User login
Caring for Muslim patients who fast during Ramadan
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
At what age should you start screening young people for anxiety?
On April 12, 2022, the US Preventive Services Task Force (USPSTF) published a draft recommendation on screening for anxiety in children and adolescents. The recommendation states that clinicians should screen for anxiety in those ages 8 to 18 years. This is a “B” recommendation, which means there is moderate certainty that screening for anxiety in these individuals has a moderate net benefit. The USPSTF felt that the evidence was insufficient to recommend for or against screening at ages 7 years and younger.1
Anxiety is common among young people in America. A survey conducted in 2018-2019 found that 7.8% of children and adolescents (ages 3 to 17 years) had a current anxiety disorder.2 The isolation created by the COVID-19 pandemic has been associated with increased rates of clinically significant psychiatric symptoms; one study suggested that in the first year of the pandemic, 20% of young people experienced elevated anxiety symptoms.3,4 Anxiety disorders in childhood and adolescence also are associated with an increased likelihood of a future anxiety disorder, or depression, in adulthood.
Therapy may improve outcomes. There is evidence that treatment of anxiety disorders can result in improved clinical outcomes. Treatment options include psychotherapy, pharmacotherapy, or a combination of both.5
However, studies showing benefit were conducted in young people whose anxiety was identified via signs or symptoms. The USPSTF could find no direct evidence that identifying anxiety in asymptomatic youth leads to better outcomes. The current draft recommendation is based on indirect evidence on the accuracy of the screening tools and the results of therapy in those who are symptomatic.
Speaking of screening tools ... There were 3 listed in the USPSTF evidence review: the Screen for Child Anxiety Related Disorders (SCARED), which assesses for generalized anxiety disorder (GAD) and any anxiety disorder6; the Patient Health Questionnaire-Adolescent, which screens for GAD and panic disorder7; and the Social Phobia Inventory.8 The SCARED and Social Phobia Inventory are the most widely used clinically.
The accuracy of the screening tests differed. For detection of GAD, sensitivity ranged from 50% to 88% and specificity from 63% to 98%; for social anxiety disorder, sensitivity ranged from 67% to 93% and specificity from 69% to 94%. False-positive results ranged from 17 to 361 per 1000 for GAD and from 104 to 254 per 1000 for social anxiety disorder.1
The USPSTF emphasized that anxiety should not be diagnosed based on a screening test alone. A positive screen should prompt further assessment and confirmation.
An unexpected rating. Given the opportunity costs to administer a screening tool, the high false-positive rates, and the lack of evidence that screening results in improved outcomes among asymptomatic youth, it is curious that this topic did not result in an “I” recommendation. Many screening interventions for children and adolescents with similar evidence profiles—including screening for suicide risk, drug abuse, eating disorders, and alcohol abuse—have previously received an “I.”9
Keep in mind that this is currently a draft recommendation that is open for public comment. The final recommendation will be published in 4 to 12 months.
1. USPSTF. Screening for anxiety in children and adolescents. Draft recommendation statement. Published April 12, 2022. Accessed May 23, 2022. www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/screening-anxiety-children-adolescents
2. US Census Bureau. 2020 National Survey of Children’s Health: Topical Frequencies. Published June 2, 2021. Accessed May 23, 2022. www2.census.gov/programs-surveys/nsch/technical-documentation/codebook/NSCH_2020_Topical_Frequencies.pdf
3. Murata S, Rezeppa T, Thoma B, et al. The psychiatric sequelae of the COVID-19 pandemic in adolescents, adults, and health care workers. Depress Anxiety. 2021;38:233-246. doi: 10.1002/da.23120
4. Racine N, McArthur BA, Cooke JE, et al. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA Pediatr. 2021;175:1142-1150. doi: 10.1001/jamapediatrics.2021.2482
5. Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr. 2019;206:256-267.e3. doi: 10.1016/j.jpeds.2018.09.021
6. Birmaher B, Brent DA, Chiappetta L, et al. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38:1230-1236. doi: 10.1097/00004583-199910000-00011
7. Johnson JG, Harris ES, Spitzer RL, et al. The Patient Health Questionnaire for Adolescents: validation of an instrument for the assessment of mental disorders among adolescent primary care patients. J Adolesc Health. 2002;30:196-204. doi: 10.1016/s1054-139x(01)00333-0
8. Antony MM, Coons MJ, McCabe RE, et al. Psychometric properties of the Social Phobia Inventory: further evaluation. Behav Res Ther. 2006;44:1177-1185. doi: 10.1016/j.brat.2005.08.013
9. USPSTF. Published recommendations: mental health conditions. Accessed May 23, 2022. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&searchterm=mental+health+conditions
On April 12, 2022, the US Preventive Services Task Force (USPSTF) published a draft recommendation on screening for anxiety in children and adolescents. The recommendation states that clinicians should screen for anxiety in those ages 8 to 18 years. This is a “B” recommendation, which means there is moderate certainty that screening for anxiety in these individuals has a moderate net benefit. The USPSTF felt that the evidence was insufficient to recommend for or against screening at ages 7 years and younger.1
Anxiety is common among young people in America. A survey conducted in 2018-2019 found that 7.8% of children and adolescents (ages 3 to 17 years) had a current anxiety disorder.2 The isolation created by the COVID-19 pandemic has been associated with increased rates of clinically significant psychiatric symptoms; one study suggested that in the first year of the pandemic, 20% of young people experienced elevated anxiety symptoms.3,4 Anxiety disorders in childhood and adolescence also are associated with an increased likelihood of a future anxiety disorder, or depression, in adulthood.
Therapy may improve outcomes. There is evidence that treatment of anxiety disorders can result in improved clinical outcomes. Treatment options include psychotherapy, pharmacotherapy, or a combination of both.5
However, studies showing benefit were conducted in young people whose anxiety was identified via signs or symptoms. The USPSTF could find no direct evidence that identifying anxiety in asymptomatic youth leads to better outcomes. The current draft recommendation is based on indirect evidence on the accuracy of the screening tools and the results of therapy in those who are symptomatic.
Speaking of screening tools ... There were 3 listed in the USPSTF evidence review: the Screen for Child Anxiety Related Disorders (SCARED), which assesses for generalized anxiety disorder (GAD) and any anxiety disorder6; the Patient Health Questionnaire-Adolescent, which screens for GAD and panic disorder7; and the Social Phobia Inventory.8 The SCARED and Social Phobia Inventory are the most widely used clinically.
The accuracy of the screening tests differed. For detection of GAD, sensitivity ranged from 50% to 88% and specificity from 63% to 98%; for social anxiety disorder, sensitivity ranged from 67% to 93% and specificity from 69% to 94%. False-positive results ranged from 17 to 361 per 1000 for GAD and from 104 to 254 per 1000 for social anxiety disorder.1
The USPSTF emphasized that anxiety should not be diagnosed based on a screening test alone. A positive screen should prompt further assessment and confirmation.
An unexpected rating. Given the opportunity costs to administer a screening tool, the high false-positive rates, and the lack of evidence that screening results in improved outcomes among asymptomatic youth, it is curious that this topic did not result in an “I” recommendation. Many screening interventions for children and adolescents with similar evidence profiles—including screening for suicide risk, drug abuse, eating disorders, and alcohol abuse—have previously received an “I.”9
Keep in mind that this is currently a draft recommendation that is open for public comment. The final recommendation will be published in 4 to 12 months.
On April 12, 2022, the US Preventive Services Task Force (USPSTF) published a draft recommendation on screening for anxiety in children and adolescents. The recommendation states that clinicians should screen for anxiety in those ages 8 to 18 years. This is a “B” recommendation, which means there is moderate certainty that screening for anxiety in these individuals has a moderate net benefit. The USPSTF felt that the evidence was insufficient to recommend for or against screening at ages 7 years and younger.1
Anxiety is common among young people in America. A survey conducted in 2018-2019 found that 7.8% of children and adolescents (ages 3 to 17 years) had a current anxiety disorder.2 The isolation created by the COVID-19 pandemic has been associated with increased rates of clinically significant psychiatric symptoms; one study suggested that in the first year of the pandemic, 20% of young people experienced elevated anxiety symptoms.3,4 Anxiety disorders in childhood and adolescence also are associated with an increased likelihood of a future anxiety disorder, or depression, in adulthood.
Therapy may improve outcomes. There is evidence that treatment of anxiety disorders can result in improved clinical outcomes. Treatment options include psychotherapy, pharmacotherapy, or a combination of both.5
However, studies showing benefit were conducted in young people whose anxiety was identified via signs or symptoms. The USPSTF could find no direct evidence that identifying anxiety in asymptomatic youth leads to better outcomes. The current draft recommendation is based on indirect evidence on the accuracy of the screening tools and the results of therapy in those who are symptomatic.
Speaking of screening tools ... There were 3 listed in the USPSTF evidence review: the Screen for Child Anxiety Related Disorders (SCARED), which assesses for generalized anxiety disorder (GAD) and any anxiety disorder6; the Patient Health Questionnaire-Adolescent, which screens for GAD and panic disorder7; and the Social Phobia Inventory.8 The SCARED and Social Phobia Inventory are the most widely used clinically.
The accuracy of the screening tests differed. For detection of GAD, sensitivity ranged from 50% to 88% and specificity from 63% to 98%; for social anxiety disorder, sensitivity ranged from 67% to 93% and specificity from 69% to 94%. False-positive results ranged from 17 to 361 per 1000 for GAD and from 104 to 254 per 1000 for social anxiety disorder.1
The USPSTF emphasized that anxiety should not be diagnosed based on a screening test alone. A positive screen should prompt further assessment and confirmation.
An unexpected rating. Given the opportunity costs to administer a screening tool, the high false-positive rates, and the lack of evidence that screening results in improved outcomes among asymptomatic youth, it is curious that this topic did not result in an “I” recommendation. Many screening interventions for children and adolescents with similar evidence profiles—including screening for suicide risk, drug abuse, eating disorders, and alcohol abuse—have previously received an “I.”9
Keep in mind that this is currently a draft recommendation that is open for public comment. The final recommendation will be published in 4 to 12 months.
1. USPSTF. Screening for anxiety in children and adolescents. Draft recommendation statement. Published April 12, 2022. Accessed May 23, 2022. www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/screening-anxiety-children-adolescents
2. US Census Bureau. 2020 National Survey of Children’s Health: Topical Frequencies. Published June 2, 2021. Accessed May 23, 2022. www2.census.gov/programs-surveys/nsch/technical-documentation/codebook/NSCH_2020_Topical_Frequencies.pdf
3. Murata S, Rezeppa T, Thoma B, et al. The psychiatric sequelae of the COVID-19 pandemic in adolescents, adults, and health care workers. Depress Anxiety. 2021;38:233-246. doi: 10.1002/da.23120
4. Racine N, McArthur BA, Cooke JE, et al. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA Pediatr. 2021;175:1142-1150. doi: 10.1001/jamapediatrics.2021.2482
5. Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr. 2019;206:256-267.e3. doi: 10.1016/j.jpeds.2018.09.021
6. Birmaher B, Brent DA, Chiappetta L, et al. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38:1230-1236. doi: 10.1097/00004583-199910000-00011
7. Johnson JG, Harris ES, Spitzer RL, et al. The Patient Health Questionnaire for Adolescents: validation of an instrument for the assessment of mental disorders among adolescent primary care patients. J Adolesc Health. 2002;30:196-204. doi: 10.1016/s1054-139x(01)00333-0
8. Antony MM, Coons MJ, McCabe RE, et al. Psychometric properties of the Social Phobia Inventory: further evaluation. Behav Res Ther. 2006;44:1177-1185. doi: 10.1016/j.brat.2005.08.013
9. USPSTF. Published recommendations: mental health conditions. Accessed May 23, 2022. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&searchterm=mental+health+conditions
1. USPSTF. Screening for anxiety in children and adolescents. Draft recommendation statement. Published April 12, 2022. Accessed May 23, 2022. www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/screening-anxiety-children-adolescents
2. US Census Bureau. 2020 National Survey of Children’s Health: Topical Frequencies. Published June 2, 2021. Accessed May 23, 2022. www2.census.gov/programs-surveys/nsch/technical-documentation/codebook/NSCH_2020_Topical_Frequencies.pdf
3. Murata S, Rezeppa T, Thoma B, et al. The psychiatric sequelae of the COVID-19 pandemic in adolescents, adults, and health care workers. Depress Anxiety. 2021;38:233-246. doi: 10.1002/da.23120
4. Racine N, McArthur BA, Cooke JE, et al. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA Pediatr. 2021;175:1142-1150. doi: 10.1001/jamapediatrics.2021.2482
5. Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr. 2019;206:256-267.e3. doi: 10.1016/j.jpeds.2018.09.021
6. Birmaher B, Brent DA, Chiappetta L, et al. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38:1230-1236. doi: 10.1097/00004583-199910000-00011
7. Johnson JG, Harris ES, Spitzer RL, et al. The Patient Health Questionnaire for Adolescents: validation of an instrument for the assessment of mental disorders among adolescent primary care patients. J Adolesc Health. 2002;30:196-204. doi: 10.1016/s1054-139x(01)00333-0
8. Antony MM, Coons MJ, McCabe RE, et al. Psychometric properties of the Social Phobia Inventory: further evaluation. Behav Res Ther. 2006;44:1177-1185. doi: 10.1016/j.brat.2005.08.013
9. USPSTF. Published recommendations: mental health conditions. Accessed May 23, 2022. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&searchterm=mental+health+conditions
From the editor: Celebrating 15 years of excellence
The inaugural issue of GI & Hepatology News was published in January 2007, and the newspaper has gone on to become part of the fabric of the AGA. This year, we celebrate the newspaper’s 15th year with a special 15th Anniversary Series that will run from June through December 2022. We will feature reflections from GIHN’s three former editors-in-chief, Dr. Charles J. Lightdale, Dr. Colin Howden, and Dr. John Allen, on the evolution of the newspaper (and the field of GI) over the past 15 years. We also will present a series of Then and Now columns, highlighting high-impact areas of GI and hepatology covered in past GIHN issues, and reflecting on how the field has changed since that time.
In this month’s issue, we are pleased to kick off the 15th Anniversary Series with reflections by Dr. Lightdale, GIHN’s inaugural editor-in-chief, as well as a Then and Now column written by Dr. Kimberly M. Persley (GIHN associate editor and longstanding AGA member) reflecting on how the demographics of gastroenterology and of the AGA as an organization have changed over the past 15 years. I hope you will find these special contributions to be engaging and thought-provoking. Other issue highlights include a lead article describing impacts of social determinants of health in driving disparities in IBD care and offering recommendations for achieving IBD health equity, a new AGA Clinical Practice Update on dietary options for our many patients with irritable bowel syndrome, and new data on the safety of anti-TNF medications prior to surgery in patients with inflammatory bowel disease.
As summer vacation season commences, I hope you will join me in taking some well-deserved time away from work demands, spending some quality time with friends and family, and seizing the opportunity to rest and recharge.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
The inaugural issue of GI & Hepatology News was published in January 2007, and the newspaper has gone on to become part of the fabric of the AGA. This year, we celebrate the newspaper’s 15th year with a special 15th Anniversary Series that will run from June through December 2022. We will feature reflections from GIHN’s three former editors-in-chief, Dr. Charles J. Lightdale, Dr. Colin Howden, and Dr. John Allen, on the evolution of the newspaper (and the field of GI) over the past 15 years. We also will present a series of Then and Now columns, highlighting high-impact areas of GI and hepatology covered in past GIHN issues, and reflecting on how the field has changed since that time.
In this month’s issue, we are pleased to kick off the 15th Anniversary Series with reflections by Dr. Lightdale, GIHN’s inaugural editor-in-chief, as well as a Then and Now column written by Dr. Kimberly M. Persley (GIHN associate editor and longstanding AGA member) reflecting on how the demographics of gastroenterology and of the AGA as an organization have changed over the past 15 years. I hope you will find these special contributions to be engaging and thought-provoking. Other issue highlights include a lead article describing impacts of social determinants of health in driving disparities in IBD care and offering recommendations for achieving IBD health equity, a new AGA Clinical Practice Update on dietary options for our many patients with irritable bowel syndrome, and new data on the safety of anti-TNF medications prior to surgery in patients with inflammatory bowel disease.
As summer vacation season commences, I hope you will join me in taking some well-deserved time away from work demands, spending some quality time with friends and family, and seizing the opportunity to rest and recharge.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
The inaugural issue of GI & Hepatology News was published in January 2007, and the newspaper has gone on to become part of the fabric of the AGA. This year, we celebrate the newspaper’s 15th year with a special 15th Anniversary Series that will run from June through December 2022. We will feature reflections from GIHN’s three former editors-in-chief, Dr. Charles J. Lightdale, Dr. Colin Howden, and Dr. John Allen, on the evolution of the newspaper (and the field of GI) over the past 15 years. We also will present a series of Then and Now columns, highlighting high-impact areas of GI and hepatology covered in past GIHN issues, and reflecting on how the field has changed since that time.
In this month’s issue, we are pleased to kick off the 15th Anniversary Series with reflections by Dr. Lightdale, GIHN’s inaugural editor-in-chief, as well as a Then and Now column written by Dr. Kimberly M. Persley (GIHN associate editor and longstanding AGA member) reflecting on how the demographics of gastroenterology and of the AGA as an organization have changed over the past 15 years. I hope you will find these special contributions to be engaging and thought-provoking. Other issue highlights include a lead article describing impacts of social determinants of health in driving disparities in IBD care and offering recommendations for achieving IBD health equity, a new AGA Clinical Practice Update on dietary options for our many patients with irritable bowel syndrome, and new data on the safety of anti-TNF medications prior to surgery in patients with inflammatory bowel disease.
As summer vacation season commences, I hope you will join me in taking some well-deserved time away from work demands, spending some quality time with friends and family, and seizing the opportunity to rest and recharge.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Using Bronchoscopy to Optimize Targeted Therapy in Non-Small Cell Lung Cancer
The expanding range of therapies and interventions available for treatment of non–small cell lung cancer (NSCLC) has brought medical oncologists and interventional pulmonologists into closer partnership.
Oncologist Dr. Joy Feliciano and interventional pulmonologist Dr. A. Christine Argento, both colleagues at Johns Hopkins University, comment on their evolving relationship as a team.
Pulmonologists now often serve as the "gatekeepers into thoracic oncology," says Dr. Argento. Recent advances in technology, including endobronchial ultrasound, navigational bronchoscopy, and robotic-assisted bronchoscopy, allow for diagnosis, staging, and collection of sufficient tissue for advanced studies into molecular markers and genetic studies necessary to guide treatment decisions for metastatic NSCLC.
In resectable disease, patients who might have gone straight to surgery in the past now can be considered for neoadjuvant treatments. Here again, says Dr. Feliciano, it is critical for oncologists to understand the biomarkers and molecular profile of the tumor before considering an intervention, and the role of bronchoscopy is key.
A multidisciplinary practice of NSCLC specialists, who have access to on-site or virtual tumor boards, sometimes on an international scale, helps ensure optimal treatment for patients in the increasingly complex NSCLC arena.
--
A. Christine Argento, MD, Associate Professor of Medicine, Department of Pulmonary and Critical Care Medicine, Johns Hopkins University; Director of Bronchoscopy, Department of Interventional Pulmonary Medicine, Johns Hopkins Hospital, Baltimore, Maryland
A. Christine Argento, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biodesix; Olympus; Cook; Intuitive; Boston Scientific
Josephine L. Feliciano, MD, Associate Professor, Clinical Director, Sidney Kimmel Cancer Center, Johns Hopkins University Hospital, Baltimore, Maryland
Josephine L. Feliciano, MD, has disclosed the following relevant financial relationships:
Received research grant from: Bristol-Myers; Pfizer; AstraZeneca.
Received income in an amount equal to or greater than $250 from: Genentech; AstraZeneca; Eli Lilly; Merck; Regeneron; Coherus; Takeda; Bristol-Myers
The expanding range of therapies and interventions available for treatment of non–small cell lung cancer (NSCLC) has brought medical oncologists and interventional pulmonologists into closer partnership.
Oncologist Dr. Joy Feliciano and interventional pulmonologist Dr. A. Christine Argento, both colleagues at Johns Hopkins University, comment on their evolving relationship as a team.
Pulmonologists now often serve as the "gatekeepers into thoracic oncology," says Dr. Argento. Recent advances in technology, including endobronchial ultrasound, navigational bronchoscopy, and robotic-assisted bronchoscopy, allow for diagnosis, staging, and collection of sufficient tissue for advanced studies into molecular markers and genetic studies necessary to guide treatment decisions for metastatic NSCLC.
In resectable disease, patients who might have gone straight to surgery in the past now can be considered for neoadjuvant treatments. Here again, says Dr. Feliciano, it is critical for oncologists to understand the biomarkers and molecular profile of the tumor before considering an intervention, and the role of bronchoscopy is key.
A multidisciplinary practice of NSCLC specialists, who have access to on-site or virtual tumor boards, sometimes on an international scale, helps ensure optimal treatment for patients in the increasingly complex NSCLC arena.
--
A. Christine Argento, MD, Associate Professor of Medicine, Department of Pulmonary and Critical Care Medicine, Johns Hopkins University; Director of Bronchoscopy, Department of Interventional Pulmonary Medicine, Johns Hopkins Hospital, Baltimore, Maryland
A. Christine Argento, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biodesix; Olympus; Cook; Intuitive; Boston Scientific
Josephine L. Feliciano, MD, Associate Professor, Clinical Director, Sidney Kimmel Cancer Center, Johns Hopkins University Hospital, Baltimore, Maryland
Josephine L. Feliciano, MD, has disclosed the following relevant financial relationships:
Received research grant from: Bristol-Myers; Pfizer; AstraZeneca.
Received income in an amount equal to or greater than $250 from: Genentech; AstraZeneca; Eli Lilly; Merck; Regeneron; Coherus; Takeda; Bristol-Myers
The expanding range of therapies and interventions available for treatment of non–small cell lung cancer (NSCLC) has brought medical oncologists and interventional pulmonologists into closer partnership.
Oncologist Dr. Joy Feliciano and interventional pulmonologist Dr. A. Christine Argento, both colleagues at Johns Hopkins University, comment on their evolving relationship as a team.
Pulmonologists now often serve as the "gatekeepers into thoracic oncology," says Dr. Argento. Recent advances in technology, including endobronchial ultrasound, navigational bronchoscopy, and robotic-assisted bronchoscopy, allow for diagnosis, staging, and collection of sufficient tissue for advanced studies into molecular markers and genetic studies necessary to guide treatment decisions for metastatic NSCLC.
In resectable disease, patients who might have gone straight to surgery in the past now can be considered for neoadjuvant treatments. Here again, says Dr. Feliciano, it is critical for oncologists to understand the biomarkers and molecular profile of the tumor before considering an intervention, and the role of bronchoscopy is key.
A multidisciplinary practice of NSCLC specialists, who have access to on-site or virtual tumor boards, sometimes on an international scale, helps ensure optimal treatment for patients in the increasingly complex NSCLC arena.
--
A. Christine Argento, MD, Associate Professor of Medicine, Department of Pulmonary and Critical Care Medicine, Johns Hopkins University; Director of Bronchoscopy, Department of Interventional Pulmonary Medicine, Johns Hopkins Hospital, Baltimore, Maryland
A. Christine Argento, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biodesix; Olympus; Cook; Intuitive; Boston Scientific
Josephine L. Feliciano, MD, Associate Professor, Clinical Director, Sidney Kimmel Cancer Center, Johns Hopkins University Hospital, Baltimore, Maryland
Josephine L. Feliciano, MD, has disclosed the following relevant financial relationships:
Received research grant from: Bristol-Myers; Pfizer; AstraZeneca.
Received income in an amount equal to or greater than $250 from: Genentech; AstraZeneca; Eli Lilly; Merck; Regeneron; Coherus; Takeda; Bristol-Myers

Can lung cancer ID be as easy as breathing into an analyzer?
A study published in May in The Lancet journal eClinicalMedicine reports that
The tool was successfully used to identify, in 84 patients, 16 lung cancer–related carcinogenic volatile compounds (VOCs), such as aldehydes, hydrocarbons, ketones, carboxylic acids, and furan – some of which are compounds used in the production of common household goods, such as furniture, carpeting, and wood floors.
“The test is anticipated to be highlighted for primary screening of lung cancer but not the final diagnosis,” according to study authors who were led by Peiyu Wang, MD, PhD, chair of social medicine and health at Peking (China) University.
While early diagnosis and treatment are critical for improving lung cancer survival, early detection of lung cancer is challenging because of the lack of clinical manifestations and specific biomarkers. Annual CT scans are costly and include radiation exposure, Dr. Wang and his associates wrote.
Breathomics testing is considered a promising method for detection and screening for lung cancer. It has been under study for years and in 2014, researchers from Belgium published a review in Cancer Epidemiology Biomarkers and Prevention documenting the use of VOCs as early diagnostic or prognostic biomarkers for mesothelioma.
Lung cancer breath biomarkers identified in various studies have been highly heterogeneous because of differing sample collection methods, varying patient conditions, testing environments, and analysis methods. As a result, there currently is no breathomics test for lung cancer screening, Dr. Wang said in an interview.
In terms of its potential as a lung cancer screening tool, “Clinicians may introduce this test for people with high risk for lung cancer, such as elderly smokers, or people with suspected symptoms. It may also be introduced for young populations with subjective or objective needs to screen for lung cancer. As the proportion of lung adenocarcinoma in nonsmoking young women is increasing, the test may be a good method for lung cancer screening in this population,” Dr. Wang said.
After adjusting for age, sex, smoking, and comorbidities, researchers found elevated levels for 16 VOCs in patients with lung cancer. A diagnostic model including the 16 VOCs achieved an area under the curve of 0.952, sensitivity of 89.2%, specificity of 89.1%, and accuracy of 89.1% in lung cancer diagnosis. A model including the top eight VOCs achieved an area under the curve of 0.931, sensitivity of 86.0%, specificity of 87.2%, and accuracy of 86.9%.
After selecting 28 VOCs as candidates through a literature review, Dr. Wang and associates conducted a prospective discovery study from Sept. 1 to Dec. 31, 2020, using high-pressure photon ionization time-of-flight mass spectrometry to evaluate their performance for lung cancer diagnosis. The validation study included 157 lung cancer patients (mean age 57.0 years; 54.1 percent female) and 368 volunteers (mean age 44.5 years; 31.3% female).
“The external validation confirmed good performance of these biomarkers in lung cancer detection,” the researchers stated. It helped, they added, to solve the heterogeneity among published studies, establishing both 16 VOCs and 8 VOCS for lung cancer screening.
The authors stated that a large gap exists between breathomics research and clinical practices in lung cancer detection and screening. While the validated 16 VOCs, mainly aldehydes and hydrocarbon, showed potential for promoting this lung cancer screening strategy, more scientific studies are warranted to investigate the underlying mechanisms of identified lung cancer VOCs.
Dr. Wang declared no competing interests.
A study published in May in The Lancet journal eClinicalMedicine reports that
The tool was successfully used to identify, in 84 patients, 16 lung cancer–related carcinogenic volatile compounds (VOCs), such as aldehydes, hydrocarbons, ketones, carboxylic acids, and furan – some of which are compounds used in the production of common household goods, such as furniture, carpeting, and wood floors.
“The test is anticipated to be highlighted for primary screening of lung cancer but not the final diagnosis,” according to study authors who were led by Peiyu Wang, MD, PhD, chair of social medicine and health at Peking (China) University.
While early diagnosis and treatment are critical for improving lung cancer survival, early detection of lung cancer is challenging because of the lack of clinical manifestations and specific biomarkers. Annual CT scans are costly and include radiation exposure, Dr. Wang and his associates wrote.
Breathomics testing is considered a promising method for detection and screening for lung cancer. It has been under study for years and in 2014, researchers from Belgium published a review in Cancer Epidemiology Biomarkers and Prevention documenting the use of VOCs as early diagnostic or prognostic biomarkers for mesothelioma.
Lung cancer breath biomarkers identified in various studies have been highly heterogeneous because of differing sample collection methods, varying patient conditions, testing environments, and analysis methods. As a result, there currently is no breathomics test for lung cancer screening, Dr. Wang said in an interview.
In terms of its potential as a lung cancer screening tool, “Clinicians may introduce this test for people with high risk for lung cancer, such as elderly smokers, or people with suspected symptoms. It may also be introduced for young populations with subjective or objective needs to screen for lung cancer. As the proportion of lung adenocarcinoma in nonsmoking young women is increasing, the test may be a good method for lung cancer screening in this population,” Dr. Wang said.
After adjusting for age, sex, smoking, and comorbidities, researchers found elevated levels for 16 VOCs in patients with lung cancer. A diagnostic model including the 16 VOCs achieved an area under the curve of 0.952, sensitivity of 89.2%, specificity of 89.1%, and accuracy of 89.1% in lung cancer diagnosis. A model including the top eight VOCs achieved an area under the curve of 0.931, sensitivity of 86.0%, specificity of 87.2%, and accuracy of 86.9%.
After selecting 28 VOCs as candidates through a literature review, Dr. Wang and associates conducted a prospective discovery study from Sept. 1 to Dec. 31, 2020, using high-pressure photon ionization time-of-flight mass spectrometry to evaluate their performance for lung cancer diagnosis. The validation study included 157 lung cancer patients (mean age 57.0 years; 54.1 percent female) and 368 volunteers (mean age 44.5 years; 31.3% female).
“The external validation confirmed good performance of these biomarkers in lung cancer detection,” the researchers stated. It helped, they added, to solve the heterogeneity among published studies, establishing both 16 VOCs and 8 VOCS for lung cancer screening.
The authors stated that a large gap exists between breathomics research and clinical practices in lung cancer detection and screening. While the validated 16 VOCs, mainly aldehydes and hydrocarbon, showed potential for promoting this lung cancer screening strategy, more scientific studies are warranted to investigate the underlying mechanisms of identified lung cancer VOCs.
Dr. Wang declared no competing interests.
A study published in May in The Lancet journal eClinicalMedicine reports that
The tool was successfully used to identify, in 84 patients, 16 lung cancer–related carcinogenic volatile compounds (VOCs), such as aldehydes, hydrocarbons, ketones, carboxylic acids, and furan – some of which are compounds used in the production of common household goods, such as furniture, carpeting, and wood floors.
“The test is anticipated to be highlighted for primary screening of lung cancer but not the final diagnosis,” according to study authors who were led by Peiyu Wang, MD, PhD, chair of social medicine and health at Peking (China) University.
While early diagnosis and treatment are critical for improving lung cancer survival, early detection of lung cancer is challenging because of the lack of clinical manifestations and specific biomarkers. Annual CT scans are costly and include radiation exposure, Dr. Wang and his associates wrote.
Breathomics testing is considered a promising method for detection and screening for lung cancer. It has been under study for years and in 2014, researchers from Belgium published a review in Cancer Epidemiology Biomarkers and Prevention documenting the use of VOCs as early diagnostic or prognostic biomarkers for mesothelioma.
Lung cancer breath biomarkers identified in various studies have been highly heterogeneous because of differing sample collection methods, varying patient conditions, testing environments, and analysis methods. As a result, there currently is no breathomics test for lung cancer screening, Dr. Wang said in an interview.
In terms of its potential as a lung cancer screening tool, “Clinicians may introduce this test for people with high risk for lung cancer, such as elderly smokers, or people with suspected symptoms. It may also be introduced for young populations with subjective or objective needs to screen for lung cancer. As the proportion of lung adenocarcinoma in nonsmoking young women is increasing, the test may be a good method for lung cancer screening in this population,” Dr. Wang said.
After adjusting for age, sex, smoking, and comorbidities, researchers found elevated levels for 16 VOCs in patients with lung cancer. A diagnostic model including the 16 VOCs achieved an area under the curve of 0.952, sensitivity of 89.2%, specificity of 89.1%, and accuracy of 89.1% in lung cancer diagnosis. A model including the top eight VOCs achieved an area under the curve of 0.931, sensitivity of 86.0%, specificity of 87.2%, and accuracy of 86.9%.
After selecting 28 VOCs as candidates through a literature review, Dr. Wang and associates conducted a prospective discovery study from Sept. 1 to Dec. 31, 2020, using high-pressure photon ionization time-of-flight mass spectrometry to evaluate their performance for lung cancer diagnosis. The validation study included 157 lung cancer patients (mean age 57.0 years; 54.1 percent female) and 368 volunteers (mean age 44.5 years; 31.3% female).
“The external validation confirmed good performance of these biomarkers in lung cancer detection,” the researchers stated. It helped, they added, to solve the heterogeneity among published studies, establishing both 16 VOCs and 8 VOCS for lung cancer screening.
The authors stated that a large gap exists between breathomics research and clinical practices in lung cancer detection and screening. While the validated 16 VOCs, mainly aldehydes and hydrocarbon, showed potential for promoting this lung cancer screening strategy, more scientific studies are warranted to investigate the underlying mechanisms of identified lung cancer VOCs.
Dr. Wang declared no competing interests.
FROM ECLINICAL MEDICINE
New test might transform male infertility
A new study suggests that, at least for certain male patients, the answer to infertility might lie with epigenetics.
According to the study, a commercially available test of epigenetic anomalies – factors that affect how genes express themselves – can grade the likelihood that sperm are viable for conception.
“The uniqueness of epigenetics is that some of the abnormalities detected have the potential to be modified with lifestyle,” said Larry I. Lipshultz, MD, head of the Division of Male Reproductive Medicine and Surgery at Baylor College of Medicine, Houston, who presented the new findings at the 2022 annual meeting of the American Urological Association.
For decades, semen analysis has been based on motility, morphology, and concentration. But these measures, while useful, are limited. Semen can still have low capacity for producing a pregnancy even when all three parameters are normal, Dr. Lipshultz told this news organization.
The test, called Path SpermQT (Inherent Biosciences) detects unstable gene promotors, which are the epigenetic markers for gene expression. In previous work with more than 1,300 gene samples, expression of the specific genes regulated by these promoters were linked to a wide variety of functions relevant to fertilization, such as spermatogenesis.
The test does not attempt to look for expression of specific unstable promoters but rather quantifies them to characterize sperm quality as excellent (≤ 10 unstable promoters), average (11-42), or poor (≥ 43).
In the studies that led to development of the SpermQT test, the number of unstable promoters correlated with pregnancy success. Pregnancy was achieved even among those in the group with poor sperm quality – but at very low rates.
Of the 172 semen samples collected so far in the ongoing analysis, sperm quality was characterized as excellent in 31%, average in 59%, and poor in 10%.
The stratifications for sperm quality were not significantly correlated with common measures of sperm viability, such as concentration, Dr. Lipshultz reported.
Certain patient characteristics were associated with greater sperm quality. These included use of antioxidant supplementation and low estrogen levels, as seen in men who had taken aromatase inhibitors.
So far, only one natural conception has occurred in the group with poor sperm versus eight in those with average or excellent quality.
The prognostic role of the test is only part of the picture.
“The exciting thing about this area of research is that epigenetics can be changed,” Dr. Lipshultz said in an interview. Based on the data so far, he said he is already starting to consider antioxidant supplementation and hormone modifications when sperm quality is poor.
The value of the Path SpermQT test for identifying treatment targets might eventually revolutionize the management of male infertility, Dr. Lipshultz said, but it has more immediate potential in helping couples decide whether to proceed with in vitro fertilization (IVF).
“We often see patients at an impasse when they are trying to decide to move to IVF,” he said. “A test like this could provide some direction. If sperm quality is good, the advice might be to keep trying. If poor, then a couple might want to move to IVF more quickly.”
A test of sperm quality on the basis of epigenetics “could change how we look at couples attempting to conceive,” agreed Peter N. Schlegel, MD, professor of urology and reproductive medicine, Weill Cornell Medicine, New York.
Dr. Schlegel praised several characteristics of the epigenetics test, including that 70%-80% of men with poor quality with SpermQT have normal results on standard assessments of semen. This finding suggests the tool is providing unique information about patients. He also noted that the studies so far indicate that sperm of poor quality for natural conception is still viable for IVF fertilization – which could be useful for couples weighing their options.
However, while the test is already available, Dr. Schlegel cautioned that much of the promise has yet to be documented.
“The results to date, despite being statistically significant, have only been gleaned from a small group of patients,” he said. “Much larger studies are needed before a change in practice is warranted.”
The value of SpermQT for identifying modifiable risks might be even further away.
“It is well recognized that environmental and lifestyle changes can affect methylation, but it is not known if the abnormalities seen so far could be influenced by lifestyle changes,” Dr. Schlegel said. Among the numerous steps needed to answer this question, he suggested that it might first be important “to evaluate why such changes in methylation occur.”
Dr. Lipshultz has financial relationships with several pharmaceutical companies, including Inherent Biosciences, which is marketing the SpermQT test. Dr. Schlegel has financial relationships with Theralogix, Posterity Health, and Roman Health.
A version of this article first appeared on Medscape.com.
A new study suggests that, at least for certain male patients, the answer to infertility might lie with epigenetics.
According to the study, a commercially available test of epigenetic anomalies – factors that affect how genes express themselves – can grade the likelihood that sperm are viable for conception.
“The uniqueness of epigenetics is that some of the abnormalities detected have the potential to be modified with lifestyle,” said Larry I. Lipshultz, MD, head of the Division of Male Reproductive Medicine and Surgery at Baylor College of Medicine, Houston, who presented the new findings at the 2022 annual meeting of the American Urological Association.
For decades, semen analysis has been based on motility, morphology, and concentration. But these measures, while useful, are limited. Semen can still have low capacity for producing a pregnancy even when all three parameters are normal, Dr. Lipshultz told this news organization.
The test, called Path SpermQT (Inherent Biosciences) detects unstable gene promotors, which are the epigenetic markers for gene expression. In previous work with more than 1,300 gene samples, expression of the specific genes regulated by these promoters were linked to a wide variety of functions relevant to fertilization, such as spermatogenesis.
The test does not attempt to look for expression of specific unstable promoters but rather quantifies them to characterize sperm quality as excellent (≤ 10 unstable promoters), average (11-42), or poor (≥ 43).
In the studies that led to development of the SpermQT test, the number of unstable promoters correlated with pregnancy success. Pregnancy was achieved even among those in the group with poor sperm quality – but at very low rates.
Of the 172 semen samples collected so far in the ongoing analysis, sperm quality was characterized as excellent in 31%, average in 59%, and poor in 10%.
The stratifications for sperm quality were not significantly correlated with common measures of sperm viability, such as concentration, Dr. Lipshultz reported.
Certain patient characteristics were associated with greater sperm quality. These included use of antioxidant supplementation and low estrogen levels, as seen in men who had taken aromatase inhibitors.
So far, only one natural conception has occurred in the group with poor sperm versus eight in those with average or excellent quality.
The prognostic role of the test is only part of the picture.
“The exciting thing about this area of research is that epigenetics can be changed,” Dr. Lipshultz said in an interview. Based on the data so far, he said he is already starting to consider antioxidant supplementation and hormone modifications when sperm quality is poor.
The value of the Path SpermQT test for identifying treatment targets might eventually revolutionize the management of male infertility, Dr. Lipshultz said, but it has more immediate potential in helping couples decide whether to proceed with in vitro fertilization (IVF).
“We often see patients at an impasse when they are trying to decide to move to IVF,” he said. “A test like this could provide some direction. If sperm quality is good, the advice might be to keep trying. If poor, then a couple might want to move to IVF more quickly.”
A test of sperm quality on the basis of epigenetics “could change how we look at couples attempting to conceive,” agreed Peter N. Schlegel, MD, professor of urology and reproductive medicine, Weill Cornell Medicine, New York.
Dr. Schlegel praised several characteristics of the epigenetics test, including that 70%-80% of men with poor quality with SpermQT have normal results on standard assessments of semen. This finding suggests the tool is providing unique information about patients. He also noted that the studies so far indicate that sperm of poor quality for natural conception is still viable for IVF fertilization – which could be useful for couples weighing their options.
However, while the test is already available, Dr. Schlegel cautioned that much of the promise has yet to be documented.
“The results to date, despite being statistically significant, have only been gleaned from a small group of patients,” he said. “Much larger studies are needed before a change in practice is warranted.”
The value of SpermQT for identifying modifiable risks might be even further away.
“It is well recognized that environmental and lifestyle changes can affect methylation, but it is not known if the abnormalities seen so far could be influenced by lifestyle changes,” Dr. Schlegel said. Among the numerous steps needed to answer this question, he suggested that it might first be important “to evaluate why such changes in methylation occur.”
Dr. Lipshultz has financial relationships with several pharmaceutical companies, including Inherent Biosciences, which is marketing the SpermQT test. Dr. Schlegel has financial relationships with Theralogix, Posterity Health, and Roman Health.
A version of this article first appeared on Medscape.com.
A new study suggests that, at least for certain male patients, the answer to infertility might lie with epigenetics.
According to the study, a commercially available test of epigenetic anomalies – factors that affect how genes express themselves – can grade the likelihood that sperm are viable for conception.
“The uniqueness of epigenetics is that some of the abnormalities detected have the potential to be modified with lifestyle,” said Larry I. Lipshultz, MD, head of the Division of Male Reproductive Medicine and Surgery at Baylor College of Medicine, Houston, who presented the new findings at the 2022 annual meeting of the American Urological Association.
For decades, semen analysis has been based on motility, morphology, and concentration. But these measures, while useful, are limited. Semen can still have low capacity for producing a pregnancy even when all three parameters are normal, Dr. Lipshultz told this news organization.
The test, called Path SpermQT (Inherent Biosciences) detects unstable gene promotors, which are the epigenetic markers for gene expression. In previous work with more than 1,300 gene samples, expression of the specific genes regulated by these promoters were linked to a wide variety of functions relevant to fertilization, such as spermatogenesis.
The test does not attempt to look for expression of specific unstable promoters but rather quantifies them to characterize sperm quality as excellent (≤ 10 unstable promoters), average (11-42), or poor (≥ 43).
In the studies that led to development of the SpermQT test, the number of unstable promoters correlated with pregnancy success. Pregnancy was achieved even among those in the group with poor sperm quality – but at very low rates.
Of the 172 semen samples collected so far in the ongoing analysis, sperm quality was characterized as excellent in 31%, average in 59%, and poor in 10%.
The stratifications for sperm quality were not significantly correlated with common measures of sperm viability, such as concentration, Dr. Lipshultz reported.
Certain patient characteristics were associated with greater sperm quality. These included use of antioxidant supplementation and low estrogen levels, as seen in men who had taken aromatase inhibitors.
So far, only one natural conception has occurred in the group with poor sperm versus eight in those with average or excellent quality.
The prognostic role of the test is only part of the picture.
“The exciting thing about this area of research is that epigenetics can be changed,” Dr. Lipshultz said in an interview. Based on the data so far, he said he is already starting to consider antioxidant supplementation and hormone modifications when sperm quality is poor.
The value of the Path SpermQT test for identifying treatment targets might eventually revolutionize the management of male infertility, Dr. Lipshultz said, but it has more immediate potential in helping couples decide whether to proceed with in vitro fertilization (IVF).
“We often see patients at an impasse when they are trying to decide to move to IVF,” he said. “A test like this could provide some direction. If sperm quality is good, the advice might be to keep trying. If poor, then a couple might want to move to IVF more quickly.”
A test of sperm quality on the basis of epigenetics “could change how we look at couples attempting to conceive,” agreed Peter N. Schlegel, MD, professor of urology and reproductive medicine, Weill Cornell Medicine, New York.
Dr. Schlegel praised several characteristics of the epigenetics test, including that 70%-80% of men with poor quality with SpermQT have normal results on standard assessments of semen. This finding suggests the tool is providing unique information about patients. He also noted that the studies so far indicate that sperm of poor quality for natural conception is still viable for IVF fertilization – which could be useful for couples weighing their options.
However, while the test is already available, Dr. Schlegel cautioned that much of the promise has yet to be documented.
“The results to date, despite being statistically significant, have only been gleaned from a small group of patients,” he said. “Much larger studies are needed before a change in practice is warranted.”
The value of SpermQT for identifying modifiable risks might be even further away.
“It is well recognized that environmental and lifestyle changes can affect methylation, but it is not known if the abnormalities seen so far could be influenced by lifestyle changes,” Dr. Schlegel said. Among the numerous steps needed to answer this question, he suggested that it might first be important “to evaluate why such changes in methylation occur.”
Dr. Lipshultz has financial relationships with several pharmaceutical companies, including Inherent Biosciences, which is marketing the SpermQT test. Dr. Schlegel has financial relationships with Theralogix, Posterity Health, and Roman Health.
A version of this article first appeared on Medscape.com.
FROM 2022 AMERICAN UROLOGICAL ASSOCIATION
How do you treat noncompliance?
Mrs. Stevens has migraines. Fortunately, they’re well controlled on nortriptyline, and she’s never had side effects from it. She’s taken it for more than 20 years now.
In that time she and I have had a strange, slow-motion, waltz.
In spite of the medicine helping her, she stops it on her own roughly twice a year, never calling my office in advance. Sometimes it’s to see if the headaches come back (they always do). Other times it’s because of something she read online, or a friend told her, or she overheard in the grocery checkout line.
Whatever the reason, her migraines always come back within a week, and then she calls my office for an urgent appointment.
I’ve never really understood this, as I know her history and am happy to just tell her to restart the medication and call it in. But, for whatever reason, the return of her migraines is something that she wants to discuss with me in person. Since it’s usually a pretty brief visit, my secretary puts her on the schedule and I get paid to tell her what could have been handled by phone. I’m not complaining. I have to make a living, too.
But still, it makes me wonder. She can’t be the only patient out there who does this. Multiply that by the number of doctors, the cost of visits, the time she takes off from work to come in ... it adds up.
So why does this happen?
Believe me, for the past 20 years I’ve spent these occasional visits reminding Mrs. Stevens about the importance of sticking with her medication and calling my office if she has questions. She agrees to, but when she’s thinking about stopping nortriptyline ... she still does it and only tells me after the fact.
I can’t change human nature, or at least not hers. And when multiplied by many like her, it creates entirely unnecessary costs on our health care system. I wish there were a way to stop it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mrs. Stevens has migraines. Fortunately, they’re well controlled on nortriptyline, and she’s never had side effects from it. She’s taken it for more than 20 years now.
In that time she and I have had a strange, slow-motion, waltz.
In spite of the medicine helping her, she stops it on her own roughly twice a year, never calling my office in advance. Sometimes it’s to see if the headaches come back (they always do). Other times it’s because of something she read online, or a friend told her, or she overheard in the grocery checkout line.
Whatever the reason, her migraines always come back within a week, and then she calls my office for an urgent appointment.
I’ve never really understood this, as I know her history and am happy to just tell her to restart the medication and call it in. But, for whatever reason, the return of her migraines is something that she wants to discuss with me in person. Since it’s usually a pretty brief visit, my secretary puts her on the schedule and I get paid to tell her what could have been handled by phone. I’m not complaining. I have to make a living, too.
But still, it makes me wonder. She can’t be the only patient out there who does this. Multiply that by the number of doctors, the cost of visits, the time she takes off from work to come in ... it adds up.
So why does this happen?
Believe me, for the past 20 years I’ve spent these occasional visits reminding Mrs. Stevens about the importance of sticking with her medication and calling my office if she has questions. She agrees to, but when she’s thinking about stopping nortriptyline ... she still does it and only tells me after the fact.
I can’t change human nature, or at least not hers. And when multiplied by many like her, it creates entirely unnecessary costs on our health care system. I wish there were a way to stop it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mrs. Stevens has migraines. Fortunately, they’re well controlled on nortriptyline, and she’s never had side effects from it. She’s taken it for more than 20 years now.
In that time she and I have had a strange, slow-motion, waltz.
In spite of the medicine helping her, she stops it on her own roughly twice a year, never calling my office in advance. Sometimes it’s to see if the headaches come back (they always do). Other times it’s because of something she read online, or a friend told her, or she overheard in the grocery checkout line.
Whatever the reason, her migraines always come back within a week, and then she calls my office for an urgent appointment.
I’ve never really understood this, as I know her history and am happy to just tell her to restart the medication and call it in. But, for whatever reason, the return of her migraines is something that she wants to discuss with me in person. Since it’s usually a pretty brief visit, my secretary puts her on the schedule and I get paid to tell her what could have been handled by phone. I’m not complaining. I have to make a living, too.
But still, it makes me wonder. She can’t be the only patient out there who does this. Multiply that by the number of doctors, the cost of visits, the time she takes off from work to come in ... it adds up.
So why does this happen?
Believe me, for the past 20 years I’ve spent these occasional visits reminding Mrs. Stevens about the importance of sticking with her medication and calling my office if she has questions. She agrees to, but when she’s thinking about stopping nortriptyline ... she still does it and only tells me after the fact.
I can’t change human nature, or at least not hers. And when multiplied by many like her, it creates entirely unnecessary costs on our health care system. I wish there were a way to stop it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Hearing, vision loss combo a colossal risk for cognitive decline
The combination of hearing loss and vision loss is linked to an eightfold increased risk of cognitive impairment, new research shows.
Investigators analyzed data on more than 5 million U.S. seniors. Adjusted results show that participants with hearing impairment alone had more than twice the odds of also having cognitive impairment, while those with vision impairment alone had more than triple the odds of cognitive impairment.
However, those with dual sensory impairment (DSI) had an eightfold higher risk for cognitive impairment.
In addition, half of the participants with DSI also had cognitive impairment. Of those with cognitive impairment, 16% had DSI, compared with only about 2% of their peers without cognitive impairment.
“The findings of the present study may inform interventions that can support older people with concurrent sensory impairment and cognitive impairment,” said lead author Esme Fuller-Thomson, PhD, professor, Factor-Inwentash Faculty of Social Work, University of Toronto.
“Special attention, in particular, should be given to those aged 65-74 who have serious hearing and/or vision impairment [because], if the relationship with dementia is found to be causal, such interventions can potentially mitigate the development of cognitive impairment,” said Dr. Fuller-Thomson, who is also director of the Institute for Life Course and Aging and a professor in the department of family and community medicine and faculty of nursing, all at the University of Toronto.
The findings were published online in the Journal of Alzheimer’s Disease Reports.
Sensory isolation
Hearing and vision impairment increase with age; it is estimated that one-third of U.S. adults between the ages of 65 and 74 experience hearing loss, and 4% experience vision impairment, the investigators note.
“The link between dual hearing loss and seeing loss and mental health problems such as depression and social isolation have been well researched, but we were very interested in the link between dual sensory loss and cognitive problems,” Dr. Fuller-Thomson said.
Additionally, “there have been several studies in the past decade linking hearing loss to dementia and cognitive decline, but less attention has been paid to cognitive problems among those with DSI, despite this group being particularly isolated,” she said. Existing research into DSI suggests an association with cognitive decline; the current investigators sought to expand on this previous work.
To do so, they used merged data from 10 consecutive waves from 2008 to 2017 of the American Community Survey (ACS), which was conducted by the U.S. Census Bureau. The ACS is a nationally representative sample of 3.5 million randomly selected U.S. addresses and includes community-dwelling adults and those residing in institutional settings.
Participants aged 65 or older (n = 5,405,135; 56.4% women) were asked yes/no questions regarding serious cognitive impairment, hearing impairment, and vision impairment. A proxy, such as a family member or nursing home staff member, provided answers for individuals not capable of self-report.
Potential confounding variables included age, race/ethnicity, sex, education, and household income.
Potential mechanisms
Results showed that, among those with cognitive impairment, there was a higher prevalence of hearing impairment, vision impairment, and DSI than among their peers without cognitive impairment; in addition, a lower percentage of these persons had no sensory impairment (P < .001).
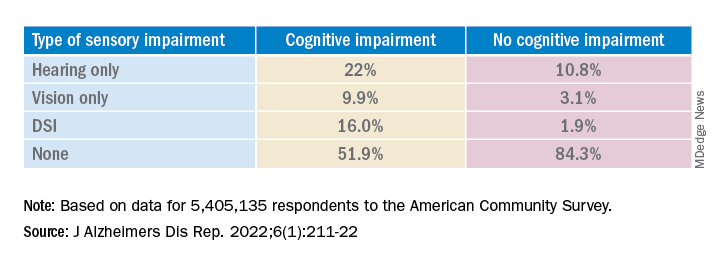
The prevalence of DSI climbed with age, from 1.5% for respondents aged 65-74 years to 2.6% for those aged 75-84 and to 10.8% in those 85 years and older.
Individuals with higher levels of poverty also had higher levels of DSI. Among those who had not completed high school, the prevalence of DSI was higher, compared with high school or university graduates (6.3% vs. 3.1% and 1.85, respectively).
After controlling for age, race, education, and income, the researchers found “substantially” higher odds of cognitive impairment in those with vs. those without sensory impairments.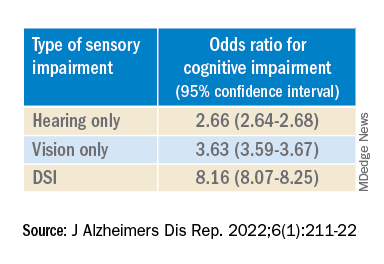
“The magnitude of the odds of cognitive impairment by sensory impairment was greatest for the youngest cohort (age 65-74) and lowest for the oldest cohort (age 85+),” the investigators wrote. Among participants in the youngest cohort, there was a “dose-response relationship” for those with hearing impairment only, visual impairment only, and DSI.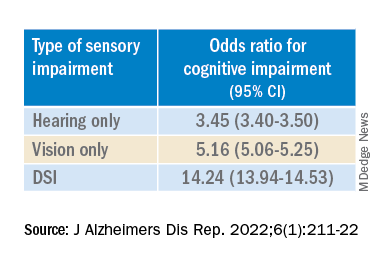
Because the study was observational, it “does not provide sufficient information to determine the reasons behind the observed link between sensory loss and cognitive problems,” Dr. Fuller-Thomson said. However, there are “several potential causal mechanisms [that] warrant future research.”
The “sensory deprivation hypothesis” suggests that DSI could cause cognitive deterioration because of decreased auditory and visual input. The “resource allocation hypothesis” posits that hearing- or vision-impaired older adults “may use more cognitive resources to accommodate for sensory deficits, allocating fewer cognitive resources for higher-order memory processes,” the researchers wrote. Hearing impairment “may also lead to social disengagement among older adults, hastening cognitive decline due to isolation and lack of stimulation,” they added.
Reverse causality is also possible. In the “cognitive load on perception” hypothesis, cognitive decline may lead to declines in hearing and vision because of “decreased resources for sensory processing.”
In addition, the association may be noncausal. “The ‘common cause hypothesis’ theorizes that sensory impairment and cognitive impairment may be due to shared age-related degeneration of the central nervous system ... or frailty,” Dr. Fuller-Thomson said.
Parallel findings
The results are similar to those from a study conducted by Phillip Hwang, PhD, of the department of anatomy and neurobiology, Boston University, and colleagues that was published online in JAMA Network Open.
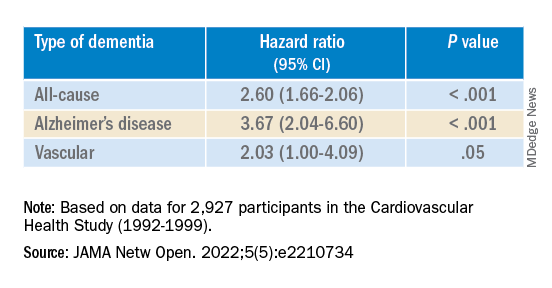
They analyzed data on 8 years of follow-up of 2,927 participants in the Cardiovascular Health Study (mean age, 74.6 years; 58.2% women).
Compared with no sensory impairment, DSI was associated with increased risk for all-cause dementia and Alzheimer’s disease, but not with vascular dementia.
“Future work in health care guidelines could consider incorporating screening of sensory impairment in older adults as part of risk assessment for dementia,” Nicholas Reed, AuD, and Esther Oh, MD, PhD, both of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
Accurate testing
Commenting on both studies, Heather Whitson, MD, professor of medicine (geriatrics) and ophthalmology and director at the Duke University Center for the Study of Aging and Human Development, Durham, N.C., said both “add further strength to the evidence base, which has really converged in the last few years to support that there is a link between sensory health and cognitive health.”
However, “we still don’t know whether hearing/vision loss causes cognitive decline, though there are plausible ways that sensory loss could affect cognitive abilities like memory, language, and executive function,” she said
Dr. Whitson, who was not involved with the research, is also codirector of the Duke/University of North Carolina Alzheimer’s Disease Research Center at Duke University, Durham, N.C., and the Durham VA Medical Center.
“The big question is whether we can improve patients’ cognitive performance by treating or accommodating their sensory impairments,” she said. “If safe and feasible things like hearing aids or cataract surgery improve cognitive health, even a little bit, it would be a huge benefit to society, because sensory loss is very common, and there are many treatment options,” Dr. Whitson added.
Dr. Fuller-Thomson emphasized that practitioners should “consider the full impact of sensory impairment on cognitive testing methods, as both auditory and visual testing methods may fail to take hearing and vision impairment into account.”
Thus, “when performing cognitive tests on older adults with sensory impairments, practitioners should ensure they are communicating audibly and/or using visual speech cues for hearing-impaired individuals, eliminating items from cognitive tests that rely on vision for those who are visually impaired, and using physical cues for individuals with hearing or dual sensory impairment, as this can help increase the accuracy of testing and prevent confounding,” she said.
The study by Fuller-Thomson et al. was funded by a donation from Janis Rotman. Its investigators have reported no relevant financial relationships. The study by Hwang et al. was funded by contracts from the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging. Dr. Hwang reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Reed received grants from the National Institute on Aging during the conduct of the study and has served on the advisory board of Neosensory outside the submitted work. Dr. Oh and Dr. Whitson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of hearing loss and vision loss is linked to an eightfold increased risk of cognitive impairment, new research shows.
Investigators analyzed data on more than 5 million U.S. seniors. Adjusted results show that participants with hearing impairment alone had more than twice the odds of also having cognitive impairment, while those with vision impairment alone had more than triple the odds of cognitive impairment.
However, those with dual sensory impairment (DSI) had an eightfold higher risk for cognitive impairment.
In addition, half of the participants with DSI also had cognitive impairment. Of those with cognitive impairment, 16% had DSI, compared with only about 2% of their peers without cognitive impairment.
“The findings of the present study may inform interventions that can support older people with concurrent sensory impairment and cognitive impairment,” said lead author Esme Fuller-Thomson, PhD, professor, Factor-Inwentash Faculty of Social Work, University of Toronto.
“Special attention, in particular, should be given to those aged 65-74 who have serious hearing and/or vision impairment [because], if the relationship with dementia is found to be causal, such interventions can potentially mitigate the development of cognitive impairment,” said Dr. Fuller-Thomson, who is also director of the Institute for Life Course and Aging and a professor in the department of family and community medicine and faculty of nursing, all at the University of Toronto.
The findings were published online in the Journal of Alzheimer’s Disease Reports.
Sensory isolation
Hearing and vision impairment increase with age; it is estimated that one-third of U.S. adults between the ages of 65 and 74 experience hearing loss, and 4% experience vision impairment, the investigators note.
“The link between dual hearing loss and seeing loss and mental health problems such as depression and social isolation have been well researched, but we were very interested in the link between dual sensory loss and cognitive problems,” Dr. Fuller-Thomson said.
Additionally, “there have been several studies in the past decade linking hearing loss to dementia and cognitive decline, but less attention has been paid to cognitive problems among those with DSI, despite this group being particularly isolated,” she said. Existing research into DSI suggests an association with cognitive decline; the current investigators sought to expand on this previous work.
To do so, they used merged data from 10 consecutive waves from 2008 to 2017 of the American Community Survey (ACS), which was conducted by the U.S. Census Bureau. The ACS is a nationally representative sample of 3.5 million randomly selected U.S. addresses and includes community-dwelling adults and those residing in institutional settings.
Participants aged 65 or older (n = 5,405,135; 56.4% women) were asked yes/no questions regarding serious cognitive impairment, hearing impairment, and vision impairment. A proxy, such as a family member or nursing home staff member, provided answers for individuals not capable of self-report.
Potential confounding variables included age, race/ethnicity, sex, education, and household income.
Potential mechanisms
Results showed that, among those with cognitive impairment, there was a higher prevalence of hearing impairment, vision impairment, and DSI than among their peers without cognitive impairment; in addition, a lower percentage of these persons had no sensory impairment (P < .001).

The prevalence of DSI climbed with age, from 1.5% for respondents aged 65-74 years to 2.6% for those aged 75-84 and to 10.8% in those 85 years and older.
Individuals with higher levels of poverty also had higher levels of DSI. Among those who had not completed high school, the prevalence of DSI was higher, compared with high school or university graduates (6.3% vs. 3.1% and 1.85, respectively).
After controlling for age, race, education, and income, the researchers found “substantially” higher odds of cognitive impairment in those with vs. those without sensory impairments.
“The magnitude of the odds of cognitive impairment by sensory impairment was greatest for the youngest cohort (age 65-74) and lowest for the oldest cohort (age 85+),” the investigators wrote. Among participants in the youngest cohort, there was a “dose-response relationship” for those with hearing impairment only, visual impairment only, and DSI.
Because the study was observational, it “does not provide sufficient information to determine the reasons behind the observed link between sensory loss and cognitive problems,” Dr. Fuller-Thomson said. However, there are “several potential causal mechanisms [that] warrant future research.”
The “sensory deprivation hypothesis” suggests that DSI could cause cognitive deterioration because of decreased auditory and visual input. The “resource allocation hypothesis” posits that hearing- or vision-impaired older adults “may use more cognitive resources to accommodate for sensory deficits, allocating fewer cognitive resources for higher-order memory processes,” the researchers wrote. Hearing impairment “may also lead to social disengagement among older adults, hastening cognitive decline due to isolation and lack of stimulation,” they added.
Reverse causality is also possible. In the “cognitive load on perception” hypothesis, cognitive decline may lead to declines in hearing and vision because of “decreased resources for sensory processing.”
In addition, the association may be noncausal. “The ‘common cause hypothesis’ theorizes that sensory impairment and cognitive impairment may be due to shared age-related degeneration of the central nervous system ... or frailty,” Dr. Fuller-Thomson said.
Parallel findings
The results are similar to those from a study conducted by Phillip Hwang, PhD, of the department of anatomy and neurobiology, Boston University, and colleagues that was published online in JAMA Network Open.

They analyzed data on 8 years of follow-up of 2,927 participants in the Cardiovascular Health Study (mean age, 74.6 years; 58.2% women).
Compared with no sensory impairment, DSI was associated with increased risk for all-cause dementia and Alzheimer’s disease, but not with vascular dementia.
“Future work in health care guidelines could consider incorporating screening of sensory impairment in older adults as part of risk assessment for dementia,” Nicholas Reed, AuD, and Esther Oh, MD, PhD, both of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
Accurate testing
Commenting on both studies, Heather Whitson, MD, professor of medicine (geriatrics) and ophthalmology and director at the Duke University Center for the Study of Aging and Human Development, Durham, N.C., said both “add further strength to the evidence base, which has really converged in the last few years to support that there is a link between sensory health and cognitive health.”
However, “we still don’t know whether hearing/vision loss causes cognitive decline, though there are plausible ways that sensory loss could affect cognitive abilities like memory, language, and executive function,” she said
Dr. Whitson, who was not involved with the research, is also codirector of the Duke/University of North Carolina Alzheimer’s Disease Research Center at Duke University, Durham, N.C., and the Durham VA Medical Center.
“The big question is whether we can improve patients’ cognitive performance by treating or accommodating their sensory impairments,” she said. “If safe and feasible things like hearing aids or cataract surgery improve cognitive health, even a little bit, it would be a huge benefit to society, because sensory loss is very common, and there are many treatment options,” Dr. Whitson added.
Dr. Fuller-Thomson emphasized that practitioners should “consider the full impact of sensory impairment on cognitive testing methods, as both auditory and visual testing methods may fail to take hearing and vision impairment into account.”
Thus, “when performing cognitive tests on older adults with sensory impairments, practitioners should ensure they are communicating audibly and/or using visual speech cues for hearing-impaired individuals, eliminating items from cognitive tests that rely on vision for those who are visually impaired, and using physical cues for individuals with hearing or dual sensory impairment, as this can help increase the accuracy of testing and prevent confounding,” she said.
The study by Fuller-Thomson et al. was funded by a donation from Janis Rotman. Its investigators have reported no relevant financial relationships. The study by Hwang et al. was funded by contracts from the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging. Dr. Hwang reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Reed received grants from the National Institute on Aging during the conduct of the study and has served on the advisory board of Neosensory outside the submitted work. Dr. Oh and Dr. Whitson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of hearing loss and vision loss is linked to an eightfold increased risk of cognitive impairment, new research shows.
Investigators analyzed data on more than 5 million U.S. seniors. Adjusted results show that participants with hearing impairment alone had more than twice the odds of also having cognitive impairment, while those with vision impairment alone had more than triple the odds of cognitive impairment.
However, those with dual sensory impairment (DSI) had an eightfold higher risk for cognitive impairment.
In addition, half of the participants with DSI also had cognitive impairment. Of those with cognitive impairment, 16% had DSI, compared with only about 2% of their peers without cognitive impairment.
“The findings of the present study may inform interventions that can support older people with concurrent sensory impairment and cognitive impairment,” said lead author Esme Fuller-Thomson, PhD, professor, Factor-Inwentash Faculty of Social Work, University of Toronto.
“Special attention, in particular, should be given to those aged 65-74 who have serious hearing and/or vision impairment [because], if the relationship with dementia is found to be causal, such interventions can potentially mitigate the development of cognitive impairment,” said Dr. Fuller-Thomson, who is also director of the Institute for Life Course and Aging and a professor in the department of family and community medicine and faculty of nursing, all at the University of Toronto.
The findings were published online in the Journal of Alzheimer’s Disease Reports.
Sensory isolation
Hearing and vision impairment increase with age; it is estimated that one-third of U.S. adults between the ages of 65 and 74 experience hearing loss, and 4% experience vision impairment, the investigators note.
“The link between dual hearing loss and seeing loss and mental health problems such as depression and social isolation have been well researched, but we were very interested in the link between dual sensory loss and cognitive problems,” Dr. Fuller-Thomson said.
Additionally, “there have been several studies in the past decade linking hearing loss to dementia and cognitive decline, but less attention has been paid to cognitive problems among those with DSI, despite this group being particularly isolated,” she said. Existing research into DSI suggests an association with cognitive decline; the current investigators sought to expand on this previous work.
To do so, they used merged data from 10 consecutive waves from 2008 to 2017 of the American Community Survey (ACS), which was conducted by the U.S. Census Bureau. The ACS is a nationally representative sample of 3.5 million randomly selected U.S. addresses and includes community-dwelling adults and those residing in institutional settings.
Participants aged 65 or older (n = 5,405,135; 56.4% women) were asked yes/no questions regarding serious cognitive impairment, hearing impairment, and vision impairment. A proxy, such as a family member or nursing home staff member, provided answers for individuals not capable of self-report.
Potential confounding variables included age, race/ethnicity, sex, education, and household income.
Potential mechanisms
Results showed that, among those with cognitive impairment, there was a higher prevalence of hearing impairment, vision impairment, and DSI than among their peers without cognitive impairment; in addition, a lower percentage of these persons had no sensory impairment (P < .001).

The prevalence of DSI climbed with age, from 1.5% for respondents aged 65-74 years to 2.6% for those aged 75-84 and to 10.8% in those 85 years and older.
Individuals with higher levels of poverty also had higher levels of DSI. Among those who had not completed high school, the prevalence of DSI was higher, compared with high school or university graduates (6.3% vs. 3.1% and 1.85, respectively).
After controlling for age, race, education, and income, the researchers found “substantially” higher odds of cognitive impairment in those with vs. those without sensory impairments.
“The magnitude of the odds of cognitive impairment by sensory impairment was greatest for the youngest cohort (age 65-74) and lowest for the oldest cohort (age 85+),” the investigators wrote. Among participants in the youngest cohort, there was a “dose-response relationship” for those with hearing impairment only, visual impairment only, and DSI.
Because the study was observational, it “does not provide sufficient information to determine the reasons behind the observed link between sensory loss and cognitive problems,” Dr. Fuller-Thomson said. However, there are “several potential causal mechanisms [that] warrant future research.”
The “sensory deprivation hypothesis” suggests that DSI could cause cognitive deterioration because of decreased auditory and visual input. The “resource allocation hypothesis” posits that hearing- or vision-impaired older adults “may use more cognitive resources to accommodate for sensory deficits, allocating fewer cognitive resources for higher-order memory processes,” the researchers wrote. Hearing impairment “may also lead to social disengagement among older adults, hastening cognitive decline due to isolation and lack of stimulation,” they added.
Reverse causality is also possible. In the “cognitive load on perception” hypothesis, cognitive decline may lead to declines in hearing and vision because of “decreased resources for sensory processing.”
In addition, the association may be noncausal. “The ‘common cause hypothesis’ theorizes that sensory impairment and cognitive impairment may be due to shared age-related degeneration of the central nervous system ... or frailty,” Dr. Fuller-Thomson said.
Parallel findings
The results are similar to those from a study conducted by Phillip Hwang, PhD, of the department of anatomy and neurobiology, Boston University, and colleagues that was published online in JAMA Network Open.

They analyzed data on 8 years of follow-up of 2,927 participants in the Cardiovascular Health Study (mean age, 74.6 years; 58.2% women).
Compared with no sensory impairment, DSI was associated with increased risk for all-cause dementia and Alzheimer’s disease, but not with vascular dementia.
“Future work in health care guidelines could consider incorporating screening of sensory impairment in older adults as part of risk assessment for dementia,” Nicholas Reed, AuD, and Esther Oh, MD, PhD, both of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
Accurate testing
Commenting on both studies, Heather Whitson, MD, professor of medicine (geriatrics) and ophthalmology and director at the Duke University Center for the Study of Aging and Human Development, Durham, N.C., said both “add further strength to the evidence base, which has really converged in the last few years to support that there is a link between sensory health and cognitive health.”
However, “we still don’t know whether hearing/vision loss causes cognitive decline, though there are plausible ways that sensory loss could affect cognitive abilities like memory, language, and executive function,” she said
Dr. Whitson, who was not involved with the research, is also codirector of the Duke/University of North Carolina Alzheimer’s Disease Research Center at Duke University, Durham, N.C., and the Durham VA Medical Center.
“The big question is whether we can improve patients’ cognitive performance by treating or accommodating their sensory impairments,” she said. “If safe and feasible things like hearing aids or cataract surgery improve cognitive health, even a little bit, it would be a huge benefit to society, because sensory loss is very common, and there are many treatment options,” Dr. Whitson added.
Dr. Fuller-Thomson emphasized that practitioners should “consider the full impact of sensory impairment on cognitive testing methods, as both auditory and visual testing methods may fail to take hearing and vision impairment into account.”
Thus, “when performing cognitive tests on older adults with sensory impairments, practitioners should ensure they are communicating audibly and/or using visual speech cues for hearing-impaired individuals, eliminating items from cognitive tests that rely on vision for those who are visually impaired, and using physical cues for individuals with hearing or dual sensory impairment, as this can help increase the accuracy of testing and prevent confounding,” she said.
The study by Fuller-Thomson et al. was funded by a donation from Janis Rotman. Its investigators have reported no relevant financial relationships. The study by Hwang et al. was funded by contracts from the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging. Dr. Hwang reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Reed received grants from the National Institute on Aging during the conduct of the study and has served on the advisory board of Neosensory outside the submitted work. Dr. Oh and Dr. Whitson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE REPORTS
Studies address ibrutinib bleeding risk in patients with CLL receiving Mohs surgery
Patients receiving , new research shows.
“Our cohort of CLL patients on ibrutinib had a two-times greater risk of bleeding complications relative to those on anticoagulants and a nearly 40-times greater risk of bleeding complications relative to those patients on no anticoagulants or CLL therapy,” Kelsey E. Hirotsu, MD, first author of one of two studies on the issue presented at the American College of Mohs Surgery annual meeting, told this news organization.
“It was definitely surprising to see this doubled risk with ibrutinib relative to anticoagulants, and certainly highlights the clinically relevant increased bleeding risk in patients on ibrutinib,” said Dr. Hirotsu, a Mohs micrographic surgery fellow in the department of dermatology, University of California, San Diego (UCSD).
With CLL associated with an increased risk for aggressive skin cancers, particularly squamous cell carcinoma, Mohs surgeons may commonly find themselves treating patients with these unique considerations. Surgical treatment of those cancers can be complicated not only because of potential underlying thrombocytopenia, which occurs in about 5% of untreated CLL patients, but also because of the increased risk for bleeding that is associated with the use of the Bruton tyrosine kinase inhibitor ibrutinib, commonly used for CLL.
While the nature of the increased bleeding-related complications among patients with CLL undergoing Mohs surgery has been documented in some case reports, evidence from larger studies has been lacking.
In one of the studies presented at the ACMS meeting, Dr. Hirotsu and her colleagues evaluated data on patients with CLL who underwent at least one Mohs surgery procedure at UCSD Dermatologic Surgery over 10 years. Of the 362 Mohs cases among 98 patients with CLL, 32 cases had at least one complication. Patients on anticoagulants, including antiplatelet agents, Coumadin, and direct oral anticoagulants (DOACs), not surprisingly, had higher rates of complications, particularly bleeding.
However, those treated with ibrutinib had the highest rates of complications among all of the patients (40.6%), with all of their complications involving bleeding-related events. In comparison, the complication rates, for instance, of patients treated with antiplatelets were 21.9%; Coumadin, 6.2%; and DOACs, 15.6%.
The incidence of bleeding-related complications among the cases in the ibrutinib-treated patients was 30.2% compared with 13.2% among those on blood thinners and no CLL therapy (relative risk [RR], 2.08; 95% confidence interval [CI], 0.85-5.11; P = .11). “Although not statistically significant, these results could trend toward significance with larger sample sizes,” Dr. Hirotsu said.
The risk for bleeding among patients on ibrutinib compared with patients on no medications, however, was significant, with a relative risk of 39.0 (95% CI, 2.35-646; P = .011).
Of note, among 12 patients on ibrutinib who experienced bleeding complications, 7 had previously undergone Mohs surgeries when they were not taking ibrutinib and no bleeding complications had occurred in those procedures. “This may further implicate ibrutinib as a cause of the bleeding-related complications,” Dr. Hirotsu said.
In investigating the role of thrombocytopenia at the time of Mohs surgery, the authors found that, among ibrutinib-treated patients who had no complications, 30% had thrombocytopenia, compared with 70% of those who did have bleeding while on ibrutinib at the time of surgery.
“It was interesting that thrombocytopenia is more common in ibrutinib patients with bleeding-related complications, but further research needs to be done to determine the clinical relevance and possible management implications,” Dr. Hirotsu said.
In a separate study presented at the meeting, 37 patients treated with ibrutinib for CLL while undergoing cutaneous surgery that included Mohs surgery and excisions had a significantly increased bleeding complication rate compared with a control group of 64 age- and sex-matched patients with CLL undergoing cutaneous surgery: 6 of 75 procedures (8%) versus 1 of 115 procedures (0.9%; P = .02).
Those with bleeding complications while on ibrutinib were all male, older (mean age, 82.7 vs. 73.0; P = .01), and had lower mean platelet counts (104 K/mcL vs. 150.5 K/mcL; P = .03).
There were no significant differences between the case and control groups in terms of anatomic site, type of procedure (Mohs versus excision), tumor diagnosis, lesion size, or type of reconstruction, while the control group was more likely to be on aspirin or other anticoagulants (P < .0001).
In an interview, senior author Nahid Y. Vidal, MD, a Mohs surgeon and dermatologic oncologist at the Mayo Clinic, Rochester, Minn., said that “the take-home message is that patients on ibrutinib should be considered higher risk for bleeding events, regardless of whether they are having a simpler surgery [excision] or more involved skin surgery procedure [Mohs with flap].”
Holding treatment
To offset the bleeding risk, Dr. Vidal notes that holding the treatment is considered safe and that the manufacturer recommends holding ibrutinib for at least 3-7 days pre- and post surgery, “depending on type of surgery and risk of bleeding.”
“In our institution, with the hematologist/oncologist’s input, we hold ibrutinib for 5 days preop and 3 days post op, and have not had bleed complications in these patients,” she said, noting that there were no bleeding events in the patients in the study when ibrutinib was held.
Likewise, Dr. Hirotsu noted that at her center at UCSD, patients on ibrutinib are asked during the preop call to hold treatment for 3 days before and after Mohs surgery – but are advised to discuss the decision with their hematologist/oncologist for approval.
The measure isn’t always successful in preventing bleeding, however, as seen in a case study describing two patients who experienced bleeding complications following Mohs surgery despite being taken off ibrutinib 3 days prior to the procedure.
The senior author of that study, Kira Minkis, MD, PhD, department of dermatology, Weill Cornell/New York Presbyterian, New York, told this news organization that her team concluded that in those cases ibrutinib perhaps should have been held longer than 3 days.
“In some cases, especially if the Mohs surgery is a large procedure with a more advanced reconstruction, such as a large flap, it might be more prudent to continue it longer than 3 days,” Dr. Minkis said. She noted that the high bleeding risk observed in the studies at ACMS was notable – but not unexpected.
“I’m not that surprised because if you look at the hematologic literature, the risk is indeed pretty significant, so it makes sense that it would also occur with Mohs surgeries,” she said.
She underscored that a 3-day hold of ibrutinib should be considered the minimum, “and in some cases, it should be held up to 7 days prior to surgery, depending on the specific surgery,” with the important caveat of consulting with the patient’s hematology team.
“Multidisciplinary decision-making is necessary for these cases, and the interruption of therapy should always be discussed with their hematology team,” she added. That said, Dr. Minkis noted that “I’ve never had a hematologist who had any concerns for withholding ibrutinib even for a week around the time of a surgery.”
Dr. Hirotsu, Dr. Vidal, and Dr. Minkis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients receiving , new research shows.
“Our cohort of CLL patients on ibrutinib had a two-times greater risk of bleeding complications relative to those on anticoagulants and a nearly 40-times greater risk of bleeding complications relative to those patients on no anticoagulants or CLL therapy,” Kelsey E. Hirotsu, MD, first author of one of two studies on the issue presented at the American College of Mohs Surgery annual meeting, told this news organization.
“It was definitely surprising to see this doubled risk with ibrutinib relative to anticoagulants, and certainly highlights the clinically relevant increased bleeding risk in patients on ibrutinib,” said Dr. Hirotsu, a Mohs micrographic surgery fellow in the department of dermatology, University of California, San Diego (UCSD).
With CLL associated with an increased risk for aggressive skin cancers, particularly squamous cell carcinoma, Mohs surgeons may commonly find themselves treating patients with these unique considerations. Surgical treatment of those cancers can be complicated not only because of potential underlying thrombocytopenia, which occurs in about 5% of untreated CLL patients, but also because of the increased risk for bleeding that is associated with the use of the Bruton tyrosine kinase inhibitor ibrutinib, commonly used for CLL.
While the nature of the increased bleeding-related complications among patients with CLL undergoing Mohs surgery has been documented in some case reports, evidence from larger studies has been lacking.
In one of the studies presented at the ACMS meeting, Dr. Hirotsu and her colleagues evaluated data on patients with CLL who underwent at least one Mohs surgery procedure at UCSD Dermatologic Surgery over 10 years. Of the 362 Mohs cases among 98 patients with CLL, 32 cases had at least one complication. Patients on anticoagulants, including antiplatelet agents, Coumadin, and direct oral anticoagulants (DOACs), not surprisingly, had higher rates of complications, particularly bleeding.
However, those treated with ibrutinib had the highest rates of complications among all of the patients (40.6%), with all of their complications involving bleeding-related events. In comparison, the complication rates, for instance, of patients treated with antiplatelets were 21.9%; Coumadin, 6.2%; and DOACs, 15.6%.
The incidence of bleeding-related complications among the cases in the ibrutinib-treated patients was 30.2% compared with 13.2% among those on blood thinners and no CLL therapy (relative risk [RR], 2.08; 95% confidence interval [CI], 0.85-5.11; P = .11). “Although not statistically significant, these results could trend toward significance with larger sample sizes,” Dr. Hirotsu said.
The risk for bleeding among patients on ibrutinib compared with patients on no medications, however, was significant, with a relative risk of 39.0 (95% CI, 2.35-646; P = .011).
Of note, among 12 patients on ibrutinib who experienced bleeding complications, 7 had previously undergone Mohs surgeries when they were not taking ibrutinib and no bleeding complications had occurred in those procedures. “This may further implicate ibrutinib as a cause of the bleeding-related complications,” Dr. Hirotsu said.
In investigating the role of thrombocytopenia at the time of Mohs surgery, the authors found that, among ibrutinib-treated patients who had no complications, 30% had thrombocytopenia, compared with 70% of those who did have bleeding while on ibrutinib at the time of surgery.
“It was interesting that thrombocytopenia is more common in ibrutinib patients with bleeding-related complications, but further research needs to be done to determine the clinical relevance and possible management implications,” Dr. Hirotsu said.
In a separate study presented at the meeting, 37 patients treated with ibrutinib for CLL while undergoing cutaneous surgery that included Mohs surgery and excisions had a significantly increased bleeding complication rate compared with a control group of 64 age- and sex-matched patients with CLL undergoing cutaneous surgery: 6 of 75 procedures (8%) versus 1 of 115 procedures (0.9%; P = .02).
Those with bleeding complications while on ibrutinib were all male, older (mean age, 82.7 vs. 73.0; P = .01), and had lower mean platelet counts (104 K/mcL vs. 150.5 K/mcL; P = .03).
There were no significant differences between the case and control groups in terms of anatomic site, type of procedure (Mohs versus excision), tumor diagnosis, lesion size, or type of reconstruction, while the control group was more likely to be on aspirin or other anticoagulants (P < .0001).
In an interview, senior author Nahid Y. Vidal, MD, a Mohs surgeon and dermatologic oncologist at the Mayo Clinic, Rochester, Minn., said that “the take-home message is that patients on ibrutinib should be considered higher risk for bleeding events, regardless of whether they are having a simpler surgery [excision] or more involved skin surgery procedure [Mohs with flap].”
Holding treatment
To offset the bleeding risk, Dr. Vidal notes that holding the treatment is considered safe and that the manufacturer recommends holding ibrutinib for at least 3-7 days pre- and post surgery, “depending on type of surgery and risk of bleeding.”
“In our institution, with the hematologist/oncologist’s input, we hold ibrutinib for 5 days preop and 3 days post op, and have not had bleed complications in these patients,” she said, noting that there were no bleeding events in the patients in the study when ibrutinib was held.
Likewise, Dr. Hirotsu noted that at her center at UCSD, patients on ibrutinib are asked during the preop call to hold treatment for 3 days before and after Mohs surgery – but are advised to discuss the decision with their hematologist/oncologist for approval.
The measure isn’t always successful in preventing bleeding, however, as seen in a case study describing two patients who experienced bleeding complications following Mohs surgery despite being taken off ibrutinib 3 days prior to the procedure.
The senior author of that study, Kira Minkis, MD, PhD, department of dermatology, Weill Cornell/New York Presbyterian, New York, told this news organization that her team concluded that in those cases ibrutinib perhaps should have been held longer than 3 days.
“In some cases, especially if the Mohs surgery is a large procedure with a more advanced reconstruction, such as a large flap, it might be more prudent to continue it longer than 3 days,” Dr. Minkis said. She noted that the high bleeding risk observed in the studies at ACMS was notable – but not unexpected.
“I’m not that surprised because if you look at the hematologic literature, the risk is indeed pretty significant, so it makes sense that it would also occur with Mohs surgeries,” she said.
She underscored that a 3-day hold of ibrutinib should be considered the minimum, “and in some cases, it should be held up to 7 days prior to surgery, depending on the specific surgery,” with the important caveat of consulting with the patient’s hematology team.
“Multidisciplinary decision-making is necessary for these cases, and the interruption of therapy should always be discussed with their hematology team,” she added. That said, Dr. Minkis noted that “I’ve never had a hematologist who had any concerns for withholding ibrutinib even for a week around the time of a surgery.”
Dr. Hirotsu, Dr. Vidal, and Dr. Minkis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients receiving , new research shows.
“Our cohort of CLL patients on ibrutinib had a two-times greater risk of bleeding complications relative to those on anticoagulants and a nearly 40-times greater risk of bleeding complications relative to those patients on no anticoagulants or CLL therapy,” Kelsey E. Hirotsu, MD, first author of one of two studies on the issue presented at the American College of Mohs Surgery annual meeting, told this news organization.
“It was definitely surprising to see this doubled risk with ibrutinib relative to anticoagulants, and certainly highlights the clinically relevant increased bleeding risk in patients on ibrutinib,” said Dr. Hirotsu, a Mohs micrographic surgery fellow in the department of dermatology, University of California, San Diego (UCSD).
With CLL associated with an increased risk for aggressive skin cancers, particularly squamous cell carcinoma, Mohs surgeons may commonly find themselves treating patients with these unique considerations. Surgical treatment of those cancers can be complicated not only because of potential underlying thrombocytopenia, which occurs in about 5% of untreated CLL patients, but also because of the increased risk for bleeding that is associated with the use of the Bruton tyrosine kinase inhibitor ibrutinib, commonly used for CLL.
While the nature of the increased bleeding-related complications among patients with CLL undergoing Mohs surgery has been documented in some case reports, evidence from larger studies has been lacking.
In one of the studies presented at the ACMS meeting, Dr. Hirotsu and her colleagues evaluated data on patients with CLL who underwent at least one Mohs surgery procedure at UCSD Dermatologic Surgery over 10 years. Of the 362 Mohs cases among 98 patients with CLL, 32 cases had at least one complication. Patients on anticoagulants, including antiplatelet agents, Coumadin, and direct oral anticoagulants (DOACs), not surprisingly, had higher rates of complications, particularly bleeding.
However, those treated with ibrutinib had the highest rates of complications among all of the patients (40.6%), with all of their complications involving bleeding-related events. In comparison, the complication rates, for instance, of patients treated with antiplatelets were 21.9%; Coumadin, 6.2%; and DOACs, 15.6%.
The incidence of bleeding-related complications among the cases in the ibrutinib-treated patients was 30.2% compared with 13.2% among those on blood thinners and no CLL therapy (relative risk [RR], 2.08; 95% confidence interval [CI], 0.85-5.11; P = .11). “Although not statistically significant, these results could trend toward significance with larger sample sizes,” Dr. Hirotsu said.
The risk for bleeding among patients on ibrutinib compared with patients on no medications, however, was significant, with a relative risk of 39.0 (95% CI, 2.35-646; P = .011).
Of note, among 12 patients on ibrutinib who experienced bleeding complications, 7 had previously undergone Mohs surgeries when they were not taking ibrutinib and no bleeding complications had occurred in those procedures. “This may further implicate ibrutinib as a cause of the bleeding-related complications,” Dr. Hirotsu said.
In investigating the role of thrombocytopenia at the time of Mohs surgery, the authors found that, among ibrutinib-treated patients who had no complications, 30% had thrombocytopenia, compared with 70% of those who did have bleeding while on ibrutinib at the time of surgery.
“It was interesting that thrombocytopenia is more common in ibrutinib patients with bleeding-related complications, but further research needs to be done to determine the clinical relevance and possible management implications,” Dr. Hirotsu said.
In a separate study presented at the meeting, 37 patients treated with ibrutinib for CLL while undergoing cutaneous surgery that included Mohs surgery and excisions had a significantly increased bleeding complication rate compared with a control group of 64 age- and sex-matched patients with CLL undergoing cutaneous surgery: 6 of 75 procedures (8%) versus 1 of 115 procedures (0.9%; P = .02).
Those with bleeding complications while on ibrutinib were all male, older (mean age, 82.7 vs. 73.0; P = .01), and had lower mean platelet counts (104 K/mcL vs. 150.5 K/mcL; P = .03).
There were no significant differences between the case and control groups in terms of anatomic site, type of procedure (Mohs versus excision), tumor diagnosis, lesion size, or type of reconstruction, while the control group was more likely to be on aspirin or other anticoagulants (P < .0001).
In an interview, senior author Nahid Y. Vidal, MD, a Mohs surgeon and dermatologic oncologist at the Mayo Clinic, Rochester, Minn., said that “the take-home message is that patients on ibrutinib should be considered higher risk for bleeding events, regardless of whether they are having a simpler surgery [excision] or more involved skin surgery procedure [Mohs with flap].”
Holding treatment
To offset the bleeding risk, Dr. Vidal notes that holding the treatment is considered safe and that the manufacturer recommends holding ibrutinib for at least 3-7 days pre- and post surgery, “depending on type of surgery and risk of bleeding.”
“In our institution, with the hematologist/oncologist’s input, we hold ibrutinib for 5 days preop and 3 days post op, and have not had bleed complications in these patients,” she said, noting that there were no bleeding events in the patients in the study when ibrutinib was held.
Likewise, Dr. Hirotsu noted that at her center at UCSD, patients on ibrutinib are asked during the preop call to hold treatment for 3 days before and after Mohs surgery – but are advised to discuss the decision with their hematologist/oncologist for approval.
The measure isn’t always successful in preventing bleeding, however, as seen in a case study describing two patients who experienced bleeding complications following Mohs surgery despite being taken off ibrutinib 3 days prior to the procedure.
The senior author of that study, Kira Minkis, MD, PhD, department of dermatology, Weill Cornell/New York Presbyterian, New York, told this news organization that her team concluded that in those cases ibrutinib perhaps should have been held longer than 3 days.
“In some cases, especially if the Mohs surgery is a large procedure with a more advanced reconstruction, such as a large flap, it might be more prudent to continue it longer than 3 days,” Dr. Minkis said. She noted that the high bleeding risk observed in the studies at ACMS was notable – but not unexpected.
“I’m not that surprised because if you look at the hematologic literature, the risk is indeed pretty significant, so it makes sense that it would also occur with Mohs surgeries,” she said.
She underscored that a 3-day hold of ibrutinib should be considered the minimum, “and in some cases, it should be held up to 7 days prior to surgery, depending on the specific surgery,” with the important caveat of consulting with the patient’s hematology team.
“Multidisciplinary decision-making is necessary for these cases, and the interruption of therapy should always be discussed with their hematology team,” she added. That said, Dr. Minkis noted that “I’ve never had a hematologist who had any concerns for withholding ibrutinib even for a week around the time of a surgery.”
Dr. Hirotsu, Dr. Vidal, and Dr. Minkis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE ACMS ANNUAL MEETING
Immunotherapy now first line for esophageal cancer
The new approval for the drug, a programmed cell death–ligand-1 inhibitor, is for use in this patient population regardless of PD-L1 status.
The indication also specifies that nivolumab is to be used together with chemotherapy (with a fluoropyrimidine- and platinum-containing regimen) or in combination with ipilimumab (Yervoy), an immunotherapy with a different mechanism of action.
“Today’s approvals bring two first-line immunotherapy-based treatment options at once ... to newly diagnosed patients with unresectable advanced or metastatic ESCC,” commented Adam Lenkowsky, a senior vice president at Bristol-Myers Squibb, which makes both nivolumab and ipilimumab.
The approval of the new indication by the Food and Drug Administration was based on improved survival shown in the phase 3 CheckMate-648 trial, which involved nearly 1,000 patients. The trial had three arms and compared nivolumab plus chemotherapy (n = 321) and nivolumab plus ipilimumab (n = 324) with chemotherapy alone (n = 324).
The results showed improved survival with both nivolumab combinations compared with chemotherapy (fluorouracil and cisplatin) alone. Overall survival was improved both in all randomized patients (a secondary endpoint) and in patients whose tumors expressed PD-L1 (≥ 1%), the primary endpoint.
For the combination of nivolumab plus chemotherapy, median overall survival was 13.2 versus 10.7 months, compared with chemotherapy alone in all randomized patients, and 15.4 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
For the combination of nivolumab plus ipilimumab, median overall survival was 12.8 versus 10.7 months with chemotherapy alone in all randomized patients and 13.7 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
However, progression-free survival did not reach statistical significance in any group.
“Unresectable advanced or metastatic ESCC is a challenging disease, and there’s a need for additional treatment options that may extend survival in the first-line setting,” commented Jaffer A. Ajani, MD, professor of gastrointestinal medical oncology at the University of Texas MD Anderson Cancer Center, Houston. He was also the lead U.S. investigator for CheckMate-648 and, in a company press release, said the “two nivolumab-based combinations showed a survival benefit compared to chemotherapy alone, offering new treatment options regardless of PD-L1 status.”
Results from the trial were presented at the 2021 annual meeting of the American Society of Clinical Oncology. At that time, trial investigator Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital in Sutton, England, told attendees that “nivolumab plus chemotherapy and nivolumab plus ipilimumab each represent a new potential first-line standard of care for patients with advanced ESCC.”
Commenting on that presentation, Samuel J. Klempner, MD, a gastrointestinal medical oncologist at the Massachusetts General Hospital Cancer Center, Boston, noted that the “prospect of a chemo-free regimen for advanced ESCC with the well-studied combination of ipilimumab and nivolumab would represent a welcome addition to our treatment armamentarium.”
No new safety signals
Dr. Chau noted there were no new safety signals with either of the immunotherapies.
Nivolumab and/or chemotherapy were discontinued in 39% of patients and delayed in 71% of patients for an adverse reaction.
Nivolumab and/or ipilimumab were discontinued in 23% of patients and delayed in 46% of patients for an adverse reaction.
The manufacturer cautioned that immunotherapy with nivolumab with or without ipilimumab has been associated with severe and fatal immune-mediated adverse reactions including pneumonitis, colitis, hepatitis and hepatotoxicity, endocrinopathies, nephritis and renal dysfunction, dermatologic adverse reactions, and infusion-related reactions.
A version of this article first appeared on Medscape.com.
The new approval for the drug, a programmed cell death–ligand-1 inhibitor, is for use in this patient population regardless of PD-L1 status.
The indication also specifies that nivolumab is to be used together with chemotherapy (with a fluoropyrimidine- and platinum-containing regimen) or in combination with ipilimumab (Yervoy), an immunotherapy with a different mechanism of action.
“Today’s approvals bring two first-line immunotherapy-based treatment options at once ... to newly diagnosed patients with unresectable advanced or metastatic ESCC,” commented Adam Lenkowsky, a senior vice president at Bristol-Myers Squibb, which makes both nivolumab and ipilimumab.
The approval of the new indication by the Food and Drug Administration was based on improved survival shown in the phase 3 CheckMate-648 trial, which involved nearly 1,000 patients. The trial had three arms and compared nivolumab plus chemotherapy (n = 321) and nivolumab plus ipilimumab (n = 324) with chemotherapy alone (n = 324).
The results showed improved survival with both nivolumab combinations compared with chemotherapy (fluorouracil and cisplatin) alone. Overall survival was improved both in all randomized patients (a secondary endpoint) and in patients whose tumors expressed PD-L1 (≥ 1%), the primary endpoint.
For the combination of nivolumab plus chemotherapy, median overall survival was 13.2 versus 10.7 months, compared with chemotherapy alone in all randomized patients, and 15.4 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
For the combination of nivolumab plus ipilimumab, median overall survival was 12.8 versus 10.7 months with chemotherapy alone in all randomized patients and 13.7 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
However, progression-free survival did not reach statistical significance in any group.
“Unresectable advanced or metastatic ESCC is a challenging disease, and there’s a need for additional treatment options that may extend survival in the first-line setting,” commented Jaffer A. Ajani, MD, professor of gastrointestinal medical oncology at the University of Texas MD Anderson Cancer Center, Houston. He was also the lead U.S. investigator for CheckMate-648 and, in a company press release, said the “two nivolumab-based combinations showed a survival benefit compared to chemotherapy alone, offering new treatment options regardless of PD-L1 status.”
Results from the trial were presented at the 2021 annual meeting of the American Society of Clinical Oncology. At that time, trial investigator Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital in Sutton, England, told attendees that “nivolumab plus chemotherapy and nivolumab plus ipilimumab each represent a new potential first-line standard of care for patients with advanced ESCC.”
Commenting on that presentation, Samuel J. Klempner, MD, a gastrointestinal medical oncologist at the Massachusetts General Hospital Cancer Center, Boston, noted that the “prospect of a chemo-free regimen for advanced ESCC with the well-studied combination of ipilimumab and nivolumab would represent a welcome addition to our treatment armamentarium.”
No new safety signals
Dr. Chau noted there were no new safety signals with either of the immunotherapies.
Nivolumab and/or chemotherapy were discontinued in 39% of patients and delayed in 71% of patients for an adverse reaction.
Nivolumab and/or ipilimumab were discontinued in 23% of patients and delayed in 46% of patients for an adverse reaction.
The manufacturer cautioned that immunotherapy with nivolumab with or without ipilimumab has been associated with severe and fatal immune-mediated adverse reactions including pneumonitis, colitis, hepatitis and hepatotoxicity, endocrinopathies, nephritis and renal dysfunction, dermatologic adverse reactions, and infusion-related reactions.
A version of this article first appeared on Medscape.com.
The new approval for the drug, a programmed cell death–ligand-1 inhibitor, is for use in this patient population regardless of PD-L1 status.
The indication also specifies that nivolumab is to be used together with chemotherapy (with a fluoropyrimidine- and platinum-containing regimen) or in combination with ipilimumab (Yervoy), an immunotherapy with a different mechanism of action.
“Today’s approvals bring two first-line immunotherapy-based treatment options at once ... to newly diagnosed patients with unresectable advanced or metastatic ESCC,” commented Adam Lenkowsky, a senior vice president at Bristol-Myers Squibb, which makes both nivolumab and ipilimumab.
The approval of the new indication by the Food and Drug Administration was based on improved survival shown in the phase 3 CheckMate-648 trial, which involved nearly 1,000 patients. The trial had three arms and compared nivolumab plus chemotherapy (n = 321) and nivolumab plus ipilimumab (n = 324) with chemotherapy alone (n = 324).
The results showed improved survival with both nivolumab combinations compared with chemotherapy (fluorouracil and cisplatin) alone. Overall survival was improved both in all randomized patients (a secondary endpoint) and in patients whose tumors expressed PD-L1 (≥ 1%), the primary endpoint.
For the combination of nivolumab plus chemotherapy, median overall survival was 13.2 versus 10.7 months, compared with chemotherapy alone in all randomized patients, and 15.4 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
For the combination of nivolumab plus ipilimumab, median overall survival was 12.8 versus 10.7 months with chemotherapy alone in all randomized patients and 13.7 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
However, progression-free survival did not reach statistical significance in any group.
“Unresectable advanced or metastatic ESCC is a challenging disease, and there’s a need for additional treatment options that may extend survival in the first-line setting,” commented Jaffer A. Ajani, MD, professor of gastrointestinal medical oncology at the University of Texas MD Anderson Cancer Center, Houston. He was also the lead U.S. investigator for CheckMate-648 and, in a company press release, said the “two nivolumab-based combinations showed a survival benefit compared to chemotherapy alone, offering new treatment options regardless of PD-L1 status.”
Results from the trial were presented at the 2021 annual meeting of the American Society of Clinical Oncology. At that time, trial investigator Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital in Sutton, England, told attendees that “nivolumab plus chemotherapy and nivolumab plus ipilimumab each represent a new potential first-line standard of care for patients with advanced ESCC.”
Commenting on that presentation, Samuel J. Klempner, MD, a gastrointestinal medical oncologist at the Massachusetts General Hospital Cancer Center, Boston, noted that the “prospect of a chemo-free regimen for advanced ESCC with the well-studied combination of ipilimumab and nivolumab would represent a welcome addition to our treatment armamentarium.”
No new safety signals
Dr. Chau noted there were no new safety signals with either of the immunotherapies.
Nivolumab and/or chemotherapy were discontinued in 39% of patients and delayed in 71% of patients for an adverse reaction.
Nivolumab and/or ipilimumab were discontinued in 23% of patients and delayed in 46% of patients for an adverse reaction.
The manufacturer cautioned that immunotherapy with nivolumab with or without ipilimumab has been associated with severe and fatal immune-mediated adverse reactions including pneumonitis, colitis, hepatitis and hepatotoxicity, endocrinopathies, nephritis and renal dysfunction, dermatologic adverse reactions, and infusion-related reactions.
A version of this article first appeared on Medscape.com.








