User login
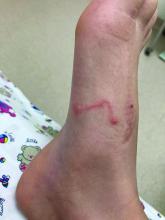
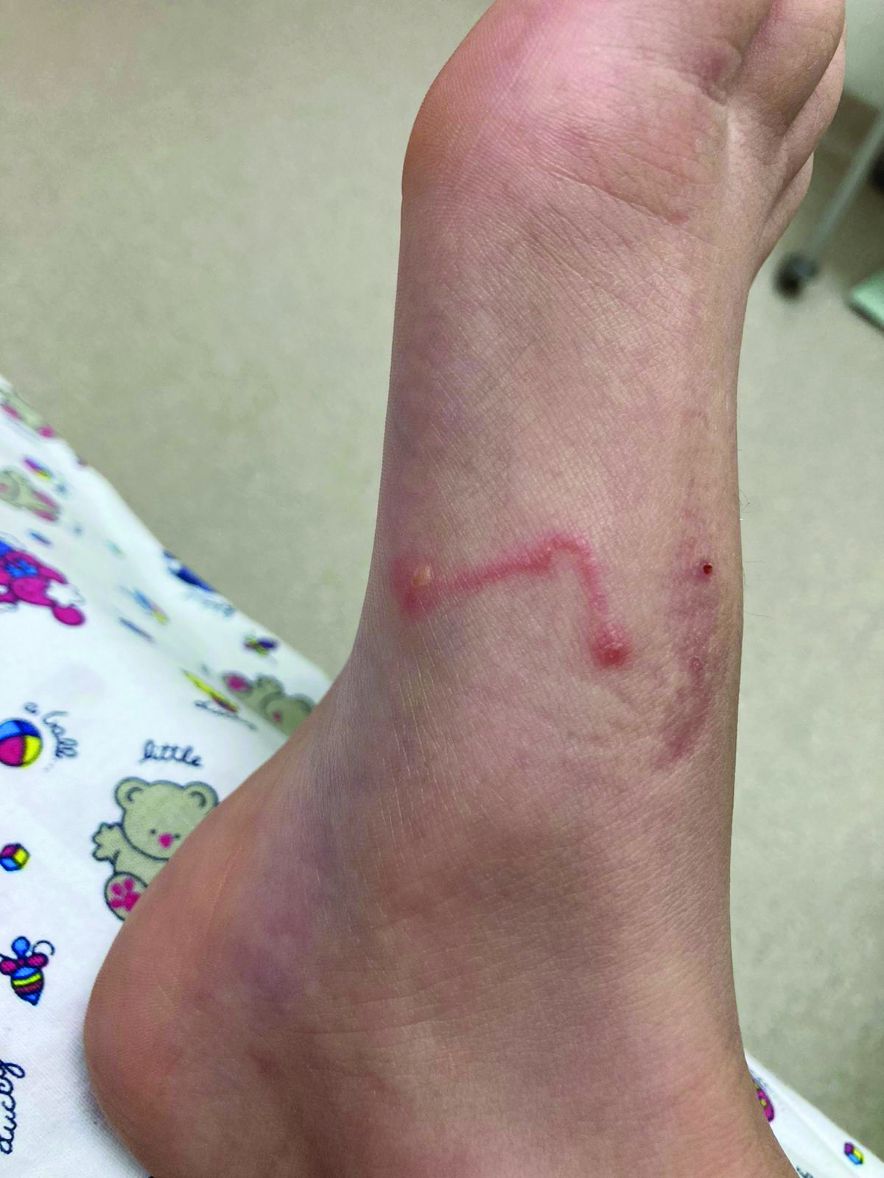
A 9-year-old girl was evaluated for a week-long history of rash on the feet
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
Her mother reported recent travel to a beachside city in Colombia. A review of systems was negative. She was not taking any other medications or vitamin supplements. There were no pets at home and no other affected family members. Physical exam was notable for an erythematous curvilinear plaque on the feet and a small vesicle.
Ultrasound on par with CT for evaluating sarcopenia in patients with cirrhosis
Using ultrasound (US) to evaluate sarcopenic obesity in patients with cirrhosis may offer accuracy on par with computed tomography (CT), according to investigators.
US-based assessment presents a more affordable point-of-care strategy that limits radiation exposure, which enables sequential monitoring, reported lead author Sukhpal Dhariwal, MBBS, MD, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India.
“Preliminary data in patients with liver disease ... suggest that US muscle assessment–derived indices, especially thigh muscle thickness, identify sarcopenia CT-skeletal muscle index (SMI) and also predict hospitalization and mortality,” the investigators wrote in Journal of Clinical Gastroenterology. “However, the applicability of US-based techniques to measure muscle mass in the high-risk group of patients with cirrhosis and sarcopenic obesity has not been evaluated.”
To address this knowledge gap, the investigators performed both US- and CT-based muscle assessments in 52 patients with obesity and evidence of cirrhosis; 40 patients were male and the mean age was 50.9 years. In all, 20 (38.5%) were diagnosed with sarcopenia based on CT-determined SMI scores of less than 39 cm2/m2 for women and 50 cm2/m2 for men.
US showed that it was similarly capable of categorizing patients. The modality significantly differentiated individuals with or without sarcopenia based on high area under the curve values in four muscle indices: quadriceps muscle thickness (0.98), quadriceps muscle feather index (0.95), forearm muscle thickness (0.85), and forearm feather index (0.80).
Direct comparison of US-based assessment against CT-based SMI revealed positive correlations, with significant r values ranging from 0.40 to 0.58. These correlations were stronger in a male-only subgroup analysis, in which r values ranged from 0.52 to 0.70. R values were not calculated in the female subgroup because of the small sample size (n = 12).
The investigators adjusted indices for height, which may pose bias for overestimating muscle mass. Another limitation is the small sample size.
“US-based assessment of sarcopenia has excellent diagnostic accuracy and correlates highly with cross-sectional imaging-based SMI in cirrhosis patients with sarcopenic obesity,” the investigators concluded. “US may serve as an easy-to-use, point-of-care tool for assessing sarcopenia in sarcopenic obesity with the advantage of repeated sequential assessment.”
According to Jamile Wakim-Fleming, MD, of the Cleveland Clinic, “US-based muscle mass assessment seems to be reliable, reproducible, and simple to perform and should be encouraged along with nutrition assessments in all patients with cirrhosis and obesity.”
In a written comment, Dr. Wakim-Fleming noted the importance of timely monitoring and intervention in this patient population.
“Considering the morbidity and the poor outcomes associated with sarcopenic obesity and its frequency in cirrhosis, it is important to make early diagnosis and institute a management plan to improve muscle mass and function,” she said.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Wakim-Fleming reported no relevant conflicts of interest.
Using ultrasound (US) to evaluate sarcopenic obesity in patients with cirrhosis may offer accuracy on par with computed tomography (CT), according to investigators.
US-based assessment presents a more affordable point-of-care strategy that limits radiation exposure, which enables sequential monitoring, reported lead author Sukhpal Dhariwal, MBBS, MD, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India.
“Preliminary data in patients with liver disease ... suggest that US muscle assessment–derived indices, especially thigh muscle thickness, identify sarcopenia CT-skeletal muscle index (SMI) and also predict hospitalization and mortality,” the investigators wrote in Journal of Clinical Gastroenterology. “However, the applicability of US-based techniques to measure muscle mass in the high-risk group of patients with cirrhosis and sarcopenic obesity has not been evaluated.”
To address this knowledge gap, the investigators performed both US- and CT-based muscle assessments in 52 patients with obesity and evidence of cirrhosis; 40 patients were male and the mean age was 50.9 years. In all, 20 (38.5%) were diagnosed with sarcopenia based on CT-determined SMI scores of less than 39 cm2/m2 for women and 50 cm2/m2 for men.
US showed that it was similarly capable of categorizing patients. The modality significantly differentiated individuals with or without sarcopenia based on high area under the curve values in four muscle indices: quadriceps muscle thickness (0.98), quadriceps muscle feather index (0.95), forearm muscle thickness (0.85), and forearm feather index (0.80).
Direct comparison of US-based assessment against CT-based SMI revealed positive correlations, with significant r values ranging from 0.40 to 0.58. These correlations were stronger in a male-only subgroup analysis, in which r values ranged from 0.52 to 0.70. R values were not calculated in the female subgroup because of the small sample size (n = 12).
The investigators adjusted indices for height, which may pose bias for overestimating muscle mass. Another limitation is the small sample size.
“US-based assessment of sarcopenia has excellent diagnostic accuracy and correlates highly with cross-sectional imaging-based SMI in cirrhosis patients with sarcopenic obesity,” the investigators concluded. “US may serve as an easy-to-use, point-of-care tool for assessing sarcopenia in sarcopenic obesity with the advantage of repeated sequential assessment.”
According to Jamile Wakim-Fleming, MD, of the Cleveland Clinic, “US-based muscle mass assessment seems to be reliable, reproducible, and simple to perform and should be encouraged along with nutrition assessments in all patients with cirrhosis and obesity.”
In a written comment, Dr. Wakim-Fleming noted the importance of timely monitoring and intervention in this patient population.
“Considering the morbidity and the poor outcomes associated with sarcopenic obesity and its frequency in cirrhosis, it is important to make early diagnosis and institute a management plan to improve muscle mass and function,” she said.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Wakim-Fleming reported no relevant conflicts of interest.
Using ultrasound (US) to evaluate sarcopenic obesity in patients with cirrhosis may offer accuracy on par with computed tomography (CT), according to investigators.
US-based assessment presents a more affordable point-of-care strategy that limits radiation exposure, which enables sequential monitoring, reported lead author Sukhpal Dhariwal, MBBS, MD, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India.
“Preliminary data in patients with liver disease ... suggest that US muscle assessment–derived indices, especially thigh muscle thickness, identify sarcopenia CT-skeletal muscle index (SMI) and also predict hospitalization and mortality,” the investigators wrote in Journal of Clinical Gastroenterology. “However, the applicability of US-based techniques to measure muscle mass in the high-risk group of patients with cirrhosis and sarcopenic obesity has not been evaluated.”
To address this knowledge gap, the investigators performed both US- and CT-based muscle assessments in 52 patients with obesity and evidence of cirrhosis; 40 patients were male and the mean age was 50.9 years. In all, 20 (38.5%) were diagnosed with sarcopenia based on CT-determined SMI scores of less than 39 cm2/m2 for women and 50 cm2/m2 for men.
US showed that it was similarly capable of categorizing patients. The modality significantly differentiated individuals with or without sarcopenia based on high area under the curve values in four muscle indices: quadriceps muscle thickness (0.98), quadriceps muscle feather index (0.95), forearm muscle thickness (0.85), and forearm feather index (0.80).
Direct comparison of US-based assessment against CT-based SMI revealed positive correlations, with significant r values ranging from 0.40 to 0.58. These correlations were stronger in a male-only subgroup analysis, in which r values ranged from 0.52 to 0.70. R values were not calculated in the female subgroup because of the small sample size (n = 12).
The investigators adjusted indices for height, which may pose bias for overestimating muscle mass. Another limitation is the small sample size.
“US-based assessment of sarcopenia has excellent diagnostic accuracy and correlates highly with cross-sectional imaging-based SMI in cirrhosis patients with sarcopenic obesity,” the investigators concluded. “US may serve as an easy-to-use, point-of-care tool for assessing sarcopenia in sarcopenic obesity with the advantage of repeated sequential assessment.”
According to Jamile Wakim-Fleming, MD, of the Cleveland Clinic, “US-based muscle mass assessment seems to be reliable, reproducible, and simple to perform and should be encouraged along with nutrition assessments in all patients with cirrhosis and obesity.”
In a written comment, Dr. Wakim-Fleming noted the importance of timely monitoring and intervention in this patient population.
“Considering the morbidity and the poor outcomes associated with sarcopenic obesity and its frequency in cirrhosis, it is important to make early diagnosis and institute a management plan to improve muscle mass and function,” she said.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Wakim-Fleming reported no relevant conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Phase 3 data: Zanubrutinib bests standard CLL treatment
At a median follow-up of 26.2 months, progression to worsening disease or death was much lower in patients with these conditions who took zanubrutinib (Brukinsa), compared with those who took bendamustine-rituximab (hazard ratio. 0.42; 95% confidence interval, 0.28-0.63; P < .00011). The study was published in The Lancet Oncology.
Researchers already knew that ibrutinib, another BTKi, improves progression-free survival, study coauthor Paolo Ghia, MD, PhD, professor of medical oncology at Vita-Salute San Raffaele University, Milan, said in an interview. “Now we confirmed that the same advantage can be seen” in zanubrutinib.
According to Dr. Ghia, bendamustine-rituximab has long been a standard treatment in blood cancers and is considered well tolerated and inexpensive. But BTKis such as first-in-line ibrutinib have shown better results, he said, “and progressively, we are going to abandon bendamustine-rituximab.”
However, ibrutinib causes significant adverse effects such as bleeding, worsening hypertension and arrhythmia, he noted. As a result, second-generation BTKi such as zanubrutinib have entered the picture. The Food and Drug Administration approved it in 2019 for mantle cell lymphoma, and it has since been approved for Waldenström’s macroglobulinemia and marginal zone lymphoma.
In 2021, an interim analysis in a trial of the drug in patients with previously treated CLL, compared with ibrutinib, found that “zanubrutinib was shown to have a superior response rate, an improved PFS, and a lower rate of atrial fibrillation/flutter.”
The drug’s manufacturer, BeiGene, launched the new open-label, multicenter study, in a bid for FDA approval of the drug as a frontline treatment for CLL and SLL. More than 150 hospitals in 14 countries participated in the trial from 2017 to 2019.
The subjects were all adults and at least 65 years old or with comorbidities; None had the genetic trait del(17)(p13.1); 241 were assigned to take zanubrutinib and 238 to bendamustine-rituximab. Another group consisted of 111 patients with CLL and del(17)(p13·1). According to the study authors, these patients are especially difficult to treat.
The vast majority of patients were White (92%-95% depending on group) and male (61%-71%); 90%-92% had CLL.
At follow-up, there was no difference in overall survival between the main zanubrutinib and bendamustine-rituximab groups; 29 (12%) of the 241 patients in the zanubrutinib group and 57 (24%) of 238 patients in the bendamustine-rituximab group had progressed or died (HR, 0.42; 95% CI, 0.27-0.66; P < .00011). Adverse events leading to discontinuation were more common in the bendamustine-rituximab group (14%) versus zanubrutinib (8%).
In the third group, which only received zanubrutinib, 14% of patients died at median follow-up of 30.5 months; 98% of patients had adverse effects, and 5% discontinued treatment.
The researchers wrote that “zanubrutinib showed superior progression-free survival versus bendamustine-rituximab in older patients or those with comorbidities with untreated CLL, with a low incidence of cardiac arrhythmia. Similar efficacy was observed in patients with del(17p)–positive disease.”
The study didn’t examine cost; zanubrutinib is quite expensive.
In an interview, hematologist-oncologist Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center in New York said the new study is important although not surprising, since other medications in the same class have shown similar results. Zanubrutinib is an alternative to ibrutinib, although the latter remains “an excellent drug,” he said.
“The era of chemotherapy being a first choice is over,” he said. “We’ve had several randomized studies that show targeted therapies are better tolerated and have better outcomes. We now need to look through the choices to decide which one of these good options are the best for our patients.”
In an interview, hematologist-oncologist Joanna Rhodes, MD, of Northwell Health in Hempstead, N.Y., highlighted the side effect profile of zanubrutinib, noting that it is low and resembles that of other BTKis, making it “another excellent treatment option.”
“We are seeing that bruising, upper respiratory tract infections, diarrhea, and arthralgias are the most common side effects,” she said. “Bleeding also is a common side effect, which is consistent across the class of BTKis, with 5% of patients developing a major bleed. Also, 3% of patients treated with zanubrutinib developed atrial fibrillation, which is consistent with data from other trials. Treatment discontinuation rates were low (8%).”
The study was funded by BeiGene. The authors reported multiple disclosures. Dr. Mato reported research or consulting relationships with BeiGene, AstraZeneca, and AbbVie. Dr. Rhodes reported multiple research or consulting relationships with Abbvie, BeiGene, Genentech, and others.
At a median follow-up of 26.2 months, progression to worsening disease or death was much lower in patients with these conditions who took zanubrutinib (Brukinsa), compared with those who took bendamustine-rituximab (hazard ratio. 0.42; 95% confidence interval, 0.28-0.63; P < .00011). The study was published in The Lancet Oncology.
Researchers already knew that ibrutinib, another BTKi, improves progression-free survival, study coauthor Paolo Ghia, MD, PhD, professor of medical oncology at Vita-Salute San Raffaele University, Milan, said in an interview. “Now we confirmed that the same advantage can be seen” in zanubrutinib.
According to Dr. Ghia, bendamustine-rituximab has long been a standard treatment in blood cancers and is considered well tolerated and inexpensive. But BTKis such as first-in-line ibrutinib have shown better results, he said, “and progressively, we are going to abandon bendamustine-rituximab.”
However, ibrutinib causes significant adverse effects such as bleeding, worsening hypertension and arrhythmia, he noted. As a result, second-generation BTKi such as zanubrutinib have entered the picture. The Food and Drug Administration approved it in 2019 for mantle cell lymphoma, and it has since been approved for Waldenström’s macroglobulinemia and marginal zone lymphoma.
In 2021, an interim analysis in a trial of the drug in patients with previously treated CLL, compared with ibrutinib, found that “zanubrutinib was shown to have a superior response rate, an improved PFS, and a lower rate of atrial fibrillation/flutter.”
The drug’s manufacturer, BeiGene, launched the new open-label, multicenter study, in a bid for FDA approval of the drug as a frontline treatment for CLL and SLL. More than 150 hospitals in 14 countries participated in the trial from 2017 to 2019.
The subjects were all adults and at least 65 years old or with comorbidities; None had the genetic trait del(17)(p13.1); 241 were assigned to take zanubrutinib and 238 to bendamustine-rituximab. Another group consisted of 111 patients with CLL and del(17)(p13·1). According to the study authors, these patients are especially difficult to treat.
The vast majority of patients were White (92%-95% depending on group) and male (61%-71%); 90%-92% had CLL.
At follow-up, there was no difference in overall survival between the main zanubrutinib and bendamustine-rituximab groups; 29 (12%) of the 241 patients in the zanubrutinib group and 57 (24%) of 238 patients in the bendamustine-rituximab group had progressed or died (HR, 0.42; 95% CI, 0.27-0.66; P < .00011). Adverse events leading to discontinuation were more common in the bendamustine-rituximab group (14%) versus zanubrutinib (8%).
In the third group, which only received zanubrutinib, 14% of patients died at median follow-up of 30.5 months; 98% of patients had adverse effects, and 5% discontinued treatment.
The researchers wrote that “zanubrutinib showed superior progression-free survival versus bendamustine-rituximab in older patients or those with comorbidities with untreated CLL, with a low incidence of cardiac arrhythmia. Similar efficacy was observed in patients with del(17p)–positive disease.”
The study didn’t examine cost; zanubrutinib is quite expensive.
In an interview, hematologist-oncologist Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center in New York said the new study is important although not surprising, since other medications in the same class have shown similar results. Zanubrutinib is an alternative to ibrutinib, although the latter remains “an excellent drug,” he said.
“The era of chemotherapy being a first choice is over,” he said. “We’ve had several randomized studies that show targeted therapies are better tolerated and have better outcomes. We now need to look through the choices to decide which one of these good options are the best for our patients.”
In an interview, hematologist-oncologist Joanna Rhodes, MD, of Northwell Health in Hempstead, N.Y., highlighted the side effect profile of zanubrutinib, noting that it is low and resembles that of other BTKis, making it “another excellent treatment option.”
“We are seeing that bruising, upper respiratory tract infections, diarrhea, and arthralgias are the most common side effects,” she said. “Bleeding also is a common side effect, which is consistent across the class of BTKis, with 5% of patients developing a major bleed. Also, 3% of patients treated with zanubrutinib developed atrial fibrillation, which is consistent with data from other trials. Treatment discontinuation rates were low (8%).”
The study was funded by BeiGene. The authors reported multiple disclosures. Dr. Mato reported research or consulting relationships with BeiGene, AstraZeneca, and AbbVie. Dr. Rhodes reported multiple research or consulting relationships with Abbvie, BeiGene, Genentech, and others.
At a median follow-up of 26.2 months, progression to worsening disease or death was much lower in patients with these conditions who took zanubrutinib (Brukinsa), compared with those who took bendamustine-rituximab (hazard ratio. 0.42; 95% confidence interval, 0.28-0.63; P < .00011). The study was published in The Lancet Oncology.
Researchers already knew that ibrutinib, another BTKi, improves progression-free survival, study coauthor Paolo Ghia, MD, PhD, professor of medical oncology at Vita-Salute San Raffaele University, Milan, said in an interview. “Now we confirmed that the same advantage can be seen” in zanubrutinib.
According to Dr. Ghia, bendamustine-rituximab has long been a standard treatment in blood cancers and is considered well tolerated and inexpensive. But BTKis such as first-in-line ibrutinib have shown better results, he said, “and progressively, we are going to abandon bendamustine-rituximab.”
However, ibrutinib causes significant adverse effects such as bleeding, worsening hypertension and arrhythmia, he noted. As a result, second-generation BTKi such as zanubrutinib have entered the picture. The Food and Drug Administration approved it in 2019 for mantle cell lymphoma, and it has since been approved for Waldenström’s macroglobulinemia and marginal zone lymphoma.
In 2021, an interim analysis in a trial of the drug in patients with previously treated CLL, compared with ibrutinib, found that “zanubrutinib was shown to have a superior response rate, an improved PFS, and a lower rate of atrial fibrillation/flutter.”
The drug’s manufacturer, BeiGene, launched the new open-label, multicenter study, in a bid for FDA approval of the drug as a frontline treatment for CLL and SLL. More than 150 hospitals in 14 countries participated in the trial from 2017 to 2019.
The subjects were all adults and at least 65 years old or with comorbidities; None had the genetic trait del(17)(p13.1); 241 were assigned to take zanubrutinib and 238 to bendamustine-rituximab. Another group consisted of 111 patients with CLL and del(17)(p13·1). According to the study authors, these patients are especially difficult to treat.
The vast majority of patients were White (92%-95% depending on group) and male (61%-71%); 90%-92% had CLL.
At follow-up, there was no difference in overall survival between the main zanubrutinib and bendamustine-rituximab groups; 29 (12%) of the 241 patients in the zanubrutinib group and 57 (24%) of 238 patients in the bendamustine-rituximab group had progressed or died (HR, 0.42; 95% CI, 0.27-0.66; P < .00011). Adverse events leading to discontinuation were more common in the bendamustine-rituximab group (14%) versus zanubrutinib (8%).
In the third group, which only received zanubrutinib, 14% of patients died at median follow-up of 30.5 months; 98% of patients had adverse effects, and 5% discontinued treatment.
The researchers wrote that “zanubrutinib showed superior progression-free survival versus bendamustine-rituximab in older patients or those with comorbidities with untreated CLL, with a low incidence of cardiac arrhythmia. Similar efficacy was observed in patients with del(17p)–positive disease.”
The study didn’t examine cost; zanubrutinib is quite expensive.
In an interview, hematologist-oncologist Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center in New York said the new study is important although not surprising, since other medications in the same class have shown similar results. Zanubrutinib is an alternative to ibrutinib, although the latter remains “an excellent drug,” he said.
“The era of chemotherapy being a first choice is over,” he said. “We’ve had several randomized studies that show targeted therapies are better tolerated and have better outcomes. We now need to look through the choices to decide which one of these good options are the best for our patients.”
In an interview, hematologist-oncologist Joanna Rhodes, MD, of Northwell Health in Hempstead, N.Y., highlighted the side effect profile of zanubrutinib, noting that it is low and resembles that of other BTKis, making it “another excellent treatment option.”
“We are seeing that bruising, upper respiratory tract infections, diarrhea, and arthralgias are the most common side effects,” she said. “Bleeding also is a common side effect, which is consistent across the class of BTKis, with 5% of patients developing a major bleed. Also, 3% of patients treated with zanubrutinib developed atrial fibrillation, which is consistent with data from other trials. Treatment discontinuation rates were low (8%).”
The study was funded by BeiGene. The authors reported multiple disclosures. Dr. Mato reported research or consulting relationships with BeiGene, AstraZeneca, and AbbVie. Dr. Rhodes reported multiple research or consulting relationships with Abbvie, BeiGene, Genentech, and others.
FROM THE LANCET ONCOLOGY
Guidelines: Convalescent plasma not recommended for most hospitalized with COVID
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Blood pressure smartphone app fails to beat standard self-monitoring
Here’s another vote for less screen time.
“By itself, standard self-measured blood pressure (SMBP) has minimal effect on BP control,” wrote lead author Mark J. Pletcher, MD, of the University of California, San Francisco, and colleagues in JAMA Internal Medicine. “To improve BP control, SMBP must be accompanied by patient feedback, counseling, or other cointerventions, and the BP-lowering effects of SMBP appear to be proportional to the intensity of the cointervention.”
While this is known, higher-intensity cointerventions demand both money and time, prompting development of new devices that link with smartphone apps, they continued.
In the prospective randomized trial, patients with hypertension were randomly assigned to self-measure their blood pressure using a standard device that paired with a connected smartphone application or to self-measure their blood pressure with a standard device alone. Both groups achieved about an 11 mm Hg reduction in systolic BP over 6 months, reported similar levels of satisfaction with the monitoring process, and shared their readings with their physicians with similar frequency.
Methods
Dr. Pletcher and colleagues enrolled 2,101 adults who self-reported a systolic BP greater than 145 mm Hg and expressed a commitment to reduce their BP by at least 10 points in their trial. The participants, who were generally middle-aged or older, were randomized in a 1:1 ratio to monitor their BP using standard SMBP or “enhanced” SMBP. The standard group used the OMRON BP monitor alone, while the enhanced group used the same BP monitor coupled with the OMRON Connect smartphone app.
After 6 months of follow-up for each patient, mean BP reduction from baseline in the standard group was 10.6 mm Hg, compared with 10.7 mm Hg in the enhanced group, a nonsignificant difference (P = .81). While slightly more patients in the enhanced group achieved a BP lower than 140/90 mm Hg (32% vs. 29%; odds ratio, 1.17; 95% confidence interval, 1.01-1.34), this trend did not extend below the 130/80 mm Hg threshold.
Other secondary outcomes were also similar between groups. For example, 70% of participants in the enhanced group said they would recommend their SMBP process to a friend, compared with 69% of participants who followed the standard monitoring approach. The smartphone app had little impact on sharing readings with physicians, either, based on a 44% share rate in the enhanced group versus 48% in the standard group (P = .22).
“Enhanced SMBP does not provide any additional reduction in BP,” the investigators concluded.
New devices that link with smartphone apps, like the one used in this trial, “transmit BP measurements via wireless connection to the patient’s smartphone, where they are processed in a smartphone application to support tracking, visualization, interpretation, reminders to measure BP and/or take medications; recommendations for lifestyle interventions, medication adherence, or to discuss their BP with their clinician; and communications (for example, emailing a summary to a family member or clinician),” the researchers explained. While these devices are “only slightly more expensive than standard SMBP devices,” their relative efficacy over standard SMBP is “unclear.”
Findings can likely be extrapolated to other apps
Although the trial evaluated just one smartphone app, Dr. Pletcher suggested that the findings can likely be extrapolated to other apps.
“Most basic BP-tracking apps have some version or subset of the same essential functionality,” he said, in an interview. “My guess is that apps that meet this description without some substantially different technology or feature would likely show the same basic results as we did.”
Making a similar remark, Matthew Jung, MD, of Keck Medicine of USC, Los Angeles, stated that the findings can be “reasonably extrapolated” to other BP-tracking apps with similar functionality “if we put aside the study’s issues with power.”
When it comes to smartphone apps, active engagement is needed to achieve greater impacts on blood pressure, Dr. Pletcher said, but “there is so much competition for people’s attention on their phone that it is hard to maintain active engagement with any health-related app for long.”
Still, Dr. Pletcher hasn’t given up on biometric apps, noting that “with the right technology and connectivity and user experience (for both patient and clinician), they still could be game-changing for managing chronic conditions like hypertension.”
To this end, he and his colleagues are exploring technologies to passively monitor health-related measurements like BP, potentially sidestepping the pitfall of active engagement.
Dr. Jung said the study is noteworthy for several reasons, including its large size, similar level of comfort with technology reported by both groups, and representation of Black and Hispanic participants, who accounted for almost one-third of the population.
Study limitations
Dr. Jung pointed out several study limitations, including the lack of standardized measurement of BP, which left more than one-third of patients unevaluated via chart review, as well as gaps in usage data, such as that one-third of the participants never confirmed receipt of a device, and less than half of the enhanced group reported using the smartphone application.
These limitations “may have detracted from its ability to identify the true efficacy of an enhanced app-based BP tracking device,” he said. “In contrast, each of these issues also helped us get a better picture for how well these devices may work in the real world.”
Dr. Jung also commented on the duration of the study, noting that only 10 weeks passed, on average, from baseline to follow-up BP measurement, which “may not have been sufficient for a possible difference between enhanced and standard BP monitoring to become noticeable.”
“This may be especially important when taking into consideration the time required to mail the devices out to patients, for patients to become familiar with usage of the devices, and for them to start using the devices in a meaningful way,” he added.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Jung, who was not involved in the study, disclosed no relevant conflicts of interest.
Here’s another vote for less screen time.
“By itself, standard self-measured blood pressure (SMBP) has minimal effect on BP control,” wrote lead author Mark J. Pletcher, MD, of the University of California, San Francisco, and colleagues in JAMA Internal Medicine. “To improve BP control, SMBP must be accompanied by patient feedback, counseling, or other cointerventions, and the BP-lowering effects of SMBP appear to be proportional to the intensity of the cointervention.”
While this is known, higher-intensity cointerventions demand both money and time, prompting development of new devices that link with smartphone apps, they continued.
In the prospective randomized trial, patients with hypertension were randomly assigned to self-measure their blood pressure using a standard device that paired with a connected smartphone application or to self-measure their blood pressure with a standard device alone. Both groups achieved about an 11 mm Hg reduction in systolic BP over 6 months, reported similar levels of satisfaction with the monitoring process, and shared their readings with their physicians with similar frequency.
Methods
Dr. Pletcher and colleagues enrolled 2,101 adults who self-reported a systolic BP greater than 145 mm Hg and expressed a commitment to reduce their BP by at least 10 points in their trial. The participants, who were generally middle-aged or older, were randomized in a 1:1 ratio to monitor their BP using standard SMBP or “enhanced” SMBP. The standard group used the OMRON BP monitor alone, while the enhanced group used the same BP monitor coupled with the OMRON Connect smartphone app.
After 6 months of follow-up for each patient, mean BP reduction from baseline in the standard group was 10.6 mm Hg, compared with 10.7 mm Hg in the enhanced group, a nonsignificant difference (P = .81). While slightly more patients in the enhanced group achieved a BP lower than 140/90 mm Hg (32% vs. 29%; odds ratio, 1.17; 95% confidence interval, 1.01-1.34), this trend did not extend below the 130/80 mm Hg threshold.
Other secondary outcomes were also similar between groups. For example, 70% of participants in the enhanced group said they would recommend their SMBP process to a friend, compared with 69% of participants who followed the standard monitoring approach. The smartphone app had little impact on sharing readings with physicians, either, based on a 44% share rate in the enhanced group versus 48% in the standard group (P = .22).
“Enhanced SMBP does not provide any additional reduction in BP,” the investigators concluded.
New devices that link with smartphone apps, like the one used in this trial, “transmit BP measurements via wireless connection to the patient’s smartphone, where they are processed in a smartphone application to support tracking, visualization, interpretation, reminders to measure BP and/or take medications; recommendations for lifestyle interventions, medication adherence, or to discuss their BP with their clinician; and communications (for example, emailing a summary to a family member or clinician),” the researchers explained. While these devices are “only slightly more expensive than standard SMBP devices,” their relative efficacy over standard SMBP is “unclear.”
Findings can likely be extrapolated to other apps
Although the trial evaluated just one smartphone app, Dr. Pletcher suggested that the findings can likely be extrapolated to other apps.
“Most basic BP-tracking apps have some version or subset of the same essential functionality,” he said, in an interview. “My guess is that apps that meet this description without some substantially different technology or feature would likely show the same basic results as we did.”
Making a similar remark, Matthew Jung, MD, of Keck Medicine of USC, Los Angeles, stated that the findings can be “reasonably extrapolated” to other BP-tracking apps with similar functionality “if we put aside the study’s issues with power.”
When it comes to smartphone apps, active engagement is needed to achieve greater impacts on blood pressure, Dr. Pletcher said, but “there is so much competition for people’s attention on their phone that it is hard to maintain active engagement with any health-related app for long.”
Still, Dr. Pletcher hasn’t given up on biometric apps, noting that “with the right technology and connectivity and user experience (for both patient and clinician), they still could be game-changing for managing chronic conditions like hypertension.”
To this end, he and his colleagues are exploring technologies to passively monitor health-related measurements like BP, potentially sidestepping the pitfall of active engagement.
Dr. Jung said the study is noteworthy for several reasons, including its large size, similar level of comfort with technology reported by both groups, and representation of Black and Hispanic participants, who accounted for almost one-third of the population.
Study limitations
Dr. Jung pointed out several study limitations, including the lack of standardized measurement of BP, which left more than one-third of patients unevaluated via chart review, as well as gaps in usage data, such as that one-third of the participants never confirmed receipt of a device, and less than half of the enhanced group reported using the smartphone application.
These limitations “may have detracted from its ability to identify the true efficacy of an enhanced app-based BP tracking device,” he said. “In contrast, each of these issues also helped us get a better picture for how well these devices may work in the real world.”
Dr. Jung also commented on the duration of the study, noting that only 10 weeks passed, on average, from baseline to follow-up BP measurement, which “may not have been sufficient for a possible difference between enhanced and standard BP monitoring to become noticeable.”
“This may be especially important when taking into consideration the time required to mail the devices out to patients, for patients to become familiar with usage of the devices, and for them to start using the devices in a meaningful way,” he added.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Jung, who was not involved in the study, disclosed no relevant conflicts of interest.
Here’s another vote for less screen time.
“By itself, standard self-measured blood pressure (SMBP) has minimal effect on BP control,” wrote lead author Mark J. Pletcher, MD, of the University of California, San Francisco, and colleagues in JAMA Internal Medicine. “To improve BP control, SMBP must be accompanied by patient feedback, counseling, or other cointerventions, and the BP-lowering effects of SMBP appear to be proportional to the intensity of the cointervention.”
While this is known, higher-intensity cointerventions demand both money and time, prompting development of new devices that link with smartphone apps, they continued.
In the prospective randomized trial, patients with hypertension were randomly assigned to self-measure their blood pressure using a standard device that paired with a connected smartphone application or to self-measure their blood pressure with a standard device alone. Both groups achieved about an 11 mm Hg reduction in systolic BP over 6 months, reported similar levels of satisfaction with the monitoring process, and shared their readings with their physicians with similar frequency.
Methods
Dr. Pletcher and colleagues enrolled 2,101 adults who self-reported a systolic BP greater than 145 mm Hg and expressed a commitment to reduce their BP by at least 10 points in their trial. The participants, who were generally middle-aged or older, were randomized in a 1:1 ratio to monitor their BP using standard SMBP or “enhanced” SMBP. The standard group used the OMRON BP monitor alone, while the enhanced group used the same BP monitor coupled with the OMRON Connect smartphone app.
After 6 months of follow-up for each patient, mean BP reduction from baseline in the standard group was 10.6 mm Hg, compared with 10.7 mm Hg in the enhanced group, a nonsignificant difference (P = .81). While slightly more patients in the enhanced group achieved a BP lower than 140/90 mm Hg (32% vs. 29%; odds ratio, 1.17; 95% confidence interval, 1.01-1.34), this trend did not extend below the 130/80 mm Hg threshold.
Other secondary outcomes were also similar between groups. For example, 70% of participants in the enhanced group said they would recommend their SMBP process to a friend, compared with 69% of participants who followed the standard monitoring approach. The smartphone app had little impact on sharing readings with physicians, either, based on a 44% share rate in the enhanced group versus 48% in the standard group (P = .22).
“Enhanced SMBP does not provide any additional reduction in BP,” the investigators concluded.
New devices that link with smartphone apps, like the one used in this trial, “transmit BP measurements via wireless connection to the patient’s smartphone, where they are processed in a smartphone application to support tracking, visualization, interpretation, reminders to measure BP and/or take medications; recommendations for lifestyle interventions, medication adherence, or to discuss their BP with their clinician; and communications (for example, emailing a summary to a family member or clinician),” the researchers explained. While these devices are “only slightly more expensive than standard SMBP devices,” their relative efficacy over standard SMBP is “unclear.”
Findings can likely be extrapolated to other apps
Although the trial evaluated just one smartphone app, Dr. Pletcher suggested that the findings can likely be extrapolated to other apps.
“Most basic BP-tracking apps have some version or subset of the same essential functionality,” he said, in an interview. “My guess is that apps that meet this description without some substantially different technology or feature would likely show the same basic results as we did.”
Making a similar remark, Matthew Jung, MD, of Keck Medicine of USC, Los Angeles, stated that the findings can be “reasonably extrapolated” to other BP-tracking apps with similar functionality “if we put aside the study’s issues with power.”
When it comes to smartphone apps, active engagement is needed to achieve greater impacts on blood pressure, Dr. Pletcher said, but “there is so much competition for people’s attention on their phone that it is hard to maintain active engagement with any health-related app for long.”
Still, Dr. Pletcher hasn’t given up on biometric apps, noting that “with the right technology and connectivity and user experience (for both patient and clinician), they still could be game-changing for managing chronic conditions like hypertension.”
To this end, he and his colleagues are exploring technologies to passively monitor health-related measurements like BP, potentially sidestepping the pitfall of active engagement.
Dr. Jung said the study is noteworthy for several reasons, including its large size, similar level of comfort with technology reported by both groups, and representation of Black and Hispanic participants, who accounted for almost one-third of the population.
Study limitations
Dr. Jung pointed out several study limitations, including the lack of standardized measurement of BP, which left more than one-third of patients unevaluated via chart review, as well as gaps in usage data, such as that one-third of the participants never confirmed receipt of a device, and less than half of the enhanced group reported using the smartphone application.
These limitations “may have detracted from its ability to identify the true efficacy of an enhanced app-based BP tracking device,” he said. “In contrast, each of these issues also helped us get a better picture for how well these devices may work in the real world.”
Dr. Jung also commented on the duration of the study, noting that only 10 weeks passed, on average, from baseline to follow-up BP measurement, which “may not have been sufficient for a possible difference between enhanced and standard BP monitoring to become noticeable.”
“This may be especially important when taking into consideration the time required to mail the devices out to patients, for patients to become familiar with usage of the devices, and for them to start using the devices in a meaningful way,” he added.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Jung, who was not involved in the study, disclosed no relevant conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Poor sleep raises risk for fatty liver disease
Sleep behaviors, both individually and combined, are associated with an increased risk of developing metabolic dysfunction–associated fatty liver disease (MAFLD), according to a Chinese analysis that suggests the effect may be independent of obesity.
Yan Liu, PhD, from the School of Public Health at Sun Yat-sen University in Guangzhou, China, and colleagues studied data on over 5,000 individuals who self-reported sleep behaviors and underwent liver ultrasound.
, increasing the risk by 37%, 59%, and 17%, respectively, whereas people with both poor nighttime sleep and prolonged daytime napping had the “highest risk for developing fatty liver disease,” said Dr. Liu in a press release.
In contrast, having any of six healthy sleep behaviors decreased the risk by 16% each, and even a “moderate improvement in sleep quality was related to a 29% reduction in the risk for fatty liver disease,” he added.
The research, published online in the Journal of Clinical Endocrinology & Metabolism, also indicated that obesity accounted for only one fifth of the effect of sleep quality on MAFLD risk.
Rise in unhealthy lifestyles leads to increase in MALFD
The authors write that MAFLD is the “leading chronic liver disease worldwide,” affecting around a quarter of the adult population, and may lead to end-stage liver diseases and extrahepatic complications, thus “posing a major health and economic burden.”
Moreover, the disease prevalence is “soaring at an unanticipated rate,” increasing from 18% to 29% in China over the past decade, because of a “rapid rise in unhealthy lifestyles,” the authors note.
Sleep disturbance is increasingly prevalent, “and an emerging contributor to multiple metabolic disorders,” with insomnia and habitual snoring, for example, positively correlated with hypertension, impaired glucose metabolism, and dyslipidemia, report the authors.
However, whether sleep quality, which includes “several metabolic-related sleep behaviors,” constitutes an independent risk for MAFLD “over and above” the effect of obesity remains unclear.
To investigate further, the researchers examined data from the baseline survey of the community-based, prospective South China Cohort study, which was conducted in four regions of Southern China and involved 5,430 individuals aged 30-79 years.
Between March 2018 and October 2019, the participants self-reported sleep behaviors on the Pittsburgh Sleep Quality Index questionnaire and underwent ultrasound examination of the liver.
MAFLD was diagnosed in those with hepatic steatosis and one of the following:
- Overweight/obesity, defined by this study as a body mass index greater than or equal to 23 kg/m2.
- Presence of diabetes.
- Evidence of metabolic dysregulation.
After excluding patients with insufficient data, and those with a history of liver cirrhosis, hepatectomy, or liver cancer, among others, the team included 5,011 individuals with an average age of 64 years and a mean body mass index of 24.31 kg/m2. Forty percent were male.
Obesity was present in 13% of participants, whereas 15% had diabetes, 58% hypertension, and 35% metabolic syndrome.
MAFLD was diagnosed in 28% of the study population. They were older, more likely to be female with a higher education, and had a higher prevalence of preexisting metabolic disorders and worse metabolic profiles, than those without the disease.
Turning to the associations between sleep and the risk of MAFLD, the researchers say that “in contrast to previous reports, neither shorter nor longer sleep duration was found to be associated with the risk for MAFLD.”
However, after adjusting for demographics, lifestyles, medication, and preexisting metabolic comorbidities including hypertension, diabetes, and obesity, they found that the risk of MAFLD was significantly associated with late bedtime (defined as after 10 p.m.), at an odds ratio of 1.37 (P < .05).
MAFLD was also linked to snoring, at an odds ratio of 1.59, and to daytime napping for longer than 30 minutes, at an odds ratio of 1.17 (P < .05 for both).
When the team compared low-risk and high-risk sleep factors, they found that participants who had an early bedtime, slept 7-8 hours per night, never or rarely had insomnia or snoring, had infrequent daytime sleepiness, and daytime napping of half-hour or less had an odds ratio for MAFLD vs. other participants of 0.64 (P < .05).
Combining those factors into a healthy sleep score, the team found that each additional increase of healthy sleep score was associated with a fully adjusted odds ratio for MAFLD of 0.84 (P < .05).
In contrast, individuals with poor nocturnal sleep patterns and prolonged daytime napping had a higher risk for developing MAFLD, compared with those with a healthy nocturnal sleep pattern and daytime napping of half-hour or less, at an odds ratio of 2.38 (P < .05).
Further analysis indicated that individuals with a sedentary lifestyle and central obesity had a higher risk of MAFLD, but that the presence of obesity accounted for only 20.8% of the total effect of sleep quality on the risk of MAFLD.
“Taken together, our results suggests that obesity only partially mediates the effect of overall sleep quality on MAFLD,” the authors write.
“Given that large proportions of subjects suffering from poor sleep quality are underdiagnosed and undertreated, our study calls for more research into this field and strategies to improve sleep quality,” Dr. Liu said.
The study was supported by the “National Key R&D Program” of China, the Fundamental Research Funds for the Central Universities (Sun Yat-sen University), Natural Science Foundation of Guangdong Province, the Key Project of Medicine Discipline of Guangzhou, and Basic Research Project of Key Laboratory of Guangzhou.
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sleep behaviors, both individually and combined, are associated with an increased risk of developing metabolic dysfunction–associated fatty liver disease (MAFLD), according to a Chinese analysis that suggests the effect may be independent of obesity.
Yan Liu, PhD, from the School of Public Health at Sun Yat-sen University in Guangzhou, China, and colleagues studied data on over 5,000 individuals who self-reported sleep behaviors and underwent liver ultrasound.
, increasing the risk by 37%, 59%, and 17%, respectively, whereas people with both poor nighttime sleep and prolonged daytime napping had the “highest risk for developing fatty liver disease,” said Dr. Liu in a press release.
In contrast, having any of six healthy sleep behaviors decreased the risk by 16% each, and even a “moderate improvement in sleep quality was related to a 29% reduction in the risk for fatty liver disease,” he added.
The research, published online in the Journal of Clinical Endocrinology & Metabolism, also indicated that obesity accounted for only one fifth of the effect of sleep quality on MAFLD risk.
Rise in unhealthy lifestyles leads to increase in MALFD
The authors write that MAFLD is the “leading chronic liver disease worldwide,” affecting around a quarter of the adult population, and may lead to end-stage liver diseases and extrahepatic complications, thus “posing a major health and economic burden.”
Moreover, the disease prevalence is “soaring at an unanticipated rate,” increasing from 18% to 29% in China over the past decade, because of a “rapid rise in unhealthy lifestyles,” the authors note.
Sleep disturbance is increasingly prevalent, “and an emerging contributor to multiple metabolic disorders,” with insomnia and habitual snoring, for example, positively correlated with hypertension, impaired glucose metabolism, and dyslipidemia, report the authors.
However, whether sleep quality, which includes “several metabolic-related sleep behaviors,” constitutes an independent risk for MAFLD “over and above” the effect of obesity remains unclear.
To investigate further, the researchers examined data from the baseline survey of the community-based, prospective South China Cohort study, which was conducted in four regions of Southern China and involved 5,430 individuals aged 30-79 years.
Between March 2018 and October 2019, the participants self-reported sleep behaviors on the Pittsburgh Sleep Quality Index questionnaire and underwent ultrasound examination of the liver.
MAFLD was diagnosed in those with hepatic steatosis and one of the following:
- Overweight/obesity, defined by this study as a body mass index greater than or equal to 23 kg/m2.
- Presence of diabetes.
- Evidence of metabolic dysregulation.
After excluding patients with insufficient data, and those with a history of liver cirrhosis, hepatectomy, or liver cancer, among others, the team included 5,011 individuals with an average age of 64 years and a mean body mass index of 24.31 kg/m2. Forty percent were male.
Obesity was present in 13% of participants, whereas 15% had diabetes, 58% hypertension, and 35% metabolic syndrome.
MAFLD was diagnosed in 28% of the study population. They were older, more likely to be female with a higher education, and had a higher prevalence of preexisting metabolic disorders and worse metabolic profiles, than those without the disease.
Turning to the associations between sleep and the risk of MAFLD, the researchers say that “in contrast to previous reports, neither shorter nor longer sleep duration was found to be associated with the risk for MAFLD.”
However, after adjusting for demographics, lifestyles, medication, and preexisting metabolic comorbidities including hypertension, diabetes, and obesity, they found that the risk of MAFLD was significantly associated with late bedtime (defined as after 10 p.m.), at an odds ratio of 1.37 (P < .05).
MAFLD was also linked to snoring, at an odds ratio of 1.59, and to daytime napping for longer than 30 minutes, at an odds ratio of 1.17 (P < .05 for both).
When the team compared low-risk and high-risk sleep factors, they found that participants who had an early bedtime, slept 7-8 hours per night, never or rarely had insomnia or snoring, had infrequent daytime sleepiness, and daytime napping of half-hour or less had an odds ratio for MAFLD vs. other participants of 0.64 (P < .05).
Combining those factors into a healthy sleep score, the team found that each additional increase of healthy sleep score was associated with a fully adjusted odds ratio for MAFLD of 0.84 (P < .05).
In contrast, individuals with poor nocturnal sleep patterns and prolonged daytime napping had a higher risk for developing MAFLD, compared with those with a healthy nocturnal sleep pattern and daytime napping of half-hour or less, at an odds ratio of 2.38 (P < .05).
Further analysis indicated that individuals with a sedentary lifestyle and central obesity had a higher risk of MAFLD, but that the presence of obesity accounted for only 20.8% of the total effect of sleep quality on the risk of MAFLD.
“Taken together, our results suggests that obesity only partially mediates the effect of overall sleep quality on MAFLD,” the authors write.
“Given that large proportions of subjects suffering from poor sleep quality are underdiagnosed and undertreated, our study calls for more research into this field and strategies to improve sleep quality,” Dr. Liu said.
The study was supported by the “National Key R&D Program” of China, the Fundamental Research Funds for the Central Universities (Sun Yat-sen University), Natural Science Foundation of Guangdong Province, the Key Project of Medicine Discipline of Guangzhou, and Basic Research Project of Key Laboratory of Guangzhou.
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sleep behaviors, both individually and combined, are associated with an increased risk of developing metabolic dysfunction–associated fatty liver disease (MAFLD), according to a Chinese analysis that suggests the effect may be independent of obesity.
Yan Liu, PhD, from the School of Public Health at Sun Yat-sen University in Guangzhou, China, and colleagues studied data on over 5,000 individuals who self-reported sleep behaviors and underwent liver ultrasound.
, increasing the risk by 37%, 59%, and 17%, respectively, whereas people with both poor nighttime sleep and prolonged daytime napping had the “highest risk for developing fatty liver disease,” said Dr. Liu in a press release.
In contrast, having any of six healthy sleep behaviors decreased the risk by 16% each, and even a “moderate improvement in sleep quality was related to a 29% reduction in the risk for fatty liver disease,” he added.
The research, published online in the Journal of Clinical Endocrinology & Metabolism, also indicated that obesity accounted for only one fifth of the effect of sleep quality on MAFLD risk.
Rise in unhealthy lifestyles leads to increase in MALFD
The authors write that MAFLD is the “leading chronic liver disease worldwide,” affecting around a quarter of the adult population, and may lead to end-stage liver diseases and extrahepatic complications, thus “posing a major health and economic burden.”
Moreover, the disease prevalence is “soaring at an unanticipated rate,” increasing from 18% to 29% in China over the past decade, because of a “rapid rise in unhealthy lifestyles,” the authors note.
Sleep disturbance is increasingly prevalent, “and an emerging contributor to multiple metabolic disorders,” with insomnia and habitual snoring, for example, positively correlated with hypertension, impaired glucose metabolism, and dyslipidemia, report the authors.
However, whether sleep quality, which includes “several metabolic-related sleep behaviors,” constitutes an independent risk for MAFLD “over and above” the effect of obesity remains unclear.
To investigate further, the researchers examined data from the baseline survey of the community-based, prospective South China Cohort study, which was conducted in four regions of Southern China and involved 5,430 individuals aged 30-79 years.
Between March 2018 and October 2019, the participants self-reported sleep behaviors on the Pittsburgh Sleep Quality Index questionnaire and underwent ultrasound examination of the liver.
MAFLD was diagnosed in those with hepatic steatosis and one of the following:
- Overweight/obesity, defined by this study as a body mass index greater than or equal to 23 kg/m2.
- Presence of diabetes.
- Evidence of metabolic dysregulation.
After excluding patients with insufficient data, and those with a history of liver cirrhosis, hepatectomy, or liver cancer, among others, the team included 5,011 individuals with an average age of 64 years and a mean body mass index of 24.31 kg/m2. Forty percent were male.
Obesity was present in 13% of participants, whereas 15% had diabetes, 58% hypertension, and 35% metabolic syndrome.
MAFLD was diagnosed in 28% of the study population. They were older, more likely to be female with a higher education, and had a higher prevalence of preexisting metabolic disorders and worse metabolic profiles, than those without the disease.
Turning to the associations between sleep and the risk of MAFLD, the researchers say that “in contrast to previous reports, neither shorter nor longer sleep duration was found to be associated with the risk for MAFLD.”
However, after adjusting for demographics, lifestyles, medication, and preexisting metabolic comorbidities including hypertension, diabetes, and obesity, they found that the risk of MAFLD was significantly associated with late bedtime (defined as after 10 p.m.), at an odds ratio of 1.37 (P < .05).
MAFLD was also linked to snoring, at an odds ratio of 1.59, and to daytime napping for longer than 30 minutes, at an odds ratio of 1.17 (P < .05 for both).
When the team compared low-risk and high-risk sleep factors, they found that participants who had an early bedtime, slept 7-8 hours per night, never or rarely had insomnia or snoring, had infrequent daytime sleepiness, and daytime napping of half-hour or less had an odds ratio for MAFLD vs. other participants of 0.64 (P < .05).
Combining those factors into a healthy sleep score, the team found that each additional increase of healthy sleep score was associated with a fully adjusted odds ratio for MAFLD of 0.84 (P < .05).
In contrast, individuals with poor nocturnal sleep patterns and prolonged daytime napping had a higher risk for developing MAFLD, compared with those with a healthy nocturnal sleep pattern and daytime napping of half-hour or less, at an odds ratio of 2.38 (P < .05).
Further analysis indicated that individuals with a sedentary lifestyle and central obesity had a higher risk of MAFLD, but that the presence of obesity accounted for only 20.8% of the total effect of sleep quality on the risk of MAFLD.
“Taken together, our results suggests that obesity only partially mediates the effect of overall sleep quality on MAFLD,” the authors write.
“Given that large proportions of subjects suffering from poor sleep quality are underdiagnosed and undertreated, our study calls for more research into this field and strategies to improve sleep quality,” Dr. Liu said.
The study was supported by the “National Key R&D Program” of China, the Fundamental Research Funds for the Central Universities (Sun Yat-sen University), Natural Science Foundation of Guangdong Province, the Key Project of Medicine Discipline of Guangzhou, and Basic Research Project of Key Laboratory of Guangzhou.
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Liver protein protects against parenteral nutrition liver injury
Hepatic protein PP2A-C-alpha may serve as a protective factor against parenteral nutrition–associated hepatic steatosis by improving liver function, according to a recent study published in Cellular and Molecular Gastroenterology and Hepatology.
Parenteral nutrition–associated hepatic steatosis likely involves the down-regulation of hepatic PP2A-C-alpha and consequent increased phosphorylation of Akt2; this in turn alters hepatic lipid metabolism, promotes triglyceride accumulation, and leads to liver injury, wrote the researchers, led by Gulisudumu Maitiabula and Feng Tian of the Research Institute of General Surgery at Jinling Hospital, Nanjing, China, and the Medical School of Nanjing University.
“Our study provides a strong rationale that PP2A-C-alpha may be involved in the pathogenesis of [parenteral nutrition–associated hepatic steatosis],” they wrote. “Further research is merited to establish whether interventions to enhance PP2A function might suppress the development of hepatic steatosis in patients receiving long-term [parenteral nutrition].”
Parenteral nutrition can be a lifesaving therapy for patients with intestinal failure caused by insufficient bowel length or function, the authors noted However, long-term use can lead to potentially fatal complications such as liver disease, but an understanding of the pathological mechanisms behind parenteral nutrition–associated hepatic steatosis limited.
The research team performed comparative proteomic/phosphoproteomic analyses of liver samples from 10 patients with parenteral nutrition–associated hepatic steatosis, as well as 8 cholelithiasis patients as controls, who were admitted to Jinling Hospital between June 2018 and June 2019. The researchers also assessed the effect of PP2A-C-alpha on liver injury from total parenteral nutrition in mice.
The research team found that PP2A-C-alpha was down-regulated in patients and mice with parenteral nutrition–associated hepatic steatosis. In addition, in patients with parenteral nutrition–associated hepatic steatosis, they found enhanced activation of serine/threonine kinase Akt2 and decreased activation of AMPK.
Mice that were given total parenteral nutrition infusion for 14 days developed hepatic steatosis, down-regulation of PP2A-C-alpha, activation of Akt2, and inhibition of AMPK. Hepatocyte-specific deletion of PP2A-C-alpha in mice given parenteral nutrition exacerbated the Akt2 activation, AMPK inhibition, and hepatic steatosis through an effect on fatty acid degradation.
On the other hand, forced expression of PP2A-C-alpha led to reductions in hepatocyte fat deposition and the pathological score for liver steatosis. Overexpression also significantly improved hepatic steatosis, suppressed Akt2, and activated AMPK. In addition, pharmacological activation of Akt2 in mice overexpressing PP2A-C-alpha led to the aggravation of hepatic steatosis.
“Collectively, these observations suggest that [parenteral nutrition] for [more than] 14 days leads to a down-regulation in PP2A-C-alpha expression that activates Akt2-dependent signaling, which would likely lead to hepatic steatosis,” the study authors wrote.
Intervention trials of PP2A-C-alpha in humans have not been performed because PP2A-C-alpha activators or effector analogs were unavailable for clinical use, they wrote. Additional clinical studies are needed to investigate the effects of PP2A-C-alpha intervention on the development of hepatic steatosis in patients receiving long-term parenteral nutrition.
The study was supported by the National Natural Science Foundation of China, the Science Foundation of Outstanding Youth in Jiangsu Province, the National Science and Technology Research Funding for Public Welfare Medical Projects, “The 13th Five-Year Plan” Foundation of Jiangsu Province for Medical Key Talents, and the Natural Science Foundation of Jiangsu Province. The study authors disclosed no conflicts of interest.
New findings may lead to novel treatments
Parenteral nutrition is a life saver for children and adults with insufficient absorptive capacity of the gastrointestinal tract. Unfortunately, up to two-thirds of patients requiring parenteral nutrition long-term develop liver disease, which can have fatal outcomes. Parenteral nutrition–associated liver disease is characterized by fibrosis and steatosis. While portal inflammation and cholestasis resolve in patients who can be weaned off parenteral nutrition, portal fibrosis and steatosis unfortunately remain in about half of the patients. The development of therapeutic strategies for this condition has thus far been hampered by the fact that the molecular mechanism of parenteral nutrition–associated liver disease was unknown.
This study by Maitiabua and colleagues from Nanjing University Medical School addresses this problem by performing a proteomic and, importantly, phospho-proteomic analysis of liver biopsies from adults treated with parenteral nutrition compared to normally-feeding controls. They discovered that levels of phosphorylated AKT2, the key signaling mediator of insulin in the liver, are increased, while protein levels of the opposing protein phosphatase 2A (PP2A) are decreased in patients receiving parenteral nutrition.
Remarkably, they could reproduce these same pathway changes in a mouse model of parenteral nutrition, which again led to a chronic activation of the insulin signaling pathway, culminating in the phosphorylation of AKT2. They show further that activation of AKT2 inhibits AMPK and alters hepatic lipid metabolism to promote triglyceride accumulation. Using the experimentally tractable mouse model, they demonstrate further that the ablation of a PP2A isoform in the liver is sufficient to cause lipid accumulation and liver injury. Conversely, restoring PP2A expression improved the hepatic phenotype in mice in the parenteral nutrition model. These findings could also be mimicked using pharmacological activation and inhibition of PP2A.
In sum, this experimental study could some day lead the way to novel treatments of parenteral nutrition-induced liver disease through the use of PP2A activators.
Klaus H. Kaestner, PhD, is with the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases, Perelman School of Medicine,University of Pennsylvania, Philadelphia.
New findings may lead to novel treatments
Parenteral nutrition is a life saver for children and adults with insufficient absorptive capacity of the gastrointestinal tract. Unfortunately, up to two-thirds of patients requiring parenteral nutrition long-term develop liver disease, which can have fatal outcomes. Parenteral nutrition–associated liver disease is characterized by fibrosis and steatosis. While portal inflammation and cholestasis resolve in patients who can be weaned off parenteral nutrition, portal fibrosis and steatosis unfortunately remain in about half of the patients. The development of therapeutic strategies for this condition has thus far been hampered by the fact that the molecular mechanism of parenteral nutrition–associated liver disease was unknown.
This study by Maitiabua and colleagues from Nanjing University Medical School addresses this problem by performing a proteomic and, importantly, phospho-proteomic analysis of liver biopsies from adults treated with parenteral nutrition compared to normally-feeding controls. They discovered that levels of phosphorylated AKT2, the key signaling mediator of insulin in the liver, are increased, while protein levels of the opposing protein phosphatase 2A (PP2A) are decreased in patients receiving parenteral nutrition.
Remarkably, they could reproduce these same pathway changes in a mouse model of parenteral nutrition, which again led to a chronic activation of the insulin signaling pathway, culminating in the phosphorylation of AKT2. They show further that activation of AKT2 inhibits AMPK and alters hepatic lipid metabolism to promote triglyceride accumulation. Using the experimentally tractable mouse model, they demonstrate further that the ablation of a PP2A isoform in the liver is sufficient to cause lipid accumulation and liver injury. Conversely, restoring PP2A expression improved the hepatic phenotype in mice in the parenteral nutrition model. These findings could also be mimicked using pharmacological activation and inhibition of PP2A.
In sum, this experimental study could some day lead the way to novel treatments of parenteral nutrition-induced liver disease through the use of PP2A activators.
Klaus H. Kaestner, PhD, is with the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases, Perelman School of Medicine,University of Pennsylvania, Philadelphia.
New findings may lead to novel treatments
Parenteral nutrition is a life saver for children and adults with insufficient absorptive capacity of the gastrointestinal tract. Unfortunately, up to two-thirds of patients requiring parenteral nutrition long-term develop liver disease, which can have fatal outcomes. Parenteral nutrition–associated liver disease is characterized by fibrosis and steatosis. While portal inflammation and cholestasis resolve in patients who can be weaned off parenteral nutrition, portal fibrosis and steatosis unfortunately remain in about half of the patients. The development of therapeutic strategies for this condition has thus far been hampered by the fact that the molecular mechanism of parenteral nutrition–associated liver disease was unknown.
This study by Maitiabua and colleagues from Nanjing University Medical School addresses this problem by performing a proteomic and, importantly, phospho-proteomic analysis of liver biopsies from adults treated with parenteral nutrition compared to normally-feeding controls. They discovered that levels of phosphorylated AKT2, the key signaling mediator of insulin in the liver, are increased, while protein levels of the opposing protein phosphatase 2A (PP2A) are decreased in patients receiving parenteral nutrition.
Remarkably, they could reproduce these same pathway changes in a mouse model of parenteral nutrition, which again led to a chronic activation of the insulin signaling pathway, culminating in the phosphorylation of AKT2. They show further that activation of AKT2 inhibits AMPK and alters hepatic lipid metabolism to promote triglyceride accumulation. Using the experimentally tractable mouse model, they demonstrate further that the ablation of a PP2A isoform in the liver is sufficient to cause lipid accumulation and liver injury. Conversely, restoring PP2A expression improved the hepatic phenotype in mice in the parenteral nutrition model. These findings could also be mimicked using pharmacological activation and inhibition of PP2A.
In sum, this experimental study could some day lead the way to novel treatments of parenteral nutrition-induced liver disease through the use of PP2A activators.
Klaus H. Kaestner, PhD, is with the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases, Perelman School of Medicine,University of Pennsylvania, Philadelphia.
Hepatic protein PP2A-C-alpha may serve as a protective factor against parenteral nutrition–associated hepatic steatosis by improving liver function, according to a recent study published in Cellular and Molecular Gastroenterology and Hepatology.
Parenteral nutrition–associated hepatic steatosis likely involves the down-regulation of hepatic PP2A-C-alpha and consequent increased phosphorylation of Akt2; this in turn alters hepatic lipid metabolism, promotes triglyceride accumulation, and leads to liver injury, wrote the researchers, led by Gulisudumu Maitiabula and Feng Tian of the Research Institute of General Surgery at Jinling Hospital, Nanjing, China, and the Medical School of Nanjing University.
“Our study provides a strong rationale that PP2A-C-alpha may be involved in the pathogenesis of [parenteral nutrition–associated hepatic steatosis],” they wrote. “Further research is merited to establish whether interventions to enhance PP2A function might suppress the development of hepatic steatosis in patients receiving long-term [parenteral nutrition].”
Parenteral nutrition can be a lifesaving therapy for patients with intestinal failure caused by insufficient bowel length or function, the authors noted However, long-term use can lead to potentially fatal complications such as liver disease, but an understanding of the pathological mechanisms behind parenteral nutrition–associated hepatic steatosis limited.
The research team performed comparative proteomic/phosphoproteomic analyses of liver samples from 10 patients with parenteral nutrition–associated hepatic steatosis, as well as 8 cholelithiasis patients as controls, who were admitted to Jinling Hospital between June 2018 and June 2019. The researchers also assessed the effect of PP2A-C-alpha on liver injury from total parenteral nutrition in mice.
The research team found that PP2A-C-alpha was down-regulated in patients and mice with parenteral nutrition–associated hepatic steatosis. In addition, in patients with parenteral nutrition–associated hepatic steatosis, they found enhanced activation of serine/threonine kinase Akt2 and decreased activation of AMPK.
Mice that were given total parenteral nutrition infusion for 14 days developed hepatic steatosis, down-regulation of PP2A-C-alpha, activation of Akt2, and inhibition of AMPK. Hepatocyte-specific deletion of PP2A-C-alpha in mice given parenteral nutrition exacerbated the Akt2 activation, AMPK inhibition, and hepatic steatosis through an effect on fatty acid degradation.
On the other hand, forced expression of PP2A-C-alpha led to reductions in hepatocyte fat deposition and the pathological score for liver steatosis. Overexpression also significantly improved hepatic steatosis, suppressed Akt2, and activated AMPK. In addition, pharmacological activation of Akt2 in mice overexpressing PP2A-C-alpha led to the aggravation of hepatic steatosis.
“Collectively, these observations suggest that [parenteral nutrition] for [more than] 14 days leads to a down-regulation in PP2A-C-alpha expression that activates Akt2-dependent signaling, which would likely lead to hepatic steatosis,” the study authors wrote.
Intervention trials of PP2A-C-alpha in humans have not been performed because PP2A-C-alpha activators or effector analogs were unavailable for clinical use, they wrote. Additional clinical studies are needed to investigate the effects of PP2A-C-alpha intervention on the development of hepatic steatosis in patients receiving long-term parenteral nutrition.
The study was supported by the National Natural Science Foundation of China, the Science Foundation of Outstanding Youth in Jiangsu Province, the National Science and Technology Research Funding for Public Welfare Medical Projects, “The 13th Five-Year Plan” Foundation of Jiangsu Province for Medical Key Talents, and the Natural Science Foundation of Jiangsu Province. The study authors disclosed no conflicts of interest.
Hepatic protein PP2A-C-alpha may serve as a protective factor against parenteral nutrition–associated hepatic steatosis by improving liver function, according to a recent study published in Cellular and Molecular Gastroenterology and Hepatology.
Parenteral nutrition–associated hepatic steatosis likely involves the down-regulation of hepatic PP2A-C-alpha and consequent increased phosphorylation of Akt2; this in turn alters hepatic lipid metabolism, promotes triglyceride accumulation, and leads to liver injury, wrote the researchers, led by Gulisudumu Maitiabula and Feng Tian of the Research Institute of General Surgery at Jinling Hospital, Nanjing, China, and the Medical School of Nanjing University.
“Our study provides a strong rationale that PP2A-C-alpha may be involved in the pathogenesis of [parenteral nutrition–associated hepatic steatosis],” they wrote. “Further research is merited to establish whether interventions to enhance PP2A function might suppress the development of hepatic steatosis in patients receiving long-term [parenteral nutrition].”
Parenteral nutrition can be a lifesaving therapy for patients with intestinal failure caused by insufficient bowel length or function, the authors noted However, long-term use can lead to potentially fatal complications such as liver disease, but an understanding of the pathological mechanisms behind parenteral nutrition–associated hepatic steatosis limited.
The research team performed comparative proteomic/phosphoproteomic analyses of liver samples from 10 patients with parenteral nutrition–associated hepatic steatosis, as well as 8 cholelithiasis patients as controls, who were admitted to Jinling Hospital between June 2018 and June 2019. The researchers also assessed the effect of PP2A-C-alpha on liver injury from total parenteral nutrition in mice.
The research team found that PP2A-C-alpha was down-regulated in patients and mice with parenteral nutrition–associated hepatic steatosis. In addition, in patients with parenteral nutrition–associated hepatic steatosis, they found enhanced activation of serine/threonine kinase Akt2 and decreased activation of AMPK.
Mice that were given total parenteral nutrition infusion for 14 days developed hepatic steatosis, down-regulation of PP2A-C-alpha, activation of Akt2, and inhibition of AMPK. Hepatocyte-specific deletion of PP2A-C-alpha in mice given parenteral nutrition exacerbated the Akt2 activation, AMPK inhibition, and hepatic steatosis through an effect on fatty acid degradation.
On the other hand, forced expression of PP2A-C-alpha led to reductions in hepatocyte fat deposition and the pathological score for liver steatosis. Overexpression also significantly improved hepatic steatosis, suppressed Akt2, and activated AMPK. In addition, pharmacological activation of Akt2 in mice overexpressing PP2A-C-alpha led to the aggravation of hepatic steatosis.
“Collectively, these observations suggest that [parenteral nutrition] for [more than] 14 days leads to a down-regulation in PP2A-C-alpha expression that activates Akt2-dependent signaling, which would likely lead to hepatic steatosis,” the study authors wrote.
Intervention trials of PP2A-C-alpha in humans have not been performed because PP2A-C-alpha activators or effector analogs were unavailable for clinical use, they wrote. Additional clinical studies are needed to investigate the effects of PP2A-C-alpha intervention on the development of hepatic steatosis in patients receiving long-term parenteral nutrition.
The study was supported by the National Natural Science Foundation of China, the Science Foundation of Outstanding Youth in Jiangsu Province, the National Science and Technology Research Funding for Public Welfare Medical Projects, “The 13th Five-Year Plan” Foundation of Jiangsu Province for Medical Key Talents, and the Natural Science Foundation of Jiangsu Province. The study authors disclosed no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Risk factors in children linked to stroke as soon as 30s, 40s
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Trials data on COPD leave primary care docs in the dark
Primary care clinicians often struggle to care for their patients with chronic obstructive pulmonary disease (COPD), thanks to a lack of real-world evidence as to which treatments work best.
As a result, potentially preventable life-threatening exacerbations are common among people with the condition. Central to the problem, some experts believe, is that the average patient bears little resemblance to participants in clinical trials of the medications used to treat COPD.
Indeed, a recent study showed that many COPD patients who were receiving maintenance therapy that should have been controlling their disease experienced severe flare-ups – a finding that caught the researchers by surprise.
“We know the benefit of COPD treatments in the context of clinical trials. However, the kinds of patients in primary care may not completely mimic those in clinical trials,” one of the authors, MeiLan Han, MD, a professor of medicine in the division of pulmonary and critical care at the University of Michigan, Ann Arbor, told this news organization. Dr. Han, a volunteer medical spokesperson for the American Lung Association, added that patients “may not be as adherent to medications in real life as they are in clinical trials.”
, who are younger than the average patient with COPD, and who typically are male. Patients are seen in resource-abundant settings designed to maximize adherence to treatment, with supports such as free medication and frequent monitoring – settings far different from those in which most primary care physicians practice.
The authors of the new article said trials conducted with typical patients in primary care settings could help physicians to optimize treatment.
Real-world evidence can shed light on physicians’ intent and on barriers to following guidelines, as well as important patient factors, such as adherence and good inhaler technique, Barbara Yawn, MD, an adjunct professor in the department of family and community health at the University of Minnesota, Minneapolis, and a coauthor of the study, said in an interview.
A window onto patient burden
According to the Centers for Disease Control and Prevention, an estimated $15 million Americans have COPD. Annual costs to the health care system approach $50 billion a year. The death rate for COPD has increased since 1969 as death rates of other major killers in the United States, such as heart disease and cancer, declined, according to a 2015 analysis of death records.
The new study, published in the July/August issue of the Annals of Family Medicine, provides a snapshot of COPD’s toll on patients.
Researchers examined electronic health records of 17,192 patients treated at primary care clinics in five states using a dataset maintained by DARTNet Institute, a nonprofit organization that supports research and quality improvement. They also analyzed self-reported assessments from 1,354 patients in the dataset who are in a registry called Advancing the Patient Experience in COPD.
Over half (56%) of patients were female, White (64%), aged 55-84 years (81%), and current or exsmokers (80%). The vast majority had three or more comorbidities, including hypertension, diabetes, and depression.
Serious flare-ups were common; 38% of patients had experienced one or more exacerbations in the previous year. Of registry respondents, half said they had had at least one exacerbation, and 20% said they had been hospitalized for COPD during that period.
Among patients in the registry, 43% reported that COPD had a high or very high impact on their health, and 45% could not walk at a normal pace without losing their breath.
Almost 90% of patients were receiving a maintenance therapy regimen. The number of exacerbations was “somewhat surprising,” the authors say. They write that the findings may indicate that patients were not receiving appropriate treatment or were not complying with their medication regimens and that there may be a need for nonpharmacologic interventions, such as smoking cessation. They also write that physician education is needed to support earlier diagnosis and treatment so as to delay declines in lung function.
The researchers say their findings highlight “the need for more real-life effectiveness trials to better support decision-making at the primary care level.”
Dr. Yawn is a coinvestigator of one such study, called CAPTURE, which is assessing a screening tool for COPD in primary care practices.
At the University of Illinois, Chicago, Jerry Krishnan, MD, PhD, pulmonologist and professor of medicine and public health, is running the RELIANCE study, which is comparing the use of azithromycin and roflumilast in preventing hospitalization and death among patients with COPD who continue to have exacerbations.
Although RELIANCE involves pulmonologists, Dr. Krishnan told this news organization, it offers a model for building real-world evidence on questions relevant to primary care. “We don’t really know if medications used by patients in my clinic are as effective as reported in clinical trials that were used to obtain regulatory approvals by the U.S. Food and Drug Administration,” he said.
Wilson Pace, MD, a family physician and chief medical officer and chief technology officer of DARTNet, said funders of research are becoming aware of the need for real-world studies along with “gold standard” efficacy trials.
Dr. Pace, who helped conduct the new study, said a remaining obstacle to improving care is “a defeatist attitude of clinicians” who are skeptical about the ability of therapy to have an effect.
Real-world evidence could remedy clinician frustrations, he said. When clinicians are shown that they can improve patients’ quality of life and maybe even reduce the cost of care, “then they will hopefully pay attention,” he said.
Some experts who were not involved in the study said the findings offer an illuminating, although incomplete, picture. Nonpharmacologic interventions, the management of other health problems, and access to specialty care are not addressed, and the researchers didn’t have data on treatment adherence, inhaler technique, and patients’ peak inspiratory flow – factors that influence the effectiveness of medications. The study also lacked information on whether patients received pulmonary rehabilitation to help their heart and lungs work better.
Nicola Hanania, MD, a professor of medicine and director of the Airways Clinical Research Center at Baylor College of Medicine, Houston, said the study “adds a lot to what we have known” but pointed out that COPD is grossly underdiagnosed.
According to one analysis of National Health and Nutrition Examination Surveys, 72% of individuals with COPD don’t know they have the condition. Such patients were not included in the study, Dr. Hanania noted.
“We need pragmatic studies over multiple years to better understand” the condition, Dr. Yawn said. Real-world evidence “based in an academic setting or specialty practices is not sufficient,” she added. “We need to see results from patients and clinics that look like what we have.”
The registry was established and funded by Optimum Patient Care Global, a nonprofit organization, and Boehringer Ingelheim. Dr. Han has consulted for Boehringer Ingelheim, GlaxoSmithKline, and AstraZeneca and has received research support from Novartis and Sunovion. Dr. Yawn has served on advisory boards for GlaxoSmithKline, Astra-Zeneca, Novartis, and Boehringer Ingelheim and has received research funds from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, and Novartis. Dr. Krishnan has disclosed no relevant financial relationshps. Dr. Hanania has received honoraria for serving as consultant or advisory board member for GSK, Boehringer Ingelheim, Novartis, Sanofi, AstraZeneca, Teva, Genentech, and Amgen. His institution has received research grant support on his behalf from GSK, Sanofi, Boehringer Ingelheim, AstraZeneca, Genentech, Teva, and Novartis. Dr. Pace is on the advisory board for Mylan and has received stock from Novo Nordisk, Pfizer, Novartis, Johnson & Johnson, Stryker, Amgen, Gilead, and Sanofi.
A version of this article first appeared on Medscape.com.
Primary care clinicians often struggle to care for their patients with chronic obstructive pulmonary disease (COPD), thanks to a lack of real-world evidence as to which treatments work best.
As a result, potentially preventable life-threatening exacerbations are common among people with the condition. Central to the problem, some experts believe, is that the average patient bears little resemblance to participants in clinical trials of the medications used to treat COPD.
Indeed, a recent study showed that many COPD patients who were receiving maintenance therapy that should have been controlling their disease experienced severe flare-ups – a finding that caught the researchers by surprise.
“We know the benefit of COPD treatments in the context of clinical trials. However, the kinds of patients in primary care may not completely mimic those in clinical trials,” one of the authors, MeiLan Han, MD, a professor of medicine in the division of pulmonary and critical care at the University of Michigan, Ann Arbor, told this news organization. Dr. Han, a volunteer medical spokesperson for the American Lung Association, added that patients “may not be as adherent to medications in real life as they are in clinical trials.”
, who are younger than the average patient with COPD, and who typically are male. Patients are seen in resource-abundant settings designed to maximize adherence to treatment, with supports such as free medication and frequent monitoring – settings far different from those in which most primary care physicians practice.
The authors of the new article said trials conducted with typical patients in primary care settings could help physicians to optimize treatment.
Real-world evidence can shed light on physicians’ intent and on barriers to following guidelines, as well as important patient factors, such as adherence and good inhaler technique, Barbara Yawn, MD, an adjunct professor in the department of family and community health at the University of Minnesota, Minneapolis, and a coauthor of the study, said in an interview.
A window onto patient burden
According to the Centers for Disease Control and Prevention, an estimated $15 million Americans have COPD. Annual costs to the health care system approach $50 billion a year. The death rate for COPD has increased since 1969 as death rates of other major killers in the United States, such as heart disease and cancer, declined, according to a 2015 analysis of death records.
The new study, published in the July/August issue of the Annals of Family Medicine, provides a snapshot of COPD’s toll on patients.
Researchers examined electronic health records of 17,192 patients treated at primary care clinics in five states using a dataset maintained by DARTNet Institute, a nonprofit organization that supports research and quality improvement. They also analyzed self-reported assessments from 1,354 patients in the dataset who are in a registry called Advancing the Patient Experience in COPD.
Over half (56%) of patients were female, White (64%), aged 55-84 years (81%), and current or exsmokers (80%). The vast majority had three or more comorbidities, including hypertension, diabetes, and depression.
Serious flare-ups were common; 38% of patients had experienced one or more exacerbations in the previous year. Of registry respondents, half said they had had at least one exacerbation, and 20% said they had been hospitalized for COPD during that period.
Among patients in the registry, 43% reported that COPD had a high or very high impact on their health, and 45% could not walk at a normal pace without losing their breath.
Almost 90% of patients were receiving a maintenance therapy regimen. The number of exacerbations was “somewhat surprising,” the authors say. They write that the findings may indicate that patients were not receiving appropriate treatment or were not complying with their medication regimens and that there may be a need for nonpharmacologic interventions, such as smoking cessation. They also write that physician education is needed to support earlier diagnosis and treatment so as to delay declines in lung function.
The researchers say their findings highlight “the need for more real-life effectiveness trials to better support decision-making at the primary care level.”
Dr. Yawn is a coinvestigator of one such study, called CAPTURE, which is assessing a screening tool for COPD in primary care practices.
At the University of Illinois, Chicago, Jerry Krishnan, MD, PhD, pulmonologist and professor of medicine and public health, is running the RELIANCE study, which is comparing the use of azithromycin and roflumilast in preventing hospitalization and death among patients with COPD who continue to have exacerbations.
Although RELIANCE involves pulmonologists, Dr. Krishnan told this news organization, it offers a model for building real-world evidence on questions relevant to primary care. “We don’t really know if medications used by patients in my clinic are as effective as reported in clinical trials that were used to obtain regulatory approvals by the U.S. Food and Drug Administration,” he said.
Wilson Pace, MD, a family physician and chief medical officer and chief technology officer of DARTNet, said funders of research are becoming aware of the need for real-world studies along with “gold standard” efficacy trials.
Dr. Pace, who helped conduct the new study, said a remaining obstacle to improving care is “a defeatist attitude of clinicians” who are skeptical about the ability of therapy to have an effect.
Real-world evidence could remedy clinician frustrations, he said. When clinicians are shown that they can improve patients’ quality of life and maybe even reduce the cost of care, “then they will hopefully pay attention,” he said.
Some experts who were not involved in the study said the findings offer an illuminating, although incomplete, picture. Nonpharmacologic interventions, the management of other health problems, and access to specialty care are not addressed, and the researchers didn’t have data on treatment adherence, inhaler technique, and patients’ peak inspiratory flow – factors that influence the effectiveness of medications. The study also lacked information on whether patients received pulmonary rehabilitation to help their heart and lungs work better.
Nicola Hanania, MD, a professor of medicine and director of the Airways Clinical Research Center at Baylor College of Medicine, Houston, said the study “adds a lot to what we have known” but pointed out that COPD is grossly underdiagnosed.
According to one analysis of National Health and Nutrition Examination Surveys, 72% of individuals with COPD don’t know they have the condition. Such patients were not included in the study, Dr. Hanania noted.
“We need pragmatic studies over multiple years to better understand” the condition, Dr. Yawn said. Real-world evidence “based in an academic setting or specialty practices is not sufficient,” she added. “We need to see results from patients and clinics that look like what we have.”
The registry was established and funded by Optimum Patient Care Global, a nonprofit organization, and Boehringer Ingelheim. Dr. Han has consulted for Boehringer Ingelheim, GlaxoSmithKline, and AstraZeneca and has received research support from Novartis and Sunovion. Dr. Yawn has served on advisory boards for GlaxoSmithKline, Astra-Zeneca, Novartis, and Boehringer Ingelheim and has received research funds from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, and Novartis. Dr. Krishnan has disclosed no relevant financial relationshps. Dr. Hanania has received honoraria for serving as consultant or advisory board member for GSK, Boehringer Ingelheim, Novartis, Sanofi, AstraZeneca, Teva, Genentech, and Amgen. His institution has received research grant support on his behalf from GSK, Sanofi, Boehringer Ingelheim, AstraZeneca, Genentech, Teva, and Novartis. Dr. Pace is on the advisory board for Mylan and has received stock from Novo Nordisk, Pfizer, Novartis, Johnson & Johnson, Stryker, Amgen, Gilead, and Sanofi.
A version of this article first appeared on Medscape.com.
Primary care clinicians often struggle to care for their patients with chronic obstructive pulmonary disease (COPD), thanks to a lack of real-world evidence as to which treatments work best.
As a result, potentially preventable life-threatening exacerbations are common among people with the condition. Central to the problem, some experts believe, is that the average patient bears little resemblance to participants in clinical trials of the medications used to treat COPD.
Indeed, a recent study showed that many COPD patients who were receiving maintenance therapy that should have been controlling their disease experienced severe flare-ups – a finding that caught the researchers by surprise.
“We know the benefit of COPD treatments in the context of clinical trials. However, the kinds of patients in primary care may not completely mimic those in clinical trials,” one of the authors, MeiLan Han, MD, a professor of medicine in the division of pulmonary and critical care at the University of Michigan, Ann Arbor, told this news organization. Dr. Han, a volunteer medical spokesperson for the American Lung Association, added that patients “may not be as adherent to medications in real life as they are in clinical trials.”
, who are younger than the average patient with COPD, and who typically are male. Patients are seen in resource-abundant settings designed to maximize adherence to treatment, with supports such as free medication and frequent monitoring – settings far different from those in which most primary care physicians practice.
The authors of the new article said trials conducted with typical patients in primary care settings could help physicians to optimize treatment.
Real-world evidence can shed light on physicians’ intent and on barriers to following guidelines, as well as important patient factors, such as adherence and good inhaler technique, Barbara Yawn, MD, an adjunct professor in the department of family and community health at the University of Minnesota, Minneapolis, and a coauthor of the study, said in an interview.
A window onto patient burden
According to the Centers for Disease Control and Prevention, an estimated $15 million Americans have COPD. Annual costs to the health care system approach $50 billion a year. The death rate for COPD has increased since 1969 as death rates of other major killers in the United States, such as heart disease and cancer, declined, according to a 2015 analysis of death records.
The new study, published in the July/August issue of the Annals of Family Medicine, provides a snapshot of COPD’s toll on patients.
Researchers examined electronic health records of 17,192 patients treated at primary care clinics in five states using a dataset maintained by DARTNet Institute, a nonprofit organization that supports research and quality improvement. They also analyzed self-reported assessments from 1,354 patients in the dataset who are in a registry called Advancing the Patient Experience in COPD.
Over half (56%) of patients were female, White (64%), aged 55-84 years (81%), and current or exsmokers (80%). The vast majority had three or more comorbidities, including hypertension, diabetes, and depression.
Serious flare-ups were common; 38% of patients had experienced one or more exacerbations in the previous year. Of registry respondents, half said they had had at least one exacerbation, and 20% said they had been hospitalized for COPD during that period.
Among patients in the registry, 43% reported that COPD had a high or very high impact on their health, and 45% could not walk at a normal pace without losing their breath.
Almost 90% of patients were receiving a maintenance therapy regimen. The number of exacerbations was “somewhat surprising,” the authors say. They write that the findings may indicate that patients were not receiving appropriate treatment or were not complying with their medication regimens and that there may be a need for nonpharmacologic interventions, such as smoking cessation. They also write that physician education is needed to support earlier diagnosis and treatment so as to delay declines in lung function.
The researchers say their findings highlight “the need for more real-life effectiveness trials to better support decision-making at the primary care level.”
Dr. Yawn is a coinvestigator of one such study, called CAPTURE, which is assessing a screening tool for COPD in primary care practices.
At the University of Illinois, Chicago, Jerry Krishnan, MD, PhD, pulmonologist and professor of medicine and public health, is running the RELIANCE study, which is comparing the use of azithromycin and roflumilast in preventing hospitalization and death among patients with COPD who continue to have exacerbations.
Although RELIANCE involves pulmonologists, Dr. Krishnan told this news organization, it offers a model for building real-world evidence on questions relevant to primary care. “We don’t really know if medications used by patients in my clinic are as effective as reported in clinical trials that were used to obtain regulatory approvals by the U.S. Food and Drug Administration,” he said.
Wilson Pace, MD, a family physician and chief medical officer and chief technology officer of DARTNet, said funders of research are becoming aware of the need for real-world studies along with “gold standard” efficacy trials.
Dr. Pace, who helped conduct the new study, said a remaining obstacle to improving care is “a defeatist attitude of clinicians” who are skeptical about the ability of therapy to have an effect.
Real-world evidence could remedy clinician frustrations, he said. When clinicians are shown that they can improve patients’ quality of life and maybe even reduce the cost of care, “then they will hopefully pay attention,” he said.
Some experts who were not involved in the study said the findings offer an illuminating, although incomplete, picture. Nonpharmacologic interventions, the management of other health problems, and access to specialty care are not addressed, and the researchers didn’t have data on treatment adherence, inhaler technique, and patients’ peak inspiratory flow – factors that influence the effectiveness of medications. The study also lacked information on whether patients received pulmonary rehabilitation to help their heart and lungs work better.
Nicola Hanania, MD, a professor of medicine and director of the Airways Clinical Research Center at Baylor College of Medicine, Houston, said the study “adds a lot to what we have known” but pointed out that COPD is grossly underdiagnosed.
According to one analysis of National Health and Nutrition Examination Surveys, 72% of individuals with COPD don’t know they have the condition. Such patients were not included in the study, Dr. Hanania noted.
“We need pragmatic studies over multiple years to better understand” the condition, Dr. Yawn said. Real-world evidence “based in an academic setting or specialty practices is not sufficient,” she added. “We need to see results from patients and clinics that look like what we have.”
The registry was established and funded by Optimum Patient Care Global, a nonprofit organization, and Boehringer Ingelheim. Dr. Han has consulted for Boehringer Ingelheim, GlaxoSmithKline, and AstraZeneca and has received research support from Novartis and Sunovion. Dr. Yawn has served on advisory boards for GlaxoSmithKline, Astra-Zeneca, Novartis, and Boehringer Ingelheim and has received research funds from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, and Novartis. Dr. Krishnan has disclosed no relevant financial relationshps. Dr. Hanania has received honoraria for serving as consultant or advisory board member for GSK, Boehringer Ingelheim, Novartis, Sanofi, AstraZeneca, Teva, Genentech, and Amgen. His institution has received research grant support on his behalf from GSK, Sanofi, Boehringer Ingelheim, AstraZeneca, Genentech, Teva, and Novartis. Dr. Pace is on the advisory board for Mylan and has received stock from Novo Nordisk, Pfizer, Novartis, Johnson & Johnson, Stryker, Amgen, Gilead, and Sanofi.
A version of this article first appeared on Medscape.com.
Polio: The unwanted sequel
Summer, since 1975, is traditionally a time for the BIG blockbusters to hit theaters. Some are new, others are sequels in successful franchises. Some anticipated, some not as much.
And, in summer 2022, we have the least-wanted sequel in modern history – Polio II: The Return.
Of course, this sequel isn’t in the theaters (unless the concessions staff isn’t washing their hands), definitely isn’t funny, and could potentially cost a lot more money than the latest Marvel Cinematic Universe flick.
Personally and professionally, I’m in the middle generation on the disease. I’m young enough that I never had to worry about catching it or having afflicted classmates. But, as a doctor, I’m old enough to still see the consequences. Like most neurologists, I have a handful of patients who had childhood polio, and still deal with the chronic weakness (and consequent pain and orthopedic issues it brings). Signing off on braces and other mobility aids for them is still commonplace.
One of my attendings in residency was the renowned Parkinson’s disease expert Abraham Lieberman. On rounds it was impossible not to notice his marked limp, a consequence of childhood polio, and he’d tell us what it was like, being a 6-year-old boy and dealing with the disease. You learn as much from hearing firsthand experiences as you do from textbooks.
And now the virus is showing up again. A few victims, a lot of virions circulating in waste water, but it shouldn’t be there at all.
We aren’t in the era when schoolchildren died or were crippled by it. Elementary school kids today don’t see classmates catch polio and never return to school, or see their grieving parents.
To take 1 year: More than 3,000 American children died of polio in 1952, and more than 21,000 were left with lifelong paralysis – many of them still among us.
When you think of an iron lung, you think of polio.
Those were the casualties in a war to save future generations from this, along with smallpox and other horrors.
But today, that war is mostly forgotten. And now scientific evidence is drowned out by whatever’s on Facebook and the hard-earned miracle of vaccination is ignored in favor of a nonmedical “social influencer” on YouTube.
So The majority of the population likely has nothing to worry about. But there may be segments that are hit hard, and when they are they will never accept the obvious reasons why. It will be part of a cover-up, or a conspiracy, or whatever the guy on Parler told them it was.
As doctors, we’re in the middle. We have to give patients the best recommendations we can, based on learning, evidence, and experience, but at the same time have to recognize their autonomy. I’m not following someone around to make sure they get vaccinated, or take the medication I prescribed.
But we’re also the ones who can be held legally responsible for bad outcomes, regardless of the actual facts of the matter. On the flip side, you don’t hear about someone suing a Facebook “influencer” for doling out inaccurate, potentially fatal, medical advice.
So cracks appear in herd immunity, and leaks will happen.
A few generations of neurologists, including mine, have completed training without considering polio in a differential diagnosis. It would, of course, get bandied about in grand rounds or at the conference table, but none of us really took it seriously. To us residents it was more of historical note. “Gone with the Wind” and the “Wizard of Oz” both came out in 1939, and while we all knew of them, none of us were going to be watching them at the theaters.
Unlike them, though, polio is trying make it back to prime time. It’s a sequel nobody wanted.
But here it is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Summer, since 1975, is traditionally a time for the BIG blockbusters to hit theaters. Some are new, others are sequels in successful franchises. Some anticipated, some not as much.
And, in summer 2022, we have the least-wanted sequel in modern history – Polio II: The Return.
Of course, this sequel isn’t in the theaters (unless the concessions staff isn’t washing their hands), definitely isn’t funny, and could potentially cost a lot more money than the latest Marvel Cinematic Universe flick.
Personally and professionally, I’m in the middle generation on the disease. I’m young enough that I never had to worry about catching it or having afflicted classmates. But, as a doctor, I’m old enough to still see the consequences. Like most neurologists, I have a handful of patients who had childhood polio, and still deal with the chronic weakness (and consequent pain and orthopedic issues it brings). Signing off on braces and other mobility aids for them is still commonplace.
One of my attendings in residency was the renowned Parkinson’s disease expert Abraham Lieberman. On rounds it was impossible not to notice his marked limp, a consequence of childhood polio, and he’d tell us what it was like, being a 6-year-old boy and dealing with the disease. You learn as much from hearing firsthand experiences as you do from textbooks.
And now the virus is showing up again. A few victims, a lot of virions circulating in waste water, but it shouldn’t be there at all.
We aren’t in the era when schoolchildren died or were crippled by it. Elementary school kids today don’t see classmates catch polio and never return to school, or see their grieving parents.
To take 1 year: More than 3,000 American children died of polio in 1952, and more than 21,000 were left with lifelong paralysis – many of them still among us.
When you think of an iron lung, you think of polio.
Those were the casualties in a war to save future generations from this, along with smallpox and other horrors.
But today, that war is mostly forgotten. And now scientific evidence is drowned out by whatever’s on Facebook and the hard-earned miracle of vaccination is ignored in favor of a nonmedical “social influencer” on YouTube.
So The majority of the population likely has nothing to worry about. But there may be segments that are hit hard, and when they are they will never accept the obvious reasons why. It will be part of a cover-up, or a conspiracy, or whatever the guy on Parler told them it was.
As doctors, we’re in the middle. We have to give patients the best recommendations we can, based on learning, evidence, and experience, but at the same time have to recognize their autonomy. I’m not following someone around to make sure they get vaccinated, or take the medication I prescribed.
But we’re also the ones who can be held legally responsible for bad outcomes, regardless of the actual facts of the matter. On the flip side, you don’t hear about someone suing a Facebook “influencer” for doling out inaccurate, potentially fatal, medical advice.
So cracks appear in herd immunity, and leaks will happen.
A few generations of neurologists, including mine, have completed training without considering polio in a differential diagnosis. It would, of course, get bandied about in grand rounds or at the conference table, but none of us really took it seriously. To us residents it was more of historical note. “Gone with the Wind” and the “Wizard of Oz” both came out in 1939, and while we all knew of them, none of us were going to be watching them at the theaters.
Unlike them, though, polio is trying make it back to prime time. It’s a sequel nobody wanted.
But here it is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Summer, since 1975, is traditionally a time for the BIG blockbusters to hit theaters. Some are new, others are sequels in successful franchises. Some anticipated, some not as much.
And, in summer 2022, we have the least-wanted sequel in modern history – Polio II: The Return.
Of course, this sequel isn’t in the theaters (unless the concessions staff isn’t washing their hands), definitely isn’t funny, and could potentially cost a lot more money than the latest Marvel Cinematic Universe flick.
Personally and professionally, I’m in the middle generation on the disease. I’m young enough that I never had to worry about catching it or having afflicted classmates. But, as a doctor, I’m old enough to still see the consequences. Like most neurologists, I have a handful of patients who had childhood polio, and still deal with the chronic weakness (and consequent pain and orthopedic issues it brings). Signing off on braces and other mobility aids for them is still commonplace.
One of my attendings in residency was the renowned Parkinson’s disease expert Abraham Lieberman. On rounds it was impossible not to notice his marked limp, a consequence of childhood polio, and he’d tell us what it was like, being a 6-year-old boy and dealing with the disease. You learn as much from hearing firsthand experiences as you do from textbooks.
And now the virus is showing up again. A few victims, a lot of virions circulating in waste water, but it shouldn’t be there at all.
We aren’t in the era when schoolchildren died or were crippled by it. Elementary school kids today don’t see classmates catch polio and never return to school, or see their grieving parents.
To take 1 year: More than 3,000 American children died of polio in 1952, and more than 21,000 were left with lifelong paralysis – many of them still among us.
When you think of an iron lung, you think of polio.
Those were the casualties in a war to save future generations from this, along with smallpox and other horrors.
But today, that war is mostly forgotten. And now scientific evidence is drowned out by whatever’s on Facebook and the hard-earned miracle of vaccination is ignored in favor of a nonmedical “social influencer” on YouTube.
So The majority of the population likely has nothing to worry about. But there may be segments that are hit hard, and when they are they will never accept the obvious reasons why. It will be part of a cover-up, or a conspiracy, or whatever the guy on Parler told them it was.
As doctors, we’re in the middle. We have to give patients the best recommendations we can, based on learning, evidence, and experience, but at the same time have to recognize their autonomy. I’m not following someone around to make sure they get vaccinated, or take the medication I prescribed.
But we’re also the ones who can be held legally responsible for bad outcomes, regardless of the actual facts of the matter. On the flip side, you don’t hear about someone suing a Facebook “influencer” for doling out inaccurate, potentially fatal, medical advice.
So cracks appear in herd immunity, and leaks will happen.
A few generations of neurologists, including mine, have completed training without considering polio in a differential diagnosis. It would, of course, get bandied about in grand rounds or at the conference table, but none of us really took it seriously. To us residents it was more of historical note. “Gone with the Wind” and the “Wizard of Oz” both came out in 1939, and while we all knew of them, none of us were going to be watching them at the theaters.
Unlike them, though, polio is trying make it back to prime time. It’s a sequel nobody wanted.
But here it is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.