User login
Pediatric vaccines & infectious diseases, 2022
Introduction
ICYMI articles featuring 9 important developments of the past year – and COVID is still here
By Christopher J. Harrison, MD
Table of contents
Antibiotics use and vaccine antibody levels
Commentary
Emerging tick-borne pathogen has spread to state of Georgia
Commentary
WHO, UNICEF warn about increased risk of measles outbreaks
Commentary
Babies die as congenital syphilis continues a decade-long surge across the U.S.
Commentary
Meningococcal vaccine shows moderate protective effect against gonorrhea
Commentary
Adolescents are undertested for STIs
Commentary
TB treatment can be shortened for most children: Study
Commentary
Nirsevimab protects healthy infants from RSV
Commentary
Norovirus vaccine candidates employ different approaches
Commentary
Introduction
ICYMI articles featuring 9 important developments of the past year – and COVID is still here
By Christopher J. Harrison, MD
Table of contents
Antibiotics use and vaccine antibody levels
Commentary
Emerging tick-borne pathogen has spread to state of Georgia
Commentary
WHO, UNICEF warn about increased risk of measles outbreaks
Commentary
Babies die as congenital syphilis continues a decade-long surge across the U.S.
Commentary
Meningococcal vaccine shows moderate protective effect against gonorrhea
Commentary
Adolescents are undertested for STIs
Commentary
TB treatment can be shortened for most children: Study
Commentary
Nirsevimab protects healthy infants from RSV
Commentary
Norovirus vaccine candidates employ different approaches
Commentary
Introduction
ICYMI articles featuring 9 important developments of the past year – and COVID is still here
By Christopher J. Harrison, MD
Table of contents
Antibiotics use and vaccine antibody levels
Commentary
Emerging tick-borne pathogen has spread to state of Georgia
Commentary
WHO, UNICEF warn about increased risk of measles outbreaks
Commentary
Babies die as congenital syphilis continues a decade-long surge across the U.S.
Commentary
Meningococcal vaccine shows moderate protective effect against gonorrhea
Commentary
Adolescents are undertested for STIs
Commentary
TB treatment can be shortened for most children: Study
Commentary
Nirsevimab protects healthy infants from RSV
Commentary
Norovirus vaccine candidates employ different approaches
Commentary
ICYMI articles featuring 9 important developments of the past year – and COVID is still here
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
Psychedelics may ease fear of death and dying
Psychedelics can produce positive changes in attitudes about death and dying – and may be a way to help ease anxiety and depression toward the end of life, new research suggests.
In a retrospective study of more than 3,000 participants,
“Individuals with existential anxiety and depression at end of life account for substantial suffering and significantly increased health care expenses from desperate and often futile seeking of intensive and expensive medical treatments,” co-investigator Roland Griffiths, PhD, Center for Psychedelics and Consciousness Research at Johns Hopkins Medicine, Baltimore, told this news organization.
“The present findings, which show that both psychedelic and non–drug-occasioned experiences can produce positive and enduring changes in attitudes about death, suggest the importance of future prospective experimental and clinical observational studies to better understand mechanisms of such changes as well as their potential clinical utility in ameliorating suffering related to fear of death,” Dr. Griffiths said.
The results were published online Aug. 24 in PLOS ONE.
Direct comparisons
Both psychedelic drug experiences and near-death experiences can alter perspectives on death and dying, but there have been few direct comparisons of these phenomena, the investigators note.
In the current study, they directly compared psychedelic-occasioned and nondrug experiences, which altered individuals’ beliefs about death.
The researchers surveyed 3,192 mostly White adults from the United States, including 933 who had a natural, nondrug near-death experience and 2,259 who had psychedelic near-death experiences induced with lysergic acid diethylamide, psilocybin, ayahuasca, or N,N-dimethyltryptamine.
The psychedelic group had more men than women and tended to be younger at the time of the experience than was the nondrug group.
Nearly 90% of individuals in both groups said that they were less afraid of death than they were before their experiences.
About half of both groups said they’d encountered something they might call “God” during the experience.
Three-quarters of the psychedelic group and 85% of the nondrug group rated their experiences as among the top five most personally meaningful and spiritually significant events of their life.
Individuals in both groups also reported moderate- to strong-lasting positive changes in personal well-being and life purpose and meaning after their experiences.
However, there were some differences between the groups.
More research needed
Compared with the psychedelic group, the nondrug group was more likely to report being unconscious, clinically dead, or that their life was in imminent danger.
The nonpsychedelic group was also more likely to report that their experience was very brief, lasting 5 minutes or less.
Both the psychedelic and nondrug participants showed robust increases on standardized measures of mystical and near-death experiences, but these measures were significantly greater in the psychedelic group.
The survey findings are in line with several recent clinical trials showing that a single treatment with the psychedelic psilocybin produced sustained decreases in anxiety and depression among patients with a life-threatening cancer diagnosis.
This includes a 2016 study by Dr. Griffiths and colleagues, which included 51 patients with late-stage cancer. As reported at the time, results showed a single, high dose of psilocybin had rapid, clinically significant, and lasting effects on mood and anxiety.
Limitations of the current survey cited by the researchers include the use of retrospective self-report to describe changes in death attitudes and the subjective features of the experiences. Also, respondents were a self-selected study population that may not be representative of all psychedelic or near-death experiences.
In addition, the study did not attempt to document worldview and other belief changes, such as increased belief in afterlife, that might help explain why death attitudes changed.
Looking ahead, the researchers note that future studies are needed to better understand the potential clinical use of psychedelics in ameliorating suffering related to fear of death.
Support through the Johns Hopkins Center for Psychedelic and Consciousness Research was provided by Tim Ferriss, Matt Mullenweg, Blake Mycoskie, Craig Nerenberg, and the Steven and Alexandra Cohen Foundation. Funding was also provided by the Y.C. Ho/Helen and Michael Chiang Foundation. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychedelics can produce positive changes in attitudes about death and dying – and may be a way to help ease anxiety and depression toward the end of life, new research suggests.
In a retrospective study of more than 3,000 participants,
“Individuals with existential anxiety and depression at end of life account for substantial suffering and significantly increased health care expenses from desperate and often futile seeking of intensive and expensive medical treatments,” co-investigator Roland Griffiths, PhD, Center for Psychedelics and Consciousness Research at Johns Hopkins Medicine, Baltimore, told this news organization.
“The present findings, which show that both psychedelic and non–drug-occasioned experiences can produce positive and enduring changes in attitudes about death, suggest the importance of future prospective experimental and clinical observational studies to better understand mechanisms of such changes as well as their potential clinical utility in ameliorating suffering related to fear of death,” Dr. Griffiths said.
The results were published online Aug. 24 in PLOS ONE.
Direct comparisons
Both psychedelic drug experiences and near-death experiences can alter perspectives on death and dying, but there have been few direct comparisons of these phenomena, the investigators note.
In the current study, they directly compared psychedelic-occasioned and nondrug experiences, which altered individuals’ beliefs about death.
The researchers surveyed 3,192 mostly White adults from the United States, including 933 who had a natural, nondrug near-death experience and 2,259 who had psychedelic near-death experiences induced with lysergic acid diethylamide, psilocybin, ayahuasca, or N,N-dimethyltryptamine.
The psychedelic group had more men than women and tended to be younger at the time of the experience than was the nondrug group.
Nearly 90% of individuals in both groups said that they were less afraid of death than they were before their experiences.
About half of both groups said they’d encountered something they might call “God” during the experience.
Three-quarters of the psychedelic group and 85% of the nondrug group rated their experiences as among the top five most personally meaningful and spiritually significant events of their life.
Individuals in both groups also reported moderate- to strong-lasting positive changes in personal well-being and life purpose and meaning after their experiences.
However, there were some differences between the groups.
More research needed
Compared with the psychedelic group, the nondrug group was more likely to report being unconscious, clinically dead, or that their life was in imminent danger.
The nonpsychedelic group was also more likely to report that their experience was very brief, lasting 5 minutes or less.
Both the psychedelic and nondrug participants showed robust increases on standardized measures of mystical and near-death experiences, but these measures were significantly greater in the psychedelic group.
The survey findings are in line with several recent clinical trials showing that a single treatment with the psychedelic psilocybin produced sustained decreases in anxiety and depression among patients with a life-threatening cancer diagnosis.
This includes a 2016 study by Dr. Griffiths and colleagues, which included 51 patients with late-stage cancer. As reported at the time, results showed a single, high dose of psilocybin had rapid, clinically significant, and lasting effects on mood and anxiety.
Limitations of the current survey cited by the researchers include the use of retrospective self-report to describe changes in death attitudes and the subjective features of the experiences. Also, respondents were a self-selected study population that may not be representative of all psychedelic or near-death experiences.
In addition, the study did not attempt to document worldview and other belief changes, such as increased belief in afterlife, that might help explain why death attitudes changed.
Looking ahead, the researchers note that future studies are needed to better understand the potential clinical use of psychedelics in ameliorating suffering related to fear of death.
Support through the Johns Hopkins Center for Psychedelic and Consciousness Research was provided by Tim Ferriss, Matt Mullenweg, Blake Mycoskie, Craig Nerenberg, and the Steven and Alexandra Cohen Foundation. Funding was also provided by the Y.C. Ho/Helen and Michael Chiang Foundation. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychedelics can produce positive changes in attitudes about death and dying – and may be a way to help ease anxiety and depression toward the end of life, new research suggests.
In a retrospective study of more than 3,000 participants,
“Individuals with existential anxiety and depression at end of life account for substantial suffering and significantly increased health care expenses from desperate and often futile seeking of intensive and expensive medical treatments,” co-investigator Roland Griffiths, PhD, Center for Psychedelics and Consciousness Research at Johns Hopkins Medicine, Baltimore, told this news organization.
“The present findings, which show that both psychedelic and non–drug-occasioned experiences can produce positive and enduring changes in attitudes about death, suggest the importance of future prospective experimental and clinical observational studies to better understand mechanisms of such changes as well as their potential clinical utility in ameliorating suffering related to fear of death,” Dr. Griffiths said.
The results were published online Aug. 24 in PLOS ONE.
Direct comparisons
Both psychedelic drug experiences and near-death experiences can alter perspectives on death and dying, but there have been few direct comparisons of these phenomena, the investigators note.
In the current study, they directly compared psychedelic-occasioned and nondrug experiences, which altered individuals’ beliefs about death.
The researchers surveyed 3,192 mostly White adults from the United States, including 933 who had a natural, nondrug near-death experience and 2,259 who had psychedelic near-death experiences induced with lysergic acid diethylamide, psilocybin, ayahuasca, or N,N-dimethyltryptamine.
The psychedelic group had more men than women and tended to be younger at the time of the experience than was the nondrug group.
Nearly 90% of individuals in both groups said that they were less afraid of death than they were before their experiences.
About half of both groups said they’d encountered something they might call “God” during the experience.
Three-quarters of the psychedelic group and 85% of the nondrug group rated their experiences as among the top five most personally meaningful and spiritually significant events of their life.
Individuals in both groups also reported moderate- to strong-lasting positive changes in personal well-being and life purpose and meaning after their experiences.
However, there were some differences between the groups.
More research needed
Compared with the psychedelic group, the nondrug group was more likely to report being unconscious, clinically dead, or that their life was in imminent danger.
The nonpsychedelic group was also more likely to report that their experience was very brief, lasting 5 minutes or less.
Both the psychedelic and nondrug participants showed robust increases on standardized measures of mystical and near-death experiences, but these measures were significantly greater in the psychedelic group.
The survey findings are in line with several recent clinical trials showing that a single treatment with the psychedelic psilocybin produced sustained decreases in anxiety and depression among patients with a life-threatening cancer diagnosis.
This includes a 2016 study by Dr. Griffiths and colleagues, which included 51 patients with late-stage cancer. As reported at the time, results showed a single, high dose of psilocybin had rapid, clinically significant, and lasting effects on mood and anxiety.
Limitations of the current survey cited by the researchers include the use of retrospective self-report to describe changes in death attitudes and the subjective features of the experiences. Also, respondents were a self-selected study population that may not be representative of all psychedelic or near-death experiences.
In addition, the study did not attempt to document worldview and other belief changes, such as increased belief in afterlife, that might help explain why death attitudes changed.
Looking ahead, the researchers note that future studies are needed to better understand the potential clinical use of psychedelics in ameliorating suffering related to fear of death.
Support through the Johns Hopkins Center for Psychedelic and Consciousness Research was provided by Tim Ferriss, Matt Mullenweg, Blake Mycoskie, Craig Nerenberg, and the Steven and Alexandra Cohen Foundation. Funding was also provided by the Y.C. Ho/Helen and Michael Chiang Foundation. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
FDA approves oral TYK2 inhibitor deucravacitinib for treating psoriasis
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
Blood test for multiple cancers: Many false positives
PARIS –
“As this technology develops, people must continue with their standard cancer screening, but this is a glimpse of what the future may hold,” commented study investigator Deborah Schrag, MD, MPH, chair, department of medicine, Memorial Sloan Kettering Cancer Center, New York.
For the PATHFINDER study, the Galleri blood test (developed by Grail) was used in 6,621 healthy individuals aged over 50, with or without additional cancer risk factors (such as history of smoking or genetic risk).
It found a positive cancer signal in 92 individuals (1.4%).
None of the individuals who tested positive was known to have cancer at the time of testing. Subsequent workup, which could include scans and/or biopsy, found cancer in 38% of those with a positive test.
“When the test was positive, the workups were typically done in less than 3 months,” Dr. Schrag commented, adding that “the blood test typically predicted the origin of the cancer.”
Dr. Schrag presented the findings at the annual meeting of the European Society for Medical Oncology (ESMO).
Approached for comment, Anthony J. Olszanski, MD, RPh, vice chair of research at the Fox Chase Cancer Center, Philadelphia, noted that the use of a blood test to “find” cancer has long been on the minds of patients. “It is not uncommon to hear oncology patients ask: ‘Why didn’t my doctor find my cancer earlier, on blood tests?’ ”
As this study suggests, finding a malignancy before it becomes apparent on imaging or because of symptoms is one step closer to becoming a reality. “But although this is an important study, it must be noted that only about 40% of patients with a positive test result were actually found to have cancer,” Dr. Olszanski said. “Conversely, about 60% of patients with a positive test result likely suffered from a considerable amount of anxiety that may persist even after further testing did not reveal a malignancy.”
Another important issue is that such testing may incur substantial health care cost. “Less than 2 participants per 100 had a positive test result, and those patients underwent further testing to interrogate the result,” he added. “It also remains unclear if detecting cancer early will lead to better outcomes.”
Whether or not the test will be cost-effective remains unknown, as Dr. Schrag emphasized they do not have a formal cost analysis at this time. “This technology is not ready for population-wide screening, but as the technology improves, costs will go down,” she said.
Dr. Schrag also added that this is a new concept and the trial shows it is feasible to detect cancer using a blood test. “It was not designed to determine if the test can decrease cancer mortality, which is obviously the purpose of screening, but it’s premature for that,” she said.
Details of the results
The Galleri test uses cell-free DNA and machine learning to detect a common cancer signal across more than 50 cancer types as well as to predict cancer signal origin.
Overall, the test detected a cancer signal in 1.4% (n = 92) of participants with analyzable samples.
A total of 90 participants underwent diagnostic testing (33 true positives and 57 false positives). Of the true positives, 81.8% underwent more than one invasive diagnostic test, as did 29.8% of false positives.
Specificity was 99.1%, positive predictive value (PPV) was approximately 40%, and 73% of those who were true positives had diagnostic resolution in less than 3 months.
Of the cancers that were diagnosed, 19 were solid tumors and 17 were hematologic cancers; 7 were diagnosed in a person with a history of cancer, 26 were cancer types without standard screening, and 14 were diagnosed at an early stage.
“What is exciting about this new paradigm is that many of these were cancers for which we don’t have standard screening,” said Dr. Schrag.
Dr. Schrag noted that given the immense interest in this study, the manufacturer is working toward refining the assay and improving the test. A reanalysis was conducted on all specimens using a refined version of the test.
“Importantly, the new analysis identified fewer patients with having positive signals, from 1.4% to 0.9%,” she said. “Specificity improved to 99.5% as did PPV – from 38% to 43.1% – and more people need to be screened to find a cancer – up to 263 from 189.”
False positives concerning
Previous, and very similar, results from the PATHFINDER trial were presented last year at the annual meeting of the American Society of Clinical Oncology.
Max Diehn, MD, PhD, associate professor of radiation oncology at Stanford (Calif.) University, was an invited discussant for the study.
He pointed out that there were more false positives than true positives and noted that “there were a significant number of invasive procedures in false positives, which could cause harm to these patients who don’t have cancer.”
Dr. Diehn also explained that most true positives were for lymphoid malignancies, not solid tumors, and it is not known whether early detection of lymphoid malignancy has clinical utility.
The Galleri test is already available in the United States and is being offered by a number of U.S. health networks. However, it is not approved by the U.S. Food and Drug Administration and is not covered by medical insurance, so individuals have to pay around $950 for it out of pocket.
Although some experts are excited by its potential, describing it as a “game-changer,” others are concerned that there are no clinical pathways in place yet to deal with the results of such a blood test, and say it is not ready for prime time.
The study was funded by Grail, a subsidiary of Illumina. Dr. Shrag has reported relationships with Grail, the Journal of the American Medical Association, and Pfizer. Several coauthors also have disclosed relationships with industry. Dr. Olszanski has reported participating in advisory boards for BMS, Merck, and Instil Bio, and running trials for them.
A version of this article first appeared on Medscape.com.
PARIS –
“As this technology develops, people must continue with their standard cancer screening, but this is a glimpse of what the future may hold,” commented study investigator Deborah Schrag, MD, MPH, chair, department of medicine, Memorial Sloan Kettering Cancer Center, New York.
For the PATHFINDER study, the Galleri blood test (developed by Grail) was used in 6,621 healthy individuals aged over 50, with or without additional cancer risk factors (such as history of smoking or genetic risk).
It found a positive cancer signal in 92 individuals (1.4%).
None of the individuals who tested positive was known to have cancer at the time of testing. Subsequent workup, which could include scans and/or biopsy, found cancer in 38% of those with a positive test.
“When the test was positive, the workups were typically done in less than 3 months,” Dr. Schrag commented, adding that “the blood test typically predicted the origin of the cancer.”
Dr. Schrag presented the findings at the annual meeting of the European Society for Medical Oncology (ESMO).
Approached for comment, Anthony J. Olszanski, MD, RPh, vice chair of research at the Fox Chase Cancer Center, Philadelphia, noted that the use of a blood test to “find” cancer has long been on the minds of patients. “It is not uncommon to hear oncology patients ask: ‘Why didn’t my doctor find my cancer earlier, on blood tests?’ ”
As this study suggests, finding a malignancy before it becomes apparent on imaging or because of symptoms is one step closer to becoming a reality. “But although this is an important study, it must be noted that only about 40% of patients with a positive test result were actually found to have cancer,” Dr. Olszanski said. “Conversely, about 60% of patients with a positive test result likely suffered from a considerable amount of anxiety that may persist even after further testing did not reveal a malignancy.”
Another important issue is that such testing may incur substantial health care cost. “Less than 2 participants per 100 had a positive test result, and those patients underwent further testing to interrogate the result,” he added. “It also remains unclear if detecting cancer early will lead to better outcomes.”
Whether or not the test will be cost-effective remains unknown, as Dr. Schrag emphasized they do not have a formal cost analysis at this time. “This technology is not ready for population-wide screening, but as the technology improves, costs will go down,” she said.
Dr. Schrag also added that this is a new concept and the trial shows it is feasible to detect cancer using a blood test. “It was not designed to determine if the test can decrease cancer mortality, which is obviously the purpose of screening, but it’s premature for that,” she said.
Details of the results
The Galleri test uses cell-free DNA and machine learning to detect a common cancer signal across more than 50 cancer types as well as to predict cancer signal origin.
Overall, the test detected a cancer signal in 1.4% (n = 92) of participants with analyzable samples.
A total of 90 participants underwent diagnostic testing (33 true positives and 57 false positives). Of the true positives, 81.8% underwent more than one invasive diagnostic test, as did 29.8% of false positives.
Specificity was 99.1%, positive predictive value (PPV) was approximately 40%, and 73% of those who were true positives had diagnostic resolution in less than 3 months.
Of the cancers that were diagnosed, 19 were solid tumors and 17 were hematologic cancers; 7 were diagnosed in a person with a history of cancer, 26 were cancer types without standard screening, and 14 were diagnosed at an early stage.
“What is exciting about this new paradigm is that many of these were cancers for which we don’t have standard screening,” said Dr. Schrag.
Dr. Schrag noted that given the immense interest in this study, the manufacturer is working toward refining the assay and improving the test. A reanalysis was conducted on all specimens using a refined version of the test.
“Importantly, the new analysis identified fewer patients with having positive signals, from 1.4% to 0.9%,” she said. “Specificity improved to 99.5% as did PPV – from 38% to 43.1% – and more people need to be screened to find a cancer – up to 263 from 189.”
False positives concerning
Previous, and very similar, results from the PATHFINDER trial were presented last year at the annual meeting of the American Society of Clinical Oncology.
Max Diehn, MD, PhD, associate professor of radiation oncology at Stanford (Calif.) University, was an invited discussant for the study.
He pointed out that there were more false positives than true positives and noted that “there were a significant number of invasive procedures in false positives, which could cause harm to these patients who don’t have cancer.”
Dr. Diehn also explained that most true positives were for lymphoid malignancies, not solid tumors, and it is not known whether early detection of lymphoid malignancy has clinical utility.
The Galleri test is already available in the United States and is being offered by a number of U.S. health networks. However, it is not approved by the U.S. Food and Drug Administration and is not covered by medical insurance, so individuals have to pay around $950 for it out of pocket.
Although some experts are excited by its potential, describing it as a “game-changer,” others are concerned that there are no clinical pathways in place yet to deal with the results of such a blood test, and say it is not ready for prime time.
The study was funded by Grail, a subsidiary of Illumina. Dr. Shrag has reported relationships with Grail, the Journal of the American Medical Association, and Pfizer. Several coauthors also have disclosed relationships with industry. Dr. Olszanski has reported participating in advisory boards for BMS, Merck, and Instil Bio, and running trials for them.
A version of this article first appeared on Medscape.com.
PARIS –
“As this technology develops, people must continue with their standard cancer screening, but this is a glimpse of what the future may hold,” commented study investigator Deborah Schrag, MD, MPH, chair, department of medicine, Memorial Sloan Kettering Cancer Center, New York.
For the PATHFINDER study, the Galleri blood test (developed by Grail) was used in 6,621 healthy individuals aged over 50, with or without additional cancer risk factors (such as history of smoking or genetic risk).
It found a positive cancer signal in 92 individuals (1.4%).
None of the individuals who tested positive was known to have cancer at the time of testing. Subsequent workup, which could include scans and/or biopsy, found cancer in 38% of those with a positive test.
“When the test was positive, the workups were typically done in less than 3 months,” Dr. Schrag commented, adding that “the blood test typically predicted the origin of the cancer.”
Dr. Schrag presented the findings at the annual meeting of the European Society for Medical Oncology (ESMO).
Approached for comment, Anthony J. Olszanski, MD, RPh, vice chair of research at the Fox Chase Cancer Center, Philadelphia, noted that the use of a blood test to “find” cancer has long been on the minds of patients. “It is not uncommon to hear oncology patients ask: ‘Why didn’t my doctor find my cancer earlier, on blood tests?’ ”
As this study suggests, finding a malignancy before it becomes apparent on imaging or because of symptoms is one step closer to becoming a reality. “But although this is an important study, it must be noted that only about 40% of patients with a positive test result were actually found to have cancer,” Dr. Olszanski said. “Conversely, about 60% of patients with a positive test result likely suffered from a considerable amount of anxiety that may persist even after further testing did not reveal a malignancy.”
Another important issue is that such testing may incur substantial health care cost. “Less than 2 participants per 100 had a positive test result, and those patients underwent further testing to interrogate the result,” he added. “It also remains unclear if detecting cancer early will lead to better outcomes.”
Whether or not the test will be cost-effective remains unknown, as Dr. Schrag emphasized they do not have a formal cost analysis at this time. “This technology is not ready for population-wide screening, but as the technology improves, costs will go down,” she said.
Dr. Schrag also added that this is a new concept and the trial shows it is feasible to detect cancer using a blood test. “It was not designed to determine if the test can decrease cancer mortality, which is obviously the purpose of screening, but it’s premature for that,” she said.
Details of the results
The Galleri test uses cell-free DNA and machine learning to detect a common cancer signal across more than 50 cancer types as well as to predict cancer signal origin.
Overall, the test detected a cancer signal in 1.4% (n = 92) of participants with analyzable samples.
A total of 90 participants underwent diagnostic testing (33 true positives and 57 false positives). Of the true positives, 81.8% underwent more than one invasive diagnostic test, as did 29.8% of false positives.
Specificity was 99.1%, positive predictive value (PPV) was approximately 40%, and 73% of those who were true positives had diagnostic resolution in less than 3 months.
Of the cancers that were diagnosed, 19 were solid tumors and 17 were hematologic cancers; 7 were diagnosed in a person with a history of cancer, 26 were cancer types without standard screening, and 14 were diagnosed at an early stage.
“What is exciting about this new paradigm is that many of these were cancers for which we don’t have standard screening,” said Dr. Schrag.
Dr. Schrag noted that given the immense interest in this study, the manufacturer is working toward refining the assay and improving the test. A reanalysis was conducted on all specimens using a refined version of the test.
“Importantly, the new analysis identified fewer patients with having positive signals, from 1.4% to 0.9%,” she said. “Specificity improved to 99.5% as did PPV – from 38% to 43.1% – and more people need to be screened to find a cancer – up to 263 from 189.”
False positives concerning
Previous, and very similar, results from the PATHFINDER trial were presented last year at the annual meeting of the American Society of Clinical Oncology.
Max Diehn, MD, PhD, associate professor of radiation oncology at Stanford (Calif.) University, was an invited discussant for the study.
He pointed out that there were more false positives than true positives and noted that “there were a significant number of invasive procedures in false positives, which could cause harm to these patients who don’t have cancer.”
Dr. Diehn also explained that most true positives were for lymphoid malignancies, not solid tumors, and it is not known whether early detection of lymphoid malignancy has clinical utility.
The Galleri test is already available in the United States and is being offered by a number of U.S. health networks. However, it is not approved by the U.S. Food and Drug Administration and is not covered by medical insurance, so individuals have to pay around $950 for it out of pocket.
Although some experts are excited by its potential, describing it as a “game-changer,” others are concerned that there are no clinical pathways in place yet to deal with the results of such a blood test, and say it is not ready for prime time.
The study was funded by Grail, a subsidiary of Illumina. Dr. Shrag has reported relationships with Grail, the Journal of the American Medical Association, and Pfizer. Several coauthors also have disclosed relationships with industry. Dr. Olszanski has reported participating in advisory boards for BMS, Merck, and Instil Bio, and running trials for them.
A version of this article first appeared on Medscape.com.
AT ESMO 2022
ILD on the rise: Doctors offer tips for diagnosing deadly disease
“There is definitely a delay from the time of symptom onset to the time that they are even evaluated for ILD,” said Dr. Kulkarni of the department of pulmonary, allergy and critical care medicine at the University of Alabama, Birmingham. “Some patients have had a significant loss of lung function by the time they come to see us. By that point we are limited by what treatment options we can offer.”
Interstitial lung disease is an umbrella term for a group of disorders involving progressive scarring of the lungs – typically irreversible – usually caused by long-term exposure to hazardous materials or by autoimmune effects. It includes idiopathic pulmonary fibrosis (IPF), a disease that is fairly rare but which has therapy options that can be effective if caught early enough. The term pulmonary fibrosis refers to lung scarring. Another type of ILD is pulmonary sarcoidosis, in which small clumps of immune cells form in the lungs in an immune response sometimes following an environmental trigger, and can lead to lung scarring if it doesn’t resolve.
Cases of ILD appear to be on the rise, and COVID-19 has made diagnosing it more complicated. One study found the prevalence of ILD and pulmonary sarcoidosis in high-income countries was about 122 of every 100,000 people in 1990 and rose to about 198 of every 100,000 people in 2017. The data were pulled from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Globally, the researchers found a prevalence of 62 per 100,000 in 1990, compared with 82 per 100,000 in 2017.
If all of a patient’s symptoms have appeared post COVID and a physician is seeing a patient within 4-6 weeks of COVID symptoms, it is likely that the symptoms are COVID related. But a full work-up is recommended if a patient has lung crackles, which are an indicator of lung scarring, she said.
“The patterns that are seen on CT scan for COVID pneumonia are very distinct from what we expect to see with idiopathic pulmonary fibrosis,” Dr. Kulkarni said. “Putting all this information together is what is important to differentiate it from COVID pneumonia, as well as other types of ILD.”
A study published earlier this year found similarities between COVID-19 and IPF in gene expression, their IL-15-heavy cytokine storms, and the type of damage to alveolar cells. Both might be driven by endoplasmic reticulum stress, they found.
“COVID-19 resembles IPF at a fundamental level,” they wrote.
Jeffrey Horowitz, MD, a pulmonologist and professor of medicine at the Ohio State University, said the need for early diagnosis is in part a function of the therapies available for ILD.
“They don’t make the lung function better,” he said. “So delays in diagnosis mean that there’s the possibility of underlying progression for months, or sometimes years, before the diagnosis is recognized.”
In an area in which diagnosis is delayed and the prognosis is dire – 3-5 years in untreated patients after diagnosis – “there’s a tremendous amount of nihilism out there” among patients, he said.
He said patients with long-term shortness of breath and unexplained cough are often told they have asthma and are prescribed inhalers, but then further assessment isn’t performed when those don’t work.
Diagnosing ILD in primary care
Many primary care physicians feel ill-equipped to discuss IPF. More than a dozen physicians contacted for this piece to talk about ILD either did not respond, or said they felt unqualified to respond to questions on the disease.
“Not my area of expertise” and “I don’t think I’m the right person for this discussion” were two of the responses provided to this news organization.
“For some reason, in the world of primary care, it seems like there’s an impediment to getting pulmonary function studies,” Dr. Horowitz said. “Anybody who has a persistent ongoing prolonged unexplained shortness of breath and cough should have pulmonary function studies done.”
Listening to the lungs alone might not be enough, he said. There might be no clear sign in the case of early pulmonary fibrosis, he said.
“There’s the textbook description of these Velcro-sounding crackles, but sometimes it’s very subtle,” he said. “And unless you’re listening very carefully it can easily be missed by somebody who has a busy practice, or it’s loud.”
William E. Golden, MD, professor of medicine and public health at the University of Arkansas, Little Rock, is the sole primary care physician contacted for this piece who spoke with authority on ILD.
For cases of suspected ILD, internist Dr. Golden, who also serves on the editorial advisory board of Internal Medicine News, suggested ordering a test for diffusing capacity for carbon monoxide (DLCO), which will be low in the case of IPF, along with a fine-cut lung CT scan to assess ongoing fibrotic changes.
It’s “not that difficult, but you need to have an index of suspicion for the diagnosis,” he said.
New initiative for helping diagnose ILD
Dr. Kulkarni is a committee member for a new effort under way to try to get patients with ILD diagnosed earlier.
The initiative, called Bridging Specialties: Timely Diagnosis for ILD Patients, has already produced an introductory podcast and a white paper on the effort, and its rationale is expected to be released soon, according to Dr. Kulkarni and her fellow committee members.
The American College of Chest Physicians and the Three Lakes Foundation – a foundation dedicated to pulmonary fibrosis awareness and research – are working together on this initiative. They plan to put together a suite of resources, to be gradually rolled out on the college’s website, to raise awareness about the importance of early diagnosis of ILD.
The full toolkit, expected to be rolled out over the next 12 months, will include a series of podcasts and resources on how to get patients diagnosed earlier and steps to take in cases of suspected ILD, Dr. Kulkarni said.
“The goal would be to try to increase awareness about the disease so that people start thinking more about it up front – and not after we’ve ruled out everything else,” she said. The main audience will be primary care providers, but patients and community pulmonologists would likely also benefit from the resources, the committee members said.
The urgency of the initiative stems from the way ILD treatments work. They are antifibrotic, meaning they help prevent scar tissue from forming, but they can’t reverse scar tissue that has already formed. If scarring is severe, the only option might be a lung transplant, and, since the average age at ILD diagnosis is in the 60s, many patients have comorbidities that make them ineligible for transplant. According to the Global Burden of Disease Study mentioned earlier, the death rate per 100,000 people with ILD was 1.93 in 2017.
“The longer we take to diagnose it, the more chance that inflammation will become scar tissue,” Dr. Kularni explained.
William Lago, MD, another member of the committee and a family physician, said identifying ILD early is not a straightforward matter .
“When they first present, it’s hard to pick up,” said Dr. Lago, who is also a staff physician at Cleveland Clinic’s Wooster Family Health Center and medical director of the COVID Recover Clinic there. “Many of them, even themselves, will discount the symptoms.”
Dr. Lago said that patients might resist having a work-up even when a primary care physician identifies symptoms as possible ILD. In rural settings, they might have to travel quite a distance for a CT scan or other necessary evaluations, or they might just not think the symptoms are serious enough.
“Most of the time when I’ve picked up some of my pulmonary fibrosis patients, it’s been incidentally while they’re in the office for other things,” he said. He often has to “push the issue” for further work-up, he said.
The overlap of shortness of breath and cough with other, much more common disorders, such as heart disease or chronic obstructive pulmonary disease (COPD), make ILD diagnosis a challenge, he said.
“For most of us, we’ve got sometimes 10 or 15 minutes with a patient who’s presenting with 5-6 different problems. And the shortness of breath or the occasional cough – that they think is nothing – is probably the least of those,” Dr. Lago said.
Dr. Golden said he suspected a tool like the one being developed by CHEST to be useful for some and not useful for others. He added that “no one has the time to spend on that kind of thing.”
Instead, he suggested just reinforcing what the core symptoms are and what the core testing is, “to make people think about it.”
Dr. Horowitiz seemed more optimistic about the likelihood of the CHEST tool being utilized to diagnose ILD.
Whether and how he would use the CHEST resource will depend on the final form it takes, Dr. Horowitz said. It’s encouraging that it’s being put together by a credible source, he added.
Dr. Kulkarni reported financial relationships with Boehringer Ingelheim, Aluda Pharmaceuticals and PureTech Lyt-100 Inc. Dr. Lago, Dr. Horowitz, and Dr. Golden reported no relevant disclosures.
Katie Lennon contributed to this report.
“There is definitely a delay from the time of symptom onset to the time that they are even evaluated for ILD,” said Dr. Kulkarni of the department of pulmonary, allergy and critical care medicine at the University of Alabama, Birmingham. “Some patients have had a significant loss of lung function by the time they come to see us. By that point we are limited by what treatment options we can offer.”
Interstitial lung disease is an umbrella term for a group of disorders involving progressive scarring of the lungs – typically irreversible – usually caused by long-term exposure to hazardous materials or by autoimmune effects. It includes idiopathic pulmonary fibrosis (IPF), a disease that is fairly rare but which has therapy options that can be effective if caught early enough. The term pulmonary fibrosis refers to lung scarring. Another type of ILD is pulmonary sarcoidosis, in which small clumps of immune cells form in the lungs in an immune response sometimes following an environmental trigger, and can lead to lung scarring if it doesn’t resolve.
Cases of ILD appear to be on the rise, and COVID-19 has made diagnosing it more complicated. One study found the prevalence of ILD and pulmonary sarcoidosis in high-income countries was about 122 of every 100,000 people in 1990 and rose to about 198 of every 100,000 people in 2017. The data were pulled from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Globally, the researchers found a prevalence of 62 per 100,000 in 1990, compared with 82 per 100,000 in 2017.
If all of a patient’s symptoms have appeared post COVID and a physician is seeing a patient within 4-6 weeks of COVID symptoms, it is likely that the symptoms are COVID related. But a full work-up is recommended if a patient has lung crackles, which are an indicator of lung scarring, she said.
“The patterns that are seen on CT scan for COVID pneumonia are very distinct from what we expect to see with idiopathic pulmonary fibrosis,” Dr. Kulkarni said. “Putting all this information together is what is important to differentiate it from COVID pneumonia, as well as other types of ILD.”
A study published earlier this year found similarities between COVID-19 and IPF in gene expression, their IL-15-heavy cytokine storms, and the type of damage to alveolar cells. Both might be driven by endoplasmic reticulum stress, they found.
“COVID-19 resembles IPF at a fundamental level,” they wrote.
Jeffrey Horowitz, MD, a pulmonologist and professor of medicine at the Ohio State University, said the need for early diagnosis is in part a function of the therapies available for ILD.
“They don’t make the lung function better,” he said. “So delays in diagnosis mean that there’s the possibility of underlying progression for months, or sometimes years, before the diagnosis is recognized.”
In an area in which diagnosis is delayed and the prognosis is dire – 3-5 years in untreated patients after diagnosis – “there’s a tremendous amount of nihilism out there” among patients, he said.
He said patients with long-term shortness of breath and unexplained cough are often told they have asthma and are prescribed inhalers, but then further assessment isn’t performed when those don’t work.
Diagnosing ILD in primary care
Many primary care physicians feel ill-equipped to discuss IPF. More than a dozen physicians contacted for this piece to talk about ILD either did not respond, or said they felt unqualified to respond to questions on the disease.
“Not my area of expertise” and “I don’t think I’m the right person for this discussion” were two of the responses provided to this news organization.
“For some reason, in the world of primary care, it seems like there’s an impediment to getting pulmonary function studies,” Dr. Horowitz said. “Anybody who has a persistent ongoing prolonged unexplained shortness of breath and cough should have pulmonary function studies done.”
Listening to the lungs alone might not be enough, he said. There might be no clear sign in the case of early pulmonary fibrosis, he said.
“There’s the textbook description of these Velcro-sounding crackles, but sometimes it’s very subtle,” he said. “And unless you’re listening very carefully it can easily be missed by somebody who has a busy practice, or it’s loud.”
William E. Golden, MD, professor of medicine and public health at the University of Arkansas, Little Rock, is the sole primary care physician contacted for this piece who spoke with authority on ILD.
For cases of suspected ILD, internist Dr. Golden, who also serves on the editorial advisory board of Internal Medicine News, suggested ordering a test for diffusing capacity for carbon monoxide (DLCO), which will be low in the case of IPF, along with a fine-cut lung CT scan to assess ongoing fibrotic changes.
It’s “not that difficult, but you need to have an index of suspicion for the diagnosis,” he said.
New initiative for helping diagnose ILD
Dr. Kulkarni is a committee member for a new effort under way to try to get patients with ILD diagnosed earlier.
The initiative, called Bridging Specialties: Timely Diagnosis for ILD Patients, has already produced an introductory podcast and a white paper on the effort, and its rationale is expected to be released soon, according to Dr. Kulkarni and her fellow committee members.
The American College of Chest Physicians and the Three Lakes Foundation – a foundation dedicated to pulmonary fibrosis awareness and research – are working together on this initiative. They plan to put together a suite of resources, to be gradually rolled out on the college’s website, to raise awareness about the importance of early diagnosis of ILD.
The full toolkit, expected to be rolled out over the next 12 months, will include a series of podcasts and resources on how to get patients diagnosed earlier and steps to take in cases of suspected ILD, Dr. Kulkarni said.
“The goal would be to try to increase awareness about the disease so that people start thinking more about it up front – and not after we’ve ruled out everything else,” she said. The main audience will be primary care providers, but patients and community pulmonologists would likely also benefit from the resources, the committee members said.
The urgency of the initiative stems from the way ILD treatments work. They are antifibrotic, meaning they help prevent scar tissue from forming, but they can’t reverse scar tissue that has already formed. If scarring is severe, the only option might be a lung transplant, and, since the average age at ILD diagnosis is in the 60s, many patients have comorbidities that make them ineligible for transplant. According to the Global Burden of Disease Study mentioned earlier, the death rate per 100,000 people with ILD was 1.93 in 2017.
“The longer we take to diagnose it, the more chance that inflammation will become scar tissue,” Dr. Kularni explained.
William Lago, MD, another member of the committee and a family physician, said identifying ILD early is not a straightforward matter .
“When they first present, it’s hard to pick up,” said Dr. Lago, who is also a staff physician at Cleveland Clinic’s Wooster Family Health Center and medical director of the COVID Recover Clinic there. “Many of them, even themselves, will discount the symptoms.”
Dr. Lago said that patients might resist having a work-up even when a primary care physician identifies symptoms as possible ILD. In rural settings, they might have to travel quite a distance for a CT scan or other necessary evaluations, or they might just not think the symptoms are serious enough.
“Most of the time when I’ve picked up some of my pulmonary fibrosis patients, it’s been incidentally while they’re in the office for other things,” he said. He often has to “push the issue” for further work-up, he said.
The overlap of shortness of breath and cough with other, much more common disorders, such as heart disease or chronic obstructive pulmonary disease (COPD), make ILD diagnosis a challenge, he said.
“For most of us, we’ve got sometimes 10 or 15 minutes with a patient who’s presenting with 5-6 different problems. And the shortness of breath or the occasional cough – that they think is nothing – is probably the least of those,” Dr. Lago said.
Dr. Golden said he suspected a tool like the one being developed by CHEST to be useful for some and not useful for others. He added that “no one has the time to spend on that kind of thing.”
Instead, he suggested just reinforcing what the core symptoms are and what the core testing is, “to make people think about it.”
Dr. Horowitiz seemed more optimistic about the likelihood of the CHEST tool being utilized to diagnose ILD.
Whether and how he would use the CHEST resource will depend on the final form it takes, Dr. Horowitz said. It’s encouraging that it’s being put together by a credible source, he added.
Dr. Kulkarni reported financial relationships with Boehringer Ingelheim, Aluda Pharmaceuticals and PureTech Lyt-100 Inc. Dr. Lago, Dr. Horowitz, and Dr. Golden reported no relevant disclosures.
Katie Lennon contributed to this report.
“There is definitely a delay from the time of symptom onset to the time that they are even evaluated for ILD,” said Dr. Kulkarni of the department of pulmonary, allergy and critical care medicine at the University of Alabama, Birmingham. “Some patients have had a significant loss of lung function by the time they come to see us. By that point we are limited by what treatment options we can offer.”
Interstitial lung disease is an umbrella term for a group of disorders involving progressive scarring of the lungs – typically irreversible – usually caused by long-term exposure to hazardous materials or by autoimmune effects. It includes idiopathic pulmonary fibrosis (IPF), a disease that is fairly rare but which has therapy options that can be effective if caught early enough. The term pulmonary fibrosis refers to lung scarring. Another type of ILD is pulmonary sarcoidosis, in which small clumps of immune cells form in the lungs in an immune response sometimes following an environmental trigger, and can lead to lung scarring if it doesn’t resolve.
Cases of ILD appear to be on the rise, and COVID-19 has made diagnosing it more complicated. One study found the prevalence of ILD and pulmonary sarcoidosis in high-income countries was about 122 of every 100,000 people in 1990 and rose to about 198 of every 100,000 people in 2017. The data were pulled from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Globally, the researchers found a prevalence of 62 per 100,000 in 1990, compared with 82 per 100,000 in 2017.
If all of a patient’s symptoms have appeared post COVID and a physician is seeing a patient within 4-6 weeks of COVID symptoms, it is likely that the symptoms are COVID related. But a full work-up is recommended if a patient has lung crackles, which are an indicator of lung scarring, she said.
“The patterns that are seen on CT scan for COVID pneumonia are very distinct from what we expect to see with idiopathic pulmonary fibrosis,” Dr. Kulkarni said. “Putting all this information together is what is important to differentiate it from COVID pneumonia, as well as other types of ILD.”
A study published earlier this year found similarities between COVID-19 and IPF in gene expression, their IL-15-heavy cytokine storms, and the type of damage to alveolar cells. Both might be driven by endoplasmic reticulum stress, they found.
“COVID-19 resembles IPF at a fundamental level,” they wrote.
Jeffrey Horowitz, MD, a pulmonologist and professor of medicine at the Ohio State University, said the need for early diagnosis is in part a function of the therapies available for ILD.
“They don’t make the lung function better,” he said. “So delays in diagnosis mean that there’s the possibility of underlying progression for months, or sometimes years, before the diagnosis is recognized.”
In an area in which diagnosis is delayed and the prognosis is dire – 3-5 years in untreated patients after diagnosis – “there’s a tremendous amount of nihilism out there” among patients, he said.
He said patients with long-term shortness of breath and unexplained cough are often told they have asthma and are prescribed inhalers, but then further assessment isn’t performed when those don’t work.
Diagnosing ILD in primary care
Many primary care physicians feel ill-equipped to discuss IPF. More than a dozen physicians contacted for this piece to talk about ILD either did not respond, or said they felt unqualified to respond to questions on the disease.
“Not my area of expertise” and “I don’t think I’m the right person for this discussion” were two of the responses provided to this news organization.
“For some reason, in the world of primary care, it seems like there’s an impediment to getting pulmonary function studies,” Dr. Horowitz said. “Anybody who has a persistent ongoing prolonged unexplained shortness of breath and cough should have pulmonary function studies done.”
Listening to the lungs alone might not be enough, he said. There might be no clear sign in the case of early pulmonary fibrosis, he said.
“There’s the textbook description of these Velcro-sounding crackles, but sometimes it’s very subtle,” he said. “And unless you’re listening very carefully it can easily be missed by somebody who has a busy practice, or it’s loud.”
William E. Golden, MD, professor of medicine and public health at the University of Arkansas, Little Rock, is the sole primary care physician contacted for this piece who spoke with authority on ILD.
For cases of suspected ILD, internist Dr. Golden, who also serves on the editorial advisory board of Internal Medicine News, suggested ordering a test for diffusing capacity for carbon monoxide (DLCO), which will be low in the case of IPF, along with a fine-cut lung CT scan to assess ongoing fibrotic changes.
It’s “not that difficult, but you need to have an index of suspicion for the diagnosis,” he said.
New initiative for helping diagnose ILD
Dr. Kulkarni is a committee member for a new effort under way to try to get patients with ILD diagnosed earlier.
The initiative, called Bridging Specialties: Timely Diagnosis for ILD Patients, has already produced an introductory podcast and a white paper on the effort, and its rationale is expected to be released soon, according to Dr. Kulkarni and her fellow committee members.
The American College of Chest Physicians and the Three Lakes Foundation – a foundation dedicated to pulmonary fibrosis awareness and research – are working together on this initiative. They plan to put together a suite of resources, to be gradually rolled out on the college’s website, to raise awareness about the importance of early diagnosis of ILD.
The full toolkit, expected to be rolled out over the next 12 months, will include a series of podcasts and resources on how to get patients diagnosed earlier and steps to take in cases of suspected ILD, Dr. Kulkarni said.
“The goal would be to try to increase awareness about the disease so that people start thinking more about it up front – and not after we’ve ruled out everything else,” she said. The main audience will be primary care providers, but patients and community pulmonologists would likely also benefit from the resources, the committee members said.
The urgency of the initiative stems from the way ILD treatments work. They are antifibrotic, meaning they help prevent scar tissue from forming, but they can’t reverse scar tissue that has already formed. If scarring is severe, the only option might be a lung transplant, and, since the average age at ILD diagnosis is in the 60s, many patients have comorbidities that make them ineligible for transplant. According to the Global Burden of Disease Study mentioned earlier, the death rate per 100,000 people with ILD was 1.93 in 2017.
“The longer we take to diagnose it, the more chance that inflammation will become scar tissue,” Dr. Kularni explained.
William Lago, MD, another member of the committee and a family physician, said identifying ILD early is not a straightforward matter .
“When they first present, it’s hard to pick up,” said Dr. Lago, who is also a staff physician at Cleveland Clinic’s Wooster Family Health Center and medical director of the COVID Recover Clinic there. “Many of them, even themselves, will discount the symptoms.”
Dr. Lago said that patients might resist having a work-up even when a primary care physician identifies symptoms as possible ILD. In rural settings, they might have to travel quite a distance for a CT scan or other necessary evaluations, or they might just not think the symptoms are serious enough.
“Most of the time when I’ve picked up some of my pulmonary fibrosis patients, it’s been incidentally while they’re in the office for other things,” he said. He often has to “push the issue” for further work-up, he said.
The overlap of shortness of breath and cough with other, much more common disorders, such as heart disease or chronic obstructive pulmonary disease (COPD), make ILD diagnosis a challenge, he said.
“For most of us, we’ve got sometimes 10 or 15 minutes with a patient who’s presenting with 5-6 different problems. And the shortness of breath or the occasional cough – that they think is nothing – is probably the least of those,” Dr. Lago said.
Dr. Golden said he suspected a tool like the one being developed by CHEST to be useful for some and not useful for others. He added that “no one has the time to spend on that kind of thing.”
Instead, he suggested just reinforcing what the core symptoms are and what the core testing is, “to make people think about it.”
Dr. Horowitiz seemed more optimistic about the likelihood of the CHEST tool being utilized to diagnose ILD.
Whether and how he would use the CHEST resource will depend on the final form it takes, Dr. Horowitz said. It’s encouraging that it’s being put together by a credible source, he added.
Dr. Kulkarni reported financial relationships with Boehringer Ingelheim, Aluda Pharmaceuticals and PureTech Lyt-100 Inc. Dr. Lago, Dr. Horowitz, and Dr. Golden reported no relevant disclosures.
Katie Lennon contributed to this report.
Crystal bone algorithm predicts early fractures, uses ICD codes
The Crystal Bone (Amgen) novel algorithm predicted 2-year risk of osteoporotic fractures in a large dataset with an accuracy that was consistent with FRAX 10-year risk predictions, researchers report.
The algorithm was built using machine learning and artificial intelligence to predict fracture risk based on International Classification of Diseases (ICD) codes, as described in an article published in the Journal of Medical Internet Research.
The current validation study was presented September 9 as a poster at the annual meeting of the American Society for Bone and Mineral Research.
The scientists validated the algorithm in more than 100,000 patients aged 50 and older (that is, at risk of fracture) who were part of the Reliant Medical Group dataset (a subset of Optum Care).
Importantly, the algorithm predicted increased fracture in many patients who did not have a diagnosis of osteoporosis.
The next steps are validation in other datasets to support the generalizability of Crystal Bone across U.S. health care systems, Elinor Mody, MD, Reliant Medical Group, and colleagues report.
“Implementation research, in which patients identified by Crystal Bone undergo a bone health assessment and receive ongoing management, will help inform the clinical utility of this novel algorithm,” they conclude.
At the poster session, Tina Kelley, Optum Life Sciences, explained: “It’s a screening tool that says: ‘These are your patients that maybe you should spend a little extra time with, ask a few extra questions.’ ”
However, further study is needed before it should be used in clinical practice, she emphasized to this news organization.
‘A very useful advance’ but needs further validation
Invited to comment, Peter R. Ebeling, MD, outgoing president of the ASBMR, noted that “many clinicians now use FRAX to calculate absolute fracture risk and select patients who should initiate anti-osteoporosis drugs.”
With FRAX, clinicians input a patient’s age, sex, weight, height, previous fracture, [history of] parent with fractured hip, current smoking status, glucocorticoids, rheumatoid arthritis, secondary osteoporosis, alcohol (3 units/day or more), and bone mineral density (by DXA at the femoral neck) into the tool, to obtain a 10-year probability of fracture.
“Crystal Bone takes a different approach,” Dr. Ebeling, from Monash University, Melbourne, who was not involved with the research but who disclosed receiving funding from Amgen, told this news organization in an email.
The algorithm uses electronic health records (EHRs) to identify patients who are likely to have a fracture within the next 2 years, he explained, based on diagnoses and medications associated with osteoporosis and fractures. These include ICD-10 codes for fractures at various sites and secondary causes of osteoporosis (such as rheumatoid and other inflammatory arthritis, chronic obstructive pulmonary disease, asthma, celiac disease, and inflammatory bowel disease).
“This is a very useful advance,” Dr. Ebeling summarized, “in that it would alert the clinician to patients in their practice who have a high fracture risk and need to be investigated for osteoporosis and initiated on treatment. Otherwise, the patients would be missed, as currently often occurs.”
“It would need to be adaptable to other [EMR] systems and to be validated in a large separate population to be ready to enter clinical practice,” he said, “but these data look very promising with a good [positive predictive value (PPV)].”
Similarly, Juliet Compston, MD, said: “It provides a novel, fully automated approach to population-based screening for osteoporosis using EHRs to identify people at high imminent risk of fracture.”
Dr. Compston, emeritus professor of bone medicine, University of Cambridge, England, who was not involved with the research but who also disclosed being a consultant for Amgen, selected the study as one of the top clinical science highlights abstracts at the meeting.
“The algorithm looks at ICD codes for previous history of fracture, medications that have adverse effects on bone – for example glucocorticoids, aromatase inhibitors, and anti-androgens – as well as chronic diseases that increase the risk of fracture,” she explained.
“FRAX is the most commonly used tool to estimate fracture probability in clinical practice and to guide treatment decisions,” she noted. However, “currently it requires human input of data into the FRAX website and is generally only performed on individuals who are selected on the basis of clinical risk factors.”
“The Crystal Bone algorithm offers the potential for fully automated population-based screening in older adults to identify those at high risk of fracture, for whom effective therapies are available to reduce fracture risk,” she summarized.
“It needs further validation,” she noted, “and implementation into clinical practice requires the availability of high-quality EHRs.”
Algorithm validated in 106,328 patients aged 50 and older
Despite guidelines that recommend screening for osteoporosis in women aged 65 and older, men older than 70, and adults aged 50-79 with risk factors, real-world data suggest such screening is low, the researchers note.
The current validation study identified 106,328 patients aged 50 and older who had at least 2 years of consecutive medical history with the Reliant Medical Group from December 2014 to November 2020 as well as at least two EHR codes.
The accuracy of predicting a fracture within 2 years, expressed as area under the receiver operating characteristic (AUROC), was 0.77, where 1 is perfect, 0.5 is no better than random selection, 0.7 to 0.8 is acceptable, and 0.8 to 0.9 indicates excellent predictive accuracy.
In the entire Optum Reliant population older than 50, the risk of fracture within 2 years was 1.95%.
The algorithm identified four groups with a greater risk: 19,100 patients had a threefold higher risk of fracture within 2 years, 9,246 patients had a fourfold higher risk, 3,533 patients had a sevenfold higher risk, and 1,735 patients had a ninefold higher risk.
Many of these patients had no prior diagnosis of osteoporosis
For example, in the 19,100 patients with a threefold greater risk of fracture in 2 years, 69% of patients had not been diagnosed with osteoporosis (49% of them had no history of fracture and 20% did have a history of fracture).
The algorithm had a positive predictive value of 6%-18%, a negative predictive value of 98%-99%, a specificity of 81%-98%, and a sensitivity of 18%-59%, for the four groups.
The study was funded by Amgen. Dr. Mody and another author are Reliant Medical Group employees. Ms. Kelley and another author are Optum Life Sciences employees. One author is an employee at Landing AI. Two authors are Amgen employees and own Amgen stock. Dr. Ebeling has disclosed receiving research funding from Amgen, Sanofi, and Alexion, and his institution has received honoraria from Amgen and Kyowa Kirin. Dr. Compston has disclosed receiving speaking and consultancy fees from Amgen and UCB.
A version of this article first appeared on Medscape.com.
The Crystal Bone (Amgen) novel algorithm predicted 2-year risk of osteoporotic fractures in a large dataset with an accuracy that was consistent with FRAX 10-year risk predictions, researchers report.
The algorithm was built using machine learning and artificial intelligence to predict fracture risk based on International Classification of Diseases (ICD) codes, as described in an article published in the Journal of Medical Internet Research.
The current validation study was presented September 9 as a poster at the annual meeting of the American Society for Bone and Mineral Research.
The scientists validated the algorithm in more than 100,000 patients aged 50 and older (that is, at risk of fracture) who were part of the Reliant Medical Group dataset (a subset of Optum Care).
Importantly, the algorithm predicted increased fracture in many patients who did not have a diagnosis of osteoporosis.
The next steps are validation in other datasets to support the generalizability of Crystal Bone across U.S. health care systems, Elinor Mody, MD, Reliant Medical Group, and colleagues report.
“Implementation research, in which patients identified by Crystal Bone undergo a bone health assessment and receive ongoing management, will help inform the clinical utility of this novel algorithm,” they conclude.
At the poster session, Tina Kelley, Optum Life Sciences, explained: “It’s a screening tool that says: ‘These are your patients that maybe you should spend a little extra time with, ask a few extra questions.’ ”
However, further study is needed before it should be used in clinical practice, she emphasized to this news organization.
‘A very useful advance’ but needs further validation
Invited to comment, Peter R. Ebeling, MD, outgoing president of the ASBMR, noted that “many clinicians now use FRAX to calculate absolute fracture risk and select patients who should initiate anti-osteoporosis drugs.”
With FRAX, clinicians input a patient’s age, sex, weight, height, previous fracture, [history of] parent with fractured hip, current smoking status, glucocorticoids, rheumatoid arthritis, secondary osteoporosis, alcohol (3 units/day or more), and bone mineral density (by DXA at the femoral neck) into the tool, to obtain a 10-year probability of fracture.
“Crystal Bone takes a different approach,” Dr. Ebeling, from Monash University, Melbourne, who was not involved with the research but who disclosed receiving funding from Amgen, told this news organization in an email.
The algorithm uses electronic health records (EHRs) to identify patients who are likely to have a fracture within the next 2 years, he explained, based on diagnoses and medications associated with osteoporosis and fractures. These include ICD-10 codes for fractures at various sites and secondary causes of osteoporosis (such as rheumatoid and other inflammatory arthritis, chronic obstructive pulmonary disease, asthma, celiac disease, and inflammatory bowel disease).
“This is a very useful advance,” Dr. Ebeling summarized, “in that it would alert the clinician to patients in their practice who have a high fracture risk and need to be investigated for osteoporosis and initiated on treatment. Otherwise, the patients would be missed, as currently often occurs.”
“It would need to be adaptable to other [EMR] systems and to be validated in a large separate population to be ready to enter clinical practice,” he said, “but these data look very promising with a good [positive predictive value (PPV)].”
Similarly, Juliet Compston, MD, said: “It provides a novel, fully automated approach to population-based screening for osteoporosis using EHRs to identify people at high imminent risk of fracture.”
Dr. Compston, emeritus professor of bone medicine, University of Cambridge, England, who was not involved with the research but who also disclosed being a consultant for Amgen, selected the study as one of the top clinical science highlights abstracts at the meeting.
“The algorithm looks at ICD codes for previous history of fracture, medications that have adverse effects on bone – for example glucocorticoids, aromatase inhibitors, and anti-androgens – as well as chronic diseases that increase the risk of fracture,” she explained.
“FRAX is the most commonly used tool to estimate fracture probability in clinical practice and to guide treatment decisions,” she noted. However, “currently it requires human input of data into the FRAX website and is generally only performed on individuals who are selected on the basis of clinical risk factors.”
“The Crystal Bone algorithm offers the potential for fully automated population-based screening in older adults to identify those at high risk of fracture, for whom effective therapies are available to reduce fracture risk,” she summarized.
“It needs further validation,” she noted, “and implementation into clinical practice requires the availability of high-quality EHRs.”
Algorithm validated in 106,328 patients aged 50 and older
Despite guidelines that recommend screening for osteoporosis in women aged 65 and older, men older than 70, and adults aged 50-79 with risk factors, real-world data suggest such screening is low, the researchers note.
The current validation study identified 106,328 patients aged 50 and older who had at least 2 years of consecutive medical history with the Reliant Medical Group from December 2014 to November 2020 as well as at least two EHR codes.
The accuracy of predicting a fracture within 2 years, expressed as area under the receiver operating characteristic (AUROC), was 0.77, where 1 is perfect, 0.5 is no better than random selection, 0.7 to 0.8 is acceptable, and 0.8 to 0.9 indicates excellent predictive accuracy.
In the entire Optum Reliant population older than 50, the risk of fracture within 2 years was 1.95%.
The algorithm identified four groups with a greater risk: 19,100 patients had a threefold higher risk of fracture within 2 years, 9,246 patients had a fourfold higher risk, 3,533 patients had a sevenfold higher risk, and 1,735 patients had a ninefold higher risk.
Many of these patients had no prior diagnosis of osteoporosis
For example, in the 19,100 patients with a threefold greater risk of fracture in 2 years, 69% of patients had not been diagnosed with osteoporosis (49% of them had no history of fracture and 20% did have a history of fracture).
The algorithm had a positive predictive value of 6%-18%, a negative predictive value of 98%-99%, a specificity of 81%-98%, and a sensitivity of 18%-59%, for the four groups.
The study was funded by Amgen. Dr. Mody and another author are Reliant Medical Group employees. Ms. Kelley and another author are Optum Life Sciences employees. One author is an employee at Landing AI. Two authors are Amgen employees and own Amgen stock. Dr. Ebeling has disclosed receiving research funding from Amgen, Sanofi, and Alexion, and his institution has received honoraria from Amgen and Kyowa Kirin. Dr. Compston has disclosed receiving speaking and consultancy fees from Amgen and UCB.
A version of this article first appeared on Medscape.com.
The Crystal Bone (Amgen) novel algorithm predicted 2-year risk of osteoporotic fractures in a large dataset with an accuracy that was consistent with FRAX 10-year risk predictions, researchers report.
The algorithm was built using machine learning and artificial intelligence to predict fracture risk based on International Classification of Diseases (ICD) codes, as described in an article published in the Journal of Medical Internet Research.
The current validation study was presented September 9 as a poster at the annual meeting of the American Society for Bone and Mineral Research.
The scientists validated the algorithm in more than 100,000 patients aged 50 and older (that is, at risk of fracture) who were part of the Reliant Medical Group dataset (a subset of Optum Care).
Importantly, the algorithm predicted increased fracture in many patients who did not have a diagnosis of osteoporosis.
The next steps are validation in other datasets to support the generalizability of Crystal Bone across U.S. health care systems, Elinor Mody, MD, Reliant Medical Group, and colleagues report.
“Implementation research, in which patients identified by Crystal Bone undergo a bone health assessment and receive ongoing management, will help inform the clinical utility of this novel algorithm,” they conclude.
At the poster session, Tina Kelley, Optum Life Sciences, explained: “It’s a screening tool that says: ‘These are your patients that maybe you should spend a little extra time with, ask a few extra questions.’ ”
However, further study is needed before it should be used in clinical practice, she emphasized to this news organization.
‘A very useful advance’ but needs further validation
Invited to comment, Peter R. Ebeling, MD, outgoing president of the ASBMR, noted that “many clinicians now use FRAX to calculate absolute fracture risk and select patients who should initiate anti-osteoporosis drugs.”
With FRAX, clinicians input a patient’s age, sex, weight, height, previous fracture, [history of] parent with fractured hip, current smoking status, glucocorticoids, rheumatoid arthritis, secondary osteoporosis, alcohol (3 units/day or more), and bone mineral density (by DXA at the femoral neck) into the tool, to obtain a 10-year probability of fracture.
“Crystal Bone takes a different approach,” Dr. Ebeling, from Monash University, Melbourne, who was not involved with the research but who disclosed receiving funding from Amgen, told this news organization in an email.
The algorithm uses electronic health records (EHRs) to identify patients who are likely to have a fracture within the next 2 years, he explained, based on diagnoses and medications associated with osteoporosis and fractures. These include ICD-10 codes for fractures at various sites and secondary causes of osteoporosis (such as rheumatoid and other inflammatory arthritis, chronic obstructive pulmonary disease, asthma, celiac disease, and inflammatory bowel disease).
“This is a very useful advance,” Dr. Ebeling summarized, “in that it would alert the clinician to patients in their practice who have a high fracture risk and need to be investigated for osteoporosis and initiated on treatment. Otherwise, the patients would be missed, as currently often occurs.”
“It would need to be adaptable to other [EMR] systems and to be validated in a large separate population to be ready to enter clinical practice,” he said, “but these data look very promising with a good [positive predictive value (PPV)].”
Similarly, Juliet Compston, MD, said: “It provides a novel, fully automated approach to population-based screening for osteoporosis using EHRs to identify people at high imminent risk of fracture.”
Dr. Compston, emeritus professor of bone medicine, University of Cambridge, England, who was not involved with the research but who also disclosed being a consultant for Amgen, selected the study as one of the top clinical science highlights abstracts at the meeting.
“The algorithm looks at ICD codes for previous history of fracture, medications that have adverse effects on bone – for example glucocorticoids, aromatase inhibitors, and anti-androgens – as well as chronic diseases that increase the risk of fracture,” she explained.
“FRAX is the most commonly used tool to estimate fracture probability in clinical practice and to guide treatment decisions,” she noted. However, “currently it requires human input of data into the FRAX website and is generally only performed on individuals who are selected on the basis of clinical risk factors.”
“The Crystal Bone algorithm offers the potential for fully automated population-based screening in older adults to identify those at high risk of fracture, for whom effective therapies are available to reduce fracture risk,” she summarized.
“It needs further validation,” she noted, “and implementation into clinical practice requires the availability of high-quality EHRs.”
Algorithm validated in 106,328 patients aged 50 and older
Despite guidelines that recommend screening for osteoporosis in women aged 65 and older, men older than 70, and adults aged 50-79 with risk factors, real-world data suggest such screening is low, the researchers note.
The current validation study identified 106,328 patients aged 50 and older who had at least 2 years of consecutive medical history with the Reliant Medical Group from December 2014 to November 2020 as well as at least two EHR codes.
The accuracy of predicting a fracture within 2 years, expressed as area under the receiver operating characteristic (AUROC), was 0.77, where 1 is perfect, 0.5 is no better than random selection, 0.7 to 0.8 is acceptable, and 0.8 to 0.9 indicates excellent predictive accuracy.
In the entire Optum Reliant population older than 50, the risk of fracture within 2 years was 1.95%.
The algorithm identified four groups with a greater risk: 19,100 patients had a threefold higher risk of fracture within 2 years, 9,246 patients had a fourfold higher risk, 3,533 patients had a sevenfold higher risk, and 1,735 patients had a ninefold higher risk.
Many of these patients had no prior diagnosis of osteoporosis
For example, in the 19,100 patients with a threefold greater risk of fracture in 2 years, 69% of patients had not been diagnosed with osteoporosis (49% of them had no history of fracture and 20% did have a history of fracture).
The algorithm had a positive predictive value of 6%-18%, a negative predictive value of 98%-99%, a specificity of 81%-98%, and a sensitivity of 18%-59%, for the four groups.
The study was funded by Amgen. Dr. Mody and another author are Reliant Medical Group employees. Ms. Kelley and another author are Optum Life Sciences employees. One author is an employee at Landing AI. Two authors are Amgen employees and own Amgen stock. Dr. Ebeling has disclosed receiving research funding from Amgen, Sanofi, and Alexion, and his institution has received honoraria from Amgen and Kyowa Kirin. Dr. Compston has disclosed receiving speaking and consultancy fees from Amgen and UCB.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2022
Usefulness of IBD biomarker may vary based on microbiome
Levels of calprotectin, a biomarker used to detect intestinal inflammation, may vary in fecal samples based on an individual’s microbiome composition, according to researchers. The results, if confirmed, might help refine its use in monitoring inflammatory bowel disease (IBD).
Researchers used a new ex vivo functional assay to identify specific bacteria that degrade calprotectin and may play a role in variations found in vivo. “Microbiome-based calibration could improve sensitivity and specificity of fecal calprotectin readouts, thereby facilitating more reliable real-time monitoring and ultimately enabling more timely interventions,” the authors wrote in their research letter, which was published in Gastro Hep Advances.
The standard for diagnosing ulcerative colitis (UC) and Crohn’s disease (CD) is endoscopy and biopsy because it allows both visual and histological examination of the severity and extent of intestinal inflammation, but this cannot be used to monitor patients on an ongoing basis.
Calprotectin is a promising biomarker for intestinal inflammation, but a meta-analysis found that it has a pooled sensitivity of 85% and a pooled specificity of 75% for the diagnosis of endoscopically active inflammatory bowel disease.
The researchers investigated whether an individual’s microbiome can metabolize calprotectin, which would complicate measurement of fecal calprotectin. They recruited 22 individuals with IBD (64% female, 73% with colonic disease), who provided stool samples. They completed a symptom questionnaire in advance of a colonoscopy. Overall, 64% had endoscopically inactive disease, and 82% had clinically inactive disease.
At a cutoff of 50 mcg/g, 9 patients had normal fecal calprotectin levels, and 13 had elevated levels. Those with clinically or endoscopically active disease had higher levels of fecal calprotectin (P < .0001).
There was a significant but poor correlation between disease activity measures and fecal calprotectin levels in CD (r = 0.62; P = .008), but there was no statistically significant association for UC (r = –0.29; P = .6). Endoscopic disease activity was also significantly correlated with fecal calprotectin in CD (r = 0.83; P < .001), but not UC (r = 0.50; P = .4).
The researchers created an ex vivo functional assay to measure calprotectin metabolism by the microbiome. They anaerobically cultured fecal samples in the presence of calprotectin and measured levels of calprotectin after 24 hours. Control samples were grown without microbes, without calprotectin, or lacking both. The researchers tested samples in both standard media and in media with low levels of amino acids, reasoning that the latter condition might encourage catabolism of calprotectin. The cultures with low amino acid content had lower calprotectin levels than those with normal amino acid content (P < .0007).
The researchers found greater calprotectin degradation in the low amino acid media, and the difference was more pronounced among samples taken from individuals with UC than CD (P < .02).
They used metagenomic sequencing data from fecal samples to identify bacterial species associated with calprotectin metabolism. Similarly to previous reports, the researchers found that Firmicutes was dominant, while Subdoligranulum correlated with calprotectin degradation in low amino acid media (P = .04).
For 5 days, they cultured Subdoligranulum variabile in low amino acid media that also contained calprotectin. Calprotectin levels were lower than a control sample with cultured Akkermansia muciniphila, which was previously shown to not be associated with calprotectin degradation in low amino acid media (P = .03). Because Subdoligranulum species were not detectable in 5 of 22 fecal samples, the authors say they are unlikely to be the only species capable of metabolizing calprotectin.
Among IBD patients, only one had both endoscopically active colitis and a low fecal calprotectin level. The patient’s micobiome had Subdoligranulum present, and their fecal sample was able to metabolize calprotectin in the functional assay.
The study was limited by its small sample size, which prevented development of a calibration model for fecal calprotectin, and the researchers called for additional studies among individuals with active colitis.
The search continues for reliable, noninvasive methods for monitoring disease activity in inflammatory bowel disease (IBD). Noninvasive disease activity measures improve quality of care by facilitating more frequent assessment of therapeutic efficacy, which for IBD otherwise depends on periodic endoscopic evaluation and biopsy. Available tools such as fecal calprotectin are valuable and widely used but are imperfect.
Of note, a patient with endoscopically active colitis and a relatively low fecal calprotectin level harbored Subdoligranulum species, which – when isolated and assayed ex vivo – degraded calprotectin. These studies suggest that individualized, patient microbiome–based calibration assays might help improve the sensitivity and specificity of fecal calprotectin levels for monitoring disease activity. As the authors note, more patients need to be studied, especially focusing on those with active disease and paradoxically low calprotectin levels.
Deborah C. Rubin, MD, AGAF, is the William B. Kountz Professor of Medicine and professor of developmental biology in the division of gastroenterology at Washington University, St. Louis. She had no conflicts of interest to disclose.
The search continues for reliable, noninvasive methods for monitoring disease activity in inflammatory bowel disease (IBD). Noninvasive disease activity measures improve quality of care by facilitating more frequent assessment of therapeutic efficacy, which for IBD otherwise depends on periodic endoscopic evaluation and biopsy. Available tools such as fecal calprotectin are valuable and widely used but are imperfect.
Of note, a patient with endoscopically active colitis and a relatively low fecal calprotectin level harbored Subdoligranulum species, which – when isolated and assayed ex vivo – degraded calprotectin. These studies suggest that individualized, patient microbiome–based calibration assays might help improve the sensitivity and specificity of fecal calprotectin levels for monitoring disease activity. As the authors note, more patients need to be studied, especially focusing on those with active disease and paradoxically low calprotectin levels.
Deborah C. Rubin, MD, AGAF, is the William B. Kountz Professor of Medicine and professor of developmental biology in the division of gastroenterology at Washington University, St. Louis. She had no conflicts of interest to disclose.
The search continues for reliable, noninvasive methods for monitoring disease activity in inflammatory bowel disease (IBD). Noninvasive disease activity measures improve quality of care by facilitating more frequent assessment of therapeutic efficacy, which for IBD otherwise depends on periodic endoscopic evaluation and biopsy. Available tools such as fecal calprotectin are valuable and widely used but are imperfect.
Of note, a patient with endoscopically active colitis and a relatively low fecal calprotectin level harbored Subdoligranulum species, which – when isolated and assayed ex vivo – degraded calprotectin. These studies suggest that individualized, patient microbiome–based calibration assays might help improve the sensitivity and specificity of fecal calprotectin levels for monitoring disease activity. As the authors note, more patients need to be studied, especially focusing on those with active disease and paradoxically low calprotectin levels.
Deborah C. Rubin, MD, AGAF, is the William B. Kountz Professor of Medicine and professor of developmental biology in the division of gastroenterology at Washington University, St. Louis. She had no conflicts of interest to disclose.
Levels of calprotectin, a biomarker used to detect intestinal inflammation, may vary in fecal samples based on an individual’s microbiome composition, according to researchers. The results, if confirmed, might help refine its use in monitoring inflammatory bowel disease (IBD).
Researchers used a new ex vivo functional assay to identify specific bacteria that degrade calprotectin and may play a role in variations found in vivo. “Microbiome-based calibration could improve sensitivity and specificity of fecal calprotectin readouts, thereby facilitating more reliable real-time monitoring and ultimately enabling more timely interventions,” the authors wrote in their research letter, which was published in Gastro Hep Advances.
The standard for diagnosing ulcerative colitis (UC) and Crohn’s disease (CD) is endoscopy and biopsy because it allows both visual and histological examination of the severity and extent of intestinal inflammation, but this cannot be used to monitor patients on an ongoing basis.
Calprotectin is a promising biomarker for intestinal inflammation, but a meta-analysis found that it has a pooled sensitivity of 85% and a pooled specificity of 75% for the diagnosis of endoscopically active inflammatory bowel disease.
The researchers investigated whether an individual’s microbiome can metabolize calprotectin, which would complicate measurement of fecal calprotectin. They recruited 22 individuals with IBD (64% female, 73% with colonic disease), who provided stool samples. They completed a symptom questionnaire in advance of a colonoscopy. Overall, 64% had endoscopically inactive disease, and 82% had clinically inactive disease.
At a cutoff of 50 mcg/g, 9 patients had normal fecal calprotectin levels, and 13 had elevated levels. Those with clinically or endoscopically active disease had higher levels of fecal calprotectin (P < .0001).
There was a significant but poor correlation between disease activity measures and fecal calprotectin levels in CD (r = 0.62; P = .008), but there was no statistically significant association for UC (r = –0.29; P = .6). Endoscopic disease activity was also significantly correlated with fecal calprotectin in CD (r = 0.83; P < .001), but not UC (r = 0.50; P = .4).
The researchers created an ex vivo functional assay to measure calprotectin metabolism by the microbiome. They anaerobically cultured fecal samples in the presence of calprotectin and measured levels of calprotectin after 24 hours. Control samples were grown without microbes, without calprotectin, or lacking both. The researchers tested samples in both standard media and in media with low levels of amino acids, reasoning that the latter condition might encourage catabolism of calprotectin. The cultures with low amino acid content had lower calprotectin levels than those with normal amino acid content (P < .0007).
The researchers found greater calprotectin degradation in the low amino acid media, and the difference was more pronounced among samples taken from individuals with UC than CD (P < .02).
They used metagenomic sequencing data from fecal samples to identify bacterial species associated with calprotectin metabolism. Similarly to previous reports, the researchers found that Firmicutes was dominant, while Subdoligranulum correlated with calprotectin degradation in low amino acid media (P = .04).
For 5 days, they cultured Subdoligranulum variabile in low amino acid media that also contained calprotectin. Calprotectin levels were lower than a control sample with cultured Akkermansia muciniphila, which was previously shown to not be associated with calprotectin degradation in low amino acid media (P = .03). Because Subdoligranulum species were not detectable in 5 of 22 fecal samples, the authors say they are unlikely to be the only species capable of metabolizing calprotectin.
Among IBD patients, only one had both endoscopically active colitis and a low fecal calprotectin level. The patient’s micobiome had Subdoligranulum present, and their fecal sample was able to metabolize calprotectin in the functional assay.
The study was limited by its small sample size, which prevented development of a calibration model for fecal calprotectin, and the researchers called for additional studies among individuals with active colitis.
Levels of calprotectin, a biomarker used to detect intestinal inflammation, may vary in fecal samples based on an individual’s microbiome composition, according to researchers. The results, if confirmed, might help refine its use in monitoring inflammatory bowel disease (IBD).
Researchers used a new ex vivo functional assay to identify specific bacteria that degrade calprotectin and may play a role in variations found in vivo. “Microbiome-based calibration could improve sensitivity and specificity of fecal calprotectin readouts, thereby facilitating more reliable real-time monitoring and ultimately enabling more timely interventions,” the authors wrote in their research letter, which was published in Gastro Hep Advances.
The standard for diagnosing ulcerative colitis (UC) and Crohn’s disease (CD) is endoscopy and biopsy because it allows both visual and histological examination of the severity and extent of intestinal inflammation, but this cannot be used to monitor patients on an ongoing basis.
Calprotectin is a promising biomarker for intestinal inflammation, but a meta-analysis found that it has a pooled sensitivity of 85% and a pooled specificity of 75% for the diagnosis of endoscopically active inflammatory bowel disease.
The researchers investigated whether an individual’s microbiome can metabolize calprotectin, which would complicate measurement of fecal calprotectin. They recruited 22 individuals with IBD (64% female, 73% with colonic disease), who provided stool samples. They completed a symptom questionnaire in advance of a colonoscopy. Overall, 64% had endoscopically inactive disease, and 82% had clinically inactive disease.
At a cutoff of 50 mcg/g, 9 patients had normal fecal calprotectin levels, and 13 had elevated levels. Those with clinically or endoscopically active disease had higher levels of fecal calprotectin (P < .0001).
There was a significant but poor correlation between disease activity measures and fecal calprotectin levels in CD (r = 0.62; P = .008), but there was no statistically significant association for UC (r = –0.29; P = .6). Endoscopic disease activity was also significantly correlated with fecal calprotectin in CD (r = 0.83; P < .001), but not UC (r = 0.50; P = .4).
The researchers created an ex vivo functional assay to measure calprotectin metabolism by the microbiome. They anaerobically cultured fecal samples in the presence of calprotectin and measured levels of calprotectin after 24 hours. Control samples were grown without microbes, without calprotectin, or lacking both. The researchers tested samples in both standard media and in media with low levels of amino acids, reasoning that the latter condition might encourage catabolism of calprotectin. The cultures with low amino acid content had lower calprotectin levels than those with normal amino acid content (P < .0007).
The researchers found greater calprotectin degradation in the low amino acid media, and the difference was more pronounced among samples taken from individuals with UC than CD (P < .02).
They used metagenomic sequencing data from fecal samples to identify bacterial species associated with calprotectin metabolism. Similarly to previous reports, the researchers found that Firmicutes was dominant, while Subdoligranulum correlated with calprotectin degradation in low amino acid media (P = .04).
For 5 days, they cultured Subdoligranulum variabile in low amino acid media that also contained calprotectin. Calprotectin levels were lower than a control sample with cultured Akkermansia muciniphila, which was previously shown to not be associated with calprotectin degradation in low amino acid media (P = .03). Because Subdoligranulum species were not detectable in 5 of 22 fecal samples, the authors say they are unlikely to be the only species capable of metabolizing calprotectin.
Among IBD patients, only one had both endoscopically active colitis and a low fecal calprotectin level. The patient’s micobiome had Subdoligranulum present, and their fecal sample was able to metabolize calprotectin in the functional assay.
The study was limited by its small sample size, which prevented development of a calibration model for fecal calprotectin, and the researchers called for additional studies among individuals with active colitis.
FROM GASTRO HEP ADVANCES
Prior psychological distress tied to ‘long-COVID’ conditions
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.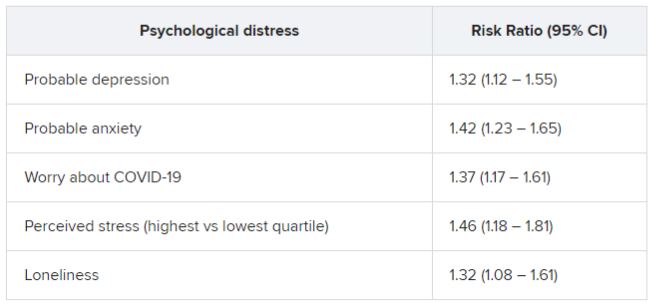
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Barriers to System Quality Improvement in Health Care
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; [email protected]
Process improvement in any industry sector aims to increase the efficiency of resource utilization and delivery methods (cost) and the quality of the product (outcomes), with the goal of ultimately achieving continuous development.1 In the health care industry, variation in processes and outcomes along with inefficiency in resource use that result in changes in value (the product of outcomes/costs) are the general targets of quality improvement (QI) efforts employing various implementation methodologies.2 When the ultimate aim is to serve the patient (customer), best clinical practice includes both maintaining high quality (individual care delivery) and controlling costs (efficient care system delivery), leading to optimal delivery (value-based care). High-quality individual care and efficient care delivery are not competing concepts, but when working to improve both health care outcomes and cost, traditional and nontraditional barriers to system QI often arise.3
The possible scenarios after a QI intervention include backsliding (regression to the mean over time), steady-state (minimal fixed improvement that could sustain), and continuous improvement (tangible enhancement after completing the intervention with legacy effect).4 The scalability of results can be considered during the process measurement and the intervention design phases of all QI projects; however, the complex nature of barriers in the health care environment during each level of implementation should be accounted for to prevent failure in the scalability phase.5
The barriers to optimal QI outcomes leading to continuous improvement are multifactorial and are related to intrinsic and extrinsic factors.6 These factors include 3 fundamental levels: (1) individual level inertia/beliefs, prior personal knowledge, and team-related factors7,8; (2) intervention-related and process-specific barriers and clinical practice obstacles; and (3) organizational level challenges and macro-level and population-level barriers (Figure). The obstacles faced during the implementation phase will likely include 2 of these levels simultaneously, which could add complexity and hinder or prevent the implementation of a tangible successful QI process and eventually lead to backsliding or minimal fixed improvement rather than continuous improvement. Furthermore, a patient-centered approach to QI would contribute to further complexity in design and execution, given the importance of reaching sustainable, meaningful improvement by adding elements of patient’s preferences, caregiver engagement, and the shared decision-making processes.9
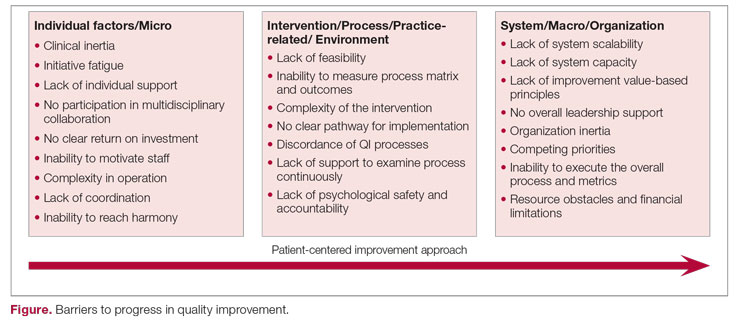
Overcoming these multidomain barriers and reaching resilience and sustainability requires thoughtful planning and execution through a multifaceted approach.10 A meaningful start could include addressing the clinical inertia for the individual and the team by promoting open innovation and allowing outside institutional collaborations and ideas through networks.11 On the individual level, encouraging participation and motivating health care workers in QI to reach a multidisciplinary operation approach will lead to harmony in collaboration. Concurrently, the organization should support the QI capability and scalability by removing competing priorities and establishing effective leadership that ensures resource allocation, communicates clear value-based principles, and engenders a psychological safety environment.
A continuous improvement state is the optimal QI target, a target that can be attained by removing obstacles and paving a clear pathway to implementation. Focusing on the 3 levels of barriers will position the organization for meaningful and successful QI phases to achieve continuous improvement.
1. Adesola S, Baines T. Developing and evaluating a methodology for business process improvement. Business Process Manage J. 2005;11(1):37-46. doi:10.1108/14637150510578719
2. Gershon M. Choosing which process improvement methodology to implement. J Appl Business & Economics. 2010;10(5):61-69.
3. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
4. Holweg M, Davies J, De Meyer A, Lawson B, Schmenner RW. Process Theory: The Principles of Operations Management. Oxford University Press; 2018.
5. Shortell SM, Bennett CL, Byck GR. Assessing the impact of continuous quality improvement on clinical practice: what it will take to accelerate progress. Milbank Q. 1998;76(4):593-624. doi:10.1111/1468-0009.00107
6. Solomons NM, Spross JA. Evidence‐based practice barriers and facilitators from a continuous quality improvement perspective: an integrative review. J Nurs Manage. 2011;19(1):109-120. doi:10.1111/j.1365-2834.2010.01144.x
7. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-34. doi:10.7326/0003-4819-135-9-200111060-00012
8. Stevenson K, Baker R, Farooqi A, Sorrie R, Khunti K. Features of primary health care teams associated with successful quality improvement of diabetes care: a qualitative study. Fam Pract. 2001;18(1):21-26. doi:10.1093/fampra/18.1.21
9. What is patient-centered care? NEJM Catalyst. January 1, 2017. Accessed August 31, 2022. https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0559
10. Kilbourne AM, Beck K, Spaeth‐Rublee B, et al. Measuring and improving the quality of mental health care: a global perspective. World Psychiatry. 2018;17(1):30-8. doi:10.1002/wps.20482
11. Huang HC, Lai MC, Lin LH, Chen CT. Overcoming organizational inertia to strengthen business model innovation: An open innovation perspective. J Organizational Change Manage. 2013;26(6):977-1002. doi:10.1108/JOCM-04-2012-0047
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; [email protected]
Process improvement in any industry sector aims to increase the efficiency of resource utilization and delivery methods (cost) and the quality of the product (outcomes), with the goal of ultimately achieving continuous development.1 In the health care industry, variation in processes and outcomes along with inefficiency in resource use that result in changes in value (the product of outcomes/costs) are the general targets of quality improvement (QI) efforts employing various implementation methodologies.2 When the ultimate aim is to serve the patient (customer), best clinical practice includes both maintaining high quality (individual care delivery) and controlling costs (efficient care system delivery), leading to optimal delivery (value-based care). High-quality individual care and efficient care delivery are not competing concepts, but when working to improve both health care outcomes and cost, traditional and nontraditional barriers to system QI often arise.3
The possible scenarios after a QI intervention include backsliding (regression to the mean over time), steady-state (minimal fixed improvement that could sustain), and continuous improvement (tangible enhancement after completing the intervention with legacy effect).4 The scalability of results can be considered during the process measurement and the intervention design phases of all QI projects; however, the complex nature of barriers in the health care environment during each level of implementation should be accounted for to prevent failure in the scalability phase.5
The barriers to optimal QI outcomes leading to continuous improvement are multifactorial and are related to intrinsic and extrinsic factors.6 These factors include 3 fundamental levels: (1) individual level inertia/beliefs, prior personal knowledge, and team-related factors7,8; (2) intervention-related and process-specific barriers and clinical practice obstacles; and (3) organizational level challenges and macro-level and population-level barriers (Figure). The obstacles faced during the implementation phase will likely include 2 of these levels simultaneously, which could add complexity and hinder or prevent the implementation of a tangible successful QI process and eventually lead to backsliding or minimal fixed improvement rather than continuous improvement. Furthermore, a patient-centered approach to QI would contribute to further complexity in design and execution, given the importance of reaching sustainable, meaningful improvement by adding elements of patient’s preferences, caregiver engagement, and the shared decision-making processes.9

Overcoming these multidomain barriers and reaching resilience and sustainability requires thoughtful planning and execution through a multifaceted approach.10 A meaningful start could include addressing the clinical inertia for the individual and the team by promoting open innovation and allowing outside institutional collaborations and ideas through networks.11 On the individual level, encouraging participation and motivating health care workers in QI to reach a multidisciplinary operation approach will lead to harmony in collaboration. Concurrently, the organization should support the QI capability and scalability by removing competing priorities and establishing effective leadership that ensures resource allocation, communicates clear value-based principles, and engenders a psychological safety environment.
A continuous improvement state is the optimal QI target, a target that can be attained by removing obstacles and paving a clear pathway to implementation. Focusing on the 3 levels of barriers will position the organization for meaningful and successful QI phases to achieve continuous improvement.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; [email protected]
Process improvement in any industry sector aims to increase the efficiency of resource utilization and delivery methods (cost) and the quality of the product (outcomes), with the goal of ultimately achieving continuous development.1 In the health care industry, variation in processes and outcomes along with inefficiency in resource use that result in changes in value (the product of outcomes/costs) are the general targets of quality improvement (QI) efforts employing various implementation methodologies.2 When the ultimate aim is to serve the patient (customer), best clinical practice includes both maintaining high quality (individual care delivery) and controlling costs (efficient care system delivery), leading to optimal delivery (value-based care). High-quality individual care and efficient care delivery are not competing concepts, but when working to improve both health care outcomes and cost, traditional and nontraditional barriers to system QI often arise.3
The possible scenarios after a QI intervention include backsliding (regression to the mean over time), steady-state (minimal fixed improvement that could sustain), and continuous improvement (tangible enhancement after completing the intervention with legacy effect).4 The scalability of results can be considered during the process measurement and the intervention design phases of all QI projects; however, the complex nature of barriers in the health care environment during each level of implementation should be accounted for to prevent failure in the scalability phase.5
The barriers to optimal QI outcomes leading to continuous improvement are multifactorial and are related to intrinsic and extrinsic factors.6 These factors include 3 fundamental levels: (1) individual level inertia/beliefs, prior personal knowledge, and team-related factors7,8; (2) intervention-related and process-specific barriers and clinical practice obstacles; and (3) organizational level challenges and macro-level and population-level barriers (Figure). The obstacles faced during the implementation phase will likely include 2 of these levels simultaneously, which could add complexity and hinder or prevent the implementation of a tangible successful QI process and eventually lead to backsliding or minimal fixed improvement rather than continuous improvement. Furthermore, a patient-centered approach to QI would contribute to further complexity in design and execution, given the importance of reaching sustainable, meaningful improvement by adding elements of patient’s preferences, caregiver engagement, and the shared decision-making processes.9

Overcoming these multidomain barriers and reaching resilience and sustainability requires thoughtful planning and execution through a multifaceted approach.10 A meaningful start could include addressing the clinical inertia for the individual and the team by promoting open innovation and allowing outside institutional collaborations and ideas through networks.11 On the individual level, encouraging participation and motivating health care workers in QI to reach a multidisciplinary operation approach will lead to harmony in collaboration. Concurrently, the organization should support the QI capability and scalability by removing competing priorities and establishing effective leadership that ensures resource allocation, communicates clear value-based principles, and engenders a psychological safety environment.
A continuous improvement state is the optimal QI target, a target that can be attained by removing obstacles and paving a clear pathway to implementation. Focusing on the 3 levels of barriers will position the organization for meaningful and successful QI phases to achieve continuous improvement.
1. Adesola S, Baines T. Developing and evaluating a methodology for business process improvement. Business Process Manage J. 2005;11(1):37-46. doi:10.1108/14637150510578719
2. Gershon M. Choosing which process improvement methodology to implement. J Appl Business & Economics. 2010;10(5):61-69.
3. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
4. Holweg M, Davies J, De Meyer A, Lawson B, Schmenner RW. Process Theory: The Principles of Operations Management. Oxford University Press; 2018.
5. Shortell SM, Bennett CL, Byck GR. Assessing the impact of continuous quality improvement on clinical practice: what it will take to accelerate progress. Milbank Q. 1998;76(4):593-624. doi:10.1111/1468-0009.00107
6. Solomons NM, Spross JA. Evidence‐based practice barriers and facilitators from a continuous quality improvement perspective: an integrative review. J Nurs Manage. 2011;19(1):109-120. doi:10.1111/j.1365-2834.2010.01144.x
7. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-34. doi:10.7326/0003-4819-135-9-200111060-00012
8. Stevenson K, Baker R, Farooqi A, Sorrie R, Khunti K. Features of primary health care teams associated with successful quality improvement of diabetes care: a qualitative study. Fam Pract. 2001;18(1):21-26. doi:10.1093/fampra/18.1.21
9. What is patient-centered care? NEJM Catalyst. January 1, 2017. Accessed August 31, 2022. https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0559
10. Kilbourne AM, Beck K, Spaeth‐Rublee B, et al. Measuring and improving the quality of mental health care: a global perspective. World Psychiatry. 2018;17(1):30-8. doi:10.1002/wps.20482
11. Huang HC, Lai MC, Lin LH, Chen CT. Overcoming organizational inertia to strengthen business model innovation: An open innovation perspective. J Organizational Change Manage. 2013;26(6):977-1002. doi:10.1108/JOCM-04-2012-0047
1. Adesola S, Baines T. Developing and evaluating a methodology for business process improvement. Business Process Manage J. 2005;11(1):37-46. doi:10.1108/14637150510578719
2. Gershon M. Choosing which process improvement methodology to implement. J Appl Business & Economics. 2010;10(5):61-69.
3. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
4. Holweg M, Davies J, De Meyer A, Lawson B, Schmenner RW. Process Theory: The Principles of Operations Management. Oxford University Press; 2018.
5. Shortell SM, Bennett CL, Byck GR. Assessing the impact of continuous quality improvement on clinical practice: what it will take to accelerate progress. Milbank Q. 1998;76(4):593-624. doi:10.1111/1468-0009.00107
6. Solomons NM, Spross JA. Evidence‐based practice barriers and facilitators from a continuous quality improvement perspective: an integrative review. J Nurs Manage. 2011;19(1):109-120. doi:10.1111/j.1365-2834.2010.01144.x
7. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-34. doi:10.7326/0003-4819-135-9-200111060-00012
8. Stevenson K, Baker R, Farooqi A, Sorrie R, Khunti K. Features of primary health care teams associated with successful quality improvement of diabetes care: a qualitative study. Fam Pract. 2001;18(1):21-26. doi:10.1093/fampra/18.1.21
9. What is patient-centered care? NEJM Catalyst. January 1, 2017. Accessed August 31, 2022. https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0559
10. Kilbourne AM, Beck K, Spaeth‐Rublee B, et al. Measuring and improving the quality of mental health care: a global perspective. World Psychiatry. 2018;17(1):30-8. doi:10.1002/wps.20482
11. Huang HC, Lai MC, Lin LH, Chen CT. Overcoming organizational inertia to strengthen business model innovation: An open innovation perspective. J Organizational Change Manage. 2013;26(6):977-1002. doi:10.1108/JOCM-04-2012-0047








