User login
Uterine cancer mortality is highest in Black women
A cohort study has found increases in mortality rates among women with non-endometrioid uterine carcinoma, despite incident rates that have stabilized. After correction with hysterectomy, mortality risk was about doubled for Black women, compared with White women, and these results could not be explained by differences in cancer subtype or cancer stage at diagnosis. Non-endometroid uterine carcinoma represents 15%-20% of uterine cancers diagnosed and carries a worse prognosis.
“We do not know why non-endometrioid subtypes are disproportionately increasing among all women, nor do we understand why they are so much more common among non-Hispanic Black women. We need more research to identify risk factors and exposures more specifically associated with non-endometrioid cancers to better understand the strong increases in this subtype among all women and the particularly high rates and recent increases in non-Hispanic black women,” said lead author Megan Clarke, PhD, MHS, the study’s lead author and a cancer epidemiologist with the National Cancer Institute.
The study was published online in JAMA Oncology.
“Physicians should be aware that both incidence and mortality rates of non-endometrioid cancers are on the rise. Because these subtypes are rarer than endometrioid uterine cancers, physicians may be less familiar with diagnosing and treating these aggressive types of cancers. Increasing awareness among clinicians and patients regarding the signs and symptoms of uterine cancer (such as postmenopausal bleeding) and the differences in histologic subtypes among racial and ethnic groups may promote earlier diagnosis and timely referral to appropriate treatment,” Dr. Clarke said.
Previous studies based on death certificates found increased mortality, especially in Black women, but they were limited by an inability to link mortality to tumor characteristics. To address this, the researchers linked mortality data to records of 208,587 women diagnosed with uterine cancer between 2000 and 2017, drawn from the U.S. Surveillance, Epidemiology, and End Results (SEER) Program.
Black women represented 9.7% of cases, but they suffered 17.7% of uterine cancer deaths. Overall, mortality from uterine corpus cancer increased by 1.8% per year (95% confidence interval, 1.5%-2.9%). Non-endometroid cancers increased at 2.7% per year (95% CI, 1.8%-3.6%), and this was higher in Asian (3.4%; 95% CI, 0.3%-6.6%), Black (3.5%; 95% CI, 2.2%-4.9%), Hispanic (6.7%; 95% CI, 1.9%-11.8%), and White women (1.5%; 95% CI, 0.6%-2.4%).
Mortality increased 1.8% per year overall for uterine cancer and 2.7% per year for non-endometrioid uterine cancer. There was no increase in mortality seen in endometrioid cancers.
“The concerning rise in deaths from non-endometrioid cancers warrants clinical attention. Our findings suggest that there may be several factors contributing to racial disparities in uterine cancer mortality. Higher mortality rates among non-Hispanic Black women are partly attributable to higher incidence of tumors with aggressive subtypes and advanced stages. However, non-Hispanic Black women in our study who were diagnosed with less aggressive subtypes and early-stage disease also had the highest mortality rates,” said Dr. Clarke.
That suggests that inequities of treatment and high-quality care may be at least partly to blame, since those factors are known to contribute to differences in uterine cancer outcomes. “Other factors including comorbidities, health care facility characteristics, treatment preferences and adherence, patient and provider communication, provider bias, discrimination and structural racism, and potential biologic differences in response to treatment need to be better understood in terms of how they influence racial disparities,” Dr. Clarke said.
Dr. Clarke reported no relevant disclosures.
A cohort study has found increases in mortality rates among women with non-endometrioid uterine carcinoma, despite incident rates that have stabilized. After correction with hysterectomy, mortality risk was about doubled for Black women, compared with White women, and these results could not be explained by differences in cancer subtype or cancer stage at diagnosis. Non-endometroid uterine carcinoma represents 15%-20% of uterine cancers diagnosed and carries a worse prognosis.
“We do not know why non-endometrioid subtypes are disproportionately increasing among all women, nor do we understand why they are so much more common among non-Hispanic Black women. We need more research to identify risk factors and exposures more specifically associated with non-endometrioid cancers to better understand the strong increases in this subtype among all women and the particularly high rates and recent increases in non-Hispanic black women,” said lead author Megan Clarke, PhD, MHS, the study’s lead author and a cancer epidemiologist with the National Cancer Institute.
The study was published online in JAMA Oncology.
“Physicians should be aware that both incidence and mortality rates of non-endometrioid cancers are on the rise. Because these subtypes are rarer than endometrioid uterine cancers, physicians may be less familiar with diagnosing and treating these aggressive types of cancers. Increasing awareness among clinicians and patients regarding the signs and symptoms of uterine cancer (such as postmenopausal bleeding) and the differences in histologic subtypes among racial and ethnic groups may promote earlier diagnosis and timely referral to appropriate treatment,” Dr. Clarke said.
Previous studies based on death certificates found increased mortality, especially in Black women, but they were limited by an inability to link mortality to tumor characteristics. To address this, the researchers linked mortality data to records of 208,587 women diagnosed with uterine cancer between 2000 and 2017, drawn from the U.S. Surveillance, Epidemiology, and End Results (SEER) Program.
Black women represented 9.7% of cases, but they suffered 17.7% of uterine cancer deaths. Overall, mortality from uterine corpus cancer increased by 1.8% per year (95% confidence interval, 1.5%-2.9%). Non-endometroid cancers increased at 2.7% per year (95% CI, 1.8%-3.6%), and this was higher in Asian (3.4%; 95% CI, 0.3%-6.6%), Black (3.5%; 95% CI, 2.2%-4.9%), Hispanic (6.7%; 95% CI, 1.9%-11.8%), and White women (1.5%; 95% CI, 0.6%-2.4%).
Mortality increased 1.8% per year overall for uterine cancer and 2.7% per year for non-endometrioid uterine cancer. There was no increase in mortality seen in endometrioid cancers.
“The concerning rise in deaths from non-endometrioid cancers warrants clinical attention. Our findings suggest that there may be several factors contributing to racial disparities in uterine cancer mortality. Higher mortality rates among non-Hispanic Black women are partly attributable to higher incidence of tumors with aggressive subtypes and advanced stages. However, non-Hispanic Black women in our study who were diagnosed with less aggressive subtypes and early-stage disease also had the highest mortality rates,” said Dr. Clarke.
That suggests that inequities of treatment and high-quality care may be at least partly to blame, since those factors are known to contribute to differences in uterine cancer outcomes. “Other factors including comorbidities, health care facility characteristics, treatment preferences and adherence, patient and provider communication, provider bias, discrimination and structural racism, and potential biologic differences in response to treatment need to be better understood in terms of how they influence racial disparities,” Dr. Clarke said.
Dr. Clarke reported no relevant disclosures.
A cohort study has found increases in mortality rates among women with non-endometrioid uterine carcinoma, despite incident rates that have stabilized. After correction with hysterectomy, mortality risk was about doubled for Black women, compared with White women, and these results could not be explained by differences in cancer subtype or cancer stage at diagnosis. Non-endometroid uterine carcinoma represents 15%-20% of uterine cancers diagnosed and carries a worse prognosis.
“We do not know why non-endometrioid subtypes are disproportionately increasing among all women, nor do we understand why they are so much more common among non-Hispanic Black women. We need more research to identify risk factors and exposures more specifically associated with non-endometrioid cancers to better understand the strong increases in this subtype among all women and the particularly high rates and recent increases in non-Hispanic black women,” said lead author Megan Clarke, PhD, MHS, the study’s lead author and a cancer epidemiologist with the National Cancer Institute.
The study was published online in JAMA Oncology.
“Physicians should be aware that both incidence and mortality rates of non-endometrioid cancers are on the rise. Because these subtypes are rarer than endometrioid uterine cancers, physicians may be less familiar with diagnosing and treating these aggressive types of cancers. Increasing awareness among clinicians and patients regarding the signs and symptoms of uterine cancer (such as postmenopausal bleeding) and the differences in histologic subtypes among racial and ethnic groups may promote earlier diagnosis and timely referral to appropriate treatment,” Dr. Clarke said.
Previous studies based on death certificates found increased mortality, especially in Black women, but they were limited by an inability to link mortality to tumor characteristics. To address this, the researchers linked mortality data to records of 208,587 women diagnosed with uterine cancer between 2000 and 2017, drawn from the U.S. Surveillance, Epidemiology, and End Results (SEER) Program.
Black women represented 9.7% of cases, but they suffered 17.7% of uterine cancer deaths. Overall, mortality from uterine corpus cancer increased by 1.8% per year (95% confidence interval, 1.5%-2.9%). Non-endometroid cancers increased at 2.7% per year (95% CI, 1.8%-3.6%), and this was higher in Asian (3.4%; 95% CI, 0.3%-6.6%), Black (3.5%; 95% CI, 2.2%-4.9%), Hispanic (6.7%; 95% CI, 1.9%-11.8%), and White women (1.5%; 95% CI, 0.6%-2.4%).
Mortality increased 1.8% per year overall for uterine cancer and 2.7% per year for non-endometrioid uterine cancer. There was no increase in mortality seen in endometrioid cancers.
“The concerning rise in deaths from non-endometrioid cancers warrants clinical attention. Our findings suggest that there may be several factors contributing to racial disparities in uterine cancer mortality. Higher mortality rates among non-Hispanic Black women are partly attributable to higher incidence of tumors with aggressive subtypes and advanced stages. However, non-Hispanic Black women in our study who were diagnosed with less aggressive subtypes and early-stage disease also had the highest mortality rates,” said Dr. Clarke.
That suggests that inequities of treatment and high-quality care may be at least partly to blame, since those factors are known to contribute to differences in uterine cancer outcomes. “Other factors including comorbidities, health care facility characteristics, treatment preferences and adherence, patient and provider communication, provider bias, discrimination and structural racism, and potential biologic differences in response to treatment need to be better understood in terms of how they influence racial disparities,” Dr. Clarke said.
Dr. Clarke reported no relevant disclosures.
FROM JAMA ONCOLOGY
Coffee drinkers – even those with a sweet tooth – live longer
Among more than 170,000 people in the United Kingdom, those who drank about two to four cups of coffee a day, with or without sugar, had a lower rate of death than those who didn’t drink coffee, reported lead author Dan Liu, MD, of the department of epidemiology at Southern Medical University, Guangzhou, China.
“Previous observational studies have suggested an association between coffee intake and reduced risk for death, but they did not distinguish between coffee consumed with sugar or artificial sweeteners and coffee consumed without,” Dr. Liu, who is also of the department of public health and preventive medicine, Jinan University, Guangzhou, China, and colleagues wrote in Annals of Internal Medicine.
To learn more, the investigators turned to the UK Biobank, which recruited approximately half a million participants in the United Kingdom between 2006 and 2010 to undergo a variety of questionnaires, interviews, physical measurements, and medical tests. Out of this group, 171,616 participants completed at least one dietary questionnaire and met the criteria for the present study, including lack of cancer or cardiovascular disease upon enrollment.
Results from these questionnaires showed that 55.4% of participants drank coffee without any sweetener, 14.3% drank coffee with sugar, 6.1% drank coffee with artificial sweetener, and 24.2% did not drink coffee at all. Coffee drinkers were further sorted into groups based on how many cups of coffee they drank per day.
Coffee drinkers were significantly less likely to die from any cause
Over the course of about 7 years, 3,177 of the participants died, including 1,725 who died from cancer and 628 who died from cardiovascular disease.
After accounting for other factors that might impact risk of death, like lifestyle choices, the investigators found that coffee drinkers were significantly less likely to die from any cause, cardiovascular disease, or cancer, than those who didn’t drink coffee at all. This benefit was observed across types of coffee, including ground, instant, and decaffeinated varieties. The protective effects of coffee were most apparent in people who drank about two to four cups a day, among whom death was about 30% less likely, regardless of whether they added sugar to their coffee or not. Individuals who drank coffee with artificial sweetener did not live significantly longer than those who drank no coffee at all; however, the investigators suggested that this result may have been skewed by higher rates of negative health factors, such as obesity and hypertension, in the artificial sweetener group.
Dr. Liu and colleagues noted that their findings align with previous studies linking coffee consumption with survival. Like those other studies, the present data revealed a “U-shaped” benefit curve, in which moderate coffee consumption was associated with longer life, whereas low or no consumption and high consumption were not.
Experts caution against drinking sweetened beverages despite new findings
Although the present findings suggested that adding sugar did not eliminate the health benefits of coffee, Dr. Liu and colleagues still cautioned against sweetened beverages, citing widely known associations between sugar consumption and poor health.
In an accompanying editorial, Christina C. Wee, MD, MPH, deputy editor of Annals of Internal Medicine, pointed out a key detail from the data: the amount of sugar added to coffee in the U.K. study may be dwarfed by the amount consumed by some coffee drinkers across the pond.
“The average dose of added sugar per cup of sweetened coffee [in the study] was only a little over a teaspoon, or about 4 grams,” Dr. Wee wrote. “This is a far cry from the 15 grams of sugar in an 8-ounce cup of caramel macchiato at a popular U.S. coffee chain.”
Still, Dr. Wee, an associate professor of medicine at Harvard Medical School, Boston, and director of the obesity research program in the division of general medicine at Beth Israel Deaconess Medical Center, Boston, suggested that your typical coffee drinker can feel safe in their daily habit.
“The evidence does not suggest a need for most coffee drinkers – particularly those who drink it with no or modest amounts of sugar – to eliminate coffee,” she wrote. “So drink up – but it would be prudent to avoid too many caramel macchiatos while more evidence brews.”
Estefanía Toledo, MD, MPH, PhD, of the department of preventive medicine and public health at the University of Navarra, Pamplona, Spain, offered a similar takeaway.
“For those who enjoy drinking coffee, are not pregnant or lactating, and do not have special health conditions, coffee consumption could be considered part of a healthy lifestyle,” Dr. Toledo said in a written comment. “I would recommend adding as little sugar as possible to coffee until more evidence has been accrued.”
Dr. Toledo, who previously published a study showing a link between coffee and extended survival, noted that moderate coffee consumption has “repeatedly” been associated with lower rates of “several chronic diseases” and death, but there still isn’t enough evidence to recommend coffee for those who don’t already drink it.
More long-term research is needed, Dr. Toledo said, ideally with studies comparing changes in coffee consumption and health outcomes over time. These may not be forthcoming, however, as such trials are “not easy and feasible to conduct.”
David Kao, MD, assistant professor of medicine-cardiology and medical director of the school of medicine at the University of Colorado at Denver, Aurora, said that the study conducted by Dr. Liu and colleagues is a “very well-executed analysis” that strengthens our confidence in the safety of long-term coffee consumption, even for patients with heart disease.
Dr. Kao, who recently published an analysis showing that higher coffee intake is associated with a lower risk of heart failure, refrained from advising anyone to up their coffee quota.
“I remain cautious about stating too strongly that people should increase coffee intake purely to improve survival,” Dr. Kao said in a written comment. “That said, it does not appear harmful to increase it some, until you drink consistently more than six to seven cups per day.”
The study was supported by the National Natural Science Foundation of China, the Young Elite Scientist Sponsorship Program by CAST, the Guangdong Basic and Applied Basic Research Foundation, and others. Dr. Toledo and Dr. Kao disclosed no relevant conflicts of interest.
Among more than 170,000 people in the United Kingdom, those who drank about two to four cups of coffee a day, with or without sugar, had a lower rate of death than those who didn’t drink coffee, reported lead author Dan Liu, MD, of the department of epidemiology at Southern Medical University, Guangzhou, China.
“Previous observational studies have suggested an association between coffee intake and reduced risk for death, but they did not distinguish between coffee consumed with sugar or artificial sweeteners and coffee consumed without,” Dr. Liu, who is also of the department of public health and preventive medicine, Jinan University, Guangzhou, China, and colleagues wrote in Annals of Internal Medicine.
To learn more, the investigators turned to the UK Biobank, which recruited approximately half a million participants in the United Kingdom between 2006 and 2010 to undergo a variety of questionnaires, interviews, physical measurements, and medical tests. Out of this group, 171,616 participants completed at least one dietary questionnaire and met the criteria for the present study, including lack of cancer or cardiovascular disease upon enrollment.
Results from these questionnaires showed that 55.4% of participants drank coffee without any sweetener, 14.3% drank coffee with sugar, 6.1% drank coffee with artificial sweetener, and 24.2% did not drink coffee at all. Coffee drinkers were further sorted into groups based on how many cups of coffee they drank per day.
Coffee drinkers were significantly less likely to die from any cause
Over the course of about 7 years, 3,177 of the participants died, including 1,725 who died from cancer and 628 who died from cardiovascular disease.
After accounting for other factors that might impact risk of death, like lifestyle choices, the investigators found that coffee drinkers were significantly less likely to die from any cause, cardiovascular disease, or cancer, than those who didn’t drink coffee at all. This benefit was observed across types of coffee, including ground, instant, and decaffeinated varieties. The protective effects of coffee were most apparent in people who drank about two to four cups a day, among whom death was about 30% less likely, regardless of whether they added sugar to their coffee or not. Individuals who drank coffee with artificial sweetener did not live significantly longer than those who drank no coffee at all; however, the investigators suggested that this result may have been skewed by higher rates of negative health factors, such as obesity and hypertension, in the artificial sweetener group.
Dr. Liu and colleagues noted that their findings align with previous studies linking coffee consumption with survival. Like those other studies, the present data revealed a “U-shaped” benefit curve, in which moderate coffee consumption was associated with longer life, whereas low or no consumption and high consumption were not.
Experts caution against drinking sweetened beverages despite new findings
Although the present findings suggested that adding sugar did not eliminate the health benefits of coffee, Dr. Liu and colleagues still cautioned against sweetened beverages, citing widely known associations between sugar consumption and poor health.
In an accompanying editorial, Christina C. Wee, MD, MPH, deputy editor of Annals of Internal Medicine, pointed out a key detail from the data: the amount of sugar added to coffee in the U.K. study may be dwarfed by the amount consumed by some coffee drinkers across the pond.
“The average dose of added sugar per cup of sweetened coffee [in the study] was only a little over a teaspoon, or about 4 grams,” Dr. Wee wrote. “This is a far cry from the 15 grams of sugar in an 8-ounce cup of caramel macchiato at a popular U.S. coffee chain.”
Still, Dr. Wee, an associate professor of medicine at Harvard Medical School, Boston, and director of the obesity research program in the division of general medicine at Beth Israel Deaconess Medical Center, Boston, suggested that your typical coffee drinker can feel safe in their daily habit.
“The evidence does not suggest a need for most coffee drinkers – particularly those who drink it with no or modest amounts of sugar – to eliminate coffee,” she wrote. “So drink up – but it would be prudent to avoid too many caramel macchiatos while more evidence brews.”
Estefanía Toledo, MD, MPH, PhD, of the department of preventive medicine and public health at the University of Navarra, Pamplona, Spain, offered a similar takeaway.
“For those who enjoy drinking coffee, are not pregnant or lactating, and do not have special health conditions, coffee consumption could be considered part of a healthy lifestyle,” Dr. Toledo said in a written comment. “I would recommend adding as little sugar as possible to coffee until more evidence has been accrued.”
Dr. Toledo, who previously published a study showing a link between coffee and extended survival, noted that moderate coffee consumption has “repeatedly” been associated with lower rates of “several chronic diseases” and death, but there still isn’t enough evidence to recommend coffee for those who don’t already drink it.
More long-term research is needed, Dr. Toledo said, ideally with studies comparing changes in coffee consumption and health outcomes over time. These may not be forthcoming, however, as such trials are “not easy and feasible to conduct.”
David Kao, MD, assistant professor of medicine-cardiology and medical director of the school of medicine at the University of Colorado at Denver, Aurora, said that the study conducted by Dr. Liu and colleagues is a “very well-executed analysis” that strengthens our confidence in the safety of long-term coffee consumption, even for patients with heart disease.
Dr. Kao, who recently published an analysis showing that higher coffee intake is associated with a lower risk of heart failure, refrained from advising anyone to up their coffee quota.
“I remain cautious about stating too strongly that people should increase coffee intake purely to improve survival,” Dr. Kao said in a written comment. “That said, it does not appear harmful to increase it some, until you drink consistently more than six to seven cups per day.”
The study was supported by the National Natural Science Foundation of China, the Young Elite Scientist Sponsorship Program by CAST, the Guangdong Basic and Applied Basic Research Foundation, and others. Dr. Toledo and Dr. Kao disclosed no relevant conflicts of interest.
Among more than 170,000 people in the United Kingdom, those who drank about two to four cups of coffee a day, with or without sugar, had a lower rate of death than those who didn’t drink coffee, reported lead author Dan Liu, MD, of the department of epidemiology at Southern Medical University, Guangzhou, China.
“Previous observational studies have suggested an association between coffee intake and reduced risk for death, but they did not distinguish between coffee consumed with sugar or artificial sweeteners and coffee consumed without,” Dr. Liu, who is also of the department of public health and preventive medicine, Jinan University, Guangzhou, China, and colleagues wrote in Annals of Internal Medicine.
To learn more, the investigators turned to the UK Biobank, which recruited approximately half a million participants in the United Kingdom between 2006 and 2010 to undergo a variety of questionnaires, interviews, physical measurements, and medical tests. Out of this group, 171,616 participants completed at least one dietary questionnaire and met the criteria for the present study, including lack of cancer or cardiovascular disease upon enrollment.
Results from these questionnaires showed that 55.4% of participants drank coffee without any sweetener, 14.3% drank coffee with sugar, 6.1% drank coffee with artificial sweetener, and 24.2% did not drink coffee at all. Coffee drinkers were further sorted into groups based on how many cups of coffee they drank per day.
Coffee drinkers were significantly less likely to die from any cause
Over the course of about 7 years, 3,177 of the participants died, including 1,725 who died from cancer and 628 who died from cardiovascular disease.
After accounting for other factors that might impact risk of death, like lifestyle choices, the investigators found that coffee drinkers were significantly less likely to die from any cause, cardiovascular disease, or cancer, than those who didn’t drink coffee at all. This benefit was observed across types of coffee, including ground, instant, and decaffeinated varieties. The protective effects of coffee were most apparent in people who drank about two to four cups a day, among whom death was about 30% less likely, regardless of whether they added sugar to their coffee or not. Individuals who drank coffee with artificial sweetener did not live significantly longer than those who drank no coffee at all; however, the investigators suggested that this result may have been skewed by higher rates of negative health factors, such as obesity and hypertension, in the artificial sweetener group.
Dr. Liu and colleagues noted that their findings align with previous studies linking coffee consumption with survival. Like those other studies, the present data revealed a “U-shaped” benefit curve, in which moderate coffee consumption was associated with longer life, whereas low or no consumption and high consumption were not.
Experts caution against drinking sweetened beverages despite new findings
Although the present findings suggested that adding sugar did not eliminate the health benefits of coffee, Dr. Liu and colleagues still cautioned against sweetened beverages, citing widely known associations between sugar consumption and poor health.
In an accompanying editorial, Christina C. Wee, MD, MPH, deputy editor of Annals of Internal Medicine, pointed out a key detail from the data: the amount of sugar added to coffee in the U.K. study may be dwarfed by the amount consumed by some coffee drinkers across the pond.
“The average dose of added sugar per cup of sweetened coffee [in the study] was only a little over a teaspoon, or about 4 grams,” Dr. Wee wrote. “This is a far cry from the 15 grams of sugar in an 8-ounce cup of caramel macchiato at a popular U.S. coffee chain.”
Still, Dr. Wee, an associate professor of medicine at Harvard Medical School, Boston, and director of the obesity research program in the division of general medicine at Beth Israel Deaconess Medical Center, Boston, suggested that your typical coffee drinker can feel safe in their daily habit.
“The evidence does not suggest a need for most coffee drinkers – particularly those who drink it with no or modest amounts of sugar – to eliminate coffee,” she wrote. “So drink up – but it would be prudent to avoid too many caramel macchiatos while more evidence brews.”
Estefanía Toledo, MD, MPH, PhD, of the department of preventive medicine and public health at the University of Navarra, Pamplona, Spain, offered a similar takeaway.
“For those who enjoy drinking coffee, are not pregnant or lactating, and do not have special health conditions, coffee consumption could be considered part of a healthy lifestyle,” Dr. Toledo said in a written comment. “I would recommend adding as little sugar as possible to coffee until more evidence has been accrued.”
Dr. Toledo, who previously published a study showing a link between coffee and extended survival, noted that moderate coffee consumption has “repeatedly” been associated with lower rates of “several chronic diseases” and death, but there still isn’t enough evidence to recommend coffee for those who don’t already drink it.
More long-term research is needed, Dr. Toledo said, ideally with studies comparing changes in coffee consumption and health outcomes over time. These may not be forthcoming, however, as such trials are “not easy and feasible to conduct.”
David Kao, MD, assistant professor of medicine-cardiology and medical director of the school of medicine at the University of Colorado at Denver, Aurora, said that the study conducted by Dr. Liu and colleagues is a “very well-executed analysis” that strengthens our confidence in the safety of long-term coffee consumption, even for patients with heart disease.
Dr. Kao, who recently published an analysis showing that higher coffee intake is associated with a lower risk of heart failure, refrained from advising anyone to up their coffee quota.
“I remain cautious about stating too strongly that people should increase coffee intake purely to improve survival,” Dr. Kao said in a written comment. “That said, it does not appear harmful to increase it some, until you drink consistently more than six to seven cups per day.”
The study was supported by the National Natural Science Foundation of China, the Young Elite Scientist Sponsorship Program by CAST, the Guangdong Basic and Applied Basic Research Foundation, and others. Dr. Toledo and Dr. Kao disclosed no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
What can we do about mass shootings?
“It must be mental illness. My mind cannot possibly conceive of an alternative. A rational healthy mind cannot be capable of this, Doc.”
These were the opening words of one of many discussions that I had with patients in the wake of yet another gut-wrenching tragedy where we saw innocent children and their teachers murdered in school.
This narrative is appealing, regardless of whether or not it is true, because we find some measure of solace in it. We are now at a point in our nation where we are not ashamed to say that we live in a mental health crisis. It is inconceivable to us that a “healthy” brain could plot and premeditate the cold-blooded execution of children.
But just because something feels true does not mean that it actually is.
I personally felt this after a shooter walked into my hospital and shot my coworkers, murdering one and injuring several others. How can this be? It didn’t make a whole lot of sense then. I don’t know if it makes any more sense now. But he had no mental illness that we knew of.
Do any mass shooters have untreated mental illness?
Could we have diagnosed those cases earlier? Intervened sooner? Offered more effective treatment? Certainly. Would that have explain away the rest of the cases? Unfortunately, no.
What is it, then?
The scary answer is that the people who are capable of doing this are not so far away. They are not the folks that we would image locking up in a “psych ward” and throwing away the key. They are, rather, people who are lonely, neglected, rejected, bullied, and broken down by life. Anger, hatred, racism, and evil may be ailments of the soul, but they are not mental illnesses. The carnage they produce is just as tangible. As a psychiatrist, I must admit to you that I do not have a good medication to treat these manifestations of the human condition.
What do we do as a society?
Gun reform is the first obvious and essential answer, without which little else is truly as impactful. We must advocate for it and fight tirelessly.
But at the time you will read this article, your disgruntled coworker will be able to walk into a local store in a moment of despair, anguish, and hopelessness and purchase a semiautomatic weapon of war.
What if we were to start seeing, as a society, that our lives are interwoven? What if we saw that our health is truly interdependent? The COVID-19 pandemic shattered many things in our lives, but one element in particular is our radical individualism. We saw that the choices you make certainly affect me and vice versa. We saw that public health is just that – a public matter, not a private one. We saw that there are some areas of our lives that force us to come together for our own survival.
Perhaps politicians will not save us here. Perhaps kindness will. Empathy can be as potent as legislation, and compassion as impactful as a Twitter hashtag. We each know a lonely coworker, an isolated neighbor, a bullied student, or someone beaten down by life.
What if some of the prevention is in fact in our hands? Together.
“Darkness cannot drive out darkness. Only light can do that. Hate cannot drive out hate; only love can do that.” – Reverend Dr. Martin Luther King, Jr.
Mena Mirhom, MD, is an assistant professor of psychiatry at Columbia University and teaches writing to public psychiatry fellows. He is a board-certified psychiatrist and a consultant for the National Basketball Players Association, treating NBA players and staff.
A version of this article first appeared on Medscape.com.
“It must be mental illness. My mind cannot possibly conceive of an alternative. A rational healthy mind cannot be capable of this, Doc.”
These were the opening words of one of many discussions that I had with patients in the wake of yet another gut-wrenching tragedy where we saw innocent children and their teachers murdered in school.
This narrative is appealing, regardless of whether or not it is true, because we find some measure of solace in it. We are now at a point in our nation where we are not ashamed to say that we live in a mental health crisis. It is inconceivable to us that a “healthy” brain could plot and premeditate the cold-blooded execution of children.
But just because something feels true does not mean that it actually is.
I personally felt this after a shooter walked into my hospital and shot my coworkers, murdering one and injuring several others. How can this be? It didn’t make a whole lot of sense then. I don’t know if it makes any more sense now. But he had no mental illness that we knew of.
Do any mass shooters have untreated mental illness?
Could we have diagnosed those cases earlier? Intervened sooner? Offered more effective treatment? Certainly. Would that have explain away the rest of the cases? Unfortunately, no.
What is it, then?
The scary answer is that the people who are capable of doing this are not so far away. They are not the folks that we would image locking up in a “psych ward” and throwing away the key. They are, rather, people who are lonely, neglected, rejected, bullied, and broken down by life. Anger, hatred, racism, and evil may be ailments of the soul, but they are not mental illnesses. The carnage they produce is just as tangible. As a psychiatrist, I must admit to you that I do not have a good medication to treat these manifestations of the human condition.
What do we do as a society?
Gun reform is the first obvious and essential answer, without which little else is truly as impactful. We must advocate for it and fight tirelessly.
But at the time you will read this article, your disgruntled coworker will be able to walk into a local store in a moment of despair, anguish, and hopelessness and purchase a semiautomatic weapon of war.
What if we were to start seeing, as a society, that our lives are interwoven? What if we saw that our health is truly interdependent? The COVID-19 pandemic shattered many things in our lives, but one element in particular is our radical individualism. We saw that the choices you make certainly affect me and vice versa. We saw that public health is just that – a public matter, not a private one. We saw that there are some areas of our lives that force us to come together for our own survival.
Perhaps politicians will not save us here. Perhaps kindness will. Empathy can be as potent as legislation, and compassion as impactful as a Twitter hashtag. We each know a lonely coworker, an isolated neighbor, a bullied student, or someone beaten down by life.
What if some of the prevention is in fact in our hands? Together.
“Darkness cannot drive out darkness. Only light can do that. Hate cannot drive out hate; only love can do that.” – Reverend Dr. Martin Luther King, Jr.
Mena Mirhom, MD, is an assistant professor of psychiatry at Columbia University and teaches writing to public psychiatry fellows. He is a board-certified psychiatrist and a consultant for the National Basketball Players Association, treating NBA players and staff.
A version of this article first appeared on Medscape.com.
“It must be mental illness. My mind cannot possibly conceive of an alternative. A rational healthy mind cannot be capable of this, Doc.”
These were the opening words of one of many discussions that I had with patients in the wake of yet another gut-wrenching tragedy where we saw innocent children and their teachers murdered in school.
This narrative is appealing, regardless of whether or not it is true, because we find some measure of solace in it. We are now at a point in our nation where we are not ashamed to say that we live in a mental health crisis. It is inconceivable to us that a “healthy” brain could plot and premeditate the cold-blooded execution of children.
But just because something feels true does not mean that it actually is.
I personally felt this after a shooter walked into my hospital and shot my coworkers, murdering one and injuring several others. How can this be? It didn’t make a whole lot of sense then. I don’t know if it makes any more sense now. But he had no mental illness that we knew of.
Do any mass shooters have untreated mental illness?
Could we have diagnosed those cases earlier? Intervened sooner? Offered more effective treatment? Certainly. Would that have explain away the rest of the cases? Unfortunately, no.
What is it, then?
The scary answer is that the people who are capable of doing this are not so far away. They are not the folks that we would image locking up in a “psych ward” and throwing away the key. They are, rather, people who are lonely, neglected, rejected, bullied, and broken down by life. Anger, hatred, racism, and evil may be ailments of the soul, but they are not mental illnesses. The carnage they produce is just as tangible. As a psychiatrist, I must admit to you that I do not have a good medication to treat these manifestations of the human condition.
What do we do as a society?
Gun reform is the first obvious and essential answer, without which little else is truly as impactful. We must advocate for it and fight tirelessly.
But at the time you will read this article, your disgruntled coworker will be able to walk into a local store in a moment of despair, anguish, and hopelessness and purchase a semiautomatic weapon of war.
What if we were to start seeing, as a society, that our lives are interwoven? What if we saw that our health is truly interdependent? The COVID-19 pandemic shattered many things in our lives, but one element in particular is our radical individualism. We saw that the choices you make certainly affect me and vice versa. We saw that public health is just that – a public matter, not a private one. We saw that there are some areas of our lives that force us to come together for our own survival.
Perhaps politicians will not save us here. Perhaps kindness will. Empathy can be as potent as legislation, and compassion as impactful as a Twitter hashtag. We each know a lonely coworker, an isolated neighbor, a bullied student, or someone beaten down by life.
What if some of the prevention is in fact in our hands? Together.
“Darkness cannot drive out darkness. Only light can do that. Hate cannot drive out hate; only love can do that.” – Reverend Dr. Martin Luther King, Jr.
Mena Mirhom, MD, is an assistant professor of psychiatry at Columbia University and teaches writing to public psychiatry fellows. He is a board-certified psychiatrist and a consultant for the National Basketball Players Association, treating NBA players and staff.
A version of this article first appeared on Medscape.com.
Where Does the Hospital Belong? Perspectives on Hospital at Home in the 21st Century
From Medically Home Group, Boston, MA.
Brick-and-mortar hospitals in the United States have historically been considered the dominant setting for providing care to patients. The coordination and delivery of care has previously been bound to physical hospitals largely because multidisciplinary services were only accessible in an individual location. While the fundamental make-up of these services remains unchanged, these services are now available in alternate settings. Some of these services include access to a patient care team, supplies, diagnostics, pharmacy, and advanced therapeutic interventions. Presently, the physical environment is becoming increasingly irrelevant as the core of what makes the traditional hospital—the professional staff, collaborative work processes, and the dynamics of the space—have all been translated into a modern digitally integrated environment. The elements necessary to providing safe, effective care in a physical hospital setting are now available in a patient’s home.
Impetus for the Model
As hospitals reconsider how and where they deliver patient care because of limited resources, the hospital-at-home model has gained significant momentum and interest. This model transforms a home into a hospital. The inpatient acute care episode is entirely substituted with an intensive at-home hospital admission enabled by technology, multidisciplinary teams, and ancillary services. Furthermore, patients requiring post-acute support can be transitioned to their next phase of care seamlessly. Given the nationwide nursing shortage, aging population, challenges uncovered by the COVID-19 pandemic, rising hospital costs, nurse/provider burnout related to challenging work environments, and capacity constraints, a shift toward the combination of virtual and in-home care is imperative. The hospital-at-home model has been associated with superior patient outcomes, including reduced risks of delirium, improved functional status, improved patient and family member satisfaction, reduced mortality, reduced readmissions, and significantly lower costs.1 COVID-19 alone has unmasked major facility-based deficiencies and limitations of our health care system. While the pandemic is not the impetus for the hospital-at-home model, the extended stress of this event has created a unique opportunity to reimagine and transform our health care delivery system so that it is less fragmented and more flexible.
Nursing in the Model
Nursing is central to the hospital-at-home model. Virtual nurses provide meticulous care plan oversight, assessment, and documentation across in-home service providers, to ensure holistic, safe, transparent, and continuous progression toward care plan milestones. The virtual nurse monitors patients using in-home technology that is set up at the time of admission. Connecting with patients to verify social and medical needs, the virtual nurse advocates for their patients and uses these technologies to care and deploy on-demand hands-on services to the patient. Service providers such as paramedics, infusion nurses, or home health nurses may be deployed to provide services in the patient’s home. By bringing in supplies, therapeutics, and interdisciplinary team members, the capabilities of a brick-and-mortar hospital are replicated in the home. All actions that occur wherever the patient is receiving care are overseen by professional nursing staff; in short, virtual nurses are the equivalent of bedside nurses in the brick-and-mortar health care facilities.
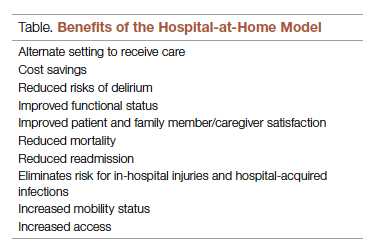
Potential Benefits
There are many benefits to the hospital-at-home model (Table). This health care model can be particularly helpful for patients who require frequent admission to acute care facilities, and is well suited for patients with a range of conditions, including those with COVID-19, pneumonia, cellulitis, or congestive heart failure. This care model helps eliminate some of the stressors for patients who have chronic illnesses or other conditions that require frequent hospital admissions. Patients can independently recover at home and can also be surrounded by their loved ones and pets while recovering. This care approach additionally eliminates the risk of hospital-acquired infections and injuries. The hospital-at-home model allows for increased mobility,2 as patients are familiar with their surroundings, resulting in reduced onset of delirium. Additionally, patients with improved mobility performance are less likely to experience negative health outcomes.3 There is less chance of sleep disruption as the patient is sleeping in their own bed—no unfamiliar roommate, no call bells or health care personnel frequently coming into the room. The in-home technology set up for remote patient monitoring is designed with the user in mind. Ease of use empowers the patient to collaborate with their care team on their own terms and center the priorities of themselves and their families.
Positive Outcomes
The hospital-at-home model is associated with positive outcomes. The authors of a systematic review identified 10 randomized controlled trials of hospital-at-home programs (with a total of 1372 patients), but were able to obtain data for only 5 of these trials (with a total of 844 patients).4 They found a 38% reduction in 6-month mortality for patients who received hospital care at home, as well as significantly higher patient satisfaction across a range of medical conditions, including patients with cellulitis and community-acquired pneumonia, as well as elderly patients with multiple medical conditions. The authors concluded that hospital care at home was less expensive than admission to an acute care hospital.4 Similarly, a meta-analysis done by Caplan et al5 that included 61 randomized controlled trials concluded that hospital at home is associated with reductions in mortality, readmission rates, and cost, and increases in patient and caregiver satisfaction. Levine et al2 found reduced costs and utilization with home hospitalization compared to in-hospital care, as well as improved patient mobility status.
The home is the ideal place to empower patients and caregivers to engage in self-management.2 Receiving hospital care at home eliminates the need for dealing with transportation arrangements, traffic, road tolls, and time/scheduling constraints, or finding care for a dependent family member, some of the many stressors that may be experienced by patients who require frequent trips to the hospital. For patients who may not be clinically suitable candidates for hospital at home, such as those requiring critical care intervention and support, the brick-and-mortar hospital is still the appropriate site of care. The hospital-at-home model helps prevent bed shortages in brick-and-mortar hospital settings by allowing hospital care at home for patients who meet preset criteria. These patients can be hospitalized in alternative locations such as their own homes or the residence of a friend. This helps increase health system capacity as well as resiliency.
In addition to expanding safe and appropriate treatment spaces, the hospital-at-home model helps increase access to care for patients during nonstandard hours, including weekends, holidays, or when the waiting time in the emergency room is painfully long. Furthermore, providing care in the home gives the clinical team valuable insight into the patient’s daily life and routine. Performing medication reconciliation with the medicine cabinet in sight and dietary education in a patient’s kitchen are powerful touch points.2 For example, a patient with congestive heart failure who must undergo diuresis is much more likely to meet their care goals when their home diet is aligned with the treatment goal. By being able to see exactly what is in a patient’s pantry and fridge, the care team can create a much more tailored approach to sodium intake and fluid management. Providers can create and execute true patient-centric care as they gain direct insight into the patient’s lifestyle, which is clearly valuable when creating care plans for complex chronic health issues.
Challenges to Implementation and Scaling
Although there are clear benefits to hospital at home, how to best implement and scale this model presents a challenge. In addition to educating patients and families about this model of care, health care systems must expand their hospital-at-home programs and provide education about this model to clinical staff and trainees, and insurers must create reimbursement paradigms. Patients meeting eligibility criteria to enroll in hospital at home is the easiest hurdle, as hospital-at-home programs function best when they enroll and service as many patients as possible, including underserved populations.
Upfront Costs and Cost Savings
While there are upfront costs to set up technology and coordinate services, hospital at home also provides significant total cost savings when compared to coordination associated with brick-and-mortar admission. Hospital care accounts for about one-third of total medical expenditures and is a leading cause of debt.2 Eliminating fixed hospital costs such as facility, overhead, and equipment costs through adoption of the hospital-at-home model can lead to a reduction in expenditures. It has been found that fewer laboratory and diagnostic tests are ordered for hospital-at-home patients when compared to similar patients in brick-and-mortar hospital settings, with comparable or better clinical patient outcomes.6 Furthermore, it is estimated that there are cost savings of 19% to 30% when compared to traditional inpatient care.6 Without legislative action, upon the end of the current COVID-19 public health emergency, the Centers for Medicare & Medicaid Service’s Acute Hospital Care at Home waiver will terminate. This could slow down scaling of the model.However, over the past 2 years there has been enough buy-in from major health systems and patients to continue the momentum of the model’s growth. When setting up a hospital-at-home program, it would be wise to consider a few factors: where in the hospital or health system entity structure the hospital-at-home program will reside, which existing resources can be leveraged within the hospital or health system, and what are the state or federal regulatory requirements for such a program. This type of program continues to fill gaps within the US health care system, meeting the needs of widely overlooked populations and increasing access to essential ancillary services.
Conclusion
It is time to consider our bias toward hospital-first options when managing the care needs of our patients. Health care providers have the option to advocate for holistic care, better experience, and better outcomes. Home-based options are safe, equitable, and patient-centric. Increased costs, consumerism, and technology have pushed us to think about alternative approaches to patient care delivery, and the pandemic created a unique opportunity to see just how far the health care system could stretch itself with capacity constraints, insufficient resources, and staff shortages. In light of new possibilities, it is time to reimagine and transform our health care delivery system so that it is unified, seamless, cohesive, and flexible.
Corresponding author: Payal Sharma, DNP, MSN, RN, FNP-BC, CBN; [email protected].
Disclosures: None reported.
1. Cai S, Laurel PA, Makineni R, Marks ML. Evaluation of a hospital-in-home program implemented among veterans. Am J Manag Care. 2017;23(8):482-487.
2. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a pilot randomized controlled trial. J Gen Intern Med. 2018;33(5):729-736. doi:10.1007/s11606-018-4307-z
3. Shuman V, Coyle PC, Perera S,et al. Association between improved mobility and distal health outcomes. J Gerontol A Biol Sci Med Sci. 2020;75(12):2412-2417. doi:10.1093/gerona/glaa086
4. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
5. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home”. Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
6. Hospital at Home. Johns Hopkins Medicine. Healthcare Solutions. Accessed May 20, 2022. https://www.johnshopkinssolutions.com/solution/hospital-at-home/
From Medically Home Group, Boston, MA.
Brick-and-mortar hospitals in the United States have historically been considered the dominant setting for providing care to patients. The coordination and delivery of care has previously been bound to physical hospitals largely because multidisciplinary services were only accessible in an individual location. While the fundamental make-up of these services remains unchanged, these services are now available in alternate settings. Some of these services include access to a patient care team, supplies, diagnostics, pharmacy, and advanced therapeutic interventions. Presently, the physical environment is becoming increasingly irrelevant as the core of what makes the traditional hospital—the professional staff, collaborative work processes, and the dynamics of the space—have all been translated into a modern digitally integrated environment. The elements necessary to providing safe, effective care in a physical hospital setting are now available in a patient’s home.
Impetus for the Model
As hospitals reconsider how and where they deliver patient care because of limited resources, the hospital-at-home model has gained significant momentum and interest. This model transforms a home into a hospital. The inpatient acute care episode is entirely substituted with an intensive at-home hospital admission enabled by technology, multidisciplinary teams, and ancillary services. Furthermore, patients requiring post-acute support can be transitioned to their next phase of care seamlessly. Given the nationwide nursing shortage, aging population, challenges uncovered by the COVID-19 pandemic, rising hospital costs, nurse/provider burnout related to challenging work environments, and capacity constraints, a shift toward the combination of virtual and in-home care is imperative. The hospital-at-home model has been associated with superior patient outcomes, including reduced risks of delirium, improved functional status, improved patient and family member satisfaction, reduced mortality, reduced readmissions, and significantly lower costs.1 COVID-19 alone has unmasked major facility-based deficiencies and limitations of our health care system. While the pandemic is not the impetus for the hospital-at-home model, the extended stress of this event has created a unique opportunity to reimagine and transform our health care delivery system so that it is less fragmented and more flexible.
Nursing in the Model
Nursing is central to the hospital-at-home model. Virtual nurses provide meticulous care plan oversight, assessment, and documentation across in-home service providers, to ensure holistic, safe, transparent, and continuous progression toward care plan milestones. The virtual nurse monitors patients using in-home technology that is set up at the time of admission. Connecting with patients to verify social and medical needs, the virtual nurse advocates for their patients and uses these technologies to care and deploy on-demand hands-on services to the patient. Service providers such as paramedics, infusion nurses, or home health nurses may be deployed to provide services in the patient’s home. By bringing in supplies, therapeutics, and interdisciplinary team members, the capabilities of a brick-and-mortar hospital are replicated in the home. All actions that occur wherever the patient is receiving care are overseen by professional nursing staff; in short, virtual nurses are the equivalent of bedside nurses in the brick-and-mortar health care facilities.
Potential Benefits
There are many benefits to the hospital-at-home model (Table). This health care model can be particularly helpful for patients who require frequent admission to acute care facilities, and is well suited for patients with a range of conditions, including those with COVID-19, pneumonia, cellulitis, or congestive heart failure. This care model helps eliminate some of the stressors for patients who have chronic illnesses or other conditions that require frequent hospital admissions. Patients can independently recover at home and can also be surrounded by their loved ones and pets while recovering. This care approach additionally eliminates the risk of hospital-acquired infections and injuries. The hospital-at-home model allows for increased mobility,2 as patients are familiar with their surroundings, resulting in reduced onset of delirium. Additionally, patients with improved mobility performance are less likely to experience negative health outcomes.3 There is less chance of sleep disruption as the patient is sleeping in their own bed—no unfamiliar roommate, no call bells or health care personnel frequently coming into the room. The in-home technology set up for remote patient monitoring is designed with the user in mind. Ease of use empowers the patient to collaborate with their care team on their own terms and center the priorities of themselves and their families.

Positive Outcomes
The hospital-at-home model is associated with positive outcomes. The authors of a systematic review identified 10 randomized controlled trials of hospital-at-home programs (with a total of 1372 patients), but were able to obtain data for only 5 of these trials (with a total of 844 patients).4 They found a 38% reduction in 6-month mortality for patients who received hospital care at home, as well as significantly higher patient satisfaction across a range of medical conditions, including patients with cellulitis and community-acquired pneumonia, as well as elderly patients with multiple medical conditions. The authors concluded that hospital care at home was less expensive than admission to an acute care hospital.4 Similarly, a meta-analysis done by Caplan et al5 that included 61 randomized controlled trials concluded that hospital at home is associated with reductions in mortality, readmission rates, and cost, and increases in patient and caregiver satisfaction. Levine et al2 found reduced costs and utilization with home hospitalization compared to in-hospital care, as well as improved patient mobility status.
The home is the ideal place to empower patients and caregivers to engage in self-management.2 Receiving hospital care at home eliminates the need for dealing with transportation arrangements, traffic, road tolls, and time/scheduling constraints, or finding care for a dependent family member, some of the many stressors that may be experienced by patients who require frequent trips to the hospital. For patients who may not be clinically suitable candidates for hospital at home, such as those requiring critical care intervention and support, the brick-and-mortar hospital is still the appropriate site of care. The hospital-at-home model helps prevent bed shortages in brick-and-mortar hospital settings by allowing hospital care at home for patients who meet preset criteria. These patients can be hospitalized in alternative locations such as their own homes or the residence of a friend. This helps increase health system capacity as well as resiliency.
In addition to expanding safe and appropriate treatment spaces, the hospital-at-home model helps increase access to care for patients during nonstandard hours, including weekends, holidays, or when the waiting time in the emergency room is painfully long. Furthermore, providing care in the home gives the clinical team valuable insight into the patient’s daily life and routine. Performing medication reconciliation with the medicine cabinet in sight and dietary education in a patient’s kitchen are powerful touch points.2 For example, a patient with congestive heart failure who must undergo diuresis is much more likely to meet their care goals when their home diet is aligned with the treatment goal. By being able to see exactly what is in a patient’s pantry and fridge, the care team can create a much more tailored approach to sodium intake and fluid management. Providers can create and execute true patient-centric care as they gain direct insight into the patient’s lifestyle, which is clearly valuable when creating care plans for complex chronic health issues.
Challenges to Implementation and Scaling
Although there are clear benefits to hospital at home, how to best implement and scale this model presents a challenge. In addition to educating patients and families about this model of care, health care systems must expand their hospital-at-home programs and provide education about this model to clinical staff and trainees, and insurers must create reimbursement paradigms. Patients meeting eligibility criteria to enroll in hospital at home is the easiest hurdle, as hospital-at-home programs function best when they enroll and service as many patients as possible, including underserved populations.
Upfront Costs and Cost Savings
While there are upfront costs to set up technology and coordinate services, hospital at home also provides significant total cost savings when compared to coordination associated with brick-and-mortar admission. Hospital care accounts for about one-third of total medical expenditures and is a leading cause of debt.2 Eliminating fixed hospital costs such as facility, overhead, and equipment costs through adoption of the hospital-at-home model can lead to a reduction in expenditures. It has been found that fewer laboratory and diagnostic tests are ordered for hospital-at-home patients when compared to similar patients in brick-and-mortar hospital settings, with comparable or better clinical patient outcomes.6 Furthermore, it is estimated that there are cost savings of 19% to 30% when compared to traditional inpatient care.6 Without legislative action, upon the end of the current COVID-19 public health emergency, the Centers for Medicare & Medicaid Service’s Acute Hospital Care at Home waiver will terminate. This could slow down scaling of the model.However, over the past 2 years there has been enough buy-in from major health systems and patients to continue the momentum of the model’s growth. When setting up a hospital-at-home program, it would be wise to consider a few factors: where in the hospital or health system entity structure the hospital-at-home program will reside, which existing resources can be leveraged within the hospital or health system, and what are the state or federal regulatory requirements for such a program. This type of program continues to fill gaps within the US health care system, meeting the needs of widely overlooked populations and increasing access to essential ancillary services.
Conclusion
It is time to consider our bias toward hospital-first options when managing the care needs of our patients. Health care providers have the option to advocate for holistic care, better experience, and better outcomes. Home-based options are safe, equitable, and patient-centric. Increased costs, consumerism, and technology have pushed us to think about alternative approaches to patient care delivery, and the pandemic created a unique opportunity to see just how far the health care system could stretch itself with capacity constraints, insufficient resources, and staff shortages. In light of new possibilities, it is time to reimagine and transform our health care delivery system so that it is unified, seamless, cohesive, and flexible.
Corresponding author: Payal Sharma, DNP, MSN, RN, FNP-BC, CBN; [email protected].
Disclosures: None reported.
From Medically Home Group, Boston, MA.
Brick-and-mortar hospitals in the United States have historically been considered the dominant setting for providing care to patients. The coordination and delivery of care has previously been bound to physical hospitals largely because multidisciplinary services were only accessible in an individual location. While the fundamental make-up of these services remains unchanged, these services are now available in alternate settings. Some of these services include access to a patient care team, supplies, diagnostics, pharmacy, and advanced therapeutic interventions. Presently, the physical environment is becoming increasingly irrelevant as the core of what makes the traditional hospital—the professional staff, collaborative work processes, and the dynamics of the space—have all been translated into a modern digitally integrated environment. The elements necessary to providing safe, effective care in a physical hospital setting are now available in a patient’s home.
Impetus for the Model
As hospitals reconsider how and where they deliver patient care because of limited resources, the hospital-at-home model has gained significant momentum and interest. This model transforms a home into a hospital. The inpatient acute care episode is entirely substituted with an intensive at-home hospital admission enabled by technology, multidisciplinary teams, and ancillary services. Furthermore, patients requiring post-acute support can be transitioned to their next phase of care seamlessly. Given the nationwide nursing shortage, aging population, challenges uncovered by the COVID-19 pandemic, rising hospital costs, nurse/provider burnout related to challenging work environments, and capacity constraints, a shift toward the combination of virtual and in-home care is imperative. The hospital-at-home model has been associated with superior patient outcomes, including reduced risks of delirium, improved functional status, improved patient and family member satisfaction, reduced mortality, reduced readmissions, and significantly lower costs.1 COVID-19 alone has unmasked major facility-based deficiencies and limitations of our health care system. While the pandemic is not the impetus for the hospital-at-home model, the extended stress of this event has created a unique opportunity to reimagine and transform our health care delivery system so that it is less fragmented and more flexible.
Nursing in the Model
Nursing is central to the hospital-at-home model. Virtual nurses provide meticulous care plan oversight, assessment, and documentation across in-home service providers, to ensure holistic, safe, transparent, and continuous progression toward care plan milestones. The virtual nurse monitors patients using in-home technology that is set up at the time of admission. Connecting with patients to verify social and medical needs, the virtual nurse advocates for their patients and uses these technologies to care and deploy on-demand hands-on services to the patient. Service providers such as paramedics, infusion nurses, or home health nurses may be deployed to provide services in the patient’s home. By bringing in supplies, therapeutics, and interdisciplinary team members, the capabilities of a brick-and-mortar hospital are replicated in the home. All actions that occur wherever the patient is receiving care are overseen by professional nursing staff; in short, virtual nurses are the equivalent of bedside nurses in the brick-and-mortar health care facilities.
Potential Benefits
There are many benefits to the hospital-at-home model (Table). This health care model can be particularly helpful for patients who require frequent admission to acute care facilities, and is well suited for patients with a range of conditions, including those with COVID-19, pneumonia, cellulitis, or congestive heart failure. This care model helps eliminate some of the stressors for patients who have chronic illnesses or other conditions that require frequent hospital admissions. Patients can independently recover at home and can also be surrounded by their loved ones and pets while recovering. This care approach additionally eliminates the risk of hospital-acquired infections and injuries. The hospital-at-home model allows for increased mobility,2 as patients are familiar with their surroundings, resulting in reduced onset of delirium. Additionally, patients with improved mobility performance are less likely to experience negative health outcomes.3 There is less chance of sleep disruption as the patient is sleeping in their own bed—no unfamiliar roommate, no call bells or health care personnel frequently coming into the room. The in-home technology set up for remote patient monitoring is designed with the user in mind. Ease of use empowers the patient to collaborate with their care team on their own terms and center the priorities of themselves and their families.

Positive Outcomes
The hospital-at-home model is associated with positive outcomes. The authors of a systematic review identified 10 randomized controlled trials of hospital-at-home programs (with a total of 1372 patients), but were able to obtain data for only 5 of these trials (with a total of 844 patients).4 They found a 38% reduction in 6-month mortality for patients who received hospital care at home, as well as significantly higher patient satisfaction across a range of medical conditions, including patients with cellulitis and community-acquired pneumonia, as well as elderly patients with multiple medical conditions. The authors concluded that hospital care at home was less expensive than admission to an acute care hospital.4 Similarly, a meta-analysis done by Caplan et al5 that included 61 randomized controlled trials concluded that hospital at home is associated with reductions in mortality, readmission rates, and cost, and increases in patient and caregiver satisfaction. Levine et al2 found reduced costs and utilization with home hospitalization compared to in-hospital care, as well as improved patient mobility status.
The home is the ideal place to empower patients and caregivers to engage in self-management.2 Receiving hospital care at home eliminates the need for dealing with transportation arrangements, traffic, road tolls, and time/scheduling constraints, or finding care for a dependent family member, some of the many stressors that may be experienced by patients who require frequent trips to the hospital. For patients who may not be clinically suitable candidates for hospital at home, such as those requiring critical care intervention and support, the brick-and-mortar hospital is still the appropriate site of care. The hospital-at-home model helps prevent bed shortages in brick-and-mortar hospital settings by allowing hospital care at home for patients who meet preset criteria. These patients can be hospitalized in alternative locations such as their own homes or the residence of a friend. This helps increase health system capacity as well as resiliency.
In addition to expanding safe and appropriate treatment spaces, the hospital-at-home model helps increase access to care for patients during nonstandard hours, including weekends, holidays, or when the waiting time in the emergency room is painfully long. Furthermore, providing care in the home gives the clinical team valuable insight into the patient’s daily life and routine. Performing medication reconciliation with the medicine cabinet in sight and dietary education in a patient’s kitchen are powerful touch points.2 For example, a patient with congestive heart failure who must undergo diuresis is much more likely to meet their care goals when their home diet is aligned with the treatment goal. By being able to see exactly what is in a patient’s pantry and fridge, the care team can create a much more tailored approach to sodium intake and fluid management. Providers can create and execute true patient-centric care as they gain direct insight into the patient’s lifestyle, which is clearly valuable when creating care plans for complex chronic health issues.
Challenges to Implementation and Scaling
Although there are clear benefits to hospital at home, how to best implement and scale this model presents a challenge. In addition to educating patients and families about this model of care, health care systems must expand their hospital-at-home programs and provide education about this model to clinical staff and trainees, and insurers must create reimbursement paradigms. Patients meeting eligibility criteria to enroll in hospital at home is the easiest hurdle, as hospital-at-home programs function best when they enroll and service as many patients as possible, including underserved populations.
Upfront Costs and Cost Savings
While there are upfront costs to set up technology and coordinate services, hospital at home also provides significant total cost savings when compared to coordination associated with brick-and-mortar admission. Hospital care accounts for about one-third of total medical expenditures and is a leading cause of debt.2 Eliminating fixed hospital costs such as facility, overhead, and equipment costs through adoption of the hospital-at-home model can lead to a reduction in expenditures. It has been found that fewer laboratory and diagnostic tests are ordered for hospital-at-home patients when compared to similar patients in brick-and-mortar hospital settings, with comparable or better clinical patient outcomes.6 Furthermore, it is estimated that there are cost savings of 19% to 30% when compared to traditional inpatient care.6 Without legislative action, upon the end of the current COVID-19 public health emergency, the Centers for Medicare & Medicaid Service’s Acute Hospital Care at Home waiver will terminate. This could slow down scaling of the model.However, over the past 2 years there has been enough buy-in from major health systems and patients to continue the momentum of the model’s growth. When setting up a hospital-at-home program, it would be wise to consider a few factors: where in the hospital or health system entity structure the hospital-at-home program will reside, which existing resources can be leveraged within the hospital or health system, and what are the state or federal regulatory requirements for such a program. This type of program continues to fill gaps within the US health care system, meeting the needs of widely overlooked populations and increasing access to essential ancillary services.
Conclusion
It is time to consider our bias toward hospital-first options when managing the care needs of our patients. Health care providers have the option to advocate for holistic care, better experience, and better outcomes. Home-based options are safe, equitable, and patient-centric. Increased costs, consumerism, and technology have pushed us to think about alternative approaches to patient care delivery, and the pandemic created a unique opportunity to see just how far the health care system could stretch itself with capacity constraints, insufficient resources, and staff shortages. In light of new possibilities, it is time to reimagine and transform our health care delivery system so that it is unified, seamless, cohesive, and flexible.
Corresponding author: Payal Sharma, DNP, MSN, RN, FNP-BC, CBN; [email protected].
Disclosures: None reported.
1. Cai S, Laurel PA, Makineni R, Marks ML. Evaluation of a hospital-in-home program implemented among veterans. Am J Manag Care. 2017;23(8):482-487.
2. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a pilot randomized controlled trial. J Gen Intern Med. 2018;33(5):729-736. doi:10.1007/s11606-018-4307-z
3. Shuman V, Coyle PC, Perera S,et al. Association between improved mobility and distal health outcomes. J Gerontol A Biol Sci Med Sci. 2020;75(12):2412-2417. doi:10.1093/gerona/glaa086
4. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
5. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home”. Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
6. Hospital at Home. Johns Hopkins Medicine. Healthcare Solutions. Accessed May 20, 2022. https://www.johnshopkinssolutions.com/solution/hospital-at-home/
1. Cai S, Laurel PA, Makineni R, Marks ML. Evaluation of a hospital-in-home program implemented among veterans. Am J Manag Care. 2017;23(8):482-487.
2. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a pilot randomized controlled trial. J Gen Intern Med. 2018;33(5):729-736. doi:10.1007/s11606-018-4307-z
3. Shuman V, Coyle PC, Perera S,et al. Association between improved mobility and distal health outcomes. J Gerontol A Biol Sci Med Sci. 2020;75(12):2412-2417. doi:10.1093/gerona/glaa086
4. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
5. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home”. Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
6. Hospital at Home. Johns Hopkins Medicine. Healthcare Solutions. Accessed May 20, 2022. https://www.johnshopkinssolutions.com/solution/hospital-at-home/
The Intersection of Clinical Quality Improvement Research and Implementation Science
The Institute of Medicine brought much-needed attention to the need for process improvement in medicine with its seminal report To Err Is Human: Building a Safer Health System, which was issued in 1999, leading to the quality movement’s call to close health care performance gaps in Crossing the Quality Chasm: A New Health System for the 21st Century.1,2 Quality improvement science in medicine has evolved over the past 2 decades to include a broad spectrum of approaches, from agile improvement to continuous learning and improvement. Current efforts focus on Lean-based process improvement along with a reduction in variation in clinical practice to align practice with the principles of evidence-based medicine in a patient-centered approach.3 Further, the definition of quality improvement under the Affordable Care Act was framed as an equitable, timely, value-based, patient-centered approach to achieving population-level health goals.4 Thus, the science of quality improvement drives the core principles of care delivery improvement, and the rigorous evidence needed to expand innovation is embedded within the same framework.5,6 In clinical practice, quality improvement projects aim to define gaps and then specific steps are undertaken to improve the evidence-based practice of a specific process. The overarching goal is to enhance the efficacy of the practice by reducing waste within a particular domain. Thus, quality improvement and implementation research eventually unify how clinical practice is advanced concurrently to bridge identified gaps.7
System redesign through a patient-centered framework forms the core of an overarching strategy to support system-level processes. Both require a deep understanding of the fields of quality improvement science and implementation science.8 Furthermore, aligning clinical research needs, system aims, patients’ values, and clinical care give the new design a clear path forward. Patient-centered improvement includes the essential elements of system redesign around human factors, including communication, physical resources, and updated information during episodes of care. The patient-centered improvement design is juxtaposed with care planning and establishing continuum of care processes.9 It is essential to note that safety is rooted within the quality domain as a top priority in medicine.10 The best implementation methods and approaches are discussed and debated, and the improvement progress continues on multiple fronts.11 Patient safety systems are implemented simultaneously during the redesign phase. Moreover, identifying and testing the health care delivery methods in the era of competing strategic priorities to achieve the desirable clinical outcomes highlights the importance of implementation, while contemplating the methods of dissemination, scalability, and sustainability of the best evidence-based clinical practice.
The cycle of quality improvement research completes the system implementation efforts. The conceptual framework of quality improvement includes multiple areas of care and transition, along with applying the best clinical practices in a culture that emphasizes continuous improvement and learning. At the same time, the operating principles should include continuous improvement in a simple and continuous system of learning as a core concept. Our proposed implementation approach involves taking simple and practical steps while separating the process from the outcomes measures, extracting effectiveness throughout the process. It is essential to keep in mind that building a proactive and systematic improvement environment requires a framework for safety, reliability, and effective care, as well as the alignment of the physical system, communication, and professional environment and culture (Figure).
In summary, system design for quality improvement research should incorporate the principles and conceptual framework that embody effective implementation strategies, with a focus on operational and practical steps. Continuous improvement will be reached through the multidimensional development of current health care system metrics and the incorporation of implementation science methods.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; [email protected]
Disclosures: None reported.
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. Washington (DC): National Academies Press (US); 2000.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001.
3. Berwick DM. The science of improvement. JAMA. 2008;299(10):1182-1184. doi:10.1001/jama.299.10.1182
4. Mazurenko O, Balio CP, Agarwal R, Carroll AE, Menachemi N. The effects of Medicaid expansion under the ACA: a systematic review. Health Affairs. 2018;37(6):944-950. doi: 10.1377/hlthaff.2017.1491
5. Fan E, Needham DM. The science of quality improvement. JAMA. 2008;300(4):390-391. doi:10.1001/jama.300.4.390-b
6. Alexander JA, Hearld LR. The science of quality improvement implementation: developing capacity to make a difference. Med Care. 2011:S6-20. doi:10.1097/MLR.0b013e3181e1709c
7. Rohweder C, Wangen M, Black M, et al. Understanding quality improvement collaboratives through an implementation science lens. Prev Med. 2019;129:105859. doi: 10.1016/j.ypmed.2019.105859
8. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296(23):2848-2851. doi:10.1001/jama.296.23.2848
9. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1(Suppl 1):i85-90. doi:10.1136/qhc.13.suppl_1.i85
10. Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501-507. doi:10.1001/jama.288.4.501
11. Auerbach AD, Landefeld CS, Shojania KG. The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608-613. doi:10.1056/NEJMsb070738
The Institute of Medicine brought much-needed attention to the need for process improvement in medicine with its seminal report To Err Is Human: Building a Safer Health System, which was issued in 1999, leading to the quality movement’s call to close health care performance gaps in Crossing the Quality Chasm: A New Health System for the 21st Century.1,2 Quality improvement science in medicine has evolved over the past 2 decades to include a broad spectrum of approaches, from agile improvement to continuous learning and improvement. Current efforts focus on Lean-based process improvement along with a reduction in variation in clinical practice to align practice with the principles of evidence-based medicine in a patient-centered approach.3 Further, the definition of quality improvement under the Affordable Care Act was framed as an equitable, timely, value-based, patient-centered approach to achieving population-level health goals.4 Thus, the science of quality improvement drives the core principles of care delivery improvement, and the rigorous evidence needed to expand innovation is embedded within the same framework.5,6 In clinical practice, quality improvement projects aim to define gaps and then specific steps are undertaken to improve the evidence-based practice of a specific process. The overarching goal is to enhance the efficacy of the practice by reducing waste within a particular domain. Thus, quality improvement and implementation research eventually unify how clinical practice is advanced concurrently to bridge identified gaps.7
System redesign through a patient-centered framework forms the core of an overarching strategy to support system-level processes. Both require a deep understanding of the fields of quality improvement science and implementation science.8 Furthermore, aligning clinical research needs, system aims, patients’ values, and clinical care give the new design a clear path forward. Patient-centered improvement includes the essential elements of system redesign around human factors, including communication, physical resources, and updated information during episodes of care. The patient-centered improvement design is juxtaposed with care planning and establishing continuum of care processes.9 It is essential to note that safety is rooted within the quality domain as a top priority in medicine.10 The best implementation methods and approaches are discussed and debated, and the improvement progress continues on multiple fronts.11 Patient safety systems are implemented simultaneously during the redesign phase. Moreover, identifying and testing the health care delivery methods in the era of competing strategic priorities to achieve the desirable clinical outcomes highlights the importance of implementation, while contemplating the methods of dissemination, scalability, and sustainability of the best evidence-based clinical practice.
The cycle of quality improvement research completes the system implementation efforts. The conceptual framework of quality improvement includes multiple areas of care and transition, along with applying the best clinical practices in a culture that emphasizes continuous improvement and learning. At the same time, the operating principles should include continuous improvement in a simple and continuous system of learning as a core concept. Our proposed implementation approach involves taking simple and practical steps while separating the process from the outcomes measures, extracting effectiveness throughout the process. It is essential to keep in mind that building a proactive and systematic improvement environment requires a framework for safety, reliability, and effective care, as well as the alignment of the physical system, communication, and professional environment and culture (Figure).

In summary, system design for quality improvement research should incorporate the principles and conceptual framework that embody effective implementation strategies, with a focus on operational and practical steps. Continuous improvement will be reached through the multidimensional development of current health care system metrics and the incorporation of implementation science methods.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; [email protected]
Disclosures: None reported.
The Institute of Medicine brought much-needed attention to the need for process improvement in medicine with its seminal report To Err Is Human: Building a Safer Health System, which was issued in 1999, leading to the quality movement’s call to close health care performance gaps in Crossing the Quality Chasm: A New Health System for the 21st Century.1,2 Quality improvement science in medicine has evolved over the past 2 decades to include a broad spectrum of approaches, from agile improvement to continuous learning and improvement. Current efforts focus on Lean-based process improvement along with a reduction in variation in clinical practice to align practice with the principles of evidence-based medicine in a patient-centered approach.3 Further, the definition of quality improvement under the Affordable Care Act was framed as an equitable, timely, value-based, patient-centered approach to achieving population-level health goals.4 Thus, the science of quality improvement drives the core principles of care delivery improvement, and the rigorous evidence needed to expand innovation is embedded within the same framework.5,6 In clinical practice, quality improvement projects aim to define gaps and then specific steps are undertaken to improve the evidence-based practice of a specific process. The overarching goal is to enhance the efficacy of the practice by reducing waste within a particular domain. Thus, quality improvement and implementation research eventually unify how clinical practice is advanced concurrently to bridge identified gaps.7
System redesign through a patient-centered framework forms the core of an overarching strategy to support system-level processes. Both require a deep understanding of the fields of quality improvement science and implementation science.8 Furthermore, aligning clinical research needs, system aims, patients’ values, and clinical care give the new design a clear path forward. Patient-centered improvement includes the essential elements of system redesign around human factors, including communication, physical resources, and updated information during episodes of care. The patient-centered improvement design is juxtaposed with care planning and establishing continuum of care processes.9 It is essential to note that safety is rooted within the quality domain as a top priority in medicine.10 The best implementation methods and approaches are discussed and debated, and the improvement progress continues on multiple fronts.11 Patient safety systems are implemented simultaneously during the redesign phase. Moreover, identifying and testing the health care delivery methods in the era of competing strategic priorities to achieve the desirable clinical outcomes highlights the importance of implementation, while contemplating the methods of dissemination, scalability, and sustainability of the best evidence-based clinical practice.
The cycle of quality improvement research completes the system implementation efforts. The conceptual framework of quality improvement includes multiple areas of care and transition, along with applying the best clinical practices in a culture that emphasizes continuous improvement and learning. At the same time, the operating principles should include continuous improvement in a simple and continuous system of learning as a core concept. Our proposed implementation approach involves taking simple and practical steps while separating the process from the outcomes measures, extracting effectiveness throughout the process. It is essential to keep in mind that building a proactive and systematic improvement environment requires a framework for safety, reliability, and effective care, as well as the alignment of the physical system, communication, and professional environment and culture (Figure).

In summary, system design for quality improvement research should incorporate the principles and conceptual framework that embody effective implementation strategies, with a focus on operational and practical steps. Continuous improvement will be reached through the multidimensional development of current health care system metrics and the incorporation of implementation science methods.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; [email protected]
Disclosures: None reported.
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. Washington (DC): National Academies Press (US); 2000.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001.
3. Berwick DM. The science of improvement. JAMA. 2008;299(10):1182-1184. doi:10.1001/jama.299.10.1182
4. Mazurenko O, Balio CP, Agarwal R, Carroll AE, Menachemi N. The effects of Medicaid expansion under the ACA: a systematic review. Health Affairs. 2018;37(6):944-950. doi: 10.1377/hlthaff.2017.1491
5. Fan E, Needham DM. The science of quality improvement. JAMA. 2008;300(4):390-391. doi:10.1001/jama.300.4.390-b
6. Alexander JA, Hearld LR. The science of quality improvement implementation: developing capacity to make a difference. Med Care. 2011:S6-20. doi:10.1097/MLR.0b013e3181e1709c
7. Rohweder C, Wangen M, Black M, et al. Understanding quality improvement collaboratives through an implementation science lens. Prev Med. 2019;129:105859. doi: 10.1016/j.ypmed.2019.105859
8. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296(23):2848-2851. doi:10.1001/jama.296.23.2848
9. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1(Suppl 1):i85-90. doi:10.1136/qhc.13.suppl_1.i85
10. Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501-507. doi:10.1001/jama.288.4.501
11. Auerbach AD, Landefeld CS, Shojania KG. The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608-613. doi:10.1056/NEJMsb070738
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. Washington (DC): National Academies Press (US); 2000.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001.
3. Berwick DM. The science of improvement. JAMA. 2008;299(10):1182-1184. doi:10.1001/jama.299.10.1182
4. Mazurenko O, Balio CP, Agarwal R, Carroll AE, Menachemi N. The effects of Medicaid expansion under the ACA: a systematic review. Health Affairs. 2018;37(6):944-950. doi: 10.1377/hlthaff.2017.1491
5. Fan E, Needham DM. The science of quality improvement. JAMA. 2008;300(4):390-391. doi:10.1001/jama.300.4.390-b
6. Alexander JA, Hearld LR. The science of quality improvement implementation: developing capacity to make a difference. Med Care. 2011:S6-20. doi:10.1097/MLR.0b013e3181e1709c
7. Rohweder C, Wangen M, Black M, et al. Understanding quality improvement collaboratives through an implementation science lens. Prev Med. 2019;129:105859. doi: 10.1016/j.ypmed.2019.105859
8. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296(23):2848-2851. doi:10.1001/jama.296.23.2848
9. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1(Suppl 1):i85-90. doi:10.1136/qhc.13.suppl_1.i85
10. Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501-507. doi:10.1001/jama.288.4.501
11. Auerbach AD, Landefeld CS, Shojania KG. The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608-613. doi:10.1056/NEJMsb070738
A Quantification Method to Compare the Value of Surgery and Palliative Care in Patients With Complex Cardiac Disease: A Concept
From the Department of Cardiothoracic Surgery, Stanford University, Stanford, CA.
Abstract
Complex cardiac patients are often referred for surgery or palliative care based on the risk of perioperative mortality. This decision ignores factors such as quality of life or duration of life in either surgery or the palliative path. Here, we propose a model to numerically assess and compare the value of surgery vs palliation. This model includes quality and duration of life, as well as risk of perioperative mortality, and involves a patient’s preferences in the decision-making process.
For each pathway, surgery or palliative care, a value is calculated and compared to a normal life value (no disease symptoms and normal life expectancy). The formula is adjusted for the risk of operative mortality. The model produces a ratio of the value of surgery to the value of palliative care that signifies the superiority of one or another. This model calculation presents an objective estimated numerical value to compare the value of surgery and palliative care. It can be applied to every decision-making process before surgery. In general, if a procedure has the potential to significantly extend life in a patient who otherwise has a very short life expectancy with palliation only, performing high-risk surgery would be a reasonable option. A model that provides a numerical value for surgery vs palliative care and includes quality and duration of life in each pathway could be a useful tool for cardiac surgeons in decision making regarding high-risk surgery.
Keywords: high-risk surgery, palliative care, quality of life, life expectancy.
Patients with complex cardiovascular disease are occasionally considered inoperable due to the high risk of surgical mortality. When the risk of perioperative mortality (POM) is predicted to be too high, surgical intervention is denied, and patients are often referred to palliative care. The risk of POM in cardiac surgery is often calculated using large-scale databases, such as the Society of Thoracic Surgeons (STS) records. The STS risk models, which are regularly updated, are based on large data sets and incorporate precise statistical methods for risk adjustment.1 In general, these calculators provide a percentage value that defines the magnitude of the risk of death, and then an arbitrary range is selected to categorize the procedure as low, medium, or high risk or inoperable status. The STS database does not set a cutoff point or range to define “operability.” Assigning inoperable status to a certain risk rate is problematic, with many ethical, legal, and moral implications, and for this reason, it has mostly remained undefined. In contrast, the low- and medium-risk ranges are easier to define. Another limitation encountered in the STS database is the lack of risk data for less common but very high-risk procedures, such as a triple valve replacement.
A common example where risk classification has been defined is in patients who are candidates for surgical vs transcatheter aortic valve replacement. Some groups have described a risk of <4% as low risk, 4% to 8% as intermediate risk, >8% as high risk, and >15% as inoperable2; for some other groups, a risk of POM >50% is considered extreme risk or inoperable.3,4 This procedure-specific classification is a useful decision-making tool and helps the surgeon perform an initial risk assessment to allocate a specific patient to a group—operable or nonoperable—only by calculating the risk of surgical death. However, this allocation method does not provide any information on how and when death occurs in either group. These 2 parameters of how and when death occurs define the quality of life (QOL) and the duration of life (DOL), respectively, and together could be considered as the value of life in each pathway. A survivor of a high-risk surgery may benefit from good quality and extended life (a high value), or, on the other end of the spectrum, a high-risk patient who does not undergo surgery is spared the mortality risk of the surgery but dies sooner (low value) with symptoms due to the natural course of the untreated disease.
The central question is, if a surgery is high risk but has the potential of providing a good value (for those who survive it), what QOL and DOL values are acceptable to risk or to justify accepting and proceeding with a risky surgery? Or how high a POM risk is justified to proceed with surgery rather than the alternative palliative care with a certain quality and duration? It is obvious that a decision-making process that is based on POM cannot compare the value of surgery (Vs) and the value of palliation (Vp). Furthermore, it ignores patient preferences and their input, as these are excluded from this decision-making process.
To be able to include QOL and DOL in any decision making, one must precisely describe these parameters. Both QOL and DOL are used for estimation of disease burden by health care administrators, public health experts, insurance agencies, and others. Multiple models have been proposed and used to estimate the overall burden of the disease. Most of the models for this purpose are created for large-scale economic purposes and not for decision making in individual cases.
An important measure is the quality-adjusted life year (QALY). This is an important parameter since it includes both measures of quality and quantity of life.5,6 QALY is a simplified measure to assess the value of health outcomes, and it has been used in economic calculations to assess mainly the cost-effectiveness of various interventions. We sought to evaluate the utility of a similar method in adding further insight into the surgical decision-making process. In this article, we propose a simple model to compare the value of surgery vs palliative care, similar to QALY. This model includes and adjusts for the quality and the quantity of life, in addition to the risk of POM, in the decision-making process for high-risk patients.
The Model
The 2 decision pathways, surgery and palliative care, are compared for their value. We define the value as the product of QOL and DOL in each pathway and use the severity of the symptoms as a surrogate for QOL. If duration and quality were depicted on the x and y axes of a graph (Figure 1), then the area under the curve would represent the collective value in each situation. Figure 2 shows the timeline and the different pathways with each decision. The value in each situation is calculated in relation to the full value, which is represented as the value of normal life (Vn), that is, life without disease and with normal life expectancy. The values of each decision pathway, the value of surgery (Vs) and the value of palliation (Vp), are then compared to define the benefit for each decision as follows:
If Vs/Vp > 1, the benefit is toward surgery;
If Vs/Vp < 1, the benefit is for palliative care.
Definitions
Both quality and duration of life are presented on a 1-10 scale, 1 being the lowest and 10 the highest value, to yield a product with a value of 100 in normal, disease-free life. Any lower value is presented as a percentage to represent the comparison to the full value. QOL is determined by degradation of full quality with the average level of symptoms. DOL is calculated as a lost time (
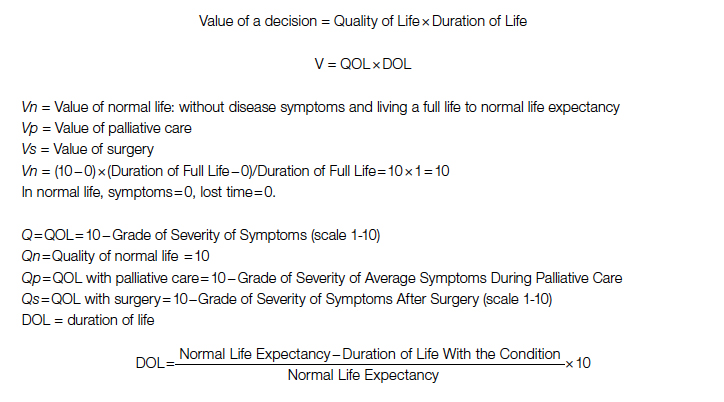
For the DOL under any condition, a 10-year survival rate could be used as a surrogate in this formula. Compared to life expectancy value, using the 10-year survival rate simplifies the calculation since cardiac diseases are more prevalent in older age, close to or beyond the average life expectancy value.
Using the time intervals from the timeline in Figure 2:
dh = time interval from diagnosis to death at life expectancy
dg = time interval from diagnosis to death after successful surgery
df = time interval from diagnosis to death after palliative care
Duration for palliative care:
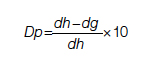
Duration for surgery:
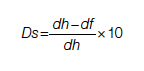
Adjustment: This value is calculated for those who survive the surgery. To adjust for the POM, it is multiplied by the 100 − POM risk.
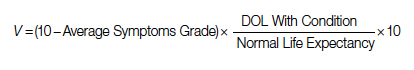
Since value is the base for comparison in this model, and it is the product of 2 equally important factors in the formula (
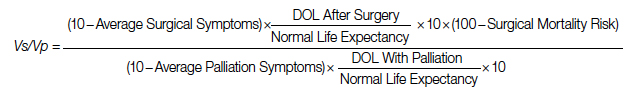
After elimination of normal life expectancy, form the numerator and denominator:
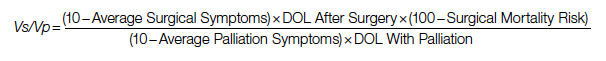
To adjust for surgical outcomes in special circumstances where less than optimal or standard surgical results are expected (eg, in very rare surgeries, limited resource institutions, or suboptimal postoperative surgical care), an optional coefficient R can be added to the numerator (surgical value). This optional coefficient, with values such as 0.8, 0.9 (to degrade the value of surgery) or 1 (standard surgical outcome), adjusts for variability in interinstitutional surgical results or surgeon variability. No coefficient is added to the denominator since palliative care provides minimal differences between clinicians and hospitals. Thus, the final adjusted formula would be as follows:
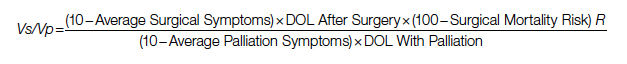
Example
A 60-year-old patient with a 10% POM risk needs to be allocated to surgical or palliative care. With palliative care, if this patient lived 6 years with average symptoms grade 4, the Vp would be 20; that is, 20% of the normal life value (if he lived 18 years instead without the disease).
Using the formula for calculation of value in each pathway:
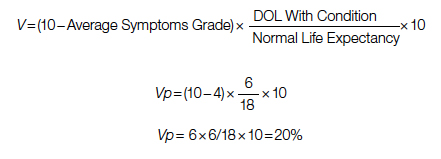
If the same patient undergoes a surgery with a 10% risk of POM, with an average grade 2 related to surgical recovery symptoms for 1 year and then is symptom-free and lives 12 years (instead of 18 years [life expectancy]), his Vs would be 53, or 53% out of the normal life value that is saved if the surgery is 100% successful; adjusted Vs with (chance of survival of 90%) would be 53 × 90% = 48%.
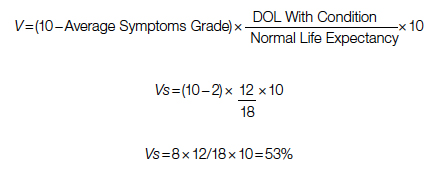
With adjustment of 90% survival chance in surgery, 53 × 90% = 48%. In this example, Vs/Vp = 48/20 = 2.4, showing a significant benefit for surgical care. Notably, the unknown value of normal life expectancy is not needed for the calculation of Vs/Vp, since it is the same in both pathways and it is eliminated by calculation in fraction.
Based on this formula, since the duration of surgical symptoms is short, no matter how severe these are, if the potential duration of life after surgery is high (represented by smaller area under the curve in Figure 1), the numerator becomes larger and the value of the surgery grows. For example, if a patient with a 15% risk of POM, which is generally considered inoperable, lives 5 years, as opposed to 2 years with palliative care with mild symptoms (eg 3/10), Vs/Vp would be 2.7, still showing a significant benefit for surgical care.
Discussion
Any surgical intervention is offered with 2 goals in mind, improving QOL and extending DOL. In a high-risk patient, surgery might be declined due to a high risk of POM, and the patient is offered palliative care, which other than providing symptom relief does not change the course of disease and eventually the patient will die due to the untreated disease. In this decision-making method, mostly completed by a care team only, a potential risk of death due to surgery which possibly could cure the patient is traded for immediate survival; however, the symptomatic course ensues until death. This mostly unilateral decision-making process by a care team, which incorporates minimal input from the patient or ignores patient preferences altogether, is based only on POM risk, and roughly includes a single parameter: years of potential life lost (YPLL). YPLL is a measure of premature mortality, and in the setting of surgical intervention, YPLL is the number of years a patient would lose unless a successful surgery were undertaken. Obviously, patients would live longer if a surgery that was intended to save them failed.
In this article, we proposed a simple method to quantify each decision to decide whether to operate or choose surgical care vs palliative care. Since quality and duration of life are both end factors clinicians and patients aspire to in each decision, they can be considered together as the value of each decision. We believe a numerical framework would provide an objective way to assist both the patient at high risk and the care team in the decision-making process.
The 2 parameters we consider are DOL and QOL. DOL, or survival, can be extracted from large-scale data using statistical methods that have been developed to predict survival under various conditions, such as Kaplan-Meier curves. These methods present the chance of survival in percentages in a defined time frame, such as a 5- or 10-year period.
While the DOL is a numerical parameter and quantifiable, the QOL is a more complex entity. This subjective parameter bears multiple definitions, aspects, and categories, and therefore multiple scales for quantification of QOL have been proposed. These scales have been used extensively for the purpose of health determination in health care policy and economic planning. Most scales acknowledge that QOL is multifactorial and includes interrelated aspects such as mental and socioeconomic factors. We have also noticed that QOL is better determined by the palliative care team than surgeons, so including these care providers in the decision-making process might reduce surgeon bias.
Since our purpose here is only to assist with the decision on medical intervention, we focus on physical QOL. Multiple scales are used to assess health-related QOL, such as the Assessment of Quality of Life (AQoL)-8D,7 EuroQol-5 Dimension (EQ-5D),8 15D,9 and the 36-Item Short Form Survey (SF-36).10 These complex scales are built for systematic reviews, and they are not practical for a clinical user. To simplify and keep this practical, we define QOL by using the severity or grade of symptoms related to the disease the patient has on a scale of 0 to 10. The severity of symptoms can be easily determined using available scales. An applicable scale for this purpose is the Edmonton Symptom Assessment Scale (ESAS), which has been in use for years and has evolved as a useful tool in the medical field.11
Once DOL and QOL are determined on a 1-10 scale, the multiplied value then provides a product that we consider a value. The highest value hoped for in each decision is the achievement of the best QOL and DOL, a value of 100. In Figure 1, a graphic presentation of value in each decision is best seen as the area under the curve. As shown, a successful surgery, even when accompanied by significant symptoms during initial recovery, has a chance (100 – risk of POM%) to gain a larger area under curve (value) by achieving a longer life with no or fewer symptoms. However, in palliative care, progressing disease and even palliated symptoms with a shorter life expectancy impose a large burden on the patient and a much lower value. Note that in this calculation, life expectancy, which is an important but unpredictable factor, is initially included; however, by ratio comparison, it is eliminated, simplifying the calculation further.
Using this formula in different settings reveals that high-risk surgery has a greater potential to reduce YPLL in the general population. Based on this formula, compared to a surgery with potential to significantly extend DOL, a definite shorter and symptomatic life course with palliative care makes it a significantly less favorable option. In fact, in the cardiovascular field, palliative care has minimal or no effect on natural history, as the mechanism of illness is mechanical, such as occlusion of coronary arteries or valve dysfunction, leading eventually to heart failure and death. In a study by Xu et al, although palliative care reduced readmission rates and improved symptoms on a variety of scales, there was no effect on mortality and QOL in patients with heart failure.12
No model in this field has proven to be ideal, and this model bears multiple limitations as well. We have used severity of symptoms as a surrogate for QOL based on the fact that cardiac patients with different pathologies who are untreated will have a common final pathway with development of heart failure symptoms that dictate their QOL. Also, grading QOL is a difficult task at times. Even a model such as QALY, which is one of the most used, is not a perfect model and is not free of problems.6 The difference in surgical results and life expectancy between sexes and ethnic groups might be a source of bias in this formula. Also, multiple factors directly and indirectly affect QOL and DOL and create inaccuracies; therefore, making an exact science from an inexact one naturally relies on multiple assumptions. Although it has previously been shown that most POM occurs in a short period of time after cardiac surgery,13 long-term complications that potentially degrade QOL are not included in this model. By applying this model, one must assume indefinite economic resources. Moreover, applying a single mathematical model in a biologic system and in the general population has intrinsic shortcomings, and it must overlook many other factors (eg, ethical, legal). For example, it will be hard to justify a failed surgery with 15% risk of POM undertaken to eliminate the severe long-lasting symptoms of a disease, while the outcome of a successful surgery with a 20% risk of POM that adds life and quality would be ignored in the current health care system. Thus, regardless of the significant potential, most surgeons would waive a surgery based solely on the percentage rate of POM, perhaps using other terms such as ”peri-nonoperative mortality.”
Conclusion
We have proposed a simple and practical formula for decision making regarding surgical vs palliative care in high-risk patients. By assigning a value that is composed of QOL and DOL in each pathway and including the risk of POM, a ratio of values provides a numerical estimation that can be used to show preference over a specific decision. An advantage of this formula, in addition to presenting an arithmetic value that is easier to understand, is that it can be used in shared decision making with patients. We emphasize that this model is only a preliminary concept at this time and has not been tested or validated for clinical use. Validation of such a model will require extensive work and testing within a large-scale population. We hope that this article will serve as a starting point for the development of other models, and that this formula will become more sophisticated with fewer limitations through larger multidisciplinary efforts in the future.
Corresponding author: Rabin Gerrah, MD, Good Samaritan Regional Medical Center, 3640 NW Samaritan Drive, Suite 100B, Corvallis, OR 97330; [email protected].
Disclosures: None reported.
1. O’Brien SM, Feng L, He X, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-statistical methods and results. Ann Thorac Surg. 2018;105(5):1419-1428. doi: 10.1016/j.athoracsur.2018.03.003
2. Hurtado Rendón IS, Bittenbender P, Dunn JM, Firstenberg MS. Chapter 8: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines. In: Transcatheter Heart Valve Handbook: A Surgeons’ and Interventional Council Review. Akron City Hospital, Summa Health System, Akron, OH.
3. Herrmann HC, Thourani VH, Kodali SK, et al; PARTNER Investigators. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134:130-140. doi:10.1161/CIRCULATIONAHA
4. Ho C, Argáez C. Transcatheter Aortic Valve Implantation for Patients with Severe Aortic Stenosis at Various Levels of Surgical Risk: A Review of Clinical Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; March 19, 2018.
5. Rios-Diaz AJ, Lam J, Ramos MS, et al. Global patterns of QALY and DALY use in surgical cost-utility analyses: a systematic review. PLoS One. 2016:10;11:e0148304. doi:10.1371/journal.pone.0148304
6. Prieto L, Sacristán JA. Health, Problems and solutions in calculating quality-adjusted life years (QALYs). Qual Life Outcomes. 2003:19;1:80.
7. Centre for Health Economics. Assessment of Quality of Life. 2014. Accessed May 13, 2022. http://www.aqol.com.au/
8. EuroQol Research Foundation. EQ-5D. Accessed May 13, 2022. https://euroqol.org/
9. 15D Instrument. Accessed May 13, 2022. http://www.15d-instrument.net/15d/
10. Rand Corporation. 36-Item Short Form Survey (SF-36).Accessed May 12, 2022. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html
11. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017:53:630-643. doi:10.1016/j.jpainsymman.2016
12. Xu Z, Chen L, Jin S, Yang B, Chen X, Wu Z. Effect of palliative care for patients with heart failure. Int Heart J. 2018:30;59:503-509. doi:10.1536/ihj.17-289
13. Mazzeffi M, Zivot J, Buchman T, Halkos M. In-hospital mortality after cardiac surgery: patient characteristics, timing, and association with postoperative length of intensive care unit and hospital stay. Ann Thorac Surg. 2014;97:1220-1225. doi:10.1016/j.athoracsur.2013.10.040
From the Department of Cardiothoracic Surgery, Stanford University, Stanford, CA.
Abstract
Complex cardiac patients are often referred for surgery or palliative care based on the risk of perioperative mortality. This decision ignores factors such as quality of life or duration of life in either surgery or the palliative path. Here, we propose a model to numerically assess and compare the value of surgery vs palliation. This model includes quality and duration of life, as well as risk of perioperative mortality, and involves a patient’s preferences in the decision-making process.
For each pathway, surgery or palliative care, a value is calculated and compared to a normal life value (no disease symptoms and normal life expectancy). The formula is adjusted for the risk of operative mortality. The model produces a ratio of the value of surgery to the value of palliative care that signifies the superiority of one or another. This model calculation presents an objective estimated numerical value to compare the value of surgery and palliative care. It can be applied to every decision-making process before surgery. In general, if a procedure has the potential to significantly extend life in a patient who otherwise has a very short life expectancy with palliation only, performing high-risk surgery would be a reasonable option. A model that provides a numerical value for surgery vs palliative care and includes quality and duration of life in each pathway could be a useful tool for cardiac surgeons in decision making regarding high-risk surgery.
Keywords: high-risk surgery, palliative care, quality of life, life expectancy.
Patients with complex cardiovascular disease are occasionally considered inoperable due to the high risk of surgical mortality. When the risk of perioperative mortality (POM) is predicted to be too high, surgical intervention is denied, and patients are often referred to palliative care. The risk of POM in cardiac surgery is often calculated using large-scale databases, such as the Society of Thoracic Surgeons (STS) records. The STS risk models, which are regularly updated, are based on large data sets and incorporate precise statistical methods for risk adjustment.1 In general, these calculators provide a percentage value that defines the magnitude of the risk of death, and then an arbitrary range is selected to categorize the procedure as low, medium, or high risk or inoperable status. The STS database does not set a cutoff point or range to define “operability.” Assigning inoperable status to a certain risk rate is problematic, with many ethical, legal, and moral implications, and for this reason, it has mostly remained undefined. In contrast, the low- and medium-risk ranges are easier to define. Another limitation encountered in the STS database is the lack of risk data for less common but very high-risk procedures, such as a triple valve replacement.
A common example where risk classification has been defined is in patients who are candidates for surgical vs transcatheter aortic valve replacement. Some groups have described a risk of <4% as low risk, 4% to 8% as intermediate risk, >8% as high risk, and >15% as inoperable2; for some other groups, a risk of POM >50% is considered extreme risk or inoperable.3,4 This procedure-specific classification is a useful decision-making tool and helps the surgeon perform an initial risk assessment to allocate a specific patient to a group—operable or nonoperable—only by calculating the risk of surgical death. However, this allocation method does not provide any information on how and when death occurs in either group. These 2 parameters of how and when death occurs define the quality of life (QOL) and the duration of life (DOL), respectively, and together could be considered as the value of life in each pathway. A survivor of a high-risk surgery may benefit from good quality and extended life (a high value), or, on the other end of the spectrum, a high-risk patient who does not undergo surgery is spared the mortality risk of the surgery but dies sooner (low value) with symptoms due to the natural course of the untreated disease.
The central question is, if a surgery is high risk but has the potential of providing a good value (for those who survive it), what QOL and DOL values are acceptable to risk or to justify accepting and proceeding with a risky surgery? Or how high a POM risk is justified to proceed with surgery rather than the alternative palliative care with a certain quality and duration? It is obvious that a decision-making process that is based on POM cannot compare the value of surgery (Vs) and the value of palliation (Vp). Furthermore, it ignores patient preferences and their input, as these are excluded from this decision-making process.
To be able to include QOL and DOL in any decision making, one must precisely describe these parameters. Both QOL and DOL are used for estimation of disease burden by health care administrators, public health experts, insurance agencies, and others. Multiple models have been proposed and used to estimate the overall burden of the disease. Most of the models for this purpose are created for large-scale economic purposes and not for decision making in individual cases.
An important measure is the quality-adjusted life year (QALY). This is an important parameter since it includes both measures of quality and quantity of life.5,6 QALY is a simplified measure to assess the value of health outcomes, and it has been used in economic calculations to assess mainly the cost-effectiveness of various interventions. We sought to evaluate the utility of a similar method in adding further insight into the surgical decision-making process. In this article, we propose a simple model to compare the value of surgery vs palliative care, similar to QALY. This model includes and adjusts for the quality and the quantity of life, in addition to the risk of POM, in the decision-making process for high-risk patients.
The Model
The 2 decision pathways, surgery and palliative care, are compared for their value. We define the value as the product of QOL and DOL in each pathway and use the severity of the symptoms as a surrogate for QOL. If duration and quality were depicted on the x and y axes of a graph (Figure 1), then the area under the curve would represent the collective value in each situation. Figure 2 shows the timeline and the different pathways with each decision. The value in each situation is calculated in relation to the full value, which is represented as the value of normal life (Vn), that is, life without disease and with normal life expectancy. The values of each decision pathway, the value of surgery (Vs) and the value of palliation (Vp), are then compared to define the benefit for each decision as follows:
If Vs/Vp > 1, the benefit is toward surgery;
If Vs/Vp < 1, the benefit is for palliative care.


Definitions
Both quality and duration of life are presented on a 1-10 scale, 1 being the lowest and 10 the highest value, to yield a product with a value of 100 in normal, disease-free life. Any lower value is presented as a percentage to represent the comparison to the full value. QOL is determined by degradation of full quality with the average level of symptoms. DOL is calculated as a lost time (

For the DOL under any condition, a 10-year survival rate could be used as a surrogate in this formula. Compared to life expectancy value, using the 10-year survival rate simplifies the calculation since cardiac diseases are more prevalent in older age, close to or beyond the average life expectancy value.
Using the time intervals from the timeline in Figure 2:
dh = time interval from diagnosis to death at life expectancy
dg = time interval from diagnosis to death after successful surgery
df = time interval from diagnosis to death after palliative care
Duration for palliative care:
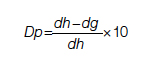
Duration for surgery:
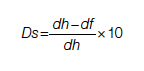
Adjustment: This value is calculated for those who survive the surgery. To adjust for the POM, it is multiplied by the 100 − POM risk.
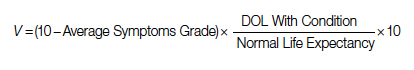
Since value is the base for comparison in this model, and it is the product of 2 equally important factors in the formula (

After elimination of normal life expectancy, form the numerator and denominator:
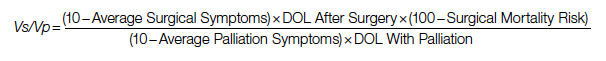
To adjust for surgical outcomes in special circumstances where less than optimal or standard surgical results are expected (eg, in very rare surgeries, limited resource institutions, or suboptimal postoperative surgical care), an optional coefficient R can be added to the numerator (surgical value). This optional coefficient, with values such as 0.8, 0.9 (to degrade the value of surgery) or 1 (standard surgical outcome), adjusts for variability in interinstitutional surgical results or surgeon variability. No coefficient is added to the denominator since palliative care provides minimal differences between clinicians and hospitals. Thus, the final adjusted formula would be as follows:
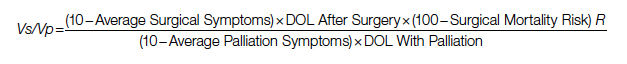
Example
A 60-year-old patient with a 10% POM risk needs to be allocated to surgical or palliative care. With palliative care, if this patient lived 6 years with average symptoms grade 4, the Vp would be 20; that is, 20% of the normal life value (if he lived 18 years instead without the disease).
Using the formula for calculation of value in each pathway:
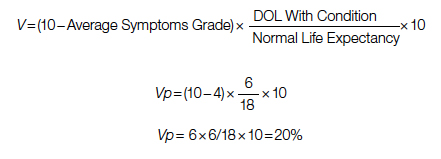
If the same patient undergoes a surgery with a 10% risk of POM, with an average grade 2 related to surgical recovery symptoms for 1 year and then is symptom-free and lives 12 years (instead of 18 years [life expectancy]), his Vs would be 53, or 53% out of the normal life value that is saved if the surgery is 100% successful; adjusted Vs with (chance of survival of 90%) would be 53 × 90% = 48%.
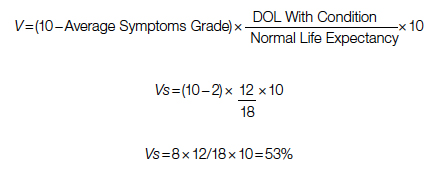
With adjustment of 90% survival chance in surgery, 53 × 90% = 48%. In this example, Vs/Vp = 48/20 = 2.4, showing a significant benefit for surgical care. Notably, the unknown value of normal life expectancy is not needed for the calculation of Vs/Vp, since it is the same in both pathways and it is eliminated by calculation in fraction.
Based on this formula, since the duration of surgical symptoms is short, no matter how severe these are, if the potential duration of life after surgery is high (represented by smaller area under the curve in Figure 1), the numerator becomes larger and the value of the surgery grows. For example, if a patient with a 15% risk of POM, which is generally considered inoperable, lives 5 years, as opposed to 2 years with palliative care with mild symptoms (eg 3/10), Vs/Vp would be 2.7, still showing a significant benefit for surgical care.
Discussion
Any surgical intervention is offered with 2 goals in mind, improving QOL and extending DOL. In a high-risk patient, surgery might be declined due to a high risk of POM, and the patient is offered palliative care, which other than providing symptom relief does not change the course of disease and eventually the patient will die due to the untreated disease. In this decision-making method, mostly completed by a care team only, a potential risk of death due to surgery which possibly could cure the patient is traded for immediate survival; however, the symptomatic course ensues until death. This mostly unilateral decision-making process by a care team, which incorporates minimal input from the patient or ignores patient preferences altogether, is based only on POM risk, and roughly includes a single parameter: years of potential life lost (YPLL). YPLL is a measure of premature mortality, and in the setting of surgical intervention, YPLL is the number of years a patient would lose unless a successful surgery were undertaken. Obviously, patients would live longer if a surgery that was intended to save them failed.
In this article, we proposed a simple method to quantify each decision to decide whether to operate or choose surgical care vs palliative care. Since quality and duration of life are both end factors clinicians and patients aspire to in each decision, they can be considered together as the value of each decision. We believe a numerical framework would provide an objective way to assist both the patient at high risk and the care team in the decision-making process.
The 2 parameters we consider are DOL and QOL. DOL, or survival, can be extracted from large-scale data using statistical methods that have been developed to predict survival under various conditions, such as Kaplan-Meier curves. These methods present the chance of survival in percentages in a defined time frame, such as a 5- or 10-year period.
While the DOL is a numerical parameter and quantifiable, the QOL is a more complex entity. This subjective parameter bears multiple definitions, aspects, and categories, and therefore multiple scales for quantification of QOL have been proposed. These scales have been used extensively for the purpose of health determination in health care policy and economic planning. Most scales acknowledge that QOL is multifactorial and includes interrelated aspects such as mental and socioeconomic factors. We have also noticed that QOL is better determined by the palliative care team than surgeons, so including these care providers in the decision-making process might reduce surgeon bias.
Since our purpose here is only to assist with the decision on medical intervention, we focus on physical QOL. Multiple scales are used to assess health-related QOL, such as the Assessment of Quality of Life (AQoL)-8D,7 EuroQol-5 Dimension (EQ-5D),8 15D,9 and the 36-Item Short Form Survey (SF-36).10 These complex scales are built for systematic reviews, and they are not practical for a clinical user. To simplify and keep this practical, we define QOL by using the severity or grade of symptoms related to the disease the patient has on a scale of 0 to 10. The severity of symptoms can be easily determined using available scales. An applicable scale for this purpose is the Edmonton Symptom Assessment Scale (ESAS), which has been in use for years and has evolved as a useful tool in the medical field.11
Once DOL and QOL are determined on a 1-10 scale, the multiplied value then provides a product that we consider a value. The highest value hoped for in each decision is the achievement of the best QOL and DOL, a value of 100. In Figure 1, a graphic presentation of value in each decision is best seen as the area under the curve. As shown, a successful surgery, even when accompanied by significant symptoms during initial recovery, has a chance (100 – risk of POM%) to gain a larger area under curve (value) by achieving a longer life with no or fewer symptoms. However, in palliative care, progressing disease and even palliated symptoms with a shorter life expectancy impose a large burden on the patient and a much lower value. Note that in this calculation, life expectancy, which is an important but unpredictable factor, is initially included; however, by ratio comparison, it is eliminated, simplifying the calculation further.
Using this formula in different settings reveals that high-risk surgery has a greater potential to reduce YPLL in the general population. Based on this formula, compared to a surgery with potential to significantly extend DOL, a definite shorter and symptomatic life course with palliative care makes it a significantly less favorable option. In fact, in the cardiovascular field, palliative care has minimal or no effect on natural history, as the mechanism of illness is mechanical, such as occlusion of coronary arteries or valve dysfunction, leading eventually to heart failure and death. In a study by Xu et al, although palliative care reduced readmission rates and improved symptoms on a variety of scales, there was no effect on mortality and QOL in patients with heart failure.12
No model in this field has proven to be ideal, and this model bears multiple limitations as well. We have used severity of symptoms as a surrogate for QOL based on the fact that cardiac patients with different pathologies who are untreated will have a common final pathway with development of heart failure symptoms that dictate their QOL. Also, grading QOL is a difficult task at times. Even a model such as QALY, which is one of the most used, is not a perfect model and is not free of problems.6 The difference in surgical results and life expectancy between sexes and ethnic groups might be a source of bias in this formula. Also, multiple factors directly and indirectly affect QOL and DOL and create inaccuracies; therefore, making an exact science from an inexact one naturally relies on multiple assumptions. Although it has previously been shown that most POM occurs in a short period of time after cardiac surgery,13 long-term complications that potentially degrade QOL are not included in this model. By applying this model, one must assume indefinite economic resources. Moreover, applying a single mathematical model in a biologic system and in the general population has intrinsic shortcomings, and it must overlook many other factors (eg, ethical, legal). For example, it will be hard to justify a failed surgery with 15% risk of POM undertaken to eliminate the severe long-lasting symptoms of a disease, while the outcome of a successful surgery with a 20% risk of POM that adds life and quality would be ignored in the current health care system. Thus, regardless of the significant potential, most surgeons would waive a surgery based solely on the percentage rate of POM, perhaps using other terms such as ”peri-nonoperative mortality.”
Conclusion
We have proposed a simple and practical formula for decision making regarding surgical vs palliative care in high-risk patients. By assigning a value that is composed of QOL and DOL in each pathway and including the risk of POM, a ratio of values provides a numerical estimation that can be used to show preference over a specific decision. An advantage of this formula, in addition to presenting an arithmetic value that is easier to understand, is that it can be used in shared decision making with patients. We emphasize that this model is only a preliminary concept at this time and has not been tested or validated for clinical use. Validation of such a model will require extensive work and testing within a large-scale population. We hope that this article will serve as a starting point for the development of other models, and that this formula will become more sophisticated with fewer limitations through larger multidisciplinary efforts in the future.
Corresponding author: Rabin Gerrah, MD, Good Samaritan Regional Medical Center, 3640 NW Samaritan Drive, Suite 100B, Corvallis, OR 97330; [email protected].
Disclosures: None reported.
From the Department of Cardiothoracic Surgery, Stanford University, Stanford, CA.
Abstract
Complex cardiac patients are often referred for surgery or palliative care based on the risk of perioperative mortality. This decision ignores factors such as quality of life or duration of life in either surgery or the palliative path. Here, we propose a model to numerically assess and compare the value of surgery vs palliation. This model includes quality and duration of life, as well as risk of perioperative mortality, and involves a patient’s preferences in the decision-making process.
For each pathway, surgery or palliative care, a value is calculated and compared to a normal life value (no disease symptoms and normal life expectancy). The formula is adjusted for the risk of operative mortality. The model produces a ratio of the value of surgery to the value of palliative care that signifies the superiority of one or another. This model calculation presents an objective estimated numerical value to compare the value of surgery and palliative care. It can be applied to every decision-making process before surgery. In general, if a procedure has the potential to significantly extend life in a patient who otherwise has a very short life expectancy with palliation only, performing high-risk surgery would be a reasonable option. A model that provides a numerical value for surgery vs palliative care and includes quality and duration of life in each pathway could be a useful tool for cardiac surgeons in decision making regarding high-risk surgery.
Keywords: high-risk surgery, palliative care, quality of life, life expectancy.
Patients with complex cardiovascular disease are occasionally considered inoperable due to the high risk of surgical mortality. When the risk of perioperative mortality (POM) is predicted to be too high, surgical intervention is denied, and patients are often referred to palliative care. The risk of POM in cardiac surgery is often calculated using large-scale databases, such as the Society of Thoracic Surgeons (STS) records. The STS risk models, which are regularly updated, are based on large data sets and incorporate precise statistical methods for risk adjustment.1 In general, these calculators provide a percentage value that defines the magnitude of the risk of death, and then an arbitrary range is selected to categorize the procedure as low, medium, or high risk or inoperable status. The STS database does not set a cutoff point or range to define “operability.” Assigning inoperable status to a certain risk rate is problematic, with many ethical, legal, and moral implications, and for this reason, it has mostly remained undefined. In contrast, the low- and medium-risk ranges are easier to define. Another limitation encountered in the STS database is the lack of risk data for less common but very high-risk procedures, such as a triple valve replacement.
A common example where risk classification has been defined is in patients who are candidates for surgical vs transcatheter aortic valve replacement. Some groups have described a risk of <4% as low risk, 4% to 8% as intermediate risk, >8% as high risk, and >15% as inoperable2; for some other groups, a risk of POM >50% is considered extreme risk or inoperable.3,4 This procedure-specific classification is a useful decision-making tool and helps the surgeon perform an initial risk assessment to allocate a specific patient to a group—operable or nonoperable—only by calculating the risk of surgical death. However, this allocation method does not provide any information on how and when death occurs in either group. These 2 parameters of how and when death occurs define the quality of life (QOL) and the duration of life (DOL), respectively, and together could be considered as the value of life in each pathway. A survivor of a high-risk surgery may benefit from good quality and extended life (a high value), or, on the other end of the spectrum, a high-risk patient who does not undergo surgery is spared the mortality risk of the surgery but dies sooner (low value) with symptoms due to the natural course of the untreated disease.
The central question is, if a surgery is high risk but has the potential of providing a good value (for those who survive it), what QOL and DOL values are acceptable to risk or to justify accepting and proceeding with a risky surgery? Or how high a POM risk is justified to proceed with surgery rather than the alternative palliative care with a certain quality and duration? It is obvious that a decision-making process that is based on POM cannot compare the value of surgery (Vs) and the value of palliation (Vp). Furthermore, it ignores patient preferences and their input, as these are excluded from this decision-making process.
To be able to include QOL and DOL in any decision making, one must precisely describe these parameters. Both QOL and DOL are used for estimation of disease burden by health care administrators, public health experts, insurance agencies, and others. Multiple models have been proposed and used to estimate the overall burden of the disease. Most of the models for this purpose are created for large-scale economic purposes and not for decision making in individual cases.
An important measure is the quality-adjusted life year (QALY). This is an important parameter since it includes both measures of quality and quantity of life.5,6 QALY is a simplified measure to assess the value of health outcomes, and it has been used in economic calculations to assess mainly the cost-effectiveness of various interventions. We sought to evaluate the utility of a similar method in adding further insight into the surgical decision-making process. In this article, we propose a simple model to compare the value of surgery vs palliative care, similar to QALY. This model includes and adjusts for the quality and the quantity of life, in addition to the risk of POM, in the decision-making process for high-risk patients.
The Model
The 2 decision pathways, surgery and palliative care, are compared for their value. We define the value as the product of QOL and DOL in each pathway and use the severity of the symptoms as a surrogate for QOL. If duration and quality were depicted on the x and y axes of a graph (Figure 1), then the area under the curve would represent the collective value in each situation. Figure 2 shows the timeline and the different pathways with each decision. The value in each situation is calculated in relation to the full value, which is represented as the value of normal life (Vn), that is, life without disease and with normal life expectancy. The values of each decision pathway, the value of surgery (Vs) and the value of palliation (Vp), are then compared to define the benefit for each decision as follows:
If Vs/Vp > 1, the benefit is toward surgery;
If Vs/Vp < 1, the benefit is for palliative care.


Definitions
Both quality and duration of life are presented on a 1-10 scale, 1 being the lowest and 10 the highest value, to yield a product with a value of 100 in normal, disease-free life. Any lower value is presented as a percentage to represent the comparison to the full value. QOL is determined by degradation of full quality with the average level of symptoms. DOL is calculated as a lost time (

For the DOL under any condition, a 10-year survival rate could be used as a surrogate in this formula. Compared to life expectancy value, using the 10-year survival rate simplifies the calculation since cardiac diseases are more prevalent in older age, close to or beyond the average life expectancy value.
Using the time intervals from the timeline in Figure 2:
dh = time interval from diagnosis to death at life expectancy
dg = time interval from diagnosis to death after successful surgery
df = time interval from diagnosis to death after palliative care
Duration for palliative care:
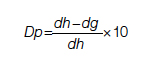
Duration for surgery:
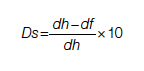
Adjustment: This value is calculated for those who survive the surgery. To adjust for the POM, it is multiplied by the 100 − POM risk.
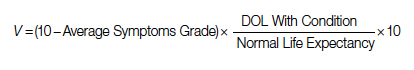
Since value is the base for comparison in this model, and it is the product of 2 equally important factors in the formula (

After elimination of normal life expectancy, form the numerator and denominator:
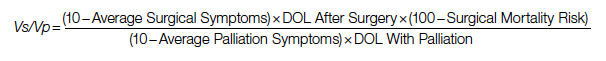
To adjust for surgical outcomes in special circumstances where less than optimal or standard surgical results are expected (eg, in very rare surgeries, limited resource institutions, or suboptimal postoperative surgical care), an optional coefficient R can be added to the numerator (surgical value). This optional coefficient, with values such as 0.8, 0.9 (to degrade the value of surgery) or 1 (standard surgical outcome), adjusts for variability in interinstitutional surgical results or surgeon variability. No coefficient is added to the denominator since palliative care provides minimal differences between clinicians and hospitals. Thus, the final adjusted formula would be as follows:
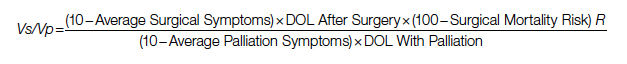
Example
A 60-year-old patient with a 10% POM risk needs to be allocated to surgical or palliative care. With palliative care, if this patient lived 6 years with average symptoms grade 4, the Vp would be 20; that is, 20% of the normal life value (if he lived 18 years instead without the disease).
Using the formula for calculation of value in each pathway:
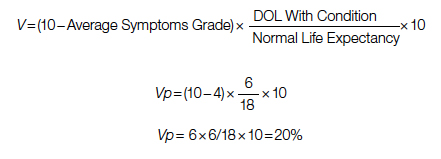
If the same patient undergoes a surgery with a 10% risk of POM, with an average grade 2 related to surgical recovery symptoms for 1 year and then is symptom-free and lives 12 years (instead of 18 years [life expectancy]), his Vs would be 53, or 53% out of the normal life value that is saved if the surgery is 100% successful; adjusted Vs with (chance of survival of 90%) would be 53 × 90% = 48%.
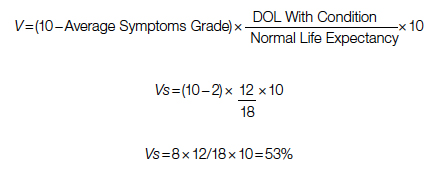
With adjustment of 90% survival chance in surgery, 53 × 90% = 48%. In this example, Vs/Vp = 48/20 = 2.4, showing a significant benefit for surgical care. Notably, the unknown value of normal life expectancy is not needed for the calculation of Vs/Vp, since it is the same in both pathways and it is eliminated by calculation in fraction.
Based on this formula, since the duration of surgical symptoms is short, no matter how severe these are, if the potential duration of life after surgery is high (represented by smaller area under the curve in Figure 1), the numerator becomes larger and the value of the surgery grows. For example, if a patient with a 15% risk of POM, which is generally considered inoperable, lives 5 years, as opposed to 2 years with palliative care with mild symptoms (eg 3/10), Vs/Vp would be 2.7, still showing a significant benefit for surgical care.
Discussion
Any surgical intervention is offered with 2 goals in mind, improving QOL and extending DOL. In a high-risk patient, surgery might be declined due to a high risk of POM, and the patient is offered palliative care, which other than providing symptom relief does not change the course of disease and eventually the patient will die due to the untreated disease. In this decision-making method, mostly completed by a care team only, a potential risk of death due to surgery which possibly could cure the patient is traded for immediate survival; however, the symptomatic course ensues until death. This mostly unilateral decision-making process by a care team, which incorporates minimal input from the patient or ignores patient preferences altogether, is based only on POM risk, and roughly includes a single parameter: years of potential life lost (YPLL). YPLL is a measure of premature mortality, and in the setting of surgical intervention, YPLL is the number of years a patient would lose unless a successful surgery were undertaken. Obviously, patients would live longer if a surgery that was intended to save them failed.
In this article, we proposed a simple method to quantify each decision to decide whether to operate or choose surgical care vs palliative care. Since quality and duration of life are both end factors clinicians and patients aspire to in each decision, they can be considered together as the value of each decision. We believe a numerical framework would provide an objective way to assist both the patient at high risk and the care team in the decision-making process.
The 2 parameters we consider are DOL and QOL. DOL, or survival, can be extracted from large-scale data using statistical methods that have been developed to predict survival under various conditions, such as Kaplan-Meier curves. These methods present the chance of survival in percentages in a defined time frame, such as a 5- or 10-year period.
While the DOL is a numerical parameter and quantifiable, the QOL is a more complex entity. This subjective parameter bears multiple definitions, aspects, and categories, and therefore multiple scales for quantification of QOL have been proposed. These scales have been used extensively for the purpose of health determination in health care policy and economic planning. Most scales acknowledge that QOL is multifactorial and includes interrelated aspects such as mental and socioeconomic factors. We have also noticed that QOL is better determined by the palliative care team than surgeons, so including these care providers in the decision-making process might reduce surgeon bias.
Since our purpose here is only to assist with the decision on medical intervention, we focus on physical QOL. Multiple scales are used to assess health-related QOL, such as the Assessment of Quality of Life (AQoL)-8D,7 EuroQol-5 Dimension (EQ-5D),8 15D,9 and the 36-Item Short Form Survey (SF-36).10 These complex scales are built for systematic reviews, and they are not practical for a clinical user. To simplify and keep this practical, we define QOL by using the severity or grade of symptoms related to the disease the patient has on a scale of 0 to 10. The severity of symptoms can be easily determined using available scales. An applicable scale for this purpose is the Edmonton Symptom Assessment Scale (ESAS), which has been in use for years and has evolved as a useful tool in the medical field.11
Once DOL and QOL are determined on a 1-10 scale, the multiplied value then provides a product that we consider a value. The highest value hoped for in each decision is the achievement of the best QOL and DOL, a value of 100. In Figure 1, a graphic presentation of value in each decision is best seen as the area under the curve. As shown, a successful surgery, even when accompanied by significant symptoms during initial recovery, has a chance (100 – risk of POM%) to gain a larger area under curve (value) by achieving a longer life with no or fewer symptoms. However, in palliative care, progressing disease and even palliated symptoms with a shorter life expectancy impose a large burden on the patient and a much lower value. Note that in this calculation, life expectancy, which is an important but unpredictable factor, is initially included; however, by ratio comparison, it is eliminated, simplifying the calculation further.
Using this formula in different settings reveals that high-risk surgery has a greater potential to reduce YPLL in the general population. Based on this formula, compared to a surgery with potential to significantly extend DOL, a definite shorter and symptomatic life course with palliative care makes it a significantly less favorable option. In fact, in the cardiovascular field, palliative care has minimal or no effect on natural history, as the mechanism of illness is mechanical, such as occlusion of coronary arteries or valve dysfunction, leading eventually to heart failure and death. In a study by Xu et al, although palliative care reduced readmission rates and improved symptoms on a variety of scales, there was no effect on mortality and QOL in patients with heart failure.12
No model in this field has proven to be ideal, and this model bears multiple limitations as well. We have used severity of symptoms as a surrogate for QOL based on the fact that cardiac patients with different pathologies who are untreated will have a common final pathway with development of heart failure symptoms that dictate their QOL. Also, grading QOL is a difficult task at times. Even a model such as QALY, which is one of the most used, is not a perfect model and is not free of problems.6 The difference in surgical results and life expectancy between sexes and ethnic groups might be a source of bias in this formula. Also, multiple factors directly and indirectly affect QOL and DOL and create inaccuracies; therefore, making an exact science from an inexact one naturally relies on multiple assumptions. Although it has previously been shown that most POM occurs in a short period of time after cardiac surgery,13 long-term complications that potentially degrade QOL are not included in this model. By applying this model, one must assume indefinite economic resources. Moreover, applying a single mathematical model in a biologic system and in the general population has intrinsic shortcomings, and it must overlook many other factors (eg, ethical, legal). For example, it will be hard to justify a failed surgery with 15% risk of POM undertaken to eliminate the severe long-lasting symptoms of a disease, while the outcome of a successful surgery with a 20% risk of POM that adds life and quality would be ignored in the current health care system. Thus, regardless of the significant potential, most surgeons would waive a surgery based solely on the percentage rate of POM, perhaps using other terms such as ”peri-nonoperative mortality.”
Conclusion
We have proposed a simple and practical formula for decision making regarding surgical vs palliative care in high-risk patients. By assigning a value that is composed of QOL and DOL in each pathway and including the risk of POM, a ratio of values provides a numerical estimation that can be used to show preference over a specific decision. An advantage of this formula, in addition to presenting an arithmetic value that is easier to understand, is that it can be used in shared decision making with patients. We emphasize that this model is only a preliminary concept at this time and has not been tested or validated for clinical use. Validation of such a model will require extensive work and testing within a large-scale population. We hope that this article will serve as a starting point for the development of other models, and that this formula will become more sophisticated with fewer limitations through larger multidisciplinary efforts in the future.
Corresponding author: Rabin Gerrah, MD, Good Samaritan Regional Medical Center, 3640 NW Samaritan Drive, Suite 100B, Corvallis, OR 97330; [email protected].
Disclosures: None reported.
1. O’Brien SM, Feng L, He X, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-statistical methods and results. Ann Thorac Surg. 2018;105(5):1419-1428. doi: 10.1016/j.athoracsur.2018.03.003
2. Hurtado Rendón IS, Bittenbender P, Dunn JM, Firstenberg MS. Chapter 8: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines. In: Transcatheter Heart Valve Handbook: A Surgeons’ and Interventional Council Review. Akron City Hospital, Summa Health System, Akron, OH.
3. Herrmann HC, Thourani VH, Kodali SK, et al; PARTNER Investigators. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134:130-140. doi:10.1161/CIRCULATIONAHA
4. Ho C, Argáez C. Transcatheter Aortic Valve Implantation for Patients with Severe Aortic Stenosis at Various Levels of Surgical Risk: A Review of Clinical Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; March 19, 2018.
5. Rios-Diaz AJ, Lam J, Ramos MS, et al. Global patterns of QALY and DALY use in surgical cost-utility analyses: a systematic review. PLoS One. 2016:10;11:e0148304. doi:10.1371/journal.pone.0148304
6. Prieto L, Sacristán JA. Health, Problems and solutions in calculating quality-adjusted life years (QALYs). Qual Life Outcomes. 2003:19;1:80.
7. Centre for Health Economics. Assessment of Quality of Life. 2014. Accessed May 13, 2022. http://www.aqol.com.au/
8. EuroQol Research Foundation. EQ-5D. Accessed May 13, 2022. https://euroqol.org/
9. 15D Instrument. Accessed May 13, 2022. http://www.15d-instrument.net/15d/
10. Rand Corporation. 36-Item Short Form Survey (SF-36).Accessed May 12, 2022. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html
11. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017:53:630-643. doi:10.1016/j.jpainsymman.2016
12. Xu Z, Chen L, Jin S, Yang B, Chen X, Wu Z. Effect of palliative care for patients with heart failure. Int Heart J. 2018:30;59:503-509. doi:10.1536/ihj.17-289
13. Mazzeffi M, Zivot J, Buchman T, Halkos M. In-hospital mortality after cardiac surgery: patient characteristics, timing, and association with postoperative length of intensive care unit and hospital stay. Ann Thorac Surg. 2014;97:1220-1225. doi:10.1016/j.athoracsur.2013.10.040
1. O’Brien SM, Feng L, He X, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-statistical methods and results. Ann Thorac Surg. 2018;105(5):1419-1428. doi: 10.1016/j.athoracsur.2018.03.003
2. Hurtado Rendón IS, Bittenbender P, Dunn JM, Firstenberg MS. Chapter 8: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines. In: Transcatheter Heart Valve Handbook: A Surgeons’ and Interventional Council Review. Akron City Hospital, Summa Health System, Akron, OH.
3. Herrmann HC, Thourani VH, Kodali SK, et al; PARTNER Investigators. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134:130-140. doi:10.1161/CIRCULATIONAHA
4. Ho C, Argáez C. Transcatheter Aortic Valve Implantation for Patients with Severe Aortic Stenosis at Various Levels of Surgical Risk: A Review of Clinical Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; March 19, 2018.
5. Rios-Diaz AJ, Lam J, Ramos MS, et al. Global patterns of QALY and DALY use in surgical cost-utility analyses: a systematic review. PLoS One. 2016:10;11:e0148304. doi:10.1371/journal.pone.0148304
6. Prieto L, Sacristán JA. Health, Problems and solutions in calculating quality-adjusted life years (QALYs). Qual Life Outcomes. 2003:19;1:80.
7. Centre for Health Economics. Assessment of Quality of Life. 2014. Accessed May 13, 2022. http://www.aqol.com.au/
8. EuroQol Research Foundation. EQ-5D. Accessed May 13, 2022. https://euroqol.org/
9. 15D Instrument. Accessed May 13, 2022. http://www.15d-instrument.net/15d/
10. Rand Corporation. 36-Item Short Form Survey (SF-36).Accessed May 12, 2022. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html
11. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017:53:630-643. doi:10.1016/j.jpainsymman.2016
12. Xu Z, Chen L, Jin S, Yang B, Chen X, Wu Z. Effect of palliative care for patients with heart failure. Int Heart J. 2018:30;59:503-509. doi:10.1536/ihj.17-289
13. Mazzeffi M, Zivot J, Buchman T, Halkos M. In-hospital mortality after cardiac surgery: patient characteristics, timing, and association with postoperative length of intensive care unit and hospital stay. Ann Thorac Surg. 2014;97:1220-1225. doi:10.1016/j.athoracsur.2013.10.040
Case study: Managing venous thromboembolism in the cancer patient
He is admitted and started on enoxaparin 1 mg/kg subcutaneously every 12 hours.
By the next morning, he is feeling better and wants to discuss discharge to home and follow-up plans.
Two months ago he presented with abdominal pain and evaluation revealed he had a pancreatic head mass with liver metastases. A liver biopsy was positive for adenocarcinoma consistent with pancreas primary. CA 19-9 level was 1,200 U/mL and he was started on FOLFIRINOX chemotherapy – which he has tolerated well thus far. CA 19-9 and follow-up CT scan show early response to chemotherapy.
Of course, this case raises many questions. Given how successful some directed biomarker-positive therapies are now, you would want to know his microsatellite instability (MSI)/progressive death–ligand 1 (PD-L1) and BRCA mutation status. A high PD-L1 positivity or MSI deficiency would suggest immunoantibody therapy and a BRCA mutation might suggest a poly (ADP-ribose) polymerase inhibitor could play a role.
However, let’s use this case to discuss his venous thromboembolism (VTE) .
Studies show that metastatic cancer patients on chemotherapy might experience a VTE episode of deep vein thrombosis (DVT) or pulmonary embolism (PE) or both as high as 20% of the time during their cancer course and therapy. This patient would be among those who experience the highest incidence of VTE because of the liver metastasis from the pancreatic adenocarcinoma.
So, what to do? Standard treatment of his pulmonary emboli would include either enoxaparin therapeutic dosing 1 mg/kg subcutaneously q12H or 1.5 mg/kg q24H for 3 months. At 3 months, repeat a CT chest scan to show resolution of pulmonary emboli and/or DVT or both, and repeat D-dimer, which should now be well under 1.
But then, there is a second decision to make: Can you stop anticoagulation if his clots have resolved? The answer is yes. If the clots were provoked and the provoking feature is gone you can stop anticoagulation. Patients with pregnancy, on a birth control pill, or on a long trip where immobilization occurred for a extended time (such as driving or flying) can have anticoagulation stopped because the provoking feature is gone, but this is not true in this case. This patient’s pancreas cancer and chemotherapy are ongoing and he will be at increased risk to clot once again if anticoagulation is stopped.
Should this patient have a hypercoagulable workup which might include protein C, protein S, and antithrombin levels? Remember this is quite rare and patients with these deficiencies usually present in their teens or 20s with increased clotting issues. The more common hypercoagulable workup would include checking for factor V Leiden and prothrombin G20210A mutations, as well as acquired antiphospholipid antibodies such as beta2 glycoprotein I, anticardiolipin, and the lupus inhibitor. However, in this 75-year-old cancer patient, these are not necessary or even relevant since his VTE was clearly provoked by metastatic cancer on chemotherapy.
Unfortunately, with metastatic active cancer, anticoagulation would need to be continued at full or possibly half therapeutic dose. Of course, enoxaparin injections can get tiresome for the patient and data suggest the same result can be achieved either with initial management or by continuing anticoagulation management using either rivaroxaban or apixaban.
Wouldn’t it have been better if this patient had never experienced VTE in the first place? Is that possible?
Yes, data suggest that it is. Higher-risk patients like this one could benefit from prophylactic anticoagulation. The Khorana predictive model gives us a simple clinical means to evaluate this and decide who might be at highest VTE risk and who could benefit from low-dose preventive anticoagulation.
In summary, cancer patients undergoing treatment for metastatic disease are at increased risk for symptomatic VTE. Once diagnosed, therapy is usually very effective, but may need to be prolonged as long as the cancer is still active or else, the VTE could recur. Preventive therapy for high-risk patients would be reasonable.
Dr. Henry is a medical oncologist with the Abramson Cancer Center at the University of Pennsylvania, Philadelphia.
He is admitted and started on enoxaparin 1 mg/kg subcutaneously every 12 hours.
By the next morning, he is feeling better and wants to discuss discharge to home and follow-up plans.
Two months ago he presented with abdominal pain and evaluation revealed he had a pancreatic head mass with liver metastases. A liver biopsy was positive for adenocarcinoma consistent with pancreas primary. CA 19-9 level was 1,200 U/mL and he was started on FOLFIRINOX chemotherapy – which he has tolerated well thus far. CA 19-9 and follow-up CT scan show early response to chemotherapy.
Of course, this case raises many questions. Given how successful some directed biomarker-positive therapies are now, you would want to know his microsatellite instability (MSI)/progressive death–ligand 1 (PD-L1) and BRCA mutation status. A high PD-L1 positivity or MSI deficiency would suggest immunoantibody therapy and a BRCA mutation might suggest a poly (ADP-ribose) polymerase inhibitor could play a role.
However, let’s use this case to discuss his venous thromboembolism (VTE) .
Studies show that metastatic cancer patients on chemotherapy might experience a VTE episode of deep vein thrombosis (DVT) or pulmonary embolism (PE) or both as high as 20% of the time during their cancer course and therapy. This patient would be among those who experience the highest incidence of VTE because of the liver metastasis from the pancreatic adenocarcinoma.
So, what to do? Standard treatment of his pulmonary emboli would include either enoxaparin therapeutic dosing 1 mg/kg subcutaneously q12H or 1.5 mg/kg q24H for 3 months. At 3 months, repeat a CT chest scan to show resolution of pulmonary emboli and/or DVT or both, and repeat D-dimer, which should now be well under 1.
But then, there is a second decision to make: Can you stop anticoagulation if his clots have resolved? The answer is yes. If the clots were provoked and the provoking feature is gone you can stop anticoagulation. Patients with pregnancy, on a birth control pill, or on a long trip where immobilization occurred for a extended time (such as driving or flying) can have anticoagulation stopped because the provoking feature is gone, but this is not true in this case. This patient’s pancreas cancer and chemotherapy are ongoing and he will be at increased risk to clot once again if anticoagulation is stopped.
Should this patient have a hypercoagulable workup which might include protein C, protein S, and antithrombin levels? Remember this is quite rare and patients with these deficiencies usually present in their teens or 20s with increased clotting issues. The more common hypercoagulable workup would include checking for factor V Leiden and prothrombin G20210A mutations, as well as acquired antiphospholipid antibodies such as beta2 glycoprotein I, anticardiolipin, and the lupus inhibitor. However, in this 75-year-old cancer patient, these are not necessary or even relevant since his VTE was clearly provoked by metastatic cancer on chemotherapy.
Unfortunately, with metastatic active cancer, anticoagulation would need to be continued at full or possibly half therapeutic dose. Of course, enoxaparin injections can get tiresome for the patient and data suggest the same result can be achieved either with initial management or by continuing anticoagulation management using either rivaroxaban or apixaban.
Wouldn’t it have been better if this patient had never experienced VTE in the first place? Is that possible?
Yes, data suggest that it is. Higher-risk patients like this one could benefit from prophylactic anticoagulation. The Khorana predictive model gives us a simple clinical means to evaluate this and decide who might be at highest VTE risk and who could benefit from low-dose preventive anticoagulation.
In summary, cancer patients undergoing treatment for metastatic disease are at increased risk for symptomatic VTE. Once diagnosed, therapy is usually very effective, but may need to be prolonged as long as the cancer is still active or else, the VTE could recur. Preventive therapy for high-risk patients would be reasonable.
Dr. Henry is a medical oncologist with the Abramson Cancer Center at the University of Pennsylvania, Philadelphia.
He is admitted and started on enoxaparin 1 mg/kg subcutaneously every 12 hours.
By the next morning, he is feeling better and wants to discuss discharge to home and follow-up plans.
Two months ago he presented with abdominal pain and evaluation revealed he had a pancreatic head mass with liver metastases. A liver biopsy was positive for adenocarcinoma consistent with pancreas primary. CA 19-9 level was 1,200 U/mL and he was started on FOLFIRINOX chemotherapy – which he has tolerated well thus far. CA 19-9 and follow-up CT scan show early response to chemotherapy.
Of course, this case raises many questions. Given how successful some directed biomarker-positive therapies are now, you would want to know his microsatellite instability (MSI)/progressive death–ligand 1 (PD-L1) and BRCA mutation status. A high PD-L1 positivity or MSI deficiency would suggest immunoantibody therapy and a BRCA mutation might suggest a poly (ADP-ribose) polymerase inhibitor could play a role.
However, let’s use this case to discuss his venous thromboembolism (VTE) .
Studies show that metastatic cancer patients on chemotherapy might experience a VTE episode of deep vein thrombosis (DVT) or pulmonary embolism (PE) or both as high as 20% of the time during their cancer course and therapy. This patient would be among those who experience the highest incidence of VTE because of the liver metastasis from the pancreatic adenocarcinoma.
So, what to do? Standard treatment of his pulmonary emboli would include either enoxaparin therapeutic dosing 1 mg/kg subcutaneously q12H or 1.5 mg/kg q24H for 3 months. At 3 months, repeat a CT chest scan to show resolution of pulmonary emboli and/or DVT or both, and repeat D-dimer, which should now be well under 1.
But then, there is a second decision to make: Can you stop anticoagulation if his clots have resolved? The answer is yes. If the clots were provoked and the provoking feature is gone you can stop anticoagulation. Patients with pregnancy, on a birth control pill, or on a long trip where immobilization occurred for a extended time (such as driving or flying) can have anticoagulation stopped because the provoking feature is gone, but this is not true in this case. This patient’s pancreas cancer and chemotherapy are ongoing and he will be at increased risk to clot once again if anticoagulation is stopped.
Should this patient have a hypercoagulable workup which might include protein C, protein S, and antithrombin levels? Remember this is quite rare and patients with these deficiencies usually present in their teens or 20s with increased clotting issues. The more common hypercoagulable workup would include checking for factor V Leiden and prothrombin G20210A mutations, as well as acquired antiphospholipid antibodies such as beta2 glycoprotein I, anticardiolipin, and the lupus inhibitor. However, in this 75-year-old cancer patient, these are not necessary or even relevant since his VTE was clearly provoked by metastatic cancer on chemotherapy.
Unfortunately, with metastatic active cancer, anticoagulation would need to be continued at full or possibly half therapeutic dose. Of course, enoxaparin injections can get tiresome for the patient and data suggest the same result can be achieved either with initial management or by continuing anticoagulation management using either rivaroxaban or apixaban.
Wouldn’t it have been better if this patient had never experienced VTE in the first place? Is that possible?
Yes, data suggest that it is. Higher-risk patients like this one could benefit from prophylactic anticoagulation. The Khorana predictive model gives us a simple clinical means to evaluate this and decide who might be at highest VTE risk and who could benefit from low-dose preventive anticoagulation.
In summary, cancer patients undergoing treatment for metastatic disease are at increased risk for symptomatic VTE. Once diagnosed, therapy is usually very effective, but may need to be prolonged as long as the cancer is still active or else, the VTE could recur. Preventive therapy for high-risk patients would be reasonable.
Dr. Henry is a medical oncologist with the Abramson Cancer Center at the University of Pennsylvania, Philadelphia.
Pilonidal disease, other conditions may benefit from laser treatment
SAN DIEGO – Pilonidal disease – a chronic inflammatory condition that can trigger the formation of cysts and sinuses in the superior portion of the intragluteal cleft or the sacrococcygeal area – remains challenging to manage, but mounting evidence supports the use of lasers to enhance treatment success.
“Draining sinuses or acute abscesses are usually associated with an underlying cyst and associated granulation tissue, fibrosis, and tufts of hair,” Catherine M. DiGiorgio, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “This is why laser hair removal can help with the treatment of these patients.”
The suspected etiology is a foreign body reaction to the entrapped hairs, which are found in the sinuses in about 75% of cases. “The treatment for that is surgery,” said Dr. DiGiorgio, a laser and cosmetic dermatologist in Boston. Laser hair reduction decreases the recurrence of cyst formation and drainage, and is usually covered by insurance, she noted.
Supportive evidence
In a comparative study, French researchers retrospectively reviewed the efficacy of laser hair removal after surgery in reducing recurrence rate of pilonidal cysts, versus surgery alone. Of the 41 study participants, 12 had laser hair removal plus surgery and 29 had surgery alone. The rate of cyst recurrence was significantly lower in the laser hair removal plus surgery group, compared with the surgery only group (8.3% vs. 51.7%, respectively; P < .001).
In another study, researchers from the United Kingdom and The Johns Hopkins Hospital, Baltimore, evaluated the use of the long-pulsed Alexandrite laser in 19 patients with recurrent pilonidal disease who had undergone multiple surgeries.They were treated with the laser for hair removal in the sinus area, requiring 4-12 sessions. The researchers found that 84.2% of patients had a reduction of hair density to less than 5 hairs/cm2, while 15.8% had a reduction of hair density to 5-10 hairs/cm2. They also noted a statistically significant increase in disease-free time in the laser-treated group compared with those treated with surgical management only (P < .01).
Lasers for pseudofolliculitis barbae, HS
Lasers also play a significant role in the treatment of pseudofolliculitis barbae, a chronic, inflammatory disease that primarily affects the bearded area of men with thick hairs, usually those with a darker Fitzpatrick skin type. This can also occur in women, particularly those with polycystic ovary syndrome, Dr. DiGiorgio said.
In people with pseudofolliculitis barbae, the hair follicle is positioned at an acute angle to the skin surface and the sharp end of shaved hair reenters the skin, which results in the formation of pustules, papules, secondary infection, and keloids. Treatment involves a variety of medical therapies including retinoids, benzoyl peroxide, antibiotics, and keratolytics, “but laser hair removal is the best way to get rid of this issue, and results in permanent reduction,” she said. “When treating male patients with laser hair removal in the bearded area, you have to tell them that they won’t be able to grow a beard going forward. Most of them are okay with that.”
A 2002 study, led by E. Victor Ross, MD, of the Naval Medical Center, San Diego, evaluated treatment of pseudofolliculitis barbae in patients with skin types IV, V, and VI with a long-pulsed Nd:YAG laser. For the first phase of the study, the investigators tested epidermal tolerance on the thighs of 37 patients and determined that the laser was safe and effective. For the second phase 2 weeks later, they treated a 15x15-mm submental area with the highest fluence tolerated in phase 1 of the trial and used an adjacent site as the control.
After 90 days, the mean papule count was 6.95 for the control site compared with 1 for the laser-treated site. The researchers observed that miniaturization and elimination of hair shafts resulted in decreased inflamed papules. “We know that this works,” Dr. DiGiorgio said.
In another study from investigators at the Naval Medical Center, San Diego, 22 patients with skin types IV, V, and VI who had pseudofolliculitis barbae underwent 5 weekly treatments with a 1,064 nm Nd:YAG laser. Topical anesthesia was not used, and 10 evaluators used a Global Assessment Scale (GAS) to assess treatment success from photos taken at baseline and at 4 weeks. At 4 weeks, 11 patients demonstrated 83% improvement on the GAS (P < .01), the investigators reported.
Laser and energy-based treatments can also be used to treat hidradenitis suppurativa (HS), a chronic condition that affects apocrine gland–bearing skin. “The hypothesized pathogenesis is that it’s an inflammatory disorder of the hair follicle, where the follicle rupture introduces its contents into the surrounding dermis,” Dr. DiGiorgio said. “The skin reacts with a chemotactic response and abscess formation. This results in inflammatory nodules and sterile abscesses, which can lead to sinus tracts and hypertrophic scars and chronic drainage, which can be foul-smelling. This frequently leads to depression and psychological distress for the patients.”
Possible laser and energy-based treatments for HS include follicular destruction with the Nd:YAG laser, the diode laser, the Alexandrite laser, microwave technology, or intense pulsed light, she said. Microwave technology or radiofrequency can be used for sweat gland destruction, while CO2 lasers can be used to debulk tissue, and the ablative fractional CO2 laser can be used to reduce scarring and improve range of motion.
In a prospective, randomized, intraindividual comparative trial conducted at eight centers in France, researchers evaluated the use of a long-pulsed Nd:YAG laser to treat 36 patients with mild to moderate HS; 27 had inguinal disease and 9 had axillary disease. They received four laser treatments at 6-week intervals; laser settings varied depending on the patient skin type.
At 1 month, there was a significant reduction in the number of inflammatory lesions on the areas treated with lasers, compared to the untreated areas, but the difference was not significant at 3 months. There was no significant difference in the number of flares between the treated and untreated sites at 1 or 3 months.
In a separate study, researchers found that the Nd:YAG laser in combination with topical benzoyl peroxide and clindamycin was significantly more effective than topical benzoyl peroxide and clindamycin alone for the treatment of HS in 22 patients with Hurley stage II disease. The patients received monthly treatments for 4 months and were followed up 2 months after the last treatment; the Hidradenitis Suppurativa Area and Severity Index was used to measure treatment response.
Statistically significant improvements were observed in the inguinal and axillary areas but not in the inframammary areas. Most patients (90%) reported less frequent breakouts while 10% reported no change. “In addition, 92% of subjects felt that the use of laser was more effective than other treatments they had tried but 8% stated it was equal to the other treatments they had tried,” said Dr. DiGiorgio, who was not affiliated with the study. “The researchers noted continued improvement with subsequent laser sessions,” she added.
According to 2019 guidelines from the United States and Canadian HS Foundations on the management of HS – in the section on light, laser, and energy sources – an Nd:YAG laser is recommended in patients with Hurley stage II or III disease on the basis of randomized, controlled trials and case series data, and in patients with Hurley stage I disease based on expert consensus. “Other wavelengths that are used for follicular destruction are recommended on the basis of lower-quality evidence,” the recommendations state.
The guidelines also state that CO2 laser excision “is recommended in patients with Hurley stage II or III disease with fibrotic sinus tracts” while “external beam radiation and PDT have a limited role in the management of patients with HS.”
Dr. DiGiorgio reported having no relevant disclosures.
SAN DIEGO – Pilonidal disease – a chronic inflammatory condition that can trigger the formation of cysts and sinuses in the superior portion of the intragluteal cleft or the sacrococcygeal area – remains challenging to manage, but mounting evidence supports the use of lasers to enhance treatment success.
“Draining sinuses or acute abscesses are usually associated with an underlying cyst and associated granulation tissue, fibrosis, and tufts of hair,” Catherine M. DiGiorgio, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “This is why laser hair removal can help with the treatment of these patients.”
The suspected etiology is a foreign body reaction to the entrapped hairs, which are found in the sinuses in about 75% of cases. “The treatment for that is surgery,” said Dr. DiGiorgio, a laser and cosmetic dermatologist in Boston. Laser hair reduction decreases the recurrence of cyst formation and drainage, and is usually covered by insurance, she noted.
Supportive evidence
In a comparative study, French researchers retrospectively reviewed the efficacy of laser hair removal after surgery in reducing recurrence rate of pilonidal cysts, versus surgery alone. Of the 41 study participants, 12 had laser hair removal plus surgery and 29 had surgery alone. The rate of cyst recurrence was significantly lower in the laser hair removal plus surgery group, compared with the surgery only group (8.3% vs. 51.7%, respectively; P < .001).
In another study, researchers from the United Kingdom and The Johns Hopkins Hospital, Baltimore, evaluated the use of the long-pulsed Alexandrite laser in 19 patients with recurrent pilonidal disease who had undergone multiple surgeries.They were treated with the laser for hair removal in the sinus area, requiring 4-12 sessions. The researchers found that 84.2% of patients had a reduction of hair density to less than 5 hairs/cm2, while 15.8% had a reduction of hair density to 5-10 hairs/cm2. They also noted a statistically significant increase in disease-free time in the laser-treated group compared with those treated with surgical management only (P < .01).
Lasers for pseudofolliculitis barbae, HS
Lasers also play a significant role in the treatment of pseudofolliculitis barbae, a chronic, inflammatory disease that primarily affects the bearded area of men with thick hairs, usually those with a darker Fitzpatrick skin type. This can also occur in women, particularly those with polycystic ovary syndrome, Dr. DiGiorgio said.
In people with pseudofolliculitis barbae, the hair follicle is positioned at an acute angle to the skin surface and the sharp end of shaved hair reenters the skin, which results in the formation of pustules, papules, secondary infection, and keloids. Treatment involves a variety of medical therapies including retinoids, benzoyl peroxide, antibiotics, and keratolytics, “but laser hair removal is the best way to get rid of this issue, and results in permanent reduction,” she said. “When treating male patients with laser hair removal in the bearded area, you have to tell them that they won’t be able to grow a beard going forward. Most of them are okay with that.”
A 2002 study, led by E. Victor Ross, MD, of the Naval Medical Center, San Diego, evaluated treatment of pseudofolliculitis barbae in patients with skin types IV, V, and VI with a long-pulsed Nd:YAG laser. For the first phase of the study, the investigators tested epidermal tolerance on the thighs of 37 patients and determined that the laser was safe and effective. For the second phase 2 weeks later, they treated a 15x15-mm submental area with the highest fluence tolerated in phase 1 of the trial and used an adjacent site as the control.
After 90 days, the mean papule count was 6.95 for the control site compared with 1 for the laser-treated site. The researchers observed that miniaturization and elimination of hair shafts resulted in decreased inflamed papules. “We know that this works,” Dr. DiGiorgio said.
In another study from investigators at the Naval Medical Center, San Diego, 22 patients with skin types IV, V, and VI who had pseudofolliculitis barbae underwent 5 weekly treatments with a 1,064 nm Nd:YAG laser. Topical anesthesia was not used, and 10 evaluators used a Global Assessment Scale (GAS) to assess treatment success from photos taken at baseline and at 4 weeks. At 4 weeks, 11 patients demonstrated 83% improvement on the GAS (P < .01), the investigators reported.
Laser and energy-based treatments can also be used to treat hidradenitis suppurativa (HS), a chronic condition that affects apocrine gland–bearing skin. “The hypothesized pathogenesis is that it’s an inflammatory disorder of the hair follicle, where the follicle rupture introduces its contents into the surrounding dermis,” Dr. DiGiorgio said. “The skin reacts with a chemotactic response and abscess formation. This results in inflammatory nodules and sterile abscesses, which can lead to sinus tracts and hypertrophic scars and chronic drainage, which can be foul-smelling. This frequently leads to depression and psychological distress for the patients.”
Possible laser and energy-based treatments for HS include follicular destruction with the Nd:YAG laser, the diode laser, the Alexandrite laser, microwave technology, or intense pulsed light, she said. Microwave technology or radiofrequency can be used for sweat gland destruction, while CO2 lasers can be used to debulk tissue, and the ablative fractional CO2 laser can be used to reduce scarring and improve range of motion.
In a prospective, randomized, intraindividual comparative trial conducted at eight centers in France, researchers evaluated the use of a long-pulsed Nd:YAG laser to treat 36 patients with mild to moderate HS; 27 had inguinal disease and 9 had axillary disease. They received four laser treatments at 6-week intervals; laser settings varied depending on the patient skin type.
At 1 month, there was a significant reduction in the number of inflammatory lesions on the areas treated with lasers, compared to the untreated areas, but the difference was not significant at 3 months. There was no significant difference in the number of flares between the treated and untreated sites at 1 or 3 months.
In a separate study, researchers found that the Nd:YAG laser in combination with topical benzoyl peroxide and clindamycin was significantly more effective than topical benzoyl peroxide and clindamycin alone for the treatment of HS in 22 patients with Hurley stage II disease. The patients received monthly treatments for 4 months and were followed up 2 months after the last treatment; the Hidradenitis Suppurativa Area and Severity Index was used to measure treatment response.
Statistically significant improvements were observed in the inguinal and axillary areas but not in the inframammary areas. Most patients (90%) reported less frequent breakouts while 10% reported no change. “In addition, 92% of subjects felt that the use of laser was more effective than other treatments they had tried but 8% stated it was equal to the other treatments they had tried,” said Dr. DiGiorgio, who was not affiliated with the study. “The researchers noted continued improvement with subsequent laser sessions,” she added.
According to 2019 guidelines from the United States and Canadian HS Foundations on the management of HS – in the section on light, laser, and energy sources – an Nd:YAG laser is recommended in patients with Hurley stage II or III disease on the basis of randomized, controlled trials and case series data, and in patients with Hurley stage I disease based on expert consensus. “Other wavelengths that are used for follicular destruction are recommended on the basis of lower-quality evidence,” the recommendations state.
The guidelines also state that CO2 laser excision “is recommended in patients with Hurley stage II or III disease with fibrotic sinus tracts” while “external beam radiation and PDT have a limited role in the management of patients with HS.”
Dr. DiGiorgio reported having no relevant disclosures.
SAN DIEGO – Pilonidal disease – a chronic inflammatory condition that can trigger the formation of cysts and sinuses in the superior portion of the intragluteal cleft or the sacrococcygeal area – remains challenging to manage, but mounting evidence supports the use of lasers to enhance treatment success.
“Draining sinuses or acute abscesses are usually associated with an underlying cyst and associated granulation tissue, fibrosis, and tufts of hair,” Catherine M. DiGiorgio, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “This is why laser hair removal can help with the treatment of these patients.”
The suspected etiology is a foreign body reaction to the entrapped hairs, which are found in the sinuses in about 75% of cases. “The treatment for that is surgery,” said Dr. DiGiorgio, a laser and cosmetic dermatologist in Boston. Laser hair reduction decreases the recurrence of cyst formation and drainage, and is usually covered by insurance, she noted.
Supportive evidence
In a comparative study, French researchers retrospectively reviewed the efficacy of laser hair removal after surgery in reducing recurrence rate of pilonidal cysts, versus surgery alone. Of the 41 study participants, 12 had laser hair removal plus surgery and 29 had surgery alone. The rate of cyst recurrence was significantly lower in the laser hair removal plus surgery group, compared with the surgery only group (8.3% vs. 51.7%, respectively; P < .001).
In another study, researchers from the United Kingdom and The Johns Hopkins Hospital, Baltimore, evaluated the use of the long-pulsed Alexandrite laser in 19 patients with recurrent pilonidal disease who had undergone multiple surgeries.They were treated with the laser for hair removal in the sinus area, requiring 4-12 sessions. The researchers found that 84.2% of patients had a reduction of hair density to less than 5 hairs/cm2, while 15.8% had a reduction of hair density to 5-10 hairs/cm2. They also noted a statistically significant increase in disease-free time in the laser-treated group compared with those treated with surgical management only (P < .01).
Lasers for pseudofolliculitis barbae, HS
Lasers also play a significant role in the treatment of pseudofolliculitis barbae, a chronic, inflammatory disease that primarily affects the bearded area of men with thick hairs, usually those with a darker Fitzpatrick skin type. This can also occur in women, particularly those with polycystic ovary syndrome, Dr. DiGiorgio said.
In people with pseudofolliculitis barbae, the hair follicle is positioned at an acute angle to the skin surface and the sharp end of shaved hair reenters the skin, which results in the formation of pustules, papules, secondary infection, and keloids. Treatment involves a variety of medical therapies including retinoids, benzoyl peroxide, antibiotics, and keratolytics, “but laser hair removal is the best way to get rid of this issue, and results in permanent reduction,” she said. “When treating male patients with laser hair removal in the bearded area, you have to tell them that they won’t be able to grow a beard going forward. Most of them are okay with that.”
A 2002 study, led by E. Victor Ross, MD, of the Naval Medical Center, San Diego, evaluated treatment of pseudofolliculitis barbae in patients with skin types IV, V, and VI with a long-pulsed Nd:YAG laser. For the first phase of the study, the investigators tested epidermal tolerance on the thighs of 37 patients and determined that the laser was safe and effective. For the second phase 2 weeks later, they treated a 15x15-mm submental area with the highest fluence tolerated in phase 1 of the trial and used an adjacent site as the control.
After 90 days, the mean papule count was 6.95 for the control site compared with 1 for the laser-treated site. The researchers observed that miniaturization and elimination of hair shafts resulted in decreased inflamed papules. “We know that this works,” Dr. DiGiorgio said.
In another study from investigators at the Naval Medical Center, San Diego, 22 patients with skin types IV, V, and VI who had pseudofolliculitis barbae underwent 5 weekly treatments with a 1,064 nm Nd:YAG laser. Topical anesthesia was not used, and 10 evaluators used a Global Assessment Scale (GAS) to assess treatment success from photos taken at baseline and at 4 weeks. At 4 weeks, 11 patients demonstrated 83% improvement on the GAS (P < .01), the investigators reported.
Laser and energy-based treatments can also be used to treat hidradenitis suppurativa (HS), a chronic condition that affects apocrine gland–bearing skin. “The hypothesized pathogenesis is that it’s an inflammatory disorder of the hair follicle, where the follicle rupture introduces its contents into the surrounding dermis,” Dr. DiGiorgio said. “The skin reacts with a chemotactic response and abscess formation. This results in inflammatory nodules and sterile abscesses, which can lead to sinus tracts and hypertrophic scars and chronic drainage, which can be foul-smelling. This frequently leads to depression and psychological distress for the patients.”
Possible laser and energy-based treatments for HS include follicular destruction with the Nd:YAG laser, the diode laser, the Alexandrite laser, microwave technology, or intense pulsed light, she said. Microwave technology or radiofrequency can be used for sweat gland destruction, while CO2 lasers can be used to debulk tissue, and the ablative fractional CO2 laser can be used to reduce scarring and improve range of motion.
In a prospective, randomized, intraindividual comparative trial conducted at eight centers in France, researchers evaluated the use of a long-pulsed Nd:YAG laser to treat 36 patients with mild to moderate HS; 27 had inguinal disease and 9 had axillary disease. They received four laser treatments at 6-week intervals; laser settings varied depending on the patient skin type.
At 1 month, there was a significant reduction in the number of inflammatory lesions on the areas treated with lasers, compared to the untreated areas, but the difference was not significant at 3 months. There was no significant difference in the number of flares between the treated and untreated sites at 1 or 3 months.
In a separate study, researchers found that the Nd:YAG laser in combination with topical benzoyl peroxide and clindamycin was significantly more effective than topical benzoyl peroxide and clindamycin alone for the treatment of HS in 22 patients with Hurley stage II disease. The patients received monthly treatments for 4 months and were followed up 2 months after the last treatment; the Hidradenitis Suppurativa Area and Severity Index was used to measure treatment response.
Statistically significant improvements were observed in the inguinal and axillary areas but not in the inframammary areas. Most patients (90%) reported less frequent breakouts while 10% reported no change. “In addition, 92% of subjects felt that the use of laser was more effective than other treatments they had tried but 8% stated it was equal to the other treatments they had tried,” said Dr. DiGiorgio, who was not affiliated with the study. “The researchers noted continued improvement with subsequent laser sessions,” she added.
According to 2019 guidelines from the United States and Canadian HS Foundations on the management of HS – in the section on light, laser, and energy sources – an Nd:YAG laser is recommended in patients with Hurley stage II or III disease on the basis of randomized, controlled trials and case series data, and in patients with Hurley stage I disease based on expert consensus. “Other wavelengths that are used for follicular destruction are recommended on the basis of lower-quality evidence,” the recommendations state.
The guidelines also state that CO2 laser excision “is recommended in patients with Hurley stage II or III disease with fibrotic sinus tracts” while “external beam radiation and PDT have a limited role in the management of patients with HS.”
Dr. DiGiorgio reported having no relevant disclosures.
AT ASLMS 2022
Fractional lasers appear to treat more than a fraction of skin, expert says
SAN DIEGO – Using the according to Molly Wanner, MD, MBA.
As a case in point, Dr. Wanner discussed the results of a trial of 48 people over aged 60 years with actinic damage, who received ablative fractional laser treatment on one arm and no treatment on the other arm, which served as the control. At 24 months, only two nonmelanoma skin cancers (NMSCs) developed on the treated arms, compared with 26 on the treated arms.
“What I find interesting is that the treated arm did not develop basal cell carcinoma, only squamous cell carcinoma,” she said at the annual meeting of the American Society for Laser Medicine and Surgery. “It appears that this is working through more than just treatment of the AK precursor lesions, for which fractional lasers are cleared for use. It appears to impact both types of NMSCs.”
The ablative fractional laser and other wounding therapies can modulate a response to UV light – a process that naturally diminishes with age, according to Dr. Wanner, a dermatologist at Massachusetts General Hospital’s Dermatology Laser and Cosmetic Center in Boston. “This ability to repair DNA is actually modulated by insulin-like growth factor 1,” she said. “IGF-1 is produced by papillary dermal fibroblasts and communicates with keratinocytes. If keratinocytes are exposed to UV light and there is no IGF-1 around, you get a mutated cell, and that keeps spreading, and you could potentially get a skin cancer.”
On the other hand, she continued, if IGF-1 is injected around the keratinocytes, they are able to respond. “Keratinocytes, which are the most superficial layer of the skin, are really active,” noted Dr. Wanner, who is also an assistant professor of dermatology at Harvard Medical School, Boston. “They’re dividing and replicating, whereas fibroblasts are more non-proliferative and more long-lived. They stick around for a long time. I think of them as the adults in the room, giving these new keratinocytes direction.”
In a review of wounding therapies for the prevention of photocarcinogenesis, she and her coauthors noted that IGF-1 increases nucleotide excision repair of damaged DNA, promotes checkpoint signaling and suppression of DNA synthesis, favors specialized polymerases that are better able to repair DNA damage, and enhances p53-dependent transcriptional responses to DNA damage.
“Older fibroblasts produce less IGF-1 and lead to a situation where keratinocytes can grow unchecked,” she said. “We can use fractional laser to help with this. Fractional laser increases fibroblast production and decreases senescent fibroblasts.”
In a 2017 review on the impact of age and IGF-1 on DNA damage responses in UV-irradiated skin, the authors noted the high levels of IGF-1 in the skin of younger individuals and lower levels in the skin of their older counterparts.
“But once older skin has been treated with either dermabrasion or fractional laser, the levels of IGF-1 are restored to that of a young adult,” Dr. Wanner said. “The restoration of IGF-1 then restores that level of appropriate response to UV light. So, what’s interesting is that fractional lasers treat more than a fraction [of skin]. Fractional lasers were developed to have an easier way to improve wound healing by leaving the skin intact around these columns [of treated skin]. It turns out that treatment of these columns of skin does not just impact the cells in that area. There is a true global effect that’s allowing us to almost normalize skin.”
Dr. Wanner now thinks of fractional lasers as stimulating a laser-cell biology interaction, not just a laser-tissue interaction. “It’s incredible that we can use these photons to not only impact the tissue itself but how the cells actually respond,” she said. “What’s going to be interesting for us in the next few years is to look at how lasers impact our cellular biology. How can we harness it to help our patients?”
She and her colleagues are conducting a trial of different wounding modalities to assess their impact on IGF-1. “Does depth matter? Does density matter? Does the wavelength matter?” she asked. “The bottom line is, it turns out that when the skin looks healthier, it is healthier. Cosmetic treatments can impact medical outcomes.”
Dr. Wanner disclosed that she is a consultant and advisor to Nu Skin. She has also received research funding and equipment from Solta.
SAN DIEGO – Using the according to Molly Wanner, MD, MBA.
As a case in point, Dr. Wanner discussed the results of a trial of 48 people over aged 60 years with actinic damage, who received ablative fractional laser treatment on one arm and no treatment on the other arm, which served as the control. At 24 months, only two nonmelanoma skin cancers (NMSCs) developed on the treated arms, compared with 26 on the treated arms.
“What I find interesting is that the treated arm did not develop basal cell carcinoma, only squamous cell carcinoma,” she said at the annual meeting of the American Society for Laser Medicine and Surgery. “It appears that this is working through more than just treatment of the AK precursor lesions, for which fractional lasers are cleared for use. It appears to impact both types of NMSCs.”
The ablative fractional laser and other wounding therapies can modulate a response to UV light – a process that naturally diminishes with age, according to Dr. Wanner, a dermatologist at Massachusetts General Hospital’s Dermatology Laser and Cosmetic Center in Boston. “This ability to repair DNA is actually modulated by insulin-like growth factor 1,” she said. “IGF-1 is produced by papillary dermal fibroblasts and communicates with keratinocytes. If keratinocytes are exposed to UV light and there is no IGF-1 around, you get a mutated cell, and that keeps spreading, and you could potentially get a skin cancer.”
On the other hand, she continued, if IGF-1 is injected around the keratinocytes, they are able to respond. “Keratinocytes, which are the most superficial layer of the skin, are really active,” noted Dr. Wanner, who is also an assistant professor of dermatology at Harvard Medical School, Boston. “They’re dividing and replicating, whereas fibroblasts are more non-proliferative and more long-lived. They stick around for a long time. I think of them as the adults in the room, giving these new keratinocytes direction.”
In a review of wounding therapies for the prevention of photocarcinogenesis, she and her coauthors noted that IGF-1 increases nucleotide excision repair of damaged DNA, promotes checkpoint signaling and suppression of DNA synthesis, favors specialized polymerases that are better able to repair DNA damage, and enhances p53-dependent transcriptional responses to DNA damage.
“Older fibroblasts produce less IGF-1 and lead to a situation where keratinocytes can grow unchecked,” she said. “We can use fractional laser to help with this. Fractional laser increases fibroblast production and decreases senescent fibroblasts.”
In a 2017 review on the impact of age and IGF-1 on DNA damage responses in UV-irradiated skin, the authors noted the high levels of IGF-1 in the skin of younger individuals and lower levels in the skin of their older counterparts.
“But once older skin has been treated with either dermabrasion or fractional laser, the levels of IGF-1 are restored to that of a young adult,” Dr. Wanner said. “The restoration of IGF-1 then restores that level of appropriate response to UV light. So, what’s interesting is that fractional lasers treat more than a fraction [of skin]. Fractional lasers were developed to have an easier way to improve wound healing by leaving the skin intact around these columns [of treated skin]. It turns out that treatment of these columns of skin does not just impact the cells in that area. There is a true global effect that’s allowing us to almost normalize skin.”
Dr. Wanner now thinks of fractional lasers as stimulating a laser-cell biology interaction, not just a laser-tissue interaction. “It’s incredible that we can use these photons to not only impact the tissue itself but how the cells actually respond,” she said. “What’s going to be interesting for us in the next few years is to look at how lasers impact our cellular biology. How can we harness it to help our patients?”
She and her colleagues are conducting a trial of different wounding modalities to assess their impact on IGF-1. “Does depth matter? Does density matter? Does the wavelength matter?” she asked. “The bottom line is, it turns out that when the skin looks healthier, it is healthier. Cosmetic treatments can impact medical outcomes.”
Dr. Wanner disclosed that she is a consultant and advisor to Nu Skin. She has also received research funding and equipment from Solta.
SAN DIEGO – Using the according to Molly Wanner, MD, MBA.
As a case in point, Dr. Wanner discussed the results of a trial of 48 people over aged 60 years with actinic damage, who received ablative fractional laser treatment on one arm and no treatment on the other arm, which served as the control. At 24 months, only two nonmelanoma skin cancers (NMSCs) developed on the treated arms, compared with 26 on the treated arms.
“What I find interesting is that the treated arm did not develop basal cell carcinoma, only squamous cell carcinoma,” she said at the annual meeting of the American Society for Laser Medicine and Surgery. “It appears that this is working through more than just treatment of the AK precursor lesions, for which fractional lasers are cleared for use. It appears to impact both types of NMSCs.”
The ablative fractional laser and other wounding therapies can modulate a response to UV light – a process that naturally diminishes with age, according to Dr. Wanner, a dermatologist at Massachusetts General Hospital’s Dermatology Laser and Cosmetic Center in Boston. “This ability to repair DNA is actually modulated by insulin-like growth factor 1,” she said. “IGF-1 is produced by papillary dermal fibroblasts and communicates with keratinocytes. If keratinocytes are exposed to UV light and there is no IGF-1 around, you get a mutated cell, and that keeps spreading, and you could potentially get a skin cancer.”
On the other hand, she continued, if IGF-1 is injected around the keratinocytes, they are able to respond. “Keratinocytes, which are the most superficial layer of the skin, are really active,” noted Dr. Wanner, who is also an assistant professor of dermatology at Harvard Medical School, Boston. “They’re dividing and replicating, whereas fibroblasts are more non-proliferative and more long-lived. They stick around for a long time. I think of them as the adults in the room, giving these new keratinocytes direction.”
In a review of wounding therapies for the prevention of photocarcinogenesis, she and her coauthors noted that IGF-1 increases nucleotide excision repair of damaged DNA, promotes checkpoint signaling and suppression of DNA synthesis, favors specialized polymerases that are better able to repair DNA damage, and enhances p53-dependent transcriptional responses to DNA damage.
“Older fibroblasts produce less IGF-1 and lead to a situation where keratinocytes can grow unchecked,” she said. “We can use fractional laser to help with this. Fractional laser increases fibroblast production and decreases senescent fibroblasts.”
In a 2017 review on the impact of age and IGF-1 on DNA damage responses in UV-irradiated skin, the authors noted the high levels of IGF-1 in the skin of younger individuals and lower levels in the skin of their older counterparts.
“But once older skin has been treated with either dermabrasion or fractional laser, the levels of IGF-1 are restored to that of a young adult,” Dr. Wanner said. “The restoration of IGF-1 then restores that level of appropriate response to UV light. So, what’s interesting is that fractional lasers treat more than a fraction [of skin]. Fractional lasers were developed to have an easier way to improve wound healing by leaving the skin intact around these columns [of treated skin]. It turns out that treatment of these columns of skin does not just impact the cells in that area. There is a true global effect that’s allowing us to almost normalize skin.”
Dr. Wanner now thinks of fractional lasers as stimulating a laser-cell biology interaction, not just a laser-tissue interaction. “It’s incredible that we can use these photons to not only impact the tissue itself but how the cells actually respond,” she said. “What’s going to be interesting for us in the next few years is to look at how lasers impact our cellular biology. How can we harness it to help our patients?”
She and her colleagues are conducting a trial of different wounding modalities to assess their impact on IGF-1. “Does depth matter? Does density matter? Does the wavelength matter?” she asked. “The bottom line is, it turns out that when the skin looks healthier, it is healthier. Cosmetic treatments can impact medical outcomes.”
Dr. Wanner disclosed that she is a consultant and advisor to Nu Skin. She has also received research funding and equipment from Solta.
AT ASLMS 2022
The whitest specialty: Bias
As Usha Lee McFarling has pointed out, the orthopedic surgeon specialty suffers from a gross underrepresentation of minorities and women, more severe than in other medical specialties. There are various reasons for this and a variety of possible paths toward improvement, but the “critical first step,” as American Academy of Orthopedic Surgeons former president Kristy Weber, MD, told Ms. McFarling, “is changing the culture.”
“Changing the culture” is a large, diffuse aspiration. The AAOS has taken a number of steps toward that end, but they have not had much success. The two of us have identified others, which may help to move the needle.
Viewed from this perspective, the cultural barriers to inclusivity are similar to those that perpetuate inequitable health care. Both are driven by ingroup/outgroup prejudices that operate below the level of consciousness and are largely unseen.In our book Seeing Patients, we examined health disparities in six “non-mainstream” groups: African Americans, Hispanic Americans, women, gays and lesbians, and the elderly. We based our work initially on the Institute of Medicine’s breakthrough 2003 compendium, Unequal Treatment, which brought together a large number of studies on health care inequities that had appeared in a variety of journals over many years, but had never generated the critical mass necessary to create a call for action or even attract serious attention.
Unequal Treatment allowed us to understand that each medical specialty, right down the line – orthopedics, cardiology, gynecology, oncology, psychiatry, to name just a few – has its own grim history of discrimination. Our sense of the medical community in the 21st century led us away from the idea that overt bias is a significant cause of these still ongoing inequities. Most physicians, we believed, consider themselves to be, and strive to be, humane, compassionate, and egalitarian caregivers. The answer then seemed to be in subconscious rather than conscious bias.
As we reviewed the literature and strove to understand the primary drivers of the discrimination that systematically affects medical care, our attention was drawn to two critical and complementary mechanisms hard-wired into our systems for parsing and responding to our environment. The first was “stereotyping,” so often used as a pejorative, but which is, in fact, a primary and essential mental function.
“We all make stereotypic judgments,” says Rice University emeritus professor of psychology David Schneider in The Psychology of Stereotyping (page 419). “It happens with race. It happens with disability. It happens ... with gender, age, and physical appearance. ... That’s just the way it is: Our mental apparatus was designed to facilitate quick decisions based on category membership.”
Differentiation – social stereotyping in our case – is a given, then; it’s innate. The content of stereotyping – of Blacks, gays, women, and others – is not innate, but it is deeply ingrained by living in a given milieu and just as impossible to ignore.
The second mechanism we focused on was the neurobiology that underlies the impact of hidden emotion on rational thought. In his seminal book Descartes’ Error, neuroscientist Antonio Damasio spells out how the mind with its cognitive functions has evolved from the body and its emotional systems, and how they function together through neuro-networks that connect the mechanisms of feeling with the brain’s decision-making centers.
“Feelings,” Dr. Damasio tells us, “come first in [brain] development and retain a primacy that pervades our mental life.” The limbic system, the part of the brain that controls our emotional responses, constitutes a “frame of reference and has “a say on how the rest of the brain and cognition go about their business. [Its] influence is immense.” (Page 185)
Dr. Damasio was not focusing on medical decisions, but his insights, we felt, had great relevance for the question of unconscious bias in health care. Various studies by physicians and medical scientists do speak directly to the issue of how affective bias influences diagnosis and treatment. Pat Croskerry, director of Dalhousie University’s Clinical Research Center, argues that “cognitive and affective biases are known to compromise the decision-making” and that commonly “these are largely unconscious mistakes.”
Harvard’s Jerome Groopman, in his book How Doctors Think (page 40), writes that most incorrect diagnoses and treatments are “mistakes in thinking. And part of what causes these cognitive errors is our inner feelings, feelings we ... often don’t even recognize.” Cognition and emotion, Dr. Groopman insists, are inseparable. The emotional landscape sets the ground for decision-making.
The underlying mechanisms that enable health care prejudice are the same that enable interpersonal prejudice generally. Unseen and largely unrecognized, they affect ingroup/outgroup relations in every field of interaction, from bias in policing, to bias in housing, to bias in employment – “powerful and universal,” in Dr. Croskerry’s words, “affecting all walks of life.”
Decision-making about acceptance into orthopedic residencies is no exception. As Prof. Schneider says, “That’s just the way it is.”
What conclusions can be drawn from understanding the deep origins of subconscious bias that might improve the inclusion of minorities and women in orthopedics? A growing interest in “debiasing” in both the medical and cognitive psychology literature has identified or suggested methods of counteracting the prejudices we all harbor. (See Bhatti’s “Cognitive Bias in Clinical Practice,” Wilson and Brekke’s “Mental Contamination and Mental Correction: Unwanted Influences on Judgments and Evaluations,” and De Neys and colleagues’ “Feeling We’re Biased: Autonomic Arousal and Reasoning Conflict.”)
Many of these debiasing techniques have to do with education regarding cognitive functions, from training in decision-making processes to “time outs,” to checklists à la Atul Gawande, to other methods of metacognition.
But the two key prerequisites to all of these approaches are more or less self-evident. “For biases to be successfully addressed,” says Dr. Croskerry, “there needs to be ... awareness as well as the motivation for change.”
In a previous article we discussed the need to heighten awareness over and above current levels, and we have suggested steps toward that end. But awareness is only the first prerequisite; the second is motivation, and the depth of motivation necessary to create change in the business of orthopedic inclusion is, for all the AAOS’s efforts, simply inadequate – the result being that the culture does not change, or it changes so glacially as to be hardly noticeable.
Ms. McFarling noted in her interviews with orthopedic leaders, clinicians, residents, and medical students simmering feelings of frustration and perplexity. We would suggest that the frustration is because of the fact that, while there is a general awareness of the problem, there has simply not been the sufficiently determined motivation to fix it. “It is not neglected truths,” as religious scholar Gregory Dix put it, “but those that are at once fully acknowledged and frustrated of their proper expression, which take the most drastic psychological revenge.”
All of this leads back to the original problem posed by Prof. Weber, the former AAOS president: changing the orthopedic culture. The question of how cultures undergo transformation has been addressed by scholars across widely diverse fields (see, for example, Thomas Kuhn’s The Structure of Scientific Revolutions, Francis Fukuyama›s The End of History and the Last Man, and many others). But we are addressing here a narrow, well-defined slice of that problem. And our own explorations have led to the conclusion that the answer here lies in the issue of motivation – namely, how can a community that is aware of a problem be sufficiently motivated to fix it?
In Seeing Patients we argued that doctoring is the paradigmatic humanitarian profession, that physicians’ whole business is to care for and alleviate the suffering of other human beings. In this sense, doctors are the carriers of the humane ideal, which is congruent also with the noblest egalitarian principles of our life as a nation. We argued also that humanitarian medicine with its egalitarian mandate is a win-win-win proposition. The patient wins, the doctor wins, the society wins.
We think arguments like these should provide plenty of motivation for change. But in reality they are not sufficient. Our arguments and those of others along the same lines (see Louis Sullivan’s Breaking Ground and David McBride’s Caring for Equality) are directed for the most part at the better angels of our nature. They appeal to personal and political values: compassion, fairness, equality – powerful yet set against custom, habituation, and the daily pressures of practice, such arguments can and do easily come up short.
But when looked at straight on, with unblinking eyes, health care disparities should provoke other more forceful emotions: anger, to begin with; chagrin, consternation. Women receive fewer heart catheterizations and reperfusions than men. (See R. Di Cecco and colleagues’ “Is There a Clinically Significant Gender Bias in Post-Myocardial Infarction Pharmacological Management in the Older Population of a Primary Care Practice?” and Jneid and coworkers’ “Sex Difference in Medical Care and Early Death after Acute Myocardial Infarction.”) Because of this, more women die.
Blacks and Hispanics receive fewer analgesics for the excruciating pain of broken bones, and they are amputated more frequently than whites for identical peripheral arterial disease. (See Knox and colleagues’ “Ethnicity as a Risk Factor for Inadequate Emergency Department Analgesia,” Bonham’s “Race, Ethnicity and Pain Treatments: Striving to Understand the Causes and Solutions to the Disparities in Pain Treatments,” and Feinglass and coworkers’ “Racial Differences in Primary and Repeat Lower Extremity Amputation: Results From a Multihospital Study.”) They suffer accordingly.
The statistical accounting of these disparities masks the faces of pain and desperation – of disabilities, often of mortality. These are hard visceral truths that derive in part from the underrepresentation of minorities in various specialties, most pronounced in orthopedics. These are the truths that, when actually absorbed rather than just registered, have the capacity to transform awareness into motivation and in so doing can begin reshaping a culture that restricts minorities and women and makes orthopedics, as Ms. McFarling calls it, “the whitest specialty.”
A version of this article first appeared on Medscape.com.
As Usha Lee McFarling has pointed out, the orthopedic surgeon specialty suffers from a gross underrepresentation of minorities and women, more severe than in other medical specialties. There are various reasons for this and a variety of possible paths toward improvement, but the “critical first step,” as American Academy of Orthopedic Surgeons former president Kristy Weber, MD, told Ms. McFarling, “is changing the culture.”
“Changing the culture” is a large, diffuse aspiration. The AAOS has taken a number of steps toward that end, but they have not had much success. The two of us have identified others, which may help to move the needle.
Viewed from this perspective, the cultural barriers to inclusivity are similar to those that perpetuate inequitable health care. Both are driven by ingroup/outgroup prejudices that operate below the level of consciousness and are largely unseen.In our book Seeing Patients, we examined health disparities in six “non-mainstream” groups: African Americans, Hispanic Americans, women, gays and lesbians, and the elderly. We based our work initially on the Institute of Medicine’s breakthrough 2003 compendium, Unequal Treatment, which brought together a large number of studies on health care inequities that had appeared in a variety of journals over many years, but had never generated the critical mass necessary to create a call for action or even attract serious attention.
Unequal Treatment allowed us to understand that each medical specialty, right down the line – orthopedics, cardiology, gynecology, oncology, psychiatry, to name just a few – has its own grim history of discrimination. Our sense of the medical community in the 21st century led us away from the idea that overt bias is a significant cause of these still ongoing inequities. Most physicians, we believed, consider themselves to be, and strive to be, humane, compassionate, and egalitarian caregivers. The answer then seemed to be in subconscious rather than conscious bias.
As we reviewed the literature and strove to understand the primary drivers of the discrimination that systematically affects medical care, our attention was drawn to two critical and complementary mechanisms hard-wired into our systems for parsing and responding to our environment. The first was “stereotyping,” so often used as a pejorative, but which is, in fact, a primary and essential mental function.
“We all make stereotypic judgments,” says Rice University emeritus professor of psychology David Schneider in The Psychology of Stereotyping (page 419). “It happens with race. It happens with disability. It happens ... with gender, age, and physical appearance. ... That’s just the way it is: Our mental apparatus was designed to facilitate quick decisions based on category membership.”
Differentiation – social stereotyping in our case – is a given, then; it’s innate. The content of stereotyping – of Blacks, gays, women, and others – is not innate, but it is deeply ingrained by living in a given milieu and just as impossible to ignore.
The second mechanism we focused on was the neurobiology that underlies the impact of hidden emotion on rational thought. In his seminal book Descartes’ Error, neuroscientist Antonio Damasio spells out how the mind with its cognitive functions has evolved from the body and its emotional systems, and how they function together through neuro-networks that connect the mechanisms of feeling with the brain’s decision-making centers.
“Feelings,” Dr. Damasio tells us, “come first in [brain] development and retain a primacy that pervades our mental life.” The limbic system, the part of the brain that controls our emotional responses, constitutes a “frame of reference and has “a say on how the rest of the brain and cognition go about their business. [Its] influence is immense.” (Page 185)
Dr. Damasio was not focusing on medical decisions, but his insights, we felt, had great relevance for the question of unconscious bias in health care. Various studies by physicians and medical scientists do speak directly to the issue of how affective bias influences diagnosis and treatment. Pat Croskerry, director of Dalhousie University’s Clinical Research Center, argues that “cognitive and affective biases are known to compromise the decision-making” and that commonly “these are largely unconscious mistakes.”
Harvard’s Jerome Groopman, in his book How Doctors Think (page 40), writes that most incorrect diagnoses and treatments are “mistakes in thinking. And part of what causes these cognitive errors is our inner feelings, feelings we ... often don’t even recognize.” Cognition and emotion, Dr. Groopman insists, are inseparable. The emotional landscape sets the ground for decision-making.
The underlying mechanisms that enable health care prejudice are the same that enable interpersonal prejudice generally. Unseen and largely unrecognized, they affect ingroup/outgroup relations in every field of interaction, from bias in policing, to bias in housing, to bias in employment – “powerful and universal,” in Dr. Croskerry’s words, “affecting all walks of life.”
Decision-making about acceptance into orthopedic residencies is no exception. As Prof. Schneider says, “That’s just the way it is.”
What conclusions can be drawn from understanding the deep origins of subconscious bias that might improve the inclusion of minorities and women in orthopedics? A growing interest in “debiasing” in both the medical and cognitive psychology literature has identified or suggested methods of counteracting the prejudices we all harbor. (See Bhatti’s “Cognitive Bias in Clinical Practice,” Wilson and Brekke’s “Mental Contamination and Mental Correction: Unwanted Influences on Judgments and Evaluations,” and De Neys and colleagues’ “Feeling We’re Biased: Autonomic Arousal and Reasoning Conflict.”)
Many of these debiasing techniques have to do with education regarding cognitive functions, from training in decision-making processes to “time outs,” to checklists à la Atul Gawande, to other methods of metacognition.
But the two key prerequisites to all of these approaches are more or less self-evident. “For biases to be successfully addressed,” says Dr. Croskerry, “there needs to be ... awareness as well as the motivation for change.”
In a previous article we discussed the need to heighten awareness over and above current levels, and we have suggested steps toward that end. But awareness is only the first prerequisite; the second is motivation, and the depth of motivation necessary to create change in the business of orthopedic inclusion is, for all the AAOS’s efforts, simply inadequate – the result being that the culture does not change, or it changes so glacially as to be hardly noticeable.
Ms. McFarling noted in her interviews with orthopedic leaders, clinicians, residents, and medical students simmering feelings of frustration and perplexity. We would suggest that the frustration is because of the fact that, while there is a general awareness of the problem, there has simply not been the sufficiently determined motivation to fix it. “It is not neglected truths,” as religious scholar Gregory Dix put it, “but those that are at once fully acknowledged and frustrated of their proper expression, which take the most drastic psychological revenge.”
All of this leads back to the original problem posed by Prof. Weber, the former AAOS president: changing the orthopedic culture. The question of how cultures undergo transformation has been addressed by scholars across widely diverse fields (see, for example, Thomas Kuhn’s The Structure of Scientific Revolutions, Francis Fukuyama›s The End of History and the Last Man, and many others). But we are addressing here a narrow, well-defined slice of that problem. And our own explorations have led to the conclusion that the answer here lies in the issue of motivation – namely, how can a community that is aware of a problem be sufficiently motivated to fix it?
In Seeing Patients we argued that doctoring is the paradigmatic humanitarian profession, that physicians’ whole business is to care for and alleviate the suffering of other human beings. In this sense, doctors are the carriers of the humane ideal, which is congruent also with the noblest egalitarian principles of our life as a nation. We argued also that humanitarian medicine with its egalitarian mandate is a win-win-win proposition. The patient wins, the doctor wins, the society wins.
We think arguments like these should provide plenty of motivation for change. But in reality they are not sufficient. Our arguments and those of others along the same lines (see Louis Sullivan’s Breaking Ground and David McBride’s Caring for Equality) are directed for the most part at the better angels of our nature. They appeal to personal and political values: compassion, fairness, equality – powerful yet set against custom, habituation, and the daily pressures of practice, such arguments can and do easily come up short.
But when looked at straight on, with unblinking eyes, health care disparities should provoke other more forceful emotions: anger, to begin with; chagrin, consternation. Women receive fewer heart catheterizations and reperfusions than men. (See R. Di Cecco and colleagues’ “Is There a Clinically Significant Gender Bias in Post-Myocardial Infarction Pharmacological Management in the Older Population of a Primary Care Practice?” and Jneid and coworkers’ “Sex Difference in Medical Care and Early Death after Acute Myocardial Infarction.”) Because of this, more women die.
Blacks and Hispanics receive fewer analgesics for the excruciating pain of broken bones, and they are amputated more frequently than whites for identical peripheral arterial disease. (See Knox and colleagues’ “Ethnicity as a Risk Factor for Inadequate Emergency Department Analgesia,” Bonham’s “Race, Ethnicity and Pain Treatments: Striving to Understand the Causes and Solutions to the Disparities in Pain Treatments,” and Feinglass and coworkers’ “Racial Differences in Primary and Repeat Lower Extremity Amputation: Results From a Multihospital Study.”) They suffer accordingly.
The statistical accounting of these disparities masks the faces of pain and desperation – of disabilities, often of mortality. These are hard visceral truths that derive in part from the underrepresentation of minorities in various specialties, most pronounced in orthopedics. These are the truths that, when actually absorbed rather than just registered, have the capacity to transform awareness into motivation and in so doing can begin reshaping a culture that restricts minorities and women and makes orthopedics, as Ms. McFarling calls it, “the whitest specialty.”
A version of this article first appeared on Medscape.com.
As Usha Lee McFarling has pointed out, the orthopedic surgeon specialty suffers from a gross underrepresentation of minorities and women, more severe than in other medical specialties. There are various reasons for this and a variety of possible paths toward improvement, but the “critical first step,” as American Academy of Orthopedic Surgeons former president Kristy Weber, MD, told Ms. McFarling, “is changing the culture.”
“Changing the culture” is a large, diffuse aspiration. The AAOS has taken a number of steps toward that end, but they have not had much success. The two of us have identified others, which may help to move the needle.
Viewed from this perspective, the cultural barriers to inclusivity are similar to those that perpetuate inequitable health care. Both are driven by ingroup/outgroup prejudices that operate below the level of consciousness and are largely unseen.In our book Seeing Patients, we examined health disparities in six “non-mainstream” groups: African Americans, Hispanic Americans, women, gays and lesbians, and the elderly. We based our work initially on the Institute of Medicine’s breakthrough 2003 compendium, Unequal Treatment, which brought together a large number of studies on health care inequities that had appeared in a variety of journals over many years, but had never generated the critical mass necessary to create a call for action or even attract serious attention.
Unequal Treatment allowed us to understand that each medical specialty, right down the line – orthopedics, cardiology, gynecology, oncology, psychiatry, to name just a few – has its own grim history of discrimination. Our sense of the medical community in the 21st century led us away from the idea that overt bias is a significant cause of these still ongoing inequities. Most physicians, we believed, consider themselves to be, and strive to be, humane, compassionate, and egalitarian caregivers. The answer then seemed to be in subconscious rather than conscious bias.
As we reviewed the literature and strove to understand the primary drivers of the discrimination that systematically affects medical care, our attention was drawn to two critical and complementary mechanisms hard-wired into our systems for parsing and responding to our environment. The first was “stereotyping,” so often used as a pejorative, but which is, in fact, a primary and essential mental function.
“We all make stereotypic judgments,” says Rice University emeritus professor of psychology David Schneider in The Psychology of Stereotyping (page 419). “It happens with race. It happens with disability. It happens ... with gender, age, and physical appearance. ... That’s just the way it is: Our mental apparatus was designed to facilitate quick decisions based on category membership.”
Differentiation – social stereotyping in our case – is a given, then; it’s innate. The content of stereotyping – of Blacks, gays, women, and others – is not innate, but it is deeply ingrained by living in a given milieu and just as impossible to ignore.
The second mechanism we focused on was the neurobiology that underlies the impact of hidden emotion on rational thought. In his seminal book Descartes’ Error, neuroscientist Antonio Damasio spells out how the mind with its cognitive functions has evolved from the body and its emotional systems, and how they function together through neuro-networks that connect the mechanisms of feeling with the brain’s decision-making centers.
“Feelings,” Dr. Damasio tells us, “come first in [brain] development and retain a primacy that pervades our mental life.” The limbic system, the part of the brain that controls our emotional responses, constitutes a “frame of reference and has “a say on how the rest of the brain and cognition go about their business. [Its] influence is immense.” (Page 185)
Dr. Damasio was not focusing on medical decisions, but his insights, we felt, had great relevance for the question of unconscious bias in health care. Various studies by physicians and medical scientists do speak directly to the issue of how affective bias influences diagnosis and treatment. Pat Croskerry, director of Dalhousie University’s Clinical Research Center, argues that “cognitive and affective biases are known to compromise the decision-making” and that commonly “these are largely unconscious mistakes.”
Harvard’s Jerome Groopman, in his book How Doctors Think (page 40), writes that most incorrect diagnoses and treatments are “mistakes in thinking. And part of what causes these cognitive errors is our inner feelings, feelings we ... often don’t even recognize.” Cognition and emotion, Dr. Groopman insists, are inseparable. The emotional landscape sets the ground for decision-making.
The underlying mechanisms that enable health care prejudice are the same that enable interpersonal prejudice generally. Unseen and largely unrecognized, they affect ingroup/outgroup relations in every field of interaction, from bias in policing, to bias in housing, to bias in employment – “powerful and universal,” in Dr. Croskerry’s words, “affecting all walks of life.”
Decision-making about acceptance into orthopedic residencies is no exception. As Prof. Schneider says, “That’s just the way it is.”
What conclusions can be drawn from understanding the deep origins of subconscious bias that might improve the inclusion of minorities and women in orthopedics? A growing interest in “debiasing” in both the medical and cognitive psychology literature has identified or suggested methods of counteracting the prejudices we all harbor. (See Bhatti’s “Cognitive Bias in Clinical Practice,” Wilson and Brekke’s “Mental Contamination and Mental Correction: Unwanted Influences on Judgments and Evaluations,” and De Neys and colleagues’ “Feeling We’re Biased: Autonomic Arousal and Reasoning Conflict.”)
Many of these debiasing techniques have to do with education regarding cognitive functions, from training in decision-making processes to “time outs,” to checklists à la Atul Gawande, to other methods of metacognition.
But the two key prerequisites to all of these approaches are more or less self-evident. “For biases to be successfully addressed,” says Dr. Croskerry, “there needs to be ... awareness as well as the motivation for change.”
In a previous article we discussed the need to heighten awareness over and above current levels, and we have suggested steps toward that end. But awareness is only the first prerequisite; the second is motivation, and the depth of motivation necessary to create change in the business of orthopedic inclusion is, for all the AAOS’s efforts, simply inadequate – the result being that the culture does not change, or it changes so glacially as to be hardly noticeable.
Ms. McFarling noted in her interviews with orthopedic leaders, clinicians, residents, and medical students simmering feelings of frustration and perplexity. We would suggest that the frustration is because of the fact that, while there is a general awareness of the problem, there has simply not been the sufficiently determined motivation to fix it. “It is not neglected truths,” as religious scholar Gregory Dix put it, “but those that are at once fully acknowledged and frustrated of their proper expression, which take the most drastic psychological revenge.”
All of this leads back to the original problem posed by Prof. Weber, the former AAOS president: changing the orthopedic culture. The question of how cultures undergo transformation has been addressed by scholars across widely diverse fields (see, for example, Thomas Kuhn’s The Structure of Scientific Revolutions, Francis Fukuyama›s The End of History and the Last Man, and many others). But we are addressing here a narrow, well-defined slice of that problem. And our own explorations have led to the conclusion that the answer here lies in the issue of motivation – namely, how can a community that is aware of a problem be sufficiently motivated to fix it?
In Seeing Patients we argued that doctoring is the paradigmatic humanitarian profession, that physicians’ whole business is to care for and alleviate the suffering of other human beings. In this sense, doctors are the carriers of the humane ideal, which is congruent also with the noblest egalitarian principles of our life as a nation. We argued also that humanitarian medicine with its egalitarian mandate is a win-win-win proposition. The patient wins, the doctor wins, the society wins.
We think arguments like these should provide plenty of motivation for change. But in reality they are not sufficient. Our arguments and those of others along the same lines (see Louis Sullivan’s Breaking Ground and David McBride’s Caring for Equality) are directed for the most part at the better angels of our nature. They appeal to personal and political values: compassion, fairness, equality – powerful yet set against custom, habituation, and the daily pressures of practice, such arguments can and do easily come up short.
But when looked at straight on, with unblinking eyes, health care disparities should provoke other more forceful emotions: anger, to begin with; chagrin, consternation. Women receive fewer heart catheterizations and reperfusions than men. (See R. Di Cecco and colleagues’ “Is There a Clinically Significant Gender Bias in Post-Myocardial Infarction Pharmacological Management in the Older Population of a Primary Care Practice?” and Jneid and coworkers’ “Sex Difference in Medical Care and Early Death after Acute Myocardial Infarction.”) Because of this, more women die.
Blacks and Hispanics receive fewer analgesics for the excruciating pain of broken bones, and they are amputated more frequently than whites for identical peripheral arterial disease. (See Knox and colleagues’ “Ethnicity as a Risk Factor for Inadequate Emergency Department Analgesia,” Bonham’s “Race, Ethnicity and Pain Treatments: Striving to Understand the Causes and Solutions to the Disparities in Pain Treatments,” and Feinglass and coworkers’ “Racial Differences in Primary and Repeat Lower Extremity Amputation: Results From a Multihospital Study.”) They suffer accordingly.
The statistical accounting of these disparities masks the faces of pain and desperation – of disabilities, often of mortality. These are hard visceral truths that derive in part from the underrepresentation of minorities in various specialties, most pronounced in orthopedics. These are the truths that, when actually absorbed rather than just registered, have the capacity to transform awareness into motivation and in so doing can begin reshaping a culture that restricts minorities and women and makes orthopedics, as Ms. McFarling calls it, “the whitest specialty.”
A version of this article first appeared on Medscape.com.







