User login
FDA Okays Subcutaneous Ocrelizumab for MS
The subcutaneous (SC) injection can be administered by a healthcare professional in approximately 10 minutes and is the first and only twice-a-year SC injection approved for both RMS and PPMS, according to a company news release.
The FDA approval is based on pivotal data from the phase 3 OCARINA II trial, which showed no clinically significant difference in blood levels of ocrelizumab when administered subcutaneously and an efficacy profile consistent with the intravenous (IV) formulation.
“The trial met its primary and secondary endpoints, demonstrating SC injection was noninferior to IV infusion based on [ocrelizumab] levels in the blood, and consistent control of clinical (relapses) and radiological (MRI lesions) disease activity,” the company said in the release.
The safety profile of SC ocrelizumab was consistent with the safety profile of IV ocrelizumab, with the exception of injection site reactions, the most common adverse event.
Injection reactions were more often reported with the first injection, with 49% of trial participants experiencing an injection reaction after the first injection. All injection reactions were mild or moderate, and none led to treatment withdrawal.
Ocrevus Zunovo “may offer greater flexibility for healthcare providers and people living with multiple sclerosis, based on their individual treatment needs,” Levi Garraway, MD, PhD, chief medical officer for Genentech, said in the press release. “We are pleased that with a new method of delivery, there is now an additional option for those who need flexibility in the route of administration or treatment time,” Natalie Blake, executive director of the MS Foundation, said in the release.
The SC formulation of ocrelizumab was approved by the European Commission in June.
Complete prescribing information is available online.
A version of this article appeared on Medscape.com.
The subcutaneous (SC) injection can be administered by a healthcare professional in approximately 10 minutes and is the first and only twice-a-year SC injection approved for both RMS and PPMS, according to a company news release.
The FDA approval is based on pivotal data from the phase 3 OCARINA II trial, which showed no clinically significant difference in blood levels of ocrelizumab when administered subcutaneously and an efficacy profile consistent with the intravenous (IV) formulation.
“The trial met its primary and secondary endpoints, demonstrating SC injection was noninferior to IV infusion based on [ocrelizumab] levels in the blood, and consistent control of clinical (relapses) and radiological (MRI lesions) disease activity,” the company said in the release.
The safety profile of SC ocrelizumab was consistent with the safety profile of IV ocrelizumab, with the exception of injection site reactions, the most common adverse event.
Injection reactions were more often reported with the first injection, with 49% of trial participants experiencing an injection reaction after the first injection. All injection reactions were mild or moderate, and none led to treatment withdrawal.
Ocrevus Zunovo “may offer greater flexibility for healthcare providers and people living with multiple sclerosis, based on their individual treatment needs,” Levi Garraway, MD, PhD, chief medical officer for Genentech, said in the press release. “We are pleased that with a new method of delivery, there is now an additional option for those who need flexibility in the route of administration or treatment time,” Natalie Blake, executive director of the MS Foundation, said in the release.
The SC formulation of ocrelizumab was approved by the European Commission in June.
Complete prescribing information is available online.
A version of this article appeared on Medscape.com.
The subcutaneous (SC) injection can be administered by a healthcare professional in approximately 10 minutes and is the first and only twice-a-year SC injection approved for both RMS and PPMS, according to a company news release.
The FDA approval is based on pivotal data from the phase 3 OCARINA II trial, which showed no clinically significant difference in blood levels of ocrelizumab when administered subcutaneously and an efficacy profile consistent with the intravenous (IV) formulation.
“The trial met its primary and secondary endpoints, demonstrating SC injection was noninferior to IV infusion based on [ocrelizumab] levels in the blood, and consistent control of clinical (relapses) and radiological (MRI lesions) disease activity,” the company said in the release.
The safety profile of SC ocrelizumab was consistent with the safety profile of IV ocrelizumab, with the exception of injection site reactions, the most common adverse event.
Injection reactions were more often reported with the first injection, with 49% of trial participants experiencing an injection reaction after the first injection. All injection reactions were mild or moderate, and none led to treatment withdrawal.
Ocrevus Zunovo “may offer greater flexibility for healthcare providers and people living with multiple sclerosis, based on their individual treatment needs,” Levi Garraway, MD, PhD, chief medical officer for Genentech, said in the press release. “We are pleased that with a new method of delivery, there is now an additional option for those who need flexibility in the route of administration or treatment time,” Natalie Blake, executive director of the MS Foundation, said in the release.
The SC formulation of ocrelizumab was approved by the European Commission in June.
Complete prescribing information is available online.
A version of this article appeared on Medscape.com.
AI-Powered Clinical Documentation Tool Reduces EHR Time for Clinicians
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Rare Case of Photodistributed Hyperpigmentation Linked to Kratom Consumption
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
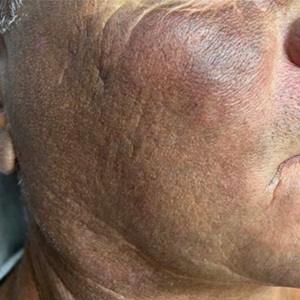
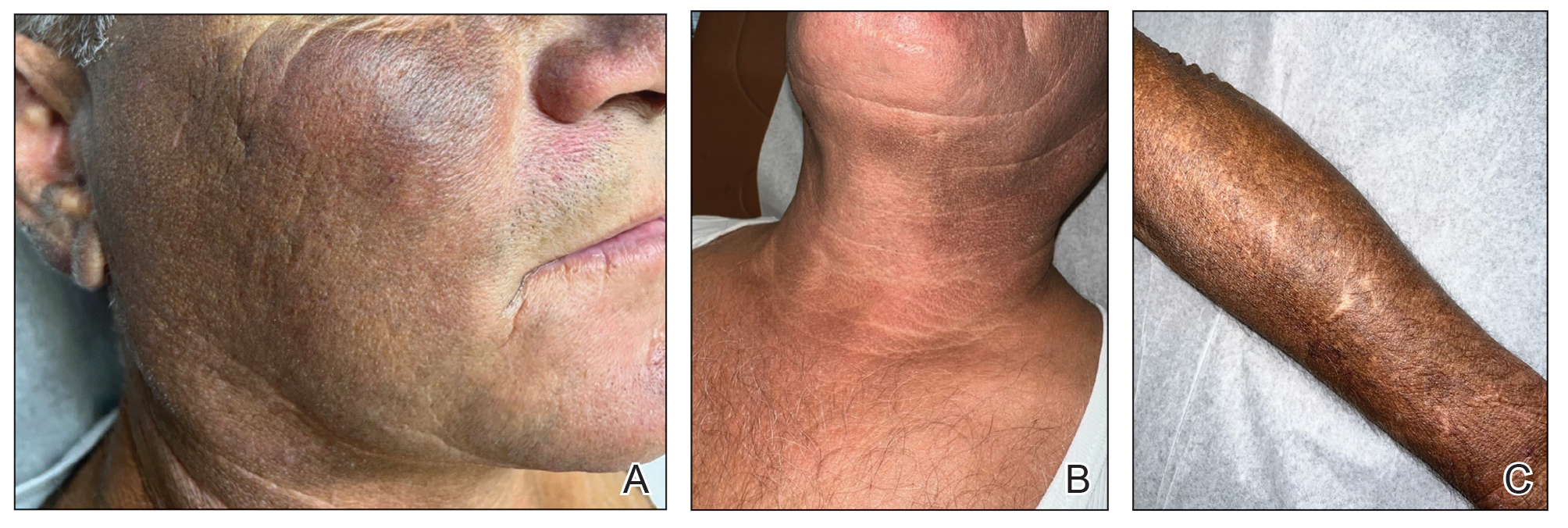
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.
When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
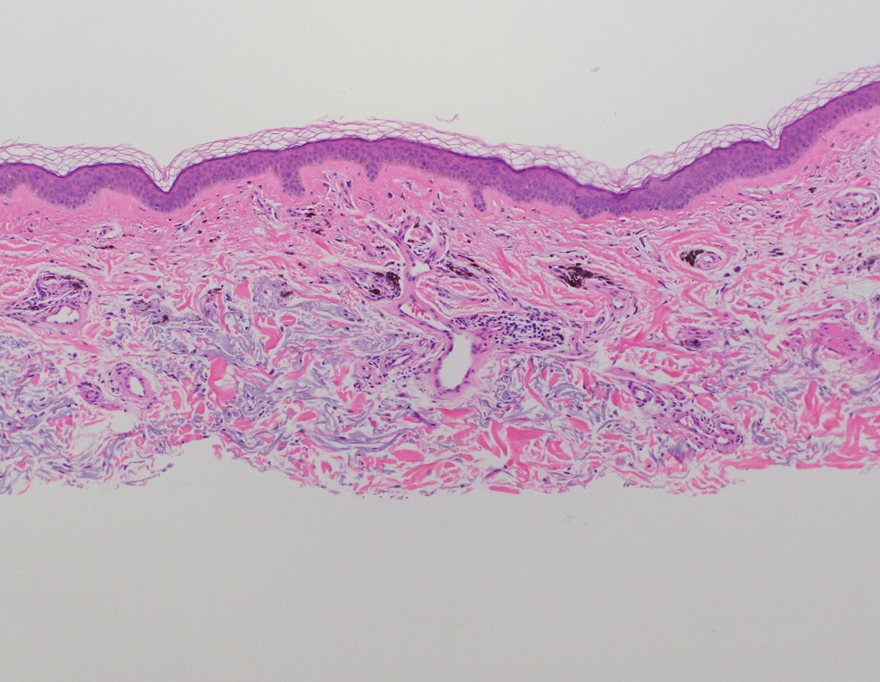
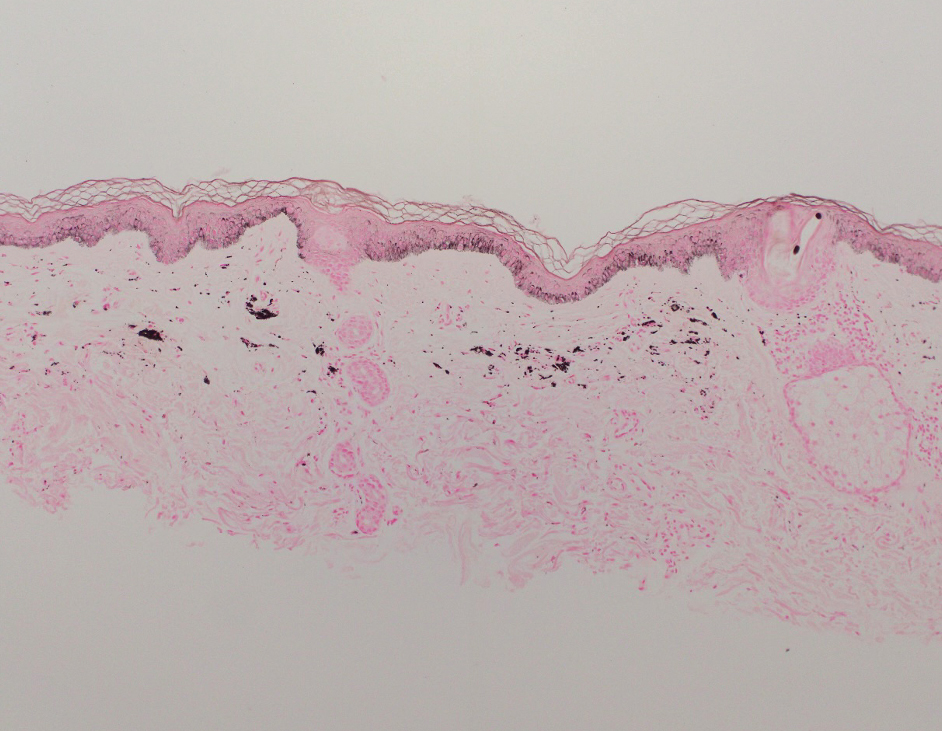
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).
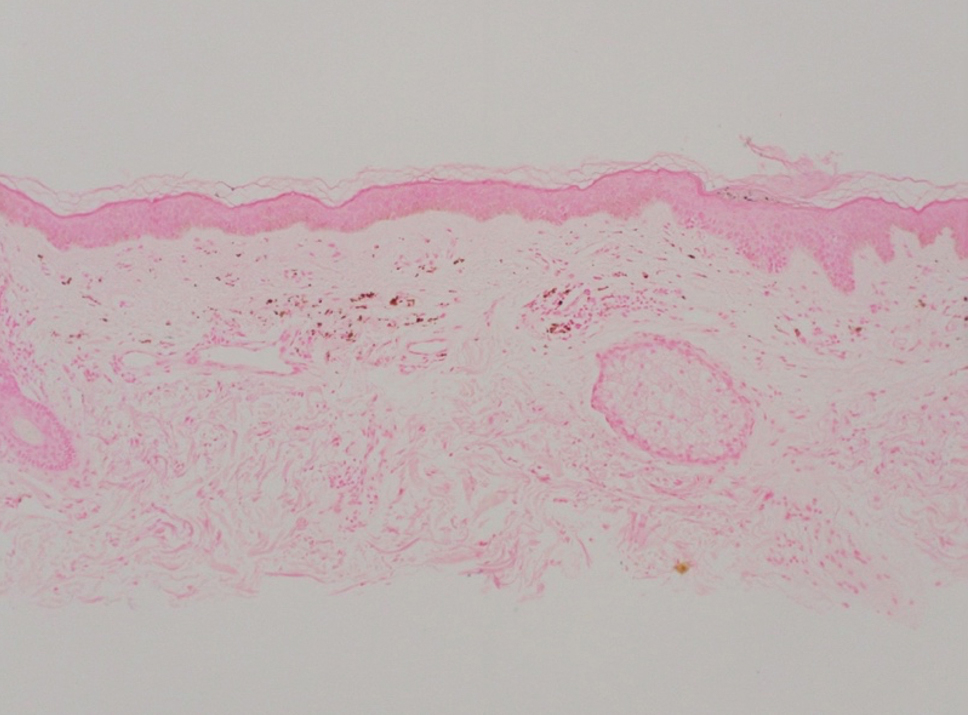
Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.

When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).



Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.

When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).



Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
Practice Points
- Clinicians should be aware of photodistributed hyperpigmentation as a potential adverse effect of kratom usage.
- Kratom-induced photodistributed hyperpigmentation should be suspected in patients with hyperpigmented lesions in sun-exposed areas of the skin following kratom use. A biopsy of lesions should be obtained to confirm the diagnosis.
- Cessation of kratom should be recommended.
Study Reports Safety Data in Children on JAK Inhibitors
TOPLINE:
which also found that acne was the most common skin-related AE in children, and serious AEs were less common.
METHODOLOGY:
- Researchers analyzed 399,649 AEs in 133,216 adult patients and 2883 AEs in 955 pediatric patients (age, < 18 years) from November 2011 to February 2023 using the US Food and Drug Administration Adverse Event Reporting System and the Canada Vigilance Adverse Reaction Online Database.
- AEs were categorized on the basis of the Medical Dictionary for Regulatory Activities system organ class.
- Five JAK inhibitors approved for use in children were included in the study: Baricitinib, upadacitinib, abrocitinib, ruxolitinib, and tofacitinib.
TAKEAWAY:
- The most frequently reported AEs in children were blood and lymphatic system disorders, including neutropenia, thrombocytopenia, and anemia (24%); viral, fungal, and bacterial infections, such as pneumonia and sepsis (17.2%); constitutional symptoms and administrative concerns, including pyrexia and fatigue (15.7%); gastrointestinal disorders, such as vomiting and abdominal pain (13.6%); and respiratory disorders, such as cough and respiratory distress (5.3%).
- In adults, the most common AEs were viral, fungal, and bacterial infections (16.8%); constitutional symptoms and administrative concerns (13.5%); musculoskeletal and connective tissue disorders (7.04%); and gastrointestinal (5.8%) and nervous system (5%) disorders.
- Acne (30.6%), atopic dermatitis (22.2%), and psoriasis (16.7%) were the most common skin and subcutaneous tissue AEs reported in children. Skin and subcutaneous AEs were more common with upadacitinib (21.1%), abrocitinib (9.1%), and tofacitinib (6.3%) in children.
- Serious AEs included in the boxed warning for JAK inhibitors — serious infection, mortality, malignancy, cardiovascular events, and thrombosis — were similar for baricitinib in children (4 of 49 patients, 8.2%) and adults (325 of 3707, 8.8%). For other JAK inhibitors, absolute numbers of these AEs in children were small and rates were lower in children than in adults.
IN PRACTICE:
“This information can support customized treatment and minimize the potential for undesired or intolerable AEs,” the authors wrote.
SOURCE:
This study was led by Sahithi Talasila, BS, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and was published online in Pediatric Dermatology.
LIMITATIONS:
Pharmacovigilance registries did not fully capture the complete range of AEs because of potential reporting bias or recall bias. Additionally, events lacking sufficient objective evidence were underreported, while common AEs associated with JAK inhibitor therapy were overreported.
DISCLOSURES:
No specific funding sources for the study were reported. One author reported being a consultant, one reported serving as a principal investigator in clinical trials, and another reported serving on data and safety monitoring boards of various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which also found that acne was the most common skin-related AE in children, and serious AEs were less common.
METHODOLOGY:
- Researchers analyzed 399,649 AEs in 133,216 adult patients and 2883 AEs in 955 pediatric patients (age, < 18 years) from November 2011 to February 2023 using the US Food and Drug Administration Adverse Event Reporting System and the Canada Vigilance Adverse Reaction Online Database.
- AEs were categorized on the basis of the Medical Dictionary for Regulatory Activities system organ class.
- Five JAK inhibitors approved for use in children were included in the study: Baricitinib, upadacitinib, abrocitinib, ruxolitinib, and tofacitinib.
TAKEAWAY:
- The most frequently reported AEs in children were blood and lymphatic system disorders, including neutropenia, thrombocytopenia, and anemia (24%); viral, fungal, and bacterial infections, such as pneumonia and sepsis (17.2%); constitutional symptoms and administrative concerns, including pyrexia and fatigue (15.7%); gastrointestinal disorders, such as vomiting and abdominal pain (13.6%); and respiratory disorders, such as cough and respiratory distress (5.3%).
- In adults, the most common AEs were viral, fungal, and bacterial infections (16.8%); constitutional symptoms and administrative concerns (13.5%); musculoskeletal and connective tissue disorders (7.04%); and gastrointestinal (5.8%) and nervous system (5%) disorders.
- Acne (30.6%), atopic dermatitis (22.2%), and psoriasis (16.7%) were the most common skin and subcutaneous tissue AEs reported in children. Skin and subcutaneous AEs were more common with upadacitinib (21.1%), abrocitinib (9.1%), and tofacitinib (6.3%) in children.
- Serious AEs included in the boxed warning for JAK inhibitors — serious infection, mortality, malignancy, cardiovascular events, and thrombosis — were similar for baricitinib in children (4 of 49 patients, 8.2%) and adults (325 of 3707, 8.8%). For other JAK inhibitors, absolute numbers of these AEs in children were small and rates were lower in children than in adults.
IN PRACTICE:
“This information can support customized treatment and minimize the potential for undesired or intolerable AEs,” the authors wrote.
SOURCE:
This study was led by Sahithi Talasila, BS, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and was published online in Pediatric Dermatology.
LIMITATIONS:
Pharmacovigilance registries did not fully capture the complete range of AEs because of potential reporting bias or recall bias. Additionally, events lacking sufficient objective evidence were underreported, while common AEs associated with JAK inhibitor therapy were overreported.
DISCLOSURES:
No specific funding sources for the study were reported. One author reported being a consultant, one reported serving as a principal investigator in clinical trials, and another reported serving on data and safety monitoring boards of various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which also found that acne was the most common skin-related AE in children, and serious AEs were less common.
METHODOLOGY:
- Researchers analyzed 399,649 AEs in 133,216 adult patients and 2883 AEs in 955 pediatric patients (age, < 18 years) from November 2011 to February 2023 using the US Food and Drug Administration Adverse Event Reporting System and the Canada Vigilance Adverse Reaction Online Database.
- AEs were categorized on the basis of the Medical Dictionary for Regulatory Activities system organ class.
- Five JAK inhibitors approved for use in children were included in the study: Baricitinib, upadacitinib, abrocitinib, ruxolitinib, and tofacitinib.
TAKEAWAY:
- The most frequently reported AEs in children were blood and lymphatic system disorders, including neutropenia, thrombocytopenia, and anemia (24%); viral, fungal, and bacterial infections, such as pneumonia and sepsis (17.2%); constitutional symptoms and administrative concerns, including pyrexia and fatigue (15.7%); gastrointestinal disorders, such as vomiting and abdominal pain (13.6%); and respiratory disorders, such as cough and respiratory distress (5.3%).
- In adults, the most common AEs were viral, fungal, and bacterial infections (16.8%); constitutional symptoms and administrative concerns (13.5%); musculoskeletal and connective tissue disorders (7.04%); and gastrointestinal (5.8%) and nervous system (5%) disorders.
- Acne (30.6%), atopic dermatitis (22.2%), and psoriasis (16.7%) were the most common skin and subcutaneous tissue AEs reported in children. Skin and subcutaneous AEs were more common with upadacitinib (21.1%), abrocitinib (9.1%), and tofacitinib (6.3%) in children.
- Serious AEs included in the boxed warning for JAK inhibitors — serious infection, mortality, malignancy, cardiovascular events, and thrombosis — were similar for baricitinib in children (4 of 49 patients, 8.2%) and adults (325 of 3707, 8.8%). For other JAK inhibitors, absolute numbers of these AEs in children were small and rates were lower in children than in adults.
IN PRACTICE:
“This information can support customized treatment and minimize the potential for undesired or intolerable AEs,” the authors wrote.
SOURCE:
This study was led by Sahithi Talasila, BS, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and was published online in Pediatric Dermatology.
LIMITATIONS:
Pharmacovigilance registries did not fully capture the complete range of AEs because of potential reporting bias or recall bias. Additionally, events lacking sufficient objective evidence were underreported, while common AEs associated with JAK inhibitor therapy were overreported.
DISCLOSURES:
No specific funding sources for the study were reported. One author reported being a consultant, one reported serving as a principal investigator in clinical trials, and another reported serving on data and safety monitoring boards of various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FDA OKs Subcutaneous Atezolizumab Formulation for Multiple Cancer Indications
Approved indications include non–small cell lung cancer (NSCLC), SCLC, hepatocellular carcinoma, melanoma, and alveolar soft part sarcoma. Specific indications are available with the full prescribing information at Drugs@FDA.
This is the first programmed death–ligand 1 inhibitor to gain approval for subcutaneous administration.
“This approval represents a significant option to improve the patient experience,” Ann Fish-Steagall, RN, Senior Vice President of Patient Services at the LUNGevity Foundation stated in a Genentech press release.
Subcutaneous atezolizumab and hyaluronidase-tqjs was evaluated in the open-label, randomized IMscin001 trial of 371 adult patients with locally advanced or metastatic NSCLC who were not previously exposed to cancer immunotherapy and who had disease progression following treatment with platinum-based chemotherapy. Patients were randomized 2:1 to receive subcutaneous or IV administration until disease progression or unacceptable toxicity.
Atezolizumab exposure, the primary outcome measure of the study, met the lower limit of geometric mean ratio above the prespecified threshold of 0.8 (cycle 1C trough, 1.05; area under the curve for days 0-21, 0.87).
No notable differences were observed in overall response rate, progression-free survival, or overall survival between the two formulations, according to the FDA approval notice.
The confirmed overall response rate was 9% in the subcutaneous arm and 8% intravenous arm.
Adverse events of any grade occurring in at least 10% of patients were fatigue, musculoskeletal pain, cough, dyspnea, and decreased appetite.
The recommended dose for subcutaneous injection is one 15 mL injection, which contains 1875 mg of atezolizumab and 30,000 units of hyaluronidase.
Injections should be administered in the thigh over approximately 7 minutes every 3 weeks. By contrast, IV administration generally takes 30-60 minutes.
A version of this article first appeared on Medscape.com.
Approved indications include non–small cell lung cancer (NSCLC), SCLC, hepatocellular carcinoma, melanoma, and alveolar soft part sarcoma. Specific indications are available with the full prescribing information at Drugs@FDA.
This is the first programmed death–ligand 1 inhibitor to gain approval for subcutaneous administration.
“This approval represents a significant option to improve the patient experience,” Ann Fish-Steagall, RN, Senior Vice President of Patient Services at the LUNGevity Foundation stated in a Genentech press release.
Subcutaneous atezolizumab and hyaluronidase-tqjs was evaluated in the open-label, randomized IMscin001 trial of 371 adult patients with locally advanced or metastatic NSCLC who were not previously exposed to cancer immunotherapy and who had disease progression following treatment with platinum-based chemotherapy. Patients were randomized 2:1 to receive subcutaneous or IV administration until disease progression or unacceptable toxicity.
Atezolizumab exposure, the primary outcome measure of the study, met the lower limit of geometric mean ratio above the prespecified threshold of 0.8 (cycle 1C trough, 1.05; area under the curve for days 0-21, 0.87).
No notable differences were observed in overall response rate, progression-free survival, or overall survival between the two formulations, according to the FDA approval notice.
The confirmed overall response rate was 9% in the subcutaneous arm and 8% intravenous arm.
Adverse events of any grade occurring in at least 10% of patients were fatigue, musculoskeletal pain, cough, dyspnea, and decreased appetite.
The recommended dose for subcutaneous injection is one 15 mL injection, which contains 1875 mg of atezolizumab and 30,000 units of hyaluronidase.
Injections should be administered in the thigh over approximately 7 minutes every 3 weeks. By contrast, IV administration generally takes 30-60 minutes.
A version of this article first appeared on Medscape.com.
Approved indications include non–small cell lung cancer (NSCLC), SCLC, hepatocellular carcinoma, melanoma, and alveolar soft part sarcoma. Specific indications are available with the full prescribing information at Drugs@FDA.
This is the first programmed death–ligand 1 inhibitor to gain approval for subcutaneous administration.
“This approval represents a significant option to improve the patient experience,” Ann Fish-Steagall, RN, Senior Vice President of Patient Services at the LUNGevity Foundation stated in a Genentech press release.
Subcutaneous atezolizumab and hyaluronidase-tqjs was evaluated in the open-label, randomized IMscin001 trial of 371 adult patients with locally advanced or metastatic NSCLC who were not previously exposed to cancer immunotherapy and who had disease progression following treatment with platinum-based chemotherapy. Patients were randomized 2:1 to receive subcutaneous or IV administration until disease progression or unacceptable toxicity.
Atezolizumab exposure, the primary outcome measure of the study, met the lower limit of geometric mean ratio above the prespecified threshold of 0.8 (cycle 1C trough, 1.05; area under the curve for days 0-21, 0.87).
No notable differences were observed in overall response rate, progression-free survival, or overall survival between the two formulations, according to the FDA approval notice.
The confirmed overall response rate was 9% in the subcutaneous arm and 8% intravenous arm.
Adverse events of any grade occurring in at least 10% of patients were fatigue, musculoskeletal pain, cough, dyspnea, and decreased appetite.
The recommended dose for subcutaneous injection is one 15 mL injection, which contains 1875 mg of atezolizumab and 30,000 units of hyaluronidase.
Injections should be administered in the thigh over approximately 7 minutes every 3 weeks. By contrast, IV administration generally takes 30-60 minutes.
A version of this article first appeared on Medscape.com.
Osimertinib/Savolitinib Combo Shows Promise in NSCLC
according to results of the phase 2 FLOWERS study.
Compared with EGFR inhibitor osimertinib alone, the combination demonstrated a clinically meaningful improvement in the objective response rate — the study’s primary endpoint — with a positive trend in progression-free survival and a manageable safety profile.
About 30% patients with EGFR-mutated NSCLC fail to respond well to EGFR–tyrosine kinase inhibitors (TKIs), explained Jin-Ji Yang, MD, with Guangdong Lung Cancer Institute, Guangzhou, China, who reported the study results at the annual meeting of the World Conference on Lung Cancer.
Data suggested that de novo MET amplification occurs in up to 5% patients with treatment-naive, EGFR-mutated advanced NSCLC, and MET overexpression occurs in up to 15% these patients.
Coexistence of EGFR mutation and MET amplification/overexpression reduces sensitivity to EGFR-TKI therapy “and is likely the mechanism for mediating primary resistance to first-line EGFR-TKI monotherapy,” Dr. Yang explained in her presentation.
Osimertinib is a third-generation EGFR-TKI recommended as the first-line treatment for EGFR-mutant advanced NSCLC. Savolitinib is a highly selective MET-TKI which has demonstrated antitumor activity in various cancers with MET alterations.
The FLOWERS study is the first to test whether combining the two agents could improve efficacy and overcome MET-driven primary resistance in these patients.
The phase 2 study enrolled 44 treatment-naive patients with de novo MET-aberrant, EGFR-mutant, stage IIIB-IV NSCLC; 23 were randomly allocated to receive oral osimertinib (80 mg once daily) alone and 21 to receive oral osimertinib (80 mg once daily) plus savolitinib (300 mg twice daily).
At a median follow-up of 8.2 months, the objective response rate was 60.9% with osimertinib monotherapy vs 90.5% with combination therapy. The disease control rate was also better with the combination therapy than with monotherapy (95.2% vs 87%).
Median duration of response (not yet mature) was 8.4 months with monotherapy vs 18.6 months with combination therapy.
Preliminary progression-free survival data also showed a trend in favor of combination therapy over monotherapy (a median of 19.3 vs 9.3 months; hazard ratio, 0.59).
Most treatment-related adverse events were grade 1 or 2, and there were no fatal adverse events.
Treatment-related adverse events of grade 3 or higher were more common with combination therapy (57.1% vs 8.7%). The most common events with monotherapy were diarrhea (56.5%), rash (52.2%), and pruritus (43.5%) and with dual therapy were rash (52.4%), thrombocytopenia (52.4%), and peripheral edema (42.9%).
The results showed that the combination therapy has the potential to become a first-line treatment option for patients who do not respond well to EGFR-TKIs alone, Dr. Yang said in a press release.
Discussant for the study Paul Paik, MD, thoracic oncologist at Memorial Sloan Kettering Cancer Center in New York City, said this study “adds to data suggesting high MET expression might be a poor prognostic or predictive marker, the outcomes of which are improved with MET inhibition.”
He cautioned, however, that there appears to be “quality of life, side-effect trade-offs with dual MET plus EGFR TKI upfront.”
Dr. Paik said he looks forward to results from FLOWERS on serial circulating tumor DNA and formal androgen receptor testing, which “might aid in further assessing clonality and characterizing MET as a co-driver in this setting.”
The study was funded by AstraZeneca China. Dr. Yang had no disclosures. Dr. Paik disclosed relationships with EMD Serono, Bicara, Novartis, and Summit.
A version of this article first appeared on Medscape.com.
according to results of the phase 2 FLOWERS study.
Compared with EGFR inhibitor osimertinib alone, the combination demonstrated a clinically meaningful improvement in the objective response rate — the study’s primary endpoint — with a positive trend in progression-free survival and a manageable safety profile.
About 30% patients with EGFR-mutated NSCLC fail to respond well to EGFR–tyrosine kinase inhibitors (TKIs), explained Jin-Ji Yang, MD, with Guangdong Lung Cancer Institute, Guangzhou, China, who reported the study results at the annual meeting of the World Conference on Lung Cancer.
Data suggested that de novo MET amplification occurs in up to 5% patients with treatment-naive, EGFR-mutated advanced NSCLC, and MET overexpression occurs in up to 15% these patients.
Coexistence of EGFR mutation and MET amplification/overexpression reduces sensitivity to EGFR-TKI therapy “and is likely the mechanism for mediating primary resistance to first-line EGFR-TKI monotherapy,” Dr. Yang explained in her presentation.
Osimertinib is a third-generation EGFR-TKI recommended as the first-line treatment for EGFR-mutant advanced NSCLC. Savolitinib is a highly selective MET-TKI which has demonstrated antitumor activity in various cancers with MET alterations.
The FLOWERS study is the first to test whether combining the two agents could improve efficacy and overcome MET-driven primary resistance in these patients.
The phase 2 study enrolled 44 treatment-naive patients with de novo MET-aberrant, EGFR-mutant, stage IIIB-IV NSCLC; 23 were randomly allocated to receive oral osimertinib (80 mg once daily) alone and 21 to receive oral osimertinib (80 mg once daily) plus savolitinib (300 mg twice daily).
At a median follow-up of 8.2 months, the objective response rate was 60.9% with osimertinib monotherapy vs 90.5% with combination therapy. The disease control rate was also better with the combination therapy than with monotherapy (95.2% vs 87%).
Median duration of response (not yet mature) was 8.4 months with monotherapy vs 18.6 months with combination therapy.
Preliminary progression-free survival data also showed a trend in favor of combination therapy over monotherapy (a median of 19.3 vs 9.3 months; hazard ratio, 0.59).
Most treatment-related adverse events were grade 1 or 2, and there were no fatal adverse events.
Treatment-related adverse events of grade 3 or higher were more common with combination therapy (57.1% vs 8.7%). The most common events with monotherapy were diarrhea (56.5%), rash (52.2%), and pruritus (43.5%) and with dual therapy were rash (52.4%), thrombocytopenia (52.4%), and peripheral edema (42.9%).
The results showed that the combination therapy has the potential to become a first-line treatment option for patients who do not respond well to EGFR-TKIs alone, Dr. Yang said in a press release.
Discussant for the study Paul Paik, MD, thoracic oncologist at Memorial Sloan Kettering Cancer Center in New York City, said this study “adds to data suggesting high MET expression might be a poor prognostic or predictive marker, the outcomes of which are improved with MET inhibition.”
He cautioned, however, that there appears to be “quality of life, side-effect trade-offs with dual MET plus EGFR TKI upfront.”
Dr. Paik said he looks forward to results from FLOWERS on serial circulating tumor DNA and formal androgen receptor testing, which “might aid in further assessing clonality and characterizing MET as a co-driver in this setting.”
The study was funded by AstraZeneca China. Dr. Yang had no disclosures. Dr. Paik disclosed relationships with EMD Serono, Bicara, Novartis, and Summit.
A version of this article first appeared on Medscape.com.
according to results of the phase 2 FLOWERS study.
Compared with EGFR inhibitor osimertinib alone, the combination demonstrated a clinically meaningful improvement in the objective response rate — the study’s primary endpoint — with a positive trend in progression-free survival and a manageable safety profile.
About 30% patients with EGFR-mutated NSCLC fail to respond well to EGFR–tyrosine kinase inhibitors (TKIs), explained Jin-Ji Yang, MD, with Guangdong Lung Cancer Institute, Guangzhou, China, who reported the study results at the annual meeting of the World Conference on Lung Cancer.
Data suggested that de novo MET amplification occurs in up to 5% patients with treatment-naive, EGFR-mutated advanced NSCLC, and MET overexpression occurs in up to 15% these patients.
Coexistence of EGFR mutation and MET amplification/overexpression reduces sensitivity to EGFR-TKI therapy “and is likely the mechanism for mediating primary resistance to first-line EGFR-TKI monotherapy,” Dr. Yang explained in her presentation.
Osimertinib is a third-generation EGFR-TKI recommended as the first-line treatment for EGFR-mutant advanced NSCLC. Savolitinib is a highly selective MET-TKI which has demonstrated antitumor activity in various cancers with MET alterations.
The FLOWERS study is the first to test whether combining the two agents could improve efficacy and overcome MET-driven primary resistance in these patients.
The phase 2 study enrolled 44 treatment-naive patients with de novo MET-aberrant, EGFR-mutant, stage IIIB-IV NSCLC; 23 were randomly allocated to receive oral osimertinib (80 mg once daily) alone and 21 to receive oral osimertinib (80 mg once daily) plus savolitinib (300 mg twice daily).
At a median follow-up of 8.2 months, the objective response rate was 60.9% with osimertinib monotherapy vs 90.5% with combination therapy. The disease control rate was also better with the combination therapy than with monotherapy (95.2% vs 87%).
Median duration of response (not yet mature) was 8.4 months with monotherapy vs 18.6 months with combination therapy.
Preliminary progression-free survival data also showed a trend in favor of combination therapy over monotherapy (a median of 19.3 vs 9.3 months; hazard ratio, 0.59).
Most treatment-related adverse events were grade 1 or 2, and there were no fatal adverse events.
Treatment-related adverse events of grade 3 or higher were more common with combination therapy (57.1% vs 8.7%). The most common events with monotherapy were diarrhea (56.5%), rash (52.2%), and pruritus (43.5%) and with dual therapy were rash (52.4%), thrombocytopenia (52.4%), and peripheral edema (42.9%).
The results showed that the combination therapy has the potential to become a first-line treatment option for patients who do not respond well to EGFR-TKIs alone, Dr. Yang said in a press release.
Discussant for the study Paul Paik, MD, thoracic oncologist at Memorial Sloan Kettering Cancer Center in New York City, said this study “adds to data suggesting high MET expression might be a poor prognostic or predictive marker, the outcomes of which are improved with MET inhibition.”
He cautioned, however, that there appears to be “quality of life, side-effect trade-offs with dual MET plus EGFR TKI upfront.”
Dr. Paik said he looks forward to results from FLOWERS on serial circulating tumor DNA and formal androgen receptor testing, which “might aid in further assessing clonality and characterizing MET as a co-driver in this setting.”
The study was funded by AstraZeneca China. Dr. Yang had no disclosures. Dr. Paik disclosed relationships with EMD Serono, Bicara, Novartis, and Summit.
A version of this article first appeared on Medscape.com.
FROM WCLC 2024
Oropouche Virus
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected]. (Also [email protected])
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected]. (Also [email protected])
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected]. (Also [email protected])
No Matched Sibling Donor? Sickle Cell Experts Debate Next-Best Option
“If there is an indication for intervention, for a curative therapy, in the absence of a matched sibling donor, gene therapy is the first choice,” Jaap-Jan Boelens, MD, PhD, of Memorial Sloan Kettering Cancer Center, New York City, argued in a presentation at the annual meeting of the Society of Hematologic Oncology (SOHO) in Houston.
“In the registries, alternative transplant outcomes are pretty poor, although there is some encouraging data coming up. The time is not there yet when this is the [best] choice.”
But Adetola Kassim, MBBS, of Vanderbilt University Medical Center in Nashville, Tennessee, said patients with sickle cell disease (SCD) who don’t qualify for a matched sibling donor transplant can still have good transplant options. And the results can be impressive.
“Once you’re engrafted, and you don’t lose your graft, the effect in transplant is lifelong,” he said. When it comes to long-lasting effects, he added, “we’re not sure yet about gene therapy.”
As Dr. Kassim noted, SCD continues to take a huge toll.
“Median survival for patients with sickle cell anemia remains stuck in the fifth decade of life with no change in 25 years,” he said. Heart, lung, and kidney complications account for 50% of identifiable causes of death, followed by about 26% attributed to cardiovascular disease, he said. “The question here is about which therapy can impact the most debilitating complication in children, which is stroke, and improve survival in adults with progressive organ dysfunction.”
Dr. Boelens said there are “huge barriers” to stem cell transplant in SCD because only 15% of patients eligible for the treatment have a matched related donor, and only 10% have a matched related or unrelated donor.
“There’s also a lack of financial and psychosocial support in many of the families. There is also parental refusal because of the mortality risk, and there’s also physician refusal because hematologists aren’t always in the same hospitals as the transplant programs.”
Dr. Boelens highlighted a 2019 study of data from 2008-2017 that found outcomes in unmatched donor transplantations are “not great,” with higher risk for mortality and graft failure.
As an alternative, he said, two gene therapies, both gene “additions,” are now approved by the US Food and Drug Administration (FDA). They are exagamglogene autotemcel (exa-cel, Casgevy) and betibeglogene autotemcel (LentiGlobin, Zynteglo). There’s also a gene “correction” option in the works, but it’s not yet ready for prime time, he said.
In the two approved gene therapy treatments, stem cells are removed from the patient, modified/manufactured in an outside facility, and then engrafted.
The advantages of gene therapy include no need to find a donor or worry about graft resistance, and there’s no need for immunosuppression, he said. However, the process takes a long time, there’s limited long-term data, and there’s a risk for loss of fertility and other chemotherapy-related adverse effects.
For his part, Dr. Kassim noted how several groups are excluded from the strong outcomes in matched sibling donor stem-cell transplants: Children with strokes and no eligible donors, others without eligible donors, and adults with severe disease and organ dysfunction who are typically excluded.
“We need transplants with less toxicity and alternative donors,” he said. Another challenge: “How do we decrease graft failure without increasing transplant-related mortality?”
Researchers are exploring several strategies to adjust drug therapy during conditioning, Dr. Kassim said, and he led a promising phase II study that explored one approach. The results of that study were recently published in the journal Blood. Graft failures were very low in both adults and children, he said, and 2-year survival among 70 patients was 94.8%. The five deaths were related to infection.
The evidence about the various strategies shows that “virtually all SCD patients, except those with severe heart, lung, or kidney disease” can benefit from a curative transplant, Dr. Kassim said.
Dr. Boelens had no disclosures. Disclosures for Dr. Kassim were not provided, but he recently reported no disclosures in a report about transplants in SCD.
A version of this article appeared on Medscape.com.
“If there is an indication for intervention, for a curative therapy, in the absence of a matched sibling donor, gene therapy is the first choice,” Jaap-Jan Boelens, MD, PhD, of Memorial Sloan Kettering Cancer Center, New York City, argued in a presentation at the annual meeting of the Society of Hematologic Oncology (SOHO) in Houston.
“In the registries, alternative transplant outcomes are pretty poor, although there is some encouraging data coming up. The time is not there yet when this is the [best] choice.”
But Adetola Kassim, MBBS, of Vanderbilt University Medical Center in Nashville, Tennessee, said patients with sickle cell disease (SCD) who don’t qualify for a matched sibling donor transplant can still have good transplant options. And the results can be impressive.
“Once you’re engrafted, and you don’t lose your graft, the effect in transplant is lifelong,” he said. When it comes to long-lasting effects, he added, “we’re not sure yet about gene therapy.”
As Dr. Kassim noted, SCD continues to take a huge toll.
“Median survival for patients with sickle cell anemia remains stuck in the fifth decade of life with no change in 25 years,” he said. Heart, lung, and kidney complications account for 50% of identifiable causes of death, followed by about 26% attributed to cardiovascular disease, he said. “The question here is about which therapy can impact the most debilitating complication in children, which is stroke, and improve survival in adults with progressive organ dysfunction.”
Dr. Boelens said there are “huge barriers” to stem cell transplant in SCD because only 15% of patients eligible for the treatment have a matched related donor, and only 10% have a matched related or unrelated donor.
“There’s also a lack of financial and psychosocial support in many of the families. There is also parental refusal because of the mortality risk, and there’s also physician refusal because hematologists aren’t always in the same hospitals as the transplant programs.”
Dr. Boelens highlighted a 2019 study of data from 2008-2017 that found outcomes in unmatched donor transplantations are “not great,” with higher risk for mortality and graft failure.
As an alternative, he said, two gene therapies, both gene “additions,” are now approved by the US Food and Drug Administration (FDA). They are exagamglogene autotemcel (exa-cel, Casgevy) and betibeglogene autotemcel (LentiGlobin, Zynteglo). There’s also a gene “correction” option in the works, but it’s not yet ready for prime time, he said.
In the two approved gene therapy treatments, stem cells are removed from the patient, modified/manufactured in an outside facility, and then engrafted.
The advantages of gene therapy include no need to find a donor or worry about graft resistance, and there’s no need for immunosuppression, he said. However, the process takes a long time, there’s limited long-term data, and there’s a risk for loss of fertility and other chemotherapy-related adverse effects.
For his part, Dr. Kassim noted how several groups are excluded from the strong outcomes in matched sibling donor stem-cell transplants: Children with strokes and no eligible donors, others without eligible donors, and adults with severe disease and organ dysfunction who are typically excluded.
“We need transplants with less toxicity and alternative donors,” he said. Another challenge: “How do we decrease graft failure without increasing transplant-related mortality?”
Researchers are exploring several strategies to adjust drug therapy during conditioning, Dr. Kassim said, and he led a promising phase II study that explored one approach. The results of that study were recently published in the journal Blood. Graft failures were very low in both adults and children, he said, and 2-year survival among 70 patients was 94.8%. The five deaths were related to infection.
The evidence about the various strategies shows that “virtually all SCD patients, except those with severe heart, lung, or kidney disease” can benefit from a curative transplant, Dr. Kassim said.
Dr. Boelens had no disclosures. Disclosures for Dr. Kassim were not provided, but he recently reported no disclosures in a report about transplants in SCD.
A version of this article appeared on Medscape.com.
“If there is an indication for intervention, for a curative therapy, in the absence of a matched sibling donor, gene therapy is the first choice,” Jaap-Jan Boelens, MD, PhD, of Memorial Sloan Kettering Cancer Center, New York City, argued in a presentation at the annual meeting of the Society of Hematologic Oncology (SOHO) in Houston.
“In the registries, alternative transplant outcomes are pretty poor, although there is some encouraging data coming up. The time is not there yet when this is the [best] choice.”
But Adetola Kassim, MBBS, of Vanderbilt University Medical Center in Nashville, Tennessee, said patients with sickle cell disease (SCD) who don’t qualify for a matched sibling donor transplant can still have good transplant options. And the results can be impressive.
“Once you’re engrafted, and you don’t lose your graft, the effect in transplant is lifelong,” he said. When it comes to long-lasting effects, he added, “we’re not sure yet about gene therapy.”
As Dr. Kassim noted, SCD continues to take a huge toll.
“Median survival for patients with sickle cell anemia remains stuck in the fifth decade of life with no change in 25 years,” he said. Heart, lung, and kidney complications account for 50% of identifiable causes of death, followed by about 26% attributed to cardiovascular disease, he said. “The question here is about which therapy can impact the most debilitating complication in children, which is stroke, and improve survival in adults with progressive organ dysfunction.”
Dr. Boelens said there are “huge barriers” to stem cell transplant in SCD because only 15% of patients eligible for the treatment have a matched related donor, and only 10% have a matched related or unrelated donor.
“There’s also a lack of financial and psychosocial support in many of the families. There is also parental refusal because of the mortality risk, and there’s also physician refusal because hematologists aren’t always in the same hospitals as the transplant programs.”
Dr. Boelens highlighted a 2019 study of data from 2008-2017 that found outcomes in unmatched donor transplantations are “not great,” with higher risk for mortality and graft failure.
As an alternative, he said, two gene therapies, both gene “additions,” are now approved by the US Food and Drug Administration (FDA). They are exagamglogene autotemcel (exa-cel, Casgevy) and betibeglogene autotemcel (LentiGlobin, Zynteglo). There’s also a gene “correction” option in the works, but it’s not yet ready for prime time, he said.
In the two approved gene therapy treatments, stem cells are removed from the patient, modified/manufactured in an outside facility, and then engrafted.
The advantages of gene therapy include no need to find a donor or worry about graft resistance, and there’s no need for immunosuppression, he said. However, the process takes a long time, there’s limited long-term data, and there’s a risk for loss of fertility and other chemotherapy-related adverse effects.
For his part, Dr. Kassim noted how several groups are excluded from the strong outcomes in matched sibling donor stem-cell transplants: Children with strokes and no eligible donors, others without eligible donors, and adults with severe disease and organ dysfunction who are typically excluded.
“We need transplants with less toxicity and alternative donors,” he said. Another challenge: “How do we decrease graft failure without increasing transplant-related mortality?”
Researchers are exploring several strategies to adjust drug therapy during conditioning, Dr. Kassim said, and he led a promising phase II study that explored one approach. The results of that study were recently published in the journal Blood. Graft failures were very low in both adults and children, he said, and 2-year survival among 70 patients was 94.8%. The five deaths were related to infection.
The evidence about the various strategies shows that “virtually all SCD patients, except those with severe heart, lung, or kidney disease” can benefit from a curative transplant, Dr. Kassim said.
Dr. Boelens had no disclosures. Disclosures for Dr. Kassim were not provided, but he recently reported no disclosures in a report about transplants in SCD.
A version of this article appeared on Medscape.com.
FROM SOHO 2024
GI-Targeted Bitter Hop Extract Curbs Hunger, Food Cravings
TOPLINE:
METHODOLOGY:
- Researchers conducted a randomized, double-blind, crossover study with 30 normal-weight women ages 18-40 with BMIs of 18.5-25. The trial design was similar to that used previously to test the intervention among men.
- Participants engaged in a water-only fast for 24 hours (6 p.m. to 6 p.m.) on three occasions and were given an ad libitum meal to break each fast.
- Participants were randomly assigned to one of three treatments: placebo, a high-dose (500 mg) bitter hop–based appetite suppressant (Amarasate), or a low dose (250 mg) of the appetite suppressant.
- Treatment capsules of half the total dose were given twice daily (16 hours and 20 hours into the fast).
- Participants recorded their subjective feelings about appetite and food cravings using a visual analog scale at 30-minute intervals starting 16 hours into the fast.
TAKEAWAY:
- Both the high-dose and low-dose treatment groups experienced a significant reduction in appetite (ie, hunger, fullness, satisfaction, thoughts of food) and food cravings compared with the placebo group.
- Energy intake at the ad libitum meal was 14.3% lower in the high-dose group than the placebo group (P < .05). It was 8.1% lower in the low-dose group, but the difference was not statistically significant.
- In the high-dose group, two participants reported loose stools and one reported heartburn. In the low-dose group, one participant reported loose stools.
IN PRACTICE:
“These data suggest that appetite suppressant co-therapy may be useful in reducing hunger during fasting in women and show that gastrointestinal delivery of bitter compounds may also be an effective method of reducing cravings for food,” the authors wrote.
SOURCE:
The study, led by Edward Walker, PhD, of the New Zealand Institute for Plant and Food Research Limited, Auckland, New Zealand, was published online in Obesity Pillars.
LIMITATIONS:
The study has limitations that affect its application for weight management. First, the study included only normal-weight individuals, which limits extension of the results for weight loss treatments. Second, it involved acute fasting and does not assess any accommodation to the intervention that may occur over longer-term use. An additional limitation is the small number of participants.
DISCLOSURES:
The research was funded by Calocurb Limited. The article processing charge was funded by the New Zealand Institute for Plant and Food Research Limited, a New Zealand government–owned Crown Research Institute. The authors declare no competing financial interests. The New Zealand Institute for Plant and Food Research Limited has licensed a hop extract as a dietary supplement to Calocurb to commercialize and currently holds a minor shareholding in this company.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a randomized, double-blind, crossover study with 30 normal-weight women ages 18-40 with BMIs of 18.5-25. The trial design was similar to that used previously to test the intervention among men.
- Participants engaged in a water-only fast for 24 hours (6 p.m. to 6 p.m.) on three occasions and were given an ad libitum meal to break each fast.
- Participants were randomly assigned to one of three treatments: placebo, a high-dose (500 mg) bitter hop–based appetite suppressant (Amarasate), or a low dose (250 mg) of the appetite suppressant.
- Treatment capsules of half the total dose were given twice daily (16 hours and 20 hours into the fast).
- Participants recorded their subjective feelings about appetite and food cravings using a visual analog scale at 30-minute intervals starting 16 hours into the fast.
TAKEAWAY:
- Both the high-dose and low-dose treatment groups experienced a significant reduction in appetite (ie, hunger, fullness, satisfaction, thoughts of food) and food cravings compared with the placebo group.
- Energy intake at the ad libitum meal was 14.3% lower in the high-dose group than the placebo group (P < .05). It was 8.1% lower in the low-dose group, but the difference was not statistically significant.
- In the high-dose group, two participants reported loose stools and one reported heartburn. In the low-dose group, one participant reported loose stools.
IN PRACTICE:
“These data suggest that appetite suppressant co-therapy may be useful in reducing hunger during fasting in women and show that gastrointestinal delivery of bitter compounds may also be an effective method of reducing cravings for food,” the authors wrote.
SOURCE:
The study, led by Edward Walker, PhD, of the New Zealand Institute for Plant and Food Research Limited, Auckland, New Zealand, was published online in Obesity Pillars.
LIMITATIONS:
The study has limitations that affect its application for weight management. First, the study included only normal-weight individuals, which limits extension of the results for weight loss treatments. Second, it involved acute fasting and does not assess any accommodation to the intervention that may occur over longer-term use. An additional limitation is the small number of participants.
DISCLOSURES:
The research was funded by Calocurb Limited. The article processing charge was funded by the New Zealand Institute for Plant and Food Research Limited, a New Zealand government–owned Crown Research Institute. The authors declare no competing financial interests. The New Zealand Institute for Plant and Food Research Limited has licensed a hop extract as a dietary supplement to Calocurb to commercialize and currently holds a minor shareholding in this company.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a randomized, double-blind, crossover study with 30 normal-weight women ages 18-40 with BMIs of 18.5-25. The trial design was similar to that used previously to test the intervention among men.
- Participants engaged in a water-only fast for 24 hours (6 p.m. to 6 p.m.) on three occasions and were given an ad libitum meal to break each fast.
- Participants were randomly assigned to one of three treatments: placebo, a high-dose (500 mg) bitter hop–based appetite suppressant (Amarasate), or a low dose (250 mg) of the appetite suppressant.
- Treatment capsules of half the total dose were given twice daily (16 hours and 20 hours into the fast).
- Participants recorded their subjective feelings about appetite and food cravings using a visual analog scale at 30-minute intervals starting 16 hours into the fast.
TAKEAWAY:
- Both the high-dose and low-dose treatment groups experienced a significant reduction in appetite (ie, hunger, fullness, satisfaction, thoughts of food) and food cravings compared with the placebo group.
- Energy intake at the ad libitum meal was 14.3% lower in the high-dose group than the placebo group (P < .05). It was 8.1% lower in the low-dose group, but the difference was not statistically significant.
- In the high-dose group, two participants reported loose stools and one reported heartburn. In the low-dose group, one participant reported loose stools.
IN PRACTICE:
“These data suggest that appetite suppressant co-therapy may be useful in reducing hunger during fasting in women and show that gastrointestinal delivery of bitter compounds may also be an effective method of reducing cravings for food,” the authors wrote.
SOURCE:
The study, led by Edward Walker, PhD, of the New Zealand Institute for Plant and Food Research Limited, Auckland, New Zealand, was published online in Obesity Pillars.
LIMITATIONS:
The study has limitations that affect its application for weight management. First, the study included only normal-weight individuals, which limits extension of the results for weight loss treatments. Second, it involved acute fasting and does not assess any accommodation to the intervention that may occur over longer-term use. An additional limitation is the small number of participants.
DISCLOSURES:
The research was funded by Calocurb Limited. The article processing charge was funded by the New Zealand Institute for Plant and Food Research Limited, a New Zealand government–owned Crown Research Institute. The authors declare no competing financial interests. The New Zealand Institute for Plant and Food Research Limited has licensed a hop extract as a dietary supplement to Calocurb to commercialize and currently holds a minor shareholding in this company.
A version of this article first appeared on Medscape.com.
Stress Management
With the changing leaves and cooling temperatures, early autumn also brings the excitement of the new school year. While returning to sports, mastering new subjects, and spending time with old and new friends is exhilarating, this season can also be a time of intense stress.
For those high school students who are especially ambitious, the school year presents the challenge of a very high stakes performance, one whose success will be measured by admission to a prized college. Not only are there classes to study for, but schedules are packed with a maximum number of subjects, a maximum number of Advanced Placement courses and a maximum number of impressive extra-curricular activities. Varsity sports practice, SAT prep, Debate Club, volunteer hours, and on and on.
What is often missing is enough time for sleep, socializing, exploring new interests, and unwinding. When you hear your patients (or parents) describing the intense stress of their overloaded schedules compounded by a sense that “I have no choice,” you have an opportunity to complicate their thinking. Introduce the idea that there are smart approaches to performing your best under stress. Pushing themselves relentlessly will inevitably lead to burnout and exhaustion. This approach will help them learn to make wise choices and will better serve their healthy development.
Start by acknowledging the stress of high-stakes performance. Telling your patients that they need to lower the temperature by not putting so much pressure on themselves is likely to be experienced as a lack of confidence in them and is unlikely to get any traction. Instead, ask your patients what matters to them the most: Is it admission to the college of their choice? Achieving a certain score or GPA? Is it their competitiveness and drive to win? There is no wrong answer, but it is helpful for them to be able to reflect on what matters to them. Are they hoping to impress someone else? Are they worried about their future financial health and convinced that getting into a certain college will secure their financial success? Do they think this matters more to their parents than to themselves? Or have they discovered an intense interest in theoretical physics and want to be able to study at Caltech? If their ambition is meaningfully connected to an authentic interest or to their emerging identity, their sense of purpose will be much deeper and able to sustain them.
Even with talent and a strong sense of purpose, performing well is very difficult and demanding. It is important to consider the cycle of performance as including preparation, performance itself, and effective rest and recovery, just as with athletic performance. Whether the performance is the SATs, an AP test, a debate or big game, there were probably hours of preparation for every hour of performance. Help them to consider the importance of this practice or preparation time, and how to use that time effectively. Are they able to work in environments where there are few distractions? Do they have the support or useful feedback they need? How are they able to know when it is time for a break or when they are ready? It can be helpful for them to appreciate whether preparation or performance is more challenging for them, as the former requires focus and patience, while the latter requires courage and tenacity. If they are aware of which is harder for them, they can be thoughtful about how to effectively handle those challenges.
What can be most valuable for your patients is hearing from their pediatricians that they need to have time protected for rest and recharging, and not only for preparation and performance. Any athlete knows that failing to do so will lead to exhaustion and injury, and performance inevitably suffers. Rest is unwinding and slowing down, and a restful activity will leave them feeling calm, relaxed, and ready for sleep. A recharging activity is one that leaves them feeling refreshed and energized. Some common restful activities are a hot bath or shower, a distracting activity such as watching a show or surfing the web, playing a simple video game or puzzle or listening to music. Some recharging activities are creative ones (making art or music), engaging in hobbies, reading, or talking with a good friend. A few activities — sleep, exercise, and mindfulness meditation, are powerful in that they pack both rest and recharge into the same activity. Your patients should be discovering and learning which activities they find restful or recharging. The college application process or preparing for a varsity tryout will both add stress and give them an opportunity to learn what rests and recharges them. They should aim to have a list of at least five effective strategies that they can turn to when it’s time to rest or to recharge. Help them turn their work ethic to building a deeper well of self-knowledge that will serve them when they face challenges in high school or when they are on their own in college. This time of stress can be a time of growth, too.
Of course, remind your patients that this is a critical time to focus on basic self-care: They need consistently adequate, restful sleep, good nutrition, and physical activity. They will benefit from regular time in nature and time spent with friends that nourish them. They can find ways to compound these activities: Go for a walk with a friend, eat dinner with family, play a relaxing game while enjoying music. Lastly, ask your patients what is the last new thing they tried. It is easy to become so focused on an ambitious project that there is no time for exploration and play. Play is important throughout life, but adolescents are actively discovering their interests, talents, tastes, and values. To do this they need to be trying things that are new and maybe less purpose-driven. I call this type of activity “senseless fun.” Splashing in the pool is senseless fun, swimming laps is purposeful exercise that my contribute to recharging, and competing in a swim meet is often more on the stressful side. As they discover new talents, deeply engaging interests, what relaxes and recharges them, they will be learning who they are. Regardless of the outcome of a test, a big game, or where they go to college, it is this emerging knowledge about themselves that will carry them into adulthood. The pediatrician’s goal: Encouraging aspiration, exploration, and self-awareness in the context of giving permission for rest, recharging, and senseless fun.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
With the changing leaves and cooling temperatures, early autumn also brings the excitement of the new school year. While returning to sports, mastering new subjects, and spending time with old and new friends is exhilarating, this season can also be a time of intense stress.
For those high school students who are especially ambitious, the school year presents the challenge of a very high stakes performance, one whose success will be measured by admission to a prized college. Not only are there classes to study for, but schedules are packed with a maximum number of subjects, a maximum number of Advanced Placement courses and a maximum number of impressive extra-curricular activities. Varsity sports practice, SAT prep, Debate Club, volunteer hours, and on and on.
What is often missing is enough time for sleep, socializing, exploring new interests, and unwinding. When you hear your patients (or parents) describing the intense stress of their overloaded schedules compounded by a sense that “I have no choice,” you have an opportunity to complicate their thinking. Introduce the idea that there are smart approaches to performing your best under stress. Pushing themselves relentlessly will inevitably lead to burnout and exhaustion. This approach will help them learn to make wise choices and will better serve their healthy development.
Start by acknowledging the stress of high-stakes performance. Telling your patients that they need to lower the temperature by not putting so much pressure on themselves is likely to be experienced as a lack of confidence in them and is unlikely to get any traction. Instead, ask your patients what matters to them the most: Is it admission to the college of their choice? Achieving a certain score or GPA? Is it their competitiveness and drive to win? There is no wrong answer, but it is helpful for them to be able to reflect on what matters to them. Are they hoping to impress someone else? Are they worried about their future financial health and convinced that getting into a certain college will secure their financial success? Do they think this matters more to their parents than to themselves? Or have they discovered an intense interest in theoretical physics and want to be able to study at Caltech? If their ambition is meaningfully connected to an authentic interest or to their emerging identity, their sense of purpose will be much deeper and able to sustain them.
Even with talent and a strong sense of purpose, performing well is very difficult and demanding. It is important to consider the cycle of performance as including preparation, performance itself, and effective rest and recovery, just as with athletic performance. Whether the performance is the SATs, an AP test, a debate or big game, there were probably hours of preparation for every hour of performance. Help them to consider the importance of this practice or preparation time, and how to use that time effectively. Are they able to work in environments where there are few distractions? Do they have the support or useful feedback they need? How are they able to know when it is time for a break or when they are ready? It can be helpful for them to appreciate whether preparation or performance is more challenging for them, as the former requires focus and patience, while the latter requires courage and tenacity. If they are aware of which is harder for them, they can be thoughtful about how to effectively handle those challenges.
What can be most valuable for your patients is hearing from their pediatricians that they need to have time protected for rest and recharging, and not only for preparation and performance. Any athlete knows that failing to do so will lead to exhaustion and injury, and performance inevitably suffers. Rest is unwinding and slowing down, and a restful activity will leave them feeling calm, relaxed, and ready for sleep. A recharging activity is one that leaves them feeling refreshed and energized. Some common restful activities are a hot bath or shower, a distracting activity such as watching a show or surfing the web, playing a simple video game or puzzle or listening to music. Some recharging activities are creative ones (making art or music), engaging in hobbies, reading, or talking with a good friend. A few activities — sleep, exercise, and mindfulness meditation, are powerful in that they pack both rest and recharge into the same activity. Your patients should be discovering and learning which activities they find restful or recharging. The college application process or preparing for a varsity tryout will both add stress and give them an opportunity to learn what rests and recharges them. They should aim to have a list of at least five effective strategies that they can turn to when it’s time to rest or to recharge. Help them turn their work ethic to building a deeper well of self-knowledge that will serve them when they face challenges in high school or when they are on their own in college. This time of stress can be a time of growth, too.
Of course, remind your patients that this is a critical time to focus on basic self-care: They need consistently adequate, restful sleep, good nutrition, and physical activity. They will benefit from regular time in nature and time spent with friends that nourish them. They can find ways to compound these activities: Go for a walk with a friend, eat dinner with family, play a relaxing game while enjoying music. Lastly, ask your patients what is the last new thing they tried. It is easy to become so focused on an ambitious project that there is no time for exploration and play. Play is important throughout life, but adolescents are actively discovering their interests, talents, tastes, and values. To do this they need to be trying things that are new and maybe less purpose-driven. I call this type of activity “senseless fun.” Splashing in the pool is senseless fun, swimming laps is purposeful exercise that my contribute to recharging, and competing in a swim meet is often more on the stressful side. As they discover new talents, deeply engaging interests, what relaxes and recharges them, they will be learning who they are. Regardless of the outcome of a test, a big game, or where they go to college, it is this emerging knowledge about themselves that will carry them into adulthood. The pediatrician’s goal: Encouraging aspiration, exploration, and self-awareness in the context of giving permission for rest, recharging, and senseless fun.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
With the changing leaves and cooling temperatures, early autumn also brings the excitement of the new school year. While returning to sports, mastering new subjects, and spending time with old and new friends is exhilarating, this season can also be a time of intense stress.
For those high school students who are especially ambitious, the school year presents the challenge of a very high stakes performance, one whose success will be measured by admission to a prized college. Not only are there classes to study for, but schedules are packed with a maximum number of subjects, a maximum number of Advanced Placement courses and a maximum number of impressive extra-curricular activities. Varsity sports practice, SAT prep, Debate Club, volunteer hours, and on and on.
What is often missing is enough time for sleep, socializing, exploring new interests, and unwinding. When you hear your patients (or parents) describing the intense stress of their overloaded schedules compounded by a sense that “I have no choice,” you have an opportunity to complicate their thinking. Introduce the idea that there are smart approaches to performing your best under stress. Pushing themselves relentlessly will inevitably lead to burnout and exhaustion. This approach will help them learn to make wise choices and will better serve their healthy development.
Start by acknowledging the stress of high-stakes performance. Telling your patients that they need to lower the temperature by not putting so much pressure on themselves is likely to be experienced as a lack of confidence in them and is unlikely to get any traction. Instead, ask your patients what matters to them the most: Is it admission to the college of their choice? Achieving a certain score or GPA? Is it their competitiveness and drive to win? There is no wrong answer, but it is helpful for them to be able to reflect on what matters to them. Are they hoping to impress someone else? Are they worried about their future financial health and convinced that getting into a certain college will secure their financial success? Do they think this matters more to their parents than to themselves? Or have they discovered an intense interest in theoretical physics and want to be able to study at Caltech? If their ambition is meaningfully connected to an authentic interest or to their emerging identity, their sense of purpose will be much deeper and able to sustain them.
Even with talent and a strong sense of purpose, performing well is very difficult and demanding. It is important to consider the cycle of performance as including preparation, performance itself, and effective rest and recovery, just as with athletic performance. Whether the performance is the SATs, an AP test, a debate or big game, there were probably hours of preparation for every hour of performance. Help them to consider the importance of this practice or preparation time, and how to use that time effectively. Are they able to work in environments where there are few distractions? Do they have the support or useful feedback they need? How are they able to know when it is time for a break or when they are ready? It can be helpful for them to appreciate whether preparation or performance is more challenging for them, as the former requires focus and patience, while the latter requires courage and tenacity. If they are aware of which is harder for them, they can be thoughtful about how to effectively handle those challenges.
What can be most valuable for your patients is hearing from their pediatricians that they need to have time protected for rest and recharging, and not only for preparation and performance. Any athlete knows that failing to do so will lead to exhaustion and injury, and performance inevitably suffers. Rest is unwinding and slowing down, and a restful activity will leave them feeling calm, relaxed, and ready for sleep. A recharging activity is one that leaves them feeling refreshed and energized. Some common restful activities are a hot bath or shower, a distracting activity such as watching a show or surfing the web, playing a simple video game or puzzle or listening to music. Some recharging activities are creative ones (making art or music), engaging in hobbies, reading, or talking with a good friend. A few activities — sleep, exercise, and mindfulness meditation, are powerful in that they pack both rest and recharge into the same activity. Your patients should be discovering and learning which activities they find restful or recharging. The college application process or preparing for a varsity tryout will both add stress and give them an opportunity to learn what rests and recharges them. They should aim to have a list of at least five effective strategies that they can turn to when it’s time to rest or to recharge. Help them turn their work ethic to building a deeper well of self-knowledge that will serve them when they face challenges in high school or when they are on their own in college. This time of stress can be a time of growth, too.
Of course, remind your patients that this is a critical time to focus on basic self-care: They need consistently adequate, restful sleep, good nutrition, and physical activity. They will benefit from regular time in nature and time spent with friends that nourish them. They can find ways to compound these activities: Go for a walk with a friend, eat dinner with family, play a relaxing game while enjoying music. Lastly, ask your patients what is the last new thing they tried. It is easy to become so focused on an ambitious project that there is no time for exploration and play. Play is important throughout life, but adolescents are actively discovering their interests, talents, tastes, and values. To do this they need to be trying things that are new and maybe less purpose-driven. I call this type of activity “senseless fun.” Splashing in the pool is senseless fun, swimming laps is purposeful exercise that my contribute to recharging, and competing in a swim meet is often more on the stressful side. As they discover new talents, deeply engaging interests, what relaxes and recharges them, they will be learning who they are. Regardless of the outcome of a test, a big game, or where they go to college, it is this emerging knowledge about themselves that will carry them into adulthood. The pediatrician’s goal: Encouraging aspiration, exploration, and self-awareness in the context of giving permission for rest, recharging, and senseless fun.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].