation in FOI assessment related to technical standards such as temperature and positioning.
However, the results support FOI as a safe and effective method of detecting early signs of joint inflammation that could predict increased risk for PsA in psoriasis patients, the researchers said.
The researchers added that more work is needed to evaluate FOI in clinical practice, but FOI has the potential to identify vascularization changes earlier than other imaging modalities and in advance of clinical symptoms.
“Accordingly, FOI may have the potential to improve patient outcomes in PsA by reducing the time to initiation of early treatment,” they concluded.
The study was supported by Fraunhofer ITMP, a nonprofit organization, and a research grant from Pfizer Germany. Some of the researchers disclosed financial relationships with many pharmaceutical companies, including Pfizer.
Fluorescence-optical imaging (FOI) identified early signs of psoriatic arthritis, based on data from 2 years of follow-up of a cohort of 389 adults at 14 rheumatology centers.
Approximately 25% of individuals with psoriasis go on to develop psoriatic arthritis (PsA), but there are no validated biomarkers to identify patients at risk for progression to PsA, Michaela Koehm, MD, of Goethe University, Frankfurt am Main, Germany, and colleagues wrote in RMD Open.
FOI is a technique that allows assessment of changes in microvascularization and subdermal skin inflammation, and because individuals with psoriasis who develop PsA have shown changes in blood vessel formation in the early stages of disease, the researchers sought to determine if FOI could be used to predict early PsA.
The researchers conducted a multicenter, two-part observational cohort study. The two parts, known as XCITING and XTEND, included 389 adults aged 18-75 years with plaque psoriasis deemed at increased risk for PsA. The patients were seen at rheumatology sites in Germany between Jan. 28, 2014, and March 16, 2017. The XTEND study included clinic visits 18-24 months after the XCITING study.
Participants underwent a complete clinical examination, with musculoskeletal ultrasound (MSUS) and FOI on both hands at a single visit. Those with positive FOI findings not seen with clinical exam or MSUS underwent MRI within 7 days. Patients with positive FOI but negative findings on clinical exam, MSUS, and MRI were followed for 2 years in the XTEND study.
The primary outcome was the ability of FOI to detect musculoskeletal inflammation, compared with clinical examination and MSUS.
Overall, 50% of the patients were diagnosed with PsA. A total of 116 (30%) had positive FOI findings; complete MRI data were available for 108 of these patients, including 68 negative MRIs and 40 positive MRIs.
In the XTEND study, another 12% of patients who were positive on FOI but not on MRI also developed PsA by the end of the 2-year follow-up. In comparison, the researchers noted that “literature data on yearly incidence rates [of PsA] in different national cohorts indicate an incidence rate of approximately 4.3% per year.”
A total of 149 of the 196 patients with PsA confirmed by either clinical exam or MSUS were also positive on FOI, yielding a sensitivity of 76.0%. The specificity of FOI was 39.5%.
The sensitive visualization of musculoskeletal inflammation possible with FOI “may exceed its ability to detect clinically manifest PsA at high sensitivity or specificity, but early visualization is arguably of greater value as other imaging methods are currently available for detection of later stages of PsA,” the researchers wrote in their discussion. “A technique allowing early identification of PsA may be especially valuable for nonrheumatologists, including dermatologists and general practitioners, and help expedite more efficient referral to specialists.”
The findings were limited by several factors, including the nonrandomized design and small subgroup numbers, the researchers noted. Other limitations include the presence of alternative conditions such as osteoarthritis that might have complicated the imaging; the focus only on the hands; and potential variation in FOI assessment related to technical standards such as temperature and positioning.
However, the results support FOI as a safe and effective method of detecting early signs of joint inflammation that could predict increased risk for PsA in psoriasis patients, the researchers said.
The researchers added that more work is needed to evaluate FOI in clinical practice, but FOI has the potential to identify vascularization changes earlier than other imaging modalities and in advance of clinical symptoms.
“Accordingly, FOI may have the potential to improve patient outcomes in PsA by reducing the time to initiation of early treatment,” they concluded.
The study was supported by Fraunhofer ITMP, a nonprofit organization, and a research grant from Pfizer Germany. Some of the researchers disclosed financial relationships with many pharmaceutical companies, including Pfizer.
Fluorescence-optical imaging (FOI) identified early signs of psoriatic arthritis, based on data from 2 years of follow-up of a cohort of 389 adults at 14 rheumatology centers.
Approximately 25% of individuals with psoriasis go on to develop psoriatic arthritis (PsA), but there are no validated biomarkers to identify patients at risk for progression to PsA, Michaela Koehm, MD, of Goethe University, Frankfurt am Main, Germany, and colleagues wrote in RMD Open.
FOI is a technique that allows assessment of changes in microvascularization and subdermal skin inflammation, and because individuals with psoriasis who develop PsA have shown changes in blood vessel formation in the early stages of disease, the researchers sought to determine if FOI could be used to predict early PsA.
The researchers conducted a multicenter, two-part observational cohort study. The two parts, known as XCITING and XTEND, included 389 adults aged 18-75 years with plaque psoriasis deemed at increased risk for PsA. The patients were seen at rheumatology sites in Germany between Jan. 28, 2014, and March 16, 2017. The XTEND study included clinic visits 18-24 months after the XCITING study.
Participants underwent a complete clinical examination, with musculoskeletal ultrasound (MSUS) and FOI on both hands at a single visit. Those with positive FOI findings not seen with clinical exam or MSUS underwent MRI within 7 days. Patients with positive FOI but negative findings on clinical exam, MSUS, and MRI were followed for 2 years in the XTEND study.
The primary outcome was the ability of FOI to detect musculoskeletal inflammation, compared with clinical examination and MSUS.
Overall, 50% of the patients were diagnosed with PsA. A total of 116 (30%) had positive FOI findings; complete MRI data were available for 108 of these patients, including 68 negative MRIs and 40 positive MRIs.
In the XTEND study, another 12% of patients who were positive on FOI but not on MRI also developed PsA by the end of the 2-year follow-up. In comparison, the researchers noted that “literature data on yearly incidence rates [of PsA] in different national cohorts indicate an incidence rate of approximately 4.3% per year.”
A total of 149 of the 196 patients with PsA confirmed by either clinical exam or MSUS were also positive on FOI, yielding a sensitivity of 76.0%. The specificity of FOI was 39.5%.
The sensitive visualization of musculoskeletal inflammation possible with FOI “may exceed its ability to detect clinically manifest PsA at high sensitivity or specificity, but early visualization is arguably of greater value as other imaging methods are currently available for detection of later stages of PsA,” the researchers wrote in their discussion. “A technique allowing early identification of PsA may be especially valuable for nonrheumatologists, including dermatologists and general practitioners, and help expedite more efficient referral to specialists.”
The findings were limited by several factors, including the nonrandomized design and small subgroup numbers, the researchers noted. Other limitations include the presence of alternative conditions such as osteoarthritis that might have complicated the imaging; the focus only on the hands; and potential variation in FOI assessment related to technical standards such as temperature and positioning.
However, the results support FOI as a safe and effective method of detecting early signs of joint inflammation that could predict increased risk for PsA in psoriasis patients, the researchers said.
The researchers added that more work is needed to evaluate FOI in clinical practice, but FOI has the potential to identify vascularization changes earlier than other imaging modalities and in advance of clinical symptoms.
“Accordingly, FOI may have the potential to improve patient outcomes in PsA by reducing the time to initiation of early treatment,” they concluded.
The study was supported by Fraunhofer ITMP, a nonprofit organization, and a research grant from Pfizer Germany. Some of the researchers disclosed financial relationships with many pharmaceutical companies, including Pfizer.
FROM RMD OPEN
‘Sugar tax’ prevented thousands of girls becoming obese
The introduction of the soft drinks industry levy (SDIL) – dubbed the ‘sugar tax’ – in England was followed by a drop in the number of older primary school girls succumbing to obesity, according to researchers from the Universities of Cambridge, Oxford, and Bath, with colleagues at the London School of Hygiene and Tropical Medicine.
The study, published in PLOS Medicine, has led to calls to extend the levy to other unhealthy foods and drinks
Obesity has become a global public health problem, the researchers said. In England, around 10% of 4- to 5-year-old children and 20% of 10- to 11-year-olds were recorded as obese in 2020. Childhood obesity is associated with depression in children and the adults into which they maturate, as well as with serious health problems in later life including high blood pressure and type 2 diabetes.
In the United Kingdom, young people consume significantly more added sugars than are recommended – by late adolescence, typically 70 g of added sugar per day, more than double the recommended 30g. The team said that sugar-sweetened beverages (SSB) are the primary sources of dietary added sugars in children, with high consumption commonly observed in more deprived areas where obesity prevalence is also highest.
Protecting children from excessive sugar
The two-tier SDIL on drinks manufacturers was implemented in April 2018 and aimed to protect children from excessive sugar consumption and tackle childhood obesity by incentivizing reformulation of SSBs in the U.K. with reduced sugar content.
To assess the effects of SDIL, the researchers used data from the National Child Measurement Programme on over 1 million children at ages 4 to 5 years (reception class) and 10 to 11 years (school year 6) in state-maintained English primary schools. The surveillance program includes annual repeat cross-sectional measurements, enabling the researchers to examine trajectories in monthly prevalence of obesity from September 2013 to November 2019, 19 months after the implementation of the SDIL.
Taking account of previous trends in obesity levels, they estimated both absolute and relative changes in obesity prevalence, both overall and by sex and deprivation, and compared obesity levels after the SDIL with predicted levels had the tax not been introduced, controlling for children’s sex and the level of deprivation of their school area.
Although they found no significant association with obesity levels in reception-age children or year-6 boys, they noted an overall absolute reduction in obesity prevalence of 1.6 percentage points (PPs) (95% confidence interval, 1.1-2.1) in 10- to 11-year-old (year 6) girls. This equated to an 8% relative reduction in obesity rates compared with a counterfactual estimated from the trend prior to the SDIL announcement in March 2016, adjusted for temporal variations in obesity prevalence.
The researchers estimated that this was equivalent to preventing 5,234 cases of obesity per year in this group of year-6 girls alone.
Obesity reductions greatest in most deprived areas
Reductions were greatest in girls whose schools were in the most deprived areas, where children are known to consume the largest amount of sugary drinks. The greatest reductions in obesity were observed in the two most deprived quintiles – such that in the lowest quintile the absolute obesity prevalence reduction was 2.4 PP (95% CI, 1.6-3.2), equivalent to a 9% reduction in those living in the most deprived areas.
There are several reasons why the sugar tax did not lead to changes in levels of obesity among the younger children, the researchers said. Very young children consume fewer sugar-sweetened drinks than older children, so the soft drinks levy would have had a smaller effect. Also, fruit juices are not included in the levy, but contribute similar amounts of sugar in young children’s diets as do sugar-sweetened beverages.
Advertising may impact consumption in boys
It’s also unclear why the sugar tax might affect obesity prevalence in girls and boys differently, they said, especially since boys are higher consumers of sugar-sweetened beverages. One explanation is the possible impact of advertising – numerous studies have found that boys are often exposed to more food advertising than girls, both through higher levels of TV viewing and in how adverts are framed. Physical activity is often used to promote junk food and boys, compared with girls, have been shown to be more likely to believe that energy-dense junk foods depicted in adverts will boost physical performance, and so are more likely to choose energy-dense, nutrient-poor products following celebrity endorsements.
Tax ‘led to positive health impacts’
“Our findings suggest that the U.K. SDIL led to positive health impacts in the form of reduced obesity levels in girls aged 10-11 years,” the authors said. However: “Additional strategies beyond SSB taxation will be needed to reduce obesity prevalence overall, and particularly in older boys and younger children.”
Dr. Nina Rogers from the MRC Epidemiology Unit at Cambridge (England), who led the study, said: “We urgently need to find ways to tackle the increasing numbers of children living with obesity, otherwise we risk our children growing up to face significant health problems. That was one reason why the U.K.’s SDIL was introduced, and the evidence so far is promising. We’ve shown for the first time that it is likely to have helped prevent thousands of children each year becoming obese.
“It isn’t a straightforward picture, though, as it was mainly older girls who benefited. But the fact that we saw the biggest difference among girls from areas of high deprivation is important and is a step towards reducing the health inequalities they face.”
Although the researchers found an association rather than a causal link, this study adds to previous findings that the levy was associated with a substantial reduction in the amount of sugar in soft drinks.
Senior author Professor Jean Adams from the MRC Epidemiology Unit said: “We know that consuming too many sugary drinks contributes to obesity and that the U.K. soft drinks levy led to a drop in the amount of sugar in soft drinks available in the U.K., so it makes sense that we also see a drop in cases of obesity, although we only found this in girls. Children from more deprived backgrounds tend to consume the largest amount of sugary drinks, and it was among girls in this group that we saw the biggest change.”
Tom Sanders, professor emeritus of nutrition and dietetics at King’s College London, said: “The claim that the soft drink levy might have prevented 5,000 children from becoming obese is speculative because it is based on an association not actual measurements of consumption.”
He added that: “As well as continuing to discourage the consumption of sugar sweetened beverages and sweets, wider recognition should be given to foods such as biscuits [and] deep-fried foods (crisps, corn snacks, chips) that make [a] bigger contribution to excess calorie intake in children. Tackling poverty, however, is probably [the] best way to improve the diets of socially deprived children.”
Government ‘should learn from this success’
Asked to comment by this news organization, Katharine Jenner, director of the Obesity Health Alliance, said: “Government should be heartened that their soft drinks policy is already improving the health of young girls, regardless of where they live. The government should learn from this success, especially when compared with the many unsuccessful attempts to persuade industry to change their products voluntarily. They must now press ahead with policies that make it easier for everyone to eat a healthier diet, including extending the soft drinks industry levy to include other less healthy foods and drinks and measures to take junk food out of the spotlight.
“The research notes that numerous studies have found that boys are often exposed to more food advertising content than girls, negating the impact of the soft drinks levy [so] we need restriction on junk food marketing now, to put healthy food back in the spotlight.”
The research was supported by the National Institute of Health and Care Research and the Medical Research Council.
A version of this article originally appeared on MedscapeUK.
The introduction of the soft drinks industry levy (SDIL) – dubbed the ‘sugar tax’ – in England was followed by a drop in the number of older primary school girls succumbing to obesity, according to researchers from the Universities of Cambridge, Oxford, and Bath, with colleagues at the London School of Hygiene and Tropical Medicine.
The study, published in PLOS Medicine, has led to calls to extend the levy to other unhealthy foods and drinks
Obesity has become a global public health problem, the researchers said. In England, around 10% of 4- to 5-year-old children and 20% of 10- to 11-year-olds were recorded as obese in 2020. Childhood obesity is associated with depression in children and the adults into which they maturate, as well as with serious health problems in later life including high blood pressure and type 2 diabetes.
In the United Kingdom, young people consume significantly more added sugars than are recommended – by late adolescence, typically 70 g of added sugar per day, more than double the recommended 30g. The team said that sugar-sweetened beverages (SSB) are the primary sources of dietary added sugars in children, with high consumption commonly observed in more deprived areas where obesity prevalence is also highest.
Protecting children from excessive sugar
The two-tier SDIL on drinks manufacturers was implemented in April 2018 and aimed to protect children from excessive sugar consumption and tackle childhood obesity by incentivizing reformulation of SSBs in the U.K. with reduced sugar content.
To assess the effects of SDIL, the researchers used data from the National Child Measurement Programme on over 1 million children at ages 4 to 5 years (reception class) and 10 to 11 years (school year 6) in state-maintained English primary schools. The surveillance program includes annual repeat cross-sectional measurements, enabling the researchers to examine trajectories in monthly prevalence of obesity from September 2013 to November 2019, 19 months after the implementation of the SDIL.
Taking account of previous trends in obesity levels, they estimated both absolute and relative changes in obesity prevalence, both overall and by sex and deprivation, and compared obesity levels after the SDIL with predicted levels had the tax not been introduced, controlling for children’s sex and the level of deprivation of their school area.
Although they found no significant association with obesity levels in reception-age children or year-6 boys, they noted an overall absolute reduction in obesity prevalence of 1.6 percentage points (PPs) (95% confidence interval, 1.1-2.1) in 10- to 11-year-old (year 6) girls. This equated to an 8% relative reduction in obesity rates compared with a counterfactual estimated from the trend prior to the SDIL announcement in March 2016, adjusted for temporal variations in obesity prevalence.
The researchers estimated that this was equivalent to preventing 5,234 cases of obesity per year in this group of year-6 girls alone.
Obesity reductions greatest in most deprived areas
Reductions were greatest in girls whose schools were in the most deprived areas, where children are known to consume the largest amount of sugary drinks. The greatest reductions in obesity were observed in the two most deprived quintiles – such that in the lowest quintile the absolute obesity prevalence reduction was 2.4 PP (95% CI, 1.6-3.2), equivalent to a 9% reduction in those living in the most deprived areas.
There are several reasons why the sugar tax did not lead to changes in levels of obesity among the younger children, the researchers said. Very young children consume fewer sugar-sweetened drinks than older children, so the soft drinks levy would have had a smaller effect. Also, fruit juices are not included in the levy, but contribute similar amounts of sugar in young children’s diets as do sugar-sweetened beverages.
Advertising may impact consumption in boys
It’s also unclear why the sugar tax might affect obesity prevalence in girls and boys differently, they said, especially since boys are higher consumers of sugar-sweetened beverages. One explanation is the possible impact of advertising – numerous studies have found that boys are often exposed to more food advertising than girls, both through higher levels of TV viewing and in how adverts are framed. Physical activity is often used to promote junk food and boys, compared with girls, have been shown to be more likely to believe that energy-dense junk foods depicted in adverts will boost physical performance, and so are more likely to choose energy-dense, nutrient-poor products following celebrity endorsements.
Tax ‘led to positive health impacts’
“Our findings suggest that the U.K. SDIL led to positive health impacts in the form of reduced obesity levels in girls aged 10-11 years,” the authors said. However: “Additional strategies beyond SSB taxation will be needed to reduce obesity prevalence overall, and particularly in older boys and younger children.”
Dr. Nina Rogers from the MRC Epidemiology Unit at Cambridge (England), who led the study, said: “We urgently need to find ways to tackle the increasing numbers of children living with obesity, otherwise we risk our children growing up to face significant health problems. That was one reason why the U.K.’s SDIL was introduced, and the evidence so far is promising. We’ve shown for the first time that it is likely to have helped prevent thousands of children each year becoming obese.
“It isn’t a straightforward picture, though, as it was mainly older girls who benefited. But the fact that we saw the biggest difference among girls from areas of high deprivation is important and is a step towards reducing the health inequalities they face.”
Although the researchers found an association rather than a causal link, this study adds to previous findings that the levy was associated with a substantial reduction in the amount of sugar in soft drinks.
Senior author Professor Jean Adams from the MRC Epidemiology Unit said: “We know that consuming too many sugary drinks contributes to obesity and that the U.K. soft drinks levy led to a drop in the amount of sugar in soft drinks available in the U.K., so it makes sense that we also see a drop in cases of obesity, although we only found this in girls. Children from more deprived backgrounds tend to consume the largest amount of sugary drinks, and it was among girls in this group that we saw the biggest change.”
Tom Sanders, professor emeritus of nutrition and dietetics at King’s College London, said: “The claim that the soft drink levy might have prevented 5,000 children from becoming obese is speculative because it is based on an association not actual measurements of consumption.”
He added that: “As well as continuing to discourage the consumption of sugar sweetened beverages and sweets, wider recognition should be given to foods such as biscuits [and] deep-fried foods (crisps, corn snacks, chips) that make [a] bigger contribution to excess calorie intake in children. Tackling poverty, however, is probably [the] best way to improve the diets of socially deprived children.”
Government ‘should learn from this success’
Asked to comment by this news organization, Katharine Jenner, director of the Obesity Health Alliance, said: “Government should be heartened that their soft drinks policy is already improving the health of young girls, regardless of where they live. The government should learn from this success, especially when compared with the many unsuccessful attempts to persuade industry to change their products voluntarily. They must now press ahead with policies that make it easier for everyone to eat a healthier diet, including extending the soft drinks industry levy to include other less healthy foods and drinks and measures to take junk food out of the spotlight.
“The research notes that numerous studies have found that boys are often exposed to more food advertising content than girls, negating the impact of the soft drinks levy [so] we need restriction on junk food marketing now, to put healthy food back in the spotlight.”
The research was supported by the National Institute of Health and Care Research and the Medical Research Council.
A version of this article originally appeared on MedscapeUK.
The introduction of the soft drinks industry levy (SDIL) – dubbed the ‘sugar tax’ – in England was followed by a drop in the number of older primary school girls succumbing to obesity, according to researchers from the Universities of Cambridge, Oxford, and Bath, with colleagues at the London School of Hygiene and Tropical Medicine.
The study, published in PLOS Medicine, has led to calls to extend the levy to other unhealthy foods and drinks
Obesity has become a global public health problem, the researchers said. In England, around 10% of 4- to 5-year-old children and 20% of 10- to 11-year-olds were recorded as obese in 2020. Childhood obesity is associated with depression in children and the adults into which they maturate, as well as with serious health problems in later life including high blood pressure and type 2 diabetes.
In the United Kingdom, young people consume significantly more added sugars than are recommended – by late adolescence, typically 70 g of added sugar per day, more than double the recommended 30g. The team said that sugar-sweetened beverages (SSB) are the primary sources of dietary added sugars in children, with high consumption commonly observed in more deprived areas where obesity prevalence is also highest.
Protecting children from excessive sugar
The two-tier SDIL on drinks manufacturers was implemented in April 2018 and aimed to protect children from excessive sugar consumption and tackle childhood obesity by incentivizing reformulation of SSBs in the U.K. with reduced sugar content.
To assess the effects of SDIL, the researchers used data from the National Child Measurement Programme on over 1 million children at ages 4 to 5 years (reception class) and 10 to 11 years (school year 6) in state-maintained English primary schools. The surveillance program includes annual repeat cross-sectional measurements, enabling the researchers to examine trajectories in monthly prevalence of obesity from September 2013 to November 2019, 19 months after the implementation of the SDIL.
Taking account of previous trends in obesity levels, they estimated both absolute and relative changes in obesity prevalence, both overall and by sex and deprivation, and compared obesity levels after the SDIL with predicted levels had the tax not been introduced, controlling for children’s sex and the level of deprivation of their school area.
Although they found no significant association with obesity levels in reception-age children or year-6 boys, they noted an overall absolute reduction in obesity prevalence of 1.6 percentage points (PPs) (95% confidence interval, 1.1-2.1) in 10- to 11-year-old (year 6) girls. This equated to an 8% relative reduction in obesity rates compared with a counterfactual estimated from the trend prior to the SDIL announcement in March 2016, adjusted for temporal variations in obesity prevalence.
The researchers estimated that this was equivalent to preventing 5,234 cases of obesity per year in this group of year-6 girls alone.
Obesity reductions greatest in most deprived areas
Reductions were greatest in girls whose schools were in the most deprived areas, where children are known to consume the largest amount of sugary drinks. The greatest reductions in obesity were observed in the two most deprived quintiles – such that in the lowest quintile the absolute obesity prevalence reduction was 2.4 PP (95% CI, 1.6-3.2), equivalent to a 9% reduction in those living in the most deprived areas.
There are several reasons why the sugar tax did not lead to changes in levels of obesity among the younger children, the researchers said. Very young children consume fewer sugar-sweetened drinks than older children, so the soft drinks levy would have had a smaller effect. Also, fruit juices are not included in the levy, but contribute similar amounts of sugar in young children’s diets as do sugar-sweetened beverages.
Advertising may impact consumption in boys
It’s also unclear why the sugar tax might affect obesity prevalence in girls and boys differently, they said, especially since boys are higher consumers of sugar-sweetened beverages. One explanation is the possible impact of advertising – numerous studies have found that boys are often exposed to more food advertising than girls, both through higher levels of TV viewing and in how adverts are framed. Physical activity is often used to promote junk food and boys, compared with girls, have been shown to be more likely to believe that energy-dense junk foods depicted in adverts will boost physical performance, and so are more likely to choose energy-dense, nutrient-poor products following celebrity endorsements.
Tax ‘led to positive health impacts’
“Our findings suggest that the U.K. SDIL led to positive health impacts in the form of reduced obesity levels in girls aged 10-11 years,” the authors said. However: “Additional strategies beyond SSB taxation will be needed to reduce obesity prevalence overall, and particularly in older boys and younger children.”
Dr. Nina Rogers from the MRC Epidemiology Unit at Cambridge (England), who led the study, said: “We urgently need to find ways to tackle the increasing numbers of children living with obesity, otherwise we risk our children growing up to face significant health problems. That was one reason why the U.K.’s SDIL was introduced, and the evidence so far is promising. We’ve shown for the first time that it is likely to have helped prevent thousands of children each year becoming obese.
“It isn’t a straightforward picture, though, as it was mainly older girls who benefited. But the fact that we saw the biggest difference among girls from areas of high deprivation is important and is a step towards reducing the health inequalities they face.”
Although the researchers found an association rather than a causal link, this study adds to previous findings that the levy was associated with a substantial reduction in the amount of sugar in soft drinks.
Senior author Professor Jean Adams from the MRC Epidemiology Unit said: “We know that consuming too many sugary drinks contributes to obesity and that the U.K. soft drinks levy led to a drop in the amount of sugar in soft drinks available in the U.K., so it makes sense that we also see a drop in cases of obesity, although we only found this in girls. Children from more deprived backgrounds tend to consume the largest amount of sugary drinks, and it was among girls in this group that we saw the biggest change.”
Tom Sanders, professor emeritus of nutrition and dietetics at King’s College London, said: “The claim that the soft drink levy might have prevented 5,000 children from becoming obese is speculative because it is based on an association not actual measurements of consumption.”
He added that: “As well as continuing to discourage the consumption of sugar sweetened beverages and sweets, wider recognition should be given to foods such as biscuits [and] deep-fried foods (crisps, corn snacks, chips) that make [a] bigger contribution to excess calorie intake in children. Tackling poverty, however, is probably [the] best way to improve the diets of socially deprived children.”
Government ‘should learn from this success’
Asked to comment by this news organization, Katharine Jenner, director of the Obesity Health Alliance, said: “Government should be heartened that their soft drinks policy is already improving the health of young girls, regardless of where they live. The government should learn from this success, especially when compared with the many unsuccessful attempts to persuade industry to change their products voluntarily. They must now press ahead with policies that make it easier for everyone to eat a healthier diet, including extending the soft drinks industry levy to include other less healthy foods and drinks and measures to take junk food out of the spotlight.
“The research notes that numerous studies have found that boys are often exposed to more food advertising content than girls, negating the impact of the soft drinks levy [so] we need restriction on junk food marketing now, to put healthy food back in the spotlight.”
The research was supported by the National Institute of Health and Care Research and the Medical Research Council.
A version of this article originally appeared on MedscapeUK.
Infant with red eyelid lesion
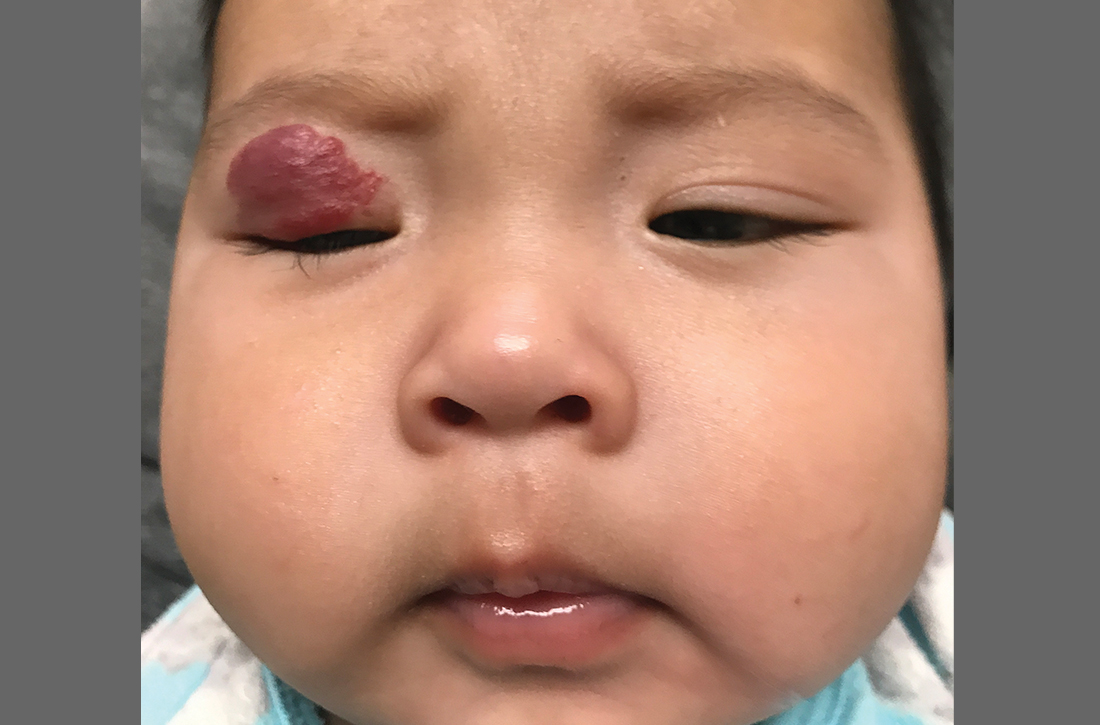
A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
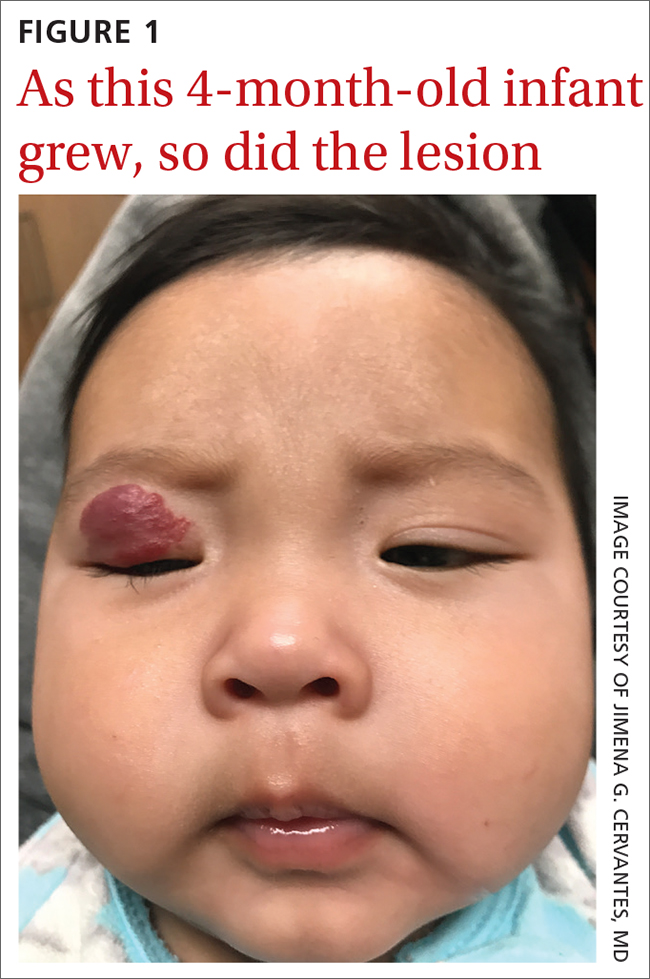
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
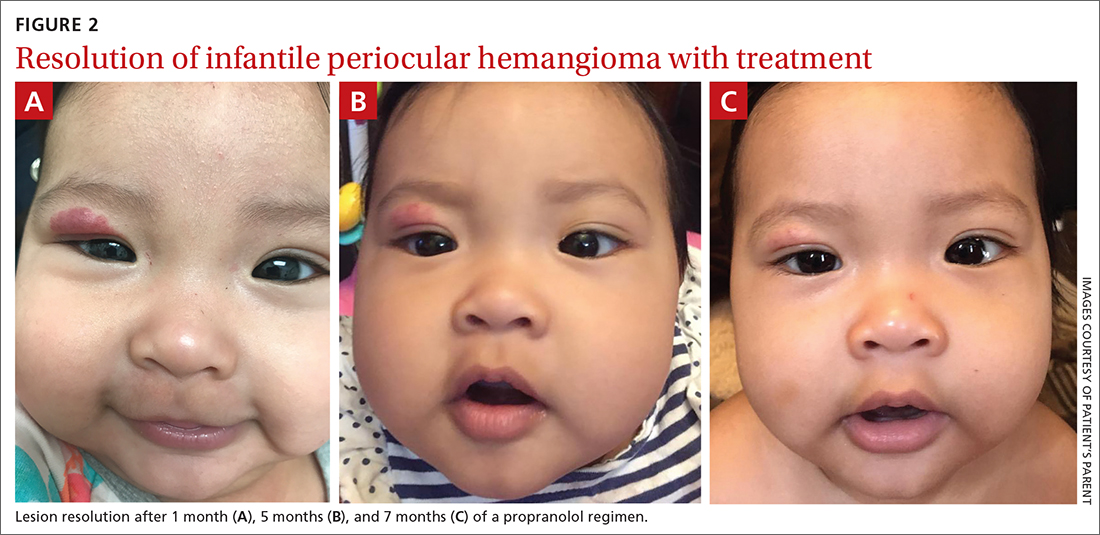
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.
1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525
A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.

A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.

1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525
1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525
New ‘reference regimen’ in metastatic pancreatic cancer?
(mPDAC) in a global phase 3 trial dubbed NAPOLI-3.
Liposomal irinotecan (Onivyde) plus 5-fluorouracil/leucovorin and oxaliplatin (together known as NALIRIFOX) extended both overall and progression-free survival compared with gemcitabine plus nab-paclitaxel when used as first-line therapy in treatment-naive patients with mPDAC.
“These results support the NALIRIFOX regimen as a new regimen for the first-line treatment of patients with metastatic PDAC,” said lead study author Zev A. Wainberg, MD, professor of medicine at University of California, Los Angeles (UCLA) and codirector of the UCLA GI Oncology Program. “This study indicates that the more aggressive chemotherapy approach should be considered for those patients who are able to tolerate it.”
The findings were presented at the ASCO Gastrointestinal Cancers Symposium 2023.
Liposomal irinotecan administered with 5-fluorouracil/leucovorin has been approved in both the United States and Europe for patients with mPDAC who experience disease progression after treatment with gemcitabine-based therapy. This new trial investigated the approach as a first-line option.
It involved 770 patients who were randomly assigned to one of two treatment arms: NALIRIFOX (liposomal irinotecan 50 mg/m2, 5-fluorouracil 2400 mg/m2, leucovorin 400 mg/m2, and oxaliplatin 60 mg/m2; days 1 and 15 of 28-day cycle) or gemcitabine 1,000 mg/m2 plus nab-paclitaxel 125 mg/m2 (days 1, 8, and 15 of 28-day cycle).
Most patients in both study arms were male (53.3% and 59.4% for liposomal irinotecan/NALIRIFOX recipient vs. the control group, respectively), had at least three metastatic sites (38.9% and 36.4%), had liver metastases (80.2% and 80.4%), and had their main tumor located outside the head of the pancreas (61.6% and 59.7%).
Most patients (65% in both arms) were treated outside of North America and East Asia.
“This is truly a global study, enrolling in over 20 countries, with a mix of both academic and community centers,” said Dr. Wainberg.
At a median follow-up of 16.1 months, 544 events had occurred.
“The study met its primary endpoint of overall survival by improving the median from 9.2 months with gemcitabine plus nab-paclitaxel to 11.1 months with NALIFIROX-treated group,” said Dr. Wainberg. “The hazard ratio was 0.84, with a P value of .04.”
Significant improvement was also observed in progression-free survival: 7.4 months for NALIFIROX vs. 5.6 months for gemcitabine plus nab-paclitaxel (hazard ratio, 0.69; P < .0001).
The overall response rate for liposomal irinotecan/NALIRIFOX vs. nab-paclitaxel/gemcitabine was 41.8% vs. 36.2%, and the complete response rate was 0.3% in both arms.
Dr. Wainberg noted that about half of the patients in both groups underwent subsequent anticancer therapy (50.5% vs. 54.4%); this included systemic treatments, surgery, and radiation therapy.
Grade 3/4 treatment-emergent adverse events reported in ≥ 10% of patients in both groups included diarrhea (20.3% vs. 4.5%), nausea (11.9% vs. 2.6%), hypokalemia (15.1% vs. 4.0%), anemia (10.5% vs. 17.4%), and neutropenia (14.1% vs. 24.5%).
“When one looks at the nuances in the patients and the toxicity profiles, we can see these two regimens have very different toxicity profiles,” Dr. Wainberg said. “There was also less peripheral neuropathy seen in the NALIRIFOX arm relative to the nab-paclitaxel/gemcitabine arm.”
The differences in the toxicity profiles were related to the mechanisms of action, Dr. Wainberg explained. “No new safety concerns with the NALIRIFOX regimen were identified.”
New reference regimen
Dr. Wainberg concluded that the NALIRIFOX regimen can be considered the new reference regimen for first-line treatment of metastatic pancreatic adenocarcinoma. “Hopefully it’s something we can build off of in the future.”
Discussant for this paper, Laura Goff, MD, MSCI, associate professor of medicine and the executive medical director for the Cancer Patient Care Center at Vanderbilt Ingram Cancer Center, Nashville, Tenn., agreed.
For “fit patients, these results support the NALIRIFOX regimen as the new reference regimen for first-line treatment of metastatic pancreatic adenocarcinoma,” she said.
“It is the new standard for fit patients,” she added.
She also agreed with the authors’ conclusion that NALIRIFOX demonstrated clinically meaningful and statistically significant improvements in overall and progression-free survival compared with nab-paclitaxel/gemcitabine.
“The safety profile of NALIRFOX was manageable and consistent with the treatment components,” she said. “Both regimens had high toxicity rates, but their toxicity profiles were different.”
Dr. Goff pointed out that high rates of toxicity were seen in both arms, “despite the reputation that gemcitabine/nab-paclitaxel is a significantly easier regimen to tolerate. I would argue that these data do not necessarily support that.”
The study was funded by Ipsen. Dr. Wainberg reported relationships with Amgen, Arcus Bioscience, AstraZeneca/MedImmune, Bayer, Bristol-Myers Squibb, Daiichi Sankyo/Astra Zeneca, Ipsen, Lilly, Merck, Novartis, Pfizer, Plexxikon, PureTech, QED Therapeutics, Seattle Genetics. Dr. Goff reported relationships with Agios, ASLAN Pharmaceuticals, AstraZeneca, Basilea, BeiGene, Boehringer Ingelheim, Bristol-Myers Squibb, Exelixis, Genentech, Merck, and QED Therapeutics. Most of the other sudy authors had disclosures.
A version of this article first appeared on Medscape.com.
(mPDAC) in a global phase 3 trial dubbed NAPOLI-3.
Liposomal irinotecan (Onivyde) plus 5-fluorouracil/leucovorin and oxaliplatin (together known as NALIRIFOX) extended both overall and progression-free survival compared with gemcitabine plus nab-paclitaxel when used as first-line therapy in treatment-naive patients with mPDAC.
“These results support the NALIRIFOX regimen as a new regimen for the first-line treatment of patients with metastatic PDAC,” said lead study author Zev A. Wainberg, MD, professor of medicine at University of California, Los Angeles (UCLA) and codirector of the UCLA GI Oncology Program. “This study indicates that the more aggressive chemotherapy approach should be considered for those patients who are able to tolerate it.”
The findings were presented at the ASCO Gastrointestinal Cancers Symposium 2023.
Liposomal irinotecan administered with 5-fluorouracil/leucovorin has been approved in both the United States and Europe for patients with mPDAC who experience disease progression after treatment with gemcitabine-based therapy. This new trial investigated the approach as a first-line option.
It involved 770 patients who were randomly assigned to one of two treatment arms: NALIRIFOX (liposomal irinotecan 50 mg/m2, 5-fluorouracil 2400 mg/m2, leucovorin 400 mg/m2, and oxaliplatin 60 mg/m2; days 1 and 15 of 28-day cycle) or gemcitabine 1,000 mg/m2 plus nab-paclitaxel 125 mg/m2 (days 1, 8, and 15 of 28-day cycle).
Most patients in both study arms were male (53.3% and 59.4% for liposomal irinotecan/NALIRIFOX recipient vs. the control group, respectively), had at least three metastatic sites (38.9% and 36.4%), had liver metastases (80.2% and 80.4%), and had their main tumor located outside the head of the pancreas (61.6% and 59.7%).
Most patients (65% in both arms) were treated outside of North America and East Asia.
“This is truly a global study, enrolling in over 20 countries, with a mix of both academic and community centers,” said Dr. Wainberg.
At a median follow-up of 16.1 months, 544 events had occurred.
“The study met its primary endpoint of overall survival by improving the median from 9.2 months with gemcitabine plus nab-paclitaxel to 11.1 months with NALIFIROX-treated group,” said Dr. Wainberg. “The hazard ratio was 0.84, with a P value of .04.”
Significant improvement was also observed in progression-free survival: 7.4 months for NALIFIROX vs. 5.6 months for gemcitabine plus nab-paclitaxel (hazard ratio, 0.69; P < .0001).
The overall response rate for liposomal irinotecan/NALIRIFOX vs. nab-paclitaxel/gemcitabine was 41.8% vs. 36.2%, and the complete response rate was 0.3% in both arms.
Dr. Wainberg noted that about half of the patients in both groups underwent subsequent anticancer therapy (50.5% vs. 54.4%); this included systemic treatments, surgery, and radiation therapy.
Grade 3/4 treatment-emergent adverse events reported in ≥ 10% of patients in both groups included diarrhea (20.3% vs. 4.5%), nausea (11.9% vs. 2.6%), hypokalemia (15.1% vs. 4.0%), anemia (10.5% vs. 17.4%), and neutropenia (14.1% vs. 24.5%).
“When one looks at the nuances in the patients and the toxicity profiles, we can see these two regimens have very different toxicity profiles,” Dr. Wainberg said. “There was also less peripheral neuropathy seen in the NALIRIFOX arm relative to the nab-paclitaxel/gemcitabine arm.”
The differences in the toxicity profiles were related to the mechanisms of action, Dr. Wainberg explained. “No new safety concerns with the NALIRIFOX regimen were identified.”
New reference regimen
Dr. Wainberg concluded that the NALIRIFOX regimen can be considered the new reference regimen for first-line treatment of metastatic pancreatic adenocarcinoma. “Hopefully it’s something we can build off of in the future.”
Discussant for this paper, Laura Goff, MD, MSCI, associate professor of medicine and the executive medical director for the Cancer Patient Care Center at Vanderbilt Ingram Cancer Center, Nashville, Tenn., agreed.
For “fit patients, these results support the NALIRIFOX regimen as the new reference regimen for first-line treatment of metastatic pancreatic adenocarcinoma,” she said.
“It is the new standard for fit patients,” she added.
She also agreed with the authors’ conclusion that NALIRIFOX demonstrated clinically meaningful and statistically significant improvements in overall and progression-free survival compared with nab-paclitaxel/gemcitabine.
“The safety profile of NALIRFOX was manageable and consistent with the treatment components,” she said. “Both regimens had high toxicity rates, but their toxicity profiles were different.”
Dr. Goff pointed out that high rates of toxicity were seen in both arms, “despite the reputation that gemcitabine/nab-paclitaxel is a significantly easier regimen to tolerate. I would argue that these data do not necessarily support that.”
The study was funded by Ipsen. Dr. Wainberg reported relationships with Amgen, Arcus Bioscience, AstraZeneca/MedImmune, Bayer, Bristol-Myers Squibb, Daiichi Sankyo/Astra Zeneca, Ipsen, Lilly, Merck, Novartis, Pfizer, Plexxikon, PureTech, QED Therapeutics, Seattle Genetics. Dr. Goff reported relationships with Agios, ASLAN Pharmaceuticals, AstraZeneca, Basilea, BeiGene, Boehringer Ingelheim, Bristol-Myers Squibb, Exelixis, Genentech, Merck, and QED Therapeutics. Most of the other sudy authors had disclosures.
A version of this article first appeared on Medscape.com.
(mPDAC) in a global phase 3 trial dubbed NAPOLI-3.
Liposomal irinotecan (Onivyde) plus 5-fluorouracil/leucovorin and oxaliplatin (together known as NALIRIFOX) extended both overall and progression-free survival compared with gemcitabine plus nab-paclitaxel when used as first-line therapy in treatment-naive patients with mPDAC.
“These results support the NALIRIFOX regimen as a new regimen for the first-line treatment of patients with metastatic PDAC,” said lead study author Zev A. Wainberg, MD, professor of medicine at University of California, Los Angeles (UCLA) and codirector of the UCLA GI Oncology Program. “This study indicates that the more aggressive chemotherapy approach should be considered for those patients who are able to tolerate it.”
The findings were presented at the ASCO Gastrointestinal Cancers Symposium 2023.
Liposomal irinotecan administered with 5-fluorouracil/leucovorin has been approved in both the United States and Europe for patients with mPDAC who experience disease progression after treatment with gemcitabine-based therapy. This new trial investigated the approach as a first-line option.
It involved 770 patients who were randomly assigned to one of two treatment arms: NALIRIFOX (liposomal irinotecan 50 mg/m2, 5-fluorouracil 2400 mg/m2, leucovorin 400 mg/m2, and oxaliplatin 60 mg/m2; days 1 and 15 of 28-day cycle) or gemcitabine 1,000 mg/m2 plus nab-paclitaxel 125 mg/m2 (days 1, 8, and 15 of 28-day cycle).
Most patients in both study arms were male (53.3% and 59.4% for liposomal irinotecan/NALIRIFOX recipient vs. the control group, respectively), had at least three metastatic sites (38.9% and 36.4%), had liver metastases (80.2% and 80.4%), and had their main tumor located outside the head of the pancreas (61.6% and 59.7%).
Most patients (65% in both arms) were treated outside of North America and East Asia.
“This is truly a global study, enrolling in over 20 countries, with a mix of both academic and community centers,” said Dr. Wainberg.
At a median follow-up of 16.1 months, 544 events had occurred.
“The study met its primary endpoint of overall survival by improving the median from 9.2 months with gemcitabine plus nab-paclitaxel to 11.1 months with NALIFIROX-treated group,” said Dr. Wainberg. “The hazard ratio was 0.84, with a P value of .04.”
Significant improvement was also observed in progression-free survival: 7.4 months for NALIFIROX vs. 5.6 months for gemcitabine plus nab-paclitaxel (hazard ratio, 0.69; P < .0001).
The overall response rate for liposomal irinotecan/NALIRIFOX vs. nab-paclitaxel/gemcitabine was 41.8% vs. 36.2%, and the complete response rate was 0.3% in both arms.
Dr. Wainberg noted that about half of the patients in both groups underwent subsequent anticancer therapy (50.5% vs. 54.4%); this included systemic treatments, surgery, and radiation therapy.
Grade 3/4 treatment-emergent adverse events reported in ≥ 10% of patients in both groups included diarrhea (20.3% vs. 4.5%), nausea (11.9% vs. 2.6%), hypokalemia (15.1% vs. 4.0%), anemia (10.5% vs. 17.4%), and neutropenia (14.1% vs. 24.5%).
“When one looks at the nuances in the patients and the toxicity profiles, we can see these two regimens have very different toxicity profiles,” Dr. Wainberg said. “There was also less peripheral neuropathy seen in the NALIRIFOX arm relative to the nab-paclitaxel/gemcitabine arm.”
The differences in the toxicity profiles were related to the mechanisms of action, Dr. Wainberg explained. “No new safety concerns with the NALIRIFOX regimen were identified.”
New reference regimen
Dr. Wainberg concluded that the NALIRIFOX regimen can be considered the new reference regimen for first-line treatment of metastatic pancreatic adenocarcinoma. “Hopefully it’s something we can build off of in the future.”
Discussant for this paper, Laura Goff, MD, MSCI, associate professor of medicine and the executive medical director for the Cancer Patient Care Center at Vanderbilt Ingram Cancer Center, Nashville, Tenn., agreed.
For “fit patients, these results support the NALIRIFOX regimen as the new reference regimen for first-line treatment of metastatic pancreatic adenocarcinoma,” she said.
“It is the new standard for fit patients,” she added.
She also agreed with the authors’ conclusion that NALIRIFOX demonstrated clinically meaningful and statistically significant improvements in overall and progression-free survival compared with nab-paclitaxel/gemcitabine.
“The safety profile of NALIRFOX was manageable and consistent with the treatment components,” she said. “Both regimens had high toxicity rates, but their toxicity profiles were different.”
Dr. Goff pointed out that high rates of toxicity were seen in both arms, “despite the reputation that gemcitabine/nab-paclitaxel is a significantly easier regimen to tolerate. I would argue that these data do not necessarily support that.”
The study was funded by Ipsen. Dr. Wainberg reported relationships with Amgen, Arcus Bioscience, AstraZeneca/MedImmune, Bayer, Bristol-Myers Squibb, Daiichi Sankyo/Astra Zeneca, Ipsen, Lilly, Merck, Novartis, Pfizer, Plexxikon, PureTech, QED Therapeutics, Seattle Genetics. Dr. Goff reported relationships with Agios, ASLAN Pharmaceuticals, AstraZeneca, Basilea, BeiGene, Boehringer Ingelheim, Bristol-Myers Squibb, Exelixis, Genentech, Merck, and QED Therapeutics. Most of the other sudy authors had disclosures.
A version of this article first appeared on Medscape.com.
AT ASCO GI 2023
SUNLIGHT shows new standard of care in refractory metastatic CRC
SAN FRANCISCO – ,” say the trial investigators. An expert not involved in the study predicted that it will change clinical practice.
The results show that adding bevacizumab to trifluridine (FTD)/tipiracil (TPI) significantly improved survival, compared with those who received FTD/TPI alone. Median overall survival was 10.8 months in the investigational arm vs 7.5 months among controls (hazard ratio, 0.61; P < .001).
Adding bevacizumab also did not increase the risk for serious adverse events or events that led to treatment discontinuation, the researchers noted.
The new data were presented by Josep Tabernero, MD, PhD, head of the department of medical oncology, Vall d’Hebron University Hospital, and director of the Vall d’Hebron Institute of Oncology, Barcelona, at the ASCO Gastrointestinal Cancers Symposium 2023. He concluded that bevacizumab added to FTD/TPI “represents a new standard of care for the treatment of patients with refractory metastatic colorectal cancer who had previously progressed after two lines of therapy.”
Discussant for the abstract, Dustin Deming, MD, an associate professor in the division of hematology, medical oncology and palliative care at the University of Wisconsin–Madison, said the findings showed very “exciting advantages in progression-free and overall survival.” He agreed that “FTD/TPI and bevacizumab should be considered the preferred nontargeted regimen in the refractory setting” and added that “this trial does change standard clinical practice.”
Dr. Deming also said that this has implications for future clinical trials because these results create a new standard for control arms.
Improvement in all endpoints
FTD/TPI, which is marketed as Lonsurf, is already approved as a monotherapy for third-line use in refractory metastatic colorectal cancer, and bevacizumab is an established anticancer agent that targets vascular endothelial growth factor and inhibits angiogenesis, he explained. The combination of bevacizumab plus FTD/TPI has previously produced encouraging results in the treatment of refractory metastatic colorectal cancer in smaller phase 2 randomized and single-arm studies.
“The phase 3 SUNLIGHT study was designed to confirm the efficacy and safety of FTD/TPI plus bevacizumab, as compared with FTD/TPI alone, in patients with refractory metastatic colorectal cancer following two chemotherapy regimens,” said Dr. Tabernero.
The cohort included 492 patients who were randomly assigned to receive either FTD/TPI plus bevacizumab (FTD/TPI 35 mg/m2 twice daily on days 1-5 and 8-12 [28-day cycle] and bevacizumab 5 mg/kg on days 1 and 15) or FTD/TPI alone.
Across both arms, most patients (72%) had already received prior treatment with bevacizumab.
At 6 months, the overall survival rate was 77% with the combination therapy versus 61% with the control therapy, and after 12 months, the overall survival rate was 43% versus 30%.
Median progression-free survival was 5.6 months in the treatment arm versus 2.4 months in the control arm (HR, 0.44; P < .001). At 6 months, progression-free survival was 43% versus 16%, respectively, and at 12 months, it was 16% versus 1%.
Both overall response rate and disease control rate were also superior in the investigational arm. The overall response rate was 6.3% versus 0.0% in the control arm, with an absolute gain of 5.4% (P = .004). Similarly, the absolute gain for disease control rate was 29.6% (76.6% vs. 47.0%; P < .001).
For quality of life, worsening in global health status in the investigational arm was significantly delayed, compared with the control arm (8.5 months vs. 4.7 months; HR, 0.50; P < .001), as was worsening to an Eastern Cooperative Oncology Group performance status of 2 or greater (9.3 months vs. 6.3 months; HR, 0.54; P < .001).
When looking at toxicity, Tabernero reported that there were no treatment-related deaths and the rates of severe adverse events were similar in both groups: 72% in the FTD/TPI plus bevacizumab group versus 70% among controls.
“The safety profile was manageable and consistent with the individual safety profiles of FTD/TPI plus bevacizumab,” said Dr. Tabernero.
Overall, adverse events were comparable in both groups, except for slightly higher rates in the bevacizumab plus FTD/TPI arm for hypertension (10% vs. 2%), nausea (37% vs. 27%), and neutropenia (62% vs. 51%).
The study was sponsored by Taiho Oncology, manufacturer of Lonsurf (trifluridine plus tipiracil). Dr. Tabernero and Dr. Deming both reported relationships with numerous pharmaceutical companies. Several of the study authors also reported conflicts of interest.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – ,” say the trial investigators. An expert not involved in the study predicted that it will change clinical practice.
The results show that adding bevacizumab to trifluridine (FTD)/tipiracil (TPI) significantly improved survival, compared with those who received FTD/TPI alone. Median overall survival was 10.8 months in the investigational arm vs 7.5 months among controls (hazard ratio, 0.61; P < .001).
Adding bevacizumab also did not increase the risk for serious adverse events or events that led to treatment discontinuation, the researchers noted.
The new data were presented by Josep Tabernero, MD, PhD, head of the department of medical oncology, Vall d’Hebron University Hospital, and director of the Vall d’Hebron Institute of Oncology, Barcelona, at the ASCO Gastrointestinal Cancers Symposium 2023. He concluded that bevacizumab added to FTD/TPI “represents a new standard of care for the treatment of patients with refractory metastatic colorectal cancer who had previously progressed after two lines of therapy.”
Discussant for the abstract, Dustin Deming, MD, an associate professor in the division of hematology, medical oncology and palliative care at the University of Wisconsin–Madison, said the findings showed very “exciting advantages in progression-free and overall survival.” He agreed that “FTD/TPI and bevacizumab should be considered the preferred nontargeted regimen in the refractory setting” and added that “this trial does change standard clinical practice.”
Dr. Deming also said that this has implications for future clinical trials because these results create a new standard for control arms.
Improvement in all endpoints
FTD/TPI, which is marketed as Lonsurf, is already approved as a monotherapy for third-line use in refractory metastatic colorectal cancer, and bevacizumab is an established anticancer agent that targets vascular endothelial growth factor and inhibits angiogenesis, he explained. The combination of bevacizumab plus FTD/TPI has previously produced encouraging results in the treatment of refractory metastatic colorectal cancer in smaller phase 2 randomized and single-arm studies.
“The phase 3 SUNLIGHT study was designed to confirm the efficacy and safety of FTD/TPI plus bevacizumab, as compared with FTD/TPI alone, in patients with refractory metastatic colorectal cancer following two chemotherapy regimens,” said Dr. Tabernero.
The cohort included 492 patients who were randomly assigned to receive either FTD/TPI plus bevacizumab (FTD/TPI 35 mg/m2 twice daily on days 1-5 and 8-12 [28-day cycle] and bevacizumab 5 mg/kg on days 1 and 15) or FTD/TPI alone.
Across both arms, most patients (72%) had already received prior treatment with bevacizumab.
At 6 months, the overall survival rate was 77% with the combination therapy versus 61% with the control therapy, and after 12 months, the overall survival rate was 43% versus 30%.
Median progression-free survival was 5.6 months in the treatment arm versus 2.4 months in the control arm (HR, 0.44; P < .001). At 6 months, progression-free survival was 43% versus 16%, respectively, and at 12 months, it was 16% versus 1%.
Both overall response rate and disease control rate were also superior in the investigational arm. The overall response rate was 6.3% versus 0.0% in the control arm, with an absolute gain of 5.4% (P = .004). Similarly, the absolute gain for disease control rate was 29.6% (76.6% vs. 47.0%; P < .001).
For quality of life, worsening in global health status in the investigational arm was significantly delayed, compared with the control arm (8.5 months vs. 4.7 months; HR, 0.50; P < .001), as was worsening to an Eastern Cooperative Oncology Group performance status of 2 or greater (9.3 months vs. 6.3 months; HR, 0.54; P < .001).
When looking at toxicity, Tabernero reported that there were no treatment-related deaths and the rates of severe adverse events were similar in both groups: 72% in the FTD/TPI plus bevacizumab group versus 70% among controls.
“The safety profile was manageable and consistent with the individual safety profiles of FTD/TPI plus bevacizumab,” said Dr. Tabernero.
Overall, adverse events were comparable in both groups, except for slightly higher rates in the bevacizumab plus FTD/TPI arm for hypertension (10% vs. 2%), nausea (37% vs. 27%), and neutropenia (62% vs. 51%).
The study was sponsored by Taiho Oncology, manufacturer of Lonsurf (trifluridine plus tipiracil). Dr. Tabernero and Dr. Deming both reported relationships with numerous pharmaceutical companies. Several of the study authors also reported conflicts of interest.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – ,” say the trial investigators. An expert not involved in the study predicted that it will change clinical practice.
The results show that adding bevacizumab to trifluridine (FTD)/tipiracil (TPI) significantly improved survival, compared with those who received FTD/TPI alone. Median overall survival was 10.8 months in the investigational arm vs 7.5 months among controls (hazard ratio, 0.61; P < .001).
Adding bevacizumab also did not increase the risk for serious adverse events or events that led to treatment discontinuation, the researchers noted.
The new data were presented by Josep Tabernero, MD, PhD, head of the department of medical oncology, Vall d’Hebron University Hospital, and director of the Vall d’Hebron Institute of Oncology, Barcelona, at the ASCO Gastrointestinal Cancers Symposium 2023. He concluded that bevacizumab added to FTD/TPI “represents a new standard of care for the treatment of patients with refractory metastatic colorectal cancer who had previously progressed after two lines of therapy.”
Discussant for the abstract, Dustin Deming, MD, an associate professor in the division of hematology, medical oncology and palliative care at the University of Wisconsin–Madison, said the findings showed very “exciting advantages in progression-free and overall survival.” He agreed that “FTD/TPI and bevacizumab should be considered the preferred nontargeted regimen in the refractory setting” and added that “this trial does change standard clinical practice.”
Dr. Deming also said that this has implications for future clinical trials because these results create a new standard for control arms.
Improvement in all endpoints
FTD/TPI, which is marketed as Lonsurf, is already approved as a monotherapy for third-line use in refractory metastatic colorectal cancer, and bevacizumab is an established anticancer agent that targets vascular endothelial growth factor and inhibits angiogenesis, he explained. The combination of bevacizumab plus FTD/TPI has previously produced encouraging results in the treatment of refractory metastatic colorectal cancer in smaller phase 2 randomized and single-arm studies.
“The phase 3 SUNLIGHT study was designed to confirm the efficacy and safety of FTD/TPI plus bevacizumab, as compared with FTD/TPI alone, in patients with refractory metastatic colorectal cancer following two chemotherapy regimens,” said Dr. Tabernero.
The cohort included 492 patients who were randomly assigned to receive either FTD/TPI plus bevacizumab (FTD/TPI 35 mg/m2 twice daily on days 1-5 and 8-12 [28-day cycle] and bevacizumab 5 mg/kg on days 1 and 15) or FTD/TPI alone.
Across both arms, most patients (72%) had already received prior treatment with bevacizumab.
At 6 months, the overall survival rate was 77% with the combination therapy versus 61% with the control therapy, and after 12 months, the overall survival rate was 43% versus 30%.
Median progression-free survival was 5.6 months in the treatment arm versus 2.4 months in the control arm (HR, 0.44; P < .001). At 6 months, progression-free survival was 43% versus 16%, respectively, and at 12 months, it was 16% versus 1%.
Both overall response rate and disease control rate were also superior in the investigational arm. The overall response rate was 6.3% versus 0.0% in the control arm, with an absolute gain of 5.4% (P = .004). Similarly, the absolute gain for disease control rate was 29.6% (76.6% vs. 47.0%; P < .001).
For quality of life, worsening in global health status in the investigational arm was significantly delayed, compared with the control arm (8.5 months vs. 4.7 months; HR, 0.50; P < .001), as was worsening to an Eastern Cooperative Oncology Group performance status of 2 or greater (9.3 months vs. 6.3 months; HR, 0.54; P < .001).
When looking at toxicity, Tabernero reported that there were no treatment-related deaths and the rates of severe adverse events were similar in both groups: 72% in the FTD/TPI plus bevacizumab group versus 70% among controls.
“The safety profile was manageable and consistent with the individual safety profiles of FTD/TPI plus bevacizumab,” said Dr. Tabernero.
Overall, adverse events were comparable in both groups, except for slightly higher rates in the bevacizumab plus FTD/TPI arm for hypertension (10% vs. 2%), nausea (37% vs. 27%), and neutropenia (62% vs. 51%).
The study was sponsored by Taiho Oncology, manufacturer of Lonsurf (trifluridine plus tipiracil). Dr. Tabernero and Dr. Deming both reported relationships with numerous pharmaceutical companies. Several of the study authors also reported conflicts of interest.
A version of this article first appeared on Medscape.com.
AT ASCO GI 2023
Weight bias affects views of kids’ obesity recommendations
Apparently, offering children effective treatments for a chronic disease that markedly increases their risk for other chronic diseases, regularly erodes their quality of life, and is the No. 1 target of school-based bullying is wrong.
At least that’s my take watching the coverage of the recent American Academy of Pediatrics new pediatric obesity treatment guidelines that, gasp, suggest that children whose severity of obesity warrants medication or surgeries be offered medication or surgery. Because it’s wiser to not try to treat the obesity that›s contributing to a child’s type 2 diabetes, hypertension, fatty liver disease, or reduced quality of life?
The reaction isn’t surprising. Some of those who are up in arms about it have clinical or research careers dependent on championing their own favorite dietary strategies as if they are more effective and reproducible than decades of uniformly disappointing studies proving that they’re not. Others are upset because, for reasons that at times may be personal and at times may be conflicted, they believe that obesity should not be treated and/or that sustained weight loss is impossible. But overarchingly, probably the bulk of the hoopla stems from obesity being seen as a moral failing. Because the notion that those who suffer with obesity are themselves to blame has been the prevailing societal view for decades, if not centuries.
Working with families of children with obesity severe enough for them to seek help, it’s clear that if desire were sufficient to will it away, we wouldn’t need treatment guidelines let alone medications or surgery. Near uniformly, parents describe their children being bullied consequent to and being deeply self-conscious of their weight.
And what would those who think children shouldn’t be offered reproducibly effective treatment for obesity have them do about it? Many seem to think it would be preferable for kids to be placed on formal diets and, of course, that they should go out and play more. And though I’m all for encouraging the improvement of a child’s dietary quality and activity level, anyone suggesting those as panaceas for childhood obesity haven’t a clue. Not to mention the fact that, in most cases, improving overall dietary quality, something worthwhile at any weight, isn’t the dietary goal being recommended. Instead, the prescription seems to be restrictive dieting coupled with overexercising, which, unlike appropriately and thoughtfully informed and utilized medication, may increase a child’s risk of maladaptive thinking around food and fitness as well as disordered eating, not to mention challenge their self-esteem if their lifestyle results are underwhelming.
This brings us to one of the most bizarre takes on this whole business – that medications will be pushed and used when not necessary. No doubt that at times, that may occur, but the issue is that of a clinician’s overzealous prescribing and not of the treatment options or indications. Consider childhood asthma. There is no worry or uproar that children with mild asthma that isn’t having an impact on their quality of life or markedly risking their health will be placed on multiple inhaled steroids and treatments. Why? Because clinicians have been taught how to dispassionately evaluate treatment needs for asthma, monitor disease course, and not simply prescribe everything in our armamentarium.
Shocking, I know, but as is the case with every other medical condition, I think doctors are capable of learning and following an algorithm covering the indications and options for the treatment of childhood obesity.
How that looks also mirrors what’s seen with any other chronic noncommunicable disease with varied severity and impact. Doctors will evaluate each child with obesity to see whether it’s having a detrimental effect on their health or quality of life. They will monitor their patients’ obesity to see if it’s worsening and will, when necessary, undertake investigations to rule out its potential contribution to common comorbidities like type 2 diabetes, hypertension, and fatty liver disease. And, when appropriate, they will provide information on available treatment options – from lifestyle to medication to surgery and the risks, benefits, and realistic expectations associated with each – and then, without judgment, support their patients’ treatment choices because blame-free informed discussion and supportive prescription of care is, in fact, the distillation of our jobs.
If people are looking to be outraged rather than focusing their outrage on what we now need to do about childhood obesity, they should instead look to what got us here: our obesogenic environment. We and our children are swimming against a torrential current of cheap ultraprocessed calories being pushed upon us by a broken societal food culture that values convenience and simultaneously embraces the notion that knowledge is a match versus the thousands of genes and dozens of hormones that increasingly sophisticated food industry marketers and scientists prey upon. When dealing with torrential currents, we need to do more than just recommend swimming lessons.
Like asthma, which may be exacerbated by pollution in our environment both outdoors and indoors, childhood obesity is a modern-day environmentally influenced disease with varied penetrance that does not always require active treatment. Like asthma, childhood obesity is not a disease that children choose to have; it’s not a disease that can be willed away; and it’s not a disease that responds uniformly, dramatically, or enduringly to diet and exercise. Finally, literally and figuratively, like asthma, for childhood obesity, we thankfully now have a number of effective treatment options that we can offer, and it’s only our societal weight bias that leads to thinking that’s anything but great.
A version of this article first appeared on Medscape.com.
Apparently, offering children effective treatments for a chronic disease that markedly increases their risk for other chronic diseases, regularly erodes their quality of life, and is the No. 1 target of school-based bullying is wrong.
At least that’s my take watching the coverage of the recent American Academy of Pediatrics new pediatric obesity treatment guidelines that, gasp, suggest that children whose severity of obesity warrants medication or surgeries be offered medication or surgery. Because it’s wiser to not try to treat the obesity that›s contributing to a child’s type 2 diabetes, hypertension, fatty liver disease, or reduced quality of life?
The reaction isn’t surprising. Some of those who are up in arms about it have clinical or research careers dependent on championing their own favorite dietary strategies as if they are more effective and reproducible than decades of uniformly disappointing studies proving that they’re not. Others are upset because, for reasons that at times may be personal and at times may be conflicted, they believe that obesity should not be treated and/or that sustained weight loss is impossible. But overarchingly, probably the bulk of the hoopla stems from obesity being seen as a moral failing. Because the notion that those who suffer with obesity are themselves to blame has been the prevailing societal view for decades, if not centuries.
Working with families of children with obesity severe enough for them to seek help, it’s clear that if desire were sufficient to will it away, we wouldn’t need treatment guidelines let alone medications or surgery. Near uniformly, parents describe their children being bullied consequent to and being deeply self-conscious of their weight.
And what would those who think children shouldn’t be offered reproducibly effective treatment for obesity have them do about it? Many seem to think it would be preferable for kids to be placed on formal diets and, of course, that they should go out and play more. And though I’m all for encouraging the improvement of a child’s dietary quality and activity level, anyone suggesting those as panaceas for childhood obesity haven’t a clue. Not to mention the fact that, in most cases, improving overall dietary quality, something worthwhile at any weight, isn’t the dietary goal being recommended. Instead, the prescription seems to be restrictive dieting coupled with overexercising, which, unlike appropriately and thoughtfully informed and utilized medication, may increase a child’s risk of maladaptive thinking around food and fitness as well as disordered eating, not to mention challenge their self-esteem if their lifestyle results are underwhelming.
This brings us to one of the most bizarre takes on this whole business – that medications will be pushed and used when not necessary. No doubt that at times, that may occur, but the issue is that of a clinician’s overzealous prescribing and not of the treatment options or indications. Consider childhood asthma. There is no worry or uproar that children with mild asthma that isn’t having an impact on their quality of life or markedly risking their health will be placed on multiple inhaled steroids and treatments. Why? Because clinicians have been taught how to dispassionately evaluate treatment needs for asthma, monitor disease course, and not simply prescribe everything in our armamentarium.
Shocking, I know, but as is the case with every other medical condition, I think doctors are capable of learning and following an algorithm covering the indications and options for the treatment of childhood obesity.
How that looks also mirrors what’s seen with any other chronic noncommunicable disease with varied severity and impact. Doctors will evaluate each child with obesity to see whether it’s having a detrimental effect on their health or quality of life. They will monitor their patients’ obesity to see if it’s worsening and will, when necessary, undertake investigations to rule out its potential contribution to common comorbidities like type 2 diabetes, hypertension, and fatty liver disease. And, when appropriate, they will provide information on available treatment options – from lifestyle to medication to surgery and the risks, benefits, and realistic expectations associated with each – and then, without judgment, support their patients’ treatment choices because blame-free informed discussion and supportive prescription of care is, in fact, the distillation of our jobs.
If people are looking to be outraged rather than focusing their outrage on what we now need to do about childhood obesity, they should instead look to what got us here: our obesogenic environment. We and our children are swimming against a torrential current of cheap ultraprocessed calories being pushed upon us by a broken societal food culture that values convenience and simultaneously embraces the notion that knowledge is a match versus the thousands of genes and dozens of hormones that increasingly sophisticated food industry marketers and scientists prey upon. When dealing with torrential currents, we need to do more than just recommend swimming lessons.
Like asthma, which may be exacerbated by pollution in our environment both outdoors and indoors, childhood obesity is a modern-day environmentally influenced disease with varied penetrance that does not always require active treatment. Like asthma, childhood obesity is not a disease that children choose to have; it’s not a disease that can be willed away; and it’s not a disease that responds uniformly, dramatically, or enduringly to diet and exercise. Finally, literally and figuratively, like asthma, for childhood obesity, we thankfully now have a number of effective treatment options that we can offer, and it’s only our societal weight bias that leads to thinking that’s anything but great.
A version of this article first appeared on Medscape.com.
Apparently, offering children effective treatments for a chronic disease that markedly increases their risk for other chronic diseases, regularly erodes their quality of life, and is the No. 1 target of school-based bullying is wrong.
At least that’s my take watching the coverage of the recent American Academy of Pediatrics new pediatric obesity treatment guidelines that, gasp, suggest that children whose severity of obesity warrants medication or surgeries be offered medication or surgery. Because it’s wiser to not try to treat the obesity that›s contributing to a child’s type 2 diabetes, hypertension, fatty liver disease, or reduced quality of life?
The reaction isn’t surprising. Some of those who are up in arms about it have clinical or research careers dependent on championing their own favorite dietary strategies as if they are more effective and reproducible than decades of uniformly disappointing studies proving that they’re not. Others are upset because, for reasons that at times may be personal and at times may be conflicted, they believe that obesity should not be treated and/or that sustained weight loss is impossible. But overarchingly, probably the bulk of the hoopla stems from obesity being seen as a moral failing. Because the notion that those who suffer with obesity are themselves to blame has been the prevailing societal view for decades, if not centuries.
Working with families of children with obesity severe enough for them to seek help, it’s clear that if desire were sufficient to will it away, we wouldn’t need treatment guidelines let alone medications or surgery. Near uniformly, parents describe their children being bullied consequent to and being deeply self-conscious of their weight.
And what would those who think children shouldn’t be offered reproducibly effective treatment for obesity have them do about it? Many seem to think it would be preferable for kids to be placed on formal diets and, of course, that they should go out and play more. And though I’m all for encouraging the improvement of a child’s dietary quality and activity level, anyone suggesting those as panaceas for childhood obesity haven’t a clue. Not to mention the fact that, in most cases, improving overall dietary quality, something worthwhile at any weight, isn’t the dietary goal being recommended. Instead, the prescription seems to be restrictive dieting coupled with overexercising, which, unlike appropriately and thoughtfully informed and utilized medication, may increase a child’s risk of maladaptive thinking around food and fitness as well as disordered eating, not to mention challenge their self-esteem if their lifestyle results are underwhelming.
This brings us to one of the most bizarre takes on this whole business – that medications will be pushed and used when not necessary. No doubt that at times, that may occur, but the issue is that of a clinician’s overzealous prescribing and not of the treatment options or indications. Consider childhood asthma. There is no worry or uproar that children with mild asthma that isn’t having an impact on their quality of life or markedly risking their health will be placed on multiple inhaled steroids and treatments. Why? Because clinicians have been taught how to dispassionately evaluate treatment needs for asthma, monitor disease course, and not simply prescribe everything in our armamentarium.
Shocking, I know, but as is the case with every other medical condition, I think doctors are capable of learning and following an algorithm covering the indications and options for the treatment of childhood obesity.
How that looks also mirrors what’s seen with any other chronic noncommunicable disease with varied severity and impact. Doctors will evaluate each child with obesity to see whether it’s having a detrimental effect on their health or quality of life. They will monitor their patients’ obesity to see if it’s worsening and will, when necessary, undertake investigations to rule out its potential contribution to common comorbidities like type 2 diabetes, hypertension, and fatty liver disease. And, when appropriate, they will provide information on available treatment options – from lifestyle to medication to surgery and the risks, benefits, and realistic expectations associated with each – and then, without judgment, support their patients’ treatment choices because blame-free informed discussion and supportive prescription of care is, in fact, the distillation of our jobs.
If people are looking to be outraged rather than focusing their outrage on what we now need to do about childhood obesity, they should instead look to what got us here: our obesogenic environment. We and our children are swimming against a torrential current of cheap ultraprocessed calories being pushed upon us by a broken societal food culture that values convenience and simultaneously embraces the notion that knowledge is a match versus the thousands of genes and dozens of hormones that increasingly sophisticated food industry marketers and scientists prey upon. When dealing with torrential currents, we need to do more than just recommend swimming lessons.
Like asthma, which may be exacerbated by pollution in our environment both outdoors and indoors, childhood obesity is a modern-day environmentally influenced disease with varied penetrance that does not always require active treatment. Like asthma, childhood obesity is not a disease that children choose to have; it’s not a disease that can be willed away; and it’s not a disease that responds uniformly, dramatically, or enduringly to diet and exercise. Finally, literally and figuratively, like asthma, for childhood obesity, we thankfully now have a number of effective treatment options that we can offer, and it’s only our societal weight bias that leads to thinking that’s anything but great.
A version of this article first appeared on Medscape.com.
Metabolic syndromes worsen outcomes in BC patients treated with neoadjuvant chemotherapy
Key clinical point: The presence of metabolic syndromes (MetS) worsened survival outcomes and increased disease recurrence risk in patients with breast cancer (BC) who received neoadjuvant chemotherapy (NAC).
Major finding: The MetS group had a significantly lower likelihood of achieving pathological complete response than the non-MetS group (odds ratio [OR] 0.316; P = .028), with the risk for death (OR 2.587; P = .004) and disease recurrence (OR 2.228; P = .007) being significantly higher in patients with vs without MetS.
Study details: Findings are from a retrospective study including 221 women with BC who received preoperative NAC, of which 22.2% of patients were included in the MetS group.
Disclosures: This study was supported by the Beijing Medical Award Foundation. The authors declared no conflicts of interest.
Source: Zhou Z et al. Metabolic syndrome is a risk factor for breast cancer patients receiving neoadjuvant chemotherapy: A case-control study. Front Oncol. 2023;12:1080054 (Jan 4). Doi: 10.3389/fonc.2022.1080054
Key clinical point: The presence of metabolic syndromes (MetS) worsened survival outcomes and increased disease recurrence risk in patients with breast cancer (BC) who received neoadjuvant chemotherapy (NAC).
Major finding: The MetS group had a significantly lower likelihood of achieving pathological complete response than the non-MetS group (odds ratio [OR] 0.316; P = .028), with the risk for death (OR 2.587; P = .004) and disease recurrence (OR 2.228; P = .007) being significantly higher in patients with vs without MetS.
Study details: Findings are from a retrospective study including 221 women with BC who received preoperative NAC, of which 22.2% of patients were included in the MetS group.
Disclosures: This study was supported by the Beijing Medical Award Foundation. The authors declared no conflicts of interest.
Source: Zhou Z et al. Metabolic syndrome is a risk factor for breast cancer patients receiving neoadjuvant chemotherapy: A case-control study. Front Oncol. 2023;12:1080054 (Jan 4). Doi: 10.3389/fonc.2022.1080054
Key clinical point: The presence of metabolic syndromes (MetS) worsened survival outcomes and increased disease recurrence risk in patients with breast cancer (BC) who received neoadjuvant chemotherapy (NAC).
Major finding: The MetS group had a significantly lower likelihood of achieving pathological complete response than the non-MetS group (odds ratio [OR] 0.316; P = .028), with the risk for death (OR 2.587; P = .004) and disease recurrence (OR 2.228; P = .007) being significantly higher in patients with vs without MetS.
Study details: Findings are from a retrospective study including 221 women with BC who received preoperative NAC, of which 22.2% of patients were included in the MetS group.
Disclosures: This study was supported by the Beijing Medical Award Foundation. The authors declared no conflicts of interest.
Source: Zhou Z et al. Metabolic syndrome is a risk factor for breast cancer patients receiving neoadjuvant chemotherapy: A case-control study. Front Oncol. 2023;12:1080054 (Jan 4). Doi: 10.3389/fonc.2022.1080054
Metastatic BC: Not worth changing the 4-weekly schedule of pegylated liposomal doxorubicin
Key clinical point: Recent phase 2 trials have recommended a 2-weekly schedule of pegylated liposomal doxorubicin (PLD) in patients with heavily treated metastatic breast cancer (BC); however, it failed to demonstrate any advantage in terms of efficacy or safety over the registered 4-weekly regimen of PLD.
Major finding: The median progression-free survival was 3.0 and 3.4 months in the 2-weekly and 4-weekly PLD schedule groups, respectively, with a weighted hazard ratio of 1.12 (P = .54). The rate of adverse events also appeared comparable between both the groups.
Study details: Findings are from a retrospective study including 191 heavily pretreated patients with metastatic BC who received a 2-weekly (n = 95) or the registered 4-weekly (n = 96) schedule of PLD.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Bischoff H et al. A propensity score-weighted study comparing a two- versus four-weekly pegylated liposomal doxorubicin regimen in metastatic breast cancer. Breast Cancer Res Treat. 2022 (Dec 23). Doi: 10.1007/s10549-022-06844-5
Key clinical point: Recent phase 2 trials have recommended a 2-weekly schedule of pegylated liposomal doxorubicin (PLD) in patients with heavily treated metastatic breast cancer (BC); however, it failed to demonstrate any advantage in terms of efficacy or safety over the registered 4-weekly regimen of PLD.
Major finding: The median progression-free survival was 3.0 and 3.4 months in the 2-weekly and 4-weekly PLD schedule groups, respectively, with a weighted hazard ratio of 1.12 (P = .54). The rate of adverse events also appeared comparable between both the groups.
Study details: Findings are from a retrospective study including 191 heavily pretreated patients with metastatic BC who received a 2-weekly (n = 95) or the registered 4-weekly (n = 96) schedule of PLD.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Bischoff H et al. A propensity score-weighted study comparing a two- versus four-weekly pegylated liposomal doxorubicin regimen in metastatic breast cancer. Breast Cancer Res Treat. 2022 (Dec 23). Doi: 10.1007/s10549-022-06844-5
Key clinical point: Recent phase 2 trials have recommended a 2-weekly schedule of pegylated liposomal doxorubicin (PLD) in patients with heavily treated metastatic breast cancer (BC); however, it failed to demonstrate any advantage in terms of efficacy or safety over the registered 4-weekly regimen of PLD.
Major finding: The median progression-free survival was 3.0 and 3.4 months in the 2-weekly and 4-weekly PLD schedule groups, respectively, with a weighted hazard ratio of 1.12 (P = .54). The rate of adverse events also appeared comparable between both the groups.
Study details: Findings are from a retrospective study including 191 heavily pretreated patients with metastatic BC who received a 2-weekly (n = 95) or the registered 4-weekly (n = 96) schedule of PLD.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Bischoff H et al. A propensity score-weighted study comparing a two- versus four-weekly pegylated liposomal doxorubicin regimen in metastatic breast cancer. Breast Cancer Res Treat. 2022 (Dec 23). Doi: 10.1007/s10549-022-06844-5