User login
Full-dose antithrombotic aids selected COVID-19 ICU patients
BARCELONA – Hospitalized patients in the ICU because of an acute COVID-19 infection had significantly fewer thrombotic events and complications when treated with full-dose anticoagulation, compared with patients who received standard-dose anticoagulation prophylaxis, but full-dose anticoagulation also triggered an excess of moderate and severe bleeding events, randomized trial results show.
The new findings from the COVID-PACT trial in an exclusively U.S.-based cohort of 382 on-treatment patients in the ICU with COVID-19 infection may lead to a change in existing guidelines, which currently recommend standard-dose prophylaxis based on results from prior head-to-head comparisons, such as guidelines posted March 2022 from the American Society of Hematology.
” after weighing an individual patient’s risk for both thrombotic events and bleeding, David D. Berg, MD, said at the annual congress of the European Society of Cardiology. Simultaneous with his report at the congress, the results also appeared online in the journal Circulation.
“What the results tell us is that full-dose anticoagulation in critically ill patients with COVID-19 is highly effective for reducing thrombotic complications,” said Dr. Berg, a cardiologist and critical care physician at Brigham and Women’s Hospital, Boston.
The report’s designated discussant agreed with Dr. Berg’s conclusions.
‘Need to replace the guidelines’
“We probably need to replace the guidelines,” said Eduardo Ramacciotti, MD, PhD, MPH, a professor of vascular surgery at Santa Casa School of Medicine, São Paulo. Dr. Ramacciotti praised the study’s design, the endpoints, and the fact that the design excluded patients at high risk for bleeding complications, particularly those with a fibrinogen level below 200 mg/dL (2 g/L).
But other experts questioned the significance of the COVID-PACT results given that the outcomes did not show that full-dose anticoagulation produced incremental improvement in patient survival.
“We should abandon the thought that intensified anticoagulation should be routine, because it did not overall increase the number of patients discharged from the hospital alive,” commented John W. Eikelboom, MBBS, a professor of hematology and thromboembolism at McMaster University, Hamilton, Ont.
“Preventing venous thrombosis is a good thing, but the money is in saving lives and stopping need for ventilation, and we haven’t been successful doing that with an antithrombotic strategy,” said Dr. Eikelboom. “It is useful to prevent venous thrombosis, but we need to look elsewhere to improve the outcomes of [critically ill] patients with COVID-19.”
Reducing thromboembolism is a ‘valid goal’
Dr. Berg took a different view. “It’s a valid goal to try to reduce venous thromboembolism complications,” the major benefit seen in his study, he said. “There is clinical significance to reducing thrombotic events in terms of how people feel, their functional status, and their complications. There are a lot of clinically relevant consequences of thrombosis beyond mortality.”
COVID-PACT ran at 34 U.S. centers from August 2020 to March 2022 but stopped short of its enrollment goal of 750 patients because of waning numbers of patients with COVID-19 admitted to ICUs. In addition to randomly assigning patients within 96 hours of their ICU admission to full-dose anticoagulation or to standard-dose antithrombotic prophylaxis, the study included a second, concurrent randomization to the antiplatelet agent clopidogrel (Plavix) or to no antiplatelet drug. Both randomizations used an open-label design.
The results failed to show a discernable effect from adding clopidogrel on both the primary efficacy and primary safety endpoints, adding to accumulated evidence that treatment with an antiplatelet agent, including aspirin, confers no antithrombotic benefit in patients with COVID-19.
The trial’s participants averaged 61 years old, 68% were obese, 59% had hypertension, and 32% had diabetes. The median time after ICU admission when randomized treatment began was 2.1 days, and researchers followed patients for a median of 13 days, including a median time on anticoagulation of 10.6 days.
The trial design allowed clinicians to use either low molecular weight heparin or unfractionated heparin for anticoagulation, and 82% of patients received low molecular weight heparin as their initial treatment. The prespecified design called for an on-treatment analysis because of an anticipated high crossover rate. During the trial, 34% of patients who started on the prophylactic dose switched to full dose, and 17% had the reverse crossover.
95% increased win ratio with full dose
The study’s primary efficacy endpoint used a win-ratio analysis that included seven different adverse outcomes that ranged from death from venous or arterial thrombosis to clinically silent deep vein thrombosis. Treatment with full-dose anticoagulation led to a significant 95% increase in win ratio.
Researchers also applied a more conventional time-to-first-event secondary efficacy analysis, which showed that full-dose anticoagulation cut the incidence of an adverse outcome by a significant 44% relative to prophylactic dosing.
The two study groups showed no difference in all-cause death rates. The efficacy advantage of the full-dose regimen was driven by reduced rates of venous thrombotic events, especially a reduction in clinically evident deep vein thrombotic events.
The primary safety endpoint was the rate of fatal or life-threatening bleeding episodes, and while life-threatening bleeds were numerically more common among the full-dose recipients (four events, compared with one event on prophylaxis dosing) the difference was not significant, and no patients died from a bleeding event.
More secondary safety bleeds
The safety difference showed up in a secondary measure of bleeding severity, the rate of GUSTO moderate or severe bleeds. These occurred in 15 of the full-dose recipients, compared with 1 patient on the prophylactic dose.
Dr. Berg highlighted that several prior studies have assessed various anticoagulation regimens in critically ill (ICU-admitted and on respiratory or cardiovascular support) patients with COVID-19. For example, two influential reports published in 2021 by the same team of investigators in the New England Journal of Medicine had sharply divergent results.
One multicenter study, which tested full-dose heparin against prophylactic treatment in more than 1,000 critically ill patients, was stopped prematurely because it had not shown a significant difference between the treatment arms. The second study, in more than 2,000 multicenter patients with COVID-19 who did not require critical-level organ support, showed clear superiority of the full-dose heparin regimen.
Notably, both previous studies used a different primary efficacy endpoint than the COVID-PACT study. The earlier reports both measured efficacy in terms of patients being alive and off organ support by 21 days from randomization.
Patients to exclude
Although Dr. Berg stressed the clear positive result, he also cautioned that they should not apply to patients excluded from the study: those with severe coagulopathies, those with severe thrombocytopenia, and patients already maintained on dual antiplatelet therapy. He also cautioned against using the full-dose strategy in elderly patients, because in COVID-PACT, those who developed bleeding complications tended to be older.
Dr. Berg also noted that heparin prophylaxis is a well-established intervention for ICU-admitted patients without COVID-19 for the purpose of preventing venous thromboembolisms without evidence that this approach reduces deaths or organ failure.
But he conceded that “the priority of treatment depends on whether it saves lives, so anticoagulation is probably not as high a priority as other effective treatments” that reduce mortality. “Preventing venous thromboembolism has rarely been shown to have a mortality benefit,” Dr. Berg noted.
COVID-PACT received no direct commercial funding. Dr. Berg has been a consultant to AstraZeneca, Mobility Bio, and Youngene Therapeutics, and he participated in a trial sponsored by Kowa. Dr. Ramacciotti has been a consultant to or speaker on behalf of Aspen, Bayer, Daiichi Sankyo, Mylan, Pfizer, and Sanofi, and he has received research support from Bayer, Esperon, Novartis, and Pfizer. Dr. Eikelboom has received honoraria and research support from Bayer.
A version of this article first appeared on Medscape.com.
BARCELONA – Hospitalized patients in the ICU because of an acute COVID-19 infection had significantly fewer thrombotic events and complications when treated with full-dose anticoagulation, compared with patients who received standard-dose anticoagulation prophylaxis, but full-dose anticoagulation also triggered an excess of moderate and severe bleeding events, randomized trial results show.
The new findings from the COVID-PACT trial in an exclusively U.S.-based cohort of 382 on-treatment patients in the ICU with COVID-19 infection may lead to a change in existing guidelines, which currently recommend standard-dose prophylaxis based on results from prior head-to-head comparisons, such as guidelines posted March 2022 from the American Society of Hematology.
” after weighing an individual patient’s risk for both thrombotic events and bleeding, David D. Berg, MD, said at the annual congress of the European Society of Cardiology. Simultaneous with his report at the congress, the results also appeared online in the journal Circulation.
“What the results tell us is that full-dose anticoagulation in critically ill patients with COVID-19 is highly effective for reducing thrombotic complications,” said Dr. Berg, a cardiologist and critical care physician at Brigham and Women’s Hospital, Boston.
The report’s designated discussant agreed with Dr. Berg’s conclusions.
‘Need to replace the guidelines’
“We probably need to replace the guidelines,” said Eduardo Ramacciotti, MD, PhD, MPH, a professor of vascular surgery at Santa Casa School of Medicine, São Paulo. Dr. Ramacciotti praised the study’s design, the endpoints, and the fact that the design excluded patients at high risk for bleeding complications, particularly those with a fibrinogen level below 200 mg/dL (2 g/L).
But other experts questioned the significance of the COVID-PACT results given that the outcomes did not show that full-dose anticoagulation produced incremental improvement in patient survival.
“We should abandon the thought that intensified anticoagulation should be routine, because it did not overall increase the number of patients discharged from the hospital alive,” commented John W. Eikelboom, MBBS, a professor of hematology and thromboembolism at McMaster University, Hamilton, Ont.
“Preventing venous thrombosis is a good thing, but the money is in saving lives and stopping need for ventilation, and we haven’t been successful doing that with an antithrombotic strategy,” said Dr. Eikelboom. “It is useful to prevent venous thrombosis, but we need to look elsewhere to improve the outcomes of [critically ill] patients with COVID-19.”
Reducing thromboembolism is a ‘valid goal’
Dr. Berg took a different view. “It’s a valid goal to try to reduce venous thromboembolism complications,” the major benefit seen in his study, he said. “There is clinical significance to reducing thrombotic events in terms of how people feel, their functional status, and their complications. There are a lot of clinically relevant consequences of thrombosis beyond mortality.”
COVID-PACT ran at 34 U.S. centers from August 2020 to March 2022 but stopped short of its enrollment goal of 750 patients because of waning numbers of patients with COVID-19 admitted to ICUs. In addition to randomly assigning patients within 96 hours of their ICU admission to full-dose anticoagulation or to standard-dose antithrombotic prophylaxis, the study included a second, concurrent randomization to the antiplatelet agent clopidogrel (Plavix) or to no antiplatelet drug. Both randomizations used an open-label design.
The results failed to show a discernable effect from adding clopidogrel on both the primary efficacy and primary safety endpoints, adding to accumulated evidence that treatment with an antiplatelet agent, including aspirin, confers no antithrombotic benefit in patients with COVID-19.
The trial’s participants averaged 61 years old, 68% were obese, 59% had hypertension, and 32% had diabetes. The median time after ICU admission when randomized treatment began was 2.1 days, and researchers followed patients for a median of 13 days, including a median time on anticoagulation of 10.6 days.
The trial design allowed clinicians to use either low molecular weight heparin or unfractionated heparin for anticoagulation, and 82% of patients received low molecular weight heparin as their initial treatment. The prespecified design called for an on-treatment analysis because of an anticipated high crossover rate. During the trial, 34% of patients who started on the prophylactic dose switched to full dose, and 17% had the reverse crossover.
95% increased win ratio with full dose
The study’s primary efficacy endpoint used a win-ratio analysis that included seven different adverse outcomes that ranged from death from venous or arterial thrombosis to clinically silent deep vein thrombosis. Treatment with full-dose anticoagulation led to a significant 95% increase in win ratio.
Researchers also applied a more conventional time-to-first-event secondary efficacy analysis, which showed that full-dose anticoagulation cut the incidence of an adverse outcome by a significant 44% relative to prophylactic dosing.
The two study groups showed no difference in all-cause death rates. The efficacy advantage of the full-dose regimen was driven by reduced rates of venous thrombotic events, especially a reduction in clinically evident deep vein thrombotic events.
The primary safety endpoint was the rate of fatal or life-threatening bleeding episodes, and while life-threatening bleeds were numerically more common among the full-dose recipients (four events, compared with one event on prophylaxis dosing) the difference was not significant, and no patients died from a bleeding event.
More secondary safety bleeds
The safety difference showed up in a secondary measure of bleeding severity, the rate of GUSTO moderate or severe bleeds. These occurred in 15 of the full-dose recipients, compared with 1 patient on the prophylactic dose.
Dr. Berg highlighted that several prior studies have assessed various anticoagulation regimens in critically ill (ICU-admitted and on respiratory or cardiovascular support) patients with COVID-19. For example, two influential reports published in 2021 by the same team of investigators in the New England Journal of Medicine had sharply divergent results.
One multicenter study, which tested full-dose heparin against prophylactic treatment in more than 1,000 critically ill patients, was stopped prematurely because it had not shown a significant difference between the treatment arms. The second study, in more than 2,000 multicenter patients with COVID-19 who did not require critical-level organ support, showed clear superiority of the full-dose heparin regimen.
Notably, both previous studies used a different primary efficacy endpoint than the COVID-PACT study. The earlier reports both measured efficacy in terms of patients being alive and off organ support by 21 days from randomization.
Patients to exclude
Although Dr. Berg stressed the clear positive result, he also cautioned that they should not apply to patients excluded from the study: those with severe coagulopathies, those with severe thrombocytopenia, and patients already maintained on dual antiplatelet therapy. He also cautioned against using the full-dose strategy in elderly patients, because in COVID-PACT, those who developed bleeding complications tended to be older.
Dr. Berg also noted that heparin prophylaxis is a well-established intervention for ICU-admitted patients without COVID-19 for the purpose of preventing venous thromboembolisms without evidence that this approach reduces deaths or organ failure.
But he conceded that “the priority of treatment depends on whether it saves lives, so anticoagulation is probably not as high a priority as other effective treatments” that reduce mortality. “Preventing venous thromboembolism has rarely been shown to have a mortality benefit,” Dr. Berg noted.
COVID-PACT received no direct commercial funding. Dr. Berg has been a consultant to AstraZeneca, Mobility Bio, and Youngene Therapeutics, and he participated in a trial sponsored by Kowa. Dr. Ramacciotti has been a consultant to or speaker on behalf of Aspen, Bayer, Daiichi Sankyo, Mylan, Pfizer, and Sanofi, and he has received research support from Bayer, Esperon, Novartis, and Pfizer. Dr. Eikelboom has received honoraria and research support from Bayer.
A version of this article first appeared on Medscape.com.
BARCELONA – Hospitalized patients in the ICU because of an acute COVID-19 infection had significantly fewer thrombotic events and complications when treated with full-dose anticoagulation, compared with patients who received standard-dose anticoagulation prophylaxis, but full-dose anticoagulation also triggered an excess of moderate and severe bleeding events, randomized trial results show.
The new findings from the COVID-PACT trial in an exclusively U.S.-based cohort of 382 on-treatment patients in the ICU with COVID-19 infection may lead to a change in existing guidelines, which currently recommend standard-dose prophylaxis based on results from prior head-to-head comparisons, such as guidelines posted March 2022 from the American Society of Hematology.
” after weighing an individual patient’s risk for both thrombotic events and bleeding, David D. Berg, MD, said at the annual congress of the European Society of Cardiology. Simultaneous with his report at the congress, the results also appeared online in the journal Circulation.
“What the results tell us is that full-dose anticoagulation in critically ill patients with COVID-19 is highly effective for reducing thrombotic complications,” said Dr. Berg, a cardiologist and critical care physician at Brigham and Women’s Hospital, Boston.
The report’s designated discussant agreed with Dr. Berg’s conclusions.
‘Need to replace the guidelines’
“We probably need to replace the guidelines,” said Eduardo Ramacciotti, MD, PhD, MPH, a professor of vascular surgery at Santa Casa School of Medicine, São Paulo. Dr. Ramacciotti praised the study’s design, the endpoints, and the fact that the design excluded patients at high risk for bleeding complications, particularly those with a fibrinogen level below 200 mg/dL (2 g/L).
But other experts questioned the significance of the COVID-PACT results given that the outcomes did not show that full-dose anticoagulation produced incremental improvement in patient survival.
“We should abandon the thought that intensified anticoagulation should be routine, because it did not overall increase the number of patients discharged from the hospital alive,” commented John W. Eikelboom, MBBS, a professor of hematology and thromboembolism at McMaster University, Hamilton, Ont.
“Preventing venous thrombosis is a good thing, but the money is in saving lives and stopping need for ventilation, and we haven’t been successful doing that with an antithrombotic strategy,” said Dr. Eikelboom. “It is useful to prevent venous thrombosis, but we need to look elsewhere to improve the outcomes of [critically ill] patients with COVID-19.”
Reducing thromboembolism is a ‘valid goal’
Dr. Berg took a different view. “It’s a valid goal to try to reduce venous thromboembolism complications,” the major benefit seen in his study, he said. “There is clinical significance to reducing thrombotic events in terms of how people feel, their functional status, and their complications. There are a lot of clinically relevant consequences of thrombosis beyond mortality.”
COVID-PACT ran at 34 U.S. centers from August 2020 to March 2022 but stopped short of its enrollment goal of 750 patients because of waning numbers of patients with COVID-19 admitted to ICUs. In addition to randomly assigning patients within 96 hours of their ICU admission to full-dose anticoagulation or to standard-dose antithrombotic prophylaxis, the study included a second, concurrent randomization to the antiplatelet agent clopidogrel (Plavix) or to no antiplatelet drug. Both randomizations used an open-label design.
The results failed to show a discernable effect from adding clopidogrel on both the primary efficacy and primary safety endpoints, adding to accumulated evidence that treatment with an antiplatelet agent, including aspirin, confers no antithrombotic benefit in patients with COVID-19.
The trial’s participants averaged 61 years old, 68% were obese, 59% had hypertension, and 32% had diabetes. The median time after ICU admission when randomized treatment began was 2.1 days, and researchers followed patients for a median of 13 days, including a median time on anticoagulation of 10.6 days.
The trial design allowed clinicians to use either low molecular weight heparin or unfractionated heparin for anticoagulation, and 82% of patients received low molecular weight heparin as their initial treatment. The prespecified design called for an on-treatment analysis because of an anticipated high crossover rate. During the trial, 34% of patients who started on the prophylactic dose switched to full dose, and 17% had the reverse crossover.
95% increased win ratio with full dose
The study’s primary efficacy endpoint used a win-ratio analysis that included seven different adverse outcomes that ranged from death from venous or arterial thrombosis to clinically silent deep vein thrombosis. Treatment with full-dose anticoagulation led to a significant 95% increase in win ratio.
Researchers also applied a more conventional time-to-first-event secondary efficacy analysis, which showed that full-dose anticoagulation cut the incidence of an adverse outcome by a significant 44% relative to prophylactic dosing.
The two study groups showed no difference in all-cause death rates. The efficacy advantage of the full-dose regimen was driven by reduced rates of venous thrombotic events, especially a reduction in clinically evident deep vein thrombotic events.
The primary safety endpoint was the rate of fatal or life-threatening bleeding episodes, and while life-threatening bleeds were numerically more common among the full-dose recipients (four events, compared with one event on prophylaxis dosing) the difference was not significant, and no patients died from a bleeding event.
More secondary safety bleeds
The safety difference showed up in a secondary measure of bleeding severity, the rate of GUSTO moderate or severe bleeds. These occurred in 15 of the full-dose recipients, compared with 1 patient on the prophylactic dose.
Dr. Berg highlighted that several prior studies have assessed various anticoagulation regimens in critically ill (ICU-admitted and on respiratory or cardiovascular support) patients with COVID-19. For example, two influential reports published in 2021 by the same team of investigators in the New England Journal of Medicine had sharply divergent results.
One multicenter study, which tested full-dose heparin against prophylactic treatment in more than 1,000 critically ill patients, was stopped prematurely because it had not shown a significant difference between the treatment arms. The second study, in more than 2,000 multicenter patients with COVID-19 who did not require critical-level organ support, showed clear superiority of the full-dose heparin regimen.
Notably, both previous studies used a different primary efficacy endpoint than the COVID-PACT study. The earlier reports both measured efficacy in terms of patients being alive and off organ support by 21 days from randomization.
Patients to exclude
Although Dr. Berg stressed the clear positive result, he also cautioned that they should not apply to patients excluded from the study: those with severe coagulopathies, those with severe thrombocytopenia, and patients already maintained on dual antiplatelet therapy. He also cautioned against using the full-dose strategy in elderly patients, because in COVID-PACT, those who developed bleeding complications tended to be older.
Dr. Berg also noted that heparin prophylaxis is a well-established intervention for ICU-admitted patients without COVID-19 for the purpose of preventing venous thromboembolisms without evidence that this approach reduces deaths or organ failure.
But he conceded that “the priority of treatment depends on whether it saves lives, so anticoagulation is probably not as high a priority as other effective treatments” that reduce mortality. “Preventing venous thromboembolism has rarely been shown to have a mortality benefit,” Dr. Berg noted.
COVID-PACT received no direct commercial funding. Dr. Berg has been a consultant to AstraZeneca, Mobility Bio, and Youngene Therapeutics, and he participated in a trial sponsored by Kowa. Dr. Ramacciotti has been a consultant to or speaker on behalf of Aspen, Bayer, Daiichi Sankyo, Mylan, Pfizer, and Sanofi, and he has received research support from Bayer, Esperon, Novartis, and Pfizer. Dr. Eikelboom has received honoraria and research support from Bayer.
A version of this article first appeared on Medscape.com.
Texas district court allows employers to deny HIV PrEP coverage
Fort Worth, Tex. – A case decision made by Texas U.S. District Judge Reed Charles O’Connor that will allow employers to deny health care insurance coverage for HIV preexposure prophylaxis (PrEP) is already provoking HIV activists, medical associations, nonprofits, and patients.
As this news organization first reported in August, the class action suit (Kelley v. Azar) has a broader goal – to dismantle the Affordable Care Act using the argument that many of the preventive services it covers, including PrEP, violate the Religious Freedom Restoration Act.
“Judge O’Connor has a long history of issuing rulings against the Affordable Care Act and LGBT individuals, and we expect the case to be successfully appealed as has been the case with his previous discriminatory decisions,” said Carl Schmid, executive director of the HIV+Hepatitis Policy Institute in Washington, in a prepared statement issued shortly after the ruling.
“To single out PrEP, which are FDA approved drugs that effectively prevent HIV, and conclude that its coverage violates the religious freedom of certain individuals, is plain wrong, highly discriminatory, and impedes the public health of our nation,” he said.
PrEP is not just for men who have sex with men. According to the Centers for Disease Control and Prevention, more than 1 million Americans could benefit from PrEP, and roughly 20% are heterosexual women – a fact both Mr. Schmid and the HIV Medicine Association pointed out in response to Judge O’Connor’s ruling.
“Denying access to PrEP threatens the health of more than 1.2 million Americans who could benefit from this potentially life saving intervention,” stated Marwan Haddad, MD, MPH, chair of the HIV Medicine Association, in a press release issued by the organization.
“This ruling is yet one more instance of unacceptable interference in scientific, evidence-based health care practices that must remain within the sanctity of the provider-patient relationship,” she said.
The ruling is also outside what is normally considered religious “conscientious objection.”
While the American Medical Association supports the rights of physicians to act in accordance with conscience, medical ethicists like Abram Brummett, PhD, assistant professor, department of foundational medical studies, Oakland University, Rochester, Mich., previously told this news organization that this ruling actually reflects a phenomenon known as “conscience creep” – that is, the way conscientious objection creeps outside traditional contexts like abortion, sterilization, and organ transplantation.
Incidentally, the case is not yet completed; Judge O’Connor still has to decide on challenges to contraceptives and HPV mandates. He has requested that defendants and plaintiffs file a supplemental briefing before he makes a final decision.
Regardless of how it plays out, it is unclear whether the U.S. Department of Health and Human Services will appeal.
A version of this article first appeared on Medscape.com.
Fort Worth, Tex. – A case decision made by Texas U.S. District Judge Reed Charles O’Connor that will allow employers to deny health care insurance coverage for HIV preexposure prophylaxis (PrEP) is already provoking HIV activists, medical associations, nonprofits, and patients.
As this news organization first reported in August, the class action suit (Kelley v. Azar) has a broader goal – to dismantle the Affordable Care Act using the argument that many of the preventive services it covers, including PrEP, violate the Religious Freedom Restoration Act.
“Judge O’Connor has a long history of issuing rulings against the Affordable Care Act and LGBT individuals, and we expect the case to be successfully appealed as has been the case with his previous discriminatory decisions,” said Carl Schmid, executive director of the HIV+Hepatitis Policy Institute in Washington, in a prepared statement issued shortly after the ruling.
“To single out PrEP, which are FDA approved drugs that effectively prevent HIV, and conclude that its coverage violates the religious freedom of certain individuals, is plain wrong, highly discriminatory, and impedes the public health of our nation,” he said.
PrEP is not just for men who have sex with men. According to the Centers for Disease Control and Prevention, more than 1 million Americans could benefit from PrEP, and roughly 20% are heterosexual women – a fact both Mr. Schmid and the HIV Medicine Association pointed out in response to Judge O’Connor’s ruling.
“Denying access to PrEP threatens the health of more than 1.2 million Americans who could benefit from this potentially life saving intervention,” stated Marwan Haddad, MD, MPH, chair of the HIV Medicine Association, in a press release issued by the organization.
“This ruling is yet one more instance of unacceptable interference in scientific, evidence-based health care practices that must remain within the sanctity of the provider-patient relationship,” she said.
The ruling is also outside what is normally considered religious “conscientious objection.”
While the American Medical Association supports the rights of physicians to act in accordance with conscience, medical ethicists like Abram Brummett, PhD, assistant professor, department of foundational medical studies, Oakland University, Rochester, Mich., previously told this news organization that this ruling actually reflects a phenomenon known as “conscience creep” – that is, the way conscientious objection creeps outside traditional contexts like abortion, sterilization, and organ transplantation.
Incidentally, the case is not yet completed; Judge O’Connor still has to decide on challenges to contraceptives and HPV mandates. He has requested that defendants and plaintiffs file a supplemental briefing before he makes a final decision.
Regardless of how it plays out, it is unclear whether the U.S. Department of Health and Human Services will appeal.
A version of this article first appeared on Medscape.com.
Fort Worth, Tex. – A case decision made by Texas U.S. District Judge Reed Charles O’Connor that will allow employers to deny health care insurance coverage for HIV preexposure prophylaxis (PrEP) is already provoking HIV activists, medical associations, nonprofits, and patients.
As this news organization first reported in August, the class action suit (Kelley v. Azar) has a broader goal – to dismantle the Affordable Care Act using the argument that many of the preventive services it covers, including PrEP, violate the Religious Freedom Restoration Act.
“Judge O’Connor has a long history of issuing rulings against the Affordable Care Act and LGBT individuals, and we expect the case to be successfully appealed as has been the case with his previous discriminatory decisions,” said Carl Schmid, executive director of the HIV+Hepatitis Policy Institute in Washington, in a prepared statement issued shortly after the ruling.
“To single out PrEP, which are FDA approved drugs that effectively prevent HIV, and conclude that its coverage violates the religious freedom of certain individuals, is plain wrong, highly discriminatory, and impedes the public health of our nation,” he said.
PrEP is not just for men who have sex with men. According to the Centers for Disease Control and Prevention, more than 1 million Americans could benefit from PrEP, and roughly 20% are heterosexual women – a fact both Mr. Schmid and the HIV Medicine Association pointed out in response to Judge O’Connor’s ruling.
“Denying access to PrEP threatens the health of more than 1.2 million Americans who could benefit from this potentially life saving intervention,” stated Marwan Haddad, MD, MPH, chair of the HIV Medicine Association, in a press release issued by the organization.
“This ruling is yet one more instance of unacceptable interference in scientific, evidence-based health care practices that must remain within the sanctity of the provider-patient relationship,” she said.
The ruling is also outside what is normally considered religious “conscientious objection.”
While the American Medical Association supports the rights of physicians to act in accordance with conscience, medical ethicists like Abram Brummett, PhD, assistant professor, department of foundational medical studies, Oakland University, Rochester, Mich., previously told this news organization that this ruling actually reflects a phenomenon known as “conscience creep” – that is, the way conscientious objection creeps outside traditional contexts like abortion, sterilization, and organ transplantation.
Incidentally, the case is not yet completed; Judge O’Connor still has to decide on challenges to contraceptives and HPV mandates. He has requested that defendants and plaintiffs file a supplemental briefing before he makes a final decision.
Regardless of how it plays out, it is unclear whether the U.S. Department of Health and Human Services will appeal.
A version of this article first appeared on Medscape.com.
Test Lp(a) levels to inform ASCVD management: NLA statement
Lipoprotein(a) (Lp[a]) levels should be measured in clinical practice to refine risk prediction for atherosclerotic cardiovascular disease (ASCVD) and inform treatment decisions, even if they cannot yet be lowered directly, recommends the National Lipid Association (NLA) in a scientific statement.
The statement was published in the Journal of Clinical Lipidology.
Don P. Wilson, MD, department of pediatric endocrinology and diabetes, Cook Children’s Medical Center, Fort Worth, Tex., told this news organization that lipoprotein(a) is a “very timely subject.”
“The question in the scientific community is: What role does that particular biomarker play in terms of causing serious heart disease, stroke, and calcification of the aortic valve?”
“It’s pretty clear that, in and of itself, it actually can contribute and or cause any of those conditions,” he added. “The thing that’s then sort of problematic is that we don’t have a specific treatment to lower” Lp(a).
However, Dr. Wilson said that the statement underlines it is “still worth knowing” an individual’s Lp(a) concentrations because the risk with increased levels is “even higher for those people who have other conditions, such as metabolic disease or diabetes or high cholesterol.”
There are nevertheless several drugs in phase 2 and 3 clinical trials that appear to have the potential to significantly lower Lp(a) levels.
“I’m very excited,” said Dr. Wilson, noting that, so far, the drugs seem to be “quite safe,” and the currently available data suggest that they can “reduce Lp(a) levels by about 90%, which is huge.”
“That’s better than any drug we’ve got on the market.”
He cautioned, however, that it is going to take time after the drugs are approved to see the real benefits and risks once they start being used in very large populations, given that raised Lp(a) concentrations are present in about 20% of the world population.
The publication of the NLA statement coincides with a similar one from the European Atherosclerosis Society presented at the European Society of Cardiology Congress 2022 on Aug. 29, and published simultaneously in the European Heart Journal.
Coauthor of the EAS statement, Alberico L. Catapano, MD, PhD, professor of pharmacology at the University of Milan, and past president of the EAS, said that there are many areas in which the two statements are “in complete agreement.”
“However, the spirit of the documents is different,” he continued, chief among them being that the EAS statement focuses on the “global risk” of ASCVD and provides a risk calculator to help balance the risk increase with Lp(a) with that from other factors.
Another is that increased Lp(a) levels are recognized as being on a continuum in terms of their risk, such that there is no level at which raised concentrations can be deemed safe.
Dr. Wilson agreed with Dr. Capatano’s assessment, saying that the EAS statement takes current scientific observations “a step further,” in part by emphasizing that Lp(a) is “only one piece of the puzzle” for determining an individuals’ cardiovascular risk.
This will have huge implications for the conversations clinicians have with patients over shared decision-making, Dr. Wilson added.
Nevertheless, Dr. Catapano underlined to this news organization that “both documents are very important” in terms of the need to “raise awareness about a causal risk factor” for cardiovascular disease as well as that modifying Lp(a) concentrations “will probably reduce the risk.”
The statement from the NLA builds on the association’s prior Recommendations for the Patient-Centered Management of Dyslipidemia, published in two parts in 2014 and 2015, and comes to many of the same conclusions as the EAS statement.
It explains that apolipoprotein A, a component of Lp(a) attached to apolipoprotein B, has “unique” properties that promote the “initiation and progression of atherosclerosis and calcific valvular aortic stenosis, through endothelial dysfunction and proinflammatory responses, and pro-osteogenic effects promoting calcification.”
This, in turn, has the potential to cause myocardial infarction and ischemic stroke, the authors note.
This has been confirmed in meta-analyses of prospective, population-based studies showing a high risk for MI, coronary heart disease, and ischemic stroke with high Lp(a) levels, the statement adds.
Moreover, large genetic studies have confirmed that Lp(a) is a causal factor, independent of low-density lipoprotein cholesterol levels, for MI, ischemic stroke, valvular aortic stenosis, coronary artery stenosis, carotid stenosis, femoral artery stenosis, heart failure, cardiovascular mortality, and all-cause mortality.
Like the authors of the EAS statement, the NLA statement authors underline that the measurement of Lp(a) is “currently not standardized or harmonized,” and there is insufficient evidence on the utility of different cut-offs for risk based on age, gender, ethnicity, or the presence of comorbid conditions.
However, they do suggest that Lp(a) levels greater than 50 mg/dL (> 100 nmol/L) may be considered as a risk-enhancing factor favoring the initiation of statin therapy, although they note that the threshold could be threefold higher in African American individuals.
Despite these reservations, the authors say that Lp(a) testing “is reasonable” for refining the risk assessment of ASCVD in the first-degree relatives of people with premature ASCVD and those with a personal history of premature disease as well as in individuals with primary severe hypercholesterolemia.
Testing also “may be reasonable” to “aid in the clinician-patient discussion about whether to prescribe a statin” in people aged 40-75 years with borderline 10-year ASCVD risk, defined as 5%-7.4%, as well as in other equivocal clinical situations.
In terms of what to do in an individual with raised Lp(a) levels, the statement notes that lifestyle therapy and statins do not decrease Lp(a).
Although lomitapide (Juxtapid) and proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors both lower levels of the lipoprotein, the former is “not recommended for ASCVD risk reduction,” whereas the impact of the latter on ASCVD risk reduction via Lp(a) reduction “remains undetermined.”
Several experimental agents are currently under investigation to reduce Lp(a) levels, including SLN360 (Silence Therapeutics), and AKCEA-APO(a)-LRX (Akcea Therapeutics/Ionis Pharmaceuticals).
In the meantime, the authors say it is reasonable to use Lp(a) as a “risk-enhancing factor” for the initiation of moderate- or high-intensity statins in the primary prevention of ASCVD and to consider the addition of ezetimibe and/or PCSK9 inhibitors in high- and very high–risk patients already on maximally tolerated statin therapy.
Finally, the authors recognize the need for “additional evidence” to support clinical practice. In the absence of a randomized clinical trial of Lp(a) lowering in those who are at risk for ASCVD, they note that “several important unanswered questions remain.”
These include: “Is it reasonable to recommend universal testing of Lp(a) in everyone regardless of family history or health status at least once to help encourage healthy habits and inform clinical decision-making?” “Will earlier testing and effective interventions help to improve outcomes?”
Alongside more evidence in children, the authors also emphasize that “additional data are urgently needed in Blacks, South Asians, and those of Hispanic descent.”
No funding declared. Dr. Wilson declares relationships with Osler Institute, Merck Sharp & Dohm, Novo Nordisk, and Alexion Pharmaceuticals. Other authors also declare numerous relationships. Dr. Catapano declares a relationship with Novartis.
A version of this article first appeared on Medscape.com.
Lipoprotein(a) (Lp[a]) levels should be measured in clinical practice to refine risk prediction for atherosclerotic cardiovascular disease (ASCVD) and inform treatment decisions, even if they cannot yet be lowered directly, recommends the National Lipid Association (NLA) in a scientific statement.
The statement was published in the Journal of Clinical Lipidology.
Don P. Wilson, MD, department of pediatric endocrinology and diabetes, Cook Children’s Medical Center, Fort Worth, Tex., told this news organization that lipoprotein(a) is a “very timely subject.”
“The question in the scientific community is: What role does that particular biomarker play in terms of causing serious heart disease, stroke, and calcification of the aortic valve?”
“It’s pretty clear that, in and of itself, it actually can contribute and or cause any of those conditions,” he added. “The thing that’s then sort of problematic is that we don’t have a specific treatment to lower” Lp(a).
However, Dr. Wilson said that the statement underlines it is “still worth knowing” an individual’s Lp(a) concentrations because the risk with increased levels is “even higher for those people who have other conditions, such as metabolic disease or diabetes or high cholesterol.”
There are nevertheless several drugs in phase 2 and 3 clinical trials that appear to have the potential to significantly lower Lp(a) levels.
“I’m very excited,” said Dr. Wilson, noting that, so far, the drugs seem to be “quite safe,” and the currently available data suggest that they can “reduce Lp(a) levels by about 90%, which is huge.”
“That’s better than any drug we’ve got on the market.”
He cautioned, however, that it is going to take time after the drugs are approved to see the real benefits and risks once they start being used in very large populations, given that raised Lp(a) concentrations are present in about 20% of the world population.
The publication of the NLA statement coincides with a similar one from the European Atherosclerosis Society presented at the European Society of Cardiology Congress 2022 on Aug. 29, and published simultaneously in the European Heart Journal.
Coauthor of the EAS statement, Alberico L. Catapano, MD, PhD, professor of pharmacology at the University of Milan, and past president of the EAS, said that there are many areas in which the two statements are “in complete agreement.”
“However, the spirit of the documents is different,” he continued, chief among them being that the EAS statement focuses on the “global risk” of ASCVD and provides a risk calculator to help balance the risk increase with Lp(a) with that from other factors.
Another is that increased Lp(a) levels are recognized as being on a continuum in terms of their risk, such that there is no level at which raised concentrations can be deemed safe.
Dr. Wilson agreed with Dr. Capatano’s assessment, saying that the EAS statement takes current scientific observations “a step further,” in part by emphasizing that Lp(a) is “only one piece of the puzzle” for determining an individuals’ cardiovascular risk.
This will have huge implications for the conversations clinicians have with patients over shared decision-making, Dr. Wilson added.
Nevertheless, Dr. Catapano underlined to this news organization that “both documents are very important” in terms of the need to “raise awareness about a causal risk factor” for cardiovascular disease as well as that modifying Lp(a) concentrations “will probably reduce the risk.”
The statement from the NLA builds on the association’s prior Recommendations for the Patient-Centered Management of Dyslipidemia, published in two parts in 2014 and 2015, and comes to many of the same conclusions as the EAS statement.
It explains that apolipoprotein A, a component of Lp(a) attached to apolipoprotein B, has “unique” properties that promote the “initiation and progression of atherosclerosis and calcific valvular aortic stenosis, through endothelial dysfunction and proinflammatory responses, and pro-osteogenic effects promoting calcification.”
This, in turn, has the potential to cause myocardial infarction and ischemic stroke, the authors note.
This has been confirmed in meta-analyses of prospective, population-based studies showing a high risk for MI, coronary heart disease, and ischemic stroke with high Lp(a) levels, the statement adds.
Moreover, large genetic studies have confirmed that Lp(a) is a causal factor, independent of low-density lipoprotein cholesterol levels, for MI, ischemic stroke, valvular aortic stenosis, coronary artery stenosis, carotid stenosis, femoral artery stenosis, heart failure, cardiovascular mortality, and all-cause mortality.
Like the authors of the EAS statement, the NLA statement authors underline that the measurement of Lp(a) is “currently not standardized or harmonized,” and there is insufficient evidence on the utility of different cut-offs for risk based on age, gender, ethnicity, or the presence of comorbid conditions.
However, they do suggest that Lp(a) levels greater than 50 mg/dL (> 100 nmol/L) may be considered as a risk-enhancing factor favoring the initiation of statin therapy, although they note that the threshold could be threefold higher in African American individuals.
Despite these reservations, the authors say that Lp(a) testing “is reasonable” for refining the risk assessment of ASCVD in the first-degree relatives of people with premature ASCVD and those with a personal history of premature disease as well as in individuals with primary severe hypercholesterolemia.
Testing also “may be reasonable” to “aid in the clinician-patient discussion about whether to prescribe a statin” in people aged 40-75 years with borderline 10-year ASCVD risk, defined as 5%-7.4%, as well as in other equivocal clinical situations.
In terms of what to do in an individual with raised Lp(a) levels, the statement notes that lifestyle therapy and statins do not decrease Lp(a).
Although lomitapide (Juxtapid) and proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors both lower levels of the lipoprotein, the former is “not recommended for ASCVD risk reduction,” whereas the impact of the latter on ASCVD risk reduction via Lp(a) reduction “remains undetermined.”
Several experimental agents are currently under investigation to reduce Lp(a) levels, including SLN360 (Silence Therapeutics), and AKCEA-APO(a)-LRX (Akcea Therapeutics/Ionis Pharmaceuticals).
In the meantime, the authors say it is reasonable to use Lp(a) as a “risk-enhancing factor” for the initiation of moderate- or high-intensity statins in the primary prevention of ASCVD and to consider the addition of ezetimibe and/or PCSK9 inhibitors in high- and very high–risk patients already on maximally tolerated statin therapy.
Finally, the authors recognize the need for “additional evidence” to support clinical practice. In the absence of a randomized clinical trial of Lp(a) lowering in those who are at risk for ASCVD, they note that “several important unanswered questions remain.”
These include: “Is it reasonable to recommend universal testing of Lp(a) in everyone regardless of family history or health status at least once to help encourage healthy habits and inform clinical decision-making?” “Will earlier testing and effective interventions help to improve outcomes?”
Alongside more evidence in children, the authors also emphasize that “additional data are urgently needed in Blacks, South Asians, and those of Hispanic descent.”
No funding declared. Dr. Wilson declares relationships with Osler Institute, Merck Sharp & Dohm, Novo Nordisk, and Alexion Pharmaceuticals. Other authors also declare numerous relationships. Dr. Catapano declares a relationship with Novartis.
A version of this article first appeared on Medscape.com.
Lipoprotein(a) (Lp[a]) levels should be measured in clinical practice to refine risk prediction for atherosclerotic cardiovascular disease (ASCVD) and inform treatment decisions, even if they cannot yet be lowered directly, recommends the National Lipid Association (NLA) in a scientific statement.
The statement was published in the Journal of Clinical Lipidology.
Don P. Wilson, MD, department of pediatric endocrinology and diabetes, Cook Children’s Medical Center, Fort Worth, Tex., told this news organization that lipoprotein(a) is a “very timely subject.”
“The question in the scientific community is: What role does that particular biomarker play in terms of causing serious heart disease, stroke, and calcification of the aortic valve?”
“It’s pretty clear that, in and of itself, it actually can contribute and or cause any of those conditions,” he added. “The thing that’s then sort of problematic is that we don’t have a specific treatment to lower” Lp(a).
However, Dr. Wilson said that the statement underlines it is “still worth knowing” an individual’s Lp(a) concentrations because the risk with increased levels is “even higher for those people who have other conditions, such as metabolic disease or diabetes or high cholesterol.”
There are nevertheless several drugs in phase 2 and 3 clinical trials that appear to have the potential to significantly lower Lp(a) levels.
“I’m very excited,” said Dr. Wilson, noting that, so far, the drugs seem to be “quite safe,” and the currently available data suggest that they can “reduce Lp(a) levels by about 90%, which is huge.”
“That’s better than any drug we’ve got on the market.”
He cautioned, however, that it is going to take time after the drugs are approved to see the real benefits and risks once they start being used in very large populations, given that raised Lp(a) concentrations are present in about 20% of the world population.
The publication of the NLA statement coincides with a similar one from the European Atherosclerosis Society presented at the European Society of Cardiology Congress 2022 on Aug. 29, and published simultaneously in the European Heart Journal.
Coauthor of the EAS statement, Alberico L. Catapano, MD, PhD, professor of pharmacology at the University of Milan, and past president of the EAS, said that there are many areas in which the two statements are “in complete agreement.”
“However, the spirit of the documents is different,” he continued, chief among them being that the EAS statement focuses on the “global risk” of ASCVD and provides a risk calculator to help balance the risk increase with Lp(a) with that from other factors.
Another is that increased Lp(a) levels are recognized as being on a continuum in terms of their risk, such that there is no level at which raised concentrations can be deemed safe.
Dr. Wilson agreed with Dr. Capatano’s assessment, saying that the EAS statement takes current scientific observations “a step further,” in part by emphasizing that Lp(a) is “only one piece of the puzzle” for determining an individuals’ cardiovascular risk.
This will have huge implications for the conversations clinicians have with patients over shared decision-making, Dr. Wilson added.
Nevertheless, Dr. Catapano underlined to this news organization that “both documents are very important” in terms of the need to “raise awareness about a causal risk factor” for cardiovascular disease as well as that modifying Lp(a) concentrations “will probably reduce the risk.”
The statement from the NLA builds on the association’s prior Recommendations for the Patient-Centered Management of Dyslipidemia, published in two parts in 2014 and 2015, and comes to many of the same conclusions as the EAS statement.
It explains that apolipoprotein A, a component of Lp(a) attached to apolipoprotein B, has “unique” properties that promote the “initiation and progression of atherosclerosis and calcific valvular aortic stenosis, through endothelial dysfunction and proinflammatory responses, and pro-osteogenic effects promoting calcification.”
This, in turn, has the potential to cause myocardial infarction and ischemic stroke, the authors note.
This has been confirmed in meta-analyses of prospective, population-based studies showing a high risk for MI, coronary heart disease, and ischemic stroke with high Lp(a) levels, the statement adds.
Moreover, large genetic studies have confirmed that Lp(a) is a causal factor, independent of low-density lipoprotein cholesterol levels, for MI, ischemic stroke, valvular aortic stenosis, coronary artery stenosis, carotid stenosis, femoral artery stenosis, heart failure, cardiovascular mortality, and all-cause mortality.
Like the authors of the EAS statement, the NLA statement authors underline that the measurement of Lp(a) is “currently not standardized or harmonized,” and there is insufficient evidence on the utility of different cut-offs for risk based on age, gender, ethnicity, or the presence of comorbid conditions.
However, they do suggest that Lp(a) levels greater than 50 mg/dL (> 100 nmol/L) may be considered as a risk-enhancing factor favoring the initiation of statin therapy, although they note that the threshold could be threefold higher in African American individuals.
Despite these reservations, the authors say that Lp(a) testing “is reasonable” for refining the risk assessment of ASCVD in the first-degree relatives of people with premature ASCVD and those with a personal history of premature disease as well as in individuals with primary severe hypercholesterolemia.
Testing also “may be reasonable” to “aid in the clinician-patient discussion about whether to prescribe a statin” in people aged 40-75 years with borderline 10-year ASCVD risk, defined as 5%-7.4%, as well as in other equivocal clinical situations.
In terms of what to do in an individual with raised Lp(a) levels, the statement notes that lifestyle therapy and statins do not decrease Lp(a).
Although lomitapide (Juxtapid) and proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors both lower levels of the lipoprotein, the former is “not recommended for ASCVD risk reduction,” whereas the impact of the latter on ASCVD risk reduction via Lp(a) reduction “remains undetermined.”
Several experimental agents are currently under investigation to reduce Lp(a) levels, including SLN360 (Silence Therapeutics), and AKCEA-APO(a)-LRX (Akcea Therapeutics/Ionis Pharmaceuticals).
In the meantime, the authors say it is reasonable to use Lp(a) as a “risk-enhancing factor” for the initiation of moderate- or high-intensity statins in the primary prevention of ASCVD and to consider the addition of ezetimibe and/or PCSK9 inhibitors in high- and very high–risk patients already on maximally tolerated statin therapy.
Finally, the authors recognize the need for “additional evidence” to support clinical practice. In the absence of a randomized clinical trial of Lp(a) lowering in those who are at risk for ASCVD, they note that “several important unanswered questions remain.”
These include: “Is it reasonable to recommend universal testing of Lp(a) in everyone regardless of family history or health status at least once to help encourage healthy habits and inform clinical decision-making?” “Will earlier testing and effective interventions help to improve outcomes?”
Alongside more evidence in children, the authors also emphasize that “additional data are urgently needed in Blacks, South Asians, and those of Hispanic descent.”
No funding declared. Dr. Wilson declares relationships with Osler Institute, Merck Sharp & Dohm, Novo Nordisk, and Alexion Pharmaceuticals. Other authors also declare numerous relationships. Dr. Catapano declares a relationship with Novartis.
A version of this article first appeared on Medscape.com.
Why some infectious disease docs are ‘encouraged’ by new bivalent COVID vaccines
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
Nocturnally pruritic rash
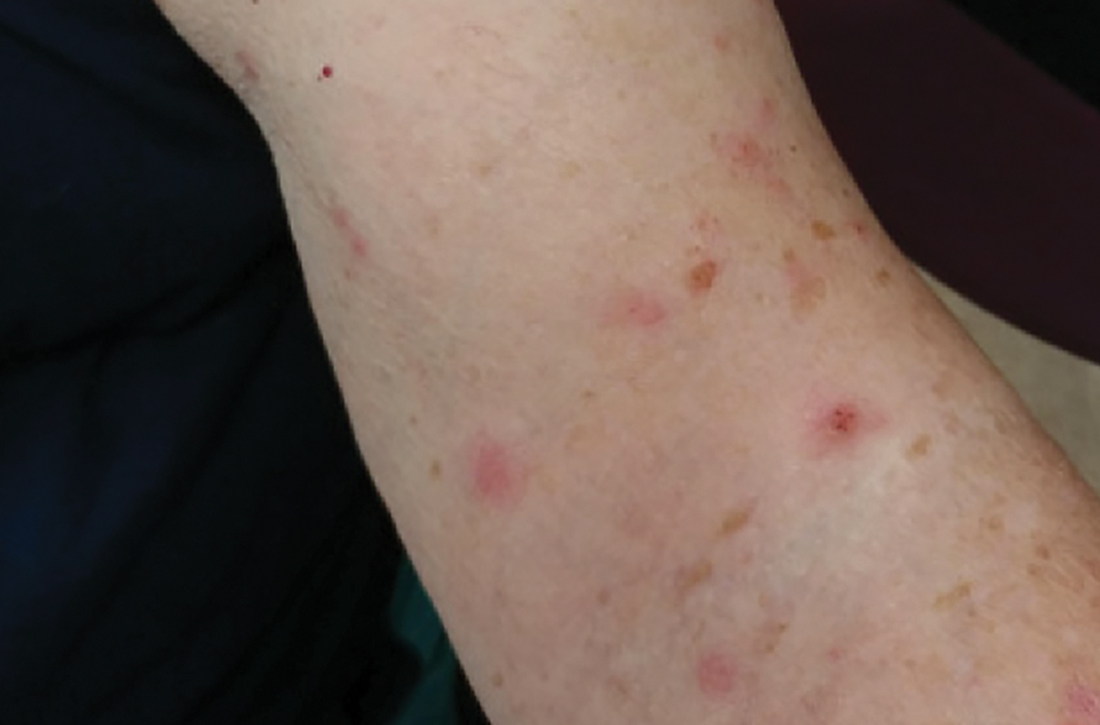
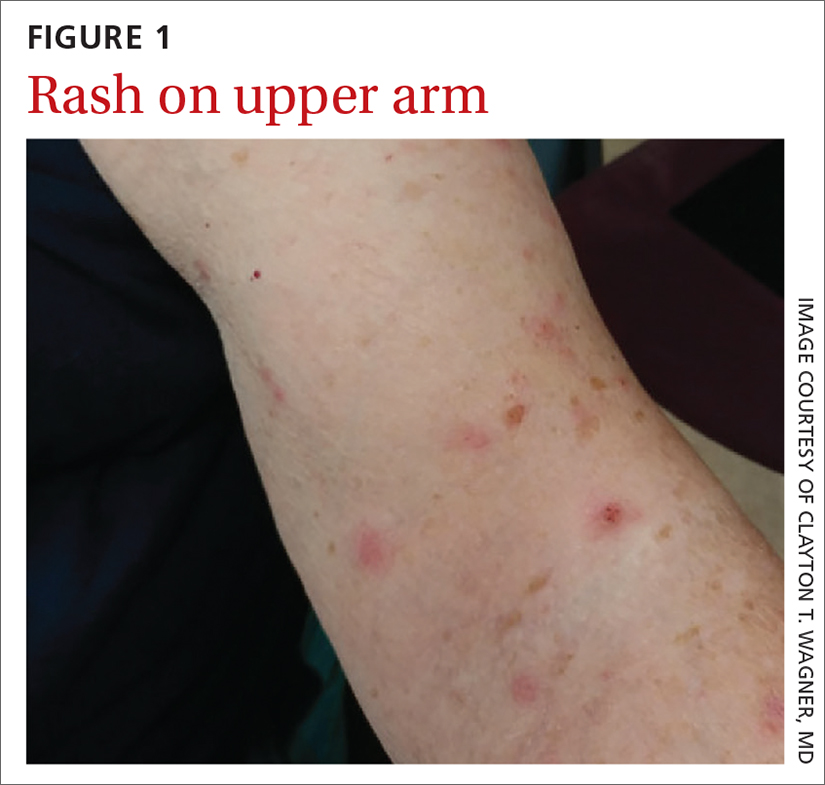
A 74-YEAR-OLD WOMAN presented with a 3-day history of an intensely pruritic rash that was localized to her upper arms, upper chest between her breasts, and upper back. The pruritus was much worse at night while the patient was in bed. Symptoms did not improve with over-the-counter topical corticosteroids.
The patient had a history of atrial fibrillation (for which she was receiving chronic anticoagulation therapy), hypertension, an implanted pacemaker, depression, and Parkinson disease. Her medications included carbidopa-levodopa, fluoxetine, hydrochlorothiazide, metoprolol tartrate, naproxen, and warfarin. She had no known allergies. She reported that she was a nonsmoker and drank 1 glass of wine per week.
There were no recent changes in soaps, detergents, lotions, or makeup, nor did the patient have any bug bites or plant exposure. She shared a home with her spouse and several pets: a dog, a cat, and a Bantam-breed chicken. The patient’s husband, who slept in a different bedroom, had no rash. Recently, the cat had been bringing its captured prey of rabbits into the home.
Review of systems was negative for fever, chills, shortness of breath, cough, throat swelling, and rhinorrhea. Physical examination revealed red/pink macules and papules scattered over the upper arms (FIGURE 1), chest, and upper back. Many lesions were excoriated but had no active bleeding or vesicles. Under dermatoscope, no burrowing was found; however, a small (< 1 mm) creature was seen moving rapidly across the skin surface. The physician (CTW) captured and isolated the creature using a sterile lab cup.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gamasoidosis
The collected sample (FIGURE 2) was examined and identified as an avian mite by a colleague who specializes in entomology, confirming the diagnosis of gamasoidosis.

Two genera of avian mites are responsible: Dermanyssus and Ornithonyssus. The most common culprits are the red poultry mite (D gallinae) and the northern fowl mite (O bursa). These small mites parasitize birds, such as poultry livestock, domesticated birds, and wild game birds. When unfed, the mite appears translucent brown and measures 0.3 to 0.7 mm in length, but after a blood meal, it appears red and increases in size to 1 mm. The mites tend to be active and feed at night and hide during the day.2 This explained the severe nighttime pruritus in this case.
Human infestation, although infrequent, can be a concern for those who work with poultry, or during the spring and summer seasons when young birds leave their nests and the mites migrate to find alternative hosts.3 The 1- to 2-mm erythematous maculopapules are often found with excoriations in covered areas.3,4 Unlike scabies, the genitalia and interdigital areas are spared.3,5
Differential for arthropod dermatoses
The differential diagnosis includes cimicosis, pulicosis, pediculosis corporis, and scabies.
Cimicosis is caused by bed bugs (from the insect Cimex genus). Bed bugs are oval and reddish brown, have 6 legs, and range in size from 1 to 7 mm. Most bed bugs hide in cracks or crevices of furniture and other surfaces (eg, bed frames, headboards, seams or holes of box springs or mattresses, or behind wallpaper, switch plates, and picture frames) by day and come out at night to feed on a sleeping host. Commonly, bed bugs will leave a series of bites grouped in rows (described as “breakfast, lunch, and dinner”). The bites can mimic urticaria, and bullous reactions may also occur.2
Continue to: Pulicosis
Pulicosis results from bites caused by a variety of flea species including, but not limited to, human, dog, oriental rat, sticktight, mouse, and chicken fleas. Fleas are small brown insects measuring about 2.5 mm in length, with flat sides and long hind legs. Their bites are most often arranged in a zigzag pattern around a host’s legs and waist. Hypersensitivity reactions may appear as papular urticaria, nodules, or bullae.2
Pediculosis corporis is caused by body lice. The adult louse is 2.5 to 3.5 mm in size, has 6 legs, and is a tan to greyish white color.6 Lice live in clothing, lay their eggs within the seams, and obtain blood meals from the host. Symptoms include generalized itching. The erythematous blue- and copper-colored macules, wheals, and lichenification can occur throughout the body, but spare the hands and feet. Secondary impetigo and furunculosis commonly occur.2
Scabies is caused by an oval mite that is ventrally flat, with dorsal spines. The mite is < 0.5 mm in size, appearing as a pinpoint of white. It burrows into its host’s skin, where it lives and lays eggs, causing pruritic papular lesions and ensuing excoriations. The mite burrows with a predilection for the finger web spaces, wrists, axillae, areolae, umbilicus, lower abdomen, genitals, and buttocks.2
Treatment involves a 3-step process
The mainstay of treatment is removal of the infested bird, decontamination of bedding and clothing, and use of oral antihistamines and topical corticosteroids.1,3,5 Bedding and clothing should be washed. Carpets, rugs, and curtains should be vacuumed and the vacuum bag placed in a sealed bag in the freezer for several hours before it can be thrown away. Eggs, larvae, nymphs, and adults are killed at 55 to 60 °F. Because humans are only incidental hosts and mites do not reproduce on them, the use of scabicidal agents, such as permethrin, is controversial.
Our patient was treated with permethrin cream before definitive identification of the mite. Once the mite was identified, the chicken was removed from the home and the patient’s bedding and clothing were decontaminated. The patient continued to apply over-the-counter topical steroids and take oral antihistamines for several more days after the chicken was removed from the home.
ACKNOWLEDGEMENT
The authors would like to acknowledge Patrick Liesch of the University of Wisconsin-Madison’s Department of Entomology, Insect Diagnostic Lab, for his help in identifying the avian mite.
1. Leib AE, Anderson BE. Pruritic dermatitis caused by bird mite infestation. Cutis. 2016;97:E6-E8.
2. Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377. doi: 10.1111/j.1365-2230.2012.04434.x
3. Baselga E, Drolet BA, Esterly NB. Avian mite dermatitis. Pediatrics. 1996;97:743-745.
4. James WD, Elston DM, Treat J, et al, eds. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. J Dermatolog Treat. 2010;21:319-321. doi: 10.3109/09546630903287437
6. Centers for Disease Control and Prevention. Parasites. Reviewed September 12, 2019. Accessed August 4, 2022. www.cdc.gov/parasites/lice/body/biology.html
A 74-YEAR-OLD WOMAN presented with a 3-day history of an intensely pruritic rash that was localized to her upper arms, upper chest between her breasts, and upper back. The pruritus was much worse at night while the patient was in bed. Symptoms did not improve with over-the-counter topical corticosteroids.
The patient had a history of atrial fibrillation (for which she was receiving chronic anticoagulation therapy), hypertension, an implanted pacemaker, depression, and Parkinson disease. Her medications included carbidopa-levodopa, fluoxetine, hydrochlorothiazide, metoprolol tartrate, naproxen, and warfarin. She had no known allergies. She reported that she was a nonsmoker and drank 1 glass of wine per week.
There were no recent changes in soaps, detergents, lotions, or makeup, nor did the patient have any bug bites or plant exposure. She shared a home with her spouse and several pets: a dog, a cat, and a Bantam-breed chicken. The patient’s husband, who slept in a different bedroom, had no rash. Recently, the cat had been bringing its captured prey of rabbits into the home.
Review of systems was negative for fever, chills, shortness of breath, cough, throat swelling, and rhinorrhea. Physical examination revealed red/pink macules and papules scattered over the upper arms (FIGURE 1), chest, and upper back. Many lesions were excoriated but had no active bleeding or vesicles. Under dermatoscope, no burrowing was found; however, a small (< 1 mm) creature was seen moving rapidly across the skin surface. The physician (CTW) captured and isolated the creature using a sterile lab cup.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gamasoidosis
The collected sample (FIGURE 2) was examined and identified as an avian mite by a colleague who specializes in entomology, confirming the diagnosis of gamasoidosis.

Two genera of avian mites are responsible: Dermanyssus and Ornithonyssus. The most common culprits are the red poultry mite (D gallinae) and the northern fowl mite (O bursa). These small mites parasitize birds, such as poultry livestock, domesticated birds, and wild game birds. When unfed, the mite appears translucent brown and measures 0.3 to 0.7 mm in length, but after a blood meal, it appears red and increases in size to 1 mm. The mites tend to be active and feed at night and hide during the day.2 This explained the severe nighttime pruritus in this case.
Human infestation, although infrequent, can be a concern for those who work with poultry, or during the spring and summer seasons when young birds leave their nests and the mites migrate to find alternative hosts.3 The 1- to 2-mm erythematous maculopapules are often found with excoriations in covered areas.3,4 Unlike scabies, the genitalia and interdigital areas are spared.3,5
Differential for arthropod dermatoses
The differential diagnosis includes cimicosis, pulicosis, pediculosis corporis, and scabies.
Cimicosis is caused by bed bugs (from the insect Cimex genus). Bed bugs are oval and reddish brown, have 6 legs, and range in size from 1 to 7 mm. Most bed bugs hide in cracks or crevices of furniture and other surfaces (eg, bed frames, headboards, seams or holes of box springs or mattresses, or behind wallpaper, switch plates, and picture frames) by day and come out at night to feed on a sleeping host. Commonly, bed bugs will leave a series of bites grouped in rows (described as “breakfast, lunch, and dinner”). The bites can mimic urticaria, and bullous reactions may also occur.2
Continue to: Pulicosis
Pulicosis results from bites caused by a variety of flea species including, but not limited to, human, dog, oriental rat, sticktight, mouse, and chicken fleas. Fleas are small brown insects measuring about 2.5 mm in length, with flat sides and long hind legs. Their bites are most often arranged in a zigzag pattern around a host’s legs and waist. Hypersensitivity reactions may appear as papular urticaria, nodules, or bullae.2
Pediculosis corporis is caused by body lice. The adult louse is 2.5 to 3.5 mm in size, has 6 legs, and is a tan to greyish white color.6 Lice live in clothing, lay their eggs within the seams, and obtain blood meals from the host. Symptoms include generalized itching. The erythematous blue- and copper-colored macules, wheals, and lichenification can occur throughout the body, but spare the hands and feet. Secondary impetigo and furunculosis commonly occur.2
Scabies is caused by an oval mite that is ventrally flat, with dorsal spines. The mite is < 0.5 mm in size, appearing as a pinpoint of white. It burrows into its host’s skin, where it lives and lays eggs, causing pruritic papular lesions and ensuing excoriations. The mite burrows with a predilection for the finger web spaces, wrists, axillae, areolae, umbilicus, lower abdomen, genitals, and buttocks.2
Treatment involves a 3-step process
The mainstay of treatment is removal of the infested bird, decontamination of bedding and clothing, and use of oral antihistamines and topical corticosteroids.1,3,5 Bedding and clothing should be washed. Carpets, rugs, and curtains should be vacuumed and the vacuum bag placed in a sealed bag in the freezer for several hours before it can be thrown away. Eggs, larvae, nymphs, and adults are killed at 55 to 60 °F. Because humans are only incidental hosts and mites do not reproduce on them, the use of scabicidal agents, such as permethrin, is controversial.
Our patient was treated with permethrin cream before definitive identification of the mite. Once the mite was identified, the chicken was removed from the home and the patient’s bedding and clothing were decontaminated. The patient continued to apply over-the-counter topical steroids and take oral antihistamines for several more days after the chicken was removed from the home.
ACKNOWLEDGEMENT
The authors would like to acknowledge Patrick Liesch of the University of Wisconsin-Madison’s Department of Entomology, Insect Diagnostic Lab, for his help in identifying the avian mite.
A 74-YEAR-OLD WOMAN presented with a 3-day history of an intensely pruritic rash that was localized to her upper arms, upper chest between her breasts, and upper back. The pruritus was much worse at night while the patient was in bed. Symptoms did not improve with over-the-counter topical corticosteroids.
The patient had a history of atrial fibrillation (for which she was receiving chronic anticoagulation therapy), hypertension, an implanted pacemaker, depression, and Parkinson disease. Her medications included carbidopa-levodopa, fluoxetine, hydrochlorothiazide, metoprolol tartrate, naproxen, and warfarin. She had no known allergies. She reported that she was a nonsmoker and drank 1 glass of wine per week.
There were no recent changes in soaps, detergents, lotions, or makeup, nor did the patient have any bug bites or plant exposure. She shared a home with her spouse and several pets: a dog, a cat, and a Bantam-breed chicken. The patient’s husband, who slept in a different bedroom, had no rash. Recently, the cat had been bringing its captured prey of rabbits into the home.
Review of systems was negative for fever, chills, shortness of breath, cough, throat swelling, and rhinorrhea. Physical examination revealed red/pink macules and papules scattered over the upper arms (FIGURE 1), chest, and upper back. Many lesions were excoriated but had no active bleeding or vesicles. Under dermatoscope, no burrowing was found; however, a small (< 1 mm) creature was seen moving rapidly across the skin surface. The physician (CTW) captured and isolated the creature using a sterile lab cup.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gamasoidosis
The collected sample (FIGURE 2) was examined and identified as an avian mite by a colleague who specializes in entomology, confirming the diagnosis of gamasoidosis.

Two genera of avian mites are responsible: Dermanyssus and Ornithonyssus. The most common culprits are the red poultry mite (D gallinae) and the northern fowl mite (O bursa). These small mites parasitize birds, such as poultry livestock, domesticated birds, and wild game birds. When unfed, the mite appears translucent brown and measures 0.3 to 0.7 mm in length, but after a blood meal, it appears red and increases in size to 1 mm. The mites tend to be active and feed at night and hide during the day.2 This explained the severe nighttime pruritus in this case.
Human infestation, although infrequent, can be a concern for those who work with poultry, or during the spring and summer seasons when young birds leave their nests and the mites migrate to find alternative hosts.3 The 1- to 2-mm erythematous maculopapules are often found with excoriations in covered areas.3,4 Unlike scabies, the genitalia and interdigital areas are spared.3,5
Differential for arthropod dermatoses
The differential diagnosis includes cimicosis, pulicosis, pediculosis corporis, and scabies.
Cimicosis is caused by bed bugs (from the insect Cimex genus). Bed bugs are oval and reddish brown, have 6 legs, and range in size from 1 to 7 mm. Most bed bugs hide in cracks or crevices of furniture and other surfaces (eg, bed frames, headboards, seams or holes of box springs or mattresses, or behind wallpaper, switch plates, and picture frames) by day and come out at night to feed on a sleeping host. Commonly, bed bugs will leave a series of bites grouped in rows (described as “breakfast, lunch, and dinner”). The bites can mimic urticaria, and bullous reactions may also occur.2
Continue to: Pulicosis
Pulicosis results from bites caused by a variety of flea species including, but not limited to, human, dog, oriental rat, sticktight, mouse, and chicken fleas. Fleas are small brown insects measuring about 2.5 mm in length, with flat sides and long hind legs. Their bites are most often arranged in a zigzag pattern around a host’s legs and waist. Hypersensitivity reactions may appear as papular urticaria, nodules, or bullae.2
Pediculosis corporis is caused by body lice. The adult louse is 2.5 to 3.5 mm in size, has 6 legs, and is a tan to greyish white color.6 Lice live in clothing, lay their eggs within the seams, and obtain blood meals from the host. Symptoms include generalized itching. The erythematous blue- and copper-colored macules, wheals, and lichenification can occur throughout the body, but spare the hands and feet. Secondary impetigo and furunculosis commonly occur.2
Scabies is caused by an oval mite that is ventrally flat, with dorsal spines. The mite is < 0.5 mm in size, appearing as a pinpoint of white. It burrows into its host’s skin, where it lives and lays eggs, causing pruritic papular lesions and ensuing excoriations. The mite burrows with a predilection for the finger web spaces, wrists, axillae, areolae, umbilicus, lower abdomen, genitals, and buttocks.2
Treatment involves a 3-step process
The mainstay of treatment is removal of the infested bird, decontamination of bedding and clothing, and use of oral antihistamines and topical corticosteroids.1,3,5 Bedding and clothing should be washed. Carpets, rugs, and curtains should be vacuumed and the vacuum bag placed in a sealed bag in the freezer for several hours before it can be thrown away. Eggs, larvae, nymphs, and adults are killed at 55 to 60 °F. Because humans are only incidental hosts and mites do not reproduce on them, the use of scabicidal agents, such as permethrin, is controversial.
Our patient was treated with permethrin cream before definitive identification of the mite. Once the mite was identified, the chicken was removed from the home and the patient’s bedding and clothing were decontaminated. The patient continued to apply over-the-counter topical steroids and take oral antihistamines for several more days after the chicken was removed from the home.
ACKNOWLEDGEMENT
The authors would like to acknowledge Patrick Liesch of the University of Wisconsin-Madison’s Department of Entomology, Insect Diagnostic Lab, for his help in identifying the avian mite.
1. Leib AE, Anderson BE. Pruritic dermatitis caused by bird mite infestation. Cutis. 2016;97:E6-E8.
2. Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377. doi: 10.1111/j.1365-2230.2012.04434.x
3. Baselga E, Drolet BA, Esterly NB. Avian mite dermatitis. Pediatrics. 1996;97:743-745.
4. James WD, Elston DM, Treat J, et al, eds. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. J Dermatolog Treat. 2010;21:319-321. doi: 10.3109/09546630903287437
6. Centers for Disease Control and Prevention. Parasites. Reviewed September 12, 2019. Accessed August 4, 2022. www.cdc.gov/parasites/lice/body/biology.html
1. Leib AE, Anderson BE. Pruritic dermatitis caused by bird mite infestation. Cutis. 2016;97:E6-E8.
2. Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377. doi: 10.1111/j.1365-2230.2012.04434.x
3. Baselga E, Drolet BA, Esterly NB. Avian mite dermatitis. Pediatrics. 1996;97:743-745.
4. James WD, Elston DM, Treat J, et al, eds. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. J Dermatolog Treat. 2010;21:319-321. doi: 10.3109/09546630903287437
6. Centers for Disease Control and Prevention. Parasites. Reviewed September 12, 2019. Accessed August 4, 2022. www.cdc.gov/parasites/lice/body/biology.html
56-year-old man • increased heart rate • weakness • intense sweating • horseradish consumption • Dx?
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; [email protected]
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; [email protected]
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; [email protected]
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
Noncardiac inpatient has acute hypertension: Treat or not?
ILLUSTRATIVE CASE
A 48-year-old man is admitted to your family medicine service for cellulitis after failed outpatient therapy. He has presumed community-acquired methicillin-resistant Staphylococcus aureus infection of the left lower extremity and is receiving intravenous (IV) vancomycin. His BP this morning is 176/98 mm Hg, and the reading from the previous shift was 168/94 mm Hg. He is asymptomatic from this elevated BP. Based on protocol, his nurse is asking about treatment in response to the multiple elevated readings. How should you address the patient’s elevated BP, knowing that you will see him for a transition management appointment in 2 weeks?
Elevated BP is common in the adult inpatient setting. Prevalence estimates range from 25% to > 50%. Many factors can contribute to elevated BP in the acute illness setting, such as pain, anxiety, medication withdrawal, and volume status.2,3
Treatment of elevated BP in outpatients is well researched, with evidence-based guidelines for physicians. That is not the case for treatment of asymptomatic elevated BP in the inpatient setting. Most published guidance on inpatient management of acutely elevated BP recommends IV medications, such as hydralazine or labetalol, although there is limited evidence to support such recommendations. There is minimal evidence for outcomes-based benefit in treating acute elevations of inpatient BP, such as reduced myocardial injury or stroke; however, there is some evidence of adverse outcomes, such as hypotension and prolonged hospital stays.4-8
Although the possibility of intensifying antihypertensive therapy for those with known hypertension or those with presumed “new-onset” hypertension could theoretically lead to improved outcomes over the long term, there is little evidence to support this presumption. Rather, there is evidence that intensification of antihypertensive therapy at discharge is linked to short-term harms. This was demonstrated in a propensity-matched veteran cohort that included 4056 hospitalized older adults with hypertension (mean age, 77 years; 3961 men), equally split between those who received antihypertensive intensification at hospital discharge and those who did not. Within 30 days, patients receiving intensification had a higher risk of readmission (number needed to harm [NNH] = 27) and serious adverse events (NNH = 63).9
The current study aimed to put all these pieces together by quantifying the prevalence of hypertension in hospitalized patients, characterizing clinician response to patients’ acutely elevated BP, and comparing both short- and long-term outcomes in patients treated for acute BP elevations while hospitalized vs those who were not. The study also assessed the potential effects of antihypertensive intensification at discharge.
STUDY SUMMARY
Treatment of acute hypertension was associated with end-organ injury
This retrospective, propensity score–matched cohort study (N = 22,834) evaluated the electronic health records of all adult patients (age > 18 years) admitted to a medicine service with a noncardiovascular diagnosis over a 1-year period at 10 Cleveland Clinic hospitals, with 1 year of follow-up data.
Exclusion criteria included hospitalization for a cardiovascular diagnosis; admission for a cerebrovascular event or acute coronary syndrome within the previous 30 days; pregnancy; length of stay of less than 2 days or more than 14 days; and lack of outpatient medication data. Patients were propensity-score matched using BP, demographic features, comorbidities, hospital shift, and time since admission. Exposure was defined as administration of IV antihypertensive medication or a new class of oral antihypertensive medication.
Continue to: Outcomes were defined...
Outcomes were defined as a temporal association between acute hypertension treatment and subsequent end-organ damage, such as AKI (serum creatinine increase ≥ 0.3 mg/dL or 1.5 × initial value [Acute Kidney Injury Network definition]), myocardial injury (elevated troponin: > 0.029 ng/mL for troponin T; > 0.045 ng/mL for troponin I), and/or stroke (indicated by discharge diagnosis, with confirmation by chart review). Monitored outcomes included stroke and myocardial infarction (MI) within 30 days of discharge and BP control up to 1 year later.
The 22,834 patients had a mean (SD) age of 65.6 (17.9) years; 12,993 (56.9%) were women, and 15,963 (69.9%) were White. Of the 17,821 (78%) who had at least 1 inpatient hypertensive systolic BP (SBP) episode, defined as an SBP ≥ 140 mm Hg, 5904 (33.1%) received a new treatment. Of those receiving a new treatment, 4378 (74.2%) received only oral treatment, and 1516 (25.7%) received at least 1 dose of IV medication with or without oral dosing.
Using the propensity-matched sample (4520 treated for elevated BP matched to 4520 who were not treated), treated patients had higher rates of AKI (10.3% vs 7.9%; P < .001) and myocardial injury (1.2% vs 0.6%; P = .003). When assessed by SBP, nontreatment of BP was still superior up to an SBP of 199 mm Hg. At an SBP of ≥ 200 mm Hg, there was no difference in rates of AKI or MI between the treatment and nontreatment groups. There was no difference in stroke in either cohort, although the overall numbers were quite low.
Patients with and without antihypertensive intensification at discharge had similar rates of MI (0.1% vs 0.2%; P > .99) and stroke (0.5% vs 0.4%; P > .99) in a matched cohort at 30 days post discharge. At 1 year, BP control in the intensification vs no-intensification groups was nearly the same: maximum SBP was 157.2 mm Hg vs 157.8 mm Hg, respectively (P = .54) and maximum diastolic BP was 86.5 mm Hg vs 86.1 mm Hg, respectively (P = .49).
WHAT’S NEW
Previous research is confirmed in a more diverse population
Whereas previous research showed no benefit to intensification of treatment among hospitalized older male patients, this large, retrospective, propensity score–matched cohort study demonstrated the short- and long-term effects of treating acute, asymptomatic BP elevations in a younger, more generalizable population that included women. Regardless of treatment modality, there appeared to be more harm than good from treating these BP elevations.
In addition, the study appears to corroborate previous research showing that intensification of BP treatment at discharge did not lead to better outcomes.9 At the very least, the study makes a reasonable argument that treating acute BP elevations in noncardiac patients in the hospital setting is not beneficial.
CAVEATS
Impact of existing therapy could be underestimated
This study had several important limitations. First, 23% of treated participants were excluded from the propensity analysis without justification from the authors. Additionally, there was no reporting of missing data and how it was managed. The authors’ definition of treatment excluded dose intensification of existing antihypertensive therapy, which would undercount the number of treated patients. However, this could underestimate the actual harms of the acute antihypertensive therapy. The authors also included patients with atrial fibrillation and heart failure in the study population, even though they already may have been taking antihypertensive agents.
CHALLENGES TO IMPLEMENTATION
Potential delays in translating findings to patient care
Although several recent studies have shown the potential benefit of not treating asymptomatic acute BP elevations in inpatients, incorporating that information into electronic health record order sets or clinical decision support, and disseminating it to clinical end users, will take time. In the interim, despite these findings, patients may continue to receive IV or oral medications to treat acute, asymptomatic BP elevations while hospitalized for noncardiac diagnoses.
1. Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352. doi: 10.1001/jamainternmed.2020.7501
2. Jacobs ZG, Najafi N, Fang MC, et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019;14:144-150. doi: 10.12788/jhm.3087
3. Pasik SD, Chiu S, Yang J, et al. Assess before Rx: reducing the overtreatment of asymptomatic blood pressure elevation in the inpatient setting. J Hosp Med. 2019;14:151-156. doi: 10.12788/jhm.3190
4. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5:473-477. doi: 10.1016/j.jash.2011.07.002
5. Gauer R. Severe asymptomatic hypertension: evaluation and treatment. Am Fam Physician. 2017;95:492-500.
6. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11:193-198. doi: 10.1002/jhm.2510
7. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2018;12:7-15. doi: 10.1177/1753944717746613
8. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12:29-33. doi: 10.1111/j.1751-7176.2009.00196.x
9. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536. doi: 10.1001/jamainternmed.2019.3007
ILLUSTRATIVE CASE
A 48-year-old man is admitted to your family medicine service for cellulitis after failed outpatient therapy. He has presumed community-acquired methicillin-resistant Staphylococcus aureus infection of the left lower extremity and is receiving intravenous (IV) vancomycin. His BP this morning is 176/98 mm Hg, and the reading from the previous shift was 168/94 mm Hg. He is asymptomatic from this elevated BP. Based on protocol, his nurse is asking about treatment in response to the multiple elevated readings. How should you address the patient’s elevated BP, knowing that you will see him for a transition management appointment in 2 weeks?
Elevated BP is common in the adult inpatient setting. Prevalence estimates range from 25% to > 50%. Many factors can contribute to elevated BP in the acute illness setting, such as pain, anxiety, medication withdrawal, and volume status.2,3
Treatment of elevated BP in outpatients is well researched, with evidence-based guidelines for physicians. That is not the case for treatment of asymptomatic elevated BP in the inpatient setting. Most published guidance on inpatient management of acutely elevated BP recommends IV medications, such as hydralazine or labetalol, although there is limited evidence to support such recommendations. There is minimal evidence for outcomes-based benefit in treating acute elevations of inpatient BP, such as reduced myocardial injury or stroke; however, there is some evidence of adverse outcomes, such as hypotension and prolonged hospital stays.4-8
Although the possibility of intensifying antihypertensive therapy for those with known hypertension or those with presumed “new-onset” hypertension could theoretically lead to improved outcomes over the long term, there is little evidence to support this presumption. Rather, there is evidence that intensification of antihypertensive therapy at discharge is linked to short-term harms. This was demonstrated in a propensity-matched veteran cohort that included 4056 hospitalized older adults with hypertension (mean age, 77 years; 3961 men), equally split between those who received antihypertensive intensification at hospital discharge and those who did not. Within 30 days, patients receiving intensification had a higher risk of readmission (number needed to harm [NNH] = 27) and serious adverse events (NNH = 63).9
The current study aimed to put all these pieces together by quantifying the prevalence of hypertension in hospitalized patients, characterizing clinician response to patients’ acutely elevated BP, and comparing both short- and long-term outcomes in patients treated for acute BP elevations while hospitalized vs those who were not. The study also assessed the potential effects of antihypertensive intensification at discharge.
STUDY SUMMARY
Treatment of acute hypertension was associated with end-organ injury
This retrospective, propensity score–matched cohort study (N = 22,834) evaluated the electronic health records of all adult patients (age > 18 years) admitted to a medicine service with a noncardiovascular diagnosis over a 1-year period at 10 Cleveland Clinic hospitals, with 1 year of follow-up data.
Exclusion criteria included hospitalization for a cardiovascular diagnosis; admission for a cerebrovascular event or acute coronary syndrome within the previous 30 days; pregnancy; length of stay of less than 2 days or more than 14 days; and lack of outpatient medication data. Patients were propensity-score matched using BP, demographic features, comorbidities, hospital shift, and time since admission. Exposure was defined as administration of IV antihypertensive medication or a new class of oral antihypertensive medication.
Continue to: Outcomes were defined...
Outcomes were defined as a temporal association between acute hypertension treatment and subsequent end-organ damage, such as AKI (serum creatinine increase ≥ 0.3 mg/dL or 1.5 × initial value [Acute Kidney Injury Network definition]), myocardial injury (elevated troponin: > 0.029 ng/mL for troponin T; > 0.045 ng/mL for troponin I), and/or stroke (indicated by discharge diagnosis, with confirmation by chart review). Monitored outcomes included stroke and myocardial infarction (MI) within 30 days of discharge and BP control up to 1 year later.
The 22,834 patients had a mean (SD) age of 65.6 (17.9) years; 12,993 (56.9%) were women, and 15,963 (69.9%) were White. Of the 17,821 (78%) who had at least 1 inpatient hypertensive systolic BP (SBP) episode, defined as an SBP ≥ 140 mm Hg, 5904 (33.1%) received a new treatment. Of those receiving a new treatment, 4378 (74.2%) received only oral treatment, and 1516 (25.7%) received at least 1 dose of IV medication with or without oral dosing.
Using the propensity-matched sample (4520 treated for elevated BP matched to 4520 who were not treated), treated patients had higher rates of AKI (10.3% vs 7.9%; P < .001) and myocardial injury (1.2% vs 0.6%; P = .003). When assessed by SBP, nontreatment of BP was still superior up to an SBP of 199 mm Hg. At an SBP of ≥ 200 mm Hg, there was no difference in rates of AKI or MI between the treatment and nontreatment groups. There was no difference in stroke in either cohort, although the overall numbers were quite low.
Patients with and without antihypertensive intensification at discharge had similar rates of MI (0.1% vs 0.2%; P > .99) and stroke (0.5% vs 0.4%; P > .99) in a matched cohort at 30 days post discharge. At 1 year, BP control in the intensification vs no-intensification groups was nearly the same: maximum SBP was 157.2 mm Hg vs 157.8 mm Hg, respectively (P = .54) and maximum diastolic BP was 86.5 mm Hg vs 86.1 mm Hg, respectively (P = .49).
WHAT’S NEW
Previous research is confirmed in a more diverse population
Whereas previous research showed no benefit to intensification of treatment among hospitalized older male patients, this large, retrospective, propensity score–matched cohort study demonstrated the short- and long-term effects of treating acute, asymptomatic BP elevations in a younger, more generalizable population that included women. Regardless of treatment modality, there appeared to be more harm than good from treating these BP elevations.
In addition, the study appears to corroborate previous research showing that intensification of BP treatment at discharge did not lead to better outcomes.9 At the very least, the study makes a reasonable argument that treating acute BP elevations in noncardiac patients in the hospital setting is not beneficial.
CAVEATS
Impact of existing therapy could be underestimated
This study had several important limitations. First, 23% of treated participants were excluded from the propensity analysis without justification from the authors. Additionally, there was no reporting of missing data and how it was managed. The authors’ definition of treatment excluded dose intensification of existing antihypertensive therapy, which would undercount the number of treated patients. However, this could underestimate the actual harms of the acute antihypertensive therapy. The authors also included patients with atrial fibrillation and heart failure in the study population, even though they already may have been taking antihypertensive agents.
CHALLENGES TO IMPLEMENTATION
Potential delays in translating findings to patient care
Although several recent studies have shown the potential benefit of not treating asymptomatic acute BP elevations in inpatients, incorporating that information into electronic health record order sets or clinical decision support, and disseminating it to clinical end users, will take time. In the interim, despite these findings, patients may continue to receive IV or oral medications to treat acute, asymptomatic BP elevations while hospitalized for noncardiac diagnoses.
ILLUSTRATIVE CASE
A 48-year-old man is admitted to your family medicine service for cellulitis after failed outpatient therapy. He has presumed community-acquired methicillin-resistant Staphylococcus aureus infection of the left lower extremity and is receiving intravenous (IV) vancomycin. His BP this morning is 176/98 mm Hg, and the reading from the previous shift was 168/94 mm Hg. He is asymptomatic from this elevated BP. Based on protocol, his nurse is asking about treatment in response to the multiple elevated readings. How should you address the patient’s elevated BP, knowing that you will see him for a transition management appointment in 2 weeks?
Elevated BP is common in the adult inpatient setting. Prevalence estimates range from 25% to > 50%. Many factors can contribute to elevated BP in the acute illness setting, such as pain, anxiety, medication withdrawal, and volume status.2,3
Treatment of elevated BP in outpatients is well researched, with evidence-based guidelines for physicians. That is not the case for treatment of asymptomatic elevated BP in the inpatient setting. Most published guidance on inpatient management of acutely elevated BP recommends IV medications, such as hydralazine or labetalol, although there is limited evidence to support such recommendations. There is minimal evidence for outcomes-based benefit in treating acute elevations of inpatient BP, such as reduced myocardial injury or stroke; however, there is some evidence of adverse outcomes, such as hypotension and prolonged hospital stays.4-8
Although the possibility of intensifying antihypertensive therapy for those with known hypertension or those with presumed “new-onset” hypertension could theoretically lead to improved outcomes over the long term, there is little evidence to support this presumption. Rather, there is evidence that intensification of antihypertensive therapy at discharge is linked to short-term harms. This was demonstrated in a propensity-matched veteran cohort that included 4056 hospitalized older adults with hypertension (mean age, 77 years; 3961 men), equally split between those who received antihypertensive intensification at hospital discharge and those who did not. Within 30 days, patients receiving intensification had a higher risk of readmission (number needed to harm [NNH] = 27) and serious adverse events (NNH = 63).9
The current study aimed to put all these pieces together by quantifying the prevalence of hypertension in hospitalized patients, characterizing clinician response to patients’ acutely elevated BP, and comparing both short- and long-term outcomes in patients treated for acute BP elevations while hospitalized vs those who were not. The study also assessed the potential effects of antihypertensive intensification at discharge.
STUDY SUMMARY
Treatment of acute hypertension was associated with end-organ injury
This retrospective, propensity score–matched cohort study (N = 22,834) evaluated the electronic health records of all adult patients (age > 18 years) admitted to a medicine service with a noncardiovascular diagnosis over a 1-year period at 10 Cleveland Clinic hospitals, with 1 year of follow-up data.
Exclusion criteria included hospitalization for a cardiovascular diagnosis; admission for a cerebrovascular event or acute coronary syndrome within the previous 30 days; pregnancy; length of stay of less than 2 days or more than 14 days; and lack of outpatient medication data. Patients were propensity-score matched using BP, demographic features, comorbidities, hospital shift, and time since admission. Exposure was defined as administration of IV antihypertensive medication or a new class of oral antihypertensive medication.
Continue to: Outcomes were defined...
Outcomes were defined as a temporal association between acute hypertension treatment and subsequent end-organ damage, such as AKI (serum creatinine increase ≥ 0.3 mg/dL or 1.5 × initial value [Acute Kidney Injury Network definition]), myocardial injury (elevated troponin: > 0.029 ng/mL for troponin T; > 0.045 ng/mL for troponin I), and/or stroke (indicated by discharge diagnosis, with confirmation by chart review). Monitored outcomes included stroke and myocardial infarction (MI) within 30 days of discharge and BP control up to 1 year later.
The 22,834 patients had a mean (SD) age of 65.6 (17.9) years; 12,993 (56.9%) were women, and 15,963 (69.9%) were White. Of the 17,821 (78%) who had at least 1 inpatient hypertensive systolic BP (SBP) episode, defined as an SBP ≥ 140 mm Hg, 5904 (33.1%) received a new treatment. Of those receiving a new treatment, 4378 (74.2%) received only oral treatment, and 1516 (25.7%) received at least 1 dose of IV medication with or without oral dosing.
Using the propensity-matched sample (4520 treated for elevated BP matched to 4520 who were not treated), treated patients had higher rates of AKI (10.3% vs 7.9%; P < .001) and myocardial injury (1.2% vs 0.6%; P = .003). When assessed by SBP, nontreatment of BP was still superior up to an SBP of 199 mm Hg. At an SBP of ≥ 200 mm Hg, there was no difference in rates of AKI or MI between the treatment and nontreatment groups. There was no difference in stroke in either cohort, although the overall numbers were quite low.
Patients with and without antihypertensive intensification at discharge had similar rates of MI (0.1% vs 0.2%; P > .99) and stroke (0.5% vs 0.4%; P > .99) in a matched cohort at 30 days post discharge. At 1 year, BP control in the intensification vs no-intensification groups was nearly the same: maximum SBP was 157.2 mm Hg vs 157.8 mm Hg, respectively (P = .54) and maximum diastolic BP was 86.5 mm Hg vs 86.1 mm Hg, respectively (P = .49).
WHAT’S NEW
Previous research is confirmed in a more diverse population
Whereas previous research showed no benefit to intensification of treatment among hospitalized older male patients, this large, retrospective, propensity score–matched cohort study demonstrated the short- and long-term effects of treating acute, asymptomatic BP elevations in a younger, more generalizable population that included women. Regardless of treatment modality, there appeared to be more harm than good from treating these BP elevations.
In addition, the study appears to corroborate previous research showing that intensification of BP treatment at discharge did not lead to better outcomes.9 At the very least, the study makes a reasonable argument that treating acute BP elevations in noncardiac patients in the hospital setting is not beneficial.
CAVEATS
Impact of existing therapy could be underestimated
This study had several important limitations. First, 23% of treated participants were excluded from the propensity analysis without justification from the authors. Additionally, there was no reporting of missing data and how it was managed. The authors’ definition of treatment excluded dose intensification of existing antihypertensive therapy, which would undercount the number of treated patients. However, this could underestimate the actual harms of the acute antihypertensive therapy. The authors also included patients with atrial fibrillation and heart failure in the study population, even though they already may have been taking antihypertensive agents.
CHALLENGES TO IMPLEMENTATION
Potential delays in translating findings to patient care
Although several recent studies have shown the potential benefit of not treating asymptomatic acute BP elevations in inpatients, incorporating that information into electronic health record order sets or clinical decision support, and disseminating it to clinical end users, will take time. In the interim, despite these findings, patients may continue to receive IV or oral medications to treat acute, asymptomatic BP elevations while hospitalized for noncardiac diagnoses.
1. Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352. doi: 10.1001/jamainternmed.2020.7501
2. Jacobs ZG, Najafi N, Fang MC, et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019;14:144-150. doi: 10.12788/jhm.3087
3. Pasik SD, Chiu S, Yang J, et al. Assess before Rx: reducing the overtreatment of asymptomatic blood pressure elevation in the inpatient setting. J Hosp Med. 2019;14:151-156. doi: 10.12788/jhm.3190
4. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5:473-477. doi: 10.1016/j.jash.2011.07.002
5. Gauer R. Severe asymptomatic hypertension: evaluation and treatment. Am Fam Physician. 2017;95:492-500.
6. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11:193-198. doi: 10.1002/jhm.2510
7. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2018;12:7-15. doi: 10.1177/1753944717746613
8. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12:29-33. doi: 10.1111/j.1751-7176.2009.00196.x
9. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536. doi: 10.1001/jamainternmed.2019.3007
1. Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352. doi: 10.1001/jamainternmed.2020.7501
2. Jacobs ZG, Najafi N, Fang MC, et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019;14:144-150. doi: 10.12788/jhm.3087
3. Pasik SD, Chiu S, Yang J, et al. Assess before Rx: reducing the overtreatment of asymptomatic blood pressure elevation in the inpatient setting. J Hosp Med. 2019;14:151-156. doi: 10.12788/jhm.3190
4. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5:473-477. doi: 10.1016/j.jash.2011.07.002
5. Gauer R. Severe asymptomatic hypertension: evaluation and treatment. Am Fam Physician. 2017;95:492-500.
6. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11:193-198. doi: 10.1002/jhm.2510
7. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2018;12:7-15. doi: 10.1177/1753944717746613
8. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12:29-33. doi: 10.1111/j.1751-7176.2009.00196.x
9. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536. doi: 10.1001/jamainternmed.2019.3007
PRACTICE CHANGER
Manage blood pressure (BP) elevations conservatively in patients admitted for noncardiac diagnoses, as acute hypertension treatment may increase the risk for acute kidney injury (AKI) and myocardial injury.
STRENGTH OF RECOMMENDATION
C: Based on a single, large, retrospective cohort study.1
Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352.
When the public misplaces their trust
Not long ago, the grandmother of my son’s friend died of COVID-19 infection. She was elderly and unvaccinated. Her grandson had no regrets over her unvaccinated status. “Why would she inject poison into her body?” he said, and then expressed a strong opinion that she had died because the hospital physicians refused to give her ivermectin and hydroxychloroquine. My son, wisely, did not push the issue.
Soon thereafter, my personal family physician emailed a newsletter to his patients (me included) with 3 important messages: (1) COVID vaccines were available in the office; (2) He was not going to prescribe hydroxychloroquine, no matter how adamantly it was requested; and (3) He warned against threatening him or his staff with lawsuits or violence over refusal to prescribe any unproven medication.
How, as a country, have we come to this? A sizeable portion of the public trusts the advice of quacks, hacks, and political opportunists over that of the nation’s most expert scientists and physicians. The National Institutes of Health maintains a website with up-to-date recommendations on the use of treatments for COVID-19. They assess the existing evidence and make recommendations for or against a wide array of interventions. (They recommend against the use of both ivermectin and hydroxychloroquine.) The Centers for Disease Control and Prevention publishes extensively about the current knowledge on the safety and efficacy of vaccines. Neither agency is part of a “deep state” or conspiracy. They are comprised of some of the nation’s leading scientists, including physicians, trying to protect the public from disease and foster good health.
Sadly, some physicians have been a source of inaccurate vaccine information; some even prescribe ineffective treatments despite the evidence. These physicians are either letting their politics override their good sense or are improperly assessing the scientific literature, or both. Medical licensing agencies, and specialty certification boards, need to find ways to prevent this—ways that can survive judicial scrutiny and allow for legitimate scientific debate.
I have been tempted to just accept the current situation as the inevitable outcome of social media–fueled tribalism. But when we know that the COVID death rate among the unvaccinated is 9 times that of people who have received a booster dose,1 I can’t sit idly and watch the Internet pundits prevail. Instead, I continue to advise and teach my students to have confidence in trustworthy authorities and websites. Mistakes will be made; corrections will be issued. However, this is not evidence of malintent or incompetence, but rather, the scientific process in action.
I tell my students that one of the biggest challenges facing them and society is to figure out how to stop, or at least minimize the effects of, incorrect information, misleading statements, and outright lies in a society that values free speech. Physicians—young and old alike—must remain committed to communicating factual information to a not-always-receptive audience. And I wish my young colleagues luck; I hope that their passion for family medicine and their insights into social media may be just the combination that’s needed to redirect the public’s trust back to where it belongs during a health care crisis.
1. Fleming-Dutra KE. COVID-19 Epidemiology and Vaccination Rates in the United States. Presented to the Authorization Committee on Immunization Practices, July 19, 2022. Accessed August 9, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/02-COVID-Fleming-Dutra-508.pdf
Not long ago, the grandmother of my son’s friend died of COVID-19 infection. She was elderly and unvaccinated. Her grandson had no regrets over her unvaccinated status. “Why would she inject poison into her body?” he said, and then expressed a strong opinion that she had died because the hospital physicians refused to give her ivermectin and hydroxychloroquine. My son, wisely, did not push the issue.
Soon thereafter, my personal family physician emailed a newsletter to his patients (me included) with 3 important messages: (1) COVID vaccines were available in the office; (2) He was not going to prescribe hydroxychloroquine, no matter how adamantly it was requested; and (3) He warned against threatening him or his staff with lawsuits or violence over refusal to prescribe any unproven medication.
How, as a country, have we come to this? A sizeable portion of the public trusts the advice of quacks, hacks, and political opportunists over that of the nation’s most expert scientists and physicians. The National Institutes of Health maintains a website with up-to-date recommendations on the use of treatments for COVID-19. They assess the existing evidence and make recommendations for or against a wide array of interventions. (They recommend against the use of both ivermectin and hydroxychloroquine.) The Centers for Disease Control and Prevention publishes extensively about the current knowledge on the safety and efficacy of vaccines. Neither agency is part of a “deep state” or conspiracy. They are comprised of some of the nation’s leading scientists, including physicians, trying to protect the public from disease and foster good health.
Sadly, some physicians have been a source of inaccurate vaccine information; some even prescribe ineffective treatments despite the evidence. These physicians are either letting their politics override their good sense or are improperly assessing the scientific literature, or both. Medical licensing agencies, and specialty certification boards, need to find ways to prevent this—ways that can survive judicial scrutiny and allow for legitimate scientific debate.
I have been tempted to just accept the current situation as the inevitable outcome of social media–fueled tribalism. But when we know that the COVID death rate among the unvaccinated is 9 times that of people who have received a booster dose,1 I can’t sit idly and watch the Internet pundits prevail. Instead, I continue to advise and teach my students to have confidence in trustworthy authorities and websites. Mistakes will be made; corrections will be issued. However, this is not evidence of malintent or incompetence, but rather, the scientific process in action.
I tell my students that one of the biggest challenges facing them and society is to figure out how to stop, or at least minimize the effects of, incorrect information, misleading statements, and outright lies in a society that values free speech. Physicians—young and old alike—must remain committed to communicating factual information to a not-always-receptive audience. And I wish my young colleagues luck; I hope that their passion for family medicine and their insights into social media may be just the combination that’s needed to redirect the public’s trust back to where it belongs during a health care crisis.
Not long ago, the grandmother of my son’s friend died of COVID-19 infection. She was elderly and unvaccinated. Her grandson had no regrets over her unvaccinated status. “Why would she inject poison into her body?” he said, and then expressed a strong opinion that she had died because the hospital physicians refused to give her ivermectin and hydroxychloroquine. My son, wisely, did not push the issue.
Soon thereafter, my personal family physician emailed a newsletter to his patients (me included) with 3 important messages: (1) COVID vaccines were available in the office; (2) He was not going to prescribe hydroxychloroquine, no matter how adamantly it was requested; and (3) He warned against threatening him or his staff with lawsuits or violence over refusal to prescribe any unproven medication.
How, as a country, have we come to this? A sizeable portion of the public trusts the advice of quacks, hacks, and political opportunists over that of the nation’s most expert scientists and physicians. The National Institutes of Health maintains a website with up-to-date recommendations on the use of treatments for COVID-19. They assess the existing evidence and make recommendations for or against a wide array of interventions. (They recommend against the use of both ivermectin and hydroxychloroquine.) The Centers for Disease Control and Prevention publishes extensively about the current knowledge on the safety and efficacy of vaccines. Neither agency is part of a “deep state” or conspiracy. They are comprised of some of the nation’s leading scientists, including physicians, trying to protect the public from disease and foster good health.
Sadly, some physicians have been a source of inaccurate vaccine information; some even prescribe ineffective treatments despite the evidence. These physicians are either letting their politics override their good sense or are improperly assessing the scientific literature, or both. Medical licensing agencies, and specialty certification boards, need to find ways to prevent this—ways that can survive judicial scrutiny and allow for legitimate scientific debate.
I have been tempted to just accept the current situation as the inevitable outcome of social media–fueled tribalism. But when we know that the COVID death rate among the unvaccinated is 9 times that of people who have received a booster dose,1 I can’t sit idly and watch the Internet pundits prevail. Instead, I continue to advise and teach my students to have confidence in trustworthy authorities and websites. Mistakes will be made; corrections will be issued. However, this is not evidence of malintent or incompetence, but rather, the scientific process in action.
I tell my students that one of the biggest challenges facing them and society is to figure out how to stop, or at least minimize the effects of, incorrect information, misleading statements, and outright lies in a society that values free speech. Physicians—young and old alike—must remain committed to communicating factual information to a not-always-receptive audience. And I wish my young colleagues luck; I hope that their passion for family medicine and their insights into social media may be just the combination that’s needed to redirect the public’s trust back to where it belongs during a health care crisis.
1. Fleming-Dutra KE. COVID-19 Epidemiology and Vaccination Rates in the United States. Presented to the Authorization Committee on Immunization Practices, July 19, 2022. Accessed August 9, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/02-COVID-Fleming-Dutra-508.pdf
1. Fleming-Dutra KE. COVID-19 Epidemiology and Vaccination Rates in the United States. Presented to the Authorization Committee on Immunization Practices, July 19, 2022. Accessed August 9, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/02-COVID-Fleming-Dutra-508.pdf
COPD inhaler therapy: A path to success
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).
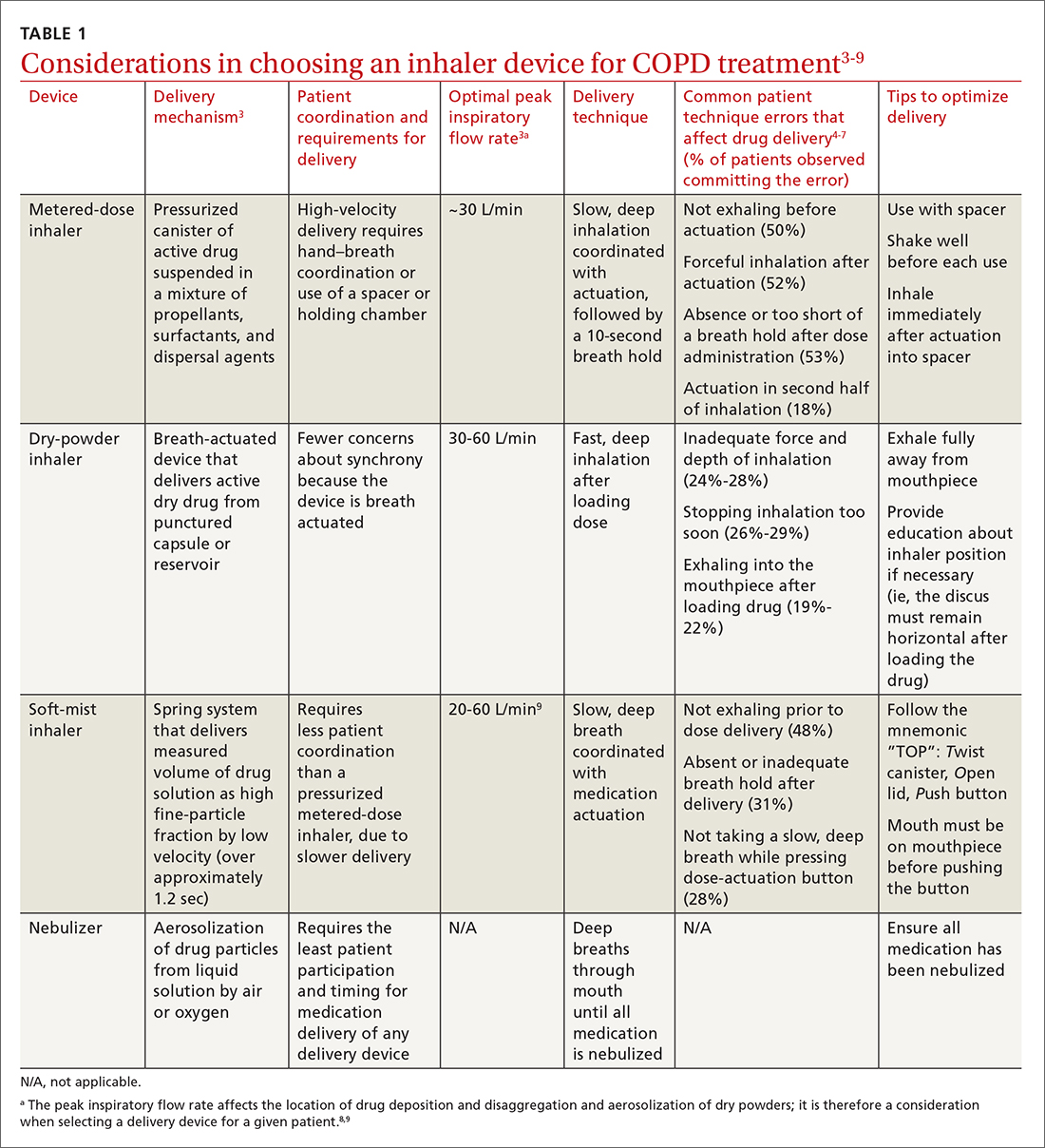
Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.
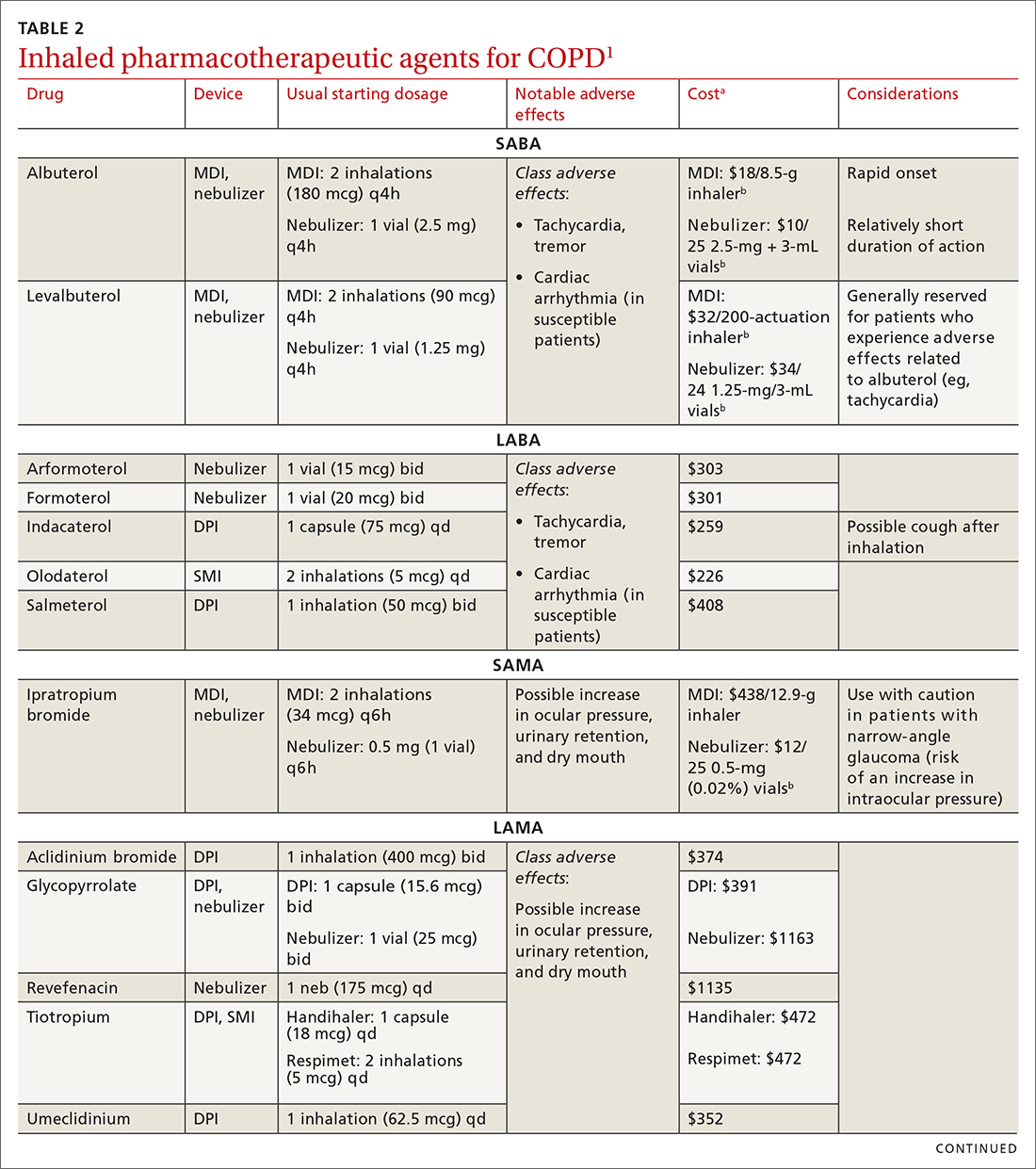
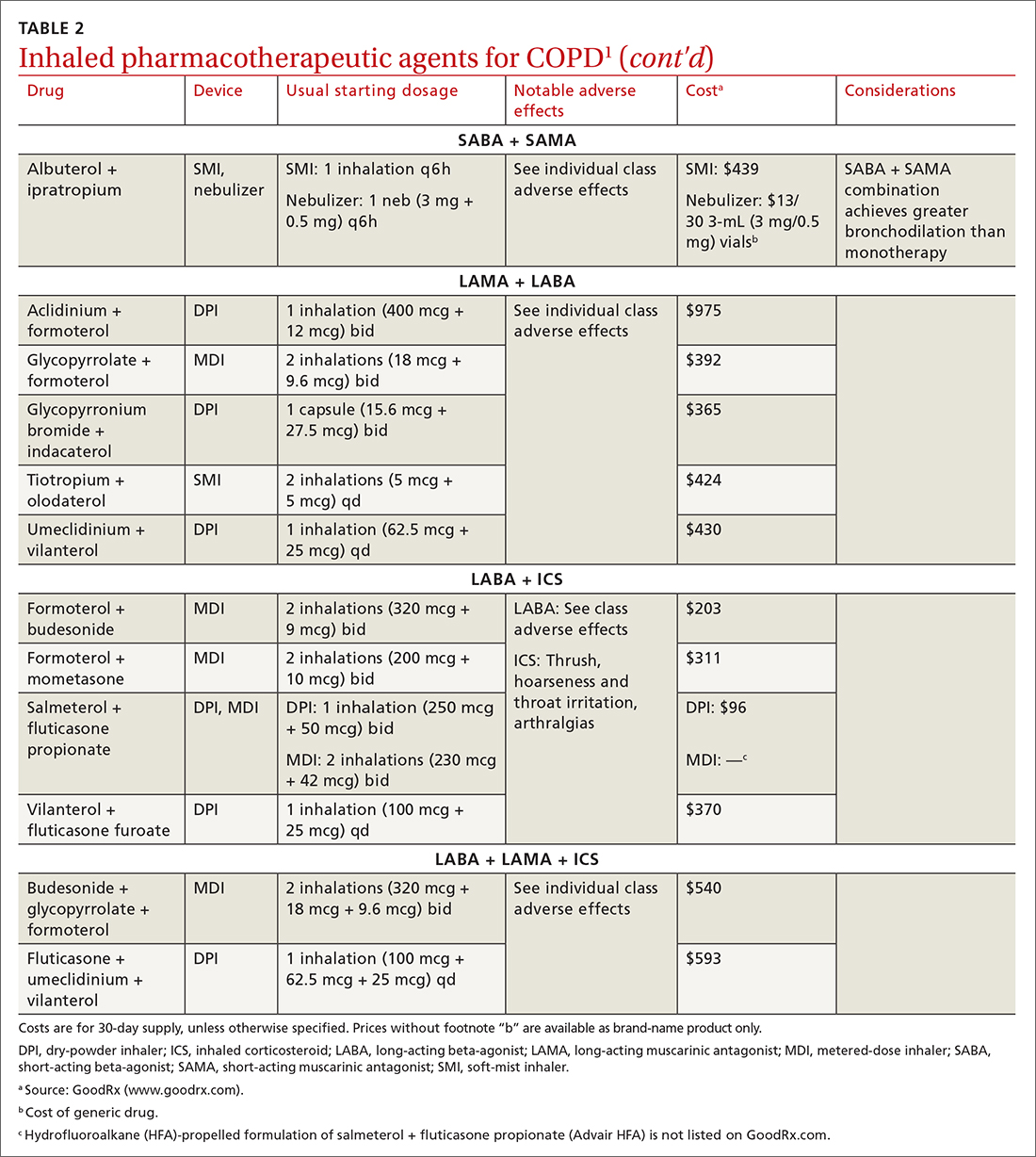
Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1
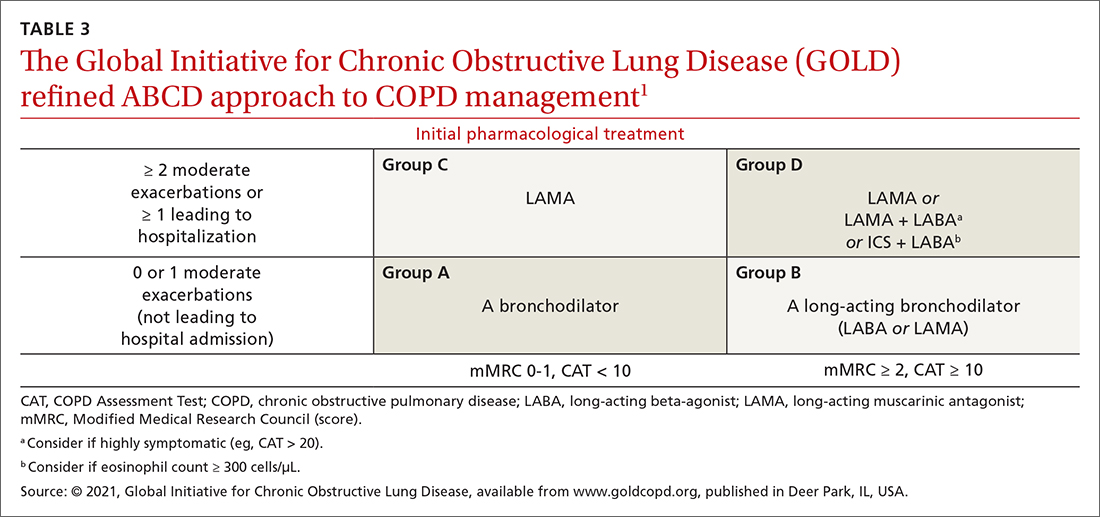
Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
a www.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
b www.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; [email protected]
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).

Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.


Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1

Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
a www.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
b www.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; [email protected]
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).

Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.


Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1

Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
a www.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
b www.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; [email protected]
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
PRACTICE RECOMMENDATIONS
› Follow guideline advice that (1) in general, short-acting beta-agonists (SABAs) are not for daily use in stable chronic obstructive pulmonary disease (COPD) but (2) agents in this class of drugs might have a role in relieving occasional COPD-associated dyspnea. C
› Prescribe albuterol over levalbuterol when a SABA is indicated because of the lower cost of albuterol, its comparative efficacy, and its lower incidence of tachycardia and palpitations, even in patients with cardiovascular disease. B
› Avoid the use of an inhaled corticosteroid, or consider withdrawing inhaled corticosteroid therapy, in patients with COPD whose blood eosinophil count is < 100 cells/μL or who have repeated bouts of pneumonia or a history of mycobacterial infection. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The LIVE CHEST Challenge Championship is back!
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.