User login
Meet Our 10 Editorial Fellows
We are excited to announce the 2024-25 participants, who will gain hands-on experience and mentorship working closely with the editors and staff at the AGA journals over the next year.
The 10 editorial fellows (2 per journal) will learn about the entire editorial process, from manuscript submission to peer review to acceptance. They will participate in discussions and conferences with the boards of editors, assist with manuscript review, and help disseminate articles via their social media platforms.

Clinical Gastroenterology and Hepatology
Robyn Jordan, MD, MPH
Johns Hopkins Hospital, Baltimore | USA
Daryl Ramai, MD, MPH, MSc
Brigham and Women’s Hospital, Boston | USA
Cellular and Molecular Gastroenterology and Hepatology
Kole H. Buckley, PhD
University of Pennsylvania Perelman School of Medicine, Philadelphia | USA
Lin Y. Hung, PhD
New York University | USA
Gastroenterology
Corey J. Ketchem, MD
University of Pennsylvania, Philadelphia | USA
Rishad Khan, MD
University of Toronto | Canada
Gastro Hep Advances
Sasha Kapil, MD
UT Southwestern Medical Center, Dallas | USA
June Tome, MD
Mayo Clinic, Rochester, Minnesota | USA
Techniques and Innovations in Gastrointestinal Endoscopy
Thomas Enke, MD
University of Colorado, Aurora | USA
Sami Elamin, MD
Harvard, Beth Israel Deaconess Medical Center, Boston | USA
We are excited to announce the 2024-25 participants, who will gain hands-on experience and mentorship working closely with the editors and staff at the AGA journals over the next year.
The 10 editorial fellows (2 per journal) will learn about the entire editorial process, from manuscript submission to peer review to acceptance. They will participate in discussions and conferences with the boards of editors, assist with manuscript review, and help disseminate articles via their social media platforms.

Clinical Gastroenterology and Hepatology
Robyn Jordan, MD, MPH
Johns Hopkins Hospital, Baltimore | USA
Daryl Ramai, MD, MPH, MSc
Brigham and Women’s Hospital, Boston | USA
Cellular and Molecular Gastroenterology and Hepatology
Kole H. Buckley, PhD
University of Pennsylvania Perelman School of Medicine, Philadelphia | USA
Lin Y. Hung, PhD
New York University | USA
Gastroenterology
Corey J. Ketchem, MD
University of Pennsylvania, Philadelphia | USA
Rishad Khan, MD
University of Toronto | Canada
Gastro Hep Advances
Sasha Kapil, MD
UT Southwestern Medical Center, Dallas | USA
June Tome, MD
Mayo Clinic, Rochester, Minnesota | USA
Techniques and Innovations in Gastrointestinal Endoscopy
Thomas Enke, MD
University of Colorado, Aurora | USA
Sami Elamin, MD
Harvard, Beth Israel Deaconess Medical Center, Boston | USA
We are excited to announce the 2024-25 participants, who will gain hands-on experience and mentorship working closely with the editors and staff at the AGA journals over the next year.
The 10 editorial fellows (2 per journal) will learn about the entire editorial process, from manuscript submission to peer review to acceptance. They will participate in discussions and conferences with the boards of editors, assist with manuscript review, and help disseminate articles via their social media platforms.

Clinical Gastroenterology and Hepatology
Robyn Jordan, MD, MPH
Johns Hopkins Hospital, Baltimore | USA
Daryl Ramai, MD, MPH, MSc
Brigham and Women’s Hospital, Boston | USA
Cellular and Molecular Gastroenterology and Hepatology
Kole H. Buckley, PhD
University of Pennsylvania Perelman School of Medicine, Philadelphia | USA
Lin Y. Hung, PhD
New York University | USA
Gastroenterology
Corey J. Ketchem, MD
University of Pennsylvania, Philadelphia | USA
Rishad Khan, MD
University of Toronto | Canada
Gastro Hep Advances
Sasha Kapil, MD
UT Southwestern Medical Center, Dallas | USA
June Tome, MD
Mayo Clinic, Rochester, Minnesota | USA
Techniques and Innovations in Gastrointestinal Endoscopy
Thomas Enke, MD
University of Colorado, Aurora | USA
Sami Elamin, MD
Harvard, Beth Israel Deaconess Medical Center, Boston | USA
Creating a Planned Gift That’s Meaningful To You
The AGA Research Foundation has helped make significant strides in advancing the treatment and cure of digestive diseases by funding talented investigators.
Planning your gift to benefit AGA Research Foundation in the future is an opportunity to express what matters to you. As an AGA member, you can work with the AGA Research Foundation to ensure that your planned gift is designated for a purpose that meets your goals for leaving a legacy—such as research awards, support for specific programs, or unrestricted gifts to help meet the Foundation’s mission.
In as little as one sentence in your will and/or trust, you can complete your gift: “I give to AGA Research Foundation, a nonprofit corporation currently located at 4930 Del Ray Avenue, Bethesda, MD 20814, or its successor thereto, _________ [written amount or percentage of the estate or description of property] for its unrestricted charitable use and purpose.”
If you have named the AGA Research Foundation in your will or trust, please let us know so we can ensure that your gift is used according to your wishes. Notifying us of your plans will enable us to plan for the use of your future gift. However, if you prefer to remain anonymous, we will keep your name and gift in strict confidence.
Please contact [email protected] for more information. If you are considering a planned gift, consult with your own legal and tax advisors.
The AGA Research Foundation has helped make significant strides in advancing the treatment and cure of digestive diseases by funding talented investigators.
Planning your gift to benefit AGA Research Foundation in the future is an opportunity to express what matters to you. As an AGA member, you can work with the AGA Research Foundation to ensure that your planned gift is designated for a purpose that meets your goals for leaving a legacy—such as research awards, support for specific programs, or unrestricted gifts to help meet the Foundation’s mission.
In as little as one sentence in your will and/or trust, you can complete your gift: “I give to AGA Research Foundation, a nonprofit corporation currently located at 4930 Del Ray Avenue, Bethesda, MD 20814, or its successor thereto, _________ [written amount or percentage of the estate or description of property] for its unrestricted charitable use and purpose.”
If you have named the AGA Research Foundation in your will or trust, please let us know so we can ensure that your gift is used according to your wishes. Notifying us of your plans will enable us to plan for the use of your future gift. However, if you prefer to remain anonymous, we will keep your name and gift in strict confidence.
Please contact [email protected] for more information. If you are considering a planned gift, consult with your own legal and tax advisors.
The AGA Research Foundation has helped make significant strides in advancing the treatment and cure of digestive diseases by funding talented investigators.
Planning your gift to benefit AGA Research Foundation in the future is an opportunity to express what matters to you. As an AGA member, you can work with the AGA Research Foundation to ensure that your planned gift is designated for a purpose that meets your goals for leaving a legacy—such as research awards, support for specific programs, or unrestricted gifts to help meet the Foundation’s mission.
In as little as one sentence in your will and/or trust, you can complete your gift: “I give to AGA Research Foundation, a nonprofit corporation currently located at 4930 Del Ray Avenue, Bethesda, MD 20814, or its successor thereto, _________ [written amount or percentage of the estate or description of property] for its unrestricted charitable use and purpose.”
If you have named the AGA Research Foundation in your will or trust, please let us know so we can ensure that your gift is used according to your wishes. Notifying us of your plans will enable us to plan for the use of your future gift. However, if you prefer to remain anonymous, we will keep your name and gift in strict confidence.
Please contact [email protected] for more information. If you are considering a planned gift, consult with your own legal and tax advisors.
Study Questions Relationship Between Crohn’s Strictures and Cancer Risk
, according to investigators.
Although 8% of patients with strictures in a multicenter study were diagnosed with CRC, this diagnosis was made either simultaneously or within 1 year of stricture diagnosis, suggesting that cancer may have driven stricture development, and not the other way around, lead author Thomas Hunaut, MD, of Université de Champagne-Ardenne, Reims, France, and colleagues reported.
“The occurrence of colonic stricture in CD always raises concerns about the risk for dysplasia/cancer,” the investigators wrote in Gastro Hep Advances, noting that no consensus approach is currently available to guide stricture management. “Few studies with conflicting results have evaluated the frequency of CRC associated with colonic stricture in CD, and the natural history of colonic stricture in CD is poorly known.”The present retrospective study included 88 consecutive CD patients with 96 colorectal strictures who were managed at three French referral centers between 1993 and 2022.
Strictures were symptomatic in 62.5% of cases, not passable by scope in 61.4% of cases, and ulcerated in 70.5% of cases. Colonic resection was needed in 47.7% of patients, while endoscopic balloon dilation was performed in 13.6% of patients.
After a median follow-up of 21.5 months, seven patients (8%) were diagnosed with malignant stricture, including five cases of colonic adenocarcinoma, one case of neuroendocrine carcinoma, and one case of B-cell lymphoproliferative neoplasia.
Malignant strictures were more common among older patients with longer disease duration and frequent obstructive symptoms; however, these factors were not supported by multivariate analyses, likely due to sample size, according to the investigators.
Instead, Dr. Hunaut and colleagues highlighted the timing of the diagnoses. In four out of seven patients with malignant stricture, both stricture and cancer were diagnosed at the same time. In the remaining three patients, cancer was diagnosed at 3 months, 8 months, and 12 months after stricture diagnosis. No cases of cancer were diagnosed later than 1 year after the stricture diagnosis.
“We believe that this result is important for the management of colonic strictures complicating CD in clinical practice,” Dr. Hunaut and colleagues wrote.
The simultaneity or proximity of the diagnoses suggests that the “strictures observed are already a neoplastic complication of the colonic inflammatory disease,” they explained.
In other words, common concerns about strictures causing cancer at the same site could be unfounded.
This conclusion echoes a recent administrative database study that reported no independent association between colorectal stricture and CRC, the investigators noted.
“Given the recent evidence on the risk of cancer associated with colonic strictures in CD, systematic colectomy is probably no longer justified,” they wrote. “Factors such as a long disease duration, primary sclerosing cholangitis, a history of dysplasia, and nonpassable and/or symptomatic stricture despite endoscopic dilation tend to argue in favor of surgery — especially if limited resection is possible.”
In contrast, patients with strictures who have low risk of CRC may be better served by a conservative approach, including endoscopy and systematic biopsies, followed by close endoscopic surveillance, according to the investigators. If the stricture is impassable, they recommended endoscopic balloon dilation, followed by intensification of medical therapy if ulceration is observed.
The investigators disclosed relationships with MSD, Ferring, Biogen, and others.
, according to investigators.
Although 8% of patients with strictures in a multicenter study were diagnosed with CRC, this diagnosis was made either simultaneously or within 1 year of stricture diagnosis, suggesting that cancer may have driven stricture development, and not the other way around, lead author Thomas Hunaut, MD, of Université de Champagne-Ardenne, Reims, France, and colleagues reported.
“The occurrence of colonic stricture in CD always raises concerns about the risk for dysplasia/cancer,” the investigators wrote in Gastro Hep Advances, noting that no consensus approach is currently available to guide stricture management. “Few studies with conflicting results have evaluated the frequency of CRC associated with colonic stricture in CD, and the natural history of colonic stricture in CD is poorly known.”The present retrospective study included 88 consecutive CD patients with 96 colorectal strictures who were managed at three French referral centers between 1993 and 2022.
Strictures were symptomatic in 62.5% of cases, not passable by scope in 61.4% of cases, and ulcerated in 70.5% of cases. Colonic resection was needed in 47.7% of patients, while endoscopic balloon dilation was performed in 13.6% of patients.
After a median follow-up of 21.5 months, seven patients (8%) were diagnosed with malignant stricture, including five cases of colonic adenocarcinoma, one case of neuroendocrine carcinoma, and one case of B-cell lymphoproliferative neoplasia.
Malignant strictures were more common among older patients with longer disease duration and frequent obstructive symptoms; however, these factors were not supported by multivariate analyses, likely due to sample size, according to the investigators.
Instead, Dr. Hunaut and colleagues highlighted the timing of the diagnoses. In four out of seven patients with malignant stricture, both stricture and cancer were diagnosed at the same time. In the remaining three patients, cancer was diagnosed at 3 months, 8 months, and 12 months after stricture diagnosis. No cases of cancer were diagnosed later than 1 year after the stricture diagnosis.
“We believe that this result is important for the management of colonic strictures complicating CD in clinical practice,” Dr. Hunaut and colleagues wrote.
The simultaneity or proximity of the diagnoses suggests that the “strictures observed are already a neoplastic complication of the colonic inflammatory disease,” they explained.
In other words, common concerns about strictures causing cancer at the same site could be unfounded.
This conclusion echoes a recent administrative database study that reported no independent association between colorectal stricture and CRC, the investigators noted.
“Given the recent evidence on the risk of cancer associated with colonic strictures in CD, systematic colectomy is probably no longer justified,” they wrote. “Factors such as a long disease duration, primary sclerosing cholangitis, a history of dysplasia, and nonpassable and/or symptomatic stricture despite endoscopic dilation tend to argue in favor of surgery — especially if limited resection is possible.”
In contrast, patients with strictures who have low risk of CRC may be better served by a conservative approach, including endoscopy and systematic biopsies, followed by close endoscopic surveillance, according to the investigators. If the stricture is impassable, they recommended endoscopic balloon dilation, followed by intensification of medical therapy if ulceration is observed.
The investigators disclosed relationships with MSD, Ferring, Biogen, and others.
, according to investigators.
Although 8% of patients with strictures in a multicenter study were diagnosed with CRC, this diagnosis was made either simultaneously or within 1 year of stricture diagnosis, suggesting that cancer may have driven stricture development, and not the other way around, lead author Thomas Hunaut, MD, of Université de Champagne-Ardenne, Reims, France, and colleagues reported.
“The occurrence of colonic stricture in CD always raises concerns about the risk for dysplasia/cancer,” the investigators wrote in Gastro Hep Advances, noting that no consensus approach is currently available to guide stricture management. “Few studies with conflicting results have evaluated the frequency of CRC associated with colonic stricture in CD, and the natural history of colonic stricture in CD is poorly known.”The present retrospective study included 88 consecutive CD patients with 96 colorectal strictures who were managed at three French referral centers between 1993 and 2022.
Strictures were symptomatic in 62.5% of cases, not passable by scope in 61.4% of cases, and ulcerated in 70.5% of cases. Colonic resection was needed in 47.7% of patients, while endoscopic balloon dilation was performed in 13.6% of patients.
After a median follow-up of 21.5 months, seven patients (8%) were diagnosed with malignant stricture, including five cases of colonic adenocarcinoma, one case of neuroendocrine carcinoma, and one case of B-cell lymphoproliferative neoplasia.
Malignant strictures were more common among older patients with longer disease duration and frequent obstructive symptoms; however, these factors were not supported by multivariate analyses, likely due to sample size, according to the investigators.
Instead, Dr. Hunaut and colleagues highlighted the timing of the diagnoses. In four out of seven patients with malignant stricture, both stricture and cancer were diagnosed at the same time. In the remaining three patients, cancer was diagnosed at 3 months, 8 months, and 12 months after stricture diagnosis. No cases of cancer were diagnosed later than 1 year after the stricture diagnosis.
“We believe that this result is important for the management of colonic strictures complicating CD in clinical practice,” Dr. Hunaut and colleagues wrote.
The simultaneity or proximity of the diagnoses suggests that the “strictures observed are already a neoplastic complication of the colonic inflammatory disease,” they explained.
In other words, common concerns about strictures causing cancer at the same site could be unfounded.
This conclusion echoes a recent administrative database study that reported no independent association between colorectal stricture and CRC, the investigators noted.
“Given the recent evidence on the risk of cancer associated with colonic strictures in CD, systematic colectomy is probably no longer justified,” they wrote. “Factors such as a long disease duration, primary sclerosing cholangitis, a history of dysplasia, and nonpassable and/or symptomatic stricture despite endoscopic dilation tend to argue in favor of surgery — especially if limited resection is possible.”
In contrast, patients with strictures who have low risk of CRC may be better served by a conservative approach, including endoscopy and systematic biopsies, followed by close endoscopic surveillance, according to the investigators. If the stricture is impassable, they recommended endoscopic balloon dilation, followed by intensification of medical therapy if ulceration is observed.
The investigators disclosed relationships with MSD, Ferring, Biogen, and others.
FROM GASTRO HEP ADVANCES
Navigating Ethical and Clinical Considerations Relating to Percutaneous Gastrostomy (PEG) Tubes
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
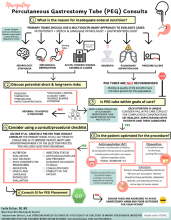
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
ANA Testing: When to Tap the Brakes
This transcript has been edited for clarity.
There are five reasons you do not want to order that notorious antinuclear antibody (ANA) test — when a patient comes into your office and you say, “Let’s just run a wellness check” and you order the ANA test, or the patient comes in and says, “Hey doc, order everything, okay?” — without really thinking these things through.
1. I’m sure you know that the ANA test, if positive, does not exclude other conditions. For instance, older women could have a positive ANA test; it’s very common in this group.
2. There’s a high false-positive rate for an ANA test. For instance, cancers and viral infections can cause an ANA test to be positive, and certain medications can cause a false-positive ANA test.
3. Context matters. If you have a patient that has particular symptoms, joint swelling, a strong family history of autoimmune disease, a luminal rash that you can’t understand, hair loss, those kind of things, then yes, when you order that ANA test, it’s going to be valuable. If the patient does not have those symptoms, you are just running down this rabbit hole that causes worry for you and your patient.
4. The ANA test on its own is not helpful until you order the subtypes. Double-stranded DNA and anti-SSA or anti-SSB antibodies are just a few examples of the subtypes of the ANA test that really help you understand what you ordered.
5. The elephant in the room: What is the pretest probability of your diagnostic test — all the symptoms, the hair loss, the malar rash, the sores in the mouth, the joint swelling, the blood in the urine? Fluid around the heart, pericarditis, pleurisy, those kinds of symptoms, right? When you have those symptoms and you order an ANA test, then you have basically put directions into your GPS. So now you know that if the test is positive, these are the things you’re going to do with the test going forward.
I hope that these five things have told you: Hey, before you order that ANA test, let’s make sure that we’re not causing unnecessary stress for our patients and also minimizing unnecessary testing.
Dr. Dada, CEO, Overlake Arthritis and Osteoporosis Center, Bellevue, Washington, disclosed ties with Horizon Pharmaceuticals.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There are five reasons you do not want to order that notorious antinuclear antibody (ANA) test — when a patient comes into your office and you say, “Let’s just run a wellness check” and you order the ANA test, or the patient comes in and says, “Hey doc, order everything, okay?” — without really thinking these things through.
1. I’m sure you know that the ANA test, if positive, does not exclude other conditions. For instance, older women could have a positive ANA test; it’s very common in this group.
2. There’s a high false-positive rate for an ANA test. For instance, cancers and viral infections can cause an ANA test to be positive, and certain medications can cause a false-positive ANA test.
3. Context matters. If you have a patient that has particular symptoms, joint swelling, a strong family history of autoimmune disease, a luminal rash that you can’t understand, hair loss, those kind of things, then yes, when you order that ANA test, it’s going to be valuable. If the patient does not have those symptoms, you are just running down this rabbit hole that causes worry for you and your patient.
4. The ANA test on its own is not helpful until you order the subtypes. Double-stranded DNA and anti-SSA or anti-SSB antibodies are just a few examples of the subtypes of the ANA test that really help you understand what you ordered.
5. The elephant in the room: What is the pretest probability of your diagnostic test — all the symptoms, the hair loss, the malar rash, the sores in the mouth, the joint swelling, the blood in the urine? Fluid around the heart, pericarditis, pleurisy, those kinds of symptoms, right? When you have those symptoms and you order an ANA test, then you have basically put directions into your GPS. So now you know that if the test is positive, these are the things you’re going to do with the test going forward.
I hope that these five things have told you: Hey, before you order that ANA test, let’s make sure that we’re not causing unnecessary stress for our patients and also minimizing unnecessary testing.
Dr. Dada, CEO, Overlake Arthritis and Osteoporosis Center, Bellevue, Washington, disclosed ties with Horizon Pharmaceuticals.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There are five reasons you do not want to order that notorious antinuclear antibody (ANA) test — when a patient comes into your office and you say, “Let’s just run a wellness check” and you order the ANA test, or the patient comes in and says, “Hey doc, order everything, okay?” — without really thinking these things through.
1. I’m sure you know that the ANA test, if positive, does not exclude other conditions. For instance, older women could have a positive ANA test; it’s very common in this group.
2. There’s a high false-positive rate for an ANA test. For instance, cancers and viral infections can cause an ANA test to be positive, and certain medications can cause a false-positive ANA test.
3. Context matters. If you have a patient that has particular symptoms, joint swelling, a strong family history of autoimmune disease, a luminal rash that you can’t understand, hair loss, those kind of things, then yes, when you order that ANA test, it’s going to be valuable. If the patient does not have those symptoms, you are just running down this rabbit hole that causes worry for you and your patient.
4. The ANA test on its own is not helpful until you order the subtypes. Double-stranded DNA and anti-SSA or anti-SSB antibodies are just a few examples of the subtypes of the ANA test that really help you understand what you ordered.
5. The elephant in the room: What is the pretest probability of your diagnostic test — all the symptoms, the hair loss, the malar rash, the sores in the mouth, the joint swelling, the blood in the urine? Fluid around the heart, pericarditis, pleurisy, those kinds of symptoms, right? When you have those symptoms and you order an ANA test, then you have basically put directions into your GPS. So now you know that if the test is positive, these are the things you’re going to do with the test going forward.
I hope that these five things have told you: Hey, before you order that ANA test, let’s make sure that we’re not causing unnecessary stress for our patients and also minimizing unnecessary testing.
Dr. Dada, CEO, Overlake Arthritis and Osteoporosis Center, Bellevue, Washington, disclosed ties with Horizon Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Diabetes Increases Injury Risk: A Troubling Trend
In 2024, a record number of people are celebrating their 65th birthdays. Increasing age is associated with a higher risk for falls, fractures, and other injuries that may require hospitalization.
In older adults with type 1 and type 2 diabetes, the risk for falls is double that seen in older people without these conditions. Increased clinician awareness of the many factors that result in this higher risk in people with diabetes, and timely implementation of strategies to prevent falls, are essential.
The annual incidence of falls in people with diabetes older than 65 years is about 39%, compared with 19% among those without diabetes. People with diabetes on insulin face an even greater increased risk for falls compared with those who are not using insulin (94% vs 27% increased risk).
Many well-known aspects of diabetes contribute to this greater risk. These include decreased sensorimotor function, musculoskeletal and neuromuscular deficits, foot and body pain, poor vision, hypoglycemic episodes, pharmacologic complications, and problems with hearing and balance.
Optimal management of diabetes and its complications is essential, and the American Diabetes Association has developed clear guidelines for clinicians to follow to reduce the risk for diabetes related complications and manage these conditions.
The prevalence of diabetic peripheral neuropathy increases with age and duration of diabetes. People with diabetic peripheral neuropathy and diminished sensation on their feet are at increased risk for loss of postural control. Loss of proprioceptive feedback (the ability to sense movement, action and location) during standing and walking leads increases the risk for falls.
In addition, less physical activity, impaired muscle strength, and suboptimal postural control all influence gait patterns and increase the risk for falling. Adults with diabetes have a two to three times higher risk for sarcopenia (decreased muscle strength and muscle mass). They also have low plantar flexion strength, causing increased displacement of their center of gravity, which in turn reduces their maximum forward stride and may result in falls and injury.
Many people with diabetes experience neuropathic foot and body pain, requiring psychotropic and other medications that may exacerbate the risk, such as amitriptyline and duloxetine. Furthermore, older adults with diabetes are more likely to take more prescription medications and may be more sensitive to effects of multiple medications than are individuals without diabetes.
A hazard of managing diabetes, particularly with insulin, is the increased risk for unexpected low blood glucose levels. These episodes can also occur in patients taking certain kinds of oral diabetes medications, but they are more common in those on insulin. Low blood glucose can cause dizziness, confusion, and postural instability, increasing the risk for falling.
Diabetic eye complications include retinopathy, macular edema, cataracts, and glaucoma. In a study of close to 10,000 middle-aged and older adults with diabetes, those with moderate eye complications had almost double the risk of falls as those without eye complications.
Another concern with diabetes is its effect on nerves and blood vessels in the inner ear, leading to a negative effect on balance and hearing loss, both of which are also associated with a higher risk for falling and injury.
Clinicians can reduce the risk for falls in patients by taking measures to improve diabetes control and reduce the risk for microvascular disease affecting the nerves, eyes, and ears.
In addition, exercises that optimize muscle mass, bone strength, gait, and balance, and use of specialized footwear in people with neuropathy, may reduce fall risk. Chair yoga and tai chi have also been shown to be helpful. Clinicians can also advise patients on commonsense strategies to implement in their homes, such as ensuring proper lighting, reducing, clutter and minimizing the use of floor rugs.
The risk for falls and the associated risk for fracture and possible hospitalization are of significant concern in older adults — particularly those with diabetes, and even more so in those with diabetes who are on insulin. It is our responsibility as clinicians to implement strategies to optimize diabetes control in our patients and monitor them for microvascular and other complications that may increase this risk, and manage them appropriately if and when these complications occur.
Madhusmita Misra, Professor, Chair, Physician-in-Chief, Department of Pediatrics, University of Virginia and UVA Health Children’s, Charlottesville, has disclosed being a key opinion leader for Lumos Pharma. Sidhartha Pani, Assistant Professor, Department of Internal Medicine, UVA School of Medicine; Medical Director, Department of General Medicine, Same Day Care Clinic, Charlottesville, disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In 2024, a record number of people are celebrating their 65th birthdays. Increasing age is associated with a higher risk for falls, fractures, and other injuries that may require hospitalization.
In older adults with type 1 and type 2 diabetes, the risk for falls is double that seen in older people without these conditions. Increased clinician awareness of the many factors that result in this higher risk in people with diabetes, and timely implementation of strategies to prevent falls, are essential.
The annual incidence of falls in people with diabetes older than 65 years is about 39%, compared with 19% among those without diabetes. People with diabetes on insulin face an even greater increased risk for falls compared with those who are not using insulin (94% vs 27% increased risk).
Many well-known aspects of diabetes contribute to this greater risk. These include decreased sensorimotor function, musculoskeletal and neuromuscular deficits, foot and body pain, poor vision, hypoglycemic episodes, pharmacologic complications, and problems with hearing and balance.
Optimal management of diabetes and its complications is essential, and the American Diabetes Association has developed clear guidelines for clinicians to follow to reduce the risk for diabetes related complications and manage these conditions.
The prevalence of diabetic peripheral neuropathy increases with age and duration of diabetes. People with diabetic peripheral neuropathy and diminished sensation on their feet are at increased risk for loss of postural control. Loss of proprioceptive feedback (the ability to sense movement, action and location) during standing and walking leads increases the risk for falls.
In addition, less physical activity, impaired muscle strength, and suboptimal postural control all influence gait patterns and increase the risk for falling. Adults with diabetes have a two to three times higher risk for sarcopenia (decreased muscle strength and muscle mass). They also have low plantar flexion strength, causing increased displacement of their center of gravity, which in turn reduces their maximum forward stride and may result in falls and injury.
Many people with diabetes experience neuropathic foot and body pain, requiring psychotropic and other medications that may exacerbate the risk, such as amitriptyline and duloxetine. Furthermore, older adults with diabetes are more likely to take more prescription medications and may be more sensitive to effects of multiple medications than are individuals without diabetes.
A hazard of managing diabetes, particularly with insulin, is the increased risk for unexpected low blood glucose levels. These episodes can also occur in patients taking certain kinds of oral diabetes medications, but they are more common in those on insulin. Low blood glucose can cause dizziness, confusion, and postural instability, increasing the risk for falling.
Diabetic eye complications include retinopathy, macular edema, cataracts, and glaucoma. In a study of close to 10,000 middle-aged and older adults with diabetes, those with moderate eye complications had almost double the risk of falls as those without eye complications.
Another concern with diabetes is its effect on nerves and blood vessels in the inner ear, leading to a negative effect on balance and hearing loss, both of which are also associated with a higher risk for falling and injury.
Clinicians can reduce the risk for falls in patients by taking measures to improve diabetes control and reduce the risk for microvascular disease affecting the nerves, eyes, and ears.
In addition, exercises that optimize muscle mass, bone strength, gait, and balance, and use of specialized footwear in people with neuropathy, may reduce fall risk. Chair yoga and tai chi have also been shown to be helpful. Clinicians can also advise patients on commonsense strategies to implement in their homes, such as ensuring proper lighting, reducing, clutter and minimizing the use of floor rugs.
The risk for falls and the associated risk for fracture and possible hospitalization are of significant concern in older adults — particularly those with diabetes, and even more so in those with diabetes who are on insulin. It is our responsibility as clinicians to implement strategies to optimize diabetes control in our patients and monitor them for microvascular and other complications that may increase this risk, and manage them appropriately if and when these complications occur.
Madhusmita Misra, Professor, Chair, Physician-in-Chief, Department of Pediatrics, University of Virginia and UVA Health Children’s, Charlottesville, has disclosed being a key opinion leader for Lumos Pharma. Sidhartha Pani, Assistant Professor, Department of Internal Medicine, UVA School of Medicine; Medical Director, Department of General Medicine, Same Day Care Clinic, Charlottesville, disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In 2024, a record number of people are celebrating their 65th birthdays. Increasing age is associated with a higher risk for falls, fractures, and other injuries that may require hospitalization.
In older adults with type 1 and type 2 diabetes, the risk for falls is double that seen in older people without these conditions. Increased clinician awareness of the many factors that result in this higher risk in people with diabetes, and timely implementation of strategies to prevent falls, are essential.
The annual incidence of falls in people with diabetes older than 65 years is about 39%, compared with 19% among those without diabetes. People with diabetes on insulin face an even greater increased risk for falls compared with those who are not using insulin (94% vs 27% increased risk).
Many well-known aspects of diabetes contribute to this greater risk. These include decreased sensorimotor function, musculoskeletal and neuromuscular deficits, foot and body pain, poor vision, hypoglycemic episodes, pharmacologic complications, and problems with hearing and balance.
Optimal management of diabetes and its complications is essential, and the American Diabetes Association has developed clear guidelines for clinicians to follow to reduce the risk for diabetes related complications and manage these conditions.
The prevalence of diabetic peripheral neuropathy increases with age and duration of diabetes. People with diabetic peripheral neuropathy and diminished sensation on their feet are at increased risk for loss of postural control. Loss of proprioceptive feedback (the ability to sense movement, action and location) during standing and walking leads increases the risk for falls.
In addition, less physical activity, impaired muscle strength, and suboptimal postural control all influence gait patterns and increase the risk for falling. Adults with diabetes have a two to three times higher risk for sarcopenia (decreased muscle strength and muscle mass). They also have low plantar flexion strength, causing increased displacement of their center of gravity, which in turn reduces their maximum forward stride and may result in falls and injury.
Many people with diabetes experience neuropathic foot and body pain, requiring psychotropic and other medications that may exacerbate the risk, such as amitriptyline and duloxetine. Furthermore, older adults with diabetes are more likely to take more prescription medications and may be more sensitive to effects of multiple medications than are individuals without diabetes.
A hazard of managing diabetes, particularly with insulin, is the increased risk for unexpected low blood glucose levels. These episodes can also occur in patients taking certain kinds of oral diabetes medications, but they are more common in those on insulin. Low blood glucose can cause dizziness, confusion, and postural instability, increasing the risk for falling.
Diabetic eye complications include retinopathy, macular edema, cataracts, and glaucoma. In a study of close to 10,000 middle-aged and older adults with diabetes, those with moderate eye complications had almost double the risk of falls as those without eye complications.
Another concern with diabetes is its effect on nerves and blood vessels in the inner ear, leading to a negative effect on balance and hearing loss, both of which are also associated with a higher risk for falling and injury.
Clinicians can reduce the risk for falls in patients by taking measures to improve diabetes control and reduce the risk for microvascular disease affecting the nerves, eyes, and ears.
In addition, exercises that optimize muscle mass, bone strength, gait, and balance, and use of specialized footwear in people with neuropathy, may reduce fall risk. Chair yoga and tai chi have also been shown to be helpful. Clinicians can also advise patients on commonsense strategies to implement in their homes, such as ensuring proper lighting, reducing, clutter and minimizing the use of floor rugs.
The risk for falls and the associated risk for fracture and possible hospitalization are of significant concern in older adults — particularly those with diabetes, and even more so in those with diabetes who are on insulin. It is our responsibility as clinicians to implement strategies to optimize diabetes control in our patients and monitor them for microvascular and other complications that may increase this risk, and manage them appropriately if and when these complications occur.
Madhusmita Misra, Professor, Chair, Physician-in-Chief, Department of Pediatrics, University of Virginia and UVA Health Children’s, Charlottesville, has disclosed being a key opinion leader for Lumos Pharma. Sidhartha Pani, Assistant Professor, Department of Internal Medicine, UVA School of Medicine; Medical Director, Department of General Medicine, Same Day Care Clinic, Charlottesville, disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What Do We Know About Postoperative Cognitive Dysfunction?
Postoperative cognitive dysfunction (POCD) is a form of cognitive decline that involves a functional deterioration of activities of the nervous system, such as selective attention, vigilance, perception, learning, memory, executive function, verbal and language abilities, emotion, visuospatial and visuomotor skills. It occurs in the absence of cranial trauma or other brain injuries, and prevalence rates range from 36.6% in young adults to 42.4% in older adults, as a consequence of significant invasive procedures such as cardiac, noncardiac, and carotid surgeries that are lengthy and intensive.
Alzheimer’s disease (AD), the most common form of dementia, accounts for about two thirds of all cases of dementia globally. It is estimated that 41 million patients with dementia remain undiagnosed worldwide, and 25% of patients are diagnosed only when they are fully symptomatic. AD is a neurodegenerative disorder defined by neuropathologic changes, including beta-amyloid (Abeta) plaques composed of aggregated Abeta and neurofibrillary tangles containing aggregated tau proteins.
Patients with AD are unaware of their condition. Dementia, especially in its early stages, is often a hidden disease. Even when suspected, patients and families may believe that the symptoms are part of normal aging and may not report them to the doctor. In these patients, surgery may unmask subclinical dementia.
The complex correlation between POCD and AD has sparked debate following numerous anecdotal reports of how older adults undergoing surgical procedures may experience long-term cognitive decline with clinical characteristics such as those of patients with dementia. Despite advances in knowledge, it is still difficult to establish a priori how much surgery and anesthesia can increase the risk or accelerate the progression of a prodromal and asymptomatic AD condition (stages I-II) to clinically evident stage III AD. The current trend of an aging population poses a challenge for anesthesiology surgery because as the age of patients undergoing surgery increases, so does the likelihood of developing POCD.
Recent research in these fields has improved knowledge of the characteristics, epidemiology, risk factors, pathogenesis, and potential prevention strategies associated with POCD. It has improved the perspectives of future prevention and treatment.
Definition and Diagnostic Criteria
POCD, according to the cognitive impairment classification in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, is characterized by mild neurologic disturbance resulting from routine surgical procedures, excluding conditions such as deafness, dementia, or amnesia. The definition of POCD involves prolonged cognitive decline that can last for weeks, months, or even years. POCD may be confused with postoperative delirium, an acute and fluctuating disorder of consciousness that typically occurs within 3 days of surgery.
The diagnosis of POCD is based primarily on neurocognitive function scales. Widely used assessments include the Montreal Cognitive Assessment, the Wechsler Memory Scale, and the Mini-Mental State Examination.
Epidemiology
POCD is prevalent among patients undergoing cardiac or orthopedic surgery. In patients undergoing aortic-coronary bypass and cardiopulmonary bypass, 50%-70% develop POCD 1 week after surgery. In addition, 10%-30% experience long-term effects on cognitive function at 6 months after the procedure. In patients undergoing hip arthroplasty, 20%-50% exhibit POCD within 1 week of surgery, with 10%-14% still presenting it after 3 months.
Risk Factors
Age
POCD is typically observed in patients older than 65 years. However, after surgery, around 30% of younger patients and about 40% of older patients develop POCD at the time of hospital discharge. Specifically, 12.7% of older patients continue to have POCD 3 months after surgery, compared with 5% of younger patients.
Type of Surgery
Hip and knee arthroplasty procedures entail a higher risk for POCD than general surgery. The same is true of cardiac surgery, especially aortic-coronary bypass and cardiopulmonary bypass.
Types of Anesthesia
Initial assessments of postoperative cognitive function in cardiac surgery did not provide significant correlations between observed changes and the type of anesthesia because of the high number of confounding factors involved. A more recent meta-analysis of 28 randomized clinical trials concluded that the incidence of POCD is lower in surgeries using intravenous anesthesia with propofol than in those using inhalation anesthesia with isoflurane or sevoflurane.
Pain
Postoperative pain is a common issue, mainly resulting from substantial surgical trauma or potential wound infection. Patient-controlled postoperative analgesia independently increases the risk for POCD, compared with oral postoperative analgesia. Meta-analyses indicate that persistent pain can lead to a decline in patients’ cognitive abilities, attention, memory, and information processing.
Evolving Scenarios
Current research on POCD has deepened our understanding of its pathogenesis, implicating factors such as central nervous system inflammation, neuronal apoptosis, synaptic plasticity damage, abnormal tau protein modification, chronic pain, and mitochondrial metabolic disorders. Several neuroprotective drugs are currently under study, but none have shown consistent benefits for the prevention and treatment of POCD. The available evidence on the subject does not unambiguously guide the practicing physician. But neither does it exclude the importance of a careful assessment of POCD risk factors and the cognitive status of an older patient before surgery to provide useful information to the patient, family, and doctors when deciding on appropriate and shared procedures.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Postoperative cognitive dysfunction (POCD) is a form of cognitive decline that involves a functional deterioration of activities of the nervous system, such as selective attention, vigilance, perception, learning, memory, executive function, verbal and language abilities, emotion, visuospatial and visuomotor skills. It occurs in the absence of cranial trauma or other brain injuries, and prevalence rates range from 36.6% in young adults to 42.4% in older adults, as a consequence of significant invasive procedures such as cardiac, noncardiac, and carotid surgeries that are lengthy and intensive.
Alzheimer’s disease (AD), the most common form of dementia, accounts for about two thirds of all cases of dementia globally. It is estimated that 41 million patients with dementia remain undiagnosed worldwide, and 25% of patients are diagnosed only when they are fully symptomatic. AD is a neurodegenerative disorder defined by neuropathologic changes, including beta-amyloid (Abeta) plaques composed of aggregated Abeta and neurofibrillary tangles containing aggregated tau proteins.
Patients with AD are unaware of their condition. Dementia, especially in its early stages, is often a hidden disease. Even when suspected, patients and families may believe that the symptoms are part of normal aging and may not report them to the doctor. In these patients, surgery may unmask subclinical dementia.
The complex correlation between POCD and AD has sparked debate following numerous anecdotal reports of how older adults undergoing surgical procedures may experience long-term cognitive decline with clinical characteristics such as those of patients with dementia. Despite advances in knowledge, it is still difficult to establish a priori how much surgery and anesthesia can increase the risk or accelerate the progression of a prodromal and asymptomatic AD condition (stages I-II) to clinically evident stage III AD. The current trend of an aging population poses a challenge for anesthesiology surgery because as the age of patients undergoing surgery increases, so does the likelihood of developing POCD.
Recent research in these fields has improved knowledge of the characteristics, epidemiology, risk factors, pathogenesis, and potential prevention strategies associated with POCD. It has improved the perspectives of future prevention and treatment.
Definition and Diagnostic Criteria
POCD, according to the cognitive impairment classification in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, is characterized by mild neurologic disturbance resulting from routine surgical procedures, excluding conditions such as deafness, dementia, or amnesia. The definition of POCD involves prolonged cognitive decline that can last for weeks, months, or even years. POCD may be confused with postoperative delirium, an acute and fluctuating disorder of consciousness that typically occurs within 3 days of surgery.
The diagnosis of POCD is based primarily on neurocognitive function scales. Widely used assessments include the Montreal Cognitive Assessment, the Wechsler Memory Scale, and the Mini-Mental State Examination.
Epidemiology
POCD is prevalent among patients undergoing cardiac or orthopedic surgery. In patients undergoing aortic-coronary bypass and cardiopulmonary bypass, 50%-70% develop POCD 1 week after surgery. In addition, 10%-30% experience long-term effects on cognitive function at 6 months after the procedure. In patients undergoing hip arthroplasty, 20%-50% exhibit POCD within 1 week of surgery, with 10%-14% still presenting it after 3 months.
Risk Factors
Age
POCD is typically observed in patients older than 65 years. However, after surgery, around 30% of younger patients and about 40% of older patients develop POCD at the time of hospital discharge. Specifically, 12.7% of older patients continue to have POCD 3 months after surgery, compared with 5% of younger patients.
Type of Surgery
Hip and knee arthroplasty procedures entail a higher risk for POCD than general surgery. The same is true of cardiac surgery, especially aortic-coronary bypass and cardiopulmonary bypass.
Types of Anesthesia
Initial assessments of postoperative cognitive function in cardiac surgery did not provide significant correlations between observed changes and the type of anesthesia because of the high number of confounding factors involved. A more recent meta-analysis of 28 randomized clinical trials concluded that the incidence of POCD is lower in surgeries using intravenous anesthesia with propofol than in those using inhalation anesthesia with isoflurane or sevoflurane.
Pain
Postoperative pain is a common issue, mainly resulting from substantial surgical trauma or potential wound infection. Patient-controlled postoperative analgesia independently increases the risk for POCD, compared with oral postoperative analgesia. Meta-analyses indicate that persistent pain can lead to a decline in patients’ cognitive abilities, attention, memory, and information processing.
Evolving Scenarios
Current research on POCD has deepened our understanding of its pathogenesis, implicating factors such as central nervous system inflammation, neuronal apoptosis, synaptic plasticity damage, abnormal tau protein modification, chronic pain, and mitochondrial metabolic disorders. Several neuroprotective drugs are currently under study, but none have shown consistent benefits for the prevention and treatment of POCD. The available evidence on the subject does not unambiguously guide the practicing physician. But neither does it exclude the importance of a careful assessment of POCD risk factors and the cognitive status of an older patient before surgery to provide useful information to the patient, family, and doctors when deciding on appropriate and shared procedures.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Postoperative cognitive dysfunction (POCD) is a form of cognitive decline that involves a functional deterioration of activities of the nervous system, such as selective attention, vigilance, perception, learning, memory, executive function, verbal and language abilities, emotion, visuospatial and visuomotor skills. It occurs in the absence of cranial trauma or other brain injuries, and prevalence rates range from 36.6% in young adults to 42.4% in older adults, as a consequence of significant invasive procedures such as cardiac, noncardiac, and carotid surgeries that are lengthy and intensive.
Alzheimer’s disease (AD), the most common form of dementia, accounts for about two thirds of all cases of dementia globally. It is estimated that 41 million patients with dementia remain undiagnosed worldwide, and 25% of patients are diagnosed only when they are fully symptomatic. AD is a neurodegenerative disorder defined by neuropathologic changes, including beta-amyloid (Abeta) plaques composed of aggregated Abeta and neurofibrillary tangles containing aggregated tau proteins.
Patients with AD are unaware of their condition. Dementia, especially in its early stages, is often a hidden disease. Even when suspected, patients and families may believe that the symptoms are part of normal aging and may not report them to the doctor. In these patients, surgery may unmask subclinical dementia.
The complex correlation between POCD and AD has sparked debate following numerous anecdotal reports of how older adults undergoing surgical procedures may experience long-term cognitive decline with clinical characteristics such as those of patients with dementia. Despite advances in knowledge, it is still difficult to establish a priori how much surgery and anesthesia can increase the risk or accelerate the progression of a prodromal and asymptomatic AD condition (stages I-II) to clinically evident stage III AD. The current trend of an aging population poses a challenge for anesthesiology surgery because as the age of patients undergoing surgery increases, so does the likelihood of developing POCD.
Recent research in these fields has improved knowledge of the characteristics, epidemiology, risk factors, pathogenesis, and potential prevention strategies associated with POCD. It has improved the perspectives of future prevention and treatment.
Definition and Diagnostic Criteria
POCD, according to the cognitive impairment classification in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, is characterized by mild neurologic disturbance resulting from routine surgical procedures, excluding conditions such as deafness, dementia, or amnesia. The definition of POCD involves prolonged cognitive decline that can last for weeks, months, or even years. POCD may be confused with postoperative delirium, an acute and fluctuating disorder of consciousness that typically occurs within 3 days of surgery.
The diagnosis of POCD is based primarily on neurocognitive function scales. Widely used assessments include the Montreal Cognitive Assessment, the Wechsler Memory Scale, and the Mini-Mental State Examination.
Epidemiology
POCD is prevalent among patients undergoing cardiac or orthopedic surgery. In patients undergoing aortic-coronary bypass and cardiopulmonary bypass, 50%-70% develop POCD 1 week after surgery. In addition, 10%-30% experience long-term effects on cognitive function at 6 months after the procedure. In patients undergoing hip arthroplasty, 20%-50% exhibit POCD within 1 week of surgery, with 10%-14% still presenting it after 3 months.
Risk Factors
Age
POCD is typically observed in patients older than 65 years. However, after surgery, around 30% of younger patients and about 40% of older patients develop POCD at the time of hospital discharge. Specifically, 12.7% of older patients continue to have POCD 3 months after surgery, compared with 5% of younger patients.
Type of Surgery
Hip and knee arthroplasty procedures entail a higher risk for POCD than general surgery. The same is true of cardiac surgery, especially aortic-coronary bypass and cardiopulmonary bypass.
Types of Anesthesia
Initial assessments of postoperative cognitive function in cardiac surgery did not provide significant correlations between observed changes and the type of anesthesia because of the high number of confounding factors involved. A more recent meta-analysis of 28 randomized clinical trials concluded that the incidence of POCD is lower in surgeries using intravenous anesthesia with propofol than in those using inhalation anesthesia with isoflurane or sevoflurane.
Pain
Postoperative pain is a common issue, mainly resulting from substantial surgical trauma or potential wound infection. Patient-controlled postoperative analgesia independently increases the risk for POCD, compared with oral postoperative analgesia. Meta-analyses indicate that persistent pain can lead to a decline in patients’ cognitive abilities, attention, memory, and information processing.
Evolving Scenarios
Current research on POCD has deepened our understanding of its pathogenesis, implicating factors such as central nervous system inflammation, neuronal apoptosis, synaptic plasticity damage, abnormal tau protein modification, chronic pain, and mitochondrial metabolic disorders. Several neuroprotective drugs are currently under study, but none have shown consistent benefits for the prevention and treatment of POCD. The available evidence on the subject does not unambiguously guide the practicing physician. But neither does it exclude the importance of a careful assessment of POCD risk factors and the cognitive status of an older patient before surgery to provide useful information to the patient, family, and doctors when deciding on appropriate and shared procedures.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The Surgeon General’s Advisory on Parental Mental Health: Implications for Pediatric Practice
As child psychiatrists and pediatricians, our mission extends beyond treating the physical health of children; it encompasses understanding the intricate web of factors that influence a child’s overall well-being. A recent advisory from U.S. Surgeon General Dr. Vivek Murthy has brought to light a critical issue that demands our attention: the declining mental health of parents and its profound impact on children. As providers who depend heavily on parental involvement to manage the needs of our pediatric patients, addressing parental mental health can be a crucial step in safeguarding the mental health of children.
The Surgeon General’s Advisory: A Call to Action
On August 28, 2024, the U.S. Surgeon General issued an advisory highlighting the significant stressors impacting parents and caregivers, and the broader implications for children’s mental health. The advisory emphasizes the bidirectional relationship between parental and child mental health, urging healthcare providers, policymakers, and communities to prioritize support for parents. It stresses that the mental health of parents is not only vital for their well-being but also plays a critical role in shaping the emotional and psychological development of their children.1
The Link Between Parental and Child Mental Health
Research shows that a parent’s mental health directly influences the child’s emotional and behavioral outcomes. Children of parents with untreated mental health conditions, such as depression, anxiety, trauma, or chronic stress, are at a significantly higher risk of developing similar conditions. This risk is mediated through various mechanisms, including genetic predisposition, compromised parent-child interactions, and exposure to adverse environments.
1. Parental Depression and Child Outcomes: Parental depression, particularly maternal depression, has been extensively studied and is strongly associated with emotional and behavioral problems in children. Children of depressed parents are more likely to experience anxiety, depression, and resulting academic difficulties. Depressed parents may struggle with consistent and positive parenting, which can disrupt the development of secure attachments and emotional regulation in children.2-4
2. Anxiety and Parenting Styles: Parental anxiety can influence parenting styles, often leading to overprotectiveness, inconsistency, or heightened criticism. These behaviors, in turn, can cultivate anxiety in children, creating a cycle that perpetuates mental health challenges across generations. Children raised in environments where anxiety is pervasive may learn to view the world as threatening, contributing to hypervigilance and stress.5
3. Impact of Chronic Stress on Parenting: Chronic stress experienced by parents, often due to financial hardship, lack of social support, or work-life imbalance, can impair their ability to engage in responsive and nurturing parenting. This, in turn, can affect children’s ability to meaningfully engage with parents to form secure attachments. Further, chronic stress can negatively impact the quality of parent-child interactions and fuel the cycle of rupture with limited opportunity for repair. The advisory stresses the need to address these systemic stressors as part of a broader public health strategy to support families.1
Implications for Pediatric Practice
Pediatricians are often the first point of contact for families navigating mental health challenges. The Surgeon General’s advisory highlights the need for pediatricians to adopt a holistic approach that considers the mental health of the entire family, not just the child. This can be challenging with the average follow-up appointment time of 16 minutes, though many of the recommendations take this logistical hurdle into consideration:
1. Screening for Parental Mental Health: Incorporating routine screening for parental mental health into pediatric visits can be a powerful tool. Questions about parental stress, depression (especially postpartum depression), and anxiety should be integrated into well-child visits, especially in families where children present with emotional or behavioral difficulties. By identifying at-risk parents early, timely referrals to mental health services can be secured.
2. Providing Resources and Referrals: Offering resources and referrals to parents who may be struggling can positively impact the entire family. This includes connecting families with mental health professionals, parenting support groups, or community resources that can alleviate stressors such as food insecurity or lack of childcare. Having a list of local mental health resources available in your practice can empower parents to seek the help they need.
3. Promoting Positive Parenting Practices: Guidance on positive parenting practices, stress management, and self-care can make a significant difference in the mental health of parents and their children. Workshops or educational materials on topics like mindfulness, managing work-life balance, and fostering healthy communication within the family can be valuable and high-yield additions to pediatric care.
4. Collaborative Care Models: Collaborative care models, where pediatricians work closely with child psychiatrists, psychologists, and social workers, can provide comprehensive support to families. This integrated approach ensures that both children and their parents receive the care they need, promoting better outcomes for the entire family unit.
Addressing Broader Systemic Issues
The advisory also calls for systemic changes that extend beyond the clinic. Policy changes such as expanding access to paid family leave, affordable childcare, and mental health services are essential to creating an environment where parents can thrive. As pediatricians, advocating for these changes at the local and national level can amplify the overall impact on families.
1. Advocating for Paid Family Leave: Paid family leave allows parents to bond with their children and attend to their own mental health needs without the added pressure of financial instability. Supporting policies that provide adequate paid leave can pave the way for a successful and healthy return to work and have long-term benefits for family health.
2. Expanding Mental Health Services: Increasing access to mental health services, especially in underserved communities, is crucial. Pediatricians can play a role by partnering with local mental health providers to offer integrated care within their practices or community settings.
3. Community Support Programs: The creation of community support programs that offer parenting classes, stress management workshops, and peer support groups can help reduce the isolation and stress that many parents feel. Pediatricians can collaborate with community organizations to promote these resources to families.
Conclusion
The Surgeon General’s advisory serves as a timely reminder of the interconnectedness of parental and child mental health. Pediatricians have a unique opportunity to influence not only the health of their pediatric patients, but also the well-being of their families. By recognizing and addressing the mental health needs of parents, we can break the cycle of stress and mental illness that affects so many families, ensuring a healthier future for the next generation.
Let us embrace this call to action and work together to create a supportive environment where all parents and children can thrive.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. American Hospital Association. Surgeon General Issues Advisory on Mental Health and Well-Being of Parents. American Hospital Association. 2024 Sep 4.
2. Goodman SH, Gotlib IH. Risk for Psychopathology in the Children of Depressed Mothers: A Developmental Model for Understanding Mechanisms of Transmission. Psychol Rev. 1999;106(3):458-490. doi: 10.1037/0033-295X.106.3.458.
3. Lovejoy MC et al. Maternal Depression and Parenting Behavior: A Meta-Analytic Review. Clin Psychol Rev. 2000;20(5):561-592. doi: 10.1016/s0272-7358(98)00100-7.
4. Weissman MM et al. Offspring of Depressed Parents: 20 Years Later. Am J Psychiatry. 2006;163(6):1001-1008. doi: 10.1176/ajp.2006.163.6.1001.
5. Smith KE, Pollak SD. Early Life Stress and Development: Potential Mechanisms for Adverse Outcomes. J Neurodev Disord. 2020;12(1):3-14. doi: 10.1186/s11689-020-09337-y.
As child psychiatrists and pediatricians, our mission extends beyond treating the physical health of children; it encompasses understanding the intricate web of factors that influence a child’s overall well-being. A recent advisory from U.S. Surgeon General Dr. Vivek Murthy has brought to light a critical issue that demands our attention: the declining mental health of parents and its profound impact on children. As providers who depend heavily on parental involvement to manage the needs of our pediatric patients, addressing parental mental health can be a crucial step in safeguarding the mental health of children.
The Surgeon General’s Advisory: A Call to Action
On August 28, 2024, the U.S. Surgeon General issued an advisory highlighting the significant stressors impacting parents and caregivers, and the broader implications for children’s mental health. The advisory emphasizes the bidirectional relationship between parental and child mental health, urging healthcare providers, policymakers, and communities to prioritize support for parents. It stresses that the mental health of parents is not only vital for their well-being but also plays a critical role in shaping the emotional and psychological development of their children.1
The Link Between Parental and Child Mental Health
Research shows that a parent’s mental health directly influences the child’s emotional and behavioral outcomes. Children of parents with untreated mental health conditions, such as depression, anxiety, trauma, or chronic stress, are at a significantly higher risk of developing similar conditions. This risk is mediated through various mechanisms, including genetic predisposition, compromised parent-child interactions, and exposure to adverse environments.
1. Parental Depression and Child Outcomes: Parental depression, particularly maternal depression, has been extensively studied and is strongly associated with emotional and behavioral problems in children. Children of depressed parents are more likely to experience anxiety, depression, and resulting academic difficulties. Depressed parents may struggle with consistent and positive parenting, which can disrupt the development of secure attachments and emotional regulation in children.2-4
2. Anxiety and Parenting Styles: Parental anxiety can influence parenting styles, often leading to overprotectiveness, inconsistency, or heightened criticism. These behaviors, in turn, can cultivate anxiety in children, creating a cycle that perpetuates mental health challenges across generations. Children raised in environments where anxiety is pervasive may learn to view the world as threatening, contributing to hypervigilance and stress.5
3. Impact of Chronic Stress on Parenting: Chronic stress experienced by parents, often due to financial hardship, lack of social support, or work-life imbalance, can impair their ability to engage in responsive and nurturing parenting. This, in turn, can affect children’s ability to meaningfully engage with parents to form secure attachments. Further, chronic stress can negatively impact the quality of parent-child interactions and fuel the cycle of rupture with limited opportunity for repair. The advisory stresses the need to address these systemic stressors as part of a broader public health strategy to support families.1
Implications for Pediatric Practice
Pediatricians are often the first point of contact for families navigating mental health challenges. The Surgeon General’s advisory highlights the need for pediatricians to adopt a holistic approach that considers the mental health of the entire family, not just the child. This can be challenging with the average follow-up appointment time of 16 minutes, though many of the recommendations take this logistical hurdle into consideration:
1. Screening for Parental Mental Health: Incorporating routine screening for parental mental health into pediatric visits can be a powerful tool. Questions about parental stress, depression (especially postpartum depression), and anxiety should be integrated into well-child visits, especially in families where children present with emotional or behavioral difficulties. By identifying at-risk parents early, timely referrals to mental health services can be secured.
2. Providing Resources and Referrals: Offering resources and referrals to parents who may be struggling can positively impact the entire family. This includes connecting families with mental health professionals, parenting support groups, or community resources that can alleviate stressors such as food insecurity or lack of childcare. Having a list of local mental health resources available in your practice can empower parents to seek the help they need.
3. Promoting Positive Parenting Practices: Guidance on positive parenting practices, stress management, and self-care can make a significant difference in the mental health of parents and their children. Workshops or educational materials on topics like mindfulness, managing work-life balance, and fostering healthy communication within the family can be valuable and high-yield additions to pediatric care.
4. Collaborative Care Models: Collaborative care models, where pediatricians work closely with child psychiatrists, psychologists, and social workers, can provide comprehensive support to families. This integrated approach ensures that both children and their parents receive the care they need, promoting better outcomes for the entire family unit.
Addressing Broader Systemic Issues
The advisory also calls for systemic changes that extend beyond the clinic. Policy changes such as expanding access to paid family leave, affordable childcare, and mental health services are essential to creating an environment where parents can thrive. As pediatricians, advocating for these changes at the local and national level can amplify the overall impact on families.
1. Advocating for Paid Family Leave: Paid family leave allows parents to bond with their children and attend to their own mental health needs without the added pressure of financial instability. Supporting policies that provide adequate paid leave can pave the way for a successful and healthy return to work and have long-term benefits for family health.
2. Expanding Mental Health Services: Increasing access to mental health services, especially in underserved communities, is crucial. Pediatricians can play a role by partnering with local mental health providers to offer integrated care within their practices or community settings.
3. Community Support Programs: The creation of community support programs that offer parenting classes, stress management workshops, and peer support groups can help reduce the isolation and stress that many parents feel. Pediatricians can collaborate with community organizations to promote these resources to families.
Conclusion
The Surgeon General’s advisory serves as a timely reminder of the interconnectedness of parental and child mental health. Pediatricians have a unique opportunity to influence not only the health of their pediatric patients, but also the well-being of their families. By recognizing and addressing the mental health needs of parents, we can break the cycle of stress and mental illness that affects so many families, ensuring a healthier future for the next generation.
Let us embrace this call to action and work together to create a supportive environment where all parents and children can thrive.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. American Hospital Association. Surgeon General Issues Advisory on Mental Health and Well-Being of Parents. American Hospital Association. 2024 Sep 4.
2. Goodman SH, Gotlib IH. Risk for Psychopathology in the Children of Depressed Mothers: A Developmental Model for Understanding Mechanisms of Transmission. Psychol Rev. 1999;106(3):458-490. doi: 10.1037/0033-295X.106.3.458.
3. Lovejoy MC et al. Maternal Depression and Parenting Behavior: A Meta-Analytic Review. Clin Psychol Rev. 2000;20(5):561-592. doi: 10.1016/s0272-7358(98)00100-7.
4. Weissman MM et al. Offspring of Depressed Parents: 20 Years Later. Am J Psychiatry. 2006;163(6):1001-1008. doi: 10.1176/ajp.2006.163.6.1001.
5. Smith KE, Pollak SD. Early Life Stress and Development: Potential Mechanisms for Adverse Outcomes. J Neurodev Disord. 2020;12(1):3-14. doi: 10.1186/s11689-020-09337-y.
As child psychiatrists and pediatricians, our mission extends beyond treating the physical health of children; it encompasses understanding the intricate web of factors that influence a child’s overall well-being. A recent advisory from U.S. Surgeon General Dr. Vivek Murthy has brought to light a critical issue that demands our attention: the declining mental health of parents and its profound impact on children. As providers who depend heavily on parental involvement to manage the needs of our pediatric patients, addressing parental mental health can be a crucial step in safeguarding the mental health of children.
The Surgeon General’s Advisory: A Call to Action
On August 28, 2024, the U.S. Surgeon General issued an advisory highlighting the significant stressors impacting parents and caregivers, and the broader implications for children’s mental health. The advisory emphasizes the bidirectional relationship between parental and child mental health, urging healthcare providers, policymakers, and communities to prioritize support for parents. It stresses that the mental health of parents is not only vital for their well-being but also plays a critical role in shaping the emotional and psychological development of their children.1
The Link Between Parental and Child Mental Health
Research shows that a parent’s mental health directly influences the child’s emotional and behavioral outcomes. Children of parents with untreated mental health conditions, such as depression, anxiety, trauma, or chronic stress, are at a significantly higher risk of developing similar conditions. This risk is mediated through various mechanisms, including genetic predisposition, compromised parent-child interactions, and exposure to adverse environments.
1. Parental Depression and Child Outcomes: Parental depression, particularly maternal depression, has been extensively studied and is strongly associated with emotional and behavioral problems in children. Children of depressed parents are more likely to experience anxiety, depression, and resulting academic difficulties. Depressed parents may struggle with consistent and positive parenting, which can disrupt the development of secure attachments and emotional regulation in children.2-4
2. Anxiety and Parenting Styles: Parental anxiety can influence parenting styles, often leading to overprotectiveness, inconsistency, or heightened criticism. These behaviors, in turn, can cultivate anxiety in children, creating a cycle that perpetuates mental health challenges across generations. Children raised in environments where anxiety is pervasive may learn to view the world as threatening, contributing to hypervigilance and stress.5
3. Impact of Chronic Stress on Parenting: Chronic stress experienced by parents, often due to financial hardship, lack of social support, or work-life imbalance, can impair their ability to engage in responsive and nurturing parenting. This, in turn, can affect children’s ability to meaningfully engage with parents to form secure attachments. Further, chronic stress can negatively impact the quality of parent-child interactions and fuel the cycle of rupture with limited opportunity for repair. The advisory stresses the need to address these systemic stressors as part of a broader public health strategy to support families.1
Implications for Pediatric Practice
Pediatricians are often the first point of contact for families navigating mental health challenges. The Surgeon General’s advisory highlights the need for pediatricians to adopt a holistic approach that considers the mental health of the entire family, not just the child. This can be challenging with the average follow-up appointment time of 16 minutes, though many of the recommendations take this logistical hurdle into consideration:
1. Screening for Parental Mental Health: Incorporating routine screening for parental mental health into pediatric visits can be a powerful tool. Questions about parental stress, depression (especially postpartum depression), and anxiety should be integrated into well-child visits, especially in families where children present with emotional or behavioral difficulties. By identifying at-risk parents early, timely referrals to mental health services can be secured.
2. Providing Resources and Referrals: Offering resources and referrals to parents who may be struggling can positively impact the entire family. This includes connecting families with mental health professionals, parenting support groups, or community resources that can alleviate stressors such as food insecurity or lack of childcare. Having a list of local mental health resources available in your practice can empower parents to seek the help they need.
3. Promoting Positive Parenting Practices: Guidance on positive parenting practices, stress management, and self-care can make a significant difference in the mental health of parents and their children. Workshops or educational materials on topics like mindfulness, managing work-life balance, and fostering healthy communication within the family can be valuable and high-yield additions to pediatric care.
4. Collaborative Care Models: Collaborative care models, where pediatricians work closely with child psychiatrists, psychologists, and social workers, can provide comprehensive support to families. This integrated approach ensures that both children and their parents receive the care they need, promoting better outcomes for the entire family unit.
Addressing Broader Systemic Issues
The advisory also calls for systemic changes that extend beyond the clinic. Policy changes such as expanding access to paid family leave, affordable childcare, and mental health services are essential to creating an environment where parents can thrive. As pediatricians, advocating for these changes at the local and national level can amplify the overall impact on families.
1. Advocating for Paid Family Leave: Paid family leave allows parents to bond with their children and attend to their own mental health needs without the added pressure of financial instability. Supporting policies that provide adequate paid leave can pave the way for a successful and healthy return to work and have long-term benefits for family health.
2. Expanding Mental Health Services: Increasing access to mental health services, especially in underserved communities, is crucial. Pediatricians can play a role by partnering with local mental health providers to offer integrated care within their practices or community settings.
3. Community Support Programs: The creation of community support programs that offer parenting classes, stress management workshops, and peer support groups can help reduce the isolation and stress that many parents feel. Pediatricians can collaborate with community organizations to promote these resources to families.
Conclusion
The Surgeon General’s advisory serves as a timely reminder of the interconnectedness of parental and child mental health. Pediatricians have a unique opportunity to influence not only the health of their pediatric patients, but also the well-being of their families. By recognizing and addressing the mental health needs of parents, we can break the cycle of stress and mental illness that affects so many families, ensuring a healthier future for the next generation.
Let us embrace this call to action and work together to create a supportive environment where all parents and children can thrive.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. American Hospital Association. Surgeon General Issues Advisory on Mental Health and Well-Being of Parents. American Hospital Association. 2024 Sep 4.
2. Goodman SH, Gotlib IH. Risk for Psychopathology in the Children of Depressed Mothers: A Developmental Model for Understanding Mechanisms of Transmission. Psychol Rev. 1999;106(3):458-490. doi: 10.1037/0033-295X.106.3.458.
3. Lovejoy MC et al. Maternal Depression and Parenting Behavior: A Meta-Analytic Review. Clin Psychol Rev. 2000;20(5):561-592. doi: 10.1016/s0272-7358(98)00100-7.
4. Weissman MM et al. Offspring of Depressed Parents: 20 Years Later. Am J Psychiatry. 2006;163(6):1001-1008. doi: 10.1176/ajp.2006.163.6.1001.
5. Smith KE, Pollak SD. Early Life Stress and Development: Potential Mechanisms for Adverse Outcomes. J Neurodev Disord. 2020;12(1):3-14. doi: 10.1186/s11689-020-09337-y.
The Silent Exodus: Are Nurse Practitioners and Physician Assistants Quiet Quitting?
While she cared deeply about her work, Melissa Adams*, a family nurse practitioner (NP) in Madison, Alabama, was being frequently triple-booked, didn’t feel respected by her office manager, and started to worry about becoming burned out. When she sought help, “the administration was tone-deaf,” she said. “When I asked about what I could do to prevent burnout, they sent me an article about it. It was clear to me that asking for respite from triple-booking and asking to be respected by my office manager wasn’t being heard ... so I thought, ‘how do I fly under the radar and get by with what I can?’ ” That meant focusing on patient care and refusing to take on additional responsibilities, like training new hires or working with students.
“You’re overworked and underpaid, and you start giving less and less of yourself,” Ms. Adams said in an interview.
Quiet quitting, defined as performing only the assigned tasks of the job without making any extra effort or going the proverbial extra mile, has gained attention in the press in recent years. A Gallup poll found that about 50% of the workforce were “quiet quitters” or disengaged.
It may be even more prevalent in healthcare, where a recent survey found that 57% of frontline medical staff, including NPs and physician assistants (PAs), report being disengaged at work.
The Causes of Quiet Quitting
Potential causes of quiet quitting among PAs and NPs include:
- Unrealistic care expectations. Ms. Adams said.
- Lack of trust or respect. Physicians don’t always respect the role that PAs and NPs play in a practice.
- Dissatisfaction with leadership or administration. There’s often a feeling that the PA or NP isn’t “heard” or appreciated.
- Dissatisfaction with pay or working conditions.
- Moral injury. “There’s no way to escape being morally injured when you work with an at-risk population,” said Ms. Adams. “You may see someone who has 20-24 determinants of health, and you’re expected to schlep them through in 8 minutes — you know you’re not able to do what they need.”
What Quiet Quitting Looks Like
Terri Smith*, an NP at an academic medical center outpatient clinic in rural Vermont, said that, while she feels appreciated by her patients and her team, there’s poor communication from the administration, which has caused her to quietly quit.
“I stopped saying ‘yes’ to all the normal committee work and the extra stuff that used to add a lot to my professional enjoyment,” she said. “The last couple of years, my whole motto is to nod and smile when administration says to do something — to put your head down and take care of your patients.”
While the term “quiet quitting” may be new, the issue is not, said Bridget Roberts, PhD, a healthcare executive who ran a large physician’s group of 100 healthcare providers in Jacksonville, Florida, for a decade. “Quiet quitting is a fancy title for employees who are completely disengaged,” said Dr. Roberts. “When they’re on the way out, they ‘check the box’. That’s not a new thing.”
“Typically, the first thing you see is a lot of frustration in that they aren’t able to complete the tasks they have at hand,” said Rebecca Day, PMNHP, a doctoral-educated NP and director of nursing practice at a Federally Qualified Health Center in Corbin, Kentucky. “Staff may be overworked and not have enough time to do what’s required of them with patient care as well as the paperwork required behind the scenes. It [quiet quitting] is doing just enough to get by, but shortcutting as much as they can to try to save some time.”
Addressing Quiet Quitting
Those kinds of shortcuts may affect patients, admits Ms. Smith. “I do think it starts to seep into patient care,” she said. “And that really doesn’t feel good ... at our institution, I’m not just an NP — I’m the nurse, the doctor, the secretary — I’m everybody, and for the last year, almost every single day in clinic, I’m apologizing [to a patient] because we can’t do something.”
Watching for this frustration can help alert administrators to NPs and PAs who may be “checking out” at work. Open lines of communication can help you address the issue. “Ask questions like ‘What could we do differently to make your day easier?’” said Dr. Roberts. Understanding the day-to-day issues NPs and PAs face at work can help in developing a plan to address disengagement.
When Dr. Day sees quiet quitting at her practice, she talks with the advance practice provider about what’s causing the issue. “’Are you overworked? Are you understaffed? Are there problems at home? Do you feel you’re receiving inadequate pay?’ ” she said. “The first thing to do is address that and find mutual ground on the issues…deal with the person as a person and then go back and deal with the person as an employee. If your staff isn’t happy, your clinic isn’t going to be productive.”
Finally, while reasons for quiet quitting may vary, cultivating a collaborative atmosphere where NPs and PAs feel appreciated and valued can help reduce the risk for quiet quitting. “Get to know your advanced practice providers,” said Ms. Adams. “Understand their strengths and what they’re about. It’s not an ‘us vs them’ ... there is a lot more commonality when we approach it that way.” Respect for the integral role that NPs and PAs play in your practice can help reduce the risk for quiet quitting — and help provide better patient care.
*Names have been changed.
A version of this article first appeared on Medscape.com.
While she cared deeply about her work, Melissa Adams*, a family nurse practitioner (NP) in Madison, Alabama, was being frequently triple-booked, didn’t feel respected by her office manager, and started to worry about becoming burned out. When she sought help, “the administration was tone-deaf,” she said. “When I asked about what I could do to prevent burnout, they sent me an article about it. It was clear to me that asking for respite from triple-booking and asking to be respected by my office manager wasn’t being heard ... so I thought, ‘how do I fly under the radar and get by with what I can?’ ” That meant focusing on patient care and refusing to take on additional responsibilities, like training new hires or working with students.
“You’re overworked and underpaid, and you start giving less and less of yourself,” Ms. Adams said in an interview.
Quiet quitting, defined as performing only the assigned tasks of the job without making any extra effort or going the proverbial extra mile, has gained attention in the press in recent years. A Gallup poll found that about 50% of the workforce were “quiet quitters” or disengaged.
It may be even more prevalent in healthcare, where a recent survey found that 57% of frontline medical staff, including NPs and physician assistants (PAs), report being disengaged at work.
The Causes of Quiet Quitting
Potential causes of quiet quitting among PAs and NPs include:
- Unrealistic care expectations. Ms. Adams said.
- Lack of trust or respect. Physicians don’t always respect the role that PAs and NPs play in a practice.
- Dissatisfaction with leadership or administration. There’s often a feeling that the PA or NP isn’t “heard” or appreciated.
- Dissatisfaction with pay or working conditions.
- Moral injury. “There’s no way to escape being morally injured when you work with an at-risk population,” said Ms. Adams. “You may see someone who has 20-24 determinants of health, and you’re expected to schlep them through in 8 minutes — you know you’re not able to do what they need.”
What Quiet Quitting Looks Like
Terri Smith*, an NP at an academic medical center outpatient clinic in rural Vermont, said that, while she feels appreciated by her patients and her team, there’s poor communication from the administration, which has caused her to quietly quit.
“I stopped saying ‘yes’ to all the normal committee work and the extra stuff that used to add a lot to my professional enjoyment,” she said. “The last couple of years, my whole motto is to nod and smile when administration says to do something — to put your head down and take care of your patients.”
While the term “quiet quitting” may be new, the issue is not, said Bridget Roberts, PhD, a healthcare executive who ran a large physician’s group of 100 healthcare providers in Jacksonville, Florida, for a decade. “Quiet quitting is a fancy title for employees who are completely disengaged,” said Dr. Roberts. “When they’re on the way out, they ‘check the box’. That’s not a new thing.”
“Typically, the first thing you see is a lot of frustration in that they aren’t able to complete the tasks they have at hand,” said Rebecca Day, PMNHP, a doctoral-educated NP and director of nursing practice at a Federally Qualified Health Center in Corbin, Kentucky. “Staff may be overworked and not have enough time to do what’s required of them with patient care as well as the paperwork required behind the scenes. It [quiet quitting] is doing just enough to get by, but shortcutting as much as they can to try to save some time.”
Addressing Quiet Quitting
Those kinds of shortcuts may affect patients, admits Ms. Smith. “I do think it starts to seep into patient care,” she said. “And that really doesn’t feel good ... at our institution, I’m not just an NP — I’m the nurse, the doctor, the secretary — I’m everybody, and for the last year, almost every single day in clinic, I’m apologizing [to a patient] because we can’t do something.”
Watching for this frustration can help alert administrators to NPs and PAs who may be “checking out” at work. Open lines of communication can help you address the issue. “Ask questions like ‘What could we do differently to make your day easier?’” said Dr. Roberts. Understanding the day-to-day issues NPs and PAs face at work can help in developing a plan to address disengagement.
When Dr. Day sees quiet quitting at her practice, she talks with the advance practice provider about what’s causing the issue. “’Are you overworked? Are you understaffed? Are there problems at home? Do you feel you’re receiving inadequate pay?’ ” she said. “The first thing to do is address that and find mutual ground on the issues…deal with the person as a person and then go back and deal with the person as an employee. If your staff isn’t happy, your clinic isn’t going to be productive.”
Finally, while reasons for quiet quitting may vary, cultivating a collaborative atmosphere where NPs and PAs feel appreciated and valued can help reduce the risk for quiet quitting. “Get to know your advanced practice providers,” said Ms. Adams. “Understand their strengths and what they’re about. It’s not an ‘us vs them’ ... there is a lot more commonality when we approach it that way.” Respect for the integral role that NPs and PAs play in your practice can help reduce the risk for quiet quitting — and help provide better patient care.
*Names have been changed.
A version of this article first appeared on Medscape.com.
While she cared deeply about her work, Melissa Adams*, a family nurse practitioner (NP) in Madison, Alabama, was being frequently triple-booked, didn’t feel respected by her office manager, and started to worry about becoming burned out. When she sought help, “the administration was tone-deaf,” she said. “When I asked about what I could do to prevent burnout, they sent me an article about it. It was clear to me that asking for respite from triple-booking and asking to be respected by my office manager wasn’t being heard ... so I thought, ‘how do I fly under the radar and get by with what I can?’ ” That meant focusing on patient care and refusing to take on additional responsibilities, like training new hires or working with students.
“You’re overworked and underpaid, and you start giving less and less of yourself,” Ms. Adams said in an interview.
Quiet quitting, defined as performing only the assigned tasks of the job without making any extra effort or going the proverbial extra mile, has gained attention in the press in recent years. A Gallup poll found that about 50% of the workforce were “quiet quitters” or disengaged.
It may be even more prevalent in healthcare, where a recent survey found that 57% of frontline medical staff, including NPs and physician assistants (PAs), report being disengaged at work.
The Causes of Quiet Quitting
Potential causes of quiet quitting among PAs and NPs include:
- Unrealistic care expectations. Ms. Adams said.
- Lack of trust or respect. Physicians don’t always respect the role that PAs and NPs play in a practice.
- Dissatisfaction with leadership or administration. There’s often a feeling that the PA or NP isn’t “heard” or appreciated.
- Dissatisfaction with pay or working conditions.
- Moral injury. “There’s no way to escape being morally injured when you work with an at-risk population,” said Ms. Adams. “You may see someone who has 20-24 determinants of health, and you’re expected to schlep them through in 8 minutes — you know you’re not able to do what they need.”
What Quiet Quitting Looks Like
Terri Smith*, an NP at an academic medical center outpatient clinic in rural Vermont, said that, while she feels appreciated by her patients and her team, there’s poor communication from the administration, which has caused her to quietly quit.
“I stopped saying ‘yes’ to all the normal committee work and the extra stuff that used to add a lot to my professional enjoyment,” she said. “The last couple of years, my whole motto is to nod and smile when administration says to do something — to put your head down and take care of your patients.”
While the term “quiet quitting” may be new, the issue is not, said Bridget Roberts, PhD, a healthcare executive who ran a large physician’s group of 100 healthcare providers in Jacksonville, Florida, for a decade. “Quiet quitting is a fancy title for employees who are completely disengaged,” said Dr. Roberts. “When they’re on the way out, they ‘check the box’. That’s not a new thing.”
“Typically, the first thing you see is a lot of frustration in that they aren’t able to complete the tasks they have at hand,” said Rebecca Day, PMNHP, a doctoral-educated NP and director of nursing practice at a Federally Qualified Health Center in Corbin, Kentucky. “Staff may be overworked and not have enough time to do what’s required of them with patient care as well as the paperwork required behind the scenes. It [quiet quitting] is doing just enough to get by, but shortcutting as much as they can to try to save some time.”
Addressing Quiet Quitting
Those kinds of shortcuts may affect patients, admits Ms. Smith. “I do think it starts to seep into patient care,” she said. “And that really doesn’t feel good ... at our institution, I’m not just an NP — I’m the nurse, the doctor, the secretary — I’m everybody, and for the last year, almost every single day in clinic, I’m apologizing [to a patient] because we can’t do something.”
Watching for this frustration can help alert administrators to NPs and PAs who may be “checking out” at work. Open lines of communication can help you address the issue. “Ask questions like ‘What could we do differently to make your day easier?’” said Dr. Roberts. Understanding the day-to-day issues NPs and PAs face at work can help in developing a plan to address disengagement.
When Dr. Day sees quiet quitting at her practice, she talks with the advance practice provider about what’s causing the issue. “’Are you overworked? Are you understaffed? Are there problems at home? Do you feel you’re receiving inadequate pay?’ ” she said. “The first thing to do is address that and find mutual ground on the issues…deal with the person as a person and then go back and deal with the person as an employee. If your staff isn’t happy, your clinic isn’t going to be productive.”
Finally, while reasons for quiet quitting may vary, cultivating a collaborative atmosphere where NPs and PAs feel appreciated and valued can help reduce the risk for quiet quitting. “Get to know your advanced practice providers,” said Ms. Adams. “Understand their strengths and what they’re about. It’s not an ‘us vs them’ ... there is a lot more commonality when we approach it that way.” Respect for the integral role that NPs and PAs play in your practice can help reduce the risk for quiet quitting — and help provide better patient care.
*Names have been changed.
A version of this article first appeared on Medscape.com.
Current Hydroxychloroquine Use in Lupus May Provide Protection Against Cardiovascular Events
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.