User login
What are growing pains? Turns out no one really knows
Just about every child hears it growing up: An ache in the leg? “Growing pains.” A dull pain in the side? “Growing pains.”
The catch-all phrase for random pains that children and teens have is so common that it even inspired the name of a 1980s sitcom. Yet when scientists dug into the evidence to find out what growing pains actually are, they found out that no one really knows. The definitions were as random and all over the place as the very pains that kids complain about, the researchers report in the journal Pediatrics.
Although some studies have suggested that up to a third of children have growing pains, the term has long seemed more like folk medicine than an actual medical diagnosis. Even so, parents, teachers, and doctors frequently use it when they have no other obvious answer to a particular pain a child or teen might describe.
A group of researchers at the University of Sydney in Australia wanted to find out if there was any research offering a more precise definition or criteria. They combed through eight databases for any papers that mentioned growing pains or growth pains in children or adolescents. They found 145 studies and set out to look for common ground: Where do growing pains occur? At what age do they start? Are there any patterns? Risk factors? Common clinical features? Relationships to particular activities?
What they found was that there is “no consensus whatsoever as to what growing pains really are, what they mean, how they’re defined, and how they should be diagnosed,” coauthor Steven J. Kamper, PhD, explained in a video about the findings. “The definitions were really variable, really vague, and sometimes downright contradictory,” he said. “Some studies would suggest growing pains happen in the arms, some in the lower limbs only. Some said it was about muscles, some about joints.”
The closest thing to consistency that they found was that exactly half the studies mentioned the pain being in the lower limbs. Nearly half (48%) described it as happening in the evening or nighttime, 42% said it was recurring, 35% reported it as occurring in youths with an otherwise normal physical exam, and 31% said the pain occurred on both sides of the body. Besides these, no other common feature was mentioned in more than 30% of the studies.
“Really curiously,” Dr. Kamper said, “more than 80% said nothing about the age at which these growing pains come on.” And 93% of the studies didn’t even mention growth as being related to the pain at all.
Several studies did acknowledge that the cause of growing pains is unknown, and several others considered it a diagnosis of exclusion – that is, it’s the diagnosis when everything else has been ruled out.
But that’s hardly a satisfactory explanation for kids and their families, so the researchers drew the only reasonable conclusion they could from what they found: “We think it’s important that the term is not used without some qualification or clarification, whether by researchers or clinicians,” Dr. Kamper said.
A version of this article first appeared on WebMD.com.
Just about every child hears it growing up: An ache in the leg? “Growing pains.” A dull pain in the side? “Growing pains.”
The catch-all phrase for random pains that children and teens have is so common that it even inspired the name of a 1980s sitcom. Yet when scientists dug into the evidence to find out what growing pains actually are, they found out that no one really knows. The definitions were as random and all over the place as the very pains that kids complain about, the researchers report in the journal Pediatrics.
Although some studies have suggested that up to a third of children have growing pains, the term has long seemed more like folk medicine than an actual medical diagnosis. Even so, parents, teachers, and doctors frequently use it when they have no other obvious answer to a particular pain a child or teen might describe.
A group of researchers at the University of Sydney in Australia wanted to find out if there was any research offering a more precise definition or criteria. They combed through eight databases for any papers that mentioned growing pains or growth pains in children or adolescents. They found 145 studies and set out to look for common ground: Where do growing pains occur? At what age do they start? Are there any patterns? Risk factors? Common clinical features? Relationships to particular activities?
What they found was that there is “no consensus whatsoever as to what growing pains really are, what they mean, how they’re defined, and how they should be diagnosed,” coauthor Steven J. Kamper, PhD, explained in a video about the findings. “The definitions were really variable, really vague, and sometimes downright contradictory,” he said. “Some studies would suggest growing pains happen in the arms, some in the lower limbs only. Some said it was about muscles, some about joints.”
The closest thing to consistency that they found was that exactly half the studies mentioned the pain being in the lower limbs. Nearly half (48%) described it as happening in the evening or nighttime, 42% said it was recurring, 35% reported it as occurring in youths with an otherwise normal physical exam, and 31% said the pain occurred on both sides of the body. Besides these, no other common feature was mentioned in more than 30% of the studies.
“Really curiously,” Dr. Kamper said, “more than 80% said nothing about the age at which these growing pains come on.” And 93% of the studies didn’t even mention growth as being related to the pain at all.
Several studies did acknowledge that the cause of growing pains is unknown, and several others considered it a diagnosis of exclusion – that is, it’s the diagnosis when everything else has been ruled out.
But that’s hardly a satisfactory explanation for kids and their families, so the researchers drew the only reasonable conclusion they could from what they found: “We think it’s important that the term is not used without some qualification or clarification, whether by researchers or clinicians,” Dr. Kamper said.
A version of this article first appeared on WebMD.com.
Just about every child hears it growing up: An ache in the leg? “Growing pains.” A dull pain in the side? “Growing pains.”
The catch-all phrase for random pains that children and teens have is so common that it even inspired the name of a 1980s sitcom. Yet when scientists dug into the evidence to find out what growing pains actually are, they found out that no one really knows. The definitions were as random and all over the place as the very pains that kids complain about, the researchers report in the journal Pediatrics.
Although some studies have suggested that up to a third of children have growing pains, the term has long seemed more like folk medicine than an actual medical diagnosis. Even so, parents, teachers, and doctors frequently use it when they have no other obvious answer to a particular pain a child or teen might describe.
A group of researchers at the University of Sydney in Australia wanted to find out if there was any research offering a more precise definition or criteria. They combed through eight databases for any papers that mentioned growing pains or growth pains in children or adolescents. They found 145 studies and set out to look for common ground: Where do growing pains occur? At what age do they start? Are there any patterns? Risk factors? Common clinical features? Relationships to particular activities?
What they found was that there is “no consensus whatsoever as to what growing pains really are, what they mean, how they’re defined, and how they should be diagnosed,” coauthor Steven J. Kamper, PhD, explained in a video about the findings. “The definitions were really variable, really vague, and sometimes downright contradictory,” he said. “Some studies would suggest growing pains happen in the arms, some in the lower limbs only. Some said it was about muscles, some about joints.”
The closest thing to consistency that they found was that exactly half the studies mentioned the pain being in the lower limbs. Nearly half (48%) described it as happening in the evening or nighttime, 42% said it was recurring, 35% reported it as occurring in youths with an otherwise normal physical exam, and 31% said the pain occurred on both sides of the body. Besides these, no other common feature was mentioned in more than 30% of the studies.
“Really curiously,” Dr. Kamper said, “more than 80% said nothing about the age at which these growing pains come on.” And 93% of the studies didn’t even mention growth as being related to the pain at all.
Several studies did acknowledge that the cause of growing pains is unknown, and several others considered it a diagnosis of exclusion – that is, it’s the diagnosis when everything else has been ruled out.
But that’s hardly a satisfactory explanation for kids and their families, so the researchers drew the only reasonable conclusion they could from what they found: “We think it’s important that the term is not used without some qualification or clarification, whether by researchers or clinicians,” Dr. Kamper said.
A version of this article first appeared on WebMD.com.
The Effect of Race on Outcomes in Veterans With Hepatocellular Carcinoma at a Single Center
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
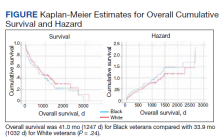
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
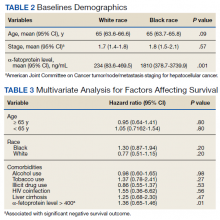
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
Two deaths from liver failure linked to spinal muscular atrophy drug
, according to a statement issued by the drug›s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment›s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported this week, according to a Novartis spokesperson.
A version of this article first appeared on Medscape.com.
, according to a statement issued by the drug›s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment›s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported this week, according to a Novartis spokesperson.
A version of this article first appeared on Medscape.com.
, according to a statement issued by the drug›s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment›s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported this week, according to a Novartis spokesperson.
A version of this article first appeared on Medscape.com.
Patients who engage in risky ‘chemsex’ benefit from appropriate treatment
Chemsex combines sex, drugs, and smartphones, and physicians know very little about it. Dedicated consultations were instituted in the fall of 2019 at the Infectious Diseases Department at the Saint-Louis Hospital in Paris. It’s estimated that 1,000 persons who were patients there practice chemsex.
Alexandre Aslan, MD, is one of the department’s physicians; he is also a sexologist and psychotherapist-psychoanalyst. At the ALBATROS International Congress of Addiction, which took place in the French capital in June, he presented the results of a study of patients who engage in chemsex and who regularly attend those consultations. Through this research, light is being shed on the phenomenon.
This news organization invited Dr. Aslan to discuss the issues connected with this practice.
Question: What exactly is chemsex, also known as party ‘n’ play (PnP)?
Dr. Aslan: Hearing the word “chemsex,” one would automatically think that it is what it sounds like it is: having sex while on drugs. That’s not really what it is. According to the definition that’s been published in the scientific literature, chemsex is a practice seen among men who have sex with men, where they take some very specific substances during sexual activity to sustain, enhance, or intensify the sexual experience, but also to “manage” issues related to intimacy, performance, and concerns about sexually transmitted infections (STIs). The substances are most commonly a cocktail of three drugs: GHB [gamma-hydroxybutyrate], cathinones, and crystal meth. In chemsex, smartphones play a central role as well, through the use of social networking and dating applications – those location-based apps that allow users to instantly find partners.
Question: In what ways does meeting through apps influence the sexual relationship and the use of substances?
Dr. Aslan: Because the plan to meet up for sex is being made through these kinds of apps, the promise to have sex is often implied – and this is before the individuals even meet up in real life. Let me explain. It’s not an encounter or a person that’s going to trigger sexual desire. Instead, it’s something within – the sexual “urge” inside of the individual – that’s going to drive them toward sexual activity. Now, finding yourself promising to have sex with someone – someone you don’t know, haven’t spoken to, and haven’t actually met – in an environment where it’s possible that you’ll meet several people and where the moments in which the sexual acts take place are predominantly characterized by pornography-related performance scripts: This can push you to take substances so you can “let go” and get to the point where you’re able to adapt to the requirements of the situation. Seeking to perform well and to not be overly inhibited, these individuals have found that this drug cocktail proves to be quite explosive, imparting a very strong capacity for experiencing excitement and even bringing about new sexual practices.
Question: Can you speak a bit about drug-enhanced sex?
Dr. Aslan: We sexologists consider it to be a very particular type of sex. People who engage in it feel that the sex is very intense, with unbelievable experiences, and that they have a deeper connection with their partner. In fact, it’s a type of sex where taking these substances does away with the very principles of sexual physiology – in other words, desire followed by excitement, plateau, orgasm, and resolution. Little by little, one’s sexual partner is no longer going to exist in the sex session, and the benefit is a succession of partners whose sole purpose is to keep the fire of excitement burning, an excitement that’s also reinforced by the substances taken. It’s “sex” under the influence rather than a sexual encounter linked to desire.
Question: What impact does it have on health?
Dr. Aslan: This practice brings with it numerous complications, such as STIs, but also physical injuries, as these sessions can last for 24 to more than 48 hours. There are also psychological complications, because these drugs can bring about depression, paranoia, self-harm, and even episodes of decompensation. And then, it should be noted that later on, the spotlight gets pulled away from the sex – the pretext from the very beginning – and shifts toward the taking of drugs: The individuals will no longer be able to separate the sexual encounter from the taking of drugs. Then, in a few years, there’s no longer the sexual encounter, only the taking of drugs. In the United States, between 2021 and 2022, there was a decrease in the number of deaths caused by heroin and prescription opioids. On the other hand, since 2020, the overdoses that have exploded in number are those related to fentanyl, nonprescription opioids, and stimulants – cocaine and methamphetamine, which can come back into the practices particularly through the seemingly “playful” arena of sex.
Question: How is it that things have gone from being a practice that’s under control to full-on drug addiction?
Dr. Aslan: You still have people who manage to keep things under control. But the kinds of drugs that are taken are highly addictive and compel the individual to take even more. It’s one big circle: The exciting sexual relationship itself, to which you add substances that cause even more dopamine to be released, and a smartphone screen with excitatory pornographic images on it all the time. In all the patients we see, we notice a trajectory that looks like the trajectory of every drug. When they’re at the beginning – in other words, the first year – after a first experience that they consider to be explosive, they may not return to the scene right away, and then they do return to it. They realize that it’s perhaps not as marvelous as the first time, but they’re going to give it another try. During this novelty phase, a strategy is pursued whereby they adapt and make adjustments in an attempt to feel again what they felt the first time. At the end of a year or two, they become disillusioned and they refocus on all activities having to do with drug use. Our hospital department conducted a survey where we asked detailed questions to over 100 individuals. It showed that people noticed the negative consequences that chemsex had on their work (60%), on their private lives and sex lives (55%), and on their relationships with friends and family (63%). This means that people are well aware of the negative effects that this practice has in very important areas of their lives. But even if they notice all of that, even if they resolve to have a certain number of sexual relations without drugs involved, these substances are so powerful in releasing a rush of dopamine that that very fact can sweep away any capacity the individual may have had to make a decision and stick to it, and they’re going to feel practically “compelled” to use. This is what’s called a craving.
Question: How do you identify patients who engage in chemsex among the patients in your infectious diseases department?
Dr. Aslan: As a rule, all patients admitted to our department are asked a series of questions. Do you use drugs to engage in sexual relations? Which drug do you prefer? How do you take it? Do you have a good time? Do you find that it’s good for you? Are you okay with how much you’re using? We also ask patients to tell us when they last had drug-free sex. It’s a very important question, because if we can identify someone who has had 10 or so partners a month but hasn’t had drug-free sex for over a month, we’ll try to steer the conversation to where they’ll come to think that it might not be such a bad idea to talk about it.
Question: Should a physician be asking younger patients whether they’re engaging in chemsex?
Dr. Aslan: Yes, but the physician has to be very careful. We often have a tendency to believe that we’re capable of speaking with our patients about relevant matters related to sex. We see ourselves as that kind of person, not to mention we’re open-minded. Now, as in all fields of medicine, we have to educate ourselves about how best to approach patients – in this case, about their sexual health. Because sometimes, despite our best intentions, we can do harm. The idea that we have of our own sexual behavior does not necessarily help provide counsel regarding another person’s sexual behavior, particularly when there are differences between the two. If you’re interested in the issue, you need to be trained on all the answers that could come up. There are training courses online. There’s a module on sexual health and chemsex at a site designed to give private practice physicians guidance about PrEP. It’s at least a place to start. This way, physicians will know what questions they can ask and when they should reach out to a specialist, such as a sexologist with training in these specific issues.
Question: What is the treatment based on?
Dr. Aslan: The traditional approach taken by addiction medicine physicians may not be comprehensive enough. Likewise, a sexologist’s approach alone can only go so far. It’s impossible to get by thinking that a single discipline can hold the solution, all the answers. So, it’s a multidisciplinary sexual health treatment. There should be a psychiatrist or addiction medicine physician who knows the drugs and is capable of navigating through this landscape of psychiatric comorbidities (such as psychoses and ADHD).
There also has to be a sexologist for the treatment of any sexual dysfunctions there may be. At Saint-Louis Hospital, 60% of patients who engage in chemsex said that engaging in the practice was related to a sexual problem that they noted – but never went to see a doctor about – before the first time they used. Be that as it may, it’s still the case that if these patients had been able to see a sexologist – who would have treated the problem – the drug may perhaps not have taken hold.
There also has to be a practitioner who can focus on risk reduction. In other words, someone capable of helping the patient get to the desired level of use where the craving, the need for instant gratification, can be kept in check.
In practice, one can sometimes, in addition to all of that, turn to medical treatments to manage the craving or medical comorbidities, an approach based on sexology to provide care for the sexual dysfunction or even to help the person learn how to evoke sexual or erotic fantasies without drugs, and an approach based on addiction medicine or psychotherapy, as some of our patients experienced sexual abuse in childhood. In the end, chemsex is just the outer layer – a problem that only seems to pertain to sex but that, in reality, covers up a wide range of issues. And not only sexual issues or issues that are related to drugs like chemsex is.
Question: What are the outcomes of this multidisciplinary treatment?
Dr. Aslan: Before we finish, I must point out and just state that the patients, when they’re cared for and when they’re provided with the appropriate treatment, change their practices. Some of our patients, even those with more advanced cases in terms of frequency, how often they’re injecting drugs – every 30 minutes over the course of 24 or 48 hours, with complications such as thrombosis, sepsis, and abscesses – they’ve completely stopped after several months of treatment. They now lead lives that, as they’ve told us, work better for them. So, those of us in the health care industry, we have to get organized and set things up in a way that will allow us to focus our efforts on treating these patients.
A version of this article first appeared on Medscape.com. This article was translated from the Medscape French edition.
Chemsex combines sex, drugs, and smartphones, and physicians know very little about it. Dedicated consultations were instituted in the fall of 2019 at the Infectious Diseases Department at the Saint-Louis Hospital in Paris. It’s estimated that 1,000 persons who were patients there practice chemsex.
Alexandre Aslan, MD, is one of the department’s physicians; he is also a sexologist and psychotherapist-psychoanalyst. At the ALBATROS International Congress of Addiction, which took place in the French capital in June, he presented the results of a study of patients who engage in chemsex and who regularly attend those consultations. Through this research, light is being shed on the phenomenon.
This news organization invited Dr. Aslan to discuss the issues connected with this practice.
Question: What exactly is chemsex, also known as party ‘n’ play (PnP)?
Dr. Aslan: Hearing the word “chemsex,” one would automatically think that it is what it sounds like it is: having sex while on drugs. That’s not really what it is. According to the definition that’s been published in the scientific literature, chemsex is a practice seen among men who have sex with men, where they take some very specific substances during sexual activity to sustain, enhance, or intensify the sexual experience, but also to “manage” issues related to intimacy, performance, and concerns about sexually transmitted infections (STIs). The substances are most commonly a cocktail of three drugs: GHB [gamma-hydroxybutyrate], cathinones, and crystal meth. In chemsex, smartphones play a central role as well, through the use of social networking and dating applications – those location-based apps that allow users to instantly find partners.
Question: In what ways does meeting through apps influence the sexual relationship and the use of substances?
Dr. Aslan: Because the plan to meet up for sex is being made through these kinds of apps, the promise to have sex is often implied – and this is before the individuals even meet up in real life. Let me explain. It’s not an encounter or a person that’s going to trigger sexual desire. Instead, it’s something within – the sexual “urge” inside of the individual – that’s going to drive them toward sexual activity. Now, finding yourself promising to have sex with someone – someone you don’t know, haven’t spoken to, and haven’t actually met – in an environment where it’s possible that you’ll meet several people and where the moments in which the sexual acts take place are predominantly characterized by pornography-related performance scripts: This can push you to take substances so you can “let go” and get to the point where you’re able to adapt to the requirements of the situation. Seeking to perform well and to not be overly inhibited, these individuals have found that this drug cocktail proves to be quite explosive, imparting a very strong capacity for experiencing excitement and even bringing about new sexual practices.
Question: Can you speak a bit about drug-enhanced sex?
Dr. Aslan: We sexologists consider it to be a very particular type of sex. People who engage in it feel that the sex is very intense, with unbelievable experiences, and that they have a deeper connection with their partner. In fact, it’s a type of sex where taking these substances does away with the very principles of sexual physiology – in other words, desire followed by excitement, plateau, orgasm, and resolution. Little by little, one’s sexual partner is no longer going to exist in the sex session, and the benefit is a succession of partners whose sole purpose is to keep the fire of excitement burning, an excitement that’s also reinforced by the substances taken. It’s “sex” under the influence rather than a sexual encounter linked to desire.
Question: What impact does it have on health?
Dr. Aslan: This practice brings with it numerous complications, such as STIs, but also physical injuries, as these sessions can last for 24 to more than 48 hours. There are also psychological complications, because these drugs can bring about depression, paranoia, self-harm, and even episodes of decompensation. And then, it should be noted that later on, the spotlight gets pulled away from the sex – the pretext from the very beginning – and shifts toward the taking of drugs: The individuals will no longer be able to separate the sexual encounter from the taking of drugs. Then, in a few years, there’s no longer the sexual encounter, only the taking of drugs. In the United States, between 2021 and 2022, there was a decrease in the number of deaths caused by heroin and prescription opioids. On the other hand, since 2020, the overdoses that have exploded in number are those related to fentanyl, nonprescription opioids, and stimulants – cocaine and methamphetamine, which can come back into the practices particularly through the seemingly “playful” arena of sex.
Question: How is it that things have gone from being a practice that’s under control to full-on drug addiction?
Dr. Aslan: You still have people who manage to keep things under control. But the kinds of drugs that are taken are highly addictive and compel the individual to take even more. It’s one big circle: The exciting sexual relationship itself, to which you add substances that cause even more dopamine to be released, and a smartphone screen with excitatory pornographic images on it all the time. In all the patients we see, we notice a trajectory that looks like the trajectory of every drug. When they’re at the beginning – in other words, the first year – after a first experience that they consider to be explosive, they may not return to the scene right away, and then they do return to it. They realize that it’s perhaps not as marvelous as the first time, but they’re going to give it another try. During this novelty phase, a strategy is pursued whereby they adapt and make adjustments in an attempt to feel again what they felt the first time. At the end of a year or two, they become disillusioned and they refocus on all activities having to do with drug use. Our hospital department conducted a survey where we asked detailed questions to over 100 individuals. It showed that people noticed the negative consequences that chemsex had on their work (60%), on their private lives and sex lives (55%), and on their relationships with friends and family (63%). This means that people are well aware of the negative effects that this practice has in very important areas of their lives. But even if they notice all of that, even if they resolve to have a certain number of sexual relations without drugs involved, these substances are so powerful in releasing a rush of dopamine that that very fact can sweep away any capacity the individual may have had to make a decision and stick to it, and they’re going to feel practically “compelled” to use. This is what’s called a craving.
Question: How do you identify patients who engage in chemsex among the patients in your infectious diseases department?
Dr. Aslan: As a rule, all patients admitted to our department are asked a series of questions. Do you use drugs to engage in sexual relations? Which drug do you prefer? How do you take it? Do you have a good time? Do you find that it’s good for you? Are you okay with how much you’re using? We also ask patients to tell us when they last had drug-free sex. It’s a very important question, because if we can identify someone who has had 10 or so partners a month but hasn’t had drug-free sex for over a month, we’ll try to steer the conversation to where they’ll come to think that it might not be such a bad idea to talk about it.
Question: Should a physician be asking younger patients whether they’re engaging in chemsex?
Dr. Aslan: Yes, but the physician has to be very careful. We often have a tendency to believe that we’re capable of speaking with our patients about relevant matters related to sex. We see ourselves as that kind of person, not to mention we’re open-minded. Now, as in all fields of medicine, we have to educate ourselves about how best to approach patients – in this case, about their sexual health. Because sometimes, despite our best intentions, we can do harm. The idea that we have of our own sexual behavior does not necessarily help provide counsel regarding another person’s sexual behavior, particularly when there are differences between the two. If you’re interested in the issue, you need to be trained on all the answers that could come up. There are training courses online. There’s a module on sexual health and chemsex at a site designed to give private practice physicians guidance about PrEP. It’s at least a place to start. This way, physicians will know what questions they can ask and when they should reach out to a specialist, such as a sexologist with training in these specific issues.
Question: What is the treatment based on?
Dr. Aslan: The traditional approach taken by addiction medicine physicians may not be comprehensive enough. Likewise, a sexologist’s approach alone can only go so far. It’s impossible to get by thinking that a single discipline can hold the solution, all the answers. So, it’s a multidisciplinary sexual health treatment. There should be a psychiatrist or addiction medicine physician who knows the drugs and is capable of navigating through this landscape of psychiatric comorbidities (such as psychoses and ADHD).
There also has to be a sexologist for the treatment of any sexual dysfunctions there may be. At Saint-Louis Hospital, 60% of patients who engage in chemsex said that engaging in the practice was related to a sexual problem that they noted – but never went to see a doctor about – before the first time they used. Be that as it may, it’s still the case that if these patients had been able to see a sexologist – who would have treated the problem – the drug may perhaps not have taken hold.
There also has to be a practitioner who can focus on risk reduction. In other words, someone capable of helping the patient get to the desired level of use where the craving, the need for instant gratification, can be kept in check.
In practice, one can sometimes, in addition to all of that, turn to medical treatments to manage the craving or medical comorbidities, an approach based on sexology to provide care for the sexual dysfunction or even to help the person learn how to evoke sexual or erotic fantasies without drugs, and an approach based on addiction medicine or psychotherapy, as some of our patients experienced sexual abuse in childhood. In the end, chemsex is just the outer layer – a problem that only seems to pertain to sex but that, in reality, covers up a wide range of issues. And not only sexual issues or issues that are related to drugs like chemsex is.
Question: What are the outcomes of this multidisciplinary treatment?
Dr. Aslan: Before we finish, I must point out and just state that the patients, when they’re cared for and when they’re provided with the appropriate treatment, change their practices. Some of our patients, even those with more advanced cases in terms of frequency, how often they’re injecting drugs – every 30 minutes over the course of 24 or 48 hours, with complications such as thrombosis, sepsis, and abscesses – they’ve completely stopped after several months of treatment. They now lead lives that, as they’ve told us, work better for them. So, those of us in the health care industry, we have to get organized and set things up in a way that will allow us to focus our efforts on treating these patients.
A version of this article first appeared on Medscape.com. This article was translated from the Medscape French edition.
Chemsex combines sex, drugs, and smartphones, and physicians know very little about it. Dedicated consultations were instituted in the fall of 2019 at the Infectious Diseases Department at the Saint-Louis Hospital in Paris. It’s estimated that 1,000 persons who were patients there practice chemsex.
Alexandre Aslan, MD, is one of the department’s physicians; he is also a sexologist and psychotherapist-psychoanalyst. At the ALBATROS International Congress of Addiction, which took place in the French capital in June, he presented the results of a study of patients who engage in chemsex and who regularly attend those consultations. Through this research, light is being shed on the phenomenon.
This news organization invited Dr. Aslan to discuss the issues connected with this practice.
Question: What exactly is chemsex, also known as party ‘n’ play (PnP)?
Dr. Aslan: Hearing the word “chemsex,” one would automatically think that it is what it sounds like it is: having sex while on drugs. That’s not really what it is. According to the definition that’s been published in the scientific literature, chemsex is a practice seen among men who have sex with men, where they take some very specific substances during sexual activity to sustain, enhance, or intensify the sexual experience, but also to “manage” issues related to intimacy, performance, and concerns about sexually transmitted infections (STIs). The substances are most commonly a cocktail of three drugs: GHB [gamma-hydroxybutyrate], cathinones, and crystal meth. In chemsex, smartphones play a central role as well, through the use of social networking and dating applications – those location-based apps that allow users to instantly find partners.
Question: In what ways does meeting through apps influence the sexual relationship and the use of substances?
Dr. Aslan: Because the plan to meet up for sex is being made through these kinds of apps, the promise to have sex is often implied – and this is before the individuals even meet up in real life. Let me explain. It’s not an encounter or a person that’s going to trigger sexual desire. Instead, it’s something within – the sexual “urge” inside of the individual – that’s going to drive them toward sexual activity. Now, finding yourself promising to have sex with someone – someone you don’t know, haven’t spoken to, and haven’t actually met – in an environment where it’s possible that you’ll meet several people and where the moments in which the sexual acts take place are predominantly characterized by pornography-related performance scripts: This can push you to take substances so you can “let go” and get to the point where you’re able to adapt to the requirements of the situation. Seeking to perform well and to not be overly inhibited, these individuals have found that this drug cocktail proves to be quite explosive, imparting a very strong capacity for experiencing excitement and even bringing about new sexual practices.
Question: Can you speak a bit about drug-enhanced sex?
Dr. Aslan: We sexologists consider it to be a very particular type of sex. People who engage in it feel that the sex is very intense, with unbelievable experiences, and that they have a deeper connection with their partner. In fact, it’s a type of sex where taking these substances does away with the very principles of sexual physiology – in other words, desire followed by excitement, plateau, orgasm, and resolution. Little by little, one’s sexual partner is no longer going to exist in the sex session, and the benefit is a succession of partners whose sole purpose is to keep the fire of excitement burning, an excitement that’s also reinforced by the substances taken. It’s “sex” under the influence rather than a sexual encounter linked to desire.
Question: What impact does it have on health?
Dr. Aslan: This practice brings with it numerous complications, such as STIs, but also physical injuries, as these sessions can last for 24 to more than 48 hours. There are also psychological complications, because these drugs can bring about depression, paranoia, self-harm, and even episodes of decompensation. And then, it should be noted that later on, the spotlight gets pulled away from the sex – the pretext from the very beginning – and shifts toward the taking of drugs: The individuals will no longer be able to separate the sexual encounter from the taking of drugs. Then, in a few years, there’s no longer the sexual encounter, only the taking of drugs. In the United States, between 2021 and 2022, there was a decrease in the number of deaths caused by heroin and prescription opioids. On the other hand, since 2020, the overdoses that have exploded in number are those related to fentanyl, nonprescription opioids, and stimulants – cocaine and methamphetamine, which can come back into the practices particularly through the seemingly “playful” arena of sex.
Question: How is it that things have gone from being a practice that’s under control to full-on drug addiction?
Dr. Aslan: You still have people who manage to keep things under control. But the kinds of drugs that are taken are highly addictive and compel the individual to take even more. It’s one big circle: The exciting sexual relationship itself, to which you add substances that cause even more dopamine to be released, and a smartphone screen with excitatory pornographic images on it all the time. In all the patients we see, we notice a trajectory that looks like the trajectory of every drug. When they’re at the beginning – in other words, the first year – after a first experience that they consider to be explosive, they may not return to the scene right away, and then they do return to it. They realize that it’s perhaps not as marvelous as the first time, but they’re going to give it another try. During this novelty phase, a strategy is pursued whereby they adapt and make adjustments in an attempt to feel again what they felt the first time. At the end of a year or two, they become disillusioned and they refocus on all activities having to do with drug use. Our hospital department conducted a survey where we asked detailed questions to over 100 individuals. It showed that people noticed the negative consequences that chemsex had on their work (60%), on their private lives and sex lives (55%), and on their relationships with friends and family (63%). This means that people are well aware of the negative effects that this practice has in very important areas of their lives. But even if they notice all of that, even if they resolve to have a certain number of sexual relations without drugs involved, these substances are so powerful in releasing a rush of dopamine that that very fact can sweep away any capacity the individual may have had to make a decision and stick to it, and they’re going to feel practically “compelled” to use. This is what’s called a craving.
Question: How do you identify patients who engage in chemsex among the patients in your infectious diseases department?
Dr. Aslan: As a rule, all patients admitted to our department are asked a series of questions. Do you use drugs to engage in sexual relations? Which drug do you prefer? How do you take it? Do you have a good time? Do you find that it’s good for you? Are you okay with how much you’re using? We also ask patients to tell us when they last had drug-free sex. It’s a very important question, because if we can identify someone who has had 10 or so partners a month but hasn’t had drug-free sex for over a month, we’ll try to steer the conversation to where they’ll come to think that it might not be such a bad idea to talk about it.
Question: Should a physician be asking younger patients whether they’re engaging in chemsex?
Dr. Aslan: Yes, but the physician has to be very careful. We often have a tendency to believe that we’re capable of speaking with our patients about relevant matters related to sex. We see ourselves as that kind of person, not to mention we’re open-minded. Now, as in all fields of medicine, we have to educate ourselves about how best to approach patients – in this case, about their sexual health. Because sometimes, despite our best intentions, we can do harm. The idea that we have of our own sexual behavior does not necessarily help provide counsel regarding another person’s sexual behavior, particularly when there are differences between the two. If you’re interested in the issue, you need to be trained on all the answers that could come up. There are training courses online. There’s a module on sexual health and chemsex at a site designed to give private practice physicians guidance about PrEP. It’s at least a place to start. This way, physicians will know what questions they can ask and when they should reach out to a specialist, such as a sexologist with training in these specific issues.
Question: What is the treatment based on?
Dr. Aslan: The traditional approach taken by addiction medicine physicians may not be comprehensive enough. Likewise, a sexologist’s approach alone can only go so far. It’s impossible to get by thinking that a single discipline can hold the solution, all the answers. So, it’s a multidisciplinary sexual health treatment. There should be a psychiatrist or addiction medicine physician who knows the drugs and is capable of navigating through this landscape of psychiatric comorbidities (such as psychoses and ADHD).
There also has to be a sexologist for the treatment of any sexual dysfunctions there may be. At Saint-Louis Hospital, 60% of patients who engage in chemsex said that engaging in the practice was related to a sexual problem that they noted – but never went to see a doctor about – before the first time they used. Be that as it may, it’s still the case that if these patients had been able to see a sexologist – who would have treated the problem – the drug may perhaps not have taken hold.
There also has to be a practitioner who can focus on risk reduction. In other words, someone capable of helping the patient get to the desired level of use where the craving, the need for instant gratification, can be kept in check.
In practice, one can sometimes, in addition to all of that, turn to medical treatments to manage the craving or medical comorbidities, an approach based on sexology to provide care for the sexual dysfunction or even to help the person learn how to evoke sexual or erotic fantasies without drugs, and an approach based on addiction medicine or psychotherapy, as some of our patients experienced sexual abuse in childhood. In the end, chemsex is just the outer layer – a problem that only seems to pertain to sex but that, in reality, covers up a wide range of issues. And not only sexual issues or issues that are related to drugs like chemsex is.
Question: What are the outcomes of this multidisciplinary treatment?
Dr. Aslan: Before we finish, I must point out and just state that the patients, when they’re cared for and when they’re provided with the appropriate treatment, change their practices. Some of our patients, even those with more advanced cases in terms of frequency, how often they’re injecting drugs – every 30 minutes over the course of 24 or 48 hours, with complications such as thrombosis, sepsis, and abscesses – they’ve completely stopped after several months of treatment. They now lead lives that, as they’ve told us, work better for them. So, those of us in the health care industry, we have to get organized and set things up in a way that will allow us to focus our efforts on treating these patients.
A version of this article first appeared on Medscape.com. This article was translated from the Medscape French edition.
U.S. tops 10,000 confirmed monkeypox cases: CDC
The United States passed the 10,000 mark on Aug. 10, with the number climbing to 10,768 by the morning of Aug. 12, according to the latest CDC data. Monkeypox cases have been found in every state except Wyoming. New York (2,187), California (1,892), and Florida (1,053) have reported the most cases. So far, no monkeypox deaths have been reported in the United States.
The numbers are increasing, with 1,391 cases reported in the United States on Aug. 12 alone, by far the most in 1 day since the current outbreak began.
“We are still operating under a containment goal, although I know many states are starting to wonder if we’re shifting to more of a mitigation phase right now, given that our case counts are still rising rapidly,” Jennifer McQuiston, DVM, the CDC’s top monkeypox official, told a group of the agency’s advisers on Aug. 9, according to CBS News.
Since late July, the United States has reported more monkeypox cases than any other nation. After the United States, Spain has reported 5,162 cases, the United Kingdom 3,017, and France 2,423, according to the World Health Organization.
Globally, 31,655 cases have been recorded, with 5,108 of those cases coming in the last 7 days, according to the WHO. There have been 12 deaths attributed to monkeypox, with one coming in the last week.
The smallpox-like disease was first found in humans in the Democratic Republic of the Congo in 1970 and has become more common in West and Central Africa. It began spreading to European and other Western nations in May 2022.
The WHO declared it a global public health emergency in late July, and the Biden administration declared it a national health emergency Aug. 4.
To fight the spread of monkeypox, the Biden administration is buying $26 million worth of SIGA Technologies Inc.’s IV version of the antiviral drug TPOXX, the company announced on Aug. 9.
U.S. health officials also modified monkeypox vaccine dosing instructions to stretch the supply of vaccine. Instead of sticking with a standard shot that would enter deep into tissue, the FDA now encourages a new way: just under the skin at one-fifth the usual dose.
A version of this article first appeared on WebMD.com.
The United States passed the 10,000 mark on Aug. 10, with the number climbing to 10,768 by the morning of Aug. 12, according to the latest CDC data. Monkeypox cases have been found in every state except Wyoming. New York (2,187), California (1,892), and Florida (1,053) have reported the most cases. So far, no monkeypox deaths have been reported in the United States.
The numbers are increasing, with 1,391 cases reported in the United States on Aug. 12 alone, by far the most in 1 day since the current outbreak began.
“We are still operating under a containment goal, although I know many states are starting to wonder if we’re shifting to more of a mitigation phase right now, given that our case counts are still rising rapidly,” Jennifer McQuiston, DVM, the CDC’s top monkeypox official, told a group of the agency’s advisers on Aug. 9, according to CBS News.
Since late July, the United States has reported more monkeypox cases than any other nation. After the United States, Spain has reported 5,162 cases, the United Kingdom 3,017, and France 2,423, according to the World Health Organization.
Globally, 31,655 cases have been recorded, with 5,108 of those cases coming in the last 7 days, according to the WHO. There have been 12 deaths attributed to monkeypox, with one coming in the last week.
The smallpox-like disease was first found in humans in the Democratic Republic of the Congo in 1970 and has become more common in West and Central Africa. It began spreading to European and other Western nations in May 2022.
The WHO declared it a global public health emergency in late July, and the Biden administration declared it a national health emergency Aug. 4.
To fight the spread of monkeypox, the Biden administration is buying $26 million worth of SIGA Technologies Inc.’s IV version of the antiviral drug TPOXX, the company announced on Aug. 9.
U.S. health officials also modified monkeypox vaccine dosing instructions to stretch the supply of vaccine. Instead of sticking with a standard shot that would enter deep into tissue, the FDA now encourages a new way: just under the skin at one-fifth the usual dose.
A version of this article first appeared on WebMD.com.
The United States passed the 10,000 mark on Aug. 10, with the number climbing to 10,768 by the morning of Aug. 12, according to the latest CDC data. Monkeypox cases have been found in every state except Wyoming. New York (2,187), California (1,892), and Florida (1,053) have reported the most cases. So far, no monkeypox deaths have been reported in the United States.
The numbers are increasing, with 1,391 cases reported in the United States on Aug. 12 alone, by far the most in 1 day since the current outbreak began.
“We are still operating under a containment goal, although I know many states are starting to wonder if we’re shifting to more of a mitigation phase right now, given that our case counts are still rising rapidly,” Jennifer McQuiston, DVM, the CDC’s top monkeypox official, told a group of the agency’s advisers on Aug. 9, according to CBS News.
Since late July, the United States has reported more monkeypox cases than any other nation. After the United States, Spain has reported 5,162 cases, the United Kingdom 3,017, and France 2,423, according to the World Health Organization.
Globally, 31,655 cases have been recorded, with 5,108 of those cases coming in the last 7 days, according to the WHO. There have been 12 deaths attributed to monkeypox, with one coming in the last week.
The smallpox-like disease was first found in humans in the Democratic Republic of the Congo in 1970 and has become more common in West and Central Africa. It began spreading to European and other Western nations in May 2022.
The WHO declared it a global public health emergency in late July, and the Biden administration declared it a national health emergency Aug. 4.
To fight the spread of monkeypox, the Biden administration is buying $26 million worth of SIGA Technologies Inc.’s IV version of the antiviral drug TPOXX, the company announced on Aug. 9.
U.S. health officials also modified monkeypox vaccine dosing instructions to stretch the supply of vaccine. Instead of sticking with a standard shot that would enter deep into tissue, the FDA now encourages a new way: just under the skin at one-fifth the usual dose.
A version of this article first appeared on WebMD.com.
Which factors fuel sexual violence in health care?
At the beginning of July, Brazilians across the country were appalled when they heard that an anesthesiologist was accused of sexually abusing a woman he had been treating during cesarean delivery. The incident was recorded on video by nurses and nurse technicians who, having become suspicious of the excessive amount of sedatives given to mothers-to-be by this particular anesthesiologist, decided to film him during a procedure. To do this, they made a last-minute change, switching delivery rooms to one in which they had hidden a cell phone in a cabinet.
What the footage showed was horrifying and the assailant, Giovanni Quintella Bezerra, was arrested on the spot. He’s a 32-year-old, White, successful physician, and he’s now accused of rape. The authorities are looking into whether there are more victims, others who may have been abused by the physician. The police are investigating about 40 surgeries in which Dr. Bezerra participated. That same month saw the arrest of another physician, gynecologist Ricardo Teles Martins, who was arrested after being accused of sexually harassing and abusing several women in Hidrolândia, in the northeastern state of Ceará.
In gathering information about these incidents, this news organization interviewed four Brazilian specialists to get their insights on the issues that have been brought to light by these recent cases and the factors that play a role in these kinds of criminal acts. Claudio Cohen, MD, PhD, is a psychiatrist, bioethicist, and professor at the University of São Paulo in Brazil. Daniela Pedroso, MA, is a psychologist who has 25 years’ experience working with victims of sexual violence. Gynecologist and obstetrician Jefferson Drezett, MD, PhD, is a professor in the field of population genetics and reproductive and sexual health at the Federal University of ABC, São Paulo, and in the department of health, life cycles, and society at the University of São Paulo School of Public Health. Maria Alice Scardoelli, MD, is a psychiatrist who also serves as vice-chair of the São Paulo Regional Council of Medicine (Cremesp).
Accusations and investigations
Not all incidents of sexual violence in health care institutions are reported, and precise numbers are difficult to obtain. The fact that there are any cases at all is troubling. In 2019, journalists from The Intercept found that over a period of 6 years (2014-2019), 1,734 such attacks were recorded in nine Brazilian states. They were able to get that information from the states’ Public Security Secretariats by using the Information Access Act, a law that regulates the right to access public information.
Efforts to determine how widespread this type of sexual violence is are further complicated by the difficulties in collating the accusations filed at each state’s regional council of medicine, police stations, and public prosecutor’s office. Which investigative steps are taken depends upon where the report was filed, and only occasionally do these entities communicate with each other. According to its data, Cremesp received 78 accusations in 2019. In 2020, that number increased to 84. In 2021, it was 83; these types of attacks were the seventh most common among the investigations opened that year. In the first 6 months of 2022, there were 36 complaints. The number includes investigations opened on the basis of press reports. In such cases, enough information must be available in the press reports make it possible to initiate an evaluation and assessment of the matter. There is no information about how many accusations became the subject of professional ethics proceedings and how many were formally adjudicated.
“Each accusation received is investigated by a technical committee made up of professionals from various specialties. There really needs to be a rigorous evaluation and assessment during the investigation. We cannot be unfair: It may turn out that there was no truth to the accusation after all, and yet someone’s career may already have been destroyed,” explained Dr. Scardoelli.
After the accusation is investigated and accepted by Cremesp, there is no deadline by which the proceedings must end. They can take up to 5 years, and sometimes longer. Since March, however, a deadline for the investigation period has been in effect, after which the proceedings can commence.
“We now have 90 days to make an evaluation and assessment in the investigation phase; that time period can be extended by 3 months, starting from the date the accusation is submitted to the council. If the case is accepted, then the proceedings are opened,” Dr. Scardoelli said.
Some incidents are not reported by victims. And there are incidents that are reported only after many years have passed. This was the case with Nina Marqueti, the actress at the center of #OndeDói — “Where It Hurts” — a campaign that was launched to raise awareness about sexual violence committed by health care professionals. When she was 16, her pediatrician sexually abused her. It wasn’t until 2019, more than a decade later, that she felt able to make this accusation known publicly.
Almost immediately, the campaign received over 4,000 posts online. Most of them were people’s accounts of acts of violence committed by physicians during appointments in their offices or during treatment in a hospital. These are available on Twitter under the hashtag #ondedoi.
Inadequate sex education?
News reports about physicians who abuse patients have a tremendous impact on the public. People are genuinely surprised when they hear the words “health care professional” and “sex attack” in the same sentence. “One of the most disturbing aspects is that health care professionals are committing these acts of violence against women who are in a vulnerable state, typically when they’re under anesthesia, they’ve fallen ill, or when the health care professional introduces an element of deception into the procedure so as to create the opportunity to abuse the patient in some way,” said Dr. Drezett.
As Dr. Cohen sees it, to perpetrate these acts of sexual violence, physicians – as well as lawyers, religious leaders, judges, politicians, police officers, and other persons in a position of trust – make use of their power to take advantage of a person’s vulnerability. “Physicians, lawyers, police officers, religious leaders, dads, bosses, husbands – the people who commit sexual abuse all have something in common,” he said. “In terms of the emotional aspect, all of them are taking advantage of both the power that their position holds in society and the asymmetrical power dynamics that exist between them and the other person.”
Indeed, anyone who knocks on a physician’s door seeking a diagnosis or treatment, anyone who knocks on a lawyer’s door seeking assistance, is putting themselves in a fragile situation. “The abuser considers the other person an object, not a human being who has rights,” said Dr. Cohen. People who fit the psychological and behavioral profile of a sexual assailant find in these “powerful” professions and in the circumstances and opportunities these professions provide a means to fulfill their desires. In medicine, however, there is yet another imbalance, one involving consent to touch a person’s body.
The age of the recently arrested anesthesiologist is something that caught Dr. Cohen’s attention. As noted in one of his many books, Bioética e Sexualidade nas Relações Profissionais [Bioethics and Sexuality in Professional Relationships], published in 1999 by the São Paulo Medical Association, age is a characteristic that repeatedly came up in his analysis of 150 sexual abuse proceedings handled by Cremesp.
“When I looked over the cases, I saw that most of the abusers were not right out of med school in their twenties – a time when sex is at the forefront of one’s life – nor were the abusers on the older end of the age spectrum. The abusers were, in fact, those who had already had several years of experience – as was the case with this 32-year-old anesthesiologist who, at a particular moment in time, breached all prohibitions and betrayed the expectations that society had of him as a physician: to care for people’s well-being and to alleviate their suffering. There was nothing that could hold him back from fulfilling his desire, not even the presence of nurses and other physicians in the operating room.” As for the findings of Dr. Cohen’s review, the majority of the 150 cases were dismissed because of lack of evidence.
To Ms. Pedroso, who has treated more than 12,000 victims of sexual harassment, it’s the questioning and intimidation that women feel in relationship to the male physician – a person who is viewed as holding knowledge about her body – that leaves them vulnerable and more subject to acts of violence, especially in more remote places. “We’re speaking, yet again, about rape culture. Not many people know what that term means, but, generally speaking, it has to do with the objectification of women’s bodies and the issue of boys growing up thinking they have the right to touch girls and women and that they will go unpunished for doing so.”
The lack of sex education and efforts to prevent sexual abuse are contributing factors for why the situation remains unchanged. “We are long overdue. We live in a country where there’s this completely mistaken belief that talking about sex education involves teaching children how to have sex, as opposed to teaching them how to protect themselves. We teach girls that they have to protect themselves from being raped, but we don’t teach boys not to rape.”
Another point highlighted by Ms. Pedroso is the fact that to carry out their actions, sexual assailants seek out-of-the-way places, places where they believe the rules can be bent and where they won’t be caught. This is what may have happened with Dr. Bezerra. During a recent press conference, the coordinator of the Health Section of the Rio de Janeiro Public Defender’s Office, Thaísa Guerreiro, stated that although the Women’s Hospital in São João do Meriti – one of the places where the assailant worked as an anesthesiologist – had adopted protocols to protect patients, it failed to enforce them. Another observation was that the health care professionals normalized violations of a woman’s right to have a companion present throughout labor and delivery, a right guaranteed by federal law. Ms. Guerreiro went on to say that the hospital’s chief of anesthesia and the state’s health coordination office did not question this, nor did they find it strange or surprising. According to witness statements, Dr. Bezerra would ask the patients’ husbands to leave the room in the middle of the procedure.
It should be clarified, Dr. Drezett mentioned, that although obstetric violence and sexual abuse overlap in places, they do not have the same root cause or definition. “There are two sets of situations that we term ‘obstetric violence.’ One involves any type of disrespectful treatment, whether comments or neglect, during pregnancy, delivery, or the postpartum period. The other refers to health care professionals’ attitudes in imposing inadequate and outdated medical procedures at the time of birth, such as keeping the woman fasting, having her pubic hair removed, and inducing labor or speeding up the delivery with oxytocin and [routine] episiotomy, among other things.”
Early education crucial
How are health care institutions dealing with this problem? “Very poorly. Sexual violence perpetrated by physicians and other health care professionals is a taboo subject that people are still afraid to talk about,” Dr. Cohen observed. “Regrettably, sexual violence happens all too often. Before, maybe we weren’t talking about it much because, from our viewpoint, health care professionals, such as physicians and nurses, weren’t likely to commit acts of violence while performing their duties,” noted Dr. Drezett.
Dr. Drezett also spoke about schools and what role they can play. “Of course, schools should discuss violence against women, especially in the field of health care. This has been done for a long time now, though it’s not in every curriculum in every medical school or nursing school, nor in every school of social work or of psychology,” said Dr. Drezett. For example, in the bioethics classes taken in the third and fourth years of the University of São Paolo’s medical degree program, Dr. Cohen asks students to reflect on the significance of being in a position where you ask a patient you’ve never met to undress so you can perform an exam, and the patient promptly and readily complies. “This is not about the physician, it’s about the power of the institution,” the professor pointed out. Sexual violence is a problem on the university campus as well. Another front in the battle has formed across various schools, where groups of students have created feminist collectives to have sexual violence and other issues related to gender-based violence added to the agenda.
Dr. Drezett said it’s very unlikely that efforts made during a degree program are going to succeed in preventing students who are prone to commit sexual violence from engaging in such behavior. “We’re talking about gender-specific lessons, where discussions about gender-based violence should be started much, much earlier – parents talking to their children, teachers talking to their pupils.” He also doesn’t believe that the molesters are dissuaded by the fact that these accusations get publicized in the media. “If they were, the Roger Abdelmassih case would have done away with the problem.”
On the other hand, Dr. Drezett suggested, publicizing these stories can help to bring very positive issues out into the open. What the Bezerra case made clear was that laws were not followed and rights were not protected. An environment was thus created in which the sexual crime could be perpetrated, with nurses coming to suspect acts of obstetric violence, such as use of sedation, which prevented the woman from having skin-to-skin contact with the newborn and from breastfeeding within the first few hours of birth – two clinical practices that are recommended the world over.
“Health care professionals who act properly, in accordance with best practices for interacting with others and performing daily duties, at all times, in public or private practice – they remove themselves from situations like those described in the Bezerra case; they don’t practice medicine in a reckless manner,” said Dr. Drezett.
Another negative aspect of all this, he said, is that the suspicion and wariness that patients feel may spread far and wide. “Among my colleagues are anesthetists and anesthesiologists with impeccable ethical and professional records. They are very upset that people are now regarding them with doubt and uncertainty. We need to make it clear that those horrifying cases are the exceptions, not the rule,” he said. There is also a need to correct the misconception that such abuse is always in some way associated with obstetrics and gynecology.
“This is not true. These incidents can happen in any doctor’s office. It all depends on the physician – whether he or she has designs on committing a criminal act,” Dr. Drezett noted. He did point out that there are few sexual molesters among health care professionals, though there are numerous cases. Yet this in no way diminishes the seriousness of the incidents. “Of course, we’re speaking again about the exceptions, but in my experience of treating victims, I’ve seen, for example, more cases where it’s been a police officer, not a physician, committing an act of sexual violence against a woman,” he stated.
The nurses and nurse technicians at São João do Meriti Hospital who reported the abuser acted very assertively. If they hadn’t gathered the evidence to back up their accusations, it’s possible that the physician wouldn’t have been caught in the act and that the case would have taken a different course – including pressure being put on them and their becoming the target of retaliation.
A version of this article first appeared on Medscape.com. This article was translated from the Medscape Portuguese edition.
At the beginning of July, Brazilians across the country were appalled when they heard that an anesthesiologist was accused of sexually abusing a woman he had been treating during cesarean delivery. The incident was recorded on video by nurses and nurse technicians who, having become suspicious of the excessive amount of sedatives given to mothers-to-be by this particular anesthesiologist, decided to film him during a procedure. To do this, they made a last-minute change, switching delivery rooms to one in which they had hidden a cell phone in a cabinet.
What the footage showed was horrifying and the assailant, Giovanni Quintella Bezerra, was arrested on the spot. He’s a 32-year-old, White, successful physician, and he’s now accused of rape. The authorities are looking into whether there are more victims, others who may have been abused by the physician. The police are investigating about 40 surgeries in which Dr. Bezerra participated. That same month saw the arrest of another physician, gynecologist Ricardo Teles Martins, who was arrested after being accused of sexually harassing and abusing several women in Hidrolândia, in the northeastern state of Ceará.
In gathering information about these incidents, this news organization interviewed four Brazilian specialists to get their insights on the issues that have been brought to light by these recent cases and the factors that play a role in these kinds of criminal acts. Claudio Cohen, MD, PhD, is a psychiatrist, bioethicist, and professor at the University of São Paulo in Brazil. Daniela Pedroso, MA, is a psychologist who has 25 years’ experience working with victims of sexual violence. Gynecologist and obstetrician Jefferson Drezett, MD, PhD, is a professor in the field of population genetics and reproductive and sexual health at the Federal University of ABC, São Paulo, and in the department of health, life cycles, and society at the University of São Paulo School of Public Health. Maria Alice Scardoelli, MD, is a psychiatrist who also serves as vice-chair of the São Paulo Regional Council of Medicine (Cremesp).
Accusations and investigations
Not all incidents of sexual violence in health care institutions are reported, and precise numbers are difficult to obtain. The fact that there are any cases at all is troubling. In 2019, journalists from The Intercept found that over a period of 6 years (2014-2019), 1,734 such attacks were recorded in nine Brazilian states. They were able to get that information from the states’ Public Security Secretariats by using the Information Access Act, a law that regulates the right to access public information.
Efforts to determine how widespread this type of sexual violence is are further complicated by the difficulties in collating the accusations filed at each state’s regional council of medicine, police stations, and public prosecutor’s office. Which investigative steps are taken depends upon where the report was filed, and only occasionally do these entities communicate with each other. According to its data, Cremesp received 78 accusations in 2019. In 2020, that number increased to 84. In 2021, it was 83; these types of attacks were the seventh most common among the investigations opened that year. In the first 6 months of 2022, there were 36 complaints. The number includes investigations opened on the basis of press reports. In such cases, enough information must be available in the press reports make it possible to initiate an evaluation and assessment of the matter. There is no information about how many accusations became the subject of professional ethics proceedings and how many were formally adjudicated.
“Each accusation received is investigated by a technical committee made up of professionals from various specialties. There really needs to be a rigorous evaluation and assessment during the investigation. We cannot be unfair: It may turn out that there was no truth to the accusation after all, and yet someone’s career may already have been destroyed,” explained Dr. Scardoelli.
After the accusation is investigated and accepted by Cremesp, there is no deadline by which the proceedings must end. They can take up to 5 years, and sometimes longer. Since March, however, a deadline for the investigation period has been in effect, after which the proceedings can commence.
“We now have 90 days to make an evaluation and assessment in the investigation phase; that time period can be extended by 3 months, starting from the date the accusation is submitted to the council. If the case is accepted, then the proceedings are opened,” Dr. Scardoelli said.
Some incidents are not reported by victims. And there are incidents that are reported only after many years have passed. This was the case with Nina Marqueti, the actress at the center of #OndeDói — “Where It Hurts” — a campaign that was launched to raise awareness about sexual violence committed by health care professionals. When she was 16, her pediatrician sexually abused her. It wasn’t until 2019, more than a decade later, that she felt able to make this accusation known publicly.
Almost immediately, the campaign received over 4,000 posts online. Most of them were people’s accounts of acts of violence committed by physicians during appointments in their offices or during treatment in a hospital. These are available on Twitter under the hashtag #ondedoi.
Inadequate sex education?
News reports about physicians who abuse patients have a tremendous impact on the public. People are genuinely surprised when they hear the words “health care professional” and “sex attack” in the same sentence. “One of the most disturbing aspects is that health care professionals are committing these acts of violence against women who are in a vulnerable state, typically when they’re under anesthesia, they’ve fallen ill, or when the health care professional introduces an element of deception into the procedure so as to create the opportunity to abuse the patient in some way,” said Dr. Drezett.
As Dr. Cohen sees it, to perpetrate these acts of sexual violence, physicians – as well as lawyers, religious leaders, judges, politicians, police officers, and other persons in a position of trust – make use of their power to take advantage of a person’s vulnerability. “Physicians, lawyers, police officers, religious leaders, dads, bosses, husbands – the people who commit sexual abuse all have something in common,” he said. “In terms of the emotional aspect, all of them are taking advantage of both the power that their position holds in society and the asymmetrical power dynamics that exist between them and the other person.”
Indeed, anyone who knocks on a physician’s door seeking a diagnosis or treatment, anyone who knocks on a lawyer’s door seeking assistance, is putting themselves in a fragile situation. “The abuser considers the other person an object, not a human being who has rights,” said Dr. Cohen. People who fit the psychological and behavioral profile of a sexual assailant find in these “powerful” professions and in the circumstances and opportunities these professions provide a means to fulfill their desires. In medicine, however, there is yet another imbalance, one involving consent to touch a person’s body.
The age of the recently arrested anesthesiologist is something that caught Dr. Cohen’s attention. As noted in one of his many books, Bioética e Sexualidade nas Relações Profissionais [Bioethics and Sexuality in Professional Relationships], published in 1999 by the São Paulo Medical Association, age is a characteristic that repeatedly came up in his analysis of 150 sexual abuse proceedings handled by Cremesp.
“When I looked over the cases, I saw that most of the abusers were not right out of med school in their twenties – a time when sex is at the forefront of one’s life – nor were the abusers on the older end of the age spectrum. The abusers were, in fact, those who had already had several years of experience – as was the case with this 32-year-old anesthesiologist who, at a particular moment in time, breached all prohibitions and betrayed the expectations that society had of him as a physician: to care for people’s well-being and to alleviate their suffering. There was nothing that could hold him back from fulfilling his desire, not even the presence of nurses and other physicians in the operating room.” As for the findings of Dr. Cohen’s review, the majority of the 150 cases were dismissed because of lack of evidence.
To Ms. Pedroso, who has treated more than 12,000 victims of sexual harassment, it’s the questioning and intimidation that women feel in relationship to the male physician – a person who is viewed as holding knowledge about her body – that leaves them vulnerable and more subject to acts of violence, especially in more remote places. “We’re speaking, yet again, about rape culture. Not many people know what that term means, but, generally speaking, it has to do with the objectification of women’s bodies and the issue of boys growing up thinking they have the right to touch girls and women and that they will go unpunished for doing so.”
The lack of sex education and efforts to prevent sexual abuse are contributing factors for why the situation remains unchanged. “We are long overdue. We live in a country where there’s this completely mistaken belief that talking about sex education involves teaching children how to have sex, as opposed to teaching them how to protect themselves. We teach girls that they have to protect themselves from being raped, but we don’t teach boys not to rape.”
Another point highlighted by Ms. Pedroso is the fact that to carry out their actions, sexual assailants seek out-of-the-way places, places where they believe the rules can be bent and where they won’t be caught. This is what may have happened with Dr. Bezerra. During a recent press conference, the coordinator of the Health Section of the Rio de Janeiro Public Defender’s Office, Thaísa Guerreiro, stated that although the Women’s Hospital in São João do Meriti – one of the places where the assailant worked as an anesthesiologist – had adopted protocols to protect patients, it failed to enforce them. Another observation was that the health care professionals normalized violations of a woman’s right to have a companion present throughout labor and delivery, a right guaranteed by federal law. Ms. Guerreiro went on to say that the hospital’s chief of anesthesia and the state’s health coordination office did not question this, nor did they find it strange or surprising. According to witness statements, Dr. Bezerra would ask the patients’ husbands to leave the room in the middle of the procedure.
It should be clarified, Dr. Drezett mentioned, that although obstetric violence and sexual abuse overlap in places, they do not have the same root cause or definition. “There are two sets of situations that we term ‘obstetric violence.’ One involves any type of disrespectful treatment, whether comments or neglect, during pregnancy, delivery, or the postpartum period. The other refers to health care professionals’ attitudes in imposing inadequate and outdated medical procedures at the time of birth, such as keeping the woman fasting, having her pubic hair removed, and inducing labor or speeding up the delivery with oxytocin and [routine] episiotomy, among other things.”
Early education crucial
How are health care institutions dealing with this problem? “Very poorly. Sexual violence perpetrated by physicians and other health care professionals is a taboo subject that people are still afraid to talk about,” Dr. Cohen observed. “Regrettably, sexual violence happens all too often. Before, maybe we weren’t talking about it much because, from our viewpoint, health care professionals, such as physicians and nurses, weren’t likely to commit acts of violence while performing their duties,” noted Dr. Drezett.
Dr. Drezett also spoke about schools and what role they can play. “Of course, schools should discuss violence against women, especially in the field of health care. This has been done for a long time now, though it’s not in every curriculum in every medical school or nursing school, nor in every school of social work or of psychology,” said Dr. Drezett. For example, in the bioethics classes taken in the third and fourth years of the University of São Paolo’s medical degree program, Dr. Cohen asks students to reflect on the significance of being in a position where you ask a patient you’ve never met to undress so you can perform an exam, and the patient promptly and readily complies. “This is not about the physician, it’s about the power of the institution,” the professor pointed out. Sexual violence is a problem on the university campus as well. Another front in the battle has formed across various schools, where groups of students have created feminist collectives to have sexual violence and other issues related to gender-based violence added to the agenda.
Dr. Drezett said it’s very unlikely that efforts made during a degree program are going to succeed in preventing students who are prone to commit sexual violence from engaging in such behavior. “We’re talking about gender-specific lessons, where discussions about gender-based violence should be started much, much earlier – parents talking to their children, teachers talking to their pupils.” He also doesn’t believe that the molesters are dissuaded by the fact that these accusations get publicized in the media. “If they were, the Roger Abdelmassih case would have done away with the problem.”
On the other hand, Dr. Drezett suggested, publicizing these stories can help to bring very positive issues out into the open. What the Bezerra case made clear was that laws were not followed and rights were not protected. An environment was thus created in which the sexual crime could be perpetrated, with nurses coming to suspect acts of obstetric violence, such as use of sedation, which prevented the woman from having skin-to-skin contact with the newborn and from breastfeeding within the first few hours of birth – two clinical practices that are recommended the world over.
“Health care professionals who act properly, in accordance with best practices for interacting with others and performing daily duties, at all times, in public or private practice – they remove themselves from situations like those described in the Bezerra case; they don’t practice medicine in a reckless manner,” said Dr. Drezett.
Another negative aspect of all this, he said, is that the suspicion and wariness that patients feel may spread far and wide. “Among my colleagues are anesthetists and anesthesiologists with impeccable ethical and professional records. They are very upset that people are now regarding them with doubt and uncertainty. We need to make it clear that those horrifying cases are the exceptions, not the rule,” he said. There is also a need to correct the misconception that such abuse is always in some way associated with obstetrics and gynecology.
“This is not true. These incidents can happen in any doctor’s office. It all depends on the physician – whether he or she has designs on committing a criminal act,” Dr. Drezett noted. He did point out that there are few sexual molesters among health care professionals, though there are numerous cases. Yet this in no way diminishes the seriousness of the incidents. “Of course, we’re speaking again about the exceptions, but in my experience of treating victims, I’ve seen, for example, more cases where it’s been a police officer, not a physician, committing an act of sexual violence against a woman,” he stated.
The nurses and nurse technicians at São João do Meriti Hospital who reported the abuser acted very assertively. If they hadn’t gathered the evidence to back up their accusations, it’s possible that the physician wouldn’t have been caught in the act and that the case would have taken a different course – including pressure being put on them and their becoming the target of retaliation.
A version of this article first appeared on Medscape.com. This article was translated from the Medscape Portuguese edition.
At the beginning of July, Brazilians across the country were appalled when they heard that an anesthesiologist was accused of sexually abusing a woman he had been treating during cesarean delivery. The incident was recorded on video by nurses and nurse technicians who, having become suspicious of the excessive amount of sedatives given to mothers-to-be by this particular anesthesiologist, decided to film him during a procedure. To do this, they made a last-minute change, switching delivery rooms to one in which they had hidden a cell phone in a cabinet.
What the footage showed was horrifying and the assailant, Giovanni Quintella Bezerra, was arrested on the spot. He’s a 32-year-old, White, successful physician, and he’s now accused of rape. The authorities are looking into whether there are more victims, others who may have been abused by the physician. The police are investigating about 40 surgeries in which Dr. Bezerra participated. That same month saw the arrest of another physician, gynecologist Ricardo Teles Martins, who was arrested after being accused of sexually harassing and abusing several women in Hidrolândia, in the northeastern state of Ceará.
In gathering information about these incidents, this news organization interviewed four Brazilian specialists to get their insights on the issues that have been brought to light by these recent cases and the factors that play a role in these kinds of criminal acts. Claudio Cohen, MD, PhD, is a psychiatrist, bioethicist, and professor at the University of São Paulo in Brazil. Daniela Pedroso, MA, is a psychologist who has 25 years’ experience working with victims of sexual violence. Gynecologist and obstetrician Jefferson Drezett, MD, PhD, is a professor in the field of population genetics and reproductive and sexual health at the Federal University of ABC, São Paulo, and in the department of health, life cycles, and society at the University of São Paulo School of Public Health. Maria Alice Scardoelli, MD, is a psychiatrist who also serves as vice-chair of the São Paulo Regional Council of Medicine (Cremesp).
Accusations and investigations
Not all incidents of sexual violence in health care institutions are reported, and precise numbers are difficult to obtain. The fact that there are any cases at all is troubling. In 2019, journalists from The Intercept found that over a period of 6 years (2014-2019), 1,734 such attacks were recorded in nine Brazilian states. They were able to get that information from the states’ Public Security Secretariats by using the Information Access Act, a law that regulates the right to access public information.
Efforts to determine how widespread this type of sexual violence is are further complicated by the difficulties in collating the accusations filed at each state’s regional council of medicine, police stations, and public prosecutor’s office. Which investigative steps are taken depends upon where the report was filed, and only occasionally do these entities communicate with each other. According to its data, Cremesp received 78 accusations in 2019. In 2020, that number increased to 84. In 2021, it was 83; these types of attacks were the seventh most common among the investigations opened that year. In the first 6 months of 2022, there were 36 complaints. The number includes investigations opened on the basis of press reports. In such cases, enough information must be available in the press reports make it possible to initiate an evaluation and assessment of the matter. There is no information about how many accusations became the subject of professional ethics proceedings and how many were formally adjudicated.
“Each accusation received is investigated by a technical committee made up of professionals from various specialties. There really needs to be a rigorous evaluation and assessment during the investigation. We cannot be unfair: It may turn out that there was no truth to the accusation after all, and yet someone’s career may already have been destroyed,” explained Dr. Scardoelli.
After the accusation is investigated and accepted by Cremesp, there is no deadline by which the proceedings must end. They can take up to 5 years, and sometimes longer. Since March, however, a deadline for the investigation period has been in effect, after which the proceedings can commence.
“We now have 90 days to make an evaluation and assessment in the investigation phase; that time period can be extended by 3 months, starting from the date the accusation is submitted to the council. If the case is accepted, then the proceedings are opened,” Dr. Scardoelli said.
Some incidents are not reported by victims. And there are incidents that are reported only after many years have passed. This was the case with Nina Marqueti, the actress at the center of #OndeDói — “Where It Hurts” — a campaign that was launched to raise awareness about sexual violence committed by health care professionals. When she was 16, her pediatrician sexually abused her. It wasn’t until 2019, more than a decade later, that she felt able to make this accusation known publicly.
Almost immediately, the campaign received over 4,000 posts online. Most of them were people’s accounts of acts of violence committed by physicians during appointments in their offices or during treatment in a hospital. These are available on Twitter under the hashtag #ondedoi.
Inadequate sex education?
News reports about physicians who abuse patients have a tremendous impact on the public. People are genuinely surprised when they hear the words “health care professional” and “sex attack” in the same sentence. “One of the most disturbing aspects is that health care professionals are committing these acts of violence against women who are in a vulnerable state, typically when they’re under anesthesia, they’ve fallen ill, or when the health care professional introduces an element of deception into the procedure so as to create the opportunity to abuse the patient in some way,” said Dr. Drezett.
As Dr. Cohen sees it, to perpetrate these acts of sexual violence, physicians – as well as lawyers, religious leaders, judges, politicians, police officers, and other persons in a position of trust – make use of their power to take advantage of a person’s vulnerability. “Physicians, lawyers, police officers, religious leaders, dads, bosses, husbands – the people who commit sexual abuse all have something in common,” he said. “In terms of the emotional aspect, all of them are taking advantage of both the power that their position holds in society and the asymmetrical power dynamics that exist between them and the other person.”
Indeed, anyone who knocks on a physician’s door seeking a diagnosis or treatment, anyone who knocks on a lawyer’s door seeking assistance, is putting themselves in a fragile situation. “The abuser considers the other person an object, not a human being who has rights,” said Dr. Cohen. People who fit the psychological and behavioral profile of a sexual assailant find in these “powerful” professions and in the circumstances and opportunities these professions provide a means to fulfill their desires. In medicine, however, there is yet another imbalance, one involving consent to touch a person’s body.
The age of the recently arrested anesthesiologist is something that caught Dr. Cohen’s attention. As noted in one of his many books, Bioética e Sexualidade nas Relações Profissionais [Bioethics and Sexuality in Professional Relationships], published in 1999 by the São Paulo Medical Association, age is a characteristic that repeatedly came up in his analysis of 150 sexual abuse proceedings handled by Cremesp.
“When I looked over the cases, I saw that most of the abusers were not right out of med school in their twenties – a time when sex is at the forefront of one’s life – nor were the abusers on the older end of the age spectrum. The abusers were, in fact, those who had already had several years of experience – as was the case with this 32-year-old anesthesiologist who, at a particular moment in time, breached all prohibitions and betrayed the expectations that society had of him as a physician: to care for people’s well-being and to alleviate their suffering. There was nothing that could hold him back from fulfilling his desire, not even the presence of nurses and other physicians in the operating room.” As for the findings of Dr. Cohen’s review, the majority of the 150 cases were dismissed because of lack of evidence.
To Ms. Pedroso, who has treated more than 12,000 victims of sexual harassment, it’s the questioning and intimidation that women feel in relationship to the male physician – a person who is viewed as holding knowledge about her body – that leaves them vulnerable and more subject to acts of violence, especially in more remote places. “We’re speaking, yet again, about rape culture. Not many people know what that term means, but, generally speaking, it has to do with the objectification of women’s bodies and the issue of boys growing up thinking they have the right to touch girls and women and that they will go unpunished for doing so.”
The lack of sex education and efforts to prevent sexual abuse are contributing factors for why the situation remains unchanged. “We are long overdue. We live in a country where there’s this completely mistaken belief that talking about sex education involves teaching children how to have sex, as opposed to teaching them how to protect themselves. We teach girls that they have to protect themselves from being raped, but we don’t teach boys not to rape.”
Another point highlighted by Ms. Pedroso is the fact that to carry out their actions, sexual assailants seek out-of-the-way places, places where they believe the rules can be bent and where they won’t be caught. This is what may have happened with Dr. Bezerra. During a recent press conference, the coordinator of the Health Section of the Rio de Janeiro Public Defender’s Office, Thaísa Guerreiro, stated that although the Women’s Hospital in São João do Meriti – one of the places where the assailant worked as an anesthesiologist – had adopted protocols to protect patients, it failed to enforce them. Another observation was that the health care professionals normalized violations of a woman’s right to have a companion present throughout labor and delivery, a right guaranteed by federal law. Ms. Guerreiro went on to say that the hospital’s chief of anesthesia and the state’s health coordination office did not question this, nor did they find it strange or surprising. According to witness statements, Dr. Bezerra would ask the patients’ husbands to leave the room in the middle of the procedure.
It should be clarified, Dr. Drezett mentioned, that although obstetric violence and sexual abuse overlap in places, they do not have the same root cause or definition. “There are two sets of situations that we term ‘obstetric violence.’ One involves any type of disrespectful treatment, whether comments or neglect, during pregnancy, delivery, or the postpartum period. The other refers to health care professionals’ attitudes in imposing inadequate and outdated medical procedures at the time of birth, such as keeping the woman fasting, having her pubic hair removed, and inducing labor or speeding up the delivery with oxytocin and [routine] episiotomy, among other things.”
Early education crucial
How are health care institutions dealing with this problem? “Very poorly. Sexual violence perpetrated by physicians and other health care professionals is a taboo subject that people are still afraid to talk about,” Dr. Cohen observed. “Regrettably, sexual violence happens all too often. Before, maybe we weren’t talking about it much because, from our viewpoint, health care professionals, such as physicians and nurses, weren’t likely to commit acts of violence while performing their duties,” noted Dr. Drezett.
Dr. Drezett also spoke about schools and what role they can play. “Of course, schools should discuss violence against women, especially in the field of health care. This has been done for a long time now, though it’s not in every curriculum in every medical school or nursing school, nor in every school of social work or of psychology,” said Dr. Drezett. For example, in the bioethics classes taken in the third and fourth years of the University of São Paolo’s medical degree program, Dr. Cohen asks students to reflect on the significance of being in a position where you ask a patient you’ve never met to undress so you can perform an exam, and the patient promptly and readily complies. “This is not about the physician, it’s about the power of the institution,” the professor pointed out. Sexual violence is a problem on the university campus as well. Another front in the battle has formed across various schools, where groups of students have created feminist collectives to have sexual violence and other issues related to gender-based violence added to the agenda.
Dr. Drezett said it’s very unlikely that efforts made during a degree program are going to succeed in preventing students who are prone to commit sexual violence from engaging in such behavior. “We’re talking about gender-specific lessons, where discussions about gender-based violence should be started much, much earlier – parents talking to their children, teachers talking to their pupils.” He also doesn’t believe that the molesters are dissuaded by the fact that these accusations get publicized in the media. “If they were, the Roger Abdelmassih case would have done away with the problem.”
On the other hand, Dr. Drezett suggested, publicizing these stories can help to bring very positive issues out into the open. What the Bezerra case made clear was that laws were not followed and rights were not protected. An environment was thus created in which the sexual crime could be perpetrated, with nurses coming to suspect acts of obstetric violence, such as use of sedation, which prevented the woman from having skin-to-skin contact with the newborn and from breastfeeding within the first few hours of birth – two clinical practices that are recommended the world over.
“Health care professionals who act properly, in accordance with best practices for interacting with others and performing daily duties, at all times, in public or private practice – they remove themselves from situations like those described in the Bezerra case; they don’t practice medicine in a reckless manner,” said Dr. Drezett.
Another negative aspect of all this, he said, is that the suspicion and wariness that patients feel may spread far and wide. “Among my colleagues are anesthetists and anesthesiologists with impeccable ethical and professional records. They are very upset that people are now regarding them with doubt and uncertainty. We need to make it clear that those horrifying cases are the exceptions, not the rule,” he said. There is also a need to correct the misconception that such abuse is always in some way associated with obstetrics and gynecology.
“This is not true. These incidents can happen in any doctor’s office. It all depends on the physician – whether he or she has designs on committing a criminal act,” Dr. Drezett noted. He did point out that there are few sexual molesters among health care professionals, though there are numerous cases. Yet this in no way diminishes the seriousness of the incidents. “Of course, we’re speaking again about the exceptions, but in my experience of treating victims, I’ve seen, for example, more cases where it’s been a police officer, not a physician, committing an act of sexual violence against a woman,” he stated.
The nurses and nurse technicians at São João do Meriti Hospital who reported the abuser acted very assertively. If they hadn’t gathered the evidence to back up their accusations, it’s possible that the physician wouldn’t have been caught in the act and that the case would have taken a different course – including pressure being put on them and their becoming the target of retaliation.
A version of this article first appeared on Medscape.com. This article was translated from the Medscape Portuguese edition.
Dermatologists share vitiligo breakthrough news with patients
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
How common are second primary lung cancers?
diagnosis, with about half occurring within 6 months of the first diagnosis. More than 80% of second primary cancers diagnosed within 2 years were stage 1, compared with about 25% when diagnosed more than 5 years later.
“With the growing adoption of lung cancer screening, more patients are being diagnosed with early-stage lung cancers and are able to achieve excellent long-term survival. After lung cancer diagnosis, these patients remain at high risk of developing a second primary lung cancer. The incidence, timing, and survival of second primary lung cancers is not well understood, particularly in a patient population with initial primary lung cancers detected via lung cancer screening,” said Alexandra Potter, who is a study coauthor.
The results were presented by Chi-Fu Jeffrey Yang, MD, at a press conference held at the World Conference on Lung Cancer sponsored by the International Association for the Study of Lung Cancer. Dr. Yang is a thoracic surgeon at Massachusetts General Hospital, Boston.
A 2012 study analyzed data from the SEER database and found that lung cancer survivors had a four- to sixfold increase in the risk of developing a second primary lung cancer, compared with the risk of lung cancer in the general population after adjusting for sex, age, race, and calendar year. “That study demonstrated that second primary lung cancers are an important risk among lung cancer survivors. However, it did not evaluate patients diagnosed with initial lung cancers detected via lung cancer screening. Thus, the incidence, timing, characteristics, and survival of lung cancers diagnosed among patients diagnosed with initial lung cancers detected via lung cancer screening remain unknown,” said Ms. Potter, who is a research assistant at Massachusetts General Hospital and president of the American Lung Cancer Screening Initiative.
To address that question, the researchers used data from the National Lung Screening Trial, which compared low-dose computed tomography to chest x-ray and found that the former led to a 15%-20% lower risk of death. The new analysis included 1,405 patients who were diagnosed with stage I-III lung cancer and treated between 2002 and 2009. Of these patients, 5.8% went on to be diagnosed with a second primary lung cancer, at a rate of 1%-2% per year. Of the second lung cancers, 54.9% were synchronous, occurring within 6 months of the diagnosis, and 45.1% were metachronous, occurring later than 6 months; 65% of synchronous secondary cancers and 81% of metachronous cancers were diagnosed at stage I; 24% of synchronous and 14% of metachronous were stage III (P = .25). The median time to diagnosis of metachronous lung cancers was 2.7 years, and 27% of the second primary tumors were diagnosed 4 or more years after the first diagnosis.
Among those with synchronous tumors, 5- and 10-year survival rates were 55.2% and 39.5%. The rates were 90.0% and 30.8% among metachronous tumors, respectively. Ms. Potter emphasized that most patients with second primary cancer were diagnosed at stage I, suggesting that it is very possible to catch these cancers early. But patients who were diagnosed with a second primary tumor 4 or more years after their first diagnosis had a greater likelihood of later-stage second cancer. Medical societies generally recommend CT screening surveillance every 6 months for 2 years following a lung cancer diagnosis, then annually thereafter. The greater frequency of later-stage cancer detected after 4 years suggests that surveillance may be flagging as time goes on. “These data highlight the importance of lifelong follow up after initial lung cancer diagnosis,” said Ms. Potter.
She also emphasized the importance of smoking cessation and ongoing abstinence following a diagnosis of lung cancer. “About 70% of patients in the NLST who developed second primary lung cancer currently smoked at the time of entry into the trial. Smoking cessation can help reduce patients’ risk of developing second primary lung cancers,” she said. Ms. Potter has no relevant financial disclosures.
diagnosis, with about half occurring within 6 months of the first diagnosis. More than 80% of second primary cancers diagnosed within 2 years were stage 1, compared with about 25% when diagnosed more than 5 years later.
“With the growing adoption of lung cancer screening, more patients are being diagnosed with early-stage lung cancers and are able to achieve excellent long-term survival. After lung cancer diagnosis, these patients remain at high risk of developing a second primary lung cancer. The incidence, timing, and survival of second primary lung cancers is not well understood, particularly in a patient population with initial primary lung cancers detected via lung cancer screening,” said Alexandra Potter, who is a study coauthor.
The results were presented by Chi-Fu Jeffrey Yang, MD, at a press conference held at the World Conference on Lung Cancer sponsored by the International Association for the Study of Lung Cancer. Dr. Yang is a thoracic surgeon at Massachusetts General Hospital, Boston.
A 2012 study analyzed data from the SEER database and found that lung cancer survivors had a four- to sixfold increase in the risk of developing a second primary lung cancer, compared with the risk of lung cancer in the general population after adjusting for sex, age, race, and calendar year. “That study demonstrated that second primary lung cancers are an important risk among lung cancer survivors. However, it did not evaluate patients diagnosed with initial lung cancers detected via lung cancer screening. Thus, the incidence, timing, characteristics, and survival of lung cancers diagnosed among patients diagnosed with initial lung cancers detected via lung cancer screening remain unknown,” said Ms. Potter, who is a research assistant at Massachusetts General Hospital and president of the American Lung Cancer Screening Initiative.
To address that question, the researchers used data from the National Lung Screening Trial, which compared low-dose computed tomography to chest x-ray and found that the former led to a 15%-20% lower risk of death. The new analysis included 1,405 patients who were diagnosed with stage I-III lung cancer and treated between 2002 and 2009. Of these patients, 5.8% went on to be diagnosed with a second primary lung cancer, at a rate of 1%-2% per year. Of the second lung cancers, 54.9% were synchronous, occurring within 6 months of the diagnosis, and 45.1% were metachronous, occurring later than 6 months; 65% of synchronous secondary cancers and 81% of metachronous cancers were diagnosed at stage I; 24% of synchronous and 14% of metachronous were stage III (P = .25). The median time to diagnosis of metachronous lung cancers was 2.7 years, and 27% of the second primary tumors were diagnosed 4 or more years after the first diagnosis.
Among those with synchronous tumors, 5- and 10-year survival rates were 55.2% and 39.5%. The rates were 90.0% and 30.8% among metachronous tumors, respectively. Ms. Potter emphasized that most patients with second primary cancer were diagnosed at stage I, suggesting that it is very possible to catch these cancers early. But patients who were diagnosed with a second primary tumor 4 or more years after their first diagnosis had a greater likelihood of later-stage second cancer. Medical societies generally recommend CT screening surveillance every 6 months for 2 years following a lung cancer diagnosis, then annually thereafter. The greater frequency of later-stage cancer detected after 4 years suggests that surveillance may be flagging as time goes on. “These data highlight the importance of lifelong follow up after initial lung cancer diagnosis,” said Ms. Potter.
She also emphasized the importance of smoking cessation and ongoing abstinence following a diagnosis of lung cancer. “About 70% of patients in the NLST who developed second primary lung cancer currently smoked at the time of entry into the trial. Smoking cessation can help reduce patients’ risk of developing second primary lung cancers,” she said. Ms. Potter has no relevant financial disclosures.
diagnosis, with about half occurring within 6 months of the first diagnosis. More than 80% of second primary cancers diagnosed within 2 years were stage 1, compared with about 25% when diagnosed more than 5 years later.
“With the growing adoption of lung cancer screening, more patients are being diagnosed with early-stage lung cancers and are able to achieve excellent long-term survival. After lung cancer diagnosis, these patients remain at high risk of developing a second primary lung cancer. The incidence, timing, and survival of second primary lung cancers is not well understood, particularly in a patient population with initial primary lung cancers detected via lung cancer screening,” said Alexandra Potter, who is a study coauthor.
The results were presented by Chi-Fu Jeffrey Yang, MD, at a press conference held at the World Conference on Lung Cancer sponsored by the International Association for the Study of Lung Cancer. Dr. Yang is a thoracic surgeon at Massachusetts General Hospital, Boston.
A 2012 study analyzed data from the SEER database and found that lung cancer survivors had a four- to sixfold increase in the risk of developing a second primary lung cancer, compared with the risk of lung cancer in the general population after adjusting for sex, age, race, and calendar year. “That study demonstrated that second primary lung cancers are an important risk among lung cancer survivors. However, it did not evaluate patients diagnosed with initial lung cancers detected via lung cancer screening. Thus, the incidence, timing, characteristics, and survival of lung cancers diagnosed among patients diagnosed with initial lung cancers detected via lung cancer screening remain unknown,” said Ms. Potter, who is a research assistant at Massachusetts General Hospital and president of the American Lung Cancer Screening Initiative.
To address that question, the researchers used data from the National Lung Screening Trial, which compared low-dose computed tomography to chest x-ray and found that the former led to a 15%-20% lower risk of death. The new analysis included 1,405 patients who were diagnosed with stage I-III lung cancer and treated between 2002 and 2009. Of these patients, 5.8% went on to be diagnosed with a second primary lung cancer, at a rate of 1%-2% per year. Of the second lung cancers, 54.9% were synchronous, occurring within 6 months of the diagnosis, and 45.1% were metachronous, occurring later than 6 months; 65% of synchronous secondary cancers and 81% of metachronous cancers were diagnosed at stage I; 24% of synchronous and 14% of metachronous were stage III (P = .25). The median time to diagnosis of metachronous lung cancers was 2.7 years, and 27% of the second primary tumors were diagnosed 4 or more years after the first diagnosis.
Among those with synchronous tumors, 5- and 10-year survival rates were 55.2% and 39.5%. The rates were 90.0% and 30.8% among metachronous tumors, respectively. Ms. Potter emphasized that most patients with second primary cancer were diagnosed at stage I, suggesting that it is very possible to catch these cancers early. But patients who were diagnosed with a second primary tumor 4 or more years after their first diagnosis had a greater likelihood of later-stage second cancer. Medical societies generally recommend CT screening surveillance every 6 months for 2 years following a lung cancer diagnosis, then annually thereafter. The greater frequency of later-stage cancer detected after 4 years suggests that surveillance may be flagging as time goes on. “These data highlight the importance of lifelong follow up after initial lung cancer diagnosis,” said Ms. Potter.
She also emphasized the importance of smoking cessation and ongoing abstinence following a diagnosis of lung cancer. “About 70% of patients in the NLST who developed second primary lung cancer currently smoked at the time of entry into the trial. Smoking cessation can help reduce patients’ risk of developing second primary lung cancers,” she said. Ms. Potter has no relevant financial disclosures.
FROM WCLC 2022
Early LV recovery after TAVR tied to 5-year mortality
Early improvement of left ventricular ejection fraction (LVEF) after transcatheter aortic valve replacement (TAVR) is associated with improved all-cause and cardiac death at 5 years in patients with severe aortic stenosis and LVEF less than 50%, new research shows.
Further analyses revealed a significant interaction by sex, with the mortality benefit largely in women.
“It’s absolutely fascinating,” senior author Sammy Elmariah, MD, Massachusetts General Hospital, Boston, said of the finding. “We know that women are more likely to have concentric hypertrophy, that they have lesser degrees of fibrosis, and smaller ventricles, and, of course, they’re in general less affected by coronary artery disease and MIs [myocardial infarctions]. All of those things in my mind, at least that’s what I assumed ahead of time, would make it more likely for women’s hearts to recover.”
“But that’s actually not what we found,” he continued. “We didn’t see a difference between the sexes in terms of likelihood of recovery. But what we saw is that the survival benefit, that associates with improvement in EF, was almost completely driven by women. So women really seem to be reaping that benefit in a manner that is unique and very different from what we saw in men.”
Dr. Elmariah noted that the reason for this benefit is unclear but points to the differences in biology for LV remodeling. “Clearly there are several details there that warrant further attention and more research.”
Suzanne J. Baron, MD, director of interventional cardiology research at Lahey Hospital and Medical Center, Burlington, Mass., said in an email that the finding of a substantial long-term survival benefit was “a bit surprising.”
Several studies have suggested that women may derive a greater benefit from TAVR versus surgical aortic valve replacement, and meta-analyses have demonstrated short and intermediate-term survival after TAVR is better in women, compared with in men, she pointed out. However, the mediating mechanism for this finding has never been clearly elucidated.
“Certainly, the sex differences in LVEF improvement after TAVR observed in this study, which could be related to sex differences in LV remodeling and LV mass regression, may now give us a clue as to why these sex-specific survival differences after TAVR persist,” Dr. Baron said.
More data amassed
Previous research in smaller cohorts with follow-up out to 1 year have shown an association between early LVEF improvement after TAVR and better survival. This includes a 2013 study by the investigators in high-risk patients in PARTNER-1 and a separate 2016 study in patients in the CoreValve extreme and high surgical risk trials.
Now, with longer follow-up amassed, the investigators examined data from 659 high- or intermediate-risk patients with severe stenosis and LVEF less than 50% who underwent transfemoral TAVR in the PARTNER 1, 2, and S3 trials and registries between July 2007 and April 2015.
Their mean age was 82.4 years, 71% were men, and 89.7% were White individuals. During the study period, 55.6% of the cohort died.
As reported in JAMA Cardiology, 32.8% of patients had early LVEF improvement, defined as an increase of at least 10% percentage points at 30 days after TAVR (mean change, 16.4%).
This compares with about 50%-60% of patients in the earlier studies, likely owing to the relatively higher baseline LVEF, especially in the intermediate-risk cohort, the authors suggested.
Independent predictors of lower likelihood of early LVEF improvement were previous MI, diabetes, cancer, higher baseline LVEF, larger LV end-diastolic diameter, and larger aortic valve area (AVA), whereas higher body mass index and higher stroke volume index predicted greater likelihood of LV recovery.
At 5 years, patients with versus without improved early LV improvement had lower risks of all-cause death (50.0% vs. 58.4%; P = .04) and cardiac death (29.5% vs. 38.1%; P = .05).
In multivariable analyses, each 5%-point increase in LVEF after TAVR was associated with a 6% lower risk of all-cause death (hazard ratio [HR], 0.94; P = .04) and 10% lower risk of cardiac death (HR, 0.90; P = .02).
Restricted cubic spline analysis demonstrated an inflection point above a 10% change in LVEF beyond which there was a steep decline in all-cause mortality with increasing degree of LVEF improvement.
There were no significant differences in rehospitalization, New York Heart Association functional class, or Kansas City Cardiomyopathy Questionnaire score at 5 years in patients with and without early LVEF improvement.
“I think what this really gets to is what is the reason behind the LV dysfunction in the first place,” said Dr. Elmariah, soon to be joining the University of California, San Francisco. “We know that TAVR cures aortic stenosis, so if the LV dysfunction is primarily related to the valve itself, hopefully those patients are going to recover.”
On the other hand, if the patient has LV dysfunction because of a prior myocardial infarction or cardiomyopathy and then developed aortic stenosis, “you can treat the aortic stenosis but the heart is still diseased from whatever process was affecting it previously and so it’s not likely to recover in those scenarios,” he added.
The results can be used for counseling patients and highlight the need to optimize goal-directed medical therapy in those with valvular heart disease, Dr. Elmariah suggested.
“Often, patients with aortic stenosis are on miniscule doses of many of the heart failure agents because people are worried about the hemodynamic consequences and they’re worried that patients won’t tolerate these medications,” he said. “But it’s very important for us to aggressively try to treat the heart failure that is affecting these patients in order to hopefully increase the chances that their left ventricles will recover and, hopefully, that they will have improved survival.”
Dr. Baron said that “this study clearly demonstrates that patients with reduced LVEF and severe aortic stenosis can benefit from TAVR and that early improvement in LVEF is an important prognostic marker for this population.”
In Dr. Baron and colleagues’ earlier analysis of 11,000 patients who underwent TAVR as part of the transcatheter valve therapy registry, only low aortic valve gradient but not LV dysfunction was associated with higher adjusted 1-year mortality. Asked about the finding, she noted that patients were evaluated based on LV function at baseline and not for a difference in outcomes based on LVEF improvement after TAVR.
“As such, I think that these two studies are actually complementary,” Dr. Baron said. “Together, they suggest that a low LVEF should not preclude a patient from receiving TAVR and if the patient does experience a 10% increase in LVEF after TAVR, then their 5-year prognosis is improved.”
Dr. Elmariah reports grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Medtronic, and Svelte Medical and has received consulting fees from Medtronic and AstraZeneca. Coauthor disclosures are listed in the paper. The PARTNER trials and registries and this analysis were supported by Edwards Lifesciences. Edwards was involved in the design and conduct of the study including collection, management, analysis, and interpretation of the data. Dr. Baron reports receiving research grant funding from Abiomed and Boston Scientific; consulting/medical advisory board fees from Boston Scientific, Shockwave and Biotronik; and speaking honoraria from Medtronic and Zoll.
A version of this article first appeared on Medscape.com.
Early improvement of left ventricular ejection fraction (LVEF) after transcatheter aortic valve replacement (TAVR) is associated with improved all-cause and cardiac death at 5 years in patients with severe aortic stenosis and LVEF less than 50%, new research shows.
Further analyses revealed a significant interaction by sex, with the mortality benefit largely in women.
“It’s absolutely fascinating,” senior author Sammy Elmariah, MD, Massachusetts General Hospital, Boston, said of the finding. “We know that women are more likely to have concentric hypertrophy, that they have lesser degrees of fibrosis, and smaller ventricles, and, of course, they’re in general less affected by coronary artery disease and MIs [myocardial infarctions]. All of those things in my mind, at least that’s what I assumed ahead of time, would make it more likely for women’s hearts to recover.”
“But that’s actually not what we found,” he continued. “We didn’t see a difference between the sexes in terms of likelihood of recovery. But what we saw is that the survival benefit, that associates with improvement in EF, was almost completely driven by women. So women really seem to be reaping that benefit in a manner that is unique and very different from what we saw in men.”
Dr. Elmariah noted that the reason for this benefit is unclear but points to the differences in biology for LV remodeling. “Clearly there are several details there that warrant further attention and more research.”
Suzanne J. Baron, MD, director of interventional cardiology research at Lahey Hospital and Medical Center, Burlington, Mass., said in an email that the finding of a substantial long-term survival benefit was “a bit surprising.”
Several studies have suggested that women may derive a greater benefit from TAVR versus surgical aortic valve replacement, and meta-analyses have demonstrated short and intermediate-term survival after TAVR is better in women, compared with in men, she pointed out. However, the mediating mechanism for this finding has never been clearly elucidated.
“Certainly, the sex differences in LVEF improvement after TAVR observed in this study, which could be related to sex differences in LV remodeling and LV mass regression, may now give us a clue as to why these sex-specific survival differences after TAVR persist,” Dr. Baron said.
More data amassed
Previous research in smaller cohorts with follow-up out to 1 year have shown an association between early LVEF improvement after TAVR and better survival. This includes a 2013 study by the investigators in high-risk patients in PARTNER-1 and a separate 2016 study in patients in the CoreValve extreme and high surgical risk trials.
Now, with longer follow-up amassed, the investigators examined data from 659 high- or intermediate-risk patients with severe stenosis and LVEF less than 50% who underwent transfemoral TAVR in the PARTNER 1, 2, and S3 trials and registries between July 2007 and April 2015.
Their mean age was 82.4 years, 71% were men, and 89.7% were White individuals. During the study period, 55.6% of the cohort died.
As reported in JAMA Cardiology, 32.8% of patients had early LVEF improvement, defined as an increase of at least 10% percentage points at 30 days after TAVR (mean change, 16.4%).
This compares with about 50%-60% of patients in the earlier studies, likely owing to the relatively higher baseline LVEF, especially in the intermediate-risk cohort, the authors suggested.
Independent predictors of lower likelihood of early LVEF improvement were previous MI, diabetes, cancer, higher baseline LVEF, larger LV end-diastolic diameter, and larger aortic valve area (AVA), whereas higher body mass index and higher stroke volume index predicted greater likelihood of LV recovery.
At 5 years, patients with versus without improved early LV improvement had lower risks of all-cause death (50.0% vs. 58.4%; P = .04) and cardiac death (29.5% vs. 38.1%; P = .05).
In multivariable analyses, each 5%-point increase in LVEF after TAVR was associated with a 6% lower risk of all-cause death (hazard ratio [HR], 0.94; P = .04) and 10% lower risk of cardiac death (HR, 0.90; P = .02).
Restricted cubic spline analysis demonstrated an inflection point above a 10% change in LVEF beyond which there was a steep decline in all-cause mortality with increasing degree of LVEF improvement.
There were no significant differences in rehospitalization, New York Heart Association functional class, or Kansas City Cardiomyopathy Questionnaire score at 5 years in patients with and without early LVEF improvement.
“I think what this really gets to is what is the reason behind the LV dysfunction in the first place,” said Dr. Elmariah, soon to be joining the University of California, San Francisco. “We know that TAVR cures aortic stenosis, so if the LV dysfunction is primarily related to the valve itself, hopefully those patients are going to recover.”
On the other hand, if the patient has LV dysfunction because of a prior myocardial infarction or cardiomyopathy and then developed aortic stenosis, “you can treat the aortic stenosis but the heart is still diseased from whatever process was affecting it previously and so it’s not likely to recover in those scenarios,” he added.
The results can be used for counseling patients and highlight the need to optimize goal-directed medical therapy in those with valvular heart disease, Dr. Elmariah suggested.
“Often, patients with aortic stenosis are on miniscule doses of many of the heart failure agents because people are worried about the hemodynamic consequences and they’re worried that patients won’t tolerate these medications,” he said. “But it’s very important for us to aggressively try to treat the heart failure that is affecting these patients in order to hopefully increase the chances that their left ventricles will recover and, hopefully, that they will have improved survival.”
Dr. Baron said that “this study clearly demonstrates that patients with reduced LVEF and severe aortic stenosis can benefit from TAVR and that early improvement in LVEF is an important prognostic marker for this population.”
In Dr. Baron and colleagues’ earlier analysis of 11,000 patients who underwent TAVR as part of the transcatheter valve therapy registry, only low aortic valve gradient but not LV dysfunction was associated with higher adjusted 1-year mortality. Asked about the finding, she noted that patients were evaluated based on LV function at baseline and not for a difference in outcomes based on LVEF improvement after TAVR.
“As such, I think that these two studies are actually complementary,” Dr. Baron said. “Together, they suggest that a low LVEF should not preclude a patient from receiving TAVR and if the patient does experience a 10% increase in LVEF after TAVR, then their 5-year prognosis is improved.”
Dr. Elmariah reports grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Medtronic, and Svelte Medical and has received consulting fees from Medtronic and AstraZeneca. Coauthor disclosures are listed in the paper. The PARTNER trials and registries and this analysis were supported by Edwards Lifesciences. Edwards was involved in the design and conduct of the study including collection, management, analysis, and interpretation of the data. Dr. Baron reports receiving research grant funding from Abiomed and Boston Scientific; consulting/medical advisory board fees from Boston Scientific, Shockwave and Biotronik; and speaking honoraria from Medtronic and Zoll.
A version of this article first appeared on Medscape.com.
Early improvement of left ventricular ejection fraction (LVEF) after transcatheter aortic valve replacement (TAVR) is associated with improved all-cause and cardiac death at 5 years in patients with severe aortic stenosis and LVEF less than 50%, new research shows.
Further analyses revealed a significant interaction by sex, with the mortality benefit largely in women.
“It’s absolutely fascinating,” senior author Sammy Elmariah, MD, Massachusetts General Hospital, Boston, said of the finding. “We know that women are more likely to have concentric hypertrophy, that they have lesser degrees of fibrosis, and smaller ventricles, and, of course, they’re in general less affected by coronary artery disease and MIs [myocardial infarctions]. All of those things in my mind, at least that’s what I assumed ahead of time, would make it more likely for women’s hearts to recover.”
“But that’s actually not what we found,” he continued. “We didn’t see a difference between the sexes in terms of likelihood of recovery. But what we saw is that the survival benefit, that associates with improvement in EF, was almost completely driven by women. So women really seem to be reaping that benefit in a manner that is unique and very different from what we saw in men.”
Dr. Elmariah noted that the reason for this benefit is unclear but points to the differences in biology for LV remodeling. “Clearly there are several details there that warrant further attention and more research.”
Suzanne J. Baron, MD, director of interventional cardiology research at Lahey Hospital and Medical Center, Burlington, Mass., said in an email that the finding of a substantial long-term survival benefit was “a bit surprising.”
Several studies have suggested that women may derive a greater benefit from TAVR versus surgical aortic valve replacement, and meta-analyses have demonstrated short and intermediate-term survival after TAVR is better in women, compared with in men, she pointed out. However, the mediating mechanism for this finding has never been clearly elucidated.
“Certainly, the sex differences in LVEF improvement after TAVR observed in this study, which could be related to sex differences in LV remodeling and LV mass regression, may now give us a clue as to why these sex-specific survival differences after TAVR persist,” Dr. Baron said.
More data amassed
Previous research in smaller cohorts with follow-up out to 1 year have shown an association between early LVEF improvement after TAVR and better survival. This includes a 2013 study by the investigators in high-risk patients in PARTNER-1 and a separate 2016 study in patients in the CoreValve extreme and high surgical risk trials.
Now, with longer follow-up amassed, the investigators examined data from 659 high- or intermediate-risk patients with severe stenosis and LVEF less than 50% who underwent transfemoral TAVR in the PARTNER 1, 2, and S3 trials and registries between July 2007 and April 2015.
Their mean age was 82.4 years, 71% were men, and 89.7% were White individuals. During the study period, 55.6% of the cohort died.
As reported in JAMA Cardiology, 32.8% of patients had early LVEF improvement, defined as an increase of at least 10% percentage points at 30 days after TAVR (mean change, 16.4%).
This compares with about 50%-60% of patients in the earlier studies, likely owing to the relatively higher baseline LVEF, especially in the intermediate-risk cohort, the authors suggested.
Independent predictors of lower likelihood of early LVEF improvement were previous MI, diabetes, cancer, higher baseline LVEF, larger LV end-diastolic diameter, and larger aortic valve area (AVA), whereas higher body mass index and higher stroke volume index predicted greater likelihood of LV recovery.
At 5 years, patients with versus without improved early LV improvement had lower risks of all-cause death (50.0% vs. 58.4%; P = .04) and cardiac death (29.5% vs. 38.1%; P = .05).
In multivariable analyses, each 5%-point increase in LVEF after TAVR was associated with a 6% lower risk of all-cause death (hazard ratio [HR], 0.94; P = .04) and 10% lower risk of cardiac death (HR, 0.90; P = .02).
Restricted cubic spline analysis demonstrated an inflection point above a 10% change in LVEF beyond which there was a steep decline in all-cause mortality with increasing degree of LVEF improvement.
There were no significant differences in rehospitalization, New York Heart Association functional class, or Kansas City Cardiomyopathy Questionnaire score at 5 years in patients with and without early LVEF improvement.
“I think what this really gets to is what is the reason behind the LV dysfunction in the first place,” said Dr. Elmariah, soon to be joining the University of California, San Francisco. “We know that TAVR cures aortic stenosis, so if the LV dysfunction is primarily related to the valve itself, hopefully those patients are going to recover.”
On the other hand, if the patient has LV dysfunction because of a prior myocardial infarction or cardiomyopathy and then developed aortic stenosis, “you can treat the aortic stenosis but the heart is still diseased from whatever process was affecting it previously and so it’s not likely to recover in those scenarios,” he added.
The results can be used for counseling patients and highlight the need to optimize goal-directed medical therapy in those with valvular heart disease, Dr. Elmariah suggested.
“Often, patients with aortic stenosis are on miniscule doses of many of the heart failure agents because people are worried about the hemodynamic consequences and they’re worried that patients won’t tolerate these medications,” he said. “But it’s very important for us to aggressively try to treat the heart failure that is affecting these patients in order to hopefully increase the chances that their left ventricles will recover and, hopefully, that they will have improved survival.”
Dr. Baron said that “this study clearly demonstrates that patients with reduced LVEF and severe aortic stenosis can benefit from TAVR and that early improvement in LVEF is an important prognostic marker for this population.”
In Dr. Baron and colleagues’ earlier analysis of 11,000 patients who underwent TAVR as part of the transcatheter valve therapy registry, only low aortic valve gradient but not LV dysfunction was associated with higher adjusted 1-year mortality. Asked about the finding, she noted that patients were evaluated based on LV function at baseline and not for a difference in outcomes based on LVEF improvement after TAVR.
“As such, I think that these two studies are actually complementary,” Dr. Baron said. “Together, they suggest that a low LVEF should not preclude a patient from receiving TAVR and if the patient does experience a 10% increase in LVEF after TAVR, then their 5-year prognosis is improved.”
Dr. Elmariah reports grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Medtronic, and Svelte Medical and has received consulting fees from Medtronic and AstraZeneca. Coauthor disclosures are listed in the paper. The PARTNER trials and registries and this analysis were supported by Edwards Lifesciences. Edwards was involved in the design and conduct of the study including collection, management, analysis, and interpretation of the data. Dr. Baron reports receiving research grant funding from Abiomed and Boston Scientific; consulting/medical advisory board fees from Boston Scientific, Shockwave and Biotronik; and speaking honoraria from Medtronic and Zoll.
A version of this article first appeared on Medscape.com.
Using wearable devices to detect AFib ‘cost effective’
Screening for atrial fibrillation with wearable devices is cost effective, when compared with either no screening or screening using traditional methods, a new study concludes.
“Undiagnosed atrial fibrillation (AFib) is an important cause of stroke. Screening for AFib using wrist-worn wearable devices may prevent strokes, but their cost effectiveness is unknown,” write Wanyi Chen, PhD, from Massachusetts General Hospital, Boston, and colleagues, in JAMA Health Forum.
The investigators used a microsimulation decision-analytic model to evaluate the cost effectiveness of these devices to screen for undiagnosed AFib.
The model comprised 30 million simulated individuals with an age, sex, and comorbidity profile matching the United States population aged 65 years or older.
The model looked at eight AFib screening strategies: six using wrist-worn wearable devices (either watch or band photoplethysmography with or without watch or band electrocardiography) and two using traditional modalities (that is, pulse palpation and 12-lead electrocardiogram) versus no screening.
The primary outcome was the incremental cost effectiveness ratio, defined as U.S. dollars per quality-adjusted life-year (QALY). Secondary outcomes included rates of stroke and major bleeding.
In the model, the mean age was 72.5 years and 50% were women.
All 6 screening strategies using wrist-worn wearable devices were estimated to be more cost effective than no screening. The model showed that the range of QALYs gained, compared with no screening, was 226 to 957 per 100,000 individuals.
The wrist-worn devices were also associated with greater relative benefit than screening using traditional modalities, as the range of QALYs gained, compared with no screening, was –116 to 93 per 100,000 individuals.
Compared with no screening, screening with wrist-worn wearable devices was associated with a reduction in stroke incidence by 20 to 23 per 100,000 person-years but an increase in major bleeding by 20 to 44 per 100,000 person years.
Overall, the preferred strategy for screening was wearable photoplethysmography, followed by wearable electrocardiography with patch monitor confirmation. This strategy had an incremental cost effectiveness ratio of $57,894 per QALY, “meeting the acceptability threshold of $100,000 per QALY,” the authors write.
The cost effectiveness of screening was consistent across multiple clinically relevant scenarios, including screening a general population aged 50 years or older with risk factors for stroke, the authors report.
“When deployed within specific AFib screening pathways, wearable devices are likely to be an important component of cost-effective AFib screening,” the investigators conclude.
Study based on modeled data
“This study is the first simulation of various screening strategies for atrial fibrillation using wearable devices and suggests that wearable devices, in particular wrist-worn wearables, in an elderly population, [are] estimated to be cost-effective,” Emma Svennberg, MD, PhD, from the Karolinska University Hospital, Stockholm, told this news organization.
“I find this study interesting, as the adoption of wearables amongst individuals is high and increasing, hence many wearers will screen themselves for arrhythmias (even if health care recommendations are discordant), and the potential costs for society have been unknown,” said Dr. Svennberg, who was not part of this study.
“Of course, no study is without its flaws, and here one must note that the study is based on modeled data alone and not RCTs of the wearable screening strategies ... hence true clinical outcome data is missing,” Dr. Svennberg added.
The large STROKESTOP study, on which she was the lead investigator, “presented data based on true clinical outcomes at ESC 2021 (European Society of Cardiology) and showed cost effectiveness,” Dr. Svennberg said.
The study authors report financial relationships with Bristol Myers Squibb, Fitbit, Medtronic, Pfizer, UpToDate, American Heart Association, IBM, Bayer AG, Novartis, MyoKardia, Boehringer Ingelheim, Heart Rhythm Society, Avania Consulting, Apple, Premier, the National Institutes of Health, Invitae, Blackstone Life Sciences, Flatiron, and Value Analytics Labs. Dr. Svennberg reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Screening for atrial fibrillation with wearable devices is cost effective, when compared with either no screening or screening using traditional methods, a new study concludes.
“Undiagnosed atrial fibrillation (AFib) is an important cause of stroke. Screening for AFib using wrist-worn wearable devices may prevent strokes, but their cost effectiveness is unknown,” write Wanyi Chen, PhD, from Massachusetts General Hospital, Boston, and colleagues, in JAMA Health Forum.
The investigators used a microsimulation decision-analytic model to evaluate the cost effectiveness of these devices to screen for undiagnosed AFib.
The model comprised 30 million simulated individuals with an age, sex, and comorbidity profile matching the United States population aged 65 years or older.
The model looked at eight AFib screening strategies: six using wrist-worn wearable devices (either watch or band photoplethysmography with or without watch or band electrocardiography) and two using traditional modalities (that is, pulse palpation and 12-lead electrocardiogram) versus no screening.
The primary outcome was the incremental cost effectiveness ratio, defined as U.S. dollars per quality-adjusted life-year (QALY). Secondary outcomes included rates of stroke and major bleeding.
In the model, the mean age was 72.5 years and 50% were women.
All 6 screening strategies using wrist-worn wearable devices were estimated to be more cost effective than no screening. The model showed that the range of QALYs gained, compared with no screening, was 226 to 957 per 100,000 individuals.
The wrist-worn devices were also associated with greater relative benefit than screening using traditional modalities, as the range of QALYs gained, compared with no screening, was –116 to 93 per 100,000 individuals.
Compared with no screening, screening with wrist-worn wearable devices was associated with a reduction in stroke incidence by 20 to 23 per 100,000 person-years but an increase in major bleeding by 20 to 44 per 100,000 person years.
Overall, the preferred strategy for screening was wearable photoplethysmography, followed by wearable electrocardiography with patch monitor confirmation. This strategy had an incremental cost effectiveness ratio of $57,894 per QALY, “meeting the acceptability threshold of $100,000 per QALY,” the authors write.
The cost effectiveness of screening was consistent across multiple clinically relevant scenarios, including screening a general population aged 50 years or older with risk factors for stroke, the authors report.
“When deployed within specific AFib screening pathways, wearable devices are likely to be an important component of cost-effective AFib screening,” the investigators conclude.
Study based on modeled data
“This study is the first simulation of various screening strategies for atrial fibrillation using wearable devices and suggests that wearable devices, in particular wrist-worn wearables, in an elderly population, [are] estimated to be cost-effective,” Emma Svennberg, MD, PhD, from the Karolinska University Hospital, Stockholm, told this news organization.
“I find this study interesting, as the adoption of wearables amongst individuals is high and increasing, hence many wearers will screen themselves for arrhythmias (even if health care recommendations are discordant), and the potential costs for society have been unknown,” said Dr. Svennberg, who was not part of this study.
“Of course, no study is without its flaws, and here one must note that the study is based on modeled data alone and not RCTs of the wearable screening strategies ... hence true clinical outcome data is missing,” Dr. Svennberg added.
The large STROKESTOP study, on which she was the lead investigator, “presented data based on true clinical outcomes at ESC 2021 (European Society of Cardiology) and showed cost effectiveness,” Dr. Svennberg said.
The study authors report financial relationships with Bristol Myers Squibb, Fitbit, Medtronic, Pfizer, UpToDate, American Heart Association, IBM, Bayer AG, Novartis, MyoKardia, Boehringer Ingelheim, Heart Rhythm Society, Avania Consulting, Apple, Premier, the National Institutes of Health, Invitae, Blackstone Life Sciences, Flatiron, and Value Analytics Labs. Dr. Svennberg reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Screening for atrial fibrillation with wearable devices is cost effective, when compared with either no screening or screening using traditional methods, a new study concludes.
“Undiagnosed atrial fibrillation (AFib) is an important cause of stroke. Screening for AFib using wrist-worn wearable devices may prevent strokes, but their cost effectiveness is unknown,” write Wanyi Chen, PhD, from Massachusetts General Hospital, Boston, and colleagues, in JAMA Health Forum.
The investigators used a microsimulation decision-analytic model to evaluate the cost effectiveness of these devices to screen for undiagnosed AFib.
The model comprised 30 million simulated individuals with an age, sex, and comorbidity profile matching the United States population aged 65 years or older.
The model looked at eight AFib screening strategies: six using wrist-worn wearable devices (either watch or band photoplethysmography with or without watch or band electrocardiography) and two using traditional modalities (that is, pulse palpation and 12-lead electrocardiogram) versus no screening.
The primary outcome was the incremental cost effectiveness ratio, defined as U.S. dollars per quality-adjusted life-year (QALY). Secondary outcomes included rates of stroke and major bleeding.
In the model, the mean age was 72.5 years and 50% were women.
All 6 screening strategies using wrist-worn wearable devices were estimated to be more cost effective than no screening. The model showed that the range of QALYs gained, compared with no screening, was 226 to 957 per 100,000 individuals.
The wrist-worn devices were also associated with greater relative benefit than screening using traditional modalities, as the range of QALYs gained, compared with no screening, was –116 to 93 per 100,000 individuals.
Compared with no screening, screening with wrist-worn wearable devices was associated with a reduction in stroke incidence by 20 to 23 per 100,000 person-years but an increase in major bleeding by 20 to 44 per 100,000 person years.
Overall, the preferred strategy for screening was wearable photoplethysmography, followed by wearable electrocardiography with patch monitor confirmation. This strategy had an incremental cost effectiveness ratio of $57,894 per QALY, “meeting the acceptability threshold of $100,000 per QALY,” the authors write.
The cost effectiveness of screening was consistent across multiple clinically relevant scenarios, including screening a general population aged 50 years or older with risk factors for stroke, the authors report.
“When deployed within specific AFib screening pathways, wearable devices are likely to be an important component of cost-effective AFib screening,” the investigators conclude.
Study based on modeled data
“This study is the first simulation of various screening strategies for atrial fibrillation using wearable devices and suggests that wearable devices, in particular wrist-worn wearables, in an elderly population, [are] estimated to be cost-effective,” Emma Svennberg, MD, PhD, from the Karolinska University Hospital, Stockholm, told this news organization.
“I find this study interesting, as the adoption of wearables amongst individuals is high and increasing, hence many wearers will screen themselves for arrhythmias (even if health care recommendations are discordant), and the potential costs for society have been unknown,” said Dr. Svennberg, who was not part of this study.
“Of course, no study is without its flaws, and here one must note that the study is based on modeled data alone and not RCTs of the wearable screening strategies ... hence true clinical outcome data is missing,” Dr. Svennberg added.
The large STROKESTOP study, on which she was the lead investigator, “presented data based on true clinical outcomes at ESC 2021 (European Society of Cardiology) and showed cost effectiveness,” Dr. Svennberg said.
The study authors report financial relationships with Bristol Myers Squibb, Fitbit, Medtronic, Pfizer, UpToDate, American Heart Association, IBM, Bayer AG, Novartis, MyoKardia, Boehringer Ingelheim, Heart Rhythm Society, Avania Consulting, Apple, Premier, the National Institutes of Health, Invitae, Blackstone Life Sciences, Flatiron, and Value Analytics Labs. Dr. Svennberg reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.